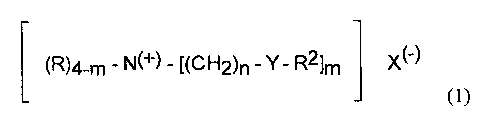Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 95/16766 PCT/US94/14255
2179007
VISCOSITY STABLE CONCENTRATED LIQUID
FABRIC SOFTENER COMPOSITIONS
TECFINICAL FIELD
The present invention relates to concentrated aqueous textile treatment .
compositions. In parl:icular, it relates to textile treatment compositions for
use in the
rinse cycle of a textilE; laundering operation to provide fabric
softening/static control
benefits, the compositions being characterized by excellent storage stability
and
excellent viscosity stalbility after freezelthaw cycling.
BACKGROUND OF THE INVENTION
Aqueous textile treatment compositions suitable for providing fabric softening
and
static control benefits during laundering are well-known in the art and have
found
wide-scale commercial application. Conventionally, aqueous, rinse-added,
fabric
. softening compositions contain, as the active softening component,
substantially
water-insoluble cationic materials having two long alkyl chains. Typical of
such
materials ~ are di-hydrogenated tallow di-methyl ammonium chloride and
imidazolinium compounds substituted with two stearyl groups. These materials
are
normally prepared in the form of a dispersion in water. It is generally not
possible to
prepare such aqueous dispersions with more than about 10% cationic materials
without encountering intractable problems of product viscosity and stability,
especially after storage at lower temperatures, such that the compositions are
unpourable and have inadequate dispensing and dissolving characteristics in
rinse
water. This physical restriction on softener concentration limits the level of
softening
performance achievable without using excessive amounts of product and also
adds
substantially to the costs of distribution and packaging. Accordingly, it
would be
highly desirable to prepare physically acceptable aqueous textile treatment
compositions containing much higher levels of substantially water-insoluble
cationic
o . 179007 ~:
2
softener materials.
Cationic softener materials are normally supplied by the manufacturer
containing about 70%-90°/~ of active material in an organic liquid such
as isopropanol
or ethanol, sometimes Containing a minor amount of water (up to 10%). Retail
fabric
softening compositions are then prepared by dispersion of the softener in warm
or hot
water under carefully controlled conditions. The physical form and
dispersibility
constraints of these industrial concentrates are such as to preclude their
direct use by
the domestic consumer; indeed, they can pose severe processing problems even
for
the industrial supplier of retail fabric softening compositions.
Many of the various solutions to the specific problem of preparing aqueous
fabric softening compositions in concentrated form suitable for consumer use
have
not been entirely satisfactory. It is generally known (for example, in U.S.
Pat. No.
3,681,241, Rudy, issued Aug. 1, 1972} that the presence of ionizable salts in
softener
compositions does help reduce viscosity, but this approach by itself is
ineffective in
preparing compositions containing more than about 12% of dispersed softener,
inasmuch as the level of ionizable salts necessary to reduce viscosity to any
substantial degree has a seriously detrimental effect on product viscosity
stability.
SUMMARY OF TI3E INVENTION
It has now been discovered that the product stability and viscosity
characteristics of concentrated fabric softener compositions containing
mixtures of
(A) biodegradable diester quaternary ammonium softening materials,
(hereinafter
referred to as "(A)"), and (13) specific co-active fabric softening materials,
(hereinafter
referred to as "(B)"), (i.e., substituted imidazoline compounds and specific
quaternary
ammonium salts which are not the same as (A)), are superior both at normal and
lower temperatures. The value of using such mixtures of fabric softening
materials
for enhancing the long term viscosity characteristics and stability of
concentrated
aqueous cationic fabric compositions especially after freeze/thaw cycling, has
hitherto
not been recognized in the art.
C
179007 _
3
DETAILED DESCRIPTION OF THE INVENTION
(A). Biode radable Ouaternized Ester-Amine Softenini? Material
The present invention contains diester quaternary ammonium material
(hereinafter refe;rred to as "DEQA") as an essential component. The
quaternary ammonium material may also be a triester. Two primary types of
DEQA are preferred.
1. The first type of DEQA preferably comprises, as the primary active,
compounds of the formula:
(R)4_m - N(~) - [(CH2)n - Y- R2lm X(-)
io (1)
wherein each R ;substituent is a short chain C1-C6, preferably C1-C3 alkyl or
hydroxyalkyl group, e.g., methyl (most preferred), ethyl, propyl,
hydroxyethyl,
and the like, benzyl or mixtures thereof; each m is 2 or 3; each n is from 1
to
about 4; each Y is -O-(O)C-, or -C(O)-O-, but not -OC(O)O-; each R2 is a
long chain Cl~;-C22 hydrocarbyl, or substituted hydrocarbyl substituent,
preferably C 15-C 19 alkyl or alkylene, most preferably C 15-C 1 ~ straight
chain
alkyl or alkylene; such that the Iodine Value (hereinafter referred to as IV)
of
the parent fatty acid of this R2 group is less than about 10, preferably less
than
2o about 5, most preferably less than about 2; and the counterion, X(-), can
be
any softener-compatible anion, preferably the anion of a strong acid, for
example, chloride, bromide, methylsulfate, formate, sulfate, nitrate and the
like. The anion can also, but less preferably, carry a double chaxge in which
case X(-) represents half a group. These materials containing a divalent
anion,
in general, are more difficult to formulate as stable concentrated liquid
compositions.
Carbonate esters, i.e., where Y = -O-C(O)O-, are unstable compounds
and are
WO 95/16766 ~ ~ ~ PCTlUS94/.14255
4
not included as (A)( 1 ) compounds.
It will be understood that substituents R and R2 can optionally be substituted
with various groups such as alkoxyl or hydroxyl groups, and can be straight,
or
branched so long as the R2 groups maintain their basically hydrophobic
character.
The preferred compounds can be considered to be diester variations of ditallow
dimethyl ammonium chloride (hereinafter referred to as "DTDMAC"), which is a
widely used fabric softener. At least 80% of the DEQA is in the diester form,
and
from 0% to about 20% can be DEQA monoester (e.g., only one of the ester groups
is hydrolyzed to yield either-Y-H, or an -OH group).
As used herein, when the diester is specified, it can include the monoester
that
is present. For softening, under no/low detergent carry-over laundry
conditions the
percentage of monoester should be as low as possible, preferably no more than
about
2.5%. However, under high, anionic detergent surfactant or detergent builder
carry-
over conditions, some monoester can be preferred. The overall ratios of
diester to
monoester are from about 100:1 to about 2:1, preferably from about 50:1 to
about
5:1, more preferably from about 13:1 to about 8:1. Under high detergent carry-
over
conditions, the di/monoester ratio is preferably about 11:1. The level of
monoester
present can be controlled in manufacturing the DEQA.
The above compounds, used as the biodegradable quaternized ester-amine
softening material in the practice of this invention, can be prepared using
standard
reaction chemistry. In one synthesis of a di-ester variation of DTDMAC, an
amine of
the formula RN(CH~CH20H)2 is esterified at both hydroxyl groups with an acid
chloride of the formula RZC(O)Cl, then quaternized with an alkyl halide, RX,
to yield
the desired reaction product (wherein R and R2 are as defined hereinbefore).
However, it will be appreciated by those skilled in the chemical arts that
this reaction
sequence allows a broad selection of agents to be prepared. The following are
non-
limiting examples (wherein all long-chain alkyl substituents are straight-
chain): '
fH0-CH(CH3)CH2][CH3]1V(+)[CH2CH20C(O)C15H31~2 Br(-)
5
[C2HSJ2 N(+)[CH2CH20C(O)C17H35J2 Cl( )
[CH3J[C21j5J N(+)[CH2CH20C(O)C13H27J2 I( )
[C3H7][Ca?HSJ N(+)[CH2CHZOC(O)C15H31J2 [S04CH3](-)
[CH3J2 N(+)_CH2CH20C(O)C15H31 Cl( )
CH2CH20C(O)C17H35
[CH2CH2OH][CH3] N(+)[CH2CH20C(O)R2]2 Cl( )
[CH3J2 N(+)[CH2CH20C(O)RZJ2 C1( )
to where -C(O)R2 is derived from hardened tallow fatty acid.
Since the foregoing materials (diesters) are somewhat labile to
hydrolysis, they ahould be handled rather carefully when used to formulate the
fabric softening composition herein. For example, stable liquid compositions
herein are formulated at a neat pH in the range of from about 2 to about 5,
preferably from about 2 to about 4.5, more preferably from about 2.5 to about
4. The pH can be adjusted by the addition of a Bronsted acid. pH ranges for
making stable softener compositions containing diester quaternary ammonium
fabric softening compounds are disclosed in U.S. Pat. No. 4,767,547,
Straathof, issued Aug. 30, 1988.
Examples of suitable Bronsted acids include the inorganic mineral acids,
carboxylic acids., in particular the low molecular weight (C1-CS) carboxylic
acids, and alkylsulfonic acids. Suitable inorganic acids include HCI, H2S04,
HN03 and H3P04. Suitable organic acids include formic, acetic, citric,
methylsulfonic and ethylsulfonic acids. Preferred acids are hydrochloric,
phosphoric, and citric acids.
2. In a second type of DEQA the primary active has the general formula:
R2-Y C H2 ~
/ CHCHZN(+)Rg X(-~
R2_Y
(2)
0
wherein each Y, R, R2, and X(-) have the same meanings as before. Such
compounds include those having the formula:
[CH3]3 N(+)[CH2CH(CH20C[O]R2)OC(O)R2] Cl(-)
where C(O)R2 i:> derived from hardened tallow fatty acid.
Preferably each R is a methyl or ethyl group and preferably each R2 is
in the range of C15 to C19. Degrees of branching and substitution can be
1o present in the alkyl chains. The anion X(-) in the molecule is the same as
in
DEQA (1) above;. As used herein, when the diester is specified, it can include
the monoester that is present. The amount of monoester that may be present is
the same as in DEQA (1).
The present invention may also contain mixtures of the two primary
types of DEQA.
These types of agents and general methods of making them are
disclosed in U.S,. Pat. No. 4,137,180, Naik et al., issued Jan. 30, 1979:
(B). The Co-Active Fabric Softening Material
Compositions prepared by the present invention contain as an essential
component a co-active fabric softening material, as described hereinafter,
which is different from the biodegradable diester quaternary ammonium
softening material.
(A):
1. One such co-active fabric softening material is a fabric softener
material with the: primary active having the formula:
(CH2)n2
% (CFi2)nz-~-R3
C
I
Rs (1)
~~0
wherein each Y2 is: -N(R4)C(O)-, in which each R4 is selected from the group
consisting of C 1 ~-C6 alkyl, alkenyl, hydroxy alkyl group or hydrogen;
-OC(O)-; or a single covalent bond; wherein each R3 is independently,
hydrocarbyl, preferably alkyl, groups containing from about 11 to about 31,
preferably from about 13 to about 17, carbon atoms, more preferably straight
chain alkyl groups, and wherein each n2 independently is from about 2 to
about 4, preferat~ly with both n2's being 2. It will be understood that each
R3
can optionally be substituted with various groups such as alkoxyl or hydroxyl,
l0 or can be branched, but such materials are not preferred herein. In
addition R3
can optionally be; unsaturated (e.g., alkenyl groups).
The above; materials used as the co-active fabric softening material in
the practice of tlhis invention are prepared using standard reaction
chemistry.
Disclosure of imidazoline fabric softener materials useful herein can be found
in U.S. Pat. Nos.: 4,661,267, Dekker, Konig, Straathof, and Gosselink, issued
April 28, 1987; 4,724,089, Konig and Buzzaccarini, issued Feb. 9, 1988;
4,806,255, Konig and Buzzaccarini, issued Feb. 21, 1989; 4,855,072, Trinh,
Wahl, Swartley, and Hemingway, issued Aug. 8, 1989; 4,933,096, DeMeyere,
Hardy, and Konig, issued June 12, 1990; and 4,954,635, Rosario-Jansen and
2o Lichtenwalter, issued Sept. 4, 1990.
A preferred co-active fabric softening material of the present invention
is the reaction product of higher fatty acids with a polyamine selected from
the
group consisting; of hydroxyalkylenediamines and dialkylenetriamines and
mixtures thereof; the process of which is disclosed in U.S. Pat. No.
5,013,846,
Walley, issued May 7, 1993. These reaction products are mixtures of several
compounds in view of the multifunctional structures of polyamines (see, for
example, the publication by H.W. Eckert in Fette-Seifen-Anstrichmittel,
September 1972., pages 527-533).
For example, in a typical synthesis of a substituted imidazoline ester
softening material of formula above, a fatty acid of the formula R3COOH is
reacted with a lhydroxyalkylenediamine of the formula NH2-(CH2)n2-NH-
(CH2)n20H to form an intermediate imidazoline precursor, which is then
reacted with a methyl ester of a fatty acid of the formula:
R3C(O)OCH3
to yield the desired reaction product (wherein R3, and n2 are as defined
1o above). It will be appreciated by those of ordinary skill in the chemical
arts
that this reaction sequence allows a broad selection of materials to be
prepared.
As illustrative, nonlimiting examples there can be mentioned the following di-
alkyl imidazoline compounds (wherein all long-chain alkyl substituents are
straight-chain): 1-stearoyl oxyethyl-2-stearyl imidazoline, 1-stearoyl
oxyethyl-2-palmityl imidazoline, 1-stearoyl oxyethyl-2-myristyl irnidazoline,
1-palmitoyl oxyc;thyl-2-palmityl imidazoline, 1-palmitoyl oxyethyl-2-myristyl
imidazoline, 1-stearoyl oxyethyl-2-tallow imidazoline, 1-myristoyl oxyethyl-
2-tallow imidazoline, 1-palmitoyl oxyethyl-2-tallow imidazoline, 1-cocyl
oxyethyl-2-coconut imidazoline, 1-tallowyl oxyethyl-2-tallow imidazoline, 1-
2o [hydrogenated tallowyl amido]ethyl-2-hydrogenated tallow imidazoline, 1-
[stearyl amido]ethyl-2-stearyl imidazoline, 1-[palmityl arnido]ethyl-2-
palmityl
imidazoline, 1-[oleyl amido]ethyl-2-oleyl imidazoline, and mixtures of such
imidazoline materials.
Other types of substituted imidazoline softening materials can also be
used
...../
WO 95/16766 ~ ~ ~ PCT/US94/14255
9
herein. Examples of such materials include:
~C H~)~2
~ -(C H2C hi20)n2H
C
I
R3 (i)
i H~)~
/N-[CH2-CH(OH)-CH2)]i.~2C(O)-R3
C
I
R3
(ii)
~C H2;
/N-[CH2-CH(OH)-CH20]~2H
C
I
R3
(iii)
wherein R3, and n2 are as previously defined. The above list is intended to be
illustrative of other types of substituted imidazoline softening materials
which can
optionaily be used in the present invention, but which are not preferred.
2. The Di(2-amidoethyl)methyl quaternary ammonium salts are also
suitable for use as Component (B) in the compositions of the
invention herein, especially those having the formula:
R8
7-O H ~ ~ H O
R C-N-C2H4- N C2H4-N-C-R
C H3
(2)
wherein each R~ is selected from the group consisting of Clq. to C20 alkyl
and alkenyl groups, wherein each R8 is selected from methyl, ethyl, and
-(CmH2m0)n3H, wherein n3 is from 1 to about 5, preferably 3, and wherein
l0 m, and X( ) havf; the same meaning as before. This class of, agents is
disclosed in ~J.S. Pat. No. 4,134,840, Minegishi et al., issued January 16,
1979.
Exemplary materials are di(2-hydrogenatedtallowamidoethyl)
ethoxylated (2-ethoxy groups) methyl ammonium methylsulfate, di(2-
oleylamidoethyl) propoxylated (3-propoxy groups) methyl ammonium
bromide, di(2-palmitoleylamidoethyl) dimethyl ammonium ethylsulfate and
di(2-stearylamidoethyl) propoxylated (2-propoxy groups) methyl ammonium
methylsulfate.
An exemplary commercial material suitable for use as Component (B)
2o herein is di(2-tallowamidoethyl) ethoxylated methyl ammonium methylsulfate
(such that the LV. Component B is about 31) sold under the trade mark
Varisoft 222, from Witco Chemical Company.
3. A co-active fabric softening material for use as Component (B) in the
composition of the invention herein can also have the formula:
1 ~g0___
O
n
R9-C OCH2CH2~ ~+)~CH3 X(-)
R9-C;-OCH2CH2 \C2H40H
(3)
wherein each R~ is C 15-C 17 alkyl group such that the IV of the parent fatty
acid of this R9 group is from about 20 to about 100, preferably from about 30
to about 70, most preferably from about 35 to about 60; and X(-) has the same
meaning as before.
A preferred fabric softening material of the present invention is prepared
according to the synthesis disclosed in U.S. Pat. No. 3,915,867, Kang et al.,
issued October :28, 1975. The fabric softening material of this invention
generally comprises the reaction of purified C 1 q.-C 1 g fatty acid
alkylester
to mixture, triethanolamine, and a quaternizing reagent, preferably dimethyl
sulfate. The select fatty acid alkylesters are preferably a mixture of
substantial
amounts of oleic;, palmitic, stearic acid alkyl esters and may include minor
amounts of other fatty substances.
4. A co-active fabric softening material suitable for use as Component (B)
in the composition of the invention can also have the formula:
R~pC(O)OCH2~
~CHCHZN(+)R3 X('
R~oC(O)O
(4)
wherein each R.10 is a C 12-C22 hYdrocarbyl or substituted hydrocarbyl
substituent, preferably C 15-C 1 g alkyl or alkylene, most preferably C 15-C
17
straight chain alkyl or alkylene such that the IV of the parent fatty acid of
this
R10 group is from about 20 to about 100, preferably from about 30 to about
<t~.a ..
~F":~.-'
~~.!s
12
70, most preferably from about 35 to about 60; and R and X(-) have the same
meaning as before.
Tallow is a convenient and inexpensive source of long chain alkyl and
alkenyl materials.
A specific example of a diester quaternary ammonium compound
suitable for use in this invention herein include:
1,2-ditallowyloxy-3-(trimethyl ammonio)propane chloride.
Other examples of suitable diester quaternary ammoniums of this
invention are obtained by, e.g.: replacing "tallowyl" in the above compounds
to with, for example, cocoyl, palmoyl, lauryl, oleyl, stearyl, palmityl, or
the like;
replacing "methyl" in the above compounds with ethyl, propyl, isopropyl,
butyl, isobutyl; t-butyl, or the hydroxy substituted analogs of these
radicals;
replacing "chloride" in the above compounds with bromide, methylsulfate,
formate, sulfate, nitrate, and the like.
In fact, the anion is merely present as a counterion of the positively
charged quaternary ammonium compounds disclosed herein. The scope of
this invention is not considered limited to any particular anion. When the
DEQA (A) material is (2), the requirement for Component (B) is not met by
this material and it is preferably not present, except in small amounts
unless, a
third softener active is present in the mixture.
The materials herein can be prepared by standard esterification and
quaternization reactions, using readily available starting materials. General
methods for preparation are disclosed in U.S. Pat. No. 4,137,180.
5. A co-active fabric softening material suitable for use as Component (B)
in the composition of the invention can also have the formula:
11
(R)4-m - NC+) - I(CH2)n - Y- R lm X(-)
(5)
W~ 95/16766 9 ~ ~ P~T/US94/14255
13
wherein each Rl 1 is .a long chain C 12-C22 hydrocarbyl, or substituted
hydrocarbyl substituent, preferalbly Clg-Clg alkyl or alkylene, most
preferably
C 15-C 17 straight chain alkyl or a.lkylene such that the IV of the parent
fatty acid
of this Rl 1 group is from about 20 to about 100, preferably from about 30 to
about 70, most prefezably from about 35 to about 60; and each m, n, 1', R and
X(-) have the same m earring as before. When the DEQA (A) material is ( 1 ),
the
requirement for component (B) i.s not met by this material and it is
preferably not
present, except in small amounts, unless, a third softener active is present
in the
mixture.
The present invention ma.y also contain mixtures of the various co-active
fabric softening materials.
'the compositions of the present invention herein comprise from about 15%
to about 35%, preferably from about 20% to about 32%, most preferably from
about
22% to about 27% of component (A) + component (B).
The ratio of component (A) to component (B) is from about 0.2:1 to about 8: l,
preferably from about 0.25:1 to about 4: l, most preferably from about 0.3:1
to about
1.5:1.
(C). The Acid Cornponent
- The present invention utilizes an acid of sufficient concentration to keep
the
pH at the desired level and optionally, to fully protonate component (B) to
the extent
that it is not already quaternized. The composition of the present invention
is
prepared using a molten premix oiF components (A) + (B), (hereinafter referred
to as
premix). The premix i;~ injected into an acid/water seat, then high shear
milling is
conducted and, electrolyte is added in no specific order. The electrolyte is
selected
from the group consisting of the Ciroup IA and IIA metals of the periodic
table of
WO 95116766 PCT/US94114255
;3 s '? C~ ; °r .rl ~
c., r ! ".:: ~..~ !
l4
elements, e.g., calcium chloride, sodium chloride, potassium bromide, and
lithium
chloride, and ammonium salts, e.g., ammonium chloride and lysine HCI.
Typically,
the acid/water seat has an acid concentration up to about 2%.
Typically the acid to (B) molar ratio is from about 0:1 to about 1.2:1.
Typically the acid to (A) molar ratio is from about 0:1 to about 0.2:1. The
neat pH
of the final composition is preferably from about 2.5 to about 4
Suitable acids include the Bronsted acids, especially inorganic mineral acids
and organic acids such as carboxylic acids. Carboxylic acids include, in
particular,
the low molecular weight (CI-CS) carboxylic acids of the formula R12-COOH (R12
being a C1-CS or H alkyl group). Suitable organic acids are selected from the
group
having the formula R13CH2S03H, wherein R13 is hydrogen or CI to C4 alkyl.
Suitable specific organic acids include fornlic, methylsulfonic,
ethylsulfonic, citric,
gluconic, and aromatic carboxylic acids like benzoic acid. Suitable inorganic
acids
include HCI, HBr, H2S04, H2S03, HN03, and H3F'0~,.
Preferred acids are phosphoric, formic, acetic, hydrochloric, citric, and
methylsulfonic acids. Mixtures of the above organic and inorganic acids are
also
suitable. ~ Typically, acids such as citric, hydrochloric, phosphoric, and
sulfi~ric are
used because of their low cost and availability.
(D). Liquid Carrier
The compositions of the present invention herein comprise from about 60% to
about 90%, preferably from about 65% to about 85% of an aqueous liquid
carrier.
The preferred aqueous carrier is water which can contain minor ingredients.
(E). Oytional Ingredients
Fully-formulated fabric softening compositions made by the process of the
present invention can optionally contain one or more of the following
ingredients.
1. Silicone Component
The fabric softening compositions herein optionally contain an aqueous
emulsion of a predominantly linear poiydialkyl or alkyl aryl siloxane in which
the
2 ~~ 15 1 7 9
alkyl groups can have from one to five carbon atoms and can be wholly, or
partially, fluoridated. These siloxanes act to provide improved fabric
benefits.
Suitable silicones are polydimethyl siloxanes having a viscosity, at
25°C, of
from about 100 ito about 100,000 centistokes, preferably from about 1,000 to
about 12,000 centistokes. In some applications as low as 1 centistoke
materials are preferred.
The fabric softening compositions herein can contain from about 0.1
to about 10%, of the silicone component.
2. Thickenin, Agent
1 o Optionally, the fabric softening compositions herein contain from 0%
to about 3%, preferably from about 0.01% to about 2%, of a thickening agent.
Examples of suitable thickening agents include: cellulose derivatives,
synthetic high nnolecular weight polymers (e.g., carboxyvinyl polymer and
polyvinyl alcohol), and cationic guar gums.
The celhulosic derivatives that are functional as thickening agents
herein can be characterized as certain hydroxyethers of cellulose, such as
Methocel, marketed by Dow Chemicals, Inc.; also, certain cationic cellulose
ether derivatives, such as Polymer JR-125, JR-400, and JR-30M, marketed by
Union Carbide.
2o Other effective thickening agents are cationic guar gums, such as
Jaguar Pluses, marketed by Stein Hall, and Gendrive~ 458, marketed by
General Mills.
Preferred! thickening agents herein are selected from the group
consisting of methyl cellulose, hydroxypropyl methylcellulose, hydroxybutyl
methylcellulose, or mixtures thereof, said cellulosic polymer having a
viscosity in 2% aqueous solution at 20°C of from about 15 to about
75,000
centipoises.
3. Soil Release Agent
In the present invention, an optional soil release agent may be added.
3o The addition of the soil release agent may occur in combination with the
premix, in combination with the acid/water seat, before or after electrolyte
addition, or afte-r the final composition is made. The softening composition
,; .
16
prepared by the process of the present invention herein can contain from 0% to
about 10%, preferably from 0.2% to about 5%, of a soil release agent.
Preferably, such a soil release agent is a polymer. Polymeric soil release
agents useful in the present invention include copolymeric blocks of
terephthalate and polyethylene oxide or polypropylene oxide, and the like.
A preferred soil release agent is a copolymer having blocks of
terephthalate and polyethylene oxide. More specifically, these polymers are
comprised of repeating units of ethylene terephthalate and polyethylene oxide
terephthalate at ;~ molar ratio of ethylene terephthalate units to
polyethylene
l0 oxide terephthalate units of from 25:75 to about 35:65, said polyethylene
oxide terephthal~ate containing polyethylene oxide blocks having molecular
weights of from. about 300 to about 2000. The molecular weight of this
polymeric soil release agent is in the range of from about 5,000 to about
55,000.
Another preferred polymeric soil release agent is a crystallizable
polyester with repeat units of ethylene terephthalate units containing from
about 10% to about 15% by weight of ethylene terephthalate units together
with from about 10% to about 50% by weight of polyoxyethylene
terephthalate units, derived from a polyoxyethylene glycol of average
2o molecular weight of from about 300 to about 6,000, and the molar ratio of
ethylene terephthalate units to polyoxyethylene terephthalate units in the
crystallizable polymeric compound is between 2:1 and 6:1. Examples of this
polymer include the commercially available materials ZelconT'''t 4780 (from
Dupont) and Milease~ T (from ICI).
Highly preferred soil release agents are polymers of the generic
formula:
O O O O
X-(OCH2CH2) (O--C-R14 C) -OR15)u(O-~-R14_OCI_p)(CH2CH20-)n-X
p
3o in which each X can be a suitable capping group, with each X typically
being
selected
::'
v.'
WO 95/16766 PCT/US94114255
17
from the group consisting of H, and alkyl or acyl groups containing from about
1 to
about.4 carbon atoms. p is selected for water solubility and generally is from
about 6
to about 113, preferably from about 20 to about 50. a is critical to
formulation in a
liquid composition having a relatively high ionic strength. There should be
very little
material in which a is greater than 10. Furthermore, there should be at least
20%,
preferably at least 40°/., of material in which a ranges from about 3
to about 5.
The R14 moieties are essentially 1,4-phenylene moieties. As used herein, the
term "the R14 moieties are essentially 1,4-phenylene moieties" refers to
compounds
where the R14 moieties consist entirely of 1,4-phenyiene moieties, or are
partially
substituted with other arylene or alkarylene moieties, alkylene moieties,
alkenyiene
moieties, or mixtures thereof. Arylene and alkarylene moieties which can be
partially
substituted for 1,4-phenylene include 1,3-phenylene, 1,2-phenylene, 1,8-
naphthylene,
1,4-naphthylene, 2,2-biphenylene, 4,4-biphenylene, and mixtures thereof.
Alkylene
and alkenylene moieties which can be partially substituted include 1,2-
propylene, 1,4-
butylene, 1,5-pentylene, 1,6-hexamethylene, 1,7-heptamethylene, 1,8-
octameth~lene,
1,4-cyciohexylene, and mixtures thereof.
For the R14 moieties, the degree of partial substitution with moieties other
than 1,4-phenyiene should be such that the soil release properties of the
compound
are not adversely affected to any great extent. Generally the degree of
partial
substitution which can be tolerated will depend upon the backbone length of
the
compound, i.e., longer backbones can have greater partial substitution for 1,4-
phylene moieties. Usually, compounds where the R14 comprise from about 50% to
about 100% 1,4-phenylene moieties (from 0% to about 50% moieties other than
1,4-
phenylene) have adequate soil release activity. For example, polyesters made
according to the present invention with a 40:60 mole ratio of isophthalic (1,3-
phenyiene) to terephtlhalic (1,4-phenylene) acid have adequate soil release
activity.
However, because most polyesters used in fiber making comprise ethylene
terephthalate units, i,t is usually desirable to minimize the degree of
partial
~8 ~ ~
substitution with moieties other than 1,4-phenylene for best soil release
activity. Preferahly, the R14 moieties consist entirely of (i.e., comprise
100%)
1,4-phenylene moieties, i.e., each R14 moiety is 1,4-phenylene.
For the R15 moieties, suitable ethylene or substituted ethylene moieties
include ethylene, 1,2-propylene, 1,2-butylene, 1,2-hexylene, 3-methoxy-1,2-
propylene, and mixtures thereof. Preferably, the R15 moieties are essentially
ethylene moieties, 1,2-propylene moieties, or mixtures thereof. Inclusion of a
greater percentage of ethylene moieties tends to improve the soil release
activity of compounds. Surprisingly, inclusion of a greater percentage of 1,2-
1o propylene moieties tends to improve the water solubility of compounds.
Therefore; the use of 1,2-propylene moieties or a similar branched
equivalent is desirable for incorporation of any substantial part of the soil
release component in the liquid fabric softener compositions. Preferably, from
about 75% to about 100%, are 1,2-propylene moieties.
The value for each p is at least about 6, and preferably is at least about
10. The value for each n usually ranges from about 12 to about 113.
Typically the value for each p is in the range of from about 12 to about 43.
A more complete disclosure of soil release agents is contained in U.S.
Pat. Nos.: 4,661,267, Decker, Konig, Straathof, and Gosselink, issued Apr.
28, 1987; 4,711.,730, Gosselink and Diehl, issued Dec. 8, 1987; 4,749,596,
Evans, Huntington, Stewart, Wolf, and Zimmerer, issued June 7, 1988;
4,818,569, Trinh, Gosselink, and Rattinger, issued April 4, 1989; 4,877,896,
Maldonado, Trinh, and Gosselink, issued Oct. 31, 1989; 4,956,447, Gosselink
et al., issued Sept. 11, 1990; and 4,976;879, Maldonado, Trinh, and
Gosselink, issued Dec. 11, 1990.
These soil release agents can also act as scum dispersants.
4. Scum Dis e,_p rsant
In the present invention, the premix can be combined with an optional
scum dispersant., other than the soil release agent, and heated to a
temperature
at or above the nnelting points) of the components.
'i
~'_
19 1
The preferred scum dispersants herein are formed by highly
ethoxylating hydrophobic materials. The hydrophobic material can be a fatty
alcohol, fatty acid, fatty amine, fatty acid amide, amine oxide, quaternary
ammonium compound, or the hydrophobic moieties used to form soil release
s polymers. The preferred scum dispersants are highly ethoxylated, e.g., more
than about 17, preferably more than about 25, more preferably more than
about 40, moles of ethylene oxide per molecule on the average, with the
polyethylene oxide portion being from about 76% to about 97%, preferably
from about 81% to about 94%, of the total molecular weight.
io The level of scum dispersant is sufficient to keep the scum at an
acceptable, preferably unnoticeable to the consumer, level under the
conditions
of use, but not enough to adversely affect softening. For some purposes it is
desirable that the: scum is nonexistent. Depending on the amount of anionic or
nonionic detergent, etc., used in the wash cycle of a typical laundering
process,
1s the efficiency of the rinsing steps prior to the introduction of the
compositions
herein, and the ~,vater hardness, the amount of anionic or nonionic detergent
surfactant and detergency builder (especially phosphates and zeolites)
entrapped in the fabric (laundry) will vary. Normally, the minimum amount of
scum dispersani: should be used to avoid adversely affecting softening
2o properties. Typically scum dispersion requires at least about 2%,
preferably at
least about 4% (at least 6% and preferably at least 10% for maximum scum
avoidance) based upon the level of softener active. However, at levels of
about 10% (relative to the softener material) or more, one risks loss of
softening efficacy of the product especially when the fabrics contain high
25 proportions of nonionic surfactant which has been absorbed during the
washing operation.
Preferred scum dispersants are: Brij'~ 700; Varonic~ U-250;
Genapol~ T-500, Genapol T-800; Plurafac~ A-79; and Neodol~ 25-50.
20
1
5. Bactericides
Examples of bactericides used in the compositions of this invention
include glutaraldehyde, formaldehyde, 2-bromo-2-nitro-propane-1,3-diol sold
by Inolex Chemiicals, located in Philadelphia, Pennsylvania, under trade mark
Bronopol, and a mixture of 5-chloro-2-methyl-4-isothiazoline-3-one and 2-
methyl-4-isothiazoline-3-one sold by Rohm and Haas Company under the
trade mark Kathon CG/ICP. Typical levels of bactericides used in the present
compositions are from about 1 to about 1,000 ppm by weight of the agent.
l0 6. Other Ot>tional Ingredients
The present invention can include optional components conventionally
used in textile treatment compositions, for example, short chain alcohols such
as ethanol, or propylene glycol, colorants, perfumes, preservatives, optical
brighteners, opacifiers, surfactants, stabilizers such as guar gum and
polyethylene glycol, anti-shrinkage agents, fabric crisping agents, spotting
agents, germicides, fungicides, anti-oxidants such as butylated hydroxy
toluene, anti-corrosion agents, and the like.
The compositions of the present invention are preferably used in the
rinse cycle of the conventional automatic laundry operations. Generally, rinse
water has a temperature of from about 15~C to about 60~C.
Fabrics or fibers are contacted with an effective amount, generally
from about 20 ml to about 300 ml (per 3.5 kg of fiber or fabric being
treated),
of the compositions herein in an aqueous bath. Of course, the amount used is
based upon the judgment of the user, depending on concentration of the
softening materials, (A) + (B), fiber or fabric type, degree of softness
desired,
and the like. Typically, from about 20 ml to about 300 ml of 9% to 40%
dispersion of thf: softening materials (A) + (B) are used in a 25 gallon
laundry
rinse bath to soften and provide antistatic
WO 95!16766 , ~ ~ ~ ~ PCTIUS94/14255
21
benefits to a 3.5 kg load of mixed fabrics. Preferably, the rinse bath
contains from
about 20 ppm to about 250 ppm of the fabric softening materials (A) + (B)
herein.
More preferably for Uoted States conditions, the rinse bath contains from
about
50 ppm ~.o about 150 ppm of the fabric softening materials (A) + (B). More
preferably for European conditions, the rinse bath contains from about 250 ppm
to .
about 450 ppm of the fabric soi~ening materials (A) + (B). More preferably for
Japanese conditions, the rinse bath contains from about 30 ppm to about 80 ppm
of
the fabric softening materials (A) + (B). These concentration levels achieve
superior
fabric softening and static control.
The invention is exemplified by the following non-limiting examples in which
all
numerical values are approximations consistent with normal experience.
EXAMPLE
I
FORMULA: 1 2 3 4 5
(fit.% )
Di(2-amidoethyl)
methyl quaternary0 ~ 6.25 12.50 18.75 25.00
ammonium salt'
DEQA2 24.5 18.75 12.50 6.25 0
Genapol~ T-1101.00 1.00 1.00 1.00 1.00
Ethanol 0.95 0.92 0.61 0.30 0.00
Isopropanol 0.00 0.68 1.39 2.08 2.78
Perfume 1.20 1.20 1.20 1.20 1.20
Silicone DC-200*0.19 0.19 0.19 0.19 0.19
Silicone Antifoam*0.32 0.32 0.32 0.32 0.32
DC-2210
Soil Release Polymer 0.50 0.50 0.50 0.50 0.50
HCl 0.03 0.03 0.03 0.03 0.03
WO 95/16766 ~ 1 l
PCTIUS94/14255
22
Lysine HC1 0.75 0.75 0.75 0.75 0.75
Deionized Water 70.56 69.40 69.01 68.63 68.23
* Product of the Dow-Corning Corporation.
1Di(2-tallowyamidoethyl)ethoxylated methyl ammonium methylsulfate (sold under
the tradename Varisoft 222).
2N,N-di( hydrogenated tallowyl-oxy-ethyl) N,N-dimethyl ammonium chlorid$
EXAMPLE I PREPARATI~N
The first three ingredients in each Formula are co-melted in a pyrex beaker,
0
covered with a concave watchglass, for three hours at about 80-85 C to form
the
premix. The water, HCI, and silicone antifoam are separately weighed into a
sealed
container and heated to about 83°C to form the water seat. The premix
is then
0
injected into the water seat over three to four minutes at about 72 C while
stirnng at
from about 1500 to about 3000 r.p.m.. A 15% aqueous solution of Lysine/HCl is
o
added to the water seat/premix dispersion over seven minutes at about 71 C
while
stirring at from about 500 to about 1000 r.p.m.. The perfume, and the silicone
DC-
200 are added to the dispersion over thirty seconds. The dispersion is milled
for two
minutes at about 70°C at from about 4000 to about 8000 r.p.m. of the
mill. A 40%
aqueous solution of soil release polymer is added to the dispersion over two
minutes
0
at about 66 C while stirnng at from about 500 to about 1000 r.p.m.. The
dispersion
is then chilled in an ice bath at about 25°C for 8 minutes while
stirring at from about
200 to about 500 r.p.m..
Formula Initial # Days 0 C 4 10
Viscosity (cP) (cP) (cP)
(CP3
1 59 145 Gel2d4 Gel3d Gel(3d
2 243 28 Gel(2d Gel3d- 275
3 281 28 Gel3d >500 248
W~ 95/16766 ~ ~ 9 ~ U,' PCT/US94I14255
23
Concentrated compositions containing rriiixtures of the two softener active
materials, Component (A) and Component (B), (Formulas 2-4), have more stable
viscosities at temperaoures down to 10~C, than compositions containing only
the
individual softener active Component (A) or Component (B), (Formulas 1 and 5).
COMPARATIVE EXAMPLE II
FORMULA -1 2 3.
(Wt.%)
Ditallow Imidazoline25 ---- 16.67
~~rnide5
DE~A6 ---- 25 8.33
Soil Release~Polym~er---- ~ 0.5 0.17
CaCl2 0.4 0.35 0.38
Perfume 1.3:5 1.35 1.35
DC-200 Silicone* 0.1 ~ 0.19 0.19
DC-2210 Silicone*~ 0.32 0.32 0.32
Antifoam
.~':~eservative(Kathon)O.O~D03 0.0003 0.0003
HCl 1.70 0.02 1.14
EtOH ---- 3.5 1.17
Deionized Water 71.0 68.8 70.3
* Product of the Dow-Corning Corporation.
1-[hydrogenated tallowyl amido]ethyl-2-hydrogenated tallow imidazoline
6N,N-di(hydrogenated tallowyl-oxy-ethyl)-N,N-dimethyl ammonium chloride
3The abbreviation "cP" denotes centipoise.
4The abbreviation °'d" denotes days.
WO 95J16766 d"~ ~ ~~ ~ ~ ~ ~ PCTT/iJS94/14255
24
EXAMPLE II PREPARATION
Formula 1 Preparation
Ditallow Imidazoline Amide in the amount of 375g is melted by heating to
0
about 93 C. This molten softener is then dispersed into 3768 of deionized
water at
0
about 82 C containing 90g of a 28.25% HCl solution and 4.8g of the DC-2210
silicone antifoam over five minutes while stirring with an IKA model RW20 DZM
stirrer at 400-600 r.p.m.. The resulting dispersion is stirred for an
additional four
minutes. A second aliquot of about 82~C deionized water in the amount of 5808
is
stirred into the thick dispersion over two and one half minutes. A blend of
20.25g of
perfume and 2.858 of DC-200 silicone fluid is then added to the dispersion
over
thirty seconds, followed by two and one half minutes of mixing with an IKA
Ultra-
Turrax T50 high shear mill at 8000 r.p.m.. The dispersion is then cooled to
room
temperature over three minutes by passing it through a small plate and frame
heat
exchanger. The preservative in the amount of 0.3g is added at room temperature
following the cool down.
Formula 2 Preparation
N;N-di(hydrogenated tallowyl-oxy-ethyl)-N,N-dimethyl ammonium chloride
in the amount of 43 lg and 24g of ethanol are melted at about 91 ~C. This
molten
0
softener is dispersed into 954g of deionized water at about 82 C containing
8.258 of
a 0.968 N HCl solution, 4.8g of DC-2210 silicone antif~am and 18.75g of a 4'0%
solution of soil release polymer over five and one half minutes while stirring
at 800-
1100 r.p.m.. Subsequently, 35g of an aqueous 15% CaCl2 solution is stirred
into the
dispersion over four and one half minutes. A blend of 20.,258 of perfume and
2.858
of DC-200 silicone fluid is then added to the dispersion over thirty seconds,
followed
by two and one half minutes of mixing with a high shear mill at 8000 r.p.m..
The
preservative in the amount of 0.38 is added just prior to cool-out. The
dispersion is
then cooled to room temperature over two and one half minutes by passing
through a
small plate and frame heat exchanger.
WO 95/16766 ~ ~ PC:T/US94114255
Formula 3 Preparation
An amount of 105g of Fonmula 2 is mixed with 214g of Formula 1 at room
temperature.
Formula Initial Visco;cityDays at 4 C Viscosity after
0
(cP) exposure to
4 C
cP)
1 38 5 >5000
2 78 3 gilled
3 43 3 gilled
The above table; demonstrates the disadvantage of the preparation process in
which the actives are not co-melteii, as described in Example II. The use of a
molten
premix of the softener actives C;orrnponent (A) and Component (B), provides a
product with superior viscosity stability.
EXAMPLE ~ _III _IV V VI
(Wt.%)
Ditallow
Imidazoline Amide?14.3 14.3 14.3 11.0
DEQAB 7.7 8.7 9. 7 12.0
CaCl2 0.375 0.375 0.375 0.375
Perfume ~ 1.35 1.35 1.35 1.35
DC-200 Silicone*0.19 0.19 0.19 0.19
DC-2210 Silicone*0.32 0.32 0.32 0.32
Preservative(Kathon)0.0003 0.0003 0.003 0.003
Antifoam
HCl 0.97 0.97 0.97 0.75
EtOH 1.25 1.42 1.58 1.95
Deionized Water73.5 72.3 71.2 72.1
WO 95/16766 PCTlUS94114255
'.a ~ .7 y'~ .j'! °;/
i
~:. . ~ ~..- ;. ~ !
26
* Product of the Dow-Corning Corporation.
I-[h~enated tallowyl amido~ethyl-2-hydrogenated tallow imidazoline
BN,N-di(hydrogenated tallowyl-oxy-ethyl)-N,N-dimethyl ammonium chloride
EXAMPLE PREPARATION
Example III
Ditallow Imidazoline Amide in the amount of 214g, 1 lS.Sg N,N-di(tallowyI-oxy-
ethyl)-N,N-dimethyl ammonium chloride and 19g of ethanol are co-melted at
about
91 ~C. This molten softener mixture is dispersed into 1,1028 of deionized
water at
about 82~C containing 14.558 of HCI and 4.88 of DC-2210 silicone antifoam over
five and one half minutes while stirring at 800-1100 r.p.m.. Subsequently,
37.58 of
an aqueous 15% CaCl2 solution is stirred into the dispersion over four and one
half
minutes. A blend of 20.258 of perfi~me and 2.858 of DC-200 silicone fluid is
then
added to the dispersion over thirty seconds, followed by two and one half
minutes of
mixing with high shear mill at 8000 r.p.m.. The dispersion is then cooled to
room
temperature over two and one half minutes by passing it through a small plate
and
frame heat exchanger.
WO 95/16766 PCT/US94/14255
~ ? ~9~~~
27
The preservative in the amount of 0.3g is added at room temperature following
cool-
out.
Examples IV. V and Vlf
These Examples are prepared in the same manner as Example III except that
the amounts of imidaz~oline, N,N-di(hydrogenated tallowyl-oxy-ethyl)-N,N-
dimethyl
ammonium chloride, etihanol, and HCl vary according to the formulas shown
above.
Example Irnitial ViscosityDays at 4 C Viscosity after
(ep~ exposure to
4 C
cP
III 18 42 60
IV 1S 23 SO
V 18 30 95
~ 18 29 33
This demonstrates the advantage of using a molten premix and the type of
high shear dispersion that is possible with a high shear mill.