Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~1~38~2
PATENT 221PUS05274
USE OF OXIDIZED WHITE LIQUOR IN CELLULOSIC PU1P PROCESSING
TECHNICAL FIELD
The present invention is directed towards white liquor
oxidation in kraft pulp mills, and in particular towards the
use of oxidized white liquor in primary or secondary pulp
processing.
BACKGROUND OF THE INVENTION
The sulfate or kraft process is widely used in the pulp
and paper industry to convert wood chips into partially
delignified cellulose pulp which is used directly in unbleached
board and other unbleached paper products, or which is further
delignified and bleached for making high brightness paper
products. In this well-known process, the chips are converted
- into pulp at elevated temperatures by chemical delignification
using an aqueous solution known as white liquor which contains
sodium hydroxide, sodium sulfide, and other dissolved salts.
The spent liquor from this process step, known as weak blac~
liquor, contains residual organics, dissolved lignin, and other
wood constituents. This weak black liquor is concentrated by
evaporation, at which point soaps, resin salts, and fatty acids
are recovered. The resulting strong black liquor is further
evaporated, sodium and sulfur in various chemical forms are
added as needed to replace sulfur losses in the process, and
the mixture is combusted in a recovery furnace to yield molten
sodium sulfide and sodium carbonate; this molten material is
then dissolved in water to give an aqueous solution known as
green liquor. The green liquor is causticized with calcium
2163852
_ - 2 -
oxide (lime) to convert the sodium carbonate to sodium
hydroxide (caustic), which yields white liquor for use in
another pulping cycle.
White liquor is a potential source of alkali for certain
process steps in a kraft pulp mill except for the presence of
sodium sulfide in the white liquor, which is undesirable in
most applications. It has become common practice in kraft
mills to oxidize white liquor with air to remove most of the
sodium sulfide by conversion to partially oxidized sulfur
compounds comprising mostly sodium thiosulfate. This yields an
aqueous alkali, commonly known as oxidized white liquor, which
contains sodium hydroxide and sodium thiosulfate as the major
constituents with lesser amounts of sodium carbonate, sodium
sulfite, and sodium sulfate, and which contains low levels of
undesirable sodium sulfide. Oxidized white liquor as defined
above is widely used as an alkali source in oxygen
delignification, a process step which removes additional lignin
from kraft pulp to produce a higher brightness pulp. The use
of oxidized white liquor helps to maintain the balance of
sodium and sulfur in the pulp mill because the residual alkali
from oxygen delignification is returned to the white liquor
cycle. Oxidized white liquor as defined above also can be used
in gas scrubbing applications, for removal of residual chlorine
or chlorine dioxide from bleach plant effluents, in the
regeneration of ion exchange columns, and for the
neutralization of various acidic streams in the pulp mill.
Oxidized white liquor as described above generally cannot be
used in bleaching stages which utilize peroxide, hypochlorite,
or chlorine dioxide because the partially oxidized sulfur
compounds consume additional bleaching chemicals in a given
stage or in subsequent stages, thus rendering the use of
oxidized white liquor uneconomical in such applications.
Oxidized white liquor as defined above also cannot be used as
an alkali source for the production of sodium hypochlorite from
chlorine and sodium hydroxide, since thiosulfate reacts with
chlorine and sodium hypochlorite.
In current kraft pulp mill operation, the term white
liquor oxidation means the oxidation of white liquor using air
2163852
or oxygen to destroy sodium sulfide by converting most of the
sulfide to sodium thiosulfate. U.S. Patent 4,053,352 discloses
a method of oxidizing white liquor with an oxygen-containing
gas, preferably air, to convert practically all sulfides to
thiosulfate. Oxidation is carried out by injecting air into
white liquor in a tank at a flow rate of 50 to 500 Nm3/(hr-m2)
whereby the air provides oxygen and agitates the liquid to
promote mixing. Oxidation is carried out between about 50C
and 130C at a pressure up to 5 bars above atmospheric
pressure. The use of oxidized white liquor as a source of
alkali is disclosed, including applications in the steps of
oxygen bleaching, flue gas scrubbing, chlorine bleaching,
treating of waste gases from bleaching processes to destroy
chlorine or chlorine dioxide, regenerating ion exchange
columns, and neutralizing acidic liquids. Several process
steps are defined for which oxidized white liquor cannot be
used as an alkali source, such as peroxide bleaching and in the
manufacture of hypochlorite.
In an article entitled "Use of White and Green Liquors as
Alkalis in the Oxygen Stage of Kraft Pulp. (1) Oxidation of
White and Green Liquors" published in Przeqlad Papier 35, No.
6, June 1979, pp. 193-195, K. Baczynska reports results of a
study on the oxidation of these liquors. The study found that
the main oxidation product of sulfide contained in these
liquors is thiosulfate; depending on the conditions of
reaction, nearly complete oxidation (99.8%) of sulfide is
possible but requires up to 5 hours of reaction time. In the
presence of pulp in an oxygen bleaching reactor, sulfide
oxidizes essentially to sulfate and very small amounts of
sulfite and thiosulfate. The article teaches that white liquor
oxidation to predominantly thiosuLfate can be accomplished
batchwise in a glass column at temperatures between 40C and
80C using a contacting time of 1.5 to 8 hours.
Soviet Union Patent SU 1146345 A discloses the oxidation
of white liquor with a gas containing oxygen with addition of
-~ 21~38~2
spent alkali from an oxygen bleaching stage to increase the
rate of oxidation. Complete oxidation of sulfide occurs in 40
minutés at 90C under an oxygen pressure of 0.2 MPa compared
with 60 minutes when no oxygen bleaching spent alkali is added.
The products formed by the oxidation of sulfide are not
described.
A. I. Novikova et al in an article entitled "Oxidation of
White Liquor by Oxygen" in Khim. Tekhnol. Ee Prorzdnykh 1985,
pp. 49-52, describe the reaction paths of sulfide oxidation in
white liquor using oxygen or air. It is postulated that the
sulfide first oxidizes rapidly to polysulfide (Na2Sx), sulfite,
and thiosulfate. Subsequent oxidation of intermediate species
to sulfate occurs slowly and catalysts are required to
accelerate the reaction. Partially oxidized white liquor
containing polysulfides is said to accelerate delignification
when used as an alkali for delignification and bleaching; for
this reason oxidation to sulfate is stated to be undesirable.
Specific operating conditions for white liquor oxidation are
not disclosed.
The use of pure oxygen instead of air for white liquor
oxidation is described in a brochure entitled "AIRCO Tech
Topics" by Airco Gases, March 1990. A pressurized pipeline
reactor with recycle is disclosed for the oxidation of sodium
sulfide in white liquor to sodium thiosulfate and sodium
hydroxide. It is stated that the oxidation chemistry is the
same whether using air or pure oxygen and that both produce a
sodium thiosulfate product.
The background art summarized above thus discloses the
oxidation of white liquor to destroy sulfide by conversion to a
partially oxidized intermediate product comprising mostly
thiosulfate. In addition, uses of such an oxidized white
liquor as an alkali source in certain process steps in a kraft
pulp mill are described. However, other applications are
listed in the background art in which such an oxidized white
liquor cannot be used as an alkali source, chiefly because it
21638~2
-- 5 --
contains thiosulfate which consumes the oxidizing compounds
used for bleaching kraft pulp. Specific methods to produce and
use an oxidized white liquor which is free of significant
amounts of thiosulfate or other partially oxidized sulfur
compounds are not known or described in the current background
art.
The invention disclosed in the following specification and
defined in the appended claims offers methods for the selective
oxidation of white liquor and the use of oxidized white liquor
for improved treatment of both primary and secondary pulps.
SUMMARY OF THE INVENTION
The invention is a method for the delignification or bleaching
of cellulosic pulp which comprises contacting the pulp with
alkali and one or more chemical treating agents selected from
the group consisting of oxygen, peroxide, hypochlorite, ozone,
chlorine dioxide, and thiourea dioxide, wherein at least a
portion of the alkali is provided by fully oxidized white
liquor. The method can be used to treat virgin cellulosic pulp
wherein these chemical treating agents are used in one or more
pulp treating processes selected from the group consisting of
oxygen delignification, alkali extraction (E), oxygen alkali
extraction (Eo)~ peroxide-enhanced alkali extraction (Ep),
peroY.ide-enhanced oxidative extraction (Eop)~ peroxide
bleaching (P), hypochlorite bleaching (H), ozone bleaching (Z),
and chlorine dioxide bleaching (D). Alternatively, the
method can be used to treat a secondary pulp prepared from
waste paper material wherein the secondary pulp is treated by
one or more processes selected from the group consisting of
oxygen delignification, oxygen bleaching (O), peroxide
bleaching (P), ozone bleaching (Z), chlorine dioxide bleaching
(D), and FAS bleaching. The secondary pulp can be prepared for
example from mixed office waste or old corrugated containers,
and also can be prepared from other types of waste paper such
2163852
as pre-consumer waste paper as well as newspapers, magazines,
and other post-comsumer wastepaper.
The fully oxidized white liquor used in the invention can
be prepared by any satisfactory method, but preferably is
prepared from a white liquor feed stream comprising one or more
oxidizable sulfur compounds selected from the group consisting
sodium sulfide, sodium sulfite, and sodium thiosulfate by
contacting the white liquor feed stream with an oxygen-
containing gas stream in a reactor at a temperature between
about 180F and about 380F utilizing an oxygen supply rate and
residence time sufficient to convert at least 80% of the
oxidizable sulfur compounds into sodium sulfate, and
withdrawing from the reactor the fully oxidized white liquor
product. Oxygen is supplied to the reactor at a rate between
about 1.0 and about 1.3 times the stoichiometric amount
required to convert at least 80% of the oxidizable sulfur
compounds into sodium sulfate.
The use of fully oxidized white liquor as the source of
alkali in the processes listed above unexpectedly gives better
bleaching and delignification performance than the use of
sodium hydroxide as the alkali source. In addition, the use of
oxidized white liquor for this purpose in a pulp mill can
improve operations by reducing requirements for makeup alkali
and allowing closer sulfur and sodium balances in the mill.
BRIEF DESCRIPTION OF THE DRAWINGS
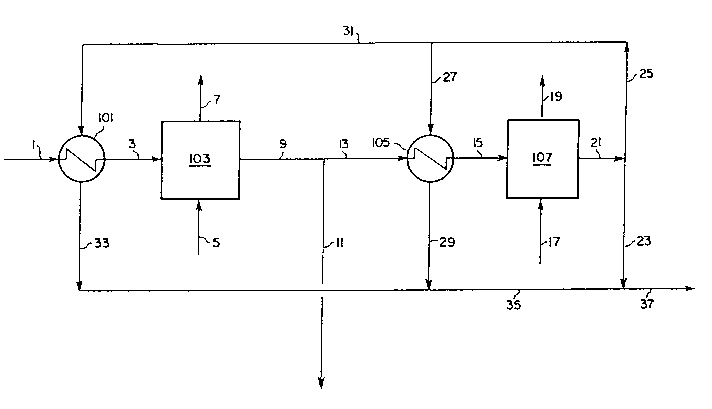
Fig. 1 is a schematic flow sheet of the process of the
present invention.
Fig. 2 is a plot describing the conversion of sulfur-
containing species as a function of the amount of oxygen added
for the process of the present invention.
Fig. 3 is a plot describing the sodium sulfate
concentration vs time for a batch oxidation of sodium
thiosulfate to sodium sulfate.
- 21G38~
Fig. 4 is a plot of relative reactor residence time vs
reactor temperature for the oxidation of sulfide at 150 psig by
the method of the present invention.
Fig. 5 is a plot of relative reactor residence time vs
reactor temperature for the oxidation of thiosulfate at 150 and
200 psig by the method of the present invention.
Fig. 6 is a plot of relative reactor residence time vs
reactor temperature for the oxidation of thiosulfate at 100
psig by the method of the present invention.
Fig. 7 is a plot of pulp yield vs Kappa number for medium
consistency oxygen delignification using unoxidized white
liquor and oxidized white liquor produced by the method of the
present invention as alkali sources.
Fig. 8 is a plot of pulp viscosity vs Kappa number for
medium consistency oxygen pulping using unoxidized white liquor
and oxidized white liquor produced by the method of the present
invention as alkali sources.
Fig. 9 is a schematic flow sheet of a typical open kraft
pulp mill which illustrates uses within the mill for oxidized
white liquor produced by the method of the present invention.
Fig. 10 is a schematic flow sheet of a closed kraft pulp
mill employing non-chlorine bleaching sequences which
illustrates uses within the mill for oxidized white liquor
produced by the method of the present invention.
- 21638~2
,_
DETAILED DESCRIPTION OF THE INVENTION
The present invention covers methods for making and using
oxidized white liquor in the bleaching and delignification of
virgin and secondary cellulosic pulps. According to the
present invention, oxidized white liquor has been found to be
an improved source of alkali to replace sodium hydroxide in
several bleaching and delignification steps for both primary
and secondary pulps.
In one embodiment, the present invention is a method for the
selective oxidation of white liquor in a pulp mill using the
kraft wood pulping process. The method comprises the steps of
(a) contacting an unoxidized white liquor feed stream
comprising sodium sulfide, sodium hydroxide, and water with a
first oxygen-rich gas stream in a first reaction zone at a
temperature between about 180F and about 325F utilizing an
oxygen supply rate and residence time sufficient to convert at
least 80% of the sodium sulfide into one or more partially
oxidized sulfur compounds and form a partially oxidized white
liquor; (b) withdrawing from the first reaction zone a portion
of said partially oxidized white liquor as a partially oxidized
white liquor product; (c) contacting the remainder of said
partially oxidized white liquor with a second oxygen-rich gas
stream in a second reaction zone at a temperature between about
300F and about 380F utilizing an oxygen supply rate and
residence time sufficient to convert at least 80% of all
unoxidized and partially oxidized sulfur compounds contained
therein into sodium sulfate; and (d) withdrawing from the
second reactor a fully oxidized white liquor product.
In an alternate embodiment, the invention is a method for
producing fully oxidized white liquor from a white liquor feed
stream comprising one or more oxidizable sulfur compounds
selected from the group consisting sodium sulfide, sodium
sulfite, and sodium thiosulfate. The method comprises the
steps of (a) contacting the white liquor feed stream with an
oxygen-containing gas stream in a reactor at a temperature
- 21638~2
;~
between about 180F and about 380F utilizing an oxygen supply
rate and residence time sufficient to convert at least 80% of
the oxidizable sulfur compounds into sodium sulfate; and (b)
withdrawing from the reactor the fully oxidized white liquor
product. The white liquor feed stream can be an unoxidized
white liquor in which the molar ratio of sulfide to total
sulfur is at least about 0.8; alternately the feed stream can
be a partially oxidized white liquor in which the molar ratio
of sulfide to total sulfur is less than about 0.2. Preferably,
oxygen is supplied to the reactor at a rate between about 1.0
and about 1.3 times the stoichiometric amount required to
convert at least 80% of the oxidizable sulfur compounds into
sodium sulfate.
The invention is also a fully oxidized white liquor
product made by (a) contacting a white liquor feed stream
comprising one or more oxidizable sulfur compounds selected
from the group consisting of sodium sulfide, sodium sulfite,
and sodium thiosulfate with an oxygen-containing gas stream in
a reactor at a temperature between about 180F and about 380F
utilizing an oxygen supply rate and residence time sufficient
to convert at least 80% of said oxidizable sulfur compounds
into sodium sulfate; and (bj withdrawing from the reactor the
fully oxidized white liquor product.
In another embodiment of the invention, fully oxidized
white liquor is used as the source of alkali in peroxide (P),
ozone (Z), hypochlorite (H), FAS, and chlorine dioxide
bleaching(D); oxygen delignification (OD); alkali extraction
(Ep); peroxide-enhanced alkali extraction (Ep); and peroxide-
enhanced oxidative extraction (Eop)~
In the background art summarized earlier, the term white
liquor oxidation pertains to the oxidation of sodium sulfide to
partially oxidized sulfur compounds, predominantly sodium
thiosulfate. The objective of the oxidation is solely to
destroy sodium sulfide. The term oxidized white liquor as-used
in the background art refers to the product of such an
21~3852
- 10 --
oxidation process. In the present specification and appended
claims, different terms are used to describe various white
liquors and the meanings of these terms are defined as follows.
White liquor (WL) is defined as a relatively unoYidized aqueous
liquor typically containing sodium hydroxide, sodium sulfide as
the major dissolved constituents, an intermediate amount of
sodium carbonate, and minor concentrations of sodium sulfite,
sodium thiosulfate, and sodium sulfate. White liquor also
contains very low concentrations of soluble metals or metal
salts derived from the wood chips fed to the pulping process.
This white liquor is obtained by causticizing green liquor as
earlier described, and typically the molar ratio of sulfide to
total sulfur in the white liquor is greater than about 0.8,
although it may be lower in some cases depending on actual mill
operation. Oxidized white liquor (OWL) is a generic term which
defines a white liquor which has been subjected to one or more
oxidation steps. Partially oxidized white liquor is defined as
white liquor in which at least 80% of the sodium sulfide
originally present has been oxidized to yield predominantly
sodium thiosulfate with smaller amounts of sodium sulfite,
sodium polysulfide, and sodium sulfate, and is alternately
defined herein as OWL(T). The molar ratio of sulfide to total
sulfur in OWL(T) is generally less than about 0.2. Fully
oxidized white liquor is defined herein as white liquor in
which at least 80% of all unoxidized or partially oxidized
sulfur compounds in partially oxidized white liquor have been
converted to sodium sulfate, and is alternately defined herein
as OWL(S). Fully oxidized white liquor made by the method of
the present invention utilizing a typical mill white liquor
feed will contain less than 15 g/l, preferably less than 10
g/l, and most preferably less than-5 g/l of oxidizable sulfur
compounds. The term oxidizable sulfur compounds as used herein
includes all unoxidized sulfur compounds (which comprise
sulfide, polysulfide, and hydrosulfide compounds) and partially
oxidized sulfur compounds (which comprise thiosulfate and
21638S2
sulfite compounds). The term oxygen-containing gas means any
gas containing oxygen, such as for example air, enriched air,
or high purity oxygen. The term oxygen-rich gas means a gas
containing at least about 80 vol% oxygen.
The use of both OWL(T) and OWL(S) as sources of alkali in
a kraft mill can improve operations by reducing requirements
for fresh alkali and allowing closer sodium and sulfur balances
in the mill. OWL(T) can be used as an alkali in oxygen
delignification, in which additional lignin is removed from
kraft pulp to produce a higher brightness pulp. The use of
OWL(T) in this process helps to maintain the balance of sodium
and sulfur in the pulp mill, and this benefit is expected to
become more important in the future as mills eliminate
chlorine-based bleaching sequences and replace them with
peroxide, ozone, and other nonchlorine sequences. OWL(T) can
be used in alkali extraction (E) or oxygen alkali extraction
(Eo) stages, preferably if these stages are not followed by
peroxide, hypochlorite, or chlorine dioxide bleaching stages.
OWL(T) also can be used for gas scrubbing applications, for
removal of residual chlorine or chlorine dioxide from bleach
plant effluents, for the regeneration of ion exchange columns,
and for the neutralization of various acidic streams in the
pulp mill. In applications in which the OWL(T) will contact an
acidic material, a sodium sulfide concentration of less than
0.5 g/l is typically required to avoid the release of any
significant amounts of hydrogen sulfide. Sodium sulfide
concentrations of less than 0.1 g/l are preferred in many
applications; such concentrations are readily achieved by the
method of the present invention, in contrast with present air
oxidation methods which typically achieve sulfide
concentrations of only 1-4 g/l.
OWL(T) is generally not economical as an alkali source in
processes which utilize oxidants which are more costly than
oxygen, since the thiosulfate and other oxidizable sulfur
compounds will consume a portion of these oxidants and thus
21638~2
.
- 12 -
adversely affect process economics. Such processes include
peroxide, ozone, hypochlorite, and chlorine dioxide bleaching
stages, as well as peroxide-enhanced alkali extraction (Ep) and
peroxide-enhanced oxidative extraction (Eop)r in which
relatively costly oxidative bleaching chemicals are utilized to
remove residual lignin and color from pulp to be used in high
quality paper products. OWL(T) also cannot be used as an
alkali source for the production of sodium hypochlorite, since
thiosulfate reacts with chlorine and sodium hypochlorite. For
such applications, OWL(T) must be further oxidized to OWL(S) by
converting a significant portion of the residual unoxidized or
partially oxidized sulfur compounds to sodium sulfate.
Practical methods for such further oxidation of white liquor to
OWL(S) were not previously available and have not been
described in the background art earlier described. The present
invention allows the efficient oxidation of partially oxidized
white liquor to a highly oxidized state for use in bleaching
and in the production of sodium hypochlorite. In an alternate
embodiment, the invention allows the efficient oxidation of
relatively unoxidized white liquor to a highly oxidized state
for use in bleaching and in the production of sodium
hypochlorite.
The oxidation of sodium sulfide and other oxidizable
sulfur compounds in aqueous solution with sodium hydroxide to a
final product of sodium sulfate proceeds through a number of
reaction steps. The overall main reactions are
2 Na2S + 2 2 + H2O ---> Na2S23 + 2 NaOH (1)
Na2S2O3 + 2 2 + 2 NaOH __-> 2 Na2SO4 + H2O (2)
and several intermediate and competing reactions also occur as
follows:
2 Na2S + 1/2 2 + H2O ---> Na2S2 + 2 NaOH ( )
21638~2
-
Na2S2 + 3/2 2 ~~~> Na2S23 (4)
Na2S2O3 + 2 + 2 NaOH ---> 2 Na2SO3 + H2O (5)
2 Na2SO3 + 2 ~~~> 2 Na2SO4 (6)
2 Na2S + 2 H2O ---> 2 NaHS + 2 NaOH (7)
2 NaHS + 3 2 + 2 NaOH ---> 2 Na2SO3 + 2 H2O (8)
Other intermediate reactions have been postulated
including the formation and direct oxidation of higher
molecular weight polysulfides (Na2Sy) to sodium thiosulfate and
sodium hydroxide. These reactions are exothermic; heats of
reaction for (1) and (2) above are -14,200 and -15,400 kJ/kg 2
consumed respectively. The kinetics and reaction equilibria of
these reactions have different temperature dependencies; in
addition, temperature affects the solubility and mass transfer
characteristics of oxygen in white liquor. The amount and
partial pressure of oxygen in the reaction zone also will
affect mass transfer rates and reaction equilibria. Further,
these reactions are readily catalyzed by various impurities and
compounds including those derived from wood in the pulping
process. For these reasons, the prediction of white liquor
oxidation reactor performance ànd operating parameters from
known background art is not possible.
A schematic flow diagram for the process of the present
invention is given in Fig. 1. In the primary mode of
operation, white liquor feed stream 1 is optionally heated in
exchanger 101 and flows as stream 3 into reaction zone 103.
Stream 1 typically has a molar ratio of sulfide to total sulfur
of at least about 0.8. Oxygen-rich gas stream 5, typically
containing at least 80 vol% oxygen, is introduced into reaction
zone 103 and contacted with the white liquor therein to
selectively oxidize the sulfide to thiosulfate and other
21638~2
- 14 -
partially oxidized sulfur compounds while minimizing the
consumption of oxygen to form sodium sulfate. This is
accomplished by controlling the flow of stream 5 such that the
molar ratio of oxygen therein to sodium sulfide in stream 1 is
between about 1.0 and about 1.3, and by controlling the
temperature in reaction zone 103. The temperature is
controlled between about 180F to 325F in reaction zone 103 by
controlling the flow of hot oxidized white liquor stream 31
through exchanger 101; the required flow of stream 31 will
depend upon the sulfide concentration in stream 1, the
temperature of stream 101, and other factors. Optionally, heat
exchange may take place within reaction zone 103 after oxygen
is in contact with the white liquor and the reaction has
commenced. Optionally, other known means for adding heat to
reaction zone 103 may be used. In certain cases, it is
possible that the combination of a high sulfide concentration
in stream 1 and a lower desired temperature in reaction zone
103 may require cooling rather than heating in exchanger 101.
Alternately, it may be desirable to operate the reaction zone
autothermally by neither heating nor cooling stream 1, in which
case the temperature in the reaction zone will reach a level
determined by the heat of reaction and the heat leak
characteristics of the reaction system. At least 80% and
preferably 95% of the sulfide in stream 1 is converted to
partially oxidized sulfur compounds, chiefly sodium
thiosulfate. Unconsumed oxygen, inert gases, and steam may be
vented from the reaction zone in stream 7.
Partially oxidized white liquor stream 9 is withdrawn from
reaction zone 103 and a portion of this stream is withdrawn as
partially oxidized white liquor product 11 (OWL(T)), which
typically has a molar ratio of sodium sulfide to total sulfur
of less than about 0.2. The remaining partially oxidized white
liquor stream 13 is heated if required in exchanger 105 by
211;3$~
indirect heat exchange with hot oxidized white liquor stream 31
and heated stream 15 flows into reaction zone 107. Partially
oxidized white liquor is contacted therein with oxygen supplied
by oxygen-rich stream 17 whereby the unoxidized and partially
oxidized sulfur compounds are further oxidized to form sodium
sulfate. The flow of stream 17 is controlled such that the
molar ratio of oxygen therein to sodium sulfide in stream 1 is
between about 1.0 and about 1.3, and the temperature in
reaction zone 107 is maintained between about 300F to 380F by
controlling the flow of hot oxidized white liquor stream 27
through exchanger 105; the required flow of stream 27 will
depend upon the temperature, flow rate, and concentration of
unoxidized sulfur compounds of stream 13, and other factors.
Optionally, heat exchange may take place within reaction zone
107 after oxygen is in contact with the white liquor and the
reaction has commenced. Optionally, other known means for
adding heat to reaction zone 107 may be used. In certain
cases, it is possible that the combination of high
concentrations of unoxidized and partially oxidized sulfur
compounds in stream 15 and the desired temperature in reaction
zone 107 will require cooling rather than heating in exchanger
105. Alternately, it may be desirable to operate the reaction
zone autothermally by neither heating nor cooling stream 13, in
which case the temperature in the reaction zone will reach a
level determined by the heat of reaction and the heat leak
characteristics of the reaction system. At least 80% and
preferably 90% of the unoxidized and partially oxidized sulfur
compounds in stream 15 are converted to sodium sulfate.
Unconsumed oxygen, inert gases, and steam may be vented from
the reaction zone in stream 19. Oxidized white liquor stream
21 is withdrawn from reaction zone 107 and split into stream
25, which supplies heat to exchangers 101 and 105, and product
stream 23, which is combined with cooled product streams 29 and
2163~2
-
- 16 -
33 via stream 35 to provide fully oxidized white liquor product
37 (OWL(S)). Reaction zones 103 and 107 are operated at
pressures between about 20 and 300 psig, preferably between
about 40 and 180 psig. Reaction zones 103 and 107 can be
contained in separate zones of a single reaction vessel or
alternately each zone can be contained in a separate reaction
vessel. Preferably, reaction zones 103 and 107 are operated in
a completely mixed gas-liquid two-phase mode using known
agitated reactor technology for contacting the respective white
liquors and oxygen-containing gas streams. Oxygen-rich gas
streams 5 and 17 contain at least 80 vol% oxygen and can be
supplied for example by vaporizing hauled-in liquid oxygen, by
an onsite cryogenic air separation system, or by an onsite
adsorptive air separation system.
The two key features of this invention are (1) specific
amounts of OWL(T) and OWL(S) can be produced to satisfy each
individual mill requirement, and (2) the reactor volumes and
oxygen requirements can be optimized to minimize reaction zone
residence time and hence reactor cost, and to minimize
operating costs such as oxygen dosage and mixing horsepower, by
control of the temperatures and oxygen addition rates to each
reactor or reaction zone. In the first reaction zone 103,
temperature is controlled between about 180F and 325F
(depending in part on feed sulfide concentration) in order to
maximize the amount of sulfide removed per unit of oxygen added
and minimize the amount of oxygen utilized to convert
thiosulfate and sulfite to sulfate. In the second reaction
zone 107, the temperature is controlled between about 300F and
380F to minimize the volume of the reaction zone; the optimum
temperature depends upon reactor pressure. These features are
discussed further in the Examples which follow. In an
alternate mode of operation as earlier described, the system of
Fig. 1 is operated without exchanger 101, reaction zone 103,
- 2163852
and associated streams, such that white liquor feed stream 1
flows directly into exchanger 105 and flows as heated stream 15
into reaction zone 107. In this mode, all of white liquor feed
stream 1 is converted into a fully oxidized white liquor
product 37 (OWL(S)), and no partially oxidized white liquor
(OWL(T)) is produced. Stream 17 is an oxygen-containing gas,
either air or enriched air, or preferably is an oxygen-rich gas
containing at least 80 vol% oxygen. Preferably, oxygen is
supplied in stream 17 to reaction zone 107 at a rate between
about 1.0 and about 1.3 times the stoichiometric amount
required to convert at least 80% of said oxidizable sulfur
compounds into sodium sulfate. In this mode, reaction zone 107
is a single reactor operating at between about 180F and about
380F (depending in part on sulfide concentrations in the
feed), and at a pressure between about 20 and 300 psig,
preferably between about 40 and 180 psig. Temperature in the
reactor is controlled as earlier described by utilizing a
portion 25 of reaction zone 107 effluent 21 to heat white
liquor feed in exchanger 105. The required flow of stream 27
will depend upon the temperature, flow rate, and concentration
of unoxidized sulfur compounds of white liquor stream 1, and
other factors. Optionally, other known means for adding heat
to reaction zone 107 may be used. In certain cases, it is
possible that the combination of high oxidizable sulfur
compound concentration in stream 1 and a lower desired
temperature in reaction zone 107 will require cooling rather
than heating in exchanger 105. Alternately, it may be
desirable to operate the reaction zone autothermally by neither
heating nor cooling stream 1, in which case the temperature in
the reaction zone will reach a level determined by the heat of
reaction and the heat leak characteristics of the reaction
system. Preferably, reaction zone 107 is operated in a
completely mixed gas-liquid two-phase mode using known agitated
~ `216 3 ~ 5 2
- 18 -
reactor technology for contacting the white liquor and oxygen-
containing gas stream. It is also possible as earlier
described to operate the process of the present invention in an
alternate mode in which the white liquor feed is split and
passed through two parallel reaction zones to yield OWL(T) and
OWL(S) products. In this mode, the oxygen addition rate and
temperature are controlled independently in each reaction zone
to yield the appropriate product and minimize the volume of
each reaction zone.
The invention is also a fully oxidized white liquor
product (OWL(S)) made by the either the primary or alternate
modes of operation described above. This OWL(S) product
comprises about 50 to 150 g/l sodium hydroxide, about 20-200
g/l sodium sulfate, and less than about 15 g/l of oxidizable
sulfur compounds. This product preferably contains less than
10 g/l and most preferably contains less than 5 g/l of
oxidizable sulfur compounds.
- In its primary mode of operation, the present invention
allows the optimum use of oxidized white liquor as a source of
alkali for a number of process steps in a kraft mill. For one
group of process applications, partially oxidized white liquor
(OWL(T)) is satisfactory as a replacement for fresh sodium
hydroxide as long as the residual sulfide concentrations are
below certain levels. These applications include oxygen
delignification, gas scrubbing applications, removal of
residual chlorine or chlorine dioxide from bleach plant
effluents, regeneration of ion exchange columns, and
neutralization of various acidic streams in the pulp mill.
OWL(T) can also be used as an alkali in alkali extraction (E)
and oxygen alkali extraction (Eo) stages in the absence of
downstream oxidative bleaching stages. Since the presence of
partially oxidized sulfur compounds such as sodium sulfite and
sodium thiosulfate are not known to be detrimental in these
` 2163852
- 19 -
applications, the white liquor can be oxidized only to the
extent needed to remove sulfides, thus minimizing reactor size
and oxygen consumption in the white liquor oxidation step as
earlier discussed. The preferred maximum residual sulfide
levels in OWL(T) for these applications depends on site-
specific process characteristics and economics, and is
typically less than 5 g/1 and most preferably between 0.1 and
0.5 g/l. In a second group of applications, the presence of
any significant level of unoxidized or partially oxidized
sulfur compounds in the oxidized white liquor is detrimental
and the use of OWL(S) is preferred. These applications include
peroxide, ozone, hypochlorite, and chlorine dioxide bleaching,
peroxide-enhanced alkali extraction (Ep), peroxide-enhanced
oxidative extraction (Eop)r FAS bleaching, and as an alkali
source in the production of sodium hypochlorite. In these
applications, residual oxidizable sulfur compounds in the
OWL(S) should generally be below about 10-15 g/l. Generally,
OWL(S) is the preferred form of alkali for use in alkaline pulp
bleaching stages, including alkali extraction (E) and oxygen
alkali extraction (Eo)~ because this use eliminates the
negative effects of residual oxidizable sulfur compounds in any
given bleaching stage or subsequent bleaching stage which uses
the expensive oxidants described earlier. OYidized white
liquor should be filtered to remove particulates prior to use
in any type of extraction stage. Also, OWL(S) may be preferred
over OWL(T) for oxygen delignification of pulps from certain
types of woods.
EXAMPLE 1
White liquor oxidation with oxygen was studied
experimentally in a kraft pulp miLl using a 850 gallon
pressurized stirred tank reactor using a 15 HP top-mounted
agitator. White liquor containing 23-38 g/l sodium sulfide, 1-
21~3852
- 20 -
4 g/l sodium thiosulfate, 0-2 g/l sodium sulfite, and 3-7 g/l
sodium sulfate was fed continuously to the reactor at 7-17 gpm
while oxygen of 99.9 vol% purity was introduced into the
reactor at different flow rates to investigate the effect of
oxygen addition rate on the extent of sulfide and thiosulfate
conversion. Liquid holdup time in the reactor was 40-118
minutes and the reactor was operated at temperatures between
263 and 329F and at total pressures between 18 and 98 psig.
Brownstock washer filtrate containing 5 wt% total dissolved
solids optionally was added as a catalyst in the range of 0-9
vol% on feed. Concentrations of sodium sulfide, thiosulfate,
sulfite, and sulfate were measured at the inlet and outlet of
the reactor for each set of operating conditions, and yield and
conversion information were calculated as defined by:
XNa2S = % conversion of sodium sulfide to any
oxidation product
YNa2S203 = % sodium thiosulfate yield expressed
as actual increase in thiosulfate
concentration divided by the
concentration of thiosulfate if all
inlet sodium sulfide were oxidized to
thiosulfate
YNa2SO4 % sodium sulfate yield expressed as
actual increase in sulfate
concentration divided by the
concentration of sulfate if all
inlet sodium sulfide were oxidized to
sulfate
` 2163852
The results of these tests are plotted in Fig. 2 as a
function of the relative oxygen addition ratio, which is
defined as the amount of oxygen added to the reactor divided by
the amount of oxygen required to oxidize all sulfide in the
reactor feed to thiosulfate. These results indicate that about
98% of the sulfide is removed at an oxygen addition ratio of
about 1.0 by conversion to thiosulfate and a small amount of
sulfate. Essentially all sulfide is removed at an oxygen
addition ratio of about 1.3 by conversion to thiosulfate and
sulfate. At an overall oxygen addition ratio of greater than
about 2.2, essentially all sulfur compounds are converted to
sulfate and the white liquor is completely oY~idized. The
catalyst was found to have no major effect on the rate or
selectivity of the reactions under these conditions.
These results illustrate that the present invention allows
the controlled oxidation of white liquor to yield any degree of
oxidation required for specific kraft mill applications. In
the primary mode of operation of the invention as earlier
described the oxidation is carried out in two reaction zones or
reactors in series; the first stage is operated preferably at
an oxygen addition ratio of between about 1.0 and 1.3 to remove
sulfide and the second stage is operated to achieve an overall
oxygen addition ratio for both stages of between about 2.0 and
2.6 in order to remove remaining oxidizable sulfur compounds.
This mode of operation provides two oxidized white liquor
products for the applications discussed above. In an alternate
mode of operation, the white liquor can be reacted with oxygen
in a single stage to a desired degree of oxidation by choosing
the appropriate oxygen addition ratio based on Fig. 2.
EXAMPLE-2
A series of experiments was carried out to understand in
more depth the oxidation of thiosulfate in white liquor. A
21638~2
-
sample of fully oxidized white liquor from Example 1 was
modified by the addition of 40 g/l sodium thiosulfate to give
an initial thiosulfate concentration of 50-55 g/l. The liquor
contained about 100 g/l sodium hydroxide, 6 g/l sodium sulfite,
-and 36 g/l of sodium sulfate. For each experiment, a sample of
the liquor was charged to a heated 4 liter stainless steel
reactor fitted with a hollow shaft turbine mixer which
circulated liquid and gas from top to bottom in the reactor.
Initially the reactor was pressurized with nitrogen to 150 psig
and mixed while being heated to about 160C. When heating was
complete, the reactor was purged with oY~ygen for about one
minute and set on pressure control wherein oxygen was added to
maintain reactor pressure as oxygen was consumed in the
reaction. Temperature was controlled at the desired
temperature by electric heaters and cooling coils. At time
zero, the mixer was set to 1800 RPM, oxygen flow was started,
and initial liquid samples were taken. As the reaction
proceeded, regular liquid samples were taken along with
measurements of oxygen addition rate and temperature. Liquid
samples were analyzed for thiosulfate, sulfate, and (in some
samples) sulfite. Several runs were made at 150 and 180C for
pressures of 120 and 150 psig. The results of these runs are
plotted as sulfate concentration vs reaction time in Fig. 3,
which demonstrates that complete oxidation at these operating
conditions can be achieved in 30-60 minutes reaction time.
EXAMPLE 3
The two-stage oxidation of white liquor to partially
oxidized white liquor, or OWL(T), and fully oxidized white
liquor, or OWL(S), was modelled using data from the literature
and from Examples 1 and 2. The purpose of the modelling was to
understand the relationship among operating parameters in the
oxidation process, particularly the effects of pressure,
- "` 2163852
temperature, oxygen addition rates, and reactor residence time.
Reaction rate constants for the oYidation of sulfide to
thiosulfate were taken from the article entitled "Kinetics of
Oxidation of Aqueous Sodium Sulfide by Gaseous Oxygen in a
Stirred Cell Reactor" by E. Alper and S. Ozturk in Chem. Eng.
Comm. 36, pp. 343-349, 1985. Reaction rate constants for the
oYidation of thiosulfate to sulfate were determined from the
data of Example 2. Expressions given by P. V. Danckwerts at
pp. 226-228 in his book entitled Gas-Liquid Reactions (McGraw-
Hill, New York, 1970) were used to model the dependencies ofthe mass transfer coefficients and interfacial area on physical
properties and process parameters. The coefficients were
determined using data from Example 1.
The model was used to calculate system operating
parameters based upon the following criteria and conditions:
(1) 98% of the sulfide is oxidized in the first stage reactor;
(2) 95% of the total sulfur in the fully oxidized white liquor
product is in the form of sulfate; (3) the molar flow of oxygen
to each reactor stage is 1.1 or 1.5 times the molar flow of
sodium sulfide in the feed; (4) the reactors are stirred tank
reactors; and (5) feed sodium sulfide concentration of 25 g/l.
The system pressure was selected as 100, 150, and 200 psig and
the temperature in each reactor was varied to observe the
reactor residence time required for the selected sulfide and
thiosulfate conversion.
The required reactor residence times were calculated at
different temperatures for an operating pressure of 150 psig
and the two oxygen to sulfide flow ratios of 1.1 and 1.5.
Results for the first stage reactor are plotted as relative
reactor residence time vs temperature in Fig. 4. The two
curves end at the temperatures at which the added oxygen is
completely consumed; this occurs because oxygen in excess of
that needed to oxidize the required fraction of sulfide to
2163852
- 24 -
thiosulfate is consumed by further oxidation of thiosulfate to
sulfate. The curves also indicate that increasing temperature
reduces reactor residence time, and that the benefits of
further increases in temperature above about 280-300F are
negligible. It may be possible in certain mills that a hot
white liquor feed (for example 200F) with a high sulfide
content (for example 50 g/l) will result in an autothermal
temperature of up to 325F in the reactor effluent. This is
the practical upper temperature limit at which the first stage
reactor should be operated, and is the basis for the upper
temperature limitation in the first stage reactor as defined
earlier in this specification. The benefit of increasing the
temperature diminishes at the higher temperatures, possibly
because (1) at constant total pressure after a certain
temperature is reached the ratio of the kinetic constant to
oxygen partial pressure declines and (2) at constant oxygen
partial pressure the solubility of oxygen decreases with
increasing temperature. Increasing the oxygen addition rate
reduces the required reactor residence time and thus capital
cost, but increases operating cost because of lower oxygen
utilization. The choice of oxygen addition rate is therefore a
balance between capital and operating costs which is determined
by the operating management of each individual mill.
The effect of temperature on reactor residence time was
calculated for the second stage reactor using a molar flow of
oxygen to the reactor of 1.1 times the molar flow of sodium
sulfide in the first stage feed, and at pressures of 100, 150,
and 200 psig. The results of relative reactor residence time
vs temperature for the two higher pressures are shown in Fig. 5
and clearly indicate sharp and unexpected minima in the
residence time vs temperature curves for the two pressures.
The minimum residence time at 200 psig is 26 minutes and occurs
at about 365F. At 150 psig, the minimum residence time is
` 21638~2
- 25 -
three times higher and occurs at about 345F. Results for a
pressure of 100 psig are plotted in Fig. 6 and indicate a less
sharp minimum and a much higher minimum reactor residence time
compared with the higher pressures of Fig. 5. These results
indicate that the two-stage white liquor oxidation system
should be operated at pressures between about 100 and 300 psig,
preferably between about 100 and 200 psig. The selection of
operating pressure is an economic tradeoff between reactor
volume and pressure rating, as well as the judgement of mill
operators regarding other equipment limitations at higher
pressures. These results suggest that the second stage reactor
should be operated at a temperature between about 300 and
380F, with a specific narrower range selected depending on the
actual operating pressure.
This Example supports a key feature of this invention in
which the each of the first and second stage reactors is
operated in different specific temperature ranges. The first
stage is operated at lower temperatures which favor the
efficient removal of sulfide to form thiosulfate while
minimizing consumption of oxygen to oxidize thiosulfate or
sulfite to sulfate. The second stage is operated at higher
temperatures required for conversion of the partially oYidized
sulfur compounds to sulfate at reasonable reactor residence
times.
EXAMPLE 4
Sodium hydroxide, white liquor (WL), partially oxidized
white liquor (OWL(T)), and fully oxidized white liquor (OWL(S))
were evaluated in the laboratory as alkali sources for oxygen
delignification and further bleaching steps using peroYide and
hypochlorite. Two sets of experiments were performed using a
softwood kraft pulp with an initial Kappa number of 34.5: (1)
-- 21638~i2
-
- 26 -
medium consisting oxygen delignification (OD), and (2) ox~ygen
delignification (OD) followed by a bleaching step.
In the first set of experiments, the kraft pulp was oYygen
delignified at the following conditions: 10~ consistency,
203F, 90 psig total pressure, reaction time of 60 minutes, and
alkali doses of 1 and 3 wt% expressed as NaOH on oven dried
pulp. Pulp viscosity (a measure of pulp strength), pulp yield,
and Kappa number were determined on each treated pulp sample.
GE brightness was measured for handsheets made from the treated
pulp. The results presented in Fig. 7 indicate that the use of
OWL(T) and OWL(S) gives better lignin removal and higher pulp
yield than WL, with OWL(S) giving slightly better results than
OWL~T). The results presented in Fig. 8 indicate that the use
of OWL(T) and OWL(S) gives higher pulp viscosity than WL, with
OWL(S) giving slightly better results than OWL(T). GE
brightness results (interpolated for a Kappa number of 12) are
presented in Table 1 for handsheets made from treated pulp, and
indicate that OWL(S) gives a brightness equivalent to that of
NaOH and slightly better than those of WL and OWL (T) .
Table 1
Brightness vs Alkali Source
Alkali Source GE Briqhtness, %
NaOH 33.4
OWL(T) 32.1
OWL(S) 33.5
WL 32.1
In the second set of experiments with a softwood sulfate
pulp, OD treatment was followed by hypochlorite bleaching. The
2163852
_
- 27 -
objective was to study the possible effect of entrained solids
and white liquor oxidation products after oxygen stage washing
on downstream brightening stages. WL, OWL(T), and OWL(S) were
used as alkali sources in the OD stage. All pulps were treated
in OD under identical conditions followed by simulated washing,
were diluted to 2% consistency, and were thickened to 10%
consistency without fresh water addition. Hypochlorite
bleaching was carried out at 3 wt% and 6 wt% dosage on pulp
using NaOH as alkali, and handsheets were made and tested for
GE brightness for all treated samples. The results of these
experiments are summarized in Table 2.
Table 2
Brightness vs OD Alkali Source
for Hypochlorite Bleaching
- OD Final Final
Alkali Brightness, % Brightness, %
Source (3 wt% Hypo) (6 wt% Hypo)
NaOH 65.6 71.1
2 5 WL 66.1 74.7
OWL(T) 69.1 73.9
OWL(S) 66.7 77.0
At the higher hypochlorite dose, OWL(S) produced the highest
brightness. At the lower dose, OWL(T) produced the brightest
pu lp .
NaOH, OWL(T), and OWL(S) were evaluated as alkali sources
for Eop and P bleaching of a softwood sulfate pulp chlorinated
to Kappa 23; the extracted pulp had a Kappa of about 14. Pulp
viscosity and handsheet brightness were determined as
`` `` 2163852
- 28 -
summarized in Table 3, which clearly indicates that OWL(S) is
the preferred alkali source.
Table 3
Viscosity and Brightness vs Alkali Source
for Oxygen Extraction with Peroxide (Eop)
Viscosity,
Alkali Source Mpa-Sec Brightness, %
NaOH 20.5 26.2
OWL(T) 24.7 22.9
15OWL(S) 25.0 25.8
The same softwood pulp was prebleached in a C Eop H sequence to
a brightness of 59.7% and treated with peroxide at 1.2 wt%
hydrogen peroxide, 158F, 10% consistency, 2 hours residence
time, 1. 8 wt% NaOH, and 0.05 wt% magnesium sulfate. The
results in Table 4 show that OWL(S) is clearly the preferred
alkali source.
2 5 Table 4
Viscosity and Brightness vs Alkali Source
for Peroxide Bleaching
Viscosity,
Alkali Source Mpa-Sec 8rightness, %
NaOH 6.1 78.2
OWL(T) 6.5 75.5
35OWL(S) 6.6 78.4
EXAMPLE 5
A mass balance for a 1000 TPD (oven-dried short tons per
day) southern pine integrated kraft mill was calculated to
` 2163852
- 29 -
illustrate the utilization of OWL(T) and OWL(S) in the mill, a
schematic flowsheet of which is given in Fig. 9. Wood chips 1,
sodium hydroxide 3 (optional), and a portion 5 of recycled
white liquor stream 6 are fed to digester 201 and cooked to
pulp and partially delignify the wood. The pulp and spent
pulping liquor as stream 7 flows to decker 203 with wash water
stream 9 in which the pulp is washed and separated from the
strong black liquor 11. Wash water stream 9 can be fresh water
or recycled filtrate from a downstream washer. The remainder
15 of recycled white liquor stream 6 at 175F is contacted with
oxygen stream 17 (99.5 vol% purity) in first stage white liquor
oxidation reactor 207 at 150 psig and 250~ to yield OWL(T)
streams 19 and 21. Unbleached pulp 13, at a consistency of 10-
12%, passes to medium consistency oxygen delignification (OD)
reactor 205 and is contacted therein with OWL(T) stream 19 and
oxygen stream 23 (99.5 vol% purity) which further delignifies
the pulp. Mixed pulp and spent liquor flow as stream 25 to
washer 209 with wash water stream 27 (which can be fresh water
or recycled filtrate from a downstream washer); OD stage
filtrate stream 29 and further delignified pulp 31 are
withdrawn therefrom. OWL(T) stream 21 is contacted with oxygen
stream 17 (99.5 vol% purity) in second stage white liquor
oxidation reactor 211 at 150 psig and 338F to yield OWL(S)
stream 35.
Oxygen-bleached pulp 31 next passes sequentially through a
five-stage bleach sequence consisting of chlorine bleaching
with chlorine dioxide substitution (CD) stage 213, peroxide-
enhanced oxidative extraction (Eop) stage 215, chlorine dioxide
(D) stage 217, alkali extraction (E) stage 219, and chlorine
dioxide (D) stage 221. The overall bleaching sequence
(including OD) is therefore O CD Eop D E D Each of these
stages includes a wash step (not shown) which utilizes wash
water stream 37, 39, 41, 43, and 45 respectively; the final
2163852
- 30 -
four bleach stages each utilize OWL(S) as an alkali source via
OWL(S) stream 49, 51, 53, and 55 respectively. Chlorine and
chlorine dioxide are added to stage 213 as stream 38; oxygen
and peroxide are added to stage 215 as streams 47 and 48
S respectively; chlorine dioxide is added to stages 217 and 221
as streams 50 and 54 respectively. Final bleached pulp product
is withdrawn as stream 57, and wash water streams (minus
recycle, not shown) from the stages are combined into waste
liquor stream 59.
Combined weak black liquor and oxygen delignification
stage filtrate stream 61 passes into evaporator system 223
which concentrates the liquor prior to recovery boiler 225 in
which the lignin and other organic wood-derived compounds are
combusted to produce steam and to yield furnace smelt 63. This
smelt is quenched and dissolved in dissolver 227 with added
water 65 to produce green liquor stream 67, which is
causticized with calcium hydroxide stream 69 in causticizer 229
to yield crude white liquor stream 71. The crude white liquor
is clarified in white liquor clarifier 231 and final white
liquor product stream 6 is recycled to the pulping process.
Precipitated calcium carbonate in streams 73 and 75 is
thickened in mud washer 233, calcined in lime kiln 235, and
slaked along with makeup lime 77 in slaker 237 to yield calcium
hydroxide stream 69. Optionally, a portion of OWL(T) stream 19
can be used to scrub lime kiln exhaust 79 (scrubbing not
shown).
The composition of the unoxidized white liquor (WL) and
oxidized white liquors are summarized in Table 5. It was
assumed that 99% of the sulfide and sulfite in the WL are
oxidized in the first stage reactor and that 99% of the
thiosulfate is oxidized to sulfate-in the second stage reactor.
2163~52
-
Table 5
White Liquor Compositions
Concentration, grams/liter
Component WL OWL(T) OWL(S)
Na2S 30 0.3 0.3
NaOH 100 100 83.5
Na2S23 3 33 0.33
Na2S3 1 0.01 0.01
Na2SO4 4 5.1 64
The required amounts of white liquor stream 15, OWL(T) stream
19, and OWL(S) stream 35 were determined using typical dosages
for the O, Eopr D, E, and D stages and are summarized in Table
6.
Table 6
Open Mill Oxidized White Liquor Requirements
Equivalent NaOH
Process StepDose, wt% on Pulp Type of WLFlow, gpm
-- OD 2.5 OWL(T)41.6
Eop 1.5 OWL(S)
29.9
D 0.6 OWL(S) 12.0
E 1.25 OWL(S) 24.9
D 0.6 OWL(S) 12.0
Total 120.4
The flow rates of oxygen streams 17 and 33 were calculated from
the required degrees of oxidation and flow rates summarized in
Tables 5 and 6, and a 20~ excess of oxygen was used. The
required amount of oxygen for the first stage reactor is 10,700
Z163852
-
- 32 -
SCFH and for the second stage is 7,760 SCFH for a total of
18,470 SCFH.
EXAMPLE 6
A mass balance was prepared for a modification of the
integrated mill of Example 5 in which all chlorine-based
bleaching stages are eliminated and the spent liquor from the
remaining non-chlorine bleaching stages is sent along with the
black liquor to the evaporation step and recovery boiler. This
modification is termed a closed mill as compared with the open
mill of Example 5, and represents the type of mill which will
be utilized by many pulp and paper producers in coming years
for its inherent environmental benefits. A schematic flowsheet
of the closed mill is shown in Fig. 10. The mill operates
essentially the same as the open mill of Fig. 9 except that (1)
the bleaching sequence CD Eop D E D is replaced by Z Eop P
where Z is ozone and P is peroxide, and (2) the spent liquors
from these bleaching steps (minus any recycled filtrate) are
recycled to the recovery system along with the black liquor.
Referring to Fig. 10, partially bleached pulp 31 from washer
209 flows with ozone stream 138 and wash water 137 to ozone
stage 301 in which the pulp is bleached and washed. The pulp
flows next to oxygen-peroxide extraction stage 303, where
oxygen 147, peroxide 148, wash water 139 (or recycled washer
filtrate), and OWL(S) 149 are added and the pulp is further
bleached. Finally, the pulp flows to peroxide stage 305 with
wash water (or recycled washer filtrate) 141, peroxide 150, and
OWL(S) 151 for final bleaching to produce pulp product 157.
Stages 301, 303, and 30S include interstage washers not
specifically shown. Spent liquor streams from these three
stages (minus recycled filtrate) are combined as stream 161
which is then combined with black liquor streams 11 and 29
prior to the chemical recovery steps described in the previous
2163852
example. A small purge stream 159 may be required to maintain
the proper chemical balance in the mill, or alternately purge
can be removed from individual bleaching stages.
White liquor was oxidized in the same manner as described
in the previous example, but different amounts of OWL(S) were
required for the final bleach stages. A mass balance was
calculated for the closed mill of Fig. 10 and the white liquor
requirements are summarized in Table 7. Oxygen requirements
were 8,000 SCFH and 4,900 SCFH for the first and second stages
respectively.
Table 7
Closed Mill Oxidized White Liquor Requirements
Equivalent NaOH
Process Step Dose, wt% on Pulp Type of WL Flow, gpm
OD 2.5 OWL(T) 41.6
Z -- -- --
Eop 1.5 OWL(S)
29.9
P 1.0 OWL(S) 19.9
Total 91.4
The closed mill bleach sequence thus requires 24% less oxidized
white liquor than the open mill bleach sequence of Example 5.
EXAMPLE 7
A comparative experimental study was conducted to compare
the effects of different sources of alkali in the chlorine
dioxide delignification of softwood kraft pulp. A softwood
kraft pulp was initially delignified with oxygen and
hypochlorite to a kappa number of 13.2, and had brightness of
55.9% and a pulp viscosity of 16.3 cps. In this and the
~163852
-
- 34 -
additional examples which follow, viscosity was measured using
the TAPPI T230 om-89 method. Synthetic fully oxidized white
liquor was prepared containing 100 g/liter NaOH and 50 g/liter
sodium sulfate. Chlorine dioxide delignification of the
initial delignified pulp was carried out in a laboratory
reactor at 70C, 1.2 wt% chlorine dioxide dosage on a dry pulp
basis, equivalent alkali dosage of 0.6 wt% as NaOH on a dry
pulp basis, and a three hour residence time. Alkali sources
were sodium hydroxide and the synthetic oxidized white liquor
described above. The brightness, pulp viscosity, kappa number,
and yield were measured for the pulp or handsheets made from
the pulp processed with each alkali source. The results are
given in Table 8.
Table 8
Chlorine Dioxide Bleaching with Different Alkali Sources
Alkali Source
Fully Oxidized
Sodium Hydroxide White Liquor
Brightness, %(GE) 72.7 76.5
Viscosity, cps10.8 12.7
Kappa No. 6.3 2.0
% Delignification 97.9 99.0
It is seen that the use of oxidized white liquor as the source
of alkali gives unexpectedly superior results compared with the
use of sodium hydroxide for chlorine dioxide bleaching.
EXAMPLE 8
- 216~8~2
- 35 -
A comparative experimental study was conducted to compare
the effects of different sources of alkali in the hypochlorite
bleaching of a previously oxygen-bleached softwood kraft pulp
having a kappa number of 24.5, a pulp viscosity of 23.9 cps,
and a brightness of 28.0%(GE). Synthetic oxidized white
liquor prepared in the previous example was used. Hypochlorite
bleaching of the initial delignified pulp was carried out in a
laboratory reactor at 65C, 3.0 wt% hypochlorite (based on the
sodium form) dosage on a dry pulp basis, equivalent alkali
dosage of 1.0 wt% as NaOH on a dry pulp basis, and a two hour
residence time. Alkali sources were sodium hydroxide and the
synthetic oxidized white liquor described above. The
brightness, pulp viscosity, and kappa number, were measured for
the pulp or handsheets made from the pulp processed with each
alkali source. The results are given in Table 9.
Table 9
Hypochlorite Bleaching with Different Alkali Sources
Alkali Source
Fully Oxidized
Sodium Hydroxide White Liquor
Brightness, %(GE) 46.2 48.9
Viscosity, cps 18.0 18.5
Kappa No. 15.4 13.8
It is seen that the use of fully oxidized white liquor as the
source of alkali gives unexpectedly better results compared
with the use of sodium hydroxide in hypochlorite bleaching.
2163852
-
- 36 -
EXAMPLE 9
White liquor was obtained from a softwood kraft mill and
oxidized in the laboratory to yield a fully oxidized white
liquor with the following composition and properties (all in
grams per liter): sodium sulfite, 1.04; sodium thiosulfate,
3.60; sodium sulfate, 84.14; sodium carbonate, 10.9; effective
alkali as sodium oxide, 70.0; and effective alkali as sodium
hydroxide, 90.2. A sample of a partially delignified softwood
pulp having a kappa number of 29.2 was obtained from the same
mill. Two portions of this sample were screened on a vibrating
flat screen and further delignified by eYtractive oYidation
with oYygen (Eo) in a laboratory reactor at 90C, an oxygen
partial pressure of 60 psig, a pulp consistency of 10%, with a
reactor contact time of 60 minutes. An effective alkali dosage
of 2.5 wt% expressed as NaOH was used based on oven-dried pulp.
NaOH and the fully oxidized white liquor described above were
used as the alkali sources in two separate experiments. The
results for the treated pulps are summarized in Table 10.
Table 10
Extractive Oxidation (Eo) with Different Alkali Sources
Alkali Source
Fully OYidized
Sodium Hydroxide White Liquor
~ Delignification 30.5 33.6
Kappa No. 20.3 19.4
It is seen that the use of fully o~idized white liquor as the
source of alkali gives unexpectedly better delignification
2163852
compared with the use of sodium hydroxide in oxidative
extraction .
EXAMPLE 10
Additional portions of the partially delignified softwood
pulp having a kappa number of 29.2 of Example 9 were further
delignified by peroxide-enhanced oxygen extraction (Eop) using
the same operating conditions and dosages as in Example 9, and
in addition a hydrogen peroxide dosage of 2.0 wt% and a
magnesium dosage of 0.25 wt% (as magnesium sulfate) on dry
pulp were used. Alkali was provided as NaOH or as oxidized
white liquor as in Example 9. The results are given in Table
11.
Table 11
Extractive Oxidation with Peroxide (Eop)
with Different Alkali Sources
Alkali Source
Fully Oxidized
Sodium Hydroxide White Liquor
% Delignification 41.4 43.8
Kappa No. 17.1 16.4
It is seen that the use of fully o~idized white liquor as the
source of alkali gives unexpectedly better delignification
compared with the use of sodium hydroxide in oxidative
extraction with peroxide.
EXAMPLE 11
"2163852
- 38 -
Partially delignified and bleached pulp was obtained from
a kraft softwood pulp mill where the pulp had been subjected to
the sequence O-D1oo-Eo~D. This pulp had an initial brightness
of 85.5% ISO. White liquor was obtained from the same mill and
fully oxidized as described in the previous examples to yield a
fully oxidized white liquor with the following composition and
properties (all in grams per liter): sodium sulfide, less than
0.1; sodium sulfite, 2.07; sodium thiosulfate, 6.55; sodium
sulfate, 85.03; sodium carbonate, 12.7; sodium hydroxide, 96.1;
effective alkali as sodium oxide, 74.4; active alkali as sodium
oxide, 74.4; and total alkali as sodium oxide, 81.8.
Two samples of this pulp were bleached with peroxide in a
laboratory reactor at the following conditions: 90C; 120
minutes contact time; 10% pulp consistency; chemical dosages as
wt% on dry pulp of 1.0% hydrogen peroxide, 0.25% magnesium
sulfate, 0.2% diethylene triamine pentacetic acid (a chelating
agent), and 1.5% effective alkali expressed as NaOH. The use
of sodium hydroxide and the fully oxidized white liquor
described above were compared as alkali sources by measuring
the brightness of handsheets made from the respective treated
pulps. The results indicated that the use of fully oxidized
white liquor yielded a brightness of 89.8% ISO while the use of
sodium hydroxide yielded a brightness of 89.1% ISO. This
increment of 0.7% ISO is a significant and unexpected
improvement achieved by the use of fully oxidized white liquor
in place of sodium hydroxide.
EXAMPLE 12
The use of fully oxidized white liquor as an alkali source
for the oxygen delignification of secondary pulps from
wastepaper was investigated in a series of laboratory
~- 2163852
- 39 -
experiments. White liquor was obtained from a kraft pulp mill
and oxidized in the laboratory to yield a fully oxidized white
liquor with the following properties and concentrations (all in
grams per liter): sodium sulfite, none detectable by the ion
chromatography (IC) method; sodium thiosulfate, 3.62 by the IC
method; sodium sulfate, 67.3 by the IC method; sodium
- hydroxide, 115.2 by the ABC method (a titration method widely
used in pulp mills) and 112.8 by the TAPPI T 624 cm-85 method;
sodium carbonate, 21.1 by the ABC method and 51.2 by the TAPPI
T 624 cm-85 method; active alkali, 110.4 by the TAPPI T 624 cm-
85 method; and T.A. total alkali, 136.4 by the ~BC method and
140.7 by the TAPPI T 624 cm-85 method.
Samples of OCC (old corrugated containers) were pulped and
the pulp or handsheet properties were determined as follows:
kappa number, 93.7; brightness as % GE, 15.8; and yellowness
parameter b , 19.7. The samples were delignified with oxygen
and alkali in an agitated laboratory reactor at 92C, 45 psig
total pressure, 45 minutes retention time, 7.5% consistency,
and 5 wt% effective alkali expressed as NaOH. Fully oxidized
white liquor as described above and sodium hydroxide were used
as alkali sources. The measured results are given in Table 12.
21638~;2
- 40 -
Table 12
Oxygen Delignification of OCC
with Different Alkali Sources
Alkali Source
Fully Oxidized
Sodium Hydroxide White Liquor
Brightness, %GE 50.6 42.3
Yellowness, b 25.5 27.3
Kappa No. 50.6 42.3
% Delignification 46 55
It is seen that the use of fully oxidized white liquor as the
source of alkali gives unexpectedly better delignification and
sheet properties compared with the use of sodium hydroxide in
oxygen delignification of OCC.
EXAMPLE 13
The experiments of Example 12 were repeated at the same
experimental conditions using a different OCC sample having the
following properties: kappa number, 76.1; brightness as % GE,
24.6; and yellowness parameter b , 16.1. Results of the
experiments are summarized in Table 13.
2163852
- 41 -
Table 13
Oxygen Delignification of OCC
with Different Alkali Sources
Alkali Source
Fully Oxidized
Sodium Hydroxide White Liquor
Brightness, %GE 24.8 27.8
Yellowness, b 18.5 19.9
Kappa No. 38.3 31.4
% Delignification 49.6 58.7
The results confirm that the use of fully oxidized white liquor
as the source of alkali gives unexpectedly better
delignification and sheet properties compared with the use of
sodium hydroxide in oxygen delignification of OCC.
EXAMPLE 14
Mixed office wastepaper was pulped and bleached with FAS
using different alkali sources to determine the benefit of
using oxidized white liquor in this application. FAS is the
notation used herein (and in the pulp industry) for the
intermediate compound formamidine sulfinic acid which is formed
from thiourea dioxide in alkaline solution; the intermediate
FAS breaks down into urea and sulfinic acid which reacts with
lignin and color bodies to remove these compounds from pulp.
Four different samples of this secondary pulp were treated in
an agitated laboratory reactor with FAS at the following
operating conditions: 75C, 60 minutes residence time, 12%
- 2163852
- 42 -
consistency, an alkali dose expressed as NaOH of 0.2 wt% on dry
pulp, and a FAS dose of 0.4 wt % on dry pulp. Alkali sources
were sodium hydroxide or oxidized white liquor as used in
Examples 12 and 13. Handsheets of the untreated and treated
pulp were prepared and used to determine GE brightness, and the
results are given in Table 14.
Table 14
FAS Bleaching of Mixed Office Waste
with Different Alkali Sources
Brightness Measurements as %GE
Alkali Source
Fully OY~idized
Sodium Hydroxide White Liquor
Sample 1 80.9 82.7
(Initial 72.2% GE)
Sample 2 79.9 79.9
(Initial 68.4% GE)
Sample 3 84.0 85.9
(Initial 75.4% GE)
Sample 4 83.2 84.9
(Initial 75.9% GE)
The results show that the use of fully oxidized white liquor as
the source of alkali gives unexpectedly better brightness for
three of the four samples compared with the use of sodium
hydro~ide in FAS bleaching of secondary pulp from office waste
paper.
EXAMPLE 15
- 2163852
- 43 -
MiYed office wastepaper was pulped and bleached with
hydrogen peroxide using different alkali sources to determine
the benefit of using oxidized white liquor in this application.
A portion of the untreated pulp was made into a handsheet and
tested to reveal a brightness of 64.4 %GE. Four different
samples of this secondary pulp were treated in an agitated
laboratory reactor with peroxide at the following operating
conditions: 80C, 60 minutes residence time, 10% consistency,
an alkali dose expressed as NaOH of 1.0 wt% on dry pulp, and a
hydrogen peroxide dose of 1.4 wt % on dry pulp. Alkali sources
were sodium hydroxide or oxidized white liquor as used in
Examples 12-14. In one comparison of the two alkali sources,
1.0 wt% magnesium sulfate was added to the pulp prior to
bleaching, which is normally done in peroxide bleaching to
improve brightness response of the peroxide. Handsheets of
the untreated and treated pulp were prepared and used to
determine GE brightness, and the results are given in Table 15.
Table 15
Peroxide Bleaching of Mixed Office Waste
with Different Alkali Sources
Brightness Measurements in %GE
Alkali Source
Fully Oxidized
Sodium Hydroxide White Liquor
Comparison A 68.4 72.2
(1.0% Mg added)
Comparison B 75.4 75.9
(No Mg added)
~1638~2
- 44 -
The results show that the use of fully oxidized white liquor as
the source of alkali gives unexpectedly better brightness than
the use of sodium hydroxide for peroxide bleaching with added
magnesium of secondary pulp from office waste paper. A slight
improvement using oxidized white liquor was realized when no
magnesium was used.
Thus an object of the present invention is the use of
fully oxidized white liquor, or OWL(S), to replace sodium
hydroxide as an alkali source in pulp processing steps which
utilize relatively costly oxidative bleaching chemicals to
remove residual lignin and color from virgin pulp such as kraft
pulp. These process steps include: oxygen delignification;
oxygen delignification followed by hypochlorite bleaching;
peroxide, ozone, hypochlorite, and chlorine dioxide bleaching
stages; oxidative alkali extraction (Eo); peroxide-enhanced
alkali extraction (Ep); and peroxide-enhanced oxidative alkali
extraction (Eop)~
Oxidized white liquor also is a preferred alkali source
for the treatment of secondary fiber from waste paper material
by oxygen delignification, FAS bleaching, ozone bleaching,
chlorine dioxide bleaching, and peroxide bleaching. OWL(S)
also can be used as an alkali source in the production of
sodium hypochlorite.
The fully oxidized white liquor for use in the present
invention can be prepared by any satisfactory method, but
preferably is prepared by the method described earlier in this
specification. A key feature of the invention is that the use
of oxidized white liquor uneYpectedly gives improved bleaching
and delignification results compared with the use of sodium
hydroxide for virgin kraft pulps and for secondary pulps
prepared from waste paper.
The essential characteristics of the present invention are
described completely in the foregoing disclosure. One skilled
2163852
in the art can understand the invention and make various
modifications thereto without departing from the basic spirit
thereof, and without departing from the scope and range of
equivalents of the claims which follow.
E: \JMF\US5274.APP