Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2l 87336
wo ss/277s6 . ~~ 2 19
r. for Treating Japanese Cedar Pollen Allergy
B ~ ' of ~h.- I
Japanese cedar (Sugi; Cryptomeria japonica) pollinosis is one of the most
important allergic diseases in Japan. The rlurnber of patients suffering from this
disease is on the increase and in some areas, more than 10% of the population are
10 affected.
The major allergen from Japanese cedar pollen has been purifled and the
amino acid sequence partially identified, and designated as Sugi basic protein
(SBP) or Cry j I (Yasueda et al. (1983) J. Allergy Clin. ImmunoL 71: 77-86; and
Taniai et al. (1988) FE~S Letters 239: 329-332). Cry j I has been cloned and the15 full length nucleic acid sequence and the full length amino acid sequence of the
Cry j I protein have been identified (WO 93/01213)
The Cry j I allergen found in Cryptomeria japonica has also been found to
be cross-reactive with allergens in the pollen from other species of trees, including
Cupressus s~ , vi,~,~. Panzani et al. (Annals of Allergy 57: 26-30 (1986~)
20 reported that cross reactivity was detected between allergens in the pollens of
Cupressus ~ . , vi, t ~..) and Cryptomeria japonica in skin testing, RAST and
RAST inhibition. A 50 kDa allc~rgen isolated from Mountain Cedar (Juniperus
sabinoides, also known as Juniperus ashei) has an NH2-terminal sequence (Gross
et al., (1978) Scand. J. ImmunoL 8: 437441) which is the same sequence as the
first five amino acids of the ~H-2 terminal end of the Cry j I allergen. The Cry j I
allergen has also been found to be ~ rg~";r~lly cross-reactive with the following
species of trees: Cupressus arL~onica, Cupressus ~ ~ ucu, ua, Juniperus
virginiana, Juniperus communis, Thuya orientalis, and ('~ ,u, is obtusa.
Treatment of Japanese cedar pollinosis by ' of Japanese cedar
pollen extract to effect ll~y./ ~ to the allergen has been attempted.
using Japanese cedar pollen extract, however, has drawbacks in
that it can elicit: . ' yl~i~ if high doses are used, whereas when low doses areused to avoid a~ yllyl~i~, treatment must be continued for several years to build
up a toierance to the extract.
WO 94/01560 discloses improved ~ llyu~;~iol~ and methods for treatment
of sensitiv~y to Japanese cedar pollen or to ar~ , 'Iy cross reactive
wo 9sr277s6 2 1 8 7 3 3 6 r ~ 2 1~ ~
pollen allergen which greatly minimiæs the potential adverse side effects
associated with .i; c ~ n therapy using Japanese cedar pollen extract. WO r
94/01560 discloses isolated antigenic fragments or peptides derived from Cry j Iwhich when ' ' to a Japanese cedar pollen-sensitive individual, or an
Sindividual allergic to an allergen cross-reacvve with Japanese cedar poUen
allergen, are capable of down regulating the allergic response of the individual to
Japanese cedar pollen or such cross reactive aUergen. Such down regulation of
the aUergic response of the in&vidual results in diminution or alleviation of the
classic symptoms of allergy including asthmatic symptoms induced by Japanese
10cedar pollen. Such antigenic fragments are disclosed as bemg capable of eliciting
a T cell response such as stimulation (i.e. T ceU proliferation or Ir , '
secretion) andlor are capable of inducing T ceU non~ .a or reduced
T cell lC;a~VI~ a when challenged with Japanese cedar pollen allergen. In
ad&tion, WO 94/01560 discloses that the most preferred C~y j I peptides suitable15for therapeutic use do not bind IgE specrfic for Cry j 1, or bind IgE to a
, lesser extent than the native Cry j I allergen, thereby reducing or
eiiminating the possibility of . ' ~l~ia in a treatment regimen which includes
such peptides. FinaUy, WO 94/01560 discloses peptides which possess the
f~ described above.
As a result of extensive ~.~,f. ' efforts, the present invention
provides novel modified Cry j I peptides and novel: ' - and r ~ . .
of modified Cry j I peptides which are optimal for preparation of a drug productsuitable for use in treating Japanese cedar poUen allergy in humans and other
mananals. Such Cry j I peptides and r ~ thereof for use as an optimiæd
human drug product have not previously been disclosed or . . '
S of thf~ Tnv~nfir n
The present invention provides novel peptides of Cry j I wbich have been
modified as a part of a fJI.,f~ ' scheme to develop an optimized drug
product for therapeutic treatment of humans suffering from allergy to Japanese
cedar pollen allergen or a pollen aUergen which is ~ , cross reactive
Japanese cedar pollen allergen. Such modified peptides p~CC~cc~f~rfq;n unique
,1. .", ~. .;~i-^cwhichrenderthem~Li~ul~ulysuitablefordrugproduct
r, The present invention further provides therapeutic ~vlll~JvaiLivllS and
35 ~il r~ r, ,~ ~;,, whichhave been optimiæd to -- - ' and
maint in the unique rhq~q~ff ricfi~c of tho modified C~y j I peptides and at the
2 1 87336
0 95127786 ~ .'0 :2 l 9
same time provide maximum therapeutic effect when used in therapeutic regimens
for the treatment of Japanese cedar pollen allergy in humans.
DPcr~?~irn of the Drawi~c
5 Fig. I shows the complete cDNA sequence for C~y j I (SEQ. ID. NO: 39) which is composed of 1312 ' " ' , including 66 nucleotides of 5' 1
sequence, an open reading frame starting with the codon for an initiating
metbionineof1122 mlrlloti~tc anda3'1 ' Iregion. Fig.Ialsoshows
the deduced amino acid sequence of C)y j I (SEQ. ID. NO: 40);
Fig. 2 shows 35 u ~- r r ~ 20mer peptides which overlap by 10 derived from
Cryjl.
Fig. 3 shows the ammo acid sequences of the novel "unique" peptides of the
15 invention.
Fig. 4 is a graphic representation depicting responses of T cell lines from twenty-
five patients primed m vitro with purified native Cr~v j I and analyzed for response
to the 35 u ~la~J~ .g 20 mer Cr,v j I peptides shown in Fig. 2, by percent of
20 responses (positive) with an S.I of at least two (shown over each bar), the mean
stimulation mdex of positive response for the peptide (shown over each bar in
parenthesis) amd the positivity mdex (Y axis);
Fig. 5 is a graphic ~ depicting T cell responses to the u.~,~ldlJ~ g Cr~v
25 j I peptides shown in Fig. 2, and the nove~ "unique" peptides of the invention
shown m Fig. 3. The mean S.l. shown above each bar (in parenthesis) as well as
the percentage of responses, the positivity index (mean S.I. multiplied by
percentage of responses) is the Y axis.
hg. 6a is a pH solubility profile of CJI-24.5 (SEQ. ID. NO: 36) (desalted) in
50mM phosphate buffer with 5% mannitol and 22C, with 95.2% estimated
peptide content and 2.7% acetdte.
Fig. 6b is a pH solubility profile of CJI-43.39 (SEQ. ID. NO: 37) m 50mM
phosphate buffer with 5% mannitol and 22C, with 835% estimated peptide
content and 1.9% acetate.
wo ss/277s6 2 1 8 7 3 3 6 ~ 749 ~ ,
Fig. 6c is a pH solubility profile of CJI-q4.8 (SEQ. ID. NO: 38)in 50mMphosphate buffer with. 5% mannitol and 22~C, with 86.~o estimated peptide
content and 1.5% acetate.
Drt7ilr~ crru~tjnn of th~ T
The present invention provides novel peptides of Cry j I which have been
modified as a part of a 1 f~ schem~ to develop an opvimized drug
product for therapeutic vreatment of humans suffering from allergy to Japanese
10 cedar pollen allergen or an allergen which is ' O "~/ cross reactive wivh
Japanese cedar pollen allergen ( e.g. Cupressus . . , v~r ~,~, Juniperus
sabinoides (also known as Juniperus ashei) Cupressus arizonica, Cupressus
,,lù~,v~u,va~ ~uniperus virginiana, Juniperus communis, Thuya orientalis, and
('I - yl~u~ obtusa). Such modified peptides possess certain unique
15 1 r ;~ which render vfl~em particularly suitable for drug product
and may be referred to herein as "unique" peptides.
In accordance with ~ ' chemistry, ~ is the
process of opvimizing a drug through ~- andlor definition of those
physical and chemical properties considered important in vhe ~ ' of a0 stable, effective, and safe dosage form. The possible wivh vhe various
intended for use in vhe final drug product are also considered.
r ~ r~n is an mtensive effort that includes the study of such parameters as
solubility, pH profile of stability, and drug-excipient onc which may have
a profound effect on a drug's ~h, Dlologh,dl availability and physical amd chemical
25 stability. The data obtained from such studies are irltegrated wivh v~ose obtamed
from I " ~ and 1,;- ' I studies of vhe active drug
component thus providmg r " that pefmits the selection of the best drug
form, and the most desirable excipients for use im its ~ v~,lu~
The d~,~. ', of an optimum I of active drug component
30 and excipients is complex and m_ny factors influence r~ - inn properties. Thehigh degree of uniformity, the ~', rD;olog;c~l availability and the therapeutic
quality expected of l l - - ", ~ ~ . .1;. .1 can only be achieved by ~. . : 1~ . -1.1~. effort
and expertise. Flexibility is also an important factor m ~ r~ nn Numerous
excipients, stabilizers counter ions and the like may have to be tested in order to
35 find those compatible with the active drug component of vhe r ~ ..
Multiple ~n~iifir7tio~c oftheactiveco ~o entmaybecomenecessalyto
21 87335
W09512M86 P~ I0I249
DU~,~.,DDrUIly formulate a drug product. Such m~ fi~ f ng must not effect the
overall therapeutic ~rr.,~ ",.,aD of the drug but at the same time, must render the
drug more suitable for '
As a part of a I r ~ ' scheme to provide an optimized drug product
S suitable for use in humans and other mammals for treating sensitivity to Cry j I, it
was determined that the active component (a peptide or candidate peptide) in such
~ ' should possess the following ~ which would render such
peptides "unique" among all of the possible peptides derived from the C~y j I
sequence. First, a unique peptide should alone or in ,f ' ' with other
10 urlique peptides comprise a sufficient percentage of the T cell reactivity of the C~y
j I protein allergen to induce T cell ~ DS or reduced T cell
~ D~ in a substantial perceûtage of the individuals sensitive to Cly j I
protein allergen. Second, the candidate peptide should possess the . I --,.. ~. . ;~lic
of "superior solubility " which is defined herein as solubility~ of greater than 5
mg/ml at a pH in the pH range of pH 6 to pH 8 in an aqueous buffer. Third, the
peptide is stable in an aqueous buffer at a pH in the pH range of pH 6 to pH 8.
Candidate peptides which have been determined to be "unique" peptides of tbe
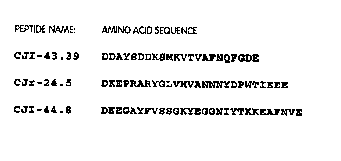
invention are CJI-24.5 (SEQ. ID. NO: 37), CJI-43.39 (SEQ. ID. NO: 36), and CJI-
44.8 (SEQ. ID. NO: 38), all as shown in Fig. 3.
In accordance with the frst ~ , those peptides found to elicit a T
cell response such as T cell ~ or Iy , ' ' secretion (i.e. comprise at
least one T cell epitope), or induce T cell non ._DIJu~ D or reduced T cell
~,J~U.,D;~ DD are understood to have T cell reactivity. T cell epitopes are
believed to be mvolved in initiation and pe~etuation of the immune response to aprotein allergen wmch is responsible for t ~ linical symptoms of allergy. These
T cell epitopes are thought to trigger early events at the level of the T helper cell
by binding to an appropriate HLA molecule on the surface of an antigen
presenting cell and stimulating the relevant T cell ~ ' r, These events
lead to T cell ~lul;r~liu--, ly . ' ' secretion, local rl y reactions,
IC~I I ' ' of additional immune cells to the site, and activation of the B cell
cascade leading to production of antibodies. One isotype of these antibodies, IgE,
is r ~ ' ~Iy important to the du~lul ' of allergic symptoms and its
production is influenced early in the cascade of events, at the level of the T helper
cell, by the nature of the ly~ hu~ ,s secreted. A T cell epitope is the basic
3~ element or smallest unit of recognition by a T cell receptor, where the epitope
comprises amino acids essential to receptor ~ ,uL. f~n It is believed that
wo9sl27786 21 87336 f~ c:2:9
exposure of Japanese cedar pollen patients to isolated C~ j I peptides which
comprise at least one T cell epitope may cause T cell non~ u.~ of
appropriate T cell ~ v~ such that they become ~ - or have
reduced l~."UUl~ ,llC;,~ to the protem allergen and do not participate in
5 stimulating an immune response upon such exposure for example, via anergy,
tolerance, or apoptosis, the ability to modify the l~ hill., secretion profile as
compared with exposure to the naturally occurring ~i,, and /or the ability
to cause induction of T suppresser cells.
To determine peptides having T cell reactivity and comprising at least one
10 T cell epitope, isolated peptides are tested by, for example, T cell biology
techniques, to determine whetller the peptides elicit a T cell response or irlduce T
cell non-lc;~,uull.,; ~ lC~ As discussed in the Examples human T cell stimulatmgactivity can be tested by culturing T cells obtained from an individual sensitive to
Japanese cedar pollen allergen, (i.e., an individual who has an IgE mediated
15 immune response to Japanese cedar pollen allergen) wit'n a peptide or modified
peptide derived from Cly j I and d~,. ., whether IJI ul;r~..~iu~l ûf T cells
occurs in response to the peptide as measured, e.g., by cellular uptake of tritiated
thymidine. Stimulation indices for responses by T cells to peptides can be
calculated as the maximum counts per minute (CPM) in response to a peptide
divided by the control CPM. A stimulation index (S.l.) equal to or greater than
two times the b~.,~- ' level is considered "positive". Positive results are usedto calculate the mean stimulation index for each peptide for the group of patients
tested. Peptides suitable as candidates for r. ~n into a fmal drug product
have a mean T cell stimulation index of greater than or equal to 2.0 and preferably
higher, (e.g. at least 2.5, more preferably at least 3.5, more preferably at least 4.0,
more preferably at least 5, even more preferably at least 7 and most preferably at
least about 9).
For therapeutic purposes, candidate peptides are recognized by at least
10%, more preferably at least 20%, more preferably at least 3û% and even more
preferably at least 40% or more of individuals in a population of individuals
sensitive to Japanese cedar pollen. In addition, preferred candidate peptides have
a positivity index (P.I.) of at least about 100, more preferably at least about 250
and most preferably at least about 350. The positivity index for a peptide is
determined by multiplying the mean T cell stimulation mdex by the percent of
individuals, in a population of individuals sensitive to Japanese cedar pollen (e.g.,
preferably at least 15 i"d;~ ~ l more preferably at least 30 mdividuals or more),
W095127786 2 1 87336 P~,l/u~ 1219
who have a T cell stimulation index to such peptide of at least 2Ø Thus, the
positivity index represents both the strength of a T cell response to a peptide (S.l.)
and the frequency of a T cell response to a peptide in a population of individuals
sensitive to Japanese cedar pollen.
To determine whether a peptide (candidate peptide) or a ~ i - of
candidate peptides are ltkely to comprise a sufficient percentage of the T cell
reactivity of the Cr,v j I to induce T cell c ~,UU.. ,.~ in a substantial
percGntage of a population of individuals sensitive to Cry j 1, an algorithm can be
used. In accordance with one such algorithm, a set of u . ~,II.T, _ peptides is
10 produced by ay ' ' '~y dividing Cry j I into at least two u . ~ J,ui~.t, peptide
regions of desired lengths (e.g., of about 12-30 a~nino acid residues in length,preferably not longer than about 25 amino acid residues in length with about 5-15
amino acid residues of overlap). For example, see Fig. 2. This division into
peptide regions can be arbitrary, can be nlade according to an algorithm, or can be
15 wholly or partially based on regions of Cry j I known to comprise at least one T
cell epitope. Preferably, at least 50% of the eDtire Cr,v j I protein sequence and
more preferably, the entire protein sequence of Cry j I is divided into two or more
peptides. A human T cell stimulation index is determined for each of the peptides
in an in vitro T cell ,u~ulif~ iu~ assay as described herein for each individual20 tested in a population of individuals sensitive to the protein antigen. For example,
see ~ig. 4. A candidate peptide or ~ ' of candidate peptides is selected
based, at least in part, on the mean human T cell stimulation index of the
candidate peptide in the set of peptides tested and the positivity index of tltecandidate peptide in the set of peptides tested. For example see, Fig. 5. The
25 human T cell stimulation index for the candidate peptide(s) is summed. For each
individual, the human T cell stimulation index for the candidate peptide(s) is
divided by the sum of the human T ceUs stimulation indices of the remaining
peptides in the set of peptides tested tû determine a percent of T cell reactivity as
shown below:
CaDdidate S.l.
( I ) S'c T Cell Reactivity of a caDdidate peptide(s) = X loo
Sum of S.l. of tDe set of
OverlappiDg pepddes
Alternatively, the presence of T cell epitopes in the candidate peptide
dependent on amino acids residues in an u . ~ll. l . & peptide located at either the
woss/27786 2 1 87 336 ~ 21~ ~
N-terminus or C-terminus of the candidate peptide in the amino acid sequence of
the protein antigen, but which epitopes are not present in the candidate peptide can
be considered in calculating the percent of T cell reactivity in the candidate
peptide by use of the following formula:
(2) % T Cell Reaebvity of a candidate peptide(s) =
NT flanl ing peptide S.l. + Carldidate peptide S.l. +:CT flanl~ing peptide S.l.
` x 100
sum of S.l. of the set of overlapping peptides
In this formula, "NT flanking peptide" refers to a peptide which comprises
15 amino acid residues which overlap with amino acid residues located at the N-
terminns of the candidate peptide m the amino acid seqnence of the protein
amtigen from which the peptide is derived; "CT flanking peptide" refers to a
peptide which comprises amino acid residues which overlap with amino acid
residues located a the C-terrstinus of the candidate peptide in the amino acid
20 sequence of the protein antigen from which the peptide is derived. In this
calculation stimnlation indices for the candidate peptide, the N-terminal flanking
peptide and the C-terminal flanking peptide are added and divided by the sum
total of the stimulation indices for the entire set of u._lL~ , peptides obtain a
percent of T cell reactivity for the candidate peptide. If a ~ of two or
25 more candidate peptides is selected each of which contains amino acid residnes
which overlap, this calculation calmot be used to determine a percent of T cell
reactivity for each candidate peptide separately. However, a total percent of T cell
reactivity for the ~" ' of candidate peptides c m be obtained. In tbis
situation, the stimulation mdices for all of the candidate peptides which overlap is
30 included in the calculation.
The values obtained for the percentage of T cell reactivity for the
candidate peptide or ~ . of peptides in each individual tested can be
expressed as a r~mge of the lower and higher values of the results of the above
described ' ' - By either of the above ~ ' ' the percent is obtained
35 for at least about twenty (20) and preferably at least about thirty (30) individuals
sensitive to the protein antigen and a mean percent is rl~PtPnrninPrl For use in the
.r.~ of the invention, the candidate peptide or c~lrnhirll~tir n of candidate
peptides has the following criteria: (I) the candidate peptide or ~ of
` 2 1 87336
wo ss/277s
can~ te peptides has a mean percent of at least about 10%, preferably at least
aboul ~0%, more preferably at least about 30%, more preferably at least about
40% and more preferably at least about 50-60% or greater; and (2) in the
population of individuals tested at least about 60%, preferably at least about 75%,
S and more preferably at least about 90-100% have positive T call responses (S.I.
equal to or greater than 2.0) in response to the camdidate peptide or, ' of
candidate peptides. A candidate peptide or ~- ' of candidate peptides
meeting the above criteria is likely to comprise a sufficient percentage of the T
cell reactivity to Cry j I to induce T cell non .~ ullai ~ a or reduced T cell
10 Ica~u...;~.,~.a in a substantial percentage of a population of individuals sensitive
to Cr~v j 1.
As an illustrative ~ ' ' of the above-described algorithm, a set of
U.~ peptides and candidate peptides, CJI-24.5, CJI-23.39 and CJ144.8,
derived from C~y j I were produced and tested. Secondary T cell cultures
15 determined to be reactive with Cry j I protein antigen were derived from 36 Cr~v j
l-allergic subjects and amalyzed for reactivity to the u ~. ' l l ~ set of peptides in
an in vitro T cell ~lulif~,l~iul~ assay as described herein. The results are shown in
Fig. 5. The highest stimulation index greater than or equal to 2.0 in response to
each peptide was recorded for each subject tested. The data were then amalyzed
20 by the equations above. The results and ' ' of the percent of T cell
reactivity for a single Cry j l-allergic subject are shown below using formulas ( I )
~d (2)
wo g~l27786 2 1 8 7 3 3 6 r ~ r l2 1s
.
T CELL REACTIVITY FOR PATIENT 1308
PEPTIDE STIMIILATION INDEX
CJl-l (SEQ. ID. NO: 1) 10.9
CJl-2 (SEQ. ID. NO: 2) 16.1
CJl-3 (SEQ. ID. NO: 3) 8.8
CJl-4 (SEQ. ID. NO: 4) 0
CJl-5 (SEQ. ID. NO: 5) 3.2
CJl-6 (SEQ. ID. NO: 6) 0
CJl-7 (SEQ. ID. NO: 7) 2.5
CJI-8 (SEQ. ID. NO: 8) 0
CJl~l (SEQ. ID. NO: 41) 8.9
CJI-ll (SEQ. ID. NO: Il) 0
CJI-12 (SEQ. ID. NO: 12) 0
CJI-13 (SEQ. ID. NO: 13) 0
CJ1-14 (SEQ. ID. NO: 14) 0
CJl-15 (SEQ. ID. NO: 15) 0
CJI-42.5 (SEQ. ID. NO: 42) 17.6
CJI-18 (SEQ. ID. NO: 18) 0
CJl-l9 (SEQ. ID. NO: 19) 0
CJl-20 (SEQ. ID. NO: 20) 0
C11-21 (SEQ. ID. NO: 21) 0
CJ1-43.39 (SEQ. ID. NO: 36) 25.6
CJ1-23 (SEQ. ID. NO: 23) 5.3
CJ1-24.5 (SEQ. ID. NO: 37) 6.9
Wl-25 (SEQ. ID. NO: 25) 9.4
CJ1-26 (SEQ. ID. NO: 26) 11.9
CJl-27 (SEQ. ID. NO: 27) 5.5
CJI-28 (SEQ. ID. NO: 28) 0
CJl-29 (SEQ. ID. NO: 39) 2.9
CJI-30 (SEQ. ID. NO: 30) 0
CJl-44.8 (SEQ. ID. NO: 38) 21.5
CJl-33 (SEQ. ID. NO: 33) 20.9
CJI-34 (SEQ. ID. NO: 34) 17.8
CJl-35 (SEQ. ID. NO: 35) 0
s
SUM OF STIMULATION INDICES: 195.7 (DENOMINATOR)
2 ~ 87336
wo ss/277s6 f ~ 2 19
% Reactivity o~ Peptide 44.8 for patient 1308
(I) al - 44.8 (S.l.) 21.5
S = _ X 100 = 11%
195.7 195.7
10 (2) C31- 30 + CJ144.8 + al-33(S.l.s) 0+21.5+209
= = x loo = 21.7
195.7 195.7
Therefore the estimated }ange of T cell reactivity for Peptide 44.8 (SEQ.
ID. N0: 38) for this patient is 11%-21.7% of the total reactivity of the Cry j Iprotein. The above calculation is repeated for any potential candidate peptides. In
the population of 36 Cry j I-allergic subjects tested the followmg results were
obtained:
Candidate Range of mean percentage Frequency of response
~ii~ T (~rll R~ ~rfivity 5~t l~ ~cr, revtide
CJl-245 + CJ143.3
+ CJ1~4.8 36-53% 97%
Thus, the .. ,., .1.: . ~ ;..., of the three candidate peptides of the mvention are well
within the desired range for possessing, in ~ ., sufficient T cell reactivity
of C~y j I, and therefore, meet the first .1. ~ of a "unique" peptide of the
invention.
For the treatment of allergy in accordance with the methods of the
invention, it is preferred that a peptide used in conj~ fi~n therewith does not bimd
i .. - ,.. ~l~.l.. l i .. E (IgE) or binds IgE to a ~ Iesser extent (i.e. at least
100-fold less binding and more preferably, at least l,000-fold less bmding) th mthe Cr~ j I protein allergen from which the peptide is derived binds IgE. The
major .. ~ of standard LhP~ry are IgE-mediated responses such
as ~~ yl~i~. T ~g' ~ ' E is a mediator of anap~ .ctic reactions which
result from the binding and cross-linking of antigen to IgE vn mast cells or
basophils and the release of mediators (e.g., histamine, serotonin, eosinophil
chemotacic factors) in allergic ("atopic") patients. Thus, ~.~l~ d~ in a
40 substantial percentage of a population of individuals sensitive to the allergen
11
- 21 ~7336
woss/277s6 r~ x, ~0~21
being treated could be avoided by the use m ' ,~y of a peptide or
peptides which do not bind IgE in a substantial percentage (e.g., at least about75%) of a population of individuals sensitive to Cr,v j I, or if the peptide binds IgE,
such binding does not result in the release of mediators from mast cells or
basophils. The risk of ~ . ' yl~iD could be reduced by the use in ' , J
of a peptide or peptides which have reduced IgE binding. IgE binding may be
tested, for example by direct ELISA or capture ELISA. Moreover, peptides which
have minimal IgE stimulating activity are desirable for therapeutic C1~ DD.
Minimal IgE stimulating activity refers to IgE production that is less than the
amount of IgE production and/or IL-4 production stimulated by the native C~ y j I
protein allergen. If a peptide binds IgE, it is preferable that such binding does not
result in the release of mediators (e.g. histamines) from mast cells or basophils.
To determine whether a peptide that binds IgE causes the release of mediators, ahistamine release assay can be performed using standard reagents and protocols
obtained for example, from Amac, Inc. (Westbrook, ME). Briefly, a buffered
solution of a peptide to be tested is combined with an equal volume of whole
~ blood from an allergic subject. After mixing amd incubation, the cdls
are pelleted and the ~u~ are processed and analyzed using a radio
~ to determine the amount of histamine released. As described in
Example 3, candidate peptides of the invention, 24.5 (SEQ. ID. NO: 37), 43.39
(SEQ. ID. NO: 36), and 44.8 (SEQ. ID. NO: 38), do not appear to bind IgE .
The second ~ I - ,.... ;-' ;~ for a unique peptide is that of "superior
solubility" which was defined earlier as being solubility of greater than 5 mglml at
a pH in the pH Mnge of pH 6 to pH 8. Solubil~ty at a pH m a IJIIJ _~
25 acceptable pH range (e.g. pH 6 to pH 8) is particularly important when
~ '- a ' ., ' theMpeutic for injection. A~' of a soluble
drug product in a ~JllJDh)logil-~lly acceptable pH range by i..L..,~,u~ ..,, or
injection provides 100% bioavailability of the drug component to
the ~ yDiolo~iu~ll system into which the drug is being introduced. Thus, it is
30 necessary that a drug product intended for injection be fluid to the extent that easy
Dy ~ ' '' h~ e~ists, and the active component be soluble as well if maximum
therapeutic effect is to be achieved. Solubility is also useful when ' '
to be r ' ' ' ' ~ via other modes of ~ ;.... such as by oMI
d ~ (tablet, aerosol, sublingual), or sustained release ~lCI~ru~liUII~ and
r ~ ,. 12
2 1 87336
wog5n7786 .!1 I~l/U~ _ 1219
Proteins and peptides may be difficult to formulate into soluble
f ~.., .l.. ~~ ' ;....~ as a peptide may not be soluble in any desirable pH range or may be
soluble in only a narrow pH range. It is y~u ~i~ul~ly difficult when multiple
peptides are being formulaîed together into a single ' i~ as
5 each peptide may be soluble in a pH range which does not overlap with those of the other peptides in the r~ As a result, it is the , . of "superior
solubility" which requires the most r ~ '' flexibility in that . . l
, ... "1;1 i. -I ;.... of the targeted candidate peptides may be necessary to ~u~ y
formulate a ' il ~ ' drug product.
The unique peptides of the invention are the product of multiple amino
acid mntlifi~ rinn~ of the original targeted candidate peptide sequence ("parent")
from which the modified unique peptides of the invention were originally derivedfor the purpose of finding a peptide which fits the ~ r ;~ of "superior
solubility". For example, the amino acid sequence of CJI-44.8 (SEQ. ID. NO: 38)
was derived from the protein sequence of Cry j I (SEQ. ID. NO: 40) by first
identifying those regions of the parent protein (Fig. I) with high T cell reactivity
using the set of ~ y~ , peptides 20-mers as discussed in Example 1, and
shown in Fig. 2, which covered the entire sequence. Two of these peptides, CJI-
31 (SEQ. ID. NO: 31) and CJ1-32 (SEQ. ID. NO: 32), individuaUy exhibited high
T-cell reactivity. Since these peptides were adjacent to each other in the native
protein sequence (Fig. I) and overlapped by 10 residues, a peptide, CJI44, was
to capture the total T ceU reactivity of both peptides. CJI44 is a
peptide 30-mer having the amino acid sequence
DEEGAYFVSSGKYEGGNIYTKKEAEFNVE (SEQ. ID. NO: 43) which contains
all of the sequence present in the two 20-mers ClI-31 (SEQ. ID. NO: 31) and CJI-32 (SEQ. ID. NO: 32). However, although CJI44 (SEQ. ID. NO: 43) possessed T
cell reactivity of the two 20-mers, CJI-31 (SEQ. ID. NO: 31) and CJI-32 (SEQ.
ID. NO: 32), when the solubility of CJI-44 (SEQ. ID. NO: 43) was tested it had asolubility much lower than the 5 mg/ml solubility required for a "unique" peptide
of the invention.
Thus, further attempts were made to increase ~olubility by trunc?~.vn at the
N-terminus portion of CJI44 which resulted in CJI-44. 1
(NGAS~;VSSGKYEGGNIYTKKEAFNVE) (SEQ. ID. NO: 44). Additional
truncation of two C-terminal residues yielded 44.2
(NGAYE~VSSGKYEGGNIYTKKEAFN) (SEQ. ID. NO: 45). However, although
solubility was improved in these sequences it still did not reach the standard of
13
21 87336
W0 95/~7786 1 ~ 2 ~9
"superior solubility". Thus, 44.2 (SEQ. ID. NO: 45) was further modified by the
addition of charged (hydrophilic residues) to the N-terminus and by ! r,~ ,l ~. ., . ,~ ..:
of the lI~d.u~llul~ic residue Val with the less IIY~r~ ' ~ residue Ala. Two of the
resulting analogs, CJI-44.5 (DENGAYFVSSGKYEGGNIYTKKEAFNAE) (SEQ.
ID. NO: 46) and CJ144.6 (DEENGAYFVSSGKYEGGNIYTKREAFNVE) (SEQ.
ID. NO: 47), showed increased solubility to usrng a "single pH point protocol
procedure" (e.g. a protocol procedure wherein d~,; of solubility were
made at a single pH in 100 mM sodium phûsphate buffer without mannitol umder
constant agitation) Two additional analogs were constructed in which the residueAsn was deleted. Of these two analogs, CJI 44.7
(DEGAYFVSSGKYEGGNIYTKKEAFNAE) (SEQ. ID. NO: 48) and CJI-44.8
(DEEGAYFVSSGKYEGGNIYTKREAFNVE) (SEQ. ID. NO: 38), CJI-44.8 was
very soluble in the "single pH point protocol" and achieved "superior solubility"
in the "pH range protocol procedure" (i.e. wherein solubility is measured as a
fumction of pH in 50 mM sodium phosphate containing 5% mannitol with no
agitation after initial mixing). CJ1-44.8 (SEQ. ID. NO: 38) was stable and soluble
at greater than 5 mg/ml over the pH range pH6-pH8 in an aqueous buffer (see,
Example 4 and Fig. 6c).
Peptide CJ144.8 (SEQ. lD. NO: 38) was deterrnined to be a "unique"
peptide after . that it also retained a T-cell reactivity similar to its
parent peptides, CJI-31 (SEQ. ID. NO: 31), CJ1-32 (SEQ. ID. NO: 32)and CJI-44
(SEQ. ID. NO: 43) (see E~ample 2), and c, ' of its stability as is
discussed below. D~ ,Iv~ r .li of the other "unique" peptides, CJ1-24.5 (SEQ. ID.
NO: 37) and CJI-4339 (SEQ. ID. NO: 36), followed a process similar to that
described abûve for CJI-44.8 (SEQ. ID. NO: 38).
The third criteria which the unique peptides of this invention must meet is
stability at a ~ ;; "y æceptable pH in the range of pH 6 to pH 8. It must
be stable umder the conditions of r ' amd storage, and under conditions of
,.. if necessary. Stability testing establishes the time period for which
the integrity, quality and purity of the drug product is preserved in its finished
dosage form. Stability testing may be performed 'y with solubility
studies as discussed in Example 4. Each of the candidate peptides of the invention
remained stable (e.g. no significant ~ ) at a ~ll,y~iulo~ y pH in a pH
range from pH 6-pH 8, at room h,.~. for at least 24 hours.
Therefore, candidate peptides CJI-245 (SEQ. ID. NO: 37), CJ1-43.39
(SEQ. ID. NO: 36), and CJ1-44.8 (SEQ. ID. NO: 38) possess each of the three
14
~ WO95/27786 21 87336 F~~ C -.9
required "unique" ~ outlined above, indicating that this Cu~lbil~oliull
of peptides is suitable for rl as an optimized therapeutic drug product for
r ~ to humans for treatment of allergy to Japanese cedar pollen
allergen.
Highly purified peptides of this invention, may be produced synthetically
by chemical synthesis using standard techniques. Various methods of chemically
D~ D;~..o peptides are known in the art such as solid phase synthesis which has
been fully or semi automated on "y available peptide D~ ' -
Synthetically produced peptides may then be purified to 1....,.~.O. Iy (i.e. at leaDt
90%, more preferably at least 95% and even more preferably at least 97% purity),free from all other l)uly~ lid~,D and . using any number of techniques
Imown in the literatnre for protein ~ ~
In accordance with one procedure for producing highly purified
peptides of the invention, a peptide produced by synthetic chemical
means (either anchored to a polymer support "solid phase synthesis" or by
Cu.~ " chemical reactions "solution synthesis") may be
purified by preparative reverse phase ' . O . ' ~. In this method, the
Dy.lll.~ ally produced peptide in "crude" form is dissolved in an appropriate
solvent (typically an aqueous buffer) and applied to a separation column (typically
a reverse phase silica based media, i~i addition, polymer or carbon based media
may be used). Peptide is eluted from the column by increasing the:
of an organic component (typically acetonitrile or methanol) in an aqueous buffer
(typically TFA, I~ hy- phosphate, acetate or similar buffer). Fractions of
the eluate will be collected and analyzed by appropriate analytical methods
(typically reverse phase HPLC or CZE .,lu~ , . ' y). Those fractions having
the required I O ~J will be pooled. The counter ion present may be
changed by additional reverse phase ~,lu~ in the salt of choice or by
ion exchange resins. The peptide may then be isolated as its acetate or other
appropriate salt. The peptide is then filtered and the water remoYed (typically by
30 Iy~Fhili7Ari~n) to give a l-,~,. - .-- - peptide ~,.l~;l;containing at least
90%, more preferably at least 95% and even more preferably at least g7% of the
required peptide ~ ~ . Optionally, or in ~ ; on with reverse phase
HPLC as described above, I ~ may be a~ ,. d by affinity
- ulu~ , ion exchange, size exclusion, counter current or normal phase
35 separation systems, or any ~-J-III-l--A~ 1 of these methods. Peptide may
wo gs~7786 2 1 8 7 3 3 6 . ~ ,. 1249
additionally be ' using ultra filtration, rotary evaporation,
.,c. .~ dialysis or other similar techniques.
The higbly purified ~ peptide . . " . .l,., ~ l is then 1 ~ ... l. .;, ;1
by any of the following techniques or ~ thereof: a) mass ~ ~
5 to determine molecular weight to check peptide identity; b) amino acid analysis to
check the identity of the peptide via amino acid . , ~ 1, c) amino acid
sequencing (using an automated protein sequencer or mamually) to confirm the
defined sequence of amino acid residues; d) HPLC (multiple systems if desired)
used to check peptide identity and purity (i.e. identifies peptide impurities); e)
10 water content to determine the water ~tiUII of the peptide ~ f)
ion content to determine the presence of salts in the peptide ~ i...., and g)
residual organics to check for the presence of residual organic reagents, starting
materials, and/or organic
A peptide of the invention may also be p}oduced by IC~,- ' ' DNA
15 techniques in a host cel1 l.. r .. fl with a nucleic acid sequence coding for such
peptide. When produced by ~ ' techniques, host cells I ~J.lll~d with
nucleic acid encoding the desired peptide are cultured in a medium suitable for the
cells and isolated peptides c;m be purified from cell culture medium, host cells, or
both using techniques known in the art for purifymg peptides and proteins
includmg ion ~ , ultra fi1tration, el~llu~,llul~L, or
with antibodies specific for the desired peptide. Peptides
produced r~ ~ ~y may be isolated and purified to hf -o ::y, free of
cellular material, other p~ly~ id~ or culture medium for use in accordance with
the methods described above for syntheticaUy produced peptides.
In certain limited ~ peptides of this mvention may also be
produced by chemical or enzymatic cleavage of a highly purified full length or
native protein of which the sites of chemical digest or enzymatic cleavage have
been i ' ~ ' and the resultmg digest is l~ ludu~ . Peptides having
defined amino acid sequences can be highly purified amd isolated free of any other
poly peptides or present in the enzymatic or chemical digest by any
of the procedures described above for highly purified, and isolated syntheticaUy or
/ produced peptides.
The present invention also pertains to therapeutic ~- mrt~ci~f~n~ and
' i~ , ' therapeutic ' ' comprising the unique peptides of the
invention. Therapeutic . ~ of the invention may comprise one or more
of the unique peptides of the invention which may be ' c~
16
~ wo gsl27786 ` 2 1 8 7 3 3 6 ~ 2 lg
or . "y as single treatment episode for treatment of allergy to Japanese
cedar pollen ailergen in a human or other mammal. Such a treatment regimen may
not necessarily be a physical mixture of more than one peptide of the invention,but does comprise a . ' of such peptides r ' ' ' ' ' ' '' ~ ~!/ or
5 sequentially as a single treatment episode in order to achieve the maximum
therapeutic effect the ~ of the unique peptides, CJI-24.5 (SEQ. ID. NO:
37), CJI-43.39 (SEQ. ID. NO: 36), and cn-44.8 (SEQ. ID. NO: 38), provide (e.g.
solubility and stability at a pH in am acceptable ~ , ' pH r~mge (preferably
pH 7.0 to pH 7.5) as well as covering 36-53% T cell reactivity in 97% of the
10 patients tested).
Therapeutic ~u,.,l...-:~i~...~ of the invention comprise one or more of
peptides CJI-24.5 (SEQ. lD. NO: 37), CJI-43.39 (SEQ. ID. NO: 36), and CJ1~4.8
(SEQ. ID. NO: 38) md aiso comprise one or more ~ y acceptable
carriers such as excipients which are compatible with peptide or peptides present
15 in a single ~ When the ~ is a ' i, r" ru~ ~u.. the
IJII~U _ '1~/ acceptable car ier must be compatible with all of the peptides in
the . . ' r~ Preferred excipients include but are not limited to
sterile water, sodium phosphate, mannitol, or both sodium phosphate and mannitolor amy ~ i. ,.. thereof. Other suitable excipients include but are not limited
20 to sorbitol, sucrose, dextrose, lactose dextran and PVP. In addition,
~,1~- "y acceptable coumter ions may be added during the preparation of
the, 1l ;l" ~1 ;,1. ' ' ' Examples of 1 ~ly acceptable counter
ions include acetate, HCI, and citrate.
A therapeutic ~ ~". q~n- ~ of the invention should be sterile, stable under
25 conditions of r ' ~ storage"' and use and should be preserved
against the ,, action of u~, such as bacteria and fungi. A
preferred means for ~ ~ a therapeutic ~ of the invention in
order to maintain the integrity of the, . (i.e. prevent
prolong storage, etc.) is to prepare the r~ ' of peptide and
30 ~ ly acceptable carrier(s) such that the ~ :l ;. ,., may be in the
form of a Iyophilized powder which is ~ in a ~I,~u. . . "~,
acceptable carrier, such as sterile water, just prior to use. In the case of sterile
powders for the preparation of sterile injectable solutions, the preferred methods
of preparation are vacuum drying, freeze-drying or spin drying which yields a
35 powder of the active ingredient plus any additional desired ingredient from a previously sterile-filtered solution thereof.
17
wo gs/27786 2 1 8 7 3 3 6 1 _llr_ ~ 1219
.
A preferred .. ~ comprises unique Cr,v3 I peptides
CJI-24.5 (SEQ. ID. NO: 37), CJ1-43.39 (SEQ. ID. NO: 3* and CJI-44.8 (SEQ.
ID. NO: 38) and sodium phosphate amd mannitol. For this c..ll ~ ' t, a suitable
counter ion such as acetate may be added during the preparation of the
5 ' ' and the ' ' is preferably prepared in the form of a
Iyophilized powder which is lc ' in a ~ . 106;~11y acceptable carrier,
such as sterile water, prior to use. One, non-limiting example of a preferred
of the inYention is described below. The Cry j I peptides
CJI-24.5 (SEQ. ID. NO: 37), CJI ~L3.39 (SEQ. ID. NO: 36) and CJ1-44.8 (SEQ.
10 ID. NO: 38) will preferably be combrned during r ' ' ~ with the
appropriate counter ion to produce a vial containing a sterile, pyrogen free,
Iyophilized powder having the following ~
Active: Cr~v j I peptides CJl-24.5 (SEQ. ID. NO: 37), CJI-43.39
(SEQ. ID. NO: 36) and CJ1-44.8 (SEQ. ID. NO: 38)
In of 0.75mg per peptide
Inactive8: 0.05 M Sodium Phosphate pH 6.0-8.0
5% w/v Manrlitol, U.S.P.
Diluent: Sterile Water for Injection, U.S.P. (initial lC "' "
0.9% Sodium Chloride for Injection
(dilution beyond initial .
Final pEI pH 7.0-pH 7.5
The ' . . ' ff nmll~if n of the rnvention may also be provided in the form of
25 a kit, including mstructions for use.
.' ofthetherapeutic.. ~ i.. and '~i,, '
~ ~ described above to an individual, preferably in non ~,
form, can be carried out usrng known procedures at dosages and for periods of
time effective to cause down regulation of the immune response to Japanese cedarpollen or an aUergen 'ogif ~lly cross reactive with Japanese cedar pollen
allergen (i.e., reduce the allergic symptoms caused by Japanese cedar pollen or
related allergen including Japanese cedar pollen induced asthma) of the individual.
Down regulation of the allergic immune response to Japanese cedar pollen in
humans may be determined clinically whenever possible (see e.g., Vamey et al,
British Medical Jourral, 302:265-269 (1990), or may be determined _ul j~,~Li~,ly
18
WO 951~7786 ' 2 1 ~ 7 3 3 6 p ~ o ,~ 9
.
(i.e. the patient feels as if some or all of the aOergic symptoms caused by Japanese
cedar pollen haYe been aOeviated).
One of the unique ~ ;rC of each peptides of the invention is that
each peptide rossesses "superior solubility". Therefore, ~ and ~ r of the invention are particularly suitable for
byinjection(e.g. or .~ J...). However,
optimized . ' and n'"1firf'rfif~f' r~ of the mvention may be
- ' l in any convenient malmer wherem solubility of the active drug
component is either desirable or acceptable, such as by injection (~
0 IIIIl~IV~IIV.~.~, etc.), oral r ' ' ' ' ' , sublingual, inhalation, i I -
r1rr1irr~ti~n rectal _ ' or any other route of ' known in
the art for r.~' ' ' ' ' ~, soluble therapeutic agents. It may be desirable to
admmister ' 'y or sequentiaOy a ~ f; ~ effective amount of
one or more of the therapeutic ~ ~ L of the invention to am mdividual as a
lS single treatment episode. Each of such ~ ;. . c for ~ ' '
! ' '' 1~ or . Ily as a single treatment episode, may comprise only
one unique peptide of the mvention or may comprise am optimi~ed ' . .
r... ,....1~l;.", in accordance with the invention.
For ~ v~ injection of one or more therapeutic ~ - and
20 ~ of the invention, preferably about I flg- 3 mg and more
preferably from about 20flg-1.5 mg, and even more preferably about 50 llg- 750
flg of each active component (peptide) per dosage unit may be: ' l. It is
especially adv~.~,.,~,... to formulate parenteral ~ . m unit dosage form
for ease of ' and uniformity of dosage. Unit dosage form as used
25 herein refers to physicaOy discrete units suited as unitary dosages for humansubjects to be treated; each unit containing a ~/lL~' ' ' ' quantity of active
compound calculated to produce the desired therapeutic effect im association with
the desired L~ carrier. The srf rifirr~fir,f~ for the novel unit dosage
forms of the invention are dictated by and directly dependent on (a) the unique
30 ~ of the active compoumd and the particular therapeutic effect to be
achieved, and (b) the limitations inherent in the art of r ~ such an active
compound for the treatment of human subjects.
To administer a . , of the invention by other than parenteral
- ' (i.e. by oral ' ) it may be necessary to coat the
35 c . ~ ;.... with, or co-administer the ~ lj with, a material to prevent its
iV.~lliUll or enhance its absorption and bioavailability. For example, a peptide
19
wo s~277s6 ' 2 1 8 7 3 3 6 r~ 2 l~
formulation may be co- ' ' with enzyme inhibitors or in liposomes.
Enzyme inhibitors include pancreatic trypsin inhibitor,
J;i ~u~ y''' ul ' . ' (DEP) and trasylol. Liposomes include water-in-oil-
in-water CGF emulsions as well as CG~ ivlldl liposûmes (Strejan et al., (1984)
S J. N~.u.., ..,..---~1 7:27). Y.~hen a peptide is suitably protected, the peptide may
be orally r ' ' ' c;d, for cxample, with an inert diluent or an assimilable edible
carrier. The peptide and other ingredients may also be enclosed m a hard or softshell gelatin capsule, .,, . ' into tablets, or ill~,U~ ' directly into the
individual's diet. For oral therapeutic r ' ' ' , the active compound may be~ ' with excipients and used in the form of ingestible tablets, buccal
tablets, troches, capsules, elixirs, solutions, gels, . . syrups, wafers, and
the like. Such ~ and 1~ should contain at least 1% by
weight of active compound The percentage of the: ... ~ ;.... and l..r.~
may, of course, be varied amd may Cull~ / be between about 5 to 80% of the
15 weight of the unit. The amount of active compound in such i' I 'Iy useful
- is such that a suitable dosage will be obtained. In addition, the
active compound may be . ' mto sustained-release or controlled release
(steady state or pulsatile release) ~ iUI.. and r~
Effective amounts of the optimized drug ~ c of the invention
20 will vary according to factors such as the degree of sensitivity of the individual to
the antigen, the age, sex, and weight of the imdividual, and the ability of peptide to
cause down regulation of the antigen specific immune response in the imdividual.Dosage regimen may be adjusted to provide the optimum therapeutic response.
For example, several divided doses may be ~ ' ' over the course of days,
25 weeks, months or years, or the dose may be IJIU~UI ~iullally increased or reduced
with each subsequent injection as indicatcd by the exigencies of the therapeuticsituation. In one preferred therapeutic regimcn, 1. injections of
therapeutic .. q.. ~ - arc given once a week for 3-6 weeks. The dosage may
remain constarlt for each mjection or may increase or decrcase with each
30 subsequent injection. A booster injection may be ' ' at intervals of
about threc months to about one year after initial treatment and may mvolve onlya single injection or may involve another series of injections similar to that of the
initial trcatment.
This invention is further illustrated by the following non-limiting
35 examples.
- - ~ 336
wossl277s6 2 1 ~7 . 1/~ c :2lg
Example 1
Japanese Cedar Pollen Allergic Patient T Cell Studies with o. . ~ Cry j
I peptides.
5Syll~hrcic of Ov~rl - P~ rri~
Japanese cedar pollen Cry j I was divided into a set of 35 peptides of 20
amino acids in length and u . ~,l' ., by 10 amino acids. Overlapping peptides
were ~y~ using standard Fmoc/tBoc synthetic chemistry and purified by
Reverse Phase HPLC. Figure 2 shows the u . . ' ., ~ C~y j I peptides used in
10 these studies. The peptide names are consistent throughout.
T Cell Responses to Ceda} Pollen Antigenic Peptides
Peripheral blood ' cells (PBMC) were purified by ly "~
separation medium (LSM) ....:,; r,."r, ;. of 60 ml of ? '1 ' blood from
15 Japanese cedar pollen-allergic patients who exhibited clinical symptoms of
seasonal rbinitis and were MAST and/or skin test positive for Japanese cedar
pollen. Long term T cell lines were established by stimulation of 2 X 106 PBL/mlin bulk cultures of complete medium (RPMI-1640, 2 mM L-glutamine, lû0 U/ml
penicilL../~ u.lly~,i.l, 5xlO-SM 2-....,., ~ , and 10 mM HEPES
20 ~u~,l ' with 5% heat inactivated human AB serum) with 20 ~Lg/ml of
partially purified native Cry j I (75% purity containing three barlds similar to the
three bands in Fig. 2) for 7 days at 37C in a humidified 5% CO2 incubator to
select for C~yj I reactive T cells. This amount of priming antigen was determined
to be optimal for the activation of T cells from most cedar pollen allergic patients.
25 Viable cells we}e purified by LSM l r ~ " and cultured in complete
medium ,,l ' ' with 5 units ' human L-2/ml and 5 units
human L-4/ml for up to three weeks until the cells no longer
responded to ly , ' I and were considered "restedn. The ability of the T cells
to proliferate to selected peptides, ' C~y j I (rC~y j 1), purified native
30 C~yjl,orl~" ' Amba I.l (rAmbaI.I) orapositivecontrol.phyto-
~,~ (PHA) was then assessed. For assay, 2 X 104 rested cells were
- - ' ' in the presence of 2 X 104 autologous Epstein-Barr virus ~BV)-
r " ,... d B cells (prepared as described below) (gamma-irradiated with 25,000
RADS) with 2-50 llg/ml of selected peptides, Cr~ j I, purified native ClyJ I or
35 rAmb a 1.1 or PHA, in a volume of 200 ,ul complete medium in duplicate or
triplicate wells in 96-well round bottom plates for 24 days. The optimal
21
2 1 87336
wo ss/277s6 ~ ~ ., u., s ~ l7 1s
incubation was found to be 3 days. Each well then received I IlCi tritiated
thymidine for 16-20 hours. The counts ~ ' were collected onto glass
fiber filter mats and processed for liquid 5~in~ n counting. Data collected
(not shown) indicated the effect of varying antigen dose in assays with
5 ' Cry j I, purified native Cr)~ j I, and ll ' Amb a I. I and
seYeral antigenic peptides synthesized as described above. Some peptides were
found to be inhibitory at high . in these assays. The titrations were
used to optimize the dose of peptides in T cell assays. The maximum response in
a titration of each peptide is expressed as the stimulation index (S.I.). The S.l. is
10 the counts per minute (CPM) i..~,u.~ ' by cells in response to peptide, divided
by the CPM ill.,GI I ' ' by cells in medium only. An S.l. value equal to or
greater than 2 times the b~L~- ' level is considered "positive" and indicates
that the peptide contains a T cell epitope. The positive results were used in
calculating mean stimulation indices for each peptide for the group of patients
15 tested. The results (data not shown) ~ .... -- -l . ,.1~ that patient #999 responds well
to IGI~I ' ' ' C~y j I, and purified native Cry j I, as well as to peptides CJ 1-2, 3,
20, and 22 but not to .c ' Amb a I.l. This indicates that C~ j I T cell
epitopes are recognized by T cells from this particular allergic patient and that
rC~vj I and peptides, 3 (SEQ ID NO: 49), 20 (SEQ ID NO: 50), and 22 (SEQ ID
20 NO: 51) contain such T cell epitopes. r, the epitopes were often not
detected with the adjacent u.. ' ., ~ peptides, and therefore probably span the
non-u.~ 3 central residues of the reactive peptides. No sigluficant cross-
reactivity was found in T cell assays using T cells primed with control antigens or
with C~y j I primed T cells against other antigens.
The above procedure was followed with a number of other patients.
Individual patient results were used in calculating the mean S.I. for each peptide if
the patient responded to the C~y j I protein at an S.I. of 2.0 or greater and the
patient responded to at least one peptide derived from C~ j I at an S.I. of 2.0 or
greater. A summary of positive ~ from twenty-five patients is shown in
Figure 4. The bars represent the positivity index. Above each bar is the percent of
positive responses with an S.I. of at least two to the peptide or protein in the group
of patients tested. In parenthesis above each bar are the mean stimulation indices
for each peptide or protein for the group of patients tested. All twenty-five T cell
lines responded to purified native Cry j I and 68.0% of the T cell hnes responded
35 to rC7y j I. These twenty-five T cell lines also responded at a Si6ir.~. .Iy Iower
level to rAmb a I.l indicating that the Amb a I allergens share a degree of
22
~ woss/277s6 2 1 87336 1~l,.,.. C1219
homology with C~ j I and that "shared" T cell epitopes might exist between C~y jI and Amb a I. This panel of Japanese cedar allergic patients responded to
peptides CJ1-1 (SEQ ID NO: 1), CJ1-2 (SEQ ID NO: 2), CJI-3 (SEQ ID NO: 3),
CJI-4 (SEQ ID NO: 4), CJ1-7 (SEQ ID NO: 7), CJ1-8 (SEQ ID NO: 8), CJI-9
(SEQ ID NO: 9), CJ1-10 (SEQ ID NO: 10), CJ1-11 (SEQ ID NO: ~1), CJI-12
(SEQ ID NO: 12), CJI-14 (SEQ ID NO: 14), CJI-15 (SEQ ID NO: 15), CJI-16
(SEQ ID NO: 16), Ul-17 (SEQ ID NO: 17), CJI-18 (SEQ ID NO: 18), CJI-I9
(SEQ ID NO: 19), CJI-20 (SEQ ID NO: 20), CJI-21 (SEQ ID NO: 21), CJI-22
(SEQ ID NO: 22), CJI-23 (SEQ ID NO: 23), CJI-24 (SEQ ID NO: 24), CJI-25
(SEQ ID NO: 25), CJI-26 (SEQ ID NO: 26), CJI-27 (SEQ ID NO: 27), CJI-28
(SEQ ID NO: 28), CJI-30 (SEQ ID NO: 30), CJI-31 (SEQ ID NO: 31), CJI-32
(SEQ ID NO: 32), CJI-33 (SEQ ID NO: 33), CJI-34 (SEQ ID NO: 34) and CJI-
35 (SEQ ID NO: 35) indicating that these peptides contain T cell epitopes.
Pl~, " of (EBV)-L ' ' B Cells for Use as Antigen Presenting
Cells
Autologous EBV-i ' ' cell lines were ~irradiated with 25,000 Rad
and used as antigen presenting cells in secondary ~lul~fc~ iu~l assays and
secondary bulk ~l; .".,l -~ ;., These EBV- ' ' cell lines were made by
incubating 5 X 106 PBL with I r~ of B-59/8 Marmoset cell line (ATCC
CRL1612, American Type Culture Collection, Rockville, MD) ' '
medium in the presence of 1 ~/ml phorbol 12-myristate 13-acetate (PMA) at
37C for 60 minutes in 12 X 75 mm ~oly~.u~,, 1.,.1~ roumd-bottom Falcon snap captubes (Becton Dickinson Labware, Lincoln Park, NJ). These cells were then
diluted to 1.25 X 106 cells/ml in RPMI-1640 as described above except
, ' with 10% h~ f ' "~ ' ~ fetal bovine serum and cultured in 200 ,ul
aliquots in flat bottom culture plates until visible colonies were detected. 7;hey
were then transferred to larger wells until the cell lines were c ' ' ' ' l.
Exarnple 2
r of, ~ ' ~ of total T cell reactivib and patient coverage of
the . ' ' of unique peptides of the invention.
Syr thrcic of unique ve~tides of thr jnv~n~ion
Peptides CJI-24.5 (SEQ. ID. NO: 37) and CJI- 44.8 (SEQ. ID. NO: 38)
were synthesized using standard Fmoc synthetic chemistry. Peptide CJI-43 39
23
wossr277s6 2187336 r~l,o. o ~219 ~
(SEQ. ID. NO: 36) was synthesized by t-Boc synthetic chemishry. All peptides
were purified to 93% or greater purity using the procedures for producing highlypurified peptides desribed e_rlier. Figure 3 shows the unique Cry j 1 peptides
used in these shndies. The peptide names are consistent throughout.
s
T r~ll rp7~ fivity of thP rP~?ti~1PC of thP i Vpntj,m-
Poptides CJI-24.5 (SEQ ID NO: 37), CJI-43.39 ~SEQ ID NO: 36), _nd C31-
44.8 (SEQ ID NO: 38) were tested for T cell reactivity (data not shown) as
described in Example l amd were determined to elicit T cell activity as did each of
10 their "parent" peptides from which they were derived amd thus are suitable ascandidate peptides for further shudy to determine if in ~, ' they possess a
sufficient percentage of the total T cell reactivity of Cry j I im a sufficient
percentage of the ~uldliu.~.. sensitive to Cry j I.
15 T rPll sh.~liP~ With ~ ;?Prti~PC ~ eP~?fi~lP ~
Secondary T cell cultures deherrnined to be reactive with Cry j I protein
amtigen were derived from 36 Cry j I-allergic subjects and analyzed for reactivity
to the ~ set of peptides (Fig. 2) 7nd the candidates peptides of the
invention (Fig.3) in am in vitro T cell l,...l;f .,.l,~... assay as described in Example
20 1. The results are shown in Fig. 5. The highest stimulation index greater than or
equal to 2.0 in response to eacb peptide was recorded for each subject tested. T_e
data were then analyzed by the equations described earlier in the ~ .~ . . r;. l ;, .. .
The, ~ on of candidate peptides CJI-24.5 (SEQ ID NO: 37), Cll-
43.39 (SEQ ID NO: 36), and CJI-44.8 (SEQ ID NO: 38) had a range of T cell
reactivity of abour 36%-53% based on am analysis of 36 patients (Fig. 5), the
frequency of response at 97% represents reactivity to at least one of the candidate
peptides, indicating that this ~ ' of peptides fits the first criteria for
"unique" peptides of the mvention im that in ~ ' peptides CJI-2~.5 (SEQ
ID NO: 37), CJI-43.39 (SEQ ID NO: 36), and CJI-44.8 (SEQ ID NO: 38),
comprising a sufficient percentage of the total T cell reactivity of Cry j I in a
substantial percentage of the population tested.
Example 3
IgE Binding Studies
To analyze IgE reactivity to candidate peptides CJI-24 5 (SEQ ID NO: 37),
ClI-43.39 (SEQ ID NO: 36), and CJI44.8 (SEQ ID NO: 38) and shown in Fig. 3,
24
i 21 87336
wo ssn77s6 ~ 2 19
a direct ELISA format was used. ELISA wells were coated with the candidate
peptides and then assayed for IgE binding. The binding results were generated
using two different pools of Cry j allergic patient plasma. Patient plasma pool A
(denoted PHP-A) was formulated by mixing equal volumes of plasma from 22
patients that were all shown to be positive for direct IgE binding to native purified
Cr,v j I by ELISA. The second pool (PHP-D) was formulated by the,
of equal plasma volnmes from 8 patients that had IgE binding by direct ELISA to
both native and denatured purified Cry j 1. This pool was generated to mcrease
the chance of detecting reactivity towards peptides. Both pools in this assay set
showed direct binding to the native purified Cr,v j I (data not shown). There was
no detectable IgE binding reactivity to any of the peptides tested at any of theplasma . . .~ ;. . used. To control for the presence of peptide coatmg the
wells, mouse polyclonal antisera was generated to the peptides. These antisera
were then used in direct ELISA binding to ~ that the peptides were
coating the wells. The results of these assays (data not shown) indicated that
peptides were coating the wells.
Example 4
c :' ' " of pII . '-' ~ und pH stability profiles of
c~ndidate peptides of the invention
1. Bl-ffPr PrPr~ nn
50 mM sodium phosphate stock solutions:
Stock solution A: 0.66 g (0.05 mol) of monobasic sodium phosphate
' ~.' was dissolved in 100 mL of WFI. The solution was filtered through
a 0.2 micron filter
Stock solution B: 0.71 g (0.05 mol) of dibasic sodium phosphate was
disolved in lOOml WFI. The solution was filtered through a 0.2 micron filter.
2. T Lpeptide (1i~rrrci.~n~
Dispersion A: 3.0 mg of each peptide CJI-24.5 (SEQ. ID. NO: 37), CJI-
- 44.8 (SEQ. ID. NO: 38)and CJI-43.39 (SEQ. ID. NO: 36) was weighed outseparately and placed in separate 1.5 rnL eppendorf vials with 600 IlL of stock
solution A. The ~ was agitated for 5 seconds to mix well.
Dispersion B: 3.0 mg of each peptide, CJI-24.5 (SEQ. ID. NO: 37~, CJI-
44.8 (SEQ. ID. NO: 38) and CJI~3.39 (SEQ. ID. NO: 36) was weighed ont
wossm7s6 2 187336 r~l~.n. ,c~ 1s
sepafately and placed in separate 1.5 rnL eppendoff vials with 600 ~LL of stock
solution B. The mixture was agitated for S seconds to mix well.
Dispersions A and B were sonicated for 2 minutes for good 1-.. f,,~. :y.
A small volurne was pipetted from each dispersion mto a labeled eppendoff vial
5 according to the fol10wing volume ratio:
Vial # Suspension A Suspension B Total Volume Estimated frnal
.,,~ . .
.,
C
The resultant svluliv.. O/~ A; were stored im the dark at 22C for 24 hours
without agitation. 11 micro centrifuge filters were labeled on the retentate cup.
10 The weight of each cup with the cap excluding the filter reservoir was measured.
The ~, 1 ' ' ' ' . ' was pipetted from each eppendoff vial into
the filer reservoir, and placed into micro centrifuge and spun for 10 minutes.
After .:. -ti ;r~ the filter reservorr was discarded and vhe weight of each
cup/cap with the collected filtrate was recorded. The net weight of the filtrate in
15 each cup was calculated by subtracting the weight of the empty cup/cap from the
weight of the cup/cap containing the filtrate. The pH of the filtrates in each cup
was measured usimg a micro, ' pH electrode. After each pH reading,
the electrode tip was rinsed with 1.0 rnL of the selected dilution buffer into each
cup. The total of diluted solution in each cup/cap was recorded. The dilution
20 factors were calculated by dividing the weight of the diluted solution by the weight of the filtrate.
The ~ of peptide in the diluted solution was determined by
HE`LC analysis. The solubility of peptide at each pH was obt,~ined by ~ulli~ g
the measured u. ~ i. ., . with the dilution factor. The extent of d~-g~ stj~ n of
26
.
wo 9sl27786 ' 2 1 8 7 3 3 6 ~ c - ,9
peptides was estimated by calculating the percent of total degradant peak area over
the total peak area.
The pH values with respect to the solubility values was plotted and are
shown in Fig. 6a-c for each respective peptide. As shown in the solubility curvefor each peptide as represented in Fig. 6a-c, peptides CJI-24.5 (SEQ. ID. NO: 37),
cn44.8 (SEQ. ID. NO: 38) and CJI43.39 (SEQ. ID. NO: 36) are each soluble at
greater than 5 mg/ml at a pH in the pH range of pH-6 to pH 8.
The pH stability profiles for each peptide cn-24.5 (SEQ. ID. NO: 37),
CJI44.8 (SEQ. lD. NO: 38) and cn-43.39 (SEQ. ID. NO: 36) were calculated
and tabulated as a function of total of degradartt peak area (data not shown). None
of the peptides showed any significant ~Ir ~" ...1 ~ ;~ .. . after a 24 hour period.
Therefore, each of peptides cn-24.5 (SEQ. ID. NO: 37), CJ144.8 (SEQ.
ID. NO: 38) and cn-43.39 (SEQ. ID. NO: 36) was determined to possess the
appropriate solubility and stability required of a unique peptide of the invention.
Although the invention has been described with reference to its preferred
, ' other ' ' can achieve the same results. Variations and
mn~iifi~ :~rinnc to the present invention will be obvious to those skilled in the art
and it is intended to cover in the appended claims all such rnn~ifir~rinn and
equi-~alents and follow in the true spirit and scope of this invention.
WO95/2?786 2 1 87336 r~~ `/.121~ ~
SEQU~NCE LISTING
5 ( 1~ GENERAL INFORNATIQN:
(i) APPLICANT: ImmuLoSTic Pl~ 1 ical Corporation
(ii) TITLE OF INVENTION: Ph^~^~ ;cal Fl lAri~-nc For
Treating ~Tapdnese Cedar Pollen Aller~y
(iii) Nl~lBER OF SEQUENCES: 51
UKK~ ADDRESS
lV ~: U_ J~
5 Inc.
(B) STREET- 610 Lincoln St
~D) STATE: MA
(B) COUNTRY: USA
(F) ZIP: 02154
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IPM PC comp~ti}~le
(c) OPERAT~MG SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: P/3tentIn Release Yl.0, Version Yl.25
(vi) CURRENT APPLICATION DATA:
(B) FAIPLILNGADATN N~MBER
(C) CLASSIFICATION:
(vii) PRIOR APPLICATION DATA:
35 (A) APPLICATION N~BER: ~T,S S 1 N . 08/226 248
(B) FILING DATE: April 8 1994erla o
(A) APPLICATION N~MBER: U S S al N 08/350 225
(B) FILING DATE: DeCember ' 6; 1egrg4 '
( ' ' ' ) ATTOR~EY/AGENT INFORNATION-
V~11 (A) NANE Darlene A, Vansto
(B) REGISTRATION N~lBER 35,729
(C) REFERENCE/DOC~ET NUMBER. 025.7 PCT(IMI-028CPC)
(iX) TT.'T. --T~M INFORNATION:
(A) TELEPHONE: (617) 466-6000
(B) TELEFAX: (617) 466-6040
( 2 ) INFORNATIQN FOR SEQ ID NO :1:
(i) SEQUENCE 'T~:~T 2-1 1 -. I .'. ' 11.:~
(A) LENGTH 20 no ac ds
(D) TOPOLOGY: linear
(ii) NOLECULE TyPE: peptide
(v) FRAGNENT TYPE: interr~l
(xi) SEQUENCE J~ ~KL~'l'lUN: SEQ ID NO:l:
65 As Asn Pro Ile AsP Ser Cys Tro ArsT Gly Asp Ser Asn Trp Ala Gln
70 Asn Arg Net L2y0s
28
W095l27786 ' ` ; ~ 2 1 87 336 ~ 9
.
(2 ) INFORMATION FOR SEQ ID NO: 2:
( i ) SEQUENCE rHARAr~TcTIcs
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
0 (ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE Lli~ l~'l'lUN: SEQ ID NO:2:
5 Asp Ser Asn Trp Ala Gln Asn Ar~ Met Lys Leu Ala Asp Cys Ala Val
, 15
Gly Phe Gly Ser
(2) IN~ --Tr,N FOR SEQ ID NO:3:
(i) SEQUENCE rT~A~A~ Ll~ -
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
30 (ii) r:OLECULE TYPE: peptide
(v) FRAGN~NT TYPE: internal
(Xi) SEQUEN-CE L~ lUN: SEQ ID NO:3:
35 Leu Ala Asp Cys Ala Val Gly Phe Gly Ser Ser Thr Met Gly Gly Lys
Gly Gly Asp Leu
( 2 ) INFORDIATIO~ POR SEQ ID NO: 4:
(i) SEQUENCE rT7AR~ iLl ~
(A) LENGTH: 20 amino ~Cids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
50 (ii) NOLECULE TYPE: peptide
( v ) FRAGD~NT TYPE: int ernal
(xi) SEQUENCE Jl;~S:~L~llUN: SEQ }D N,0:4:
ser Thr Met Gly Gly Lys Gly Gly Asp Leu Tyr Thr Val Thr Asn Ser
0 Asp Asp Asp Pro
5 (2) INFORMATION. FOR SEQ ID NO:5:
( i ) SEQUE~CE rT~A~A~oT CTICS:
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
( ii ) MOLECULE TYPE: peptide
29
W0 95127786 2 1 8 7 3 3 6 ~ g
(v) FRAG~ENT TYPE: interllal
(xi) SEQUENCE J~L~'l'lUW: SEQ ID NO:5:
S Tyr Thr Val Thr Asn Ser Asp Asp Asp Pro Val Asn Pro Ala r
Pio Gly
Thr Leu Ar~ Tyr
(2) INFORMATION FOR SEQ ID NO:6:
~1 ) SEQUENCE ~'UAR~
(A) LENGTH 20 ~mino acids
(B) TYPE am~o ac'd
(D) TOPOLOGY linear
(ii) r~OLECULE TYPE: peptide
(v) FRAGYENT TYPE: i~terral
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:6:
Val Asn Pro Ala Pro Gly Thr Leu Ar5j Tyr Gly Ala Thr Ara As,o Ar~
Pr Leu T Ile
o rp
(2) INFORUATION FOR SEQ ID NO:7:
( i ) SEQUENCE rT~ S:
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) NOLECULE TYPE: peptide
(v) FRAG~OENT TYPE: i~ternal
(xi ) SEQUENCE l~ l~'l'lUN: SEQ ID NO: 7:
Gly Ala Thr Ar~r Asp Arg Pro Leu Trp Ile Ile Phe Ser Gly Asn Xet
Asn Ile Lys Leu
(2) I~FOR~ATION FOR SEQ ID NO:8:
( i ) SEQUENCE ~
(A) LENGTH: 20 aminc acids
( B ) TYPE: amino ~c i d
( D ) TOPOLOGY: 1 i~ear
(ii) DIOLECULE TYPE: peptide
(v) FRAG~5ENT TYPE: internr~l
(xi) SEQUENCE L/~ lU~: SEQ ID NO:8:
65 Ile Phe Ser Gly Asn let Asn Ile Lys Leu Lys ;~et Pro l~et Tyr Ile
Ala Gly Tyr L2ys
( 2 ) INFOR~ATION FOR SEQ ID NO: 9:
:` ~ 1 7336
WO95l27786 2 8 r~J/~J~ 719
( i ) SEQUENCE r~r~ R A~'~R T .cTIcs:
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
10 (v) FF,AGMENT TYPE: internal
(xi) SEQUENCE 3i~:b~Kl~'1'1611~: SEQ ID NO:g:
Ly6 Net Pro Met Tyr Ile Ala Gly Tyr Lys Thr Phe As~ Gly Arg Gly
1 s lo 15
Ala Gln Val Tyr
(2) INFORMATION FOR SEQ ID NO:10:
(i) SEQUENCE rTT~R~
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
( i i ) MOLECULE TYPE: pep tide
(V) FF~AGMEN~ TYPE: intern 1
(xi) SEQUENCE ~b~n~ : SEQ ID NO:10:
Thr Phe Asp Gly Ar~ Gly Ala Gln Val l'yr Ile Gly Asn Gly Gly Pro
Cy6 Val Phe Ile
(2) INFOFMATION FOR SEQ ID NO:ll:
(i) SEQUENCE rl~R~r~r~RTcTIcs:
(A) LENGTH: 20 amino acids
(B) TYPE: bmino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE IJl;b~:~L~'l'l~_ll~: SEQ ID NO:ll:
Ile Gly Asn Gly Gly Pro Cy6 Val Phe Ile Ly6 Arg Val Ser A6n Val
Ile Ile Bi6 Gly
( 2 ) INFORMATION FOR SEQ ID NO :12:
(i) SEQUENCE rTl~R~ b:
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
7 (v) FRAGMENT TYPE: internal
(xi) SEQUENCE Jl;b:.:Kl~ N: SEQ ID NO:12:
31
` ` 336
WO 9S/27786 2 1 8 7 A ~ 1/ 1)~. S. _ 2 l~
Lys Arg Val Ser Asn Val Ile Ile His Gly Leu Tyr Leu I~r Gly Cys
Ser Thr Ser Val
5 20
( 2 ) INFOR~ATION FOR SEQ ID NO :13:
0 (i) SEQUENCE rTTARA( ~
(A) LENGTH: 2 0 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: li~ear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE IJl:iS~J:"l'lUDI: SEQ ID NO:13:
Leu Tyr Leu Tyr Gly Cys Ser Thr Ser Val Leu Gly Asn Val Leu Ile
Asn Glu Ser Phe - ~~
(2) INFORYATION FOR SEQ ID NO:14:
(i) SEQUENCE r~TLRA~T~RTcTIcs
(A) LENGTH: 20 amino acids
(El) TYPE: amino acid
( D ) TOPOLOGY: l inear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(Xi) SEQUENCE ~ ~l~'l'lUl~: SEQ ID NO:14:
Leu Gly Asn Val Leu Ile Asn Glu Ser Phe Gly Val Glu Pro Val }lis
Pro Gln Asp GlY
(2) INFORMATION FOR SEQ ID NO:lS:
(i) SEQUENCE rT~ARA~ C~i:
(A) LENGTH: 20 amino acids
(~3) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE IJ~ Cl~'l'lUDI: SEQ ID NO:lS:
Gly Val Glu Pro Val His Pro Gln Asp Gly Asp Ala Leu Thr Leu Ar
5 Thr Ala Thr Asn
(2~ INFOR10ATION FOR SEQ ID NO:16:
(i) SEQUENCE rT~ARP.r~PRTqTICS:
(~ LEN,~H: 20 amino ac~ds
; . =;V_ 2 1 87336
WO 95~27786 1 ~ J.. , C.'1~2 l9
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
Iv) FRAGMENT TYPE: internal
(xi) SEQUENCE J$::1~1~11UN: SEQ ID NO:1~:
0 Asp Ala Leu Thr Leu Arg Thr Ala Thr Asn Ile Trp Ile Asp Pis Asn
Ser Phe Ser Asn
ao
(2) INFORMATION FOR SEQ ID NO:17:
(i) SEQUENCE rT~R~
(A) LENGT~I: 20 amino ~cids
(B) TYPE: ~ino xcid
(D) TOPOLOGY: linear
25 (ii) MûLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE L~;~U~l'lUN: SEQ ID NO:17:
30 Ile Trp Ile Asp His Asn Ser Phe Ser A n Ser Ser Asp Gly Leu Val
As Val Thr Leu
P 2 0
(2) INFOR~ATION FOR SEQ ID NO:18:
(i) SEQUENCE ~ R~ , XLlU~'
(A) LENGTP~: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE U~ Kl~llUN: SEQ ID NO:18:
Ser Ser As~ Gly I.eu Val Asp Val Thr Leu Thr Ser Thr Gly Val Thr
5 10 15
Ile Ser As~ Asn
55 20
(2) INFORI!0ATION FOR SEQ ID NO:19:
(i) SEQUENCE ~pAR~`T~TcTIcs
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
( ii ) MOLECULE TYPE: peptide
(v) FR~AGMENT TYPE: internal
7û (xi) SEQUENCE l)$~ '1'1Ul~: SEQ ID NO:19:
Thr Ser Thr Gly Val Thr Ile Ser Asn Asn Leu Phe Phe Asn E~is ~is
5 10 15
33
W095/27786 2187336 r_l,. 5~0t~49 ~
y3 Val Net Leu
5 2 ) INFoRMATIr~N FOR SEQ ID NO: 2 0:
(i) SEQUENCE ruARA~ . .nr.~
(~) LENGTH: 20 amino acids
0 (B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) NOLECULE TYPE: peptide
15 (v) FRAGNIYT TYPE: internal
(xi ) SEQUENCE L~ Kl~rlUlY: SEQ ID NO: 2 0:
20 Leu Phe Phe Asn His His Lys Val Net Leu Leu Gly His Asp Asp Ala
Tyr Ser Asp Asp
(2) INFORNATION FOR SEQ ID NO:21:
(i) SEQUENCE c~ARA~ b:
(A) L~NGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) NOLECULE TYPE: peptide
35 (v) FRAGNEIYT TYPE: interral
(xi~ SEQUENCE ~ iUKL~'l'lUlY: SEQ ID NO:21:
Leu Gly His Asp Asp Ala Tyr Ser Asp Asp Lys Ser Net I.ys Val Thr
40 1 5 10 15
Val Ala Phe Asn
(2) INFORMATION FOR SEQ ID NO:22:
(i ) SEQUENCE r-~ARA~
(A) LENGTH: 20 amino acids
(B) TYPE: ~mino acid
(D) TOPOLOGY: linear
55 (ii) NOLECUL13 TYPE: peptide
(v) FKAGNEIYT TYPE: internal
(xi) SEQUENCE u~:S~KL~llulY: SEQ ID NO:22:
Ly~ Ser Net Lys Val Thr Val Ala Phe Asn Gln Phe GlY Pro Asn Cy~
5 10 15
Gly Gln Arg Net
65 20
( 2 ) INFORMATION FOR SEQ ID NO: 2 3:
(i) SEQUENCE rTTARA~
(A) LENGTH: 20 amino acids
(B) TYPE: ~AinO acid
34
- 21 87336
WO 95127786 , .~ 17 19
(D) TOPOLOGY: linear
(ii~ MOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE Ll~ lU~: SEQ ID NO:23:
0 Gln Phe Gly Pro Asn Cys Gly Gln Arg !et Pro Arg Ala Ar~ Tyr Gly
Leu Val Ris Val
(2) INFORMATION FOR SEQ ID NO:24:
(i) SEQUENCE rRARA,, rr~ 5
(B3 TYPE aminOamind acids
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(v) FRAGMRNT TYPE: illterrlal
(xi) SEQUENCE ~ U~Lrll~N: SEQ ID NO:24:
Prlo Arg Ala Ar~ Tyr Gly Leu Val His Val Ala Asn Asn Asn Tyr Asp
Pro Trp Thr Ile
(2) INFOR~ATION FOR SEQ ID NO:25:
(i) SEQUENCE rRARA~lr~RTcTIcs
(A) LENGTE~: 20 amino acids
(B) TYPE: amino ~cid
( D ) TOPOLOGY: 1 inear
(ii) IIOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE l~ ~l~'''lUN: SEQ ID NO:25:
50 Alla Asn A6n Asn Tyr As~ Pro Trp Thr Ile Tyr Ala Ile Gly Gly Ser
Ser Asn Pro Thr
(2) INFOR~ATIQN FOR SEQ ID NO:26:
(i) SEQUENCE ~ARA~'~.RTqTICS:
(A) LENGTH: 20 amino acids
(3) TYPE: amino acid
(D) TOPOLOGY: linear
3 (ii) MOLECULE TYPE: Peptide
(v) FRAGMENT mE: intern~l
(xi) SEQUENCE ~ ~LLL1UIY: SEQ ID NO:26:
70 Tyr Ala Ile Gly Gly Ser Ser Asn Pro Thr Ile Leu Ser Glu G y Asn
W095/27786 21~7336 ~ 5~0~249 ~
Ser Phe Thr Ala
5 (2) INFOR~ATION FOR SEQ ID NO:27:
(i) SEQUENCE rl~p~ RT~TIcs:
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
0 (D) TOPOLOGY: linear
(ii) NOLECULE TYPE: ~eptide
(v) FRAGNENT TYPE: internal
(xi) SEQUENCE JB~ "l'lUI~: SEQ ID N-O:27:
Ile Leu Ser Glu Gly Asn Ser Phe Thr Ala Pro A~n Glu Ser Tyr L
20 1 S 10 15 YS
Lys Gln Val Thr
25 (2) INFOR~ATION FOR SEQ ID NO:28:
(i) SEQUENCE ~'UPT~PI I r~ I .`.llC:7:
(A) LENGTH: 20 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) NOLECULE TYPE: ~e~tide
(v) FRAGD3ENT TYPE: internal
(xi) SEQUENCE NNa:_~L~l.LUN: SEQ ID NO:28:
Pro Asn Glu Ser ~ryr Lys Lys Gln Val Thr Ile Arg Ile Gly Cys Lys
Thr Ser Ser Ser
(2) INFORNATION FOR SEQ ID NO:29:
( i ) SEQUENCE CHMA~ ~b:
(A) LENGTH: 2 0 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) NOLECULE TYPE: ~e~tide
(V) FRAGNENT TYPE: internal
(xi) SEQUENCE L~lrl'lUN: SEQ ID No:29:
Ile ArS~ Ile Gly Cys Lys Thr Ser Ser Ser CYB Ser Asn Trp Val Trp
Gln Ser Thr Gln
65 (2) INFORNATION FOR SEQ ID NO:30:
(~) SEQUENCE ~ P~P~ TSTIcS -
(A) LENGTH: 20 amino ~cids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
36
W095/27786 2187336 ~ S~QI~>49
.
(ii~ MOLECULE TYPE: peptidc
(v) FRAGMENT TYPE: internal
S (xi ) SEQUENCE L~S~ rLUl~: SEQ ID NO: 3 0:
Cys Ser Asn Trp Val Trp Gln Ser Thr Gln Asp Val Phe T~r Asn Gly
5 10 15
0 Ala Tyr Phe Val
15 (2) INFORMATION FOR SEQ ID N0:31:
~i) SEQUENCE rHAR~ llU~
IA) LENGTH: 20 amino acids
(B) TYPE: amino ~cid
20 ~D) TOPOLOGY- linear
~ii) MOhECULE TYPE: peptide
~v) FRAGMENT TYPE: interr~l
~xi) SEQUENCE U~:~U~ UN: SEQ ID NO:31:
Asp Val Phe Tyr Asn Gly Ala Tyr Phe Val Ser Ser Gly Lys Tyr Glu
5 10 15
0 Gly Gly Asn Ile
~2) INFOR0$ATION FOR SEQ ID NO:32:
i ) SEQUENCE rT~ARA(, ...~ lCS:
~A) LENGTH: 20 amino acids
~B) TYPE: amino acid
40 ~D) TOPOLOGY: linear
~ii) MOLECULE TYPE: peptide
~v) FRAGMENT TYPE: internal
~xi) SEQUENCE U~:bU~l~TlU81: SEQ ID NO:32:
Ser Ser Gly Lys Tyr Glu Gly Gly Asn Ile Tyr Thr Lys Lys Glu Ala
0 Phe Asn Val Glu
~2) ~ mTr,N FOR SEQ ID No:33:
~i) SEQUENCE rHARArm~RTcTIcs
~A) LENGTH: 20 a~ino acids
~B) TYPE: a~ino acid
~D) TOPOLOGY: linear
~ii) MOLECULE TYPE: peptide
~v) FRAGMENT TYPE: interIlal
~xi) SEQUENCE LI~UKL~ lU~: SEQ ID NO:33:
70 Tyr Thr Lys Lys GlU Ala Phe A8n Val Glu A8n Gly Asn Ala Thr Pro
37
W0951277~6 2 1 87336 1~l/ c--~g ~
Gln Leu Thr Lys
5 (2) INFORMATION FOR SEQ ID NO:34:
( i ) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 2 0 amino acids
(B) TYPE: amino acid
0 (D) TOPOLOGY: linear
(ii) NOLECULE TYPE: peptide
(v) FRAG~ T TYPE: internal
(xi) SEQUENCE L~ h~: SEQ ID NO:34:
Asn Gly Asn Ala Thr Pro Gln Leu Thr Lys Asn Ala Gly Val Leu Thr
20 C Ser Leu Ser
ys
(2) ~ rIrN FOR SEQ ID NO:35:
(i) SEQUENCE rTTp~Ar~TcTIcs
(A) LENGTH: 13 amino acias
(P,) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) NOLECULE TYPE: ~eptide
(v) FRAGNENT TYPE: internal
35 (xi ) SEQUENCE L/~:~,~l'lU~: SEQ ID NO: 3 5:
Asn Ala Gly Val Leu Thr Cys Ser Le~l Ser Ly~ Arg Cys
( 2 ) INFORNATION FOR SEQ ID NO: 3 6:
(i) SEQUENCE r~AD~1 . ..rl I .`'11~.:j:
(A) LENGTH: 22 amino scids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
(ii) NOLECULE TYPE: pe~tide
(v) FRAGNENT TYPE: internal
(xi) SEQUENCE ~ r~ : SEQ ID NO:36:
Asp Asp Ala Tyr Ser Asp Asp Lys Ser Net Lys Val Thr Val Ala Phe
1 5 ~ 10 15
Asn Gln Phe Gly Asp Glu
(2) lNI~ TrN FOR SEQ ID NO:37:
(i) SEQUENCE rTTA'RA~
(A) LENGTH: 26 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: line~r
( ii ) NOLECULE TYPE: peptide
(v) FRAGNENT TYPE: i~ter~al
38
WO9S/27786 "~ 2 1 87336 .~ ?49
(xi) SEQUENCE L~ .K~ lUN: SEQ ID NO:37:
A~p Lys Glu Pro ~rg Ala Arg Tyr Gly Leu Val His Val Ala Asn Asn
5 10 15
Asn Tyr Asp Pro Trp Thr Ile Glu Glu Glu
(2) INFORMATION FOR SEQ ID NO:38:
(i) SEQUENCE rlJAp~ RTcTIcs:
(A) LENGTX: 28 amino acids
( B ) TYPE: ilmino acid
(P) TOPOLOGY: linear
(ii) NOLECULE TYPE: peptide
(v) FRAGMENT TYPE: internal
(xi) SEQUENCE LllS'i~l~llUl~: SEQ ID NO:38:
As~ Glu Glu Gly Ala Tyr Phe Val Ser Ser Gly Lys Tyr Glu Gly
Gly
5 10 15
Asn Ile Tyr Thr Lys Lys Glu Ala Phe Asn Val Glu
20 25
(2) INFORMATION FOR SEQ ID NO:39:
(i) SEQUENCE rTlA~
(A) LENGTH: 1337 base pairs
(B) TYPE: nucleic acid
(c) ~ ~: single
( D) TOPOLOGY: linear
(ii) MOLECUI.E TYPE: cDNA to mRNA
( vi ) ORIGINAL SOURCE:
(A) ORGANISN: Crytpomeria japonica
(ix) FEATURE:
(A) NAME/KEY: CDS
(B) LûCATION: 66..... 1187
( ix ) FEATUKE:
(A) NAME/REY: mat_,pe~tide
5 (B) LOCATION: 129.. 1187
(xi) SEQUENCE Jl;~U~U~'l'lUN: SEQ ID NO:39:
AGTCAATCTG CTCATAATCA ~ArrA~rAr.cr r~rA~ A~" AAATTCTACA CTCTGCTACC
60 60
AA~AA ATG GAT TCC CCT TGC TTA GTA GCA TTA CTG GTT TTC TCT TTT
107
- Net Asp Ser Pro CYs Leu Val Ala Leu Leu Val Phe Ser Phe
-21 -2D -15 -10
GTA ATT GGA TCT TGC TTT TCT GAT AAT CCC AT~ GAC AGC TGC TGG AGA
155
39
2 1 87336
WO95/27786 1~ . 5. l219
Val Ile Gly Ser Cy6 Phe Ser Asp Asn Pro Ile Asp Ser Cys Trp Arg
GGA GAC TCA AAC TGG GCC CAA AAT AGA ATG AAG CTC GCA GAT TGT GCA
203
Gly Asp Ser Asn Trp Ala Gln Asn Arg Met Lys Leu Ala Asp Cys Ala
lO 15 20 25
GTG GGC TTC GGA AGC TCC ACC ATG GGA GGC AAG GGA GGA GAT CTT TAT
Val Gly Phe Gly Ser Ser Thr Net Gly Gly Lys Gly Gly Asp Leu Tyr
30 35 - 40
ACG GTC ACG AAC TCA GAT GAC GAC CCT GTG AAT CCT GCA CCA GGA ACT
299
Thr Val Thr Asn Ser Asl~ Asp Asp Pro Val Asn Pro Ala Pro Gly Thr
CTG CGC TAT GGA GCA ACC CGA GAT AGG CCC CTG TGG ATA ATT TTC AGT
Leu Ar~ Tyr Gly Ala Thr Arg Asp Arg Pro Leu Trp Ile Ile Phe Ser
GGG AAT ATG AAT ATA AAG CTC A~A ATG CCT ATG TAC }~TT GCT GGG TAT
395
Gly Asn Net Asr. Ile Lys Leu Lys Net Pro Net Tyr Ile Ala Gly Tyr
AAG ACT TTT GAT GGC AGG GGA GCA CAA GTT TAT ~T GGC AAT GGC GGT
Lys Thr Phe Asp Gly Arg Gly Ala Gln Val Tyr Ile Gly Asn Gly Go5y
CCC TGT GTG TTT ATC A~G AGA GTT AGC AAT GTT ATC ATA CAC GGT TTG
Pro Cys Val Phe 1e Lys Arg Val Ser Asn Val Ile Ile His Gly Leu
TAT CTG TAC GGC TGT AGT ACT AGT GTT TTG GGG AAT GTT TTG ATA AAC
53 9
Tyr Leu Tyr Gly Cys Ser Thr Ser Val Leu Gly Asn Val Leu Ile Asn
125 130 135
GAG AGT TTT GGG GTG GAG CCT GTT CAT CCT CAG GAT GGC GAT GCT CTT
Glu Ser lP4heO Gly Val Glu Pro Val His Pro Gln Asp Gly Asp Ala Leu
ACT CTG CGC ACT GCT ACA AAT ATT TGG ATT GAT CAT AAT TCT TTC TCC
Thr Leu Arg Thr Ala Thr Asn Ile Trp Ile Asp His Asn Ser Phe Ser
155 160 165
AAT TCT TCT GAT GGT CTG GTC GAT GTC ACT CTT ACT TCG ACT GGA GTT
Asn Ser Ser Asp Gly Leu Val Asp Val Thr Leu Thr Ser Thr Gly Val
ACT ATT TCA AAC AAT CTT TTT TTC AAC CAT CAT AAA GTG ATG TTG TTA
Thr Ile Ser Asn Asn Leu Phe Phe Asn His His Lys Val Net Leu L
190 195 200
GGG CAT GAT GAT GCA TAT AGT GAT GAC AAA TCC ATG AAG G G AC. G G
779 T A T
Gly His Asp Asp Ala Tyr Ser Asp Asp Lys Ser Net Lys Val Thr Val
0 GCG TTC AAT CAA TTT GGA CCT AAC TGT GGA CAA AGA ATG CCC AGG GCA
Ala Phe Asn Gln Phe Gly Pro Asn Cys Gly Gln Arg~ Net Pro Arg Ala
W095117786 2 1 ~ 7 3 3 6 ~ 21~
.
CGA TAT GGA CTT GTA CAT GTT GCA AAC AAT AAT TAT GAC CCA TGG ACT
875
Arg Tyr Gly Leu Val His Val Ala Asn Asn Asn Tyr Asp Pro Trp Thr
235 240 245
ATA TAT GCA ATT GGT GGG AGT TCA AAT CCA l~CC ATT CTA AGT GAA GGG
923
Ile Tyr Ala Ile Gly Gly Ser Ser Asn Pro Thr Ile Leu Ser Glu Gly
0 250 255 260 265
AAT AGT TTC ACT GCA CCA AAT GAG AGC TAC AAG AAG CAA GTA ACC ATA
Asn Ser Phe Thr Ala Pro Asn Glu Ser Tyr Lys Lys Gln Val Thr Ile
270 275 280
CGT ATT GGA TGC AAA ACA TCA TCA TCT TGT TCA A~T TGG GTG TGG CA~
1019
Arg Ile Gly CyS Lys Thr Ser Ser Ser Cys Ser Asn Trp Val Trp Gln
TCT ACA CAA GAT GTT TTT TAT AAT GGA GCT TAT TTT GTA TCA TCA GGG
Ser Thr Gln As~ Val Phe Tyr Asn Gly Ala Tyr Phe Val Ser Ser Gly
300 305 310
AAA TAT GAA GGG GGT AAT ATA TAC ACA AAG AAA GAA GCT TTC AAT GTT
Lys Tyr Glu Gly Gly Asn Ile Tyr Thr Lys Lys Glu Ala Phe Asn Val
315 320 325
GAG AAT GGG AAT GCA ACT CCT CAA TTG ACA AAA AAT GCT GGG GTT TTA
Glu Asn Gly Asn Ala Thr Pro Gln Leu Thr Lys Asn Ala Gly Val Leu
ACA TGC TCT CTC TCT AAA CGT TGT TGATGATGCA TATATTCTAG
CATGTTGTAC 12 17
Thr Cys Ser Leu Ser Lys Arg Cys
350
TATCTA ATT AACATCAACA ~rn7~ ~AlrA TCATGATGTA TATTGTTGTA
45 ~ a~r~ ,,, ~C TATTAAAAAA AAAAATGATC r.A~CG ~Ar'G~.
~rt~ 1337
50 ~2) INFORNATION FOR SEQ ID NO:40:
(i~ SEQUENCE ~ T~ i,S:
(A) LENGTH: 374 amlno ~cids
(D) TOPOLOGY- linear
( ii ) NOLECllLE TYPE: protein
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:40:
Met Asp Ser Pro Cys Leu Val Ala Leu Leu Val Phe Ser Phe Val Ile
--21 -2 0 -15 --10
Gly Ser Cys Phe Ser Asp Asn Pro Ile Asp Ser Cys Trp Arq Gly Asp
Ser Asn Trp Ala Gln Asn Arq Met Lys Leu Ala Asp Cys Ala Val Gly
Phe Gly Ser Ser Thr Met Gly Gly Lys Gly Gly Asp Leu Tyr Thr Val
30 35 40
41
-
W0 95/27786 2 1 ~ 7 3 3 6 ~ 49
.
Thr Asn Ser Asp Asp A6p Pro Val Asn Pro Ala Pro Gly Thr Leu Arg
Tyr Gly Ala Thr Arg Asp Arg Pro Leu Trp Ile Ile Phe Ser Gly Asn
60 65 70 75
Net Asn Ile Lys Leu Lys Net Pro Net Tyr Ile Ala Gly Tyr Lys Thr
Phe Asp Gly Arg Gly Ala Gln Val Tyr Ile Gly Asn Gly Gly Pro Cys
95 100 105
Val Phe Ile Lys Arg Val Ser Asn Val Ile Ile His Gly Leu Tyr Leu
15 Tyr Gly Cys Ser Thr Ser Val Leu Gly Asn.Val Leu Ile Asn Glu Ser
2 Phe Gly Val Glu Pro Val His Pro Gln Asp Gly Asp Ala Leu Thr Leu
Arg Thr Ala Thr Asn Ile Trp Ile Asp Hi6 Asn Ser Phe
160 165 Ser Asn Ser
25 Ser ~8p Gly Leu Val Asp Val Thr Leu Thr Ser Thr Gly Val Thr Ile
19 0 19 5 2 0 0
Asp As5p Ala Tyr Ser Asp Asp Lys Ser Net Lys Val Thr Val Ala Phe
Asn Gln Phe Gly Pro Asn Cys Gly Gln Arg Net Pro Arg Ala Ar
220 225 230 g 2Ty35r
Gly Leu Val His Val Ala Asn Asn Asn Tyr Asp Pro T
2 4 0 2 4 5 rp Thr l e Tyr
40 Ala Ile Gly Gly Ser Ser Asn Pro Thr Ile Leu Ser G
255 260 lu G15Y Asn Ser
Phe Thr Ala Prc> Asn Glu Ser Tyr Lys Lys Gln Val T8hrO Ile Arg Ile
Gly Cys Lys Thr Ser Ser Ser Cys Ser Asn Trp Val Trp Gln Ser Thr
50 Gln Asp Val Phe Tyr Asn Gly Ala Tyr Phe Val Ser Ser Gly Lys Tyr
Glu Gly Gly Asn Ile Tyr Thr Lys Lys Glu Ala Phe Asn Val Glu Asn
320 325 330
55 Gly Asn Ala Thr Pro Gln Leu Thr Lys Asn Ala Gly Val Leu Thr Cys
Ser Leu Ser Lys Arg Cys
350
(2) INFOF~ATION FOR SEQ ID NO:41:
(i~ SEQU~NCE ~'MI~l?~l I ..r ) .'~ I lCS:
(A) LENGTH: 30 amino acids
(B) TYPE: amino acid
( D) TOPOLOGY: linear
(ii) NQLECULE TYPE: peptide
(v) FRAGNENT TYPE: internal
42
WO 95/27786 . 2 1 8 7 3 3 6 r~ 219
.
(xi) SEQUENCE l.:~S~l~l~llUI~I: SEQ ID NO:41:
Lys Met Pro ~et Tyr Ile Ala Gly Tyr Lys Thr Phe Asp Gly Ar~
5 10 15
Ala Gln Val TYr Ile Gly Asn Gly Gly Pro Cys Val Phe Ile
( 2 ) INFORMATION FOR SEQ ID NO: 42:
(i) SEQUENCE rT~RA~
A LENGTH: 21 amino acids
B TYPE: amino acid
C S~Rr : singl
D TOPOLOGY: linear
(ii) NOLECULE TYPE: peptide
(xi) SEQUENCE J~ u~(lrllu~: SEQ ID NO:42:
Asp Glu Ars Thr Ala Thr Asn Ile Trp Ile Asp Uis A~n Ser Ph
25 1 s lo 1S
Asn Ser Ser Asp Asp
30 ~2) INFORMATION FOR SEQ ID NO:43:
(i) SEQUENCE rT~R~
(A) LENGTE~: 30 amino dcids
(B) TYPE: amino acid
35 (D) TOPOLOGY: linear
( ii ) NOLECULE TYPE: peptide
(v) FRAGMENT ~YPE: internal
(xi) SEQUENOE ~ u~rllur~: SEQ ID NO:43:
Asp Val Phe Tyr Asn Gly Ala Tyr Phe Val Ser Ser Gly Lys Tyr Glu
Gly Gly Asn Ile Tyr Thr Lys Lys Glu Ala Phe Asn Val
2 0 2 5 3Golu
50 (2) INFORNPTION FOR SEQ ID NO:44:
(i) SEQUENCE r~T~R~ 5
(A) LENGT~: 2 6 amino acids
(B) TYPE: amino acid
( D ) TOPOLOGY: l inear
( ii ) D~OLECULE TYPE: peptide
(v) FRAGNENT TYPE: internal
(xi) SEQUENCE l/~ ~l~LlU~: SEQ ID NO:44:
Asn Gly Ala Tyr Phe Val Ser Ser Gly Lys Tyr Glu Gly Gly Asn Ile
Tyr Thr Lys Lys Glu Ala Phe Asn Val Glu
43
W0 95127786 2 1 8 7 3 3 6 ~ 2 19
20 as
~2) INFORNATION FOR SEQ ID NO:45:
(i) SEQUENCE rlTARA~
(A) LENGTH: 24 amino acids
(B) TY~E: amino acid
(D) TOPOLOGY: Linear
0 (ii) MOLECULE TYPE: pe~tide
(v) FRAGNENT TYPE: inter~al
(xi) SEQUENCE L)~ UKl~ ll JN: SEQ ID NO 45
Asn Gly Ala Tyr Phe Val Ser Ser Gly Lys TYr Glu Gly Gly Asn Ile
Tyr Thr Lys Lys Glu Ala Phe Asn
(2) INFORNATION FOR SEQ ID NO:46:
(i) SEQUENCE 'TTARA~ ll~S:
(A) LENGTH: 28 amino acids
(B) TYPE: amino acid
(D) TOPOLOGY: linear
( ii ) NOLECULE TYPE: pe~tide
(v) FRAGNENT TYPE: internal
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:46:
Asp Glu Asn Gly Ala Tyr Phe Val Ser Ser Gly Lys Tyr Glu Gly Gl
5 10 15
Asn Ile Tyr Thr Lys Lys Glu Ala Phe Asn Ala Glu
(2) lNrU___.'lLUN FOR SEQ ID NO:47:
( i ) SEQUENCE rT~ ~ R ~ I .Y l L~_S:
(A) LENGTH: 2 9 amino acids
(B) TYPE: amino acid
( D) TOPOLOGY: linear
(ii) NOLECULE TYPE: ~eptide
(v) FRAGNENT TYPE: internal
(xi) SEQUENCE llr;~L~llul~: SEQ ID NO:47:
As~ Glu Glu Asn Gly Ala Tyr Phe Val Ser Ser Gl Ly Gl
1 5 10 Y s Tyr u G y
Gly Asn Ile Tyr Thr Lys Lys Glu Ala Phe Asn Val Glu
60 (2) INFORNATION FOR SEQ ID NO:48:
(i) SEQUENCE f'T~ARA( ~ b:
(A) LENGTH: 27 amino acids
(B) TYPE: amino acid
65 (D) TOPOLOGY: linear
44
~ WO 95l27786 ~ 2 1 8 7 3 3 6 r~ 49
(ii) MOLECULE TYPE: peptide
(v~ FRAGMENT TYPE: internal
(xi~ SEQUENCE IJli~ N: SEQ ID NO:48:
Asp Glu Gly Ala Tyr Phe Val Ser Ser Gly Lys Tyr Glu Gly Gly Asn
0 l 5 10 15
Ile Tyr Thr Lys Lys Glu Ala Phe Asn Ala Glu
5 ~2~ INFORNATION FOR SEQ ID No:49:
(i~ SEQUFNCE rTT~ LlU ~ '
~A~ LENGTH: 20 e~mino aclds
(D~ TOPOLOGY linear
(ii~ ~OLECULE TYPE: peptide
(v~ FE?AG~T TYPE: internal
(xi~ SEQUENCE Lli il I~LI"l'lUN SEQ ID NO:49:
Leu Ala ASp Cys Ala Val Gly Phe Gly Ser Ser Thr Met Gly Gly Lys
Gly Gly Asp Leu
35 (2~ mTr)N FOR SEQ ID NO:50:
(i~ SEQUENCE i~U~RAI rr ~
(A~ LENGTH: 20 amuiI10 ~cids
( B ~ TYPE: a~ino acid
(D~ TOPOLOGY: linear
(ii~ NOLECULE TYPE: pe~tide
(v~ FRAGMENT TYPE: intern~l
(xi~ SEQUENCE ~ : SEQ ID NO:50:
Leu Phe Phe Asn His His Lys Val Det Leu Leu Gly His As~ Asp Ala
Tyr Ser ~Gp Asp
55 (2~ INFORNATION FOR SEQ ID NO:51:
( i ~ SEQUENCE t~u~
(A) LENGTH: 20 a_ino acids
(~ TYPE- a.m~
(D~ TOPOLOGY linear
(ii~ I!OLECULE TYPE: peptide
(v~ FRAGNENT TYPE: internal
(xi~ SF,OU~NCE J~SS~l'~ lUN SEQ ID NO:51:
W0 95127786 2 1 8 7 3 3 6 r~ 749
Ly~ Ser Net I,y6 Val ~hr Val ~la Phe Asn G1n Phe Gly Pro Asn
5 10 Cys
5 Gly Gln Arg ~et
2(~
46
.