Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
wo 95/03293 2 1 6 7 7 1 4 PCT/HU94/00028
~ .
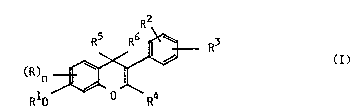
ISOFLAVONE DERIVATIVES
The present invention relates to isoflavone, isoflavan-4-one and isoflavane
derivaLhves of the general forrnula (I),
R2
S R5 R6 --~ R3 ( I ),
RI~ R4
their salts, ph~ eelltic~l compositions co~ g the compounds of the general
formula (I), and to a process for ~,e~aling ~he same.
The isoflavone derivatives of ~e general formula
R
Rlo~l ~ (IA),
the isoflavane-4-one derivatives of ~he general formula
R2
(R)lo 3 R3 (I13),
~e isoflavane derivatives of the general formula
R2
~ R3 (IC)
wo ss/032s3 2 2 1 6 7 7 1 4 PCT/~IU94/00028
form a narrower group of the compounds of the general formula (I).
In the general formula (I~
if n is 0, R5 and R6 together stand for an oxo group and the dotted line
means a double bond,
5 Rl represents Cl l8aLkyl s~bstih~ted by aLkylcarbonyl, carboxy, sulfonicacid, hydroxy, phenoxy, piperidino, morpholino or piridino or by a
(Cl-4alkYl)2N-(cH2)mco(cH2)p- or by
(Cl 4alkyl)2N-(cH2)moco(cH2)p- group; or st~nds for
C3 6cycloaLkyl or cycloaLkenyl; or
10 if n is 1, Rs and R6 together stand for an oxo group and the dotted line means a
double bond,
Rl represents Cl l8aLkyl optionally substituted by alkyl-carbonyl,
alkoxycarbonyl, carboxy, sulfonic acid, hydroxy, phenoxy, piperidino,
morpholino or piridino or by a (Cl 4alkyl)2N-(CH2)mCO(CH2)p- group; or
stands for C3 6-cycloalkyl or cycloaLIcenyl or C2 6aLkenyl; or
if n is O or 1, R5 and R6 together stand for an oxo group or stand s~lely
for hydrogen and the dotted line does not mean a çhemic~l bond,
Rl represents Cl l8aLlcyl optionally substitnte~l by aLkyl-carbonyl,
aLkoxycarbonyl, carboxy, s -lfonic acid, hydroxy, alkoxy, phenyl optionally
substituted by a halo atom, phenoxy, piperidino, morpholino or piridino or
by a (Cl 4alkyl)2N-(CH2)mCO(CH2)p- group; or stands for C3 6-cycloaLkyl
or C2 6alkenyl;
R stands for Cl 8aLkyl, halogen, Cl 4aLkoxymethyl, C2 s-acyloxymethyl, or
hydroxymethyl;
25 R4 stands for hydrogen or Cl 4aLkyl;
R2 and R3 stand for hydrogen or Cl 6aLkoxy;
R5 and ~6 together stand for an oxo group or separately stand for hydrogen;
the dotted line means a double bond being optionally present;
n isOorl;
30 m is an integer from 1 to 4; and
p is an integer from 1 to 4.
The compounds of the general formula (I) can be used for the prevention and
tre~tment of osteoporosis.
5 According to the invention the compounds of the general formula (IA) can be
d by reacting ketones of the general formula
WO 95/03293 PCT/HU94/00028
32167714
0 R2~ (III),
n ~X c - CH2
RlO OH
wherein R, n, Rl, R2 and R3 are as defined for the general formula (1),
a) with aLkyl orthoformate in the presence of a basic catalyst, or
b) with hydrogen cyanide and/or cyanic salts in the presence of
hydrohalides; or
c) with alkyl formiate in the presence of an alkali metal; or
d) with aLkyloxalylhalide, and the isoflavone ester thus obtained is,
if desired, saponified and/or decarboxylated; or
e) with organic carboxylic anhydride; or
f) with N,N-dialkyl acid amide in the presence of phosphorous chloride; or
g) by dehydrating 2-hydroxy-isoflavanone derivatives of the general
formula0
R2
R10~ C~ R3 (IV),
and if desired, converting an Rl group into another Rl group,
or forming an R group in a compound of the general formula (I), wherein R stands30 for hydrogen, and, if desired, converting a compound of the general formula (I)
thus obtained into its salt or setting it free from its salt.
According to process variant a) of the present invention a suitably substituted
ketone is reacted with alkyl orthoformiate, preferably ethyl ester, in an aprotic
35 solvent having a h~gh boiling point. As a solvent pyrrolidine, dimethyl formamide
or diet~ylene glycol dimethyl ether is used. As a basic catalyst ~lerel~bly
piperidine, morpholine, pyrrolidine and other secondary amines may be used.
WO 95/03293 4 2 1 6 7 7 1 4 PCT/I~U94/OllU8
According to process variant b) of the present invention the ketones are reactedwith hydrogen cyanide in an aprotic solvent in the presence of dry gaseous
hydrochloric acid or other hydrohalogenic acids or Lewis acids. Non-basic aprotic
solvents may also be used in the reaction, preferably diethyl ether or other diaLkyl
5 ethers. As catalyst zinc chloride or other Lewis acids may be used.
The reaction is carried out with hydrogen cyanide or an a~plu~liate salt thereof,
~rer~lably with zinc cyanide. The ll~ tule may be ~alul~L~d with dry gaseous
hydrochloric acid and the substituted a-fon~iminn-2-hydroxyphenylbenzyl-ketone
10 chlorohydrates thus obtained are decomposed with aqueous trçatrnent
- According to process variant c) of the present invention the ketones of the
formula (m) are reacted with aLkyl formiates in the presence of an aLkali metal.One preferably proceeds by adding dropwise suitably sub~liluLed 2-hydroxyphenyl
15 -benzyl-ketone dissolved in ethyl formiate onto pulverized metallic sodium, then
by decomposing the reaction ll~ ure with water and separating the isoflavone
thus-obtained.
.
According to process variant d) of the present invention suitably substituted
20 2-hydroxy-phenyl benzyl k~tonçs are reacted with alkyl oxalyl h~litles
A 2-alkoxycarbonyl-isoflavone delivilLiv~ is obt~ined, which is, if desired,
converted into an isoflavone derivative unsubstituted in position 2 by hydrolyzing
the ester group and by subsequent decarboxylation. This process variant can
prt;~lably be calried out with methyl- or ethyl oxalyl chloride in the presence of a
25 basic acid binding agent in an a~rop,iate aprotic solvent, ~rerel~bly pyridine or
another tertiary arr~ine.
According to process variant e) of the present invention the suitably substituted
2-hydroxy-phenyl benzyl ketone is reacted with organic acid anhydrides in the
30 presence of a basic catalyst. As an organic acid anhydride acetic, propionic or
ben~oic anhydride can be used. The anhydride is heated in the presence of a basic
catalyst, suitably an alkali salt of the acid component of the acid anhydride, or in
the presence of tertiary amines, without solvent or in an aprotic solvent havinghigh boiling point, such as pyridine or dimethyl formamide.
According to process variant f) of the present invention the ketone is reacted with
N,N-dialkyl acid arnides in the presence of phosphorus oxychloride, ple~,ably
by heating the suitably sub~l ;t,lle~l 2-hydroxy-phenyl benzyl ketone wi~
wo 95/03293 5 2 1 6 7 7 1 4 PCT/HU94/00028
N,N-dialkyl acid amid (e.g. dime~yl formamide or dimethyl acetamide) and
phosphorous oxychloride, using as solvent the N,N-diaLkyl acid amide itself.
According to process variant g) of the present invention 2-hydroxy-isoflavones of
S the formula (IV) are dehydrated by he~ting or by w~ g in an acidic medium in
polar solvent.
In the first step of the process according to the invention such derivatives may be
obtained from the compounds of the formula (m) or aV) in which Rl stands for
10 hydrogen or it is not the Rl group which is required in the target product.
In these cases the Rl group is introduced into the place of the hydrogen atom or,
respectively, an Rl group is converted into another Rl group.
This step can be carried out by the partial or total alkylation of the mono- or
polyh~ydroxy-isoflavones, which aLkylation can plerel~bly be carried out by
15 reacting with aLkyl h~lides or substituted aLkyl halides, alkyl sulfonic lactones,
aLkyl sulfates, olefines or epoxydes, preferably by heating the aLkylating agent in a
suitable solvent, e.g. ketones, dimethyl fonn~mi~le or ethers co~ g a higher
number of carbon atoms with the isoflavones to be aLkylated. In case of halogenspreferably an acid binding agent, such as alkali carbonate, and in case of aLkyl20 bromides and aLkyl chlorides preferably aLkali iodide is present.
This step can be carried out by the partial or total desacylation or the partial and
total d!esaLtcylation of acyloxy and polyacyloxy, aLkyloxy and polyaLkyloxy
isofla~ones. Acyloxy or polyacyloxy isoflavones are formed when process
varia~lt e) is carried out with di- or polyhydroxy phenyl benzyl ketones cont~inin~;
25 a hydroxy group in position 2. The desacylation is ~l~f~ bly carried out in an
acidic or basic medium in the presence of a polar solvent. This step can also becarried out by decarboxylating isoflavone-2-carboxylic acids. Isoflavone-2-
carbo~ylic acids are formed during process variant d) and their decarboxylation is
preferably carried out by heating with or without the presence of a catalyst, such as
30 copper dust.
The compounds of the general formula (IB), wherein R, n, Rl, R2, R3 and R4 are
as defined for general formula (I) are prepared by the reduction of the compounds
of the general formula (IA), wherein R, n, Rl, R2, R3 and R4 are as defined for
35 general formula (I). The reduction is carried out by catalytic hydrogenation or by
using metal hydrides.
2167714
WO 95/03293 6 PCT/HU94/000-8
In the case of the catalytic hydrogenation a nobel metal catalyst, l,lere.~bly ap~ lm on charcoal catalyst is used and the reduction is carried out in an
organic solvent, preferably in acetone.
As a comrle~ metal hydride, preferably diisobutyl ~l,.. ~il.. hydride is used and
the reduction is carried out at a low temperature (-70 C).
The compounds of the general form~ (IC), wherein R, n, Rl, R2, R3 and R4 are
as defined for general formula (I) are ~re~ed aby the catalytic hydrogenation ofthe compounds of the general form~ (IB), wherein R, n, Rl, R2, R3 and R4 are as
10 defined for general formula (I), in the presence of a noble metal or nickel catalyst.
The reduction is preferably carried out in a polar solvent, preferably acetic acid or
ethyl acetate.
The compounds of the general formula (I), wherein Rl stands for aL~cyl substituted
15 by carboxy are prepared by hydrolysing the ester group of the compounds
co..~ as Rl an aLkyl group substituted by alkoxyc~bullyl. The hydrolysis is
preferably carried out in an acidic medium, ~rert.~bly with lower organic acids in
the presence of a strong acid catalyst.
20 The compounds of the general formula (IA) co~ a methyl group
in position 6 are ~rep~ed by the reduction of halomethyl isoflavones obtained
from the compounds of the general formula (LA) co.~ a hydrogen atom in
position by h~lomethylation. The reduction is carried out preferably in the
presence of metals, ~i~r~lably zinc.
The compounds of the general formula (I) co~ an aLkoxy or hydroxymethyl
group in position 6 are pie~d either by replacing the halo atom of the
halomethyl isoflavones prepared as described above with an aLkoxy group by the
aid of alcohols or by replacing said halo atom with an 0-acetyl group by the aid of
30 sodium acetate and by subsequently converting the acetûxy group into an OH
group.
We have found ~at the compounds of ~e general formula (I) and salts thereof can
effectively be used for the prophylaxis and the tre~l~nent of osteoporosis.
35 It is known that Ipriflavone (7-Isopropoxy-isoflavone) is able to inhibit bone
resorption either in vitro or in vivo (Notoya, K. et al. Inhibitory effect of
Ipriflavone on pit formation in mouse unfractionated bone cells, CalciTissue Int.
51, (Supl. l) 53-56 (1992); Notoya, K. et al. Inhibitory effect of Ipriflavone on
osteoclast-me~ te~l bone resorption and new osteoclast formation in long-term
~ WO 95/03293 7 2 1 6 7 7 1 4 PCT/~IU94/00028
cultures of mouse unfractionated bone cells, Calcif Tissue Int. 50, 314-319 (1992).
On the other hand, it is known that Ipriflavone also could increase the
miner~li7~tinn of the extracellular matrix of human bone cell cultures (Ref. Ecsedi,
G.G. Model for in vitro investigation of bone mineralization, Agents and Actions41, 84~85 (1994.)
To estim~tç the effectiveness of the compounds of the general Form~ (I) on bone
r~ l;on an in vitro mineralization model was developed. Under certain
cirCl~rnct~nces cultures of partially selected (osteoblast-enriçhed) hllm~n bone cells
origin~te~1 from either nasal bone of adults or foetus femur produce Type I
coll~gene, bone specific proteins (e.g. osteocalcin), prostanoides (PGE2, PGF2a,PGI2, etc. and accnm~ te calcium into the synthesi7erl matrix (Re: Ecsedi, G.G.,
Chara~ on of cells of human nasal bone cell cultures, 4th Int. Symposium on
Osteoporosis, 27 March- 2 April, 1993 Hong Kong; Abstr. no. 534).
Method, Cells of subcultures 8-12 (usually 8th or 9th) were drop-inoc~ te~ at a
density of 2*10A4 cells per well 96-well plates. On the day 3 the tre~tment~ were
started with the compound of the Formula I at two concentrations,
lOA-8 and lOA-10 M. Because ethanolic loA 5 and loA 7 M stock solutions of the
compounds were used in the tre~tmentc all culture media cont~ined 0.1 % ethanol
in~ lin~ the Controls. Media were changed on each 2-3 day. The tre~1mentc were
fini~hed on the day 21, ~e total c~lcil~m (Ca) and DNA content of the 6-parallels~mples were me~nred by Boehringer Test Combination (~R3) and the
spectrofluorometric 3~5-~ minobenzoic acid (DA~A~ method, respectively, then
the ratios, Ca/DNA were calculated.
In the table of the compounds of Formula I below, data are given in
percentages compared to the average of the Control value (100%)
Nameof Concentration Ca DNA Ca/DNA
Compound [-log M] % % %
Ipriflavone 8 121 100 121
` (7-isopropoxy- 10 111 108 103
isoflavone)
CH-16693 8 110 97 113
(7-(-1 cyclohex- 10 131 107 124
2-enyloxy)-isoflavone)
2167714
WO 95/03293 8 PCT/HU94100028
The compounds of the general formula (I) may be lltili7t?(1 in the therapy in the
form of ~r~alions co,.~ g the active ingredient together with inert, non-toxic,
ph~ reutically acceptable solid or liquid ~ n~ntc or carriers.
If desired, the ~ ~alions can contain biologically active known substances such
S as vi~llil-c, amino acids, choline chloride, salts of mineral acids, trace elemen~c
etc. As carriers talc, ~el~tine, calcium carbonate, magnesium stearate, starch,
water, polyaLkylene glycols etc. may be used. The compositions may be
fonm~l~tçd as solid (e.g. tablets, dragées, capsules, suppositories etc.) or liquid
(e.g. solution, suspension or emulsion) preparations.
The invention is elucidated in more detail in the following non-limitin~ examples.
Example 1
10 g of 7-hydroxy-isoflavone, 10 ml of chloro~setone and 8 g of pot~csillm
carbonate are stirred in 120 ml of acetone and the ~ e is boiled for 5 hours.
The reaction ~~ is diluted with water, the precipil~le is filtered off and
recryst~lli7e-1 from acetic acid. 8.5 g of 7-(2-oxopropyl)-isoflavone are obtained,
m.p.: 174-175 C.
7-(2,3-dihydroxy-1-propyloxy)-isoflavone (FL 230), m.p.: 164-165 C,
7-(3-ethoxycarbonyl-propyloxy~isoflavone (FL 283), m.p.: 124-125 C,
7-(2-pheno~cyethoxy~isoflavone (FL 273), m.p. 195-197 C,
and
7-(1-ethoxycarbonyl-1-decyloxy~isoflavone (FL 279), m.p.: 97-99 C
are ~ ed in a similar way from 7-hydroxy-isoflavone and the corresponding
aLkyl halide or sul)~Liluled aLkyl halide.
7-(3-methyl-1-butyloxy)-isoflavone (FL 191), m.p.: 107-108 C, is ~repa~ed
from 7-hydroxy-3',4'-dimethoxy-isoflavone by using 3-methyl-1-butylbromid.
7-ethoxy-8-methyl-isoflavone (FL 315), m.p.: 129-130 C,
7-(carbethoxymethoxy)-8-methyl-isoflavone (FL 316), m.p.: 137-139 C, and
7~4-oxo-1-pentyloxy)-isoflavone (FL 501), m.p.: 143-145 C, are obtained from
7-hydroxy-8-methyl-isoflavone.
Example 2
A ~ e of 16 g of 6-n-hexyl-7-hydroxy-isoflavone, 14 ml of
isopropylbromide and 70 ml of dimethylformamide are stirred for 4 hours at a
temperature of 90 C in the presence of 16 g of potassium carbonate.
The reaction ~i~ e is poured into 500 ml of water, the product is separated, then
recryst~ e.l from 80% aqueous medlanol. 15 g of 6-n-hexyl-7-(1-methyle~oxy)-
~ WO 95/03293 9 2 1 6 7 7 1 4 PCT/HU94100028
-isoflavone are obtained, m.p. 37-39 C.
6-n-hexyl-7-ethoxy-isoflavone (FL 319), m.p.: 57-59 C, and 6-n-hexyl-7-
-(2-methyl-1-propyloxy)-isoflavone (FL 321), m.p.: 65-67 C, are prepared in a
similar way.
S By reacting 6-chloro-7-hydroxy-isoflavones with alkyl h~lides with the
following compounds are prepared:
7-ethoxy-6-chloro-isoflavone (FL 322), m.p.: 162-164 C,
7-(1-methylethoxy)-6-chloro-isoflavone (FL 323), m.p.: 156-158 C,
7-(2-methyl-1-propyloxy)-6-chloro-isoflavone (FL 324), m.p.: 170-172 C,
7-(2-propen-1-yloxy)-isoflavan-4-one (FL 238), m.p.: 76-78 C and
7-(4-nitro-benzyloxy)-isoflavan-4-one (FL 239), m.p.: 100-102 C.
Example 3
6.5 g of 7-n-hex~ecyloxy-isoflavone are hydrog~n~te~l in 1200 ml of
~cetone in the presence of 3.0 g of 10 % palladium on charcoal catalyst until a
hydrogen uptake of 1.2 equimolar amount. The catalyst is filtered off and the
solution is cv~or~Lcd. The residue is recryst~lli7e~1 from a ~llixLu~c of methanol
and ~t~etone to obtain 5.3 g of 7-n-hçx~-lecyloxy-isoflavon~one, m.p.: 90-92 C.7-et_oxy-5-methyl-isoflavan~-one (FL 299), m.p.: 97-98 C, is l)l~cd in
a similar way from 7-ethoxy-5-methyl-iso~avone.
7-cyclohexyl-isoflavan-4-one (FL 312), m.p.: 119-120 C, is p~arcd from
7-(1-cyclohex-2-enyloxy)-isoflavone (FL 286) by hydrogenation until a hydrogen
ptake of 2.2 equimolar amount.
Example 4
A solution of 14 g of 7-isopropyloxy-isoflavone in 160 ml of acetic acid is
hydrogenated in the presence of 5% p~ lm on charcoal catalyst until a
hydrogen uptake of 3 equimolar amount. The catalyst is filtered off, the solvent is
evaporated and the residue is recryst~lli7e~1 from methanol. 10 g of 7-(1-
methylethoxy)-isoflavane (FLl99) is obtained, m.p.: 93-95 C
7-(2-methyl-1-propyloxy)-isoflavane (FL 248), m.p.: 97-99 C, 7-(n-
h~ (lecyloxy)-isoflavan, m.p.: 90-92 C, are ~icpa.cd in a similar way from the
- collcspollding isoflavones.
7-cyclohexyl-isoflavane, m.p.: 90-92 C, is prepared from 7-(1-
-cyclohex-2-enyloxy)-isoflavone by hydrogenation until a hydrogen uptake of
4 equimolar amount.
wo 95/03293 2 1 6 7 7 1 4 PCT/HUg4/00028
Example S
2.38 g of 7-hydroxy-isoflavone and 1.94 g of propane sulfone are dissolved
in 25 ml of 1% methanolic sodium methylate. The nLixLule is let stand for 48
hours, then the precipiL~l~d product is separated by suction and recryst~lli7e~1 from
S water to obtain 3.0 g of 7-(3-sulfonyl-1-propyloxy)-isoflavone sodium salt which
melts above 350 C
7-(3-sulfonyl-1-propyloxy)-8-methyl-isoflavone sodium salt (FL 318),
m.p.: above 350 C,
6-chloro-7-(3-sulfonyl-1-propyloxy)-isoflavone (FL 346),
m.p.: above 350 C,
5-methyl-7-(3-sulfo-1-propyloxy)-isoflavone sodiurn salt (FL 502),
m.p.: above 320 C, and
7-(3-sulfo-1-propyloxy)-2-methyl-isoflavone sodium salt (FL 291),
m.p.: above 350 C
are ~ t;d in a similar way from the corresponding 7-hydroxy-isoflavone
derivatives.
Example 6
16.5 g of 7-(3-carbomethoxy-1-proplyoxy)-isoflavone are boiled for 9 hours
under reflux in a ~Lult; of 165 ml of glacial acetic acid, 8.5 ml of water and
1.0 ml of conc~ ed slllfilric acid. The free acid (m.p.: 188-190 C) preci~ lt;swhen the ~ixlult; is cooled, said acid is remo~ed by suction, dissolved in 300 rnl
of methanol and the solution is nel~tr~li7e~l to pH-8 with lN sodium methylate
solution. The pl~cipil~led 7-(3-carboxy-1-propyloxy)-isoflavone sodium salt is
separated by snction in an amount of 13.1 g, m.p.: above 320 C.
7-(1-carboxy-1-propyloxy)-isoflavone, m.p.: 197-200 C and its sodium salt
(FL 282) and
7-(1-carboxy-1-decyloxy)-isoflavone, m.p.: 124-126 C, and its sodium salt
(FL 280)
are prepared in a similar way from the corresponding esters.
Example 7
9 g of 7-isopropyloxy-isoflavone and 3.2 g of paraformaldehyde are stirred
for 3 hours at a temperature of 70 C in a mixture of 80 ml of glacial acetic acid
and 40 ml of concentrated hydrochloric acid under continuous introducing of
anhydrous gaseous hydrochloric acid. On the next day the solution is partially
evaporated, the pre~ is separated by suction and recryst~ e~l from
methanol. To the solution of the 7-isopropoxy-8-chloromethyl-isoflavone,
m.p.: 123-124 C, thus obtained with 50 ml of benzene an equivalent amount of
wo 9S/03293 11 2 1 6 7 7 1 4 PCT/HU94/00028
lN sodium methylate is added under boiling. The cooled solution is ch~kçn several
times with water and evaporated. The residue is recrysf~ e~l from methanol to
obtain 7 g of 7-isopropoxy-8-methoxymethyl-isoflavone, m.p.: 92-93 C.
7-methoxy-8-methoxymethyl-isoflavone (FL 308) is prepared from
7-methoxy-isoflavone in a similar way.
Example 8
To a suspension of 7.5 g of 7-methoxy-8-chloromethyl-isoflavone with
45 ml of glacial acetic acid 3.0 of zinc dust is added within 3 hours. After a further
stirling for 8 hours the reaction ~ e is diluted with warm water, the precipilat~
is separated by suction and recryst~ l from ethanol. 5.1 g of 7-methoxy-8-
-methyl-isoflavone are obtained, m.p.: 133-135 C.
Example 9
36.2 g of 2-hydroxy-4-(3-phenoxy-1-propyloxy)-phenylbenzyl-ketone, 22 g
of ethyl orthoformiate and S g of morpholine are boiled in 200 ml of dimethyl
form~mi-le for 8 hours. The ethanol formed during the reaction is removed through
a fr~c~tion~hnp~ ~tt~t~hmen~, then a great part of the solvent is evaporated in vacuo
and the residue is diluted with diluted aqueous hydrochloric acid.
The raw product is filtered offand recryst~lli7P~1 from ~cetone to obtain 32 g of
7 (3-plh~noxy-1-propyloxy)-isoflavone (FL 230), m.p.: 123-125 C.
Example 10
9.8 g of 7-(3-chloro-1-propyloxy)-isoflavone are boiled with 4.1 ml of
piperidine in 55 ml of 2-bnt~none in the presence of 5.5 g of pot~ccinm carbonate
and 0.5 g of pot~ccillm iodide for 14 hours. The inorganic salts are filtered off
while hot and after cooling the precipi~l~:d product is se~aled by suction and
recryst~lli7e~1 from methanol. 7-[3-(1-piperidine)-propyloxy]-isoflavone (FL 118)
is obtained in an amount of 6.0 g, m.p.: 138-139 C.
7-[3-(1-morpholinyl)-propyloxy~-isoflavone (FL 117) is obtained in a
simil~ way, m.p.: 162-163 C.
Example 11
18.5 g of 7-(10-ethoxycarbonyl-1-decyloxy)-isoflavone are boiled in a
n~ e of 180 ml of glacial acetic acid, 10 ml of water and 3 ml of concentrated
sulfuric acid for 4 hours. The next day the precipilal~d 7-(10-carboxy-1-decyloxy)-
isoflavone, m.p.: 118-120 C, is separated by suction, dissolved in a 4: 1 mixture of
acetone and methanol and the solution is adjusted to pH 8 by the aid of 10%
wo 95/03293 12 2 1 6 7 7 ~ 4 PCT/~IU94/00018
sodium hydroxyde. The precipitated salt is separated by suction and washed with
the solvent mixture. 7-(10-carboxy-1-decyloxy)-isoflavone sodium salt (FL 295) is
obtained in an amount of 10.6 g, which melts above 360 C.
7-(5-carboxy-1-pentyloxy)-isoflavone, m.p.: 146-148 C, is obtained from
7-(5-carbethoxy-1-pentyloxy)-isoflavone in a similar way and then the
co,lesponding sodium salt (FL 302) which melts above 360 C.
Example 12
3.0 g of 7-methoxy-isoflavone are dissolved in 30 ml of chloroforrn and
then 2.0 g of sulfurylchloride are added to the solution. The l~ Lul~ is boiled for
an hour, evaporated, then the residue is recryst~lli7P~1 from a 1~ c; of
chlorofo,m and ethanol. 8-chloro-7-methoxy-isoflavone (FL 501) is obtained in anamountof22.5g,m.p.: 181-182C.
In a similar way 7-ethoxy-isoflavone, m.p.: 144-145 C, is ~r~ ed from 7-
ethoxy-isoflavone, 8-chloro-7-(2-propyloxy)-isoflavone, m.p.: 167-169 C, from 7-
(2-propyloxy)-isoflavone and 8-chloro-2-methyl-7-methoxy-isoflavone (FL 517),
m.p.: 17~178 C, from 2-methyl-7-methoxy-isoflavone.
Example 13
2.0 g of 7-(carbethoxymethoxy)-isoflavone are dissolved in 10 ml of
diethylamino ethanol, 2.0 g of pot~cillTn carbonate are added to the solution and
the ~ix LLue is boiled for 5 hours under st~ ng then poured into a ~ e of ice
and 2% hydrochloric acid. The product is sepa~aled by s-~ction and recryst~lli7e~1
from a ~ e of methanol and acetone. 7-(N,N-diethylaminoethoxy-
-carbonylmethoxy)-isoflavone (FL 105) is obtained in an amount of 1.5 g,
m.p.: 227-228 C.
7 (N,N-diethylaminoethoxy-carbonylmethoxy)-2-methyl-isoflavone
(FL 104), m.p.: 190-192 C, is plep~ed in a similar way from
7-(carbethoxymethoxy)-2-methyl-isoflavone .
Example 14
16.0 g of 8-chloromethyl-7-methoxy-isoflavone and 11.4 g of anhydrous
sodium acetate are boiled in 80 ml of acetic anhydride for 4 hours. The reactione is poured onto water, the pre~ aled product is filtered off and
recryst~lli7;ç-1 from acetic acid. 8-acetoxymethyl-7-methoxy-isoflavone (FL 509) is
obtained in an amount of 11.7 g, m.p.: 195-197 C.
8-acetoxymethyl-7-(2-propyloxy)-isoflavone (FL 521), m.p.: 107-109 C,
is ~ ,~ed from 8-chloromethyl-7-(2-propyloxy)-isoflavone in a similar way.