Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ WO96/10441 - 2 2 ~ 1 3 7 ~ PCT~S9~/12634
.1O~O~ETTC DRUG DELIVERY SY~ INCL~DING
DISPOSABLE PATCH AND REUSABLE, REMOVABLE CONTROL~ER
.
s
FIELD OF TRE lNVh'L. ~ 1ON
The present invention generally relates to
iontophoretic systems for delivering drugs or medicines
to patients transdermally, i.e., through the skin, and
more specifically relates to an iontophoretic drug
delivery system including a disposable drug-filled patch,
and a removable, reusable controller.
BACRGROUND OF THE lNV~. llON
Transdermal drug delivery systems have, in recent
years, become an increasingly important means of
administering drugs. Such systems offer advantages
clearly not achievable by other modes of administration
such as avoiding introduction of the drug through the
gastro-intestinal tract or punctures in the skin to name
a few.
Presently, there are two types of transdermal drug
delivery systems, i.e., "Passive" and "Active." Passive
systems deliver drug through the skin of the user
unaided, an example of which would involve the
application of a topical anesthetic to provide localized
relief, as disclosed in U.S. Patent No. 3,814,095
(Lubens). Active systems on the other hand deliver drug
through the skin of the user using, for example,
iontophoresis, which according to Ste~m~n's Medical
35 Dictionary, is defined as "the introduction into the
tissues, by means of an electric current, of the ions of
a chosen medicament."
W O 96/10441 ~ 2 2 0 ~ ~ 7 ~ PCTrU~95/12634 ~
Conventional iontophoretic devices, such as those
described in U.S. Patent Nos. 4~,820,263 (Spevak et: al.),
4,927,408 (Haak et al.) and 5,084,008 (Phipps), the
disclosures of which are hereby incorporated by
reference, for delivering a drug or medicine
transdermally through iontophoresis, basically consist of
two electrodes, i.e., an anode and a cathode. Usually,
electric current is driven from an external supply into
the skin at the anode, and back out at the cathode.
Accordingly, there has been considerable interest in
iontophoresis to perform delivery of drugs for a variety
of purposes.
However, several disadvantages and limitations have
been associated with the disposability and reuse of such
devices, including cost restraints. For example, as a
result of the energy needs, conventional silver oxide and
alkaline batteries of the size and cost rec~uired clo not
provide sufficient energy for multiple uses of the
system, and lithium batteries, while able to provi.de
sufficient energy, are presently cost prohibitive for use
in a single use and/or disposable device.
In addition, several disadvantages and limitations
have been associated with the flexibility of the clevice,
especially in situations where the device is worn for
extended periods of time. For example, as a result
prolonged use of the such devices, e.g., over a 24 hour
period, they were prone to peeling and uneven
distribution of current over the applied area. In
addition, the bulkiness of such devices made them
cumbersome and noticeable.
Thus, there has been a need for an iontophoretic
drug delivery system that would eliminate the problems
and limitations associated with the prior devices
discussed above, most significant of the problems being
~WO96110441 -- 2 2 0 ~ ~ 7 ~ PCTtUS95112634
flexibility disposability and ~eusability at acceptable
energy levels. In addition, there has been a need for a
device, that could utilize low cost conventional
batteries while achieving sufficient energy levels, and
that includes a reusable controller, to provide a compact
and inconspicuously worn device.
BllN~$~RY OF THE lNv~ ON
In contrast to the prior devices discussed above, it
has been found that a iontophoretic drug delivery system
particularly suited for single use and/or disposable use,
while providing sufficient energy, and which is
particularly suited for application to the body, while
providing direct contact even during prolonged use, can
be constructed in accordance with the present invention.
In addition, the controller for the system of the present
invention can be reused to further reduce costs.
The iontophoretic drug delivery system of the
present invention for delivering at least one active
agent to an applied area of a patient, such as the skin,
mucous membrane and the like, includes housing means for
containing first power means for supplying a high rate of
current and sufficient energy to drive the active agent
into the patient, electrode assembly means including at
least two electrodes for driving the at least one active
agent into the applied area of the patient along
electrical field lines generated by the electrical
current, a first reservoir situated in electrical
communication with a first one of the electrodes and the
first reservoir containing the at least one active agent
- to be delivered to the applied area of the patient, and a
second reservoir situated in electrical communication
35 with a second one of the electrodes and the second
reservoir, and controller means being removably
engageable with the housing means for controlling and
WO96/10441 ~ 2 0 ~ ~ 7 8 PCT~Sg5112634 ~
monitoring the electrical energy delivered during
operation so that the at least one active agent is
delivered to the applied area of the patient proximate
the first reservoir, whereby upon delivery of the active
agent, the controller means and the housing means may be
disengaged for reuse of the controller and for disposal
of the housing means.
In the preferred embodiment of the iontophore1:ic
drug delivery system, the controller means include~; a
second power means having a high current density for
powering the controller means. Also, the at least one
active agent includes a local anesthetic and a
vasoconstrictor, with the local anesthetic being
Lidocaine and the vasoconstrictor being Epinephrine. In
addition, the controller means further includes timing
means for controlling the amount of electrical current
delivered and the first power means is selected from the
group including silver oxide batteries, alkaline
batteries, zinc air batteries and the like, and the
second power means is selected from the group including
lithium batteries and the like.
In an alternative embodiment, the iontophoretic drug
delivery system for delivering at least one active agent
to an applied area of a patient, such as the skin, mucous
membrane and the like, includes a single use patch
including first power means for supplying a high rate of
current and sufficient energy to drive the medicament
into the patient, electrode assembly means situated
within the disposable patch including at least two
electrodes for driving the at least one active agent into
the applied area of the patient along electrical field
lines generated by the electrical current, a first
reservoir situated in electrical communication with a
first one of the electrodes and the first reservoir
containing the at least one active agent to be del:Lvered
WO96/10441 - 2 ~ 7 ~ PCT~S95/12634
to the applied area of the patient, a second reservoir
situated in electrical communication with a second one of
the electrodes and the second reservoir, and reusable
controller means being removably engageable with the
s patch and including a second power means having a high
current density for powering the controller means to
provide sufficient energy for controlling and monitoring
the electrical energy delivered during operation so that
the at least one active agent is delivered to the applied
area of the patient proximate the first reservoir,
whereby upon delivery of the active agent, the controller
means and the patch may be disengaged for reuse of the
controller and for disposal of the patch.
In an alternative embodiment of the present
invention, the iontophoretic drug delivery system for
delivering at least one active agent to an applied area
of a patient, such as the skin, mucous membrane and the
like, includes housing means having two or more
electrically interconnected pods for containing electrode
assembly means including at least two electrodes for
driving the at least one active agent into the applied
area of the patient along electrical field lines
generated by the electrical current, a first reservoir
situated in electrical communication with a first one of
the electrodes and the first reservoir containing the at
least one active agent to be delivered to the applied
area of the patient, and a second reservoir situated in
electrical communication with a second one of the
electrodes and the second reservoir, with at least one of
the pods for containing power means for supplying
sufficient energy to drive the medicament into the
- patient, and controller means contained in one of the
pods for controlling and monitoring the electrical energy
: 35 delivered during operation so that the at least one
active agent is delivered to the applied area of the
- 2 ~ ~ 3 7 ~
WO96/10441 PCT~S95/12634
patient proximate the first reservoir, whereby the
housing conforms to the contours of the applied area.
In the preferred embodiment of the system, the pods
are interconnected by a flexible web. In addition, the
housing means includes an upper portion, a lower portion
and an intermediate portion, with the intermediate
portion including at least one tab for electrical
interconnection with the power means.
A preferred embodiment of the iontophoretic drug
delivery system, as well as other embodiments, objects,
features and advantages of this invention, will be
apparent from the following detailed description, which
is illustrated in the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
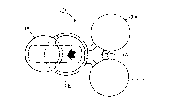
Figure l is a perspective view of the iontophoretic
system of the present invention;
Figure 2 is a plan view of the iontophoretic system
illustrated in Figure l;
Figure 3 is a partly exploded view of the
iontophoretic system illustrated in Figure l showing
connection/disconnection of the controller; and
Figure 4 is a perspective view of an alternative
embodiment of the iontophoretic system of the present
invention.
Figure 5 is an exploded view of the iontophoretic
system illustrated in Figure l;
~ i
~ WO96/10441 ~ 2 2 ~ 1 3 1 8 PCT~S95112634
Figure 6 is a cross-sectional view of the
iontophoretic system illustrated in Figure l taken along
lines 6-6; and
s Figure 7 is a partly exploded view of an alternative
embodiment of the iontophoretic system of the present
invention utilizing a reusable controller.
DET~TT~n DB8CRIPTION OF THE PREFERRED EMBODINENT
The iontophoretic drug delivery system of the
present invention is illustrated in Figures 1-7, with the
device generally designated l0.
Referring now to Figures l and 2, the device l0 of
the preferred embodiment of the present invention
includes a housing 12, a controller 14 having an
electronic array 15 (Figure 5), a controller power source
16, a drug delivery power source 18 electrically
connected to an electrode assembly 20 having two or more
electrodes for establishing an electric field between the
electrodes for use in delivering at least one active
agent iontophoretically to an applied area of the
patient. It should be appreciated that the electrodes
2s may be combined in the electrode assembly 20 or
separately provided as is well known in the art. In
addition, the housing 12 may include a drug filled patch
22, by integrally forming the two.
Referring to Figures l, 2 and 4, the housing 12
includes at least two pods, and preferably three pods 24,
26, 28 as illustrated in Figures l and 2, with the
electrode assembly 20 divided or otherwise separated into
two portions, the first portion 30 includes an electrode
32 and a reservoir 34, with the reservoir 34 being
situated adjacent to and in electrical communication with
the electrode 32. The second portion 36 also includes an
WO96/10441 ~ 2 a ~ 3 7 ~ PCT~S95/12634
electrode 38 and a reservoir 40, with the reservoir 40
being situated adjacent to and in electrical
communication with the electrode 38.
In the preferred embodiment, the pod 24 includes the
drug delivery power source 18, such as, for example, a
silver oxide, alkaline or zinc air battery, connected in
a circuit with electrodes 32 and 38. The other pod 28,
removably contains the controller 14 and the controller
power source 16, such as, for example a small lithium
battery. In this way, the lithium battery 16 has a long
shelf life and has a high current density but it is not
nec~s~rily capable of high current discharge. On the
other hand, since the patch is only used once, i.e~, to
apply a "single shot," the need for a lithium battery to
supply the drug delivering current is eliminated, with a
resulting reduction in cost. Also, since the silver
oxide, alkaline or zinc air battery is provided in the
housing 12, the housing 12, along with the patch, c:an be
disposed of after a single use. While, the controller 14
can be disengaged or otherwise disconnected from the
housing, as illustrated in Figure 3, and reused, along
with the corresponding lithium battery.
The controller 14 and the battery 16 are connected
in a circuit, with the controller 14 preferably including
an electronic array, such as for example, a
mi~Lo~ocessor, a dc/dc converter to increase the battery
supply to approximately 30 volts, a current regulator
which is controlled by the microprocessor and a timer for
monitoring the period of time the electrical current
flows in a particular direction and/or the amount o~
current applied. In addition, current flowing through
the reservoirs 34, 40 and the applied area can be
3~ controlled with a compliance voltage sufficient to
account for variations in skin impedance and losses
within the reservoirs.
- 2 2 0 ~ 3'78
wos6/10441 PCT~S95112634
In the preferred embodiment, the pod 28 houses the
controller and includes the electronic array 15 as well
as the power source 16 connected in a circuit with
electrodes 32 and 38.
s
It should be appreciated that the particular
electrode assembly and the electronic array are not
essential to the present invention and may include, for
example, those disclosed in co-pending U.S. Application
S.N. 08/129,887, filed September 30, 1993, the disclosure
of which is hereby incorporated by reference in its
entirety.
In the preferred embodiment, the controller 14
includes means for controlling the level of current to be
applied over time and also for varying the current.
Accordingly, the device lO can be utilized, for example,
to vary the current I1 during time period T1, current I2
during time period T2, current I3 during time period T3,
current I4 during time period T4, and current I5 during
time period T5 and additional currents and time intervals
as needed. Also, the controller may be adapted to
include means for controlling the voltage V or the power
I-V as well.
As is well known within the field, the device can be
situated on the area of the patient to which the active
agent is to be applied (the applied area) such as the
skin and a voltage impressed across the electrodes 32 or
34 of the electrode assembly 20 to cause electrical
current to flow through the skin of the patient to drive
or otherwise transport the ionic active agent into the
skin and the tissue to be absorbed by the body of the
patient. The electric field lines are sufficiently long,
3~ however, so that the active agent is transported to the
desired depth within the skin, and possibly to the
vasculature, to provide the desired effect, e.g.,
WO96/10441 ~ 2 2 0 1 3 7 8 PCT~S95/12634 ~
anesthetic, therapeutic or diagnostic. It should also be
appreciated that the device of the present invention can
be applied to other areas of the body such as mucous
membranes depending upon the desired therapy and drugs to
be delivered.
An alternative embodiment of the system 50 is
illustrated in Figure 4 showing two pods 52 and 54, with
the power source 56 being contained with pod 52 and the
controller 58 being interconnectable with pod 54.
Referring now to Figures 5-7, an additional feature
of the present invention will now be described. A~s
illustrated in Figure 5, the housing 12 includes an upper
portion 60, a base or lower portion 62, and a
intermediate portion 64. As previously mentioned, the
housing 12 includes a number of pods, and preferably
three pods 24, 26, 28 interconnected by a web 66
containing the circuit connecting the three pods. In the
preferred embodiment, the intermediate portion 64
includes one or more locking tabs 68, 70 for engaging the
power source, which may be split equally between t.he pods
24, 26 leaving only the electronic array, including logic
circuit, in the other pod 28. Likewise, the power source
may be incorporated within the electronic array.
Also, the electrode assembly 20 is divided or
otherwise separated into two portions, with the first pod
24 including an electrode 32 and a reservoir 34, with the
reservoir 34 being situated adjacent to and in electrical
communication with the electrode 32 as illustrated in
Figure 6. The second pod 28 also includes an electrode
38 and a reservoir 40, with the reservoir 40 being
situated adjacent to and in electrical communicati.on with
the electrode 38. In this way, a lower surface of the
intermediate portion 64 may include an electrode l.ayer as
disclosed, for example, in U.S. Patent Application S.N.
~ WO96/104~1 - 2 2 0 1 3 7 8 PCT~S95/12634
08/012,168, filed February 2, 1993, the disclosure of
which is hereby incorporated by reference in its
entirety.
In one embodiment of the invention, Lidocaine (a
local anesthetic) and Epinephrine (a vasoconstrictor)
are utilized such that the device can be used for
anesthetizing the applied area to minimize sensation from
the insertion of a needle or the like. In the preferred
embo~;~ent, the first reservoir 34 holds at least one
active agent, formulation, medication or drug 42,
preferably in an ionized or ionizable form, to be
delivered iontophoretically to the applied area of the
patient. However, electrodes 32 and 38 may include an
electrolyte, with the particular electrolyte not being
essential to the present invention and merely a matter of
choice. However, in this embodiment the electrolyte may
include sodium chloride in an aqueous solution, matrix or
the like. In situations where a polymer material or
another material is used, it may also act as an adhesive,
eliminating the need in prior devices for an adhesive
layer or the like. However, it should be apprecaited
that in certain clinical applicantions it may be
desireable to provide an adhesvie layer under the web 66
in which case the pods can still be in planes that rotate
slightly with respect to one another. Likewise, it may
be desireable to forgo the adhesive under the web so that
the planes can rotate and also flex in the distance
between the centers of the pods to accommodate the skin
as it stretches or muscles flex. Also, some elasticity
or excess length can be incorporated. In this way,
increased reliability of skin contact is achieved and
maintained by the patient when wearing the device lO,
especially for prolonged periods of time.
The active agent can have either a negative charge
or a positive charge, but the active electrode must also
WO96/10441 2 2 ~ 1 3 7 8 PCT~95112634 ~
be negatively or positively charged, respectively.
Accordingly, where the active agent contained in the
reservoir 34 or 40 is positively charged, the electrical
current flows from the first electrode 32 to the second
S electrode 38, and the first electrode 32 acts as the
active electrode and the second electrode 38 acts as the
return electrode, with the drug being delivered to the
applied area of the skin approximate the first electrode
32 and the first reservoir 34.
In the preferred embodiment, the drug reservoir 34
includes the medicament 42 for delivery, which may contain,
for example, either Alfentanil, Baclofen, Beclomethasone,
Betamethasone, Buspirone, Cromolyn sodium, Bromocriptine,
Calcitonin, Diclofenac, Diltiazem, Doxazosin, Droperidol,
Encainide, Fentanyl, Granisetron, Haloperidol,
Hydrocortisone, Indomethacin, Insulin, Isosorbide
dinitrate, Ketoprofen, Ketorolac, Lidocaine, Lisinopril,
LMW heparin, Melatonin, Methotrexate, Metocloprami~e,
Miconazole, Midazolam, Nicardipine, Oxybutynin, PGE l,
Piroxicam, Pramipexole, Prazosin, Scopolamine, Seglitide,
Sufentanil, Terbutaline, Testosterone, Tetracaine,
Tropisetron, Verapamil, Warfarin, Zacropride and
Zatosetron, including derivatives, analogs and the like,
which varying in duration for delivery from minutes to
hours. In this way, the device can be used for delivering
the medicament to the applied area for a short period of
time or for extended periods of time. In addition, it
should be appreciated that the dose of the medication can
be varied depending upon the substance used.
Active agent, drug, formulation, medication,
medicament and active compound have been used herein to
mean any pharmaceutical agent, such as therapeutic
compounds, diagnostic agents, anesthetic agents and the
like.
-
WO96/10441 ~ 2 2 0 1 3 7 ~ PCT~S95112634
.
Referring now to Figure 7, an embodiment of the device
lO is illustrated in which the housing 12 includes three
pods 24, 26 and 28, with the electronic array being
contained in a controller 14 removably engageable by a
sliding action in a receiving portion 2l formed in the
housing. Likewise, referring again to Figure 4, another
embodiment of the device lO is illustrated in which the
housing includes two pods 52 and 54, with the electronic
array being contained in a controller 14 removably
engageable by a sliding action in a receiving portion 21
formed in the housing similar to that shown in Figure 3.
Accordingly, it should be appreciated that the pods
can be arranged radially as illustrated in Figures 1-7 or
some other orientation such as linearly, e.g., two or three
in a row to accommodate the specific requirements of the
desired body location on which the device is to be applied.
The operation of the system of the present invention
will now be explained with reference to Figures 1-7,
specifically that the device lO including the various
pods 24, 26, 28 interconnected by the flexible web 66
allows the device to be applied to the body with each of
the pods not being influenced by the plane of attachment
of the other pods. Thus, if the device were to be
applied on a spherical surface, each pod would only have
to be flexible enough to give good contact under itself.
In addition, while the device has been described in
30 connection with iontophoresis, i~ should be appreciated
that it may be used in connection with other principles
of active introduction, i.e., motive forces, such as
electrophoresis which includes the movement of particles
in an electric field toward one or other electric pole,
35 anode, or cathode and electro-osmosis which includes the
transport of uncharged compounds due to the bulk flow of
water induced by an electric field. Also, it should be
-
~01 ~78
WO96/10441 PCT~S95/12634
appreciated that the patient may include humans as well
as ~n;~ s.
While the preferred embodiments of the present
invention have been described so as to enable one skilled
in the art to practice the device of the present
invention, it is to be understood that variations and
modifications may be employed without departing from the
concept and intent of the present invention as defined in
lo the following claims. The preceding description is
intended to be exemplary and should not be used to limit
the scope of the invention. The scope of the invention
should be determined only by reference to the following
claims.
14