Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02204744 1997-0~-07
Lè A 30 708-~orei~n Countries / S/m/S-P
~T Tr,~N~LA~I~N
DNA se~uences and their use
The present invention relates to a novel DNA sequence and its use for
5 transforming vectors, host org~nism.~ and plants and for producing novel plants
which are male-sterile and which exhibit an altered flower colour.
Male-sterile plants play an important role in plant breeding, in particular in hybrid
breeding. A variety of methods for producing male-sterile plants have already been
disclosed, which methods involve, for example, eliciting cell damage specifically,
10 for example in the anthers, interfering in mitochondrial functions, using antisense
DNA to create opportunities for chemicals to exert a sterilizing effect or inhibiting
chalcone synthesis (cf. WO 90/08830, WO 90/08831, WO 89/10396, EP-A-
0 329 308 and EP-A-0 335 451). However, the methods which have hitherto been
available for producing male-sterile plants do not, in many cases, lead to
15 completely satisfactory results. In addition to this, plants are frequently obtained
which exhibit a considerably increased susceptibility towards fungal pathogens,
m~king it substantially more difficult to handle them in practice. There is,
therefore, a great need for other methods of producing male-sterile plants whichdo not suffer from these disadvantages.
20 The production of plants which exhibit an altered flower colour is of particular
interest for ornamental plant breeding, so that there is considerable interest in new
methods in this field as well.
The novel DNA sequence, which is termed DNA sequence I below, has now been
found, which sequence consists of the following components, which are
25 sequentially ordered in the 5'-3' direction:
a) a promoter, which is heterologous in relation to component b), which is
strongly active in plants and/or which is anther-specific or tapetum-speci~1c,
and which is, where appropriate, located downstream of an amplifying
element (enhancer);
30 b) a DNA sequence encoding stilbene synthase; and
c) a 3 ' polyadenylation sequence;
~ CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
with the term DNA sequence I also encompassing the derived DNA sequences
which still exhibit the features which are essential for implementing the invention.
It has furthermore been found that plants which harbour DNA sequence I in their
genome are, surprisingly, male-sterile and, in addition to this, exhibit a flower
colour which is altered as compared with the corresponding plants which do not
contain the DNA sequence I.
These novel plants additionally possess an increased resistance towards microbial
plant pathogens, in particular towards phytopathogenic fungi. In many cases, thealtered flower colour makes it easier to identify the male-sterile plants readily in a
mixed population, something which can be of considerable practical relevance.
The present invention consequently also relates to novel plants (including parts of
these plants and their replicative material, such as protoplasts, plant cells, calli,
seeds, tubers or cuttings, etc.) which harbour the DNA sequence I in their genome
and which are male-sterile and/or exhibit a flower colour which is altered as com-
pared with the corresponding plants which do not harbour the DNA sequence I.
Promoters which are strongly active in plants and which can be used, in
accordance with the invention, as component a) of the DNA sequence I have been
disclosed. The promoter of the gene of the small subunit of ribulose- 1,5-
bisphosphate carboxylase (rbcS) may be mentioned as an example (cf., e.g.,
EM130 Journal, Vol. 5, No. 9, 2063-2071 (1986)). Furthermore, plant virus
promoters which are strongly active in plants may also be employed. Such
promoters have been disclosed, and the CaMV 35S promoter (cf., e.g., Science
250: 959-960 (1990)) may be mentioned by way of example.
Anther-specific and/or tapetum-specific promoters may also be used as component
a) of the DNA sequence I. Such promoters, which display their activity
particularly strongly in the anthers or in the anther site termed the tapetum, have
been disclosed. The TA29 promoter may be mentioned as an example (cf., e.g.,
Nature 347, 737-741 (1990)). The known anther-specif1c promoters, which have
been isolated from tobacco, of the TA26 and TA13 genes are also suitable for usein accordance with the invention.
' CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
-- 3 --
According to the invention, the CaMV 35S promoter is preferably used as
component a) of the DNA sequence I.
It can be advantageous to place a suitable amplifying element (enhancer) upstream
of the promoter in order to amplify the desired effect of the''promoter. Such
enhancer/promoter constructs have been disclosed. The known CaMV 35S
enhancer, for example, may particularly advantageously be employed as the
enhancer.
According to the invention, the CaMV 35S promoter is particularly preferably
used as component a) of the DNA sequence I. Very particularly preferably, a
construct is employed which consists of the CaMV 35S enhancer and, which
follows it in the CaMV 35S promoter the 5'-3' direction.
The promoter which is to be used in accordance with the invention is heterologous
with regard to component b), i.e. is different from promoters which are found innatural stilbene synthase genes.
The isolation of suitable promoters and enhancers has been disclosed or can be
effected using known processes and methods with which the skilled person is
familiar.
Any DNA which encodes the enzyme stilbene synthase may be used as component
b) in the DNA sequence I. Stilbene synthase is understood to mean any enzyme
which is able (in a suitable environment, in particular in plant cells) to produce
stilbenes. The term stilbenes describes a group of chemical substances which arefound in plants and which contain the stilbene skeleton (trans-
1,2-diphenylethylene) as their common basic structure. This basic skeleton can
also be augmented by the addition of further groups. Two important and preferredstilbenes are 3,5-dihydroxy-stilbene (pinosylvine) and 3,4',5-trihydroxy-stilbene
(resveratrol).
DNA sequences which encode stilbene synthase have been disclosed, for example,
in European Patent Applications EP-A-0 309 862, EP-A-0 464 461 and EP-A-
0 533 010. These patent applications describe the isolation of stilbene synthasegenes and their use for producing transgenic plants which exhibit an increased
resistance to pathogens. The stilbene synthase-encoding DNA sequences which are
' CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
-- 4 -
described in these patent applications are preferably employed in accordance with
the invention, with particular preference being given to the sequences which
encode resveratrol synthase. In addition, preference is given to employing the
stilbene synthase-encoding DNA sequences from groundnut plants (Arachis
hypogaea) and vine (Vitis vinifera) which are described in the said European
patent applications. The DNA sequences which encode stilbene synthase may be
present in the form in which they are contained in the corresponding natural plant
genes ("genomic form") including the noncoding regions (such as introns) which
may be present, or in a form which corresponds to the cDNA (copy DNA) which
can be obtained from mRNA using reverse transcriptase/polymerase and no longer
contains any introns. The sequences may also be present in a form which is
partially or completely synthetic or be assembled from moieties of differing origin.
The stilbene synthase-encoding DNA sequences which are contained in the
plasmid p~S828.1 (EP-A-0 309 862), the plasmid pin5-49 (EP-A-0 533 010) and,
very particularly preferably, the plasmids pVstl, pVst2 and pVstl2t3
(EP-A-0 464 461) are particularly preferably employed in accordance with the
invention, as are the additional stilbene synthase-encoding DNA sequences which
can be isolated from plants with the aid of these DNA sequences (which are used
as probes). Particular emphasis is given to the stilbene synthase-encoding sequence
which is contained in plasmid pVstl (EP-A-0 464 461).
The isolation of the DNA sequences which can be used as component b) of the
DNA sequence I has been disclosed and/or can be effected using the processes andmethods which are known and which are familiar to the skilled person. The regionencoding stilbene synthase may, for example, be isolated from plasmids pVstl,
pVst2 pVstl2t3 or pGS828.1 using the polymerase chain reaction technique (PCR
technique).
The amplification can be effected by PCR using, e.g., the following programmes:
lx 95~C 180 sec
72~C hold (addition of polymerase)
25x 95~C 45 sec
55~C 45 sec
72~C 90 sec
-
~ CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
1x 95~C 45 sec
55~C 45 sec
72~C 300 sec
The Vstl and Vst2 stilbene synthase genes from Vitis vinifera (var. optima) and
5 the stilbene synthase gene from Arachis hypogaea (A. hyp.) can be amplified
using the following primers:
Primer 1 Vstl: see SEQ ID NO: 1
Primer 1 Vst2: see SEQ ID NO: 2
Primer 1 A. hyp.: see SEQ ID NO: 3
10 Primer 2 Vstl: see SEQ ID NO: 4
Primer 2 Vst2: see SEQ ID NO: 5
Primer 2 A. hyp.: see SEQ ID NO: 6
All the coding regions, which have thus been amplified, of the individual genes
can be ligated into the appropriate restriction cleavage sites of customary vectors.
15 In addition, the coding and the termin~ting sequence can also be isolated together
from pSSVstl (cf. below) using the enzymes EcoRI and PstI and also EcoRI and
SphI.
The 3' polyadenylation sequence which is contained in the DNA sequence I as
component c) may be varied to a large extent, so that all the appropriate sequences
20 can be used which do not have a detrimental effect on the expression of the
stilbene synthase in plants. It can also be expedient to employ several (e.g. two)
polyadenylation sequences, where appropriate of differing origin, which are
inserted one after the other, in particular when this ensues as a result of the
techniques which are used on a particular occasion (cf. moiety c) in SEQ ID NO:
25 7). For the sake of simplicity, use is preferably made of the 3' polyadenylation
sequence which is contained in natural stilbene synthase genes, with this sequence
expediently being isolated from the stilbene synthase ~;enes together with the
stilbene synthase-encoding sequence. Consequently, stilbene synthase genes from
which only the natural promoter has been removed may also be employed,
30 according to the invention, as components b) and c). In this case, it is only
,
' CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
-- 6 --
necessary to add on component a) of the DNA sequence I, that is the heterologouspromoter and, where appropriate, the enhancer, upstream.
Suitable 3 ' polyadenylation sequences can be isolated using processes and
methods which are generally customary and which are familiar to the skilled
5 person.
The DNA sequences according to SEQ ID NO: 7, either individually or in the
existing combination, are very particularly preferably used as components a) to c)
of DNA sequence I. In SEQ ID NO: 7, nucleotides 1 to 720 constitute the double
35S CaMV RNA promoter, which consists of the CaMV 35S enhancer and the
CaMV 35S promoter (component a)). Nucleotides 721 to 730 are a synthetic
linker sequence. Nucleotides 731 to 2265 of SEQ ID NO: 7 represent the moiety
encoding stilbene synthase (component b)) and nucleotides 2266 to 2485 representthe polyA moiety (component c)) of the stilbene synthase gene. The nucleotides
from 2486 to 2728 represent the moiety of component c) which is derived from
15 CaMV 359 RNA, with polylinker sequences being present at the end.
The term DNA sequence I also includes all the derived DNA sequences which
still exhibit the features which are essential for implementing the invention, which
sequences consequently elicit male sterility, and may elicit a change in flower
colour, in plants. In such derived sequences, individual DNA's, codons and/or
20 constituent sequences may be lacking (for example due to the use of restriction
enzymes) and/or replaced by other DNA's, codons and/or constituent sequences.
These modifications may be present due to the degeneracy of the genetic code or
arise during manipulation of the DNA sequences. The novel DNA sequences
and/or their components a) to c) may also contain DNA's and/or DNA sequences
25 which make them easier to handle, for example so-called linkers or those of these
linkers which remain after manipulating (for example after cutting with restriction
enzymes). Components a) to c) of the DNA sequence I can be of natural origin or
be present in a form which is partially or completely synthesized.
Components a~ to c) can be joined to form the DNA sequence I, which can also
30 be regarded as a "chimeric gene", using the processes and methods which are
generally customary and which are familiar to the skilled person.
CA 02204744 1997-OF7-07
Le A 30 708-Forei~n Countries
In a particular embodiment of the invention, the DNA sequence I consists of (a)
the so-called CaMV 35S double promoter, which is made up of the CaMV 35S
promoter and the appurtenant CaMV 35S enhancer, and (b) the sequence encoding
stilbene synthase (resveratrol synthase), together with the following
3' polyadenylation sequence, as is present in plasmid pVstl (cf. EP-A-0 464 461).
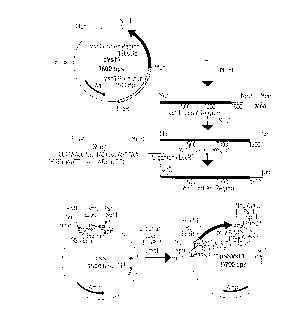
This DNA sequence is co~-ained in the novel plasmid pSSVstl, whose
construction is shown in Fig. 1. The coding region of the stilbene synthase geneVstl can, accordingly, be isolated, as a 2.1 kB MunI fragment, from plasmid
pVstl, which contains the complete stilbene synthase gene (Vstl gene) as a
10 4.9 kB EcoRI fragment. However, this MunI fragment lacks the first 4 codons at
the 5 ' end of the coding region. Expedientl~, the purified MunI fragment is
subsequently digested with restriction enzyme NruI and the resulting 1.7 kB
NruI/MunI fragment is fused to an oligonucleotide linker which encodes the firstfour amino acids. Since the protruding ends of the EcoRI and MunI restriction
15 cleavage sites are identical and it is necessary to prevent a MunI/Eco:RI fusion, the
oligonucleotide linker is designed such that the EcoRI cleavage site is only formed
by a subsequent restriction digestion. The resulting NruI/EcoRI fragment is ligated
between the SmaI and EcoRI cleavage sites of the shuttle vector pSS such that the
complete coding region of the Vstl stilbene synthase gene is under the control of
20 the double 35S promoter. However, corresponding constructs can be prepared,
using the customary methods, by the skilled person on the basis of his specialist
knowledge and the information contained in the present text, and then put to use.
The Escherichia coli strain RH pSSVstl harbours plasmid pSSVstl. This E. coli
strain, RH pSSVstl, was deposited in the Deutsche Sammlung von Mikro-
25 organismen (DSM) [German collection of microorg~ni~cm~], Mascheroder Weg lB,D-3 8124 Braunschweig, Federal Republic of Germany, in conformity with the
requirements of the Budapest treaty on the international deposition of micro-
org~ni.~m~ for the purposes of patent processes, on 18 October 1994, and was
given the deposition number DSM 9501.
30 Plasmid pSSVstl, and E. coli strain RH pSSVstl, and its mutants which still
exhibit the features of the deposited strain which are essential for implementing
the invention, are likewise part of the present invention.
CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
E. coli strain RH: pSSVstl can be replicated using the methods which are
generally customary. Plasmid pSSVstl can likewise be isolated from this E. coli
strain using the methods which are generally customary. It is also an easy matter
for the skilled person to isolate the DNA sequence I which is contained in plasmid
pSSVstl. Thus, the DNA sequence I which is contained in plasmid pSSVstl can,
for example, be isolated from this plasmid, in the form of an approximately 2700bp (base pair)-sized DNA fragment, using the restriction enzymes SphI and PstI.
It is possible, using the methods which are customary and which are familiar to
the skilled person, to incorporate the DNA sequence I once or more than once
(e.g. tandem arrangement), preferably once, as "foreign" DNA, into any proka-
ryotic (preferably bacterial) or eukaryotic (preferably plant) DNA. The recombi-nant DNA which has thus been "modified", and which can be used, for example,
for transforming plants or plant cells, and which, after the transformation, is
contained in plants or plant cells, is a constituent part of the present invention.
The DNA sequence I, and the recombinant DNA, can be contained, as "foreign"
DNA, in vectors (in particular plasmids, cosmids or phages), in transformed
microorganisms (preferably bacteria, in particular Gram-negative bacteria, such as
E. coli) and also in transformed plant cells and plants, or in their DNA. Such
vectors, transformed microorganisms (which may also harbour these vectors) and
also the transformed plant cells and plants, and their DNA, represent constituent
parts of the present invention. As already intimated, the DNA sequence I is,
according to the invention, incorporated once or more than once (at the same or
different sites in the genome) into the natural plant genome.
The present invention consequently also relates to a process for preparing
transgenic plant cells (including protoplasts) and plants (including plant parts and
seeds), where these plants are male-sterile and may exhibit an altered flower
colour, which process is characterized in that
(a) the DNA sequence I and/or novel recombinant DNA is/are inserted, once
or more than once7 into the genome of plant cells (including protoplasts)
and, where appropriate,
~ CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
g
(b) complete, transformed plants are regenerated from the transformed plant
cells (including protoplasts) and, where appropriate, replicated, and, where
appropriate,
(c) the desired plant parts (including seeds) are isolated from the resulting
transgenic plants of the parental generation or other generations which are
obtained therefrom.
Process steps (a), (b) and (c) can be carried out, in a customary manner, using
known processes and methods.
Transgenic plant cells (including protoplasts) and plants (including plant parts and
10 seeds) which harbour, once or more than once, the DNA sequence I, as "foreign"
DNA, and the descendants thereof, and also those transformed plant cells and
plants which can be obtained using the above processes, and the descendants
thereof, likewise belong to the present invention.
The following are also parts of the present invention:
15 (a) the use of the DNA sequence I and/or the novel recombinant DNA and/or
the novel recombinant vectors and/or the novel transformed micro-
org~ni~m~ for transforming plant cells (including protoplasts) and plants
(including plant parts and seeds),
(b) the use of the novel transgenic plant cells (including protoplasts) and plants
(including plant parts and seeds) for producing replicative material and also
for producing new plants and their replicative material,
(c) the use of the novel DNA sequence I and/or the novel recombinant DNA
for producing male sterility and, where appropriate, an altered flower
colour in plants,
25 (d) the use of the DNA sequence I, which is contained in plasmid pSSVstl, for
detecting the presence of the DNA sequence I in plants and also (generally)
in the production of transgenic plant cells (including protoplasts) and plants
(including plant parts and seeds), and also
~ CA 02204744 1997-0~-07
Le A 30 708-Foreign Countries
- 10 -
(e) the use of the stilbene synthase-encoding DNA sequence for producing
transgenic plants which are male-sterile and/or exhibit a flower colour
which is altered as compared with corresponding plants which do not
harbour this DNA in their genome.
A number of different methods are available for incorporating the DNA sequence
I, as "foreign" DNA, into the genetic material of plants or plant cells. The gene
transfer can be effected using the generally customary, known methods, with it
being possible for the skilled person to ascertain without difficulty the suitable
method in each case.
The Ti plasmid of Agrobacterium tumefaciens is available as a particularly
favourable and widely applicable vector for transferring foreign DNA into the
genomes of dicotyledonous and monocotyledonous plants. For this, the DNA
sequence I is inserted, in an appropriate manner, into the T-DNA of suitable Ti
plasmids (e.g. Zambryski et al., 1983) and transferred by infecting the plant,
infecting plant parts or plant tissues, such as leaf discs, stems, hypocotyles,
cotyledons or meristems, and tissues derived therefrom, such as secondary
embryos and calli, or by coculturing protoplasts with Agrobacterium tumefaciens.
An alternative is to incubate the DNA sequence I, or recombinant DNA, with
plant protoplasts (e.g. Hain et al., 1985; Krens et al., 1982; Paszkowski et al.1984) in the presence of polycations or calcium salts and polyethylene glycol.
The DNA uptake can also be additionally assisted by means of an electrical field(electroporation) (e.g. Fromm et. al., 1986).
The DNA can also, in a known manner, be introduced by way of plant pollen, for
example by "bombarding" pollen or plant tissue with physically accelerated
particles which are carrying the DNA (cf. EP-A 0 270 356).
The plants are regenerated in a known manner using suitable nutrient media (e.g.Nagy and Maliga 1976).
In a preferred embodiment of the novel process (according to the method from
EP-A 116 718), the DNA sequence I, as contained in plasmid pSSVstl, is cloned
into a suitable intermediate E. coli vector, for example pGV700 or pGV710 (cf.
-
~ CA 02204744 1997-0~-07
Le A 30 708-~orei~n Countries
11
EP-A-116 718), or preferably derivatives thereof which additionally contain a
reporter gene such as nptII (Herrera-Estrella et al. 1983) or hpt (Van den Elzen et
al. 1986).
The plasmid which has been constructed in this way is transferred, using
5 customary methods (e.g. Van Haute et al. 1983), into Agrobacterium tumefacienswhich harbours pGV 3850, for example, or derivatives thereof (Zambryski et al.
1983). Alternatively, the DNA sequence I can be cloned into a shuttle vector, for
example PCV001 or PCV002 (e.g. Koncz and Schell 198~) and transferred, as
described above, into a suitable Agrobacterium strain (Koncz and Schell 1986).
10 The resulting Agrobacterium strain, which harbours the DNA sequence I in a form
which is transferrable to plants, is then used for the plant transformation. Plasmid
pSSVstl can also be introduced directly into a suitable A. tumefaciens strain (cf.,
e.g., Koncz and Schell (1986)).
In another preferred embodiment, plasmid pSSVstl, which contains a kanamycin-
15 resistance reporter gene for plant cells (e.g. Herrera-Estrella et al . 1983), is
transferred, by direct gene transfer, in a customary manner, into plant protoplasts
(e.g. Hain et al., 1985). While plasmid pSSVstl can be in circular form for thispurpose, it is preferably in linear form. When pSSVstl containing the reporter
gene is used, kanamycin-resistant protoplasts are then examined for expression of
20 stilbene synthases.
Transformed (transgenic) plants or plant cells are produced in accordance with
known methods, for example by means of transforming leaf discs (e.g. Horsch et
al., 1985), by means of coculturing regenerating plant protoplasts or cell cultures
with Agrobacterium tumefaciens (e.g. Marton et al., 1979, Hain et al., 1985) or by
25 means of direct transfection with DNA. Resulting transformed plants are detected
either by selecting for expression of the reporter gene, for example by the
phosphorylation of kanamycin sulphate in vitro (Reiss et al., 1984; Schreier et al.
1985) or by screening for expression of nopaline synthase (in accordance with
Aerts et al. 1983) or stilbene synthase by means of Northern blot analysis and
30 Western blot analysis. The stilbene synthase, and the stilbenes, can also be
detected in transformed plants, in a known manner, with the aid of specific anti-
bodies. Stilbene synthase can also be detected by means of an enzyme activity test
(Rolfs et al., Plant Cell Reports 1, 83-85, 1981).
' CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
Cultivation of the transformed plant cells and regeneration into complete plants are
carried out in accordance with the generally customary methods, using the nutrient
media which are suitable in each case.
Both the transformed plant cells and the transformed plants which harbour the
5 novel DNA sequence I, and which are constituent parts of the present invention,
exhibit a substantially greater resistance to pathogens, in particular phytopatho-
genic fungi.
In connection with the present invention, the term "plants" denotes complete
plants, plant parts, such as leaves, stems or roots, and replicative material, such as
10 seeds, tubers, cuttings, etc. "Plant cells" encompasses protoplasts, cell lines, plant
calli, etc.
As has already been explained, plants which harbour the novel DNA sequence I in
their genome exhibit male sterility and may also exhibit a flower colour which is
altered as compared with the corresponding plants which do not harbour the DNA
15 sequence I.
In the case of ornamental plants and fiowers for cutting, for example roses,
carnations, freesias, gerbera, etc., the flower colour is of considerable commercial
importance. Influencing flower colours in a specific manner, and achieving stable
flower colours, is frequently a difficult and elaborate matter. The present invention
20 makes it possible, in a relatively simple manner, to alter the flower colour of all
plants which have coloured flowers and which possess flower pigments, in
particular anthocyanins. As a rule, the flowers become lighter, and frequently
completely white, as a result of incorporatior~~ of the DNA sequence I. In general,
a change cannot be identified, or can only be identified with difficulty in the case
25 of plants which do not have coloured flowers.
The male sterility of plants plays a very important role in plant breeding with
regard to the production of hybrid lines and hybrid seeds. Unfortunately, many
hybrid lines are very susceptible to phytopathogenic fungi, thereby greatly
restricting their usability. Male-sterile plants can be produced relatively simply
30 with the aid of the present invention. These plants additionally exhibit an
increased resistance towards microbial plant pathogens such as phytopathogenic
fungi, bacteria and/or viruses, in particular towards phytopathogenic fungi, and are
CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
consequently superior to male-sterile plants which are obtained using other
rnethods.
Practically all plants are included in the plants which can be rendered male-sterile
by incorporation (transformation) of the novel DNA sequence I. ~aturally, there is
a particular need in this regard in the case of cultivated plants such as plantswhich supply foodstuffs and raw materials, for example cereal plants (in particular
wheat, rye, barley, oats, millet, rice and maize), potatoes, leguminosae (such as
pulse crops and, in particular, alfalfa and soya beans), vegetables (in particular
cabbage varieties and tomatoes), fruit (in particular apples, pears, cherries, grapes,
citrus fruits, pineapples and bananas), oil palms, tea, cocoa and coffee bushes,tobacco, sisal and cotton, and also in he case of medicinal plants, such as
rauwolfia and digitalis. Rice, wheat, barley, rye, maize, sugar beet, rape and soya
may be mentioned as being particularly preferred.
The following exemplary embodiments are intended to clarify the present
1 5 invention:
I) Transformation of plants
1. Construction and description of vector pSSVstl
The construction of plasmid pSSVstl has already been explained in detail
above and is depicted in Fig. 1 in such a way that it can be readily
comprehended by the skilled person.
Plasmid pSSVstl is a derivative of pSS. pSS is a derivative of PCV001
(Koncz and Schell, 1986), which contains an expression cassette which is
based on plasmid pRT101 (Topfer et al., 1987) and in which the CaMV
35S RNA enhancer has been duplicated by cloning the Ddel/EcoRV
fragment into the Hincl 1 cleavage site. pSSVstl contains the coding
sequence and the polyA sequence of pVstl stilbene synthase (cf. Fig. 1).
pSSVstl contains a plant kanamycin resistance and a bacterial ampicillin
resistance. In addition, pSSVstl contains border sequences from the
Agrobacterium tumefaciens Ti-plasmid and a replication start for A.
tumefaciens and E. coli (Koncz and Schell, 1986). Plasmid pSSVstl can be
CA 02204744 1997-05-07
Le A 30 708-Forei~n Countries
- 14 -
mobilized directly into a suitable Agrobacterium tumefaciens strain (e.g.
Koncz and Schell 1986) using the strain E. coli RH pSSVstl.
~ 2 Transformation of tobacco
a) Culturing tobacco shoots and isolation of tobacco protoplasts:
Nicotiana tabacum (Petit Havana SR1) is replicated as a sterile
shoot culture on hormone-free LS medium (Linsmaier and Skoog
1965). At intervals of approx. 6-8 weeks, shoot segments are
transferred to fresh LS medium. The shoot cultures are kept in a
culture room at 24-26~C while being exposed to 12 h of light
(1000-3000 lux).
In order to isolate leaf protoplasts, approx. 2 g of leaves (approx.
3-5 cm in length) are cut into small pieces (0.5 cm x 1 cm) using a
fresh razor blade. The leaf material is incubated, at room
temperature for 14-16 h, in 20 ml of enzyme solution, consisting of
K3 medium (Nagy and Maliga 1976), 0.4 m sucrose, pH 5.6, 2%
R10 cellulase (Serva), 0.5% R10 Macerozyme (Serva). After that,
the protoplasts are separated from cell residues by filtration through
0.30 mm and 0.1 mm steel sieves. The f1ltrate is centrifuged at
100 x g for 10 minutes. During this centrifugation, the intact
protoplasts float and collect in a band at the upper margin of the
enzyme solution. The pellet, consisting of cell residues, and the
enzyme solution are sucked off using a glass capillary. The
prepurif1ed protoplasts are made up to 10 ml with fresh K3 medium
(0.4 M sucrose as osmotic agent) and floated once again. The wash
medium is sucked off and the protoplasts are diluted to 1-2 x
105/ml for culture and subsequent infection with agrobacteria
(coculture). The protoplast concentration is determined in a counting
chamber.
b) Transformation of regenerating tobacco protoplasts by coculture with Agrobacterium tumefaciens:
CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
In that which follows, the method of Marton et al. 1979 is used
with slight modifications. The protoplasts are isolated as described
and incubated, at 26~C and at a density of 1-2 x 105/ml, in K3
medium (0.4 m sucrose, 0.1 mg/l NAA, 0.2 mg of kinetin) for 2
days in the dark and for from 1 to 2 days under-weak light (500
lux). As soon as the first divisions of the protoplasts appear, 30 ~11
of a suspension of agrobacteria, which harbour the sequence I in
their T-DNA or harbour plasmid pSSVstl, in minimal A (Am)
medium (density, approx. 109 agrobacteria/ml), are added to 3 ml of
regenerating protoplasts. The duration of the coculture, at 20~C and
in the dark, is 3-4 days. After that, the tobacco cells are loaded into
12 ml centrifuge tubes, diluted to 10 ml with sea water (600
mOsm/kg) and pelleted at 60 x g for 10 minutes. This washing
procedure is repeated a further 1-2 x in order to remove the
majority of the agrobacteria. The cell suspension is cultured, at a
density of 5 x 104/ml, in K3 medium (0.3 m sucrose) cont~ining 1
mg of NAA (naphthyl-1-acetic acid) per l, 0.2 mg of kinetin per l
and 500 mg of the cephalosporin antibiotic cefotaxime per l. Each
week, the cell suspension is diluted with fresh K3 medium and the
osmotic volume of the medium is gradually reduced by 0.05 m
sucrose (approx. 60 mOsm/kg) per week. Selection with kanamycin
(100 mg/l kanamycin sulphate (Sigma), 660 mg/g active Km) is
started in agarose bead-type culture (Shillito et al. 1983) 2-3 weeks
after the coculture. 3-4 weeks after beginning the selection, it is
possible to distinguish kanamycin-resistant colonies from the
background of retarded colonies.
c) Direct transformation of tobacco protoplasts with DNA. Calcium
nitrate/PEG transformation
Approx. 106 protoplasts in 180 !11 of K3 medium are carefully
mixed, in a petri dish, with 20 ,ul of aqueous DNA solution which
contains 0.5 llg of plasmid pSSVstl, or the isolated DNA sequence
I from pSSVstl, per 1ll, as the DNA fragment, and 0.5 Ill of
pLGVneo2103 per 1ll (Hain et al. 1985). 200 1ll of fusion solution
(0.1 m calcium nitrate, 0.45 M mannitol, 25% polyethylene glycol
(PEG 6000), pH 9) are then added carefully. After 15 minutes, 5 ml
' CA 02204744 1997-0~-07
~e ~ 30 708-Forei~n Countries
- 16 -
of wash solution (0.275 M calcium nitrate, pH 6) are added and,
after a further 5 minutes, the protoplasts are transferred into a
centrifuge tube and pelleted at 60 x g. The pellet is taken up in a
small quantity of K3 medium and cultured as described in the next
section. Alternatively, the protoplasts can be transformed as
described by Hain et al. 1985.
The transformation with the DNA sequence I from pSSVstl can
also be carried out without adding the 0.5 ,ug of pLGVneo2103 per
,ul. Since no reporter gene is employed in this case, dot blot
hybridization is used to examine the resulting calli for the presence
of the DNA sequence I gene unit. The coding sequence from
pSSVstl can be used as the hybridization probe. Naturally, other
detection methods, such as tests with antibodies or an enzyme test
for stilbene synthase, can also be employed.
d) Culturing the protoplasts which have been incubated with DNA and
selecting kanamycin-resistant calli:
A modified bead-type culture technique (Shillito et al. 1983) is used
for the culture and selection of kanamycin-resistant colonies
described below. One week after treating the protoplasts with DNA
(cf. c), 3 ml of the cell suspension are mixed, in 5 cm petri dishes,
with 3 ml of K3 medium (0.3 M sucrose + hormones; 1.2%
(Seaplaque) LMT agarose (low-melting agarose, Marine colloids).
For this purpose, agarose is autoclaved in the dry state, and, after
K3 medium has been added, is boiled briefly in a microwave oven.
After the agarose has solidified, the agarose beads containing the
embedded tobacco microcalli are transferred, for further culture and
selection, into 10 cm petri dishes and in each case 10 ml of K3
medium (0.3 M sucrose, 1 mg/l NAA, 0.2 mg/l kinetin) and
100 mg/l kanamycin sulphate (Sigma) are added. The liquid
medium is changed each week. In association with this, the osmotic
value of the medium is lowered stepwise.
The sucrose concentration in the replacent medium (K3 + Km) is
reduced by 0.05 m (approx. 60 mOsm) each week.
-
CA 02204744 1997-OF7-07
Le A 30 708-Forei~n Countries
Scheme for selecting kanamycin-resistant tobacco colonies
following DNA transformation:
0.4 M 0.3 M 0.25 M 0.20 M 0.15 M 0.10 M Sucrose in the
liquid medium
U ES K
1 2 3 4 5 6 weeks after DNA
uptake
(K3 mediurn, 1 m~, of NAA, 0.2 mg of kinetin)
U = DNA uptake
E= embeddin~ in agarose
S = selection with kanamycin (100 mg of kanamycin sulphate/l)
K= kanamycin-resistant colonies can be clearly distinguished from the
1 0 background
e) Regeneration of kanamycin-resistant plants:
As soon as the kanamycin-resistant colonies have reached a dia-
meter of approx. 0.5 cm, half of them are placed on regeneration
medium (LS medium, 2% sucrose, 0.5 mg/l benzylaminopurine
BAP) and kept at 24~C in a culture room while being exposed to
12 h of light (3000-5000 lux). The other half is propagated as a
callus culture on LS medium cont~ining 1 mg/l NAA, 0.2 mg/1
kinetin, 0.1 mg/l BAP and 100 mg/l kanamycin sulphate. When the
regenerated shoots are approx. 1 cm in size, they are cut off and
placed, for rooting, on 1/2 LS medium (1% sucrose, 0.8% agar)
without growth regulators. The shoots are rooted on 1/2 MS
medium containing 100 mg/l kanamycin sulphate and subsequently
transplanted into soil.
-- --
~ CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
f) Transformation of leaf discs with Agrobacterium tumefaciens
For the transformation of leaf discs (Horsch et al. 1985), leaves of
approx. 2-3 cm in length from sterile shoot cultures are punched
into discs of 1 cm in diameter and incubated, for approx. 5 minutes,
with a suspension (approx. 109/ml) (cf. b) of appropriate agro-
bacteria, which harbour plasmid pSSVstl or the DNA sequence I
from this plasmid in their T-DNA, in Am medium (see below). The
infected leaf pieces are kept at approx. 24~C for 3-4 days on MS
medium (see below) without hormones. During this time,
Agrobacterium overgrows the leaf pieces. The leaf pieces are then
washed in MS medium (0.5 mg/ml BAP, 0.1 mg/ml NAA) and
placed on the same medium (0.8% agar) containing 500 g/ml
cefotaxime and 100 g/ml kanamycin sulphate (Sigma). The medium
should be renewed after two weeks. Transformed shoots are visible
after a further 2-3 weeks.
Biochemical method for detectin~ transformation
Neomycin phosphotransferase (NPT II) enzyme test:
NPT II activity in plant tissue is detected by the in-situ phosphorylation of
kanamycin, as described in Reil3 et al. (1984) and modified by Schreier et
al. (1985), as follows. 50 mg of plant tissue are homogenized, on ice and
in the presence of added glass powder, in 50 !11 of extraction buffer (10%
glycerol, 5% 2-mercaptoethanol, 0.1% SDS, 0.025% bromophenol blue,
62.5 mM Tris, pH 6.8) and centrifuged, at 4~C for 10 minutes, in an
Eppendorf centrifuge. 50 1ll of the supernatant are loaded onto a native
polyacrylamide gel (145 x 110 x 1.2 mm; resolving gel: 10% acrylamide,
0.33% bisacrylamide, 0.375 M tris, pH 8.8, stacking gel: 5% acrylamide,
0.165% bisacrylamide, 0.125 M tris, pH 6.8) and electrophoresed overnight
at 4~C and 60 V. As soon as the bromophenol blue marker has run out of
the gel, the latter is washed twice with distilled water for 10 min and once
with reaction buffer (67 mM Tris-maleate, pH 7.1, 42 mM MgCl2,
400 mM ammonium chloride) for 30 min. The gel is laid on a glass plate
of the same size and overlaid with 40 ml of 1% agarose in reaction buffer
which contains the substrates kanamycin sulphate (20 g/ml) and 20-200 Ci
CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
- 19 -
of 32p ATP (Amersham). The sandwich gel is incubated at room
temperature for 30 min and a sheet of phosphocellulose P81 paper
(Whatman) is then laid on the agarose. Four layers of 3 mm filter paper
(Whatman) and some paper towels are piled on top. The transfer of in-situ
phosphorylated, radioactive kanamycin phosphate to thë P8 1 paper is
stopped after 3-~ h. The P81 paper is incubated, at 60~C for 30 min., in a
solution of proteinase K and 1% sodium dodecyl sulphate (SDS) and then
washed, at 80~C, 3-4 times in 250 ml of 10 mM phosphate buffer, pH 7.5,
dried and autoradiographed (Kodak XAR5 film) at-70~C for 1-12 h.
The presence of the DNA sequence encoding stilbene synthase in the plant
cells and plants (tobacco) which were obtained in accordance with the
above examples was conf1rmed by Southern blot analysis. Expression of
the sequence encoding stilbene synthase was demonstrated by Northern
blot analysis, while stilbene synthase and resveratrol were demonstrated
with the aid of specific antibodies. Transformed plants and nontransformed
plants (for comparison) were cultivated in a greenhouse through to
flowering. The transformed plants exhibited a flower colour which was
altered (as compared with the nontransformed comparison plants) and were
male-sterile.
The media employed in transforming plants and plant cells are described,
inter alia, in EP-A 0 309 862:
All the percentage values in the above examples and in the example below
- refer to percentages by weight, unless otherwise indicated.
II) Checking the transgenic plants for altered flower colour and for male
sterility.
Example A
The transgenic tobacco plants which were obtained in accordance with the above
examples are preraised in tissue culture and then raised, through to flowering, in a
greenhouse at 23~C and 70-80% relative atmospheric humidity. They are supplied
30 with fertilizer and water as required.
CA 02204744 1997-0~-07
Le A 30 708-~orei~n Countries
- 20 -
All the plants which were transformed in accordance with Example I) exhibited a
white or whitish-pink flower colour which was retained in the F1 generation evenafter back-crossing with the wild type, whereas the corresponding control plants,
which had not been transformed, exhibited a strong red, dark pink or crimson
5 colour.
All the transformed plants were also male-sterile, with this sterility being retained
in the F1 generation as well.
The following publications may be cited with regard to the transformation of
plants:
10 Fraley RT, Rogers SG, Horsch RB, Sanders PR, Flick JS, Adams SP, Bittner ML,
Brand LA, ~ink CL, Fry JS, Fallupi GR, Goldberg SB, Hoffmann NL, Woo SC
(1983). Expression of bacterial genes in plant cells. Proc. Natl. Acad. Sci. USA80:4803 -4807.
Fromm ME, Taylor LP, Walbot V (1986) Stable transformation of maize after
gene transfer by electroporation. Nature 319: 791-793
Hain R, Stabel P, Czernilofsky, AP, Steinbil3, HH, Herrera-Estrella, L Schell, J(1985) Uptake, integration, expression and genetic transmission of a selectable
chimeric gene by plant protoplasts. Molec Gen Genet 199: 161-168
Hain R, Bieseler B, Kindl H, Schroder G, Stocker R (l990) Expression of a
20 stilbene synthase gene in Nicotiana tabacum results in synthesis of the phytoalexin
resveratrol. Plant Mol, Biol. 15:325-336.
Hain R, Reif HJ, Krause E, Langbartels R, Kindl H, Vornam B, Wiese W,
Schnetzer E, Schreier PH, Stocker RH, Stenzel K (1993) Discase resistance results
from foreign phytoalexin expression in a novce plant. Nature 361: 153-156
25 Hernalsteens JP, Thia-Tong L, Schell J, Van Montagu M (1984) An
Agrobacterium-transformed Cell culture from the monocot Asparagus officinalis.
EMBO J 3:3039-3041
-
CA 02204744 1997-0~-07
~ Le A 30 708-Forei~n Countries
- 21 -
Herrera-Estrella L, De Block M, Messens E, Hernalsteens JP, van Montagu M,
Schell J (1983) EMBO J. _: 987-995.
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A
simple and general method for transferring genes into plants. Science 277: 1229-1231
Krens FH, Molendijk, Wullems GJ, Schilperoort RA (1 982) In vitro
transformation of plant protoplasts with Ti-plasmid DNA. Nature 296: 72-74
Koncz C, Schell J (1986) The promoter of TL-DNA gene 5 controls the tissue-
specific expression of çhim~eric genes carried by a novel type of Agrobacterium
linary vector. Mol. Gen. Genet. (1986) 204: 338-396
Linsmaier DM, Skoog F (1965) Organic growth factor requirements of tobacco
tissue cultures. Physiol Plant 18: 100-127
Marton L, Wullems GJ, Molendijk L, Schilperoort PR (1979) In vitro
transformation of cultured cells from Nicotiana tabacum by Agrobacterium
tumefaciens. Nature 277: 1229-131
Melchior F, Kindl H (1990) Grapevine stilbene synthase cDNA only slightly
differing from chalcone synthase cDNA is expressed in Escherichia coli into a
catalytically active enzyme FEBS 268:17-20
Nagy JI, Maliga P (1976) Callus induction and plant regeneration from mesophyll
protoplasts of Nicotiana sylvestris. Z Pflanzenphysiol 78: 453-455
Otten LABM, Schilperoort RA (1978) A rapid microscale method for the detection
of Lysopin and Nopalin dehydrogenase activities. Biochim biophys acta 527: 497-
5Q0
Paszkowski J, Shillito RD, Saul M, Mandak V, Hohn T, Hohn B, Potrykus I
(1984) Direct gene transfer to plants. EMBO J 3:2717-2722
Rolf CH, Fritzemeier KH and Kindl H (1981) Cultured cells of Arachis hypogaea
susceptible to induction of stilbene synthase (resveratrol forming) Plant Cell. Rep.
1 :83-85
' CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
Schroder G, Brown JWS and Schroder J (1988) Molecular analysis of resveratrol
synthase: cDNA, genomic clones and relationship with chalconsynthase. Eur. J.
Biochem. 172, 161-169
Shillito RD, Paszkowski J, Potrykus I (1983) Agarose plating-and Bead type
5 culture technique enable and stimulate development of protoplast-derived colonies
in a number of plant species. Pl Cell Rep 2: 244-247
Van den Elzen PJM, Townsend J, Lee KY, Bedbrook JR (1985) A chimaeric
resistance gene as a selectable marker in plant cells. Plant Mol. Biol. 5, 299-302
Velten J, Velten L, Hain R, Schell J (1984) Isolation of a dual plant promoter
fragment from the Ti Plasmid of Agrobacterium tumefaciens. EMBO J 12: 2723-
2730
Van Haute E, Joos H, Maes M, Warren G, Van Montagu M, Schell J (1983)
Intergenic transfer and exchange recombination of restriction fragments clones in
pBR 322: a novel strategy for the reversed genetics of Ti plasmids of
/Agrobacterium tumefaciens. EMBO J 2: 411-418
Zambryski P, Joos H, Genetello C, van Montagu M, Schell J (1983) Ti-plasmid
vector for the introduction of DNA into plant cells without altering their normal
regeneration capacity, EMBO J 12: 2143-2150
Reiss, B, Sprengel, Will H and Schaller H (1984) A new sensitive method for
20 qualitative and quantitative assay of neomycin phosphotransferase in crude cell
tracts, GENE 1081: 211-217
Schreier PH, Seftor EA, Schell J and Bohnert HJ (1985) The use of nuclear
encoded sequences to direct the light-regulated synthesis and transport of a foreign
protein into plant chloroplasts, EMBO J Vol. 4, No. 1: 25-32
25 In addition, the following published patent applications may be listed:
EP-A 116 718 EP-A-126 546
EP-A 159 418 EP-A-164 597
EP-A 120 515 EP-A-175 966
EP-A- 120 516 WO 847~2913
EP-A-172 112 WO 84/02919
EP-A-140 556 WO 84/02920
CA 02204744 1997-05-07
Ee A 30 708-Forei~n Countries
- 23 -
EP-A- 174 166 WO 83/01176
EP-A-122 791
EP-A-0 309 862
EP-A-0 464 461
EP-A-0 533 010 .
' CA 02204744 1997-05-07
Le A 30 708-Forei~n Countries
- 24 -
SEQUENCE LISTING
(1) GENERAL INFORMATION:
(i) APPLICANT:
(A) NAME: Bayer AG
S (B) STREET: Bayerwerk
(C) CITY: Leverkusen
(D) COUNTRY: Germany
(E) POSTAL CODE: D-51368
(F) TELEPHONE: 0214/30 66400
(G) FAX: 0214/30 3482
(H) TELEX: 85 101-265 by d
(ii) TITLE OF APPLICATION: DNA sequences and their use
(iii) NUMBER OF SEQUENCES: 7
(iv) COMPUTER READABLE FORM:
(A) MEDIUM TYPE: Floppy disk
(B) COMPUTER: IBM PC compatible
(C) OPERATING SYSTEM: PC-DOS/MS-DOS
(D) SOFTWARE: PatentIn Release #1.0~ Version #1.25 (EPA)
(2) INFORMATION FOR SEQ ID NO: 1:
(i) SEQUENCE CHARACTERISTICS:
(A) LENGTH: 33 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 1:
CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
TCCCCCGGGA TCCATGGCTT CAATTGAGGA AAT
(2) INFORMATION POR SEQ ID NO: 2:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 33 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(xi) SEQUENCE DESCRIPTION: SEQ. ID NO: 2:
TCCCCCGGGA TCCATGGCGT CTGTGGAGGA AAT
(2) INFORMATION FOR SEQ ID NO: 3:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 33 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 3:
TCCCCCGGGA TCCATGGTGT CTGTGAGTGG AAT
(2) INFORMATION FOR SEQ ID NO: 4:
~ CA 02204744 1997-05-07
Le A 30 708-~orei~n Countries
- 26 -
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 32 base pairs
(B) T~PE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 4:
TGAATTCCCG GGTCAATTTG TAACCATAGG AA
10 (2) INFORMATION FOR SEQ ID NO: 5:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 31 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: NO
(xi) SEQUENCE DESCRIPTION: SEQ ID NO: 5:
CGGATCCCGG GTCAATTGGA ATCCCTAGGA A
20 (2) INFORMATION FOR SEQ ID NO: 6:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 2728 base pairs
(B) TYPE: nucleic acid
CA 02204744 1997-O~i-07
Le A 30 708-Forei~n Countries
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: No
(xi) SEQUENCE DESCRIPTION: SEQ. ID NO: 6:
CGGATCCCGG GTCTTCGCAT AACGAATTAA CT
(2) INFORMATION FOR SEQ ID NO: 7:
(i) SEQUENCE CHARACTERISTICS
(A) LENGTH: 2728 base pairs
(B) TYPE: nucleic acid
(C) STRANDEDNESS: single
(D) TOPOLOGY: linear
(ii) MOLECULE TYPE: DNA (genomic)
(iii) HYPOTHETICAL: No
(xi) SEQUENCE DESCRIPTION: SEQ. ID NO: 7:
AAGCTTGCAT GCCTGCAGGT CTCAGAAGAC CAGAGGGCTA TTGAGACTTT TCAACAAAGG 60
GTAATATCGG GAAACCTCCT CGGATTCCAT TGCCCAGCTA TCTGTCACTT CATCGAAAGG 120
ACAGTAGAAA AGGAAGATGG CTTCTACAAA TGCCATCATT GCGATAAAGG AAAGGCTATC 180
GTTCAAGAAT GCCTCTACCG ACAGTGGTCC CAAAGATGGA CCCCCACCCA CGAGGAACAT 240
20CGTGGAAAAA GAAGACGTTC CAACCACGTC TTCAAAGCAA GTGGATTGAT GTGATAACAT 300
GGTGGAGCAC GACACTCTCG TCTACTCCAA GAATATCAAA GATACAGTCT CAGAAGACCA 360
GAGGGCTATT GAGACTTTTC AACAAAGGGT AATATCGGGA AACCTCCTCG GATTCCATTG 420
CCCAGCTATC TGTCACTTCA TCGAAAGGAC AGTAGAAAAG GAAGATGGCT TCTACAAATG 480
CA 02204744 1997-05-07
Le A 30 708-Forei~n Countries
- 28 -
CCATCATTGC GATAAAGGAA AGGCTATCGT TCAAGAATGC CTCTACCGAC AGTGGTCCCA 540
AAGATGGACC CCCACCCACG AGGAACATCG TGGAAAAAGA AGACGTTCCA ACCACGTCTT 600
CAAAGCAAGT GGATTGATGT GATATCTCCA CTGACGTAAG GGATGACGCA CAATCCCACT 660
ATCCTTCGCA AGACCCGTCC TCTATATAAG GAAGTTCATT TCAmGGAG AGGACCTCGA 720
GAATTCCACC ATGGCTTCAA TTGAGGAAAT TAGAAACGCT CAACGTGCCA AGGGTCCGGC 780
CACCATCCTA GCCATTGGCA CAGCTACTCC CGACCACTGT GTCTACCAGT CTGATTATGC 840
TGATTACTAT TTCAGAGTCA CTAAGAGCGA GCACATGACT GAGTTGAAGA AGAAGTTCAA 900
TCGCATATGT AAGTATATAT ATTCATGCAT TAATTCTTAC ATTCACAACA mCTATACA 960
TATACGAGTG TGCTATTAAG TGAGGGTCAC CTCCAAGTGA ATGAATGm CAAGCTTAGA 1020
GAATAGCTTT TAGCTAAATT ACmAGGAA ACTTGAAAAT CATmACAT CAGTAACCGA 1080
TATTCCTTTC ATTTGATTGT AAGGGCTTGA AGAGCTGTTC mGAATCAT GTAGCATTGC 1140
TAGC'l'ATAAT TAAGAATAAC CTTTTATAAT TTCTTCAATG TTAAATGCAT GTTGATCATC 1200
TTCAAGAATA TACTATATGA CTAGTCGTTG GAAAACTAAT GTGTTCATCT TAl"l'l~'l'lll' 1260
ACAGGTGACA AATCAATGAT CAAGAAGCGT TACATTCATT TGACCGAAGA AATGCTTGAG 1320
GAGCA~CCAA ACATTGGTGC TTATATGGCT CCATCTCTCA ACATACGCCA AGAGATTATC 1380
ACTGCTGAGG TACCTAAACT TGGTAAAGAA GCAGCATTGA AGGCTCTTAA AGAATGGGGT 1440
CAACCAAAGT CCAAGATCAC CCATCTTGTA TmGTACAA CCTCCGGTGT AGAAATGCCC 1500
GGTGCAGATT ACAAACTCGC TAATCTCTTA GGCCTTGAAA CATCGGTTAG AAGGGTGATC 1560
TTGTACCATC AAGGTTGCTA TGCAGGTGGA ACTGTCCTTC GAACTGCTAA GGATCTTGCA 1620
GAAAATAACG CAGGAGCACG AGTTCTTGTG GTGTGCTCTG AGATCACTGT TGTTACATTT 1680
CGTGGGCCTT CCGAAGATGC TTTGGACTCT TTAGTAGGTC AAGCCCTTTT TGGTGATGGG 1740
TCAGCAGCTG TGATTGTTGG ATCAGATCCA GATGTCTCCA TTGAACGACC CCTCTTCCAA 1800
CTTGTTTCAG CAGCACAAAC GTTTATTCCT AATTCAGCAG GTGCTATTGC GGGTAACTTA 1860
CGTGAGGTGG GACTCACCTT TCACTTGTGG CCTAATGTGC CTACTTTGAT TTCCGAGAAC 1920
ATAGAGAAAT GCTTGAATCA GGCTTTTGAC CCACTTGGTA TTAGCGATTG GAACTCGTTA 1980
TTTTGGATTG CTCACCCTGG TGGCCCTGCA ATTCTTGATG CAGTTGAAGC AAAACTCAAT 2040
TTAGAGAAAA AGAAACTTGA AGCAACAAGG CATGTGTTAA GTGAGTATGG TAACATGTCT 2100
AGTGCATGTG TCTTGTTTAT TTTGGATGAG ATGAGAAAGA AATCCCTAAA GGGGGAAAAA 2160
~ = ~
CA 02204744 1997-0~-07
Le A 30 708-Forei~n Countries
- 29 -
GCTACCACAG GTGACGGATT GGATTGGGGN GTACTATTCG GTTTTGGGCC AGGCTTGACC 2220
ATTGAGACCG TTGTGCTGCA TAGCGTTCCT ATGGTTACAA ATTGAGTGGA AAACGGTAAG 2280
AGAAATGATA TAGGGGACAT GTCTTATTGT ATTACAGAGG AGGTGCTACG AAAGATATGT 2340
ACATGTATCT TCAAAGTTAA TAATAGTACT CCTAAATCTT TTATTCCTAT CCTAACATTG 2400
AGGGATTGTA ATTTAGTGAT TGTTGGAGGG TGCAGTCACG TCAGGCAAGT GGATGAAACT 2460
GCAAGTGCTT GTCATTCTGT TATCGGGGGA TCCTCTAGAG TCCGCAAAAA TCACCAGTCT 2520
CTCTCTACAA ATCTATCTCT CTCTATTTTT CTCCAGAATA ATGTGTGAGT AGTTCCCAGA 2580
TAAGGGAATT AGGGTTCTTA TAGGGTTTCG CTCATGTGTT GAGCATATAA GAAACCCTTA 2640
GTATGTATTT GTATTTGTAA AATACTTCTA TCAATAAAAT TTCTAATTCC TAAAACCAAA 2700
ATCCAGTGAC CTGCAGGCAT GCAAGCTT 2728