Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
005~/44896
The present invention relates to carbamoylu~Lbol,~dLazides of the
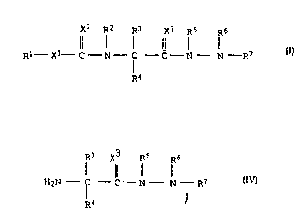
5 general formula I
x2 R2 ~2 X3 Rs ~6
Rl - ,'.' C - ~ - C - C - N - N - R7
~4
15 and their salts, where the radicals have the following meanings:
Rl is Cl-C8-alkyl, C2-C9-alkenyl or C2-C8-alkynyl, it being pos-
sible for these radicals to be partly or completely
halogenated and~or to carry one to three of the following
groups: cyano, Cl-C4-alkoxy, Cl-C4- h~ln~lkn~y~ Cl-C4 - alkyl-
thio, Cl-C4-alku~y~aLbullyl~ C3-Ct-cycloalkyl, C3--C7--cyclo-
alkenyl, aryl, aryloxy and heteroaryl, it being possible for
the cyclic radicals in turn to carry one to three of the fol-
lowing substituents: halogen, cyano, Cl-C4-alkyl, C1-C~-alk-
oxyalkyl, Cl-C4-haloalkyl, Cl-C~-alkoxy, C1-C4-h~lo~l~nRy,
C1-C4-alkylthio, Cl-C4-alku~y~dLLu..ylr aryl, aryloxy and
heteroaryl,
C3 - C~ - cycloalkyl or C3-C7-cycloalkenyl, it being po3sible for
these radicals to be partly or completely h~log~nRt~d and/or
to carry one to three ot the ~ollowing groups: cyano,
Cl-C4-alkyl, Cl-C~-alkoxyalkyl, Cl-C4-haloalkyl, cl-C4-alkoxy,
Cl - C4 ~-ln~lkn~y~ CL-C4-alkylthio, C1-C4-alku~yu,lLLu~lyl, aryl,
aryloxy and aryl-(Cl-C4)-alkyl, it being possible for the
cyclic groups to carry one to three of the following sub-
stituents: halogen, cyano, C~-C~-alkyl, Cl-C~-alkoxyalkyl,
Cl-C~-haloalkyl, C1-C~-alkoxy, Cl-C4-h~lo~l~oxy, Cl-C4-alkyl-
thio, Cl-C4-alkoxycarbonyl, aryl and aryloxy,
gO a nvn ~L~ ' ir' 4- to 8 ~d ring, which, as ring members,
in addition to carbon can also contain one or two of the
he~oat~ oxyqen, sulfur and nitrogen, it being possible
for the carbon atoms in the ring to carry one or two of the
follow;nq groups: halogen, cyano, Cl-C4-alkyl, Cl-C4-alkoxy-
~5 alkyl, Cl-C4-haloalkyl, Cl - C4 lkoxy, Cl - C4 - h~1n~1knRyr
Cl-C4-alkylthio, Cl-C4-alku~y~Lbu-.yl, aryl and aryloxy and
~ 0050~44896
219199~
the second and each further nitrogen atom as a heteroatom in
~ the ring carrying hydrogen or a Cl-C4-alkyl group,
aryl or heteroaryl, it being possible for these radicals to
carry one to three of the following groups: halogen, cyano,
Cl-C~-alkyl, Cl-Cc-~qlkoxyalkyl, cl-c~ qlkyl~ C1-C~-alkoxy,
~ Cl-C~-hqlnalk~Yy, Cl-C4-alkylthio, Cl- Cq -alku~y-~-Lo.. yl, aryl,
aryloxy and heteroaryl, in which the cyclic substituents in
turn can carry one to three of the following substituents:
lo halogen, cyano, C1-C~ alkyl, Cl-C~-alkoxyalkyl, C1-C4-halo-
alkyl, Cl-C~-alkoxy, Cl-c4-h~lo~lk~Ty~ Cl-C4-alkylthio and
Cl-C4-alku~y~Lbv..yl or
WlW2C=N-, where
Wl is Cl-C8-alkyl which can be partly or ~ ly halo-
genated and/or can carry one to three of the following
groups: cyano, Cl-C~-alkoxy, Cl-c4-hql~l k~Yy~
C1-C4-alkylthio, aryl, aryloxy and heteroaryl, it being
zo possible for the cyclic groups in turn to carry one to
three o~ the following substituents: halogen, cyano,
Cl - C4 - alkyl, CL - Cq - d1kOXYa1kY1, Cl - C~ ~-1 oqlkyl,
Cl-C4-alkoxy, Cl-c4-halnAlk~yy~ C1-Cq-alkylthio,
C1-C4-alkoxy~Lbu.,yl, aryl and aryloxy,
C2-C~ alkenyl or C2-C~-alkynyl, it being possible for
these radicals to be partly or completely halogenated
and/or to carry one to three of the following groups:
cyano, C1-Cc-alkoxy~ C1-C4-hqlo-qlk~y, C1-Cq-Rlkylthio,
C1-C4 alkù~y~Lbu~yl~ aryl and aryloxy, it being possible
for the cyclic groups in turn to carry one to three of
the following substituents: halogen, cyano, C1-C4-alkoxy,
Cl--C~--h~lo~ Yy, Cl-C4--alkylthio,Cl--C4--alkoxycarbonyl,
aryl and aryloxy,
C~-C7-cycloalkyl or C3-C~-cycloalkenyl, it being possible
for these radicals to carry one to three of the following
groups: halogen, cyano, C~ - C4 - alkyl, C1-C4-alkoxyalkyl,
Cl-c4-haloalkyl~ C1-C4-alkoxy, Cl-C4-ha~ k~y,
C1-C~-alkylthio, C1-C4-alku~cdrbu-,yl, aryl and
aryl-(cl-c4)-alkyl~ it being possible for the groups
which contain aryl to carry one to three o~ the following
substituents: halogen, cyano, Cl-c~-alkyl, Cl-C4-alkoxy-
alkyl, C1-C4-haloalkyl, Cl-C4-alkoxy, C1-C4- h~ln~lk~y~
C1- Cq -alkylthio, C1-4-alkoxycarbonyl, aryl and aryloxy or
' ' o~50/44896 2 1 9 1 9 ~ 9
~ 3
~ryl or heteroaryl, it being possible for these radicals
to carry one to three of the following groups: halogen,
cyano, Cl-C4-alkyl, Cl-C4-alkoxyalkyl, Cl-C4-haloalkyl,
Cl-C~-alkoxy, Cl-c4-h~ln~lknyy~ C1-C4-alkylthio,
Cl-C~-alhu~r.aL~ullyl, aryl and aryloxy and
W2 is hydrogen or one of the groups Wl;
R2 is hydrogen or Cl-C8-alkyl or C3-C7-cycloalkyl, which can be
partly or ~ _letP1y h~ n~t~;
R3 is Cl-C8-alkyl, it being pos3ible for this radical to carry
one to three of the following groups: halogen, cyano,
Cl-C4-alkoxy, C1-C4-h~ knYy~ C1-C4-alkylthio and
1~ C1-C4-alhu~calL~
C3-C7-cycloalkyl or phenyl-(Cl-C4)-alkyl, it being possible
for the rings of these radicals to carry one to three of the
following groups: halogen, cyano, Cl-c~-alkyl, Cl-C4-alkoxy-
ZO alkyl, Cl-C~-haloalkyl, Cl-C~-alkoxy, Cl-C4-haloalkoxy,
Cl-C4-alkylthio, Cl-C~-alku~y~Lb~n~l, aryl and aryloxy;
R4 is hydrogen or one of the radicals R3 or
ZS R3 and R4, together with the C atom to which they are bonded,
are a 4- to 8- ~d ring which, as ring members, in
addition to carbon can also contain one or two of the
heteroatoms oxygen, sulfur and nitrogen, it being possible
for the carbon atoms in the ring to carry one or two of the
following groups: halogen, cyano, Cl-C4-alkyl, Cl-C4-alkoxy-
alkyl, C1-C4-haloalkyl, C1-C4-alkoxy, C1-C4- halo~lknxy~
Cl-C4-alkylthio, Cl-C~-~lkoxycarbonyl, aryl and aryloxy and
nitrogen as a heteroatom carrying hydrogen or a C1-C4-alkyl
group;
R5 is one of the radicals R2;
R6 is Cl-C8-alkyl, C2-C8-alkenyl or C2-C8-alkynyl, it being pos-
sible for these radials to be partly or completely halo-
genated and/or to carry one to three of the following groups:cyano, Cl-C4-alkoxy, Cl-C~-h~lo~lknxy, Cl-C4-alkylthio,
C3-C7-oycloalkyl, C3-C7-cycloalkenyl, aryl, arylaxy and
heteroaryl, it being possible for the cyclic radicals in turn
to carry one to t!-ree of the following substituents: halogen,
cyano, Cl-C4-alkyl, Cl-C4-alkoxyalkyl, C1-C4-haloalkyl,
, . _ . .. _ _ . . _ .. ... .. ... . _ _ _ _ _ . _
~- 0o5of44896 2 1 9 1 9 9 ~
.
Cl-C~-alkoxy, Cl-C4-h~loAlkrYy~ Cl-Cq alkylthio, aryl, aryloxy
and h~ vhLlrl,
C3 - C7 - cycloalkyl or C3-C7-cycloalkenyl, it being possible for
S these radicals to be partly or completely halogenated andJor
to carry one to three of the ~ollowing groups: oyano,
Cl-C~ alkyl, Cl-C4-alkoxyalkyl, Cl-C4-haloalkyl, C1-C~-alkoxy,
Cl-C~ h ln-l~nYy~ Cl-C4-alkylthio, aryl, aryloxy and
aryl-(Cl-C~)-alkyl, it being possibLe for the cyclic groups to
carry one to three of the following substituents: halogen,
cyano, Cl--C4--alkyl,Cl-C4--alkoxyalkyl,Cl--C4--haloalkyl,
Cl-C~-alkoxy, Cl-C4-h~oAlkn~y, Cl-C4-alkylthio, aryl and
aryloxy,
a non-aromatic 4- to 8 ~ ed ring, which, as ring members,
in addition to carbon can also contain one or two of the
heLe.~a~ oxygen, sulfur and nitrogen, it being possible
for the carbon atoms in the ring to carry one or two of the
following groups halogen, cyano, C1-Ci-alkyl, Cl-C4-alkoxy-
alkyl, Cl-C4-haloalkyl, C1-C4-alkoxy, Cl - C4 - h~ 1 nR ~ koxy,
Cl-C~-alkylthio,'aryl and aryloxy and the second and each fur-
ther nitrogen atom as a heteroatom in the ring carrying
hydrogen or a Cl-C~-alkyl group,
aryl or heteroaryl, it being possible for these radicals to
carry one to three of the following groups: halogen, cyano,
Cl-C4-alkyl, Cl-C4-alkcxyalkyl, C1-C~-haloalkyl, Cl-C4-alkoxy,
Cl-C~-h-lo~l~nYy, Cl-C4-alkylthio, aryl, aryloxy and hetero-
aryl, in which the cyclic substituents in turn can carry one
to three of the following substituents: halogen, cyano,
Cl-C~-alkyl, C1-C4-alkoxyalkyl, Cl-C4-haloalkyl, Cl-C~-alkoxy,
Cl - C4 - h~lr)~lknyy and Cl-C4-alkylthio or
if R3 is isopropyl, hydrogen;
R7 is one of the radicals R6, excluding hydrogen;
Xl is oxygen or sulfur,
X2 i5 oxygen or sulfur,
40 X3 is oxygen or ~ulfur;
e rr~lnrling the . ~ ds according to the following definition of
the radicals:
45 Ia-d ~Rl = Ph-CH2; R2 = ~; R3 = C~3, (c~2l2c~3r c~(C~3)2 or
Ph--CE~2; R4,Rs = }~: R5,R7 = CE~2CE~zCl) ~
, . . , _
~ 00~0~44896 2 1 9 1 9 g ~
~ 5
Ie-h ~Rl = C(CH333; R2 5 H; R3 = CH3, CH(CH3)2, CH(CH3)CH2CH3,
CH2CH(CH3)2 or Ph-CH2; R4,R5 = H; R6,R7 = CR2CH2Br),
Ii tRI 5 Ph-CH2; R2 = H; R3 = CB3; R4,RS - H; R6 = CH3; R7 =
Ph),
5 Ij-k (Rl = Ph-CH2; RZ = H; R3 = CH(CH3)~ or CHlCH(C~3)2; R~,R5 =
H; R5,R7 ~ Ph),
Il (Rl = C(CH3)3; R2 = H; R3 = CH(CH3)2; R4,R5,R6 5 H; R7 =
Ph),
-Im (Rl = Ph-CM2; R2 = ~; R3 s Ph-C~2; R4, R5 = H; R5,R7 =
C112CH3 ) ~
In o (RL = Ph-CH2; R2 = N; R3 ~ CH25CH3 or CH2CH2SCM3; R4,Rs =
11; Rs,R7 = CH2CH2Cl).
The invention additionally relates to ~L~cs3a3 for preparing the
15 ~ _..ds } and their salts, compositions containing these sub-
stances, and a method for controlling harmful fungi and the use
of these substances therefor.
In addition, the present invention relates to amino acid hydra-
20 zide i~ tes of the general formula IV
R3 X3 Rs R6
H2N - C - C - N - N - R7 IV
30 where X3 is oxygen and R3 is a group CH(CH3)2, CH2CH(CH3)2 or
C~C~3)C2H5 and R4, R5, R6 and R7 have the meaning given in claim
1, ~Y~Iu~;ng the compounds according to the following definition
of the radicals:
35 IVa-b (R3 = CH(CH3)2 or (CH2)2CH3; R4,Rs = H; R6,R7 = CH2CH2Cl),
~Vc-e (R3 = CH(CH3)2, CE(CH3)CH2CH3 or C~2CH(CH3)2; R4,Rs = H;
R6,R7 = CH2CH2Br),
IVf (R3 ~ C~tCH3)2; R4,R5,R6 = H; R7 = Ph),
IVg (R3 = CH(CH3)2; R4,R5 = H; R5,R7 = Ph).
~0
Compositions are known from D~-B 1 l9g 5~0 which contain alkyl-
caLLol-~d~zideq, eg. the compound of the following formula
~ 0050/44896
2191999
o
H2-c~-c~2 l NH--N~
s
and which have an action against powdery mildew fungi.
JP - A 69/27 997 additionally describes the use of 4-methoxy-
10 carbcnylterephthalic hydrazide or terephthalic bishydrazide fcr
controlling rice blast.
However, these agents are still not satisfactory as fnn7ic~ s.
15 Further ~Lboh~v'Lazides are additionally known from the litera-
ture; nothing has been repcrted, however, of a fnntJi~idal action
of these ~ ,u~-ds (cf. Pharmazie 44, ~1988) page 608 to page
611; Pharmezie 44, (1989) page 316 to page 317; J. Org. Chem 36,
(1971) page 1580 to page 1584; Farm~ Pol. 28, (1972) page 615;
20 EP-a 361 977; Collect. Czech. Ccmmun. 49, (1989) page 2551 tc
page 2561).
It is an object of the present invention to provide novel
carbamoylcarbohydrazides having improved action against harmful
25 fungi.
we have found that this object is achieved by the ~, ~ 'z I and
their salts described at the cutset.
30 FULtl1 U, we have found i vL~an~ intermediates of the general
formula rv ~or preparing these substances.
we have additionally found compositions containing them, pro-
cesses for preparing the c ~,v~..d~ I and their salts and the com-
35 pcsitions containing them and in addition a process for control-
ling harmful fungi and the use of the c _ '~ I and their salts
therefor.
~he c ~u--~s I and their salts can be prepared starting from the
40 uuLL~a~uu..ding carbamoylcarboxylic acids II. Preferably, they are
obtained by the yLu~ S A, B and C described in the fcllowing
tthe ~ouben-Weyl refer~nces relate to: Buu'v~l~ t7_yl~ Methoden der
org~n;c~h~n Chemie (Methcds of Organic Chemistry), 4th editicn,
Thieme Verlag, Stuttgart).
4~
0050/44896
- ' 2i91993
t
Process A
The carbamoyl~L~L~d~zides I are obtained by reacting the
carbamoylcarboxylic acids II with the hydrazines III.
s
X7 R2 R3 X3 Rs Rs
Il l l 11 1 1
Rl--Xl--C--N--C --C--011 + Ei--N-- N -- R7 ~
II III
X2 ~2 R3 X3 Rs R6
Rl--xl--C--N--C --C--N-- N--R7
R4
I
The carbamoylcarboxylic acids II are known or obtainable by known
methods, r~peri~l7y starting from the amino acids on which they
are based ~cf~, for example, n~ubell~eyl UMethoden der organi-
schen Chemie~ [Methods o~ Organic Chemistryl, Volume I5/1, 4th
25 Edition, Thieme Verlag Stuttgart 1977; 3P 53148530; JP 52151146;
J. Org. Chem. 43 11978], 2g30-2932; J. Chem. Soc., Perkin Trans
1, 1972, 1983-1985; Chem. Ber. 104 tl971], 31S6-3167; J. Org.
Chem. 36 [19711, 49-59; ~elv. Chim. Acta 52 [1969], 282-291;
Tetrahedron 34 11978], 2763-2766; Chem. Pharm. Bull. 19 [1971],
30 912-929, 3. Chem. Soc. 1952, 2076-2079~.
The hydrazines III are likewise known or easily obtainable (cf.
nouben-Weyl, volume 10~2, page 1 to page 71 and page 169 to page
409, ~p~riAlly page 396 to page 399 and page 402 to page 405).
This process A is preferably carried out by first converting the
carbamoylcarboxylic acids II to carboxyl-activated derivatives,
esperi~lly to acyl cyanides or anhydrides (cf. PharmaZie 4~,
(1989) page 316 to page 317 and page 608 to page 611; Tetrahedron
40 Letters, volume 18, (1973) page 1595 to page 1598 and ~ouben-
~eyl, volume 15/1, page 28 to page 32). These derivatives are
then reacted with the hydrazines III in the presence of bases.
~ 0050~44896
2~91~99
A suitable reaction for preparitg the carboxyl-activated acyl-
cyanides is eg. the reaction of the carbamoylcarboxylic acids II
with diethyl ~y~ r~ h~n~te~ Pcp~ lly in an inert solvent
such as tetral.~lLuCuLdll, toluene or dichloromethane.
To prepare the carboxyl-activated anhydrides, the reaction of the
carbamoylcarboxylic acids II with carbonyl ~hl~rid~c such as iso-
butyl chluLvCuL~.~Le in the presence of bases and in the presence
or absence of an inert solvent such as toluene or tetraLyd
10 is preferred.
The reaction of the hydrazines III wlth the carboxyl-activated
carbamoyloarboxylic acids II is preferably carried out in a sol-
vent such as dichloromethane, tetrahydLuruLdn or toluene.
In particuiar, the bases used can be the hydrazines III them-
selves, the bases customarily being L~u~ td from the crude
product.
20 In a preferred -'i L of this stage of the process, the
carbamoylcarboxylic acid II, the hydrazine III, the reagent
suitable for generating the carboxyl-activated derivative o~ the
carbamoylcarboxylic acid II and the base are reacted in a one-eot
process, if appropriate in an inert solvent, and the crude
25 product is then worked up to the carbamoylcaLbGI.yl~zide I in a
manner known per se.
Process B
30 The carbamoylcarbohydrazides I, in which Rl is a group -WIW2C=N-,
are also obtained by converting the carbamoyLudLbol-ydLdzides I,
in which Xl, x2 and X3 are in each case oxygen, and the group
RL-Xl-(CO) is a protective group which can be removed in a manner
known per se, to carbohydrazides IV and reacting these with chlo-
35 roformyl oximes V in the presence of bases.
Stage ~a: Preparation of the carbohydrazides IV
't
ooSOf44896
219199~
XZ R2 R3 X3 R5 ~6
Il I I 11 1 1 ~
R1 - Xl - C - N - C - C - W - N - R7
R4
I
R2 R3 X3 R~ R6
~ - N - f - C - N - N - R7
R4
IV
15 The removal of the group Rl-XL-~C0) from the carbamoylcarbohydra-
zides I can be carried out in a manner known per se (cf ~ouben-
Weyl, volume 15/1, page 46 to page 305, ~cpe~iAlly page 126 to
page 129; Pharmazie 4~, (1989) page 316 to page 317).
20 Suitable removable groups r~cpr?~;~lly contain the tert-butyl group
znd additionally the benzyl group as a radical R1.
If R1 = LeL~ bu8yl, for example, removal ~as~ rily takes place
by reaction with an acid, in particular a protic acid such as
25 hydrochloric acid, hYdLUb1~ r' acid or trifluoroacetic acid (loc.
cit.).
~he carbamoylcarbohydrazides I suitable as ~tarting substances
are obtainable by known ~LVU~55eS (cf. Pharmazie 44, ~1989) page
30 316 to page 317 and page 608 ~o page 611; ~ouben-Weyl, volume
15Jl, page 28 to page 32~ or in particular by process A according
to the invention.
Stage Bb: Preparation of the carbamoylcaL'vGl.~dLdzides I
0050/44896
~191999
~ 10
wl O R2 R3 X3 R5 R6
li I 1 11 1 ~
C=~--O--C - Cl I H - ~ - f _ c _ N - N - R7 -
w7 R~
v IV
Wl o R2 ~3 X3 Rs R6
C=~ - o - C - N - C - C - N - N - R7
w2
lS
The carbohydrazides IV resulting ~rom the synthesis stage 13a)
are reacted ~ith the chloroformyl oximes V in the presence of
bases.
20 The chloroformyl oximes V are known or obtainable by known pro-
cesses, eg. by phosgenation of oximes (cf. Z. Chem. 9, (19671
page 344 to page 3451.
The reaction is preferably carried out in an organic solvent,
25 ~speciAlly in toluene, methylene chloride, tetral.~d VfUl-l~ or
mi~tures of these.
suitable bases are equally inorganic and organic bases, organic
bases and, among these in turn, tertiary amines such as triethyl-
30 amine, pyridine and N-methylpiperidine being preferred.
As a rule, the reaction is carried out at from -40 to 50 C, pre-
ferably from -10 to 20-C.
35 The carrying-out of this reaction is otherwise famiLiar to the
person skilled in the art, so no further explanations are needed
(c~. ~ouben-Weyl, volume lS/I, page 117 to page 125; ~ev.
Endocrinol. 13 (11_~L~L~v~h~eal Pept. norm. Other 3iol. Act-
Pept.1, (19811 page 37 to page 47).
Process C
The carbamoylcarbohydrazides o~ the general formula I according
to claim 1, in which R6 is C~-C8-alkyl, C4-C7-cycloalkyl or
~5 aryl-(Cl-C4)-alkyl, are also obtainable by reacting carbamoyl-
carbohydrazides of the general formula I according to claim 1, in
which R6 is hydrogen, with a compound of the general formula R6-X
; OoSoJ4~896 ~ ' ~ 1 9
~ 11
in which R6 is Cl-Ca--alkyl,C4-C7-cycloalkyl or aryl-(C1-C~)-alkyl
and X is a negative leaving group, with additional use of a base.
The carbamoyl~a-LvllylL~zides of the general formula I, in which ~6
5 is hydrogen, are ~h~lin~hlo in a manner known per se ~cf.
D~-A 1 089 390; zh. Org. Khim. 14 (1978), page 1086), or in par-
ticular by process a according to the invention.
A suitable negative leaving group X ifi the methylsulfonyl or the
10 4-methylphenylsulfonyl anion and preferably halide anions such as
iodide and in particular chloride and bromide.
The bases used are ~po~i~lly alkali metal carbonates and
~ Y;~~ such as sodium o~rbnn~o, 30dium methoxide and sodium
15 ethoxide, in addition alkaline earth metal carbonates such as
calcium carbonate, alkali metal or alkaline earth metal
hydroxides, eg. 60dium hydroxide and calcium hydroxide, hydrides,
eg. sodium hydride, hydluJ-~ tes, eg. sodium hydrogen-
carbonate, alkanes, eg. n-butyllithium, or tertiary amines such
20 as triethylamine.
The reaction can be carried out without solvent or in a solvent
which is inert under the reaction conditions, such as dimethyl-
f~rr~ o, tetrallyJLvfuL~n~ hl. ~hane or alkanols such as
25 methanol or ethanol.
As a rule, the reaction is carried out at from 10 to 60 C and
under a; ~ ic pressure.
30 Other~ise, the process is carried out in a manner known per se
(cf. ~30uben-Weyl, volume 10/2, page 402 to page 40~).
The reaction mixtures obtained by pLv~e~es A, B and C are
customarily worked up to the c _ ~- I in the customary manner,
35 eg. by mixing with water, separating the phases and, if neces-
sary, purifying the crude products by ~ LOYL~PI.Y~ The inter-
mediates and final products are obtained in some cases in the
form of cnl~r30ss or slightly brownish, viscous oils whioh can be
freed from the volatiLe , Ls under reduced pressure and at
40 moderately elevated ~ ~ULe. If the Llt ~-~tes and final
products are obtained as solids, they can also be purified, for
example, by recrystallizing or digesting.
4'i
0050/~4896 - 2 1 9 1 9 ~ ~
12
Depending on the nature of the substituents, the ~ ' of the
~ormula I can, if approeriate, be present as ge~metrical and/or
optical isomers or isomer mixtures. ~oth the pure isomers and the
mixtures of the isomers have fnn~ir;f~l action.
The salts are also part of the invention, in particular of the
acid-stable c _ '- } which contain basic centers, f~pff~;~lly
ba~3ic nitrogen atoms, ie. in particular the salts with mineral
acids such as sul~uric acid and plos~hvLic acid or Lewis acids
10 such as zinc chloride. Customarily, the nature of the salts does
not matter. In the context of the invention, those salts are pre-
ferred which do not damage the plants, sur~aces, materials or
spaces to be kept free o~ harmful fungi and do not adversely
affect the action of the ~ , ' I.
The salts of the ~ ,ds I are ~f~ csih]e in a manner known per
se, espef~i~lly by reacting the CULLI~ ;ng carbamoylcarbo-
hydrazides I with said acids in water or an inert organic solvent
at from -30 to 120 C, preferably from 0 to 60'C.
In the preparation o~ the carbamoylcaLb~hydL~zides I according to
process B, there are also cbtained novel int -i~t~c of the
general formula IV
~.3 X3 E~.S R6
Il l I
~2~ C - N - N - R7 IV
~4
where X3 is oxygen and R4, R5, R6, and R7 have the meanings given
in claim 1 and R1 is a group C~( C~3 ) 2 ~ CH2C~ ~ C~3 ) 2 or C~( C~3 ) C2~5 ~
35 the ~ ~ according to the following definition of the radi-
cals already being known:
IVa-b (R3 = C~(CR3)2 or (C~212C~3; R4,Rs = ~; R5,R7 = C~zC~2Cl),
IVc-e (R3 = C~(C~3)2, C~(c~3lC~2C~3 or CH2c~(cs3)2; R~,R5 = H;
R6,R7 - C~2C82~r),
IVf (R3 = C~(C~3)2; R~,R5,R6 = ~; R7 = Ph),
IVg (R3 = CH(C~3)2; R~,R5 = ~; R6,R7 = Ph)
0050J44896 2 1 9 1 ~ ~ ~
~ 13
(cf. Pharmazie, 44, (1989) page 608 to page 611; Pharmazie, 44,
(1989) page 316 to page 317; EP-A 0 361 977; Int. J. Pept.
Protein Res. 21 (19B3) page 406; ~arm. Pol. 28, (1972) page 615
to page 619).
s
Among the novel amino acid hydrazides IV, those are preferred in
which R3 is a group CH(C~3)2, R4 and R5 have the meanings given in
claim 1,
lo R6 is Cl-C8-alkyl, it being possible for this radical to be
partly or completely hAlng~nA~d and/or to carry one to three
of the following groups: cyano, C1-C4-alkoxy, C1-C~-halo-
alkoxy, Cl--Cj--alkylthio,C3--C7--cycloalkyl,C3--C7--cycloalkenyl,
aryl, aryloxy and heteroaryl, it being possible for the
cyclic radicals in turn to carry one to three of the
following substituents: halogen, cyano, C1-C4 alkyl,
C1-C~-alkoxyalkyl, Cl- C4 ~lnAlkyl~ C1-C4-alkoxy, Cl-C~-halo-
alkoxy and C1-C~ alkylthio, aryl, aryloxy and heteroaryl and
20 R7 is aryl, it being possible for this radical to carry one to
three of the following groups: halogen, cyano, C1-C4-alkyl,
Cl - C4 alkoxyalkyl, C1-C4-haloalkyl, C1- C4 alkoxy, C1-C4-halo-
alkoxy, C1-C4-alkyl~hio, aryl, aryloxy and heteroaryl, in
which the cyclic substituents in turn can carry one to three
25 of the following substituentsl: halogen, cyano, C1-C~-alkyl,
Cl-C~-alkoxyalkyl, C1-4 -haloalkyl, Cl-C4 alkoxy, Cl-C4-halo-
alkoxy and Cl-C~-allcylthio.
~ith respect to their use for preparing carbamoylcarbohydra-
30 zides I, the : ~- IV compiled in the following tables are
particularly preferred.
Table 1
C\ / C~3
C~ ~ R5 R6
l l 11 1 1
~ - N - C - C - ~ - N - R7
~o. Rs R5 R7
45 1 ~ C~3 C6~5
2 ~} Ci~3 4--Cl--C6Ei4
3 H c~3 4-c~3-c6~q
0050,4~896 2i9~9~
.
14
~o. R~ R~ R7
4 B CH3 4-OCH3-c6H4
H CH3 3,4-(0CH3)2-C6H3
5 6 C1i3 CH3 4-cl-c6H4
8 H CH3 4-Cl-C6Bq
9 H CH3 4-CH3-C6H4
H CH(CH3)2 CH2-(4-Cl-C6H4)
11 H CH~CH3)2 4-Cl
12 B CR~CH312 cH(c6Hs)(4-cl-c6H4)
13 H Cn(CH3l2 4-cl-cGH4
14 c~3 CH(CH312 4-CB3-C6H~
H CH(CH3)2 4-OCH3-C6H4
15 16 H CH(CH3)z 3~4-(ocH3)2-c6B3
17 H CH2CH3 CH2(4-Cl-C6H~)
18 B CH2CH3 CHCH3(4-Cl-C6H4)
19 H CH2CH3 cH(c6Hs)(4-cl-c6H4)
20 20 CH3 CH2CH3 4-Cl-C6B4
21 H CH2CH3 4-CH3-C6R4
22 H CB2CH3 4_oCH3-C6H4
23 H CH2CR3 3~4-(ocH3)2-c6H3
H cyclc-CgHg CH2(4-cl-c6H~)
H cyclo-C5Hg CH(CH3)(4-Cl-C6B4)
26 H cyclo-C5Hg CH(C6H5)(4-Cl-C6H4)
27 H cyclc-C5B9 4-C1-C6B4
2S B cycl~-C5H9 4--CB3--C6H4
29 H cyclr~-C5H9 4--0Ca3--C6H4
H cyclo-C5H9 3~4-(ocH3)2-c6H3
31 H cycl~-C6Hll CB2(4-cl-c6H4)
32 ~ cyclo-C6HIl CH(CH3)(4-Cl-C6H4)
35 33 B cyclo-C6Hll CB(C6Hs)(4-Cl-C6H4)
34 B cyclo-C6Hll 4--Cl--C6B4
H cycl~--C6H11 4--CB3--C6H4
36 H cyclo-C6Hll 4-OCB3-C6H4
40 37 H cyclo-C6Hll 3,4-(OCH3)2-C6H3
3S CH3 CB3 C6H5
39 CH3 CH3 4-Cl-C6H4
CH3 CH3 4-CB3-C6H~
gl CH3 CH3 4-OCH3-C6~4
~5 CH3 CH3 3,4-(OCH3)2-C6H3
0050~44896 ~ 21919~
~ 15
In the definition of the ~ ' ~ given at the outset, collec-
tive terms were used which are L~lesa..~ative of the following
substituents:
5 halogen: fluorine, chlorine, bromine and iodine;
alkyl: straight-chain or branched alkyl groups having 1 to 8
carbon atoms, eg. Cl-C6-alkyl such as methyl, ethyl, n-propyl,
1-methylethyl, n-butyl, 1-methylpropyl, 2-methylpropyl,
lO l,1-dimethylethyl, n-pentyl, 1-methylbutyl, 2-methylbutyl,
3_methylbutyl, l,l-dimethylpropyl, 2,2-dimethylpropyl,
1,2-dimethylpropyl, l-etllylpropyl, n-hexyl, l-methylpentyl,
2-methylpentyl, 3-methylpentyl, ~-methylpentyl, 1,1-dimethyl-
butyl, 2,2-dimethylbutyl, 3,3-dimethylbutyl, l,2-dimethylbutyl,
15 1,3-dimethylbutyl, 2,3-dimethylbutyl, l-ethylbutyl, 2-ethylbutyl,
1,1,2-trimethylpropyl, 1,2,2-trimethylpropyl, 1 othyl-l-methyl-
propyl and 1 cthyl-2-methylpropyl;
haloalkyl or partly or ~ ~let~ly halogenated alkyl: straight-
20 chain or branched alkyl groups having 1 to 4 or 8 carbon atoms
(as mentioned above), it being possible in these groups for the
hydrogen atoms to be partly or completely replaced by halogen
atoms (as mentioned above), eg. Cl-C2-haloalkyl such as chloro-
methyl, dichloromethyl, trichloromethyl, fluoromethyl, difluoro-
25 methyl, trifluoromethyl, chlorofluoromethyl, dichlorofluoro-
methyl, chlorodifluoromethyl, l-fluoroethyl, 2-fluoroethyl,
2,2-difluoroethyl, 2,2,2-trifluoroethyl, 2-chloro-2-fluoroethyl,
2-chloro-2,2-difluoroethyl, 2,2-dichloro-2-~1uoroethyl,
2,2,2-trichloroethyl and pentafluoroethyl;
alkoxy: straight-chain or branched alkoxy groups having l to 4
carbon atoms, eg. Cl-C3-alkoxy such as methoxy, ethoxy, propoxy
and l-methylethoxy;
35 alkoxyalkyl: straight-chain or branched alkyl groups having 1 to
8 carbon atoms (as mentioned above), which in any desired posi-
tion carry a straight-chain or branched alkoxy group (as
mentioned above) having, in the case of C~-C4-alkoxy, 1 to 4
carbon atoms, such as methoxymethyl, ethoxymethyl, n-propoxy-
40 methyl, n-~Lv~ Ll-yl, 1-methoxyethyl, 2 '~ y~Lhyl, 1-ethoxy-
ethyl, 2-ethoxyethyl, 2-n-y.u~u~Lhyl and 2-butoxyethyl;
bAl ~Al kn~y: straight-chain or branched alkoxy groups having 1 to
4 carbon atoms tas mentioned above~, it being possible in these
g5 groups for the hydrogen atoms to be partly or completely replaced
by halogen atoms (as mentioned above), eg. C1-C2-h~l~Alkn~y such
as chluL~ ~hu~y, dichloL Lllu~y, trichlu-~ Lhu~y~ fluoro-
00s0~44896 2 1 9 1 ~ 9 ~
.
16methoxy, diiluoromethoxy, trifluo.~ ~h~y, chloro~luoromethoxy,
dichlorofluoromethoxy, chlorodifluoromethoxy, 1-fluoroethoxy,
2-fluuLv~ yt 2,Z-difluulve~L~y, 2,2,2-trifluoroethoxy,
2-chloro-2-fluoroethoxy, 2-chloro-2,2-di~1uoroethoxy,
5 2,2-dichloro-2-~lu~.o~Lhu~y~ 2,2,2-trichlolceU.v~y and penta-
f 1UVL V~ l.hu~y;
alkylthio: straight-chain or branched alkyl groups having 1 to 4
carbon atoms (as r ion~d above), which are bonded to the struc-
10 ture via a suliur atom (-,S-), eg. Cl-C~-alkylthio such as methyl-
thio, ethylthio, propylthio, 1-methylethylthio, n-butylthio and
tert-butylthio;
alkoxycarbonyl: straight-chain or branched alkoxy groups haring 1
15 to 4 C atoms (as mentioned above), ~hich are bonded to the struc-
ture via a carbonyl group (-C0-);
alkenyl: straight-chain or branched alkenyl groups having 2 to 8
carbon atoms and a double bond in any desired position, eg.
20 C2-C6-alkenyl such as ethenyl, l-prcpenyl, 2-propenyl, 1-methyl-
ethenyl, 1-butenyl, 2-butenyl, 3-butenyl, 1-methyl-1-propenyl,
2-methyl-1-propenyl, 1-methyl-2-propenyl r 2-methyl-2-propenyl,
2-methyl-1-prcpenyl, 1-methyl-2-propenyl, 1-pentenyl, 2-pentenyl,
3-pentenyl, 4-pentenyl, l-methyl-l-butenyl, 2-methyl-1-butenyl,
25 3-methyl-1-butenyl, 1-methyl-Z-butenyl, 2-methyl-2-butenyl,
3-methyl-2-butenyl, 1-methyl-3-butenyl, 2-methy1-3-butenyl,
3-methyl-3-butenyl, 1,1-dimethyl-2-propenyl,
1,2-dimethyl-1-prcpenyl, 1,2-dimethyl-2-propenyl,
l-ethyl-l-propenyl, l-ethyl-2-propenyl, l-hexenyl, 2-hexenyl,
30 3-hexenyl, 4-hexenyl, 5-hexenyl, l-methyl-l-pentenyl,
2-methyl-1-pentenyl, 3-methyl-1-pentenyl, 4-methyl-1-pentenyl,
1-methyl-2-pentenyl, 2-methyl-2-pentenyl, 3-methyl-2-pentenyl,
4-methyl-2-pentenyl, 1-methyl-3-pentenyl, 2-methyl-3-pentenyl,
3-methy1-3-pentenyl, 4-methyl-3-pentenyl, 1-methyl-4-pentenyl,
35 2-methyl-4-pentenyl, 3-methyl-4-pentenyl, 4-methyl-4-pentenyl,
1,1-dimethyl-2-butenyl, 1,1-dimethyl-3-butenyl,
1,2-dimethyl-1-butenyl, 1,2-dimethyl-2-butenyl,
1,2-dimethyl-3-butenyl, 1,3-dimethyl-1-butenyl,
1,3-dimethyl-2-butenyl, 1,3-dimethyl-3-butenyl,
40 2,2-dimethyl-3-butenyl, 2,3-dimethyl-1-butenyl,
2,3-dimethyl-2-butenyl, 2,3-dimethyl-3-butenyl,
3,3-dimethyl-1-butenyl, 3,3-dimethyl-2-butenyl,
1 cthyl-l-butenyl, 1-ethyl-2-butenyl, 1-ethyl-3-butenyl,
2-ethyl-1-butenyl, 2-ethyl-2-butenyl, 2-ethyl-3-butenyl,
45 1,1,2-trimethyl-2-propenyl, 1-ethyl-1-methyl-2-propenyl,
l-ethyl-2-methyl-1-propenyl and 1-ethyl-2-methyl-2-propenyl;
0050/g4896 17 2 1 ~ 1 ~ 9 ~
alkynyl: straight-chain or branched alkynyl groups having 2 to 8
carbon atoms and a triple bond in any desired position, eg.
C2-C6-alkynyl such as ethynyl, 1-propynyl, 2-propynyl, 1-butynyl,
2-butynyl, 3-butynyl, 1-methyl-2-propynyl, 1-pentynyl,
5 2-pentynyL, 3-pentynyl, 4-pentynyl, 1-methyl-2-butynyl,
1-methyl-3-butynyl, 2-methyl-3-butynyl, 3-methyl-1-butynyl,
1,1-dimethyl-2-propynyl, 1 cthyl-2-propynyl, 1-hexynyl,
2-hexynyl, 3-hexynyl, 4-hexynyl, S-hexynyl, 1-methyl-2-pentynyl,
l-methyL-3-pentynyl, 1-methyl-4-pentynyl, 2-methyl-3-pentynyl,
10 2-methyL-4-pentynyl, 3-methyl-1-pentynyl, 3-methyl-4-pentynyl,
4-methyl-1-pentynyl, 4-methy1-2-pentynyl, 1,1-dimethyl-2-butynyl,
1,1-dimethyl-3-butynyl, 1,2-dimethyl-3-butynyl,
2,2-dimethyl-3-butynyl, 3,3-dimethyl-1-butynyl,
1 ethyl-2-butynyl, 1-ethyl-3-butynyl, 2 ethyl-3-butynyl and
I5 1-cthyl-1-methyl-2-propynyl;
cycloalkyl: monocyclic a7kyl groups having 3 to 7 carbon ~ing
members, eg. C3-C7-cycloalkyl such as cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl and cycloheptyl;
2~
cycloalkenyl: monocyclic alkyl groups having 5 to 7 carbon ring
members which contain one cr more double bonds, eg.
C5-C7-aycloalkenyl such as cyclopentenyl, cyclohexenyl and cyclo-
heptenyl;
non-aromatic 4- to 8-membered rings, which, as ring members, in
addition to carbon also contain one or two oxygen, sulfur or
nitrogen atoms, such as saturated 5- or 6 r ~ ~d rings having 1
or 2 nitrogen and~or oxygen atoms such as 3-tetrahydLofuLduyl,
3~ l-piperidinyl, 2-piperidinyl, 3-piperidinyl, 4-piperidinyl,
2-tetrahydL~yyLdllyl~ 3-tetral.ydL~yyL~..yl~ 4-tetral.yd~yyLdnyl,
2-morpholinyL and 3-morpholinyl:
aryl: monocyclic or polycyclic aromatic groups having 6 to 10
35 carbon atoms, such as phenyl and naphthyl;
arylal~yl: aryl groups ~as mentioned above), which in the case of
aryl-(Cl-C~)-alkyL are bonded to the ~ù~uLe via aLkyl groups
having 1 to 4 carbon atoms (as mentioned above), eg.
40 phenyl-(CI-4)-alkyl such as benzyl, 2-phenylethyl, 3-phenyl-
propyl, 4-phenylbutyl, 1-phenylethyl, 1-phenylpropyl and
1-phenylbutyl;
aryloxy: aryl groups (as mentioned above), which are bonded to
45 the ~LU~LuL~ via an oxygen atom (-o-), such as phenoxy, 1-naph-
thoxy and 2-naphthoxy;
.. .. , .. , . , . , , . , . _ _ _ _ _ _ _ , .
~050~44896 ~ 1 9 1 ~ 9 9
.
18
Le~e~vaLyl: aromatic mono- or polycyclic radicals, which in addi-
tion to carbon ring members can additionally contain 1 to 4
nitrogen atoms or 1 to 3 nitrogen atoms and an oxygen or a sulfur
atom or an oxygen or a sulfur atom, eg.:
- 5 ~_ .d heteroaryl, cnn~ining 1 to 3 nitrogen atoms:
5 - ' ed ring-heL~LvaLyl groups, which in addition to
carbon atoms can contain 1 to 3 nitrogen atoms as ring
members, eg. 2-pyrrolyl, 3-pyrrolyl, 3~pyrazolyl,
4-pyrazolyl, 5-pyrazolyl, 2-imidazolyl, 4-imidazolyl,
1,2,4-triazol-3-yl and 1,3,4-triazol-2-yl;
- s ed heteroaryl, con~;n;ng 1 to 4 nitrogen atoms or 1
to 3 nitrogen atoms and 1 sul~ur atom or oxygen atom or 1
1~ oxygen or 1 sulfur atom: 5 ' -~d ring heteroaryl groups,
which in addition to carbon atoms can contain 1 to 4 nitrogen
atoms or I to 3 nitrogen atoms and 1 sulfur or oxygen atom or
1 oxygen or sulfur atom as riny members, eg. 2-furyl,
3-furyl, 2-thienyl, 3-thienyl, 2-pyrrolyl, 3-pyrrolyl,
3-isoxazolyl, 4-isoxazolyl, S-isoxazolyl, 3-isothiazolyl,
4 - ifi othiazolyl, S-isothiazolyl, 3-pyrazolyl, 4-pyrazolyl,
5-pyrazolyl, 2-oxazolyl, 4-oxazolyl, S-oxazolyl, 2-thia201yl,
4-thiazolyl, 5-thiazolyl, 2-imidazolyl, 4-imidazolyl,
1,2,4-oxadiazol-3-yl, 1,2,4-oxadiazol-S-yl, 1,2,4-thia-
diazol-3-yl, 1~2~4-eh~ Ql-5-yl~ 1,2,4-triazol-3-yl,
1,3,4-ox~ 7nl-2-yl, 1~3~4-th;7~ nl-2
1,3,4-triazol-2-yl;
- benzo-fused S ~ L~d heteroaryl, containing 1 to 3 nitrogen
atoms or 1 nitrogen atom and/or an oxygen or sulfur atom:
' ~el ring heteroaryl groups which in addition to carbon
atoms can contain 1 to 4 nitrogen atoms or 1 to 3 nitrogen
atoms and 1 sul~ur or oxygen atom or 1 oxygen or one sulfur
atom as ~ing members, and in which 2 adjacent carbon ring
members or 1 nitrogen and 1 adjacent carbon ring member can
be bridged by a buta-1,3-diene-1,4-diyl group;
- 5 '- ed heteroaryl bonded via nitrogen, containing 1 to 4
nitrogen atoms, or benzo-fused 5 - ' ~d heteroaryl bonded
via nitrogen, oontaininy 1 to 3 nitrogen atoms: 5 ~ ~d
ring heteroaryl groups which, in addition to carbon atoms,
can contain 1 to 4 nitrogen atoms or 1 to 3 nitrogen atoms a~
ring members, and in which 2 adjacent carbon ring members or
one nitrogen and one adjacent carbon ring member can be
bridged by a buta-1,3-diene-1,4-diyl group, these rings being
bonded to the s~Lu~5ùLe via one of the nitr~gen ring members;
_ _ _ _ , . _ _, _ . _ . _ .... . .. . _ _ _ _ _ _ _ _ _
0050/44896
2I91999
~ 19
- 6 ed he~odl~l, contai~ing 1 to 3 or 1 to 4 nitrogen
atoms: 6 ~ ~d ring heteroaryl groups which, in addition
to carbon atoms, can contain 1 to 3 or l to 4 nitrogen atoms
as ring members, eg. 2-pyridinyl, 3-pyridinyl, 4-pyridinyl,
3-pyridazinyl, 4-pyridazinyl, 2-pyrimidinyl, 4-pyrimidinyl,
5-pyrimidinyl, 2-pyrazinyl, 1,3,5-triazin-2-yl,
1,2,4-triazin-3-yl and 1,~,4,5-tetrazin-3-yl;
- ben~o Lused ~ ~d heteroaryl, con~aining 1 to 4 nitrogen
atoms: 6 ~ ed ring heteroaryl groups, in which 2 ad~acent
carbon ring members can be bridged by a
buta-1,3-diene-1,4-diyl group, eg. quinoline, isoquinoline,
~n;n~70line and q.linn~lin~
15 ~he statement partly or l~ly halogenated is intended to
express that in the groups characterized in this way the hydrogen
atoms can be partly or completely replaced by identical or di~-
ferent halogen atoms, as mentioned above.
20 with respect to their biological action against harmful fungi,
I are preferred in which
~7 i5 C3-C7-cyoloalkyl or C3-C7-cycloalkenyl, it being possible
for these radicals to be partly or completely halogenated
and/or to carry one to three of the ~ollowing groups: cyano,
C1-C4-alkyl, Cl-C4-alkcxyalkyl, Cl-C4-haloalkyl, Cl-C4-alkoxy,
Cl-C4-hAlo~ xy~ Cl-C4-alkylthio, aryl, aryloxy and
aryl-(Cl-C4)-alkyl, it being possible for the cyclic groups to
carry one to three of the ~ollowing substituents: halogen,
cyano, Cl-C4-alkyl, Cl-C4-alkoxyalkyl, Cl-C4-haloalkyl,
Cl-C4-alkoxy, Cl-C4-h~lo~lk~y, Cl-C4-alkylthio, aryl and
aryloxy or
R7 is a non-arcmatic 4- tc ~ _d ring which, as ring
members, in additicn to carbon can also contain one or t~o of
the heteroatoms oxygen, sulfur and nitrogen, it being pO8-
sible for the carbon atom3 in the ring to carry one or two of
the following groups: halogen, cyano, Cl-C4-alkyl,
Cl--C4--alkoxyalkyl,Cl-C4--haloalkyl,Cl--C4--alkoxy,Cl--C4--halo-
alkoxy, Cl-C4-alkylthio, aryl and arylo~y and the second and
each ~urther nitrogen atom as a heteroatom in the ring carry-
ing hydrogen or a C~-C4-alkyl group or
~7 is aryl or heteroaryl, it being possible for these radicals
to carry cne tc three of the ~olLcwing groups: halogen,
cyano, Cl-C4-alkyl, Cl-C4-alkoxyalkyl, Cl--C4--haloalkyl,
C~-C4- alkoxy~ Cl-C4 ~Alo~lk~y~ C1-C~-alkylthio, aryl, aryloxy
~ 0050/44896
~ 2191999
and heteroaryl, in which the cyclic substituents in turn can
carry one to three of the following substituents: halogen,
cyano, cl-C4-alkyl, Cl-C~-alkoxyalkyl, Cl-C~-haloalkyl,
C1-C4-alkoxy, Cl-C4-h loalkr~y and CL_C4 - alkylthio.
Particularly ~Lefe.L~d . ~ I are those in which the
radicals have the follcwing meanings, namely per se or in com-
bination:
10 Rl i8 Cl-C6-alkyl, it being possible for this radical to carry
one to three of the following groups: halogen, Cl-C~-~lkoxy,
phenyl and phenoxy, ~sper;ally Cl-C4-alkyl and benzyl,
Rl i9 C3-C7-cycloalkyl, it being possible for this radical to
1~ carry one to three of the following groups: halogen,
C1-C4-alkoxyalkyl, phenyl and phenoxy and espe~iAlly cyclo-
propyl, oyclopentyl and cyclohexyl,
Rl is aryl, it being possible for this radical to carry one to
three of the following groups: halogen, cl-C4-alkyl and
C1-C4-alkoxy, especially phenyl and naphthyl, which can be
unsubstituted and/or in turn can carry one to three of the
following groups: fluorine, chlorine, bromine, Cl-C4-alkyl,
methoxy, ethoxy, n-propoxy, iso~ o~y, n-butoxy and
isobutoxy and ~eLL Lu~u~, and in particular unsubstituted
phenyl, l-naphthyl and 2-naphthyl,
R7 is hydrogen,
30 R3 is Cl-C6-alkyl, rsreriAlly Cl-C4-alkyl,
R3 is C3-C6-cycloalkyl, especially cyclopropyl, cyclopentyl or
cyclohexyl,
35 R4 is hydrogen,
R4 is Cl-C~-alkyl, ~qperi~lly Cl-C~-alkyl,
R4 i5 C3-C6-cycloalkyl, ~rerially cyclopropyl, cyclopentyl or
cyclohexyl,
R4 is hydrogen,
R3 is Cl-C6-alkyl and R4 is hydrogen,
RS is hydrogen,
; ' ooso/44896
2i919~9
21
R3 is C3-C6-cycloal~yl and R4 is hydrogen,
Rs is Cl-4-alkyl, esp~ ly methyl or ethyl,
5 R5 is C3-C6-cycloalkyl, e~ci~lly cyclopropyl,
R6 i8 Cl-Cs-al~yl, ~ r~ y methyl, ethyl, n-propyl or iso-
propyl,
10 Rfi is C3-C~-cycloalkyl,
~6 is aryl, it being possible for this radical to be unsubsti-
tuted or to carry one to three of the following groups: fluo-
rine, chlorine, bromine, cyano, Cl-C4-alkyl, trifluoromethyl,
methoxy, ethoxy, n-propoxy, iso~L~uu~yr n-butoxy, isobutoxy,
te~-Lu~u~y, trifluor~ ~h~y~ phenyl and phenoxy,
R7 is phenyl, it being ~ossible for this radical to be unsubsti-
tuted or to carry one to three of the following groups: fluo-
rine, chlorine, bromine, cyano, Cl-C~-alkyl, trifluoromethyl,
methoxy, ethoxy, n-propoxy, isu~uLU~uu~y~ n-butoxy, isobutoxy,
teL~ ~u~u~y, trifluoromethoxy, phenyl and phenoxy,
~7 i5 naphthyl, it being possible for this radical to be unsub-
z5 stituted or to carry one to three of the following groups:fluorine, chlorine, bromine, cyano, Cl-C~-alkyl, trifluoro-
methyl, methoxy, ethoxy, n-propoxy, isu~Lu~U~y~ n-butoxy,
isobutoxy, tert-butoxy, trifluoromethoxy, phenyl and phenoxy,
~0 R7 is C3-C7-cycloalkyl, it being possible for this radical to be
unsubstituted or to carry one to three of the following
groups: halogen, Cl-C4-alkyl and Cl-C~-alkoxy, especially sub-
stituted C5-C7- and in particular C6-cycloalkyl of this type.
35 With regard to the meaning of Xl, x2 and X3, ~ __ ' I are par-
ticularly ~Lef~LL~d where X', x2 and X3 are oxygen. I~ one or more
of Xl, x2 and X3 are sul~ur, those ~ .ullds I are particularly
preferred vhere Xl and x2 are sulfur, especially those where only
xl is sulfur. Hovever, those '- I can also be preferred
40 where only x2 is sulfur
The ~ __ ' I complled in the ~ollowing tables are very
particularly preferred wi~h respect to their use.
0050/44'.896
~.~ 22 - 21919~
Table 2
~ H R3 0 Rs Rc
Rl--Xl _ C ~ C --C--U -- tl --R7
%1 is oxygen or ~3ul~ur.
No. Rl R3 RS R6 R7
C(C53)3 Cd(CH3)2 CH3 CH3 c6~s
15 2 C(CN3 3 Cl7(CH3)C2ll5 H CH3 C6715
3 C CN3 3 CH2cH(cH3l2 N CH3 C6H5
4 C'CH313 C!l(CH3)2 H CH3 4--Cl--C6N~
S CIC~3313 CHICHI 2 H CH3 4--CH3--C6~4
6 CIC113 3 CHICH312 H CH3 4--OCH3--c6N4
20 7 C(CH3'3 CHICH312 H CH3 3,4--(OCH3~2--C6H3
8 CH(CH3)2 cyc70-C3H5 H CHI 4--cl-c6E~4
9 CH(CH3)2 Cyclo--C~H~ H CH3 4--Cl--C6N~
10 CN(CH3)2 Cyclo-C5H9 El CH3 4--Cl--C6Hq
25 1l CH(CH3)2 cyclr~C6HIl H CH3 4--Cl--C6
12 CH(cH3)2 CH2c6Hs H CH3 c6~s
l3 CH(CH3 2 CH(CN3)C2El5 n CN3 C6H5
14 CH(CH312 Cll2cH(cH3)2 H CH3 C6H5
15 CH(CH3 2 CH(CH3)2 CH3 CN3 4--cl--C6U4
30 16 CH~cH3l2 Cl{~C6Hs H CH3 4--CH3--C6H4
17 CH(CH3 2 CN(CH3l2 H CH3 4--OCH3--CCH~
18 Cll(CH3,12 CH(CB312 H CH3 3~4-(ocH332-c6H3
19 C5Hs CH(CH3 2 H CH3 C6N5
35 20 C6H5 CH~CH31C2H5 H CH3 C6H5
21 C6H5 CH2CH(CH3)2 N CH3 C6N5
22 C6H5 CH(CH3)2 CH3 CH3 4--Cl--C6~
23 c6~s CH2C6Hs ~ CH3 4--CH3--C6H4
24 C6N5 CH(CH3)2 H CH3 4_oCH3--C6E~4
40 25 C6H5 CH(CH3)2 H CH3 3r4-(ocH3)2-c6H3
26 CHICH3l2 CH(CN3)2 H CH(CH3)2 cH2(4-cl-c6Eq)
27 CHICH3'2 CH(CH3)2 H CH(C83)2 Cli(CH3)(4--Cl--C6N4)
28 CH CH3 2 CH(CH3)2 71 CH(CH3)2 Cll(c6Hs)(4-cl-c6H4)
45 29 CHICT13)2 CH(CH3)2 H CN(CH3)2 4--Cl--C6H~
30 CH CH3)2 CN(CH3)2 CH3 CH(CH3)2 4--CH3--C6Hq
31 CHICH3)2 CH(CN3)2 H CH(CH312 4{7CH3--C6HI
0050~448g6 - 2 1 9 ~
.
23
Wo. R~ R3 R5 R6 R7
32 CH~CH31z CH(CN3)2 H CH(CH3)2 3,4--(OCH3)2--4E3
33 CH(CH31z CH(CH3)2 H CH2CH3 CH~(4--Cl--C6Hq)
34 CH(C8312 Cl~(CH3)2 H CH2CH3 CN cH3)(4-cl-c6H4)
35 CH(CH3 2 CH(CH3 2 H CH2CN3 CHlC6B5)(4--Cl--C6Hq)
36 CH(CH3 2 CHICH3 2 CH3 CN2CH3 4{83--c6H4
37 CH(CH3 2 CH~CH312 H CN2CH3 CH2(4--CR2--C6H5)
38 CH(CH312 CrrlCH3 2 H CH2CH3 4--oCH3--C6i~4
39 CH(CH3l2 CHICH3 2 N CH2CH3 3,4--(OCH3)ff6H3
40 CH(CH3 2 CHICH3 2 N Cyclo-C5H9 cH2(4-cl-c6H~)
41 CH(CN312 CH CH3)2 H cyclo-c5!~9 CH(CH3)(4--Cl--C6HI)
42 CH(CN3 2 CLI~CH3)2 H cyclo-CsBg CN(C6Hs)(4-cl-c6H4)
43 CH(CN312 CH(CH3)z H cyclo-csH9 4--Cl--C6H~
44 CH(CH3)2 CH(CH3)2 H cyclo-C5H9 4--CH3--C6H4
45 CH(CH3 2 CH CH3)2 H cyclo-csH9 4--OCN3_C6H;
46 CH(CH312 CiilCH3)2 H cyclo-csH9 3,4--(OCH3)2--C6H3
47 CN(CH312 CHICH3)2 H cyclo-C6Nl~ CH?(4-cl-c6H~)
48 CH(CH3~2 CH CH3)2 H Cyclo-c6Nll CH CH3~(4-cl-c6H4)
49 CH(CH3~2 CN CH3)2 H cyclo--C6Nll CH C6H5)(4--Cl--C6Hq~
CN(CH3~2 CNICH3~2 H cyclo-c6Hll 4{ L--C6H4
51 CN(CH3)2 CHICH3 2 H cyclo-c6Hll 4--CH3--C6H4
52 CH(CH3)2 CHICH3 2 H cyclo--C6H11 4--OCH3--C6H4
53 CH(CN312 CH CH3 2 H cyclo-C6H11 3,4--(0CH3)2--C6H3
54 CH(CH3 2 CillCH3 2 H CE3 4--C6H5--C6H-
CH(CH3 2 CH CH31C2H5 N CH3 4--c6Hs-c6H4
56 CH~CH3 2 CH2CH(CH3)2 H CH3 4--C6Hs--C6H-
57 CH(CH312 CH(CH3)2 N CH3 q--CF3--C6H4
58 CH(CH312 CH(CH3)2 H CH3 4--CW--C6H4
59 CN(CH3 2 CHICH3)2 H CH3 4--OC2HffcH4
CH(CH3 2 CHICH3)2 H CH3 4_OC6H5--C6H4
61 CH(CH3 2 CH CH3)2 H CH3 4--~--C6H4
62 CH(CH3~2 CHlcH3~2 H CH3 4--Br-C6H~
63 CN(CH3~2 CN CH3)2 H CH3 4--C(CH3~3--C6N4
64 CH(CH3~2 CH CH3~2 H CH3 Z--Cl--C6H4
CH(CH3~2 CHICH3~2 H CN3 3--Cl--C6H4
40 66 CH(CH3~2 CHICH3)2 H CH3 3~5-cl2-c6H3
67 CH(CH3~2 Cll CH3)2 H CN3 2,4--cl2--C6H3
68 CH(CH3)2 CH CH3~2 H CN3 2,6--C12--C6N3
69 CH(CH3~2 CH~CH3)2 H CH3 2--CN3--C6Hi
CH(CH3)2 CH(CH3)2 H CH3 3--CN3--C6N4
45 7L CH(cH3)2 CH(CH3)2 H CH3 2,3--(CH3)2--4H3
7Z CH(CL13)2 CH(CH3)2 H CH3 2,4--(CH3)2--C6H3
' 0050/44896 - 2 1 9 1 9 3 ~
.
24
No. Rl R3 R5 R6 R7
73 CHICH3 z cH(cu3 2 H CH3 3~s-(cH3l2-c6H3
74 CH CH3 2 CHICH312 H CH3 2,6-(CH3)2-C633
75 CHI C~J 2 CHICH3 2 H CHI 2-OCH3-C6H~
76 CNICH3 2 CHICH3 2 H CH3 3_oCH3-C6H~
77 CH CH3 2 CHICH3 2 H CH3 4-OC2H5-C6H4
78 CHICH3)2 CNICH3)2 H CH3 3-cc2Hs-c6H~
79 CH~CH3 2 CH(CH3)2 H CH3 2-OC2H5- 4 H1
10 80 CH(Ca3 2 CH~CH3)2 H CH3 3~4-(cc2Hs)2-c6H3
81 CH CH3 2 CH(CN3 2 H CH3 2-F-C6Hq
82 CHICH3 2 CH(CH3 2 H CN3 3-F-C6H4
83 CHICH3l2 CHICH3 2 H CH3 3-CH(CH3)2
84 CHICH312 CHICH3 2 H CN3 4-CH~CH3)~
85 CNlCH31z CHIC83 2 H CH3 2-CP3-C6H4
86 CHlcH3 2 C81CH312 H CH3 3-cF3-c6H4
87 CE(C83 2 CNICH3l~ CN3 CH3 C6H5
88 CH(CH3 2 CHICH31C2H5 CH3 CH3 C6H5
Z0 89 CH(CH3 2 CH2CN(CH3)2 CH3 CH3 C6Hs
90 CH(CH3 2 CH(CH3)2 CH3 CH3 4-Cl-C6H~
91 CH(CH3)2 CH(CH3)2 CH3 CH3 4-CH3-C6H4
92 CH~CH3)2 CHiCH3)2 CH3 CH3 4-OCH3-C6N4
93 CH(CH3)2 CH~CH3)2 CH3 CH3 3 4-(CCH3)2-C6H3
25 94 CN(CH3)c2Ys CN CH3)2 H CH3 4-Cl~C6Hq
9S CH CH3)C2H5 CHlcH3)c2H5 H CH3 4-cl-C6H4
96 CHICH3)C2H5 CH;CH(CH3)2 H CH3 4-Cl-C6H~
97 CH(CH3)C2H5 CH CH3~2 CH3 CH3 4-CH3-C6H~
30 98 CHICH3)C2H5 CHICH3 2 H CH3 4_oCH3-C6H4
99 CHICH3 C2H5 CHICH312 H CH3 3 4-(OCH3)2-C6N3
100 CH(CH31C2n5 CHICH3 2 H CH3 cH2(4-cl-c6H4)
101 CH(CH31C2H5 CHICH312 H CH3 CH(C6H5)(4-Cl-C6H4)
102 CH~CH3 C2H5 CH CH3 C2H5 H CH3 4-Cl-C6H4
103 CH2-C6E5 CH;CH(CH3)2 H CH3 4-cl-C6H4
104 CE2-C6H5 CHICH3J2 CH3 CH3 4-Cl-C6H4
lOS CH2-(4-CH30-C6H~) CHICH3 2 H CH3 4-Cl-C6H~
106 CH2-(4-cH3-c6H~) CHICH312 H CH3 4-Cl-C6H~
~o 107 CH2-(4-cl-c6H~) CH CH312 H CH3 4-Cl-C6Hq
L08 4-Cl-C6H~ CHICH3 2 H CHJ 4~Cl-C6Hq
109 4-CH3-C6H4 C3 CH312 H CH3 4-Cl-C6Hq
110 4-CH30-C6a4 CH CH3 2 H CH3 4-Cl-C6H~
111 4-C6F5-C6Bq C31CH3 2 H CH3 4-Cl-C~H~
112 Cyclo-csH9 CH CH3)2 H CH3 4-Cl-C5Hq
113 cyclo-c6Hll CH CH3)2 H CH3 4-Cl-C6H~
_ ~
0050/44896
~ 25 2~g~ g39
No. Rl R3 R5 RC R7
114 C~Hs CH'CH3 z H CH3 4--CI--C6H4
115 CHzCH2CH3 CH CN3 2 H CH3 4--CI--C6H4
116 CH~CH2CH2CH3 CH1CH3'2 H CH3 4--CI--C6H~
117 CHlCH(CH3)2 CY CN3 i Y CH3 9--CI--C6H4
118 (CHz)~CH3 CHICH3 ~ H CN3 4--cI--C6H4
117 (cH2)scH3 CY1CN3 2 H CH3 4--CI--C6H
120 ClCH2CHz CHICH3)i H CH3 4--Cl--C6H~
121 C13CCH2 CH CH3)2 H CN3 4--Cl--C6H~
122 CH30CH2CH2 CHICH3)2 H CH3 4--CI--C6H4
lZ3 CHz-CH CH1CH3 2 H CN3 4--CI--C6Hq
I24 CH30CH2CEi~CH3) CB CR3 2 H CH3 4--CI--C6H~
125 Cl(CH3)CH CH CE13 2 H CH3 4--CI--C6H~
126 C6RsocH2cH2 CHlcH3~2 H CH3 4--Cl--C6H,
127 CH2=C(CH3) CH1CH3 2 H CH3 4--C1--C6H~
128 CH(CH3)2 CEi~CY3 2 H C6Ei5 cH2(4-cl-c6H4)
129 CH(cH3)2 CH(CH312 H C6H5 CH(CH3)(4--CI--C6H~)
130 CH~CH3)2 CH(CH3 2 N C6H5 cN(c6H5)(4-cl-c6H4)
131 CH(CH3)2 CH(CH3 2 H C6H5 4--Cl--C6H4
132 cHtca3)2 C8(CE13)2 H C6H5 4--CH3--C6H4
133 CH CH3)2 CH(CH3)2 H C6H5 4--oCH3--C6H4
134 CH~CH3)2 cycIo--C3H5 H C6H5 4--Cl--C6H4
135 CH~ CH3)2 cyclo-c~H7 H C6H5 4_cl--C6H4
136 CNI CH3~2 cycI~C5H9 H C6Hs 4--CI--C6Ei~
137 CHICH312 cycIo-C6Hl~ H C6Hs 4--CI--C6E!4
138 CHICH3 C2H5 CH(cN3)2 H CH1CH3)2 4--CI--C6H~
139 CH~CH3 C2H5 CH(CHl)C2Hs H CH~CEi3)2 4--CI--C6H-
lgO CHICH3 C2Hs CH2cH(cH3)2 li CH CH3)2 4--Cl--C6H4
141 CH(CH31C2Hs CH(CEil)2 H CHICH3)2 4--Cl--C6H4
142 CH1'CH3 C2Hs CHICH3)2 El CH1CH3)2 4--cl-c6H4
143 CH CH31C2Hs CHICE}3)2 H CH CH3)2 4--CI--C6H,
144 CN CH3 C2H5 CHICE13)2 H CH1CH3)2 4--CI--C6H4
145 CHICH31c2Ns CH(CH3)2 H CHICN3)2 4--CI--C6H~
146 CHICH3 C2Hs CH(cH3)c2Hs H CHICH3)2 4-cl-c6H~
I4 7 C H2--C6E5 CH2CH(CH3)2 H CB CH3)2 4--C 1--C6H~
148 CH~--C6H~ CH(CH3)2 H CH(CH3)2 4--CI--C6Hq
149 CH2--l 4--CH3O--C6Eil) CH(CH3)2 H CH(CH3)2 4--CI--C6H4
150 CH2--14--CH3--C6HG) CH(CH312 H CH(CH3)2 4--CI--C6H4
151 CH2--14--Cl--C6HG) CH(CH312 H CH CH3)2 4--Cl--C6H4
152 4--Cl--C6H~ CH(CFi3~2 H CH CH3)2 4--C1--C6H~
4'i 153 4--CH3--C6H4 CH(CH3)2 H CH CH3)2 4--Cl--C6H4
154 4--CH3O--C6HG CH(CEi3)2 H CH CH3)2 4--Cl--C6H~
. _ _ _ _ _ _ . .. . . . . : . . . . _ . _ .
0050~44896 2 1 9 ~ 9 9 9
26
~o. R1 R3 R5 R6 R7
lSS 4-C6Hs-C6H~ CH~CH3)2 H CN CH3I~ 4-Cl-C6H4
156 cyclo-C5Hg CHICH3)2 H CHICH3 2 4-Cl-C6H~
LS7 cyclo-C6NIl CHICH3)2 H CHIC~3l2 4-Cl-C6H~
158 C2Hs CHICH3)2 H CHICH3 2 4-Cl-C6H6
lS9 CH2CHzCH3 CHICH3 2 H CNICH3 2 4-Cl-C6H~
160 CH1CH~CH2CHI CH~CH3 2 H CHICH3)2 4-cl-c6H~
161 CH2cH(cH3)2 CH(CH3 2 H CH CH3)2 4-Cl-C6H~
10 162 (CN2)~CH3 CH(cH3 2 H CHIcH3 2 4-Cl-C6H~
163 (CH2)sCH3 CHICH3 2 H CH CH312 4-Cl-C6H~
164 ClCH2CH2 CHICH3 2 H CHICH3 2 4-Cl-C6H~
165 C13CCN2 CHICH3 2 H CHICa3 2 4-Cl-C6N~
166 CH3OC~2Ca2 CHICH3 2 N CH CH3I2 4-Cl-C6H4
15 167 CH2=C~ CHICH3 2 H CH~cH3l2 4-Cl-C6H~
168 C~3OC~2C~(CH3) CHICH3 2 N CaICH3l2 4-Cl-C6H~
169 Cl(CH3)CH CHICH3 2 H CBICE3I2 4-Cl-C6H~
170 C6HsOCH2CH2 CHICa3)2 N Ca~Cà3 2 4-cl-c6a~
20 171 CH2=c(cH3) CH CH3)i H CH CH3)2 4-C1-C6H~
172 C6Hs CH CH3)2 H cyclo-C3H5 C6H5
173 C6H5 CHICH3)2 H cyclo-C3Hs 4-cl-c6a~
174 C6Hs CHlCH3 2 ~ cyclo-C3Ns 4-CH3-C6H4
175 C6Hs CH(CH3 2 H cyclo-C3Hs 4-OCH3-C6H~
176 C6Hs ca CH3 2 H cyclo-c3Hs 3 4-(oc03)2-c6H3
172 CH(CH3)2 CH CH3~2 H cyclo-C3H5 C6H5
173 CH(CH3)~ CHICH3)~ H cyclo-C3H5 4-cl-c6a~
174 CH(CH3)2 CHICH3)2 a cyclo-C3H5 4-CH3-C6
175 CH(CH3)2 CHICH3)2 H cyclo-C3~s 4-CCH3-C6H~
176 ca(cH3)2 CHICH3)2 ~ cyclo-C3as 3,4-(ocH3)2-c6H3
177 CH(CH3)2 CH~CH3)2 a CE2CH(CH312 4-Cl-C6H~
178 CH~CH3)2 CH(CH3)2 N CH3cH2cH2 4-CH3-C6H-
179 CHICH3)~ CH(CH3 2 N CH3(CX2)3 4-Cl-C6H-
180 cnlcH3)2 CH(CH3 2 H CH~CH2CH3)2 4-CH3-C6H-
181 ClCH3)3 CH~CH3I2 H CH3 C6Hs
182 CH~CH3)z CH~Cal 2 B CH3 C6H5
163 C6H5 CH6CH3~2 H CH3 C6H5
184 CH~CH3)2 CH CH3)2 H CH3 4-CH3-C
189 C6H5 CH CH3)2 H CH3 4-CH ff 6a~
186 CN(Cn3)C2Hs CHlCH3)2 N CH3 4-CH3-C6N~
186 cn2cH(cH3)2 CHICH3)2 H CH3 4_CH3-C6H-
188 CH2C6H5 CHIC~3)2 H CH3 4-CH3-C6n-
189 CH(CH3)2 CH(CH3)2 H CH3 4-Cl-C
190 C6H5 CH~CH3)2 H CH3 4-Cl-C
0050/4~896
27 ~ 2 1 9 1 9 9
N~. Rl R3 R5 R6 ~7
191 CH2C6Hs CH~C~3~2 H C~3 4-Cl-C5Hq
l9Z C(CH1~3 C~(C~3)2 ~ C2H~ 4-CH3-C6
193 CH(CH3~1 C~(C~312 ~ C2H5 4-CH3-C6
194 CB(CH3lC2Hs CH~C~3 2 H C2Hs 4-CH3-C6H~
195 C(CH3~3 CN(CH3 2 H C2Hs 4-cl-C6H4
l9G CH(CH3~2 CH CH312 H C2Hs 4-Cl-CGHq
197 CH(C~3~C~Hs CHlcH3 12 H C2Hs 4-Cl-C6H~
10 198 CH(CH3~2 cH~aHl~2 ~ CN(C~J~2 4-CH3-C6H
199 CH(CH3~ClHS CHICH3 2 H C8(C~3)2 4-CH3-C6H~
200 C(CH3~3 CH(CH3~2 ~ H 4-Cl-C6H~
201 C(CH3~3 c~(cN3 2 H H 4-CR3-C6H
15 202 C(CH3)3 CH~CH312 H CH(CH2CH3)2 4-CH3-C6H4
203 C(CH3)3 CH(CH312 ~ (CH2)3cH3 4-CH3-C6H~
204 C(CH3)3 CH(CHI)2 ~ cyclo-C5H9 4-CH3-C6H~
205 C(CH3)3 CH(CH3~z ~ (C~l)3Cl73 4-cl-C6R4
286 C(cH3)3 CH(CH3)2 ~ cyclo-C5H9 4-Cl-C6H~
20~ C(CB3)2 CH(CH3)2 H n-CsHll 4-CH3-C6H~
208 C~CH3)2 CH(CH3)2 ~ n-C6HIl 4-CH3-C6B4
209 C C~3)2 CH~CH3)2 H n-C7Nl5 4-CH3-C6Hq
210 C~cH3)2 CH~CH3)2 8 n-ct~l7 4-CH3-C6H~
z9 211 C(C~3)2 CH CH3~2 H CH2-C(CH313 4-c~3-c6H4
212 C(CH3)2 CH~CH3)2 H C(CH312C2Hs 4-CHI-C6Hq
213 C(CH3~2 CH(CH3)2 H CH(CH3lC3H7 4-CH3-C6H4
214 C(CH3)2 CH~CH3 2 ~ n-C5~ll 4-cl-c6H4
30 215 C(CH3)2 CB(CH3 2 H n-C6H11 4-Cl-C6H~
216 C(CH3)2 CHICH3 2 ~ n-C7NIs 4-Cl-C6H~
217 C(CH3)2 CH~CH3 2 H n-CaH,7 4-Cl-C6H
218 C(CH3)2 CBlCH3)2 H CH2-C(CH3~3 4-Cl-C6H4
219 C(CH3)2 CHIC~3)2 H c(cH3~2c2Ns q-cl-c6H~
220 C(C~3)2 CH~CH3)2 ~ CH~cH3)c3H7 4-Cl-C6H4
0050~4489~
21 ~199~
28
~a~le 3
0 H R3 ~ R5 R5
Rl_xl--C--N--C --C--~-- N--X{~
Xl is oxygen or sul~ur.
No. R~ R3 Rs R~ X Z
C(CE~3)3 CH(CH3)2 H CH3 -- 4--Cl
15 2 C(CH3)3 CH(CH3)2 n C~3 -- 4--CE~3
3 C(C1~3 13 CH(CH3)2 H CH3 4--OCE13
4 c(cHl)l CH(CK3 2 H CH3 8 4--(OCH3)2
CHICH3 2 CH(CH3l2 H C~3 -- H
20 6 CHiCH3l2 CH(CH3l2 H CH3 -- 4--Cl
2 CH~CH3 2 CH(CH3)2 H CH3 -- 4--CH3
8 CHiCH3l2 CH(CH3)2 H CH3 -- 4--OCH3
g CH~CH3 12 CH(CH3)2 H CH3 -- 3,4--(OCH3)
C6H5 CH(CH3)2 H CH3 -- H
11 C6H5 CHlcH3)2 H CH3 -- 4--C1
I2 C6H5 CH~CH3)2 H CH3 -- 4--CH3
13 C6H5 CH,CH3)2 H CH3 -- 4--OCH3
14 C6H5 CHICH3)2 H CH3 -- 3 4--(OCH3)2
30 15 CH(cH3)2 CHICH3)2 H CH(CH3)2 CH2 4--Cl
16 CH(CH3)2 CHI~CH3 2 H CH(CH3)2 -- 4--Cl
17 CH(CH3)2 CH~CH3l2 H CH2CH1 CHCH3 4--CL
18 CH(C~3)2 CHICH3l2 H CH2CH3 CHC6H5 4--Cl
35 19 CHICH3)2 CHICH3 2 H CH2CH3 ~ 4_Cl
cnrCH3) 2 CHICH3)2 H cyclo-c5H9 -- 4_Cl
21 CH CH3)2 CH~CH3)2 N cyclo--C6H11 -- 4--Cl
22 CHiCH3)2 CHIC83)2 H CE~3 -- 4--CH(CH3)2
CH(CH3)2 CH~CH3)2 CH3 CH3 -- 4--Cl
24 CH(CE13)2 CHICH3)2 CH3 CE~3 -- 4_CH3
CH(C113)2 CHICH3)2 CH3 CH3 -- 4--OCH3
26 CH(CH3)2 CH~CH3)2 CH3 CH3 -- 3,4--(OCH3)2
27 CH2C6Hs CHICH3)2 H CH3 -- 4--Cl
2B cyclo--C6Hll CHICH3~2 1; C~3 -- q--Cl
29 CH2C~(CH3)2 CHICH3~2 H CH3 -- 4--Cl
' 0050r44896 2 1 ~ 1 ~ 9 ~
.
29
No. Rl R3 Rs R6 X Z
30 CH(CH332 CHICH3J2 H C6H5 - 4-Cl
31 CH(CH31~ CHICH3)2 H C6Hs - 4-CH3
5 32 CH(CH3)2 CH~CHI)2 H C6Hs - 4-OCH3
Table 4
Wl\ O H ~3 ~ Rs R6
C=N--O--C - N - C - C - N - N - R7
w2
Ls
No. W1 W2 R3 Rs R6 R7
1 CH3 CH3 CH(cH3)2 H CH3 OCH3-C6H~
2 CH3 CH3 CH~CH3 2 H CH3 3,4-(OCH3)2-C6H3
20 3 CH3 C6H5 CH(CH3 2 H CH3 C6H5
4 CH3 C6H5 CH(CH3 12 H CH3 4-Cl-C6H4
CH3 C6H5 CH(Cc3 2 H CH3 4-CH3-C6H4
6 C~3 C6H5 CH(cH3 2 ~ CH3 4_ocH3-c6Hç
25 7 CH3 C6R5 CH(CH3 '2 H CH3 3,4-(OCH3)2-C6H3
~3 CH3 4-CH3-C6H4 CH(CH3~2 H CH3 C6Hs
9 CH3 4-CH3-C6H4 cn(cH3l2 H CH3 4-Cl-C6H4
CH3 4-CB3-C6H4 CH(CH3~2 H CH3 4-CH3-C6H4
30 11 CH3 4-CH3-C6H4 CH(CH3 2 H CH3 4_ocH3-C6H4
12 CH3 4-CH3-C6H4 C~(CH3 2 H CH3 3,4-(OCH3)2-C6H3
13 CH3 4-CH3-C6H4 CH(CE3 2 H CH(CH3)2 CH2(4-Cl-Cc
14 CR3 4-CH3-C6H4 CH(CH3 2 H CH(CH3)2 4 - Cl - C6H4
lS CH3 4-CH3-C6H6 CH(CH3 12 H CH2CH3 CH(CH3)(4-Cl-C6H6)
16 CH3 4-CH3-C6H4 CH(CH3 2 H CH2CH3 CH(c6Hsl(4
17 CH3 4-Cl-C6H4 CH(CH3 '2 H CH3 C6H5
18 CH3 4-cl-C5H4 CH(CH3 2 H CHl 4-C1-C6H~
19 CH3 4-Cl-C6H4 CH(CH3)2 U C~3 4-CH3-C6H4
4~ 20 CH3 4-Cl-C6H~ CH(CH312 H CH3 4_OCH3-C6H4
21 CH3 4-Cl-C6H4 CH(CH3)2 H CH3 3,4-(0CH3)2-C6H3
22 CH3 4-C1-C6H4 CH(CH3)2 H CH(CH3)2 CH2(4-Cl-4 H4)
23 CH3 4-Cl-C6H4 CH(CH3)2 H CH(CH3)2 4-C1-C6H4
45 24 CH3 4-Cl-C6H4 CH(CH112 H CH2CH3 cH(cH3l(4-cl-c6~4l
CH3 4-Cl-C6H~ CH(CH312 H CH2CH3 CE(C6Hs)(4-Cl-C6H4)
0050~44~96
~ 30
No. W~ w2 R3 Rs R6 R7
26 CH3 CH3 CH~CH3)2 H CH3 4-Cl-C6H4
27 CH3 CH3 CH(CH312 H CH3 4-CH3-C6H~
~able 5
S H (cH3)2cH o H R5
Rl----S--C--N--C--C--N--N ~ R3
No. Rl R5 R8
l CH(CH3)2 c~3 CH3
2 CH(CH3) 2 CH3 Cl
8 CH(CH3)C2~s CH3 CH3
20 4 CH(CH3)C2~s c~3 Cl
S CH2C6H5 CH3 CH3
6 CH2C6H5 CH3 Cl
7 C~C~3)2 C2~5 c~3
25 8 CH~CH3)2 C2H5 Cl
g CHICH3)2 CH(CH3)2 CB3
CHICH3)2 CH(CH3)2 Cl
11 CH~cH3)2 n-4Hg c~3
30 12 CH(C83)2 CH2CH(CH3)2 Cl
13 CH(CH3 2 n-C~Hg CH3
14 CH(CH3~2 CH2CH(CH3)z Cl
CH(CH3.'2 CH(Cz~s)2 CH3
16 CH(CH3)2 CH(C2Hs)2 Cl
35 17 CH(CH3)2 CH2-CsHS CH3
18 CH(CH3) 2 CHZ-c6Hs Cl
l9 c(CH3)3 Cyclo-C5Hg CH3
CH(CH3)2 cyclo-C5H9 CH3
40 21 CH(CH3)C2H5 cyclo-C5H9 CH3
22 C(CH3)3 cyclo-C5Hg Cl
23 CH(cH3)2 cyclo-C5Hg C1
2G CH(CH3)C2Hs cyclo-C5Hg Cl
C(C~3)3 cyclo-C6Hl1 CH3
26 CH(CH3)2 cyclo-C6Hll CH3
27 c~(cH3)c2Hs cyclO-c6Hll CH3
. 0050/4~896 ' 2 1 5 ~
31
No. Rl R6 R8
28 C~CH3)3 cyclo-c6Hll Cl
29 CH(CHJ)2 cyclo-c6Hll Cl
5 30 CH(CHl~QH5 cyclo-C6Hll Cl
31 CH(CH3 2 C6Hs CH3
32 CH(CH3l2 CH(CH3)C2HS Cl
33 CH~CH3)2 C6H5 CH3
34 CH(CH3)2 CH(CH3)C2HS Cl
Table 6
S H (CH3)2CH o H R6
R~O--C ~ C --C_ 17 _ N ~=~ R
H
No Rl R5 RB
CH(CH3)2 CH3 CH3
2 CH(CH3 12 CH3 Cl
25 3 CH(CH3 C2H5 CH3 CH3
4 CH(cHl)c2~s CH3 Cl
CH2C6Hs CH3 CH3
6 CH,C6Hs CH3 Cl
7 CH CH3 2 C2H5 CH3
3o 8 CH CH3 2 CzHs Cl
g CHICH3l2 CH CH3)2 CH3
CH(CH3 2 CHICHJ)2 Cl
11 CHICH3 2 n-C4Hg CH3
3S 12 CH(CB3 2 n-C4Hg Cl
13 CH~CH3)2 CH2CH~CH3)2 CH3
14 CH(CH3)2 CH2CH~CH312 Cl
CH(CH3)2 CH(C2~s)2 CH3
40 16 CHICH3l2 CH(C2Hs)2 Cl
17 CHICH3~2 CH2-C6H5 CH3
18 CH~CH3~2 CH2-C6H5 Cl
19 C~CH3)3 cyclo-csH? CH3
CH~CH3)2 cyclo-C5Hg CH3
21 CH~CH3)C2~s CyClO-CgHg CH3
22 C(CH3)3 cyclo-c5H? Cl
0050/44896 2 1 9 1 9 ~ ~
.
32
No. Rl R6 ~3
23 CH(CH3)2 cyclo-CsHg Cl
24 CH(CH3)CzHs cyalo-Csag Cl
5 25 C(CH3)3 CyclC-c6Hll CH3
26 C~ICH3)2 cyclo-c6Hll CH3
27 CH~C~3)C2H5 cyclo-c6HLl CH3
28 C(CH3)3 cyclo-c6Hll C1
29 CHlCH3)2 cyclo-C6Hll C1
CH~CH3~C2Hs CyclO-C6HIl C1
31 CH CH3)2 C6HS CH3
32 CHICH3)2 C6H5 Cl
33 CH(CH3)2 CH(cH3)c2Hs CH3
15 34 CH(CH3)2 CH(CH3)C2~5 Cl
The novel ~iullds of the formula I are suitable as fungicides.
The novel ~ ~ , or the compositions containing them, can be
20 applied for example by bpraying, atomizing, dusting, scattering
or watering in the form of directly sprayable solutions, powders,
suspensions, even high-percentage aqueous, oily or other suspen-
sions or dispersions, lci~n~, oil dispersions, pastes, dusting
compositions, scattering compositions or granules. ~he applica-
25 tion forms depend on the intended uses; in each case they shouldif possible ensure the finest dispersion of the active compounds
according to the invention.
~ormally, the plants are sprayed or dusted with the active com-
30 pounds or the seeds of the plants are treated with the active
Ulllg .
The formulations are prepared in a Icnown manner, eg. by extending
the active compound with solvents and/or carriers, if desired
3!i using : l~;f;~r5 and dispersants, where if water is used as a
diluent other oryanic solvents can also be used as auxiliary sol-
vents. Suitable auxiliaries for this purpose are mainly: solvents
such as aromatics (eg. xylene), chlorinated aromatics (eg
chlolobD~ " ,), paraffins (eg. petroleum fractions), alcohols
40 (eg. methanol, butanol), Icetones (eg. cycl~h~n~n~), amines (eg~
e~h~n~l~min~, dimethylformamide) and water; carriers such as
ground natural minerals (eg. kaolins, aluminas, talc, chalk) and
ground synthetic minerals (eg. highly disperse silica,
silicates), l5ifi~rs such as nonionic and anionio emulsifiers
g5 (eg. polyoxyethylene fatty alcohol ethers, allcanesulfonates and
.. . _ . . . . . . _ _ _ _ . _ _ _ _ _ . . . .
0050/44896 ~ 9 9 ~
~ 33
arylsulfonates) and dispersant9 6uch as lignin-sulfite waste
liquors and methylce~ lose.
suitable surface-active substances are the alkali metal, alkaline
5 earth metal and ammonium salts of aromatic sulfonic acids, eg.
l;gn~ 1fonic, rhorol~ fo~i~, naphthalenesulfonic and dibutyl-
naphthalenesulfonic acid, and also of fatty acids, alkyl- and
alkylarylsnlf~n~ , alkyl-, lauryl ether and fatty alcohol
sulfates, as well as salts of sulfated hexa-, hepta- and octa-
1~ decanols, and also of fatty alcohol glycol ethers, condensationproducts of sulfonated naphthalene and its derivativeg with
formaldehyde, condensation products of naphthalene or of the
naphthalenesulfonic acids with phenol and formaldehyde, polyoxy-
ethylene octylphenol ether, ethoxylated isooctyl-, octyl- or
15 nonylphenol, allcylphenol- or tributylphenylpolyglycol ethers,
alkylaryl polyether alcohols, isotridecyl alcohol, fatty alcohol
ethylene oxide ~ delsates, ethoxylated castor oil, polyoxyethy-
lene alkyl ethers or polyu~y~Lu~ylene tsicj, lauryl alcohol
polyglycol ether acetate, sorbitol esters, lignin-sulfite waste
20 liquors or methylcellulose.
Powder, scattering and dusting compositions can be prepared by
mixing or joint grinding of the active substances with a solid
carrier.
Granules, eg. coated, impregnated and h~ , granules, can
be prepared by binding the active ~ _ ' to solid carriers
Solid carriers are mineral earths such as silica gel, silicic
acids, silica gels [sic~, silicates, talc, kaolin, limestone,
30 lime, chalk, bole, loess, clay, dolomite, dial ~L earth,
calcium sulfate and magnesium sulfate, m-gn~ m oxide, ground
synthetic materials, fertilizers, such as ammcnium sulfate, ammo-
nium i?h~sphlte, ammonium nitrate, ureas and vegetable products,
such ~ cereal flour, tree bark meal, wood meal and nutshell
35 meal, co~ lo8e powder or other solid carriers.
Examples of such preparations are:
I. a solution of 90 parts by weight of a compound I accord-ing to the invention and 10 parts by weight of
N-methyl-2-pyrrolidone, which is suitable for application
in the form of very small drops
II. a mixture of 10 parts by weight of a compound I according
to the invention, 70 parts by weight of xylene, 10 parts
by weight of the addition product of from 8 to 10 mol of
ethylene oxide to 1 mol of oleic acid ~-monoe~h~nolam~
_ _ _ , , , . . , ... , . , . _ . , _ ,,, , . _, ,
005~/44896 2 1 9 ~ 9 9 9
~ 34
5 parts by weigh~ of calcium salt of dodecylbenzene-
sulfonic acid, 5 parts by weight of the addition product
of q0 mol of ethy~lene oxide to l mol of castor oil, a
dispersion is obtained by finely dispersing the solution
s in water.
III. an aqueous dispersion of l0 parts by weight of a oompound
I according to the invention, 40 parts by weight of
cyc1nh-Y~,--..r, 30 parts by weight of isobutanol, 20 parts
by weight of the addition product of 40 mol of ethylene
oxide to l mol of castor oil
IV. an aqueous dispersion of l0 parts by weight o~ a compound
I according to the invention, 25 parts by weight of
cyr3~h~nnl, 55 parts by weight of a petroleum fraction
of boiling point from 210 to 280'C, and l0 parts by
weight of the addition product of 40 mol of ethylene
oxide to l mol of castor oil;
20 V. a mixture, ground in a hammer mill, of 80 parts by
weight, preferably of a solid compound I according to the
invention, 3 parts by weight of the sodium salt of diiso~
butylnaphthalene-2-6-~1fonic acid, l0 parts by weight of
the sodium salt of a lignosulfonic acid from a sulfite
waste liquor and 7 parts by weight of powdered silica
gel; a spray liquor is obtained by finely dispersing the
mixture in water;
VI. an intimate mixture of 3 parts by weight of a compound I
according to the invention and 97 parts by weight of
finely divided kaolin; this dusting . i~;nn contains
3i by weight of active compound;
VII. an intimate mixture of 30 parts by weight of a compound I
according to the invention, 62 parts by weight of
powdered silica gel and 8 parts by weight of liquid
paraffin which has been sprayed ODto the surface of this
silica gel; this preparation gives the active compound
good adhesion;
gO
VIII. a stable aqueous dispersion of 40 parts by weight of a
compound I according to the invention, l0 parts by weight
of the sodium salt of a phenolc--1fon;c acid/urea/formal-
dehyde condensate, 2 parts by weight of silica gel and 48
parts by weight o~ water, which can be further diluted;
_ _ _ _ _ . _ _ _ _ . .
~ - 2 ~
' OOSO/44896
.
IX. a stable oily dispersion of 20 parts by weight of a com-
pound I ~cnr~ing to the invention, 2 parts by weight of
the calcium salt of dodecylh~ n-~ lfonic acid, 8 parts
by weight of fatty alcohol polyglycol ether, 20 parts by
S weight of the sodium salt of a rh~nnlc~llfoni~ acid/urea/
fnr~ hyde condensate and S0 parts by weight of a
paraffinic mineral oil.
The novel , ~c are distinguished by an outstanding activity
10 against a broad spectrum of phytopathogenic fungi, in particular
from the class of D~hLel~ e3, As~ I eLe~, ~hi~ ~ ~Les and
Bas;~;~ y~_Les. They are systemically active in some cases and
can be employed as foliar and soil fnngir;~c
l'i They are of particular - ~ ~a~ for the contrcl of a multi-
plicity of fungi on various crop plants such as wheat, rye,
barley, oats, rice, corn, lawns [sic], cotton, soybeans, co~fee,
sugar cane, grapes, fruit and decorative plants and vegetable
plants such as ~u- a, beans and cucurbits, and on the seeds
Z0 of these plants.
~he , ' are applied by treating the fungi or the seeds,
plants, materials or the soil to be ~l~-e~-ed from fungal attack
with a fungicidally effective amount of the active c ~c.
2ri
They are applied before or after the infection of the materials,
plants or seeds by the fungi.
The novel c ~ n~C are specifically suitable for the control of
30 the following plant diseases:
Erysiphe graminis (powdery mildew) in cereals, Erysiphe
cichoracearum and Sphaerotheca fuliginea on cucurbits,
po~n5rh~ra leucotricha on apples, uncinula necator on vines,
35 Puccinia ~pecies on cereals, Rhizoctcnia species cn cotton and
lawns lsic], Ustilago species on cereals and sugar cane, Venturia
inaequalis (scab) on apples, Helminthosporium species on cereals,
Septoria nodorum on wheat, Botrytis cinerea ~gray mcld) on straw-
berries, vines, decorative plants and vegetables, C~1~0~L~
40 ara~hi~i~nl~ on groundnuts, Pseudo~ y~,~lla herpo~ri~hni~ec
on wheat, barley, Pyricularia oryzae on rice, ~hytcphthora
infestans on potatoes and tomatoes, Eusarium and ver~ -m
species on various plants, ~lasmopara viticola on vines,
~ rn~r;~ species on vegetables and fruit.
' 0050/~4896 2 ~ 9 ~
.
~ 6
The novel ~ ' can also be employed in the proteation o~
materials ~UL.3aIvation of wood), eg. against PRe~ 5
variotii.
5 The iungicidal compositions in general contain from ~.1 to 95,
preferably from C.5 to 90, % by weight of active compound.
Depending on the type of effect desired, the application rates
are from 0.025 to 2, preferably from 0.1 to 1, kg of active com-
10 pound per ha.
In seed treatment, amounts of active compound of from 0.001 to50 g, preferably 0.01 to 10 g, per kilogram of seed are in
gener~l needed.
The compositions according to the invention can also be present
as fungicides together with other active ~ '- in the
application form, eg. with h~rhic;~, insecticides, growth regu-
lators, fungicides or alternatively with fertilizers.
Cn mixing with f~lng;~ s, in many cases an increase in the fun-
gicidal spectrum of action is obtained here.
The following list of f-~n5;~ with which the ~ ~ -
Z5 according to the invention can be applied together is intended to
illustrate the combination possibilities, but not restrict them:
sulfur, dithiocArh-~t~c and their derivatives, such as ferric
dimethyldi~hionR-- ~, zinc dimethylrlilhi~ - Le, zinc
30 ethyl~n~h;~;thiocarbamate, manganese ethyl~r~hic~;thiocRrh~-te,
manganese zinc ethyl~n~liRm;nr~ bisdilhinnR-' ~e, tetramethyl-
thiuram disulfide, ammonia complex of zinc N,N-ethyl~n~hi~ithi
c~ e, ammonia complex of zinc N~N~-propylenebisdilh;ocRrha
mate, zinc N,N'-propyl~n~hi~ hinc~--'- Le, N,N~-polypropylene-
35 bis(thiocarbamoyl) disulfide;
nitro derivatives, such as dinitro~l-methylheptyl)phenyl croto-
nate, 2-sec-butyl-4,6-dini~rophenyl-3,3-dimethylacrylate, 2-~ec-
butyl-4,6-dinitrophenyl isopropyl carbonate, diisopropyl
40 5-nitroisophthalate;
heterocyclic subs~ances, such as 2-heptadecyl-2-imidazoline
acetate, 2,4-dichloro-6-~o-chloroanilino)-s-triazine, 0,C-diethyl
phShAl;mi~nphn~rhnnothioate, S-amino-l-[bis~dimethylamino)-phos-
g5 phinyl]-3-phenyl-1,2,4-triazole, 2,3-dicyano-1,4-di~h;nAnthra-
quinone, 2-thio-l~3-dithiolo[4rs-bl~ll;nn~Al;n~r methyl l-~butyl-
carbamoyl)-2--b~n?;m;~lA?Ol~n~ 2-methv.. ,~ L~,n~lamino-
0050~g48~6 ~ 1 9 1 9 3 3
bDn7i~;~7nl~, Z-(fur-2-yl)b~n7imi~701e,
2-[thiazolyl-4-yl)b~n7im;~A7Qle, N-(1,1,2,2-tetrachloroethyl-
thio)tetrahydrophthRlimi~D~ N-trichlor~ethylthiotetra-
hydroph~halimi~D, N-trichloromethylthiophthRli~i~P,
N-dichlorofluoromethylthio-N~N~-dimethyl-N-phenyl ~nl f~ r~
5 cthoxy-3-trichloromethyl-1,2,3-~hi--~iR7nl~, 2-thiocyanato-
methyl~hioh~n7othiazole~ 1,q--dichloro-2,5-dimethoxybenzene,
4-(2-chloropheny~lhydrazono)-3-methyl~5-icnvR7ol~ --, 2-thiopyri-
10 dine-1-oxide, 8-LydLu~y~uinoline or its copper salt,
2,3-dihydro-5-carboY~nili~n-6-methyl-l~4-oxathiin~
2,3-dihydro-5-rRrhov~nili~n-6-methyl-1,4-oxathiin-4,4-dicxide,
2-methyl-5,6-dihydro-4H-pyran-3-carhnYRnilide~ 2-methyl-
f uran--3--c arbnY R ni 1 i rl~ ~ 2,5-dimethylfuran-3_~rhnYRnili,~,
15 2~4~5-trimethylfuran-3-carhnyRnilir~ N-cyclohexyl-2,5-dimethyl-
furnn-3-ca.b ~1D, N-cyclohexyl-X-methoxy-2,5-dimethyl-
furan-3-ca~' d~, 2-methylhDn7Rnil;,lD, 2-in~ r~,
U-formyl ll ~holine-2,Z,2-trichloroethyl acetal, pipera-
zine-1,4-diylbis(1-(2,2,2-trichloroethyl))formamide,
20 1-(3,4-dichloroanilino)-1-formylamino-2,2,2-trichloroethane,
2,6-dimeLhyl-N-tridecylmorpholine or it5 saLts, 2,6-dimethyl-N-
cyclododecylmorpholine or its salts, N-[3-(p-tert-butyl-
phenyl)-2-methylpropyll-cis-2,6-dimethylmorpholine,
N-[3-(p-LelL ~uLylphenyl)-2-methylpropyll-piperidiner
25 1-[2-(2,4-dichlorophenyl)-4-ethyl-1,3-dioxolan-2-yl-
ethyll-l~-1,2,4-tria_ole, 1-t2-(2,4-dichloro-
phenyl)-4-n-propyl-1,3-dioxolan-2-ylethyl]-lh-1,2,4-triazole,
N-(n-propyl)-N-~2,4,6-trichluLu~L_..u~y~Lhyl)-N'-imidazolylurea,
1-(4-ohlvLupl,eno~y)-3,3-dimethyl-1-(1~-1,2,4-tria-
30 zol-1-yl)-2-butanone, (2-chlorophenyl)-(4-chlorophenyl)-5-pyrimi-
dinemethanol tsic]r S-butyl-2-dimethyl_mino-4-hydroxy-6-methylpy-
rimidine, bis(p-chlorophenyl)-3-pyridinemethanol, 1,2-bis(3-
eth~y~.bu..yl-2-thioureido)benzene, 1,2-bis(3 . Lho~y~lbvl,yl-
2-thioureido)benzene, t2-(4-chlorophenyl)ethyl]-(1,1-dimethyl-
35 ethyl)-1~-1,2,4-triazole-1-ethanol ~sicl, 1-t3-(2-chlorophenyl)-
1-(4-fluu~u~ nyl)oxiran-2-ylmethyll-lH-1,2,4-triazole, and
various fungicides, such as dodecylguanidine acetate,
3-[3-(3,5-dimethyl-2-oxycyclohexyl)-2-1.yd.u~y~Ll.yllglutarimide,
~o hDY~hlnrobenzene, DL-methyl-N-(2~6-dimethylphenyl)-N-2-furoyl
alaninate, DL-N-(2,6-dimethylphenyl)-N-(2'-methoxyacetyl)alanine
methyl ester, N-(2~6-dimethylphenyl)-N-~hlnroRcetyl-D~L-2-amino-
butyrolactone, DL-U-(2,6-dimethylphenyl)-N-(phenylacetyl)alanine
methyl ester, 5-methyl-S-vinyl-3-(3,5-dichloro-
45 phenyl)-2,4-dioxo-1,3-~Y~7oli~inr~, 3-t3~5-dichlorophenyl-(5-meth
yl-5 Lhu~y Lhyl]-1,3-~Y~ 701 i ~ i nD-2, 4-dione, 3-(3,5-dichloro-
phenyl)-l-isopropylcR ' ,ylhydantoin, N-(3,5-dichloro-
0o50~448g6 2 1 9 1 9 9 9
.
38phenyl)-1,2-dimethylcycluyLvydl~s 1,2-~;rArhnJrimirlr~,
2-cyano-[N-ethyl n~ -~b~yl-2-melhor;minolacetamide~
1-12-(2,4-dichlorophenyl~pentyl]-1~-1,2,4-triazole, 2,4-difluoro-
~~ 1,2,4-triazoLyl-l-methyl)b~nzl.ydLyl alcohol,
5 N - (3-chloro-2,6-dinitro-4-trifluoromethylphenyll-5-trifluoro-
methyl-3-chloro 2 'nopyridine, 1-((bis(4-fl~vLupll~l.yl)methyL-
silyl1methyl)-1~-1,2,4-triazole,
strobilurins such as methyl ~ ~ minr~[rl_(o-tolyl-
10 oxy)-o-tolyllacetate, methyl E-2-{2-t6-(2-cyAn~~ n~ry)pyrimi
din-4-yloxy]phenyl}-3-methoxyacrylate, methyl E-methox-
imino~o-(2,5-dimethoxy)-c-tolyllacetamide.
~nilinopyrimidines such as N-(4,6-dimethylpyrimidin-2-yl)aniline,
15 N - ~ 4-methyl-6-(L-propynyl)pyrimidin-2-yllaniline,
N - ( 4-methyl-6-cyclopropylpyrimidin-2-yl)aniline.
Phenylpyrroles such as 4-(2,2-difluoro-1,3-b~n70rl;oxol-4-yl)pyr-
role-3-carbonitrile.
C;nn: dr~ such as 3-(4-chlorophenyl)-3-(3,4-dimethoxy-
phenyl)acrylic acid morpholide.
Synthesis examples
The ~uLou.luLes given in the synthesis examples below can be used
with modification of the starting ~ ~,u~--ds to obtain further
L~yleserLatives of the ~ ~ullds I~ and I. The physical data of
the ~uLL~ul-dingly prepared products are given in the tables
30 attached in each case.
Example 1
L-Valine (2-methyl-2-(4-methylphenylhydrazide) ~sic]
10 ml of trifluoroacetic acid were added with cooling to 1 g
(3 mmol) of N~ ku~o~arbonyl)-1-valine (2-methyl-
2-(4-methylphenyl)hydrazide [sic] and the mixture was subse-
r~uently stirred at O-C for one hour. It was then warmed to 20 C,
40 the trifluoroacetic acid was largely removed by distillation and
the residue was taken up in dichloromethane. ~he organic phase
was washed in each case with 40 ml of 10~ strength by weight
sodium hydroxide solution and water and dried. The solvent was
then removed. 0.7 g of L-valine 2-methyl-2-(4-methylphenylhydra-
45 zide) ~sic] remained, which was used in the following stage with-
out puri~ication.
0050r44896 2 I 9 I 9 9 9
The i--l ';ates IV of the type [sicl listed in the following
table were preeared in a similar manner.
Table 7
;
H3C CH3
H CH ~ Rs R6
11 1 1
~ - N - C - C -~N - N - R7
~~ R5 R6 R7 M.p. ~ C)/IR tcm-l)
1 H CH3 4-Cl-C6H4 oil
2 H CH3 4-CH3-C6B4 oil
3 H C2H5 4-Cl-C6H~ 3245,2964,2934,2872,1669,1493
4 H CH~cH3)z 4-CH3-C6B4 3250,2963,2931,2871,1673,1512
~xample 2
NQ-(sec-~u~u~yo~LLu~lyl~-~-valine 2-methyl-2-~4-methylphenyl)-
hydrazide
0.39 g (2.9 mmol) of sec-butyl chluLufoL-,,dLe was added dropwise
at 0 C to 0.7 g ~3 mmol) of L-valine 2-methyl-2-(4-methyl-
phenyl)hydrazide and 0.32 g (3.2 mmol) of triethylamine in 10 ml
of toluene. After 15 hours at 20 C, the organic phase was washed
in each case with 20 ml of 10% strength by weight hydrochloric
acid, sodium l,~dluJ~,c~rh~n~te solution and water. The organic
phase was dried and the solvent was then removed. 0.6 g of the
title compound remained as an oil (compound 6 in Table 6).
~xample 3
N"-(LeLL ~u~y~.rbonyl)-1-valine 2-methyl-2-(4-chloro-
phenyl)hydrazide
S g (o~os mol) of triethylamine were added dropwise at -15 C to
10.9 g (0.05 mol) of Ce~-b~Lu~y~b~.,yl-L-valine in 200 ml of
dimethyl~ d~ and the whole was ~h~e~ n~ly stirred for
15 minutes. 6.8 g (0.05 mol) of isobutyl chloroc~rh~n~to were
then added and the mixture was left for 10 minutes at -15 C. 7 8 g
(0.05 mol) of N-methyl-N-(4-chlorophenyl)hydrazine in 50 ml of
dimethylformamide were then added to this solution. ~t was subse-
0~50/44896
219199~
40~uently stirred at -15~C for 1 hour and the reaction mixture was
left at 20 C for a further 2 hours. ~he solvent was then removed
by distillation, the residue was taken up in ethyl acetate and
the organic phase was washed in EUCc~Csion in each case with
5 10 ml of 5% ~trength by weight citric acid, 5~ strength by weight
sodiu~ 1~d u~ Ate solution and water. The organic phase
was dried and .o~l~eY.LL-Led, and the residue which remained was
.L , -LoyL~hcd on silica gel ~methyl ~er~ ~u~yl ether:n-hexane =
1:1). 5.5 g of the title compound were obtained as an orange-red
10 resin (compound 27 in Table 6).
Example 4
~ tert-~utoxycarbonyl)-L-valine 2-methyl-2-~4-methyl-
15 phenyl)hydrazide
5.1 g (0.051 mol) of triethylamine and then, at O-C, 8.31 g
(0.051 mol) of diethyl uy~r~ph~ te were added t~ a solution
of 10.85 g (0.05 mol) of N-t~.L buLu~y.~Lbullyl-L-valine and
20 5.95 g ~0.05 mol) of 4-methylphenylhydrazine in 100 ml of tetra-
hydrofuran. The mixture was stirred at O~c for one hour and at
20 C for a further 15 hours, and after removing the solvent the
residue was taken up with ethyl acetate. The organic phase was
washed in each case with 40 ml of 10% strength by weight sodium
25 hydroxide solution, 10% strength by weight hydrochloric acid,
saturated sodium l.ydlug~ ., Ate solution and water. The
organic phase was dried and the solvent was then removed. 9.8 g
of N~-(telL buLu~yualbullyl)-L-valine 2-~4-methylphenyl)hydrazide
remain (m.p.: 163-164-C, compound 21 in Iable 6).
0.48 g (3.4 mmol) of methyl iodide and 0.47 g (3.4 mmol) of
potassium carbonate were added at 20-C to 1 g (3.1 mmol) of this
compound in 10 ml of dimethy1 forr~ , The mixture was left at
this t~ , ~LuLe until starting compound was no longer detectable
35 and the solvent was then removed by distillation. The residue was
taken up in ethyl acetate. The organic phase was washed with
water, dried and conce~ d~ed. The oil which remained was chroma-
tographed on silica gel (cy~7nh~Y~n~:ethyl acetate = 2:1) and
0.3 g of the title oompound was obtained as a yellow oil (com-
40 pound 28 in Table 6).
~. 0050/44896 ' ' 2 ~ g 1 9 g 9
-
Table 8
5O H R3 ~ R5 R5
R~ - O - C - N - C - C - N - N - R7
H
No. Rl Rl Rs R5 R7 M-p-(~C)/
rR (c~
1 C(CH3)3 CH~Call2 M CH3 C6H5 108
15 2 CH(CH3)2 CHICH312 H Ca3 C6H5 115
3 C6Hs CHICH3lz E CE33 C6H5 138-140
4 CR(CH3)2 CH~CH312 M CH3 4-CH3-C6N4 118-120
C6Hs CH(CH3,l2 H CH3 4-CH3-C6H4 154
6 Ch(cH3)c2Hs CH(CH3)2 H CH3 4-CH3-C5H4
20 7 CH2CH(CH3)2 CH(CH3)2 H CM3 4-CH3-C6H4 oil
8 CH2C6H5 CH(CH3)2 H CH3 4-CH3-C6H4 163
9 cn(cH3)2 CH(CH3)2 H CH3 4-Cl-C6Hc 154
C6H5 CH(CH3)2 H CH3 4-Cl-C6H~ 178-180
25 11 CH2C6Hs CH(CH3)2 H CH3 4-Cl-C6H4 161
12 C(CH3)3 CH(CH3'2 H C2H5 4-CE3-C6H4 84-86
13 CH(CH3)2 CH(CM3 2 H CzH5 4-CH3-CGH4 137-139
14 CH(CH3)C2H5 CHICH3 2 H C2H5 4-CH3-C6H4 118-120
30 15 C(CH3)3 CHICH3 2 H C2H5 4-Cl-C6H4 156
16 CH(CH3)2 CHIcH3t2 H C2H5 4-cl-c6H4 163-165
17 CH~CH3lC2H5 CHfCH3 2 H C2M5 4-Cl-C6~4 168
18 CH CH3 2 CH CH3 2 H CH(CH3)2 4-CH3-C6H~ 118-12035 19 CH CMI,C2H5 CH CH3l2 H CH(CH3)2 4-CH3-C6H4 124-126
CtCH313 CH,CH3t2 H H 4-CL-C6H4 162
21 CICH~3l3 CH~CH3 2 H E 4-CH3-C6B4 163-164
22 CE2CHtCH3)3 CHICH3l2 H CH3 4-cl-C6H4 133
23 C(CH3~3 CHICH3 '2 H H 4-CH30-C6H4 171
24 CICH3 3 CH~,CH3 2 H CM2CH(CE33)2 4-cl-c6H4 171
CICH3l3 CH(cH3)2 El CH2CH(CH3)z 4-CH3-C6H4 154-185
26 C~,C~3l3 CH(CH3)2 H C6H5 C6H5 189-193
27 C(CH3)3 CH(CH3)2 H CH3 4-Cl-C6H~ 2)
45 28 C(CH3)3 CH(CE3~2 H CH3 4-CH3-C6H~ 3)
29 C(C~3~3 CH(CH3)2 H CH(CH2CH3)2 4-CH3-C6H4 130
oo50/44896 2 1 9 ~ ~ 9 9
~ 42
No. Rl R3 Rs R6 R7 M-p-(~C)/
IR (cmr
C CH3 3 CH,CH3)2 M ~CH2~3CH3 4-CH3-C6H4 137-139
31 CICH3l3 CH CH3 2 H cyclo-C5H9 4-CH3-C6H~ 141-142
32 CICH3~3 Ca~CH312 H (CH2)3cH3 4-cl-c6H4 160-161
33 C CH3 3 CHICH3)2 H cyclo-C5Hg 4-cl-c6H4 164-166
34 C~CH3)3 CHICH3)2 H CE{2c6Hs 4-Cl-C6Hg 168-172
CE(CH3~2 CH(CH3)2 H CH2-C6HS 4-Cl-C6H4 171-173
10 36 CH(CH3)C2H5 CH(CH3)2 H CH2-C6H5 4-cl-c6H4 161-171
37 CH(CH3)2 CH(CH3)2 H cyclo-C5H9 4-Cl-C6Hg 221-223
38 CH(CH3)2 CEI~CH3)2 H (CH2)3c~3 4-Cl-C6H~ 193-194
39 CH(CH3)C2Hs CH(CH3)2 H (CH2)3CH3 4-Cl-C6Hg 166-168
lS 40 C(CH3)Z CHlcH3)2 H C(CH3)2 4-C1-C6EI 152
41 CHICH3~2 CH~CH3 2 H CH(CH3)C2Hs 4-Cl-C6H 162
42 CH CH3~C2Hs CHICH312 M CH(CH3)C2Hs 4-Cl-C5H4 136
43 CHICH3l2 CH(CH3 i H cyclo-C6H11 4-CH3-C6H4 193-195
20 44 CHICH3l2 CH(CH312 H (CH2)3cH3 4-CH3-C6H 176-177
CHICB3)C2H5 CH(CH3 ~2 H (CH2)3CH3 4-CH3-C6H4 142
46 C(CH3)3 CH(CH3 2 H CH(CH3)2 4-CH3-C6H4 139
47 CH(CH3)2 CH(CH3 '2 H cyclo-C5H9 4-CH3-C6H~ 188
48 CH(CH3 CzHs CH~CH3 2 H cyclo-C5H9 4-CH3-C6Hg 160
25 49 CH(cH3 '2 CH CH3 2 ~ CH2-C6HS 4-CH3-CsH4 146-148
SO CH(CH3 2 CH~CH3 2 H cyclo-C6H11 4-CH3-C6H4 216
51 C(CH3)3 CHICH3 2 H (CH2)3CH3 4-CH30-C6H4 oil
52 C(CH3)3 CHICH312 H cyclo-C5H9 4-CH30-C6Hg 130-132
30 53 CH(CH3)2 CH(CH3)2 H cyclo-C5H9 4-CH30-C6H~ 172-175
54 CH(CH3)2 CH(CH3l2 H ~CH2)3CH3 4-CH3O-C6H4 158-160
CH(CH3)C2H5 CH(CH3l2 H Cyclo-csH9 4-CH3O-C6Bg 161
56 CH(CH3)C2Hs CH(CH3 2 H (CH2)3Ca3 4-CH3O-C6Hg 128-130
35 57 CH(CH3)2 CH(cH3l2 H CH(CH3)2 4-CH3-C6H4 160
S8 CH(cH3)c2Hs CH(C83 2 H CHz-C6H5 4-cH3-c6H4 139
59 CH(CH3)2 CH(CH3)2 H CH(C2Hs)2 4-CH3-C6H4 oil
CH(CH3)2 CH(CH3)2 H C2H$ cyclo-C6H11 173-17S
I) IR (cmrl): 3280, 2969, 293S, 1674, lS15, 1243
2) lH-NMR (CDC13): 0.98 (dd,2H); 1.4 (s,SH); 2.12 (m,l~); 3.8
(s,3EI); 3.95 (t,lEl~; 5.35 (d,lH,~H); 6.8 (d,2H); 7.18 (d,2H)
3) lH-NMR (CDC13): 1.98 (dd,6H); 1.4S (s,9H); 2.13 (~,lH); 2-25
(s,3El); 3.12 (s,3H); 3.87 (t,lH); 5.2 (d,lH,NH); 6.74 (d,2H);
7.00 (d,2H); 8.13 ~s,lH,~H)
0050,44896 2 1 9 1 9 9 9
43
Table 9
Wl ~ H ~3 ~ R5 R6
\ ~ T
C=N----O----C--~--C--C--~--W--R7
lq2 ~1
No . Wl w2 R' Rs R6 R7 M.p. ~~C )
1 CH3 c~3 CH(C~3)2 H Ch3 4-Cl-C5H4 104
Z CH3 c~3 CH~CH3~2 H CH3 4-CH3-C6H~ 84
15 Use example5
It was possible to show the ~ungicidal action of the ~ ' of
the general formula I by the following te3ts:
The active ~ d., were prepared as a 20% strength by weight
Dlmulsion in a mixture of 70% by weight of cyr-lr~hr~YRnr~l, 20% by
weight of WekanilD LW (Lutensol'~ AP6, wetting agent with emulsi-
~ier and dispersant action based on ethoxylated alkylphenols) and
10~ by weight of Emulphor~ EL (Emulan~ EL, c lc;~;r7r based on
ethoxylated fatty alcohols) and diluted with water according to
the desired cu~ ,,LL~ion.
Plasmopara viticola
Leaves of potted vines of the variety ~uller-Thurgau were sprayed
with aqueous spray liquor which contained 80% by weight of active
compound and 20% by weight of emulsifier in the dry substance. In
order to assess the duration of action of the active ~ , ' ,
the plants were placed in a greenhouse for 8 days after the spray
coating had dried on. Only then were the leaves infected with a
~ospore sncpDncion of Plasmopara viticola (vine PeLVLIU~UL L). The
vines were first placed for 48 hours in a chamber containing
water vapor-saturated air at 24~C and then for 5 days in a green-
40 house at from 20 to 30'C. After this time, the plants were placedin the moist chamber again for 16 hours to accelerate escape from
the spor~ngiorhr~re~ The extent of fungal outbreak on the bottoms
of the leaves was then assessed visually.
. 0050/44896
2191999
~ 44
In this test, the plants treated with an aqueous preparation con-
taining 250 ppm of the ~ ~c 4, 6, 9, 10, 12, 13, 14, 15, 16,
17, 18, 19, 20 and 22 from Table 6 in each case showed a maximum
attack o~ 15*, while ul,LL~aLed plants were attacked to 70~.