Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02176946 2003-12-11
WO 95117830 ~ PCTlCA94/0072(
PROCESS FOR MAKING UNDENATURED WHEY PROTF~IN
CONCENTRATE
This invention relates to an improved process for producing a whey
protein concentrate having a serum albumin content of about 9 q6 or more.
10 Background of the Invention and Objectives
As early as 1982 Bounous et al (1) showed that dietary whey protein
concentrate (WPC) improved the active systemic humoral immune response in
a mammal, as measured by sheep red blood cell injections. It was however
found that the use of high temperature pasteurization of milk [in 1988 and
subsequent years following a Salmonellosis epidemic in Europe) greatly
reduced the effectiveness of commercially available whey protein concentrates
in improving the immune response.
United States Patent 5,230,902 dated July 27, 1993 described a method
of improving the humoral immune response or increasing the concentration
levels of glutathione in mammals, comprising administering orally to a
mammal a therapeutically or prophylactically effective amount of undenatured
whey protein concentrate. International Publication No. WO 94/13148,
publication
date June 23, 1994 as continuation in part of previous related U.S.
applications
provided an improved method for making undenatured whey protein concentrate.
In said related applications the discovery that undenatured whey protein
concentrate had an enhanced immunological effect was presented. It was
furthermore explained that in the conventional high temperature treatment of
milk the thermosensitive protein serum albumin were partially heat denatured
and hence precipitated in the curd. Said related applications describe
experiments where WPC was prepared using the lowest level of heat treatment
of milk compatible with safety standards, so as to obtain a whey protein
distribution having a high content of the thermolabile serum albumin. It was
1
WO 95117830 PCTICA9.1I00726
2176946
found that the presence of the 6-glutamylcysteine (Glu-Cys) group/molecule in
the serum albumin (BSA) and the specific intramolecular disulfide bond in
cystine
related to the undenatured conformation of the molecule, were a key factor in
the glutathione (GSH) promoting activity of WPC (enhancement of GSH levels
in tissues). They are believed to represent the common denominator . '
underlying the beneficial effect of WPC (2,3).
Dietary W.P.C. produced with lower levels of heat treatment improves
systemic humoral immune response, increases the rrsistance of target cells
against the effect of carcinogens including chemical carcinogens such as
dimethylhydrazine, improves'resistance to pneumococca~l infection, and
provides a sustained increase of tissue (intracellular) glutathione.
The object of this invention is to provide an improved process for
prepauing a whey protein concentrate that has adequately low bacterial levels
without excessive levels of protein denaturation. It is a particular object to
provide a process that will achieve a whey protein concentrate having a serum
albumin of about 99& and adequate bacterial reduction. This approximately
9 % level of serum albumin was found in our studies to be important for the
achievement of sustained increase of tissue glutathione for the properties
such
as improved systemic humoral immune response described above (References
2 and 3).
It is a further object to provide a process that will achieve a whey
protein concxntrate having not only a high serum albumin content but also a
high content of other Glu-Cys containing proteins or Glutathione homologues
such as (Glu-Cys-Ala). The use of process conditions to give. a high serum
albumin coolant will also give a high content of such other Glu-Cys containing
proteins.
Serum albumin is the most easily denaauod serum protein since its
denaturation is not as reversible as that of alpha-lactalbumin (4). It
therefore
is a further object of this invention to maintain rigorous control of
temperature
and other conditions to minimize denaturation of serum albumin.
The casein in raw milk occurs in the form of a colloidal dispersion.
The particles of this dispersion range from 20 to 200 nm (nanometers) in
2
.. -.'va
W O 95/ I 7830 217 6 9 4 6
PCTICA94/00726
diameter and are generally referred to as casein micelles.
The industrial methods of separation of the casein are based on the
destabilization of these proteins either by lowering the pH of milk to the
isoelectric point (pH 4.~ ai 20°C or by enzymatic (rennet) hydrolysis
of the
Kappa casein which stabilizes the micelles.
The first procedure is not suitable for the recovery of native whey
proteins since low pH has been shown to denature Bovine serum albumin,
apparently because of repulsion of acidic amino acids (Haurowitz, 1963) (11).
Furthermore, if excessively high temperatures are used in this procedure,
there would be a considerable increase in the denaturing effect of a low pH.
The second procedure is less economically feasible because the caseins
recovered are less functional for dairy industry use.
Considering those facts and other economical aspects, our objective is
to develop a combination of method~steps which are compatible with cheese
making. The cheese represents roughly 1096 ('by weight) of milk being
treated and the whey represents approumately 9096, of which 0.6 to 0.796
consists of whey proteins.
A recent study (5) has shown that a microfiltration membrane
containing pores with average diameter of 0.2 micron will selectively retain
the casein micelles from skim milk. However, that study concentrated on the
separation of casein.
T6e Invention
We propose to utilize low temperature lenient techniques to achieve the
objectives of this invention.
In accordance with this invention a process is provided for producing
an undenatured whey protein concentrate having a serum albumin content of
about 9 96 or more as a by-product of a process for making cheese, preferably
cheddar cheese, comprising:
(1) cold standardisation of the fat content in milk at a temperature
not greater than about 4°C. By this approach we reduce the outgrowth of
bacteria; furthermore this method reduces fat losses and breakdown of fat
globules which would lead to further reactions involving enzymes and
3
WO 95/17830 2 ~ 7 6 9 4 6 PCT/CA94/00726 _.
bacterial metabolism;
(2) pasteurization under conditions that will avoid any substantial
denaturing of the protein in the milk;
(3) chilling preliminary to cheese production to a temperature of
about 30°C.
(4) making curd and whey at a temperature not greater than about
40°C;
(5) separating the curd firom the whey;
(6) removing excess fat from the whey;
(7) pasteurisation, if needed, at a high temperature for a time short
enough to avoid denaturing of most of the protein in the whey;
(8) ultrafiltration at a temperature close to but not substantially in
excess of 40°C. to provide a retentate; which may provide an end
product in
liquid form.
(9) where a dry powder is desired, drying said retentate, preferably
by freeze drying at a temperature and for a time that will not denature the
protein.
The pH is maintained at not Iess than 6 throughout steps 1 to 8.
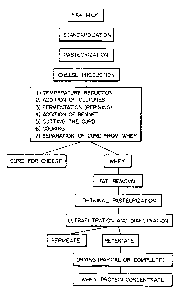
grief descriytion of the drawing
rigure 1 is a schematic representation of the process of this invention
for producing a whey protein concentrate with immunoenhancing properties.
Detailed descri_cition of the invention
Materi and Methods
Individual whey proteins were measured by polyacrylamide gel
electrophoresis. Samples of concentrated whey were applied on 1696
polyacrylamide at pH 8 (Iaemmli- buffer-system) after the samples were
reduced with 1096 2-mercaptoethanol. Samples were applied so that each slot
received 10-20 ~g (micrograms) of protein. Electrophoresis was performed at
200 volts for 70 minutes. The results were also confirmed by
chromatography.
Extent of protein denaturation by the process was determined in
triplicate by the nitrogen solubility index (NSI) at pH 4.6 and 3000 g.
4
WO 95!17830 ~ ~ PCT/CA94/00726
(Association of Official Analytical Chemists (AOAC) 1985 ref. 6). The
method utilized in these experiments differs from that described in Reference
2 and represents a more accurate reflection of the undenatured state of
protein.
Protein content (N X 6.38) and total lipids of samples were determined
in duplicate respectively by the standard method of Kjeldahl and the method of
Moj onnier.
Moisture content was determined in duplicate by AOAC method (7).
Total coliforms count was determined following incubation at 37°C
for
18 h in brilliant green using the most probable number method. Total bacteria
count (aerobic mesophiles) was determined following incubation at 32 °
C for
48 h in PCA medium. Both methods are approved by the International Dairy
Federation and American Public Health Association.
Lactose was measurod by the enrymatic method.
~I~aa~s~l~,i,Qp
keferring now to Figure 1, the first step is the standardization of the
milk. This involves skimming the milk to a desired fat content. This
procedure is used for two reasons. The first is to attain the legal percentage
of fat on a dry basis of the fatal cheese. The second is to achieve the best
yield 2nd quality (body and texture) of cheese.
For cheddar cheese the optimum fat to casein ratio in milk is one part
fat to 0.7 part of casein (Kosikowsld)(9).
The usual practice has been to use a temperature in the range of
50°C
to 65 °C as this is the most efficient range for skimming to a level of
0.05 ~
of fat. However, we prefer in accordance with this invention to maintain the
temperature at a lower level, preferably not greater than about 4°C.
This low
temperature during the fat removal process is necessary to avoid oxidation of
lipids which would then tend to continue over time during storage and produce
rancidity (liberation of fatty acids) which could in turn damage the
conformation without affecting nutritional efficiency of the labile proteins.
The raw milk used in the examples had the following composition:
Composition
5
WO 95/17830 217 b 9 4 6 PCT/CA94/00726
g6 protein 3.2
9b fat 3.65
96 lactose 4.7
The pH of the composition was 6.65.
In the examples described in this application standardization of milk
was carried out with a cold separator (Alfa Laval, CMRPX 714-HG~
coupled with an automatic standardizes (Alfa Laval, Alfast Model 110). All
standardization steps were carried out at 4°C. (Figure 1) and for all
examples
the fat content was adjusted to 3.58% of total milk.
The fat content of 3.589 was in accordance with standardization
according to the following calculation:
9& fat - 9~ protein X 1.12
(in final product) (in raw milk)
3~ fat - 3.2 X 1.12
P
The next step according to Figure 1 is pasteurisation. In the examples
in this application pasteurization of the milk was achieved on a heat
exchanger
type HTST (Alfa Laval H-10) being set at 72.6°C with a retention time
of 16
sec. , followed by flash cooling to 30 ° C .
Most countries do not allow the production of dairy products without
pasteurization. Different types of pasteurization can be used:
HTST high temperature short time - 72.8°C, 16 sec.
LTLT low temperature long time - 65.6°C, 30 min.
UHT ultra high temperature - I20°C for a few seconds;
but the effect on denaturation of protein and especially serum albumin
are mostly evident with LTLT and UHT.
The introduction of high temperature pasteurization of milk was
prompted by two different objectives. The first was to obtain a near
sterilization of milk following the cheese related infection diseases reported
during 1988 in Europe. The second objective was to improve the yield during
cheese making.
In fact, cheese makers have noticed that at these temperatures, or
6
WO 95/17830 217 6 9 4 6 PCT/CA94/0072b
increasing by five degrees or less above pasteurizing temperature, yield could
be increased in certain types of cheese.
In accordance with the teaching of this invention ultra high
temperatures should be avoided, contrary to present practice. Long time
ezposure to a lowest temperature of the order of 65.6°C should also be
avoided. Even a temperature as low as 55°C causes unfolding of the
bovine
serum albumin molecule over a period of time (along & Call. 1988).
However, this invention may use high temperatures in the range
63°C to
75°C and preferably about 72°C for a short time up to about 20
seconds.
The conditions such as time and temperature must avoid denaturing of the
protein in the milk, particularly the heat labile serum albumin, and other Glu-
Cys containing proteins, however such conditions of pasteurization should be
such that pathogens are killed or reduced to an acceptable level.
Cheese Production - Temoe,~ture RedLdion
The next step according to Figure 1 is cheese production.
The preliminary step in cheese production is to reduce the temperature.
In accordance with the examples of this invention the temperature is
accordingly immediately reduced, such as by flash cooling to a temperature of
30°C for cheese malting.
Cheese ~roductioL-~additison of cultures
Since cheese malting is a fermentation process, we add lactic acid
cultures. Those cultures and their metabolites are mainly responsible for the
acidification and repining of cheese. For cheddar choese we use a commercial
culture of mesophilic organisms which in our ezamples are provided by CH.
Hansen Laboratory. Suitable cultures are readily available from other sources
such as Miles Laboratory. The amount of cultures that has been added was
196 of total milk being transformed in cheddar.
These cultures are added after chilling to about 30°C, which
allows the
necessary growth of mesophilic lactic acid bacteria for cheese making. The
activity of these bacteria will be controlled in such a way that the pH of the
milk will never drop below pH6. Since bacteria are living organisms their
rate of metabolism may vary. If it is too rapid the amount of bacteria added
7
WO 95/17830 217 6 9 4 6 PCTlCA94/00726
to the milk can be reduced, eg. from 196 of total milk to 0.7596. With less
bacteria less acid is produced.
Another way of controlling the rate of metabolism is to reduce the
fermentation temperature. A reduction of 2°C will normally be used for
this
purpose.
At this preliminary stage of cheese making calcium chloride is usually
added to milk to increase the firmness of the curd during the manufacture of
cheese. The net result is an increase in cheese yield due to better
procipitation
and less losses of cheese curd particles in the whey. However, we have found
that denaturation of BSA is enhanced by the prcsencx of calcium ions.
Therefore we avoid the addition of calcium ions, contrary to normal practice
in cheese manufacture. Reference is also made to the report of Shimada &
Matsushita 1981 (8).
Additives of this sort are avoided during production of cheese if the
whey generated is being used for this invention. Yield can also be increased
in conventional practice by the addition of milk derivatives (casein, whey
protein) to milk being transformed into cheese. This procedure is generally
avoided by us because it influences the quality of the protein content of the
whey. The various fraction found in normal whey will be modified resulting
in a whey protein concentrate having less undenatured bovine serum albumin
per unit of total protein.
Cheese Production - fermentation
The fermentation period (repining period) was 1.5 hour for our
ezamples at a temperature of 30°C. The pH decreased from 6.b5 to 6.55.
The pH drop was thus 0.1 before rennet was added.
Cheese producing - Addition of rennet
Rennet was added at a rate of 20 m1/100 liters of milk. The time
required for curd formation was 25 minutes.
The rennet used was a pure calf rennet single strength from CH.
Hansens Laboratory. The quantity was 20 ml./100 liters of milk. The
temperature is maintained at 30°C. The rennet was diluLai in 10 times
its
volume with water before adding to milk. The clotting time was 25 minutes.
8
WO 95/17830 217 6 9 4 6 PCT/CA94100726
Cheese rroduction - culturing the curd
The curd was then cut using 5 mm. cheese knives at a temperature of
30°C to pmvide 6 mm cubes and stirred for 15 minutes before it was
cooked.
In our examples the cooking period lasted 75 min and continuous
agitation was used. The peak temperature of 38°C was reached in 30
minutes
( 1.3 ° C per 5 minutes). After this cooking period agitation was
maintained for
1 hour.
The pH of the whey after cooking was 6.5.
It is highly desirable to avoid any cheese malting steps that involves a
temperature in excess of 40°C. In fact some cheese production such as
Emmental requires a temperature of more than 50°C. Raising the
temperature
to this level before separation of the whey and maintaining such temperature
would adversely affect the serum albumin content.
Following post stirring the curd is s$parated from the whey.
The curd should be separated from the whey at this level.
No additional whey is collected for the purposes of this invention
during shaping and final pressing of the curd as acidification during this
period would result in a pH drop below 6.
The whey should be chilled to about 4°C as soon as it is separated
from the curd if it is to be kept for more than 1/2 hour. By this procedure,
the metabolism of the lactic acid bacteria is reduced and acidity does not
increase. No additives (such as HZO~ should be used as antibacterial agents.
Instead we use low temperature inhibition of bacterial metabolism. The pH of
the whey should never be below pH=6 before it is concentrated.
Whey - fat removal
The whey that has been collected is first pumped at 38°C in a
centrifugal separator to take out excess fat (Alfa-Laval M~iIviR.PX-214TG~
that was present during cheese production. The amount of fat is reduced to a
level of 0.06 96 in the resulting whey. The Ph in our examples was at 6.48.
The whey is then chilled to 4°C and stored till ultrafiltration
(LT.F.) is
9
WO 95117830 217 6 9 4 6 PCT/CA94/00726
carried out. The temperature is then raised at 40°C for the
IJltrafiltration
(using Romicon cartridge with a cut off of 50,000 Dalton). If necessary it can
be given a socond pasteurization as shown in Figure 1 under conditions similar
to the pasteurization previously described.
W6e~ ~r~,eurization
In our examples, whey was pasteurized. This heat treatment was
mainly applied to control the activity of lactic acid bacteria that was
previously added to milk. It also serve to control post pasteurization
contamination that could occur during cheese malting.
1 rafiltray~
During ultrafiltration the retentate is submitted to diafiltration by
adding water so as to reduce the lactose level (4. 6 9b ) so that the
retentate
from ultrafiltration contains less than 19b of lactose. The retentate
following
completion of ultrafiltration has a total solids of 19-20 % .
The conditions of ultrafiltration are set forth below in Table 1.
TEMPERATURE <_ 40 ° C
Transmembrane pressure ('T'MP) 1.0 BAR
TANGFNZTAL
VELOCTTY > 7m/s
Concentration factor (CF) 29
FLUX 24.5 1 /m2/h
The membrane flux increases 2-2'h per degree centigrade, giving a
similar increase in capacity of a plant. This means that without special
reasons for operations at low temperature, it is an advantage in conventional
practice to operate at as high a temperature as possible.
The temperature utilized in most other commercial methods during this
procedure is 50°C. This level of temperature facilitates a higher flux
through
the membranes hence morc retentate production per unit of time and per unit
of membrane. In our method, the draw-back of less production per unit of
membrane is compensated by increasing the membrane surface.
The objective of not exceeding 40°C is obtained throughout the
system
WO 95/17830 21 l 6 9 4 ~ PCT/CA94/00726
by fine tuning the points of input and output in the system so as to avoid a
heat producing unbalance between the two. After ultrafiltration the
temperature of the retentate is then lowered to 4°C and kept at that
temperature till freeze drying is started.
The composition of the retentate is about 19-2096 total solids. A
typical composition is:
Fat 2.09 96
True pmtein 15.91
Non protein nitrogen 0.04
Lactose 0.84
Ash 1.0
Total Solids 19.8896
Vitamins and flavours may, if desired, be added to the retentate after
ultrafiltration. The retentate may be regarded as a final product and sold in
liquid form. Alternatively, the retentate can be concentrated to provide a dry
product as described below.
Whey - Freeze dca~
Concentration to produce a dry product by lyophilization (freeze
drying) is performed at temperatures under O°C for 15 to 18 hours. This
does not denature the thermolabile proteins.
In our examples, the retentate was subjected to a blast freeze at -
25°C
before entering in the freeze dryer. The temperature of the condenser were
maintained at -50°C during the 17 hours of the freeze drying period.
The microbial counts of the retentate compare favourably with
standards applicable to conventional pasteurization. These standards differ in
each jurisdiction. As an example, the Province of Quebec, Canada, requires
that total bacteria count (aerobic mesophiles (32°C) be maintained
below
50,004 (log 4.69), both in the factory and in the final product in the case of
powdered milk products. Coliforms are to be below 10. The Province of
Quebec has a standard of a bacteria count of 25,000 (log 4.39) and a coliform
count of 5 in the factory for milk products that have not been pasteurized or
11
WO 95117830 21 l 6 9 4 6
PCT/CA94/00726
fermented.
Table 2 illustrates the composition of whey protein concentrate powder
obtained using the principles described above.
Certain factors cannot be controlled during normal production of whey
concentrate powders. Seasonal variation of milk composition and bacterial
metabolism that occurs in milk at each step of the cheese making process will
be mainly responsible for the differences observed in the composition of the
concentrate. However, the practice of the principles of this invention may be
expected to produce a consistently high level of thermolabile proteins such as
serum albumin and to avoid substantial loss of the glutamylcysteine groups in
the whey proteins.
12
WO 95/1?83~ 2 l l 6 9 4 6 pCT~CA94100726
TABI~
COMPOSITION OF WILY PROTEIN CONCENTRATE POWDER
F~cample 1 Example 2 Example 3
PROTEIN ( % ) 77. 5 77.04 78.08
a Lactalbumin ( % 23 .68 23. 85 22.20
)
~ Lactoglobulin ( 59.90 60. 37 61.40
% )
Serum Albumin ( % 11.11 9.67 11.35
)
Others ( % ) 5 . 31 6.14 5.05
(immunoglobulin,
lactoferrin, etc.)
FAT ( % ) 10. 9. 76 8.12 i
LACTOSE ( % ) 4.25 4. 6 4.9
MOISTURE ( % ) 4. _ 4. 4.
(maximum)
OTHER S
(mfg)
Ca 5.06 4.78 4.84
Mg 0.66 0.65 0.66
Cu 3.05 2.48 3.05
Zn 8.76 7.12 9.35
Na 4.23 4.67 3.53
K 6.45 6.35 4.05
C1 10.82 11.70 9.59
MICROBIOLOGY
Salmonella (/ 100g) No growth No growth No growth
Coli (/ 100g) < 50 < 50 < 50
Staphyloccoccus (/g) < 50 < 50 < 50
Total count (/g) < 10000 < 10000 < 10000
NITROGEN SOLUBILITY 99 % 98.9 % 99.5 %
INDEX
-13-
WO 95/1783b 217 6 9 4 6 p~pA94100726
The protein composition and solubility of the final product in powder
form after concentration by ultrafiltration and lyophilization (Table 2) meets
the requirements previously identified by us as essential for the development
of immunoenhancing activity and tissue GSH promotion: serum albumin
concentration around 99b and minimal degree of denaturation. In each of the
examples the serum albumin is over 9.5%. In Table 3 and Table 4 are
presented for comparison the concentrations of serum albumin in current
commercially available W.P.C.'s and the nitrogen solubility indez determined
by De Wit in some W.P.C. products.
TABLE 3
WHEY PROTEIN BOVll~IE SERUM ALBUMIN
~ONCEN1'RA~ - in qb of Total Whey Protein
Promod' 4 + 1
Alacen 855' 4 t 1
Lacprodan-80' 4.8 ~ 2
Sapro' 4 0.1
Savorpor-75' 4 t 1
Bioisolate' S + 1
Promix' 3 t I
Mean t SD
*trade-marks
14
WO 95/17830 217 6 9 4 6 PCT/CA94100726
w~Y r~to'rEn~1
CONCIE~A~ NsI at ~iH
Normal CTF WPC a.G
83 %
Neutral UF-DF-WPC 78 96
Acid UF-DF-WPC 42 %
De-fatted OF WPC 91 %
Spherosil* ' OMA' 79 %
Spherosil* 'S' WPC 35 %
Vistec* WPC 35 96
Demin. de-last. WPC 72 %
From De Wit J.N. et al
Neth. Milk Dairy J. 37 (1983) pp. ~7-49
The process of this invention therefore provides a practical procedure
for making undenatured whey protein concentrate. Furthermore it has the
advantage of using a by-product of cheese production which is otherwise
considered to be a troublesome waste product, and a potential pollutant. It
should solve what was wntil now a continuing financial problem for the dairy
industry responsible for the disposal of this major waver pollutant.
In conclusion, it is the objective of this invention to preserve intact the
conformation of the labile whey proteins in the W.P.C. This objective of
leniency is obtained through several inter-dependent steps involving
temperature, ions content, ultrafiltration flux and drying techniques.
* Trademarks
WO 95117830 217 6 9 4 6
PCT/CA9a/00726
REFERENCFS
(which are incorporated by reference in their entirety)
1. Bounous G., Konshavn P.A.C. "Influence of Dietary Proteins on the
Immune System of Mice". J.Nutr. 112, 1747, 1982.
2. Bounous G., Gold P. "The Biological Activity of Undenatured Dietary
Whey Proteins: Role of Glutathione" Clin.Invest.Med. 14: 296-309, 1991.
3. Bounous G., Batist. G; Gold P. : "Immunoenhancing Property of
Dietary Whey Protein in Mice: Role of Glutathione". Clin. Invest. Med. 12:
154-61, 1989.
4. Brown R.T. "Milk Coagulation and Protein Denatiuation in
'Fundamentals of Dairy Chemistry'", 3rd Edition, N.P. Wong (Ed) Van
Nostrand Reynold C. (Publ.) New York, 1988, pp.583-607.
5. Fauquant, J.; Maubois, J.L.; Pierre, A. "Microfiltration du fait sur
Membrane Minerale" Tech.Lait 1028, 21-23, 1988.
6. AOAC 1980,' "Official Methods of Analysis" 13 Edition Association of
Official Analytical Chemists, Washington, D.C.
7. AOAC 1985, "Official and Tentative Methods of the American Oil
Chemist Society, Official Methods", Ball-65, Revised Edition.
8. Shimada K, Matsushita S.J. Agric. Food Chem. 1981, 29, 15-20.
9. Kosikowsld F. (1977), Cheese and Fermented milk Foods, 2d Edition,
F.V. Kosikowski & Associates, N.Y.
16
WO 95/1783(1 2 ~ ? 6 9 4 6 pCT/CA9a/00726
10. along N.P., Jenness R., Kenney M; Marth E.H. 1988, Fundamentals
of Dairy Chemistry, 3d Edition, Van Nostrand Reinbold, N.Y.
11. Haurowitz F. "Albumins, globulins and other proteins" in The
Chemistry and Functions of Proteins, (Academic Press NY 1963).
17