Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ wo 95118799 ~ 2 1 8 0 6 5 1 PCT/CA94hllO688
TITLE OF THE INVFNTION
- PBNYL HETEROCYCLES AS COX-2 INHIBITORS
BACKGR~UNI; OF THI~ INVENTION
This invention relates to c~ o~"~l~ and r~
cnmrociti-~nC for the treatment of cyclooAy~ .ase mediated diseases and
methods of treatment thereof.
Non-steroidal, A"l;i"ll,.""",.l"~y drugs exert most of their
A,,l;;,,llA~,,,,,AIu,~, analgesic and antipyretic activity and inhibithormone-
0 iLduced uterine contractions and certain types of cancer growth throllgh
inhibition of prostAgl~n-lin G/H synthase, also known as .,y~lou~y~ Lla
Up until recently, only one form of cyclouAy~G--.~se had been
Cl~alaL,t~ Gd, this cull~,~ulldillg to cyclooAy~,",-asc-l or the cv..aLi~ ive
enzyme, as origin.~lly identified in bovine serninal vesicles. Recently the
5 gene for a second inducible form of .,y. lou~y~ ase (cyclouA~I,_l-ase-2)
has been cloned, 5~ ;1 and . ~ d from chicken, murine and
human sources. This enzyme is distinct from the CYCIOOAY~ aG-1
which has now also been cloned, s~ d and ~ t~ . ;,. d from
sheep, murine and human sources. The second form of cycluuAy~G-~Iaae,
20 CyClOuA~ , làSC-2, is rapidly and readily inducible by a number of agents
including rnitogens, ....lu~ .. hnrmrnPc, cytokines and growth facl.ors.
As prost~] ~nrlinc have both physiological and p~thnl~gi~AI roles, we
have ~ d that the COIlaliluli~, enzyme, cyclooAy~,l.ase-l, is
responsible, in large part, for Pnrlr.gPnn~lc basal release of prr~ct~glAn~linc
2~ and hence is i---l,u-l~u-l in their physiological functions such as the
IIIA;II~ .~AI~ e of ~;aal,lllillt _1;"~1 integrity and renal blood flow. In
contrast, we have .~ d that the inducible form, cyclouA~ I.asc-2, is
mainly IL,a~ l for the pathological effects of ~u~ wh~re
rapid induction of the enzyme would occur in response to such agents as
infl,.. ~ agents,hrrrnnnPs growthfactors,andcytokines. Thus,a
selective irihibitor of .~y.~lou~y~,_.làsG-2 will have similar
A. ~ y, antipyretic and analgesic properties to a conventional
non-steroidal A~ y drug, and in addition would inhibit
hol,l.u.- -induced uterine cl~ntr~rti~nc and have potential anti-cancer
WO 95118799 ! ", i 1 2 ~ 8 0 6 5 ~ PCTICA94100688
2 -
effects, but will have a (~ lf d ability to induce some of the
,llr~ lll-based side effects. In particular, such a compound should
have a reduced potential for ~iL~ l toxicity, a reduced potential
for renal side effects, a reduced effect on bleeding times and possibly a
5 lessened ability to induce asthma attacks in aspirir~-sensitive asthmatic
subjects.
SUMM~RY OP TTTF. INV~NlION
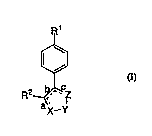
The invention C.IC~ f ~ novel compounds of Formula I
useful in the treatment of ~ y~,_.,ase-2 mediated diseases.
R1
¢~
R2 ~, CZ
ax - y
The invention also ~ `f C certain l~
C-)' "1'0`'1 ;O!~C and methods for treatment of ~ y~lo~y~_.lase-2 mediated
diseases ~ the use of c~ 'i of Formula I.
WO 95/18799 PCT/C~94/00688
~ ` 2 ~ 8 0 6 5 1
- 3 -
DF.TATT .Fn DESCRIPTION OF T~TF INVF.NTION
The invention on~omr~ the novel compound of Formula
I useful in the treatment of cyclo~ as~-2 mediated diseases
~,
R2_~
ax - y
I
or ~ "~ r~lly acceptable salts thereof wherein:
X-Y-Z-is selected from the group c~ of:
(a) -CH2CH2CH2-,
(b) -C(O)CH2CH2-,
(c) -CH2CH2C(O)-,
(d) -CR5(R5 )-O-C(O)-,
(e) -C(o)-o-CR5(R5 )-,
(f) -CH2-NR3-CH2-.
(g) -CR5(R5 )-NR3-C(o)-
(h) -CR4=CR4 -S-,
(i) -S-CR4=CR4 -,
(j) -S-N=CH-,
(k) -CH=N-S-
(1) -N=CR4-o-,
(m) -O-CR4=N-,
(n) -N=CR4-NH-,
(o) -N=CR4-S-, arld
(p) -S-CR4--N-;
(q) -C(o)-NR3-CR5(R5 )-,
(r) -R3N-CH=CH-, provided Rlis not -S(0)2Me.
(s) -CH=CH-NR3-, provided Rlis not -S(0)2Me,
WO 95/18799 PCT/CA94/00688
~ - 2~80651
- 4 -
when side b is a double bond, and sides a an c are single bonds; and
X-Y-Z~is selected from the group r,~".~;.~l;.,~ of:
(a) =CH-0-CH=, and
(b) =CH-NR3-CH=,
(c) ---N-S-CH=,
(d) =CH-S-N=,
(e) =N-0-CH=,
(f) =CH-0-N=,
0 (g) =N-S-N=,
(h) =N-0-N=,
when sides a and c are double bonds and side b is a single bond;
Rl is selected from the group c~mgictin~ of:
(a) S(0)2CH3,
(b) S(0)2NH2.
(c) S(0)2NHC(O)CF3,
(d) S(O)(NH)CH3,
(e) S(O)(NH)NH2,
(f) S(O)(NH)NHC(O)CF3,
(g) P(O)(CH3)0H, and
(h) P(0)(CH3)NH2,
R2 is selected from the group Gl ~ of:
(a) C1 6aLlcyl,
(b) C3, C4, C5, C6, and C7, cycloaL~yl,
(c) mono-, di- or tri-~ t' ;l phenyl or naphthyl wherein the
1 i I .. Il iS selected from the group ~ of:
(1) hydrogen,
(2) halo,
(3) Cl 6alkoxy, t
(4) Cl 6aL~ylthio,
(5) CN,
(6) CF3,
(7) Cl 6alkyl,
(8) N3,
WO 95/18799 ~ 8 0 6 5 1 PCT/CA94/~lK88
- 5 -
(9) -C02H,
(10) -C02-Cl 4alkyl,
(11 ) -C(RS)(R6)-OH,
(12) -C(RS)(R6)-O-C1 4alkyl, and
(13) -Cl 6alkyl-C02-R5;
(d) mono-, di- or tri-~ 1 h~lualyl wherein the
l~t~,luclLyl iS a monocyclic aromatic ring of S atoms, said
ring having ûne hetero atom which is S, O, or N, and
optionally 1, 2, or 3 ~rlrlition~lly N atoms; or
the h~ u~yl is a lllullo~y-,Lc ring of 6 atoms, said ring
having one hetero atom which is N, and optionally 1, 2, 3, or
4 ~ itinn~l N atoms; said D~ are selected from the
group CullD-DLillg of:
(1) hydrogen,
(2) halo, including fluoro, cloro, bromo and iodo,
(3) C1 6aLIcyl~
(4) C1 6aLIcoxy,
(S) Cl-6alkylthi
(6) CN,
(7) CF3,
(8) N3,
(9) -C(RS)(R6)-OH, and
(10) -C(R5)(R6):0-Cl 4alkyl;
(e) 1,. . ., ~ ,l .. ~ . ucuyl which includes the benzo fused analogs l~f
25 (d);
R3 is selected from the group C--ngictin~ of:
(a) hydrogen,
(b) CF3,
(c) CN,
(d) Cl 6alkyl~
(e) hydroxyC1 6aLlcyl,
(f) -C(O)-Cl 6alkyl,
(g) optionally D~ t~ d
(1) -Cl s alkyl-Q,
WO95118799 ~ j S 2 1 8 0 6 5 1 PCTICA94100688
-- 6 -
(2) -Cl 3aL~yl-O-Cl 3aL1~yl-Q,
(3) -Cl 3aL1cyl-S-CI 3aLl~yl-Q,
(4) -Cl 5 aL~yl-O-Q, or
(5) -Cl 5 aLIcyl-S-Q,
wherein the s. .l ,~ jl, lrl ,l resides on the aLlcyl and the
~,~1.,~1;ll,..~1isCl 3alkyl;
(h) -Q;
R4 and R4 are each ;".1~ .,lly selected from the group c~ncictin~ of:
(a) hydrogen,
(b) CF3.
(c) CN,
(d) C1 6aLlcyl,
(e) -Q,
(f j) -O-Q;
g) -S-Q. and
(h) optionally i,. ,I .~,l i 1. it~ ;
(1) -Cl s a~cyl-Q.
(2) -0-Cl 5 aL~yl-Q,
(3) -S-C1-5 alkyl-Q,
(4) -Cl 3alkyl-0-Cl 3alkyl-Q,
(5) -Cl 3alkyl-S-Cl 3alkyl-Q,
(6) -Cl-5 aL~cyl-O-Q,
(7) -Cl-5 aLIcyl-S-Q,
wherein the ~ .l resides on the aLlcyl and the
~"1,~1;ll,.. ~1 is Cl 3aUcyl, and
R5, R5, R6, R7 and R8 are each in~ p~nrllonfly selected from tbe group
Cl-ngictin~ Of
(a) hydrogen,
(b) Cl 6alkyl,
or R5 and R6 or R7 and R8 together with the carbon to which they
are attached form a saturated monocyclic carbon ring of 3, 4,
5, 6 or 7 atoms;
WO 95118799 Pcr/CA94/00688
2 1 8 0 6 5 1
~, . ...
Q is C02H, C02-Cl 4aL~yl, tetrazolyl-5-yl, C(R7)(R8)(oH), or
C(R7)(R8)(0-Cl~aLIcyl);
provided that when X-Y-Z is -S-CR4= CR4, then R4 and R4 are otller
5 than CF3 .
One Class within this t~ )Ul~ CII~ are the :UlI-I)UUl~d:~ of
Formula I
Rt
¢J
R2_ '$`CZ
ax_y
or r~ "~ y ~rcept~lhl~ salts thereof wherein:
X-Y-Z- is selected from the group C~ ~"~ of -C(o)-o-CR5(RS )-
when side b is a double bond, and sides a and c are single bonds; and
20Rl is selected frûm the group c~ of:
(a) S(o)2cH3~
(b) S(0)2NH2,
R2 is selected from the group Cull~ lg of:
(a) C1 6aLlcyl,
25(b) C3, C4, C5, C6, and C7, cycloaLI~yl,
(c) heteroaryl,
(d) 1,. .,~I.h. ~u~yl,
(e) mono- or di ~ ,l,~l ;l l l~rd phenyl wherein the .~,l,~l i l l l- ,l is
selected from the group c~",~;~l;"g of:
30(1) hydrogen,
(2) halo,
(3) C1 6aL~oxy,
(4) C1 6alkylthio,
(5) CN,
WO 95/18799 PCTICA94/00688 ~
`.1` ` 1 '; 2 1 80~51
(6) CF3,
(7) C I -6alkY
(8) N3,
(9) -C02H,
(10) -C02-Cl 4alkyl,
(I 1) -C(R5)(R6)-oH,
(12) -C(RS)(R6)-0-C1 4alkyl, and
(13) -Cl 6aL~yl-C02-R5;
R5, R5 and R6 are each ;~ J~ lly selected from the group
o consisting of:
(a) hydrogen,
(b) C 1 -6aLlcyl,
or R5 and R6 together with the carbon to which they are attached
form a saturated monocyclic carbon ring of 3, 4, 5, 6 or 7
atoms.
For purposes of this srerifir~tion aLIcyl is defined to include
linear, branched, and cyclic structures, with C1 6aLIcyl including
including methyl, ethyl, propyl, 2-propyl, s- and t-butyl, butyl, pentyl,
20 hexyl, 1,1-dimethylethyl, ;y-,lo~lulJyl, cyclobutyl, cyclopentyl and
cyclohexyl. Similarly, C1 6aLIcoxy is intended to include alkoxy groups
of from I to 6 carbon atoms of a strai~ht, branched, or cyclic
cnnfi~llr~tinn FY:mnrlPs of lower alkoxy groups include methoxy,
ethoxy, propo%y, iS~Iu~Ju~y, ,y~,lu~lul~yloxy, cyclohexyloxy, and the
2s like. I~kewise, C1 6aLI~ylthio is intended to include alkylthio groups of
from I to 6 carbon atoms of a straight, branched or cyclic configuration.
Examples of lower alkylthio groups include ~ ylLllio, propylthio,
isu~lu~yliLio, cycloll.,~,lyllllio, etc. By way of illl-~tratinn, the propylthiogroup signifies -SCH2CH2CH3.
Ileh,.u~uyl includes furarl, thiophene, pyrrole, icnY~7~
i~othi~7nl-, pyra701e, oxazole, thia_ole, imitl~7nl- 1,2,3-nY~ 7nlP,
1,2,3-thi~ 7nlP, 1,2,3-tria_ole, I,3,4 oYz~rli~7nl~, 1,3,4-thi
1,3,4-tria_ole, l,2,5 nY~ 7~l-, 1,2,5-thiS~ 7nl~ pyridine, pyrida_ine,
WO 9~11#799 PCT/CA94/00688
~ 2 1 8 0 6 5 1
g
pyrimidine, pyrazine, 1,2,4-triazine, 1,3,5-triazine, 1,2,4,5-tetrazine, and
the like.
B~ul~,t~,lu~uyl includes the above heteroaryl rings to
which it is possible to fuse a benzene ring.
Exell".liryi.,g the invention are:
(a) 3-(4-(Aminosulfonyl)phenyl)-2-(4-fluO,u~ c;"yl)-5-(2-
hydroxy-2-propyl)thioph~n~,
(b) 3-(4-(Aminosulfonyl)phenyl)-2-(4-fluu,u~l~c,.yl)thiophene,
(c) 3-(4-(Arninosulfonyl)phenyl)-2-(4-fluulu~ ,llyl)-5-(2-
o propyl)thiophene,
(d) 3-(4-(AIlullo~ulrullyl)phenyl)-2-~y~loll~yltl~iophene~
(e) 5-(4-C~ubu~y~ ;llyl)-4-(4-(methylsulfonyl)phenyl)-
~1,;..~1,. ..~-2-carboxylic acid,
(f) 4-(4-Fluulu~ l,yl)-2-methyl-5-(4-(methylsulfonyl)phenyl)-
thiazole,
(g) 2-(4-I~luu~u~ llyl)-3-(4-(methylsulfonyl)phenyl)-2-
,y-,lu~,llt.,.l- 1-one
(h) 4-(4-(Methylsulfonyl)phenyl)-5-(4-fluo,u~l,.,l,yl)-
i~othi71~l ~
(i) 3-(4-Fluu,u~ ;,.yl)~(4-(methylsulfonyl)phenyl)-2-(SH)-
furanone,
(1') 3-(4-Fluu~u~ yl)-4-(4-(~ 11 rul1yl)phenyl)-2-(SH)-
furanone,
(k) 3-(4-Fluv~u~ yl)-4-(4-(methylsulfonyl)phenyl)furan,
(1) 5,5-Dimethyl-3-(4-nuulu~ ,.lyl)~(4-methylsulfonyl)-
phenyl)-2-(SH)-furanone,
(m) 2-(4-(Aminosulfonyl)phenyl)-3-(4-nuulu~ ,lyl)thioFhlon
and
(n) 3-(4-(T~ifluuluac~yl~.~.;o:~ -lfonyl)phenyl)-2-(4-
- 30 nuolu~ l,yl)thiophene,
(o) 3-(3-Fluu~u~ yl)-4-(4-(methylsulfonyl)phenyl)-2-(SH)-
furanone,
(p) 5,5-Dimethyl-3-(3-nuc"u~ yl)-4-(4-methylsulfonyl)-
phenyl)-2-(SH)-furanone,
WO 9~/18799 PCTIC~94/00688
2 1 8 0 6 5 1
- 10-
(q) 5,5-Dimethyl-3-(3-chlorophenyl)-4-(4-methylsulfonyl)-
phenyl)-2-(SH)-furanone,
(r) 3-(3,4-Dillu~lvL~ lyl)-4-(4-(methylsulfonyl)phenyl)-2-
(SH)-furanone,
S (s) 3-(3,4-Dicnlul~,pll~llyl)~(4-(methylsulfonyl)phenyl)-2-
(SH)-furanone,
(t) 5,5-Dimethyl-3-(3,4-di~lu~ ,llyl)-4-(4-methylsulfonyl)-
phenyl)-2-(5~)-furanone,
(u) 5,5-Dimethyl-3-(3,4-dichl~ pll~,l,yl)-4-(4-methylsulfonyl)-
phenyl)-2-(SH)-furanone,
(v) 5,5-Dimethyl-3-(4-chlorophenyl)~(4-methylsulfonyl)-
phenyl)-2-(SH)-furanone,
(w) 3-(2-Naphyhyl)-4-(4-(methylsulfonyl)phenyl)-2-(5~)-
furanone,
(x) 5,5-Dimethyl-3-(2-naphyhyl)~(4-(methylsulfonyl)phenyl)-
2-(SH)-furanone,
(y) 3-phenyl~(4-(1LIclllyli,ul~,llyl)phenyl)-2-(SH)-furanone.
Some of tne c~mroun~1s described herein contain one or
more a~y,Lull.~ ; centers and may thus give rise to ~' ~,VLU~ and
optical isomers. The present invention is meant to culll~ ,llclld such
possible L~;~t~ vlll~,l, as well as their racemic and resolved,
rn:mti~ 7 ir~11y pure forms and ~ "~ ;rz~lly ac~ - salts
thereof.
2s Some of the c ~ u~ ",~1~ described herein contain olefinic
double bonds, and unless specified otherwise, are meant to include both E
and Z geomet~ic isomers.
In a second embodiment, the invention . .~
r~ ",~ ;r:~l C~JLII~OSiliVUS for inhibiting cycl~o~ llaSC~ and for
3 treating ~loo~y~ lla~ mediated diseases as disclosed herem
c~ ....l..; ~;, .~ a ~ l l ;r~lly ~ l r carrier and a non-toxic
th~relltir~lly effective amount of comro~n-l of Formula I as described
above.
WO 9S118799 r ; ~ 2 1 8 0 6 5 I PCT/CA94/1~0688
- 11 -
Within this embodiment the invention ~ .""~ ses
".~ ir~l compositions fo} inhibiting cyclooxygenase-2 and for
treating CyCIOUAy~ .as~-2 mediated diseases as disclosed herein
C-:-"-l--;.~;"~a~,l.-."u~r~ lly acct;~blf carrierandanon-toxic
5 thPri~relltir~lly effective amount of compound of Formula I as described
above.
In a third embodiment, the invention ~ 5 a method
of inhihitin~ cycluuAy~ se and treating cyclo~Ayy~llasf mediated
diseases, adv~nt~Pollcly treated by an active agent that selectively
inhibits COX-2 in ~I-,f~ lcc to COX-l as disclosed herein cnmrricin~
aLI""i~ldlion to a patient in need of such treatment of a non-toxic
Il.~ .;.I.~..I;rz-lly effective amount of a cnmrounfl of Formula I as disclosed
herein.
For purposes of this ~ ic n a compound is said to
5 scl~li~,.,ly inhibit COX-2 in ~I~,f~ c to COX-l if the ratio of the IC50
c~ rl 1l l ~l ;f~n for COX- 1 inhihifiol7 to COX-2 inhihitinn is 100 or greater. The ~ r~ ir~l compositions of the present invention
comprise a c~ ,I.uu.,fl of Formula I as an active i~ lll or a
A ~ II;cSllly acceptable salt, thereof, and may also contain a
2 0 l.l ,_ . " ,~f ~. ll; r~lly orc ~Ppt~l - carrier and optionally other Il l~ ,- l l ;r
ingredients. The term ''rll= ,,,~.~f .~l;r~lly acceptable salts" refers to saltsprepared from ~ .,l ;r~lly o~ cept~hlP non-toxic bases includirlg
inorganic bases and organic bases. Salts derived from inorganic bases
irliclude ~1"",;"."", i""",.",;".", calicium, copper, ferric, ferrous, ]iithium,25 ",_~". .;""" m~ng71nir sa]its, "~ p~ ", sodium, zinc, arid
the like. Particularly preferred are the i- "",...,;"". ca]icium, mqgr-P~illm,
pU~s~iuLil~ and sodium sa]its. Salts derived from 1~ "~r~ lly
~c~ hle organic non-toxic bases include sa]its of primary, secondaly,
and tertialiy amines, ~ lrd amines includir~g naturally occurring
- 3 0 :,. ,1 .~l ;l,, ~ d amines, cyclic amirles, and basic ion exchange resins, such as
arginiine, betaine, caffeine, choline, N,N--dibenzylethyll .,~.l;-.,.;..f,
- diethylamine, 2-diethyl-l,,;.,f.~ll,-.,nl, 2-dimietbiyl~minneth~nnl
ethonflqminf- ethyl~llr~l;-lllillf ~ N-ethylmorpholine, N elljlylll;llrl;~ P
~11....III;llf, y,l~ O~i1lll;11f, histidine, Ilylll~ f ~ isopropylamine, Iysine,
WO 95/18799 ,, ~ 2 1 8 0 6 ~ ~ PCT/CA94/00688
- 12-
methyl~ r~lminf, morpholine, ~ f-, rirfritlinf~ polyamine resins,
procaine, purines, Lll~ol,lullPille, triethylamine, trimethylamine,
t}ipropylamine, Llul,l ~ ";"f, and the like.
It will be ImtlPrctood that in the fliccucgi~n of methods of
5 treatment which follows, I-,r~ S to the compounds of rormula I are
meant to also include the l.1. . " IA~ lly ~rcf-pt~hle salts.
The Cf~mrounfl of Formula I is useful for the relief of pain,
fever and infl lmm~tif n of a variety of cnnflitif nc including rhPIlm~tir
fever, ~ylll~JI(Jl.ls ~ccori~tf-d with influenza or other viral infections,
common cold, low back and neck pain, dy~l ". . ,~ " ,1,- ~ hf ~ rh~o
toothache, sprains and strains, myositis, nfllrAAl~i~ synovitis, arLhritis,
including ~ "~ arthritis (lf ~ ;v~ joint diseases (osteoar~ritis),
gout and ankylosing spondylitis, bursitis, burns, injuries, following
surgical and dental ~,lucf~1ules. In addition, such a ~ ., . .l.u~, . ,fl may
15 inhibit cellular neoplastic Llall~rf~l IIIAI;( III.C and metastic tumor growth and
hence can be used in the treatment of cancer. CO1ll,UUUII1S of Formula I
may also be useful for the treatment of dementia including pre-senile and
senile dementia, and in particular, dementia ~o~ ..1 with Alzheimer
Disease (i.e., Al~ll~,;lll~,l's dementia).
Cu~ll.u~ of Formula I will also inhibit L.Iu~L~Ioid-
induced smooth muscle cf n~r~rtif n by ~ v~.lLillg the synthesis of
contractile 1,IU. ' "lC and hence may be of use in the treatment of
dy ~ ,. ", I ,f ~ ,~alul~ labor arld asthma
By virtue of its high cyclou"y~;~llasc-2 (COX-2) activity
and/or its ~ ,LiviLy for cyclouA~ se-2 over ~ loo~y~.,.lase-l (COX-
1) as defined above, culll~uu~L of Formula I will prove useful as an
alternative to cull~ lLiulldl non-steroidal ~ y drugs
(NSAID'S) particularly where such non-steroidal ,.,,I ;;,,n-.,,,,. ~. " ~ drugs
may be contra-indicated such as in patients with peptic ulcers, gastritis,
30 regional erlteritis, ulcerative colitis, diverticulitis or with a recurrent
history of E,a~ f ' 1 lesions; GI bleeding, coa~;ul~Liull disorders
including anemia such as lly~ulJIuLlu~JllllJ;ll. ."i~ haemophilia or other
bleeding problems (inrl-lflin~ those relating to reduced or impaired
platelet function); kidney disease (e.g., impaired renal function); those
~ WO 951~8799 ~ - r - 2 l ~ 0 6 5 I PCTrcAg4~068i
- 13-
prior to surgery or ta'King ~lntir~ gul:lnfg and those susceptable to NSAID
induced asthma.
Similarly, compounds of Formula I, will be useful as a
partial or complete substitute for conventiona'l NSAID'S in pre-p~. afirmc
5 wherein they are presently co ~ 1",;";~ t . ~,~ with other agents or
ingredients. Thus in further aspects, the invention F..l~ ...S
,.""~ CC~ J0~7iliUIIS fortreatingcyclooAy~5~l,~e-2mediated
diseases as defined above ~ a non-toxic ~ lly
effective amount of the compound of Formula I as defined above and one
or more ingredients such as another pain reliever including
a ~ l or ~ ; a pu~llLir.lul including caffeine; an H2-
~nf~rnict, s~ minllnn or m l~nPgillm hydroxide, gimPfhirrmP. a
cnn~Fcf~nf including phenylephrine, phc;-~yl~lù~llrJl ";"r,
rS~Pllr1~ r-~ u~y~ nP~ III;llr~ F, n~rh~7r7linp~
5 xyl~lmPt~7rllinP propylhP~PA~inP~ or levo-deso~yr~ r-~ P, an
LiiLussive including codeine, hydrocodone, c .,~ ll;lll. ~, c~'L,~fAl, ,l ..~,
or d~ I "." ,. .l~,r~ h In; a diuretic; a sedating or non-sedating ~nfihi gf~nninP
In addition the invention I .,r.""~ a method of treating
cyclou~y~,_,la..e mediated diseases co~ ;on to a
20 patient in need of such treatment a non-toxic li,. ..,,l.~,.,I;r~sllly effect amount of the compûund ûf Formula I, optionally co-~ ";";~ ,d with
one or more of such ingredients as listed imm~ ~ ly above.
CIJIII~UUII~, of the present invention are inhibitors of
cyclou~y~ la~7c-2 and are thereby useful in the treatment of
25 ;ycloo~y~,_l-a.c-2 mediated diseases as i s' above. This acti~rity
is illllcfr~fr~ by their ability to sclc~,Li~,ly inhibit "y.;louJiy~,~.l-ase-2 over
CyClou~yE~,Ildsc-l. Acc~ ,ly, in ûne assay, the abi'lity of the
c..,,.l.ulll~l1c of this invention to treat u~ u~ as~ mediated diseases
can be d.,lll~ll. Ll ale;d by " ~ the amount of ~ in E2
30 (PGE2) synthesized in the presence of ~r~ '^ acid, ~y~ o~ as~-
cyclou~y~;.,l.a e-2 and a c~....l.u",.~1 of Formula I. The IC50 va'lues
represent the c~ " ,r ~. ,l . ;.l ;r~n of innibitor required to retu~in PGE2 synthesis
to 50% of that obt~ined as compared to the ...;..I,;hi~.d control.
lllllc~r~fin~ this aspect. we have found that the ~ ul~ of the
WO 95/18799 ~ ~ 2 1 ~ 0 6 5 I PCT/CA94/00688
- 14-
Examples are mo}e than lO0 times more effective in inhibiting COX-2
than they are at inhibiting COX-l. In addition they all have a COX-2
IC50 of l nM to l ~LM. By way of eomrAricr,n, Ibuprofen has an IC50
for COX-2 of 1 ~, and ln~lr,m~thqrin has an IC50 for COX-2 of
~i d~JlJI V~illld~ y 1 00 nM. For the treatment of any of these eyclooxygenase
mediated diseases, compounds of Formula I may be AAminic~Pred orally,
topically, parenterally, by inh,qlAtion spray or rectlly in dosage unit
forml-lAtinng c~.l.l;.;.l;ll~ conventional non-toxic rhArn~Arel-tirAlly
acceptable carriers, adjuvants and vehicles. The term parenteral as used
herein includes ~ l n~ ~f v~ ~ injectjr~ng. intravenous, i~ n ~ l Ar,
illLl d' ~ 11&l injection or infusion tf rhniq~ g In addition to the treatment
of warm-blooded animals such as mice, rats, horses, eattle sheep, dogs,
cats, etc., the crlmrolln~l of the invention is effective in the treatment of
humans.
5 As indicated above, pl.,.. IIIA~`~ .II;rAI compositions for
treating ~,y-,loo~ &~e-2 mediated diseases as defined rnay optionally
include one or more i-~ ,di~ as listed above.
The l~lln~ ".-- ~ ..I;rAl c~ .r.~ .,c c.~"~ the active
irlgredient may be in a form suitable for oral use, for example, as tablets,
20 troches, lozenges, aqueous or oily ~ li 5I' ' ' - powders or
granules, emlllciong, hard or soft capsules, or syrups or elixirs.
Comrogitinng intended for oral use may be prepared according to any
method lcnown to the art for the " .~ .... r~ of ~ . ", - ~ . ~1 ;. nl
comrocitiong and such c-,,,.l.~.~ li-...c may contain one ormore agents
25 selected from the group consistirlg of ~"t~,lPillg agents, flavoring
agents, coloring agents and L,l~ l vi--g agents in order to provide
llllA. ~IlAr~ .ll;rAlly elegant and palatable prep~q-tiong Tablets contain the
active ingredient in &dll~~ with non-toxic rhqrmq~elltirAlly
acceptable ~-ririf ntC which are suitable for the ~ of tablets.
3 These rYriri~ntg may be for example, inert diluents, such as calcium
carbonate, sodium carbonate, lactose, calcium rhr,grhAt~ or sodium
rhogrhA~f ~I~....IlAI;II~ and .1;~;..~ ~.2,li.,~ agents, for example, corn
starch, or alginie aeid; binding agents, for example st. rch, gelatin or
acacia, and l.,l., ;c - i"p~ agents, for example, -,.n~". .~;ll", stearate, stearic
WO 9S/18799 ~ ; 2 ~ 8 0 6 5 I PCT/CA94/00688
- 15-
acid or talc. The tablets may be uncoated or they may be coated by
known tP.~hniq~ c to delay ~ ;"t~ ;on and absorption in the
gd~LL..;,,Ir~l;.,Al tract and thereby provide a sustained action over a longer
period. For example, a time delay material such as glyceryl " ,I " ,o~l ~,. .
5 or glyceryl distearate may be employed. They may also be coated by the
technique described in the U.S. Patent 4,256,108; 4,166,452; and
4,265,874 to form osmotic ~ ;r tablets for control release.
Forml11Afi~-ng for oral use may also be presented as hard
gelatin capsules wherein the active ingredient is mixed with an inert solid
diluent, for example, cAlcium carbonate, calcium ~ o~ r or kaolin, or
as soft gelatin capsules wherein the active ingredients is mixed with
water or an oil medium, for example peanut oil, liquid paraffin, or olive
oil.
Aqueous ~ contain the active material in
15 d~LUl~ with eY~iri~ntC suitable for the m~ lf?~tllre of aqueous
cllcplonsionc Such excipients are ~ 1;"E agents, for example sodium
cdll.u..y~ ,;l-yl-cellulose, methylr-o]llllos~, hydroxy-~lu~Jyhll~,;lly-
cellulose, sodium alginate, polyvinyl-pyrrolidone, gum tragacanth and
gum acacia; ~ E or wetting agents may be a naturally-occurring
20 l,llo~l,lls"i~l~, for example, lecithin, or coll~ ion products of an
alkylene oxide with fatty acids, for example polyo~,LI-ylene stearate, IDr
c- .",~ ;on products of ethylene oxide with long chain aliphatic
alcohols, for example h~pto~1P~ thylene-u~y~,e~lol, or con-l~ng~tion
productg of ethylene oxide with partial esters derived from fatty acids ~nd
25 a hexitol such as polyu~y~l~ylene sorbitol mono~l or c. .".1. ..,~ "~
products of ethylene oxide with partial esters derived from fatty acids and
hexitol anhydrides, for example, pol~,lhyl~ , sorbitan Illvl.oc~l~d~. The
aqueous C-lcpl~ncjong may also contain one or more ~ aLiv~s, for
example ethyl, or n-propyl, p-llyJlu~.~b~,llLcl ~, one or more coloring
3 agents, one or more flavoring agents, and one or more ~ illg agents,
such as sucrose, sacchann or ~~ ~lIC.
Oily ~ ,c may be f~rmlll 1 by ~J~Jrl~ E the
active ill~51~;_11l in a vegetable oil, for example, arachis oil, olive oil,
sesame oil or coconut oil, or in mineral oil such as liquid paraffin. The
woss/ls7sg ~ 2 1 8065 l PcrlcA94loo688
- 16-
oily suspensions may contain a thi~k.-nin~ agent, for example, beeswax,
hard paraffin or cetyl alcohol. S~ t.,lPillg agents such as those set forth
above, and flavoring agents may be added to provide a palatable oral
preparation. These cu~ ,o~iL,ulls may be preserved by the addition of an
5 anti-oxidant such as ascorbic acid.
Di*,., '1.1~ powders and granules suitable for preparation of
an aqueous s~lsp~ncion by the addition of water provide the active
ingredient in admixture with a ~licp~.rcin~ or wetting agent, Sl~crPnf1in~
agent and one or more ~ . vdliV~s. Suitable ~licprrcin~ or wetting
agents and ~ agents are .-Y~-mplifi.-d by those already mentioned
above. Additional ~ for example, sweetening, flavoring and
coloring agents, may also be present.
The ~ r~ I compositions of the invention may also
be in the form of an oil-in-water .omlllcir~nc The oily phase may be a
5 vegetable oil, for example olive oil or arachis oil, or a mineral oil, for
example, liquid paraffin or mixtures of these. Suitable emulsifying
agents may be naturally-occurring rh--crh~ti~lP.c, for example, soy bean,
lecithin, and esters or partial esters derived from fatty acids and hexitol
arlhydrides, for example sorbitan monooleate, and c~ ...c,.~ n products
20 Of the said partial esters with ethylene oxide, for example, polyoxy-
ethylene sorbitan luull~cl The P."..~ may also contain
,t~ lg and flavuuliulg agents.
Syrups and elixirs may be fi~rmlll~-od with ~ g
agents, for example, glycerol, propylene glycol, sorbitol or sucrose. Such
25 f~lrmlll~firlnc may also contain a rl~mllh~ont, a ~ I va~iv~; and flavoring
and coloring agents. The r~ "-~ l c.. ~ C may be in the
form of a sterile injectable aqueous or r~ sllcr~ncir~n This
may be r~ rd according to the known art using those
suitable ~licrl-rcin~ or wetting agents and ~ agents which have
30 been .~ .I;....~ d above. The sterile injectable preparation may also be a
sterile irljectable solution or, ~ l in a non-toxic parenterally-
acceptable diluent or solvent, for example as a solution in 1,3-butane diol.
Among the ~rc~ptr~' vehicles and solvents that may be employed are
water, Ringer's solution and isotonic sodium chloride solution. In
wo 9~118799 . ~ 2 t 8 ~ 6 5 t PCr/CA94~688
- 17-
addition, sterile, fixed oils are conventionally employed as a solvent o,r
$11cpen(lin~ medium. For this purpose any bland fixed oil may be
employed including synthetic mono- or diglycerides. In addition, fatt~
acids such as oleic acid find use in the preparation of il.je, 31~
CuLu~uulld~ of Formula I may also be h.1",;";~ ,d in the
form of a ~U~O~;~UIi~s for rectal ~.l",;";~l".l;(m of the drug. These
compositions can be prepared by mixing the drug with a suitable non-
irritating excipient which is solid at ordinary l~ ;s but liquid at
the rectal l~ ."l,~..,.l",e and will therefore melt in the rectum to release Ihedrug. Such materials are cocoa butter and polyethylene glycols.
For topical use, creams, ointm~ntc jellies, solutions or
Ilr.ll~ , etc., C..~ ;";"~ the compound of Formula I alre employed.
(For purposes of t~us application, topical application shall include mouth
washes and gargles.)
Dosage levels of the order of from about O.Ol mg to about
140 mg~kg of body weight per day are useful in the treatment of the
above-indicated cnn-1itionc~ or alt~ ,Iy about 0.5 mg to about 7 g per
patient per day. For example, infl~rnm~til~n may be effectively treatedl by
20 the administration of from about 0.01 to 50 mg of the compound per
kilogram of body weight per day, or alt~ ly about 0.5 mg to abollt
3.5 g per patient per day.
The amount of active ingredient that may be combined with
the carrier materials to produce a single dosage form will vary l~r.lJ. ..
25 upon the host treated and the ~ uLu mode of ~-1",;1l;~"~ For
example, a frrm~ tion intended for the oral = 1,~,;";~ n of humans
may contain from 0.5 mg to 5 g of active agent c~ vullded with arl
a~ ,.i..t., and Cvll~.,lfi~,lll amount of carrier material which may vary
from about S to about 95 percent of the total cnmr~ citi~-n Dosage unit
30 forms will generally contain between from about l mg to about 500 mg
of an active ingredient, typically 25 mg, 50 mg, lO0 mg, 200 mg, 300 Img,
- 400 mg, 500 mg, 600 mg, 800 mg, or lO00 mg.
It will be understood, however, that the specific dose level
for any particular patient will depend upon a variety of factors including
WO 95/18799 ~ C~ 2 1 8 0 6 5 1 PCT/C~94/OOfi88
- 18-
the age, body weight, general health, sex, diet, time of a~~ fi~LId~ion,
route of a.l.l~ ation, rate of excretion, drug combination and the
severity of the particular disease undergoing therapy.
5 Methods of Sy~thesis
The culll~uu~lds of the present invention can be prepared
according to the following methods.
Mt~thrl~ A:
The ~-chlorovinylaldehyde III can be obtained from the
ketone II and the Vilsmeier reagent (DMF-POC13) using the general
method described by Weissenfels (Z Chem. 1966, 6, 471). The
thiorh.on~ compound IV is obtained from III using the general method
described by Weissenfels (Z Chem. 1973, 13, 57). The thiol compound
5 V can be obtained after oxidation of c~ mro~n~ IV (Ra = -SMe) with one
equivalent of m-CPBA followed by treatment of the resulting sulfoxide
with TFAA at reflux. The ~ulrullalllide group (VI) can then be formed by
the method of Kharash (J. Amer. Chem. Soc. 1951, 73, 3240). The
hydrolysis of compound VI and dc~ l,ul~ylation with Cu bronze in
20 quinoline provides ~ullll~uulld VII. Compound VII (R4 = H) can be
treated with hqlog~nqtin~ agent such as bromine in acetic acid to allow
the ~ JalaLioll of the 5-~ . (VII, R4 = Br). When it is
desired to have a nitrile group at C-5, this can be qrct~mrlich~d from Vl
via amide formation using the Weinreb mP.th~ldtllogy (Tetrahedron
25 Le~ers, 1977, 4171) followed by dehydration with TFAA. The CF3
group can be ulLIuduced at C-5 of VII via the method of Girard (J. Org.
Chem. 1983, 48, 3220).
The introduction of an alkyl group at C-5 can be achieved
via a Friedel-Crafts reaction on VII (R4 = H) and an acyl chloride, Cl-
30 CO-lower alkyl and a catalyst such a TiC14, followed by reduction. For
R4=Me, this can be achieved from the ester (R4=C02Me) via a DIBAL,
H reduction followed by d~u~ laLiull using the method of Lau (J. Org.
Chem. 1986, 51, 3038). Tertiary alcohols (R4= - C(C~13)20H) can be
obtained from VI and MeMgBr. These tertiary alcohols can also be
WO 95118799 i ~ 2 1 8 0 6 5 1 PCT/CA941~0688
- 19-
d~ y~lld~d using the method of Lau. Similarly, the Illiu~h~lle IX car
be prepared from ketone VIII.
METHOD A
~ DMF-POCI3 H~Ra
R2 1 ,2-di~ lul u~ll ldl;e Cl R2
Il Ra=-SMe or-SO2Me 111
Ra
~ when Ra=SO2Me
1~ OMe R2~-- and R2 =PhCO2Me
Py/Et N IV ( R4~CO2Me)
3 Ra=sO2Me
R2 =PhCO2H
20 when Ra is SMe/ R4 =CO2H
/1. m-CPBAlCH,Cl2 H2N~S"
1 .CI2/HOAc ~ R4
R S R Vl (R4=co2M~e)
V ( R4=-CO2Me) H2N~ ,0
O//S~
1.HO ~~
Vl 2. Cu/quinoline R2~S~R4
Vll ( R4=H)
WO 95/18799 .. _ ~ 2 1 8 0 6 5 1 PCTICA94/00688
- 20 -
METHOD A CONT'D
[R4]+ Vll R4= Br
R4 = CF3
1.amidation Vl R4 CN
2. TFAA
Vl MeMgBr Vl (R4=-C(CH3)2oH)
~ ~~ R4
R2 iX
Vlll
20 Mlothn~l B:
Ketone X can be converted to the thiophene comrol~n~l XI
using general methods already described in Method A. The Illio~ c
XII can be prepared by m-ot~ tion of XI with n-BuLi, ~ with
methyl ~ lirhl~ and addition of water or am~monia (X' =
25 OH or NH2). Similarly, the other regioisomer XIV can be prepared from
ketone XIII.
.
~ WO 95/18799 ' i . '., I ~ 2 1 8 3 6 5 1 pcrlcAs4lao6ss
- 21 -
METHOD B
s~Br Br~
X Xl
1.n-BuLi /
2. Me(CI)2PO xf ~
3. H20 orNH3 R2~_R4
Xll X'=OHorNH2
20 ~ H C ~ l~ R4
o XIV
Xlll
X' = OH or NH2
~p,tht-~i C:
Bromin~tit~n of ketone II gives the o~ ... ,ol r~ p XV
which is then converted to the thiazole XVI afte~ treatlnent with a
thirl~nnirl~. Similarly, ketone VIII can be converted to thiazole XVII.
WO 95/18799 ~ t ~ ~ 5 5 f Pcrl~94/00688 ~
- 22 -
METHOD C
S
o R2 R4J~ NH2
Il Br2
Ra
R~ XV
~S
N R4
1~
XVI
R~l NH ~--R4
XVII
2s ~.oth-~-l D:
Ketone XV can be converted to the jmirlq7~l- compound
XVIII after treatment with f~rmqmi~1.o. using the ~ iull of Brederick
et al, Chem. Ber. 1953, p. 88.
? ~ ~
~WO 95118'799 J ' ~ 2 ~ ~ 0 6 5 1 PCT/CA94/00688
- 23 -
METHOD D
_~NH~N~ R4
XVIII
Methl-d E-
Pyrole compound XX can be obtained from diketone XIX
using the general procedures of Friedman et al., J. Org. Chem. 1965, 30,
p. 854, K. Dimroth et al., Ber. 1956, 56, 2602, K.Dimroth et al., Ann.
1961, 634, 102. The free NH of the pyrole can be acylated with Cl-C~-
lower alkyl in the presence of a base such as Et3N. Also alkylated
5 products can be prepared using aLkyl halides as reagents with a base slllch
as NaH.
METHOD E
R1_~ MeO~N~J~MeO
XIX NaH/DMF OC to r.t
lR
HO ~ 2 \~ 2
30 180 C [ ]~ ~R
XX
WO 95118799 ' ~ ~ 8 3 6 5 I PcrlCAg4100688
- 24 -
Me~odF: -
The compounds of type XXV can be prepared from readilyavailable 4-substituted phenylacetyl chlorides XXIa. Reaction of di(3-
butenyl)r~innil7nn with a 4-cll~stitlltrd pl~ yl~ yl chloride provides
5 ketone XXI. Ozonolysis of XXI affords keto aldehyde XXIb which is
cyclized by base to give cycloprnt~nnnr XXII. Addition of
arylm~n~ m bromide or aryllithium to XXII gives allylic alcohol
~IV. Oxidation of XXIV with pyridinium chlu,o-,l,,u,n~.L~ affords the
desired 2,3-~ llhstiblt~d .,~ "1. ,..1l~ XXV. For preparation of
compound X~V (Rl=S02Me), 4-methylthiophenyllithium is used
fûllowed by oxidation with the ~ salt of mu"~ ,v~y~ Ll-alic
acid (MMPP) or m-chlo,~ ,.o..yl,.,l~uic acid (mCPBA) to introduce the
required methylsulfonyl group in XXV.
~ WO 9511~799 . 2 1 8 ~ 6 5 ~ PCT~C~94/(~0688
- 25 -
METHOD F
CIJ~' R , ~ Jl , 2 3
XXla XXI
o R~
15 O J~, NaOMe
XXlb XXII
R1 R1
HO ~ F'CC ~3
R2--~ R2~
XXIV
XXV
~ethod G:
The sequence of Method G is the same as in Method F
except Rl c~.,,l~;l.;,,~ acid chloride is used as starting material. R2 is
WO 95/18799 ~ , 2 1 8 0 6 5 I PCTICA94/00688
- 26 -
introduced at a later stage via a carbonyl addition reaction, followed by
PCC oxidation.
METHOD G
Ci~ (~--)~d ~=
2 NsOMe _~ O R2M
PCC 2 C
25 R~
XXVI
Method H:
The 4~5~ rd icothi~7c~ and isothiazol-3(2H)-one-
1,1-dioxides can be prepared by the general method described by B.
Schulze et al., ~lelvetica Chimica Acta, 1991, 74, 1059. l~us, aldehyde
m (Ra=S02Me) or XXVII is treated with excess NH4SCN in refluxing
acetone to proyide the corresponding 4,5-,li~"l ,~l i 1 . Ilrd i~othi~7~
WO 95118799 ~ ~ s~~ 2 1 8 0 6 5 I PCT/CA94/00688
- 27 -
and XXVIII, oxidation of which with hydrogen pe~oxide yields XXXI
and XXIX.
- METHOD H
5 Meo2s SO2Me
v~ NH4SCN ~J H2O2, AcOH
R2 CHO R2. ~ ,S
XXVII N
XXVIII
SO2Me
R2~ MeO2S~[~CHO NH4SCN
111 (Ra = SO2Me)
XXIX
SO2Me SO2Me
¢~
T H202, AcOH
R2`--~1 ' R2~ ~7~o
S--N ~S-NH
XXX
XXXI
WO 95/18799 ~ 8 0 6 5 1 PCT/CA94/00688
- 28 -
hn~
An d~ u~lid~ly ~ub~ ul~d aryl bromomethyl ketone is
reacted with an d~ lidl~ly ~ .d aryl acetic acid in a solvent such
as acetonitrile in the presence of a base such as triethylamine and then
5 treated with 1,8-diazabicyclo[~.4.0]undec-7-ene (DBU) to afford either
the lactone XXXIII or XXXV.
METHOD I R
1 0 Br~3 Base R
O
XXXII XXXIII
R
20 ~ R2J~Br
Base R2~0
HO2C \--0
XXXIV XXXV
/ R2 is a mono- or r~ic~ 1' ' ' l'^~ phenyl or
~ amono or~iie~ lhQteroar I
Methnrl J
Either of the lactones X~III or XXXV in a solvent such as
THF is reacted with a reducing agent such as diisobutyl ~ minillm
hydride or lithium bul~lly~Lide at -78C, to yield the furan XXXVI.
WO 95/18799 i . ~ " ~ 2 1 8 0 6 5 1 PCT/CA94100688
- 29 -
METHOD J
R1
XXXIII 1. DIBAL-H [C~
2. H~ R2--6~o
XXXVI
Method K-
The preparation of lactams XXXVII and XXXIX caD be
achieved by the same reaction as described irl Method I, except an
~lupli~le amide is used.
-30
WO 95/18799 . . ~ 2 ~ 8 0 6 5 ~ PCT/CA94/00688
- 30 -
METHOD K
R1 R1
~I R2'`CoNHR3 ¢~
. Base
Br~ ~O R2 <~
10 XXXII o,~NR3
XXXVII
R1
R2~Br ~1
Base R2~O
20 CoNHR3 NR3
XXXVII XXXIX
M~hr~rl !
Methyl 2-hydroxy i~ub~lLyld~ is silylated with TMSCl to
25 give the ~MS ether XX~I, which is treated with 4-~ llyll~liu~ yl-
lit~uum to provide ketone XXXXII. Desilylation followed by acylation
yields keto-ester XXXXIV, which can be cyclized to lactone XX~V by
base catalysis. Oxiddtion of XXXXV with MMPP or mCPBA affords the
desired product XXXXVI.
'W095/18799 ` ; ~ Q65 I PCT/CA94/1~0688
- 31 -
METHOD L
SMe
5 OH OTMS
~OMe TMSCI ~ ~OMe
o imidazole O
XXXX XXXXI
J N-Bu4NF ~ ~3
XXXXII XXXXIII
~F
Cl~ ~ SMe
Pyridine, DMAP
XXXXIV
~F
~ ~
XXXXV SMe XXXXVI SO2Me
WO 95/18799 ~ 2 1 ~ 0 6 5 l PCT/CA91/00688
- 32 -
METHOD M
SMe OAq. bsaOslve nt OH SMe
~h~ge
O transfer O
XXXXIV catalyst XXXXIII
An alternative preparation of the hydroxy ketone XXXXIII
is the oxidation of the known (J. Org. Chem. 1991 56, 5955-8; Sulfur
Lett. 1991, 12, 123-32) ketone ~XXXIV. A mixture of XXXXIV,
aquous base, such as NaOH, organic solvents such as carbon
5 ~ ., irlP./toluene and a phase transfer catalyst such as ALIQUAT
336 is stirred in air at room ~r~ - d~ to provide XXXXIII. Compound
is also described in U.S. 4,321,118 and Org. Coat. 1986, 6,
175-95.
20 R~ ",~tiv~ Con~l7ountl~
Tables I and II illustrate cuul~oulld~i of Formula I.
~ WO 95/18799 , ~ r - 2 ~ 8 0 6 5 1 PCT/CA94/00688
- 33 -
Table I
Example Method
~0~SO2NH2 1 A
~,
OH F
0 ~SO2NH2 A
~ 2
ls56~so2NH2
Me ,~ 3 A
Me F
~SO2NH2
~ 4 A
~SO2Me
HO2C ~CO2H A
N~F C
Me~S~
SO2Me
WO 95/18799 ~ 3 i ~ 2 1 8 0 6 5 1 PCTIC~94/00688
- 34 -
Table I (continued~
~F Example Method
5 ~ 7 F
SO2Me
~F
NS~ 8 H
SO2Me
~F
0~ 9
~SO2Me
~F
0~ 10
S2NH2
WO 95/18799 ~ 0 6 5 1 PCT/CA94/00688
- 35 -
Table I (contin~ed)
Example Method
F
0~ 1 11 J
~SO2Me
~F
~SO2Me 12 L
)~F
~`SO2NH2 A
~,SO2NHC(O)CF3
~ 14 A
F
- 30
WO 9~;/18799 - ~ . 2 ~ 8 0 6 5 1 PCTIC,~94/00688
- I
- 36 -
Table I (continued)
O~ Example Method
0_~ 15
~ SO2Me
l O ~F
0~ 16
~SO2Me
O~F
0~ 1 7
'~SO2Me
O~-F
0~ 18
~SO2Me
O~F
0~,~ 1 9
~SO2Me
! " ! l; .i
~ WO 9S~18799 2 ~ 8 0 6 5 I PCT/CA94/OD688
- 37 -
Table I (c-mtim~
~ Example Method
`~~ SO~Me
O O~OSO~e
~OMe
0~ 22
~SO2Me
O~3
O~q 23
~SO2Me
o Cl~
~,
0~ 24
SO2Me
2~80651
WO 95/18799 ~ , "~ PCT/CA94100688
~, ,J C" ~
- 38 -
Table I (continued)
0~ Exarnple Method
SO2Me
Br~CI
10 o~b,~
o~,~q 26
~SO2Me
F~CI
O~
0/~ 27
SO2Me
~ F
O~JI`Br
0 ~ 28
SO2Me
~ P-cl
0~ 29
SO2Me
.
WO 95/18799 2 1 8 0 6 5 ~ PCT/CA9~1On688
- 39 -
Table I (continued~
O~ ~F Example Method
01~ 30
~SO2Me
Cl~CI
100~
O ~q 31
~SO2Me
~CI
lSO~CI
0~)~ 32
~SO2Me
Cl~
20~CI
0~ 33
~SO2Me
o~--~CI
0~ 34
~ SO2Me
-30
WO 95118799 ~ - ' ` 2 1 8 0 ~ 5 1 PCTICA94100688
- 40 -
Table I (c~ntin~
~ Example Method
0~ 35
SO2Me
~OMe
O~F
36
~SO2Me
OMe
5 O~cl
0~ 37
'~SO2Me
2 o ~\~OMe
38
SO2Me
F~
0~,~ 39
SO2Me
wo 95/18799 -; ~ ; 2 1 8 0 6 5 1 PCT/CA9410(D688
-41 -
- Table I (continued)
~ Example Method
o~,Q~ 40
~ SO2Me
o O~JlF
0~ 41
SO2Me
Cl~
O~ 42
SO2Me
~Me
O~Br
SO2Me -
F~Br
0~
O~q 44
~SO2Me
WO 95118799 ' ' ' I - 2 ~ 8 0 6 5 1 PCT/C~94100688
- 42 -
Table I (continued~
~Br Example Method
O~Br
SO2Me
~,CI
o O~F
O~q 46
~SO2Me
~Br
O~F
0~ 47
SO2Me
o\~ ~Br
01~ 48
SO2Me
o~
0_~ 49
SO2Me
WO 9S118799 ~ .S 2 1 8 0 6 5 ~ PCT/CA94/00688
-43 -
. .
Table I (continued)
5 ~ Example Method
SO Me
~CI 2
10, ~CI
o~q 5
~SO2NH2
5 0~
1~ 52
S2NH2
O\fOMe
~CI
0~ 53
~SO2NH2
~OMe
~Br
O~q 54
~SO2NH2
~ - n r ~
WO 95/18799 ~ ~ 2 1 8 0 6 5 1 PCT/CA94100688
- 44 ~
Table I (continl-
Example Method
5~F
6~ 55 H
N~sJ~3~
SO2Me
~SO2Me
56 L+M
O~CI
~SO2Me
L+M
~ 57
~3
~SO2Me
~ 58 L+M
2s O~F
O F
WO 95/18799 ; . ! . i' I I I i 2 1 8 0 6 5 1 PCT/CA94/00688
- 45 -
Table I (continued~
Example Method
~SO2Me
59 L+M
O~CI
Cl
SO2Me
~ 60 L+M
15 O~3`C
WO 95/18799 ~ 2 t 8 0 6 5 I PCTICA94/00688
-46 -
~ Me~
~,SO2NH2 '
OH
~SO2NH2 ,~,F
2 0 F3C S~ F3C ~6
S2NH2
[~
Q~'
OMe
WO 95118799 . ~ 2 1 8 0 ~ 5 1 PCT/CA94100688
-47 -
Table II (continued)
~OMe ~3,F
S~`502NH2 0~ ~SO2NH2
F3C ~,NH2 SO2NH2
~SO2NH2 ~3,SO2NH2
S~ F3
30
WO 95/18799 ` ~ 2 1 8 0 6 ~i I PCT/CA94/00688
- 48 -
Table IT (continued)
OH
~SO2NH2 ~
F3C ~ ~ ~SO2NH2
OH
F3C~ NHz
S2NH2
,~SO2NH2 ~CO2H
~,CO2H ~SO2NH2
OH
~CO2H
3 0 S~ Me S~3~
SO2NH2 SO2NH2
WO 95/18799 ~ 2 ~ 8 ~ 6 5 l PCTICA94/00688
-49 -
Table II (cDn~in
OH
N~ Mel~ NH2
S2NH2
OH
~F ~SO2NH2
WO 95/18799 - ' ~ I i X 2 ~ 8 0 6 5 I PCT/CA94100688
-50-
Table TT (contin~-~d)
~,F
~ SO2NH2
,~SO2NH2 ~SO2NH2
0/~ O~--CI
~,SO2Me
HN~3~F
-
~ WO 95118799 ` ~ , ~ 2 ~ 8 0 6 5 1 PCT/CA94/00688
-51 -
Table Il (continued)
~[~F ~SO2NH2
HN/~`S02NH2 h~F
~SO2NH2
HN~3`F
~SO2NH2 ~3~so2NH2
0,~3~ o~3~F
WO 95/18799 ~ 2 1 8 0 6 5 I PCT/CA94/00688
- 52 -
Table IT (C~J"I;""~
S2NH2 SO2Me
1~ ~SO2Me ~.SO2NH2
HN~3~ HN~
qNH2 ~SO2NH2
~SO2NH2 Br
~Br
~VO 95/18799 i ~`' ) ' ~` 2 ~ 8 0 6 5 1 PCT/CA94100688
- 53 -
Table II (continued~
~3,SO2NH2 ~0,SO2NH2
~--F ~F
~S~2NH2 ~2NH2
F~F ~F
~SO2NH2 ~FSO2NH2
WO 95/18799 ` - ' 2 t ~ 0 6 5 ~ PCT/CA94/00688
-54 -
Table TT (concluded~
2NH2 ~F ~,SO2NH2
~`F ~SO2NH2
NH2 $~ Me ~ O~NH2
,~3,SO2Me ~SO2NH2 ~l,SO2Me
25 S~ ~ `'~3
30 SO2Me ,~SO2NH2 _~,SO2NH2
~ ~o~
~ W0 95/18799 / . .~ ~J ~ ~' 2 1 8 0 6 5 1 PCT/CA94/00688
_ 5~ _
Table II (c--nrl~ d)
SO2NH2 SO2Me ~SO2NH2
0~ Me~ O>
SO2Me SO2Me SO2NH2
\~F ~ Me~
?~SO2Me ~,SO2NH2 ?~ ~ Me
~, ~,SO2Me ~,SO2NH2 ~,SO2NH2
~ ~ ~CI
Cl Cl
WO 9~/18799 ;, ~ ; 2 1 ~ 0 6 5 I PCT/CA94/00688
- 56 -
Table TT (concluded)
O
~SO2Me ~ Me ~ ,S~NH2
~,SO2Me ,~,SO2NH2 ~SO2Me
25 ~ ~ O~
F F OMe
~SO2Me ~,SO2NH2 ~,SO2Me
~OMe
Cl Cl
WO 95/18799 ~ 2 1 8 0 6 5 I PCT/CA94/00688
- 57 -
Table Il (concluded)
Me~; Me
F F OMe
~SO2Me SO2Me ~SO2NH2
Me~ Me>~ Me~
~ O~L`OMe Ol\le
OMe
~SO2M;~,SO2NH2 ~SO2Me
2 5
Me Me Me
M~ "~``
Me
WO 95/18799 ~ 2 1 8 0 6 5 1 PCT/CA94/00688
-58 -
Assavs for D~ iolo~ical Activity
The compound of Formula I can be tested using the
followingassaysto.1~.",;.,~ theircyclou,.y~i..a;,~-2inhibitingactivity.
5 TnhihitionofCYCIO~AY~ f Activity
Cull~oul~d~ were tested as inhibitors of cyclooxygenase
activity in whole cell and ~ us~---al cyclou~ lasc; assays. Both of
these assays measured proct~ n~lin E2 (PGE2) synthesis irl response to
hi(lnnir acid, usimg a .~.lin;"""~ .ns~c~y Cells used for whole cell
assays, and from which microsomes were prepared for microsomal
assays, were human osteosarcoma 143 cells (which specifir llly express
cyclo~"~y~ ase-2) and human IJ-937 cells (which spe~rifif- ~lly express
cyclo~,~y~cllase-l)~ In these assays, 100% activity is defined as the
iirr~ l- llce between prnst~ nflin E2 synthesis in the absence and
5 presence of ar~rhi~1nn~t~- addition. IC50 values represent the
c. " ,~ l of putative inhibitor required to return PGE2 synthesis to
50% of that obtained as compared to the unirlhibited control.
Rl~,oc~l~livti results are shown in Table III.
20 R~ ive Rat Paw Fti~-nn~ Assay - Protocol
Male Sprague-Dawley rats (150-200 g) were fasted
overnight and were given po either vehicle (5% tween 80 or 1%
methocel) or a test compound at 9 - 10 am. One hr later, a line was
drawn using a p~ - "~ l marker at the level above the ankle in one hind
25 paw to define the area of the paw to be lu~ilu~d. The paw volume
(VOh) was measured using a ~ lllu~ t~ l (IJgo-Basile, Italy) based
on the principle of water ~ f , - " The animals were then injected
subplantarly with 50 ul of a 1% c A ~ I i.r,~ ' '' I '' ' solution in saline (FMC
Corp, Maine) into the paw using an insulin syringe with a 25-gauge
30 needle (i.e. 500 ug ~ 1 c,4 1 ~1~ per paw). Three hr later, the paw volume
(V3h) was measured and the increases in paw volume (V3h - Voh) were
r~1r~ tf~fl The animals were ~ .,;,..d by C02 ~llyAiali~JII and the
absence or presence of stomach lesions scored. Stomach scores were
expressed as the sum of total lesions in mm. Paw edema data were
WO 95/18799 ' t ' 2 ~ 8 0 6 5 ~ PCT/CA94/all688
_ 59 _
compared with the vehicle-control group and percent inhibition
cal~ t~d taking the values in the control group as 100%. Since a
m~ximllm of 60 - 70% irlhibition (paw edema) was obtained with
standard NSAIDs, ED30 values were used for c.~ All treatment
5 groups were coded to eliminate observer bias. With this protocol, the
ED30 for Tntl....~r~ ... is 1.0 mg/kg. R~ a~ tivt~ results are shown
in Table IV.
Table III*
Whole Cells Microsor~es
Example Conc. COX-2 COX-1 Conc. COX-2 COX-l
(nM) % inhib. % inhib. (nM) % irlhib. % inhib.
100 96 12 100 53 8
2 10 69 0 10 49 2~
3 10 42 10 33 19
3 100 100 100 76 12
4 10 47 2
5 10 0 0 10 43 31
20 6 100 78 100 19 16
7 100 74 0 1000 58 16
8 10 41
8 100 89
9 100 83 100 37 9
25 10 100 95 100 71 12
1 1 100 39 100 46 7
12 100 54
13 10 41 10 52 7
13 100 84 10 58 10
30 14 10 73 10 45 29
14 100 89 100 63 0
14 1000 101 1000 69 0
WO 95118799 ~ 8 0 6 5 1 PCTICA94100688
- 60 -
Example Conc. COX-2 COX- l Conc. COX-2 COX- 1
(nM) % inhib. % inhib. (nM) % inhib. % inhib.
1520 39
1580 76
15160 95
1620 41
1640 50
16160 85
1740 41
17160 77
1840 24
18160 58
19 40 21
19 160 59
91
21 10 . 50
21 40 94
22 20 39
22 160 98
23 20 50
23 160 88
24 40 43
24 160 78
160 40
26 80 27
26 160 39
27 20 38
27 160 97
~ WO 95118799 ~ 2 1 8 0 6 5 ¦ PCT/CA94/00688
- 61 -
Example Conc. COX-2 COX- 1 Conc. COX-2 COX- 1
(nM) % inhib. % inhib. (nM) % inhib. % inhib.
28 20 48
28 160 69
29 20 78
29 160 85
30 160 . 30
o 31 20 49
31 160 87
32 S 43
32 10 73
32 40 92
32 80 99
33 160 6
34 10 30
34 40 80
34 160 102
32
57
160 83
36 10 11
36 40 S0
36 160 89
37 10 53
37 40 82
37 160 93
- 30 38 10 25
38 40 63
38 160 88
39 10 17
WO 95/18799 ; ~ 2 1 8 0 6 5 1 PCT/CA94/00688
-62-
Example Conc. COX-2 COX- 1 Conc. COX-2 COX- 1
(nM) % inhib. % inhib. (nM) % inhib. % inhib.
39 160 84
540 10 43
40 40 72
40 160 96
41
o 41
42 20 10
42 160 44
43 10 78
43 40 101
44 20 14
44 40 55
44 160 106
45 10 16
45 40 61
160 101
46 10 76
46 40 94
46 160 97
47 10 61
47 40 74
47 160 101
48 10 7
48 160 47
49 10 53
49 40 91
49 80 99
42
~ W095118799 - 21 8 0651 pcr/cAs4/on6ss
- 63 -
Example Conc. COX-2 COX- 1 Conc. COX-2 COX- I
(nM) % inhib. % inhib. (nM) % inhib. % inhib.
51 5 49
5 51 20 95
51 40 102
52 10 50
52 40 82
52 160 102
53 10 54
53 40 96
53 160 102
54 10 81
54 80 91
54 160 99
48
59
160 65
* In the whole cell assay Ibuprofen has an IC50 for COX-1 of 1000 nM,
and an IC50 for COX-2 of 3000 nM. Sirnilarly, Il-d~",c~,&~;ihl has ~n
IC50 for COX-1 of 100 nM, and an IC50 for COX-2 of 10 nM.
~,~ 21 80651
WO 95/18799 ~ PCTICA94/00688
Table IV
ED30(mg/lcg) STRUCTURE
~3.00
~SO2Me
S'~
>10.00 SO2Me
2 S~
F
~ WO 95118799 ; ~ 2 1 8 0 6 5 1 PCT/CA94/~10688
- 65 -
1.40 SO2NH2
.S \~F
2 80 SO2Me
5 me~ocel) ~ ~
0.72 b~F
0-43 SO2Me
F
WO 95/18799 ~ ' P~ 2 ~ 8 0 6 5 1 PCT/CA94/00688
-66-
~3.00
~,SO2NH2
>3.00
53-00 S~SO2NH2
~3~
F
1.10
~SO2Me
N`S~,
F
WO 95118799 ~ 2 1 8 0 6 5 1 PCTICA94/00688
- 67 -
<0.30
/~
SO2NH2
0.42
~,SO2Me
~3,
F
0.034
~SO2NH2
\0\F
wo 95118799 ~ 2 ~ 8 0 6 5 I PCr1CA94100688
- 68 -
03 ~SO2Me
O~
~3'F
1.49
~SO2Me
0~
F)~
.
0.35 SO2Me
~`
F
wo 95/18799 ;~ ~ ~ 0 6 5 l PCT/CA94101D688
- 69 -
0.33
~,SO2Me
Br
0.90 SO2Me
~
~CI
0.38 SO2Me
~0
WO 95118799 . ~ 2 1 8 0 6 5 1 PCT/CA94100688
- 70 -
0.88 SO2Me
o
sX~`c
0-47 SO2Me
o~
F~ I
C
0.71 SO2Me
0~
o ~3
Cl
2 1 8~5 1
WO 95/18799 ` ~ PCT/CA94100688
- 71 -
-1 .00
~SO2Me
~Br
F
1.85
O~ S2Me
Cl~3\
0.22
0.23 rJ~xSc02Me
3 ~ Cl
~ .; f ,~
r
WO 95/18799 2 1 8 0 6 5 l PCT/CA94/00688
- 72 -
0.43
SO2Me
~(
2.17 SO2Me
o~
~3,
CF3
0.81
O~ ,SO2Me
3 ~(OMe
WO 95118,f99 2 1 8 0 ~ 5 t PCT/CA94100688
f~`
0.68 b,~
OMe
0.16 SO2Me
\~
~1.00 SO2Me
3~ SM2
WO 95/18799 ' i~ PCTICA94100688
2 1 8~65 1
- 74-
0.33 SO2Me
O/~
F
0.46 SO2Me
?`~,~
CH3
Br
0.~6 SO Me
WO 95/18799 2 1 8 0 6 5 ~ PCTICA94/01~688
- 75 -
0.48
"~SO2NH2
\~F
F
0.46
o~3~0,SO2NH2
\~\CI
Cl
0.26
~SO2Me
~\~
F
T/CA94/00688
WO 95/18799 ~. ,J j ~ 2 1 8 0 6 5 I PC
-76-
0
F
0.25 ~SO2Me
~~3
ED30~ 0.30 ~SO2Me
EDgo= 1.47 0~
o \3
WO 95/18799 2 1 8 0 6 5 1 PCTICA94/OlD688
; ., ,"
- 77 -
0.1-.3
~,SO2Me
~0.10
~,SO2Me
b ~F
F
WO 95/18799 `. ~ .` PCT/CA94/00688
21 80651
- 78 -
0.13 SO2Me
O~
\~CI
Cl
0.07
SO2Me
0~
~
The invention will now be illll~tr~t.sd by the following non-
limiting examples in which, unless sta~ed uLII~- wise~
(i) all C~f~ were carried out at room or ambient
, that is, at a t` ~ ; in the range 18-25C; evaporation of
3 0 solvent was carried out using a rotary c v a~ alui urJder reduced pressure
(600-4000 pascals: 4.5-30 rnm Hg) with a bath t~ ; of up to
60C; the course of reactions was followed by thin layer chromatography
(TLC) and reaction times are given for illustration only; melting points
are ullcull~ d and 'd' indicates d~co~ .u~;lion; the melting points given
are those obtained for the ~naterials prepared as d~ A poly~
2 1 8065 1
~ WO 9~/18799 PCT/CA94/00688
-79 -
may result in isolation of materials with different melting points in some
preparations; the structure and purity of all final products were assured by
at least one of the following ~ f~ TLC, mass ~c~ vl~ y,
nuclear magnetic ~ u~ ,c: (NMR) ~ vlll"~y or microanalytical data;
5 yields are given for illllctrAtit)n only; when given, NMR data is in the
form of delta (o) values for major r~ noctir protons, given in parts per
million (ppm) relative to t~ illylsilar~e (TMS) as internal standard,
tf rminf d at 300 MHz or 400 MHz using the indicated solvent;
conventional al~l,..,vi~ions used for signal shape are: s. singlet; d.
doublet; t. triplet; m. multiplet; br. broad; ç~.: in addition "Ar" signifies
an aromatic signal; chemical symbols have their usual ",. ~.,il.r,.~, the
following abbreviations have also been used v (volume), w (weight), b.p.
(boiling point), m.p. (melting point), L (liter(s)), mL (millilit~r.s), g
(gram(s)), mg (milli~Amc(s)), mol (moles), mmol (millim~' ), eq
(equivalent(s)).
The following ~b~,vid~iu~ have the indicated mPAnin~
Ac = acetyl
Bn = benzyl
DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene
DIBAL = diisobutylAl-lmin-lm hydride
DMAP = 4-(dimethylamino)pyridine
DMF = N~N-dil~ ylr(" "~ ;tlr.
Et3N = trlcll~LulPi-l~,
LDA = lithium diisv~u~ylamide
m-CPBA = III~ llU~ lLViC acid
MMPP = ~vllu~,.v~y~ lic acid
MPPM = mvlluL,~,.u.. y~l.ll.alic acid, IIIA~ IIII salt
6H20
30 Ms = ~"~ f.,.~lrV~yl = mesyl = SO2Me
MsO = ",. l~ f7~lrvll~t~=mesylate
NSAID = non-steroidal anti-i,lrl~.. ""~ .. y drug
OXONE(~ = 2KHSOs-KHSO4-K2SO4
PCC = pyridinium ~I-Iv-v-,l.. u---.
WO 95/18799 ' PCT/CA94/00688
21 80651
- 80 -
PDC = pyridinium dichromate
Ph = phenyl
Phe = b~ P~liyl
Pye = pyridinediyl
r.t. = room ~
rac. = racemic
SAM = ;.",;.. ~ r~ yl or sulfona-mide or So2NH2
TBAF = tetra-n-butyl~mm~-nil-m fluoride
Th = 2- or 3-thienyl
0 TFAA = Lliflu~ ,a.,~,Lic acid anhydride
THF = t~,LIallyllloLu
Thi = thiu~llcllediyl
TLC = thin layer chrtlm~t~ rhy
TMS-CN = trimethylsilyl cyanide
5 Tz = lH (or 2H)-tetrazol-5-yl
C3H5 = allyl
,~lkyl Grou~ ~bbreviatio~c
Me = methyl
2 o Et = ethyl
n-Pr = normal propyl
i-Pr = isopropyl
n-Bu = normal butyl
i-Bu = isobutyl
s-Bu = se~ull&ly butyl
t-Bu = tertiary butyl
c-Pr = cyclopropyl
c-Bu = cyclobutyl
c-Pen = ~,y~,lo~,llLyl
c-Hex = cyclohexyl
'iVO 95118799 . ~ .2 1 8 ~ 6 5 1 PCT/C~94100688
- 81 -
EXAMPLE 1
3-(4-Aminosulfonyl)phenyl)-2-(4-nu~,lu~h~.lyl)-5-(2-hydroxy-2-
5 Prpyl)thiophene
?l; 1-(4-Flu~lv~ .lyl)-2-(4-(1ll.,lllyllllio)phenyl)ethanone
To 4-nuu ul,~ hyde (5.40 g) in 1,2-dichloroethane
(43.50 mL) were added TMS-CN (4.32 g) and ZnI2 (44 mg). After 0.5 h
at r.t., the solvent was removed in vacuo. To the resulting TMS
cyanohydrin (9.20 g) in THF (42.0 mL) at -78C was added dropwise a
solution of LDA 0.51M in THF (88.9 mL). After a period of 0.5 h, a
THF solution (30.0 mL) of 4-(chlololllc~lyl)th~ ni~ (9.93 g) was
added dropwise over 0.5 h. After 18 h at +5C, the resulting mixture was
treated with TBAF (57.5 mL) followed by a 25% aqueous solution o:~
5 NH40Ac (100 mL) and extracted with EtOAc (2 x 150 mL). After
evaporation, a 10:1 mixture of Et2O and hexane (200 mL) was added to
the crude ketone. After stirring for 10 h and filtration, the title product
was obtained as a solid by filtration (2.40 g).
lH NMR (CD3COCD3): o 2.45 (3H, s), 4.34 (2H, s), 7.19-7.29 (6H, m),
20 8-14 (2H, q).
Cis,trans-3-chloro-3-(4-nuulu~ .yl)-2-(4-(methylthio)-
phenyl)propenal
To asolutionof 1-(4-rlu~ yl)-2-(4-(ll~ y' ' )phenyl
25 ethanone (2.50 g) in 1,2-dichloroethane (27.0 mL) were introduced t~he
Vilsmeier reagent (Aldrich catalog, 1992-1993) 3.3M (11.6 mL) and
DMAP (1.17 g). After a period of 4 h at 80C, the reaction mixture ~was
extracted with EtOAc and 25% aqueous solution of NH40Ac. After
evaporation in vacuo arld drying for a few hours, the title product was
3 used as such for the next step.
1H NMR (CD3COCD3): o 2.40 and 2.48 (3H, 2s), 6.90-7.80 (8H, m),
9.55 (lH, s).
WO95/18799 i ~ 2 1 8 0 6 5 1 PCTICA94/00688
- 82-
~; 5-(4-Fluolu~ yl)-4-(4-(methylthio)phenyl)thiophene-2-
carboxvlic acid methyl ester
To a solution of cis,trans 3-chloro-3-(4-nuuluL,llcllyl)-2-(4-
(methylthio)phenyl)propenal (3.00 g) m pyridine (12.0 mL) were added
methyl thioglycolate (1.16 mL) and Et3N (4.09 mL). The resulting
mixture was then heated at 80C for 2 h. After extraction with EtOAc
and washing with 3N HCI, the title product was purified by flash
,Llulllal~?~slaplly (30% EtOAc in hexane) (2.00 g).
lH NMR (CD3COCD3): o 2.48 (3H, s), 3.88 (3H, s), 7.11 (2H, t), 7.21
(4H, s), 7.37 (2H, q), 7.80 (lH, s).
SteR 4: 5-(4-Fluulu~h.,llyl)-4-(4-(methylsulfinyl)phenyl)thiophene-
2-~`~rbûxylic acid m~th,yl ester
To a solution ûf 5-(4-lluulu~llwlyl)-4-(4-(methylthio)-
phenyl)thioph~ r~P 2 carboxylic acid methyl ester (5.60 g) in CH2C12
(84.0 mL) at 0C was added portionwise m-CPBA 50 to 60% (5.39 g).
After TLC showed comrl~tion (50% EtOAc in hexarle), the reaction
mixture was extracted with saturated NaHCO3, dried over Na2SO4,
filtered and ~ uldt~,d to dryness to provide the title CUIl~l~C ulld as a
20 white foam (5.00 g)-
lH NMR (CD3COCD3): o 2.75 (3H, s), 3.92 (3H, s), 7.15 (2H, t), 7.40
(2H, q), 7.52 (2H, d), 7.66 (2H, d), 7.90 (lH, s).
~ 4-(4-(.~ r~ yl)phenyl)-5-(4-fluorophenyl)thiophene-
2-carbox~ylic ~,~i,l m~th~yl ester
5-(4-Fluulû~h~ 1)-4-(4-(methylsulfinyl)phenyl`' ?plll,llC-
2-carboxylic acid methyl ester (0.500 g) was dissolved in TFAA (10.0
mL) and refluxed for 0.5 h. The sûlvent was then removed in vacuo and
the resulting residue was co e~a~ul..~ 10 times with a Et3N-MeOH
30 solution (1:1) (100.0 mL) to provide a viscous oil after pumping for a few
hours. The oil was dissolved in HOAc (10.0 mL) and treated at +10C
with C12 in HOAc (1.9M) (3.5 _L). After 20 min, the solvent was
removed under reduced pressure and after pumping, THF (20.0 mL) was
added to the resulting mass of product. After bubbling NH3 through for a
~ W09511~3799 ~ ' J~ PC'r/C~94/00688
2180651
- 83 -
few minutes at 0C, the reaction mixture was stirred for 0.5 h at r.t. After
extraction with EtOAc - 25% NH40Ac solution and flash
chromatography (30 to 40% EtOAc in hexane), the title product was
obtained as a white solid (0.210 g).
lH NMR (CD3COCD3): o 3.90 (3H, s), 6.55 (2H, bs), 7.13 (2H, t), 7.40
(2H, q), 7.46 (2H, d), 7.83 (2H, d), 7.90 (lH, s).
Step 6: 3-(4-Arninosulfonyl)phenyl)-2-(4-fluc,lu~l~c~.yl)-5-(2-
hydroxy-2-pro~yl)thiophene
To 4-(4-~1ninnsll1fonyl)phenyl)-5-(4-nuulu~h~llyl)-
thiophene-2-carboxylic acid methyl ester (0.460 g) in THF (5.70 mL) at
0C was added MeMgBr (1.4M) in toluene-THF solution (5.00 mL). The
mixture was then stirred at r.t. for a few hours. The reaction was
quenched by the addition of 25% NH40Ac solution, extracted with
5 EtOAc and dried over with Na2SO4. The title compound was purified by
flash ~lu~ lly (40 to 50% EtOAc in hexane) (0.300 g).
lH NMR (CD3COCD3): o 1.65 (6H, s), 4.52 (lH, s), 6.55 (2H, bs), 7.09
(3H, m), 7.34 (2H, dd), 7.30 (2H, m), 7.43 (2H, d), 7.82 (2H, d).
Analysis calcd. for C19H18FNO3S2
C, 58.31; H, 4.60; N, 3.58
Found: C, 57.94; H, 4.66; N, 3.44
FXAMPT.F. 2
3-(4-(Arninosulfonyl)phenyl)-2-(4-nuulu~ i)thiophene
4-(4-(Aminosulfonyl)phenyl)-5-(4-nuulu~h.,llyl)ll.;..~.l,...,~.-
2-carboxylic acid
30 To a solution of 4_(4-(i1",;".. ~"1 rul.yl)phenyl)-5-(4-fluoro-
phenyl, ' r1 ~ --2-~ u--ylic acid methyl ester (Example 1, Step 5)
(0.210 g) in THF (2.0 mL) were added MeOH (1.0 mL), NaOH lN (1.0
mL) and a few drops of NaOH 10N. The resulting mixture was heated at
WO 95/18799 2 1 8 Q 6 5 I PCT/CA94/00688
- 84-
45C for 2 h and the reaction was then partitioned between EtOAc and
HCl (3N) to provide the title product as a white solid (0.200 g).
IH NMR (CD3COCD3) o 6.60 (2H, s), 7.15 (2H, t), 7.35 (2H, q), 7.45
(2H, d), 7.82 (2H, d), 7.87 (lH, s).
~; 3-(4-(Aminosulfonyl~phenyl)-2-(4-fluorophenyl)thiophene
To a solution of 3-(4-(~mino,slllfonyl)phenyl)-2-(4-
nuu.~ yl)thiophene-2-carboxylic acid (0.280 g) in quinoline (4.0 mL)
was added Cu bronze (0.300 g). After 0.5 h at 1 80C under nitrogen, the
reaction rnixture was extracted with EtOAc and HCI 3N, dried over
Na2SO4 and purified by flash chromatography (30% EtOAc in hexane)
to give the title compound as a white solid (0.180 g).
lH NMR (CD3COCD3): o 6.60 (2H, bs), 7.15 (2H, t), 7.29 (lH, d), 7.35
(2H, q), 7.45 (2H, d), 7.60 (lH, d), 7.83 (2H, d).
Analysis calcd for Cl6Hl2FNo2s2
C, 57.65; H, 3.60; N, 4.20
Found: C, 57.62; H, 3.59; N, 4.15
2 o F.~AMPJ F. 3
3-(4-(.A~ rollyl)phenyl)-2-(4-fluu~ "yl)-5-(2-propyl)thiophene
lH NMR (CD3COCD3) o 1.40 (6H, d), 3.25 (lH, septuplet), 6.58 (2H,
25 bs), 7.05 (lH, s), 7.15 (2H, t), 7.32 (2H, dd), 7.46 (2H, d), 7.80 (2H, d).
Analysis calcd. for C19H18FNO2S2
C, 60.80; H, 4.80; N, 3.73
Found: C, 60.59; H, 4.45; N, 3.60
'W0 9S~18799 ~ 2 ~ 8 0 6 5 ~ PCIIC~94/00688
.
- 85 -
FXAMPT.F 4
3-(4-(Amin- slllfmu~l~rh~orlyl~-2-cvc~ Ayl~ . ,r
1~ NMR (CD3)2)CO) o 1.24-1.40 ~3X m), 1.40-1.56 (2H, m), 1.65-
1.85 (3H, m), l.g0-2.0 (2H, m), 3.18 (lH, m), 6.58 (2H, bs), 7.05 (lH, d),
7.37 (lH, d), 7.58 (2H, d), 7.97 (2H, d).
~X~IPT.F, S
5-(4-C~.bu~y~ yl)-4-(4-(methylsulfûnyl)phenyl)thiophene-2-
r~rbo~cyl~ rifl
~tep 1: 4-(2-(4-(M~ylllPio)phenyl)-l-oxo-ethyl)benzoic acid
methyl ester
To methyl 4-formylbenzoate (10.30 g) in 1,2-dichl<,lu~ tll~ne
at r.t. were added TMS-CN (6.58 mL) and ZnI2 (2.00 g), after 0.5 h ,~t
r.t., the solvent was removed in vacuo. To the resulting TMS ,i.yal~O~
(5.00 g) in THF (22.0 mL) at -78C was added dropwise a solution of
20 IDA 0.87 M in THF (26.2 mL). After a period of 0.5 h, a THF solu~ion
(10.0 rnI,) of 4-(~ c,lvlll.,;l~l)thi~r~ was added dropwise over 0.5 h.
The t~ "~ , was then brought slowly to -20DC then to 5C for 2 h
and TBAF lM in THF (50.0 mL) was added. After the addidon of 2.5%
aqueous solution of NH40Ac, the reaction mixture was extracted wi~h
25 EtOAc, dIied over NASO4, ~a~uldt~ in vacuo and purified by flasl
~.h~ h ~`~I nl~l Iy (20 to 30% EtOAc in hexane) t~ afford the tit~e
culll~uulld âS a white solid (7.00 g).
~ 4-(1-Oxo-2-(4-(methylsulfonyl)phenyl)ethyl) benzoic a~id
}~ thyl loch~r
To 7.10 g of 4-(2-(4-(rnethylthio)phenyl)-1-oxo-ethyl)
benzoic acid methyl ester in MeOH (100 mL) was added oxone (21.0 g)
in H20 (20.0 mL) at 0C. After a few hours at r.t., the reaction mixh~re
WO 95/18799 , ~ ~ 2 1 8 0 6 5 1 PCTICA94/00688
- 86-
was extracted with EtOAc and H20 to afford after flash ~iLIuluc~u~a~hy
(50 to 100% EtOAc in hexane), the tltle product as a white solid (3.20 g).
lH NMR (CD3COCD3) o 3.10 (3H, s), 3.95 (3H, s), 4.65 (2H, s), 7.60
(2H, d), 7.96 (2H, d), 8.20 (4H, q).
~; Cis,trans 4-(1-Chloro-3-oxo-2-(4-(methylsulfonyl)phenyl)-
l-~ropenyVh~n7~i~ acid rnPtllyl ester
To a solutlon of 4-(1-oxo-2-((4-methylsulfonyl)phenyl)-
ethyl) benzoic acid (1.70 g) in 1~2-~;r~hln.~ l.S~ (15.0 mL) were added
the Vilsmeier reagent 3.3 M (6.2 mL) and DMAP (0.624 g). The
resulting m~xture was heated at 80C for 4 h. The reaction rnixture W&S
then extracted with 25% aqueous solution of NEI4O~c and EtOAc. After
drymg over Na2S04 and evaporation the title comrolm~l was obtained as
an oil and used as such for the next step
S~? 4: 5-(4-(Mv;l.ui~y~a-hu..yl)phenyl)-4-(4-(lueLl.yl~ulru -yl)-
~h~.ru~l)th;~hene-2-~ ~rboxyli~ slrirl mPfllyl ester
Prepared from 4-(1-chloro-3-oxo-2-(4-methylsulfonyl)-
phenyl)-l-propenyl)benzoic acid methyl ester as for Example 1, Step 3.
20 lH NMR (CD3COCD3) o 3.13 (3H, s), 3.85 and 3.92 (6H, 2s), 7.50 (2H,
d), 7.55 (2H, d), 7.g0 (2H, d), 7.92 (lH, s), 7.92 (2H, d).
5-(4-(C~ulvu~y~L~"~yl)-4-(~(methylsulfonyl)phenyl)-
llllo~h~ ,r-2-r~rboxyli~ acid
25 Prepared from 5-(4-(--.. ,ll.u~y.i,ul,G.. yl)phenyl)-4-(4-
(methyl)sulfonyl)phenyl) ~ ,h~ue-2-~l,u~ylic acld methyl ester as for
Example 2, Step 1.
lH NMR (CD3COCD3) o 3.15 (3H, s), 7.5û (2H, d), 7.62 ~2H, d), 7.95
30 (2H, d), 7-98 (lH, s), 8.05 (2H, d).
~nalysis calcd. for ClgH14O6S2-0~1 H2O
C, 56.46; H, 3.51
Found: C, 56.18; H, 3.51
WO 95/18799 ~ ' 2 1 8 0 6 5 1 PCTICA94/00688
- 87 -
EXA~PLE 6
4-(4-Fluoro~henvl)-2-methyl-5-(4-(methylsulfonyl)pher~yl)thiazQle
5 ~Ç~ 1-(4-Fluvlu~ lyl~-2-(4-(~Pth~y~lllfor~yl)phenyl)~thslnore
To 1-(4-Fluv,u~llc;l,yl)-2-(4-(methylthio)phenyl)ethanone of
Exarnple 1, Step I (17.9 g) in a solution of CH2C12-MeOH (272.0
mT /27.0 mL) at 0C was added MPPM (28.0 g). The cooling bath was
then removed and the reaction mixture stirred at r.t. for I h. At 0C,
10 sl~1rlitinn~1 MPPM (28.0 g) was added and the reaction mixture kept for
l.S h at r.t. The insoluble material was filtered followed by evaporation
of the solvents, the residue was then extracted with CH2C12-NaHCO3.
After ~V~pV~Liull in vacuo, the resulting solid was washed with ether-
hexane (1:1) and filtered to provide the title compound 16.8 g.
5 IH NMR (CD3COCD3) o 3.13 (3H, s), 3.58 (2H, s), 7.29 (2H, t), 7.5S
(2H, d), 7.88 (2H, d), 8.20 (2H, dd).
2-Bromo-1-(4-nuv,u~ ,.,yl)-2-(4-(methylsulfonyl)phenyl)-
~th~nnnl~
To 1-(4-Fluu~upl~ yl)-2-(4-(methylsulfonyl)phenyl)-
ethanone (1.00 g) in CH2C12 c~-nt~inin~ CHC13 (1.0 mL) and CC14 (1.0
mL) was added bromine (0.614 g). After shining light for I h, the
reaction was quenched with Na2S2O4, extracted with CH2C12, dried
over Na2S04 and ev~ to yield the title ~v~ ùulld which was u~ed
25 as such for the next step (1.10 g).
lH NMR (CD3COCD3) o 3.10 (3H, s), 7.05 (lH, s), 7.30 (2H, t), 7.87
(2H, d), 7.95 (2H, d), 8.25 (2H, dd).
~tep 3: 4-(4-Fluv~u~ ,llyl)-2-methyl-5-(4-(methylsulfonyl)phenyl)-
~hiS17~
To 2-bromo-1-(4-nuo,v~ ,l,yl)-2-(4-(methylsulfonyl)-
phenyl) L,~ lUII~, (1.10 g) in ethanol (15.0 mL) were added
- r~ (0.266 g) and pyridine (0.300 mL). After refluxing for 2 h,
the reaction mixture was extracted with EtOAc, 25% NH40Ac and
wo ss/ls7ss , 1~ 2 t 8 Q 6 5 1 Pcr/cAs4/00688
- 88 -
purified by flash chromatography (50% EtOAc in hexane then 90% Et2O
in hexane) to yield the title compound (0.320 g).
lH NMR (CD3COCD3) o 2.72 (3H, s), 3.15 (3H, s), 7.09 (2H, t), 7.52
(2H, dd), 7-60 (2H, d), 7.92 (2H, d).
Analysis calcd. for C17H14FNO2S2
C, 58,78; H, 4.03; N, 4.03
Found: C, 58.71, H, 4.17; N, 3.85
EXAMPLE 7
2-(4-F~uo-v~ yl)-3-(4-(m~thylsulfonyl~phenyl)-2-cyclopenten-l-one
1-(4-Fluvlv~1..,1l~1)-5-hexen-2-one
To a ~ :-." of 14.6 g (80 mmol) of CdC12 in 200 mL of
ether cooled to 0C was added 115 mL of 1.3M solution of 3-butene-1-
m~n.oCil-m bromide dropwise. The mixture was refluxed for 1 h and
ether was then removed by ~ligtill~ n Benzene (500 mL) was
introduced, followed by a solution of 17.5 g (100 mmol) 4-fluulv~ lyl-
acetyl chloride. After refluxing for 1 h, the reaction mixture was
quenched with 200 mL of saturated aqueous NH4CI, 50 mL of lN HCI,
and extracted with 200 mL of 1:1 hexane/EtOAC. The organic phase
was dried over MgSO4 and Cf- 1 The residue was purified by
flash clu~ ~ , ' y eluted with 4:1 hexane/EtOAc to give 15 g of the
title product.
1H NMR (CDC13) o 2.40 (2H, t), 2.53 (2H, t), 3.63 (2H, s), 4.90-4.98
(2H, m), 5.67-5.78 (lH, m), 6.98 (2H, t), 7.13 (2H, m).
~ 1-(4-E~lucllv~ yl)-S-oxo-2-p~ vl~e
A solution of 14 g of 1-(4-nu.,lv~ l)-5-hexen-2-one in
200 mL of 3:1 CH2C12/MeOH was cooled to -78C and treated with
excess ozone. The resulting mixture was treated with 15 g of
L~ o~llll;ll~ andstirredatroom t`.1ll~ for1h. Thereaction
WO 95/18799 2 1 8 ~ 6 5 1 PCT/CA94/00688
- 89 -
mixture was cul~c~lLIdl~d and flash chromatographed with 3:1
hexane/EtOAc to give 8 g of the title kFtoq~ hyde
lH NMR (CDC13) o 2.72 (4H, s), 3.71 (2H, s), 6.99 (2H, t), 7.14 (2H,
m), 9.73 (lH, s).
~; 2-(4-FIUC)Iu~ 1)-2-cyclopenten-1-one
A solution of 8 g of 1-(4-fluulu~ yl)-S-oxo-2-p~.llh.~)llF
in 300 mL of MeOH was treated with 2 g of NaOMe. The mixture was
sti~red for 2 h and then quenched with S mL of HOAc. The solvent was
~, ~ul~t~,d and the residue purified by flash chr -m~t -gr~rhy, eluting
with 3:1 hexane/EtOAc to give 7 g of the title product.
lH NMR (CDC13) o 2.57 (2H, m), 2.68 (2H, m), 7.04 (2H, J=8.8 Hz, t),
7.67 (2H, J=8.8, 5.5 Hz, dd), 7.77 (lH, m).
5 Step 4: 1-(4-(Methylthio)phenyl)-2-(4-fluolupl~ yl)-2-cyclo-
penten-l-ol
To a solution of 3.86 g (19 mmol) of 4-bl. ", ,~ ,;û .~ in
90 mL of Et2O cooled at -78C, was added 22 mL of 1.7M solution of t-
BuLi in pentane (38 mmol) dropwise. The reaction mi%ture was stirred
20 for lS min at -78C and a solution of 2.23 g of 2-(4-Fluulupll.,.lyl)-2-
~yo,lu~ t~.l-l-one in 10 mL of Et20 was added. After sti~ring for lS
min at -78C, the reaction mixt~Te was warmed to 0C, and quenched
with 50 mL of sat. NH4CI. The product was extracted with 100 mL
EtOAc, dried over Na2SO4, and purified by flash ,lu~ " "~ , nl~l Iy, eluted
25 with 4:1 hexanelEtOAc to give 3.4 g of the desired product.
lH NMR (CDC13) o 2.12 (lH, s), 2.34 (2H, m), 2.44 (3H, s), 2.45-2.52
(lH, m), 2.56-2.65 (lH, m), 6.37 (lH, m), 6.84 (2H, J=8.7 Hz, t), 7.1
(2H, J=8.3 Hz, d), 7.24-7.33 (4H, m).
30 ~ 2-(4-Flu~lu~ yl)-3-(4-(methylt-h-io)phenyl)-2
~enten-l-one
To a ~ -. of PCC (4.5 g, 20.9 mmol) and 10 g of
alll.y~llvu~ 4A mol~c~ r sieves in 150 mL of CH2C12 was added a
solution of 2.2 g (7.3 mmol) of 1-(4-(1llcLllyllluo)phenyl)-2-(4-
WO 9S/18799 ' ~; PCTICA94/00688
2180651
- 90 -
fluo~ yl)-2-cyclopenten- 1-ol in 20 mL CH2C12. The mixture was
stirred fo} I h at r.t. and then diluted with 300 mL of Et2O. After
filtration and ~,n-l~r~ iOn the residue was flash ~hlvlllalu~ lled with
2:1 hexane/EtOAc to give 1.5 g of the title product.
lH NMR (CDC13) o 2.45 (3H, s), 2.68 (2H, m), 3.00 (2H, m), 7.02 (2H,
J=8.6 Hz, t), 7.11 (2H, J=8.6 Hz, d), 7.15-7.23 (4H, m).
$tep 6: 2-(4-Fluolupll~,lyl)-3-(4-(methylsulfonyl)phenyl)-2-
cyclQpenten- I -one
To a solution of 50 mg (0.17 mmol) of 2-(4-nu~lu~
3-(4-lll~ ylLluo)phenyl)-2-cyclopenten-l-one in 8 mL of 10:1
CH2C12/MeOH was added 124 mg (0.2 mmol) of MPPM. The reaction
mixture was stirred at room ~. .,,I.r,i ~,,,e for 2 h and then diluted with 10
mL of 1:1 hexane/EtOAc. After filtration and co~ I ion, the residue
was purified by flash chrf rn~tc~ rhy eluted with 2:1 EtOAcrnexane to
give 45 mg of the title product.
lH NMR (acetone-d6) o 2.67 (2H, m), 3.14 (3H, s), 3.16 (2H, m), 7.05-
7.10 (2H, m), 7.20-7.25 (2H, m), 7.63 (2H, d), 7.93 (2H, ~).
FXAMPT F 8
4-(4-(Iv~r.ll~yl~ulr~llyl~pherlvl)-s-(4-nll~ yll~llyl)-is~thi~7r~le
To a solution of 338 mg (I rnmol) of cis,trans 3-chloro-3-(4-
nuul~ "lyl)-2-(4-(methylsulfonyl)phenyl)propenal in 5 mL of acetone
25 was added 230 mg (3 mmol) of NH4SCN. The reaction mixture was
refluxed for 3 h, and then quenched with 20 mL of saturated NaHCO3.
The product was extracted with 100 mL of EtOAc, dried over Na2SO4,
Cl~ r~ ' il and purified by flash ~ y eluted with 3:2
hexane/EtOAc to give 250 mg of the title product.
30 lH NMR (CDC13) o 8.57 (lH, s), 7.93 (3H, d), 7.50 (2H, d), 7.30 (2H, t),
7.08 (2H, t).
WO 95/18799 2 1 8 ~ 6 5 1 PCT/CA,94/0~)688
- 91 -
EXAMPLE 9
3-(4-Flu~luy~ )-4-(4-(methylsulfonyl)phenyl)-2-(5~l)-furanQne
Step 1: 2-Bromo-1-(4-(methylsulfonyl)phenyl)eth~n(mP
A solution of 197 g of 4-(Methylthio)a~.,tuL,l,~,Ilone (ref:
JACS, 1952, 74, p. 5475) in 700 mL of MeOH and 3500 mL of CH2C12
was added 881 g of MMPP over a period of 30 min. After 3 h at room
t~ the reaction mixture was filtered and the filtrate was washed
with 2L of saturated aqueous solution of NaHCO3 ar~d lL of brine. The
aqueous phase was further extracted with 2L of CH2C12. The cnmhin~-d
extracts was dried over Na2SO4 ."~ t~ d to give 240 g of 4-
(methylsulfonyl)a~,.,tu~ ,,llul,c as a white solid.
To a cooled (-5C) solution of 174 g of 4-(methylsulfonyl)-
5 ac~tu~ ,l,u"~ in 2.5L of CHC13 was added 20 mg of AIC13, followecl bya solution of 40 mL of Br2 in 300 mL CHC13. The reaction mixture was
then treated with 1.5L of water and the CHC13 was s~p~r:ltorl The
aqueous layer was extracted with lL of EtOAc. The combined extracts
was dried over Na2SO4 arld cU"r ~ l l . t. ..1 The crude product was
20 recystalized from 50/50 EtOAclhexane to give 210 g of 2-bromo-1-(4-
(methylsulfonyl)phenyl)ethanone as a white solid.
~tep 2:
To the product of Step 1 (216 mg) dissolved irl ~etr~ni
25 (4 mL) was added Et3N (0.26 mL), followed by 4-fluuIu~ ,llylac~Lic
acid (102 mg). After 1.5 h at room ~ 0.23 mL of DBU was
added. The reaction mixture was stirred for another 45 min and then
treated with 5 mL of lN HCl. The product was extracted with EtOAc,
dried over Na2SO4 and CullC~ The residue was purified by flash
30 ullluI~ v~ lly (40% EtOAc in hexane) to yield 150 mg of the title
cc.lll~,uul~d as a solid.
lH NMR (CD3COCD3) o 3.15 (3H, s), 5.36 (3H, s), 7.18 (2H, J=8.9 Hz,
t), 7.46 (2H, m), 7.7 (2H, J=8.65 Hz, d), 7.97 (2H, J=8.68, d).
WO 95/18799 i PCT/CA94/00688
21 806~1
-92 -
FX~MPT F 10
3-~4-Fluc~lu,~ yl~-4-(4-f~minoslllfollyl)~h~rlyl) 2 !2~-fi-r~nnn~.
lH NMR (CD3COCD3) ~ 5.34 (2H, s), 6.67 (2H, bd), 7.18 (2H, m), 7.46
(2H, m), 7.61 (2H, m), 7.90 (2H, m).
M.P. 187-188~C (d).
FXAMPLF 1 1
~o
3-(4-FI~ uv~ yl)-4-(4-(~n~ot~ylglllf ~r~ hellyl)filr~n
. ~ .
Using the product of Example lQ, (0.2 g) in THF (5 rnL) ~nd
5 toluene (3 mL) was added slowly at -78C a solution of DIBAL (0.72
mL, IM in toluerle). After 15 min, the solution was w~med up to 0C
for another 15 min. This mixture was then poured into a chi~led aqueous
solution of sodium pv~ tartrate and EtOAc. The organic layer was
stirred for 0.5 h with a few crystals of carnphor sulfonic acid. This
20 solution was then ~ t~d ~nfl purified by flash ~ LvLllalu~y to
yi~ld the title c. r(~ ~l
lH NMR (CDC13) 8 3.1 (3H, s), 7.02 (2H, J=8.9, t), 7.~8 (2H, m), 7.4
(2H, J=8.8 Hz, d), 7.58 (lH, s), 7.68 (lH, s), 7.85 (2H, J=8.8 Hz, d).
F.XAMPr F. 1'~
5~5-Dimethyl-3-(4-nuulu~ yl~-~(~(methylsulfonyl)phenyl)-2-(s~)
filr~nnnl~
30 ~ç~ M~-t~yl 2-trim~t~ yloxyis~u~
To a solution of 1.2 rnL (10.4 mmol) of methyl 2-hydroxy-
iSoL7U~y~ in 50 mL of CH2C12 were added 1.2 g (17.6 rnmol~ of
7nl~ and 2.1 mL (16.6 rnmol~ of TMSCl. The rnixture was stirred at
r.t. for 1.5 h and querlched with 20 mL of H2~. The organic layer was
WO 95/18799 ` ~ _, 2 1 8 0 6 5 1 PCT/CA941C0688
- 93 -
dried over MgSO4, cullc~l.Ll~L~d and passed through a short plug of silica
gel eluted with 9:1 hexane/EtOAc. Evaporation of solvent afforded 1.27
g of fhe title compound as a colorless oil.
lH NMR (CD3COCD3) ~ 0.08 (9H, s), 1.38 (6H, s), 3.67 (3H, s).
2-Trim~fhylsilyloxy-4'-(methylthio)isbl,.lly,upll~ oll~
A solution of 204 mg (1.0 mmol) of 4-bromothioanisole in
2.5 mL of THF was cooled to -78C and treated with 0.42 mL of 2.5M n-
BuLi solution in hexane. After stirring at -78C for 1 h, a solution of 380
mg (2.0 mmol) of methyl 2-trimethylsilylu~yi~buly-~ in 2 mL of TI~F
was added. The mixture was stirred at -78C for 2 h and then quenched
with NH40Ac buffer. The product was extracted with EtOAc, dried over
MgSO4 and c~ .. ,. - . ,1, ~ .1 The residue was purified by flash
chromatography, eluting with 19:1 hexane/EtOAc to give 95 mg of the
title product.
lH NMR (CD3COCD3) o 0.05 (9H, s), 1.52 (6H, s), 2.53 (3H, s), 7.33
(2H, d), 8.12 (2H, d).
Step 3: 2-Hydroxy-4'-(m~the,vlthio)isobu~y-ù ~ VIIC
To a solution of 40 mg (0.14 mmol) of 2-trimethylsilyloxy-
4'-(lll.,Ll~ylLlfio)isob~llylu~ ulle in 2 mL THF was added 0.2 mL of lM
n-Bu4NF in THF. The resulting mixture was stirred for 30 min and then
quenched with 10 mL of NH40Ac buffer. The product was extracted
with EtOAc, dried over MgSO4 and c~ t~ The residue was
2 5 purified by flash .,1~, u. ~ l .y, eluting with 4:1 hexane/EtOAc to give
25 mg of the title product.
lH NMR (CD3COCD3) o 1.50 (6H, s), 2.54 (3H, s), 4.68 (lH, s), 7.30
(2H, d), 8.15 (2H, d).
0 $te~ 4: 2-(4-Fluùlu~ ylacetoxy)-4l-(~ l;o)iso~ulyluy~ ulle
To a solution of 72 mg (0.34 mmol) 2-hydroxy-4'-
(lu~,LllylLlliO)is~uLylu~ ,llul~ in 1.7 mL of CH2C12 were added 0.2 mLof pyridine and 140 mg (0.81 mmol) of 4-nuulu~ ,llylacetyl chloride.
The mixture was stirred at room ~. ."l,~ c overnight and then
WO 95/18799 ~ 2 1 8 0 6 5 1 PCT/C~94/00688
-g4-
quenched with NH40Ac buffer. The product was extracted with EtOAc,
dried over MgSO4 and ~ . rl The crude product was purified by
flash ch~omalugl~l,y eluting with 8:1 hexane/EtOAc to give 95 mg of
the title produet.
lH NMR ~CD3COCD3) o 1.62 (3H, s), 1.67 (3H, s), 2.48 (3H, s), 3.79
(2H, s), 7.0-7.3 (fiH, m), 7.78 (2H, d).
Step 5; 5,5-Dimethyl-3-(4-nuu.u~ yl-4-(4-(u~ yl~Lo)phenyl)-2-
(SH)-filrPnr~nP.
To a solution of 95 mg of 2-(4-fluu.u~ ylacctu~y)4'-
yl~liû)-isobuLylu~ lùllc in 4 mL of CH2C12 was added 0.2 mL of
1,8-diazabicyelo(5.4.0)undec-7-ene. The mixture w~s stirred for 4 h and
diluted with NH40Ac buffer. The product was extracted with EtOAc,
dried over MgS04 and c~ d The residue was purified by flash
5 CIIL~ tu~ln~ y~ eluting with 20:1 toluene/EtOAe to give 75 mg of the
title produet.
lH NMR (CD3COCD3) o 1.58 (6H, s), 2.50 (3H, s), 7.03 (2H, dd), 7.25-
7.35 (4H, m), 7.41 (2H, dd).
20 ~; 5~s-Dimethyl-3-(4-fluulupll~lyl)-4-(4-(u~tl~yl~ l{u-lyl)-
~ h.o.r~yl)-2 fS~)-filr~nnn.~
To a solution of 81 mg of 5,5-dimethyl-3-(4-nuv,u~ .-yl)4-(4-(methyl-
thio)phenyl)-2-oxo-2H-dihydLuru~all in 1.8 mL of CH2C12 and 0.2 mL of
MeOH was added 250 mg of MPPM. The reaetion mixture was stirred at
25 room t~ n~ ~c for 1 h and then quenehed with aqueous NaHCO3.
The product was extraeted with EtOAe, dried over MgSO4 and
co~c~ ~e"' The erude product was purified by flash ~ uu~-v~la~lly
duting with 1:1 hexaneJEtOAc to give 73 mg of the title product.
lH NMR (CD3COCD3) o 1.62 (6H, s), 3.15 (3H, s), 7.02 (2H, dd), 7.40
3 (2H, dd), 7.65 (2H, d), 8.03 (2H, d).
5,5-Dimethyl-3-(3-nuo.ù~ .yl)-4-(4-(Lu~l,yla.ll~lyl)phenyl)-2-
(5.rl)-furanone was prepared in an analogous manner (m.p. 172.7C).
Analysis: C~ C, 63.32; H, 4.75;
Found: C, 63.50; H, 4.79;
WO95/18799 ` ` j~ 21 8a65l PC11CA94/C0688
_ 95 _
lEXAMPl.F 13
2-((4-n~ rullvl)phenvl)-3-(4-nu~lvL)~ yl)thiophene
lH NMR (CD3COCD3) o 6.60 (2H, bs), 7.12 (2H, t)l 7.25 (lH, d), 7.35
(2H, m), 7.45 (2H, d), 7.65 (lH, d), 7.85 (2H, d).
Analysis r~ Atl-d for Cl6Hl2FNs2o2
C, 57.65, H, 3.60; N, 4.20
Found: C, 57.55; H, 3.79; N, 4.03
F.X~MPT.F. 14
3-(4-(T~i~luo~u~c~,lyl,.~ cl~lfonyl)phenyl)-2-(4-nuùl~yl~ yl)thioph~ne
lH NMR (300 MHz, CD3COCD3) o 7.15 (2H, t), 7.30 (3H, m), 7.45
(2H, d), 7.65 (lH, d), 7.95 (2H, d).
F.XAMPT.F lS
3-(2.4-D;n~ uyll. Iyl)~(4-(m.-.th,ylsulfonyl)pher~yl)-2-(SH)-fur~nonP
Analysis c~ 1 for C17H12F204S
C, 58.28; H, 3.45; S, 9.15
Found: C, 58.27; H, 3.50; S, 9.27
FXAMPl F 16
3-(3.4-Dinuu~u~lJ~,llyl)-4-(4-(methyl~lllfonyl)phenyl)-2-(5H)-furanone
To a solution of 3,4-dinuoluj~ .,.lylac~lic acid (ALDRICH
CHIMICAL) (10 g) and 2-bromo-1-(4-(~,,.,~hyl~ulrullyl)phenyl)ethanone
(Example 9, Step 1) (17.3 g) in sretnnitril~ (200 mL) at room ~ LiUl~
was added slowly trlcll.yl~i.l~ (20.2 mL). After 1 h at room
t. ~ , the mixture was cooled in an ice bath and treated with 17.4
-
WO 95118799 - ~ ~i 2 1 8 0 6 5 1 PCT/CA94100688
-96-
mL of DBU. After 2 h at 0C, the mixture was treated with 200 mL of
lN HCI and the product was extracted with EtOAc, dried over Na2S04
and cnn~Pntr~t~-~1 The residue was applied on top of a silica gel plug
(sintered glass funnel) eluted with 75% EtOAc/hexane, giving after
5 evaporation of the so~vent and swish in ethyl acetate, 10 g of the title
compound.
Analysis c~ tpd for C17H12F204S
C,58.28;H,3.45;S,9.15
o Found: C, 58.02; H, 3.51; S, 9.35
EXAMPLE 17
3-(2.6-Dinuu~ lyl)-4-(4-(methylsulfonyl~phenyl)-2-(5H)-furanone
Analysis c~ t~d for C17H12F204S
C, 58.28; H, 3.45; S, 9.15
Found: C, 58.18; H, 3.50; S, 9.44
FXAMPLE 18
3-(2.5-Dinu~ .,llyl)-4-(4-(methylsulfonyl)phenyl)-2-(SH)-furanone
Analysis ~ t~d for C17H12F204S
C, 58.28; H, 3.45; S, 9.15
Found: C, 58.89; H, 3.51; S, 9.11
~ WO 95118799 ~ ; 2 1 8 0 6 5 1 PCTICA94/00688
- 97 -
EXAMPT~F 19
3-(3.5~Dinl~n~u~ llyl)-4-(4-(m~othylsulfonvl)phenyl)-2-(5H)-furanone
Analysis r~lrul~t~d for C17H12F204S
C, 58.28; H, 3.45; S, 9.15
Found: C, 58.27; H, 3.62; S, 9.32
EXAMPT F. 20
3-(4-Bromophenyl)-4-(4-(mP.~hyl~--lfonyl)phenvl)-2-(5~-furanone
Analysis c~lr~ tPd for C17H13BrO4S
C, 51.94; H, 3.33; S, 8.16
15 Found: C, 51.76; H, 3.42; S, 8.21
FX~MPT.F 21
3-(4-~hl~., uv~ yl)-4-(4-(l~ llr~ ,vl)phenyl)-2-(SHl-furanone
lH NMR (300 MHz, CDC13) o 7.93 (2H, d), 7.49 (2H, d), 7.35 (4H, m),
5.16 (2H, s), 3.06 (3H, s).
pX~MPT.F 22
3-(~ M~lllu~y~ l)-4-(4-(m~hylsulforlyl)pher~yl)-2-(SH)-furanone
Analysis c~lr~~l! ~ for C1gH160sS
C, 62.78; H, 4.68; S, 9.31
Fou~d: C, 62.75; H, 4.72; S, 9.39
WO 95/18799 ~ C 2 1 8 0 6 5 1 PCTICA94/00688
- 98 -
EXAMPLE 23
3-(Phenyl)-4-(4-(methylsulfonyl)phenvl)-2-(5H)-furanone
To a solution of phenylacetic acid (27.4 g, 201 mmol) and 2-
5 bromo-1-(4-(methylsulfonyl)phenyl)ethanone (Example g, Step 1) (60 g,
216 mmol, 1.075 eq.) in P~.tt)nitnlP (630 mL) at 25C was added slowly
tric;~lylalllill~ (30.8 mL, 1.1 eq.). The mixture was sti~red for 20 min at
room t~,llllJ.,IaLLllci and then cooled in an ice bath. DBU (60.1 mL, 3 eq.)
was slowly added. After stirring fo} 20 min in the ice bath, the reaction
o was complete and the mixture was acidified with IN HCI (color changes
from dark brown to yellow). Then 2.4 L of ice and water were added,
stirred for a few minutes, then the ~JI~il)iLal~ was filtered and rinsed with
water (giving 64 g of crude wet product). The solid was dissolved in 750
mL of dichlululll~lllall~ (dried over MgSO4, filtered) and 300 g of silica
5 gel was added. The solvent was ~va~ to near dryness (silica gel a
bit sticky) and the residue was applied on top of a silica gel plug (sintered
glass funr~el) eluted with 10% EtOAc/CH2C12, giving after ~va~OIa~i
of the solvent and swish in ethyl acetate, 36.6 g (58%) of the title
CO~rolln~
Analysis ~ r~llqtod for C17H1404S
C, 64.95; H, 4.49; S, 10.20
Found: C, 64.63; H, 4.65; S, 10.44
F.X~IPLE 24
3-(2-t~hlo~ yl)~(4-(1l~ lr~llyl)phenyl)-2-(5H)-furanone
Analysis c~ t~d for C17H13C104S
C, 58.54; H, 3.76; S, 9-19
Found: C, 58.59; H, 3.80; S, 9.37
WO 95/18~99 j~ ~ 2 1 8 0 6 5 1 PCT/CA94/00688
_ 99 _
FXAMP! F 25
3-(2-Bromo-4-nu~l u~llellyl)4-(4-(1llc;lllyl~ulr(JIlyl)phenyl)-2-(S~)-
fur~n~-ne
Analysis calculated for C17H12BrF04S
C, 49.75; H, 2.93
Found: C, 49.75; H, 3.01
XAMP! F 2~
3-(2-Bromo-4-Chlo~v~ ,.lyl)-4-(4-(methylsulfonyl)phenyl)-2-(5~)-
filranone
5 lH NMR (300 MHz, acetone-d6) ~ 7.95 (2H, d), 7.85 (lH, d), 7.63 (2]1,
dd), 7.55 (lH, dd), 7.45 (lH, d), S.S0 (2H, s), 3.15 (3H, s).
FxAMPT.F. 27
2 3-(4-Chloro-2-flu~l u~l~c;-lyl)-4-(4-(methylsulfonyl)phenyl)-2-(5H)-
furanone
lH NMR (300 MHz, acetone-d6) ~ 8.0 (2H, d), 7.70 (2H, d), 7.50-7.30
(3H, m), 5.35 (2h, s), 3.15 (3H, s).
EXAMPT F 28
3-(3-Bromo-4-nuulv~l.el.yl)~(4-(methylsulfonyl)phenyl)-2-(5H)-
filr~n~ne
Analysis c~1rlll~t~d for C17H12BrF04S
C, 49.75; H, 2.93
Found: C, 49.44; H, 2.98
WO 95/18799 ~ 2 1 8 0 6 ~ 1 PCT/CAg4/00688
- 100-
EXAMPLE 29
3-(3-Chlvlv~ lyl)~(~(methylsulfonyl)phenyl)-2-(5~)-furanorle
5Analysis rAlr-llAtPd for C17H13CIO4S
C, 58.54; H, 3.76
Found: C, 58.29; H, 3.76
EXAMPLE 30
3-(2-Chloro-4-nu~lv~ ;llyl)-4-(4-(methylsulfonyl)phenyl)-2-(5H)-
furAnrne
Analysis CAlr~lAtPd for C17H12CIFO4S
C, 55.67; H, 3.30
Found: C, 55.67; H, 3.26
EXAMPLE 31
20 3-(2.4-Dirhl~lu~ lyl)-4-(4-(ll~ ylDul~llyl)phenyl)-2-(5H)-furanone
Anlysis rAlr~ d for C17H12C1204S
C, 53.28; H, 3.16; S, 8.37
Found: C, 52.89; H, 3.23; S, 8.58
FX~MPT.F 32
3-(3.~Di~hl~-uyll~,.l,yl)-4-(4-(rnPth~ylsulfonyl)phenyl)-2-(5H)-furanone
30 Analysis cAlt ' ' for Cl7Hl2cl2o4s
C, 53.28; H, 3.16; S, 8.37
Found: C, ~3.07; H, 3.32; S, 8.51
~ WO 95/18799 ~ 2 ~ 8 0 6 5 1 PC'r1CA941C0688
- 101 -
FXAMPLE 33
3-(2.6-D;chl- " upll~ Iyl)-4-(4-(methylsulfonyl~phenyl)-2-(5H)-furanon~
Analysis r~lr~ d for C17H12C12O4S
C, 53.28; H, 3.16; S, 8.37
Found: C, 52.99; H, 3.22; S, 8.54
FXAMpT F 34
3-(3-Chloro-4-fluorophenyl)-4-(4-(methylsulfonyl)phenyl)-2-(5H)-
fur~nr,nP
H NMR (300 MHz, acetone-d6) d 8.0 (2H, d), 7.70 (2H, d), 7.60 (IH,
5 d), 7.25-7.40 (2H, m), 5.35 (2H, s), 3.15 (3H, s).
FXAMPT F 35
3-(4-Trifluu~u~ hyl~ yl)-4-(4-(methylsulfonyl)phenyl)-2-(5H)
20 fur~lnr~ne
lH NMR (CD3COCD3) o 8.10 (2H, d), 7.82-7.93 (4H, m), 7.75 (2H, d),
5.55 (2H, s), 3.30 (3H, s).
FXAMPT F 36
3-(3-Fluoro-4-~ u~y~ ,l.yl)~(4-(methylsulfonyl)phenyl)-2-(5~)-
filr~nr~n-o
30 Analysis ~lrlllo~l for ClgH15FO5S
C, 59.66; H, 4.17
Found: C, 59.92; H, 4.37
WO 95/18799 ' `; ~ ~ ~ 2 1 8 0 6 5 1 PCT/CA94/00688
- 102-
EXAMPLE 37
3-(3-Chloro-4-metlluAy~ yl)-4-(4-(methylsulfonyl)phenyl)-2-(5H)-
furanone
Analysis calculated for ClgH15ClO5$
C, 57.07; H, 3.99
Found: C, 57.29; H, 4.15
0 FX~MPT F 38
3-(3-Bromo-4-meth~Ay~ yl)-4-(4-(methylsulfonyl)phenyl)-2-(5H)-
fur~n~-n.
5 Analysis c~ for ClgH15BrO5S
C, 51.08; H, 3.57
Found: C, 51.38; H, 3.62
EXAMPLE 39
3-~2-Fluu~u~ -4-(4-(methylsulfonyl)phenyl~-2-(5}0-furanone
Analysis cal~ fPd for C17H13F04S
C, 61.44; H, 3.94
Found: C, 61.13; H, 3.85
FXAMPT F 40
3-(~ yl~ h~llyl)-4-(4-(methylsulfonyl)~henyl)-2-(5H)-filr~nOne
lH NMR (300 MHz, acetone-d6) d 8.0 (2H, d), 7.70 (2H, d), 7.35 (2H,
d), 7.25 (2H, d), 5.35 (2H, s), 3.15 (3H, s), 2.~5 (3H, s).
WO 9U18799 ~ 2 ~ 8 0 6 5 ~ PCT/CA941C0688
- 103-
FXAMPLF 41
3-r3-Fluulu~ yl~-4-(4-(rn~thylslllfonyl)phenyl)-2-(5~)-furanone
lH NMR (300 MHz, CDCl3) d 7.93 (2H, d), 7.49 (2H, d), 7.35 (lH, m),
7.12 (3H, m), 5.18 (2H, s), 3.06 (3H, s).
FXAMPT F 42
3-(2-Chloro-6-nuuluyl~c~llyl)-4-(4-(methylsulfonyl)phenyl)-2-(5
fur~n~ne
lH NMR (300 MH~, acetone-d6), d 8.0 (2H, d), 7.70 (2H, d), 7.55-7065
(lH, m), 7.40 (lH, d), 7.30 (lH, m), 5.60 (2H, s), 3.15 (3H, s).
FXAMPLE 43
3-(3-Bromo4-1ll~,lllylL,1l~ 1)~(4-(methylsuLfonyl)phenyl)-2-(S~)-
filr~none
Analysis c~ for ClgHlsBrO4S
C, 53.08; H, 3.71
Found: C, 53.06; H, 3.83
2 5 FXA~rPT F 44
3-(4-Bromo-2-nuulu~l~llyl)-4-(4-(methylsuLfonyl)phenyl)-2-(5H)-
furanone
30 AnalysiS rzllrlll~t~d forC17H12BrFO4S
C, 49.65; H, 2.94
Found: C, 49.76; H, 3.00
2 1 8 0 6 5 1 CT/CAg4/00688
WO 9S/18799 P
- 104-
EXAMP~ F 45
3-(3.4-DibrQmo~henyl)-4-(4-(methylsulfonyl)phenyl)-2-(SH)-furanone
lH NMR (300 MHz, acetone-d6) o 8.0 (2H, d), 7.80 (lH, d), 7.75 (3H,
m), 7.25 (lH, d), 5.35 (2H, s), 3.15 (sH, s)
FXAMPJ F 46
3-(4-Chloro-3-nuulu~ ,.,yl)4-(4-(methylsulfonyl)phenyl)-2-(SH)-
fi-ranone
Analysis ~1r~ t~d for C17H12ClF04S
C, 55.67; H, 3.30
Found: C, 55.45; H, 3.30
EXAMPT F 47
3-(4-Bromo-3-fluulu~ ,l,yl)-4-(4-(methylsulfonyl)phenyl)-2-(SH)-
filr5~non~
Analysis c~ for C17H12BrF04S
C, 49.66; H, 2.94; S, 7.80
Found: C, 49.79; H, 3.01; S, 7.51
FX~MPT F 48
3-(4-Bromo-2-~,hlu-ul,ll.,.,yl)4-(4-(methylsulfonyl)phenyl)-2-(SH)-
filr~n~ n.-
An~lysis cz~lr~ for C17H12BrC104S
C, 47.74; H, 2.83; S, 7.50
Found: C, 47.92; H, 2.84; S, 7.42
wo 95118799 _ 2 1 8 0 6 5 I PCr1C~9410~688
- 105-
F,XAMPT .F. 49
3-(2-l~T~htllyl)-4-(~mAth,ylglllf~myl)rht~ ) 2_~5~)-filr~nnn~.
Analysis ~ ul~t~d for C21H160~S
C, 69.22; H, 4.43
Found: C, 69.22; H, 4.46
FXAMPT,F, 50
3-~7-Qllinolir~yV 4 (4 (m~.tllylglllforly~ hlsrlyl)-2-(5~)-fur~nnnp
Analysis ç~l~ul~tcd for C20Hl5NO4S
C, 65.74; H, 4.14; N, 3.83
5 Found: C, 65.34; H, 4.40; N, 3.80
M.S. (DCI, CH4) r~ tpd for M+, 365
Found for M+ I 1, 366
FXAMPT,P. 51
3-~3.4-Di~hlo~ vl)-4~ r~ yl)~?hl~r~yl)-2-(s~)-filr~nn~
lH NMR (400 MHz, CD3COCD3) 8 7.92 (2H, dd), 7,64 (3H, dm), 7.60
(lH, dd), 7.32 (lH, dd), 6.70 (lH, bs), 5.38 (2H, s).
FXAMPT,F. 52
3-(3.4-Dill~ yl)-4-(4-(~ ru,lyl~phc~-yl)-2-(5Fr~-f lr~non~
30 lH NM~. (400 MHz, CD3COCD3) o 7.92 (2H, dd), 7,64 (2H, dd), 7.30-
7.45 (2H, m), 7.22 (lH, m), 6.68 (2H, bs), 5.37 (2H, s).
PCT/CA9~/00688
Wo9S/l879, 2 1 8 0 6 5 1
- 10~-
FXAM~'L F 53
3-(3-Chloro-4-me~o"y~lle,..,yl)~-(4-(~Tn;nnc~llf~.nyl)phenyl~-2-(5H)-
filr~nr.n~
Analysis c~lrlll~t~d for C17H14CIN05S
C, 53.76; H, 3.72, N, 3.69
Found: C, 53.32 H 3.84, N, 3.59
M.S. (DCI, CH4) c~;~llint~d for M+, 37g
o ~ound for M++l, 380
FX.~MPT F 54
3-(3-Bromo-4-~ y~ yl)~(4-(~minoslllf~nyl)phenyl)-2-(5H~-
5 filrrn-~nP
An~lysis c~1rlll~t~d for C17H14BrN05S
C, 48.13; X 3.33. N, 3.30
Found: C, 48.26- H 3.40, N, 3.28
20 M S. (DCI, CH4) ~irlli~tPd for M+, 423
Found for M++l, 424