Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
21540~1
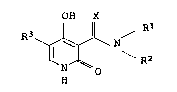
Hydroxypyridonecarboxamides, their preparation and use
The invention relates to hydroxypyridonecarboxamides of the
5 general formula I
OH X
R3 ~J~ ~ ( I)
N
15 where the substituents have the following meanings:
X is oxygen or sulfur;
R1 is hydrogen, C1--C10--alkyl,C3--Clo--alkenyl, C3--Clo--alkynyl~~0 C3-C8-cycloalkyl or phenyl, it being possible for these groups
to carry one to five halogen atoms and/or one to three of the
following radicals: cyano, C3--C8--cycloalkyl, Cl--C4--alkoxy,
Cl--C4--haloalkoxy, Cl--C4--alkylthio, Cl--C4--haloalkylthio,di--
Cl--C4--alkylamino, phenyl, phenylthio or phenoxy, it being
possible for the phenyl radicals in turn to carry one to
three of the following groups: Cl--C4--alkyl, Cl--C4--alkoxy~
Cl--C4--haloalkyl, C1--C4--haloalkoxy,halogen, cyano or nitro;
R2 is hydrogen, hydroxyl, Cl--C8--alkoxy,C3--C8--alkenyloxy,di--
Cl--C4--alkylamino, C1--C10--alkyl,C3--Clo--alkenyl, C3--Clo--alkynyl
or C3--C8--cycloalkyl, it being possible for the groups to carry
one to five halogen atoms andtor one to three of the
following radicals: cyano, C3--C8--cycloalkyl, Cl--C4--alkoxy,
Cl--C4--haloalkoxy, C1~4--alkylthio,Cl--C4--haloalkylthio,
di-C1-C4-alkylamino, phenyl, phenylthio or phenoxy, it being
possible for the phenyl radicals in turn to carry one to
three of the following groups: C1-C4-alkyl, C1-C4-alkoxy,
C1-C4-haloalkyl, C1-C4-haloalkoxy, halogen, cyano or nitro;
40 R2 further is substituted Cl--C8--alkoxy,C3--C8--alkenyloxy,
di-C1--C4--alkylamino,C1--Cl0--alkyl,C3--Clo--alkenyl,
C3--Clo--alkynyl or C3--C8--cycloalkyl, these groups being
substituted by one to five halogen atoms and/or one or two
5- to 6-membered heterocyclic, aliphatic or aromatic
radicals, containing one to three heteroatoms selected from
the group consisting of oxygen, sulfur and nitrogen, which
"" - 21540 11
can carry one or two of the following substituents: halogen,
Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkyl or Cl-C4-haloalkoxy;
or
Rl and R2 together are an alkylene chain having 4 to ~ members,
which can be interrupted by oxygen, sulfur or N-methyl;
R3 is hydrogen, halogen, nitro, Cl-C10-alkyl, C2-C10-alkenyl,
C2--Clo--alkynyl, C3--C8-~ycloalkyl, C7--Cl5--arylalkyl,
Cl-C6-alkoxy, Cl-C6-haloalkoxy, Cl-C6-alkylthio,
Cl-C6-haloalkylthio, di-Cl-C6-alkylamino, phenyl, phenylthio,
phenoxy or a 5- or 6-membered heterocyclic, aliphatic or
aromatic radical containing one to three heteroatoms selected
from the group consisting of oxygen, sulfur and nitrogen,
said aromatic or heteroaromatic radicals being unsubstituted
or substituted by halogen, cyano, nitro, Cl-C4-alkyl,
Cl-C4-haloalkyl, C3-C8-cycloalkyl, Cl-C4-alkoxy,
Cl-C4-haloalkoxy, Cl-C4-alkylthio, Cl-C4-haloalkylthio or
di-Cl-C4-alkylamino;
and their environmentally compatible salts.
The invention additionally relates to processes for preparing
25 these compounds, their use as herbicides and for regulating plant
growth and to herbicidal compositions which contain these
compounds as active substances.
Herbicidaily active hydroxypyridonecarboxamides have already been
30 disclosed in EP-A 544 151. Tbe compound~ described there all
carry a substituent, eg. a methyl radical, in the 6-position of
the pyridone ring. Hydroxypyridone-3-carboxamides unsubstituted
in the 6-position were previously unknown.
35 The herbicidal properties and the compatibility to crop plants of
the known compounds are satisfactory, however, only to a limited
extent.
It is an object of the present invention to make available novel
40 hydroxypyridonecarboxamides having improved properties.
We have found that this object is achieved by the compounds
described at the outset, and processes for their preparation and
herbicidal compositions which contain the compounds I.
- ~ls~n4~
Particularly preferred hydroxypyridonecarboxamides are those
where X = oxygen. The radical R1 is preferably hydrogen.
R2 is preferably hydrogen, C1-Cl0-alkyl, C3-C10-alkenyl,
C3-Cl0-alkynyl, C3-C8-cycloalkyl or Cl-C8-alkoxy, it being
possible for said radicals to be substituted by halogen,
Cl-C4-alkoxy, Cl-C4-haloalkoxy or phenyl, it being possible
for the phenyl radical in turn to carry one to three of the
following groups:
Cl-C4-alkyl, Cl-C4-alkoxy, C1-C4-haloalkyl, halogen or
Cl-C4-haloalkoxy;
R3 is hydrogen, Cl-Cl0-alkyl, Cl-Cl0-haloalkyl, C2-Cl0-alkenyl,
C2-Cl0-haloalkenyl, C2-Cl0-alkynyl, C3-Cl0-cycloalkyl,
C7-Cl5-arylalkyl or halogen.
The hydroxypyridonecarboxamides I can exist in the following
tautomeric formulae:
OH O OH X
R8 ~ ~ R2 ~ R3 ~ ~ N
H H
-
~y'~bO R R ~ N
The hydroxypyridonecarboxamides I are prepared by reaction of
compounds of the formula II
: ` - 21~0 11
OH X
~ ~ Y (II)
~ N
H
where X and R3 have the meanings indicated in claim 1 and Y is a
10 halogen atom, an OH group or an alkoxy group, with an amine of
the formula III
H2N ~ Rl (III)
where Rl and R2 have the meanings indicated above, under suitable,
20 generally known conditions.
Particularly advantageously, alkyl esters IIa (Y = O-alkyl), eg.
methyl or ethyl esters, are reacted with the amines of the
general formula III.
This amidation is in general carried out in the temperature range
50 - 250 C, preferably 110 - 160 C, in a stirring process or in an
autoclave.
30 Suitable solvents are alcohols such as methanol, ethanol or
propanol, and hydrocarbons such as benzene or toluene, water,
ethers such as diethyl ether or tetrahydrofuran, and also aprotic
dipolar solvents such as dimethylformamide, dimethyl sulfoxide or
pyridine.
Mixtures of these substances can also be used as solvents and
diluents.
The starting substances are customarily reacted in stoichiometric
40 amounts. It may be advantageous, for example to increase the
yield, to use one of the starting substances, preferably the
amine, in an excess, eg. in an amount from 0.1 to 10 mol
equivalents, based on II.
45 The amidation is in general carried out at atmospheric pressure.
However, depending on the type of amine or solvent used, it may
be advantageous to carry out the reaction at elevated pressure,
21540~ ~
,,
in particular under autogenously elevated pressure, in an
autoclave.
The starting compounds of the formula IIa can be prepared by
5 generally known chemical processes ~J. Org. Chem. 51 (1986),
1374ff) from aminoacrylic esters IV and malonic esters V.
The intermediates of the general formula IIa are additionally
obtained by thermal cyclization of the acylation products VII
10 obtained from vinyl isocyanates VI and malonic esters V by known
chemical processes (J. Heterocyclic Chem., 22 ~1985), 985ff).
NH2 0 0
R4 ~ + R4~ Jl ~ O~
`o O
2~ ~3 ~ ~ /
H IIa
o O
~ `NCO ~ R4~ ~ ~ ~ R4 ~ O
VI V VII
R3
35 The aminoacrylic esters IV required for the reactions are known
or can be prepared by known chemical processes (Annales de Chimie
18 (1932), 81ff).
The vinyl isocyanates VI are likewise known or can be prepared by
40 known chemical processes (Angew. Chem. 91 (1979), 334ff; J. Chem.
Soc. Perkin Trans. I (1988), 2185ff).
The amines III and malonic esters V required for the reactions
are known, commercially available or can be prepared by generally
45 known chemical processes.
"` - 21~40~1
On account of their acidic character, the hydroxypyridone-
carboxamides I according to the invention can form salts of
alkali metal or alkaline earth metal compounds and also
tetraalkylammonium salts, benzyltrialkylammonium salts,
5 trialkylsulfonium salts or trialkylsulfoxonium salts.
Alkali metal salts of the compounds I can be obtained by treating
I with sodium hydroxide or alkoxide or potassium hydroxide or
alkoxide in aqueous solution or in an organic solvent such as
10 methanol, ethanol, acetone or toluene.
Other metal salts, eg. the manganese, copper, zinc, iron,
calcium, magnesium and barium salts or salts with organic
cations, can be prepared from the sodium salts in a customary
15 manner.
R4 in the formulae IIa, IV, V and VII i9 Cl-C6-alkyl such as
methyl, ethyl, propyl, 1-methylethyl, butyl, 1-methylpropyl,
2-methylpropyl, 1,1-dimethylethyl, pentyl, 1-methylbutyl,
2-methylbutyl, 3-methylbutyl, 1,1-dimethylpropyl,
1,2-dimethylpropyl, 2,2-dimethylpropyl, l-ethylpropyl, hexyl,
1-methylpentyl, 2-methylpentyl, 3-methylpentyl,
4-methylpentyl, l,l-dimethylbutyl, 1,2-dimethylbutyl,
1,3-dimethylbutyl, 2,2-dimethylbutyl, 2,3-dimethylbutyl,
3,3-dimethylbutyl, l-ethylbutyl, 1,1,2-trimethylpropyl,
1,2,2-trimethylpropyl, 1-ethyl-1-methylpropyl or
1-ethyl-2-methylpropyl, in particular methyl and ethyl.
With respect to the intended use of the hydroxypyridone-
30 carboxamides of the general formula I, suitable substituents are
the following radicals:
Rl is hydrogen;
unbranched or branched C1-C10-alkyl, such as methyl, ethyl,
propyl, 1-methylethyl, butyl, 1-methylpropyl, 2-methylpropyl,
1,1-dimethylethyl, pentyl, l-methylbutyl, 2-methylbutyl,
3-methylbutyl, 1,1-dimethylpropyl, 1,2-dimethylpropyl,
2,2-dimethylpropyl, 1-ethylpropyl, hexyl, 1-methylpentyl,
2-me~hylpentyl, 3-methylpentyl, 4-methylpentyl,
1,1-dimethylbutyl, 1,2-dimethylbutyl, 1,3-dimethylbutyl,
2,2-dimethylbutyl, 2,3-dimethylbutyl, 3,3-dimethylbutyl,
1-ethylbutyl, 1,1,2-trimethylpropyl, 1-ethyl-1-methylpropyl,
1-ethyl-2-methylpropyl, heptyl, l-methylheptyl, octyl, nonyl
and decyl,
-` - 2l~n4l
in particular Cl-C6-alkyl such as methyl, ethyl,
l-methylethyl, 1-methylpropyl, 2-methylpropyl,
l,l-dimethylethyl and l,l-dimethylpropyl;
unbranched or branched C3 -C1O-alkenyl such as 2-propenyl,
2-butenyl, 3-butenyl, 1-methyl-2-propenyl,
2-methyl-2-propenyl, 2-pentenyl, 3-pentenyl, 4-pentenyl,
3-methyl-2-butenyl, 1-methyl-2-butenyl, 2-methyl-2-butenyl,
l-methyl-3-butenyl, 2-methyl-4-butenyl, 3-methyl-3-butenyl,
1,1-dimethyl-2-propenyl, 1,2-dimethyl-2-propenyl,
l-ethyl-2-propenyl, 2-hexenyl, 3-hexenyl, 4-hexenyl,
5-hexenyl, 1-methyl-2-pentenyl, 2-methyl-2-pentenyl,
3-methyl-2-pentenyl, 4-methyl-2-pentenyl,
l-methyl-3-pentenyl, 2-methyl-3-pentenyl,
3-methyl-3-pentenyl, 4-methyl-3-pentenyl,
1-methyl-4-pentenyl, 2-methyl-4-pentenyl,
3-methyl-4-pentenyl, 4-methyl-4-pentenyl,
1,1-dimethyl-2-butenyl, 1,1-dimethyl-3-butenyl,
1,2-dimethyl-2-butenyl, 1,3-dimethyl-3-butenyl,
2,2-dimethyl-3-butenyl, 2,3-dimethyl-2-butenyl,
2,3-dimethyl-3-butenyl, 1-ethyl-2-butenyl, 1-ethyl-3-butenyl,
2-ethyl-2-butenyl, 2-ethyl-3-butenyl,
1,1,2-trimethyl-2-propenyl, 1-ethyl-1-methyl-2-propenyl and
ethyl-2-methyl-2-propenyl,
in particular l-methyl-2-propenyl, 1-methyl-2-butenyl,
1,1-dimethyl-2-propenyl and 1,1-dimethyl-2-butenyl,
C3 -C1O-alkynyl such as propargyl, 2-butynyl, 3-butynyl,
2-pentynyl, 3-pentynyl, 4-pentynyl, 1-methyl-3-butynyl,
2-methyl-3-butynyl, 1-methyl-2-butynyl,
1,1-dimethyl-2-propynyl, 1-ethyl-2-propynyl, 2-hexynyl,
3-hexynyl, 4-hexynyl, 5-hexynyl, 1-methyl-2-pentynyl,
1-methyl-3-pentynyl, 1-methyl-4-pentynyl,
3-methyl-4-pentynyl, 4-methyl-2-pentynyl,
1,1-dimethyl-2-butynyl, 1,1-dimethyl-3-butynyl,
1,2-dimethyl-3-butynyl, 2,2-dimethyl-3-butynyl,
l-ethyl-2-butynyl, 1-ethyl-3-butynyl, 2-ethyl-3-butynyl and
l-ethyl-l-methyl-2-propynyl;
in particular propargyl and 1,1-dimethyl-2-propynyl;
C3 - C8 - cycloalkyl such as cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, in particular
cyclopropyl and cyclohexyl;
`~ ` 2154041
.
it being pos~ible for the abovementioned organic groups to
carry one to five halogen atoms such as fluorine, chlorine,
bromine and iodine, preferably fluorine, chlorine and
bromine, in particular fluorine and chlorine,
and/or one to three of the following radicals:
cyano;
10 C3 - CB -cycloalkyl such as cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, cyclooctyl, in particular
cyclopropyl and cyclohexyl;
Cl-C4-alkoxy such as methoxy, ethoxy, n-propoxy,
2-methylethoxy, n-butoxy, 1-methylpropoxy, 2-methylpropoxy
and 1,1-dimethylethoxy, in particular Cl-C3-alkoxy such as
methoxy, ethoxy and i-propoxy;
Cl-C4-haloalkoxy such as chloromethoxy, dichloromethoxy,
trichloromethoxy, fluoromethoxy, difluoromethoxy,
trifluoromethoxy, chlorodifluoromethoxy,
dichlorofluoromethoxy, 1-fluoroethoxy, 2-fluoroethoxy,
2,2-difluoroethoxy, 1,1,2,2-tetrafluoroethoxy,
2,2,2-trifluoroethoxy, 2-chloro-1,1,2-trifluoroethoxy and
pentafluoroethoxy, in particular Cl-C3-haloalkoxy such as
2,2,2-trifluoroethoxy and 2-chloro-2,2-difluoroethoxy;
Cl - C4 -alkylthio such as methylthio, ethylthio, n-propylthio,
l-metnylethylthio, n-butylthio, 1-methylpropylthio,
2-methylpropylthio and l,l-dimethylethylthio, in particular
methylthio;
Cl - C4 -haloalkylthio such as difluoromethylthio,
trifluoromethylthio, chlorodifluoromethylthio,
l-fluoroethylthio, 2-fluoroethylthio, 2,2-difluoroethylthio,
2,2,2-trifluoroethylthio, 2-chloro-2,2-difluoroethylthio,
2,2-dichloro-2-fluoroethylthio, 2,2,2-trichloroethylthio and
pentafluoroethylthio, in particular trichloromethylthio;
di-Cl-C4-alkylamino such as dimethylamino, diethylamino,
dipropylamino, dibutylamino, N-methyl-N-ethylamino, N-methyl-
N-propylamino, N-methyl-N-l-methylethylamino, N-methyl-
N-1,1-dimethylethylamino, di-1-methylethylamino, N-ethyl-
N-l-methylethylamino and N-ethyl-N-l,1-dimethylethylamino;
- ~ 21~40~11
g
phenyl, phenylthio or phenoxy, it being possible for the
phenyl radicals in turn to carry one to five halogen atoms
such as fluorine, chlorine, bromine and iodine, in particular
fluorine and chlorine,
and/or one to three of the following groups:
cyano or nitro;
Cl-C4-alkyl, as mentioned above, in particular methyl, ethyl,
propyl, 1-methylpropyl and 1,1-dimethylpropyl;
Cl-C4-alkoxy, as mentioned above, in particular methoxy and
ethoxy;
Cl - C4 - haloalkyl, as mentioned above, in particular
trifluoromethyl;
Cl-C4-haloalkoxy, as mentioned above, in particular
trifluoromethoxy;
R2 is hydrogen, hydroxyl;
Cl-C8-alkoxy, eg. methoxy, ethoxy, n-propoxy, 2-methylethoxy,
n-butoxy, 1-methylpropoxy, 2-methylpropoxy and
1,l-dimethylethoxy, in particular Cl-C4-alkoxy such as
methoxy, ethoxy, propoxy and butoxy;
C3 - C8 - alkenyloxy such as 2-propenyloxy, 2-butenyloxy,
3-butenyloxy, 1-methyl-2-propenyloxy, 2-methyl-2-propenyloxy,
2-pentenyloxy, 3-pentenyloxy, 4-pentenyloxy,
3-methyl-2-butenyloxy, 1-methyl-2-butenyloxy,
2-methyl-2-butenyloxy, 1-methyl-3-butenyloxy,
2-methyl-3-butenyloxy, 3-methyl-3-butenyloxy,
1,1-dimethyl-2-propenyloxy, 1,2-dimethyl-2-propenyloxy,
1-ethyl-2-propenyloxy, 2-hexenyloxy, 3-hexenyloxy,
4-hexenyloxy, 5-hexenyloxy, 1-methyl-2-pentenyloxy,
2-methyl-2-pentenyloxy, 3-methyl-2-pentenyloxy,
4-methyl-2-pentenyloxy, 1-methyl-3-pentenyloxy,
2-methyl-3-pentenyloxy, 3-methyl-3-pentenyloxy,
4-methyl-4-pentenyloxy, 1-methyl-4-pentenyloxy,
2-methyl-4-pentenyloxy, 3-methyl-4-pentenyloxy,
4-methyl-4-pentenyloxy, 1,1-dimethyl-2-butenyloxy,
1,1-dimethyl-3-butenyloxy, 1,2-dimethyl-2-butenyloxy,
1,2-dimethyl-3-butenyloxy, 1,3-dimethyl-2-butenyloxy,
1,3-dimethyl-3-butenyloxy, 2,2-dimethyl-3-butenyloxy,
2,3-dimethyl-2-butenyloxy, 2,3-dimethyl-4-butenyloxy,
215~0~1
1-ethyl-2-butenyloxy, l-ethyl-3-butenyloxy,
2-ethyl-2-butenyloxy, 2-ethyl-3-butenyloxy, 1,1,2-tri-
methyl-2-propenyloxy, 1-ethyl-1-methyl-2-propenyloxy and
ethyl-2-methyl-2-propenyloxy, in particular 2-propenyloxy;
di-Cl-C4-alkylamino, Cl-C10-alkyl, C3-C10-alkenyl and
C3-Cl0-alkynyl and also C3 - C~ -cycloalkyl, in each case as
mentioned above for Rl in general and in particular,
it being possible for said organic groups to carry one to
five halogen atoms such as fluorine, chlorine, bromine and
iodine, preferably fluorine, chlorine and bromine, in
particular fluorine and chlorine,
and/or one to three of the following radicals:
cyano,
C3-C~-cycloalkyl, Cl-C4-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-alkylthio, Cl-C4-haloalkylthio and di-Cl-C4-alkylamino,
in each case as specifically indicated above,
phenyl, phenylthio or phenoxy, it being possible for the
phenyl radicals in turn to carry one to five halogen atoms
such as fluorine, chlorine, bromine and iodine, in particular
fluorine and chlorine,
and/or one to three of the following groups:
cyano or nitro,
Cl-C4-alkyl, Cl-C4-alkoxy, Cl-C4-haloalkyl and
Cl-C4-haloalkoxy, in each case as mentioned above;
35 R2 further is substsituted Cl-C8-alkoxy, C3-C8-alkenyloxy,
di-Cl-C4-alkylamino, Cl-C1O-alkyl, C3-C10-alkenyl,
C3 - Cl o-alkynyl or C3-C8-cycloalkyl, these groups being
substituted by one to five halogen atoms and/or one or two 5-
to 6-membered heterocyclic, aliphatic or aromatic radicals,
containing one to three heteroatoms selected from the group
consisting of oxygen, sulfur and nitrogen; examples which may
be mentioned are the following heterocyclic radicals:
2-tetrahydrofuranyl, 3-tetrahydrofuranyl, 2-tetrahydro-
thienyl, 3-tetrahydrothienyl, 2-tetrahydropyranyl,
3-tetrahydropyranyl, 4-tetrahydropyranyl, 2-furyl, 3-furyl,
2-thienyl, 3-thienyl, 2-pyrrolyl, 3-pyrrolyl, 3-isoxazolyl,
4-isoxazolyl, 5-isoxazolyl, 3-isothiazolyl, 4-isothiazolyl,
215~0~ 1
11
5-isothiazolyl, 3-pyrazolyl, 4-pyrazolyl, 5-pyrazolyl,
2-oxazolyl, 4-oxazolyl, 5-oxazolyl, 2-thiazolyl, 4-thiazolyl,
5-thiazolyl, 2-imidazolyl, 4-imidazolyl,
1,2,4-oxadiazol-3-yl, 1,2,4-oxadiazol-5-yl,
1,2,4-thiadiazol-3-yl, 1,2,4-thiadiazol-5-yl,
1,2,4-triazol-3-yl, 1,3,4-oxadiazol-2-yl,
1,3,4-thiadiazol-2-yl, 1,3,4-triazol-2-yl, 2-pyridinyl,
3-pyridinyl, 4-pyridinyl, 3-pyridazinyl, 4-pyridazinyl,
2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl, 2-pyrazinyl,
1,3,5-tria~in-2-yl and 1,2,4-triazin-3-yl;
it being possible for these heterocyclic radicals to carry
one or two of the following substituents:
halogen such as fluorine, chlorine, bromine and iodine, in
particular fluorine or chlorine, Cl-C4-alkyl, Cl-C4-alkoxy,
Cl-C4-haloalkyl or Cl - C4 -haloalkoxy as mentioned above;
Rl and R2 together are an alkylene chain having 4 to 7
members, which can be interrupted by oxygen, sulfur or
N-methyl, such as -(CH2)4-, -(CH2)5-, -(CH2)6-,
~H2~H2~CH2--CH2--, --CH2--CH2--S{:H2--CH2--,
-CH2-CH2-(NCH3)-CH2-CH2-, in particular -(CH2)5- and
-cH2-cH2-o-cH2-cH2-;
R3 is hydrogen;
halogen such as fluorine, chlorine, bromine and iodine, in
particular fluorine or chlorine;
nitro;
Cl-C1o-alkyl, C2-C10-alkenyl, C2-C10-alkynyl, C3-C8-cycloalkyl,
Cl-C6-alkoxy, Cl-C6-haloalkoxy, Cl-C6-alkylthio,
Cl-C6-haloalkylthio, di-Cl-C6-alkylamino, in each case as
mentioned above for Rl in detail;
C7-Cl5-arylalkyl, in particular phenyl-Cl-C4-alkyl such as
benzyl, l-phenylethyl, 2-phenylethyl, 1-phenylpropyl,
2-phenylpropyl, 3-phenylpropyl, 1-methyl-1-phenylethyl,
l-methyl-2-phenylethyl, 1-phenylbutyl, 2-phenylbutyl,
3--phenylbutyl, 4-phenylbutyl, particularly preferably benzyl;
phenyl, phenylthio, phenoxy or a 5- or 6-membered
heterocyclic, aliphatic or aromatic radical containing one to
three heteroatoms selected from the group consisting of
oxygen, sulfur and nitrogen, as specifically mentioned above
215~041
.
12
for R2, said aromatic or heteroaromatic radicals being
unsubstituted or substituted by halogen, cyano, nitro,
Cl-C4-alkyl, Cl-C4-haloalkyl, C3-C8-cycloalkyl, Cl- C4 -alkoxy,
Cl - C4 - haloalkoxy, Cl-C4-alkylthio, Cl- C4 -haloalkylthio or
di-Cl-C4-alkylamino, in each case as specifically mentioned
above for Rl.
Examples of particularly preferred compounds of the general
formula I are compiled in the following Table. The groups
10 mentioned therein for a substituent are additionally considered
per se, independently of the specific combination with other
substituents in which they are mentioned, to be a particularly
preferred definition of the substituents concerned.
15 Table: Compounds of the structure I
OH X
R3 ~ ~ / (I)
N 0
25 I R2 R3 X
H Methoxy Methyl O
H Methoxy Ethyl o
H Methoxy Phenyl o
3 0 H Methoxy H o
H I~opropyl H o
H tert-Butyl H o
H 1, 1 - Dimethyl-2-propenyl H O
H 2-Phenoxyethyl H o
35 H Benzyl H o
H n-Propyl H O
H n-sutyl H o
H Methyl H o
40 H Ethyl H o
H 1 - Methyl-l-ethylpropyl H o
H 2-Methyl-n-butyl H o
H l-(Cyclopropyl)ethyl H o
H Cyclopropyl H o
45 H l-Phenylethyl H o -
H 2-Phenylethyl H o
`` ` ~154041
13
Rl R2 R3 X
H 1 - ( 3-Thienyl)ethyl H O
H 1 - ( Methoxymethyl)ethyl H O
H 1,1, 3,3-Tetramethylbutyl H O
H 1 - Ethylpropyl H O
H Cyclopropylmethyl H O
H Cyclopentyl H O
H Cyclohexyl H o
10 H 1-Methylpropyl H o
H 2-Methylpropyl H O
H 2-Methylbutyl H O
H 1 - Methylbutyl H O
H 4 - Phenylbutyl H O
15 H 1,1-Dimethyl-2-propynyl H O
H 2-~Dimethylamino)-l-methyl- H o
ethyl
H 1-Methyl-2-methylthioethyl H O
H Decyl H o
H 2-Propynyl H O
H 1-Methyl-2-butynyl H O
H 1-ffethyl-1-phenylethyl H O
H 2-Methylthioethyl H o
~5 H 2-Fluoroethyl H O
H 2-Fluoro-l-methylethyl H O
1,1-Dimethyl-2-fluoroethyl H O
H 2-Chloroethyl H O
H 2-Chloro-1-methylethyl H O
H 2-Chloro-1,1-dimethylethyl H O
H 2-Cyanoethyl H o
H 2-Cyano-l,l-dimethylethyl H o
H Dimethylamino H O
35 H 2-Chlorobenzyl H o
H 2 - Fluorobenzyl H O
H 3-Fluorobenzyl H O
H 3-(Trifluoromethyl)benzyl H O
H 4-Methoxybenzyl H O
H 2, 2 - Dimethylbutyl H O
H l, l - Dimethylbutyl H O
H l,l-Dimethylpentyl H O
H 1-Methylcyclopropyl H O
H 1 - ( 2-Fluorophenyl)ethyl H O
H 1-(4-~ethoxyphenyl)ethyl H O
` ~ 21~40~1
14
Rl R2 R3 X
H 1 - ( 3-Trifluoromethylphenyl) H O
ethyl
H l - ( 4-Ethylphenyl~ethyl H 0
5 H Cyclohexylmethyl H O
H Methoxy Methyl O
H Ethoxy Methyl o
H Benzyloxy Methyl O
lO H I~opropyl Methyl O
H tert-Butyl Methyl O
H l, 1 - Dimethyl-2-propenyl Methyl O
H 2-Phenoxyethyl Methyl O
H ~enzyl Methyl O
15 H n-Propyl Methyl O
H n-Butyl Methyl O
H Methyl Methyl O
H Ethyl Methyl O
20 H 1 - Methyl-1-ethylpropyl Methyl O
H 2-Methyl-n-butyl Methyl O
H 1 - ( Cyclopropyl)ethyl Methyl
H Cyclopropyl Methyl O
H l - Phenylethyl Methyl O
25 H 2-Phenylethyl Methyl O
H 2-(3-Thienyl)ethyl Methyl o
H l - ( Methoxymethyl)ethyl Methyl O
H 1,1,3,3-Tetramethylbutyl Methyl o
H 1 - Ethylpropyl Methyl O
H Cyclopropylmethyl ~ethyl O
H Cyclopentyl Methyl O
H cyclohexyl Methyl o
H 1-Methylpropyl Methyl o
3 5 H 2-Methylpropyl Methyl o
H 2-Methylbutyl Methyl o
H 3-Methylbutyl Methyl o
H 4-Phenylbutyl Methyl o
H 1,1 - Dimethyl-2-propynyl Methyl o
H 2-(Dimethylamino)- Methyl O
l-methylethyl
H l-Methyl-2-methylthioethyl Methyl o
H Decyl Methyl o
H 2-Propynyl Methyl O
45 H l-Methyl-2-butynyl Methyl O
H l-Methyl-l-phenylethyl Methyl o
2 1 ~
R1 R2 R3 X
H 2-Methylthioethyl Methyl O
H 2-Fluoroethyl Methyl O
H 2-Fluoro-l-methylethyl Methyl O
H 1,1-Dimethyl-2-fluoroethyl Methyl O
H 2-Chloroethyl Methyl O
H 2-Chloro-1-methylethyl Methyl o
H 2-Chloro-l,l-dimethylethyl Methyl O
10 H 2-Cyanoethyl Methyl O
H 2-Cyano-1,1-dimethylethyl Methyl o
H Dimethylamino Methyl O
H 2-Chlorobenzyl Methyl O
H 2-Fluorobenzyl Methyl O
15 H 3-Fluorobenzyl Methyl o
H 3-(Trifluoromethyl)benzyl Methyl o
H 4-Methoxybenzyl Methyl O
H 2,2-Dimethylbutyl Methyl O
20 H l,l-Dimethylbutyl Methyl O
H l,l-Dimethylpentyl Methyl O
H l-Methylcyclopropyl Methyl O
H 1-(2-Fluorophenyl)ethyl Methyl o
H 1-(3-Trifluoromethylphenyl)- Methyl o
ethyl
H 1-(4-Methoxyphenyl)ethyl Methyl O
H 1-(4-Ethylphenyl)ethyl Methyl O
Methyl Methyl Methyl O
Methyl Ethyl Methyl o
Methyl Isopropyl Methyl O
Methyl tert-Butyl Methyl O
Methyl ~enzyl Methyl o
Ethyl Ethyl Methyl O
35 Ethyl I~opropyl Methyl O
~thyl tert-Butyl Methyl O
Ethyl Benzyl Methyl O
Benzyl ~enzyl Methyl O
H I~opropyl Ethyl Q
H tert-Butyl Ethyl O
H 1,1-Dimethyl-2-propenyl Ethyl o
H l-(Cyclopropyl)ethyl Ethyl O
H Cyclopropyl Ethyl O
45 H l-Phenylethyl Ethyl O
H l-(Methoxymethyl)ethyl Ethyl O
H Isopropyl Benzyl O
2ls4n il
i
16
R1 R2 R3 X
H tert-Butyl Isopropyl 0
H 1,1-Dimethyl-2-propenyl Isopropyl 0
H 1-(Cyclopropyl)ethyl Isopropyl 0
H Cyclopropyl I~opropyl 0
H l-Phenylethyl Isopropyl 0
H l-(Methoxymethyl)ethyl I~opropyl 0
H Isopropyl Phenyl 0
10 H tert-Butyl Phenyl 0
H 1,1-Dimethyl-2-propenyl Phenyl 0
H l-(Cyclopropyl)ethyl Phenyl 0
H Cyclopropyl Phenyl 0
H l-Phenylethyl Phenyl 0
15 H l-(Methoxymethyl)ethyl Phenyl o
H H Methyl 0
H H Ethyl 0
H H I~opropyl 0
20 H H Phenyl o
H H H 0
H Isopropyl Methyl S
H tert-Butyl Methyl S
H 1,1-Dimethyl-2-propenyl Hethyl S
25 H l-(Cyclopropyl)ethyl Hethyl S
H Cyclopropyl Methyl S
H l-Phenylethyl Hethyl S
H l-(Methoxymethyl)ethyl Methyl S
30 H I~opropyl Benzyl 0
H tert-Butyl Benzyl 0
H I~opropyl Trifluoromethyl 0
H tert-Butyl Trifluoromethyl 0
H I~opropyl 3-Chlorophenyl 0
35 H tert-Butyl 3-Chlorophenyl o
H I~opropyl 3-Methylisoxazol-5-yl 0
H tert-Butyl 3-Methyli~oxazol-5-yl 0
H I~opropyl 3-Thienyl 0
40 H tert-Butyl 3-Thienyl 0
H Isopropyl 2-M;thyl-1,3, 4 - O,YA~ i azol- 0
H tert--Butyl 2-Methyl-1,3, 4 - OYA~; A ZO 1 - O
5-yl
45 H I~opropyl 3-Pyridyl 0
H tert-Butyl 3-Pyridyl 0
` ` 21~40 11
- 17
R1 R2 R3 X
H Isopropyl Cyclopropyl O
H tert-~utyl Cyclopropyl O
The compounds I or the herbicidal compositions containing them
and their environmentally compatible salts of, for example,
alkali metals, alkaline earth metal~ or amines or the herbicidal
compositions containing them can control broad-leaved weeds and
10 grass weeds highly effectively in crops such as wheat, rice,
maize, soybeans and cotton without noticeably damaging the crop
plants. This effect occurs especially at low application rates.
Taking into account the versatility of the application methods,
1 the compounds I or compositions containing them can also be
employed in a further number of crop plants for the elimination
of undesired plants. Examples of suitable crops are the
following:
Allium cepa, Ananas comosus, Arachis hypogaea, Asparagus
officinalis, Beta vulgaris spp. altissima, Beta vulgaris spp.
rapa, ~rassica napus var. napus, Brassica napus var.
napobrassica, Brassica rapa var. silvestris, Camellia sinensis,
Carthamus tinctorius, Carya illinoinensis, Citrus limon, Citrus
sinensis, Coffea arabica (Coffea canephora, Coffea liberica),
Cucumis sativus, Cynodon dactylon, Daucus carota, Elaeis
guineensis, Fragaria vesca, Glycine max, Gossypium hirsutum,
(Gossypium arboreum, Gossypium herbaceum, Gossypium vitifolium)~
Helianthus annuus, Hevea brasiliensis, Hordeum vulgare, Humulus
lupulus, Ipomoea batatas, Juglans regia, Lens culinaris, Linum
usitatissimum, Lycopersicon lycopersicum, Malus spp., Manihot
esculenta, Medicago sativa, Musa spp., Nicotiana tabacum (N.
rustica), Olea europaea, Oryza sativa, Phaseolus lunatus,
Phaseolus vulgaris, Picea abies, Pinus spp., Pisum sativum,
Prunus avium, Prunus persica, Pyrus communis, Ribes sylvestre,
Ricinus communis, Saccharum officinarum, Secale cereale, Solanum
tuberosum, Sorghum bicolor (S. vulgare), Theobroma cacao,
Trifolium pratense, Triticum aestivum, Triticum durum, Vicia
faba, Vitis vinifera, Zea mays.
O Moreover, the compounds I can also be employed in crops which are
tolerant to the action of I or other herbicides as a resul-t of
breeding and/or use of genetic engineering methods.
The application of the herbicidal compositions or of the active
5 compounds can be carried out preemergence or postemergence. If
the active compounds are less tolerable for certain crop plants,
application techniques can be used in which the herbicidal com-
positions are sprayed with the aid of the spray equipment such
" 21~4041
.
18
that the leaves of the sensitive crop plants are not affected if
possible, while the active compounds reach the leaves of
undesired plants growing under them or the uncovered soil surface
(post-directed, lay-by).
The compounds I or the herbicidal compositions containing them
can be applied, for example, by spraying, atomizing, dusting,
scattering or watering in the form of directly sprayable aqueous
solutions, powders, suspensions, even high-percentage aqueous,
lO oily or other suspensions or dispersions, emulsions, oil disper-
sions, pastes, dusting compositions, scattering compositions or
granules. The application forms depend on the intended uses; if
possible they should in each case guarantee the finest dispersion
of the active compounds according to the invention.
Suitable inert auxiliaries for the preparation of directly spray-
able solutions, emulsions, pastes or oil dispersions are essen-
tially: mineral oil fractions of medium to high boiling point
such as kerosene or diesel oil, further coal tar oils and oils of
20 vegetable or ~n;~l origin, aliphatic, cyclic and aromatic
hydrocarbons, eg. paraffins, tetrahydronaphthalene, alkylated
naphthalenes or their derivatives, alkylated benzenes and their
derivatives, alcohols such as methanol, ethanol, propanol,
butanol and cyclohexanol, ketones such as cyclohexanone or
25 strongly polar solvents, eg. amines such as N-methylpyrrolidone,
or water.
Aqueous application forms can be prepared from emulsion concen-
trates, suspensions, pastes, wettable powders or water-
30 dispersible granules by addition of water. To prepare emulsions,pastes or oil dispersions, the substrates can be homogenized in
water, as such or dissolved in an oil or solvent, by means of
wetting agents, adhesives, dispersants or emulsifiers. However,
concentrates consisting of active substance, wetting agent,
35 adhesive, dispersant or emulsifier and possibly solvents or oil
which are suitable for dilution with water can also be prepared.
Suitable surface-active substances are the alkali metal, alkaline
earth metal or ammonium salts of aromatic sulfonic acids, eg.
40 lignosulfonic, phenolsulfonic, naphthalenesulfonic and dibutyl-
naphthalenesulfonic acid, as well as of fatty acids, alkyl- and
alkylarylsulfonates, alkyl-, lauryl ether and fatty alcohol
sulfates, and also salts of sulfated hexa-, hepta- and octa-
decanols as well as of fatty alcohol glycol ethers, condensation
45 products of sulfonated naphthalene and its derivatives with
formaldehyde, condensation products of naphthalene or of the
naphthalenesulfonic acids with phenol and formaldehyde,
`~` 21540~1
19
polyoxyethylene octyl phenol ethers, ethoxylated isooctyl-,
octyl- or nonylphenol, alkylphenyl or tributylphenylpolyglycol
ethers, alkylaryl polyether alcohols, isotridecyl alcohol, fatty
alcohol-ethylene oxide condensates, ethoxylated castor oil, poly-
5 oxyethylene alkyl ether or polyoxypropylene alkyl ether, laurylalcohol polyglycol ether acetate, sorbitol esters, lignin-sulfite
waste liquors or methylcellulose.
Powder, scattering and dusting compositions can be prepared by
10 mixing or joint grinding of the active substances with a solid
carrier.
Granule~, eg. coated, impregnated and homogeneous granules, can
be prepared by binding of the active compounds to solid carriers.
15 Solid carriers are mineral earths such as silicic acids, silica
gels, silicates, talc, kaolin, limestone, lime, chalk, bole,
loess, clay, dolomite, diatomaceous earth, calcium sulfate and
magnesium sulfate, magnesium oxide, ground synthetic materials,
fertilizers, such as ammonium sulfate, ammonium phosphate,
20 ammonium nitrate, ureas and vegetable products such as cereal
flour, tree bark meal, wood meal and nutshell meal, cellulose
powder or other solid carriers.
In general, the formulations contain from 0.01 to 95% by weight,
25 preferably from 0.5 to 90% by weight, of active compound. The
active compounds are employed here in a purity of from 90% to
100%, preferably from 95~ to 100% (according to NMR spectrum).
The compounds I according to the invention can ke formulated, for
example, as follows:
I. 20 parts by weight of the compound No. 2.006 are dissolved in
a mixture which consists of 80 parts by weight of alkylated
benzene, 10 parts by weight of the addition product of from 8
to 10 mol of ethylene oxide to 1 mol of oleic acid N-mono-
ethanolamide, 5 parts by weight of calcium salt of dodecyl-
benzenesulfonic acid and 5 parts by weight of the addition
product of 40 mol of ethylene oxide to 1 mol of castor oil.
By pouring out the solution and finely dispersing it in
100,000 parts by weight of water, an aqueous dispersion is
obtained which contains 0.02~ by weight of the active com-
pound.
II. 20 parts by weight of the compound No. 2.006 are dissolved in
a mixture which consists of 40 parts by weight of cyclo-
hexanone, 30 parts by weight of isobutanol, 20 parts byweight of the addition product of 7 mol of ethylene oxide to
1 mol of isooctylphenol and 10 parts by weight of the
21S~O 11
.
addition product of 40 mol of ethylene oxide to 1 mol of
castor oil. By pouring the solution into and finely dis-
persing it in 100,000 parts by weight of water, an aqueous
dispersion is obtained which contains 0.02% by weight of the
active compound.
III.20 parts by weight of the active compound No. 2.006 are di~-
solved in a mixture which consists of 25 parts by weight of
cyclohexanone, 65 parts by weight of a mineral oil fraction
of boiling point from 210 to 280 C and 10 parts by weight of
the addition product of 40 mol of ethylene oxide to 1 mol of
castor oil. By pouring the solution into and finely dis-
persing it in 100,000 parts by weight of water, an aqueous
dispersion is obtained which contains 0.02% by weight of the
active compound.
IV. 20 parts by weight of the active compound No. 2.006 are
thoroughly mixed with 3 parts by weight of the sodium salt of
diisobutylnaphthalene-~-sulfonic acid, 17 parts by weight of
the sodium salt of a lignosulfonic acid from a sulfite waste
liquor and 60 parts by weight of powdered silica gel and
ground in a hammer mill. By finely dispersing the mixture in
20,000 parts by weight of water, a spray mixture is obtained
which contains 0.1% by weight of the active compound.
V. 3 parts by weight of the active compound No. 2.006 are mixed
with 97 parts by weight of finely divided kaolin. In this
way, a dusting composition is obtained which contains 3% by
weight of the active compound.
VI. 20 parts by weight of the active compound No. 2.006 are inti-
mately mixed with 2 parts by weight of calcium salt of
dodecylbenzenesulfonic acid, 8 parts by weight of fatty
alcohol polyglycol ether, 2 parts by weight of sodium salt of
a phenol-urea-formaldehyde condensate and 68 parts by weight
of a paraffinic mineral oil. A stable oily dispersion is
obtained.
To widen the spectrum of action and to achieve a synergistic
40 effect, the hydroxypyridonecarboxamides I can be mixed with
numerous representatives of other herbicidal or growth-regulating
active compound groups and applied jointly. For example, suitable
mixture components are diazines, 4H-3,1-benzoxazine derivatives,
benzothiadiazinones, 2,6-dinitroanilines, N-phenylcarbamates,
45 thiocarbamates, halocarboxylic acids, triazines, amides, ureas,
diphenyl ethers, triazinones, uracils, benzofuran derivatives,
cyclohexane-1,3-dione derivatives which carry eg. a carboxyl or
" 21~0~11
21
carbimino group in the 2-position, qui,nolinecarboxylic acid
derivatives, imidazolinones, sulfonamides, sulfonylureas, aryl-
oxy- or heteroaryloxyphenoxypropionic acids and their salts,
esters and amides and others.
Additionally, it may be useful to apply the compounds I on their
own or together in combination with other herbicides,
additionally mixed with further crop protection agents, for
example with agents for controlling pests or phytopathogenic
lO fungi and bacteria. Further of interest is the miscibility with
mineral salt solutions, which are employed for the elimination of
nutritional and trace element deficiencies. Non-phytotoxic oils
and oil concentrates can al~o be added.
15 Depending on the aim of control, time of year, target plants and
stage of growth, the application rates of active compound are
from 0.001 to 3.0, preferably from 0.01 to 1.0, kg/ha of active
substance (a.s.).
20 Synthesis Examples
Example 1
Preparation of the starting compounds
25 Preparation of ethyl S-ethyl-4-hydroxypyrid-2-one-3-carboxylate
4.2 g (0.17 mol) of sodium hydride are suspended in 500 ml of
toluene and 27 g (0.17 mol) of diethyl malonate are added
dropwise. The suspension is stirred at room temperature for 2
30 hours and then added dropwise to a solution of 16 g (0.17 mol) of
l-butenyl isocyanate in 300 ml of toluene. The reaction mixture
is stirred overnight at room temp~rature and then adjusted to
pH 6 with ammonium chloride solution. The organic phase is
separated off, dried with sodium sulfate and concentrated. The
35 residue is recrystallized from cyclohexane. 32 g of white
crystals (m.p.: 85C) are obtained.
15 g of these crystals are heated at 200C for 3 minutes. The
remaining brown residue is separated by column chromatography on
40 silica gel. After recrystallization from diisopropyl ether, 2.4 g
of ethyl 5-ethyl-4-hydroxypyrid-2-one-3-carboxylate (m.p.:
116 - 117C) are obtained.
Further intermediates IIa were prepared in a corresponding manner
45 with modification of the starting substances. The compounds thus
obtained are listed with physical data in Table 1 below.
21S~O~l
22
Table 1: Starting compounds IIa
OH O
R3~ ~ ~ oR4 tIIa
H
No. R3 R4 Melting point ~C] or lH-NMR
1.002 Methyl Ethyl 2~4
1.003 Phenyl Ethyl 1H-NMR(DMSO-d6) ~ (ppm): 13.8
(bs, lH), 11.8 tbs, lH), 7.65 (s,
lH), 7.59 - 7.31 (m, 5H), 4.37
(q, 2H), 1.33 (t, 3H)
Isopropyl Ethyl 1K-NMR (CDCl3) ~ (ppm): 13.9 (bs,
1.004 lH), 12.6 (bs, lH), 7.30 (s, lH),
4.51 (q, 2H), 3.07 (m, lH), 1.46
(t, 3H); 1.19 (d, 6H)
Example 2
Preparation of 5-ethyl-4-hydroxy-N-isopropylpyrid-2-one-
25 3-carboxamide (No. 2.002 of Table 2)
1.9 g (9 mmol) of ethyl 5-ethyl-4-hydroxypyrid-2-one-
3-carboxylate (Ia) are stirred under autogenous pressure at 150C
for 8 hours with 0.6 g (9 mmol) of isopropylamine in 30 ml of
30 ethanol in a mini autoclave. After cooling, the residue is
filtered off, and the mother liquor is concentrated and purified
by column chromatography on silica gel.
Yield: 0.75 g (37% of theory), m.p.: 117C.
Further compounds I were prepared in a corresponding manner with
modification of the starting substances. The compound~ thus
obtained are listed with physical data in Table 2 below.
40 Table 2: Hydroxypyridone-3-carboxamides I
OH X
R3 ~ ~ / (I)
H
` 21~40~1
;
23
No. R1 R2 R3 X Melting
point
[C]
2.001 H tert-~utyi Phenyl o 247
5 2.002 ~ Isopropyl Ethyl o 117
2.003 H l-Phenylethyl Ethyl o 111
2.004 H l-Cyclopropylethyl Ethyl o 121
2.005 H tert-sutyl Ethyl o 141
2.006 H tert-~utyl Methyl 0 220
2.007 H tert-~utyl Isopropyl o 142
2.008 H Isopropyl Methyl o 180
2.009 - H l-(Methoxymethyl)ethyl Methyl o 112
2.0010 H l-Cyclopropylethyl Methyl o 163
15 2.0011 ~ l-Phenylethyl Methyl o 221
2.0012 H H Ethyl o 263
2.0013 H l-Cyclohexylethyl Methyl o 159
2.0014 H 1,1 - Dimethyl-2-propynyl Methyl o 217
2.0015 H 1, 1 - Dimethyl-2-propenyl Methyl o 200
2. 0016 H 1-(Dimethoxymethyl)ethyl Methyl o 111
2. 0017 H Cyclopropyl Methyl O 221
- 2. 0018 H 3-Trifluoromethylphenyl Methyl o 264
2.0019 H 1-Ethynylcyclohexyl Methyl o 200
25 2.0020 H 1-Methyl-2-phenoxyethyl Methyl 0 178
2.0021 H 2-Fluorobenzyl Methyl o 204
2.0022 H 1-Methyl-2-(4-methoxy- Methyl o 163
phenyl)ethyl
30 Example 3
Preparation of N-tert-butyl-5-methyl-4-hydroxypyrid-2-one-
3-carboxamide, Na salt (No. 3.002 of Table 3)
35 0.63 g (2.8 mmol) of N-tert-butyl-5-methyl-4-hydroxypyrid-2-one-
3-carboxamide is suspended in methanol and treated with a
solution of 150 mg (2.8 mmol) of sodium methoxide in methanol.
The solution is stirred at room temperature for one hour and then
concentrated, and the residue is dried.
Yield: 0.68 g (98% of theory), m.p. 280C
Further salts of compounds I were prepared in a corresponding
manner with modification of the starting substances. The salts
45 thus obtained are listed with physical data in Table 3 below.
21~0 ll
,
24
Table 3: Salts of hydroxypyridone-3-carboxamides I
0~ X
N
N OH
No. R1 R2 R3 X Counterion Melting
point [C]
3.001 H tert-Butyl Methyl O K~ 275
3.002 H tert-Butyl Methyl O Na~ 280
Use examples
It was possible to show the herbicidal action of the
hydroxypyridonecarboxamides of the formula I by greenhouse tests:
The cultivation vessels used were plastic flowerpots cont~i n; ~g
loamy sand with about 3.0% humus as a substrate. The seeds of the
test plants were sown separately according to species.
25 In the case of preemergence treatment, the active compounds
suspended or emulsified in water were applied directly after
sowing by means of finely dispersing nozzle~. The vessels were
lightly watered in order to promote germination and growth, and
then covered with transparent plastic hood~ until the plants had
30 taken root. Thi~ covering causes a uniform germination of the
test plants if this was not adversely affected by the active
compounds.
For the purpose of postemergence treatment, the test plants were
35 first raised, according to growth form, to a height of growth of
from 3 to 15 cm and only then treated with the active compounds
suspended or emulsified in water. For this purpose, the test
plants are either sown directly and raised in the same vessels or
they are first raised separately as seed plants and transplanted
40 into the test vessels a few days before the treatment. The
application rate for postemergence treatment was 0.5 or
0.25 kg/ha of a.s.
` ` 21~0~1
The plants were kept species-specifically at 10 - 25 C or
20 - 35 C. The test period extended over 2 to 4 weeks. During this
time, the plants were tended and their reaction to the individual
treatments was assessed.
Rating was carried out on a scale of from 0 to 100. 100 in this
case means no emergence of the plants or complete destruction of
at least the above-ground parts and 0 means no damage or normal
course of growth.
The plants used in the greenhouse tests were made up of the
following species:
15 Abbreviation Botanical name Common name
ECHCG Echinochloa crus-galli Barnyard grass, common
ABUTH Abutilon theophrasti Velvetleaf
POLPE Polygonum persicaria Lady' 8 thumb
SINAL Sinapis alba Mustard, white
The result showed that undesired plants can be controlled very
effectively using compound No. 2.006.
In comparison with the structurally closest compound of the prior
25 art, the comparison substance A disclosed in EP-A 544 151
OH
H3C ~ ~ NHc(cH3)3 A
H3 N O
H
35 compound No. 2.006, in particular at low application rates of
0.25 kg/ha, has a better herbicidal action, as the results
compiled in Table I below show.
Table I
40 Comparison of results from greenhouse tests postemergence
Example No. 2.006 A
Application rate 0.5 ¦0.25 0.5 ¦0.25
45 (kg/ha of a.s.) l l
21~4~ 11
26
Test plants
( Damage in % )
ECHCG 90 90 30 40
ABUTH 100 100 85 70
POLPE 100 100 70 10
SINAL 100 100 100 8G