Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
g~2
BOEHRINGER MANNHEIM GMBH 3973/OA
3'-(4'-)non-radioactively labelled nucleosides and
nucleotides with aminocarboxylic acid, peptide or
carboxylic acid spacer~
The invention concerns nucleosides, nucleotides and
oligonucleotides modified in the 3'(4') position with
non-radioactively labelled groups, a process for their
production as well as their use.
Extremely many uses have been found for labelled
nucleotides in genetic engineering since it is easier to
use them than the DNA probes conventionally used as
hybridization probes which are produced from native gene
material by restriction digestion.
Modified oligonucleotides which are used in the form of
so-called "antisense" DNA oligonucleotides can intervene
in a regulatory manner in cellular processes and are
thus becoming increasingly important for example for the
in vivo investigation of the expression of proteins as
well as in cancer, viral and gene therapy. The mechanism
in this case involves DNA-DNA, DNA-RNA and RNA-RNA
interactions but still requires a complete detailed
elucidation.
Labelled oligonucleotides serve in vitro for example to
identify gene fragments within a gene bank by screening
and identifying blotted gene samples of the gene bank
with the aid of a labelled oligonucleotide.
Apart from radioactive labelling by means of suitable
- 213~2
isotopes, derivatised fluorescent dyes have for example
been used as a form of non-radioactive labelling which
enable easier and safer handling.
Previously such a technique has been used successfully
for the non-radioactive sequencing of DNA. In this case
approaches which are essentially based on the method of
Sanger [F. Sanger, S. Nicklen and S. Coulsen, Proc.
Natl. Acad. Sci. USA 74, 5463 (1977)] have been carried
out.
In this method the fluorescent label was either attached
to the 5' end of the nucleotide [L.E. Hood, L.M. Smith
and C. Heiner, Nature 321, 674 (1986)] or to the
nucleobase [J.M. Prober, G.L. Trainor and R.J. Dam,
Science 238, 336 (1987)] [G.L. Trainor, Anal. Chem. 62,
418 (1990)]. This is associated with the disadvantages
that only particular polymerases can be used for the
synthesis, the triphosphates are accepted less by the
polymerases and that in addition a large substrate
excess is necessary.
Chemical sequencing according to Maxam-Gilbert using
fluorescent labels is also known [H. Voss, C. Schwager,
U. Wirkner, B. Sproat, J. Zimmermann, A. Rosenthal, H.
Erfle, J. Stegmann and W. Ansorge, Nucl. Acids. Res. 17,
2517 (1989)].
In addition it is known that a fluorescent dye can be
directly coupled via an amino or thiol group in the 3'
position of a nucleoside, nucleotide or oligonucleotide
and that this compound can be used advantageously for
the synthesis of complementary strands in the presence
of a template strand as well as for the detection of
- 21g~g2
-- 3
genetic material in vivo and in vitro (EP O 490 281).
However, a disadvantage of these compounds is that the
quantum yield is relatively low and the synthesis of the
compounds involves tedious and time-consuming
chromatographic purification steps.
Therefore the object of the present invention was to
provide new non-radioactively labelled nucleosides and
nucleotides which do not have the disadvantages of the
previously known compounds.
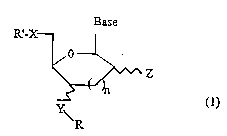
This object is achieved by nucleosides and nucleotides
modified at the 3' or 4' position having the following
general formula:
Base
R'-X- ~
~Z
~ (1)
\ R
in which R': represents hydrogen, mono-, di- or
triphosphate, alkylphosphonate, dialkyl-
phosphinate or phosphoramidite
X: oxygen, nitrogen, carbon or sulphur
Y: oxygen, nitrogen or sulphur
Z: a hydrogen, hydroxyl, amino or thiol
group
R: denotes a spacer group to which a non-
radioactive, detectable group or an
effector molecule is bound
base: represents a purine or pyrimidine base or
a deazapurine or derivatives thereof
n: is o or 1.
- ~l9~g82
The spacer group is preferably composed of a protected
or unprotected bi-, tri- or polyfunctional carboxylic
acid, aminocarboxylic acid or a peptide or corresponding
derivatives or salts thereof. All naturally occurring
amino acids represent particularly preferred spacers
such as for example glycine or aspartic acid, carboxylic
acids or aminocarboxylic acids with 2 to 20 atoms, but
also diamines, thiols and aminoalcohols.
Di- and tetramers of amino acid units have proven to be
especially suitable for spacer groups; particularly when
the spacer group has a length which corresponds to a
carbon chain of 2 to 12 atoms. All chemically,
physically or biologically detectable groups come into
consideration as non-radioative, detectable groups, so-
called reporter molecules. Such groups are known to a
person skilled in the art. In this case so-called
effector molecules are also suitable which are capable
of chemical, physical or biological interactions with
particular target molecules either directly or after
chemical, physical or biological activation (such as
alkylation agents, ~ systems of aromatic compounds,
enzymes etc.). The intercalation with DNA/RNA by acetyl-
aminofluorenes, cleavage of DNAtRNA by nucleases or the
partial or complete covalent binding of alkyl or aryl
groups to DNA/RNA by alkylation agents or psoralens and
for example metal clusters which lead locally to a
strong increase in the capture cross-section of
therapeutically effective electromagnetic radiation are
mentioned here as examples. Luminescent dyes which emit
in the wavelength range of ca. 630 to 670 nm, enzymes
such as peroxidase or alkaline phosphatase and haptens
such as for example biotin/iminobiotin or digoxigenin
come into particular consideration as reporter
molecules. In this connection fluorophores have proven
21~0~82
-- 5 --
to be particularly suitable for example for sequencing
according to Sanger. Compounds substituted with effector
groups are of particular value especially for antisense
therapy.
The OH group, amino or thiol group located at the 3'
and/or 4' position of a nucleoside, nucleotide or
oligonucleotide is coupled to a spacer group such as for
example an aminocarboxylic acid, carboxylic acid or a
peptide and this is subsequently linked to a reporter or
effector molecule. The 3'- or 4'- hydroxyl-, amino- or
thiol-modified nucleosides, nucleotides and
oligonucleotides that are formed can then be used for
the synthesis of complementary strands in the presence
of a natural or synthetic template strand as well as for
the detection of particular sequences or to localize an
effector molecule at particular sequences in the genetic
material. They have in particular the advantage that the
modification is no longer attached to the 5' end of the
nucleotide or to the nucleobase as in previously known
labelling techniques.
The invention in addition concerns a process for the
production of the 3'(4'-)modified nucleosides and
nucleotides according to the general formula (1).
Compounds of the general formula (2)
B~e
carrier - ~ker)-X - l
- '/0~~
~ (2)
21gO~82
-- 6 --
in which: linker: represents a selectively cleavable
anchor group
X: represents oxygen, nitrogen, carbon
or sulphur
Y: represents oxygen, nitrogen or sulphur
Z: represents a hydrogen, hydroxyl, amino
or thiol group
R: represents hydrogen
base: represents purine or pyrimidine base
or a deazapurine or derivatives
thereof
n: is O or 1.
are bound via the linker group to a solid carrier a
bifunctional or polyfunctional compound provided if
necessary with protecting groups is added, subsequently
a detectable signal component which is reactivated if
necessary is added, the nucleoside derivative is cleaved
from the carrier matrix and, if desired, it is
derivatized in the 5' position.
The reaction to form nucleosides bound via a linker in
the 5'(6') position to a solid carrier can in addition
be achieved by other methods known to a person skilled
in the art. All selectively cleavable anchor groups can
be used as the linker group [e.g. G.B. Fields and R.L.
Noble, Int. J. Peptide Prot. Res. 35 (1990), 165 - 171].
Groups which can be cleaved under mild conditions such
as for example photochemically, by hydrogenation or in
the acid or alkaline range have proven to be
particularly suitable. Preferred linker groups are
trityl groups which can be substituted if desired.
Methoxy and halogen residues are suitable substituents
in this case. Dimethoxy and o-chlorotrityl groups have
proven to be particularly suitable in this case. In
2 ~ 8 2
addition the basic procedure for linking the linker
group to the 5' or 6' position of nucleosides is well-
known to a person skilled in the art (M.J. Gait:
Oligonucleotide synthesis; a practical approach; IRL
Press, Oxford, Washington DC (1984), p. 12 ff).
Any solid, chemically inert material which is insoluble
in the solvent used in the respective case and which
should be as easy to filter as possible is suitable
according to the invention as the carrier material.
Preferred carrier materials are inorganic polymeric
materials (resins) based on polystyrene or polyethylene
glycol.
Nucleosides bound in this manner to a solid carrier are
subsequently admixed with an appropriate carboxylic
acid, aminocarboxylic acid or peptide derivative in the
presence of an activation reagent such as for example
condensation agents such as DCC, DIC or HOBT.
After linking the aminocarboxylic acid, peptide or
carboxylic acid or their derivatives or salts to the
nucleoside or analogue thereof bound to the solid phase,
one or several, identical or different reporter/effector
molecules are introduced covalently and selectively into
the side chain or C or N terminus, for example by means
of esterification, amidation, sulfonamidation, sulfonic
acid esterification or by reaction with isothiocyanates
or N-hydroxysuccinimide esters. Appropriate methods are
known to a person skilled in the art.
Compounds and classes of compounds which come into
consideration as reporter molecules or effector
molecules are those which can be covalently linked to a
219~g~2
-- 8
hydroxy, amino or thiol group (see above).
The cleavage of the nucleoside derivatives from the
linker group is preferably carried out under weakly
acidic, basic, photochemical or reductive conditions.
Nucleoside derivatives which have one or several
protecting groups or side chains on the spacer group can
be produced in a corresponding manner.
The nucleoside derivatives produced in this manner can
be subsequently converted into the corresponding 5' or
6' mono-, di- or triphosphates (nucleotides), alkyl-
phosphonates, dialkylphosphinates or phosphoramidite
according to methods known to a person skilled in the
art.
The synthesis according to the invention of the 5' and
6' modified nucleosides is particularly advantageous
because - in contrast to the well-known processes - a
large excess of the dye components can be used without
problems and easily separated. In the case of the
previously known processes tedious and expensive
chromatographic purification steps follow the actual
production process in order to separate the non-reacted
dye.
The compounds according to the invention having the
general formula (1) in which residue R' represents a
triphosphate group can be used advantageously as
substrates for DNA/RNA polymerases with the aid of a
primer and a template strand and in the presence of the
four nucleoside triphosphates. Suitable polymerases in
this case are T7 polymerase, Taq polymerase, DNA
8 2
g
polymerase I and reverse transcriptases. In addition the
compounds are suitable as terminators in enzymatic
DNA/RNA sequencing. In this case termination of the
synthesis can be specifically determined in each case by
the use of a 3'-(4'-)modified A, C, G or T nucleotide of
formula (1). This is of particular importance for the
synthesis of DNA complementary strands in the presence
of a template strand (and thus also for the sequencing
of DNA strands) since the use of a modified nucleotide
ensures a very base-specific termination of the
reaction.
The synthesis of RNA nucleosides and oligonucleotides is
achieved in an analogous manner.
In addition it is also possible to synthesize
derivatizable oligonucleotides with the aid of a start
nucleotide which carries a spacer group at its 3'(4')
end and which has been amino-modified or thio-modified
and immobilized on a polymeric carrier.
Oligonucleotides are understood as all DNA and RNA
nucleotides produced in a conventional manner
preferably, however, having a length of 2 to 100,
particularly preferably of 12 - 50 nucleotides (chemical
synthesis) or having a length of up to ca. 3000
nucleotides (enzymatic synthesis) depending on the
efficiency of the polymerase used.
The oligonucleotide synthesis is carried out starting
with the start nucleotide-carrier complex in the
conventional sense i.e. in the 3' and 5' direction and
enables the synthesis of an oligonucleotide with a
defined sequence.
-- 21~82
-- 10 --
The immobilization of the start nucleoside to commercial
carriers is achieved via a connecting arm (spacer) which
can be cleaved after the synthesis; for example by means
of the succinic acid linkage known in the literature or
by means of a linkage with urethane (Efimov et al.,
Nucl. Acids. Res. 11, 8369, 1983).
After the synthesis is completed the oligonucleotide
must be cleaved from the carrier using suitable
reagents. Derivatization with any desired fluorescent
dye is carried out directly afterwards.
It is also possible to synthesize oligonucleotides in
the opposite direction (5'~ 3') by linking the start
nucleoside via the 5' OH group to the linker group of
the carrier material and then proceeding according to
conventional oligonucleotide synthesis. In this case the
labelling of the oligonucleotide with dye can be
achieved on the solid phase.
The oligonucleotides modified at their 3'(4') end and
synthesized in this manner can then be used to detect
for example complementary oligonucleotides or nucleic
acids and corresponding derivatives.
The compounds according to the invention are, however,
also suitable as in vivo and in vitro sensors for
analysis and as gene probes in gene therapy.
The invention is elucidated further by the following
examples:
- 11- 21~82
ExamPle 1:
Esterification of Fmoc-amino acid derivatives with 2-
chlorotrityl resin
1 gram resin which is loaded with 1.1-1.6 mmol 2-chloro-
trityl chloride linker [K. Barlos, O. Chatzi, D. Gatos,
G. Stauropoulos, Int. J. Peptide Prot. Res. 37, (lg91),
513 - 520] is shaken for several minutes in absolute
DCM. After addition of 3 mmol/l DIEA, 1.2 mmol Fmoc-
protected amino acid is added. The reaction period is 90
minutes. After addition of a further 3 mmol DIEA, the
unesterified linker function is etherified with 5 ml
absolute methanol within approximately 30 minutes. The
coated resin is filtered and washed several times with
DMF, DCM, isopropanol and finally with diethyl ether.
After drying in an oil-pump vacuum, the amino acid
loading is determined using a quantitative ninhydrin
test (example 7b) or by using W spectrometric
determination of the Fmoc cleavage. Amino acid loadings
between 0.05 and 1.1 mmol/g is obtained by varying the
amount of amino acid.
Example 2:
Na-linking of fluorescent dyes to resin-bound amino
acids or peptides
For dyes with carboxy groups the following applies:
3 equivalents dye, 3.3 equivalents DIC and 4.5
equivalents HOBt are added per equivalent of resin-bound
amino acid or peptide in a shaking flask and shaken for
- 2~ gV9~2
- 12 -
50 hours at room temperature in DMF/DCM in the dark.
Subsequently it is washed several times with pure DMF,
DCM, methanol, isopropanol and ether and the amino acid
or peptide conjugate is cleaved from the solid carrier
as described below.
3 equivalents of the fluorescent dye, 1.2 equivalents
DIEA and a catalytic amount of DMAP (4,4'-dimethylamino
pyridine) are reacted per equivalent of the resin-bound,
N-terminally-deprotected peptide or amino acid to be
labelled in a solution of DMF/DCM (v/v 4:1) in a shaking
flask with a frit for 48 hours. In order to avoid
decomposition reactions of the dyes, it is shaken in the
dark. The reaction course can be monitored using the
ninhydrin reaction or TLC. If free amino functions are
still present, then it can be acetylated with Ac20/DIEA
(equivalents/equivalents 1:2). Non-reacted dye is
removed by filtration and it is washed several times
with 10 ml in each case of DMF, DCM, DMSO, MeOH and
finally with diethyl ether. After drying in an oil-pump
vacuum the amino acid-peptide-dye conjugates can be
stored in a refrigerator.
Orthogonality of the protecting groups also allows
one or several identical or different fluorescent dyes
to be introduced selectively into the side chain or
N-terminus. The introduction of fluorescent dyes into
the side chain of trifunctional amino acids is carried
out analogously to the N~-linking, but the proportion of
DMAP has to be increased.
- 21gV~82
- 13 -
Example 3
Cleavage from 2-chlorotrityl resin to prepare
fluorescent-labelled amino acids or fluorescent-labelled
peptides
The ester cleavage is carried out under weakly acidic
conditions. Approximately 20 ml of a mixture of glacial
acetic acid/TFE/DCM in a ratio of (v/v/v 1:2:7) is used
per 1 g peptide resin [K. Barlos, O. Chatzi, D. Gatos,
G. Stauropoulos, Int. J. Peptide Prot. Res. 37, (1991),
513 - 520]. The cleavage period is usually approximately
90 minutes. If no His(Nim Trt) residue is present, then
it can be cleaved with a mixture of DCM/TFE (v/v 1:1).
Subsequently the resin is removed by filtration and the
peptide solution is filled up with approximately 100 ml
water. The added water prevents the concentration of
acetic acid in the subsequent removal of the readily
volatile components by distillation on a rotary
evaporator. The hydrophobic amino acid and peptide
derivatives are precipitated during the rotary
evaporation and are then dried in a freeze dry
apparatus.
Example 4:
Etherification of nucleosides via the 5'-OH group using
the 2-chlorotrityl resin
1 gram resin which is loaded with 1.1-1.6 mmol 2-chloro-
trityl chloride linker is shaken for several minutes in
a mixture of CHCl3/DMSO (v/v 1:2). After addition of 2.5
mmol DIEA, 0.52 mmol nucleoside is added. The reaction
period is ca. 120 minutes. After addition of a further
21g~
- 14 -
3 mmol DIEA the non-etherified linker function is
etherified with methanol within 30 minutes using 5 ml
absolute methanol. The coated resin is filtered and
washed several times with DMF, DCM, isopropanol and
finally with diethyl ether. After drying in an oil-pump
vacuum, the loading of the resin is determined using the
quantitative ninhydrin test, in the case of the 3'-amino
nucleotides (example 7b) by W spectrometric
determination after suitable derivatization of the 3'
function of the nucleoside or by weighing the resin. By
varying the amount of nucleoside, resin loadings between
0.1 and 1 mmol/g is obtained.
Example 5:
Coupling of amino acids, peptides and carboxylic acids
and their derivatives to carrier-bound nucleosides
The coupling is carried out according to the activation
methods known from peptide chemistry (period: ca. 12
hours). The use of base-labile protecting groups enables
the extension of the amino acid chains or fragment
condensations according to the well-known methods [M.
Bodanszky Principles of Peptide Syntheses, 2nd Edition,
Springer 1993, G.B. Fields & R.L. Noble, Int. J. Peptide
Prot. Res. 35, (1990), 161 - 214].
,~ .
21gO98~
- 15 -
Exam~le 6:
Cleavage of nucleosides, nucleoside-amino acids,
nucleoside-peptides and their dye derivatives from
2-chlorotrityl resin
The ether cleavage is carried out under acidic
conditions. Approximately 10 ml of a solution of
dichloroacetic acid/DCM in a ratio of (v/v 3:97) is used
per l g coated resin. The cleavage period is ca. 1
minute. Subsequently the resin is removed by filtration
and the product solution is neutralized immediately with
an equimolar solution of DIEA/DCM in a ratio of (v/v
63:937). The resin is washed several times with a small
amount of ACN. Ca. 10 ml water is added. The product is
now dried in a freeze drying apparatus.
Example 7:
Ninhydrin test
The following three solutions are required to carry out
the ninhydrin test [I. Kaiser, R.L. Colescott, C.D.
Bossinger, P.I. Cook, 3. Anal. Biochem. 34, (1970),
595]
Solution 1: A solution of 80 g phenol in 20 ml ethanol
Solution 2: A 2 % solution of 33 mg potassium cyanide
KCN in 50 ml water in pyridine.
Solution 3: A solution of 500 mg ninhydrin in 10 ml
ethanol.
21g~82
- 16 -
a) Qualitative ninhydrin test
In order to test whether an acylation has
proceeded quantitatively, two to three
drops of the three solutions are reacted
in each case with a microspatula-tip full
of the coated resin in an Eppendorf tube.
The reaction mixture is heated for about
5 minutes in a water-bath to 100C. If
the acylation reaction is not completed
then the solution has a deep dark-blue
colour whereas in the case of
quantitative coupling reactions the
reaction mixture retains its yellow
colour.
b) Quantitative ninhydrin test
In order to quantitatively determine the
loading of the resin with amino acid, the
Fmoc-amino acid derivatives are
deprotected for 40 minutes with
piperidine/DMF (v/v 40:60).
After washing with DMF, isopropanol and
DCM, the resin is dried. A sample of
about 3 - 5 mg is heated to 100C with
solutions 1 (4 drops), 2 (8 drops) and
3 (4 drops) in a thermo-heating block for
7 minutes. The reaction mixture is
diluted immediately with 60 ~ ethanol and
transferred to a measuring flask of
suitable size.
After calibrating the W spectrometer with
60 ~ ethanol, the absorbance is determined
~2lsa~s2
-
- 17 -
and the resin loading in ~mol/g is
determined by entering the appropriate
values in the following equation.
[absorbance x dilution (ml)]
Loading ~mol/g] = x 106
extinction coefficient x weighed mass (mg)
The compounds of examples 8-23 set forth in the
following were synthesized according to the general
conditions of examples 1 - 6.
Amino acid/peptide-dye conjugates
2190~8~
- 18 -
Example 8:
Fluorescein-5(6)-aminothiono-N-glycine (FlTC-Gly(OH))3
HO~N\~S
~ OOH
O ~ OH
Preparation:
0.17 mmol (673 mg) resin-gly-NH2
0.5 mmol (193 mg) FITC
Yield (77.9 mg) 91 %
HPLC ana lYS i S:
Column Nucleosil RP18, 5 ~m, 300A, 4 x 250 mm
Gradient 20 - 100 % ACN (0.1 ~ TFA) 40'
Flow rate 1 ml/min. Abs. at 235.0 nm and 225.0 nm.
Retention time: 10.05 /11.00 minutes.
'~lg~gB~
-- 19 --
ExamPle 9:
Fluorescein-5(6)-aminothiono-N-glycyl-glycine (FlTC-Gly2(0H))4
HO~ N~S x = 2
H~ `
~ OOH
O ~ OH
Preparation:
0.43 mmol resin-gly2-NH2
1.3 mmol (504 mg) FITC
Yield (220.4 mg) 89 %
HPLC analYsis:
Column Nucleosil RP18, 5 ~m, 300A, 4 x 250 mm
Gradient 20 - 100 % ACN (0.1 % TFA) 40'
Flow rate 1 ml/min. Abs. at 287.5 and 425.0 nm.
Retention time: 8.00 minutes.
Z190$8Z
- 20 -
Exam~le 10:
Fluorescein-5(6)-aminothiono-N-triglycyl-glycine (FlTC-Gly4(0H))5
~ OOH
O ~ OH
Preparation:
0.2 mmol resin-gly4-NH2
0.5 mmol (195 mg) FITC
Yield (77 mg) 74 %
HPLC ana 1YS i S:
Column Nucleosil RP18, 5 ~m, 300A, 4 x 250 mm
Gradient 0 - 80 % ACN (0.1 % TFA) 40'
Flow rate 1 ml/min. Abs. at 287.5 nm.
Retention time: 17.5 minutes.
9 8 Z
-
- 21 -
Exam~le 11:
Rhodamine MR 200-N methylcarbonyl-N'-triglycyl-glycine
o o
HotC~,NH~ IC2H5
=~<
Cl~COOH
CI~CI
Cl
Preparation:
44.4 mg resin-gly4-NH2 (corresponding to ca. 0.02 mmol)
30 mg dye MR 200, free carboxylic acid
8.0 mg HOBT
6.3 ~l DIC
Yield: quantitative
HPLC ana lYs i s:
Column: Nucleosil RP18, 5 ~m, 300 A, 4 x 250 mm
Gradient: 0-80 % acetonitrile (0.1% TFA) in 40 min
Flow: 1 ml/min
Detection: Abs. at 617 nm
Retention time: 19.5 min
TLC: silica gel, CHCl3/MeOH l:l:Rf 0.55
Mass: calculated 943.7
found 943.5
s~ectral data: ~maX,EX615 nm, ~aX,Em642 nm (in MeOH)
219~2
- 22 -
Example 12:
Rhodamine JA 51-N-propylcarbonyl-N'-triglycyl-glycine
O o
HOtC NHtC~ Cl 02CH3
4 (CH2)3 (CH2)3
,t
CF3
Pre~aration:
0.047 mmol resin-gly4-NH2
0.141 mmol dye JA 51
0.2 mmol HOBT
0.2 mmol DIC
Yield: nearly quantitative
HPLC analysis:
Column: Nucleosil RP18, 5 ~m, 300 A, 4 x 250 mm
Gradient: 0-80 % acetonitrile (0.1% TFA) in 40 min
Flow: 1 mllmin
Detection: Abs. at 631 nm
Retention time: 16.5 min
Mass: calculated 774.8
found 774.6
219~
_
- 23 -
Example 13:
Digoxigenin 3-0-methylcarbonyl-triglycyl-glycine
o
~o
OH
1 C~/
CH
O
HO ~ ~ ~ OH
Retention time: 15 minutes
Preparation:
0.2 mmol resin-gly4-NH2
0.5 mmol digoxigenin-3-O-acetic acid-N-hydroxy-
succinimide ester
Yield (94 mg) 72 %
HPLC analysis:
Column: Nucleosil RP 18, 5 ~m, 300 A, 4 x 250 mm
Gradient: 0-80 ~ acetonitrile (0.1~ TFA) in 40 min
Flow: 1 ml/min
Detection: Abs. at 215 nm
Retention time: 15 min
Nucleoside/nucleotide dye conjugates
- 21g~982
- 24 -
Example 14:
3'-0-[S-triphenylmethyl-Na-(9-fluorenylmethoxycarbonyl)-cysteinyl]-2'-
deoxy thymidine (thymidine-Cys(Trt)(Fmoc)) 6
~ CH3
o~N~
HO -
O
(Trt) ~S ~CH2 -f 6
~moc)
Preparation:
0.25 mmol resin-thymidine
0.5 mmol (Fmoc)Cys(Trt)OH
Retention time: 28.5 min
Yield: Cleavage was achieved on an analytical scale
HPLC analysis of the crude product:
Column Vydac RP 18, 5 ~m, 300 A, 4 x 250 mm
Gradient 0-60 % ACN (0.1 % TFA) 20', 60-100 % 25' 100 %
30', 0 % 35'
Flow rate 1 ml/min
Abs. at 265 nm
Retention time: 28.5 min
- 2~g~82
- 25 -
ExamPle 15:
3'-0-N-[N-pentamethylchromyl-Na-(9-fluorenylmethoxycarbonyl)-
arginyl]-2'-deoxy thymidine (thymidine-Arg(Pmc)(Fmoc)) 7
~ H3
o~NJ
HO~
~mc) H
EDN- C -~nH - (CH~)3 -
~nH NH
I
~moc)
Preparation:
0.25 mmol resin-thymidine
0.5 mmol (Fmoc)Arg(Pmc)OH
Yield: Cleavage was achieved on an analytical scale
HPLC analysis of the crude product:
Column Vydac RP 18, 5 ~m, 300 A, 4 x 250 mm
Gradient 0-60 % ACN (0.1 % TFA) 20', 60-100 % 25' 100 %
30', 0 % 35'
Flow rate 1 ml/min
Abs. at 265 nm
Retention time: 26.7 min
- 2~98~
Example 16:
3'-0-lNa-(9-fluorenylmethoxycarbonyl)-leucinyl]-2'-deoxy-thymidine
(thymidine-Leu(Fmoc)) 8
~CH3
ol~NJ
HO
O
(CH3)2 --CH--CH2 --Cl ~
~moc)
Preparation:
0.25 mmol resin-thymidine
O.5 mmol (Fmoc)LeuOH
Yield: Cleavage was achieved on an analytical scale
HPLC analysis of the crude product:
Column Vydac RP 18, 5 ~m, 300 A, 4 x 250 mm
Gradient 0-60 % ACN (0.1 % TFA) 20', 60-100 % 25' 100 %
30', 0 % 35'
Flow rate 1 ml/min
Abs. at 265 nm
Retention time: 25.5 min
- 2~9~g~
- 27 -
Example 17:
Example 17, compound 9 was successively synthesized on a
carrier (1. Coating of the resin with thymidine, 2.
Coupling a ~Fmoc)-amino acid, 3. Deprotecting the amino
acid, 4. Coupling the dye).
3'-0-glycyl~N-thiono-[5(6)-amino-fluoresceinyl]}-2'-deoxy-thymidine
(thymidine-gly-FlTC) 9
~ H3
HO o~N
O ~S
r OOH
O ~ OH
Preparation:
0.5 mmol resin-thymidine
0.5 mmol (Fmoc)gly(OH)
1.0 mmol FITC
Yield: Cleavage was achieved on an analytical scale
Rf value (silica gel 60, F254, Merck): 0.35; CHC13/MeOH
(v/v 9: 1) .
The following examples 18-23 were also synthesized on a
carrier, subsequently cleaved from the resin and further
~ 19~
- 28 -
reacted in solution to form the triphosphates.
Triphosphate synthesis was carried out according to
[Ludwig, Eckstein, J. Org. Chem. 54, 631 - 635, (1989)].
- 29 -
Example 18:
Fluorescein-5(6)-amino-thiono-~N-glycyl)-[(3'-amino-2',3'-dideoxy)-
thymidine-5'-triphosphate] (3'-amino-Gly-FlTC, 3'-deoxy, 5'-
triphosphate-thymidine) 1 0
O
~ _,CH3
H~
(4 ) 09P30~
HN~N~S
H ~
~ OOH
O ~ OH
Preparation:
0.031 mmol resin-3'amino-3'-deoxy-thymidine
0.041 mmol fluorescein-gly(OH)
Yield: over all steps (17.4 mg) 60.1 %
Rf value (silica gel 60, F254 Merck): 0.59; isoprop./
NH40H/water (v/v/v 7:1:4)
HPLC analysis of the crude Product:
Column Supersphere RP18(e); 3 ~m; 2 x 125 mm Gradient
0 - 2 % B 20'; 2 - 4 % B 25'; 4 - 20 % B
A = TEAA, pH 7.0; B = ACN Flow rate 0.15 ml/min
Abs. at 265 mn
Retention time: 11.95 min.
Z~9~98~
- 30 -
ExamPle 19:
Fluorescein-5(6)-amino-thiono-(N-diglycyl)-1(3'-amino-2',3'-dideoxy)-
thymidine-5'-triphosphate] (3'-amino-Gly2-FlTC,3'-deoxy,5'-
triphosphate-thymidine) 1 1
~ _,CH3
(¢)OgP3O ~ '~
H~N ~ ~ S x=2
~`~
`r OOH
O~OH
Preparation:
0.031 mmol resin-3'amino-3'-deoxy-thymidine
0.052 mmol fluorescein-gly2(OH)
Yield: over all steps (19.9 mg) 54 %
Rf value (silica gel 60, F254 Merck): 0.29; CHCl3/MeOH
(v/v 1: 1)
HPLC analysis of the crude Product:
Column Supersphere RP18 (e); 3 ~m; 2 x 125 mm
Gradient 0 - 2 % B 20'; 2 - 4 % B 25'; 4 - 20 % B
A = TEAA, pH 7.0; B = ACN Flow rate 0.15 ml/min
Abs. at 265 mn
Retention time: 12.8 min.
- ~g~98~
-- 31 --
Example 20:
Fluorescein-5(6)-amino-thiono-(N-tetraglycyl)-[(3'-amino-2',3'-
dideoxy)-t]-thymidine-5'-triphosphate]
(3'-amino-Gly4-FlTC,3'-deoxy,5'-triphosphate-thymidine) 1 2
(¢) gP30~
~HN~bS X = 4
H~ ,
~COOH
O~OH
Preparation:
0.031 mmol resin-3'amino-3'-deoxy-thymidine
0.045 mmol fluorescein-gly4(OH)
Yield: over all steps (21.9 mg) 64.2 %
Rf value (silica gel 60, F254 Merck): 0.53 isoprop./
NH40H/water (v/v/v 7:1:4)
Rf value (silica gel 60, F254 Merck): 0.26 CHCl3/MeOH
(v/v 1: 1)
HPLC analYsis of the crude product:
Column Supersphere RP18 (e); 3 ,um; 2 x 125 mm
Gradient O - 2 % B 20'; 2 - 4 % B 25'; 4 - 20 % B
A = TEAA, pH 7.0; B = ACN Flow rate 0.15 ml/min
Abs. at 265 mn
Retention time: 12.8 min.
9 ~
- 32 -
Example 21:
Rhodamine MR 200-N-methylcarbonyl-(N'-tetraglycyl)-1(3'-amino-2',3'-
dideoxy)-adenosine-5'-triphosphate]
NH2
. ~NJ
09PJO~
HN~C NH~
~CH2 C2H~
~'
C~COOH
C~CI
Preparation:
0.027 mmol resin-3'-amino-2',3'-dideoxy-N6-benzoyl-
adenosine
0.050 mmol Gly4-MR200
0.1 mmol HOBT
0.1 mmol DIC
After cleavage from the carrier resin and subsequent
triphosphate synthesis, the benzoyl protecting group was
removed with concentrated ammonia solution.
Yield over all steps: 49 % of theory
` ~l9~g82
- 33 -
HPLC ana lYs i s:
Column: Hypersil ODS, 5 ~m, 120 A, 4x2S0 mm
Flow: 1 ml/min
Gradient: 0-20 % B in 10 min
80 % B in 20 min
100 % B in 25 min
Solvent: A: 0.1 M TEAA in water
B: acetonitrile
Detection: Abs. 617 nm
Retention time: 18.3 min
Mass: calculated 1411.9
found 1411.5
Example 22:
Rhodamine MR 200-N-methylcarbonyl-(N'-tetraglycyl)-1(3'-amino-2'.3'-
dideoxy)-guanosine-5'-triphosphate] l
~ NH2
gP30~o
\J
O O
HI~C NHt4C~ C2H5
Cl~COOH
C~CI
Cl
Preparation:
0.027 mmol resin-3'-amino-2',3'-dideoxy-N2-isobutyryl-
2 1 ~
-
- 34 -
guanosine
0.050 mmol Gly4-MR200
0.1 mmol HOBT
0.1 mmol DIC
After cleavage from the carrier resin and subsequent
triphosphate synthesis, the isobutyryl protecting group
was removed with concentrated ammonia solution.
Yield over all steps: 41 % of theory
HPLC analysis:
Column: Hypersil ODS, 5 ~m, 120 A, 4x250 mm
Flow: 1 ml/min
Gradient: 0-20 % B in 10 min
80 % B in 20 min
100 % B in 25 min
Solvent: A: 0.1 M TEAA in water
B: acetonitrile
Detection: Abs. 617 nm
Retention time: 18.0 min
Mass: calculated 1427.9
found 1427.3
~19~98~
Example 23:
Digoxigenin 3-0-methylcarbonyl-(N'-tetraglycyl)-~(3'-amino-2',3'-
dideoxy)-thymidine-5'-triphosphate]
0~ ~0
~ CH ~
O ~
H ~ ~ O
Preparation:
0.03 mmol resin-3'-amino-2',3'-dideoxy-thymidine
0.06 mmol digoxigenin 3-O-methylcarbonyl-triglycyl-
glycine
0.1 mmol HOBT
0.1 mmol DIC
After the condensation was completed it was cleaved from
the carrier resin as in examples 21 and 22 and the 5'-
triphosphate was obtained after the phosphorylation.
Yield over all steps: 58 % of theory
21g~g~2
- 36 -
HPLC analysis:
Column: Hypersil ODS, 5 ~m, 120 A, 4x250 mm
Flow: 1 ml/min
Gradient: 0-20 % B in 10 min
80 % B in 20 min
100 % B in 25 min
Solvent: A: 0.1 M TEAA in water
B: acetonitrile
Detection: Abs. 260 nm
retention time: 15.2 min
Mass: calculated 1139.9
found 1139.1
Example 24:
Use of 3'-modified nucleotideQ for enzymatic DNA
sequencing
The following nucleotides were used that had been
prepared according to examples 1 to 6:
H3C a
~r ~
~N~_
4-o9p30_1 o - J U
OOC ~C)
~\NH3'n NH
l:n=O (\ ~
2:n=l ~o
3:n=2
4:n=4
Z1~0~8Z
1 ~g M13mpl8 single-stranded DNA (5 ~1), 2 ~1
fluorescein-labelled universal primer (1 pmol, Pharmacia
LKB), 2 ~1 Mn buffer I (311 mM Tris HCl, pH 7.5), 2 ~1
Mn buffer II (177 mM DTT) and 2 ~1 Mn buffer III (62 mM
MnC12, 460 mM sodium isocitrate) are mixed together and
heated to 70C and then cooled to 25C (ca. 40 min).
2 ~1 of a diluted T7 DNA polymerase (4 units, Pharmacia)
are then added to the template and primer heated to
37C. In the meantime 3 ~1 of a termination mixture (T-
mix 1: 150 ~l/compound 1; T-mix compound 2: 200 ~M II;
T-mix compound 3: 300 ~M compound 4; each mixture in
addition contains: 1 mM dATP, 1 mM dGTP, 1 mM dCTP, 1 mM
dTTP, 50 mM NaCl, 40 mM Tris HCl, pH 7.5) is pipetted
into four reaction vessels and heated for at least 1
minute to ca. 37C. Subsequently 3.8 ~1 of the first
mixture (annealing mixture) is immediately pipetted into
the preheated termination mixtures. After an incubation
of ca. 10 minutes at 37C, 4 ~1 of a stop reagent
(deionised formamide solution, Dextran blue) is added to
each reaction solution. The reaction solutions are
heated for 2 minutes at ca. 90C and placed in the
individual slots (6 ~1 of each) of a 6 % denaturing
sequencing gel. Subsequently the individual DNA
fragments were detected and identified using a
fluorescence detection instrument.
Figure 1 shows the quotient of fluorescence emission
F (~ex = 488 nm; ~em = 520 nm) and absorbance A (~abs =
265 nm) which was determined for the compounds 1 - 4
during the RP-HPLC analysis.
According to this the fluorescence intensity was lowest
for compound 1. The intensity for compounds 2 to 4
significantly increases with the length of the spacer
function at the 3'(4') position.
Z1 9~2
- 38 -
All four modified nucleotides are accepted as a
substrate by the DNA polymerase used and they have a
termination quality that is comparable for example to
ddTTP. This is remarkable since, due to the bulky
residues at the 3'(4') position of the nucleotides of
compounds 2 to 4 according to the invention the
compounds would not have been expected to be recognized
and converted as substrates by DNA polymerases.
The triphosphates of rhodamine and digoxigenin prepared
as described in examples 20 and 21 are used accordingly.
~9~2
- 39 -
Abbreviation~
According to the proposals of the IUPAC-IUB commission
for biochemical nomenclature (J. Biochem. 138, (1984),
9; J. Biol. Chem. 247, (1972), 977; Biochemistry 9,
(1970), 3471).
ACN acetonitrile
AcOH glacial acetic acid
AA amino acid
CDCl3 deuterochloroform
CH30H methanol
CHCl3 trichloromethane
TLC thin layer chromatography
DCA dichloroacetic acid
DCC N-N'-dicyclohexylcarbodiimide
DCM dichloromethane
ddTTp 2',3'-dideoxy,5'-triphosphate
DIC N-N'-diisopropylcarbodiimide
DIEA diisopropylethylamine
DMAP 4,4'-dimethylaminopyridine
DMF N,N-dimethylformamide
DMTr 4,4'-dimethoxytrityl
DNA deoxynucleic acid
Et20 diethyl ether
FITC fluoroescein isothiocyanate (5-isomer)
Fmoc 9-fluorenylmethoxycarbonyl
HOBt 1-hydroxybenzotriazole
HPLC high performance liquid chromatography
KCN potassium cyanide
linker anchor group between AA and the resin
MeOH methanol
MW molecular weight
ml millilitre
mM millimolar
219~g8~
- 40 -
Mn manganese
nm nanometre
NMR nuclear magnetic resonance
Pmc pentamethylchromane
Rf relative migration path of the sample in TLC
RNA ribonucleic acid
RP reversed phase
RT room temperature
Rt retention time
SPPS solid phase peptide synthesis
T thymidine
T7 Escherichia coli phage T7
Taq Thermococcus aquaticus
TBTU 2-(lH-benzotriazol-lyl)-1,1,3,3-tetramethyl-
uroniumtetrafluoroborate
TEAA tetraethylammonium acetate
TFA trifluoroacetic acid
TFE trifluoroethanol
Tris Tris(hydroxymethyl)aminomethane
Trt triphenylmethyl(trityl)
TTp 5'-triphosphate-thymidine
W ultraviolet
VIS visible
~1 microlitre