Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
21~82
--1--
UCFl COMPOUNDS DERIVATIVES THEREOF AND
PROCESSES FOR THEIR PREPARATION
This is a division of copending Canadian Patent
Application Serial No. 2,041,237 filed April 25, 1991.
The present invention relates to compounds
hereinafter collectively designated UCFl, derivatives
thereof and processes for their preparation. The compounds
and derivatives of the present invention exhibit anti-
bacterial and anti-tumour activities and may be used for the
preparation of anti-bacterial and anti-tumour agents.
The following known compounds have anti-bacterial
and anti-tumour activities:
(1) Manumycin:
HN ~
C~13 C~13 CH3 ~
0~ Nff~
[disclosed in J. Antibiotics, 40 (11) 1530 (1987)].
(2) Asukamycin and U-56407:
R
H t'~
-- , NH~
~ HO
8~g2
--2--
{wherein R is either
,'1 ~
s lJ
[Asukamycin disclosed in J. Antibiotics, 29 (9) 876
(1976)]
or
"1 ,~
[U-56407, ibid. 36 (8) 950 (1983)~}.
- (3) MT36531:
O
H~NHCOCH 3
o'l
H--~T/~H
O
_ [disclosed in J. Antibiotics, 36 (12) 1631 (1983)]
The present invention is based upon the discovery
that new compounds having high anti-bacterial and anti-
tumour activities may be obtained by fermentation of a
microorganism of the genus Streptomyces, which we have
isolated from the soil at Numazu-shi, Shizuoka-ken,
Japan.
Therefore the present invention is directed to
provide new compounds and derivatives having high
anti-bacterial and anti-tumour activities and processes
for their preparation.
- 21~82
--3--
According to one aspect of the present invention,
there are provided compounds represented by the
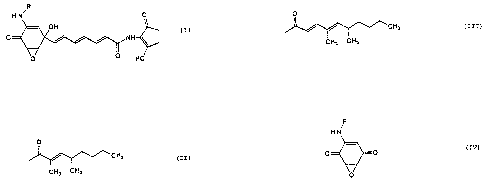
following formula (I):-
,R
HN ~
~=\ OH \~
0 ~ JH~ (I)
[wherein R represents either
0
CH3 (II)
CH3 CH3
20or
CH3 (III)
_ CH3 C~3
] -
Compounds of the formula (I) wherein R represent (II)
and (III) are respectively designated UCF1-A and
UCF1-B hereinafter.
It has been found that the compounds of the
formula (I) are new compounds and may be used for the
preparation of anti-bacterial and anti-tumour agents
by virtue of their physiological activities.
2~8~82
Another aspect of the present invention, claimed in
a divisional application, provides derivatives of UCF1,
represented by the following formula (IV):-
H N
O ~ ~ O (IV)
[wherein R represents either
~ CH3
(V)
CH3 CH3
or
~ ~ ~ - C~3 (VI)
CH3 CH3
~ ] -
Compounds of the formula (IV) wherein R represent (V)
and (VI) are designated respectively UCFl-AX and
UCFl-~X hereinafter.
The above-mentioned derivatives may be used for
the preparation of anti-bacterial and anti-tumour
agents by virtue of their anti-bacterial and anti-tumour
activities.
1 8 2
-
--5--
The compounds UCF1 of the present invention may
be produced by fermentation of a microorganism of the
genus Streptomyces capable of producing UCFl in a
medium to accumulate the desired compounds in the
cultured broth and the cells, and recovering the
desired compounds therefrom.
UCF1-AX and UCF1-BX, the derivatives of UCF1
of the present invention may respectively be obtained
by subjecting cr ~w ~d UCF1-A and UCFl-~ to oxidation
in an organic solvent in a conventional manner.
The physico-chemical characteristics of UCF1-A,
UCF1-~, UCF1-AX and UCF1-B~ are as follows:-
UCF1-A:
1) Appearance: yellow powder
2) Melting point: >96~C (decomposed)
3) Molecular formula: C28H34N207
4) Secondary ion mass spectrum (m/z): 511 (M+1)+
5) Elemental analysis:
Calculated (+1/2 H2O): C 64.73, H 6 79, N 5.3g
Found C 64.69, H 6.74, N 5.27
6) Specific rotation: ta ]D = -159
(c=0.1 in chloroform)
7) W absorption spectrum (in methanol):
_ ~ max nm ( ) 265(29,600), 282(30,300), 313(31,100)
8) IR absorption spectrum (KBr tablet method):
cm 1 3398, 1672, 1624, 1603, 1514, 1367, 1005
9) Colour reàction:
Positive in the reactions with vanillin/sulfuric
acid, Ehrlich's reagent, iodine and ferric chloride.~0 10) Solubility in various solvents:
Soluble in chloroform, dimethylsulfoxide, ethyl
acetate, methanol and acetone.
Insoluble in n-hexane and water.
1 8 ~
--6--
11) lH-NMR spectrum (in CDC13): ~ (ppm)
13.62 t1H, br), 7.95 (lH, s), 7.85 (lH, brs),
7.38 (lH, d), 7.32 (lH, dd), 6.54 ~ 6.64 (2H, m),
6.41 (lH, dd), 6.21 (lH, dd), 6.12 (lH, d), 5.85
(lH, d), 3.71 (lH, dd), 3.65 (lH, d), 2.57
(4H, brs), 2.43 ~ 2.53 (lH, m), 1.8g (3H, d),
1.16~ 1.48 (6H, m), 1.00 (3H, d), 0.88 (3H, t)
12) 13C-NMR spectrum (in chloroform): ~ (ppm)
197.4 (s), 189.0 (s), 174.4 (s), 168.1 (s),
165.6 (s), 144.8 (d), 143.4 (d), 139.5 (d),
136.5 (d), 131.7 (d), 131.5 (d), 129.2 (s),
128.0 (s), 126.5 (d), 121.7 (d), 115.1 (s),
71.2 (s), 57.4 (d), 52.9 (d), 36.6 (t), 33.3 (d),
32.2 (t), 29.7 (t), 25.9 (t), 22.8 (t), 20.1 (q),
14.0 (~), 12.6 (~)
UCFl-B:
1) Appearance: yellow powder
2) Melting point: ~188 ~ (decomposed)
3) Molecuiar formula: C30H36N2O7
4) Secondary ion mass spectrum (m/z): 537 (M+l)
5) Elemental analysis:
Calculated (+1/2 H2O): C 66.04, H 6.84, N 5.13
Found C 65.64, H 6.84, N 4.92
7) Speci~ic rotation: [ a ] 24= +101 ~
(c=0.1 in chloroform)
8) W absorption spectrum (in methanol):
~ max nm( ) 279(54,500), 315(38,800)
9) IR absorption spectrum (KSr tablet method):
cm 1 3354, 1664, 1630, 1616, 1597, 1522, 1363,
1014
10) Colour reaction:
Positive in the reactions with vanillin/sulfuric
acid, Ehrlich's reagent, iodine and ferric chloride.
~1~8~2
--7--
11) Solubility in various solvents:
Soluble in chloroform, dimethylsulfoxide,ethyl
acetate, methanol and acetone.
Insoluble in n-h~n~ and water.
12) lH-NMR spectrum (in dimethylsulfoxide-d6): ~ (ppm)
9.72 (lH, brs), 9 14 (lH, s), 7.19 (lH, dd),
7 13 (lH, d), 7.08 (lH, d), 6.72 (lH, dd),
6.54 (lH, dd), 6.51 (lH, d), 6.50 (lH, dd),
6.40 (lH, d), 6.26 (lH, brs), 5.98 (lH, d),
5.68 (lH, d), 3.69 (lH, dd), 3.66 (lH, d),
2.45~ 2.55 (lH, m), 2 40 (4H, s), 1.74 (3H, s)
1.12~ 1.38 (6H, m), 0.94 (3H, d), 0.83 (3H, t)
13) 13C-NMR spectrum (in dimethylsulfoxide-d6):~ (ppm)
189 5 (s), 186.g (s), 165.6 (s), 165.2 (s),
146.9 (d), 145.g (d), 141.6 (d), 139.2 (d),
139.1 (d), 131.4 (d), 131.2 (s), 130.0 (d),
128.8 (d), 128.1 (s), 123.0 (d), 119.5 (d),
114 3 (s), 70.7 (s), 56.5 (d), 52.4 (d),
36.4 (t), 32.5 (d), 29.2 (t), 29.0 (t),
22.2 (t), 20.4 (q), 13.9 (q), 12.4 (q)
UCFl-AX:
1) Appearance: yellow oil
2) Molecular formula: C16H21NO4
- 3) Electron ionization mass spectrum (m/z): 291 (M)
4) High resolution mass spectrum (m/z):
Calculated as C16H21NO4
291.1470
Found: 291.1436
5) Specific rotation: [ a ]D4= +16~
(c= 0 2 in chloroform)
6) W a~sorption spectrum in methanol:
~ max nm( ~ ) 326(3,900),
- ~lS8~82
--8--
7) IR absorption spectrum (KBr tablet method):
cm~l 3386, 1676, 1608, 1504,
8) Colour reaction:
Positive in the reactions with vanillin-sulfuric
acid, Ehrlich's rea~ent, iodine and ferric chloride.
9) Solubility in various solvents:
Soluble in chloroform, methanol and diethyl ether.
Insoluble in water.
10) lH-NMR spectrum (in CDC13): ~ (ppm)
8.25 (lH, brs), 7.58 (lH, d), 6.27 (lH, dd),
3.93 (lH, d), 3.84 (lH, dd), 2.46 ~ 2.57 (lH, m),
1.92 (3H, d), 1.18~ 1.48 (6H, m), 1.03 (3H, d),
0.89 (3H, t)
11) 13C-NMR spectrum (in CDC13): ~ (ppm)
191.1 (s), 188.5 (s), 167.7 (s), 146 3 (d),
139.0 (s), 129.3 (s), 115.1 (d), 53.9 (d), 52.6 (d),
36.5 (t), 33.5 (d), 29.7 (t), 22.8 (t), 20.0 (q),
14.0 (q), 12.6 (q)
UCFl-BX:
1) Appearance: yellow oil
2) Molecular formula: C18H23NO4
3) Electron ionization mass spectrum (m/z): 317 (M)+
4) Hi~h resolution mass spectrum (m/z):
Calculated as C18H23N04
317.1627
Found: 317.1614
5) Specific rotation: [~ ]D4= -14~
(c=0.2 in chloroform)
6) W absorption spectrum (in methanol):
~ max nm (~ ) 270(16,500), 327(14,300)
7) IR absorption spectrum (KBr tablet method):
cm~l 3313, 1695, 1670, 1604, 1506
2~8'1~2
8) Colour reaction:
Positive in the reactions with vanillin-sulfuric
acid, Ehrlich's reagent, iodine and ferric chloride.
9) Solubility in various solvents:
Soluble in chloroform, methanol and diethyl ether.
Insoluble in water.
10) lH-NMR spectrum (in CDC13): ~ (ppm)
7.86 (lH, brs), 7.63 (lH, d), 7.36 (lH, d),
5.~5 (lH, d), 5.78 (lH, d), 3.g2 (lH, d),
3.83 (lH, dd), 2.50 ~ 2.60 (lH, m), 1.80 (3H, d),
1.14~ 1.43 (6H, m), 1.00 (3H, d), 0.87 (3H, t)
11) 13C NMR spectrum (in CDC13):~ (ppm)
191.0 (s), 188.3 (s), 165.2 (s), 151.1 (d), 150.5
(d), 139.1 (s), 131.0 (s), 116.5 (d), 115.2 (d),
53.9 (d), 52.5 (d), 36.9 (t), 33.5 (d), 29'.7 (t),
22.8 (t), 20.4 (q), 14.0 (q), 12.5 (q)
- The physiological characteristics of the present
compounds and derivatives are as follows.
(A) Anti-bacterial activity:
Mi n i ~ inhibitory conc~ntration (~ g/ml) was
measured by the agar dilution method using ~ medium
(pH=7.0) which was prepared by dissolving Bacto-
tLy~tone medium (3 g; commercial product of Difco,
U.S.A.), meat e2tract (3 g), yeast e2tract (1 g),
glucose (1 g) and agar (16 g) in water (1 ¢ ). The
results are shown in the following Table 1.
21~g~182
-- --10--
TABLE 1
Notes:-
A..UCF1-A; B..UCF1-B; AX..UCF1-AX; BX..UCF1-BX;
Ma..Manumycin (control);
l..Staphylococcus aureus ATCC 6538P;
2..Enterococcus faecium ATCC 10541;
3..Bacillus subtilis No.10707;
4..Klebsiella pneumoniae ATCC 10031;
5..Escherichia coli ATCC 26;
6..Pseudomonas aeruginosa Bin H No.1;
7..Salmonella typhi ATCC 9992;
8..Shigella sonnei ATCC 9290;
9..Candida albicans ATCC 10231
Organism A B AX BX Ma
1 2.6 2.6 10 8.3 1.3
2 10 10 10 0.65 2.6
3 10 1.3 1.30.16 0.3
4 >100 >100 21 >100 >100
>100 >100 21 >100 >100
6 >100 >100 83 83 >100
7 >100 >100 21 >100 >100
8 >100 >100 10 >100 >100
9 >100 >100 10 42 21
215~ 82
(B) Anti-tumour activity:
MCF7 cells (ATCC HTB22) was add to RPMI-1640
medium (commercial product of Gibco, U.S.A.) cont~ining
10 % fetal calf serum, insulin (10~ g/ml) and estradiol
(10 8M) (hereinafter referred to as medium A) to
prepare a cell suspension containing 4.5 X 104 cell/ml.
On each occasion, 0.1 ml of the suspension was
poured into each well of a 96 well microtitre plate
and incubated at a temperature of 37~C for 20 hours in
a carbon dioxide incubator. Then a test compound
(each 0.05 ml) was added to each well, followed by
incubation similarly for 72 hours. After removal of
the the supernatant, another medium (0.1 ml) containing
medium A and 0.0Z% neutral red was added to the material
for incubating similarly for one hour to dye the cells.
The supernatant was removed, and the residue was washed
once with physiological Saline. Then the dyestuff was
removed by using an aqueous solution of ethanol (30 %)
containing 0.001N hydrochloric acid.
The absorptivity at 550 nm of the material was
measured by the use of a microplate reader. The
absorptivity of the treated cells was c~ ~-red with
the absorptivity of untreated cells to measure the IC50
value viz. the concentration of the test compound
required to inhibit the growth of the cells by 50%.
The results are shown in the following table.
TABLE 2
Compound IC50 (~ g/ml)
UCFl-A 12.5
UCFl-B 7.3
UCF1-AX 18.9
UCF1-BX 7.5
Manumycin (control) 8.3
2 ~ '1 8 2
-12-
Preparation of compounds UCF1:
Compounds UCFl of the present invention may be
obtained by culturing a microorganism belonging to
the genus Streptomyces capable of producing UCFl in
a medium to accumulate UCFl in the cultured broth,
and recovering the desired compounds therefrom.
For the purpose of the present invention, it is
possible to use any and all strains of the genus
Streptomyces such as, for example, naturally-occurring
or artificially-induced mutant strains thereof so far
as they are capable of producing the desired compounds
UCFl by fermentation.
In one preferred embodiment, a strain designated
by us as Streptomyces sp. UOFl is used.
The following mycological characteristics of
strain UOF1 were investigated using the
E.B. Sherling method, recom~-n~ by the ISP
-- (International Streptomyces Project) for identifying
the characteristics of the strains of Streptomyces as
well as by using the D. Gottlieb method disclosed in Int.
J. Syst. Bacteriol., 16, 313-340 (1966~. Diaminopimellic
acid isomers were obtained by hydrolysis of the cell
wall and identified with reference to the method of
B. Becker et al [Appl. Microbiol., 12, 421-423 (lg64)].
The morphology of the cell surfaces was observed by
_ using an optical microscope, and the surfaces of the
spore was observed by a sC~nni ng electron microscope.
The colour names were designated with reference to
Color Harmony M~nll~l, the 4th Edition, published by
the Container Corpn. of America (1958).
(l) Morphology:
Aerial mycelium: branched.
Vegetative hypha: br~nch~. No fragment.
Spore: growing as a curly or loopy long chain having
at least 10 to 30 arthrospores on the aerial
mycelium.
~1J8482
-13-
Surface of the spore: smooth.
Shape and size of the spore: oval, 0.5 X 0.7 ~ m.
Sclerotium and sporangium: not found.
(2) Colour tone:
Aerial mycelium: gray.
Vegetative hypha: light yellow.
Melanoid pigment: not found.
(3) Chemical composition of the cell wall:
Stereotype of diaminopimellic acid: LL type
(4) Assimilability of carbon sources:
Assimilable:
glucose, xylose, mannitol, arabinose, rhamnose,
inositol, lactose, galactose.
Not assimilable:
raffinose, salicin, sucrose.
Liquefaction of gelatin: negative.
Hydrolysis of starch: positive.
Coagulation of skimmed milk: negative.
Decomposition of cellulose: positive.
Growth temperature*: 16 to 37 ~C (optimal 28 to 32~C )
(* Measured after culturing for 2 days).
The actions of this strain for gelatin, skimmed
milk and cellulose were observed after culturing at a
temperature of 28 ~ for one month. Other characteristics
were observed after culturing at a temperature of 28~C
- for two weeks.
( 5 ? Growth on various agar media:
The following table indicates the results which
were obtained by culturing strain UOF1 on various media
at a temperature of 28~C for two weeks.
2lsgll~2
-14-
TABLE 3
Agar medium Results
Sucrose-nitrate G : Poor
AM: Moderate. White(a)
SM: Colourless
P : Not found
Glucose-asparagine G : Good
AM: Abundant. Natural(3dc)~ white(a)
SM: Pearl pink(3ca) ~ light tan(3gc)
P ; Found. Light yellow.
Glycerol- G : Good.
asparagine AM: Abundant. Grieg(lfe)~ pearl(3ba)
SM: Cork tan(4ie) ~ light apricot(4ea)
P : Found. Light yellow.
Starch G : Good.
AM: Abundant. Grieg(lfe)~ pearl(3ba)
SM: Dusty coral(6gc)~ cedar(61e)
- P : Not found.
Tyrosine G : Good.
AM: Abundant. Grieg(lfe)
SM: Dusty coral(6gc)~ copper tan(5ie)
P : Found. Light yellow.
Nutrient - G : Good.
AM: Moderate. Grieg(lfe)
SM: Copper tan(5ie) ~ dark redwood
_ (61g)
P : Found. Light yellow.
Yeast-malt-extract G : Good.
AM: Moderate. Fresh pink(6ca)
SM: Light persimmon(5ic)
P : Found. Light yellow.
Oatmeal G : Good.
AM: Abundant. Natural(3dc)
SM: Cocoa brown(51g)
P : Found. Light yellow.
~1 ~,8~2
_ -15-
TABLE 3 (continued)
Agar medium Results
Peptone-yeast G : Good.
extract-iron AM: Not found.
SM: Peach tan(5gc)
P : Not found.
Notes:-
G... Growth degree.
AM.. Formation and colour tone of aerial mycelia.
SM.. Colour tone of vegetative hyphae.
P... Colour tone of soluble pigment.
(6) Identification of strain UOFl:
With respect to the existence of LL-type diamino-
pimellic acid, strain UOF1 had preliminarily been
classified into the Cell wall I-type according to the
classification of Actinomycetes by M.P. Lechevalier
- and H.A. Lechevalier [Int. J. Syst. Bacteriol., 20,
435-443 (1970)]. As a result of further studies of
the morphological characterisitics of UFOl, this strain
has been classified into Streptomyces.
With regard to the identification of the species
of strain UOF1, various species recognized by learned
societies [Int. J. Syst. Bacteriol. 30, 225-420 (1980)
authored by V.D.B. Skerman et al.] were studied to discover
_ a candidate on the basis of various characterisitics
such as the aerial mycelium coloured in gray to white,
the spore chain in the form of a curl or loop, the
smooth surface of spore, the productivities of
melanoid pigment and soluble pigment as well as the
assimilability of carbon sources, with reference to
the reports by ISP [ibid., 18, 69-189 (1968); ibid.,
I8, 279-392 (1968); ibid., 19, 391-512 (1969); and
ibid., 22, 265-394 (1972)] and to Bergy~s ~n~ Of
Determinative Bacteriology, [8th Edition, edited
by R.E. BuchAn~n and N.E. Gi hhonc ] . As a result, it
- 21~3~182
_ -16-
was noted that Streptomyces olivaceiscleoticus is a
relevant species, but it was impossible to identify
the species of strain UOF1. Thus strain UOF1 has been
designated as Streptomyces sp.
Strain UOFl has been filed with the Fermentation
Research Institute, Agency of Industrial Science and
Technology, located at 1-1, Higashi l-chome, Tsukuba-shi,
Ibaraki-ken, Japan on 30th March 1990 on the basis of
the Budapest Treaty, the deposition number being FERM
~P-2844.
The microorganisms which may be used for the
process of the present invention may be cultured in
a conventional manner applicable to fermentation of
microorganisms of Actinomycetes. Thus, both organic
and synthetic media may be used so far as they contain
appropriate amounts of assimilable sources of carbon,
nitrogen and inorganic substances.
- Preferred e~amples of carbon sources which may
be used for this process include glucose, starch,
de~trin, mannose, fructose, sucrose, lactose, xylose,
arabinose, mannitol and molasses. If desired, it is
possible to use, for e~ample, various hydrocarbons,
alcohols and organic acids, depen~i ng upon the
assimilability of the microorganism used. The carbon
sources may be used solely or in combination.
_ Preferred examples of nitrogen sources which may
be used solely or in combination for this process
include ammonium chloride, ammonium sulfate, ~mmonium
nitrate, sodium nitrate, urea, peptone, meat e~tract,
yeast e~tract, dried yeast, corn steep liquor, soyabean
powder and cazamino acid.
It is also possible, if desired, to add to the
medium various substances such as, for example,
sodium chloride, potassium chloride, magnesium sulfate,
calcium carbonate, potassium dihydrogen phosphate,
dipotassium hydrogen phosphate, ferrous sulfate,
21~ 8 L1 8 2
-17-
calcium chloride, manganese sulfate, zinc sulfate and
copper sulfate. If desired, it is also possible to add
to the medium suitable substances capable of promoting
the growth of the microorganism or the productivity of
the desired compounds UCF1 such as for example, vitamin
Bl and biotin.
For fermentation, submerged culturing in liquid
medium with sh~ki ng is preferred. Culturing may be
effected, at a temperature of from 16 to 37 ~C ,
preferably from 25 to 32~C and at a pH of from 4 to 10,
preferably from 6 to 8, usually for a period of from
1 to 7 days to accumulate the desired compounds UCF1
in the cultured broth and the cells. During culturing,
the pH of the medium may be adjusted by using, for
example, an aqueous solution of ammonia or ammonum
carbonate.
When the amount of the desired product rP~rhes
the maximum, the culturing is discontinued. The
isolation of the desired compounds from the cultured
broth and cells and the following purification may
be effected in a conventional m~nner which may be used
for isolation and purification of metabolic products
obtained by fermentation of microorganism, for example,
as follows:-
The cells are separated from the cultured broth
_ by filtration and extracted with chloroform or acetone.
The e~tracted solution is combined with the filtrate.
The combined solutions are passed through a column
packed with a polystyrene-type adsorbing agent (Diaion
HP20, commercial product of Mitsubishi Kasei K.K.,
Japan) to adsorb the active material. Elution may be
effected by the use of ethyl acetate, acetone and the
like. The eluate is concPntrated and subjected to
silica gel chromatography, high performance liquid
chromatography and the like to obtain UCF1 in the form
of yellow powders.
-18-
In the course of isolation and purification, the
desired compounds may be detected, for example, by
bioassay using Bacillus subtilis No. 10707, thin layer
chromatography and absorption of ultraviolet rays and
S appropriate colour reactions.
(7) Preparation of UCFl-AX and UCF1-BX:
It is possible to obtain UCF1-AX and UCF1-B~ in
a conventional manner by subjecting the compounds UCFl-A
and UCF1-B respectively to oxidation with a suitable
oxidizing agent such as, for example, chromium (VI),
lead tetraacetate (IV) and mercury oxide in a solvent
such as, for esample, acetic acid, benzene,
dimethylsulfoside and pyridine. The solvents may be
used solely or in combination.
The amount of the oxidizing agent is preferably
from 1 to 10 mol on the basis of the amount of the
starting compound. The reaction may be effected at a
temperature of from 0 to 50 ~ for a suitable period of
time (usually l to 24 hours) which may vary, dep~n~ing
upon, for esample, the amount of the oxidizing agent
employed and the reaction temperature.
Progress of the reaction may be monitored, for
e~ample, by thin layer or colllmn chromatography. The
reaction may preferably be continued until the starting
compound is not found in the reaction solution.
_ The isolation of the desired derivatives from the
starting compound and the following purification may
be effected by conventional methods of organic chemistry,
for example, solvent extraction, colllmn chromatography,
thin layer chromatography and preparative high performance
liquid chromatography.
The following non-limiting esamples illustrate
the invention.
EXAMPLE 1
Streptomyces sp. UOF1 (FERM BP-2844) was used as
a seed strain. The seed strain was cultured in a seed
~ 3~2
--19--
medium (300 ml) put in a 2Q Erlenmeyer flask at a
temperature of 30 JC for 48 hours with shaking (200
r.p.m.). The medium contained Bacto-tryptone (5g/Q ;
commercial product of Difco. U.S.A.), yeast extract
(5g/¢ ), meat extract (3g/¢ ), soluble starch (lOg/¢ ),
glucose (10g/¢ ) and calcium carbonate (5g/¢ ) and
had an adjusted pH of 7.2 after sterilization.
A main medium (15 Q ) was put in a 30 ¢ jar
fermenter for culturing the resultant seed (10 v/v %
on the basis of the main medium) at a temperature
of 28 ~ with sh~king and aeration (200 r.p.m.;15 ¢ /
min). The composition of the main medium was as
follows:-
soluble starch (50 g); corn steep liquor (30 g);
potassium phosphate (0.5 g); magnesium sulfate- 7H2O
(0.5 g) and calcium carbonate (5 g) [per ¢ ].
The pH of the medium was adjusted to 7.0 with NaOH
~ before sterilization and was left during culturing
which was effected for 80 hours. After completion of
fermentation, n-propanol (15¢ ~ was added to the
cultured broth (28¢ ) with 5h~king, which was then
fiLtered to separate the cells and precipitates.
The filtrate was conrentrated and diluted with
water. The material was passed through a col-lmn packed
with a polysLyLelL~-type adsorbing agent (10 ¢ ; Diaion*
- HP20, commercial product of Mitsubishi Kasei K.K.,
Japan). After removal of impurities by elution with
deionized water and 50% methanol successively, elution
was further effected successively by using methanol
and ethyl acetate. The eluate was concentrated and
water was added thereto, followed by extraction with
ethyl acetate. The e~tracted solution was dehydrated
using anhydrous sodium sulfate and conc~ntrated.
Then the material was transferred to a coln~n packed
with silica gel (BW300, commercial product of Fuji
Devison Kagaku K.K., Japan) and developed by using
* TRADE-MARK
1 8 ~
-20-
a solvent system of chloroform/methanol (100:1 v/v).
The eluate was collected and concentrated.
The concentrated material was subjected to high
performance liquid chromatography using a reversed
phase silica gel colnmn (YMC R-335-20, commercial
product of YMC K.K.,Japan) and a solvent system of
methanol/50mM potassium phosphate (~:2 v/v, pH=7). The
eluate was divided into small fractions (each 1~ ml).
Fraction Nos. 20-21 were collected, combined and passed
through a column packed with Diaion*HP20 to adsorb the
active substance onto the adsorbing agent. The colllmn
was washed with water and eluted with methanol to yield
UCF1-A (63 mg) in the form of yellow powders.
Separately, fraction Nos 23-32 were treated in a
similar manner to that described above to obtain UCFl-B
(51 mg) in the form of yellow powders.
UCFl-A and UCFl-B gave an Rf value of 0.3~ by thin
layer chromatography on silica gel (Art. 5715; commercial
product of Merck A.G., Germany) in methanol. The retention
times of UCFl-A and UCFl-B were respectively 4.68 and
6.57 minutes when subjected to high performance liquid
chromatography using a silica gel column (YMC AM312 ODS,
commercial product of YMC K.K., Japan) and a solvent
system of 50mM potassium diphosphate/potassium monophosphate
(7:3 v/v, pH=7) at a flow rate of 1 ml/min.
_ EXAMPLE 2
UCFl-A (55 mg) obtained by the method of Example
1 was dissolved in acetic acid (2.5 ml). A 60% aqueous
solution ~2.5 ml in total) containing chromium (VI)
(34 mg) was added to the UCF1-A solution five times
every hour at room temperature while stirring the
mixture for six hours. After ~ing 2N hydrochloric acid
(22ml), the reaction mixture was extracted five times
with ether (125 ml). The organic layer was collected
and dried by using anhydrous sodium sulfate, followed
by filtration. The residue was conc~ntrated under
* TRADE-MARK
~1~8~2
-21-
reduced pressure. The resultant crude product was
subjected to column chromatography using a silica gel
column and chloroform to obtain purified UCFl-AX (5.4 mg)
in the form of a yellow oil.
EXAMPLE 3
UCFl-B (43 mg) obtained by the method of E~ample
1 was dissolved in acetic acid (4.0 ml). A 60% aqueous
solution (2.0 ml in total) cont~ining chromium (VI)
(29 mg) was added to the UCFl-B solution four times
every hour at room temperature while stirring the
mi~ture for six hours. After ~ddi n~ 2N hydrochloric
acid (20ml), the reaction mi~ture was estracted five
times with ether (150 ml). The organic layer was
collected and dried by using ~nhydrous sodium sulfate,
followed by filtration. The residue was con~ntrated
under reduced pressure. The resultant crude product
was chromatographed in a similar to that described in
Example 2 to obtain purified UCFl-B~ (4.6 mg) in the
form of a yellow oil.