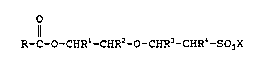Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Zl ~932
HOECHST ARTIENGESELLSCHAFT HOE 95/F 059 Dr.Rl
Description
Process for the preparation of foreign-salt-free alkoxy-
lated acyloxyalkanesulfonates
The invention relates to a process for the preparation of
foreign-~alt-free alkoxylated acyloxyalkanesulfonates of
the formula 1 below
~-C-O-CE~-CH~2-C~-CEIR3-CEIP~;-aO~X ( 1)
in which:
R is a hydrocarbon radical having 5 to 31 carbon
atoms,
X is an alkali metal or ammonium,
R1 and R2 are identical or different and are hydrogen, a
C1- to C4-alkyl or a hydroxy-C1- to C4-alkyl,
R3 and R4 are identical or different and are hydrogen or
a C1- to C4-alkyl.
Al koxylated acyloxyalkanesulfonates are valuable anionic
surfactants, which are primarily used for the preparation
of syndet soaps, cosmetic compositions and cle~n; ng
formulations. In comparison to non-alkoxylated acyloxy-
alkanesulfonates, they have a higher foaming capacity,higher hard water stability and improved skin compatibil-
ity.
In EP-A-O 585 071 (US-A-5 384 421), the preparation of
non-alkoxylated acyloxyalkanesulfonatesby esterification
of fatty acids with non-alkoxylated hydroxyalkane-
sulfonates is described (direct esterification). In this
process, the fatty acid and the salt of the hydroxy-
alkanesulfonic acid are reacted in the presence of an
esterification catalyst and a consistency regulator at a
2~ 72932
temperature of 180 to 240C with simultaneous removal of
water present. As consistency regulator, use is made of
certain paraffins. The use of such compounds is neces-
sary, because the reaction mixture becomes highly viscous
with advancing esterification. With consistency regula-
tors, a lower reaction mixture viscosity is achieved and
the reaction is facilitated, but the esterification
product contains the compounds used, as a result of which
the target acyloxyalkanesulfonate is obtained diluted to
a greater or lesser extent.
In EP-A-0 544 478, alkoxylated acyloxyalkanesulfonates
and a two-step process for their preparation are de-
scribed. In the first step, alkoxylated hydroxyalkane-
sulfonates are prepared by sulfonation of the correspond-
ing chlorine compounds with sodium bisulfite, whichalkoxylated hydroxyalkanesulfonates are then reacted in
a second step with fatty acid chloride or fatty acid to
give alkoxylated acyloxyalkanesulfonates. The following
reaction equations involving 2-(2-chloroethoxy)ethanol
and lauric acid are intended to illustrate this in more
detail:
HO-CH2CHzOCH2CH2-Cl + Na2SO3 > HO-CH2CH20CH2CH2-SOINa + NaCl
CH3 (CH2) lo~C~OH + HO-CH2CH20CH2CHz-SO~Na >
o
> CH3(CH2) ,o-c-ocH2cH2ocH2cH2-so3Na
The alkali metal halide NaCl arising in the first step i8
also present in the end product (sodium lauroyldiethoxy-
isethionate). Separation of the NaCl from the intermedi-
ate 2-(2-sulfoethoxy)ethanol could only be achieved with
a high expenditure on equipment. The alkoxylated acyloxy-
alkanesulfonates thus prepared therefore generally
contain a certain amount of the foreign salt alkali metal
halide. The known process has the further disadvantage
21 72~
-- 3
that it is scarcely suitable for the industrial
preparation of foreign-salt-free products because of the
high expenditure on foreign-salt separation.
The object of the invention is to propose a process which
can even be carried out industrially, by which foreign-
æalt-free and thus highly pure alkoxylated acyloxyalkane-
sulfonates are obtained.
The process of the invention comprises
a) reacting a glycol compound of the formula 2
H0-CHR1-CHR2-OH (2), in which Rl and R2 have the
specified me~n;ngs~ with a hydroxyalkanesulfonate of
the formula 3 Ho-CHR3-CHR4-So3X (3), in which R3, R4
and X have the specified me~n;n~s~ in the presence
of an alkaline catalyst for the formation of an
alkoxylated hydroxyalkanesulfonate of the formula 4
HO - CHRl - CHR2 - O - CHR3 - CHR4 - SO3X ( 4) and
b) reacting the alkoxylated hydroxyalkanesulfonate
obtained in step a) with a fatty acid of the formula
5 R-COOH (5), in which R has the ~pecified me~n;ng,
in the presence of an esterification catalyst for
the formation of an alkoxylated acyloxyalkane-
sulfonate of the specified formula 1.
R1 and R2 are preferably each H or R1 is H and R2 is CH3,
80 that -CHR1-CHR2- is the ethylene radical or the iso-
propylene radical. R3 and R4 are preferably each H. X is
preferably sodium, potas~ium or ammonium. In regard to
the fatty acid RCOOH, R is a hydrocarbon radical having
5 to 31 carbon atoms, which can be saturated or unsatu-
rated, ~traight-chain or br~nche~, straight-chain
(unbr~nche~) being preferred. R can also be a mixture of
such hydrocarbon radicals. R is preferably C5- to
C21-alkyl or C5- to C2l-alkenyl or a mixture thereof. The
alkyl and alkenyl radicals are preferably unbranched. The
alkenyl radicals are further, preferably, monounsaturated
to triunsaturated. Example~ of fatty acids which may be
mentioned are caproic acid, capric acid, lauric acid,
2 i 72932
-
-- 4
- myristic acid, stearic acid, arachic acid, oleic acid,
linoleic acid, linolenic acid, coconut fatty acid and
tallow fatty acid and mixtures thereof.
In the process of the in~ention, an alkoxylated hydroxy-
alkanesulfonate is first prepared in such a manner that
a glycol compound of the formula 2, preferably ethylene
glycol (ethane-1,2-diol) or propylene glycol (propane-
1,2-diol), i8 reacted with a hydroxyalkanesulfonate
compound of the formula 3, preferably with a potassium,
sodium or ammonium hydroxyethanesulfonate (isethionate),
in the presence of a basic catalyst, preferably NaOH or
KOH. The first step of the process of the invention, that
is a base-catalyst con~en~ation reaction, is carried out
at a temperature of generally 150 to 250C, preferably
180 to 210C, and at atmospheric pressure, water present
(that is the water possibly introduced into the reaction
mixture and the water formed in the reaction) is removed,
preferably by continuous distillation. The glycol com-
pound of the formula 2, the hydroxyalkanesulfonate of the
formula 3 and the base-catalyzing compound are generally
used in a molar ratio of 2 to 7:1:0.1 to 0.2, preferably
in a molar ratio of 2 to 5:1:0.1 to 0.2. The reaction
time is about 2 to 10 hours. The more or less strongly
alkaline reaction product containing the glycol compound
used in excess is preferably further treated in such a
manner that, in step a), a product having a high content
of alkoxylated hydroxyalkanesulfonate and without inter-
fering contents for the subsequent esterification reac-
tion is obtained. This treatment is preferably carried
out as follows: the alkaline reaction product is first
neutralized with a hydroxy~lk~nesulfonic acid which
corresponds to the hydroxyalkanesulfonate prepared by the
con~en~ation reaction. If, therefore, the compound HO-
CH2CH2OCH2CH2-SO3Na has been prepared by reaction of, for
example, ethylene glycol (HocH2cH2oH) and sodium
isethionate (HOCH2CH2SO3Na) in the presence of sodium
hydroxide, this reaction product is neutralized with
HO-CH2CH2OCH2CH2-SO3H. The glycol compound used in excess
~i 72932
-- 5
~ is then separated off from the neutralized reaction
product, for example using a thin-film evaporator,
depen~; ng on the glycol compound, a temperature of about
120 to 250C and a pressure of about 10 to 500 mbar being
maintained. The product thus obtained essentially com-
prises one or more alkoxylated hydroxyalkanesulfonates.
In step b) of the process of the invention, the product
obtained in step a) is reacted with one or more fatty
acids in the presence of a catalyst. Suitable esterifica-
tion catalysts are described extensively in saidEP-A-0 585 071, which is incorporated herein by refer-
ence. These are alkanesulfonic acids, hydroxyalkane-
sulfonic acids, arylsulfonic acids, inorganic acids such
as sulfuric acid, phosphoric acid, phosphorous acid,
boric acid or anhydrides thereof, heavy metal salts such
as zinc sulfate, zirconium sulfate, zinc isethionate,
zinc borate, aluminum sulfate, titanium sulfate or
tungsten phosphate, metal oxides such as zinc oxide,
aluminum oxide, magnesium oxide, cerium oxide, zirconium
oxide or lanthanum oxide, in addition mixtures of two or
more of said catalysts and soaps which are formed from
heavy metals and metal oxides. A particularly preferred
esterification catalyst is zinc oxide. The esterification
catalyst is used in an amount of generally 0.05 to 2% by
weight, preferably 0.05 to 1% by weight, percentages by
weight based on fatty acid and alkoxylated hydroxyAlkAne-
sulfonic acid salt.
The reaction of fatty acid and alkoxylated hydroxyalkane-
sulfonic acid salt, generally in a molar ratio of 1:1 to
2:1, preferably about 1:1, is carried out according to
the invention at a temperature of 180 to 250C, prefera-
bly 200 to 230C. The water possibly introduced into the
reaction mixture with the starting components and the
water formed by the esterification reaction is continu-
ously discharged from the reaction mixture. The reactionmixture to the end of the reaction, even at 100% conver-
sion, is homogeneous, of relatively low viscosity and
- 2 1 7293;~
_ -- 6
- readily stirrable, 80 that consistency regulators for
further reduction of the viscosity are generally not
required. Suitable products therefore would be, for
example, paraffins and esters of fatty acid in an amount
of about 5 to 20% by weight, based on the total reaction
mixture. The time up to the sought-after conversion of
fatty acid or of alkoxylated hydroxyalkanesulfonate i8
about 4 to 8 hours. Generally, for example for reasons of
time, 100% conversion will not be attempted, but the
esterification reaction will be interrupted at a lower
percentage, for example 70 to 90% by weight of alkoxy-
lated acyloxyalkanesulfonate.
The esterification step of the invention, in detail, can
be carried out, for example, in such a manner that - at
atmospheric pressure - the fatty acid, the salt of the
alkoxylated hydroxy~lkAnesulfonic acid and the esterifi-
cation catalyst are introduced into a reaction vessel and
the mixture is heated to the specified temperature with
stirring. Water present distills off continuously as
early as during the heating of the reaction mixture and
then further during the esterification reaction. The
esterification can also be carried out by the method
described in EP-A-0 585 071. Here, the reaction is
carried out partly at atmospheric pressure and partly
with application of a vacuum for more rapid removal of
the water. After the sought-after degree of conversion
has been achieved, the esterification reaction is termi-
nated, for example, by cooling. The reaction product
obtained is liquid or solid at room temperature. Formula-
tion of the product which is solid at room temperaturecan be carried out, for example, using a flaking roll or
a cooling belt.
By means of the process of the invention, which can also
be carried out industrially, foreign-salt-free alkoxy-
lated acyloxyalkanesulfonates can be prepared with highpurity. The products have a high content of alkoxylated
acyloxyalkanesulfonate and, as further advantageous
- 2 1 7~32
~_ - 7
properties, good water solubility, high foam formation
and good hard water stability and a beneficially acting
feel on the skin. The products obtained according to the
invention are therefore especially also suitable for
aqueous formulations. Owing to the direct esterification
(direct co~en~ation), the use of fatty acid chlorides
can be dispensed with, which would otherwise have to be
prepared from fatty acid in a separate step. The use of
consistency regulators and/or diluents, which do not
generally represent valuable materials in, for example,
cle~n;ng formulations and cosmetic preparations, is not
necessary. The solid or liquid reaction product generally
contains, as mentioned above, 70 to 90% by weight of
alkoxylated acyloxyalkanesulfonate of the specified
formula 1, percentages by weight based on the solid or
liquid total product.
The invention is now described in still more detail by
means of examples. Percentage~ are by weight, unless
stated otherwise.
Example 1
a) Preparation of an alkoxylated hydroxyalkane-
sulfonate:
310 g (5 mol) of ethane-1,2-diol, 148 g (1 mol) of
2-hydroxyethanesulfonic acid sodium salt (sodium
isethionate) and 4 g (0.1 mol) of sodium hydroxide
are introduced into a 1 1 flat flange reaction
vessel equipped with an anchor agitator, desc~n~;ng
distillation bridge and internal thermometer. The
reaction mixture is heated to a temperature of 190
to 195C. In the course of 3 hours, about 18 g of
water distill off from the reaction mixture. After
cooling to room temperature, the alkaline product
solution is neutralized by ~;ng approximately 17 g
(0.1 mol) of2-(2-hydroxyethoxy)ethanesulfonic acid.
The ether sulfonate i~ recovered from the neutral
2 1 72q32
-- 8
glycol-contA;n;ng product solution by means of thin-
film evaporation. (The jacket heater of the evapora-
tor tube i8 kept at about 200C and the vacuum at
about 10 mbar.) The sodium 2-(2-hydroxyethoxy)-
ethanesulfonate (sodium diglycol sulfonate) arises
as a colorless distillation residue having a melting
range of 140 to 150C. The purity of the product is
98% and the glycol content is ~ 1%.
b) Esterification of an alkoxylated hydroxyalkane-
sulfonate with fatty acid to give the alkoxylated
acyloxy~lk~nesulfonate:
285 g (1 mol) of stearic acid, 196 g (1 mol) of the
above described sodium 2-(2-hydroxyethoxy)ethane-
sulfonate and 0.5 g of zinc oxide are introduced
into a 1 1 flat flange reaction vessel equipped with
an anchor agitator, descen~; ng distillation bridge,
internal thermometer and nitrogen inlet. The batch
is heated to 200C and the water formed in the
direct condensation is distilled off. The reaction
is interrupted at a sodium 2-(2-stearoyloxyethoxy)-
ethanesulfonate content of 70% and the product is
poured onto a metal sheet to cool. The end product
essentially consists of 70% of sodium 2-(2-stearoyl-
oxyethoxy)ethanesulfonate, 17% of stearic acid and
13% of sodium 2-(2-hydroxyethoxy)ethanesulfonate.
The above described sodium 2-(2-hydroxyethoxy)ethane-
sulfonate is also used in the other examples.
Example 2
200 g (1 mol) of lauric acid, 196 g (1 mol) of sodium
2-(2-hydroxyethoxy)ethanesulfonate and 0.5 g of zinc
oxide are introduced into the device of Example 1. The
mixture is heated to 200C and the water formed in the
direct co~Pn~ation is distilled off. The reaction is
interrupted at an active compound content of 79% and the
21 7 29932
- product iB poured onto a metal sheet to cool. The reac-
tion product essentially consists of 79% of sodium
2-(2-lauroyloxyethoxy)ethanesulfonate (active compound),
9% of lauric acid and 12% of sodium 2-(2-hydroxyethoxy)-
ethanesulfonate.
Example 3
158 g (0.8 mol) of coconut fatty acid having a mean
molecular weight of 205, 158 g (0.8 mol) of sodium
2-(2-hydroxyethoxy)e~hAnesulfonate and 0.5 g of zinc
oxide are introduced into the device of Example 1. The
batch is heated to 200C and the water formed in the
direct condensation is distilled off. The reaction is
interrupted at an active compound content of 80% and the
product is poured onto a metal sheet for cooling. The end
product essentially consists of 80% of sodium 2-(2-
cocoyloxyethoxy)ethanesulfonate, 9% of coconut fatty acid
and 11% of sodium 2-(2-hydroxyethoxy)ethane-sulfonate.
Example 4
289 g (1.3 mol) of coconut fatty acid having a mean
molecular weight of 218, 196 g (1 mol) of sodium
2-(2-hydroxyethoxy)ethanesulfonate and 0.8 g of zinc
oxide are introduced into the device of Example 1. The
mixture is heated to 220C and the water formed in the
direct condensation i8 distilled off. The reaction is
interrupted at an active compound content of 75% and the
product is poured onto a metal sheet for cooling. It
essentially consists of 75% of æodium 2-(2-cocoyloxy-
ethoxy)ethanesulfonate, 20% of coconut fatty acid and 5%
of sodium 2-(2-hydroxyethoxy)ethane8ulfonate.
Example 5
43 kg (224 mol) of sodium 2-(2-hydroxyethoxy)ethane-
sulfonate, 50 kg (250 mol) of lauric acid and 0.2 kg of
zinc oxide powder are introduced into a 360 l stirred
2~ 72~2
- 10 -
vessel equipped with an anchor agitator, desc~n~; ng
distillation bridge, nitrogen inlet and temperature
measurement in the vessel, and the mixture is then heated
to 200C with distillation of water. After an active
compound content of 73% has been achieved, the reaction
product is cooled to 130C and formulated via a flaking
roll. The flaked product essentially consists of 73% of
sodium 2-(2-lauroyloxyethoxy)ethanesulfonate, 17% of
lauric acid and 10% of sodium 2-(2-hydroxyethoxy)ethane-
sulfonate.