Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ WO 96/04233 2 l q 6 1 9 3 . ~ ~ 0~7
ARYLOXY AND ARYLTHIOPROPANOLAMINE DERIVATIVES USEFUL AS BETA 3-ADRENO~t~E~lU~
AGONISTS AND ANTAGONISTS OF THE BETA 1 and BETA Z-ADRENORECEPTORS AND
PHARMACEUTICAL COMPOSITION THEREOF
This invention relates to novel c- ,. "~ to a process for preparing such
5 c..,.,l...~l,..l~ tol,l,--",~ ;.Alc..,.,l,.~ ;n,\ccontaining5uch~ l.u,..,.1~andtothe
use of such c~ ll.ull~r1c and c,.."l.r~;l;u,.~ in medicine and agriculture.
European Patent ArplirAtinn Publication Number 0328251 discloses certain
2-(2-hydroxy-3-1,h~,lluA~,.ulJyla.lli..v)~,Lll~l~}~l.uA ~ ,La~llidcs which are stated to be
useful in the treatment of obesity and related conditions.
It has now surprisingly been discovered that a particular series of nûvel
arylûxy and arylthio ~ "; . .~ derivatives have good ,B3~ I r~ p ~ agonist
activity and in particular show good selectivity for ,~3-ad,c..vlG.,~,~,Lvl, over the ~1- or
,~2-a~ullvlG~,G~Lvl~, to the extent that these CVIII~lUUlldS are --n~ of the .~1- and
,~2-adlGllul G~ u~ ~. These ~ ~ I" ,l"J" ". l~ are indicated to have good anti-
h~ ;ly~,a~,llli~, and/or anti-obesity activity coupled with especially good selectivity
from cardiac and tremorigenic side effects
These C~ v~ lc are also indicated to have potential in the treatment of
~,a~L ui.l.~,ALh,al disorders such as peptic ulceration, oesorh ~gitic~ gastritis and
duodenitis, intestinal nlr.-rA~innc, including ~ y bowel disease, and irritable
bowel syndrome and also for the treatment of ~ Ll~ I nlr~r~tinnc especiallywhen induced by non-steroidal anti-i,,ll- ".,~n~.,y drugs or.,ul~ u~t~lu;v~.
These ~..,,,I.,.,,,.,lc may also be of use in increasing the high-density-
lipoprotein (HDL) cholesterol ~ n,~ ~ . n I A l ;~ II I and decreasing the triglyceride
~.11.. lln AH~I.. in human blood serum and are therefore of potential use in the
treatment and/or ~lu~h~l_A is of aLh.,.usclclusis. They are also indicated to be useful
forthetreatmentofll~l,~.;..~..l;.,-- ,.;l Theyarealsoindicatedtobeusefulforthe
treatment of depression.
These ~ u~ Ic also have potential as growth promoters for livestock and
for decreasing birth mortality rate and increasing the post-natal survival rate in
livestock.
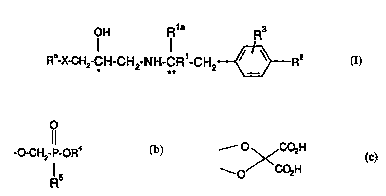
Accordingly the present invention provides a compound of formula (I):
~ OH R1a R
Ro-x-cH2-cH-cH2-NH-cR-cH2~Rz
(I)
-1-
WO 96/04233 2 1 q 6 1 9 3
or a ~ ",- ,~ lly acceptable salt thereof, or a l~h~."~ ~,n;, ~lly acceptable solvate
thereof,
wherein,
R~ represents an aryl group optionally substituted with one, two or three ~ ;n,a "
5 selected from the list consisting of: hydroxy, Lydlu~y~ yl, nitro, amino,
alkylamino,dialkylamino,aLkyl,,.l~ n"~ ln ~llyl~ 1n,fnrrnq~ n,
halogen, alkoxy and allyl;
X represents O or S;
Rl and Rla each 1...1. 1.. ",l. ., lly represents hydrogen or an alkyl group;
10 R2 represents OCH2CO2H, or an ester or amide thereof, or R2 represents a moiety of
formula (b):
-o-CH2-P-CR4
Rs
(b)
wherein R4 represent hydrogen, alkyl, hydroxyalkyl, arylalkyl, alyloxyalkyl,
aralkyloxyalkyl or cycloalkyl and R5 represent hydroxy, alkoxy, arylalkyloxy,
hydroxyalkyloxy, alkoxyalkyloxy, aryloxyalkyloxy, arylalkoxyalkyloxy or
cycloalkyloxy or R5 represents hydrogen, alkyl, substituted alkyl, cycloalkyl, aryl,
20 arylalkyl, aryloxyalkyl, arylalkyloxyalkyl or R5 together with oR4 representsO(CH2)nO wherein n is 2, 3 or 4; and
R3 represents hydrogen, halogen, alkyl or alkoxy or R3 together with R2 represents a
moiety of formula (c):
O ~CO2H
--O--co2H
(c)
or an ester or amide thereof; providing that 4-[2-[2-hydroxy-3-(4-
h y~u~yl~h.,~lu~y)lulUIJyialll;llO]prOpyl] IJL~ u~ ,liC acid and salts and esters thereof
and the compounds of examples 1 to 36 disclosed in EP0328251 are excluded from
30 the scope of formula (I)
Suitable aryl groups include phenyl or naphthyl groups, especially phenyl
groups
Suitable optional ,"l.~l;n.n ..l~ for R~ include one, two or three ~ ;n.~
selectedfromthelistconsistingof:hydroxy,l,yd. w-ylll~ yl~ alkyls.,ll.l... -".;~nand
35 halogen.
--2--
~w096/04233 2 1 9 6 ~ q 3 r~l".,~/Q~7
Suitably, R~ represents a phenyl group optionally substituted with hydroxy
andlor ~ l andlor halogen~ especially fluoro andlor alky~ h~
Examples of R~ include 4-hydroxy-3-hydlu-~yl.l~,.llyl~ lyl, 3- and 4-
h~u~y~h~llyl, 3-fluoro-4-hy,llu,~y~ ,.lyl and 4-hydroxy-3-methylc~
phenyl groups.
Suitably, R 1 is an alkyl group and R la represents hydrogen.
Suitably, R 1 and R la each represents hydrogen.
When Rlis alkyl, it is favourably a C1 6 alkyl group, especially a methyl
group.
Suitably, Rla represents hydrogen.
In one aspect, R2 represents OCH2C02H, o} an ester or amide thereof.
Suitably, R3 together with R2 represents a moiety of formula (c) or R2
represents a moiety of formula (b) and R3 represents hydrogen, halogen, alkyl oralkoxy.
In one aspect, R2 represents a moiety of formula (b).
In one aspect of the invention, R3 together with R2 represents a moiety of
formula (c).
Preferably, R2 is a moiety of formula (b).
Favorably, R3 represents hydrogen, halogen, alkyl or alkoxy.
Preferably, R3 is hydrogen.
Suitably, R4 represent hydrogen, alkyl, hydroxyalkyl, phenylalkyl,
benzyloxyalkyl or cycloalkyl.
When R4 represents alkyl, especially C1 6 alkyl, examples include ethyl and
butyl, especially n-butyl.
When R4 represents hydroxyalkyl, an example is hy~u~iy~lulJyl.
When R4 represents arylalkyl, an example is pllellyllJlu~yl
When R4 represents arylalkyloxyalkyl, an example is b~ ylo~y~ yl.
Favourably, R4 represent hydrogen or alkyl, especially hydrogen.
When R5 represents substituted alkyl, suitable ~ are selected from:
hydroxy, alkoxy and arylalkoxy.
Suitably, RS represents hydroxy, alkoxy, arylalkyloxy, hydroxyalkyloxy,
alkoxyalkyloxy, arylalkoxyalkyloxy or cycloalkyloxy, especially alkoxy,
- hydroxyalkyloxy or arylalkoxyalkyloxy.
When R5 represents alkoxy, especially Cl-6 alkoxy, examples include ethoxy
~ 35 and n-butoxy.
When R5 represents arylalkyloxy an example is p'.~ yl~lu,~ylu~y.
When R5 represents arylalkoxyalkyloxy an example is b~ L~lu~y~lu~yloxy.
21 96~ 93
w0 96/0~233 ~ r~
Suitably, in the hydroxyalkyloxy group represented by R5 the hydroxy group
is substituted on the terminal carbon atom of the alkyl group, for example as in a 2-
hyJIw~y~ yloxy group and a 3-hyJIu~y~lu~Jyloxy group.
Favourably, R5 represents hydrogen, alkyl, substituted alkyl, cycloalkyl or
5 aryl.
When RS represents cycloalkyl an example is cyclohexyl.
Preferably, R5 represents alkyl for example n-hexyl. Preferably, RS
represents aryl for example phenyl.
When R5 represents alkyl examples include n-hexyl.
Preferably, R4 represent alkyl, especially Cl 6 alkyl, for example ethyl, and
R~ represent alkoxy, especially C1 6 alkoxy, for example ethoxy.
In another aspect, R4 is alkyl, for example ethyl, and R5 is hydrogen.
Preferably, X represents 0.
In one aspect the invention provides a subgroup of the culll~Juullds of formula
(I) wherein R~, R1, Rla, R2, R3 and X are as defined in relation to formula (I),providing that formula (I) does not include 4-[2-[2-hydroxy-3-(4-
Ly~llu~y~h~,llu~y)~u~yl~lllillo]propyl] ~h~ u~y~CcLiC acid and the salts and esters
thereof or 4-[2-[2-hydroxy-3-phenoxyl lu,uyl~nillo]ethyl] phenoxyacetic acid and an
amide thereof.
~ 20 In a further aspect the invention provides a subgroup of the ~ v ~ of
formula a) wherein R~ and X are as defined in relation to formula ~I), R2 represents
OCH2C02H or an ester or amide thereof, R3 represents hydrogen and R1, R1a are asdefined in reladon to formula (I) providing that at least one of R1 or R1a represents
alkyl.
In one particular aspect the invention provides a subgroup of the compounds
of formula (I) wherein R~, Rl, Rla and X are as defined in relation tO forrnula (I) and
R2 represents a moiety of formula (b) and R3 represen~s hydrogen, halogen, alkyl or
alkoxy or R3 together with R2 represents a moiety of formula (c), such compoundsshall hereinafter be referred to as cnrnpol~n~lc of formula (IA).
The l;uu,l)uul,J~ of formula (I) have one or two a~yl~ ,uic carbon atoms,
marked with an asterisk (*) or two asterisks (**) in the formula. These c.,ll~ lc
may therefore exist in up to four st~,.cuisu.ll~,.ic forms. The present invention
"" ~ all ~ uiavlu~ of the compounds of the general formula (I) whether
free from other isomers, or admixed with other isomers in any proportion, such as
35 mixtures of ,li~ u;sv,~ and racemic mixtures of ~ . ~, .l i. ,,, ,. . ~
In addition when the ~I b~ on the phosphorous atom of moiety (b) are
different and other than OH the ~Jhb~lluluu~ atom is chiral: The invention extends to
mixed and separated isomers of such cu.,ll,uuuds in an analogous fashion to thatdiscussed for chiral carbon atoms.
--4--
~ Wo 96/0~233 2 1 9 6 l 9 3 P~ 7J/
Preferably, the ~y~ ic carbon atom indicated by a single asterisk (~) is in
the S-cnnfigllrAtinn
Preferably, the A~ylll~ ,il;C carbon atom indicated by two asterisks (**) is in
the R-configuration
One suitable form of a compound of formula (I) is a mixture of the SR and RS
One favoured form of a compound of formula (I) is the SR cllauLiu~
The term 'alkyl' when used alone or when forming part of other groups (such
as the 'alkoxy' group) includes straight- or branched-chain alkyl groups containing 1
10 to 12 carbon atoms, suitably I to 6 carbon atoms, examples include methyl, ethyl,
n-propyl, isopropyl, n-butyl, isobutyl or tert-butyl group.
The term 'cycloalkyl' includes C3 8 cycloalkyl groups, especially Cs or C6
cycloalkyl groups.
When used herein the term "halogen" refers to fluorine, chlorine, bromine and
15 iodine, preferably fluorine or chlorine.
When used herein the term "cnl~ n..~".i.l.~" refers to the moiety '-SO1-NH-',
for example Ul~llly~ 0 refers to the moiety 'CH3-SOl-NH-'.
Suitable l .l , -, . . ~- ~, . 1 ;. .AIly acceptable esters of carboxyl groups include alkyl
esters, especially C 1-6 alkyl esters such as methyl.
~ 20 Suitable l,l .A ", ~ y acceptable amides are those of formula -coNRsRt
wherein Rs and Rt each ' r ~.e 'ly represent hydrogen, alkyl or alkoxyalkyl.
Suitable ~ Ally acceptable salts include acid addition salts, salts of
carboxy groups and salts of ph~ h~ acid groups. Salts of phosphinic acids are
also suitable l 1 l- " ~ ly acceptable salts of the invention.
Suitable Ill,A, ." -- ~.,n. Ally acceptable acid addition salts include salts with
inorganic acids such, for example, as hydrochloric acid, hydlublulllic acid,
u. i' , ' . ' - acid or sulphuric acid, or with organic acids such, for example as
",. ~ acid, tnl~.~ n~ n";n acid, acetic acid, propionic acid, lactic acid,
cittic acid, fumaric acid, malic acid, succinic acid, salicylic acid, maleic acid or
acetylsalicylic acid.
Suitablel,l,A""=~ nnAIlyacceptable5alt50fcarboxygroup5,~ acid
or phosphinic acid groups include metal salts, such as for example All~mininm aLkali
- metal salts such as sodium or potassium and lithium, alkaline earth metal salts such as
calcium or mAgn~ cillm and Ammnninm or substituted ammnnhlm salts, for example
those with C1 6 alkylamines such as triethylamine, hydroxy-Cl 6 alkylamines suchas 2-l~yd~uAy~,~llyl,ll~l;lle, bis-(2- hyd~uA~.,illyl)-amine or t~i-(2-hy~uA~"llyl)-amine,
cycloalkylamines such as l,i-;y-,loh.,Aylamine, or with procaine,
21 961 ~3
w0 96/04233 r~
1,4-dibenzylpiperidine, N-benzyl-,~-phenethylamine, d.,l,y.lludl,h,~ le,
N,N'-bi~d~ ydludlJ;~Lylamine, glucamine, N-methylglucamine or bases of the
pyridine type such as pylidine, cûllidine or quinoline.
Suitable ~ .u;, ~lly acceptable solvates are conventional solvates,
5 prefesbly hydrates.
In a further aspect the invention also provides a process for the ~ J~aLiOll of
a cûmpound of formula (I) or a ~ " l ;. ,.liy acceptable acid addition salt or a
"~ ly acceptable solvate thereof, which process comprises reacting a
compound of formula (II):
R~ -X-CH2--CH--CH2
(II)
wherein X is as defined in relation to formula (I) and R~ represents R~ as defined in
15 relation to formula (1) or a protected form thereof, with a compound of formula (III):
R'~
T~NH--CR1 CH2~R2
~
aII)
wherein Rl, Rla, R2 and R3 are as def,ned in relalion to formula (l) and T~
represents a hydrogen or a protecting group; and thereafter, if required, carrying out
one or more of the following optional steps:
(i) converting a compound of formula (I) to a further compound of formula (I);
(ii) removing any protecting group;
(iii) preparing a pl. -- ", -- ~.u ~ lly. acceptable salt of the compound of formula (1)
and/or a plla~ lly acceptable solvate thereof.
The reaction between ~.v~ u~ of formulae (II) and (III) may be carried out
in any suitable solvent, such as methanol, at any ~U~ ,.dlUlC providing a suitable ste
of formation of the required product, generally at an elevated Ltlll~ Lulc such as the
reflux ~ ulc of the solvent; preferably under an inert aLIllO~ c such as
nitrogen or argon, alternatively the reaction between compounds of formulae (II) and
(III) may be carried out in a chlorinated solvent such as dichlu~wll~,illdlle or in an
aprotic solvent such as acetonitrile; suitably the reaction is carried out in the presence
of a catalyst such as ytterbium triflate as described in Tetshedron Letters, 1994,
35(3), 433 or a perchlorate such as lithium perchlorate.
-6-
21 961 93
~wo 96/04233 r~~ 7~!r~ 7
Suitably R~ represents a protected form of R~, suitable protected forms being
as defined herein.
Suitable protecting groups represented by T~ are benzyl or p-ul~Lllu~rb._u~yl
groups.
- 5 A compound of formula (Il) may be prepared by reacting an activated form of
a compound of formula (IV):
R~-XH (IV)
wherein R~ and X are as defined in relation to formula (II), with a compound of
formula (V):
L~- CH2--CH--~CH2
(V)
wherein L~ represents a leaving group.
A suitable activated form of a compound of formula (IV) is an ionic form,
such as an alkali metal salted form, for example a potassium salted form.
An activated form of a compound of formula (IV) may be prepared by use of
the .I~JlUJrl;..;~, cul~ lLiulldl procedure, for example a salted form may be prepared
20 by treating the compound of forrnula (IV) with a base such as an alkali carbonate, for
example potassium carbonate.
Suitably, L~ represents a tosylate or a 3-1-iLr uL,~ h. -~ ~ylu~y group.
The reaction between the crlmpo~ c of formulae (IV) and (V) may be carried
out in an aprotic solvent such as acetone or d;lll~,Lhylr~r~ -ll;lc at any t~ Lulc
25 providing a suitable rate of formation of the required product, generally at an ambient
to elevated t~ y.,~aLul~, suitably an elevated L~iul~ tulc, such as the reflux
t~ UI~i of the solvent.
L~ also represents OH.
When L~ represents OH, the compound of formula (V) is oxiranyl-methanol
30 and the reaction between it and the compound of formula (IV) is uul~ lLly
effected using a Mitsunobu reaction, according to methods disclosed in Tetrahedron
Letters., 1994, 35, 5997-6û00 and Organic Reactions 1992, 42, 335-656.
A compound of formula (III), wherein Rl is not hydrogen, is suitably
- prepared by the hydlu~,~.loly~;~ of a compound of formula (VI):
Wo96/04233 2 1 9 6 1 ~ 3 r~ n~7
CH3 IR
(VI)
wherein Rl, R2 and R3 are as defined in relation to formula (I), Y represents
hydrogen or a moiety -B(OH)2 and the **CH carbon and ~**CH carbon atoms are
5 chiral carbon atoms.
Suitably, catalytic hyd~ lvly~;~ is used, using for example 10% palladium
on charcoal in the presence of ammonium formate, suitably in an alkanolic solvent
such as methanol, at any ~ Ul c prvviding a convenient rate of formation of the
required prvduct, for example at ambient t~ ,-aLulc, preferably the reaction is
10 carried out in an inert dL.IIo"JI,~,.C, generally under nitrogen .
A compound of formula (VI) wherein Y is a moiety B(OH)2 may be prepared
from a cullc~lJu~ lg compound of formula (VI) wherein Y is H, by treatment with
boron tribromide in an inert solvent such as methylene chloride at ambient
t~ ulc, preferably in an inert d~ V ,~h~.lc such as argon, followed by rcmoval of
15 Y using catalytic hy~Lv~,~,llvlya;~, using for example a palladium on carbon catalyst.
A compound of formula (VI) wherein Y is H may be prepared by
.cu~le~ivt; reduction of a compound of formula (VII):
CH3 R' R3
~CH--N=C--CHz~R2
(VII)
wherein R1, R2 and R3 are as deflned in relation to formula (I) and the
***C carbon is a chiral carbon.
The reduction of the compound of formula (VII) may be carried out using
25 catalytic reduction in the presence of hydrogen.
A preferred catalyst is platinum oxide.
Suitable reduction conditions include using an alkanol solvent such as
methanol or ethanol, at any LCII~ UIC providing a convenient rate of formation of
the required product, e~ e.~ ly at ambient ~clll~,lalulc using a pressure of 1-530 al,.,o~ ,.c~ of hydrogen.
The compound of formula (VII) may be prepared by reacting a compound of
formula (VIII):
-8-
~ wos6/04233 21 9 6 l 9 3 ~ ,S/~'~m7
R' R3
O=l--CH2~RZ
(VIII)
wherein Rl, R2 and R3 are as defined in relation to formula (1), with
S R-a-ll..,LI-yl~ y
The reaction between cu~ of formulae (VIII) and
R-a-l..~,Lhy~ yL~ e may be carried out under ~wllv~,~lLiullal amination conditions,
for example in a solvent such as methanol or toluene.
G~uv~ lLly~ the compound of formula (VII) is prepared in-situ by reacting a
10 compound of the above deflned formula (VIlI) with R-a-methylbenzyl amine and
thereafter reducing the compound of formula (VII) so formed using reaction
conditions and catalysts as described above.
The ~ J""~1~ of formula (VIII) wherein R2 represents OCH2CO2H or an
ester or amide thereof or wherein R2 represents a moiety of the above defined
15 formula (b) wherein R5 represent hydroxy, alkoxy, hydroxyalkyloxy or
cycloalkyloxy or R5 together with oR4 represents O(CH2)nO, are known
,u l~ or they may be prepared by processes analogous to those used to prepare
such ~ , for example they may be prepared according to methods disclosed
in European Patent Application, Publication Number 0023385 or T,ll~", ~l;..1l;~1 Application number WO 94/02493.
A compound of formula (VIII) such as those wherein R2 represents a moiety
of the above defined formula (b) wherein RS represent hydrogen, alkyl, substituted
alkyl7 cycloalkyl or aryl may be prepared by reducing a compound of formula (IX):
R2~ <N O
R3
(IX)
wherein Rl and R3 are as defined in relation to formula (1) and as stated R2 is as
defmed in relation to the required uu~ /uunds of formula (VIII).
The reduction of the compound of formula (IX) may ~;ou~ ly be carried
out using iron powder in the presence of acetic acid in an aqueous solvent such as
aqueous methanol, at any lc~ aLulc providing a suitable rate of formation of therequired product, generally at an elevated L~ Lulc and cul.~c.l;,,.l~ly at the reflux
Ll,lll~ ulc of the solvent.
-9-
. _ .... _ .... . . ... . .
wo s6/04233 2 1 ~ 6 ~ q 3 P~ ~ 7'/"~n~7
A compound of formula (IX) may be prepared by reacting a compound of
formula (X):
RZ~CHO
S (X)
wherein, R2 and R3 are as defined in relation to formula (IX), with a nitroalkane,
such as uiLIu~ ,dlall~ or l,iL~. '
Generally, the carbon atom of the -C~iO group in the compound of formula
10 (X) is in an activated form, a suitable activated form being provided by forming an
imine of the said carbonyl group: The imine may be prepared by reacting the
compound of formula (X) with an amine, suitably a primary alkyl amine such as n-
butylamine. The reaction of the compound of formula (X) and the amine may be
carried out in any suitable solvent, such as toluene, at any t~ ,la~ulc providing a
15 suitable rate of formation of the required product, generally at an elevated
t~ ,IalUlc such as the reflux ~ ,.atulc of the solvent; and preferably in the
presence of a catalytic amount of ~ acid.
The reaction between the compound of formula ~X), and when it is in the
form of an imine and nitroalkane may be carried out in glacial acetic acid, preferably
20 in the presence of an ammonium acetate catalyst, generally at an elevated t~,lu~ aLu~c;
such as in the range of from 60~C to 1 20~C, for example 1 00~C
A compound of formula (X) may be prepared from a compound of formula
(Xl):
L~CH0
R
(XI)
whGrein R3 is as defined in relation to formula (IX) and L~ is a leaving group or
atom, generally a fluorine atom, with an activated form of a compound of formula
~XII):
HO-CH2-P-OR~
Rs
(XII)
-10-
21 '~6I q3
6/04233 r ~ ;l ,s. Q~n~7
wherein R4 and R5 are as defined in relation to formula (I).
A suitable activated form of a compound of formula (XII) is an ionic form,
such as a salted form, for example an alkali metal salted form.
An activated form of a compound of formula (Xi'I) may be prepared by use of
S the a~ ul r u~v~,.lLiul~al procedure, for example a salted form may be prepared
by treating the compound of formula (XII) with a base such as an alkali metal
hydride, for example sodium hydride.
The reaction between the c~ vu~ c of formulae (Xl) and (XII) may be
carried out in any suitable solvent, generally an aprotic solvent such as
o ~ r~ or N~ hyl~ylluli~ lune at a low to ambient L~ alulc~ for
example in the range of from -15~C to 20~C, such as 5~C
~ nmpountlc of formula (III) wherein R2 together with R3 represent a moietyof above defined formula (c), or an ester or amide thereof, are prepared from a
protected form of a sub-set of the ~,ulll~uuuds of formula (III) of formula (XIII):
OH
TtNH--CR--CH2~0H
(XIII)
wherein Rl and Rla are as defined in relation to formula (I) and Tl represents aprotecting group, such as a t-bulu~y~,~ulJullyl group, by reaction with a compound of
20 formula (XIV):
LXCO.T
~2 Co.T3 (XIV)
wherein Ll and L2 each represents a leaving group or atom, suitably a halogen atom
such as bromine atom, and T2 and T3 each represents a protecting group; and
25 thereafter if rer~uired removing any protecting group.
Suitably T2 and T3 each represent a Cl 6 alkoxy group, for example an
ethoxy group.
Preferably, the compound of formula (XIII) is in an activated form
A suitable activated form of a compûund of formula (XIII) is an ionic form,
30 such as an alkali metal salted form, for example a potassium salted form.
An activated form of a compound of formula (XIII) may be prepared by use
of the alJIJIul~l iaL~; COII ~, 1 procedure, for example a salted form may be prepared
by treating the compound of formula (XIII) with a base such as an alkali carbonate,
for example potassium carbonate.
2 l 9 6 l 93
w0 96104233 l ~ 0
In the above mentioned reactions the compound of formula (XIII) is usually in
an activated form, such as an anionic form. The activated form is ~;u~ ic.~Lly
preparcd in~i~ prior to addition of the compound of formula (XIV).
The reaction between the .~ ,u~ lc of fommula (xnI) and (XIV) may be
S carried out in an aprotic solvent, such as acetone, at any t~ Lul~ which provides a
suitable rate of formation of the required product but usually at an elevated
t~,."~ ;, such as the reflux ~ Lulc of the solvent, preferably in the presence
of a base such as potassium carbonate and preferably under an inert ~ such
as argon.
The ~,UllllJuulldS of formula (XIII) are known UUI~I~JUUII-15 or they are prepared
according to methods used to prepare known ~.~ ,.. ,l.., ..k, such as those disclosed in J.
Med. Chem. 1973, 16(5), 480.
The '"~ Of formula (XIV) are known cullllll~,lu;ally available
U~ 1. ,llc
Ct~mrolln~1c of formula (III), wherein R2 is OCH2C02H or an ester or amide
thereof or a moiety of the above defined formula (b) and R3 is hydrogen, halogen,
alkyl or alkoxy are ~,u..~ ,.,Lly prepared from a protected form of a sub-set of the
CUIIIIJUUIId~ of formula (111) of formula (XV):
R 1~ _~ O H
~A
whcrein Rl, Rla, R3 and Tl are as defined in relation to formula (XIII):
a) for ~,UIIIIVUUlld:~ of formula (III) wherein R2 is OCH2C02H or an ester or amide
thereof, by reaction with a compound of formula (XVI):
L3-CH2-Co-T4 (XVI)
wherein L3 is a leaving group or atom, suitably a halogen atom such as a bromineatom, and T4 is a protecting group; or
b) for ~,ull~uuuds of formula (Ill) wherein R2 is a moiety of the above defined
30 formula (b), by reaction with a compound of formula (XVII):
o
L4 CH2--P--oR4
Rs (XVII)
wherein R4 and RS are as defined in relation to formula (I) and L4 is a leaving group
35 or atom; and thercafter, as necessary removing any protecting group.
-12-
2l 961 93
~wo9~i/04233 r. "~ 0~7
Suitably, Tl is a t-bu~u~yl albullyl group.
Suitably, T4 is a C1 6 alkoxy group such as a methoxy group.
Suitably, L4 rep}esents a tosylate group, a 4-chlu,uhf .,,. ~f '''Irhr~nylOxy
group or a 3-~ ul,c..,,- ,~ hrl.~yloxy group.
In the above mentioned reactions the compound of formula (XV) is usually in
an activated form, such as an anionic form. The activated form is ~.u.. ~c n;.,~lly
prepared in-situ prio} to addition of the compound of formula (XVI) or (XVII).
Preferably, the activated form of the compound of formula (XV) is prepared
by reaction of the compound of formula (XV) with a base such as sodium hydride.
The reaction between the c ". . ,l~u~ k of formulae (XV) and (XVI) is suitably
carried out in an aprotic solvent, such as acetone, at any u,l~ rllulc which provides a
suitable rate of rc.".",l-l;.." of the recluired product usually an elevated tc~ lu.~i
such as the reflux l~,..-,o~..~Lu.c of the solvent, preferably in the presence of a base such
as potassium carbonate and preferably under an inert a~lllo~ c such as argon.
The reaction between ~ u~ .k of formulae (XV) and (XVII) is carried out
in an aprotic solvent, such as dimethyl[u.l..,lll.;dc or dimeth)~ iAf at any
t~,llllJ~,I~Lulc which provides a suitable rate of reaction, uull~ d~ ly at ambient
t~ Ulc.
The c..ll,.l,u~ of formula (XV) wherein Rl and R1a each represent
20 hydrogen are known c~ u"~llk of are prepared according to methods used to
prepare known CUlll~uulld~, such as those disclosed for such c ~ u~..k when T1 is t-
bU~ y~ )ullyl in Can. J. Chem. 1985, 63,153.
The uullll!uullds of formula (XV) wherein either Rl or Rla is hydrogen are
prepared by hydrogenolysis of a compound of formula (XIX):
~CH-NY--CR~CH2~0H
wherein R1, R3, Y and the **CH and ***CH carbon atoms are as defined in relationto formula (VI).
The hydrogenolysis of colllluuu-ld~ of formula (XIX) is carried out under
analogous conditions to the hydrogenolysis of the CUUIIJUUIU1~ of formula (VI).
The .. ".,l,,.""Ac of formula (XIX) wherein Y is a moiety -B(OH)2 are
prepared from ~...I.l~lullllAc of formula (XIX) wherein Y is the H, using analogous
methods to those described above for co~l,u,."-lc of formula (VI) wherein Y is a35 moiety-B(OH)2
-13-
21 961 ~3
wo 96/04233 . ~ ,s/r~
The cnmro~n~i~ of formula (XIX) wherein Y is H are known cullluuuuds or
they are prepared using analogous methods to those used to prepare known
..~,,,I,,,...,Ik for example those disclosed in J~ Med. Chem. 1973, 16(5), 480.
A compound of formula (XVII) may be prepared by l-y~Lu~ylll~,.hyl.l60n of a
5 compound of formula (XX):
101
Rs (XX)
wherein R4 and R5 are as defined in relation to the c ~ u~ l c of formula (1), to
provide a compound of the above defined formula (Xll); and thereafter reacting the
10 compound so formed with a source of leaving group L4.
The Lydlu~yll~ hylation is carried out using formaldehyde, generally in the
form of ~ ldcl.y.l~, using ~ ull~ iullal procedures depending upon the exact nature
of the substrate, such as those disclosed by Houben-Weyl in Phosphor Verbinungenp28, J. Amer. Chem.-Soc. 1955, 77, 3522, PLO~I~LUIUS and Sulphur 1978, .~, 455 or in
Aust. J. Chem. 1979, 32, 463.
The conditions of reaction of the hy(llu~y~ klL~d compound of formula
(XII) with the source of the leaving group will depend upon the nature of the leaving
group L4 bul the a~},.ul conventional conditions are employed. For example
when L4 represents a 4-chlu,. ,i,... ,. n. ~ .lrh~7nyloxy group the literature method of J.
Cornforth et al (J.C S. Perkin 1, 1994, 1897) may be employed.
A compound of formula (1), wherein Rla represents hydrogen, or a
pl.~ y acceptable salt7 ester or amide thereof or a l)~ lly
acceptable solvate thereof, may also be prepared by reducing a compound of formula
XXI):
OH R' R~
R~-X-CH2-CH-CH2-N=C-CH2 ~R2~
(XXI)
wherein R~, Rl, R3 and X are as defined in relation to formula (I) and R2 represents
R2 as defined in relation to formula (1) or a protected form thereof; and thereafter, if
necessary, carrying out one or more of the following optional steps:
(i) converting one compound of formula (I) to another compour~d of formula (I);
(ii) removing any protecting group; and
(iii) preparingal~ ".,~ e.,~ llyacceptablesalt,esteroramidethereofofa
compound of formula (I) or a l.l,~u 1,,~. ..,li ~lly acceptable solvate thereof.
-14-
21 q6l q3
wo g6/04233 r~
The reduction of the compound of formula (XXI) may be carried out using
any suitable reduction procedure, for example by using catalytic reduction.
Suitable catalysts include platinum oxide or 10% palladium on charcoal.
Suitable reduction conditions include using an alkanolic solvent such as
S methanol, at any t~ ,Ia~ul~ providing a convenient rate of formation of the required
product, for example when using the platinum catalyst the reaction may u~ Lly
be carried out at ambient t~ r, or when using the palladium catalyst the
reaction may be carried out at a medium t~,llllJ~,.~.Lul~ such as 50~C, under a pressure
of 1-5 .ILl~.u~JL~ of hydrogen.
For C ~ of formula (I) wherein R2 represents a moiety of the above
defined formula (b), R2 generally represents a protected form of R2, for example a
benzylated form, which may be removed by use of any conventional method, thus the
benzylated form may be removed by use of hyl~ lfJlysis using,, . " ". " ,; " ", formate
in the presence of a 10% palladium on carbon catalyst.
The compound of formula (XXI) may be prepared by reacting a compound of
formula (XXII):
OH
R~ -X-CH2-CH-CH2-NH2
(XXII)
wherein R~ and X are as defined in relation to formula (II) with a compound of the
20 above defined formula (VIII).
The reaction between (.. " . .~ c of formulae (VlIl) and (XXII) may be
carried out under conventional amination conditions, for example in a solvent such as
toluene or, preferably, methanol.
Cuu~,ui~,uLly, the compound of formula (XXI) is prepared ~by reacting
25 f..,."ll~"",.k of the above defined formulae (VIII) and (XXII) under reductive
amination conditions which includes reaction in an alkanolic solvent, such as
methanol, in the presence of a suitable reduction catalyst. for example those described
above for the reduction of the compound of formula (XXI).
In a further aspect of the present invention there is provided a process for
30 preparing a compound of formula (1) or a l~l ,,., ... -~, .. ;~.~lly acceptable salt thereof, or
a ~ f . ,.; ;. .,~lly acceptable solvate thereof, which process comprises reacting a
compound of formula (XXIII):
OH Ts R1~ R~
R~--X--CH2 CH-CH2N-lR' CI~R2~
(XXIII)
-15-
wo 96/04233 2 1 9 ~ ~ ~ 3 . ~
wherein Rl, Rla and X are as defined in relation to formula (I), R~ is as defined in
relation to formula (II), T5 is a protecting group, R2a represent R2 or a group or atom
convertible into R2 and R3a represents R3 or a group or atom convertible into R3,
wherein R2 and R3 are each as defned in relation to formula (1), with a reagent
5 capable of converting R2a into R2 and/or a reagent capable of converting R3a into
R3; and thereafter, if required, carrying out one or more of the following optional
steps:
(i) converting a compound of formula (I) to a further compound of formula (I);
(ii) removing any protecting group;
10 (iii) preparing a p~ 1Y acceptable salt of the compound of formula (I)
and/or a l~h ~ y acceptable solvate thereof.
Suitably, when R3 in the required compound of formula (1) is hydrogen,
halogen, alkyl or alkoxy R3a is R3.
Suitably, when R3 together with R2 in the required compound of forrnula (1)
15 represents a moiety of the above defined formula (c), or an ester or amide thereof,
then R2a and R3a each represent OH.
Suitably, when R2a and R3a each represent OH they may be converted into a
moiety of formula (c) by treating the compound of formula (XXIII) with a compound
of the above defined formula (XIV) and thereafter as required forming an ester or
20 arnide of the resulting compound of formula (1).
The reaction conditions for the reaction between .~ u~ 1, of formulae
(XXm) and (XIV) are analogous to those for the reaction between UUIII~)UUlld~ offormulae (xm) and (XIV).
When R2 in the required compound of formula (1) represents OCH2CO2H or
25 an ester or amide thereof, then R2a is suitably an OH group.
When R2a is OH, then a compound of formula (I) wherein R2 represents
OCH2CO2H or an ester or amide thereof, may be prepared by reacting a compound
of formula (XXIII) with a compound. of the above defined formula (XVI).
The reaction conditions for the reaction between the compounds of fortnulae
30 (XXIII) and (XVI) are analogous to those for the reaction between the compounds of
forrnulae (XV) and (XVI).
When R2 in the required compound of formula (1) represents a moiety of the
above mentioned formula (b), then R2a is suitably an OH group.
When R2 is OH, then a compound of formula (I) wherein R2 represents a
35 moiety of formula (b) may be prepared by reacting a compound of formula (XXIII)
with a compound of the above defined formula (XVII).
The reaction conditions for the reaction between the cnmpolln~l~ of formulae
(XXIII) and (XVII) are analogous to those for the reaction between the compounds of
formulae (XV) and (XVII).
-16-
21 q61 93
WO 96/04233 P~, J l~; ,.,/ ~7
The ..~ lull,~,k of formula (XXII) are known compounds or they may be
prepared according to methods used to prepare known ~ . for example those
methods disclosed in Swiss Patent number 1549945 (1976).
The ~ ,. " " ,~k of formula (XXIII) are prepared according to uu.~ Liullal
5 procedures depending upon the value of R2a and R3a. For example, when R2a and
R3a each represents OH or when R2a is OH and R3a is hydrogen, halogen, alkyl or
alkoxy then they may be prepared by reaction of a compound of above defined
formula ~I) with a compound of above defined formula (XIII) or (XV) as a~ ul)lia~
using conditions analogous to those used in the reaction between ...,.,I.u,,,,.l~ of
10 formulae (II) and (m).
~ lmroun~lc of formula (III) including those of formula (XIII) or (XV)
wherein Rl and R1a each; 1~ ~,...,.1~ ..~ly represent alkyl are known ~. " ,.llu~"l~ or
they may be prepared according to processes used to prepare known ~ l-,-ll,u 1~ such
as those disclosed by B. Renger in Arch. Pharm. (Weinheim)., 1983, 316(3), 193-201.
The c ~ ,,, Il .u~ " "l~ of formula (IV) are either known commercially availablecullllJvu,,d~ or they are prepared according to published methods or by use of
analogous methods to the published methods, for example those disclosed J.C.S.
Perkin 1; 1974, 1353.
Thec~...,l.."".rlcofformula(V)areknowncommerciallyavailable
20 ~...,.,I.v ~
The cnmronnl1c of formula (Xll) are known u"~ u~ c or they may be
preparcd by proccsses analogous to those used to prepare known cu,,ll,uu..d~, for
example the c~mrvllnflc of formula (XII) may be preparcd according to methods
disclosed in Phu~hv~u~ and Sulphur, 1978, ~, 455.
Suitable uu--~ iUII~ of one compound of formula (I) into another compound
of formula (I) include converting one group oR4 into another group oR4 and/or
converting one group R5 into another group R5; or when R2 is OCH2CO2H or an
ester or amide thereof, converting one R2 into another R2; or when R3 together with
R2 represents a moiety of the above defined formula (a) or an ester or amide thereof,
by converting one (a) into another (a).
Suitable conversions of one group oR4 into another group oR4 include:
(i) converting oR4 as hydroxy into oR4 as alkoxy;
(ii) converting oR4 as alkoxy into oR4 as hydroxy;
(iii) converting oR4 as alkoxy into oR4 as another alkoxy group.
~ 35 The abov~m~n~ir n~1 conversion (i) may be carried out under cull~ iùllal
I ' I ' alkylation methods, using for example the alJIJI U~JI ' alcohol (R40H)
in the presence of hydrogen chloride, altematively, the appropriate alcohol may be
usedwith b~ uL,ia~Jlc-l-yloxy-tris-(dimethylamino)l~
L~Aarluvlupllu~ ~ in dil~ ylrullllalll;d~ in the presence of dii~ulJIu~yll,illylaullill~,.
-17-
wo 96/04233 2 t q 6 1 9 3 . ~ll~l ,~ n~o~7
The abov~ , ,. d conversion (ii) may be carried out using cv,l v ~,."iv,.al
c hydrolysis methods, for example by treating the appropriate compound
of formula (I) with an alkaline metal hydroxide, such as sodium hydroxide.
The abu~..,,,...l;ollrd conversion (iii) may be carried out by first converting
5 oR4 as alkoxy into oR4 as hydroxy using the conditions set out in respect of the
abov~ 1 conversion (ii), followed by converting the hydroxy group so formed
into another alkoxy group, using the conditions set out in respect of the
abu.. ' conversion (i).
The abu. ~.., ,. "~ ....1 conversion (iii) is of particular use for prepating
s - , _ ' of formula (1) wherein oR4 represents methoxy: such UULII~JVUlld~t aregenerally prepared from ( nmponnfl~ of formula (I) wherein oR4 represents an
alkyloxy group other than methoxy (suitably ethoxy) by first hydrolysing the relevant
oR4 group (via converSion (ii)) to prepare a compound of formula (I) wherein oR4represents hydroxy and thereafter methylating (via conversion (i~) to provide the
required compound wherein oR4 represents methoxy.
Suitable CUll~ iVUS of one group R5 into another group R5, when R5
reprcsents hydroxy, alkoxy, arylalkyloxy, hydroxyalkyloxy, alkoxyalkyloxy,
arylalkoxyalkyloxy or cycloalkyloxy, include analogous CVll~ iVlls to those
mentioned above in regard to converting one group oR4 into another group oR4.
When R2 is OCH2CO2H or an ester or amide thereof, suitable cv"~ ;v"~ of
one R2 into another R2 include converting OCH2CO2Re wherein CO2Re is an ester,
irlto OCH2CO2H, usually by conventional carboxylic acid hydrolysis, using for
example basic hydrolysis with sodium hydroxide in an aprotic solvent such as 1,4-
dioxan, at room ~ Jcla~ulc and preferably in an inert aullO~ ,.c such as argon.
Other suitable cv"~ ;v"s include hltcll,vl~ illg the respective acids, esters and
amides, such conversions being ~rcnmpli~he~l by the a~y~lu~Jliatc conventional
procedure including those described herein.
When R3 together with R2 represents a moiety of the above defined forrnula
(a) or an ester or amide thereof suitable cuu~ iuns of one (a) into another (a)
include hydrolysing esters to acids using an appropriate conventional procedure, such
as treating the ester with lithium hydroxide in dioxan or methanol at ambient
Lclll~,la~ulc, preferably in an inert a~ulv~ ,lc such as argon. Other suitable
Cull~ ivlls include ill~Cl~,Ull~ illg the respective acids, esters and amides using an
appropriate cv"~,u~iv"al procedure including those described herein.
The protection of any reactive group or atom, may be carried out at any
appropriate stage in the arw~ ;u,.~ d proCesSeS. Suitable protecting groups include
those used conventionally in the art for the particular group or atom being protected.
Protecting groups may be prepared and removed using the a~ v~lk.:~ cvll~llLivllal
procedure, for example OH groups, including diols, may be protected as the silylated
-18-
.. ... . ... . . . . .... . . _ . .. . _ . . . _ .. .... .. _ , . .. .. .. .
~Wo 96/04233 2 1 9 6 1 9 3 ~l/r~ _/0~0~7
derivatives by Lreatment with an appropiate silylating agent such as di-tert-
butylsilylbis(L~ifuu~ " ,.,lfonate): The silyl group may then be removed using
cu~ ,..liuual procedures such as Lreatment with hydrogen fluoride, preferably in Lhe
form of a pyridine complex. AlternaLively benzyloxy groups may be used to protect
5 phenoxy groups, the benzyloxy group may be removed using catalytic hydrogenolysis
using such catalysts as palladium (II) chloride or 10% palladium on carbon.
Amino groups may be protected using any ccn,~ iullal protecting group, for
example ~-butyl esters of carbamic acid may be formed by LreaLing the amino group
wiLh di-~-bu~ldi~.~ubulld~:, the amino group being ~ tcd by hydrolysing the
10 ester under acidic condiLions, using for example hydrogen chloride in ethyl acetate or
L-inuuluac~,~ic acid in methylene dichloride. The amino group also may be protected
as an ~ hlobuloll;c ~id, prepared from the a~,lu~ , amine and boron nribromide
followed by work up with iced water. The Au..hlobulul.i-, acid may be removcd using
catalyLic hydrogenolysis, using for example a palladium on carbon catalyst. In
15 addition, an amino group may be protected as a benzyl derivative, prepared from the
appropriate amine and a benzyl halide under basic conditions, the benzyl group being
removed by catalyLic hydrogenolysis, using for example a palladium on carbon catalysL
A leaving group or atom is any group or atom that will, under the reacLion
conditions, cleave from the starting material, thus promoting reacLion at a specified
20 site. Suitable examples of such groups unless otherwise specified are halogen atoms,
mesyloxy groups and tosyloxy groups.
The salts, esters, amides and solvates of the .. ," .I,u~ mentioned herein may
be produced by methods coll~ iulldl in the art: For example, acid addition saltsmay be prepared by Lreating a compound of formula (I) with the ~ lu~liat~ acid.
Estersofcarboxylicacidsmaybepreparedby~;Ull~ ioual ~I rl;l;~ AI;~
procedures, for example alkyl esters may be prepared by treating the required
carboxylic acid with the _~ u,ul;_Lc alkanol, generally under acidic conditions.Amides may be preparcd using cull~cl-lional amidation procedures, for
example amides of formula CONRsRt may be prepared by treating the relevant
carboxylic acid wiLh an amine of formula HNRsRt~ wherein Rs and Rt are as defined
above. Alternatively, a Cl 6 alkyl ester such as a methyl ester of the acid may be
treated with an amine of the above defined formula HNRsRt to provide the required
amide.
('nmpm-nrl~ of formula (I) and P~ IY accepLable acid addition salts
~ 35 Lhereof; or a ~ 111;1 A1IY acceptable solvate thereof, produced by the above
processes, may be recovered by conventional methods.
If required mixtures of isomers of the compounds of the invention may be
separated into individual at~ v;aulll~,la and di~la~lcùisulll~la by ~,uu~a,~ltiul~al means,
for example by the use of an optically active acid as a resolving agent. Suitable
_19_
w096/04233 21 q ~ l 93 r~ 3~7
optically active acids which maybe used as resolving agents are described in ~opics
in S.a,lcul h~ y', Vol. 6, Wiley T~ ce, 1971, Allinger, N.L. and Eliel, W.L.
Eds.
Alternatively, any enantiomer of a compound of the invention may be
5 obtained by ~,t.,lcoa~e.,irlc synthesis using optically pure starting materials of known
c..".''i~,,..~l;....
The absolute cnnfiellrPtif~n of uv~ Juu~lds may be determined by cull~ iondl
X-ray cryct~llf grPrhin techniques.
As previously indicated, the c. ~ U~ fl~ of formula (I) have been discovered
10 to possess valuable rh ~ rl~icdl properties.
The present invention accordingly provides a compound of formula (I) or a
C I~ y acceptable acid addition salt thereof, or a ~,l,,.. " ~ ~ .,l;, ~lly
acceptable solvate thereof, for use as an active therapeutic substance.
In one aspect, the present invention provides a compound of formula (I), or a
5 rl,~, - - C ~ y acceptable acid addition salt thereof, or a ph- ", - .~ lly
acceptable solvate thereof, for use in the treatment of hyp~ ly~a~,l..;d in human or
non-human animals.
The present invention further provides a compound of formula (1), or
pllfU - -- ~ t;~ ~lly acceptable acid addition salt thereof, or a pl ~ -lly
20 acceptable solvate thereof, for use in the treatment of obesity in human or non-human
animals.
In addition the present invention provides a compound of formula (1), or
~hl" l l l - ~ ;, Ally acceptable acid addition salt thereof, or a ph~ ld~ ,allyacceptable solvate thereof, for use in the treatment of ~fl~lluhl.~,~lilldl disorders such
2~i as peptic ulceration, orQorhpgitic~ gastritis and duodenitis, intestinal ulcerations,
including i. . .'~ ., y bowel disease~ and irritable bowel syndrome and also for the
treatment of ~a~lluhla,~lh~al nlrf~rPtif nc especially when induccd by non-steroidal
anti- rl y drugs or uu. Iiuu~lwu;J~.
Finally, the present invention provides a compound ûf formula (1), or
30 l~h-, ~ .n ;. ~lly acceptable acid addition salt thcreof, or a l~ llyacceptable solvate thereof, for use in increasing the high-density-lipoprotein (HDL)
cholesterol.,...-~....n~l;..,~anddecreasingthetriglyceridec...."..,.l,..li.,,.inhuman
blood serum, in particular in the treatment andlor J..~,~l,.,laAi~ of atherosclerosis, and
in the treatment of hyll. . ;,.~.,l;..~- ..,;~ or depression.
A compound of formula ~1), or a pl - . .,~. ~ .,l;. ,,lly acceptable acid addition
salt thereof, or a pl.,ll . " - . ., l i. ,.lly acceptable solvate thereof, may be aJ...;..i~lc.cd
per se or, preferably, as a l~l, - ", - . . l l ;, ~l cf~mrf~citif~n also comprising a
- c...;, ~lly acceptable carrier.
-~0-
21 961 q3
WO 96/~4233 p~ llrl 5/ï ~n~7
Accordingly~ the present invention also provides a l~h~
cnmrrJcitir~n comprislng a compound of formula (I)~ or a ~ y acceptable
acid addition salt thereof, or a pl,O ~ lly acceptable solvate thereof, and a
pl ," . " . ~ lly acceptable carrier therefor.
S As used herein the term "~ " "~ lly acceptable" embraces cu~ ,uul-ds,
and ingrcdients for both human and veterinary use: for example the
term "1,l ~ ;. ,,lly acceptable salt" embraces a veterinarily acceptable salt.
The ,. .. - .~ ;-," may, if desired, be in the form of a pack anc~ by
written or printcd hl~LI U~.~iUlls for use.
Usuallythel.l.--.. ~. . .n;~l c~.. ,.l.. ~;~;.-ncofthepresertinvention willbe
adapted for oral ~lminictr~ nn~ although c~ n~ for al~ iull by other
routes, such as by injection, are also envisaged.
P~u~icukuly suitable ,~.. ,l.. ,~:li.. ,.~ fororal ;Idlllillia~ iOn are unit dosage
forms such as tablets and capsules. Other fixed unit dosage forms, such as powders
15 presented in sachets, may also be uscd.
In ~cr,r~nrc- with conventional pl.~",,~ ~..I;r~l practice the carrier may
comprise a diluent, filler, f' ,, ~, wetting agent, lubricant, colourant, flavourant
or other cul.v~,..tiullal adjuvant.
Typical carriers include, for example, l~iwu~,ly~dlline cellulose, starch,
20 sodium starch glycollate, pol~vi,lyl,uyl.ulidul,c, polyvinylpolypyrrolidone,
~IAgn. ~ stcarate or sodium lauryl sulphate.
Most suitably the Cuul~)u~iLiull will be formulated in unit dose form. Such unitdose will normally contain an amount of the active ingredient in the range of from 0.1
to 1000 mg, more usually 2-100 mg or 0.1 to 500 mg, and more especially 0.1 to 250
mg.
The present invention further provides a method for treating hy,u,,. ~Iy1a~.lll;à
in a human or non-human mammal, which comprises A~ h ~ h ~g an effective,
non-toxic,amountofacompoundofformula(l),oraplla.,..~ lyacceptable
acidadditionsaltthereof,oral-h,.,l-,~.";~llyacceptablesolvatethereof,toa
II~ IY~U~ ;C human or non-human mammal in need thereof.
The present invention further provides a method for treating obesity or for the
treatment and/or prophylaxis of atherosclerosis in a human or non-human mammal,
- which comprises a~ h~g an effective, non-toxic, amount of a compound of
formula (I), or a phd~ ir~lly acceptable acid addition salt thereof, or a
l~n~ "l;r~lly acceptable solvate thereof, to a human or non-human mammal in
need thereof.
The present invention further provides a method for treating ga~tlu;ll~c~
disorders such as peptic ulceration, o~crrh~gi~ic~ gastritis and duodenitis, intestinal
nlrerltinnc including h,n~ y bowel disease, and irritable bowel syndrome and
-21-
21 961 93
wo 96/04233 ~ 0~7
also for the treatment of ~a ~lluilllcalhlal ulcerations, especially when induced by non-
steroidal anti-i ~- n~ 1 y drugs or ~:UI liCU~IClUills, in a human or non-human
mammal, which comprises :~.l,-,;.,i~.. ;"g an effective, non-toxic, amount of a
compound of formula (1), or a 1~ lly acceptable acid addition salt thereof,
S or a 1~ lly acceptable solvate thereof, to a human or non-human mammal in need thereof.
In addition the present invention provides a method for treating for increasing
the high-density-lipoprotein (HDL) cholesterol rnnm~n~r~rion and decreasing the
ly~ ide . . . u n ;n. . in human blood serum, in particular in the treatment and/or
lû ~JIulJhyla~ i~ of aLlL,~u~,L,~Iai~, and in the treatment of Ly~ . " ordepression, in a human or non-human mammal, which comprises ~.1,..;,.;~.... ;,.g an
effective, non-toxic, amount of a compound of formula (1), or a pLal 1 l l ~ U ~ ,.lly
acceptable acid addition salt thereof, or a 1~ ;;l A11Y acceptable solvate thereof,
to a human or non-human mammal in need thereof.
15The present invention also provides the use of a compound of formula (1), or a
,uhallll-~11;.Allyacceptableacidadditionsaltthereof~orapl~ u;~lly
acceptable solvate thereof, for the ,llallural,iul c of a ., . ~ for the treatment of:
Ly~J.,.~;lyca~"ll;d, obesity, Lai~LIu;llt~ illdl disorders such as peptic ulceration,
u., ,~ gastritis and duodenitis, intestinal nlrer~ltinn~, including ;"n ~"~"~ y
20 bowel disease, and irtitable bowel syndrome and also for the treatment of
~a~LIv;.lt~ Lhldl nlm~rsltinn~, especially when induced by non-steroidal anti-
'' y drugs or cu~ ,u~Lcluhl~, for increasing the high-density-lipoprotein
(HDL) cholesterol, .."~. .U,,,Ii~a and decreasing the triglyceride culll,~,.lllaLiull in
human blood serum, in particular in the treatment andlor ~JIupllyld~i~ of
25 d~L~,~U~,L,~U:~;s~ and in the treatment of hyperin~ n ~ mi~ or depression.
Cull~ ,...ly, the active ingredient may be adllli,li~.clcd as a 1~
l;l"' IICIC;III~ UIC defined, and this forms a particular aspect of the present
invention.
In treating hy~ ;ly~,a~,lll;~, or obese humans the compound of formula (I), or apllal l ~lly acceptable salt, ester or amide thereof; or a ~ ly
acceptable solvate thereof, may be taken in doses, such as those described above, one
to six times a day in a manner such that the total daily dose for a 70 kg adult will
generally be in the range of from 0.1 to 60no mg, and more usually about I to 1500
mg.
The treatment regimens for treating the abov~ o ~ed ~a~lluillt~,~til~al
disorders ~lh~.lu~cl~,luais~ hyp~ . ;,.~,.1;.,-. .. Il;A and depression are generally as described
for hyperglycaemia.
-22-
21 96l q3
wo 96/04233 J ~ .r75/~ 7
In treating non-human mammals, especially dogs, the active ingredient may
be ad~ Lclcd by mouth, usually once or twice a day and in an amount in the rangeof from about 0.025 mg/kg to 25 mg/kg, for example 0.1 mg/kg to 20 mg/lcg.
In a further aspect the present invention also provides a method for increasing
5 weight gain andlor improving the feed utilisation efficiency and/or increasing lean
body mass andlor decreasing birth mortality rate and increasing post/natal survival
rate; of livestock, which method comprises the ~ u, l ;. ", to livestock of an
effective non-toxic amount of a compound of formula (I) or a veterinarily acceptable
acid addition salt thereof, or a veterinarily acceptable solvate thereof.
Whilst the ~,UIIIIJuulldS of formula (I) and the veterinarily acceptable acid
addition salts thereof or a veterinarily acceptable solvate thereof, may be A~ , cd
to any livestock in the abu~ ,.. u;~ d method, they are ~JalLiculally suitable for
increasing weight gain and/or feed utilisation efficiency andlor lean body mass andlor
decreasing birth mortality rate and increasing post-natal survival rate; in poultry,
15 especially turkeys and chickens, cattle, pigs and sheep.
In the preceding method the ~:ullltJuul~ds of formula (I) or veterinarily
acceptable acid addition salts thereof will normally be alllllh~i~t~,lcd orally although
non-oralmodesofA,ll.,;ll;~l,,lli.".,forexampleinjectionorimrlAn~qti-~n,arealso
envisaged. Suitably the CulllJJuulldS are administered in the feed-stuff or drinking
20 water provided for the livestock. CU~ ,.ILIY these are adl~ c,cd in the
feed-stuff at from lû-3 ppm - 500ppm of total daily fed intake, more usually 0.01ppm
to 250ppm, suitably less than 100ppm.
The particular fr~rm~ ticmc used will of course depend upon the mode of
adlllill; ~LaLiull but will be those used cu~l~cllLiullally in the mode of A.~
25 chosen. For ad"li..i~L,aLion in feed-stuff the drugs are conveniently formulated as a
premix in association with a suitable carrier.
Accordingly, the present invention also provides a veterinarily acceptable
premix formulation comprising a compound of formula (I), or a veterinarily
acceptable acid addition salt thereof; or a veterinarily acceptable solvate thereof, in
30 association with a veterinarily acceptable carrier therefore.
Suitable carriers are inert conventional agents such as powdered starch. Other
conventional feed-stuff premix carriers may also be employed.
No ~ qrceprqhle trl~icr~lflgi~Al effects are expected when r..,..llu."..1~ of the
invention are adll,i.,;~lt;lcd in accordance with the present invention.
The following Examples and Procedures illustrate the invention but do not
limit it in any way.
-23-
~=
wo 96/04233 2 1 9 6 1 9 3 P~ ~ n~7 ~
Procedure 1: (S)-Glycidyl-2-l~...L.ylu~p'T
0~~
BnO~ O
S A mixture of 2-1,. Il~yluAy~ ,nol (9OOmg, 4.5 mMol) and potassium carbonate
(1.87g, 135 mMol) in acetone (45 ml) was heated under reflux for 15 mins. (S)-
Glycidyl-3-...1".1l..,,..,. ~ r (l.Og, 4.5 mMol) was added and the reactionmixture was heated under reflux for 23 hours. After cooling, the reaction mixture was
filtered and the solvent was Cva~ The residue was partitioned between edhyl
10 acetate and water. The organic fractions were combined, washed with water and brine, dried and evaporated to give the tide compound as an oil.
~lH (270MHz, CDC13~: 7.36 (5H, m), 6.88 (4H, m), 5.10 (2H, s), 4.26 (lH, dd.
J=11.4, 3.3Hz), 4.20 (lH, dd, J=11.4, 5.5Hz), 3.36 (lH, m), 2.85 (lH, dd, J=5.0, 4.1Hz), and 2.73 (lH, dd, J=5.0, 2.5Hz) ppm.
Procedure2- (S,R)-Methyl-4-[2-[2-hydroxy-3-(2-
,lu~ ' y)propylarnino]propyl]r
Me
H
BnO~ OH
O~,CO2Me
A mixture of (S)-glycidyl-2-benzyloxyphenol (666mg, 2.59 mMol) and (R)-methyl-
4-(2-dlldllu~lu,uyl)~)h~,llu~yac~,Ld~e (SOlmg, 2.25 mMol) in MeOH (lSml) was heated
25 under reflux under argon for 24 hours. After cooling, the solvent was evaporated and
the residue was partitioned between dichl,,.~""~lLa,le and water. The organic layer
was washed with water and brine, dried and evaporated. The residue was purified by
column ~,L~ y eluting with 0-10% methanol in di-,lllolu,,,~,il,alle giving the
tide compound as an oil.
-24-
, ... . ... ..... . . ... . .
21 96l 93
WO 96/04_33 PCT/EP95/03037
olH (270MHz, CDC13): 7.5-7.25 (5H, m), 7.05 (2H, d, J=8.6Hz), 6.92 (4H, m),
6.79 (2H, d, J=8.6Hz), 5.08 (2H7 s), 4.~9 (2H, s), 4.2-4.0 (3H, m), 3.80 (3H, s), 3.0-
2.4 (SH, m) and 1.04 (3H, d, J=6.3Hz) ppm.
Procedure 3: (S,R)-Methyl-4-[2-[2-hydroxy-3-(2-
I.,~d,UAYI' y),u.u~ ]propyl]
Me
O--NH~
HO ~ OH
O~CO2Me
(s~R)-Methyl-4-[2-[2-hydroxy-3-(2-benzylu~ylu~ uAy)lulu~ylal~ ]propyl]
phvllu~y~ dLv (270mg, 0.56mMol) was dissolved in methanol (40ml), palladium on
charcoal (5~o, 40mg) was added and the mixture was hyvllu~ vd at room
LCIIIIUVI_~J.-V and pressure for 18 hours. The suspension was filtered through a pad of
filter aid, the filter pad was washed with methanol and the combined filtrates were
evaporated giving a dark residue. Purification by column vhl. ." .A~ ;, Apl Iy eluting
with 0-10 % methanol in dichlulu.l.viha.lc gave the title compound as an oil.
olH (270MHz, CDC13): 7.08 (2H, d, J=8.8Hz), 6.79 (6H, m), 4.61 (2H, s), 4.1-
3.9 (3H, m), 3.BO (3H, s), 3.0-2.7 (5H, m) and 1.12 (3H, d, J6.1Hz) ppm.
Procedure 4: (S)-Glycidyl-3 t_..LylUAyl '~
0~~ .
"~3 ~
BnO
A rnixture of 3-benzyloxyphenol (9OOmg, 4.5 mMol) and potassium carbonate
(1.87g, 13.5 mMol) in acetone (45 ml) was heated under reflux for 15 mins. (S)-
Glycidyl-3-,.,nulv~ .,,. .,~ ,.,lphon~îl- (l.Og, 4.5 mMol) was added and the reaction
mixture was heated under reflux for 23 hours. After cooling the reaction mixture was
30 filtered and the solvent was evaporated. The residue was partitioned between ethyl
-25 -
2l 96~ 93
W0 96/04233 r~ 7 . .
acetate and water. The organic fractions were combined, washed with water and
brine, dried and evaporated to give the title compound as an oil.
olH (270M Hz,CD C13~ 7.25 (SH, m), 7.15 (lH, m~, 6.50 (3H, m), 5.14 (2H, s),
4.10 (IH, dd, J=11.0, 33~1z), 3.80 (IH, dd, J=11.0, 5.8Hz), 3.40 (lH, m), 2.80
(lH,dd, J=5.0, 4.1Hz) and 2.70 (IH, dd, J=5.0, 2.5Hz) ppm.
Procedure5: (S,R)-Methyl-4-[2-[2-hydroxy-3-(3-
.ylw~ ' y)propylarnino]propyl]
10 1~
Me
O--N~
3nO ~
0~ CO2Me
A mixture of (S)-glycidyl-3-benzyloxyphenol (580mg, 2.27 mMol) and (R)-methyl-
4-(2~ hlu~lu~yl)phenoxyacetate (640mg, 2.87 mMol) in MeOH (ISml) was heated
under reflux under argon for 24 hours. After cooling, the solvent was evaporated and
the residue was partitioned between dichlu.u.ll~ih.lne and water. The organic layer
was washed with water and brine, dried and evaporated. The residue was purified by
column ~,Ll, , ' y eluting with 0-20% methanol in dichlulu.~ , giving the
20 title compound as an oil.
~IH (270M}lz, CD C13): 7.5-7.08 (8H, m), 6.83 (2H, d, J=8.5Hz), 6.7-6.5 (3H,
m), 5.03 (2H, s), 4.60 ~2H, s), 3.90 (3H, m), 3.80 (3H, s), 2.9-2.5 (5H, m) and 1.06
(3H, d, J=6.3Hz) ppm.
Procedure6: (S,R)-Methyl-4-[2-[2-hydroxy-3-(3-
il~ d~ o.~y 1 ' y)propylamino]propyl]
r~ ..t~l~
-26-
21 96~ ~3
W O 96/04Z33 P(~rAEP9S/03037
M e
O~\ H
HO~
0~ CO2Me
(S,R)-Methyl-4-[2-[2-hydroxy-3-(3-benzylu.cy~ ,..u~y)propylamino]propyl]
phenoxyacetate (540mg, 1.13 mMol) was dissolved in methanol (50ml), palladium oncharcoal (5%, 75mg) was added and the mixture was hydrogenated at room
t~ ,.atul~i and pressure for 24 hours. The suspension was filtered through a pad of
filter aid, the filter pad was washed with methanol and the combined filtrates were
evaporated giving a dark residue. Purification by column chr ~m~t--gr~phy eluting
with 0-20 % methanol in dichlu,u.ll~l.dne gave the title compound as an oil.
olH (270MHz, d6-DMSO/D20): 7.2-7.0 (3H, m), 6.79 (2H, d, J=8.8Hz), 6.4-6.3
(3H, m), 4.73 (2H, s), 3.95-3.75 (3H, m), 3.69 (3H, s), 2.9-2.6 (4H, m), 2.45-2.35
(lH, m) and 0.92 (3H, d, J=6.0Hz) ppm.
Procedure7: (S)-Glycidyl 1 t ,~
o~o
BnO
A mixture of 4-b~ ylu~y~llenol (2.0g, 10 mMol) and potassium carbonate (4.14g, 30
mMol) in acetone (50 ml) was heated under reflux for 15 mins. (S)-Glycidyl-3-
Il;LIUb' ~ h~ . (2.23g, 10 mMol) was added and the reaction mixture was
heated under reflux for 18 hours. After cooling, the reaction mixture was filtered and
the solvent was cva~ ' The residue was partitioned between ethyl acetate and
water. The organic fractions were combined, washed with water and brine, dried and
evaporated to give the title compound as an oil.
-27-
21 ~61 93
WO 96/04233 P~
~lH (270MHz, CDC13): 7.35 (SH, m), 6.87 (4H, m), 5.01 (2H, s), 4.16 (IH, dd,
J=11.0, 3.3Hz), 3.91 (IH, dd, J=l l .0, 5.8Hz), 3.34 (lH, m), 2.89 (IH, dd, J=5.0,
4.1Hz) and 2.74 (IH, dd, J=5.0, 2.8Hz) ppm.
S Procedure 8: (S,R)-Methyl-4-[2-[2-hydroxy-3-(4-
~lu~ )t .u~ ~]propyl]
a~le
Me
O--~N~
- H
OH [~ ~
BnO O~CO2Me
A mixture of (S)-glycidyl-4-l,. ll~ylu~ypllcllol (330mg, 1.29 mMol) and (R)-methyl-
4-(2-alllhlulJlu~lyl)ph~,nu~y . (380mg, 1.47 mMol) in MeOH (lSml) was heated
under reflux under argon for 24 hours. After cooling, the solvent was evaporated and
the residue was partitioned between dichlululll~,dlalle and water. The organic layer
15 was washed with water and brine, dried and l,va~ The residue was purified by
column .,1....,., ~ y eluting with 0-15% methanol in diulllululll~,llla~le giving the
title compound as an oil.
~lH (270MHz, CDC13): 7.26(5H, m), 7.08 (2H, m), 6.8û (6H, m), 5.01 (2H, s),
20 4.61 (2H, s), 3.90 (3H, m), 3.80 (3H,s), 2 75 (5H, m) and 1.08 (3H, d, J=6.3Hz) ppm.
Procedureg: (S,R)-Methyl-4-[2-[2-hydroxy-3-(4-
~ . w-~t ,~ u~.y' ~ -]propyl]
r ~a~ le
Me
O--NH~
[~3 OH
HO O~CO2Me
~28-
~ Wo 96/04233 2 1 9 6 1 9 3 ~ n~7
(S,R)-Methyl-4-[2-[2-hydroxy-3-(4-benzylw~y~l,c.luAy)propylamino]propyl]
IJh~ w~a~,~,LdL~ (200mg, 0.42mMol) was dissolved in methanol (25ml), palladium on
charcoal (5~o, 20mg) was added and the mixture was hydluO ' at room
a~ and pressure for 18 hours. The suspension was filtered through a pad of
5 filter aid, the filter pad was washed with methanol and the combined filtrates were
evaporated giving a dark residue. Purification by column Chl~ )gl .~ y eluting
with 0-10 ~o methanol in dichlulu~ l.allc gave the title compound as an oil.
~lH (270MHz, d6-DMSO/D20): 7.10 (2H, d, J=8.5Hz), 6.80 (2H, d, J=8.5Hz),
6.72 (2H, d, J=8.9Hz), 6.65 (2H, d, J=8.9Hz), 4.73 (2H, s), 3.8-3.75 (3H, m), 3.70
(3H, s), 2.9-2.4 (5H, m) and 0.90 (3H, d, J=6.1Hz) ppm.
Procedure 10: 2,2-Di-tert-butyl-4H-1,3,2-t ' ' 6 ol
OH
o~ ,~
Amixtureof2,2-di-tert-butyl-6-(benzyloxy)-4H-1,3,2-l,~..,,..,ii..~ilin:~ne(2g,
5.41mMol) and lO'ro palladium on charcoal (SOmg) in dichlu.ulll,,lldl.e (20ml) was
hy~, ' at atmospheric pressure. After 6 hours the reaction mixture was filtered
through a short pad of celi~e and Ihe solvent evaporated to yield a clear oil.
H (2~0MHz, CDC13): 6.80 (IH, d, J=8); 6.67 (IH, dd, J=8.1Hz and 2.4Hz);
6.45 (IH, d, J=2.4Hz); 4.90 (2H, s); 1.14 (18H, s).
-29-
wo96/04233 21 96193 r~ slr~7
Procedure 11: 2~2-Di-tert-butyl-6-(benzyloxy)-4H-1,3,2-
t ~ ~
O Ph
oxsi~<
Lithium aluminium hydride (0.235g, 6.2mMol) was suspended in t~ ahydlurulan
(25ml) and cooled to 0~C. S-Benzyloxy-2-hydroxy benzoic acid methyl ester (2g,
7.75mMol) in tetrahydrofuran (lOml) was added dropwise, via cannula. The mixturewas warmed to room L..IIIJ~,I aLul c~ and stirred for 20 minutes. The reaction was then
10 cooled to 0~C and cautiously quenched by the addition of water (O.Sml), 2M sodium
hydroxide solution (0.5ml), and water (lml). The resulting mixture was stirred at
room t~ J~d~Ulc for 30 minutes and filtered. The filtrate was evaporated in vacuo to
yield 4-benzyloxy-2-l,y~Lu,~y.ll~,.l,yl phenol as a clear oil which was used in the next
stepwithoutfurtherl."l;ri. ~l;....
To a solution of 4-benzyloxy-2-hydlu,.y..l~il.yl phenol in chloroform (lOml) wasadded 2,6-lutidine (2.49g, 23.25mMol) at room ~Clll~ Ul~i under argon. Di-tert-
butylsily bis(~li[luu.~.,., .ll~.,..,,,lfonate) (4.1g, 9.3mMol) was added and the mixture
stirred at room t~,Ul~ Ul~ for 18 hours. The solvent was evaporated irl vacuo and
20 the residue purified by normal phase column ~hlulll~Lu~ hy, eluting with 50~~c
hexane in ether to give the title product as pale yellow oil.
olH (250MHz, Cl)Cl3): 7.34-7.48 (SH, m); 6.85 (2H, m); 6.61 (IH, m), S.O
(2H, s); 4.78 (2H, s); 1.14 (18H, s).
-30-
21 ~61 93
~ wo96/04233 ~ ~ 7 1r~f~7
Procedure 12: ~S)-2,2-Di-tert-butyl-6-(oxiran-2-ylmethoxy)-4H-1,3,2-
L
o~
~<5'X
To a solution of 2,2-di-tert-butyl-4H- 1,3,2-l.~ n-6-ol (1.4g, SmMol) inacetone (40ml) at room t~ UI~ under argon was added potassium carbonate
(2.07g, 15mMol). (2S)-(+)-glycidyl-3-uiLIvl.f .,,~ .,., ,lfonate (1.43g 5.5mMol) was
added pulLiu~.w;~e and the reaction mixture was healed at reflux for 48 hours. The
10 solvent was removed under reduced pressure. The residue was taken into ethyl
acetate and washed with water (2xl5ml). The organic extracts were dried (Na2SO4)and the solvent evaporated in vacuo. The crude product was purified by
~,h.~ hy over normal phase silica eluting with 50f~o hexane in ether to give
(S)-2,2-Di-tert-butyl-6-(oxiran-2 ylmethoxy)-4H 1,3,2-1,. ..~ in~,1f
olH (250MHz, CDC13): 6.75-6.90 (2H, m), 6.7 (lH, d, J=2.5Hz); 4.97 (2H, s);
4.16 (lH, dd, J=ll, 3Hz); 3.88 (lH, dd, J=l l, 5.7Hz); 3.35 (lH, m); 2.89 (lH, dd,
J=5.0, 4.1 Hz); 2.73 (lH, dd, J=5, 2.4Hz); 1.14 (18H, s).
Procedure 13: (SR)-4-{2-[3-(2,2-Di-tert-butyl-4H-1,3,2-' " "
6-yl-oxy)-2-hydroxY p~ vt~ ' -]propyl}"' ~ rls . ~ '~ acid
diethyl ester
O - N~
~OH
lI,OEt
O~ ,O ~~ 'OEt
A mixture of (S)-2,2-di-tert-butyl-6-(oxiran-2-ylmethoxy)-4H-1,3,2-benzodio-
xasilinane (0.622g, 1.85mMol) and (R)-diethyl 4-(2-GIuillu~JIulJ.yl)~uh~,uO~ylll~ yl
-31-
w0 96104233 2 1 9 6 1 9 3 P~ n~7 ~
ph~rh~ (0.55~, 1.83mMol) was dissolved in methanol (lOml) and reiluxed
under a argon aLlllu~ c for 20 hours. The methanol was evaporated and the
residue taken up into dichloromethane (75ml), washed with water (3xSOml) and dried
with anhydrous sodium sulfate. After evaporation of the solvent in vacuo, the crude
5 product was purified by column 1hlull.atv~lalully over normal phase silica, eluting
with lO~o methanol in ethyl acetate to give the title compound as a dark oil.
~lH (250MHZ, CDC13): 7.15 (2H, d, J=9.3Hz); 6.09 (2H, d, J=9.0Hz); 6.85 (IH,
d, J=7.9Hz); 6.72 (lH, dd, J=7.9Hz and 2.7Hz); 6.50 (IH, dd, J=2.6Hz); 4.93 (2H, s);
4.25 (6H, m); 3.9 (3H, m); 2.5-2.9 (SH, m); 1.36 (6H, t, J=6.6Hz); 1.07 (3H, d,
J=6.8Hz); 1.04 (18H, s)
Procedure 14: (SR)-4-{2-[3-(2,2-Di-fer~-butyl-4H-1,3,2-B~ .-, .,,' ' -
6-yl-oxy)-2-h~d. u,.y,ul o~ ]propyl}~ ' .yl carboxylic acid methyl
ester
~OH
~'Si~~ O~COzMe
>< X
A solu!ion of (S)-2,2-Di-tert--bulyl-6-(oxiran-2-ylmethoxy)-4~-1,32,-benzodio-
xasilinane (0 65g, 1.93mMol) in acetonitrile (25ml) was treated with lithium
perchlorate (0.205g, 1.93mMol), then stirred until complete solution of the salt. To
the resulting stirred solution was added (R)-methyl 4-(2-alllh)u~lu~Jyl) pL~"lo,~ylll~,ill~l
carboxylic acid methyl ester (0.43g, 1.93mMol). The mixture was heated at 80~C for
20 hours, then cooled, diluted ~ith ethyl acetate and washed with water (2x50ml).
The dried (Na2S04) extracts were ~ ~"l ~ . l u A ~ in vacllo, and the crude product
purified by column ~.hlulllaLu~laiully over normal phase silica, eluting with 59'o
methanol in ethyl acetate to give the title compound as an oil.
olH (250MHz, CDC13) 7.12 (2H, d, J=8.7Hz); 6.87 (3H, m); 6.75 (IH, dd,
J=8.8Hz and 2.7Hz); 6.53 (IH, d, J=2.6Hz); 4.93 (2H, s); 4.60 (2H, s); 3.87 (3H, m);
3.81 (3H, s); 2.95-2.50 (5H, m); 1.07 (3H, d, J=6.7Hz); 1.03 (18H, s)
Procedure 15: 3,4-Dii,.n,.~lu~
-32-
21 ~6lf~3
96/04233 . ~. 1/1~1 7_"~ 7
~nO~OH
BnO
A solution of 3,4-dibenzyluAy~f (5.18g, 20mMol) in acetic acid (25ml),
chloroform (8ml), water (4ml) and peracetic acid (36-40 Wt~cJ in acetic acid, 18ml)
S was stirred at 40~C for 4 hours. After cooling to room ~ ~ 'l" " n l l r, saturated sodium
thiosulfate solution was added and the product was extracted into ethyl acetate. The
organic extracts were separated, washed with saturated sodium b;~ Jf ' solution,water and brine. The organic solution was dried and evaporated. A solution of the
residue in methanol (25ml) was treated with sodium hydroxide solution (2M, 8ml)
10 and the reaction was stirred at room t~ UI~ for 16 hours. The solvent wasevaporated and the residue was dissolved in water (lOml) and the pH of the solution
was adjusted to 9 with lM hydrochloric acid. The solvent was evaporated and the
residue was partitioned between ethyl acetate and water. The organic extracts were
separated, dried and evaporated giving the title compound as a coloorless solid.
~(CDCl3): 7.25 (lOH, m), 6.77 (IH, d, J = 8.5Hz), 6.48 (IH, d, J = 2.8Hz), 6.27 (lH,
dd, J = 8.5, 2.8Hz), 5.10 (2H, s), 5.06 (2H, s), 4.65 (lH, br, exchanges with D2O).
20 Procedure lG: ~S)-3-(3,4-Di~ ~IVA~ 2-e~,~/A~IJI ~, ~
0~~
f~l ~
BnO ;~
OBn
The title compound was prepared from 3,4-div~ ylw~ ,llOI and (S)-glycidyl 3-
~ lvb~,.l4~,lle. sulfonate according to the method described in Procedure 12.
~(CDCl3): 7.36 (lOH, m), 6.85 (IH, d, J = 8.8Hz), 6.60 (lH, d, J = 2.9Hz), 6.38 (IH,
dd, J = 8.8, 2.9Hz), 5.13 (2H, s), 5.08 (2H, s), 4.14 (lH, dd, J = I l, 3.3Hz), 3.85
(lH, dd, J = I 1, 5.8Hz), 3.31 (IH, m), 2.88 (IH, dd, J = 4.9, 4.1Hz), 2.71 (lH, dd, J =
4.9, 2.6Hz).
21 q6, q3
WO 96/04233 1~ n~7
Procedure 17: (SR)-4-{2-[3-(3,4-Dib...~,ylu~ ' y)-2-
U~ U~J.Y a]propyl~phenoxy acetic acid, methyl ester
Me
O--H~
J~ ~
OBn O~CO2Me
The title compound was prepared from (S)-3-(3,4-dibenzyluAy~ cllol~y)-ll2-
o~UAylJlulJallG and (R)-4-(2-alllhlu~lu~yl)pl~ lw-ya~ ic acid methyl ester according
to the method described In Procedure 13.
~(CDC13): 7.5-7.3 (lOH, m), 7.09 (ZH, d, J = 8.8Hz), 6.85-6.8û (3H, m), 6.58 (IH, d,
J = 3.0Hz), 6.35 (lH, dd, J = 8.8, 3.0Hz), 5.12 (2H, s), 5.07 (2H, s), 4.60 (2H, s), 3.9-
3.83 ~3H, m), 3.80 (3H, s), 3.0-Z.5 (5H, m), 1.06 (3H, d, J = 6.3Hz).
Procedure 18: (R)-3-(3~4-l)ihyd~u~yl '- yl)-2-propylamine hydrobromide
Me HBr
H2N~OH
OH
A solution of (R)-3-(3,4-di~uc~hu~yl~hc~yl)-2-propylamine hydrochloridel (500 mg,
2.15 mMol) in hydrogen bromide (48%, 5 ml) was stirred at 100~C under an argon
a~ u~ ,.c for 20 hours. After cooling, the solvent was evaporated and the residue
was dried giving the title compound.
o(D6DMSO + D20): 6.9 - 6.4 (3H, m), 3.5 - 2.4 (3H, m), 1.3 (3H, d, J = 7 Hz) ppm.
1 D.E Nichols, C.F. Barfknecht and D.B. Rusterholz. J.Med.Chem., 1973, 16(5),
480.
Procedure 19: (R)-2-(3,4-Dihyd. U~.y~ I),u~ ui~,rl al; ~ ~ acid, f-butyl ester.
-34-
21 ~61 53
~ WO 96/04z33 A .~ 0~7
Me
BOCNH ~ OH
OH
A solution of (R)-3-(3,4-dillydlu~y~ilc~lyl)-2-~lu~ylal~ Ly~Lub~u~llide (480 mg,
1.9 mMol) in di~ yl~ullllallli ic (5 ml) containing L.i~,~l.yld...;..e (3 equiv, 586 mg,
5 5.7 mMol) was stirred at 5~C under an argon al~ h~,lc for 15 minutes.
Di-t-butyHliualL (414 mg, 1.9 mMol) was added and the reaction mixture was
stirred at 5~C for 1 hour and then at ambient ~ .,la~ul~i for I hour. The solvent was
evaporated. Ethyl acetate (100 ml) and water (50 ml) were added and the organic
layer was separated, washed with water (50 ml) and brine (50 ml), dried (MgSO4)
10 and cv l ' Purification of the residue by chlul~alu~-a,uhy on silica gel eluting
with 25% ethyl acetate in n-hexane gave the title compound, m.p. 116-118~C;
o(CDCl3): 6.76 (IH, d, J = 7.9 Hz), 6.70 (IH, d, J = 2 Hz), 6.55 (lH, dd, J = 7.9, 2
Hz), 6.25-5.90 (2H, br, exchanges with D2O), 4.45 (lH, br, exchanges with D2O),
3.8 (lH, b), 2.75 - 2.5 (2H, m), 1.43 (9H, s), 1.07 (3H, d, J = 6.6 Hz) ppm.
Procedure 20: (R)-5-[N-(t-Bul~lu~ al b ~ 2-: , u~,~l]-1,3 t " ' -
2,2-dicarboxylic acid, diethyl ester
Me
BoCNH~ CO2Et
CO2Et
A solution of (R)-2-(3,4-dil-yd~u~ypl-~ yl)propylcarbamic acid, /-butyl ester.
(1.07g, 4 mMol) in acetone (25 ml) containing potassium carbonate (3 equiv, 1.66 g,
25 12 mMol) was stirred at 60~C under an argon alua~,L~,lc for I hour. After cooling to
ambient tel~ laLLl~;~ a solution of diethyl dibrom--m~lon:~t~ (1.27 g, 4 mMol) in
acetone (7 ml) was acided and the reaction was stirred at ambient t~ u~,laLul~i for 18
hours. The suspension was filtered and the residue was washed with ethyl acetate.
The filtrates were combined, evaporated and the residue was partitioned between
ethyl acetate (200 ml) and dilute hydrochloric acid (100 ml, pH5). The organic layer
was separated, washed with water (2 x 100 ml) and brine (100 ml), dried (MgSO4)
and evaporated. The residue was purified by column chromatography on silica gel
eluting with 25~o ethyl acetate in n-hexane giving the title compound as an oil;
-35-
21 961 93
w0 96/04~33 r~
o(CDCi3): 6.86 (IH, d, J = 8 Hz), 6.78 (IH, d, J = 1.3 Hz), 6.71 (IH, dd, J = 8, 1.3
Hz), 4.41-4.32 (SH, m), 3.8 (IH, br, exchanges with D20),2.76 (IH, dd, J = 13.5, 5.6
Hz), 2.60 (lH, dd, J = 13.5, 7.2 Hz),1.43 (9H, s), 1.36 - 1.31 (6H, m), 1.07 (3H, d, J
S = 6.6 Hz) ppm.
Procedure21: (R)-5-(2~ uu.~ L3 1~ -2,2-d;~.i~u~' acid,
diethyl ester, hydrochloride salt.
Me HCI
H2N ~ J<CO2Et
CO2Et
A solution of (R)-5-[N-(r-butylu~y~ d,u~lyl)-2-~ u~u~ul,yl]-l~3-~ e-2~2-
di~,rliJu~ylic acid diethyl ester (3.0 g, 7 mMol) in ethyl acetate (40 ml) and hydrogen
15 chloride solution in diethyl ether (IM, 56 ml, 56 mMol) was stirred at ambient
tU~ rllUl~ under an argon atmosphere for 48 hours. The solvent was evaporated
and the residue was dried giving the title compound as a glass.
~(d6-DMSO): 8.07 (3H, br, exchanges with D2O), 7.10 - 7.06 (2H, m), 6.85 (IH, dd,
J = 8, 1.4 Hz), 4.33 (4H, q, J = 7.1 Hz), 3.5 - 3.4 (IH, m), 2.93 (lH, dd, J = 13.4, 5.8
Hz), 2.66 (lH, d, J = 135, 8.2 Hz), 1.24 (6H, t, J = 7.1 Hz), 1.12 (3H, d, J = 6.3 Hz)
ppm.
Procedure 22: (R)-5-(2-~ ~ , u~Jyl)-1~3-t~~--~ '- '~-2,2-d;.". i~u~.~l;c acid,
diethyl ester
Me
~=~
H2N~<CO2Et
CO2Et
A solution of (R)-5-(2-~lldllu~-uuyl)-1,3-b~ ule~2~2-dicarboxylic acid diethyl
ester hydrochloride (646 mg, 2 mMol) in dichlulul,.~ ,le (80ml) was shaken with a
saturated solution of sodium hydrogen carbonate (20 ml) for 30 seconds. The organic
layer was separatcd and the aqueous layer was extracted with dichlululll.,tl,~ , (2 x
-36-
2 1 96 1 93
WO 96/04233 P~ 7
50 ml). The combined organic extracts were washed with water (50 ml) and brine
(50 ml), dried (MgS04). The solvent was evaporated giving the title compound
which was used ' '~/.
Procedure 23: (SR)-5-{Z-[3-(2,2-Di-f-buiyl-4H-1,3,2-t ' " - 6 yloxy)-
2 h,~P ur.y~.O~ ~]propyl}-1,3-L~ e-2,2-d;~a-l,u,.y' -acid,diethyl
ester
Me
~~~
'S 0 7LCO2Et
10t-Bu~ t-Bu CO2Et
The title compound was prepared from (R)-5-(2-au~ ulJ~ulJyl)-1,3-b ~ .-2,2-
di~,~l)u~ylic acid diethyl ester and (S)-2,2-di-r-butyl-6-(oxiran-2-ylmethoxy)-4H-
1,3,2-b~ c;lir ~ by heating in ethanol as solvenl according to the method
described in Procedure 13.
o(CDC13): 6.86-6.49 (6H, m), 4.94 (2H, s), 4.36 (4H, q, J = 7.2Hz), 4.1-3.8 (3H, m),
3.0-2.5 (SH, m), 1.34 (6H, t, J = 7.2Hz), 1.08 (3H, d, J = 6.3Hz), 1.03 (18H, s).
Procedure 24: 4-(2-f-BU~ hyl)~ ... Ih~l ph p~ '-
acid, diethyl ester
BOCNH'----~O~P(OEt)2
~ A solution of 2-(4-hyd.uAyl,l,.,.lyl)ethylcarbamic acid, t-butyl esterl (4.0g, 16.9
mMol) in dry DMSO (37.5 ml) was cooled in an ice-bath and treated with sodium
~ hydride (80~o in mineral oil 0.557g, I.lequiv) with stirring under argon according to
the method described by Cornforth2. When effervescence ceased a solution of
4-~ h~ ulh "- "- ~lfo,.yluAy...~.l'yllll'o~ r diethyl ester (6.07g, 1.05 equiv) in
dry DMSO (I lOml) was added and the resulting pale yellow solution stirred at room
t~ Ulc overnight. The mixture was then poured into water (550ml) and
-37 -
21 961 93
wo s6/04~33 r~ 5~ 0~7
extracted with dieIhyl ether/ethyl acetate (1: 1, 3xl50ml) and finally ethyl acetate (3x
lOOml). The combined extracts were washed with brine, dried over anhydrous
sodium sulfate and evaporated to dryness. The resulting oil was purifed by
.1Iy on silica gel with a gradient of 3:2 pentane: ethyl acetate rising to
S lOO''Yo ethyl acetate to give the title compound as a colourless viscous oil.
vlH (2SOMHz, CDC13): 7.12 (2H, d), 6.90 (2H, d), 4.51 (IH, br s), 4.30-4.15 (6H,m), 3.33 (2H, br. q), 2.74 (2H, t), 1.43 (9H, s), 1.37 (6H, t).
I F. Houlihan et. al. Can. J. Chem., 1985, 63, 153.
2 J. Cornfonh and J.R.H Wilson. J.C.S. Perkin 1., 1994, 1897.
Procedure25: 4-(2-Aminoethyl)t ' ~Y~ - Yl! ' , ' - acid,
15 diethyl ester
H2N~ o~P(OEt)2
4~(2-t-Bu~w~y~,~ubullyl~ u~.illyl)pl~ lu/~ylll~,dlylll~u~ acid, diethyl ester
(2.856g, 9.95 mMol) in methylene chloride (300ml) and nirl.. ,,.. ~.~;r acid (16ml)
was stirred at room ~ ul~ for 5h. The solution was: ' under reduced
pressure and product dried under in vacuo. The ~illuu~ ,Lic acid salt was neutralized
with aqueous sodium carbonate and exuacted with dichlu-uu-~.laLllc containing a
small proportion of methanol (5xlOOml). The combined organic layers were dried
25 over sodium sulfate and evaporated to dryness to give the title compound as a pale
yellow gum.
olH (2501VllHz, CDC13): 7.12 (2H, d), 6.9 (2H, d), 4.30-4.15 (6H, m), 3.00-2.55
(4H, m) and 1.37 (6H, t~.
Procedure 2G: (S)-4-{2-[3-(2,2-Di-t-butyl-4H-1,3,2-v~. " " G yl-oxy)-2-
h,~v7~v~ ,rvtJ.r, ~]ethyl),n' yl...ll~yl ~'~;,' ~acid,diethylester
-38-
~ WO 96104233 219 619 3 r~ r ~o~7
~ --H~
OH
O ~ , 0 O ~ P'
Usingsimilara~l,. .;.". .,lAimethodtothatofProcedure 13,thetitlecompoundwas
obtained from 4-(2-aminoethyl)pl-~,.-v,-y-~ yl~ r acid diethyl ester
and (S)-2,2-di-t-butyl-6-(oxiran-2-ylmethoxy)-4H-1,3,2-1,~ .~u~ lin~ as an oil.
v1H (250MHz, CDC13): 7.1 (2H, d, J=8.7Hz), 6.87 (2H, d, J=8.9Hz), 6.84 (IH, m);
6.75 (IH, dd, J=8.3Hz and 3.1Hz), 6.52 (IH, d, J=3.0Hz), 4.94 (2H, s), 4.25 (6H, m),
3.88 (3H, m), 2.5-2.85 (7H, m), 1.37 (6H, 1, J=6.7Hz), 0.99 (18H, s);
[a]DZ2 - 18.5~ (c = 0.2, CHC13).
Procedure27: (RR)-2-(4-H~rv~v~p'l yl)-l-methylethyl~ phenyl-
ethyl) ' ~ acid
Me Me
~/ \~
HO OH
OH
(RR)-[2-( i hl"Ll~u~y~l~.,"yl)- l -methylethyl] -( l -phenylethyl)amine hydrochloride
saltl (lOg, 0.0327 Mol) in dichlu,u.,..,il,a,,e (SOml) was treated with boron tribromide
(lN in CH2C12, 72 ml) under argon and stirring continued overnight at room
LUIC~ The mixture was then evaporated to dryness and ice added to hydrolyse
the complex. The resulting white solid was collected and dried to give the titlecompound.
~lH (250MHz, CDC13 + CD30D): 7.50 (SH, m), 6.83 (2H, d), 6.72 (2H, d), 4.38
(lH, q), 3.23 (IH, dd), 3.00 (IH, m), 2.67 (IH, dd), 1.78 (3H, d), 1.24 (3H, d)
m/z: FAB MH+: 300 (S~o), 256 (100)
-39-
21 961 93
wo 96/04A~33 ~ 0~7
1 D.E. Nichols, C.F. Barfknech~ and D.B. Rusterholz. J.Med.Chem., 1973, IG(~),
480.
Procedure 28: (R)-4-(2-~ l~ o~ l)phenol
Me
-
~"
10 (RR)-2-(4-Hy~Lui~y~hc.~l)-l-methylethyl-(l-phenylethyl)~ lol)ulullic acid
(9.73g, 0.0325 Mol) was dissolved in methanol (120ml) and hy~ug~,.ldl~l at S0 psi
and 50~C with 10% palladium on charcoal (lg) for 2 days. The mixture was allowedto cool, then filtered through Kieselguhr and evaporated to dryness to give the title
compound as a pale yellow solid.
~lH (250MHz, CDC13): 7.06 ~2H, d), 6.80 (2H, d), 4.12 (3H, br s), 3.12 (IH, m),
2.96 (IH, dd), 2.73 (IH, dd), 1.30 (3H, d).
Procedure 29: (R)-2-(4-H~dl u~ l)-l-methylethylcarbamic acid, t-butyl
ester
Me
E,OCNH ~
OH
(R)-4-(2-A~lhlv~u~yl)phenol (4.91g, 0.0325 mol) in dichlulullll,ll,a,lc (240 ml) and
dry di.,.~,L;.~lru.."a."ide (50ml) was treated with triethylamine (7.59 ml) and di-t-
butyl,li~ (11.77g, 1.2 equiv.) and the mixture stirred at room r~ r for
1 day. After CVcl~JuldLiu~ of volatile material in vacuo, the residue was washed with
diethyl ether. The combined portions of diethyl ether (500ml) were washed with
-40-
21 96t ~3
wo 96/04.33 , ~ ~ 7
water (3 x 100ml) and dried over anhydrous sodium sulfate. After evaporation to
dryness the residue was chromatographed on silica gel with 0-3~o methanol in
v;~hlolu~ ,Lllallc to give the title compound as a gum that slowly solidified.
olH (250MHz, CDC13): 7.00 (2H, d), 6.76 (2H, d), 6.25 (IH, br s), 4.44 (IH, br s),
3.83 (lH, m), 2.73 (lH, m), 2.57 (IH, dd), 1.43 (9H, s), 1.07 (3H, d).
Procedure 30: (R)-4-(2-~-Butoxycal l,v..~ r - o~.~l)p ' .~ a.~tic acid,
methyl ester
MG
BOCNH~
O ~ CO2M~
Potassium carbonate (1.95g, 14.2 mMol) was added to a solution of (R)-2-(4-
hyvl~v~y~ yl)- I-lll~allyl~,~llyL,allJalllic acid, t-butyl ester (2.96g, 11.8 mMol) in
acetone (soml) at room [~ aLul~i under argon. Methyl lvl~ (1.81g~ 11.8
mMol) was added dropwise and the reaction mixture was heated at reflux for 3 hours.
The solvent was removed under reduced pressure and the residue was taken into ethyl
acetate and washed with water (2x30ml). The organic extracts were dried with
20 sodium sulfate and the solvent evaporated in vacuo. The crude product was purified
by cl.., ~,l a~hy on Kieselgel 60 (eluting with 20% ethyl acetate in pentane) to give the title compound as an oil.
olll (250MHz., CDC13): 7.1 (2H, d, J=7.3Hz), 6.85 (2H, d, J=7.3Hz), 4.53 (2H, s),
4.35 (IH, br s), 3.85 (IH, m), 3.8 (3H, s), 2.5-2.8 (2H, m), 1.42 (9H, s), 1.07 (3H, d,
J=6.6Hz); [~]D22 7 9~ (c=0.49, MeOH)
Procedure 31: H~v~ Nlyl~,l , ' - acid, bis-(3-benzyloxy-propyl)ester.
o
HO~P ~~O~Ph)2
Pl~ l.v..ic acid bis-(3-benzyloxypropyl) ester was prepared by the genera' method
of Houben-Weyl, Phosphor Verbinungen, p28 and J. Amer. Chem. Soc., 1955, 77,
-41 -
2l q~ q3
w0 96104233 P~~
3522. A mixture of this crude phosphite (Sg, 0.012 Mol based on 85'~o purity),
paraformaldehyde (0.36Sg, 1 equiv.) and triethylamine (0.17ml, 0.1 equiv.) was
heated under argon in an oil bath to 90~C. Further triethylamine (2ml in total) was
added to promote reaction. After ca 0.5h. the mixture was allowed to cool and then
5 cl,~ ,hrtl on silica gel with 0-5~0 methanol in dicl-lulul~ lld-~e to give the
title compound as a colourless oil.
~1H (250MHz, CDC13): 7.32 (IOH, m), 4.49 (4H, s), 4.22 (4H, m), 3.85 (2H, t),
3.58 (4H, t), 3.08 (IH, m), and 1.97 (4H, m).
Procedure 32: 4 C~ 'fu~ lur.~ ' . ' , bis-(3-
t ,~lv~tJ~o~Jyl) ester
5Cl~ I--O 111~ ~O~Ph)z
The title compound was prepared in a similar manner to the literature procedure
from hYdIUA.YIII~IIIY~ ;r acid, bis-(3-benzyloxy-propyl) ester
as an oil.
~lH (250MHz, CDCl3): 7.82 (2H, d), 7.50 (IOH, m), 4.48 (4H, s), 4.30-4.10 (6H,
m), 3.53 (4H, t), 1.93 ~4H, m).
lJ. Cornforth and J.R.H. Wilson, JC.S Perkin 1, 1994, 1897.
Procedure 33: (R)-4-(2-t-ButoxycarbonyB~ ~ r UI
p' ~acid, bis-(3 t ,~lv~ o!~yl) ester
Me
BOCNH~
~ .
~~
o~P'(~~~~~Ph)2
The title compound was prepared as a viscous oil from 4-ChIU~UIJC
-42-
WO 96/04233 ~ ~ 9 fi 1 ~ 3 1~ 7
~ulruu()~y~ yll~llo~ f)n~t~ bis-(3-benzyloxypropyl) ester and (R)-2-(4-
Ly~u~y~uL.,.~yl)-l-methylethylcarbamic acid, t-butyl ester according to the method
described in Procedure 24.
~lH (200MHz, CDC13): 7.30 (IOH, m), 7.09 (2H, d), 6.83 (2H, d), 4.48 (4H, s),
4.40-4.15 (7H, m), 3.83 (lH, br m), 3.57 (4H, t), 2.78 (IH, dd), 2.59 (lH, dd), 1.98
(4H, m), 1.52 (9H, s) and 1.05 (3H, d).
Procedure 34: (R)-4-(2-~ ' ~r ~IJYI)P~ .~ 'h~ ~ acid, bis-(3-
th,..L,tlh~y,ul U,u,~l) ester
Me
H2N~
~0
o~p~O~~O Ph)2
(R)-4-(2-t-BIliuAy~,d~l~ollyldlllillu~ Jyl)~Jllcllv~ylll~.illy~ acid, bis-(3-
~ ULYIW~Y~IU~JYI) ester (3.2g, 4.99 mMol) was converted into the title compound
using the method described in Procedure 25.
olH (200MHz, CDC13): 7.30 (lOH, m), 7.10 (2H, d), 6.85 (2H, d), 4.47 (4H, s),
4.35-4.15 (6H, m), 3.56 (4H, t), 3.22 (lH, m), 2.70 (2H, d), 2.60 (2H, br s), 1.98 (4H,
m), 1.18 (3H, d).
Procedure 35: (SR)-4-{2-[3-(2,2-Di-t-butyl-4H-1,3,2-t~~- - ' ' 6 yl-oxy)-
25 2-hydroxy p-u~"~ ~ ~]propyl}p'- yl ' y~ acid, bis-(3-
t....lLy~ JI u~uil)ester
Me
o~
0~ P~O~--O Ph)2
-43-
_ _ . . . . . ....
w096104233 21 9 61 93 r~ 7 ~
(R)-4-(2-A~Ilh~u~vl~yl)~ loi~ylll~ dlyl~ho~ ullic acid, bis-(3-benzyloxypropyl)
ester (2.507g, 4.6 mMol) was reacted with (S)-2,2-di-1-butyl-6-(oxiran-2-ylmethoxy)-
4H-1,3,2-l.~ lin~ne (1.557g, 1 equiv.) using the method described in
5 Procedure 14 to yield the title compound as a colourless gum.
~1H (200MHz, CDC13): 7.30 (IOH, m), 7.09 (2H, d), 6.83 (3H, m), 6.70 (IH, dd),
6.49 (IH, d), 4.93 (2H, s), 4.47 (4H, s), 4.35 - 4.20 (6H, m), 3.99 (IH, m), 3.87 (2H,
d), 3.55 (4H, t), 3.1 - 2.5 (5H, m), 2.32 (2H, br s), 1.98 (4H, m), 1.11 (3H, d), 1.03
10 (18H, s).
Procedure 36: (SR)-4-{2-[3-(2,2-Di-t-butyl-4H-1,3,2-b~ 6 yl-oxy)-
2~ u~ u~ amino]propyl}l ' Y~ 'Ylr . ' ~ acid, bis-(3-
15 1. ~ d. ~".y~.. utJ yl)ester.
Me
O~--H~i
~3 OH ~3
0~, ,0 O~P--(O~\--OH)2
(SR)-4-(2-[3-(2,2-Di-t-butyl- 4H-1,3,2-b~ Acilin~n-6-yl-oxy)-2-hydro
20 l~lul~yL~ u]propyl}pllc.~u~y~ hyl~ in acid, bis-(3-benzyloxypropyl)
ester (Ig, 1.14mmol) was hydrogenated at 50~C and 50psi for 2 days in methanol
(120ml) in the presence of 10% palladium on charcoal (I.Og). After allowing the
mixture to cool, it was filtered through Kieselguhr, evaporated to dryness and purified
by column chromatography on silica gel eluting with 0- 15% methanol
25 dichloromethane. The title compound was obtained as a clear gum.
olH (2COMHz, CDC13): 7.13 (2H, d), 6.90 (2H, d), 6.82 (IH, d), 6.70 (IH, dd), 6.49
(lH, d), 4.93 (2H, s), 4.40 - 4.25 (6H, m), 4.07 (lH, m), 3.88 (2H, m), 3.73 (4H, t),
3.40 - 2.60 (9H, overlapping m + br. s), 1.90 (4H, m), 1.18 (3H, d), 1.02 (18H, s).
~ 30
Procedure 37: Hyd.u.~r...~lb~lt '- ylr' i, ' - acid, ethyl ester
-44-
21 96 1 93
~wos6/04233 r, ~,~
Ph--P OH
OEt
The title compound was prepared by the general method of Procedure 31 by
L~Lur.yll~ iullofpL~ y~ h~;~ acidethylesterl(10.136g,0.059Mol). The
5 product was obtained as a colourless viscous oil after cl.., O ~ ' y.
H (250MHz, CDC13): 7.83 (2H, m), 7.65 - 7.42 (3H, m), 4.26 - 3.90 ~5H, m),
1.32 (3H, t).
ID.G. Hewitt. Aust. J. Chem., 1979, 32, 463.
Procedure 38: 4-Cr ' .' l '~ùu~.~lo~ 1, ' yll '- , ' ' '. acid ethylester
Ph--P ~O--S ~ Cl
OEt O
The title compound was prepared as a white crystalline solid, m.p. 70-72~C, fromL.ydlu~.yl~ yl~h~,~lyl~ u~ acid, ethyl ester (9.525g, 0.0476 Mol) by a method
20 similar to that of Procedure 32.
~lH (250MHz, CDC13): 7.83 - 7.58 (5H, m), 7.58 - 7.40 (4H, m), 4.40 (IH, dd),
4.28 - 4.00 (3H, m), 1.35 (3H, t).
Procedure 39: (R)-4-(2-~-B~tv~ r Ul~rl)
phenyl! ' - . ' ' ~~ acid, ethyl ester.
Me
EOCNH~
~0
O ~ P--OEt
Ph
-45-
wo 96/04233 2 1 9 6 1 9 3 1 ~.,~ 75/~-~77
The title compound was prepared as a colourless gum from
4-chlul..b~ fonoxymethylphenylrh~rhini~ acid ethyl ester (3.91g,
10.4mMol) and (R)-2-(4-hydroxyphenyl)-1-methylethylcarbamic acid, t-butyl ester
(2.5g, 9.96mMol) by the method described in Procedure 24 .
olH (200MH.., CDC13): 7.93 (2H, m), 7.52 (3H, m), 7.07 (2H, d), 6.82 (2H, d),
4.52-4.0 (5H, m), 3.81 (IH, br), 2.76 (IH, dd), 2.57 (IH, dd), 1.42 (9H, s), 1.38 (3H,
t), 1.03 (3H, d).
Procedure 40: (R)-4-(2-A ~ r uuyl)~ Y~ ' Yl! ~, I ~ acid,
ethyl ester
Me
~0
o ~ P--OEt
Ph
The title compound was prepared by a method similar to that described in Procedure
25 from (R)-4-(2-t-butoxycarbonyl~ ,yl)phenoxymethyl-phenylrho~rhinin
acid, ethyl ester (2.647g, 6.1mMol).
olH (250MHz, CDC13): 7.93 (2H, m),7.65 - 7.44 (8H, m),7.07 (2H, d), 6.83 (2H,
d), 4.44 (IH, dd), 4.36 - 4.02 (3H, m), 3.09 (IH, m), 2.63 (IH, dd), 2.43 (IH, dd),
1.38 (3H, t), 1.08 (3H, d).
Procedure41: (SR)-4-{2-[3-(2,2-Di-t-butyl-4H-1,3,2-b~",.vvh ' 6-yl-oxy)-
2-L~v~u/~yt~O,u~'~ ]propyl}l3h~ y ''yll' Yi~ ,' eacid,ethyl
ester
-46-
21 96l 93
0 96/04233 r~ l ,5.'~ 7
Me
~~~ H '~
~ OH ~;3
O~ ~0 O~P--OEt
The title compound was prepared as a colourless gum by a method similar to tha~
described in Procedure 13 from (R)-4-(2-a~ lu~yl)~lltllu~ylll~,illyl~h.,llyl
5 phosphinic acid, ethyl ester (lg, 3mMol) and (S)-2,2-di-t-butyl-6-(oxiran-2-
ylmethoxy)-4H- 1,3,2-1,., ., .~ ;lin~ln~ (1.009g, 3mMol.)
~lH (400MHz, CDC133: 7.92 (2H, m), 7.59 (lH, m), 7.51 (2H, m), 7.07 (2H, d),
6.82 (3H, m), 6.69 (IH, dd), 6.48 (IH, d), 4.93 (2H, s), 4.43 (IH, dd), 4.30 (lH, m),
4.25 - 4.05 (2H, m), 3.98 (lH, m), 3.88 (2H, m), 3.03 - 2.35 (7H, m), 1.38 (3H, t),
1.10 (3H, d), 1.03 (18H, s).
Procedure42:3-Bc..,"~ t,.~,t.~ acid.
H--[~\--O/~
OH
The title compound was prepared from allylbenzyl ether and 50% aqueous phosphinic
acid by an analogous procedure to that described in J. Inorg. Nucl. Chem., 1965, 27,
20 697.
~(CDC13): 10.83(1H, s, exchanges with D2O); 7.36-7.18(5H, m.); 7.10(1H, d, J =
546.57Hz.); 4.49(2H, s.); 3.52(2H, t, J = 5.77Hz.); 1.94-1.80(4H, m.).
Procedure 43: 3-B..l~"~lu~ " ot~ylr' ~ acid, n-butyl ester
H--P~\~O ~3
OC4H~
-47 -
wo 96/04233 2 l 9 6 1 9 3 ~ 5/ ~n~7
The title compound was prepared from 3-benzyloxypropylpho~phini~ acid and n-
butanol according the general procedure described in European Palent 0093010. The
compound was used without further purification.
s
;;(CDC13): 7.39-7.15(5H, m.); 7.09(1H, ddd, J = 532.27, 1.92, 1.65Hz.); 4.51(2H,s.); 4.17-3.91(2H, m.); 3.53(2H, t, J = 5.77Hz.); 1.99-1.72(4H, m.); 1.69-1.58(2H,
m.); 1.47-1.33(2H, m.~; 0.94(3H, t, J = 7.15Hz.).
Procedore 44: 3-Bc.. ~.ylu ~yl~l o~ylhydroxyrn~ y'~ acid, n-butyl ester
o
Ho~P~--o~3
OC~H~
lS The title compound was prepared from 3-benzyloxypropyll,h.~ ;";~ acid n-butylester and ~J~uarulllldl l~llyd~ according to the method described in Procedure 31.
Pu.ir~,a~ion by chromatography, eluting with dichlv-u...~hane containing 5~o
methanol, gave an oil.
3(CDC13): 7.38-7.25(5H, m.); 4.50(2H, s.); 4.09-3.97(2H, m.); 3.83(2H, t, J =
4.95Hz.); 3.77-3.71(1~, m. exchanges with D2O); 3.54-3 51(2H, m.); 1.99-1.86(4H,m.); 1.68-1.60(2H, m.); 1.48-1.34(2H,m.); 0.92(3H, t, J = 7.42Hz.).
Procedure 45: 3-B~IU"YIUA~ ,u. ("U,~ I (4
~hlo.., l ~ ylu~ yl),ullu~r - acid, n-butyl ester
Cl~~~
30 The title compound was prepared from 3-benzyloxypropylhydroxy-~ .hyll,l,o~ .;";n
acid, n-butyl ester and 4-chlu.ub~ .." n. ~,llftmyl chloride according to the method
described in Procedure 32. The crude compound was used without further
-48-
21 96l 93
W0 96/04233 1~ 5 nl~ ~7
o(CDC13): 7.84(2H, d, J = 8.8ûHz.); 7.52(2H, d, J = 8.79Hz.); 7.36-7.26(5H, m.);4.50(2H, s.); 4.27-3.78(4H, m.); 3.51(2H, t, J = 6.05Hz.); 1.97-1.83(4H, m.); 1.67-
1.55(2H, m.); 1.42-1.26(2H, m.); 0.91(3H, t, J = 7.15Hz.).
Procedure46: 4-(2-f E~ arbu.. ~ .... lhyl-(3-
t ylu~ acid, n-butyl ester
BOCNH ~
'
O
o~lll~ o~,3
OC~Hg
The title cûmpound was prepared from 3-b~ rluAy~lvluyl-(4-chloro-
brn7rn~snlfonyluAy,.,~,Ll,yl)rhf)~rhini~ acid, n-butyl ester and 2-(4-
l~yd~u~y~ yl)ethylcarbamic acid, t-butyl ester according to the procedure described
in Procedure 24. The crude product was purified by, I,, . ~ g, ,,~ y, eluting with
15 dichlulul..~il,ane containing 3~O methanol, to give an oil.
~(CDC13): 7.37-7.24(5H, m.); 7.11 (2H, d, J = 8.80Hz.); 6.87(2H, d, J = 8.80Hz.);
4.49(2H, s.); 4.27-3.95(4H, m.); 3.55(2H, t, J = 6.05Hz.); 3.36-3.32(2H, m.);
2.74(2H, t, J = 6.88Hz.); 2.06-1.79(5H, m.); 1.71-1.60(2H, m.); 1.47-1.31(2H, m.);
1.25(9H, s.); 0.91(3H, t, J = 7.15Hz.).
Procedure 47: 4-(2-A yl)l ~,.. ~til~1(3 ~.. ~!lu~ rù~ l)p,L, L~ ~_
acid, n-butyl ester
H2N ~
OC,H9 /~3
The title compound was prepared from 4-(2-r-butoxycarbonylaminoethyl)
-49-
wo 96/042.~,3 2 1 9 b 1 ~ 3 P~ 7
}~h~"~uAyl~ llyl(3-benzyloxypropyl)phosphinic acid, n-butyl ester according to the
method described in Procedure 25. The crude product was used without further
purification.
o(CDC13~: 7.36-7.26~5H, m.); 7.12(2H, d, J = 8.80Hz ); 6.87t2H, d, J = 8.53Hz.);4.49(2H, s.); 4.23-4.19(2H, m.); 4.15-3.97(2H, m.); 3.54(2H, t, J = 5.78Hz.);
2.94(2H, t, J = 6.60Hz.); 2.70(2H, t, J = 6.60Hz ); 2.05-1.95(4H, m.) 1.67-1.59(4H,
m. 2H exchange with D2O); 1.43-1.35(2H, m.); 0.91(3H, t, J = 7.42Hz.),
Procedure 48: (S) 4-{2-[3-(2,2-Di-t-butyl-4H-1 ~ '' - " 6 yloxy)-2-
tJIU,.~ ]~ }P~ 3-b~ t~
p ' . ' ~ ~ - acid, n-butyl ester
~J~3 OH
~S~ OC~Ha ~3
15t-Bu t-Bu
The title compound was prepared from 4-(2-aminoethyl)~l.c"u~-yl.-~,Lllyl(3-
ylu-~y"lu~yl)phosphinic acid, n-butyl ester and (S)-2,2-di-t-butyl-6-(oxiran-2-
ylmethoxy)4H-1,3,2-b~n7,~ fY~cilin~nP according to the method described in
Procedure 13. The crude product was purified by ~,hlulllalu~,la~ y over silica gel
eluting with dichlu~u~ hO~Ie containing 3% methanol to give a viscous gum.
~(CDC13 + D2O): 7.36-7.26(5H, m.); 7.13(2H, d, J = 8.80Hz.); 6.86(2H, d, J =
8.8ûHz.); 6.83(1H, d, ~ - 8.80Hz.); 6.72(1 H, dxd, J = 8.80 & 3.03Hz.); 6.50(1H, d, J
= 3.02Hz.); 4.94(2H, s.); 4.49(2H, s.); 4.22-3.88(7H, m.); 3.54(2H, t, J = 6.05Hz.);
2.92-2.70(6H, m.); 2.06-1.93(4H, m.); 1.46-1.30(2H, m.); 1.02(18H, s.); 0.91(3H, t, J
= 7.14Hz.).
Procedure49: (R)-4-(2-t-Butoxyca.l,ûu,r rl~ 'n~ tll~1-(3-
b~ YIUA~I u~ acid, n-butyl ester
-50-
~ Wo 96/04233 2 1 9 6 1 9 3 r ~ ~ n~7
Me
BOCNH
~0
o ll/~~o~
OC4Hg
The title compound was prepared from 3-benzyloxypropyl-(4-
chlv~ 1 ro~lyloxymethyl)pllva~h~ c acid, n-butyl ester and (R)-2-(4-
S hydlvA~y~ ,.lyl)-1-methylt;~ L~A,l,d,,,;c acid, t-butyl ester according to the method
described in Procedure 24. The crude product was purified by u h~v~aLu~ Yily,
eluting with dichlu.ul..~il.a"c containing 3% methanol, to give an oil.
o(CDC13 + D2O): 7.34-7.26(5H, m.); 7.11 (2H, d, J = 8.53Hz.); 6.86(2H, d, J =
8.52Hz.); 4.50(2H, s.); 4.36-3.85(5H, m.); 3.56(2H, t, J = 5.91Hz.); 2.80(1H, dd, J =
13.48, 3.49Hz.); 2.60(1H, dd. J = 13.48, 7.43Hz); 2.17-2.00(4H, m.); 1.69-1.62(4H,
m.); 1.43(9H, s.); 1.06(3H, d, J = 6.60Hz.); 0.91(3H, t, J = 6.60Hz.).
Procedure 50: (R)-4-(2-~ P~I)F ~ lhyl-~3-
IUA~ I u~ l)p~ , ' ~ ~- ncid, n-butyl ester
Me
-
O
T 11~ o~3
OC4H9
20 The title compound was prepared from (R)-4-(2-r-butoxy~ IJv"yla,ldnoprûpyl)
IJh~"lvA~yl~l~,ihyl(3-benzyloxypropyl)pllva~hillic acid, n-butyl ester according to the
method described in Procedure 25. The crude producl was used without further
"~",
o(CDC13 + D2O): 7.36-7.26(5H, m.); 7.12(2H, d, J = 8.8()Hz.); 6.87(2H, d, J =
8.80Hz.); 4.50(2H, s.); 4.21(2H, d, J = 6.88Hz.); 4.17-3.97(2H, m.); 3.54(2H, t, J =
51_
wo 96/04233 2 1 9 6 l 9 3 , ~ I ,~ s. . 1 7
3.55Hz.); 3.25-3.10(1H, m.); 2.74-2.53(2H, m.); 2.11-1.93(4H, m.); 1.67-1.59(2H,m.); 1.43-1.35(2H, m.); 1.13(3H, d, J = 6.32Hz.); 0.91(3H, t, J = 7.42Hz.).
Procedure 51: (SR)-4-{2-[3-(2,2-Di-t-butyl-4H-1,3,2 b~ o~ -6-yloxy)-
2-h.~d.u,.y~,.u,u.~- ~ ~]propyl}p' y~ yl (3-b...~ lu~,~t~u~
acid, n-butyl ester.
Me
O~--N~
~ OH
t-Bu/ ~t-Bu OC~Hg
The title compound was prepared from (R)-4-(2-alldllu~ulu~yl)~ u~ylllGLllyl
-(3-b~ ylur.y~lu,oyl)phn~rhinir acid, n-butyl ester and (S)-2,2-di-t-butyl-6-(oxiran-
2-ylmethoxy)-4H-1,3,2-l,~ .,,..,li..~ilinon-~ according to the method described in
Procedure 13. The crude product was purified by chromatography over silica gel
15 eluting with dichlu~u~ d~ G containing 3% methanol to give a viscous gum.
o(CDC13): 7.37-7.31 (5H, m.); 7. I l (2H, d, J = 8 52Hz.); 6.86(2H, d, J = 8.60Hz.);
6.83(1H, J = 8.8ûHz.); 6.72(1H, dd, J = 8.80, 3.30Hz.); 6.50(1H, d, J = 2.75Hz.);
4.94(2H, s.); 4.50(2H, s.); 4.21-3 88(8H, m.); 3 54(2H, m.); 2.92-2.66(5H, m.);
2.57(1H, dd, J = 13.47, 6.5ûHz.); 2.09-1.90(4H, m.); 1.77-1.60(2H, m.); 1.57-
1.38(2H, m.); 1.02(18H, s.); 1.06(3H, d, J = 6.25Hz.); ().92(3H, t, J = 7.14Hz.).
Procedure ~2: C,~ cl - ' ~1, ' , ' ~ ~ ~ acid, n-butyl ester
H--P~O
OC~H~
The title compound was prepared from cyclohexylrh()crhinin acid and n-butanol
according the the method described in Procedure 43. The compound was used without
30 furtherl.,.,;ri~li.."
-52-
~ wo 96/04233 21 ~ 3 P~ n~7
~(CDC13): 6.82(1H, d, J = 517.97Hz.); 4.17-3.92(2H, m.); 1.92-1.22(15H, m,);
0.94(3H, t, J = 7.43Hz.).
Procedure 53: Cyclohexyll. ~ l. u,.~ y', ' . ' ' - acid, n-butyl ester
H O ~ I 'O
OC~H~
10 The title compound was prepared from ~:yulollw~yl~ ' acid, n-butyl ester and
paraformaldehyde according to the method described in Procedure 31. Purification by
Y~ eluting with dichlulu~ lldne containing 5% methanol, gaYe an oil.
~(CDC13 + D2O): 4.13-4.93(2H, m.); 3.88-3.65(2H, m.); 1.97-1.22(15H, m.);
0.93(3H, t, J = 7.15Hz~).
Procedure 54: (4 f~1 ' ,1, ~ r yloxy).,~ - acid, n-butyl
ester
C1'[~11~~~ 11~
The title compûund was prepared frûm cyclohexyll~yd~w~ylll~.lly~ ln~ acid, n-
butyl ester and 4-chlol- ~b . ,,e"~ lforyl chloride according to the method described
25 in Procedure 32. The crude compound was used without further purifcation.
~(CDC13): 7.87(2H, d, J = 8.80Hz.); 7.57(2H, d, J = 8.80Hz.); 4.19(2H, d, J =
- 7.70Hz.); 4.12-3.81(2H, m.); 2.05-1.2û(15H, m.); 0.91(3H, t, J = 7.15Hz.).
Procedure 55: 4-(2-t-B.,lu,~y~rL ~' ~ ' ' yl)~ .ll4~ 1
.~. lc' ,~1, ' , ' - acid, n-butyl ester
-53-
wog6/0S233 21 961 q3
BOCNH /~
~f~
OC~Hg
The title compound was prepared from (4-Chlol- ~b ., ,. . IF ~ fonyloxy)
S ~,y~.loll~,~.yl~ l.h;~ acid,n-butylester and2-~4-hydroxyphenyl)ethylcarbamic
acid, t-butyl ester according to the method described in Procedure 24. The crudeproduct was purified by chromatography, eluting with dichlulu~ Lalle containing
3~O methanol, to give an oil.
~(CDC13): 7.13(2H, d, J = 8.g0Hz.); 6.88~2H, d. J = 8.80Hz.); 4.50(1H, s. exchanges
with D2O); 4.32-3.95(4H, m.); 3.34(2H, q, J = 7.15Hz.); 2.74(2H, t, J = 7.15Hz.);
2.07-1.47(15H, m.); 1.44(9H, s.); 0.92(3H, t, J = 7.43Hz.);
15 Procedure56:4.(2-Aminoethyl)r' ~ yl~,~cloh~ y','~ "'' '-acid,n-
butyl ester
H2N~
OC~H~
20 The title compound was prepared from 4-(2-t-butoxycdll)ullyldl..i',u~,l,yl)
ph~,u~ yll~,ihylcyclohexylrho~rhillic acid, n-butyl esler according to the method
described in Procedure 25. The crude product was used without further ~Julirh,d~
~(CDC13 + D2,O): 7.14(2H, d, J = 8.8()Hz.); 6.68(2H, d, J = 8.80Hz.); 4.26-3.98(4H,
m.); 2.96-2.90(2H, m.); 2.75-2.70(2H, m.); 2.12-1.26(15H, m.): 0.92(3H, t, J =
7.15Hz.);
-54-
21 q61 93
~ wo 96/04233 r ~ I / 1 7
Procedure 57: (S)- 4-{2-[3-(2,2-Di-t-butyl-4H-1,3,2-b~..zodiuA,I .ili--an-6-yloxy)-2-
Il~Jl~ JtJ,~]ethyl}, ' ~ n;racjd"z-butyl
ester
~ ---H~
~3 OH [~1
S~ OC~Hg
t-Bu t-Bu
The title compound was prepared from 4-(2-aminoethyl)phenoxypropyl
methylcycloh~,.yll,l,r.~l,l.;";~ acid, n-butyl ester and (S)-2,2-di-r-butyl-6-(oxiran-2-
ylmethoxy)-4H-1,3,2-b~n7~ ilin~n- according to the method described in
10 Procedure 13. The crude product was purified by chromatography over silica gel
eluting with dichlu.ul.l~.l.a..e containing 3% methanol to give a viscous gum.
o(CDC13 + D2O): 7.14(2H, d, J = 8.56Hz.); 6.88(2H, d, J = 8.65Hz.); 6.82(1H, d, J
= 8.79Hz.); 6.72(1H, dd, J = 8.78, 3.00Hz.); 6.50(1H, d, J = 2.95Hz.); 4.95(2H, s.);
4.26-4.11(4H, m.); 4.09-3.95(3H, m.); 3.89(2H, d, J = 5. I lHz.); 2.92-2.83(2H, m.);
2.77-2.72(2H, m.); 2.03-1.93(3H, m.); 1.93-1.83(2H, m.); 1.72-1.61(4H, m.); 1.51-
1.40(4H, m.); 1.39-1.21(2H, m.); 1.03(18H, s.); 0.91(3H, t, J =7.39Hz.).
Procedure 58: (S)-4-{2-[3-(4-B~--,,~, IUA,~ I ' y)-2-
II~J.oA~y~J-u~ ~ ]ethyl}, '~ ~ acid, n-butyl
er,ter
OH
OC4Hg
The title compound was prepated from 4-(2-aminoethyl)phenoxy
W0 96/04233 2 19 6 19 3 r~ Q~7 --
ll~cLLylcy~,lullcr.~ .o~ . acid, n-butyl ester and (S)-2-(4-
benzyloxyphenoxymethyl)oxirane according to the method described in Procedure
13.
~(CDCl3): 7.44-7.26 (5H, m), 7.14 (2H, d, J = 8.8Hz), 6.91-6.80 (6H, m), 5.01 (2H,
s), 4.25-4.0 (5H, m), 3.91 (2H, d, J = SHz), 3.0-2.75 (6H, m), 2.1-1.25 (15H, m), 0.92
(3H, t, J =7.4Hz)
Procedure59: (S)-4-{2-[3-(3-B~ lu/~yl ' y)-2-hyJlu~ u~ ]
ethyl}l~ tl-yl~.~cr ' yl~! , ' ~ ~ acid, n-butyl ester
~--H~
,~ OH
OC4Hg
15 The title compound was prepared from 4-(2-aminoethyl)l,l,~v~y~ yl--,.l,yl
cyclohexy~ acid, n-butyl ester and (S)-2-(3-
benzylu~y~ .,u,~y,.,~,~l.yl)oxirane according to the method described in Procedure
13.
o(CDC13): 7.5-7.3 (SH, m), 7.2-7.1 (3H, m), 6.88 (2H, d, J = 8.5Hz), 6.65-6.45 (3H,
m), 5.04 (2H, s), 4.25-3 9 (7H, m), 2.95-~7s (6H, m), 2.1-1.25 (ISH, m), 0.92 (3H,
t, J = 7.2Hz).
Procedure GO: (R)-4-~2-t-Butoxya~ - upyl)l h y yl-
cyclohexyl, ' , ' ~ ~ acid, n-butyl ester
-56-
~ Wo 96/04233 2 1 9 6 1 ~ 3 ~ s~ ~n~7
Me
BOCNH ~,
OC~H~
The title compound was prepared from (4-chlorophenylsulfonyloxy)
~,loL~.yl~ l;n acid, n-butyl ester and (R)-2-(4-hydroxyphenyl)-1-
5 methyL~ ylcallJd~ acid, t-butyl ester according to the method describcd in
Procedure 24. The crude product was purified by l,hlulll.l~u~,ldlllly, eluting with
di~ lululll~,,lldllc containing 39'o methanol, to give an oil.
o(CDC13): 7.10(2H, d, J = 8.53Hz.); 6.87(2H, d, J = 8.60Hz.); 4.34-3.85(6H, m. IH
lû exchanges with D2O); 2.78(1H, dd, J = 13.74, 5.49Hz.); 2.60(1H, dd, J = 13.48,
7.43Hz.); 2.04-1.13(15H, m.); 1.42(9H, s.); 1.07(3H, d, J = 6.87Hz.); 0.92(3H, t, J =
7.42Hz.).
15 Procedure 61: (R)-4-(2-~ r Ul~l)t~l y '' ~ lc' ,~,ll ' ~, ' ~ ~ acid,
n-butyl ester
Me
H2N
OC~H~
20 The title compound was prepared from (R)-4-(2-t-butoxycarbon~ u-iln)~,-ul,yl)phcu~)~y~ ,d~ylcyclohexylphosphillic acid, n-butyl ester according to the methoddescribed in Procedure 25. The crude product was used without further ~ul;fi~,dLiull.
o(CDC13): 7.13(2H, d, J = 8.8()Hz.); 6.88(2H, d~ J = 8.80Hz.); 4.23-4.02(4H, m.);
3.20-3.12(1H, m.); 2.68(1H, dd, J = 13.48, 5.50Hz.); 2.54(1H, dd, J = 13.47,
-57-
21 96l 93
wo 96/04233 ~ n~7
7.70Hz.); 2.06-1.21(17H, m. 2H exchanged with D2O); 1.14(3H, d, J = 632Hz.);
0.92(3H, t, J = 7.15Hz.).
Procedure 62: (sR)-4-{2-[3-(272-d~ Butyl-4H-l~3~2-ben7F)~ ' 6 yloxy)-2-L.~d~v/~.~ylv~]propyl}~ th.~l.. r~'e' .rlp!T ,' -acid, n-
butyl ester
Me
~--H~
OH ~
\ /0 O~P
/ ~ OC4Hg
t-Bu t-Bu
The title compound was prepared from (R)-4-(2-aul;llv~lu~u.yl)
pll~.lv~-y~ ,llyl~y~,lOll~ yl,ullua~llillic acid, n-butyl ester and (S)-2,2-di-t-butyl-6-
(oxiran-2-ylmethoxy)-4H-1,3,2-bF, ,.-(I;v~c;lin~nF according to the method
described in Procedure 13. The crude product was purified by clll~ t( l~;. ,.lll ly over
silica eluting with dichl~ J(IlcLha~o, containing 3% methanol to give a viscous gum.
~(CDC13 + D2O): 7.10~2H, d, J = 8.53Hz.); 6.87(2H, d, J = 8.80Hz.); 6.83(1H, d, J
= 8.80Hz.); 6.70(1H, dxd, J = 8.80 & 3.û2Hz.); 6.50(1H, d, J = 3.02Hz.); 4.94(2H,
s.); 4.26-3.88(7H, m.); 2.91-2.53(5H, m.); 2.05-1.26(15H, m.); 1.06(3H, d, J =
6.32Hz.); 1.02(18H, s.~, 0.92(3H, t, J = 7.43Hz.).
Procedure 63: n-Hexylp' , ' ~ - ncid
1~l
H--I--C6H~3
OH
The title compound was prepared from n-hexene and 50% aqueous phosphinic acid
by an analogous procedure to that described in J. Inorg. Nucl. Chem., 1965, 27, 697.
-~8-
21 961 93
~ WO 96104233 P~ l 75'l'~n~7
~CDCI3): 12.10(1H, s, exchanges with D2O); 7.08(1H, dd, J = 540.10, 1.93Hz.);
I.X2-1.51(4H, m.); 1.42-1.23(6H, m.); 0.87(3H, t, J = 6.87Hz.).
S Procedure 64: n-HcA~lpl , ' ~ acid, n-butyl ester
1~l
H--P--C6H
OC4H3
The title compound was prepared from n-h~;~yll)h~ l;r acid and n-butanol
10 according the the method described in Procedure 43. The compound was used without
further purification.
o(CDC13): 7.08(1H, d, J = 525.92Hz.); 4.12-3.98(2H, m.); 1.80-1.26(14H, m.); 0.97-
0.86(6H, m.).
Procedure65: n-Hc,~lh~dlu~.ll..h,~l~ I . ' ' ' e acid, n-butyl ester
HO~ I--CCHl3
OC~Hg
The title compound was prepared from n-he~y~ hl~;n acid, n-butyl ester and
paraformaldehyde according to the method described in Procedure 31. Purification by
.,I"~ alJhy, eluting with dichloromethane containing 5% methanol, gave an oil.
o(CDCI3): 4.09-3.99(3H, m.); 3.89-3.79(2H, m.); 1.83-1.75(2H, m.); 1.69-1.46(4H,m.); 1.43-1.29(8H, m.); 0.96-0.86(6H, m.).
Procedure 66: 4-Chlol ob~ onyloxymethyl-rl-hexylphosphinic acid,
30 n-butyl ester
f~ O
Cl~ ~--O~ I--C6H13
~ OC4H3
Wo 96/04 A73 2 l 9 6 ~ 9 3 ~ 5 ~7
The title compound was prepared from n-hexylhydroxymethylphosphinic acid,
n-butyl ester and 4-chlu-ubt ", . .~ I rullyl chloride and according to the procedure
described in procedure 32. The crude compound was used without further
purification.
o(CDC13): 7.89(2H, d, J = 8.88Hz.); 7.57(2H, d, J = 8.80Hz.); 4.25-3.84(4H, m.);2.04-1.23(14H, m.); 0.97-0.86(6H, m.).
Procedure 67: (R)-4-(2-t-BuluA~ iJu~ ' ' , u~7.Tl)pl y".ell,.~l-n-
~'j ' . ' ~ acid, n-butyl ester
Me
BOCNH ~
1~l
o B--c H
OC~Ha
15 The title compound was prepared from 4-chlw,~ ,lfonyloxymethyl-n-
hc~.yl~ acid, n-butyl ester and (R)-2-(4-hyd-u~-y~ht,-~yl)-l-methylethyl-
carbamic acid, t-butyl ester according to the method described in Procedure 24. The
crude product was purified by chromatography, eluting with dichlulul.l.d
containing 3~0 methanol, to give an oil.
o(CDC13):7.11(2H,d,J=8.8()Hz.);6.87(2H,d,J=8.52Hz.);4.21-4.00(4H,m.):
3.83(1H, s.); 3.41(1H, m.); 2.77(1H, dd, J = 13.83, 5.58Hz.); 2.58(1H, dd, J = 13.76,
7.12Hz.); 1.89-1.84(2H, m.); 1.68-1.60(6H, m.); 1.44-1.39(2H, m.); 1.43(9H, s.);1.38-1.25(4H, m.); 1.07~3H, d, J = 6.95Hz.); 0.94-0.85(6H, m )
Procedure 68: (R)-4-(2-Aminopl-opyi)l]lu .r---.lbyl ,7l-b ~ - acid,
n-butyl ester
-60-
2l 96~ 93
~ Wo 96/04233 P~~ 7
Me
-
~ 1~l
0~ 1--C6H13
OC4HG
The title compound was prepared from (R)-4-(2-t-l,utuAyu~bu,.ylaminopropyl)
IJh~,lluAy~ .illyl-n-l..,Ayl~ l;o acid, n-butyl ester according to the method
S described in Procedure 25. The crude product was used without further ~.u, ;r~ ivn.
~(CDC13): 7.12(2H, d, J = 8.53Hz.); 6.89(2H, d, J = 8.80 Hz.); 4.22-3.86(6H, m. 2H
exchanges with D2O); 3.24(1H, q, J = 6.59Hz.); 2.70(2H, d, J = 6.88Hz.); 1.95-
1.84(2H, m.); 1.71-1.58(4H, m.); 1.47-1.25(8H, m.); 1.18(3H, d, J = 6.32Hz.); 0.95-
10 0.84(6H, m.).
Procedure 6g: (SR)-4-{2-[3-(2,2-Di-f-butyl-4H-1,3,2-t " ' 6 yloxy~-
2 h.~diur.~l UiJ,~; ~ -]propyl}p' .~ '' yl n ' .~ , ' ' - acid, n-butyl
15 ester
Me
~~ H~
~ OH
O~ ,0 0~ 1--C6H,3
~S, OC~H2
t-Bu t-Bu
The title compound was prepared from (R)-4-(2-aminopropyl)~ ,noAy",.,il,yl-n-
20 h~,Ayl~ ;";~~ acid n-butyl esler and (S)-2,2-di-t-butyl-6-(oxiran-2-ylmethoxy)-
4H-1,3,2-b.-n7-)~1ioY~c;lin~n~- according to the method described in Procedure 13. The
crude product was purified by ~i,.,.",~ ,..rl,Y over silica gel eluting with
dichlu,..."~ ..c containing 3~o methanol to give a viscous gum.
-61 -
21 961 93
wo 96/04233 r ~ ,s,~ n~
~(CDC13): 7.12(2H, d, J = 8.53Hz.); 6.87(2H, d, J = 8.80Hz.); 6.72(1H, d, J =
8.80~z.); 6.72(1H, dd, J = 8.80, 3.03Hz.); 6.50(1H, d, J = 3.03Hz.); 4.92(2H, s.);
4.27-3.86(8H, m. 2H exchange with D2O); 2.93-2.86(1H, m.); 2.84-2.63(2H, m.);
2.57(1H, dd, J = 13.47, 6.59Hz.); 1.96- 1.84(4H, m.); 1.71 - 1.58(4H, m.); I .47-
1.15(8H, m.); 1.06(3H, d, J = 6.32Hz.); 1.03(18H, s.); 0.97-0.85(6H, m.).
Procedure70: (S)-1-(4-B~ .ylu~ p'll y)-3-[N-2-(4-
L.r~l.o..~l ' yl)ethylamino]propan-2-ol.
~--H
OBn OH
The title compound was prepared from ~yramine and (S)-2-(4-
benzyloxy~ ,.,uAy~ Ll,yl)oxirane according to the method described in Procedure
15 13.
~(d6-DMSO): 9.3-8.9 (lH, b, exchanged with D2O)7 7.5-7.25 (5H, m), 6.98 (2H, d, J
= 8.6Hz), 6.92 (2H, d, J - 9Hz), 6.83 (2H, d, J = 9Hz), 6.65 (2H, d, J = 8.6Hz), 5.02
(2H, s), 4.9 (lH, b, exchanged with D2O), 3.9-3.75 (3H, m), Z.75-2.55 (6H, m).
Procedure 71: (S)-N-Benxyl-1-(4-benxyloxyphenoxy)-3-[N'-Z-(4-
d- u~ I)ethylamino]propan-2-ol.
O----N
~3 OH
OBn OH
A solution of (S)-1-(4-benzyloxyphenoxy)-3-[N-2-(4-hydroxyphenyl)
ethylamino]propan-2-ol (1.9g, 4.8mMol) and benzyl bromide (0.57ml, 4.8mMol) in
dill~ hylrullllalll;de (lOml) containing sodium carbonate (770mg, 7.2mMol) was
30 stirred at room t~ ,. alu,e for 18 hours. The mixture was filtered and the residue
was washed with ethyl acetate. The f ltrates were combined, washed with water and
-62-
21 961 93
~wo 96/04233 ~ 5/(~ 7
brine, dried and evaporated. Purification of the residue by flash chromatography(silica gel, 50% ethyl aceta~e in hexane) gave the title compound.
o(CDC13 + D2O): 7.5-7.25 (IOH, m), 6.96 (2H, d, J = 8.5Hz). 6.88 (2H, d, J =
9.1Hz), 6.79 (2H, d, J = 9.1Hz), 6.71 (2H, d, J = 8.5Hz), 5.01 (2H, s), 4-3.78 (4H,
m), 3.59 (IH, d, J = 13.5Hz), 2.9-2.6 (6H, m).
Procedure72: (S)-N-Benzyl-4-{2-[-3-(1 t ~lu~ )-2-
l.~.' u.. ~,.o,.. ,' ~]ethyl~r ~..... lh.yl n '~ 5pl ~ -~acid, n-butyl
ester
O--~N
Bn
~3 OH [~1
OBn o~ I--C6H13
OC4H9
15 The title compound was prepared from (S)-N-benzyl-1-(4-benzylvAy~l.c.lv~ y)-3-[N-
2-(4-h~y,l,v~y~henyl)ethylamino]propan-2-ol and 4-chlvlvb~ ,.,. ." ~ Ifonyloxy
methyl-n-h~,~y~ v~ acid, n-butyl ester according to the method described in
Procedure 24.
~(CDC13 + D2O): 7.45-7.25 (IOH, m), 7.04 (2H, d, J = 8.5Hz), 6.9-6.78 (6H, m),
5.01 (2H, s), 4.2-3.83 (8H, m), 3.54 (IH, d, J = 12.3Hz), 2.9-2.6 (6H, m), 1.9 (2H,
m), 1.65 (4H, m), 1.5-1.25 (8H, m), 0.95-0.85 (6H, m).
Procedure 73: P' ~ p~ ~ acld, bis-(2-phenylethyl) ester
~0~ ,H
~ a
--2
The title compound was prepared from 2-pl~ yl~ ol and pllvalJllvl us tribromide
according to the method described in Procedure 31. Purification by Uh~ hY
on silica-gel eluling with 5% methanol in dichloromethane gave the title compound as
an oil.
-63-
, .. . . .
wo96/04233 21 9 61 q3 r~ I,rl~ 7 ~
o(CDC13): 7.92-5.32 ~lH, d.); 7.16-7.33 (I()H, m.); 4.10-4.28 (4H, m.); 2.92-3.04
(4H, t.)
Procedure 74: H~d~u~ .Ylt 'm . ' - acid, bis-(2-phenylethyl) ester
- ~~'e~OH
--2
10 The title compound was prepared from pho~B,ù,,ic acid, bis-(2-phenylethyl) ester and
paraformaldehyde according to the method described in Procedure 31. Purification by
column ulllu~ ;u~ ll,y on silica-gel in 2-5% methanol in dichlulu-l.. d,~l.,e gave the
title compound as an oil.
o(CDC13): 7.17-7.33 (IOH, m.); 4.15-4.30 (4H, m.); 3.70-3.74 (2H, t.); 2.86-2.96(4H, m.)
Procedure 75: (4_('1 - ub.. ~ ~lru~yloxymethyl)l ' ' ~ acid, bis-(2-
~- yl~ yl) ester
- _ lol,~CI
~~ e~o'll
-~ -2~
The title compound was prepared from hydroxymethylphosphonic acid, bis-(2-
~LC~YI~LIIYI) ester and 4-chloroben~ nr~lllrhr~nyl chloride according to the method
described in Procedure 32. The crude product was used in the next stage without
further pllrifir~til)n
o(CDCl3): 7.75-7.90 (2H, d.); 7.49-i.52 (2H, d.); 7.13-7.33 (lOH m.); 4.15-4.23
(4H, m.); 4.02-4.05 (2H, d.); 2.88-2.95 (4H, m.)
Procedure 76: (S)-4-(2-t-BI.lu~.~wr- J r o,u,rl)pl yu.cthyl
p'l~ - acid, bis-(2-phenylethyl) ester
-64-
21 961 q3
~ WO 96104233 ~ ''Q~Q~7
Me
EOCNH~
The title compound was prepared from (4-chlul-,b~ fonyloxymethyl)5 r~o~ acid,bis-(2-phenylethyl)esterand (R)-2-(4-hydlu~-ylul~ yl)-1-
methy~ lyl~ c acid, ~-batyl ester accûrding to the method described in
Procedure 24. Purification by column ~.hl~ ,hy on silica-gel in 1-2% methanol
in dichlululll~llallc gave the title compound as a gum.
10 o(CDCl3): 7.19-7.27 (lOH, m.); 7.07-7.17 (2H, d.); 6.78-6.81 (2H, d.); 4.26-4.30
(4H, m.); 4.03-4.06 (2H, d); 3.75-3.90 (IH, s. exchanges with D20); 2~93-2.98 (4H,
t.); 2.52-2.81 (3H, complex m.); 1.43 (9H,s.); 1.05-1.07 (2H, d.).
Procedure 77: (R)-4-(2-A ~ . acid, bis-(2-
p! yl.lh~l) ester
Me
H2N~
o~3
20 The title compound was prepared frûm (R)-4-(2-t-butoxycarbonyl
alll;.lu~lul,yl)phenoxymethyl pho~pllo"ic acid, bis-(2-phenylethyl) ester according to
the method described in Procedure 25. The crude product was used in the next stage
without further purification.
25o(CDC13): 7.07-7.30 (12H, complex m.); 6.78-6 83 (2H, d); 4.22-4.32 (4H, m); 4.03-
4.07 (2H, d.); 2.65-3.25 (9H, complex m, 2H exchange with D20); 1.17-1.20 (3H, d).
-65-
, _,, . , .. . . , _ . , , _ .. ... .. .
wo 9~/04233 2 l , 6 l p . /~1 ~7 ~
Procedure 78: (SR)-4-{2-[3-(2,2-di-~-Butyl-4H-1,2,3 b~n7~ n 6-yloxy)-
2-h,~d~ y~o~ l"mino]propyl}l)~ y ''Ylr' .' :~acid,bis-(2-
5 p~ yl.tl,yl) ester
Me
H
[~3 1~l ~J3
~'Si'O ~~P'O _2
The title compound was prepared from (R)-4-(2-~ul~h~ u~yl)phenoxymethyl
phocrhr~nir acid, bis-(2-phenylethyl) ester and (S)-2,2-di-r-butyl-6-(oxiran-2-
ylmethoxy)4H-1,3,2-l ..,,..,li-,~ ~;lin5~nr~ according to the method described in
Procedure 13. The crude product was purified by . hlullldtc.~ lly on silica-gel in 1-
5r~c methanol in dichl~.lvlll~nlla.,-, to give the title compound as a gum.
~(CDCl3): 7.20-7.30 (IOH, complex m.); 7.07-7.18 (2H, d.); 6.68-6.84 (4H, complex
m.); 6.49-6.50 IIH, d.); 4.94 (2H, s.); 4.22-4.33 (4H, m.); 4.04-4.08 (2H, d.); 3.97-
4.04 (IH, m.); 3.86-3.89 (2H, m.); 2.93-~98 (4H, m.); 2.56-2.89 (3H, complex m.);
1.07-1.10 (3H, d.); 1.03 (18H, s.)
Procedure 79: Bern~,yl! ' , ' ' '- acid, n-butyl ester
1~l ,l3
H--P
OC~H~
A mixture of ~mmr\ninm p~ l,;r".l~ (9.18g) and hexamethyldisilazane (25mL) was
heated at 110~C for 2 hours. The mixture was cooled in ice, dissolved in dry
dichlu,o,l,~"l"l"c (120mL), ben_yl chloride (20g; 14mL) was added and the mixture
allowed to warm to room le~ ul~ and stirred 18 hours. The solution was filtered,the solvent evaporated, the residue azeotroped with methanol (2x70mL), dissolved in
toluene (ISOmL) containing n-butanol (30mL) and the solution was boiled under
-66-
.... ..... ... . .. ...... .. . . .. ... . ..... .. . . . _ . _ . . _ ..
21 961 93
w0 96/04233
reflux in a Dean and Stark water trap for 5 hours. The solvent was evaporated, the
residue slurried with dichloromethane (120mL), filtered and evaporated and the
residue l,Ll~ dLv~la~ cd on silica-gel in 1-2% methanol in dichl.Jl.,ll,~,~h~llc to give
the title compound as an oil.
S
o(CDC13): 8.05-6.03 (IH, d.); 7.24-7.36 (5H, complex m.); 3.88-4.15 (2H, dd.);
3.16-3.24 (2H,d.); 1.57-1.67 (2H, m.); 1.27-1.41 (2H, m.); 0.87-0.93 (3H, t.)
10 Procedure 80: Bc..~ yJl o,.y 'byl~ '~ acid, n-butyl ester
HC~ P~ ~3
OC4Hg
The title compound was prepared from benzyirhrJcrhinir acid, n-butyl ester and
15 ~ ar~llllaldcllyde according to the method described in Procedure 31.
o(CDC13): 7.21-7.35 (5H, s.); 3.5-4.5 (lH, s, exchanges with D2O); 3.93-3.98 (2H,
q.); 3.77-3.78 (2H, d.); 3.22-3.28 (2H, d.); 1.52-1.63 (2H, m.); 1.25-1.39 (2H, m.);
0.86-0.91 (3H, t.)
Procedure81: Benzyl(4-. ' '~ ,b ~ hyl)~ -acid,
n-butyl ester
Cl ~ ll OC,Hg
The title compound was prepared from benzylhydroxylll~dly~ nir acid, n-butyl
ester and 4-chlul.l.- .,,. ~r ~,lfonyl chloride according to the method described in
Procedure 32. The resulting white solid (mp 87-88~C) was used in the next stage
30 without further purification.
o(CDC13): 7.81-7.85 (2H, d.); 7.59-7.62 (2H, d.); 7.19-7.28 (5H, m.); 3.85-4.18 (4H,
complex m.); 3.19-3.26 (2H, d.); 1.52-1.63 (2H, complex m.); 1.25-1.39 (2H,
complex m.); 0.87-0.92 (3H, t.)
-67-
2l 961 93
wo 96/04233 A ~ Q~~
Procedure 82: 4-[2-(Sl-(2-t-Butoxycarbonylarnino)propyl]-
p~ lb~l.,"~l, ' . '' ~~ acid, n-butyl ester
Me
BOCNH
~ 8~3
o~, I
S OC~Hg
The title compound was prepared from benzyl(4-chlulub~ ", .,.~~~..lfonyloxy
methyl)l.l.u,~,hi,,ic acid, n-butyl ester and (R)-2-(4-hydroxyphenyl)-1-
methyl~,ll,yl~alL,al,li~ acid, t-butyl ester according to the procedure described in
10 Procedure 24. The crude product was chromatographed on silica-gel in 2% methanol
in dichlu.u,.l~..L~A"c to give a gum.
~(CDCl3): 7.21-7.29 (SH, s.); 7.09-7.13 (2H, d.); 6.83-6.87 (2H, d.); 4.01 -4.11 (3H,
m.); 3.30-3.38 (2H, dd.); 2.88-2.96 (2H, d.); 2.61-2.96 (2H, complex m.); 1.59-1.65
lS (2H, m.); 1.34-1.43 (IIH, complex m.); l.OS-I.09 (3H, d.); 0.87-0.92 (3H, t.)
Procedure 83: (R)-4-(2-Aminopropyl)~ .... lb,~ l- , ' ' .~ acid,
n-butyl ester
Me
H2N ~
OC4Hg
The title compound was prepared from (R)-4-[2-(2-t-butoxycarbonylamino)
propyl]phenoxymethylbenzylphocphini.~ acid, n-butyl ester according to the method
25 described in Procedure 25. The crude product was used in the nexl stage without
further ~uliric.~Aliull.
-68-
21 961 93
wo 96/04233
o(CDC13): 7.22-7.29 (5H, s.); 7.11-7.]9 (2H, d.); 6.84-6.88 (2H, d.); 3.99-4.12 (3H,
complex m.); 3.30-3.38 (2H, complex m.); 3.10-3.17 (2H, complex m.); 2.44-2.70
(2H, complex m.); 1.57-1.65 (2H, complex m.); 1.23-1.44 (2H, complex m.); 1.10-
1 13 (3H, d.); 0.87-0.94 (3H, t.)
Procedure84: (SR)-4-[2-[3-(2,2-di-t-Butyl-4H-1,3,2-b. _ ' " ~~ 6 yloxy)-
2-IIY~I~U~Y~J~U~ ~]propyl~p'~ y 'ylbenzylp'- ,'' ' acid,n-butyl
ester
Me
~--N~3
~~Si~O O~P
~ ~< OC~Hg
The title compound was prepared from (R)-4-(2-a~ o~ Jyl)l)ll~llu~ylll~illyl
b~ yl~ n acid n-botyl ester and (S)-2,2-di-t-butyl-6-(oxiran-2-ylmethoxy)-
4H-1,3,2-L.~ cilin~ln~ according to the procedure described in Procedure 13.
The crude product was porified by ~ , 0 a~hy on silica-gel in 2-5% methanol in
dichloromethane to give a gum~
o(CDC13): 7.26-7.28 (5H, s.); 7.10-7.13 (2H, d.); 6.65-6.86 (4H, complex m.); 6.50
(lH, d.); 4.94 (2H, s.); 4.07-4.19 (4H, complex m.); 3.89 (2H, s.); 3.27-3.35 (2H,
dd.); 2.55-3.08 (4H, complex m.); 1.60-1.72 (2H, m.); 1.28-1.45 (2H, m.); 1.03-1.08
(23H, complex m.); 0.86-0.91 (3H, t.).
Procedure85: 4~(2-fert-Butoxycarbonyl~ ~ ' yl)l ' y ' ~', ' ~1-
p~ acid, ethyl ester
-69-
21 9~1 93
wo 96/04Z33 -
Boc
~ 1~l
O~ P--OEt
Ph
The title compound was prepared from 4-chl~,-ut,f .,,..,. ~ fullyloxymethylphen
phosphinic acid, ethyl ester (4.97g, 13.3mMol) and 2-(4-hyd~u~yluh~"lyl)ethyl-
carbamic acid, tert-butyl ester (3.0g, 12.7 mMol) by the method described in
5 Procedure 24 as a colourless gum.
~IH (200MHz, CDCI3): 7.92 (2H, m), 7.66-7.42 (3H, m), 7.08 (2H, d), 6.83 (2H, d),
4.6-4.0 (SH, m), 3.31 ~f2H, m), 2.71 (2H, t), 1.42 (9H, s), 1.38 (3H, t, partially
overlapping the signal at 1.42).
Procedure 86: 4-(2-AminoethYl)r: ,~, ' ylphenylp'~ acid, ethyl
ester
H2N ~
l~
~ 10
O~P--OEt
Ph
The title compound was prepared by a method similar to that described in Procedure
2~ from4-(2-tert-l~ulu:~y~ ollylaminoethyl)phenoxy~ lylp~ y~ f acid,
ethyl ester (3.197g, 7.63mMol), giving a very pale yellow gum.
~'H (250MHz, CDCI,): 7.93 (2H, m), 7.68-7.42 (3H, m), 7.09 (2H, d), 6.83 (2H, d),
4.5-4.0 (4H, m), 2.90 (2H, t), 2.67 (2H,1), 1.38 (3H, t with overlapping 2H).
Procedure 87: (S)-4-{2-[3-(2,2-Di-tert-butyl-4H-1,3,2-t . ' "' 6 yloxy)-
2-L,~IIu~.~t~up~' ' -]ethyl}phenoxymethylphenylFI . ' ~ acid,ethyl ester
-70-
2 1 96 1 93
~ WO 96/04233 r~ I ~ 5!~ ~7
~~~H~
3 1~l OE
0~ ~0 O~P~
xsJ<
The title compound was prepared as a colourless gum by a method similar to tha
described in Procedure 13 from 4-(2-aminoethyl)~h~,.uA y.ll~,.l,yll~he,lyll~h. '~l .h h. i~
acid, ethyl ester (2.32g, 8.06mMol) and (S)-2,2-Di-ser/-butyl-6-(oxiran-2-
S ylmethoxy)-4H-1~3~2-brn7o~tir~YA~ilhA~ (Ig, 2.98mMol).
~'H (250MHz, CDCI,~: 7.93 (2H, m), 7.65-7.44 (3H, m), 7.10 (2H, d), 6.88-6.77
(3H, m), 6.71 (IH, dd), 6.50 (IH, d), 4.93 (2H, s), 4.49-3.92 (5H, m), 3.88 (2H, d),
2.94-2.68 (6H, m), 2.04 (2H, br s), 1.38 (3H, t), 1.02 (I 8H, s).
Procedure 88:(S)-4-{2-[3-(4-BL.,~ lUA~y,Ullenoxy)-2-
d~UA;.~ o~ laminû]ethyl}~l- y -'hylr' ,~ -acid,ethylester
O--N~
[~1 OH ¢~
Il ,Ph
OBn O~ 'OEt
The title compound was prepared from 4-(2-aminoethyl)phenoxymelhyl-
pll~,lyl~ h;~ acid, ethyl ester and (S)-2-(4-benzyloxyphenoxy)methyloxirane
according to the method described in Procedure 13.
o'H (250MHz, CDC13): 7.9-7.8 (2H, m); 7.55 (3H, m); 7.5-7.25 (5H, m); 7.10 (2H,
d, J=8.0Hz); 6.90-6.70 (6H, m); 4.98 (2H, s); 4.5-4.0 (5H, m); 3.89 (2H, d, J=6Hz);
- 3.0-2.75 (6H, m); 1.45 (3H, t, J=7.3Hz).
Procedure 89: (S,1~)-4-{2-[3-(4-BenixyloAyl~!~ y)-2-
ll~dluA~ur(~t~Y' ]propyl}p'- y~ lhylphenylpl , ' - acid, ethyl ester
2 ~ 9 6~ ~, 3
WO 96/04233 ~ 1 1 ~ 7 IA'~ 7
Me
O--H~
Il ,OEt
OBn O~--P~Ph
The title compound was prepared from (R)-4-(2-aminopropyl)phenoxy-,--~,il,~ll.l-.,-~l
phosphinic acid, ethyl ester and (S)-2-(4-benzyloxyphenoxy)-methyloxirane
according to the method described in Procedure 14.
~IH (250MHz, CDC13): 7.92 (2H, m); 7.55 (3H, m); 7.4 (3H, m); 7.35 (SH, m); 7.07(2H, d, J=8.2Hz); 6.82 (3H, m); 5.02 (2H, s); 4.5-4.07 (4H, m); 3.91 (3H, m); 2.95-
2.49 (5H, m); 1.37 (3H, t, J=7.1Hz); 1.07 (3H, d, J=7.4 Hz).
Procedure 90: 1-(4-Benzyloxy-3-nitrophenyl)ethanone
Oq/
~NO2
OBn
A mixture of 1-(4-hydroxy-3-nitrophenyl)ethanone (lOg, 55.2mMol) andpotassium
carbonate (11.5g, 82.8 mMol) in acetone (150 ml) was heated at reflux for 10 mins.
Bc..~lbl~,l..;de (6.6 ml, 55.2 mMol) was added and the mixture heated at reflux for
48 hours. After cooling, the reaction mixture was filtered and the solvent evaporated
15 in vacr~o. The residue was dissolved in ethyl acetate. washed with water and brine,
dried and the solvent evaporated to give the title compound as an oil.
olH (250MHz, CDC13): g.42 (IH, d, J=1.4Hz); 8.11 (IH, dd, J=8.2Hz and 1.4Hz);
7.4 (SH, m); 7.18 (IH, d, J=8.3Hz); 5.32 (2H, s); 2.60 (3H, s).
Procedure 91: Acetic . cid, ~ I b.n~.~lo~ -3-nitrophenyl)ester
21. 96~ 93
~ w0 96/04~33 r~ 7
NO2
OBn
A mixture of 1-(4-benzyloxy-3-nitrophenyl)ethanone (6g, 22.1mMol) and 3-
chlu~uy~,lu~;yb~ Lu;c acid (19.1g, 110.7mMol) in dichloromethane (ISOml) was
heated at reflux for 72 hours. After cooling, the reaction mixture was filtered and the
S organic phase washed with saturated sodium carbonate (2x25ml), water (30ml) and
brine (30ml). The solution was dried and the solvent evaporated in vaCuo. The
residue was purified by normal phase column chromatography eluting with 40%
diethyl ether in hexane to give the title compound as a clear oil.
o'H (250MHz, CDCI,): 7.68 (IH, d, J=1.6Hz); 7.4 (5H, m); 7.26 (IH, dd, J=8.2Hz
and 1.4Hz); 7.10 (IH, d, J=8.2Hz); 5.24 (2H, s); 2.31 (3H, s).
Procedure92: Acetic acid,(3-~ ; 1 b .~lu~ yl)ester
O~
~ NH2
OB~
Acetic acid, (4-benzyloxy-3-nitrophenyl)ester (3g, 10.45mMol) was dissolved in
methanol (30ml) and hyd~u5,~l~. t~,d at aLulO~yll~ c pressure and room tc~llyl ~a~ulc~
with platinum (IV) oxide for 30 houfs. The mixture was fillered through ftlteraid and
the solvent evaporated in vacuo to yield a dark oil.
o'H (200MHz, CDC13): 7.35 (7H, m); 6.82 (IH, d, J=8.8Hz); 6.48 (IH, d, J=1.5Hz);6.40 (lH, dd, J=6.6Hz and 1.3Hz); 5.05 (2H, s); 2.23 (3H, s).
Procedure 93: Acetic acid (4-benzyloxy-3 ~tt- -- l~ F' ~I)ester
- 25
w0 96/04233 ~ l ~ 6 1 Cj 3 F~ n~7 ~
MeSOz~N $
H OBn
Acetic acid, (3-amino-4-benzyloxyphenyl)ester (1.70g, 6.61mMol) in
di~ u~ àlle (35ml) was treated with triethylamine (0.802g, 7.93mMol) and
rullyl chloride (0.832g, 7.27mMol) and the mixture stirred at room
S l~ ,.aiLllc under argon for 20 minutes. The mixture was washed with water
(3xlOml), dried and the solvent evaporated in vacuo. Trituration with diethyl ether
gave the tide compound as an off-white solid.
o'H (t50MHz, CDC13): 7.40 (SH, m); 7.33 (IH, d, J=2.2Hz); 6.96 (IH, d, J=8.5Hz);6.88 (IH, br s); 6.84 (lH, dd, J=8.2Hz and 2.1Hz); 5.08 (2H, s); 2.94 (3H, s); 2.31
(3H, s).
Procedure 94: Acetic acid, 4-benzyloxy-3-(N-tert-~ ~.dl l,ul,~l)methane-
yl ester
1~
MeSOz~ ~
BOC OBn
To a solution of acetic acid, (4-benzyloxy-3-mprh~npslllfonylaminophenyl) ester
(0.9g, 2.69mMol) in dichluluul~;ll.anc (lOml) was added di-tcrt-butyldicarbonate(0.704g, 3.23mMol) and 4-dimethylaminopyridine (0.065g, 0.54mMol) at room
20 ~ laLul~ under argon. The mixture was stirred at room l~ul~ a~ul~ for I hour
after which the solvent was evaporated in vac~o. Purification by normal phase
column ~hl"". ~ hy eluting with diethyl ether gave the title compound as a whitefoam.
o'H (250MHz, CDC13): 7.38 (SH, m); 7.15 (2H, m); 6.96 (IH, d, J=8.6Hz); 5.08
(2H, s); 3.24 (3H, s); 2.27 (3H, s); 1.4 (9H, s).
-74-
21 961 93
~wos6/o4~33 .~l/r
Procedure 95: 4-Benzyloxy-3-(N-tert-butoxycarbonyl)mA~llan~cl~lfonyl-
,
OH
MeSO2~N~)
BOC OBn
S To a solution of acetic acid, 4-benzyloxy-3-(N-tert-bu~u~y-,a bunyl)-
mP~h~np~nlrully~ ;llvpll~ ,.yl ester (1.16g, 2.67mMol) in a mixture of methanol
(lOml) and water (5ml) was added I M sodium hydroxide solution (3.20mMol) at
room lc..l~,.dLulG. The mixture was stirred for 10 minutes and citric acid added to
adjust to pH 6-7. The solvent was evaporated i~ vacllo and the residue taken up into
~i.,hlulull~llalle (30ml), washed with water (3xl5m1) and dried. Evaporation of the
solvent gave the title compound as â white foam.
o'H (250MHz, CDC13): 7.37 (5H, m); 6.82 (3H, m); 5.09 (IH, br s); 5.20 (2H, s);
3.24 (3H, s); 1.42 (9H, s).
Procedure 9G: (S)-2-[4-Benzyloxy-3-(N-Jert-butoxycarbonyl)-
' - '~ ylamino]p~ - ,yl..cthyloxirane
~0
MeSO2~N~J
BOC OBn
The title compound was prepared from l4-benzyloxy-3-(N-tert-butoxycarbonyl)-
Irullylamino]phenol and (2S)-(+)-glycidyl-3-"illul,~,a~lle sulfonate
according to the method described in Procedure 12.
~'H (250MHz7 CDCI,): 7.35 (5H, m); 6.94 (3H, m); 5.û5 (2H, s); 4.20 (IH, dd,
J=llHz and 2.7Hz); 3.90 (IH, m); 3.35 (IH, m); 3.25 (3H, s), 2.92 (IH, m); 2.77
(IH, m); 1.42 (9H, s).
Procedure ~7: (S~R)-4 {2-[3-(4-Benzyloxy-3-met'h7~ on~la-.. n rl~ -2-
d~w~rlJlu~ a ~]ProPYI}F~ ylpl ~ b ~ ~nir acid, ethyl ester
-75-
21 961 93
Wo 96/04233 1 ~llr~ 0~7
Me
O~--NH~
2 N J~ 3 0
Il ,OEt
H OBn O~P~
A solution of (S)-2-[4-benzyloxy-3-(N-tert-bul~lAy~,~ubul-yl)-
''cnlyb..l-;llv]phenoxymethyloxirane (0.45g, lmMol) in acetoniLrile (25ml)
was treated with lithium perchlorate (0.107g, ImMol), then stirred until complete
S dissolution of the salt. To the resulting stirred solution was added (R)-4-(2-~ ul~u~yl)p'.~ uAy~l~eLIlylphenylrhû~rhini(~ acid, ethyl ester (0.40g, 1.2mMol).The mixture was stirred at room telllL~ Iul~ for 110 hours, and the solvent
evaporated in vaCuo. The residue was dissolved in dichlulull-~,.hduc (25ml) and IM
hydlu~ hlolic acid (2ml) added. The mixture was stirred for 30 minutes at room
10 t~,.llL,~.~.I~,I~; then washed with saturated sodium bi~,Jll)Ull.Ut~ (3xl0ml), water (ISml)
and brine (15ml). The dried extracts were concentrated in vaCuo, and the crude
product purified by normal phase column chromatography, eluting with 10%
methanol in dichlwu-l,~,il-.ll-c to give the title compound as a white foam.
lS o~H (200MHz, CDCl, + D,O): 7.85 (2H, m); 7.57 (3H, m); 7.38 (SH, m); 7.12 (3H,
m); 6.85 (3H, m); 6.62 (IH, m); 5.07 (2H, s); 4.5-4.35 (2H, m); 4.2 (3H, m); 3.~2
(2H, m), 3.4-2.9 (3H, m~; 2.85 (3H, s); 2.78 (2H, m); 1.37 (3H, t, J=7.2Hz); 1.30
(3H, d, J=7.8Hz).
Procedure 98: (S)-4-{2-[3-(4-tert-Butyldil.. ll-y ' ~1~ lu~yLuhenoxy)-2-
llydlu~y~,.oLuylamino]ethyl}plu y~ Lhyl(3 t y h".y~"u~ acid,
7l-butyl ester.
- H
t-BuMe25iO O~P------O~
The title compound was prepared from 4-(2-aminoethyl)pl,~,.lo~y-l-.,illyl(3-
benzyloxypropyl)phosphinic acid, n-butyl ester and (S)-2-(4-tert-
-76-
21 ~61 93
~W0 96/04233 r~
butyldimethylsilyloxyphenoxy)melhyloxirane' according to the method described inProcedure 13.
ulH (CDC13 + D20): 7.35-7.25 (SH, m), 7.1 (2H, d, J = 8.5Hz), 6.78-6.7 (4H, m),
6.55 (2H, d, J = 8.5Hz), 4.70 (2H, s), 4.25-3.75 (7H, m), 3.5-2.75 (8H, m), 2.05-1.9
(4H, m), 1.7-1.6 (2H, m), 1.45-1.35 (2H, m), 0.96 (9H, s), 0.93 (3H, t, J = 7.1Hz),
O. IS (6H, s).
' European patent EP 0611003.
Procedure9g: Methyl5-acetyl-2-l~ lv~v..l~.v~le.
0~
~0~
O OBn
lS Methyl 5-acetylsalicylate (ISg, 0.077Mol), benzyl bromide (9.2ml) and potassium
carbonate (11.7g) were heated at re~flux in acelone (lOOml) for 2 hours. After
cooling, the solids were filtered off and the filtrale uo~lc~ d on a rotary
evaporator. The crude product was chromatographed on silica gel eluting with ethyl
~ (3:7) to afford the product as a white solid.
vlH (CDCI3): 8.44-7.05 (8H, m), 5.27 (2H, s), 3.94 (3H, s), 2.58 (3H s).
Procedure 100: Methyl S-acetoxy-2-b...,..~ Iv~.~b...~.u~
O~
~0~
0 OBn
A solution of methyl S-acetyl-2-benzyloxybenzoate (15.6g, O.OSSMol) and 3-
chlulu~ u~ybc~l~uic acid (42g~ in dh,hlwuu~,d~d~e (250ml) was stirred overnight at
ambient t~,u~ ul~. The reaction mixture was washed with saturated aqueous
30 sodium m~t~hisl~lfile solution (2x250ml), saturated sodium hydrogen carbonate(3x250)and dried over anhydrous ~ f ~ h ~ ~ ~ l sulfate. Filtration and removal of
solvent under reduced pressure gave a white solid.
-77-
wo96/04233 2 1 9 6 1 9 3 ~ 7 ~
~H (CDCI,): 7.59-6.99 (8H, m), 5.18 (2H, s), 3.89 (3H, s), 2 27 (3H, s).
Procedure 101: 3-Hydroxymethyl-4-l~ ,ylu~.y. ~ ~'
,~3
OH OBn
Lithium aluminium hydride (5.7g) was added to a solution of methyl 5-acetoxy-2-
b~ ylc)~ylJ~ ua~ (16g, O.û53Mol) in dry diethylether (270ml) under an argon
10 du.lua~ ,.c and at ice bath Lc~ a~ulc. The reaction mix~ure was allowed to warm
to ambient t~ ,ldlulC and stirring continued for 3 hours. Saturated aqueous
:Imm~nillm chloride (530ml) was cautiously added and the solids filtered off. The
filtrate was extracted with ethyl acetate and the organic extracts dried over anhydrous
,". h ", l sulfate. Filtration and removal of solvent gave a white solid.
olH (d~-DMSO): 8.85 (IH, br, exchanges with D,O), 7.44-6.51 (8H, m), 4.96 (3H, mcollapses to 2H after addition of D2O), 4.48 (2H, d, collapses to singlet after addition
of D2O).
20 Procedure lO2: (S~-2-(4-Benx.yloxy-3-llyd. ur.~ ~- ylphenoxy)-m~ lu~i. ..n~.
0 ~o
,,~3
OH OBn
To a solution of 3-hydroxymethyl-4-benzyloxyphenol ( I 0.4g) in dimethylru ula~ lc
25 (55ml) at ice-bath lelll,u.,ldLult; was added sodium hydride (60% dispersion in mineral
oil, 1.8g). The reaction was stirred for 5 minutes and (2S)-glycidyl-3-
ni~lub~ ,.,..,. ,~ll~ulldte (11.7g) was added in a single portion. The L~llJp~ldLulc was
allowed to warm to ambient and stirring continued overnigh~. Ethyl acetate (370ml)
was added and the organic phase washed with water (2x560ml), saturated brine
30 solution (370ml) and dried over anhydrous "l~g". ~;l"" sulfate. Filtration followed by
removal of solvent gave the crude product as an oil. Purifcation by flash
ulll~ r . ' y (silica gel, 20r~ad.,cLunc/lle~dne) gave an oil, which slowly solidified
on standing. :::
21 961 ~3
096/04233 ~ r1,~7/~10~7
olH (CDC1,): 7.40-6.78 (8H, m), 5.07 (2H, s),4.69 (2H, d), 4.18 (IH, dd), 3.91 (IH,
dd), 3.33 (IH, m), 2.90 (lH, m), 2.75 (IH, m), 2.28 (IH, t).
Procedure 103: (S~R) 4-{2-[3-(4-t-Butyldimethylsil~luayl h~ y)-2-
S ll,rJluay~.Op~ ]propyl}p~ hyl-(3-b,,,,,~1u~.y~l-up~
acid, n-butyl ester,
_ 3
O--H~
~ OH ~
t~BuMe2SiO O~P--(CH2)30Bn
OC4Hg
10 The tit]e cûmpound was prepared from (R) 4-(2-dlldnu,ululJyl)~ lu~y~ yl-(3-
ylùAy~JIuluyl)l~lu~ ;r acid, n-butyl ester and (S)-2-[(4-fen-
butyldimethylsilyloxyphenoxy)methyl]oxirane according tû the method described inProcedure 13.
o~H (CDCl,+ D,O): 7.40-6.75 (13H, m), 4.50 (2H, s),4.25-3.80 (7H, m), 3.55 (2H,
m), 2.95-2.50 (3H, m), 2.00 (6H, m), 1.60, (2H, m), 1.40 (2H, m), 1.06 (3H, d), 0.97
(9H, s),0.95 (3H, t), 0.01 (6H, s).
Procedure 104: (S,R) 4-{2-[3-(4-B~,".~luay, ' .y)-2-
20 Il,~JI uay~ti U,~ ~ ] propyl}r ' ,~ 1l,cthylc,r. ~ol- yl rh~ , ' ~ ~ r acid, n-blltyl
ester.
CH3
O--H~
[~3 [~ 11
OBn O~ l
OC,Hg
25 The title compound was prepared from (R)-4-(2-
alldllu~)lv~ ,lv~y~ ahylcyclohexylrh~l~rhilli( acid,n-butyl esterand (S)-2-~(4-
benzylvay~h~".u/~y)melhyl]oxirane according to the method described in Procedure13.
-79 -
2 I 9 ~1 9 3 f ~l/~ Jyo~n.t7 ~
wo 96/04233
olH (CDCI, + DIO): 7.50-6.75 (13H, m), S.00 (2H, s), 4.27-3.85 (7H, m), 3.10-2.60
(SH, m), 2.20-l.10 (18H, m), 0.94 (3H, t).
Procedure 105: (S,R) 4-{2-[3-(4-Benzyloxypllenoxy)-2-
S h~J~ l.,mino]propyl~p' ~ ~ ylhexylp~ , ' ' '- acid, n-butyl ester.
CH3
1~ ---H
OH
OBn O~P--(CH2)sCH3
OC~Hg
Tbe title compound was prepared from ~R)-4-(2-
10 ~ h~ JlulJyl)~he,lu~yl~ ylhexylphosphinic acid, n-butyl ester and (S)-2-~(4-
benzyloxyphenoxy)methyl]oxirane according to the method described in Procedure
13.
~i~H (CDCI3 + D,O): 7.40-6.75 (13H, m), 5.00 (2H, s), 4.25-3.90 (7H, m), 3.20-2.68
(5H, m), 1.97-1.30 (14H, m), 1.15 (3H, d), 0.95 (6H, m).
Procedure IOG: Aceticacid, ( I 1~ 1u~y-3-~ yl)ester.
O~
F~/
OBn
The title compound was prepared from 4-benzyloxy-3-flu~ .,onc~ accûrding
to the method described in Prûcedure 100.
~'H (CDC13): 7.45-7.32 (5H, m), 7.00-6.75 (3H, m), 5.12 (2H, s), 2.27 (3H, s).
' J. Med. Chem., 1983, 26 (11), 1570-6.
Procedure 107: 4 B...,", lu~.y-3-tluorophelloL
-80-
21 9~ 93
WO 96/04233 P~~ 7
OH
F
OBn
A solution of acetic acid, (4-benzyloxy-3-nuu- ulJh~ yl)ester (5.96g, 23mMol) inmethanol (80ml) and sodium hydroxide (1.0g, 25mMol) in water (20ml) was heated
5 under reflux for 90 minutes. After cooling, the solution was CullCI,.lL..:~,d and the
residue was partitioned between ethyl acetate and IM hydlo~hlu~ic acid. The organic
extracts were separated, washed with water and brine, dried and -- I giving
the title compound.
olH (CDC13): 7.43-7.25 (SH, m), 6.85 (IH, t, J = 9.1Hz), 6.63 (IH, dd, J = 12.1,3Hz), 6.46 (IH, m), 5.06 (2H, s), 4.65 (IH, br, exchanges with D2O).
Procedure 108: (S)-2-(4-Benzyloxy-3~ . nl ' y)~ lùAi
o~o
F~3
OBn
The title compound was prepared from 4-benzyloxy-3-fluorophenol and (S)-glycidyl-
3-~ ub~ nc sulfonate according to the method described in Procedure 102.
olH (CDC13): 7.44-7.25 (SH, m), 6.90 (IH, t, J = 9.2Hz), 6.72 (IH, dd, J = 12.5,3Hz), 6.58 (lH, ddd, J = 9.2, 3, 1.6Hz), 5.07 (2H, s), 4.17 (IH, dd, J = I 1, 3Hz), 3.86
(lH, dd, J = I l, 5.8Hz), 3.35-3.29 (lH, m), 2.90 (IH, dd, J = 5, 4.1Hz), 2.73 (IH, dd,
J = 4.1, 2.5Hz).
Procedure 109: (S)-4-{2-[3-(4-Benzyloxy-3-lluv~-r- y)-2-
UA,~U~U,U,~' ' ~]ethyl~,~' yl~tllylc~ u~ acid, n-butyl
ester.
W0 96/04233 2 l 9 6 l 9 3 r~
~--H~
,~3 OH
OBn O~
OC~H9
The title compound was prepared from 4-(2-aminoethyl)phenoxy
hyl~,y~,lull~,Ayl~ c~ acid, n-butyl ester and (S)-2-(4-benzyloAy-3-
S nuulolJh~,lloAy)~ llyluAil~lllt according to the method described in Procedure 13.
olH (CDC13): 7.44-7.31 (SH, m), 7.14 (2H, d, J = 8.5Hz), 6.9-6.86 (3H, m), 6.69
(lH, dd, J = 12.5, 2.8Hz), 6.55 (lH, ddd, J = 8.9, 3, 1.5Hz), 5.06 (2H, s), 4.25-3.93
(SH, m~ 3.88 (2H, d, J = 4.9Hz), 2.93-2.71 (6H, m), 2.2-1.2 (17H, m), 0.91 (3H, t, J
10 = 7.3Hz).
Procedure 110: 3-~' y~., upylp~ acid
H--P ~\~O J3
OH
The title compound waS prepared from phosphinic acid and O-allylphenol accordingto the method described in Procedure 42. The crude product was used without further
ir~l;U,.
o'H (CDC13): 9.06(1H, s.); 7.31-7.15(2H, m.); 7.19(1H, d, J = 548.72Hz.); 6.94(1H,
t, J = 7.4Hz.); 6.89-6.81(2H, m.); 4.01(2H, t, J = 6.04Hz.); 2.14-1.84(4H, m).
Procedure 111: 3-P. y~ lph. , ' ~ - acid, ethyi ester
Ol ~
H--P~\~O~
OEt
The title compound was prepared from 3-phenoxyl .u~ylllllo~llll;ll;e acid and ethanol
according to the method described in Procedure 43. The crude product was used
without further p~ "; l ;, A nl l l l
-82-
.
~ wo 96/04233 2 1 9 6 1 9 3 1 ~ 7
o'H (CDC13): 7.32-7.20(2H, m); 7.18(1H, dt, J = 533.65, 1.65Hz); 6.95(1H, t, J =7.43Hz); 6.88(2H, t, J = 7.78Hz); 4.28-4.06(2H, m); 4.03(2H, t, J = 5.49Hz); 2.15-
1.93(4H, m); 1.37(3H, t, J = 7.87Hz).
5 Procedure 112: H~dlUA~ ~~hyl(3 "~ ~urvpt~ . ' '~ acid, ethyl ester
HO--p--/~O ~3
OEt
The title compound was prepared from 3-phenuAy~Jlu~,yll~h~ hh -;r acid, ethyl ester
10 and paraformaldehyde according to the method described in Procedure 31.
Ch~ IY over silica gel eluting with dichlu.ulll~,.l,a.le containing 5%
methanol gave a colourless oil.
o'H (CDC13): 7.30-7.24(2H, m); 6.94(1H, t, J = 7.43Hz); 6.88(2H, d, J = 8.25Hz);4.30(1H, s, exchanges with D2O); 4.20-4.07(2H, m); 4.02(2H, t, J = 5.33Hz);
3.88(2H, d, J = 5.50Hz); 2.15-1.95(4H, m); 1.32(3H, t, J = 7.15Hz).
Procedure 113: 4~ L ~ ~IUA~ lhYI(3-r' ~,.u~
p~ .l ' '~ acid, ethyl ester
Cl~11--~~ ~~
O OEt
The title compound was prepared from hydroxymelhyl(3-~he~luAylJlu~Jyl)
phosphinic acid, ethyl ester and 4-chlul.,1,..,,. ,.. ~"lruuyl chloride according to the
25 method described in Procedure 32. The crude product was used without further
r; r A l, . ",
o'H (CDC13): 7.87 (2H, d, J = 8.00Hz); 7.54 (2H, d. J = 8.45Hz); 7.29-7.26 (2H, m);
6.96 (IH, t, J = 7.42Hz); 6.87 (2H, d, J = 7.70Hz); 4.26-3.98 (6H, m); 2.08-2.00 (4H,
m); 1.31 (3H, t, J = 7.15Hz).
Procedure 114: 4-(2-terl-Butu~wl l)v.,~ laminoethyl)~ h yl..~lll~l
(3-~ ,u. u~ . I 'A, ethyl ester
-83-
wo96104233 21 q6 1 S 3 ~ ,51~ 7
BOCNH ~
O~~ 0~
OEt
The title compound was prepared from 4-chlulub~ .,,- .,~.,lfnnyloxymethyl (3-
pl~ u~ u~ u~ ; acid, ethyl ester and 2-(4-hydroxyphenyl)ethyl carbamic
5 acid, tert-butyl ester according to the method described in Procedure 24. The crude
product was purified by ~,LlulllaLu~ laluhy over silica eluting with dichlulul~ hallc
containing 5% methanol.
o'H (CDC13): 7.29-7.23 (3H, m); 7.13 (2H, d, J = 8.52Hz); 6.97-6.85 (4H, m); 4.50
(IH, s); 4.25-4.10 (4H, m); 4.05 (2H, t, J = 5.50Hz); 3.37-3.32 (2H, m); 2.74 (2H, t, J
= 7.15Hz); 2.16-2.06 (4H, m); 1.43 (9H, s); 1.34 (3H, t, J = 7.15Hz).
Procedure 115: 4-(2~ 1(3-ph .~ o,uri~
acid, ethyl ester
H2N ~
0~
OEt
The title cûmpound was prepared from 4-(2-tert-butoxy~allJollylalllillû~ lyl)
2Q ~JL~l~v~yl~l~,lllyl(3-pll~,llu~ ul~yl)phosphinic acid, ethyl es~er according to the
method described in Procedure 25. The crude product was used without further
o'H (CDC13): 7.31-7.23 (3H, m); 7.15 (2H, d, J = 8.80Hz); 6.97-6.85 (4H, m); 4.31-
4.00 (6H, m); 2.95 (2H, t, J = 6.88Hz); 2.71 (2H, d, I = 6.87Hz); 2.22-2.06 (4H, m);
1.79 (2H, s, exchanges with D2O); 1.34 (3H, t, J = 7.14Hz).
Procedure 116: (S)-4-{2-[3-(4-Benzyloxy-3-l,~ll. u,..~ ..l.lllyl)phenoxy-2-
~ d~ P. -]ethyl}~'- y.~.;llyl(3-~ Ulu~u.rl)p~ql ~acid,
30 ethyl ester
-84-
2 1, 6 1 9 3
W O 9 6/04233
H ~
OH O:n O~ p ~~OJ3
OEt
The title compound was prepared from 4-(2-aminoethyl)pl,cnuAy~ llyl
(3-ph~ u~y,ulu~Jyl)~ u~ ic acid, ethyl ester and (S)-2-(4-benzyloxy-3-
hydlu~y~ llyl,ull."lu;~y)methyloxirane according to the method described in
Procedure 13. The crude product was purifed by .;hll l~j.dlJI'ly over silica eluting
with dichlulu~ Lalle containing 5% methanol.
o'H (CDC13+D20 ): 7.39-7.26 (5H, m); 7.15 (2H, d, J = 8.79Hz); 6.91-6.75 (IOH,
m); 5.06 (2H, s); 4.69 (2H, s); 4.22-3.90 (7H, m); 2.90-2.77 (6H, m); 2.12-2.05 (2H,
m); 1.82-1.54 (4H, m); 1.34 (3H, t, J = 6.88Hz)m); 1.61-1.53 (2H, m).
Procedure 117: 3-P ~Ip~u,u,~ . " acid
H--P~~3
The title compound was prepared from phosphinic acid and allylbenzene according to
the method described in Procedure 42. The crude product was used without further
~u.ir;. ,;,
o'H (CDCl3): 11.28 (IH, s, exchanges with D20); 7.31-7.14 (5H, m); 7.05 (IH, dt, J
= 542.72, 1.92Hz); 2.71 (2H, t, J = 7.15Hz); 1.99-1.84 (2H, m); 1.79-1.68 (2H, m).
Procedure 118: 3-P ,~ up~ . ~ ~ acid"~-butyl ester
0
H--P~3
OC~Hg
The title compound was p}epared from 3-phenylprop~ ,hv~l,;uic acid and n-butanolaccording to the method described in Procedure 43. The crude product was used
30 without further purification.
21 q61 93
wo 96/04233 1 ~ ll~l ~sl. ~0
o'H (CDCl3): 7.32-7.15 (5H, m); 7.06 (IH, dt, J = 548.72, 1.92Hz); 4.01-3.97 (2H,
m); 2.73 (2H, t, 7.15Hz); 1.95-1.62 (6H, m); 1.44-1.35 (2H, m); 0.94 (3H, t, J =7.15Hz).
5 Procedure 119: H~d.u,.y...c~ 1(3-rl- ~l".o~yl)~ acid, n-butyl ester
HO--p~
OC~HD
The dtle compound was prepared from 3-phenylp-u~Jy~ m~l,h; nir acid, n-butyl ester
10 and paraformaldehyde according to the method described in Procedure 31.
Chl, ~, ayhy over silica gel eluting with dichlulvlu~.il,.llle containing 5%
methanol gave a colourless oil.
o'H (CDC13): 7.32-7.16 (5H, m ); 4.13-3.95 (3H, m, IH exchanges with D2O); 3.90-lS 3.77 (2H, m); 2.70 (2H, t, J = 6.87Hz); 2.02-1.74 (4H, m); 1.67-1.56 (2H, m); 1.43-
1.25 (2H, m); 0.92 (3H, t, 7.43Hz).
Procedure l20: 4-C~ ony lv~y, ~ ' .rl(3 ~'- yl,~ ,- u~.yl)
pl- . ' - acid, n-butyl ester
OO
Cl~~ 0~
The title compound was prepared from hydlv~-y~ ,.llyl(3-phenylpropyl)
phosphinic acid, n-butyl ester and 4-chlvl~ . ,I ru~yl chloride according to the
25 method described in Procedure 32. The crude product was used without further
~"";ri~ -li"l,
oiH (CDc13): 7.82 (2H, d, J = 9.07Hz); 7.54 (2H, d, J = 8.79Hz); 7.33-7.13 (5H, m);
4.25-3.82 (4H, m);2.70 (2H, t, J = 6.05Hz); 1.99-1.73(4H, m); 1.66-1.54 (2H, m);30 1.43-1.23 (2H, m);0.91 (3H, t, J = 7.42Hz).
Procedure 121: 4-(2-tert-Bull~ ...- I,uu~ ' ' yl
(3-l~ ' yl~). UtJ~ acid, n-butyl ester
-g6-
2 1 9b ~ ~3
~ WO 96/04233 . ~ r/0;~0~7
BOCNH ~
1~l
~\~
OC~H~
The title compound was prepared from 4-chlu~ ruuylu~yl~ lyl(3-
PI~ UIV~ acid, n-butyl ester and 2-(4-Lydlu~y~ ,..yl)ethyl carbamic
5 acid, t-butyl ester according to the method described in Procedure 24. The crude
product was purified by ~,hlv~ y over silica eluting with dichlulul~ lc
containing 5% methanol.
~IH (CDC13~: 7.28-7.11 (7H, m); 6.85 (2H, d, J = 8.80Hz); 4.50 (lH, s); 4.21-3.99
(4H, m); 3.45-3.30 (2H, m); 2.77-2.72 (4H, m); 1.95-1.80 (3H, m); 1.75-1.62 (3H,m); 1.51-1.30 (2H, m);l.43 (9H, s); û.91 (3H, t, J = 7.42Hz).
Procedure 122: 4-(2-Aminoethyl), L ,~ (3-phenylpropyl)~ h ,)1
acid, n-butyl ester
OC~H~
The title compound was prepared from 4-(2-/ert-l,ulu,~yu~ll L,ouylaminoethyl)
~ L~ vAyll~,l,yl(3-1~h~,lylulvuyl)phosphinic acid, n-butyl esler according to the
method described in Procedure 25. The crude product was used without further
,u.irl~ u.l.
~'H (CDC13): 7.28-7.12 (7H, m); 6.86 (2H, d, J = 8.79Hz); 4.21-3.90 (4H, m); 3.00-
2.87 (2H, m); 2.74-2.71 (4H, m); 1.95-l.X0 (4H, m); 1.76-1.51 (4H, m, 2H exchanges
with D2O); 1.48-1.34 (2H, m); 0.91 (3H, t, J = 7.42Hz).
Procedure 123: (S)- 4-{2-[3 (~i Bc..~.ylo~.y-3-hydroxymethyl)phenoxy-2-
hydl UAyUl ouyl....lil.o]ethyl}p'- yll~.~hyl(3-p '- yl~l vpyl)~ h , ' - acid, n-
30 butyl ester
-87-
21 961 93
W0 96/04233 l U ~ n-i7
0--/~ N ~j
OC,H~
The title compound was prepared from 4-(2-aminoethyl)phenoxymethyl-(3-
~h.,.~yl~ulu~yl)rhr~rhinic acid, n-butyl ester and (S)-2-(4-benzyloxy-3-
S hydiw~y~ ",vJ~y)methyloxirane according to the method described in
Procedure 13. The crude product was purified by uluuul~laJ~ hy over silica eluting
with dichlolulll.,lhG~Ie containing 5% methanol.
o'H (CDC13+D2O ): 7.43-7.11 (13H, m); 6.92-6.74 (4H, m); 5.30 (2H, s); 5.06 (2H,s); 4.69-3.91 (7H, m); 2.94-2.70 (8H, m); 2.03-1.77 (6H, m); 1.67-1.46 (2H, m); 0.91
(3H, t, J = 7.42Hz).
Procedure 124: r , h acid, '7is-cyclohexyl ester
~0 ~ ,H
~J O
-- _2
The title compound was prepared from cyclohexanol and pho~ u-u~ tribromide
according to the method described in Procedure 31. purh~lcation by .,LIUIII4LU~;IaiUI
on silica gel eluting with dichlulul~ alle to 2riO methanol in dichlululll~,.llall~ gave
20 the title cûmpûund as an oil.
o~H(CDC13): 8.16-5.61 (lH, d), 4.38-4.51 (2H, m), 1.19-l.g6 (20H, m).
Procedure 125: H~ o~ , ' r I '- acid, bis-cyclohexyl ester
O' IPI ~OH
_2
T.'.e title compound was prepared from phr,~rhrnir acid, bis-cyclohexyl ester and
paraformaldehyde according to the method described in Procedure 31. Pul;rlu,lLiull by
30 ulll~ g ~ hy on silica-gel eluting with 2% methanol in dichlulull-.,.hall~, gave the
title compound as an oil.
~H(CDC13 + D2O): 4.81-4.42 (2H, m), 3.86-3.84 (2H, d), 1.21-1.96 (20H, m).
-88-
2~ 961 93
wo 96/04233 P~ll~ n~7
Procedure 126: (4-Cl.h"ut ~-~ Ifoll~luAy ~~hyl)~ 1- , I ~ acid, bis-
cyclohexyl ester
O
_~~2~ ~CI
The title cûmpound was prepared from hydlul~ylllwily~ ollie acid, bis-
cyclohexyl ester and 4-chlu-ub~ ru.,yl chloride according to the method
describcd in Proccdure 32 as a colourless oil following chlullla~u~lal!lly on silica gel
10 eluting with 10-20% ethyl acetate in hexane. The oil thus obtained solidifiled to give a
white solid, mp SS-57~C.
o~H(CDC13): 7.85-7.88 (2H, d), 7.53-7.57 (2H, d), 4.40-4.49 (2H, m), 4.08-4.18
(2H, m), 1.20-1.86 (20H, m).
Procedure 127: (R)-4-(2-tert-Bulo,.,~ al L.vu,~' -r U~uy~ h ,~ .lh,~l
p~ - . h~ - acid, bis-cyclohexyl ester
Me
i30CNH~
~P~
The title compound was prepared from (4-chlu-ub ..,., ,~llrhonyloxymethyl)
,I,u~uhu..iu acid, bis-cyclohexyl ester and (R)-2-(4-hydroxyphenyl)-1-
methylethylcarbamic acid, tert-butyl ester according to the method describcd in
Procedure 24. Pulir~caliull by column chromatography on silica gel eluting with 2~o
25 methanol in dh,lllu u~ ,iLàll~. gave the title compound as a gum.
olH(CDC13): 7.12-7.14 (2H, d), 6.88-6 g2 (2H, d), 4.49-4.60 (2H, m), 4.21-4.24
(2H, d), 3.75-3.90 (IH, broad s), 2.52-2.81 (3H, m), 1.22-2.05 (20H, m), 1.42 (9H,
s), 1.05-1.07 (3H, d).
Procedure 128: (R)-4-(2-~ -r uu,rl)p!- ~ ~r . - acid, bis-
cyclobexyl ester
-89-
21 96~ 93
WO 9G/0~233 ~ 5 A'~ 37
Me
H2N
The title compound was prepared from (R)-4-(2-tert-l,ulu,-y.all,o.)ylamino
propyl)y:.~,..u,~y.u.,ll.ylyl~u~ rlrir acid, bis-cyclohexyl ester according to the method
5 described in Procedure 25. Purification by column ~LlulllaLu~ldyhy on silica gel
eluting with lO~o methanol in dichlu.ul...,.l..l-le gave the title compound as a gum.
~'H(CDC13): 7.07-7.09 (2H, d), 6.88-6.90 (2H, d), 4.48-4.58 (2H, m), 4.22-4.25
(2H, d), 2.45-3.20 (3H, m), 1.22-2 û5 (22H, m), I . I l -1.13 (3H, d).
Procedure 129: (S~ 4-{2-[3-(4-B~ lu~.yl '- .y)-2-hydl u.~,u. u,u~' ~ ]
propyl}l ~ acid, bis-cyclohexyl ester
Me
O --N~
~3 1~ ~0~
BnO ~~P' 2
The title compound was prepared from (R)-4-(2-aminopropyl)yl-e.-u~y~ ylpLo~ o..i., acid, bis-cyclohexyl esteF and (S)-glycidyl-4-benzyloxyphenol according
to the method described in Procedure 13. The crude product was purified by
,. I-t.y on silicagel eluting with 2r/o methanol in dichloromethane to give the
20 title compound as a gum.
~lH(CDC13): 7.25-7.48 (5H, m),7.13-7.16 (2H, d), 6.76-6.90 (6H, m), 5.00 (2H, s),
4.48-4.62 (2H, m), 4.18-4.22 (2H, d), 3.85-4.00 (2H, m), 2.62 3.22 (6H, m), 1.20-
2.02 (20H, m), 1.18-1.20 (3H, d).
Procedure 130: Phosphonic acid, bis-(2,2-diphenylethyl) ester
-90 -
21 ~61 93
~ wo 96/04233 ~ n ~7
- 3-
~,O~p,H
-- --2
The title compound was prepared from 2,2~ h~ ,illallul and pho~ul.u.us
tribromide according to tbe method described in Procedure 31. PulircaLion by
5 Clll.)~ t.~ l,y on silica gel eluting with 29~o methanol in dichlu.u--.~,.l,u..~. gave the
itle compound as an oil.
~'H(CDC13): 7.78-5.18 (lH, d), 7.08-7.35 (20H, m), 4.18-4.49 (6H, m).
Procedure 131: H~dl~A.~ .. 11.. ~', '- P~ - acid, bis-(2,2- ';,h ,~1.11,.~1) ester
~ 'e--OH
_2
The title compound was obtained from ~ l,c ";c acid, bis-(2,2-diphenylethyl) ester
15 and paraformaldehyde according to the method described in Procedure 31 as a solid
(mp 95-97~C) following ~ -U~ h,y on silica gel eluting with 2% methanol in
dichlululll~,i11allc.
o~H(CDCl3): 7.15-7.31 (20H, m), 4.25-4.51 (6H, m), 3.52-3.57 (2H, t), 2.07-2.14
20 (IH, t).
Procedure 132: (4-(~1 ' .h '~. YIUAYI..~ I '- acid, bis-(2,2-
diphenylethyl) ester
,~ _
_~~~p~O,S~
-91-
~ 961 q3
w0 96/04233 ~ n~0
The title compound was prepared from hyd-u~-y~ ,tl.ylphosphonic acid, bis-(2,2-
diphenylethyl) ester and 4-chlvl..b .,,~ "lphonyl chloride according to the method
described in Procedure 32. Purification by ~,L~ g~ y on silica gel eluting with
10% ethyl acetate in hexane followed by trituration oFthe residue with diethyl ether-
S hexane gave the title compound as a solid (mp 75-76~C).
o~H(CDC13): 7.62-7.67 (2H, d), 7.41-7.45 (2H, d), 7.12-7.31 (20H, m), 4.31-4.45
(4H, m), 4.20-4.25 (2H, t), 3.83-3.87 (2H, d).
Procedure 133: 4-(2-tert-Bulu~y~rl,v.,ylaminoethyl)t ' y yi p' , '
acid, bis-(2,2-~ yl~Lhyl) ester
BOCNH ~
~_ _
1~l ~q
O~P~o~
~5 The title compound was prepared from (4-chlulvliJ~ llrlv~y~ Lllyl)
acid, bis-(2,2-diphenylethyl) ester and 2-(4-h~/vlu~ylJhcllyl) ethyl
carbamic acid, tert-butyl ester according to the method described in Procedure 24.
Pulir~ ivu by column ~ .t;~ hy on silica gel eluting with 1 % methanol in
di~,l-lvlulll~ ; gave the title compound as a gum.
ol(CDCI, + D,O): 7.04-7.29 (22H, m), 6.70-6.73 (2H, d), 4.41-4.55 (4H, m), 4.26-4.31 (2H, t), 3.87-3.90 (2H, d), 3.31-3.45 (2H, t), 2.70-2.75 (2H, t), 1.43 (9H, s).
Procedure 134: 4-(2-Aminoethyl)~ , ' yl~ h p'~ - acid, bis-(2,2-
25 diphenylethyl) ester
-92-
~ wo 96/04233 2 1 9 6 l 9 3 . ~l/~z ,. ~0~7
H2N ~
~1~l- -
o~P~0~3
1~1 _2
The title compound was made from 4-(2-ter/-buLu~iy~,alvu..ylà~ lo~llyl)
IJL~,.lur.yl~ lyl~ u~ acid, bis-(2,2-diphenylethyl) ester according to the
5 method described in Procedure 25 and was used in the next stage without further
ri. ,i""
olH(CDCl3): 7.05-7.29 (22H, m), 6.70-6.74 (2H, d), 4.41-4.51 (4H, m), 4.26-4.31
(2H, t), 3.87-3.91 (2H, dd), 3.31-3.44 (IH, m), 2.90-2.96 (lH, t), 2.67-2.75 (2H, q),
1.56-1.70 (2H, broad s, exchanges with D2O).
Procedure 135: (S)-4-{2[3-(4-Bc..~lu~ JI- y)-2-h~dlu,.~"u~ lamino]
ethyl}p~ h~l~ ' , ' ~e acid, bis-(2,2-~i, ' yl~lhyl) ester
OH ~3
BnO o~p~O~l
_ [3 -2
The title compound was prepared from 4-(2-aminoethyl)~JL~,I.v~ylll~,lhyl l,l,c,~l,l,.".;o
acid, bis-(2,2-diphenylethyl) ester and (S)-glycidyl-4-benzylv~cy l)h~,..ol according to
the method described in Procedure 13. The crude product was purified by
20 ~ o~alJhy on silica gel eluting with 5~o methanol in dichlv,v..-~LLa,,e to give
the title compound.
olH(d'-DMSO + D2O): 6.69-7.40 (33H, m), 5.01 (2H, m), 4.28-4.47 (7H, m), 3.86-
4.18 (4H, m), 2.74-2.95 (6H, m).
-93-
wos6/04233 2 1 9 6 1 93 P~
Procedure 136: (R)-2,2-Di-~ert-butyl-6-(oxiran-2-ylmethoxy)-4H-1,3,2-
o~o
[~1
xS~,<
The title compound was prepared from 2,2-di-tert-butyl4H-173,2-lJ.. ,,.. ~ lin-6-
ol (700mg, 2.50mMol) and (2R)-(-)-glycidyl-3-niL~ fonate (780mg,
3.0mMol) employing a method similar to that described in Procedure 12.
~'H (250MH7., CDQ,): 6.72-6.88 (2H, m); 6.52 (IH, d); 4.95 (2H, s); 4.16 (IH, dd);
3.89 (IH, dd); 3.34 (IH, m); 2.90 (IH, t); 2.73 (IH, dd) and 1.04 (18H, s).
Procedure 137: (RR).4.{2-[3-(2,2-Di-lert-butyl-4H-1,3,2-t~~~~ ' ' 6 yl-
oxy)-2-~ 1,u"~,~,.u~ ..J]propyi}p~ 'r - . ~ acid,
ester.
Me
? O~, P-OEt
The title compound was prepared from (R)-2,2-di-tert-butyl-6-(oxiran-2-ylmethoxy)-
4H-1,3,2-~,..,,...1;..~ ~c;lin~ne (240mg, 0.71mMol) and (R)-4-(2-
c~ ul~lul~yl)phenoxymethyl-l~h~,~lyl~ acid, ethyl ester (238mg, 0.71mMol)
20 following a method similar to that in Procedure 14.
olH (250MHz, CDCl,): 7.93 (2H, m); 7.46-7.62 (3H, m); 7.08 (2H, d); 6.83 (3H, m);
6.69 (IH, dd); 6.48 (IH, d); 4.93 (2H, s); 4.48-3.99 (4H, m); 3.88 (2H, d); 3.04-2.46
(8H, m); 1.38 (3H, t); 1.11 (3H, d) and 1.03 (18H, s).
2~
Exarnple 1: (S,R)- Sodium-4-~2-[2-hydroxy-3-(2-
h~.ll v,.,~ ,. u~ ; ~ ~]propyl]
-94-
21 961 93
o 96/0~233 P~,5/ )30~7
pher~ ~....t~te
Me
O--N~
HO ~[~3 OH
O~CO2Na
A solution of (S,R)-methyl-4-[2-[2-hydroxy-3-(2-hy~ .x.yl,ll~,.lo1cy)~ yl ]
propyl]~Jh~ Ay~ la~ (120mg, 0.31mMol~ in dioxan (8ml) and sodium hydroxide
solution (2M, Sml) was stirred at room ~ dLUI~ under an argon a~ o~ "e for 18
hours. The pH of the solution adjusted to pH9 with 2M hydrochloric acid and the
solvent was evaporated.The residue was purified by reverse phase chn~m ~ grS~phy10 eluting with 0-5~c isopropanol in water giving the title compound as a colourless
solid; m.p. 130~C; ra]D25 -13~ (c = 0.35, water).
olH(270MHz, d6-DMSO/D20): 7.0-6.7 (8H, m), 4.18 (2H, s), 4.2-3.9 (3H, m),
3.1-2.8 (4H, m), 2.40 (IH, m) and 0.90 (3H, d, J=6.3Hz) ppm.
Example2: (S,R)-.Sr-' ~ [2-[2-hydroxy-3-(3-
]propyl]
F ~ ~ ~
Me
O NH ~
HO~ ~
O~CO2Na
A solution of (S,R)-methyl-4-[2-[2-hydroxy-3-(3-hydroxyphenoxy)propylamino]
propyl]phenoxyacetate (140mg, 0.36mMol) in dioxan (8ml) and sodium hydroxide
solution (2M, 5ml) was stirred at room ~ ,.aiw~; under an argon aLll.o~ for 5
25 hours. The pH of the solution adjusted to pH9 with 2M hydrochloric acid and the
solvent was evaporated.The residue was purified by reverse phase ~,hl~ O . ' y
eluting with 0-5% i~ allol in water giving the title compound as a colourless
solid;m.p. 136~C;[cc]D25-11~~c=0.16,50~oisopropanol/50~owater).
-95 -
wos6/04~33 2 ~ 3 .~ n~7 ~
~111(270MHz, d~-DMSO/D2O): 7.1-6.9 (3H, m), 6.70 (2H, d, J=8.5Hz), 6.35-
6.25 (3H, m), 4.19 (2H, s), 4.0-3.7 (3H, m), 2.9-2.3 (SH, m) and 0.91 (3H, d,
J=6.3Hz) ppm.
Example 3: (S,R)- Sodium-4-[2-[2-hydroxy-3-(4-
11~ d- UA~ )t~l o~ ]propyl]
r- ~ ~
Me
O N
~3 OH
HO O~CO2Ncl
A solution of (S,R)-methyl4-[2-[2-hydroxy-3-(4-hy lluAy~ v~y)lJlulJyla~ v]
propyl]~ lvA~aLLlat~, (140mg, 0.36mMol) in dioxan (8ml) and sodium hydroxide
solution (2M, Sml) was stirred at room Lc.lllJ~la~lc under an argon atlllvs~ for 18
15 hours. The pH of the solution adjusted to pH9 with 2M hydrochloric acid and the
solvent was evaporated.The residue was purifed by reverse phase LL~ hy
eluting with 0-20% isopropanol in water giving the title compound as a colourless
solid; m.p. 119~C; [~]D25 -15~ (c = 0.34, 70~o isopropanol / 30Yo water).
~1H(270MHz, d6-DMSO/D20): 6.93 (2H, d, J=8.0Hz), 6.8-6.6 (6H, m), 4.22
(2H, s), 4.1-3.8 (3H, m), 2.95-2.75 (4H, m~, 2.5-2.4 (IH, m) and 0.90 (3H, d,
J=6.3Hz) ppm.
Example4: (S,R)-4-~2-[2-Hydroxy-3-(4-hydroxy-3-h.~d.uA.~, ' Y',' y)
I~u"~ ]propyl}~ .... lhyl ~ ( acid diethyl ester.
~ ' ~ H
~ ~
HO OH O~P~
OEt
-96-
~ w096/04233 2 1 ~ 6 1 93 F ~ 037
To a solution of (S,R)-4-{2-[3-(2,2-Di-tert-butyl-4H-1.3,2-b~ nsln-6-
yloxy)-2-Ly.llu,.y~lul,yL..Ili,.~,]propyl)phenoxymethyl pllosl~holl;e acid diethyl ester
(270mg, 0.424mMol) in l~llahydlul UI~Ul (lOml) in a plastic container at room
Lul~; under argon was added hydrogen ftuoride pyridine complex (S drops).
S After IS minutes, alumina (220mg) was added and the stirring was continued for a
further 30 minutes. The reaction mixture was filtered through a short pad of celite
and the solvent evaporated ~n vacuo. The crude product was purified by reverse
phase ..h.- -- " ~ my ~ ,y over C18 silica, eluting with 20% ethanol in water to give the
title compound as a beige coloured foam.
olH (400MHz, d6-DMSO): 8.81 (s, br, exchanges with D20; 7.18 (2H, d,
J=10.7Hz); 6.95 (2H, d, J=10.7Hz); 6.92 (IH, d, J=2.4Hz); 6.70 (lH, d, J=9.6Hz);6.65 (IH, d, J=9.6Hz and 2.4Hz); 4.47 (2H, s); 4.4û (2H, d, J=10.7Hz); 4.15 (4H, q,
J=6.4Hz); 4.0 (IH, m); 3.85 (2H, d, J=3.2Hz); 2.8-3.1 (SH, m); 1.27 (6H, t, J=6.4Hz);
lS 1.04 (3H, d, J=7.5Hz)
Example 5: (S,R)-T -'- '4 ~2-[2-hydroxy-3-(4-hydroxy-3-hydlu~-y '' ,~1-
phenoxy),u.u,u,~; ~ ~]propyl)ph.,w~y....lhyl) ethyl l ' -, ' '
-
O--Hi~
~ ~
HO OH O~P'OEt
(S,R)~{2-[2-hydroxy-3-(4-hydroxy-3-hydlu~ylll~,illyl phenoxy) ~-u~yLIlillo)-
propyl) phenoxymethyl ph~ ,-in acid diethyl ester (220mg, 0. 443mMol) and lM
lithium hydroxide (Sml) in 1,4-dioxan (Sml) was stirred at room lGll~ldlUlC; for 24
25 hours under argon. The solution was adjusted to pH 9 by addition of solid carbon
dioxide, and the solvents evaporated. The residue was purified by reverse phase
I,lllUll~iU~ )Ly over C18 silica eluting with O-lO~o ethanol in water to give a white
powder. mpt. 120-21~C.
~lH (400MHz, d6-DMSO): 9.25 (s, br, exchanges with D20); 7.07 (2H, d,
J=10.7Hz); 6.9 (lH, d, J=2.0Hz); 6.78 (2H, d, ~=10.7Hz); 6.71 (IH, d, J=lOHz,); 6.5
(IH, d, J=lO.SHz and 2.1Hz); 5.2 (s, br, exchanges with D20); 4.45 (2H, s); 4.07(IH, m); 3.8 (6H. m); 2.8-3.2 (5H, m); 1.14 (3H, t, J=6.5Hz); 0.94 (3H, d, J=7.5Hz)
-97-
Wo 96/04233 21 9 61 9 3 r~l~ 73,~ 7
Example 6: (S~R)-4-{2-[2-hydroxy-3-(4-hydroxy-3-hydroxymethyl-phenoxy)
u~"r ~ ]Pr~PYl}F~ lhyl carboxylic acid methyl ester
~ H
~OH
HO OH O~CO2M~
Toasolutionof(s~R)4-{2-[3-2~2-di-tert-butyl-4H-l73~2-~ 7~ iSln-6-yl-oxy)-
2-hylloAy~Jlu~lylamine]propyl}pll~ Ay~ Lllyl carboxylic acid methyl ester (0.262g,
0.469mMol) in t~alally~hufLllall (lOml) in a plastic container at room temperature
under argon was added hydrogen fluoride pyridine complex (5 drops). After 10
minutes, aluminia (250mg) was added and the stirring was continued for a further 30
minutes. The reaction mixture was filtered through a short pad of celite and thesolvent evaporated in vaeuo. The crude product was used in the next step.
olH (400MHz, d6-DI\lSO): 8.83 (s, br, exchanges with D20); 7.15 (2H, d,
J=9.8Hz); 6.89 (IH, d, J=2.6Hz); 6.83 (2H, d, J=9.6Hz); 6.66 (2H, m); 4.95 (s, br,
exchanges with D20); 4.73 (2H, s): 4.46 (2H, s); 3.96 (lH, m); 3.82 (2H, m); 3.68
(3H, s); 3.30-2.75 (5H, m); 1.07 (3H, d, J=7.8Hz)
Example 7: (SR) Sodium-(4-{2-[2-hydroxy-3-(4-hydroxy-3-
11~ 1, y) plv~ ,.. ;.. o]propyl}~ 1 a~ JA~rlale
~--H~
OH
HO OH O~CO2Na
(SR)-4-{2-[2-hydroxy-3-(4-hydroxy-3-l,y;L~Ay~ .hylphenoxy)propylamino]prop-
yl}ph~,.,OA~ ,Ll,yl carboxylic acid methyl ester (0.16g, 0.382mMol) and 2M sodium
hydroxide (5ml) in 1,4-dioxane (5ml) was stirred at room Lcul~ dLul~ for 20 hours
under argon. The solution was adjusted to pH 9 by addition of solid carbon dioxide,
and the solvents evaporated. The residue was purifed by reverse phase
-98-
21 ~61 '~3
~0 96/04233 ~ n~7
~hl~ o alJLy over Cl g silica eluting with 0-20% ethanol in water to give white
crystals. mpt. 140-2~C.
olH(400MHz, d6-DMSO): 9.0 (s, br, exchanges with D2O); 6.94 (3H, m); 6.81-
6.62 (4H, m); 4.48 (2H, s); 4.41 (2H, s); 4.18 (IH, m); 3.87 (2H, m); 3.25 (2H, m);
2.94 (IH, m); 2.82 (IH, m); 2.34 (IH, m); 0.94 (3H, d, J=7.5Hz).
Example8: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-h~d~ ' y)-
]propyl}l ' ,~c~t;c acid
Me
O~ N~
HO~J~ OH [~
OH OJ~OH
The title compound is prepared from (S,R)-4-{2-[2-hydroxy-3-(4-hydroxy-3-
hydlu~y~ h~ v~y)~u~u~ylf~ lo]propyl~ "lu/~yul~illyll,l,o~ ,,;f acid,methyl
15 esteraccordingtoa.,l.,~l;.',.AI;.".oftheproceduredescribedinExample7.
Af iAifif ~ fln to pH 7 with IM hydrochloric acid followed by reverse phase
.,1..~ O , ' y and freeze drying provides the title compound.
Example 9: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-hydroxymethylphenoxy)-
I~-ù~ -]propyl}l ' y ' y~ - acid, ethyl ester
Me
O--N~
HO~ ,~ OH
OH o~P--O
OH
The title compound is prepared from (S,R)-4-~2-[2-hydroxy-3-(4-hydroxy-3-
hydlu~y~ hylphenoxy)~JIv~ylalll;llo]propyl~ ,llu~yll~ yl~ o~ onif acid diethyl
ester according to a ,-.o,:l;r~,alion of the procedure described in Example 5.
99 ~
21 961 93
w0 96104233 r~ l0~ 7
A~ ifin~ti~-n to p~ 7 w;th IM dilute hydrochloric acid followed by reverse phase~I-IU-I-~LU~ IIY and freeze drying provides ~he title compound.
Example 10: (SR)-4-{2-[3-(3,4-Dil-y-l- u~y~,henoxy)-2-hydrûxy-
I,.u~,,, ' -]propyl~r'~ ,~ lic acid, methyl ester, hydr,L ' ' ;de
Me
H~ HCI
OH O~CO2Me
A solution of (SR)-4-(2-[3-(3,4-dibenzyloxyphenoxy)-2-
Lydlu~yl,,u~yLul~illo]propyl)phenoxyacetic acid, methyl ester (230mg, 0 4mMol) in
methanol (30ml) containing palladium(ll)chloride (71mg, 0.4mMol) was
Lydlu~ldtv;l at room t~ ul~; and pressure for 18 hours. The mixture was
filtered through a pad of filter aid, the filter pad was washed with methanol and the
combined filtrates were evaporated. The residue was dissolved in water and the
solution was freeze dried giving the title compound as a colourless solid.
~(D2O): 7.28 (2H, d, J = 8.6Hz), 6.94 (2H, d, J = 8 6Hz), 6 85 (lH, d, J = 8.7Hz),
6.51 (IH, d, J = 3Hz), 6.40 (lH, dd, J = 8.7, 3Hz), 4.72 (2H, s), 4.3-4.25 (IH, m),
4.05-3.97 (2H, m), 3.82 (3H, s), 3.68 (IH, dd, J = 13.8, 6.9Hz), 3.4-3.36 (2H, m),
3.05 (IH, dd~ J = 13.1, 7Hz), 2.94 (IH~ dd, J = 13.8, 7.6Hz), 1.34 (3H~ d, J = 6.6Hz).
Example 11: (SR)-4-{2-[3-(3,4-Dih,~dlu?.yl ' y)-2-
dLU~JY~ ]prûpyl}ph~ y.-.. licacid,hydrochlûride
Me
O~H~ HCI
,~ OH [~
OH O~CO2H
-100-
~ wo s6/04233 2 1 9 6 1 9 3 P(,~ 7
A solution of (SR)-4-{2-[2-hydroxy-3-(3,4-
diLyLu~y~ullclluAy)propylamino]propylJphenoxyacetic acid, methyl ester,
hydlu~,lllu~ide (78mg, û.l7mMol) in water (2ml) containing hydrochloric acid (IM,
0.5ml, 0.51mMol) was heated at 100~C under argon for 3 hours. After cooling to
S room ~ ul~ the solution was freeze dried giving the title compound as a
colourless solid.
o(dfi-DMS0 + D20): 7.16 (2H, d, J = 8.6Hz), 6.86 (2H, d, J = 8.6Hz), 6.64 (IH, d, J
= 8.5Hz), 6.42 (IH, d, J = 3Hz), 6.21 (IH, dd, J = 8.5, 3Hz), 4.65 (2H, s), 4.25 (lH,
m), 3.9-3.8 (2H, m), 3.5 (lH, m), 3.3-3.2 (2H, m), 3.05 (IH, dd, J = 12.5, 9.7Hz),
2.60 (IH, dd, J = 12.5, 1 lHz), 1.10 (3H, d, J = 6.3Hz).
Example 12: (SR)-5-~2-[3-(4-Hydroxy-3-hy.l.u,.y.l..llly', ' y)-2-
5 h~dlù.~ JIv,uJ' ~ ~]propyl}-1,3 h~ d- ~-2,2-' ~L,ux.~licacid,diethyl
ester
Me
O--H~
OH ~
HO OH O 7LCO2Et
C0zEt
The title compound was prepared from (SR)-5-12-[3-(2,2-di-~-butyl-4H-1,3,2-
I;n:~n 6-YIOXY)-2-IIYd~U~Y~U~UIJYIaminO]PrOPYI ) 1 ,3-l~ - .1 .-2,2
diu~ulJu~ylic acid, diethyl ester according to the procedure described in Example 4.
o(d6-DMS0 + D20): 7.1-6.6 (6H, m), 4.44 (2H, s), 4.32 (4H, q, J = 7Hz), 4.1-3.8
(3H, m), 3.2-2.6 (SH, m), 1.24 (6H, t, J = 7Hz), 1.02 (3H, d, J = 6.5Hz).
Example 13: (SR)-5-{2-[3-(4-Hydroxy-3-llyd.u~y ~ ' y).2-
dl U~ J- o~,J - - ~ ]propyl}-1,3-t .... ' '- 2,2-dicarboxylic acid, dilithium
. 30 salt
-101-
Wos6/o~233 2 ~ q 6 1 9 3 P~ ,S/~ 7 ~
Me
0~--N~
CO Li
CO2Li
A solution of (SR)-5-(2-[3-(4-Hydroxy-3-hyd~ y~ ,.l.yl~ c.u~;~y)-2-
Ly~ AylJlulJylo~ll;llO]propyl}-l~3-~ n~ lf-2~2-di~albw~ylic acid, diethyl ester
5 (520mg, mMol) in dioxan (lOml), water (3ml) and lithium hydroxide solution (lM,
6ml, 6mMol) was stirred at room t~ cl,l~ulc under argon for 24 hours. The pH of
the solution was adjusted to 9 with dilute hydrochloric acid and the solvent wasevaporated. The residue was purifled by reverse phase chromatography eluting with
water-methanol mixtures to give the title compound as a colourless solid.
~i(d6-DMSO + D20): 6~90 (lH, d, J = 2.2Hz), 6.76-6.58 (4H, m), 6.49 (lH, d, J =
7.7Hz), 4.42 (2H, s), 4.1-4.0 (lH, m), 3.84-3.81 (2H, m), 3.2 ~8 (4H, m), 2.45-2.38
(IH, m), 0.98 (3H, d, J = 6.3Hz).
Example 14: (S)-4-~2-[2-Hydroxy-3-(4-hydroxy-3-
A~ 'Y)~ Jy;-]ethyl}l)! y - hy~FI~ -acid,
diethyl ester
~~ ---H~
[3 o
HO OH ~~P~
OEt
The title compound was prepared from (S)-4-(2-[3-(2,2-di-t-butyl-4H-1,3,2-
~n7n~ ciiin~n-6-yl-oxy)-2-hy~ y~ ,yl~,lli~lo]ethyl}~ lloAylll~lllyl
l~lu~ ..'r acid, diethyl ester using an ~ ~pr~ procedure similar to that
~s described in Example 4. The title compound was prepared and isolated as a white
foam.
-102-
~w096/04233 2 1 9 6 l 9 3 ~l/rl7~ 0~7
olH (200MHz, d6-DMSO): 7.20 ~2H, br d), 6.95 (3H, m), 6.67 (2H, m), 5.10 (IH,
br t), 4.45 (4H, m), 4.15 (7H, m), 3.88 (2H, m), 2.8 - 3.2 (4H, m), 1.25 (6H, t,J=6.5Hz)
Example 15: (S)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-l~ Ar ~ Yl-
phenoxy~ u~ ' ]ethyl}p ~ fe) ethyl ester, mûno
lithium salt
~ ~
~ ~
HO OH ~~P~
~L'
The title compound was prepared from (S)-4-{2-[2-hydroxy-3-(4-hydroxy-3-
lly~lluA~ ,Ll~yl~ lv~y)~lu~ylul~ u]ethyl3~ v~ylll~illy~ acid, diethyl
ester using a procedure similar to that employed for Example 5 and isolated as a solid
15 after freeze drying.
olH (250MHz, d6-DMSO): 7.0 (2H, d, J=8Hz), 6.70 (4H, m), 6.25 (lH, dd, J=8Hz
and 2.3Hz), 4.42 (2H. s), 4.20 (lH, br s), 3.75 (5H, m); 3.55 (lH, m), 2.4-2.8 (5H,
m), 0.95 (3H, t, J=6.7Hz)
Example 16: (SR)-4-{2-r2-Hydroxy-3-(4-hydroxy-3-i-~d~o~ '' .rl-
phenoxy)~,~ u~l~r6~ll;llo]propyl}ph ,~ , '~ ~ acid, bis-(3-
t ~IUA~ 1) ester
Me
O~--,7~
~3 ~3 p_p~\--O Ph~2
(SR)-4-~2-[3-(2,2-Di-t-butyl-4H-1,3,2-bcn7~ io~ ~ilin~n-6-yl-oxy)-2-hydroxy-
-u~lu~-~hlo]propyl~ vAy~ ho~ acid, bis-(3-benzyloxypropyl)ester
-103-
-
W096/04~33 21 9 61 9 3 ~ slQ~n~7
(0.49g, 0.56 mMol) was converted into the title compound by a method similar to
Example 4.
olH (250MHz, CDCl3): 7.25 (10~, m), 7.05 (2H, d), 6.73 (2H, d), 6.62 (IH, d),
6.55 - 6.35 (2H,m), 4.50 (2H, br), 4.42 (4H, s), 4.30 - 4.00 (8H, m, ), 3.70 (2H, br),
3.58 - 3.30 (SH, m), 3.30 - 2.85 (3H, overlapping br.), 2.71 (IH, br), 1.92 (4H, m),
1.18 (3H, d).
Example 17: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-hyd. v,.~ '' yl-
phenoxy)~ ",J ~ ~]propyl~pl~ .y~ lpt . ' acid, (3-
t,.,.,iylo~,. UpJI) ester, lithium salt.
Me
~~--H~
~ ~ O O Lii
HO OH O~P--O--~O~Ph
The title compound was prepared by a similar method to that of Example 5 from
(SR)-4-{2-r2-hydroxy-3-(4-hydroxy-3-hydroxymethyl-phenoxy)propylamino]-2-
propyl}pl.~,..u~ ,nin acid, bis-(3-benzyloxypropyl) ester (0.314g,
0 43mMol).
H (400MHz., CD30D): 7.15 (5H, m), 6.97 (2H, d), 6.74 (3H, m), 6.55 (lH, d),
6.48 (lH, dd), 4.49 (2H, s), 4.29 (2H, s), 4.00 - 3.80 (5H, m), 3.71 (2H, m), 3.47 (2H,
t), 2.82 - 2.54 (4H, m), 2.42 (IH, dd), 1.78 (2H, m), 0.92 (3H, d).
Example 18: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-l~.~d- u~, ' ' yl-
phenoxy)~.o~JI ~ -]Pr~PYI}F' .~ ' , ' - acid, bis-(3-
u~ u~ l) ester.
-104-
~ W0 96/04233 Me 2 1 9 6 l 9 3 p~ 7~lp~nl7
o~ H~
HO OH O~P~O~~OH)2
The title compound was prepared from (SR)~-{2.2-di-t-butyl-4H-1,3,2-
1r ~ -6-yl-oxy)-2-lly~ u;~yul u~ylamino]propyl ~ uAy lllcthyl-
p~ u~pll~ acid, bis-(3-hy~hu~y~u~yl) ester (0.248g, 0.36mMol) by a method
similar to that of Example 4.
olH (200MHz, CD30D): 7.25 (2H, d), 7.10 -6.90 (3H, m), 6.71 (2H, m), 4.64 (2H,
s), 4.42 (2H, d), 4.30 (5H, m), 3.97 (2H, m), 3.68 (4H, t), 3.54 (lH, m), 3.25 (3H,
m), 2.74 (IH, m), 1.91 (4H, m), 1.24 (3H, d).
Example 19: (SR)-4-{2-r2-Hydroxy-3-(4-hydroxy-3-llyJ. u~
phenoxy)~ JtJJ' ~]propyl}r ~ lhy~ - acid, mono-(3-
I-y-ll . ,~y~J. ., yl) ester, lithium salt.
Me
O ~~ H~
[~ O O U
HO OH O~P--O--~OH
The title compound was obtained by hydrolysis of (SR)-4-(2-[2-hydroxy-3-(4-
20 hydroxy-3-hydl u~ylll.,.hyl~llc.lul~y)propyl~mino]propyl ) phenoxy-methyl)pho~rh~nir
acid, bis-(3-hy.lluAy,ulu~,yl) ester (0.198g, 0.356mMol) using a method similar to that
~ of Example 5.
~ ~lH (250MHz, CD30D): 7. I l (2H, d), 6.90 (3H, m), 6.70 (lH, d), 6.60 (lH, dd),
4.62 (2H, s), 4.15 - 3.92 (5H, m) 3.85 (2H, d), 3.69 (2H, t), 3.05 - 2.68 (4H, m), 2.57
(lH, dd), 1.83 (2H, m), 1.08 (3H, d).
-1()5-
wo 96/04233 21 9 61 q 3 P~l/ra /~n~7
Example 20: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-hy(ll u~.y, '' yl-
phenûxy)propyl annino]prOpyl}F~ yl~.cthylphenylrh~ . ~ acid, ethyl
ester.
Me
H
~ OH
HO OH O~P--OEt
Ph
The title compound was prepared from (SR)-~2-[3-(2,2-di-r-butyl-4H-1,3,2-
in~ 6-yl-oxy)-2-l,y~Lu,.y~".",yl~ ino]propyl)phenoxy-
u~ hyllJln,~lyl~ ;ll;n acid, ethyl ester (().906g, 1.35mMol) by a method similar to
10 that of Example 4 and was obtained as a colourless gum.
m/z: FAB MH+ 530 (149~o)
Example 21: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-
I~y~ y ''~ ' y)propyl-amino]propyl~F'~ yl~ lyl-phen.
acid, lithium salt.
Me
O--H
~3 OH
HO OH O~P--OLi
Ph
The title compound was prepared as a white foam after freeze-drying, from (SR)-4-
{ 2-[4-hydroxy-3-(4-hydroxy-3-hydroxymethylphenoxy)propyl
amino]propyl]pl..,.lu,,y~ llyl~ lylrh~rhinir acid, ethyl ester (0.715g, 1.35mMol)
by a method similar to that of Example 5, except that methanol was used as co-
25 solvent instead of 1,4-dioxan.
-106-
21 961 93
96/04233 ~ 037
olH (250MHz, CD30D): 7.89 (2H, m), 7.40 (3H, m), 7.08 (2H, d), 6.91 (lH, d),
6.84 (2H, d), 6.70 (lH,d), 6.64 (IH, dd), 4.62 (2H, s), 4.20 - 4.00 (3H, m), 3.91 (2H,
m), 3.37 - 2.85 (4H, m), 2.62 (IH, dd), 1.15 (3H, d).
Example 22: (S)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-ll~dl u,.~...cthyl-
phenoxy)L.u~ l}r ~ .~1(3 I~ lu~
acid, n-butyl ester.
O--H~
1~3 ~ 11,
OH OH O~l o~3
OC~Hg
The title compound was prepared from (S)-4-{2-[3-(2,2-di-t-butyl-4H-1.3,2-
b..,,.,J;..~;l;"-- 6-yloxy)-2-hydluAy~u~ylamino]ethyl}l)h~.,u~y~ yl-(3-
benzyloxypropyl)pho~hi"ic acid, n-butyl ester according to the procedure described
15 inExample4, thecrudeproductwasusedwithoutfurthcr~,,,,iri,~li...
o(d6-DMSO + D2O): 7.43-7.28(5H, m.); 7.27(2H, d, J = 8.86Hz); 7.04(2H, d, J =
8.8ûHz.); 6.99(1H, d, J = 2.2ûHz.); 6.74-6.71 (2H, m.); 4.53(2H, s.); 4.44-4.42(2H,
m.); 4.40-3.91(7H, m.); 3.56(2H, t, J = 6.05Hz.); 3.25-2.90(6H, m.); 1.93-1.79(4H,
m.); 1.66-1.61(2H, m.); 1.44-1.39(2H, m.); 0.94(3H, t, J = 7.41Hz.).
Example 23: (S)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-
UA.~ ')L'I ~'IJ.~; ]ethyl}r ~ ~1 (3-
J.. ~lu.~ l,. u~ , acid.
- H ~1
~ 11 o~3
-107-
W0 96/04233 2 ~ ~;1 b 1 ~ 3 A ~ n~7 ~
The title compound was prepared from (S)-4-~2-12-hydroxy-3-(4-hydroxy-3-
lly~Lu~yl~ lyl-phenoxy)}~lu~yla~ o]ethyl~phenoxymethyl-(3-
b~ ylu~y~)luiJyl)~ho~llilli~, ~id, n-butyl ester according to a mr7~1ifir~tion of the
procedure described in Example 5. Ani~lifi~slrir~n to pH 3.5 with IM hydrochloric acid
followed by C18 reverse phase ~Llulllà~u~7~1ly, eluting with water-methanol (30%)
and freeze drying of the resultant foam gave the title compound as a solid.
~(d6-DMSO + D20)
7.31-?.21(5H, m.); 7.06(2H, d, J = 8.40Hz.); 6.88(1H, d, J = 2.97Hz.); 6.81(2H, d, J
= 8.40Hz.); 6.66(1H, d, J= 8.65Hz.); 6.59(1H, dd, J = 8.68, 2 99Hz.); 4.42( 2H, s.);
4.38(2H, s.); 4.124.09(1H, m.); 3.87-3.70(2H, m.); 3.72(2H, d, J = 8.00Hz.)
3.38(2H, t, J = 6.57Hz.); 3.40-2.95(4H, m.); 2.84-2.82(2H, m.); 1.73-1.67(2H, m.);
1.46-1.38(2H, m.).
Exarnple 24: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-
I-yd-u~.y~.ll-y', ' y)propylarnino]propyl}F y~l.;hyl(3-
t~lL,ylU.~ )}~- acid, n-butyl ester
Me
~ - H~
l I ~ o
OH OH O~ I ~~
OC4Hg
The title compound was prepared from (SR)-4- ~ 2-[3-(2,2-di-t-butyl-4H- 1,3,2-
b~n7n~1ioy~ in~n-6-yloxy)-2-hydroxy~lulJyla~ o]propyl}pll~llu;cylll~llyl-(3-
benzyloxypropyl)phosphinic acid, n-butyl ester according to the procedure described
25 in Example 4. The crude product was used without further ~Ju~irl~-alion~
o(CDC13 + CD30D): 7.44-7.30(5H, m.); 7.17(2H, d, J = 8.53Hz.); 6.82(2H, d, J =
8.57Hz.); 6.76-6.68(2H, m.); 6.65-6.59(1H, m.); 4.75(2H, s.); 4.51(2H, s.); 4.20-
3.86(7H, m.); 355(2H, t, J = 5.77Hz.); 3.28-3.09(4H, m.); 2.81-2.73(1H, m); 2.12-
1.91(4H, m.); 1.75-1.55(2H, m.); 1.48-1.37(2H, m.); 1.24(3H, d, J = 5.50Hz.);
O.gl(3H, t, J = 7.15Hz.);
-108-
21 96! 93
~wo s6l04z33 r~ /"'0'7
Example 25: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-h~d- v~y ' ' ~ lphenoxy)
p-UI~J -]Pr~PYI}F ~ n:lllyl(3-b~ YlUAYlUlU
acid, hydrochloride salt.
Me
O--H~ HCI
~ 11/~ o
OH OH O~ I --
OH
The title compound was prepared from (SR)~{2-[2-hydroxy-3-(4-hydroxy-3-
Ily~u1cyl~ y~ cllu~cy)~lul~ylà~ lo]propy~ v~y~ ,Lllyl(3-
10 benzyloxypropyl)~l,v~,~,l,i,d., acid, n-butyl ester according to a, ~ li ril A ~ of the
procedure described in Example 5. Acidification to pH 3.5 with IM hydrochloric acid
followed by C18 reverse phase l hlullla~ublalJlly, eluting with water-methanol (30%)
and freeze drying of the resultant foam gave the title compound as a solid.
~(d6-DMSO + D2O): 7.33-7.23(5H, m.); 7.00(2H, d, J = 8.31Hz.); 6.81(1H, d, J =
2.94Hz.); 6.75(2H, d, J = 8.32Hz.); 6.70(1H, d, J = 8.62Hz.); 6.28(1H, dd, J = 8.52.
2.69Hz.); 4.46(2H, s.); 4.41(2H, s.); 3.73-3.66(5H, m.); 3.42(2H, t, J = 6.58Hz.);
2.88-2.72(2H, m.); 2.67-2.52 (3H ,m), 1.79-1.69(2H, m.); 1.45-1.37(2H, m);
0.97(3H, d, J = 6.12Hz.).
Example 26: (S)-{2-[2-Hydroxy-3-(4-hydroxy-3-L.~d, UAy ' ' ,~ 1-
phenoxy),u, ~ ]ethyl}p~ y ~ acid, n-butyl
ester.
H~
OC4Hg
-109-
: :::
wo96104z~3 21 9 6 1 93 ~ s/r~ 7
The title compound was prepared from (S)-4-{2-[3-(2,2-di-t-butyl-4H-1,3,2-
t~l~n7~ y~ inAn-6-yloxy)-2-hyd}o~y~ Jylal~ o]ethyl}ph~ yl~ Lllyl
~,y~ lohc~.yl~ acid, n-butyl ester according to the procedure described inExample 4. The crude product was used without further ,IJUI irll,.:l6U~I
S
o(d6-DMSO + D2O): 7.18(2H, d, J = 8.25Hz.); 6.96(2H, d, J = 8.52Hz.); 6.89(1H,
s.); 6.70-6.66(2H, m.); 4.45(2H, s.); 4.31 (2H, d, J = 6.87Hz); 4.05-3.88(2H, m.);
3.78(D20 obscuring IH signal), 2.87-2.77(3H, m.); 2.54-2.52(3H, m.); 2.00-
1.04(15H, m.); 0.86(3H, t, J = 7.43Hz.).
Example 27: (S)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-
1~.' u.~.. Lh~5~ ,.u~ ]ethyl}pl ~.. ethyl.~r.'c' .vl
F'- . - acid, lithium salt.
~ - H~
~ 11
OH OH O~p
OLi
The title compound was prepared from (S)-4-(2-[2-hydroxy-3-(4-hydroxy-3-
llu~y~ Ll~yl-phenoxy)LJl~ llo]ethyl}p}~ ylll~llyl cyclohexylrh~-crh~ r
acid, n-butyl ester according the ptocedure described in Example 5 as a solid afler
C18 reverse phase ~ hy~ eluting with water-methanol (30%), and freeze
drying of the resultant foam.
o(CD30D): 7.24(2H, d, J = 8.56Hz.); 7.01(2H, d, J = 8.56Hz.); 6.90(1H, d, J =
3.09Hz.); 6.78(1H, dd, J = 8.76, 3.16Hz.); 6.70(1H, d, J = 8.75Hz.); 4.62(2H, s.);
4.04(2H, s.); 4.09-4.00(1H, m); 3.97(1H, dd, J = 10.45, 6.70Hz); 3.90(1H, dd, J =
10.46, 6.24Hz.); 2.91-2.71(6H, m.); 1.92-1.65(6H, m.); 1.33-1.15(SH, m.).
Example 28: (S)-4-~2-[2-Hydroxy-3-(4-
U~ Y),U~UIJ,~' -]ethyl}pt y~ h~l~yc's~ t ,' eacid,
n-butyl ester.
-I 10-
~wo s6104233 2 1 9 6 l 9 3 ~ 03n~7
H~
~ 11 ~
OH O~P
OC~,H~
A solution of (S)-4-(2-[2-hydroxy-3-(4-benzyluAyl~h~ uAy)propylamino]
ethyl}~JL~lluAyll~ yl~y~do~ Ay~ u~ l;r acid, n-butyl ester (I.OOg, 1.64mMol) in
S methanol (lOOml) containing lor~ palladium on charcoal (50mg) was l.y~ at~,d
at 40~C and 40 p.s.i. for 24 hours. After cooling to room t~,lu~ a~ulci the suspension
was filtered through a pad of filter aid and the filtrate was evaporated giving the title
compound.
o(dfi-DMSO + D20): 7.14 (2H, d, J = 8.8Hz), 6.93 (2H, d, J = 8.8Hz), 6.73 (2H, d, J
= 9Hz), 6.66 (2H, d, J = 9Hz), 4.31 (2H, d, J = 7.2Hz), 4.1 -3.7 (5H, m), 2.8-2.55 (6H,
m), 2.0-1.2 (15H, m), 0.86 (3H, t, J = 7.2Hz).
Example29: (S)-4-{2-[2-Hydroxy-3-(4-h.~d.uAy, ' Ay)~ rJ~ ~ -]ethyl}
r - ~ yl~c~~~ y~ , I c acid, lithium salt
o~ H~l
11
OLi
The title compound was prepared from (S)-4-~2-[2-hydroxy-3-(4-
hYdlUAYI ' y)lulo~y~ lolethyl~phenoxymethylcyclohexylrhrJ~rhinir acid, n-
butyl ester according to the procedure described in Example 5.
~(d6-DMSO + D20): 7.07 (2H, d, J = 8.5Hz), 6.81 (2H, d, J = 8.5Hz), 6.67 (2H, d, J
.25 = 9.1Hz), 6.60 (2H, d, J = 9.1Hz), 3.85-3.6 (5H, m), 2.8-2.5 (6H, m), 1.95-1.0 (llH,
m).
-111-
w0 96/04233 2 1 9 6 ~ '~ 3 ~ S/A~n~7
Example 30: (S)-4-[2-[2-Hydroxy-3-(3-
Ay~ y)~,O~y' ~]ethyl)F'L J?.y~ yl~y~ Ylr' , ' - acid,
n-butyl ester.
- H~
1 1
OC4Hg
The title compound was prepared from (S)~- (2-[2-hydroxy-3-(3-
b~ .ylvAy~ Ay)~u~yl.l~d~u]ethyl ~phenoxymethylcyclohexylllh. ~ h; l :r acid, n-
butyl ester according to the procedure described in Example 28.
o(d6-DMSO + D2O): 7.19 (2H, d, J = 8.5Hz), 7.05-6.98 (3H, m), 6.4-6.35 (3H, m),
4.33 (2H, d, J = 6.9Hz), 4.0-3.88 (SH, m), 3.25-2.8 (6H, m), 2.0-1.2 (lSH, m), 0.87
(3H, t, J = 6.4Hz).
Example 31: (S) 4-{2-[2-Hydroxy-3-(3-1.,~ d~ .y p' y)~.. v~,y ' - ]ethyl}
'Y~ Yl! , ' - acid, lithium salt
~ - H~
Ho~
OLi
The title compound was prepared from (S)-4-[2-[2-hydroxy-3-(3-
hy~vAy~h~v~y)~ Jy~ lo]ethylJphenoxylll~ lyL~y~lohexylllhl~ acid, n-
butyl ester according to the procedure described in Example S.
o(d6-DMSO + D2O): 7.01 (2H, d, J = 8.5Hz), 6.98 (IH, t, J = 8Hz), 6.78 (2H, d, J =
8.5Hz), 6.48 (lH, d, J = 2.2Hz), 6.3-6.25 (2H, m), 4.0-3.75 (SH, m), 2.85-2.55 (6H,
m), 1.9- 1.0 (I IH, m).
-112-
21 961 93
~wos6/04233 P~l/ILI._.
Example32: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-l-~l-u~yl~elllyl-
phenoxy)~,.u~.y -]propyl}pl y "yl~-' ' yl~ acid,n-butyl
ester.
Me
~ - H~
~3 OH
OH OH O~ I
OC4H3
The title compound was prepared frvm (SR)-4-[2-[3-(2,2-di-t-butyl-4H-1,3.2-
b..l,...l,..~-cilin ~ 6-yloxy)-2-hydroxypropylamino]propyl)ph~,..v~yl~ llyl
cy~,lul..,~.yl~ acid, n-butyl ester according to the procedure described in
Example 4. The crude product was used without further purification.
o(CDC13 + D2O): 7.19(2H, d, J = 8.80Hz.); 6.90(2H, d, J = 8.52Hz.); 6.78(1H, d, J
= 2,74Hz.); 6.72(1H, d, J = 8.80Hz.); 6.65(1H, dd, J = 8.80, 2.75Hz.); 4.68(2H, s.);
4.17(2H,d,J = 11.82Hz.);4.13-3.91(3H,m.).3.48-3.42(1H,m.);3.24(2H,d,J=
14.03Hz.); 3.10(1H, t, J = 9.90Hz.); 2.72(1H, t, J = 10.17Hz.); 2.01-1.06(15H, m.);
1.25(3H, d, J = 6.33Hz.); 0.93(3H, t, J = 7.15Hz.).
Example 33: (SR)-4-{2-[2-Hydroxy-3-14-hydroxy-3-l~yd- u~y ' yl-
phenoxy)~ ]propyl}FI- y~ ylc~kl Ylr . - acid, lithium
salt.
Me
~--H~
~3 OH
OH OH o~p
OLi
The title compound was prepared from (SR)-4 { 2-[2-hydroxy-3-[(4-hydroxy-3-
Lydlu~y~ ..llyl~h~,.lv7.y)propylamino~propyl } phenoxymethylcyclo
-113-
wo 96/04233 2 1 9 6 1 9 3 . . 11 . 7.~. 0 7
h~Ayll~h~ l;(. acid, n-butyl este} ascording the procedure described in Example 5
as a solid after C18 reverse phase ~hlollld~ y, eluting with water methanol
(30%), and freeze drying of the resultant foam.
o(D2O): 7.20(2H, d, J = 8.56Hz ); 6.96(2H, d, J = 8.64Hz.); 6.91(1H, d, J =
5.37Hz.); 6.80(1H, d, J = 8.76Hz.); 6.75(1H, dd, J = 8.75, 5.15Hz.); 4.63(2H, s.);
4.08-3.86(3H, m.); 4.01(2H, d, J = 7.55Hz.); 3.04-2.98(5H, m.); 1.87-1.67(7H, m.);
1.29-1.14(4H, m.); 1.09(3H, t, J = 6.17Hz.).
Example 34: (SR)-4-~2-[2-Hydroxy-3-(4-hydroxy-3-
]propyl}r~ hyl-n-hexyl F
acid, n-butyl ester.
Me
H--
~ OH ~
OH OH O~ I--CCH13
OC4H9
The title compound was prepared from (SR)-4-{2-r3-(2,2-di-r-butyl-4H-1,3,2-
n~n-6-yloxy)-2-hy~ y~ )yl~ lo)propyl)phenoxy-methyl-n-
L~y~ ,i.;,.,. acid, n-butyl ester according to the procedure described in Example
4. The crude product was used without further purification.
o(d6-DMSO+D20): 7.15(2H, d, J = 8.50Hz.); 6.94(2H, d, J = 8.63Hz.); 6.90(1H, d,
J = 2.84Hz.); 6.68(1H, d, J = 8.61Hz.); 6.63(1H, dd, J = 8.68, 2.97Hz.); 4.45(2H, s.);
4.35-4.28(2H, m.); 4.05-3.90(4H, m.); 3.86-3.81(1H, m.); 2.97-2.90(1H, m.);
2.89(3H, m.); 2.50-2.45(1H, m.); 1.86-1.79(2H, m.); 1.59-1.48(4H, m.); 1.38-
1.21(8H, m.); 0.97(3H, d, J = 6.25Hz.); ().86(3H, t, J = 7.28H~); 0.84(3H, t, J =
6.87Hz.).
Example 3~5: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-
1.~ y)~,O~ ]propyl}~ ",~II,y
acid, lithium salt.
-114-
~wos6/04233 2 1 9 6 1 q3
Me
~~--H~
~3 OH
OH OH o~ I--C6H~3
OLI'
The title compound was prepared from (SR)-4-l2-[2-hydroxy-3-(4-hydroxy-3-
hyJ~u~.y~ ,.Lyl~hc.~ y)~lu~yl~ o]propyl}phenoxymethyl-n-hexyl phosphinic
5 acid, n-butyl ester according the procedure described in Example 5 as a solid after
C18 reverse phase ~hIUI~IaLO~ IY~ eluting with water-methanol (30~o), and freezedrying of the resultant foam
o(d6-DMSO+D20): 7.09(2H, d, J = 8.48Hz.); 6.85(2H, d, J = 8.45Hz.); 6.89(1H, d,
J = 3.00Hz.); 6.68(1H, d, J = 8.62Hz.); 6.52(1H, dd, J = 8.62, 3.00Hz.); 4.47(2H, s.);
3.84(1H, t, J = 5.32Hz.); 3.82-3.73(4H, m.); 2.81-2.73(2H, m.) 2.71-2.61(2H, m.);
2.52-2.43(1H, m.); 1.44-1.41(4H, m.); 1.28-1.15(6H, m.); 0.95(3H, d, J = 6.24Hz.);
0.81(3H, t, J = 6.70Hz.).
Example 36: (S)-4-{2-[2-Hydroxy-3-(4-
I-~d~u-~ Y)IJ~U~ i ]ethyl}r .~.yl r~ acid, n-
butyl ester.
~---~ H--
~3 OH ¢~
OH O~ I--C6H13
OC~Hg
The title compound was prepared from (S)-N-benzyl-4-~2-[2-hydroxy-3-(4-
benzyll~,.yl ' y)~ yl~llllillo]ethyl)p~ lo~ylll~illyl-n-hexy~ acid, n-
butyl ester according to the procedure described in Example 28.
-115-
w096/04233 2 ~ q 6 ~ q 3 ~ J ~ 037
o(d6-DMSO + D2O~: 7.14 (2H, d, J = 8.5Hz), 6.92 (2H, d, J = 8.5Hz), 6.74 (2H, d, J
= 8.6Hz), 6.66 (2H, d, J = 8.6Hz), 4.35-3.75 (7H, m), 2.75-2.6 (6H, m), 1.85-1.75
(2H, m), 1.6-1.45 (4H, m), 1.4-1.25 (8H, m), 0.9-0.8 (6H, m).
Example 37: (S) 4-{2-[2-Hydroxy-3-(4-
L,.~ ur~ Y)lJ~rJ~ ~ ]ethyl]pl~ 1 n ' ,~', '~ . - ~ acid.
~ ~ H~
OH
OH O~P--C6H
OH
The title compound was prepared from (S)-4-12-[2-hydroxy-3-(4-
h~v;u~y~ uAy)~ul~yL~ v]ethyl}ph~ v~y~ d~yl-n-hexyl phosphinic acid, n-
butylesteraccordinga",-.,l;l;. ~01lll of IheproceduredescribedinExample25.
ifir~tinn to pH 6 with lM hydrochloric acid followed by C18 reverse phase
15 cL~ Jhy and freeze drying gave the title compound as a solid.
~(d6-DMSO + D2O): 7.05 (2H, d, J = 8.5Hz), 6.79 (2H, d, J = 8.5Hz), 6.65 (4H, b),
3 9-3 7 (5H, m), 2.85-2.6s (6H, m), 1.4 (4H, m), 1.2 (6H, m), 0.80 (3H, t, J = 7.0Hz).
Example 38: (SR)-4-{2-[2-Hydroxy-3-(4-hydro1~:y 3 llydl u~ yl
~- Y~ ~~ ]propyl}p- ~ acid~ bis~(2
F~I~LIIyl) ester
Me
H~3
OH OH O~P~O
The title compound was prepared from (R)-4-12-[3-(2,2-di-t-butyl-4H-1,2,3-
"-' 6-yloxy)-2-(S)-l.yd~u"y~lu}~yl~ o]propyl]phenoxy
-116-
~ W0 96/04z33 2 1 9 6 1 9 3 . ~ 17~ n~7
methyl~ ,l,. ,";o acid, bis-(2-phenylethyl) ester according to the procedure
described in Example 4. The crude product was used in the next stage without further
Example 39: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-h~d. u~.,..lhyi-
p' ~)~ u~~]propyl},l' ~ .; . ' acid, 2-1~' yhlllyl
ester, lithium salt
Me
O--N'~
~3 11
OH OH O~ I _o~
OLi
The title compound was prepared from (SR)-4-(2-[2-hydroxy-3-(4-hydroxy-3-
I~y~llu~ y~ u~y)propylamino]propyl}phenox~ y~ acid, bis-(2-
phenylethyl) ester according to the method described in Example 5 as a solid
following el."-" ".~ ,l,y on Cl8 reverse phase silica-gel, eluting with 40~o
methanol-water, and freeze drying of the resultant foam.
~(d6-DMSO + D2O): 9.44 (IH, s, exchanges with D2O); 7.15-7.26 (5H, complex
m.); 7.03-7.05 (2H, d.), 6.81-6.82 (IH, d.); 6.67-6.78 (2H, d.); 6.67-6.69 (2H, d.);
6.35-6.38 (IH, dd.); 5.08 (IH, t.); 4.43-4.44 (2H, d.); 3.94-3.99 (2H, q.); 3.65-3.76
(5H, complex m.); 2.78-2.83 (2H, t.); 2.45-2.76 (3H, complex m.); 0.95-0.96 (3H,d.).
Example 40: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-h.y i- u,.~---.lhyl-
u,u.r' ~ -]propyl}rl- .r ' ylbenzylr'- , ' - acid, n-butyl
ester
-117-
21 961 93
wo s6/04233 ~ 0
Me
~U ~
OC~H~
The title compound was prepared from (SR)-4-(2-[3-(2,2-di-s-butyl-4H-1,3,2-
YAciljn~.~ 6-yloxy)-2-hydroxypropylamino]propyl]phenoxy
S ~ ,Ll~ylb~.lLyl~ uo acid, n-butyl ester according to the procedure described in
Example 5. The compound was used in the next stage without further ~ ul irl~"lLi~
o(CDC13+D2O): 7.24-7.26 (5H, s.); 7.05-7.09 (2H, d.); 6.65-6.72 (3H, complex m.);
6.40-6.62 (3H, complex m.); 4.58 (2H, s.); 3.82-4.08 (4H, complex m.); 3.68-3.72(2H, d.); 3.25-3.30 (2EI, d.); 2.52-3.00 (5H, complex m.); 1.50-1.63 (2H, m.); 1.27-
1.39 (2H, m.); 1.03-1.15 (3H, d.), 0.86-0.93 (3H,t.)
Example41: (SR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-hydl~ lphenoxy)-
~r~ -]Pr~PYl}F y ' ' yl1...~,yl~u I~ , ' ~ ~ ~ acid.
Me
~J3 OH ~3
OH OH O~P
OH
The title compound was prepared from (SR)-4-(2-[2-hydroxy-3-(4-hydroxy-3-
llydl~J~ylll~,Lllyl-phenoxy)lJlu~yla,ll;llo]propyl)phenoxymethylbenzyll.h~ acid,
n-butyl ester according to the method described in Example 5 followed by
~ni~ ." to pH 3 5 with lM hydrochloric acid~ as a solid (mp l8o-l83oc)
following l,hl~ ,lly on C18 reverse phase silica-gel, eluting with 40~c
methanol in water.
o(d6-DMSO + D2O): 7.00-7.24 (7H, complex m.); 6.94-6.95 (IH, d.); 6.79-6.81
(2H, d.); 6.70-6.72 (lH, d.); 6.61-6.69 (IH, m.); 4.46 (2H, s.); 4.14-4.17 (IH, m.);
-118-
21 961 93
~wos6/o~33 r~ Q~7
3.83-3.91 (IH, m.); 3.64-3.69 (2H, d.); 3.25-3.30 (IH, m).; 2.98-3.29 (SH, complex
m.); 2.92-3.00 (2H, d.); 1.03-1.23 (3H, d.)
Example 42: (S)-4-{2-[3-(4-Hydroxy-3-hyd- UAy ' ~ ylphenoxy)~rouy ~ -]
S ethyl}~ Y I Yl! ~ . ' ~ acid,ethyl ester
~ ~~ ~
HO OH O~P~
The tit e compound was prepared from (S)-4-~2-[3-(2,2-di-ser~-butyl-4H-1,3,2-
I.~.,"l.l;.,~ciiin~- 6-yloxy)-2-hyd~uAyl~u,~yla~hlo]ethyl~ cllu:~yllleLllylphenyl
phosphinic acid, ethyl ester (0 926g, 14 1mMol) by a method similar to that ûf
Example 4 and was obtained as a colourless gum.
rn/z: MH~ 516 (609'o).
Example43: (S)-4-{2-[3-(4-Hydroxy-3-h~ h UAy " ylphenoxy)-2-
L,~ UA.~,~,. U,U,~ ]ethyl}p' y '' y'~ '- , ' ~ acid, lithium salt
O ~1
OH
Il ,OLi
HO OH O~P~
The title compound was prepared as a white foam (after freeze-drying) from (S)-4-
(2-[3-(4-hydroxy-3-hydluAyl.l~,~l,ylphenoxy)-2-hydroxy~Jlu,uyldl~ o]ethyl3-
luL~,lluAyl~ llyllulle.lylllh.l~ ;lli. acid, ethyl ester (483mg, 0.94mMol) by a method
similar to that of Example 21.
o'H (2~0MHz, CD30D): 7.89 (2H, m), 7.41 (3H, m), 7.09 (2H, d), 6.90 (IH, d), 6.82
(2H, d), 6.69 (IH, d), 6.63 (IH, dd), 4.62 (2H, s), 4.05 (3H, m), 3.87 (2H, m), 3.05-
2.63 (6H, m).
Example 44: (S)-4-{2-[3-(4-Hyd, UAy~- y)-2-hyd. uAy,urù,u~; ~]ethyl}-
p' y~,,.lllylphenyl! ' ~ -' ' ', acid, ethyl ester
_119_
21 96l 93
wo 96/04233 r.,~ 7r.'A~7
H
[~ OH
ll ,OEt
OH O~P~
The title compound was prepared from (S)-4-(2-[3-(4-b~ ylu~y~ ,.lu7y)-2-
L~LoAytJlu~Jyla.~ o]ethyl)lJh~.~uAy~ ,8lyl~ lyll.hc~ acid ethyl ester
according to the procedure described in Example 28.
o'H (250MHz, CDJOD): 7.92 (2H, m); 7.65 (3H, m); 7.14 (2H, d, J=8.6Hz); 6.87
(2H, d, J=8.6Hz); 6.74 (4H, m); 4.51 (2H, m); 4.20 (2H, m); 4.0~ (IH, m); 3.93 (2H,
d, J=6.5Hz); 2.9-2.7 (6H, m); 1.38 (3H, t, J=7.1Hz).
Example 45: (S)-4-{2-[3-(4-H~dl uAyt '- y)-2-hydrûxyp~ ]ethyl}
~: J by', Ylr ~ acid, lithium salt
O~--H
OH
Il ,
OHO~P~
The title compound was prepared from (S)-4- 12-[3-(4-hydroxyphenoxy)-2-
15 hy~LuAy~u~ylalllillo]ethyl~ lluAy~ Jlylpheny~ acid, ethyl cster
according to the procedure described in Example 5.
c~t'H (250MHz, CD,OD): 7.94 (2H, m); 7 41 (3H. m); 7.08 (2H. d. J=8.4Hz); 6.82
(2H, d, J=8.3Hz); 6.75 (4H, m); 4.07 (3H, m); 3.85 (2H, d, J=6Hz); 2.9-~7 (6H, m).
Example46:(S,R)-4-{2-[3-(4-H,~dlo..y~' y).2-hydroxypropylaminû]propyl}-
~_y~ yll ~- ' yl~ r acid, ethyl ester
Me
[~) OH
ll ,OEt
OHO~P~
-120-
21 961 93
wo 96/04233 r ~ I " . r ~ ~7
The title compound was prepared from (S,R)-4- { 2-[3-(4-benzyloxyphenoxy)-2-
Ly~Lu~y~Jlu~ylalll;no]propyllphenoxymethylpheny]~ acid, ethyl ester
according to the procedure described in Example 28.
o'H (250MHz, CD30D): 7.89 (2H, m); 7.64 (3H, m); 7.18 (2H, d, J=8.8Hz); 6.92
~ (2M, d, J=8.0Hz); 6.75 (4H, m); 4.52 (2H, m); 4.18 (3H, m); 3.92 (2H, m); 3.45 (IH,
m); 3.35-3.08 (3H, m); 2.7 (lH, m); 1.4 (3H, t, J=7.4Hz); 1.22 (3H, d, J=7.7 Hz).
Example 47: (S,R)~4-{2-t3-(4-Hyd, UA~ ' y)-2-14~d, UA~.~t~,. u,u,~' ' ]propyl}
10 P' ~ - Ylr~ ~, '~ . ' ~ acid, lithium salt
Me
O--NH~
OH [~
Il OLi
OH O~P~
The title compound was prepared from (S,R)-4-~2-[3-(4-hydroxyphenoxy)-2-
h.~u~y~lu~,ylà~ lo]propylJphenoxymeth~ ,h~ lllu~llllill;~ acid, ethyl ester
15 according to the procedure described in Example 5.
o'H (250MHz, CD50D): 7.9 (2H, m); 7.42 (3H, m); 7.08 (2H, d, J=8.4Hz); 6.84
(2H, d, J=8.3Hz); 6.75 (4H, s); 4.08 (3H, m); 3.82 (2H, m); 3.1-2.55 (5H, m); 1.08
(3H, d, J=7.7Hz).
Example48: (S,R)-4-{2-[3-(4-Hydroxy-3 -~I rull~ y)-2-
UA~ ~ ~]PrOPYI}rh~ l ' ii I ~ acid, ethyl ester
Me
--H~l
The title compound was prepared from (S,R)-4-~2-[3-(4-benzyloxy-3-
mPti' ~lPCIl I rl,"~ y)--2--ily~ A YYI l~)yldll .illol propyl J ~ ,. Iy ¦ I, I
acid, ethyl ester according to the procedure described in Example 28.
-121-
W0 96/04233 2 1 q 6 1 9 3 r~
o'H (250MHz, d'-DMSO): 7.8 (2H, m); 7.68 (lH, m); 7.61 (2H, m); 7.16 (2H, d,
J=8.2Hz); 7.01 (IH, d, J=1.40Hz); 6.94 (2H, d, J-7.9); 6.84 (lH, d, J=8.4Hz~; 6.71
(lH, m); 4.52 (2H, m); 4.17 (3H, m); 3 96 (2H, m); 3.3-3.14 (4H, m); 2.94 (3H, s);
2.71 (IH, m); 1.38 (3H, t, J=7.4Hz); 1.21 (3H, d, J=7.1Hz)
Example49: (S,R)-4-{2-[3-(4-Hydroxy-3-- ~r~ y)-2-
~ dIUA.~ U~U~- -]propyl}r - .t ~YlF'~ p~- ~~ acid,
I.y~ ul~. ~ ' ' salt
Me
O--N'~
2 N ~ 3 0
H OH HBr o p,OH
The title compound was prepared from (S.R)-4-(2-[3-(4-hydroxy-3-methane
~ulrullyl~ y)-2-hydroxypropylamino]propyl~ clAylll~ ylphenyl-
phosphinic acid, ethyl ester and trimethylsilyl bromide using the procedure described
by D.M. Walker et. al. J. Chem. Soc. Chem. Commun., (1987) 22, 1710.
H (250MHz, CD30D): 7.9 (2H, m); 7.48 (3H, m); 7.13 (2H, d); 7.0 (IH, d); 6.89
(2H, d); 6.83 (IH, d); 6.68 (IH, dd); 4.20 (3H, m); 3.95 (2H, m); 3.5-2.9 (4H, m);
2.93 (3H, s); 2.72 (IH, m); 1.23 (3H, d); m/z: [M-HI 563 (2846).
Example 50: (S) 4-{2-[3-(4-llyd~ u.~y ~ ' ~)-2-
~ d~u~ ,u~ut~ ]ethyl~FI. ~, ' yl(3 b~ lut.,~,ur(J~ h(, ' ~ - acid,
n-butyl ester, ll,~d- ~ ~ ' ;dc salt.
O--N~ HCI
[~3 OH
OH O~P 0~~3
OC~Hg
A solution of (S) 4-~2-[3-(4-t-butyldimethylsilyloxyphenoxy)-2-
hy~w~y~ yL~ ;llo]ethyl}phc~lu~yll~ lyl(3-benzyloxypropyl)phb~ in;c acid. n-
-122-
... . . . ... . . _ _ . . ... .. . . _ .. ..
~wos6/~)4233 2 1 9 6 l 93 ~ 7
butyl ester ~199mg, 0.28mMol) in dichlvlu~ ,Llldlle (lOml) and IM hydrogen chloride
in diethylether (Iml) was stirred at room ~ullJ~,laLul~ for 2 hours. The solution was
~JI....,I"u~l giving the title compound as a solid.
~IH (d6~DMSO + D20): 7.4-7.25 (SH, m)7 7.19 (2H, d, J = 8.8Hz), 6.98 (2H, d, J =8.6Hz), 6.77 (2H, m), 6.68 (2H, m), 4.45 (2H, s), 4.35-3.8 (7H, m), 3.5-2.8 (8H, m),
1.95-1.75 (4H, m), 1.6-l.S (2H, m), 1.4-1.25 (2H, m), 0.86 (3H, t, J = 7.4Hz).
Example 51: (S) 4-{2 [3-(4-H~I~ UA~ y)-2-
L~d~uA,~,.u~,~k.minû]ethyl}.~ hyl(3-l;~ luA~,,û~ k, ~ -acid,
lithium salt.
O--o/~ H ~
OH O~ --~3
The title compound was prepared from (S) 4-12-[3-(4-hydroxyphenoxy)-2-
lly~UAy~lul~yla~ lo]ethyl]~ v~ylll~ lyl(3-l~ yluA~lu~yl)~/llo~ acid, n-
butyl ester, hy~ . h~ . according to the procedure described in Example S.
olH (d6-DMSO + D20): 7.36-7.26 (5H, m), 7.09 (2H, d, J = 8.5Hz), 6.82 (2H, d, J
= 8.5Hz), 6.7-6.6 (4H, br), 4.42 (2H, s), 3.8-3.6 (SH, m), 3.41 (2H, t, J = 6.7Hz), 2.7-
2.55 (6H, m), 1.75-1.65 (2H, m), 1.45-1.30 (2H, m).
Example 52: (S,R) 4-{2-[4-Hydroxyphenoxy)-2-
h ~1. UA~ ' U,U,r ]Pr~PYI}t .Y Yl~(3 t ~YIUA~ u,').~l)t-l~ ---r ~
25 acid, n-butyl ester, h~r.l. . ' ' ;dc.
=CH3
O--N~
OH ~,
OH O~P--(cH2~3oBn
OC~Hg HCI
A solution of (S,R) 4-(2-[4-t-ButyldimethylsilyluAypllc.luAy)-2-
30 hy~u~y~u~ylaulh~o]propyl)phenoxymethyl-(3-benzylol~y~lolJyl)~ht~ h~;~ acid, n-
-123-
wo s6/04233 2 l 9 6 ~ 9 3 ~ 1,5. .
butyl ester (525mg, 0.74mMol) in methanol (50ml) was treated with acetyl chloride
(2.70ml). The reaction was stirred ~or 8 hours at ambient L~ dLul~; and solvent
removed to give the title compound.
~'H (d'-DMSO): 7.37-6.67 (13H, m), 4.45 (2H, s), 4.33 (2H,m), 4.18 (IH brs), 4.04-
3.83 (4H, m), 3.48 (2H, t), 3.35 (IH,m), 3.18 (2H, m), 3.06 (lH, m), 2.60 (lH,
t),1.95-1.81 (4H, m),l.53 (2H, m), 1.32 (2H, m), 1.08 (3H, d), 0.86 (3H, t).
Example53: (S,R)4-{2-[4-H~d.vJ~ )-2-
10 i-~. 0~ ut~; ~]propyl}p .~ yl-(3 t ~lu~yl~ro,~ h p~ -
acid, lithium salt.
CH3
H~
[~3 OH ~
OH O ~ P--(CH2)30Bn
OLi
The title compound was prepared from (S,R) 4-(2-[4-hydroxyphenoxy)-2-
Lyvlu~ nul~yla~ u]propyl}phenoxymethyl-(3-benzyloxypropyl)~h.~L~h;";n acid, n-
butyl ester, hydrochloride according to procedure desctibed in Example 5.
~IH (d'-Dl\l[SO): 7.32 650 (13H, m), 4.41 (2H, s), 3.80-3.69 (5H, m), 3.45 (2H, t),
2.95-2 50 (5H, m), 1.75 (2H, m), 1.44 (2H, m), 0.95 (3H, d).
E~ample 54: (S,R) 4-{2-[4-Hyvl u~,y~ 2-
llyJIu~y~ ' ~]propyl}FL y -'h~ ,' ' ylph~ pL -acid,n-butyl
ester.
CH3
~ - H~
OH ~~,P O
OC~H~
The title compound was prepared from (S,R) 4- ~ 2-~4-benzyloxyphenoxy)-2-
hydlu~y~lu~ylâmino]propyl~ .lu~yl~ llylcyclohexylrhmcrhinir acid, n-butyl ester
30 according to the procedure described in Example 28.
-124-
2~ q61 93
wo 96/04233
olH (CDCI,): 7.10-6.45 (8H, m), 4.25-3.80 (7H, m), 3.20-2.75 (5H, m), 2.15-1.15
(18H, m), 0.93 (3H, d).
S Example 55: (S,l'c) 4-{2-[4-Hy~l~ u~y~ 2-
1~ .J~-UU.~ ]propyl},~ yle~ C ~ 5, ' ~ ~- acid, lithium
s~dlt.
~ CH3
H~
OH
OH O~ P;~{~
The title compound was prepared from (S,R) 4- { 2-[4-hydroxyphenoxy)-2-
I~y~llu~y~Jlu,uyld~ lo]propyl}~ Ay~ lylc~ lollc~y~ hini~ acid,r~-butylester
according to the procedure described in Example S.
IS olH (d'-DMSO + D,O): 7.20 (2H, d), 6.80 (2H, d), 6.71 (4H, m), 4.11 (IH, m),3.84-3.77 (4H, m), 3.08-2.82 (SH, m), 1.88-1.23 (I IH, m), 0.98 (3H, d).
Example 56: (S,R) 4-{2-[4-H.~ druAy~ )-2-
1.. ~ d u~y,u~ - ]propyl}p~ hylhexylph~ , ~ ~ ic acid, n-butyl e~ster.
CHa
~ ---H~
OH
OH O~P--(CH2)sCHa
OC~H9
The title compound was prepared from (S,R) 4-(2-[4-benzyloxyphenoxy)-2-
Ly~Lu~yp~o,uylamino]propyl ~phenoxymethylhexylphosphinic acid, n-bulyl ester
25 according to the method described in Example 28.
o~H (d'-DMSO + DIO): 7.15-6.64 (8H, m), 5.25 (2H, s), 4.30 (2H, m), 4.02-3.80
(SH, m), 3.10-2.75 (4H, m), 2.45 (IH, m), 2.40-1.70 (13H, m) 0.96 (3H, d), 0.86
(6H, m).
-125-
. .
wos6/04233 2 1 9 6 1 93 ~ r,~7 ~
Example57: (S,R)4-{2-[4-Hyd.v~yl' y)-2-
A,~ u,~ ]Pr~PYl}r ,~ ylhe%ylr~ , L -~ acid lithium salt.
CH3
O--N
[~ ~ 8
OH O ~ IF--(CH2~scH3
OLi
The title compound was prepared from (S,R) 4-{2-[4-hydroxyphenoxy)-2-
hyd~vAy~lu~ylamino]propyl}ph~.lu~y~ ylh-,~.yl~ u~ lir acid, n-butyl ester
according to the procedure described in Example S.
o'H (d'-DMSO): 7.04-6.41 (8H, m), 3.80-3.63 (5H, m), 2.90-2.50 (5H, m), l.SO-
1.20 (IOH, m), 0.97 (3H, d), 0.83 (3H, t).
Example 58: (S)-4-{2-[3-(3-Fluoro-4-hydroxyphenoxy)-2-
L.~d.uAy,u,~ ]~:hyl}l ' y hyl~ 'e-- yl~ ' . ' ~ ~- acid, n-butyl
15 ester.
-- H~
~3 OH [~
OC4HG
A solution of (S)-4-[2-[3-(4-benzyloxy-3-lluu-u~ .,oxy)-2-
20 hydlv~y~Jlu~yla~ u]ethy~ cllv~ylll~dlylcyclohexyl phosphinic acid, n-butyl ester
(0.88g, 1.36mMol) in methanol (SOml) containing palladium (Il) chloride (20mg) was
l.ydlub_..dLt;d atroom t~ ,.dlUl~i and dLluvs~Jh~,lic pressure for 4 hours. The
suspension was filtered through a pad of filter aid and the filtrate was evaporated
giving the title compound.
H (CDC13 + D20): 7.10 (2H, d, J = 8.5Hz), 6.88-6.78 (3H7 m), 6.54 (IH, dd, J =
12.2, 2.8Hz), 6.36-6.32 (lH, m), 4.16-3.98 (SH, m), 3.84 (2H, d, J = S.OHz), 3.06-
2.77 (6H, m), 2.02-1.23 (ISH, m), 0.92 (3H, t, J = 7.3Hz).
-126-
21 9bi 93
WO 96104233 F~ 7
Example 59: (S)-4-{2-[3-(3-Fluoro-4-hyJ- ù~yl '- y)-2-
hyd.(.~y,u.u,.Jl.. mino]ethyl}p'~ .~ .lhyl~ Y~l r ~ acid, litbium
5 salt
O--~ H~
~3 OH
OLi
The title compoand was prepared from (S)-4-{2-[3-(3-fluoro-4-hydroxyphenoxy)-2-
10 hydlu~y~u~yL~ h~u]ethyl)p~ o~y~ Lllylcyclohexylrh~srhinir acid, n-butyl ester according to the procedure described in Example 5.
~'H (d6-DMSO + D2O): 7.04 (2H, d, J = 8.6Hz), 6.89 (IH, dd, J = 10.2, 8.9Hz),
6.75 (2H, d, J = 8.6Hz), 6.60 (lH, dd, J = 12.9, 2.9Hz), 6.08 (IH, ddd, J = 8.9, 2.8,
1.3Hz), 3.91-3.63 (3H, m), 3.67 (2H, d, J = 7.7Hz), 2.78-2.58 (6H, m), 1.85-1.11(1 lH, m).
Example 60: (S)-4-{2-[3-(4-Hydroxy-3-h~dl u~y ' ~ l)phenoxy-2-
hYLU~.Y~.~U~J~ ~]ethyl}p'- y -~hyl(3-p- yl~lu~yl)~ - ~-acid,n-
20 butyl ester
~ '------H~
~,~ OH ~3
The title compound was prepared from (S)-4-{2-[3-(4-benzyloxy-3-
25 h~u~yl~,.hyl)phenoxy-2-hydlu~ lv~)ylamino]ethylJphenoxymethyl(3-
~vh~ yl~lu~yl)pl~ .l;n acid, n-butyl ester according to the method described in
Example 28. The crude product was used without further l,u, ir~cd~iuu.
Mass Spectrum m/z 586(92%)MH~
-1~7-
wo 96/04233 2 1 9 6 1 9 3 1 ~11~1 ~ o ~7 ~
Example 61: (S)-4-{2-[3-(4-Hydroxy-3-hyd, u7.y ' yl)pbenoxy-2-
~ J. u~ t~ ]ethyl}p~ ~ ~ yl(3-phenylpropyl)l ' . ' s acid,
lithium salt
~ H
~ ~ OH ~,3
The title compound was prepared from (S)-4-{2-[3-(4-hydroxy-3-
Lyd~w~y~ yl)phenoxy-2-llydlu~y~lulJylamino]ethyl3phenoxymethyl(3-
!JL~ ulJyl)phosphinic acid, n-butyl ester according to the method described in
10 Example 5. The crude product was purified by chromatography over C-18 reverse phase silica eluting with wdLt~ ,dlallul mixtures.
ulH (d6-DMSO + D2O): 7.26-7.22 (2H, m); 7.16-7.12 (3H, m);7.06 (2H, d, J =
8.63Hz); 6.89 (IH, d, J = 2.95Hz); 6.79 (2H, d, 8.63Hz); 6.69 (lH, d, J = 8.67Hz.);
6.55 (IH, dd, J = 8.67, 3.07Hz); 4.45 (2H, s); 4.104.00 (IH, m); 3.82 (2H, t, J =
5.35Hz); 3.71 (2H, d, J = 8.00Hz); 2.96-2.90 (3H, m); 2.85-2.74 (3H, m); 2.58 (2H, t,
J = 7.48Hz); 1.79-1.73 (2H, m); 1.45- 1.23 (2H, m)
Exarnple 62: (S)-4-{2-[3-(4-Hydroxy-3-hy-lrv~y -'hyl)phenoxy-2-
l~d~v~ v~]ethyl}p~ ~.... ethyl(3 p~ ~t~VPy~ acid,
ethyl ester
0~\H/~
11
OH OH O ~ p ~\--o
OEt
The title compound was prepared from (S)-4-(2-[3-(4-benzyloxy-3-
hydlvAylll~.illyl)phenoxy-2-llydlu~y,ulu~Jyl.llllillu]ethyl}phenoxymethyl(3-
I ~ yl lu~yl)phosphinic acid, ethyl ester according to the method described in
Example 28. The crude product was used without further pu~irl~ iull.
-128-
~ wo 96/04233 ;~ ~ 9 ~ 7 ~ 3 ~ 7
ôlH (CDC13+D2O): 7.30-7.24 (3H, m.); 7.12 (2H, d, J = 8.52Hz); 6.97-6.74 (6H,
m); 6.53 (lH, d, J = 8.52Hz); 4.73 (2H, s); 4.21-3.98 (7H, m); 3.79-3.73 (2H, m);
2.96-2.77 (6H, m); 2.35-2.10 (4H, m); 1.34 (3H, t, J = 7.15Hz)
S Example63: (S)-4-{2-[3-(4-Hydroxy-3-h~J-uA~ ~'hyl)phenoxy-2~
~ I.. ~d uA~.~,u~,U~ ]ethyl}~ yl(3-p'~ ~t~ropyl)p~ ,' -acid,
h.~ d. . ' ' ;dc salt
O ~\ H ~ HCI
OH OH O ~ P ~ O J3
OH
The title compound was prepared from (S)-4-(2-[3-(4-hydroxy-3-
llr~llu~yl~ lyl)phenoxy-2-lly~lluAyl)lul~ylamino]ethyl~phenûxymethyl(3-
I)h~ ùAy~JlulJyl)l~h~ h~;. acid, ethyl ester according to the method described in
Example 25. The crude product was purified by ~hIUIIIaIO~ Y over C-18 reverse
phase silica eluting with vvu;~ LL~ ol mixture.
~'H (d6-DMSO + D2O): 7.23 (2H, t, J = 7.7Hz); 6.94 (2H, d, J = 8.8Hz); 6.93 (IH,s); 6.89-6.86 (3H, m); 6.72 (2H, d, J = 8.6Hz); 6.67 (IH, d, J = 8.64Hz); 6.56 (IH,
dd, J = 2.9, 8.6Hz); 4.44 (2H, s); 4.18-4.16 (IH, m); 3.97 (2H, t, J = 6.5Hz); 3.87-
3.79 (4H, m); 3.08 (IH, d, J = 10.3Hz); 2.97-2.90 (3H, m); 2.83-2.81 (2H, m); 1.97-
1.87 (2H, m), 1.61-1.53 (2H,m).
Example 64: (S,R)-4-{2-[3-(4-H~-I- UA~ ' y)-2-hyd- UA.~U~ uu~lamino]propyl}
r . - acid, bis-cyclohexyl ester, hydrochloride salt
Me
~--NH ~3
OH O~P~
HCI
The title compound was prepared from (S,R)-4-{ 2-[3-(4-benzyloxyphenoxy)-2-
L~d~w~y~lul~ylamino]propyl}phenoxymethyll~h~ acid, bis-cyclohexyl ester
according to the method described in Procedure 9. The crude product was purified by
-129-
W0 96/04_33 2 1 9 6 1 9 3 P~ C/Q~n~7 ~1
crystallisation of the hydrochloride salt from dichlulu~ ,d~dne to give a solid (mp
187-189~C).
~'H(CDC13 + D2O): 7.18-7.21 (2H, d), 6.87-6.90 ~2H, d), 6.25-6.28 (2H, d), 6.20-6.22 (2H, d), 4.68-4.72 (IH, m), 4.45-4.58 (2H, m), 4.18-4.22 (2H, d) 4.00-4.06 (lH,
m), 3.88-3.93 (lH, m), 3.11-3.48 (4H, m), 3.28-3.33 (lH, dd), 1.27-2.00 (20H, m),
1.35-1.37 (3H, d).
Example65: (S)-4-{2-[3-(4-H,~I~u~ '- y) 2-h.~d~uA~ulûu.~ ].. l.yl}
1 ,~ acid, bis-(2,2-diphenylethyl)ester
- H~
~ 11
OH O~P~o ~
_ ~ _2
The title compound was prepared from (S)-4-(2-[3-(4-benzylv~y~ ,..v;~y)-2-hydroxy
15 l~lu~yl~ hlv]ethyl~L~ u~y~ .d~yl~ h~ acid, bis-(2,2-diphenylethyl) ester
according to rhe method described in Procedure 9. The title compound was obtained
as an off-white solid.
olH(d6-DMSO + D~O): 6.72-7.41 (28H, complex); 4.32-4.66 (7H, m); 3.81-4.20
20 (4H, m); 2,72-2.99 (6H, m).
Example66:(S)-4-{2-[3-(4-Hyd-u,.~l y)-2-ll.~d~u~yl~lo~ldminû]ethyl}
Ip'~ acid, bis-(2-phenylethyl) ester
- H~
O ~ ~ P ~3
The title compound was prepared from (S)-4-{2-[3-(4-benzylu~y~ ",u~y)-2-
llydlu~y~lu~yla~h~O]ethyl~phenoxylll~..llyll.h~ vl6n acid, bis-(2-phenylethyl) ester
-130-
~1 961 ~3
wo96/04233 r~ /o~n~7
according to the method described in Procedure 9. The solid thus obtained was
crystallised from methanol/dichlu~ .h~lne (1:1) to give the title compound (mp 75-
76~C).
S olH(d6-DMSO+D20): 6.83-7.40 (18H, m); 3.80-4.72 (9H, m); 2.66-3.05 (IOH, m).
Example67: (RR)4-{2-[2-Hydroxy-3-(4-hydroxy-3-l-,~diu~y 'hylp!~ y)
I ~ ]propyl}P' ~ -~hylphen.~ acid, ethyl ester.
Me
o~f H~
~3~OH ~ 8
OH OH O~ IP--O~t
Ph
The title compound was prepared from (RR)-4-{2-[3-(2,2-di-tert-butyl-4H-1,3,2-
~n7ntlinY~cilirqn 6-yl-oxy)-2-hydro;~y~lul~y~ o]propyl}~ y~ tllyl~ lyl-
phosphinic acid, ethyl ester (140mg, 0.21mMol) by a method similar to that in
Example 4. The crude product, obtained as a grey gum, was used without further
15 L....;ri.-l;n.,
m/z: MH~ 530 (18%).
Example68: (RR)-4-{2-[2-Hydroxy-3-(4-hydroxy-3-h.~dlu~.y~ll.lllylphenoxy)
20 ~ u~ ' ~]propyl}p' . y~lhylphenylr'~ ~ acid, lithium salt.
Me
~NH~ 8
OH OH O~ IP-OLi
Ph
The title compound was prepared from (RR)-4- { 2-[2-hydroxy-3-(4-hydroxy-3-
hydlu~ylll~l.ylphenoxy)propylamino~propyl)phenoxymethyl~ .lyl~ u~ acid,
ethyl ester (I lOmg, 0.21mMol) by a method similar to that used in Example 21; the
prûduct was isolated as a white powder after freeze-drying.
-131-
-
WO Y6/04233 2 1 9 6 ~ 9 3 1 ~ ;1 7...................... ~0~7 ~
o'H (250MH~, CD30D): 7.90 (2H, m); 7.42 (3H, m); 7.11 (2H, d); 6.g5 (lH, d);
6.87 (2H, d); 6.69 (2H, d); 4.64 (2H, s); 4.19 (IH, m); 4.08 (2H, d); 3.97 (2H, m);
3.48 (lH, m); 3.36 (IH, m, partially obscured by MeOH signal); 3.20-3.02 (2H, m);
2.69 (IH, m) and 1.23 (3H, d).
Example69: (S)-4-~2-[3-(4-Hyd.u,~y~' y)-2-hydru~.ylJ~u~yL~ illo]ethyl}
r y ~h,~ ' acid,2-p' yyl~tllylester,l.ydl, ' idesalt
dihydrate
- H~
$H ~ 8 , _~
O
OH
HCI. HzO
The title compound was prepared from (S)-4-{2-[3-(4-hydroxyphenoxy)-2-
hydlu~y~lu~ylamino]ethyl~pllcllv~cyll~ yl~ u~ r~l~lr acid, bis-(2-phenylethyl ester
according to the method described in Example 25, as a white solid.
olH(d6-DMSO+D2O~: 7.15-7.26 (5H, m); 7.04-7.08 (2H, d); 6.75-6.82 (4H, m);
6.69-6.72 (2H, d); 4.11-4.16 (IH, m), 3.95-3.99 (2H, q); 3.77-3.95 (4H, ); 2.79-2.87
(4H, m), 2.99-3 18 (4H, m).
Example70: (S)-4-{2-[3-(4-H~ u~yl ' y)-2-hydlw~y~.. u~,ylal.,h,u]ethyl~
'- y~; ~ rl~ ~~ acid, (2,2-r~iF~ ylcthyl) ester, hy.lr~ ~ ' idc salt
~ _ H~l
OH ~3
OH ~~ 1~0~3
OH
HCI
The title compound was prepared from (S)-4-~2-~3-(4-hydroxyphenoxy)-2-
Lydlu~y~lu~ylamino]ethyl]pll~ u~yl~ llylphsphonic acid, bis- (2,2-diphenyl ethyl)
ester according to the method described in Example 25,.as a white solid.
-132-
~wos6/04233 2 1 q 61 9 3 r~.,r, s~Q~n~7
m/z: MH+ 578.
Bhd. .~ v~ Data: The activity of the present ~ "yv~ k is tested by use of
the following ylu~ cJu.~
s
A ~ ~ and Agonist Activity at Human ~ 3 2~ and ~ 3-Adi. ~ c ~ ~-
Subclones of CHO cells are stably transfected with each of the human ~ 2
and ~ 3-aJ.G.lv.,~.Lvl~1. Cells are then disrupted by immersion in ice-cold Iysis
buffer (10 mM TRIS, 2mM EDTA, pH 7.4) containing protease inhibitors leupeptin
10 and 1,..., .";.i;..~ (S llg / ml) and soyabean trypsin inhibitor (10 ~Lg / ml). M ...l ,.,..,~,
are prepared by the method of Bouvier et. al.2 and stored in 1 ml aliquots in liquid N2
for future use.
~ 3-Ad. ~ - . t - Mediated Adenylyl Cyclase Activity
lS Adenylyl cyclase activity is assayed by the method of Kirkham et. al.3 by the
addition of 40 111 (70 -80 lag protein) to the incubation medium of the above CHO cell
plasma ..,. "l,.,- ,. i transfected with the human ~3-adrenoceptor . cAMP produced
over 20 minutes is separated from ATP by the method of Salomon et al.4. Agonist
ECso values and intrinsic activities are expressed as the rr,nrrn~ir,n of agonist
20 producing 50 % activation of adenylyl cyclase and the maximum response produced
by each agonist relative to that produced by (-) isoprenaline respectively.
Antagonist Bindin~ at ~1~ and 13 2-A il ~ - ?~ ~ a
D;~yl irr ~"- ~ of [1251]-iodo~ y~n~y;~lrir~lrl from CHO cell plasma mrmhr:~nPs
25 transfectedwitheitherthehuman,~1,or ,f~2-adlGllo~y~ iscarriedoutbythe
method of Blin et. al.5. Ki values (nM) are calculated from the binding ICso values
for each agonist, using the Cheng -Prusoff equation.
Results
Example Beta-3 Beta- I Beta-2
EC50 (IA) uM Ki uM Ki uM
17 1.1 (0.7) 21 10
21 1.26 ~>1.0) 155 15
29 1.7 (0.72) 288 269
-133-
W096/04233 2 ~ 9 61 93 r~
References
1. T. Frielle et~ al., Proc. Natl. Acad. Sci., 1987, 84, 7920; B. Kobilka,
Proc. Natl. Acad. Sci., 1987, 84, 46; S. Liggett and D. Schwinn, DNA
S Sequence, 1991, 2, 61.
2. M. Bouvier et. al., Mol. Pharmacol., 19X7, 33, 133.
3. D. Kirkham et. al., Biochem. J., 1992,284, 301.
4. Y. Salomon et al., Anal. Biochem., 1974, S8, 541.
5. N. Blin, et. al., Br. J. Pharmacol., 1994, 112, 911.
-134-