Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
2 1 8980 1
-- 1 --
METHQD FOR PRODUCTION
OF MTcR0rAPsur~ T~lPE CONDIrCTIVE FTrrrR
BA~KuuNL~ OF THE INVENTION
This application is a divisional application of CAnA-liAn
Patent Application Serial No. 2,081,222 filed October 23, 1992.
1. Field of the Invention
This invention relates to a method for the production of
a microcapsule (MC) type conductive filler and more
particularly to a method for coating the surface of minute
con~ t; ve particles with an insulating polymer and to an MC
type adhesive agent having dispersed in an adhesive agent the
coated MC type conductive f iller.
2. Description of the Related Art
In the conventional method of adhesion, the adhesion
effected by soft soldering or welding where the interface
produced by this adhesion requires conductivity. The
conventional method is effectively applicable only to a limited
number of materials because of the heat factor. In contrast,
the organic-inorganic composite conductive adhesive agent that
is composed of a binder using a synthetic resin as a main
-nPnt thereof and a conductive filler using a metal powder
as a main component thereof finds utility in a wide variety of
applications that involving different kinds of materials
subject to adhesion. This adhesive agent, therefore, is an
indispensable medium for conductive adhesion of plastic
substances tsuch as epoxy and phenol resins) that do not adhere
by soft soldering, for adhesion of NESA glass used in liquid
crystal display devices, for adhesion of phosphor bronze with
a carbon brush used in micrometers, and ~or adhesion of lead
wires as in quartz oscillators and sdc meters, for exam~le.
Particularly, in the sPmi rnn~l~ctor industry, whic~i h ls
been enjoying significant growth recently, IC's and LSI's of
increasingly high quality have been developed and mass
produced. For the A-~heC; nn o~ these semiconductor chips
(silicon wafers) to lead
- 2 - 2l8980l
frames, though the method involving to an Au-Sn
eutectic once prevailed, conductive a&esive agents
formed by kn~r~ng an epoxy resin with silver powder
now have multiple applications utility owing to their
ability to lower cost and enhance productivity.
As a resin binder for conductive adhesive
agents, while epoxy resin is generally used, polyimide
type, phenol type, and polyester type resins are also
used, though only partially. As a conductive filler,
minute particles of such metals as gold, silver, and
copper and amorphous carbon and graphite powder are
generally used as well as metal oxides, though only
partially. Silver powder is preferably used over the
conductive f illers cited above because it is
inl~7rrPn~ive, reliable and effective.
~he conductive adhesive agent is
advantageous in various respects compared with
conventional applications such as soft soldering and
welding though it is not perfectly free from fault.
When this conductive adhesive agent is used between an
LSI chip and patterns for mounting component parts,
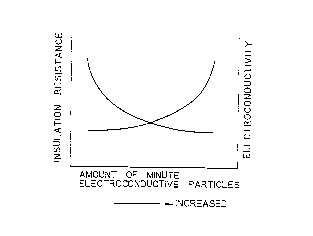
for example, an increase in the amount of minute
. conductive particles that are incorporated in the
conductive adhesive agent lowers insulation resistance
as illustrated in Fig. 1 and increases the possibility
of ad~acent patterns forming electric continuity. A
reduction in the amount of minute conductive particles
reduces the electric continuity between the LSI and
the patterns. Data indicate the necessity for rigidly
3 0 controlling the amount of minute conductive particles
to be used in the conductive adhesive agent. And at
the same time, reveal the fact that the minute
conductive particles cannot be used in large amounts.
It is believed possible that this problem
can be solved by a procedure that comprises preparing
an ~C type conductive adhesive agent having dispersed
in an adhesive agent, an MC type conductive filler
f ormed by coating the surf ace of minute conductive
particles with an insulating polymer, applying the MC
.
. ... .... .... .... . _ _ _ _ _ _ _ _ _ . .
_ 3 _ 2 l 898o t
type conductive adhesive agent to the entire surface
of the substrate of an IC or LSI chip, exerting
pressure to bear on the interface between the chip and
patterns deposited thereon, thereby rupturing the
coating layer of the capsules and establishing
electric continuity between the chip and the patterns,
and meanwhile allowing the encapsulated minute
conductive particles Lnterposed between the adjacent
patterns to remain intact and continue to insulate
these patterns from one another.
The insulating resins that are usable for
coating the surface of minute conductive particles
include thermoplastic resins and thermosetting resins
as classified by kind. In terms of resistance to
moisture absorption and electric insulating
properties, thermosetting resins definitely excel
thermoplastic resins. Since thermocompression bonding
of a chip to a substrate is generally carried out at
an elevated temperature of at least 170C, the
insulating resin to be used is required to be stable
enough to resist this elevated temperature though few
thermoplastic resins can endure this temperature. In
- contrast, most thermosetting resins can tolerate
temperatures in the neighborhood of 200C.
For use as an insulating resin in the MC
type conductive filler, thermosetting resins that are
advantageous in varlous respects over thermoplastic
resins are suitable.
~or the application of an insulating resin
coating to the surface of minute conductive particles,
however, the procedure that involves dissolving the
resin in a solvent, spraying the solution on the
surface of the minute conductive particles, and drying
the applied coating of the solution is predominant
though since thermosetting resins are insoluble in
solvents, this procedure applied conventionally is
dif f icult and the application of a thermosetting resin
coating to the surf ace of minute conductive particles,
therefore, necessitates development of a novel coating
~ 4 ~ 21 898G l
procedure .
~he prior techniques pert~ining to the MC
type conductive a&esive agent have been disclosed by
Japanese IJnp~mined Patent Publications
No. 176,139/1987, No. 76,215/1987, No. 47,943/1988,
No. 54,796/1988, No. 103,874/lg90, and
No. 103,875/1990, for example.
~irst, the disclosures of Japanese
JJnP~minP~ Patent Publications No. 176,139/1987,
No. 76,215/1987, No. 47,943/1988, and No. 54,796/1988
will be described. These patent publications
disclose, as conductive adhesive agents, those
produced by forming an int~ te conductive layer
on s~hPri~l cores of resin and coating the
int~ -rliAte layer ~ith a surface layer of an
insulating thermoplastic resin and those prQduced by
coating the surf ace of minute spherical conductive
particles with an insulating thermoplastic resin.
Actual mounting of a chip on a substrate for a printed
circuit by using such a conductive adhesive agent is
attained by a procedure that comprises applying the
conductive adhesive agent to the substrate and
:- thermocompression bonding the chip to the substrate so
~rat the int~ i ate layer or the minute conductive
particles will discharge a conductive function and the
insulating thermoplastic resin an adhesive function
and an insulating f unction . The techniques disclosed
by these patent publications differ from the method
using the MC type conductive adhesive agent of the
present invention and these patent publications do not
mention using a the~mosetting resin as an insulating
resin f or coating the surf ace of the minute conductive
particles .
Now, the disclosure of Japanese T7nP~mined
Patent Publication No. 103,874~1990 will be described
below. The invention of this patent publication
pertains to an MC type conductive adhesive agent
produced by dispersing in a film of an insulating
adhesive agent serving as a binder an MC type
_ _ _ _ _ _ _ _ _ . _ _ _ . . . . .. . . . . .. . . . _ . . .
-- 5 --
conductive filler havlng minute conductive Qarticles
coated with an insulating thermoplastic resin or
thermosetting resin. Conductive union of two given
members using this MC type conductive adhesive agent
is accomplished by depositing this adhesive agent on
the two members and pressing the two members against
each other while being heated state. Thus, in the
part expected to form electric continuity, the impact
of the pressure exerted as described above ruptures
the insulating resin layer of the MC filler and
establishes the desired electric continuity, whereas
in the part requiring insulation, the MC type
conductive filler is allowed to remain intact and,
therefore, retain stable insulation. Incidentally,
this NC type conductive filler is manufactured by
plasma polymerization or plasma CVD polymerization and
there are times when the insulating f ilm of the NC
type filler may be formed of a thermosetting resin.
The number of kinds of thermosetting resins that can
be manufactured by the plasma polymerization and the
plasma CVD polymerization is very small because the
number of kinds of gases usable for in~ection during
. the polymerization is not large. Further in
accordance with this method of plasma polymerization
or plasma CVD polymerization, the cost is sufficiently
high to render the manufacturing thereof impracticable
and productivity is inferior because the amount of MC
type filler to be manufactured is small.
The disclosure of Japanese T~n~ Ami ned Patent
Publication No. 103,875/1990 will be described below.
The inventLon of ~his patent publication pertains to
the use of an MC type conductive adhesive agent
produced by coating minute conductive particles with
an insulating thermoplastic resin or thermosetting
resLn. Actual mounting of a chip on a substrate for a
printed circuit using this MC type conductive adhesive
agent is attained by applying the conductive adhesive
agent to the substrate and thermocompression bonding
the chip to the substrate, with the in~ i Ate layer
_ _ _ _ _ . . ..... .. . . .... . .. _ .... ....... . .. _ _ _
2189801
-- 6 --
or the minute conductive particles discharging a conductive
function and the insulating resin on the surface of the minute
conductive particles an adhesive function and an insulating
function. Incidentally, this MC type conductive filler is
manufactured by either plasma polymerization or plasma CVD
polymerization. Thus, these prior techrliques are described as
allowing what is formed by coating the surface of minute
conductive particles with a thermosetting resin. In spite of
these disclosures, thermosetting resins should be unusable for
the purpose of coating because they do not melt with heat and,
therefore, are incapable of functioning as an adhesive. Even
if a th~ ~etting resin is used, the method of manufacturing
the MC type conductive filler entails a serious drawback as
pointed out in Japanese rTn~Y~min~tl Patent Publication No.
103, 874/1990.
Practically all the prior techniques pertaining to the
manufacture of an MC type conductive filler or the conductive
adhesive agent using this filler invariably use a thermoplastic
resin. Even when the patent publications mention usability of
a th~ ~setting resin, methods of manufacturing using such a
1-hr ~ ~ ~ctting resin are not disclosed with suf f icient
specificity or are devoid of practicability and thus, these
method cannot be actually used.
SUMMARY OF THE INVENTION
In accordance with an embodiment of the present invention
there is provided a method for production of an MC type
conductive filler comprising (a) a step of immersing minute
conductive particles in an affinity agent thereby treating the
surface of the minute conductive particles, (b) a step of
ersing and dispersing the surface treated minute conductive
-
2~898~1
-- 7 --
particles in an epoxy monomer thereby forming a suspension, and
(c) a step of polymerizing the monomer in the suspension
thereby forming a th~ -ctting insulating polymer on the
surface of the minute conductive particles.
In accordance with another embodiment of the present
invention there is provided an MC type conductive adhesive
agent having dispersed in an adhesive agent an MC type
conductive filler coated with a ~h,~ tting resin, the
th. - setting resin coating having a thickness of not more than
3 ,~lm and having no pinholes.
The term "reactive substance" as used in this
specification refers to a substance that is capable of forming
an insulating polymer on the surface of a filler either by
itself or through reaction with another reactive substance.
The substances that answer this description include monomer
_ ~nf~nts, oligomer s~)mrrmf~nts, and polymer components that
form an insulating polymer, for example.
BRIEF DESCRIPTION OF THE DRAWINGS
The invention will be better understood and objects and
advantages other than those set forth above will become
~pparent when consideration is given to the following detailed
description thereof. The description makes reference to the
annexed drawings wherein:
Fig. 1 is a graph showing the relation between insulation
resistance and conductivity with the amount of minute
conductive particles as a parameter, Fig. 2 is a flow sheet of
the production of a microcapsule type conductive filler, Fig.
3 is a type diagram of the microcapsule type conductive filler,
Fig. 4 is a type diagram illustrating one example of a
substrate, Fig. 5 is a typ-- diagram illust~-~ting one example
2189801
-- 8 --
of a glass chip, Fig. 6 is a type diagram illustrating sites
for determination of electric continuity resistance and
insulation resistance, Fig. 7 is a partially magnified diagram
of Fig. 6, Fig. 8 is a photomicrograph of the microcapsule type
conductive filler (15,000 magnifications), Fig. 9 is a
photomicrograph of a part of union between a bump and a pad
(504 magnifications), Fig. 10 is a type diagram illustrating
the state of u~ ion between a chip and a
9 21~980t
substrate, Fig. 11 is a fl:ow sheet of the production
of a microcapsule type conductive filler as the second
aspect of this invention, Fig. 12 is a type diagram
illustrating a growth model of a triazine thiol film
5 on the surface of metal, and Fig. 13 is a type diagram
illustrating the reaction ^ll;~ni~m of an epoxy
monomer with triazine thiol.
D~Tr.~n DESCRIPTION OF TEIE PREFER~ED EMBODI~ENTS
The principle for the production of an MC
conductive filler will be described below.
~Production using one kind of monomer~
~ suspension is produced by dispersing minute
conductive particles having the surface thereof
treated with a coupling agent in a solution of a
15 monomer and a reaction initiator (oil phase) and
adding the resultant dispersion dropwise to water
having an emulsifier and a viscosity t~n~ncf~r
dissolved therein (aqueous phase). By applying heat
to this suspension, for example, the monomer is
20 polymerized in situ on the surface of the minute
conductive particles and allowed to f orm a coating
thereon. Examples of the monomer that is usable
singly herein are divinyl benzene and acryl. A
~hermosetting polymer is obtained from divinyl benzene
25 monomer and a thermoplastic polymer from acryl
monomer .
(Production using two or more kinds of r~
A suspension is produced by dispersing minute
conductive particles having the surface thereof
30 treated with a coupling agent in the solution of a
monomer in a solvent (oil phase) and adding the
resultant dispersion dropwise to water having another
monomer, an emulsifier, and a viscosity enhancer
dissolved therein (a~ueous phase). sy applying heat
35 or adding a catalyst to this suspension, the
are interfacially polymerized on the surface of the
minute conductive particles and allowed to coat the
minute conductive particles. The coating can be
alternatively effected by preparing a suspension
-- 10 --
2189801
having at least two kinds of ~ , dissolved in the
oil phase and subjecting the - I to in situ
polymerization. Examples of the r-~ ~ that are
usable in the form of a combination of two t -
herein are epoxy/amine and bismaleimide/amine (both
producing a thermosetting polymer).
In the production of the MC conductive filler,
the following points must be taken into consideration.
( l ) The minute conductive particles should be
treated in advance with a coupling agent. ~2) The sp
value of the coupling agent to be used f or this
treatment should be within +10 (cal/cm~)3S of that of
the monomer to be used. (3) The viscosity of the
aqueous phase should be in the range between 20 and
10,000 cps. (4) The suspension should be stirred at a
rate in the range between 50 and 250 rpm to effect the
reaction of the monomer. The reason for (l) is that
since the monomolecular f ilm of the coupling agent on
the surface of the minute conductive particles and the
monomer molecules are intertwined, the monomer is
retained on the surface of the minute conductive
particles and the coating is ef f ected unif ormly . The
. reason for (2) is that if the sp value deviates from
t~re range of +lO (cal/cm~)~, the monomer is not
thoroughly intertwined with the coupling agent and it
is retained on the surf ace of minute conductive
particles with difficulty. The reason for (3) is that
the minute conductive particles settle and agglomerate
if the viscosity is less than 20 cps and the
separation of the MC type conductive filler after
completion of the coating is not obtained if the
viscosity exceeds 10,000 cps. The reason for (4) is
that the minute conductive particles settle and
agglomerate during the reaction of the monomer if
agitation is omitted.
The minute conductive particles to be used for
this method of production of the filler are only
required to be made of a conductive metallic material.
The kind of metallic material is irrespective. For
=
2 t ~q8~ t
example, minute Cu particles having the surface thereof coated
with Ag or minute Ag particles are preferably used.
The minute conductive particles are preferably spheres or
psF~ srh Dres in shape . These minute conductive particles
preferably have a diameter of not more than 50 ~m.
The insulating layer of a thermosetting resin for the MC
type conductive filler is preferably made of a cured
epoxy/amine or bismaleimide/amine type resin. The insulating
layer of ~h- --etting resin of the Mc type conductive filler
preferably has a thickness of not more than 3 ,um.
This invention pertains in one aspect of an MC type
conductive adhesive agent having dispersed in an adhesive agent
the filler obtained as described above. The adhesive agent
that can be effectively used in the MC type conductive adhesive
agent is the same as mentioned above. For example, an epoxy
type one-component polyimide or polyester adhesive agent is
preferably used.
The viscosity of the adhesive agent mentioned above is
preferably not more than 150,000 cps. The content of the MC
type conductive filler in the MC type conductive adhesive agent
is preferably not more than 50% by volume.
One preferred method comprises forming a suspension by
uniformly dispersing minute conductive particles allowing the
presence of a solvent and a monomer (monomer A) on the surface
thereof in water having another monomer (monomer B) dissolved
therein and applying heat to the suspension thereby inducing
the two monomers to react on the surface of the minute
conductive particles and form an insulating polymer and
conse~luently producing a microcapsule type filler. In this
method, the monomer A and the monomer B are monomer c, ~ ~ents
that are intended to form an insulating polymer. When a
polyamide is intended to form the insulating polymer, for
example, adipic acid dichloride serves as the monomer A and
hexamethyIene diamine as the monomer B. Where polyurethane is
intended to form the insulating polymer, for example,
2189801
..
-- 12 --
tetramethylene diisocyanate serves as the monomer A and
methamethylene glycol as the monomer B.
The solvents that are effectively usable for dissolving
the monomer A include dichloroethane, chloroform, carbon
tetrachloride, xylene, toluene, benzene, dichloromethane,
alcohol and ethyl acetate, for example. The suspension is
heated for the purpose of promoting the reaction of the
monomers therein. The temperature of this heating is in the
range between normal room temperature and boiling point of the
solvent. It is selected in accordance with the particular
quality of the suspension to be heated.
In the method described above, the minute conductive
particles must be treated with a coupling agent before using.
This treatment serves the purpose of f ixing the monomer A on
the minute conductive particles.
Further, in the method described above, the viscosity of
the aqueous phase having the monomer B dissolved therein is
preferably adjusted so as to fall in the range between 20 and
10,000 cps by the addition of a viscosity PnhAn~ r. During the
application of heat to the suspension mentioned above, the
suspension must be stirred at a rate in the range between 50
and 250 rpm for reacting the two monomers.
The monomers are preferably used in an amount that is at
least sufficient for the monomers to form a film of not less
than 0 . 05 ~Lm in thickness on the surface of the minute
conductive particles.
Now, the present invention will be described in detail
below with reference to working examples. Of course, this
invention is not limited to the working examples.
The affinity enhancer such as a triazine thiol, which is
used at the step (a) in the method, allows effective
polymerization of the monomers because it is capable of
inducing uniform adhesion of the epoxy resin monomer to the
surface of the minute metallic particles and opening the
heterocycles in the resin. As a re~ult, the heretofore
2 1 8980 ~
-- 13 --
difficult coating of the surface of the minute conductive
particles with the thermosetting resin can be easily attained
by the method of this invention. Further, since the coating
film of the thermosetting resin is superior to the coating film
of a thermoplastic resin in strength, the MC type conductive
filler can be incorporated in a large amount in the adhesive
agent and the MC type conductive adhesive agent consequently
produced can effect an adhesive union o~ two given members with
higher reliability than the conventional technique.
Now, this invention will be described more specifically
below with reference to working examples, which are
illustrative of and not limitative in any sense of this
invention .
Examples 1 to 13 and Comparative Examples 1 to 3 cited
hereinbelow pertain to the first and second aspects of this
invention .
~~ le 1
A microcapsule type conductive adhesive agent was produced
with the following materials.
Minute conductive particles: Minute pq~ nqrheres of Cu
having the surface thereof plated with Ag tAg/Cu,
average ~ r ~ ~r 5 ,~lm) .
Dispersant: Titanate type coupling agent.
Mnnl `:.: Bisphenol A type epoxy (BPA) and tetraethylene
pentamine (TEPA).
Adhesive agent: Epoxy type one-component adhesive agent.
(l) Production of microcapsule type conductive filler
(using a monomer and a solvent respectively in oil phase and
3 0 aqueous phase) .
Coating of silver powder with cured BPA and
- 14 - 2 1 ~9 80 1
TEPA
An aqueous phase was prepared by dissolving
25 g of polyvinyl alcohol, 2 g of an emulsifier, and
10 g of TEPA in 400 ml of water. An oil phase was
prepared by dissolving 7 g of BPA in 15 ml of
dichloroethane and adding to the resultant solution
15 g of silver powder treated with a titanate type
coupling agent in Acc~)r~An~e with the flow chart
illustrated in ~ig. 2. By exposing the oil phase to
an ultrasonic wave for 20 minutes, the silver powder
agglomerated therein was dispersed. Then, the a~ueous
phase was stirred with a homogenizer at a rate of
3,000 rpm and, at the same time, the oil phase was
~rAC~llA 1 1 y added dropwise to the stirred aqueous phase
to produce a suspension allowing the presence of the
oil phase on the surface of the silver powder. This
suspension was kept at 60C and stirred with a three-
one motor at a ra~e of 180 rpm for four hours.
Thereafter, a microcapsule type conductive filler A
having the surface of minute conductive particles
(silver powder~ coated with a polymer as illustrated
in Fig. 3 was separated and dried at 60C for
:- 30 minutes, to afford an NC type conductive filler.
Since the production of this NC type
conductive filler forms the subject matter of this
invention, the production of the MC type filler set
forth in Example 1 above will be described more
specifically below (in the following description, the
amounts of part o~ the raw materials are different
from those of the preceding paragraph).
1.1 Treatment of f ine metallic particles with a
coupling agent
To ensure retention of the monomer on the
surace of f ine metallic particles, the f ollowing
treatment with a coupling agent was carried out. In
50 ml of ethanol, 0 . 3 g of a titanate type coupling
agent and 6 g of minute Ag/Cu particles were retained
at 60C and sub~ected to ultrasonic dispersion for
10 minutes. Then, by keeping the solution at 60C and
- 15 - 2 1 89 8 0 1
.
11 in~ ethanol by dist~llation, the treatment of
the suriace of minute metallic particles with the
coupling agent was effected. Incidentally, the amount
of coupling agent to be used must be in the range
between 0.1 and 1096 by weight and is preferably 5~ by
weight, based on the amount of the minute metallic
particles. The reason for the particular range is
that the surface of the minute metallic particles
cannot be uniormly coated with the coupling agent if
the amount is less than O.196 by weight and the minute
metallic particles cohere if the amount exceeds 10~6 by
weight. Further, the solubility parameter of the
coupling agent is desired to be within ilO ~cal/cm3)llz
of that of the monomer to be used in the oil phase.
This range is important f or the purpose of improving
the molecular intertwining of the coupling agent and
the monomer.
1. 2 Preparation of aqueous phase
An aqueous phase was prepared by dissolving
1.5 g of an ~ml~lR;fier, 14.5 g of PVA (viscosity of
the aqueous phase 20 cps), and 10 g of TEPA in 200 ml.
Here, the amount of PVA to be added must be controlled
:- 60 as to adjust the viscosity of the aqueous phase in
-the range between 1 and 1,000 cps and preclude the
otherwise possible sedimentation of the minute
metallic particles.
1. 3 Preparation of oil phase
An oil phase was prepared by dissolving 10 g
of BPA in 30 ml of ethyl acetate and adding 7 g of
minute Ag~Cu particles to the resultant solution. The
solvent to be used for the oil phase must exhibit
solubility of not less than O.1~ in water. If a
solvent not satisfying this condition is used, the
solvent in the produced NC filler intervenes between
35 the polymer and the minute metallic particles and,
when this MC filler is used in the conductive adhesive
agent, the entrapped solvent causes corrosion of the
product oE union. The solubility of the solvent to be
used is preferably about 396 in water.
. , ....... . . . .... . .. .. .... . . . _ _ _ _ _ _ _ _
2~8~80~
1. 4 Dispersion of minute Ag/Cu part~cles
The oil phase was exposed to an ultrasonic
wave for 10 minutes to effect thorough dispersion of
the minute Ag/Cu particles therein. Though the minute
5 Ag/Cu particles used in this example were spheres in
shape, the coating is equally effected when these
particles are pseudospheres or fish scales in shape.
When the ~C filler is intended for use in the ~qC type
conductive adhesive agent, the particles in the shape
of fish scales are not used advantageously because
they do not serve as spacers between the bump and the
pad as shown in Table 8.
1. 5 Preparation of suspension
A suspension was prepared by stirring the
agueous phase with a homogenizer at a rate of
4,000 rpm and, at the same time, adding the oil phase
gradually to the stirred aqueous phase dropwise. The
operating speed of the homogenizer must be in the
range between 500 and 10,000 rpm. The reason for the
particular range is that no homogeneous suspension is
obtained if the speed is less than 500 rpm and the
minute Ag/Cu particles are damaged if the speed
. exceeds 10,000 rpm.
l . 6 Interfacial polymerization reaction
The suspension prepared in 1. 5 above was
stirred with a three-one motor at 150 rpm and heated
at 60C to Lnduce a reaction for four hours. The
stLrring must be carrLed out with an operatLonal speed
kept in the range between 50 and 250 rpm., whLch
prevents sedLmentatLon of the mLnute metallLc
partLcles ( to whLch occurs Lf the speed Ls less than
50 rpm), cohesion (whLch occurs Lf the speed Ls larger
than 250 rpm) durLng the LntPr~a~ polymerization
reaction .
(2) Observation of cross section of microcapsule
type conductor filler
The microcapsule type conductive filler
produced as described above was buried Ln an epoxy
resLn, allowed to set thereLn, and cut wLth a
_ _ _ _ _ _ . . . . .... ..
2189801
microtome to expose the cross section of the filler
for visual observation.
(3) C~nfinr~tion of insulation of microcapsule
type conductive f iller
The filler was dispersed between two opposed
glass substrates having the surface thereof coated
with ITO and tested for insulation between the glass
substrates .
( 4 ) Preparation of conductive adhesive agent
The microcapsule type conductive filler
prepared in ( 1 ) above was mixed in a voluminal
proportion of 2096 with an epoxy type one-~ nt
adhesive agent. The resultant mixture was thoroughly
stirred to effect dispersion of the filler therein to
afford a microcapsule type conductive adhesive agent.
( 5 ) Bonding of chip to substrate
A 40 ,um conductive adhesive agent prepared
in (2) above was uniformly applied to a substrate
(number of pads 128, interval between pads 100 llm, and
size pad 200 ,~Lm Cl) illustrated in Fig. 4. The
substrate and a glass chip ( 128 pins ) illustrated in
Fig. 5 to which the substrate was tacked by bumping
were sub~ected to thermocompression bonding at a
temperature of 170C, 30 sec, and 35 g/bump. In the
diagram of Fig. 4, 2 represents an electrode and 4
represents an electrode to be used for such
evaluations as a test f or electric continuity .
( 6 ) Test for electric continuity and test for
insulation
3 0 The product of union obtained by bonding in
( 3 ) above was tested f or electric - continuity by the
four-t-~nm;n~l method using the sites of measurement
illustrated in Fig . 6 and Fig . 7 and was tested f or
insulation by using a high-resistance meter
(insulation resistance meter).
Incidentally, the measurement of electric
continuity was made at circuit 1, circuit 2, circuit
3, and circuit 4 and that of insulation resistance at
insulation part 1, insulation part 2, and insulation
_ _ _ _ _ _ _ _ _ _ _ . . .. . .. ..... . _ . _ . .. . _ .
- 18 ~ ~ ~ 8 98 0 ~
part 3 as illustrated in Eig. 7.
( 7 ) Observation of state of adhesion of chip to
substrate
The product of union obtained by bonding in
( 3 ) above was sectioned and the cross section
consequently exposed was visually ~ m~n~d to
determine the state of adhesion of the filler to the
chip and the substrate.
(Results )
~1) Observation of cross section of microcapsule
type conductive f iller
Fig. 8 is a photograph o a cross section of
the microcapsule type conductive filler. It is
clearly noted f rom the photograph that an insulating
polymer was present on the surface of a minute
conductive particle, indicating that the particle was
completely coated.
( 2 ) Conf irmation of insulation with microcapsule
type conductive f iller
The two opposed glass substrates were found
to be insulated from each other, indicating that the
microcapsule type conductive f iller served to ef f ect
. insulation.
( 3 ) Measurement of electric continuity
The results of the test f or electric
continuity are shown in Table 1. All the circuits
used for the test invariably showed highly
satisf actory results of electric continuity not
exceeding 1.5 n (not more than 0.2 Q per ~oint).
To be specific, the chip and the substrate
were joined as illustrated in Fig. 10 and the electric
continuity resistance was not more than 0 . 2 Q per
~oint and, in spite of the high filler content of 2096
by volume, the adjacent patterns showed highly
satisfactory insulation in th~ order of 1 x 10ll Q.
;
~ - 19 2 1 89 80 1
Table 1 Electric continuity resistance
Side of measurement Circuit 1 Circuit 2 Circuit 3 Circuit 4
A 1.1034 1.1298 1. 0865 1. 2051
5 s 1.1298 l . 2114 1.1695 1.1326
C 1.2365 1.1511 1.1233 1.1519
D 1.2562 1.1145 1.2314 1.1413
In: Q
(4) Measurement of insulation resistance
Table 2 shows the results of the test for
insulation resistance. Even though the amount of
filler incorporated was as large as 20~ by volume
(substantially equal to the amount of silver paste for
a die bond ), the ad~acent patterns displayed highly
satisfactory insulation of not less than 101l Q.
Table 2 Insulation resistance
Side of measurement Insulation l Insulation 2 Insulation 3
A 3.6 2.5 2.8
B 2.1 2.6 3.0
C 1.5 2.7 3.0
- . D 1.8 2.0 3.0
In: 101l n
( 5 ) Observation of state of union between chip
and substrate (bump and pad)
Fig. 9 is a photograph showing a cross
section of the joint between the bump and the pad. It
is clearly noted f rom this photograph that the
microcapsule type conductive f iller was amply present
between the bump and the pad.
Example 2
A microcapsule type conductive filler was
produced by faithfully following the procedure of
Example 1, excep~ that minute Ag particles ( average
diameter 0.1 ~Lm) were used instead as minute
conductive particles. It was evaluated in the same
4 0 manner as in Example 1.
(Results )
_ 20 --
2 1 8980 1
( 1 ) Observation of cross section of microcapsule
type conductive f iller
Similarly to the filler illustrated in
Fig. 8, an insulating polymer was found to have
uniformly coated the surface of agglomerated minute
conductive particles.
(2) Cnnfir~-tion of insulation with microcapsule
type conductive flller
The filler showed the same degree of
insulation as found in Example l.
( 3 ~ ~easurement of electric continuity
resistance
The electric continuity resistance was
substantially the same as in Example 1.
( 4 ) Neasurement of insulation resistance
The insulatLon resistance was substantially
the same as in Example 1.
( 5 ) Observation of state of union between chip
and substrate (bump and pad)
Similarly to the product of union
illustrated in Fig. 9, the microcapsule type
conductive filler was amply p~esent between the pad
and the bump.
~ Exam~le 3
~icrocapsule type conductive f iller and a&esive
agent were produced by faithfully following the
procedure- of Example l, except that 10 g of
h~q~ l~ (BDqI) and 0.1 g of rl;;~ohicyclo~ln~iPc~
were used in place of the monomer sP~. They were
evaluated in the same manner as in Example 1.
(Results )
(1) Observation of cross section of microcapsule
type conductive filler
Similarly to the product of union
illustrated in Fig. 8, an insulating polymer was found
to have coated minute conductive particles completely.
(2) Cnnfirr~tion of insulation with microcapsule
type conductive filler
The filler showed the same degree of
- 21 - 2t89801
insulation as in Example ~.
( 3 ) ~eas~rement o~ electric continuity
resistance
The f iller showed the same degree of
electric continuity resistance as in Example l.
( 4 ) Measurement of insulation resistance
The f iller showed the same degree of
insulation resistance as in Example l.
( 5 ) Observation of state of union between chip
and substrate (bump and pad)
The state of union was the same as that
found in Example 1.
Com~arative Exam~le l
~ l ) Preparation of microcapsule type conductive
filler
A microcapsule type conductive f iller was
produced by the coating method described below using
the following materials.
Minute conductive particles: 30 g of minute Ag/Cu
particles (same as those of Example 1)
Polymer: PMNA ( average particle aiameter 0 .15
,um) (m.p. 135C)
A microcapsule type conductive filler coated
wi-th PM~A was produced by dissolving 5 g of P~MA in
100 ml of xylene, spraying the resultant solution into
minute conductive particles, and drying the particles
(for expulsion of xylene).
( 2 ) Observation of cross section of microcapsule
type conductive filler
3 0 ~ 3 ) ~on f ~ tion of insulation with microcapsule
type conductive filler
t 4 ) Preparation of conductive adhesive agent
( 5 ) Bonding of chip to substrate
(6) Test for electric continuity and test for
insulation
( 7 ) Observation of state of union between bump
and pad
The operations of ( 2 ) to ( 7 ) indicated above
were carried out in the same manner under the same
_ _ , . . . .. . . .. _ . . .. . _
- 22 ~ 218980;
conditions as those of ( 2 r to ( 7 ) of Example 1.
(Results ~
( 1 ) Observation of cross section of microcapsule
type conductive filler
Similarly to the minute conductive particles
of ( 7 ), Example 1 illustrated in Fig . 8, the filler
particles were found to be completely coated with
PMN~. ''
( 2 ) Conf irmation of insulation with microcapsule
type conductive filler
5jm~lArly to the filler of Example 1, the
microcapsule type conductive f iller retained
insulation .
( 3 ) l~easurement of electric continuity
resistance
All the circuits, ~;m~ li3rly to those of
Example 1, showed highly satisfactory electric
continuity resistance of not more than 1. 5 Q .
( 4 ) ~easurement of insulation resistance
Table 3 shows the results of the
mea~uL. L. Of the total of 12 insulation parts, two
insulation parts showed electric continuity, probably
because the bonding was made at a temperature of 200C
an-d the PlD!SA was conseguently ~ sed or fused to
establish contact between the minute conductive
particles .
Table 3 Insulation resistance
Side of mea:,uL~ L Insulation 1 Insulation 2 Insulation 3
A 1.5 2.5 x 10 510
B 2.1 x 101l 8 150
C 20 2. 7 x 101l 26
D 35 10 3 . 0 x 10
In: Q
( 5 ) State of union between bump and pad
Similarly to the results of ( 5 ) in
Example 1, the microcapsule type conductive filler was
amply present between the bump and the pad.
_ . _ , . . .,, : . ,, _ _ _ _ _ _ . ,
21898
- 23 -
~omParative Example 2
Preparation of microcapsule type conductive
f iller
A mi~luu~ ule type conductive filler was
produced by faithfully following the procedure of
Example, except that Cu particles 60 llm in diameter
were used as minute cûnductive particles.
The produced microcapsule type conductive f iller
was evaluated in the same manner under the same
conditions as described in (2) to (7) of Example l.
(Results )
The produced f iller having the surf ace thereof
completely coated with a polymer showed insulation.
The electric continuity resistance and the state of
union between the bump and the pad were equal tû those
obtained in Example l. No insulation was retained
between the ad~acent pads.
ComParative Example 3
A microcapsule type conductive filler produced by
following the procedure of Example l was mixed with an
epoxy type adhesive agent having a viscosity of 210 ,-
'~. 000 cps.
(Results )
The filler could not be dispersed in the adhesive
agent because the viscosity of the adhesive agent was
unduly high.
Example 4
The use of two kinds of ~ - , a thermosetting
resin, and a solvent was omitted and a monomer were
used in both the oil phase and the a~[ueous phase.
A microcapsule type conductive filler and a
microcapsule type conductive adhesive agent were
produced by faithfully following the Procedure of
Example l, except that an oil phase obtained by
dispersing 7 g of conductive particles treated with a
coupling agent in lO g of sPA in accordance with the
flow sheet shown in Fig. 2 was used dichloroethane
instead of ethyl acetate solvent in the oil phase.
They were evaluated in the same manner as in
.. .. _ _ . _ ........ .. . . .
- 24 - 2 ~ 8 ~ 8 0 1
Example 1.
( Results ~
In all the items of evaluation, the results were
equal to those obtained in Example ~.
Exam~le 5
One kind of monomer was used and a thermosetting
resin and a solvent were used, and one kind of monomer
was used in the oil phase.
A microcapsule type conductive filler and a
microcapsule type conductive adhesive agent were
produced by following the procedure of Example 1,
except that an aqueous phase was prepared by
dissolving 12 g of polyvinyl alcohol and 1. 5 g of an
emulsifier in 200 ml of water and a solution of 10 g
of divinyl benzene and 0.1 g of benzoyl peroxide in 15
ml of ethyl acetate was used as an oil phase.
~Results )
In all the items of evaluation, the results were
almost the same as those obtained in Example 1.
ExamPle 6
Two kinds of - r.~ were used, including a
thermosetting resin and a solvent, and two kinds of
: r ~ ~ were used in the oil phase.
- A microcapsule type conductive filler and a
microcapsule type conductive adhesive agent were
produced by f ollowing the procedure of Example 1,
except that an aqueous phase was prepared by
dissolving 12 g of polyvinyl alcohol and 1. 5 g of an
emulsifier in 200 ml of water and an oil phase was
prepared with 15 ml of ethyl acetate and 5 g of
imidazole. They were evaluated in the same manner as
in Example 1.
(Results )
In all the items of evaluation, the results were
nearly the same as those obtained in Example 1.
Example 7
Two kinds of monomers and a thermosetting resin
were used only in the oil phase and no solvent was
us ed .
. . . _ _ _ _ . .
-
- 25 - 21898~1
.
An MC f iller and an ~C conductive adhesive agent
were produced by faithfully following the procedure of
Example 6, except that the use of ethyl acetate was
omitted. They were evaluated in the same manner as in
Example 6.
(Results )
In all the items of evaluation, the results were
nearly the same as those obtained in Example 1.
ExamPle 8
One kind of monomer and a thermosetting resin
were used and no solvent was used. The monomer was
used in the oii phase.
A microcapsule type conductive filler and a
microcapsule type conductive adhesive agent were
produced by following the procedure of Example 2,
except that an aqueous phase was prepared by
dis801ving 12 g of polyvinyl alcohol and an emulsifier
in 200 ml of water and an oil phase was prepared by
dispersing in 10 g of divinyl benzene 0.1 g of benzoyl
peroxide and 7 g of minute conductive particles
treated with a coupling agent in accordance with the
flow sheet illustrated in Fig. 2 without using ethyl
-- acetate ( solvent ) . They were evaluated in the same
manner as in Example 2.
(Results )
In all the items of evaluation, the results were
nearly the same as those obtained in Example 1.
Example 9
A blend of a thermoplastic resin and a
thf ,seLLing resin and a solvent were used. ~he
monomer was used in the oil phase.
A microcapsule type conductive filler and a
microcapsule type conductive adhesive agent were
produced by following the procedure of Example 1,
except that 5 g of methyl methacrylate, 5 g of
f ~r--olf~;mide, and 0.1 g of azoisobutyronitrile were
used as nl ~ in place of BPA and TEPA. They were
evaluated in the same manner as in Example 1.
(Results )
,, , ,, . _ .. . . . . .
- 26 - 218q8(}1
In all the items of evaluation, the results were
nearly the same as those obtained in Example 1.
ExamPle 10
A blend of a thermoplastic resin and a
~h~ LLing resin and a solven~ were used. The
monomer was used in the oil phase and the aqueous
phase .
A microcapsule type conductive filler and a
microcapsule type conductive adhesive agent were
produced by following the procedure of Example 1,
except that an agueous solution was prepared by
dissolving 12 g of polyvinyl alcohol, 1. 5 g of an
emulsifier, and 15 g of hexamethylene diamine in
200 ml of water and a solution of 7 g of adipic acid
and 7 g of BPA in 15 ml of ethyl acetate was used as
an oil phase. They were evaluated in the same manner
as in Example 1.
( Results )
In all the items of evaluation, the results were
nearly the same as those obtained in Example l.
Example 11
A blend of - I - was used in the oil phase and
. no solvent was used.
An MC filler and an MC type conductive adhesive
agent were produced by following the procedure of
Example lO, except that ethyl acetate was omitted.
They were evaluated in the same manner as Example 10.
(Results )
In all the items of evaluation, the results were
nearly the same as those obtained in Example 1.
Example 1 2
A blend of , rs was used in the oil phase and
the aqueous phase and no solvent was used.
An MC type filler and an MC type conductive
adhesive agent were produced by following the
procedure of Example 11, except that ethyl acetate was
omitted. They were evaluated in the same manner as in
Example 11.
(Results )
. _ ........ . .
_ 27 - 2 1 a980 1
In all the items of evaluation, the results were
nearly the same as those obtained in Example l.
Example 13
An NC type filler produced by the procedure of
Example 1 was tested for the following items.
(l) Effect of sp (solubility parameter) value of
coupling agent on production of MC type f iller
Table 4 shows the results of the test
performed on MC fillers prepared using coupling agents
of different sp values with respect to electric
continuity .
Table 4 Results of test of MC type f iller
for insulation
Difference of sp values of Results of test for
coupling agent and monomer insulation
o Insulatlon
5 Insulation
10 Insulation
11 Electric continuity
The results indicate that the dif f erence
~ between the sp value of the monomer (epoxy resin)
25 . and the sp value of the coupling agent must be
within 10 (cal/cm~) 112. The possible reason for
this limit is that the monomer molecules and the
coupling agent molecules are intertwined with
dif f iculty and retention of the monomer on the
surface of the minute conductive particles is not
attained. Incidentally, the sp value o the
epoxy resin is 10.9 (cal/cm3)l/2.
(2) Effect of viscosity of agueous phase on
stability of suspension
Table 5 shows the results of the test
performed involving the effect of changes in the
viscosity of the aqueous phase on the stability
Of the suspension-
- 28 - 2 t 8 98 0 7
Table 5 Relatian between viscosity of
aqueous phase and suspension
viscosity
5 Of aqueous lo 20 loo looo loOOO 11, ooo
phase ~cps)
Stability Sediment- Suspension not
of ation of producible and
suspension minute i Stable stable Stable stab1e~c filler after
par~icles reaction not
observed effectible
The results indicate that the viscosity of
the a~ueous solution is proper in the range between 20
and 10,000 cps.
( 3 ) Ef f ect of stirring speed on stability of
suspension
Table 6 shows the ~esults of the test
performed involving the effect of the stirring speed
(30, 50, 250, and 300 rpm~ on the stability of the
20 suspension.
Table 6 Relation of speed of stirring and
stability of suspension
~ stirring 30 50 250 300
Stability Sedimentation Adhesion of minute
of of minute conductive particles to
suspension conductive Stable Stable beaker wall observed
particles
obs erved
The results indicate that the stirring must
be carried out at a rate in a range between 5 0 and
250 rpm.
( 4 ) Relation of particle diameter and insulation
resistance of minute conductive particles
Table 7 shows the results of the test
performed on minute conductive particles of diameters
10, 30, 50, and 70 ~m for insulation.
- 29 _ 2 1 8 9~ t
Table 7 RelatiOn between particle diameter
and insulating property of coated minute
conductlve particles
Particle diameter (um) of Results of test for
5minute conductive particles insulation
Insulation
3 o Insulation
Insulation
7 0 l~lectric continuity
The results indicate that the minute
conductive particles to be used should have a
diameter of not more than 5 0 ,um .
( 5 ) Relation between shape and electric
continuity resistance of minute conductive
particles .
Table 8 shows the results o the test
performed on minute conductive particles having
different shapes of spheres, pseudospheres, and fish
scales with respect to electric continuity.
Table 8 Relation between shape and
conductivity of minute conductive particles
Shape of minute Conductivity (l~umber of deective
conductive particles portions/number of sites of measurement)
Spheres 0 /10 0
Pseudospheres 0 /10 0
l~i sh s ca l es 2 3 /10 0
In the case of a filler using minute
particles of the shape of fish scales, the
surface completely coated with a polymer, the
insulation was satisfactory, and the ad~acent
pads were insulated from each other. Absolutely
no electric continuity was es~h~ . Thouyh
the filler was present between the bump and the
pad, it failed to serve as a medium for union
4 0 thereo . The results indicate that the minute
conductive particles should be in the shape of
. . . .
_ 30 _ 2 1 898 ~ 1
either spheres or pseudospheres.
6 ) Relation between thickness and electric
continuity resistance of an insulating resin
layer
Table 9 shows the results of the test
performed on insulating resin layers formed of
the MC type conductive filler with different
thicknesses with respect to electric continuity.
Table 9 Relation between thickness and
conductivity of insulating resin
Thickness (,um) of Resistance per site of measureInent
insulating resin ( Q )
0.1 0.1
2.0 0.4
3.0 0.5
4.0 1.5
It is noted from Table 9 that the resistance
to electric continuity was high and points of poor
electric continuity were detected when the thickness
of the insulating resin layer (coating layer) was 4.0
,um. The results indicate that the thickness of the
insulating resin layer is desired to be not more than
3 ~
( 7 ) Content of MC type ~iller
Table lO shows the relation between the MC
type ~iller and the state of curing of the adhesive
3 0 agent .
21 89801
-- 31 --
.
Table 10 Content of ~IC type conductLve
filler and state of adhesive agent
Content ( % ) 1 10 30 50 55 65 70
State of Good Good Good Good Good Poor Poor
curing adhesion adhesion
Poor A~lhPfiir n: Complete wetting of filler with
adhesive agent not obtained because of excess
amount of filler.
The results indLcate that the content of the
~C type conductive filler must not be more than 609; by
volume. ~ow, the third and fourth aspects of this
invention will be described specifically below with
reference to Examples 14 to 16.
Exam~le 1 4
An l~C ty~e conductive adhesive agent was produced
with the following materials.
~inute conductive particles: ~inute Cu
pseudospheres having the surface plated with Ag
(Ag~Cu, average particle diameter 5 ,um~.
Adhesive agent: A composition consisting of an
~ epoxy resin as the main component and an acid
~ anhydride as the curing agent ) .
Affinity agent: ~ri~zinR thiol (RTD).
~onomer: Bisphenol A type epoxy resin (BPA)
(produced by Shell and marketed under ~ri~lcl ~rk
designation of "Epikote 828 " ) .
(1) ~Iethod for production of ~C type conductive
filler
The minute metallic particles were subjected
to a surface treatment. ~irst, the minute metallic
particles were washed with an acid and then with an
alkali, and pretreated with Triclene to defat and
clean the surface thereof. The cleaned minute
metallic particles were immersed in a triazine thiol
solution to be coated with a film of t.ri~7inr~, thiol.
This solution was prepared by dissolving tr~7~nP
thiol in acetone in a concentration of 10-4 mol/lit.
~o uniform film is obtained if the concentration is
- 32 - 2 ~ ~ ~8 0 1
.
lower than this level and the speed of treatment is
too high to be controlled as re~uired if the
concentration exceeds 10 ~ mol/l. The temperature of
this treatment is not lower than 17C. It is desired
to be in the range of 20 + 3C because the speed of
the treatment is too high to be controlled as desired
if the temperature is unduly high. The time of
treatment is desired to be in the range of 30 +
5 minutes in due consideration of the relation between
the concentration mentioned above and the temperature.
It goes without saying ~hat for such conditions as
concentration, temperature, and time of the treatment,
the magnitudes thereo to be selected should be
optimum for obtaining a film having a suitable
thickness and a suitable constitution depending on the
purpose or use thereof. Then, the minute metallic
particles were washed with the solvent used and
methanol and the wet minute metallic particles were
dried to complete the surf ace treatment . In a
solution of 10 g of epoxy monomer (BPA) in 15 ml of
ethyl acetate, 10 g of the surface-treated minute
metallic particles were stirred with a homogeni~er at
- 150 rpm as illustrated in Fig. 11 to form a suspension
a~d induce a reaction to ef fect the coating of the
surface of Ag/Cu particles with an insulating resin
layer .
Here, the principle of the production of the
MC type conductive filler will be described below.
When a suspension is formed by disperslng
minute metallic particles in a solution of tr;A7;
thiol in an organic solvent, this tr;~7;nl~ thiol
reacts with the OH group on the surface of the
metallic particles to form a relevant salt. As a
result, the surf ace of the minute metallic particles
is coated with a tr;A7in~ thiol film. When a
suspension is formed by dispersing the minute metallic
particles treated will tr~7;ne thiol in a solution of
the monomer, the surface of the minute metallic
particles undergoes a reaction. Conseguently an l!~C
_
21 89~û 1
-- 33 --
type conductive filler having the surface of minute
metallic particles coated with the poLymer is
obtained .
Now, the reaction ~h;~n i ~m lnvolved herein
5 will be described below.
The ~r;~7lnP thiol is a compound having a
structural formula I shown below.
R
N N-H
s,l~ ~is
N
H
(wherein R stands for a group represented by -SH,
-N(CH~)2, -NHC6H5, -N(C4Hg~z, -N(C8H~7)2, ~N(CI2H2s)2~
N ( CH2CH=CH2 ) 2, -NHC8H~6CH=CHC8HI7, -NCH2C6H4CH=CH2 ( C8HI7 ), or
2 0 -NHC6H4 ) .
~7hen the minute metallic particles are
sub~ected to a surface treatment with this ~ri~7in~
thiol, there ensues a reaction path in which a
monomolecular film of triazine thiol is formed on the
- surface of the minute metallic particles in the first
s~ep and the monomolecular film develops into a
polymolecular film in the second step as illustrated
in Fig. 12, with the result that the surface of the
minute metallic particles will be coated with the
triazine thiol film. ~.7hen the minute metallic
particles that have undergone the surf ace treatment
are mixed with an epoxy monomer, the triazine thiol
acts as a cross-linking agent for the epoxy monomer to
undergo a reaction illustrated in Fig. 13 and gives
rise to a cured product of epoxy. Consequently, an MC
type conductive filler having a surface of the minute
metallic particles coated with the epoxy resin i5
obtained .
Here, it is necessary tb pay attention to
the following points.
( 1 ) The production of the triazine thiol f ilm
mu5t be carried out in an atmosphere of nitrogen . ( 2 )
.. _ .. .. ... .. , . _ _ _
2 t 8q80t
The fr;~in~ thiol concen~ration must be not more than
lO ~ mol/liter. (3) The reaction of the monomer must
be carried out with the suspension stirred at a rate
in the range between 50 and 250 rpm. The reason for
( 1 ) is that the minute metallic particles readily
undergo corrosion in the presence of air because they
have a large surface area. The reason for (2) is that
the concentration of RTD (~r;;~;nr thiol) must be kept
below 10 3 mol/liter because the amount of film i8
calculated from the amount of unreacted RTD. The
reason for ( 3 ) is that the minute metallic particles
settle and agglomerate during the reaction of the
monomer when stirring is omitted.
( 2 ) Observation of cross section of capsule type
minute metallic particles
The produced f iller was embedded in the
epoxy resin, allowed to cure, and cut with a microtome
to expose a cross section of the capsule type minute
metallic particle to visual observation.
( 3 ) Conf irmation of insulation with capsule type
minute metallic particles
. The produced MC type conductive f iller was
' agglomerated into a crh~r;n~ mass and tested for
insulation resistance with an insulation resistance
meter used at freely selected points of measurement.
( 4 ) Production of conductive adhesive agent
An MC type conductive adhesive agent was
produced by mixing an MC type conductive adhesive
agent with 20% by volume of the MC type conductive
filler prepared in ( l ) above . The results of the test
indicate the viscosity of the produced adhesive agent
was so high as to jeopardize the workability if the
voluminal proportion ~re~r~d 20 %, the produced
adhesive agent was barely usable if the voluminal
proportion was up to 6096 of the MC type conductive
filler, and the adhesive agent included parts allowing
no electric continuity if the voluminal proportion was
unduly small. Thus, the optimum content of the MC
type filler is fixed at 20% by volume. Here, the
.. .. , . , . . .. _ _ = .
2t'~q8~1
-- 35 --
adhesive agent used herein was a one-component type
for facilitating the process of production.
(5) Union of chip and substrate
A substrate illustrated in Fig. 4 to which
the conductive adhesive aqent produced in ( 4 ) above
was applied and a glass chip ( 12~ pins, 300 um pitch,
and electrode interval 100 um) illustrated in Fig. 6
on which stud bumps were formed were subjected to
~he c~ ~ ~ssion bonding at 175C, 30 s, and
20 g/bump.
(6) Test for electric continuity and test for
insulation
Samples of the product of union indicated in
(5) above.were tested for electric continuity
resistance by the four-~ n~l method using the
points of measurement illustrated in Fig. 6 and Fig. 7
and tested for resistance with a resistance meter.
(Results )
( 1 ) Observation of cross section of microcapsule
type conductive filler
The condition of the surface of minute
- conductive particles coated uniformly with an
i~sulating resin as illustrated by a type diagram
of Fig. 3 was cnn f~
( 2 ) Insulation resistance of microcapsule type
conductive f iller
The magnitudes of insulation measured at all
the points invariably exceeded a high level of
1 x 101l Q.
(3) ~easurement of electric continuity
resistance and insulation resistance between
bonded chip and substrate
The union between the chip and the substrate
was obtained as illustrated by a type diagram in
Fig. 10. The magnitudes of electric continuity
resistance were satisfactory, invariably falling ~elow
0 . 2 Q per point of contact . Though the f iller was
incorporated in such a large proportion as 20% by
volume, highly satisfactory insulation of 1 x 101l Q
.. , , ., _, ,, .. .. _ _ _ _ _ _ _ _ , . ~ ~ . ,
-36- 2~8~
was found between the ad~acent patterns.
~his example represents one case of using
tiazine thiol as an affinity agent. This invention is
not limited to this particular affinity agent.
5 Naturally, any compound possessing a reactive group
that exhibits affinity for both the metal and the
monomer i nt~ntlf~ to coat the metal can be used as an
af f inity agent .
ExamPle 15
In the production of capsule type minute metallic
particles by the procedure of Example 14, the stirring
of the suspension was carried out at varying rates of
30, 50, 250, and 300 rpm to determine the effect of
the stirring speed on the stability of the suspension.
~Results )
Table 11 shows the effect of the stLrring speed
(30, 50, 250, and 300 rpm) on the stability of the
suspension. The results indicate that the stirring
speed must be in the range between 5 0 and 25 0 rpm f or
20 the sake of suspension stability.
Table 11 Relation between stirring speed
and suspension stability
Speed of
st rring 30 50 250 300
( rpm)
suSpension Sedimentation of Adheslon of ~inute
stability minute conductive Stable Stable conductive particles
particles observed to beaker wall
observed
ExamPle 1 6
An ~C type conductive filler and a capsule type
conductive adhesive agent were produced by following
the procedure of Example 14, except that alcohol was
used in the place of acetone. They were evaluated in
the same manner as in Example 14.
(Results )
In all the items of evaluation, the results we~e
nearly the same as those obtained in Example 14.
This invention is constructed as described above,
.... .. _ . . .. , _ , ,,
- 37 - 2 1 ~ 9 ~ O ~
it enables an MC type conductive filler coated with a
thermosetting resin possessed of better characteristic
properties than a thermoplastic resin to be produced
easily at a low cost. Thus, this invention realizes a practica~ MC type conductive adhesive agent excellent
liAhl~;ty ~ p~ fon~n~.