Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~` ~ WO 94/11/)90 .~ 1 4 9 3 1 ~ PCI/NO93/00166
,` . .
5~ 1
:
~ ~`
: .
~ :
: ~
Pre-treatment of natural gas to be condensed to liquefied natural
aas (LNG) ~- -
~ : ~ ; : `
`The present invention relates to pre-treatment of natural gas~to
be condensed to liquefi~ed natural gas~(LNG). The said treatment
relates to removal of water and C02 from the natural gas in order
to meet the specification for LNG, regarding water content and
2~
Removal of contaminants ~from natura~ gas~ is an old problem.
Already in the 1920'g it~was known to use amines for binding CO2.
In the thirties processes were developed~for~simultaneous removal
f~C2 and water,~and~also H25 if present.~Th;is gas treatment was
;aimed at meeting~pipeline specifications.~ These processes are
described in US~ patents 2,177,068,~ 2,435,089, 2,518,752 and~
2,547,278, and they all relate'~to application of diethylene
~lycol-monoethanol amine (DEG-MEA~;~;and a~few per~cent~;of water~
This mixture was applicable for treatment of gas~ to be trans~-
ported in pipelines. The content of C02 in the gas could;thereby~
be~brought down t~o;~2-2;.5~mol%, while~thé water~content~would vary
according,to the~temperature the~pipeline,would be exposed to.~A
dew point of~0-10C~was~ mostly use~d~as~standard.
When the natural~gas;is-~to be~condensed ~to~LNG,~ C02~ls usually ~ ~ 3'~
f;irs;t~removed by;~ab50rptl0n~ in an~aqueous solution of~MEA,
diethanol' amine~ DEA~ or similar chemicals.~ Subsequent to this
treatment water~ls absorbed;~on mole~cular;~sleves~which~also~can;be
W094/11090 2~ ~93 ~ PCT/NO~3~00166
~ ,
applied for final removal of CO~ But it is costly and requires
large process units to reach the required specifications for LNG
by such a process, and inherent problems will arise. More than l
ppm H20 in LNG may result in formation of ice in the heat
exchangers, and too much CO2~will result in crystallization of
¦ C2 either in the heat exchangers or in the liquid natural gas
I when the temperature becomes sufficiently low.
i It is further known from the so-called Drizo processj US patent
I No. 3,349,544, to remove water by absorption in for instance
triethylene glycol (TEG) to~an extent that a dew point of -80C
can be attained. This process requires, however, subsequent
stripping of water in several steps. A further disadvantage of
this process is that CO2 is not removed.
The object of the invention was to arrive at a s1mple and
economicaI process for lowering both the water and CO2 content of
natural gas down to the respective LN~ specifications. It was
further desired to lower the water content to such an extent
~ that a dew point of -80C could be atta1ned.
: :~
In order to arrive at an integrated process by which both CO2 and
water were removed from the natural gas! it soon became evident
that the selection of absorption ;solution would be of~ great
importance. In view of that known~ from previous ~attempts to
~ purify natural gas, the inventor found that a new approach to the
¦ problem was necessary. Tests were then performed using an
absorption soluti:on having a double function and comprising two
main components,~one acting as a solvent whi~ch can be a mixture
of solven~s for~èmoving water ~and the~ other component being
compatible w~ith the solvent and ~having the ability~ to react
reversibly with CO2.
The solvent constltutes~the;~ma~or~ part~ of the ~absorpti~n
solution, and it~s~essential property is great affinity for water
~ WO94/11090 21~93~ PCT/NO93/00166
in order to obtain the re~uired drying of the natural gas. Useful
solvents will be ethylene glycols, glycol ethers and normal
methyl pyrrolydone (NMP). TEG is preferred because it can be
applied at temperatures as high as 172-200C without being
degraded. For removal of CO2 (the second component~ alkanolamines
were found to be useful. Also for~the second component the
operating temperature proved to be a selectlon criteria, and MEA
and DEA were found to be most suitable. The ratio between the two
components, solvent: alkanolamine, should~be~in the range of 2-1:
50-1. However, further tests sh~owed that this ratio was not very
critical.
The basic process according to the invention comprises bringing
the crude natural gas in contact with the two-component absorp-
tion solution for simultaneous removal ~of water and C02 and
regenerate the absorption solution by stripping with a large
amount of stripping gas (methanej containing substantlally no
water and C02.
` : ~ ~ :
The treatment with absorption solution is preferably performed at
40-80 bar and 30-50C. Desorption`of C02 from used absorption
solution is preferably performed at 130-200C.
The scope of the inventi~on is as~defined by the attached claims.
: ~: :
. ::
The invention is further described and explained in the following
description of the figures and in the examples.
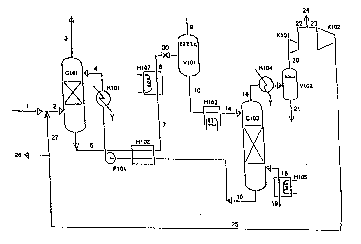
;~ Fig. 1 shows a flow~sheet of the bas~ic process àccording
to the invention. ~ ~
Fig. 2~ shows a~flow~sheet~for a process according to the
invention~comprising split~ absorption and one
desorption~column.~
I WO94/1l090 ~ PCT/NO93/00166 ~ ~
.i ,
'
Fig. 3 shows a process according to the invention com-
prising split absorption and two desorption
columns.
The basic process shown in Fig. 1 comprises one complete
absorption saction. If the raw natural gas~ contains condensed
water, this should be removed in a scrubber prior to the process
described in Fig. 1.
, ~ :
~Natural gas 1 containing water and CO2 is fed to an absorption
column C101. Optionally, this stream 1 might be mixed with
recirculation stream 27 and then fed as stream 2 to C101. In
column C101 water and CO2 are removed by absorption in an
absorption solution 4 fed to the top of said column. Purified
natural gas 3 having desired LNG specifications leaves column
C101 at its top. Used absorption solution 6 is removed from the
~! bottom of C101 and is heat exchanged with regenerated absorption
solution 15 before the latter solutio~ `is pumped by PlO1 and
cooled in Hl01 before its return to~ column C101. The used
absorption solution 7 is further heated in H107 before its
pressure is released over valve 30 and it is fed to the phase
separator V101 where CO2 is removed at the top as a stream 9.;The
bottom fraction 10 from separator Vl01~ is heated in heat
exchanger H103 and then as stream 14 fed to a desorption column
Cl03 where the remaining CO2 and substantially all the water are
removed by means of hot stripping gas 18. The heat exchanger H103
is optional. The bdttom fraction from column C103 is heat
exchanged in H10?~ and H101 and~ returned~to column C101 as~-
previously described. The~used stripping gas 16 leaving~ :column
C103 at its top can~be used as fuel gas~24 or 26. However, it may
~ also be cooled with subsequent separation~of water in V102 and
i`~ ` pressurized by fan K101. This gas~22~;can~then be supplied to the
uel gas net 24 or be~lng compressed in~K102~and recirculated as
st~eam 25 to the crude~natural gas 5tream~l. A bleed 26 can~be
used as high pressure fuel~gas. ~
~ WO94/llO90 214 9 31~ : PCT/NO93/00166
In Fig. 2 the process according to the invention applies split
absorption and one desorption column. Natural gas containing
water and C02 is fed to an absorption column ClOl~ This~natural
gas feed might be mixed with recirculating gas 27 and then fed to
the column ClOl a5 stream 2. In~ column Cl~0~1 water and CO2 are
removed by absorption in the absorption solution which is
supplied as a split feed as streams 5 and 4. Purified gas leaves
at the top of column ClOl. Used absorption solution 6 is removed
from the bottom of column C101 and heat exchanged with regener-
ated absorption solutions 15 and 12 in the;heat exchangers Hl02,
and Hl08 respectively, before the regenerated absorption
solutions are returned to column ClOl as streams 4 and 5. The
used absorption solution is further heated ~in H107 before its~
pressure is released over valve 30. It is then transferred to
phase separator ~101 for removal of CO2 which Ieaves at the top
as stream 9. The bottom fraction 10 from~ separator ~lOl is
separated in a stream 12 which is returned to co~umn C101 through
pump Pl02 and heat exchanger H106, ;and ~ ~stream~13 which can'be
heated in a heat exchanger H103 and then transferred as a~stream
14 to the top of a desorption column Cl03 ln which the remaining
C02~and substantiall~ all the water are r~emoved by means of hot
stripping gas 18. The bottom fraction~ from column C103 is
returned through pipe 15 back to column Cl~Ol. The used stripping
gas 16 can be applied~as fuel gas~ 24,~ 264 or 28, or cooled and
separated from water as~described f~or;;~Fig.~
Fig. 3 describes a process according to the inYention co~prislng
split, absorption and~ two'desorption,~colu,mns~. The ~absorption~ '
sect~ion is'basically~run as~shown~in Fig. 2~. In this embodiment
of;~the process~according to~the~invention two;desorption columns
are used- The bottom~fraction lO from the~separator VlOl is fed
to desorption column ClO2 where most~ of~the~rema ming~Co2;ln the
absorpticn solution~is stripped by the~gas supplied to the column
through~;pipe~17 from the top~of column~C103~. The bottom fraction
from column C102`is split in~one stream 12 which is returned
W094~ll090 ` 2 1 49 3 1 4 PCT/NO93/00166
~ '6
~
j.
to the absorption column C101 and a stream 13 which optionally -" ¦
can be heated in heat exchanger H103 and then fed to the top f ! `
desorption column C103 where the remaining C02 and substantially ''
all the water are removed by the hot stripping gas 18. The used
stripping gas 16 from the top of desorption column Cl02 is then
passed through V102 ~or removal of water and subsequently
pr,essurized in fans KlOl and Kl02 and returned to the feed gas or
alternatively used as fuel gas as explained in connection with
Figs~ 1 and 2.
,
ExamPl e 1
This example refers to the basic process described in Fig. 1.
100,000 Nm3/h of natural gas at 60 bar and:35C and containing
7.5 mol~ C02 is fed to:column ClOl:and brought in contact with an
absorption solution of 340 m3/h. The absorption solution contains
2.5 M MEA and has a: water content of 5 ppm (weight) and a CO2
content corresponding to 0.00001 mol~CO2/mol MEA. Used absorption
solution is heated to 180C and its pressure is released over a :
valve 30, whereupon it is brought:to a separator V101 where the :
pressure is 1.2 bar. The CO2 content in the solution was thereby~ -
reduced from 0.4 mol C02/mol MEA to 0,13 mol C02/mol MEA. The
~temperature was reduaed to 122C during the pressure release. The
solution was then~preheated to 200bC in a heat exchanger H103;and
thereupon fed to the desorption column Cl03 where it was stripped
by 13,000 Nm3/h gas,which did:not contain any water or CO2. The
content of water and C02~in the absorption~solution:was:~th~ereby
reduced down to the level;stated~for~the absorption~solution feed
to the column C101.~
The natural gas~pur,ified:~ as described~above contains less than 3:
lOO ppm~C02 and l~ess~than l~ppm~:water as~it leaves the absorption~
coluDn~_O.
i~
~ WO94/llO90 21 i9314 ~ PCT/NO93/00166
Exa,mPle 2
This example refers to 'the embodiment described Ln Fig. 2.
Natural gas in an amount of 100,000 Nm3/h at 70 bar and 3SC and
containing 6.4 mol% CO2 was`fed to the absorption column C101 in
which it was brought in contact with,an àbsorption solution '
primarily consisting of TEG in an amount of 340 m3/h split in
two streams 4 and 5 as shown in Fig. 2. The MEA concentration was
2.5 M in the absorptlon solution. In the absorption stream 4
-the water content was 5 ppm (weight) and the CO2 content cor-
responding to 0.05 mol CO2/mol MEA~ while the absorption stream
5 had a water content of 3000 ppm (weight) and the CO2 content
corresponding to 0.21 mol/mol MEA. Used absorption solution was
heated to'160C and pressure released and then fed to separator ' ,-
V101 in which the pressure was 1.2 bar. The CO2 content in the
solution was thereby reduced from 0.4 mol CO2/mol MEA to 0.21 mol ~
CO2/mol MEA. During the pressure release the temperature was ~ ,
reduced to 1070C The solution was then spllt ~in two~streams 12
and 13, where the stream 12 was cooled and pumped back to ClOl as
stream 5, while stream 13 was transferrsd`~without being preheated~
to desorption column C103 in which it was~stripped with 10,~000
Nm3/h pre-heated gas being water and CO2 free gas. The content of
water and CO2 in th~e absorption solution was thereby reduced to~ ,
the same level as ~or 5tream 4. The~liquld was cooled and pumped
back to the absorption column.
When the natural gas was purified according to this embodiment of
the invention, the gas leaving column Cl01 contained~less than 50
ppm CO2~and 1 ~ppm ~water. A further ad~antage Qf~this embodiment
of the invention is that smaller~amounts~of stripplng~gas~ls
necessary fox ~obtaining pure~ LNG ~or it can be used~ the~ sam~e
Do-nt ~ 5tripping ga5~ but les~hea=lng~n~H~l~7 and/or;H 103.
WO94/11OgO ` ~ ` PCT/NO93/00166 ~
~ 8 ~`
Example 3
This example refers to the embodiment described in Fig 3
100,000 Nm3/h of natural gas at a pressure of 65 bar and 35C and
having a C02 content of 6 9 mol% was fed to column C101 in which
it was brought in contact with an absorption solution pre-
dominantly consisting of TEG Thi5 solution o~ 340 m3/h comprises
both streams 4 and 5 and it contains MEA in~an amount of 2 5 M
In stream 4 the water content was 3 ppm (weight) and the C02
content corresponding to 0 01 mol CO2/mol MEA, while stream 5 had
a water content of 2750 ppm (weight) and a C02 content corres-
ponding to 0 1 mol/mol MEA
Used absorption solution was heated to 160C and pressure re-
leased over valve 30 and then fed to separator V101 in which the
pressure was 1 2 bar The C02 content in the absorption solution
was thereby reduced from 0 4 mol C02/mol MEA~to 0 21 mol C02/mol
MEA During the pressure release the t~mperature was reduced to
107C The solution was then fed to a desorption column C102 ln
which C02 and water were stripped by gas which had already been
used as stripping gas in desorption column C103 The bottom
fraction from C102 is split in two~streams 12 and 13 where the
stream 12 is cooled and pumped back to the absorption column C101
as stream 5, while stream 13 without being preheated~was fed to
the desorption column C103 in which it was further stripped by
preheated gas which did not contain any water or CO2 The water
and C02 content of the absorption~solution was thereby reduced to
a level as stated for stream 4 The liquid was ~hen cooled~and ~ t
pumped bac~ to the~absorption column
When` the crude natural gas~was treated as ~described in this
example the gas~leaving~column C1~01 had~a~maximum of~SO ppm~C02
and 1 ppm water ~Thus`the advantage by~using two desorptiQn
columrs~r-sult- :n l--- C0z nd~zO in~s-rea- 12, es~ecially~C5~2
:~! ~ WO 94/11090 21~13 ~1~ PCT/NO93/00166
'
.
i~; Accordingly a smaller ClOl unit can be applied for obtaining the
required LNG specifications without increasing the amount of
stripping gas.
,!;
*
As shown by the above examples, water and CO2 can be removed
simultaneously from natural gas by using the process according to
the invention.
The desired LNG specification can also easily be met by the
invention. Thus purified natural gas can also be exposed to
temperatures as low as -80C without getting serious problems
with regard to i~ce or crystallization due to the CO2 or water
content.
: :
: :: ~: ;` : :
The process according to the invention requires only standard
equipment and smaller units than the conventional processes in
which removal of the contaminants has to be performed in several
steps. In fact the equipment specifications required allows it to~
be placed on board ships and platforms. The invention also
utilizes available gas free of water and~CO2 ~for being used as
~lash gas and fuel gas on board. It is also part of this
invention that the fuel gas can be recirculated to the process if
it i9 not required as ~uel gas. The~flexibility of the~process
makes it suitable for application ashore`where the~ requirement
for space to the process equipment is less~lmportant.
The process according to the invention makes~it also possible to
utilize flash gas~from the LNG condensatlon~unit to~purify~the
natural gas prior to the condensation step. This synergy effect~
impro~es the overall~economy~