Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
wo9snna~ ~Z~~J~$
PCTfUS95103689
METHOD FOR DISPOSING CARBON DIOXIDE IN A COALBED AND SIMULTANEOUSLY RECOVERING
METHANE FROM THE COALBED
__
F1,~1 OF THE INVENTION
The present invention relates. to methods which utilize a solid
carbonaceous subterranean formation's ability to preferentially sorb gases to
1 0 fractionate a mixture of gaseous fluids within the formation and to
dispose of
strongly adsorbing gases within the formation.
~yUND OF THE INVENTION
Numerous industrial processes discharge streams which contain a
1 5 mixture of various gaseous fluids. There is increasing concern that some
of the
constituents of the effluent streams may cause significant environmental
problems, and that these streams therefore should not be released into the
atmosphere. Carbon dioxide is a coompound which is a constituent of many of
the effluent streams released from lindustrial processes and whose release
into
20 the atmosphere is causing increasing concern.
R is hypothesized that carbon dioxide released into the atmosphere acts
as a green-house gas and that too high a concentration of green-house gases
in the atmosphere will cause global warming. In response to this potential
threat, many governmental bodies have either enacted or plan to enact
25 regulations limiting the quantity of carbon dioxide which can be released
into
the atmosphere. These regulation s can hamper many industries because the
combustion of virtually any hydrocarbon fuel with sir produces an effluent
containing carbon dioxide, nitrogen, and other gaseous combustion products.
The mixture of gases which n3sutts from the combustion of a hydrocarbon
30 with oxygen or air is hereinafter referred to as "flue gas." The chemical
composition of flue gas depends on many variables, including but not limited
to,
the combusted hydrocarbon, the combustion process oxygen-to-fuel ratio, and
the combustion temperature. In addition to carbon dioxide and nitrogen, flue
gas may contain compounds such ass carbon monoxide, oxides of sulfur, oxides
35 of nitrogen, and other constituents. The release of these compounds to the
W0 95/27123 2 PCT/US95l03689
.
atmosphere also is coming under increasing public scrutiny and is the subject
of increasing govemrnentai regulation.
In addition to being a hydrocarbon. combustion product, carbon dioxide
can be produced by natural processes and released to the environment during
a non-combustion process. For example, carbon dioxide is produced by the
thermal and biogenic processes which are believed to form hydrocarbons such
as oil, natural gas, or coal. Carbon dioxide often is recovered with these
hydrocarbons and released to the atmosphere by various post-production
steps.
There are several types of commercially available systems which may be
used for removing carbon dioxide from gas streams. One of the most
commonly-used systems utilizes a selective amine absorption solution to strip
the carbon dioxide from the gas stream. Unfortunately, this type of system
will
not tolerate high levels of particuiates or oxides of sulfur. Particulates
cause
plugging, contamination, and erosion or corrosion of the treating process,
while
oxides of sulfur, such as sulfur dioxide (S02), react irreversibly with the
amine
solution utilized in the system to form non-regenerabie by-products.
Therefore,
if particulates or oxides of sulfur are present, extra process steps are
required to
remove the oxides of sulfur and particulates prior.to stripping the carbon
dioxide
from a gas stream. These extra process steps add complexity and cost to the
system.
The increasing concern over the atmospheric release of carbon dioxide
and other compounds demands methods by which to dispose of the
compounds. Because the waste compounds often are a constituent of a
volumetrically larger effluent stream, it is preferred that the disposal
methods
somehow utilize the larger effluent streams to enhance the efficiency of the
overall process andlor to facilitate the recovery of a valuable product using
the
process in addition to providing for the disposal of carbon dioxide contained
therein.
Preferably, the methods also should be capable of disposing of both
carbon dioxide and other contaminants together, without the need to use a
process step to dispose of carbon dioxide and another separate process step to
dispose of other contaminants, such as oxides of sulfur and oxides of
nitrogen.
As used herein, the following terms shall have the following meanings:
(a) 'adsorbate' is that portion of a mixture of gaseous fluids
which is preferentially adsorbed by a carbonaceous matrix of the solid .
~~~s~ss
W0 95127123 3 PCTlITS95103689
carbonaceous subterranean formation and which is recovered from the
formation when the total pressure within the formation is reduced;
(bj "cleats' or 'cleat system" is the natural system of fractures
within a solid carbonaceous subterranean formation;
(c) a 'coalbed" comprises one or more coal seams in fluid
communication with each other;
(d) ~desorbing fluid" includes any fluid or mixture of fluids
which is capable of causing methane to desorb from a solid carbonaceous
subterranean formation;
1 0 (e) "formation parting pressure" and "parsing pressure' mean
the pressure needed to open a foirmation and propagate an induced fracture
through the formation;
(f) "fracture half-length' is the distance, measured along the
fracture, from the wellbore to the fracture tip;
(g) 'preferentially sorbing", 'preferentially sorbs', and
"preferential sorption" refer to processes which occur within a solid
carbonaceous subterranean formation that alter the relative proportions of the
components of a gaseous fluid. These processes may after the relative
proportions of the components of a gaseous.fiuud by equilibrium separation,
kinetic separation, steric separation, andlor any other physical or chemical
processes or combination of processes which within a solid carbonaceous
subterranean formation will selectively alter the relative proportions of the
components of a mixture of gaseou> fluids. Within the formation, the gases
sorbed to the carbonaceous malter?al of the formation will be enriched in
relatively stronger adsorbing fluid components;
(h) "raffrnate" refers to that portion of a mixture of gaseous fluids
injected into a solid carbonaceous subterranean formation which is not
preferentially sorbed by the formation;
(i) 'recovering' means a controlled collection andlor
3 0 disposition of a fluid, such as storing the fluid in a tank or
distributing the fluid
through a pipeline. "Recovering' spec:i~caliy excludes venting the fluid into
the
atmosphere;
(j) "reservoir pressure' means the pressure of a productive
formation near a well during shut-in of that well. The reservoir pressure can
vary throughout the formation. Al;ao, the reservoir pressure of the formation
WO 95/27123 4 PCTIUS95103689
may change over time as desorbing fluid is injected into the formation and
fluids are produced from the formation;
(k) "solid carbonaceous subterranean formation" refers to any
substantially solid carbonaceous, methane-containing material located below
the surface of the earth. It is believed that these methane-containing
materials
are produced by the therm8l and biogenic degradation of organic matter. Solid
carbonaceous subterranean formations include but are not limited to coalbeds
and other carbonaceous formations such as antrium, carbonaceous, and
devonian shales. The. formations utilized by the invention include formations
which are depleted of recoverable methane;
(I) 'sorption" refers to a process by which a gas is held by a
carbonaceous material, such as coal, which contains micropores. The gas is
held on the carbonaceous material in a condensed or liquid-like phase within
the micropores, or the gas may be chemically bound to the carbonaceous
1 5 material; and
(m) "well spaang' or "spacing" is the straight-line distance
between the individual wellbores of a production well and an injection well.
The distance is measured from where the wellbores intercept the formation of
interest.
SUMMARY OF THE INVENTION
One object of this invention is to provide a method for fractionating a
mixture of gaseous fluids within a solid carbonaceous subterranean formation.
Another object of the invention is to provide a method for disposing of a
strongly adsorbing fluid within a solid carbonaceous subterranean formation.
Yet another object of the invention is to provide a method for disposing of
carbon dioxide within a solid carbonaceous subterranean formation.
Still another object of the invention is to provide a method for disposing
of carbon dioxide within a solid carbonaceous subterranean formation while
3 0 simultaneously recovering methane from the formation.
Another object of the invention is to provide a method for fractionating a
mixture of gaseous fluids containing relatively stronger adsorbing fluids and
relatively weaker adsorbing fluids within a solid carbonaceous subterranean
formation, and for recovering a gaseous eifiuent enriched in relatively weaker
3 5 adsorbing fluids from the formation.
WO 95127123 5 ~ ~ ~ PCl1US95103689
Another further object of the invention is to utilize the recovered gaseous
effluent enriched in relatively weaker adsorbing fluids to enhance the
recovery
of methane from a solid carbonaceous subterranean formation.
Yet another further object ~of the invention is to provide a method for
disposing of unwanted gaseous fluids within a solid carbonaceous
subterranean formation that has been at least partially depleted of
recoverable
methane.
Still yet another further object of the invention is to provide a method for
disposing of flue gas w'tthin a solid carbonaceous subterranean formation.
The above objects of the invention are satisfied by the following aspects
of the invention:
The first aspect of the invemiion is a method for fractionating a mixture of
gaseous fluids within a coal seam, lthe method comprising the steps of:
a) introducing a mixture of gaseous fluids comprising a
1 5 weaker adsorbing fluid component and a stronger adsorbing fluid
component into the coal seam: and
b) recovering a naffinate, enriched in the weaker adsorbing
fluid component, from the coal seam.
In a second aspect of the irnrention, a method is disclosed for recovering
methane from a solid carbonaceous subterranean formation penetrated by an
injection well and a production well" the method comprising the steps of:
a) injecting a desorbing fluid having a volume peroentage of
carbon dioxide equal to A into the solid carbonaceous subterranean
formation through the injection well;
b) recovering an effluent comprising methane from the
production well;
c) monitoring the composition of the effluent produced in step
b); and
d) ceasing recovery of the effluent produced in step bj when a
3 0 volume percentage of carbon dioxide in the effluent recovered in step b)
is greater than 0.5 A.
In a third aspect of the invention is, a method is disclosed for recovering
methane from a solid carbonaceous subterranean formation penetrated by an
injection well and a production well, the method comprising the steps of:
R'O 95127123 6 PCT/US95/03689
21'~GS~~
a) injecting a desorbing fluid, having a volume ratio of carbon
dioxide to other injected desorbing fluid components equal to B, into the
solid carbonaceous subterranean formation through the injection well;
b) recovering an effluent comprising injected desorbing fluid
and methane from the production well;
c) monitoring the volume ratio of the carbon dioxide to other
injected desorbing fluid components contained in the effluent recovered
at the production well; and
d) ceasing recovery of the effluent from the production well
1 0 when the volume ratio of carbon dioxide to other injected desorbing fluid
components within the effluent recovered at the production well is greater
than 0.5 B and at least 70 percent of the methane available to the
production well has been recovered.
In a fourth aspect of the invention, a method is disclosed for disposing
carbon dioxide within a solid carbonaceous subterranean formation, the
method comprising the steps of:
a) injecting a desorbing fluid, having a volume ratio of carbon
dioxide to other injected desorbing fluid components equal to B, into the
solid carbonaceous subterranean formation;
b) withdrawing a gaseous effluent having a volume ratio of
carbon dioxide to other desorbing fluids of less than B from the formation;
and
c) ceasing to withdraw the gaseous effluent from the formation
when a volume ratio of carbon dioxide to other injected desorbing fluid
components within the gaseous effluent withdrawn in step b) is greater
than 0.5 B.
In a fifth aspect of the invention is a method for disposing of an unwanted
gaseous fluid component within a solid carbonaceous subterranean formation,
the method comprising the steps of:
a) introducing a gaseous fluid, comprising the unwanted
gaseous fluid component, into the formation to sorb the unwanted
gaseous fluid component to the formation; and
b) maintaining disposal conditions within the formation to
ensure that at least 10 peroent of a disposal saturation level of an
unwanted gaseous fluid component remains sorbed to the formation.
276588
WO 95/27123 PCT/US9S/03689
7
In a sixth aspect of the invention, a method is disclosed for disposing of
an unwanted gaseous fluid component within a coal seam, the method
comprising the steps of:
a) introducing a gaseous fluid, containing the unwanted
gaseous fluid component, inl;o the coal seam; and
b) ceasing to introduce the gaseous fluid into the coal seam
when the coal seam becomes saturated to a desired degree with the
unwanted gaseous fluid cornponent.
In a seventh aspect of the invention, a method is disclosed for disposing
1 0 of an unwanted gaseous fluid component, the method comprising the step of:
injecting a gaseous fluid, comprising the unwanted gaseous fluid component,
into a coal seam which is at least partially depleted of methane under
disposal
conditions to cause the unwanted gaseous fluid component to sorb to the coal
seam and to minimize the release of unwanted fluid component to the
atmosphere.
In some aspects, the invention provides a means for disposing of large
quantities of an unwanted gaseous fluid within a solid carbonaceous
subterranean formation. Some a;:pacts allow fluids such as flue gas, which
may contain oxides of nitrogen, oxides of sulfur, carbon monoxide and/or
carbon dioxide, to be introduced into a solid carbonaceous subterranean
formation to enhance the recovery of methane from the formation. The
invention further provides an efficient means for producing a nitrogen-
enriched
effluent stream which can be utiliza,i to enhance the recovery of methane from
a
solid carbonaceous subterranean formation. Other aspects allow fluids such as
flue gas to be disposed of within a solid carbonaceous subterranean formation
without requiring separate processing systems for the carbon dioxide and the
oxides of sulfur.
Numerous other advantage:: and features of the present invention will
become readily apparent from i:he following detailed description of the
invention, the embodiments described therein, the claims, and the
accompanying drawings.
BRI F D FiIPTION OF THE D AWIN =
FIG. 1 is a graph of the total gas production rate over time from a fully
3 5 methane-saturated coalbed into which several different gaseous
compositions
have been introduced.
R'O 95/27123 $ PGT/US95I03689
2 ~-~ ~ ~ g ~ FIG. 2 is a graph of the cumulative methane predicted to be
recovered
from the coaibed of FIG. 1.
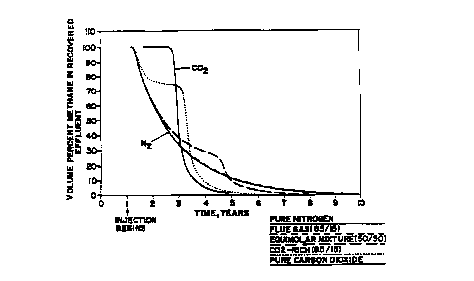
FIG. 3 is a graph of the volume percent of methane predicted to be
present in the effluents recovered from the coalbed of FlG. 1.
FIG. 4 is a graph of the volume peroent of nitrogen predicted to be
present in the effluents recovered from the coalbed of FIG. 1.
FIG. 5 is a graph of the volume peroent of carbon dioxide predicted to be
present in the effluents recovered from the coalbed of FIG. t.
FIG. 6 is a graph of predicted methane recovery rates for the coalbed of
FIG.1.
FIG. 7 is a graph of cumulative methane recovered versus cumulative
desorbing fluid injected into the coalbed of FIG. 1.
FIG. 8 is a graph of the cumulative volume of nitrogen recovered from a
methane-depleted coalbed which is used to fractionate a mixture of gaseous
fluids containing 15 volume percent carbon dioxide and 85 volume peroent
nitrogen.
FIG. 9 is a graph of the cumulative mixture of gaseous fluids which are
injected over time into the depleted coalbed of FIG. 8.
FIG. 10 is a graph of the recovery rate of nitrogen from the depleted
coalbed of FIG. 8.
FIG. 11 is a graph of the total gas recovery rate over time from a fully
methane-saturated coalbed. The graph compares the total gas recovery rate
when pure carbon dioxide is injected into the bed to the recovery rate when a
mixture containing 70 volume percent carbon dioxide and 30 volume peroent
methane is injected into the coalbed.
FIG. 12 is a graph of the cumulative volume of methane predicted to be
recovered from the cosibed of FIG. 11.
FIG. 13 is a graph of the volume percent of methane predicted to be
present in the effluents recovered from the coalbed of FIG. 11.
FIG. 14 is a graph of the volume peroent of carbon dioxide predicted to
be present in the effluents recovered from the coaibed of FIG. 11.
FIG. 15 is a graph of the predicted methane recovery rates for the
coalbed of FIG. 11.
FIG. 16 is a graph of the predicted carbon dioxide recovery rates far the
3 5 coalbed of FIG. 11.
2~7~588
W0 9512T123 PGT/US95/03689
S
F1G. 17 is a graph of the prei9icted total gas production rates for coalbed
of FIG. 11.
FIG. 18 is a graph of predicted cumulative methane recovered from the
coalbed versus cumulative desorbing fluid injected for the coaibed of FIG. 11.
DES(;RIPTION_OF THE EMBODIM NTS
While this invention is suscepltible of embodiment in many different forms,
there is shown in the FIGS., and will herein be described in detail, specific
embodiments of the invention. It should be understood, however, that the
present disclosure is to be considered an exemplification of the prinaples of
the
invention and is not intended to lima the invention to the speafic embodiments
illustrated.
Solid carbonaceous subterranean formations, such as coal seams, are
comprised of carbonaceous material. The carbonaceous material includes a
1 5 matrix, having an extensive system of micropores, and a system of
fractures
which penetrate the matrix, commonly refereed to as "cleats". The system of
micropores provides a large internal surface on which gaseous fluids can
adsorb. The present invention exploits the differing adsorption strengths of
various gaseous fluid molecules on the carbonaceous material of the formation,
and the ability of large quantities of unwanted gaseous fluid components to
sorb
to the micropores of a carbonaceous matrix.
jpjs~ed ~a eo ~c FI sid
In general, a gaseous fluid molecule that has a relatively stronger
2 5 adsorption strength will preferentially sorb to the carbonaceous material
of the
formation over a gaseous fluid molecule that has a weaker adsorption strength.
The amount of any gaseous fluid which will sorb to the carbonaceous material
of a formation is dependent in part on the relative adsorption strength of the
gaseous fluid for the carbonaceous matrix, the capacity of the carbonaceous
3 0 matrix for holding the gaseous fluid of interest, the tendency of the
gaseous fluid
molecules of interest to chemically ~~eact w'tth the carbonaceous material and
thereby chemisorb to the material, and the pressure and temperature present
within the formation.
An important factor in the operi~tion of the present invention is the relative
35 adsorption strengths of the components of an injected mixture of gaseous
fluids
2 i~~~gg
PCTIUS95103689
~~/~123
to one another and to any fluids, such as methane, which may already be
present w'tthin the formation.
For a carbonaceous material such as coal, it is believed that the
atmospheric boiling point of a fluid is indicative of the relative adsorption
strength of the molecules or compounds which make up the fluid. Table 1 lists
the atmospheric boiling point of several common fluids.
TABLE 1
Helium (He) -269C Weaker Adsorption Strength
Hydrogen (H2) -253C
Nitrogen (N2) -196C
Carbon Monoxide (CO) -192C
Argon (Ar) -186C
Oxygen (02) -183C
Methane (CH4) -162C
Nitric Oxide (NO) -151 C _ .
Xenon (Xe) -108C
Ethane (C2Hg) -88C
Carbon Dioxide (C02) -78C
Suifur Hexafiuoride -64C
(SF6)
Hydrogen Sulfide (H2S) -60C
Propane (C3Hg) -42C
Suifur Dioxide (S02) -10C
Nitrogen Dioxide (NOZ) 21C
Sulfur Trioxide (S03) 44C Stronger Adsorption suength
ft is believed that, in general, the stronger adsorbing fluids have higher
boiling points and the weaker adsorbing fluids have relatively lower boiling
points. Therefore, the relative adsorption strength of one fluid component to
another within a gaseous mixture and to other gaseous fluids within the
formation can be determined by comparing their relative atmospheric boiling
points. For example, carbon dioxide, with an atmospheric boiling point of
276588
i WO 95127123 _ _ _ P~~pS95I03689
11
-78°C, is relatively more strongly adsorbing to carbonaceous material
than
methane or nitrogen, which have atmospheric boiling points of -162°C
and
-196°C respectively. The relative atmospheric boiling points of a fluid
will
provide one of ordinary skill in the art with general information relating to
the
relative adsorption strength of various gaseous fluids. However, the relative
adsorption strength of various gaaeous fluids on a particular carbonaceous
' material of interest should be determined empirically where possible.
Further, the relative adsorption strength of various gaseous fluids will
provide one of ordinary skill in the art with general information relating to
the
disposal of an unwanted gaseous fluid within a solid carbonaceous
subterranean formation. However, the quantity of an unwanted gaseous fluid
which can adsorb to a particular carbonaceous material should be determined
empirically where possible. The empirical data will allow the quantity of
gaseous fluid which can be disposed of within a solid carbonaceous
1 5 subterranean formation to be predicted more accurately. If chemisorptiom
of a
particular gaseous fluid component t~ the carbonaceous material of the
formation is believed to be a significant factor, it also should be taken into
account when determining the amount of unwanted gaseous fluid which can be
disposed of within a formation.
The gaseous fluid may be introduced into the formation in either a
gaseous or liquid state. A detailed description of suitable methods for
injecting
gaseous fluids in accordance with the invention may be found in the discussion
relating to the injection of a nitrogen-enriched raffinate discussed below.
Other
suitable ways of introducing gaseous fluids into a solid carbonaceous
subterranean formation are known to one of ordinary skill in the art. If a
gaseous fluid containing carbon dioxide is injected in a liquid state, it
typically
will change to a gaseous fluid within the formation. Aftematively, the gaseous
fluid may be introduced into the fon~nation as a super-critical fluid.
Depending
on the temperatures and pressures within the formation, the gaseous fluid may
3 0 either be maintained in the formation as a super-critical fluid or it may
become a
liquid, a gaseous fluid, or a co-existing liquid and vapor.
If the gaseous fluid utilized is near its critical temperature and pressure,
it
may be necessary to operate any production wells in a manner which will
minimize the preapitation and/or ca~ndensation of solids and liquids within
the
3 5 formation. A more detailed description of how to operate the production
wells in
such a situation is described below.
R'O 95127123 PCTlU59S103689
12
~1'~~383
In this embodiment, a desorbing fluid containing a strongly adsorbing
fluid is introduced into the solid carbonaceous subterranean formation through
an injection well in fluid communication with the formation and an effluent
containing methane is recovered from one or more production wells. The
injection well preferably penetrates the formation.
The desorbing fluid typically is comprised of carbon dioxide and other
fluid components, for. example nitrogen andlor methane. Because carbon
dioxide is a strongly adsorbing fluid, it will preferentially adsorb to the
carbonaceous material of the formation over other weaker adsorbing fluids such
as nitrogen or methane.
During the conversion of organic matter to coal and other solid
carbonaceous substances, methane is produced. The methane exists within
1 5 the formation both as free gas in the cleats and fractures of the
formation and as
sorbed gas within the carbonaceous matrix of the formation.
A carbon dioxide-containing desorbing fluid mobilizes both the free
methane and the sorbed methane within the formation. The mobilization of the
methane sorbed to the carbonaceous matrix occurs as a resuR of a lowering of
the partial pressure of methane within the cleats and because carbon dioxide
and other injected desorbing fluids competitively sorb to the carbonaceous
matrix of the formation.
The partial pressure of methane within the cleats is lowered duo to the
presence of injected desorbing fluid within the cleats near methane sorption
sites. As the partial pressure of methane within the cleats is Powered,
methane
sorbed to the carbonaceous matrix will desorb from the matrix and diffuse to
the
cleats.
The competitive sorption of carbon dioxide and other injected desorbing
fluids to the carbonaceous matrix will also cause methane to desorb from the
carbonaceous matrix into the cleats. Once methane is within a cleat, the
pressure gradient developed between the formation and a production well
and/or weAs will move the methane to the production well or wells where it can
be recovered.
Since methane is not as strongly adsorbed to a carbonaceous matrix as
carbon dioxide, it will move faster through a solid carbonaceous subterranean
formation than more strongly adsorbed gaseous fluids.
2i763~8
WO 93127123 - PCT/US93/03689
13. _ :r .
The preferential sorption of stronger adsorbing fluids, such as carbon . ~ I
dioxide, within the carbonaceous matrix of the formation causes the injected
desorbing fluid to be fractionated within the formation. The stronger
adsorbing
fluids will be preferentially sorbed to the region of the carbonaceous matrix
surrounding the injection well into which the desorbing fluid is being
introduced.
The stronger adsorbing fluids will continue to sorb to the matrix in the
region
. until the matrix is saturated with stronger adsorbing fluid. The relatively
weaker
adsorbing fluids will not sorb as strongly to the matrix and therefore will
move
faster through the formation than the stronger adsorbing fluids.
In general, as desorbing fluid is injected into the formation, the region
within the formation which is saturated with stronger adsorbing fluid is being
continually advanced forvvard toward a production well or wells. Therefore, it
can be said that the stronger adsorbing fluids will forth what approximates a
concentration front that advances within the formation. As desorbing fluid is
1 5 injected into the formation, the concentration front is Continually being
swept
from an injection well toward a region of lower pressure within the formation,
ss.~~ as a production well.
Ahead of the front, the concentration of the stronger adsorbing fluids
sorbed to the matrix is small relative td the concentration of stronger
adsorbing
fluids within the front or behind the front. Behind the front, the relative
concentration of stronger adsorbing fluids, such as carbon dioxide, sorbed to
the matrix approaches a steady-state value as desorbing fluid is continually
injected into the formation through l:he injection well.
The steady-state value is dependent on several factors including: the
relative adsorption strength of the stronger adsorbing fluids to the
carbonaceous matrix as compared to the adsorption strength of the other fluids
within the formation; and the relatiive concentration of the stronger
adsorbing
fluids contained within the injected desorbing fluid introduced into the
formation.
Since the stronger adsorbing fluid components will be preferentially
3 0 sorbed by the formaflon, a desorE~ing fluid containing carbon dioxide can
be
injected into a solid carbonaceous subterranean formation while an effluent
having a bwer carbon dioxide volume percent is recovered from a production
well. The volume percentage of carbon dioxide within a mixture of gaseous
fluids is sometimes hereafter referred to as a volume peroernage A.
3 5 Aiso, because the stronger adsorbing fluid components will move more
slowly through the formation than llhe weaker adsorbing fluid components, an
R'O 95/27123 PCT/US95103689
effluent, having a volume ratio of carbon dioxide to other injected desorbing
fluid components which is less than the volume ratio of carbon dioxide to
other
injected fluid components within the desorbing fluid introduced into the
formation, can be recovered from a production well. The volume ratio of carbon
dioxide to other injected desorbing fluid components is sometimes referred to
hereafter as a ratio B.
One type of carbon dioxide containing gaseous fluid which may be
utilized in the invention is flue gas. Typically, the volume ratio B of carbon
dioxide to other injected desorbing fluid components within flue gas is from
1/11
1 0 to 2I8. Another example of a cafion dioxide-containing gaseous fluid which
may be utilized in the invention is a mixture of gaseous fluids which is
rejected
by a nitrogen rejection unit or a membrane separator which is separating
carbon dioxide from a natural gas production stream. Typically, the rejected
stream contains a volume ratio B of carbon dioxide to methane and other gases
1 5 of from 1/1 to 95/5.
It is believed the volume peroentage of carbon dioxide in the effluent
recovered from a production well will be less than the volume peroentage of
carbon dioxide in the injected desorbing fluid introduced into the formation
until
the region of the formation between an injection well and the production well
20 becomes saturated with stronger adsorbing fluid components. Additionally,
it is
believed the volume ratio of carbon dioxide to other injected desorbing fluid
components contained in an effluent recovered from a production well will be
less than the volume ratio of carbon dioxide to other injected desorbing fluid
components within the desorbing fluid introduced into the formation, until the
25 region of the formation between an injection well and the production well
becomes saturated with stronger adsorbing fluid components.
In an ideal situatiori, the solid carbonaceous subterranean formation is
uniform, and a carbon dioxide concentration front will move radially outwardly
from an injection well into the formation. However, there are very few solid
30 carbanacaous subterranean formations that show such uniformity. Most
formations have regions through which the injected desorbing fluid will
rapidly
pass. These so-called "stringers" include regions of relatively higher
permeability compared with the majority of the carbonaceous material.
Stringers also include regions that are comprised of materials to which the
35 desorbing fluid components do not readily adsorb. Examples of stringer
regions that do not readily adsorb fluids include sandstone, carbonaceous
2~'~6~88
WO 95127123 --' PCT/US95/03659
shale, and other similar types of miateriais known to one of ordinary skill in
the
art, i
The injected desorbing fiuici which passes through the stringers will at
least partially bypass the carbonaceous material of the formation and is said
to
5 "streak' within the formation toward a production well. The streaking will
increase the relative amount of desorbing fluid present within an effluent
recovered from a production well.
Additionally, injected desorbing fluid which travels through a stringer to a
production wail does not contact as much of the carbonaceous matrix of the
10 formation as injected desorbing fluids which do not travel through
stringers.
Therefore, injected desorbing fluid which travels through a stringer to a
production well will not be as effectively fractionated into its respective
components. Consequently, the volume ratio of carbon dioxide to other injected
desorbing fluid components within the effluent recovered from the production
1 5 well will be increased above what vvoukl be expected in an idealized
formation,
but the ratio should still be reduced relative to the initial ratio B
contained in the
injected desorbing fluid.
The preferential sorbtion of the stronger adsorbing fluid components
within the formation, which causes the stronger adsorbing fluid components to
move more slowly through the formation than the weaker adsorbing fluid
components, allows stronger adsorbing fluids, such as carbon dioxide, to be
disposed of within the formation. AAethods for disposing of carbon dioxide and
other stronger adsorbing fluids within a solid carbonaceous subterranean
formation acre discussed more fully below.
The ability of a solid carbonaceous subterranean formation to fractionate
the injected desorbing fluid, with the majority of the carbon dioxide reaching
the
production well andlor wells at a later time than methane and/or other fluid
having weaker adsorption strengths, provides a method for recovering a
substantial portion of the methansr from a solid carbonaceous subterranean
formation while simultaneously disposing of carbon dioxide within the
formation.
As the method is carried out, the ratio of carbon dioxide to other injected
desorbing fluids within the recovered effluent is preferably monitored at a
' production well or wells using methods known to one of ordinary skill in the
art,
such as a gas chromatography. This monitoring should provide a relative
R'O 95/27123 ' 6 PCTIUS95103689
2~.'~6~$8 .
;.
indication of how the injected desorbing fluids are moving within the
formation
and whether the formation is becoming saturated with stronger adsorbing
fluids.
As discussed earlier, the volume ratio of carbon dioxide to other injected
desorbing fluid components within the effluent recovered at a production wail
will be reduced relative to the volume ratio of carbon dioxide to other
injected
desorbing fluid components within the injected desorbing fluid introduced into
the solid carbonaceous subterranean formation.
The ratio of carbon dioxide to other injected desorbing fluid components
recavered.at a production well should rapidly increase once a carbon dioxide
1 0 front reaches the weilbore of a production well. See for example FIGS. 4,
5, 13,
and 14. Also, the majority of the methane originally sorbed within the
carbonaceous matrix located along the flow path of the injected desorbing
fluid
between an injection well and a production well will have been desorbed from
the formation once the carbon dioxide concentration front has traveled from an
1 5 injection well to a production well (i.e. the carbonaceous matrix within
the
formation between an injection well and a production well along the flow path
of
the injected desorbing fluid will have been swept of the methane contained
therein.)
Also, as discussed earlier, actual solid carbonaceous subterranean
20 formations have stringers which can increase the volume percentage of the
desorbing fluid contained within the effluent recovered from the formation.
Additionally, the amount of injected desorbing fluid required to sweep a
formation can be increased due to the stringers.
Ono method which the inventors believe can effectively reduce the
25 amount of streaking which occurs within the formation utilizes the
intermittent
injection of a liquid such as water into an injection well. The injected water
should selectively enter the higher permeability regions. Once the liquid
enters
the highor permeability regions it should reduce the flow of desorbing fluids
through these regions. This will cause the injected desorbing fluid to be
3 0 redirected into regions of lower permeability, thereby increasing the
vertical and
area) swoop within the formation. By redirecting the injected desorbing fluids
into regions of lower permeability, the time it takes for those fluids to
travel from
an injection well to a production well is increased. Also, because the
redirected
injected desorbing fluid may come into contact with more carbonaceous
35 material of the formation, the volume percentage of carbon dioxide within
the
effluent recovered from the production well and the volume ratio of carbon
CA 02176588 2000-07-06
17
dioxide to other injected desorbing fluid components within the effluent
recovered from a
production well may be reduced.
Whether or not to cease recovery of effluent from a production well depends in
part
on the percentage of the available methane which has been recovered from the
area of the
formation drained by the production well. The available methane is that
methane which is
available for recovery from a production well. It should be noted that the
methane available
within the formation undergoing enhanced recovery may not be depleted, but the
methane
available for a specific production well may have been depleted. The quantity
of methane
contained in a solid carbonaceous subterranean formation can be determined by
methods
known to one of ordinary skill in the art. Examples of methods for calculating
the quantity
of methane within a formation are set forth in Yee et al. "Gas Sorption on
Coal and
Measurement of Gas Content", Chapter 9, pages 203-217, Hydrocarbon from Coal,
published
by the American Association of Petroleum Geologists (1993).
One of ordinary skill in the art who knows the quantity of methane contained
in a
formation and the composition of the injected desorbing fluid can calculate
the available
methane which can be recovered form the formation.
The decision of whether to continue to recover effluent from a production well
also
should take into consideration the value of the effluent. When determining the
value of the
effluent, it is important to take into consideration the processing costs
required to further
utilize the effluent stream. For example, if the effluent stream is to be sent
to a natural gas
pipeline the effluent stream may have to be processed to lower the percentage
of inert gases
contained therein to an acceptable level. The acceptable level for inert gases
contained in
natural gas is governed by natural gas pipeline specifications. If the value
of the effluent is
not great enough to justify further recovery of the effluent, then recovery of
the effluent
should be ceased or the method should be modified in an attempt to change the
composition
of the effluent stream to a point where further recovery is justified.
A methane containing solid carbonaceous subterranean formation under
development
may have several injection wells and several production wells. Due to the
heterogeneity of
most solid carbonaceous subterranean formations, a carbon dioxide
concentration front from
one injection well may reach a production well before the carbon dioxide
concentration front
from a second
2~'~6 ~~8
R'O 95/27123 PCTlUS95/03689
18
injection well reaches the same production well. This could cause the volume
ratio of carbon dioxide to other injected desorbing fluid components within
the
effluent recovered from a production well to increase and approach or exceed
the initial ratio B before the formation between the injection wells and a
production well has been swept of available methane.
As can be seen from FIGS. 2, 3, 5-7, and 11-15, A substantial peroentage
of the available methane may be recovered from a formation before the ratio of
carbon dioxide to other injected desorbing fluid components within the
effluent
recovered from a production well reaches a value equal to the initial ratio B.
R is
1 0 believed that in some situation it is preferable to cease recovering
effluent from
the production welt when the ratio B is from 0.5 to 0.9 the value of the
initial ratio
B. For example, when the ratio of carbon dioxide to other injected desorbing
fluid components within the recovered effluent is less than B, but a
substantial
percentage of the available methane has been recovered from the production
1 5 wail, ft may be preferable to cease recovery of effluent from the
production well.
If the volume ratio of carbon dioxide to other injected desorbing fluid
components within the effluent recovered from a production well is greater
than
the initial ratio B and a substantial percentage of the available methane has
bean produced from that well, recovery from that production well preferably is
2 0 ceased.
If the volume ratio of carbon dioxide to other injected desorbing fluid
components within the effluent recovered from a production well is greater
than
the initial ratio B, but there is a substantial percentage of methane still
available
to be produced from that production well, the recovery of effluent from that
25 production well is not preferably ceased. Instead, the flow of affluent
from the
production well should be restricted.
The restriction of flow from a production welt will increase the pressure
within the well. This increase in pressure near the production well will cause
the injected desorbing fluids to be redirected to areas of the formation with
30 relatively tower pressure. This restriction will improve the sweep of the
formation end also reduce the streaking of injected desorbing fluid toward the
production well from which flow has been restricted. Additionally, ft is
believed
that by increasing the pressure in the formation surrounding a production
well,
the preferential adsorption of carbon dioxide over methane to the carbonaceous
35 matrix will be enhanced. ft is believed this will reduce the volume
percentage of
carbon dioxide contained in the effluent recovered from the production well.
W0 95127123 ~ ~ ~ PGT/US95/03689
19
Several procedures may be utilized to restrict the flow of effluent from a
production well. One procedure utilizes the introduction of a flow restricting
material into the subterranean formation adjacent the. production well from
which flow is desirably restricted. E;Kamples of materials useful for
restricting the
flow of effluent from a production well include, for example, carbon dioxide,
acetone, pyridene diesel oil, polymers, epoxy, surtactant, foam, cement and
mixtures thereof. The above listed materials reduce the flow of desorbing
fluid
within the formation by plugging or (binding the fracture system of the
formation,
thereby reducing the permeability of the affected area of the formation. In
addition to the above listed materials, any material which causes the
carbonaceous matrix to swell, theireby reducing its permeability, or plugs or
binds the fracture system of the formation can be utilized.
Another method for restricting the flow of effluent from a production well
involves operating a valve in fluid communication with the production well in
such a manner that the flow of effluent from that production well is
restricted. As
with the earlier described techniques, the restriction of flow of effluent
from the
production well will increase the pressure within the well near the formation
and
provide the earlier discussed benefits which are believed to result from an
increase in the pressure within the formation near a production well.
The above discussed procedures should assist in allowing the remaining
methane available to the production well of interest to be recovered.
Any carbon dioxide which is recovered with the effluent is readily
separable from the methane and ovther fluids present in the effluent, such as
nitrogen. Examples of processes for separating carbon dioxide from an affluent
stream include:
separation of carbon dioxide from a gaseous mixture using a
membrane separator;
separation of carbon dioxide from a gaseous mixture using an
adsorptive type separator, such as a pressure swing adsorptive
separator; and
cryogenic separation afi carbon dioxide from a gaseous mixture
using a nitrogen rejection type unit.
The carbon dioxide containing stream which is produced by tho above
processes also typically contains methane and/or nitrogen. If desired, the
carbon dioxide-containing stream can be reinjected into the solid carbonaceous
subterranean formation.
21'~G~8~
WO 95127123 PCTIU595103689
ft has been discovered by the inventors that it may be desirable in some
instances to continue to recover effluent from a production well and reinject
a
substantial portion of the effluent stream back into the formation. Examples
of
situations where this may be advantageous are where there is a substantial
5 amount of available methane to be recovered from a production well, but the
volume percentage of the carbon dioxide in the effluent stream is high. In
this
type of situation, the cost of separating the inert gases, such as carbon
dioxide
and nitrogen from the methane using conventional separation means, may be
prohibitive.
10 In these situations, the inventors have discovered that it may be more
advantageous to use the effluent stream to enhance the recovery of methane
from another solid carbonaceous subterranean formation or a different region
of
the same solid carbonaceous subterranean formation. Preferably, the formation
into which the effluent is injected is still capable of adsorbing a large
quantity of
15 carbon dioxide.
The reinjected stream will be fractionated by the formation. The carbon
dioxide contained in the reinjected stream will move slowly through the
formation toward the production wells. As discussed earlier, the carbon
dioxide
will displace the methane from the formation. The methane contained in the
20 reinjeded stream shoukf travel faster through the formation toward a
production
well. It is believed the methane contained in the reinjected stream will also
help
in maintaining the reservoir pressure of the formation and therefore assist in
recovering methane from the formation. FIGS. 11 through 18 graphically
illustrate how in some circumstances a mixture of gaseous fluids containing
methane and carbon dioxide advantageously can be used to recover methane
from a solid carbonaceous subterranean formation.
pia_p~r_aai of Caseo~c Fluidc_
In this embodiment, a gaseous fluid which contains an unwanted
3 0 gaseous fluid component is introduced into a solid carbonaceous
subterranean
formation. The gaseous fluid is introduced into the formation through an
injection well in fluid communication with the formation, preferably, through
an
injection well which penetrates the formation. The gaseous fluid is introduced
into the formation at a pressure higher than the reservoir pressure of the
3 5 formation and may be introduced into the formation in either a gaseous or
liquid
state. Preferably, the gaseous fluid is introduced at a pressure below the
j.. r_ 4~ ~~ .L t
21'6588
R'O 95127123 PGT/US95/03689
21
formation parting pressure of the fiortnation. If the injection pressure is
too high
and the formation fractures, the injected gaseous fluid may leak out of the
solid
carbonaceous subterranean formation into surrounding formations.
The gaseous fluid typically <;ontalns carbon dioxide and/or other gaseous
fluid components which are n3latively more strongly-adsorbing to the
carbonaceous material of the formation than methane. Examples of other
gaseous fluid components which are typically contained in the introduced
gaseous fluid include oxides of sulfur, oxides of n'ttrogen, and hydrogen
sulfide.
These relatively more strongly-adsorbing gaseous fluids will preferentially
sorb
1 0 to the carbonaceous material of the formation over any methane which may
be
present within the formation.
Flue gas is an example of a gaseous fluid which may be disposed of in
accordance with the invention. Flue gas typically contains 10 to 25 percent
carbon dioxide by volume, from about 75 to 90 percent by volume nitrogen, and
small volume peroentages of oxides of nitrogen and oxides of sulfur. Another
example of a gaseous fluid which may be utilized in the invention is the
mixture
of gaseous fluids which is rejectedl by a separation system which is
separating
carbon dioxide from a natural gas. production stream. Typically,.the rejected
stream contains about 50 to 95 percent by volume carbon dioxide with the rest
of the gaseous fluid being comprised mainly of methane. The rejected stream
may also contain some hydrogen sulfide, oxides of nitrogen, and oxides of
sulfur.
A solid carbonaceous subterranean formation utilized to dispose of
unwanted gaseous fluid components, in accordance with this embodiment,
preferably is depleted of recoverable methane, more preferably substantially
depleted of recoverable methane, most preferably essentially depleted of
recoverable methane. A formation which is depleted of recoverable methane is
preferably utilized because the preferential sorption of fluids such as carbon
dioxide to a solid carbonaceous subterranean formation will be enhanced
within a formation which has an initially lower concentration of methane
sorbed
to its carbonaceous matrix. Also, if the pressure of the formation is reduced
and
the formation is depleted of recoverable methane, substantial quantities of
gaseous fluids which are relatively weaker adsorbing than methane may be
eff~cientiy disposed of within the fonmation.
In other situations, it may be preferable to utilize solid carbonaceous
subterranean formations from which methane has never been produced. ft may
2~~~58~
W0 95127123 PCfIUS95103689
22
not be attractive to produce methane from such formations. Examples of such
formations include formations with lour original methane in place and
formations
with low permeability.
A formation which is depleted of recoverable methane still contains some
methane, but the methane is at such concentration that it is not economical to
recover it from the formation. A formation which is depleted of recoverable
methane has had at least 25 volume percent of the original methane in place
removed from the formation. A formation which is substantially depleted of
recoverable methane, has had at least 50 volume percent of the original
methane in place removed from the formation. A formation which is essentially
depleted of recoverable methane has had at least 70 volume peroent of the
original methane in place removed from the formation.
One method for recovering methane from a formation utilizes pressure
depletion of the formation. The reduction of pressure within the formation
causes methane to desorb from the carbonaceous material and flow to a
production well where it can be recovered. A coal seam which is produang
methane through a production well using primary depletion will typically be
abandoned when from 259'° to about 70°~ of the original methane
in place has
been recovered. The typical abandonment.pressures for such primary
depletion wells range from 689,476 Pascais (Pa) to about 2,068,427 Pa.
Methane can also be recovered from coal seams using enhanced
recovery techniques. An example of an enhanced recovery technique which
can efficiently remove methane from a coal seam is the use of a nitrogen
enriched stream to desorb methane from a coal seam. For a coal seam which is
using nitrogen enhanced recovery techniques, the percentage of methane
recoverable from a seam is primarily dependent on the volume percentage of
nitrogen contained in the production stream recovered from the formation. The
production wells are typically abandoned once the percentage of nitrogen
becomes too high and/or the percentage of methane becomes too low to justify
further recovery. With current nitrogenlmethane separation technology, a
production well will typically be abandoned when the volume percentage of
methane in the effluent recovered from the formation is from 25~° to
about 50%.
This corresponds to recovery of from 45~° to about 70% of the original
methane
in place w'tthin the formation. It should be noted that as more efficient
methods
are developed to separate methane from nitrogen, the amount of methane
recoverable from a formation will increase. When a formation has used
2~'~6588
WO 95127123 PCT/US95/03689
23
nitrogen to enhance the recover)r of methane from the formation, it may be
preferable to reduce the pressure in the formation prior to disposing of an
unwanted gaseous fluid component within the seam.
Another enhanced recovery technique which can effectively recovery
methane from a solid carbonaceous subterranean formation is carbon dioxide
enhanced recovery, which is more fully discussed above.
As discussed earlier, the stronger adsorbing fluids will be preferentially
sorbed to the region of the carbonaceous matrix surrounding the injection well
over relatively weaker adsorbing fluids. The stronger adsorbing fluids will
continue io sorb to the matrix in the region until the matrix is saturated
with
stronger adsorbing fluid. Any relalJVely weaker adsorbing fluids, which may be
presets within the formation, will not sorb as strongly to the matrix and
therefore
will migrate within the formation toward regions of lower pressure. In
general,
as gaseous fluid is injected into the formation, the region within the
formation
which is saturated with stronger adsorbing fluid is continually expanding away
from the injection well.
Tho saturation level of any gaseous fluid component on the
carbonaceous material of a formation is dependent on several factors
including:
the relative adsorption strength of the stronger adsorbing fluids to the
carbonaa~ous matrix as compared to the adsorption strength of the other fluids
w'tthin the formation, the relative amcentration of the stronger adsorbing
fluids
contained within the injected gaseous fluid introduced into the formation, the
capacity of a carbonaceous material for sorbing a particular gaseous fluid
component, and the pressure and temperature prevalent within the formation.
For example, a typical San Juan Fruitland formation anal, which is totally
depleted of methane, will sorb approximately 0.0246 standard cubic meters
(SCM) of gas per kilogram of coal at 10,342,136 Pa and 46.1 °C, when
the coal
is allowed to reach saturation wiith a gaseous fluid containing 85 volume
peroeM carbon dioxide and 15 volume peroent nitrogen. The sorbed phase on
the coal will comprise approximately 99 volume percent carbon dioxide and
approximately 1 volume pera3nt nitrogen. When the coal is allowed to reach
saturation with a gaseous fluid cointaining 50 volume peroent a;rbon dioxide
and 50 volume pera3nt nitrogen at the same temperature and pressure
conditions, the coal will sorb approximately 0.0219 SCM of gas per kilogram of
anal. The sorbed phase will comprise approximately 93 volume percent a~ubon
dioxide and approximately 7 volume peroent nitrogen. For a gaseous fluid
PCT/US95103689
24
..
containing 15 volume peroent carbon dioxide and 85 volume percent nitrogen
at the same temperature and pressure, the coal will sorb approximately 0.0153
SCM of gas per kilogram of coal, the sorbed phase being comprised of
approximately 70 volume peroent carbon dioxide and approximately 30 volume
percent nitrogen. For a gaseous fluid containing 70 volume percent carbon
dioxide and 30 volume percent methane at the same temperature and pressure,
the coal will sorb approximately 0.0233 SCM of gas per kilogram of coal, with
the sorbed phase comprising approximately S6 volume percent carbon dioxide
and approximately 14 volume percent methane. The above calculated
saturation levels are developed with the assumption that an unlimited quantity
of gaseous fluid is available to the coal and that the weaker adsorbing
gaseous
fluid components are continually flowing by the sample and being replaced by
fresh gaseous fluid so that additional stronger adsorbing gaseous fluid
components can be preferentially sorbed to the coal. The enrichment of
1 5 strongly adsorbing fluid in the sorbed phase is a result of the
preferential
sorption which occurs within a solid carbonaceous subterranean formation. A
gal which is totally depleted of methane correlates to a coal seam which has
less than approximately 10 volume percent of the original methane in place
still
remaining within the seam.
The above listed saturation levels for a particular unwanted gaseous fluid
component are hereinafter referred to as 'disposal saturation levels.' The
disposal. saturation levels for a particular unwanted gaseous fluid component
are ratculated for a given temperature and pressure. The pressure and
temperature, along with other operating parameters which are utilized to
dispose of unwanted gaseous fluid components within the formation are
referred to as disposal conditions. The disposal conditions are manipulated to
maximize the quantity of unwanted gaseous fluid component which is sorbed to
the formation. Typically, the disposal conditions are such that between 10 and
99 volume peroent of an introduced unwanted gaseous fluid component is
disposed of within the formation. In some instances, ft is believed that
greater
than 99 volume percent of an unwanted gaseous fluid component can be
disposed of within the formation. By disposing the unwanted gaseous fluid
component within the formation, the unwanted gaseous fluid component's
release to the atmosphere is prevented. Typically, maintaining disposal
conditions requires only closing andlor controlling effluent paths from the
formation to prevent the unwanted gaseous fluid component from being
CA 02176588 2000-07-06
released from the formation while maintaining the pressure within the
formation preferably
below the parting pressure of the formation. In some instances, it may be
advantageous to
periodically dewater the formation to maintain or increase the formations
capacity to sorb an
unwanted gaseous fluid component. In some instances it may be advantageous to
raise the
5 temperature prevalent within the formation. An example of a situation where
it can be
preferable to raise the temperature within the formation is where the unwanted
gaseous fluid
component chemically reacts with the formation and the reaction becomes more
favorable as
temperatures within the formation increase.
For a given solid carbonaceous subterranean formation, the disposal saturation
levels
10 for a particular unwanted gaseous fluid component can be calculated by
using an extended
Langmuir adsorption isotherms model and the required empirical data for the
given formation.
A description of an extended Langmuir adsorption isotherm model and how to
utilize it to
produce a model similar to the one used by the inventors' is disclosed in L.
E. Arri, et. al,
"Modeling Coalbed Methane Production with Binary Gas Sorption," SPE 24363,
pages 459-
15 472, (1992) Published by the Society of Petroleum Engineers.
It is believed that the disposal saturation levels can be approached within a
solid
carbonaceous subterranean formation if there exists a method for removing the
relatively
weaker adsorbing fluid components from the formation so that more of the
stronger adsorbing
components can be introduced into the formation. The additional stronger
adsorbing
20 components will continue to sorb to the formation until the disposal
saturation levels are
approached within the sorbed phase of the matrix. One way of removing the
weaker
adsorbing components from the formation would be to intermittently or
continuously vent the
weaker adsorbing fluids from the formation. Another way to remove the weaker
adsorbing
components from the formation would be to recover them through a production
well. It is
25 believed that the formation can be saturated with unwanted gaseous fluid
components to from
10 to 99 percent of the disposal saturation levels during the operation of the
current invention;
preferably, from 50 to 95 percent; more preferably, from 70 to 90 percent.
In general, the pressure utilized by the invention is selected so as to
optimize the
sorption of the unwanted gaseous fluid component to the carbonaceous matrix of
the
formation. In general, the higher the pressure utilized, the more gas which
can be sorbed by
the carbonaceous matrix.
W0 95f27123 PC1YUS95103689
26
2~."~~a~8 .
As the gaseous fluid is introduced into a solid carbonaceous
subterranean formation, the posttion of the unwanted gaseous fluid components
within the formation, the relative concentration of unwanted gaseous fluid
components within the formation, and the ratio of unwanted gaseous fluid
components to other injected gaseous fluids are preferably monitored. One
method of monitoring the formation involves obtaining samples of effluent from
a monitor well. The samples are analyzed using methods known to one of
ordinary skill in the art, such as gas chromatography. This monitoring will
provide a relative indication of how the injected gaseous fluids are moving
within the formation and the degree to which the formation is becoming
saturated wfth the unwanted gaseous fluid component.
If the unwanted gaseous fluid component is a relatively stronger
adsorbing fluid than the other injected gaseous fluid components, then the
volume ratio of unwanted gaseous fluid component to other injected gaseous
1 5 fluid components within the effluent sampled at a monitor well will be
reduced
relative to the volume ratio of unwanted gaseous fluid component to other
injected gaseous flu'~d components within the injected gaseous fluid
introduced
into the solid carbonaceous subterranean formation.
It believed that this reduction in the ratio of unwanted gaseous fluid
components to other.injected gaseous fluid components is a result of the
preferential sorption of stronger adsorbing fluid components, such as carbon
dioxide, within the carbonaceous matrix of the formation. It is believed that
the
preferential sorption causes the relatively stronger adsorbing fluid
components
to move more slowly through the formation than weaker adsorbing fluid
components. As discussed earlier, as gaseous fluid is introduced into the
formation, the region within the formation which is saturated with stronger
adsorbing fluid is being expanded away from the injection well. The stronger
adsorbing fluid form what approximates a concentration front that advances
within tho formation. As the gaseous fluid is introduced into the formation,
the
concentration front is continually being swept from the injection well toward
a
region of bwer pressure within the formation. The enrichment of the sampled
effluent with other injected fluids will continue until the concentration
front
reaches a monitor well.
The ratio of the unwanted gaseous fluid component to other injected
gaseous fluid components collected at a monitor well should rapidly increase
once the concentration front reaches the region of the formation which the
WO 95!27123 ~ ~ ~ ~ p~117S95103689 ,
27
monitor well drains. Due to the heterogeneity of most solid carbonaceous
subterranean, the unwanted gaseous fluid component can move unevenly
within the formation. This may cause the unwanted gaseous fluid component to
become unevenly distributed within the formation. Therefore it is preferably
to
utilize more than one well to monitor the formation.
In one aspect of the invention, the introduction of gaseous fluid is
continued until the formation is saturated to a desired degree with the
unwanted
gaseous fluid component. One of ordinary skill in the art will be able to
determine the degree of saturation of a particular region within the formation
by
obtaining a gaseous effluent sample from a monitor well which penetrates the
region of the formation.
The chemical composition of the obtained sample together with
information relating to the pressure within the formation near the monitor
well
will enable one of ordinary skill in the art to determine the relative
concentrations of each of the gaseous components sorbed within the
carbonaceous matrix in the region of the formation from which the sample is
obtained. This will allow one of ordinary skill in the art to determine if the
unwanted gaseous fluid component of the injected gaseous fluid has reached
the region from which the sample is obtained. It should also allow one of
ordinary skill in the art to determine the degree to which the region of the
formation is saturated with the unwanted gaseous fluid component. The
desired degree of saturation within the formation is described more fully
above
in the description relating to disposal saturation levels.
As discussed earlier, if the unwanted gaseous fluid component is a
relatively stronger adsorbing fluid, venting the formation will allow a larger
quantity of unwanted gaseous fluid component to be sorbed to the formation for
any given disposal pressure. The venting can take place through any well
which is in fluid communication wish the formation. Venting, if utilized, may
be
pertormed continuously or interm'ttllentiy, and it may take place
simultaneously
3 0 with injection of gaseous fluid or it may Occur after the injection of
gaseous fluid
has ceased.
If gaseous fluid components, such as hydrogen sulfide, oxides of sulfur,
and oxides of nitrogen, are being disposed of, it may be preferable to reduce
the pressure within the formation sufftcientiy to desorb carbon dioxide from
~~,:~
formation, but not enough to cause hydrogen sulfide, oxides of nitrogen, ~~~0
oxides of sulfur to desorb from the carbonaceous matrix. This venting of the
W0 95127123 2$ PGTIUS95/03689
formation will allow a greater quantity of stronger adsorbing constituents,
such
as hydrogen sulfide and some oxides of nitrogen and sulfur, to be disposed of
within the formation.
The injection of gaseous fluid into the formation can be intermittent or
continuous. The injection of gaseous fluid is typically continued until the
desired pressure is reacheii. After a desired quantity of gaseous fluid has
been
introduced into the formation or the formation has attained a desired
pressure,
the injection well is shut-in and the formation is preferably maintained at
sufficient disposal conditions to maintain from 40 to 80 volume percent of the
unwanted gaseous fluid components sorbed to the formation; preferably to
maintain the unwanted gaseous fluid components sorbed to the fom~ation for at
least one year after ceasing to introduce gaseous fluids into the solid
carbonaceous subterranean formation.
Aftematively, the method of the invention is ceased after the formation
1 5 has bean saturated with the unwanted gaseous fluid to a desired degree. In
carrying out the invention, it is preferable that less than 50 volume percent
of the
total quantity of unwanted gaseous fluid component introduced into the
formation be allowed to escape to the atmosphere; more preferably less than 10
volume percent, most preferably less than one percent. The unwanted gaseous
fluid components preferably are maintained within the formation for at least
one
year, more preferably at least five years, most preferably at least ten years.
Fra-ctionation of a Mixtere of Cac_eo~c Fluids
In a further embodiment of the invention, a mixture of gaseous fluids
containing relatively stronger adsorbing fluid components and relatively
weaker
adsorbing fluid components is introduced into a solid carbonaceous
subterranean formation through an injection well in fluid communication with
the formation. The relatively stronger adsorbing fluid components of the
gaseous mixture will be preferentially adsorbed to the carbonaceous matrix of
the formation. The current invention takes advantage of this preferential
adsorption of the stronger adsorbing fluid components to the formation to
provide a method for fractionating the mixture of gaseous fluids into a first
fraction enriched in relatively weaker adsorbing fluids and a second fraction
enriched in relatively stronger adsorbing fluids. Examples of mixtures of
3 5 gaseous fluids which may be fractionated include, but are not limited to:
air, flue
gas, the gaseous mixtures produced from various industrial processes, and the
- 2~7~588
i WO 95127123 PCTIUS95I03689
29
gaseous mixtures that are discharged from a separation unit which is
separating nonflammable gases and condensible liquids from a natural gas
production stream.
In this embodiment of the invention, the mixture of gaseous fluids
typically is introduced into a solid carbonaceous subterranean formation
through an injection well'which penetrates the formation. Preferably, the
formation already has been deipleted of recoverable methane. Using a
depleted formation should provide for better fractionation of the injected
gaseous fluid. The pressure established on the formation will enhance the
fractionation of the mixture of gassoous fluids into a fraction enriched.in
stronger
adsorbing fluids and a fraction enriched in relatively weaker adsorbing
fluids. In
general, the higher the pressure on the formation, the more gas which can
adsorb to the carbonaceous matrix of the formation.
The fraction enriched in weaker adsorbing fluids (hereinaffer sometimes
1 5 referred to as the raffinatej is typii;ally withdrawn from the formation
through a
production well. The rafHnate will be enriched in relatively weaker adsorbing
fluids because the relatively stronger adsorbing fluids which are
preferentially
adsorbed by the carbonaceous matrix will travel slower through the formation
as described previously. -
The raffinate is typically recovered from the formation until the
concentration of stronger adsorbing fluids in the raffinate increases above an
acceptable level. For a mixture of gaseous fluids containing carbon dioxide,
the
volume peroentage of carbon dioxide in the raffinate is preferably maintained
less than 50 peroent, more preferably less than 20 percent, most preferably
less
than 5 percent. In some situations, it may be possible to maintain the volume
percer>tage of carbon dioxide in the raffinate at less than 1 percent.
Alternatively, the injection a~f the mixture of gaseous fluids is continued
until a desired saturation of the formation is achieved. The desired
adsorptive
saturation of the formation can be determined by routine experimentation. For
example, the mixture of gaseous fluids can be injected until the volume ratio
of
the relatively stronger adsorbing fluids within the raffinate increases above
an
acceptable level as described above. Once the desired adsorptive saturation of
the formation is reached, the carbonaceous matrix's adsorptive capaaty can be
regenerated by reducing the total pressure on the formation. A desorbed
adsorbate, which is enriched in relativeiy stronger adsorbing fluids, is
released
from the carbonaceous matrix of the formation as the total pressure of the
W 0 95l 7123 PCTIUS95103689
2~'~~~8~ ao,
formation is reduced. This desorbed adsorbate may be recovered from the
formation through an injection and/or a production well.
If the mixture of gaseous fluids which is fractionated within the formation
contains carbon dioxide, for example, flue gas, the desorbed adsorbate will be
enriched in carbon dioxide. If the gaseous mixture contains oxygen, for
example, air, the desorb~ed adsorbate will be enriched in oxygen. The
recovered desorbed adsorbate can be reinjected into a solid carbonaceous
subterranean formation. For example, if a mixture of gaseous fluids containing
carbon dioxide is fractionated within a solid carbonaceous subterranean
formation, the recovered desorbed adsorbate would be enriched in carbon
dioxide. The recovered desorbed adsorbate enriched in carbon dioxide could
be used to enhance the recovery of methane from a solid carbonaceous
subterranean formation.
It may be desirable to maintain the relatively strongly adsorbing fluids
1 5 within the formation. In this situation, the pressure on the formation is
not
reduced and the adsorptive capacity of the carbonaceous matrix of the
formation is not regenerated. Alternatively, the adsorptive capacity of the
carbonaceous matrix of the formation can be partially regenerated without
lowering the total pressure to the point where undesirable components such as
carbon dioxide, hydrogen sulfide, or carbon monoxide, if present, may be
desorbed and released from the matrix.
In general, the pressure utilized during the fractionation of the gaseous
mixture of fluids is selected so as to optimize the fractionation of the
fluid. In
general, the higher the pressure utilized, the more gas which can be adsorbed
by the carbonaceous matrix of the formation. For a given system, the faster
the
removal of raffinate from the system, the higher the volume percentage of the
relatively strongly adsorbing fluids within the raffinate.
If the gaseous mixture of fluids to be fractionated contains a large volume
peroentage of nitrogen, the resulting raffinate will be enriched in nitrogen.
Examples of gaseous mixtures of fluids which contain a large volume
percentage of nitrogen include air and flue gas. The nitrogen-enriched
raffinate
produced from these gaseous mixtures of fluids can be utilized to enhance the
recovery of methane from a solid carbonaceous subterranean formation. if flue
gas is used, it should preferably be de-watered before it is injected into the
formation. It is believed de-watering will reduce potential corrosion problems
WO 95127123 ~ ~ ~ pCT/US95/03689
31
that can result in injection equipment and welibores as a result of injecting
flue
gas into a formation.
The nitrogen-enriched raffinate is injected into a solid carbonaceous
subterranean formation at a pressure higher than the reservoir pressure of
the formation. Preferably, the nitrogen-enriched raffinate is injected at a
pressure of from about 3',447,378 Pascais (Pa) to about 10,342,136 Pa
above the reservoir pressure of this formation. If the injection pressure is
below or equal to the reservoir pressure, the nitrogen-enriched raffinate
typically cannot be injected bec~iuse it cannot overoome the reservoir
pressure. The nitrogen-enriched rafBnate is injected preferably at a pressure
below the formation parting pressure of the solid carbonaceous
subterranean formation. If the injection pressure is too high and the
formation extensively fractures, injected nitrogen-enriched raffinate may be
lost and less methane may be produced.
1 5 However, based on studies of other types of reservoirs, it is believed
that nitrogen-enriched raffinate may be injected into the formation at
pressures
above the formation parting pressure as long as induced fractures do not
extend from an injection well to a production well. In fact, injection above
formation parting pressure may be required in order to achieve sufficient
injection and/or recovery rates to make the process economical or, in other
cases, may be desired to achieve improved financial results when it can be
done without sacrificing overati performance. Preferably, the fracture half-
length of the induced fractures witlhin the formation is less than from about
20% to about 30% of the spacing between an injection well and a production
well. Also, preferably, the induced fractures should be maintained within the
formation.
Parameters important to the recovery of methane. such as fracture half-
length, fracture azimuth, and height growth, can be determined using
formation modeling techniques which are known in the art. Examples of the
techniques are discussed in Johni L. Gidley, et sl., Recent Advances in
Hydraulic Fracturing, Volume 12, Scxiety of Petroleum Engineers Monograph
Series, 1989, pp. 25-29 and pp. 76-77; and Schuster, C. L., "Detection Within
the Welibore of Seismic Signals Creiated by Hydraulic Fracturing", paper SPE
7448 presented at the 1978 Society of Petroleum Engineers' Annual
Technical Conference and Exhilbition, Houston, Texas, October 1-3.
Alternatively, the fracture half-lengith and impact of its orientation can be
PCTlUS95103689
W O 95127 23
3z .
assessed using a combination of pressure transient analysis and reservoir
flow modeling such as described in paper SPE 22893, 'Injection Above-
Fracture-Parting Pressure Pilot, Valhal Feld, Norway; by N. Ali et al., 69th
Annual Technical Conference and Exhibition of the Society of Petroleum
Engineers, Dallas, Texas, October 6-9, 1991. While it should be noted that the
above reference describes'a method for enhanang oil recovery by injection of
water above fracture-parting-pressure, it is believed that the methods and
techniques discussed in SPE 22893 can be adapted to enhance the recovery
of methane from a solid carbonaceous subterranean formation.
In general, the deeper the solid carbonaceous subterranean
formation, the higher the pressure necessary to inject the nitrogen-enriched
raffinate into the formation. Typically, an injection pressure of from about
2,757,903 to 13,789,514 Pa will be sufficient to inject nitrogen-enriched
raffinate into a majority of the formations from which ft is desirable to
recover
methane.
The nitrogen-enriched raffinate is injected into the solid carbonaceous
subterranean formation through an injection well in fluid communication with
the formation. Preferably, the injection well penetrates the methane-
containing formation, but the injection welt need not penetrate the formation
as long as fluid communication exists between the formation and the
injection well. The injection of nitrogen-enriched raffinate may be either
continuous or discontinuous. The injection pressure may be maintained
constant or varied.
A fluid comprising methane is recovered from a production well in fluid
communication with the formation. As with the injection well, the production
well preferably penetrates the methane-containing formation, but the
production well need not penetrate the formation as long as fluid
communication exists between the formation and the production well. The
production well or wells are operated in the same manner as conventional
coalbed methane recovery wells. It may be desirable to minimize the
backpressure on a production well during recovery of fluids comprising
methane through that production well. The reduction of back pressure on the
production well will assist the movement of the fluid, comprising methane,
from the formation to the wellbore.
Preferably, a production well is operated so that the pressure in the
production well at a wellbore location adjacent the methane-producing
R'O 95127123 ~ g g PCT/OS95/03689
33
formation is less than the initial reservoir pressure of the formation. The
wellbore location adjacent to the methane producing formation is within the
welibore, not the formation. The initial reservoir pressure is the reservoir
pressure near the production well ~of interest at a time before the initial
injection
of nitrogen-enriched raffinate into the formation. The reservoir pressure may
increase during the injection of nitrogen-enriched raf~nate, but 'tt is
believed that
the pressure in the production wait near the formation preferably should be
maintained less than the initial ireservoir pressure. This will enhance the
rrwvement of fluid from the formation to the welibore. Most preferably, the
pressure in a production well at a wellbore location adjacent the methane
produang formation should be less than about 2,757,903 Pa.
In some instances, back-pressure on a production welts wellbore may
be preferable, for example, when it is desirable to maintain a higher
reservoir
pressure to minimize the influx o11 water into the formation from surrounding
aquifers. Such an influx of water into the formation could reduce the methane
recovery rate and also complicate the operation of a production well.
Another situation where it csin be preferable to maintain back-pressure
on a production well's welibore is 'when there is concern over the
precipitation
and/or condensation of solids and/or liquids within the formation near the
weilbore or in the welibore itseH. The precip'ttation andlor
condensatio°~ of
solids or liquids in or near the welibore could reduce the methane recovery
rate
from a production well. Examplles of materials which may precipitate or
condense out near the welibore sand present a problem are wax containing
occluded oils which may be mobilized from the matrix and carried toward a
production wellbore. tt is believed that a higher pressure in the production
well's w~Ilbore at a location adjacent to the formation will minimize such
precipitation and/or condensation of solids or liquids in or near the
welibore.
Therefore, if precipitation and condensation in the wellbore are a problem, it
may ba profarable to increase the pressure in the production well's wellbore
to
3 0 a value as high as practicable.
The timing and magn'ttude of the increase in the rate of methane recovery
from a production well will depend on many factors, including, for example,
well
spacing, thickness of the solid cs~rbona~ous subterranean formation, cleat
porosity, injection pressure and injection rate, injected gaseous fluid
3 5 composition, sorbed gas composition, reservoir pressure, permeability of
the
R'0 95127123 PCTlITS95103689
34
2~.'~~~~8
formation, and cumulative production of methane prior to injection of nitrogen-
enriched raffinate.
When the foregoing parameters are generally held constant, a smaller
spacing between an injection well and a production well will result in a
faster
observable production well response (both an increase in the recovery rate of
methane and a shorter time before injected nitrogen-enriched raffinate appears
at a production well) than the response which occurs with an injection well
and
a production well separated by a larger spaang. When spacing the wells, the
desirability of a fast increase in the rate of methane production must be
balanced against other factors such as earlier nitrogen breakthrough when
utilizing a reduced well spacing and the quantity of nitrogen-enriched
raffinate
utilized to desorb the methane from the formation for any given spaang.
if desired, methane produced in accordance with this invention can be
separated from co-produced gases, such as nitrogen or mixtures of nitrogen
1 5 and any other gas or gases which may have been injected or produced from
the
solid carbonaceous subterranean formation. Such co-produced gases will, of
course, include any gases that occur naturally in solid carbonaceous
subterranean formations together with the methane. These gases which occur
together naturally with the methane are commonly referred to as coalbed
methane. These naturally-occurring gases can include, for example, hydrogen
sulfide, carbon dioxide, ethane, propane, butane, and heavier hydrocarbons in
lesser amounts. If desired, the methane produced in accordance with this
invention can be blended with methane from other sources which contain
relatively fewer impurities.
2 5 Examral9-1.
This example shows the predicted response of a coalbed when various
desorbing fluids are injected into the coaibed to enhance the recovery of
methane from the coalbed. In this Example injection is commenced at the one
year point. All the desorbing fluids in this Example are injected into the
coalbed
at an injection pressure of 13,789,514 Pa. The desorbing fluids injected into
the formation include:
pure nitrogen;
flue gas having 85 volume percent nitrogen and 15 volume percent
carbon dioxide;
an equimotar mixture of carbon dioxide and nitrogen;
CA 02176588 2000-07-06
r
a desorbing fluid having 85 volume percent carbon dioxide and 15 volume
percent
nitrogen; and
pure carbon dioxide.
The data graphed in FIGS. 1-7 are generated from a model which was developed
to
S describe a hypothetical coalbed which is 3.05 meters thick and is
homogeneous throughout
in both the vertical and horizontal directions. The data graphed are corrected
to a temperature
of 15.6° and a pressure of 101,353 Pa. The hypothetical coalbed has the
following
characteristics:
permeability = 10 millidarcies;
10 porosity = 0.5%
reservoir pressure of 10,342,136 Pa prior to injection of desorbing fluid; and
reservoir temperature = 46.1 °C.
The coalbed is saturated with methane and the area drained by the production
well
is a 186,155 square meter region of the formation. In the model, it was
assumed that the
15 production well was surrounded by four injection wells which are arranged
in a five-spot
formation. It is assumed that each injection well affects the production well
in the same
manner and that therefore one quarter of the response in the production well
is attributable
to each injector. The cumulative desorbing fluid injected into the formation
being drained
by the production well comes from the four injection wells. Each injection
well contributes
20 a quarter of the total desorbing fluid injected. FIGS. 1 through 7 show the
predicted gas
recovery rate (thousand standard cubic meters per day (MSCM/Day) and the
predicted
cumulative gas recovered (million standard cubic meters (MMSCM)).
The model utilized was developed using two-dimensional Virial equations of
state.
A description of the Virial Equations of State and how to utilize them to
produce a model
25 similar to the one used by the inventors' is disclosed in defiance,
"Multicomponent high-
pressure adsorption equilibrium on carbon substrates: theory and data," Fluid
Phase
Equilibria, 78, pages 99-137, (1992) Elsevier Science publishers B.V.,
Amsterdam.
As can be seen from FIGS. 1 and 5, the volume percentage of carbon dioxide in
the
fluid recovered from a production well is maintained at levels below the
volume percentage
30 of carbon dioxide contained in the injected desorbing fluid for an extended
period after
injection of desorbing fluid is
~.~ ~ 127123 PCTlUS95f03689
36
commenced. The volume percentage of carbon dioxide in the recovered
effluent starts to increase substantially at approximately the same time as
the
volume peroentage of methane in the recovered effluent starts to decrease. As
can be seen from FIGS. 2 and 5, a substantial percentage of the available
methane contained in the formation will have been recovered by the time the
volume peroentage of carbon dioxide in the recovered effluent increases to
above the volume percentage, of carbon dioxide in the injected desorbing
fluid.
Also, since methods are available to economically separate carbon dioxide
from methane, nitrogen, and other gases, carbon dioxide can be separated from
1 0 the effluent recovered from a production well and injected back into the
coalbed
andl or another nearby coalbed.
FIGS. 1,-7 also show that a substantial percentage of the available
methane can be recovered from the region being drained by a production well
before the volume ratio of carbon dioxide to other injected desorbing fluid
1 5 components contained in the effluent recovered from a production well
reaches
the magnitude of the volume ratio of carbon dioxide to other injected
desorbing
fluid components contained within the injected desorbing fluids.
It should be noted that since the model in the above example and the
example that follows are idealized, they cannot take account of
heterogeneities
20 present in an actual solid carbonaceous subterranean formation. Therefore,
this model and the model described in Examples 2 and 3 do not predict the
streaking which may oxur within the formation. However, the examples
disclosed together with the earlier discussion regarding the. mitigation of
streaking within a formation will enable one of ordinary skill in the art to
practice
2 5 the invention.
Eusmgls32
This example shows the predicted response of a coalbed when
desorbing fluids containing carbon dioxide are injected into the coalbed to
enhance the recovery of methane from the coalbed. Injection of desorbing
3 0 fluids was commenced in this Example after four years of primary
depletion. All
the desorbing fluids in this Example are injected into the coalbed at an
injection
pressure of 13,789,514 Pa through injection wells having a skin of -3. The
production well had a downhole production pressure of 689,476 Pa and a skin
of -3. Skin is a measure of the permeability of the near wellbore formation. A
3 5 positive skin indicates near wellbore formation damage and a negative skin
CA 02176588 2000-07-06
.,
37
indicates near wellbore formation stimulation. The desorbing fluids injected
into the formation
include:
pure carbon dioxide; and
a desorbing fluid having 70 volume percent carbon dioxide and 30 volume
percent
methane.
The data graphed in FIGS. 11-18 were generated from a model which was
developed to
describe a hypothetical coalbed which is 15.24 meters thick and is homogeneous
throughout in
both the vertical and horizontal directions. The data graphed are corrected to
a temperature of
15.6°C and apressure of 101,353 Pa. The hypothetical coalbed has the
following characteristics:
permeability = 5 millidarcies;
reservoir pressure of 10,342,136 Pa prior to injection of desorbing fluid; and
reservoir temperature = 46.1 °C.
The coalbed is saturated with methane and the area drained by the production
well is a
647,497 square meter region of the formation. In the model it is assumed that
the production
well is surrounded by four injection wells which are arranged in a five-spot
formation. It is
assumed that each injection well affects the production well in the same
manner and that
therefore one quarter of the response in the production well is attributable
to each injector. The
cumulative desorbing fluid injected into the formation being drained by the
production well
comes from the four injection wells. Each injection well contributes a quarter
of the total
desorbing fluid injected.
The model utilized was developed using the extended Langmuir adsorption
isotherms
model. A description of an extended Langmuir adsorption isotherm model and how
to utilize
it to produce a model similar to the one used by the inventors' is disclosed
in L. E. Arri, et. al,
"Modeling Coalbed Methane Production with Binary Gas Sorption, " SPE 24363,
pages 459-472,
( 1992) Published by the Society of Petroleum Engineers.
This example illustrates that in some situations, which would be known to one
of ordinary
skill in the art from the foregoing disclosure, that a desorbing fluid
comprising carbon dioxide
and methane is preferably utilized to recover methane from a solid
carbonaceous subteranean
formation, especially if the cost of separating methane from a recovered
effluent stream is not
economically viable. In this situation, the effluent is reinjected into a
formation which will
W0 95127123 PCT/US95/03689
38
C
2 ~-~ ~ '1~~ rb the carbon dioxide components, of the scream and allow the
methane to
pass to a production well where it is recovered.
This example uses the same type of modeling techniques and
parameters as utilized in Example 2. However, in this example an unsaturated
coalbed is utilized and a mixture of gaseous fluids having 15 volume peroent
carbon dioxide and 85 volume peroent nitrogen is injected into the coalbed.
The injection of gaseous fluids was commenced at time zero. FIGS. 8-10 of the
example show that a solid carbonaceous subterranean formation can effectively
provide a nitrogen-enriched effluent when a mixture of gaseous fluids
containing nitrogen and carbon dioxide is introduced into the formation. The
model predicted that the volume percent of nitrogen in the recovered effluent
would be 100% during the entire period graphed by FIGS. 8-10 that the volume
percent of carbon dioxide in the effluent recovered would not increase to
above
0.01 % during the entire period graphed by FIGS. 8-10.
It fs believed that a soled carbonaceous subterranean formation will also
provide a nitrogen rich effluent when air is introduced into the formation
through
an injection well and removed through a production well.
From the foregoing description, it will _be observed that numerous
variations, aftematives and modifications will be apparent to those skilled in
the
art. Accordingly, this description is to be construed as illustrative only and
is for
the purpose of teaching those skilled in the art the manner of carrying out
the
invention. Various changes may be made and materials may be substituted for
those described in the application. For example, it is believed that a gaseous
fluid which will chemisorb to the carbonaceous material of a formation may be
disposed of in the formation in a similar method as disclosed for the disposal
of
a strongly adsorbing fluid within the formation.
Thus, it will be appreciated that various modifications, aftematives,
variations, etc., may be made without departing from the spirit and scope of
the
invention as defined in the appended claims. It is, of course, intended that
all
such modifications are covered by the appended claims.