Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02203636 1997-04-24
W O96/13162 PCT~N095/00198
"METHOD AND MEANS FOR CONTROLLING INSECT SPECIES."
The present invention relates to analogues of pheromones and their use for controlling
courtship behaviour of insect species. The invention also relates to a method for controlling
the courtship behaviour of insect species by using a analogue of the natural pheromone of
the insect.
The use of conventional insecticides for controlling insect species is becoming a growing
concem world wide. The reasons for this are costs, natural and genetic resistance and
environmental factors. As a consequence there is public pressure to reduce the quantity of
incecticides used.
Semiochemicals are 'signalling chemicals' which are responsible for highly effective
communication systems between and within the plant and animal kingdoms. These
semiochemicals can be subdivided into two main groups, pheromones and alleochemicals.
An alleochemical is a substance released by an individual causing a behavioural or
physiological response by an individual of a different species. A pheromone is a substance
released by an individual causing a behavioural or physiological response by an individual
of the same species. Pheromones play an important role in animal behaviour and because
of their particular significance in affecting reproductive behaviour of insects, there has been
considerable interest in using pheromones for pest managements. Research has
concentrated on use of the pheromone itself or analogues of the pheromone which
stimulate the same type of behavioural response in the target species. Pheromones may be
subdivided into groups such as sex pheromones, aggregation pheromones and alarm
pheromones. Sex pheromones are usually sent from the females to attract the males, and
are involved in increasing the probability of courtship or mating. They may either act over a
short range as aphrodisiacs or over a long distance causing attraction. Many pheromones
are long chain aliphatic compounds with one or two carbon-carbon double bonds. Alcohol,
aldehyde or acetate is usually a terminal functional group.
SU~STITUTE SH~ET (RULE 26)
CA 02203636 1997-04-24
W O96/13162 PCT~NO9~/00198
Several methods for disrupting or inhibiting of mating of insects and crop pest are known.
From US-A-4,291,051 the use of 1,5-dimethyl-6,7-dioxabicyclo (3.2.1)octane, which is an
analogue of pine beetle natural pheromone, for controlling infestation by pine beetles is
known. 1,5-dimethyl-6,7-dioxabicyclo (3.2.1)octane is a pheromone analogue whichdisrupts the natural pheromone (frontalin) activity of pine beetles. It functions (1) as an
aggregation pheromone in a manner similar to that of frontalin and (2) binds to the frun~
receptor sites of the insects and thereby blocks the sites. 1,5-Dimethyl-6,7-dioxabicyclo
(3.2.1)octane is somewhat unstable and as it slowly diffuses away from the release site in
the target area, it slowly rearranges to frontalin.
It is well established that the naturally occurring cyclopropene fatty acid sterculic acid (A) or
its esters inhibit the enzyme ag- desaturase which converts stearic acids into oleic acid (B),
A. C. Fogerty et al., Lipids, vol. 4, 1969, pp. 265 and R. Jeffcoat et al., Lipids, vol. 1Z, 1977,
Pp. 480.
A H
CH~(CH2)~ C02~ C~ H2)~ H
(~) (B)
Compound (A) is identical to (B) except that a Z-alkene has been repiaced structurally by a
cyclopropene. It is also well established that diets containing sterculic acid can alter the
development of a number of animal species. Thus, M. Benzoa et al., J. Econ. Entomal.,
1967, pp. 196, show that on feeding the housefly Musca domestica with a diet containing
2.5% sterculic foctida oil, the females produced no eggs. The reason that the cyclopropene
is an effective inhibitor may be that it is close in structure to the product of enzyme action
and therefore fit into the enzyme site, but is much more reactive than the product.
L. Gosalbo et al., Insec Biochem.Molec.Biol. Vol.22, No. 7, pp. 687-690, 1992, "Inhibition of
Sex Pheromone Biosynthesis in Spodoptera littoralis by Cyclopropene Fatty Acids.", have
also shown that treatment of females with a cyclopropene derivative inhibits the action of
enzymes which are needed for changing the fatty acids to diflerent pheromone
components. Normally it is the females which send out pheromones to attract the males.
The females do not produce the pheromones when they are treated with the cyclopropene
~UBSTITUT~ SHEET (RllL~ 26~
CA 02203636 l997-04-24
W O96/13162 PCT~N095/00198
compounds 10,11-methylene- hexadec-10-enoic acid and
11 ,12-methylenehexadec-1 1-enoic acid.
~ A. R. Jutsum et al., Crop Protection, Vol. 4, pp. 501-506, 1984, "Inhibition of response of
Heliothis virescens to its natural pheromone by antipheromones.", describes four derivatives
of pheromones which have been shown to inhibit the response of Heliothis virescens to its
natural pheromone. This inhibition was demonstrated to be reversible and has been
suggested to be based on an associative interaction between the receptor site and the
carbonyl group of the antipheromone.
The main object of this invention is to arrive at a method of controlling insect species which
is less detrimental to the environment than standard procedures. Another object of the
invention is to arrive at a mixture of cyclopropene analogues of pheromones which
comprises at least one cyclopropene analogue of a pheromone and the use of theseanalogues for controlling the courtship behaviour of insect species.
There have been a number of studies of cyclopropane analogues. The chemistry of the
cyclopropanes in a number of cases resembles that of a carbon-carbon double bond, for
example they are susceptible to electrophilic attack, although the geometry of the
cyclopropane is rather different of that of the double bond.
Cyclopropane analogues of pheromones in the early studies did show an effect on the
pheromone receptor. A cyclopropene in itself contains a double bond and the geometry
about the carbon-carbon double bond of the cyclopropene is similar to that of a normal
Z-carbon- carbon double bond. However the cyclopropene is highly strained, i.e. of high
energy, and is often more reactive than a simple Z-carbon-carbon double bond in reactions
with electrophiles and, in particular, nucleophiles. The three membered ring is often opened
in these reactions
releasing the strain. Cyclopropene analogues of a pheromone may therefore have the right
geometry to reach the receptor site, but once there may react in such a way that the
receptor site is irreversibly blocked. Studies of the effect of cyclopropene analogues of
pheromones where the analogue (C) is identical to the pheromone (D) except that an
alkene group has been replaced by a cyclopropene having two hydrogens at the 3-position,
or where the 3-position bears substituents other than hydrogen, have not been reported.
SUBSTITlJTE SHEET ~RUl~ 2~
CA 02203636 1997-04-24
W O96/13162 PCT~NO95/00198
~H
A R
\R~
R (C~ R' ~D)
R and R' are selected such that D is made to a pheromone.
With these ideas in mind it was assumed that an analogue of a pheromone in which a
Z-carbon-carbon double bond was replaced by a cyclopropene would 'mimic' the
pheromones, block the pheromone receptor site to the insect, disrupt mating, and thus
selectively control insect populations. Pheromones are either a single compound or a
combination of components. If the use of these analogues is to be of practical importance,
the blocking of the receptor site should ideally be irreversible or at least last long enough to
cause an effect during life time of the insect. Insects that are exposed to analogues which
block the receptor site, would not respond normally to each other. The mating would be
disrupted and the population reduced.
In the present invention, it is proposed that a cyclopropene analogue of a pheromone may
act directly at the site on the insect which recognises that pheromone and therefore the
analogue may interfere with the behaviour of the insect.
Different insect species have been studied. The inventors have chosen Musca domestica
L., the housefly as a representant of the order Diptera, or two-winged flies and Plutella
xylostella, the diamondback moth and Ephesfia elutella, the warehouse moth both as a
representant to the order Lepidoptera.
Musca domestica L., the housefly is a dangerous world-wide pest to animals and man
because it breeds in manure, rubbish and fermenting crops. It carries and spreads typhoid,
dysentery, diarrhoea, cholera, yaws, trachoma and many other diseases. It serves also as
an intermediate host of roundworms and tapeworms.
Plutella xylostella, the diamondback moth has become one of the most destructive insects
to cruciferous plants throughout the world. Few effective natural enemies of thediamondback moth exist. Broad-spectrum insecticides have been used to control
diamondback moth. These may also cause ~estruction of natural enemies. The continual
SUBSTITUTE SHEET (RULE 26)
CA 02203636 1997-04-24
WO96/13162 PCT~N095/00198
use of these insecticides has also promoted insecticide resistance. Alternative control
methods are therefore urgently required for these insects.
Ephestia elutella, the warehouse moth is a serious pest of cocoa beans, chocolate
confectionery, dried fruit and nuts. Many other substances may become infested such as
tobacco, wheat or other grain stored in bulk, oilseeds, and oilseed products andmanufactured animal feeding stuffs.
Because the housefly, the diamondback moth and warehouse moth are harmful against
animals, crops and food stuffs, the inventors have been studying these three insects and
their behaviour when treated with cyclopropene analogues of their natural sex pheromones.
The sex pheromone of the housefly is (Z)-9-tricosene (I) with trivial name rnuscalure.
(Z)-9-Tricosene attracts the male housefly to the female over a short distance. As it
approaches, the male leaps onto the female - the mating strike - and, if the female is
receptive copulation will follow. Synthetic (Z)-9-tricosene may be used to increase
aggregation by flies at a target location where they may be controlled by other means. The
inventors have been studying a cyclopropene pheromone analogue,
1-octyl-2-tridecylcyclopropene (Il), of (Z)-9-tricosene, which is thought to be sufficiently
similar in shape and physical properties to reach receptor sites.
A
~ ~,
CH3(CH2)12 (CH2~7CH3 CH3(CH2)~2 (CH~7CH3
(I) tll~
The sex pheromone for the diamondback moth consists of three components,
(Z)-1 1-hexadecenal (Ill), (Z)-1 1-hexadecenyl acetate (IV) and (Z)-1 1-hexadecenyl alcohol
(V), where (Z)-1 1-hexadecenal and (Z)-1 1-hexadecenyl acetate are the major components.
The inventors have been studying the cyclopropene analogue,
10-(2-butylcycloprop-1-enyl)decanal (Vl) and 10-(2-butylcycloprop-1-enyl)decanyl acetate
(Vll), of the major components. The male diamondback moth courtship behaviour, such as
wing-fanning and circling, involves a complex sequence of events initiated by a 'calling
female'. The 'calling female' releases a pheromone blend from a terminal abdominal gland
which attracts receptive males.
SU3STITUTE SHEET (RU~E 26)
CA 02203636 1997-04-24
W O96/13162 PCT~N095/00198
.
CH3(CH2)3 (CH2)9CH~ CH3(CH2)3 (Vl) (CH2)9CHO
CH3(CH2)3 (IV) (Cff2)100Ac CH3(CH2)3 (CH2~iOoAc
CH3(CH2)3 (CH2)~
The sex pheromone of the warehouse moth consists of two components, (Z,E)-9,12-tetra-
decadienyl acetate (Vlll), which is the major component, and (Z,E)-9,12-tetra- decadien-1-ol
(IX). The inventors have been studying the cyclopropene analogue 8-(2-(E-but-2-en-1-yl)-
cycloprop-1-enyl)octan-1-yl acetate (X) and 8-(2-(E-but-2-en-1-yl)-cycloprop-1-enyl)octanol
(Xl) of the pheromones of the warehouse moth.
\~--~\ (CH2)80Ac ~ _ (cH2)8oAc
(Vlll) (X)~
(CH2)80H ~ ~ (CH2)80H
(IX) (xl)
The inventors found that the cyclopropene analogues of the pheromones have a dramatic
effect on courtship behaviour of each of the three insect species. Treatment of houseflies
with cyclopropene analogue, 1-octyl-2-tridecylcyclopropene (Il) of (Z)-9-tricosene (I), which
is the sex pheromone of the housefiy, resulted in a reduced number of mating strikes.
When diamondback moth and warehouse moth were treated with cyclopropene analogues
of their respective sex pheromones, their courtship behaviour like wing-fanning and circling
were dramatically decreased.
SU~STlTUTi- S~iE~T (RUL- 26~
CA 02203636 1997-04-24
W O96/13162 PCTA~095/00198
Thus the inventors have arrived at a new mixture of analogues of pheromones comprising
at least one cyclopropene analogue of a pheromone in which the analogue is identical to
the pheromone except that an alkene group has been replaced by a cyclopropene having
either two hydrogens at the 3-position or one or two s~ ~hstit~ lents other than hydrogen at
the 3-position. In particular the invention relates to a mixture of analogues of pheromones
where a Z- or cis-alkene is replaced by a cyclopropene. The invention further comprises use
of said pheromone analogues for controlling insect species and a method for changing the
mating behaviour of insect species, where a pheromone analogue in which the analogue is
identical to the pheromone except that an alkene group has been replaced by a
cyclopropene having either two hydrogens at the 3-position or one or two substituents other
than hydrogen at the 3-position, or a mixture of such analogues, are applied to insect
species, separately or distributed in a carrier, for controlling the mating behaviour of the
insect species.
The scope of the invention and its special features are as defined by the attached claims.
In the following the invention will be further explained by examples.
Figure 1 shows mating strikes made by male houseflies on female targets.
Figure 2 shows mating strikes made by male houseflies on female targets.
Figure 3 shows mating strikes made by male houseflies on female targets.
Figure 4 shows the EAG dose-response curves of male Plutella xylostella
Figure 5 shows the amount of circling and walking.
Figure 6 Graphs to show behavioural responses of five male Plutella xylostella to
treatment.
Figure 7 Graphs to show behavioural responses of five male Plutella xylostella to
treatment.
Figure 8 Graphs to show behavioural responses of five male Plutella xylostella to
treatment.
Figure 9 shows the EAG dose-response curves of male Ephestia elutella
~ Figure 10 Graphs to show behavioural responses of five male Ephestia elutella to
treatment.
~ Figure 11 Graphs to show behavioural responses of five male Ephestia elufella to
treatment.
SUBSTITUTE SHEET (RUL~ 26)
CA 02203636 1997-04-24
W O96/13162 PCTAN095/00198
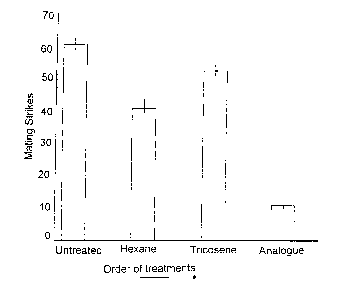
Figure 1 shows graphically the number of mating strikes made on live, three days old
females before and after treatment of females with 1 ~LI hexane, 1 1l9 of (Z)-9-tricosene (I) in
1 ~Li hexane, or 1~Lg of cyclopropene analogue (Il) in 1 1ll hexane. Vertical bars represent
the Standard Error (SE) of the mean.
Figure 2 shows graphically the number of mating strikes made on live, three days old
females before and after treatment of females with 1 llg of cyclopropene analogue (Il) in 1 !11
hexane. Vertical bars represent the Standard Error (SE) of the mean.
Figure 3 shows graphically the number of mating strikes made on females before and after
treatment of males with 1~1g of cyclopropene analogue (Il) in 1 ~I hexane and then retested
after 24 hours and 48 hours of the treatment. Vertical bars represent the Standard Error
(SE) of the mean.
Figure 4 shows the EAG dose-response curves of male Plutella xylostella to (Z)-1 1-hexa-
decenal (Ill) and (Z)-11-hexadecenyl acetate (IV) and 10-(2-butylcycloprop-1-enyl)- decanal
(Vl) and 10-(2-butylcycloprop-1-enyl)decanyl acetate (Vll). Vertical bars represent the
Standard Error (SE) of the mean.
Figure 5 shows the amount of circling (5a) and walking (5b) in the arm treated with
pheromone and analogue.
Figure 6 shows graphically circling (6a) and wing fanning (6b) responses of five male
Plutella xylostella to treatment with no chemical, treatment with a 1:1 mixture of
(Z)-1 1-hexadecenal (Ill) and (Z)-1 1-hexadecenyl acetate (IV) (two times), and finally
treatmentwith a 1:1 mixture of 10-(2-butylcycloprop-1-enyl)decanal (Vl) and
10-(2-butylcycloprop-1-enyl)decanyl acetate (Vll).
Figure 7 shows graphically circling (7a) and wing fanning (7b) responses of five male
Plutella xylostella to treatment with no chemical, treatment with 1:1 mixture of(Z)-1 1-hexadecenal (Ill) and (Z)-1 1-hexadecenyl acetate (IV) (two times), treatment with 1:1
mixture of 10-(2-butyl- cycloprop-1-enyl)decanal (Vl) and
10-(2-butylcycloprop-1-enyl)decanyl acetate Vll), and finally a newtreatmentwith a 1:1
mixture of (Z)-1 1-hexadecenal (Ill) and (Z)-1 1-hexadecenyl acetate (IV).
SU~STIT~E SHEET (RULE 26)
CA 02203636 1997-04-24
WO96/13162 PCT~N095/00198
Figure 8 shows y~dphically circling (8a) and wing fanning (8b) responses of five male
Plutella xylosfella to treatment with no chemical, treatment with hexane and a 1:1 mixture
of (Z)-1 1-hexadecenal (Ill) and (Z)-1 1-hexadecenyl acetate (IV),
10-(2-butylcycloprop-1-enyl)- decanal (Vl) and 10-(2-butylcycloprop-1-enyl)decanyl acetate
(Vll).
Figure 9 shows the EAG dose-response curves of male Ephestia elufella to (Z,E)-9,12-tetra-
decadienyl acetate (Vlll) and 8-(2-(E-but-2-en-1-yl)-cycloprop-1-enyl)octanyl acetate (X).
Vertical bars represent the Standard Error (SE) of the mean.
Figure 10 shows graphically behavioural responses of five male Ephestia elutella to
treatmentwith no chemical, treatmentwith a 1:1 mixture of (Z,E)-9,12-tetradecadienyl
acetate (Vlll) and (Z,E)-9,12-tetradecadien-1-ol (IX), and a 1:1 mixture of
8-(2-(E-but-2-en-1-yl)-cycloprop-1-enyl)- octanyl acetate (X) and
8-(2-(E-but-2-en-1-yl)-cycloprop-1-enyl)octanol (Xl).
Figure 11 shows graphically responses of five male Ephestia elutella to treatment with no
chemical, treatmentwith a 1:1 mixture of (Z,E)-9,12-tetradecadienyl acetate (Vlll) and
(Z,E)-9,12-tetradecadien-1-ol (IX), a 1:1 mixture of
8-(2-(E-but-2-en-1-yl)-cycloprop-1-enyl)octanyl acetate (X) and
8-(2-(E-but-2-en-1-yl)-cycloprop-1-enyl)octanol (Xl) and finally a new treatment with a 1:1
mixture of (Z,E)-9,12-tetradecadienyl acetate (Vlll) and (Z,E)-9,12-tetradecadien-1-ol (IX).
Example 1.
Musca domestica L., the housefly.
This example shows treatment of houseflies to determine the effect of cyclopropene
pheromone analogue, 1-octyl-2-tridecylcyclopropene (Il) of (Z)-9-tricosene (I) on male
mating strikes on different treatments and targets~
In previous studies of the pheromone the number of mating strikes observed has been
taken as indicative of potency of the pheromone. The tests were conducted in a glass
chamber 6 cm high and 9.5 cm in diameter divided by glass partitions into four quadrants. In
the tests, targets, treated in different ways, were exposed to groups of five virgin male flies
SUBST~TUTE SHEET (RULE 26)
CA 02203636 1997-04-24
W O96/13162 PCT~N095/00198
in a test chamber, the interactions were recorded on video tape for 15 minutes, and then
analysed to assess the numbers of strikes observed on each target. In the series of tests
live, virgin female flies of known age were used as the targets. In some cases the male
flies copulated with the target, thus making it unavailable for further mating strikes. The
inventors have assumed that during copulation a mating strike would have occurred every
15 seconds.
Targets were fixed to a piece of sealing wax on a glass cover slip. The targets were tested
before and after treatment enabling a paired comparison between the same flies, before
and after treatment. In some of the tests the female target were treated in one of three
ways:
(1) With 1~1 of hexane (the solvent)
(2) With 1 ul of a solution of (Z)-9-tricosene (I) in hexane (1 mg in 1 ml)
(3) With 1 ul of a solution of analogue (Il) in hexane (1 mg in 1 ml)
In other tests the female targets were untreated, but the male were treated with 1 ul of
analogue (Il) solution and were either tested immediately or after a specific period.
Response of male housefly to targets of live, virgin female, 3 days old, before and after
treatments were recorded by the number of mating strikes made by five males at the target
over a fifteen minute period (each test result in the sum of four replicates). The recorded
results are shown in table A - C. Each value in the column Test is the average of four
replicates.
A. Number of strikes made on females before and after treatment of females with 1 ul of
hexane. 1 ua of (Z)-9-tricosene (I) in 1 ul hexane. or 1 uq of analoaue (Il) in 1 ul hexane.
Percent reduction of matinq shown in brackets.
TestBeforetreatmentHexane (Z)-9-tricosene Analogue
60.5 38.25 (39) 49 (19) 16.25 (73)
2 60.5 51.75 (14) 60 ( 1) 15.50 (74)
3 60.75 36.75 (39) 52 (15) 7.50 (88)
4 62.0 37.75 (37) 56 (16) 7.25 (88)
SUBSTITUTE SHEET (RUl~ 26)
CA 02203636 l997-04-24
W O96/13162 PCT~N095/00198
11
B. Number of strikes made on females before and after treatment of females with 1 !lq ~f
analoque (Il) in 1ul hexane.
Test Before treatment Treated with analogue Percent reduction in mating
24 3 82
2 25 4 86
3 8 3 67
4 13 3 80
6 38 4 92
7 31 5 85
C. Number of strikes made on female tarqet before and after treatment of males with 1 uq
of analoque (Il) in 1 ul hexane and then retested 24 hours an 48 hours after recovery of the
-reatment.
Test Un- Ana- % Analogue % Analogue %
treated loguereduction+ 24 h reduction +48 h reduction
76 1 99 7 90 - -
2 10 0 100 2 80
3 37 - - - - 1.5 96
These data shows that treatment with hexane, (Z)-9-tricosene (I) and pherornone analogue
(Il) all reduced the number of mating strikes. When applied to female target (Z)-9-tricosene
(I) caused a small, but significant reduction in the number of strikes made by males when
compared with their response to untreated females. Treatment with hexane led to a larger
reduction. The pheromone analogue (Il) reduced the number of strikes by more than 70%
(Figures 1 and 2). When the pheromone analogue (Il) was applied to male flies there was
an even greater reduction in the number of strikes. There is evidence that this effect
declined with increasing time after treatment (Figure 3), but even after 24 hours you still
have considerable effect.
~ ExamPle 2.
Plufella xylosfella L., the diamondback moth.
This example shows the biological activity of (Z)-11-hex~decenal (Ill), (Z)-11-hexadecenyl
acetate (IV) and the cyclopropene analogues, 10-(2-butylcycloprop-1-enyl)decanal (Vl) and
SUBSTITUTE SHEET !RULE 26)
CA 02203636 1997-04-24
W O96/13162 PCTAN095/00198
12
10-(2-butyicycloprop-1-enyl)-decanyl acetate (Vll) electrophysiologically by an electro-
antennogram assay, and the effect on courtship behaviour of diamondback moth exposed
to a mixture of the cyciopropene pheromone analogues,
10-(2-butylcycloprop-1-enyl)decanal (Vl) and 10-(2-butylcycloprop-1-enyl)decanyl acetate
(Vll), which are the analogues of (Z)-1 1-hexa- decenal (Ill) and (Z)-1 1-hexadecenyl acetate
(IV) which are the major components of the sex pheromone of the diamondback moth.
Electroantennograms (EAG's) were recorded from excised heads of Plutella xylostella
males with Ag/AgCI electrodes. The indifferent electrode (glass capillary, innerdiameter=0.86 mm), filled with Beadle Ephrussi Ringer (BER) solution was inserted into the
base of the head and the BER-filled recording electrode was brought into contact with the
cut end of an antenna. Signals were amplified and recorded by standard methods. The
stimulus was delivered into a purified air stream (1 litre/min) that flowed continuously over
the preparation using a filter paper in a disposable Pasteur pipette cartridge. The impulse
frequency was determined as the number of impulses that were elicited during the first 1
sec after stimulus application. To compensate for antennal fatigue, responses were
normalised using a standard stimulus (0.1 9 hexen-1-ol) before and after each experimental
stimulus. Control values (solvent only) were subtracted from the normalised values to give
the final, corrected absolute EAG vaiues. The results for the EAG of (Z)-1 1-hexadecenal
(Ill) and (Z)-1 1-hexadecenyl acetate (IV) and for the cyclopropene analogues (Vl) and (Vll)
are shown in Figure 4. The cyclopropene analogues are being detected by the antenna and
therefore there is a response, although at a lower level than for (Ill) and (IV). It is not
possible to deduce from this electrophysiology whether the cyclopropenes are acting as
inhibitors and binding to the same receptor site as that of the natural pheromones,
~ reversibly or irreversibly. It can be concluded however, that they are acting as analogues to
pheromones.
The experiments on courtship behaviour were performed by applying the above stated
chemicals in a solvent to a piece of filter paper in a side-arm of a four way olfactometer.
The olfactometer (29.5 cm X 29.5 cm X 2.5 cm), a modification of Pettersson olfactometer,
was constructed of four perspex crescents forming a four-pointed-star-shape. The males
were able to move freely throughout the olfactometer. When the moth remained in one of
the four fields, it was recorded as having made a preferential choice. After the first choice
was recorded, the time and position of each subsequent change to a different direction and
particular movement was recorded. Once they entered the Pheromone plume, the amount
of time and males exhibited "looking on" behaviour (i.e. circling and wing fanning) was
SUBSTITUTE S~EET (RULE 26)
CA 02203636 1997-04-24
W 096/13162 PCT~N095/00198 . 13
recorded. Few male responses were observed when no treatment was present in any of the
arms of the olfactometer. However, if the pheromone was present, the males remai ned in
the treated arm and repeated the mating dance which included circling and wing fanning.
Five 3 day old virgin males were exposed to a variety of chemical treatments applied to filter
paper in the bioassay chamber. The responses were divided into the five categories as
outlined below:
1) Circling
2) Flying
3) Still
4) Walking
5) Wing-fanning
Four odour fields were created by sucking air out through a removable joint in the centre
ceiling of the chamber. This hole was also used to introduce test insects into the chamber.
After each experiments the test chamber was thoroughly washed in water with detergent,
rinsed with hot water, rinsed again with distilled water, and then dried.
For each set of experiments an initial run was perfommed in which no chemical was applied
to the olfactometer. This ensured that there was no deviation from a random distribution of
insects throughout the chamber.
The odour source was provided in one of the side-arms by a piece of filter paper dosed with
either solvent, pheromone in solvent or analogue in solvent. The chemical treatments
included hexane (1~1), a 1:1 mixture of (Z)-11-hexadecenal (Ill) (0.1 ng) and
(Z)-11-hexadecenyl acetate (IV) (0.1 ng) and a 1:1 mixture of
10-(2-butylcycloprop-1-enyl)decanal (Vl) (0.1 ng) and 10-(2-butylcycloprop-1-enyl)-decanyl
acetate (Vll) (0.1ng). The responses to these treatments were then compared with the
responses of untreated 3 day old virgin male moths without any chemical present. The
tested materials (0.1 ng) were applied to filter paper strips in 1 !11 HPLC grade hexane.
A video camera allowed insect behaviour to be recorded with a video cassette recorder.
Each test was recorded for 15 minutes and then discontinued. The tapes were analysed at
one second intervals.
' SUBSTITUTE SHET (RlILE 26)
CA 02203636 1997-04-24
W 096/13162 PCT~N095/00198 14
Ali experimental data were analysed using a two-way analysis of variance (ANOVA), then
by Duncan's multiple-range test when significant differences were found in the ANOVA.
The amount of circling and walking is shown in Figure 5. Figure 5 shows that the greatest
amount of circling and walking occurs in the arm treated with the pheromone and this
activity was observed continuously throughout the 15 minute experimental period. However,
there was a small amount of circling observed in the analogue treated arm and this activity
occurred very early in the observation period. Walking was less in the analogue treated arm
than in the pheromone treated arm.
As walking is less specifically associated with courtship, wing fanning and circling were
chosen as behaviours to indicate a response to pheromone. The effect of treatment with
the analogue following a previous treatment with the pheromone is shown in Figure 6. Wing
fanning and circling were reduced on addition of the analogues. When synthetic pheromone
treated moth were compared to analogue treated moths, significantly more males exhibit
circling (P<0.001) and wing fanning (P<0.05) with the pheromone. The wing fanning
response reduced by 84% from the pheromone to the analogue. The male initially was
attracted to the analogue treated arm and within the first four minutes there was
considerable movement which mainly involved walking and very little wing fanning and
circling. The moths then either remained still in the analogue source or moved away from it
into a different section of the chamber.
Immediate reapplication of the pheromone after the atmosphere had been treated with the
analogue provided no restoration of wing fanning or circling behaviour (P>0.05) This effect
is illustrated in figure 7 and provides an initial indication that the analogue had been bound
to the same receptor site as that of the pheromone. Pheromone was presented in
conjunction with the analogue, to observe whether the pheromone would mask the
analogue or vice versa and to see if there was an appreciable reduction in male response.
The side-arm was initially treated with the solvent hexane. No siy~ liricanl effect with wing
fanning and c7rcling (P>0.05) was observed by the males on addition of hexane. The
amount of time spent walking did however increase. By comparison with a treatment with a
1:1 pheromone blend of (Z)-11-hexadecenal (Ill) and (Z)-11-hexadecenyl acetate (IV) in
treatment with mixed atmosphere of pheromone and analogue caused significantly fewer
males to exhibit circling ~P<0.002) but there was no effect on wing fanning (P'0.2). Also, on
co",pa,ing the treatment of a pheromone and analogue mixture to moths in atmosphere of
a analogues, sig"iricar,Lly more males exhibit circling (P<0.05) with the Pheromone present,
SUBSTITUTE SHEET ~RULE 26)
CA 02203636 1997-04-24
W 096/13162 PCTAN095/00198
but no effect on wing fanning (P<0.44) was observed. Pheromone receptor blocking may
have occurred in these case. These results are illustrated in Figure 8.
The olfactometer experiments show that physiological levels of hexane did have a small
effect, i.e. increasing the activity of the males, but not as much as with the addition of the
sex pheromone. The overall results showed that, the average amount of time spent circling
and wing fanning was greatest when the males were in the plume of the synthetic
pheromone. This was significantly longer than any of the other treatments. It was found to
be substantially less when the analogue was added, and least when no chemical was
applied to the filter paper. The cyclopropene analogue had a dramatic effect on decreasing
the amount of time spent by the moths on circling and wing fanning.
Field tests, where traps were loaded with either no chemical, pheromone mix, analogue mix
or analogue mix and pheromone mix in combination, have been performed. The number of
moths were greatest in the pheromone baited lures, and significantly less in all other
treatments. Traps loaded with the analogue mix caught very few moths. When pheromone
mix and analogue mix were used in combination, trap catch was significantly reduced
compared to pheromone loaded traps.
Example 3.
Ephestia elutella Hbn, the warehouse moth.
This example shows the biological activity of (Z,E)-9,12-tetradecadienyl acetate (Vlll) and
the cyclopropene analogue, 8-(2-(E-but-2-en-1-yl)cycloprop-1-enyl)octanyl acetate (X)
electro- physiologically by an electroantennogram assay, and the effect on courtship
behaviour of warehouse moth exposed to a mixture of the cyclopropene pheromone
analogues, 8-(2-(E-but-2- en-1-yl)cycloprop-1-enyl)octanyl acetate (X) and
8-(2-(E-but-2-en-1-yl)cycloprop-1-enyl)octanol (Xl), which are the analogues of
(Z,E)-9,12-tetradecadienyl acetate (Vlll) and (Z,E)-9,12-tetra- decadien-1-ol (IX) which are
the sex pheromone of warehouse moth.
Electroantennograms (EAG's) were recorded from excised heads of Ephestia elutella males
with Ag/AgCI electrodes. The set up was the same as that used for Plutella xylostella in
example 2. The results forthe EAG of (Z,E)-9,12-tetradecadienyl acetate (Vlll) and the
SalBSTITUTE SHEET (RUEE 26~
CA 02203636 1997-04-24
W O96/13162 PCT~N095/00198
16
cyclopropene analogue, 8-(2-(E-but-2-en-1-yl)cycloprop-1-enyl)octanyl acetate (X) are
shown in Figure 9. The cyclopropene analogues are being detected by the antenna and
therefore there is a response, although at a lower level than for the pheromone. It is not
possible to deduce from the electrophysiology whether the cyclopropene are acting as an
inhibitor, binding reversibly or irreversibly to the same receptor site as that for the natural
pheromones. It can be concluded however, that they are acting as analogues to
pheromones.
The experiments on courtship behaviour were performed by applying the above stated
chemicals to a piece of filter paper in a side-arm of a four way olfactometer. The same
experimental procedure was adopted and the same olfactometer was used throughout this
study as described for diamondback moth in example 2. The chemical treatments included
the individual majorpheromone component, (Z,E)-9,12-tetradecadienyl acetate (Vlll) and
then a 1:1 mixture of (Z,E)-9,12-tetradecadienyl acetate (Vlll) and
(Z,E)-9,12-tetradecadien-1-ol (IX) and finally, the cyclopropene analogues
8-(2-(E-but-2-en-1-yl)cycloprop-1-enyl)octan-1-yl (X) acetate and
8-(2-(E-but-2-en-1-yl)cycloprop-1-enyl)octanol (Xl). The tested materials (0.1 ng initially,
followed by 1~9) were applied to filter paper strips in 1~1 HPLC grade hexane. The
responses to these treatments were then compared with the responses of untreated three
day old virgin male moths without any chemicals present.
All experimental data were analysed using a two-way analysis of variance (ANOVA), then
by Duncan's multiple-range test when significant differences were found in the ANOVA.
As walking is less specifically associated with courtship, wing-fanning and circling were
chosen as behaviours to indicate a response to pheromone. The effect of treatment with
the analogue following a previous treatment with the pheromone is shown in Figure 10.
Wing-fanning and circling were reduced on addition of the analogue. When synthetic
pheromone treated moths were compared to analogue treated moths, significantly more
males exhibited circling (P<0.001) and wing-fanning (P<0.01) with the pheromone. The
wing-fanning response reduced by 96% from the pheromone to the analogue. The male
initially was attracted to the analogue treated arm and within the first four minutes there was
a great deal of movement which mainly involved walking and very little wing-fanning and
circling. The moths then more commonly moved away from it into a different section of the
chamber.
SllBSTlTUTE SHEET (RULE 26)
CA 02203636 1997-04-24
W O96/13162 PCTAN095/00198
17
Immediate reapplication of the pheromone after the atmosphere had been treated with the
analogue provided no restoration of wing-fanning or circling behaviour (P>0.05). This effect
is illustrated in Figure 11 and provides an initial indication that the analogue had bound
irreversibly to the same receptor site as the pheromone, and saturated the receptors and
possibly stopped them firing on the antennae.
I
The olfactometer experiments show that the average amount of time spent circling and
wing-fanning was greatest when the males were in the plume of the synthetic pheromone.
This was significantly longer than any of the other treatments. Activity was found to be
substantially less when the analogue was added, and at least when no chemical was
applied to the filter paper. The cyclopropene analogue had a dramatic effect, decreasing
the overall amount of time spent circling and wing-fanning and causing the insects to move
away from the analogue plume. However, a time-analysis of the results showed that in the
early stages of the experiment the insects were attracted towards the analogue plume and
showed considerable short-term activity including wing-fanning and circling. This suggests
that the analogue may be binding to and blocking the receptor site.
During the above experiments a solvent was used to place the pheromone analogues in the
test chambers. The solvent was used for practical reasons only and did not interfere with
the effect of the pheromone itself. When the pheromone analogues are used in practice for
controlling insect species they may be mixed with a carrier which can be a gas or solvent.
Said carrier shall not interfere with the effect of the pheromone analogue and shall not be
environmentally detrimental.
Thus according to the present invention it has been demonstrated that cyclopropene
analogues of natural pheromones applied to insects can be useful in controlling the mating
behaviour in insects. All the behavioural experiments, with the housefly, the diamondback
moth and the warehouse moth, showed that the respective cyclopropenated pheromone
analogues had a dramatic effect on the courtship behaviour of the males. The number of
mating strikes of the housefly and the circling and wing fanning, which are associated with
courtship of diamondback moth and warehouse moth, were decreased significantly. In the
case of diamondback moth and warehouse moth the analogues cause a response at the
antenna in the EAG studies. When the insects were treated with cyclopropene pheromone
analogue and after a while exposed to the natural sex pheromone, the courtship behaviour
STITUTE SHEET (RULE 26)
CA 02203636 1997-04-24
- W O96/13162 PCT~N09S/00198
18
was dramatically reduced by the first treatment and showed only a small retum to normal
values after the second treatment. The cyclopropene analogue therefore seem to be
targeting the same receptor site as the natural pheromone, and blocking it.
SU~SllT~TE SH~ET (RULE 26)