Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 94/15018 ?~ 15 1~ 1~ PCT/US93112135
MET}~OD AND APPAR~TUS FOR PF~T '`'rING WOOD PU~P RT~ UTNG
BACKGROUND OF TF~ lNV~:r. 1 ~ ON
This invention relates generally to bleaching of wood
pulp for paper making and more particularly to ble~c~;ng of
high consistency wood pulp using a gaseous ble~Ghing
reagent.
A typical high consistency pulp bleaching reactor
system for use with a gaseous reagent has pulp at 30-45~
consistency fed from a dewatering press to be compacted in a
screw feeder to form a gas-tight plug. As the leading face
of the gas-tight plug leaves the feeder it encounters a high
speed fluffer which breaks or shreds the pulp into very
small particles and deposits those particles evenly on the
surface of a bed type reactor. The gaseous reagent, as part
of a contacting gas made up of the gaseous reagent, a
carrier gas, and a mixture of reaction products and other
gases and vapors, which constitutes the environment within
the bleAching system, is fed into the fluffer and passes
from there with the pulp particles into the bed type reactor
where it reacts with the pulp to substantially complete the
bleaching reaction. In some cases, the fluffer is located
above the bed type reactor so that the fluffed pulp just
falls into the bed; and the contacting gas flows downward
through the pulp bed, where the bleAsh;ng reagent reacts
with the pulp, and is then removed from the reactor and
reprocessed to remove impurities and reaction products and
to replenish the concentration of gaseous reagent in the
contacting gas. After reprocessing and replenishment, the
gaseous reagent/contacting gas mixture is again fed into the
fluffer to continue the bleaching cycle.
Nhen the fluffer is located next to the bed type
reactor, the fluffed pulp must be transported to the top of
the reactor for deposit on the pulp bed within the reactor.
wo ~/1~18 rCT~S93/~5
21~1813
This is usually accomplished by blowing with a sufficient
volume flow rate of contacting gas mixture to transport the
fluffed pulp upward to the top of the reactor. In both
cases, the bleAch;ng reaction begins in the fluffer and
continues into and through the bed reactor, although, in the
first case, there is clearly less reaction time before the
pulp fluff is deposited in the bed reactor.
There are also some systems in which the pulp passes
from the pulp fluffer into a mixer in which it is agitated
to enhance contact between the fluff and the gas mixture.
It then is deposited in the bed reactor to substantially
complete the reaction. Pulp residence time in the mixer is
commonly of the order of 60 seconds, or less. Usually such
mixers have an auger, biased paddles, or other mechanical
conveying provision to transport the pulp fluff, at
substantially the same speed as the gas mixture, through the
mixer. The reaction is completed in the bed reactor, as in
the preceding cases.
Even though the pulp fluffer divides the pulp into very
small particles, each particle still contains a great number
of intertwined individual fibers so that access of gaseous
reagent to the fibers within each particle i8 not uniform
and outer fibers are more thoroughly bleached than are inner
ones. This may result in a salt and pepper effect in the
reactor bed and a consequent decrease in bleaching
effectiveness. The result is a degradation of pulp quality.
Generally, achievement of uniform bleAch;ng in high
consistency pulp using gaseous reagents is very difficult.
This is due to the combination of channeling of the gas
between pulp particles, the time required for dissolution of
the reagent gas in the liquor which surrounds the fibers in
the pulp particle, and the relatively slow diffusion of the
WO ~/1~18 PCT~S93/~5
21~1813
dissolved reagent to the centers of the pulp particles. The
result may be overbleached outer fibers, weakened outer
fibers, and underbleached interior fibers.
Chlorine and chlorine dioxide are the most commonly
applied gas for pulp bleaching, but there is increasing
interest in ozone as a bleAching reagent. Due to
difficulties in obt~;n;ng chlorine dioxide which has no free
chlorine, environmental considerations have dictated a
preference for ozone as a gaseous bleA~h;ng reagent to
simplify waste treatment and to reduce the potential for
objectionable discharges from mills.
Additional background of the pulp bleaching technology
is to be found in U.S.Patent Nos. 3,814,664; 3,917,176; and
3,964,962; all commonly assigned and incorporated herein by
reference; as well as application serial number 07/953,321,
filed September 29, 1992 and also commonly assigned.
In addition to being relatively expensive, gaseous
bleaching reagents are very aggressive bleaching agents
which have reaction rates which are directly proportional to
concentration. This aggravates the non-uniformity of
bleaching and increases the severity of pulp damage due to
overble~ching. These characteristics, and the difficulties
they entail, have delayed widespread application of ozone
bleaching in the pulping industry; because reagents which
are cheaper and which are less sensitive to operating
conditions have been available, even though such reagents
have been more difficult to treat and to render
unobjectionable in effluent disposal operations.
The foregoing illustrates limitations known to exist in
present wood pulp bleaching operations. Thus, it is
apparent that it would be advantageous to provide an
,'J~ 3 5
21~8Q~
alternative directed to overcoming one or more of the
limitations set forth above. Accordingly, a suitable
alternative is provided including features more fully
disclosed hereinafter.
SUMMARY OF THE INVENTION
In one aspect of the present invention, this is
accomplished by providing a method for mitigating the degree
of non-uniformity of bleached wood pulp in a wood pulp
bleaching system having a mechanically agitated pulp
contactor, comprising the steps of: supplying a quantity of
fresh bleaching gas F comprising a gaseous ble ~hing reagent
in a carrier gas, the bleaching gas having a first
concentration of the gaseous bleaching reagent in the
carrier gas; determining a contact time for the pulp in the
contactor for a desired production rate; and regulating a
reaction rate between the gaseous bleaching reagent and the
pulp. The regulating step comprises the steps of:
mixing the quantity of fresh bleaching gas F with a
controlled quantity of recirculated untreated contacting gas
R from a gas outlet of the contactor to create a mixture of
gases F + R having a second concentration of the gaseous
bleaching reagent in the carrier gas, the second
concentration being less than the first concentration; and
introducing the mixture of gases F + R into the contactor
along with the pulp, wherein the step of introducing causes
a reduced reaction rate of the gaseous bleaching reagent and
the pulp during an initial portion of the determined contact
time relative to that which would occur during the same
period of time with the wood pulp and the bleaching gas
having the first concentration.
Another aspect of the present invention is directed to
an improvement in combination with a mechanically agitated
~ME~DE~ E~-T
v
21~1813
~ J ' , _ ~
4a
contactor of a wood pulp bleaching apparatus in which wood
pulp passes through a fluffer to be fluffed, gaseous
bleaching reagent is introduced into the fluffer with the
wood pulp, and fluffed pulp and gaseous reagent passes
through the mechanically agitated contactor. The
improvement comprises means for mixing fresh gaseous
bleaching reagent in its carrier gas F with a controlled
quantity of recirculated untreated contacting gas R, from a
gas outlet of the contactor, and for introducing the mixture
F + R into the contactor.
The foregoing and other aspects will become apparent
from the following detailed description of the invention
when considered in conjunction with the accompanying drawing
flgures.
BRIEF DESCRIPTION OF THE DRAWING FIGURES
Fig. 1 is a sectional elevation view schematically
illustrating one embodiment of a typical high consistency
Wo ~/1~18 PCT~Sg3/12~5
21~1813
ozone bleaching reactor of the prior art;
Fig. 2 is a fragmentary sectional elevation view this
time illustrating an embo~ir~nt of the bleaching apparatus
disclosed in co-pending U.S. application number 07/953,321;
Fig. 3 is a fragmentary view, similar to Figs. 1 & 2,
in which one embodLment of the present invention is
illustrated;
Fig. 4 is a graphic representation of the relationship
among ozone concentration, retention time of ozone on pulp
fiber, ozone consumption, and degree of dilution of the
ozone concentration;
Fig. 5 is another graph, similar to Fig 4, representing
the relationship between ozone concentration, dilution rate,
retention time, and ozone consumption per pulp unit weight;
and
Fig. 6 is another fragmentary sectional elevation view
showing an alternative arrangement of the gas handling
system of the present invention.
D~TAII.ED D~SCRIPTION
To avoid confusion, all detailed description will be
conducted with reference to ozone gas even though other
ga~eous ble~ch;~g reagents, such as chlorine monoxide,
chlorine dioxide~ and others are possible. Moreover, the
expression "bleAch;~g gas" will refer to the mixture of
ozone in carrier gas (oxygen) plus the other gases and
vapors present, at equilibrium, in various sections of the
reactor apparatus. This is the same as the "contacting gas"
previously described.
Fig. 1 shows a typical high consistency bleaching
reactor of the current or prior art. Pulp 25 is supplied at
30% to 45% consistency from a dewatering press 20 and is
compacted in a screw feeder 30 to form a gas-tight plug 32.
~CT, ~S 3 . ~ i ~ 1 3 S
215 p~ I ~r
Plug 32 prevents backward flow of bleaching reagent gas
through the screw feeder 30. The leading face of pulp plug
32 enters fluffer 40, at the top of bed reactor 50, where it
is fluffed and deposited on pulp bed 52. Ozone inlet 75,
through which the ozone bleaching gas is admitted is near
the fluffer outlet at the top of the bed reactor. The
bleaching gas at inlet 75 is about 6% concentration of ozone
in oxygen carrier gas coming out of ozone generator 70.
After flowing downward through pulp bed 52 and reacting with
the pulp there, the bleaching gas concentration is usually
of the order of 0.1% ozone, or less, and the reaction rate
is almost zero. The gas exits the bed reactor 50 through
oxygen carrier gas return 60 and passes into oxygen cleaner
and dryer 65 where vapors and reaction product gases are
removed from the oxygen. From oxygen cleaner and dryer 65,
the oxygen passes through feed tube 66 to ozone generator
70. Make-up oxygen is supplied through oxygen tube 67 into
feed tube 66 to replace any oxygen consumed in the bleaching
reaction, consumed in ozone generation, or otherwise lost in
circulating through the reactor. Below the dirty carrier
gas return 60, the pulp continues downward to where it is
diluted to about 4% consistency, by adding liquor through
dilution inlet 100, and removed from the bed reactor 50
through pulp outlet 110. Because of the very aggressive
nature of ozone at a 6% concentration, and because of the
short exposure time (about 1 second or less) of the pulp to
the bleaching gas between the fluffer and the bed reactor;
only about 30 - 50~ of the available ozone is consumed
before the pulp is deposited on the pulp bed 52.
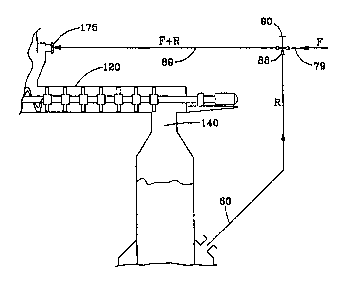
The reactor of Fig. 2 provides a contactor 120 between
the fluffer 40 and bed reactor 50. Up to the point where
the pulp enters fluffer 40, the operation is identical to
that of the reactor in Fig. 1. However fluffer 40, now
located at the inlet of contactor 120, has inlet 175 through
~MENGE~ tET
-C.T '~S ~ 2 1 3 5
2 1 S l ~ 3 ~
which bleaching gas is admitted from ozone generator 70.
The gas recycling is the same as described for Fig. 1 except
that the replenished gas is supplied to the fluffer 40 at
the inlet of contactor 120. Fluffed pulp particles fall
into contactor feed chamber 45 below which is auger 125 to
transport the pulp into and through mixer 120. Even without
any other driving force, the auger 125 would keep the pulp
moving. Paddles 135 are intended for agitating the pulp to
improve uniformity of exposure of the pulp to the bleaching
gas. The paddles 135 also may have a bias so they drive the
pulp forward in contactor 120 toward discharge 140 where it
falls onto pulp bed 52 in bed reactor 50. This bed reactor
has no bleaching gas inlet except through discharge 140,
contactor 120, feed chamber 45, and fluffer 40 as fed by
inlet 175. A great deal of the ozone present reacts before
the pulp enters the bed reactor 50. Since the ozone
concentration in the bleaching gas is reduced, the reaction
speed is decreased, and there is more time for dissolution
and diffusion of ozone to occur. The bleaching reaction is,
therefore, more uniform. Although this represents an
improvement, it does not completely eliminate non-uniformity
of color and fiber damage due to excessive bleaching.
Fig. 3, in conjunction with Figs. 4 & 5, illustrates an
embodiment of the invention in which the operation is
identical up to the point where the pulp enters fluffer 40.
In this case, however, fluffer 40 receives a mixture of
fresh bleaching gas (which may come from a dryer/cleaner and
generator, or it may be supplied from cylinders or some
other source as determined by economics), together with a
quantity of untreated contacting gas taken from the gas
outlet of the contactor 120, through gas inlet 175. Note
that this embodlment does not ~ecessarily include a bed type
reactor as is shown in Fig. 3. I~ one were desired, it
would be incorporated substantlally as described.
"C~!'l~ " -` ~ 1 2 1 3 5
2 1 ~ 1 8 1 3 03 R~?~
Fluffer 40 and contactor 120 operate as described
above. Here, however, recirculating gas R (untreated
contacting gas), is fed through pipe 88 from gas outlet 87
and is mixed with fresh gas F in pipe 79 in proportioning
valve 90. (Fresh gas F has typically passed through
cleaner/dryer 65 and ozone generator 70 or has been supplied
from some other source.) The gas mixture F + R travels
through pipe 89 and enters fluffer 40 through gas inlet 175.
Proportioning valve 90 regulates the relative amounts of
recirculating gas R and fresh gas F in the mixture going to
fluffer 40, thereby controlling the total volume and initial
concentration of gaseous bleaching reagent acting upon the
pulp in the system.
Ozone generator 70 is operated to produce the amount of
fresh gas F (For example, 6% ozone in oxygen carrier gas)
required for bleaching the pulp at the desired pulp feed
rate.
Recirculating gas R dilutes the fresh gas F and reduces
the bleaching reaction rate, thereby mitigating the degree
of non-uniformity of the bleaching reaction in contactor
120. This is better illustrated by reference to Fig 4, in
which the relationship between ozone concentration,
recirculation rate, and pulp residence time in contactor 120
is qualitatively illustrated for recirculation rates of 0,
1, 4, and 10 times the flow rate of the fresh gas. It is
clear that recirculation flow rates up to 10 times the flow
rate of fresh gas produce remarkably little change in ozone
consumption in contactor 120, but they do reduce reaction
rates significantly during the first few seconds of exposure
WO ~/1~18 2 1 5 1 8 1 3 PCT~S93/~
of the pulp to the gas and thereby significantly reduce the
danger of overbleaching otherwise experienced.
More than 50% of the initial ozone concentration, as
seen in Fig. 4, is consumed during the first part of the
reaction with a recirculation rate of 0, while a
recirculation rate of 4 times the flow rate of fresh gas
yields a consumption of about half of that quantity in the
same tIme period. After a short time, the rate of change of
ozone concentration is virtually identical regardless of
recirculation rate.
This behavior is even more clearly seen in Fig. 5, in
which the ozone tran~fer rate is plotted as a function of
re-~idence time for recirculation rates of 0, l, 4, and lO
times the fresh gas flow rate. In this case, the rate of
change of the ozone transfer rate is virtually identical for
all recirculation ratios after even less time than described
for Fig. 4.
The result of the behavior seen in Figs. 4 & 5 is that,
since the initial reaction rates are slower with higher
proportions of recirculation gas added to the fresh gas,
pulp degradation due to overbleaching is significantly
reduced; because the ozone has time to diffuse into the
interior portion of the fibers and flocs to react with the
lignin there. Since ozone concentration and consumption
rate decrease only slightly from beginning to end of the
residence of the pulp in contactor 120 at high recirculation
rates, gas channeling is a less important cause of
non-uniform bleaching than it is without such recirculation.
If ble~ch;ng efficiency is defined as brightness increase or
30 kappa number reduction per unit of applied ozone, bleaching
efficiency is higher at lower ozone concentrations.
Recirculation also provides multiple pass reaction
WO ~/1~18 2 1 5 1 8 1 3 PCT~S93/~5
opportunities for unreacted ozone in the contacting gas. Of
course, these benefits are more pronounced at higher
temperatures because reaction rates are higher at elevated
temperatures. It should be noted that counter-current or
co-current flow is contemplated for this application. The
determination depends on pulp characteristics and on mill
operating constraints.
In addition to the above-mentioned benefits, gas
recirculation permits adjustment of the total gas flow rate
without changing the amount of ozone entering the reactor.
This allows variation of total gas flow to adjust contact
time of pulp with gas through contactor 120 without
deviating from the ozone quantity required by the bleAch; ng
reaction stoichiometry.
Fig. 6 shows another arrangement of the system in which
a fluffer/blower 240 is mounted apart from the contactor 120
and in which recirculation to fluffer/blower 240 can be
adjusted independen~ly of the recirculation through the
contactor. Fluffer/blower 240 feeds fluffed pulp to cyclone
140 above the inlet of contactor 120 by blown gas transport.
The blowing gas is made up of fresh gas from the
cleaner/dryer 65 and ozone generator 70, recirculating gas
taken from gas outlet 87 of contactor 120, and additional
recirculating gas ta~en from port 187 of cyclone 140.
Recirculation from cyclone 140 permits supply of adequate
blowing gas for operation of the fluffer/blower
independently of requirements of the contactor operation.
This invention may also be applied in conjunction with
bed type reactors as shown in Figs. 1 & 2, but it is
preferable to complete the bleaching reaction in the
agitated contactor in which the highest degree of uniformity
of ble~ch;ng is attained. In combination with the above, it
~T~IJS~ 213 5
21S1~13 03 Re~ rT~ 0~ 1
11
improves performance of pulp bleaching systems by providing
adjustability of bleaching gas flow and concentration
without sacrifice of efficiency. The required amount of
ozone is supplied for the pulp production rate, but its
concentration is decreased to reduce the initial reaction
rate with the pulp. Pulp degradation due to overbleaching
is greatly reduced and bleaching uniformity is improved
without any decrease of production rate. Gas flow is thus
adjustable for purposes of concentration adjustment and
total reagent contact time.
Depending upon pulp characteristics and operating
constraints, the bleaching contacting gas may flow co-
currently or counter-currently through the contactor with
the pulp. Of course a counter-current reagent flaw can
retard flow of the pulp to a greater or lesser degree,
depending on the volumetric flow rate of the gas and the
size of the contactor chamber.
~lC~,F~Ht~-,