Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 0220441~ 1997-0~-02
WO 96/14300 1 ~ S/145g7
N-Aryl and N-Heteroaryl-1,2-D1am~nocyclobutene-3,4-dlones w~th smooth muscle
relaxlng act~v~tles
Back~round of Invention
The present invention relates to novel 1, 2-diamino derivatives of cyclobutene
3-4-diones having pharmacological activity, to a process for their preparation, to
pharmaceutical compositions containing them, and to their use in the treatment of
disorders associated with smooth muscle contraction; via potassium channel
modulation. Such disorders include, but are not limited to: urinary incontinence,
hypertension, asthma, premature labor, irritable bowel syndrome, congestive heart
failure, angina, and cerebral vascular disease.
Stemp et al. disclose a class of amino substituted cyclobutenedione derivatives
of chromans described as having blood pressure lowering activity and
bronchodilatory activity in EP-426379-A2. Several series of 1-amino-2-
phenylalkylamino-cyclobutene-3,4-diones are reported as H-2 receptor antagonists by
Algieri et al. in US Patent 4,390,701 and its numerous divisionals and CIPs. Several
related 1-amino-2-phenoxyalkylamino derivatives are disclosed by Nohara et al. in
US Patent 4,673,747.
The synthesis of representative 1,2-diamino-cyclobutene-3,4-diones are
described in the following publications: Tietze et al., Chem Ber. 1991, 124, 1215;
Tietze et al., Bioconjugate Chem. 1991, 2, 148; Ehrhardt et al., Chem. Ber. 1977,
110, 2506, and Neuse et al., Liebigs Ann. Chem. 1973, 619.
Description of The ~nvention
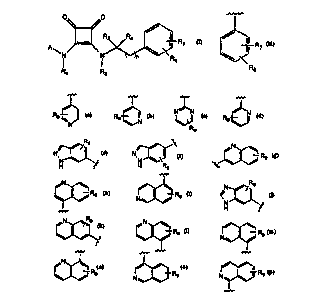
Accordingly, the present invention discloses compounds represented by the
30 formula (I):
CA 0220441=. 1997-0=.-02
W O96/14300 PCTnUS9S/14Sg7
0~, ~0
N ~ R R5
R 1 R2 (I)
whereln:
R1 is hydrogen, Cl 1o straight or branched chain alkyl, C3 10 cyclic
or bicyclic alkyl, alkanoyl of 2 to 7 carbon atoms,
alkylsulfonyl of 1 to 7 carbon atoms, aroyl of 7 to 12 carbon
atoms, arylalkenoyl of 9 to 20 carbon atoms, arylsulfonyl of 6
to 12 carbon atoms, arylalkanoyl of 8 to 12 carbon atoms or
arylalkylsulfonyl of 7 to 12 carbon atoms;
R2 is hydrogen, C1 1o straight or branched chain alkyl or
C3 10 cyclic or bicyclic alkyl;
A is a group of the following formula:
~r
l R7
~<
R8
wherein:
R7 and R8, independent from each other, are selected from the
following: cyano, nitro, amino, C1 6 alkyl,
CA 02204415 1997-05-02
WO g6/14300 PCl'lUSg5/14Sg7
C1 6perfluoroalkyl,C1 6alkoxy,C1 6perfluoroalkoxy,
amino, C1 12mono-ordialkylamino, sulfon~mi-le,
C1 6 alkylsulfon~mi~o, C6 12 arylsulfonamido,
C2 6 alkylcarbox~mi~o, C7 12 arylcarboxamido,
S C1 6 alkylsulfonyl, C 1-6 perfluoroalkylsulfonyl,
C6 12 arylsulfonyl, chloro, bromo, fluoro, iodo, 1-imi~1~7.01yl,
carboxyl, carboalkoxy of 2 to 7 carbon atoms, hydroxyl or
hydrogen;
or, A is Het where Het is selected from the following:
~N
Rg
H sSS H R9 ~ X
~r
Rg NlX Rg < ~
Rg N~ Rg
~--Rg N~--Rg N~ 9
whereln:
CA 0220441~ 1997-0~-02
Wo 96/14300 PCr/US95/14597
Rg is hydrogen, C1 6 alkyl, C1 6 perfluoroalkyl, C1 6 alkoxy,
C1 6 perfluoroalkoxy, amino, C1 12 mono- or dialkylarnino,
C1 6 alkylsulfonamido, C2 6 alkylcarboxamido, nitro, cyano,
carboxyl, chloro, bromo, fluoro, iodo;
s
n is an integer from 0 to 6;
R3 and R4 are, independent from each other, hydrogen, C1 1o straight
or branched chain alkyl, or C3 10 cyclic or bicyclic alkyl;
C1 1o perfluoro alkyl, C1 1o hydroxyalkyl, C2 l0 alkoxyalkyl,
fluoro; or, when taken together, form a spirocyclic ring
containing a total of 3-7 carbon atoms;
Rs and R6, independent from each other, are selected from the
following: cyano, nitro, amino, C1-6 alkyl,
C1 6perfluoroalkyl,C1 6alkoxy,C1 6perfluoroalkoxy,
amino, C1 12mono-ordialkylamino, sulfon~mide,
C1 6 alkylsulfonamido, C6 12 arylsulfonamido,
C2 6 alkylcarboxamido, C7 12 arylcarboxamido,
C1 6 alkylsulfonyl, C1 6 perfluoroalkylsulfonyl,
C6 12 arylsulfonyl, chloro, bromo, fluoro, iodo, 1-imid~7olyl,
carboxyl, C2-7 carboalkoxy, hydroxyl, or hydrogen;
or a pharmaceutically acceptable salt thereof.
A preferred aspect of this invention includes compounds of formula (I)
wherein:
Rl and R2 are as stated above;
A is a group of the following formula:
CA 02204415 1997-05-02
WO 96/14300 PCI~/US9S1145g7
~r
R7
R8
wherein:
R7 and Rg, independent from each other, are selected from the
S following: cyano, nitro, amino, chloro, bromo, fluoro, iodo, 1-imidazolyl, carboxyl, hydrogen;
or A is Het where Het is selected from the following:
Rg ~, ~3 9 ~N~ ~S5s
,~--Rg N~ Rg N~
~ ~--Rg
wherein:
R9 is as stated above;
n=O;
CA 0220441~ 1997-0~-02
WO g6/14300 PcrluS9Stl4S97
R3 and R4 are, independent from each other, hydrogen, C1 1o straight
or branched chain alkyl, Cl lo perfluoro alkyl,
C1 1o hydroxyalkyl or fluoro;
S Rs and R6, independent from each other, are selected from the
following: cyano, nitro, amino, C1 6 alkyl,
C1 6perfluoroalkyl,C1 6alkoxy,C1 6perfluoroalkoxy,
amino, chloro, bromo, fluoro, iodo, carboxyl,
C2 7 carboalkoxy, hydroxyl, hydrogen;
or a pharmaceutically acceptable salt thereof.
It is understood that the definition of the compounds of formula (I), when Rl
R2, R3, R4, Rs, or R6 contain asymmetric carbons, or when R3 is dirre~ent from R4,
lS encompass all possible stereoisomers and mixtures thereof. In particular, it
encompasses racemic modifications and optical isomers. The optical isomers may be
obtained in pure form by standard separation techniques. The pharmaceutically
acceptable salts are those derived from such organic and inorganic acids as: lactic,
citric, acetic, tartaric, succinic, maleic, malonic, hydrochloric, hydrobromic,
20 phosphoric, nitric, sulfuric, methanesulfonic, and similarly known acceptable acids.
Where R3, R4, Rs or R6 are carboxyl groups, salts of the compounds of this
invention may be formed with bases such as alkali metals (Na, K, Li) or the alkaline
earth metals (Ca or Mg).
The acyl groups representing R1 are derived from such acids as acetic,
propionic, butyric, valeric, caproic, methanesulfonic, ethanesulfonic, benzoic, toluic,
cinnamic, phenylsulfonic, phenylacetic, naphthylacetic, benzylsulfonic and the like.
Examples of alkyl as a group or part of a group, e.g. alkoxy, aryl are alkyl
groups of 1-4 carbon atoms such as methyl, ethyl, propyl and butyl.
The term aryl when used for a group or part of a group, e.g. arylalkyl, aroyl,
includes carbocyclic aromatic groups of 6 to 10 carbon atoms, e.g. phenyl and
naphthyl such as 1-naphthyl.
CA 0220441~ 1997-0~-02
WO 96/14300 Pcr/usgs/l4sg7
Examples of perfluoroalkyl group are such groups having 1-4 carbon atoms,
e.g. perfluoromethyl and perfluoroethyl.
The present invention also provides processes for the preparation of
S compounds of formula (I). Accordingly, this invention provides a process for
preparing a co.llpound of formula I which comprises:
a) reacting a compound of the formula:
0~
Rl~ X
A (II)
wherein X is a leaving group and A and R I are as defined above, with a compound of
15 formula:
H IN~ R R5
R2 (m)
wherein n and R2 6 are as defined above to give a compound of formula I; or
b) reacting a compound of formula I wherein Rl is hydrogen with an
alkylating or acylating agent containing Rl where Rl is as defined above expecting
hydrogen in the presence of a base to give a compound of formula I where Rl is other
than hydrogen; or
CA 0220441~ 1997-0~-02
WO g6/~4300 ~ tl4Sg7
c) converting an acidic compound of formula I wherein at least one of Rs-Rg
is a carboxy group to a salt with a base, e.g. alkali metal, alkaline earth metal or
optionally substituted ammonium salt; or
d) converting a basic compound of formula I to a pharmaceutically
acceptable acid addition salt; or
e) isolating an optically active isomer of a compound of formula I from a
~l~ibc~ure of isomers; or
f) reacting a compound of formula I having a reactive site or substituent
group to give a different compound of formula I.
Conveniently the compounds of formula I may be plel)ared by reacting a
compound of formula II.
As mentioned previously, the compounds of formula (I) have been found to
relax smooth muscle. They are therefore useful in the treatment of disorders
associated with smooth muscle contraction, disorders involving excessive smooth
muscle contraction of the urinary tract (such as incontinence), or of the gastro-
intestinal tract (such as irritable bowel syndrome), asthma, and hair loss.
Furthermore, the compounds of formula (I) are active as potassium channel activators
which render them useful for treatment of peripheral vascular disease, congestive
heart failure, stroke, anxiety, cerebral anoxia and other neurodegenerative disorders.
The present invention accordingly provides a pharmaceutical composition
which comprises a compound of this invention and a pharmaceutically acceptable
carrier. In particular, the present invention provides a pharmaceutical composition
which comprises an effective amount of a compound of this invention and a
pharmaceutically acceptable carrier.
The compositions are preferably adapted for oral ~Aministration. However,
they may be adapted for other modes of a~ministration, for example parenteral
~tlmini~tration for patients suffering from heart failure.
CA 0220441~ 1997-0~-02
WO g6/14300 1 ~ ,3~ 4S97
In order to obtain consistency of admini~tration, it is preferred that a
composition of the invention is in the form of a unit dose. Suitable unit dose forms
~ include tablets, capsules and powders in sachets or vials. Such unit dose forms may
contain from 0.1 to 100 mg of a compound of the invention and preferably from 2 to
5 50 mg. Still further preferred unit dosage forms contain S to 25 mg of a compound of
the present invention. The compounds of the present invention can be a~lmini.ctered
orally at a dose range of about 0.01 to 100 mg/kg or preferably at a dose range of 0.1
to 10 mg/kg. Such compositions may be aclministered from 1 to 6 times a day, more
usually from 1 to 4 times a day.
The compositions of the invention may be formulated with conventional
excipients, such as a filler, a disintegrating agent, a binder, a lubricant, a flavoring
agent and the like. They are formnl~ted in conventional manner, for example, in a
manner similar to that used for known antihypertensive agents, diuretics and ,~-15 blocking agents.
The present invention further provides a compound of the invention for use as
an active therapeutic substance. Compounds of formula (I) are of particular use in
inducing smooth muscle relaxation.
The present invention further provides a method of treating smooth muscle
disorders in m~mm~ls including man, which comprises ~lmini~tering to the afflicted
m~mm~l an effective amount of a compound or a pharmaceutical composition of the
mventlon.
- The following examples are presented to illustrate rather than limit the
methods for production of representative compounds of the invention.
.
FXAMP~,F 1
4-r3.4-Dioxo-2-((R)-l-Dhenvl-ethylamino)-cvclobut-l-envlaminol-benzonitrile
Step 1) Preparation of 4-(3,4-Dioxo-2-ethoxy-cyclobut-1-enylamino)-
benzonitrile
CA 02204415 1997-0~-02
WO g6/14300 P~ ,9~114S97
- 10-
4-Aminobenzonitrile (3.47 g, 29.4 mmol) was added to a solution of 3,4-
diethoxy-3-cyclobutene-1, 2-dione (5.00 g, 29.4 mmol) in absoh)te ethanol (100 mL).
The Illixlule was heated at reflux overnight. The mixture was cooled, and the
resul~ing yellow pleci~ilate was collected by vacuum filtration. Yield: 2.60 g (37%):
5 mp 218-222~C; IH NMR (DMSO-d6): ~ 11.07 (s, lH), 7.81 (d, 2H), 7.56 (d, 2H),
4.79 (q, 2H), 1.46 (t, 3H).
Step 2) P~p~tion of 4-[3,4-dioxo-2-((R)-1-phenyl-ethylamino)-
cyclobut- 1 -enylamino]-benzonitrile
To the above squarate (0.50 g, 2.06 mmol) in ethanol (10 mL) was added (R)-
a-methylbenzylamine (0.27 mL, 2.1 mmol). The l~lixlule was heated at reflux for 16
hours and vacuum filtered. The precipitate was recryst~lli7e~1 from methanol to
afford 0.17 g (26%) of product as a pale yellow solid: mp 273-274~C; [a]25D -53.20
(DMSO); IH NMR (DMSO-d6): ~ 9.91 (s, lH), 8.21 (d, lH), 7.72 (d, lH), 7.79-7.31
(m, 9H), 5.29 (m, lH), 1.59 (d, 3H). IR (KBr): 3200, 2230, 1790, 1670, 1600 cm-l;
MS (m/z) 317 (M+).
Elemental analysis for ClgHlsN3O2
Calc'd: C, 71.91; H, 4.76; N, 13.24.
Found: C, 71.26; H, 4.86; N, 13.49.
EXAMPLE 2
3-(5-Bromo-Dvridin-3-vlamino~-4-((R)-1-Dhenvl-ethvlamino)-
cvclobut-3-ene- 1 .2-dione
Step 1) Preparation of 3-(5-bromo-pyridin-3-ylamino)-4-ethoxy-cyclobut-
3-ene- 1 ,2-dione)
3-Amino-5-bromopyridine (1.92 g, 11.3 mmol) was added to a solution of
3,4-diethoxy-3-cyclobutene-1,2-dione (2.24 g, 11.1 mmol) in absolute ethanol (30mL). The mixture was heated at reflux for 18 hours, cooled and filtered. The filtrate
was concentrated and the resulting residue chromatographed (CH3OH/CH2Cl2) to
CA 0220441~ 1997-0~-02
WO 96/14300 1 ~,-1/I)i~9~ 4Sg7
afford 2.06 g (62%) of product as an off-white solid: lH NMR (DMSO-d6): â 11.00
(s, lH), 8.56 (s, lH), 8.42 (s, lH), 8.09 (s, lH), 4.79 (q, 2H), 1.42 (t, 3H).
Step 2) Preparation of 3-(5-Bromo-pyridin-3-ylamino)-4-((R)-l-phenyl-
ethylamino)-cyclobut-3-ene- 1,2-dione
To the above squarate (0.815 g, 2.74 mmol) in ethanol (25 mL) was added
(R)-a-methylbenzylamine (0.36 mL, 2.8 mmol). The mi~cLule was heated at reflux for
23 hours. The precipitate was filtered off and rinsed with ethanol to afford 0.92 g
(90%) of product as an off-white solid: mp 268-271~C (dec); [~]25D +6.57 (DMSO);H NMR (DMSO-d6): ~ 9.85 (s, lH), 8.44-8.15 (m, 4H), 7.43-7.27 (m, 5H), 5.29 (m,
lH), 1.59 (d, 3H). IR (KBr): 3200, 1790, 1670, 1590 cm-l; MS (m/z) 372 (MH+).
F.le,..~ l analysis for Cl7HI4BrN3O2
Calc'd: C, 54.86; H, 3.79; N, 11.29.
Found: C, 54.88; H, 3.67; N, 11.20.
FXAMPI.E 3
3-(2-Methoxy-S-tri~luoromethvl-~henylamino)-4-((R)-l-Dhenvl-
ethvlamino)-cvclobut-3-ene-1.2-dione
Step 1) Preparation of 3-ethoxy-4-(2-methoxy-5-trifluoromethyl-
phenylamino)-cyclobut-3-ene- 1,2-dione
2-Methoxy-5-trifluoromethylaniline (5.62 g, 29.4 mmol) was added to a
solution of 3,4-diethoxy-3-cyclobutene-1,2-dione (5.00 g, 29.4 mmol) in absoluteethanol (100 mL). The IlliXIUl~ was heated at reflux for 66 hour, cooled and filtered.
The plccipilate was purified by chromatography (CH30H/CH2CI2) to afford 1.88 g
(20%) of product as a yellow solid: lH NMR (DMSO-d6): ~ 10.42 (s, lH), 7.64-
7.20 (m, 3H), 4.69 (q, 2H), 3.90 (s, 3H), 1.34 (t, 3H).
Step 2) Preparation of 3-(2-methoxy-5-trifluoromethyl-phenylamino)-4-
((R)- 1 -phenyl-ethylamine)-cyclobut-3-ene- 1,2-dione
CA 0220441~ 1997-0~-02
WO 96tl4300 ~ g~/14S97
To the above squarate (0.806 g, 2.56 mmol) in ethanol (10 rnL) was added
(R)-a-methylbenzylamine (0.33 mL, 2.6 mmol). The ~ e was heated at reflux for
23 hours. The clear yellow solution was concentrated and the res-llhng foarn purified
by chromatography (CH3OH/CH2Cl2)to afford 0.84 g (84%) of product as a white
solid: mp 115-124~C; [a]25D -33.61 (DMSO); lH NMR (DMSO-d6): ~ 9.37 (s,
lH), 8.72 (d, lH), 8.24 (s, lH), 7.42-7.19 (m, 7H), 5.33 (m, lH), 3.97 (s, lH), 1.59 (d,
3H). IR (KBr): 3250, 1790, 1690, 1610 cm~l; MS (m/z) 391 (MH+).
F.lemen~l analysis for C2oHl7F3N2o3
Calc'd: C, 61.54; H, 4.39; N, 7.18.
Found: C, 61.42; H, 4.26; N, 7.23.
FXAMPLE 4
3-((R~-l-Phenyl-ethvlamino)-4-~Dyridin-4-vlamino)-cvclobut-3-ene-1.~-dione
Step 1) Preparation of 3-ethoxy-4-(pyridin-4-ylamino)-cyclobut-3-ene-
1,2-dione
To a solution of 3,4-diethoxy-3-cyclobutene- 1,2-dione (5.00 g, 29.4 mmol) in
ethanol (100 mL) was added a suspension of 4-aminopyridine (2.77 g, 29.4 mmol) in
ethanol (50 mL). The reaction mixture was heated at reflux for 4 hours.
Concentration and chromatography (EtOAc) of the resulting residue afforded 0.632 g
(10%) of product as a white solid: lH NMR (DMSO-d6): ~ 11.18 (br s, lH), 8.45
(d, 2H), 7.40 (d, 2H), 4.80 (q, 2H), 1.43 (t, 3H).
Step 2) Preparation of 3-((R)-l-phenyl-ethylamino)-4-(pyridin-4-ylamino)-
cyclobut-3-ene- 1,2-dione
To the above squarate (0.850 g, 3.90 mmol) in ethanol (25 rnL) was added
(R)-~-methylbenzylamine (0.51 mL, 4.0 mmol). The mixture was heated at reflux for
23 hours. The precipitate was filtered off and rinsed with ethanol. Chromatography
(CH3OH/CH2Cl2) afforded 0.276 g (24%) of product as an off-white solid: mp 252-
254~C (dec); [a]25D -31.45 (DMSO); IH NMR (DMSO-d6): ~ 9.82 (s, lH), 8.40 (d,
CA 0220441~ 1997-0~-02
WO g6/14300 rcr/usssll4ss7
2H), 8.22 (d, lH), 7.45-7.38 (m, 7H), 5.28 (m, lH), 1.59 (d, 3H). IR (KBr): 3200,
1800, 1675, 1590 cm-l; MS (m/z) 293 (MH+).
F~ f l~t~l analysis for C17H15N3O2
Calc'd: C, 69.61; H, 5.15; N, 14.33.
Found: C, 69.49; H, 5.06; N, 14.18.
EXAMPLE 5
10 4-r3.4-Dioxo-2-(~S)-l-Dhenvl-ethylamino)-cvclobut-l-enyl~minol-benzonitrile
To the squarate of Example 1, step 1 (0.50 g, 2.06 mmol) in ethanol (10 rnL)
was added (S)-a-methylbenzylamine (0.27 mL, 2.1 mmol). The mixture was heated
at reflux for 16 hours and vacuum filtered. The precipitate was recrystallized from
methanol to afford 0.17 g (26%) of product as a pale yellow solid: mp 269-270~C;[a]25D +46.47 (DMSO); IH NMR (DMSO-d6): ~ 9.91 (s, lH), 8.21 (d, lH), 7.72 (d,
lH), 7.79-7.31 (m, 9H), 5.29 (m, lH), 1.59 (d, 3H). IR (KBr): 3200, 2230, 1790,
1670, 1600 cm-l; MS (m/z) 317 (M+).
20 Elemental analysis for Cl9HlsN3o2
Calc'd: C, 71.91; H, 4.76; N, 13.24.
Found: C, 71.17; H, 4.83; N, 13.34.
EXAMPLF. 6
3-(4-Tritluoromethoxv-~henvlamino)-4-((R~(-)-1 -~henvl-
ethvlamino)-cvclobut-3-ene-1.2-dione
Step 1~ Preparation of 3-ethoxy-4-(4-trifluoromethoxy-phenylamino)-
cyclobut-3-ene-1,2-dione
4-Trifluoromethoxyaniline (5.00 g, 28.2 mmol) was added to a solution of
3,4-diethoxy-3-cyclobutene-1,2-dione (5.00 g, 29.4 mmol) in absolute ethanol (50mL). The mixture was heated at reflux overnight, then vacuum filtered hot. The
35 filtrate was reduced in volume and the resulting precipitate was filtered to afford 4.50
CA 02204415 1997-0~-02
Wo 96/14300 PCrlUS9S/14S97
- 14 -
g (53%) of white solid: mp 145-146~C; lH NMR (DMSO-d6): ~ 10.87 (s, lH),
7.45 (d, 2H), 7.36 (d, 2H), 4.75 (q, 2H), 1.41 (t, 3H).
Step 2) Preparation of 3-(4-trifluo~ eLhoxy-phenylamino)-4-
S ((R)- 1 -phenyl-ethylamine)-cyclobut-3-ene- 1,2-dione
To the above squarate (0.330 g, 1.10 mmol) in ethanol (25 rnL) was added
(R)-a-methylbenzylamine (0.135 mg, 1.11 mmol). The reaction was refluxed for 23
hours. Upon cooling, the product precipitated as a white solid 0.350g (84%): mp
258-260~C; [~]25D -17.52 (DMSO); lH NMR (DMSO-d6): ~ 9.70 (s, lH), 8.12 (d,
lH), 7.51-7.28 (m, 9H), 5.28 (m, lH), 1.59 (d, 3H). IR (KBr): 3250, 1800, 1675,
1600 cm-l; MS (m/z) 377 (MH+).
Elemental analysis for C1gHlsF3N2O3
lS Calc'd: C, 60.64; H, 4.02; N, 7.44.
Found: C, 60.40; H, 4.01; N, 7.21.
FXAMPLE 7
(R)-4-r2-rl-(4-Nitro-Dhenyl~-ethylaminol-3.4-dioxo-
cvclobut-l-envlamino~-ben70nitrile
To the squarate of Example 1, step 1 (0.598 g, 2.47 mmol) in ethanol (S0 mL)
was added (R)-a-methyl-4-nitrobenzylamine hydrochloride (O.S0 g, 2.5 mmol) and
N,N-diisopropylethylamine (0.43 g, 2.5 mmol). The Illi~C~UlC was heated at reflux for
16 hours. After cooling the precipitate was filtered off to afford 0.70 g (78%) of
product as an orange solid: mp 290-295~C; [~]25D -100.52 (DMSO); lH NMR
(DMSO-d6): ~ 9.98 (s, lH), 8.32 (d, lH), 8.25 (d, 2H), 7.79 (d, 2H), 7.68 (d, 2H),
7.57 (d, 2H), 5.42 (m, lH), 1.61 (d, 3H). IR (KBr): 3200, 2220, 1790, 1670, 160030 cm-l; MS (m/z) 362 (M+).
Elemental analysis for ClgHI4N4O4
Calc'd: C, 62.98; H, 3.89; N, 15.46.
Found: C, 62.38; H, 3.73; N, 14.95.
CA 0220441~ 1997-0~-02
WO g6/14300 PCr/US9S/14S97
F.XAMP~ F 8
3-r3.4-Dioxo-2-((R)-l-~henvl-ethyl~mi~lo)-cvclob~t-l-
envl~minol-benzonitrile
Step 1) Preparation of 3-(3,4-dioxo-2-ethoxy-cyclobut-1-enylamino)-
benzonitrile
3-Aminobenzonitrile (2.06 g, 17.4 mmol) was added to a solution of 3,4-
diethoxy-3-cyclobutene-1,2-dione (2.97 g, 17.5 mmol) in absolute ethanol (S0 mL).
The mixture was heated at reflux overnight. The mixture was cooled and the
resulting yellow precipitate was collected by vacuum filtration. Yield: 3.40 g (81%):
lH NMR (DMSO-d6): ~ 10.95 (s, lH), 7.75-7.40 (m, 4H), 4.73 (q, 2H), 1.39 (t, 3H).
~S Step 2) Preparation of 3-[3,4-dioxo-2-((R)-1-phenyl-ethylamino)-
cyclobut- 1 -enylamino]-benzonitrile
To the above squarate (1.00 g, 4.13 mmol) in ethanol (100 mL) was added
(R)-a-methylbenzylamine (0.53 mL, 4.1 mmol). The mixture was heated at reflux
20 overnight and vacuum filtered. The precipitate was triturated twice with hot methanol
to afford 0.80 g (61%) of product as a pale yellow solid: mp 289-290~C (dec);
[a]25D-13.9 (DMSO); lH NMR (DMSO-d6): ~ 9.72 (s, lH), 8.25 (d, lH), 7.90-7.28
(m, 9H), 5.30 (m, lH), 1.60 (d, 3H). IR (KBr): 3200, 2220, 1790, 1650, 1600 cm-l;
MS (m/z) 317 (M+).
Elemental analysis for Cl9HlsN3o2
Calc'd: C, 71.91; H, 4.76; N, 13.24.
Found: C, 71.80; H, 4.61; N, 13.33.
CA 0220441~ 1997-05-02
Wo 96/14300 PcrluS95114S97
- 16-
FXAMP~ F 9
4-r3.4-Dioxo-2-~1 -methvl-l-~henyl-etb,vl~mino)-
cvclobut-l-enyl~minol-bem- nitrile
s
To the squarate of Example 1, step 1 (0.81 g, 3.3 mmol) in ethanol (30 mL)
was added a,a-dimethylbenzylamine (0.45 g, 3.3 mmol). The ll~ Ul~ was heated at
reflux for 20 hours. After cooling the precipitate was filtered off and
chromatographed (CH3OH/CH2CI2) to afford 0.41 g (37%) of product as yellow
solid: mp >300~C; lH NMR (DMSO-d6): ~ 10.08 (s, lH), 8.38 (s, lH), 7.79 (d,
2H), 7.61 (d, 2H), 7.48-7.27 (m, SH), 1.78 (s, 6H). IR (KBr): 3200, 2230, 1790,
1675, 1600 cm-l; MS (m/z) 331 (M+).
Elemental analysis for C2oHl7N3o2.(o. l CH30H) (0 05 CH2Cl2)
Calc'd: C, 71.43; H, 5.21; N, 12.40.
Found: C, 71.30; H, 5.33; N, 12.69.
EXAMP~ F 10
4-r3~4-Dioxo-2-((R)-1-Dhenvl-Dropvlamino~-
cvclobut- I -enylaminol-ben~onitrile
To the squarate of Example 1, step 1 (1.79 g, 7.39 mmol) in ethanol (30 rnL)
was added (R)-1-phenyl-propylamine (1.00 g, 7.40 mmol). The mixture was heated
at reflux for 18 hours, cooled slightly and vacuum filtered to afford 1.76 g (72%) of
product as a yellow solid: mp 242-243~C; I~]25D -52.73 (DMSO); lH NMR
(DMSO-d6): ~ 9.84 (s, lH), 8.12 (br d, lH), 7.76 (d, 2H), 7.S6 (d, 2H), 7.42-7.27 (m,
5H), 5.06 (m, lH), 1.94 (m, 2H), 0.90 (t, 3H). IR (KBr): 3200, 2220, 1790, 1670,1600 cm-l; MS (m/z) 331 (M+).
Elemental analysis for C20H17N3~2
Calc'd: C, 72.49; H, 5.17; N, 12.68.
Found: C, 72.42; H, S.01; N, 12.73.
CA 0220441~ 1997-0~-02
Wo 96/14300 PCrlUS9S/14S97
FXAMPI,F 11
4-r3.4-Dioxo-2.f(S)-l.Dhen~l.Dro~yla~n;no).
cvclob--t-l-e~ minol-benzonitrile
s
To the squarate of Example 1, step 1 (1.79 g, 7.39 mmol) in ethanol (30 mL)
was added (S)- 1 -phenyl-propylamine (1.00 g, 7.40 mmol). The ~ ul~ was heated at
reflux for 18 hours, cooled slightly and vacuum filtered to afford 1.61 g (66%) of
product as a yellow solid: mp 241-243~C; [cc]25D +52.33 (DMSO); lH NMR
(DMSO-d6): ~ 9.84 (s, lH), 8.21 (br d, lH), 7.76 (d, 2H), 7.56 (d, 2H), 7.42-7.27 (m,
5H), 5.06 (m, lH), 1.94 (m, 2H), 0.90 (t, 3H). IR (KBr): 3200, 2220, 1790, 1670,1600 cm-l; MS (m/z) 331 (M+).
Elemental analysis for C2oHl7N3o2
Calc'd: C, 72.49; H, 5.17; N, 12.68.
Found: C, 72.17; H, 5.04; N, 12.80.
EXAMPLE 12
4-r3.4-Dioxo-2-(benzylamino)-cyclobut-1-envlaminol-benzonitrile
To the squarate of Example 1, step 1 (1.00 g, 4.13 mmol) in ethanol (30 mL)
was added benzylamine (0.45 mL, 4.1 mmol). The ~ ule was heated at reflux for
18 hours, cooled slightly and vacuum filtered. The precipitate was triturated with hot
methanol to afford 0.78 g (62%) of product as yellow solid: mp 288-290~C (dec); lH
NMR (DMSO-d6): ~ 9.91 (s, lH), 8.10 (m, lH), 7.79 (d, 2H), 7.75 (d, 2H), 7.55 (d,
2H), 7.91-7.78 (m, SH), 4.82 (d, 2H). IR (KBr): 3190, 2220, 1790, 1660, 1575 cm~l;
MS (m/z) 303 (M+).
Elemental analysis for C18Hl3N3~2
Calc'd: C, 71.28; H, 4.32; N, 13.85.
Found: C, 71.07; H, 4.16; N, 12.89.
CA 0220441~ 1997-0~-02
Wo g6/14300 PCTIUS9S/14S97
- 18-
FXAMP~ F. 13
(R)-4-r2-rl-(4-Methvl-Dhe~ ethvl~lninol-3.4-dioxo-cvclobl-t-
I -enylamino~-be~2~"~ilrile
s
To the squarate of Example 1, step 1 (1.00 g, 4.13 mmol) in ethanol (30 mL)
was added (R)-1-~D -tolyl)-ethylamine (0.56 g, 4.1 mmol). The lllixlulc was heated at
reflux for 18 hours, cooled slightly and vacuum filtered to afford 1.02 g (75%) of
product as a yellow solid: mp >300~C; [a]25D -56.01 (DMSO); lH NMR (DMSO-
d6): ~ 9.81 (s, lH), 8.10 (m, lH), 7.76 (d, 2H), 7.55 (d, 2H), 7.29 (d, 2H), 7.19 (d,
2H), 5.26 (m, lH), 1.57 (d, 3H). IR (KBr): 3200, 2220, 1790, 1670, 1600 cm-1; MS(m/z) 331 (M+).
Elemental analysis for C20HI7N3~2
Calc'd: C, 72.49; H, 5.17; N, 12.68.
Found: C, 72.42; H, 5.07; N, 12.82.
EXAMPLE 14
(R)-4-r2-rl-(4-Methoxv-~henvl)-ethylaminol-3.4-dioxo-cvclobut-
I -en vlamino~-benzonitrile
To a solution of (lR, l'R)-N-(1'-phenylethyl)-1-(4"-methoxyphenyl)-
ethylamine (1.37 g, 5.36 mmol; prepared as in J. Med Chem. 1992, 35, 2327) and
amrnonium formate (1.01 g, 16.0 mmol) in methanol (125 mL) was added 10%
palladium on activated carbon. The suspension was refluxed for 2 h, filtered through
Celite and concentrated. The squarate of Example 1, step 1 (1.00 g, 4.13 mmol) was
added to a solution of the resulting residue in ethanol (30 mL). The mixture washeated at reflux for 18 h, cooled slightly and vacuum filtered. The precipitate was
chromatographed (CH3OH/CH2CI2) and recrystallized (CH3OH/CH2CI2) to afford
0.21 g (15%) of product as a yellow solid: mp >300~C; [a]25D -46.95 (DMSO); lH
NMR (DMSO-d6): ~ 9.89 (s, lH), 8.13 (d, lH), 7.77 (d, 2H), 7.56 (d, 2H), 7.34 (d,
2H), 6.95 (d, 2H), 5.23 (m, lH), 3.73 (s, 3H), 1.57 (d, 3H). IR (KBr): 3200, 2200,
1800, 1670, 1575 cm-l; MS (mlz) 347 (M+).
CA 0220441~ 1997-0~-02
Wo 96/14300 PCr/US95/14S97
- 19-
Fl~m~nt~l analysis for C2~H17N303.(0.03 CH2Cl2)
Calc'd: C, 68.75; H, 4.91; N, 12.01.
Found: C, 68.40; H, 4.74; N, 11.89.
FXAMPT,F 15
(R~-4-r3.4-Dioxo-2-r1-f4-trifluoromethoxv-Dbenyl)-ethyl~minol-
-cyclobut-1 -envlamino~-ben~onitrile
To a solution of (lR, l'R)-N-(1'-phenylethyl)-1-(4"-trifluoromethoxyphenyl)-
ethylamine (1.92 g, 6.21 mmol; prepared as in J. Med Chem. 1992, 35, 2327) and
ammonium formate (1.17 g, 18.6 mmol) in methanol (150 mL) was added 10%
palladium on activated carbon. The suspension was refluxed for 2 h, filtered through
Celite and concentrated. The squarate of Example 1, step 1 (1.00 g, 4.13 mmol) was
added to a solution of the resulting residue in ethanol (35 mL). The mixture washeated at reflux for 18 h, cooled slightly and vacuum filtere~. The precipitate was
combined with a second crop of solid obtained from the cooled filtrate,
chromatographed (CH3OH/CH2CI2) and recrystallized (CH3OH/CH2CI2) to afford
0.74 g (45%) of product as a white solid: mp 281-284~C (dec); [(x]25D -55-94
(DMSO); lH NMR (DMSO-d6): ~ 9.94 (s, lH), 8.22 (d, lH), 7.78 (d, 2H), 7.60-7.51
(m, 4H), 7.40 (d, 2H), 5.33 (m, lH), 1.60 (d, 3H). IR (KBr): 3200, 2200, 1800, 1670,
1560 cm~l; MS (m/z) 401 (M+).
Elemental analysis for C20Hl4F3N3O3
Calc'd: C, 59.85; H, 3.52; N, 10.47.
Found: C, 59.94; H, 3.38; N, 10.43.
EXAMPLE 16
4-r3.4-Dioxo-2-(2~2~2-trifluoro-1-phenvl-ethvlamino)-
cvclobut- 1 -envlaminol-ben~onitrile
To a solution of N-2,2,2-trifluoro-1-phenylethyl-N-1'-(phenyl)ethylamine
(1.65 g, 6.22 mmol; prepared as in J. Org. Chem. 1977, 42, 2436) and ammonium
forrnate (1.17 g, 18.6 mmol) in methanol (150 mL) was added 10% palladium on
CA 0220441~ 1997-0~-02
Wo 96114300 PCrlUS9S/14Sg7
- 20-
activated carbon. The suspension was refluxed for 4 h, filtered through Celite and
conce~ ted to a volume of ~ llately 10 mL. The squarate of Example 1, step 1
(1.00 g, 4.13 mmol) and ethanol (20 mL) were added and the ll~ e was heated atreflux for 18 h, cooled slightly and vacuum f1ltered to remove a small amount of5 solid. The filtrate was chromatographed (CH3OH/CH2Cl2) and the resulting yellow
solid cryst~lli7erl from chlorofc.llll and ether to afford 0.72 g (47%) of product as a
pale yellow solid: mp 206-207~C; IH NMR (DMSO-d6): 8 9.99 (s, lH), 8.88 (d,
lH), 7.82 (d, 2H), 7.60-7.46 (m, 9H), 5.98 (m, lH), 3.73 (s, 3H). IR (KBr): 3200,
2200, 1800, 1690, 1570 cm~l; MS (m/z) 371 (M+).
Elemental analysis for C19HI2F3N3~2
Calc'd: C, 61.46; H, 3.26; N, 11.32.
Found: C, 61.26; H, 3.16; N, 11.23.
lS EXAMPLE 17
~R~-4-r3~4-Dioxo-2-(1-Dhenvl-ethvlamino)-cvclobut-l-envlaminol-
3-meth vl-ben~onitrile
Step 1) Preparation of 4-(3,4-Dioxo-2-ethoxy-cyclobut-1-enylamino)-3-
methylbenzonitrile
4-Amino-3-methylbenzonitrile (1.94 g, 14.7 mmol) was added to a solution
of 3,4-diethoxy-3-cyclobutene-1,2-dione (2.53 g, 14.9 mmol) in acetonitrile (S mL).
After refluxing the mixture for 24 h a second portion of 3,4-diethoxy-3-cyclobutene-
1,2-dione (l.lS g, 6.76 mmol) was added and heating was continued for an additional
48 h. The reaction mixture was diluted with ethyl acetate (50 mL), stirred vigorously
and filtered free of undissolved solid. The filtrate was chromatographed
(CH3OH/CH2Cl2) to afford 0.90 g (24%) of product as a yellow solid: lH NMR
(DMSO-d6): ~ 10.50 (s, lH), 7.76-7.63 (m, 2H), 7.31 (d, lH), 4.71 (q, 2H), 2.33 (s,
3H), 1.38 (t, 3H).
Step 2) Preparation of (R)-4-[3,4-Dioxo-2-(1-phenyl-ethylamino)-cyclobut-1-
enylamino] -3-methyl-benzonitrile
CA 0220441~ 1997-0~-02
WO g6/14300 PCrlUS95114Sg7
- 21 -
To the above squarate (0.90 g, 3.51 mmol) in ethanol (40 mL) was added (R)-
a-methylbenzylamine (0.45 mL, 3.49 mmol). The mixture was heated at reflux for
18 h. The resulting clear solution solution was concentrated and the residue
chromatographed (CH30H/CH2Cl2) to afford 0.98 g (85%) of product as a yellow
S solid: mp 110-130~C (dec); [a]25D -40.91 (DMSO); lH NMR (DMSO-d6): ~ 8.95
(s, lH), 8.58 (d, 2H), 7.70-7.28 (m, 8H), 5.36 (m, lH), 2.33 (s, 3H), 1.61 (d, 3H). IR
(KBr): 3250, 2220, 1790, 1690, 1590 cm-l; MS (m/z) 332 (MH+).
Elemental analysis for C2oHl7N3o2.(o.lo CH2Cl2)
Calc'd: C, 71.03; H, 5.10; N, 12.36.
Found: C, 71.37; H, 5.09; N, 12.61.
FXAMPLE 18
(R)-4-r3.4-Dioxo-2-(1-Dhenvl-ethylamino)-cvclobut-l-envlaminol-
3-ethvl-ben~onitrile
Step 1) Prepalation of 4-(3,4-Dioxo-2-ethoxy-cyclobut-1-enylamino)-3-ethyl- benzonitrile
4-Amino-3-ethylbenzonitrile (2.00 g, 13.7 mmol) was added to a solution of
3,4-diethoxy-3-cyclobutene-1,2-dione (2.30 g, 13.5 mmol) in acetonitrile (5 mL).After refluxing the mixture for 24 h a second portion of 3,4-diethoxy-3-cyclobutene-
1,2-dione (1.15 g, 6.76 mmol) was added and heating was continued for an additional
25 24 h. The reaction mixture was diluted with ethyl acetate (45 mL), stirred vigorously
and filtered free of undissolved solid. The filtrate was concentrated and the resulting
residue was purified by chromatographed (CH3OH/CH2CI2) and trituration with ether
to afford 0.86 g (24%) of product as a light yellow solid: IH NMR (DMSO-d6):
10.57 (s, lH), 7.77-7.66 (m, 2H), 7.31 (d, lH), 4.71 (q, 2H), 2.73 (q, 2H), 1.37 (t,
3H), 1.13 (t, 3H).
Step 2) Preparation of (R)-4-[3,4-Dioxo-2-(1-phenyl-ethylamino)-cyclobut-1-
enylamino] -3-ethyl-benzonitrile
CA 0220441~ 1997-0~-02
WO g6/14300 1 ~ 4S97
To the above squarate (0.85 g, 3.14 mmol) in ethanol (25 mL) was added (R)-
oc-methylbenzylamine (0.41 mL, 3.18 mmol). The l~lixlure was heated at reflux for
18 h, cooled slightly and suction filtered. The filtrate was cooled by gradual
evapora~on of solvent and the preçipit~t~ which formed was collected in two crops to
afford 0.76 g (70%) of product as an off-white solid: mp 206-207~C (dec); [a]25D-45.25 (DMSO); lH NMR (DMSO-d6): ~ 9.98 (s, lH), 8.55 (d, 2H), 7.68-7.29 (m,
8H), 5.37 (m, lH), 2.69 (q, 2H), 1.61 (d, 3H), 1.20 (t, 3H). IR (KBr): 3200, 2200,
1800, 1670, 1570 cm-l; MS (m/z) 345 (M+).
10 Flemsnt~l analysis for C21HIgN3O2
Calc'd: C, 73.03; H, 5.54; N, 12.17.
Found: C, 72.69; H, 5.52; N, 12.18.
F.XAMPLE 19
(R)-N-(4-Cvano-Dhenvl)-N-r3.4-dioxo-2~ henvl-ethvlamino)-
cvclobut- 1 -en vll-acetamide
To a stirred solution of the squarate of Example 1, step 2 (1.77 g, 5.58 mmol)
in N,N-dimethylfonn~mi~e (50 rnL) was added, in one portion, sodium hydride (as a
60% dispersion in mineral oil; 0.252 g, 6.30 mmol). The frothy suspension was
stirred at rt for 15 min and then at 0~C for an additional 1 h. Acetic anhydride (0.58
mL, 6.15 mmol) was added and the reaction mixture was stirred at 0~C for 1.5 h and
then allowed to warm to rt. After an additional 1 h of stirring the reaction solution
was concentrated. The resulting yellow solid was washed with succes~ive portions of
acetone, methylene chloride and ethyl acetate. The combined washings were
concentrated and the resulting residue chromatographed to afford 0.48 g (24%) ofproduct as an off-white solid: mp 240-243~C; [a]25D -94.66 (DMSO); lH NMR
(DMSO-d6): ~ 8.29 (d, lH), 7.96 (d, 2H), 7.69 (d, 2H), 7.45-7.25 (m, 5H), 5.49 (m,
lH), 2.06 (s, 3H), 1.59 (d, 3H). IR (KBr): 3340, 2230, 1800, 1740, 1690, 1610 cm-l;
MS (m/z) 359 (M+).
Elemental analysis for C2lHl7N3o3.(o.os CH2Cl2)
Calc'd: C, 69.53; H, 4.74; N, 11.56.
Found: C, 69.16; H, 4.74; N, 11.53.
CA 0220441~ 1997-0~-02
Wo 96/14300 Pcrlusssll4ss7
- 23 -
Smooth muscle relaxing activity of the compounds of this invention was
established in accordance with standard pharmaceutic~lly accepted test procedures in
se-nl~tive compounds as follows:
s
Sprague-Dawley rats ( 150-200 g) are rendered unconscious by CO2
asphyxiation and then euth~ni7e-1 by cervical dislocation. The bladder is removed into
warm (37 deg.C) physiological salt solution (PSS) of the following composition
(mM): NaCl, 118.4; KCl, 4.7; CaCl2, 2.5; MgSO4, 4.7; H2O, 1.2; NaHCO3, 24.9;
KH2PO4, 1.2; glucose, 11.1; EDTA, 0.023; gassed with 95% ~2; 2/5% CO2; pH 7.4.
The bladder is opened and then cut into strips 1-2 mm in width and 7-10 mm in
length. The strips are subsequently suspended in a 10 mL tissue bath under an initial
resting tension of 1.5 g. The strips are held in place by two surgical clips one of which
is ~tt~hed to fixed hook while the other is attached to an isometric force tr~nsclucer.
15 The preparations, which usually exhibit small spontaneous contractions, are allowed
to recover for a period of 1 hour prior to a challenge with ~.1 uM carbachol. The
carbachol is then washed out and the tissue allowed to relax to its resting level of
activity. Following 1 further 30 min period of recovery an additional 15 mM KCl are
introduced into the tissue bath. This increase in KCl concentration results in a large
20 increase in the amplitude of spontaneous contractions (and initiation of contractions
in previously quiescent strips) superimposed upon a small increase in basal tone.
Following stabilization of this enhanced level of contractile activity, incremental
increases in the concentration of test compound or vehicle are introduced into the
tissue bath. Contractile activity is measured for each compound or vehicle
25 concentration during the last min of a 30 min challenge.
Isometric force developed by the bladder strips is measured using a
concentration required to elicit 50% inhibition of pre-drug contractile activity (ICso
concentration) is calculated from this concentration-response curve. The maximum30 percentage inhibition of contractile activity evoked by a test compound is also
recorded for concentrations of test compound < or equal to 30 ~M.
CA 02204415 1997-05-02
Wo 96/14300 PcrluS9Sl14597
- 24-
The results of this study are shown in Table I.
Table I
S Inhibition of Contraction~ in l~olated 12~t Rl~dder ~triDs
Compound n IC~QInhibition of Force
(%) at (x) ~LM
Example 1 6 0.05611M
Example 4 3 2.3 ~M
F.Y~mple 5 3 - 38% (30 ~lM)
Example 9 4 - 22% (30~M)
Examplell 4 - 28% (30~M)
Hence, the compounds of this invention have a pronounced effect on smooth
10 muscle contractility and are useful in the tre~sment of urinary incontinence, irritable
bladder and bowel disease, ~cthm~, hypertension, stroke, and similar diseases asmentioned above, which are amenable to treatment with potassium channel activating
compounds by ~-lminictration, orally, parenterally, or by aspiration to a patient in
need thereof.