Note : Les revendications sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- 68-
THE EMBODIMENTS OF THE INVENTION IN WHICH AN EXCLUSIVE
PROPERTY OR PRIVILEGE IS CLAIMED ARE DEFINED AS FOLLOWS:
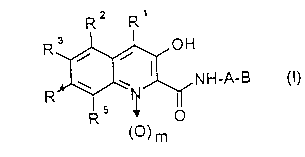
1. A compound of the formula (I)
<IMG> (I)
in which
A is (C1-C4)-alkylene, which is optionally substituted
by one or two substituents from the series consist-
ing of halogen, cyano, nitro, trifluoromethyl,
(C1-C6)-alkyl, (C1-C6)-hydroxyalkyl, (C1-C6)-alkoxy,
-O-[CH2]X-CfH (2f+1-g) Halg, (C1-C6)-alkylmercapto,
(C1-C6)-alkylsulfinyl, (C1-C6)-alkylsulfonyl,
(C1-C6)-alkylcarbonyl, (C1-C6)-alkoxycarbonyl,
carbamoyl, N-(C1-C4)-alkylcarbamoyl, N,N-di-(C1-C4)-
alkylcarbamoyl, (C1-C6)-alkylcarbonyloxy, (C -3C )8-
cycloalkyl, phenyl, benzyl, phenoxy, benzyloxy,
anilino, N-methylanilino, phenylmercapto,
phenylsulfonyl, phenylsulfinyl, sulfamoyl,
N-(C1-C4)-alkylsulfamoyl and N,N-di-(C1-C4)-
alkylsulfamoyl, or
by (C6-C12)-aryloxy, (C7-C11)-aralkyloxy, (C6-C12)-
aryl or (C7-C11)-aralkyl radical, which carries, in
the aryl part, 1, 2, 3, 4 or 5 identical or
different substituents from the series consisting of
halogen, cyano, nitro, trifluoromethyl, (C1-C6)-
alkyl, (C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Halg,
(C1-C6)-alkylmercapto, (C1-C6)-alkylsulfinyl,
(C1-C6)-alkylsulfonyl, (C1-C6)-alkylcarbonyl,
- 69 -
(C1-C6)-alkoxycarbonyl, carbamoyl, N-(C1-C4)-
alkylcarbamoyl, N,N-di-(C1-C4)-alkylcarbamoyl,
(C1-C6)-alkylcarbonyloxy, (C3-C8)-cycloalkyl,
sulfamoyl, N-(C1-C4)-alkylsulfamoyl and N,N-di-
(C1-C4)-alkylsulfamoyl, or
by one or more substituents Rx of the .alpha.-C atom of an
.alpha.-amino acid, being a naturally occuring L-amino
acid or a D isomer thereof;
B is an acid grouping from the series comprising
-CO2H, -CONHCOR"', -CONHSOR"', CONHSO2R"', -NHSO2CF3,
tetrazolyl, imidazolyl and 3-hydroxyisoxazolyl, in
which R"' is aryl, heteroaryl, (C3-C7)-cycloalkyl or
(C1-C4)-alkyl, optionally monosubstituted by
(C6-C12)-aryl, heteroaryl, OH, SH, (C1-C4)-alkyl,
(C1-C4)-alkoxy, (C1-C4)-thioalkyl, -sulfinyl or
-sulfonyl, CF3, Cl, Br, F, I, NO2, -COOH, (C2-C5)-
alkoxycarbonyl, NH2, mono- or di-(C1-C4-alkyl)-amino
or (C1-C4)-perfluoroalkyl,
R1, R2, R3, R4 and R5 are identical or different and are
hydrogen, hydroxyl, halogen, cyano, trifluoromethyl,
nitro, carboxyl, (C1-C20)-alkyl, (C3-C8)-cycloalkyl,
(C3-C8)-cycloalkyl-(C1-C12)-alkyl, (C3-C8)-cyclo-
alkoxy, (C3-C8)-cycloalkyl-(C1-C12)-alkoxy, (C3-C8)-
cycloalkyloxy-(C1-C12)-alkyl, (C3-C8)-cycloalkyloxy-
(C1-C12)-alkoxy, (C3-C8)-cycloalkyl-(C1-C8)-alkyl-
(C1-C6)-alkoxy, (C3-C8)-cycloalkyl-(C1-C8)-alkoxy-
(C1-C6)-alkyl, (C3-C8)-cycloalkyloxy-(C1-C8)-alkoxy-
(C1-C6)-alkyl, (C3-C8)-cycloalkoxy-(C1-C8)-alkoxy-
(C1-C8)-alkoxy, (C6-C12)-aryl, (C7-C16)-aralkyl,
(C7-C16)-aralkenyl, (C7-C16)-aralkynyl, (C2-C20)-
alkenyl, (C2-C20)-alkynyl, (C1-C20)-alkoxy, (C2-C20)-
alkenyloxy, (C2-C20)-alkynyloxy, retinyloxy,
(C1-C20)-alkoxy-(C1-C12)-alkyl, (C1-C12)-alkoxy-
(C1-C12)-alkoxy, (C1-C12)-alkoxy-(C1-C12)-alkoxy-
(C1-C8)-alkyl, (C6-C12)-aryloxy, (C7-C16)-aralkyloxy,
(C6-C12)-aryloxy-(C1-C6)-alkoxy, (C7-C16)-aralkoxy-
- 70 -
(C1-C6)-alkoxy, (C1-C16)-hydroxyalkyl, (C6-C16)-
aryloxy-(C1-C8)-alkyl, (C7-C16)-aralkoxy-(C1-C8)-
alkyl, (C6-C12)-aryloxy-(C1-C8)-alkoxy-(C1-C6)-alkyl,
(C7-C12)-aralkyloxy-(C1-C8)-alkoxy-(C1-C6)-alkyl,
(C2-C20)-alkenyloxy-(C1-C6)-alkyl, (C2-C20)-
alkynyloxy-(C1-C6)-alkyl, retinyloxy-(C1-C?)-alkyl,
-O-[CH2-]xCfH(2f+1-g)Halg, (C1-C6)-chlorofluoroalkoxy,
(C1-C20)-alkylcarbonyl, (C3-C8)-cycloalkylcarbonyl,
(C6-C12)-arylcarbonyl, (C7-C16)-aralkylcarbonyl,
cinnamoyl, (C2-C20)-alkenylcarbonyl, (C2-C20)-
alkynylcarbonyl,
(C2-C20)-alkoxycarbonyl, (C1-C12)-alkoxy-(C1-C12)
alkoxycarbonyl, (C6-C12)-aryloxycarbonyl, ( C7 -C16)-
aralkoxycarbonyl, (C3-C8)-cycloalkoxycarbonyl,
(C2-C20)-alkenyloxycarbonyl, retinyloxycarbonyl,
(C2-C20)-alkynyloxycarbonyl, (C6-C12)-aryloxy-
(C1-C6)-alkoxycarbonyl, (C7-C16)-aralkoxy-(C1-C6)-
alkoxycarbonyl, (C3-C8)-cycloalkyl-(C -C1)-alkoxy-
carbonyl, (C3-C8)-cycloalkoxy-(C1-C6)-alkoxycarbonyl,
(C1-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkyl-
carbonyloxy, (C6-C12)-arylcarbonyloxy, (C7-C16)-
aralkylcarbonyloxy, cinnamoyloxy, (C2-C12)-alkenyl-
carbonyloxy, (C2-C12)-alkynylcarbonyloxy,
(C1-C12)-alkoxycarbonyloxy, (C1-C12)-alkoxy (C1-C12)-
alkoxycarbonyloxy, (C6-C12)-aryloxycarbonyloxy,
(C7-C16)-aralkyloxycarbonyloxy, (C3-C8)-cycloalkoxy-
carbonyloxy, (C2-C12)-alkenyloxycarbonyloxy,
(C2-C12)-alkynyloxycarbonyloxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl,
N-(C1-C10)-alkyl-N- (C3-C8) -cycloalkylcarbamoyl,
N-((C3-C8)-cycloalkyl-(C1-C6)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
- 71 -
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N-(C7-C16)-
aralkylcarbamoyl, N-(C1-C10)-alkyl-N-(C-C6)?6
arylcarbamoyl, N-(C1-C10)-alkyl-N-(C7-C16)-
aralkylcarbamoyl, N-((C1-C12)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-((C6-C16)-aryloxy-(C1-C10)-
alkyl)carbamoyl, N-((C7-C16)-aralkyloxy-(C1-C10)-
alkyl)carbamoyl, N-(C1-C10)-alkyl-N-((C1-C10)-alkoxy-
(C1-C10)-alkyl)carbamoyl, N-(C1-C10)-alkyl-N-
((C6-C12)-aryloxy-(C1-C10)-alkyl)carbamoyl,
N-(C1-C10)-alkyl-N-((C7-C16)-aralkyloxy-(C1-C10)-
alkyl)carbamoyl, CON(CH2)h, in which a CH2 group can
be replaced by O, S, N-(C1-C8)-alkylimino, N-(C3-C8)-
cycloalkylimino, N-(C3-C8)-cycloalkyl-(C1-C12)-alkyl-
imino, N-(C6-C12)-arylimino, N-(C7-C16)-aralkylimino
or N-(C1-C12)-alkoxy-(C1-C6)-alkylimino and h is 3 to
7, or a carbamoyl radical of the formula J
<IMG>
in which
Rx is the substituent of an L- or D-.alpha.-amino acid,
s is 1, 2, 3, 4 or 5 and
T is OH, OR or NR*R**, in which
R*, R** and R*** are identical or different and are
hydrogen, (C6-C12)-aryl, (C7-C11)-aralkyl,
(C1-C8)-alkyl, (C3-C8)-cycloalkyl, (+)-dehydro-
abietyl, (C1-C8)-alkoxy-(C1-C8)-alkyl, (C7-C12)-
aralkoxy-(C1-C8)-alkyl, (C6-C12)-aryloxy-
(C1-C8)-alkyl, (C1-C10)-alkanoyl, optionally
substituted (C7-C16)-aralkanoyl or optionally
- 72 -
substituted (C6-C12)-aroyl, or
R* and R** together are -[CH2]h, in which one CH2
group can be replaced by O, S, SO, SO2,
N-acylamino, N-(C1-C10)-alkoxycarbonylimino,
N-(C1-C8)-alkylimino, N-(C3-C8)-cyclo-
alkylimino, N-(C3-C8)-cycloalkyl-(C1-C4)
alkylimino, N-(C6-C12)-arylimino, N-(C7-C16)-
aralkylimino or N-(C1-C4)-alkoxy-(C-C1)-6
alkylimino and h is 3 to 7,
or further wherein R1 R2 R3 R4 and R5 are
identical or different and are:
carbamoyloxy, N-(C1-C12)-alkylcarbamoyloxy, N,N-di-
(C1-C12)-alkylcarbamoyloxy, N-(C3-C8)-cycloalkyl-
carbamoyloxy, N-(C6-C12)-arylcarbamoyloxy,
N-(C7-C16)-aralkylcarbamoyloxy, N-(C ?C ?-alkyl-N-
(C6-C12)-arylcarbamoyloxy, N-(C -C1)-?alkyl-N-
(C7-C16)-aralkylcarbamoyloxy, N-((C1-C10) -
alkyl)carbamoyloxy, N-((C6-C12)-aryloxy-(C1-C10)-
alkyl)carbamoyloxy, N-((C7-C16)-aralkyloxy-(C1-C10)-
alkyl)carbamoyloxy, N-(XC1-C10)-alkyl-N-((C1-C10)
alkoxy)-(C1-C10)-alkyl)carbamoyloxy, N-(C1-C10)-
alkyl-N-((C6-C12)-aryloxy)- (C1-C10)-alkyl)-
carbamoyloxy, N-(C1-C10)-alkyl-N-((C7 -C16) -
aralkyloxy-(C1-C10)-alkyl)carbamoyloxy,
NRYRZ, wherein RY and RZ are independently selected
from (C1-C12)-alkyl, (C ?-C ?-cycloalkyl, (C ?-C 12-
alkenyl, (C3-C12)-alkynyl, (C6-C12)-aryl, (C7-C11)-
aralkyl, (C1-C12)-alkoxy,(C7-C12)-aralkoxy,
(C1-C12)-alkanoyl, (C3-C8)-cycloalkanoyl, (C6-C12)-
aroyl, (C7-C16)-aralkanoyl,
and further wherein RY and RZ together are -[CH2]h-,
wherein one CH2-group can be replaced by O, S, N-
(C1-C4)-alkylcarbonylimino or N-(C1-C4)-
alkoxycarbonylimino, and h is 3 to 6,
- 73 -
(C1-C12)-alkanoylamino-(C1-C8)-alkyl, (C3-C8) -
cycloalkanoylamino-(C1-C8)-alkyl, (C6-C12)-
aroylamino-(C1-C8)-alkyl, (C7-C16)-aralkanoylamino-
(C1-C8)-alkyl, amino-(C1-C10)-alkyl, N-(C1-C10)
alkylamino-(C1-C10)-alkyl, N,N-di(C1-C10)-alkyl-
amino-(C1-C10)-alkyl, (C3-C8)-cycloalkylamino-
(C1-C10)-alkyl, (C1-C20)-alkylmercapto, (C1-C20)-
alkylsulfinyl, (C1-C20)-alkylsulfonyl, (C6-C12)-
arylmercapto, (C6-C12)-arylsulfinyl, (C6-C12)-aryl-
sulfonyl, (C7-C16)-aralkylmercapto, (C7-C16)-aralkyl-
sulfinyl, (C7-C16)-aralkylsulfonyl, (C1-C12)-alkyl-
mercapto-(C1-C6)-alkyl, (C1-C12)-alkylsulfinyl-
(C1-C6)-alkyl, (C1-C12)-alkylsulfonyl-(C1-C6)-alkyl,
(C6-C12)-arylmercapto-(C1-C6)-alkyl, (C6-C12)-aryl-
sulfinyl-(Cl-C6)-alkyl, (C6-C12)-arylsulfonyl-
(C1-C6)-alkyl, (C7-C16)-aralkylmercapto-(C1-C6)-
alkyl, (C7-C16)-aralkylsulfinyl-(C1-C6)-alkyl,
(C7-C16)-aralkylsulfonyl-(C1-C6)-alkyl,
sulfamoyl, N-(C1-C10)-alkylsulfamoyl, N,N-di-
(C1-C10)-alkylsulfamoyl, (C3-C8)-cycloalkylsulfamoyl,
N-(C6-C12)-arylsulfamoyl, N-(C7-C16)-aralkylsulf-
amoyl, N-(C1-C10)-alkyl-N-(C6-C12)-arylsulfamoyl,
N-(C1-C10)-alkyl-N- (C7-C16) -aralkylsulfamoyl,
(C1-C10)-alkyl-sulfonamido, N-((C1-C10)-alkyl)-
(C1-C10)-alkylsulfonamido, (C7-C16)-aralkyl-
sulfonamido, or N-(C1-C10)-alkyl-(C7-C16)-aralkyl-
sulfonamido,
where the radicals which contain an aryl radical can
in turn be substituted on the aryl by 1 to 5 ident--
ical or different radicals from the series
comprising:
hydroxyl, halogen, cyano, trifluoromethyl, nitro,
carboxyl, (C1-C16)-alkyl, (C3-C8)-cycloalkyl,
(C3-C8)-cycloalkyl-(C1-C12)-alkyl, (C3-C8)-cyclo-
alkoxy, (C3-C8)-cycloalkyl-(C1-C12)-alkoxy, (C3-C8)-
cycloalkyloxy-(C1-C12)-alkyl, (C3-C8)-cycloalkyloxy-
(C1-C12)-alkoxy, (C3-C8)-cycloalkyl-(C1-C8)-alkyl-
(C1-C6)-alkoxy, (C3-C8)-cycloalkyl-(C1-C8)-alkoxy-
- 74 -
(C1-C6)-alkyl, (C3-C8)-cycloalkyloxy-(C1-C8)-alkoxy-
(C1-C6)-alkyl, (C3-C8)- cycloalkoxy-(C -?)?alkoxy-
(C1-C8)-alkoxy, (C6-C12)-aryl, (C7-C16)-aralkyl,
(C2-C16)-alkenyl, (C2-C12)-alkynyl, (C1-C16)-alkoxy,
(C1-C16)-alkenyloxy, (C1-C12)-alkoxy-(C1-C12)-alkyl,
(C1-C12)-alkoxy-(C1-C12)-alkoxy, (C1-C12)-alkoxy-
(C1-C8)-alkoxy-(C1-C8)-alkyl, (C6-C12)-aryloxy,
(C7-C16)-aralkyloxy, (C6-C12)-aryloxy-(C1-C6)-alkoxy,
(C7-C16)-aralkoxy- (C1-C6)-alkoxy (C1-C8) -
hydroxyalkyl, (C6-C16)-aryloxy-(C1-C8)-alkyl,
(C7-C16)-aralkoxy-(C1-C8)-alkyl, (C6-C12)-aryloxy-
(C1-C8)-alkoxy-(C1-C6)-alkyl, (C7-C12)-aralkyloxy-
(C1-C8)-alkoxy-(C1-C6)-alkyl, -O-[CH2-]xCfH(2f+1-g) Fg,
-OCF2Cl, -OCF2-CHFCl,
(C1-C12)-alkylcarbonyl, (C3-C?-cycloalkylcarbonyl,
(C6-C12)-arylcarbonyl, (C7-C16)-aralkylcarbonyl,
(C1-C12)-alkoxycarbonyl, (C1-C12)-alkoxy-(C1-C12)-
alkoxycarbonyl, (C6-C12)-aryloxycarbonyl, (C7-C16)-
aralkoxycarbonyl, (C3-C8)-cycloalkoxycarbonyl,
(C2-C12)-alkenyloxycarbonyl, (C2-C12)-alkynyloxy-
carbonyl, (C6-C12)-aryloxy-(C1-C6)-alkoxycarbonyl,
(C7-C16)-aralkoxy-(C1-C6)-alkoxycarbonyl, (C3-C8)-
cycloalkyl-(C1-C6)-alkoxycarbonyl, (C3-C8)-cyclo-
alkoxy-(C1-C6)-alkoxycarbonyl,
(C1-C12)-alkylcarbonyloxy, (C3-C8)-cycloalkyl-
carbonyloxy, (C6-C12)-arylcarbonyloxy, (C7-C16)-
aralkylcarbonyloxy, cinnamoyloxy, (C2-C12)-alkenyl-
carbonyloxy, (C2-C12)-alkynylcarbonyloxy,
(C1-C12)-alkoxycarbonyloxy, (C1-C12)-alkoxy (C1-C12)-
alkoxycarbonyloxy, (C6-C12)-aryloxycarbonyloxy,
(C7-C16)-aralkyloxycarbonyloxy, (C3-C8)-cycloalkoxy-
carbonyloxy, (C2-C12)-alkenyloxycarbonyloxy,
(C2-C12)-alkynyloxycarbonyloxy,
- 75 -
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo-(C3-C8)-alkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C3-C8)-cycloalkylcarbamoyl,
N-((C3-C8)-cycloalkyl-(C1-C6)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N- (C7-C16 ) -aralkyl-
carbamoyl, N-(C1-C10)-alkyl-N-(C6-C16)-arylcarbamoyl,
N-(C1-C10)-alkyl-N-(C7-C16)-aralkylcarbamoyl,
N ((C1-C16)-alkoxy-(C1-C10)-alkyl)carbamoyl,
N-((C6-C16)-aryloxy-(C1-C10)-alkyl)carbamoyl,
N-((C7-C16) -aralkyloxy-(C1-C10)-alkyl)carbamoyl,
N-(C1-C10)-alkyl-N-((C1-C10)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-(C1-C10)-alkyl-N-((C6-C12)-
aryloxy-(C1-C10)-alkyl)carbamoyl, N-(C1-C10)-alkyl-N-
((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyl,
CON(CH2)h, in which one CH2 group can be replaced by
O, S, N-(C1-C8)-alkylimino, N-(C3-C8)-cycloalkyl-
imino, N-(C3-C8)-cycloalkyl-(C1-C4)-alkylimino,
N-(C6-C12)-arylimino, N-(C -? ?aralkylimino or
N-(C1-C4)-alkoxy-(C1-C6)-alkylimino and h is 3 to 7,
carbamoyloxy, N-(C1-C12)-alkylcarbamoyloxy, N,N-di-
(C1-C12)-alkylcarbamoyloxy, N-(C3-C8) -cycloalkyl-
carbamoyloxy, N-(C6-C16)-arylcarbamoyloxy,
N-(C7-C16)-aralkylcarbamoyloxy, N-(C1-C10)-alkyl-N-
(C6-C12)-arylcarbamoyloxy, N-(C1-C10)-alkyl-N-
(C7-C16)-aralkylcarbamoyloxy, N-((C1-C10)-
alkyl)carbamoyloxy, N-((C6-C12)-aryloxy-(C1-C10)-
alkyl)carbamoyloxy, N-((C7-C16)-aralkyloxy-(C1-C10)-
alkyl)carbamoyloxy, N-(C1-C10)-alkyl-N-((C1-C10)-
alkoxy-(C1-C10)-alkyl)carbamoyloxy, N-(C1-C10)
alkyl-N-((C6-C12)-aryloxy-(C1-C10)-alkyl)carba-
moyloxy, N-(C1-C10)-alkyl-N-((C7-C16)-aralkyloxy-
(C1-C10)-alkyl)carbamoyloxy,
- 76 -
amino, (C1-C12)-alkylamino, di-(C1-C12)-alkylamino,
(C3-C8)-cycloalkylamino, (C3-C12)-alkenylamino,
(C3-C12)-alkynylamino, N-(C6-C12)-arylamino,
N-(C7-C11)-aralkylamino, N-alkyl-aralkylamino,
N-alkyl-arylamino, (C1-C12)-alkoxyamino, (C IC ?-
alkoxy-N-(C1-C10)-alkylamino,
(C1-C12)-alkanoylamino, (C3-C8)-cycloalkanoylamino,
(C6-C12)-aroylamino, (C7-Cl6)-aralkanoylamino,
(C1-C12)-alkanoyl-N-(C1-C10)-alkylamino, (C3-C8)-
cycloalkanoyl-N-(C1-C10)-alkylamino, (C6-C12)-aroyl-
N-(C1-C10)-alkylamino, (C7-C11)-aralkanoyl-N-(C1-C10)-
alkylamino,
(C1-C12)-alkanoylamino-(C1-C8)-alkyl, (C3-C8)-cyclo-
alkanoylamino-(C1-C8)-alkyl, (C6-C12)-aroylamino-
(C1-C8)-alkyl, ( C7-Cl6) -aralkanoylamino-(C1-C8)-
alkyl, amino-(C1-C10)-alkyl, N-(C1-C10)-alkylamino-
(C1-C10)-alkyl, N,N-di-(C1-C10)-alkylamino-(C1-C10)-
alkyl, (C3-C8)-cycloalkylamino-(C1-C10)-alkyl,
(C1-C12)-alkylmercapto, (C1-C12)-alkylsulfinyl,
(C1-C12)-alkylsulfonyl, (C6-C16)-arylmercapto,
(C6-C16)-arylsulfinyl, (C6-C16)-arylsulfonyl,
(C7-C16)-aralkylmercapto, (C7-C16)-aralkylsulfinyl
and (C7-C16)-aralkylsulfonyl, or optionally carries
up to 3 of said radical substituents for said aryl
and two adjacent carbon atoms of an arylalkoxy,
radical recited in the definition of R1-R5 together
carry a chain -[CH2]- and/or -CH=CH-CH=CH, where one
CH2 group of the chain is optionally replaced by O,
S, SO, SO2 or NR',
R1 and R2, R2 and R3, R3 and R4, or R4 and R5 form a chain
[CH2]o, in which one or two CH2 groups of the chain,
which is saturated or unsaturated with a C=C double
bond, are optionally replaced by O, S, SO, SO2 or
NR', in which o is 3, 4 or 5, and
- 77 -
R" is hydrogen, (C6-C12)-aryl, (C1-C8)-alkyl, (C1-C8)-
alkoxy-(C1-C8)-alkyl, (C7-C12)-aralkoxy-(C1-C8)-
alkyl, (C6-C12)-aryloxy-(C1-C8)-alkyl, (C1-C10)-
alkanoyl, optionally substituted (C7-C16)-aralkanoyl
or optionally substituted (C6-C12)-aroyl, and
m is 0 or 1,
f is 1 to 8,
g is 0 or 1 to (2f + 1),
x is 0 to 3 and
h is 3 to 6, or a physiologically active salt thereof.
2. A compound of the formula I as claimed in claim 1,
in which
A is (C1-C3)-alkylene, which is optionally mono-
substituted by halogen, cyano, trifluoromethyl,
(C1-C6)-alkyl, (C1-C6)-hydroxyalkyl, (C1-C6)-alkoxy
or -O-[CH2]x-CfH(2f+1-g)Fg or
A is -CHRX-, in which Rx is one of the substituents of
the .alpha.-C atom of an .alpha.-amino acid, in particular of a
naturally occuring L-amino acid or its D isomer,
B is -CO2H,
R1 and R5 are identical or different and are hydrogen,
(C1-C12)-alkyl, (C1-C12)-alkenyl, chlorine, fluorine,
bromine, trifluoromethyl, (C1-C12)-alkylsulfonyl,
(C1-C12)-alkylsulfinyl, phenylsulfonyl or phenyl-
sulfinyl; where phenyl is optionally substituted by
fluorine, chlorine or (C1-C5)-alkoxy, (C1-C10)-
alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg, (C1-C6)-alkoxy-
(C1-C6)-alkoxy or (C3-C8)-cycloalkoxy,
R2, R3 and R4 are identical or different and are hydro-
gen, hydroxyl, (C1-C20)-alkyl, (C2-C20)-alkenyl,
(C2-C20)-alkynyl, (C1-C20)-alkoxy, (C2-C20)-alkenyl-
oxy, (C2-C20)-alkynyloxy, retinyloxy, (C1-C20)-
- 78 -
alkoxy-(C1-C8)-alkyl, (C2-C20)-alkenyloxy-(c -?)8
alkyl, retinyloxy-(C1-C6)-alkyl, (C2-C20)-alkynyloxy-
(C1-C8)-alkyl, halogen, cyano, trifluoromethyl,
(C1-C12)-hydroxyalkyl, (C1-C20)-alkanoyl, (C7-C16) -
aralkanoyl, (C6-C12)-aroyl, (C6-C12)-aryl, (C7-C16)
aralkyl, -O-[CH2]x-CfH(2f+1-g)Fg, (C1-C18)-alkyl-
mercapto, (C1-C18)-alkylsulfinyl, (C1-C18)-alkyl-
sulfonyl, (C6-C12)-arylmercapto, (C6-C12)-aryl-
sulfinyl), (C6-C12)-arylsulfonyl, (C7-C12)-aralkyl-
mercapto, (C7-C12)-aralkylsulfinyl, (C7-C12)-aralkyl-
sulfonyl, (C6-C12)-aryloxy, (C7-C16)-aralkyloxy,
carboxyl, (C1-C20)-alkoxycarbonyl, (C -? )12alkoxy-
(C1-C12)-alkoxycarbonyl, (C6-C12)-aryloxycarbonyl,
(C7-C16)-aralkoxycarbonyl, (C3-C8)-cycloalkoxy-
carbonyl, (C2-C2? -alkenyloxycarbonyl, retinyloxy-
carbonyl, (C2-C20)-alkynyloxycarbonyl, (C3-C8)-cyclo-
alkyl-(C1-C6)-alkoxycarbonyl, (C3-C8)-cycloalkoxy-
(C1-C6)-alkoxycarbonyl, (C6-C12)-aryloxy-(C1-C6)-
alkoxycarbonyl, (C7-C16) -aralkoxy-(C -C1)-6
alkoxycarbonyl,
(C1-C12)-alkoxy-(C1-C12)-alkyl, (C1-C )-?alkoxy-
(C1-C12)-alkoxy, (C1-Cl2)-alkoxy-(C -C1)-?alkoxy-
(C1-C6)-alkyl, (C7-C11)-aralkyloxy, (C3-C8)-cyclo-
alkyl, (C3-C8)-cycloalkyl-(C1-C12)-alkyl, (C3-C8)-
cycloalkyloxy, (C3-C8)-cycloalkyl-(C -C1 )-?alkoxy,
(C3-C8)-cycloalkyloxy-(C1-C12)-alkyl, (C3-C8)-cyclo-
alkyloxy-(C1-C8)-alkoxy, (C3-C8)-cycloalkyl-(C1-C12)-
alkyl-(C1-C6)-alkoxy, (C3-C8)-cycloalkyl-(C1-C6)-
alkoxy-(C1-C6)-alkyl, (C3-C8)-cycloalkoxy-(C1-C6)-
alkoxy-(C1-C6)-alkyl, NRYRZ, substituted (C6-C12)-
aryloxy-(C1-C6)-alkyl, (C7-C11)-aralkoxy-(C1-C6)-
alkyl, (C6-C12)-aryloxy-(C1-C6)-alkoxy-(C1-C6)-alkyl,
(C7-C11)-aralkyloxy-(C1-C6)-alkoxy-(C1-C6)-alkyl,
(C6-C12)-aryloxy-(C1-C6)-alkoxy or (C7-C11)-aralkoxy-
(C1-C6)-alkoxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
- 79 -
carbamoyl, N,N-dicyclo(C3-C8)-alkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C3-C8)-cycloalkylcarbamoyl,
N-(C3-C8)-cycloalkyl-(C1-C16)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N- (C7 -C16 ) -
aralkylcarbamoyl, N-(C1-C10)-alkyl-N-(C6-C16)-
arylcarbamoyl, N-(C1-C10)-alkyl-N- (C7 -C16 ) -
aralkylcarbamoyl, N-((C1-C12)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-((C6-C16)-aryloxy-(C1-C10)
alkyl)carbamoyl, N-((C7-C16)-aralkyloxy-(C ?C )6-
alkyl)carbamoyl, N-(C1-C10)-alkyl-N-((C1-C10)-
alkoxy-(C1-C10)-alkyl)carbamoyl, N-(C1-C10)-alkyl-N-
((C6-C12)-aryloxy-(C1-C10)-alkyl)carbamoyl,
N-(C1-C10)-alkyl-N-((C7-C16)-aralkyloxy-(C1-C10)-
alkyl)carbamoyl, or CON(CH2)h, in which one CH2
group can be replaced by O, S, N-(C1-C16)-alkyl-
imino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-cyclo-
alkyl-(C1-C12)-alkylimino, N-(C6-C12)-arylimino,
N-(C7-C16)-aralkylimino or N-(C1-C12)-alkoxy-(C1-C6)-
alkylimino and h is 3 to 7, where an aromatic rad-
ical carries 1, 2, 3, 4 or 5 identical or different
substituents from the series comprising of hydrogen,
halogen, cyano, nitro, hydroxyl, trifluoromethyl,
(C1-C16)-alkyl, (C2-C16)-alkenyl, (C1-C6)-hydroxy-
alkyl, (C1-C16)-alkoxy, (C1-C16)-alkenyloxy, -O-
[CH2]xCfH(2f+1-g)Fg, -OCF2Cl, -O-CF2-CHFCl, (C1-C6)-
alkylmercapto, (C1-C6)-alkylsulfinyl, (C1-C6)-alkyl-
sulfonyl, (C1-C6)-alkylcarbonyl, (C -?)?-alkoxy-
carbonyl, carbamoyl, N-(C1-C4)-alkylcarbamoyl, N,N-
di-(C1-C4)-alkylcarbamoyl, (C1-C6)-alkylcarbonyloxy,
(C3-C8)-cycloalkylcarbamoyl, phenyl, benzyl,
phenoxy, benzyloxy, phenylmercapto, phenylsulfonyl,
phenylsulfinyl, sulfamoyl, N-(C1-C4)-alkylsulfamoyl
and N,N-di-(C1-C4)-alkylsulfamoyl, or optionally
carries up to 3 of the abovementioned identical or
different substituents and two adjacent carbon atoms
of the aralkyloxy radical recited in the definition
- 80 -
of R1-R5 together carry a chain -[CH2]- and/or -
CH=CH-CH=CH-, where one CH2 group of the chain is
optionally replaced by O, S, SO, SO2 or NR',
RY and RZ are identical or different and are hydrogen,
(C6-C12)-aryl, (C1-C10)-alkyl, (C3-C10)-cycloalkyl,
(C1-C8)-alkoxy-(C1-C8)-alkyl, (C7-C12)-aralkoxy (C1-
C8)-alkyl, (C6-C12)-aryloxy-(C1-C8)-alkyl, (C1-C10)-
alkanoyl, optionally substituted (C7-C16)-
aralkanoyl, or optionally substituted (C6-C12)-
aroyl, or
RY and RZ together are -[CH2]h-, wherein one CH group can
be replaced by O, S, N-(C1-C4)-alkanoylimino or
N-(C1-C4)-alkoxycarbonylimino,
m is 0 or 1,
f is 1 to 8,
g is 0 or 1 to (2f + 1),
h is 3 to 6 and
x is 0 to 3, or a physiologically active salt thereof.
3. A compound of the formula I as claimed in claims 1
or 2, in which
m is 0,
A is a -CH2- group, which can be substituted by a
methyl group,
B is -CO2H,
R1 is hydrogen, (C1-C4)-alkyl, (C1-C4)-alkoxy, chlorine
or bromine,
R5 is hydrogen, fluorine, chlorine or methyl and
R2, R3 and R4 are identical or different and are hydro-
gen, (C1-C18)-alkyl, (C2-C18)-alkenyl, (C2-C18)-
alkynyl, phenyl, chlorine, fluorine, bromine,
hydroxyl, trifluoromethyl, (C1-C18)-alkylsulfinyl,
(C1-C18)-alkylsulfonyl, phenylsulfinyl, phenyl-
sulfonyl, naphthylsulfinyl, naphthylsulfonyl,
(C1-C18)-alkoxy, (C3-C8)-cycloalkoxy, (C1-C8)-alkoxy-
- 81 -
(C1-C8)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg, phenyl-
(C1-C4)-alkoxy, phenoxy, (C1-C12)-alkanoyl, phenyl-
(C1-C4)-alkanoyl or benzoyl, where, in substituents
with a phenyl or naphthyl ring, this optionally
carries up to 5 identical or different substituents
from the series comprising fluorine, chlorine,
bromine, nitrile, trifluoromethyl, (C1-C6)-alkyl,
(C1-C6)-alkoxy, -o-[CH2]x-CfH(2f+l-g)Fg or (C1-C6)-
alkylsulfonyl,
or a physiologically active salt thereof.
4. A compound of the formula I as claimed in claims 1
to 3, in which
m is 0,
A is a -CH2- group,
B is -CO2H,
R1 and R5 are hydrogen and
R2, R3 and R4 are identical or different and are hydro-
gen, (C1-C18)-alkyl, (C1-C18)-alkenyl, phenyl, chlor-
ine, fluorine, bromine, trifluoromethyl, (C1-C18)-
alkylsulfinyl, (C1-C18)-alkylsulfonyl, phenyl-
sulfinyl, phenylsulfonyl, naphthylsulfinyl,
naphthylsulfonyl, (Cl-C18)-alkoxy, (C3-C8)-cyClo-
alkoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy, -O-[CH2]x-
CfH(2f+l-g)Fg, phenyl-(C1-C4)-alkoxy, phenoxy,
(C1-C12)-alkanoyl, phenyl-(C1-C4)-alkanoyl or
benzoyl, where, in substituents having a phenyl or
naphthyl ring, this optionally carries up to 5
identical or different substituents from the series
comprising fluorine, chlorine, bromine, nitrile,
trifluoromethyl, (C1-C )?alkyl, (C -? )?alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg and (C1-C6)-alkylsulfonyl, or
a physiologically active salt thereof.
5. A compound of the formula I as claimed in claims 1
to 4, in which
m is 0,
A is a -CH2- group,
R1 and R5 are hydrogen,
- 82 -
one of the substituents R2, R3 or R4 is hydrogen and the
other two are identical or different and are hydrogen,
(C1-C16)-alkyl, fluorine, chlorine, bromine, trifluoro-
methyl, (C1-C16)-alkylsulfonyl, phenylsulfonyl, (C1-C16)-
alkoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy, -O-[CH2]x-
CfH(2f+1g)Fg, benzyloxy or phenoxy, where, in substituents
which contain a phenyl ring, this optionally carries up
to 3 substituents from the series comprising fluorine,
chlorine, bromine, trifluoromethyl, (C1-C6)-alkyl,
(C1-C6)-alkoxy, -O-[CH2]X-C ? (2f+1-g? g or (C-?C )4-alkyl-
sulfonyl, or a physiologically active salt thereof.
6. A compound of the formula I as claimed in claims 1
to 5, in which
m is 0,
A is a -CH2- group,
R1, R2 and R5 are hydrogen and
R3 and R4 are identical or different and are hydrogen,
(C1-C16)-alkyl, fluorine, chlorine, trifluoromethyl,
(C1-C16)-alkylsulfonyl, phenylsulfonyl, naphthylsulfonyl,
(C1-C16)-alkoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy, -O-[CH2]x-
CfH(2f+1-g)Fg, benzyloxy or phenoxy, where, in substituents
which contain a phenyl ring, this optionally carries up
to 3 substituents from the series comprising fluorine,
chlorine, trifluoromethyl, (C1-C6)-alkyl, (C1-C6)-alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg and (C1-C4)-alkylsulfonyl, or a
physiologically active salt thereof.
7. A compound of the formula I as claimed in claims 1
to 6, in which
m is 0,
A is a -CH2- group,
R1, R2, R3 and R5 are hydrogen and
R4 is hydrogen, (C1-C12)-alkyl, fluorine, chlorine,
bromine, trifluoromethyl, (C1-C12)-alkylsulfonyl, phenyl-
sulfonyl, naphthylsulfonyl, (C1-C12)-alkoxy, (C1 -C6)-
alkoxy-(C1-C6)-alkoxy, -O-[CH2]X-CfH(2f+1-g)Fg, benzyloxy or
phenoxy, where, in substituents which contain a phenyl or
naphthyl ring, this is optionally monosubstituted by
- 83 -
fluorine, chlorine, bromine, trifluoromethyl, (C1-C6)-
alkyl, (C1-C6)-alkoxy, -O-[CH2]X-CfH(2f+1-g)Fg or (C1-C4)-
alkylsulfonyl, or a physiologically active salt thereof.
8. A compound of the formula I as claimed in claims 1
to 6, in which
m is 0,
A is a -CH2- group,
R1, R3, R4 and R5 are hydrogen and
R2 is hydrogen, (C1-C12)-alkyl, fluorine, chlorine,
bromine, trifluoromethyl, (C1-C12)-alkylsulfonyl, phenyl-
sulfonyl, naphthylsulfonyl, (C1-C12)-alkoxy, (C1-C6)-
alkoxy-(C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg, benzyloxy or
phenoxy, where, in substituents which contain a phenyl or
naphthyl ring, this is optionally monosubstituted by
fluorine, chlorine, bromine, trifluoromethyl, (C1-C6)-
alkyl, (C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg or (C1-C4)-
alkylsulfonyl, or a physiologically active salt thereof.
9. A compound of the formula I as claimed in claims 1
to 6, in which
m is 0,
A is a -CH2- group,
R1, R2 and R5 are hydrogen,
R4 is hydrogen or chlorine and
R3 is hydrogen, fluorine, chlorine, (C1-C12)-alkoxy,
(C1-C4)-alkoxy-(C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg,
phenylsulfonyl, naphthylsulfonyl or phenoxy, where, in
substituents which contain a phenyl or naphthyl ring,
this optionally carries up to 3 identical or different
substituents, preferably one substituent, from the series
comprising fluorine, chlorine, bromine, trifluoromethyl,
trifluoromethoxy, (C1-C6)-alkyl and (C1-C6)-alkoxy, or a
physiologically active salt thereof.
10. A compound of the formula I as claimed in claims 1
to 6 and 9, in which
m is 0,
A is a -CH2- group,
- 84 -
R1, R2, R4 and R5 are hydrogen and
R3 is hydrogen, fluorine, chlorine, (C1-C12)-alkoxy,
(C1-C4)-alkoxy-(C1-C6)-alkoxy, -O-[CH2]y-CfH(2f+1-g)Fg,
phenylsulfonyl, naphthylsulfonyl or phenoxy, where, in
substituents which contain a phenyl or naphthyl ring,
this optionally carries up to 3 identical or different
substituents, preferably one substituent, from the series
consisting of fluorine, chlorine, bromine, trifluoro-
methyl, trifluoromethoxy, (C1-C6)-alkyl and (C1-C6)-
alkoxy, or a physiologically active salt thereof.
11. A compound of the formula I as claimed in claims 1
and 2, in which
A is (C1-C3)-alkylene, which is optionally mono-
substituted by halogen, cyano, trifluoromethyl,
(C1-C6)-alkyl, (C1-C6)-hydroxyalkyl, (C1-C6)-alkoxy
or -O-[CH2]x-CfH(2f+1-g)Fg or
A is -CHRx-, in which Rx is one of the substituents of
the .alpha.-C atom of an .alpha.-amino acid, in particular of a
naturally occuring L-amino acid or its D isomer,
B is -CO2H,
R1 and R5 are identical or different and are hydrogen,
(C1-C12)-alkyl, (C2-C12)-alkenyl, chlorine, fluorine,
bromine, trifluoromethyl, (C1-C12)-alkylsulfonyl,
(C1-C12)-alkylsulfinyl, phenylsulfonyl or phenyl-
sulfinyl; where phenyl is optionally substituted by
fluorine, chlorine or (C1-C5) -alkoxy, (C1-C10)-
alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg, (C1-C6)-alkoxy-
(C1-C6)-alkoxy or (C3-C8)-cycloalkoxy,
R2 is hydroxyl, (C1-C20)-alkyl, (C2-C20)-alkenyl,
(C2-C20)-alkynyl, (C1-C20)-alkoxy, (C2-C20)-alkenyl-
oxy, (C2-C20)-alkynyloxy, retinyloxy, (C1-C20)-
alkoxy-(C1-C8)-alkyl, (C2-C20)-alkenyloxy-(C1-C8)-
alkyl, retinyloxy-(C1-C6)-alkyl, (C2-C20)-alkynyloxy-
- 85 -
(C1-C8)-alkyl, halogen, cyano, trifluoromethyl,
(C1-C12)-hydroxyalkyl, (C ?C 2)-alkanoyl, (C ?C 1?-
aralkanoyl, (C6-C12)-aroyl, (C6-C12)-aryl, (C7-C16)-
aralkyl, -O-[CH2]x-CfH(2f+1-g)Fg, (C1-C18)-alkyl-
mercapto, (C1-C18)-alkylsulfinyl, (C1-C18)-alkyl-
sulfonyl, (C6-C12)-arylmercapto, (C -C6 )-aryl-
sulfinyl, (C6-C12)-arylsulfonyl, (C7-C12)-aralkyl-
mercapto, (C7-C12)-aralkylsulfinyl, (C7-C12)-aralkyl-
sulfonyl, (C6-C12)-aryloxy, (C7-C16)-aralkyloxy,
carboxyl, (C1-C20)-alkoxycarbonyl, (C1-C12)-alkoxy-
(C1-C12)-alkoxycarbonyl, (C6-C12)-aryloxycarbonyl,
(C7-C16)-aralkoxycarbonyl, (C3-C8)-cycloalkoxy-
carbonyl, (C2-C20)-alkenyloxycarbonyl, retinyloxy-
carbonyl, (C2-C20)-alkynyloxycarbonyl, (C3-C8)-cyclo-
alkyl-(C1-C6)-alkoxycarbonyl, (C3-C8)-cycloalkoxy-
(C1-C6)-alkoxycarbonyl, (C6-C )-?ryloxy-(C -C1)-6
alkoxycarbonyl, (C7 -C16)-aralkoxy-(C1-C6)-
alkoxycarbonyl,
(C1-C12)-alkoxy-(C1-C12)-alkyl, (C1-C12)-alkoxy-
(C1-C12)-alkoxy, (C1-C12)-alkoxy-(C1-C12)-alkoxy-
(C1-C6)-alkyl, (C7-C11)-aralkyloxy, (C3-C8)-cyclo-
alkyl, (C3-C8)-cycloalkyl-(C1-C12)-alkyl, (C3-C8)-
cycloalkyloxy, (C3-C8)-cycloalkyl-(C1-C8)-alkoxy,
(C3-C8)-cycloalkyloxy-(C1-C12)-alkyl, (C3-C8)-cyclo-
alkyloxy-(C1-C8)-alkoxy, (C3-C8)-cycloalkyl-(C1-C12)-
alkyl-(C1-C6)-alkoxy, (C3-C8)-cycloalkyl-(C1-C6)-
alkoxy-(C1-C6)-alkyl, (C3-C8)-cycloalkoxy-(C1-C6)-
alkoxy-(C1-C6)-alkyl, NRYRZ, substituted (C -?C ?-
aryloxy-(C1-C6)-alkyl, (C7-C11)-aralkoxy-(C1-C6)-
alkyl, (C6-C12)-aryloxy-(C1-C6)-alkoxy-(C1-C6)-alkyl,
(C7-C11)-aralkyloxy-(C1-C6)-alkoxy-(C1-C6)-alkyl,
(C6-C12)-aryloxy-(C1-C6)-alkoxy or (C7-C11)-aralkoxy-
(C1-C6)-alkoxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C ) -?ycloalkyl-
carbamoyl, N,N-dicyclo(C3-C8)-alkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C3-C8)-cycloalkylcarbamoyl,
- 86 -
N-(C3-C8)-cycloalkyl-(C1-C16)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N- (C7-C16) -aralkyl-
carbamoyl, N-(C1-C10)-alkyl-N-(C6-C16)-arylcarbamoyl,
N-(C1-C10)-alkyl-N-(C7-C16)-aralkylcarbamoyl,
N-((C1-C12)-alkoxy-(C1-C10)-alkyl)carbamamoyl,
N-((C6-C16)-aryloxy-(C1-C10)-alkyl)carbamamoyl,
N-((C7-C16)-aralkyloxy-(C1-C6)-alkyl)carbamoyl,
N-(C1-C10)-alkyl-N-((C1-C10)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-(C1-C10)-alkyl-N-((C6-C12)-
aryloxy-(C1-C10)-alkyl)carbamoyl, N-(C1-C10)-alkyl-
N-((C7-C16)-aralkyloxy-(C1-C10)-alkyl)carbamoyl, or
CON(CH2)h, in which one CH2 group can be replaced by
O, S, N-(C1-C16)-alkylimino, N-(C3-C8)-cycloalkyl-
imino, N-(C3-C8)-cycloalkyl-(C1-C12)-alkylimino,
N-(C6-C12)-arylimino, N-(C7-C16)-aralkylimino or
N-(C1-C12)-alkoxy-(C1-C6)-alkylimino and h is 3 to 7,
where an aromatic radical carries 1, 2, 3, 4 or 5
identical or different substituents from the series
comprising hydrogen, halogen, cyano, nitro,
hydroxyl, trifluoromethyl, (C1-C16)-alkyl, (C2-C16)-
alkenyl, (C1-C6)-hydroxyalkyl, (C1-C16)-alkoxy,
(C1-C16)-alkenyloxy, -O-[CH2]XCfH(2f+1-g) Fg, -OCF2Cl,
-O-CF2-CHFCl, (C1-C6)-alkylmercapto, (C1-C6)-
alkylsulfinyl, (C1-C6)-alkylsulfonyl, (C -C1)-6
alkylcarbonyl, (C1-C6)-alkoxycarbonyl, carbamoyl, N-
(C1-C4)-alkylcarbamoyl, N,N-di-(C1-C4)-
alkylcarbamoyl, (C1-C6)-alkylcarbonyloxy, (C3-C8)-
cycloalkylcarbamoyl, phenyl, benzyl, phenoxy,
benzyloxy, phenylmercapto, phenylsulfonyl,
phenylsulfinyl, sulfamoyl, N-(C1-C4)-alkylsulfamoyl
and N,N-di-(C1-C4)-alkylsulfamoyl, or optionally
carries up to 3 of the abovementioned identical or
different substituents and two adjacent carbon atoms
of the aralkyloxy radical together carry a chain
-[CH2]- and/or -CH=CH-CH=CH-, where one CH2 group of
- 87 -
the chain is optionally replaced by O, S, SO, SO2 or
NR',
R3 is hydroxyl, (C1-C20)-alkyl, (C2-C20)-alkenyl,
(C2-C20)-alkynyl, (C11-C20)-alkoxy, (C7-C20)-alkenyl-
oxy, (C2-C20)-alkynyloxy, retinyloxy, (C1-C20)-
alkoxy-(C1-C8)-alkyl, (C2-C20)-alkenyloxy-(C1-C8)-
alkyl, retinyloxy-(C1-C6)-alkyl-(C2-C20)-alkynyloxy-
(C1-C8)-alkyl, fluorine, cyano, trifluoromethyl,
(C1-C12)-hydroxyalkyl, (C1-C20)-alkanoyl, (C7-C16)-
aralkanoyl, (C6-C12)-aroyl, (C6-C12)-aryl, (C7-C16)-
aralkyl, (C1-C18)-alkylmercapto, (C1-C18)-alkyl-
sulfinyl, (C1-C18)-alkylsulfonyl, (C6-C12)-aryl-
mercapto, (C6-C12)-arylsulfinyl, (C7-C12)-aralkyl-
mercapto, (C7-C12)-aralkylsulfinyl, (C7-C12)-aralkyl-
sulfonyl, (C6-C12)-aryloxy, (C7-C16)-aralkyloxy,
apart from benzyloxy, carboxyl, (C1-C20)-alkoxy-
carbonyl, (C1-C12)-alkoxy-(C1-C12)-alkoxycarbonyl,
(C6-C12)-aryloxycarbonyl, (C7-C16)-aralkoxycarbonyl,
(C3-C8)-cycloalkoxycarbonyl, (C2-C20)-
alkenyloxycarbonyl, retinyloxycarbonyl, (C2-C20)-
alkynyloxycarbonyl, (C3-C8) -cycloalkyl-(C1-C6)-
alkyloxycarbonyl, (C3-C8)-cycloalkoxy- (C1-C6)-
alkoxycarbonyl, (C6-C12)-aryloxy-(C1-C6)-
alkoxycarbonyl, (C7-C16) -aralkoxy-(C1-C6)-
alkoxycarbonyl,
-O-[CH2]x-CfH(2f+1-g)Halg apart from trifluoroethoxy,
pentafluoropropoxy and heptafluorobutoxy, (C1-C12)-
alkoxy-(C1-C12)-alkoxy, (C1-C12)-alkoxy-(C1-C12)-
alkoxy-(C1-C6)-alkyl, (C7-C11)-aralkyloxy, (C3-C8)-
cycloalkyl, (C3-C8)-cycloalkyl-(C1-C12)-alkyl,
(C3-C8)-cycloalkyloxy, (C3-C8)-cycloalkyl-(C1-C )?
alkoxy, (C3-C8)-cycloalkyloxy-(C1-C12)-alkyl,
(C3-C8)-cycloalkyloxy-(C1-C8)-alkoxy, (C3-C8)-cyclo-
alkyl-(C1-C12)-alkyl-(C1-C6)-alkoxy, (C3-C8)-cyclo-
alkyl-(C1-C6)-alkoxy-(C1-C6)-alkyl, (C3-C8)-
cycloalkoxy-(C1-C6)-alkoxy-(C1-C6)-alkyl, NRYRZ,
substituted (C6-C12)-aryloxy-(C1-C6)-alkyl, (C7-C11)-
- 88 -
aralkoxy-(C1-C6)-alkyl, (C6-C12)-aryloxy-(C1-C6)
alkoxy-(C1-C6)-alkyl, (C7-C11)-aralkyloxy-(C1-C6)-
alkoxy-(C1-C6)-alkyl, (C6-C12)-aryloxy-(C1-C6)-alkoxy
or (C7-C11)-aralkoxy-(C1-C6)-alkoxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8) -cycloalkyl-
carbamoyl, N,N-dicyclo(C3-C8)-alkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C3-C8) -cycloalkylcarbamoyl,
N-(C3-C8)-cycloalkyl-(C1-C16)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N- (C7 -C16)-
aralkylcarbamoyl, N-(C1-C10)-alkyl-N-(C6-C16)-
arylcarbamoyl, N-(C1-C10)-alkyl-N-(C7-C16)-
aralkylcarbamoyl, N-((C1-C12)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-((C6-C16)-aryloxy-(C1-C10)
alkyl)carbamoyl, N-((C7-C16)-aralkyloxy-(C1-C6)-
alkyl)carbamoyl, N-(C1-C10)-alkyl-N-((C1-C10)-
alkoxy-(C1-C10)-alkyl)carbamoyl, N-(C1-C10)-alkyl-N-
((C6-C12)-aryloxy-(C1-C10)-alkyl)carbamoyl,
N-(C1-C10)-alkyl-N-((C7-C16)-aralkyloxy-(C1-C10)-
alkyl)carbamoyl, or CON(CH2)h, in which one CH2
group can be replaced by O, S, N-(C1-C16)-alkyl-
imino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-cyclo-
alkyl-(C1-C12)-alkylimino, N-(C6-C12)-arylimino,
N-(C7-C16)-aralkylimino or N-(C1-C12)-alkoxy-(C1-C6)-
alkylimino and h is 3 to 7, where an aromatic rad-
ical carries 1, 2, 3, 4 or 5 identical or different
substituents from the series comprising hydrogen,
halogen, cyano, nitro, hydroxyl, trifluoromethyl,
(C1-C16)-alkyl, (C2-C16)-alkenyl, (C1-C6)-hydroxy-
alkyl, (C1-C16)-alkoxy, (C1-C16)-alkenyloxy, -O-
[CH2]xCfH (2f+1-g) Fg, -OCF2Cl, -O-CF2-CHFCl, (C1-C6)-
alkylmercapto, (C1-C6)-alkylsulfinyl, (C1-C6)-alkyl-
sulfonyl, (C1-C6)-alkylcarbonyl, (C1-C6)-alkoxy-
carbonyl, carbamoyl, N-(C1-C4)-alkylcarbamoyl, N,N-
di-(C1-C4)-alkylcarbamoyl, (C1-C6)-alkylcarbonyloxy,
- 89 -
(C3-C8)-cycloalkylcarbamoyl, phenyl, benzyl,
phenoxy, benzyloxy, phenylmercapto, phenylsulfonyl,
phenylsulfinyl, sulfamoyl, N-(C1-C4)-alkylsulfamoyl
and N,N-di-(C1-C4)-alkylsulfamoyl, or optionally
carries up to 3 of the abovementioned identical or
different substituents and two adjacent carbon atoms
of the aralkyloxy radical together carry a chain
-[CH2]- and/or -CH=CH-CH=CH-, where one CH2 group of
the chain is optionally replaced by O, S, SO, SO2 or
NR', and
R4 is hydroxyl, (C1-C20)-alkyl, (C2-C20)-alkenyl,
(C2-C20)-alkynyl, (C1-C20)-alkoxy apart from
1-butoxy, (C2-C20)-alkenyloxy, (C 2-C 20) -alkynyloxy,
retinyloxy, (C1-C20)-alkoxy-(C 1-C 8)-alkyl, (C 2-C 20) -
alkenyloxy-(C1-C8)-alkyl, retinyloxy-(C1-C6)-alkyl,
(C2-C20)-alkynyloxy-(C1-C8)-alkyl, halogen, cyano,
trifluoromethyl, (C1-C12)-hydroxyalkyl, (C1-C20)-
alkanoyl,(C7-C16)-aralkanoyl, (C6-C12)-aroyl,
(C6-C12)-aryl, (C7-C16)-aralkyl, -o-[CH2]x-
CfH(2f+l-g)Fg, (C1-C18)-alkylmercapto, (C1-C18)-alkyl-
sulfinyl, (C1-C18)-alkylsulfonyl, (C6-C12)-aryl-
mercapto, (C6-C )?arylsulfinyl, (C -C )6-aryl-
sulfonyl, (C7-C12)-aralkylmercapto, (C7-C12)-aralkyl-
sulfinyl, (C7-C12)-aralkylsulfonyl, (C6-C12)-aryloxy,
(C7-C16)-aralkyloxy apart from benzyloxy, carboxyl,
(C1-C20)-alkoxycarbonyl, (C1-C12)-alkoxy-(C1-C12)-
alkoxycarbonyl, (C6-C12)-aryloxycarbonyl, (C7-C16)-
aralkoxycarbonyl, (C3-C8)-cycloalkoxycarbonyl,
(C2-C20)-alkenyloxycarbonyl, retinyloxycarbonyl,
(C2-C20)-alkynyloxycarbonyl, (C3-C8)-cycloalkyl-
(C1-C6)-alkoxycarbonyl, (C3-C8)-cycloalkoxy- (C1-C6)-
alkoxycarbonyl, (C6-C12)-aryloxy-(C1-C6)-
alkoxycarbonyl, (C7-C16)-aralkoxy-(C1-C6)-
alkoxycarbonyl,
(C1-C12)-alkoxy-(C1-C12)-alkyl, (C1-C12)-alkoxy-
(C1-C12)-alkoxy, (C1-C12)-alkoxy-(C1-C12)-alkoxy-
(C1-C6)-alkyl, (C7-C11)-aralkyloxy, (C3-C8)-cyclo-
- 90 -
alkyl, (C3-C8)-cycloalkyl-(C1-C12)-alkyl, (C3-C8)-
cycloalkyloxy, (C3-C8)-cycloalkyl-(C1-C8)-alkoxy,
(C3-C8)-cycloalkyloxy-(C1-C12)-alkyl, (C3-C8)-cyclo-
alkyloxy-(C1-C8)-alkoxy, (C3-C8)-cycloalkyl-(C1-C12)-
alkyl-(C1-C6)-alkoxy, (C3-C8)-cycloalkyl-(C1-C6)-
alkoxy-(C1-C6)-alkyl, (C3-C8)-cycloalkoxy-(C1-?)-?
alkoxy-(C1-C6)-alkyl, NRYRZ, substituted (C6-C12)-
aryloxy-(C1-C6)-alkyl, (C7-C11)-aralkoxy-(C1-C6)
alkyl, (C6-C12)-aryloxy-(C1-C6)-alkoxy-(C1-C6)-alkyl,
(C7-C11)-aralkyloxy-(C1-C6)-alkoxy-(C1-C6)-alkyl,
(C6-C12)-aryloxy-(C1-C6)-alkoxy or (C7-C11)-
aralkyloxy-(C1-C6)-alkoxy,
carbamoyl, N-(C1-C12)-alkylcarbamoyl, N,N-di-
(C1-C12)-alkylcarbamoyl, N-(C3-C8)-cycloalkyl-
carbamoyl, N,N-dicyclo(C3-C8)-alkylcarbamoyl,
N-(C1-C10)-alkyl-N-(C3-C8)-cycloalkylcarbamoyl,
N-(C3-C8)-cycloalkyl-(C1-C16)-alkyl)carbamoyl,
N-(C1-C6)-alkyl-N-((C3-C8)-cycloalkyl-(C1-C6)-
alkyl)carbamoyl, N-(+)-dehydroabietylcarbamoyl,
N-(C1-C6)-alkyl-N-(+)-dehydroabietylcarbamoyl,
N-(C6-C12)-arylcarbamoyl, N- (C7 -C16 ) -
aralkylcarbamoyl, N-(C1-C10)-alkyl-N-(C6-C16)-
arylcarbamoyl, N-(C1-C10)-alkyl-N-(C7-C16)-
aralkylcarbamoyl, N-((C1-C12)-alkoxy-(C1-C10)-
alkyl)carbamoyl, N-((C6-C16)-aryloxy-(C1-C10)-
alkyl)carbamoyl, N-((C7-C16)-aralkyloxy-(C1-C6)-
alkyl)carbamoyl, N-(C1-C10)-alkyl-N-((C1-C10)-
alkoxy-(C1-C10)-alkyl)carbamoyl, N-(C1-C10)-alkyl-N-
((C6-C12)-aryloxy-(C1-C10)-alkyl)carbamoyl, N-(C1-
C10)-alkyl-N-((C7 -C16) -aralkyloxy-(C1-C10)-
alkyl)carbamoyl, or CON(CH2)h, in which one CH2
group can be replaced by O, S, N-(C1-C16)-
alkylimino, N-(C3-C8)-cycloalkylimino, N-(C3-C8)-
cycloalkyl-(C1-C12)-alkylimino, N-(C6-C12)-arylimino,
N-(C7-C16)-aralkylimino or N-(C1-C12)-alkoxy-(C1-C6)-
alkylimino and h is 3 to 7, where an aromatic rad-
ical carries 1, 2, 3, 4 or 5 identical or different
substituents from the series comprising hydrogen,
- 91 -
halogen, cyano, nitro, hydroxyl, trifluoromethyl,
(C1-C16)-alkyl, (C2-C16)-alkenyl, (C1-C6)-hydroxy-
alkyl, (C1-C16)-alkoxy, (C1-C16)-alkenyloxy, -O-
[CH2]xCfH(2f+1-g)Fg, -O-CF2Cl, -OCF2-CHFCl, (C1-C6)-
alkylmercapto, (C1-C6)-alkylsulfinyl, (C1-C6)-alkyl-
sulfonyl, (C1-C6)-alkylcarbonyl, (C1-C6)-alkoxy-
carbonyl, carbamoyl, N-(C1-C4)-alkylcarbamoyl, N,N-
di-(C1-C4)-alkylcarbamoyl, (C1-C6)-alkylcarbonyloxy,
(C3-C8)-cycloalkylcarbamoyl, phenyl, benzyl,
phenoxy, benzyloxy, phenylmercapto, phenylsulfonyl,
phenylsulfinyl, sulfamoyl, N-(C1-C4)-alkylsulfamoyl
and N,N-di-(C1-C4)-alkylsulfamoyl, or optionally
carries up to 3 of the abovementioned identical or
different substituents and two adjacent carbon atoms
of the aralkyloxy radical together carry a chain
-[CH2]- and/or -CH=CH-CH=CH-, where one CH2 group of
the chain is optionally replaced by O, S, SO, SO2 or
NR',
RY and RZ are identical or different and are hydrogen,
(C6-C12)-aryl, (C1-C10)-alkyl, (C3-C10)-cycloalkyl,
(C1-C8)-alkoxy-(C1-C8)-alkyl, (C7-C12)-aralkoxy (C1-
C8) alkyl, (C6-C12)-aryloxy-(C1-C8)-alkyl, (C1-C10)-
alkanoyl, optionally substituted (C7-C16)-
aralkanoyl, or optionally substituted (C6-C12)-
aroyl, or
RY and RZ together are -[CH2]h-, wherein one CH2 group can
be replaced by O, S, N-(C1-C4)-alkanoylimino or
N-(C1-C4)-alkoxycarbonylimino,
m is 0 or 1,
f is 1 to 8,
g is 0 or 1 to (2f + 1),
h is 3 to 6 and
x is 0 to 3, or a physiologically active salt thereof.
12. A compound of the formula I as claimed in claims 3
and 11, in which
- 92 -
m is O,
A is a -CH2- group, which can be substituted by a
methyl group,
B is -CO2H,
R1 is hydrogen, (C1-C4)-alkyl, (C1-C4)-alkoxy, chlorine
or bromine,
R5 is hydrogen, fluorine, chlorine or methyl,
R2 is (C1-C18)-alkyl, (C2-C18)-alkenyl, (C2-C18)-
alkenyloxy, (C2-C18)-alkynyl, phenyl, chlorine,
fluorine, bromine, hydroxyl, trifluoromethyl,
(C1-C18)-alkylsulfinyl, (C1-C18)-alkylsulfonyl,
phenylsulfinyl, phenylsulfonyl, naphthylsulfinyl,
naphthylsulfonyl, (C1-C18)-alkoxy, (C3-C8)-
cycloalkoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg, phenyl-(C1-C4)-alkoxy,
phenoxy, (C1-C12)-alkanoyl, phenyl-(C1-C4)-alkanoyl
or benzoyl, where, in substituents with a phenyl or
naphthyl ring, this optionally carries up to 5
identical or different substituents from the series
comprising fluorine, chlorine, bromine, nitrile,
trifluoromethyl, (C1-C6) -alkyl, (C1-C6) -alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg or (C1-C6)-alkylsulfonyl, and
R3 is (C1-C18)-alkyl, (C -? )?alkenyl, (C -? ?
alkenyloxy, (C2-C18)-alkynyl, phenyl, fluorine,
hydroxyl, trifluoromethyl, (C1-C18)-alkylsulfinyl,
(C1-C18)-alkylsulfonyl, phenylsulfinyl, naphthyl-
sulfinyl, naphthylsulfonyl, (C11-C18)-alkoxy,
(C3-C8)-cycloalkoxy, (C ?C )?-alkoxy-(C -? )?-alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg apart from trifluoroethoxy,
pentafluoropropoxy and heptafluorobutoxy, phenyl-
(C2-C4)-alkoxy, phenoxy, (C1-C12)-alkanoyl, phenyl-
(C1-C4)-alkanoyl or benzoyl, where, in substituents
with a phenyl or naphthyl ring, this optionally
carries up to 5 identical or different substituents
from the series comprising fluorine, chlorine,
bromine, nitrile, trifluoromethyl, (C1-C6)-alkyl,
(C1-C6)-alkoxy, -O-[CH2]X-CfH(2f+1-g)Fg or (C1-C6)-
alkylsulfonyl, and
- 93 -
R4 is (C1-C18)-alkyl, (C2-C18)-alkenyl, (C2-C18)-
alkenyloxy, (C2-C18)-alkynyl, phenyl, chlorine,
fluorine, bromine, hydroxyl, trifluoromethyl,
(C1-C18)-alkylsulfinyl, (C1-C18)-alkylsulfonyl,
phenylsulfinyl, phenylsulfonyl, naphthylsulfinyl,
naphthylsulfonyl, (C1-C18)-alkoxy apart from
1-butoxy, (C3-C8)-cycloalkoxy, (C1-C8)-alkoxy-
(C1-C8)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg, phenyl-
(C2-C4)-alkoxy, phenoxy, (C1-C12)-alkanoyl, phenyl-
(C1-C4)-alkanoyl or benzoyl, where, in substituents
with a phenyl or naphthyl ring, this optionally
carries up to 5 identical or different substituents
from the series consisting of fluorine, chlorine,
bromine, nitrile, trifluoromethyl, (C1-C6)-alkyl,
(C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg or (C1-C6)-
alkylsulfonyl,
or a physiologically active salt thereof.
13. A compound of the formula I as claimed in claims 4
and 12, in which
m is 0,
A is a -CH2- group,
B is -CO2H,
R1 and R5 are hydrogen and
R2 is (C1-C18)-alkyl, (C1-C18)-alkenyl, phenyl,
chlorine, fluorine, bromine, trifluoromethyl,
(C1-C18)-alkylsulfinyl, (C1-C18)-alkylsulfonyl,
phenylsulfinyl, phenylsulfonyl, naphthylsulfinyl,
naphthylsulfonyl, (C1-C18) -alkoxy, (C3-C8)-cyclo-
alkoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy, -O-[CH2]x-
CfH(2f+1-g)Fg, where, in substituents having a phenyl
or naphthyl ring, this optionally carries up to 5
identical or different substituents from the series
consisting of fluorine, chlorine, bromine, nitrile,
trifluoromethyl, (C1-C6)-alkyl, (C1-C6)-alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg and (C1-C6)-alkylsulfonyl, and
R3 is (C1-C18)-alkyl, (C1-C18)-alkenyl, phenyl,
- 94 -
fluorine, trifluoromethyl, (C1-C18)-alkylsulfinyl,
(C1-C18)-alkylsulfonyl, phenylsulfinyl, naphthyl-
sulfinyl, naphthylsulfonyl, (C11-C18)-alkoxy,
(C3-C8)-cycloalkoxy, (C1-C ?-alkoxy-(C ?C ?-alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg, apart from trifluoroethoxy,
pentafluoropropoxy and heptafluorobutoxy, phenyl-
(C2-C4)-alkoxy, phenoxy, (C1-C12)-alkanoyl, phenyl-
(C1-C4)-alkanoyl or benzoyl, where, in substituents
having a phenyl or naphthyl ring, this optionally
carries up to 5 identical or different substituents
from the series comprising fluorine, chlorine,
bromine, nitrile, trifluoromethyl, (C1-C6)-alkyl,
(C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg and (C1-C6)-
alkylsulfonyl, and
R4 is (C1-C18)-alkyl, (C1-C18)-alkenyl, phenyl,
chlorine, fluorine, bromine, trifluoromethyl,
(C1-C18)-alkylsulfinyl, (C1-C18)-alkylsulfonyl,
phenylsulfinyl, phenylsulfonyl, naphthylsulfinyl,
naphthylsulfonyl, (C1-C18)-alkoxy apart from
1-butoxy, (C3-C8)-cycloalkoxy, (C1-C8)-alkoxy-
(C1-C8)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg, phenyl-
(C2-C4)-alkoxy, phenoxy, (1l-C12)-alkanoyl, phenyl-
(C2-C4)-alkanoyl or benzoyl, where, in substituents
with a phenyl or naphthyl ring, this optionally
carries up to 5 identical or different substituents
from the series comprising fluorine, chlorine,
bromine, nitrile, trifluoromethyl, (C1-C6)-alkyl,
(cl-c6)-alkoxyl, -O-[CH2]x-CfH(2f+1-g)Fg or (C1-C6)-
alkylsulfonyl,
or a physiologically active salt thereof.
14. A compound of the formula I as claimed in claims 5
and 13, in which
m is 0,
A is a -CH2- group,
R1 and R5 are hydrogen,
one of the substituents R2, R3 or R4 is hydrogen and the
other two are identical or different and are hydrogen,
- 95 -
(C1-C16)-alkyl, fluorine, chlorine, bromine, trifluoro-
methyl, (C1-C16)-alkylsulfonyl, phenylsulfonyl, (C1-C16)-
alkoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy, -O-[CH2]x-
CfH(2f+1g)Fg, benzyloxy or phenoxy, where, in substituents
which contain a phenyl ring, this optionally carries up
to 3 substituents from the series comprising fluorine,
chlorine, bromine, trifluoromethyl, (C1-C6)-alkyl,
(C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg or (C1-C4)-alkyl-
sulfonyl, or a physiologically active salt thereof.
15. A compound of the formula I as claimed in claims 6
and 14, in which
m is 0,
A is a -CH2- group,
R1, R2 and R5 are hydrogen and
R3 is (C1-C16)-alkyl, fluorine, trifluoromethyl,
(C1-C16)-alkylsulfonyl, naphthylsulfonyl, (C11-C16)-
alkoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy, -O-[CH2]x-
CfH(2f+1-g)Fg apart from trifluoroethoxy, pentafluoro-
propoxy and heptafluorobutoxy or phenoxy, where, in
substituents which contain a phenyl ring, this
optionally carries up to 3 substituents from the
series comprising fluorine, chlorine, trifluoro-
methyl, (C1-C6)-alkyl, (C1-C6)-alkoxy, -O-[CH2]x-
CfH(2f+1-g)Fg and (C1-C4)-alkylsulfonyl, and
R4 is (C1-C16)-alkyl, fluorine, chlorine, trifluoro-
methyl, (C1-C16)-alkylsulfonyl, phenylsulfonyl,
naphthylsulfonyl, (C1-C16)-alkoxy apart from
1-butoxy, (C1-C8)-alkoxy-(C1-C8)-alkoxy or -O-[CH2]x-
CfH(2f+1-g)Fg, where, in substituents which contain a
phenyl ring, this optionally carries up to 3 sub-
stituents from the series consisting of fluorine,
chlorine, trifluoromethyl, (C1-C6)-alkyl, (C1-C6)-
alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg and (C1-C4)-alkyl-
sulfonyl, or a physiologically active salt thereof.
16. A compound of the formula I as claimed in claims 7
and 15, in which
- 96 -
m is 0,
A is a -CH2- group,
R1, R, R3 and R5 are hydrogen and
R4 is (C1-C12)-alkyl, fluorine, chlorine, bromine, tri-
fluoromethyl, (C1-C12)-alkylsulfonyl, phenylsulfonyl,
naphthylsulfonyl, (C1-C12)-alkoxy apart from 1-butoxy,
(C1-C6)-alkoxy-(C1-C6)-alkoxy, -O-[CH2]X-CfH(2f+1-g)Fg, or
phenoxy, where, in substituents which contain a phenyl or
naphthyl ring, this is optionally monosubstituted by
fluorine, chlorine, bromine, trifluoromethyl, (C1-C6)-
alkyl, (C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg or (C1-C4)-
alkylsulfonyl, or a physiologically active salt thereof.
17. A compound of the formula I as claimed in claims 8
and 16, in which
m is 0,
A is a -CH2- group,
R1, R3, R4 and R5 are hydrogen and
R is (C1-C12)-alkyl, fluorine, chlorine, bromine, tri-
fluoromethyl, (C1-C12)-alkylsulfonyl, phenylsulfonyl,
naphthylsulfonyl, (C1-C12)-alkoxy, (C1-C6)-alkoxy-(C1-C6)-
alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg, benzyloxy or phenoxy,
where, in substituents which contain a phenyl or naphthyl
ring, this is optionally monosubstituted by fluorine,
chlorine, bromine, trifluoromethyl, (C1-C6)-alkyl,
(C1-C6)-alkoxy, -O-[CH2]x-CfH(2f+1-g)Fg or (C1-C4)-alkyl-
sulfonyl, or a physiologically active salt thereof.
18. A compound of the formula I as claimed in claims 9
and 17, in which
m is 0,
A is a -CH2- group,
R1, R and R5 are hydrogen,
R4 is hydrogen or chlorine and
R3 is hydrogen, fluorine, (C1-C4)-alkoxy-(C1-C6)-alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg apart from trifluorethoxy,
pentafluoropropoxy and heptafluorobutoxy, naphthyl-
sulfonyl or phenoxy, where, in substituents which
contain a phenyl or naphthyl ring, this optionally
- 97 -
carries up to 3 identical or different substituents,
preferably one substituent, from the series
comprising fluorine, chlorine, bromine,
trifluoromethyl, trifluoromethoxy, (C1-C6)-alkyl and
(C1-C6)-alkoxy, or a physiologically active salt
thereof.
19. A compound of the formula I as claimed in claims 10
and 18, in which
m is 0,
A is a -CH2- group,
R1, R2, R4 and R5 are hydrogen and
R3 is hydrogen, fluorine, (C1-C4)-alkoxy-(C1-C6)-alkoxy,
-O-[CH2]x-CfH(2f+1-g)Fg apart from trifluoroethoxy,
pentafluoropropoxy and heptafluorobutoxy, naphthyl-
sulfonyl or phenoxy, where, in substituents which
contain a phenyl or naphthyl ring, this optionally
carries up to 3 identical or different substituents,
preferably one substituent, from the series
comprising fluorine, chlorine, bromine,
trifluoromethyl, trifluoromethoxy, (C1-C6)-alkyl and
(C1-C6)-alkoxy, or a physiologically active salt
thereof.
20. A prodrug to a compound of the formula I as claimed
in claims 1 to 19.
21. A process for the preparation of a compound of the
formula I as claimed in claims 1 to 19, which comprises
1.i1.) reacting a quinoline-2-carboxylic acid of the
formula II (R11 = H) with an amino ester of the
formula III to give an amide ester of the formula
IV, or
1.i2.) reacting a quinoline-2-carboxylic acid ester of
the formula II (R11 = lower alkyl) under the
conditions of aminolysis to give a compound of
the formula IV; where R10 is PG (Protecting
- 98 -
Group) and A-B is (CH2)1-4-CO2H
<IMG> <IMG>
<IMG>
II IV
1.ii) liberating a compound of the formula I or V from
its ester of the formula IV or VI; and/or
<IMG> <IMG> <IMG>
VI I
<IMG> <IMG> <IMG>
IV V
1.iii) obtaining a compound of the formula I or VI from
a compound of the formula V or IV by splitting
off the hydroxyl-protecting group R10,
and if appropriate
- 99 -
<IMG> <IMG> <IMG>
V I
<IMG> <IMG> <IMG>
IV VI
1.iv) oxidizing a compound of the formula I, IV, V or
VI to give a compound of the formula Ia, IVa, Va
or VIa.
<IMG> <IMG> <IMG>
I Ia
22. A compound as claimed in claims 1 to 19 for use for
inhibiting collagen biosynthesis.
23. A compound as claimed in claims 1 to 19 as an
inhibitor of prolyl hydroxylase.
24. A compound as claimed in claims 1 to 19 for use as
a fibrosupressant.
- 100 -
25. A compound as claimed in claims 1 to 19 for the
preparation of a medicament against fibrotic diseases.
26. A compound as claimed in claims 1 to 19 for the
preparation of a medicament against fibrotic diseases of
the liver.
27. A compound as claimed in claims 1 to 19 for the
preparation of a medicament against fibrotic diseases of
the lung.
28. A compound as claimed in claims 1 to 19 for the
preparation of a medicament against fibrotic diseases of
the skin.
29. The use of a compound of the formula I as claimed in
claims 1 to 19 as an inhibitor of prolyl hydroxylase and
as a fibrosupressant.
30. A medicament comprising a compound of the formula I
as claimed in claims 1 to 19.
31. An intermediate product of the formula II
<IMG>
II
in which
R1 to R5 have the meanings which apply to a compound of
the formula I as claimed in claims 1 to 19,
R10 is hydrogen or an HO-protecting group and
R11 is hydrogen, (C1-C8)-alkyl or benzyl,
- 101 -
with the exception of compounds of the formula II in
which R10 is benzyl or methyl if R1 to R5 and R11 are
hydrogen, and those compounds of the formula II in which
R10 is hydrogen, benzyl or p-toluenesulfonyl if R1 to R5
are hydrogen and R11 is ethyl and those compounds of
formula II in which R3 is hydrogen or methoxy, if R1, R2,
R3 R4 R5 R10 and R11 are hydrogen.
32. An intermediate of the formula X
<IMG> (X)
in which
m is 0 or 1 and
R2 to R5 have the meanings which apply to a compound of
the formula I as claimed in claims 1 to 19,
with the exception of compounds of the formula X in which
m is 0, R3 is hydrogen, methyl or chlorine if R2, R4 and
R5 are hydrogen.