Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ W094/2~080 1 2 1 6 1 7 8 S PCT~S94/04710
INJECTABLE POLY~C~TnE-CELL CONPOSITIONS
Background of the Invention
The present invention is generally in the
area of using polysaccharide hydrogel-cell
compositions in the area of medical treatments, and
specifically relates to a method for correcting
vesicoureteral reflux, incontinence and other
defects.
Craniofacial contour deformities
Craniofacial contour deformities, whether
traumatic, congenital, or aesthetic, currently
require invasive surgical tPrhn;ques for
correction. Furthermore, deformities requiring
augmentation often necessitate the use of
alloplastic prostheses which suffer from problems
of infection and extrusion. A ~;n;~lly invasive
method of delivering additional autogenous
cartilage or bone to the craniofacial skeleton
would minimize surgical trauma and eliminate the
need for alloplastic prostheses. If one could
transplant via injection and cause to engraft large
numbers of isolated cells, one could augment the
craniofacial osteo-cartilaginous skeleton with
autogenous tissue, but without extensive surgery.
Unfortunately, attempts to inject
dissociated cells subcutaneously or to implant
dissociated tissues within areas of the body such
as the peritoneum have not been successful. Cells
are relatively quickly removed, presumably by
phagocytosis and cell death.
Cells can be implanted onto a polymeric
matrix and implanted to form a cartilaginous
structure, as described in U.S. Patent No.
5,041,138 to Vacanti, et al., but this requires
surgical implantation of the matrix and shaping of
the matrix prior to implantation to form a desired
anatomical structure.
2 1 ~ 5
W094/25080 PCT~S94/04710
Vesicoureteral reflux.
Vesicoureteral reflux is a condition
wherein there is an abnormal development of the
ureteral bud as it enters the bladder during
embryologic development. The shortened course of
the ureter through the bladder musculature
decreases the ureteral resistance and allows for
urine to reflux from the bladder reservoir back up
into the ureter and into the kidney. With this
condition, bacteria which may occasionally be
present in the bladder through retrograde urethral
transport, can reach the kidneys and cause
recurrent pyelonephritis. In addition, the
constant back pressure of the urine into the
calyces and renal pyramids results in mechanical
damage to the renal parenchyma. If untreated,
urinary vesicoureteral reflux can cause loss of
renal parenchyma, and in some instances, renal
failure, as reviewed by Atala and Casale,
Infections in Uroloqy 39-43 (March/April l990). In
1960, 70% of the patients with renal failure were
described as having vesicoureteral reflux as the
primary etiology. With the advent of new
diagnostic and treatment modalities, patients with
vesicoureteral reflux now account for less than 1%
of the renal failure population.
In the past, vesicoureteral reflux was
usually diagnosed with a voiding cystogram after
the child presented with repeated episodes of
pyelonephritis. With the increased use of prenatal
and postnatal sonography, hydronephrosis is more
detectable, prompting further radiologic workup and
earlier detection, as reported by Atala and Casale.
Vesicoureteral reflux is graded depending on the
severity. Grade l reflux signifies that urine is
seen refluxing from the bladder up to the ureter
only; in grade 2 reflux, urine refluxes into the
~ W094/25080 21 6 1 7 8 5 PCT~S94/04710
--3--
ureter and calyceal dilatation. Grade 4 and 5
reflux are more severe, showing ureteral tortuosity
and further calyceal blunting and dilatation,
respectively.
The treatment of vesicoureteral reflux has
been well established over the last decade.
Initially it was believed that all patients with
reflux would require surgery. Another school of
management soon proposed that only medical therapy
with antibiotics was required. It is now well
established that the treatment of reflux depends on
many factors, including the severity of reflux,
associated congenital abnormalities, and the social
situation of the child (parental compliance with
medical treatment). Medical treatment is usually
recommended for patients with grade 1 and 2 reflux,
which usually resolve on their own as the
bladder/ureteral configuration changes with growth.
Grade 3 reflux is generally managed with medical
therapy unless it persists or breakthrough
infections occur while on antibiotic suppression.
Surgical treatment is usually required for grade 4
and 5 reflux.
Medical treatment implies that the patient
is treated with daily suppressive antibiotics. A
close follow-up is required in these patients,
generally consisting of a catheterized urine
culture every three months, an ultrasound exam and
serum analysis every six months, a fluoroscopic or
nuclear voiding cystourethrogram every year, and a
DMSA renal scan every two years. Surgical treatment
consists of an open surgery wherein a low abdominal
incision is made, the bladder is entered, the
ureters are mobilized and new ureteral submucosal
tunnels are created; thereby extending the muscular
backing of the ureter which increases their
resistance. These patients require a general
W094/25080 PCT~S94/04710
--4--
endotracheal anesthetic for a four to five hour
surgery, an epidural catheter for both
intraoperative and postoperative pain control, a
bladder catheter for drainage, a perivesical drain,
and a five to six day hospital stay. Antibiotic
therapy and bladder antispasmodics are required
post-operatively.
Although open surgical procedures for the
correction of reflux have excellent results in the
hands of experienced surgeons, it is associated
with a well recognized morbidity, including pain
and immobilization of a lower abdominal incision,
bladder spasms, hematuria, and post-operative
voiding frequency in some children. In an effort
to avoid open surgical intervention, widespread
interest was initiated by Matouschek's clinical
experience with the endoscopic injection of TeflonTM
(polytetrafluoroethylene) paste subureterally in
1984, as reported in Matouschek, E.: Die
Behandlung des vesikorenalen Refluxes durch
transueterale Einspritzung von
polytetrafluoroethylenepast. Uroloqe, 20:263
(1981). With this technique, a cystoscope is
inserted into the bladders, a needle is inserted
through the cystoscope and placed under direct
vision underneath the refluxing ureter in the
submucosal space, and TeflonTM paste is injected
until the gaping ureteric orifice configuration
changes into a half-moon slit. The TeflonTM paste,
injected endoscopically, corrects the reflux by
acting as a bulking material which increases
ureteral resistance. However, soon after the
introduction of this treatment, a controversy
regarding the use of TeflonTM paste ensued. Malizia
et al. "Migration and granulomatous reaction after
periurethral injection of polymer
(polytetrafluoroethylene)" JAMA, 251:3277 (1984),
r
~W094125080 21617 ~ 5 PCT~S94/04710
~5~
showed granuloma formation and particulate
migration to the brain, lungs, and lymph nodes in
animal studies. Polytetrafluoroethylene migration
and granuloma formation have also been reported in
5 humans by Claes et al., "Pulmonary migration
following periurethral polytetrafluoroethylene
injection for urinary incontinence" J. Urol.,
142 821 (1989)~ The safety of Teflon~ for human
use was questioned, and the paste was thereafter
banned by the FDA.
However, there are definite advantages in
treating vesicoureteral reflux endoscopically. The
method is simple and can be completed in less than
fifteen minutes, it has a success rate of greater
15 than 85% with low morbidity and it can be performed
in an outpatient basis, as reported by Atala et al,
"Endoscopic treatment of vesicoureteral reflux with
a self-detachable balloon system" J. Urol. 148 724
(1992) ~ The goal of several investigators has been
to find alternate implant materials which would be
safe for human use.
Bovine dermal collagen preparations have
been used to treat reflux endoscopically. However,
only 58 ~ 5% of the patients were cured at one year
25 follow-up, as described by Leonard et al,
"Endoscopic injection of glutaraldehyde cross-
linked bovine dermal collagen for correction of
vesicoureteral reflux" J. Urol. 145 115 (1991).
The collagen implant volume decreases with time,
30 which results in high percentage of recurrence of
reflux, over 90% within 3 years. The high failure
- rate with this substance presents a high risk to
the unaware patient of developing renal damage
- after treatment.
A paste consisting of textured
microparticles of silicone, suspended in a
hydrogel, has been injected subureterally to
S
W094/25080 - PCT~S94/04710
~ . .
--6--
correct reflux with an initial success rate of 91%
in one European study, as reported by Buckley et
al., "Endoscopic correction of vesicoureteric
reflux with injectable silicone microparticles" ~.
Urol. 149: 259A (1993). However, distant particle
migration has been observed in animal models, as
reported by Henly et al., "Particulate silicone for
use in periurethral injections: a study of local
tissue effects and a search for migration" J. Urol.
147:376A (1992). Approximately thirty percent of
the silicone particles have a diameter which is
less than lOO ~m. This suggests that thirty
percent of the silicone particles have a potential
for distant organ migration through the macrophage
system. The manufacturer of this technology tried
unsuccessfully to obtain FDA approval, and
subsequently filed for bankruptcy.
Laparoscopic correction of reflux has been
attempted in both an animal model (Atala et al,
"Laparoscopic correction of vesicoureteral reflux"
J. Urol. 150:748 (1993)) and humans (Atala,
"Laparoscopic treatment of vesicoureteral reflux"
Dial. Ped. Urol. 14:212 (1993)) and is technically
feasible. However, at least two surgeons with
laparoscopic expertise are needed, the length of
the procedure is much longer than with open
surgery, the surgery is converted from an
extraperitoneal to an intraperitoneal approach, and
the cost is higher due to both increased operative
time and the expense of the disposable laparoscopic
equipment.
Despite the fact that over a decade has
transpired since the TeflonTM controversy, little
progress has been made in this area of research.
The ideal substance for the endoscopic treatment of
reflux should be injectable, non-antigenic, non-
~ W094/2~080 21617 8 5 PCT~S94/04710
--7--
migratory, volume stable, and safe for human use(Atala et al, 1992).
Urinary incontinence.
Urinary Incontinence is the most common and
the most intractable of all GU maladies. Urinary
incontinence, or the inability to retain urine and
not void urine involuntarily, is dependent on the
interaction of two sets of muscles. One is the
detrusor muscle, a complex of longitudinal fibers
forming the external muscular coating of the
bladder. The detrusor is activated by
parasympathetic nerves. The second muscle is the
smooth/striated muscle of the bladder sphincter.
The act of voiding requires the sphincter muscle be
voluntarily relaxed at the same time that the
detrusor muscle of the bladder contracts. As a
person ages, his ability to voluntarily control the
sphincter muscle is lost in the same way that
general muscle tone deteriorates with age. This
can also occur when a radical event such as
paraplegia "disconnects" the parasympathetic
nervous system causing a loss of sphincter control.
In different patients, urinary incontinence
exhibits different levels of severity and is
classified accordingly.
The most common incontinence, particular in
the elderly, is urge incontinence. This type of
incontinence is characterized by an extremely brief
warning following by immP~; ate urination. This
type of incontinence is caused by a hyperactive
detrusor and is usually treated with "toilet
training" or medication. Reflex incontinence, on
the other hand, exhibits no warning and is usually
the result of an impairment of the parasympathetic
nerve system such as a spinal cord injury.
Stress incontinence is most common in
elderly women but can be found in women of any age.
216~
W094125080 PCT~S94104710
, 8-
It s also commonly seen in pregnant women. This
type of incontinence accounts for over half of the
total number of cases. It is also found in men but
at a lower incidence. Stress incontinence is
characterized by urine leaking under conditions of
stress such as sneezing, laughing or physical
effort. There are five recognized categories of
severity of stress incontinence, designated as
types as O, l, 2a, 2b, and 3. Type 3 is the most
severe and requires a diagnosis of intrinsic
Sphincter Deficiency or ISD (Contemporary Urology,
March 1993). There are many popular treatments
including weight loss, exercise, medication and in
more extreme cases, surgical intervention. The two
most common surgical procedures involve either
elevating the bladder neck to counteract leakage or
constructing a lining from the patient's own body
tissue or a prosthetic material such as PTFE to put
pressure on the urethra. Another option is to use
prosthetic devices such as artificial sphincters to
external devices such as intravaginal balloons or
penile clamps. For treatment of type 3 stress
incontinence, there has been a recent trend toward
injection of Teflon~ or collagen paste around the
sphincter muscle in order to "beef up" the area and
improve muscle tone. None of the above methods of
treatment, however, are very effective for periods
in excess of a year.
Overflow incontinence is caused by
anatomical obstructions in the bladder or
underactive detrustors. It is characterized by a
distended bladder which leads to frequent urine
leakage. This type of incontinence is treated
acutely by catheterization and long-term by drug
therapy. Enuresis or bed-wetting is a problem in
pediatrics and is controlled by various alarming
devices and pads with sensors. Enuresis is not
~ W094/25080 21 G 1 7 8 ~ PCT~S94/04710
_g_
considered a serious problem unless it lasts beyond
the age of four or five. Finally, there is true
functional incontinence which occurs in patients
with chronic impairment either of mobility or
mental function. Such patients are usually treated
by the use of diapers, incontinence pads or
continuous catheterization (BBI, lg85 Report 7062).
Accordingly, it is an object of the present
invention to provide a method and compositions for
injection of cells to form cellular tissues and
cartilaginous structures.
It is a further object of the invention to
provide compositions to form cellular tissues and
cartilaginous structures including non-cellular
material which will degrade and be removed to leave
tissue or cartilage that is histologically and
chemically the same as naturally produced tissue or
cartilage.
It is another object of the present
invention to provide a method and material for
treating vesicoureteral reflux, incontinence, and
other defects which results in a natural and
permanent cure to the defect.
It is a further object of the present
invention to provide a method and material for
treating vesicoureteral reflux, incontinence, and
other defects which is quick, simple, safe, and
relatively non-invasive.
8ummary of the Invention
Slowly polymerizing, biocompatible,
biodegradable hydrogels have been demonstrated to
be useful as a means of delivering large numbers of
isolated cells into a patient to create an organ
equivalent or tissue such as cartilage. The gels
promote engraftment and provide three dimensional
W094/2~080 216 ~ ~ ~ 5 - - PCT~S94/04710
--10--
templates for new cell growth. The resulting
tissue is similar in composition and histology to
naturally occurring tissue. In one embodiment,
cells are suspended in a hydrogel solution and
injected directly into a site in a patient, where
the hydrogel hardens into a matrix having cells
dispersed therein. In a second embodiment, cells
are suspended in a hydrogel solution which is
poured or injected into a mold having a desired
anatomical shape, then hardened to form a matrix
having cells dispersed therein which can be
implanted into a patient. Ultimately, the hydrogel
degrades, leaving only the resulting tissue.
This method can be used for a variety of
reconstructive procedures, including custom molding
of cell implants to reconstruct three dimensional
tissue defects, as well as implantation of tissues
generally.
A method of treatment of vesicoureteral
reflux, incontinence and other defects is described
wherein bladder muscle cells are mixed with a
liquid polymeric material, to form a cell
suspension, which is injected into the area of the
defect, in an amount effective to yield a tissue
that corrects the defect, for example, which
provides the required control over the passage of
urine. As described in the examples, human bladder
muscle specimens or chondrocytes are obtained and
processed, the cells are mixed with alginate, which
is designed to solidify at a controlled rate when
contacted with calcium salts, and the cells are
then injected at the desired site where they
proliferate and correct the defect. Examples
demonstrate efficacy in mice and pigs.
~ W094/25080 21617 8 5 PCT~S94104710
--11--
Brief Description of the Drawings
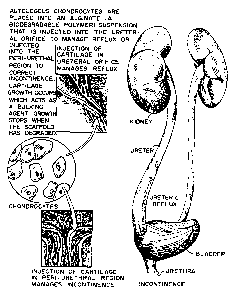
Figures la-ld are a schematic of injection
of a polymer-chondrocyte suspension into a region
of the ureteral orifice for control of
vesicoureteral reflux or into the peri-urethral
region to manage incontinence; with
photomicrographs showing the alginate polymer
suspension, new cartilage formed following
injection of the suspension, and a cartilage cell
control injected in the absence of polymer.
Detailed Description of the Invention
Techniques of tissue engineering employing
biocompatible polymer scaffolds hold promise as a
means of creating alternatives to prosthetic
materials currently used in craniomaxillofacial
surgery, as well as formation of organ equivalents
to replaced diseased, defective, or injured
tissues. However, polymers used to create these
scaffolds, such as polylactic acid,
polyorthoesters, and polyanhydrides, are difficult
to mold and hydrophobic, resulting in poor cell
attachment. Moreover, all manipulations of the
polymers must be performed prior to implantation of
the polymeric material.
Calcium alginate and certain other polymers
can form ionic hydrogels which are malleable and
can be used to encapsulate cells. In the preferred
embodiment described herein, the hydrogel is
produced by cross-linking the anionic salt of
alginic acid, a carbohydrate polymer isolated from
seaweed, with calcium cations, whose strength
increases with either increasing concentrations of
calcium ions or alginate. The alginate solution is
mixed with the cells to be implanted to form an
D SHEET (RULE 91
P
W094l2508o 2 ~ 6 ~ 7 ~ 5 PCT~S94/04710 ~
-12-
alginate suspension. Then, in one embodiment, the
suspension is injected directly into a patient
prior to hardening of the suspension. The
suspension then hardens over a short period of
time. In a second embodiment, the suspension is
injected or poured into a mold, where it hardens to
form a desired anatomical shape having cells
dispersed therein.
Source of Cells
Cells can be obtained directed from a
donor, from cell culture of cells from a donor, or
from established cell culture lines. In the
preferred embodiment, cells of the same species and
preferably immunological profile are obtained by
biopsy, either from the patient or a close
relative, which are then grown to confluence in
culture using standard conditions, for example, as
described below in Example l and used as needed.
If cells that are likely to elicit an immune
reaction are used, such as human muscle cells from
immunologically distinct individual, then the
recipient can be immunosuppressed as needed, for
example, using a schedule of steroids and other
immunosuppressant drugs such as cyclosporine.
However, in the most preferred embodiment, the
cells are autologous.
In the preferred embodiments, cells are
obtained directly from a donor, washed and
implanted directly in combination with the
polymeric material. The cells are cultured using
techniques known to those skilled in the art of
tissue culture.
Cells obtained by biopsy are harvested and
cultured, passaging as necessary to remove
cont~min~ting cells. Isolation of chondrocytes and
muscle cells are demonstrated in the examples.
~ W094125080 2 ~ 617 8 5 PCT~S94104710
-13-
Cell attachment and viability can be
assessed using scanning electron microscopy,
histology, and quantitative assessment with
radioisotopes. The function of the implanted cells
can be determined using a combination of the above-
te~-h~;ques and functional assays. For example, in
the case of hepatocytes, in vivo liver function
studies can be performed by placing a cannula into
the recipient's common bile duct. Bile can then be
collected in increments. Bile pigments can be
analyzed by high pressure liquid chromatography
looking for underivatized tetrapyrroles or by thin
layer chromatography after being converted to
azodipyrroles by reaction with diazotized
azodipyrroles ethylanthranilate either with or
without treatment with P-glucuronidase.
Diconjugated and monoconjugated bilirubin can also
be determined by thin layer chromatography after
alkalinemethanolysis of conjugated bile pigments.
In general, as the number of functioning
transplanted hepatocytes increases, the levels of
conjugated bilirubin will increase. Simple liver
function tests can also be done on blood samples,
such as albumin production. Analogous organ
function studies can be conducted using techniques
known to those skilled in the art, as required to
determine the extent of cell function after
implantation. For example, islet cells of the
pancreas may be delivered in a similar fashion to
that specifically used to implant hepatocytes, to
achieve glucose regulation by appropriate secretion
of insulin to cure diabetes. Other endocrine
tissues can also be implanted. Studies using
labelled glucose as well as studies using protein
assays can be performed to quantitate cell mass on
the polymer scaffolds. These studies of cell mass
can then be correlated with cell functional studies
W094l2508o 2 1 6 1 7 8 5 PCT~S94/04710 ~
-14-
to determine what the appropriate cell mass is. In
the case of chondrocytes, function is defined as
providing appropriate structural support for the
surrounding attached tissues.
This technique can be used to provide
multiple cell types, including genetically altered
cells, within a three-dimensional scaffolding for
the efficient transfer of large number of cells and
the promotion of transplant engraftment for the
purpose of creating a new tissue or tissue
equivalent. It can also be used for
immunoprotection of cell transplants while a new
tissue or tissue equivalent is growing by excluding
the host immune system.
Examples of cells which can be implanted as
described herein include chondrocytes and other
cells that form cartilage, osteoblasts and other
cells that form bone, muscle cells, fibroblasts,
and organ cells. As used herein, "organ cells"
includes hepatocytes, islet cells, cells of
intestinal origin, cells derived from the kidney,
and other cells acting primarily to synthesize and
secret, or to metabolize materials.
Addition of Biologically Active Materi~ls
to the hydrogel.
The polymeric matrix can be combined with
humoral factors to promote cell transplantation and
engraftment. For example, the polymeric matrix can
be combined with angiogenic factors, antibiotics,
antiinflammatories, growth factors, compounds which
induce differentiation, and other factors which are
known to those skilled in the art of cell culture.
For example, humoral factors could be mixed
in a slow-release form with the cell-alginate
suspension prior to formation of implant or
transplantation. Alternatively, the hydrogel could
be modified to bind humoral factors or signal
~ W094/25080 21617 8 5 -- PCT~S94l04710
--15--
recognition sequences prior to combination with
isolated cell suspension.
Polymer Solutions
In the preferred embodiment described
herein, calcium alginate and certain other polymers
that can form ionic hydrogels which are malleable
are used to encapsulate cells. The hydrogel is
produced by cross-linking the anionic salt of
alginic acid, a carbohydrate polymer isolated from
seaweed, with calcium cations, whose strength
increases with either increasing concentrations of
calcium ions or alginate. The alginate solution is
mixed with the cells to be implanted to form an
alginate suspension. Then the suspension is
injected directly into a patient prior to hardening
of the suspension. The suspension then hardens
over a short period of time due to the presence in
vivo of physiological concentrations of calcium
ions.
The polymeric material which is mixed with
cells for implantation into the body should form a
hydrogel. A hydrogel is defined as a substance
formed when an organic polymer (natural or
synthetic) is cross-linked via covalent, ionic, or
hydrogen bonds to create a three-dimensional open-
lattice structure which entraps water molecules to
form a gel. Examples of materials which can be
used to form a hydrogel include polysaccharides
such as alginate, polyphosphazines, and
polyacrylates, which are crosslinked ionically, or
block copolymers such as PluronicsTM or TetronicsTM,
polyethylene oxide-polypropylene glycol block
copolymers which are crosslinked by temperature or
pH, respectively. Other materials include proteins
such as fibrin, polymers such as
polyvinylpyrrolidone, hyaluronic acid and collagen.
W094/25080 2 ~ 61~ 8 5 PCT~S94/04710 ~
-16-
In general, these polymers are at least
partially soluble in aqueous solutions, such as
water, buffered salt solutions, or aqueous alcohol
solutions, that have charged side groups, or a
monovalent ionic salt thereof. Examples of
polymers with acidic side groups that can be
reacted with cations are poly(phosphazenes),
poly(acrylic acids), poly(methacrylic acids),
copolymers of acrylic acid and methacrylic acid,
poly(vinyl acetate), and sulfonated polymers, such
as sulfonated polystyrene. Copolymers having
acidic side groups formed by reaction of acrylic or
methacrylic acid and vinyl ether monomers or
polymers can also be used. Examples of acidic
groups are carboxylic acid groups, sulfonic acid
groups, halogenated (preferably fluorinated)
alcohol groups, phenolic OH groups, and acidic OH
groups.
Examples of polymers with basic side groups
that can be reacted with anions are poly(vinyl
amines), poly(vinyl pyridine), poly(vinyl
imidazole), and some imino substituted
polyphosphazenes. The ammonium or quaternary salt
of the polymers can also be formed from the
backbone nitrogens or pendant imino groups.
Examples of basic side groups are amino and imino
groups.
Alginate can be ionically cross-linked with
divalent cations, in water, at room temperature, to
form a hydrogel matrix. Due to these mild
conditions, alginate has been the most commonly
used polymer for hybridoma cell encapsulation, as
described, for example, in U.S. Patent No.
4,352,883 to Lim. In the Lim process, an aqueous
solution containing the biological materials to be
encapsulated is suspended in a solution of a water
soluble polymer, the suspension is formed into
~vo 94/2s08n 2 1 6 1 7 8 5 ~CT~S941~4710
droplets which are configured into discrete
microcapsules by contact with multivalent cations,
then the surface of the microcapsules is
crosslinked with polyamino acids to form a
semipermeable membrane around the encapsulated
materials.
Polyphosphazenes are polymers with
backbones consisting of nitrogen and phosphorous
separated by alternating single and double bonds.
Each phosphorous atom is covalently bonded to two
side chains ("R"). The repeat unit in
polyphosphazenes has the general structure (1):
-(-P = N~)n~
where n is an integer.
The polyphosphazenes suitable for cross-
linking have a majority of side chain groups whichare acidic and capable of forming salt bridges with
di- or trivalent cations. Examples of preferred
acidic side groups are carboxylic acid groups and
sulfonic acid groups. Hydrolytically stable
polyphosphazenes are formed of monomers having
carboxylic acid side groups that are crosslinked by
divalent or trivalent cations such as Ca2+ or Al3+.
Polymers can be synthesized that degrade by
hydrolysis by incorporating monomers having
imidazole, amino acid ester, or glycerol side
groups. For example, a polyanionic
poly[bis(carboxylatophenoxy)]phosphazene (PCPP) can
be synthesized, which is cross-linked with
dissolved multivalent cations in aqueous media at
room temperature or below to form hydrogel
matrices.
Bioerodible polyphosphazines have at least
two differing types of side chains, acidic side
groups capable of forming salt bridges with
W094l25080 21~ ~ ~ 8 ~ PCT~S94/04710 ~
-18
multivalent cations, and side groups that hydrolyze
under in vivo conditions, e.g., imidazole groups,
amino acid esters, glycerol and glucosyl. The term
bioerodible or biodegradable, as used herein, means
a polymer that dissolves or degrades within a
period that is acceptable in the desired
application (usually in vivo therapy), less than
about five years and most preferably less than
about one year, once exposed to a physiological
solution of pH 6-8 having a temperature of between
about 25C and 38OC. Hydrolysis of the side chain
results in erosion of the polymer. Examples of
hydrolyzing side chains are unsubstituted and
substituted imidizoles and amino acid esters in
which the group is bonded to the phosphorous atom
through an amino linkage (polyphosphazene polymers
in which both R groups are attached in this manner
are known as polyaminophosphazenes). For
polyimidazolephosphazenes, some of the "R" groups
on the polyphosphazene backbone are imidazole
rings, attached to phosphorous in the backbone
through a ring nitrogen atom. Other "R" groups can
be organic residues that do not participate in
hydrolysis, such as methyl phenoxy groups or other
groups shown in the scientific paper of Allcock, et
al., Macromolecule 10:824-830 (1977).
Methods for synthesis and the analysis of
various types of polyphosphazenes are described by
Allcock, H.R.; et al., Inorq. Chem. 11, 2584
(1972); Allcock, et al., Macromolecules 16, 715
(1983); Allcock, et al., Macromolecules 19, 1508
(1986); Allcock, et al., Biomaterials, 19, 500
(1988); Allcock, et al., Macromolecules 21, 1980
(1988); Allcock, et al., Inor~. Chem. 21(2), 515-
521 (1982); Allcock, et al., Macromolecules 22, 75
(1989); U.S. Patent Nos. 4,440,921, 4,495,174 and
4,880,622 to Allcock, et al.; U.S. Patent No.
~ W094l2~080 21617 8 5 - PCT~S94/04710
--19--
4,946,938 to Magill, et al.; and Grolleman, et al.,
J. Controlled Release 3, 143 (1986), the teachings
of which are specifically incorporated herein by
reference.
Methods for the synthesis of the other
polymers described above are known to those skilled
in the art. See, for example Concise EncYclo~edia
of Polymer Science and Polymeric Amines and
Ammonium Salts, E. Goethals, editor (Pergamen
Press, Elmsford, NY 1980). Many polymers, such as
poly(acrylic acid), are commercially available.
The water soluble polymer with charged side
groups is crosslinked by reacting the polymer with
an aqueous solution containing multivalent ions of
the opposite charge, either multivalent cations if
the polymer has acidic side groups or multivalent
anions if the polymer has basic side groups. The
preferred cations for cross-linking of the polymers
with acidic side groups to form a hydrogel are
divalent and trivalent cations such as copper,
calcium, al~lm;nllm, magnesium, strontium, barium,
and tin, although di-, tri- or tetra-functional
organic cations such as alkylammonium salts, e.g.,
R3N+ -\/\/\/-+NR3 can also be used. Aqueous
solutions of the salts of these cations are added
to the polymers to form soft, highly swollen
hydrogels and membranes. The higher the
concentration of cation, or the higher the valence,
the greater the degree of cross-linking of the
polymer. Concentrations from as low as 0.005 M
have been demonstrated to cross-link the polymer.
Higher concentrations are limited by the solubility
of the salt.
The preferred anions for cross-linking of
the polymers to form a hydrogel are divalent and
trivalent anions such as low molecular weight
dicarboxylic acids, for example, terepthalic acid,
W094/250g0 2 ~ 61~ ~ 5 ~ PCT~S94/04710
-20-
sulfate ions and carbonate ions. Aqueous solutions
of the salts of these anions are added to the
polymers to form soft, highly swollen hydrogels and
membranes, as described with respect to cations.
A variety of polycations can be used to
complex and thereby stabilize the polymer hydrogel
into a semi-permeable surface membrane. Examples
of materials that can be used include polymers
having basic reactive groups such as amine or imine
groups, having a preferred molecular weight between
3,000 and 100,000, such as polyethylen;~ine and
polylysine. These are commercially available. One
polycation is poly(L-lysine); examples of synthetic
polyamines are: polyethyleneimine,
poly(vinylamine), and poly(allyl amine). There are
also natural polycations such as the
polysaccharide, chitosan.
Polyanions that can be used to form a semi-
permeable membrane by reaction with basic surface
groups on the polymer hydrogel include polymers and
copolymers of acrylic acid, methacrylic acid, and
other derivatives of acrylic acid, polymers with
pendant SO3H groups such as sulfonated polystyrene,
and polystyrene with carboxylic acid groups.
Cell Suspensions
Preferably the polymer is dissolved in an
aqueous solution, preferably a 0.1 M potassium
phosphate solution, at physiological pH, to a
concentration forming a polymeric hydrogel, for
example, for alginate, of between 0.5 to 2% by
weight, preferably 1%, alginate. The isolated
cells are suspended in the polymer solution to a
concentration of between 1 and 50 million cells/ml,
most preferably between 10 and 20 million cells/ml.
Methods of Implantation.
The techniques described herein can be used
for delivery of many different cell types to
~ W094/25080 216 17 8 ~ , PCT~S94/04710
-21-
achieve different tissue structures. In the
preferred embodiment, the cells are mixed with the
hydrogel solution and injected directly into a site
where it is desired to implant the cells, prior to
hardening of the hydrogel. However, the matrix may
also be molded and implanted in one or more
different areas of the body to suit a particular
application. This application is particularly
relevant where a specific structural design is
desired or where the area into which the cells are
to be implanted lacks specific structure or support
to facilitate growth and proliferation of the
cells.
The site, or sites, where cells are to be
implanted is determined based on individual need,
as is the requisite number of cells. For cells
having organ function, for example, hepatocytes or
islet cells, the mixture can be injected into the
mesentery, subcutaneous tissue, retroperitoneum,
properitoneal space, and intramuscular space. For
formation of cartilage, the cells are injected into
the site where cartilage formation is desired. One
could also apply an external mold to shape the
injected solution. Additionally, by controlling
the rate of polymerization, it is possible to mold
the cell-hydrogel injected implant like one would
mold clay.
Alternatively, the mixture can be injected
into a mold, the hydrogel allowed to harden, then
the material implanted.
W094/25080 ~ 785 PCT~S94/04710
-22-
The suspension can be injected via a
syringe and needle directly into a specific area
wherever a bulking agent is desired, i.e., a soft
tissue deformity such as that seen with areas of
muscle atrophy due to congenital or acquired
diseases or secondary to trauma, burns, and the
like. An example of this would be the injection of
the suspension in the upper torso of a patient with
muscular atrophy secondary to nerve damage.
The suspension can also be injected as a
bulking agent for hard tissue defects, such as bone
or cartilage defects, either congenital or acquired
disease states, or secondary to trauma, burns, or
the like. An example of this would be an injection
into the area surrounding the skull where a bony
deformity exists secondary to trauma. The
injunction in these instances can be made directly
into the needed area with the use of a needle and
syringe under local or general anesthesia.
The suspension could also be injected
percutaneously by direct palpation, such as by
placing a needle inside the vas deferens and
occluding the same with the injected bulking
substance, thus rendering the patient infertile.
The suspension could also be injected through a
catheter or needle with fluoroscopic, sonographic,
computed tomography, magnetic resonance imaging or
other type of radiologic guidance. This would
- allow for placement or injection of this substance
either by vascular access or percutaneous access to
specific organs or other tissue regions in the
body, wherever a bulking agent would be required.
Further, this substance could be injected
through a laparoscopic or thoracoscope to any
intraperitoneal or extraperitoneal or thoracic
organ. ~or example, the suspension could be
injected in the region of the gastro-esophageal
~ W094/25080 21617 8 ~ PCT~S94/04710
-23-
junction for the correcting of gastroesophageal
reflux. This could be performed either with a
thoracoscope injecting the substance in the
esophageal portion of the gastroesophageal region,
or via a laparoscope by injecting the substance in
the gastric portion of the gastroesophageal region,
or by a combined approach.
Vesicoureteral reflux is one of the most
common congenital defects in children, affecting
approximately 1% of the population. Although all
patients do not require surgical treatment, it is
still one of the most common procedure performed in
children. Over 600 ureteral reimplants are
performed yearly at Children's Hospital in Boston,
Massachusetts. This translates to an approximately
saving of 3600 inpatient hospital days per year at
this institution alone, if the endoscopic treatment
described herein is used instead of open surgery.
In addition to its use for the endoscopic
treatment of reflux, the system of injectable
autologous muscle cell may also be applicable for
the treatment of other medical conditions, such as
urinary and rectal incontinence, dysphonia, plastic
reconstruction, and wherever an injectable
permanent biocompatible material is needed.
As described herein, an injectable
biodegradable polymer as a delivery vehicle for
muscle cells or chondrocytes is useful in the
treatment of reflux and incontinence. In the
preferred embodiment, a biopsy is obtained under
anesthesia from a patient with vesicoureteral
reflux, the isolated cells are mixed with alginate,
and the cell-alginate solution is injected
endoscopically in the sub-ureteral region to
correct reflux, as shown in Figure la. The time to
solidification of the alginate-cell solution may be
manipulated by varying the concentration of calcium
R~CT~i~lED S~EET (RU~
!~rA ~P
'~6~ ~5
W094/25080 PCT~S94/04710
-24-
as well as the temperature at which the cells are
added to the algina~te. The use of autologous cells
precludes an immunologic reaction. Solidification
of the alginate impedes its migration until after
it is degraded. The suspension can be injected
through a cystoscopic needle, having direct visual
access with a cystoscope to the area of interest,
such as for the treatment of vesico-ureteral reflux
or urinary incontinence. In addition to the use of
the cell-polymer suspension for the treatment of
reflux and incontinence, the suspension can also be
applied to reconstructive surgery, as well as its
application anywhere in the human body where a
biocompatible permanent injectable material is
necessary. The suspension can be injected
endoscopically, for example through a laryngoscope
for injection into the vocal chords for the
treatment of dysphonia, or through a hysteroscope
for injection into the fallopian tubes as a method
of rendering the patient infertile, or through a
proctoscope, for injection of the substance in the
perirectal sphincter area, thereby increasing the
resistance in the sphincter area and rendering the
patient continent of stool.
This technology can be used for other
purposes. For example, custom-molded cell implants
can be used to reconstruct three dimensional tissue
defects, e.g., molds of human ears could be created
and a chondrocyte-hydrogel replica could be
fashioned and implanted to reconstruct a missing
ear. Cells can also be transplanted in the form of
a thee-dimensional structure which could be
delivered via injection.
The present invention will be further
understood by reference to the following non-
limiting examples.
~ W094/25080 2 1 61 7 8 5 PCT~594/n47l0
Example 1: Preparation of a Calcium-Alginate-
chondrocyte mixture and injection into
mice to form cartilaginous structures.
A calcium alginate mixture was obtained by
S combining calcium sulfate, a poorly soluble calcium
salt, with a 1% sodium alginate dissolved in a 0.1
M potassium phosphate buffer solution (pH 7.4).
The mixture remained in a liquid state at 4C for
30-45 min. Chondrocytes isolated from the
articular surface of calf forelimbs were added to
the mixture to generate a final cellular density of
1 x 107/ml (representing approximately 10% of the
cellular density of human juvenile articular
cartilage).
The calcium alginate-chondrocyte mixture
was injected through a 22 gauge needle in 100 ~l
aliguots under the pannus cuniculus on the dorsum
of nude mice.
The nude mice were ~A~; ned 24 hours post-
operatively, and all injection sites were firm topalpation without apparent diffusion of the
mixture. Specimens were harvested after 12 weeks
of in vivo incubation. On gross ~A~ ination, the
calcium alginate-chondrocyte specimens exhibited a
pearly opalescence and were firm to palpation. The
specimens weighed 0.11 + 0.01 gms (initial weight
0.10 gms). The specimens were easily dissected
free of surrounding tissue and exhibited minimal
inflammatory reaction. Histologically, the
specimens were stained with hematoxylin and eosin
and demonstrated lacunae within a basophilic ground
glass substance.
Control specimens of calcium alginate
without chondrocytes had a doughy consistency 12
weeks after injection and had no histologic
evidence of cartilage formation.
This study demonstrates that an injectable
calcium alginate matrix can provide a three
W094/25080 21617~5 PCT~S94/04710 ~
-26-
dimensional scaffold for the successful
transplantation and engraftment of chondrocytes.
Chondrocytes transplanted in this manner form a
volume of cartilage after 12 weeks of in vivo
incubation similar to that initially injected.
Ex~mple 2: Effect of cell density on cartilage
formatio~.
Varying numbers of chondrocytes isolated
from the articular surface of calf forelimbs were
mixed with a 1.5% sodium alginate solution to
generate final cell densities of O.O, 0.5, l.O, and
5.0 x 1O6 chondrocytes/ml (approximately O.O, 0.5,
1.0, and 5.0% o~ the cellular density of human
juvenile articular cartilage). An aliquot of the
chondrocyte-alginate solution was transferred to a
circular mold 9 mm in diameter and allowed to
polymerize at room temperature by the diffusion of
a calcium chloride solution through a semi-
permeable membrane at the base of the mold. The
gels formed discs measuring 2 mm in height and 9 mm
in diameter.
Discs of a fixed cellular density of 5 x 1o6
cells/ml were also formed in which the
concentration of the sodium alginate and the
molarity of the calcium chloride solutions were
varied.
All discs were placed into dorsal
subcutaneous pockets in nude mice. Samples were
harvested at 8 and 12 weeks and examined for gross
and histological evidence of cartilage formation.
~ m;n~tions of 8 and 12 week specimens
revealed that a minimum cell density of 5 x 106
chondrocytes/ml was required for cartilage
production which was observed only 12 weeks after
implantation. On gross e~m;nation, the specimens
were discoid in shape and weighed 0.13 + 0.01 gms
(initial weight 0.125 gms). The specimens were
easily dissected free of surrounding tissue and
~ W094/2~080 2 1 6 1 7 8 5 PCT~S94/04710
-27-
exhibited minimal inflammatory reaction.
Histologically, the specimens were stained with
hematoxylin and eosin and demonstrated lacunae
within a basophilic ground glass substance.
Cartilage formation was independent of
calcium chloride concentration used in gel
polymerization. Cartilage was observed in
specimens with alginate concentrations varying from
0.5% to 4.0%; however, the lowest alginate
concentration tested (0.5%) showed only microscopic
evidence of cartilage.
Cartilage can be grown in a subcutaneous
pocket to a pre-determined disc shape using calcium
alginate gel as a support matrix in 12 weeks.
Cartilage formation is not inhibited by either
polymerization with high calcium concentrations or
the presence of high alginate concentrations but
does require a minimum cellular density of 5 x 106
cells/ml.
The ability to create a calcium alginate-
chondrocyte gel in a given shape demonstrates that
it is possible to use this technique to custom
design and grow cartilaginous scaffolds for
craniofacial reconstruction. Such scaffolds have
the potential to replace many of the prosthetic
devices currently in use.
Ex mple 3: Preparation of Implantable Premolded
Cell-polymer mixtures.
250 ~l aliquots of an isolated chondrocyte
suspension was mixed with 750 ~ls of a 2% (w/v)
sodium alginate solution (0.1 M K2HPO4, 0.135 M
NaCl, pH 7.4). A 125 ~l aliquot was placed into 9
mm diameter cell culture inserts with 0.45 ~m pore
size semipermeable membranes. The cell-alginate
mixture was placed into contact with a 30 mM CaCl2
bath and allowed to polymerize for 90 minutes at
37C. After 90 minutes, the cell-alginate gel
constructs were removed from the mold and had a
W094/25080 2 i 6 ~ ; PCT~S94/04710
-28-
diameter of 9 mm and a height of 2 mm. The discs
were placed into the wells of 24-well tissue
culture plates and incubated at 37C in the
presence of 5% CO2 with 0.5 ml of a solution
contA;n;ng Hamm's F-12 culture media (Gibco, Grand
Island, N.Y.) and 10% fetal calf serum (Gibco,
Grand Island, N.Y.) with L-glutamine (292 ~g/ml),
penicillin (100 U/ml), streptomycin (100 ~g/ml) and
ascorbic acid (5 ~g/ml) for 48 hrs.
Using this method, bovine chondrocyte-
alginate discs were prepared, then implanted in
dorsal subcutaneous pockets in athymic mice using
standard sterile technique. After one, two, and
three months, athymic mice were sacrificed, and the
gel/chondrocyte constructs removed, weighed and
placed in appropriate fixative. The cell-polymer
complexes were studied by histochemical analysis.
Cartilage formation was observed
histologically after three months of in vivo
incubation at an initial chondrocyte density of 5 x
106 cell/ml.
The above protocol was modified by using a
range of CaCl2 concentration and a range of sodium
alginate concentrations. Cartilage formation was
observed using 15, 20, 30, and 100 mM CaCl2 baths
and 0.5, 1.0, 1.5, 2.0, and 4.0% sodium alginate
solutions.
By changing the mold within which the cell-
alginate construct is created, the shape of the
implant can be customized. Additionally, the mold
need not be semipermeable as calcium ion can be
directly mixed with the cell-alginate solution
prior to being placed within a mold. The key
feature is that the construct can be fashioned into
a given shape prior to implantation.
~ W094/25080 21617 8 S PCT~S94104710
-29-
Example 4: Preparation of injectable osteoblasts-
hydrogel mixtures.
Using the methodology described above,
bovine osteoblasts have been substituted for
chondrocytes and injected into animals using a
hydrogel matrix.
Histology after 12 weeks of in vivo
incubation showed the presence of early bone
formation.
Ex~mple 5: ~se of the hydrogel matrix to form an
immunoprotective matrix around the
implanted cells.
By fashioning a cell-alginate construct as
described above, one can use the hydrogel matrix to
sterically isolate the encapsulated cells from the
host immune system, and thereby allow allogenic
cell transplants to form new tissues or organs
without immunosuppression.
Bovine chondrocytes in an alginate
suspension were transplanted into normal immune-
competent mice. Histology after six weeks of in
vivo incubation shows the presence of cartilage
formation. Gross examination of the specimens does
not demonstrate features of cartilage. Literature
states that similar chondrocyte xenografts without
alginate do not form cartilage.
The following examples demonstrate that
human bladder muscle cell-alginate suspensions are
injectable, non-migratory, and appear to conserve
their volume and that autologous bladder muscle
cells are useful in the endoscopic treatment of
vesicoureteral reflux.
- Example 6: Isolation and Char~cterization of
Human Bladder Muscle Cells ~p~n~ed In
Vitro.
Cell Culture:
Human Tissue Origin. Human bladder tissue
specimens were obtained and processed within one
hour after surgical removal at Children's Hospital,
W094/25080 2 ~ 617 8 3 PCT~S94/04710
-30-
Boston. The specimens varied in size, ranging from
one cm2 to four cm2. The specimens were transported
to Hanks balanced salt solution containing lO mM
HEPES and lOO KIU apoprotein.
8mooth Muscle Cells. Muscle cells were
obtained by mincing small detrusor muscle fragments
to approximately 0.5 mm diameter and using these as
explants in lO0 mm tissue culture dishes containing
10 mL of DMEM supplemented with 10% fetal calf
serum. Approximately 30 explants of similar size
were added to each dish. Medium was changed twice
a week. Outgrowth was routinely observed at 72 hr
after explants were placed in culture. When
cultures were 80% confluent, the cells were
trypsinized and passaged. Cell populations highly
enriched in elongated, striated cells were
routinely obtained using this method. Cell strains
were stained with a-actin antibody to verify their
muscle phenotype.
Results. Populations of fusiform, striated
cells could be obtained from the bladder biopsy
specimens by dissection of the muscle layer
followed by explant culture of the muscle fragments
in DMEM supplemented with 10% calf-serum.
Immunostaining with a monoclonal antibody which
specifically recognized a-actin verified the muscle
phenotype. These data indicate that highly
proliferative populations of smooth muscle can be
obtained from small biopsy specimens. The muscle
cells can also be grown transiently in serum-free
defined media which renders the cells free from any
impurities.
Ex~mple 7: Iniection of cell suspensions into
~ice.
Using a 22 gauge needle, 20 nude mice were
injected with a 500 microliter solution of muscle
cells and alginate. Each mouse had two injection
sites consisting of either control, or a solution
~ W094/2~080 2 1 6 1 7 8 5 ~ PiC~S94/04710
-31-
of ten million human bladder cells per cc of
alginate (32 injection sites). Injections of
alginate alone or bladder muscle cells alone served
as controls. Animals were sacrificed at two, four,
six and eight weeks after implantation. Histologic
examination of the injection areas demonstrated
evidence of muscle formation in the muscle/alginate
injection sites. Immunohistochemical analysis
using an anti-desmin antibody indicated that the
cells maintained a program of muscular
differentiation. Examination of the injection
sites with increasing periods of time, showed that
the alginate was progressively replaced by muscle.
The size of the muscle-alginate complex appeared to
be uniform and stable. In both control groups
(alginate alone or muscle cells alone) there were
no muscle cells evident. Histologic analysis of
distant organs showed no evidence of bladder muscle
cells or alginate migration or granuloma formation.
Ex~mple 8: Correction of vesicoureteral reflux in
pigs using bladder muscle cells
implanted in an alginate gel.
Materials and Methods
An i r - 7 model of vesicoureteral reflux.
The pig was used for this study because of the
similarities between porcine and human bladders and
kidneys. The Hanford mini-pig was used for the
convenience of its smaller size. Bilateral
vesicoureteral reflux was created in four mini-
swine using the open bladder technique, which
consists of unroofing the entire intravesical
ureter, as described by Vacanti, et al., "Synthetic
polymers seeded with chondrocytes provide a
template for new cartilage formation" Plastic and
Recon. Surq. 88:753 (1991).
Three months after the procedure, the
presence of bilateral reflux was assessed by
conventional radiographic cystography using an
W094/25080 2 1 6 1 ~ 8 ~ " ~ ~ " ,~ PCT~S94/04710 ~
-32-
iodinated contrast agent, and by sonography using
sonicated albumin, as described by Vacanti, et al.,
"Tissue engineered growth of new cartilage in the
shape of a human ear using synthetic polymers
seeded with chondrocytes" Mat. Res. Soc. Proc.
252:367 (1992). Excretory urography was performed
to detect any evidence of obstruction.
Cell Narvest. A segment of bladder muscle
was obtained from each animal. Muscle cells were
harvested and plated separately in vitro. After
expansion, the cells were individually quantitated
and concentrated to 20 x 1o6 cells per cc.
Autologous bladder muscle cell-calcium
alginate suspension. Two percent weight/volume
sodium alginate (O.l M K2PO4, 0.135 M NaCl, pH 7.4,
Protan, Portsmouth, NH) was made and sterilized in
ethylene oxide. A 1.5 ml aliquot of 20 x 106
cells/ml bladder muscle cell suspension was added
to an equal volume of sodium alginate solution for
a final alginate concentration of 1%. The bladder
muscle cell-sodium alginate suspension was kept at
32OC. Immediately prior to injection, calcium
sulfate (0.2 g/ml) was added to the bladder muscle
cell-sodium alginate suspension. The mixture was
vortexed and stored in ice until injection. The
gelling process was initiated with the addition of
calcium sulfate, which allowed the suspension to
remain in a liquid state for approximately 40
minutes.
3 0 Experimental study . Mini-pigs were
anesthetized with intramuscular injections of 25
ml/kg ketamine and 1 ml/kg acylpromazine.
Additional anesthesia was obtained with an
intramuscular administration of 25 mg/kg ketamine
and 10 mg/kg of xylazine. Animals were placed in a
supine position. With a 15.5 French cystoscope
introduced into the bladder, a 21 gauge needle was
~ W094/25080 2 1 6 1 7 8 S ~ -PC~S9;4/04710
-33-
inserted in the subureteral region of the right
refluxing ureter. Approximately 2 to 3 ml of the
autologous bladder muscle cell-alginate suspension
(40-60 x 106 bladder muscle cells) were injected
through the needle, while lifting of the ureteral
orifice was endoscopically visualized. The left
ureteral orifice remained untreated and served as a
control. Serial cystograms, cystoscopy, and
excretory urographic studies were performed at
eight week intervals until sacrifice. The mini-
pigs were sacrificed at eight (1), 16 (1), and 26
(2) weeks after treatment. The bladder injection
sites were resected and ~;ned macroscopically
and microscopically. Specimens were stained with
hematoxylin and eosin, and alcian blue at a pH of
1.0 and 2.5. Histological analyses of the bladder,
ureters, regional lymph nodes, kidneys, liver, and
spleen were performed.
Results
Four mini-swine underwent bilateral
creation of reflux. All four were found to have
bilateral reflux without evidence of obstruction at
three months following the procedure. Bladder
muscle cells were from each mini-swine and expanded
in vitro. The animals then underwent endoscopic
repair of reflux with the injectable autologous
bladder muscle cell-alginate gel solution on the
right side only.
Cystoscopic and radiographic examinations
were performed at two, four, and six months after
treatment. Cystoscopic examinations showed a
smooth bladder wall. Cystograms showed no evidence
of reflux on the treated side and persistent reflux
in the uncorrected control ureter in all animals.
All animals had a successful cure of reflux in the
repaired ureter without evidence of hydronephrosis
on excretory urography.
W094/25080 2161~ ~ 5 PCT~S94/04710 ~
; -34-
At the time of sacrifice, gross e~min~tion
of the bladder injection site showed the presence
of bladder muscle cell-alginate composites in the
subureteral region. Microscopic analyses of the
tissues surrounding the injection site showed no
inflammation. Tissue sections from the bladder,
ureters, lymph nodes, kidneys, liver and spleen
showed no evidence of alginate migration or
granuloma formation.
SummarY of ExPerimental Data
Autologous bladder muscle cells can be
readily harvested, expanded in vitro, and injected
cystoscopically. The cells survive and form a
muscle nidus which is non-antigenic. This system
is able to correct reflux without any evidence of
obstruction.
The following examples demonstrate that
chondrocyte-polymer suspensions are injectable,
non-migratory, and appear to conserve their volume,
and are useful in the endoscopic treatment of
vesicoureteral reflux. As demonstrated in Example
1, alginate-bovine chondrocyte cell allografts were
found to contain viable cartilage cells after
implantation times for as long as 90 days in
athymic mice. The new cartilage formed retains the
approximate configuration and dimensions of the
injected template. The cell-polymer construct is
essential in that injection of free chondrocytes or
alginate alone does not result in cartilage
formation.
Example 9: Implantation of chondrocytes in
alginate gel into mice.
Naterials ~nd Methods
Hyaline cartilage was obtained from the
3S articular surfaces of calf shoulders and
chondrocytes were harvested. Chondrocyte
suspensions were concentrated to 20, 30, and 40 X
106 cells per cc and mixed with dry alginate powder
~ W094/2~080 2161~ 8 5 PCT~S94/04710
-35-
to form a gel. Twelve athymic mice were injected
subcutaneously with a chondrocyte/alginate
solution. Each mouse had four injection sites
consisting of control, 10, 15, and 20 X 1O6
chondrocyte cells (48 injection sites). Mice were
sacrificed at 2, 4, 6, and 12 weeks after
injection.
Histologic ~Am;n~tion of the injection
sites demonstrated evidence of cartilage formation
in 34 of the 36 experimental injection sites.
Gross ~ ;nation of the injection sites with
increasing periods of time, showed that the polymer
gels were progressively replaced by cartilage. The
ultimate size of the cartilage formed was related
to the initial chondrocyte concentration injected
and appeared to be uniform and stable within each
category. There was no evidence of cartilage
formation in the 12 controls. Histologic analyses
of distant organs showed no evidence of cartilage
or alginate gel migration or granuloma formation.
Materials an~ Methods
An;r~7s - Young adult athymic nu/nu mice
were used as cell recipients. The animals were
housed individually, allowed access to food and
water as desired, and maintained on 12 hours of
light and dark intervals. Anesthesia was performed
with methoxyflurane by cone administration.
Polymers - Dry alginate impression powder
(Dentsply International; Milford, DE) was used as
the delivery vehicle. Alginate, a copolymer of
gluronic and mannuronic acid, is designed to gel at
a controlled rate when mixed with calcium salts and
water. Calcium phosphates and sulfates are
included in the pure polymer powder to control the
gelation kinetics. The powder was sterilized in
ethylene oxide and sealed in aluminum foil until
injection.
W094/25080 21~178 ~ PCT~S94/04710 ~
. ~.
~ -36-
. ... . .
Cell Harvest - Hyaline cartilage was
obtained from the articular surfaces of calf
shoulders within six hours of sacrifice. The
shoulders were washed in providine-iodine 10
percent solution and chondrocytes were harvested
under sterile conditions using a technique
described by Klagsbrun, "Large scale preparation of
chondrocytes" Methods in EnzymoloqY, 58:560
(1979). The isolated cells were quantitated using
a hemocytometer, and the chondrocyte suspension was
concentrated to 20, 30, and 40 X lo6 cells per cc.
Cell Delivery - The chondrocyte cell
suspensions were mixed with dry alginate powder to
form a gel. Using a 21 gauge needle, 12 nude mice
were injected with a 600 microliter
chondrocyte/alginate solution. Each mouse had four
injection sites consisting of control, 10, 15, and
20 X l06chondrocytes (48 injection sites).
Injection of alginate gel alone served as control
in six mice. As another control six mice were
injected subcutaneously in the same region with 600
microliters cell suspensions containing 10, 15, and
20 X 1o6 chondrocytes alone, without alginate.
Implant Recovery - Mice were sacrificed at
2, 4, 6, and 12 weeks after injection. The
implants were excised following a tissue plane that
easily separated the implant from the surrounding
tissue, weighted, fixed in 10 percent neutral
buffered formalin, and imbedded in Paraffin.
Tissue sections were also obtained from the
regional lymph nodes, kidneys, bladder, ureters,
lungs, spleen, and liver. Tissue sections were
stained with hematoxylin and eosin. Gross and
histologic examination were performed.
Results
Figure la is a schematic of the general
method which was used. Histologic examination of
RECT~ SH~ET(Ri ~E
I~;AiFP
W094/2~080 ~ 16 1 7 8 S PCT~S94/04710
-37-
injection sites demonstrated evidence of cartilage
formation in 34 of the 36 chondrocyte/alginate
implants. A mild inflammatory response appeared to
be resolving by four weeks. This consisted of an
inflammatory response that exhibited an acute phase
and a chronic foreign body reaction. Fibroblast
infiltration were seen up to two weeks after
injection. ~mi n~tion of the injection sites with
increasing periods of time, showed that the polymer
gels were progressively replaced by cartilage.
Gross ~A~i nation showed normally appearing rubbery
to hard cartilage structures. The ultimate size of
the cartilage formed appeared to be related to the
initial volume and chondrocyte concentration
injected and appeared to be uniform within each
category. The weight of the retrieved cartilage
structures appeared to be stable over time. In the
six polymer gel control injections (not containing
chondrocytes) there was no visual evidence of
cartilage formation. In the second control group
(chondrocyte suspension alone) cartilage formation
was not evident in any area. Histologic analysis
of the peri-injection site and distant organs
showed no evidence of cartilage or alginate gel
migration.
Example lO: Correction of veQicoureteral reflux in
pigs using chondrocytes implanted in
an alginate gel.
Materials and Nethoas
,~n i rn~ 7 model of vesicoureteral reflux .
The pig was used for this study because of the
similarities between porcine and human bladders and
kidneys. The Hanford mini-pig was used for the
convenience of its smaller size. Bilateral
vesicoureteral reflux was created in four mini-
swine using the open bladder technique, which
consists of unroofing the entire intravesical
ureter, as described by Vacanti, et al., "Synthetic
W094/25080 21~1~ 8 5 : PCT~S94/04710 ~
-38-
polymers seeded with chondrocytes provide a
template for new cartilage formation" Plastic and
Recon. Surq. 88:753 (1991).
Three months after the procedure, the
presence of bilateral reflux was assessed by
conventional radiographic cystography using an
iodinated contrast agent, and by sonography using
sonicated albumin, as described by Vacanti, et al.,
"Tissue engineered growth of new cartilage in the
shape of a human ear using synthetic polymers
seeded with chondrocytes" Mat. Res. Soc. Proc.
252:367 (1992). Excretory urography was performed
to detect any evidence of obstruction.
Cell Harvest. Hyaline cartilage was
obtained from the auricular surfaces of each mini-
swine. The ears were washed with providine-iodine
10% solution and chondrocytes were harvested under
sterile conditions using the technique, Atala, et
al., "Endoscopic treatment of vesicoureteral reflux
with a self-detachable balloon system" J. Urol.
148:724 (1992).
The isolated cells were expanded in vitro
in a solution of Hamms F-12 media (Gibco, Grand
Island, NY) with 10% fetal calf serum (Gibco), 5
micrograms/ml ascorbic acid, 292 micrograms/ml
glutamine, 100 micrograms/ml streptomycin, 40
nanograms/ml vitamin D3 and lO0 units/ml
penicillin. The cells were incubated at 37C in
the presence of 5% CO2. Five to eight weeks after
initial harvest, the chondrocytes were trypsinized
and quantitated using a hemocytometer. The
chondrocyte suspension from each mini-swine was
concentrated to 40 X 106 cells/ml in minimal
essential media - 199 (Gibco).
Autologous chondrocyte-calcium alginate
suspension. Two percent weight/volume sodium
alginate (O.1 M K2PO4, 0.135 M NaCl, pH 7.4, Protan,
2i6~785 - `
W094/25080 PCT~S94/04710
Portsmouth, NH) was made and sterilized in ethylene
oxide. A 1.5 ml aliquot of 40 x 1O6 cells/ml
chondrocyte suspension was added to an equal volume
of sodium alginate solution for a final alginate
concentration of 1%. The chondrocyte-sodium
alginate suspension was kept at 32C. Immediately
prior to injection, calcium sulfate (0.2 g/ml) was
added to the chondrocyte-sodium alginate
suspension. The mixture was vortexed and stored in
ice until injection. The gelling process was
initiated with the addition of calcium sulfate,
which allowed the suspension to remain in a liquid
state for approximately 40 minutes.
Experimental study. Mini-pigs were
anesthetized with intramuscular injections of 25
ml/kg ketamine and 1 ml/kg acylpromazine.
Additional anesthesia was obtained with an
intramuscular administration of 25 mg/kg ketamine
and lO mg/kg of xylazine. A~;m~ls were placed in a
supine position. With a 15.5 French cystoscope
introduced into the bladder, a 22 gauge needle was
inserted in the subureteral region of the right
refluxing ureter. Approximately 2-3 ml of the
autologous cartilage-alginate suspension (40-60 x
1O6 chondrocytes) were injected through the needle,
while lifting of the ureteral orifice was
endoscopically visualized. The left ureteral
orifice remained untreated and served as a control.
Serial cystograms, cystoscopy, and excretory
urographic studies were performed at eight week
intervals until sacrifice. The mini-pigs were
sacrificed at eight (1), 16 (1), and 26 (2) weeks
after treatment. The bladder injection sites were
resected and e~Am;ned macroscopically and
microscopically. Specimens were stained with
hematoxylin and eosin, and alcian blue at a pH of
l.O and 2.S. Histological analyses of the bladder,
wO 94/250802 1 6 1 ~ r; PCT~S94/04710 ~
-40-
ureters, regional lymph nodes, kidneys, liver, and
spleen were performed.
Result~
Four mini-swine underwent bilateral
creation of reflux. All four were found to have
bilateral reflux without evidence of obstruction at
three months following the procedure. Chondrocytes
were harvested from the left auricular surface of
each mini-swine and expanded in vitro for 5-8
weeks, with a final concentration of 50-150 X 106
viable cells per animal. The animals then
underwent endoscopic repair of reflux with the
injectable autologous chondrocyte-alginate gel
solution on the right side only.
Cystoscopic and radiographic e~;nAtions
were performed at two, four, and six months after
treatment. Cystoscopic e~A~;nations showed a
smooth bladder wall. Cystograms showed no evidence
of reflux on the treated side and persistent reflux
in the uncorrected control ureter in all animals.
All animals had a successful cure of reflux in the
repaired ureter without evidence of hydronephrosis
on excretory urography.
At the time of sacrifice, gross eXAm;nAtion
of the bladder injection site showed a well defined
rubbery to hard cartilage structure in the
subureteral region. Histologic exA~;n~tion of
these specimens using hematoxylin and eosin stains
showed evidence of cartilage formation. The
polymer gels were progressively replaced by
cartilage with increasing time. Aldehyde fuschin-
alcian blue staining suggested the presence of
chondroitin sulfate. Microscopic analyses of the
tissues surrounding the injection site showed no
inflammation. Tissue sections from the bladder,
ureters, lymph nodes, kidneys, liver and spleen
2161785 :
W094/25080 PCT~S94/04710
-41-
showed no evidence of chondrocyte or alginate
migration, or granuloma formation.
SummarY of ExPerimental Data
Chondrocytes can be readily grown and
r S e~rAn~ed in culture. Neocartilage formation can be
achieved in vitro and in vivo using chondrocyte
cultured on synthetic biodegradable polymers. In
these experiments, the cartilage matrix replaced
the alginate as the polysaccharide polymer
10 underwent biodegradation. Six mini-swine underwent
bilateral creation of reflux. All six were found
to have bilateral reflux without evidence of
obstruction at three months following the
procedure. Chondrocyte were harvested from the
15 left auricular surface of each mini-swine and
expanded to a final concentration of 50-150 X 106
viable cells per animal. The animals then
underwent endoscopic repair of reflux with the
injectable autologous chondrocyte-alginate gel
20 solution on the right side only.
Cystoscopic and radiographic ~m; nAtions
were performed at two, four, and six months after
treatment. Cystoscopic examinations showed a
smooth bladder wall. Cystograms showed no evidence
25 of reflux on the treated side and persistent reflux
in the uncorrected control ureter in all animals.
All animals had a successful cure of reflux in the
repaired ureter without evidence of hydronephrosis
on excretory urography. The harvested ears had
30 evidence of cartilage regrowth within one month of
chondrocyte retrieval.
At the time of sacrifice, gross ~mination
of the bladder injection site showed a well defined
rubbery to hard cartilage structure in the
35 subureteral region. Histologic ~AminAtion of
these specimens using hematoxylin and eosin showed
evidence of normal cartilage formation. The
2 1 ~ 5
W094/2~080 PCT~S94/04710
-42-
polymer gels were progressively replaced by
cartilage with increasing time. Aldehyde fuschin-
alcian blue staining suggested the presence of
chondroitin sulfate. ~icroscopic analyses of the
tissues surrounding the injection site showed no
inflammation. Tissue sections from the bladder,
ureters, lymph nodes, kidneys, lungs, liver and
spleen showed no evidence of chondrocyte or
alginate migration, or granuloma formation.
Modifications and variations of the
compositions and methods of the present invention
will be obvious to those skilled in the art from
the foregoing detailed description. Such
modifications and variations are intended to come
within the scope of the following claims.