Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
21 98333
TITLE OF THE I NVENT I ON
M~THOD OF AND APPARATUS FOR REFORMING FUEL AND FUEL CELL SYSTEM
WITH FUEL-REFORMING APPARATUS INCORPORATED THEREIN
BACKGROUND OF THE INVENTION
5 1. Field of the Invention
The present invention relates to a method of and an apparatus
for reforming a fuel and a fuel cell system with the fuel-reforming
apparatus incorporated therein. More specifically, the present
invention pertains to a fuel-reforming apparatus that reforms a
hydrocarbon supplied as a raw fuel to a hydrogen-rich gaseous fuel,
which is then supplied to fuel cells. The present invention further
pertains to a method of reforming a fuel and a fuel cell system with
such a fuel-reforming apparatus incorporated therein.
2. Description of the Prior Art
Fuel cells are a device in which the chemical energy of a fuel
is converted, not via mechanical energy or thermal energy, but
directly into electrical energy. The fuel cells can thus realize
a favorably high energy efficiency. A well-known structure of the
fuelcell includes apairofelectrodes arrangedacross anelectrolyte
layer. While a gaseous fuel cont~;n;ng hydrogen is supplied to one
electrode (cathode), an oxidizing gas cont~;n;ng oxygen is fed to
the other electrode (anode). An electromotive force is obtained by
taking advantage of electrochemical reactions proceeding at the
respective electrodes. Equations representing the electrochemical
reactions occurring in the fuel cell are given below. Equations (1)
and (2) respectively represent the reaction at the anode and the
reaction at the cathode; the reaction expressed as Equation (3)
21 98333
accordingly proceeds as a whole in the fuel cell:
H2 -~ 2H+ + 2e~ (1)
(1/2)O2 + 2H + 2e -~ H20 (2)
H2 + (1/2)02 -~ H20 ( )
Fuel cells are generally classified by the type of the
electrolyte used therein, the operation temperature, and the other
parameters. Among the various fuel cells, Polymer Electrolyte Fuel
Cells, Phosphoric Acid Fuel Cells, and Molten Carbonate Fuel Cells
allow supplies of the oxidizing gas and the gaseous fuel cont~;ning
lo carbondioxide, becauseofthecharacteristics oftheirelectrolytes.
In these fuel cells, the air is generally used as the oxidizing gas,
and the hydrogen-cont~;n;ng gas obt~;ne~ by steam reforming a raw
hydrocarbon fuel,such as methanolor natural gas as the gaseous fuel.
The fuel cell system having such fuel cells is accordingly
15 provided with a reformer, which reforms the raw fuel to generate a
gaseous fuel. The followinggives anexemplified reforming reaction
of the rawfuel proceeding in the reformer. Inthis example, methanol
is supplied as the raw fuel and steam reformed:
CH30H -~ C0 + 2H2 - 90.O (kJ/mol) (4)
CO + H20 -~ C~2 + H2 + 40-5 (kJ/mol) (5)
CH30H + H20 -~ CO2 + 3H2 - 49.5 (kJ/mol) (6)
In the process of steam reforming methanol, the decomposition
of methanol expressed as Equation (4) and the converting reaction
of carbon monoxide expressed as Equation (5) occur simultaneously;
25 the reactionexpressedasEquation(6) accordingly proceeds as awhole
21 98333
in the reformer. Since the process of steam reforming the raw fuel
is an endothermic reaction, the conventional reformer is typically
provided with a burner or a heater in ordertosupply arequired amount
of heat for the reforming reaction.
In thestructure includingthe burnerto supply a requiredamount
of heat for the reforming reaction, however, the burner itself
attached to the reformer and additional conduits to feed supplies
of a fuel and the air to the burner for combustion make the whole
fuel cell system rather complicated and bulky. This is especially
lo unsuitable when the fuel cell system having the fuel cells and the
reformer is located in a limited space, for example, when the fuel
cell system is mounted on the vehicle as a power source for driving
the vehicle. The structure including the heater, on the other hand,
requires extra energy for driving the heater, in addition to having
the above drawbacks, that is, the complicated and bulky fuel cell
system. This leads to a decrease in energy efficiency of the whole
fuel cell system. By way of example, in the structure that supplies
part of electricpower generated bythe fuel cells to the heaterwhich
is used for heating the reformer, the fuel cells are required to have
a sufficiently large capacity.
The conventional reformer can not be favorably applied to the
case in which an increase in supply of gaseous fuel is required with
the enhanced loading of the fuel cells. The reforming reaction
expressed as Equation (6) is an endothermic reaction as discussed
above. Theendothermic reactiongenerally has aslower reactionrate,
and it is accordingly difficult to abruptly increase the amount of
the raw fuel processed by the reforming reaction. The endothermic
21 98333
reforming reaction can be activated by increasing the amount of heat
appliedtothe reformer. Anextreme increase intemperature, however,
deteriorates the catalyst packed in the reformer and causes other
problems. Application of a small amount of heat to prevent
5 deterioration of the catalyst leads to an insufficient effect of
activating the reforming reaction. As another possible solution,
a reformer of a sufficiently large capacity may be used to readily
generate an estimated maximum amount of the reformed gas. This,
however, makes the reformer undesirably bulky.
lo In the structure of heating the reformer with an external heat
source, such as a heater, another problem arises; that is, the
temperature distribution curve in the reformer has smaller values
in the vicinity of the inlet of the reformer and greater values in
the vicinity of the outlet. Fig. 21 is a graph showing a temperature
15 distribution in a conventional reformer with a heater. In the
conventional reformer, the inside temperature decreases with the
progress of the endothermic reforming reaction at the inlet, through
which steam and methanol as the raw fuel are introduced. Although
the heater continues supplying heat, the temperature in the reformer
20 iS decreasing while the endothermic reforming reaction is vigorous
to consume alarge amount of heat. Asthe progressof theendothermic
reforming reaction becomes gentle with consumption of the raw fuel,
the amount of heat supplied by the heater reaches and then exceeds
the amount ofheat required for theendothermic reaction. The inside
25 temperature of the reformer accordingly starts increasing. A
temporary decrease in temperature in the vicinity of the inlet of
the reformer lowers the rate of the endothermic reforming reaction
2 1 ~8333
and thereby the efficiency per unit volume of the reformer. An
increase in temperature inthe vicinity ofthe outlet interferes with
the exothermic shift reaction of Equation (5), thereby undesirably
increasing the concentration of carbon monoxide included in the
5 gaseous fuel obtained by the reforming reaction.
SUMMARY OF THE INVENTION
One object of the present invention is thus to generate agaseous
fuel having a low content of carbon monoxide.
Another object of the present invention is to provide a
sufficiently compact fuel-reforming apparatus and simplify
structure of a fuel cell system with a fuel-reforming apparatus
incorporated therein.
Still another object of the present invention is to reform a raw
fuel gas without lowering an energy efficiency of the whole fuel cell
15 system.
At least part of the above and the other related objects is
realized by a method of reforming a hydrocarbon supplied as a raw
fuel to generate a hydrogen-contA;ning gaseous fuel through a
reforming reaction occurring in a predetermined reformer. The
method includes the steps of: feeding a supply of oxygen to a raw
fuel gas contA;n;ng the raw fuel andenabling an exothermic oxidation
reaction to proceed for a specified component of the raw fuel gas;
and enabling an endothermic reforming reaction of the raw fuel to
proceed with heat generated by the exothermic oxidation reaction of
the specified component.
The method of the present invention feeds a supply of oxygen to
a rawfuelgascontA;n;ngthe rawfuel, sothat anexothermic oxidation
21 98333
reaction proceeds to oxidize a specified component of the raw fuel
gas. The required amount of heat externally supplied for the
reforming reaction of the raw fuel can be lessened significantly by
combining the exothermic oxidation reaction with the endothermic
5 reforming reaction andutilizing theheat generated bytheexo~herr;c
reaction for the endothermic reaction. The principle of this method
applied to a fuel-reforming apparatus effectively reduces the size
ofaheatsource arranged inthe fuel-reforming apparatus forsecuring
the amount of heat required for the reforming reaction as well as
o the size of the whole fuel-reforming apparatus.
In accordance with one preferable application, the specified
component subjected to the exothermic oxidation reaction is the raw
fuel, and the exothermic oxidation reaction represents an oxidizing
reforming reaction that oxidizes the raw fuel to reform the raw fuel.
With a supply of oxygen to the raw fuel gas, the oxidizing
reforming reaction proceeds to oxidize the raw fuel. The heat
generated by the oxidizing reforming reaction is utilized for the
endothermic reforming reaction of the raw fuel. The exothermic
oxidization reaction for supplying the amount of heat required for
20 the endothermic reforming reaction of the raw fuel also reforms the
raw fuel to generate hydrogen. Even when the reaction other than
the endothermic reforming reaction occurs to supply the amount of
heat required for the reforming reaction, the generation of hydrogen
effectively prevents the hydrogen partial pressure of the resulting
gaseous fuel from being lowered.
In accordance with one preferable application, the method ofthe
present invention further includes the steps of: deterr;n;ng a
21 98333
proportion of oxygen added to the raw fuel-cont~;n;ng raw fuel gas
in the process of the reforming reaction, based on an amount of heat
generated by the exothermic oxidation reaction of the specified
component of the raw fuel gas and an amount of heat required for the
5 endothermic reforming reaction; and supplying oxygen corresponding
to the proportion thus determined being mixed with the raw fuel gas
prior to being subjected to the exothermic oxidation reaction.
This structure enables the amount of heat required for the
reforming reaction to besufficiently generated inside the reformer.
lo In case that a predetermined amount of oxygen is added to the raw
fuel gas in order to supply all the required amount of heat for the
reforming reaction, there is no need of externally supplying heat
to secure the required amount of heat for the reforming reaction.
When the principle of the method is applied to a fuel-reforming
15 apparatus, no requirement for the heat source effectively reduces
the size of the whole fuel-reforming apparatus. Since there is no
requirement for not only the heat source itself but piping for
supplying a fuel to drive the heat source and wiring for supplying
energy, the system with the fuel-reforming apparatus has the
preferably simplified structure.
In this preferable structure, the supply of oxygen added to the
raw fuel gas is determined, based on the amount of heat generated
by the exothermic oxidation reaction and the amount of heat required
for the endothermic reforming reaction. This structure enables the
amount of heat required for the endothermic reforming reaction to
be sufficiently generated and balances the amount of heat generated
by the exothermic oxidation reaction of the specified component of
21 98333
the raw fuel gas with the amount of heat required for the endothermic
reforming reaction, thereby not causing excess heat. This prevents
an unnecessary increase in temperature of the catalyst and thereby
the possible energy loss due to heat dissipation.
The proportion of oxygen to theraw fuel gas is determined, based
on the amount of heat generated by the oxidation reaction and the
amount of heat required for the reforming reaction. This structure
effectively prevents the ratio of the oxidation reaction from being
unnecessarily expanded. Even in case that the oxidation reaction
lo proceeds to generate components other than hydrogen or in case that
the number of hydrogen molecules generated by the oxidation reaction
per one molecule of methanol is less than the number of hydrogen
molecules generated by the reforming reaction, this structure
prevents the hydrogen partial pressure of the resulting gaseous fuel
from being undesirably lowered.
In accordance with another preferable structure, a first
catalyst having a predetermined heat resistance and being packed in
the reformer accelerates at least the exothermic oxidation reaction
of the specified component of the raw fuel gas. The raw fuel gas
that has undergone the oxidation reaction subsequently comes into
contact with a second catalyst that is packed in the reformer for
accelerating at least the endothermic reforming reaction.
The first catalyst having a predeter~;ned heat resistance
accelerates the exothermic oxidation reaction. The first catalyst
accordingly does not deteriorate even when the temperature in the
active area of the oxidation reaction rises with the progress of the
exothermic oxidation reaction. Since the first catalyst is not
21 98333
easily deteriorated by the temperature increase in the active area
of the exothermic oxidation reaction, the oxidation reaction can be
activated to a further extent and the size of the reformer can thus
be reduced. An improvement in heat generation efficiency per unit
volume in the area filled with the first catalyst ensures generation
of a sufficient amount of heat even when the area filled with the
first catalyst is narrowed.
The raw fuel gas that has undergone the oxidation reaction comes
into contact with the second catalyst. The endothermic reforming
o reaction procee~ on the second catalyst using the heat generated
by the preceding oxidation reaction. The reforming reaction, which
produces hydrogen from a hydrocarbon, generally includes the
reaction of producing carbon dioxide and hydrogen from carbon
monoxide and water. ThiS reaction is the exother~;c shift reaction,
andthe lowersurroundingtemperatures accelerate the shift reaction.
As the reforming reactionconsuming the heat proceeds in the presence
of the second catalyst to lower the temperature in the reformer, the
shift reaction is accelerated to reduce the concentration of carbon
monoxide and thereby generate a gaseous fuel having a low content
of carbon monoxide.
The present invention is further directed to a method of
reforming a hydrocarbon supplied as a raw fuel to generate a
hydrogen-con~;n;ng gaseous fuel through a reforming reaction
occurring inapredetermined reformer. The method includesthesteps
of: extending the exothermic oxidation reaction activating area to
be arranged in such a way that the exothermic oxidation reaction
activating area and the endothermic reforming reaction area overlap
21 98333
each other in awide range, whereinthe exothermic oxidationreaction
of a specified component of a raw fuel gas cont~;n;ng the raw fuel
is enabled to proceed.
Still another preferable method representing the prevent
5 invention is a method of reforming a hydrocarbon supplied as a raw
fuel to generate a hydrogen-cont~i n; ng gaseous fuel through a
reforming reaction occurring in a predetermined reformer. The
method includes the steps of: feeding a supply of oxygen to a raw
fuel gas cont~;n;ng the raw fuel andenabling an exothermic oxidation
lo reaction to proceed for a specified component of the raw fuel gas;
and diffusing heat widely over the endothermic reforming reaction
area, the heat being generated by the exothermic oxidation reaction
of the specified component; and enabling an endothermic reforming
reaction of the raw fuel to proceed with the diffused heat.
This structure lowers the peak of the temperature distribution
in the reformer and thus prevents a variety of problems caused by
an extreme increase in temperature in the reformer. These problems
due to an increase in temperature of the reformer to and above a
predetermined level include deterioration of the catalyst that is
packed in the reformer for accelerating the reforming reaction and
generation of non-required by-products by the undesirable reactions
other than the oxidation reaction and the reforming reaction. This
structure also s~p~n~ the area having temperatures for activating
the endothermic reforming reaction, thus improving the efficiency
of the reforming reactionperunit volume ofthe reformer andreducing
the size of the reformer.
~ 1 98333
The present invention is also directed to an apparatus for
reforming a hydrocarbon supplied as a raw fuel to generate a
hydrogen-cont~;n;ng gaseous fuel through a reforming reaction. The
apparatus of the invention includes: a reformer unit in which the
s reforming reaction proceeds; raw fuel supply means for feeding a
supply of a raw fuel gas cont~;n;ng the raw fuel to the reformerunit;
and oxygen supply means for feeding a supply of oxygen to the raw
fuel gas. The reformer unit includes: a first catalyst for
accelerating an exothermic oxidation reaction of a specified
o component of the raw fuel gas with the supply of oxygen fed by the
oxygen supply means; and a second catalyst for accelerating an
endothermic reforming reaction with heat generated by the oxidation
reaction of the specified component of the raw fuel gas.
The apparatus of the present invention feeds a supply of oxygen
to a raw fuel gas cont~;n;ng the raw fuel, so that an exothermic
oxidation reaction proceeds fora specified component of the raw fuel
gas. Since the exo~herr;c reaction proceeds inside the apparatus,
the endothermic reforming reaction of the raw fuel can be carried
out with the heat generated in the apparatus. The required amount
of heat externally supplied for the reforming reaction of the raw
fuel is thus lessened significantly. Thisstructure caneffectively
reduce thesize ofaheatsource arranged inthe apparatus forsecuring
the amount of heat required for the reforming reaction as well as
the size of the whole apparatus.
2s In accordance with one preferable application, the specified
component subjected to the exothermic oxidation reaction is the raw
fuel, and the exothermic oxidation reaction represents an oxidizing
2t 98333
reforming reaction that oxidizes the raw fuel to reform the raw fuel.
In the apparatus of this preferable structure, the oxidizing
reforming reaction proceeds with a supply of oxygen to the raw fuel
gas, so as to oxidize the raw fuel. The heat generated by the
5 oxidizing reforming reaction is utilized for the endothermic
reforming reaction of the raw fuel. The exo~herric oxidization
reactionforsupplyingthe amount ofheat requiredfortheendothermic
reforming reaction of the raw fuel also reforms the raw fuel to
generate hydrogen. Even when the reaction other than the reforming
lo reaction occurs to supply the amount of heat required for the
reforming reaction, the generation of hydrogen effectively prevents
the hydrogenpartial pressure ofthe resultinggaseous fuel frombeing
lowered.
In accordance with another preferable application of the
15 apparatus of the invention, the oxygen supply means includes: oxygen
supply regulation means for determining a proportion of oxygen
supplied to the raw fuel gas, based on an amount of heat generated
by the exothermic oxidation reaction of the specified component of
the raw fuel gas and an amount of heat required for the endothermic
reforming reaction, and feeding a supply of oxygen corresponding to
the proportion thus determined to the raw fuel gas.
In the apparatus of this preferable structure, the supply of
oxygen added to the raw fuel gas is determined, based on the amount
of heat generated by the exothermic oxidation reaction of the
2s predetermined component of the raw fuel gas and the amount of heat
required for the endothermic reforming reaction. This structure
enables the amount of heat required for the endothermic reforming
21 98333
reaction to be sufficiently generated inside the reformer unit. In
case that a predeterr;ne~ amount of oxygen is added to the raw fuel
gas in order to supply all the required amount of heat for the
endothermic reforming reaction, there is no requirement for a heat
5 source that supplies the required amount of heat for the reforming
reaction, thereby enabling the size of the whole apparatus to be
desirably reduced. Since there is no requirement for not only the
heat source itself but piping for supplying a fuel to drive the heat
source and wiring for supplying energy, the system with the
lo fuel-reforming apparatus has the preferably simplified structure.
In this preferable structure, the supply of oxygen added to the
raw fuel gas is determined, based on the amount of heat generated
by the exothermic oxidation reaction of the predetermined component
of the raw fuel gas and the amount ofheat required fortheendothermic
15 reforming reaction as discussed above. This structure enables the
amount of heat required for the endothermic reforming reaction to
be sufficiently generated and balances the amount of heat generated
by the exothermic oxidation reaction of the predetermined component
of the raw fuel gas with the amount of heat required for the
endothermic reforming reaction, thereby not causing excess heat.
This prevents an unnecessary increase in temperature of the catalyst
and thereby the possible energy loss due to heat dissipation.
The proportion of oxygen to the raw fuel gas is determined, based
on the amount of heat generated by the exothermic oxidation reaction
25 andthe amount ofheat requiredfortheendothermic reforming reaction
This structure effectively prevents the ratio of the oxidation
reaction from being unnecessarily exp~n~ed. Even in case that the
21 98333
exothermic oxidation reaction proceeds to generate components
foreign to the cell reaction of the fuel cells or in case that the
number of hydrogen molecules generated by the oxidation reaction per
one molecule of methanol is less thanthe number ofhydrogen molecules
5 generated by the reforming reactionl this structure prevents the
hydrogen partial pressure of the resulting gaseous fuel from being
undesirably lowered.
In the apparatus of the present invention, it is also preferable
that the first catalyst and the second catalyst are identical with
lo each other, and the reformer unit has a homogeneous catalyst layer
composed of the first catalyst and the second catalyst.
In this preferable structure, the single catalyst functions to
accelerate both the exothermic oxidation reaction of the specified
component of the raw fuel gas and the reforming reaction. This
simplifies the structure of the apparatus and accordingly eases the
manufacturing process ofthis apparatus. The reforming reactioncan
proceed simultaneously in the active area of the oxidation reaction.
The endothermic reaction occurring simultaneously with the
e~othermic reaction improves the efficiency of the reforming
reaction per unit volume of the apparatus. The improvement in
efficiency of the endothermic reforming reaction enables the size
of the apparatus to be reduced desirably. In the active area of the
exo~herr;c oxidation reaction and the simultaneous endothermic
reforming reaction, the heat generated by the exothermic oxidation
reaction is consumed immediately by the endothermic reforming
reaction. This structure effectively prevents the possible energy
loss duetotransmissionof heat generatedby theexothermic oxidation
2 1 98333
reaction to the active area of the endothermic reforming reaction.
In accordance with another preferable application, the first
catalyst has both or either of a predeterr;ne~ heat resistance and
durability at high temperatures, and is arranged at a positioncloser
5 to an inlet of the raw fuel gas fed into the reformer unit than a
position of arrangement of the second catalyst.
Even when the oxidation reaction of the specified component of
the raw fuel gas proceeds to generate a large amount of heat, the
first catalyst does not deteriorate. In the reformer unit, a large
lo quantity of oxygen can thus be supplied to the area filled with the
first catalyst inordertoactivatetheexothermic oxidationreaction.
This improves the efficiency of the exothermic oxidation reaction
per unit volume in the area filled with the first catalyst andthereby
reduces the size of the reformer unit and the whole apparatus. This
15 structure also improves the durability of the apparatus.
The first catalyst isarrangedat the positionclosertothe inlet
of the reformer unit than the position of the second catalyst. The
exothermic oxidation reaction accordingly proceeds prior to the
endothermic reforming reaction. Theendothermic reformingreaction
20 lowers the temperature in the vicinity of the outlet of the reformer
unit. The reforming reaction, which produces hydrogen from a
hydrocarbon, generally includes the reaction of producing carbon
dioxide and hydrogen from carbon monoxide and water. ThiS reaction
is the exothermic shift reaction, and the lower surrounding
25 temperatures accelerate the shift reaction. As the reforming
reaction consuming the heat proceeds in the vicinity of the outlet
of the reformer unit to lower the temperature in the reformer unit,
21 98333
the shift reaction is accelerated to reduce the concentration of
carbon monoxide and thereby generate a gaseous fuel having a low
content of carbon monoxide.
In accordance with one preferable application, the apparatus of
the invention further includes temperature distribution averaging
means for lowering a peak of a temperature distribution caused by
heat generated by the exothermic oxidation reaction of the specified
component of the raw fuel gas in the reformer unit and expanding an
area having temperatures for activating the endothermic reforming
lo reaction.
In this preferable structure, the amount of heat generated by
the exothermic oxidation reaction is averaged and distributed in the
reformer unit. This structure prevents the temperature in the
reformer unit from rising locally, and the catalyst packed in the
reformer unit accordingly does not deteriorate. Averaging the
temperature in the reformer unit raises the temperature of the
non-active area ofthe exothermic oxidizing reformingreaction. The
endothermic reformingreactionoccurs insuch anareaand is activated
with an increase in temperature. The process of averaging the
temperature in the reformer unit improves the efficiency of the
reforming reaction per unit volume of the reformer unit and thereby
reduces the size of the whole apparatus.
In accordance with one preferable structure, the temperature
distribution averaging means may include a plurality of the oxygen
supply means arranged along a flow of the raw fuel gas in the reformer
unit. In this structure, oxygen is supplied from a plurality of
different positions into the reformer unit, and the activity curve
21 98333
of the exothermic oxidation reaction accordingly has a plurality of
peaks. Upon condition that the supply of oxygen to the reformerunit
is fixed, the structure of supplying oxygen from plural positions
effectively lowers the peak temperature and increases the number of
5 peaks of the temperature increase, compared with the structure of
supplying oxygen from only one position. ThiS expands the area
having temperatures e~ual to or greater than a predetermined level.
This structure prevents the temperature in the reformer unit from
rising locally, andthereby protectsthecatalyst fromdeterioration.
lo Thee~pAn~ionofthe active areaoftheendothermic reformingreaction
improves the efficiency of the reforming reaction proceeding in the
reformer unit.
Inaccordance withanother preferablestructure,thetemperature
distribution averaging means may include heat dispersion means for
15 dispersing heat generated by the exothermic oxidation reaction of
the predetermined component through heat transmission in the
reformer unit. This structure disperses the heat generated in the
area where the exothermic oxidation reaction reaches its peak,
thereby preventing the temperature in the reformer unit from rising
20 locally and protecting the catalyst from deterioration. The heat
generated by the exothermic oxidation reaction is dispersed in the
reformer unit and raises the temperature of the non-active area of
the exothermic oxidation reaction, so as to active the endothermic
reforming reaction in this area. This improves the efficiency of
25 the reforming reaction per unit volume of the reformer unit and
reduces the size of the whole apparatus.
2 1 98333
In accordance with still another preferable application, the
temperature distribution averaging means includes the reformer unit
having a first portion close to inlets of the raw fuel gas and oxygen
and a second portion close to an outlet of the gaseous fuel generated
5 by the reforming reaction, wherein a total surface area of the
catalyst existingin the first portion issmallerthan atotalsurface
area of the catalyst existing in the second portion. This structure
depresses the rate of the exothermic oxidation reaction in the first
portion that is close to the inlets of the raw fuel gas and oxygen
lo and has the smaller total surface area. The rate of the exothermic
oxidation reaction is generally higher than the rate of the
endothermic reforming reaction. In the first portion close to the
inlet of oxygen, the amount of heat generated by the exothermic
oxidation reaction exceeds the amount of heat required for the
15 endothermic reforming reaction to raise the temperature in the
reformer unit. The change of the total surface area of the catalyst
in the first portion close tothe inlets ofthe raw fuelgas andoxygen
to depress the rate of the exothermic oxidation reaction relieves
the temperature increase due to the exothermic oxidation reaction
20 and accordingly averages the temperature distribution inside the
reformer unit. A simple process of, for example, varying the
particle size of the pellets of the catalytic metal packed in the
reformer unit between the inlet of the raw fuel gas and the outlet
of the resulting gaseous fuel averages the temperature distribution
25 in the reformer unit without making the structure of the apparatus
or piping undesirably complicated. This effectively prevents
deterioration of the catalyst due to the high temperatures in the
21 ~8333
reformer unit and improves the efficiency of the reforming reaction
per unit volume of the reformer unit.
In accordance with another preferable application, the
temperature distribution averaging means includes the reformer unit
5 having a first portion close to inlets of the raw fuel gas and oxygen
and a second portion close to an outlet of the gaseous fuel generated
by the endothermic reforming reaction, wherein the first portion has
a greater flow sectional area than that of the second portion. The
flow rate of the raw fuel gas is lowered in the first portion that
lo is close tothe inlet ofthe raw fuel gas and hasthe greatersectional
area of the flow path, whereas the flow rate of the resulting gaseous
fuel is heightened in the second portion that is close to the outlet
of the gaseous fuel and has the smaller sectional area of the flow
path. This structure depresses the rate of the exothermic oxidation
15 reaction proceeding in the first portion of the reformer unit close
to the inlet of the raw fuel gas, which flows relatively slowly. The
depression of the rate of the exothermic oxidation reaction reduces
the relative rate oftheoxidationreactiontothe reforming reaction.
This relieves the temperature increase due to the exo~herr;c
oxidation reaction and accordingly averages the temperature
distribution inside the reformer unit. A simple process of, for
example, making the flow section of the first portion close to the
inlet of the raw fuel gas greater than that of the second portion
close to the outlet of the gaseous fuel averages the temperature
25 distribution in the reformer unit without making the structure of
the apparatus or piping undesirably complicated. This effectively
prevents deterioration of the catalyst due to the high temperatures
19
21 98333
in the reformer unit and improves the efficiency of the reforming
reaction per unit volume of the reformer unit.
The flowsectionofthe reformerrepresents aplaneperpendicular
to the direction of the gas flow in the reformer unit. As long as
5 the area of the plane perpendicular to the flow direction of the gas
is gradually decreased, it is not required to fix the direction of
the gas flow in the reformer unit where the first portion close to
the inlet of the raw fuel gas has the greater flow sectional area
than that of the second portion close to the outlet of the gaseous
o fuel. By wayofexample,the reformerunit may be formed in acolumnar
shape, wherein the raw fuel gas is introduced from the outer face
of the column and the resulting gaseous fuel is discharged to the
central axis of the column. This structure can gradually decrease
the area of the plane perpendicular to the direction of the gas flow.
The present invention is further directed to a fuel cell system,
which includes a fuel-reforming apparatus of the present invention
discussed above and a fuel cell for receiving a supply of gaseous
fuel from the fuel-reforming apparatus and generating electrical
energy.
In the fuel cellsystem ofthe present invention, theendothermic
reforming reactionproceeds with theheat generated bytheexothermic
oxidation reaction of the specified component of the raw fuel gas.
The required amount of heat externally supplied for the reforming
reaction of the raw fuel is thus lessened significantly. Especially
when the supply of oxygen fed to the reformer unit of the fuel-
reforming apparatus is determined, based on the amount of heat
generated by the exothermic oxidation reaction andthe amount of heat
21 ~8333
required for the endothermic reforming reaction, all the required
amount of heat for the reforming reaction can be sufficiently
generated by the oxidation reaction. There is accordingly no
requirement for a heat source that supplies the required amount of
5 heat for the reforming reaction, thereby enabling the size of the
apparatus incorporatedinthe fuelcellsystemtobedesirably reduced
No requirement for not only the heat source itself but piping for
supplying a fuel to drive the heat source and wiring for supplying
energy simplifies the structure ofthe fuelcell system ofthe present
lo invention and improves the energy efficiency in the system~
In accordance with one preferable application, the fuel-
reforming apparatus incorporated in the fuel cell system may have
heat dispersion means for dispersing heat through heat transmission
in the reformer unit, in order to lower the peak of the temperature
distribution and expand the area having temperatures equal to or
greater than a predetermined level. ThiS structure effectively
deals with an abrupt increase in loading of the fuel cell arranged
in the fuel cell system without delay. In case that an abrupt
activation ofthe reforming reactionis desired inthe fuel-reforming
apparatus, for example, at the time of starting the fuel cell system,
even when a large amount of oxygen is supplied corresponding to the
large amount of raw fuel gas, the heat dispersion means disperses
the heat generated by the exothermic oxidation reaction and prevents
deterioration of the catalyst and other problems due to a temporary
25 or local extreme increase in temperature in the reformer unit. This
structure enables alarge amount ofraw fuel gasto be reformedwithin
a short time period and supplies the required amount of resulting
2 1 98333
gaseous fuel to the fuel cell without delay.
Other possible applications of the present invention are given
below. In the apparatus and the method according to the present
invention, the predetermined component subjected to the oxidation
5 reaction may be carbon monoxide and/or hydrogen generated by the
decomposition of the raw fuel.
Decomposition of a hydrocarbon generally produces carbon
monoxide and hydrogen. A wide range of hydrocarbons can be used as
the raw fuel in the method and the apparatus of the present invention
by utilizing the heat generated by the oxidation reaction of carbon
monoxide and/or hydrogen.
As another application, the fuel cell system of the present
invention may be mounted on an electric vehicle as a power source
for driving the vehicle.
The fuel cell system of the present invention can be used
preferably as a portable power source when the allowable space and
weight are strictly restricted. Generation of the heat required for
the simultaneous reforming reaction in the reformer unit simplifies
the structure ofthesystem andreducesthe weight ofthe wholesystem.
The electric vehicle with such a fuel cell system has various
advantages, such as easy maintenance, the reduced number of parts,
simplified assembly, and reduced manufacturing cost.
These and other objects, features, aspects, and advantages of
the present invention will become more apparent from the following
2s detailed description of the preferred embodiments with the
accompanying drawings.
2 1 98333
BRIEF DESCRIPTION OF THE DRAHINGS
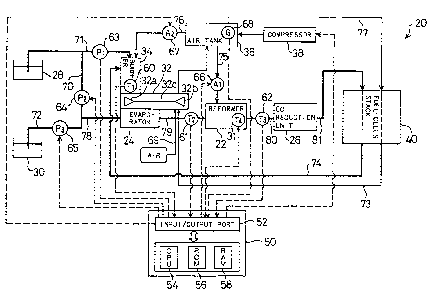
Fig. 1 is a block diagram schematically illustrating structure
of a fuel cell system 20 embodying the present invention;
Fig. 2 is a cross sectional view illustrating structure of each
5 unit cell 48 included in a fuel-cells stack 40;
Fig. 3schematically showsstructure of areformer22 ofthe first
embodiment;
Fig. 4 is a graph showing a temperature distribution in the
reformer 22;
Fig. 5 schematically shows structure of another reformer 22a;
Fig. 6 is a graph showing a temperature distribution in the
reformer 22a;
Fig. 7 schematically shows structure of still another reformer
22b;
Fig. 8 schematically shows structure of another reformer 22c;
Fig. 9 is a graph showing a temperature distribution in the
reformer 22c;
Fig. 10 schematically shows structure of another reformer 22d;
Fig. 11 schematically shows structure of still another reformer
22e;
Fig. 12 schematically shows structure of another reformer 22f;
Fig. 13 is a graph showing variations in temperature of the
catalyst in a reformer filled with large pellets and in a reformer
filled with small pellets;
Fig. 14 is a graph showing a temperature distribution in the
reformer 22f;
2t 98333
Fig. 15 schematically shows structure of another reformer 22g;
Fig. 16 is a graph showing a temperature distribution in the
reformer 22g;
Fig. 17 schematically shows structure of still another reformer
22h;
Fig. 18 schematically shows structure of another reformer 22i;
Fig. 19 schematically shows structure of still another reformer
22j;
Fig. 20is ablock diagram illustrating structure of another fuel
cell system 20A; and
Fig. 21 is a graph showing a temperature distribution in a
conventional reformer with a heater.
DESC~IPTION OF THE PREF~ EMBODINENTS
Modes of carrying out the present invention are described below
as preferred embodiments. Fig. 1 is a block diagram schematically
illustrating structure of a fuel cell system20 embodying thepresent
invention. The fuel cell system 20 includes as primary constituents
a methanol tank 28 for storing methanol, a water tank 30 for storing
water, a burner 34 for generating a combustion gas, a compressor 32
for compressing the air, an evaporator 24 with the burner 34 and the
compressor 32 mounted thereon, a reformer 22 for generating agaseous
fuel through the reforming reaction, a CO reduction unit 26 for
reducing theconcentration of carbon monoxide included inthe gaseous
fuel, a fuel-cells stack 40 for generating an electromotive force
through the electrochemical reaction, an air tank 36 for storing the
compressed air, a compressor 38 for feeding an a~ ry supply of
the compressed air, and a control unit 50 utilizing a computer. The
24
21 98333
structure of the fuel-cells stack 40 working as a generator in the
fuel cell system 20 is described first.
The fuel-cells stack 40 used in this embodiment is a stack of
Polymer Electrolyte Fuel Cells and includes a number of unit cells
5 48 layered one over another. Fig. 2 is a cross sectional view
illustratingstructure ofeachunitcell48 included inthe fuel-cells
stack 40. The unit cell 48 includes an electrolyte membrane 41, an
anode 42, a cathode 43, and separators 44 and 45.
The anode 42 and the cathode 43 are gas diffusion electrodes
lo arranged across the electrolyte membrane 41 to construct a
sandwich-like structure. The separators 44 and 45 are disposed
outside the sandwich-like structure and combined with the anode 42
and thecathode 43to formflowpaths ofagaseous fuel andano~i~;7ing
gas. The anode 42 and the separator 44 define flow paths 44P of
gaseous fuel, whereas the cathode 43 and the separator 45 define flow
paths 45P of oxidizing gas. Although the separators 44 and 45
respectively form the flow paths on their single side faces in the
drawing of Fig. 2, ribs are formed on either side faces of each
separator inthe actualstate. Namely one side face ofeachseparator
combined with the anode 42 forms the flow paths 44P of gaseous fuel,
while the otherside face combinedwith the cathode 43 of an adjoining
unit cell forms the flow paths 45P of oxidizing gas. The separators
44 and 45 are combined with the gas diffusion electrodes to define
gas flow paths andseparate flows ofthe gaseous fuel andthe oxidizing
gas in the adjoining unit cells. In the process of laying a number
of unit cells 48 one upon another to form a stack structure, the two
separators located on both ends of the stack structure may have ribs
2 1 98333
only on their single side faces coming into contact with the gas
diffusion electrodes.
The electrolyte membrane 41 is a proton-conductive ion-ezchange
membrane composed of a solid polymer material, such as fluororesin,
5 and shows favorable electrical conductivity in the wet state. In
this embodiment, a Nafion membrane (manufactured by du Pont) is
applied for the electrolyte membrane 41. The surface of the
electrolyte membrane 41 is coated with platinum or a platinum-
cont~;n;ng alloy functioning as a catalyst. The process adopted in
lo this embodiment to apply the catalyst prepares carbon powder with
platinum or a platinum-cont~in;~g alloy carried thereon, disperses
the catalyst-carried carbon powder into an appropriate organic
solvent, adds adesired amount ofanelectrolytesolution(forexample,
Nafion solution manufactured by Aldrich Chemical Corp.) to the
15 dispersion to form a paste, and screen-prints the paste on the
electrolyte membrane41. Anotherpreferably applicable methodforms
the paste cont~;n;ng the catalyst-carried carbon powder to a sheet
and presses the sheet onto the electrolyte membrane 41.
The anode 42 and the cathode 43 are made of carbon cloth, which
is woven of yarns consisting of carbon fibers. Although the anode
42 and thecathode 43 arecomposed of carboncloth in thisembodiment,
carbon paper or carbon felt consisting of carbon fibers are also
favorably applicable for the material of the anode 42 and the cathode
43.
The separators 44 and45 are made of a gas-impermeableconductive
material, for example, gas-impermeable, dense carbon obt~;neA by
compressing carbon. Each of the separators 44 and 45 has aplurality
21 98333
of ribs arranged in parallel and formed on both side faces thereof.
As discussed previously, each separator is combined with the surface
of the anode 42 to define the flow paths 44P of gaseous fuel and with
the surface of the cathode 43 of the adjoining unit cell to define
5 the flow paths 45P of oxidizing gas. In accordance with another
possible structure, the ribs formedon one side face ofeachseparator
may be arranged perpendicular to or at a certain angle with those
formed onthe otherside face ofthe separator. As long asthe gaseous
fuel and the oxidizing gas can be supplied to the gas diffusion
lo electrodes, the ribs may not be formed as parallel grooves.
As discussed above, each unit cell 48 has the separator 44, the
anode 42, the electrolyte membrane 41, the cathode 43, and the
separator 45, whichare arranged in this order. The fuel-cellsstack
40 is obtained by stacking plural sets of such unit cells 48 (100
sets in this embodiment) and arranging current collector plates (not
shown), which are made of dense carbon or copper plates, on both ends
of the stack structure.
Referring back to the drawing of Fig. 1, the following describes
the constituents of the fuel cell system 20 other than the fuel-
cells stack 40 and their connections. The evaporator 24 receivessupplies of methanol and water respectively fed from the methanol
tank 28 and the water tank 30 and vaporizes the supplied methanol
and water. The evaporator 24 is provided with the burner 34 and the
compressor 32 as mentioned previously. The heat of combustion is
supplied from the burner 34 via the compressor 32 to a heat exchanger
(not shown) included in the evaporator 24, so as to boil and vaporize
methanol and water fed to the evaporator 24, which will be described
21 98333
in detail later.
A methanol flow path 70, through which a supply of methanol is
fed as the raw fuel from the methanol tank 28 to the evaporator 24,
is providedwithasecondpump 64that functions toadjust the quantity
of methanol supplied to the evaporator 24. The second pump 64 is
electrically connected to the control unit 50 and driven by signals
output from the control unit 50 to regulate the quantity of methanol
supplied to the evaporator 24.
A water flow path 72, through which a supply of water is fed from
lo the water tank 30 to the evaporator 24, is provided with a third pump
65 that functions to adjust the quantity of water supplied to the
evaporator 24. Like the second pump 64, the third pump 65 is
electrically connected to the control unit 50 and driven by signals
output from the control unit 50 to regulate the quantity of water
supplied to the evaporator 24. The methanol flow path 70 and the
water flow path 72 meet each other to form a first fuel supplyconduit
78, which is connected with the evaporator 24. A mixture including
a certain amount of methanol regulated by the second pump 64 and a
certain amount of water regulated by the third pump 65 is accordingly
fed via the first fuel supply conduit 78 to the evaporator 24.
The compressor 32 mounted on the evaporator 24 receives an
oxidizing exhaust gas discharged from the fuel-cells stack 40,
compresses the oxidizing exhaust gas, and supplies the compressed
exhaust gas to the air tank 36. The compressor 32 has a turbine
25 element 32a and a compressor element 32b, which are formed as
impellers. The turbine element 32a and the compressor element 32b
are linked with each other via a co~ l shaft 32c, so that rotation
28
2 1 98333
of the turbine element 32a leads to a rotation of the compressor
element 32b. The evaporator 24 is also provided with the burner 34,
which gives the high temperature combustion gas to the compressor
32 to drive the turbine element 32a.
The compressor element 32b rotates with the rotation of the
turbine element 32a and compresses the oxygen-con~;n;ng gas
circulated through the fuel cell system 20. The compressor element
32b receives the fresh air via an air feed conduit 69 as well as the
oxidizing exhaust gas, which isdischarged from the oxygen electrodes
lo of the fuel-cells stack 40, via an oxidizing exhaust gas conduit 73.
The oxidizing exhaust gas passing through the fuel-cells stack 40
has a predetermined raised temperature and a predetermined pressure.
The oxidizing e~h~ t gas having such energy advantage is taken into
the fuel cell system 20 again and reused as the oxygen-cont~;n;ng
gas. Since a predeterr;ned amount of oxygen has already been
consumed in the fuel-cells stack 40, the oxygen content of the
oxidizing exhaust gas is naturally less than that of the air. A
certain amount of oxygen included in the oxygen-cont~; n; ng gas
circulated through the fuel cell system 20 is consumed by the burner
20 34 and the reformer 22 as discussed later. The compressor element
32b accordingly receives the fresh air as well as the oxidizing
exhaust gas to assure the sufficient supplies of oxygen fed to
predetermined portions of the fuel cell system 20. Although the
gaseous mixture of the fresh air and the oxidizing exhaust gas is
25 actually compressedbythe compressorelement 32b, the gascompressed
by the compressor element 32b is hereinafter simply referred to as
the compressed air.
2 1 98333
The turbine element 32a, which is driven to rotate by the high
temperature combustion gas fed from the burner 34, is composed of
a material having sufficient heat resistance and durability, such
as super alloy or ceramics. In this embodiment, the turbine element
5 32a is made of a nickel-based alloy (Inconel 700 manufactured by
Inconel Corp.) The compressor 32b is composed of a light-weight
aluminum alloy.
The pressurized, compressed air is sent to the air tank 36 and
subsequently supplied to the burner 34, the oxygen electrodes of the
lo fuel-cells stack 40, and the reformer 22 as discussed later. The
air tank 36 is provided with a pressure sensor 68 for measuring the
air pressure in the air tank 36 and with a compressor unit 38 for
supplying the air when the amount of the air in the air tank 36 is
insufficient. The pressure sensor 68 is electrically connected to
15 the control unit 50. The control unit 50 determines whether or not
the amount of the air in the air tank 36 is sufficient based on the
input signal sent from the pressure sensor 58, and outputs a driving
signal to the compressor unit 38 when determining that the amount
of the air is insufficient, so as to allow an adequate amount of the
20 compressed air to be supplied into the air tank 36. While the fuel
cell system 20 is driven in the stationary state, the sufficient
amount of the compressed air is supplied from the compressor 32 to
the air tank 36. The compressor unit 38 is thus mainly used at the
time of starting the system or on other required occasions.
The burner 34 for driving the turbine element 32a receives the
fuel for combustion from the cathodes of the fuel-cells stack 40 and
the methanol tank 28. The fuel-cells stack 40 receives the
2 1 98333
hydrogen-rich gas, which is obtained by reforming methanol by the
reformer 22, as a fuel and carries out the electrochemical reaction.
All the hydrogen supplied to the fuel-cells stack 40 is not consumed,
but afuelexhaust gascont~;n;ngthe remaininghydrogen is discharged
5 from a fuel exhaust conduit 74. The burner 34, which is connected
withthe fuelexhaustconduit 74, receivesthe discharged fuele~h~ust
gas andcompletelycombuststhe r~in;nghydrogen inthe fuelexhaust
gas, so as to improve the utilization factor of the fuel. When the
fuel component inthe fuel exhaust gas is notsufficient or whenthere
lo is no supply of fuel exhaust gas from the fuel-cells stack 40, for
example, at the time of starting the fuel cell system 20, the burner
34 receives a supply of methanol from the methanol tank 28. A
methanol branch path 71 is arranged to supply methanol to the burner
34. The methanol branch path 71 is branched from the methanol flow
path 70 for supplying methanol from the methanol tank 28 to the
evaporator 24.
The burner 34 receives oxygen required for combustion, in
addition to the above fuel. The oxygen required for combustion is
fed as the compressed air from the air tank 36 via a second air supply
conduit 76. The second air supply conduit 76 has a second flow
regulator 67, which receives a driving signaloutput from thecontrol
unit 50 and regulates the amount of the compressed air supplied to
the burner 34.
The burner 34 is provided witha first temperature sensor 60that
2s measures the temperature of the heat of combustion in the burner 34
and outputs the result of measurement as an electric signal to the
control unit 50. The control unit 50 outputs driving signals to a
2 1 98333
first pump 63 and the second flow regulator 67 based on the input
data from the first temperature sensor 60 and regulates the amount
of methanol and the amount of the compressed air fed to the burner
34, soas to keepthe temperature of combustion inthe burner34 within
a predetermined range (that is, approximately 800~C to 1000~C). The
combustion gas supplied from the burner 34 drives and rotates the
turbine element 32a and is subsequently led into the evaporator 24.
Since the heat exchange efficiency in the turbine element 32a is not
significantly high (less than approximately 10%), the temperature
o ofthecombustiongas led intotheevaporator24reaches approximately
600 to 700~C, which is sufficient as the heat source of the evaporator
24. The high temperature combustion gas of the burner 34 supplied
to the evaporator 24 vaporizes the solution mixture of methanol and
water supplied via the first fuel supply conduit 78. The raw fuel
gas including methanol and water vaporized by the evaporator 24 is
then fed into the reformer 22 via a second fuel supply conduit 79.
The reformer 22 reforms the raw fuel gas including the vaporized
methanol and water to a hydrogen-rich gaseous fuel. The structure
of the reformer 22 and the reforming reaction occurring in the
reformer 22 are essential parts of the present invention and will
be described in detail later. The second fuel supply conduit 79 for
supplying the raw fuelgas including the vaporized methanol andwater
to the reformer 22 is provided with a second temperature sensor 61
that measures the temperature of the raw fuel gas. The result of
measurement is input as an electric signal into the control unit 50
via a predetermined conducting line. The control unit 50 receives
21 98333
the signal from the first temperature sensor 60 that measures the
temperature of the heat of combustion in the burner 34 as well as
the signal from the second temperature sensor 61, determines the
internal state of the evaporator 24 based on these input signals,
5 and drives the second pump 64 and the third pump 65 to regulate the
quantities of methanol and water supplied to the evaporator 24 and
thereby regulate the temperature of the raw fuel gas vaporized in
the evaporator 24. The raw fuel gas supplied from the evaporator
24 generally has the raised temperature of approximately 250~C.
o As discussed later, oxygen is involved in the reformingreaction
proceeding in the reformer 22. In order to supply oxygen required
for the reforming reaction, the compressed air can be fed as the
oxidizing gas from the air tank 36 to the reformer 22 via a first
air supply conduit 75. The first air supply conduit 75 is provided
15 with afirst flowregulator66, whichreceives a drivingsignal output
from the control unit 50 via a predetermined conducting line and
regulates the amount of the oxidizing gas supplied to the reformer
22.
The hydrogen-rich gaseous fuel generated by the reformer 22 is
led into the CO reduction unit 26 via a third fuel supply conduit
80. A third temperature sensor 62 disposed in the third fuel supply
conduit 80 measures the temperature of the gaseous fuel discharged
from the reformer22 andgives the result of measurement as anelectric
signal to the control unit 50 via a predetermined conducting line.
The control unit 50 determines the reaction temperature in the
reformer 22 based on the input signal from the third temperature
21 98333
sensor 62 and outputs the driving signal to the first flow regulator
66, so as to regulate the amount of the air fed into the reformer
22. As discussed later, the regulation of the amount of the air
supplied to the reformer 22 controls the state of the reforming
5 reaction proceeding in the reformer 22, thereby regulating the
internal temperature of the reformer 22.
The CO reduction unit 26 is a device for reducing the
concentration of carbon monoxide included in the gaseous fuel
supplied from the reformer 22 via the third fuel supply conduit 80.
lo The typical reforming reaction of methanol is expressed as Equations
(4) through (6) above. In the actual state, however, the reforming
reaction expressed as these Equations does not proceed ;~e~lly in
the reformer 22, but the gaseous fuel generated by the reformer 22
contains a certain amount of carbon monoxide. The CO reduction unit
15 26 then functions to reduce the concentration of carbon monoxide
included in the gaseous fuel supplied to the fuel-cells stack 40.
The fuel-cells stack 40 of this embodiment is a stack of Polymer
Electrolyte Fuel Cells with platinum or a platinum-cont~;n;ng alloy
carried thereon as the catalyst, as described previously. In this
embodiment, the platinum catalyst is applied on the surface of the
electrolyte membranes 41. In case that carbon monoxide is included
in the gaseous fuel, the carbon monoxide is adsorbed by the platinum
catalyst and lowers the catalytic function of platinum. This
interferes with the reaction expressed as Equation (1) at the anode
and thereby lowers the performance of the fuel cells. When the
Polymer Electrolyte Fuel Cells like the fuel-cells stack 40 are used
for generating electrical energy, it is accordingly essential to
34
21 98333
reduce the concentration of carbon monoxide included in the supply
of gaseous fuel to a predetermined or lower level and thus prevent
the performance of the fuel cells from being lowered. In thePolymer
Electrolyte Fuel Cells, the allowable limit of carbon monoxide
included in the supply of gaseous fuel is not greater than several
ppm.
The gaseous fuel led to the CO reduction unit 26 is the
hydrogen-rich gas cont~;n;ng a certain level of carbon monoxide as
discussed above. The CO reduction unit 26 oxidizes carbon monoxide
lo in preference to hydrogen in the gaseous fuel. The CO reductionunit
26 is filled with a carrier with a platinum-ruthenium alloy catalyst
that works as a selective oxidizing catalyst of carbon monoxide. The
final concentration of carbon monoxide included in the gaseous fuel
after the treatment in the CO reduction unit 26 depends upon the
operation temperature of the CO reduction unit 26, the concentration
of carbon monoxide included in the gaseous fuel fed into the Co
reduction unit 26, the flow of gaseous fuel per unit volume of the
catalyst into the COreduction unit 26, and other relatedparameters.
The CO reduction unit 26 is provided with a carbon monoxide
concentration sensor (not shown). The operation temperature of the
CO reduction unit 26 and the flow of supplied gaseous fuel are
regulated according to the result of measurement by the carbon
monoxide concentration sensor, so that the final concentration of
carbon monoxide included in the treated gaseous fuel is controlled
to be not greater than several ppm.
The gaseous fuel treated by the CO reduction unit 26 to have the
reduced concentration of carbon monoxide is led into the fuel-cells
2t 98333
stack 40 via a fourth fuel supply conduit 81 and consumed by the cell
reaction at the anodes. The fuel ex~ st gas after the cell reaction
in the fuel-cells stack 40 is discharged to the fuel exh~ust conduit
74 and led into the burner 34, which consumes the remaining hydrogen
included in the fuel exhaust gas as the fuel for combustion. The
oxidizing gas involved in the cell reaction at the cathodes of the
fuel-cells stack 40 is, on the other hand, supplied as the compressed
air from the air tank 36 via a third air supply conduit 77. The
oxidizing exhaust gas afterthe cell reaction in the fuel-cells stack
40 is discharged to the oxidizing exhaust gas conduit 73 and led into
the compressor 32, so as to be compressed by the compressor element
32b and recycled to the air tank 36.
The control unit 50 is constructed as a logic circuit including
a microcomputer. Concretely the control unit 50 includes a CPU 54
for executing a variety of operations according to preset control
programs, a ROM 56 inwhich control programs and control data required
for the various operations by the CPU 54 are stored in advance, a
RAM 58 from and into which a variety of data required for the various
operations by the CPU 54 are temporarily read and written, and an
input/output port 52 that receives detection signals from the
temperature sensors and pressuresensors and outputs drivingsignals
to the pumps and flow regulators based on the results of operations
by the CPU 54.
The following describes the structure of the reformer 22, which
is an essential part of the present invention. Fig. 3 schematically
shows structure of the reformer 22 of the first embodiment. The
reformer 22 has a reformer unit 23 filled with pellets of a Cu-Zn
2 1 98333
catalyst, which is a catalytic metal for accelerating the reforming
reaction. The second fuel supply conduit 79, which sends the raw
fuel gas including methanol and water vaporized in the evaporator
24 to the reformer 22, joins the first air supply conduit 75 before
5 the joint with the reformer 22. The raw fuel gas including the
vaporized methanoland water is accordingly mixed withthecompressed
air given as the oxidizing gas to contain a certain level of oxygen,
before being fed into the reformer 22. The reformer unit 23 of the
reformer 22 reforms the oxygen-cont~;n;ng raw fuel gas to a gaseous
lo fuel. The gaseous fuel thus obtained is then supplied via the third
fuel supply conduit 80 to the fuel-cells stack 40 and consumed by
the cell reaction.
The pellets of the catalytic metal, that is, Cu-Zn catalyst, are
particles of 3to 7 mmin diameter obtainedby preparing thecatalytic
15 metal through coprecipitation of copper and zinc oxide, adding a
binder, such as alumina, to the catalytic metal, and extruding the
mixture of the catalytic metal and the binder. The pellets used in
this embodiment have the dimensions of approximately 3 mm x 3 mm x 3
mm. The pellets of the Cu-Zn catalyst are homogeneously packed into
20 the reformer unit 23. The oxygen-cont~;n;ng raw fuel gas led into
the reformer unit 23 comes into contact with the catalytic metal on
the surface of the pellets to undergo the reforming reaction, while
moving toward the outlet of the reformer 22 connecting with the third
fuel supply conduit 80. The catalyst-cont~;n;ngpellets packed into
25 the reformer unit 23 may be prepared by impregnation or any adequate
process other than the coprecipitation.
21 98333
The reforming reaction occurring in the reformer 22 is described
in detail. Steam reforming of methanol in the presence of Cu-Zn
catalyst generally follows the reactions defined by Equations (4)
through (6) given above, and an endothermic reaction proceeds as a
5 whole. In this embodiment, however, oxygen is further added to
methanol and steam. In this case, an exothermic reforming reaction
defined as Equation (7) given below proceeds in the presence of the
Cu-Zn catalyst, in addition to the reforming reaction defined by
Equations (4) through (6):
CH30H + (l/2)02 -~ CO2 + 2H2 + 189.5 (kJ/mol) (7)
When the oxygen-cont~;n;ng raw fuel gas is supplied to the
reformer 22 as shown in Fig. 3, the exothermic reaction defined by
Equation (7) continues proceeding in the reformer 22 until oxygen
is used up. While the exothermic reaction defined by Equation (7)
proceeds, the general steam reforming reaction defined by Equation
(6) is carried out. After oxygen has been consumed to stop the
exothermic reaction defined by Equation (7), only the endothermic
reforming reaction defined by Equation (6) proceeds. The amount of
oxygen added to the raw fuel gas supplied to the reformer 22 should
thus be determined according to the amount of methanol included in
the raw fuel gas, in order to allow the amount of heat required for
the general reforming reactiondefined by Equation (6) to besupplied
by the exothermic reaction defined by Equation (7). When the amount
of oxygen included inthe compressed air introduced into the reformer
22 is set equal to 10 to 20% of the amount of methanol fed into the
reformer 22 by taking into account the possible energy loss due to
21 98333
the partial heat dissipation outside the high-temperature reformer
22, the amounts of heat absorbed and generated by the reactions
balance with each other. Namely the amount of heat required for the
endothermic reaction can be supplied by the amount of heat generated
5 by the exothermic reaction.
Fig. 4 is a graph showing a temperature distribution in the
reformer 22 when the oxygen-cont~;n;ng raw fuel gas is supplied to
the reformer 22 and the endothermic and exothermic reforming
reactions proceed as discussed above. In the vicinity of the inlet
of the reformer 22, the endothermic reforming reaction defined by
Equation (6) and the exothermic reaction defined by Equation (7)
proceed simultaneously. The exothermic reaction generally has the
higher reaction rate than that of the endothermic reaction, so that
the amount of heat generated exceeds the amount of heat absorbed to
increase the temperature in the reformer 22. Oxygen fed to the
reformer 22 isused up sometime after thetemperature in the reformer
22 reaches a peak, and only the endothermic reaction defined by
Equation (6) proceeds afterwards. The amount of heat required for
the endothermic reaction is supplied by the heat generated by the
exothermic reaction. After the temperature in the reformer 22
reaches its peak, the temperature thus gradually decreases toward
the outlet of the reformer 22.
The reformer 22 of this embodiment discussed above can reduce
the amount of heat supplied externally to the reformer 22. Since
the mixture of the oxygen-cont~'n;ng compressed air and the raw fuel
gas including the vaporized methanol and steam is fed into the
reformer 22, the exothermic reforming reaction expressed asEquation
2 1 98333
(7) occurs in the reformer 22, in addition to the endothermic
reforming reaction expressed as Equation (6). The reformer 22
includes the reformer unit 23 that is homogeneously filled with the
Cu-Zn catalyst functioning as the reforming catalyst. The heat
5 generated by the exothermic reaction of Equation (7) is diffused in
the reformer unit 23 and thereby utilized as the heat required for
the endothermic reaction of Equation (6). ThiS structure can thus
reduce the amount of heat supplied externally to the reformer 22 for
the endothermic reaction.
lo In this embodiment, the amount of oxygen added to the raw fuel
gas supplied to the reformer 22 is determined according to the amount
of methanol included in the raw fuel gas, in order to allow the amount
of heat required for the endothermic reaction to be supplied by the
exothermic reaction. The structure of the embodiment does not
accordingly require any additional heating device for supplying the
heat required for the endothermic reaction. This effectively
prevents the whole fuel cell system from being undesirably
complicated or bulky. No extra energy is consumed for heating the
reformer 22 in the structure of the embodiment, so that the energy
efficiency ofthe whole fuelcell system is not lowered. As discussed
previously, the amount of heat required for the endothermic reaction
of Equation (6) proceeding in the reformer 22 is supplied by the
exother~;c reaction of Equation (7) proceeding in the reformer 22.
Compared with the structure that supplies the heat from an external
heat source arranged a predetermined distance apart, the structure
of theembodiment has asmallerenergy loss due tothe heat dissipation
and thereby prevents the energy efficiency of the whole system from
21 98333
being undesirably lowered.
In this embodiment, the oxygen-cont~;n;ng compressed air is
added to the methanol-cont~;n;ng raw fuel gas at the inlet of the
reformer 22. The peak of the temperature distribution in the
reformer 22 is thus observed at the position closer to the inlet of
the reformer 22. As discussed previously, after the temperature in
the reformer 22 reaches the peak and the supply of oxygen is used
up, only the endothermic reforming reaction expressed by Equation
(6) proceeds. The temperature in the reformer 22 thus continues
lo decreasing toward the outlet connecting with the third fuel supply
conduit 80. In the reforming reaction, the lower temperature
generally accelerates the shift reaction defined as Equation (5)
given above and reduces the concentration of carbon monoxide. In
this embodiment, the exothermic reaction that increases the
15 temperature is vigorously carried out at the inlet of the reformer
22, whereas the temperature decreases at the outlet. The shift
reaction is accordingly accelerated at the outlet of the reformer
22, so as to reduce the concentration of carbon monoxide included
in the reformed fuel gas.
The reduced concentration of carbon monoxide included in the
gaseous fuel decreases the quantity of carbon monoxide to be treated
by the CO reduction unit 26. The required capacity of the CO
reduction unit 26 can thus be lessened. In the structure that does
not utilize the heat generated in the process of reducing carbon
monoxide in the CO reduction unit 26 but discharges the heat out of
the system, the decreased amount of carbon monoxide to be treated
by the CO reduction unit 26 leads to a decrease in amount of heat
41
2 1 98333
not utilized but discharged, thus improving the energy efficiency
of the whole system.
As discussed previously, when the amount of oxygen included in
the compressed air introduced into the reformer 22 is set equal to
10 to 20% of the amount of methanol fed into the reformer 22, the
amounts of heat absorbed and generated by the reactions balance with
each other. Under such conditions, 20 to 40% of the amount of
methanol supplied to the reformer 22 is used for the exothermic
reaction expressed as Equation (7). Since the exother~;c reaction
o is faster than the endothermic reaction, the rate of the reforming
reaction occurring in the reformer 22 is heightened in case that the
exothermic reaction of Equation(7) proceeds simultaneously withthe
endothermic reaction of Equation(6), compared withthe case inwhich
only the endothermic reaction of Equation (6) proceeds. In this
15 embodiment, the exothermic reaction of Equation (7) is carried out
with the certain ratio of raw fuel gas. This structure effectively
reduces the volume of the reformer unit 23 required for generating
a desired amount of gaseous fuel within a predetermined time period.
The ratio of the amount of oxygen to the amount of methanol can be
lessened by improving the heat insulation of the reformer 22 and
reducing the possible energy loss of the reformer 22 due to the heat
dissipation.
In theconventionalstructurethat carries out only the reforming
reaction defined by Equations (4) through (6), the amount of water
(steam) two to three times as much as the amount of methanol is fed
into the reformer. The excess amount of steamvaries theequilibrium
of the reaction expressedas Equation (5)to reduce theconcentration
42
2 1 98333
of carbon monoxide, thereby accelerating the total reaction defined
by Equation (6). Extra energy is, however, required to vaporize the
excess amount of water, whichleads to adecrease inenergy efficiency
of the whole system. In thestructure ofthe embodiment,on the other
5 hand, the reaction of Equation (7) which does not require water is
carried out at the predetermined ratio, so that the amount of water
supplied to the reformer 22 can be decreased. When 20 to 40% of
methanol fed into the reformer 22 is consumed by the reaction of
Equation (7), for example, the amount of water to be added is 1.2
lo to 2 times as much as the amount of methanol. This structure
effectively decreasesthe amount ofwater vaporized intheevaporator
24 and reduces the amount of energy required for vaporizing water
in the evaporator 24, thus improving the energy efficiency of the
whole system. The structure of the embodiment further enables the
15 evaporator 24,the watertank30, andthe piping systemforconnecting
them with each other to be desirably space-saving and compact.
Another desirable structure of the embodiment has means for
lowering the peak of the temperature distribution in the reformer
22 and maint~;n;ng the internal temperature ofthe reformer 22 within
20 an appropriate range. As discussed above, the amount of oxygen fed
into the reformer 22 can be determined according to the supply of
methanol. In accordance with the desirable structure, the amount
of oxygen fed into the reformer 22 is finely regulated, based on the
state of the reforming reaction procee~;ng in the reformer 22. This
25 enables the state of the temperature distribution in the reformer
22 to be kept in a favorable range. A concrete structure for
regulating the supply of oxygen, for example, includes a fourth
43
21 98333
temperature sensor 31 disposed in the reformer 22. The control unit
50 receives a detection signal from the fourth temperature sensor
31 and outputs a driving signal to the first flow regulator 66 based
on the input data. In case that the internal temperature of the
5 reformer 22 rises with an increase insupply of oxygen, thisstructure
enables the control unit 50 to immediately control the first flow
regulator 66, in order to reduce the amount of oxygen and maintain
the temperature in the reformer 22 within a suitable range.
This desirable structure of regulating the supply of oxygen
lo accordingtothestateofthetemperature distribution inthe reformer
22 can effectively prevent the internal temperature of the reformer
22 from temporarily or partially rising too high and causing various
problems. The major problems caused by the unintentional increase
in internal temperature of the reformer 22 include deterioration of
15 the catalyst and generation of non-required by-products.
The first drawback is deterioration of the catalyst. The
reformer unit 23 of the reformer 22 is filled with the Cu-Zn catalyst
as described above. In case that the Cu-Zn catalyst is exposed to
the temperatures of about 300~C or higher, there is a possibility
of lowering its durability and sintering the Cu-Zn catalyst to
deteriorate its catalytic effect. The term 'sintering' herein
implies the phenomenon of aggregating the catalyst carried on the
surface of the carrier. In the normal state of the Cu-Zn catalyst,
fine particles of copper are dispersed on the surface of zinc
particles. Sintering, however, agglomerates the fine particles of
copper to large particles on the surface of the carrier. This
44
21 98333
phenomenon decreases the surface area of the copper particles and
reduces the active area of the catalyst, thereby lowering the
performance of the reformer 22.
The second drawback is generation of by-products. At the
5 predetermined high temperatures, while the normal reforming
reactions proceed, undesirable side reactions are carried out to
generate, for example, methane or nitrogen oxides (the latter is
generated by a reaction of nitrogen gas included in the pressurized
air). These by-products are not decomposed in the temperature range
lo of the normal reforming reactions occurring in the reformer 22 and
are accordingly fed to the fuel-cells stack 40. Especially an
increase in methane undesirably leads to a decrease in hydrogen
partial pressure in the gaseous fuel. The desirable structure of
this embodiment can prevent these drawbacks.
In the above structure, the control unit 50 receives a detection
signal representing the internal temperature of the reformer 22 and
controls the supply of oxygen based on the input ~ata. Another
available structure regulates the supply of oxygen without the
operation of the control unit 50. In this available structure, the
20 first flowregulator 66 iscomposed ofabimetal orshape-memory alloy
and disposed at a site that adjoins to the reformer 22 and reflects
the internal temperature of the reformer 22. This structure enables
the first flow regulator 66 to directly detect the temperature in
the reformer22 andregulatethe amount ofthe compressedairsupplied
25 to the reformer22. Thestructure of controllingthe amount of oxygen
fed into the reformer 22 according to the internal temperature of
the reformer 22 enables the inside of the reformer 22 to be kept in
21 98333
the desirable temperature range of 250 to 300~C, and effectively
prevents the drawbacks discussed above, that is, deterioration of
the catalyst and generation of by-products.
In the above embodiment, the first air supply conduit 75 joins
5 the second fuel supply conduit 79 immediately before the reformer
unit 23 of the reformer 22, in order to add the compressed air to
the raw fuel gas cont~;n;ng methanol. As long as the raw fuel gas
cont~;n;ng oxygen can reach the reforming catalyst, however, the
compressed air may be added at any desirable position. By way of
o example, the first airsupplyconduit 75 withthe first flowregulator
66 may join the first fuel supply conduit 78, in order to mix oxygen
with the raw fuel gas prior to the evaporator 24.
Alternatively, the first air supply conduit 75 with the first
flow regulator 66 may be connected directly with the reformer 22,
15 in stead of joining the second fuel supply conduit 79 as shown in
Fig. 3, so as to introduce the compressed air alone into the reformer
unit 23. The inlet of the compressed air into the reformer unit 23
may be close to or a certain distance apart from the inlet of the
raw fuel gas. In this alternative structure, the peak of the
20 temperature increase by the exothermic reaction of Equation (7) is
shifted toward the outlet of the reformer 22. A sufficient increase
in temperature ofthe raw fuel gas supplied tothe reformer 22enables
the endothermic reforming reaction to proceed at the inlet of the
reformer 22. Means for supplementing the heat required for the
25 reforming reaction may be disposed in the vicinity of the inlet of
the reformer 22. In order to reduce the concentration of carbon
46
2l q8333
monoxide included in the gaseous fuel, it is desirable to arrange
the inlet of the compressed air at an adequate position to allow a
sufficient decrease in temperature at the outlet of the reformer 22.
The reformingcatalyst used in the reformer 22Of this embodiment
5 iS the Cu-Zn catalyst, which accelerates both the steam reforming
reaction defined by Equations (4) through (6) and the exothermic
reforming reaction expressed as Equation (7). This single catalyst
realizes the above functions required for the reformer unit 23 and
thereby simplifies the structure of the reformer unit 23. The
o catalyst packed into the reformer unit 23 may, however, be any other
catalyst that can accelerate both the steam reforming reaction and
the exothermic reforming reaction, for example, a Pd-Zn catalyst.
In the reformer 22 of this embodiment, the reformer unit 23 is
filled with the pellets of the Cu-Zn catalyst. The reformer unit
23 may alternatively have a honeycomb structure. In this case, the
catalytic metal is carried on the surface of the honeycomb structure
of the reformer unit 23.
In the first embodiment, the required amount of oxygen is all
added in advance to the methanol-cont~;ning raw fuel gas prior to
the supply into the reformer 22. Another desirable structure given
as a second embodiment adds a certain amount of oxygen in the course
of the reactions occurring inthe reformer22. Fig.5shows structure
of a reformer 22a incorporated in a fuel cell system according to
the second embodiment. The fuel cellsystem ofthe second embodiment
25 has the same constituents as those of the fuel cell system 20 of the
first embodiment shown in Fig. 1, except the reformer 22a. The like
elements are accordingly not described here and are shown by the like
21 98333
numerals. Reformers of third through sixth embodiments described
after thesecondembodiment are respectively incorporated inthe fuel
cell system having the same structure as that of the fuel cell system
20 of the first embodiment.
Like the reformer 22 of the first embodiment shown in Fig. 3,
in the structure of the second embodiment, the second fuel supply
conduit79 forsupplyingthe rawfuel gas includingvaporized methanol
and steam to the reformer 22a joins the first air supply conduit 75
prior to the joint with the reformer 22a. The raw fuel gas including
lo vaporized methanol and water is mixed with the compressed air to
contain acertain amountofoxygen, before being fed intothereformer
22a. An air supply branch path 82 is connected to the reformer 22a
of the second embodiment at a position closer to the inlet of the
reformer 22a, in order to supply an additional amount of the
compressed air to the reformer 22a. The air supply branch path 82
is branched from the first air supply conduit 75 and receives the
compressed air from the air tank 36.
Like the reformer 22 of the first embodiment, a reformer unit
23a of the reformer 22a is filled with pellets of the Cu-Zn catalyst
20 functioning as the reforming catalyst. In the same manner as the
first embodiment, the gaseous fuel generated by the reformer 22a is
supplied to the fuel-cells stack 40 via the third fuel supply conduit
80.
Fig. 6 is a graph showing a temperature distribution in the
25 reformer 22a when the reforming reactions proceed, upon condition
that the oxygen-cont~;n;ng raw fuel gas is supplied to the inlet of
the reformer 22 and the compressed air is further added to the middle
48
2 1 98333
of the reformer 22a. The amount of oxygen added to the middle of
the reformer 22a is identical with that added to the inlet of the
reformer 22a. Like the first embodiment, the exo~herr;c reaction
expressed as Equation (7) proceeds in the vicinity of the inlet of
5 the reformer 22a. This exothermic reaction raises the temperature
in the reformer 22a and accelerates the endothermic reaction
expressed as Equation (6). Since the exothermic reaction is faster
than the endothermic reaction as discussed above, the internal
temperature of the reformer 22a continues increasing.
Oxygen introduced with the raw fuel gas is used up to conclude
the exothermic reaction of Equation (7) some time after the internal
temperature of thereformer 22areaches apeak. Only theendothermic
reaction expressed as Equation (6) continues proceeding afterwards
byutilizingthe heat generatedbytheexothermic reactionofEquation
15 (7), so as to lower the internal temperature of the reformer 22a,
in the same manner as the first embodiment. In the structure of the
second embodiment, however, the additional amount of the compressed
air issuppliedthroughthe air supply branch path82. The additional
supply of the compressed air initiates the exothermic reaction of
20 Equation (7) and raises the internal temperature of the reformer 22a
again. After the additional supply of oxygen is used up, only the
endothermic reaction of Equation (6) proceeds in the reformer 22a
and the temperature thus gradually decreases toward the outlet of
the reformer 22a.
The structure of the second embodiment feeds the oxygen-
cont~ining compressed air tothe reformer 22aat two different times.
This leads to two separate peaks of the exotherr;c reaction of
49
21 98333
Equation (7) among the reforming reactions occurring in the reformer
22a. While the total amount of oxygen supplied to the reformer 22a
is identical with that of the first embodiment, the structure of the
second embodiment can lower the peak of the temperature distribution
5 in the reformer 22a. The lowered peak temperature in the reformer
22a effectively prevents the catalyst from being sintered and
deteriorating its catalytic effect, due to a partial or temporary
extreme increase in temperature in the reformer 22a. The lowered
peak temperature in the reformer 22a also decreases the possible
lo energy loss due to heat dissipation, which is ascribed to the
temperature difference between the inside of the reformer 22a and
the surrounding air. Thesmaller energy loss due to heat dissipation
enables the greater amount of heat generated by the exothermic
reaction to be used for the endothermic reaction, thereby enabling
15 a further reduction in size of the reformer 22a and improving the
energy efficiency of the whole system.
It is preferable that the additional supply of the oxygen-
cont~;n;ng air is given to the reformer 22a at a position closer to
its inlet, or more specifically at a position defined by d/c of 1
to 4 in the drawing of Fig. 5. This structure gives two separate
peaks oftheexothermic reactiontoexert theeffects discussed above,
and enables the internal temperature to be sufficiently lowered in
the vicinity of the outlet of the reformer 22a. The sufficiently
low temperature accelerates the shift reaction of Equation (5) at
25 the outlet of the reformer 22a and thus significantly lowers the
concentration of carbon monoxide included in the gaseous fuel fed
through the third fuel supply conduit 80.
2 1 98333
In thestructure ofthe secondembodiment, the two separatepeaks
of the exothermic reaction widen the area having temperatures equal
to or higher than a predetermined level in the reformer 22a, thus
increasing the active area of the endothermic reaction. The
5 endothermic reaction is accelerated sufficiently when the
surrounding temperature is raised toapredetermined or higher level.
On the assumption that the total amount of heat generated by the
exothermic reaction is fixed, even if the peak temperature of the
exothermic reaction is low, the wider area having temperatures equal
to or higher than a predetermined level increases the efficiency of
the endothermic reaction as a whole. The increase in active area
of the endothermic reaction improve~ the reforming efficiency per
unit volume in the reformer 22a, therebyenabling a further reduction
in size of the reformer 22a.
In the second embodiment, two equal portions of the air are
supplied to the reformer 22a separately. The supply of the air may,
however, be divided at a variable ratio, in order to regulate the
temperature distribution in the reformer 22a. This alternative
structure is readily realized by arranging regulating valves, which
20 are controllable by the control unit 50, in the first air supply
conduit 75 and the air supply branch path 82. Even when the amount
processed by the reforming reaction in the reformer 22a is varied
with a variation in amount of electrical energy generated by the
fuel-cells stack 40, the structure of controlling the divisions of
oxygen supply can adjust the peak temperature in the reformer 22a
and the active area of the endothermic reaction. The regulation of
the oxygen supplies from predeterr;ne~ positions based on the amount
51
21 ~8333
of methanol included in the raw fuel gas fed into the reformer 22a
adequately controls the temperature distribution in the reformer22a
and regulates the concentration of carbon monoxide in the gaseous
fuel generatedbythe reformingreactionwithin anappropriate range.
5 This structureenables the amount processedby the reformingreaction
in the reformer 22a to be regulated according to the output state
of the fuel-cells stack 40 without delay, thereby facilitating the
regulation of the gas flow supplied to the fuel-cells stack 40.
When the supply of oxygen to the reformer 22a is controlled by
lo the regulating valves arranged in the first air supply conduit 75
and the air supply branch path 82 as discussed above, sensors should
be disposed at the related portions of the fuel cell system. The
control unit 50 receives detection signals from these sensors and
controls the regulating valves based on the input information. This
15 enables the supply of oxygen to be regulated with high accuracy. By
way ofexample, the reformer22a isprovided with atemperaturesensor
for measuring the temperature in the reformer 22a. In case that the
peaktemperature ofthe reformingreactions risestoo high,thesupply
of oxygen through either or both of the two regulating valves is
20 decreased to discourage the exothermic reforming reaction and lower
the temperature. In case that the temperature in the reformer 22a
starts decreasing, on thecontrary, the supply of oxygen is increased
to encourage the exothermic reforming reaction.
As another example, a CO sensor for measuring the concentration
25 of carbon monoxide included in the gaseous fuel generated by the
reforming reaction is arranged in the third fuel supply conduit 80.
This structure enables a variation in concentration of carbon
52
2 1 98333
monoxide included in the reformed gas to be monitored. When the
concentration of carbon monoxide becomes equal to or greater than
a predetermined level, the supply of the air through the air supply
branch path 82 is decreased to lower the temperature in the vicinity
5 of the outlet of the reformer 22a and reduce the concentration of
carbon monoxide included in the gaseous fuel. As still another
example, a flow sensor for measuring the flow of the raw fuel gas
supplied to the reformer 22a is arranged in the second fuel supply
conduit 79. This structure enables the supply of oxygen to be
lo regulated to an appropriate level, in response to a variation in
amount of the gas to be reformed by the reformer 22a.
In the second embodiment discussed above, the oxygen-cont~;n;ng
compressed air can be supplied from two different positions into the
reformer 22a. Another desirable structure given as a modification
15 has three or more different positions, from which the compressed air
is supplied. Fig. 7 shows structure of a reformer 22b having three
or more air-supply positions (five air-supply positions in the
drawing). In the second embodiment shown in Fig. 5, the compressed
air flowing through the air supply branch path 82 is subjected
20 directly to the reforming reactions occurring in the reformer 22a.
In the modified structure shown in Fig. 7, on the other hand, the
compressed air flowing through the air supply branch path 82 is fed
into a reformer unit 23b via a plurality of regulating apertures 83
formed inside the reformer 22b. The reformer unit 23b is filled with
25 pellets of the Cu-Zn catalyst in the same manner as the first and
the second embodiments.
21 98333
This structure of feeding divisions of the compressed air from
a plurality of different positions decreases the amount of the air
to be supplied from each posi~ion, thus further lowering the peak
of the temperature increase by the exothermic reforming reaction.
The increase in number of the air-supply positions further widens
the area having temperatures equal to or higher than a predetermined
level in the reformer 22b and thereby increases the active area of
the endothermic reaction. It is preferable that the position of the
last regulating aperture 83 is a predetermined distance apart from
lo the outlet of the reformer 22b connecting with the third fuel supply
conduit 80. The predeterr;neA distance enables the internal
temperature of the reformer 22b to be sufficiently lowered in the
vicinity of the outlet, thus accelerating the shift reaction of
Equation (5) and sufficiently reducing the concentration of carbon
monoxide included in the reformed fuel gas.
When the plurality of regulating apertures 83 have an identical
diameter, the compressed air flowing through the air supply branch
path 82 is fed into the reformer 22b equally from the respective
regulating apertures 83. This uniformly disperses the temperature
increase in the reformer 22b. In accordance with another desirable
structure, the diameter of the regulating apertures 83 in the rear
portion is made smaller than that of the regulating apertures 83 in
the front portion. This decreases the amount of the air supplied
to the rear portion of the reformer 22b and lowers the peak of the
temperature increase in the rear portion, thereby lowering the
temperature in the vicinity of the outlet of the reformer 22b and
reducing the concentration of carbon monoxide.
54
21 98333
The regulating apertures 83 may be constructed as regulating
valves driven by the control unit 50. This structure allows the
supply of the air to be finely controlled according to the various
conditions surrounding the reformer 22b. By way of example, the
5 controlunit 50receives adetectionsignal fromatemperaturesensor,
which measures the temperature distribution in the reformer 22b, and
controls the open and close conditions of the respective regulating
apertures 83 based on the input data of temperature distribution to
varythesupply ofthe airthroughthe respective regulatingapertures
o 83, thus keeping the temperature distribution in the reformer 22b
in a desired state with high accuracy. In accordance with another
desirable structure, the control unit 50 also receives detection
signals from various sensors for measuring the concentration of
carbon monoxide included in the gaseous fuel flowing into the third
15 fuel supply conduit 80 and the flow of the raw fuel gas supplied
through the second fuel supply conduit 79, other than the internal
temperature of the reformer 22b, and finely controls the open and
close conditions of the respective regulating apertures 83 based on
the input data, thereby maint~;n;ng the high reforming efficiency
20 in the reformer 22b.
The regulating apertures 83 may alternatively be composed of a
bimetal or shape-memory alloy. The open and close conditions of the
respective regulatingapertures83 arethenvaried withatemperature
change in the surrounding areas of the regulating apertures 83. This
25 structure regulates the supply of the air and enables thetemperature
distribution in the reformer 22b to be kept in a desired range.
Compared with the above structure of the regulating apertures 83
21 98333
controlled by the control unit 50, the elements and wiring can be
remarkably simplified in this alternative structure.
In the second embodiment discussed above, the temperature inthe
reformer is controlled by regulating the supply of the air used for
the reforming reaction. Another preferable structure described as
a third embodiment has heat tr~n~ sion means disposed in the
reformer for regulating the reaction temperature. Fig. 8 shows
structure of another reformer 22c having heat pipes 84 incorporated
therein. Like the reformer 22 of the first embodiment shown in Fig.
lo 3, in the structure of the third embodiment, the second fuel supply
conduit 79 joins the first air supply conduit 75 in the vicinity of
the inlet of the reformer 22c. The methanol-cont~;n;ng raw fuel gas
is mixed with oxygen, before being fed into the reformer 22c. The
reformer 22c is filled with pellets of the Cu-Zn catalyst, in the
same manner as the first and the second embodiments. The heat
generated by the exothermic reforming reaction is transmitted in the
reformer 22c via the heat pipes 84 arranged inside the reformer 22c.
Fig. 9 is a graph showing a temperature distribution in the
reformer 22c when the reforming reactions proceed in the reformer
22c. The raw fuel gas fed into the reformer 22c undergoes the
reforming reactions, while moving toward the outlet of the reformer
22c. Since the raw fuel gas contains a large quantity of oxygen at
the inlet of the reformer 22c, the exothermic reforming reaction
defined by Equation (7) vigorously proceeds in the vicinity of the
inlet. Because of the heat generated by the exothermic reaction,
the temperature in the reformer 22c continues increasing and reaches
a peak. The heat pipes 84 disposed in the reformer 22c transmit the
56
21 98333
heat generated by the exothermic reaction to the rear portion of the
reformer 22c. The peak temperature in the reformer 22c with the heat
pipes 84 for heat transmission is accordingly lower than the same
in the reformer without any heat pipes. ThiS structure effectively
5 prevents the internal temperature of the reformer 22c from
temporarily or partially rising too high, thereby being free from
the drawbacks discussedabove, suchas deterioration ofthecatalyst.
The heat pipes 84 transmit the heat from the high-temperature
area to the low-temperature area. In the reformer 22c of the third
lo embodiment, while the peak temperature is lowered, the temperature
level other than the peak is totally heightened. This widens the
area having temperatures equal to or greater than a predetermined
level and increases the active area of the endothermic reforming
reaction defined by Equation (6), thereby improving the efficiency
of the reforming reaction per unit volume in the reformer 22c. The
reformer 22c of thethird embodiment does not require anycomplicated
mech~n;~m for controlling the supply of the air, but exerts the
effects discussed above with the heat pipes disposed therein.
In the structure of the third embodiment, the heat generated by
20 the exothermic reaction is dispersed inside the reformer 22c. This
lowers thepeakofthe temperature increase bythe exothermic reaction
and thus enables a qreater quantity of oxygen to be supplied at one
time in order to accelerate the exothermic reaction. This
characteristic is especially advantageous when the load abruptly
25 increases, for example, at the time of starting the system, as
discussed below.
21 98333
When an abrupt increase in amount of the raw fuel gas treated
by the reforming reaction is required, for example, at the time of
starting the fuel cell system, it is desirable to heighten the ratio
of the exothermic reaction that has the higher reaction rate than
5 that of the endothermic reaction, in order to reform the required
amount of the raw fuel gas within a shorter time period. In case
that the excess amount of oxygen is supplied at one time to enhance
the ratio of the exothermic reaction, however, the peak temperature
rises too highandthe problems,suchas deteriorationofthecatalyst,
lo arise. Even when the supply of oxygen is increased temporarily to
enhance the ratio of the exothermic reaction, for example, at the
time of starting the system, the structure of the third embodiment
effectively prevents the internal temperature of the reformer 22c
from locally rising too high. This structure accordingly enables
15 the exothermic reforming reaction to be accelerated and generate the
required amount of gaseous fuel within a short time period. In the
general state, the supply of oxygen is 10 to 20% of the amount of
methanol applied for the reforming reaction, and the exothermic
reaction expressed as Equation (7) proceeds with 20 to 40% of the
20 total amount of methanol. In the reformer 22c, on the other hand,
the supply of oxygen is 12.5 to 30% of the amount of methanol, and
the exothermic reaction proceeds with 25 to 60% of the total amount
of methanol.
The reformer 22c with the heat pipes 84 incorporated therein for
25 transmitting the heat inside the reformer 22c accelerates the
endothermic reforming reaction as well as the exothermic reaction
havingthe higherreactionrate. While loweringthe peaktemperature,
58
21 ~8333
the reformer 22c expands the active area of the endothermic reaction
having temperatures equal to or higher than a predetermined level
as shown in Fig. 9. The acceleration of the endothermic reaction
defined byEquation (6) increasesthe processed amount bythe reformer
5 22c, thus improving the reforming efficiency per unit volume.
The dispersion of the heat by the heat transmission as shown in
the third embodiment not only decreases the peak temperature to
prevent deterioration of thecatalyst but interferes with generation
oftheundesiredby-products inthe process ofthe reforming reaction.
o As discussed previously, incase that thetemperature rises too high,
for example, 400~C or higher, even temporarily or partially in the
reformer 22c, methane and nitrogen oxides may be generated. A
temporary or partial decrease in temperature to or below a
predetermined level may also cause some by-products, such as formic
acid, methyl formate, and formaldehyde. These by-products are
decomposed with a subsequent increase in temperature to hydrogen and
carbon dioxide. The by-products generated at high temperatures are,
on the contrary, not decomposed once being generated. The reformer
22c of this embodiment with the heat pipes 84 for dispersing the heat
and averaging the internal temperature of the reformer 22c
effectively prevents such by-products from being generated with an
extreme increase in reaction temperature.
In case that the heat dispersiontransmits the heat to the outlet
of the reformer 22c, an increase in température at the outlet
undesirably raises the concentration of carbon monoxide included in
the reformed fuel gas. The shift reaction expressed as Equation (5)
21 98333
does not sufficiently proceed and the concentration of carbon
monoxide included in the reformed fuel gas is heightened, unless the
temperature at the outlet of the reformer 22c decreases to or below
a predetermined level. In the reformer 22c of the third embodiment,
5 the heat pipes 84 do not extend to the outlet of the reformer 22c.
This structure prevents the heat from beingtransmitted to the outlet
of reformer 22c, and the endotherric reforming reaction thus
vigorously proceeds to lower the temperature in the vicinity of the
outlet of the reformer 22c. The sufficiently low temperature at the
lo outlet of the reformer 22c accelerates the shift reaction expressed
as Equation (5) and reduces the concentration of carbon monoxide
included in the gaseous fuel.
Fig. 10 shows structure of still another reformer 22d which is
given as a modification of the reformer 22c of the third embodiment.
Whereas the reformer 22c has the heat pipes 84 disposed therein for
heat transmission, the cont~;ner of the reformer 22d functions as
means of heat transmission in this modified structure. A plurality
of reforming pipes 85 are arranged inside a stainless-steel heat
conductive unit 86. Each reforming pipe 85 is filled with pellets
20 of the Cu-Zncatalyst. The first air supplyconduit 75 and thesecond
fuel supply conduit 79 meet each other to add oxygen to the raw fuel
gas. The reforming pipes 85 receive a supply of the oxygen-
cont~;n;ng raw fuelgas and reformthe raw fuel gas to ahydrogen-rich
gaseous fuel. The heat generated bythe oxygen-consuming, reforming
25 reactionofEquation(7)proceeding inthe reformer22d istransmitted
to lower-temperature areas inthe reformer22dviathe heat conductive
unit 86, which is the cont~;ner of the reformer 22d.
21 98333
In a similar manner as în the reformer 22c, the heat generated
by the exothermic reaction is transmitted to the surrounding
lower-temperature areas in the reformer 22d. This leads to a
decrease in peak temperature and thereby prevents drawbacks, such
5 as deterioration of the catalyst. ThiS structure is especially
advantageous when a larger amount of oxygen is supplied to heighten
the ratio of theexothermic reforming reactionwith a viewto abruptly
increasing the amount of gaseous fuel generated throughthe reforming
reaction, for example, at the time of starting the fuel cell system.
o Like the structure of the reformer 22c, this modified structure
enables the heat generatedby the exothermic reaction to be dispersed
inside the reformer 22d, thus expanding the active area of the
endothermic reaction and preventing the non-required by-products
from being generated by an extreme increase or decreased in
temperature in the process of the reforming reaction.
As shown in Fig. 10, the heat conductive unit 86 is not arranged
in the vicinity of the outlet of the reformer 22d connecting with
the third fuel supply conduit 80. Like the structure of the reformer
22c, this structure prevents the heat from being transmitted to the
outlet of the reformer 22d, and the endothermic reforming reaction
thus vigorously proceeds to lower the temperature in the vicinity
of the outlet of the reformer 22d. The sufficiently low temperature
at the outlet of the reformer 22c accelerates the shift reaction
expressed as Equation (5) and reduces the concentration of carbon
2s monoxide included in the gaseous fuel.
In both the reformers 22c and 22d, the heat is not transmitted
to the outlet connecting with the third fuel supply conduit 80, in
2 1 98333
order to reduce the concentration of carbon monoxide included in the
resulting gaseous fuel. As discussed previously, the gaseous fuel
is transported viathethirdfuelsupply conduit 80totheCOreduction
unit 26, which further reduces the concentration of carbon monoxide.
As long as the CO reduction unit 26 has the capacity of sufficiently
lowering the concentration of carbon monoxide, the reformers 22c and
22d may have the structure of transmitting the heat generated by the
exothermic reaction to the outlet and not specifically accelerating
the shift reaction of Equation (5).
o As described above, the peak of the temperature increase by the
exothermic reaction is lowered to prevent deterioration of the
catalyst by regulating the supply of oxygen in the reformer 22 of
the first embodiment, by dividing the supply of the air required for
the exothermic reaction in the reformers 22a and 22b of the second
15 embodiment, and by transmitting the heat in the reformers 22c and
22d of the third embodiment. In still another reformer 22e given
below as a fourth embodiment according to the present invention, a
catalyst with excellent heat resistance is used to accelerate the
exo~herric reaction and thereby prevent deterioration of the
catalyst. Fig. 11 shows structure of the reformer 22e of the fourth
embodiment.
The reformer 22e includes a first catalyst layer 87 filled with
pellets of a palladium catalyst having excellent heat resistance and
a second catalyst layer 88 filled with pellets of the Cu-Zn catalyst
discussed above. The first air supply conduit 75 joins the second
fuel supply conduit 79 to add oxygen to the raw fuel gas. The
oxygen-cont~ining rawfuel gas first flows through the firstcatalyst
62
21 98333
layer 87 for the reforming reaction. The palladium catalyst held
on the carrier packed in the first catalyst layer 87 does not
deteriorate at high temperatures as 500~C and has better heat
resistance than the Cu-Zn catalyst. With a supply of the raw fuel
5 gas cont~;n;ng methanol and oxygen, the palladium catalyst
accelerates the oxidizing reforming reaction of methanol expressed
as Equation (7) and the decomposition of methanol expressed as
Equation (4). The palladium pellets packed into the first catalyst
layer 87 are prepared by impregnating zinc oxide with palladium
o nitrate in this embodiment, although any other suitable method may
be adopted.
Compared with the Cu-Zn catalyst, the palladium catalyst more
vigorously accelerates the exothermic reaction of Equation (7) and
thereby leads to generation of a greater amount of heat. While the
15 endothermic reaction expressed as Equation (4) is carried out
simultaneously, the internal temperature of the reformer 22e
continues increasing since the heat consumed by the endo~herr;c
reaction of Equation (4) is less than the heat generated by the
exothermic reaction of Equation (7). The increase in internal
temperature of the reformer 22e further accelerates the endothermic
reaction of Equation (4). Decomposition of methanol included in the
raw fuel gas accordingly proceeds in the reformer 22e with the
increase in temperature.
After the decomposition of methanol through the reactions of
25 Equations (4) and (7) in the first catalyst layer 87, the raw fuel
gas is moved into the secondcatalyst layer 88, in which the reactions
21 98333
expressed as Equations (5) and (6) proceed. The heat generated by
the exothermic reaction of Bquation (7) proceeding in the first
catalyst layer87 isutilizedfortheendothermic reactionofEquation
(6). In the second catalyst layer88, thetemperature thus gradually
5 decreases toward the outlet of the reformer 22e. When the internal
temperature of the second catalyst layer 88 decreases to a
predetermined or lower level, the exothermic shift reaction of
Equation (5) is accelerated to reduce the concentration of carbon
monoxide including in the gaseous fuel generated by the reforming
lo reaction.
The reformer 22e of the fourth embodiment has the first catalyst
layer 87 that is composed of thecarrier with the high-heat-resistant
palladium catalyst held thereon. Even if the exothermic reaction
of Equation (7) abruptly increases the temperature in the first
15 catalyst layer 87, the palladium catalyst exposed to the high
temperatures does not deteriorate significantly. ThiS enables a
larger amount of oxygen to be supplied to the first catalyst layer
87 and accelerate the exothermic reaction of Equation (7). The
acceleration of the exo~herr;c reaction of Equation (7) generates
the greater amount of heat and thereby accelerates the endothermic
reaction of Equation (4) occurring simultaneously. This improves
the reforming efficiency per unit volume in the reformer 22e. The
improvement in reformingefficiency realizes theefficient reforming
reaction in a smaller volume, thus enabling reduction in size of the
25 reformer 22e. When the processed amount by the reforming reaction
is to be abruptly increased, for example, at the time of starting
the system with the reformer 22e incorporated therein, the structure
64
2 1 98333
of the fourth embodiment allows for the increased supply of oxygen
in order to increase the amount of gaseous fuel thus generated.
The second catalyst layer 88 is filled with the Cu-Zn catalyst
having the poorerheat resistancethanthe palladiumcatalyst. Since
5 the endothermic reaction occurs primarily in the second catalyst
layer 88, the temperature decreases toward the outlet of the reformer
22e. The second catalyst layer 88 is accordingly free from
deterioration of the catalyst due to an extreme increase in
temperature. The Cu-Zn catalyst has the activity of accelerating
the shift reaction expressed as Equation (5). In the vicinity of
the outlet ofthecatalyst22e withthe loweredtemperature, oxidation
of carbon monoxide proceeds to reduce the concentration of carbon
monoxide included in the resulting gaseous fuel.
Although the first catalyst layer 87 in the reformer 22e of the
lS fourth embodiment is filled with the palladium catalyst, any other
catalyst that has a certain heat resistance and can accelerate the
oxidation reaction of methanol defined by Equation (7) may be
applicable for the first catalyst layer 87. The available examples
other than the palladium catalyst include metals, such as platinum,
20 nickel, rhodium,chromium,tungsten, rhenium, gold,silver, and iron,
and alloys of such metals and other metals.
As discussed previously, in the fuel cell systems of the first
through the fourth embodiments, each reformer adopts a specific
structure to prevent the heat generated by the oxidation reaction
25 of methanol included in the raw fuel gas from raising the temperature
of the catalyst in the reformer too high and thereby causing problems
like sintering of the reforming catalyst. One of the adopted
2 1 98333
structures monitors the temperature of the catalyst and decreases
the supply of oxygen with an extreme increase in temperature to
interfere with the exothermic reaction. Another adopted structure
has a plurality of oxygen-supply positions or a mechanism for heat
5 transfer to disperse the heat generated by the oxidation reaction
in the reformer. Still another adopted structure utilizes the
high-heat-resistant oxidation catalyst to prevent its deterioration
with an increase in temperature. An extreme increase in temperature
of the catalyst causes problems because the oxidation reaction of
lo methanol expressed as Equation (7) proceeds much faster than the
reforming reaction of methanol defined by Equations (4) and (6) in
the reformer. The exothermic reaction generally has the higher
reaction rate than that of the endothermic reaction. When the
endothermic reforming reaction of methanol and the exothermic
oxidation reaction of methanol are carried out simultaneously, the
higher rate of the oxidation reaction of methanol leads to a gradual
increase in temperature of the catalyst. Another effective method
to prevent deterioration of the catalyst due to an extreme increase
in temperature is to suppress the exothermic oxidation reaction
relative to the endothermic reforming reaction. This structure is
given below as a fifth embodiment accordingto the present invention.
Fig. 12 shows structure of another reformer 22f as the fifth
embodiment. The reformer 22f of the fifth embodiment includes two
reformer units 89 and 90 that are both filled with the pellets of
the reforming catalyst, that is, the Cu-Zn catalyst. The catalytic
pellets packed in the two reformer units 89 and 90 have different
particle sizes. The first reformer unit 89 arranged at the inlet
2 1 98333
of the raw fuel gas and the oxidizing gas is filled with the pellets
of the reforming catalyst having the size of approximately 3 mm x 3
mm x 6 mm (hereinafter referred to as large pellets). The second
reformer unit 90 arranged at the outlet of the gaseous fuel generated
5 through the reforming reaction is filled with the pellets of the
reforming catalyst having the size of approximately 3 mm x 3 mm x 3
mm (hereinafter referredtoassmall pellets). Thecatalytic pellets
packed in the respective reformers of the first through the fourth
embodiments discussed above correspond to the small pellets of the
lo fifth embodiment. In the fifth embodiment, the raw fuel gas fed
through the second fuel supply conduit 79 is mixed in advance with
the compressed air fed through the first air supply conduit 75, and
the oxygen-cont~;n;ng raw fuel gas supplied into the reformer 22f
first passes through the surface of the large pellets packed in the
15 first reformer unit 89 and then through the surface of the small
pellets packed in the second reformer unit 90.
The following describes the relationship between the rates of
the reforming reaction and the oxidation reaction of the raw fuel
gas and the size of the pellets of the reforming catalyst, that is,
20 the total surface area of the catalyst. Fig. 13 is a graph showing
variations in temperature of the catalyst plotted against the
direction of the flow of a modeled raw fuel gas (methanol 0.2 mol/min
and water 0.4 mol/min), whichpasses througheitherone ofthe vessels
that havethe sameshape as the reformer 22fandare filledwitheither
25 the large pellets or the small pellets. The air is introduced as
the oxidizing gas into the raw fuel gas from two different positions.
21 98333
The first position is just before the inlet of the raw fuel gas into
the reforming catalyst, and the second position is approximately one
third the lengthofthe reformer fromthe inletthereof (seethebottom
drawing of Fig. 13). The air is introduced from these two positions
into the raw fuel gas respectively at the flow of 1.75 l/min. As
shown in Fig. 13, when the reformer is filled with the small pellets
of the reforming catalyst, which are used in the first through the
fourth embodiments, the temperature of the catalyst has a peak in
the vicinity of the inlet of the reformer. When the reformer is
lo filled with the large pellets of the reforming catalyst to have the
smaller total surface area of the catalyst per unit volume than the
reformer filled with the small pellets, on the other hand, no such
a peak is observed in the varied temperature of the catalyst.
In the reformer filled with the small pellets to have the larger
total surface area of the catalyst per unit volume, the temperature
variation curve of the catalyst has a peak in the vicinity of the
inlet of the reformer. ThiS may be ascribed to the higher rate of
the oxidation reaction than that of the reforming reaction, which
leads to an increase in temperature of the catalyst. In the reformer
20 filled with the large pellets to have the smaller total surface area
of the catalyst per unit volume, on the other hand, the temperature
variation curve of the catalyst does not have any significant peak.
When the reformer is filled with the large pellets, it is accordingly
assumed thatthe rate ofthe oxidation reactiondoes notsignificantly
25 exceed the rate of the reforming reaction. This substantially
balances the heat generated by the oxidation reaction with the heat
consumed by the reforming reaction, which results in a gentle
21 98333
temperature variation and a practically uniform temperature
distribution in the reformer. According tothese experimentaldata,
when the reformer is filled the large pellets of the reforming
catalyst, that is, when the catalyst has the small total surface area
5 per unit volume that can be in contact with the raw fuel gas, the
rate of the oxidation reaction is slowed relative to the reforming
reaction of methanol. The relative decrease in rate of theoxidation
reaction with a decrease in total surface area of the catalyst may
be ascribed to the following.
lo For oxidation of methanol, methanol is required to come into
contact with oxygen on the catalyst. The smaller total surface area
of the catalyst decreases the possibility of making these three
essential components existsimultaneously, thereby loweringthe rate
oftheoxidationreaction. The reformingreaction, onthe other hand,
requires a supply of heat energy simultaneously with bringing
methanol in contact with the catalyst. The supply of heat energy
is the rate-deterrin;ng step as discussed below, and the decrease
in total surface area of the catalyst to some extent does not
significantly lower the rate of the reforming reaction of methanol.
While the total amount of heat required for the reforming reaction
occurring in the reformer can be supplied by the heat transmitted
from the evaporator 24 and the heat generated by the oxidation
reaction, the transmission rate of heat energy required for the
reforming reaction is slower than the rate of the reforming reaction
on the molecular level. The condition of transmitting heat energy
accordingly determines the rate of the reforming reaction. In this
state, the decrease in total surface area of the catalyst does not
69
2~ 98333
significantly affect the rate of the endothermic reformingreaction.
The decrease in total surface area of the catalyst slows the relative
rate of the exothermic oxidation reaction and substantially balances
the exothermic reaction with the endothermic reaction, thus
5 preventing an extreme increase in temperature of the catalyst.
The following gives another possible reason why the decrease in
total surface area of the catalyst slows the relative rate of the
oxidation reaction to the reforming reaction. The exothermic
oxidation reaction proceeds only on the surface of the catalyst,
lo whereas the reforming reaction is accelerated not only by thesurface
of the catalyst but by the catalytic metal included in the pellets.
Based on this idea, the decrease in total surface area of thecatalyst
depresses the rate of the oxidation reaction proceeding only on the
surface of the catalyst, but does not significantly affect the rate
of the reformingreaction as long as the total amount of the available
catalytic metal is substantially unchanged.
By taking into account these experimental results, in the
reformer 22f of the fifth embodiment, the first reformer unit 89
filled with the large pellets is arranged in the upstream portion
of the flow path of the raw fuel gas and the second reformer unit
90 filled with the small pellets in the downstream portion of the
flow path. The smaller total surface area of the catalyst existing
in the upstream portion of the reformer 22f slows the rate of the
oxidation reaction of the raw fuel gas in the upstream portion and
thereby prevents thetemperatureofthe catalyst fromrisingtoohigh.
The larger total surface area of the catalyst existing in the
downstream portion of the reformer 22f, on the other hand, secures
2 1 98333
the activity of the reforming reaction in the downstream portion.
In the reformer 22f of the fifth embodiment, the ratio of the area
filled with the large pellets to that filled with the small pellets
is approximately one to two. Fig. 14 is a graph showing comparison
5 between variations in temperature of the catalyst against the flow
of the gas when the reforming reaction proceeds in the reformer 22f
of the fifth embodiment and in a reference reformer filled with only
the small pellets. As shown in Fig. 14, compared with the reforming
reaction occurring in the reference reformer filled with only the
o small pellets, the reforming reaction occurring in the reformer 22f
of the fifth embodiment results in a gentler temperature increase
in the vicinity of the inlet of the reformerand a gentlertemperature
decrease toward the outlet.
As discussed above, the reformer 22f of the fifth embodiment has
15 the upstream portion filled with the large pellets of the catalyst
and the downstream portion filled with the small pellets. This
structure changes the surface area of the catalyst per unit volume
in the reformer 22fanddepresses therate of theexothermic oxidation
reaction proceeding in the upstream portion relative to the rate of
the endothermic reforming reaction. This effectively prevents the
temperature of the catalyst from rising too high due to the heat
generated by the oxidationreaction that hasthe higher reaction rate
than that of the endothermic reaction. Since the structure of the
embodiment prevents an extreme increase in temperature of the
25 catalyst, the catalytic metal (Cu-Zn catalyst in this embodiment)
is not exposed to the undesired high temperatures and is thereby free
fromthe problems,such asdeterioration ofthe catalyst andsintering
21 98333
This structure also prevents the reactions other than the normal
reforming reaction from proceeding due to an extreme increase in
temperature ofthecatalyst andgeneratingtheundesiredby-products.
The downstream portion of the reformer 22f is filled with the small
5 pellets as described previously. This secures the surface area of
the reforming catalyst involved in the reforming reactionproceeding
in the downstream portion of the reformer 22f, thus preventing the
rate ofthe reformingreaction fromunnecessarily beinglowered. The
depression of the rate of the oxidation reaction proceeding in the
lo upstream portion of the reformer 22f e~p~n~ the active area of the
oxidation reaction to the downstream portion, and thereby spreads
the area, which is heated to the temperatures that sufficiently
activate the endothermic reforming reaction, to the further
downstream portion. This leads to an improvement in efficiency of
lS the reforming reaction per unit volume in the reformer 22f.
The reformer 22f of the fifth embodiment has the catalytic
pellets of the different particle sizes in the upstream portion and
the downstream portion, in order to prevent an extreme increase in
temperature of the catalyst. The structure of the fifth embodiment
does not require a plurality of different catalysts like the fourth
embodiment, but the reformer 22f is filled with the single catalytic
metal having the activities of accelerating both the reforming
reaction and the oxidation reaction. Even when the whole amount of
the compressed air corresponding to the required amount of oxygen
is added in advance to the raw fuel gas at the inlet of the reformer
22f, thetemperature distribution inthe reformer22fcanbeuniformed
sufficiently. There is no need to introduce the oxidizing gas from
72
2 1 ~8333
a plurality of different positions. This prevents the piping system
from being complicated and thereby simplifies the structure of the
whole reformer 22f.
As discussed above, in the reformer 22f of the fifth embodiment,
5 the upstream portion and the downstream portion thereof are filled
with the pellets ofthe catalytic metalhaving the different particle
sizes. Any other structure may, however, be applied to the reformer
22f as long as the total surface area of the catalyst in the upstream
portion is different from the same in the downstream portion. By
lo way of example, the reformer 22f may have a honeycomb structure,
instead of being filled with the catalytic pellets. In this case,
the respective cells constituting the honeycomb structure have
different cross sectional areas in the upstream portion and the
downstream portionof the reformer;that is,thecells inthe upstream
portion have larger crosssectional areas andthose in the downstream
portion have smaller cross sectional areas.
In the reformer 22f of the fifth embodiment, the ratio of the
area filled with the large pellets to that filled with the small
pellets is approximately one to two. AS long as the same effects
can be exerted to lessen the surface area of the catalyst in the
upstream portion and thereby prevent an extreme increase in
temperature of the catalyst, the area filled with the large pellets
and that filledwiththesmall pellets may bedefined by anotherratio.
The reformer 22f of the fifth embodiment is filled with the pellets
having the different particle sizes specified above. The sizes of
the pellets are, however, not restricted to these values. In
accordance with another preferable structure, the surface area of
2 1 98333
the catalyst varies in three or more steps in the reformer.
The structure of the fifth embodiment discussed above changes
the surface area of the catalyst in the upstream portion and the
downstream portion of the reformer, so as to depress the rate of the
5 exothermic oxidation reaction relative to the rate of the reforming
reaction and hence prevent an extreme increase in temperature of the
catalyst. Still another structure given as a sixth embodiment
according to the present invention changes the flow rate of the raw
fuel gas passing through the upstream portion and the downstream
o portion in the reformer, so as to depress the rate of the exothermic
oxidation reaction relative to the rate of the reforming reaction
and hence prevent anextreme increase intemperature of thecatalyst.
Fig. 15 illustrates structure of another reformer 22g as the
sixth embodiment. The reformer 22g of the sixth embodiment includes
15 a reformer unit 91 filled with the pellets of the reforming catalyst,
that is, the Cu-Zn catalyst. A supply of the raw fuel gas is fed
via the second fuel supply conduit 79 into the reformer 22g, whereas
the oxidizing gas or the compressed air is supplied via the first
air supply conduit 75 that joins the second fuel supply conduit 79
20 before thejoint ofthesecond fuelsupplyconduit 79withthe reformer
22g. The reformer 22g of the sixth embodiment has a truncated
cone-like shape. The second fuel supply conduit 79 connects with
the bottom face of the reformer 22g and the third fuel supply conduit
80 with the top face thereof. Namely the reformer 22g isconstructed
25 to form the flow path having the decreasing sectional area in the
direction from the upstream portion to the downstream portion. The
oxygen-cont~in;ng raw fuel gas fed into the reformer 22g is
74
2~ 98333
pressurized to a predeter~ine~ level, and accordingly passesthrough
the upstream portion having the greater flow section at a slower rate
and through the downstream portion having the smaller flow section
at a higher rate. The reformer 22g of the sixth embodiment has the
5 specific shape to vary the flow rate of the raw fuel gas, so as to
depress the rate of the exothermic oxidation reaction relative to
the rate of the reforming reaction and hence prevent an extreme
increase in temperature of the catalyst. The relationship between
the flow rate ofthe gas andthe reactionrate will be discussedlater.
lo In this embodiment, the flow rate of the raw fuel gas is regulated
by the specific shape of the reformer 22g, and the reformer unit 91
can thus be filled with the catalytic pellets formed in a homogeneous
particle size. As one preferable example, the reformer 22g of the
sixth embodiment is filled with the small pellets specified in the
15 fifth embodiment.
[0148 ]
The slower flow rate of the raw fuel gas in the upstream portion
ofthe reformer22gdepressesthe progress oftheexo~herr;c oxidation
reaction. This is ascribed tothe following reaso~. Theslower flow
20 rate of the raw fuel gas decreases the chance of collision between
methanol molecules and water molecules and lowers the probability
of enabling both methanol and water to be supplied to the oxidation
reaction proceeding on the surface of the catalyst, thus suppressing
the progress of the oxidation reaction. The endothermic reaction
25 of Equation (4) among the reforming reaction decomposes methanol
molecules alone on the surface of the catalyst. A large excess of
methanol molecules exist even when the flow rate of the raw fuel gas
2~ 98333
is slowed to some extent. The supply of methanol molecules onto the
surface of the catalyst is hence sufficient for the ability of the
reforming catalyst that accelerates the reforming reaction. The
rate of the reforming reaction is thus hardly affected by the slower
flow rate of the raw fuel gas. The slower flow rate of the raw fuel
gas in the upstream portion of the reformer accordingly slows the
rate of the oxidation reaction relative to the rate of the reforming
reaction in the upstream portion of the reformer.
Fig. 16 is a graph showing comparison between variations in
o temperature of the catalyst against the flow of the gas when the
reforming reaction proceeds in the reformer 22g of the sixth
embodiment and in a reference reformer forming the flow path of a
uniform sectional area. In case of the reference reformer having
the uniform flow section, that is, in case that the flow rate of the
15 raw fuel gas is fixed in the reformer, the temperature curve of the
catalyst gradually increases from the inlet of the reformer to form
a peak. In case of the reformer 22g of this embodiment, on the other
hand, the temperature curve of the catalyst shows a gentle increase
in the upstream portion having the slower flow rate of the raw fuel
gas and has no significant peak that is observed in case of the fixed
flow rate. The temperature curve shows a gentle decrease toward the
outlet of the reformer after the gentle increase.
In the reformer 22g of the sixth embodiment discussed above, the
greater cross sectional area of the flow path in the upstream portion
slows the flow rate of the raw fuel gas and thereby slows the relative
rate of the oxidation reaction in the upstream portion. Like the
fifth embodiment, the structure of the sixth embodiment accordingly
76
21 98333
prevents the temperature of the catalyst from rising too high due
to the heat generated by the oxidation reaction that has the higher
reaction rate than that of the endothermic reaction. Since the
structure of the embodiment prevents an extreme increase in
temperature of the catalyst, the catalytic metal (Cu-Zn catalyst in
this embodiment) is not exposed to the undesired high temperatures
and is thereby free from the problems, such as deterioration of the
catalyst and sintering. This structure also prevents the reactions
other than the normal reforming reaction from proceeding due to an
lo extreme increase in temperature of the catalyst and generating the
undesired by-products. The depression of the rate of the oxidation
reaction proceeding in the upstream portion of the reformer 22g
expands the active area of the oxidation reaction to the downstream
portion, and thereby spreads the area, which is heated to the
temperatures that sufficiently activate the endothermic reforming
reaction, to the further downstream portion. This leads to an
improvement in efficiency of the reforming reaction per unit volume
in the reformer 22g.
The reformer 22g of the sixth embodiment has the varying flow
section in the upstream portion and the downstream portion, in order
to prevent an extreme increase in temperature of the catalyst. The
structure of the sixth embodiment does not require a plurality of
different catalysts like the fourth embodiment, but the reformer 22g
is filled with the single catalytic metal having the activities of
accelerating both the reformingreaction and the oxidation reaction.
The structure of the sixth embodiment further saves the labor of
preparing the pellets of the catalytic metal since they have the
21 98333
uniform particle diameter. Even when the whole amount of the
compressedaircorrespondingtothe requiredamount ofoxygen isadded
in advance to the raw fuel gas at the inlet of the reformer 22g, the
temperature distribution in the reformer 22g can be uniformed
5 sufficiently. There is no need to introduce the oxidizing gas from
a plurality of different positions. This prevents the piping system
from being complicated and thereby simplifies the structure of the
whole reformer 22g.
The reformer 22g of the sixthembodiment is formed in atruncated
o cone-like shape to gradually decrease the sectional area of the flow
path in the direction from the upstream portion to the downstream
portion and thereby slow the flow rate of the raw fuel gas in the
upstream portion. The reformer may have any other shape as long as
the flow rate oftheraw fuel gas canbeslowed intheupstreamportion.
15 Fig. 17 shows a reformer 22h having another possible structure for
slowing the flow rate of the raw fuel gas in the upstream portion.
The reformer 22h of Fig. 17 has a substantially columnar shape and
includes a raw fuel gas introduction conduit 92 formed on the
circumference thereof and a reformer unit 94 arranged inside the raw
fuel gas introduction conduit 92 and filled with pellets of the
reforming catalyst. The raw fuel gas introduction conduit 92 formed
on the outer face of the reformer 22h is open to the bottom face (in
the drawing of Fig. 17) of the reformer 22h having the substantially
columnar shape. The circular opening formed in the bottom face of
25 the reformer 22h connects with the second fuel supply conduit 79,
through which the raw fuel gas previously mixed with the compressed
air is supplied. In the reformer 22h, the boundary between the
78
2 1 98333
reformer unit 94 and the raw fuel gas introduction conduit 92 is made
of a metal mesh that can hold the catalytic pellets. The raw fuel
gas is accordingly introduced from the raw fuel gas introduction
conduit 92 formed on the circumference of the reformer 22h via the
5 metal mesh into the reformer unit 94.
The raw fuel gas introduced from the outer face of the reformer
unit 94 into the reformer 94 is subjected to the reforming reaction
while passing through thesurface of the reforming catalyst andbeing
flown to the central axis of the reformer 22h. The reformer 22h has
lo a gaseous fuel discharge conduit 93 formed along the central axis
thereof. The gaseous fuel generated by reforming the raw fuel gas
in the reformer22h is flown out to the gaseous fuel dischargeconduit
93. The gaseous fuel discharge conduit 93 is open to the top face
(in the drawing of Fig. 17) of the reformer 22h, which is opposite
15 to the opening of the raw fuel gas introduction conduit 92. The
opening of the gaseous fuel discharge conduit 93 connects with the
third fuel supply conduit 80, and the gaseous fuel generated by the
reforming reaction in the reformer 22h is flown into the third fuel
supply conduit 80 via the gaseous fuel discharge conduit 93.
The reformer 22h of Fig. 17 has the substantially columnar shape
and receives the raw fuel gas from the raw fuel gas introduction
conduit 92 formed on the outerface of the reformer 22h anddischarges
the resulting gaseous fuel to the gaseous fuel discharge conduit 93
formed along the central axis thereof. This varies the flow rate
25 of the gas in the reformer 22h; that is, the slower flow rate in the
upstream portion and the faster flow rate in the downstream portion.
It is assumed that the plane perpendicular to the flow direction of
79
2 1 98333
the gas represents the section of the reformer. The section of the
reformer 22h has the columnar shape having the varying area; that
is, the larger sectional area in the place closer to the raw fuel
gas introduction conduit 92 and the smaller sectional area in the
5 place closer to the gaseous fuel discharge conduit 93. Namely the
gas flowing in the reformer 22h has the higher flow rate when
approaching the gaseous fuel discharge conduit 93. The reformer 22h
of Fig.17 accordinglyexertsthesame effects as those ofthe reformer
22g of the sixth embodiment. Compared with the reformer 22g formed
lo in the truncated cone-like shape, the reformer 22h formed in the
substantially columnar shape has a smaller dead space in
installation.
The reformer may have a rectangular cross section as still
another possible structure to realize the slower flow rate in the
15 upstream portion than that in the downstream portion. Fig. 18
illustrates structure of another reformer 22i having the rectangular
cross section. The reformer 22i of Fig. 18 has a similar structure
to that of the reformer 22g of the sixth embodiment, except that the
reformer 22i is formed in a truncated quadrilateral pyramid-like
20 shape instead of the truncated cone-like shape. In the reformer 22i
formed in the truncatedquadrilateral pyramid-like shape, the bottom
face thereof having the larger area connects with the second fuel
supply conduit 79, and the top face thereof having the smaller area
with the third fuel supply conduit 80. The sectional area of the
25 flow path in the reformer 22i thus constructed gradually decreases
in the direction fromthe upstream portionto the downstreamportion,
so that the gas passing through the reformer 22i has the slower flow
2 1 98333
rate intheupstreamportion. The reformer 22iofFig. 18accordingly
exerts the same effects as those of the reformer 22g of the sixth
embodiment.
In accordance with another preferable application, the reformer
5 may include a plurality of reformer units that have the structure
of varying the flow rate of the gas in the upstream portion and the
downstream portion and are laid one upon another. Fig. 19
illustrates still another reformer22j as anexample of suchstacking
structure. The reformer 22j includes two reformer units 9S and 96
lo that are respectively formed in a truncated quadrilateral
pyramid-like shape like the reformer 22i and are laid one upon the
other to have the gas flows in the opposite directions. The reformer
units 95 and 96 respectively receive a supply of the raw fuel gas
from the bottom face thereof having the greater flow section and
15 discharge the resulting gaseous fuel from the top face thereofhaving
the smaller flow section.
The reformer22j ofFig. 19 hasthe followingeffects, in addition
to the same effects as those of the reformer 22g of the sixth
embodiment and the reformer 22i of Fig. 18. In the reformer 22j,
the two reformer units 95 and 96 are laid one upon the other to have
the gas flows in the opposite directions. This structure further
uniforms the temperature distribution in the reformer 22j. In the
respective reformer units 95 and 96, the gas has the slower flow rate
in the upstream portion. This depresses the rate of the oxidation
reaction of the raw fuel gas and prevents an abrupt increase in
temperature in the upstream portion of each reformer unit. The
oxidation reaction proceeds more vigorously and actively in the
81
2 1 ~8333
upstream portion where the raw fuel gas has the higher content of
oxygen than in the downstream portion where the raw fuel gas has the
lower content of oxygen. The temperature in the upstream portion
is accordingly a little higher than that in the downstream portion.
s In the reformer 22j, since the two reformer units 95 and 96 are laid
one upon the other to have the gas flows in the opposite directions,
the higher-temperature upstream portion and the lower-temperature
downstream portion adjoin eachother to exchange the heat and further
uniform thetemperature distribution inthe whole reformer22j. This
lo structure effectively prevents the temperature in the reformer from
rising too high due to the heat generated by the oxidation reaction.
The increase in temperature in the downstreamportion of the reformer
22j activates the reforming reaction in the downstream portion and
thereby improves the reaction efficiency of the whole reformer 22j.
The structure ofheaping the reformerunits 95 and96 of thetruncated
quadrilateral pyramid-like shape to form the whole reformer 22j in
a quadratic prism-like shape reduces the size of the whole reformer
22j and lessens the dead space in installation.
In the reformer of the sixth embodiment, from reformer 22g to
20 22j, the varying sectional area in the upstream portion and the
downstream portion depresses the rate of the oxidation reaction in
the upstream portion. Such effect can be attained with the pellets
of the uniform particle diameter packed into the reformer. In the
reformer of the sixth embodiment, from reformer 22g to 22j, however,
the upstream portion of the gas flow may be filled with the catalytic
pellets of the greater particle diameter and the downstream portion
with those of the smaller particle diameter. ThiS further depresses
2 1 98333
the rate of the oxidation reaction in the upstream portion and
uniforms the temperature distribution in the reformer.
The reformers of the first through the sixth embodiments
according to the present invention can be incorporated in the fuel
cell system 20 constructed as shown in Fig. 1. These reformers may,
however, be disposed in another fuel system having a different
configuration. Fig. 20 is a block diagram illustrating another fuel
cell system 20A having the structure different from that of Fig. 1.
The elements ofthe fuel cellsystem 20A that are identical withthose
lo of the fuel cell system 20 of the first embodiment are shown by like
numerals and are not described here.
In the fuel cell system 20A shown in Fig. 20, the oxidizing
exhaust gas discharged from the oxygen electrodes of the fuel-cells
stack 40 is flown via the oxidizing exhaust gas conduit 73 to a
condensate recovery unit 39, instead of to the compressor 32 as in
the first embodiment. The electrochemical reaction of Equation (2)
occurring onthe side ofthe oxygenelectrodes of the fuel-cellsstack
40 generates water. In the fuel cell system 20A, the oxidizing
exhaust gas cont~in;ng water generated by the cell reaction is led
20 into the condensate recovery unit 39, in which water in the oxidizing
exhaust gas is condensed and recovered for recycle. Water recovered
in the condensate recovery unit 39 is supplied to the water tank 30
via a water recovery conduit 35 and then sent via the evaporator 24
to the reformer 22 to undergo the steam reforming of the raw fuel
25 in the reformer 22.
After the recovery of water in the condensate recovery unit 39,
the oxidizing exhaust gas is supplied through an exhaust gas recovery
83
2t~8333
conduit 33 into the burner 34 mounted on the compressor 32. As
discussed previously, oxygen remains in the oxidizing exhaust gas
discharged after the electrochemical reaction in the fuel-cells
stack 40. The oxidizing exhaust gas supplied to the burner 34
5 accordingly functions as the oxidizing gas required for the
combustion reaction in the burner 34. In the fuel cell system 20A,
the o~;~;7ing exhaust gas from the fuel-cells stack 40 is led into
the condensate recovery unit 39, whereas only the fresh air is
supplied to the compressor element 32b of the compressor 32, which
lo feeds the compressed air to the air tank 36.
As discussed above, in the fuel cell system having any one of
the reformers of the first through the sixth embodiments, the amount
of heat required for the reforming reaction is generated inside the
reformer. ThiS reduces the size of the whole fuel cell system
including the reformer and simplifies the structure of the fuel cell
system. The structure of the fuel cell system according to the
present invention is especially advantageous when the allowable
space is strictly limited, for example, when the fuel cell system
is mounted as a power source for driving the electric vehicle.
The reformers of the first through the sixth embodiments
discussed above reform the methanol-cont~;n;ng raw fuel gas to
generate a hydrogen-rich gaseous fuel. Among the available
hydrocarbons as the raw fuel, methanol can be subjected to the steam
reforming reaction under a relatively mild condition. Thisproperty
25 prevents the reformer from being undesirably bulky and is especially
advantageous when the fuel cell system is used as a power source for
driving the vehicle. The principle of the present invention is,
84
21 98333
however, applicable to the reformer for reforming another
hydrocarbon-cont~;ning fuel. The followingdescribes the reactions
carried out to steam reform other hydrocarbons.
As one example, the natural gas may be used as the raw fuel.
5 Methane, which is the primary constituent of the natural gas, is
subjected to the following reforming reaction. Equation (8)
represents the decomposition of methane occurring in the process of
steam reforming methane, and Equations (9) and (10) respectively
represent the oxidationreaction ofcarbon monoxide andthe oxidation
lo reaction of hydrogen carried out by addition of oxygen in the
reforming reaction:
CH4 + H20 -~ CO + 3H2 - 206.2 (kJ/mol) (8)
CO + (l/2)02 -~ C02 + 279.5 (kJ/mol) (9)
H2 + (l/2)~2 -~ H20 + 240 (kJ/ ~ 1) (10)
In the process of the steam reforming reaction of methane,
methane is first decomposed by the endothermic reaction expressed
as Equation (8). At the moment, methane reacts with water (that is,
steam) to generate carbon monoxide and hydrogen. Carbon monoxide
thus generated reacts with water according to the shift reaction
expressed as Equation (5) given above to generate carbon dioxide and
hydrogen. A supply of oxygen enables the reaction of Equation (9)
to proceed and change carbon monoxide to carbon dioxide. Part of
hydrogen generated by the decomposition of methane defined by
Equation (8) is subjected to the oxidation reaction of Equation (10)
to generate water. Water generated by the oxidation reaction
expressed asEquation(10) is consumedbythe decompositionof methane
2 ~ 98333
defined by Equation (8) or the shift reaction of Equation (5) for
oxidizing carbon monoxide. Methane subjected to these reactions is
finally reformed to a carbon dioxide-cont~;n;ng hydrogen rich gas.
These reactions are accelerated by a catalyst, such as nickel.
In case of the reforming reaction of the methanol-cont~;n;ng raw
fuel gas in the presence of oxygen, the amount of heat required for
the endothermic reaction can be supplied by the exothermic reaction
proceeding first. In case of the reforming reaction of the
methane-cont~;ning fuel gas, on the other hand, the endothermic
decomposing reaction of methane proceeds first and the required heat
can thus not be supplied by the exo~herr;c reforming reaction. In
this case, however, the structure of sufficiently heating the
methane-cont~;n;ng raw fuel inthe evaporator priorto being fed into
the reformer is favorably applied to initiate the decomposing
15 reaction of methane expressed as Equation (8) in the reformer with
the heat supplied by the methane itself. Once the decomposition of
methane defined by Equation (8) is initiated, the exothermic shift
reaction of Equation (5) and the exothermic oxidation reactions of
Equations (9) and (10) immediately occur tosupply the requiredheat.
20 The decomposing reactionof methaneshown by Equation(8) accordingly
continues proceeding with the newly supplied heat. The raw fuel can
thus be reformed in the reformer while the heat generated by the
exothermic reactions balances with the heat consumed by the
endothermic reactions.
In the process ofsteamreformingthe methane-cont~;n;ng rawfuel
gas to generate a hydrogen-rich gaseous fuel, a supply of oxygen fed
to the reformeractivates the exothermic oxidation reactiontosupply
86
2t 98333
the heat required for the endothermic decomposing reaction. Like
the reforming reaction of methanol described in the first through
the sixth embodiments, the amount of heat externally supplied for
theendothermic reformingreactioncanthus bereducedsignificantly.
5 Especially when the amount of oxygen supplied to the reformer and
the position of oxygen supply are controlled according to the state
of the temperature distribution in the reformer and the rates of the
shift reactionofEquation (5) andthe exothermic oxidationreactions
of Equations (9) and (10) are regulated, the required amount of heat
o can be supplied sufficiently. In this case, an external heat source
for heating the reformer is not required. Inaccordance withanother
possible structure, a heat source may be placed in the vicinity of
the inlet of the reformer to accelerate the decomposing reaction of
methane defined by Equation (8). In case that the town gas is used
as the natural gas, it is preferable to arrange a desulfurizer prior
to the reformer, in order to remove the organic sulfur oxides added
as an odorant.
The principle of the present invention is also applicable to the
raw fuels other than the natural gas, for example, LPG or liquefied
petroleum gas (propane as the rawfuel component), gasoline (n-octane
or isooctane as the raw fuel component), and gas oil (n-hexadecane
or cetane as the raw fuel component). When these raw fuels are
subjected to the reforming reaction, the heat required for the
endothermic reforming reaction can be supplied by the exothermic
oxidation reaction occurring in the reformer. Equations (11),(12),
and (13) given below respectively represent the decomposing
reactions of propane, octane, and cetane:
87
2 1 98333
C3H8 + 3H2O -~ 3CO + 7H2 - 498.0 (kJ/mol) (11)
C~Hl8 + 8H2~ -~ 8CO + 17H2 - 1260 (kJ/mol) (12)
Cl6H34 + 16H2~ -~ 16CO + 33H2 - 2475 (kJ/mol) (13)
Like the process of steam reforming methane, when any one of the
5 above raw fuels is subjected to the reforming reaction, a supply of
oxygen to the reformer enables the shift reaction of carbon monoxide
expressed as Equation (5) and the oxidation reactions of Equations
(9) and (10) to proceed after the decomposition of the raw fuel.
Because of the exothermic oxidation reactions proceeding inside the
lo reformer, the amount of heat externally supplied for the endothermic
reforming reaction in the reformer can be reduced significantly.
once the raw fuel gas supplied to the reformer is heated in advance
to a predetermined or higher temperature or a heat source is disposed
in the vicinity of the inlet of the reformer to supply the amount
of heat required for the endothermic reaction, the subsequent
reforming reaction can proceed without any external supply of heat.
In the above embodiments, the gaseous fuel obtained by the
reforming reaction isutilized inthePolymer Electrolyte FuelCells.
The method of and the apparatus for reforming the fuel according to
the present invention are also applicable to the fuel cell system
including other types of fuel cells, such as Phosphoric Acid Fuel
Cells, which can receive a supply of the carbon dioxide-contAin;ng
gaseous fuel. Especially when the fuel cells are used as a portable
power source, the principle of the present invention is
advantageously appliedtosimplifythestructure ofthesystem. When,
for example, methanol is supplied as the raw fuel to the low-
88
2 1 ~8333
temperature fuel cells which can not receive carbon monoxide as the
fuel, the principle of the present invention is favorably applied
toreduce theconcentrationofcarbon monoxide included inthe gaseous
fuel.
The present invention is not restrictedtothe aboveembodiments,
but there may be many modifications,changes, andalterations without
departing from the scope or spirit of the main characteristics of
the present invention.
It should be clearly understood that the above embodiments are
lo only illustrative and not restrictive in any sense. The scope and
spirit of the present invention are limited only by the terms of the
appended claims.
89