Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 95/11960 PCT/AU94100671
' 2175184
CELL $FPARAR'TO DEVTCE
Technical Field
The present invention relates to a method and
apparatus for separation of cells. The apparatus includes
a semi-permeable substrate to which a ligand is attached
to its surface which is adapted to bind a desired cell
type. The invention also relates to cells that are
separated by the method or apparatus of the invention.
Background Art
Separation of specific cells from a mixed cell
population is important for cell biological and
immunological studies and for use in medical cell
therapy. Small-scale ligand based separation techniques
(less than 109 cells) which include fluorescent
activated cell droplet sorting and cell panning are
unsuitable for medical therapies. Non-ligand based
separation techniques such_as synthetic fibre leucocyte
filters and counter-flow elutriation are not selective
or adaptive for separation of specific cell subtypes.
Current large-scale ligand based separation techniques
such as column chromatography, magnetic bead or
microsphere adsorption cannot separate easily and
quickly purified cell subtypes for clinical therapies.
Cell depletion techniques for clinical applications
have been adequate for large-scale removal of pathogenic
cell sub-types, while cell enrichment and expansion
culture technologies still require further development.
Cell affinity separations are based on the.selective
absorption of cell phenotype using antibody, lectins, or
other moieties specific for cell surface markers.
Affinity techniques include plate panning, column
chromatography, and magnetic bead or micro-particle
absorption. Depletion of cell subsets (>1,000-fold) is
possible using magnetic beads. Recovery of adherence
cells involves the use of mechanical agitation and may
require proteolytic enzymes. High gradient magnetic cell
WO 95/11960 PCT/AU94/00671
2175184
2
separation (MACS) can enrich cell populations by greater
than 100-fold. Magnetic microparticles (<80nm), which
remain attached to the positive cell population, do not
interfere with proliferation assays or flow cytometry.
Affinity methods offer the selectivity of monoclonal
antibody based separations and are required for large-
scale clinical applications.
Large-scale affinity separation techniques
(>109 cells) have played an important role in the
development of bone marrow transplant and adoptive
cellular immunotherapy. If there is homogeneous
expression of tumour markers, then magnetic bead
absorption can purge tumour cells from autologous grafts.
Enrichment strategies are useful for the purification of
autologous haemopoietic progenitor cells using the CD34
marker or collection of lymphoid subsets for adoptive
immunotherapy. About 1% ofa bone marrow graft is CD34
positive. Purities of CD34 positive cells exceeding 90%
require greater than 900-fold enrichment factors. Work
has therefore concentrated on improving the efficiency of
cell enrichment using affinity cell separation.
A method for the separation of cells from a mixed
population of cells has been reported by Bigalow et al
1989, Journal of Immunological Methods 211: 289-293.
These authors have reported the development of a hybrid of
two separation methods, cellular adhesion chromatography
(AC) and field-flow fractionation (FFF) that achieves
effective separation of rat mesenteric B and T
lymphocytes. This method combines the selective adhesion
of AC and the control displacement forces of FFF, it also
yields quantitative estimates of the binding forces of B
and T lymphocytes to the adhesion surface of the system.
This method uses an apparatus comprising two parallel
glass plates and utilises the different binding affinities
to these plates by different cell types. This method has
a major problem in that it utilises the inherent binding
WO 95/11960 2175184- PCTIAU94100671
~ 3
properties of cells to the particular glass surfaces and
that it cannot be scaled up to separate a large number of
cells.
The present inventors have developed a method for
the separation of a desired cell type from a population of
cells that has the ability to select any given cell type
and also may be scaled up for use in clinical
applications.
Disclosure of Invention
Accordingly, in a first aspect the present invention
consists in a method for removing a desired cell type from
a sample containing cells including the desired cell type,
the method comprising the steps of:-
(a) loading the sample into a device including a
semi-permeable substrate provided with a ligand reactive
with the desired cell type,
(b) incubating to allow deposition and binding of
the desired cell type to the ligand,
(c) treating the semi-permeable substrate in a
manner such that the cells not bound to the ligand are
removed, and optionally
(d) treating the semi-permeable substrate in a
manner such that the cells bound to the ligand are
removed.
In a preferred embodiment of the first aspect, the
semi-permeable substrate is cellulose and is in the form
of a hollow fibre(s). Where the semi-permeable substrate
is in the form of hollow fibres in order to obtain the
optimum separation of any given cell type, it is preferred
that the permeability of the substrate is such that the
fluid loss across the substrate is less than 5%.
Other types of hollow fibres may also be used
including ultrafiltration hollow fibre membranes made of
polyamide. When such ultrafiltration membranes are used
it will typically be necessary to use the membranes under
WO 95/11960 PCT/AU94/00671
217 ~4 4
conditions such that the permeability of the membrane is
reduced below its normal level.
In a further preferred embodiment of the first
aspect, the ligand is selected from the group consisting
of an antibody, lectin, growth factor and receptor. More
preferably, the ligand is an antibody and still more
preferably the antibody is a monoclonal antibody.
In a further preferred embodiment of the present
invention the treatment in steps (c) and (d) comprises
shear stress. The cells not bound to the semi-permeable
substrate are removed by low shear stress and cells bonnd
to the semi-permeable substrate are removed by higher
shear stress. After removal of the unbound cells, the
cells bound to the semi-permeable substrate may be pre-
treated with a cell-releasing agent prior to removal by
treatment with shear stress. Preferably the cell-
releasing agent is an enzyme and more preferably and
enzyme is chymopapain. If the bound cells are pre-
treated, then the shear stress used to remove the pre-
treated bound cells may be lower, the same or higher than
the shear stress used to remove the unbound cells.
In the preferred embodiment where the semi-permeable
substrate is in the form of a hollow fibre, the treatment
to remove the cells is by shear stress generated by the
flow of liquid through the hollow fibre. Shear stress may
be generated by increasing the flow of fluid through the
fibre or by increasing the viscosity of the fluid or by
utilising a mixture of both procedures.
In this form, the cells that are not bound to the
semi-permeable substrate are eluted by low shear stress
and the cells bound to the semi-permeable substrate are
eluted by high shear stress. Alternatively, the cells not
bound to the semi-permeable substrate are removed by low
shear stress and the cells bound to the semi-permeable
substrate are pre-treated with a cell-releasing agent
prior to removal by treatment with shear stress.
WO 95/11960 2175la4 PCTlAU94100671
= 5
Preferably the cell-releasing agent is an enzyme and more
preferably the enzyme is chymopapain. The shear stress
used to remove the pre-treated bound cells may be lower,
the same or higher than the shear stress used to remove
the unbound cells.
In yet a further preferred embodiment of the first
aspect of the present invention the method includes
growing the desired cells in the device after separation.
In this embodiment after step (c) the bound cells are
maintained under conditions in which the cells may divide
and multiply.
In a second aspect, -the present invention consists
in an apparatus for removing a desired cell type from a
sample including the desired cell type comprising a
semi-permeable substrate in the form of an array of hollow
fibres provided internally with a ligand reactive to the
desired cell type.
In a preferred embodiment of the second aspect, the
ligand is selected from the group consisting of antibody,
lectin, growth factor and receptor. More preferably, the
ligand is an antibody and still more preferably the
antibody is a monoclonal antibody.
In a further preferred embodiment of the second
aspect, the hollow fibres are cellulose and the fluid loss
across the wall of the hollow fibre is less than 5%.
Other types of hollow fibres may also be used
including ultrafiltration hollow fibre membranes made of
polyamide. When such ultrafiltration membranes are used
it will typically be necessary to use the membranes under
conditions such that the permeability of the membrane is
reduced below its normal level.
In a still further preferred embodiment of the
second aspect, the hollow fibres are held within a
cylindrical module. The module contains ports to allow
buffer to be circulated around the outside of the fibres
and also inlet and outlet ports to allow flow through the
WO 95/11960 PCT/AU94/00671
21~51b 4 6 0
fibres. This arrangement of the hollow fibres allows the
inside to be sealed from the outside so that flow along
the inside of the fibre can be controlled independently
from the flux across the walls of the fibre. The fluid
flow to the hollow fibre module is preferably achieved by
a pump means.
In further aspects the present invention consists in
cells obtained using the method of the first aspect of the
present invention or the apparatus of the second aspect of
the present invention.
in order that the nature of the present invention
may bemore clearly understood, preferred forms thereof
will be described with reference to the following examples
and drawings.
Brief-Description of Drawings
Figure 1 shows a schematic representation of laminar
flow observed in a porous tube;
Figure 2 shows the streamlines determined for
laminar flow in a porous tube;
Figure 3 shows the results of the fractionation of
cells using an apparatus of the present invention;
Figure 4 shows a schematic representation of an
apparatus of the present invention indicating the valve
numbering as referred to in Table 2;
Figure 5 is a schematic representation of cell
selection and subsequent expansion of the cells using a
hollow fibre apparatus of the present invention;
Figure 6 shows the non-specific absorption of
mononuclear cells by an immunoadsorbent module;
Figure 7 shows the separation of KGla (CD34+) from
NALM-6 (CD4-) cells using experimental details set out in
Table 7;
Figure 8 shows the results of separation and
recovery of KGla cells from buffy coat;
Figure 9 shows the results of recovery of attached
CD34+ cells using fluid shear stress;
WO 95/11960 217~ 19/j PCT/AU94/00671
7 Y
Figures 10(a) and 10(b) show the purity and
enrichment factor, respectively of cells in experiment No
3 according to the details set out in Table 9;
Figure 11 shows flow cytometric analysis of
mononuclear cells before separation carried out in
experiment 3;
Figure 12 shows the flow cytometric analsyis of a
highly enriched cell fraction from experiment 3 where
fraction collection was between 150-200 dines/cm2; and
Figure 13 shows the flow cytometric analysis of CD34
antigen expression in collected fractions.
Best Modes for Carrying out the Invention
The present inventors have utilised mathematical
modelling to determine the preferred permeability
requirements of the semi-permeable substrates that may be
best used in the invention. In particular, the
permeability of hollow fibres has been calculated to
ensure efficient cell deposition and recovery.
DEPOSITION OF CELLS WITHIN PERMEABLE HOLOW-FIBRE MODULES
AND RECOVERY USING UNIFORM SHEER STRESS
The permeable hollow-fibre separation process may be
divided into 3 stages:
1. Cell deposition within the device using either
closed-ended filtration or open-ended flow.
2. Elution of marker negative populations at low
shear stress (open-ended flow).
3. Elution of adherent populations (marker
positive) at higher shear stress (open-ended flow).
Very low flow rates are required for cell deposition
using open-ended flow. Closed-ended filtration has the
advantage of higher volume flow rates, and more complete
capture of cells by the device. Hydraulic permeability
must be carefully selected so that adequate volume flow
rates may be generated using closed-ended filtration
during the cell deposition phase (stage 1), as well as
wV y~/11yoU CA 02175184 2005-06-21 Y(:l%AUy4/UUb'/1
8
generating uniform shear stress during the open-ended flow
cell recovery phase (stages 2 and 3).
Comparison of open-ended and closed-ended flow for cell
deposition
For open-ended flow the outlet of the hollow-fibre
array is open to atmospheric pressure. Cells will pass
directly through the hollow-fibre system and may attach to
the walls of the fibres if the shear stress is very low
(<0.2 dynes/cm2). A 1m2 device has 8000 fibres (inner
fibre diameter 200 m, length 20cm) in parallel. The wall
shear stress may be calculated using the Poiseuille-Hagen
law for laminar tube flow.
z= 4Q,u
nn r3
where Q is the flow-rate, p the fluid viscosity, r
the tube radius, and n the number of fibres in the
parallel array. Using the dimensions of a 1m2 device,
flows less than 0.lml/sec are required for.cell
deposition. For processing of clinical samples such as
blood apheresis collections (>200ml), the deposition
process will be time consuming (>30 minutes).
It is possible to generate larger fluxes for cell
deposition if the fibres are permeable to water and the
end of the hollow-fibre array is sealed using a stop flow
valve. A "Bioflux" Cuprophan*membrane (supplied by AKZO
FASER AG, Obernburg, Germany. 63785) has.a hydraulic flux
of greater than 50m1/(min.m2.l00mmHg). A volume of 200m1
could be deposited within a device within 2 minutes
(filtration pressure = 200mmHg).
Selection of hollow-fibre hydraulic_permeability for
uniform shear elution
The selection of fibre hydraulic permeabilities for
uniform shear elution affinity cell separation will be a
compromise. Highly permeable fibres may be used for rapid
*Trade-mark
WO 95/11960 ~1/ J 1 a'4 PCT/AU94l00671
9
loading of cells using closed-ended filtration. if the
fibre is too permeable, then during open-ended mode and
cell recovery, the net radial flux will result in a drop
in axial flow and surface shear stress along the fibre.
Non-uniform shear stress will result in reduced separation
purity and recovery.
Set out below is a summary of the derivation of the
velocity field for a porous hollow tube (1). This may be
used to calculate the fluid losses across the fibre
membrane for open-ended filtration . For fluid losses
across the membrane to be less than 5%, then
MXL (0.3
where M is the non-dimensional hydraulic
permeability and XL is the non-dimensional fibre length.
For large-scale hollow-fibre module dimensions
(fibre inner radius = 100 m, fibre length = 20cm), the
hydraulic permeability should be less than 1.4 x 10-llcm.
Typical Cuprophan dialysis membranes have a hydraulic
permeability of about 1.5 x 10-12cm, well below the
maximum'hydraulic permeability required for uniform shear
elution (<5% drop along fibre). Whilst these membranes
are suitable for uniform shear elution using open-ended
flow, rapid loading of cells is not possible using blind
ended filtration. The high flux Cuprophan membrane
"bioflux" is more suitable for this purpose (hydraulic
permeability >6.4 x 10-12cm). Such a membrane can
generate filtration flows of >50ml.min-1.m-2.100mmHg and
will generate uniform shear stress along the fibre length
(<5% drop) during open ended flow.
VELOCITY FIELD FOR A POROUSHOLLQW TUBE
The solution of the Navier-Stokes equation for
laminar flow in a porous tube has been derived by Granger
and coworkers (1). The flow-field may be used to derive
trajectories for particles which follow streamlines.
WO 95/11960 PCT/AU94/00671
Diffusion, sedimentation and inertial effects will-result
in deviation away from this purely convective model.
The undisturbed velocity flow-field U is derived by
applying the Navier-Stokes equations. Symmetry results in
5 simplifying the problem to that of two dimensions where
axial and radial velocities (trõtry) vary with axial and
radial position (x,y) (see Fig. 1). Pressure p forms a
scalar field inside the tube, whilst po, the pressure
outside the tube, is constant.
10 Boundary conditions are assumed to be as follows:
Radial fluid velocity across the wall of the tube radius
R is given by Darcy's Law.
_k
tfy(}.=R) P(y_R) -Po) . . .A4. 1
where k is the hydraulic permeability and p the
viscosity of the fluid. -
Furthermore it is assumed that there are "no slip"
conditions at the walls (us(y_R)=O). The radial velocity at
the centre of the tube is zero (ux(>-R)=0). Tube
hydrodynamic end-effects are neglected. -
The Navier-Stokes equations using cylindrical
coordinates simplifies to
( att tt JI a~ atr 1 aZtt l
x-momentum p vz ax +t{'' a a ) ax ap+ u a aY x)+ axZ
Y1 Y Y
at,x at~,.l a a (i a l a~t,,,
y-momentum p ( l tts ax +try Y ~ 1= a p+ ~ a (yu ) I+ xz }
l JJ Y YIIY Y yll
atl i a
continuity x+(y )=0 ...A4.1
ax yay y
WO 95/11960 PCT/AU94100671
c17,;g4
~ u
where p is the fluid density and u the fluid
viscosity.
The mathematical formulation is simplified with non-
dimensional variables:
x L ~
X=n;XL=R;Y=R;T=1fxR t
ux y=c==L> tly P- Po
Uz = ; UL = _ i Uy = ; P=
us(:=o) u=(==o) Ft :(~- 0) P.
M=4 C;Re=2pF'0)R ...A4.3
where t is time.
Granger obtained an iterative solution by first
assuming that the velocity profile is parabolic, and then
using the expression for U. to obtain a first iteration
for Uy from the continuity equation. Expressions for P
were found using the Y-momentum equation. A new
expression for U. was derived using the X-momentum
expression and then reintroduced into the continuity
equation and so on iteratively. What results is a
development in M, the coefficients of which are
polynomials in Y.
Z
Ux=2K(1-Y2)+MKG72Re (29-36Y2+9Y4 -2Y6~+ (3-4Y2 +Y4)
M'KG Re
+ 4608 (119-96Y=-36Y4 +16I6 -3Y8) +... .A4.4
Z
~
U', 2G'2Y-Y3) + 288e(K2 +GZ)(58Y-36Y'+6Y5-Y7
3
48G(9Y-6Y3 +YS) +... ...A4.5
WO 95/11960 PCT/AU94/00671
12
where G=-(BcoshMX+sinhMX), K=coshMX+BsinhtWX,
UL-coshMXL
and B = sinh MXL
Microfiltration and ultrafiltration membranes have
hydraulic permeabilities which range from
10-8cm<k<10-12cm. M is relatively small and only the
first term of each development is significant.
U=2K(1-Y2)i+ ZC'(2Y-Y3)j ...A4.6
where U is the flow velocity vector; i and j are
unit vectors in the X and Y direction.
A particle which follows streamlines will-have the
same velocity vector V as the flow-field.
, .
U=V= dX ~T~+ dY d7,1 ...A4.7
Substitution of equation A4.6 into A4.7 gives
dX _ 2dY
dT=2K(I-YZ) MG(2Y-Y') = = =A4/8
Separation of variables and integration gives the
trajectory equation in K and Y.
c
K Y=(2-Y2) ...A4.9
where c=KYP(2-Yr) and (Kp(Xt,),YP) is a point on the
trajectory.
Figure 2 shows the streamline pattern for low
(k=10-'Zcm) and high (k=10-8cin) permeability membranes with
Uc=O (blind-ended filtration). Streamlines are more
closely spaced proximally for a highly permeable fibre.
An important result of this model is the effect of
WO 95/11960 2 , 7 J 134 PCT/AU94J00671
~ 13
permeability on the transmembrane fluid flux along the
length of the tube. If the fibre has high permeability,
then the drop in pressure along the tube due to viscous
flow is enough to drive a greater transmembrane flux
proximally.
SELECTION OF FIBRE PERMFABIL.ITY FOR UNIFORM SHEAR rmTON
For cell recovery, the end of the porous tube is
open to the outside pressure, pe. Even though the end of
the tube is open, there will be a pressure gradient along
the tube (due to viscous resistance), and this will drive
filtration (open-ended filtration).
Therefore the selection of fibre hydraulic
permeabilities for uniform shear elution affinity cell
separation will be a compromise. For cell deposition
using blind-ended filtration, cells may be rapidly loaded
into fibres which have a high hydraulic permeability. On
the other hand, for cell recovery (open-ended filtration),
high fibre permeability will result in a significant drop
in axial flow along the fibre with a drop in fluid surface
shear stress.
Granger et al (1) derives an expression for the non-
dimensional mean pressure P as a function of non-
dimensional axial distance X.
M(sinhtYfX+BcoshMX) . . .A4.10
where P=P-P
Pa
If there is open ended flow, then p=po at x=L. The
= 30 mean non-dimensional fractional outflow velocity UL can
be calculated from B with P=0 at XL.
0=sinhMXL +BcoshMXL;
WO 95/11960 ' PCT/AU94/00671
14
217~~~4
coshZ tLIXL -sinh' MXL
U' cosh MXL
UL=1/coshMXL ...A4.11
For the fluid losses across the membrane to be less
than 5% (UL )0.95), then
MXL (0.3 . . .A4.12
For typical hollow-fibre module dimensions
(R=100pnt, L=20cm) k(1.4x10-11cm. A typical cuprophan dialysis
membrane has a k=-1.5x10-'Zcnr. A suitable membrane would
have a hydraulic permeability about 10-fold greater than
dialysis cuprophan.
1. Granger J, Dodds J, Midoux N. Laminar flow in
Channels with Porous Walls. The Chemical
Enaineerina Journal. -1989; 42:193-204.
JHE USE OF ULTRAFILTRATION HOLLOW FIBRE MEMBRANES FOR
UNIFORM SHEAR ELUTION AFFINITY CELL SEPARATION
For cell fractionation using uniform shear stress,
there must be uniform axial flow rate along the fibre
length. The preceding discussion defines a permeability
below which there is minimal leak across the fibre
membrane. Whilst ultrafiltration membranes have a
permeability well above this value, a process will be
described whereby membrane pores may be temporarily sealed
for uniform shear elution.
The separation process is as follows:
1. Loading of cells using blind-ended filtration.
2. Drainage of fluid surrounding hollow fibres
(extra-capillary space).
3. Application of extra-capillary gas pressure
greater than intracapillary pressure and less than the
bubble point or collapsing pressure of the fibre.
Membrane pores are sealed by formation of a gas-water
WO 95/11960 2175 18 4 PCTlAU94100671
~ 15
interface. Surface tension prevents gas from entering the
fibre lumen.
4. Recovery of cells not bound to the ligand by
treating with low shear stress.
5. Recovery of cells bound to the ligand by
treating with higher shear stress.
The technique for sealing membrane pores using an
extra capillary gas pressure and a gas-water interface
seal, has been demonstrated using nylon microporous hollow
fibre modules. Table 1 gives the relevant specifications
for this fibre type (supplied by ARZO Fazer, Fibres
division, Wuppertal, Germany).
TABLEI
Ca illa Membrane PA-386c
Polymer polyamide
Inner diameter 300t10 m
Wall thickness 110t10 m
Maximum pore size <0.43t10 m
Hydraulic permeability (25C) 2.2x10'9cm
Implosion pressure >105Pa
Bubble point >3.2x105Pa
Without application of extracapillary gas pressure,
flow along this fibre results in significant leakage of
fluid across the membrane (hydraulic permeability
>1.4x10-11cm). Application of 50kPa gas pressure to the
extra-capillary space prevented leakage of fluid across
the membrane. This pressure is well below the bubble
point and collapsing pressure of this hollow-fibre
membrane.
Flow from the intra to extra-capillary space is
prevented by a positive pressure in the extra-capillary
space. Flow from the extra-capillary space into the
intra-capillary space will cease as soon as a gas-liquid
interface forms at the membrane pore entrance. Surface
tension prevents gas from entering the fibre.
WO 95111960 PCT/AU94/00671
16
The pressure drop inside the tube due to viscous
flow may be calculated from the Hagen-Poiseuille law:
L
Ap=2rR
where 4p is the pressure drop, is the wall shear
stress, L is the tube length, and R is the tube radius.
Therefore, flow generating 100 dynes/cm2 (sufficient for
cell recovery) will result in a pressure drop of 25kPa
along a 20cm fibre. This is below the applied extra-
capillary gas pressure (50kPa), the bubble point (320kPa)
and collapsing pressure (100kPa) of this fibre type.
In summary, ultrafiltration membrane pores may be
sealed using a gas-fluid interface provided:
1. Gas on the outside of the hollow fibre, fluid on
the inside.
2. Extra-capillary gas pressure is greater than the
intra-capillary pressure. - -
3. The bubble point of the fibre is less than the
extra-capillary gas pressure.
4. Extra-capillary gas pressure is less than the
collapsing pressure of the hollow fibre.
SEPARATION OF NALM-6 CELLS FROM CEM CELLS
A positive cell population was formed by coating
NALM-6 cells with a mouse monoclonal antibody by
incubating the cells with the anti-CD9 antibody FMC56.
Negative cells were CEM cells prestained with the
nuclear fluorescent stain H33342 so that they could be
distinguished from the positive cells by UV
fluorescence. Equal numbers of the negative and
positive cells were mixed before separation.
A cellulose hollow fibre cell separation module
was constructed by coupling polyclonal antibody (raised
in sheep) against mouse immunoglobulin to the inner
surface of the hollow fibres using periodate reaction.
WO95/I1960 2 PCT/AU94100671
~ 17 f
The coupling reaction was carried out by activation of
the cellulose hollow fibres with 0.5M sodium periodate
for two hours at room temperature and then binding the
polyclonal antimouse IgG antibody at 200 g/ml overnight
at 4 C. The hollow fibre module was then washed with
buffer to remove unbound antibody and all traces of the
coupling chemicals.
The cells were loaded into the hollow fibres by
applying a 20 l drop of cell suspension to the open end
of the module. The cells were drawn into the fibre
lumen under the action of gravity and surface tension
prevented air from entering the fibres. The header port
was replaced and closed and the device was rotated to a
horizontal position to facilitate contact between the
cells and the inner surface of the fibres. The cell
sediment under gravity at about 4 m-sec so that full
deposition was completed in 60 seconds. Attachment
kinetics were rapid and 4 minutes was long enough for
the completion of binding.. The device was rotated a
further 90 (inverted) the negative cells were
fractionated from positive cells using shear stress
(proportional to flow rate). A flow rate of 2.5 ml per
minute (1.5 DYNES/cm2) was sufficient to remove most of
the negative cells from the device. A flow greater than
50m1 per minute (30 DYNES/cm2) was sufficient to recover
all of the bound positive cells.
Figure 2 shows absolute numbers of positive and
negative cells eluted from successive fractions.
Fraction 0 shows a total number of positive and negative
cells eluted from the device and has a similar ratio to
the initial mix. All of the cells applied to the device
were recovered using flow elution. Most of the negative
cells (filled bar) and some positive cells were eluted
in fraction 1 at low shears (2.5ml per minute).
Fractions 2-6 have relatively few cells and the
remaining bound positive cells were recovered in
W O 95111960 18 PCT%AU94/00671
fraction 7 where higher flow rates (greater than 50m1
per minute) were applied. Approximately 70% of the
positive cells were recovered in this fraction. This
fraction was 99.7% pure. Removal of the header and drop
wise elution of cells from the inverted device-
dramatically reduced flow dispersion with substantial
improvements enrichment purity.
A schematic representation of the apparatus for
removing a desired cell type from a sample containing a
mixed cell population including the desired cell type is
given in Figure 3. The apparatus can be operated in a
batch mode as summarized in Table 2.
TABLE 2
Flow control s stem for cell s aration device.
Phase Valve I Valve 2 Valve 3 Valve 4 Valve 5 Valve 6 Flow
Cell Open Closed Closed Open Closed Closed 1 to 4
loading
Inlet Open Open Closed Closed Closed Closed 2 to I
header
flus
Adhesion Closed Closed Closed Closed Closed Closed Nil
Recovery Open Closed Closed Closed Open Closed 1 to 5
of Low
negaUve flows
cells
Outlet Closed Closed Closed Closed Open Open 6 to 5
header
flush
Recovery Open Closed Closed Closed Open Closed 1 to 5
of High
posifive flows
cells
Cells are loaded using blind ended filtration
(flow from valve 1 to 4). Most of the cell population
WO 95/11960 -? 1 ~ ~ ~ ~ ~ PCT/AU94100671
19L
is concentrated within the hollow fibres. The inlet
vortex header is flushed (flow from valve 2 to 1). All
valves are closed for cell deposition and adhesion
(device in horizontal position). The negative cell
population is eluted at low shear (flow from valve 1 to
5). Residual negative cells are flushed from the outlet
vortex header (flow from valve 6 to 5). Finally the
positive cell population is recovered using high shear
(flow from valve 1 to 5).
The vortex headers prevent contamination of
purified negative and positive cell populations. The
loading of cells into the hollow fibre module using
blind ended filtration minimizes cell losses in the
header space.
As will be appreciated the present invention
provides a novel process of the separation of cells.
The method of the present invention is believed to
provide an easier and highly selective method of cell
separation than that of the techniques currently in use.
The present invention also is adaptive for automation
and large scale production of given cell populations by
a device similar in configuration to a hollow fibre
hemodialyser.
A2PLICATION OF HOLLOW-FIBRECELLSEPARATION DEVICE TO
CELL EXPANSION CULTURE SYSTEMS
Instead of recovering theselectively adsorbed
cell population, cells which have been adsorbed to the
lumenal surface of the hollow fibre may be left in situ,
and allowed to proliferate. The hollow-fibre membrane
will be permeable to growth factors and metabolites, so
that the cells may be supported by perfusing culture
media around the outside of the fibres. Once cells have
grown to optimal density, they may be harvested by fluid
flow along the lumen of the fibres.
Cells will consume anabolites (e.g., oxygen,
glucose, amino acids), and produce catabolites (lactate,
W 0 95/11960 PCTlAU94l00671
720
C02, H+). In addition cells will consume growth
factors, and produce inhibitory factors. Thus for
maximal growth, the culture media must be continually
replaced with fresh media. Hollow-fibre perfusion
systems may be used to continually exchange components
of this culture medium.
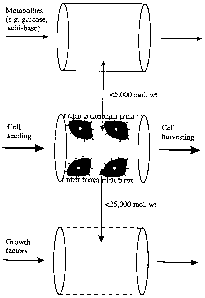
Low molecular weight substance (<5,000) are
involved in the metabolism of the cell (e.g., glucose,
amino acids, 02, C02, H + , lactate etc.) are consumed and
produced at a greater rate than larger molecular weight
substance such as growth and inhibitory factors
(molecular weight 5,000-25,000). The low molecular
weight fraction may be exchanged using dialysis
membranes (impermeable to molecules with a molecular
weight >5,000). The fraction containing growth factors
may be exchanged independently of the former using semi-
permeable membranes with a higher molecular_weight cut-
off. Figure 5 shows a schematic representation of a
system which combines hollow-fibre cell selection and
expansion.
This system has the application for the
development of cell therapies which involve cell
selection and expansion culture. For example, the
duration of life threatening pancytopenia associated
with conventional bone marrow transplant (2-3 weeks)
could be shortened to a few days by administration of
granulocyte and platelet precursors derived from bone
marrow stem cells. Haemopoietic stem cells may be
isolated using antibody to CD34 (stem cell marker).
These cells may be expanded and matured in culture to
produce granulocyte and platelet precursors. Both
primitive haemopoietic cells and more mature precursors
would be given immediately after myeloablative
chemoradiotherapy for cancerl resulting in short and
long term regeneration of haemopoiesis.
0 WO 95111960 21 2 p e 7-5 184 PCT/AU94100671
SEPARATION OF CD34 CELLS
Antibody couA.ing chemistrv
A method for antibody oxidation, and the
derivatisation of Cuprophan hollow-fibre modules with
adipic dihydrazide has been used to couple the antiCD34
monoclonal antibodies, #9069 (clone 9 C5) or #9079 (clone
HPCA-2), to Cuprophan hollow-fibre modules.
Hydrazide derivatisation of Cuprophan modules
Blind-ended filtration was used to deposit cells within
modules and therefore the antibody coupling process should
not significantly reduce the hydraulic permeability of
Cuprophan modules.
Heat annealing of modules was required to prevent
alkali-induced stress cracking of polycarbonate module
shells. Dehydration of Cuprophan membrane was associated
with a significant drop in hydraulic permeability (see
Table 3). Therefore polycarbonate modules were heat
annealed in the hydrated state by autoclaving them in
water for 20 minutes at 121 C.
The reaction with 50% ethylene glycol diglycidyl
ether (EGDGE) also resulted in a significant decline in
hydraulic permeability (see Table 3). The level of cross-
linking was reduced by lowering the concentration of EGDGE
(10%).
TABLE 3
The effect of dehydration and cross-linkin of Cu ro han h draulic ermeabili
Process Hydraulic permeabifity Percentage of
ml/min1100mmH m2 ini6al value
Unmodified Cu ro han F1 5.2 100
Dry autoclaving 1.6 31
Wet autoclavin 4.3 83
50% EGDGE (after wet autoclaving) 0.67 16
10% EGDGE + Adipic dihydrazide 3 58
WO 95111960 PCT/A1J94/00671
f~.
22
Method
Modules were heat annealed fully hydrated in an
autoclave (121 C for 20 minutes) prior to reaction with a
10% solution of EGDGE. After extensive washing of
Cuprophan modules in distilled water, 20ml of the EGDGE
solution is recirculated (5m1/min) through Cuprophan
hollow fibre modules immersed in a 50 C water bath for one
hour. The modules were then washed thoroughly with
distilled water. 20m1 of filtered 10% adipic dihydrazide
is recirculated at lml/min overnight. The next day the
module is washed and stored in PBS (phosphate buffered
saline) + 0.1% Na Azide.
Antibody oxidation and coupling to modules
Antibody carbohydrate moieties were oxidised using
sodium periodate. Oxidised antibody solution was
recirculated through hydrazide derivatised modules
overnight at room temperature, or over the weekend at 4 C.
The two different oxidation and coupling protocols are
shown below:
Method 1
Antibody solution (0.5-2mg Ig) is desalted against
0.2M sodium acetate buffer pH 5.0 using a PD10 column
(Pharmacia). 40 1 of 1 M Na104 solution is added to 2m1
of antibody solution to make a final concentration of 20mM
NaIOy. Antibody is oxidised at 4 C for 1 hour in the dark
with gentle agitation. The reaction is quenched with
glycerol (final concentration 15mM). The quenched
solution is immediately desalted against sodium acetate
buffer. The optical density at 280nm of the oxidised
antibody solution is measured, and Gml recirculated (@
200 1/min) through the bore of hydrazide derivatised
modules over the weekend at 4 C. The optical density of
the oxidation solution is measured again after completion
of coupling. Modules are washed and stored in PBS + 0.1%
Na azide at 4 C.
WO 95/11960 PCT/AU94/00671
M 2i7~;84
23
Method 2
Antibody is oxidised for 1 hour at room temperature
in the dark. 20 1 of 1M Na104 concentration of lOmM.
After quenching, the solution is desalted against 0.1M
acetate buffer (pH 4.5), and recirculated overnight
through the bore of hydrazide derivatised modules are room
temperature.
Flow protocols for cell separation
A microcomputer controlled system was used to
generate flow for shear fractionation of cell populations.
The flow protocol file is read by software which generates
stepper motor speeds and screen instructions for
separation experiments. Table 4 shows the separation
protocol which was used in subsequent experiments.
With the module in the vertical position, cells are
injected slowly into the outlet port of the hollow fibre
module (-2.5ml in 4 minutes), with the inlet valve closed
(blind-ended filtration). About two void volumes is
sufficient to fill the module with cells resulting in an
even distribution of cells along the module length, with
no cells in the inlet header, and relatively few cells in
the outlet header.
The module is then rotated into the horizontal
position for cell sedimentation and attachment.
Deposition distances are less than 200 m (deposition time
<60 seconds), and after a period of120 seconds, the
module is slowly rotated along its long axis (90
increments every 120 seconds). This creates a probability
of cell attachment along the entire inner circumference of
fibres. After incubation with the membrane surface, the
module is returned to the vertical position, and lOml cell
fractions are sequentially eluted, with flow-rate
incremented to a higher value for each subsequent
fraction.
WO 95/11960 PCT/AU94/00671
24
TABLE 4
Flow protocol for cell se aration
Action Duration (seconds)
10m1 module flush 10.24
Refill feed s rin e 10m1 25.61
Draw up 2m] of cell suspension 20.49
Inject 2.4m] from cell s rin e 204.89
De osi6on of cells (module horizontal 120
Rotate 90 degrees
De osition of cells (120 seconds) 120
Rotate 90 degrees
De osition of cells (120 seconds) 120
Rotate 90 degrees
10m1 fraction at 1 d nelcm2 512.22
Refill feed s rin e 10m1 25.61
10m1 fraction at 2 d nelcm2 256.11
Refill feed s rin e 10m1 25.61
10m1 fraction at 5 d nelcm2 102.44
Refill feed s rin e 10m1 25.61
10m1 fraction at 10 d nelcm2 51.22
Refill feed s rin e 10m1 25.61
10m1 fraction at 25 d nelcmz 20.49
Refill feed s rin e 10m1 25.61
10m1 fraction at 50 tl nelcm2 10.24
Refill feed s rin e 10m1 25.61
10m1 fraction at 75 d nelcmz 6.83
Refill feed s rin e 10m1 25.61
1 Oml frac6on at 100 d nelcmz 5.12
Refill feed s rin e 10m1 25.61
10ml fraction at 150 d nelcm2 3.41
Refill feed s rin e 10m1 25.61
1 0m1 fraction at 200 d nelcm2 2.56
=t V JJlJ,1JVV a va.~vJ-.ivvvi a
CA 02175184 2005-06-21
The hollow fibre wall shear stress z is calculated
using:
4Qfu
f1T173
5
where Q is the device flow-rate, p, the fluid
viscosity, n, the number of fibres per device and r the
fibre internal radius. The dimensions of the modules used
are shown in Table 5.
10 The elution shear stresses for this flow protocol
were 1, 2, 5, 10, 25, 50, 75, 100, 150, 200 dynes/cm2.
TABLE 5
Device dimensions
Fibre type Cu ro han Fl (dialysis membrane)
Inner fibre radius 100 m
Number of fibres 348
Fibre length 20cm
Lumenal voild volume 2.2m1
Lumenal surface area 437cm2
Viscosity (PBS + 0.5% BSA) 1.4 centi oise (measured)
Hydraulic permeability -3mI/min/100mmH /m2
Adsorption of mononuclear cells by immunoadsorbent modules
15 The level of non-specific adsorption of mononuclear
cells by immunoadsorbent modules was measured. The cells
were collected by apheresis from a patient undergoing stem
cell mobilisation. Red cells were removed from the
concentrate by centrifuging cells through a Ficol*layer.
20 Cells were used within 24 hours of collection and Table 6
gives the experimental details.
*Trade-mark
W O 95111960 PCT/AU94/00671
26
TABLE 6
Non-specific adso tion of mononuclear cells collected by apheresis
Membrane type Cu ro han Fl
Antibody coupling #9079 oxidised at 20mM Na104. Final oxidised
antibod concentration before cou lin = 270 Iml
Device surface area 437cm2
Cell loading densi 210,000 cells/cm2
Flow pro See Table 3
The number of cells collected in each fraction was
counted using a haemocytometer. Cells were dual stained
with antiCD45-FITC and antiCD14-PE (Leukogate, Becton
Dickinson) to obtain the relative ratio of lymphocytes and
monocytes. A Becton Dickinson Facstar plus was used to
analyse cell fractions.
Figure b shows the recovery of mononuclear cells as
a function of wall shear stress. Cells were incubated
with the membrane for 8 minutes before shear
fractionation. Recovery was calculated by dividing the
number of cells recovered in a faction by the total number
of cells eluted in all fractions (i.e. 0-200 dynes/cm2)
and expressed as a percentage. About 98% of the total
injected population were recovered at 1 dyne/cm 2 (lOml).
The relative ratio of lymphocytes to monocytes was about
4:6. In subsequent fractions this ratio was inverted,
suggesting that monocytes have relative greater affinity
for the membrane.
Non-specific adsorption in factions recovered above
10 dynes/cm2 was about 0.1-0.15% for monocytes and less
than 0.01% for lymphocytes (1 in 10,000). The purity of
CD34+ cell fractions will depend on the background of non-
specific adsorption. Since most of the cells eluting
above 10 dynes/cm2 will be CD14+, purity may be improved
by depletion of this subset.
WO 95/11960 PCT/AU94100671
~
27 2175184
Separation of cell lines
Initial experiments examined the separation of CD34+
cell lines. A CD34+ cell-line (KGla) was separated from
NALM-6 (CD34-). NALM-6 cells were stained with
fluorescein isothiocyanate before mixing with equal
numbers of KGla cells. Figure 7 shows the numbers of KGla
and NALM-6 cells which were recovered by shear
fractionation. Table 7 shows the experimental details for
this separation run.
TABLE 7
Se aration of KGI a from NALM-6
Membrane type Cu ro han Fl
Antibody coupling #9079 oxidised at 20mM Na104. Final oxidised
antibod concentraticn before cou lin = 240 /ml
Device surface area 219cm2
Cell loadindensity 27,000cells/cm2. KG1a:NALM-6=1.09:1
Flow rotocol 8 minutes membrane contact before shear elution
Almost all of the NALM-6 cells and only about 2% of
KGla cells were i=ecovered with a shear stress of
1 dyne/cmz. There were relatively few cells recovered in
the shear stress range 1-10 dyne/cmZ. KGla cells could be
recovered above 10 dynes/cmZ. The purity of these
fractions was greater than 99.5%. Not all of the KGla
cells were recovered even with vigorous flushing of the
module. The fraction of KGla still bound was estimated by
subtraction of the number of KG1a cells recovered from the
estimated number of KGla cells injected.
1
Nrrrrrber= of CD34' cells rr jected _- CD34'
l ) xCD34lo~rre~,wõed
(CD34- Whdmi.
The number of injected CD34+ cells may be estimated
using the ratio of CD34+/CD34- from the initial mix.
WO 95/11960 PCT/AU94/00671
28 =
This experiment demonstrates that the developed
immunoadsorbent has high specific affinity for KGla cells.
To simulate bone marrow, KGla was added to human
buffy coat (-1:100). Table_8 shows the experimental
details for this separation. The amount of immobilised
antibody (#9079) was similar to that shown in the prior
experiment (Table 7), whilst the cell loading density was
an order of magnitude greater (290,000 cells/cmZ).
TABLE 8
Se aretion of KG1 a from NALM-6
Membrane type Cu ro han Fl
Antibody coupling #9079 oxidised at 10mM Na104 (room
temperature). Final oxidised antibody
concentration before cou lin = 270 !ml
Device surface area 437cm2
Cell loading density 290,000 cellslcm2. 0.64% KG1 a ositive
Flow protocol 8 minutes membrane contact before shear elution
Figure 8 shows the recover of KGla cells. Once
again there was strong binding of KGla cells. The
attachment probability of KGla was reduced from 0.98 to
0.85. At higher cell loading densities, the blocking of
binding by CD34- cells is more likely.
Separation of mobilised stem cells GDC 34+1
Mononuclear concentrates have been obtained from
patients undergoing peripheral stem cell mobilisation
using colony stimulating factors at the Royal Adelaide
Hospital. Cells are separated within 24 hours of
collection. About 200 million mononuclear cells were
donated from each harvest ("'2x1010 cells).
Table 9 shows the experimental details for the first
three separation runs. In successive experiments the
immunoadsorbent affnity was reduced by lowering the
antibody coupling concentration and affinity. #9079
WO 95/Il1960 PCTIAU94100671
~ 51
29
(HPCA-2) has a dissociation constant which is about 10-
fold lower than #9069 (9 C5).
TABLE 9
Se aration of stem cells from mononuclear cell concentrates
Ex eriment 1 2 3
Mobilisa6on method SCF+G-CSF G-CSF G-CSF
Male:myeloma Female:Breast Ca Female:Breast Ca
Anfibody coupling concentra6on #9079 #9069 #9069
270 /ml 135 /ml 60 /ml
Cell loading densi 210,000 cells/cmz 390,000 cellslcmz 950,000 cells/cmz
Flow protocol Table 4 Table 4 Table 4
Incidence of CD34{ cells 0.2% 0.56% 0.61%
Attachment probability of CD34+ 0.8 0.58 0.39
cells
% of attached CD34+ cells 0.9% 17.5% 34.2%
recovered
The probability of attachment of CD34+ cells is
defined as the proportion of CD34+ cells which remain
bound after exposure to a shear stress of 1 dyne/cm2.
This is calculated from the incidence of CD34+ cells found
in the depleted fraction (0-1 dyne/cm2) and before
separation.
%CD34 ;,;nar -%CD34a~lemd
Attacl7ed probability =
%CD341+rnd
In fractions where there was significant recovery of
CD34+ cells, there was a purity of 70-80% with high
enrichment factors (400-600 fold). CD34+ cells were
stained with antiCD34-PE (HPCA-2, Becton Dickinson).
Figure 11 shows the flow cytometric analysis of the
initial cell population from the experiment number 3. The
stem cell population is clearly resolved by plotting CD34
''"a""" CA 02175184 2005-06-21 A\ 11AV)y/VVV/1
fluorescence versus side scatter (see region 2). Regions
7 and 6 were determined using Leukogate * (Becton
Dickinson), and correspond to the side and forward light
scatter properties of lymphocytes (CD45+, CD14-) and
5 monocytes (CD45 CD14+). The percentage of CD34+ cells
is expressed as a percentage of the total number of
mononuclear cells (R6+R7). The incidence of stem cells
was 0.61%.
Figure 12 shows the flow cytometric analysis of a
10 highly enriched fraction from the same experiment. The
fraction analysed was collected between 150-200 dynes/cm2.
The incidence of CD34+ cells was 80%. The enrichment
factor for this fraction was calculated as follows:
0 0
15 Ein=ichnteirt factor = ( g0 /o _ (0.61 /o ) = 652
'20% recovared jractlaf 99.39% in/hal
Cuprophan is a suitable substrate for affinity cell
'separation. It has a low.level of non-specific cell
adsorption, and has a chemistry which is suitable for
20 covalent immobilisation of antibody: The developed
immunoadsorbents had selective affinity for CD34+ cells
(both KGla and haemopoietic stem cells). Highly.enriched
CD34} cell populations may be recovered using shear
fractionation.
25 Attachment probability was lower at high cell
loading densities (106 cells/cm2). Interaction of CD34+
cells with the immunoadsorbent membrane may be blocked by
CD34- cells. Attachment probability at high cell loading
densities may be increased by increasing membrane contact
30 times and by slowly rotating the device along its central
axis. The rate of antibody-antigen-bond formation may
also influence cell attachment probability, and a high
affinity antibody (e.g. HPCA-2) may give rise to more
rapid cell attachment.
*Trade-mark
WO 95/11960 PCT/AU94100673
~ 31 21 75184
SEPARATION OF STEM CELLS (CD34+) FROM MOBILIZED BL=OOD
USING SHEAR FRACTTONATTON AND PRE-TREAmMFNT WTTH
CHYMOPAPAIN
Cells bound to the semi-permeable substrate of the present
invention may be pre-treated with a cell-releasing agent
prior to removal by shear stress. The proteolytic enzyme
chymopapain has been used to assist the removal of CD34+
cells bound to a hollow fibre apparatus of the present
invention.
Cell source
A mononuclear cell concentrate was collected from a
patient undergoing peripheral stem cell mobilisation with
cyclophosphamide and G-CSF. The initial population had an
incidence of 2.74% CD34+ cells.
Module chemistry -
A Cuprophan hollow-fibre module (binding area
"500cm2) was derivatised with antiCD34 monoclonal antibody
(clone 9e5) using the hydrazide method as described
previously with the following change in protocl.
Cuprophan was derivatisedwith 25% ethylene glycol
diglycidyl ether for 2 hours at room temperature.
Cell loading
A total of 1.7 x 10a cells in 4mls of phosphate
buffered saline with 0.5% human Ig and 0.5% bovine serum
albumin added was injected into the vertically aligned
module using blind-ended filtration. Residual cells in
the inlet tubing were flushed with a further 0.5m1 of
buffer (blind-ended filtration). The entire loading
process took less than 8 minutes. Cells were loaded at
approximately 340,000 cells/cmZ.
Cell deposition
For cell deposition within the fibre lumen, the
module was rotated into the horizontal position. After.4
minutes of settling time, the module was rotated around
its long axis over a 12 minute period (2 full
revolutions).
WO 95/11960 PCT/AU94/00671
2175~~~ 32
Cell recovery
For cell recovery the module was returned to the
vertical position. The CD34 depleted cell population was
recovered in successive lOml fractions by incrementing
shear stress up to 100 dynes/cm 2 (Fraction numbers 1-5).
Following this, attached cells were incubated with
the proteolytic enzyme Chymopapain (DISCASETM) at
400pKat/ml. The incubation period lasted 20 minutes.
During this time released cells were collected at low
shear stress (0.2 dynes/cm2, Fraction 6). Shear
fractionation was then used to further purify treated
cells. lOml fractions were collected at shear stresses of
10, 50 and 100 dynes/cm2.
TABLE 10
Separation of CD34+ cells from mobilised blood
Tube ID Elution conditions Total number DC34} Number of
of cells incidence CD34 cells
M
Before N.A. 1.67 x 101 2.74 4,550,000
se aration
Fraction 1 <5 d nes/cm2 1.66 x 108 0.77 1,280,000
Fraction 2 5 dynes/cm2 1.380,000 1.29 17,800
Fraction 3 10 d nes/cm2 540,000 3.58 19,300
Fraction 4 50 d nes/cm2 1,720,000 6.89 119,000
Fracfion 5 100 dynes/cm2 807,000 24.2 195,000
Fraction 6 chymopapain + 0.2 687,000 93.1 640,000
dynes/cm2
Fraction 7 10 d neslcm2 2,060,000 86.1 1,770,000
Fraction 8 50 d nes/cm2 2,360,000 1.91 45,100
Fraction 9 100 dynes/cm2 1,040,000 1.19 12,400
Fraction 10 100 d neslcm2 567,000 0.54 3,060
Fraction 11 -1000 dynes/cmz (3% 660,000 0.31 2,050
dextran T2000
WO 95/11960 2 1"7 51Q /~ pCTJAU94100673
~ (I "~
33
Table 10 shows the absolute cell numbers collected
in fractions 1-11. Most of the cells were recovered in
fraction 1. This fraction was depleted of CD34+ cells
(CD34+ = 0.77%). The incidence of CD34+ cells increased
as the shear stress was increased (fractions 2-5). Most
of the CD34+ cells were recovered in fractions 6 and 7.
The purity of these fractions was at least 90%. Fractions
8-11 have very few CD34+ cells.
Attachment probability of CD34+ cells was 72%. When
fractions 6 and 7 are pooled the separation yield is 53%
(CD34+ cells recovered/CD34+ cells input).
CD34 antigen expression
Figure 13shows the level of CD34 antigen expression
in each fraction as determined by flow cytometry (8G12-
PE). A uniform gated region was used for determination of
the incidence of CD34+ cells (see Table 10). CD34+ cells
collected in fractions 4 and 5 had low levels of antigen
expression (channels 100-1000). Chymopapain released
cells had higher antigen expression (channels 150-1600).
In fraction 7 there were two distinct populations of CD34+
cells (channels 50-120 and 120-1600). The lightly
staining population appears to be distinct from the
typical fluorescence range for CD34+ cells (channels 10-
100). Therefore the purity of fractions 6 and 7 may have
been underestimated by the uniform CD34 gating region
adopted (low side scatter and PE fluorescence
> channel 105).
It will be appreciated by persons skilled in the art
that numerous variations and/or modifications may be made
to the invention as shown in the specific embodiments
without departing from the spirit or scope of the
invention as broadly described. The present embodiments
are, therefore, to be considered in all respects as
illustrative and not restrictive.