Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
Wo95/32051 2 ~ 8 7')33 p ",,~ .Q
PERVAPORATION PROCESS WITH REDUCED DRMNG FORCE
FIELD OF THE INV~TION
The invention relntes to ~ More pnrticularly, tbe inV0tion relates to improved
5 ~ ~ ., processes in which the advase effects of polnrization are reduced
BACKGROUND OF THE INVENTION
is n -I~J process used to separnte solutions on the basis of diffaences
m the volnlilities or diffusion ~ of the comporlents. A liquid feed solution contacts one side
of a merrbrnne; tbe pamente is removed as a vnpor from the otha side. Transport tbrough the membrane
10 is induced by the difference in partial pressure between the liquid feed solution and the perlnente vnpor.
This pnrtial vapor pressure diffaence cnn be maintained in sevaal wnys, such as drawing n vacuum on
the permeate side of the systen~, Sweeping the permente sidk with n carrier gas, or simply cooling the
permente vapor, cnusing it to condense.
Whether carrying out ~ , experiments in the Inborntory or op~rnting larger-scale
15 systems, it is conventi~l to wk with n low pressure on the pamente side, and it is also common to heat
the feedstrenm to some extent, to increase the driving force across the me~nbrarle. Tnbk I lists some
typical conditions identified in n vnriety of refaences.
TABLE 1.
RefSep-r tion Membr ne Feed Perme te Pressure
temp. preAure r tio
T~lpe Thickness (C~
20 1~ ' ' ' PVA 10 llm 60 up to 200 mbar
(20 kPn)
2I;~w~ MTR100 40 15 torr 4
MTR200 (2 Wn~
3; ~/~. PDMS 70 10mbnr (I kPn) 30
4aroma/wnter PDMS 10 ,um 30-60 5x10 2 - 50 mb 4-400
<5 IiPn)
. ` 5 wnta/lPA TexSep3D 70-95 2-189 mmHg
(0.3-25 kPn)
25 6~ ~,' ' 100 -12C
7TCE/wata various 80-150 ~,lm 25 <3 rnb 10
i ' '~ dastomer (<0.3 kPn)
8 ~ ' ' ' GFT 70-93 -20C
`933
WO 951320SI ~ O
RefSep ration Membr~me Feed Perme te Pres~ure
temp. pressure r~tio
Type Thicknegg (nC~
9 ~PDMS 35 20 mb (2 kPa) 3
10aromatic hc/water PDMS 1-170 l~m 0.5-2 mb 13
Pebax 50 1-10 mb (<I kPa)
PDMS165 pm 23 <I tolT(0.1 kPa) 20
512 ,''/~ Pebax 42 pm 45 13Pa 700
13 1 ' "~ Pebax 1-2 mil 50 5-lO tolr 10-20
(0.6-1.3 kPa)
14 ~'gl$ ' PVA 80 5 tolr (0.6kPa)
15 , . A . PVA 65 5tolT (0.6kPa)
16 water/ PVA 60 21olr (0.3kPa)
MEK-tolucne
10 17v~lrious various 1-11 mil 25-70 <0.1 tcrr 200-
(0.01 kPa) 2,000
18I~w/~ 40 15torr (2kPa) ~4
19TCE/water MTR 100 30-58 5-40 tC~ 30
. ' ' ~ MTR 200 (0.6-5.3 kPa)
~, ~
20various MTR 100 40 10-15 torr >4
'~MTR 200 (1.3-2 kPa)
21ethanol/MEK various 25 9.5-50 torr
(1.3-6.7 kPa)
22 ~ PDMS 2 llm 30 up to 6cmHg 1-15
(8 kPa)
23 various butadiene 200-300 30 1~3 mmHg 1-40
U'L '~ copol$mer pm (0.1-5.7kPa)
24TCE/water PDMS 165 pm 20 3 to~r (0.4 kPa) 6
25 , ~PDMS 2.2 ~un 30 0.4-4cmHg 1.5-15
',' '~ I pm 30 1.5-3 cmHg
(up to 5.3 kPa)
26 ~ PEI 25 133 Pa
27 ~ ' ' oil 50 120 mb (12 kPa)
28 TCE/watcr PDMS 165 pm 25 <I tolr (0.1 kPa) 24
29 TCE/watcr various 5-70 pm 25 <3 mb (0.3 kPa) 10
.. ~ . . . . . ..
1 7933
WO 95/32051 2 8 ~ r~
l~rom tbe table, it can be seerl tbat typicaily, permeate pressures are low, such as n few torr, anci
a typiced feed tempersture migilt be 60C. Typicai pressure ratios for wori~able processes are up to 100
or more. Ti~e c~... ' belief in tile art is that the iower the permeate pressure, the better is tbe
separation I r Many references can be identified that support tilis beiie For e~cample:
5 1. A paper by Brurl et d. (J.-P. Brun, C. Larchet, R Melet and G. Buivestre, "Mo~ielling of the
P~. ~ ~ of Birlary Mixtures tilrougb Moderately Swelling, Non-Reacting Membrarles", Journai of
Membrane Scierlce, Vol. 23, 1985, pp. 257-283) modeis ti~e ~ v~ aLull process. Figure 2 shows a
cdcuiated plot of ~ va~vldLvll selectivity (separation factor) as a function of permeate pressure. The
f gure sbows a sharp deciine in separation factor with increasing i~neate pressure. Figure 3 dso shows
10 tilat bigi~er pameate pressures resuit in poora separabon.
2. A pap by Rautenbach et ai. (nThe Separabon Potenbai of P~ ~alJvl~vu. Part 1. Discussion of
Transport Equatirms and Comparison witb Reverse Osmosisn, Journd of Membrane Scierlce, Vol. 25
(1985presentsadiscussionofthe~ . transporte~inaborlsandagaininclu~iesgraphs(Figures
8 and 9) that sbow separaborl factor faliing with increasing permeate pressure.
3. US. Patent 4,941,976 recommends a permeate pressure of 10 tr~rr (1.3 icPa), U.S. Pntent 4,935,144
' 5 torr (0.7 icPa), and US. Patent 5,004,861 recr~nmencis 2 torr (0.3 icPa).
4. A paper by T ' ' (Reference 1) ddscusses factors that determine ovali mass transport in
~, and concludes, amongst otber conclusions, that the permeate pressure shouid be as low as
possible (Figure 20).
20 5. A paper by Lamer d ai. (Reference 4) repc rting on the ~ . of arc~na compounris shows a
decrease of selecbvity ~om over 160 to d~out 25 as drlwnstream permeate pressure rises from 0.1 mbar
to 50 mbar (0.01-5 icPa).
6. A paper by Rcde et d. (Rcfi ~ce 5) r~n rernovai of water or methanol from r)rgaluc compounds SilOWs
in Figure 7 how the permeate of isopropanol (the rejected component) increases with
25 _ ~ pressure and si~ows that a signif cant loss in paformance occurs beginning at about
40 torr (5.3 icPa).
7. A paper by Neel et ai. (niniuence of Downstream Pressure on the r~. . of Water-
T ~, . r Mixtures tbrwgh a Regenerated Ceiiuiose Mennbrane (Cupropi~an), Jrlurnd of Membrane
Science, Vol 2? (1985 exanunes the infiuence of dc~ms~ream pressore on clehydrabon of: , r
30 thrc)ugh celiuiosern~nes. ~n this case, membrane sweiiing plays a big part in determining separatir~
properties. Agam, ho~vev, loss of perforrrlance as perrneate pressure increases is repc~rted (Figure 4).
In di membrane processes, other factors than the membrane properties may infiuence the
21 87~33
Wo 95132051 i ~~ 0
separation As with any fluid fiowing across A surface, the velocity proSle irl a feed solution subjected to
r~i~ i8 not constant acro8s tbe tbickness of the solution layer, because of friction at the
interface. The feed solution velocity decreases as tbe distance from the membranc
surfuce decredses and a stagndnt bourldary layer is present near the membrane surface. The solution
, is urlifo~m o~side tbe stagrldnt boundary Idyer, because the 'dow is turbulent. However, the
flow in the bourldary layer is laminar, producing a profile across this layer as tbe faster
perrncating components are removed 1 6 '~ tbrougb the membrane. The boundary layer acts as
an additional resistance, in series with the rnembrane, to trarlsport of material from the bulk feed to thc
perlneate side of the membrane. If the membrane has the capacity to transport a component faster tharl
the diffusion rate of that component into the bourldary layer, tne depleted boundary layer may in fact be
the d~nant rcsistarlce to mass transport, thereby diminishing the driving force across the membrane arld
reducing rr~embrdne separation performance sigrlificantly. The effect of polarization is that
complents that are euriched in the permeate are depleted in the boundary layer, arld cornponents that are
depleted in the permeate are enriched in the boundary layer. hn other wards, - polarizatior
warks against the separation achieved by the membrane, and should be avoided as much as possible.
Boundary layer polarization problerns are exacerbated by a low feed flOw rate, a
low feed ~ of the faster permeating component and a high separation factor het veen the
~npcn~ts to be separalal ~ , " separations, such as the removal of smaD
amoumts of organic from water, meet orle or several of these criteria. Since the water
is high both in the bulk fecd and in the boundary l~yer region, bourlddry layer effects do not
affect ~ i . adv~ely. However, the ~ separation factor is of len very high, such as
irl the hur~cds or ths~sands, arld the: of the orgarlic in the water is often low, such as in the
ppm range, so the ef~cts of ~ polarization on the orgal~ic camponents that are to he removed
are often very severc.
The stdndard way of mirmnizing polarization is to maxirnize mixing at the
merrlbrane surfilce, that is to mir~irnize tbe tbichless of the boundary layer. This can be done by inereasing
the fe~d nuid velocity, ' , I turbulence. In practical systems, howcv, there is a limit to the
extent to which ~lis can be done.
ln additian, recent references that discuss polarizatian in ~A_ . .."~Lu-l malcc the
30 point that, if the feed nOw rate cannot he made large enough to ovcrcome . polarization
problems, as is ofterl tbe case, another tdCtiC is to use a tbiclrer membrane. F example, ~Mass TrarJsfer
1'1 of ~ New P~ ~, Module for Water Purification", ~ papcr presented by Goading,
,
~ ~ 87933
WO 9S/32051 ~ '~Q
Hickey arld Crowder at tbe Fifth Internatiorlal Corlference on P ,. ~ Processcs in the Chernical
Industly, lists the factors thnt nffect removal of organics from dilute nqueous streams ns: permente
presQ~; Her~s Law coefficient; feed ~ rnembrarle thickness; and liquid pbase mass transfer
cceffcient. It furthcr states thnt:
5 (n) Permcnte pressure must be maintained at a low Ynlue,
(b) A large Henry's Law coeffcient increases drivirlg force for ~ l of that component and
reduces membrarle resistance,
(c) Thc membrnne should be reasorlably thin, but, unless thc mass transfer ccefficient in the liquid
boundary Inyer is large, the bourldary layer carl ensily dominate the total mass transfer resistance, so thnt
10 making the membrane thirmer or more ~ " I ' ' is fruit1ess.
An earlier paper erltitled by Coté and Lipski (Reference 11 ) expresses similar thoughts. If one
is dealing with volatile, hydrophobic compounds (that is, the Herlry's Law coefficient is largc) the
separation is generally ~ ' and, if it carmot be made ' ' ' by increasing
feed f ow rate and turbulerlce, cne is better off using a thick membranc than a thin rnembrane, bccause the
15 thick membrane will give a better separation factor for the same mass transfer rate. These ideas are
reiterated m "The Use of F~ ~ . f the Removal of Or~uuc C~ from Watcr~ (Refercnce
28), again by Lipski and Coté; in "A T ' EA ' Evaluation of P~ . ., for Water
Treatment~, a paper presented by Coté and lipski at the Fcurth International Conference on ~ a~ul aL;wl
Prccesses in tbe Chernical h~dustry; and in U.S. Patent 5,167,825. Each of these teaches that a relatively
20 i' ' ' yields bet~er separâtion performance of organics from water because the thick mcmbranc
decreases the water f~ux to a greater extent than it decrcases the VOC flux, thereby improving the
separation factor. As is pointed out in 5,167,825, rubbery membranes are preferred for separating
~lrgsnics fr~m water and very thick rubbery membranes are difficult to make. hn '825, the solution to this
problem is to irnpregnate the rubbery selective polymcr irlto tbe pores of a microporous support, thereby
25 producing a selective layer with an effective tbickness of as much as 200 llm.
In thcse references and other analyses of polarization effects in ~ at~ulaLiuu~
permeate prcssure is generally ignored, or its effect on polarization is not recognized For
example, a poster by H.H. Nijhuis et al. (I ' Symposium cn Syrlthetic Membrancs in Science
and Industry, T~bir~, Germany, 1989) givcs an analysis of mass i ' on removal of trace
30 d from aqueous solutions that assumes that the permeate prcssurc is zero. Likewise, the doctoral
thesis of H.H. Nijhuis (Reference 29) lists factors affecting ~ . - I r as feed
boundary layer mass transfer coefficient, membrane permeability and membrane tbickncss,
2 1 ~7~33
Wo 95~ 51
but does rlot list or discuss d~iviog forec effe~s. In two of tbe three chapters that mskc up the
C r ' ' ~ porbon of the thesis, permeate pressure is not even menboned A paper by Psaume
(Reference 24~ focuses on tbe noporlance of poluizabon in removing orgamcs fron~ water,
but ignores the effects of permeate pressure in the analysis.
To ourh~ an, ' ' _ ofthe imptant effect of pelmeate pressure on
polu zabon in certain ~ separabons has not previousb been tvailable to the ut.
SUMMARY OF THE INV~TION
The invenbon concerns separabon of liquids with high relabve volablibes. One example is the
separsbon of vola~le ganic compounos (VOCs) from water. Other examples include separabon of low
molecular weight, high volablity organics from high molecular weight, lower volablity organics,
dehydrabon of hydrophobic or low volatility orgaoics, and removal of dissolved gases, parbcularly air or
oxygen, from water or other liquids.
~ such processes, we hsve discovered that reducing the ' oriving force, by raisnng
the permeate pressure andlor loweling the feed temperature, actually increases the separabon factor. This
is a surprising result, which is contrary to convenbonal teachings about membrane proresses, as will be
explained in the detaileo descripbon that follows.
This discovery providcs a useful and beoeficial advance in }~ . tec~nology. Thesc
teachings can be adopted in an improved ~ u~ ativ~ process by carryiog out the process at higher
permeate prcssure or lower feed temperature than was previously n~mal pracbce. Io parbcular, thc
inveobon iovolves ca[lyiog out the ~ . . process at a pemmeate pressure greater thao about 50 torr
(6.7 kPa), aod preferably Nen higher, such as 8reater than about 60 torr (8 kPa) or 100 torr (13.3 kPa).
In another aspect, thc invenbon involves carryiog out the ~ . proccss at a feed
temperature no greater than about 60C, and preferably no greater than about 50C.
In another aspect, the noveobon nnvolves c~yiog out the ~ . process at a lower
pressure rsbo than was previously normal pracbce. ho parbcular, the invenbon involves carrying out the
~. proccssatapressurerabolowerthannbout4,preferablylower,suchasbelow3,andmore
preferabb eveo lower, such as below 2. Depending on the vapor pressura of the compooents to be
se~ed, pr~ssure rabos within tbae raoga csn be obtained by using a permeate pressure greater than
~bout 35 torr (4.7 kPa) in conjuncbon with a fe~d temperature lowcr than about 55C, a permeate
pressure greater than about 50 torr (6.7 kPa) with a feed tempersture lower than about 60C, or a
permeate pressure greater than about 70 torr (9.3 kPa) with a feed temperature lower than about 50 C,
for example.
21 87933
WO 95/32051 F~~ Q
iA~ . _, processes in accordance viith the invention have the following advantages:
1. ('. Dolarization Droblems can be ameliorated
Tbe ir~veDtiOn provides a simple te~'DDique tokeep bouDda~y layer resistance effec,ts under control
in certain ~ v . processes.
5 2. Fn~ r~v efflcicencv
(a) A low feed mav be used
Tbe lo v d~iving force required to esrTy out tbe process can be achieved by opernting at low feed
temperature, high permeate pressure or a combination of both. It is not unusual for ' ' feed
for ~~~ _. to be as high as 80C or even higber. By operating under mild feed
10 temperature conditions, the energy required to heat tbe feed liquid is - -~ small.
A second advantage is tbat many polym membranes are not resistant to high , By
operating at . below about 60 C, even the most . . _ membrane materials
can be used
A tbird advantage is tbat feed streams containing labile compounds, sucb as may be encountered
15 in tbe food or r- " ~ industries, for example, may be treated
m A higher D0meate Dressure mav be used
~ n tbe past, ~~ ~, processes have often been carried out at very low permeate pressures,
such as 20 torr (2.7 kPa), 10 torr (1.3 kDa) or below. For laboratory-scale reseDrch , low
pressure on tbe permeate side is often obtained by means of a vacuum pump. Tbis pumD removes the
20 totality of tbe pen~ate stAeam, including condeDsable vapors and rn~: ' ' ' gases, sucb as air, tbat
may have been dissc,lved in tbe fi ed liquid or have leaked into tbe apparatus. Tbe need to pass everytbing
tbrougb tbe v= pump makes tbis mode of operation difficult except for small-scale laboratory use.
I~rger systems often obtain partial vaA~wm conditions on the p0meate side by using a condenser
to liquefy tbe coDd0Dsable vapor, so tbat tbe pressure on tbe pemmeate side is determined by tbe
25 equilibrium vapor pressure at tbe cond01ser temp0ature. Such systems still need a v= pump to
remove DU..: ' ' ' gases, v~bich are almost always present to wme ext01t~ but tbe load on tbe
vacuurn pump is much reduced ~' ' ' to reach a pressure as low as a few torr, tbe condeDser
~ A bas to be substan~allybelow ambient t0Dperature, w tbat cbilling or ev0n refrigeration
may need to be empl~l In tbe case of aqueous soh~s, tbe p0meate D~mally camlot be cbilled below
30 0C. In tbe case of solutions v~itb lower freezing points, sncb as ' "~ mixtures, for example,
lower condensation i . sucb as dovin to -20 C, can be and have been employed For example,
~ paper by JL Rapin, eDtitled "Tbe B0beniville P~ ~ r " Unit for tbe First Large-Scale Prn,ductive
21 8~3
WO 95/32051 .~ u
Plant for tbe Dehydration of Ethanol" (Reference 8) deseribes tbe first GFT ethanol debvdration plant in
ichthree condensers operating at i , of 10C, -5^C and -20C v~ere used and in v,lhich tbe
viere reached by refrigeration v~ith Freon 22. The lov~er tbe c^wling temperatD tbat is
used, the greater is the energy expenditD.
Anotber issue is the presence of the w : ' ' '~ gases. Suppose, for exAmple, tbat the
desircd permeate pressD is 20 torr (2.7 L~Pa). At a particular e~,milibrium conditiorl deterrnined by
temperature, this total permeate pressD my be maoe up of 19 torr (2.5 kPn) partial pressD of
' ' and I tvrr (0.01 kra) of non~densable gases. In tbat case, however, tbe off-gas vented
frvm the vacuum pump that is removing tbe non ' ' ' will wntain 19 palts of vapor to every I
10 part of l.v~ ' ' ' gas. To prevent tbis vapor loss, it my be necessary to sct the
tempaature even lower, so tbat tbe ~ e~ntributcs a s naller fraclion of tbe tvtsl pressure. This
my mean that, to obtain a total permeate pressure of 20 torr (2.7 Icra), tbe condenscr temperature my
have to be set to correspond to an e~uilibrium vapor pressure of only 10 torr (1.3 kra) or less, for
e~A~ample.
In contrast, permeate pressures of 50 torr (6.7 kPa), 60 torr (8 I~Pa), or above can usually be
obtained by wnd~ion at i , of S-25 C. Bosides saving ener~y, tbis substantially sunplifes
tbe eq~2~4 si^ce large refrigeration urlits or large vacuum pumps are not needed, and thereby imprvves
operating reliability and decreAses evsts.
3. rh~ fr^^^~:^nisn ~ . n as "
Tbe principal product of a ~ , prwsss may be the residue stream, or the permeatesheam, or both may be importar~ If tbe goal of tbe process is to remove an unwanted ~ such
as an org~nic solvent from an i~ial wastewater stream4 tben tbe, elean residue stream is tbe prw~uct,
and tbe 1~ : ' ' permeate is a secondary waste stream. The more concentratcd in solvent is tbis
strear4 the easi it oftcn becomes to carry out further treatmcnt, such as to recover usable prc,ducts. If
tbe st,^~am has to be &sposed of, tbe4 generally, tbe smaUer the volume, tbe lower are tbe &sposal cvsts.
If the product is the permeate stre^r4 then it is usually tbe case tbat the higher tbe purity, tbe
g~ater is the value of the product, so bigh in a srnall volume is again desirable.
4. Ve~v ' ' I r~i r.^t be l.c. A
As &scussed in the Baclc~round section above, one way to handle polarizatioh
problems is to use tbick mernbranes. To separate organic wmpo,unds frvm water, rubbery mer. branes
wilhaneffectivetbicknessasmuchas200,umarcadvocatedinU.S.Patcnt5,167,8;aS. Inaprvgramthat
r,ueG,ed ~ large number of dastnmeric membrûne materials for tbeir VOC/water separating properties,
2~ ~f7~
Wo 95/32051 ~ Q
~ NIJbuis et at. (Refereuce 7) used merrlbrane stamps with a tbickness of 60-80 ,um for silicoAe rubber
and 80-150 ,um for the otber materials.
The processes of the invention provides a tecbnique tbat relies on driving force, rQther tban
membr~me thich~ss, to circumvent ' polarization problems. The processes of the invention
5 ean, tberefore, be carried out using ar~y membrane of any convenient thickness tbat otben-vise fits ttle
. ' of the separation.
It is an object of the inverdion to provide ~ . . . ' proccsses in wbich the adverse effects of
' polarization are reduccd
It is an object of tbe invention to provide ~,~.. v r '- processes in which very low pameate
10 pressurcs need not be used.
It is an object of tbe invention to provide ~ . . . ' processcs in wbich very low condenser
need not oe used
It is an object of tbe inverdion to ,m~vide ~, . ' processes in which bigh feed i
necd not be used.
It is Jn object of the invention to provide ~ " processes suitable f~ VOCs
from water.
Otber objects and advantages of ttle inv0tion will be apparent from tbe description of tbe
invention to tbose of ordinary skill in the art.
It is to be ur~stood tbat the above sumrnary and the foltowing detailed dcscription are intendcd
20 to e~plsul and iltustrate the invention without rcstricting its scope.
BRIEF l~k~ OF THE DRAWINGS
Figure I is a graph of separation factor as a function of pressure ratio for separation of i ' ' ' ", '
(TCE) from water.
25 Figure 2 is 8 plot of separation fsctor ss a function of fecd temperature for separation of toluene from
water at tbree different fecd ftow rstes.
Figure 3 is a graph of separstion fsctor as a function of feed tcmperature for separation of toluene from
wster,
Figure 4 is a graph of TCE reduction factor ss a function of stage cut at tbree different feed
30 for separstion of TCE wster.
Figure 5 is a graph of sepsrstion fsctor ss 8 function of feed tempersture for sepsrstion of TCE from
water.
~ ~7~33
WO 95/32051 r~ ... 'C-'IX
Flgure 6 is a ~rnph of separatioD factor as a fuDction of pumeate pressure far separation of tolueDe fiom
water d tbree differeDt feed flow rates.
Figure 7 is ~ yraph of TCE reductioD factor ns a fuDction of sta~e cut at t vo differeDt driviDg forces for
separation of TCE from water.
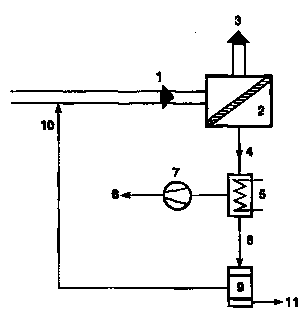
5 Figure 8 is a scheD~atic of the process desigD used for calculatioDs iD Exarnples 18-21.
Figure 9 is a set of gas ' ~ ~ of fecd, residue aDd permeate stream snmples f am a BTEX
removd experimeDt.
Figure 10 is a graph of permeate as a fUDCtioD of feed far BTEX removal
expimeDts at 10 torr (1.3 Wa) aDd 70 torr (9.3 kPa) permoate pressure.
Figure 11 is a graph of BTEX f~ux as a fUDCtioD of feed solutioD for BTEX removal
eXperim~eDtS at 10 torr (1.3 I~Pa) and 70 torr (9.3 kPa) penDeate pressure.
Figure 12 is n set of graphs shoviDg feed and permeate far n set of ~.va~at;experirnents carried out under different driving forces.
DETAILED DESCRIPTION OF THE INV~TION
The invenion is ~ process tbat is npplicable to ~ 5 " sepnrations
' by n higb relntive volntility between tbe components to be separated, and n higb sepnraion
factor. The irlvention is particularly uselùl im tbe separntion of VOCs from wnter, nnd for simplicity, tbe
dacription of tbe invention bdow defmcs one component ns VOC, tbe otb as water. Howev, tbe
20 ir~on is useful for ar~ higb relative volntility set of components and is not to bo construed as limited
to VOC/water separations.
A corne~t way to und~land ~ ~ ~ processes is to nssume tbnt tbe process is divided
imto two sequential steps. Tbe first is evnparntion from tbe feed liqlud to n snturntod v5nor phase; tbe
second is permeaticn tb~ugb tbe membrnne under tbe driving force of tbe vnpor pressure difference across
25 tbc rnembrnne. Tbe snturnted fe~d vnpor phase is n ' '~ tool; no sucb phse actunlly exists.
1. . ' ' tbis npproncb is l; '~ "~ equivalent to conventional treatments, nnd aliows tbe
drivmg force f~. I to be exprcssed as n ~ . I difference acrass tbe membrAne. Tbe fe~d
liquid is in equihbrium witb tbe hypotbctical fecd vnpor wbich, in tum, is im equilibrium witb tbe feed side
of tbe membrlme. According to tbis defirlitian of ~ . _, tbe overall separation uhieved by the
30 process, ,lp~,~, is given by
~ r~
whe ~"~, is tbe evaporative separatian telm, whicb c~n be obtamod fram publisbed vapor-liquid
21 ~933
WO 95/32051 ll I ~1l~., .'C -lx
equilibrium data, and ~ " is the meTnbrane permeation term derived rrom the standard ~ ~i ffilcir~n
model for gas separation using Fick's Law. If tbe separation is inf~uenced by the boundaTy layer, this
GYpression can be written:
The relative volatility of a set of components such as VOC/water is defmed as:
CV ~ tC'",,
where p vx and p ",," are the partial vapor pressures of tbe components of the feed, and c 'vx and c'v,,, are
10 the feed liquid From the Henry's Law ' ' .
P x = Hvx c ~x and p ~,", = H~ . c ",~
the relative volatility is also given by:
Relativevolatility = HVX/H~
Relative volatility is a convenient .,~ . for the ~,~,p term irl the separation factor
15 equation above, because the quantities required for the relative volatility calculation are readily availablG
R~ative volatility and p .~.p ~re identical ir the following equations for the partial vapor pressurGs of the
components of the feed liquid (indicated as quantities) are valid:
P vx = Pvx c vx / c"l and p ~ p".~" c ,,"~,
In separations of interest in the context of tbe invention, the relative volatility of the components
20 tobe separated is bigb, so the p,q, term is large and the overall separation factor p,~,q, is large also. By
high relative volatility, we mean having a relative volatility greater than about 300, more particularly
greater than about 1,000 and Gspecially above 3,000.
We have discovered that, in separations ~ by high relative volatility arld bigh
separation ractor, reducing the i ' driving rorce, T)y raising the permeate pressure and/or
25 lowering the fG~d temperature, actually increases the separation factor. This is an unexpected result,
beeTuse roducing the driving force lowers both the partial prC-csure difTerences and the pressure ratio across
the membrane and, ~ .. 'l~, this would be GYpected to have a negative efrcct on both fiux and
separation I '
According to Fick's Law, loWeTing the component partial pressure difference across the membrane
30 should Iead to a directly proportiorlal decrease in fiux of that component
As desen~ed in U.S. Patent 5,089,033, Figure I and col= 11 and 12, for GYaTnple, there is a
relationship between membrane separation and pressure ratio ~ (total feed I 't.,~l permeate
pT~ssure), such tbat at low pressure ratios, tbat is when ~ << , the enricbment obtained in a separation
93~
Wo 95/32051 12 Y~ Q
is pressure ratio-ljn??ted and is essentiaily iu?ependent of the membrane sepi2rdtion cdpability. Conversely,
at high pressure ratios, that is when ~ ~> 1~, the enrichment obtained is membrane separation-limited and
is esscntially independent of the pressure ratio. Thus, to take advantage of high membrane separation
capabiiity, it is normal to operate at the highest convenient pressure ratios. Yet we have discovered that
5 in some cases, even though the separation factor in ~ lL.U.. process3 can be very high, such as in
tile hundreds or thousands, the separation perfom?ance is not t . ~ but is actuaiiy improved, wher
the pressure ratio is smail compared with the separation factor.
Our strange results appear to arise in situations where poiarization, discussed
4..dl;L~ in the Background section, is a serious problem and v~here the relative volatility of the
10 components to be separated is high. We believe that these unusual effects are seen because reducing the
drivil?g force reduces the tOtZd pcrmcdte f uY thr~?ugh the membrane, which in turn alleviatcs
polarization.
Without wishing to be bound by theory, we suggest the following eYplanation. The equation
commonly used to describe the efTect of the boundary layer in membrane processes iS:
1
A~y ~n
where k is a mass transfer coefficient and the subscripts ov, m and bl refer to overall, membrane and
boundary layer. The polarization equation, derived for _' ~' and revcrse osmosis,
but equally valid for ~ iull is:
~ -- cxp ~/ ")
C -- C (V
where c is , the subscripts b, m and p refer to the buik feed, the mcmbrane feed surface and
the pclmcate, and vp is the vclocity ~ ~.di~ to the membrane surrace ~encrated in the l?oundary layer
by the permeate fiow. Combining these two basic equations, we obtained thc eYpression:
~v,/~u )
xp
~;~v l~n ~ ?~p (3)
v~here E ~ c,/cb is thc cnrichment achieved in the ~ Vll~ iUII proccss. The velocity vp in cmls is relatcd
to thc totai pcrmeatc fiux, ~ in molc/cm2.s by:
vp = J~ P'
30 where p ' is the density in mole/em' of the fecd solution, so that Equation 3 cdn aiso bc written as:
xp / " ) _ I
n ~; Jlol /P I (4)
.....
WO 95132~51 ~ 3 ~ J ~ 8
The boumdary layer resistance term m Equation 4 is, through the term l-I/E, a function of the
separation achieved. If no separation is achieved, E=l and (I-l/E)=0. If E~l, in other words tbat
component is enriched m the permeate, then (I-l/E) is positive and permeation is slowed down by the
boundary layer t~nL Conversely, if E<l, m other words that component is depleted in the permeate, then
5 (I-IIE) is negative amd permeation is enhanced by the boundary layer tcnn. Also, it can be seen tbat the
boundarv layer resistaoce term increases with mcreasmg J~O~
r ~, we have found that, by increasing the membrane thiclmess, it is possible to'~ ' the boundary layer resistance. This is a different conclusion from that obtained from the
basic resistaoce model eqoation. Accordiog to this equatio4 mcreasmg the thickoess of the membraoe
10 iocreases the rolative contribution of the membraoe resistaoce term but has no absolute effect on the
boundaly lay term. We have also fouod that, for enrichiog separatioos such as those of mterest io the
cootext of the mveotio4 reduciog J~O~ reduces the slowiog effect of the boundary lay term.
Reduciog J~0, by re~uciog the pressure differeoce across the membraoe cao be aehieved by raismg
the pem eate pressure, by lowering the feed temperature (aod heoce the feed side vapor pressure) or by a
15 mi~c of the two.
Our preferred method is to raise the permeate pressure, that is to operate the ~. A
process at a permeate pressure higher thao was previously normal practice fcr logh volatility, logh
soparatioofactorscparatioos. hopalticular,theioveotioomvolvescanyiogoutthe~a,uu.aLu..process
at a permeate pressure greater thao about 50 torr (6. 7 kPa), aod prefably oveo hogher, such as greater
thao about 60 torr (8 ~Pa), 80 torr ( 10.7 I~Pa), 100 torr (13.3 kPa), 120 torr ( 16 I~Pa), or oveo 150 tolr
(20 I~Pa). r ~ it is preferred that these prossures be generated, at least ' '~, by
ca~eosiog the permeaLe at a suitable ten~e, aod usiog a vacuum pump only as necessaTy to roloove
' gases. The temperature to which the condeoser must bo clolled to acloeve permeate
pressures of this order depeods, of course, on the vapor pressures of the pormeate . . but will
typically be no lower thao about 5C. It is preferred to operate the condenser at . no lower
tham about 10C, more preferably no lower th~m about 15 C aod most preferabb oo low thao about
20 C. A particular advamtage of operatmg m tlos mode is the low eoergy reqluremeot compared with
caoveotional ~ ~ . For example, if a coodenser temperature of 20 C or above is uscd, this cao
normally he achieved by meaos of am air cooled condenser. If a condenser temperaolre of 10-20C is
used, this cao normalb be achieved by means of coohog with available water.
As am alteroative or .. ' ~ method to reduce the driviog for~e, the process of the
~ 8Y~33
wo 9S/32051 ~ 5 ~ "9
invention a~n be opated under mild feed t~e conditions. It is not unusual for ' ' fevd
for ~ , to be as lugh as 60C, 80C or even higher. The preferred feed
tempahlrefop~a~ngtbeprov~ssvsoftbeinventionisbelo~v60C,morepreferablybelowSOCand
most preferably below 40C.
Of course, tbe permeate pressure and tbe feed temperature should be selected based on
the vapor pressures of the components of interest to give an appropriate separation perfr~mance taking
into account ary otber relevant ~ ' sucb as energy , or costs. In many cases,
bowever, the provssses of the invention are ~ by n low pressure ratio, wbere the pressure ratio
is v*ined as the ratio of tbe total ~ . I on tbe feed side to the total pressure on the permeate side.
P~c~ses ofthe inver~vn may, for example, be charac~rized by pressure ratios as low as 2 or even lower,
sucb as 1.8, 1.6 or even lower.
Anotber aspect of tbe inYention is tbat it provides go3d ~ r r ' ' for heat mtegration. For
example, tf the incoming feed hq ud is cool, tbe incoming feed strvlun may be warmed and tbe permeate
vapor cooled by flowing the streams against one anv~her m a heat exchanger. Since only moderate heating
~nd cooling is needed to carry out tbe processes of tbe mvention, it m~y be possible to provide ~
substantisl portion of tbe drivmg force tbis way, tbereby reducnng expenditure on external heating or
coohng. As an alternative to direct beat excbamge, a beat pump or otber indirect I . ' _ Ig
mecbanism can be used. As just one specific example, tbe feed strcam clm be used to cool an electrical
cbiller, whicb m tum provio~s tbe codant for permeate ' The feed stream is tben fed tnto tbe
~ ~ . r ' system via a heat recovery heat exch~mger and a heater.
The memhranes used m tbe invention m~y take tbe form of a I _ membrame, an
asyn~ric membrane, a multilayer composite membrane, a matrix . a gel or hqmd layer, or
ary odl^vr form known m the arL Ary ' material m~y be used. F separating orgamc
compourlds from water, rubbery I ' .v Iayers are prefer ed. Suitable rubbery materials are
a~iscussed, for example, nn ~Sdection of Elastomeric Membranes f tbe Removal of Volatile Orgamc
C~rnents from Water~ (Reference n For orgamc . ~/~ separations, we prefer membranes
made from siliconv rubb^vr ', ' r ~ ylv~v copolymers. For otber separations, ;ubbery or glassy
membranes as appropriate to tbe separation mar be ured.
Tbe membrames oan be incorporated into membrane modulvs of ary convenivnt type, such as
spiral-wour~, potted hollow-f ber or ~ ~ , r To carrr out tbe procvss of tbe invention ~e
i~odsh~n is introduced mto an array of one or more membrane modules and flows across tbe feed svrface
of tbe membr~mc. The nv~ portion of tbe feedstrcam ts removcd as a hq~ud residuc stream,
2 I P~7933
WO 95/32051 15
which is depleted in the ~ (c) Permcate Yapor, emiched in the facter-
permeating , ~O, is withdrawn from the pe~meate side of the membrane. Depending on the
mutudi solubilities of the cornplents to be separated, it is frequently the case tbat the permeate wiil form
a two-pbdse rluxture after ' In the case of separation of VOCs from water, for exdmple, an
5 orgaluc phase and an aqueous phase saturated with the orgaDic will typicaily for~ If the orgaluc phase
is the desired product, this may be decarlted offand the aqueous phase may WA.. '.~ be recycied to
the ~ r ' process, as described in U.S. Patent 5,030,356.
Preferably, the 1--. . . process shouid remoYe at ieast 80% of the faster permeating
~c), more preferably at least 90%, and most preferably 95% or more.
The array of membrare moduies used to carly out the process may form a singie-stage unit, a two-
or muiti-stdge unit, in which the permedte from one stage becomes the feed to the next, a two- or mniti-
step unit, in which the residue from one step bewmes the feed to the next, or; ' thereof.
The invention may be appGed to any solution contaiDing components ~ by a high
relative volatiGty. Exdmples include separation of low molecuiar weight, high volatility organics from
high molecuiar weight, lower volatiGty orgaDics, dehydration of hydrophobic or low volatiiity organics,
and removai of dGssolved gases, particuldriy air or oxygen, from water or other Gquids. We particuiariy
iDtend the invention to apply to separations where an organic component is l '` ".~ removed either
from water or from another orgaDic component. It is believed that the invention wiil be found to be
especiaily usefui in the removai of VOCs from water, snch as as is needed in surface- or groundwater
remelGation or industriai wastewater treatment, for example.
r . . orgamc materiais that may be separated from water or each other by the process
ofthe mvenlion inciude, but are not hmited to, aiiphdtic 1~1 ' such as snch rts hexane, octane or
decdne; aromatic l" ' ' , such as benzene, toluene and xylerle; haiOgeDdted I, ' ' such as
' ' ', ' , i ' ' ~ , i ' ' ' or chiorinated " ' esters, such as ethyl
acetate or bn~yl acetdte; i~etones, snch as methyl ethyl i~etone; aicohols, such as butanol, hexanol or
octanol; naphthas; terpenes; and the hke.
if the separation tDdt is to be canied out is tDe removai of a VOC from water, the relative volatiGty
may be expressed by means of the HcDry's Law ccefficient. The process of the invention is particniariy
usefui for removing organic components having a Hemy's Law coefficient at 25C greater than about
2 x 10~ l/L 1, especiaily ~ose hdving a Hemy's Law ccefficient at 25C gredter thdn about
6xlO~l 'IL 1,moreespeciailythosehdvingaHer~sLawccefficientat25Cgreaterthanabout2 x 10~ /L
~1 ~7~3~
WO 95/3205~ /u,,,~
16
Thc processes of the inventian can d~ be carried out in conjurlcti~n v~ith the
processes descrbed in a co-owned and copending application entitled "Higb Flux
E~ ., Plocess" (seriai number not yet assigned), the spccification of v~hicb is incorporated herein
by reference irl its entirety. bl tbat case, the inventions togetber wili provide processes tbat reduce
polari~ation effi~cts both by v~i~ing in an optimum membrane thickness range and by usirlg
rno~iest i ' driving forces. In tbis case, it is prefelred tbat tbe membrane tbickness be selected
tobeofsuchathicknesstoyieidai ' ~ofthemorevoiatiieeomponenttbatisatleast70%
of tbe maximum flux of tbat component, more preferably at least 80% and most prefersbly at least 90/O
of the maximum fiux of that component.
The invention is now further illustrsted by the following examples, which are intende~i to show
certain aspects of the invention, but arc not intended to hmit the scope or underiying principles of the
invention in any way.
F~AMPT .F.~
15 EXA~PiFI: i~ELATlVEVOLATlLiTYCALCULAT~iONS
We cala~ated the reiative voiatiiitics of eight different VOCs representing four classes of organic
arnpo~is: aromatic ~ ' ' chiorinatcd ':r ' ' , esters and i~etones. The relntive volatilities
were caicuiated from ~e pure VOC vapor pressure ut 40C and the solubiiity in water according to the
20 e~iuation:
Dlative volatiiity - r.O. / (po.,.,., c~
where p,", and p,",~ are the pUD VOC and pure water vapor pressnres an~i c,O, is the solubility i it of
the VOC in water in mole fraction. The Dsuits are given in Table 2.
Tabie 2
25 ~ b`~ TI
Toluene 0.07 S.9 (7.9) 8,150
T ~ .. ,. 0.11 13.8 (18-4) 16,600
1,1,2-i '' ' 0.48 4.8 (6.4) 1,320
i3utyl acetate 0 57 2.4 (3.2) 490
30 Metbylenechioride 1.32 76.6 (102.1) 4,950
Ethyl acetate 8.0 18.8 (25.1) 210
r~ ,' ! 25 17.7 (23.6) Sl
Acetone 100 56.6 ~75.5) 10
2~ 87933
WO95132051 17 r~l~u.,._. '1
EXAMPLES 2-10: EFFECT OF FEED TEMPERATURE
EXAMPLE 2
A set of ~ ~ exp~iments to measure the removal of toluene from water was carried out.
5 A sp~ module e~taining 1.1 m2 of composite merr~ane with a 10 ,um silicone rubber
sele~tivelayerwasusel Thefeedtemperaturewas60Carldthepermeatepressure,providedbymeaos
of a condenser and a hquid ring vacuum pump, was 50 torr (6.7 kPa). The feed flow rate was 3 gpm
(11.4L/min). Theexpain~twasrepeatedforvariousfeed. Theresultsoftheexperiment
are summari2ed m Table 3.
Table 3
(`~ Permeate f~ux Stage cut (D/D) Separation
Feed (ppm) Residue Permeate (%) (kg/m2.h) factor
(ppm)
0.40.04 0.8 0.13 640
13 6.2 0.7 0.6 0.10 780
1543 21 1.9 0.7 0.11 630
270 130 9.8 0.7 0.12 570
The separation factors were reasorlably corlstant m the experiment, even though the feed
varied by 270-fold.
EXAMPLE 3
E~ ~, expannents were carried out as m Example 2, but tbis time at a feed tcmperature
of 45C. The results are given m Table 4.
Ta e4
~ - Permeate flux Stage cut (n/O) Separation
Feed (ppm) Residue Permeate (%) (kg/m2.h) factor
(ppm)
I 1563 1 1 0.2 0.04 1,490
30270160 24 0.3 0.05 1,5 12
The ! A - f ~ was around 1,500 m both cases. Comparmg the data m Tables 3 and 4, it
rn~y be seen tbat the separation factor appears to be improved by a factor of 2-3 by operating at a lower
feed temperature. Table 5 repeats the data for a feed of 270 ppm at 60C and at 45 C.
33
WO 95/32051 ~ u
18
Ta eS
c-tpPm) Permeatc flux Stage cut (%) Separation
tFeed 270 ppm) tkg/m2.h) factor
5 T . tC) Residue Permcate t%)
emp tppm)
45 160 24 0.3 O.OS 1,512
60 130 9.8 0.7 0.12 570
EXAMPLE 4
The expiments of Example 2 to measure the removal of toluene from water were rcpeated at a
f~wrate of S.S Apm t20.8 L/min). The feed tern~aaturc was 60C and the permeate pressurc was 50 torr
(6.7 kPa). The results are summarized in Table 6.
Tabe6
IS C~ - Pelmeate flux Stage cut (/O) Separation
Feed tppm) Residue Permeate (%) tkgfm2.h) factor
tppm)
0.40.07 0.7 0.06 1,280
12 7.6 0.7 0.7 0.06 745
50 32 3 3 0.7 0.06 838
20235135 13 0.8 0.07 843
The separation factor varied in the range 745-1,280.
EXAMPLE 5
P~ ., exp ments were carried out as in Example 4, but this time at a feed temperature
of 45C. The results are given m Table 7.
Table 7
~ Permeate flux Stage cut t%) Separation
Fecd tppm) Residue Permeate (/0) tkg/m2-h) factor
tppm)
73 45 9.0 0.3 0.02 1,700
235155 30 0.3 0.03 2,170
The .,eparation factors were again morc than doubled by operating at a lowcr feed temperature.
Table8repeatsthedataforafecd of235ppmat60Candat45C.
Wo 95/320sl 2 ~ 8 7 9 3 3 ~ rs~l~
Table 8
C~ (ppm) Permcate nux Stage cut (/O) Separation
(Feed 235 ppm) (kg/m2.h) factor
STemp. (C) Residue Permeate (/o)
45155 30 0.3 0.03 2,170
60135 13 0.8 0.07 843
EXAMPLE 6
The exp~iments of Exrmple 2 to measure the removal of toluene from water were repeated at a
nOw rate of 8 gpm (30.3 L!min). Tbe feed temperature was 60C and tbe p3meate pressure was 50 torr
(6.7 kPa). The results are summarized in Table 9.
Ta e9
C~ Permeate flux Stage cut (/O) Separation
Feed (ppm) Residue Permeate (/O) (kg/m2.h) factor
(ppm)
0.60.3 0.07 0.7 0.04 1,820
127.3 1.3 0.7 0.04 1,390
- 200107 16 0.8 0.05 1,320
The separation factor varied in the range 1,320-1,820.
EXAMPLE 7
~ ~ . experiments were carried out as in Exnmple 6, but this time at a feed ternperature
of 45C. The results are given in Tabk 10.
Tab e 10
C~ Permeate flux Stage cut (/0) Separation
30Feed (ppm) Residue Permeate (o/0) (I~glm2 h) factor
(ppm)
100 67 19 0.3 0.02 2,860
200 160 32 0.3 0.02 2,500
The separation factors were about doubled by operating at a lower feed temp3ature. Table 11
repeats the data frlr a feed ! ' " of 200 ppm at 60C and at 45C.
9~
WO gS/3205~ Q
Tab.~ l I
r.(ppm) Pameate flux Stage cut (/O) Separation
(Feed 200 ppm) (lcglm~h) factor
STemp. (C) (ppm) Permeate (/O)
45 160 32 0.3 0.02 2,500
60 107 16 0.8 O.OS 1,323
E~AMPLE 8
Additianal experimlts of tbe type describ~d in ExDmples 2-7 were carried out. This time the data
wae plotted graphically. Tbc resu]ts are sbown in Figure 2. As ean be seen, ' ~ bigher
separation factors were always obtained at the lower feed temperature.
15 EXAMPLE 9
A set of ~ . . experin~ts to measure the ra~val of toluene from a solution of 600 ppm
toluene in water wns carried out. In ~is ease, four . ' . ' membrane modules with a total
membrane area of 4 m2 Of 20 ,um silicone rubber membrnne were used. The pcrmeate pressure was 100
torr (13.3 kPa) Qnd the feed temperature was varied from 60-70C. P, results of the
20experimant are summarized in Table 12 and plotted graphically in Figurc 3.
Tablc 12
r.(ppm) Permeate fiux Separation
~g/m2.h) factor
25Tarlp (C) Residue(ppm) Permeate(/O)
60139.6 0.2 780
656.0 5.6 0.3 450
705.0 3.6 0.5 300
It cnn be s~en that, ova n 10C ta~lperature c~ange, tbc separation factor incr~ases ov 2.5 fold.
EXAMPLE 10
A sct of ~ ~ . experiments to mensure the rcmoval of i ' ' ', ' (TCE) from
solutions containing about 200 ppm TCE in water was earried out, using a spiral-wound membranc
module containing 0.2 m2 Of composite membrane with a 3.5 ,um silicone rubbcr selective la~er. The
2 1 87933
Wo 95/32051 ~ ~7Q
21
"n~-7te prcssure was mairdai~d at 10 tr (1.3 Wa) us~ng 8 liquid nitrogen trap to remove the VOC and
r7 small vacuum pump to remove non-condensable gases. The parameters varied in the expaiment were
feed temperature and feed fio v rate. Evaluation of the membrane pelform~-7nce was standardized by
o~aring tbe stage cut reqlured to reduce the TCE fecd by a certain reduction factor. The
S results f n feed flow rate of 1.8 gpm (6.8 L/min) are given in Figure 4. As can be seen, the lower tbe feed
temperature, tbe better is the TCE ~nova7.. The ~ sults are replotted in Figure 5 in ter7ns of the separation
factor obtained st different i , using the lowest stage c~t data.
EXAMPLES 11-15: EFFECT OF PEI~MEATE PRESSURE
10 EXAMPLE 11
r of tbe type described in Example 2 for i ' '~. _ separations were pe7folmed,
usingafeedflowrateoflgprn(3.8L/min),arldthistimefxingthefeedtemperatureat50C,butvarying
the permeate pressure. The stage cut varied from 0.1-0.3%. The results are summarized in Table 13.
Table 13
15 Pelmeate C~ . Pe7meate flux Separation
pressure ~cg/m2.h) factor
(torr) (kPa) Feed(ppm) P~ '~,, ) Pe7meate(/O)
40 (5.3) 0.7 0.2 0.02 0.6 410
60 (8) 0.6 0.2 0.02 0.5 500
20 60 (8) 123 38 6.0 0.3 830
80 (10.7) 0.3 0.1 0.01 0.3 800
80 (10.7) 62 20 4.3 0.2 1,200
The separabon factor varied in the range 410-1,200. The ratio between the worst separation
factor at 40 torr (5.3 Wa) and the best at 80 torr (10.7 Wa) is about 3.
FXAMPLE 12
Toluene/water exp~iments of the type described in Example 11 were repeated at a feed flow rate
of 2 gpm (7.6 L/min). The resu.7ts are summarized in Table 14.
9~3
Wo 95132051 r~ s ~ --18
22
Tnble 14
Pelmeate rl Parneate flux Separation
pressure ~glm2.h) factor
(toTr) t~Pa) FeOdtppm) Residuetppm) Pelmeatetn/o)
5 40 (5.3) 0.3 0.1 0.02 0.4 1,190
60 (8) 0.2 0.1 0.02 0.3 1,490
60 (8) 82 31 7.0 0.3 1,440
80 (10.7) 0.2 0.1 0.06 0.2 4,030
80 (10.7) 33 13 4.6 0.2 2,200
EXAMPLE 13
Toluene/waterexperiments ofthe typedegcribed in Exa~nple 11 wae repeated at n feed flow rate
of 3 gpm ( 11.4 L/min). The results are sumTna~i2ed m Table 15.
T~ble 15
Perlneate r: Permeate Separation
pressure flux factor
(torr) tl~Pa) Feed Residue Permeate tkg/m2.h)
(ppm) tppm) tn/~)
20 40 (5.3) 0.4 0.2 0.04 0.4 1,400
40 (5.3) 72 30 3.7 0.4 800
60 (8) 0.4 0.2 0.05 0.3 1,700
60 (8) 74 311 6.5 0.4 1,400
80 tl0.7) 0.3 0.2 0.06 0.2 2,420
The separation factor varied fiom a low of 800 to a high of 2,420 at 80 toTr (10.7 IcPa).
EXAMPLE 14
Ad~itwnd experi~ts ofthe type described in Examples 11-13 were caTried out. This time the
30 data were plotted graphicnlly. The results are shown m Figure 6. As can be seen, ' ~!~ higber
sepaTation factorg were dways obtained at the higha permeatc pressure.
EXAMPLE 15
One of the experimcnts of Exnmple 9 with J 600 ppm toluene feed was repeated usmg 8 feed
temperature of 60C and a pameate pressure of 150 torr ao kPa). The compaTison with tbe previous
3 5 at 100 torr (13.3 I~Pa) is hsted m Table 16.
21 ~7933
WO 95132051 ~ v~ Q
23
Table 16
Feed tarlp. (C) / C~ ' tppm) Pameate flux Separation
Permeate pressure tkg/m2.h) factor
(tvrr) tkPa) Residue tppm) Pe meate t/~)
560 /100 (13.3) 13 9.6 0.2 690
60 / 150 (20) 24 60 0.02 8,320
The process worked, dtbough the very bigb vdue for the separation factor at 150 tvrr (20 kPa)
is suspect.
EXAMPLE 16 AND 17: EFFECT OF DRIVING FORCE AND PRESSURE RATIO
EXAMPLE 16
The previous exarnples sb~v the beneficid ef~ect of reducing driving fvrce eitber by reducmg feed
temperature at constant permeate pressure vr by raising pameate pressure at constant feed temperature.
In this example, twv experiments with diffacnt feed i , and pameate pressures are
vJ^mpared. Eoth experirnents measured tbe reD~al of i ' ' ,1~. tTCE) frvm solutions contairliDg
about 200 ppm TCE in vater, usmg a . ' . ' membraDe module corltaining 0.2 m2 Of compvsite
membrane vitb a 2 ,um ~ ,.v~,yl~ copo~mer selective laya. Tbe feed nOw rate was 1.8 gpm
(6.8 L/mm) m each case. Evduation of the membraûe performarlce was ' " ' by comparirlg the
stage cut ~quired to reduce tbe TCE feed ~ ' by a r ertain reduction factor. In o. e case, tbe feed
temperaturewas40Carldthepermeatepressurewas lOtorr(1.3kPa);intheother,tbefeedtarlpaature
was 30C and the pameate pressure was 20 torr (2.7 kPa). Tbe results are plotted irl FiOure 7. As c,an
be seen, the reduced drivmg force provided by both raising the pelmeate pressure arld lowering the feed
temperature results irl better TCE removd, The results are replotted m Figure 8 m tams of thc separation
factorobtainedatdiffaent-pressureratios,usmgtheloweststagecutdata Afeedtemperatureof40C
and a pameate pressure of 10 torr (1.3 kPa) prvvides a ' '/, pressure ratio of 6; a feed
temperatureof30Candapemleatepressureof20torr(2.7kPa)prvvidesaf~'l pressureratioof 2. The separation factor increases almost fourfold as the pressure ratio drvps.
- EXAMPLE 17
A saies of experimants was paformed to measure the ramoval of benzene from water m~der
different drivmg forces. A banch-scale ~~ ~ ., unit containing a sir~gle membrane module viith a
membrane area of 0.18 m2 was used to paform the tests. The system could treat about 2-3 gallons
(7.6-11.4 L) of water ova a paiod of 2-3 hours. A small pump was used to circulate feed solution
between a feed tar~ and the test mo~lulG A vacuum pump and a dual pameate condalser system provided
WO 95132051 ~ 8
2~
tbe Jpproprinte permeate pressure. The pemmette stream fiow wts switched from ono condcnser to the
otha from time to timc, tllowing stmpling of the condensed permette hqlud witbout mterrupting
operation of the unit.
The experiments woro performed at vtrigble perrrleate pressures, but at t fixed feed solution
temperature of 55 C. A 50-100 ppm bonzenc solution wts used as the test mixturc. At 55 C, this
solution hts an tvage vtpor pressure of 118 torr (15.7 kPt).
The offe~t of changes in the penneate prcssure, tnd hence driving forco, is shown in Figures 12J-
12d, in which tho feed tnd permcate benzene tre plotted ts t function of sttge cut. The
ttrget in tll ctscs was 95/ benzcno romoval from the feod, so that the fecd solution is
reduced to 1-5 ppm. As the pemleate pressure rises, or the oriving force falls, the stage cut required to
rneet this target falls. The rcducod sttgc cut at high permcato pressures is extremely ..J~ . _ sincc
it reduces the heat load on the permeatc condenser. Also the overall cmichment obtamed increases from
..~",., 1~ 250 fold at 10 torr (1.3 kPa) to more than 1,000 fold at 95 torr (12.7 kPt). The
~1/1 pressureratioisll.8ttlOtorr(1.3kPa),3.4at35torr(4.7kPa),1.7at70torr(9.3kPa),
and 1.2 at 95 torr (12.7 kPt).
EXAMPLES 18-21: COMPARATIVECALCULATIONS
EXAMPLE 18
A set of ctlc~ations was performed using tn in-house computer modeling program that simulttes
tho perfonnancc of the ~ ~ _. process shown in Figure 8. Referring now to this figure, feedstrcam,
20 1, comprising two con~poncnts to bo scparated, is brought into conttct with membrtne urlit, 2. The non-
pcrmeating portion of the feedstream is removed as a liquid residue stream, 3. The permeate vapor is
o~oled nn conderlser, 5. The pemleate vtpor stream, 4, should cont~in the components m proportions such
that, after ' , phase separation ttkcs place. The non-condenscd fraction, 6, of the pmeate
vapor, ' ' ~ ' ' ' gases, is lemoved by a small vacmm~ pump, 7. The condensed permette
25 hquid, 8, ptsses to t de~anter, 9. The proouct phtse, I l, is withdrawn from the decanter. The other phtsc,
10, is mixed with the iD~nung feedstrcam to the system tnd rcp ocesscd tbrough the ~ ~a~Jul~liuu unit.
The calculations were performed using TCE . r ' ~ data for total permeate f~ux and
separttion factor at 50C. The experimQltal data had been gathered at different pameate pressures frc;n
experiments such as those reported in Exan~ples 11-15. The data were then used to calculate the attributes
30 ofn~ , , 'toreducethe~ ofaTCE~ 'waterstre~lmfrom
100 ppm to I ppn~, that is 99% removal of TCE. The feed flow rate was assumed to be 10 gpm
(37.9 L/min). The calculation was repeated for carried out for three permeate pressures, 40, 60 and 80
SU9STITUTE SHEET (RULE 26~
~ ~79~3
Wo 95/3205~
torr (5.3, 8, and 10.7 kPa). The resulb are shown nn Table 17.
Tab e 17
Permeate Permeate f~ux Separation Permeate Membrane Conderlser
pressure (I g/m2.h) factor area (m2) load
5(torr) (kPa) (%) (104Btulh)
40 (5.3) 0.42 1,140 0.36 21 2.0
60 (8) 0.33 1,450 0.45 21 1.5
80 (10.7) 0.19 2,120 0.66 25 1.0
As can be seen from the table, operating at permeate pressure of 80 torr (10.7 kPa) ratber than
40 torr (5.3 kPa) ahmost doubles the permeate and halves the permeate Yolume, thereby
reducing the load on the conderlser to 50/O of its value at 40 torr (5.3 kPa). The pcrmeate, stream 8 in
Figure 8, is sufficiently concentrated to phase separate, producing a pure TCE prodnct phase and an
aqueous phase that is recycled. Although more membrane area is reqnired at lo~ver driving force, the
15 increase in membrane area requirement is modest.
EXAMPLE 19
The calculatioos of Example 18 were repeated using ~. , fiux and separation factor data
obtainedfortolueneatfeedternperaboresof45ocand6ooc Thefcedf~owratcwasonccagainassuoned
to be 10 gpm (37.9 Llmin), the feed 100 ppm and the rcsidue - I ppb. The
20 pcrmeatc prcssurc was assumed to bc 40 torr (5.3 kPa). The rcsults of the calculations arc shown in Table
18.
Table 18
FecdPermeatc f~ux Scparation Permeatc Mcmbranc Condcrlser
25tcmpcraturc (kg/m2h) factor : area (m2) load
(C) (/O) (104Btu/h)
450.29 2,700 1.16 13 0.8
600.68 1,450 0.63 1 1 1.6
Thc rcsults agam show a two phasc peroleatc. The pmeatc ~ is rlcarly doubled and
the volumc is morc than halved by lowcring the feed temperaturc 15 DC. The conderlscr load is again
halvcd.
EXAMPLE 20
The calcula~s of Example 18 for TCE were repeated assuming a fced of 10 ppm
anda i ~ ` . of 0.1 ppm, ~ud is 99%~moval. The feed nowrate WaS again aSsUm~l
21 87933
WO 9S/320SI . ~ )... r
26
tobel0gpm(37.9L/min)andthcfeedtempernturc50C. Theresultsofthecalculationsareshownin
Table 19.
Table 19
P tePelmeate flux Separation P= Membrane Condenser
pressure ~g/m2.h) fact0 ; - area (m2~ load
(tor.r) ~cPn) (/.) (104Btn/h)
40 (5.3) 0.42 1,140 0.34 23 2.1
10 60 (8) 0.33 1,450 0.41 23 1.6
80 (10.7) 0.19 2,120 0.55 27 1.1
Again, the substantial berlefits of operation at relatively high permeate presure are clear.
15 EXAMPLE 21
The calculations of Ex~mple 20 were repeated, the cnly difference being that the feed
was assnmed to be I ppm and the residue 0.01 ppm. The results are shawn in Table 20
Table 20
Pelmeate P te f~ux Separation Permeate Membrane C~ndenser
pressure (kg/m2h) factor , area (m2) load
(torr) (kPa) (/.) (104Btulh)
25 40 (5.3) 0.42 1,140 0.13 30 2.8
60 (8) 0.33 1,450 0.13 29 2.1
80 (10.7) 0.19 2,120 0.15 32 1.3
Once again, the results strongly favor operation at high per~neate pressure.
EXAMPLE 22: REMOVAL OF BTEX AROMATICS
A series of tests w~s perfonned with a produced water sample from an oilfield. Benzene, tolnene
~nd xylenes were the major VOC ~ and the of these components was summed
' during tbe course of the experiment. A bench-scsle ~ ~ L;uu unit contanung a single
35 membranemodulewithamembraneareaof0.18m2wasusedtoperformthetests. Thesystemcouldtreat
about 2-3gallons (7.6-11.4 L) of water over a period of 2-3 hours. A small pump was nsed to circulate
feed wlution between a feed tank and the test module. A vacuum pump and a dual permeate conderlser
System provided the appropriste permeate pressure. The pem~eate stream nOw was switched from one
21 87933
Wo 95132051 ~ 3!
27
condenser to the other from time to time, allowing sampGng of the condensed permeate Gquid witbout
inter upting operation of the unit
Two sets of exp~iments were performed, one nt a permeate pressure of 10 torr (1.3 kPa), the
o~er at 70 tolr (9.3 kPa), bdh at 55 C feed temperature. The feed, residue and pameate stream sampla
5 were analyzedbygas ' ~ . ', . Figure 9 shows a . i- ~ set oftraces. Me~anol was added
to the penneate sample to ensure complete dissolution of the BTEX compounds.
Figl)re 10 shows the of the permeate solution as a fur ction of fe~d
The average enrichment, measured as the slope of the ~rap4 obtained at a pameate prcssure of 10 torr
(1.3 kPa) was 250. When the pressure was raised to 70 torr (9.3 kPa), the enrichment increased to about
10 600. This significant . . in separation did not de~rease the i ' BTEX f~ux. As
Figure 11 shows, the BTEX flux dec,reases with decreasing feed solution At the same feed
. however, the BTEX fluxes at 10 and 70 torr ( 1.3 and 9.3 kPa) were the same.
REFERENCES
I RN T ' ' ' r nTI ~S andTransp~rtPropertiesforF~.. ProcessDesignn,
Proccedings of Fourth I ' Confaence on r~,~ v r ' Process in the Chemical Industry, Ft.
Lauderdale, FL, December 1989, pp. 138-155.
2. J. ~ J. G. Wijmans dnd R W. Baker, "Removal of Org~nuc Solvent C~ from
Industrial Eflluent Streams by P,_ v r " ~ Proceedings of Fourth ' ' Conference on
P~ VU~UldtiUII Precess in the Chemical Industry, Ft. Lauderdale, Fl" Dec~nber 1989, pp. 321-331.
3. H E. A. Bruschke and W. Schneider, "Modified PDMS Membranes for Solvent Removal from Water",
Proceedings of Fifth I ' Conference on P~ ~ _. Process in the Chemical Industry,Heidelberg, Germany, March 1991, pp. 54-66.
4. T. L~uner and A. VoiOey, "InQuence of Different Par~uneters on the P~ . _. of Arolna
C, . ' ", Proceedings of Fifth I ' Conference on P~ ~ . . Process in the Chemical
Industry, Heidelbag, Germany, March 1991, pp. 110-122.
5. J. Reale, Jr., V. M. Shah and C. R Bartels, "The Use of Spiral Wound Modules m P~ v ~ u.~,
Proceedings of Fifth I ' Confaenr~e on P~.. Process in the Chernical Industry,
Heidelberg, Gernuuy, March 1991, pp. 231-236.
. 35
6. G. Sodeck and F. Harmdc, "Expcrience with Pw~ _. Plants--A Case Samplen, Proceedings of
First Intanational Ccnf~ence on P~ . _. Process in the Chen~ical Industry, Atlanta, GA, February
1986, pp. 154-162.
40 7. H. H. Nijhuis, M. H. V. Mulder and C. A. Smolders, "Sele~tion of Elastomaic Membranes f the
Removal of Volstile Organic Components from Watern, Prooeedings of Third International Conference
93~
Wo 95132051 ~ c ~ rlQ
28
onP~-r ProcessintheChemicallndustry,Nuncy,France,Septemberl988,pp.239-251.
8 J. L. Rapin, "The Bethuniville ~, Unit--The First Lurge-Scale Productive Plânt for the
Dehydralion of Ethunol", Prooeedings of Third hnternutional Conference on r~. ~al/uluLull Process in the
Chennical hndustry, Nancy, Frunce, Septernber 1988, pp. 364-378
9. J. L. Escoudier, M Ibl3Ouar, ~ Ma~Nmet, C. Jo~et snd J. M. Barillere, "Applicution nnd Evaluation
of P~ a~ldLull for the Production of Low Alcohol Wines", Proceedings of Third International
Conference on P~ ~, Process in the Chen~ical Industly, Nancy, France, September 1988, pp. 387-
397.
10. G.Bengtsonund K.W. Boddeker, I~_, of LowVolatilesfr~nWuter", ProceedingsofThird International Conference on P~_, Process in the Chennical h~dustry, Nancy, Fruoce,
September 1988, pp. 439-448.
I l. P. Cote Jnd C. Lipski, "Mass Transfer Limitations im P~ r for Wâter and Wastewater
Treatment", Proc~edings of Third hnterrultional Conference on P~ ~ . Process in the Chenucal
Industly, Narlcy, France, Septe~nber 1988, pp. 449-462.
12. R J. Ray and D. D. Newbold, "Hybrid Membrane Separation Systems", U. S. Patent 4,944,882, July
31, 1990.
13. K. W. Boddeker, I ., ~wutiw. of PhenolsU, U. S. Pateot 4,806,245, February 21, 1989.
14. C. R Bartels and J. Reale, Jr., "Debydration of Glycolsn, U. S. Patent 4,941,976, July 17, 1990.
15. M.Pasternak, C.RBartelsandJ.Reale,Jr.,n~ of W_ v~ C~, ,U.S.
Plltent4,935,144,June 19, 1990.
16. M. Pastemak, C. R Bartels snd J. Reale, Jr., "Process for P~ . Using Membrane
Separating Meaos", U. S. Patent 5,004,861, April 2, 1991.
17. E. Perry, "Membrane Separation of Orga~ucs frw-n Aqueous Solutionsn, U. S. Patent 4,311,594,
Janualy 19, 1982.
18. L Bhnme and R W. Balcer, "Process for Recovering Orgaruc Con ponents frwn Liquid Streamsn, U.
S. Patent 5,030,356, July 9, 1991.
19. J. ~ ' ' t, J G. wijmans, R W. Baker and 1. Pinnau, "Separation of Organics frwn Water
Using P~ . _, , Proceedings of Third Intematiooal Conference on P~. ., Process in the
Chemical Industry, Nancy, France, September 1988, pp. 405-412 .
20. ~ G. Wijrrlaos, J. ~ J. E. Davidson and R W. Baker, "Treatment of Organic-
C~ ' Wastewater St~uns by p~., . -, r ~ ~ pr~ co Vol. 9, No. 4, November
1990, pp. 262-268.
21. R W. Bal;er, "Sepaltion of Orgamc Azeotropic Mixtures by r~ , , Progress Report to U.
S.DepartmentofEner~yunderGrantNo.DE-FG03-89ER14067,March 1990.
2~ ~7933
WO 9~/32051 ~ 1
29
22. I. Blume, J. G. Wijmans ~nd R W. Baker, "The Separation of Dissolved Organics from Water by
r~ nr~l Qf ~ ' ' S~ Vol. 49, No. 3, 1990, pp. 253-286.
23. J.-P. Brun, C. Larchet, G. Bulvestre aod B. Auclair, "Sclption and P.. ~ .Ij of Dilute Aqueous
5 Solutions of Organic Compounds through Polymer Membranes, I ~ nf r ~ 1 Sri~ vOI. 25,
No. 1, September 1985, pp. 55-100.
24. R Psaume, Ph. Aptel, Y. Aurelle, J. C. Mora and J. L Bersillon, 1 ~lva~/ulaliull~ Importance of
C~ Polarization in the E~ctraction of Trace Organics from Water",
~j~, Vol. 36, March 1988, pp. 373-384.
25. I. Blume and R W. Baker, "Separation aQd G of Organic Solvents from Water Using
F~ ~ I ' Procecdings of Second h~naticnal Conference on P~ ~ _, Process in the Chemiall
Industry, 1987,pp. 111-125.
26. R Y. M. Huang and X. Feng, "Dehydration of Isopropanol by P~ ~ ., - Using Aromatic
P~ 5 ' ' Memblanes", Se~aration ~ ' T ' ' . Vol. 28, No. I l-12, August 1993, pp.
2035-2048.
20 27. P. PfrQmm and F. J. Zimmer, "Residue Oil Recovery from I r ~ C~ by Means of
P~val,u.-.Liul- ,Dissertation,April 1986.
28. C. Lpski and P. Cote, "The Use of P~ . , for the Removal of Organic C~ from
Water", r pr~cC vOI. 9,No 4,November1990,pp.254-261.
29. H H Nijhuis, "Removal of Trace Organics from Water by P~ v, ~, PhD. Thesis, Univer~Qty
of T~vente, Octobcr 1990.