Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 95/18381 ~ PCT/US94/14902
1
IMMUNOASSAYS FOR PROSTATE SPECIFIC ANTIGEN
FIELD OF THE INVENTION
The present invention relates to the field of cancer immunoassay.
Specifically, a new immunoassay method for prostate specific antigen (PSA) is
presented. Also presented is a PSA-anti-PSA complex which resembles a
complex of PSA and al-antichymotrypsin (ACT) that can be used as a
calibrator and/or control in an immunoassay for PSA. Further presented is a
method for fractionating anti-PSA polyclonal antibodies, into those which bind
epitopes that are masked by the binding of PSA to ACT and those which do
not bind such epitopes.
BACKGROUND OF THE INVENTION
Prostate specific antigen (PSA) was first described by Wang, M. C., et
al., Invest Urol. 17:159 (1979). It is a secretion of prostate epithelium and
is
also produced by prostate cancer cells. PSA was characterized as a
glycoprotein monomer of 33-34,000 dalton molecular weight with protease
activity (Wang, M.C., et al., supra and Ban, Y, et al., Biochem BiophXs Res
Commun. 123:482 ( 1984)). More recently the amino acid sequence of the
antigen has been reported (Watt, W.K., et al., Proc Natl Acad Sci USA.
$x:3166 (1986)) and the gene for PSA has been cloned (Lundwall, A.,
Biochem Biophys Res Commun. 161:1 151 (1989)). Development of an
enzyme immunoassay by Kuriyama, et al., made it possible to detect low
concentrations of PSA in the blood of patients with malignant and benign
prostate disease and a significant proportion of normal males (Kuriyama, M. et
al., Cancer Res. 40:4658 (1980)).
PSA testing can have significant value in detecting metastatic or
persistent disease in patients following surgical or medical treatment of
prostate cancer (Lange, P.H., et al., Urology 33(6 suaal):13, (June, 1989);
and Killian, C.S., et al., Cancer Res 45:886 (1985)). Persistent elevation of
PSA following treatment or an increase in the post-treatment baseline PSA
level is indicative of recurrent or residual disease (Brawer, M.K., et aL,
rolo
33 5 Suaoll:l 1 (May, 1989); Siddal, J.K., et al., Eur Urol 12:1:3 (1986);
Stamey, T.A., et al., N Engl J Med 317:909 ( 1987); Lange, P.H. et al., J
Urol.
WO 95/18381 L~ ~ j~ ~ ~ 4 PCT/U594114902
2
141:873 (1989); Stamey, T.A., et al., J Urol. 141:1076 (1989); Stamey,
T.A., et al., J Urol 141:1084, ( 1989); Stamey, T.A., et al., J Urol 141:1088
(1989); and Chan, D.W., et al., Clin Chem 33:1916 (1987)).
S PSA testing alone is not recommended as a screening procedure in the
general population nor as a guide in disease staging. Instead, it is widely
accepted as an adjunctive test in the management of prostate cancer
patients (Kuriyama, M. et aL, J Natl Cancer Inst 68:99 (1982); Stamey, T.A.,
et al., N Enql J Med 317:909 (1987); Lange, P.H. et al., J Urol. 141:873
( 1989); Stamey, T.A., et al., J Urol. 141:1076 ( 1989); Stamey, T.A., et al.,
J_
rol 141:1084 (1989); Stamey, T.A., et al., J Urol 141:1088 (1989); Chan,
D.W., et al., Clin Chem 33:1916 (1987); and Oesterling, J.E., et al., rol
~: 766 (1988)).
Measurements of the serum concentration of PSA have now found
widespread use in monitoring of patients with prostate cancer, although
increased serum concentrations of PSA have also been reported in benign
prostatic hyperplasia and secondary to surgical trauma of the prostate (Duffy,
Ann Clin Biochem, (1989); Brawer, et al., Urology Sup_pl. (1989)).
PCT patent application WO 92/01936, "Assay of Free and Complexed
Prostate Specific-Antigen (PSA)" to Lilja, H. et al., published February 6,
1992, discloses immunoassays for free PSA as well as PSA as a proteinase
inhibitor complex. The free PSA and the PSA complex are measured by a non-
competitive immunoassay employing at least two different monoclonal
antibodies. The invention is further characterized in that the PSA proteinase
inhibitor complex of interest is formed either with al-antichymotrypsin (ACT),
al-protease inhibitor (API) or a2-macroglobulin. Moreover, the invention is
characterized in that free PSA, the PSA-proteinase inhibitor complex and their
ratio to total PSA are applied in the diagnosis of patients with prostate
cancer.
The patent application discloses three monoclonal antibodies ("MAB").
PSA complexed to ACT ("PSA-ACT complex" ) and free PSA were identified by
MAB 2E9 and 2H11. MAB 5A10 recognizes free PSA but not PSA-ACT
complex. MAB 2E9 is the only anti-PSA MAB that readily identified free PSA
and PSA-ACT complex on immunoblots. None of the anti-PSA MAB 2E9, 2H1 1
or the 5A10 significantly blocked the binding of each other to solid phase-
bound PSA.
CA 02178504 2006-05-29
WO 95/18381 PCT/US94/14902
3
By using different combinations of the MAB in non-competitive
immunoassays of human sera, the application found that increased clinical
specificity is achieved by measuring bath free PSA and PSA-ACT complex and
that the ratios between free PSA/total PSA and free PSA/PSA-ACT complex
are significantly different between benign prostatic hyperplasia and prostate
cancer patients.
MAB specific for free PSA and those reactive with PSA-ACT complex
are commercially available, for example, from CanAg Diagnostics AB,
Gothenburg, Sweden. Through inhibition studies and analyses of dose-
response of different combinations of their MAB, CanAg Diagnostics AB found
at least 9 major antigenic determinant groups on the PSA molecule. One
group of its MAB-defined epitopes was exposed both on uncomplexed PSA
and PSA-ACT complex, and another group of its MAB-defined epitopes was
exposed only in free PSA. (CanAg Diagnostics AB, Nilsson et al., E i o a
mapping of PSA, and development of assays for determination of different
isoforms of PSA. and the abstract of the same title, Abstract P 38, J. Tumor
Marker Oncology, 10th Int'l Conference on Human Tumor Markers, Sept. 8-11,
1993, Bonn, Germany).
Competitive radioimmunoassays for PSA are commercially available (e.
g., from PROS-CHECK PSA, Yang Laboratories, Inc., Bellevue, Washington).
PROS-CHECK PSA uses polyclonal rabbit antibodies to PSA, and PSA labelled
with lodine~ 25,
Currently, there are two types of PSA non-competitive sandwich
immunoassays on the market. The first type are sandwich assays which use
two sets of MAB specific for PSA: (1 ) one set of MAB ("capture antibodies")
is bound onto a solid phase to capture PSA in a sample, and (2) the other set
of MAB ("probe antibodies") is labelled and in free solution to bind the
captured PSA for its detection. These assays are herein referred to as "MONO
assays", Generally, the binding of these MAB to PSA is not prevented by the
binding of ACT to PSA. That is, generally, these MAB can bind both free PSA
and the PSA-ACT complex. Examples of such assays are the Hybritech
Tandem-E and Tandem-R PSA Assays (Hybritech, La Jolla, CA). Tanc~n is a trace-
mark.
The second type of sandwich assays uses MAB specific for PSA on the
solid phase, and polyclonal antibodies to PSA as probe antibodies. Generally,
WO 95/18381 PCT/US94114902
4
in these assays, the MAB can bind both free PSA and the PSA-ACT complex.
In contrast, the pool of polyclonal antibodies contains antibodies which can
bind both free PSA and the PSA-ACT complex, and antibodies which can bind
free PSA but not the PSA-ACT complex. In the latter case, the epitopes
bound by these antibodies are blocked by the binding of the ACT to PSA.
Examples of these assays are the Abbott IMxO PSA Assay (Abbott
Laboratories, Abbott Park, IL), and the ACSTM PSA Assay (Ciba-Corning
Diagnostics Corporation, East Walpole, MA).
It has been found that the second type of sandwich assays
preferentially detects free PSA over that of PSA-ACT complex (this
phenomenon is herein referred to as "bias"). On the other hand, some MONO
assays do not exhibit such a bias. See Bluestein, B., et aL, J. Tumor Marker
ncolo 7(4) 41 (1992).
It has been found that in the serum of patients with benign prostatic
hyperplasia (BPH), there are more free PSA than PSA-ACT complexes. On the
other hand, in the serum of prostate cancer patients, there are more PSA-ACT
complexes than free PSA. (Lilja, H., et al., Clin. Chem., ~: 1618 ( 1991 );
Stenman, U., et al., Cancer Res., 51: 222 ( 1991 ); Lilja, H., et al., ancer
Suppl., 7~: 230 (1992); Christensson, A., et al., rolo , 15 : 100
(1993)).
SUMMARY OF THE INVENTION
One aspect of the invention presents new MOLY and MONO assays for
detecting and quantitating PSA in a sample, wherein the sample is treated
with HEpSA Antibodies which bind free PSA but not a complex of PSA and
ACT ("PSA-ACT complex"). The addition of HEpSA Antibodies to the assay s
reduces or eliminates bias in these assays. Also presented are kits for
conducting these assays.
Another aspect of the invention presents a complex of PSA and
HEpSA Antibodies ("PSA-HEpSA Antibodies complex"), which resembles PSA-
ACT complex. This complex is useful as a calibrator and/or control for PSA
immunoassays.
CA 02178504 2005-09-19
-$-
Another aspect of the invention presents a method for fractionating polyclonal
antibodies to PSA to obtain fractions containing antibodies which bind
epitopes that are
masked ("hidden epitopes") by the binding of PSA to ACT and those which do not
bind
such epitopes. Also presented are affinity columns useful for such
fractionations.
The above aspects of the invention can be applied generally to any analyte
which can
exist in a free state or complexed to a binding molecule.
Thus in accordance with one aspect of the invention there is provided an
immunoassay
method for detecting or quantitating an Analyte A in a biological sample, the
Analyte A
being capable of existing in the sample in a free form as free Analyte or
bound to a
Binding Molecule to form a complex {(A)(Binding Molecule)}of the Analyte A and
the
Binding Molecule, the method comprising the steps of
(a) contacting the Analyte A to an antibody aHE specific for a particular
class of hidden
epitopes of the Analyte A, a capture antibody ariHE specific for a different
class of non-
hidden epitopes of the Analyte A, and a probe antibody aA* specific for the
Analyte A,
providing that if the probe antibody is a polyclonal antibody then the Analyte
A is
contacted to the capture antibody before the probe antibody;
(b) correlating the presence or amount of aA* which is bound to the Analyte A,
with the
presence or amount of the Analyte A in the sample, or correlating the amount
of
unbound aA* with the absence or amount of the Analyte A in the sample; wherein
ariHE can bind both the free Analyte A and said complex{(A)(Binding
Molecule)}; and
aHE can bind the free Analyte A but not said complex {(A)(Binding Molecule)}.
In accordance with another aspect of the invention there is provided a kit for
conducting
an assay for Analyte A in a sample, wherein the Analyte A can exist in the
sample as a
free Analyte A or bound to a Binding Molecule to form a complex Analyte-
Binding
Molecule Complex, the kit comprising the following:
(a) an antibody aHE which recognises a specific hidden epitope specific to the
Analyte
A which can bind to the free Analyte A but not Analyte-Binding Molecule
Complex;
DOCSMTL: 1889845\1
CA 02178504 2005-09-19
-Sa-
(b) a capture antibody specific for non-hidden epitopes of the Analyte A in
both the free
form and the complexed form; and
(c) a probe antibody specific for the Analyte A.
In a specific embodiment of the invention there is provided a kit for
conducting an assay
for Analyte in a sample, wherein the Analyte can exist in the sample as a free
Analyte or
bound to a Binding Molecule to form a complex Analyte-Binding Molecule
Complex,
the kit comprising the following:
(a) a first container containing an antibody aHE specific to the Analyte which
can bind
to the free Analyte but not Analyte-Binding Molecule Complex;
(b) a second container containing a capture antibody specific for non-hidden
epitopes of
the Analyte and can bind to both the free Analyte and the Analyte-Binding
Molecule
Complex ; and
(c) a third container containing a probe antibody specific for the Analyte.
In a further specific embodiment of the invention there is provided a kit for
conducting
an assay for Analyte in a sample, wherein the Analyte can exist in the sample
as a free
Analyte or bound to a Binding Molecule to form a complex of Analyte-Binding
Molecule Complex, the kit comprising the following:
(a) a first container containing: (i) a capture antibody specific for non-
hidden epitopes
of the Analyte and can bind to both the free Analyte and the Analyte-Binding
Molecule
Complex, and (ii) an antibody aHE specific to the Analyte which can bind the
free
Analyte but not the Analyte-Binding Molecule Complex; and
(b) a second container containing a probe antibody specific for the Analyte.
In a still further specific embodiment of the invention there is provided. a
kit for
conducting an assay for Analyte in a sample, wherein the Analyte can exist in
the
sample as a free Analyte or bound to a Binding Molecule to form a complex of
Analyte-
Binding Molecule Complex, the kit comprising the following:
DOCSMTL: 1889845\l
CA 02178504 2005-09-19
-Sb-
(a) a first container containing a capture antibody specific for non-hidden
epitopes of
the Analyte and can bind to both the free Analyte and the Analyte-Binding
Molecule
Complex; and (b) a second container containing:
(i) an antibody aHE specific to the Analyte which can bind the free Analyte
but not the
Analyte-Binding Molecule Complex, and (ii) a probe antibody specific for the
Analyte.
In yet another specific embodiment of the invention there is provided. an
immunoassay
method for detecting or quantitating an Analyte A in a sample, the Analyte A
being
capable of existing in the sample in a free form as free Analyte A or bound to
a Binding
Molecule to form a complex {(A)(Binding Molecule)}, the method comprising the
steps
of:
(a) incubating the sample with polyclonal capture antibodies aA which contain
antibodies which bind the free Analyte A and antibodies which bind said
complex
{(A)(Binding Molecule)};
(b) incubating the sample with an antibody aHE specific for a particular class
of hidden
epitopes of the Analyte A and capable of binding free analyte but not said
complex
{(A) (Binding Molecule)} for a sufficient time for the aHE to bind to the
Analyte A;
(c) incubating the sample with a probe antibody anHE* specific for a different
class of
non-hidden epitopes of the Analyte A and capable of binding both the free
Analyte A
and said complex {(A)(Binding Molecule)}; and
(d) correlating the presence or amount of anHE* bound to the Analyte A with
the
presence or amount of Analyte A in the sample; or correlating the amount of
unbound
ar~HE* with the absence or amount of the Analyte A in the sample.
In still another specific embodiment of the invention there is provided an
immunoassay
method for defecting or quantitating Analyte A in a sample, the Analyte A
being
capable of existing in the sample in a free form as free Analyte A or bound to
a Binding
Molecule to form a complex {(A)(Binding Molecule)}, the method comprising the
steps
o~
DOCSMTL: 1889845\1
CA 02178504 2005-09-19
-SC-
(a) incubating the sample with a capture antibody anHE specific for a class of
non-
hidden epitopes of the Analyte A and which can bind both free Analyte A and
said
complex {(A)(Binding Molecule)}, for a sufficient time for the capture
antibody to bind
to any Analyte A and complex {(A)(Binding Molecule)} that may be present in
the
sample to form complexes {(cmHE)(A)} and
{(ar~HE)(A)(Binding Molecule)}, respectively;
(b) incubating the sample with an antibody aHE specific for a particular class
of hidden
epitopes' of the Analyte A and which can bind the free Analyte A but not said
complex
{(A) (Binding Molecule)} for a sufficient time for the aHE to bind to the
Analyte A;
(c) incubating the sample with a probe antibody aA* specific for the Analyte A
for a
sufficient time to bind to the Analyte A;
(d) detecting the presence or amounts of the complexes {(ariHE)(A)(aHE)( a*)}
and
{(ariHE)(A)(Binding Molecule)(aA*)}, or free aA*; and
(e) correlating the presence or amounts of {(anHE)(A)( aHE)(aA*)} and {(
ariHE)(A)(Binding Molecule)( aA*)} with the presence or amount of the Analyte
A in
the sample; or correlating the amount of free aA* with the absence or amount
of the
Analyte A in the sample.
BRIEF DESCRIPTION OF THE DRAWINGS
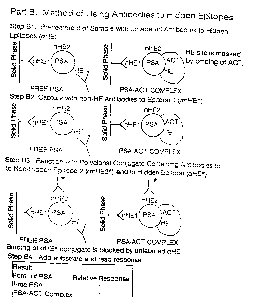
FIG. 1 compares the conventional method (Part A) and the method of the present
invention (Part B) for assaying PSA and shows how HE.psp Antibodies modify
assay
specificity for free PSA and PSA-ACT complex; and
FIG. 2 illustrates the method for obtaining polyclonal antibodies to hidden
and non-
hidden epitopes on PSA.
DETAILED DESCRIPTION OF THE INVENTION
As used herein, the term "antibodies" includes both polyclonal and monoclonal
antibodies, whole immunoglobulins and antigen binding fragments of the
DOCSMTL: 1889845\1
CA 02178504 2005-09-19
-Sd-
immunoglobulins. Examples of these fragments are Fab, F(ab')<sub>2</sub> and Fv.
Such
fragments can be produced by methods known in the art.
As used herein, the term "HE Antibodies" or "aHE" denotes antibodies whose
binding
to their target antigen ("Analyte") would be prevented by the binding of
another
molecule ("Binding Molecule") to the Analyte. The term "Non-HE Antibodies" or
aHE"
denotes antibodies whose binding to the Analyte is not prevented by the
binding of the
Binding Molecule to the Analyte. The epitope on the Analyte which is masked by
the
binding of the Binding Molecule to the Analyte is termed "hidden epitope"
("HE").
Similarly, the epitope on the Analyte which is not masked by the binding of
the Binding
Molecule to the Analyte is termed "non-hidden epitope" ("nHE"). The term
"aAnalyte"
or "aA" denotes any antibody Which is directed to the Analyte, whether it is
anaHE
orar~HE.
Analyte is preferably an antigen found in a biological sample, and is
preferably
endogenous to the sample. The Binding Molecule is preferably
DOCSMTL: 1889845\1
WO 95/18381 ~ PCT/US94/14902
6
endogenous to the sample and is often associated with the Analyte.
Preferably, the Binding Molecule and the Analyte are proteins. Examples of
Analytes are serum proteases and their Binding Molecules are serum protease
inhibitors. Some of the serum proteases and their inhibitors (in parentheses)
are: protein C (protein C inhibitor); elastase (anti-trypsin); cathepsin G
(ACT);
PSA (ACT); PSA (a2-macroglobulin); thrombin (anti-thrombin III ); C~-
esterase (C~-inhibitor); t-PA (PAI-1 ); uPA (PAI-1 ); plasmin (a2-antiplasmin
);
PSA (a~-protease inhibitor); and PSA (protein C inhibitor). "PAI-1" denotes
plasminogen activator inhibitor Type 1. "t-PA" denotes tissue plasminogen
activator. "uPA" denotes urinary plasminogen activator. One skilled in the art
would realize that the Binding Molecule can be serum proteases and the
Analyte can be serum protease inhibitors.
The term "MOLY assay" describes an immunoassay in which the
capture antibodies are MAB and the probe antibodies are polyclonal
antibodies, or vice versa.
The present invention discloses an immunoassay for an Analyte in a
test sample. The sample is generally a biological sample. The biological
sample can be biological fluids such as blood, serum, plasma, prostatic fluid,
seminal fluid, urine, lymph and spinal fluid.
If a sample contains Analytes which are not bound to the Binding
Molecule (these unbound Analytes are herein also referred to as "free
Analytes") and Analytes which are complexed to the Binding Molecules
("complexed Analytes" or "Analyte-Binding Molecule complexes"), a MOLY
assay and some MONO assays for the Analytes would exhibit a bias in that a
sample without complexed Analytes will have a higher reading than a sample
with complexed Analyte. This can occur if, e.g., some members of the
polyclonal antibodies used to detect the Analyte can bind free Analyte but
not complexed Analyte.
The present invention discovers that the bias found in MOLY and some
MONO assays for Analytes can be corrected by adding free HE Antibodies into
the reaction mixture. Other. than the addition of the free HE Antibodies, the
MOLY and MONO assays for a particular Analyte can be conducted according
to methods known in the art for such assays. Additionally, if the Analyte in a
sample is capable of being directly bound to a solid phase when the sample is
incubated with the solid phase, then a capture antibody is not necessary.
PCT/US94/14902
WO 95/18381
7
Probe and capture antibodies can be produced according to methods known in
the art. The probe antibodies can be labelled according to methods known in
the art with labels such as chemilumiscent, fluorescent, enzyme and
radioactive labels, and the assays accordingly tailored to the labels
involved.
An example of luminescent-labelled immunometric assays is Wood, W. G., et
al., Eur. J. Clin. Chem. Clin. Biochem.. 29: 787 (1991 ). The HE and Non-HE
Antibodies can be produced using methods known, such as those used for
producing HEpSA and Non-HEpSA Antibodies discussed below. The HE
Antibodies are preferably monoclonal antibodies or specific polyclonal HE
Antibodies as described below.
One aspect of this invention thus presents a sandwich noncompetitive
immunoassay for an Analyte in a biological sample. A capture antibody
("anHE") which is directed to a non-hidden epitope of the Analyte is attached
to a solid phase to capture any Analyte that may be present in the biological
sample. The capture antibody is incubated with HE Antibodies ("aHE")
simultaneously with or after the addition of the biological sample.
Alternatively, aHE may also be incubated with the sample before it is exposed
to the capture antibody. Preferably, the aHE is a MAB. The incubation lasts
for a sufficient time to allow the complex of {(anHE)(Analyte)(aHE)} to form.
Next, any reagents that are not bound to the solid phase are removed from
the reaction mixture. This can be done by a washing step known to those
skilled in the art. For example, the unbound reagents are dissolved in an
aqueous medium and washed away from the solid phase, leaving the complex
of {(anHE)(Analyte)(aHE)} on the solid phase.
Next, polyclonal anti-Analyte antibodies ("aAnalyte*") are added.
aAnalyte* are preferably labeled for detection, e.g. with enzyme, radioactive,
fluorescent, or chemiluminiscent labels.
The reaction mixture is incubated for a sufficient time for the
formation of {(anHE)(Analyte)(aHE)(aAnalyte*)} complex and
{(anHE)(Analyte)(Binding Molecule)(aAnalyte*)} complex. Note that in the
complex of {(anHE)(Analyte)(aHE)(aAnalyte*)}, both aHE and aAnalyte* are
bound to the Analyte in the complex. Note that if a Binding Molecule is
present in the sample, another complex may also be formed, i.e. the complex
of {(anHE)(Analyte)(Binding Molecule)(aAnalyte*)}.
WO 95/18381 C ~ i PCT/US94/14902
8
Next, the unbound reagents are separated. The formations of the
complexes of {(anHE)(Analyte)(aHE)(aAnalyte*)} and
{(anHE)(Analyte)(Binding Molecule)(aAnalyte*)} on the solid phase are
detected by detecting the labeled aAnalyte*. If Analyte is not present in the
S sample, these complexes will not be present. The presence of these
complexes is directly proportional to the Analyte concentration in the sample.
Alternatively, one can assay for the presence of the remaining unbound
labelled aAnalyte*, the amount of which is inversely proportional to the
presence of the Analyte in the sample.
The above assay format can also be used in MONO assays which exhibit
bias. In a MONO assay, the above discussion will apply, except that the
aAnalyte* is labelled anHE (i.e. "anHE*").
In the case where the capture antibody is a polyclonal antibody and the
probe antibody is a MAB, the above discussion will apply except that the
capture antibody is aAnalyte or anHE (anHE can be fractionated from
polyclonal antibodies, e.g. by the fractionation methods described below), and
the probe antibody is anHE*. If the capture antibodies, aAnalyte, contain a
subpopulation of aHE, there will also be an additional complex of
{(aHE)(Analyte)(anHE*)} resulting from the above assay steps.
The above discussion presumes that there is only one HE on the
Analyte. However, it shall be noted that if a particular aHE does not mask all
the HE on the Analyte, then additional HE Antibodies directed to other HE
sites on the Analyte are to be used.
One skilled in the art would realize that a probe antibody need not be
labeled if it can be specifically bound by another molecule which is labeled.
Further, if the Analyte can be directly bound to the solid phase, then a
capture antibody need not be used.
Also presented in this application are assay kits for conducting the
above assays. Preferably, the assay kit has a container containing unlabelled
HE Antibodies; and another container containing capture antibodies directed
to the Analyte, preferably these antibodies are directed to non-HE, more
preferably they are MAB, most preferably, they are bound to a solid phase.
The unlabelled HE Antibodies can be in a separate container or in the same
container as the capture antibodies or the probe antibodies. For a MOLY
PCT/US94/14902
WO 95/18381
9
Assay, the kit may additionally have: a container containing probe polyclonal
antibodies to Analyte and preferably the antibodies are labeled for detection;
and another container containing reagents which would react with the labels
on the antibodies to emit a signal. For example, the polyclonal antibodies can
be labeled with an enzyme such as alkaline phosphatase and the reagent
which would react with it will be the enzyme substrate. In the case of
alkaline
phosphatase, 4-methylumbelliferyl phosphate (MUP) has been found to be
convenient and the reaction is described in Example II below. Alternatively,
the probe antibodies can be monoclonal antibodies, and the capture
antibodies can be polyclonal antibodies. In such a case, the monoclonal probe
antibodies are preferably Non-HE Antibodies. For a MONO assay, the probe
and capture antibodies are MAB directed to the Analyte. Preferably, the
probe and capture MAB are non-HE Antibodies. In the above kits, the
antibodies are preferably in a solution, such as a buffer, which has no
adverse
effect on immunoassay. The above kits may additionally have containers)
containing calibrators) and/or containers) contianing control(s). For an
Analyte Assay, the preferred calibrator and control contain free Analyte, the
complex of Analyte-HE Antibodies, or the complex of Analyte-Binding Molecule
described below.
In the preferred invention, the Analyte is PSA, and the Binding Molecule
is ACT. The HE Antibodies to PSA are those which bind free PSA but not PSA-
ACT complex. These antibodies are herein also referred to as "HEpSA
Antibodies". The corresponding Non-HE Antibodies are also referred to as
"Non-HEpSA Antibodies".
The methods for obtaining HEpSA Antibodies (and Non-HEpSA
Antibodies) are known in the art. For example, they are described in PCT
patent application WO 92/01936, to H. Lilja et al., supra. and CanAg
Diagnostics AB, Nilsson et al., ~itope mapping of PSA~and development of
assays for determination of different isoforms of PSA (1993) and its Abstract
of the same title, ~aora.. Examples of HEpSA Antibodies are: (1 ) MAB 5A10
as described in WO 92/01936; (2) MAB 9810 which is disclosed in K.
Pettersson et al., Development of Immunofluorometric Methods for PSA to
Improve the Discrimination Between Early Prostate Cancer and Benian
Prostatic Hyperplasia. XV International Congress of Clinical Chemistry,
Melbourne, Australia, Nov. 14-19 (1993) which also discloses MAB H117 and
H50; and (3) MAB PSA6, PSA30, PSA17, PSA19, PSA20, and PSA25 which
are commcercially available from CanAg Diagnostics AB, Gothenburg, Sweden.
CA 02178504 2005-09-19
It is known in the art that one may use
both MAB 5A10 and 9810, their production
and characteristics; the hybridomas secreting these MABs are therein
designated 5A1 OE7F11 H4 and 9B1 OA9H3, respectively. These hybridomas
5 were deposited with the European Collection of Animal Cell Cultures (ECACC),
Public Health Laboratory Service, Centre for Applied Microbiology and
Research, Porton Down, Salisbury, Wiltshire SP4 OJG, Great Britain on March
12, 1993, and given ECACC accession Nos. 93031201 and 93031202,
respectively.
Thus, in particular, the present invention presents three inventions
useful for PSA assays: new PSA assays using HEpSA Antibodies; a method for
selecting specific polyclonal HEpSA Antibodies and antibodies for non-hidden
1 S epitopes; and PSA-HEpSA Antibody complex which resembles PSA-ACT
complex. The first two inventions avoid or reduce bias present in the prior
art
MOLY assays. The last invention is useful as calibrator and/or control for
both the MONO and MOLY PSA assays. The specific polyclonal Non-HEpSA
Antibodies selected by the second method can also be used in both MOLY (as
either probe or capture antibodies, or both) and competitve (as capture
antibodies) immunoassays for PSA. On the other hand, HEpSA Antibodies
could be used in assays specific for free PSA or they could be used in PSA
immunoassays (whether MOLY or MONO) suffering from a bias to alleviate or
eliminate such bias, as described above.
The present application uses PSA, ACT, and HEpSA Antibodies and
Non-HEpSA Antibodies to illustrate the invention. However, one skilled in
the art would understand that the invention can be applied to any Analyte,
Binding Molecule, and HE Antibodies and Non-HE Antibodies, respectively.
I. f~EW PSA IMMLlfIOASSAY METHOD
Figure 1 compares the conventional PSA MOLY assay (Part A) and the
PSA MOLY assay of the present invention (Part B) to illustrate how'HEpSA
Antibodies modify assay specificity for free PSA and PSA-ACT complex.
WO 95/18381 PC"T/US94114902
11
Part A presents the conventional MOLY assay which suffers from bias.
The procedural steps in Part A are the same as Part B except that Part B has
the additional step of Step B1.
The steps in Part A are as follow:
Step A1: A Non-HE Antibody (anHE1 ) which is specific for a non-hidden
epitope (designated nHE1 ) on the PSA molecule is linked to a
solid phase. When a sample containing either free PSA or PSA
ACT complex is allowed to react with the solid phase bound
anHEl, both free PSA and PSA-ACT complex can bind exclusively
through the nHE1 epitope. On the other hand, the HE is still
available on free PSA for subsequent reaction with other
antibodies.
Step A2: The subsequent addition of labelled polyclonal antibodies to PSA
("labelled polyclonals" ), which contain both labelled antibodies
specific for HE (aHE*) and antibodies specific for non-HE2
(anHE2*), will result in the binding of both types of antibodies
to the free PSA. In contrast, since HE is masked by ACT in the
PSA-ACT complex, the same labelled polycionals can only bind to
the non-hidden epitope nHE2. These labelled polyclonals are
conjugated to a label for detection purpose. Examples of labels
are: chemiluminiscent labels, radioactive labels, and enzyme
labels which are known in the art. In this example, the label is an
enzyme. The unbound reagents are removed from the solid
phase, e.g., by washing the solid phase using methods known in
the art.
Step A3: Then the enzyme substrate is added to the solid phase and the
enzymatic product generates a signal which is monitored. The
free PSA which has bound two labelled polyclonals generates
twice the signal as the PSA-ACT complex, which has bound only
one labelled polyclonal resulting in a positive bias for the free
form of PSA.
Part B presents the assay of the present invention in which unlabelled
HEpsA Antibodies are added (in Step B1 ) to the assay described in Part A and
this results in an assay without bias.
WD 95/18381 ~ PCT/US94/14902
12
The steps in Part B are as follow:
Step B1: The sample is pretreated with an unlabelled HEpSA Antibody
(aHE). This antibody reacts with HE on the free PSA, but it does
not bind to PSA-ACT complex. Alternatively, the unlabelled aHE
can be added in Step B2 or Step B3, respectively.
Step B2: The sample is added to solid phase bound anHE 1 antibody as in
Part A.
Step B3: The subsequent addition of labelled polyclonals containing
labelled antibodies which recognize HE (aHE*) and antibodies
which recognize non-HE (anHE2*) will result in the binding of
anHE2* to both free PSA and PSA-ACT complex. aHE* cannot
bind free PSA nor PSA-ACT complex, because HE is masked by
unlabelled aHE in the free PSA and by ACT in the PSA-ACT
complex.
Step B4: The substrate is added and the signal monitored. Both free PSA
and PSA-ACT complex generate equivalent signal, because both
have only bound the anHE2*. This results in an assay without
bias to free PSA compared to the PSA-ACT complex.
Materials for the solid phase can be any of those used for
immunoassays. Natural, synthetic or naturally occurring materials that are
synthetically modified can be used. They include: polysaccharides, e.g.,
cellulose materials including paper, cellulose and cellulose derivatives such
as
cellulose acetate and nitrocellulose; silica; fiberglass; inorganic materials
such
as deactivated aluminium, diatomaceous earth or other inorganic finely divided
material uniformly dispersed in a porous polymer matrix made of polymers
such as vinyl chloride, vinyl chloride-propylene copolymer, and vinyl chloride-
vinyl acetate copolymer; cloth, both naturally occurring (e.g., cotton) and
synthetic (e.g., nylon); porous gels such as silica gel, agarose, dextran and
gelatin; polymeric films such as polyacrylamide; magnetic particles;
microtitre
plates; polystyrene tubes; protein binding membranes; agarose; Sephadex
(Pharmacia Fine Chemicals, Inc., Piscataway, N.J.); Trisacryl (Pointet-Girard,
France); sillicon particles; porous fibrous matrixes etc. The solid phase is
WO 95/18381 ~ PCT/US94l14902
13
preferably synthetic microparticles preferably made of polystyrene, vinyl
chloride or latex of 0.1 to 10 microns in diameter.
S II. METHODS FOR FRACTIONATING POLYCLONAL ANTIBODIES TO
ANALYTES
The present invention also presents methods for fractionating
polyclonal antibodies to Analytes to yield fractions containing HE Antibodies
and Non-HE Antibodies, respectively. Polyclonal antibodies to Analytes can be
produced using methods known in the art. For example, the antibodies can be
produced by injecting a host animal such as a rabbit, rat, goat, mouse, etc.,
with the Analyte or fragments thereof, alone or conjugated to an appropriate
carrier, if required, to elicit an antibody response.
This method comprises exposing the polyclonal antibodies to a Analyte
which has its HE site masked ("masked Analyte") by either a Binding Molecule
or an HE Antibody. The antibodies that bind the masked Analyte, and which
can be eluted, are Non-HE Antibodies which bind both free Analyte and the
complex of Analyte-Binding Molecule. Thus, immunoassays using Non-HE
Antibodies to detect the Analyte, which may be present in free or complexed
forms, will not exhibit a bias. The Non-HE Antibodies thus obtained can be
used in both MOLY (as either probe or capture antibodies, or both) and
competitve (as capture antibodies) immunoassays. On the other hand, the
polyclonal antibodies that do not bind the masked Analyte are HE Antibodies.
HE Antibodies could be used in assays specific for free Analyte or as
described above for addition in Analyte assays (whether MOLY or MONO
immunoassays) suffering from a bias to alleviate or eliminate such bias.
The above fractionation is preferably conducted using affinity
chromatography. Preferably, the masked Analyte is cross-linked to the
Binding Molecule, HE Antibodies, or Non-HE Antibodies. The cross-linking can
be achieved by methods known in the art or modification of such which is
within the knowledge of one skilled in the art. Examples of publications which
3 S disclose methods for cross-linking molecules are: Ji, T. H., Methods in
Enz~rmoloav, X1:580 (1983); Wong, S. S., et al., in Chapter 22, 266-282 of
Biocatalyst Desian for Stability and Specificity. Eds. M.E. Himmel and G.
Georgiou, American Chemical Society, Washington, DC (1993); M. N. Gupta in
Chapter 26, 307-326 of Biocatalvst Design for Stability and Specificity, Eds.
CA 02178504 2003-12-02
WO 95/18381 PCT/US94/14902
14
M.E. Himmel and G. Georgiou, su a; chemical ReaoerLts for Protein
Modification, 2nd ed., R.L. Lundblad, CRC Press, Boston.
Preferably, the resulting complex is bound to a solid, directly or by
means of a binding agent such as a HE Antibody which is immobilized on the
solid phase, and used in affinity chromatography to select for the HE
Antibodies. The general methods for affinity chromatograpy, such as the
preparations of the antibodies and solid phase, and the procedures are known
in the art, see e.g. Bio-Rad Bulletin 1099, lmmunoaffinity Chromatoar~~hy
with Affinity Supports (1990); Pharmacia LKB Biotechnology, Af nit
Chromatography. Princiales & Methods (1993); and Methods in Molecular
iolo , vol. 10, "Immunochemical Protocols", ed: M. M. Manson, p. 89-91
(1992); Immunochemistp~ in Practice, A. Johnstone, et al., Chapter 10, 202-
232, Scientific Publications, Oxford (1982); and Current O inion in
Biotechnology, vol. 2, "Affinity Chromatography for Protein Isolation", W.H.
Scouten, 37-43 (199i).
Figure 2 illustrates the three methods for fractionating polyclonal
antibodies into HE Antibodies and Non-HE Antibodies using PSA, ACT and
HEpSA Antibodies as examples. The methods are as follow:
Part A: Use of ACT to Bind PSA
ACT is conjugated to a solid phase, such as cyanogen bromide-
activated Sepharose-4B (commercially available from Pharmacia
LKB Biotechnology, Sweden). Purified PSA is allowed to react
with the ACT to form a PSA-ACT complex that masks the hidden
epitopes (HE). The complex can be further stabilized by
chemical cross-finking. When polyclonal antibodies to PSA are
incubated with the PSA-ACT-solid phase, HEpSA Antibodies will
be unbound. Non-HEpSA Antibodies specific for non-hidden
epitopes, such as nHE1 and nHE2, will be bound and can be
eluted with standard methods, such as low pH. Thus, the
polycfonal anti-PSA antibodies are separated into HEpSA and
Non-HEpSA Antibodies.
i 7:~5 ~ 4 pCT/US94I14902
WO 95/18381
Part B: Use of HEpSA Antibodies (aHE) to Bind PSA
HEpsA Antibodies (aHE) are conjugated to a solid phase, such
as cyanogen bromide-activated Sepharose-4B. Purified PSA is
S allowed to react with the aHE to form a PSA-aHE complex that
masks the hidden epitopes (HE). The complex can be further
stabilized by chemical cross-linking. When polyclonal antibodies
to PSA are incubated with the PSA-aHE solid phase, polyclonal
HEpSA Antibodies will not be bound to the solid phase. On the
10 other hand, polyclonal Non-HEpSA Antibodies will be bound and
can be eluted with standard methods, such as tow pH. It shall be
noted that if a particular aHE does not mask all the HE on the
PSA, then additional HE Antibodies directed to other HE sites
can be used simultaneously or preferably, in sequential
1 S fractionation columns.
Part C: Use of Non-HEpSA Antibodies (anHE1 ) to Bind PSA Followed by
Use of HEpSA Antibody (aHE) to Block HE
Non-HEpsA Antibodies (anHEl) directed to non-hidden
epitope 1 (nHEl ) are conjugated to a solid phase, such as
cyanogen brorriide-activated Sepharose-4B. Purified PSA is
allowed to react with the anHEl to form a PSA-anHEl
complex. An HEpsA Antibody (aHE) is allowed to react with
the PSA-anHEl to form a complex that masks the hidden
epitopes (HE). The complex can be further stabilized by
chemical cross-linking. When polyclonal antibodies to PSA
are incubated with the solid phase-anHEl-PSA-aHE,
antibodies to HE and nHEl will not bind to the solid phase.
Non-HEpsA Antibodies to non-hidden epitopes other than
nHEl will be bound to the solid phase and can be eluted
with standard methods, such as low pH. The antibodies
that do not bind to the solid phase can be further separated
by methods A or B. Thus, the polyclonal anti-PSA
3 S antibodies are separated into HEpsA Antibodies, Antibodies
to nHEl and nHE other than nHEl. It shall be noted that if a
particular aHE does not mask all the HE on the PSA, then
additional HE Antibodies directed to other HE sites can be used
simultaneously or in sequential fractionation columns.
WO 95/18381 PCT/US94I14902
16
III. COMPLEXES OF ANALYTE-HE ANTIBODIES USEFUL AS CALIBRATORS
AND CONTROLS FOR PSA ASSAYS
Another aspect of the invention presents a complex of Analyte-HE
Antibody which can serve as a calibrator and control in an assay for Analytes.
The complex can be formed by adding an excess of HE Antibodies to the
Analyte. The HE Antibodies are preferably at a 2-fold to 100-fold, and a more
preferred 10-fold excess to the Analyte. Alternatively, the Analyte can be
cross-linked to the HE Antibodies. The method described above for cross-
linking Analyte and Binding Molecule are similarly applicable for cross-
linking
Analyte and HE Antibodies. The complex is preferably stored in an inert
buffer such as phosphate buffered saline, Tris-HCI, or HEPES. The storage
temperature preferably ranges from 2°C to 25°C. The pH is
preferably
between 5 to 9. A cross-linked complex of the Analyte and its Binding
Molecule useful as a calibrator or control in an assay for the Analyte can
also
be similarly produced.
The following examples illustrate the present invention and are not to
be construed as limiting it.
Examples
Example I. New PSA Assay Method
The following experiment was conducted using the reagents for IMx~
PSA Assay and conducted on the IMx~ instrument (Abbott Laboratories,
Abbott Park, IL) following the protocol in the IMxO PSA Assay's accompanying
package insert, except that in this invention, the Assay Diluent additionally
contained 0 to 20 ug/mL of MAB 9810 or 5A10 (discussed above) which
bind free PSA but not PSA-ACT complex. The IMxO PSA Assay reagents
include the following: ( 1 ) microparticles coated with capture antibody MAB
H50 ("Anti-PSA Coated Microparticles") which bind and capture both free and
complexed PSA (i.e. PSA complexed to ACT) onto the microparticles; and (2)
goat polyclonal antibodies to PSA (which serve as probe antibodies) which are
labelled with alkaline phosphatase for detection ("goat polyclonal Anti-PSA:
Alkaline Phosphatase Conjugate" or "probe polyclonal antibodies" ).
PC"T/US94/14902
WO 95/18381
17
The samples tested were pooled serum samples from patients with
prostate cancer.
S The descriptions of the IMxO instrument, its operation and general
protocol can be found in Barnes et al., J. Clin. Imm., 14 (2): 115-119 (1991 )
and EP-A-288,793; Ludington et al., Clin. Chem., ~(9), 1726-1732 (1988)).
The components of the reaction cells are shown in Figure 2(a) of Clin. Chem.,
,~(9), at 1727. In this case, the reaction scheme is that of a microparticle
enzyme immunoassay (MEIA), as follows:
(1 ) The probe/electrode assembly of the IMxO instrument delivers the
sample, Anti-PSA Coated Microparticles and Assay Diluent to the incubation
well of the reaction cell. During the incubation of this reaction mixture, the
free and complexed PSA in the sample bind to the Anti-PSA Coated
Microparticles forming an antibody-antigen complex. MAB 9810 in turn binds
the PSA (which is not complexed to ACT) that is bound to the Anti-PSA
Coated Microparticles, to form an antibody-antigen-antibody complex.
(2) An aliquot of the reaction mixture is transferred to the glass fiber
matrix of the IMxO instrument. The microparticles bind irreversibly to the
glass fiber matrix.
(3) The matrix is washed to remove unbound materials, e.g. serum
proteins, Assay Diluent, and MAB 9810 that are not bound to the
microparticles.
(4) The goat polyclonal Anti-PSA: Alkaline Phosphatase Conjugate is
dispensed onto the matrix and binds to the antibody-antigen-antibody
complex.
(5) The matrix is washed to remove unbound materials.
(6) The substrate, 4-methylumbelliferyl phosphate (MUP), is added to
the matrix and the surface-bound alkaline phosphatase converts the
nonfluorogenic MUP to 4-methylumbelliferone (MU), whose
fluorescence is measured by the MEIA optical assembly of the IMx~
instrument.
WO 95/18381 PCT/US94/14902
18
The above assay steps were run separately but concurrently using
calibrators and controls containing free PSA, instead of samples. The readings
for the samples (i.e. panels) were read off the calibrators (of Table 1 below)
to arrive at the PSA values reported in Tables 2 and 3 below.
WO 95/18381 PCT/US94114902
19
Table 1
Calibrators MAB 9810
(Free PSA) Concentration
(ng/ml) in Assay
Diluent
(ug/ml)
0 5 10 20
0 7.0 6.3 6.4 6.1
2 90.9 58.0 58.3 57.4
373.6 248.6 248.8 247.1
30 877.3 640.1 623.4 606.3
60 1394.8 1084.8 1084.8 1053.7
100 1834.3 ~ 1486.1 ~ 1520.8 ~ 1493.8
The readings were taken in c/s/s.
Table 2
Controls MAB 9810
(Free PSA) Concentration
in Assay
Diluent
(ug/ml)
0 5 10
20
Low (3-5 n /ml) 3.98 4.14 4.17 3.86
Medium ( 12-18 n /ml) 15.18 15.02 15.12 14.95
High (36-54 ng/ml) 46.90 42.85 45.87 J 48.20
The readings are reported in ng/ml.
Table 3
MAB 9810
Panels Concentration
(Pooled Prostate Cancer in Assay
Patient Sera) Diluent
(ug/ml)
0 5 10
20
JP1 50.49 77.88 83.71 85.72
C8 1.37 2.01 2.02 2.08
C9 3.25 5.06 4.97 5.16
C10 13.02 18.99 19.21 19.69
C11 36.65 60.88 62.62 64.93
92-297-0384 26.59 48.15 47.71 50.13
The readings are reported in ng/ml.
The above Tables show that increasing amounts of free MAB 9810 in
the assay reduced the calibration curve signals and increased sample panel
values. The effect of MAB 9B 10 on panel values appears to plateau at about
10 ug/ml. The above experiments were repeated using MAB 5A10 in place of
MAB 9810 and the results were similar.
WO 95/18381 PCT/US94I14902
In the following experiment, the above plateau level of 10 ug/ml of
MAB 9810 in Assay Diluent was used to demonstrate bias between free and
complexed PSA. A sample containing free PSA was mixed with purified ACT
S and allowed to form complexes. Purified PSA and ACT were obtained from Dr.
Hans Lilja, University of Malmo. Purification of PSA and ACT and formation of
complexes of PSA and ACT have been previously described (A. Christensson,
et al., Eur. J. Biochem. 1_~4: 755-763, 1990). Purified seminal fluid PSA in
0.1 M phosphate buffer, pH 7.0 containing 7.5% bovine serum albumin and
10 0.05% sodium azide was mixed with a 100 molar excess of purified ACT. A
control sample of PSA was mixed with buffer instead of ACT. These samples
were allowed to incubate overnight at 35 °C.
Following the incubation, these samples were assayed in the IMxO PSA
15 assay with the following modifications:
a. Abbott IMxO PSA Assay (no modifications)
b. Abbott IMx~ PSA Assay with 9810 in Assay Diluent (i.e. with
the addition of 10 ug/mL of 9810 to the Assay Diluent)
c. Abbott IMx~ PSA Assay with MAB H50 probe and MAB H1 17
20 capture antibodies (i.e. with the use of H 117 coated
microparticles and labelled H50)
The assay was performed as described above. Table 4 shows the
amounts of PSA obtained for the free PSA samples and for the PSA samples
which had ACT added. Bias is indicated by the reduction of the amount of
PSA detected in the sample containing ACT as compared to the PSA control.
WO 95118381 PCT/US94114902
21
Table 4
Assay Tested Free PSA + ACT % Bias
PSA
Abbott IMx~ PSA Assa 64.7 40.9 36.8
Abbott IMx~ PSA Assay
with
9B10 in Assa Diluent 67.2 61.3 8.8
Abbott IIvIx~ PSA Assay
with
IvIAB H50 probe antibodies
and MAB H117 capture 68.8 70.1 -1.8
antibodies
The readings were taken in ng/ml
The data in Table 4 shows that the MONO assay (with MAB H50 as
probe antibodies and MAB H117 as capture antibodies) has no bias. In
contrast, the MOLY assay (Abbott IMxO PSA Assay) has a 36.8% bias which
, is dramatically reduced by the addition of free MAB 9810 to the assay.
The above experiment was repeated, wherein the MONO assay used
MAB H50 as capture antibody and MAB H1 17 as probe antibody. It was
observed that such a MONO assay also suffered from bias which can be
eliminated by the addition of free 9810 in the Assay Diluent.
Example II
PSA-HE Antibody Complex Useful As Calibrators and Controls
The mixture of HEpsA Antibodies with PSA results in the formation of a
PSA-HEpSA Antibodies complex in which the hidden epitopes are blocked
similarly to PSA-ACT complex. This material can be used as a calibrator or
control in PSA assays.
Seminal fluid PSA in 0.01 M phosphate buffered saline, pH 7.4 is mixed
with 9810 at a 10-fold excess and incubated overnight at 2-8 degrees C.
Following incubation, the PSA-HEpSA Antibody complex is diluted in IMx~ PSA
Calibrator Diluent at levels of 0, 2, 10, 30, 60 and 100 ng/mL. These
calibrators can be used in the IMx~ PSA assay and mimic PSA-ACT complexes
by masking the HE on PSA.
WO 95/18381 PCT/US94/14902
22
Example III
Cross-Linking HE Antibody to Free PSA for Selecting Polyclonal Antibodies to
PSA HE and PSA non-HE
This example describes the implementation of the fractionation
method shown in Part B of Figure 2 discussed above. MAB 5A10 is used to
illustrate the method though any HEpSA Antibody can be used in its place,
such as MAB 9810.
In the initial step, 5A10 is cross-linked to cyanogen bromide-activated
Sepharose-4B based on manufacturer's recommendations. (Affinity
Chromatograph,~ia~les and Methods, Pharmacia LKB Biotechnology,
Sweden, 23-30 (1993)).
The procedure is as follows:
The 5A10 for Affinity Column is mixed with 0.1 M sodium phosphate
dibasic (Na2HP04) and the pH is adjusted to 9Ø 5A10 is then mixed with
cyanogen bromide-activated Sepharose-4B gel at a ratio of 2 mg 5A10 per
mL of gel. The mixture is incubated at 2° - 8°C overnight with
gentle shaking.
Following the coupling of 5A10 to the Sepharose gel, the gel is washed
with 0.1 M Na2HP04, pH 8.0, to remove any unbound ligand, and then mixed
with 1 M ethanolamine, pH 8.0, at 2° - 8°C to block any active
groups
remaining in the gel. The gel is washed with distilled or deionized water
followed by two alternating cycles of 0.1 M sodium acetate buffer with 1 M
NaCI pH 4.0 and 0.1 M Na2HP04 with 1 M NaCI, pH 8Ø A column is poured,
washed with PBS solution (0.01 M NaHP04, 0.15M NaCI, pH 7.2) and then
eluted with 0.1 M glycine, 0.1 M NaCI, pH 2.5. The column is then washed with
PBS at pH 7.2 containing 0.1 % sodium azide. The 5A10 affinity column is
stored at 2° - 8°C.
The next step in the procedure involves the binding of PSA to the
Sepharose 4B-5A10 followed by chemical crosslinking to stabilize the 5A10-
PSA complex. Binding of PSA to 5A10 blocks the HE on the PSA so that the
HE cannot bind other HEpSA antibodies. The procedure for cross-linking
insoluble proteins with the cross-linking agent, dimethylpalemidate (DMP) has
CA 02178504 2003-12-02
WO 95/18381 PCT/US941I4902
23
previously been described. (C. Schneider, et al., J. 8iol. Chem. 25, 10766-
10769, ( 1982)).
The next procedure is as follows:
Remove the Sepharose 4B-5A10 gel from the column and place it in a
graduated cylinder. After the gel has settled, remove the residual buffer. For
each mL of packed gel, add 10 mg of purified PSA at an initial concentration
of 2 mg/mL in 0.01 M phosphate buffer, pH 7.4. Transfer to an Erlenmeyer
flask and incubate at 2-8°C overnight with~gentle shaking.
Following overnight incubation, wash the gel twice in 10 volumes of
0.2M triethanolamine, pH 9.0 (TEA) on a Buchner funnel. Transfer the gel to
a beaker and add 5 parts of 40mM DMP in TEA, pH 9Ø Incubate with shaking
1 S for 1 hour at room temperature. Using a Buchner funnel wash with 10-20
volumes of TEA, pH 9.0 and 10-20 volumes of 0.01 M phosphate buffered
saline, pH 7.4 with 0.0296 sodium azide. Transfer gel to column.
The final step of the process is to purify polyclonal antibodies to PSA
over the Sepharose 4B-5A10-PSA column by standard affinity procedures as
follows:
Dilute the anti-PSA polyclonal antibodies 1:1 with column buffer (0.01
M phosphate buffered saline, pH 7.4). (Examples of polyclonal antibodies are
goat, rabbit, sheep, mouse, and rat polyclonal antibodies.) Slowly, load the
sample onto the column. Collect the protein that does not bind to the
column. This fraction contains the HE antibodies. Wash the column with
column buffer and monitor the OD at 280 nm. When the absorbance falls to
baseline, rapidly elute the bound non-HE Antibodies with 0.1 M glycine-HCI, pH
2.5. Collect fractions into an equal volume of 0.1 M Tris-HCI, pH 8.5 to
rapidly adjust the pH to neutrality. Both the purified HE and non-HE
antibodies may be dialyzed into the desired buffer and stored at 2-8°C
following filtration through a 0.2 micron sterile filter. Alternatively, the
antibodies may be frozen.
WO 95/18381 PCT/US94/14902
24
Although the foregoing invention has been described in some detail by
way of illustration and example for purposes of clarity and understanding, it
will be obvious that various modifications and changes which are within the
skill of those skilled in the art are considered to fall within the scope of
the
S appended claims. Future technological advancements which allow for obvious
changes in the basic invention herein are also within the claims.