Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 94117218 ~ ~ ~ ~~ ~ j'" ~ PCTICA94/00027
1
Production of Particle-Stabilized Metal Foams
Technical Field
This invention relates to the production of particle-
stabilized metal foams. More particularly, the invention
relates to a particle-stabilized metal foam having a
unique cellular structure produced via a precursor which
can be subsequently "foamed". The invention also relates
to the metal foam precursor itself.
Background Art
Foamed metals have high strength-to-weight ratios and
are extremely useful as load-bearing materials and as
thermal insulators. Metallic foams are characterized by
high impact energy absorption capacity, low thermal
conductivity, good electrical conductivity and high
absorptive acoustic properties.
Foamed metals have been described previously, e.g. in
U.S. Patent Nos. 2,895,819, 3,300,296 and 3,297,431. In
general such foams are produced by adding a gas-evolving
compound to a molten metal. The gas evolves to expand and
foam the molten metal. After foaming, the resulting body
is cooled to solidify the foamed mass thereby forming a
foamed metal solid. The gas-forming compound can be metal
hydride, such as titanium hydride, zirconium hydride,
lithium hydride, etc. as described in U.S. Patent no.
2,983,597.
A recent development in the production of lightweight
foamed metal is described by Jin in U.S. Patent No.
4,973,358, issued November 27, 1990. In that patent, a
precursor composite of a metal matrix and finely divided
solid stabilizer particles was heated above the liquidus
temperature of the metal matrix and gas bubbles were
discharged into the molten metal composite below the
surface to thereby form a foamed melt on the surface of
the molten metal composite. When this foam was cooled, it
formed a solid foamed metal having a plurality of closed
cells and the stabilizer particles dispersed within the
metal matrix.
WO 94/17218 PCT/CA94/000:
2
The metal matrix precursor composite used in the
process of U.S. Patent 4,973,358 was one in which the
contained stabilized particles were quite uniform in size
and were fully wetted by the matrix material. One such
product that was used was the aluminum matrix composite
sold under the trademark DURALCAN by Alcan Aluminum
Corporation. However, such composites are expensive to
produce and result in a relatively expensive foam product.
Metal foam generated from such precursor materials
have as stabilizing particles monolithic or blocky
particles, typically SiC or alumina. These precursor
composites are also produced under reduced pressures or
even under vacuum conditions. Foam generated from such
material has been found to be difficult to cut except for
the lowest density material.
An object of this invention is to provide metal foams
having desirable properties using a relatively inexpensive
procedure.
A further object of this invention is to provide an
2o alternative to standard metal matrix precursor composites
used for producing metal foams.
Another further object is to provide a relatively
inexpensive precursor for the production of foamed metals.
A further object is to provide a stabilized metal
foam which is easier to machine than previous stabilized
foam materials.
A still further object is to provide a stabilized
metal foam with modified and adjustable crush properties.
Disclosure of the Invention
According to the present invention, there is provided
a process for producing a metal foam, which comprises:
heating a matrix metal above a liquidus temperature of the
metal to form a liquid matrix metal, adding to said liquid
matrix metal stabilizer particles capable of remaining
dispersed within the matrix metal, mixing the liquid
matrix metal and stabilizer particles under a covering gas
in such a way that bubbles of said gas, as well as said
2~~~~~a
WO 94/17218 PCT/CA94/00027
3
particles, are dispersed throughout the matrix metal to
form a composite which is a precursor for a particle-
stabilized metal foam (referred to hereinafter as a
"precursor composite"), introducing a gas into said
precursor composite in molten form to form a liquid metal
foam, and solidifying said liquid metal foam with closed
cells substantially filled with gas to form a solid
particle-stabilized metal foam.
The invention also relates to the resulting metal
foams and to a process for producing a precursor composite
suitable for foaming and the precursor composite thus
produced.
According to an aspect of the present invention,
there is provided a process for producing a metal foam,
which comprises: heating a matrix metal above a liquidus
temperature of the metal to form a liquid matrix metal,
adding to said liquid matrix stabilizer particles, mixing
the liquid matrix metal and stabilizer particles under a
covering gas in such a way that said particles are
dispersed throughout the matrix metal to form a precursor
composite for a particle stabilized metal foam, wherein
said precursor composite may incorporate some of the said
gas in pores within the precursor composite, introducing a
gas into said precursor composite in molten form to form a
liquid metal foam with closed cells substantially filled
with gas, and solidifying said liquid metal foam to form a
solid particle-stabilized metal foam.
The foam as produced by the above method may contain
pores within some of the walls of the foam. The number of
these pores may be controlled by altering the method of
mixing of the precursor composite, for example, more
vigorous mixing will result in more pores.
According to another aspect of the present invention,
there is provided a process for producing a metal foam,
' 35 which comprises: heating a matrix metal above a liquidus
temperature of the metal to form a liquid matrix metal,
adding to said liquid matrix stabilizer particles
WO 94/17218 PCTICA94/OOOI
4
consisting of a mixture of particles mixing the liquid
matrix metal and stabilizer particles under a covering gas
in such a way that said particles are dispersed throughout
the matrix metal to form a precursor composite for a
particle stabilized metal foam, introducing a gas into
said precursor composite in molten form to form a liquid
metal foam, and solidifying said liquid metal foam to form
a solid particle-stabilized metal foam, where the walls of
the foam contain a distribution of stabilizer particles
with the finer particles preferentially located at the
interfaces between the cell walls and the gas filled
cells.
The walls of the foam produced by this embodiment may
contain pores. The number of such pores may be varied, as
in the previous embodiment, by varying the mixing
conditions in the precursor composite production. The gas
pressure during the production of precursor composite by
this embodiment may also be varied, for example, by
operating the process in a closed vessel under reduced
pressure. Gas pressures of less than 10 Torr may be used
while still generating pores within the precursor and
final foam product, and still ensuring that fine particles
are still preferentially located adjacent to the surfaces
of the closed cell walls. Nevertheless it is most
convenient in this embodiment to operate at atmospheric
pressure.
The mixtures of stabilizer particles preferentially
have a specific surface area as measured by BET nitrogen
adsorption of at least 2.0 mZ/gm and more preferably at
least 10 m2/gm. Mixtures of particles with specific
surface areas in this range may consist of a broad range
of particles sizes, including a substantial number of fine
particles, and therefore differ from the narrow
distribution of blocky or monolithic particles of the
prior art. It is preferred that the mixtures of
stabilizer particles contain aggregates or agglomerates of
finer particles, and it is most preferred that such
2:~~~w
WO 94117218 PCTIC.A94./00027
mixtures consist almost entirely of such agglomerates.
The aggregates preferably are sufficiently weak that the
stirring action of the mixer used in the precursor
composite preparation is sufficient to break up a
5 substantial number of these agglomerates to produce fine
particles within the final foam product. These fine
particles may have a wide size distribution but often
contain appreciable quantities of material having particle
sizes of less than 1 ~Cm. The amount of shear which has to
be introduced to break up these agglomerates is typically
similar to that used to ultrasonically disperse materials
for particle size distribution by sedigraph, etc. The
breakup may be assisted by the wetting or partial wetting
of the particles by the matrix alloy.
Suitable stabilizer particles may be alumina with
high surface area (calcined or activated), MgO, feldspar,
calcined bauxite and many others. A particularly useful
stabilizer particle is Mg0 in a form which has a
substantial specific surface area, for example at least
25 m2/gm. By comparison, a blocky alumina as used in the
prior art method of producing foam would have a specific
surface are of about 0.5 m2/gm.
Because of the high surface area of the stabilizer
particles most suitable for the present invention, the
particles frequently require calcination prior to mixing
in the precursor composite to drive adsorbed water and
other gases.
The foam generated from precursor composite produced
under a gas has specific crush strength properties that
can be adjusted by varying the mixing method and the gas
pressure. With the addition of stabilizer particles that
are mixtures containing agglomerates, the full advantages
of the new material, including improved machinability can
be developed.
Because the process can be varied by changing the
mixing in the precursor composite production step and by
altering the gas pressure in the same step, it must be
WO 94117 1 PCT/CA94/00027
~~.~42~
6
appreciated that the number distribution of the pores can
be varied within the structure. It is possible to reduce
the number of pores to the extent that about 10~ of cell
walls contain pores or to increase the number to the
extent that over 50~ of cell walls contain significant
number of pores.
The foamed product obtained by the process using
stabilizer particles which contain a distribution of
particles sizes including agglomerates also is
characterized by the presence of a distribution of
particles throughout the cell walls with fine particles
preferentially located at the interfaces between the cell
walls and the gas filled cells within the foam structure.
This differs from the prior art foam wherein the
stabilizer particles were generally mono-sized and located
essentially at the interfaces only.
While not wishing to be bound by any theory, it is
believed that refractory particles may be used which are
capable of undergoing an interaction with the matrix
metal, thus forming a compound at the particle/matrix
interface. It is further believed that this interaction
may predominate over contact angle in keeping the
particles dispersed in the molten matrix. While a variety
of refractory particles may meet these requirements, a
particularly effective material is MgO.
A particularly effective combination is an aluminum
matrix with stabilizing particles of Mg0 mixed in an open
vessel so that air forms the gas component of the
composite. The aluminum and Mg0 interact to form spinel
(MgA1204) at the interface. This spinel forms a coating on
the Mg0 particles and thereby keeps the particles
dispersed in the matrix.
The matrix metal may consist of a wide variety of
metals capable of being mixed in the molten state by
vortex mixing. Examples of these include aluminum,
magnesium, steel, zinc, lead, nickel, copper and alloys
thereof. Of particular interest are standard wrought,
~~~4~~~
WO 94/17218 PCT/CA94100027
7
cast or other aluminum alloys, for example alloys
available under Aluminum Association (AA) designations
6163, 2024, 7075, 7079 and A356. A particularly useful
matrix alloy has been found to contain at least 5% Si and
up to 3% Mg. For example, the foundry alloy A356 with 3~
Mg added is particularly useful with MgO. The matrix
alloy may enhance the machinability properties of the
foam.
For mixing the stabilizer particles with the metal
matrix it is preferable to use an impeller in such a
manner that a vortex is formed in the molten metal. The
stabilizer particles are added to the molten metal with
mixing and preferably the impeller is first rotated at a
lower speed in order to subduct the refractory particles
beneath the liquid metal surface using the vortex. Once
this is accomplished, the impeller is rotated at a higher
speed in order to create high shear conditions which
intimately disperse the stabilizer particles throughout
the liquid metal matrix. In one preferred embodiment, the
impeller is first slowly rotated (e.g. at 500-900 rpm) and
then rotated more quickly (e.g. at 800-1200 rpm). The gas
component of the precursor composite is entrained in the
melt at both low and high impeller speeds.
The gas component of the~precursor composite enters
the open vessel and is entrained into the molten metal
vortex caused by the rotation of the impeller. The shear
forces on the metal break the entrained gas into small
bubbles which are stabilized by the ceramic particles
within the metal matrix composite. The gas for this
component can be selected from a group consisting of air,
CO2, 02, N2, inert gases, etc. Because of its convenience,
air is usually preferred.
There are several important advantages in this
invention over the prior art. Firstly, because the
stabilizing particles can be in the form of a bulk
chemical of random sizes and some non-wetting properties
(presence of adhering gas bubbles) can be accepted, and
WO 94117218 PCTICA941000I
8
2~.5~~~~
because the entire mixing process may take place in an
open vessel (no vacuum being required), the precursor
composite is cheap and easy to make. Secondly, the foamed
product obtained from the precursor composite is more
machinable and is easier to cut than traditional particle
stabilized foam metals. In summary, the use of foam
precursor composite of the present invention to produce
metal foam materials results in a unique cellular
structure, lowers the production costs of the material and
eases both the machinability and cutability of the foamed
products. A desirable strength of the foam product may be
achieved by varying the porosity within the cell walls.
Brief Description of the Drawings
Methods and apparatus for performing the present
invention will now be more particularly described by way
of example with reference to the accompanying drawings in
which:
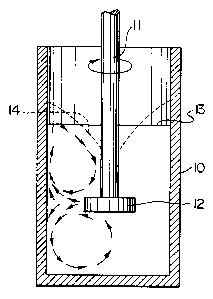
Figure 1 illustrates schematically an apparatus for
carrying out vortex mixing, suitable for production of
precursor composites of this invention.
Figure 2 is a photomicrograph of a cross-section
through a precursor composite of the invention at 50X
magnification. The precursor composite was produced using
Mgo and air at atmospheric pressure.
Figure 3 is a photomicrograph of a cross-section
through a precursor composite of the invention at 50X
magnification. The precursor composite was produced using
Mg0 and a low pressure of gas.
Figure 4 shows a 4X magnification of a cross-section
through a metallic foam structure produced using the foam
precursor composite illustrated in Figure 2.
Figure 5 shows a 100X magnification a further cross-
section through a metallic foam structure of the
invention, using the foam precursor composite illustrated
in Figure 2.
Figure 6A is a 200X magnification of a cell wall from
2~.~~~~~a'
WO 94117218 PCTICA94100027
9
the foam product of the invention produced using the foam
precursor composite illustrated in Figure 2 showing the
stabilizer particle distribution characteristic of the
invention;
Figure 6B is a 200X magnification of a cell wall from
a foam product produced using a foam precursor composite
formed by incorporating blocky alumina into the matrix
metal under reduced pressure conditions, and represents
the prior art foam.
Figure 7 is a plot of cutter tip wear versus number
of cuts for foams of the invention compared to prior art
foam.
Best Modes For CarryinQ Out the Invention
In the apparatus shown in Figure 1, a crucible 10
contains a rotatable shaft 11 provided with an impeller 12
including two shear bars. In operation, molten metal is
filled to the level 13 and the impeller is rotated at a
speed of about 500-900 rpm to form a vortex 14. The
stabilizer particles according to the invention are added
2o to the molten metal with mixing at an impeller speed of
500-900 rpm and the resulting vortex subducts the
stabilizer particles beneath the surface of the molten
metal. This slower speed mixing is continued until all of
the stabilizer particles are beneath the metal surface.
Then the rotational speed of the impeller is then
increased to the range of 800-1200 rpm to create high
shear conditions whereby the stabilizer particles are
intimately dispersed within the liquid metal and the
entrained gas is sheared and stabilized to form fine gas
bubbles.
The material thus formed comprises a foam precursor
composite which can be foamed to produce lightweight
foamed metal products. This foam precursor composite
- contains the ceramic particles of widely varying sizes
unevenly dispersed throughout the metal matrix. Some of
these ceramic particles have adhering gas bubbles, and
there are also a number of stabilized fine gas bubbles or
WO 94/17218 PCT/CA941000
to
pores throughout the matrix. Since the initial ceramic
particles are unsized, there is also a tendency for clumps
and aggregates of particles to exist within the precursor
composite. These do not seem to affect the subsequent
foaming procedure.
It is believed that the combination of aggregates
along with finer particles present in the original
particles or generated during mixing of the precursor
composite provide the unique structure and properties of
the material produced by this process. The fine particles
present assist in stabilizing the foam by migrating to the
surfaces of the cell walls. The proportion of
agglomerates or clumps in the particles added to the
precursor composite, the type of mixing used in forming
the precursor composite, and the pressure of gas used in
the precursor composite mixing vessel will all therefore
affect the fine porosity in the final foam product and
hence the properties.
The following non-limiting examples illustrate
certain preferred embodiments of the invention.
Example 1
Using the crucible of Figure 1, A356 aluminum alloy
in the form of auto scrap was melted and 3% by weight of
free magnesium was added thereto. Then with the impeller
rotating at a speed in the range of 500-900 rpm, 11.6% by
volume of bulk Mgo was added to the molten metal. The Mg0
had particles of average size 17 ~,m as measured by
Sedigraph without vigorous dispersion but which generated
a broad distribution of sizes in the range from less than
1 ~Cm to 60 ~,m following ultrasonic agitation for l0
minutes in the presence of a dispersant. During the
mixing, the molten alloy was maintained at a temperature
of 725°C and the Mg0 was preheated to that temperature
before being added to the melt.
The mixing at 500-900 rpm was continued for about 10
minutes, after which the speed was increased to the range
800-1200 rpm and continued for a further 20 minutes. The
WO 94/17218 PCTICA94/00027
11
product obtained was a precursor composite for producing a
foamed metal product.
The precursor composite formed was allowed to
solidify and the solidified cast was sectioned and
examined microscopically. The results obtained are shown
in Figure 2 and it will be seen that there is substantial
porosity and that there is a wide range of Mg0 particle
sizes. Figure 3 shows a cross-section of a precursor
composite prepared from the same bulk Mg0 as the present
l0 example, but where the precursor mixing was done under a
reduced pressure (approximately 0.5 Torr). The number of
gas bubbles present in the precursor composite is
substantially less than the precursor composite produced
under one atmosphere, but the wide range of particle sizes
of Mg0 are present.
A metal foam was produced by a known technique from
the precursor composite prepared as above, and cross-
sections are shown in Figures 4 and 5. The microporosity
in the large cell walls is particularly apparent from
Figure 5.
Figure 6A is a micrograph at 200X of a cell wall
where few pores are visible. The stabilizer particles are
visible distributed throughout the wall, but with the fine
particles preferably located at the interfaces. Figure 6B
is a micrograph of a foam wall where stabilizer particles
are blocky alumina mixed into the precursor composite
under reduced pressure. The particles in the structure
are almost entirely located at the interfaces.
Example 2
The material produced by the method of Example 1 (and
referred to as Material A) was subject to compressive
strength tests. Foam (Material B) was also prepared from
the same starting materials as Material A but the
precursor was mixed to generate fewer gas bubbles in the
precursor composite. Material A had pores present in
approximately 50% of the cell walls whereas Material B has
pores present in approximately 10 to 200 of the walls.
~1~~2~~
~VtfJ ~4~1~1~ PCT/CA94/OOOi
12
Finally foam (Materials C and D) were prepared
respectively using 10~ blocky alumina (0.5 m2/g specific
surface area) with AA6061 matrix alloy, and 10$ SiC of
approximately the same specific surface area with A356
matrix alloy, both however mixed under a reduced gas
pressure (approximately 3 Torr). Materials C and D were
therefore typical of prior art foam material. The
compressive strength at 20% reduction was compared at a
foam density of 0.23 g/cm2. The compressive strength of
Material A was found to be 0.37~0.22 MPa, the compressive
strength of Material B was found to be 0.75 MPa and the
compressive strength of Materials C and D were found to
both be 0.93 MPa. This indicates the adjustment of
compressive strength possible in the present invention by
combination of precursor composite mixing method and use
of particles of high surface area or consisting of
agglomerates.
Example 3
Material B of the previous example (Mg0 stabilizer
particles, air mixed and foamed to give product with 10-
20% pores in the foam walls) was tested for machinability
and compared to Material C of the previous example (blocky
alumina stabilizer particles, precursor produced under
reduced gas pressure) and a foam product (Material E)
prepared as for Material C except that precursor mixing
was carried out under one atmosphere of air in order to
introduce a degree of porosity into the foam walls similar
to Material B. Machinability was tested by passing slabs
of foam material through a single blade milling machine
and measuring the amount of blade wear versus the number
of cuts completed by the machine. Results of these tests
are shown plotted in Figure 7. The slopes of the wear
curves after initial machining passes (open symbols) were
completed represent the characteristic wear behaviour of
the sample (solid symbols on Figure 7). For these tests,
the prior art foam (triangles, Material C) had a slope of
4.5 ~m/cut, the foam produced from the same blocky alumina
WO 94/17218 PCT/CA94/00027
13
mixed under an atmosphere of air (squares, material E) had
a slope of 3.7 ~,m/cut, and the foam produced by the method
of Example 1 with limited porosity in the walls (circles,
Material B) had a slope of 0.95 ~m/cut. The lowest slope
represents the easiest machinability, and demonstrates
that there was a benefit of using agglomerates in the
present invention in terms of machinability.