Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
21 68939
-
TITLE OF INVENTION
Synthesis of Pharmaceutically Useful Pyridine Derivatives.
FIELD OF INVENTION
This invention relates to the manufacture of Omeprazole,
intermediates suitable for the manufacture of Omeprazole and the use thereof to
manufacture Omeprazole. This invention in its broadest aspects is directed to
the manufacture of medicines such as Omeprazole, Pantoprazole, and
Lansoprazole, intermediates suitable for the use to manufacture each of the
medicines and the processes using those intermediates to manufacture the
medicines.
BACKGROUND OF INVENTION
Omeprazole was discovered by Hassel chemists, and is derived from
the oxidation of intermediate 1'.
S--<~ ~OCH3 H3~S--<~ ~OCH3
T - '- 1' O
Intermediates 2' and 3' are coupled to give 1.
H3CO
~` ~OCH3
IntPnnPAi~tP 2' IntermPtli~tP 3
(See for example Canadian Letters Patent No. 1,127,158)
-2- 216g9~,~
-
Because the intermediates leading to the pyridine entity were very unstable,
Hassel came up with the following starting intermediate, w~ere the oxygen on
the nitrogen is eliminated at the stage when X is converted from methyl to
hydroxymethyl.
H3CO
~\~X
o
Tn~ , 4
(See Canadian Letters Patent No. 1,234,118)
EP 484,265 (Esteve) on the other hand, carried the synthesis with
either of chloro or nitro at the 4 position. Once the skeleton was built, Esteveeither substituted at the 4 position with methoxy and then reduced the nitroso or
vice-versa.
US 5,374,730 (Torcan) purports to teach the manufacture of
15 Omeprazole free from highly coloured impurities. Torcan achieves that result by
making a solid intermediate, that can be crystallized. To this end, Torcan
oxidized their substituted thioether and obtained a water soluble crystalline
intermediate which upon decarboxylation yielded pure water insoluble
Omeprazole.
Applicant is also aware of new and efficient oxidizing agents used
for converting the thioether to S=O purportedly taught by recent Takeda (CA
1,263,119) and Hassel's (U.S. 5,386,032) patents.
Applicant has now discovered a novel method for the manufacture
of Omeprazole, Pantoprazole and Lansoprazole and related medicines which
Applicant believes is efficient and suitable to produce these medicines.
3 21 6~9~
-
These methods are to be used to build substituted pyridines (useful
pharmaceutical intermediates), which could be used as ~recursors for the
synthesis of Omeprazole, Pantoprazole or Lansoprazole and related medicines.
In all the published synthesis covering Omeprazole or
5 Lansoprazole, the appropriately substituted pyridine was reacted with A, B or C
synthons.
R I ~f NH~_OCH3
N A
cB
Applicant believes that the following approach would be highly
suitable for use to make pyridines which are intermediates that could be used tomake medicines. Applicant proposes that the following pyridine compound:
x~ ~R2
~N R3
A' B'
20 could be prepared by the following scheme of reaction (in suitable solvents):
4 2 i 6g~,9~
-
Scheme 1:
+ ~;~R R~R3
n ~ III
Rl~R2
R~N~R3
IV
(Intermediate II is available. Intermediate I is generally known and may be
prepared using methods known in the literature such as:
1. Lou, J.-D.; Lou, W.-X. Synthesis, 1987, 179 (and references cited therein).2. E. Breitmaier; S. Gassenmann, Chem. Ber., 1971, 104, 665.
3. Kalina, N.N.; Klimko, V.T.; Protopopova, T.-V; Skoldinor, A.P. Z h .
Obshch. Khim. 1962, 32, 2146, C.A., 58, 7825 g.
4. Klimko, V.T.; Protopopova, T.-V.; Smirnova, N.V.; Skoldinov, A.P. Zh.
Obshch. Khim. 1962, 32, 2961.
5. Kalinina, N.N.; Klimko, V.T.; Protopopova; Skoldinov, A.P. J. Gen. Chem.
USSR (Engl. Transl.), 1962, 32, 2116.
6. Klimko, V.T.; Protopopova, N.V.; Smirnova, N.V.; Skoldinov, A.P. J. Gen.
Chem. USSR (Engl. Transl.), 1962, 32, 2913.
7. Wang, Chia-Lin J.; Salvino, J.M., Tetrahedron Lett. 1984, 25(46), 5243-6.
8. Seebach D., Chem. Ber. 1972, 102, 487.
-5- 2 1 68939
-
9. Solladié, G.; Ruiz, P.; Colobert, F.; Carreno, M.C.; Garcia Ruano, J.L.
Synthesis 1991, 1011.
10. Thummel, R.P.; Kohli, D.K. J. Org. Chem. 1977, 42, 2742.
11. Moller, R.; Engel, N.; Steglich, W. Synthesis, 1978, 621.
12. Ullrich, F.-W.; Breitmaier Synthesis, 1983, 641.
13. Menicagli, R.; Malanga, C.; Guidi, M.; Lardicci, L. Tetrahedron, 1987, 43(1),
171 (and rererellces cited therein).
14. Breitmaier, E.; Ullrich, F.W.; Potthoff, B.; Bohme, R.; Bastian, H. Synthesis,
1987, 1 (Ubersicht).
15. Hertenstein, U.; Hunig, S.; Oller, M. Chem. Ber 1980, 113, 3783.
16. Ruegg, R.; Lindlar, H.; Montavon, M.; Saucy, G.; Schaeren, S.F.; Schwieter,
U.; Isler, O. Helv. Chim. Acta 1959, 42, 847.
17. Nair, V.; Vietti, D.E.; Cooper, C.S. J. Am. Chem. Soc., 1981, 103, 3030.
18. Gagan, J.M.F.; Lloyd, D. Chem. Comm. 1967, 1043.
19. Weibenfels, M.; Schurig, H.; Huhsam, G. Chem. Ber., 1967, 100, 584.
20. Todoriki, R.; Ono, M.; Tamura, S. Heterocycles, 1986, 24(3), 775.
21. Eskenazi, P.C.; Maitte, P. Bull. Soc. Chim. 1976, 995.
22. Farina, F.; Gomez, M.J.; Martin, M.V. An. Quim. 1974, 70(12), 900-4.
23. Farina, F.; Victory, P. Tetrahedron Lett. 1969, 38, 3219-22.)
Intermediates I and II are selected to form A' and B' (the halves of
the pyridine molecule). III is converted to the final product IV by oxidation [O].
The substituents R, Rl, R2, R3 and X are chosen having regard to the substituents
on the medicines. Thus, the following combinations present themselves:
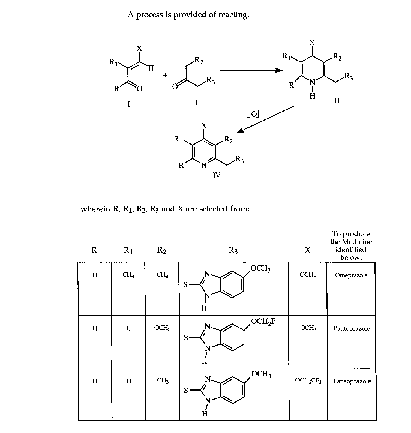
R R1 R2 R3 X Medicine
OCH
H CH3 _~ ~3/ OCH3 Omeprazole
HN
- 6 - 2 1 ~
-
H H S~ OCH2F OCH3 Pantoprazole
H H S--</ ~/OCH3 OCH2CF3 Lansoprazole
H
or R may be selected from:
H
Alkyl (1-3C)
Carboxyl acid
Esters
Cyano
Rl may be selected from:
H
Alkyl (1-3C)
Carboxyl acid
Esters
Cyano
R2 may be selected from:
H
Alkyl (1-3C)
Carboxyl acid
Esters
Cyano
20 R3 may be selected from:
Alkoxy
Hydroxy
7 2 l 6~3q
Halogen
Activated Ester
Tosylate
Mesylate
Thiol
Xanthyl
and X may be selected from:
Alkoxy
Halogen
Nitro
Cyano
Carboxyl
Alkyl
H
Compound III is novel and the precursor for the medicines
identified above (Omeprazole, Pantoprazole and Lansoprazole).
Compound III may also be an intermediate where R3 is a leaving
group such as Halogen (for example chlorine, bromine, fluorine and the like) or
a protected oxygen (OP where P is a protecting group). In this regard,
intermediate "A"", useful to make the above medicines, may be made from
intermediate IIIA where R3 is OP (where P= a protecting group).
The following synthesis based on building intermediate "A"" set
out below presents itself:
-8- 21 68939
-
X
~H ~, Base ~ ~
P_P~olecting Group mA
IIA
[O]
X X
Rl~R2 chlorinatingRl~R2
RJ~N~ ~ agentRJ~Nl~OH
IVA
A"
(Wherein the process X is eliminated during the reaction(s), X may be added to
Intermediate "A" or to the intermediates during the final stages of preparing the
5 medicine by methods known to persons skilled in the art.)
Compound IIA may be prepared from the corresponding alcohol
and a suitable protecting group (e.g. tetrahydropuranyl, tert-butyldimethyl silyl,
etc.). Other protecting groups like esters, carbonates and substituted methyl,
10 ethyl, benzyl or silyl esters can also be used.
Intermediate A" is then used to manufacture one of the three
medicines, as follows:
g ~ q3~
R~ + HS~
Rl~ N~
R N
~ N~
wherein R, Rl, R2 and X are defined above in the chart and L is selected from
OCH3 and 0CH2F.
Additionally, the pyridine may be built last so that all constituents
of the molecules are attached to a skeleton first, and ~hen the pyridine is
completed last. For example, the following scheme presents itself. (Synthesis
based on building the pyridine last):
-lo- 2168~3~
HS--<' ~L
~R2 TsCl ~R2 H
o~OH Chloride) o
R2
+ ~S~
VI
~5 ~NH~L
~NH VII
~[o]
~S~
N
wherein R, Rl, R2, X and L are defined as previously.
In another scheme, the Benzimidazole is built last:
2 t 6~39
- 11 -
R2 R2 J' R2 OEt
KS OEt ~ S~ (Et is ethyl)
~OH ~OTs ~/ S
V VII
R~ H
H~O
~ I
X R2 ~ OEt X R2 OEt
~/ ~ ~ [] R ~ ~S
R X R IX
H2N ~3~L
H2N
Rl~ NH~L
N
R
wherein R, Rl, R2, X and L are as previously described.
Compounds IIA, III, IIIA, VI, VII, VIl[I, IX and X following, are new:
o~P
P=Protecting Group
IIA
-12- 21 68~3~
Rl ~R2
R)~N~R3
III
Rl~R2
R N~
H IIIA
o~S~\
VI
Rl~ ~NH~L
NH VII
R2 OEt
~S~s
o vm
X R2 OEt
Rl~/S~`s
~NH
R IX
-13- 2 1 68~39
Rl ~ ~, ~SOEt
R N X
R, Rl, R2, R3, and X are as defined above.
The invention will now be illustrated with reference to the
following proposed examples.
Example 1:
Synthesis of the chloro pyridine (scheme relating to building intermediate "A")
A base (e.g. Potassium t-Butoxide) (1.0 eq) will be added to a cooled
solution (-20 to OC) of the protected hydroxy ketone (1.0 eq) in dry
tetrahydrofuran (THF). A THF solution of the a"~ unsaturated carbonyl (1.0 eq),
would then be added dropwise. At the end of the reaction, ammonium
chloride/ammonia (3.0 eq) will be added and the reaction mixture stirred at
room temperature. Water may then be added to the mixture and the organic
product extracted in toluene. The crude dihydropyridine will then be extracted
with an acidic aqueous solution (sulfuric acid).
To the aqueous solution, nitric acid will be added and the mixture
heated to reflux until the oxidation is complete. The solution will then be slowly
cooled to OC. Crushed ice will then be added followed by ammonium
hydroxide until the mixture is alkaline. The solid is then isolated and washed
with cold water. The crude product will be recrystallized from alcohol.
Other oxidizing agents could be used to oxidize the dihydropyridine
to the pyridine, e.g. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).
-14- 2 1 68939
-
The 2-Chloromethyl pyridine "Intermediate A" can be prepared by
reacting the 2-hydroxymethylpyridine with thionylchloride (according to Arch.
Pharm. Vol. 26 pp. 448-451 (1956)).
5 Example 2:
Based on the scheme for building the pyridine last.
Tosylchloride (1.0 eq) is added to a solution of the hydroxy ketone
(1.0 eq) and base (e.g. triethylamine) (1.0 eq) in a suitable solvent (e.g. toluene,
methylene chloride). The mercaptobenzimidazole sodium salt (1.0 eq) will be
10 added to the tosylate solution. At the end of the reaction, the mixture will be
washed successively with water, a saturated solution of sodium bicarbonate and
brine. The organic extract will be dried over sodium sulfate, filtered and will be
rotary evaporated to yield the crude product.
15 Example 3:
Benzimidazole formation (synthesis based on building the imidazole last)
Xanthate (1.0 eq) and the tosylate (1.0 eq) will be reacted in a solvent
(e.g. ethanol) at reflux. When the reaction is complete, the solvent will be
replaced with toluene and the organic layer will be washed with water and brine.20 The toluene is then rotary evaporated, THF will be added, and the solution
cooled (-20 to OC).
A base (e.g. Potassium t-Butoxide) (1.0 eq) is added to the cooled
solution of the xanthate adduct. A THF solution of the oc,~ unsaturated carbonyl(1.0 eq), is then added dropwise. At the end of the reaction, ammonium
25 chloride/ammonia (3.0 eq) will be added and the reaction mixture stirred at
room temperature. Water will be added to the mixture and the organic product
extracted in toluene. Toluene will be rotary evaporated and the crude product
will be used for the next step.
- 15- ~ 9 ~
-
m-Chloroperbenzoic acid (2.0 eq) will be dissolved in chloroform,
cooled to OC and added to the cooled chloroform solution (OC) of the
dihydropyridine. The mixture will be stirred overnight at room temperature,
filtered, and washed with 10% NaHCO3, and dried over sodium sulfate.
Filteration and rotary evaporation afford the crude product.
The crude product and 5-substituted phenylenediamine (1.0 eq) will
be dissolved in toluene that contained TFA (1.0 eq). The mixture will be refluxed
until the reaction is complete. At the end of the reaction, the mixture will be
cooled, 10% NaOH will then be slowly added until the mixture is just alkaline.
The crude benzimidazole will be then filtered, washed with water and
recrystallized from alcohol.
Example 4:
Intermediate I which is 3-methoxy, 2-methyl, 2-propenal, where X is
methoxy, R1 is methyl and R is hydrogen, may be prepared as follows:
Methanesulfonyl chloride (1 eq.) will be added to a solution of
methylmalondialdehyde sodium salt (prepared by a literature procedure
involving the Vilsmeier-Haack-Arnold acylation of propionaldehyde
diethyl acetal: Nair, V.; Vietti, D.E.; Cooper, C.S. J. Am. Chem. Soc. 1981,
103, 3030-3036) (1 eq.) in a suitable solvent (e.g. methylene chloride,
toluene). The mixture will be stirred at room temperature until the
reaction is complete. At the end of the reaction, the product will be
concentrated on the rotary evaporator and dissolved in anhydrous
methanol. The mesylate methanol solution will then be added to a
sodium methoxide (1-5 eq.) solution in the same solvent. The mixture
will be stirred at room temperature until the reaction is complete. The
product will be concentrated on the rotary evaporator, dissolved in
methylene chloride (or other suitable solvent, e.g. toluene) and washed
consecutively with saturated aqueous ammonium chloride, water, and
Z 1 ~ 9
- 16-
brine. The solution will then be dried over anhydrous sodium sulfate,
filtered, and rotary evaporated to yield the crude 3-methoxy, 2-methyl, 2-
propenal (I).
Other specific intermediate (I) compounds can be prepared by persons skilled in
5 the art having regard to the articles referred to herein and the above teachings.
As many changes can be made to the invention without departing
from the scope of the invention, it is intended that all material herein be
inl~l~reled as illustrative of the invention and not in a limiting sense.