Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02172053 2004-11-26
23940-887
1
PROCESS (I) FOR THE PREPARATION OF POWDERED MEDICAMENT
Background of invention
The invention relates to a method and an apparatus for making a powder
consisting of particles having a particle size smaller than lOllm free-flowing
by forcing the particles to create agglomerates.
Powders coi.sisting of very small particles are commonly used in the
inhalation therapy where the size of the particles are of utmost importance.
The diameter of particles which are to be inhaled must be less than 10 i.im
to ensure the adequate penetration of the particles into the bronchial area of
the lungs.
Most finely divided powdered medicament, such as micronized powders,
are light, dusty and fluffy and they often create problems during handling,
processing and storing. For particles having a diameter less than 10 ~.mn the
van der Wahls forces are generally.greater than the force of gravity and
consequently the material is cohesive. The particles tend to adhere to each
other forming non-defined agglomerates. Such powders have very poor
free-flowing properties which often make handling and precise metering. .
problematic.
One pflssible method of making these powders free-flowing or of least
improve their flowing properties is to force, in a controlled manner, the
primary particles to form larger particles, agglomerates. Non-defined
agglomerates are formed at random when this finely divided powdered
medicament is handled, for instance during storage, conveying, sieving,
sifting, mixing or grinding.
WO 95/09615
2
PCTISE94/00896
It is common knowledge that spherical agglomerates flow freely, packs
easily and uniformly, have an ideal form for coatings and are therefore .
commonly used in drug formulations.
The flow of very cohesive powders can be improved by vibration-induced
.agglomeration. Depending on the type of powder, liquid (often water) or
solid binders are added during the agglomeration, but it is also possible to
agglomerate without binders.
The method of agglomeration is applicable in principle to all materials,
including mixtures of various powders. Any powder, provided it is fine
enough, can be granulated or pelletized without a binder by systematic
agitation or rolling of the particulate material.
The common principle of performing this agglomeration is to let the
individual particles be exposed to a systematic movement of a powder bed,
without changing the physical and chemical properties of the primary
particles.
The agglomerated powders consist of relatively large, more dense and
compact spheres which exhibit the normal flow properties, but at the same
time the spheres should have sufficient low internal coherence to break up
into small primary particles of medicament of a therapeutically effective
size during inhalation in an inhalation device.
The inhaled route of administration enables the dose to be delivered
directly to the airways. By this type of administration it is possible to give
a
small dose and thereby minimizing unwanted side effects which for
.
example could occur if the substance ends up in another part of the body,
e.g. the gastrointestinal tract or the oro-laryngeal tract.
CA 02172053 2004-04-27
23940-887
3
Prior art
Methods of controlled agglomeration are known in the prior art. For
example, Claussen and Petzow Qournal of Materials Technology, vol 4(3),
I48-156 (1973)) have described a dry agglomeration method where no
binders are intentionally added for preparation of spheres in the size range
0.1 - 3 mrn by tumbling in a cylinder tilted at an angle to the horizontal
axis
of rotation. According to the authors a dry agglomeration process for small
particles requires nuclei in order to start, but almost all powders consist of
natural agglomerates that function as nuclei. They also conclude that
agglomerates from commonly used devices such as a rotating drum or a
granulating pan form a wide size distribution of the agglomerates that
makes frequent sieving necessary. The product formed often exhibit bad
spheriaty and low density.
US-A-5143 126 describes a vibratory conveyor for forming flowable
agglomerates from previously poorly flowable fine-grained powder by
using a nnethod wherein the poorly flowable poyvder is subjected to a
mechanical vibration step prior to transport and metering.
GB-A-1 569 611 describes a process for agglomeration of a drug into soft
pellets. In this process moist is used as a binder to provide a doe which by
extrusion is pressed through a sieve to create agglomerates.
G&A-2 187 952 describes a method in which crystalline ibuprofen is
compacted by kneading as it is conveyed by conveying screws through an
extruder. The obtained agglomerates can also be passed through an
extruder plate affixed to the end of the extruder.
CA 02172053 2004-04-27
23940-887
The invention
The present invention provides a method ~f. contro~3:ed
agglomeration of finely divided powdered medicament having a primary
particle size smaller than 10 ~.im, preferably smaller than 5 um, for example
such as micronized powders, in which no binders are needed and in which
the obtained agglomerates are of a uniform size having a shvcture which
provides sufficient flowability for the transport and metering of such
powders and which nevertheless have sufficient low internal coherence to
break up; within an inhalation device,-such as a dry powder inhalator, into
particles of medicament of a therapeuticaDy effective size, e.g, having a
particle size which is snnaller than 10 pun.
The method according to the invention provides a process for facilitating
the technical handling and significantly increase the medical value of the
substance. It has been found that this method produces agglomerates
having excellent handling properties, which have sufficient strength to
withstand packaging and storage, but which are sufficiently soft so that
they will break down into primary particles when they are expelled from an
inhalator during inhalation therapy.
According to the present invention there is provided a method for the
manufacture of agglomerates, which comprises subjecting the finely divided
particles of the medicament, which could be in admixture with any other
ingredient desired to be incorporated into the agglomerates, to mechanical
unit operations under certain conditions. More specifically there is prtwided
a method of treatment of a finely divided powdered medicament having a
particle size smaller than 10 Iun and poor flowing properties to form, in a
controlled manner, agglomerates or pellets which are free flowing and
CA 02172053 2004-04-27
23940-887
which are capable of breaking down to provide the finely divided
medicament, comprising the steps of
a) agglomerating the powdered medicament having particles sizes being
smaller than l0um by feeding the material to a screw feeder, letting the
5 material pass through the screw feeder thereby obtaining agglomerates,
b) spheronizing the resulting agglomerates in order to provide agglomerates
which are more spherical, more dense and more compact than the
agglomerates obtained from the agglomeration process in the screw feeder,
c) sizing the agglomerates to obtain a uniform size of the final product.
According to the invention there is also' provided an apparatus for
performing the method of treatment of a~finely divided powdered
medicament having a particle size smaller than 10 lim and poor flowing
properties to form, in a controlled manner, agglomerates or pellets which
are free flowing and which are capable of breaking down to provide the
finely divided medicament, wherein the apparatus comprises a screw feeder
with at least trvo co-operating screws which are rotating, a spheronizing .
device for spheronizing the resulting agglomerates andwa sizing device for
sizing the agglomerates to obtain a uniform size of the final product. ,
I i
CA 02172053 2004-04-27
23940-887
5a
In a method aspect, the invention provides a
method for treating a finely divided powdered medicament
haying a particle size smaller than l0 um and poor flowing
properties to form, in a controlled manner, agglomerates or
pellets which are free flowing and which are capable of
breaking down to provide the finely divided medicament,
comprising the steps of: (a) agglomerating the finely
divided powdered medicament by feeding the finely divided
powdered medicament through a screw feeder comprising twin
concave screws, wherein the pitch of the screws is
about 2 - 20 mm, to thereby obtain agglomerates: (b) sizing
the agglomerates obtained in step -(a)-to produce
agglomerates of a uniform size; (c) spheronising the
resulting agglomerates in a tilted granulating container in
order to provide. agglomerates which are more spherical, more
dense and more compact than the agglomerates obtained from
the agglomeration process in step (~); and (d) sizing the
agglomerates in a sieve to obtain a uniform size of the
final product. Suitably, the tilted granulating containe r
has one or more scrapers. Preferably, the particle size of
the finely divided powdered medicament is smaller than 10 um
and the size of the agglomerates after the agglomeration.
process in step (a) is less than or equal to 2 mm.
Preferably, the pitch of the screws is about 5 - 15 mm. The
method may further comprise: (c1) additional sizing and
spheronising after steps (a), (b) and (c), and wherein step
(cl) for the additional sizing a further sieve is used and
for the additional spheronising a further granulating
container. is used, and wherein the further granulating
container has one or more scrapers. Preferably, the
periphery speed of the tilted granulating container
is 0.5 - 1.0 m/s, and the spheronisation time is 2 - 20 min.
i a
CA 02172053 2004-04-27
23940-8$7
5b
In an apparatus aspect, the invention provides an
apparatus for forming a finely divided powdered medicament
having a particle size smaller than 10 ~zm and poor flowing
properties into agglomerates or pellets which are free
flowing and which are capable of breaking down to provide
the finely divided medicament, comprising: (a) a screw
feeder comprising twin concave screws, wherein the pitch, of
the screws is about 2 - 20 mm through which the finely
divided powdered medicament passes to obtain agglomerates;
(b) a sieve for sizing the agglomerates obtained in (a) to
produce agglomerates of a uniform size: (c) a spheronising
device comprising a tilted granulating container for
spheronising the resulting agglomerates; and (d) a sizing
device comprising a further sieve for sizing the
agglomerates to obtain a uniform size of the final product:
Suitably, the tilted granulating container has one or more
scrapers. Preferably, the pitch of the screws is about 5
to 15 mm. The apparatus may further comprise an additional
sieve for further sieving the agglomerates and an additional
tilted granulating container for further spheronising the
agglomerates, wherein the additional tilted granulating
container has one or more scrapers. Preferably, the
apertures of the sieves have a size between 0.2 - 2.0 mm.
More preferably, the apertures of the sieves have a size
between 0.3 - 1.0 mm.
The invention also provides use of an apparatus
according to the invention for carrying out a method
according to the invention.
The invention also provides an agglomerate
manufactured in accordance with a method of the invention by
using an apparatus according to the invention for use in a
breath-actuated dry powder inhaler. The breath-actuated dry
powder inhaler is, e.g., Turbuhaler~.
i a
CA 02172053 2004-04-27
28940-887
6
Brief description of the drawings
The agglomeration of the powder is now described with reference to the.
drawings which show a prefered embodiment of the apparatus as well as
diagrams from the results of experiments made in accordance with the
invention, wherein
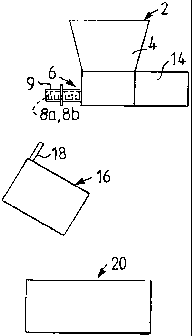
Fig. 1 shows a schematic view of a first embodiment of an apparatus
according to the invention,
Fig. 2a shows a schematic view of the screws in the screw feeder,
Fig. 2b shows a schematic view of the screws mounted within the housing,
Fig. 3 shows a schematic view of a second embodiment of an apparatus
according to the invention, '
Fig. 4a and 4b show the differences in agglomerates obtained from a saew
feeder having long-pitch screws and short-pitch screws respectively,
Fig. 5 is a diagram showing a comparison of the size of the agglomerated
' particles as a function of different screws,
Fig. 6 is a diagram showing size distribution of spheres as a function of
different screws,
Fig. 7 is a diagram of a sieving analysis of miuonised terbutaline sulphate
processed in an apparatus according to the invention, and
W O 95/09615
PCT/SE94/00896
7
Detailed description of the drawings
The apparatus used to carry out the method according to the invention is
shown in fig.l. The apparatus includes an agglomerator in the form of a
screw feeder apparatus 2 comprising a receiving vessel 4 and a screw
feeder device 6. The screw feeder device 6 comprises at least two screws 8a,
8b being surrounded by a housing 9. The finely divided powdered
medicament is supplied to the screw feeder device 6 through the vessel 4.
The vessel 4 is provided with a mechanical stirring device (not shown) to
facilitate the feeding of the cohesive powder to the screws. The stirring
device could be of any known type, for example having L-shaped arms
extending form a shaft provided perpendicular to the vertical axis of the
container.
The powder is transported through the screw feeder device 6 and due to
the pressure which is created between the at least two screws 8a, 8b by the
twisting movement of the screws 8a, 8b, which is created by a motor 14, the
powder particles will be pressed together and form soft agglomerates of
different sizes. The agglomerated particles which are obtained from the
agglomeration process in the screws 8a, Sb of the screw feeder 6 have a size
between 0.1 mm - 2 mm, are flowable, due to their size, and comparatively
soft in their structure.
A screw feeder device suitable to be used for the agglomeration according
to the invention is a device having so-called Twin-concave screws whereby
the screws have identical pitches. The agglomeration procedure of the finely
divided powdered medicament is obtained as the powder is mechanical
forced into the grooves 10a, lOb between the pitches 12a, 12b of the co-
operating screws 8a, 8b as they rotate. As the screws rotate the pitches 12a
of one screw 8a will move into the groove lOb of the other screw 8b and
WO 95/09615 PCT/SE9.1i0089G
thereby removing attached powder on the screw and forcing the powder
forward at the same time in a form of cleaning process. In this manner the
dearung process of the screws will create agglomerates of the powder
which is forced into the distance between the screws, see especially fig.2a.
Tests have shown that Twin-concave screws having a short pitch will give
the most uniform agglomerates having the best properties for meeting the
requirements for powders for inhalation. The tests have also shown that the
length and the rotation speed of the screws are of minor importance for the
result of the agglomeration procedure and that mainly the interval of the
pitch of the screws and the groove between the pitches are of importance.
More dense and uniform agglomerates are obtained from screws where the
distance between the pitches of the two screws is as small as possible. The
measure between the outer diameter and the inner diameter of the screws
should be between 1 - 10 mm, preferably between 1 - 5 mm. If this distance
is too big the agglomerates will not have the well defined dimensions
which are required. In a preferred embodiment the outer diameter of the
screws is 20 mm and the inner diameter of the screws is between 10 - 19
mm, preferably between 15 - 19 mm. It is also important for the
characteristics of the obtained agglomerates that the housing 9 around the
screws in the screw feeder fits around the screws in a tight manner leaving
only a minimal groove between the walls of the housing and the screws,
see fig. 2b. If there is a distance between the wall and the screws finely
divided powdered medicament will be compacted in this area during the
rotation of the screws and the agglomeration procedure will result in a Iess
uniform product.
In a preferred embodiment of the invention the pitches of the screws are
between 2 - 20 mm, preferably between 5 - 15 mm. Suitable screw feeders
CA 02172053 2004-04-27
23940-887
9
include the K-TRON SODERT"'standard twin shaft feeder without
agitator and the Brabender twin-screw feeder type DDSR/20.
After the agglomeration procedure the agglomerates can be transported to a -
. sieving device in order to obtain agglomerates within a certain range of
size
if desired.
The agglomerates obtained from the screw feeders have different sizes and
are comparatively soft and need to be fwther treated to obtain the desired
characteristics. The agglomerates are therefore collected in a spheronizing
device, preferably a rotating container, such as a pan or drum 16 and which
is preferably provided with one or more scrapers IS (only shown
schematically in the drawings). The container 16 is tilted and rotating. The
rotating movement of the container 16 will make the agglomerates rotate
, and tumble due to the tilting of the container. During the rotation the
agglomerates will obtain a stronger,. more spherical, dense, compact and
uniform form and a smoother outer surface. The characteristics achieved in
the rotating container will further improve the flowability and the resistans
against breaking during handling and storage. The speed of the container
determines the characteristics of the agglomerates after this spheronization:
Tests have shown that the optimal periphery speed of the container is
between 0.2 - 2.0, preferably between 0.4 - 1.0 m/s. The spheronization time
is preferably between 1 - 20 min. Tests have shown that after 3 - 10 min the
agglomerates often, have obtained required optimal size, capability of
breald.ng down to provide the finely divided medicament and density for
their future use. These characteristics are; as already mentioned above, of
utmost importancy when the agglomerates are to be used in the inhalation
therapy.
CA 02172053 2004-04-27
23940-887
Tests have shown that the most optimate tilting angle of the container 16 is
between 1~° - 8fl° f~ron~ the vertical, preferably between
3(~° - ~~° as an angle
chosen herebetween gives the best densifying and growing effect to the
a gglomera tes.
5
The granulating container is ra~ade of a material which is inert and do not
contaminate the powder, such as for example ~netai, plastic or any other
suitable material. In order to avoid electrostatic forces to build up during
the spheronization ~prflcess die container could be grounded.
~fl
After the spheronization in the tilted container l~ the agglomerates are
supplied to a sieve 2~ having an aperture size which is between i1.2 - 2.~
mrn, preferably between f).3 - L~ ran: The sieving is used in order to obtain
a uniform sine of the agglomerates. The requarernents for the use of this
operation is strongly depending on the type of in halation device to be used.
The requarerr~ents of uniform size and proper density is hlghe~ if a
agglomerates are to be used in a dry powder inhaler or in a metered dose
inhaler. During inhalation it is of utmost importance that agglonnerates are
2a~ broken up into a high amount of primary particles having a particle size
smaller than ~~ Via.
To utilize the process according to a invention in the anost efficient and
economic manner and to rnini~nize the a3nount of aggloareerates which are
2~ too big and therefore have to be recycled into the process it would be
advantageous to incorporate further steps of sieving and spheronization
into the prs~cess. 'bests have shown that the most efficient manner to carry
out the agglomeration process according to this invention is to incorporate
two f~zrt'~er steps of sieving and one further step of spheronization. A
3f3 fur~er sieving step is hereby incorporated into the process directly after
the
WO 95/09615
PCTISE94/00896
11
agglomeration process in the screw feeder. After this sieving the
agglomerates are spheronized in the granulating container and a second
further sieving step is carried out after this spheronization. A second
spheronization step is then carried out and the whole process is ended by
the final sieving step. These further steps of sieving and spheronization will
provide a more effective process and the agglomerates obtained after the
second spheronization are uniform and have the required characteristics.
An apparatus according to this embodiment of the invention is shown in
fig. 3.
According to this figure the finely divided powdered medicament is
agglomerated in the screw feeder device 6' and the resulting agglomerates
are supplied to a sieve 22. The sifted agglomerates are thereafter supplied
to the tilted granulating container 16'. After the spheronization in the
container 16' the agglomerates are supplied to a second sieve 24 to obtain a
more uniform size. After this second sieving the agglomerates are
spheronized a second time in a second tilted granulating container 26. This
second granulating container 26 is of the same type as the first container
and the periphery speed and the spheronization time are defined above for
the first step of spheronization. After this second spheronization the
agglomerates are sifted through the final sieve 20' to obtain a uniform size
of the final product. The sieving after the spheronization is necessary as in
some case the agglomerates might grow too much during the spheroniza-
tion and therefore the final product could contain agglomerates having a
size being bigger than the required size, e.g. 0. 2 - 2 mm, preferably 0.3 - 1
The agglomerates obtained from the process according to the invention are
to be used in a dry-powder inhalator, and preferably in a dry-powder
~1 l.- t' i ~.v
WO 95/09615 ' PCT/SE94/00896
~~. ~~~J3 12
breath-actuated inhalator. The hardness of the agglomerates are therefore of
utmost importanry. T'he required hardness of agglomerates which are
capable of breaking down into primary particles during inhalation has been
measured by a MH'T-4 Microhardness tester. (A.Paar, Austria) and has been
found to vary between 0.5 to 20 mN for agglomerates having good
deagglomeration properties and which break down into the required
primary particles in an inhalator during inhalation. With values above 20
mN the deagglomeration of the agglomerates will be less and above 100
mN very little deagglomeration of the agglomerates will occur.
The agglomeration process according to the invention will now be
described by experiments which are intended to illustrate but not limit the
scope of the invention as described in the appended claims.
Example 1
Properties of agglomerates of three different powders have been determined
and is to be seen in the table below. The powder consisted of finely divided
particles which was passed through the steps of the method according to
the invention:
SUBSTANCE MASS MEDIUM SURFACE AREA BULKDENSITY
DIAMETER (um) (m2/~~) (l;/ml)
Terbutaline 1.7 9 0.25
Budesonide 2.0 6 0.24
Lactose 3.0 6 0.32
Typically the bulk density for agglomerated powders consisting of finely
divided particles can be determined to vary between 0.2 mg to 0.4 g/ml for
particles having a particle size of less than 10 lim. The surface area varies
WO 95/09615 ~ PC'1'ISE94/00896
13
between substances but there is no difference between micronised and
micronised and agglomerated (and spheronised) powder. The area is
between 2 20 m2/g, preferably 3 - 12 m2/g.
Example 2
In order to examine the form of the agglomerates obtained from the method
according to the invention a high-speed video camera was installed at the
end of the screws. It was then possible to visualize the resulting product
from the .screw feeder and compare the formed agglomerates with non-
treated powder under different experimental conditions. Samples was taken
and was further examined under a microscope.
In the experiment micronised terbutaline sulphate with a mass medium
diameter (MMD) of 1.2 um was added to a K-tron Soder twin-shaft feeder
(speed 15 gram/min) using long-pitch twin concave-profile screws. The
resulting agglomerates were collected and examined under a microscope.
Fig. 4a shows a picture of the irregular and soft agglomerates which was
obtained.
In a further experiment the long-pitch screws were changed to short-pitch
twin concave-profile screws of the same type and the same substance was
added to the same equpiment and under the same experimental conditions.
Fig 4b shows a picture of the agglomerates which are more regular.
Example 3
The importance of the dimension of the screws was further established in
order to examine the size of the agglomerates and especially the uniformity
of the agglomerates - an important parameter for dosing accuracy. The
WO 95/09615 , ~ PCT/SE94/00896
~4
results clearly indicate the necessity of using well-controlled agglomerating
procedures.
In the experiments a Brabender twin-screw feeder type DDSR/20 was used.
Microrused lactose (MMD < 4 um) was added to the feeder with different
twin-screw sizes (20/11, 20/12, 20/20; (length of pitch according to
Brabender technical data sheet)). The soft agglomerates from the feeder was
added to a gyro-vibrating sieve with a net size of 0.5 mm. The velocity of
feeding on the sieve was adjusted so as the powder passed the sieve at
once, i.e. no agglomeration was to occur on the sieve. Two experiments for
each screw were performed. The result shows that screws with longer pitch
(e.g. 20/20) results in less defined and larger agglomerates. As a
comparative experiment micronised powder was slowly added by a spoon
directely on the sieve, i.e. without passing the screw feeder. Great variation
of the size of the agglomerates was obtained, which indicates the necessity
of using a screw feeder to obtain more regular agglomerates and a
satisfactory yield.
A summary of these experiments is to be seen in fig. 5. The reproducibility
of the formation of the agglomerates and their properties like size, bulk
density and distribution is thereby obtained by the present invention, which
is of utmost importance for the accuracy of a dose, something which is
especially important when the agglomerated and spheroruzed powder is to
be used in a volume dosing inhalation device.
Example 4
In order to determine the size of the agglomerates as a function of the
screw dimension the following measures were taken. The agglomerates
obtained in a Brabender twin-screw feeder type DDSR/20 were rotated at
WO 95/09615 - PCT/SE94/00896
35 rpm in a pan (diameter 320 mm) for 5 min. The agglomerates formed
were sized in different fractions (< 0.315, 0.315-0.5, 0.5-071, 0.71-1 and >1
mm) by passing a gyro-vibrating sieve (Russel Finex). This sieving shows
that the size of the spheres is strongly depending on the size and form of
5 the screws. The final hardness of the agglomerates is determined by the
spheronization procedure. The results are shown in fig. 6.
Example 5
10 In a further experiment it was determined that the size of the agglomerates
depends less on the speed of the screws than the size of the screws.
Microrused terbutaline sulphate (MNID 1.2 lun) was fed to a K-Tron twin-
screw feeder with different screws and speeds. The feeding speed 30 for the
short pitch screws is equal to 10 for the long pitch screws (15 gram/min).
15 The agglomerates were sized through 3 net dimensions (0.3, 0.5, 0.7 mm).
The result is shown in fig. 7.
Example 6
In a further experiment it was shown that by getting a uniform size of the
agglomerates resulting from the agglomeration process in the screw feeder,
the yield will increase in the subsequent steps as well as a more narrow
distribution range of spheres will be obtained. This is further improved by
using the multistep procedure as shown in fig. 3. In the experiment
micronised terbutaline sulphate was agglomerated and spheronized. The
resulting agglomerates were thereafter sized into different fraction and each
fraction was Filled into a powder inhaler. The metered dose was therafter
determined for each fraction. The results shown in the diagram below
which shows the relationship between different fractions of the substance
and the metered dose give about 20% difference in dose when using
WO 95/09615 ~ PCT/SE9~/00896
~2Q~3 16
different agglomerate sizes which clearly indicates the necessity of using a
uniform size of the agglomerates in order to obtain a constant dose and
small batch to batch variations.
SIZE FRACTION METERED DOSE (m /dose)
< 0.14 0.65
0.14 - 0.3 0.62
0.3 - 0.5 0.59
> 0.5 0.55
The agglomeration process described in the present invention gives a high
yield of the total operation and acceptable batch to batch variations to the
final product.
Possible modifications
The method as well as the apparatus according to the invention could of
course be modified within the scope of the appended claims.
Thus the construction and the size of the screw feeders as well as the speed
and the length of the screws could be modified. The size of the apertures in
the sieves which have been used could of course also be modified.
Additional screws may also be used in the same manner.
It is also possible to modify the size, shape, speed and tilting angle of the
granulating container thereby changing the size of the final agglomerates.
The spheronization could also be done in a so called marumerizer which is
a commercially available apparatus for spheronization or granulation. The
spheronization could also be done in any other suitable way using a
W O 95/09615
PCT/SE9410089G
17
rotation symmetrical receptacle or container, which could be rotated, such
as any container being cylindrical or barrel formed.