Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 94/17778 215 5 8 6 6 PCTrUS94/01202
Description
METHOD OF ENHANCIN~ HAIR GROWTH AND HAIR REVITALIZING
COMPOSITIONS COMPRISING LUTZOMYIA PROTEIN
1. Field of the Invention
The present invention relates to a hair revitalization
composition having excellent hair revitalizing effects, such
as the prevention of epilation, and excellent trichogenous
effects. The invention is particularly useful in the
preparation of drugs, quasi-drugs, and in the field of
cosmetics. The composition comprises a peptide found in the
sand fly Lutzo~r~ria longipalpis.
2. Backqround of the Invention
Several possible causes of baldness and epilation have
been explored as investigators have attempted to better
understand and to develop means of alleviating these
conditions. Abnormalities of the scalp, such as activation
of androgen in the organs, for example, hair roots and sebum
glands, have been considered. In addition, decrease in the
blood flowing to hair follicles, hypersecretion of sebum, and
the formation of peroxides have all been considered as
possible causes of baldness and epilation. Conventional hair
revitalizing compositions, therefore, have been formulated
with the aim of alleviating or reducing the occurrence of
these events.
Formulations using vit~m' n~, such as B vitamins and
vitamin E; amino acids, such as serine and methionine;
vasodilators, such as swertiae extract and acetylcholine
derivatives; anti-inflammatory agents, such as
lithosperiradix extract and hinokitiol; female hormones, such
as estradiol; and skin-hyperactive agents, such as
cepharanthine, have been developed and utilized for
prophylaxis and as a cure for alopecia.
- Recently, compositions containing chemical inhibitors
for glycanases have been disclosed in U.S. Patent No.
5,015,470, compositions containing growth factors such as
transforming growth factors alpha and beta have been
disclosed in U.S. Patent No. 5,037,643, and compositions
containing mixtures of nicotinates and hydrochloride have
WO94/17778 2 ~ S S ~ 6 5 PCT~S94/0~202
-
-- 2 --
been disclosed in U.S. Patent No. 5,043,162. These
compositions have a certain efficacy in promoting hair
growth.
In spite of these various attempts, the conventional
hair revitalizing/enhancement compositions have not been
satisfactory in the prevention or tre;atment of alopecia,
epilation and trichogenous effects. This is likely due to
the fact that revitalization of hair and enhancement of hair
growth are very complicated processes and may involve
numerous or interrelated causes.
Consequently, there remains a need in the art for more
diverse and effective means of revitalizing hair or enhancing
hair growth. Such means would have beneficial effects in
several applications. For example, hair growth enhancement
or revitalization could be used in the cosmetic industry for
increasing hair growth rates, in the ph~r~ceutical industry
for the treatment of baldness, and in the animal breeding
industry for stimulating wool growth and fur growth in
commercially important animals, such as sheep and rabbits.
sTn~M~T~ OF ln~ INVENTION
The present inventors have researched substances
effective for the treatment of epilation and possessing other
excellent hair revitalizing effects, such as the prevention
of trichogenous effect. As a result, it has been discovered
that specific peptides that can be found in the salivary
glands of the sand fly Lutzomyia longipalpis exhibit
characteristics useful for revitalizing hairs, thereby making
this invention possible.
Accordingly, this invention aids in fulfilling the need
in the art by providing a hair revitalizing composition
comprising, as effective components, peptides that can be
fcund in the salivary glands of the sand fly Lutzomyia
longipalpis. The peptides exhibit vasodilation activity in
mammals. The hair revitalizing composition according to the
present invention provides excellent trichogenous effects and
WO94/17778 21~ ~ 8 6 6 PCT~S94/01202
hair revitalization effects by topical application and
subcutaneous injection.
More particularly, this invention provides a method for
inducing ~ maintA i n ing or increasing hair growth in a mammal.
The method comprises administering a hair growth inducing,
maint~i n i ng or increasing amount of Lutzomyia protein to the
skin or hair of the mammal. In a preferred embodiment of the
invention, the Lutzomyia protein is topically applied to the
mammal. A preferred Lutzomyia protein is maxadilan.
This invention also provides a composition suitable for
topical application to mammalian skin or hair. The
composition comprises Lutzomyia protein in a hair growth
inducing, maint~i n i ng or increasing amount in a
pharmaceutically acceptable vehicle.
The present invention is useful for the enhancement of
hair growth utilizing compounds belonging to a category
different from the group of substances hitherto used. The
present invention is also useful for increasing production of
wool or fur in animal breeding industries. The invention is
thus beneficial for inducing, maint~ining or increasing hair
growth in mammals.
BRIEF DESCRIPTION OF THE DRAWING
This invention will be described in greater detail with
reference to the drawing in which:
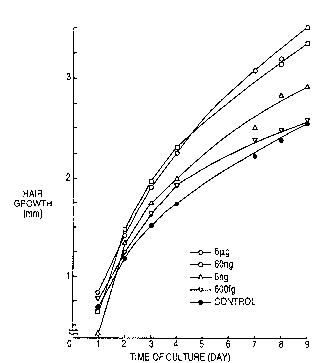
The Figure shows a graph in which the hair growth of
organ cultures of mouse whiskers is plotted against
cultivation day to show the action of Lutzomyia longipalpis
on these cultures.
DE~ATT.~n DESCRIPTION OF THE INVENTION
The present invention resulted from research efforts
directed to the identification of substances which
effectively prevent epilation and the trichogenous effect.
Applicants' efforts led to the discovery that specific
peptides that can be found in the salivary glands of the sand
fly Lutzomyia
WO94/17778 PCT~S94101202
2~S5866
-- 4
longipalpis exhibit strong effects, which result in the
enhancement of hair growth.
1. LutzomYia Proteins.
Peptides from salivary gland lysates o`f the sand fly
were previously identified and shown to be capable of
vasodilation and of temporary immune suppression in mammals.
(See, International Patent Publication No. WO91/00293 of
Lerner E. A., et al.; and Ribeiro et al., Science, Vol. 243
pp. 212-214 (1989)). These peptides are exemplified by the
~Lutzomyia protein" (LP), which, in one embodiment, has been
referred to as "maxadilan". Maxadilan has a molecular weight
of about 6800 daltons, and elutes prior to calcitonin gene-
related peptide in an acetonitrile-H2O-trifluoracetic acid-
reverse phrase column. (See, International Patent Pub-
lication No. WO91/00293 of Lerner E. A., et al.). LP can be
obtained by conventional purification chromatography from
surgically excised salivary glands of Lutzomyia longipalpis.
One pair of salivary glands contains about 10-15 ng LP. The
Lutzomyia protein constitutes about 1% of the total protein
in the salivary glands of the sand fly Lutzomyia longipalpis.
Applicants have discovered new properties possessed by
Lutzomyia protein, exemplified by maxadilan. In addition to
their vasodilator activity and their ability to suppress the
immune system of mammals, percutaneous injection or topical
administration of the protein results in the enhancement of
hair growth.
Peptides suitable for use in the methods and
compositions of the present invention include those peptides
extracted and purified from the lysate of salivary glands of
sand fly, active analogs and active fragments thereof,
described in International Patent Publication No. WO91/00293
or recombinantly produced peptides. The term Lutzomyia
protein as used herein refers to all such peptides, active
analogs, and active fragments. Maxadilan or active fragments
thereof are examples of suitable Lutzomyia proteins for use
in the present invention. Maxadilan will be used as a
WO94/17778 21 5 ~ 8 6 6 PCT~S94/01202
representative peptide in the following description of the
invention.
There are at least two va-iants of Lutzomyia protein.
The nucleotide sequence of the cDNA and the deduced amino
acid sequence of one form of mature Lutzomyia protein is as
follows:
TGT GAT GCA ACA TGC CAA TTT CGC AAG G(T)CC ATA GAT GAC
TGC GAG AAG 48
Cys Asp Ala Thr Cys Gln Phe Arg Lys Ala Ile Asp Asp
Cys Gln Lys
CAG GCG CAT CAT AGC AAT GTT TTG CAG ACT TCT GTA CAA ACA
ACT GCA 96
Gln Ala His His Ser Asn Val Leu Gln Thr Ser Val Gln Thr
Thr Ala
ACA TTC ACA TCA ATG GAT ACC TCC CAA CTA CCT GGA AAT AGT
GTC TTC l44
Thr Phe Thr Ser Met Asp Thr Ser Gln Leu Pro Gly Asn Ser
Val Phe
AAA GAA TGT ATG AAG CAG AAG AAA AAG GAA TTT AAG GCA GGA
AAG TAA l92
Lys Glu Cys Met Lys Gln Lys Lys Lys Lys Phe Lys Ala Gly
Lys (SEQ ID NO:2)
AATGATTGAA GAAAATTGTA GCCGAGGAGA GAAAGAAAGA AAGTCCCATA
CCATATTTTG 252
~ ~llAATT GTAACGAATT TTCCGAAAAA ATAA~AATATT ATGCACTCAA
TT~AAAAAAA 3l2
AAA (SEQ ID NO:l) 315
The genomic Lutzomyia protein DNA sequence is as
follows:
ATG AAA TAT TCT TTA AAT AAT CTC CAT TTT CTT GTA GAC GTT
GCT GAG 4
Met Lys Tyr Ser Leu Asn Asn Leu His Phe Leu Val Asp Val
Ala Glu
GGC TGT GAT GCA ACA TGT CAA TTT CGC AAG GCC ATA GAA GAC
TGC AGG 96
Gly Cys Asp Ala Thr Cys Gln Phe Arg Lys Ala Ile Glu Asp
Cys Arg
AAG AAG GCG CAT CAT AGC GAT GTT TTG CAG ACT TCT GTA CAA
ACA ACT l44
Lys Lys Ala His His Ser Asp Val Leu Gln Thr Ser Val Gln
Thr Thr
WO94/17778 215 5 ~ 6 6 PCT~S94/01202
GCA ACA TTT ACA TCA ATG GAT ACC TCC CAA CTA CCT GGA AGT
GGT GTT l92
Ala Thr Phe Thr Ser Met Asp Thr Ser Gln Leu Pro Gly Ser
Gly Val
TTC AAA GAA TGC ATG AAG GAG AAA GCT AAG GAA TTT AAG GCA
GGA AAG 240
Phe Lys Glu Cys Met Lys Gln Lys Ala Lys Lys Phe Lys Ala
Gly Lys (SEQ ID NO:4)
TAG (SEQ ID NO:5) ~c 243
The genomic Lutzomyia protein~DNA sequence varies
somewhat from the Lutzomyia protein cDNA sequence and is
believed to represent a variant Lutzomyia protein gene. The
genomic Lutzomyia protein DNA sequence includes the DNA
sequence and deduced amino acid sequence of a l7 amino acid
leader peptide. The signal sequence of Lutzomyia protein is
also given in the genomic DNA sequence (nucleotides 1-51).
Compositions rich in Lutzomyia protein or its active
analogs and fragments can be used in carrying out this
invention. The active analogs and fragments of Lutzomyia
protein are typically proteins or peptides comprising an
amino acid sequence sufficiently duplicative of the sequence
of the active portion of the Lutzomyia protein such that the
proteins or peptides are capable of inducing, maintaining or
increasing hair growth in a mammal. The peptides of the
present invention need not be identical to those disclosed in
International Patent Publication No. WO9l/00293. Variations
can be attributable to local mutations, which do not
substantially detract from the hair revitalizing properties
of the peptide. Such variations can be found in peptides
isolated from lysates as well as chemically or recombinantly
constructed peptides.
Knowledge of the Lutzomyia protein sequence enables the
skilled artisan to produce large quantities of the protein
for therapeutic use. The Lutzomyia protein can be
synthesized using conventional chemical solid or solution
phase peptide synthesis techniques. In addition, knowledge
of the sequence permits expression of the DNA encoding
Lutzomyia protein in various types of host cells, including
WO94/17778 2 1 5 5 8 6 6 PCT~S94/01202
both prokaryotes and eucaryotes, to produce large quantities
of the protein.
Instead of its primary amino acid structure, Lutzomyia
protein suitable for use in this invention can be
characterized by its chemical and biological properties.
Specifically, the Lutzomyia protein suitable for use in this
invention is capable of inducing vasodilation or temporary
immune suppression in a mammal. The vasodilatory activity of
the Lutzomyia protein is shown by relaxation of at least
about 100% of a constricted rabbit aortic ring as described
in International Patent Publication No. WO91/00293.
Temporary immune suppression using salivary gland extract is
demonstrated by the assay of H2O2 production by macrophages
as a marker of immune stimulation as described in
International Patent Publication No. WO91/00293 such that
H2O2 production is depressed by at least about 75~ in the
assay.
In one embodiment of the invention, the Lutzomyia
protein has a molecular weight of about 6839 daltons as
determined by mass spectrometry. In another embodiment, the
Lutzomyia protein employed in this invention is characterized
by reference to calcitonin gene-related peptide (CGRP). That
is, the Lutzomyia protein is characterized by elution prior
to CGRP in an acetonitrile-H2O-trifluoroacetic acid elution
in a reverse-phase high performance liquid chromatography
column. The Lutzomyia protein can also be characterized as
having vasodilation activity as measured by erythema
induction in animal skin of at least about 80-100 times that
of CGRP as measured by the assay described in International
Patent Publication No. WO91/00293.
An example of expression of the Lutzomyia protein by
recombinant techniques can be found in the article entitled
~Expression of Recombinant Maxadilan", "Maxadilan, Cloning
and Functional Expression of the Gene Encoding This Potent
Vasodilator Peptide", Journal of Biological Chemistry, Vol.
267, No. 2, Issue of January 15, pp. 1063, Ethan Lerner and
Charles Shoemakers authors.
W094/17778 215 5 8 6 G ~ PCT~S94/01202
There is a difference in the maxadilan peptide
synthesized chemically, the peptide produced by recombinant
techniques, and the natural peptide. The differences can be
summarized as follows.
WO94/17778 2 1 5 5 8 6 6 PCT~S94/01202
MAXADILAN
SYnthetic Recombinant Natural
- Amino 63 aa's 63 aa's + 2 or 3 61 aa's + C
acid aa's at N- terminus is
sequence terminus (whether amidated
2 or 3 is
dependent upon
plasmid used)
biological 2 - 5 x more
properties active in
vasodilation than
synthetic
(probably because
of folding of
peptides)
It will be understood that each of these peptides can be
employed in practicing this invention.
2. Enhancement of Hair Growth
The present invention also provides a method for
inducing, maintAin;ng or increasing hair growth comprising
administering Lutzomyia protein at the desired site of hair
growth. The Lutzomyia protein can be applied to the desired
site by topical application or by subcutaneous injection.
The Lutzomyia protein can be applied to the hair of a m~mm~ 1
the skin of a mammal, or both the hair and the skin to
induce, maintain, or increase hair growth in the mammal. It
is preferable that the subject is a human, although the
compositions and method of the invention can be employed with
hair and fur bearing animals, such as sheep, rabbits, mice,
rats and other mammalia. The composition of the invention
can be applied to a location proximate hair pellicles in
order to obtain the beneficial effects of the invention.
(a) ComPosition for Topical APPlication. The
composition of the invention can be formulated into a form
suitable for topical application by incorporating the
Lutzomyia protein in a suitable vehicle. The vehicle can be
a solid, semi-solid or liquid vehicle that is cosmetically
acceptable, pharmaceutically acceptable, or physiologically
acceptable, and which enables the Lutzomyia protein to be
WO94/17778 PCT~S94/0~202
2~5s866
-- 10 --
conveyed to the skin at an appropriate dilution. The nature
of the vehicle will depend upon the method chosen for topical
administration of the composition. The vehicle can itself be
inert or it can impart physiological or pharmaceutical
benefits to the composition. i
The vehicle for topical application is a substance that
acts as a diluent, dispersant, or æ~olvent for the Lutzomyia
protein and other reagents in the ~omposition so that the
composition can be applied to and distributed substantially
evenly over the hair, the scalp or both the hair and scalp at
an appropriate concentration. The vehicle is preferably one
that aids penetration of the Lutzomyia protein into the skin
to reach the immediate environment of hair pellicles.
The vehicle for topical application of the composition
of this invention can be based on water or at least one
cosmetically acceptable vehicle other than water. It will be
understood that non-aqueous cosmetically acceptable vehicles
can also be combined with water to provide a composition
suitable for topical application. Vehicles that can be
employed in the composition of the invention include solids
or liquids, such as emollients, solvents, humectants,
thickeners, and powders.
Lutzomyia protein can be utilized in the methods and
compositions of the present invention either alone, or in
combination with other known treatment agents. For example,
peptides of the present invention can be used in combination
with one or more of the following agents: vasodilators, such
as carpronium chloride, swertiae extract and acetylcholine
derivatives; amino acids, such as serine and methionine;
vit~mi ~s, such as vitamin B6, vitamin E and derivatives
thereof, and biotin, pantothenic acid and derivatives
thereof; gly~ylrhetinic acid and derivatives thereof;
nicotinates, such as benzyl nicotinate; skin-hyperactive
agents, such as female sex hormones, such as estradiol;
cepharanthine and minoxidil.
Moreover, other additives that are typically used in the
hair revitalizing compositions can be combined with the
WO94117778 PCT~S94/01202
2155866
Lutzomyia protein according to the present invention. For
example, anti-inflammatories such as hinokitiol,
hexachlorophene, benzalkonium chloride, cetylpyridinium
chloride, undecylenic acid, trichlorocarbanilide, and
bithionol; refrigerants such as menthol; agents such as
salicylic acid, zinc, and derivatives thereof, lactic acid
and alkyl esters thereof, organic acids (such as citric
acid), amino acids (such as arginine), oils (such as olive
oil), squalene, liquid paraffin, isopropyl myristate, higher
fatty acids and higher alcohols, polyhydric alcohols (such as
glycerol), propylene glycol, as well as surfactants,
perfumes, antioxidants, ultraviolet absorbers, pigments,
ethanol, water, humectants, propellants, and thickeners, can
optionally be employed in the methods and compositions of
this invention to the extent that they do not deteriorate the
effect of the invention.
Although the hair revitalizing composition according to
the present invention can be incorporated with an extremely
low level of the Lutzomyia protein, generally, the protein is
employed in the amount of about l x lO 5 to about 5% by
weight per total weight of the composition, and preferably in
an amount of about l x lO 5 to about 3% by weight per total
weight of the composition, in topical application.
The hair revitalizing composition according to the
invention can be either directly applied or sprayed on the
skin or applied by percutaneous injection. The dosage of the
hair revitalizing composition according to the invention
varies depending on age, individual differences, symptoms,
etc., but in the case of an adult, it is generally in the
range of 500 fg to 500 mg, and preferably in the range of lO0
pg to lO0 mg, Lutzomyia protein per kg of body weight per
day.
In one embodiment, maxadilan can be applied to humans in
an amount ranging from lO0 fg to lO0 mg, and preferably in
the range from lO0 pg to lO0 mg, per square cm of surface
area. Maxadilan is present in the topical composition of the
present invention in an amount of about 5.0 x lO lO to about
WO94/17778 21 S 5 8 6 6 PCT~S94/01202
5.0 x lO l percent by weight, preferably about 5.0 x lO 8 to
about 5.0 x lO 3 percent by weight. Generally, to stimulate
and enhance hair growth the composition is administered to an
adult in an amount of about lO0 fg to about lO0 mg,
preferably about lO0 pg to about lOO~mg, per kg of host body
weight per day. ~ -
Preferably, the hair pellicle~of the mammal is treateddaily between l and 4 times pe ~ ay. The exact regime used
will depend on several factors such as the condition of the
individual, but are readily determin~hle by the treating
physician. Treatment should be continued at least until the
desired effect is achieved.
The composition of this invention can be in any form
that it can be applied to the hair pellicle. For example,
the composition can be formulated as a shampoo, a
conditioner, a hair spray, a hair gel product, or a
moisturizing lotion.
(b) ComPosition for Subcutaneous Iniection. In
addition to topically applying the composition of this
invention to the subject, the composition of the invention
can also be administered to the subject by subcutaneous
injection to induce, maintain or increase hair growth.
Lutzomyia protein has an extremely good hair revitalizing
effect so that it has an efficacy dose in animals of at least
l pg per kg of host body weight per day by subcutaneous
injection. The Lutzomyia protein will not be used in an
amount exceeding about l mg per kg of body weight per day.
WO94/17778 21 5 5 8 6 6 PCT~S94/01202
- 13 -
* * *
= Maxadilan was found to be an effective hair revitalizing
agent preventing epilation and trichogenous effects. Both in
vitro and in vivo results showed that maxadilan enhances hair
growth. Organ cultures of hair follicles from mouse whisker
were used to demonstrate the effectiveness of the present
invention in vitro. -Topical application on mice and humans,
and subcutaneous injections of maxadilan, demonstrated the
effectiveness of the present invention in vivo.
The present invention will now be described in greater
detail by way of the working examples, which however, do not
in any way limit the invention. All of the amounts to be
formulated are expressed as % by weight, but Lutzomyia
protein is used in a 1 mg/10 ml strength aqueous solution as
a stock solution in Examples 1 to 5, and Lutzomyia protein is
used in the form of purified powders thereof.
EXAMPLE I
PREPARATION OF HAIR ~R~ATMENT
COMPOSITIONS CONTAINING LUT8QMYIA PROTEIN
Lutzomyia protein and an ethylene oxide (40 mol) adduct
of hardened castor oil were added to 95% ethanol, and dis-
solved by stirring. Deionized water was added and mixed to
obtain the liquid hair revitalizing composition according to
Ex. 1 below. Hair tonics according to Ex. 2-5 and
Comparative Ex. 1 were obtained similarly. The proportion of
the formulation in each case is shown below in Table 1.
W094/17778 2 ~ 5 5 8 6 6 PCT~S94/01202
- 14 -
TABLE 1
Hair Treatment Compoæitions
Cont~i n i n~ MA~A~ i 1 An As LutzomYia Protein
Ex. 1 Ex. 2 Ex~ 3 Ex. 4 Ex. 5 Comp.
` Ex. 1
L.P. * 0.01% 0.001% 0.5% 0.5% 2.5% 0%
95% Ethanol 61.0 61.9 57.0 61.5 59.5 62.0
Hardened 2.0 2.0 2.0 2.0 2.0 2.0
castor oil-
ethylene
oxide (40
mol) adduct
* L.P. represents Lutzomyia Protein, which was maxadilan
in each Ex.
** The formulation made to 100% by deionized water.
WO94/17778 2 1 5 5 8 6 6 PCT~S94/01202
EXANPLE II
DEMONSTRATION OF ~ATR GRO~T~
STTMT~.A~ION IN A TRI~UL 'CUS TEST
The hair growth-stimulating effects of compositions
prepared as described in Example I were determined in a test
termed a trichogenous test. The trichogenous tests for the
hair revitalizing compositions of Example I were carried out
using C3H/HeNCrJ mice whose hair cycle was at a resting
stage. The method used was that of Ogawa et al. ("Normal
and Abnormal Epidermal Differentiation", M. Seiji and I.A.
Berstein eds. (1982) pp. 159-170, Todal Press.)
More specifically, each test group was comprised of 10
mice. One test group received no application, while other
groups were treated with the formulations of Ex. 1 to 5 and
Comparative Ex. 1. The backs of the mice were shaved by
means of a pair of hair clippers and a shaver. Each sample
in an amount of 0.1 ml was daily topically applied. The
trichogenous (hair growth) portions at the backs of the mice
were measured, and the trichogenous effects of respective
samples were compared with each other by means of proportion
of area. The time required for 50% trichogenous rate in each
sample is shown in Table 2.
wo 94~17778 ~55~ ~ ~ PCT~S94/01202
- 16 -
TABL~ 2
~air Growth St ,ulation as
Determined in Mouse Trichoqenous Test
Sample as Described Number of~Dàys Number of
in Example I, supra Confirming 50% Accelerated Days
Trichogenous
Action
No aPPlication 60
Ex. 1 40 20
Ex. 2 50 lO
Ex. 3 30 30
Ex. 4 45 15
Ex. 5 35 25
Comparative 60 0
Ex. l
The "number of accelerated days" in Table 2 is the
difference between the number of days confirming 50%
trichogenous action for "no application" and the measured
value for the respective Ex., and is a measure of the hair
growth-stimulating effect of the composition. The greater
the number of accelerated days, the more effective the
composition is in stimulating hair growth in the trichogenous
test. Thus, with reference to Table 2, the stimulation of
hair growth was the same in test groups in which no
application was made and in the Comparative Ex. l using a
composition that did not contain Lutzomyia protein. In Ex. 1
to 5, which utilized compositions of the invention containing
Lutzomyia protein, hair growth was stimulated as evidenced by
the increase in the "number of accelerated days" when
compared with the group that received "no application" and
the group that received the composition of Comparative Ex. 1,
which did not contain Lutzomyia protein.
W094/17778 21 S 5 8 6 6 PCT~S94/01202
EXANPLE III
DEMONSTRATION OF HAIR
REVITALIZATION IN A TRIr~AM TEST
In order to ex~ine the hair revitalizing activities,
such as preventing epilation and trichogenous effect,
trichogram tests were carried out for humans. The tests were
conducted for hair revitalizing compositions of Ex. 1-5 and
Comparative Ex. 1 prepared as described in Example I.
Trichoqram Test. The hair roots of removed hairs were
microscopically observed before and after the use of the hair
revitalizing compositions, and the number of hair roots at
the resting stage was counted by observing the form of the
hair roots. The hair revitalizing activities of the
compositions were compared by the increase or decrease in the
rate of growth. The "hair roots" at the resting stage are
hair roots of the hairs whose growth has stopped, and the
proportion of the hair roots at the resting stage is
confirmed to be higher in the person suffering from epilation
than in a person not suffering from epilation.
Each of the hair revitalizing compositions according to
Ex. 1-5 and Comparative Ex. 1 was continuously applied twice
daily on the scalps of ten male subjects in an amount of 2 ml
over a period of 6 months. One hundred hairs were removed
per subject immediately before the application and 6 months
after the application, and each of the hair roots was
ex~;ned. The results are shown below in Table 3.
WO94/17778 PCT~S94/01202
2~ss~66
- - 18 -
TABLE 3
HATR REVITALIZATION AS
DE~RRMTN~n IN TRTc~or-~AM TEST
Variation of [Proportion of Rested Hair
Root After the Application]/[Proportion
of non-rested Hair Root at the Resting
State] Unit [Person] Evalu-
Sample as ation of
Described Effect
in 30 15-30% 15-30% 30% or
Example I or more decrease +15% increase more
decrease increase
Ex. 1 1 5 3 1 0 Yes
Ex. 2 1 3 5 1 0 Yes
Ex. 3 3 4 2 1 0 Yes
Ex. 4 1 4 4 1 0 Yes
Ex. 5 2 5 2 1 0 Yes
Comp. 0 1 7 2 0 No
Example 1
From the above, it is clear that the present invention
is effective in the enhancement of hair growth.
EXAMPLE TV
OIL-IN-WATER EMnLSION HAIR TONIC
An emulsion hair tonic was prepared from phase A, phase
B, phase C, phase D, and phase E having the following
compositions:
WO94/17778 215 5 8 6 6 PCT~S94/01202
-- 19 --
Phase A
Lutzomyia protein (maxadilan) 1.0
Polyoxyethylene (60 mol) adduct hardened castor oil 2.0
Glycerol lO.0
Dipropylene glycol lO.0
1,3-Butylene glycol 5.0
Polyethylene glycol 1500 5.0
Phase B
Cetylisooctanate 10.0
Squalene 5.0
Vaseiine 2.0
Propylparaben 2.0
Phase C
Aqueous 1% solution of carboxyvinyl polymer 30.0
Sodium hexametaphosphate 0.03
Deionized water 8.35
Phase D
Deionized water 4.5
Phase E
Potassium hydroxide 0.12
Deionized water 5.0
Phase A and phase B were separately and thermally melted
at 60C and then mixed with the homomixer to produce a gel.
Phase D was gradually added thereto and dispersed by means of
the homomixer.
Subsequently, the dispersed phase C was added thereto,
and finally E phase was added followed by homomixer treatment
to obtain an oil-in-water type emulsion hair tonic.
This hair tonic was applied to a person as set forth in
Example II. The hair tonic was confirmed to have excellent
hair revitalizing effects.
WO 94/17778 PCTtUS94tO1202
2~ss866
-- 20 --
E2~A~D?LE: V
CREAN EIAIR TONIC
A cream hair tonic was prepared from phase A and phase B
having the following compositions:
Phase A
Lutzomyia protein (maxadilan) 5.0
Liquid paraffin 5.0
Cetostearyl alcohol 5.5
Glyceryl monostearate 3.0
EO (20 mol)-2-octyldodecyl ether 3.0
Propylparaben 0.3
Perfume 0.1
Phase B
Glycerol 8.0
Dipropylene glycol 20.0
Polyethylene glycol 4000 5.0
Sodium hexametaphosphate 0.005
Deionized water 49.095
Phase A and phase B were each separately and thermally
dissolved and were then mixed together. The mixture was
emulsified by means of a homomixer to obtain a creamy hair
tonic. Using this hair tonic, a practical test was carried
out on a person as described in Example II. The hair tonic
was confirmed to have excellent hair revitalizing effects.
WO 94/17778 215 5 8 B 6 PCT/US94/01202
-- 21 --
E~AMPLE VI
CREAM HAIR TONIC
A cream hair tonic was prepared by mixing two phases
having the following compositions:
Phase A
Lutzomyia protein (maxadilan) 0.5
Liquid paraffin 10.5
Glyceryl monostearate 3.0
EO ( 20 mol)-2-octyldodecyl ether 3.0
Propylparaben 0.3
Perfume 0.1
Phase B
Glycerol 10.0
Dipropylene glycol 18.0
Polyethylene glycol 4000 5.0
Sodium hexametaphosphate 0.005
Deionized water 54.595
Phase A and phase B were separately and thermally
dissolved. After combining the phase A and the phase B, the
mixture was emulsified by means of a homomixer to obtain a
creamy hair tonic. Using this hair tonic, a practical test
was carried out for person as described in Example II. The
hair tonic was confirmed to have excellent hair revitalizing
effects.
WO 94/17778 PCTIUS94/01202
2lss866
-- 22 --
E~AMPLE VII
HAIR GEL
A hair gel was prepared by-combining five phases having
the following compositions: ~'
Phase A
Lutzomyia protein (maxadilan) 3.0
Polyoxyethylene (60 mol) adduct hardened castor oil 2 . 0
Glycerol 10.0
1,3-Butylene Glycol 5.0
Polyethylene glycol 1500 5.0
Phase B
Cetylisooctanate 10.0
Vaseline 8.0
Propylparaben 2.0
Phase C
Aqueous 1~ solution of carboxyvinyl polymer30.0
Sodium hexametaphosphate 0.03
Deionized water 8.35
Phase D
Deionized water 4.5
Phase E
Potassium hydroxide 0.12
Deionized water 12 . 0
Phase A and phase B were separately and thermally melted
at 60C and then mixed with homomixer to produce a gel.
Phase D was gradually added thereto and dispersed by means of
homomixer. Subsequently, the dispersed phase C was added
thereto, and finally phase E was added followed by the
homomixer treatment to obtain an oil-in-water type emulsion
hair tonic.
Using this hair tonic, a practical test was carried out
for person as described in Example II. The hair tonic was
confirmed to have excellent hair revitalizing effects.
O~ ;R rrr~ OF LUT8t~IA PROTEIN
WO94/17778 -~ 215 5 8 6 6 PCT~S94/01202
Effect on Oraan Culture of Hair Follicle of Mouse
Whisker. The Figure shows plots of the results, from which
each sample prepared from dissolving l mg of Lutzomyia
protein into physiological saline, followed by dilution as
described below, was added to the following culture medium
and hair growth was measured from incubation day by an
inserted microscope.
Orqan Culture of Hair Follicle of Mouse Whiskers. The
tissue of the whisker portion of a C3H mouse was aseptically
extracted and a hair follicle at a growth stage was isolated
by means of a microblade under a stereoscopic microscope.
Using a 24 well micro-plate and placing a membrane filter
produced by Nucleopore in l ml of a culture (DMEM:Ham Fl2 =
l:l), the isolated hair follicle was cultured in a CO2
incubator. Here, DMEM is Dulbecco's Modified Eagle Medium,
and Ham Fl2 is culture broth of Ham Fl2.
Promotion of hair growth by Lutzomyia protein depends
substantially on the amount applied. A significant effect is
achieved if the amount is over 6 ~g.
Trichoqenous Effect of Maxadilan b~ Percutaneous
Iniection. Each sample prepared by dissolving 5 ~g of the
Lutzomyia protein maxadilan into 5 ml of physiological saline
was percutaneously injected in C3H mice as described below.
Control sample was physiological saline without Lutzomyia
protein.
The backs of 58 days old C3H mice were shaved by means
of a pair of hair clippers. Each test group was comprised of
5 animals. The samples to be tested were intradermally or
percutaneously injected daily in an amount of 0.05 ml per
day, and similar injections were continued over a period of
20 days (except for Saturdays and Sundays). Thereafter, the
hair growth on the mice was observed every week over a period
of about 40 days.
The results of the evaluation are summarized in Table 4,
in which (-) means that there is no hair gro~,7th, (+) means
that there is less than 50% of the hair growth, and (+) means
that there is more than 50% of the hair growth.
WO94/17778 PCT~S94/01202
2lss866
- 24 -
Table 4
Trichogenous Effect of Lutzomyia
Protein bY ~el~uLaneous Iniection
(1) Reference ~ 2) Lutzomyia Protein
-;-` (maxadilan)
Day after Injection 43 57Day after Injection 43 57
(DaY) (DaY)
Sample No. 1 - +Sample No. 11 + +
2 - - 12 + +
3 - - 13 + +
4 _ _ 14 - +
_ +
The foregoing examples, clearly show that the present
invention has excellent trichogenous effects and hair
revitalization effects.
* * *
While the foregoing invention has been described in some
detail for purposes of clarity and understAn~ing, it will be
clear to one skilled in the art from a reading of this dis-
closure that various changes in form and detail can be made
without departing from the true scope of the invention.
i~l 21SS866
W094/17778 PCT~S94/01202
- 25 -
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: Ribeiro, Jose M.
Lerner, Ethan A.
Remold, Heinz G.
Titus, Richard G.
Tsuji, Yoshiharu
Hansawa, Chika
Uzuka, Makota
(ii) TITLE OF lNv~NlION: Method for Enhancing Hair Growth and
Hair Revitalizing Compositions Comprising Lutzomyia
Protein
(iii) NUMBER OF SEQ~NC~:S: 4
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: Finnegan, Henderson, Farabow, Garrett &
Dunner
(B) STREET: 1300 I Street, N.W., Suite 700
(C) CITY: Washington
(D) STATE: D.C.
(E) COUNTRY: USA
(F) ZIP: 20005-3315
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COM~Ul~:~: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.25
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: 08/017,061
(B) FILING DATE: February 12, 1993
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: Meyers, Kenneth J.
(B) REGISTRATION NUMBER: 25,146
(C) REFERENCE/DOCKET NUMBER: 05136-0002-00000
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: 202-408-4000
(B) TELEFAX: 202-408-4400
W094/17778 PCT~S94/01202
- 26 -
(2) INFORMATION FOR SEQ ID NO:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 315 base pairs
(B) TYPE: nucleic acid ~.
(C) STRANDEDNESS: single ~.
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: cDNA
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:1:
TGTGATGCAA CATGCCAATT TCG~AAG~CC AT~Ar~A~GAcT GCGAGAAGCA GGCGCATCAT 6
AGCAATGTTT TGCAGACTTC TG~ACAAAC~ ACTGCAACAT TCACATCAAT GGATACCTCC 12
CAACTACCTG GAAA~AGTGT CTTCAAA~-A~ TGTATGAAGC AGA~r-AAAAA GGAATTTAAG 18
GCAGGAAAGT AAAATGATTG AAGAAAATTG TAGCCGAGGA GAGAAAGAAA GAAAGTCCCA 24
TACCATATTT l~-LllGllAA TTG~AAC~A~ TTTTCCGAAA AAA~AAAATA TTATGCACTC 30
AATTTAAAAA AAAAA 31
(2) INFORMATION FOR SEQ ID NO:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 63 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:2:
Cys Asp Ala Thr Cys Gln Phe Arg Lys Ala Ile Asp Asp Cys Gln Lys
1 5 10 15
Gln Ala His His Ser Asn Val Leu Gln Thr Ser Val Gln Thr Thr Ala
Thr Phe Thr Ser Met Asp Thr Ser Gln Leu Pro Gly Asn Ser Val Phe
Lys Glu Cys Met Lys Gln Lys Lys Lys Lys Phe Lys Ala Gly Lys
WO94/17778 21 5 5 8 6 6 PCT~S94/01202
- 27 -
(2) INFORMATION FOR SEQ ID NO:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 243 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(xi) SEQU~C~: DESCRIPTION: SEQ ID NO:3:
ATGAAA~TT CTTTAAA~AA TCTCCATTTT CATGTAGACG TTGCTGAGGG CTGTGATGCA 61
ACATGTCAAT TTCGCAAGGC CATAr-AAGAC TGCAGGAAGA AGGCGCATCA TAGCGATGTT 12J
TTGCAGACTT CTGTACAAAC AACTGCAACA TTTACATCAA TGGATACCTC CCAACTACCT 18i
GGAAGTGGTG TTTTCA~r~ ATGCATGAAG GAGAAAGCTA AGGAATTTAA GGCAGGAAAG 241
TAG 24:
(2) INFORMATION FOR SEQ ID NO:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 80 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:4:
Met Lys Tyr Ser Leu Asn Asn Leu His Phe Leu Val Asp Val Ala Glu
1 5 10 15
Gly Cys Asp Ala Thr Cys Gln Phe Arg Lys Ala Ile Glu Asp Cys Arg
Lys Lys Ala His His Ser Asp Val Leu Gln Thr Ser Val Gln Thr Thr
Ala Thr Phe Thr Ser Met Asp Thr Ser Gln Leu Pro Gly Ser Gly Val
Phe Lys Glu Cys Met Lys Gln Lys Ala Lys Lys Phe Lys Ala Gly Lys