Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ 2I54133
PROCESS FOR THE RECOVERY OF MOLYBDENUM FROM IMPURE CALCIUM
MOLYBDATE RESULTING FROM THE TREATMENT OF URANIFEROUS ORES
.
DESCRIPTION
The present invention relates to a process for the
recovery of molybdenum from impure calcium molybdate
resulting from the treatment of uraniferous ores.
In the uranium industry and in particular in hydro-
metallurgical processes, carbonated solutions are producedwhich contain uranium and various metals such as molyb-
denum, which it is necessary to separate from the uranium.
This separation can be performed after acidification
by precipitation with lime, which leads to the formation of
an impure calcium molybdate precipitate, which more partic-
ularly contains impurities such as Si, P and/or V liable to
form complexes of the heteropolyanionic type with
molybdenum.
In orfler to recover the molybdenum from this precipi-
tate, it is necessary to dissolve the latter in a sulphuric
solution and then extract the molyb~emlm present in said
solution, particularly in the form of heteropolyanions
and/or isopolyanions, by means of an appropriate organic
solvent.
FR-A-2 691 373 describes a process for the recovery of
molybdenum using these stages of dissolving and extracting
by solvent. According to said document, the impure calcium
molybdate is firstly dissolved in an aqueous sulphuric acid
solution at approximately 0.25 mole/e, i.e. a pH-value
close to 2 after dissolving and then the molyb~Pnl]m
dissolved in the aqueous solution is extracted in an
organic solvent comprising a first extractant constituted
by a dialkyl phosphoric acid such as di-(2-ethylhexyl)-
phosphoric acid and a second extractant chosen from
tertiary amine salts, quaternary ~mmo~;um salts, tributyl
phosphate and phosphine oxides.
B 11985.3 MDT
215~133
- 2
This process suffers from the disadvantage of requir-
ing the use of an organic solvent incorporating two ex-
tractant types in order to achieve satisfactory extraction
yields.
- Thus, due to the presence of heteropolymolybdic
components in the aqueous dissolving solution, firstly
there is a transfer of molybdenum heteropolyanions into the
organic phase by means of the second extractant in anion or
acid form as a function of the nature of the second ex-
tractant and then the proton form of the polynuclearanionic molybdenum complex in the organic phase into a di-
alkyl phosphoric (MO2 2 + ) acid complex by means of the
first extractant, whilst releasing the phosphoric acid,
vanadic acid and/or orthosilicic acid, as well as the water
in the aqueous phase.
US-A-3 751 555 and US-A-4 275 039 also illustrates the
use of an organic solvent having two extractant types, such
as di-(2-ethylhexyl)-phosphoric acid and tributyl phosphate
in order to recover ~he molybdenum from molvbdite or
scheelite.
The present invention specifically relates to a pro-
cess for the recovery of molybdenum from impure calcium
molybdate by dissolving and then extracting in an organic
solvent, which avoids the formation of heteropolyanion com-
plexes between the molybdenum and the impurities such as
silicon, phosphorus and/or vanadium present in the starting
calcium molybdate, whilst as a result simplifying the
molyhA~nllm extraction procedure and at the same time
obtaining very good extraction yields.
According to the invention, the process is character-
ized in that it comprises the following steps:
a) dissolving the calcium molybdate in an aqueous
sulphuric acid solution having a sulphuric acid concentra-
tion of at least two mole/~,
b) contacting the aqueous dissolving solution from
step a) with an organic solvent incorporating a dialkyl
phosphoric acid, and
B 11985.3 MDT
2154133
- 3
c) separating from the aqueous solution the organic
solvent which has extracted the molybdenum.
In this process, the use of an aqueous solution having
at least 2 mole/~ of sulphuric acid, e.g. 2 to 4 mole/~ of
sulphuric acid for dissolving the calcium molybdate avoids
the formation of heteropolymolybdic components in the
dissolving solution. Therefore it is no longer necessary
to then use for the extraction of the molyb~nllm two
extractants in order to ensure on the one hand the transfer
of the heteropolymolybdic components into the solvent and
on the other their transformation into the dialkyl phos-
phoric (MoO22+) acid complex.
Thus, it has been found that the existence range in
aqueous solution of heteropolymolybdic components was in
the pH range 1 to 3, which corresponds to sulphuric acid
concentrations of 0.2 to 2 mole/e and that the degradation
of the components formed by the subsequent acidification of
the solution was very slow.
In addition, by performing in accordance with the
present invention the dissolving of the calcium molybdate
with a sulphuric acid concentration above 2 mole/~,
troublesome heteropolybdic components are not formed.
After dissolving the calcium molybdate in the aqueous
sulphuric acid solution, said solution is contacted with an
organic solvent incorporating a dialkyl phosphoric acid.
In general, the organic solvent is constituted by a
dialkyl phosphoric acid solution in an appropriate organic
diluent.
The dialkyl phosphoric acids which can be used are
those in which the alkyl radicals are straight or branched
radicals with 4 to 16 carbon atoms.
As an example of such an acid reference can be made to
di-(2-ethylhexyl)-phosphoric acid, which is hereinafter
designated by the abbreviation HDEHP.
The organic diluents used are e.g. aliphatic or
aromatic hydrocarbons, e.g. hydrogenated tetrapropylene
(TPH).
B 11985.3 MDT
21S 1133
- 4
The dialkyl phosphoric acid concentration of the
- organic solvent is generally high, because the extractionrate increases with the extractant content. Preferably,
- -the dialkyl phosphoric acid concentration is 15 to 40
vol.%.
According to the invention after contacting the or-
ganic solvent with the dissolving solution, an organic
solvent is obtained which contains the extracted molyb-
denum.
0 Preferably said solvent is washed with an aqueous,
dilute sulphuric acid solution, e.g. having a sulphuric
acid concentration of 0.5 to 1 mole/e. The molyb~nllm can
then be reextracted from the organic solvent by means of an
aqueous ~mmo~acal solution.
The process according to the invention can be per-
formed in conventional cocurrent or countercurrent ex-
traction installations such as mixer-settlers, pulsed
columns, etc.
It is possible to carry out contacting at ambient
pressure and temperature, but it is also possible to use
higher temperatures, particularly for improving the
extraction kinetics.
It is also possible to operate continuously by
bringing about a countercurrent circulation of the organic
solvent and the aqueous solutions and recycling the aqueous
washing solution of the organic solvent with the starting
aqueous solution containing the molyb~Pn-lm to be extracted.
Other features and advantages of the invention can be
better gathered from the following non-limitative descrip-
tion with reference to the attached drawings, wherein show:Fig. 1 A diagram representing the heteropolymolybdic
complex quantities present in a sulphuric medium
as a function of the sulphuric acid concentration
of the medium (in mole/~).
Fig. 2 A diayLallullatic representation of an exemplified
embodiment of the process of the invention.
B 11985.3 MDT
,, 215~133
Fig. 3 A diagrammatic representation of another embodi-
ment of the process of the invention.
Fig. 1 illustrates the behaviour of the following
heteropolymolybdic complexes:
- phosphomolybdic complex (Mo:P atomic ratio 20:1)
absorbing at 400 nm,
- phosphov~n~Aomolybdic complex (Mo:P:V atomic ratio
11:1:1) absorbing at 490 nm, and
- silicomolybdic complex (Mo:Si atomic ratio 12:1)
absorbing at 430 nm,
at a concentration of 0.1 mole/e of Mo(VI) in an aqueous
sulphuric acid solution, as a function of the H2 S04 concen-
tration (in mole/e).
The abundance of the complex in solution is repre-
g sented by the optical density of the solution at the wave-
length corresponding to the absorption peak of the con-
sidered complex.
In Fig. 1, curve 1 relates to the phosphomolybdic
complex, curve 2 to the phosphov~n~Aomolybdic complex and
curve 3 to silicomolybdic complex.
Thus, it can be seen that beyond a sulphuric acid con-
centration of 2 mole/e, there is no longer any complex in
solution.
This is why, according to the invention, use is made
for the purpose of dissolving the calcium molybdate of an
aqueous sulphuric acid solution having a H2 S04 concentra-
tion of at least 2 mole/e.
Thus, the dissolving solution incorporates virtually
no heteropolymolybdic components. This result cannot be
achieved rapidly by carrying out the dissolving with a
dilute H2 S04 solution and then acidifying the dissolving
solution, because it is found that these heteropolymolybdic
components and in particular silicomolybdic components have
an excessively slow degradation kinetics in concentrated
acid medium. For example, a period of 15 days is necessary
B 11985.3 MDT
,, ` 215~133
- - 6
at ambient temperature in order to degrade silicomolybdic
- components in a 3M sulphuric medium
The following examples illustrate the results obtained
- with the process of the invention
EXAMPLE 1: DISSOLVING CALCI~M MOLYBDATE
In this example, lg of calcium molybdate having the
composition given in the following Table 1 is dissolved in
20ml of an aqueous solution containing 3 mole/e of sul-
phuric acid, accompanied by stirring, at ambient tempera-
ture and the composition of the dissolving solution is
checked by periodically taking samples and analyzing them
by spectrophotometry and emission spectroscopy JCP for
determining their Mo, P, V, Si and U contents
After stirring for 45 minutes, the dissolving yield no
longer undergoes any significant variation and a dissolving
solution is obtained having the Mo, P, V, Si and U concen-
trations given in Table 1
This Table includes for comparison purposes the
results obtained when carrying out the dissolving under the
same conditions, but using a 0 25 mole/e sulphuric acid
solution (pH = 2) as in FR-A-2 691 373
TABLE 1
CALCIUM DISSOLVING DISSOLVING
ELEMENTMOLYBDATESOLUTION SOLUTION
(wt ~)3 mole/e H2 S04 H2 S04 (pH=2)
(in mole/e) (in mole/e)
Mo 33 660 0 212 0 159
P 1 360 5 99-10- 3 1 . 10-10- 2
V 1 610 1 69-10- 2 1. 49-10- 2
Si 0 760 1 81-10- 3 5 80-10- 3
U 0 378 7 04-10- 4 7-10- 4
B 11985 3 MDT
2154133
- 7
The results of Table 1 show that according to the
- invention an increased molybdenum dissolving is obtained.
EXAMPLE 2: MOLYBDEN~M EXTRACTION
Contacting takes place, accompanied by stirring, of
one volume of a dissolving solution obtained as in example
1 either with 2M H2 S04 or with 3M H2 S04, four days after it
was obtained, with 1 volume of an organic solvent consist-
ing of 30 vol.~ di-(2-ethylhexyl)-phosphoric acid and 70
vol.~ of hydrogenated tetrapropylene.
After stirring for 5 minutes at 23C, the two phases
are allowed to settle and their respective molybdenum con-
tents are determined. This operation is repeated twice
contacting the aqueous solution separated from the organic
solvent resulting from the preceding contact with 1 volume
of new organic solvent.
The composition of the starting solution and the
results obtained are given in Table 2. In this Table, the
extraction yields correspond to the cumulative values ob-
tained after 1, 2 or 3 contacts.
This Table gives for comparison purposes the results
obtained when working in the same way with a dissolving
solution having a H2 S04 concentration of 1 mole/e.
B 11985.3 MDT
,, 2154133
- 8
TABLE 2
- DISSOLVING H2 S04 ( in 1 2 3
SOLUTION mole/~)
Mo(VI) in 0.161 0.165 0.15
mole/~
After 1 contact Organic Mo(VI)0.07530.135 0.129
(in mole/ e )
Aqueous Mo(VI)0.08570.030 0.021
(in mole/~)
Extraction
yield (~) 46.7~ 81.8~ 86~
After 2 contacts Organic Mo(VI)0.03150.0164 0.0129
(in mole/~)
Aqueous Mo(VI)0.05420.0136 0.0081
(in mole/~)
Extraction
yield (%) 66.3~ 91.7~ 94.6~
After 3 contacts Organic Mo(VI)0.02360.0028 0.0017
(in mole/~)
Aqueous Mo(VI)0.03060.0108 0.0061
(in mole/~)
Extraction
yield (~) 80.9~ 93.4~ 95.9
The results of Table 2 show that better results are
obtained with the dissolving solutions obtained according
to the process of the invention.
EXAMPLE 3:
In this example the molybdenum is recovered from a
dissolving solution obtained according to the invention, by
using the installation operating in continuous manner and
in countercurrent form, as is diay~auu--atically shown in
Fig. 2.
B 11985.3 MDT
215~133
g
This installation comprises an extraction stage 21 and
- a washing stage 23 in which the organic solvent flows con-
tinuously, being introduced by the pipe 25. In this ex-
- traction stage, the organic solvent is countercurrent con-
tacted with the dissolving solution of the calcium molyb-
date stored in the tank 27. This solution is introduced by
the pipe 29 in the extraction stage 21.
On leaving the extraction stage pipe 31 recovers an
aqueous efflue~t containing P, Si, V and U and virtually no
molybdenum and by the pipe 33 the organic solvent contain-
ing molybdenum, as well as a few impurities.
At the washing stage 23, this organic solvent is
brought into countercurrent contact with an aqueous washing
solution, e.g. a 0.5 mole/e sulphuric acid solution, intro-
duced by the pipe 35.
On leaving the washing stage pipe 37 recovers the
molybdenum-containing organic solvent and pipe 39 an
aqueous effluent containing the impurities deextracted from
the solvent.
In this installation it is possible to use circulation
flow rates such that the volume ratio of the organic phase
to the aqueous phase is 2 in the extraction stage and in
the washing stage.
This stage can be constituted by several individual
stages each constituted by a mixer-settler. For example,
the extraction stage can have 12 individual stages and the
washing stage 4 individual stages.
In the following Table 3 are given the results
obtained in this installation with a 3 mole/~ sulphuric
acid dissolving solution. For comparison purposes this
Table also gives the results obtained with a dissolving
solution in a weakly acid medium (pH 2), then acidified in
order to have a 3 mole/~ sulphuric acid concentration. In
this Table, the extraction yields are calculated on the
basis of concentrations measured on the different aqueous
phases.
B 11985.3 MDT
TABLE 3 f `
Mo(VI) concentration Extraction Reflux E/S(.)
(mole/l) yield ratio(*)
~o
~ DissolvingOn entry Extraction Washing Organic (%) (%)
r 3 solution (in 29) effluent effluent solvent
(3 mole/l) (in 31) (in 39) (in 37)
H2S04
dissolving
solution
(3 mole/l~ 0.212 6.1.10 2.2.10 0.10 99~7 0 05 1.056
Dissolving
solution (pH2)
acidified to
3 mole/l in
H2S04 0.138 1.5.10 2.90.10 35.55.10 88.1 1.0 1.08 t~
~n
Mo flow (mole/h) in the aqueou~ phase to be treated
(*) Reflux ratio = ~-~
Mo flow (mole/h) in the dissolving solution entering the extraction operation C~
(.) E/S = Entering Mo flow
~departing Mo flow
2154133
11 --
The results of Table 3 ~emonstrate that an extraction
yield of 99.7~ is obtained with the process according to
the invention, whereas it is well below this figure (88~)
when the dissolving is carried out at pH 2, followed by an
aci~dification to 3 mole/l of H2 S04 .
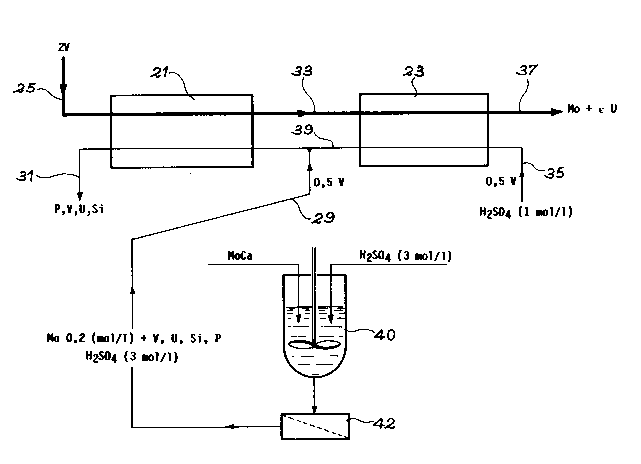
EXANPLE 4:
In this example, the dissolving and then the extrac-
tion of the molybdenum take place from calcium molybdate,
using the installation diagrammatically shown in Fig. 3.
Fig. 3 uses the same references as in Fig. 2 for designat-
ing the components common to both installations.
In this installation, firstly the calcium molybdate is
dissolved in the container 40 using 20 m~ of 3M H2 S04 per
tonne of calcium molybdate. Dissolving is carried out,
accompanied by stirring, for 1 hour and at 23C and then
the solution is separated from solid materials by filtra-
tion in the filter 42.
The separated solution containing approximately 0.2
mole/~ of molybdenum and impurities (V, U, Si, P) is
supplied by the pipe 29 to the extraction stage 21, which
consists of six individual stages with the washing solution
leaving the washing stage 23.
The organic solvent (30~ HDEHP - 70~ THP) is intro-
duced by the pipe 25 and circulates in countercurrent
manner with the aqueous solution.
On leaving the extraction stage 21, it is introduced
into the washing stage 23 consisting of four individual
stages, where it is countercurrent contacted with a 1
mole/~ H2 S04 aqueous solution introduced by the pipe 35.
On leaving the washing stage 23, the organic solvent,
which virtually contains no molybdenum, is treated with a
view to recovering the molybdenum by deextraction in an
o~;acal medium (pH 8.5 to 9) and then undergoes decon-
tamination with respect to uranium in a carbonate medium.
It is then reacidified by H2 S04 and recycled to extraction
stage 21. However, it is possible to avoid this
B 11985.3 MDT
215~1~3
- 12 -
reacidification of the solvent, because the latter can be
performed by the aqueous solution in the first extraction
stage.
- The molybdenum can be separated from the ammoniacal
solution by ammonium molybdate precipitation in the acid
medium (pH _ 1).
Fig. 3 indicates the volumes of solutions introduced
at this stage.
With this installation, molybdenum is recovered with a
yield of 99.7~ using a single extractant.
B 11985.3 MDT