Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
DEMANDES OU BREVETS VOLUMINEUX
LA PRÉSENTE PARTIE DE CETTE DEMANDE OU CE BREVET
COMPREND PLUS D'UN TOME.
CECI EST LE TOME _ L DE ,~
NOTE: Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
~ 1 5 0
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE
THAN ONE VOLUME
THIS IS VOLUME I OF 2
NOTE: For additional volumes please contact the Canadian Patent Offics
wo 95/09159 2 1 5 0 3 4 5 PCT/JP94/OlSS9
DESCRI PTION
QUINOXALINE DERIVAlIVE AS ANTIDIA~ElIC AGENT
Technical Field
The present invention relates to an antidiabetic agent.
Background Art
Various quinoxaline derivatives have been known. For example,
BE-764998 (= JP-A-46-4377) discloses that the following sulfonylureas are
useful as hypoglycemic agents.
/=\
R-CO-NH-CH2CH2 ~ SO2NHCONHR2
wherein R is a group of the formula:
~X ~
and R2 is alkyl, alkenyl, cycloalkyl, etc. Said compounds are similar to the
compounds of this invention in the basic structure and also in the
pharmacological activity but are distinguished from the compounds of this
invention in the substituent on the phenyl ring in the substituent -CoNR3R4 at 2 -
20 position of quinoxaline ring.
DD-273254-A discloses selective reduction of quinoxaline-di-N -
oxide derivatives of the formula:
0~
R ~ ~N COR2
o
wherein R1 is H, Cl, methyl, or methoxy, and R2 is OH, alkoxy or ~ -
hydroxyethylamino, to give the corresponding N-4-monoxides which are useful
30 as an intermediate for pharmaceuticals and pesticides.
DT-2410852 (= JP-A-50-29583) discloses N-tetrazolyl -
quinoxaline-2-carboxamides of the formula:
WO 95/09159 o ~ 45 PCT/JI'94/01559
- 2 -
R6~C ~CONH~/ "N
R7 H
wherein A is -N=CR2- etc., R2 is H, halogen, alkyl, OR3, NR4R5, etc., and R6
and R7 are H, halogen, alkyl, OR3, NR4R5, etc., which are useful for treating
disorders such as asthma, hay-fever, urticaria, eczema or atopic dermatitis.
EP-23785 discloses substituted alkoxy-phenoxy-quinoxaline(s), N -
oxide(s) thereof of the formula:
A ()k
Y ~ X--CR1R2--(CH2)n W
()m
1 5 wherein A, B, D, E, J, U and V are H, halogen, nitro, CN, amino, mono- or di -
substituted amino, alkyl, alkoxy, carbamoyl, etc.; Y and X are O or S; R1 and R2are H, alkyl, alkenyl, etc.; and W is CN, CSNH2, etc., which are useful as pre-
and post-emergence selective herbicides. EP-26622 discloses also similar
quinoxalinyl-amino-phenoxyalkanoic acids which are useful as pre- and post-
20 emergence selective herbicides.
Heterocycles, Vol. 26, No. 3, 1987, pp. 699-711 discloses
substituted 2-quinoxalinecarboxamides and their N-oxides of the formula:
()I
~N CONH(CH2)n~
()m
wherein R is H or methyl, n is 0 or an integer of 1 to 4,1 and m are 0 or 1, but no
30 pharmacological activity of these compound is mentioned.
J. Chem. Eng. Data, vol. 29, No. 2, pp. 229-231 (1984) discloses
quinoxaline compounds of the formula:
WO 95/09159 ~ t 5 ~ 3 4 5 PCI/JP94/01559
(~)1
[~N CO NH (CH2)2- R
( )m
wherein I and m are 0 or 1, and R is 2-imidazolyl, 3-indolyl, etc. which have
antibacterial activity.
DD-284585-A discloses quinoxaline-1,4-di-N-oxides of the
formula:
[~ R2
wherein R1 is H or optionally substituted alkyl, and R2 is H, optionally
substituted alkyl, or hydroxyethylcarbamoyl, which are useful as a medicinal
feedstuff to protect piglets from gastro-intestinal disorders.
BE-721725 discloses 3-carboxamidoquinoxaline-di-N-(1,4) -
oxides of the formula:
R2
~NXCON--
~N CH2XCOR4
wherein R1 is H, alkyl, alkoxy, or Cl; R2 and R3 are H, optionally substituted
alkyl, or may form with N a 5- or 6- membered heterocyclic ring; X is O or S; and
R4 is optionally substituted alkyl or optionally substituted phenyl, which have
antibacterial activity. Similar compounds are also disclosed in many literaturessuch as BE-721726, BE-721728, BE-738246, BE-742970, DT-2012743, NL -
7305048, BE-846532, GB-1308370, JP-B-46-23264, JP-B-45-24988, and JP-B-
45-24989.
2lSo345
WO 95tO9159 PCI/JP94/OlS59
NL-7206031 discloses 2-formyl-3-carbamoyl-quinoxaline 1,4-
dioxides of the formula:
0~ .
~N XC i l-Z
o
wherein R1 and R2 are H, optionally substituted aliphatic or cycloaliphatic
group, or form a 5- to 7-membered ring optionally containing O or S, Z is NOH
or NNHC(Y)R3, which have antimicrobial activity. Similar compounds are also
disclosed in many literatures such as BE-856771, DT-2639429, DT-2656783,
EP-1618, EP-73390, DE-3230273-A, BE-824065, DT-2501492, BE-828745,
US-3948911, US-4039540, DD-268127, DD-268942-A, JP-A-62-120371, JP-A-
62-123178, EP-288628-A, JP-A-60-120815, BE-856771, and BE-753582.
DE-3324908-A discloses 2-(N-(2-hydroxyethyl)carbamoyl)-3 -
methylquinoxaline 1,4-di-N-oxide of the formula:
o
~N~CONHCH2CH20H
~ N"~CH
which is useful as animal growth promoters. Similar compounds are also
disclosed in other many literatures such as EP-142093-A, DT-2907174, JP-A -
50-082217, GB-2038824, JP-A-62-174061, and JP-A-62-149670.
NL-7206601 discloses 2,3-disubstituted quinoxaline-1,4-dioxides
of the formula:
o
~ ~`X R~
wherein R1 is phenyl optionally substituted by alkyl, alkoxy, halogen or CF3,
and R2 is -CoNR3R4 where R3 and R4 are H or alkyl or form together alkylene
wo gs/ogls9 2 1 5 0 ~ 4 ~ PCI1JP94/01559
optionally containing O, S or N heteroatom, which are useful as bactericides
and amoebicides. Similar compounds are also disclosed in BE-763377, DT -
2228802, and BE-904482.
EP-12725 discloses quinoxaline di-N-oxides of the formula:
O
NXCONR1-A-R4
~N CH2CH2N R2R3
wherein R1 is H or alkyl; R2 and R3 are alkyl or NR2R3 forms 4-5C heterocyclic
ring optionally substituted by alkyl, and R4 is H, methoxy, methylthio, OH, F, Cl,
Br or CN, which are useful as broad-spectrum antimicrobial agents, esp. as
veterinary medicaments, and animal growth promoters. Similar compounds
are also disclosed in DT-2701707.
CH-619456 discloses 6-phenylthio-quinoxaline-1,4-dioxide
derivatives of the formula:
O+
wherein one of A and B is methyl, and the other is -CONHCH2CH2OH, which
are useful as animal growth stimulants. Similar compounds are also disclosed
in CH-619457 and CH-630908.
DT-2052359 discloses quinoxaline 1,4-dioxides of the formula:
,~ O
R2 ~X ~'X ~ R3
wherein Rl and R2 are each H, alkyl or alkoxy, or form together
methylenedioxy; R3 is H, optionally unsaturated aliphatic residue (optionally
substituted by CN, COOH, carbamoyl, alkylamino, etc.), a 5- or 6-membered
WO 9~;/09159 PCT1JP94/01559
~S~3 45
- 6 -
cycloaliphatic residue (optionally substituted by alkyl), arylalkyl or furfuryl; R4 is
H, or NR3R4 is optionally unsaturated 5- or 6-membered heterocyclic ring
optionally substituted by alkyl; and R5 is optionally substituted and optionallyunsaturated aliphatic, cycloaliphatic, araliphatic aromatic or heterocyclic
5 residue, which have antiprotozoal and antibacterial activity. Similar
compounds are also disclosed in DT-2052279.
DT-2120501 discloses 3-substituted quinoxaline-2-carboxamido-
1,4-dioxides of the formula:
o
X~¢ 1~1 CO N R' R "
wherein X is H, methyl, methoxy, CF3, F, Cl, or Br; Y is alkylthio, alkylsulfinyl, or
alkylsulfonyl; R' is H or alkyl; and R" is H or alkyl optionally substituted by amino
alkylamino, dialkylamino, pyrrolidino, piperidino, etc., which are useful as
broad spectrum antibacterial agents, growth stimulants especially for pigs and
poultry. Similar compounds are also disclosed in BE-764088, BE-773396, and
DT-2212932.
US-3185688 discloses quinoxaline derivatives of the formula:
X~N~COZ
H2N J~N NH2
wherein Z is NR(CH2)nNR1R2, NR(CH2)nOalkyl, NR(CH2)nSalkyl; X and R are
H or alkyl; R1 and R2 are alkyl, or NR1 R2 is morpholino, piperidino, pyrrolidino;
and n is 2 - 4, which are useful as tranquilizers. Similar compounds are also
disclosed in US-3192212 and FR-2211006.
Disclosure of Invention
The antidiabetic agent of the present invention comprises as an
active ingredient at least one of quinoxaline derivatives of the formula (1):
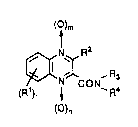
WO 95/091592 1 5 0 3 ~ ~ PCT1Jr94/01559
( l)m
~NXR2 (1)
(R1) ~ ~ R4
()n
wherein R1 is hydrogen atom, a halogen atom, a lower alkyl group, a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent,
R2 is hydrogen atom, a lower alkyl group having optionally a halogen
substituent, phenyl group, a morpholino-substituted lower alkyl group or an
imidazolyl-substituted lower alkyl group,
nandmareeachOor1,
ris 1 or2,
R3 and R4 are the same or different and each a) hydrogen atom; b) a
lower alkyl group; c) a phenyl-lower alkoxycarbonyl group; d) a lower
alkanoyloxy-substituted lower alkyl group; e) a lower alkanoyl group; f) a loweralkoxycarbonyl group; g) a lower alkoxy-lower alkyl group; h) a
phenoxycarbonyl group; i) a lower alkanoyl-substituted lower alkyl group; j) a
20 lower alkoxycarbonyloxy-substituted lower alkyl group; k) a benzoyl-substituted
lower alkyl group having optionally a halogen substituent on the phenyl ring; I)a group of the formula: -E-N(R52)(R53) (in which R52 and R53 are the same or
different and each hydrogen atom, a lower alkyl group, a lower alkoxycarbonyl
group or phenyl group, or R52 and R53 may combine together with the nitrogen
25 atom to which they bond to form a 5- or 6-membered saturated heterocyclic
group with or without being intervened with another nitrogen atom or oxygen
atom, E is a lower alkylene group, a group of the formula: -CO-, or a group of
the formula: -CO-A- (in which A is a lower alkylene group)); m) a group of the
formula:
WO 95/09159 ~ S ~3 ~S PCI/JP94/01559
- 8 -
A Rs4
0~0
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); n) a group of the formula:
(Rs)p
A~
10 (in which A is the same as defined above, p is an integer of 1 to 3, R5 is
hydrogen atom, a lower alkoxy-substituted lower alkoxy group, a lower alkoxy
group, an amino group having optionally a lower alkyl substituent, a halogen
atom, nitro group, hydroxy group, a lower alkyl group having optionally a
hydroxy substituent, a lower alkenyloxy group, a carboxyl-substituted lower
15 alkoxy group, a lower alkoxycarbonyl-substituted lower alkoxy group, a lower
alkoxycarbonyl group, a halogen-substituted lower alkoxy group, a hydroxy -
substituted lower alkoxy group, a phenyl-lower alkoxy group having optionally
a substituent selected from a lower alkyl group and a lower alkoxy group on the
phenyl moiety, a 1,3-dioxolanyl group having optionally a lower alkyl
20 substituent, a lower alkanoyl group, a morpholino-substituted lower alkoxy
group, a morpholino-substituted lower alkyl group, a morpholinocarbonyl group
or a group of the formula: -Y-A1-CONR6R7 (in which A1 is a lower alkylene
group, Y is a group of the formula: -O- or a group of the formula: -NH-, R6 and
R7 are the same or different and each hydrogen atom, a lower alkyl group
25 having optionally a hydroxy substituent, a phenyl-lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, a furyl-substituted
lower alkyl group, or a lower alkoxy-substituted lower alkyl group, or R6 and R7may combine together with the adjacent nitrogen atom to which they bond to
form a 5- or 6-membered saturated heterocyclic group with or without being
30 intervened with nitrogen atom or oxygen atom, said heterocyclic group having
optionally 1 to 3 substituents selected from hydroxy group, a lower alkyl group
and a phenyl-lower alkyl group)); o) a phenyl-lower alkenyl group having
optionally a substituent selected from a lower alkoxy group, a halogen atom, an
WO 95/09159 2 1 5 0 3 ~ 5 PCTIJP94/01559
amino group having optionally a substituent selected from a lower alkanoyl
group and a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted
lower alkoxy group, a tetrazolyl group having optionally a lower alkyl
substituent on the tetrazole moiety, hydroxy group, a group of the formula: -O -A4-CO-NR40R41 (in which A4 is a lower alkylene group, R40 and R41 are the
same or different and each hydrogen atom or a lower alkyl group, or R40 and
R41 may combine together with the adjacent nitrogen atom to which they bond
to form a 5- or 6-membered saturated heterocyclic group with or without being
intervened with another nitrogen atom or oxygen atom), a lower alkenyloxy
group, nitro group and a lower alkyl group having optionally a halogen
substituent on the phenyl moiety; p) an alkenyl group; q) a cycloalkyl-lower
alkyl group; r) a naphthyl-lower alkyl group; s) a phenylthio-substituted lower
alkyl group having optionally a lower alkoxy substituent on the phenyl moiety; t)
a phenylsulfinyl-substituted lower alkyl group having optionally a lower alkoxy
substituent on the phenyl moiety; u) a phenylsulfonyl-substituted lower alkyl
group having optionally a lower alkoxy substituent on the phenyl moiety; v) a
phenoxy-substituted lower alkyl group; w) a group of the formula:
(R8)q
~
(in which q is an integer of 1 to 3, a group of the formula:
is a lower alkyl group substituted by a 5- to 1 4-membered saturated or
25 unsaturated heteromonocyclic, heterobicyclic or heterotricyclic group having 1
to 4 heteroatoms selected from nitrogen atom, oxygen atom and sulfur atom, R8
substitutes on the above heterocyclic group, and is hydrogen atom, oxo group,
a lower alkyl group having optionally a hydroxy substituent, a halogen atom,
nitro group, a lower alkoxy group, cyano group, a lower alkoxycarbonyl group,
30 a phenyl-lower alkoxy group having optionally an amino group having
optionally a lower alkanoyl substituent on the phenyl moiety, a carboxyl -
substituted lower alkoxy group, carboxyl group, a lower alkoxycarbonyl -
substituted lower alkoxy group, hydroxy group, a lower alkoxy-substituted lower
WO 95/09159 PCT/JP94/01559
,2150345
- 10-
alkoxy group, a lower alkenyloxy group, a lower alkanoyloxy-substituted lower
alkyl group, a halogen-substituted lower alkyl group, a lower alkanoyl group, a
phenyl group having optionally a substituent selected from a lower alkyl group,
a lower alkoxy-substituted lower alkoxy group, hydroxy group, a halogen atom
5 and a lower alkoxy group on the phenyl moiety, a lower alkenyl group, a
morpholinocarbonyl-lower alkoxy group, a lower alkylsufinyl group, an amino -
substituted lower alkyl group having optionally a substituent selected from a
lower alkylsulfonyl group and a lower alkanoyl group, a lower alkylthio group, alower alkylsulfonyl group, a lower alkanoyloxy group, a 1,3-dioxolanyl -
10 substituted lower alkyl group having optionally a lower alkyl substituent, alower alkanoyl-substituted lower alkyl group, an aminocarbonyl-substituted
lower alkyl group having optionally a lower alkyl substituent, a lower
alkoxycarbonyl-substituted lower alkenyl group, an aminocarbonyl-substituted
lower alkenyl group having optionally a lower alkyl substituent, a carboxyl -
15 substituted lower alkenyl group, benzoyl group, a lower alkoxy-lower alkyl
group, a group of the formula:
(R45)s
20 (in which s is an integer of 1 to 3, a group of the formula:
is a 5- to 6-membered saturated or unsaturated heterocyclic group having 1 to
4 hetero atoms selected from nitrogen atom, oxygen atom and sulfur atom, R45
25 bonds to said heterocyclic group and is hydrogen atom, a lower alkyl group, alower alkoxy-substituted lower alkyl group, phenyl group or oxo group), a group
of the formula:
(R46)
~<
~)
(in which t is an integer of 1 to 3, a group of the formula:
WO 95/09159 2 1 5 0 3 ~ ~ PCI/JP94/01559
is a lower alkyl group substituted by a 5- to 6-membered saturated or
unsaturated heterocyclic group having 1 to 4 hetero atoms selected from
nitrogen atom, oxygen atom and sulfur atom, R46 bonds to said heterocyclic
group and is hydrogen atom, a lower alkyl group or oxo group), or a group of
the formula: -(C=O)INR9R10 (in which I is 0 or 1, R9 and R10 are the same or
different and each hydrogen atom, a lower alkanoyl group, a lower alkyl group,
a morpholinocarbonyl-lower alkyl group, a cycloalkylcarbonyl group, a phenyl -
lower alkenylcarbonyl group, a lower alkyisulfonyl group, an aminocarbonyl
group having optionally a lower alkyl substituent, a phenylsulfonyl group
having optionally a lower alkyl substituent on the phenyl moiety, a phenyl-loweralkenyl group, a benzoyl group having optionally 1 to 3 substituents selected
from a halogen atom, a lower alkoxy group, an amino group having optionally a
lower alkanoyl substituent and hydroxy group on the phenyl moiety, an amino -
substituted lower alkanoyl group having optionally a lower alkanoyl substituent,an amino-substituted sulfonyl group having optionally a lower alkyl substituent,a phenyl-lower alkyl group, phenyl group, or an amino group having optionally
a lower alkanoyl substituent, or R9 and R10 may combine together with the
adjacent nitrogen atom to which they bond to form a 5- or 6-membered
saturated heterocyclic group with or without being intervened with another
nitrogen atom or oxygen atom)); x) a group of the formula: -AS-CR42R43R44 (in
which A5 is a lower alkylene group, R42 and R43 combine together to form a
group of the formula: =O, or =N-OH or a lower alkylenedioxy group, and R44 is
a phenyl group having optionally a lower alkoxy substituent on the phenyl
moiety); y) a 2,3-dihydro-1H-indenyl-substituted lower alkyl group having
optionally a substituent selected from oxo group, hydroxy group and a silyloxy
group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring; or z)
a group of the formula:
(R47)
(in which u is an integer of 1 to 3, a group of the formula:
WO 95109159 PCT/JP94101559
t ~1.S34S
- - 12-
{~
is a lower alkenyl group substituted by a 5- to 1 4-membered saturated or
5 unsaturated, heteromonocyclic, heterobicyclic or heterotricyclic group having 1
to 4 hetero atoms selected from nitrogen atom, oxygen atom and sulfur atom,
R47 bonds on said heterocyclic group and is hydrogen atom, a halogen -
substituted lower alkyl group, oxo group, a halogen atom, a lower alkoxy group,
a lower alkyl group, a lower alkoxycarbonyl group, carboxyl group, an
10 aminocarbonyl group having optionally a lower alkyl substituent, an amino
group having optionally a lower alkanoyl substituent, a phenyl group having
optionally a substituent selected from a lower alkoxy group and a halogen atom
on the phenyl moiety, or a group of the formula:
(R45)s
~
(in which A6, R45 and s are the same as defined above)),
or R3 and R4 may combine together with the adjacent nitrogen atom to
which they bond to form 1,2,3,4-tetrahydroisoquinolyl group, said heterocyclic
20 group having optionally a lower alkoxy substituent,
or a salt thereof.
The present invention provides also novel quinoxaline derivatives
of the formula (1 ) as described above wherein all the symbols are the same as
defined above except that n is 0 and the group R5 excludes hydrogen atom and
25 further provided that when R1 is hydrogen atom, R2 is methyl group, R3is
hydrogen atom, and m is 0, then R4is not 2-(imidazol-2-yl)ethyl, 2-(indol-3 -
yl)ethyl, or sec-butyl,
and a salt thereof.
According to the diseases determination by WHO, diabetes is
30 caused by the absolute lack of the secretion of insulin and arises either acutely
or subacutely, and includes, insulin dependent diabetes mellitus (IDDM) which
requires the insulin-treatment, non insulin dependent diabetes mellitus
(NIDDM) which does not always require insulin-treatment, malnutrition-related
WO 95/OglS9 2 1 5 0 3 4 5 PCT/JP94/01559
diabetes mellitus (MRDM), other complications accompanied with other
diseases.
Among the above, a cause of IDDM is estimated to be the
destruction of pancreatis 13-cells by auto-immune system. The pancreatis ~ -
5 cells are considered to be destroyed by HLA antigen, cytokine virus, etc. (cf.Koji NAKANISHI, Tetsuro KOBAYASHI, Mitsuru HARA; Tonyobyogaku
(Diabetology) 1989: edited by Mikinori KOSAKA, Yasuo AKANUMA, Shindan-
to-Chiryo sha, 1989, pages 226-244). On the other hand, the cause of NIDDM
is estimated to be (i) congenital anomaly in pancreatin, i.e. anomaly in
10 adaptability to the increase in insulin consumption, or (ii) disorder in insulin
activity induced by various factors such as aging, obesity, stress, etc. (Hiroo
IMURA; Tonyobyogaku-no-Shinpo (Progress in Diabetology) 1989, No. 23,
edited by Japan Diabetology Association, Shindan-to-Chiryo sha, 1989, pages
1 to12).
However, the crisis of diabetes is not simple but happens in
complicated situation, such as hereditary factors, environmental factors, etc.,
and it has not been clarified yet.
In viewpoint of the tissue of the patient of NIDDM, the important
causes of hyperglycemia is considered to be the decrease in the uptake of
glucose at the peripheral tissue, especially at the muscle, and the increase in
glucose secretion at the liver. Hitherto, the most common drug therapy for
diabetes is treatment with insulin or sulfonylurea agent (agent for promotion ofinsulin secretion), which are both based on the supplement of insulin, but thesetreatment have difficulty in strict blood glucose level control, and sometimes
they induce hyperinsulin serum or hypoglycemia. Accordingly, it has been
considered that a compound which promotes the uptake of glucose at the
muscle without promoting the secretion of insulin would be a new kind of
hypoglycemic agent without hyperinsulinism nor hypoglycemia and would be
useful for treatment of diabetes.
The quinoxaline derivatives of the above formula (1 ) and salts
thereof (hereinafter referred to as the compounds of the present invention)
promote the uptake of 2-deoxyglucose (2DG) against L6 cells, cell line of rat
WO 95tO91S9 PCT/JP94101559
?t~503 ~5
- 14-
striated muscle (muscular cells) and also promote the consumption of glucose
by which they show hypoglycemic activity. Especially, the compounds of the
present invention show hypoglycemic activity in db/db mice and KK-Ay mice,
which are diabetic animal model (L. Herberg, D.L. Coleman: Metabolism, vol.
26, No. 1 (January), 1977, pp. 59-99). The compounds of the present invention
promote the uptake of glucose at the muscle and do not affect the insulin
secretion and the glucose release at the liver so that they do not show acute
hypoglycemic activity and do not affect oral glucose tolerance test.
Accordingly, the antidiabetic agent of the present invention is
useful for treatment of diabetes and diabetic complications such as diabetic
blood vessel disorder, diabetic retinopathy, diabetic nephropathy, diabetic
neuropathy, etc.
Each group in the above formula (1 ) specially means the
following groups.
The lower alkyl group includes a straight chain or branched chain
alkyl group having 1 to 6 carbon atoms such as methyl, ethyl, propyl, isopropyl,butyl, tert-butyl, pentyl, hexyl, etc.
The halogen atom is fluorine atom, chlorine atom, bromine atom
or iodine atom.
The lower alkyl group having optionally a halogen substituent
includes, for example, in addition to the above mentioned lower alkyl groups, a
straight chain or branched chain alkyl group having 1 to 6 carbon atoms having
optionally 1 to 3 halogen substituents, such as trifluoromethyl, trichloromethyl,
chloromethyl, bromomethyl, fluoromethyl, iodomethyl, difluoromethyl, dibromo -
methyl, 2-chloroethyl, 2,2,2-trifluoroethyl, 2,2,2-trichloroethyl, 3-chloropropyl,
2,3-dichloropropyl, 4,4,4-trichlorobutyl, 4-fluorobutyl, 5-chloropentyl, 3-chloro-2 -
methylpropyl, 5-bromohexyl, 5,6-dichlorohexyl, 5-bromohexyl, 5,6-dichloro-
hexyl, etc.
The morpholino-substituted lower alkyl group includes a
morpholino-substituted alkyl group wherein the alkyl moiety is a straight chain
or branched chain alkyl group having 1 to 6 carbon atoms, for example,
morpholinomethyl, 2-morpholinoethyl, 1-morpholinoethyl, 3-(2-morpholinyl)-
propyl, 4-(3-morpholinyl)butyl, 1,1-dimethyl-2-(2-morpholinyl)ethyl, 5-
WO 95109159 2 1 ~ O ~ 4 ~ PCTIJP94101559
- 15-
morpholinopentyl, 6-morpholinohexyl, 2-methyl-3-morpholinopropyl, etc.
The imidazolyl-substituted lower alkyl group includes an
imidazolyl-substituted alkyl group wherein the alkyl moiety is a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms, for example, (1 -
imidazolyl)methyl, 2-(1-imidazolyl)ethyl, 1-(2-imidazolyl)ethyl, 3-(4-imidazolyl)-
propyl, 4-(5-imidazolyl)butyl, 1,1-dimethyl-2-(2-imidazolyl)ethyl, 5-(4-
imidazolyl)pentyl, 6-(1-imidazolyl)hexyl, 2-methyl-3-(1-imidazolyl)propyl, etc.
The lower alkylene group includes a straight chain or branched
chain alkylene group having 1 to 6 carbon atoms, for example, methylene,
ethylene, trimethylene, 2-methyltrimethylene, 2,2-dimethyltrimethylene, 1 -
methyltrimethylene, methylmethylene, ethylmethylene, tetramethylene,
pentamethylene, hexamethylene, etc.
The lower alkoxy group includes a straight chain or branched
chain alkoxy group having 1 to 6 carbon atoms, for example, methoxy, ethoxy,
propoxy, isopropoxy, butoxy, tert-butoxy, pentyloxy, hexyloxy, etc.
The amino group being optionally substituted by a lower alkyl
group includes an amino group which may optionally be substituted by 1 to 2
substituents of a straight chain or branched chain alkyl group having 1 to 6
carbon atoms, for example, amino, methylamino, ethylamino, propylamino,
isopropylamino, butylamino, tert-butylamino, pentylamino, hexylamino,
dimethylamino, diethylamino, dipropylamino, dibutylamino, dipentylamino,
dihexylamino, N-methyl-N-ethylamino, N-ethyl-N-propylamino, N-methyl-N-
butylamino, N-methyl-N-hexylamino, and the like.
The lower alkyl group having optionally a hydroxy substituent
includes a straight chain or branched chain alkyl group having 1 to 6 carbon
atoms which may optionally have 1 to 3 hydroxy substituents, for example,
hydroxymethyl, 2-hydroxyethyl, 1-hydroxyethyl, 1-hydroxyisopropyl, 3-hydroxy-
propyl, 2,3-dihydroxypropyl, 4-hydroxybutyl, 1,1-dimethyl-2-hydroxyethyl, 5,5,4 -
trihydroxypentyl, 5-hydroxypentyl, 6-hydroxyhexyl, 2-methyl-3-hydroxypropyl,
2,3-dihydroxyethyl, 3,4-dihydroxybutyl, 5,6-dihydroxyhexyl, and the like.
The lower alkenyloxy group includes a straight chain or branched
chain alkenyloxy group having 2 to 6 carbon atoms, for example, allyloxy, 2 -
butenyloxy, 3-butenyloxy, 1-methylallyloxy, 2-pentenyloxy, 2-hexenyloxy, and
W095/09159 . 215~1~4S pCTlJP94/01559
- 16-
the like.
The carboxy-substituted lower alkoxy group includes a carboxy -
alkoxy group wherein the alkoxy moiety is a straight chain or branched chain
alkoxy group having 1 to 6 carbon atoms, for example, carboxymethoxy, 2 -
carboxyethoxy, 1-carboxyethoxy, 3-carboxypropoxy, 4-carboxybutoxy, 5 -
carboxypentyloxy, 6-carboxyhexyloxy, 1,1-dimethyl-2-carboxyethoxy, 2-methyl -
3-carboxypropoxy, and the like.
The lower alkoxycarbonyl-substituted lower alkoxy group includes
a straight chain or branched chain alkoxy group having 1 to 6 carbon atoms
which is substituted by a straight chain or branched chain alkoxycarbonyl
group having 1 to 6 carbon atoms in the alkoxy moiety, for example, methoxy -
carbonylmethoxy, 3-methoxycarbonylpropoxy, ethoxycarbonylmethoxy, 3-
ethoxycarbonylpropoxy, 4-ethoxycarbonylbutoxy, 5-isopropoxycarbonyl-
pentyloxy, 6-propoxycarbonylhexyloxy, 1,1-dimethyl-2- butoxycarbonylethoxy, 2-
1 5 methyl-3-tert-butoxycarbonylpropoxy, 2-pentyloxycarbonylethoxy, hexyloxy - carbonylmethoxy, and the like.
The lower alkoxycarbonyl group includes a straight chain or
branched chain alkoxycarbonyl group having 1 to 6 carbon atoms in the alkoxy
moiety, for example, methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
isopropoxycarbonyl, butoxycarbonyl, tert-butoxycarbonyl, pentyloxycarbonyl,
hexyloxycarbonyl, and the like.
The halogen-substituted lower alkoxy group includes a straight
chain or branched chain alkoxy group having 1 to 6 carbon atoms which has 1
to 3 halogen substituents, for example, trifluoromethoxy, trichloromethoxy,
chloromethoxy, bromomethoxy, fluoromethoxy, iodomethoxy, difluoromethoxy,
dibromomethoxy, 2-chloroethoxy, 2,2,2-trifluoroethoxy, 2,2,2-trichloroethoxy, 3 -
chloropropoxy, 2,3-dichloropropoxy, 4,4,4-trichlorobutoxy, 4-fluorobutoxy, 5 -
chloropentyloxy, 3-chloro-2-methylpropoxy, 5-bromohexyloxy, 5,6-dichloro-
hexyloxy, 5-bromohexyloxy, 5,6-dichlorohexyloxy, and the like.
The hydroxy-substituted lower alkoxy group includes a straight
chain or branched chain alkoxy group having 1 to 6 carbon atoms which is
substituted by 1 to 3 hydroxy groups, for example, hydroxymethoxy, 2-hydroxy -
ethoxy, 1-hydroxyethoxy, 1-hydroxypropoxy, 3-hydroxypropoxy, 2,3-dihydroxy-
WO 95/09159 2 1 5 0 3 ~ 5 PCT/JP94101559
propoxy, 4-hydroxybutoxy, 1,1-dimethyl-2-hydroxyethoxy, 5,5,4-trihydroxy-
pentyloxy, 5-hydroxypentyloxy, 6-hydroxyhexyloxy, 1-hydroxyisopropoxy, 2-
methyl-3-hydroxypropoxy, 2,3-dihydroxyethoxy, 3,4-dihydroxybutoxy, 5,6 -
dihydroxyhexyloxy, and the like.
The phenyl-lower alkoxy group having optionally a substituent
selected from a lower alkyl group and a lower alkoxy group on the phenyl
moiety includes a phenylalkoxy group wherein the alkoxy moiety is a straight
chain or branched chain alkoxy group having 1 to 6 carbon atoms which may
optionally have 1 to 3 substituents selected from a straight chain or branched
chain alkoxy group having 1 to 6 carbon atoms and a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms on the phenyl moiety,
for example, benzyloxy, 2-phenylethoxy, 1-phenylethoxy, 3-phenylpropoxy, 4 -
phenylbutoxy, 1,1-dimethyl-2-phenylethoxy, 5-phenylpentyloxy, 6-phenyl-
hexyloxy, 2-methyl-3-phenylpropoxy, 2-(3-methoxyphenyl)ethoxy, 1-(4-
methoxyphenyl)ethoxy, 2-methoxybenzyloxy, 3-(2-ethoxyphenyl)propoxy, 4-(3-
ethoxyphenyl)butoxy, 1,1-dimethyl-2-(4-ethoxyphenyl)ethoxy, 5-(4-isopropoxy-
phenyl)pentyloxy, 6-(4-hexyloxyphenyl)hexyloxy, 3,4-dimethoxybenzyloxy,
3,4,5-trimethoxybenzyloxy, 2,5-dimethoxybenzyloxy, 3-methoxybenzyloxy, 4 -
methoxybenzyloxy, 2,4-diethoxybenzyloxy, 2,3-dimethoxybenzyloxy, 2,4 -
dimethoxybenzyloxy, 2,6-dimethoxybenzyloxy, 2-methylbenzyloxy, 4-ethyl -
benzyloxy, 2-(3-methylphenyl)ethoxy, 1-(4-methylphenyl)ethoxy, 3-(2-ethyl -
phenyl)propoxy, 4-(3-ethylphenyl)butoxy, 1,1-dimethyl-2-(4-ethylphenyl)ethoxy,
5-(4-isopropylphenyl)pentyloxy, 6-(4-hexyphenyl)hexyloxy, 3,4-dimethyl-
benzyloxy, 3,4,5-trimethylbenzyloxy, 2,5-dimethylbenzyloxy, 2-methoxy-3 -
methylbenzyloxy, and the like.
The 1,3-dioxolanyl group having optionally a lower alkyl
substituent includes a 1,3-dioxolanyl group which may optionally be substituted
by 1 to 3 straight chain or branched chain alkyl groups having 1 to 6 carbon
atoms, for example, 1,3-dioxolanyl, 2-methyl-1,3-dioxolanyl, 4-ethyl-1,3 -
dioxolanyl, 2-propyl-1,3-dioxolanyl, 4-butyl-1,3-dioxolanyl, 2-pentyl-1,3-
dioxolanyl, 4-hexyl-1,3-dioxolanyl, 2,4-dimethyl-1,3-dioxolanyl, 2,4,5-trimethyl -
1,3-dioxolanyl, and the like.
The lower alkanoyl group includes a straight chain or branched
WO 95tO91S9 PCTIJP94/OlS59
2~,~o3~s
- 18-
chain alkanoyl group having 1 to 6 carbon atoms, for example, formyl, acetyl,
propionyl, butyryl, isobutyryl, pentanoyl, t-butylcarbonyl, hexanoyl, and the like.
The morpholino-substituted lower alkoxy group includes a
morpholino-substituted alkoxy group wherein the alkoxy moiety is a straight
5 chain or branched chain alkoxy group having 1 to 6 carbon atoms, for example,
morpholinomethoxy, 2-morpholinoethoxy, 1-morpholinoethoxy, 3-(2-
morpholinyl)propoxy, 4-(3-morpholinyl)butoxy, 1,1-dimethyl-2-(2-morpholinyl) -
ethoxy, 5-morpholinopentyloxy, 6-morpholinohexyloxy, 2-methyl-3-morpholino-
propoxy, and the like.
The phenyl-lower alkyl group includes a phenyl-alkyl group
wherein the alkyl moiety is a straight chain or branched chain alkyl group
having 1 to 6 carbon atoms, for example, benzyl, 2-phenylethyl, 1-phenylethyl,
3-phenylpropyl, 4-phenylbutyl, 5-phenylpentyl, 6-phenylhexyl, 1,1-dimethyl-2-
phenylethyl, 2-methyl-3-phenylpropyl, and the like.
The phenyl-lower alkyl group having optionally a lower alkoxy
substituent on the phenyl moiety includes a phenyl-alkyl group wherein the
alkyl moiety is a straight chain or branched chain alkyl group having 1 to 6
carbon atoms and may optionally have 1 to 3 straight chain or branched chain
alkoxy substituents having 1 to 6 carbon atoms on the phenyl moiety, for
example, in addition to the above mentioned phenyl-lower alkyl group, 2-(3 -
methoxyphenyl)ethyl, 1-(4-methoxyphenyl)ethyl, 2-methoxbenzyl, 3-methoxy-
benzyl, 4-methoxybenzyl, 3-(2-ethoxyphenyl)propyl, 4-(3-ethoxyphenyl)butyl,
1,1-dimethyl-2-(4-ethoxyphenyl)ethyl, 5-(4-isopropoxyphenyl)pentyl, 6-(4-hexyl-
oxyphenyl)hexyl, 3,4-dimethoxybenzyl, 2,4-dimethoxybenzyl, 3,4,5-trimethoxy-
benzyl, and the like.
The furyl-substituted lower alkyl group includes a furyl-substituted
alkyl group wherein the alkyl moiety is a straight chain or branched chain alkylgroup having 1 to 6 carbon atoms, for example, (2-furyl)methyl, 2-(3-furyl)ethyl,
1-(2-furyl)ethyl, 3-(2-furyl)propyl, 4-(3-furyl)butyl, 5-(2-furyl)pentyl, 6-(3-furyl) -
hexyl, 1,1-dimethyl-2-(2-furyl)ethyl, 2-methyl-3-(3-furyl)propyl, and the like.
The lower alkoxy-lower alkyl group includes a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms which is substituted by
a straight chain or branched chain alkoxy group having 1 to 6 carbon atoms, for
WO 9S/09159 2 1 5 0 3 4 5 PCT/JP94101S59
,,
- 19-
example, methoxymethyl, 2-ethoxyethyl, 1-methoxyethyl, 3-methoxypropyl, 4 -
ethoxybutyl, 6-propoxyhexyl, 5-isopropoxypentyl, 1,1-dimethyl-2-butoxyethyl, 2-
methyl-3-tert-butoxypropyl, 2-pentyloxyethyl, hexyloxymethyl, and the like.
The phenyl-lower alkenyl group having optionally a substituent
selected from a lower alkoxy group, a halogen atom, an amino group having
optionally a substituent selected from a lower alkanoyl group and a phenyl -
lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy group, a
tetrazolyl group having optionally a lower alkyl substituent on the tetrazole ring,
hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (A4, R40 and R41
are the same as defined above), a lower alkenyloxy group, nitro group and a
lower alkyl group having optionally a halogen substituent on the phenyl moiety
includes a phenylalkenyl group wherein the alkenyl moiety is a straight chain
or branched chain alkenyl group having 2 to 6 carbon atoms and having 1 to 2
double bonds, and the phenyl moiety may opitionally be substituted by 1 to 3
groups selected from a straight chain or branched chain alkoxy group having 1
to 6 carbon atoms, a halogen atom, an amino group having optionally 1 to 2
substituents selected from a straight chain or branched chain alkanoyl group
having 1 to 6 carbon atoms and a phenylalkenylcarbonyl group wherein the
alkenylcarbonyl moiety is a straight chain or branched chain alkenylcarbonyl
group having 3 to 6 carbon atoms in the alkenylcarbonyl moiety, a straight
chain or branched chain alkoxy group having 1 to 6 carbon atoms which is
substituted by a straight chain or branched chain alkoxy group having 1 to 6
carbon atoms, a tetrazolyl group having optionally a straight chain or branched
chain alkyl group having 1 to 6 carbon atoms on the tetrazole ring, hydroxy
group, a group of the formula: -O-A4-Co-NR4R41 (A4 is a straight chain or
branched chain alkylene group having 1 to 6 carbon atoms, R40 and R41 are
the same or different and each hydrogen atom or a straight chain or branched
chain alkyl group having 1 to 6 carbon atoms, or R40 and R41 may combine
together with the adjacent nitrogen atom to which they bond to form a 5- or 6 -
membered saturated heterocyclic ring with or without being intervened with
another nitrogen atom or oxygen atom), a straight chain or branched chain
alkylenyloxy group having 2 to 6 carbon atoms, nitro group, and a straight
chain or branched chain alkyl group having 1 to 6 carbon atoms having
WO9S/09159 , ~,,,ts~34S PCT/JP94/01559
- 20 -
optionally 1 to 3 halogen substituents, for example, styryl, cinnamyl, 4-phenyl-3 -
butenyl, 4-phenyl-2-butenyl, 5-phenyl-4-pentenyl, 5-phenyl-3-pentenyl, 5-
phenyl-2-pentenyl, 6-phenyl-5-hexenyl, 6-phenyl-4-hexenyl, 6-phenyl-3-
hexenyl, 6-phenyl-2-hexenyl, ~-methyl-4-phenyl-3-butenyl, ~-methyl-cinnamyl,
~-methyl-cinnamyl, 5-phenyl-2,4-pentadienyl, 4-phenyl-1,3-butadienyl, 6-
phenyl-2,4-hexadienyl, 6-phenyl-2,4-hexadienyl, 6-phenyl-3,5-hexadienyl, 6-
phenyl-1,3-hexadienyl, 5-phenyl-1,3-pentadienyl, 3-methoxycinnamyl, 4-
methoxycinnamyl, 2-methoxycinnamyl, 4-methoxystyryl, (2-ethoxyphenyl)-2 -
butenyl, 5-(3-ethoxyphenyl)-4-pentenyl, a,a-dimethyl-4-ethoxycinnamyl, 5-(4-
isopropoxyphenyl)-2,4-pentadienyl, 6-(4-hexyloxyphenyl)-2-hexenyl, 3,4-
dimethoxycinnamyl, 3,4,5-trimethoxystyryl, 2,5-dimethoxystyryl, 3-methoxy -
styryl, 4-methoxystyryl, 2,4-diethoxystyryl, 2,3-dimethoxycinnamyl, 2,4 -
dimethoxycinnamyl, 2,6-dimethoxycinnamyl, 2-nitrocinnamyl, 3-nitrocinnamyl,
4-nitrocinnamyl, 4-(2-nitrophenyl)-3-butenyl, 4-(3-nitrophenyl)-2-butenyl, 5-(2-nitrophenyl)-2-pentenyl, 6-(3-nitrophenyl)-3-hexenyl, 3,4-dinitrocinnamyl, 3,4,5-
trinitrocinnamyl, 2-nitrostyryl, 3-nitrostyryl, 4-nitrostyryl, 3-methylcinnamyl, 2 -
methylstyryrl, 4-methylcinnamyl, 2-ethylcinnamyl, 4-isopropylcinnamyl, 4-(3 -
ethylphenyl)-3-butenyl, a-methyl-4-isopropylcinnamyl, 5-(4-isopropylphenyl)-2-
pentenyl, 6-(4-hexylphenyl)-2-hexenyl, 3,4-dimethylcinnamyl, 3,4,5-trimethyl-
cinnamyl, 2,5-dimethylcinnamyl, 2-chlorocinnamyl, 3-chlorostyryl, 2-fluoro-
cinnamyl, 4-chlorocinnamyl, 2-fluorostyryl, 4-(3-fluorophenyl)-2-butenyl, 5-(4 -fluorophenyl)-2-pentenyl, a,a-dimethyl-2-bromocinnamyl, 6-(3-bromophenyl)-2-
hexenyl, 4-bromostyryl, 2-iodocinnamyl, 3-iodostyryl, 3,4-dichlorocinnamyl, 3,5 -
dichlorocinnamyl, 2,6-dichlorostyryl, 2,3-dichlorocinamyl, 2,4-dichlorostyryl, 3,4-
difluorocinnamyl, 3,5-dibromocinnamyl, 3,4,5-trichlorocinnamyl, 2-methoxy-3-
chlorocinnamyl, 3-(4-acetylaminophenyl)-2-butenyl, 3-(2-trifluoromethylphenyl)-
2-butenyl, 3-[4-(1-methyltetrazol-5-yl)phenyl]-2-butenyl, 3-(4-cinnamoylamino-
phenyl)-2-butenyl, 3-(3-methoxymethoxyphenyl)-2-butenyl, 3-(2-methoxy-
phenyl)-2-butenyl, 3-(3-methoxyphenyl)-2-butenyl, 3-(4-trifluoromethylphenyl)-
2-butenyl, 3-(3-trifluoromethylphenyl)-2-butenyl, 3-(3-acetylaminophenyl)-2-
butenyl, 3-(3-hydroxyphenyl)-2-butenyl, 3-(3-morpholinocarbonylmethoxy-
wo 9S/O9lS9 ~ 1 5 0 3 ~ ~ PCT/JP94/OlSS9
.. _
- 21 -
phenyl)-2-butenyl, 3-(3-diethylaminocarbonylmethoxyphenyl)-2-butenyl, 3-[3-(2-
methyl-2-propenyloxy)phenyl]-2-butenyl, 4-chloromethylstyryl, 3-bromomethyl-
cinnamyl, 4-(2-iodomethylphenyl)-3-butenyl, 4-[4-(2,2,2-trichloroethyl)phenyl]-2-
butenyl, 5-(4-aminophenyl)-4-pentenyl, 5-(3-propionylaminophenyl)-2-
pentenyl, 6-(2-butyrylaminophenyl)-5-hexenyl, 6-(4-pentanoylaminophenyl)-4-
hexenyl, 6-(3-hexanoylaminophenyl)-3-hexenyl, 6-(2,4-diaminophenyl)-2-
hexenyl, 2,4,6-triaminocinnamyl, 4-(3-butenoylamino)styryl, 3-(2-pentenoyl -
amino)cinnamyl, 4-[2-(4-hexenoylamino)phenyl]-3-butenyl, 4-[4-(4-ethoxy-
butoxy)phenyl]-2-butenyl, 3-[4-(N-acetyl-N-cinnamoylamino)phenyl]-2-butenyl,
1 0 5-[2-(6-propoxyhexyloxy)phenyl]-4-pentenyl, 6-[3-(2-pentyloxyethoxy)phenyl]-2 -
pentenyl, 6-(4-hexyloxymethoxyphenyl)-5-hexenyl, 6-[2-(1,1-dimethyl-2-butoxy-
ethoxy)phenyl]-3-hexenyl, 3-(2,4-dimethoxymethoxyphenyl)-2-butenyl, 3-(2,4,6-
trimethoxyphenyl)-2-butenyl, 3-[4-(1-ethyltetrazol-5-yl)phenyl]-2-butenyl, 3-[3-(2-
propyltetrazol-5-yl)phenyl]-2-butenyl, 3-[2-(1-butyltetrazol-5-yl)phenyl]-2-
butenyl, 3-[4-(2-pentyltetrazol-5-yl)phenyl]-2-butenyl, 3-[3-(1-hexyltetrazol-5-yl)-
phenyl]-2-butenyl, 2-hydroxycinnamyl, 3-hydroxycinnamyl, 4-hydroxycinnamyl,
4-(2-hydroxyphenyl)-3-butenyl, 5-(2-hydroxyphenyl)-2-pentenyl, 6-(3-hydroxy-
phenyl)-3-hexenyl, 2,4-dihydroxycinnamyl, 3,4,5-trihydroxycinnamyl, 4-hydroxy-
cinnamyl, 4-allyloxystyryl, 3-(2-butenyloxy)cinnamyl, 4-[2-(3-butenyloxy)phenyl] -
3-butenyl, 5-[3-(2-pentenyloxy)phenyl]-2-pentenyl, 6-[4-(2-hexenyloxy)phenyl]-
4-hexenyl, 2,4-diallyloxycinnamyl, 2,4,6-triallyloxystyryl, 3-(2-dimethylamino-
carbonylethoxyphenyl)-2-butenyl, 4-[4-(3-butylaminocarbonylpropoxy)phenyl]-
3-butenyl, 5-[2-(4-pentylaminocarbonylbutoxy)phenyl]-3-pentenyl, 6-[3-(5-hexyl-
aminocarbonylpentyloxy)phenyl]-5-hexenyl, 4-[6-(N-methyl-N-propylamino)-
carbonylhexyloxy]styryl, 4-methylaminocarbonylmethoxycinnamyl, 4-(1 -
piperidinyl)carbonylmethoxycinnamyl, 3-(1-piperazinyl)carbonylmethoxystyryl,
4-[3-(1-pyrrolidinyl)carbonylmethoxyphenyl]-3-butenyl, 3-(2-methoxy-5-chloro-
phenyl)-2-butenyl, 3-(2-methoxymethoxy-5-chlorophenyl)-2-butenyl, 3-(2-
hydroxy-5-chlorophenyl)-2-butenyl, and the like.
The alkenyl group includes a straight chain or branched chain
alkenyl group with 1 to 3 double bonds having 2 to 12 carbon atoms, for
example, vinyl, allyl, 3-methyl-2-butenyl, 2-butenyl, 3-butenyl, 1-methylallyl, 2 -
pentenyl, 2-hexenyl, 1-heptenyl, 1-octenyl, 1-nonenyl, 1-decenyl, 1-undecenyl,
3 ~ PCTJJ~94tO1559
wo gS/oglS9 2~ ~
- 22 -
2-dodecenyl, 2-heptenyl, 3-heptenyl, 3-methyl-4-heptenyl, 2-methyl-5-heptenyl,
4-methyl-2-heptenyl, 3-methyl-1-heptenyl, 1,3-heptadienyl, 1,4-heptadienyl, 1,5-heptadienyl, 1,6-heptadienyl, 2,4-heptadienyl, 2-methyl-2,4-heptadienyl, 2,6-
dimethyl-2,4-heptadienyl, 2,5-dimethyl-1,3-heptadienyl, 2,4,6-trimethyl-2,4-
heptadienyl, 2-octenyl, 3-octenyl, 4-octenyl, 2-methyl-5-octenyl, 3-methyl-6 -
octenyl, 2-methyl-7-octenyl, 1,3-octadienyl, 1,4-octadienyl, 1,5-octadienyl, 1,6 -
octadienyl, 1,7-octadienyl, 2,4-octadienyl, 3,7-octdienyl, 4,8-dimethyl-3,7 -
octadienyl, 2,4,6-trimetyl-3,7-octadienyl, 3,4-dimethyl-2,5-octadienyl, 3,7 -
dimethyl-2,6-octadienyl, 4,8-dimethyl-2,6-octadienyl, 2-nonenyl, 3-nonenyl, 4-
nonenyl, 2-methyl-5-nonenyl, 2-methyl-6-nonenyl, 2-methyl-7-nonenyl, 2 -
methyl-8-nonenyl, 1,3-nonadienyl, 1,4-nonadienyl, 1,5-nonadienyl, 1,6-
nonadienyl, 1,7-nonadienyl, 1,8-nonadienyl, 2,4-nonadienyl, 3,7-nonadienyl,
4,8-dimethyl-3,7-nonadienyl, 2,4,6-trimethyl-3,7-nonadienyl, 3,4-dimethyl-2,5-
nonadienyl, 4,8-dimethyl-2,6-nonadienyl, 2-decenyl, 3-decenyl, 4-decenyl, 5 -
decenyl, 2-methyl-6-decenyl, 3-methyl-7-decenyl, 4-methyl-8-decenyl, 5-methyl -
9-decenyl, 1,3-decadienyl, 1,4-decadienyl, 1,5-decadienyl, 1,6-decadienyl, 1,7-
decadienyl, 1,8-decadienyl, 1,9-decadienyl, 2-methyl-2,4-decadienyl, 3-methyl -
2,5-decadienyl, 4,8-dimethyl-2,6-decadienyl, 2,4,6-trimethyl-3,7-decadienyl,
2,9-dimethyl-3,7-decadienyl, 2-undecenyl, 3-undecenyl, 4-undecenyl, 5-
undeceyl, 2-methyl-6-undecenyl, 3-methyl-7-undecenyl, 4-methyl-8-undecenyl,
5-methyl-9-undecenyl, 2-methyl-10-undecenyl, 1,3-undecadienyl, 1,4-
undecadienyl, 1,5-undecadienyl, 1,6-undecadienyl, 1,7-undecadienyl, 1,8-
undecadienyl, 1,9-undecadienyl, 1,10-undecadienyl, 2-methyl-2,4-
undecadienyl, 3-methyl-2,5-undecadienyl, 4,8-dimethyl-2,6-undecadienyl,
2,4,6-trimethyl-3,8-undecadienyl, 2,9-dimethyl-3,8-undecadienyl, 2-dodecenyl,
3-dodecenyl, 4-dodecenyl, 5-dodecenyl, 6-dodecenyl, 2-methyl-7-dodecenyl, 3-
methyl-8-dodecenyl, 4-methyl-9-dodecenyl, 5-methyl-10-dodecenyl, 6-methyl-
11-dodecenyl, 2-methyl-2,4-dodecadienyl, 3-methyl-2,5-dodecadienyl, 4,8-
dimethyl-2,6-dodecadienyl, 2,4,6-trimethyl-2,7-dodecadienyl, 2,10-dimethyl-2,8-
dodecadienyl, 2,5-dimethyl-3,7-dodecadienyl, 4,8,12-trimethyl-3,7,11-dodeca-
trienyl, 1,3,5-heptatrienyl, 2,4,6-octatrienyl, 1,3,6-nonatrienyl, 2,6,8 -
dodecatienyl, 1,5,7-undecatrienyl, and the like.
The cycloalkyl-lower alkyl group includes a straight chain or
WO 95/O91S9 2 1 5 0 3 4 5 PCT/JP94/01559
- 23 -
branched chain alkyl group having 1 to 6 carbon atoms, which is substituted by
a cycloalkyl group having 3 to 8 carbon atoms, for example, cyclohexylmethyl,
2-cyclopropylethyl, 1-cyclobutylethyl, 3-cyclopentylpropyl, 4-cyclohexylbutyl,
2,2-dimethyl-3-cycloheptylpropyl, 5-cyclooctylpentyl, 6-cyclohexylhexyl, and
5 the like.
The naphthyl-lower alkyl group includes a naphthyl-alkyl group
wherein the alkyl moiety is a straight chain or branched chain alkyl group
having 1 to 6 carbon atoms, for example, a-naphthylmethyl, ~-naphthylmethyl,
2-(a-naphthyl)ethyl, 1-(~-naphthyl)ethyl, 3-(~-naphthyl)propyl, 4-(a-naphthyl)-
10 butyl, 2-methyl-3-(a-naphthyl)propyl, 5-(~-naphthyl)pentyl, 6-(a-naphthyl)hexyl,
1,1-dimethyl-2-(~-naphthyl)ethyl, and the like.
The phenylthio-substituted lower alkyl group having optionally a
lower alkoxy substituent on the phenyl moiety includes a phenylthio-alkyl group
wherein the alkyl moiety is a straight chain or branched chain alkyl group
15 having 1 to 6 carbon atoms and may optionally have 1 to 3 substituents of
straight chain or branched chain alkoxy group having 1 to 3 carbon atoms on
the phenyl moiety, for example, phenylthiomethyl, 2-phenylthioethyl, 1 -
phenylthioethyl, 3-phenylthiopropyl, 4-phenylthiobutyl, 5-phenylthiopentyl, 6-
phenylthiohexyl, 1,1-dimethyl-2-phenylthioethyl, 2-methyl-3-phenylthiopropyl,
20 (2-methoxyphenylthio)methyl, (3-methoxyphenylthio)methyl, 2-(4-methoxy-
phenylthio)ethyl, 1-(2-ethoxyphenylthio)ethyl, 3-(4-isopropoxyphenylthio)-
propyl, 4-(3-pentyloxyphenylthio)butyl, 5-(4-hexyloxyphenylthio)pentyl, 6-(2-
butyloxyphenylthio)hexyl, (3,4-dimethoxyphenylthio)methyl, (3-ethoxy-4-
methoxyphenylthio)methyl, 2-(2,3-dimethoxyphenylthio)ethyl, 1-(2,6-dimethoxy-
25 phenylthio)ethyl, 2-(3,4,5-trimethoxyphenylthio)ethyl, and the like.
The phenylsulfinyl-substituted lower alkyl group having optionally
a lower alkoxy substituent on the phenyl moiety includes a phenylsulfinylalkyl
group wherein the alkyl moiety is a straight chain or branched chain alkyl grouphaving 1 to 6 carbon atoms, and may optionally have 1 to 3 substituents of
30 straight chain or branched chain alkoxy group having 1 to 6 carbon atoms on
the phenyl moiety, for example, phenylsulfinylmethyl, 2-phenylsulfinylethyl, 1-
W095/O91S9 o~4S PCTtJP94/015S9
- 24 -
phenylsulfinylethyl, 3-phenylsulfinylpropyl, 4-phenylsulfinylbutyl, 5-phenyl-
sulfinylpentyl, 6-phenylsulfinylhexyl, 1,1-dimethyl-2-phenylsulfinylethyl, 2-
methyl-3-phenylsulfinylpropyl, (2-methoxyphenylsulfinyl)methyl, (3-methoxy-
phenylsulfinyl)methyl, 2-(4-methoxyphenylsulfinyl)ethyl, 1-(2-ethoxyphenyl-
5 suflinyl)ethyl, 3-(4-isopropoxyphenylsulfinyl)propyl, 4-(3-pentyloxyphenyl-
sulfinyl)butyl, 5-(4-hexyoxyphenylsulfinyl)pentyl, 6-(2-butyloxyphenylsulfinyl)-hexyl, (3,4-dimethoxyphenylsulfinyl)methyl, 3-ethoxy-4-methoxyphenylsulfinyl)-
methyl, 2-(2,3-dimethoxyphenylsulfinyl)ethyl, 1-(2,6-dimethoxyphenylsulfinyl)-
ethyl, 2-(3,4,5-trimethoxyphenylsulfinyl)ethyl, and the like.
The phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety includes a
phenylsulfonyl-alkyl group wherein the alkyl moiety is a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms, which may optionally
have 1 to 3 substituents of straight chain or branched chain alkoxy group
15 having 1 to 6 carbon atoms on the phenyl moiety, for example, phenylsulfonyl -
methyl, 2-phenylsulfonylethyl, 1-phenylsulfonylethyl, 3-phenylsulfonylpropyl, 4-phenylsulfonylbutyl, 5-phenylsulfonylpentyl, 6-phenylsulfonylhexyl, 1,1-
dimethyl-2-phenylsulfonylethyl, 2-methyl-3-phenylsulfonylpropyl, (2-methoxy-
phenylsulfonyl)methyl, (3-methoxyphenylsulfonyl)methyl, 2-(4-methoxyphenyl-
20 sulfonyl)ethyl, 1-(2-ethoxyphenylsulfonyl)ethyl, 3-(4-isopropoxyphenylsulfonyl)-
propyl, 4-(3-pentyloxyphenylsulfonyl)butyl, 5-(4-hexyloxyphenylsulfonyl)pentyl,
6-(2-butyloxyphenylsulfonyl)hexyl, 3,4-dimethoxyphenylsulfonylmethyl, 3-
ethoxy-4-methoxyphenylsulfonyl)methyl, 2-(2,3-dimethoxyphenylsulfonyl)ethyl,
1-(2,6-dimethoxyphenylsulfonyl)ethyl, 2-(3,4,5-trimethoxyphenylsulfonyl)ethyl,
25 and the like.
The 5- to 14-membered saturated or unsaturated hetero -
monocyclic, heterobicyclic or heterotricyclic group having 1 to 4 heteroatoms
selected from nitrogen atom, oxygen atom and sulfur atom includes, for
example, pyrrolidinyl, piperidinyl, piperazinyl, morpholino, pyridyl, thienyl,
30 quinolyl, 1,4-dihydroquinolyl, benzothiazolyl, pyrazinyl, pyrimidyl, pyridazinyl,
pyrrolyl, carbostyril, 3,4-dihydrocarbostyril, 1,2,3,4-tetrahydroquinolyl, indolyl,
isoindolyl, indolinyl, benzimidazolyl, benzoxazolyl, imidazolidinyl, isoquinolyl,
quinazolidinyl, quinoxalinyl, cinnonyl, phthalazinyl, chromanyl, isoindolinyl,
WO 95/09159 PCT/JP94101559
21~03~
- 25 -
isochromanyl, pyrazolyl, imidazolyl, pyrazolidinyl, 2,3-dihydrobenzofuryl,
perhydrobenzofuryl, benzofuryl, benzothienyl, 4H-chromenyl, 1,3,4 -
oxadiazolyl, 1,2,4-triazolyl, 1,2,3,4-tetrazolyl, 1,3,4-triazolyl, 1,2,4-oxadiazolyl,
2,3-dihydrobenzofuryl, perhydrobenzofuryl, 5-1H-indazolyl, furyl, pyrrolinyl,
5 nolyl, oxazolyl, isoxazolyl, thiazolyl, thiazolidinyl, 1,2,3,5-oxathiadiazolyl,
isothiazolyl, pyranyl, pyrazolidinyl, quinuclidinyl, 1,4-benzoxazinyl, 3,4-dihydro-
2H-1,4-benzoxazinyl, 1,4-benzothiazinyl, 1,2,3,4-tetrahydroquinoxalinyl, 1,3-
dithia-2,4-dihydronaphthalenyl, 1,4-dithianaphthalenyl, furo[3,2-c]pyridyl,
furo[2,3-g~quinolyl, 3,4-dihydrofuro[2,3-g]quinolyl, 1,2,3,4-tetrahydrofuro[2,3-
g]quinolyl, 1,4-benzodioxanyl, 1,2,4-triazinyl, naphtho[2,1-b]furyl, imidazo[1,2-
a]pyridyl, and the like.
The lower alkyl group which is substituted by a 5- to 14 -
membered saturated or unsaturated heteromonocyclic, heterobicyclic or
heterotricyclic group having 1 to 4 heteroatoms selected from nitrogen atom,
oxygen atom and sulfur atom includes, for example, pyrrolidinylmethy, 2 -
piperidinylethyl, 3-piperazinylpropyl, 4-morpholinobutyl, (2-pyridyl)methyl, (3-pyridyl)methyl, (2-thienyl)methyl, (3-quinolyl)methyl, 5-(6-quinolyl)pentyl, 6-(1,4-
dihydro-2-quinolyl)hexyl, (2-benzothiazolyl)methyl, 2-(3-pyrazinyl)ethyl, 1-(2-
pyrimidyl)ethyl, 3-(3-pyridazinyl)propyl, 4-(2-pyrrolyl)butyl, 5-(3-carbostyril) -
pentyl, 6-(3,4-dihydrocarbostyril-6-yl)hexyl, (1,2,3,4-tetrahydroquinolyl-8-
yl)methyl, (2-indolyl)methyl, (3-indolyl)methyl, 2-(3-indolyl)ethyl, (4-isoindolyl)-
methyl, 2-(3-indolinyl)ethyl, (2-benzoimidazolyl)methyl, 3-(5-benzoxazolyl)-
propyl, 4-(4-imidazolidinyl)butyl, 5-(1-isoquinolyl)pentyl, 6-(7-quinazolidinyl)-
hexyl, (8-quinoxalinyl)methyl, 1-(4-cinnolinyl)ethyl, 3-(5-phthalazinyl)propyl, 4-
(6-chromanyl)butyl, 5-(4-isoindolinyl)pentyl, 6-(7-isochromanyl)hexyl, (3-
pyrazolyl)methyl, 2-(2-imidzolyl)ethyl, 3-(3-pyrazolidinyl)propyl, (2-benzofuryl)-
methyl, (3-benzofuryl)methyl, 4-(6-benzofuryl)butyl, (2-benzothienyl)methyl, (3-benzothienyl)methyl, 5-(5-benzothienyl)pentyl, [6-(4H-chromenyl)]methyl, (2,3-
dihydro-2-benzofuryl)methyl, (2-perhydrobenzofuryl)methyl, (5-1H-indazolinyl)-
methyl, thienylmethyl, 1-(5-isoindolinyl)ethyl, 3-(2-imidazolinyl)propyl, 4-(2 -pyrrolinyl)butyl, (2-furyl)methyl, (4-oxazolyl)methyl, (5-oxazolyl)methyl, 5-(4-oxazolyl)pentyl, 6-(3-isoxazolyl)hexyl, (4-thiazolyl)methyl, (2-thiazolyl)methyl, 2-
(3-isothiazolyl)ethyl, (2-pyranyl)methyl, 3-(3-pyrazolidinyl)propyl, 4-(2-
WO 95/09159 PCT/JP94/01559
,2lso345
- 26 -
pyrazolinyl)butyl, 5-(2-quinuclidinyl)pentyl, (1,4-benzoxazin-6-yl)methyl, (3,4-dihydro-2H- 1 ,4-benzoxazin-2-yl)methyl, ( 1 ,4-benzothiazin-5-yl)methyl, ( 1 ,2,3,4 -
tetrahydroquinoxalinyl-6-yl)methyl, (1,3-dithia-2,4-dihydronaphthalen-6-yl)-
methyl, (1,4-dithianaphthalen-7-yl)methyl, (5-thiazolyl)methyl, (1,3,4-
oxadiazolin-5-yl)methyl, (1,2,4-triazol-5-yl)methyl, (1,2,3,4-tetrazol-5-yl)methyl,
(1,3,4-triazol-5-yl)methyl, (1,2,4-oxadiazol-5-yl)methyl, (1,2,4-triazin-3-yl)-
methyl, (thiazolidin-5-yl)methyl, (1,2,3,5-oxathiazolin-4-yl)methyl, (3-furyl)-
methyl, (2-furyl)methyl, (2-imidazolyl)methyl, 2-(5-thiazolyl)ethyl, 1-(1,3,4-
oxadiazolin-2-yl)ethyl, 3-(1,2,4-triazol-3-yl)propyl, 4-(1,2,3,4-tetrazol-5-yl)butyl,
6-(1,3,4-triazol-2-yl)hexyl, 2-(1,2,4-oxadiazol-3-yl)ethyl, 1-(1,2,4-triazin-5-yl) -
ethyl, 3-(thiazolidin-2-yl)propyl, 4-(1,2,3,5-oxathiadiazolin-4-yl)butyl, 5-(furo[3,2-
c]pyridin-2-yl)pentyl, 6-(furo[2,3-g]quinolin-7-yl)hexyl, (3,4-dihydrofuro[2,3-g] -
quinolin-8-yl)methyl, 2-(1,2,3,4-tetrahydrofuro[2.3-g]quinolin-4-yl)ethyl, (1,4-benzodioxan-2-yl)methyl, 1-(1,4-benzodioxadin-3-yl)ethyl, (2,3-dihydrobenzo-
furan-2-yl)methyl, (perhydrobenzofuran-2-yl)methyl, naphtho[2,1-b]furylmethyl,
4-(naphtho[2,1-b]furyl)pentyl, imidazo[1,2-a]pyridylmethyl, 2-(imidazo[1,2-a] -
pyridyl)ethyl, 1-(imidazo[1,2-a]pyridyl)ethyl, and the like.
The phenoxy-substituted lower alkyl group includes a
phenoxyalkyl group wherein the alkyl moiety is a straight chain or branched
chain alkyl group having 1 to 6 carbon atoms, for example, phenoxymethyl, 2 -
phenoxyethyl, 1-phenoxyethyl, 3-phenoxypropyl, 4-phenoxybutyl, 5-phenoxy-
pentyl, 6-phenoxyhexyl, 1,1-dimethyl-2-phenoxyethyl, 2-methyl-3-phenoxy-
propyl, and the like.
The phenyl-lower alkoxy group which may optionally have an
amino substituent having optionally a lower alkanoyl substituent on the phenyl
moiety includes a phenylalkoxy group, wherein the alkoxy moiety is a straight
chain or branched chain alkoxy group having 1 to 6 carbon atoms, which may
optionally have 1 to 3 substituents of amino group having optionally a straight
chain or branched chain alkanoyl group having 1 to 6 carbon atoms, for
example, benzyloxy, 2-phenylethoxy, 1-phenylethoxy, 3-phenylpropoxy, 4 -
phenylbutoxy, 5-phenylpentyloxy, 6-phenylhexyloxy, 1,1-dimethyl-2-phenyl-
ethoxy, 2-methyl-3-phenylpropoxy, 4-acetylaminobenzyloxy, 2-(2-propionyl -
aminophenyl)ethoxy, 1-(3-butyrylaminophenyl~ethoxy, 3-(4-pentanoylamino-
WO 95/09159 2 1 5 0 3 4 ~ PCT/JP94/01559
- 27 -
phenyl)propoxy, 4-(3-tert-butylcarbonylaminophenyl)butoxy, 5-(4-hexanoyl -
aminophenyl)pentyloxy, 6-(3,4-bisacetylaminophenyl)hexyloxy, 3,4,5-triacetyl-
aminobenzyloxy, 2,4-bisacetylaminobenzyloxy, 4-aminobenzyloxy, 2,3-
diaminobenzyloxy, 2,4,6-triaminobenzyloxy, 2-(3-aminophenyl)ethoxy, 3-(2 -
5 aminophenyl)propoxy, and the like.
The lower alkoxy-substituted lower alkoxy group includes a
straight chain or branched chain alkoxy group having 1 to 6 carbon atoms
which is substituted by a straight chain or branched chain alkoxy group having
1 to 6 carbon atoms, for example, methoxymethoxy, 3-methoxypropoxy, 4 -
ethoxybutoxy, 6-propoxyhexyloxy, 5-isopropoxypentyloxy, 1,1-dimethyl-2-
butoxyethoxy, 2-methyl-3-tert-butoxypropoxy, 2-pentyloxyethoxy, hexyloxy-
methoxy, and the like.
The lower alkanoyloxy-substituted lower alkyl group includes a
straight chain or branched chain alkyl group having 1 to 6 carbon atoms which
is substituted by a straight chain or branched chain alkanoyloxy group having 2
to 6 carbon atoms, for example, acetyloxymethyl, 2,2-dimethylpropionyloxy -
methyl, propionyloxymethyl, 2-propionyloxyethyl, 1-acetyloxyethyl, 1-butyryl -
oxyethyl, 3-acetyloxypropyl, 4-isobutyryloxybutyl, 5-pentanoyloxypentyl, 6-tert -
butylcarbonyloxyhexyl, 1,1-dimethyl-2-hexanoyloxyethyl, 2-methyl-3-acetyloxy-
propyl, and the like.
The halogen-substituted lower alkyl group includes a straight
chain or branched chain alkyl group having 1 to 6 carbon atoms which is
substituted by 1 to 3 halogen atoms, for example, trifluoromethyl, trichloro -
methyl, chloromethyl, bromomethyl, fluoromethyl, iodomethyl, difluoromethyl,
dibromomethyl, 2-chloroethyl, 2,2,2-trifluoroethyl, 2,2,2-trichloroethyl, 3-
chloropropyl, 2,3-dichloropropyl, 4,4,4-trichlorobutyl, 4-fluorobutyl, 5-chloro-pentyl, 3-chloro-2-methylpropyl, 5-bromohexyl, 5,6-dichlorohexyl, 5-bromo -
hexyl, 5,6-dichlorohexyl, and the like.
The tetrazolyl group having optionally a lower alkyl substituent on
the tetrazole ring includes a tetrazolyl group having optionally a straight chain
or branched chain alkyl group having 1 to 6 carbon atoms on the tetrazole ring,
for example, tetrazolyl, 1-methyltetrazolyl, 2-methyltetrazolyl, 5-ethyltetrazolyl, 5 -
propyltetrazolyl, 1-butyltetrazolyl, 2-pentyltetrazolyl, 1-hexyltetrazolyl, and the
WO 9S/09159 PCI/JP94/01559
2,~5a34s
- 28 -
like.
The phenyl group having optionally a substituent selected from a
lower alkyl group, a lower alkoxy-substituted lower alkoxy group, hydroxy
group, a halogen atom and a lower alkoxy group includes a phenyl group
which may optionally have 1 to 3 substituents selected from a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms, a straight chain or
branched chain alkoxy group having 1 to 6 carbon atoms which is substituted
by a straight chain or branched chain alkoxy group having 1 to 6 carbon atoms,
hydroxy group, a halogen atom and a straight chain or branched chain alkoxy
group having 1 to 6 carbon atoms, for example, phenyl, 2-methylphenyi, 3 -
methylphenyl, 4-methylphenyl, 2-ethylphenyl, 3-ethylphenyl, 4-ethylphenyl, 3 -
isopropylphenyl, 4-hexylphenyl, 3,4-dimethylphenyl, 2,5-dimethylphenyl, 3,4,5-
trimethylphenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2-
ethoxyphenyl, 3-ethoxyphenyl, 4-ethoxyphenyl, 4-isopropoxyphenyl, 4-pentyl -
oxyphenyl, 2,4-dimethoxyphenyl, 4-hexyloxyphenyl, 3,4-dimethoxyphenyl, 3-
ethoxy-4-methoxyphenyl, 2,3-dimethoxyphenyl, 3,4-diethoxyphenyl, 2,5 -
dimethoxyphenyl, 2,6-dimethoxyphenyl, 3,5-dimethoxyphenyl, 3,4-dipentyloxy-
phenyl, 3,4,5-trimethoxyphenyl, 2-chlorophenyl, 3-chlorophenyl, 4-chloro-
phenyl, 2-fluorophenyl, 3-fluorophenyl, 4-fluorophenyl, 2-bromophenyl, 3-
bromophenyl, 4-bromophenyl, 2-iodophenyl, 3-iodophenyl, 4-iodophenyl, 3,4-
dichlorophenyl, 3,5-dichlorophenyl, 2,6-dichlorophenyl, 2,3-dichlorophenyl, 2,4-dichlorophenyl, 3,4-difluorophenyl, 3,5-dibromophenyl, 3,4,5-trichlorophenyl, 2 -
methoxy-5-chlorophenyl, 3-chloro-4-methoxyphenyl, 3-methoxy-5-iodophenyl,
3,4-dimethoxy-5-bromophenyl, 3,5-diiodo-4-methoxyphenyl, 2-hydroxy-5-
chlorophenyl, 2-methoxymethoxy-5-chlorophenyl, 2-hydroxyphenyl, 3-hydroxy-
phenyl, 4-hydroxyphenyl, 2,3-dihydroxyphenyl, 3,4-dihydroxyphenyl, 3,4,5-
trihydroxyphenyl, 2-methoxymethoxyphenyl, 3-(3-methoxypropoxy)phenyl, 4-(4-
ethoxybutoxy)phenyl, 2-(6-propoxyhexyloxy)phenyl, 3-(5-isopropoxypentyloxy)-
phenyl, 4-(1,1-dimethyl-2-butoxyethoxy)phenyl, 2-(2-methyl-3-tert-butoxy-
propoxy)phenyl, 3-(2-pentyloxyethoxy)phenyl, 4-hexyloxymethoxyphenyl, 2,3-
dimethoxymethoxyphenyl, 3,4,5-trimethoxymethoxyphenyl, and the like.
The lower alkenyl group includes a straight chain or branched
chain alkenyl group having 2 to 6 carbon atoms, for example, vinyl, allyl, 2-
WO 95/09159 ~ 1 S 0 3 4 5 PCI/JP94/01559
- 29 -
butenyl, 3-butenyl, 1-methylallyl, 2-pentenyl, 2-hexenyl, and the like.
The morpholinocarbonyl-lower alkoxy group includes a
morpholinocarbonylalkoxy group wherein the alkoxy moiety is a straight chain
or branched chain alkoxyl group having 1 to 6 carbon atoms, for example,
5 morpholinocarbonylmethoxy, 2-morpholinocarbonylethoxy, 1-morpholino-
carbonylethoxy, 3-(2-morpholinocarbonyl)propoxy, 4-(3-morpholinocarbonyl)-
butoxy, 1,1-dimethyl-2-(2-morpholinylcarbonyl)ethoxy, 5-morpholinylcarbonyl-
pentyloxy, 6-morpholinocarbonylhexyloxy, 2-methyl-3-morpholinocarbonyl-
propoxy, and the like.
The morpholinocarbonyl-lower alkyl group includes a
morpholinocarbonylalkyl group wherein the alkyl moiety is a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms, for example,
morpholinocarbonylmethyl, 2-morpholinocarbonylethyl, 1-morpholinocarbonyl-
ethyl, 3-(2-morpholinocarbonyl)propyl, 4-(3-morpholinocarbonyl)butyl, 1,1 -
dimethyl-2-(2-morpholinocarbonyl)ethyl, 5-morpholinocarbonylpentyl, 6-
morpholinocarbonylhexyl, 2-methyl-3-morpholinocarbonylpropyl, and the like.
The cycloalkylcarbonyl group includes a cycloalkylcarbonyl group
having 3 to 8 carbon atoms in the cycloalkyl moiety, for example, cyclopropyl -
carbonyl, cyclobutylcarbonyl, cyclopentylcarbonyl, cyclohexylcarbonyl,
cycloheptylcarbonyl, cyclooctylcarbonyl, and the like.
The phenyl-lower alkenylcarbonyl group includes a phenylalkenyl -
carbonyl group wherein the alkenylcarbonyl moiety is a straight chain or
branched chain alkenylcarbonyl group having 3 to 6 carbon atoms in the
alkenylcarbonyl moiety, for example, cinnamoyl, 4-phenyl-2-butenoyl, 4-phenyl -
3-butenoyl, 5-phenyl-4-pentenoyl, 5-phenyl-3-pentenoyl, 5-phenyl-2-
pentenoyl, 6-phenyl-5-hexenoyl, 6-phenyl-4-hexenoyl, 6-phenyl-3-hexenoyl, 6-
phenyl-2-hexenoyl, 2-methyl-4-phenyl-3-butenoyl, 2-methyl-cinnamoyl, 1 -
methyl-cinnamoyl, and the like.
The 5- to 6-membered saturated heterocyclic group which is
formed by combining R6 and R7, R9 and R10, R40 and R41 or R52 and R53
together with the adjacent nitrogen atom with or without being intervening with
nitrogen atom or oxygen atom, for example, pyrrolidinyl, piperidinyl,
piperazinyl, morpholino, and the like.
WO 9S/09159 PCI/JI'94/OlS59
~S03~5
- 30-
The above heterocyclic group having 1 to 3 substituents selected
from hydroxy group, a lower alkyl group and a phenyl-lower alkyl group
includes the above mentioned heterocyclic groups having 1 to 3 sustituents
selected from hydroxy group, a straight chain or branched chain alkyl group
5 having 1 to 6 carbon atoms, and a phenylalkyl group wherein the alkyl moiety
is a straight chain or branched chain alkyl group having 1 to 6 carbon atoms,
for example, 3-hydroxypyrrolidinyl, 2-hydroxypyrrolidinyl, 4-hydroxypiperidinyl,3-hydroxypiperidinyl, 2-hydropiperidinyi, 3-hydroxypiperazinyl, 2-hydroxy-
piperazinyl, 3-hydroxymorpholino, 2-hydroxymorpholino, 4-benzylpiperazinyl,
3-(2-phenylethyl)pyrrolidinyl, 2-(3-phenylpropyl)pyrrolidinyl, 4-(4-phenylbutyl)-
piperidinyl, 3-(5-phenylpentyl)morpholino, 2-(6-phenylhexyl)piperazinyl, 4-
methylpiperazinyl, 3,4-dimethylpiperazinyl, 3-ethylpyrrolidinyl, 2-propyl-
pyrrolidinyl, 3,4,5-trimethylpiperidinyl, 4-butylpiperidinyl, 3-pentylmorpholino, 2-
hexylpiperazinyl, 3-methyl-4-benzylpiperazinyl, 3-ethyl-4-hydroxypiperidinyl, 3-methyl-4-benzylpyrrolidinyl, and the like.
The amino group having optionally a substituent selected from a
lower alkanoyl group and a phenyl-lower alkenylcarbonyl group includes an
amino group having optionally 1 to 2 substituents selected from a straight chainor branched chain alkanoyl group having 1 to 6 carbon atoms and a
phenylalkenylcarbonyl group wherein the alkenylcarbonyl moiety is a straight
chain or branched chain alkenylcarbonyl group having 3 to 6 carbon atoms in
the alkenylcarbonyl moiety, for example, amino, formylamino, acetylamino,
propionylamino, butyrylamino, isobutyrylamino, pentanoylamino, t-butyl -
carbonylamino, hexanoylamino, cinnamoylamino, 4-phenyl-3-butenoylamino,
4-phenyl-2-butenoylamino, 5-phenyl-4-pentenoylamino, 5-phenyl-3-pentenoyl-
amino, 5-phenyl-2-pentenoylamino, 6-phenyl-5-hexenoylamino, 6-phenyl-4-
hexenoylamino, 6-phenyl-3-hexenoylamino, 6-phenyl-2-hexenoylamino, 2-
methyl-4-phenyl-3-butenoylamino, 2-methyl-cinnamoylamino, 1-methyl-
cinnamoylamino, N-acetyl-N-cinnamoylamino, and the like.
The amino group having optionally a lower alkanoyl substituent
includes an amino group having optionally a substituent of a straight chain or
branched chain alkanoyl group having 1 to 6 carbon atoms, for example,
amino, formylamino, acetyamino, propionylamino, butyrylamino, isobutyryl-
WO 95/09159 2 1 5 0 3 1 5 PCT1JP94/OlS59
- 31 -
amino, pentanoylamino, tert-butylcarbonylamino, hexanoylamino, and the like.
The alkylsufinyl group includes a straight chain or branched chain
alkylsulfinyl group having 1 to 6 carbon atoms, for example, methylsulfinyl,
ethylsulfinyl, isopropylsulfinyl, butylsulfinyl, tert-butylsulfinyl, pentylsulfinyl,
5 hexylsulfinyl, and the like.
The lower alkylthio group includes a straight chain or branched
chain alkylthio group having 1 to 6 carbon atoms, for example, methylthio,
ethylthio, propylthio, isopropylthio, butylthio, tert-butylthio, pentylthio, hexylthio,
and the like.
The lower alkylsulfonyl group includes a straight chain or
branched chain alkylsulfonyl group having 1 to 6 carbon atoms, for example,
methylsulfonyl, ethylsulfonyl, isopropylsulfonyl, butysulfonyl, tert-butysulfonyl,
pentylsulfonyl, hexylsulfonyl, and the like.
The lower alkanoyloxy group includes a straight chain or
branched chain alkanoyloxy group having 1 to 6 carbon atoms, for example,
formyloxy, acetyloxy, propionyloxy, butyryloxy, isobutyryloxy, pentanoyloxy, tert -
butylcarbonyloxy, hexanoyloxy, and the like.
The amino-substituted lower alkyl group having optionally a
substituent selected from a lower alkylsulfonyl group and a lower alkanoyl
group includes a straight chain or branched chain alkyl group having 1 to 6
carbon atoms which is substituted by an amino group having optionally 1 to 2
groups selected from a straight chain or branched chain alkylsulfonyl group
having 1 to 6 carbon atoms and a straight chain or branched chain alkanoyl
group having 1 to 6 carbon atoms, for example, aminomethyl, 2-aminoethyl, 1 -
aminoethyl, 3-aminopropyl, 4-aminobutyl, 5-aminopentyl, 6-aminohexyl, 1,1 -
dimethyl-2-aminoethyl, 2-methyl-3-aminopropyl, formylaminomethyl, 1-acetyl -
aminoethyl, 2-propionylaminoethyl, 3-butyrylaminopropyl, 4-pentanoylamino-
pentyl, 5-hexanoylaminohexyl, 6-isobutyrylaminohexyl, 1,1-dimethyl-2-acetyl-
aminoethyl, 2-methyl-3-formylaminopropyl, methylsulfonylaminomethyl, 2-
ethylsulfonylaminoethyl, 1-isopropylsulfonylaminoethyl, 3-butylsulfonylamino-
propyl, 4-tert-butylsulfonylaminobutyl, S-pentylsulfonylaminopentyl, 6-hexyl -
sulfonylaminohexyl, 1,1-dimethyl-2-methylsulfonylaminoethyl, 2-methyl-3-
ethylsulfonylaminopropyl, N-methylsulfonylamino-N-acetylaminomethyl, and
WO 9S/09159 ~ 4~ pCI/JP94/01559
- 32 -
the like.
The 1,3-dioxolanyl-substituted lower alkyl group having optionally
a lower alkyl substituent includes a 1,3-dioxolanyl-substituted alkyl group
wherein the alkyl moiety is a straight chain or branched chain alkyl group
5 having 1 to 6 carbon atoms, which may optionally have 1 to 3 substituents of astraight chain or branched chain alkyl group having 1 to 6 carbon atoms, for
example, (1,3-dioxolan-2-yl)methyl, 2-(1,3-dioxolan-4-yl)ethyl, 1-(1,3-dioxolan-2-yl)ethyl, 3-(1,3-dioxolan-4-yl)propyl, 4-(1,3-dioxolan-2-yl)butyl, 2-(1,3-
dioxolan-2-yl)propyl, 5-(1,3-dioxolan-2-yl)pentyl, (4-hexyl-1,3-dioxolan-2-yl)-
10methyl, (2,4-dimethyl-1,3-dioxolan-2-yl)methyl, 6-(1,3-dioxolan-3-yl)hexyl, 4-(2-
propyl-1,3-dioxolan-2-yl)butyl, 5-(4-butyl-1,3-dioxolan-2-yl)pentyl, 6-(2-pentyl-
1,3-dioxolan-2-yl)hexyl, 1,1-dimethyl-2-(1,3-dioxolan-2-yl)ethyl, 2-methyl-3-(1,3-
dioxolan-2-yl)propyl, (2-methyl-1,3-dioxolan-2-yl)methyl, 2-(4-ethyl-1,3-
dioxolan-2-yl)ethyl, 3-(2,4,5-trimethyl-1,3-dioxolan-2-yl)propyl, and the like.
15The alkanoyl-substituted lower alkyl group includes an
alkanoylalkyl group wherein the alkanoyl moiety is a straight chain or branched
chain alkanoyl group having 1 to 6 carbon atoms and the alkyl moiety is a
straight chain or branched chain alkyl group having 1 to 6 carbon atoms, for
example, formylmethyl, acetylmethyl, 2-propionylethyl, 1-butyrylethyl, 3 -
20 isobutyrylpropyl, 4-pentanoylbutyl, 5-hexanoylhexyl, 6-tert-butylcarbonylhexyl,
1,1-dimethyl-2-acetylethyl, 2-methyl-3-acetylmethyl, and the like.
The aminocarbonyl-substituted lower alkyl group which may
optionally have a lower alkyl substituent includes a straight chain or branched
chain alkyl group having 1 to 6 carbon atoms which is substituted by an
25 aminocarbonyl having optionally 1 to 2 substituents of a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms, for example,
aminocarbonylmethyl, 2-aminocarbonylethyl, 1-aminocarbonylethyl, 3-
aminocarbonylpropyl, 4-aminocarbonylbutyl, 5-aminocarbonylpentyl, 6-
aminocarbonylhexyl, 1,1-dimethyl-2-aminocarbonylethyl, 2-methyl-3-amino-
30 carbonylpropyl, methylaminocarbonylmethyl, 1-ethylaminocarbonylethyl, 2-
propylaminocarbonylethyl, 3-isopropylaminocarbonylpropyl, 4-butylamino-
carbonylbutyl, 5-pentylaminocarbonylpentyl, 6-hexylaminocarbonylhexyl,
dimethylaminocarbonylmethyl, 2-diethylaminocarbonylethyl, 2-dimethylamino-
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 33 -
carbonylethyl, (N-ethyl-N-propylamino)carbonylmethyl, 2-(N-methyl-N-hexyl-
amino)carbonylethyl, and the like.
The lower alkoxycarbonyl-substituted lower alkenyl group
includes a straight chain or branched chain alkenyl group having 2 to 6 carbon
5 atoms which is substituted by a straight chain or branched chain alkoxy -
carbonyl group having 1 to 6 carbon atoms in the alkoxy moiety, for example, 2 -methoxycarbonylvinyl, 2-ethoxycarbonyvinyl, 3-propoxycarbonylallyl, 4-butoxy-
carbonyl-2-butenyl, 4-pentyloxycarbonyl-3-butenyl, 3-hexyloxycarbonyl-1-
methylallyl, 5-isopropoxycarbonyl-2-pentenyl, 6-tert-butoxycarbonyl-2-hexenyl,
10 and the like.
The aminocarbonyl-substituted lower alkenyl group which may
optionally have a lower alkyl substituent includes a straight chain or branched
chain alkenyl group having 2 to 6 carbon atoms which is substitued by an
aminocarbonyl group having optinally have 1 to 2 substituents of a straight
15 chain or branched chain alkyl group having 1 to 6 carbon atoms, for example, 2 -
aminocarbonylvinyl, 3-aminocarbonylallyl, 4-aminocarbonyl-2-butenyl, 4-
aminocarbonyl-3-butenyl, 3-aminocarbonyl-1-methylallyl, 5-aminocarbonyl-2-
pentenyl, 6-aminocarbonyl-2-hexenyl, 2-methylaminocarbonylvinyl, 3-ethyl-
aminocarbonylallyl, 4-propylaminocarbonyl-2-butenyl, 4-isopropylamino-
20 carbonyl-3-butenyl, 3-butylaminocarbonyl-1-methylallyl, 5-pentylamino-
carbonyl-2-pentenyl, 6-hexylaminocarbonyl-2-hexenyl, 2-dimethylamino-
carbonylvinyl, 2-diethylaminocarbonylvinyl, 3-(N-ethyl-N-propylaminocarbonyl)-
allyl, 4-(N-methyl-N-hexylaminocarbonyl)-2-butenyl, and the like.
The carboxy-substituted lower alkenyl group includes a straight
25 chain or branched chain alkenyl group having 2 to 6 carbon atoms, for
example, 2-carboxyvinyl, 3-carboxyallyl, 4-carboxy-2-butenyl, 4-carboxy-3-
butenyl, 3-carboxy-1-methylallyl, 5-carboxy-2-pentenyl, 6-carboxy-2-hexenyl,
and the like.
The aminocarbonyl group having optionally a lower alkyl
30 substituent includes an aminocarbonyl group which may optionally have 1 to 2
substituents of a straight chain or branched chain alkyl group having 1 to 6
carbon atoms, for example, aminocarbonyl, methylaminocarbonyl, ethyl -
aminocarbonyl, propylaminocarbonyl, isopropylaminocarbonyl, butyl-
WO 95/09159 PCT/JP94/01559
~ts~34~
- 34 -
aminocarbonyl, tert-butylaminocarbonyl, pentylaminocarbonyl, hexyl -
aminocarbonyl, dimethylaminocarbonyl, diethylaminocarbonyl, dipropyl-
aminocarbonyl, dibutylaminocarbonyl, dipentylaminocarbonyl, dihexyl -
aminocarbony!, N-methyl-N-ethylaminocarbonyl, N-ethyl-N-propylamino-
carbonyl, N-methyl-N-butylaminocarbonyl, N-methyl-N-hexylaminocarbonyl,
and the like.
The phenylsulfonyl group having optionally a lower alkyl
substituent includes a phenylsulfonyl group having optionally 1 to 3
substituents of a straight chain or branched chain alkyl group having 1 to 6
carbon atoms, for example, phenylsulfonyl, 2-methyl phenylsulfonyl, 3 -
methylphenylsulfonyl, 4-methylphenylsulfonyl, 2-ethylphenylsulfonyl, 3-
ethylphenylsulfonyl, 4-ethylphenylsulfonyl, 3-isopropylphenylsulfonyl, 4-
hexylphenylsulfonyl, 3,4-dimethylphenylsulfonyl, 2,5-dimethylphenylsulfonyl,
3,4,5-trimethylphenylsulfonyl, and the like.
The phenyl-lower alkenyl group includes a phenylalkenyl group
wherein the alkenyl moiety is a straight chain or branched chain alkenyl group
having 2 to 6 carbon atoms, for example, styryl, cinnamyl, 4-phenyl-3-butenyl, 4 -
phenyl-2-butenyl, 5-phenyl-4-pentenyl, 5-phenyl-3-pentenyl, 5-phenyl-2-
pentenyl, 6-phenyl-5-hexenyl, 6-phenyl-4-hexenyl, 6-phenyl-3-hexenyl, 6-
phenyl-2-hexenyl, 2-methyl-4-phenyl-3-butenyl, 2-methyl-cinnamyl, 1-methyl -
cinnamyl, and the like.
The benzoyl group which may optionally have 1 to 3 substituents
selected from a lower alkoxy group, a halogen atom, an amino group having
optionally a lower alkanoyl substituent, and hydroxy group on the phenyl
moiety includes a benzoyl group which may optinally have 1 to 3 substituents
selected from a straight chain or branched chain alkoxy group having 1 to 6
carbon atoms, a halogen atom, an amino group having optionally a straight
chain or branched chain alkanoyl substituent having 1 to 6 carbon atoms and
hydroxy group on the phenyl moiety, for example, benzoyl, 2-chlorobenzoyl, 3 -
chlorobenzoyl, 4-chlorobenzoyl, 2-fluorobenzoyl, 3-fluorobenzoyl, 4-fluoro-
benzoyl, 2-bromobenzoyl, 3-bromobenzoyl, 4-bromobenzoyl, 2-iodobenzoyl, 4-
iodobenzoyl, 3,5-dichlorobenzoyl, 2,6-dichlorobenzoyl, 3,4-dichlorobenzoyl,
3,4-difluorobenzoyl, 3,5-dibromobenzoyl, 3,4,5-trichlorobenzoyl, 2-methoxy-
WO 9S/09159 PCItJP94/OlS59
2150345
- 35 -
benzoyl, 3-methoxybenzoyl, 4-methoxybenzoyl, 2-ethoxybenzoyl, 3-ethoxy-
benzoyl, 4-ethoxybenzoyl, 4-isopropoxybenzoyl, 4-hexyloxybenzoyl, 3,4-
dimethoxybenzoyl, 3,4-diethoxybenzoyl, 3,4,5-trimethoxybenzoyl, 2,5 -
dimethoxybenzoyl, 3-methoxy-4-chlorobenzoyl, 2-chloro-6-methoxybenzoyl, 2-
5 methoxy-5-chlorobenzoyl, 2-aminobenzoyl, 3-aminobenzoyl, 4-aminobenzoyl,
2-hydroxybenzoyl, 3-hydroxybenzoyl, 4-hydroxybenzoyl, 2,5-diaminobenzoyl,
3,4,5-triaminobenzoyl, 2-formylaminobenzoyl, 3-acetyaminobenzoyl, 4-acetyl-
aminobenzoyl, 2-acetylaminobenzoyl, 3-propionylaminobenzoyl, 4-butyryl-
aminobenzoyl, 2-isobutyrylaminobenzoyl, 3-pentanoylaminobenzoyl, 3-tert-
10 butylcarbonylaminobenzoyl, 4-hexanoylaminobenzoyl, 2,6-diacetylamino-
benzoyl, 2,4-dihydroxybenzoyl, 2,4,6-trihydroxybenzoyl, 2-hydroxy-5-chloro-
benzoyl, and the like.
The amino-substituted lower alkanoyl group having optionally a
lower alkanoyl substituent includes a straight chain or branched chain alkanoyl
15 group having 2 to 6 carbon atoms which is substitued by an amino group
having optionally 1 to 2 substituents of a straight chain or branched chain
alkanoyl group having 1 to 6 carbon atoms, for example, 2-aminoacetyl, 3 -
aminopropionyl, 2-aminopropionyl, 4-aminobutyryl, 5-aminopentanoyl, 6-
aminohexanoyl, 2,2-dimethyl-3-aminopropionyl, 2-methyl-3-aminopropionyl, 2-
20 acetylaminoacetyl, 2-acetylaminopropionyl, 3-propionylaminopropionyl, 3-
isopropionylaminopropionyl, 4-butyrylaminobutyryl, 5-pentanoylamino-
pentanoyl, 6-hexanoylaminohexanoyl, 2-formylaminoacetyl, and the like.
The amino-substituted sulfonyl group having optionally a lower
alkyl substituent includes an aminosulfonyl group which may optionally have 1
25 to 2 substituents of a straight chain or branched chain alkyl group having 1 to 6
carbon atoms, for example, aminosulfonyl, methylaminosulfonyl, ethylamino -
sulfonyl, propylaminosulfonyl, isopropylaminosulfonyl, butylaminosulfonyl, tert-butylaminosulfonyl, pentylaminosulfonyl, hexylaminosulfonyl, dimethylamino-
sulfonyl, diethylaminosulfonyl, dipropylaminosulfonyl, dibutylaminosulfonyl,
30 dibutylaminosulfonyl, dipentylaminosulfonyl, dihexylaminosulfonyl, N-methyl-N-
ethylaminosulfonyl, N-ethyl-N-propylaminosulfonyl, N-methyl-N-butylamino-
sulfonyl, N-methyl-N-hexylaminosulfonyl, and the Iike.
The lower alkeneldioxy group includes a straight chain or
WO 9S/09159 I'CT1JP94/OlS59
3 4~ 36 -
branched chain alkylenedioxy group having 1 to 4 carbon atoms, for example,
methylenedioxy, ethylenedioxy, trimethylenedioxy, tetramethylenendioxy, and
the like.
The phenyl group having optionlly a lower alkoxy substituent
5 includes a phenyl group which may optionally have 1 to 3 substituents of a
straight chain or branched chain alkoxy group having 1 to 6 carbon atoms, for
example, phenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4-methoxyphenyl, 2-
ethoxyphenyl, 3-ethoxyphenyl, 4-ethoxyphenyl, 4-isopropoxyphenyl, 4-pentyl -
oxyphenyl, 2,4-dimethoxyphenyl, 4-hexyloxyphenyl, 3-ethoxy-4-methoxy-
phenyl, 2,3-dimethoxyphenyl, 3,4-dimethoxyphenyl, 3,4-diethoxyphenyl, 2,5-
dimethoxyphenyl, 2,6-dimethoxyphenyl, 3,5-dimethoxyphenyl, 3,4-dipentyloxy-
phenyl, 3,4,5-trimethoxyphenyl, and the like.
The 2,3-dihydro-1 H-indenyl-substituted lower alkyl group which
may optionall have a substituent selected from oxo group, hydroxy group and
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring includes a straight chain or branched chain alkyl group having 1 to 6
carbon atoms which is substituted by a 2,3-dihydro-1 H-indenyl group having
optionally 1 to 3 substituents selected from oxo group, hydroxy group and a
silyloxy group having 3 substituents of a straight chain or branched chain alkylgroup having 1 to 6 carbon atoms, for example, (2,3-dihydro-1 H-inden-2-yl) -
methyl, 2-(2,3-dihydro-1 H-inden-1 -yl)ethyl, 1 -(2,3-dihydro-1 H-inden-3-yl)ethyl,
3-(2,3-dihydro-1H-inden-4-yl)propyl, 4-(2,3-dihydro-1H-inden-5-yl)butyl, 5-(2,3-dihydro-1 H-inden-6-yl)pentyl, 6-(2,3-dihydro-1 H-inden-7-yl)hexyl, (1 -oxo-2,3 -
dihydro-1H-inden-2-yl)methyl, (1-hydroxy-2,3-dihydro-1H-inden-2-yl)methyl, (1 -
dimethyl,tert-butylsilyloxy-2,3-dihydro-1H-inden-2-yl)methyl, (1,3-dihydroxy-2,3-
dihydro-1H-inden-2-yl)methyl, [1,3-bis(trimethylsilyloxy)-2,3-dihydro-1H-inden-
2-yl)methyl, (1,3,7-trihydroxy-2,3-dihydro-1H-inden-2-yl)methyl, [1,3,4-tri-
(dimethyl,ethylsilyloxy)-2,3-dihydro-1H-inden-2-yl)methyl, and the like.
The silyloxy group having a lower alkyl substituent includes a
silyloxy group being substituted by three straight chain or branched chain alkylgroups having 1 to 6 carbon atoms, for example, trimethylsilyloxy, triethyl -
silyloxy, triisopropylsilyloxy, tributylsilyloxy, tri-tert-butylsilyloxy, tripentylsilyloxy,
trihexylsilyloxy, dimethyl,tert-butylsilyloxy, and the like.
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
-
The phenyl group having optionally a substituent selected from a
lower alkoxy group and a halogen atom on the phenyl ring includes a phenyl
group which may optionaly be substituted by 1 to 3 groups selected from a
straight chain or branched chain alkoxy group having 1 to 6 carbon atoms and
5 a halogen atom, for example, phenyl, 2-methoxyphenyl, 3-methoxyphenyl, 4 -
methoxyphenyl, 2-ethoxyphenyl, 3-ethoxyphenyl, 4-ethoxyphenyl, 4-
isopropoxyphenyl, 4-pentyloxyphenyl, 2,4-dimethoxyphenyl, 4-hexyloxyphenyl,
3,4-dimethoxyphenyl, 3-ethoxy-4-methoxyphenyl, 2,3-dimethoxyphenyl, 3,4-
diethoxyphenyl, 2,5-dimethoxyphenyl, 2,6-dimethoxyphenyl, 3,5-dimethoxy-
phenyl, 3,4-dipentyloxyphenyl, 3,4,5-trimethoxyphenyl, 2-chlorophenyl, 3-
chlorophenyl, 4-chlorophenyl, 2-fluorophenyl, 3-fluorophenyl, 4-fluorophenyl, 2-bromophenyl, 3-bromophenyl, 4-bromophenyl, 2-iodophenyl, 3-iodophenyl, 4-
iodophenyl, 3,4-dichlorophenyl, 3,5-dichlorophenyl, 2,6-dichlorophenyl, 2,3-
dichlorophenyl, 2,4-dichlorophenyl, 3,4-difluorocphenyl, 3,5-dibromophenyl,
3,4,5-trichlorophenyl, 2-methoxy-5-chlorophenyl, 3-chloro-4-methoxyphenyl, 3-
methoxy-5-iodophenyl, 3,4-dimethoxy-5-bromophenyl, 3,5-iodo-4-methoxy-
phenyl, and the like.
The saturated or unsaturated 5- to 6-membered heterocyclic
group having 1 to 4 hetero atoms selected from nitrogen atom, oxygen atom
20 and sulfur atom includes, for example, pyrrolidinyl, piperidinyl, piperazinyl,
morpholino, pyridyl, thienyl, pyrazinyl, pyrimidinyl, pyridazinyl, pyrrolyl,
imidazolidinyl, pyrazolyl, imidazolyl, pyrazolidinyl, 1,3,4-oxadiazolyl, 1,2,4-
triazolyl, 1,2,3,4-tetrazolyl, 1,3,4-triazolyl, 1,2,4-oxadiazolyl, furyl, pyrrolinyl,
oxazolyl, isoxazolyl, thiazolyl, thiazolidinyl, 1,2,3,5-oxathiadiazolyl, isothiazolyl,
25 pyranyl, pyrazolidinyl, 1,2,4-triazinyl, and the like.
The lower alkyl group substitued by a 5- to 6-membered saturated
or unsaturated heterocyclic group having 1 to 4 hetero atoms selected from
nitrogen atom, oxygen atom and sulfur atom includes a straight chain or
branched chain alkyl group having 1 to 6 carbon atoms which is substituted by
30 the above mentioned heterocyclic group, for example, pyrrolidinylmethyl, 2 -
piperidinylethyl, 3-piperazinylpropyl, 4-morpholinobutyl, (2-pyridyl)methyl, (3-pyridyl)methyl, (2-thienyl)methyl, 2-(3-pyrazinyl)ethyl, 1-(2-pyrimidyl)ethyl, 3-(3-
pyridazinyl)propyl, 4-(2-pyrrolyl)butyl, 4-(4-imidazolidinyl)butyl, (2-imidazolyl)-
WO 95/09159 PCTIJP94/01559
21503~5
- 38 -
methyl, (3-pyrazolyl)methyl, 2-(2-imidazo!yl)ethyl, 3-(3-pyrazolidinyl)propyl,
thienylmethyl, 3-(2-imidazolinyl)propy~1, 4`-(2-pyrrolinyl)butyl, (2-furyl)methyl, (4-
oxazolyl)methyl, (5-oxazolyl)methyl, 5-(4-oxazolyl)pentyl, 6-(3-isooxazolyl)-
hexyl, (4-thiazolyl)methyl, (2-thiazolyl)methyl, 2-(3-isothiazolyl)ethyl, (2-pyranyl) -
5 methyl, 3-(3-pyrazolidinyl)propyl, 4-(2-pyrazolinyl)butyl, (5-thiazolyl)methyl,
(1,3,4-oxadiazolin-5-yl)methyl, (1,2,4-triazol-5-yl)methyl, (1,2,3,4-tetrazol-5-yl)-
methyl, (1,3,4-triazol-5-yl)methyl, (1,2,4-oxadiazol-5-yl)methyl, (1,2,4-triazin-3-
yl)methyl, (thiazolidin-5-yl)methyl, (1,2,3,5-oxathiadiazolin-4-yl)methyl, (2-furyl)-
methyl, (3-furyl)methyl, 2-(5-thiazolyl)ethyl, 1-(1,3,4-oxadiazolin-2-yl)ethyl, 3-
(1,2,4-triazol-3-yl)propyl, 4-(1,2,3,4-tetrazol-5-yl)butyl, 6-(1,3,4-triazol-2-yl) -
hexyl, 2-(1,2,4-oxadiazol-3-yl)ethyl, 1-(1,2,4-triazin-5-yl)ethyl, 3-(thiazolidin-2 -
yl)propyl, 4-(1,2,3,5-oxathiadiazolin-4-yl)butyl, and the like.
The lower alkenyl group substituted by a 5- to 14-membered
saturated or unsaturated heteromonocylic, heterobicyclic or heterotricyclic
group having 1 to 4 hetero atoms selected from nitrogen atom, oxygen atom
and sulfur atom includes the above mentioned heterocyclic group-substituted
alkenyl group wherein the alkenyl moiety is a straight chain or branched chain
alkenyl group with 1 to 2 double bounds having 2 to 6 carbon atoms, for
example, 2-benzofurylvinyl, 3-benzofuryl-2-propenyl, 4-benzofuryl-3-butenyl, 3-
benzofuryl-2-butenyl, 2-methyl-3-benzofuryl-2-propenyl, 5-benzofuryl-4-
petenyl, 5-benzofuryl-2-pentenyl, 4-benzofuryl-3-pentenyl, 6-benzofuryl-5 -
hexenyl, 6-benzofuryl-4-hexenyl, 6-benzofuryl-3-hexenyl, 6-benzofuryl-2-
hexenyl"B-methyl-4-benzofuryl-3-butenyl, 5-benzofuryl-2,4-pentadienyl, 4-
benzofuryl-1,3-butadienyl, 6-benzofuryl-2,4-hexadienyl, 6-benzofuryl-3,5-
hexadienyl, 6-benzofuryl-1,3-hexadienyl, 5-benzofuryl-1,3-pentadienyl, 2-
benzothienylvinyl, 3-benzothienyl-2-propenyl, 4-benzothienyl-3-butenyl, 3-
benzothienyl-2-butenyl, 2-methyl-3-benzothienyl-2-propenyl, 5-benzothienyl-4-
petenyl, 5-benzothienyl-2-pentenyl, 6-benzothienyl-5-hexenyl, 6-benzothienyl-
4-hexenyl, 6-benzothienyl-3-hexenyl, 6-benzothienyl-2-hexenyl, ~-methyl-4-
benzothienyl-3-butenyl, 5-benzothienyl-2,4-pentadienyl, 4-benzothienyl-1,3-
butadienyl, 6-benzothienyl-2,4-hexadienyl, 6-benzothienyl-3,5-hexadienyl, 6-
benzothienyl-1,3-hexadienyl, 5-benzothienyl-1,3-pentadienyl, 2-(furo[3,2-c]-
WO 95/09159 2 1 5 0 3 4 S PCI/JP94/01559
- 39 -
pyridyl)vinyl, 3-(furo[3,2-c]pyridyl)-2-propenyl, 4-(furo[3,2-c]pyridyl)-3-butenyl, 3 -
(furo[3,2-c]pyridyl)-2-butenyl, 2-methyl-3-(furo[3,2-c]pyridyl)-2-propenyl, 5 -
(furo[3,2-c]pyridyl)-4-petenyl, 5-(furo[3,2-c]pyridyl)-2-pentenyl, 6-(furo[3,2-
c]pyridyl)-5-hexenyl, 6-(furo[3,2-c]pyridyl)-4-hexenyl, 6-(furo[3,2-c]pyridyl)-3-
hexenyl, 6-(furo[3,2-c]pyridyl)-2-hexenyl"B-methyl-4-(furo[3,2-c]pyridyl)-3 -
butenyl, 5-(furo[3,2-c]pyridyl)-2,4-pentadienyl, 4-(furo[3,2-c]pyridyl)-1,3-
butadienyl, 6-(furo[3,2-c]pyridyl)-2,4-hexadienyl, 6-(furo[3,2-c]pyridyl)-3,5-
hexadienyl, 6-(furo[3,2-c]pyridyl)-1,3-hexadienyl, 5-(furo[3,2-c]pyridyl)-1,3-
pentadienyl, 2-quinolylvinyl, 3-(1,4-dihydroquinoly)1-2-propenyl, 4-benzo-
thiazolyl-3-butenyl, 3-carbostyril-2-butenyl, 2-methyl-3-(3,4-dihydrocarbostyril)-
2-propenyl, 5-(1,2,3,4-tetraquinolyl)-4-petenyl, 5-indolyl-2-pentenyl, 6-indolinyl-
5-hexenyl, 6-indolinyl-4-hexenyl, 6-benzoimidazolyl-3-hexenyl, 6-benzoxazolyl-
2-hexenyl, ~-methyl-4-isoquinolyl-3-butenyl, 5-quinazolidinyl-2,4-pentadienyl,
4-cinnolyl-1,3-butadienyl, 6-quinoxalinyl-2,4-hexadienyl, 6-phthalazinyl-3,5-
hexadienyl, 6-chromanyl-1,3-hexadienyl, 5-isoindolinyl-1,3-pentadienyl, 2-(4H-
chromenyl)vinyl, 3-(2,3-dihydro-2-benzofuryl) -2-propenyl, 4-(2-perhydrobenzo-
furyl)-3-butenyl, 3-(1,4-benzoxadinyl)-2-butenyl, 2-methyl-3-(3,4-dihydro-2H -
1,4-benzoxazinyl)-2-propenyl, 5-(1,4-benzothiazinyl)-4-pentenyl, 5-(1,2,3,4-
tetrahydroquinoxalinyl)-2-pentenyl, 6-(1,3-dithia-2,4-dihydronaphthalenyl)-5-
hexenyl, 6-(1,4-dithianaphthalenyl)-4-hexenyl, 6-(1,4-benzodioxanyl)-3-
hexenyl, 2-pyrrolidinylvinyl, 3-pyrrolidinyl-2-propenyl, 4-pyrrolidinyl-3-butenyl,
3-pyrrolidinyl-2-butenyl, 2-methyl-3-pyrrolidinyl-2-propenyl, 5-pyrrolidinyl-4-
pentenyl, 5-pyrrolidinyl-2-pentenyl, 6-pyrrolidinyl-5-hexenyl, 6-pyrrolidinyl-4-hexenyl, 6-pyrrolidinyl-3-hexenyl, 6-pyrrolidinyl-2-hexenyl, ~-methyl-4-
pyrrolidinyl-3-butenyl, 5-pyrrolidinyl-2,4-pentadienyl, 4-pyrrolidinyl-1,3-
butadienyl, 6-pyrrolidinyl-2,4-hexadienyl, 6-pyrrolidinyl-3,5-hexadienyl, 6-
pyrrolidinyl-1,3-hexadienyl, 5-pyrrolidinyl-1,3-pentadienyl, 2-piperidinylvinyl, 3-
piperidinyl-2-propenyl, 4-piperidinyl-3-butenyl, 3-piperidinyl-2-butenyl, 2-
methyl-3-piperidinyl-2-propenyl, 5-piperidinyl-4-pentenyl, 5-piperidinyl-2-
pentenyl, 6-piperidinyl-5-hexenyl, 5-piperidinyl-2-pentenyl, 6-piperidinyl-5-
hexenyl, 6-piperidinyl-4-hexenyl, 6-piperidinyl-3-hexenyl, 6-piperidinyl-2-
WO 95/09159 2 ~S O 3 ~S PCI/JP94/015S9
- 40 -
hexenyl, ~-methyl-4-piperidinyl-3-butenyl, 5-piperidinyl-2,4-pentadienyl, 4-
piperidinyl-1,3-butadienyl, 6-piperidinyl-2,4-hexadienyl, 6-piperidinyl-3,5-
hexadienyl, 6-piperidinyl-1,3-hexadienyl, 5-piperidinyl-1,3-pentadienyl, 2-
piperazinylvinyl, 3-piperazinyl-2-propenyl, 4-piperazinyl-3-butenyl, 3-
piperazinyl-2-butenyl, 2-methyl-3-piperazinyl-2-propenyl, 5-piperazinyl-4-
pentenyl, 5-piperazinyl-2-pentenyl, 6-piperazinyl-5-hexenyl, 6-piperazinyl-4-
hexenyl, 6-piperazinyl-3-hexenyl, 6-piperazinyl-2-hexenyl, ~-methyl-4-
piperazinyl-3-butenyl, 5-piperazinyl-2,4-pentadienyl, 4-piperazinyl-1,3-
butadienyl, 6-piperazinyl-2,4-hexadienyl, 6-piperazinyl-3,5-hexadienyl, 6-
piperazinyl-1,3-hexadienyl, 5 piperazinyl-1,3-pentadienyl, 2-morpholinovinyl, 3-pyridyl-2-propenyl, 4-thienyl-3-butenyl, 3-pyradinyl-2-butenyl, 2-methyl-3-
pyrimidyl-2-propenyl, 2-pyridazinylvinyl, 3-pyrrolyl-2-propenyl, 4-imidazolyl-3-
butenyl, 3-imidazolyl-2-butenyl, 2-methyl-3-imidazolyl-2-propenyl, 5-imidazolyl-4-pentenyl, 5-imidazolyl-2-pentenyl, 6-imidazolyl-5-hexenyl, 6-imidazolyl-4-
hexenyl, 6-imidazolyl-3-hexenyl, 6-imidazolyl-2-hexenyl, ~-methyl-4-imidazolyl-
3-butenyl, 5-imidazolyl-2,4-pentadienyl, 4-imidazolyl-1,3-butadienyl, 6-
imidazolyl-2,4-hexadienyl, 6-imidazolyl-3,5-hexadienyl, 6-imidazolyl-1,3-
hexadienyl, 5-imidazolyl-1,3-pentadienyl, 2-imidazolidinylvinyl, 3-pyrazolyl-1,3-
pentadienyl, 2-imidazolidinylvinyl, 3-pyrazolyl-2-propenyl, 4-pyrazolidinyl-3-
butenyl, 3-perhydrobenzofuryl-2-butenyl, 2-methyl-3-(1,3,4-oxadiazolyl)-2-
propenyl, 5-(1,2,4-triazolyl)-4-petenyi, 5-(1,2,3,4-tetrazolyl)-2-pentenyl, 6-(1,3,4 -
triazolyl)-5-hexenyl, 6-(1,2,4-oxadiazolyl)-4-hexenyl, 6-(2,3-dihydro-2-
benzofuryl)-3-hexenyl, 6-pyrrolinyl-2-hexenyl, ,B-methyl-5-nonyl-3-butenyl, 5-
isoxazolyl-2,4-pentadienyl, 4-thiazolyl-1,3-butadienyl, 6-thiazolidinyl-2,4-
hexadienyl, 6-(1,2,3,5-oxathiadiazolyl)-3,5-hexadienyl, 6-isothiazolyl-1,3-
hexadienyl, 5-pyranyl-1,3-pentadienyl, 2-oxazolylvinyl, 3-oxazolyl-2-propenyl,
4-oxazolyl-3-butenyl, 3-oxazolyl-2-butenyl, 2-methyl-3-oxazolyl-2-propenyl, 5-
oxazolyl-4-pentenyl, 5-oxazolyl-2-pentenyl, 4-oxazolyl-3-pentenyl, 6-oxazolyl-5-hexenyl, 6-oxazolyl-4-hexenyl, 6-oxazolyl-3-hexenyl, 6-oxazolyl-2-hexenyl, ,B-
methyl-4-oxazolyl-3-butenyl, 5-oxazolyl-2,4-pentadienyl, 4-oxazolyl-1,3-
butadienyl, 6-oxazolyl-2,4-hexadienyl, 6-oxazolyl-3,5-hexadienyl, 6-oxazolyl-
Wo 9S/O9lS9 2 1 5 0 3 4 ~
- 41 -
1,3-hexadienyl, 5-oxazolyl-1,3-pentadienyl, 2-pyrazolylvinyl, 3-quinuclidinyl-2-propenyl, 4-benzothiazolyl-3-butenyl, 3-carbostyril-2-butenyl, 2-methyl-3-(3,4-
dihydro[2,3-g]quinolyl)-2-propenyl, 5-(1,2,3,4-tetrahydrofuro[2,3-g]quinolyl)-4-pentenyl, 2-(naphtho[2,1-b]furyl)vinyl, 3-(naphtho[2,1-b]furyl)-2-propenyl, 4 -
(naphtho[2,1-b]furyl)-3-butenyl, 3-(naphtho[2,1-b]furyl)-2-butenyl, 2-methyl-3-
(naphtho[2,1-b]furyl)-2-propenyl, 5-(naphtho[2,1-b]furyl)-4-pentenyl, 5-(naphtho-
[2,1-b]furyl)-2-pentenyl, 4-(naphtho[2,1-b]furyl)-3-pentenyl, 6-(naphtho[2,1-b] -
furyl)-5-hexenyl, 6-(naphtho[2,1-b]furyl)-4-hexenyl, 6-(naphtho[2,1-b]furyl)-3-
hexenyl, 6-(naphtho[2, 1 -b]furyl)-2-hexenyl"~-methyl-4-(naphtho[2, 1 -b]furyl)-3 -
butenyl, 5-(naphtho[2,1-b]furyl)-2,4-pentadienyl, 4-(naphto[2,1-b]furyl)-1,3-
butadienyl, 6-(naphtho[2,1-b]furyl)-2,4-hexadienyl, 6-(naphtho[2,1-b]furyl)-3,5-hexadienyl, 6-(naphtho[2,1-b]furyl)-1,3-hexadienyl, 5-(naphtho[2,1-b]furyl)-1,3-pentadienyl, 2-(imidazo[1,2-a]pyridyl)vinyl, 3-(imidazo[1,2-a]pyridyl)-2-
propenyl, 4-(imidazo[1,2-a]pyridyl)-3-butenyl, and the like.
The phenyl-lower alkoxycarbonyl group includes a phenylalkoxy -
carbonyl group wherein the alkoxycarbonyl moiety is a straight chain or
branched chain alkoxycarbonyl group having 1 to 6 carbon atoms in the alkoxy
moiety, for example, benzyloxycarbonyl, 2-phenylethoxycarbonyl, 1-phenyl -
ethoxycarbonyl, 3-phenylpropoxycarbonyl, 4-phenylbutoxycarbonyl, 5-phenyl-
pentyloxycarbonyl, 6-phenylhexyloxycarbonyl, 1,1-dimethyl-2-phenylethoxy-
carbonyl, 2-methyl-3-phenylpropoxycarbonyl, and the like.
The lower alkoxycarbonyloxy-substituted lower alkyl group
includes a straight chain or branched chain alkyl group having 1 to 6 carbon
atoms, which is substituted by alkoxycarbonyloxy group wherein the alkoxy
moiety is a a straight chain or branched chain alkoxy group having 1 to 6
carbon atoms, for example, methoxycarbonyloxymethyl, ethoxycarbonyloxy -
methyl, 2-ethoxycarbonyloxyethyl, 1-ethoxycarbonyloxyethyl, 3-methoxy-
carbonyloxypropyl, 4-ethoxycarbonyloxybutyl, 6-propoxycarbonyloxyhexyl, 5-
isopropoxycarbonyloxypentyl, 1,1-dimethyl-2-butoxycarbonyloxyethyl, 2-methyl-
3-tert-butoxycarbonyloxypropyl, 2-pentyloxycarbonyloxyethyl, hexyloxy-
carbonyloxymethyl, and the like.
The benzoyl-substituted lower alkyl group having optionally a
WO 95/09159 PCTIJP94/OlSS9
2~s0345
- 42 -
halogen substituent on the phenyl ring includes a straight chain or branched
chain alkyl group having 1 to 6 carbon atom, which is substituted by a benzoyl
group wherein the phenyl ring may opti~nally have 1 to 3 halogen substituents,
for example, benzoylmethyl. 1-(2-chlorobenzoyl)ethyl, 2-(3-chlorobenzoyl) -
ethyl, 3-(4-chlorobenzoyl)propoyl, 4-(2-fluorobenzoyl)butyl, 1,1-dimethyl-2-(3-
fluorobenzoyl)ethyl, 5-(4-fluorobenzoyl)pentyl, 6-(2-bromobenzoyl)hexyl, 2-
methyl-3-(3-bromobenzoyl)propyl, (4-bromobenzoyl)methyl, 2-(2-iodobenzoyl)-
ethyl, 1-(4-iodobenzoyl)ethyl, (3,5-dichlorobenzoyl)methyl, 2-(2,6-dichloro-
benzoyl)ethyl, 1-(3,4-dichlorobenzoyl)ethyl, 3-(3,4-difluorobenzoyl)propyl, (3,5-
dibromobenzoyl)methyl, (3,4,5-trichlorobenzoyl)methyl, and the like.
The amino group having optionally a lower alkanoyl substituent
includes an amino group which may optionally have a substituent of a straight
chain or branched chain alkanoyl group having 1 to 6 carbon atoms, for
example, amino, formylamino, acetylamino, propionylamino, butyrylamino,
isobutyrylamino, pentanoylamino, t-butylcarbonylamino, hexanoylamino, and
the like.
The present invention specifically includes the following
compounds.
(1 ) A quinoxaline derivative of the formula (1 ) wherein R1 is hydrogen
atom and R2, R3, R4, m, n and r are the same as defined above, or a salt
thereof.
(2) A quinoxaline derivative of the formula (1 ) wherein R1 is a
halogen atom and R2, R3, R4, m, n and r are the same as defined above, or a
salt thereof.
(3) A quinoxaline derivative of the formula (1 ) wherein R1 is a lower
alkyl group and R2, R3, R4, m, n and r are the same as defined above, or a salt
thereof.
(4) A quinoxaline derivative of the formula (1 ) wherein R2 is hydrogen
atom and R1, R3, R4, m, n and r are the same as defined above, or a salt
thereof.
(5) A quinoxaline derivative of the formula (1) wherein R2 is a lower
alkyl group having optionally a halogen substituent, and R1, R3, R4, m, n and r
are the same as defined above, or a salt thereof.
WO 95/O91S9 2 I 5 0 3 ~ 5 PCT/JP94/OlS59
- 43 -
(6) A quinoxaline derivative of the formula (1 ) wherein R2 is phenyl
group and R1, R3,R4, m, n and r are the same as defined above, or a salt
thereof.
(7) A quinoxaline derivative of the formula (1 ) wherein R2 is a
5 morpholino-substituted lower alkyl group and R1, R3,R4, m, n and r are the
same as defined above, or a salt thereof.
(8) A quinoxaline derivative of the formula (1 ) wherein R2 is an
imidazolyl-substituted lower alkyl group and R1, R3,R4, m, n and r are the same
as defined above, or a salt thereof.
10 (9) A quinoxaline derivative of the formula (1 ) wherein R3iS hydrogen atom, R4is a group of the formula:
(R5)p
A~
15 (wherein A, R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(10) A quinoxaline derivative of the formula (1 ) wherein R3iS a lower
alkyl group, R4is a group of the formula:
(R5)p
A ~
(wherein A,R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(11) A quinoxaline derivative of the formula (1 ) wherein R3is hydrogen
25 atom, R4iS a phenyl-lower alkenyl group which may optionally have a
substituent on the phenyl moiety selected from a lower alkoxy group, a halogen
atom, an amino group having optionally a substituent selected from a lower
alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower alkoxy -
substituted lower alkoxy group, a tetrazolyl group having optionally a lower
30 alkyl substituent on the tetrazole ring, hydroxy group, a group of the formula:
-O-A4-Co-NR40R41 (in which A4, R40 and R41 are the same as defined above),
a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and R1, R2, m, n and r are the same as defined above, or
WO 95/09159 ~ 1 S 3 4 5 PCT/JP94/01559
- 44 -
a salt thereof.
(12) A quinoxaline derivative~of the formula (1) wherein R3iS a lower
alkyl group, R4is a phenyl-lower alkenyl group which may optionally have a
substituent on the phenyl moiety selected from a lower alkoxy group, a halogen
5 atom, an amino group having optionally a substituent selected from a lower
alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower alkoxy -
substituted lower alkoxy group, a tetrazolyl group having optionally a lower
alkyl substituent on the tetrazole ring, hydroxy group, a group of the formula:
-O-A4-Co-NR40R41 (in which A4, R40 and R41 are the same as defined above),
10 a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and R1, R2, m, n and r are the same as defined above, or
a salt thereof.
(13) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a lower alkenyl group, and R1, R2, m, n and r are the
15 same as defined above, or a salt thereof.
(14) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a lower alkenyl group, and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(15) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a cycloalkyl-lower alkyl group, and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(16) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a cycloalkyl-lower alkyl group, and R1, R2, m, n and r are thesame as defined above, or a salt thereof.
25 (17) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a naphthyl-lower alkyl group, and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(18) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a naphthyl-lower alkyl group, and R1, R2, m, n and r are the
30 same as defined above, or a salt thereof.
(19) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a phenylthio-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
21503~
WO 9S/09159 - PCI~/JP94/OlS59
- 45 -
n and r are the same as defined above, or a salt thereof.
(20) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a phenylthio-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
5 n and r are the same as defined above, or a salt thereof.
(21 ) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a phenylsulfinyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
10 (22) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a phenylsulfinyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
(23) A quinoxaline derivative of the formula (1), wherein R3is
15 hydrogen atom, R4is a phenylsulfonyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
(24) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a phenylsulfonyl-substituted lower alkyl group which may
20 optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
(25) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a phenoxy-substituted lower alkyl group, and R1, R2, m, n
and r are the same as defined above, or a salt thereof.
25 (26) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a phenoxy-substituted lower alkyl group, and R1, R2, m, n and
r are the same as defined above, or a salt thereof.
(27) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a group of the formula:
(R8)q
(wherein the symbols are the same as defined above), and R1, R2, m, n and r
WO 95/O91S9 PCI`/JP94/OlS59
~ls~34s
- 46 -
are the same as defined above, or a salt thereof.
(28) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a group of the formula:
(R8)q
~
(in which the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(29) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a group of the formula: -A5-CR42R43R44(A5,R42,R43
and R44 are the same as defined above), and R1, R2, m, n and r are the same
as defined above, or a salt thereof.
(30) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a group of the formula: -A5-CR42R43R44 (A5,R42,R43 and
15 R44 are the same as defined above), and R1,R2, m, n and r are the same as
defined above, or a salt thereof.
(31) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a 2,3-dihydro-1 H-indenyl group-substituted lower alkyl
group which may optionally have a substituent selected from oxo group,
20 hydroxy group and a silyloxy group having a lower alkyl substituent on the 2,3 -
dihydro-1 H-indenyl ring, and R1, R2, m, n and r are the same as defined above,
or a salt thereof.
(32) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkyl group, R4is a 2,3-dihydro-1 H-indenyl group-substituted lower alkyl group
25 which may optionally have a substituent selected from oxo group, hydroxy
group and a silyloxy group having a lower alkyl substituent on the 2,3-dihydro -1 H-indenyl ring, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
(33) A quinoxaline derivative of the formula (1), wherein R3is
hydrogen atom, R4is a group of the
(R47)uformula:
{~
WO 95tO9159 2 1 5 0 3 ~ 5 PCT/JP94/OlSS9
- 47-
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(34) A quinoxaline derivative of the formula (1), wherein R3 is a lower
5 alkyl group, R4 is a group of the formula:
(R47)u
(in which the symbols are the same as defined above),
10 and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(35) A quinoxaline derivative of the formula (1), wherein R3 and R4
combine together with the nitrogen atom to form 1,2,3,4-tetrahydroisoquinolyl
group which may optionally have a lower alkoxy group, and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
15 (36) A quinoxaline derivative of the formula (1), wherein R1 is a
halogent atom or a lower alkyl group, R2 is hydrogen atom, R3 is hydrogen
atom or a lower alkyl group, R4 is a group of the formula:
(R5)
A~
(in which A, R5 and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
(37) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkenyl group, R2 is hydrogen atom, R3 is hydrogen
25 atom or a lower alkyl group, R4 is a phenyl-lower alkenyl group which may
optionally have a substituent on the phenyl moiety selected from a lower alkoxy
group, a halogen atom, an amino group having optionally a substituent
selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
30 optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula: -O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as
defined above), a lower alkenyloxy group, nitro group and a lower alkyl group
having optionally a halogen substituent, and m, n and r are the same as
WO 95/09159 PCT/JP94/01559
2~50345
- 48 -
defined above, or a salt thereof.
(38) A quinoxaline derivative of thQ formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3is hydrogen atom
or a lower alkyl group, R4is a lower alkenyl group, and m, n and r are the same
5 as defined above, or a salt thereof.
(39) A quinoxaline derivative of the formula t1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3is hydrogen atom
or a lower alkyl group, R4is a cycloalkyl-lower alkyl group, and m, n and r are
the same as defined above, or a salt thereof.
10 (40) A quinoxaline derivative of the formula (1), wherein R1is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3is hydrogen atom
or a lower alkyl group, R4is a naphthyl-lower alkyl group, and m, n and r are
the same as defined above, or a salt thereof.
(41 ) A quinoxaline derivative of the formula (1), wherein R1 is a
15 halogen atom or a lower alkyl group, R2is hydrogen atom, R3is hydrogen atom
or a lower alkyl group, R4is a naphthyl-lower alkyl group, and m, n and r are
the same as defined above, or a salt thereof.
(42) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3is hydrogen atom
20 or a lower alkyl group, R4iS a phenylthio-substitued lower alkyl group which
may optinally have a lower alkoxy substituent on the phenyl moiety, and m, n,
and r are the same as defined above, or a salt thereof.
(43) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3is hydrogen atom
25 or a lower alkyl group, R4is a phenylsulfinyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and m, n and r are the same as defined above, or a salt thereof.
(44) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3is hydrogen atom
30 or a lower alkyl group, R4is a phenylsulfonyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and m, n and r are the same as defined above, or a salt thereof.
(45) A quinoxaline derivative of the formula (1), wherein Rlis a
WO 95/09159 2 1 5 0 3 4 5 PCT1JP94/OlS59
^ 49 -
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3 is hydrogen atom
or a lower alkyl group, R4 is a phenoxy-substituted lower alkyl group, and m, n
- and r are the same as defined above, or a salt thereof.
(46) A quinoxaline derivative of the formula (1), wherein R1 is a
5 halogen atom or a lower alkyl group, R2 is hydrogen atom, R3 is hydrogen atom
or a lower alkyl group, R4 is a group of the formula:
(R8)q
10 (in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(47) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3 is hydrogen atom
or a lower alkyl group, R4 is a group of the formula: -A5-CR42R43R44 (A5, R42,
15 R43 and R44 are the same as defined above), and m, n and r are the same as
defined above, or a salt thereof.
(48) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3 is hydrogen atom
or a lower alkyl group, R4 is a 2,3-dihydro-1 H-indenyl group-substituted lower
20 alkyl group which may optionally have a substituent selected from oxo group,
hydroxy group and a silyloxy group having a lower alkyl substituent on the 2,3 -dihydro-1 H-indenyl ring, and m, n and r are the same as defined above, or a
salt thereof.
(49) A quinoxaline derivative of the formula (1), wherein R1 is a
25 halogen atom or a lower alkyl group, R2 is hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:
(R47)u
30 (in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(50) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is hydrogen atom, R3 and R4 combine
WO 95/09159 PCI/JP94/01559
2~503~S
- 50 -
together with the nitrogen atom to form 1,2,3,4-tetrahydroisoquinolyl group
which may optionally have a lowenalkoxy group), and m, n and r are the same
as defined above, or a salt thereof.
(51 ) A quinoxaline derivative of the formula (1), wherein R1 is a
5 halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally
a halogen substituent, R3 is hydrogen atom or a lower alkyl group, R4 is a
group of the formula:
(R5)p
A~
1 0
(in which A, R5 and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
(52) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally15 a halogen substituent, R3 is hydrogen atom or a lower alkyl group, R4 is a
phenyl-lower alkenyl group which may optionally have a substituent on the
phenyl moiety selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
20 group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole ring, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are the same as defined above), a lower alkenyloxy
group, nitro group and a lower alkyl group having optionally a halogen
substituent, and m, n and r are the same as defined above, or a salt thereof.
25 (53) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionallya halogen substituent, R3 is hydrogen atom or a lower alkyl group, R4 is a loweralkenyl group, and m, n and r are the same as defined above, or a salt thereof.
(54) A quinoxaline derivative of the formula (1), wherein R1 is a
30 halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally
a halogen substituent, R3 is hydrogen atom or a lower alkyl group, R4 is a
cycloalkyl-lower alkyl group, and m, n and r are the same as defined above, or
a salt thereof.
WO 95/09159 2 1 5 0 3 4 ~ PCT/JP94/01559
(55) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionallya halogen substituent, R3is hydrogen atom or a lower alkyl group, R4is a
naphthyl-lower alkyl group, and m, n and r are the same as defined above, or a
5 salt thereof.
(56) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionallya halogen substituent, R3is hydrogen atom or a lower alkyl group, R4is a
phenylthio-substituted lower alkyl group which may optionally have a lower
10 alkoxy substituent on the phenyl moiety, and m, n and r are the same as
defined above, or a salt thereof.
(57) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionallya halogen substituent, R3is hydrogen atom or a lower alkyl group, R4is a
15 phenylsulfinyl-substituted lower alkyl group which may optionally have a lower
alkoxy substituent on the phenyl moiety and m, n and r are the same as defined
above, or a salt thereof.
(58) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally20 a halogen substituent, R3is hydrogen atom or a lower alkyl group, R4is a
phenylsulfonyl-substituted lower alkyl group which may optionally have a lower
alkoxy substituent on the phenyl moiety, and m, n and r are the same as
defined above, or a salt thereof.
(59) A quinoxaline derivative of the formula (1), wherein R1 is a
25 halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally
a halogen substituent, R3is hydrogen atom or a lower alkyl group, R4is a
phenoxy-substituted lower alkyl group, and m, n and r are the same as defined
above, or a salt thereof.
(60) A quinoxaline derivative of the formula (1), wherein R1 is a
30 halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally
a halogen substituent, R3is hydrogen atom or a lower alkyl group, R4is a
group of the formula:
WO 95/09159 PCT/JP94/OlSS9
~ls~345
- 52 -
(R8)
(in which the symbols are the same as defined above), and m, n and r are the
5 same as defined above, or a salt thereof.
(61 ) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionallya halogen substituent, R3 is hydrogen atom or a lower alkyl group, R4 is a
group of the formula: -A5-CR42R43R44 (A5, R42, R43 and R44 are the same as
10 defined above), and m, n and r are the same as defined above, or a salt
thereof.
(62) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionallya halogen substituent, R3 is hydrogen atom or a lower alkyl group, R4 is a 2,3 -
15 dihydro-1 H-indenyl group-substituted lower alkyl group which may optionally
have a substituent selected from oxo group, hydroxy group and a silyloxy group
having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and m, n
and r are the same as defined above, or a salt thereof.
(63) A quinoxaline derivative of the formula (1), wherein R1 is a
20 halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally
a halogen substituent, R3 is hydrogen atom or a lower alkyl group, R4 is a
group of the formula:
(R47)u
~<
~J
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(64) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a lower alkyl group having optionally30 a halogen substituent, R3 and R4 combine together with the nitrogen atom to
form 1,2,3,4-tetrahydroisoquinolyl group which may optionally have a lower
alkoxy group, and m, n and r are the same as defined above, or a salt thereof.
(65)A quinoxaline derivative of the formula (1), wherein R1 is a
21~03 1~
WO 9S/O91S9 PCI/JP94/015S9
halogen atom or a lower alkyl group, R2 is phenyl group, R3is hydrogen atom
or a lower alkyl group, R4is a group of the formula:
A~ (R5)p
(in which A,R5 and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
(66) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3is hydrogen atom
10 or a lower alkyl group, R4is a phenyl-lower alkenyl group which may optionally
have a substituent on the phenyl moiety selected from a lower alkoxy group, a
halogen atom, an amino group having optionally a substituent selected from a
lower alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower
alkoxy-substituted lower alkoxy group, a tetrazolyl group having optionally a
15 lower alkyl substituent on the tetrazole ring, hydroxy group, a group of the
formula: -O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as defined
above), a lower alkenyloxy group, nitro group and a lower alkyl group having
optionally a halogen substituent, and m, n and r are the same as defined
above, or a salt thereof.
(67) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3is hydrogen atom
or a lower alkyl group, R4is a lower alkenyl group, and m, n and r are the same
as defined above, or a salt thereof.
(68) A quinoxaline derivative of the formula (1), wherein R1 is a
25 halogen atom or a lower alkyl group, R2 is phenyl group, R3is hydrogen atom
or a lower alkyl group, R4is a cycloalkyl-lower alkyl group, and m, n and r are
the same as defined above, or a salt thereof.
(69) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3is hydrogen atom
30 or a lower alkyl group, R4is a naphthyl-lower alkyl group, and m, n and r are the same as defined above, or a salt thereof.
(70) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3is hydrogen atom
WO 9S/09159 PCT/JP94/015S9
2lso34s
- 54 -
or a lower alkyl group, R4 is a phenylthio-substituted lower alkyl group which
may optionally have a lower alkoxy substituent on the phenyl moiety, and m, n
and r are the same as defined above, or a salt thereof.
(71 ) A quinoxaline derivative of the formula (1), wherein R1 is a
5 halogen atom or a lower alkyl group, R2 is phenyl group, R3 is hydrogen atom
or a lower alkyl group, R4 is a phenylsulfinyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety and
m, n and r are the same as defined above, or a salt thereof.
(72) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group,R2 is phenyl group, R3 is hydrogen atom
or a lower alkyl group, R4 is a phenylsulfonyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and m, n and r are the same as defined above, or a salt thereof.
(73) A quinoxaline derivative of the formula (1), wherein R1 is a
15 halogen atom or a lower alkyl group, R2 is phenyl group, R3 is hydrogen atom
or a lower alkyl group, R4 is a phenoxy-substituted lower alkyl group, and m, n
and r are the same as defined above, or a salt thereof.
(74) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3 is hydrogen atom
20 or a lower alkyl group, R4 is a group of the formula:
(R8)q
{~
(in which the symbols are the same as defined above), and m, n and r are the
25 same as defined above, or a salt thereof.
(75) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3 is hydrogen atom
or a lower alkyl group, R4 is a group of the formula: -A5-CR42R43R44 (A5, R42,
R43 and R44 are the same as defined above), and m, n and r are the same as
30 defined above, or a salt thereof.
(76) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3 is hydrogen atom
or a lower alkyl group, R4 is a 2,3-dihydro-1 H-indenyl group-substituted lower
WO 95/09159 PCI~/JP94/01559
21503~5
- 55 -
alkyl group which may optionally have a substituent selected from oxo group,
hydroxy group and a silyloxy group having a lower alkyl substituent on the 2,3 -dihydro-1 H-indenyl ring, and m, n and r are the same as defined above, or a
salt thereof.
5 (77) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3 is hydrogen atom
or a lower alkyl group, R4 is a group of the formula:
(R47)
~<
~)
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(78) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is phenyl group, R3 and R4 combine
15 together with the nitrogen atom to form 1,2,3,4-tetrahydroisoquinolyl group
which may optionally have a lower alkoxy group), and m, n and r are the same
as defined above, or a salt thereof.
(79) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
20 group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:
(R5)p
A~
(in which A, R5 and p are the same as defined above),
25 and m, n and r are the same as defined above, or a salt thereof.
(80) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenyl-lower alkenyl
group which may optionally have a substituent on the phenyl moiety selected
30 from a lower alkoxy group, a halogen atom, an amino group having optionally asubstituent selected from a lower alkanoyl group and a phenyl-lower alkenyl -
carbonyl group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl
group having optionally a lower alkyl substituent on the tetrazole ring, hydroxy
PCT/~4101559
WO95/09159
2~S o3 4S -56-
group, a group of the formula: -O-A4-CO-NR40R41 (in which A4, R40 and R41
are the same as defined above), a lower alkenyloxy group, nitro group and a
lower alkyl group having optionally a halogen substituent, and m, n and r are
the same as defined above, or a salt thereof.
5 (81) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3is hydrogen atom or a lower alkyl group, R4is a lower alkenyl group,
and m, n and r are the same as defined above, or a salt thereof.
(82) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3is hydrogen atom or a lower alkyl group, R4is a cycloalkyl-lower alkyl
group, and m, n and r are the same as defined above, or a salt thereof.
(83) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
15 group, R3is hydrogen atom or a lower alkyl group, R4iS a naphthyl-lower alkyl group, and m, n and r are the same as defined above, or a salt thereof.
(84) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3iS hydrogen atom or a lower alkyl group, R4is a phenylthio -
20 substituted lower alkyl group which may optionally have a lower alkoxysubstituent on the phenyl moiety, and m, n and r are the same as defined
above, or a salt thereof.
(85) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
25 group, R3iS hydrogen atom or a lower alkyl group, R4is a phenylsulfinyl -
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety and m, n and r are the same as defined
above, or a salt thereof.
(86) A quinoxaline derivative of the formula (1), wherein R1 is a
30 halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3is hydrogen atom or a lower alkyl group, R4 is a phenylsulfonyl -
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety, and m, n and r are the same as defined
WO 9S/09159 PCI/JP94101559
21503~
- 57 -
above, or a salt thereof.
(87) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenoxy-substituted
5 lower alkyl group, and m, n and r are the same as defined above, or a salt
thereof.
(88) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:
1 0 (R8)q
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(89) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:-A5-CR42R43R44 (A5, R42, R43 and R44 are the same as defined above), and m,
n and r are the same as defined above, or a salt thereof.
(90) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a 2,3-dihydro-1 H -
indenyl group-substituted lower alkyl group which may optionally have a
substituent selected from oxo group, hydroxy group and a silyloxy group having
a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and m, n and r are
the same as defined above, or a salt thereof.
(91 ) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula: (R47)
~,
(in which the symbols are the same as defined above), and m, n and r are the
WO 95/O91S9 2~S o3 45 PCT/JP94101559
same as defined above, or a salt thereof.
(92) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is a morpholino-substituted lower alkyl
group, R3 and R4 combine together with the nitrogen atom to form 1,2,3,4 -
5 tetrahydroisoquinolyl group which may optionally have a lower alkoxy group),and m, n and r are the same as defined above, or a salt thereof.
(93) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:1 0 (R5)p
A~
(in which A, R5 and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
15 (94) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a phenyl-lower alkenyl
group which may optionally have a substituent on the phenyl moiety selected
from a lower alkoxy group, a halogen atom, an amino group having optionally a
20 substituent selected from a lower alkanoyl group and a phenyl-lower alkenyl -carbonyl group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl
group having optionally a lower alkyl substituent on the tetrazole ring, hydroxygroup, a group of the formula: -O-A4-CO-NR4R41 (in which A4, R40 and R41
are the same as defined above), a lower alkenyloxy group, nitro group and a
25 lower alkyl group having optionally a halogen substituent, and m, n and r are the same as defined above, or a salt thereof.
(95) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a lower alkenyl group,
30 and m, n and r are the same as defined above, or a salt thereof.
(96) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a cycloalkyl-lower alkyl
WO 95/09159 2 1 5 0 3 ~ 5 PCI/JP94/01559
_
- 59 -
group, and m, n and r are the same as defined above, or a salt thereof.
(97) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a naphthyl-lower alkyl
5 group, and m, n and r are the same as defined above, or a salt thereof.
(98) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a phenylthio -
substituted lower alkyl group which may optionally have a lower alkoxy
10 substituent on the phenyl moiety, and m, n and r are the same as defined
above, or a salt thereof.
(99) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a phenylsulfinyl -
15 substituted lower alkyl group which may optionally have a lower alkoxysubstituent on the phenyl moiety and m, n and r are the same as defined
above, or a salt thereof.
(100) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkyl20 group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenylsulfonyl -
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety, and m, n and r are the same as defined
above, or a salt thereof.
(101 ) A quinoxaline derivative of the formula (1), wherein R1 is a
25 halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenoxy-substituted
lower alkyl group, and m, n and r are the same as defined above, or a salt
thereof.
(102) A quinoxaline derivative of the formula (1), wherein R1 is a
30 halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:
WO 9S/09159 PCT/JP94/OlS59
2l503~
- 60 -
(R8)q
~-,.
(in which the symbols are the same as defined above), and m, n and r are the
5 same as defined above, or a salt thereof.
(103) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:-A5-CR42R43R44 (A5, R42, R43 and R44 are the same as defined above), and m,
10 n and r are the same as defined above, or a salt thereof.
(104) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a 2,3-dihydro-1 H -
indenyl group-substituted lower alkyl group which may optionally have a
15 substituent selected from oxo group, hydroxy group and a silyloxy group having
a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and m, n and r are
the same as defined above, or a salt thereof.
(105) A quinoxaline derivative of the formula (1), wherein Rl is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkyl20 group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:
(R47)u
~<
(in which the symbols are the same as defined above), and m, n and r are the
25 same as defined above, or a salt thereof.
(106) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group, R2 is an imidazolyl-substituted lower alkylgroup, R3 and R4 combine together with the nitrogen atom to form 1,2,3,4 -
tetrahydroisoquinolyl group which may optionally have a lower alkoxy group),
30 and m, n and r are the same as defined above, or a salt thereof.
(107) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3 is hydrogen atom, R4 is a group of the formula:
WO 95/09159 2 1 ~ 0 3 ~ 5 PCT/JP94/01559
- 61 -
A ~ (R5)p
(wherein A,R5 and p are the same as defined above),
and R2, m, n and r are the same as defined above, or a salt thereof.
(108) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3is a lower alyl group, R4is a group of the formula:
(Rs jp
A
(wherein A,R5 and p are the same as defined above),
and R2, m, n and r are the same as defined above, or a salt thereof.
(109) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3is hydrogen atom, R4is a phenyl-lower alkenyl group which
may optionally have a substituent on the phenyl moiety selected from a lower
alkoxy group, a halogen atom, an amino group having optionally a substituent
selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula: -o-A4-Co-NR4R41 (in which A4, R40 and R41 are the same as
defined above), a lower alkenyloxy group, nitro group and a lower alkyl group
having optionally a halogen substituent, and R2, m, n and r are the same as
defined above, or a salt thereof.
(110) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3iS a lower alkyl group, R4is a phenyl-lower alkenyl group
which may optionally have a substituent on the phenyl moiety selected from a
lower alkoxy group, a halogen atom, an amino group having optionally a
substituent selected from a lower alkanoyl group and a phenyl-lower alkenyl -
carbonyl group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl
group having optionally a lower alkyl substituent on the tetrazole ring, hydroxygroup, a group of the formula: -O-A4-CO-NR40R41 (in which A4, R40 and R41
are the same as defined above), a lower alkenyloxy group, nitro group and a
lower alkyl group having optionally a halogen substituent, and R2, m, n and r
WO gS/Ogl59 2 ~S O 3 45 PCT/JPg4/01559
- 62 -
are the same as defined above, or a salt thereof.
(111 ) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3is hydrogen atom, R4is a lower alkenyl group, and R2, m, n
and r are the same as defined above, or a salt thereof.
(112) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3is a lower alkyl group, R4is a lower alkenyl group, and R2,
m, n and r are the same as defined above, or a salt thereof.
(113) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3is hydrogen atom, R4is a cycloalkyl-lower alkyl group, and
R2, m, n and r are the same as defined above, or a salt thereof.
(114) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3is a lower alkyl group, R4is a cycloalkyl-lower alkyl group,
and R2, m, n and r are the same as defined above, or a salt thereof.
(115) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3is hydrogen atom, R4is a naphthyl-lower alkyl group, and
R2, m, n and r are the same as defined above, or a salt thereof.
(116) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3is a lower alkyl group, R4is a naphthyl-lower alkyl group,
and R2, m, n and r are the same as defined above, or a salt thereof.
(117) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3is hydrogen atom, R4is a phenylthio-substituted lower alkyl
group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R2, m, n and r are the same as defined above, or a salt thereof.
(118) A quinoxa ine derivative of the fornula (1), wherein R1is
hydrogen atom, R3is l lower alkyl group, R4is a phenylthio-substituted lower
alkyl group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R2, m, r. and r are the same as defined above, or a salt thereof.
(119) A quinoxaline derivative of the formula (1), wherein R1is
hydrogen atom, R3 is hydrogen atom, R4is a phenylsulfinyl-substituted lower
alkyl group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R2, m, n and r are the same as defined above, or a salt thereof.
(120) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen ator " R3is a lower alkyl group, R4is a phenylsulfinyl-substituted
WO 95/09159 2 1 5 0 3 ~ 5 PCI/JP94/OlS59
- 63 -
lower alkyl group which may optionally have a lower alkoxy substituent on the
phenyl moiety, and R2, m, n and r are the same as defined above, or a salt
thereof.
(121) A quinoxaline derivative of the formula (1), wherein R1 is
5 hydrogen atom, R3 is hydrogen atom, R4 is a phenylsulfonyl-substituted lower
alkyl group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R2, m, n and r are the same as defined above, or a salt thereof.
(122) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3 is a lower alkyl group, R4 is a phenylsulfonyl-substituted
10 lower alkyl group which may optionally have a lower alkoxy substituent on thephenyl moiety, and R2, m, n and r are the same as defined above, or a salt
thereof.
(123) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3 is hydrogen atom, R4 is a phenoxy-substituted lower alkyl
15 group, and R2, m, n and r are the same as defined above, or a salt thereof.
(124) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3 is a lower alkyl group, R4 is a phenoxy-substituted lower
alkyl group, and R2, m, n and r are the same as defined above, or a salt thereof.
(125) A quinoxaline derivative of the formula (1), wherein R1 is
20 hydrogen atom, R3 is hydrogen atom, R4 is a group of the formula:
(Ra)q
(wherein the symbols are the same as defined above), and R2, m, n and r are
25 the same as defined above, or a salt thereof.
(126) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3 is a lower alkyl group, R4 is a group of the formula:
(R8)q
~
(wherein the symbols are the same as defined above), and R2, m, n and r are
the same as defined above, or a salt thereof.
(127) A quinoxaline derivative of the formula (1), wherein R1 is
wo gs/ogl592 15 03 4~ PCT/JP94/01559
- 64 -
hydrogen atom, R3is hydrogen atom, R4is a group of the formula:
-A5-CR42R43R44(A5,R42,R43 and R44 are the same as defined above), and
R2, m, n and r are the same as defined above, or a salt thereof.
(128) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3is a lower alkyl group, R4is a group of the formula:
-A5-CR42R43R44(A5,R42,R43 and R44 are the same as defined above), and
R2, m, n and r are the same as defined above, or a salt thereof.
(129) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3is hydrogen atom, R4is a 2,3-dihydro-1 H-indenyl group -
substituted lower alkyl group which may optionally have a substituent selected
from oxo group, hydroxy group and a silyloxy group having a lower alkyl
substituent on the 2,3-dihydro-1 H-indenyl ring, and R2, m, n and r are the sameas defined above, or a salt thereof.
(130) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3is a lower alkyl group, R4is a 2,3-dihydro-1 H-indenyl group -
substituted lower alkyl group which may optionally have a substituent selected
from oxo group, hydroxy group and a silyloxy group having a lower alkyl
substituent on the 2,3-dihydro-1 H-indenyl ring, and R2, m, n and r are the sameas defined above, or a salt thereof.
(131) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3is hydrogen atom, R4is a group of the formula:
(R47)u
,~
,~,
(in which the symbols are the same as defined above), and R2, m, n and r are
the same as defined above, or a salt thereof.
(132) A quinoxaline derivative of the formula (1), wherein Rlis
hydrogen atom, R3is a lower alkyl group, R4is a group of the formula:
(R47)u
~
(in which the symbols are the same as defined above), and R2, m, n and r are
the same as defined above, or a salt thereof.
WO95109159 2 1 5 ~ 3 4 ~ PCT/JP94101559
- 65 -
(133) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom, R3 and R4 combine together with the nitrogen atom to form
1,2,3,4-tetrahydroquinolyl group which may optionally have a lower alkoxy
group), and R2, m, n and r are the same as defined above, or a salt thereof.
5 (134) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group and R2, R3,R4, m, n and r are the same as defined above, or a
salt thereof.
(135) A quinoxaline derivative of the formula (1),wherein R1 is an amino
group having optionally a lower alkyl substituent and R2, R3,R4, m, n and r are
10 the same as defined above, or a salt thereof.
(136) A quinoxaline derivative of the formula (1),wherein R1 is an
aminocarbonyl group having optionally a lower alkyl substituent and R2, R3,R4,
m, n and r are the same as defined above, or a salt thereof.
(137) A quinoxaline derivative of the formula (1),wherein R3is a phenyl -
15 lower alkoxycarbonyl group and R4is a group of the formula:
(R5)p
A~
(in which A, R5 and p are the same as defined above), and R1, R2, m, n and r
20 are the same as defined above, or a salt thereof.(138) A quinoxaline derivative of the formula (1),wherein R3is a lower
alkanoyloxy-substituted lower alkyl group and R4is a group of the formula:
A~ (R5)p
(in which A, R5 and p are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(139) A quinoxaline derivative of the formula (1),wherein R3is a phenyl -
lower alkoxycarbonyl group and R4is a phenyl-lower alkenyl group which may
optionally have a substituent on the phenyl ring selected from a lower alkoxy
group, a halogen atom, an amino group having optionally a substituent
selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
WO 95/09159 PCT/JP94/OlSS9
~2l~03~
- 66 -
optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula: -o-A4-Co-NR4R41 (in which A4, R40 and R41 are the same as
defined above), a lower alkenyloxy group, nitro group and a lower alkyl group
having optionally a halogen substituent, and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(140) A quinoxaline derivative of the formula (1),wherein R3is a lower
alkanoyloxy-substituted lower aikyl group and R4is a phenyl-lower alkenyl
group which may optionally have a substituent on the phenyl ring selected from
a lower alkoxy group, a halogen atom, an amino group having optionally a
substituent selected from a lower alkanoyl group and a phenyl-lower alkenyl -
carbonyl group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl
group having optionally a lower alkyl substituent on the tetrazole ring, hydroxygroup, a group of the formula: -O-A4-CO-NR4R41 (in which A4, R40 and R41
are the same as defined above), a lower alkenyloxy group, nitro group and a
lower alkyl group having optionally a halogen substituent, and R1,R2, m, n and
r are the same as defined above, or a salt thereof.
(141) A quinoxaline derivative of the formula (1),wherein R3is a phenyl -
lower alkoxycarbonyl group and R4is a lower alkenyl group, and R1, R2, m, n
and r are the same as defined above, or a salt thereof.
(142) A quinoxaline derivative of the formula (1),wherein R3is a lower
alkanoyloxy-substituted lower alkyl group and R4is a lower alkenyl group, and
R1, R2, m, n and r are the same as defined above, or a salt thereof.
(143) A quinoxaline derivative of the formula (1),wherein R3is a phenyl -
lower alkoxycarbonyl group and R4is a cycloalkyl-lower alkyl group, and R1,
R2, m, n and r are the same as defined above, or a salt thereof.
(144) A quinoxaline derivative of the formula (1),wherein R3is a lower
alkanoyloxy-substituted lower alkyl group and R4is a cycloalkyl-lower alkyl
group, and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(145) A quinoxaline derivative of the formula (1),wherein R3iS a phenyl -
lower alkoxycarbonyl group and R4is a naphthyl-lower alkyl group, and R1, R2,
m, n and r are the same as defined above, or a salt thereof.
(146) A quinoxaline derivative of the formula (1),wherein R3is a lower
alkanoyloxy-substituted lower alkyl group and R4is a naphthyi-lower alkyl
wogs/ogl59 2 1 S 0 3 4 ~ PCT/JP94/OlSS9
- 67 -
group, and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(147) A quinoxaline derivative of the formula (1),wherein R3is a phenyl -
lower alkoxycarbonyl group and R4is a phenylthio-substituted lower alkyl
group having optionally a lower alkoxy substituent on the phenyl ring, and R1,
5 R2, m, n and r are the same as defined above, or a salt thereof.
(148) A quinoxaline derivative of the formula (1),wherein R3is a lower
alkanoyloxy-substituted lower alkyl group and R4 is a phenylthio-substituted
lower alkyl group having optionally a lower alkoxy substituent on the phenyl
ring, and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(149) A quinoxaline derivative of the formula (1),wherein R3is a phenyl -
lower alkoxycarbonyl group and R4is a phenylsulfinyl-substituted lower alkyl
group having optionally a lower alkoxy substituent on the phenyl ring, and R1,
R2, m, n and r are the same as defined above, or a salt thereof.
(150) A quinoxaline derivative of the formula (1), wherein R3is a lower
15 alkanoyloxy-substituted lower alkyl group, R4is a phenylsulfinyl-substituted
lower alkyl group which may optionally have a lower alkoxy substituent on the
phenyl moiety, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
(151) A quinoxaline derivative of the formula (1), wherein R3is a phenyl -
20 lower alkoxycarbonyl group, R4is a phenylsulfonyl-substituted lower alkyl
group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R1, R2, m, n and r are the same as defined above, or a salt thereof.(152) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyloxy-substituted lower alkyl group, R4is a phenylsulfonyl-substituted
25 lower alkyl group which may optionally have a lower alkoxy substituent on thephenyl moiety, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
(153) A quinoxaline derivative of the formula (1), wherein R3is a phenyl -
lower alkoxycarbonyl group, R4is a phenoxy-substituted lower alkyl group, and
30 R1, R2, m, n and r are the same as defined above, or a salt thereof.
(154) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyloxy-substituted lower alkyl group, R4is a phenoxy-substituted lower
alkyl group, and R1, R2, m, n and r are the same as defined above, or a salt
WO 95/09159 PCI/JP94/OlS59
2~so3 ~
- 68 -
thereof.
(155) A quinoxaline derivative of the formula (1), wherein R3is a phenyl -
lower alkoxycarbonyl group, R4is a group of the formula:
(R8)q
~
(wherein the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(156) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyloxy-substituted lower alkyl group, R4is a group of the formula:
(R8)q
(in which the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(157) A quinoxaline derivative of the formula (1), wherein R3is a phenyl -
lower alkoxycarbonyl group, R4is a group of the formula: -A5-CR42R43R44 (A5,
R42,R43 and R44 are the same as defined above), and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(158) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyloxy-substituted lower alkyl group, R4is a group of the formula:
-A5-CR42R43R44 (A5, R42, R43 and R44 are the same as defined above), and
R1, R2, m, n and r are the same as defined above, or a salt thereof.
(159) A quinoxaline derivative of the formula (1), wherein R3is a phenyl -
lower alkoxycarbonyl group, R4is a 2,3-dihydro-1 H-indenyl group-substituted
lower alkyl group which may optionally have a substituent selected from oxo
group, hydroxy group and a silyloxy group having a lower alkyl substituent on
the 2,3-dihydro-1H-indenyl ring, and R1, R2, m, n and r are the same as defined
above, or a salt thereof.
(160) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyloxy-substituted lower alkyl group, R4is a 2,3-dihydro-1 H-indenyl
group-substituted lower alkyl group which may optionally have a substituent
selected from oxo group, hydroxy group and a silyloxy group having a lower
WO 95/09159 2~ 1 5 0 3 4 ~i PCI/JP94/01559
- 69 -
alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(161 ) A quinoxaline derivative of the formula (1), wherein R3is a phenyl -
lower alkoxycarbonyl group, R4is a group of the formula:
(R47)
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(162) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyloxy-substituted lower alkyl group, R4is a group of the formula:
(R47)
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(163) A quinoxaline derivative of the formula (1) wherein R3is a lower
alkanoyl group, R4is a group of the formula:
(R5)p
A ~
(wherein A,R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(164) A quinoxaline derivative of the formula (1) wherein R3is a lower
alkoxycarbonyl group, R4is a group of the formula:
(R5)p
A~
(wherein A, R5 and p are the same as defined above),
30 and R1, R2 and r are the same as defined above, or a salt thereof.
(165) A quinoxaline derivative of the formula (1) wherein R3is a lower
alkanoyl group, R4is a phenyl-lower alkenyl group which may optionally have
a substituent on the phenyl moiety selected from a lower alkoxy group, a
WO95/09159 rcT/Jrg4/olssg
2~a3~s
- 70 -
halogen atom, an amino group having optionally a substituent selected from a
lower alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower
alkoxy-substituted lower alkoxy group, a tetrazolyl group having optionally a
lower alkyl substituent on the tetrazole ring, hydroxy group, a group of the
formula: -o-A4-CO-NR40R41 (in which A4, R40 and R41 are the same as defined
above), a lower alkenyloxy group, nitro group and a lower alkyl group having
optionally a halogen substituent, and R1, R3,R4, m, n and r are the same as
defined above, or a salt thereof.
(166) A quinoxaline derivative of the formula (1) wherein R3is a lower
alkoxycarbonyl group, R4is a phenyl-lower alkenyl group which may optionally
have a substituent on the phenyl moiety selected from a lower alkoxy group, a
halogen atom, an amino group having optionally a substituent selected from a
lower alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower
alkoxy-substituted lower alkoxy group, a tetrazolyl group having optionally a
lower alkyl substituent on the tetrazole ring, hydroxy group, a group of the
formula:
-O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as defined above),
a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and R1, R3,R4, m, n and r are the same as defined
above, or a salt thereof.
(167) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4is a lower alkenyl group, and R1, R2, m, n and r are the
same as defined above, or a salt thereof.
(168) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a lower alkenyl group, and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(169) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4is a cycloalkyl-lower alkyl group, and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(170) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a cycloalkyl-lower alkyl group, and R1, R2, m, n and
r are the same as defined above, or a salt thereof.
(171) A quinoxaline derivative of the formula (1), wherein R3is a lower
WO 95/O91S9 PCI~/JP94/OlSS9
- 21~0345
alkanoyl group, R4is a naphthyl-lower alkyl group, and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(172) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a naphthyl-lower alkyl group, and R1, R2, m, n and
5 r are the same as defined above, or a salt thereof.
(173) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4is a phenylthio-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
(174) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a phenylthio-substituted lower alkyl group which
may optionally have a lower alkoxy substituent on the phenyl moiety, and R1,
R2, m, n and r are the same as defined above, or a salt thereof.
(175) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkanoyl group, R4is a phenylsulfinyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
(176) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a phenylsulfinyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(177) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4is a phenylsulfonyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
(178) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a phenylsulfonyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(179) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4is a phenoxy-substituted lower alkyl group, and R1, R2, m, n
and r are the same as defined above, or a salt thereof.
(180) A quinoxaline derivative of the formula (1), wherein R3is a lower
WO 95tO9lS9 PCI/JP94/01559
~5~3~ - 72 -
alkoxyca nyl group, R4is a phenoxy-substituted lower alkyl group, and R1,
R2, m, n and r are the same as defined above, or a salt thereof.
(181) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4is a group of the formula:
(R8)
(wherein the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(182) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a group of the formula:
(R8)q
(in which the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(183) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4is a group of the formula: -A5-CR42R43R44 (A5, R42, R43 and
R44 are the same as defined above), and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(184) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a group of the formula: -A5-CR42R43R44 (A5, R42,
R43 and R44 are the same as defined above), and R1, R2, m, n and r are the
same as defined above, or a salt thereof.
(185) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl group, R4iS a 2,3-dihydro-1H-indenyl group-substituted lower alkyl
group which may optionally have a substituent selected from oxo group,
hydroxy group and a silyloxy group having a lower alkyl substituent on the 2,3 -dihydro-1 H-indenyl ring, and R1, R2, m, n and r are the same as defined above,
or a salt thereof.
(186) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a 2,3-dihydro-1H-indenyl group-substituted lower
alkyl group which may optionally have a substituent selected from oxo group,
WO 95/09159 2 1 5 0 3 ~ ~ PCT/JP94/01559
- 73-
hydroxy group and a silyloxy group having a lower alkyl substituent on the 2,3 -dihydro-1 H-indenyl ring, and R1, R2, m, n and r are the same as defined above,
or a salt thereof.
(187) A quinoxaline derivative of the formula (1), wherein R3is a lower
5 alkanoyl group, R4is a group of the formula:
(R47)u
~,
(in which the symbols are the same as defined above),
10 and R1,R2, m, n and r are the same as defined above, or a salt thereof.
(188) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyl group, R4is a group of the formula:
(R47)u
~
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(189) A quinoxaline derivative of the formula (1) wherein R3is a lower
alkoxy-lower alkyl group, R4is a group of the formula:
(R5)p
A~
(wherein A, R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(190) A quinoxaline derivative of the formula (1 ) wherein R3is
phenoxycarbonyl group, R4is a group of the formula:
(R5)p
A~
\=J
(wherein A, R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(191) A quinoxaline derivative of the formula (1) wherein R3is a lower
alkoxy-lower alkyl group, R4is a phenyl-lower alkenyl group which may
WO95/09159 PCT/JPg4/olSS9
2~S o3 4S
optionally have a substituent on the phenyl moiety selected from a lower alkoxy
group, a halogen atom, an amino group having optionally a substituent
selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
5 optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula:
-O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as defined above),
a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and R1, R3,R4, m, n and r are the same as defined
10 above, or a salt thereof.
(192) A quinoxaline derivative of the formula (1) wherein R3is
phenoxycarbonyl group, R4is a phenyl-lower alkenyl group which may
optionally have a substituent on the phenyl moiety selected from a lower alkoxy
group, a halogen atom, an amino group having optionally a substituent
15 selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula:
-O-A4-Co-NR4R41 (in which A4, R40 and R41 are the same as defined above),
20 a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and R1, R3,R4, m, n and r are the same as defined
above, or a salt thereof.
(193) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a lower alkenyl group, and R1, R2, m, n and r are25 the same as defined above, or a salt thereof.
(194) A quinoxaline derivative of the formula (1), wherein R3is phenoxy
carbonyl group, R4is a lower alkenyl group, and R1, R2, m, n and r are the
same as defined above, or a salt thereof.
(195) A quinoxaline derivative of the formula (1), wherein R3iS a lower
30 alkoxy-lower alkyl group, R4is a cycloalkyl-lower alkyl group, and R1, R2, m, n
and r are the same as defined above.
(196) A quinoxaline derivative of the formula (1), wherein R3 is
phenoxycarbonyl group, R4 is a cycloalkyl-lower alkyl group, and R1, R2, m, n
WO 95/09159 PCT/JP94/01559
2150345
- 75 -
and r are the same as defined above, or a salt thereof.
(197) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a naphthyl-lower alkyl group, and R1, R2, m, n
and r are the same as defined above, or a salt thereof.
(198) A quinoxaline derivative of the formula (1), wherein R3is
phenoxycarbonyl group, R4is a naphthyl-lower alkyl group, and R1, R2, m, n
and r are the same as defined above, or a salt thereof.
(199) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a phenylthio-substituted lower alkyl group which
may optionally have a lower alkoxy substituent on the phenyl moiety, and Rl,
R2, m, n and r are the same as defined above, or a salt thereof.
(200) A quinoxaline derivative of the formula (1), wherein R3is
phenoxycarbonyl group, R4is a phenylthio-substituted lower alkyl group which
may optionally have a lower alkoxy substituent on the phenyl moiety, and R1,
R2, m, n and r are the same as defined above, or a salt thereof.
(201 ) A quinoxaline derivative of the formula (1), wherein R3is a lower alkoxy -
lower alkyl group, R4is a phenylsulfinyl-substituted lower alkyl group which
may optionally have a lower alkoxy substituent on the phenyl moiety, and R1,
R2, m, n and r are the same as defined above, or a salt thereof.
(202) A quinoxaline derivative of the formula (1), wherein R3is
phenoxycarbonyl group, R4is a phenylsulfinyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(203) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a phenylsulfonyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(204) A quinoxaline derivative of the formula (1), wherein R3is
phenoxycarbonyl group, R4is a phenylsulfonyl-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and Rl, R2, m, n and r are the same as defined above, or a salt thereof.
(205) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a phenoxy-substituted lower alkyl group, and
WO 95/09159 PCT/JP94/01559
2l5034s
- ~6 -
R1, R2, m, n and r are the same as defined above, or a salt thereof.
(206) A quinoxaline derivative of the formula (1), wherein R3is
phenoxycarbonyl group, R4is a phenoxy-substituted lower alkyl group, and R1,
R2, m, n and r are the same as defined above, or a salt thereof.
(207) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a group of the formula:
(R8)q
(wherein the symbols are the same as defined above), and R1,R2, m, n and r
are the same as defined above, or a salt thereof.
(208) A quinoxaline derivative of the formula (1), wherein R3is
phenoxycarbonyl group, R4is a group of the formula:
tR8)q
~
(in which the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(209) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a group of the formula: -A5-CR42R43R44 (A5,
R42,R43 and R44 are the same as defined above), and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(210) A quinoxaline derivative of the formula (1), wherein R3is
phenoxycarbonyl group, R4is a group of the formula: -A5-CR42R43R44 (A5,
R42, R43 and R44 are the same as defined above), and R1, R2, m, n and r are
the same as defined above, or a salt thereof.
(211 ) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxy-lower alkyl group, R4is a 2,3-dihydro-1 H-indenyl group-substituted
lower alkyl group which may optionally have a substituent selected from oxo
group, hydroxy group and a silyloxy group having a lower alkyl substituent on
the 2,3-dihydro-1H-indenyl ring, and R1, R2, m, n and r are the same as defined
above, or a salt thereof.
(212) A quinoxaline derivative of the formula (1), wherein R3is phenoxy-
WO 9S/09159 2 1 5 0 3 4 S PCI~/JP94/01559
- 77-
carbonyl group, R4 is a 2,3-dihydro-1 H-indenyl group-substituted lower alkyl
group which may optionally have a substituent selected from oxo group,
hydroxy group and a silyloxy group having a lower alkyl substituent on the 2,3 -dihydro-1 H-indenyl ring, and R1, R2, m, n and r are the same as defined above,
or a salt thereof.
(213) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkoxy-lower alkyl group, R4 is a group of the formula:
(R47)u
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(214) A quinoxaline derivative of the formula (1), wherein R3 is phenoxy -
carbonyl group, R4 is a group of the formula:
1 5 (R47)u
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(215) A quinoxaline derivative of the formula (1) wherein R3 is a lower
alkanoyl-substituted lower alkyl group, R4 is a group of the formula:
(R5)p
A~
(wherein A, R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(216) A quinoxaline derivative of the formula (1) wherein R3 is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4 is a group of the formula:
(R5)p
A~
\=/
(wherein A, R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
WOgS/09159 PCT/~4/01559
345 78-
(217) A quinoxaline derivative of the formula (1) wherein R3is lower
alkanoyl-substituted lower alkyl group, R4is a phenyl-lower alkenyl group
which may optionally have a substituent on the phenyl moiety selected from a
lower alkoxy group, a halogen atom, an amino group having optionally a
5 substituent selected from a lower alkanoyl group and a phenyl-lower alkenyl -
carbonyl group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl
group having optionally a lower alkyl substituent on the tetrazole ring, hydroxygroup, a group of the formula: -O-A4-CO-NR4R41 (in which A4, R40 and R41
are the same as defined above), a lower alkenyloxy group, nitro group and a
10 lower alkyl group having optionally a halogen substituent, and R1,R3,R4, m, n and r are the same as defined above, or a salt thereof.
(218) A quinoxaline derivative of the formula (1) wherein R3is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4is a phenyl-lower alkenyl
group which may optionally have a substituent on the phenyl moiety selected
15 from a lower alkoxy group, a halogen atom, an amino group having optionally asubstituent selected from a lower alkanoyl group and a phenyl-lower alkenyl -
calbonyl group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl
group having optionally a lower alkyl substituent on the tetrazole ring, hydroxygroup, a group of the formula: -O-A4-Co-NR4R41 (in which A4, R40 and R41
20 are the same as defined above), a lower alkenyloxy group, nitro group and a
lower alkyl group having optionally a halogen substituent, and R1, R3,R4, m, n
and r are the same as defined above, or a salt thereof.
(219) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl-substituted lower alkyl group, R4is a lower alkenyl group, and R1, R2,
25 m, n and r are the same as defined above, or a salt thereof.
(220) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4is a lower alkenyl group,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(221) A quinoxaline derivative of the formula (1), wherein R3iS a lower
30 alkanoyl-substituted lower alkyl group, R4is a cycloalkyl-lower alkyl group, and
R1, R2, m, n and r are the same as the same, or a salt thereof.
(222) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4is a cycloalkyl-lower alkyl
WO 95/09159 2 1 ~ 0 3 4 5 PC~IJP94/OlS~9
- 79 -
group, and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(223) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkanoyl-substituted lower alkyl group, R4 is a naphthyl-lower alkyl group, and
R1, R2, m, n and r are the same as defined above, or a salt thereof.
5 (224) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkoxycarbonyloxy-substituted lower alkyl group group, R4 is a naphthyl-lower
alkyl group, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
(225) A quinoxaline derivative of the formula (1), wherein R3 is a lowe
10 alkanoyl-substituted lower alkyl group, R4 is a phenylthio-substituted lower
alkyl group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R1, R2, m, n and r are the same as defined above, or a salt thereof.(226) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4 is a phenylthio-substituted
15 lower alkyl group which may optionally have a lower alkoxy substituent on thephenyl moiety, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
(227) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkanoyl-substituted lower alkyl group, R4 is a phenylsulfinyl-substituted lower20 alkyl group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R1, R2, m, n and r are the same as defined above, or a salt thereof.(228) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4 is a phenylsulfinyl -
substituted lower alkyl group which may optionally have a lower alkoxy
25 substituent on the phenyl moiety, and Rl, R2, m, n and r are the same as
defined above, or a salt thereof.
(229) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkanoyl-substituted lower alkyl group, R4 is a phenylsulfonyl-substituted loweralkyl group which may optionally have a lower alkoxy substituent on the phenyl
30 moiety, and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(230) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4 is a phenylsulfonyl -
substituted lower alkyl group which may optionally have a lower alkoxy
WO 9S/09lS9 ~ 3 ~s~ PCT/JP94/OlSS9
- 80 -
substituent on the phenyl moiety, and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(231) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl-substituted lower alkyl group, R4is a phenoxy-substituted lower alkyl
group, and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(232) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4is a phenoxy-substituted
lower alkyl group, and R1, R2, m, n and r are the same as defined above, or a
salt thereof.
1 0 (233) A quinoxaline derivative of the formula (1), wherein R3iS a lower
alkanoyl-substituted lower alkyl group, R4is a group of the formula:
(R8)q
(wherein the symbols are the same as defined above), and R1,R2, m, n and r
are the same as defined above, or a salt thereof.
(234) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4is a group of the formula:
(R8)q
~
~,
(in which the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(235) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl-substituted lower alkyl group, R4is a group of the formula:
-A5-CR42R43R44 (A5, R42, R43 and R44 are the same as defined above), and
R1, R2, m, n and r are the same as defined above, or a salt thereof.
(236) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4iS a group of the formula:
-A5-CR42R43R44 (A5, R42, R43 and R44 are the same as defined above), and
R1, R2, m, n and r are the same as defined above, or a salt thereof.
(237) A quinoxaline derivative of the formula (1), wherein R3is a lower
alkanoyl-substituted lower alkyl group, R4is a 2,3-dihydro-1H-indenyl group-
WO 95/09159 2 1 S 0 3 4 5 PCT/JP94/01559
substituted lower alkyl group which may optionally have a substituent selected
from oxo group, hydroxy group and a silyloxy group having a lower alkyl
substituent on the 2,3-dihydro-1H-indenyl ring, and R1, R2, m, n and r are the
same as defined above, or a salt thereof.
5 (238) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4 is a 2,3-dihydro-1 H -
indenyl group-substituted lower alkyl group which may optionally have a
substituent selected from oxo group, hydroxy group and a silyloxy group having
a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and R1, R2, m, n
10 and r are the same as defined above, or a salt thereof.
(239) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkanoyl-substituted lower alkyl group, R4 is a group of the formula:
(R47)u
,7<
15 ~)
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(240) A quinoxaline derivative of the formula (1), wherein R3 is a lower
alkoxycarbonyloxy-substituted lower alkyl group, R4 is a group of the formula:
(R47)u
~,
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(241 ) A quinoxaline derivative of the formula (1 ) wherein R3 is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4 is a group of the formula:
(R5)
A~
(wherein A, R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(242) A quinoxaline derivative of the formula (1 ) wherein R3 is a group
WO 95/09159 PCT/JP94/OlS59
2,~S~3 4S - 82 -
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a group of the formula:
(R5)p
A ~
(wherein A,R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
(243) A quinoxaline derivative of the formula (1 ) wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a phenyl-lower alkenyl group which may
optionally have a substituent on the phenyl moiety selected from a lower alkoxy
group, a halogen atom, an amino group having optionally a substituent
selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula: -O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as
defined above), a lower alkenyloxy group, nitro group and a lower alkyl group
having optionally a halogen substituent, and R1, R3,R4, m, n and r are the
same as defined above, or a salt thereof.
(244) A quinoxaline derivative of the formula (1) wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4iS a phenyl-lower alkenyl group which may optionally have a
substituent on the phenyl moiety selected from a lower alkoxy group, a halogen
atom, an amino group having optionally a substituent selected from a lower
alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower alkoxy -
substituted lower alkoxy group, a tetrazolyl group having optionally a lower
alkyl substituent on the tetrazole ring, hydroxy group, a group of the formula:
-O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as defined above),
a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and R1, R3,R4, m, n and r are the same as defined
above, or a salt thereof.
(245) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
wo gS/oglS9 2 1 ~ 0 3 4 ~ PCT/JP94/01559
substituent on the phenyl ring, R4is a lower alkenyl group, and R1, R2, m, n andr are the same as defined above, or a salt thereof.
(246) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a lower alkenyl group, and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(247) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a cycloalkyl-lower alkyl group, and R1, R2,m, n and r are the same as defined above, or a salt thereof.
(248) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a cycloalkyl-lower alkyl group, and R1, R2, m, n and r are the
same as defined above, or a salt thereof.
(249) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a naphthyl-lower alkyl group, and R1, R2,
m, n and r are the same as defined above, or a salt thereof.
(250) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a naphthyl-lower alkyl group, and R1, R2, m, n and r are the same
as defined above, or a salt thereof.
(251) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a phenylthio-substituted lower alkyl group
which may optionally have a lower alkoxy substituent on the phenyl moiety,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(252) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a phenylthio-substituted lower alkyl group which may optionally
have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(253) A quinoxaline derivative of the formula (1), wherein R3is a
WO9S/09159 PCT1~4/01559
~S~3~5
- 84-
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a phenylsulfinyl-substituted lower alkyl
group which may optionally have a lower alkoxy substituent on the phenyl
moiety, and R1, R2, m, n and r are the same as defined above, or a salt thereof.(254) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a phenylsulfinyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
1 0 (255) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a phenylsulfonyl-substituted lower alkyl
group which may optionally have a lower alkoxy substituent on the phenyl ring,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
1 5 (256) A quinoxaline derivative of the formula (1), wherein R3is a groupof the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a phenylsulfonyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and R1, R2, m,
n and r are the same as defined above, or a salt thereof.
(257) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a phenoxy-substituted lower alkyl group,
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(258) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a phenoxy-substituted lower alkyl group, and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(259) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring R4is a group of the formula:
WO 95/09159 2 1 5 0 3 ~ 5 PCTIJP94/01559
(R8)q
{~
(wherein the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(260) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a group of the formula:
(R8)q
(in which the symbols are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(261 ) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a haiogen
substituent on the phenyl ring, R4is a group of the formula: -A5-CR42R43R44
(A5,R42,R43 and R44 are the same as defined above), and R1, R2, m, n and r
are the same as defined above, or a salt thereof.
(262) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a group of the formula: -A5-CR42R43R44 (A5,R42,R43 and R44
are the same as defined above), and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(263) A quinoxaline derivative of the formula (1), wherein R3is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4is a 2,3-dihydro-1 H-indenyl group-substitutedlower alkyl group which may optionally have a substituent selected from oxo
group, hydroxy group and a silyloxy group having a lower alkyl substituent on
the 2,3-dihydro-1 H-indenyl ring, and R1, R2, m, n and r are the same as definedabove, or a salt thereof.
(264) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula: -E-NR52R53 (in which E, R52 and R53 are the same as defined
above), R4is a 2,3-dihydro-1 H-indenyl group-substituted lower alkyl group
WO 95109159 PCTIJP94101559
3~
- 86 -
which may optionally have a substituent selected from oxo group, hydroxy
group and a silyloxy group having a lower alkyl substituent on the 2,3-dihydro -1 H-indenyl ring, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
5 (265) A quinoxaline derivative of the formula (1), wherein R3 is a
benzoyl-substituted lower alkyl group which may optionally have a halogen
substituent on the phenyl ring, R4 is a group of the formula:
(R47)u
~
(in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(266) A quinoxaline derivative of the formula (1), wherein R3 is a group
of the fomula: -E-NR52N53 (in which E, R52 and R53 are the same as defined
15 above), R4 is a group of the formula:
(R47)u
~,
(in which the symbols are the same as defined above),
20 and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(267) A quinoxaline derivative of the formula (1 ) wherein R3 is a group
of the formula:
A~R54
`n'
(in which A and R54 are the same as defined above), R4 is a group of the
formula:
(R5)p
A~
(in which A, R5 and p are the same as defined above),
and R1, R2 and r are the same as defined above, or a salt thereof.
WO9S/09159 2l~n345 PCI/JP94/01559
(268) A quinoxaline derivative of the formula (1) wherein R3is a group
of the formula:
- --A~R54
O~O
o
(in which A and R54 are the same as defined above), R4is a phenyl-lower
alkenyl group which may optionally have a substituent on the phenyl moiety
selected from a lower alkoxy group, a halogen atom, an amino group having
10 optionally a substituent selected from a lower alkanoyl group and a phenyl -
lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy group, a
tetrazolyl group having optionally a lower alkyl substituent on the tetrazole ring,
hydroxy group, a group of the formula: -O-A4-CO-NR40R41 (in which A4,R40
and R41 are the same as defined above), a lower alkenyloxy group, nitro group
15 and a lower alkyl group having optionally a halogen substituent, and R1,R3,
R4, m, n and r are the same as defined above, or a salt thereof.
t269) A quinoxaline derivative of the formula (1), wherein R3iS a group
of the formula:
A R54
~
`n'
o
(in which A and R54 are the same as defined above), R4is a lower alkenyl
group, and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(270) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula:
0~,~0
O
(in which A and R54 are the same as defined above), R4is a cycloalkyl-lower
alkyl group, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
WO95/09159 ~ 34'j PCT/JP94/01559
- 88 -
(271 ) A quinoxaiine derivative of the formula (1), wherein R3 is a group
of the formula:
A Rs4
S O~lfO
(in which A and R54 are the same as defined above), R4 is a naphthyl-lower
alkyl group, and R1, R2, m, n and r are the same as defined above, or a salt
thereof.
(272) A quinoxaline derivative of the formula (1), wherein R3 is a group
of the formula:
A Rs4
0~,0
O
(in which A and R54 are the same as defined above), R4 is a phenylthio -
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety, and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(273) A quinoxaline derivative of the formula (1), wherein R3 is a group
of the formula:
--A R54
0~0
O
(in which A and R54 are the same as defined above), R4 is a phenylsulfinyl -
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety, and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(274) A quinoxaline derivative of the formula (1), wherein R3 is a group
of the formula:
WO 95/09159 ~ 2 1 5 0 3 ~ S PCT/JP94/01559
- 89 -
--A Rs4
~1'
O
(in which A and R54 are the same as defined above), R4 is a phenylsulfonyl -
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety, and R1, R2, m, n and r are the same as
defined above, or a salt thereof.
(275) A quinoxaline derivative of the formula (1), wherein R3 is a group
of the formula:
A Rs4
O O
(in which A and R54 are the same as defined above), R4 is a phenoxy -
substituted lower alkyl group, and R1, R2, m, n and r are the same as defined
above, or a salt thereof.
(276) A quinoxaline derivative of the formula (1), wherein R3 is a group
of the formula:
--A R54
`n'
o
(in which A and R54 are the same as defined above), R4 is a group of the
25 formula:
(R8)q
~<
(wherein the symbols are the same as defined above), and R1, R2, m, n and r
30 are the same as defined above, or a salt thereof.
(277) A quinoxaline derivative of the formula (1), wherein R3 is a group
of the formula:
WO 95tO9159 PCT1JP94101559
3 ~
90 -
A Rs4
0~0
O
5 (in which A and R54 are the same as defined above), R4is a group of the
formula: -A5-CR42R43R44 (A5,R42,R43 and R44 are the same as defined
above), and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(278) A quinoxaline derivative of the formula (1), wherein R3is a group
of the formula:
A Rs4
0~0
o
(in which A and R54 are the same as defined above), R4is a 2,3-dihydro-1 H -
15 indenyl group-substituted lower alkyl group which may optionally have a
substituent selected from oxo group, hydroxy group and a silyloxy group having
a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and R1, R2, m, n
and r are the same as defined above, or a salt thereof.
(279) A quinoxaline derivative of the formula (1), wherein R3is a group
20 of the formula:
`n'
o
25 (in which A and R54 are the same as defined above), R4is a group of the
formula:
(R47)u
30 (in which the symbols are the same as defined above),
and R1, R2, m, n and r are the same as defined above, or a salt thereof.
(280) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
WO 95/09159 2 1 ~ 0 3 ~ ~ PCT/JP94101559
- 91 -
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3is hydrogen atom or a lower alkyl group, R4is a group of the
formula:
(R5)p
A ~
(in which A, R5 and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
(281) A quinoxaline derivative of the formula (1), wherein R1 is a lower
10 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3is hydrogen atom or a lower alkyl group, R4is a phenyl -
lower alkenyl group which may optionally have a substituent on the phenyl
moiety selected from a lower alkoxy group, a halogen atom, an amino group
having optionally a substituent selected from a lower alkanoyl group and a
phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole ring, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are the same as defined above), a lower alkenyloxy
group, nitro group and a lower alkyl group having optionally a halogen
substituent, and m, n and r are the same as defined above, or a salt thereof.
(282) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3is hydrogen atom or a lower alkyl group, R4is a lower
alkenyl group, and m, n and r are the same as defined above, or a salt thereof.
(283) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
30 hydrogen atom, R3is hydrogen atom or a lower alkyl group, R4iS a cycloalkyl -lower alkyl group, and m, n and r are the same as defined above, or a salt
thereof.
(284) A quinoxaline derivative of the formula (1), wherein R1 is a lower
WO 95/09159 PCI/JP94/01559
3 ~
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower aikyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a naphthyl -
lower alkyl group, and m, n and r are the same as defined above, or a salt
5 thereof.
(285) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a naphthyl -
10 lower alkyl group, and m, n and r are the same as defined above, or a saltthereof.
(286) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
15 hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a phenylthio -
substitued lower alkyl group which may optinally have a lower alkoxy
substituent on the phenyl moiety, and m, n and r are the same as defined
above, or a salt thereof.
(287) A quinoxaline derivative of the formula (-i ), wherein R1 is a lower
20 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a
phenylsulfinyl-substituted lower alkyl group which may optionally have a lower
alkoxy substituent on the phenyl moiety, and m, n and r are the same as
25 defined above, or a salt thereof.
(288) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a
30 phenylsulfonyl-substituted lower alkyl group which may optionally have a lower
alkoxy substituent on the phenyl moiety, and m, n and r are the same as
defined above, or a salt thereof.
(289) A quinoxaline derivative of the formula (1), wherein R1 is a lower
WO 95/09159 2 1 ~ o 3 4 5 PCT/JP94/01559
- 93 -
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a phenoxy -
substituted lower alkyl group, and m, n and r are the same as defined above, or
a salt thereof.
(290) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the
1 0 formula:
(R8)q
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(291 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the
formula: -A5-CR42R43R44 (A5, R42, R43 and R44 are the same as defined
above), and m, n and r are the same as defined above, or a salt thereof.
(292) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a 2,3-dihydro -1H-indenyl group-substituted lower alkyl group which may optionally have a
substituent selected from oxo group, hydroxy group and a silyloxy group having
a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and m, n and r are
the same as defined above, or a salt thereof.
(293) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
hydrogen atom, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the
WO gS/O9159 PCI`/JI'94tO1559
34~
- 94 -
formula:
(R47)
5 (in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(294) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is
10 hydrogen atom, R3 and R4 combine together with the nitrogen atom to form
1,2,3,4-tetrahydroisoquinolyl group which may optionally have a lower alkoxy
group), and m, n and r are the same as defined above, or a salt thereof.
(295) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
15 aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a group of the formula:
(R5)p
A~
(in which A, R5 and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
(296) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
25 aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a phenyl-lower alkenyl group which may optionally
have a substituent on the phenyl moiety selected from a lower alkoxy group, a
halogen atom, an amino group having optionally a substituent selected from a
30 lower alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower
alkoxy-substituted lower alkoxy group, a tetrazolyl group having optionally a
lower alkyl substituent on the tetrazole ring, hydroxy group, a group of the
formula: -o-A4-Co-NR4R41 (in which A4, R40 and R41 are the same as defined
wo 9S/09159 2 1 5 0 3 9 ~ PCT/JPg4/OlSS9
- 95 -
above), a lower alkenyloxy group, nitro group and a lower alkyl group having
optionally a halogen substituent, and m, n and r are the same as defined
above, or a salt thereof.
(297) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent or anaminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a lower alkenyl group, and m, n and r are the same as
defined above, or a salt thereof.
(298) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a cycloalkyl-lower alkyl group, and m, n and r are the
15 same as defined above, or a salt thereof.
(299) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
20 lower alkyl group, R4 is a naphthyl-lower alkyl group, and m, n and r are the same as defined above, or a salt thereof.
(300) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
25 alkyl group having optionally a halogen substituent, R3 is hydrogen atom or alower alkyl group, R4 is a phenylthio-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and m, n and
are the same as defined above, or a salt thereof.
(301 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
30 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a phenylsulfinyl-substituted lower alkyl group which
WO gS/O9159 PCT/JP94/OlS59
3 4~
- 96 -
may optionally have a lower alkoxy substituent on the phenyl moiety and m, n
and r are the same as defined above, or a salt thereof.
(302) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
5 aminocarbonyl group having optionally a lower alkyl substituent, R2 is a loweralkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a phenylsulfonyl-substituted lower alkyl group which
may optionally have a lower alkoxy substituent on the phenyl moiety, and m, n
and r are the same as defined above, or a salt thereof.
(303) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a phenoxy-substituted lower alkyl group, and m, n and rare the same as defined above, or a salt thereof.
(304) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a group of the formula:
(R8)q
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(305) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a group of the formula: -A5-CR42R43R44 (A5, R42, R43
and R44 are the same as defined above), and m, n and r are the same as
defined above, or a salt thereof.
(306) A quinoxaline derivative of the formula (1), wherein R1 is a lower
21503~
WO 95/09159 - PCI`/JP94101559
- 97-
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a 2,3-dihydro-1 H-indenyl group-substituted lower alkyl5 group which may optionally have a substituent selected from oxo group,
hydroxy group and a silyloxy group having a lower alkyl substituent on the 2,3 -dihydro-1 H-indenyl ring, and m, n and r are the same as defined above, or a
salt thereof.
(307) A quinoxaline derivative of the formula (1), wherein R1 is a lower
10 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 is hydrogen atom or a
lower alkyl group, R4 is a group of the formula:
(R47)u
/~
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(308) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a lower
alkyl group having optionally a halogen substituent, R3 and R4 combine
together with the nitrogen atom to form 1,2,3,4-tetrahydroisoquinolyl group
which may optionally have a lower alkoxy group, and m, n and r are the same
as defined above, or a salt thereof.
(309) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula: (R5)p
A~
(in which A, R5 and p are the same as defined above),
W095/0915;~3~ PCI/JP94/01559
- 98 -
and m, n and r are the same as defined above, or a salt thereof.
(310) A quinoxaline derlvative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenyl-lower alkenyl
group which may optionally have a substituent on the phenyl moiety selected
from a lower alkoxy group, a halogen atom, an amino group having optionally a
substituent selected from a lower alkanoyl group and a phenyl-lower alkenyl -
carbonyl group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl
group having optionally a lower alkyl substituent on the tetrazole ring, hydroxygroup, a group of the formula: -O-A4-CO-NR4R41 (in which A4, R40 and R41
are the same as defined above), a lower alkenyloxy group, nitro group and a
lower alkyl group having optionally a halogen substituent, and m, n and r are
the same as defined above, or a salt thereof.
(311 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a lower alkenyl group,
and m, n and r are the same as defined above, or a salt thereof.
(312) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a cycloalkyl-lower alkyl
group, and m, n and r are the same as defined above, or a salt thereof.
(313) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a naphthyl-lower alkyl
group, and m, n and r are the same as defined above, or a salt thereof.
(314) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenylthio-
WO 95tO9159 2 1 5 0 3 4 ~ PCT/JP94/01559
99
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety, and m, n and r are the same as defined
above, or a salt thereof.
(315) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent or anaminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenylsulfinyl -
substituted lower alkyl group which may optionally have a lower alkoxy
substituent on the phenyl moiety and m, n and r are the same as defined
10 above, or a salt thereof.
(316) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a phenylsulfonyl -
15 substituted lower alkyl group which may optionally have a lower alkoxysubstituent on the phenyl moiety, and m, n and r are the same as defined
above, or a salt thereof.
(317) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
20 aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenylgroup, R3 is hydrogen atom or a lower alkyl group, R4 is a phenoxy-substituted
lower alkyl group, and m, n and r are the same as defined above, or a salt
thereof.
(318) A quinoxaline derivative of the formula (1), wherein R1 is a lower
25 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:
~ (R8)q
~)
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(319) A quinoxaline derivative of the formula (1), wherein R1 is a lower
WO95/09159 ~ 3~ PCI/Jl'94/01559
- 100-
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:-A5-CR42R43R44 (A5, R42, R43 and R44 are the same as defined above), and m,
S n and r are the same as defined above, or a salt thereof.
(320) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a 2,3-dihydro-1 H -
10 indenyl group-substituted lower alkyl group which may optionally have a
substituent selected from oxo group, hydroxy group and a silyloxy group having
a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl ring, and m, n and r are
the same as defined above, or a salt thereof.
(321 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
15 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenyl
group, R3 is hydrogen atom or a lower alkyl group, R4 is a group of the formula:(R47)
~)
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(322) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
25 aminocarbonyl group having optionally a lower alkyl substituent, R2 is phenylgroup, R3 and R4 combine together with the nitrogen atom to form 1,2,3,4 -
tetrahydroisoquinolyl group which may optionally have a lower alkoxy group),
and m, n and r are the same as defined above, or a salt thereof.
(323) A quinoxaline derivative of the formula (1), wherein R1 is a lower
30 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula:
WO 95/09159 2 1 5 0 3 ~ ~ PCT/JP94/OlS59
- 101 -
~, (R5)p
(in which A, Rs and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
(324) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenyl-lower alkenyl group which may optionally have a
substituent on the phenyl moiety selected from a lower alkoxy group, a halogen
atom, an amino group having optionally a substituent selected from a lower
alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower alkoxy -
substituted lower alkoxy group, a tetrazolyl group having optionally a lower
alkyl substituent on the tetrazole ring, hydroxy group, a group of the formula:
-O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as defined above),
a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and m, n and r are the same as defined above, or a salt
thereof.
(325) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a lower alkenyl group, and m, n and r are the same as defined
above, or a salt thereof.
(326) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a cycloalkyl-lower alkyl group, and m, n and r are the same as
defined above, or a salt thereof.
(327) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
:.
WO 9S/09159 PCT/JP94/OlS59
~3~
- 102-
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a naphthyl-lower alkyl group, and m, n and r are the same as
defined above, or a salt thereof.
5 (328) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenylthio-substituted lower alkyl group which may optionally
10 have a lower alkoxy substituent on the phenyl moiety, and m, n and r are the
same as defined above, or a salt thereof.
(329) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
15 morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenylsulfinyl-substituted lower alkyl group which may optionallyhave a lower alkoxy substituent on the phenyl moiety and m, n and r are the
same as defined above, or a salt thereof.
(330) A quinoxaline derivative of the formula (1), wherein R1 is a lower
20 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenylsulfonyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and m, n and r
25 are the same as defined above, or a salt thereof.
(331 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
30 group, R4 is a phenoxy-substituted lower alkyl group, and m, n and r are the
same as defined above, or a salt thereof.
(332) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 103-
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula:
(R8)q
~
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(333) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula: -A5-CR42R43R44 (A5, R42, R43 and R44 are
the same as defined above), and m, n and r are the same as defined above, or
a salt thereof.
(334) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a 2,3-dihydro-1H-indenyl group-substituted lower alkyl group
which may optionally have a substituent selected from oxo group, hydroxy
group and a silyloxy group having a lower alkyl substituent on the 2,3-dihydro -1 H-indenyl ring, and m, n and r are the same as defined above, or a salt
thereof.
(335) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
morpholino-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula:
(R47)
(in which the symbols are the same as defined above), and m, n and r are the
WO 95/O91S9 PCT/JP94/01559
? ~3 - 104 -
same as defined above, or a salt thereof.
(336) A qulnoxa!ine derivative of the formula t1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is a
5 morpholino-substituted lower alkyl group, R3 and R4 combine together with the
nitrogen atom to form 1,2,3,4-tetrahydroisoquinolyl group which may optionally
have a lower alkoxy group), and m, n and r are the same as defined above, or a
salt thereof.
(337) A quinoxaline derivative of the formula (1), wherein R1 is a lower
10 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula:
(R5)p
A~
(in which A, R5 and p are the same as defined above),
and m, n and r are the same as defined above, or a salt thereof.
(338) A quinoxaline derivative of the formula (1), wherein R1 is a lower
20 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenyl-lower alkenyl group which may optionally have a
substituent on the phenyl moiety selected from a lower alkoxy group, a halogen
25 atom, an amino group having optionally a substituent selected from a lower
alkanoyl group and a phenyl-lower alkenylcarbonyl group, a lower alkoxy -
substituted lower alkoxy group, a tetrazolyl group having optionally a lower
alkyl substituent on the tetrazole ring, hydroxy group, a group of the formula:
-O-A4-CO-NR4R41 (in which A4, R40 and R41 are the same as defined above),
30 a lower alkenyloxy group, nitro group and a lower alkyl group having optionally
a halogen substituent, and m, n and r are the same as defined above, or a salt
thereof.
(339) A quinoxaline derivative of the formula (1), wherein R1 is a lower
WO 95/09159 2 1 5 ~ 3 ~ ~ PCT/JP94/01559
- 105-
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a lower alkenyl group, and m, n and r are the same as defined
above, or a salt thereof.
(340) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a cycloalkyl-lower alkyl group, and m, n and r are the same as
defined above, or a salt thereof.
(341 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a naphthyl-lower alkyl group, and m, n and r are the same as
defined above, or a salt thereof.
(342) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenylthio-substituted lower alkyl group which may optionally
have a lower alkoxy substituent on the phenyl moiety, and m, n and r are the
same as defined above, or a salt thereof.
(343) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenylsulfinyl-substituted lower alkyl group which may optionallyhave a lower alkoxy substituent on the phenyl moiety and m, n and r are the
same as defined above, or a salt thereof.
(344) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
WO95/09159 ~ 34~ PCT/JPg4/015S9
- 106 -
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a phenylsulfonyl-substituted lower alkyl group which may
optionally have a lower alkoxy substituent on the phenyl moiety, and m, n and r
5 are the same as defined above, or a salt thereof.
(345) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
10 group, R4 is a phenoxy-substituted lower alkyl group, and m, n and r are the
same as defined above, or a salt thereof.
(346) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
15 imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula:
(R8)q
20 (in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(347) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
25 imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula: -A5-CR42R43R44 (A5, R42, R43 and R44 are
the same as defined above), and m, n and r are the same as defined above, or
a salt thereof.
(348) A quinoxaline derivative of the formula (1), wherein R1 is a lower
30 alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a 2,3-dihydro-1H-indenyl group-substituted lower alkyl group
WO 95/09159 PCT/JP94/01559
21S03~S
- 107-
which may optionally have a substituent selected from oxo group, hydroxy
group and a silyloxy group having a lower alkyl substituent on the 2,3-dihydro -1 H-indenyl ring, and m, n and r are the same as defined above, or a salt
thereof.
5 (349) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 is hydrogen atom or a lower alkyl
group, R4 is a group of the formula:
1 0 (R47)u
(in which the symbols are the same as defined above), and m, n and r are the
same as defined above, or a salt thereof.
(350) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent or an
aminocarbonyl group having optionally a lower alkyl substituent, R2 is an
imidazolyl-substituted lower alkyl group, R3 and R4 combine together with the
nitrogen atom to form 1,2,3,4-tetrahydroisoquinolyl group which may optionally
20 have a lower alkoxy group), and m, n and r are the same as defined above, or a
salt thereof.
Wo95/09159 ?,~a3~ PCT/JP94/01559
- 108-
(351 ) A quinoxaline derivative of the forrnula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
5 alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
1 0 A~R54
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
15 defined above); R4 is a group of the formula:
(R47)u
.~
(in which the group {~), R47 and u are as defined above), and R2, m, n,
20 and r are as defined above, or a salt thereof.
(352) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
25 alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
WO 95/09159 PCT/JP94/01559
,.
2.......................................... 1 '~ O 3 4 S
A~=~R54
`n'
o
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
--A~
10 (in which A, R5 and p are as defined above), and R2, m, n, and r are as defined
above, or a salt thereof.
(353) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
15 group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
20 the formula:
A~R54
0~,~0
o
25 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
30 group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -O-A4-Co-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
WO 95/09159 ? ~ 3 45 PCT/JP94101559
- 110-
phenyl moiety, and R2, m, n, and r are as defined above, or a salt thereof.
(354) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
5 group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
10 the formula:
A Rs4
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group; and R2, m, n, and r are as defined
above, or a salt thereof.
(355) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
R54
--A~
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 iS a cycloalkyl-lower alkyl group; and R2, m, n, and r are asdefined above, or a salt thereof.
WO 95/09159 ~ 1 S (1 3 ~ S PCTIJP94/01559
- 111 -
(356) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
5 alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
15 defined above); R4 is a naphthyl-lower alkyl group; and R2, m, n, and r are as
defined above, or a salt thereof.
(357) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
20 group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
25 the formula:
A Rs4
- 0~0
o
30 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety; and R2, m, n, and r
are as defined above, or a salt thereof.
W095/09159 21 S03~5 PCI/JP94/01559
- 112-
(358) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
5 alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
1 0 A~R54
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety; and R2, m, n, and r
are as defined above, or a salt thereof.
(359) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A~ R54
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety; and R2, m, n, and r
WO9S/09159 ~1 S 0 ~ 4 5 PCI1JP94/OlS59
- 113-
are as defined above, or a salt thereof.
(360) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A~ R54
O O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group; and R2, m, n,
and r are as defined above, or a salt thereof.
(361 ) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
- A Rs4
0~0
0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
WO 95/09159 PCI'1JP94/01559
O~S
- 114-
(R8)q
(in which the group ~), R3 and q are as defined above); and R2, m, n,
5 and r are as defined above, or a salt thereof.
(362) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
10 alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above); and R2, m, n, and r are as defined
above, or a salt thereof.
(363) A quinoxaline derivative of the formula (1), wherein R1 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
wo 9S/O9lS9 2 1 5 0 3 ~ ~
- 115-
--A R54
0~0
O
5 tin which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
having optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and R2, m, n, and r are as defined above, or a salt thereof.
(364) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
15 group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R47)u
(in which the group {~), R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(365) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
WO 95/09159 2 1 5 0 3 4 5 PCI/JP94/01559
- 116-
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
5 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-NtR52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
O~O
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
A~
(in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(366) A quinoxaline derivative of the formula (1), wherein R1 is a
20 halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
25 group, a benzoyl-substituted lower alkyl group having otionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
E, R52 and R53 are as defined above), or a group of the formula:
--A R54
O~O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
WO 95/O91S9 2 1 ~ 0 3 4 5 PCT/JP94/OlSS9
- 117-
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-iower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
5 tetrazole moiety, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(367) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
15 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A~R54
~
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(368) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
WO 95/09159 PCT/JP94/01559
~ 21~03~5
- 118-
A~R54
0~0
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(369) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
10 alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
15 substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
E, R52 and R53 are as defined above), or a group of the formula:
A>=~R54
O O
~
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(370) A quinoxaline derivative of the formula (1), wherein R1 is a
25 halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
30 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
wo gS/oglS9 ~ ~ ~ o ~ 4 ~ PCT/JP94/01559
- 119-
A Rs4
- 0~0
O
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(371 ) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
15 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A~ R54
O~O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
25 as defined above, or a salt thereof.
(372) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
30 alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
WO 9S/O91S9 PCT/JP94/OlSS9
~o3 45
- 120-
E, R52 and R53 are as defined above), or a group of the formula:
A~R54
0~,~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(373) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~(
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
25 are as defined above, or a salt thereof.
(374) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxy -
30 carbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a
phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl group, a
benzoyl-substituted lower alkyl group having optionally a halogen substituent
on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and
WO 9S/09159 2 1 ~ 0 3 4 ~ PCI/JP94/01559
- 121 -
R53 are as defined above), or a group of the formula:
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R8)q
f~<
10 ~J
(in which the group ~, R8 and q are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(375) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxy -
carbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a
phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl group, a
benzoyl-substituted lower alkyl group having optionally a halogen substituent
on the phenyl ring, a group of the formula: -E-N(R52)(R53) ~in which E, R52 and
R53 are as defined above), or a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(376) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is hydrogen atom; R3 is a lower
W095'09l59 2 1 5 ~3 ~ pCI/JP94/01559
- 122-
aikanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxy -
carbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a
phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl group, a
5 benzoyl-substituted lower alkyl group having a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
A Rs4
1 0 0~,0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
having optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
(377) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A~=~
0~,0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
WO 95/O91S9 2 1 ~ 0 3 4 ~ PCI/JP94/01559
- 123-
(R47)u
(in which the group ~3, R47 and u are as defined above), and m, n, and r
5 are as defined above, or a salt thereof.
(378) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
10 alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
15 the formula:
A~R54
0~,0
o
20 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
A~
25 (in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(379) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
30 a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
WO 95/09159 PCT/JP94/01559
21S03~
- 124-
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
--A R54
O O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(380) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula: -E -
N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of the
formula:
O O
~f
W095/09159 2l5n31l5 PCI/JP94/01559
- 125-
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(381 ) A quinoxaline derivative of the formula (1), wherein R1 is a
5 halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionally
a halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
10 substituted lower alkyl group, a benzoyl-substituted lower alkyl group havingoptionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
~
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
20 defined above, or a salt thereof.
(382) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
25 alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
30 the formula:
WO 95/09159 PCI`/JP94/01559
215~3~5
- 126-
0~0
O ~
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(383) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionally10 a halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
15 optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A~R54
~
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
25 as defined above, or a salt thereof.
(384) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
30 alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
_ WO 95/09159 2 1 5 0 3 4 ~ PCr/JP94/OlS59
- 127-
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A~R54
O~O
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
10 as defined above, or a salt thereof.
(385) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionally
a halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
15 alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
20 the formula:
A~R54
0~0
o
25 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(381 ) A quinoxaline derivative of the formula (1), wherein R1 is a
30 halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionally
a halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
WO 95tO9lS9 2 1 S 0 3 ~ S pCT/Jp94/OlSS9
- 128-
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
5 the formula:
A~R54
0~0
o
10 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
(387) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionally15 a halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
20 optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R8)q
~<
(in which the group ~), R8 and q are as defined above), and m, n, and r
WO 95/09159 2 1 5 0 3 ~ 5 PCT/JP94101559
- 129-
are as defined above, or a salt thereof.
(388) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
O~fO
0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(389) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a lower alkyl group having optionallya halogen substituent; R3 is a lower alkanoyloxy-substituted lower alkyl group,
a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower
alkyl group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a
lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
WO 95/09159 PCI/JP94/015S9
21S034S
- 130-
A Rs4
0~0
o
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
having optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
(390) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
0~,~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R47)u
~ ,
(in which the group {~, R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(391 ) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
WO 95/O91S9 2 1 5 0 ~ 4 5 PCI/JP94/01559
- 131 -
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
5 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A Rs4
1 0 0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(Rs)p
--A~
(in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(392) A quinoxaline derivative of the formula (1), wherein R1 is a
20 halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
25 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
E, R52 and R53 are as defined above), or a group of the formula:
O~O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
WO 95/09159 PCI'1JP94/01559
2~ 03 4S
- 132-
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
5 tetrazole moiety, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(393) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
15 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A~ R54
~f
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(394) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
_ WO 95/09159 2 1 ~ 0 3 4 ~ PCI/JP94/01559
- 133-
A Rs4
0~,~0
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(395) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
10 alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
15 substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
E, R52 and R53 are as defined above), or a group of the formula:
A~R54
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(396) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
WO 9S/09159 PCIIJP94/OlSS9
z~so34s
- 134-
A Rs4
O O
~ .
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(397) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
15 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
25 as defined above, or a salt thereof.
(398) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
30 alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 135-
E, R52 and Rs3 are as defined above), or a group of the formula:
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(399) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
25 are as defined above, or a salt thereof.
(400) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
30 alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
WO 95/09159 PCT/JP94/01559
2l~03~5
- 136-
E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
- O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R8)q
~
(in which the group ~), R8 and q are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(401 ) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
15 alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a benzoyl-substituted lower alkyl group having optionally a halogen
20 substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which
E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(402) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is phenyl group; R3 is a lower
WO 95/09159 2 1 ~ 0 3 ~ S PCT/JP94/01559
- 137-
alkanoyloxy-substituted lower alkyl group, a lower alkoxycarbonyl group, a
lower alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower
alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
5 group, a benzoyl-substituted lower alkyl group having optionally a halogen
substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in whichE, R52 and R53 are as defined above), or a group of the formula:
A Rs4
1 0 0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
having optionally a substituent selected from oxo group, hydroxy group and a
15 silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1H-indenyl ring, and m, n, and r are as defined above, or a salt thereof.
(403) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
20 alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
25 (in which E, R52 and R53 are as defined above), or a group of the formula:
A R54
0~0
o
30 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
WO 95/O91S9 PCT/JP94/OlS~9
3 45
- 138-
(R47)u
~ .
(in which the group {~, R47 and u are as defined above), and m, n, and r
5 are as defined above, or a salt thereof.
(404) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
10 a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
1 5 A~R54
`n'
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
20 defined above); R4 is a group of the formula:
(R5)p
A~
\=/
(in which A, R5 and p are as defined above), and m, n, and r are as defined
25 above, or a salt thereof.
(405) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
30 a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
WO 95/09159 2 ~ 5 0 3 4 5 PCT/JP94/01559
- 139-
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
0~.~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
10 substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -o-A4-CO-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(406) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
20 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
25 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
WO 95/09159 2~5 03 ~S PCT1JP94/015S9
- 140-
(407) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-subs-tituted lower alkyl group, a lower alkoxy -
carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
5 phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
~
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
15 defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(408) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
20 alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
25 (in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
o
30 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(409) A quinoxaline derivative of the formula (1), wherein R1 is a
WO 95/09159 2 1 ~ 0 3 4 S PCTtJP94/01559
- 141 -
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
5 lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
--A Rs4
O O
~f
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
15 optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(410) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
20 alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
25 (in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
- ' ~
0~0
O
30 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
wo 95/09l59 2l5 03 4S PCT/JP94/01559
- 142-
(411 ) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
5 a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
15 defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(412) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
20 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
25 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
--A R54
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
WO 95/O91S9 2 I 5 0 3 ~ S PCTIJP94/OlSS9
- 143-
(413) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
5 a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
~=~
O O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
15 defined above); R4 is a group of the formula:
(R8)q
.~
(in which the group ~), R3 and q are as defined above~, and m, n, and r
20 are as defined above, or a salt thereof.
(414) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
25 a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
WO 9S/09159 PCT/JP94101S59
~so34~
- 144-
A~R54
0~0
O -
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(415) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group; R2 is a morpholino-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
15 alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A>=~R54
~f
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
having optionally a substituent selected from oxo group, hydroxy group and a
25 silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
(416) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
30 alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
WO 9StO9159 2 15 l) 3 ~ 5 PCTIJP94/01559
- 145-
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A R54
5 ~n~
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R47)u
f~<
(in which the group ~), R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(417) A quinoxaline derivative of the formula (1), wherein R1 is a
15 halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
20 alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
.~
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
A~
WO95/09159 2lso345 PCT/JP94/01559
- 146-
(in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(418) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkyl5 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
10 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
20 a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxygroup, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -O-A4-Co-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
25 phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(419) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
30 a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
WO 95/09159 21 ~ 0 3 4 ~ PCT/JP94/01559
- 147-
(in which E, R52 and R53 are as defined above), or a group of the formula:
X
0~0
0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(420) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A~R54
V
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(421) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
WO 9S/09159 2 I S ~3 4S PCT/JP94/OlSS9
- 148-
A~R54
O~O
o
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(422) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkyl10 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
15 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A~R54
O~.~O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(423) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
WO 95/09159 PCT/JP94/01559
21S034~
- 149-
A Rs4
0~0
O
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(424) A quinoxaline derivative of the formula (1), wherein R1 is a
10 halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
15 alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A~R54
~
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
25 as defined above, or a salt thereof.
(425) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
30 a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
WO 95/09159 ~34~ PCI/JP94/015S9
- 150-
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~,0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
(426) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R8)q
~
(in which the group ~, R8 and q are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(427) A quinoxaline derivative of the formula (1), wherein R1 is a
30 halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy-
WO 95/09159 ~ 1 5 0 3 1 5 PCT/JP94/01559
- 151 -
carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
5 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~,~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(428) A quinoxaline derivative of the formula (1), wherein R1 is a
halogen atom or a lower alkyl group; R2 is an imidazolyl-substituted lower alkylgroup; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower
alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group,
a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
20 lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~(
`n'
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
30 having optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
(429) A quinoxaline derivative of the formula (1), wherein R1 is a lower
WO 9S/09159 PCT/JP94/01559
3 4~
- 152-
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
5 group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula: -E -
N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of the
1 0 formula:
A~R54
0~0
o
15 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R47)u
20 (in which the group ~, R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(430) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
25 hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
30 optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
WO 95/09159 21 ~ 0 3 4 ~ PCT/JP94/01559
- 153-
the formula:
A Rs4
0~,~0
O
- (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
- A~
1 0 \~/
(in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(431 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
15 aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
20 substituted lower alkyl group, a benzoyl-substituted lower alkyl group havingoptionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
~
0~0
- O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
30 substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
WO 9S/O91S9 PCT/JP94101559
2lS0~5
- 154-
tetrazole moiety, hydroxy group, a group of the formula: -O-A4-Co-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
5 (432) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
10 group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
15 the formula:
A~R54
0~0
o
20 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(433) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
25 aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
30 substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
wo 9S/O9lS9 21 ~ 0 3 4 5 PCTtJP94/OlSS9
- 155-
A~R54
`n'
O
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(434) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
10 aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
15 substituted lower alkyl group, a benzoyl-substituted lower alkyl group havingoptionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
~
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
25 defined above, or a salt thereof.
(435) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
30 lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkylgroup, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
WO 95/09159 PCT/JP94/01559
2~034s
- - 156-
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
--A R54
~
`n'
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
10 optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(436) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
15 hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
20 optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A~R54
`r~
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(437) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
WO 95/09159 2 1 S 0 3 ~ 5 PCT/JP94/01559
- 157-
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
5 substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
- optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A Rs4
0~,0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
15 optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(438) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
20 hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
25 optionally a halogen substituent on the phenyl ring, a group of the formula: -E -
N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of the
formula:
``n'
WO 95/09159 PCT/JP94/01559
2~5~3~
- 158-
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
(439) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
10 alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
1 5 A~R54
O O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
20 defined above); R4 is a group of the formula:
(R8)q
{~
(in which the group ~3, R3 and q are as defined above), and m, n, and r
25 are as defined above, or a salt thereof.
(440) A quinoxaline derivative of the formula (1), wherein Rl is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
30 lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkylgroup, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
WO 95/09159 2 1 5 0 3 4 S PCT/JP94/01559
- 159-
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
5 the formula:
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(441 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is
hydrogen atom; R3 is a lower alkanoyloxy-substituted lower alkyl group, a
lower alkoxycarbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl
group, a phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower
alkoxy-lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl -
substituted lower alkyl group, a benzoyl-substituted lower alkyl group having
optionally a halogen substituent on the phenyl ring, a group of the formula:
-E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or a group of
the formula:
A~R54
- `n'
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
30 defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl grouphaving optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
WO 95/09159 PCT1JP94/01559
2l50345
- 160-
(442) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
R54
--A~
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R47)
~
(in which the group ~, R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(443) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
25 aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower
alkoxycarbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxy -
carbonyl group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a
30 phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl group, a
benzoyl-substituted lower alkyl group having optionally a halogen substituent
WO 95/09159 2 1 ~ 0 3 4 ~ PCT/JP94/01559
- 161 -
on the phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and
R53 are as defined above), or a group of the formula:
- A R54
`n'
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(Rs)p
A~
(in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(444) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
--A R54
0~,0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
30 defined above); R4 is a phenyl-lower alkenyl group having optionally a
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
WO 9S/09159 2 1 5 3 4 5 PCI/JP94/OlS59
- 162-
group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -o-A4-Co-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(445) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
A>~R54
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(446) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
WO 9S/09159 21 5 0 3 ~ 5 PCTtJP94/01559
- 163-
are as defined above), or a group of the formula:
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(447) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
A Rs4
0~,0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
25 defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(448j A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
30 alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -
substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy-
WO 95/09159 PCT/JP94/01559
2150345
- 164-
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
A R54
`n'
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
10 defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(449) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
15 aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
20 carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
A Rs4
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
30 optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(450) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
WO 95/09lS9 2 1 5 0 ~ 4 5 PCT/JP94/01559
- 165-
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
5 group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
A Rs4
O~fO
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
15 defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(451 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
20 aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
25 carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
--A R54
~
`n'
WO 95/09159 PCT/JP94/01559
215034~
- 166-
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
(452) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
10 group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
1 5 A~R54
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
20 defined above); R4 is a group of the formula:
(R8)q
,~
~,
(in which the group ~, R8 and q are as defined above), and m, n, and r
25 are as defined above, or a salt thereof.
(453) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -
30 substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
wo gS/oglS9 2 1 5 û 3 ~ ~ PCT/JP94/01559
- 167-
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
5 are as defined above), or a group of the formula:
A Rs4
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(454) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a lower
alkyl group having optionally a halogen substituent; R3 is a lower alkanoyloxy -substituted lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxy -
carbonyloxy-substituted lower alkyl group, a phenyl-lower alkoxycarbonyl
group, a lower alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxy -
carbonyl group, a lower alkanoyl-substituted lower alkyl group, a benzoyl -
substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-N(R52)(R53) (in which E, R52 and R53
are as defined above), or a group of the formula:
--A R54
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
30 defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
having optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
WO 95/09159 PCT/JP94/OlS59
~2,iS 03 Q~S - 168 -
(455) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -
5 carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
10 (in which E, R52 and R53 are as defined above), or a group of the formula:
A~R54
0~,~0
o
15 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R47)u
20 (in which the group {~), R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(456) A quinoxaline derivative of the formula (1), wherein Rl is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
25 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -
carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
30 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
WO 95/09159 21 5 0 3 ~ ~ PCT/JP94/01559
- 169-
A Rs4
O~fO
O
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
A~
10 (in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(457) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
15 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -
carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
20 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
WO 9S/09159 PCT/JP94/01559
~,~S~34S
- 170-
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(458) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
5 aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
10 alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A~ R54
1 5 `n'
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(459) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
0 A Rs4
0~0
WO 9S/09159 2 1 ~ 0 3 4 ~ PCTIJP94/01559
- 171 -
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(460) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
10 lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
O O
~f
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
20 defined above, or a salt thereof.
(461 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -
25 carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
30 (in which E, R52 and R53 are as defined abovej, or a group of the formula:
WO 9S/09159 PCT/JP94/015S9
~S~34~
- - 172-
A Rs4
0~0
o
5 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(462) A quinoxaline derivative of the formula (1), wherein R1 is a lower
10 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
15 lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
O O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
25 optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(463) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
30 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -
carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
WO 95/09159 ~ 1 5 0 3 4 ~ - PCT/JP94/01559
- 173-
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
10 optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(464) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
15 group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -
carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
20 halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
(465) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
WO 95/O91S9 PCTIJP94/01559
2~So3 4S
- 174-
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
5 (in which E, R52 and R53 are as defineq ~above)~ or a group of the formula:
--A~R54
`n'
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R8)q
{~
(in which the group ~), R8 and q are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(466) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
`n'
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
WO 95/09159 2 1 5 0 3 4 5 PCTtJP94/01559
- 175-
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(467) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is phenyl
group; R3 is a lower alkanoyloxy-substituted lower alkyl group, a lower alkoxy -carbonyl group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a
phenyl-lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxy -
10 lower alkyl group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower
alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which E, R52 and R53 are as defined above), or a group of the formula:
A Rs4
~
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl group
20 having optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
(468) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
25 aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
30 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
WO 95/O91S9 PCT/JP94/01559
2~5~1345 - 176-
A Rs4
0~,0
O
5 (in which R54 is hydrogen atom or a iower alkyi group and A is the same as
defined above); R4 is a group of the formula:
(R47)u
10 (in which the group ~, R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(469) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
15 morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
20 group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
--A R54
O~O
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
A~
WO 9S/09159 2 1 ~ 0 3 4 ~ PCT/JP94101S59
- 177-
(in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(470) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
5 aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
10 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
--A R54
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(471 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
WO 95/09159 ~ S ~3 ~S PCT/JP94/01559
- 178-
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
5 a group of the formula:
R54
A~
0~0
o
10 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(472) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
15 aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
20 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
R54
--A~
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
30 defined above, or a salt thereof.
(473) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/OlS59
- 179-
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
5 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
~(
0~,0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(474) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
O~f O
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
WO 9S/09159 PCT/JP94/015S9
~,~So3~S
- - 180-
as defined above, or a salt thereof.
(475) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
5 morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
10 group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
1 5 O~O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(476) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
WO 95/09159 2 1 5 0 3 ~ 5
- 181 -
--A~=~R54
- `n'
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(477) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
25 defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
(478j A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
30 morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
WO 95/09159 PCT/JP94/01559
~3 ~JS
- - 182-
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
0~,~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R8)q
(in which the group ~), R3 and q are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(479) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
21503~5
WO 95/09159 PCT/JP94/01559
- 183-
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(480) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is a
morpholino-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
10 alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
--A~= ~R54
O O
~f
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
20 defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl grouphaving optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
(481 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
25 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
30 alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
WO gS/O9159 PCT/JP94/01559
~3~
- 184-
a group of the formula:
--A~= ~RS4
0~0
O
(in which Rs4 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R47)u
~
(in which the group ~, R47 and u are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(482) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
15 aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
20 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
--A R54
~
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R5)p
A~
WO 95/09159 _ 2 1 S 0 3 ~ S PCT/JP94101~59
- 185-
(in which A, R5 and p are as defined above), and m, n, and r are as defined
above, or a salt thereof.
(483) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
5 aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
10 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
~(
0~,~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenyl-lower alkenyl group having optionally a
substituent selected from a lower alkoxy group, a halogen atom, an amino
group having optionally a substituent selected from a lower alkanoyl group and
a phenyl-lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy
group, a tetrazolyl group having optionally a lower alkyl substituent on the
tetrazole moiety, hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in
which A4, R40 and R41 are as defined above), a lower alkenyloxy group, nitro
group and a lower alkyl group having optionally a halogen substituent on the
phenyl moiety, and m, n, and r are as defined above, or a salt thereof.
(484) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
WO 95109159 PCT/JP94101559
3 ~ -186-
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
5 a group of the formula:
A Rs4
0~0
o
10 (in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a lower alkenyl group, and m, n, and r are as defined
above, or a salt thereof.
(485) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
15 aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
20 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
~(
O~.~O
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a cycloalkyl-lower alkyl group, and m, n, and r are as
30 defined above, or a salt thereof.
(486) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
WO 9S/09159 2 t ~ 0 3 ~ 5 PCT/JP94/01559
- 187-
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
5 lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
~
0~0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same.as
defined above); R4 is a naphthyl-lower alkyl group, and m, n, and r are as
defined above, or a salt thereof.
(487) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylthio-substituted lower alkyl group having
optionally a lower alkoxy-substituent on the phenyl moiety, and m, n, and r are
WO 95/09159 PCT/JP94101559
3 188-
as defined above, or a salt thereof.
(488) A quinoxaline derivative of the formula (1), wherein R1 is a iower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
5 imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
10 group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A~ R54
1 5 ~n'
o
(in which R54 is hydrogen atom or a lower alkyl group ànd A is the same as
defined above); R4 is a phenylsulfinyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(489) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
WO 95109159 21 5 0 3 ~ 5 PCT/JP94/01559
- 189-
A Rs4
O O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a phenylsulfonyl-substituted lower alkyl group having
optionally a lower alkoxy substituent on the phenyl moiety, and m, n, and r are
as defined above, or a salt thereof.
(490) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
--A R54
0~,0
o
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
25 defined above); R4 is a phenoxy-substituted lower alkyl group, and m, n, and r
are as defined above, or a salt thereof.
(491 ) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
30 imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
WO 95/09159 PCT/JP94/OlS59
~34~j
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above); R4 is a group of the formula:
(R8)q
.~
(in which the group ~, R3 and q are as defined above), and m, n, and r
are as defined above, or a salt thereof.
(492) A quinoxaline derivative of the formula (1), wherein R1 is a lower
alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
alkanoy! group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A Rs4
0~0
O
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
WO 95/09159 2 1 5 0 3 ~ S PCT/JP94/01559
- 191 -
defined above); R4 is a group of the formula: -A5-CR42R43R44 (in which A5,
R42, R43 and R44 are as defined above), and m, n, and r are as defined above,
or a salt thereof.
(493) A quinoxaline derivative of the formula (1), wherein R1 is a lower
5 alkoxy group, an amino group having optionally a lower alkyl substituent, or an
aminocarbonyl group having optionally a lower alkyl substituent; R2 is an
imidazolyl-substituted lower alkyl group; R3 is a lower alkanoyloxy-substituted
lower alkyl group, a lower alkoxycarbonyl group, a lower alkoxycarbonyloxy -
substituted lower alkyl group, a phenyl-lower alkoxycarbonyl group, a lower
10 alkanoyl group, a lower alkoxy-lower alkyl group, a phenoxycarbonyl group, a
lower alkanoyl-substituted lower alkyl group, a benzoyl-substituted lower alkyl
group having optionally a halogen substituent on the phenyl ring, a group of
the formula: -E-N(R52)(R53) (in which E, R52 and R53 are as defined above), or
a group of the formula:
A~ R54
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
20 defined above); R4 is a 2,3-dihydro-1 H-indenyl-substituted lower alkyl grouphaving optionally a substituent selected from oxo group, hydroxy group and a
silyloxy group having a lower alkyl substituent on the 2,3-dihydro-1 H-indenyl
ring, and m, n, and r are as defined above, or a salt thereof.
WO 95/09159 PCT/JP94/01559
3~'j
- 192-
The compound of the present invention also inciudes the
compounds of the formula (1 ) wherein both m and n are simultaneously 1, or m
is 1 and n is 0, or m is O and n is 1, or both m and n are simultaneously 0, butthe more preferable compounds are compounds of the formula (1) wherein m is
1 andnisO.
The heterocyclic group represented by the formula:
includes, for example, pyridyl, thiazolyl, furyl, benzimidazolyl, benzothiazolyl,
oxazolyl, quinolyl, indolyl, 1,4-benzodioxanyl, 3,4-dihydrofuro[2,3-g]quinolyl,
1,2,3,4-tetrahydrofuro[2,3-g]quinolyl, furo[3,2-c]pyridyl, furo[2.3-g]quinolyl,
benzofuryl, benzothienyl, 2,3-dihydrobenzofuryl, perhydrobenzofuryl,
imidazolyl, 1,2,3,4-tetrazolyl, imidazo[1,2-a]pyridyl, and the like.
The heterocyclic group represented by the formula:
includes, for example, 1,2,3,4-tetrazolyl, thiazolyl, pyridyl, oxazolyl, 1,2,3,5 -
oxathiadiazolyl, 1,3,4-triazolyl, 1,2,4-oxadiazolyl, 1,2,4-triazinyi, imidazolyl,
1,3,4-oxadiazolyl, pyrimidinyl, 1,2,3,4-tetrazolyl, 3,4-dihydrofuro[2,3-g]quinolyl,
1,2,3,4-tetrahydrofuro[2,3-g]quinolyl, naphtho[2,1-b]furyl, oxazolyl, furo[2,3 -g]quinolyl, and the like.
The heterocyclic group represented by the formula:
includes, for example, thiazolyl, 1,2,3,4-tetrazolyl, thiazolidinyl, and the like.
The heterocyclic group represented by the formula:
includes, for example, benzofuryl, benzothiazolyl, carbostyril, 3,4 -
dihydrocarbostyril, furo[3,2-c]pyridyl, benzothienyl, and the like.
The compounds of the present invention of the formula (1 ) may be
prepared by various processes, but preferably prepared by the following
WO 9S/09159 PCT/JP94/01559
21~U34~
- 193-
processes.
Reaction Scheme-1
(f )m ~ R3 ~l )m
R2 HN 4 (3) ~; R2
()n ()n
(2) (1)
[wherein R1, R2, R3, R4, r, m and n are the same as defined above]
The reaction of the compound (2) and the compound (3) is carried
out by a conventional amido bond producing reaction. The amido bond
producing reaction can be carried out under the same conditions as those of
15 the conventional amino bond producing reaction, for example,
(a) a mixed acid anhydride process, i.e. a process of reacting the
carboxylic acid compound (2) with an alkyl halocarbonate to form a mixed acid
anhydride and reacting the resultant with the amine compound (3),
(b) an activated ester process, i.e. a process of converting the
20 carboxylic acid compound (2) into an activated ester such as p-nitrophenyl
ester, N-hydroxysuccinimide ester, 1-hydroxybenzotriazole ester, etc., and
reacting the resultant with the amine compound (3),
(c) a carbodiimide process, i.e. a process of condensing the
carboxylic acid compound (2) and the amine compound (3) in the presence of
25 an activating agent such as dicyclohexylcarbodiimide, carbonyldiimidazole,
etc.,
(d) other processes, i.e. a process of converting the carboxylic acid
compound (2) into a carboxylic anhydride by treating it with a dehydrating
agent such as acetic anhydride, and reacting the resultant with the amine
30 compound (3); a process of reacting an ester of the carboxylic acid compound
(2) with a lower alcohol and the amine compound (3); a process of reacting an
acid halide compound of the carboxylic acid compound (2), i.e. a carboxylic
WO 9S/09159 PCT/JP94/OlSS9
3~ej
- 194-
acid halide, with the amine compound (3); a process of activating the carboxylicacid (2) with a phosphorus compound such as triphenylphosphine, diethyl
chlorophosphate, etc., and reacting the resultant with the amine compound (3);
a process of converting the carboxylic acid compound (2) into a N -
5 carboxyamino anhydride with a phosgene or chloroformic acid trichloromethylester, and reacting the resultant with the amine compound (3); a process of
activating the carboxylic acid compound (2) with an acetylene compound such
as trimethylsilylethoxyacetylene, etc., and reacting the resultant with the amine
compound (3), and the like.
The mixed acid anhydride used in the above mixed acid
anhydride process (a) is obtained by the known Schotten-Baumann reaction,
and the reaction product is used without isolating from the reaction mixture forthe reaction with the amine compound (3) to give the desired compound (1 ) of
the present invention. The Schotten-Baumann reaction is usually carried out in
the presence of a basic compound. The basic compound is any conventional
compounds used for the Schotten-Baumann reaction and includes, for
example, organic basic compounds such as triethylamine, trimethylamine,
pyridine, dimethylaniline, N-methylmorpholine, 4-dimethylaminopyridine, 1,5-
diazabicyclo[4.3.0]nonene-5 (DBN), 1,8-diazabicyclo[5.4.0]undecene-7 (DBU),
1,4-diazabicyclo[2.2.2]octane (DABCO), etc., and inorganic basic compounds
such as potassium carbonate, sodium carbonate, potassium hydrogen
carbonate, sodium hydrogen carbonate, etc. The reaction is usually carried
out at a temperature from about -20C to about 1 00C, preferably at a
temperature of 0C to about 50C, for about 5 minutes to about 10 hours,
preferably for 5 minutes to about 2 hours.
The reaction between the mixed acid anhydride thus obtained
and the amine compound (3) is usually carried out at a temperature of -20C to
about 1 50C, preferably at a temperature of 1 0C to about 50C, for 5 minutes
to about 10 hours, preferably for about 5 minutes to 5 hours. The mixed acid
anhydride process is usually carried out in a solvent or without a solvent, and
the solvent may be any conventional solvents which are usually used in the
mixed acid anhydride process and includes, for example, halogenated
hydrocarbons (e.g. methylene chloride, chloroform, dichloroethane, etc.),
WO 95/09159 21 ~ 0 3 4 ~ PCT/JP94/01559
- 195-
aromatic hydrocarbons (e.g. benzene, toluene, xylene, etc.), ethers (e.g. diethyl
ether, dioxane, diisopropyl ether, tetrahydrofuran, dimethoxyethane, etc.),
esters (e.g. methyl acetate, ethyl acetate, etc.), aprotic polar solvents (e.g.
1,1,3,3-tetramethylurea, N,N-dimethylformamide, dimethylsulfoxide,
hexamethylphosphoric triamide, etc.), or a mixture of these solvents. The alkyl
halocarbonate used in the mixed acid anhydride process includes, for
example, methyl chloroformate, methyl bromoformate, ethyl chloroformate,
ethyl bromoformate, isobutyl chloroformate, and the like. In said process, the
carboxylic acid compound (2), the alkyl halocarbonate ester and the amine
compound (3) are usually used in each equimolar amount, but preferably, the
alkyl halocarbonate ester and the amine compound (3) are used each in an
amount of about 1 to 1.5 mole to 1 mole of the carboxylic acid compound (2).
The activated ester process (b), for example, a process using N -
hydroxysuccinic acid imide ester is carried out in a suitable solvent which doesnot affect the reaction, in the presence or absence of a basic compound.
Besides, the reaction may be carried out with addition of a condensing agent
such as dicyclohexylcarbodiimide, carbonyldiimidazole, 1-ethyl-3-(3'-dimethyl -
aminopropyl)carbodiimide, etc. The basic compound may be any basic
compounds for the above mentioned Schotten-Baumann reaction, and alkali
metal carboxylates (e.g. sodium acetate, sodium benzoate, sodium formate,
potassium acetate, lithium benzoate, cecium acetate, etc.), alkali metal halides(e.g. potassium fluoride, cecium fluoride, etc.), and the like. The solvent
includes, for example, halogenated hydrocarbons (e.g. methylene chloride,
chloroform, dichloroethane, etc.), aromatic hydrocarbons (e.g. benzene,
toluene, xylene, etc.), ethers (e.g. diethyl ether, dioxane, tetrahydrofuran,
dimethoxyethane, etc.), esters (e.g. methyl acetate, ethyl acetate, etc.), aprotic
polar solvents (e.g. N,N-dimethylformamide, dimethylsulfoxide, hexamethyl -
phosphoric triamide, etc.), or a mixture of these solvents. The reaction is
carried out at a temperature of 0 to 1 50C, preferably at a temperature of 10 to
100C for 5 to 30 hours. The amine compound (3) and N-hydroxysuccinic acid
imide ester are used each at least in equimolar amount, preferably in an
amount of 1 to 2 moles to 1 mole of the carboxylic acid compound (2).
Besides, the amido bond producing reaction in Reaction Scheme-
wogs/ ~ts~3~S
- 196-
1 may also be carried out by reacting the carboxylic acid compound (2) and the
amine compound (3) in the presence of a condensing agent such as
phosphorus compounds (e.g. triphenylphosphine, triphenylphosphine-2,2' -
dipyridyldisulfide, diethyl chlorophosphate, diphenylphosphinyl chloride,
5 phenyl-N-phenylphosphoramide chloridate, diethyl cyanophosphate, bis(2-oxo-
3-oxazolidinyl)phosphinic chloride, etc.). The reaction is usually carried out in
the presence of the solvent and the basic compound. The basic compound
includes, for example, in addition to the basic compounds used for the above
mentioned Schotten-Baumann reaction, sodium hydroxide, potassium
10 hydroxide, etc. The solvent includes, for example, in addition to the solvents
used in the mixed acid anhydride process (a), pyridine, acetone, acetonitrile, or
a mixture of two or more above solvents. The reaction is usually carried out at
a temperature of -20C to about 1 50C, preferably at a temperature of 0C to
about 1 00C, for about 5 minutes to about 30 hours. The condensing agent
15 and the amine compound (3) are used at least in equimolar amount, preferably
in an amount of about 1 to 2 moles, to 1 mole of the carboxylic acid compound
(2).
The amido bond producing reaction is also carried out by reacting
the amine compound (3) and the carboxylic acid compound (2) in the presence
20 of a condensing agent. The reaction is carried out in a suitable solvent in the
presence or absence of a catalyst. The solvent includes, for example,
halogenated hydrocarbons (e.g. dichloromethane, dichloroethane, chloroform,
carbon tetrachloride, etc.), acetonitrile, dimethylformamide, etc. The catalyst
includes, for example, organic bases such as dimethylaminopyridine, 4 -
25 piperidinopyridine, etc., salts such as pyridinium tosylate, etc., camphorsulfonicacid, mercury oxide, and the like. The condensing agent includes, for example,
acetylene compounds such as trimethylsilylethoxyacetylene, etc., and is used
in an amount of 1 to 10 moles, preferably in an amount of 2 to 6 moles, to 1
mole of the amine compound (3). The carboxylic acid compound (2) is usually
30 used at least in equimolar amount, preferably in an amount of 1 to 2 moles, to 1
mole of the amine compound (3). The reaction is usually carried out at a
temperature of 0 to about 150C, preferably at a temperature of room
temperature to about 100C, for about 1 to about 10 hours.
WO 95/09159 2 1 ~ 0 3 ~ ~ PCT/JP94/01559
- 197-
Among the above other processes (d), in case of the process of
reacting the carboxylic acid halide with the amine compound (3), the reaction isusually carried out in the presence of a de-hydrogen halogenating agent in an
appropriate solvent. The de-hydrogen halogenating agent is any conventional
5 basic compounds and includes, for example, in addition to the basic
compounds used for the above mentioned Schotten-Baumann reaction,
sodium hydroxide, potassium hydroxide, sodium hydride, potassium hydride,
and the like. The solvent includes, for example, in addition to the solvents used
in the mixed acid anhydride process, alcohols (e.g. methanol, ethanol,
10 propanol, butanol, 3-methoxy-1-butanol, ethylcellosolve, methylcellosolve,
etc.), pyridine, acetone, acetonitrile, or a mixture of two or more these solvents,
and the like. The amount of the amine compound (3) and the carboxylic acid
halide is not critical, but the amine compound (3) is usually used at least in
equimolar amount, preferably about 1 to 5 moles, to 1 mole of the carboxylic
15 acid halide. The reaction is usually carried out at a temperature of about
-20C to about 1 80C, preferably at a temperature of about 0C to about 1 50C,for about 5 minutes to about 30 hours.
The carboxylic acid halide used in the above reaction may be
prepared, for example, by reacting the carboxylic acid compound (2) with a
20 halogenating agent in a solvent or without a solvent. The solvent may be any
one which does not affect the reaction, for example, aromatic hydrocarbons
(e.g. benzene, toluene, xylene, etc.), halogenated hydrocarbons (e.g.
chloroform, methylene chloride, carbon tetrachloride, etc.), ethers (e.g. dioxane,
tetrahydrofuran, diethyl ether, etc.), dimethylformamide, dimethylsulfoxide, and25 the like. The halogenating agent may be any conventional one which convert
the hydroxy group in carboxyl group into a halogen, for example, thionyl
chloride, phosphorus oxychloride, phosphorus oxybromide, phosphorus
pentachloride, phosphorus pentabromide, etc. The amount of the carboxylic
acid compound (2) and the halogenating agent is not critical, but the
30 halogenating agent is usually used in an excess amount to the carboxylic acidcompound (2) when the reaction is carried out without a solvent, and when the
reaction is carried out in a solvent, the hydrogenating agent is usually used atleast in equimolar amount, preferably in an amount of 2 to 4 moles, to 1 mole of
WO 95109159 ~3 ~ PCT1JP94/01559
- 198-
the carboxylic acid compound (2). The reaction temperature and the reaction
period are not necessarily specified, but the reaction is usually carried out at a
temperature of room temperature to about 1 00C, preferably at a temperature
of 50 to 80C, for 30 minutes for about 6 hours.
In the process of reacting the amine compound (3) with an ester of
the carboxylic acid compound (2) and a lower alcohol, the reaction is carried
out in a suitable solvent or without a solvent. The solvent may be any ones
which are used in the above mentioned reaction of the carboxylic acid halide
and the amine compound (3). The amine compound (3) is usually used at least
in equimolar amount, preferably in an amount of 1 to 15 moles, to 1 mole of the
ester of the carboxylic acid compound (2) and a lower alcohol. The reaction is
usually carried out at a temperature of room temperature to 1 50C, preferably
at a temperature of room temperature to about 1 20C, for one to about 20
hours.
Reaction Scheme-2
(t)m (t)m
j~ ~X R3bX (4a) or ~N`X R
y CoNHR3a R3CCoR3d (4b) (R1) CO ~ R3b
()n ()n
(1a) (1 b)
[wherein R1, R2, r, m and n are the same as defined above, X is a halogen
25 atom, R3a is hydrogen atom, a lower alkyl group, a phenyl-lower alkoxy -
carbonyl group, a lower alkanoyloxy-substituted lower alkyl group, e) a lower
alkanoyl group, a lower alkoxycarbonyl group, a lower alkoxy-lower alkyl
group, a phenoxycarbonyl group, a lower alkanoyl-substituted lower alkyl
group, a lower alkoxycarbonyloxy-substituted lower alkyl group, a benzoyl -
30 substituted lower alkyl group having optionally a halogen substituent on the
phenyl ring, a group of the formula: -E-NR52R53 (in which R52 and R53 are the
same or different and each hydrogen atom, a lower alkyl group, a lower
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 199-
alkoxycarbonyl group or phenyl group, or R52 and R53 may combine together
with the nitrogen atom to which they bond to form a 5- or 6-membered
saturated heterocyclic group with or without being intervened with another
nitrogen atom or oxygen atom, E is a lower alkylene group, a group of the
5 formula: -CO-, or a group of the formula:
-CO-A- (in which A is a lower alkylene group)), a group of the formula:
A Rs4
0~0
(in which R54 is hydrogen atom or a lower alkyl group and A is the same as
defined above), a group of the formula:
(R5)p
A~
(in which A, R5 and p are the same as defined above), a phenyl-lower alkenyl
group which may optionally have a substituent on the phenyl moiety selected
from a lower alkoxy group, a halogen atom, an amino group having optionally a
substituent selected from a lower alkanoyl group and a phenyl-lower
20 alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy group, a
tetrazolyl group having optionally a lower alkyl substituent on the tetrazole ring,
hydroxy group, a group of the formula: -O-A4-CO-NR4R41 (in which A4, R40
and R41 are the same as defined above), a lower alkenyloxy group, nitro group
and a lower alkyl group having optionally a halogen substituent, an alkenyl
25 group, a cycloalkyl-lower alkyl group, a naphthyl-lower alkyl group, a
phenylthio-substituted lower alkyl group having optionally a lower alkoxy
substituent on the phenyl moiety, a phenylsulfinyl-substituted lower alkyl grouphaving optionally a lower alkoxy substituent on the phenyl moiety, a
phenylsulfonyl-substituted lower alkyl group having optionally a lower alkoxy
30 substituent on the phenyl moiety, a phenoxy-substituted lower alkyl group, a
group of the formula:
WO 9S/O91S9 PCIIJP94/OlSS9
3 45 - 200 -
(R8)q
~A ~ -
(in which a group of the formula: ~, R3 and p are the same as defined
above), a group of the formula: -A5-CR42R43R44 (in which A5, R42, R43 and R44
are the same as defined above), a 2,3-dihydro-1 H-indenyl-substituted lower
alkyl group having optionally a substituent on the 2,3-dihydro-1 H-indenyl ring
selected from oxo group, hydroxy group and a silyloxy group having a lower
alkyl substituent, or a group of the formula:
1 0 (R47)u
.~
(in which a group of the formula: ~, R47 and u are the same as defined
above, R3b is the same groups for the above mentioned R3a except hydrogen
atom, and R3C and R3d are each hydrogen atom or a lower alkyl group]
The reaction of the compound (1a) and the compound (4a) is
usually carried out in the presence or absence of a basic compound in a
suitable inert solvent. The inert solvent includes, for example, aromatic
hydrocarbons (e.g. benzene, toluene, xylene, etc.), ethers (e.g. tetrahydrofuran,
dioxane, diethylene glycol dimethyl ether, etc.), alcohols (e.g. methanol,
ethanol, isopropanol, butanol, etc.), ethyl acetate, acetone, acetonitrile,
dimethylsulfoxide, dimethylformamide, hexamethylphosphoric triamide, or a
mixture of these solvents. The basic compound includes, for example, an alkali
metal carbonate (e.g. sodium carbonate, potassium carbonate, sodium
hydrogen carbonate, potassium hydrogen carbonate, etc.), an alkali metal
hydroxide (e.g. sodium hydroxide, potassium hydroxide, etc.), sodium hydride,
potassium, sodium, sodium amide, an alkali metal alcoholate (e.g. sodium
methylate, sodium ethylate, etc.), organic basic compounds (e.g. pyridine, N -
ethyldiisopropylamine, dimethylaminopyridine, triethylamine, DBN, DBU,
WO 95/09159 5 0 3 4 S ~CT/~4/01559
- 201 -
DABCO, etc.), and the like. The amount of the compound (1a) and the
compound (4a) is not critical, but the compound (4a) is used at least in
equimolar amount, preferably in an amount of 1 to 10 moles, to 1 mole of the
compound (1 a). The reaction is usually carried out at a temperature of 0 to
about 200C, preferably at a temperature of 0 to about 1 70C, for 30 minutes to30 hours. The reaction may be carried out by adding an alkali metal halide
compound such as sodium iodide, potassium iodide, etc., into the reaction
system. Further, the reaction may be carried out by adding a halogenated
ammonium such as tetra-n-butylammonium iodide, tetra-n-butylammonium
bromide, n-butyl triethyl ammonium iodide, tetraethylammonium iodide, tri-n -
butyl methyl ammonium iodide, etc., into the reaction system.
The reaction of the compound (1a) and the compound (4b) is
usually carried out in the presence of a reducing agent in a suitable solvent orwithout a solvent. The solvent includes, for example, water, alcohols (e.g.
methanol, ethanol, isopropanol, butanol, etc.), acetonitrile, formic acid, acetic
acid, ethers (e.g. dioxane, diethyl ether, diglyme, tetrahydrofuran, etc.),
aromatic hydrocarbons (e.g. benzene, toluene, xylene, etc.), or a mixture of
these solvents. The reducing agent includes, for example, formic acid, alkali
metal salts of fatty acid (e.g. sodium formate, etc.), hydrogenating-reducing
agents (e.g. sodium borohydride, sodium cyanoborohydride, lithium aluminum
hydride, etc.), catalytic reducing agents (e.g. palladium-black, palladium -
carbon, platinum oxide, platinum black, Raney-nickel, etc.), and the like.
When formic acid is used as a reducing agent, the reaction is
usually carried out at a temperature of room temperature to about 200C,
preferably at a temperature of ~0 to about 1 50C, for 1 to about 10 hours. The
formic acid is used in an excess amount to the compound (1a).
When a hydrogenating agent is used, the reaction is usually
carried out at a temperature of -30 to about 1 00C, preferably at a temperatureof 0 to about 70C, for 30 minutes to about 15 hours. The reducing agent is
used in an amount of 1 to 20 moles, preferably in an amount of 1 to 6 moles, to
1 mole of the compound (1a). When lithium aluminum hydride is used as an
reducing agent, the solvent may preferably ethers (e.g. diethyl ether, dioxane,
tetrahydrofuran, diglyme, etc.) and aromatic hydrocarbons (e.g. benzene,
WO 95/09159 PCT/JP94/01559
,a~ 202-
toluene, xylene, etc.).
When a catalytic reducing agent is used, the reaction is usually
carried out under atmospheric pressure or 20 atms. of hydrogen gas, preferably
under atmospheric pressure or about 10 atms. of hydrogen gas, or in the
5 presence of a hydrogen-donor such as formic acid, ammonium formate,
cyclohexene, hydrazine hydrate, etc., at a temperature of -30 to about 1 00C,
preferably at a temperature of 0 to about 60C, for 1 to 12 hours. The catalyticreducing agent is usually used in an amount of 0.1 to 40 % by weight,
preferably 1 to 20 % by weight, to the compound (1a).
The compound (4b) is usually used at least in equimolar amount,
preferably in an amount of 1 to excess amount, to 1 mole of the compound (1 a).
Reaction Scheme-3
o
5~N~X O ~R3 ~N'X ,R3
(Rl)r ~ ~ R4 (R1)r ~ ~ R4
()n ()n
(1c) (1 d)
~l )m ( j~ )m
X ~X _R '5~:7X ,R3
(1e) (1f)
[in which R1, R2, R3, R4, r, m and n are the same as defined above]
The reaction of converting the compound (1c) into the compound
(1d), and the reaction of converting the compound (1e) into the compound (1f)
are carried out in the presence of an oxidizing agent in a suitable solvent. The
21503~
WO 9S/O91S9 - PCT/JP94/01559
- 203 -
solvent includes, for example, water, organic acids (e.g. formic acid, acetic acid,
trifluoroacetic acid, etc.), alcohols (e.g. methanol, ethanol, etc.), halogenated
hydrocarbons (e.g. chloroform, dichloromethane, etc.), or a mixture of these
solvents. The oxidizing agent includes, for example, peracids (e.g. performic
5 acid, peracetic acid, pertrifluoroacetic acid, perbenzoic acid, m-chloro -
perbenzoic acid, p-carboxyperbenzoic acid, etc.), hydrogen peroxide, sodium
metaperiodate, dichromic acid, dichromates (e.g. sodium dichromate,
potassium dichromate, etc.), permanganic acid, permanganates (e.g.
potassium permanganate, sodium permanganate, etc.), lead salts (e.g. Iead
10 tetraacetate, etc.), and the like. The oxidizing agent is usually used at least in
equimolar amount, preferably in an amount of 1 to 2 moles, to 1 mole of the
starting compound. The above reaction is usually carried out at a temperature
of -10 to 40C, preferably at a temperature of -1 0C to room temperature, for
about 1 to 100 hours.
WO 95/09159 PCT/JP94/01559
3~
- 204 -
Reaction Scheme-4
~l )m
~N~XR (R~)ql
()n (C)IN H R9a
(19) \R OH (5) ()m
R10bX (6a) \ ~N~xR
Rl0CcoR10d (6b) ~N CON_~
(1h)
~;'XCO N~ ~ (R8)q/Rgb
2û ()n lCI )IN~R~ob
(1i)
[wherein R1, R2, R3a~ R8, a group of the formula: ~, r, m, n, X and I are
the same as defined above, R9a is hydrogen atom, a lower alkanoyl group, a
lower alkyl group, a morpholinocarbonyl-lower alkyl group, a cycloalkyl -
25 carbonyl group, a phenyl-lower alkenylcarbonyl group, a lower alkylsulfonyl
group, an aminocarbonyl group which may optionally have a lower alkyl
substituent, a phenylsulfonyl group which may optionally have a lower alkyl
substituent on the phenyl moiety, a phenyl-lower alkenyl group, a benzoyl
group which may optionally have 1 to 3 substituents on the phenyl moiety
30 selected from a halogen atom, a lower alkoxy group, an amino group having
optionally a lower alkanoyl substituent and hydroxy group, an amino-
WO 95/09159 2 1 5 0 3 ~ 5 PCT/JP94/01559
- 205 -
substituted lower alkanoyl group which may optionally have a lower alkanoyl
substituent, an amino-substituted sulfonyl group which may optionally have a
- lower alkyl substituent, a phenyl-lower alkyl group, phenyl group and an amino
group which may optionally have a lower alkanoyl substituent, R10a is a lower
alkanoyl group, a cycloalkylcarbonyl group, a phenyl-lower alkenylcarbonyl
group, a benzoyl group which may optionally have 1 to 3 substituents on the
phenyl moiety selected from a halogen atom, a lower alkoxy group, an amino
group having optionally a lower alkanoyl substituent and hydroxy group, and
an amino-substituted lower alkanoyl group which may optionally have a lower
alkanoyl substituent, R10b is a lower alkyl group, a morpholinocarbonyl-lower
alkyl group, a lower alkenylsulfonyl group, an aminocarbonyl group which may
optionally have a lower alkyl substituent, a phenylsulfonyl group which may
optionally have a lower alkyl substituent, a phenyl-lower alkenyl group, an
amino-substituted sulfonyl group which may optionally have a lower alkyl
substituent, or a phenyl-lower alkyl group, q' is 1 or 2, R10C and R10d are eachhydrogen atom or a lower alkyl group]
The reaction of the compound (1g) and the compound (5) is
carried out under the same conditions as those of the reaction of the compound
(2) and the compound (3) in the above mentioned Reaction Scheme-1.
The compound (1h) wherein R10a is a lower alkanoyl group may
be derived from the compound (19) by subjecting the compound (19) to lower
alkanoylization with using a compound of the formula: (R11)2O (7) or R11X (8)
(in which R1 l is a lower alkanoyl group, and X is the same as defined above).
The lower alkanoylization reaction is carried out in the presence or absence of
a basic compound. The basic compound includes, for example, an alkali metal
(e.g. sodium, potassium, etc.), hydroxides, carbonates and hydrogen
carbonates of these alkali metals, or organic basic compounds (e.g. N,N -
dimethylaminopyridine, pyridine, piperidine, etc.), and the like. The reaction is
carried out in a solvent or without a solvent. The solvent includes, for example,
ketones (e.g. acetone, methyl ethyl ketone, etc.), ethers (e.g. diethyl ether,
dioxane, etc.), aromatic hydrocarbons (e.g. benzene, toluene, xylene, etc.),
water, pyridine, and the like. The compound (7) or the compound (8) is used at
WO9S/09159 2~Sa34S PCT/JP94/01559
- 206 -
least in equimolar amount, preferably in an amount of 1 to excess amount, to 1
mole of the starting compound (19). The reaction is carried out at a
temperature of 0 to 200C, preferably at a temperature of 0 to 1 50C, for about5 minutes to about 5 days.
The reaction of the compound (19) and the compound (6a) or the
compound (6b) is carried out under the same conditions as those of the
reaction of the compound (1a) and the compound (4a) or the compound (4b) in
the above mentioned Reaction Scheme-2.
Reaction Scheme-5
(~ )m
~)n (cRo)oRl2
(1 j) \
~drolysis ()m
5~ 'X _R~ COOH
( ~~)m R HN\ (9) (1k)
~N'XCON'~ (R8)q,
()n CON\
R10
(1 I)
[wherein R1, R2, R8, R3a~ m, n, q', R9, R10, r and the group of the formula:
WO 95/O91S9 PCT1JP94/01559
- ~ 2lso3~s
- 207 -
~) are the same as defined above, and R12 is a lower alkyl group]
The hydrolysis of the compound (1j) is carried out in the presence
of an acid or a basic compound in a suitable solvent or without a solvent. The
solvent includes, for example, water, lower alcohols (e.g. methanol, ethanol,
5 isopropanol, etc.), ketones (e.g. acetone, methyl ethyl ketone, etc.), ethers (e.g.
dioxane, tetrahydrofuran, ethylene glycol dimethyl ether, etc.), fatty acids (e.g.
acetic acid, formic acid, etc.), dimethylformamide, or a mixture of these solvents.
The acid includes, for example, inorganic acids (e.g. hydrochloric acid, sulfuric
acid, hydrobromic acid, etc.), and organic acids (e.g. formic acid, acetic acid,10 aromatic sulfonic acid, etc.). The basic compound includes, for example, alkali
metal salts (e.g. sodium carbonate, potassium carbonate, etc.), alkali metal or
alkaline earth metal hydroxides (e.g. sodium hydroxide, potassium hydroxide,
calcium hydroxide, etc.), and the like. The reaction is usually carried out at atemperature of room temperature to about 200C, preferably at a temperature
of room temperature to about 1 50C, for 10 minutes to about 25 hours.
The reaction of the compound (1 k) and the compound (9) is
carried out under the same conditions as for the reaction of the compound (2)
and the compound (3) in the above mentioned Reaction Scheme-1.
WO 95/09159 PCT/JP94/01559
34~
- 208 -
Reaction Scheme-6
()m
X N`X R (RR1a) q~
(10)
\ (t )m R2
X)x R3 OH
15 (t )m R13X (10) (1 m)
~ N`X R2
()n oR13
(1 n)
[wherein R1, R2, R3a~ R8, m, n, q', X and the group of the formula: ~3 are
the same as defined above, R13 is a lower alkyl group, a phenyl-lower alkyl
25 group which may optionally have an amino substituent having optionally a
lower alkanoyl substituent on the phenyl moiety, a carboxy-substituted lower
alkyl group, a lower alkoxycarbonyl-substituted lower alkyl group, a lower
alkoxy-substituted lower alkyl group, a lower alkenyl group, a lower alkanoyl
group, or a morpholinocarbonyl-lower alkyl group, and Rl4 is a lower alkoxy
30 group, a phenyl-lower alkoxyl group which may optionally have an amino
substituent having optionally a lower alkanoyl substituent on the phenyl moiety,
WO 9S/09159 PCI/JP94/OlSS9
21~034~
- 209 -
a lower alkanoyloxy group or a lower alkoxy-substituted lower alkoxy group]
The reaction of converting the compound (10) into the compound
(1m) is carried out by reducing the compound (10) when R14 is a phenyl-lower
alkoxy group. The reduction reaction is carried out by subjecting the
5 compound (1 m) to catalytic hydrogenation in the presence of a catalyst in a
suitable solvent . The solvent includes, for example, water, acetic acid,
alcohols (e.g. methanol, ethanol, isopropanol, etc.), hydrocarbons (e.g. hexane,cyclohexane, etc.), ethers (e.g. dioxane, tetrahydrofuran, diethyl ether, ethylene
glycol dimethyl ether, etc.), esters (e.g. ethyl acetate, methyl acetate, etc.),10 aprotic polar solvents (e.g. dimethylformamide, etc.), or a mixture of these
solvents. The catalyst is, for example, palladium, palladium-black, palladium -
carbon, platinum, platinum oxide, copper chromite, Raney-nickel, etc. and used
in an amount of 0.02 to 1 part by weight to 1 part by weight of the compound
(7). The reaction is usually carried out at a temperature of -20 to 1 00C,
preferably at a temperature of 0 to about 80C, under 1 to 10 atms of hydrogen
gas, for 0.5 to 20 hours.
The reaction of converting the compound (1 O) into the compound
(1m) is carried out by subjecting the compound (10) to hydrolysis when R14 is a
lower alkoxy group or a lower alkoxy-substituted lowre alkoxy group. The
hydrolysis is carried out in the presence of an acid in a suitable solvent. The
solvent includes, for example, water, lower alcohols (e.g. methanol, ethanol,
isopropanol, etc.), ethers (e.g. dioxane, tetrahydrofuran, etc.), halogenated
hydrocarbons (e.g. dichloromethane, chloroform, carbon tetrachloride, etc.),
polar solvents (e.g. acetonitrile, etc.), or a mixture of these solvents. The acid
includes, for example, inorganic acids (e.g. hydrochloric acid, sulfuric acid,
hydrobromic acid, etc.), fatty acids (e.g. formic acid, acetic acid, etc.), Lewis
acids (e.g. boron trifluoride, aluminum chloride, boron tribromide, etc.), iodides
(e.g. sodium iodide, potassium iodide, etc.), a mixture of the above Lewis acid
and a iodide, and the like. The reaction is usually carried out at a temperatureof 0 to 150C, preferably at a temperature of room temperature to 100C, for 0.5to about 50 hours.
The reaction of converting the compound (1 O) into the compound
WO 95/09159 3 ~ PCT/JP94/OlS59
- 210 -
(1 m) is carried out in the same manner as in the hydrolysis of the compound
(1j) in the above mentioned Reaction Scheme-5, when R14 is a lower
alkanoyloxy group.
The reaction of the compound (1 m) into the compound (10) is
5 carried out under the same conditions as those of the reaction of the compound(19) and the compound (6) in the above mentioned Reaction Scheme-4. The
compound (1 n) wherein R13 is a lower alkanoyl group is also prepared by
reacting the compound (1m) and the compound (7) under the same conditions
as those of the reaction of the compound (19) and the compound (7) in the
10 above mentioned Reaction Scheme-4.
Reaction Scheme-7
~l )m ~l )m
~, N~ R Halogenation ~N`y R2
CON--R (R8) ~ ~N CON~ (R8)
()n ()n X
(1 p) (1q)
~l )m ~l )m
~N~R Halogenation ~N~R2
~N CON (R5)p~ ~N CON (R5)p~
(R1), ~ `A~ (R1)r ~ A~
()n ()n X
(1E) (1F)
[wherein R1 R2, R3a, R5, R8, m, n, q', A, X, r and the group of the formula:
are the same as defined above, and p' is 1 or 2]
The halogenation reaction of the compound (1p) or the compound
30 (1 E) is carried out in the presence of a conventional halogenating agent. The
halogenating agent may be any conventional ones, for example, a halogen
WO 95tO9159 _ 2 1 ~ O ~ ~ S PCTIJP94tO1559
- 211 -
atom (e.g. bromine, chlorine, etc.), or halogenating agents such as iodine
monochloride, sulfuryl chloride, N-halogenosuccinimides (e.g. N-bromo-
succinimide, N-chlorosuccinimide, etc.). The halogenating agent is used at
least in equimolar amount, preferably in an amount of 1 to 1.5 mole, to 1 mole o5 the compound (1 p) or the compound (1 E). The solvent includes, for example,
halogenated hydrocarbons (e.g. dichloromethane, dichloroethane, chloroform,
carbon tetrachloride, etc.), acetic acid, propionic acid, water, or a mixture ofthese solvents. The reaction is carried out at a temperature of 0C to a boilingpoint of the solvent to be used, preferably at a temperature of 0 to 100C, for 1
10 to about 10 hours.
WO 95/09159 PCT/JP94/01559
~3~
? i - 21 2 -
Reaction Scheme-8
()m ( l)m
(R )q ~ N,XcoN~(~(Rh)
(1u) (1r)
(R~ ~ ~N~(Rh)q,
~N~R ()n R16
~N~ CON_~(R8)ql (1s)
()n C= C H- R18a ¦ R MgX (11 )
(1v) R18b (l)m
~N~X ,R3a (RH)q,
()n C- R17
18
(1t) R
~wherein R1, R2, R3a~ R8, m, n, q', X, r, and the compound of the formula:
~ are the same as defined above, R15 is a lower alkyl group having a
hydroxy substituent, R16 is a lower alkanoyl group, R17 and R13 are each a
lower alkyl group, R19 is a lower alkanoyloxy-substituted lower alkyl group,
R18a is a hydrogen atom or a lower alkyl group, R13C is phenyl group, and R13b
is a lower alkyl group]
The reaction of converting the compound (1 r) into the compound
(1 s) is carried out in the presence of an oxidizing agent in a suitable solvent.
wo 95/og1S9 2 1 5 0 3 4 5 PCT/JP94/01559
- 213-
The oxidizing agent includes, pyridinium chromates (e.g. pyridinium chloro -
chromate, pyridinium dichlorochromate, etc.), dimethylsulfoxide-oxazolyl -
chloride, manganese dioxide, DDQ, chromic acid, chromites (e.g. sodium
chromite, potassium chromite, etc.), permanganic acid, permanganates (e.g.
5 potassium permanganate, sodium permanganate, etc.), and the like. The
solvent includes, for example, water, organic acids (e.g. formic acid, acetic acid,
trifluoroacetic acid, etc.), alcohols (e.g. methanol, ethanol, etc.), halogenated
hydrocarbons (e.g. chloroform, dichloromethane, etc.), ethers (e.g.
tetrahydrofuran, diethyl ether, dioxane, etc.), dimethylosulfoxide, dimethyl -
10 formamide, or a mixture of these solvents. The oxidizing agent is usually usedat least in equimolar amount, preferably in an amount of 1 to 30 moles, to 1
mole of the starting compound. The reaction is carried out at a temperature of 0to 150C, preferably at a temperature of 0 to 100C, for 1 to about 10 hours.
The reaction of converting the compound (1 r) into the compound
15 (1s) is carried out by reacting the compound (1r) with an oxidizing agent in the
presence of a co-oxidizing agent in a suitable solvent. The solvent includes,
pyridine, ethers (e.g. dioxane, tetrahydrofuran, diethyl ether, etc.), aromatic
hydrocarbons (e.g. benzene, toluene, xylene, etc.), halogenated hydrocarbons
(e.g. dichloromethane, dichloroethane, chloroform, carbon tetrachloride, etc.),
20 esters (e.g. ethyl acetate, etc.), water, alcohols (e.g. methanol, ethanol,
isopropanol, t-butanol, etc.), or a mixture of these solvents. The co-oxidizing
agent used therein includes, for example, N-oxides of organic amine such as
pyridine N-oxide, N-ethyldiisopropylamine N-oxide, N-methylmorpholine N -
oxide, trimethylamine N-oxide, triethylamine N-oxide, etc. The oxidizing agent
25 includes, for example, tetra(n-propyl)ammonium perruthenate, and the like.
The co-oxidizing agent is used at least in equimolar amount, preferably in an
amount of 1 to 5 moles, to 1 mole of the starting compound. The oxidizing
agent is used in a catalytic amount. The reaction is carried out at a temperature
of -20 to 1 50C, preferably at a temperature of 0 to 1 00C, for 1 to about 20
30 hours. The reaction may proceed more advantageously by adding Molecular
Shieves into the reaction system.
The reaction of converting the compound (1s) into the compound
(1 r) is carried out by a reduction reaction using a hydrogenating agent. The
WO 95/O91S9 ~ c~ j PCT/JP94/01559
- 214 -
hydrogenating agent includes, for example, lithium aluminum hydride, diboran,
aluminum diisobutyl hydride, sodium borohydride, lithium borohydride,
tetrabutylammonium borohydride, calcium borohydride, aluminum hydride, and
the like, and is used at least in 0.1 mole amount, preferably in an amount of 0.1
5 to 15 moles, to 1 mole of the starting compound. The reduction reaction is
usually carried out in a suitable solvent, for example, water, lower alcohols (e.g.
methanol, ethanol, isopropanol, etc.), ethers (e.g. tetrahydrofuran, diethyl ether,
diisopropyl ether, diglyme, etc.), aromatic hydrocarbons (e.g. benzene, toluene,xylene, etc.), or a mixture of these solvents, at a temperature of -60 to 1 50C,
preferably at a temperature of -30 to 1 00C, for about 10 minutes to about 20
hours.
The reaction of the compound (1s) and the compound (11) is
carried out in a suitable solvent. The solvent includes any solvents used for the
Grignard reaction, and preferable ones are, for example, ethers (e.g. diethyl
ether, dioxane, tetrahydrofuran, etc.), aromatic hydrocarbons (e.g. benzene,
toluene, etc.), saturated hydrocarbons (e.g. pentane, hexane, heptane,
cyclohexane, etc.), and the like. The compound (11 ) is usually used at least inequimolar amount, preferably in an amount of 1 to 2 moles, to 1 mole of the
compound (1s). The reaction is carried out at a temperature of -70 to 1 00C,
preferably at -30 to about 70C, for 1 to about 50 hours.
The reaction of converting the compound (1 r) into the compound
(1 u) is carried out by reacting the compound (1 r) with the compound (7) or thecompound (8) under the same conditions as those of the reaction of the
compound (1 h) and the compound (7) or the compound (8) in the above
mentioned Reaction Scheme-4.
The reaction of converting the compound (1 u) into the compound
(1 r) is carried out under the same conditions as those of the hydrolysis of thecompound (1j) in the above mentioned Reaction Scheme-5.
The reaction of the compound (1s) and the compound (11a) is
carried out in the presence of a basic compound in a suitable solvent. The
basic compound includes, for example, inorganic bases such as metal sodium,
metal potassium, sodium hydride, sodium amide, sodium hydroxide, potassium
hydroxide, sodium carbonate, potassium carbonate, sodium hydrogen
WO 9S/09159 PCT/JP94/01559
,_ ~ 2~S03~
- 21 5 -
carbonate, and organic bases such as alkali metal alcoholates (e.g. sodium
methylate, sodium ethylate, potassium t-butoxide, etc.), an alkyl lithium, aryl
- lithium or lithium amide (e.g. methyl lithium, n-butyl lithium, phenyl lithium,
lithium diisopropylamide, etc.), pyridine, piperidine, quinoline, triethylamine,- 5 N,N-dimethylaniline, and the like. The solvent may be any one which does not
affect the reaction, for example, ethers (e.g. diethyl ether, dioxane,
tetrahydrofuran, monoglyme, diglyme, etc.), aromatic hydrocarbons (e.g.
benzene, toluene, xylene, etc.), aiiphatic hydrocarbons (e.g. n-hexane,
heptane, cyclohexane, etc.), amines (e.g. pyridine, N,N-dimethylaniline, etc.),
aprotic polar solvents (e.g. N,N-dimethylformamide, dimethylsulfoxide,
hexamethylphosphoric triamide, etc.), alcohols (e.g. methanol, ethanol,
isopropanol, etc.), or a mixture of these solvents. The reaction is usually
carried out at a temperature of -80 to 1 50C, preferably at a temperature of -80
to about 120C, for 0.5 to about 15 hours. The compound (1 1a) is used at least
in equimolar amount, preferably in an amount of 1 to 10 moles, to 1 mole of the
compound (1s).
Reaction Scheme-9
(l )m ()m
~N XR2 (R5)p ~ NXR2 (R5)p
N CON ~ N CON /~/
(R )r ~ A~R21 (R )r ~ `A~`OH
()n ()n
(1x) / (1xl)
()m / R20X (12)
`X (R5)p~
1w)
WO 95/09159 2 1 ~; 0 3 ~5 PCT/JP94/01559
-216-
[wherein R1, R2, R3a~ R5, m, n, p', A, r and X are the same as defined above,
R20 is a lower alkyl group, a lower alkoxy-substituted lower alkyl group, a lower
alkenyl group, a lower alkoxycarbonyl-substituted lower alkyl group, a hydroxy -substituted lower alkyl group, a carboxy-substituted lower alkyl group, a phenyl -
5 lower alkyl group which may optionally have a substituent selected from alower alkyl group and a lower alkoxy group on the phenyl moiety, a morpholino -
substituted lower alkyl group, or a group of the formula: -A1-CO-NR6R7 (in
which A1, R6 and R7 are the same as defined above), and R21 is a lower alkoxy
group, a lower alkoxy group-substituted lower alkoxy group, or a phenyl-lower
10 alkoxy group which may optionally have a substituent selected from a lower
alkyl group and a lower alkoxy group on the phenyl moiety]
The reaction of converting the compound (1x) into the compound
(1x') is carried out under the same conditions as those of the reaction of
converting the compound (10) into the compound (1m) in the above mentioned
15 Reaction Scheme-6.
The reaction of the compound (1x') and the compound (12) is
carried out under the same conditions as those of the reaction of the compound
(1m) and the compound (10) in the above mentioned Reaction Scheme-6.
WO 95/09159 21 5 0 3 4 5 PCT/JP94/01559
- 21 7 -
Reaction Scheme-10a
(l )m (t )m
(R3)q ~ R (R3)q
(1Y) /' (1z)
(1A)
Reaction Scheme-10b
(1 )m ()m
~ N CON (R )p ~ 5~ ~X ~ R3a (R5)p
~1, A~ R22 ( )r ~1, A~ R23
(1B) ~ (1C)
25()m / ~ R7
~ NXR2 (R5)p
(R )r J~ A~R25
()n
30(1D)
[wherein R1, R3a' R8, m, n, q', R5, p', r and the group of the formula:
WO 95/O91S9 2 1 5 0 3 4 5 PCT/JP94/015S9
- 218 -
are the same as defined above, R22 is a lower alkoxycarbonyl-substituted
lower alkoxy group, R23 is a carboxy-substituted lower alkoxy group, R24 is a
morpholinocarbonyl-lower alkoxy group, R25 is a group of the formula:
-O-A1-CO-NR6R7 (in which A1, R6 and R7 are the same as defined above)]
The reaction of converting the compound (1y) into the compound
(1z) and the reaction of converting the compound (1B) into the compound (1C)
are carried out under the same conditions as those of the hydrolysis of the
compound (11) in the above mentioned Reaction Scheme-5.
The reaction of the compound (1z) and the compound (13) and
10 the reaction of the compound (1C) and the compound (14) are carried out
under the same conditions as those of the reaction of the compound (2) and the
compound (3) in the above mentioned Reaction Scheme-1.
Reaction Scheme-11
()m ()m
l 2 l
~ ~X ~R (Ra)q ~ ~ ~X ~R (Ra)q~
()n COOR12 ()n CH2OH
(1i) (1G)
(l )m (l )m
~ NXR2 (R5)p ~ NX R3a (R5)
(R1)r ~ `A~ (R1)r ~ A~
()n COOR1 ()n CH2OH
(1 H) (11)
[wherein R1, R2, R8, R3a~ m, n, q', R12, R5, p', A, r and the group of the formula:
~ are the same as defined above]
The reaction of converting the compound (1j) into the compound
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 21 9 -
(1 G) and the compound of converting the compound (1 H) into the compound
(11) are carried out under the same conditions as those of the reaction of
converting the compound (1s) into the compound (1r) in the above mentioned
Reaction Scheme-8.
5 Reaction Scheme- 12
(~)m (l)m
ça~ N CON ~' ~ ~X (R5)p
10 (R )r ~ A~o BCOOR12 (R )r ~ A~O--B--CH20H
(lJ) / (1K)
()m
(t)m ~
~N CON ()p (R )r '1, A~(R )p
(~n A~O--B--CH2X ()n O-B-CH2N~ O
(1 M)
(1L)
[wherein R1, R2, R3a~ R5, A, m, n, p', R12, r and X are the same as defined
above and B is a lower alkylene group]
The reaction of converting the compound (1J) into the compound
25 (1 K) is carried out under the same conditions as those of the reaction of
converting the compound (1s) into the compound (1r) in the above mentioned
Reaction Scheme-8.
The reaction of converting the compound (1 K) into the compound
(1 L) is carried out by reacting the compound (1 K) with a halogenating agent in30 a suitable solvent or without a solvent. The halogenating agent includes, forexample, inorganic acids (e.g. hydrochloric acid, hydrobromic acid, etc.), N,N -diethyl-1,2,2-trichlorovinylamide, phosphorus pentachloride, phosphorus
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
.~.
- 220 -
pentabromide, phosphorus oxychloride, thionyl chloride, mesyl chloride, tosyl
chloride, etc., and a basic compound, carbon tetrachloride or carbon
tetrabromide and triphenylphosphine. The basic compound may be the same
as the above mentioned basic compounds for the reaction of the carboxylic
5 halide and the amine compound in Reaction Scheme-1. The solvent includes,
for example, ethers (e.g. dioxane, tetrahydrofuran, etc.), halogenated
hydrocarbons (e.g. chloroform, methylene chloride, carbon tetrachloride, etc.),
aromatic hydrocarbons (e.g. benzene, toluene, xylene, etc.), and the like.
When a phenyl-lower alkyl halide (e.g. tosyl chloride, etc.) and a basic
10 compound are used as a halogenating agent, the halogenating agent is used
at least in equimolar amount, preferably in 1 to 2 moles, to 1 mole of the
compound (1K). When other halogenating agents are used, the halogenating
agent is used at least in equimolar amount, preferably in an excess amount, to
1 mole of the compound (1 K). The reaction is usually carried out at a
15 temperature of room temperature to 1 50C, preferably at a temperature of room
temperature to 80C, for 1 to about 80 hours.
The reaction of the compound (1L) and the compound (13) is
carried out under the same conditions as those of the reaction of the compound
(1a) and the compound (4a) in the above mentioned Reaction Scheme-2.
WO 95/09159 2 1 5 0 3 ~ 5 PCT/JP94/01559
- 221 -
Reaction Scheme-13
()m ()m
t
5~ N~X , R3a ~ NXR2
(R )r ~ ~R26 (R )r ~ ~R27
()n \ ()n
(1N) \ (10)
~)m
\,~ ~ NXR2
(R1) ¦ `R28
1 5 ()n
(1P)
[wherein R1, R2, R3a~ r, m and n are the same as defined above, R26 is a
phenylthio-substituted lower alkyl group which may optionally have a lower
alkoxy substituent on the phenyl moiety, R27 is a phenylsulfinyl-substituted
20 lower alkyl group which may optionally have a lower alkoxy substituent on the
phenyl moiety, and R28 is a phenylsulfonyl-substituted lower alkyl group which
may optionally have a lower alkoxy substituent on the phenyl moiety]
The reaction of converting the compound (1 N) into the compound
(10) and the reaction of converting the compound (10) into the compound (1 P)
25 are carried out under the same conditions as those of the reaction of converting
the compound (1c) into the compound (1d) in the above mentioned Reaction
Scheme-3.
- The reaction of converting the compound (1 N) into the compound
(1 P) is carried out under the same conditions as those of the reaction of
30 converting the compound (1 N) and the compound (10) except that the
oxidizing agent is used at least in an amount of 2 moles, preferably in an
amount of 2 to 4 moles, to 1 mole of the compound (1 N).
2150~5
WO 95/09159 PCI'1JP94101S59
- 222 -
Reaction Scheme-14
(t )m
S ~[CON' ~Y--A~-
(1a) R6bX ~, 5~z
RBC_I_R6d (15b) (R~ CON ~Y--A~--CON
~t)m (1 R)
1 5 ~ NXR ( R5)p
(R1)r ~ A~NHR2g
()n
(lS) \ (~)m
R30X (1 6a)~, N~ R2
R30a_C_R30b (16b) (R ), ~ CON ~(RR52)9p~
()n
\ R30
(1~)
[wherein R1, R2, R3a~ R5, m, n, p', X, A, A1, r and Y are the same as defined
25 above, R6a is hydrogen atom, a lower alkyl group which may optionally have a
hydroxy substituent, a phenyl-lower alkyl group which may optionally have a
lower alkoxy substituent on the phenyl moiety, a furyl-lower alkyl group, or a
lower alkoxy-substituted lower alkyl group, R6b is the same groups for the
above mentioned R6a except hydrogen atom, R6C and R6d are each hydrogen
30 atom or a lower alkyl group, R29 is hydrogen atom or a lower alkyl group, R30 is
a lower alkyl group or a group of the formula: -A1 CONR6R7 (in which A1, R6
WO 95/09159 2 1 S 0 3 9 ~ PCr/JP94/01559
- 223 -
and R7 are the same as defined above), R30a and R30b are each hydrogen
atom or a lower alkyl group, provided that when R30 is a group of the formula:
-A1CoNR6R7, R29 is hydrogen atom]
The reaction of the compound (1Q) and the compound (15a) and
5 the reaction of the compound (1S) and the compound (16a) are each carried
out under the same conditions as those of the reaction of the compound (1 a)
and the compound (4a) in the above mentioned Reaction Scheme-2 The
reaction of the compound (1Q) and the compound (15b) and the reaction of the
compound (1 S) and the compound (1 6b) are each carried out under the same
10 conditions as those of the reaction of the compound (1 a) and the compound
(4b) in the above mentioned Reaction Scheme-2.
The starting compound (2) or (3) may be prepared by the
following processes.
Reaction Scheme-15
R3bCH2N~3 ~ R3bCH2NH2
(17) (3a)
[wherein R3b is the same as defined above, provided that the total number of
carbon atoms of no-cyclic parts of a group of the formula: R3bCH2- in the
compound (3a) is not over 6]
The reaction of converting the compound (17) into the compound
25 (3a) is carried out by reacting the compound (17) with hydrazine in a suitable
solvent or by subjecting the compound (17) to hydrolysis. The solvent used for
the reaction of the compound (17) and hydrazine includes, for example, in
addition to water, the same solvents for the reaction of the compound (2) and
the compound (3) in the above mentioned Reaction Scheme-1. The reaction is
30 usually carried out at a temperature of room temperature to about 1 20C,
preferably at a temperature of 0 to about 1 00C, for 0.5 to about 10 hours.
Hydrazine is used at least in equimolar amount, preferably in an amount of 1 to
WO 95/Ogl59 2 1 5 0 3 4 5 PCT/JP94101559
- 224 -
5 moles, to 1 mole of the compound (17).
The hydrolysis is carried out in the presence of an acid or a basic
compound in a suitable solvent or without a solvent. The solvent includes, for
example, water, lower alcohols (e.g. methanol, ethanol, isopropanol, etc.),
ketones (e.g. acetone, methyl ethyl ketone, etc.), ethers (e.g. dioxane,
tetrahydrofuran, ethylene glycol dimethyl ether, etc.), fatty acids (e.g. aceticacid, formic acid, etc.), or a mixture of these solvents. The acid includes, forexample, inorganic acids (e.g. hydrochloric acid, sulfuric acid, hydrobromic
acid, etc.), organic acids (e.g. formic acid, acetic acid, aromatic sulfonic acid,
etc.), and the like. The basic compound includes, for example, alkali metal
carbonates (e.g. sodium carbonate, potassium carbonate, etc.), alkali metal or
alkaiine earth metal hydroxides (e.g. sodium hydroxide, potassium hydroxide,
calcium hydroxide, etc.), and the like. The reaction is usually carried out at atemperature of room temperature to about 200C, preferably at a temperature
of room temperature to about 1 50C, for 10 minutes to about 25 hours.
Reaction Scheme-16
R3e--C--R31 R32NH4 (19) R3e--C H--R3
O NH2
(18) (3b)
[wherein R31 is hydrogen atom, a lower alkyl group or a lower alkenyl group,
R3e is hydrogen atom, a lower alkyl group, a group of the formula: -(D)r'-R33 (Dis a lower alkylene group, r' is 0 or 1, R33 is a group of the formula:
(R5)p (R5 and p are the same as defined above), a lower alkanoyloxy -
substituted lower alkyl group, a lower alkoxy-lower alkyl group, a lower
alkanoyl-substituted lower alkyl group, a lower alkoxycarbonyloxy-substituted
lower alkyl group, a benzoyl-substituted lower alkyl group having optionally a
halogen substituent on the phenyl ring, a group of the formula: -E-N(R52)(R53)
(in which R52 and R53 are the same as defined above, and E is a lower
alkylene group), a group of the formula:
WO 9S/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 225 -
A Rs4
0~0
O
5 (in which Rs4 is hydrogen atom or a lower alkyl group and A is the same as
defined above), a cycloalkyl group; naphthyl group; a group of the formula:
(R8)q (R8 and q are the same as defined above, a group of the
.~
formula: ~ is a 5- to 14-membered saturated or unsaturated
heteromonocyclic, heterobicyclic or heterotricyclic group having 1 to 4
10 heteroatoms selected from nitrogen atom, oxygen atom and sulfur atom); a
phenyl group which may optionally have a substituent selected from a lower
alkoxy group, a halogen atom, an amino group having optionally a substituent
selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
15 optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula: -o-A4-CO-NR4R41 (A4, R40 and R41 are the same as defined
above), a lower alkenyloxy group, nitro group and a lower alkyl group having
optionally a halogen substituent on the phenyl ring; a phenyl-lower alkenyl
group which may optionally have a substituent selected from a lower alkoxy
20 group, a halogen atom, an amino group having optionally a substituent
selected from a lower alkanoyl group and a phenyl-lower alkenylcarbonyl
group, a lower alkoxy-substituted lower alkoxy group, a tetrazolyl group having
optionally a lower alkyl substituent on the tetrazole ring, hydroxy group, a group
of the formula: -O-A4-CO-NR4R41 (A4, R40 and R41 are the same as defined
25 above), a lower alkenyloxy group, nitro group and a lower alkyl group having
optionally a halogen substituent on the phenyl ring; a phenyltio-substituted
lower alkyl group having optionally a lower alkoxy substituent on the phenyl
ring; a phenylsulfinyl-substituted lower alkyl group having optionally a lower
wo gs/09159 2 1 S 0 3 4 5 PCTIJP94/015S9
- 226 -
alkoxy substituent on the phenyl ring; a phenylsulfonyl-substituted lower alkyl
group having optionally a lower alkoxy substituent on the phenyl ring; a
phenoxy-substituted lower alkyl group, a group of the formula: -A5-CR42R43R44
(A5, R42, R43 and R44 are the same as defined above); a 2,3-dihydro-1 H -
5 indenyl-substituted lower alkyl group which may optionally have a substituent
selected from oxo group, hydroxy group a silyloxy group having a lower alkyl
substituent on the 2,3-dihydro-1 H-indenyl ring; a group of the formula:
(R47) ( ~, R47 and u are the same as defined above), a group of
the formula: (R47)u (R47 and u are the same as defined above, a group
10 oftheformula: ~ isa5-tol4-memberedunsaturated
heteromonocyclic, heterobicyclic or heterotricyclic group having 1 to 4 hetero -atoms selected from nitrogen atom, oxygen atom and sulfur atom), provided
that wherein R3e is a phenyl group which may optionally have a substituent
selected from a lower alkoxy group, a halogen atom, an amino group having
15 optionally a substituent selected from a lower alkanoyl group and a phenyl -
lower alkenylcarbonyl group, a lower alkoxy-substituted lower alkoxy group, a
tetrazolyl group having optionally a lower alkyl substituent on the tetrazole ring,
hydroxy group, a group of the formula:
-o-A4-Co-NR4R41 (A4, R40 and R41 are the same as defined above), a lower
20 alkenyloxy group, nitro group and a lower alkyl group having optionally a
halogen substituent on the phenyl ring, or a group of the formula:
(R47)u~ then R31 is a lower alkenyl group, and that the total number of
wo 9S/O9lS9 2 1 S 0 3 4 5 PCT/JP94/01559
- 227 -
carbon atoms in non-cyclic part of the group of the formula: (R3e)(R31)CH- in
the compound (3b) is not over 6, and R32 is a lower alkanoyloxy group]
The reaction of the compound (18) and the compound (19) is
carried out under the same conditions as those of the reaction of the compound
5 (1a) and the compound (4b) in the above Reaction Scheme-2.
The starting compound (17) is prepared by the following
processes.
Reaction Scheme-17
R3b - CH3
(20)
M N~ (22a)
R3b--CH2X
~ R3~-CH2N~,3
~ (1~
R3b- CH2O H ~
(24) H N~J (22b)
~ ~ O (22a)
R3b cHo R3b-CooR12 R(236)H
(23) (25)
25 [wherein R3b, R12 and X are the same as defined above, M is an alkali metal
such as potassium, sodium, etc., provided that the total number of carbon
atoms of non-cyclic part of the group of the formula: -CH2R3b in the compound
(17) is not over 6]
The reaction of converting the compound (20) into the compound
30 (21) is carried out in the same manner as in the halogenation reaction of thecompound (1p) in the above mentioned Reaction Scheme-7, but it is preferably
carried out by adding to the reaction system a radical initiator such as 2-(4-
WO 95/09159 2 1 S 0 3 4 ~i PCT/JP94101559
- 228 -
biphenylyl)-5-phenyloxazole, azobisisobutyronitrile perbenzoic acid, etc., and asuitable amount of water (e.g. 3 mole %).
The reaction of the compound (21 ) and the compound (22a) is
carried out under the same conditions as thosè of the reaction of the compound
5 (1a) and the compound (4a) in the above Reaction Scheme-2.
The reaction of converting the compound (23) into the compound
(24) is carried out under the same conditions as those of the reaction of
converting the compound (1s) into the compound (1r) in the above Reaction
Scheme-8.
The reaction of the compound (24) and the compound (22b) is
carried out in a suitable solvent in the presence of an azodicarboxylic acid
derivative such as dialkyl azodicarboxylate (e.g. diethyl azodicarboxylate,
dibutyl azodicarboxylate, etc.) and a dialkylazodicarboxyamide (e.g. 1,1' -
azodicarbonyldipiperidine, etc.), and a phosphorus compound such as a
15 trialkylphosphine (e.g. trimethylphosphine, etc.) and a triarylphosphine (e.g.
triphenylphosphine, etc.). The solvent may be the same solvents for the
reaction of the compound (1a) and the compound (4a) in the above Reaction
Scheme-2 except lower alcohols. The azodicarboxylic acid derivative,
phosphorus compound and the compound (22) are each used at least in
20 equimolar amount, preferably in an amount of 1 to 1.5 mole, to 1 mole of the
compound (24). The reaction is usually carried out at a temperature of 0 to
1 00C, preferably at a temperature of 0 to about 70C, for 1 to about 15 hours.The reaction of converting the compound (25) into the compound
(24) is carried out under the same conditions as those of the reaction of
25 converting the compound (1s) into the compound (1 r) in the above Reaction
Scheme-8.
The reaction of the compound (26) and the compound (22a) is
carried out in a suitable solvent in the presence of formaldehyde and an acid.
The solvent includes, for example, halogenated hydrocarbons (e.g.
30 dichloromethane, dichloroethane, chloroform, carbon tetrachloride, etc.), water,
alcohols (e.g. methanol, ethanol, isopropanol, etc.), alkanoic acids (e.g. acetic
acid, propionic acid, etc.), acid anhydrides (e.g. acetic anhydride, etc.), polar
soivents (e.g. acetone, dimethylformamide, etc.), or a mixture of these solvents.
WO 95/09159 21 5 0 3 4 5 - - PCT/JP94/01559
- 229 -
The acid includes, for example, inorganic acids such as hydrogen chloride gas,
hydrochloric acid, hydrobromic acid, etc. Formaldehyde includes, for example,
an aqueous 20 to 40 % by weight formaldehyde solution, trimer of
formaldehyde, polymer of formaldehyde, i.e. para-formaldehyde, and the like.
- 5 The compound (22a) is usually used at least in equimolar amount, preferably in
an amount of 1 to 2 moles, to 1 mole of the compound (26). Formaldehyde is
usually used at least in equimolar amount, preferably in an excess amount, to 1
mole of the compound (26). The reaction is carried out at a temperature of 0 to
about 200C, preferably at a temperature of room temperature to 150C, for 0.5
to about 24 hours.
Reaction Scheme-18
R3b-C N . R3b-CH2NH2
(27) (3c)
15 [wherein R3b is the same as defined above]
The reaction of converting the compound (27) into the compound
(3c) is carried out under the same conditions as those of the reaction of
converting the compound (1s) into the compound (1 r) in the above Reaction
Scheme-8.
20 Reaction Scheme-19
R3sH
R34--A--X ~R34--A- R35 H2N--A- R35
(28) (30) (3d)
25 [wherein R34 is a group of the formula:
--N~
or a group of the formula: -NR36R37 (in which R36 and R37 are each a phenyl -
lower alkyl group), R35 is a phenylthio group having optionally a lower alkoxy
substituent on the phenyl ring, and A is the same as defined above]
WO 95/09159 2 1 ~ 0 3 ~ 5 PCT/JP94/01559
- 230 -
The reaction of the compound (28) and the compound (29) is
carried out under the same conditions as those of the reaction of the compound
(1 a) and the compound (4a) in the above Reaction Scheme-2.
The reaction of converting the compound (30) into the compound
5 (3d) is carried out under the same conditions as those of the reaction of
converting the compound (17) into the compound (3a) in the above Reaction
Scheme-15, when R34 of the compound (30) is a group of the formula:
--N~
o
When R34 of the compound (30) is a group of the formula:
-NR36R37 the reaction of converting the compound (30) into the compound
(3d) is carried out under the same conditions as those of the reduction of the
compound (10) into the compound (1m) in the above Reaction Scheme-6
wherein R14 is a phenyl-lower alkoxy group.
Reaction Scheme-20
o o
~,N~ R2 CH2CO2Rl2 ~NXR2
N (32) N C02R
(R )r ~ (R )r
O / 3O
,' ~
~N C02R12 ~NXco2R12 ~N'xco R12
(R1)r (R1)r (R1)r
(33d) (33c) (33b)
WO 9S/09159 PCT/JP94/01559
-- 2150345
- 231 -
[wherein R1, R2, R12 and r are the same as defined above]
The reaction of the compound (31 ) and the compound (32) is
carried out in the presence of a basic compound. The basic compound
includes the same basic compounds as used for the reaction of the compound
- 5 (1a) and the compound (4a) in the above Reaction Scheme-2. The compound
(32) is used at least in equimolar amount, preferably in an amount of 1 to 5
moles, to 1 mole of the compound (31). The reaction is carried out at a
temperature of 0 to 1 00C, preferably at a temperature of 0 to about 70C, for 1
hour to about 5 day.
The reaction of converting the compound (33a) into the
compound (33b) is carried out in the presence of a halogenated phosphorus
compound such as phosphorus tribromide, phosphorus trichloride, etc. in a
suitable solvent. The solvent includes, for example, ethers (e.g. dioxane,
tetrahydrofuran, etc.), halogenated hydrocarbons (e.g. chloroform, methylene
chloride, carbon tetrachloride, etc.), and the like. The halogenated phosphorus
compound is used in an amount of 1 to 2 moles, to 1 mole of the compound
(33a). The reaction is usually carried out at a temperature of 0 to 1 00C,
preferably at a temperature of 0 to about 70C, for 0.5 to 5 hours.
The reaction of converting the compound (33a) into the
compound (33c) is carried out by reacting the compound (33a) with sodium
hydrosulfate in the presence of an acid in a suitable solvent. The solvent
includes, for example, alcohols (e.g. methanol, ethanol, propanol, butanol, 3 -
methoxy-1-butanol, ethylcellosolve, methylcellosolve, etc.), water, and the like.
The acid includes, for example, mineral acids such as hydrochloric acid, etc.
Sodium hydrosulfate is used at least in equimolar amount, preferably in an
amount of 1 to 5 moles, to 1 mole of the compound (33a). The reaction is
usually carried out at a temperature of 0 to 1 00C, preferably at a temperatureof 0 to abol~t 70C, for 1 to about 10 hours.
The reaction of converting the compound (33c) into the compound
(33d) is carried out under the same conditions as those of the reaction of
converting the compound (1c) into the compound (1d) in the above Reaction
Scheme-3.
WO 9S/09159 2 1 5 0 3 4 5 pCTtJP94/01559
- 232 -
The reaction of converting the compound (33a) into the
compound (33d) is carried out under the same conditions as those of the above
mentioned reaction of converting the compound (33a) into the compound (33d)
except a trialkylphosphite such as trimethylphosphite is used instead of a
5 halogenated phosphorus compound, and the solvent used therein may be an
alcohol such as 1-propanol, etc., and the reaction is carried out at a
temperature of 0 to 1 50C, preferably at a temperature of 0 to 1 00C.
Reaction Scheme-21
()m ()m
t
~N~y R Hydrolysis ~N~, R2
~NlCOOR12 ~ ~N COOH
(R )r ~ Csl~ nfica~l~ tR1)r
()n ()n
(33) (2)
[wherein R1, R2, R12, r, m and n are the same as defined above]
The hydrolysis of the compound (33) is carried out under the
same conditions as those of the hydrolysis of the compound (1j) in the above
Reaction Scheme-5.
The esterification of the compound (2) is carried out by reacting
the starting compound with an alcohol (e.g. methanol, ethanol, isopropanol,
etc.) in the presence of an inorganic acid (e.g. hydrochloric acid, sulfuric acid,
etc.) and a halogenating agent such as thionyl chloride, phosphorus
oxychloride, phosphorus pentachloride, phosphorus trichloride, etc., at a
temperature of 0 to 1 50C, preferably at a temperature of 50 to 1 00C, for 5
minutes to about 10 hours.
The intermediate compound (24a) is prepared by the following
Reaction Scheme-22.
WO 95tO9159 PCT/JP94/01559
-- 21503~5
- 233 -
Reaction Scheme-22
(R8)q~ (R8)q~
- 5 \~CH3 ~CH3
(20a)
(20b)
(R8)q~ (R8)q~
1 0 ~`CH2OCOR12 ~CH2OH
(20c) (24a)
[wherein R8, q' and R12 are the same as defined above]
The reaction of converting the compound (20a) into the
compound (20b) is carried out in the presence of an oxidizing agent in a
suitable solvent. The solvent and the oxidizing agent used therein are the
same solvents and oxidizing agents as used for the reaction of the converting
the compound (1c) into the compound (1d) in the above Reaction Scheme-3,
20 respectively. The oxidizing agent is usually used in an excess amount to the
compound (20a). The reaction is usually carried out at a temperature of room
temperature to 150C, preferably at a temperature of room temperature to
about 120C, for 1 to about 20 hours.
The reaction of converting the compound (20b) into the
25 compound (20c) is carried out by heating the compound (20b) in the presence
of a compound of the formula: R12COOCOR12 (R12 is the same as defined
above) at a temperature of room temperature to about 200C, preferably at a
temperature of room temperature to 150C, for 1 to 10 hours.
The reaction of converting the compound (20c) into the compound
30 (24a) is carried out under the same conditions as those of the hydrolysis of the
compound (1j) in the above Reaction Scheme-5.
The compound (1) wherein R5 or R8 is an amino group is
WO 95/09159 2 ~ 5 3 4 5 PCT/JP94/OlSS9
- 234 -
prepared by reducing the corresponding compound (1) wherein R5 or R8 is
nitro group.
The reduction reaction is carried out, for example, (a) by using a
catalyst in a suitable solvent, or (b) by using ~as a reducing agent a mixture of a
metal or a metal salt with an acid, a mixture of a metal or a metal salt and an
alkali metal hydroxide, an alkali metal sulfite or an alkali metal ammonium saltin an inert solvent.
When the above (a) is employed, the solvent includes, for
example, water, acetic acid, alcohols (e.g. methanol, ethanol, isopropanol,
etc.), hydrocarbons (e.g. hexane, cyclohexane, etc.), ethers (e.g. dioxane,
tetrahydrofuran, diethyl ether, diethylene glycol dimethyl ether, etc.), esters (e.
ethyl acetate, methyl acetate, etc.), aprotic polar solvents (e.g. N,N -
dimethylformamide, etc.), or a mixture of these solvents. The catalyst includes,for example, palladium, palladium-black, palladium-carbon, platinum, platinum
oxide, copper chromite, Raney-nickel, and the like. The catalyst is used in an
amount of 0.02 to 1 mole, to 1 mole of the starting compound. The reaction is
carried out at a temperature of -20 to 1 50C, preferably, at a temperature of 0 to
1 00C, under 1 to 10 atms of hydrogen gas, for 0.5 to 10 hours. An acid (e.g.
hydrochloric acid) may be added to the reaction system.
When the method (b) is employed, there is used as a reducing
agent a mixture of iron, zinc, tin or stannous chloride and a mineral acid (e.g.hydrochloric acid, sulfuric acid, etc.), or a mixture of iron, iron sulfide, zinc or tin
and an alkali metal hydroxide (e.g. sodium hydroxide, etc.), sulfide (e.g.
ammonium sulfide, etc.), aqueous ammonia, ammonium salt (e.g. ammonium
chloride, etc.). The inert solvent includes, for example, water, methanol,
ethanol, dioxane, acetic acid, and the like. The conditions for reduction can beselected according to the kinds of the reducing agent to be used. For example,
when a mixture of stannous chloride and hydrochloric acid is used as a
reducing agent, the reaction is preferably carried out at a temperature from 0Cto about room temperature, for 0.5 to about 10 hours. The reducing agent may
be used at least in equimolar amount, usually in an amount of 1 mole to 5
moles, to 1 mole of the starting compound.
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 235 -
Reaction Scheme-23
()m
S ~N'Xco ~(RB)q
(R )r ~ CHO
(1 U) ~ ()m
(R48)2PCH2(D),,R49 N~ R2
1 0 (34) ~ --l ~ R3a (R8)
()n ~ CH=CH(D)r'R49
(~)m R / (1V)
15~N~XcO ~R (R8)
(R1 ~
()n ~~ CH=CH(D)~'COOH
()m
R50 ,~ t
~Rs~ ,~N CO ~( )q ~Rso
()n CH=CH(D)r CON~
(1
[wherein R1 R2 R3a~ R8, R39, m, n, q', D, r, r' and the group of the formula:
are the same as defined above, R48 is a lower alkoxy group, R49 is a
lower alkoxycarbonyl group, and R50 and R51 are the same or different and
each hydrogen atom or a lower alkyl group]
The reaction of the compound (1 U) and the compound (34) is
30 carried out in the presence of a basic compound in a suitable solvent. The
basic compound includes, for example, inorganic bases (e.g. metal sodium,
WO 95/09159 215 0 3 4 S PCT/JP94/01559
- 236 -
metal potassium, sodium hydride, sodium amide, sodium hydroxide, potassium
hydroxide, sodium carbonate, potassium carbonate, sodium hydrogen
carbonate, etc.), organic bases such as an alkali metal alcoholate (e.g. sodium
methylate, sodium ethylate, potassium t-butoxide, etc.), an alkyl lithium, aryl
lithium or lithium amide (e.g. methyl lithium, n-butyl lithium, phenyl lithium,
lithium diisopropylamide, etc.), pyridine, piperidine, quinoline, triethylamine,N,N-dimethylaniline, and the like. The solvent may be any one which does not
affect the reaction, and includes, for example, ethers (e.g. diethyl ether,
dioxane, tetrahydrofuran, monoglyme, diglyme, etc.), aromatic hydrocarbons
(e.g. benzene, toluene, xylene, etc.), aliphatic hydrocarbons (e.g. n-hexane,
heptane, cyclohexane, etc.), amines (e.g. pyridine, N,N-dimethylaniline, etc.),
aprotic polar solvents (e.g. N,N-dimethylformamide, dimethyl sulfoxide,
hexamethylphosphoric triamide, etc.), alcohols (e.g. methanol, ethanol,
isopropanol, etc.), and the like. The reaction is usually carried out at a
temperature from -80C to 1 50C, preferably at a temperature from -80 to about
120C, for 0.5 to about 15 hours.
The reaction of converting the compound (1V) into the compound
(1 W) can be carried out under the same conditions as those of the hydrolysis ofthe compound (1j) in the above Reaction Scheme-5.
The reaction of the compound (1W) and the compound (35) can
be carried out under the same conditions as those of the reaction of the
compound (2) and the compound (3) in the above Reaction Scheme-1.
Reaction Scheme-24
()m ()m
~ R2
~X R3CoH (38) ~ XN~ 3a
CoNHR3a N CON
(Rl)r ~ (Rl)r ~ ~ R3
()n ()n
(1 a) (1 y)
[wherein R1, R2, m, n, r and R3a are the same as defined above, R3C' is a phenyl -
lower alkoxycarbonyl group, a lower alkanoyl group, a lower alkoxycarbonyl
WO 95/09159 21~ 0 3 4 S PCT/JP94/01559
- 237 -
group, a phenoxycarbonyl group, or a group of the formula: -Co-A-NRs2R53 (in
which A, R52, R53 are the same as defined above)]
The reaction of the compound (1a) and the compound (38) is
carried out under the same conditions as those of the reaction of the compound
5 (2) and the compound (3) in Reaction Scheme-1. In said reaction, when the
compound (38) is used in the form of an acid anhydride, the reaction of the
carboxylic acid anhydride and the amine compound is carried out in the same
manner as in the reaction of the compound (2) and the compound (3) when the
active ester method is employed, i.e. in the presence of a basic compound in a
1 0 solvent at a temperature of 0 to 1 50C, preferably at a temperature of 10 to
100C, for 5 to 30 hours. The carboxylic acid anhydride compound is used at
least in equimolar amount, preferably in an amount of 1 to 2 moles, to 1 mole ofthe compound (1a).
The compound (1 ) wherein R3 is a lower alkanoyl group is
15 prepared by reacting the corresponding compound (1 ) wherein R3 is cyano
group with a compound of the formula: R17MgX (11 ) (wherein R17 and X are
the same as defined above), followed by subjecting the resultant to hydrolysis.
The reaction of the compound (1 ) and the compound (11 ) is carried out under
the same conditions as those of the reaction of the compound (1s) and the
20 compound (11 ) in the above Reaction Scheme-8. The subsequent hydrolysis
is carried out under the same conditions as those of the hydrolysis of the
compound (1j) in the above Reaction Scheme-5. The reaction is preferably
carried out in the presence of an acid.
The compound (1) wherein R5 is a 1,3-dioxolanyl group having
25 optionally a lower alkyl substituent or R8 is a 1,3-dioxolanyl-substituted lower
alkyl group having optionally a lower alkyl substituent is prepared by reacting
the corresponding compound (1 ) wherein R5 is a lower alkanoyl group or R8 is
a lower alkanoyl-substituted lower alkyl group with a compound of the formula:
(R38)v
,L, (36)
HO OH
WO 95/09159 2 1 ~ 0 3 4 5 PCI/JP94/01559
- 238 -
(wherein R33 is a lower alkyl group, v is 0 or an integer of 1 to 3). The reaction
is carried out in the presence of an acid in a suitable solvent. The solvent
includes, for example, in addition to the same solvents for the above mentioned
reaction of the compound (1a) and the compound (4a) in the above mentioned
Reaction Scheme-2, halogenated hydrocarbons (e.g. methylene chloride,
dichloroethane, chloroform, etc.). The acid includes, for example, mineral acids(e.g. hydrochloric acid, sulfuric acid, hydrobromic acid, etc.), organic acids (e.g.
p-toluenesulfonic acid, etc.), and the like. The compound (36) is used at least
in equimolar amount, preferably in an amount of 1 to 10 mole, to 1 mole of the
starting compound. The reaction is usually carried out at a temperature of room
temperature to 1 50C, preferably at a temperature of room temperature to
about 1 00C, for 1 to about 24 hours.
The compound (1 ) wherein R5 is a lower alkanoyl group or R3 is a
lower alkanoyl-substituted lower alkyl group is prepared by hydrolysis of the
corresponding compound (1 ) wherein R5 is a 1 ,3-dioxolanyl group having
optionally a lower alkyl substituent, or R3 is a 1,3-dioxolanyl-substituted lower
alkyl group having optionally a lower alkyl substituent. The hydrolysis is
carried out in the presence of an acid in a suitable solvent. The solvent
includes, for example, water, lower alcohols (e.g. methanol, ethanol,
isopropanol, etc.), ketones (e.g. acetone, methyl ethyl ketone, etc.),
halogenated hydrocarbons (e.g. dichloromethane, dichloroethane, chloroform,
carbon tetrachloride, etc.), or a mixture of these solvents. The acid includes, for
example, inorganic acids (e.g. hydrochloric acid, sulfuric acid, hydrobromic
acid, etc.) and organic acids (e.g. p-toluenesulfonic acid, etc.). The reaction is
carried out at a temperature of 0 to 70C, preferably at a temperature of 0C toroom temperature, for 1 to 10 about hours.
The compound (1 ) wherein R3 is a tetrazolyl group having a
substituent selected from a lower alkyl group and a lower alkoxy-lower alkyl
group is prepared by reacting the corresponding compound (1 ) wherein R3 is a
tetrazolyl group with a compound of the formula: R39X (37) (wherein R39 is a
lower alkyl group or a lower alkoxy-lower alkyl group, and X is the same as
defined above) in the same manner as in the reaction of the compound (10)
WO 95/09159 21 5 0 3 4 5 PCT/JP94/01559
- 239 -
with the compound (4a) in the above mentioned Reaction Scheme-2.
The compound (1 ) wherein R8 is unsubstituted tetrazolyl group is
prepared by subjecting the compound (1) wherein R8 is a tetrazolyl group
having a lower alkoxy-lower alkyl substituent, to hydrolysis in the same manner
as in the hydrolysis of the compound (10) wherein R14 is a lower alkoxy -
substituted lower alkoxy group in the above mentioned Reaction Scheme-6.
The compound (1) wherein R2 is a morpholino-substituted lower
alkyl group or an imidazolyl-substituted lower alkyl group is prepared by
reacting the corresponding compound (1 ) wherein R2 is a lower alkyl group
having a halogen substituent with morpholine or imidazole in the same manner
as in the reaction of the compound (1a) and the compound (4a) in the above
mentioned Reaction Scheme-2.
The compound (1) wherein R2 is a halogen-substituted methyl
group is prepared by reacting the corresponding compound (1) wherein R2 is
methyl group in the same manner as in the reaction of converting the
compound (20) into the compound (21) in the above mentioned Reaction
Scheme-17. Further, the compound (1) wherein R2 is a halogen-substituted
methyl group (in which halogen atom is fluorine atom ) is also prepared by
reacting the corresponding compound (1) wherein R2 is a halogen-substituted
methyl group (in which halogen atom is other than fluorine atom) with silver
fluoride.
Among the desired compounds (1 ) of the present invention, the
compounds having a basic group can easily be converted into acid addition
salts thereof by treating them with a pharmaceutically acceptable acid. The
acid includes, for example, inorganic acids (e.g. hydrochloric acid, sulfuric acid,
phosphoric acid, hydrobromic acid, etc.), and organic acids (e.g. oxalic acid,
acetic acid, succinic acid, malonic acid, methanesulfonic acid, maleic acid,
fumaric acid, malic acid, tartaric acid, citric acid, benzoic acid, etc.). These salts
show as well excellent antidiabetic activity as the free compounds (1).
Besides, among the desired compounds (1 ) of the present
invention, the compounds having an acidic group can easily be converted into
WO 95tO9159 2 l S O 3 4 ~ PCT/JP94/01559
- 240 -
salts by treating them with a pharmaceutically acceptable basic compound.
The basic compound includes, for example, sodium hydroxide, potassium
hydroxide, calcium hydroxide, sodium carbonate, potassium hydrogen
carbonate, and the like.
The desired compound of each process can easily be isolated
and purified by conventional isolation methods. The isolation methods are, for
example, extraction with solvent, dilution method, recrystallization method,
column chromatography, preparative thin layer chromatography, and the like.
In addition, the compounds (1 ) of the present invention include
stereoisomers and optical isomers, and these isomers are also useful as
antidiabetic agents.
The desired compounds (1 ) of the present invention and salts
thereof are useful as antidiabetic agent and are used in the form of a
conventional pharmaceutical preparation. The preparation is prepared by
using conventional diluents or carriers such as fillers, thickening agents,
binders, wetting agent, disintegrators, surfactants, lubricants, and the like. The
pharmaceutical preparations can be selected from various forms in accordance
with the desired utilities, and the representative forms are tablets, pills,
powders, solutions, suspensions, emulsions, granules, capsules,
suppositories, injections (solutions, suspensions, etc.), and the like. In order to
form in tablets, there are used carriers such as vehicles (e.g. Iactose, white
sugar, sodium chloride, glucose, urea, starch, calcium carbonate, kaolin,
crystalline cellulose, silicic acid, etc.), binders (e.g. water, ethanol, propanol,
simple syrup, glucose solution, starch solution, gelatin solution, carboxymethylcellulose, shellac, methyl cellulose, potassium phosphate, polyvinyl -
pyrrolidone, etc.), disintegrators (e.g. dry starch, sodium alginate, agar powder,
laminaran powder, sodium hydrogen carbonate, calcium carbonate,
polyoxyethylene sorbitan fatty acid esters, sodium laurylsulfate, stearic
monoglyceride, starches, lactose, etc.), disintegration inhibitors (e.g. white
sugar, stearin, cacao butter, hydrogenated oils, etc.), absorption promoters (e.g.
quaternary ammonium base, sodium laurylsulfate, etc.), wetting agents (e.g.
glycerin, starches, etc.), adsorbents (e.g. starches, lactose, kaolin, bentonite,
colloidal silicates, etc.), lubricants (e.g. purified talc, stearates, boric acid
WO95/09159 21 $ o 3~ S PCT/JP94/01559
- 241 -
powder, polyethylene glycol, etc.), and the like. Moreover, the tablets may alsobe in the form of a conventional coated tablet, such as sugar-coated tablets,
gelatin-coated tablets, enteric coated tablets, film coating tablets, or double or
multiple layer tablets. In the preparation of pills, the carriers may be
conventional ones, and include, for example, vehicles (e.g. glucose, lactose,
starches, cacao butter, hydrogenated vegetable oils, kaolin, talc, etc.), binders
(e.g. gum arabic powder, tragacanth powder, gelatin, ethanol, etc.),
disintegrators (e.g. Iaminaran, agar, etc.), and the like. In the preparation ofsuppositories, the carriers may be conventional ones, and include, for
example, polyethylene glycol, cacao butter, higher alcohols, higher alcohol
esters, gelatin, semi-synthetic glycerides, and the like. Capsules can be
prepared by charging a mixture of the compound of the present invention and
the above carriers into hard gelatin capsules or soft capsules in usual manner.
In the preparation of injections, the solutions, emulsions and suspensions are
sterilized and are preferably made isotonic with the blood. In the preparation of
these solutions, emulsions and suspensions, there are used conventional
diluents, such as water, ethyl alcohol, macrogol, propylene glycol, ethoxylated
isostearyl alcohol, polyoxylated isostearyl alcohol, polyoxyethylene sorbitan
fatty acid esters, and the like. In this case, the pharmaceutical preparations
may also be incorporated with sodium chloride, glucose, or glycerin in an
amount sufficient to make them isotonic, and may also be incorporated with
conventional solubilizers, buffers, anesthetizing agents. Besides, the
pharmaceutical preparations may optionally be incorporated with coloring
agent, preservatives, perfumes, flavors, sweetening agents, and other
medicaments, if required.
The amount of the desired compound of the present invention to
be incorporated into the pharmaceutical preparation is not specified but may be
selected from a broad range, but usually, it is preferably in the range of 1 to 70
% by weight.
The pharmaceutical preparation of the present invention
containing as an active ingredient the compounds (1) of the present invention
or a salt thereof may be administered in any method, and suitable method for
administration may be determined in accordance with various forms of
WO 95109159 PCT/JP94101559
2~s0~4~
- 242 -
preparations, ages, sexes and other conditions of the patients, the degree of
severity of diseases, and the like. For example, tablets, pills, solutions,
suspensions, emulsions, granules and capsules are administered orally. The
injections are intravenously administered à~one or together with a conventional
5 auxiliary liquid (e.g. glucose, amino acid solutions), and further are optionally
administered alone in intramuscular, intracutaneous, subcutaneous, or
intraperitoneal route, if required. Suppositories are administered in intrarectal
route.
The dosage of the pharmaceutical preparation of the present
10 invention may be selected in accordance with the usage, ages, sexes and
other conditions of the patients, the degree of severity of the diseases, and the
like, but it is usually in the range of about 0.2 to 200 mg of the active compound
of the present invention per 1 kg of body weight of the patient per day.
wo gS/oglS9 2 1 5 0 ~ 4 S ~Cr/JP94101S59
- 243 -
Best Mode for CarryinQ Out the Invention
Examples
The present invention is illustrated in more detail by the following
Preparations of antidiabetic agent, Reference Examples of processes for
5 preparing the starting compounds to be used for preparing the desired
compounds of the present invention, and Examples of processes for preparing
the desired compounds, and Experiments of the activities of the desired
compounds of the present invention.
Preparation 1
Tablets are prepared from the following components.
Components Amount
2-(2-Benzofuranylmethylaminocarbonyl)-
3-methylquinoxalin-4-oxide 5 mg
Starch 132 mg
Magnesium stearate 18 mg
Lactose 45 mg
Totally 200 mg
In the conventional manner, these are obtained tablets each
containing the above components.
Preparation 2
Film coated tablets are prepared from the following components.
Components Amount
2-[3-(4-Methoxyphenyl)propylaminocarbonyl]-
3-methylquinoxalin-4-oxide 150 mg
Avicel (trade name of microcrystalline cellulose
manufactured by Asahi Chemical Industry, Co.,
Ltd. Japan) 40 9
Corn starch 30 9
Magnesium stearate 2 9
Hydroxypropyl methylcellulose 10 g
Polyethylene glycol-6000 3 9
Castor oil 40 9
Methanol 40 9
The active compound of the present invention, Avicel, corn starch
35 and magnesium stearate are mixed and kneaded and the mixture is tabletted
W095/09159 2 lS 03 45 pCI/JP94/01559
- 244 -
using a conventional pounder (R 10 mm) for sugar coating. The tablets thus
obtained are coated with a film coating agent consisting of hydroxypropyl
methylcellulose, polyethylene glycol-6000, castor oil and methanol to give film
coated tablets.
Reference Example 1
A solution of 2-ethoxycarbonyl-3-methylbenzofuran (5.0 9) in
anhydrous diethyl ether (10 ml) is added dropwise to a suspension of lithium
aluminum hydride (0.93 9) in diethyl ether (30 ml) under ice-cooling, and the
mixture is stirred at room temperature for 30 minutes. The mixture is cooled,
and decomposed with saturated aqueous sodium sulfate solution. The mixture
is filtered through celite, and dried over anhydrous sodium sulfate, and the
residue is evaporated to remove the solvent to give 2-hydroxymethyl-3 -
methylbenzofuran (3.7 9) as white powder.
1 H-NMR (CDCI3) ~ ppm: 1.82 (1 H, t, J=6 Hz), 2.27 (3H, s), 4.76 (2H, d,
J=6 Hz), 7.20-7.55 (4H, m)
Reference Example 2
In tetrahydrofuran (40 ml) are suspended 2-hydroxymethyl-3 -
methylbenzofuran (3.7 9), triphenylphosphine (6.6 9) and phthalimide (3.7 9),
and thereto is added dropwise a solution of diethyl azodicarboxylate (4.4 9) in
tetrahydrofuran (10 ml) under ice-cooling. The mixture is stirred at room
temperature overnight, and evaporated to remove the solvent, and the resulting
residue is purified by silica gel column chromatography (solvent; methylene
chloride), and crystallized from diethyl ether. The crystals ~re collected by
filtration, and dried to give 3-methyl-2-phthalimidomethylbenzofuran (4.6 9) as
white powder.
1H-NMR (CDCI3) ~ ppm: 2.40 (3H, s), 4.97 (2H, s), 7.10-7.30 (2H, m),
7.40 (1 H, d, J=7 Hz), 7.48 (1 H, d, J=7 Hz), 7.65-7.75 (2H, m), 7.80-7.90 (2H, m)
Reference Example 3
To methanol (80 ml) is added 3-methyl-2-phthalimidomethyl -
benzofuran (4.6 9), and thereto is added hydrazine hydrate (1.2 9), and the
mixture is refluxed for three hours. The mixture is evaporated to remove the
solvent, and to the residue is added diluted aqueous sodium hydroxide
solution, and then the mixture is extracted with chloroform. The chloroform
WO 95/09159 2 1 5 0 3 ~ 5 pcrt~4/0l559
- 245 -
layer is washed with saturated brine solution, and dried over anhydrous
sodium sulfate. The residue is evaporated to remove the solvent to give 2 -
aminomethyl-3-methylbenzofuran (3.0 9) as colorless transparent liquid.
1H-NMR (CDCI3) ~ ppm: 1.55 (2H, brs), 2.21 (3H, s), 3.93 (2H, s), 7.15 -
7.30 (2H, m), 7.35-7.50 (2H, m)
Reference Example 4
To carbon tetrachloride (50 ml) are added 2-methylbenzo -
thiophene (4.0 9), N-bromosuccinimide (4.8 9) and azobisisobutyronitrile (0.3
g), and the mixture is refluxed for five hours. After cooling, the insoluble
1 0 materials are removed by filtration, and the filtrate is concentrated to give 2 -
bromomethylbenzothiophene (6.7 g) as brown powder.
1H-NMR (CDCI3) ~ ppm: 4.79 (2H, s), 7.30-7.45 (2H, m), 7.36 (1 H, s),
7.70-7.85 (2H, m)
Reference Example 5
1 5 In dimethylformamide (40 ml) is dissolved 2-bromomethyl -
benzothiophene (6.7 9), and thereto is added potassium phthalimide (5.0 9),
and the mixture is stirred at 60C for two hours. The mixture is evaporated to
remove the dimethylformamide, and the residue is extracted with chloroform.
The extract is washed with water and saturated brine solution, dried over
anhydrous sodium sulfate, and evaporated to remove the solvent. To the
residue is added diethyl ether, and the precipitated crystals are collected by
filtration, and dried to give 2-phthalimidomethylbenzothiophene (4.5 9) as pale
brown powder.
1H-NMR (CDCI3) ~ ppm: 5.10 (2H, s), 7.20-7.40 (3H, m), 7.70-7.80 (4H,
m), 7.80-7.95 (2H, m)
Reference Example 6
3-Formylbenzofuran (4.3 9) is dissolved in methanol (50 ml), and
thereto is added gradually sodium borohydride (1.1 g) under ice-cooling. The
mixture is stirred at the same temperature for one hour, and evaporated to
remove the methanol. The residue is extracted with chloroform, and the extract
is washed with water, and dried over anhydrous sodium sulfate. The residue is
evaporated to remove the solvent to give 3-hydroxymethylbenzofuran (4.1 9) as
WO 95/09159 2 1 5 0 3 4 5 PCI`/JP94/01559
- 246 -
pale yellow liquid.
1H-NMR (CDCI3) ~ ppm: 1.62 (1H, t, J=5 Hz), 4.85 (2H, d, J=5 Hz), 7.20 -
7.45 (2H, m), 7.53 (1 H, d, J=8 Hz), 7.62 (1 H, s), 7.68 (1 H, d, J=8 Hz)
Reference Example 7 ~
A solution of ethyl 2-benzofuranacrylate (3.46 g) in dry toluene is
cooled to -50C, and thereto is added dropwise alminum diisobutyl hydride (1 M
toluene solution, 37 ml). The mixture is stirred at -20C for one hour, and
thereto is added methanol (30 ml), and the mixture is stirred at room
temperature overnight. The precipitates are removed by filtration, and the
filtrate is concentrated. The residue is dissolved in ethyl acetate, and filtered
through Florisil (an activated magnesium silicate), and concentrated to give 2 -(3-hydroxy-1-propenyl)benzofuran (2.6 9) as colorless liquid.
1H-NMR (CDCI3) ~ ppm: 4.39 (2H, brs), 6.60 (3H, m), 7.17-7.32 (2H, m),
7.43 (1 H, d, J=7 Hz), 7.52 (1 H, d, J=7 Hz)
Reference Example 8
Into a mixture of benzothiophene (13.4 9) and 37 % aqueous
formaldehyde solution (15 ml) is blown hydrogen chloride gas for about 20 to
30 minutes under ice-cooling. The mixture is stirred at room temperature for
two hours. The reaction mixture is poured into ice-water, and extracted with
diethyl ether. The extract is washed with saturated sodium hydrogen carbonate
solution, and dried over anhydrous sodium sulfate. The resultant is evaporated
to remove the solvent. and the resulting residue and potassium phthalimide
(18.5 9) are dissolved in diemthylformamide (100 ml). The mixture is heated at
60C for 1.5 hour, and after cooling, the mixture is poured into ice-water. The
precipitated crystals are collected by filtration, washed with diisopropyl ether to
give 3-phthalimidomethylbenzothiophene (13.4 9) as pale brown crystals.
1H-NMR (CDCI3) ~ ppm: 5.08 (2H, s), 7.3-7.5 (2H, m), 7.61 (1 H, s), 7.6 -
7.9(5H,m),8.15(1H,d,J=8Hz)
Reference Example 9
2-Acetylbenzofuran (3.2 9) is dissolved in methanol (60 ml), and
thereto are added ammonium acetate (15 9) and sodium cyanoborohydride
(1.26 9). The mixture is stirred at room temperature overnight, and thereto is
WO95/09159 21 5 03 ~ S ` ~ PCT/JP94/01559
- 247 -
added diluted aqueous hydrochloric acid solution to make the solution acidic.
The mixture is washed with ethyl acetate, and the aqueous layer is basified
with aqueous sodium hydroxide solution, and extracted with chloroform. The
chloroform layer is washed with water, dried over anhydrous sodium sulfate,
5 and evaporated to remove chloroform to give 2-(1-aminoethyl)benzofuran (1.8
g) as colorless liquid.
1 H-NMR (CDCI3) ~ ppm: 1.52 (3H, d, J=6 Hz),1.83 (2H, brs), 4.20 (1 H, q,
J=6 Hz), 6.50 (1H, s), 7.15-7.30 (2H, m), 7.43 (1H, d, J=8 Hz), 7.51 (1H, d, J=8Hz)
Reference Example 10
5-Ethyl-2-methylpyridine (25 9) is dissolved in acetic acid (200
ml), and thereto is added 30 % aqueous hydrogen peroxide solution (25 ml),
and the mixture is heated at 100C. Four hours later, to the mixture is added 30% aqueous hydrogen peroxide solution (25 m), and the mixture is heated with
1 5 stirring at the same temperature for 14 hours. After cooling, the mixture is
concentrated several times with adding water thereto. The final residue is
neutralized with saturated aqueous potassium carbonate solution, and
extracted with chloroform. The extract is dried over anhydrous potassium
carbonate, and evaporated to remove the solvent. The resulting residue is
dissolved in acetic anhydride (200 ml), and the mixture is heated at 120C for 4hours. The mixture is evaporated to remove the solvent, and thereto is added
saturated aqueous sodium hydrogen carbonate solution, and extracted with
ethyl acetate. The extract is dried over anhydrous sodium sulfate, and
evaporated to remove the solvent. The residue is dissolved in methanol (200
ml), and thereto is added potassium carbonate (57 g), and the mixture is stirredat room temperature for 12 hours. The mixture is evaporated to remove the
solvent, and thereto is added water. The mixture is extracted with chloroform,
- and dried over anhydrous potassium carbonate. The resultant is evaporated to
remove the solvent, and the residue is purified by silica gel column
chromatography (solvent; ethyl acetate: n-hexane=1: 4) to give 5-ethyl-2 -
hydromethylpyridine (20.4 9) as pale brown liquid.
1H-NMR (CDCI3) ~ ppm: 1.26 (3H, t, J=8 Hz), 2.66 (2H, q, J=8 Hz), 3.67
(1H, br), 4.73 (2H, s), 7.18 (1H, d, J=8 Hz), 7.52 (1H, d, J=8 Hz), 8.41 (1H, s)
WO 9S/09159 PCI/JP94/01559
~ 2150~45
- 248 -
Reference Example 11
4-(2-Methyl-1,3-dioxolan-2-yl)benzonitrile (1.9 9) is dissolved in
diethyl ether (20 ml), and thereto is added lithium aluminum hydride (400 mg)
at 0C. The mixture is reacted at room ternperature for 14 hours, and thereto
5 are added water (1 ml) and 8M aqueous sodium hydroxide solution (3 ml). To
the mixture is added magnesium sulfate, and the insoluble materials are
removed by filtration. The filtrate is concentrated to give [4-(2-methyl-1,3 -
dioxolan-2-yl)benzyl]amine (2.1 9) as colorless oil.
1H-NMR (CDCI3) ~ ppm: 7.45 (2H, d, J=8 Hz), 7.29 (2H, d, J=8 Hz), 4.04
(2H, m),3.87 (2H, s), 3.77 (2H, m),1.77 (2H, br),1.65 (3H, s)
Reference Example 12
N-Bromoethylphthalimide (13 9) and p-methoxythiophenol (7.9 9)
are dissolved in dimethylformamide (70 ml), and thereto is added potassium
carbonate (10 9). The mixture is stirred at 70C overnight, and evaporated to
15 remove the solvent. The residue is extracted with diethyl ether, washed with
water, and dried over anhydrous sodium sulfate. The residue is evaporated to
remove diethyl ether, and crystallized from n-hexane. The crystals are
collected by filtration, and dried to give N-[2-(4-methoxyphenylthio)ethyl] -
phthalimide (13.9 9) as white powder.
1H-NMR (CDCI3) â ppm: 3.14 (2H, t, J=7 Hz), 3.75 (3H, s), 3.89 (2H, t,
J=7 Hz), 6.80 (2H, d, J=8 Hz), 7.42 (2H, d, J=8 Hz), 7.65-7.75 (2H, m), 7.75-7.90
(2H, m)
Reference Example 13
N-(2-Chloroethyl)dibenzylamine hydrochloride (25 9) and
phenol (8.0 9) are dissolved in dimethylformamide (100 ml), and thereto is
added potassium carbonate (30 9). The mixture is stirred at 70C for 6 hours,
and evaporated to remove dimethylformamide. The residue is extracted with
diethyl ether, and the extract is washed with water, and dried over anhydrous
sodium sulfate. The residue is evaporated to remove diethyl ether to give N-(2 -phenoxyethyl)dibenzylamine (25.5 9) as colorless oil.
1H-NMR (CDCI3) ~ ppm: 2.90 (2H, t, J=6 Hz), 3.72 (4H, s), 4.03 (2H, t,
J=6 Hz), 6.80 (2H, d, J=8 Hz), 6.85-7.00 (1H, m), 7.15-7.45 (12H, m)
WO 95/O91S9 2 1 5 0 3 ~ 5 PCI~/JP94/015S9
- 249 -
Reference Example 14
N-(2-Phenoxyethyl)dibenzylamine (25.5 9) is dissolved in ethanol
(500 ml), and thereto is added 10 % palladium-carbon (3.0 g). The mixture is
subjected to hydrogenation at 50C under 1 atm of hydrogen gas. The catalyst
5 is removed by filtration, and the filtrate is evaporated to remove ethanol to give
2-phenoxyethylamine (11.0 g) as colorless oil.
1H-NMR (CDCI3) ~ ppm: 1.47 (2H, brs), 3.08 (2H, t, J=5 Hz), 4.01 (2H, t,
J=5 Hz), 6.85-7.00 (3H, m), 7.25-7.35 (2H, m)
Reference Example 15
To triethylamine (1.5 liter) are added benzofuroxane (216 g) and
ethyl acetoacetate (207 g), and the mixture is stirred at room temperature for
four days. The precipitated crystals are collected by filtration, washed with
water, dried, and crystallized from ethyl acetate to give 2-ethoxycarbonyl-3 -
methylquinoxaline-1,4-dioxide (115 g) as pale yellow needles.
M.p. 137-138C
Reference Example 16
2-Ethoxycarbonyl-3-methylquinoxaline-1,4-dioxide (77 g) is
dissolved in ethanol (3.5 liters) and conc. hydrochloric acid (200 ml), and to the
mixture is added dropwise aqueous solution of sodium hydrosulfite (200 g) in
20 water (1 liter) with stirring at room temperature. The mixture is stirred at the
same temperature for four hours, and the reaction mixture is neutralized with
sodium hydrogen carbonate, and evaporated to remove ethanol. To the
residue is added water, and the precipitated crystals are collected by filtration,
washed with water, dried, and recrystallized from n-pentane to give 2-ethoxy -
25 carbonyl-3-methylquinoxaline (67 9) as colorless needles.
M.p. 74-75C
Reference Example 17
2-Ethoxycarbonyl-3-methylquinoxaline (64 g) is dissolved in
methylene chloride (700 ml), and thereto is added gradually m-chloro -
30 perbenzoic acid (70 g) under ice-cooling. The mixture is stirred at room
temperature overnight, and the reaction solution is washed successively with
diluted aqueous sodium thiosulfate solution, saturated sodium hydrogen
carbonate solution, and water, and dried over anhydrous sodium sulfate. The
WO 95/09159 2 1 5 0 3 ~ 5 PCTIJP94/01559
- 250 -
resultant is evaporated to remove the solvent, and to the residue is added n -
hexane. The precipitated crystals are collected by filtration, dried, and
recrystallized from n-hexane/diethyl ether to give 2-ethoxycarbonyl-3 -
methylquinoxalin-4-oxide (45 9) as colorless needles.
M.p. 91 -93C
Reference Example 18
2-Ethoxycarbonyl-3-methylquinoxaline-1,4-dioxide (105 9) is
dissolved in chloroform (500 ml), and thereto is added dropwise gradually
phosphorus tribromide (44 ml) under ice-cooling. The mixture is stirred at room
temperature for one hour, and evaporated to remove the solvent. The residue
is poured into ice-water, and neutralized with potassium carbonate. The
mixture is extracted with chloroform, washed with water, and dried over
anhydrous sodium sulfate. The resultant is evaporated to remove the solvent,
and the residue is purified by silica gel column chromatography (solvent; n -
hexane: ethyl acetate = 2: 1), and recrystallized from ethyl acetate/n-hexane togive 2-ethoxycarbonyl-3-methylquinoxalin-1-oxide (35 9) as colorless prisms.
M.p. 85-87C
Reference Example 19
2-Ethoxycarbonyl-3-methylquinoxalin-4-oxide (4.0 9) is
suspended in methanol (80 ml) and 5N aqueous sodium hydroxide solution
(10 ml), and the mixture is stirred at room temperature for three hours. The
mixture is evaporated to remove the solvent, and the residue is dissolved in
water. The aqueous layer is washed with ethyl acetate, and acidified with
hydrochloric acid. The precipitated crystals are collected by filtration, washedwith water, and dried to give 2-carboxy-3-methylquinoxaline-4-oxide (3.2 9) as
white powder.
M.p. 143-145C (decomposed)
Reference Example 20
2-Carboxyquinoxaline (2.0 9) is dissolved in methanol (20 ml),
and thereto is added dropwise thionyl chloride (1.3 ml) under ice-cooling. The
mixture is refluxed for 15 minutes, and evaporated to remove the solvent. The
residue is extracted with chloroform, and the extract is washed with saturated
aqueous sodium hydrogen carbonate solution, and dried over anhydrous
WO 95/09159 2 1 5 0 3 4 5 PCT/JP94/01559
- 251 -
sodium sulfate. The residue is evaporated to remove the solvent, and the
resulting 2-methoxycarbonylquinoxaline is dissolved in methylene chloride (40
ml). To the mixture is added m-chloroperbenzoic acid (2.9 9) with stirring at
room temperature, and the mixture is stirred at room temperature overnight.
5 The reaction solution is washed with diluted aqueous sodium thiosulfate
solution and saturated sodium hydrogen carbonate solution, and dried over
anhydrous sodium sulfate. The resultant is evaporated to remove the solvent,
and to the residue is added n-hexane. The precipitated crystals are collected
by filtration, and dried to give 2-methoxycarbonylquinoxalin-4-oxide (2.0 9) as
10 yellow powder.
M.p. 154-1 55C
Reference Example 21
2-Ethoxycarbonyl-3-methylquinoxaline (2.2. 9) is dissolved in
carbon tetrachloride (40 ml), and thereto are added N-bromosuccinimide (2.7
9) and perbenzoic acid (0.2 9), and the mixture is refluxed for 8 hours. The
mixture is evaporated to remove the solvent, and thereto is added water. The
mixture is extracted with dichloromethane, and the extract is dried over
anhydrous sodium sulfate, and evaporated. The residue is dissolved in
isopropanol (50 ml), and thereto is added imidazole (2.8 9). The mixture is
20 refluxed for 10 hours, and evaporated to remove the solvent. To the residue is
added water, and the mixture is extracted with chloroform, and dried over
anhydrous sodium sulfate. The resultant is evaporated to remove the solvent,
and the resulting residue is purified by silica gel column chromatography
(solvent; dichloromethane: methanol = 16: 1) to give 2-ethoxycarbonyl-3-(1 -
25 imidazolyl)methylquinoxaline (1.33 9) as yellow powder.
1H-NMR (CDCI3) â ppm: 1.47 (3H, t, J=7 Hz), 4.55 (2H, q, J=7 Hz), 5.82
(2H, s), 7.04 (2H, d, J=5 Hz), 7.70 (1 H, s), 7.8-8.0 (2H, m), 8.0-8.1 (1 H, m), 8.2
8.3 (1 H, m)
By using the suitable starting compounds, there are obtained the
30 following compounds as listed in Table 1 in the same manner as in Reference
Examples 3, 9, 11 and 14.
WO 95/09159 PCT/JP94/01559
2150~S
- 252 -
Table 1
M.p. ~;rystalline
No.Structure C Forrr~ ~ 1H-NMR (CDCI3) ~ ppm:
H3C Colorless 1.55 (2H, brs), 2.21 (3H, s),
H2N`~)~ oil 3.93 (2H, s), 7.15-7.30 (2H, m),
O 7.35-7.50 (2H, m)
2 Cl Yellow 1.31 (2H, brs), 4.14 (2H, s),
H2N~J~ powder 7.07 (1H, s), 7.2-7.3 (1H, m),
7.6-7.7 (2H, m)
1.61 (2H, brs), 4.15 (2H, s),
3 ~ 65 Yellow 7.14 (1 H, s), 7.25-7.40 (2H, m),
H2NJ~s~ powder 7.72 (1H, d, J=8 Hz), 7.82 (1H,
d, J=8 Hz)
1.2/ (2H, brs), 4.01 (2H, s),
4 o~, Pale yelow 7.20-7.40 (2H, m), 7.50 (1 H, d,
H2NJ~J oil J=8 Hz), 7.55 (1H, s), 7.60
(1H, d, J=8 Hz)
1.56 (2H, brs), 3.9/ (2H, s),
5~,~CI Pale yellow 6.48 (1 H, s), 7.20 (1 H, d, J=8
H2NJ~o~ oil Hz) 7.34 (1H, d, J=8 Hz), 7.48
1.54 (2H, brs), 3.95 (2H, s),
6 O Pale yellow 6.74 (1 H, d, J=2 Hz), 7.25 (1 H,
H2N~ oil d, J=8 Hz), 7.49 (1H, d, J=8
Hz), 7.54 (1H, s), 7.63 (1 H, d,
J=2 Hz)
1.58 (2H, brs), 4.04 (2H, s),
7 NO2 Yellow 6.69 (1 H, s), 7.50 (1 H, d, J=9
H2N~J~ powder Hz) 8.21 (1H, d, J=9 Hz), 8.46
8 ~ Pale yellow 1.61 (2H, brs), 4.01 (2H, s),
H2NJ~J oil 6.58 (1H, s), 7.13 (1H, m), 7.25
Cl (1H, d, J=8 Hz), 7.42 (1H, d,
J=8 Hz)
WO 95/O91S9 2 1 5 0 3 4 ~ PCT/JP94/01559
- 253 -
1.5~ (~H, ~rs), ~ H, s)~
9 _ ~,OCH3 Pale yellow 3.94 (2H, s), 6.46 (1 H, s),
H2N~ 3~ oil 6.84 (1H, d, J=8 Hz), 6.98
(1 H, s), 7.31 (1 H, d, J=8 Hz)
Yellow 1.49 (2H, brs), 3.75 (3H, s),
J~ powder 4.03 (2H, s), 6.38 (1 H, s),
H2N N 7.04-7.24 (2H, m), 7.30 (1 H,
CH3 d, J=8 Hz), 7.58 (1 H, d, J=8
Hz)
1.58 (2H, brs), 4.06 (2H, s),
11 ~ Yellow 6.32 (1 H, s), 7.03-7.20 (2H,
H2NJ~N~J powder m), 7.34 (2H, d, J=8 Hz),H 7.54 (2H, d, J=8 Hz), 8.50
(1H, brs)
12 OCH3 Pale yellow 1.54 (2H, brs), 3.9 1(3H, s),
H2N~J~ powder 3.94 (2H, s), 6.46 (1H, s),
Br 7.01 (1H, s), 7.63 (1H, s)
1.51 (2H, brs), 3.56 (2H,
13~NH2 Yellow brs), 3.91 (2H, s), 6.36 (1H,
H2NJ~o~J powder s), 6.62 (1H, d, J=8 Hz),
6.79(1H,s),7.22(1H,d,
J=8 Hz)
14 ~ Pale yellow 1.57 (2H, brs), 3.98 (2H, s),
H2NJ~oJ~ oil 4.01 (3H, s), 6.52 (1H, s),
OCH3 6.74-6.83 (1 H, m), 7.08-7.18
(2H, m)
15CN Pale yellow 4.02 (2H, s), 6.61 (1H, s),
H2NJ~ powder 7.52 (2H, brs), 7.86 (1H, brs)
3.94 (3H, s), 4.00 (2H, s),
16CO2CH3 Yellow 6.60 (1H, s), 7.45 (1H, d,
H2N~ powder Hz) 8 26 (r1H s)
WO 95/091592 1 5 0 3 4 ~ pCT/JP94/01559
- 254 -
H, ~rs), ~-Y;~ ~H, S),
17 ,CH3 Pale 3.93 (2H, s), 6.42 (1H, s),
~N~CH3 yel!ow oil 6.80 (1 H, d, J=8 Hz), 6.85
H2N O ~ (1 H, s), 7.34 (1 H, d, J=8 Hz)
. 1.42 (3H, t, J=/ Hz), 1.53
18 ' Pale (2H, brs), 4.00 (2H, s), 4.40
~CO2C2Hs yellow oil (2H, q, J=7 Hz), 6.60 (1 H,
H2N~o~ s), 7.47 (1 H, d, J=8 Hz),
8.00(1H,d,J=8Hz),8.26
(1H, s)
1.5/ (2H, brs), 3.9/ (2H, s),
19 Pale 7.15-7.30 (2H, m), 7.44 (1H,
H2N J~ yellow oil d, J=7 Hz), 7.52 (1 H, d, J=7
1.52 (2H, brs), 2.18 (2H, s),
H3C Pale 3.05 (3H, s), 3.89 (2H, s),
H ~)~:l yellow oil 5.20 (2H, s), 6.95 (1 H, d,
2N O OCH2OCH3 J=8 Hz), 7.14 (1H, s), 7.32
(1H, d, J=8 Hz)
1.51 (2H, brs), 2.34 (2H, s),
21 OCH2OCH3 Pale 3.52 (3H, s), 3.89 (2H, s),
H3C ,~ yellow oil 5.28 (2H, s), 6.81 (1H, d,
H2NJ~o~J J=8 Hz), 7.05-7.16 (2H, m)
0.92 (3H, t, J=/ Hz), 1.08 -
22 H3C Colorless 1.18 (2H, m), 1.40-2.05 (9H,
H2N`~ oil m), 2.45-2.62 (1 H, m), 2.62 -
2.90 (2H, m), 3.80-3.95 (2H,
m)
1.92 (2H, m), 2.82 (2H, t, J=
23 ~ Pale Hz), 2.84 (2H, t, J=7 Hz),
H2N J~O~ yellowoil 6.41 (1H, s), 7.13-7.25 (2H,
m), 7.35-7.50 (2H, m)
24 Pale 1.83 (2H, brs), 3.56 (3H, s),
H2N~_J~ yellow oil 3.40 (2H, s), 5.37 (2H, s),
OCH2OCH3 6.55 (1H, s), 7.04 (1H, d,
J=8 Hz), 7.05-7.21 (2H, m)
2 1 5 0 3 4 S pCTJJP94101559
WO 95/09159
._ ~
- 255 -
H3C White 1.51 (2H, brs), 2.17 (3H, s),
~q powder 3.89 (2H, s), 5.10 (2H, s),
H2N~o~OCH2~ 6.94 (1H, d, J=8 Hz), 7.02
(1 H, s), 7.27-7.50 (6H, m)
1.26 (3H, t, J_/ Hz). 2.92
Pale (2H, q, J=7 Hz), 3.95 (3H,
26 CH3CH2~,CO2CH3 yellow s), 4.24 (2H, s), 7.41 (1H, d,
H2N~'`o oil J=9 Hz), 8.00 (1H, d, J=9
Hz), 8.25 (1 H, s)
27 S Yellow 1.89 (2H, brs), 4.13 (2H, s),
H2N~)~ dl 7 2-7.5 (3H, m), 7.7-7.9 (2H,
Pale 1.97 (2H, brs), 4.05 (2H, s),
H~ yellow 7.2-7.6 (9H, m)
1.59 (2H, brs), 4.10 (2H, s),
Brown 7.54 (1 H, dd, J=8 Hz, 8 Hz),
~ oil 7.69 (1 H, dd, J=8 Hz, 8 Hz),
H2N~ 7.80 (1H, d, J=8 Hz), 8.0-8.2
(2H, m), 8.89 (2H, s)
2.2/ (3H, s), 3.9/ (3H, s),
30 H3C CO2CH3 Pale 4.52 (2H, d, J=6 Hz), 7.42
~ yellow (1 H, d, J=8 Hz), 7.98 (1 H, d,
H2N~O~ oil J=8 Hz~, 8.20 (1 H, s),
1.52 (3H, d, J=6 Hz), 1.83
31 Color - (2H, brs), 4.20 (1 H, q, J=6
~q less oil Hz), 6.50 (lH, s), 7.15-7.30
H2N~o ~ (2H, m~, 7.43 (1 H, d, J=8CH3 Hz), 7.51 (1H, d, J=8 Hz)
2.20 (3H, brs), 4.00 (2H, s),
Pale 5.08 (1H, s), 5.34 (1H, brs),
H2N ~CCH--3CH2 oil 7 56 (lH, s)
WO 95/091S9 2 1 5 0 3 4 5 pCT/JP94/01559
- 256 -
Wttlte l.W (~H, ~rS), ;~.Y~ H, S),
33~CO2CH3 powder 4.17 (2H, s), 7.21 (1H, s),
H2N~J~S~ 7.84 (1H, d, J=8 Hz), 7.94
(1 H, d, J=8 Hz), 8.40 (1 H, s)
1.60 (2H, brs), 2.18 (3H, s),
34H3C~OCH2OCH3 Pale 3.51 (3H, s), 3.91 (2H, s),
H2N~o~W yellow 5.20 (2H, s), 6.95 (1H, d,
oil J=8 Hz), 7.10 (1H, s), 7.29
(1H, d, J=8 Hz)
35CH3 Yellow 1.14 (3H, s),1.44(3H, s), 2.8 -
H3C~ oil 3.1 (2H, m),4.2-4.3 (1H, m),
H2N ~o ~ 6.7-7.2 (4H, m)
1.35 (2H, brs), 2.8-3.1 (3H,
36~, Yellow m), 3.2-3.4 (1 H, m),4.7-4.9
H2N~J~o~ oil (1H, m), 6.7-6.9 (2H, m), 7.0 -
7.2 (2H, m)
1.5/ (3H, d, J=8 Hz). 1.~9
37R Pale (2H, brs), 2.07 (3H, s), 3.97
OCCH3 yellow (2H, s), 5.97 (1 H, q, J=8
~CH3 oil Hz), 6.52 (1 H, s), 7.25 (3H,
H2N~o~ d, J=8 Hz), 7.40 (1H, d, J=8
Hz), 7.52 (1 H, s)
1.45 (3H, t, J=/ Hz), 1.74
38~ Pale (2H, s), 4.05 (2H, s), 4.46
H2N~o~fJ brown (2H, q, J=7 Hz), 6.58 (1H,
CO2C2Hs oil s), 7.20- 7.35 (1 H, m), 7.70
(1 H, d, J=8 Hz), 7.89 (1 H, d,
J=8 Hz)
39~ Pale 1.60 (2H, brs), 4.03 (2H, s),
H2NJ~oJ~J brown 6.61 (1 H, s), 7.20-7.35 (1 H,
CF3 oil m), 7.48 (1 H, d, J=8 Hz),
7.69 (1 H, d, J=8 Hz)
40CF3 Pale 1.56 (2H, brs), 4.01 (2H, s),
H2N~J~ brown 6.60 (1H, s), 7.50 (2H, s),
oil 7.81 (1H, s)
wo gS/oglS9 2 1 5 0 3 ~ 5
- 257 -
~.14 (;~H, S), ;~.4;~ 11, S),
41 H3C ,~, Pale 6.39 (1H, brs), 6.59 (1H, s),
H2N~J;~o~J yellow 7.15-7.25 (2H, m), 7.40-7.50
oil (2H, m)
2.04 (3H, s), 3.54 (2H, d,
42~ Pale J=7 Hz), 6.44 (1H, t, J=7
H2N ~O~ yellow Hz), 6.60 (1 H, s), 7.1 5-7.30
CH oil (2H, m), 7.46 (1H, d, J=73 Hz), 7.51 (1 H, d, J=7 Hz)
43N-N Pale 3.53 and 3.60 (3H, s), 4.02
- ~N,' yellow and4.04 (2H, s), 5.89 and
H2NJ~o~ CH20CH3 oil 5.72 (2H, s), 6.62and 6.66
(1H,s),7.54and7.62(1H,d,
J=9 Hz), 8.00 and 8.02 (1 H,
(1- or 2-rrlethoxymethyl group ) d, J=9 Hz), 8.37 (1 H, s)
44~ Pale 3.54 (2H, d, J=5 Hz), 6.43 -
H2N~o~ yellow 6.63 (3H, m), 7.13-7.30 (2H,
oil m), 7.40-7.55 (2H, m)
1.39 (2H, brs), 1.67-1.80
45OCH3 Pale (2H, m), 2.60 (2H, t, J=6 Hz),
H2N~ yellow 2.72 (2H, t, J=6 Hz), 3.79
oil (3H, s), 6.82 (2H, d, J=8
Hz), 7.09 (2H, d, J=8 Hz)
1.28 (2H, brs), 1.15-1.30
46/CH3 Pale (2H, m), 2.56 (2H, t, J=7 Hz),
~ N\ yellow 2.72 (2H, t, J=7 Hz), 2.91
H2N ~,!J CH3 oil (6H, s), 6.69 (2H, d, J=8
Hz), 7.07 (2H, d, J=8 Hz)
- 1.2/ (2H, brs), 1.61 (3H, s),
47 Pale 1.63 (3H, s), 1.71 (3H, s),
- H2N~ ~CH3 brown 1.95-2.15 (4H, m), 3.27 (2H,
CH3 CH3 oil d, J=7 Hz), 5.05-5.15 (1H,
m), 5.20-5.31 (1 H, m)
WO 95/O91S9 PCT/JP94101559
21503~5
- 258 -
48 CH3 N Brown 2.50 (3H, s), 3.90 (2H, s),
H2N~S>~ ` ' 7 3-7.5 (3H, m), 7.8-8.0 (2H,
~ 1.26 (2H, br), 3.49 (2H, d,
OCH Yellow J=6 Hz), 3.88 (3H, s), 3.90
1~ oil (3H, s), 6.2-6.3 (1 H, m), 6.42
H2N~ `OCH3 (1H, d, J=16 Hz), 6.82 (1H,
d, J=8 Hz), 6.9-7.0 (2H, m)
Yellow 1.30 (2H, br), 3.53(2H, d,
~ oil J=6 Hz), 6.2-6.4 (1 H, m),
H2N~ 6.90 (1H, d, J=16 Hz), 7.1 -
Cl 7.3 (2H, m), 7.3-7.5 (2H, m)
51 Pale 1.53 (2H, brs), 4.03 (2H, s),
1~1 brown 7.35-7.50 (3H, m), 7.70-7.92
H2N~ powder (4H, m)
1.18 (2H, brs), 4.31 (2H, s),
52 Brown 7.30-7.50 (2H, m), 7.88 (1 H,
H2N~J`~I oil d, J =7 Hz), 7.97 (1H, d, J=7
1.30 (2H, brs), 3.45 (2H, d,
53 Yellow J=6 Hz), 3.81 (3H, s), 6.0 -
OCH3 oil 6.2 (1 H, m), 6.48 (1 H, d,
H2N~J J=16 Hz), 6.82 (2H, d, J=8
Hz), 7.20 (2H, d, J=8 Hz)
1.25 (2H, br), 3.48 (2H, d,
54 Yellow J=6 Hz), 3.86 (3H, s), 6.3 -
~ oil 6.5 (1 H, m), 6.7-6.9 (3H, m),
H2N ~ 7.20 (1 H, m), 7.42 (l H, d,
OCH3 J=8 Hz)
Brown 1.36 (2H, brs), 3.54 (2H, d,
~jl oil J=6 Hz), 6.2-6.4 (lH, m),
H2N~J 6.98 (1H, d, J=16 Hz), 7.37
NO2 (1H, m), 7.5-7.7 (2H, m), 7.90
(1H, d, J=8 Hz)
WO 95/O91S9 2 1 5 0 ~ 4 S PCr/JP94/0l55
- 259 -
56 White 2.37 (3H, s), 3.74 (2H, s),
CH3~_o ~=\ powder 7.4-7.5 (3H, m), 7.95-8.05
H2N ~N>~ (2H, m)
1.23 (2H, t, J=8 Hz), 1.49
57,~CH2CH3 Yelllow oil (2H, brs), 2.63 (2H, q, J=8
H2N~ Hz), 3.82 (2H, s), 7.16 (2H,
d, J=8 Hz), 7.22 (2H, d, J=8
Hz)
1.39 (2H, brs), 3.40 (2H, d,
58 ~ Pale yellow J=6 Hz), 5.85-6.00 (1 H, m),
H2N ~1~ oil 6.32 (1 H, dd, J=16 Hz, 10
Hz),6.52(1H,d,J=16Hz),
6.78 (1H, dd, J=16 Hz, 10
Hz). 7.15-7.42 (5H, m)
1.24 (3H, t, J=8 Hz), 1.91
59 ,~ C2H5 Brown oil (2H, brs), 2.64 (2H, q, J=8
H2NJ~ ~ Hz), 3.94 (2H, s), 7.19 (1H,
N d, J=8 Hz), 7.48 (1 H, d, J=8
Hz), 8.40 (1 H, s)
/.24 (2H, d, J=8 Hz), /.20
CH3 Colorless oil (2H, d, J=8 Hz), 3.83 (2H,
~CH3 bp.80C/ s), 2.90 (1H, sep, J=7 Hz),
H2N ~ 0.3 mmHg 1.40 (2H, br), 1.25 (6H, d,
J=7 Hz)
61CH3 N White 2.22 (3H,s), 3.92 (2H, s), 7.4 -
H2N ~0>~ powder 7 5 (3H, m), 8.00-8.05 (2H,
/.45 (2H, d, J=8 Hz), /.29
62 ~ Colorless oil (2H, d, J=8 Hz), 4.04 (2H,
m), 3.87 (2H, s), 3.77 (2H,
CH3 m), 1.77 (2H, br), 1.65 (3H,
H2N ~ S)
WO 95/09159 2 15 0 3 ~ 5 PCT/JP94/01559
- 260 -
1.~() (;~H, t, J=~ HZ), ~
63 CH3 o Pale (3H,s),2.65(2H,q,J=8
~ \ ~ C2H5 yellow Hz),2.82(2H, brs), 3.58
H2N~ N powder (2H,s),7.33(2H, d, J=8
Hz),8.06(2H, d, J=8 Hz)
1.62(2H, brs), 1.l-1.9(2H,
64 ~ r_~ Yellow m), 2.3-2.5(6H,m),2.63(2H,
H2N'~ N~_~O oil t, J=8 Hz),3.72(4H, t, J=5
Hz),3.83(2H,s),7.15(2H,
d, J=8 Hz),7.23(2H, d, J=8
Hz)
/.29(2H,d,J=8 Hz),/.16
CH3 Color - (2H,d,J=8 Hz),6.48(1H, d,
~CH3 less oil J=16 Hz),6.28(1H, dt, J=16
H2N ~ Hz,6 Hz),3.47(2H, d, J=6
Hz),2.88(1H, sep, J=7 Hz),
1.72(2H, br), 1.23(6H, d,
J=7 Hz)
/.15(2H,d,J=8 Hz),/.11
66CH3 Color - (2H,d,J=8 Hz),2.88(1H,
~CH3 less oil sep, J=7 Hz),2.74(2H, t,
H2N ~ J=7 Hz),2.63(2H, t, J=7
Hz),1.77(2H,qui,J=7 Hz),
1.24(6H, d, J=7 Hz),1.55
(2H, br)
2.//(2H,t,J=6 Hz),3.12
67 OCH3 Pale (2H,t,J=6 Hz),3.78(3H,s),
HN~ yellow 3.95(2H,s),6.63(1H, brs),
oil 6.69(1H, brd, J=8Hz),6.92
(1H, d, J=8 Hz)
/.20(4H,s),6.41(1H, brs),
68 CH3 Color - 3.39(2H,s),2.90(1H,sep,
CH ~ lessoil J=7 Hz),2.09(2H, br), 1.91
H2N~ CH3 (3H, brs), 1.25(6H,d,J=7
1.38(2H, brs), 2.80-2.95
69~,OCH3 Color - (4H,m),3.80(3H,s),6.84
H2N S~ less oil (2H, d, J=8 Hz),7.38(2H,d,
WO 95/09159 2 1 S 0 3~4 5 PCTIJP94/OlSS9
- 261 -
H, ~rs), Z.~ U
,~, Color - (2H, m), 2.90-3.05 (2H, m),
H2N S~ less oil 7.10-7.40 (5H, m)
1.4~ (2H, brs), 3.08 (2H, t,
71 ~ Color - J=5 Hz), 4.01 (2H, t, J=
H2N o,l~ lessoil 5Hz), 6.85-7.00 (3H, m), 7.25 -
7.35 (2H, m)
72 S~ Pale 7.9-8.0 (2H, m), 7.4-7.5 (3H,
H2N-CH2~N yellow m), 7.08 (1 H, s), 4.05 (2H, s)
73 CH3 S Pale 7.85-7.95 (2H, m), 7.4-7.5
~ ~>~ yellow (3H, m), 3.88 (ZH, s), 2.45
H2N-CH2 N oil (3H, s)
/.5-/.6 (1H, m), /.4-/.5 (1H,
74 Yellow m), 7.2-7.3 (2H, m), 6.69 (1H,l~q oil s), 5.gO (1 H, s), 5.23 (1 H, s),
H2N ~f o ~ 2.98 (2H, t, J-6.0 Hz), 2.62
CH2 (2H, t, J=6.0 Hz)
N--N Pale 7.58 (5H, brs), 4.17 (2H, s~
H2NCH2 ~N ' yellow
76 CH Pale 3.88 (2H, s), 2.59 (3H, s~,
~S yellow 2.53 (3H, s)
H2N-CH2
77 ,N Pale 4.16 (2H, s), 4.23 (3H, s)
N~ N yellow
H2N-CH2 , oil
CH3
WO 95/O91S9 21 S ~ 3 ~ 5 PCT/JP94/015S9
- 262 -
~ H,~r),~.U4(~H,
78 ~ Pale s), 3.55(2H, d, J=7 Hz),
yellow 6.51(1H, t, J=7 Hz),
o C,=CH-CH2NH2 oil 6.62(1H, d, J=3 Hz),
F CH3 6.90-7.15(2H,m),7.40-
7.60(2H,m)
1.41(2H, br), 2.04(3H,
79 White s), 3.56(2H, d, J=7 Hz),
solid 6.46(1H, t, J=7 Hz),
S ~ 6.64(1H,s),7.30(1H,
=cHcH2NH2 d, J=3 Hz),7.46(1H, d,
CH3 J=9 Hz),7.85(1H, d,
J=2 Hz),7.86(1H, dd,
J=9 Hz,3 Hz),8.13
(1H, d, J=2 Hz)
1.30(3H, t, J=/Hz),
C2H5 Pale 1.56(2H, br), 2.02(3H,
O N~ yellow s), 2.64(2H, t, J=7 Hz),
ll oil 2.96(2H, t, J=7Hz),
~`o ~ C,=CHCH2NH2 3.54(2H, d, J=7 Hz),
CH3 4.04(2H,q,J=7 Hz),
6.41(1H, t, J=7Hz),
6.56(1H,s),7.12(1H,
s),7.21(1H,s)
2.13(3H,s),3.41(2H,
81 Yellow d, J=7 Hz),6.05(1H, dt,
powder J=15 Hz,7 Hz),6.57
~`O C,=CH-CH-CHCH2NH2 (1H, dd, J=15 Hz,12
CH3 Hz),6.65(1H,s),6.97
(1H, d, J=12 Hz),7.15-
7.42(2H,m),7.43(1H,
d, J=8 Hz),7.51(1H, d,
J=8 Hz)
2.05(3H,s),2.52(3H,
82 CH S CH3 Pale s), 2.69(3H,s),3.56
yellow (2H, d, J=7 Hz),6.46
N~ powder (1H, t, J=7 Hz),6.62
O ~ C=cHcH2NH2 (1H,s),7.45(1H, d, J=8
CH3 Hz),7.51(1H,d,J=8
Hz),7.73(lH,s)
WO 95/09159 2 1 5 0 3 4 S PCTIJP94/01559
- 263 -
83 N~ Yellow 2.07 (3H, s), 3.58(2H,
N ~ /N powder d, J=7 Hz), 4.20 (3H, s),
/ ~ ~ 6.52 (1 H, t, J=7 Hz),
CH3 ~O C=cHcH2NH2 6.68 (1H, s), 7.59 (2H,
CH s), 7.89 (1 H, s)
2.11 (3H, s), 3.~/ (2H,
84 Pale d, J=7 Hz), 6.47 (1 H, t,
yellow J=7 Hz), 7.10 (1H, s),
powder 7.43-7.62 (3H, m), 7.69
(1 H d, J=8 Hz), 7.93
O/c=cHcH2NH2 (1H d, J=8 Hz), 8.11
CH3 (1H, d, J=8 Hz)
Yellow 1.78 (2H, brs), 2.23 (3H,
CH3
oil s), 2.52 (3H, s), 2.70
s~NI CH3 (3H, s), 3.96 (2H, s), 7.4 -
~( 7.5 (2H, m), 7.70 (1 H, s)
CH3 ~o CH2NH2
1.53 (2H, brs), 2.3/ (3H,
86 CH3 Yellow s), 2.43 (3H, s), 3.48
)~N powder (3H, s), 3.97 (2H, s),
CH3--N _~ 6.52 (1H, s), 7.4-7.6
(2H, m), 7.72 (1 H, s)
CH3 ~O CH2NH2
87 CH3 Yellow 1.56 (2H, brs), 2.47 (3H,
kN oil s), 2.51 (3H, s), 3.98
~ (2H, s), 6.54 (1H, s), 7.4 -
CH3 ~O CH2NH2 7 6 (2H, m), 7.77 (1 H
1.56 (2H, brs), 2.54 (3H,
88 CH3 Color - s), 2.71 (3H, s), 4.17
)~ less oil (2H, s), 7.33 (1 H, s),
S~,S~ CH2NH2 7.61 (1 H, d, J=8 Hz),
CH3 ~ ~ 7.90 (1 H, d, J=8 Hz),
S 8.01 (1 H, s)
WO 95/O91S9 5 03 45 PCT/JP94/01559
- 264 -
89 OC2H5 White 1.46 (3H, t, J=7 Hz), 1.57
~N powder (2H, brs), 3.98 (2H, s), 4.57
(2H, q, J=7 Hz), 6.57 (1 H, d,
J=6 Hz), 6.59 (1 H, s), 7.49
O CH2NH2 (1H, d, J=9 Hz), 8.38 (1H, d,
J=9 Hz), 8.48 (1 H, d, J=6
Hz), 8.61 (1H, s)
~N Brown 1.62 (2H, brs), 4.03 (2H, s),
~CH H oil 6.75 (1 H, dd, J=7 Hz, 7 Hz),
N 2N 2 7.15(1H,dd,J=7Hz,8Hz),
7.48 (1 H, s), 7.54 (1 H, d, J=8
Hz), 8.07 (1 H, d, J=7 Hz)
91 CH3 Pale 1.29 (2H, brs), 2.06 (3H, s),
yellow 2.50 (3H, s), 3.50 (2H, d, J=7
N~c:cH_cH2NH2 powder Hz), 6.05 (1H, t, J=7 Hz), 7.4 -
CH3 7.5 (3H, m), 7.9-8.1 ( 2H, m) -
92 CH3 Color - 1.2-1.5 (2H, br), 2.27 (3H, s),
~\N~ less oil 3.71 (3H, s), 3.88 (2H, s), 7.3 -
H2NCH2 N--\~Y 7.7 (5H, m)
CH3
93 CH3 ,CH3 Pale 1.55 (2H, brs), 2.26 (3H, s),
~N ~ yellow 3.56 (3H, s), 3.78 (2H, s), 7.3 -
H2NCH2--~N~<~ oil 7.6 (5H, m)
1.25 (2H, brs), 2.01 (3H, s),
94 F~ Pale 3.54 (2H, d, J=7 Hz), 6.43
L 11 ~J~ yellow (1 H, t, J=7 Hz), 6.55 (1 H, s),
o Ç=CHCH2NH2 powder 6.9-7.0 (1H, m), 7.15 (1H, d,
CH3 J=9 Hz), 7.2-7.4 (1H, m)
wo 9S/O9lS9 215 0 3 ~ 5 PCT/JP94101SS9
- 265 -
CH3 Paie 1.65 (2H, brs), 2.48 (3H, s),
J~N yellow 2.51 (3H, s), 4.03 (2H, s), 7.4 -
~ oil 7.6 (3H, m), 7.87 (1 H, s)
CH3 ~ ~CH2NH2
b~`oli
Pale DMSO-d6:
C=O yellow 1.96 (3H, s), 2.04 (3H, s),
N H powder 3.36 (2H, d, J=7 Hz), 6.31
(1H, t, 7 Hz), 6.82 (1 H, s),
7.31 (1H, dd, J=2 Hz, 9 Hz),
--o C, =CHCH2NH2 7.42 (1 H, d, 9 Hz), 7.90 (1 H,
CH3 d, J=2 Hz), 9.91 (1H, s)
97 F~_ Yellow 1.31 (2H, brs), 2.02 (3H, s),
powder 3.55 (2H, d, J=7 Hz), 6.51
-- o C, =CHCH2NH2 (1 H, t, J=7 Hz), 6.58 (1 H, d,
F CH3 J=3 Hz), 6.77 (1 H, dd, J=10
Hz, 10 Hz), 6.97 (1H, d, J=8
Hz)
Using suitable starting compounds, there are obtained the
compounds as listed in Table 2 in the same manner as in Reference Examples
15-21 .
WO 9S/09159 PCTIJP94/01559
215034S
- 266 -
Table 2
m.p. Crystalline
No.Structure C Form 1H-NMR (CDCI3) ~ pm:
N CF3 Pale 1.48 (3H, t, J=7 Hz), 4.58
[~ ~X yellow (2H, q, J=7 Hz), 7.95-8.05
N CO2C2Hs powder (2H, m), 8.20-8.32 (2H, m)
2 ~ 64- Pale 1.18 (3H, t, J=7 Hz), 4.34
N J~ 65 yellow (2H, q, J=7 Hz), 7.45-7.55
`~ powder (3H, m), 7.70-7.80(2H, m),
~N~CO2c2Hs 7.80-7.90 (2H, m), 8.15-8.25
(2H, m)
3 o 154- Yellow 4.12 (3H, s), 7.80-7.95 (2H,
N 155 powder m), 8.34 (1H, d, J=8 Hz), 8.61
~NlCO2CH3 (1 H, d, J=8 Hz), 9.04 (1 H, s)
4 O Pale 1.48 (3H, t, J=7 Hz), 1.52
N J~ yellow (6H, d, J=8 Hz), 3.6-3.8 (1H,
CH3 powder m), 4.54 (2H, q, J=7 Hz), 7.7 -
~N CO2C2Hs 7.9 (2H, m), 8.0-8.2 (1 H, m),
8.5-8.6 (1 H, m)
o Yellow 1.38 (3H, t, J=7 Hz), 3.27
powder (2H, q, J=7 Hz), 4.09 (3H, s),
~,N~C2Hs 7.7-7.9 (2H, m), 8.1-8.3 (1H,
~NJ`CO2CH3 m), 8.5-8.7 (1H, m)
6 CH3 Yellow 1.42 (3H, d, J=7 Hz), 1.49
~~N~J`cH powder (3H, t, J=7 Hz), 3.6-3.8 (1 H,
-l m), 4.56 (2H, q, J=7 Hz), 7.7 -
N CO2C2Hs 7.9 (2H, m), 8.0-8.2 (2H, m)
wo 95/og1S9 2 15 0 3 ~ 5 PCT/JP94/01559
- 267 -
1.4~ H, t, J=/ HZ), ;~.~U
7 ~ N~C2Hs Pale brown (2H, q, J=7 Hz), 4.09 (3H, s),
'l powder 7.7-7.9 (2H, m), 8.09 (1 H, dd,
2CH3 J=2Hz,8Hz),8.18(1H,dd,
J=2 Hz, 8Hz)
L~MS~-d6:
8 O ,~ Pale yellow 7.45-7.60(5H, m), 7.85-8.05
N~J~J powder (2H, m), 8.21 (1H, d, J=8 Hz),
~NlCO2H 8.48 (1H, d, J=8 Hz)
9 O 106 - Pale yellow 1.03 (3H, t, J=7 Hz), 4.18
108 powder (2H, q, J=7 Hz), 7.45-7.60
~,N~ (5H, m), 7.75-7.95 (2H, m),
~NJ~CO2C2Hs 8 25 (1 H, d, J-8 Hz), 8.64
O Yellow 1.49 (3H, t, J=7 Hz), 2.78
N CH3 flakes (3H, s), 4.56 (2H, q, J=7 Hz),
~ `~' (diisopropyl 7.73 (1 H, dd, J=2 Hz, 9 Hz),
CI~N~CO2C2Hs ether) 8.18 (1H, d, J=2 Hz), 8.53
(1 H, d, J=9 Hz)
11 o Yellow oil 1.49 (3H, t, J=7 Hz), 3.0-3.2
~ ~ (4H, m), 3.8-4.0 (4H, m),4.56
N J (2H, q, J=7 Hz), 4.56 (2H, s),
7.8-7.9 (2H, m), 8.0-8.1 (1H,
~N CO2C2Hs m), 8.2-8.3 (1 H, m)
12 ~N Yellow 1.47 (3H, t, J=7 Hz), 4.55
N~ powder (2H, d, J=7 Hz), 5.82 (2H, s),
N J 7.04 (2H, d, J=5 Hz), 7.70
"~ (1H,s),7.8-8.0(2H,m),8.0-
~N'~CO2C2Hs 8.1 (1H, m), 8.2-8.3 (1H, m)
WO 95/09159 21 S Q 3 4 5 PCT/JP94/01559
- 268 -
;~ ~;olorless
13 O '` 91-93 needles 1.49 (3H, t, J=7 Hz), 2.81
CH (n-hexane/ (3H, s), 4.56 (2H, q, J=7 Hz),
-X diethyl 7.75-7.85 (2H, m), 8.15-8.25
~N CO2C2Hs ether) (1 H, m), 8.55-8.65 (1 H, m)
1.50 (3H, t, J=/ Hz), 2.96
14 ~N CH3 74-75 Colorless (3H, s), 4.57 (2H, q, J=7 Hz),
1 1~ needles 7.70-7.90 (2H, m), 8.05 (1H,
--N~CO2C2Hs (n-pentane) d, J=8 Hz), 8.19 (1H, d, J=8
Hz)
O 137 - Pale yellow 1.48 (3H, t, J=7 Hz), 2.60
138 needles (3H, s), 4.59 (2H, q, J=7 Hz),
~N~,CH3 (ethyl 7.30-7.46 (2H, m), 8.53-8.67
~N~CO2C2Hs acetate) (2H, m)
o
16 O 143 - White 2.87 (3H, s), 7.75-7.90 (2H,
145 powder m), 8.15-8.25 (1H, m), 8.54 -
~NXCH3 (dec) 8.65 (1H, m)
Colorless 1.50 (3H, t, J-/ Hz), 2.68
17 N CH3 85-87 prisms (3H, s), 4.58 (2H, q, J=7 Hz),
'X (n-hexane/ 7.6S-7.90 (2H, m), 8.04 (1 H,
--N~ CO2C2Hs ethyl d, J=8 Hz), 8.53 (1H, d, J=8
O acetate) Hz)
18 /~ Yellow 3.0-3.2 (4H, m), 3.9-4.0 (4H,
~,N~CH2-N~JO powder m), 4.56 (2H, s), 7.8-7.9 (2H,
~NlCOOH m), 8.0-8.1 (1H, m), 8.2-8.3
(1 H, m)
21503~5
WO 95/09159 PCTIJP94/01559
- 269 -
19 O Yellow 1.48 (3H, t, J=7 Hz), 2.56
N CH3 powder (3H, s), 4.00 (3H, s), 4.59
~ `~' (2H, q, J=7 Hz), 7.46 (1 H,
CH3O~N~`CO2C2Hs dd, J=3 Hz, 10 Hz), 7.86
- O (1H, d, J=3 Hz), 8.51 (1 H, d,
J=10 Hz)
O Yellow 1.49 (3H, t, J=7 Hz), 2.78
N CH3 powder (3H, s), 3.96 (3H, s), 4.56
~ `~' (2H, q, J=7 Hz), 7.3-7.5 (2H,
CH3O~N~`CO2C2Hs m), 8.48 (1 H, d, J=9 Hz)
21 O Brown 1.26 (6H, t, J=7 Hz), 1.47
N CH3 powder (3H, t, J=7 Hz), 2.52 (3H, s),
~ `~' 3.52(4H,q,J=7Hz),4.57
(C2Hs)2N~N~CO2C2Hs (2H, q, J=7 Hz), 7.27 (1H,
O dd, J=3 Hz, 10 Hz), 7.42
(1H,d,J=3Hz),8.39(1H,d,
J=10 Hz)
22 o Brown 1.25 (6H, t, J=7 Hz), 1.47
N CH3 powder (3H, t, J=7 Hz), 2.73 (3H, s),
~ `~ 3.50 (4H, q, J=7 Hz), 4.53
(C2H5)2N~N~CO2C2Hs (2H, q, J=7 Hz), 7.08 (1 H, d,
J=3 Hz), 7.29 (1 H, dd, J=3
Hz,10Hz),8.38(1H,d,
J=10 Hz)
23 O Brown 1.0-1.4 (6H, br),1.48 (3H, t,
- (c2Hs)2N--C N~ CH3 powder J=7 Hz), 2.60 (3H, s), 3.1 -
~ ' 3.7 (4H, br), 4.59 (2H, q, J=7
~N CO2C2Hs Hz), 7.8-7.9 (1 H, m), 8.5-8.6
O (2H, m)
W095/09159 2~SO~ PCT1JP94/01559
- 270 -
:
O Brown 1.0-1.4 (6H, br), 1.49 (3H, t,
Il ~ powder J=7 Hz), 2.81 (3H, s), 3.2 -
(C2Hs)2N--C~ ~X 3.8 (4H, br), 4.56 (2H, q, J=7
~N CO2C2Hs Hz), 7.80 (1 H, dd, J=2 Hz, 9
Hz), 8.24 (lH, d, J=9 Hz),
8.58(1H,d,J=2Hz)
Using the suitable starting compounds, there are obtained the
compounds as listed in Table 3 in the same manner as in Reference Example
3,9,11 adn14.
Table 3
Crystalline
No. Structure Form 1H-NMR (CDCI3) ~ppm:
CH3 ~,Co2cH3 Pale 1.61 (2H, br), 2.23 (3H, s), 3.95
,~ J yellow (5H, s), 4.05 (3H, s), 7.50 (1 H,
H2NCH2 ~f solid s), 7.85 (1H, s)
OCH3
2 /CH3 Pale 1.56 (2H, br), 2.87 (3H, s), 3.36
~,N\ orange (3H, s), 3.98 (2H, s), 6.53 (1 H,
ll I SO2CH3 solid s), 7.24 (1 H, d, J=9 Hz), 7.43
H2NCH2 (1 H, d, J=9 Hz), 7.54 (1 H, s)
3 H2NCH2 CF3 Pale 1.43 (2H, br), 2.47 (3H, s), 3.95~ yellow oil (2H, s), 7.40-7.60 (2H, m), 7.85
CH3 O~~ (1H, s)
4H2NCH2 Pale 1.44 (2H, br), 2.50 (3H, s), 3.94~ yellow oil (2H, s), 7.20-7.35 (1 H, m), 7.45
CH3--o-`f (1H, d, J=9 Hz), 7.75 (1H, d,
CF3 J=9 Hz)
&OCH3 Pale 1.12 (3H, t, J=7 Hz), 1.67 (2H,
~ N~ yellow oil br), 1.82 (3H, s), 3.77 (2H, q,
H NCH ~ C2H5 J=7 Hz), 4.01 (2H, s), 6.56 (1H,
2 2 S), 7.03 (1 H, d, J=9 Hz), 7.30
(1 H, s), 7.46 (1 H, d, J=9 Hz)
WO 95/09159 2 1 5 0 3 4 5 PC'r/JP94/OlSS9
- 271 -
VM~ 6
6 Cl Yellow 2.18 (3H, s), 4.13 (2H, s), 7.43
)~ powder (1H, d, J=9 Hz), 7.63 (1 H, d,
H2NCH2 S NHCOCH3 J=9 Hz), 8.38 (1H, s), 9.42 (1H,
br)
- 1.60 (2H, br), 2.21(3H, s), 4.13
7NHCOCH3 White (2H, s), 7.09 (1H, s), 7.15-7.30
~ powder (2H, m), 7.70 (1 H, d, J=9 Hz),
H2NCH2 S 8.03 (1H, s)
8 Pale 1.56 (2H, br), 2.83 (2H, t, J=7
H2N(CH2)2~ brown Hz), 3.05 (2H, t, J=7 Hz), 7.20
o~ oil 7.40 (2H, m), 7.40-7.65 (3H, m)
9 ~ Pale 1.58 (2H, br), 3.97 (2H, s), 4.00
O O brown 4.25 (4H, m), 5.89 (1 H, s), 6.53
~H oil (1 H, s), 7.37 (1 H, d, J=8 Hz),
H2NCH2 7 44 (1 H, d, J=8 Hz), 7.65 (1 H,
O Pale 1.54 (2H, br), 4.04 (2H, s), 7.45
H2NCH2 C~ yellow 7.70 (5H, m), 7.75-7.90 (3H, m),
~ oil 8.12(1H,s)
11 H2NCH2 Cl White 1.48 (2H, br), 2.27 (3H, s), 3.97
powder (2H, s), 7.31 (1 H, s), 7.55 (1 H,
``f s), 7.61 (1H, br), 8.25 (1H, s)
NHCOCH3
12 H2NCH2 CN Pale 1.48 (2H, br), 4.04 (2H, s), 7.50
yellow 7.65 (2H, m), 7.68 (1 H, s), 8.01
0~~ powder (1H, s)
13 CH3 Br Orange 1.58 (2H, br), 2.17 (3H, s), 3.92
,~ solid (2H, s), 7.20-7.40 (2H, m), 7.57
H2NCH2 (1H, s)
WO9S/09159 215 0 3 4 5 PCT/JP94/OlSS9
- 272 -
14 CH3 ~,CI Pale 1.58 (2H, br), 2.17 (3H,
yellow s), 2.27 (3H, s), 3.95
H2NCH2 ~ - powder (2H, s), 7.15 (1H, s),
NHCOCH3 7.70 (1H, br), 8.21 (1H,
s)
H2NCH2 Br Pale 1.51 (2H, br), 3.97 (2H,
yellow s),7.30-7.45 (2H, m),
~~ oil 7.55 (1 H, s), 7.75 (1 H,
s)
16 ~,Br Pale 1.58 (2H, br), 3.97 (2H,
~ ~J yellow s), 6.53 (lH, s), 7.20 -
H2NCH2 oil 7.60(3H, m)
17 O Pale 1.61 (2H, br),4.00(2H,
Br~ ~NHCCH=CH~ brown s), 6.58 (1H, d, J=16
J~ 1 1 oil Hz), 7.30-7.90 (10H, m)
H2N C H2
18 Br NHCOCH3 Pale 1.61 (2H, br), 2.20 (3H,
~ brown s), 4.00 (2H, s), 7.35
H2NCH2 O solid (2H, s), 7.46 (1H, br),
7.68 (1 H, s)
19 O Pale 1.61 (2H, br),2.75 (3H,
yellow s), 4.01 (2H, s), 6.62
~SCH3 solid (1 H, s),7.49 (1 H, d,
H2NCH2 J=9 Hz), 7.57 (1 H, d,
J=9 Hz), 7.89 (1H, s)
WO 95/09159 2 1 S 0 3 4 S PCT/JP94/01559
- 273 -
1 ~/ (;~H, S), ~./4 (~H~
~ Pale red s), 3.94 (2H, s), 4.78
& \=/ oil (2H, s), 6.46 (1H, s),
~[~ SO2N(CH3)2 7.00 (1 H, d, J=9 Hz),
H2NCH2 7.15-7.32 (5H, m), 7.32
(1H, d, J=9 Hz), 7.41
(1H, s)
1.5/ (2H, s), 3.95 (3H,
21 H2NCH2 CO2CH3 Pale s), 4.03 (2H, s), 4.05
yellow (3H, s), 7.53 (1 H, s),
~ oil 7.62 (1 H, s), 7.97 (1 H,
OCH3 s)
1.35 (3H, t, J=/ Hz),
22 White 1.60 (2H, br), 3.99 (2H,
~,CH=CH-CO2C2H5 solid s), 4.27 (2H, q, J=7
H2NcH2 Hz), 6.41 (1 H, d, J=16
Hz), 6.55 (1H, s), 7.40
(2H, m), 7.68 (1 H, s),
7.77 (1H, d, J=16 Hz)
1.00-1.20 (6H, m), 1.58
23 CH2CON(C2H5)2 Pale (2H, br), 3.20-3.45 (4H,
~f brown m), 3.76 (2H, s), 3.96
H2NCH2--O--~ oil (2H, s), 6.47 (1H, s),
7.13 (1H, d, J=8 Hz),
7.37 (2H, d, J=8 Hz),
7.41 (1 H, s)
1.5/ (2H, br), 2.45 (3H,
24 CH3 N Brown s), 2.69 (3H, s), 3.96
~ \~CH3 oil (2H, s), 6.47 (1H, s),
J~ S 7.10 (1H, d, J=8 Hz),
H2NCH2 0--~ 7.30-7.55 (2H, m)
1.32 (3H, s), 1.5/ (2H,
~CH~2 CH3 Color - br), 2.99 (2H, s), 3.70 -
J~ ~ X lessoil 3.95 (4H, m), 3.96 (2H,
H2NCH2 O q 1 s), 6.48 (1H, s), 7.16
CH2--CH2 (1H, d, J=8 Hz), 7.34
(1 H, d, J=8 Hz), 7.41
(1H, s)
r PCr/JP94/015S9
WO 9S/091592 1 5 0 3 4 ~
- 274 -
l .~U (~H, ~r), ;~-U~ H,
26 SO2CH3 White s), 4.04 (2H, s), 6.67
~[~ powder (1H, s), 7.58 (1H, d,
H2NCH2 J=9 Hz), 7.84 (1 ff, d,
J=9 Hz), 8.16 (1H, s)
1.5/ (2H, brl, 2.51 (3H,
27 SCH3 White s), 3.97 (2H, s), 6.47
~ solid (1 H, s), 7.22 (1 H, d,
H2NCH2 O J=9 Hz), 7.35 (1 H, d,
J=9 Hz), 7.47 (1 H, s)
UM~ d6:
28 CH2NHSO2CH3 Pale 2.84 (3H, s), 4.24 (4H,
,1~ yellow s), 7.04 (1 H, s), 7.33
H2NCH2 powder (1H, d, J=9 Hz), 7.57
(HCI) (1H, d, J=9 Hz), 7.64
(1H, s), 8.78 (1H, br)
1.54 (2H, br), 1.59 (3H,
29 /OCOCH3 Pale d, J_8 Hz), 2.07 (3H,
H2NCH2 CH yellow s), 4.02 (2H, s), 6.00
CH3 oil (1 H, q, J=8 Hz), 7.32
(1 H, d, J=1 0 Hz), 7.45
(1H, d, J=10 Hz), 7.56
(1H, s), 7.60 (1H, s)
1.53 (2H, br), 4.0/ (2H,
N--N White s), 4.41 (3H, s), 7.57
H2NCH2~~ (1 H, s), 8.11 (1 H, d,
O J=9 Hz), 8.41 (1H, s)
1.52 (2H, br), 3.95 (3H,
31 Pale s), 4.05 (2H, s), 7.50
H2NCH2~,CO2CH3 yellow (1H, d, J=10 Hz), 7.62
O~ powder (1H, s), 8.04 (1H, d,
J=10 Hz), 8 35 (1 H, s)
wo gS/oglS9 2 I 5 0 3 ~ S PCT/JP94/OlSS9
- 275 -
~) ~4 (;~H, t, J=/ HZ),
32 ~ (CH2)2CH3 Pale yellow 1.50-1.62(2H,m),2.40-
/ oil 2.60(2H, m), 3.55(2H,
~`O C=CHCH2NH2 - d,J=7 Hz),5.70(1H,t,
J=7 Hz),6.63(1H,s),
7.17-7.29(2H, m), 7.40-
7.56(2H, m)
33 o Pale yellow 4.02(2H,s),6.61(1H,
oil s),7.46-7.63(4H, m),
7.77-8.01(3H, m), 8.01
~`O CH2NH2 (1H,s)
0.88(3H,t,J=/ Hz),
34 OCOCH3 Pale yellow 1.80-2.00(2H, m), 2.07
C2H5 - CH amorphous (3H,s),3.96(1H,s),
5.73(1H,t,J=7 Hz),
~`o CH2NH2 6.51(1H,s),7.23(1H,
dd,J=8 Hz,2 Hz),7.39
(1H,d,J=8 Hz),7.48
(1H,d,J=2 Hz)
1.4/(3H,d,J=/ Hz),
OCH3 Colorless oil 3.22(3H,s),3.96(2H,
CH3 - CH s), 4.37(1H,q,J=7
Hz),6.51(1H,s),7.20
o CH2NH2 (1H,dd,J=8 Hz,2 Hz),
7.40(1H,d,J=8 Hz),
7.45(1H,d,J=2 Hz)
1.53(3H,d,J=/ Hz),
36 NHCOCH3 Yellow 1.99(3H,s),3.96(2H,
CH3 - CH amorphous s), 5.21(1H,qui,J=7Hz),5.7(1H, br), 6.65
~'`O CH2NH2 (1H,s),7.23(1H,d,
J=8 Hz),7.40(1H,d,
J=8 Hz),7.50(1H,s)
WO 95/O91S9 21 S Q 3 4 5 PCT/JP94/015S9
- 276 -
~.U;~ H, s), ~.4~ H,
37 CH3~ Pale s), 3.53 (2H, d, J=7
yellow Hz), 6.41 (1H, t, J=7
--o C=CHCH2NH2 oil Hz), 6.52 (1H, s), 7.04
CH3 (1H, d, J=8 Hz), 7.28
(1H,s),7.30(1H,d,
J=8 Hz)
2.04 (3H, s), 2.52 (3H,
38 ~ Pale s), 3.55 (2H, d, J=7
yellow Hz), 6.47 (1H, t, J=7
--0 C=CH-CH2NH2 oil Hz), 6.58 (1H, s), 7.04
CH3 CH3 (1H, d, J=8 Hz), 7.08
(1 H, t, J=8 Hz), 7.33
(1H, d, J=8 Hz)
39 ~ Pale 3.99 (2H, s), 6.57 (1 H,
yellow s), 7.30-7.48 (5H, m),
~3~ powder 7.59-7.62 (2H, m), 7.71
o CH2NH2 (1H, s)
4.02 (2H, s), 6.60 (1H,
40 ~ Pale s), 7.34-7.55 (3H, m),
N~ yellow 7.72 (1H, d, J=5 Hz),
T 1~ powder 7.90 (1 H, d, J=7 Hz),
--0 CH2NH2 8.58 (1H, d, J=5 Hz),
8.87(1H,s)
41 N CH3 Pale 2.39 (3H, s), 2.49 (3H,
CH3~/--~ yellow s), 4.00 (2H, s), 6.56
~ powder (1H, s), 7.47 (2H, s),
~o~cH2NH2 7.70 (1 H, s)
_ Wo95/09159 2 1 5 0 3 4 5 PCT/JP94/OlS59
-277-
~.~U~H,S),~.4~(~H,~,
42 ~ Pale yellow J=7 Hz),3.82(3H,s),
C=CHCH2NH2 oil 5.55(1H,t,J=7Hz),6.85-
OCH3 CH3 6.93(2H,m),7.13(lH,
dd,J=8 Hz,2 Hz),7.22
(1H,dd,J=8 Hz,2 Hz)
2.04(3H,s),3.49(2H,d,
43 ~ Colorless J=7 Hz),3.82(3H,s),
CH3O~C=CHCH2NH2 oil 5.86(1H,t,J=7 Hz),6.79
CH3 (1 H,dd,J=8 Hz,2 Hz),
6.93(1H,t,J=2 Hz),6.99
(1H,d,J=8 Hz),7.23
(1H,t,J=8 Hz)
44 Colorless 2.06(3H,s),3.52(2H,d,
F3C~CI=CHCH2NH2 oil J=7 Hz),5.92(1H,t,J=7
CH3 Hz),7.47-7.61(4H,m)
2.0/(3H,s),3.52(2H,d,
~ Colorless J=7 Hz),5.90(1H,t,J=7
~ C~=CHCH2NH2 oil Hz),7.39-7.58(3H,m),
F3C CH3 7.63(1H,s)
46 ~ Colorless 2.06(3H,s),3.49(2H,d,
Cl=CHCH2NH2 oil J=7 Hz),5.85(1H,t,J=7
CH3 Hz),7.21-7.42(5H,m)
2.04(3H,s),2.18(3H,s),
47 ~ Pale yellow 3.48(2H,d,J=7 Hz),
~ Cl=CHCH2NH2 oil 5.86(1H,t,J=7Hz),7.15
CH3CONH CH3 (1H,d,J=8 Hz),7.26
(1H,t,J=8 Hz),7.39(1H,
d,J=8 Hz),7.51(1H,s),
7.20(1 H, br)
48 o Yellow DMSO-d6:
CH3O2C ~ ~ amorphous 2.05(3H,s),3.38(2H,d,
=CHCH2NH2 J=7 Hz),3.90(3H,s),
H 5 86(1 H, t,J=7 Hz), 7.50-
C 3 7.78(4H,m)
PCT/JI'94101559
WO95/09159 2lS03 ll~
- 278 -
H, s~ .5~ ~H, ~,
49 N-N White J=7 Hz),4.40(3H,s),5.96
' ~C=CHCH2NH2 powder (1 H,t,J=7 Hz),7.53(2H,d,
I CH3 J=8 Hz),8.09t2H,d,J=8
CH3 Hz)
1.9/(3H,s~,3.44(2H,d,
Colorless J=7 Hz),5.41(1H,t,J=7
C=CHCH2NH2 oil Hz),7.22(1H,d,J=8 Hz),
CH 7.37(1H,t,J=8 Hz),7.48
CF3 3 (1H,t,J=8 Hz),7.63(1H,d,
J=8 Hz)
2.03(3H,s),2.1/(3H,s),
Colorless 3.48(2H,d,J=7 Hz),5.83
CH3CONH ~ Cl=CHCH2NH2 oil (1H,d,J=7 Hz),7.35(2H,
CH3 d,J=8 Hz),7.45(2H,d,
J=8 Hz),7.30(1H, br)
2 09(3H,s),2.44(3H,s),
52 Colorless 3 50(2H,d,J=6Hz),5.82
CH3 O~ oil (1H,t,J=6 Hz),6.34(1H,
,C=CHCH2NH2 s),7.25(1H;dd,J=8 Hz,
7.46(1H,d,J=2 Hz)
2.31(2H,t,J=/Hz~,2./2
Colorless (2H,t,J=7 Hz),3.82-3.91
53 ~ oil (2H,m),3.88(3H,s),3.98-
~ CH2CH2NH2 4.07(2H,m),6.90(1H,t,
CH30 q q J=8 Hz),6.93(1H,t,J=8
CH2-CH2 Hz),7.29(1H,t,J=8 Hz),
7.47(1H,d,J=8 Hz)
54 Pale 3.86(2H,s),6.47(1H,s),
yellow 7.20-7.55(5H,m),7.64~1H,
needles s)
O CH2NH2
-WO 95/09159 2 1 5 0 ~ 4 5 PCI/JP94/01559
- 279 -
O Pale yellow 2.03 (3H, s), 3.49 (2H, d,
~CH-CHCNH-- oil J=7 Hz), 5.84 (1H, t, J=7
Hz), 6.59 (lH, d, J=16Hz),
~C=CHCH2NH2 7.35-7.68 (10H, m), 7.75
CH3 (1H, d, J=16 Hz)
2.14(3H,s),3.54(2H,d,
56 Yellow J=7 Hz), 5.96 (1H, t, J=7
~<N~ solid Hz), 7.45-7.60(4H, m), 7.91
\=/ S~c=cHcH2NH2 (1H, d, J=2 Hz), 8.00 (1H,
CH3 d, J=8 Hz), 8.07-8.11 (2H,
m)
57 OCH3 Pale yellow 2.43 (3H, s), 3.99 (3H, s),
h~<N_~ 3 powder 4.05 (2H, s), 6.93 (1 H, d,
W S~ J=8 Hz), 7.28 (1H, dd, J=8
Cl CH2NH2 Hz, 3 Hz), 8.32 (1H, d, J=3
Hz)
2.14(3H,s),3.54(2H,d,
58 OCH3 Yellow J=7 Hz), 4.06 (3H, s), 5.96
~<,N~ amorphous (1H, t, J=7 Hz), 7.00 (1H, d,
Cl S~,C=CHCH2NH2 J--8 Hz), 7.39 (1H, dd, J=8
J=8 Hz, 2 Hz), 7.92 (1 H, d,
J=2 Hz), 8.0 1(1H, d, J=8
Hz), 8.53 (1 H, d, J=3 Hz)
59 Brown oil 3.97 (2H, s), 6.52 (1 H, d,
~ J=2 Hz), 7.25-7.55 (6H, m)
o CH2NH2
WO 95/09159 2 1 5 0 ~ 4 ~ PCr/JP94/01559
- 280 -
H, s)~ H, t,
H Yellow J=7 Hz), 2.98 (2H, t, J=7
O~,N~ amorphous Hz), 3.53 t2H, d, J=7 Hz),
~J~ 5.94 (1H, t, J=7 Hz), 6.72
,C=CHCH2NH2 (1 H, d, J=8 Hz), 7.23 (2H,
CH3 m), 8.06 (1 H, br)
2.44 (3H, s), 3.52 (3H, s),
61 OCH OCH Yellow oil 4.06 (2H, s), 5.35 (2H, s),
2 3 7.17 (1H, d, J=8 Hz), 7.26
~<N~CH3 (1 H, dd, J=8 Hz, 2 Hz),
S CH2NH2 8.34 (1H, d, J=2 Hz)
Cl
2.04 (3H, s), 3.48 (2H, d,
62 ~ Colorless J=7 Hz), 3.49 (3H, s), 5.19
~,C=CHCH2NH2 oil (2H, s), 5.86 (1 H, t, J=7
CH3 Hz), 6.93 (1H, dd, J=8 Hz,
OCH2OCH3 3 Hz), 7.05 (1H, dd, J=8
Hz, 3 Hz), 6.95 (1 H, s),
7.23 (1 H, t, J=8 Hz)
2.98 (2H, dd, J=6 Hz, 2Hz),
63 Colorless 4.01 (1 H, dd, J=11 Hz, 7
~~ oil Hz),4.13(1H,m),4.27(1H,
~OlCH2NH2 dd, J=11 Hz, 2 Hz), 6.81 -
1.84 (3H, s), 2.04 (3H, s),
~ Colorless 3.49 (2H, d,J=7 Hz), 4.44
~f=CHCH2NH2 oil (2H, s), 4.99 (1H, s), 5.10
CH2= ICCH2O CH3 (1 H, s), 6.82 (1 H, d, J=8
CH3 Hz), 6.97 (1 H, s), 6.99 (1 H,
d, J=8 Hz), 5.86 (1 H, t, J=7
Hz), 7.22 (1 H, t, J=8 Hz)
- WO 95/09lS9 2 1 S 0 3 4 5 PCT/JP94/015S9
- 281 -
~.11(~H,S),~ H,S),
66 N~ Colorless 3.52(2H, d, J=7 Hz),5.91
CH3~/ 11 1 amorphous (1H,t,J=7 Hz),7.50(1H,
S~ `c=cHcH2NH2 dd, J=8 Hz,2Hz),7.82(1H,
CH3 d, J=2 Hz),7.87(1H, d,
J=8 Hz)
67O~ ,CH3 Pale yellow 3.41(3H,s,),3.96(2H,s),
S-N oil 7.06(1H, d, J=2 Hz),7.14
`N~Br (1H, dd, J=8 Hz,2 Hz),
~ o ~ CH2NH2 7.43(1H, d, J=8 Hz)
68 \\S ,CH3 Pale yellow 3.35(3H,s),3.97(2H,s),
~\ oil 6.68(1H,s),7.03(1H, dd,
`N~ J=8 Hz,2 Hz),7.17(1H,d,
oJ~cH2NH2 J=2 Hz),7.41(1H,d,J=8
Hz)
69 ~ Yellow 1.59(2H, brs), 3.92(2H,
powder brs), 3.96(2H,s),6.48(1H,
o CH2NH2 s),6.61(1H, d, J=7 Hz),
NH2 6.9-7.1(2H,m)
Br Yellow 1.61(2H, brs), 4.14(2H,s),
powder 7.06(1H,s),7.37(1H,d,
S CH2NH2 J=8 Hz),7.64(1H,d,J=8
Hz),7.82(1H,s)
WO95/09159 5034~ PCI`/JP94/01559
- 282 -
71 CH3 Yellow 1.51 (2H, brs), 2.34 (3H, s),
~( powder 4.09 (2H, s), 7.2-7.4 (2H,
S CH2NH2 m), 7.63 (1 H, d, J=8 Hz),
7.79 (1 H, d, J=8 Hz)
72 CH2NH2 Yellow oil 1.46 (3H, t, J=7 Hz), 1.68
(2H, brs), 4.25 (2H, s), 4.48
o CO2C2Hs (2H, q, J=7 Hz), 7.3-7.5
(2H, m), 7.58 (1 H, d, J=8
Hz), 7.77 (1H, d, J=8 Hz)
73 CH2NH2 Pale yellow 1.46 (2H, brs), 2.44 (3H, s),
[~ oil 3.91 (2H, s), 7.1-7.3 (2H,
O CH3 m), 7.3-7.4 (1 H, m), 7.5-7.6
t1H, m)
74 NH2 Yellow 1.53 (2H, brs), 3.87 (2H,
,~ powder brs), 3.95 (2H, s), 6.45 (1H,
J~ s), 6.49 (1 H, d, J=8 Hz),
O CH2NH2 6.90 (1 H, d, J=8 Hz), 7.05
(1 H, dd, J=8 Hz, 8 Hz)
Yellow 1.54 (2H, brs), 3.71 (2H,
,~ powder brs), 3.90 (2H, s), 6.38 (1H,
H2N O CH2NH2 s), 6.60 (1H, d, J=8 Hz),
6.77 (1 H, s), 7.26 (1 H, d,
J=8 Hz)
76 H2N CH2NH2 Orange 1.44 (2H, brs), 2.38 (3H, s),
~( powder 3.56 (2H, brs), 3.83 (2H, s),
o CH3 6.59 (1 H, d, J=8 Hz), 6.83
(1H, s), 7.17 (1H, d, J=8
Hz)
-WO 95/09159 2 1 ~ 0 3 ~ 5 PCT/JP94/01559
- 283 -
77 SCH3 Orange 1.57 (2H, brs), 2.35 (3H, s),
~( oil 4.10 (2H, s), 7.2-7.3 (2H,
o CH2NH2 m), 7.4-7.5 (1H, m), 7.6-7.7
(1H, m)
- 78 CH3 White 1.64 (2H, brs), 2.52 (3H, s),
~=N powder 2.69 (3H, s), 3.99 (2H, s),
S~ 6.55 (1H, s), 7.4-7.6 (2H,
CH3 ~oJ`cH2NH2 m), 7.74 (1 H, s)
79 ~ Orange 1.59 (2H, brs), 4.00 (2H, s),
\~N powder 6.60 (1 H, s), 7.3-7.5 (5H,
m), 7.9-8.1 (3H, m), 8.22
S ~ (1H, s)
~ 0 CH2NH2
~ White 1.61 (2H, brs), 2.61 (3H, s),
~=~S ~,CH3 powder 4.00 (2H, s), 6.58 (1 H, s),
N~ 7.3-7.6 (4H, m), 7.61 (1 H, d,
J=8 Hz), 7.85 (1 H, s), 7.9 -
o CH2NH2 8.0 (2H, m)
L1MSO-d6:
81 O S CH Brown 3.0-3.1 (1H, m), 3.4-3.6
~-- >'~ 2~ powder (1H, m), 4.09 (2H, s), 4.7 -
HN ~ ~0 CH2NH2 4.8 (1H, m), 6.85 (1H, s),
7.18 (1H, d, J=9 Hz), 7.4 -
7.6 (2H, m)
wo gs~0925~ 5 o 3 4 5 PCIIJP94/OlSS9 ~
- 284 -
82 CH3 S Yellowoil 1.58(2H, brs), 2.79(3H,s),
~NI ~ 3.99(2H,s),6.56(1H,s),
7.26(1H,s),7.45(1H,d,
H2NH2 J=8 Hz),7.75(1H,d,J=8
Hz),8.06(1H,s)
83 ~ N Brown 1.53(2H, brs), 4.00(2H,s),
~S~ powder 6.59(lH,s),7.30(1H, d,
~ J=3 Hz),7.48(1H,d,J=9
i~,~o CH2NH2 Hz),7.8-7.9(2H,m),8.14
(1H,s)
84 CH3 ~S CH3 Yellow oil 1.56(2H, brs), 2.52(3H,s),
N ~ CH2NH2 2.70(3H,s),4.03(2H,s),
~ 74-7.6(3H,m),7.85(1H,
N~N Yellow oil 1.57(2H, brs), 3.95(2H,s),
N /~ CH2 4.29(3H,s),4.30(2H,s),
CH3' N ~ 6.47(1H,s),7.21(1H,d,
'`o CH2NH2 J=8 Hz),7.36(1H,d,J=8
Hz),7.46(1H,s)
86 CH2NH2 Yellow oil 1.49(2H, brs), 4.05(2H,s),
7.33(1H,dd,J=8 Hz,8
Hz),7.56(1H,d,J=8 Hz),
CF3 7.66(1H,s),7.81(1H,d,
J=8 Hz)
87 CH3 Yellow oil 1.61(2H, brs), 2.24(3H,s),
3.97(2H,s),7.28(1H,dd,
o CH2NH2 J=8 Hz,8 Hz),7.49(1H,d,
CF3 J=8 Hz),7.63(1H,d,J=8
Hz)
215034~
- WO 95/09159 PCI/JP94101559
- 285 -
88 I N Yellow 1.45 (2H, brs), 4.06 (2H, s),
~S ~ CH2NH2 powder 7.32 (1 H, d, J=3 Hz), 7.52
(1 H, d, J=9 Hz), 7.60 (1 H,
s), 7.8-8.0 (2H, m), 8.24
(1H, s)
89 ~N Yellow 1.57 (2H, brs), 3.96 (2H, s),
CH2 powder 4.42 (2H, s), 6.49 (1 H, s),
7.1-7.3 (2H, m), 7.49 (1H, d,
--0 CH2NH2 J=8 Hz), 7.46 (1 H, s), 7.70
(1 H, d, J=3 Hz)
CH3 Yellow 1.54 (2H, brs), 2.51 (3H, s),
\~ly powder 3.99 (2H, s), 6.57 (1 H, s),
S'~ 6.84 (1 H, s), 7.45 (1 H, d,
~o ~CH2NH2 J=9 Hz), 7.83 (1 H, d, J=9
Hz), 8.11 (1 H, s)
91 CH2NH2 Yeilow oil 1.53 (2H, brs), 4.05 (2H, s),
,~ 7.52 (1 H, d, J=8 Hz), 7.6 -
F3C O 7.8 (3H, m)
92 CH3 Yellow 1.56 (2H, brs), 2.24 (3H, s),
~( powder 3.97 (2H, s), 7.4-7.6 (2H,
F3C~--0 CH2NH2 m), 7.67 (1 H, s)
93 CH3~ Yellow 1.54 (2H, brs), 3.97 (3H, s),
N--N powder 3.98 (2H, s), 6.58 (1 H, s),
~N~ 7.48 (1H, d, J=9 Hz), 8.01
H2NH2 s), 8.26 (1H, s)
WO 95tO9159 2 ~S O 3 4~ PCrlJP94101559
- 286 -
94 ,CH3 Yellow 1.61 (2H, brs), 4.01 (3H, s),
N--N powder 4.02 (2H, s), 6.62 (1 H, s),
N ~ 7.5-7.6 (2H, m), 7.83 (1 H,
H2NH2 s), 7.95 (1H, s)
S Brown oil 1.37 (2H, brs), 2.34 (3H, s),
/ ~ 2.71 (3H, s), 3.54 (2H, s),
CH3~N 7.15 (1H, d, J=7 Hz), 7.33
~CH2NH2 (1H, dd, J=7 Hz, 8 Hz),
~ 750(1H,d,J=8Hz),7.55
96 ~ Yellow oil 1.54 (2H, brs), 2.54 (3H, s),
J~ 2.69 (3H, s), 3.99 (2H, s),
~ O CH2NH2 6.54 (1 H, s), 7.4-7.6 (2H,
CH3 S CH3 m), 7.67 (1 H, s)
CH3 N~N Pale 1.54 (2H, brs), 2.61 (3H, s),
ll yellow 2.72 (3H, s), 4.01 (2H, s),
CH3 N--~ powder 6.63 (1 H, s), 7.54 (1 H, d,
O CH2NH2 J=9 Hz), 8.46 (1 H, d, J=9
Hz), 8.70 (1 H, s)
98 CH3 Yellow oil 1.49 (2H, brs), 2.47 (3H, s),
S~ 2.71 (3H, s), 3.97 (2H, s),
~N 6.69 (1 H, s), 7.2-7.5 (3H,
CH3 l m)
(~CH2NH2
- wo 95/09159 2 1 5 0 3 4 5 PcrtJps4lol5ss
- 287 -
99 ,~ Yellow 0.16 (3H, s), 0.20 (3H,
oil s), 0.95 (9H, s), 1.61
--`CH2NH2 (2H, brs), 2.3-2.4 (1H,
OSi(t-C4Hg)(CH3)2 m), 2.5-2.6 (1 H, m), 2.7 -
2.9 (1 H, m), 3.0-3.2
(2H, m), 4.94 (1 H, d,
J=7 Hz), 7.1-7.3 (4H,
m)
100 ~ ,CH2NH2 Yellow 1.50 (2H, brs), 2.54
ll ll oil (3H, s), 2.70 (3H, s),
CH3~ ~'' 4.04 (2H, s), 7.5-7.7
S CH3 (3H, m), 7.73 (1 H, s)
101 Cl White 2.17 (3H, s), 3.88 (2H,
~ powder d, J=6.8 Hz), 6.51 (1 H,
H2NCH2CH=C\ O t, J=6.8 Hz), 6.63 (1H,
CH3 s), 7.20 (1 H, d, J=8.7
Hz), 7.33 (1 H, d, J=8.7
Hz), 7.49 (1 H, s)
102 CONHCH3 Yellow 2.97 (3H, s), 4.00 (2H,
J~ oil brs), 6.27 (1 H, brs),
H2NCH2 O' 7.42 (1 H, brd, J=8 Hz),
7.65 (1 H, brs), 7.76
(1 H, brd, J=8 Hz), 8.05
(1H, brs)
- 103 CH3 Yellow 2.39 (3H, s), 2.42 (3H,
J~,COOCH3 oil s), 3.92 (3H, s), 4.05
J~ (2H, s), 6.65 (1 H, s),
H2NCHz O ~ CH3 7.12 (1H, s)
WO 95/09159 PCT/JP94/01559
2~5034s
- 288 -
104 ,CH3 Pale 1.27 (6H, d, J=6.9 Hz),
~,,CH~ ~ ~ yellow 2.99 (1H, sept. J=6.9
~ ~ CH3 oil Hz), 3.99 (2H, s), 6.52
H2NCH2 (1H, s), 7.12 (1H, d,
J=8.7 Hz), 7.34 (1H, d,
J=8.7 Hz), 7.36 (1 H, s)
105 ~ Yellow 2.13 (3H, brs), 3.53
J~ ~ oil (2H, d, J=6.6 Hz), 6.10
H2NCH2CH=C\ S (1H, t, J=6.6 Hz), 7.19
CH3 (lH, s), 7.2-7.4 (2H, m),
7.6-7.8 (2H, m)
106 ~ Yellow 1.55 (3H, d, J=7.4 Hz),
~ ~J oil 4.44 (1H, q, J=7.4 Hz),
H2N-CH S 7.13 (1H, s), 7.2-7.4
CH3 (2H, m),7.68(1H,d,
J=7.3 Hz), 7.80 (1H, d,
J=7 3 Hz)
107 H2N(CH2)2 ~ Yellow 2.92 (2H, t, J=6.5 Hz),
~J oil 3.10 (2H, t, J=6.5 Hz),
6.46 (1H, s), 7.1-7.3
(2H, m), 7.4-7.6 (2H, m)
108 CO2CH3 Yellow 2.19 (3H, s), 3.91 (2H,
,[~ oil d, J=6.5 Hz), 3.94 (3H,
H2NCH2CH=C\ O s), 6.54 (1H, t, J=6.5
CH3 Hz), 6.74 (1H, s), 7.43
(1 H, d, J=8.6 Hz), 8.00
(1 H, d, J=8.6 Hz), 8.27
(1H, s)
109 NHCOCH3 Yellow 2.21 (3H, s), 4.01 (2H,
~ oil s), 6.59 (1H, s), 7.42
H2NCH2 ~f (1H, s), 8.06 (1H, s)
CF3
2tS03~5
-- WO 95/09159 PCT/JP94/01559
- 289 -
110 ~ Yellow 4.02 (2H, s), 4.15 (3H,
~ oil s), 6.69 (1 H, s), 7.40
H2NCH2 ~f (1H, t, J=8.1 Hz), 7.58
N~N~CH3 . (1H, d, J=8.1 Hz), 7.76
N N (1 H, d, J=8.1 Hz)
111 Yellow 4.10 (2H, s), 4.49 (3H,
,~q oil s), 6.64 (1 H, s), 7.34
H2NCH2 ~f (1 H, t, J=7.7 Hz), 7.65
N~N (1H, d, J=7.7 Hz), 8.05
N--N (1 H, d, J=7.7 Hz)
CH3~
112 ~OCH3 Pale 2.02 (3H, brs), 3.54
J~ ~J yellow (2H, d, J=6.8 Hz), 3.84
H2NCH2CH=C\ O powder (3H, s), 6.41 (1H, t,
CH3 J=6.8 Hz), 6.54 (1H, s),
6.84 (1 H, dd, J=8.8 Hz,
2.5Hz),6.98(1H,d,
J=2.5 Hz), 7.30 (1 H, d,
J=8.8 Hz)
113 CH3 Yellow 2.12 (3H, brs), 2.35
~q oil (3H, s), 3.55 (2H, d,
H2NCH2CH=C~`O~ J=6.7 Hz), 6.11 (1H, t,
CH3 J=6.7 Hz), 7.2-7.3 (2H,
m), 7.4-7.5 (2H, m)
114 Yellow 2.05 (3H, brs), 3.56
,~q oil (2H, d, J=7.1 Hz), 6.53
H2NCH2CH=C\ O~ (1H, t, J=7.1 Hz), 6.64
CH3 CF3 (1 H, s), 7.25 (1 H, t,
J=6.9 Hz), 7.48 (1H, d,
J=6.9 Hz), 7.68 (1 H, d,
J=6.9 Hz)
WO 9S/09159 ~3 45 PCT/JP94/OlS59
- 290 -
115 ,N N, Yellow 4.03 (2H, s), 4.20 (3H,
N~ N~ oil s), 6.69 (1 H, s), 7.4-7.5
r CH3 (2H, m), 7.6-7.7(1H, m)
H2NCH2
116 ,CH3 Yellow 4.05 (2H, s), 4.45 (3H,
,N, N, oil s), 7.33 (1 H, s), 7.37
N~N (1 H, t, J=7.6 Hz), 7.55
~ (1H, d, J=7.6 Hz), 8.06
H2NCH2 (1H, d, J=7.6 Hz)
117 H Yellow 2.44 (2H, t, J=7.5 Hz),
~ ~ oil 2.96 (2H, t, J=7.5 Hz),
H2NCH2 4.00 (2H, s), 6.68 (1 H,
s), 7.00 (1 H, s), 7.37
(1H, s), 10.11 (1H, s)
118 CH3 Yellow 2.42 (3H, s), 3.95 (2H,
~ oil s), 6.45 (1 H, s), 7.0-7.2
H2NCH2 (1H, m), 7.3-7.5 (2H, m)
119 , ~, Yellow 2.51 (3H, s), 3.98 (2H,
J~ ~ d oil s), 6.51 (1H, s), 7.06
H2NCH2 1' (1H, d, J=7.3 Hz), 7.10
CH3 (1 H, t, J=7.3 Hz), 7.34
(1 H, d, J=7.3 Hz)
120 N Yellow 4.02 (2H, s), 6.61 (1H,
,~ oil s), 7.39 (1 H, d, J=5.7
H2NCH2 0 Hz), 8.46 (1 H, d, J=5.7
Hz), 8.86 (1H, s)
- wo 95/09159 2 ~ 5 o ~ 4 ~ PCr,JP94,0l559
- 291 -
121 ~N Yellow 2.05 (3H, s), 3.56 (2H,
J~ ~J oil d, J=6.6 Hz), 6.50 (1H,
H2NCH2CH=C\ O t, J=6.6 Hz), 6.64 (1H,
CH3 s), 7.37 (1 H, d, J=5.7
Hz), 8.45 (1 H, d, J=5.7
Hz), 8.84 (1H, s)
122 Cl 2H5 Yellow 1.29 (3H, t, J=7.1 Hz),
,~N~ ~O oil 2.64 (2H, t, J=8.2 Hz),
2.97 (2H, t, J=8.2 Hz),
H2NCH2 4.04 (2H, q, J=7.1 Hz),
4.12 (2H, s), 6.71 (1H,
s), 7.16 (1H, s), 7.27
(1H, s)
123 N=N Yellow 2.21 (3H, s), 3.91 (2H,
~N,N~CH3 oil d, J=6.6 Hz), 4.41 (3H,
ll I ~ s), 6.54 (1H, t, J=6.6
H2NCH2CH=C\ O Hz), 6.76 (1H, s), 7.52
CH3 (1 H, d, J=8.5 Hz), 8.06
(1H, d, J=8.5 Hz), 8.33
(1H, s)
124 ~_CH2 Yellow 2.39 (3H, s), 2.61 (3H,
~ I ~S oil s), 3.98 (2H, s), 4.12
H2NCH2 CH3--N--CH3 (2H, s), 6.47 (1H, s),
7.07 (1H, d, J=8.4 Hz),
7.30 (1 H, s), 7.47 (1 H,
d, J=8.4 Hz)
125 ~ Yellow 2.41 (3H, s), 2.72 (3H,
J~ ~J oil s), 3.97 (2H, s), 6.58
H2NcH2 T (1H, s), 7.3-7.7 (3H, m)
~N
H3C S CH3
WO 9S/09159 ` PCT/JP94/01559
~5~ - 292 -
126 H2NCH2 Yellow 2.41 (3H, s), 2.72 (3H,
oil s), 4.04 (2H, s), 7.3-7.7
O~ (4H, m)
~N
H3C--~S~CH3
Using the suitable starting compounds, there are obtained the
compounds as listed in Table 4 in the same manner as in Reference Examples
15-21.
Table 4
m.p. Crystalline
No. Structure C Form 1H-NMR (CDCI3) ~ppm:
o Yellow 1.47 (3H, t, J=7 Hz), 2.51
powder (3H, s), 2.53 (3H, s), 2.57
CH3 ~N`~XcH3 (3H, s), 4.58 (2H, q, J=7
CH3~N co2c2Hs Hz), 8.31 (1H, s), 8.36 (1H,
O s)
2 CH3 ~,~N CH3 Orange 1.49 (3H, t, J=7 Hz), 2.48
ll ~X powder (3H, s), 2.50 (3H, s), 2.93
CH3~--N CO2C2Hs (3H, s), 4.55 (2H, q, J=7
Hz), 7.78 (1 H, s), 7.91 (1 H,
s)
3 O Pale yellow 1.48 (3H, t, J=7 Hz), 2.48
powder (3H, s), 2.53 (3H, s), 2.80
CH3~N~,CH3 (3H, s), 4.54 (2H, q, J=7
CH3~NJ`CO2c2Hs Hz), 7.93 (1H, s), 8.34 (1H,
s)
2 1 S 0 3 4 S PCr/JP94/01559
- WO 95/09159
- 293 -
~;rystalllne ~orm
No. Structure m.p. C (solvent for recystal.)
4 N ,CH2Br 83-95 Colorless needles (dichloro -
, ~ methane/
`N~ `CO2C2Hs n-hexane)
NMR analysis:
,~ N CH2F 96-97 White powder
(dichlor~."~elhane/
~N~C02C2H5 n-hexane)
6 O 109-1 10 Colorless flakes
(dichloromethane/
~NXco2c2Hs n-hexane)
7 O 140-141 Brown powder
(chlorofor"~)
~N XCH2F
~: 1H-NMR (CDCI3) ppm:
1.53 (3H t J=7 Hz) 4.61 (2H d J=7 Hz) 5.14 (2H s) 7.81-7.93 (2H m)
8. 1 1 (1 H, m) 8.23 (1 H m)
WO 95/09159 PCI/JP94/01559
~ ,~5~ 4S ~ 294 -
Example 1
2-Ethoxycarbonyl-3-methylquinoxalin-4-oxide (1.5 9) is mixed
with 3-aminomethylbenzofuran (1.9 9) under nitrogen atmosphere, and the
mixture is stirred at 60C overnight. The reaction mixture is purified by silica5 gel column chromatography (solvent; n-hexane: ethyl acetate = 3: 1), and
recrystallized from ethanol to give 2-[(3-benzofuranyl)methylaminocarbonyl]-3 -
methylquinoxalin-4-oxide (1.3 9) as colorless needles.
M.p. 143-1 44C
Example 2
2-Carboxy-3-methylquinoxalin-4-oxide (0.8 9) and 2-aminomethyl -
5-dimethylaminobenzofuran (0.9 9) are dissolved in dimethylformamide (10
ml), and thereto are added dropwise successively diethyl cyanophosphate (0.8
g) and triethylamine (0.8 9) under ice-cooling. The mixture is stirred at room
temperature overnight, extracted with ethyl acetate, and washed with water.
The mixture is dried over anhydrous sodium sulfate, and evaporated to remove
the solvent. The residue is purified by silica gel column chromatography
(solvent; n-hexane: ethyl acetate = 1: 1), and recrystallized from acetonitrile to
give 2-[(5-dimethylamino-2-benzofuranyl)methylaminocarbonyl]-3-
methylquinoxalin-4-oxide (0.6 9) as yellow plates.
M.p. 155-1 57C
Example 3
2-Quinoxalinecarboxylic acid (0.5 9) is dissolved in
dichloromethane (10 ml), and thereto is added dicyclohexylcarbodiimide (0.7
9). The mixture is stirred for 30 minutes, and thereto is added 2 -
aminomethylbenzofuran (0.5 9), and the mixture is stirred overnight. The
insoluble materials are removed by filtration, and the organic layer is washed
with saturated sodium hydrogen carbonate solution and water, and dried over
anhydrous sodium sulfate. The resultant is evaporated to remove the solvent,
and the residue is purified by silica gel column chromatography (solvent; n -
hexane: ethyl acetate = 3: 1), and recrystallized from n-hexane/ethyl acetate togive 2-[(2-benzofuranyl)methylaminocarbonyl]quinoxaline (0.43 9) as colorless
flakes.
M.p. 155-1 56C
- WO 9S/09159 2 1 5 0 3 4 ~ PCT/JP94/01559
- 295 -
Example 4
2-[(4-Morpholinocarbonylmethoxybenzyl)aminocarbonyl]-3 -
methylquinoxaline (2.2 9) is dissolved in methylene chloride (30 ml), and
thereto is added m-chloroperbenzoic acid (1.3 9) at room temperature, and the
mixture is stirred for one day. To the mixture is added chloroform, and the
organic layer is washed successively with aqueous sodium thiosulfate solution,
saturated aqueous sodium hydrogen carbonate solution, and water, and dried
over anhydrous sodium sulfate. The mixture is evaporated to remove the
solvent, and the residue is purified by silica gel column chromatography
1 0 (solvent; ethyl acetate), and recrystallized from acetonitrile to give 2-[(4 -
morpholinocarbonylmethoxybenzyl)aminocarbonyl]-3-methylquinoxalin-4 -
oxide (1.5 9) as colorless needles.
M.p. 142-143C
Using the suitable starting compounds, there are obtained the
compounds of Examples 28-103, 125, 126, 128, 130-135, 137, 138, 141, 143 -
146, 148,150, 152, 154-157, 159, 161, 163, 165, 167, 169, 171, 173, 175, 177-
184, 187-189, 191, 192, 194, 196-201, 203-205, 207, 209-213, 216, 21g, 220,
224, 226, 228-230, 233, 235, 236, 239, 240 and the following Examples 435 -
440, 442-479 and 484-487 in the same manner as in Example 4.
Example 5
2-Cinnamylaminocarbonyl-3-methylquinoxaline (1.b 9) is
dissolved in dimethylformamide (15 ml), and thereto is added sodium hydride
(160 mg) under ice-cooling. The mixture is stirred at the same temperature for
30 minutes, and thereto is added methyl iodide (0.52 g), and the mixture is
stirred at room temperature for two hours. The mixture is poured into ice-water,- and extracted with diethyl ether. The extract is washed with water, dried over
anhydrous sodium sulfate. The resultant is evaporated to remove the solvent,
and the residue is purified by silica gel column chromatography (solvent; n -
hexane: ethyl acetate = 2: 1) to give 2-(N-cinnamyl-N-methylaminocarbonyl)-3 -
methylquinoxaline (0.8 9) as pale yellow liquid.
1H-NMR (CDCI3) ~ ppm: 2.78, 2.79 (3H, s), 2.92, 3.24 (3H, s), 3.98, 4.43
(2H, d, J=6 Hz), 6.10-6.40 (1H, m), 6.40, 6.70 (1H, d, J=16 Hz), 7.20-7.50 (5H,
WO 95tO9159 PCT/Jl'94/01559
~,~S~3 4S ~ 296 -
m), 7.70-7.85 (2H, m), 8.00-8.15 (2H, m)
Using the suitable starting compounds, there are obtained the
compounds of Examples 44, 66, 107, 110, 135, 138,150, 151, 152, 153,175,
176,178, 186, 187, 191 and 192 in the same manneras in Example 5.
Example 6
2-[(5-Aminobenzofuran-2-yl)methylaminocarbonyl]-3 -
methylquinoxalin-4-oxide (0.4 9) is suspended in pyridine (6 ml), and thereto isadded dropwise with stirring acetic anhydride (0.26 9) under ice-cooling. The
mixture is stirred at room temperature for one day, and poured into ice-water.
The precipitated crystals are collected by filtration, washed with water, and the
resulting crude crystals are recrystallized from acetonitrile to give 2-[(5 -
acetylaminobenzofuran-2-yl)methylaminocarbonyl]-3-methylquinoxalin-4-oxide
(0.4 9) as pale yellow needles.
M.p. 183-184C
Using the suitable starting compounds, there are obtained the
compounds of Examples 84 and 85 in the same manner as in Example 6.
Example 7
2-[(5-Aminobenzofuran-2-yl)methylaminocarbonyl]-3-methyl -
quinoxalin-4-oxide (0.8 g) is dissolved in dimethylformamide (15 ml), and
thereto are added potassium carbonate (0.5 9) and N-chloroacetylmorpholine
(0.37 9). The mixture is stirred at 70C for four hours, and evaporated to
remove the solvent. To the residue is added water, and the mixture is extracted
with chloroform, washed with water, and dried over anhydrous sodium sulfate.
The resultant is evaporated to remove the solvent, and the residue is purified
by silica gel column chromatography (solvent; ethyl acetate), to convert the
resultant to an oxalate thereof, and recrystallized from ethanol to give 2-[(5 -morpholinocarbonylmethylaminobenzofuran-2-yl)methylaminocarbonyl]-3 -
methylquinoxalin-4-oxide (40 mg) as pale brown granules.
M.p. 159-167C (decomposed)
1 H-NMR (CDCI3) ~ ppm: 3.09 (3H, s), 3.40-3.55 (2H, m), 3.55-3.85 (6H,
m), 3.90 (2H, s), 4.78 (2H, d, J=6 Hz), 6.60 (1 H, s), 6.65 (1 H, d, J=7 Hz),6.67
(1H, s), 7.28 (1H, d, J=7 Hz), 7.75-7.85 (2H, m), 8.05-8.15 (lH, m), 8.47 (1H,
-- WO 95109159 2 1 5 0 3 4 5 PCI/JP94101SS9
- 297 -
brs), 8.50-8.60 (1 H, m)
Example 8
2-[(5-Ethoxycarbonylbenzofuran-2-yl)methylaminocarbonyl]-3 -
methylquinoxalin-4-oxide (4.2 9) is added to a solution of sodium hydroxide
(1.4 9) in methanol (50 ml) and water (14 ml), and the mixture is stirred at room
temperature overnight. The mixture is evaporated to remove methanol, and to
the resultant is added water and acidified with hydrochloric acid, and the
precipitated crystals are collected by filtration, washed with water, and dried to
give 2-[(5-carboxybenzofuran-2-yl)methylaminocarbonyl]-3-methylquinoxalin-4-
oxide (3.8 9) as white powder.
M.p. 251-252C (decomposed)
Example 9
2-[(6-Methoxymethoxy-3-methylbenzofuran-2-yl)methylamino -
carbonyl]-3-methylquinoxalin-4-oxide (2.1 g) is dissolved in tetrahydrofuran (20ml) and methanol (20 ml), and to the mixture is added 6N hydrochloric acid (10
ml), and the mixture is refluxed for one hour. The mixture is evaporated to
remove the solvent, and the residue is extracted with chloroform. The extract iswashed with saturated aqueous sodium hydrogen carbonate solution, water
and brine, and dried over anhydrous sodium sulfate. The solvent is evaporated
to remove the solvent, and the residue is recrystallized from dimethyl -
formamide/water to give 2-[(6-hydroxy-3-methylbenzofuran-2-yl)methylamino -
carbonyl]-3-methylquinoxalin-4-oxide (1.6 9) give as yellow needles.
M.p. 222-223C (decomposed)
Example 10
2-[(4-Hydroxy-3-methylbenzofuran-2-yl)methylaminocarbonyl]-3-
methylquinoxalin-4-oxide (0.55 9) is dissolved in dimethylformamide (4 ml),
and to the mixture are added potassium carbonate (0.41 9) and methyl iodide
(0.19 ml), and the mixture is stirred at 60C for four hours. To the mixture is
added water, and the precipitated crystals are collected by filtration, and
washed with water. The crude crystals are recrystallized from ethyl acetate/n -
hexane to give 2-[(4-methoxy-3-methylbenzofuran-2-yl)methylaminocarbonyl] -
3-methylquinoxalin-4-oxide (0.41 9) as yellow prisms.
M.p. 196-1 97C
WO 95/09159 PCI~1JP94/OlS59
2~S03 4~ - 298 -
Example 1 1
2-[(Benzofuran-2-yl)methylaminocarbonyl]-3-methylquinoxalin-4 -
oxide (0.8 9) is dissolved in chloroform (10 ml), and thereto is added N -
bromosuccinimide (0.47 9), and the mixture is refluxed for 7 hours. The mixture
5 is evaporated to remove the solvent, and the residue is purified by silica gel column chromatography (solvent; n-hexane: ethyl acetate = 2: 1), and
recrystallized from ethyl acetate/n-hexane to give 2-[(3-bromobenzofuran-2 -
yl)methylaminocarbonyl]-3-methylquinoxalin-4-oxide (0.44 g) as colorless
needles.
1 0 M.p. 170-171 C
Using the suitable starting compounds, there are obtained the
compounds of Examples 31, 34, 38, 88, 93, 98, 119, 124, 125, 131 and 139 in
the same manner as in Example 11.
Example 12
2-[(4-Methoxybenzyl)aminocarbonyl]-3-methylquinoxaline (10.5
g) is dissolved in dry methylene chloride (150 ml), and thereto is added
dropwise a 1M solution of boron tribromide in methylene chloride (105 ml)
under ice-cooling. The mixture is stirred at room temperature for two days,
cooled, decomposed with methanol, and evaporated to remove the solvent. To
the residue is added water, and the mixture is neutralized with sodium
hydrogen carbonate, and the precipitated crystals are collected by filtration,
washed with water, and dried. The residue is recrystallized from ethanol to
give 2-[(4-hydroxybenzyl)aminocarbonyl]-3-methylquinoxaline (10.0 g) as
colorless needles.
M.p. 21 1-21 4C
Example 13
2-{[5-(1 -Acetoxyethyl)benzofuran-2-yl]methylaminocarbonyl}-3 -
methylquinoxalin-4-oxide (4.5 g) is dissolved in methanol (90 ml), and thereto
is added potassium carbonate (1.8 9), and the mixture is stirred at room
temperature overnight. The mixture is evaporated to remove the solvent, and to
the residue is added water. The mixture is extracted with chloroform, washed
with water, and dried over anhydrous sodium sulfate. The mixture is
evaporated to remove the solvent, and the crude crystals are recrystallized from
- WO 95/09159 2 1 5 û 3 4 S PCT/JP94/01559
- 299 -
ethyl acetate/n-hexane to give 2-{[5-(1-hydroxyethyl)benzofuran-2-yl]methyl -
aminocarbonyl}-3-methylquinoxalin-4-oxide (3.4 9) as colorless granules.
M.p. 112-114C
Example 14
- 5 2-{[5-(1-Hydroxyethyl)benzofuran-2-yl]methylaminocarbonyl}-3-
methylquinoxalin-4-oxide (0.8 9) is dissolved in chloroform (30 ml), and theretois added manganese dioxide (3.6 9), and the mixture is refluxed for 6 hours.
The reaction solution is filtered through celite, and concentrated. The residue
is recrystallized from ethyl acetate/n-hexane to give 2-[(5-acetylbenzofuran-2 -yl)methylaminocarbonyl]-3-methylquinoxalin-4-oxide (0.5 9) as pale yellow
granules.
M.p. 205-206C
Example 15
2-[(5-Acetylbenzofuran-2-yl)methylaminocarbonyl]-3 -
methylquinoxalin-4-oxide (0.7 9) is dissolved in anhydrous tetrahydrofuran (20
ml), and thereto is added dropwise a 1 M solution of methylmagnesium bromide
in diethyl ether (2.5 ml) under ice-cooling. The mixture is stirred at room
temperature for three hours, and poured into ice-aqueous ammonium chloride
solution. The mixture is extracted with chloroform, and the extract is washed
with water and brine, and dried over anhydrous sodium sulfate. The resultant
is evaporated to remove the solvent, and the residue is purified by silica gel
column chromatography (solvent; n-hexane: ethyl acetate = 1: 1), and
recrystallized from n-hexane/ethyl acetate to give 2-{[5-(1-hydroxy-1 -
methylethyl)benzofuran-2-yl]methylaminocarbonyl}-3-methylquinoxalin-4-oxide
(0.1 9) as pale yellow prisms.
- M.p. 147-1 50C
Example 16
2-{[5-(5-Tetrazolyl)benzofuran-2-yl]methylaminocarbonyl}-3 -
methylquinoxalin-4-oxide (1.4 9) is dissolved in dimethylformamide (15 ml),
and thereto are added potassium carbonate (0.97 9) and methyl iodide (0.44
9), and the mixture is stirred at room temperature for four hours. The reaction
solution is poured into ice-water, and the precipitated crystals are collected by
filtration, and recrystallized from dimethylformamide/ethanol to give 2-{[5-(2-
WO 95/09159 PCT/JP94/01559
2~so34~
- - 300 -
methyl-5-tetrazolyl)benzofuran-2-yl]methylaminocarbonyl}-3-methylquinoxalin -
4-oxide (0.48 9) as white powder.
M.p. 206-209C
Using the suitable starting compounds, there are obtained the
compounds of Examples 36 and 161-164 in the same manner as in Example
16.
Example 17
2-[(4-Methoxycarbonylmethoxybenzyl)aminocarbonyl]-3 -
methylquinoxaline (3.65 9) is dissolved in methanol (50 ml), and thereto is
added 2N sodium hydroxide (6 ml), and the mixture is stirred at room
temperature for two hours. The mixture is evaporated to remove the solvent,
and to the resultant is added water. The mixture is acidified with hydrochloric
acid, and the precipitated crystals are collected by filtration, washed with water,
and dried to give 2-[(4-carboxymethoxybenzyl)aminocarbonyl]-3-methyl -
quinoxaline (3.40 9) as white powder.
1H-NMR (DMSO-d6) ~ ppm: 2.83 (3H, s), 4.48 (2H, d, J=6 Hz), 4.66 (2H,
s), 6.91 (2H, d, J=9 Hz), 7.33 (2H, d, J=9 Hz), 7.8-8.0 (2H, m), 8.0-8.2 (2H, m),
9.33 (1 H, t, J=6 Hz)
Using the suitable starting compounds, there is obtained the
compound of Example 49 in the same manner as in Example 17.
Example 1 8
2-[(4-Carboxymethoxybenzyl)aminocarbonyl]-3 -
methylquinoxaline (1.05 9) is dissolved in dimethylformamide (10 ml), and
thereto is added furfurylamine (0.35 9), and thereto are added dropwise
successively diethyl cyanophosphate (0.6 9) and triethylamine (0.6 9) under ice -
cooling. The mixture is stirred at room temperature overnight, and poured into
ice-water, and the precipitated crystals are collected by filtration. The crystals
are recrystallized from acetone/n-hexane to give 2-[(4-furfurylaminocarbonyl -
methoxybenzylaminocarbonyl]-3-methylquinoxaline (0.92 9) as white powder.
M.p. 175-1 76C
Example 19
2-[(4-Methoxycarbonylmethoxybenzyl)aminocarbonyl]-3-
DEMANDES OU BREVETS VOLUMINEUX
LA PRÉSENTE PARTIE DE C~TTE DEMANDE OU CE BREVET
COMPREND PLUS D'UN TOME.
CECI EST LE TOME / DE_ ~
NOTE: Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
~ ~ S ~c~t5
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLlCATlON/PATENT CONTA1NS MORE
THAN ONE VOLUME
THIS IS VOLUME ¦ OF *~
NOTE: For additional volumes please contact the Canadian Patent Offic~