Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
-
CA 02204491 1997-0~-0~
WO 96/14331 PCI~/US95/14377
TITLE OF THE INVENTION
MODIFIED NEUROP~l ll)E Y RECEPTORS
BACKGROUND OF THE INVENTION
S This application is a contiml~ion-in-part of U.S. Serial No.
08/335,017, filed November 7, 1994, the contents of which are hereby
incorporated by reference.
Neuropeptide Y (NPY) is a 36 residue, amidated peptide.
It is anatomically co-distributed and co-released with norepinephrine in
10 and from sympathetic postganglionic neurons ([1], [2], [3], [4], [5], [6]).
Stimulation of the sympathetic nervous system under physiological
circumstances such as exercise ([7], [8]) or exposure to the cold ([9],
[10]) promotes an elevation of both norepinephrine and NPY.
NPY is believed to act in the regulation of appetite control
15 ([11], [12]) and vascular smooth muscle tone ([13], [14]) as well as
regulation of blood pressure ([6], [15], [16], [17]). NPY also decreases
cardiac contractility ([18], [19], [20], [21], [22]). Congestive heart
failure and cardiogenic shock are associated with probable releases of
NPY into the blood ([23], [24], [25]). Regulation of NPY levels may be
20 beneficial to these disease states [26].
At the cellular level, neuropeptide Y binds to a G-protein
coupled receptor ([27], [28], [29], [30]). Neuropeptide Y is involved in
reg~ tin.~ eating behavior and is an extremely potent orixigenic agent
([11], [12], [31]). When 2-1rnini~tered intracerebroventricularly or
25 injected into the hypoth~l~mic paraventricular nucleus (PVN) it elicits
eating in satiated rats ([32], [33], [34]) and intraventricular injection of
antisera to NPY decreases eating ([11], [31]). It has been shown to
stimulate appetite in a variety of species and at different stages of
development ([12]). Other effects on energy metabolism include
30 decreased thermogenesis, body temperature and uncoupling protein, and
increased white fat storage and lipoprotein lipase activity ([9], [35], [36],
[37], [38], [39]). NPY levels in the PVN increase upon fasting ([40],
[41], [42], [43], [44]), before a scheduled meal ([31], [36], [40]), and in
both streptozotocin-induced and spontaneous diabetes ([36], [45], [46],
35 [47], [48], [49]). Also, NPY levels are increased in genetically obese
CA 02204491 1997-0~-0~
WO 96/14331 PCr/US95/14377
and hyperphagic Zucker rats ([36], [50], [51]). Thus, a specific
centrally acting antagonist for the ~l~rop~iate NPY receptor subtype
may be therapeutically useful for treating obesity and diabetes. Other
disorders which might be targeted therapeutically include anxiety,
5 hypertension, cocaine withdrawal, congestive heart failure, memory
enhancement, cardiac and cerebral vasospasm, pheochromocytoma and
ganglioneuroblastoma, and Huntington's, Alzheimer's and Parkinson's
diseases ([26], [52]).
At least four receptor subtypes of the NPY family have
10 been proposed based on ph~ cological and physiological properties.
The Y1 receptor is stimulated by NPY or PYY (peptide YY) and
appears to be the major vascular receptor ([16], [53], [54], [55]). The
Y2 receptor is stimulated by C-terminal fragments of NPY or PYY and
is abundantly expressed both centrally and peripherally ([55], [56], [57],
15 [58]). A third receptor (Y3) is exclusively responsive to NPY and is
likely present in adrenal medulla, heart, and brain stem ([27], [59]). In
addition, other subtypes of this receptor family are known to exist,
based on pharmacological and physiological characterization ([60], [61],
[62], [63]). The feeding behavior is stimulated potently by NPY, NPY2-
20 36 and the Y1 agonist [Leu31, Pro34]NPY, but is not stimulated by theY2 agonist NPY13-36 ([11], [64], [65], [66]). This pharmacology is not
characteristic of the defined Y1, Y2 or Y3 receptors and can thus be
attributed to a unique receptor, termed "atypical Y1 " ([11], [65], [66]),
that is responsible for evoking the feeding response. In addition, data
25 indicate the existence of additional members of this receptor family
including one subtype specific for peptide PP ([62], [63]), one with
affinity for short C-terminal fragments of NPY which induce
hypotension when ~dmini~tered systemically ([15], [17], [30], [67], [68]),
and one associated with binding of NPY and PYY to brain sigma and
30 phencyclidine binding sites ([61]).
The DNA encoding the Y 1 receptor has been cloned and
shown to be a G protein coupled receptor ([53], [69], [70]). G-protein
coupled receptors are well-known to share substantial sequence
homology to each other (71). Recently, DNA encoding the Y4 receptor
CA 02204491 1997-05-05
WO g6/14331 PCI~/US95/14377
has been isolated using Yl DNA probes [72]. In addition, DNA
encoding the Y2 receptor has been isolated by expression cloning ([73],
~74]). The cDNAs encoding these receptors are at least 45% identical at
~e DNA level and 30% at the protein level. Other NPY receptors
5 have not been cloned.
References
1. DeQuidt, M.E. and P.C. Emson, Distribution of neuropeptide Y-
10 like immunoreactivity in the rat central nervous system~Immunohistochemical analysis. Neuroscience, 1986. 18(3): p. 545-618.
2. Lundberg, J.M., et al., Co-release of neuropeptide Y and
catecholamines during physical exercise in man. Biochem Biophys Res
15 Commun, 1985. 133(1): p. 30-6.
3. Morris, M.J., et al., Increases in plasma neuropeptide Y
concentrations during sympathetic activation in man. J Auton Nerv Syst,
1986. 17(2): p. 143-9.
4. Pemow, J., Co-release andfunctional interactions of neuropeptide
Y and noradrenaline in peripheral sympathetic vascular control. Acta
Physiol Scand Suppl, 1988. 568(1): p. 1-56.
25 5. Sawchenko, P.E., et al., Colocalization of neuropeptide Y
immunoreactivity in brainstem catecholaminergic neurons that project
to the paraventricl~lar nucleus of the hypothalamus. J Comp Neurol,
1985. 241(2): p. 138-53.
30 6. Wahlestedt, C., et al., Norepinephrine and neuropeptide Y:
vasoconstrictor cooperation in vivo and in vitro. Am J Physiol, 1990.
258: p. R736-R742.
7. Kaijser, L., et al., Neuropeptide Y is released together with
35 noradrenaline from the human heart during exercise and hypoxia. Clin
Physiol, 1990. 10(2): p. 179-88.
8. Lewis, D.E., et al., Intense exercise and food restriction cause
similar hypothalamic neuropeptide Y increases in rats. Am J Physiol,
40 1993. 264: p. E279-E284.
CA 02204491 1997-0~-0~
WO 96/14331 PCI/US9S/14377
9. McCarthy, H.D., et al., Widespread increases in regional
hypothalamic Neuropeptide- Y levels in acute Cold-Exposed rats.
Neuroscience, 1993. 54(1): p. 127-132.
10. Zukowska, G.Z. and A.C. Vaz, Role of neuropeptide Y (NPY) in
cardiovascular responses to stress. Synapse, 1988. 2(3): p. 293-8.
11. Stanley, B.G., et al., Evidence for neuropeptide Y mediation of
0 eating produced by food deprivation and for a variant of the Yl
receptor mediating this peptide's effect. Peptides, 1992. 13: p. 581-587.
12. Stanley, B.G., Neuropeptide Y in multiple hypothalamic sites
controls eating behavior, endocrine, and autonomic systems for body
15 energy balance, in Neuropeptide Y, W.F. Colmers and C. Wahlestedt,
Editor. 1993, Hllm~n~ Press: Totowa, NJ. p. 457-509.
13. Abel, P.W. and C. Ha~, Effects of neuropeptide Y on contraction,
relaxation, and membrane potential of rabbit cerebral arteries. J
20 Cardiovasc Pharmacol, 1989. 13(1): p. 52-63.
14. Han, C. and P.W. Abel, Neuropeptide Y potentiates contraction
and inhibits relaxation of rabbit coronary arteries. J Cardiovasc
Pharmacol, 1987. 9(6): p. 675-81.
15. Grundemar, L., et al., Biphasic blood pressure response to
neuropeptide Y in anesthetized rats. Eur J Pharmacol, 1990. 179(1-2):
p. 83-7.
30 16. Grundemar, L., et al., Characterization of vascular neuropeptide
Y receptors. Br J Pharmacol, 1992. 105(1): p. 45-50.
17. Shen, S.H., et al., C-terminal neuropeptide Y fragments are mast
cell-dependent vasodepressor agents. Eur. J. Pharmacol., 1993. 204: p.
35 249-256.
18. Tseng, C.J., et al., Cardiovascular effects of neuropeptide Y in rat
brainstem nuclei. Circ Res, 1989. 64(1): p. 55-61.
40 19. Carter, D.A., M. Vallejo, and S.L. Lightman, Cardiovascular
effects of neuropeptide Y in the nucleus tractus solitarius of rats:
CA 02204491 1997~ -0~
WO 96/14331 PCT/US95/14377
relationship with noradrenaline and vasopressin. Peptides, 1985. 6(3):
p. 421-5.
20. Grundemar, L., C. Wahlestedt, -nd D.J. Reis, Neuropeptide Y
5 acts at an atypical receptor to evoke cardiovascular depression and to
inhibit glutamate responsiveness in the brainstem. J Ph~ col Exp
Ther, 1991. 258(2): p. 633-8.
21. Grundemar, L., C. Wahlestedt, a nd D.J. Reis, Long-lasting
0 inhibition of the cardiovascular responses to glutamate and the
baroreceptor reflex elicited by neurop~ptide Y injected into the nucleus
tractus solitarius of the rat. Neurosci Lett, 1991. 122(1): p. 135-9.
22. Zukowska-Grojec, Z. and C. Wallestedt, Origin and actions of
15 neuropeptide Y in the cardiovascular ~ystem, in Neuropeptide Y, W.F.
Colmers and C. Wahlestedt, Editor. 1993, Hllm~n~ Press: Totowa, NJ.
p. 315-388.
23. Edvinsson, L., et al., Congestive heart failure: involvement of
20 perivascular peptides reflecting activi~ in sympathetic, parasympathetic
and afferentfibres. Eur J Clin Invest, 990. 20(1): p. 85-9.
24. Franco, C.A., et al., Release of nPuropeptide Y and noradrenaline
from the human heart after aortic occlusion during coronary artery
25 surgery. Cardiovasc Res, 1990. 24(3): ?. 242-6.
25. Maisel, A.S., et al., Elevation of ~lasma neuropeptide Y levels in
congestive heartfailure. Am J Med, lC89. 86(1): p. 43-8.
30 26. Wahlestedt, C. and D.J. Reis, Neuropeptide Y-related peptides
and their receptors - are the receptors potential therapeutic drug
targets? Annu. Rev. Ph~ col. Toxicol., 1993. 32: p. 309-352.
27. Wahlestedt, C., S. Regun~th~n, and D.J. Reis, Identification of
35 cultured cells selectively expressing Y1-, Y2-, or Y3-type receptors for
neuropeptide Y/peptide YY. Life Sciences, 1992. 50: p. PL7-PL12.
28. Feth, F., W. Rascher, and M.C. Michel, G-protein coupling and
signalling of Y1-like neuropeptide Y receptors in SK-N-MC cells.
40 Naunyn Schmiedebergs Arch Pharmaccl, 1991. 344(1): p. 1-7.
CA 02204491 1997~ -0~
WO 96/14331 PCT/US9S/14377
29. Motulsky, H.J. and M.C. Michel. Neuropeptide Y mobilizes Ca2+
and inhibits adenylate cyclase in human erythroleukemia cells. Am J
Physiol, 1988. 255: p. E880-E885.
5 30. Wahlestedt, C., et al., Neuropeptide Y receptor subtypes, Yl and
Y2. Ann N Y Acad Sci, 1990. 611(7): p. 7-26.
31. Sahu, A. and S.P. Kalra, Neurop~ptidergic regulation of feeding-
behavior - neuropeptide-Y. Trends In Endocrinology And Metabolism,
10 1993. 4(7): p. 217-224.
32. Clark, J.T., et al., Neuropeptide Y and human pancreatic
polypeptide stimulate feeding behavior in rats. Endocrinology, 1984.
115(1): p. 427-429.
33. Stanley, B.G. and S.F. Leibowitz, Neuropeptide Y injected in the
paraventricular hypothalamus: a powerful stimulant offeeding
behavior. Proc. Natl. Acad. Sci. USA, 1985. 82: p. 3940-3943.
20 34. Stanley, B.G. and S.F. Leibowitz, Neuropeptide Y: stimulation of
feeding and drinking by injection into the paraventricular nucleus. Life
Sci, 1984. 35(26): p. 2635-42.
35. Zarjevski, N., et al., Chronic intracerebroventricular
25 neuropeptide-Y administration to normal rats mimics hormonal and
metabolic changes of obesity. Endocrinology, 1993. 133(4): p. 1753-
1758.
i
36. Billington, C.J. and A.S. Levine, Hypothalamic neuropeptide Y
30 regulation offeeding and energy metabolism. Current Opinion in
Neurobiology, 1992. 2: p. 847-851.
37. Leibowitz, S.F., Brain neuropeptide Y: an integrator of
endocrine, metabolic and behavioral processes. Brain Research Bulletin,
35 1991. 27: p. 333-337.
38. Billington, C.J., et al., Effects of intracerebroventricular injection
of neuropeptide Y on energy metabolism. Am. J. Physiol., 1991. 260:
p. R321-R327.
CA 02204491 1997-0~-0~
WO 96/14331 PCI'/US95/14377
39. Billington, C.J., et al., Neuropeptide-Y in hypothalamic
paraventricular nucleus - a center coordinating energy-metabolism.
American Journal Of Physiology, 1994. 266(6): p. R1765-R1770.
5 40. Kalra, S.P., et al., Neuropeptide Y secretion increases in the
paraventricular nucleus in association with increased appetite for food.
Proc. Natl. Acad. Sci. USA, 1991. 88: p. 10931-10935.
41. Beck, B., et al., Rapid and localized alterations of neuropeptide Y
0 in discrete hypothalamic nuclei with feeding status. Brain Res, 1990.
528(2): p. 245-9.
42. Brady, L.S., et al., Altered expression of hypothalamic
neuropeptide mRNAs in food-restricted and food-deprived rats.
15 Neuroendocrinology, 1990. 52(5): p. 441-7.
43. Calza, L., et al., Increase of neuropeptide Y-like
immunoreactivity in the paraventricular nucleus offasting rats.
Neurosci Lett, 1989. 104(1-2): p. 99-104.
44. Sahu, A., P.S. Kalra, and S.P. Kalra, Food deprivation and
ingestion induce reciprocal changes in neuropeptide Y concentrations in
the paraventricular nucleus. Peptides, 1988. 9(1): p. 83-6.
25 45. Abe, M., et al., Increased neuropeptide Y content in the arcuato-
paraventricular hypothalamic neuronal system in both insulin-dependent
and non-insulin-dependent diabetic rats. Brain Res, 1991. 539(2): p.
223-7.
30 46. Sahu, A., et al., Neuropeptide-Y concentration in microdissected
hypothalamic regions and in vitro release from the medial basal
hypothalamus-preoptic area of streptozotocin-diabetic rats with and
without insulin substitution therapy. Endocrinology, 1990. 126(1): p.
192-8.
47. White, J.D., et al., Increased hypothalamic content of
preproneuropeptide-Y messenger ribonucleic acid in streptozotocin-
diabetic rats. Endocrinology, 1990. 126(2): p. 765-72.
40 48. Willi~m.~, G., et al., Increased hypothalamic neuropeptide Y
concentrations in diabetic rat. Diabetes, 1988. 37(6): p. 763-72.
CA 02204491 1997-o~-o~
WO 96/14331 PCT/US95/14377
49. Willi~m.~, G., et al., Increased neuropeptide Y concentrations in
specific hypothalamic regions of streptozocin-induced diabetic rats.
Diabetes, 1989. 38(3): p. 321-7.
50. Beck, B., et al., Hypothalamic neuropeptide Y (NPY) in obese
Zucker rats: implications in feeding and sexual behaviors. Physiol
Behav, 1990. 47(3): p. 449-53.
10 51. Sanacora, G., et al., Increased hypothalamic content of
preproneuropeptide Y messenger ribonucleic acid in genetically obese
Zucker rats and its regulation by food deprivation. Endocrinology,
1990. 127(2): p. 730-7.
15 52. Wahlestedt, C., R. F.km~n, and E. Widerlov, Neuropeptide Y
(NPY) and the central nervous system: distribution effects and possible
relationship to neurological and psychiatric disorders. Prog
Neuropsychoph~ col Biol Psychiatry, 1989. 13(1-2): p. 31-54.
20 53. Larh~mm~r, D., et al., Cloning and functional expression of a
human neuropeptide Y/peptide YY receptor of the Yl-type. J. Biol.
Chem., 1992. 267: p. 10935- 10938.
54. Sheikh, S.P., et al., Localization of Y] receptors for NPY and
25 Pn on vascular smooth muscle cells in rat pancreas. Arn J Physiol,
1991. 260: p. G250-G257.
55. Wahlestedt, C., N. Y~n~ih~ra, and R. Hakanson, Evidence for
diJ~ferent pre-and post-junctional receptors for neuropeptide Y and
30 related peptides. Regul Pept, 1986. 13(3-4): p. 307-18.
56. Jorgensen, J.C., J. Fuhlendorff, and T.W. Schwartz, Structure-
function studies on neuropeptide Y and pancreatic polypeptide--evidence
for two PP-fold receptors in vas deferens. Eur J Ph~ Gol, 1990.
35 186(1): p. 105-14.
57. Cox, H.M. and J.L. Krstenansky, The effects of selective amino
acid substitution upon neuropeptide Y antisecretory potency in rat
jejunum mucosa. Peptides, 1991. 12(2): p. 323-7.
CA 02204491 1997-0~-0~
WO 96114331 PCI/US95/14377
58. Aicher, S.A., et al., Receptor-selective analogs demonstrate
NPYIPYY receptor heterogeneity in rat brain. Neurosci Lett, 1991.
130(1): p. 32-6.
5 59. Balasubr2m~ni~m, A., et al., Characterization of neuropeptide Y
binding sites in rat cardiac ventricular membranes. Peptides, l 990.
11(3): p. 545-50.
60. Li, X.J., et al., Cloning, functional expression, and developmental
0 regulation of a neuropeptide Y receptor from Drosophila melanogaster.
J Biol Chem, 1992. 267(1): p. 9-12.
61. Roman, F.J., et al., Neuropeptide Y and peptide YY interact with
rat brain sigma and PCP binding sites. Eur J Pharmacol, 1989. 174(2-
15 3): p. 301-2.
62. Schwartz, T.W., S.P. Sheikh, and M.M. O'Hare, Receptors on
phaeochromocytoma cells for two members of the PP-fold family--NPY
and PP. Febs Lett, 1987. 225(1-2): p. 209-14.
63. Schwartz, T.W., et al., Signal epitopes in the three-dimensional
structure of neuropeptide Y. Interaction with Yl, Y2, and pancreatic
polypeptide receptors. Ann N Y Acad Sci, 1990. 611(35): p. 35-47.
25 64. Wahlestedt, C., et al., Modulation of anxiety and neuropeptide Y-
Yl receptors by antisense oligodeoxynucleotides. Science, 1993. 259: p.
528-531.
65. Jolicoeur, F.B., et al., In vivo structure activity study supports the
30 existence of heterogeneous neuropeptide Y receptors. Brain Res Bull,
1991. 26(2): p. 309-11.
66. Leibowitz, S.F. and J.T. Alexander, Analysis of neuropeptide Y-
inducedfeeding: dissociation of Yl and Y2 receptor effects on natural
35 meal patterns. Peptides, 1991. 12(6): p. 1251 -60.
67. Inui, A., et al., Characterization of peptide YY receptors in the
brain. Endocrinology, 1989. 124(1): p. 402-9.
CA 02204491 1997-0~-0~
WO 96/14331 PCI~/US95/14377
- 10-
68. Boublik, J., et al., Neuropeptide Y and ne~lropeptide Yl~-36.
Structural and biological characterization. Int J Pept Protein Res, 1989.
33(1): p. 11-5.
5 69. Eva, C., et al., Molecular cloning of a novel G protein-coupled
receptor that may belong to the neuropeptide receptor family. FEBS
Lett., 1990. 271: p. 81-84.
70. Herzog, H., et al., Cloned human neuropeptide Y receptor couples
1O to two di~erent second messenger systems. Proc Natl Acad Sci U S A,
1992. 89: p. 5794-5798.
71. Strader, C.D., I.S. Sigal, and R.A. Dixon, Structural basis of
beta-adrenergic receptor function. FASEB-J, 1989. 3(7): p. 1825-1832.
72. Bard, J.A., Walker, M.W., Brancheck, T., Wein~h~nk, R. DNA
encoding a human neuropeptide Ylpeptide YYIpancreatic polypeptide
receptor (Y4) and uses thereof. PCT International Application
Publication No. WO 95/17906, published 6 July 1995.
73. Gerald, C., Walker, M.W., Branchek, T., and Weinshank, R.
Nucleic acid encoding Neuropeptide YlPeptide YY (Y2( receptors and
uses thereof. PCT International Application Publication No.
WO95/21245, published 10 August 1995.
74. Rose, P. A. et al., Cloning and functional expression of a cDNA
encoding the human type 2 neuropeptide Y receptor. J. Biol. Chem.,
270:22661 -22664.
30 SUMMARY OF THE INVENTION
Modified neuropeptide Y receptors having deletions,
replacements or additions in the third intracellular domain are identified
and methods of making the modified receptors are provided. The
invention includes the modified receptors, assays employing the
35 modified receptors, cells expressing the modified receptors, compounds
identified through the use of the modif1ed receptors, including
modulators of the receptors, and the use of the compounds to treat
conditions, including obesity, diabetes, anxiety, hypertension, cocaine
withdrawal, congestive heart failure, memory enhancement, cardiac and
CA 02204491 1997-0~-0~
WO 96/14331 PCr/US95114377
cerebral vasospasm, pheochromocytoma and ganglioneuroblastoma, and
Huntington's, Alzheimer's and Parkinson's diseases.
BREF DESCRIPTION OF THE DRAWINGS
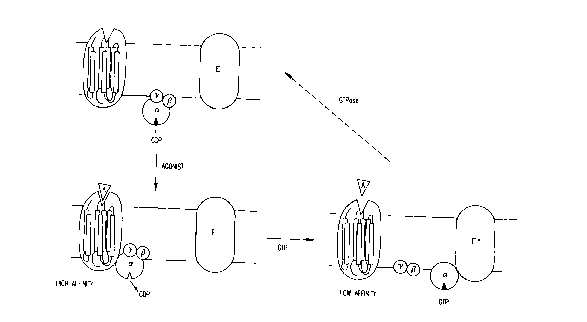
5 Figure 1. Schematic diagram of G-protein signal transduction system.
The receptor is shown as a seven-helical bundle. a, ~, and ~ indicate
the three subunits of the G protein. E indicates an effector enzyme,
such as adenylyl cyclase. The agonist ~A) binding with high affinity to
the receptor-G protein complex and with low affinity to the receptor
10 alone is shown.
Figure 2. Schematic diagram of the hamster ~2 adrenergic receptor.
The third intracellular loop comprises residues 221-273. The proximal
and distal segments of this loop are drawn in cylinders.
Figure 3 shows the amino acid sequence of the human NPY1 receptor
subtype aligned with that of the hamster ~2-adrenergic receptor. The
transmembrane helices are underlined.
20 DETAILED DESCRIPTION OF THE INVENTION
Modified neuropeptide Y receptors having deletions,
replacements or additions in the third intracellular domain are identified
and methods of m~king the modified receptors are provided. The
invention includes the modified receptors, assays employing the
25 modified receptors, cells expressing the modified receptors, compounds
identified through the use of the modified receptors, including
modulators of the receptors, and the use of the compounds to treat
conditions, including obesity, diabetes, anxiety, hypertension, cocaine
withdrawal, congestive heart failure, memory enhancement, cardiac and
30 cerebral vasospasm, pheochromocytoma and ganglioneuroblastoma, and
Huntington's, Alzheimer's and Parkinson's diseases. Modulators, as
described herein, include but are not limited to agonists, antagonists,
suppressors and inducers.
CA 02204491 1997-0~-0~
WO 96/14331 PCI/US95tl4377
- 12-
Neuropeptide Y receptors belong to a class of receptors
known as "G-protein coupled receptors." The term "G-protein coupled
receptor" refers to any receptor protein that mediates its endogenous
signal transduction through activation of one or more guanine
nucleotide binding regulatory proteins (G-proteins). These receptors
share common structural features, including seven hydrophobic
transmembrane domains. G-protein coupled receptors include receptors
that bind to small biogenic amines, including but not limited to beta-
adrenergic receptors (~AR), alpha-adrenergic receptors (ocAR) and
muscarinic receptors, as well as receptors whose endogenous ligands are
peptides, such as neurokinin, neuropeptide Y and glucagon receptors.
Examples of ~AR include beta-1, beta-2, and beta-3 adrenergic
receptors.
G-protein coupled receptors are cell surface proteins that
mediate the responses of a cell to a variety of environmental signals.
Upon binding an agonist, the receptor interacts with one or more
specific G proteins, which in turn regulate the activities of specific
effector proteins. By this means, activation of G-protein coupled
receptors amplifies the effects of the environmental signal and initiates a
cascade of intracellular events that ultimately leads to defined cellular
responses. G-protein coupled receptors function as a complex
information processing network within the plasma membrane of the
cell, acting to coordinate a cell's response to multiple environmental
signals.
G-protein coupled receptors are characterized by the ability
of agonists to promote the formation of a high affinity ternary complex
between the agonist, the receptor and the G-protein (Figure 1). The a-
subunit of the G protein contains a guanine nucleotide binding site
which, in the high affinity ternary [G protein-receptor-agonist]
complex, is occupied by GDP. In the presence of physiological
concentrations of GTP, the GDP molecule in the guanine nucleotide
binding site of the G protein is displaced by a GTP molecule. The
binding of GTP dissociates the a subunit of the G protein from its ~ and
y subunits and from the receptor, thereby activating the G-protein to
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
- 13 -
stimulate downstream effectors (adenylyl cyclase in the case of the ,B-
adrenergic receptor (~AR)) and propagating the intracellular signal.
Thus, the ternary complex is transient in the presence of physiological
concentrations of GTP. Because the affinity of the agonist for the
5 receptor-G protein complex is higher than its affinity for the
uncomplexed receptor, one consequence of the destabilization of the
ternary complex is a reduction in the affinity of the receptor for the
agonist. Thus, the affinity of agonists for G-protein coupled receptors
is a function of the efficiency with which the receptor is coupled to the
10 G-protein. In contrast, antagonists bind with the same affinity to the
receptor in the presence or absence of G-protein coupling.
The observation that agonist affinity can be reduced by
conditions under which a receptor is not optimally coupled to its G-
protein has important implications for the identification of agonists of
15 G-protein coupled receptors, particularly identification based on ligand
binding. If a receptor is not optimally coupled to the G-protein under
the conditions of binding assays, an agonist will bind to the receptor
with relatively low affinity. Thus, a screen that relies on a binding
assay based on displacement of a radiolabeled ligand, although attractive
20 for its ease and the potential for high throughput, poses the risk that a
promising partial agonist might be overlooked because the agonist
would bind predominantly to the low affinity state of the receptor, and
thus would have low affinity in the binding assay. Consequently,
functional assays are frequently used to screen for agonists of G-protein
25 coupled receptors. However, functional assays (ranging from ex vivo
muscle contraction assays to determination of second messenger levels
in cells expressing exogenous cloned G-protein coupled receptors) are
tedious and more time-consuming than ligand binding assays, and hence
are not readily adapted to high-throughput screens. Because the
30 modified receptors of the present invention bind agonists with high
affinity in the presence or absence of the G-protein, they can be used in
high throughput radioligand binding assays to screen for high affinity
ligands, regardless of whether the ligands are agonists or antagonists.
CA 02204491 1997-0~-0~
WO 96/14331 PCIIUS9S/14377
- 14-
G-protein coupled receptors consist of seven hydrophobic
domains connecting eight hydrophilic domains. The hydrophobicity or
hydrophilicity of the domains may be determined by standard
hydropathy profiles, such as Kyte-Doolittle analysis (Kyte, J. and
5 Doolittle, R.J.F. J. Mol. Biol. 157: 105 (1982)). The receptors are
thought to be oriented in the plasma membrane of the cell such that the
N-terminus of the receptor faces the extracellular space and the C-
terminus of the receptor faces the cytoplasm, so that each of the
hydrophobic domains crosses the plasma membrane. The receptors
10 have been modeled and the putative boundaries of the extracellular,
transmembrane and intracellular domains are generally agreed (for a
review, see Baldwin, EMBO J. 12:1693, 1993). In general, the
transmembrane domains are comprised of stretches of 20-25 amino
acids in which most of the amino acid residues have hydrophobic side
15 chains (including cysteine, methionine, phenyl~l~nine, tyrosine,
tryptophan, proline, glycine, ~l~nine, valine, leucine, isoleucine),
whereas the intracellular and extracellular loops are defined by
contiguous stretches of several amino acids that have hydrophilic or
polar side chains (including aspartate, glutamate, asparagine, glutamine,
20 serine, threonine, histidine, lysine, and arginine). Polar amino acids,
especially uncharged ones (such as serine, threonine, asparagine, and
gll-t~mine) are found in both transmembrane and extramembrane
regions.
The extramembrane regions are characterized by
25 contiguous stretches of three or more hydrophilic residues. In contrast,
hydrophilic residues are found only in groups of 1-2, surrounded by
hydrophobic residues, in the transmembrane domain. Thus, the
transmembrane and extramembrane regions can be identified by the
number of contiguous hydrophilic or hydrophobic amino acids in the
30 primary sequence of the receptor, in addition to the constraints on the
length of the hydrophobic segments given above. The boundaries
between the transmembrane and extramembrane regions are often
defined by the presence of charged or polar residues at the beginning or
end of a stretch of hydrophobic amino acids. The locations of the
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95114377
mutations in the receptors of the present invention are described on the
basis of these models and can be specifically defined by the specific
amino acid numbers of the residues being mllt~ted.
By these criteria, the third intracellular loop is defined as
5 the hydrophilic loop connecting the hydrophobic, putative
tr~n~membrane domains V and VI. For example, in hamster ,~2
adrenergic receptor, the third intracellular loop would refer to amino
acids 221 through 273. In accordance with the principles described
above, the beginning of this loop is defined by the presence of Arg22
10 (a charged residue at the end of the hydrophobic stretch of residues
198-220) and Lys273 (a charged residue at the beginning of the
hydrophobic stretch of residues 274-298). In the human NPY1 receptor
(PCT International Application Publication Nos. W093/09227 published
13 May 1993 and WO93/24515 published 9 December 1993, the
15 contents of both of which are hereby incorporated by reference), the
third intracellular loop refers to amino acids #233-260 (Figure 3). In
accordance with the principles described above, the beginning of this
loop is defined by the presence of Lys233 (a charged residue at the end
of the long stretch of hydrophobic residues comprising helix 5) and
20 Arg260 (a charged residue at the beginning of the long stretch of
hydrophobic residues comprising helix 6). In the rat NPY1 receptor,
the third intracellular loop refers to amino acids #232-259 (Eva, C., et
al., FEBS Lett. 271:81, 1990). In accordance with the principles
described above, the be~inning of this loop is defined by the presence of
25 Lys232 (a charged residue at the end of the long stretch of hydrophobic
residues comprising helix 5) and Arg259 (a charged residue at the
beginning of the long stretch of hydrophobic residues comprising helix
6). In the human and rat NPY2 receptors, the third intracellular loop
refers to amino acids #241-268 (Gerald, C., et al., PCT International
30 Application Publication No. WO95/21245, the contents of which are
hereby incorporated by reference). In accordance with the principles
described above, the be~innin~ of this loop is defined by the presence of
Arg241 (a charged residue at the end of the long stretch of hydrophobic
residues comprising helix 5) and Lys268 (a charged residue at the
CA 02204491 1997-o~-o~
WO 96/14331 PCT/US95tl4377
- 16 -
beginning of the long stretch of hydrophobic residues comprising helix
6). In the human NPY4 receptor, the third intracellular loop refers to
amino acids #236-263 (Bard, J.A., et al., PCT International Application
Publication No. WO95/17906, the contents of which are hereby
5 incorporated by reference). In accordance with the principles described
above, the beginning of this loop is defined by the presence of Arg236
(a charged residue at the end of the long stretch of hydrophobic residue.s
comprising helix 5) and Gln263 (a polar residue at the beginning of the
long stretch of hydrophobic residues comprising helix 6). In the rat
10 NPY4 receptor, the third intracellular loop refers to amino acids #236-
263 (Bard, J.A., et al., PCT International Application Publication No.
WO95/17906). In accordance with the principles described above, the
beginning of this loop is defined by the presence of Arg236 (a charged
residue at the end of the long stretch of hydrophobic residues
15 comprising helix 5) and Arg263 (a charged residue at the beginning of
the long stretch of hydrophobic residues comprising helix 6).
The present invention pertains to modified neuropeptide
Y receptors having deletions, replacements or additions in the third
intracellular domain. Methods of designing and making modified
20 receptors are provided. The modified receptors are uncoupled from
or are poorly coupled to their respective neuropeptides. However,
these modif1ed receptors bind agonists with high affinity in the
absence of G protein coupling. Because of their high intrinsic
affinity for agonists, these modified receptors may be used in high
25 throughput binding assays to identify compounds that bind to the
receptor with high affinity, regardless of whether these compounds
are agonists or antagonists. The invention includes the DNA
encoding the modif1ed receptors, the modified receptors, assays
employing the modified receptors, cells expressing the modified
30 receptors, substances identified through the use of the modified
receptors including specific modulators of the modified receptors,
and the use of these substances in treating diseases, including obesity,
diabetes, cardiovascular, and neurological disorders. Modulators
identified in this process are useful as therapeutic agents.
CA 02204491 1997-0~-05
WO 96/14331 PCT/US95/14377
Modulators, as described herein, include but are not limited to
agonists, antagonists, suppressors and inducers.
Modified receptors may include genetic variants, both
natural and induced. Induced modified receptors may be derived by a
S variety of methods, including but not limited to, site-directed
mutagenesis. Techniques for nucleic acid and protein manipulation are
well-known in the art and are described generally in Methods in
Enzymology and in Sambrook et al., Molecular Cloning: A Laboratory
Manual, Cold Spring Harbor Laboratory (1989).
It is known that there is a substantial amount of
redundancy in the various codons which code for specific amino
acids. Therefore, this invention is also directed to those DNA
sequences which contain alternative codons which code for the
eventual translation of the identical amino acid. For purposes of this
specification, a sequence bearing one or more replaced codons will
be defined as a degenerate variation. Also included within the scope
of this invention are mutations either in the DNA sequence or the
translated protein which do not subst~nti~lly alter the ultimate
physical properties of the expressed protein. For example,
substitution of valine for leucine, arginine for Iysine, or asparagine
for glutamine may not cause a change in functionality of the
polypeptide.
It is known that DNA sequences coding for a peptide
may be altered so as to code for a peptide having properties that are
different than those of the naturally-occurring peptide. Methods of
altering the DNA sequences include, but are not limited to site
directed mutagenesis. Examples of altered properties include but are
not limited to changes in the affinity of an enzyme for a substrate or
a receptor for a ligand.
As used herein, a "functional derivative" of a modified
receptor is a compound that possesses a biological activity (either
functional or structural) that is substantially .simil~r to the biological
activity of the modified receptor. The term "functional derivative"
is intended to include the "fragments," "variants," "degenerate
CA 02204491 1997-0~-0~
WO 96114331 PCT/US95/14377
- 18 -
variants," "analogs" and "homologues" or to "chemical derivatives"
of modified receptors. The term "fragment" is meant to refer to any
polypeptide subset of modif1ed receptors. The term "variant" is
meant to refer to a molecule subst~nti~lly similar in structure and
function to either the entire modified receptor molecule or to a
fragment thereof. A molecule is "subst~nti~lly similar" to a
modified receptor if both molecules have subst~nti~lly similar
structures or if both molecules possess similar biological activity.
Therefore, if the two molecules possess subst~nti~lly similar activity,
they are considered to be variants even if the structure of one of the
molecules is not found in the other or even if the two amino acid
sequences are not identical.
The term "analog" refers to a molecule substantially
similar in function to either the entire modifed receptor molecule or
to a fragment thereof.
"Substantial homology" or "substantial similarity", when
referring to nucleic acids means that the segments or their
complementary strands, when optimally aligned and compared, are
identical with a~pro~liate nucleotide insertions or deletions, in at least
50% of the nucleotides. Alternatively, substantial homology exists when
the segments will hybridize to a strand or its complement.
The nucleic acids claimed herein may be present in whole
cells or in cell lysates or in a partially purified or subst~nti~lly purified
form. A nucleic acid is considered substantially purified when it is
purified away from environmental cont~min~nt,s. Thus, a nucleic acid
sequence isolated from cells is considered to be substantially purified
when purified from cellular components by standard methods while a
chemically synthesized nucleic acid sequence is considered to be
substantially purified when purified from its chemical precursors.
Nucleic acid compositions of this invention may be derived
from genomic DNA or cDNA, prepared by synthesis or by a
combination of techniques.
The natural or synthetic nucleic acids encoding the
modified G-coupled protein receptors of the present invention may be
CA 02204491 1997-0~-0~
WO 96/14331 PCr/US95/14377
- 19-
incorporated into expression vectors. Usually the expression vectors
incorporating the modified receptors will be suitable for replication in a
host. Examples of acceptable hosts include, but are not limited to,
prokaryotic and eukaryotic cells.
The phrase "recombinant expression system" as used
herein means a subst~nti~lly homogenous culture of suitable host
org~ni~m~ that stably carry a recombinant expression vector. Examples
of suitable hosts include, but are not limited to, bacteria, yeast, fungi,
insect cells, plant cells and m~mm~ n cells. Generally, cells of the
10 expression system are the progeny of a single ancestral transformed
cell.
The cloned modified receptor DNA obtained through
the methods described herein may be recombinantly expressed by
molecular cloning into an expression vector cont~inin~ a suitable
15 promoter and other appropriate transcription regulatory elements,
and transferred into prokaryotic or eukaryotic host cells to produce
recombinant modified receptor. Techniques for such manipulations
are fully described in Sambrook, J., et al., supra, and are well known
in the art.
Expression vectors are defined herein as DNA sequences
that are required for the transcription of cloned copies of genes and
the translation of their mRNAs in an appropriate host. Such vectors
can be used to express eukaryotic genes in a variety of hosts such as
bacteria, bluegreen algae, plant cells, insect cells, fungal cells and
25 ~nim~l cells.
Specifically designed vectors allow the shllttling of DNA
between hosts such as bacteria-yeast or bacteria-~nim~l cells or
bacteria-fungi or bacteria-invertebrate cells. An appropriately
constructed expression vector should contain: an origin of replication
30 for autonomous replication in host cells, selectable markers, a
limited number of useful restriction enzyme sites, a potential for
high copy number, and active promoters. A promoter is defined as a
DNA sequence that directs RNA polymerase to bind to DNA and
initi~te RNA synthesis. A strong promoter is one which causes
CA 02204491 1997-0~-0~
WO 96/14331 PCIIUS95/14377
- 20 -
mRNAs to be initiated at high frequency. Expression vectors may
include, but are not limited to, cloning vectors, modified cloning
vectors, specifically designed plasmids or viruses.
A variety of m~mm~ n expression vectors may be used
to express recombinant modified receptor in m~mm~ n cells.
Commercially available m~mm~ n expression vectors which may
be suitable for recombinant modified receptor expression, include
but are not limited to, pcDNA3 (Invitrogen), pMClneo (Stratagene),
pXTl (Stratagene), pSG5 (Stratagene), EBO-pSV2-neo (ATCC
37593) pBPV-1(8-2) (ATCC 37110), pdBPV-MMTneo(342-12)
(ATCC 37224), pRSVgpt (ATCC 37199), pRSVneo (ATCC 37198),
pSV2-dhfr (ATCC 37146), pUCTag (ATCC 37460), and ~ZD35
(ATCC 37565), pCI-neo (Promega).
A variety of bacterial expression vectors may be used to
express recombinant modified receptor in bacterial cells.
Commercially available bacterial expression vectors which may be
suitable for recombinant modified receptor expression include, but
are not limited to pET1 la (Novagen), lambda gtl 1 (Invitrogen),
pcDNAII (Invitrogen), pKK223-3 (Pharmacia).
A variety of fungal cell expression vectors may be used
to express recombinant modified receptor in fungal cells.
Commercially available fungal cell expression vectors which may be
suitable for recombinant modified receptor expression include but
are not limited to pYES2 (Invitrogen), Pichia expression vector
(Invitrogen).
A variety of insect cell expression vectors may be used
to express recombinant receptor in insect cells. Commercially
available insect cell expression vectors which may be suitable for
recombinant expression of modified receptor include but are not
limited to pBlue Bac III (Invitrogen).
An expression vector cont~ining DNA encoding
modified receptor may be used for expression of modified receptor
in a recombinant host cell. Recombinant host cells may be
prokaryotic or eukaryotic, including but not limited to bacteria such
CA 02204491 1997-0~-0~
WO 96/14331 PCr/US95114377
- 21 -
as E. coli, fungal cells such as yeast, m~mm~ n cells including but
not limited to cell lines of human, bovine, porcine, monkey and
rodent origin, and insect cells including but not limited to Drosophila
and silkworm derived cell lines. Cell lines derived from m~mm~lian
species which may be suitable and which are commercially available,
include but are not limited to, L cells L-M(TK-) (ATCC CCL 1.3),
L cells L-M (ATCC CCL 1.2), 293 (ATCC CRL 1573), Raji (ATCC
CCL 86), CV-l (ATCC CCL 70), COS-1 (ATCC CRL 1650), COS-7
(ATCC CRL 1651), CHO-K1 (ATCC CCL 61), 3T3 (ATCC CCL
92), NIH/3T3 (ATCC CRL 1658), HeLa (ATCC CCL 2), C127I
(ATCC CRL 1616), BS-C-1 (ATCC CCL 26) and MRC-5 (ATCC
CCL 171).
The expression vector may be introduced into host cells
via any one of a number of techniques including but not limited to
transformation, transfection, lipofection, protoplast fusion, and
electroporation. The expression vector-cont~ining cells are clonally
propagated and individually analyzed to determine whether they
produce modified receptor protein. Identification of modified
receptor expressing host cell clones may be done by several means,
including but not limited to immunological reactivity with anti-
modified receptor antibodies.
Expression of modified receptor DNA may also be
performed using in vitro produced synthetic mRNA or native
mRNA. Synthetic mRNA or mRNA isolated from modified receptor
producing cells can be efficiently translated in various cell-free
systems, including but not limited to wheat germ extracts and
reticulocyte extracts, as well as efficiently translated in cell based
systems, including but not limited to microinjection into frog
oocytes, with microinjection into frog oocytes being preferred.
The term "substantial homology", when referring to
polypeptides, indicates that the polypeptide or protein in question
exhibits at least about 30% homology with the naturally occurring
protein in question, usually at least about 40% homology.
CA 02204491 1997-0~-0~
WO 96/14331 PCI'/US95114377
- 22 -
The modified receptors may be expressed in an
appropriate host cell and used to discover compounds that affect the
modified receptor. Preferably, the modified receptors are expressed in
a m~mm~ n cell line, including but not limited to, COS-7, CHO or L
5 cells, or an insect cell line, including but not limited to, Sf9 and Sf21,
and may be used to discover ligands that bind to the receptor and alter
or stim~ te its function. The modified receptors may also be produced
in bacterial, fungal or yeast expression systems.
The expression of the modified receptor may be detected
10 by use of a radiolabeled ligand specific for the receptor. For example,
for the ,~2 adrenergic receptor, such a ligand may be 125I-
iodocyanopindolol (125I-CYP). For the NPY receptor, such a ligand
may be 125I-NPY, 125I-Peptide YY (PYY) or 125I-Pancreatic
polypeptide.
The specificity of binding of compounds showing affinity
for the modified receptors is shown by measuring the affinity of the
compounds for cells transfected with the cloned modified receptor or
for membranes from these cells. Expression of the cloned modified
receptor and screening for compounds that inhibit the binding of
20 radiolabeled ligand to these cells provides a rational way for rapid
selection of compounds with high affinity for the receptor. These
compounds may be agonists or antagonists of the receptor. Because the
modified receptor does not couple well to G proteins, the agonist
activity of these compounds is best assessed by using the wild-type
25 receptor, either natively expressed in tissues or cloned and exogenously
expressed.
Once the modified receptor is cloned and expressed in a
m~mm~ n cell line, such as COS-7 cells or CHO cells, the recombinant
modified receptor is in a well-characterized environment. The
30 membranes from the recombinant cells expressing the modified
receptor are then isolated according to methods known in the art. The
isolated membranes may be used in a variety of membrane-based
receptor binding assays. Because the modified receptor has a high
affinity for agonists, ligands (either agonists or antagonists) may be
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
identified by standard radioligand binding assays. These assays will
measure the intrinsic affinity of the ligand for the receptor.
The present invention provides methods of generating
modified NPY receptors. Such methods generally comprise the deletion
5 of at least one nucleotide from the third intracellular domain of the
receptor. Additional methods include, but are not limited to, enzymatic
or chemical removal of amino acids from the third intracellular domain
of the receptor. One method of generating modified NPY receptors
comprlses:
(a) isolating DNA encoding an NPY receptor;
(b) altering the DNA of step (a) by deleting at least one
nucleotide from DNA encoding the third intracellular domain of the
NPY receptor or disrupting the amphipathic helix at the N- or C-
terminus of the third intracellular domain by replacement with
15 nucleotides or addition of nucleotides coding for non-helical protein
sequence;
(c) isolating the altered DNA;
(d) expressing the altered DNA; and
(e) recovering the modified NPY receptor.
20 The third intracellular domain of a G-protein coupled receptor is
located between the fifth and sixth hydrophobic transmembrane domains
of the receptor.
The present invention provides methods of identifying
compounds that bind to modified NPY receptors. Methods of
25 identifying compounds are exemplified by an assay, comprising:
a) cloning a neuropeptide Y receptor;
b) altering the DNA sequence encoding the third
intracellular domain of the cloned receptor;
c) splicing the altered receptor into an expression
30 vector to produce a construct such that the altered receptor is operably
linked to transcription and translation signals suff cient to induce
expression of the receptor upon introduction of the construct into a
prokaryotic or eukaryotic cell;
CA 02204491 1997-0~-0~
WO 96/14331 PCI~/US95/14377
- 24 -
d) introducing the construct into a prokaryotic or
eukaryotic cell which does not express the altered receptor in the
absence of the introduced construct; and
e) incubating cells or membranes isolated from cells
5 produced in step c with a quantifiable compound known to bind to the
receptors, and subsequently adding test compounds at a range of
concentrations so as to compete the quantifiable compound from the
receptor, such that an IC50 for the test compound is obtained as the
concentration of test compound at which 50% of the quantifiable
10 compound becomes displaced from the receptor.
The present invention is also directed to methods for
screening for compounds which modulate the expression of DNA or
RNA encoding modified receptors or which modulate the function of
modified receptor protein. Compounds which modulate these
15 activities may be DNA,RNA, peptides, proteins, or non-
proteinaceous organic molecules. Compounds may modulate by
increasing or attenuating the expression of DNA or RNA encoding
modified receptor, or the function of modified receptor protein.
Compounds that modulate the expression of DNA or RNA encoding
20 modified receptor or the function of modified receptor protein may
be detected by a variety of assays. The assay may be a simple
"yes/no" assay to determine whether there is a change in expression
or function. The assay may be made quantitative by comparing the
expression or function of a test sample with the levels of expression
25 or function in a standard sample.
Kits cont~inin.~ modified receptor DNA, antibodies to
modified receptor, or modified receptor protein may be prepared.
Such kits are used to detect DNA which hybridizes to modif1ed
receptor DNA or to detect the presence of modified receptor protein
30 or peptide fragments in a sample. Such characterization is useful for
a variety of purposes including but not limited to forensic,
taxonomic or epidemiological studies.
The DNA molecules, RNA molecules, recombinant
protein and antibodies of the present invention may be used to screen
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
- 25 -
and measure levels of modified receptor DNA, modified receptor
RNA or modified receptor protein. The recombinant proteins, DNA
molecules, RNA molecules and antibodies lend themselves to the
forrnnl~tion of kits suitable for the detection and typing of modified
5 receptor. Such a kit would comprise a compartmentalized carrier
suitable to hold in close confinement at least one container. The
carrier would further comprise reagents such as recombinant
modified receptor protein or anti-modified receptor antibodies
suitable for detecting modified receptor. The carrier may also
10 contain a means for detection such as labeled antigen or enzyme
substrates or the like.
Ph~rm~ceutically useful compositions comprising
modulators of modified receptor activity, may be form~ ted
according to known methods such as by the admixture of a
15 pharmaceutically acceptable carrier. Examples of such carriers and
methods of formulation may be found in Remington's
Ph~rm~ceutical Sciences. To form a pharmaceutically acceptable
composition suitable for effective ~lrnini~tration~ such compositions
will contain an effective amount of the protein, DNA, RNA, or
20 modulator.
Therapeutic or diagnostic compositions of the invention
are ~lmini~tered to an individual in amounts sufflcient to treat or
diagnose disorders. The effective amount may vary according to a
variety of factors such as the individual's condition, weight, sex and
25 age. Other factors include the mode of ~lmini.~tration.
The ph~rrn~ceutical compositions may be provided to
the individual by a variety of routes such as subcutaneous, topical,
oral and intramuscular.
The term "chemical derivative" describes a molecule
30 that contains additional chemical moieties which are not normally a
part of the base molecule. Such moieties may improve the solubility,
half-life, absorption, etc. of the base molecule. Alternatively the
moieties may ~ttenll~te undesirable side effects of the base molecule
or decrease the toxicity of the base molecule. Exarnples of such
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
- 26 -
moieties are described in a variety of texts, such as Remington's
Pharmaceutical Sciences.
Compounds identified according to the methods disclosed
herein may be used alone at appropriate dosages. Alternatively, co-
5 ~lmini~tration or sequential ~dmini~tration of other agents may bedesirable.
The present invention also has the objective of providing
suitable topical, oral, systemic and parenteral pharmaceutical
formulations for use in the novel methods of treatment of the present
10 invention. The compositions containing compounds identified
according to this invention as the active ingredient can be
~(lmini~tered in a wide variety of therapeutic dosage forms in
conventional vehicles for ~lmini~tration. For example, the
compounds can be ~lmini~tered in such oral dosage forms as tablets,
15 capsules (each including timed release and sustained release
formulations), pills, powders, granules, elixirs, tinctures, solutions,
suspenslons, syrups and emulsions, or by injection. Likewise, they
may also be ~tlmini~tered in intravenous (both bolus and infusion),
intraperitoneal, subcutaneous, topical with or without occlusion, or
20 intramuscular form, all using forms well known to those of ordinary
skill in the pharmaceutical arts.
Advantageously, compounds of the present invention
may be ~lmini~tered in a single daily dose, or the total daily dosage
may be ~clmini~tered in divided doses of two, three or four times
25 daily. Furthermore, compounds for the present invention can be
~clmini~tered in intranasal form via topical use of suitable intranasal
vehicles, or via transdermal routes, using those forms of transdermal
skin patches well known to those of ordinary skill in that art. To be
~dmini.~tered in the form of a transdermal delivery system, the
30 dosage a-lmini~tration will, of course, be continuous rather than
intermittent throughout the dosage regimen.
For combination treatment with more than one active
agent, where the active agents are in separate dosage formulations,
-
CA 02204491 1997-0~-0~
WO 96/14331 PCI/US95/14377
the active agents can be ~lmini~tered concurrently, or they each can
be ~-lmini~tered at separately staggered times.
The dosage regimen utilizing the compounds of the
present invention is selected in accordance with a variety of factors
5 including type, species, age, weight, sex and medical condition of the
patient; the severity of the condition to be treated; the route of
~clmini~tration; the renal and hepatic function of the patient; and the
particular compound thereof employed. A physician or veterinarian
of ordinary skill can readily determine and prescribe the effective
10 amount of the drug required to prevent, counter or arrest the
progress of the condition. Optimal precision in achieving
concentrations of drug within the range that yields efficacy without
toxicity requires a regimen based on the kinetics of the drug's
availability to target sites. This involves a consideration of the
15 distribution, equilibrium, and elimin~tion of a drug.
The modified G-protein coupled receptors of the present
invention are exemplified herein by the neuropeptide Y receptors.
Deletion mutagenesis of the ~2-adrenergic receptor has
shown that none of the hydrophobic clusters of amino acids (the putative
20 transmembrane helices) could be deleted without substantial loss of
binding. In contrast, most of the connecting loops could be deleted
without affecting the ligand binding properties of the receptor. This
indicates that these hydrophilic loops are not required for ligand
binding to the receptor, suggesting that the ligand binding pocket is
25 located predomin~ntly within the transmembrane domain of the protein
(Strader, et al. FASEB J .3: 182-183 (1989)). Deletions in the
connecting loops that were large enough to encompass the entire loop
led to steric problems, resulting in incorrect processing of the protein
(Dixon, et al. EMBO J. 6: 3269-3275 (1987)). Certain connecting loop
30 deletion mutations, however, led to loss of functional activation of
adenylyl cyclase by the receptor. For example, deletion of the carboxy
terminal region of the third intracellular loop attenuated the ability of
the receptor to activate adenylyl cyclase, and deletion of the amino
terminal portion of this loop abolished adenylyl cyclase activation
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
- 28 -
(Strader, et al. J. Biol. Chem. 262: 16439-16443 (1987)). Moreover,
the agonist binding isotherms for these modified receptors displayed a
single affinity site, suggesting altered G protein interactions. Since
these modified receptors also retain their functional activation of Na+-
5 H+ exchange, which is mediated through a different G protein (Barber,et al. Mol. Pharm. 41: 1056-1060 (1992)), the deletions appear not to
result in gross structural perturbations of the receptor, suggesting that
the changes seen in adenylyl cyclase activation are due to alteration of a
specific G protein interaction. Subsequent amino acid replacements in
10 the third intracellular loop confirmed the role of this region in G
protein interaction (Cheung, et al. Mol. Pharm. 41: 1061 - 1065 (1992)).
Modified NPY1 receptors lacking between 6 and 12 amino
acids in the N termin~l portion of the third intracellular loop
(connecting transmembrane helices 5 and 6) may be synthesized. The
15 bottom of transmembrane helix 5 is defined by the presence of a
charged amino acid (human NPY1 Lys233, rat NPYl Lys232) at the
end of a series of hydrophobic amino acids. The modified receptors
include the deletion of 6-12 residues following Lys233 (human) or
Lys232 (rat) (i.e., I234YIRLKRRNNMM; Seq. I.D. No. 1).
20 Alternatively, this sequence could be disrupted by deletion of one or
more of the charged residues (ie., K238, R239 or R240), or
replacement of such residues with alanine or a helix-disrupting residue
such as proline.
A second group of modified NPY1 receptors encompass the
25 deletion of 6-13 residues at the C termin~l end of the third intracellular
loop of the receptor. The C terminus of this loop is defined by the
bottom of helix 6, defined by the presence of the charged residue
Arg260 (human NPY1) or Arg259 (rat NPY1) preceding a stretch of
hydrophobic amino acids. The modified receptors of this group have
30 deletions of 6-13 residues preceding Arg 260 in hllm~n NPY1 (i.e.,
KMRDNKYRSSETK259; Seq. I.D. No. 2) and proceeding Arg259 in rat
NPY1 (i.e., KIRDSKYRSSETK258; Seq. I.D. No. 4). Alternatively,
this sequence could be disrupted by deletion of one or more of the
CA 02204491 1997-0~-0~
WO 96/14331 PCI'/US95/14377
- 29 -
charged residues (ie., R249, D250, K252), or replacement of such
residues with ~l~nine or a helix-disrupting residue such as proline.
Modified NPY2 receptors lacking between 6 and 12 amino
acids in the N terminal portion of the third intracellular loop
5 (connecting transmembrane helices 5 and 6) may be synthesized. The
bottom of transmembrane helix 5 is defined by the presence of a
charged amino acid (Arg241 in rat and hl1m~n) at the end of a series of
hydrophobic amino acids. The modified receptors include the deletion
of 6-12 residues following Arg241 (i.e., I242WSKLKNHVSPG; Seq.
10 I.D. No. 5). Alternatively, this sequence could be disrupted by deletion
of one or more of the charged residues (ie., K244, K246 or H248), or
replacement of such residues with ~l~nine or a helix-disrupting residue
such as proline.
A second group of modified NPY2 receptors encompass the
15 deletion of 6-13 residues at the C terminal end of the third intracellular
loop of the receptor. The C terminus of this loop is defined by the
bottom of helix 6, defined by the presence of the charged residue
(Lys268 in human and rat) preceding a stretch of hydrophobic amino
acids. The modified receptors of this group have deletions of 6-13
20 residues preceding Lys268 in human NPY2 (i.e.,
ANDHYHQRRQK1~267; Seq. I.D. No. 6) and proceeding Lys268 in rat
NPY2 (i.e., AASDHYHQRRHKl-r267; Seq. I.D. No. 7). Alternatively,
this sequence could be disrupted by deletion of one or more of the
charged residues (ie., D257, H258, H260, R262, R263, H264, K265), or
25 replacement of such residues with ~l~nine or a helix-disrupting residue
such as proline.
Modified NPY4 receptors lacking between 6 and 12 amino
acids in the N terminal portion of the third intracellular loop
(connecting transmembrane helices 5 and 6) may be synthesized. The
30 bottom of transmembrane helix 5 is defined by the presence of a
charged amino acid (Arg236 in rat and human) at the end of a series of
hydrophobic amino acids. The modified receptors include the deletion
of 6-12 residues following Arg236 (i.e., I237YRRLQRQGRVF in
hllm~n NPY4 (Seq. I.D. No. 8) and I237YQRLQRQRRAF in rat NPY4
CA 02204491 1997-0~-0~
W0 96/14331 ~C~IUS95/14377
- 30 -
(Seq. I.D. No. 9)). Alternatively, this sequence could be disrupted by
deletion of one or more of the charged residues (ie., R238, R239, R242,
R245), or replacement of such residues with ~l~nine or a helix-
dis~ g residue such as proline.
A second group of modified NPY4 receptors encompass the
deletion of 6-13 residues at the C terrnin~l end of the third intracellular
loop of the receptor. The C terminus of this loop is defined by the
bottom of helix 6, defined by the presence of the charged residue
(Gln263 in hllm~n and Arg263 in rat) preceding a stretch of
hydrophobic amino acids. The modified receptors of this group have
deletions of 6-13 residues preceding Gln263 in human NPY4 (i.e.,
HKGTYSLRAGHMK263; Seq. I.D. No. 10) and proceeding Arg263 in
rat NPY4 (i.e., HTHTCSSRVGQMK263; Seq. I.D. No. 11).
Alternatively, this sequence could be disrupted by deletion of one or
more of the charged residues (ie., H251, K252, R258, H261 ), or
replacement of such residues with ~l~nine or a helix-di~ g residue
such as proline.
Other modified receptors encompass the deletion of 6-13
residues at either the N or C terminal end of the third intracellular loop,
or replacement of residues within this region, of other members of the
family of NPY receptors. The N terminus of the third intracellular
loop (connecting transmembrane helices 5 and 6) is defined by the
presence of a charged or polar amino acid at the end of the fifth series
of hydrophobic amino acids in the sequence of the receptor (helix 5).
The C te~nimls of this loop is located at the bottom of helix 6, defined
by the presence of a charged or polar residue preceding the sixth stretch
of hydrophobic amino acids.
Other modified receptors encompass the addition of 5 to 10
residues at either the N or C terminal end of the third intracellular loop
of the NPY1, 2 or 4 receptors such that the amphipathic nature of these
regions is disrupted.
The following examples are provided to further define the
invention without, however, limiting the invention to the particulars of
the examples.
CA 02204491 1997-0~-05
W O96114331 PCTrUS95/14377
EXAMPLE 1
Deletion of 6-13 amino acids at the N-terminal portion of the third
5 intracellular loop of the hllm~n Neuropeptide Y1 receptor
Modified receptor is constructed by site-directed
mutagenesis of the human neuropeptide Y1 receptor cDNA by standard
molecular biological techniques.
The modified DNA sequence encodes a hllm~n neuropepide
10 Yl receptor lacking between 6 and 13 amino acid residues at the N-
terminal portion of the third intracellular loop. The nucleotide
sequence of the modified receptor is confirmed by DNA sequencing~
As with modified ~2 receptors, the modified NPY receptor is designed
so as to dismpt the proximal portion of the third intracellular loop,
15 without affecting the adjacent fifth transmembrane helix. Thus, the
charged amino acid that delineates the bottom of helix 5 (Lys233) is left
intact in the modified receptor, while the six to thirteen amino acids
which follow it are deleted. The size of the deletion in the present
invention may vary from six to 13 amino acids in this region, beginning
20 immediately after the charged residue at the bottom of transmembrane
helix 5, for example D(234-241)NPYl receptor.
EXAMPLE 2
25 Deletion of 6-13 amino acids at the C-terminal portion of the third
intracellular loop of the hllm~n Neuropeptide Y1 receptor
Modified human NPY1 receptor, lacking 13 residues at the
C-terminal portion of the third intracellular loop (D(247-259)NPYI
receptor), is prepared by standard mutagenesis procedures. The
30 nucleotide sequences of the modified receptors are confirmed by DNA
sequencing. This modified human NPY1 receptor is designed so as to
disrupt the distal portion of the third intracellular loop, without
affecting the adjacent sixth transmembrane helix. Thus, the charged
amino acid that defines the bottom of helix 6 (Lys260) is left intact,
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
- 32 -
while the nearby proximal residues are deleted. The size of the deletion
in the present invention may vary from six to 13 amino acids in this
region, ending immediately before the charged residues at the bottom of
helix 6.
s
EXAMPLE 3
Expression and characterization of the altered Neuropeptide Y1
receptor
COS-7 cells are transfected with the modified receptor
cDNA subcloned into a eukaryotic expression vector such as the
eukaryotic expression vector pcDNA I/neo (Invitrogen). Cells are
harvested after incubation for about 60-72 h. Membranes cont~ining
the expressed receptor protein are prepared as described (C. D. Strader
et al., Proc. Natl. Acad. Sci. U.S.A. 84, 4384-4388 (1987).
Binding reactions are performed in a final volume of 250
,ul of buffer A (50 ,uM Tris, pH 7.4 cont~ining 20 mM CaC12, 5 mM
KCI, 0.2% bovine serum albumin, 10 ,uM phosphoramidon, 40 ~lg/ml
bacitracin and 2 ,ug/ml leupeptin). 125I-NPY or 125I-PYY (0.1 nM) is
incubated with membranes for 2 hr at 25~C before filtration over GF/C
filters presoaked in 0.1% polyethyleneimine. Filters are washed with
ice cold buffer A before analysis of the bound radioactivity by
scintill~tion counting.
Membranes prepared from the COS-7 cells transfected with
a vector cont~ining either the wild type or the modified receptor cDNA
specifically bind a radiolabeled neuropeptide Y receptor radioligand.
The modified receptor is characterized by an absence of coupling to G
proteins, an inability to mediate the activation of second messenger
systems, and an increased affinity for agonists.
The modified neuropeptide Y receptor, when expressed in
m~mm~ n cells, does not stimulate G protein activation in response to
the agonist NPY. In contrast, when the wild type receptor is expressed
in the same cell line, activity is stimulated.
CA 02204491 1997-0~-0~
~ WO 96tl4331 PCT/US95/14377
These modified receptors have increased affinity for
agonists when compared to the wild type receptor. The wild type NPYl
receptor can be described pharmacologically by the relative potency of
peptide ligands: neuropeptide Y= peptide YY > [Leu31Pro34]NPY >
5 NPY[2-36]>> NPY[13-36], with the affinity of NPY in the range of 0.1-
10 nM, and NPY[13-36] having an affinity in the ~M range. The
mutant receptor binds the agonists with the same relative order of
potency. The high affinity of the agonist for the modified receptor is
not affected by agents that uncouple the receptor from the G protein;
10 such agents include the nonhydrolyzable GTP analog GppNHp, sodium
fluoride, and the detergent digitonin. In contrast, the wild type receptor
binds agonists with two affinity states: a high affinity state, indicative of
binding to the receptor-G protein complex, and a low affinity state,
reflecting binding to the uncoupled receptor alone. When the receptor
15 is not optimally coupled to the G protein, a binding assay using the
modified receptor will detect agonists with more sensitivity than will the
identical binding assay using the wild-type receptor.
EXAMPLE 4
Screening Assay usin~ modified Neuropeptide Y1 receptors
Transfected cells expressing recombinant modified receptor
may be used to identify compounds that bind to the receptor with high
affinity. This may be accomplished in a variety of ways, such as by
25 incubating the test compound in a final volume of 0.25 ml of buffer A
with membranes cont~ining 5-7 pM of the modified neuropeptide Y
receptor and 100 pM 125I-PYY or 125I-NPY for 2 hour at 25~. The
reaction is stopped by filtration over GF/C glass fiber filters presoaked
in 0.1% polyethyleneimine, washing with 3 x 5 ml of cold buffer A, and
30 counting the filters in a gamma counter to measure bound radioactivity.
This assay will detect a compound that has a high intrinsic affinity for
the receptor. Such compounds may be either agonists or antagonists.
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
- 34 -
EXAMPLE 5
Deletion of 6-13 amino acids at the N-terrnin~l portion of the third
intracellular loop of Neuropeptide Y receptor subtypes
Modified NPY receptor subtypes (e.g., NPY2, NPY4)
having deletions at the N terminal region of the third intracellular loop
are constructed by site-directed mutagenesis of the neuropeptide Y
receptor cDNA by standard molecular biological techniques.
The modified DNA sequence encodes a neuropepide Y
receptor lacking between 6 and 13 amino acid residues at the N-terminal
portion of the third intracellular loop. The nucleotide sequence of the
modified receptor is confirmed by DNA sequencing. The modified
NPY receptor is designed so as to disrupt the proximal portion of the
third intracellular loop, without affecting the adjacent fifth
transmembrane helix. Thus, the charged amino acid that delineates the
bottom of helix 5 is left intact in the modified receptor, while the six to
thirteen amino acids which follow it are deleted. The size of the deletion
in the present invention may vary from six to 13 amino acids in this
region, be~innin~ imrnediately after the charged residue at the bottom
of transmembrane helix 5.
EXAMPLE 6
Deletion of 6-13 amino acids at the C-terminal portion of the third
intracellular loop of the Neuropeptide Y receptor
Modified NPY receptor subtypes (e.g., NPY2, NPY4)
having deletions at the C termin~l region of the third intracellular loop
are constructed by site-directed mutagenesis of the neuropeptide Y
receptor cDNA by standard molecular biological techniques. The
nucleotide sequences of the modified receptors are confirmed by DNA
sequencing. These modified NPY receptors have disruptions in the
distal portion of the third intracellular loop, without affecting the
adjacent sixth transmembrane helix. Thus, the polar amino acid that
defines the bottom of helix 6 is left intact, while the nearby proximal
CA 02204491 1997-0~-0~
WO 96/14331 PCI'IUS95/14377
residues are deleted. The size of the deletion in the present invention
may vary from six to 13 amino acids in this region, ending immediately
before the polar residues at the bottom of helix 6.
EXAMPLE 7
Expression and characterization of modified NPY Receptor
The modified receptor is subcloned into an expression
vector such as pRC/CMV (Invitrogen,San Diego, CA) and expressed in
10 m~mm~ n cells bytransfection. Approximately 72hours after
transfection, cells are harvested for radioligand binding assays.
For binding assays, the membranes are prepared by
harvesting the cells in ice-cold lysis buffer (5 mg Tris, pH 7.4; 2 mM
EDTA), followed by 15 min centrifugation at 38,000 x g. The
15 membrane pellet is then resuspended in buffer A. Equilibrium binding
to the wild type or modified NPY receptor is performed in a final
volume of 0.25 ml cont~ining membranes, 100 pM 125I-PYY, and
serial dilution of the competing ligands. Binding reactions are
incubated for 2 hr at 25~C, and terminated by rapid filtration over
20 GF/C filters pre-soaked in 0.1% polyethyleneimine. The radioactivity
is quantified with a Packard gamma counter.
These modified receptors have increased affinity for
agonists when compared to the wild type receptor. The wild type
"atypical NPY1" or NPY4 receptor that mediates feeding behavior can
25 be described phar~nacologically by the high affinity of neuropeptide Y,
peptide YY, NPY[2-36], and [Leu31Pro34]NPY, and the lower affinity
of more truncated analogs NPY[13-36] and NPY [20-36], and
structurally by its sequence homology (>45% at the DNA level) to the
NPY1 receptor. The affinity of NPY for the atypical Y1 receptor
30 subtype is in the range of 0.01-10 nM, and that for NPY[13-36] is in the
0.1-10 ~M range. The mutant receptor binds the agonists with the same
relative order of potency as the wild type receptor. The high affinity of
the agonist for the modified receptor is not affected by agents that
uncouple the receptor from the G protein; such agents include the
35 nonhydrolyzable GTP analog GppNHp, sodium fluoride, and the
CA 02204491 1997-0~-05
WO 96/14331 PCI/US95114377
- 36 -
detergent digitonin. In contrast, the wild type receptor binds agonists
with two affinity states: a high affinity state, indicative of binding to the
receptor-G protein complex, and a low affinity state, reflecting binding
to the uncoupled receptor alone. When the receptor is not optimally
S coupled to the G protein, a binding assay using the modified receptor
will detect agonists with more sensitivity than will the identical binding
assay using the wild-type receptor. Other NPY receptor subtypes
(NPY2, NPY3, and others) are also defined pharmacologically by the
relative potencies of peptide ligands for these receptors and str~cturally
10 by their sequence similarity to the NPY 1 receptor. Mutant receptors
having deletions in the third intracellular loop have similar orders of
potency as the corresponding wild type receptor, but with higher
affinity than the wild type receptor in the absence of G protein
coupling.
These modified NPY receptors are readily used in a
screening assay to detect compounds that bind with high affinity to the
NPY receptor subtype, regardless of whether these compounds are
agonists or antagonists.
EXAMPLE 8
Cloning and Expression of Modified NPY Receptor cDNA into
Bacterial Expression Vectors
Recombinant modified receptor is produced in a bacterial
expression system such as E. coli. The modified receptor expression
25 cassette is transferred into an E. coli expression vector; expression
vectors include but are not limited to, the pET series (Novagen). The
pET vectors place modified receptor expression under control of the
tightly regulated bacteriophage T7 promoter. Following transfer of this
construct into an E. coli host which contains a chromosomal copy of the
30 T7 RNA polymerase gene driven by the inducible lac promoter,
expression of modified receptor is induced by addition of an
approl,liate lac substrate (IPTG) is added to the culture. The levels of
expressed modified receptor are determined by the assays described
herein.
CA 02204491 1997-0~-0~
WO 96/14331 PCT/US95/14377
- 37 -
EXAMPLE 9
Cloning and Expression of Modified NPY Receptor cDNA into a
Vector for Expression in Insect Cells
Baculovirus vectors derived from the genome of the
AcNPV virus are designed to provide high level expression of cDNA in
the Sf9 line of insect cells (ATCC CRL# 1711). Recombinant
baculovirus expressing modified receptor cDNA is produced by the
following standard methods (InVitrogen Maxbac Manual): the modified
receptor cDNA constructs are ligated into the polyhedrin gene in a
variety of baculovirus transfer vectors, including the pAC360 and the
BlueBac vector (InVitrogen). Recombinant baculoviruses are generated
by homologous recombination following co-transfection of the
baculovirus transfer vector and linearized AcNPV genomic DNA [Kitts,
P.A., Nuc. Acid. Res. 18, 5667 (1990)] into Sf9 cells. Recombinant
pAC360 viruses are identified by the absence of inclusion bodies in
infected cells and recombinant pBlueBac viruses are identified on the
basis of ~-galactosidase expression (Summers, M. D. and Smith, G. E.,
Texas Agriculture Exp. Station Bulletin No. 1555). Following plaque
purification, modified receptor expression is measured.
Authentic modified receptor is found in association with the
infected cells. Active modified receptor is extracted from infected cells
by hypotonic or detergent lysis.
Alternatively, the modified receptor is expressed in the
Drosophila Schneider 2 cell line by cotransfection of the Schneider 2
cells with a vector cont~ining the modified receptor DNA downstream
and under control of an inducible metallothionin promoter, and a vector
encoding the G418 resistant neomycin gene. Following growth in the
presence of G418, resistant cells are obtained and induced to express
modified receptor by the addition of CuSO4. Identification of
modulators of the modified receptor is accomplished by assays using
either whole cells or membrane preparations.
CA 02204491 1997-0~-0~
WO 96/14331 PCI~/US9~/14377
- 38 -
EXAMPLE 10
Cloning of Modified NPY Receptor cDNA into a yeast expression
vector
S Recombinant modified receptor is produced in the yeast S.
cerevisiae following the insertion of the modified receptor cDNA
cistron into expression vectors designed to direct the intracellular or
extracellular expression of heterologous proteins. In the case of
intracellular expression, vectors such as EmBLyex4 or the like are
ligated to the modified receptor cistron [Rinas, U. et al., Biotechnology
8, 543-545 (1990); Horowitz B. et al., J. Biol. Chem. 265, 4189-4192
(1989)]. For extracellular expression, the modified receptor cistron is
ligated into yeast expression vectors which fuse a secretion signal. The
levels of expressed modified receptor are determined by the assays
described herein.
EXAMPLE 11
Purification of Recombinant Modified NPY Receptor
Recombinantly produced modified receptor may be
purified by a variety of procedures, including but not limited to
antibody affinity chromatography.
Modified receptor antibody affinity columns are made by
adding the anti-modified receptor antibodies to Affigel-10 (Biorad), a
gel support which is pre-activated with N-hydroxysuccinimide esters
such that the antibodies form covalent linkages with the agarose gel bead
support. The antibodies are then coupled to the gel via amide bonds
with the spacer arm. Thè rem~ining activated esters are then quenched
with 1 M ethanolamine HCI (pH 8). The column is washed with water
followed by 0.23 M glycine HCl (pH 2.6) to remove any non-conjugated
antibody or extraneous protein. The column is then equilibrated in
phosphate buffered saline (pH 7.3) together with appropriate membrane
solubilizing agents such as detergents, and the cell culture supernatants
or cell extracts cont~ining solubilized modified receptor or modified
CA 02204491 1997-0~-0~
WO 96/14331 PCI/US9Stl4377
- 39 -
receptor subunits are slowly passed through the column. The column is
then washed with phosphate-buffered saline (PBS) supplemented with
detergents until the optical density (A280) falls to background; then the
protein is eluted with 0.23 M glycine-HCI (pH 2.6) supplemented with
S detergents. The purified modified receptor protein is then dialyzed
against PBS.
EXAMPLE 12
10 Cloning and Expression of Modified NPY Receptor in M~mm~ n
Cell System
A modified receptor is cloned into a m~mm~ n expression
vector. The m~mm~ n expression vector is used to transform a
m~mm~ n cell line to produce a recombinant m~mm~ n cell line.
15 The recombinant m~mm~ n cell line is cultivated under conditions that
permit expression of the modified receptor. The recombinant
m~mm~ n cell line or membranes isolated from the recombinant
m~mm~lian cell line are used in assays to identify compounds that bind
to the modified receptor.
EXAMPLE 13
Screening Assay
Recombinant cells co-lt~ DNA encoding a modified
25 NPY receptor, membranes derived from the recombinant cells, or
recombinant modified receptor preparations derived from the cells or
membranes may be used to identify compounds that modulate modified
NPY receptor activity. Modulation of such activity may occur at the
level of DNA, RNA, protein or combinations thereof. One method of
30 identifying compounds that modulate modified NPY receptor,
comprises:
(a) mixing a test compound with a solution cont~ining
modified NPY receptor to form a mixture;
CA 02204491 1997-05-0~
WO 96114331 PCT/US95tl4377
- 40 -
(b) measuring modified NPY receptor activity in the
mixture; and
(c) comparing the modified NPY receptor activity of the
mixture to a standard.
EXAMPLE 14
Formulation of Pharmaceutical Compositions
Compounds identified by the method of Example 13 are
10 formulated into pharmaceutical compositions according to standard
method.s. The compounds or pharmaceutical compositions are used
either alone or in combination with other compounds or compositions
for the treatment of ~nim~l.s (including humans) in need of treatment.
Conditions requiring treatment include but are not limited to obesity,
15 regulation of apetite, congestive heart failure, diabetes, anxiety,
hypertension, cocaine withdrawal, congestive heart failure, memory
enhancement, cardiac and cerebral vasospasm, pheochromocytoma and
ganglioneuroblastoma, and Huntington's, Alzheimer's and Parkinson's
dlseases.
EXAMPLE 15
Methods of Treatment
~nim~ (including hllm~ns) having a condition, the
25 condition being characterized by factors selected from altered levels of
neuropeptide Y, altered activities of neuropeptide Y, altered levels of
neuropeptide Y receptor activity, altered neuropeptide Y receptor
activity, and combinations thereof, are treated with compounds or
derivatives of compounds identified by the screening method or
30 pharmaceutical compositions comprising the compounds or derivative.s
of compounds identified by the screening method.
~ nim~l.s (including humans) having a condition selected
from obesity, diabetes, anxiety, hypertension, cocaine withdrawal,
congestive heart failure, memory enhancement, cardiac vasospasm,
CA 02204491 1997-0~-0~
WO 96/14331 PCI/US95/14377
- 41 -
cerebral vasospasm, pheochromocytoma and ganglioneuroblastoma,
Huntington's Disease, Alzheimer's Disease, Parkinson's disease, and
combinations thereof, are treated with a therapeutically effective
amount of compounds or derivatives of compounds identified by the
5 screening method or pharmaceutical compositions comprising the
compounds or derivatives of compounds identif1ed by the screening
method.
While the foregoing specification teaches the principles of
10 the present invention, with examples provided for the purpose of
illustration, it will be understood that the practice of the invention
encompasses all of the usual variations, adaptions, or modifications, as
come within the scope of the following claims and its equivalents.
CA 0220449l 1997-0~-0~
W O 96/14331 PCTrUS95/14377
- 42 -
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT: STRADER, CATHERINE D.
CASCIERI, MARGARET A.
MACNEIL, DOUGLAS J.
(ii) TITLE OF INVENTION: MODIFIED NEUROPEPTIDE Y RECEPTORS
(iii) NUMBER OF SEQUENCES: 11
(iv) CORRESPONDENCE ADDRESS:
(A) ADDRESSEE: MARY A. APPOLLINA
(B) STREET: 126 EAST LINCOLN AVENUE
(C) CITY: RAHWAY
(D) STATE: NEW JERSEY
(E) COUNTRY: US
(F) ZIP: 07065-0900
(v) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0, Version #1.30
(vi) CURRENT APPLICATION DATA:
(A) APPLICATION NUMBER: US 08/335,017
(B) FILING DATE: 07-NOV-1994
(C) CLASSIFICATION:
(viii) ATTORNEY/AGENT INFORMATION:
(A) NAME: APPOLLINA, MARY A.
(B) REGISTRATION NUMBER: 34,087
(C) REFERENCE/DOCKET NUMBER: 19339Y PCT
(ix) TELECOMMUNICATION INFORMATION:
(A) TELEPHONE: (908) 594-3462
(B) TELEFAX: (908) 594-4720
(2) INFORMATION FOR SEQ ID NO:1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
CA 0220449l l997-0~-0~
W O 96/14331 PCTrUS95/14377
- 43 -
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:l:
Ile Tyr Ile Arg Leu Lys Arg Arg Asn Asn Met Met
1 5 10
(2) INFORMATION FOR SEQ ID NO:2:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 13 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:2:
Lys Met Arg Asp Asn Lys Tyr Arg Ser Ser Glu Thr Lys
1 5 10
(2) INFORMATION FOR SEQ ID NO:3:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 411 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:3:
Met Asn Ser Thr Leu Phe Ser Gln Val Glu Asn His Ser Asp Phe Leu
1 5 10 15
Val His Ser Asn Phe Ser Glu Lys Asn Ala Gln Leu Leu Ala Phe Glu
Asn Asp Asp Cys His Leu Pro Leu Ala Met Ile Phe Thr Leu Ala Leu
Ala Tyr Gly Ala Val Ile Ile Leu Gly Val Ser Gly Asn Leu Ala Leu
Ile Ile Ile Ile Leu Lys Gln Lys Glu Met Arg Asn Val Thr Asn Ile
Leu Ile Val Asn Leu Ser Phe Ser Asp Leu Leu Val Ala Ile Met Cys
Leu Pro Leu Thr Phe Val Tyr Thr Leu Met Asp His Trp Val Phe Gly
100 105 110
CA 0220449l l997-0~-0~
WO 96/14331 PCI/US95/14377
- 44 -
Glu Ala Met Cys Lys Leu Asn Pro Phe Val Gln Cys Val Ser Ile Thr
115 120 125
Val Ser Ile Phe Ser Leu Val Leu Ile Ala Val Glu Arg His Gln Leu
130 135 140
Ile Ile Asn Pro Arg Gly Trp Arg Pro Asn Asn Arg His Ala Tyr Val
145 150 155 160
~ly Ile Ala Val Ile Trp Val Leu Ala Val Ala Ser Ser Leu Pro Phe
165 170 175
~eu Ile Tyr Gln Val Met Thr Asp Glu Pro Phe Gln Asn Val Thr Leu
180 185 190
Asp Ala Tyr Lys Asp Lys Tyr Val Cys Phe Asp Gln Phe Pro Ser Asp
195 200 205
Ser His Arg Leu Ser Tyr Thr Thr Leu Leu Leu Val Leu Gln Tyr Phe
210 215 220
Gly Pro Leu Cys Phe Ile Phe Ile Cys Tyr Phe Lys Ile Tyr Ile Arg
225 230 235 240
~eu Lys Arg Arg Asn Asn Met Met Asp Lys Ser Glu Gly Arg Phe His
245 250 255
~er Pro Asn Leu Gly Gln Val Glu Gln Asp Gly Arg Ser Gly His Gly
260 265 270
Leu Met Arg Asp Asn Lys Tyr Arg Ser Ser Glu Thr Lys Arg Ile Asn
275 280 285
Ile Met Leu Leu Ser Ile Val Val Ala Phe Ala Val Cys Trp Leu Pro
290 295 300
Leu Thr Ile Phe Asn Thr Val Phe Asp Trp Asn His Gln Ile Ile Ala
305 310 315 320
~hr Cys Asn His Asn Leu Leu Phe Leu Leu Cys His Leu Thr Ala Met
325 330 335
~le Ser Thr Cys Val Asn Pro Ile Phe Tyr Gly Phe Leu Asn Lys Asn
340 345 350
Phe Gln Arg Asp Leu Gln Phe Phe Phe Asn Phe Cys Asp Phe Arg Ser
355 360 365
Arg Asp Asp Asp Tyr Glu Thr Ile Ala Met Ser Thr Met His Thr Asp
370 375 380
Val Ser Lys Thr Ser Leu Lys Gln Ala Ser Pro Val Ala Phe Lys Lys
385 390 395 400
CA 02204491 1997-0~-0~
W O 96/14331 PCTrUS95/14377
- 45 ~
Ile Asn Asn Asn Asp Asp Asn Glu Lys Ile Xaa
405 410
(2) INFORMATION FOR SEQ ID NO:4:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 13 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:4:
Lys Ile Arg Asp Ser Lys Tyr Arg Ser Ser Glu Thr Lys
l 5 l0
(2) INFORMATION FOR SEQ ID NO:5:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:5:
Ile Trp Ser Lys Leu Lys Asn His Val Ser Pro Gly
l 5 l0
(2) INFORMATION FOR SEQ ID NO:6:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 13 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:6:
Ala Asn Asp His Tyr His Gln Arg Arg Gln Lys Thr Thr
l 5 l0
CA 0220449l l997-0~-0~
W O96/14331 PCTtUS9Stl4377
- 46 -
(2) INFORMATION FOR SEQ ID NO:7:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 14 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:7:
Ala Ala Ser Asp His Tyr His Gln Arg Arg His Lys Thr Thr
1 5 10
(2) INFORMATION FOR SEQ ID NO:8:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:8:
Ile Tyr Arg Arg Leu Gln Arg Gln Gly Arg Val Phe
1 5 10
(2) INFORMATION FOR SEQ ID NO:9:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 12 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:9:
Ile Tyr Gln Arg Leu Gln Arg Gln Arg Arg Ala Phe
1 5 10
CA 02204491 l997-0~-0~
WO 96/14331 PCT/US95114377
- 47 -
(2) INFORMATION FOR SEQ ID NO:10:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 13 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:10:
His Lys Gly Thr Tyr Ser Leu Arg Ala Gly His Met Lys
1 5 10
(2) INFORMATION FOR SEQ ID NO:ll:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 13 amino acids
(B) TYPE: amino acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: peptide
(xi) SEQUENCE DESCRIPTION: SEQ ID NO:ll:
His Thr His Thr Cys Ser Ser Arg Val Gly Gln Met Lys
1 5 10