Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
FILE, P~. I IN ~'r:r~ ~215 ~ 36 g
- _ WO 94/21248 ~E~TRANSLATION PCT/EP94/00773
~se of substituted alkyl nitrates for the treatment of
pathologically raised intraocular pressure.
Field of application of the invention
The invention relates to the novel use of substituted
alkyl nitrates for the preparation of medicaments for the
- treatment of eye disorders.
Rnown technical backqround
European Patent Application EP-A-0 359 335 describes
nitrate esters which are intended to be employed for the
- 10 treatment of cardiovascular disorders. - J.A. Nathanson
[Journal of Pharmacology and Experimental Therapeutics
260, 956 (1992)] describes the topical application of
nitrovasodilators (such as nitroglycerin or isosorbide
dinitrate) to the eye for reducing the intraocular
pressure.
Description of the invention
It has now been found that the compounds known from
EP-A-0 359 335, which are described below in greater
detail, are outstandingly suitable for the treatment of
(pathologically raised) intraocular pressure.
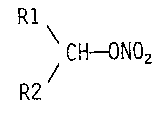
The invention relates to the use of compounds of the
formula I (see accompanying formula sheet), in which
Rl - is hydrogen (H), 1-4C-alkyl, -CH2-R8 or
-CH2-OCH2-R8, or
- together with R2 and including the CH group to
which Rl and R2 are bonded is a cyclohexyl or
bicyclo[4.4.0]decyl radical substituted by a
hydroxyl group or by a nitryloxy group, or a 2,6-
dioxabicyclo[3.3.0]oct-4-yl radical substituted in
the 8-position by the radical R3-O-,
R2 - is straight-chain or branched 1-12C-alkyl which
is substituted by a substituent selected from the
~- 2158~68
- ~_ WO 94/21248 - 2 - PCT/EP94/00773
group consisting of NH2, R3, R3-O, R4, R4-O,
R4-S, R4-NH, R4-C0-0, R4-CO-NH, R5 and R6, or
which is substituted by the two substituents R3
and R5, or which is substituted by the two
substituents R4-O and R5, or which is substituted
by the two substituents R4 and R5,
- is 2-7C-alkenyl which, if desired, is substituted
by R3,
- has the me~n; ng of R3,
- is 3-7C-cycloalkyl which, if desired, is
substituted by a substituent from the group
consisting of R4 and R5,
- is a dibenzo-5-7C-cycloalkanyl radical, or
- together with R1 and including the CH group to
which R1 and R2 are bonded is a cyclohexyl or
bicyclo[4.4.0]decyl radical substituted by a
nitryloxy group, or a 2,6-dioxabicyclo[3.3.0]oct-
4-yl radical substituted in the 8-position by the
radical R3-O-,
R3 is a methyl radical substituted by R7 and R8
i [-CH(R7)R8],
R4 - is phenyl,
- 1-4C-alkyl,
- 3-7C-cycloalkyl,
- substituted phenyl having one, two or three
identical or different substituents selected from
the group consisting of 1-4C-alkyl, 1-4C-alkoxy,
nitro, halogen, nitryloxy-1-4C-alkyl, hydroxyl
and carboxyl,
30. - naphthyl or
- thienyl which, if desired, is substituted by
halogen,
R5 is nitryloxy-1-4C-alkyl, hydroxy-1-4C-alkyl or
nitryloxy,
R6 is nitryloxy-1-4C-alkyl-tetramethylphenyl, dibenzo-
5-7C-cycloalkanyl, dibenzocycloheptenyl,
thioxanthenyl, if desired substituted by halogen,
benzo-5-7C-cycloalkanyl, if desired substituted in
the benzo moiety by nitro, or benzyloxy,
- _ WO 94/21248 _ 32 15 ~ 6 8 PCT/~P94/00773
R7 - is phenyl,
- substituted phenyl having one or two identical or
different substituents selected from the group
consisting of 1-4C-alkyl, 1-4C-alkoxy, nitro and
halogen,
- pyridyl or
- thienyl which, if desired, is substituted by
halogen, and
R8 - is phenyl or
- substituted phenyl having one or two identical or
different substituents selected from the group
consisting of 1-4C-alkyl, 1-4C-alkoxy, nitro and
halogen,
and their pharmacologically tolerable salts for the
production of medicaments for the treatment of
pathologically raised intraocular pressure, the compounds
1,9-nonanediol dinitrate and 1,10-decanediol dinitrate
being excluded.
1-4C-alkyl is straight-chain or branched alkyl radicals
20 ~ having 1 to 4 carbon atoms. Examples which may be
mentioned are the butyl, isobutyl, sec-butyl, tert-butyl,
propyl, isopropyl, ethyl and, in particular, the methyl
radical.
The 2,6-dioxabicyclo[3.3.0]oct-4-yl radical substituted
by -ONO2 in the 4-position, formed jointly including the
CH group of R1 and R2 and substituted in the 8-position
by the radical R3-O- can also be designated as an
isosorbide monon;trate radical substituted by R3.
Straight-chain or branched 1-12C-alkyl is various
straight-chain or branched alkyl radicals, of which the
radicals methyl, ethyl, propyl, isopropyl, butyl, pentyl,
hexyl, heptyl, 2-ethyl-2-butylpropyl and dodecyl may be
mentioned by way of example.
2-7C-alkenyl is straight-chain or branched alkenyl
radicals having 1 to 7 carbon atoms. Examples which may
- _ WO 94/21248 4 21 S 8 3 6 8 PcT/EP94/oo773
~ be mentioned are the vinyl, allyl, l-propenyl, l-butenyl,
2-butenyl, 3-butenyl and isopropenyl radical.
3-7C-cycloalkyl is cycloalkyl radicals having 3 to 7
carbon atoms, i.e. the cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl or cycloheptyl radical.
Dibenzo-5-7C-cycloalkanyl radicals which may be mentioned
are the dibenzocyclopentyl, dibenzocyclohexyl and, in
particular, the dibenzocycloheptyl radical.
In addition to the oxygen atom, 1-4C-alkoxy radicals
contain one of the abovementioned 1-4C-alkyl radicals.
The methoxy radical is preferred.
Halogen in the context of the present invention is
bromine, chlorine or fluorine.
Of the nitryloxy-1-4C-alkyl radicals, the nitryloxymethyl
radical (-CH2-O-NO2) and the nitryloxyethyl radical
(-CH2CH2-O-NO2) are preferred.
Benzo-5-7C-cycloalkanyl is the benzocyclopentyl (= 1-, 2-
or 3-indanyl), benzocyclohexyl (= 1-, 2-, 3- or 4-tetra-
hydronaphthyl) or the benzocycloheptyl radical.
Suitable pharmacologically tolerable salts of compounds
of the formula I are preferably all acid addition salts
with inorganic and organic acids customarily used in
pharmacy. Those suitable are water-soluble and water-
insoluble acid addition salts with acids such as, for
example, hydrochloric acid, hydrobromic acid, phosphoric
acid, nitric acid, sulfuric acid, acetic acid, citric
acid, D-gluconic acid, benzoic acid, 2-(4-hydroxyben-
zoyl)benzoic acid, butyric acid, sulfosalicylic acid,
maleic acid, lauric acid, malic acid, fumaric acid,
succinic acid, oxalic acid, tartaric acid, embonic acid,
stearic acid, toluenesulfonic acid, methanesulfonic acid
or 3-hydroxy-2-naphthoic acid, the acids being employed
~,
.- 2158368
_ WO 94/21248 - 5 - PCT/EP94/00773
in salt preparation - depending on whether a mono- or
polybasic acid is concerned and depending on which salt
is desired - in an equimolar ratio or a quantitative
ratio differing therefrom.
The compounds of the formula I are used, in particular,
in the form of those medicaments which are suitable for
the treatment of eye disorders. For the production of the
medicaments, the compounds of the formula I and/or their
ph~rm~cologically tolerable salts (= active compounds)
are preferably mixed with suitable pharmaceutical
auxiliaries and additionally processed to give suitable
pharmaceutical formulations. Suitable pharmaceutical
formulations which may be mentioned, for example, are
emulsions, suspensions, ointments or solutions (e.g. eye
drops), in which the active compound content is
advantageously between 0.1 and 99 %.
The person skilled in the art is familiar with which
auxiliaries are suitable for the desired pharmaceutical
~ $ formulations on the basis of his expert knowledge. In
addition to solvents and other active compound carriers
it is possible to use for example antioxidants,
dispersants, emulsifiers, preservatives, solubilizers or
- permeation promoters.
Compounds of the formula I which can be used according to
the invention and are to be emphasized are mentioned in
the claims.
The invention further relates to the novel compounds
coming under the formula I which are mentioned in the
claims.
The compounds of the formula I are prepared in a manner
known per se to the person skilled in the art, e.g. as
described in EP-A-0 359 335. Otherwise, the compounds can
be prepared, for example, by one of the following
methods:
~ _ WO 94/21248 - 6 _ Z 1 5 8 3 6 ~CT/EP94/00773
Method 1:
Compounds of the formula I in which R2 is 1-12C-alkyl
which is substituted by R4-CO-O and R4 is methyl are
prepared as follows: A solution of 10 mmol of the
corresponding diol in ethyl acetate is added to a
mixture, which is cooled to 0 to 5C, of 40 mmol of
acetic anhydride and 20 mmol of nitric acid. After
complete addition of the diol, the reaction mixture is
poured into an aqueous solution cont~;n;ng 100 mmol of
sodium carbonate. After extraction with ethyl acetate,
drying of the organic phase with magnesium sulfate and
concentration, the residue is purified by chromatography.
Method 2:
Compounds of the formula I which, apart from one or more
nitroxy groups, contain no other reactive groups, are
prepared from corresponding alcohols, diols or triols as
follows: A solution of the alcohol, diol or triol in
ethyl acetate is added to a mixture of acetic anhydride
and nitric acid which contains 3 equivalents of acetic
20~ anhydride and 3 equivalents of nitric acid per aliphatic
hydroxyl group of the alcohol, diol or triol. After
completion of the addition, the reaction mixture is
washed with aqueous sodium carbonate solution, dried over
magnesium sulfate and concentrated. The residue is
purified by chromatography and/or crystallization.
Method 3:
Compounds of the formula I in which R2 is 1-12C-alkyl
which is substituted by R4-CO-NH are prepared following
the method of Bodansky and Bodansky, The Practice of
peptide synthesis, page 107 (Springer-Verlag, Berlin,
1984), for example as follows: 10 mmol of the compound I
in which R2 is 1-12C-alkyl which is substituted by NH2
are dissolved in dichloromethane and added to a mixture
of 10 mmol of the carboxylic acid R4-COOH, 10 mmol of
triethylamine and 10 mmol of ethyl chloroformate in
dichloromethane. After completion of the addition, the
mixture is heated to boiling under reflux. After 30 min,
~583(~8
- _ WO 94/21248 - 7 - PCT/~P94/00773
~ the reaction mixture is allowed to cool to room temper-
ature and is then washed successively with dilute hydro-
chloric acid and aqueous sodium carbonate solution. The
organic phase is dried over magnesium sulfate and concen-
trated. The residue is purified by chromatography and/or
crystallization.
Method 4:
Compounds of the formula I in which R2 is 1-12C-alkyl
which is substituted by NH2 are prepared following the
method of Berth~nn and Ratz, Houben-Weyl, Methoden der
organischen Chemie (Methods of Organic Chemistry) 6/2,
page 357, for example as follows: A nitrate salt of the
amino~lk~nol is added to absolute nitric acid at
temperatures between -10 and -5C. After completion of
the addition, the mixture is poured into the 5-fold
volume of diethyl ether. The precipitate which is
deposited is filtered off and recrystallized in ethanol.
Method 5:
~ The compounds prepared according to Method 1 can be
hydrolyzed to hydroxyalkyl nitrates by the following
method: 10 mmol of the acyloxyalkyl nitrate obtained
according to Method 1 in 10 ml of tetrahydrofuran are
heated to 50C with 100 ml of a lN potassium hydroxide
solution. The end of the reaction is monitored by
continuous TLC checking. The reaction mixture is
concentrated and extracted with ethyl acetate. After
drying over magnesium sulfate and concentrating, the
residue is purified by chromatography.
Method 6:
Compounds of the formula I in which R1 is 1-12C-alkyl
which is substituted by R4-S are obtained, for example,
as follows: 10 mmol of the compounds R4-SH are reacted
with 10 mmol of the appropriate bromoalkyl nitrate
[prepared according to M.L. Wolfrom et al., J. Org. Chem.
35 25, 1079-1082 (1960)] at a temperature below 60C in the
presence of 10 mmol of sodium hydride in dimethyl-
` 21S8368
WO 94/21248 - 8 - PCT/EP94/00773
~ formamide. After concentrating the reaction solution, the
residue is purified by chromatography.
Method 7:
Alternatively to Method 5, hydroxyalkyl nitrates can be
prepared directly from the correspo~; n~ diols as
followæ: 10 mmol of a diol in ethyl acetate are added to
a mixture of 15 mmol of acetic anhydride and 10 mmol of
nitric acid. The reaction mixture is worked up as
described in Method 2.
Examples
The following compounds were prepared according to one of
the methodæ described above:
1. 5-Acyloxy-1-pentanol nitrate, oil, NMR data (CDCl3):
1.24-1.95 ppm, m, 6.0 H -(C-(CH2)3-C); 2.04 ppm, s, 3.0 H
15 (COCH3); 4.05 ppm, t, 2.0 H, J=6.1 Hz, 2.0 H (CH2OCO);
4.42 ppm, t, J=6.2 Hz, 2.0 H (CH2ONO2) (Method 1).
2. 1.3-Di(4-nitrophenyl)-2-propanol nitrate, m.p. 116-
121C (Method 2).
3. 6,7,8,9-Tetrahydro-5H-3-nitrobenzocycloheptyl-5-
20 ethanol nitrate, oil, NMR data (CDCl3): 1.40-2.58 ppm, m,
8.1 H (aliphatic H); 2.67-3.30 ppm, m, 2.9 H (phenyl-
CH2+phenyl-CH); 4.30-4.70 ppm, m, 1.9 H (CH2-O);
7.06-7.42 ppm, m, 1.6 H (arom. H); 2.82-8.07 ppm, m, 1.6
H (arom. H) (Method 2).
4. 2-Phenoxyethanol nitrate, oil, NMR data (CDCl3):
4.25 ppm, t, J=4.5 Hz, 2.0 H (Ph-O-CH2); 4.8 ppm, t,
J=4.5 Hz, 2.0 H (CH2-ONO2); 6.74-7.56 ppm, m, 5.0 H (arom.
H) (Method 2).
-
5. 3-(4-Hydroxyphenyl)propanol nitrate, oil, NMR data
(CDCl3): 1.86-2.30 ppm, m, 4.0 H, (C-CH2(C-ONO2);
2.76 ppm, t, J=8.1 Hz, 2.0 H (Ph-CH2-); 4.44 ppm, t,
_ WO 94/21248 2 15 8 36p8cT/Epg4/oo773
J=6.3 Hz, 2.0 H, (CH2-O-); 7.00-8.00 ppm, m, 4.0 H,
(arom. H); 10.44 ppm, 1.0 H, (HO) (Method 2).
6. 1,7-Heptanediol dinitrate, oil, NMR data (CDCl3):
1.27-2.07 ppm, m, 10.0 H (C-(CH2)5-C); 4.45 ppm, t,
5 J=6.2 Hz, 4.0 H (2xCH2ONO2) (Method 2).
7. 9-Acetoxy-l-nonanol nitrate, oil, NMR data (CDCl3):
1.24-1.95 ppm, m, 14.0 H (C-(CH2)7-C); 2.04 ppm, s, 3.0 H
(COCH3); 4.05 ppm, t, 2.0 H, J=6.1 Hz, 2.0 H (CH2OCO);
4.42 ppm, t, J=6.2 Hz, 2.0 H (CH2ONO2) (Method 1).
8. 2-(3-Methylphenyl)ethanol nitrate, oil, NMR data
(CDCl3): 2.32 ppm, s, 3.0 H (CH3); 3.04 ppm, t, J=7.2 Hz,
2.0 H, (Ph-CH2-); 4.60 ppm, t, J=6.3 Hz, 2.0 H (CH2-O);
6.88-7.30 ppm, m, 4.0 H (arom. H) (Method 2).
9. 1,2-Octanediol dinitrate, oil, NMR data (CDCl3):
0.76-2.10 ppm, m, 13.4 H (-(CH2)5-CH3); 4.30-4.86 ppm, m,
2.0 H (CH2ONO2); 5.02-5.42 ppm, m, 1.0 H, (CHONO2)
(Method 2).
10. 2-(2-Methylphenyl)ethanol nitrate, oil, NMR data
(CDCl3): 2.32 ppm, s, 2.9 H (CH3); 3.00 ppm, t, J=7.2 Hz,
1.9 H (phenyl-CH2); 4.58 ppm, t, J=7.2 Hz, 1.9 H,
(CH2ONO2); 6.94-7.28 ppm, t, J=7.2 Hz, 1.9 H (CH2-ONO2);
6.94-7.28 ppm, m, 4.0 H (arom. H) (Method 2).
11. 2-(4-Nitrophenoxy)ethanol nitrate, m.p. 97.0-98.6C
(Method 2).
12. 3-Benzyl-1,5-pentanediol dinitrate, oil, NMR data
(CDC13): 1.50-2.14 ppm, m, 5.5 H (CH2-CH-CH2); 2.66 ppm,
d, J=6.7 Hz, 1.8 H (phenyl-CH2); 4.40 ppm, t, J=6.3 Hz,
3.7 H (2xCH2-O); 7.01-7.41 ppm, m, 5.0 H (arom. H)
(Method 2).
13. N-(2-Nitroxyethyl)benzamide, m.p. 71-72C (Method 3).
` 2158368
_ WO 94/21248 - 10 - PCT/EP94/00773
14. 2-Aminoethanol nitrate, m.p. 103C (Method 4).
15. 4-Penten-1-ol nitrate, oil, NMR data (CDC13):
1.62-2.32 ppm, m, 4.1 H, (CH2-CH2); 4.44 ppm, t, J=6.3 Hz,
1.9 H (CH2ONO2); 4.92-5.16 ppm, m, 1.9 H (=CH2); 5.54-6.02
ppm, m, 1.0 H (=CH) (Nethod 2).
16. 1,8-Octanediol dinitrate, oil, NMR data (CDCl3): 1.2-
1.94 ppm, m, 12.1 H (-(CH2)6-); 4.48 ppm, t, J=6.3 Hz, 4.0
H (2xCH2ONO2) (Method 2).
17. 1,6-Hexanediol ~o~o~;trate, oil, NMR data (CDCl3):
1.18-2.00 ppm, m, 8.0 H, (C-(CH2)4-C); 2.78 ppm, broad
signal, 1.0 H (OH); 3.62 ppm, t, J=6.1 Hz, 2.0 H (CH2-
hydroxyl); 4.45 ppm, t, J=6.5 Hz, 2.0 H (CH2-ONO2) ~Method
5).
18. 2-(N-Methylamino)ethanol nitrate, m.p. 74-75C
(Method 4).
19. 1-(4-Nitrophenoxy)-2,3-propanediol dinitrate, m.p.
128-131C (Method 2).
20. 1-(2-Nitrophenoxy)-2,3-propanediol dinitrate, m.p.
91-93C (Method 2).
21. 2-(4-Fluorophenoxy)ethanol nitrate, oil, NMR data
(CDCl3): 4.18 ppm, t, 2.0 H, J=3.6 Hz, phenoxy-CH2); 4.76
ppm, t, 2.0 H, J=3.6 Hz, (CH2-ONO2); 6.56-7.12 ppm, m, 4.0
H (arom. H) (Method 2).
22. 2-(3-Nitrophenoxy)ethanol nitrate, oil, NMR data
(CDCl3): 4.34 ppm, t, 2 H, J=5.4 Hz, (phenoxy-CH2); 4.86
ppm, t, J=5.4 Hz, 2.0 H, (CH2-ONO2); 7.14-7.98 ppm, m, 4 H
(arom. H) (Method 2).
23. 2-(4-t-Butylphenyl)-1,3-propanediol dinitrate, m.p.
38.1-40.9C (Method 2).
2158368
~ WO 94/21248 - 11 - PCT/EP94/00773
24. 3-(3,5-Dinitro-4-methoxyphenoxy)-1,2-propanediol
dinitrate, m.p. 122.4-123.7C (Method 2).
25. 2-Phenylmercaptoethanol nitrate, oil, NMR data
(CDCl3): 3.17 ppm, t, J=7.2 Hz, 2.0 H (SCH2); 4.52 ppm, t,
J=7.2 Hz, 2.0 H (CH2-O); 7.06-7.52 ppm, m, 5.3 H (arom.
H) (Method 6).
26. Cyclohexylmethanol nitrate, oil, NMR data, (CDCl3):
0.70-2.42 ppm, m, 11 H, (cyclohexyl-H); 4.26 ppm, d,
J=6.3 Hz, 2 H (CH2-ONO2) (Method 2).
27. cis-1,2-Di(hydroxymethyl)cyclohexane dinitrate, oil,
NMR data (CDCl3): 1.12-2.44 ppm, m, 10.0 H (cyclohexyl-
H); 4.44 ppm, d, J=7.2 Hz, 4.0 H (2xCH2ONO2) (Method 2).
28. Cyclobutylmethanol nitrate, oil, NMR data (CDCl3):
1.54-3.02 ppm, m, 7.0 H, (cyclobutyl-H); 4,44 ppm, d,
J=7.2 Hz, 2.0 H (CH2ONO2) (Method 2).
-~ 29. 2-Cyclohexylethanol nitrate, oil, NMR data (CDCl3):
0.66-2.00 ppm, m, 13.0 H, (cyclohexyl-H); 4.52 ppm, t,
J=6.3 Hz, 2.0 H, (CH2ONO2) (Method 2).
30. 2-(4-Methoxyphenoxy)ethanol nitrate, oil, NMR data
(CDCl3): 3.76 ppm, s, 3.1 H, (O-CH3); 4.08-4.34 ppm, m,
2.0 H, (phenoxy-CH2); 4.66-4.94 ppm, m, 1.9 H, (CH2-ONO2);
6.82 ppm, s, 4.0 H, (arom. H) (Method 2).
31. 2-Nitroxyethyl 4-hydroxy-3-nitrobenzoate, m.p.
64-65C (Method 2).
32. 2-Nitroxyethyl 4-hydroxybenzoate, m.p. 88-91C
(formed as a further reaction product in the preparation
of the compound of Example 31 and isolated by
chromatography).
33. trans-1,2-Di(hydroxymethyl)cyclohexane dinitrate,
30 oil, NMR data (CDCl3): 0.94-2.20 ppm, m, 10 H,
215~368
_ WO 94/21248 - 12 - PCT/EP94/00773
(cyclohexyl-H); 4.46 ppm, double d, 4 H (2xCH2-ONO2)
(Method 2).
34. 2-Nitroxyethyl 2-hydroxy-3-methylbenzoate, oil, NMR
data (CDCl3): 2.26 ppm, s, 3.0 H (CH3); 4.52-4.95 ppm, m,
4.1 H (CH2CH2); 6.70-6.96 ppm, m, 1.0 H (arom. H);
7.25-7.49 ppm, m, 1.0 H (arom. H); 7.61-7.83 ppm, m,
1.0 H, (arom. H); 10.80 ppm, s, 1.0 H (OH) (Method 2).
35. 2-Nitroxyethyl 4-hydroxy-3-methyl-5-nitrobenzoate,
m.p. 91-93C (Method 2).
36. 2-Nitroxyethyl 2-hydroxy-5-nitrobenzoate, m.p.
88.7-89.9C (Method 2).
37. 2-Nitroxyethyl 2-hydroxybenzoate, oil, NMR data
(CDCl3): 4.44-4.98 ppm, m, 4.1 H, (CH2-CH2);
6.74-7.96 ppm, m, 4.1 H, (arom. H); 10.50 ppm, s, 0.9 H,
(OH) (Method 2).
38. 2-Nitroxyethyl 2-hydroxy-3-nitrobenzoate, m.p.
77.7-78.2C (Method 2).
39. 2-Nitroxyethyl 5-hydroxy-2-nitrobenzoate, m.p.
83.1-83.5C (Method 2).
40. 2-Nitroxyethyl 3-hydroxybenzoate, oil, NMR data
(CDCl3): 3.66-4.20 ppm, m, 3.9 H (CH2-CH2); 5.56-7.00 ppm,
m, 5.1 H, (arom. H, OH) (Method 2).
41. 2-Nitroxyethyl 3-hydroxy-4-nitrobenzoate, oil, NMR
data (CDCl3): 3.76-4.14 ppm, m, 3.7 H (CH2-CH2);
6.14-7.38 ppm, m, 4.3 H, (arom. H, OH) (Method 2).
42. trans-1,4-Di(hydroxymethyl)cyclohexane mononitrate,
oil, NMR data (CDCl3): 0.82-2.06 ppm, m, 11.0 H,
(cyclohexyl-H, OH); 3.50 ppm, d, J=5.8 Hz, 2.0 H
(hydroxy-CH2); 4.30 ppm, d, J=6.8 Hz, 2.0 H (CH2ONO2)
(Method 7).
.- 21~31~8
_ WO 94/21248 - 13 - PCT/EP94/00773
43. 2-Nitroxyethyl benzoate, oil, NMR data (CDCl3):
4.42-4.92 ppm, m, 4.0 H (2xCH2); 7.31-7.72 ppm, m, 3.0 H,
(arom. H); 7.89-8.18 ppm, m, 2.0 H (arom. H) (Method 2).
44. 2-Nitroxyethyl 4-hydroxy-3-methoxy-5-nitrobenzoate,
m.p. 94.3-95.4C (Method 2).
45. 2,4-Dinitro-N-(2-nitroxyethyl)aniline, m.p.
132.5-135.9C (Method 2).
46. 4-(2-Nitroxyethoxy)benzoic acid, m.p. 156.7-157.3C
(Method 2).
47. 3-(2-Nitroxyethoxy)benzoic acid, m.p. 106.3-106.6C
(Method 2).
48. 2-(2-Nitroxyethoxy)benzoic acid, m.p. 36.6-37.7C
(Method 2).