Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 94/26215 2 1 ~ 3 1 3 1 - ~ ~ PCT/US94/05536
EXPANDABLE URETHRAL PLUG
BACKGROUND OF THE INVENTION
Field of the invention
; The present invention relates to a novel plug which is in-
serted into the urethra to control urinary incontinence.
Description of the Prior Art
Urinary stress incontinence is defined as the involuntary
loss of urine when the pressure within the urethra exceeds the
maximum urethral pressure required for maint~; ni ng closure.
While the problem of urinary incontinence occurs in men and
women, it is an affliction especially common in women of child
bearing age and beyond.
There are in existence many methods used to address the
problem of incontinence. Bladder neck suspension surgery,
wherein the neck of the bladder is reduced by suspending the
bladder, is perhaps the most desirable way to treat incontinence,
especially in younger patients. However, there are numerous
risks associated with such surgery, notwithstanding the expense.
For some patients, surgery is not recommPn~ed for medical or
other reasons, and for those with mild incontinence surgery is
not an appropriate solution.
Also in existence are a variety of devices for controlling
urinary incont;nence. Many of these devices require surgery for
implantation, and of these surgically implanted devices, there
are two distinct types: non-manipulable devices and manipulable
devices. One such non-manipulable device, described in United
States Patent No. 4,019,499, is a capsule filled with a variable
amount of fluid. The capsule is surgically implanted between
supporting tissue and the urethra to exert an occluding force
thereon. A similar, non-manipulable capsule implant is
described in United States Patent No. 3,789,828. However, this
device has ties extending therefrom to aid in fiber ingrowth,
thus providing mechanical stability to the capsule. One problem
associated with this device is the risk of fluid leakage. In ad-
WO94/26215 21 6 3 l 31 ~ PCT~S94105536
dition to problems with leakage, severe tissue damage may resultfrom the unnatural method in which such devices regulate incon-
tinence.
Other surgically implanted devices exist which are manipul-
able. These devices provide the wearer with the ability to
selectively control the operation of the device via manually
operable elements implanted in the tissue surrounding the
urethra. United States Patent No. 4,428,365, and United States
Patent No. 4,846,784 each disclose an indwelling device having
an inflatable chamber with an attached tubing and an inflation
bulb. The wearer may manually adjust the pressure exhibited by
the inflatable member on the urethra, simply by squeezing the
tissue encasing the bulb. These devices, however, often produce
thickening and scarring of surrounding tissue, making their
usefulness questionable. Additional adverse effects associated
with surgically implanted indwelling devices, whether non-
manipulable or manipulable in nature, are encrustation,
irritation and infection.
There are also known in the art certain indwelling devices
that do not require surgical implantation. These devices are in-
serted by a physician through the urethral orifice and allow the
wearer to void either past or through the device. An example of
such a device is disclosed in United States Patent No. 4,850,963
in which a physician inserts a bolus of ferromagnetic material
through the urethra and into the bladder. The bolus rests at the
juncture of the bladder and urethra and is moved for bladder
evacuation, by the relative positioning of a magnet across the
body of the wearer. However, the bolus may become lodged in an
area beyond the reaches of the magnetic force exhibited by the
magnet, making the device inoperative. Another example of this
type of indwelling device is the prestressed capsule disclosed
in United States Patent No. 4,457,299. The capsule is inserted
by a physician within the lower interior of the urethra and is
set at a prestressed pressure slightly above involuntary
pressure. When the urine pressure exceeds the preset pressure
of the capsule, the capsule deforms allowing urine to flow around
the device. This device, however, has no feature to prevent
W09412621~ 2 1 6 3 1 3 1 PCT~S94/OS536
3 ' - ~C
migration of the device into the bladder. In United States
Patent No. 4,553,533 there is shown a prosthetic urethral
sphincter valve which is placed in the urethra and anchored in
the bladder. The patient increases his bladder pressure by means
of a valsalva maneuver, and holds this pressure while the valve
; activates. Urine may then pass through the valve with the valve
later returning to its closed position. This device is very
complicated, expensive, difficult to manufacture and
uncomfortable. Another physician-inserted device is disclosed
in United States Patent No. 3,797,478. This device has an
expandable collar which is inflated after insertion, by an
injection of fluid therein. When it is desired to remove the
device, the inflated collar is ruptured or serrated, thus
expelling the fluid into the wearer's body. Notwithstanding the
cumbrous use of this device, there is a risk of infection
associated with the release of injection fluid upon removal.
Similarly, United States Patent No. 3,841,304 discloses a plug
which is inserted by a physician into the urethra and
subse~uently inflated to block the flow of urine. This device
may be left in the body for extended periods. After insertion,
the device merely requires repositioning in the urethra to permit
bladder evacuation. Such a device leaves the wearer susceptible
to infection, as bacteria may be introduced into the urethra
during repositioning, or during indwelling time. Also, serious
complications can occur upon removal, when a separate wire must
be inserted therein. These devices being indwelling, are often
cumbersome to the wearer and often cause numerous complications
such as encrustation, irritation and infection.
Also known in the art are devices capable of being inserted
by the wearer into the urethra. Such devices are removed for
voiding, and then reintroduced into the urethra upon completion
of bladder evacuation. An example of such a device is the
solid-type urethral plug, described by Neilsen, Kurt K. et al.,
in "The Urethral Plug: A New Treatment Modality for Genuine Uri-
nary Stress Incontinence in Women" J. Urology, vol. 44, p. llOO
(l990). This device consists of one or two solid spheres located
along a soft shaft, and a thin, soft plate located at the end of
WO94/26215 ~. ~ PCT~S94105536
21 63~ 31 4 ~
the shaft. One sphere is located upstream of the maximum
urethral closing pressure point, corresponding to the location
of the sphincter. In the two sphere embodiment, the second
sphere is located with its midpoint at the bladder neck, and is
used to assist in reducing urinary flow and pressure transmission
to the urethra so that the sphincter can operate. When the
patient wants to evacuate the bladder, the plug is removed,
evacuation occurs, and a fresh plug is inserted. One problem
associated with this device is that the patient must have three
urethral closure pressure profiles performed as well as other
exAmln~tions, before the device is made for the wearer.
Additional problems associated with this device include placement
difficulties, lack of sealing capabilities associated therewith,
inade~uate retention thereby allowing expelling and inade~uate
anchoring by the plate at the meatus. In addition to such
problems of inadequate placement, sealing, and retention, is the
discomfort associated with insertion and removal, due to the size
profile and rigidity of the spheres. The spheres of this device
maintain a constant diameter during insertion, and removal.
Another "remove-to-void" device is disclosed in United States
Patent No. 5,090,424, which comprises a conformable urethral
plug. The body of the plug forms a cavity which is in fluid com-
munication with another cavity via a check-valve. Thus, fluid
may be pumped into the cavity within the urethra to provide a
custom fit. This device, like many others relying on liquids or
gels for expansion, relies heavily on a fluid-tight valve in or-
der to maintain retention. Should valve failure occur, evacua-
tion would immediately follow. There is also a chance of fluid
leakage into the body of the wearer should rupture of the plug
occur.
In view of the above problems associated with the prior art,
an easily usable, expandable plug device of a non-fluid construc-
tion, which is mechanically actuated, would be desirable to those
afflicted with urinary incontinence.
OBJECTS OF THE INVENTION
One object of the invention is to provide a urethral plug
wo94l26~ls 2 ~ 6 3 1 3 I rcT~ss4lo5s36
which is easily manipulated by the wearer.
Another object of the invention is to improve the degree of
comfort associated with insertion and removal of a urethral plug.
- A further object of the invention is to enhance the sealing
ability of a urethral plug with the urethra, bladder neck or
; bladder wall.
Another object of the invention is to stabilize the place-
ment of a urethral plug at the urethral meatus, such that migra-
tion into the bladder will not occur.
Still another object of the invention is to reduce the risk
of infection to the wearer of a urethral plug .
Still another object of the invention is to provide a method
of using a manually deployable urethral plug by patients suffer-
ing from urinary incontinence.
It is yet another object of the present invention to provide
a method of using a removable-to-void urethral plug which expands
automatically without user intervention for retention in the body
and to block the flow of urine in the urethra.
These and other objects of the invention are carried out by
an expandable urethral plug having a contracted diameter for in-
sertion and removal through the orifice of the urethra, and a
larger, expanded diameter for blocking the flow of urine in the
urethra, bladder neck and bladder. In a first embodiment, the
plug comprises a cooperating housing and inner member lying in
coaxial engagement. A larger diameter is achieved by mechanical
deployment of the inner member, resulting in the changing of the
shape of the housing, or in another embodiment, the changing of
the shape of the inner member. In an alternative embodiment the
urethral plug comprises a member comprised of material which un-
dergoes automatic expansion when exposed to temperature or li-
~uid. The urethral plug assumes a non-expanded first condition
when exposed to a temperature approximately less than that of a
m~mm~l ian body, and assumes an expanded second condition when ex-
posed to a transition temperature of approximately the body tem-
perature of a m~mm~ 1 . In yet another embodiment, the expanded
second condition is achieved when the urethral plug is exposed
to natural bodily conditions such as, but not limited to, body
WO94/26215 , f-~ PCT~S94/05~36
21 63l 31
moisture.
In each embodiment, the expansion of the plug causes a bal-
loon to expand, which seals the plug to the urethral, bladder
neck and bladder wall. The plug further has a meatal plate for
anchoring the plug in the urethra, which preven:ts migration of
both the plug and the cord into the bladder.-~ Removal of the
plug for bladder evacuation, may be easily accomplished by pull-
ing a cord. In accordance with a further feature of the inven-
tion, there is provided a method for controlling incontinence in
humans.
BRIEF DESCRIPTION OF THE DRAWINGS
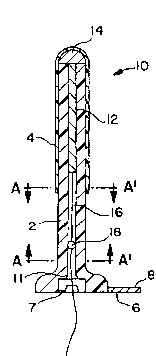
Figure lA shows a first embodiment of the urethral plug
which has an expansible outer tube, in its contracted configura-
tion.
Figure lB shows the first embodiment of the urethral plug
in its expanded configuration.
Figure 2A shows a second embodiment of the urethral plug
which has a braided mesh, in its contracted configuration.
Figure 2B shows the second embodiment of the urethral plug
in its expanded configuration.
Figure 3A shows a third embodiment of the urethral plug in
its contracted configuration.
Figure 3B shows the third embodiment of the urethral plug
in its expanded configuration.
Figure 4A shows a fourth embodiment of the urethral plug,
which is thermally expandable, in its contracted configuration.
Figure 4B shows a fourth embodiment of the urethral plug,
which is thermally expandable, in its expanded configuration.
Figure 5A shows a fifth embodiment of the urethral plug,
comprising a hydrophillic material, in its contracted
configuration.
Figure 5B shows a fifth embodiment of the urethral plug,
comprising a hydrophillic material, in its expanded
configuration.
Figure 6 shows an end view of the meatal plate of the
urethral plug of each of the above embodiments.
wo94l262ls 2 1 6 3 1 3 1 -~ PCT~S94/05536
Figure 7A shows an cross sectional view of the urethral
plug.
Figure 7B shows a cross sectional view of an alternate em-
bodiment of the urethral plug.
DETAILED DESCRIPTION OF THE INVENTION
Figure lA shows the urethral plug of the first embodiment
10, in its contracted configuration. The plug housing comprises
a hollow, cylindrical tube 2 which is sized to be easily inserted
through the orifice of the urethra. The tube 2 is made from a
biocompatible material having characteristics ofcompressibility.
Attached on the periphery thereof either by thermal bonding,
laminating or other means, is a sealing membrane, or balloon, 4
which is adapted to rest against the outer tube 2. At the distal
end of the tube 2 is a meatal plate 6. The meatal plate 6 is
adapted to anchor the urethral plug 10 at the meatus urinarius.
To carry out this function of anchoring, the meatal plate 6 is
of a thickness sufficient to withstand bodily compression during
wear, preferably on the order of 1 millimeter or greater. The
meatal plate 6 prevents the plug 10 from passing through the
orifice in the urethra ultimately leading into the bladder neck
or bladder.
Figure 6 shows an end view of the meatal plate 6, which is
the same as the meatal plates to be shown in future embodiments.
A portion of the meatal plate 6 is extended so as to form a tab
8 which may be grasped by the wearer for ease of removal. The
meatal plate may further aid in maintaining the plug's expanded
configuration during wear, by providing means for securing, such
as a ball retention socket (not shown). The meatal plate 6 also
has an opening 11 therein, lying within the plane of the opening
of the outer tube 2.
Referring again to Figure lA, enclosed within the tube 2 is
a support rod 12, which may be a hollow or a solid member. The
support rod 12 has a bulb 14 at one end thereof, abutting the
proximal end of the tube 2. The bulb 14 functions to hold the
support rod 12 within the tube 2. The support rod 12 has a cord
16 attached at its end opposite the bulb 14, which extends
WO94/26215 - - ~; PCT~S94/05536
2 1 6 3 1 3 1 ~
through the tube 2 and beyond the meatal plate 6, thus ensuring
that a wearer will always be able to reach the cord 16. On the
cord 16 there is preferably formed a knot 18. Although the knot
18 has been used, the attachment of any member having a diameter
greater than ball retention socket 7 would suffice. The support
rod 12 is preferably stainless steel, the o~ter tube 2 is
preferably formed of a biocompatible thermoplastic material and
the balloon 4 is preferably a biocompatible~thermoplastic elas-
tomer, such as that sold under the trademark KRATON. However,
any biocompatible material may be used for each of the aforemen-
tioned elements, as the invention is not to be limited to those
named above. Lines A-A' represents the cross sectional view of
the tube 2, which will be discussed further with reference to
Figures 7A and 7B.
A user inserts the plug 10 while it is in the configura-
tion shown in Figure lA. Once the plug 10 has been inserted and
the meatal plate 6 abuts the meatus urinarius, the plug 10 may
be deployed by the wearer, upon which it achieves an expanded
configuration, as set forth in Figure lB. To deploy, the wearer
pulls on the cord 16 depending from the support rod 12. By pull-
ing, a downward force is exerted on the cord 16 in the vertical
direction, forcing the support rod 12 to slide downwardly in the
tube 2 and exert a compressive force against the proximal end of
the outer tube 2. The tube 2 thus expands outwardly in the
horizontal direction, causing the balloon 4 to expand until the
balloon forms a seal with the wall of the urethra, bladder neck
or bladder. The wearer then secures the cord by sliding it
through a slit (not shown) in the ball retention socket 7 located
on the meatal plate 6. This causes the knot 18 to act as a stop,
as the knot 18 rests within the socket 7, thereby preventing the
tube 2 from returning to its contracted state (Figure lA).
The plug 10 in an expanded form as shown in Figure lB,
functions to retain and block the flow of urine. When the wearer
wishes to remove the plug, a simple tug on the cord 16 in a
direction away from the socket 7 will cause the knot 18 to be
released therefrom, thus causing the tube 2 to retract to its
contracted state of Figure lA. The tube 2 thereby returns to its
WO94/26215 2 ¦ 6 3 1 ~ ~PCT~S94/05536
original diameter prior to insertion, making plug removal a
comfortable task. Thus, the tube 2 and balloon 4 cooperatively
provide an expandable housing and the plug includes means for
mechanically expanding the housing and selectively returning the
housing to its non-expanded condition.
Figure 2A shows a second embodiment of the urethral plug
110 in its contracted configuration. Tube 102 is formed by a
flexible braided mesh 103. Attached on the periphery thereof
either by thermal bonding, laminating or other means, is a seal-
ing membrane, or balloon, 104 which is adapted to rest against
the tube 102. Enclosed within the tube 102 is a support rod 112,
which may be a hollow or a solid member. The support rod has a
bulb 114 at one end thereof, fixed to the proximal end of the
tube 102. The bulb 114 functions to secure the support rod 112
within the tube 102. The support rod 112 has a cord 116 attached
at its end opposite the bulb 114, which extends through the tube
102 and beyond the meatal plate 106, thus ensuring that a wearer
will always be able to reach the cord 116. The cord 116
preferably has formed therein a knot 118. Although a knot 118
has been used, the att~chment of any member having a diameter
greater than ball retention socket 107 would suffice. The sup-
port rod 112 is preferably stainless s,teel, the outer tube 102
is preferably formed of a biocompatible thermoplastic material
and the balloon 104 is preferably a biocompatible thermoplastic
elastomer, such as that sold under the tr~em~rk KRATON.
However, any biocompatible material may be used for each of the
aforementioned elements, as the invention is not to be limited
to those named above. Lines A-A' represent the cross sectional
view of the tube 102, which will be discussed further with
reference to Figures 7A and 7B.
The user inserts the plug 110 while it is in the
configuration shown in Figure 2A. Once the plug has been
inserted and the meatal plate 106 abuts the orifice of the
urethra, the plug is deployed by the wearer, upon which it
achieves the expanded configuration as set forth in Figure 2B.
To deploy, the wearer pulls down on the cord 116, in a direction
opposite the direction of initial insertion of the device. Thus,
WO94126215 2 t 6 31 3 l : ~ PCT~S94/05~36
a compressive force is exerted in the vertical direction by the
cord 116 on the bulb 114, which is transmitted from the bulb 114
to the tube 102. This force causes the braided mesh 103 to
expand outwardly in the horizontal direction. The expansion of
the braided mesh forms an oval projection, which projection
causes the balloon 104 to expand therewith. The wearer then
secures the cord 116 by sliding it through the slit (not shown)
in the ball retention socket 107 in the meatal plate 106. This
causes the knot 118 to act as a stop, as the knot is brought to
rest within the socket 107, thereby preventing the braided mesh
103 from returning to its contracted state as shown in Figure
2A). The balloon thus retains its seal with the urethral,
bladder neck or bladder wall and functions to block the flow of
urine.
When the wearer wishes to remove the plug , a simple tug on
the cord 116 in a direction away from the socket 107 will cause
the knot 118 to be released therefrom, thus causing the braided
mesh 103 to retract. The tube 102 thereby returns to its
original diameter prior to insertion, making plug removal a com-
fortable task.
Figure 3A shows the plug 210 of the third embodiment of the
present invention in its contracted state. An outer tube 202 has
a plurality of elongated apertures 201 formed therein. Line A-A'
represents the cross sectional view of the outer tube 202 which
will be discussed further with reference to Figures 7A and 7B.
Attached to the periphery of the outer tube 202, is a sealing
membrane, or balloon 204 which is its pre-insertion configuration
(Figure 3A), is adapted to rest against the outer tube 202. At
the proximal end of the outer tube 202 is an end cap 214. At the
distal end of the outer tube 202 is a meatal plate 206 which has
a thickness sufficient to prevent compression thereof while the
plug is worn. The meatal plate 206 further has a tab 208 and a
a groove 207 within its upper portion, which acts as a retaining
means, to be described in further detail below. The meatal plate
206 also has an opening 211 therein, lying within the plane of
the opening of the outer tube 202, through which an inner tube
212 passes.
WO94/26215 2 1 6 3 1 ~ I PCT~S94/05536
;, .. I~ ,~
11
Referring again to Figure 3A, inner tube 212 fits within the
outer tube 202, and is preferably longer than the outer tube 202.
The inner tube 212, has a plurality of cuts 213 defining a
- plurality of elongated segments 215 therein. The inner tube 212
is made from a biocompatible material having a property of com-
pressibility. The inner tube 212 further has a flange 218 at its
distal end which aids in securing the inner tube 212 within the
outer tube 202 after deployment. The inner tube 212 has a cord
216 attached to its bottom end, which aids in the removal of the
plug 210.
The user inserts the plug 210 while it is in the configura-
tion shown in Figure 3A. Once the plug has been inserted and the
meatal plate 206 abuts the meatus urinarius, the plug 210 may be
deployed by the wearer, whereupon it achieves the expanded con-
figuration set forth in Figure 3B. To deploy, the wearer pushes
the inner tube 212 into the outer tube 202, thus causing the
proximal end of the inner tube 212 to abut the end cap 214 of the
outer tube 202. The wearer continues to push in the vertical
direction until the elongated segments 215 of the inner tube 212
expand in the horizontal direction. The elongated segments 215,
thus flare-out, until each elongated segment 215 pops through one
of the elongated apertures 201 in the outer tube. Upon popping
through the apertures 201, the elongated segments 215 cause the
balloon 204 to expand. The wearer continues to push until the
flange 218 of the inner tube 212 is received in the groove 207
of the outer tube 202, whereupon the two members form a snap-fit
thereby locking the elongated segments 215 in a flared configura-
tion.
The balloon 204, having expanded with the flaring of the
elongated segments 215, thus forms a seal with the urethral,
bladder neck or bladder wall, and thus functions to retain the
device and block the flow of urine. When the wearer wishes to
remove the plug 210, a simple tug on the cord 216 breaks the snap
fit connection between the flange 218 and the groove 207, thereby
releasing the flange. This releasing action will cause the elon-
gated segments 215 of the inner tube 212 to retract as the distal
end of the inner tube 212 is pulled down and away from abutment
WO94/26215 ~ PCT~S94/05536
2.~ 63~3~ 12
with the end cap 214. Thus, the elongated segments 215 will
again lie flush within the outer tube, as shown in Figure 3A,
making removal of the plug a comfortable and easy procedure.
The urethral plug assemblies of Figures 4A, 4B, are com-
prised of a temperature sensitive compound, more particularly a
plastic polymer compound, even more particularly, a polyurethane-
based polymer compound. The compound isise~ected for its expan-
sion properties when subjected to temperatures of up to about and
including 37 C, which temperatures encompass the range of
temperature of a mAmmAlian body, more particularly, a human body.
The compound further exhibits properties of shape memory.
The plug of this embodiment is formed from a mold having
a hollow center. The shape memory polymer material is blow
molded into the maximum expanded shape desired for the plug. The
plug in this maximum expanded shape is then drawn through a
tubular shaped die heated beyond the transition temperature of
the shape memory polymer material, thereby reducing the diameter
of the to form a smaller diameter tubular shape, hereinafter
referred to as the 'pre-insertion'. Immediately after
withdrawing the plug from the die, it is cooled to a temperature
below the transition temperature of the shape memory polymer
material to maintain its pre-insertio~ shape. This process
produces a plug suitable for insertion into a subject's urethra.
The plug in its pre-insertion shape is now ready for packag-
ing. Packaging means includes encasing the plug in a suitable
plastic molded tray designed to maintain the pre-insertion
diameter during shipping, as h~n~l;ng and temperature fluctua-
tions above the transition temperature of the shape memory
polymer material may follow. Encasing the plug in a gelatin
material will also maintain the pre-insertion diameter while the
plug is in storage or transit. For those plugs packaged in
gelatin, the gelatin simply dissolves when exposed to moisture
in the body when inserted by the subject.
Upon insertion of the plug into the urethra of a mAmm~l, the
plug is exposed to a temperature gradient which triggers
automatic expansion of the device. R~memhering the original
diameter inherent to its mold shape, the plug begins to expand
~ WO94/26215 2 1 6 3 1 3 1 i~ i; PCT~Sg4/05536
13
from its pre-insertion shape to its mold shape. As it expands
toward this end, the plug conforms to the shape and size of the
urethra, especially upstream of the sphincter toward the bladder
neck. The plug continues to expand diametrically as it continues
to realize its mold shape, with the outer limits of expansion
defined by the walls Qf the urethra. Under no circumstances,
however, can the plug expand beyond the ~imen.~ions of its
original mold shape. The shape memory polymer comprising the
plug is only capable of expanding and conforming to the environ-
ment into which it is placed; it is incapable of exerting a
resistive force by itself.
There is no need to custom make the plug for each in-
dividual; the subject's urethra is simply measured by a physician
to ensure that the proper length and size plug is used. The plug
may be manufactured in several lengths and sizes in order to ac-
commodate males and females, adults and children. In its active
and operational state, after having been properly sized and used,
the plug forms a secure seal with the urethral, bladder neck or
bladder wall. The flow of urine through the urethra is thus
blocked.
Figure 4A shows the urethral plug 410 in its contracted con-
figuration. Tube 420 is a hollow, thin-walled cylindrical tube
which is sized to be easily inserted through the orifice of the
urethra. The tube 420 is made from a biocompatible material
having characteristics of expansion and compressibility. At the
distal end of the tube 420 is a meatal plate 406. The meatal
plate 406 is a flanged-type member which is adapted to anchor the
urethral plug at the meatus urinarius. To carry out this func-
tion of anchoring, the meatal plate 406 is of a thickness suffi-
cient to withstand bodily compression during wear, preferably on
the order of l millimeter or greater. The meatal plate 406 will
prevent the plug from passing through the orifice of the urethra
and into the proximal urethra, bladder neck or bladder.
The tube 420 is preferably formed of a biocompatible ther-
moplastic material. In a preferred embodiment, the tube 420 is
made of a known polyurethane-based polymer which provides the
plug with shape memory. The unique characteristic of the plas-
WO94/26215 ~ ~3 l 3 ~ PCT~S94/05536
14
tic polymer is its thermally triggered shape memory, which allowsthe tube 420 constructed of the shape memory polymer to be in-
serted into the urethra in a relatively compressed and elongated
state, and regain a useful shape at a selected temperature, such
as human body temperature. The two interchangeable shapes are
possible because the shape memory polymer-has "elastic memory",
that is, a large reversible change in el~stic modulus across the
glass transition temperature (Tg). Thus, the shape memory
polymer offers the unique characteristic of changing from a
glassy, more rigid condition to a softer, rubbery condition
across the Tg temperature. Such a large change in elastic
modulus around the Tg temperature allows for significant deforma-
tion in response to temperature changes. An increase in tempera-
ture allows the shape memory polymer to become more flexible and,
therefore, easily deformable into a new shape. The glass transi-
tion of a polymer, such as the shape memory polymer of the
preferred embodiment, is depicted below. The diagram
demonstrates a first transition from a glassy state to a rubbery
state as the temperature increases, and a second transition from
a rubbery state to a fluid state as the temperature is allowed
to further increase. It is the first transition from the glassy
state to the rubbery state that is exploited in the present
invention.
Any compound with thermally-triggered shape memory and
having a glass transition temperature approximately that of mam-
malian body temperature can be used in the device of the present
invention. A preferred compound is the polyurethane-based shape
memory polymer as described above, developed by Mitsubishi Heavy
Industries, Ltd. and available from Memry Technologies in Brook-
field, CT.
Accordingly, when the urethral plug shown in Figure 4A is
subjected to a transition temperature, the relatively rigid plug
changes to a second condition in which it is flexible and easily
deformable. The plug is now pliable and, remembering its " mold
shape plug", able to expand significantly in diameter to conform
to the shape of the wearer's urethra. A tight seal with the
urethra, bladder neck or bladder wall is formed and the plug is
WO94/~6~15 2 1 6 3 ~ ~ 1 PCT~594/05536
retained in the wearer's urethra to block the flow of urine.
In accordance with the above discussion, the user inserts
the urethral plug of the present invention into the urethra while
~ it is in the configuration of Figure 4A. Once the plug has been
inserted into the urethra and the meatal plate 406 abuts the
meatus urinarius, the plug is exposed to the heightened tempera-
ture of the human body. The temperature increase causes the
shape memory polymer comprising the tube 420 to automatically ex-
pand outwardly and achieve a protrusion 422 to conform to the
size and shape of the wearer's urethra. The shape memory polymer
is able to freely adapt and conform to its environment - here,
the urethra - because, as already discussed, it is only capable
of expanding and conforming to the environment into which it is
placed; it is incapable of exerting a resistive force by itself.
This important characteristic of the shape memory polymer
prevents displacement of the urethra, bladder neck or bladder by
the shape memory polymer material.
The plug is now in its expanded configuration 422 as set
forth in Figure 4B. As urine accumulates in the bladder, pres-
sure from the accumulating urine builds until the bladder is suf-
ficiently full to exert a downward force on the urine in the
bladder neck and urethra. The downward force in turn bears down
on the proximal portion of the expanded member of the plug,
furthering the diametrical expansion of the proximal portion of
the member. The expansion of the plug, in its expanded form,
provides a tight seal with the wall of the urethra, bladder neck
or bladder to retain the plug in the wearer's body. When the
wearer wishes to remove the plug to void, a continuous tug on tab
408 of the meatal plate 406 will cause the rubbery, diametrically
expanded member to elongate. The tube 420 is then returned to
a smaller diameter and is simply withdrawn from the body. Other
means for removal of the plug are contemplated, such as but not
limited to, a pulling means, such as a cord, whereby the plug is
simply removed by pulling on a cord attached to the plug. The
ease with which the shape memory polymer plug allows removal
prevents discomfort potentially associated with plug removal.
Figure 5A shows a second embodiment of the automatically ex-
O94/26215 2 1 G 3 1 3 l PCT~S94/05536t
16
pandable urethral plug 510 of the present invention in its con-
tracted configuration. Similar to the first embodiment discussed
above, tube 502 comprises a hollow, thin-walled cylindrical
shaft which is sized to be easily inserted through the orifice
of the urethra. In an alternative embodiment, the tube 502 may
comprise a solid cylindrical shaft. The tube'`'502 may be made of
any inert material suitable for insertion into a m~mm~lian body.
The tube 502 is made from a biocompatible' material, preferably
from a biocompatible thermoplastic elastomer, more preferably
from a biocompatible polyurethane-based polymer. The most
preferred tube is injection molded Kraton G, a non-toxic, biocom-
patible thermoplastic elastomer. Other suitable materials in-
clude polyethylene and nylon polymers, and other copolymers
similar thereto.
At the proximal end 504 of tube 502, there is an expandable,
deformable member 507 which, upon insertion into a m~mm~lian
body, is exposed to normal bodily conditions. Exposure to the
normal bodily conditions in turn causes the member 507 to expand
and achieve its expanded configuration, as shown in Figure 5B.
The member 507 may be sponge or any suitable absorbent
hydrophillic material. The expandable, deformable member 507 may
be attached to the tube 502 by an adhesive, a collar, thermal
bonding, or any attaching means suitable for the materials
selected for the member 507. The bodily conditions which affect
the member 507 are temperature (as in the shape memory material
device); moisture; pH gradations; and/or other such conditions
that act on and expand the member 507.
At the distal end 505 of the tube 502 is a meatal plate 506
which, as in the aforementioned embodiment, is a flanged-type
mem.~ber with a thickness sufficient to prevent compression by the
urethra during insertion and wear, preferably on the order of 1
millimeter or greater. Additionally on the meatal plate is a tab
508 for ease of removal. Tab 508 is instructive only and can be
substituted by other removal means such as, but not limited to,
a cord 510 attached to the inside of the tube 502 and extending
downwardly through the opening 509 in the meatal plate. Any
other such adaptation sufficient to allow removal of the device
~ . ~ r .~
S ~r~~ ~
WO94/2621~ 2 1 6 ~ 1 3 1 PCT~S94/05536
by a simple, continuous pulling by the subject, without tools or
undue force, is e~ually contemplated.
The expandable, deformable member 507 has a pre-insertion
shape which is sufficiently sized to allow easy insertion into
the urethra of a m~mm~lian body, more particularly, a human body.
once inserted, natural conditions in the body cause expansion,
preferably diametrical~expansion, of the member 507. In one em-
bodiment, the member would be a sponge secured to the tube 502
by any of, but not limited to, the aforementioned attaching
means. Upon insertion of the plug into the body, the sponge is
exposed to and absorbs moisture naturally present in the body and
expands diametrically, thereby forming a secure seal with the
urethral, bladder neck or bladder wall of the body. As in the
urethral plug comprised of the shape memory polymer material, the
urethral plug of this embodiment also expands diametrically until
it meets with resistance from the walls of the urethra, bladder
neck or bladder. The member 507 maintains its expanded state
until acted upon by the wearer, for instance, when voiding is
desired. The diametrically expanded member is sufficiently soft
and deformable so as to respond to the downward pressure exerted
by the wearer's pulling on the tab 508 or other pulling means.
A continuous tug on tab S08 of the meatal plate 506, or on cord
510, will cause the expanded member 507 to elongate, as it meets
tissue resistance. The plug is then returned to a smaller
diameter and is simply withdrawn from the body.
Figure 7A shows a cross sectional view of the urethral plug
along line A-A of the preferred embodiments set forth above.
Tube 300 represents the diameter of outer tubes 2, 202 of Figures
lA and 3B, and as applicable to the embodiments of Figures 2A,
4A, 5A the diameter of the tubes 102, 420, and 502, respectively.
Figure 7B shows an alternate embodiment, along line A-A, of the
above mentioned tubes, in cross section. As shown, the diameter
of tube 700 is not constant but variant as shown by the curved
indentations 702 on the periphery. The indentations 702 provide
enhanced surface area by which the plug may more readily adapt
to the urethral wall. Such enhanced sealing ability of the plug,
means a better fit for the wearer.
2l63131
WO94/26215 l ,~f I ~., PCT~S94/05536
18
The above and other features of the invention, including
various novel details of construction and combinations of parts,
will now be more particularly described with reference to the ac-
companying drawings and pointed out in the claims. It will be
understood that the particular devices embodying the invention
are shown by way of illustration only and not as limitations of
the invention. The principles and features of this invention may
be employed in various and numerous embodiments without departing
from the scope of the invention.