Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 0220~66~ 1997-0~-21
WO 96~16027 PCT/US9S/15530
MATRIX METAI,LOPROTEASE T~TRTTORS
FIELD OF l~IE lNV~Nl I ON
The present invention is directed to compounds and their
pharmaceutically acceptable salts, which inhibit matrix
metalloproteases, and are therefore useful in the treatment of
mAmm~ls having disease-states alleviated by the inhibition of
such matrix metalloproteases.
R~ OUND OF THE lNv~ lON
Matrix metalloproteases ~"MMPs)~ are a family of
proteases (enzymes) involved in the degradation and remodeling
of connective tissues. Members of this ~amily of
endopeptidase enzymes are present in various cell types that
reside in or are associated with connective tissue, such as
fibroblasts, monocytes, macrophages, endothelial cells, and
invasive or metastatic tumor cells. MMP expression is
stimulated by growth factors and cytokines in the local tissue
environment, where these enzymes act to specifically degrade
protein components of the extracellular matrix, such as
collagen, proteoglycans (protein core), fibronectin and
l~m; n; n . These ubiquitous extracellular matrix components are
present in the linings of joints, interstitial connective
tissues, basement membranes, and cartilage. Excessive
degradation of extracellular matrix by MMPs is implicated in
the pathogenesis of many diseases, including rheumatoid
arthritis, osteoarthritis, periodontal disease, aberrant
angiogenesis, tumor invasion and metastasis, corneal
ulceration, and in complications of diabetes. MMP inhibition
i35 is, therefore, recognized as a good target for therapeutic
intervention.
The MMPs share a number of properties, including zinc and
calcium dependence, secretion as zymogens, and 40-50~ amino
acid sequence homology. The MMP family includes collagenases,
stromelysins, gelatinases, and matrilysin, as discussed in
greater detail below.
SUBSTInn~S~EET(RUL~26)
CA 0220~66~ 1997-0~-21
wos6ll6o27 PCT~S9S/lSS30
Interstitial collagenases catalyze the initial and rate-
limiting cleavage of native collagen types I, II, III and X.
Collagen, the major structural protein of m~mm~l S, iS an
essential component of the matrix of many tissues, for
example, cartilage, bone, tendon and skin. Interstitial
collagenases are very specific matrix metalloproteases which
cleave collagen to give two fragments which spontaneously
denature at physiological temperatures and therefore become
susceptible to cleavage by less specific enzymes. Cleavage by
the collagenase results in the 1088 of structural integrity of
the target tissue, essentially an irreversible process.
The gelatinases include two distinct, but highly related,
enzymes: a 72-kD enzyme secreted by fibroblasts and a wide
variety of other cell types, and a 92-kD enzyme released by
mononuclear phagocytes, neutrophils, corneal epithelial cells,
tumor cells, cytotrophoblasts and keratinocytes. These
gelatinases have been shown to degrade gelatins (denatured
collagens), collagen types IV (basement membrane) and V,
fibronectin and insoluble elastin.
The stromelysins (1 and 2) have been shown to cleave a
broad range of matrix substrates, including l~m; n; n,
fibronectin, proteoglycans, and collagen types IV and IX in
their non-helical domains.
Matrilysin (putative metalloprotease or PUMP) is a
recently described member of the matrix metalloprotease
family. Matrilysin has been shown to degrade a wide range of
matrix substrates including proteoglycans, gelatins,
fibronectin, elastin, and l~m; n; n . Its expression has been
documented in mononuclear phagocytes, rat uterine explants and
sporadically in tumors.
Inhibitors of MMPs provide useful treatments for diseases
associated with the excessive degradation of extracellular
matrix, such as arthritic diseases (rheumatoid arthritis and
osteoarthritis), bone resorptive diseases (such as
osteoporosis), the enhanced collagen destruction associated
with diabetes, periodontal disease, corneal ulceration,
ulceration of the skin, tumor invasion and metastasis, and
SUBSTITUTE SHEET (RULE 26)
_ _ _
CA 02205665 1997-05-21
WO 96/16U27 PCTlUSgS/lS530
aberrant angiogene~is.
The de~ign and uses of MMP inhibitors is described, for
example, in J. Enzyme Inhibition (19~7), Vol. 2, pp. 1-22;
Drug New~ & Prospective~ (1990), Vol. 3, No. 8, pp. 453-458;
~ 5 Arthriti~ and Rheumatism (1993), Vol. 36, No. 2, pp. 181-189;
Arthritis and Rheumatism (1991), Vol. 34, No. 9, pp. 1073-
1075; s~minA~g in Arthritis and Rheumatism (1990), Vol. 19,
No. 4, Supplement 1 (February), pp. 16-20; Drugs of the Fhture
(1990), Vol. 15, No. 5, pp. 495-508; and J. Enzyme Inhibition
(1987), Vol. 2, pp. 1-22. MMP inhibitors are also the subject
of variou8 patents and patent applications, for example,
U.S. Patent Nos. 5,189,178 (Galardy) and 5,183,900 (Galardy),
European Published Patent Applications 0 438 223 (Beecham) and
0 276 436 (F. Hoffmann-La Roche), and Patent Cooperation
Treaty International Applications 92/21360 (Merck), 92/06966
(Beecham) and 92/09563 (Glycomed).
.-
,,~
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/1S530
~ SUMMARY OF THE lNv~NllON
The invention provides new compounds which are useful as
inhibitors of matrix metalloproteases and which are effective
in treating disease-states characterized by excessive activity
of matrix metalloproteases.
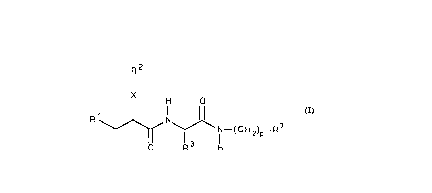
Accordingly, one aspect of the invention is directed to
compounds of formula (I):
R2
I
H O
R1~ N--(CH2)p -R7
formula (I)
wherein:
R1 is mercapto, acetylthio, carboxy, hydroxycarbamoyl,
N-hydroxyformylamino, alkoxycarbonyl,
aryloxycarbonyl, aralkoxycarbonyl,
benzyloxycarbamoyl or a group of the formula
Ii S
' ~R6
OH
where R6 is aryl or heteroaryl;
R2 is alkyl, cycloalkyl, aryl, heterocycloalkyl, or
heteroaryl;
R3 is alkyl, cycloalkyl, aralkyl, or heteroaralkyl; ,=
R7 is aryl, heteroaryl or heterocycloalkyl;
X is a group of the formula -(CH2)m-Y-(CH2)D-, where:
Y is O, S, or a single bond,
m is an integer from O to 4,
n is an integer from O to 4, and
SUBSlll~llt SHEr (RUIE ~C~
... . . . . . . . .
CA 02205665 1997-05-21
WO 96116027 PCrrUS9SIlSS30
m + n is an integer from O to 4;
p i~ an integer from O to 4, provided that R2-X is
biphenylalkyl when p is not o;
and the pharmaceutically acceptable salts thereof.
Another aspect of the invention provides proces~eR for
synthesizing the compounds and ~alts of formula (I).
In another aspect, the invention is directed to a sub-
genus of formula (I), i.e., the compoundR of formula (II), as
f ollows:
R2 H o
R1 ~ I ~ ~ R4
formula (II)
wherein:
R1 is mercapto, acetylthio, carboxy, hydroxycarbamoyl,
alkoxycarbonyl, aryloxycarbonyl, aralkoxycarbonyl,
benzyloxyaminocarbonyl or a group of the formula
/ I ~S~R6
OH
where R6 is aryl or heteroaryl;
R2 is alkyl, aralkyl or cycloalkylalkyl;
R3 is cycloalkyl, alkyl (optionally substituted by
cycloalkyl, hydroxy, mercapto, alkylthio, aralkoxy,
carboxy, amino, alkylamino, guanidino, carbamoyl,
pyridyl or indolyl), or aralkyl (optionally
substituted by hydroxy, carboxy, alkyl or alkoxy);
R4 is nitro, amino, cyano, hydroxy, alkoxy, carboxy,
alkoxycarbonyl, alkyl~ulfonyl, haloalkyl,
alkoxycarbonylalkyl, tetrazolyl, carbamoyl
(optionally substituted by alkyl or
SUBSTITUTE Sl l~ET (RULE 26~
CA 0220~66~ 1997-0~-21
WO96tl6027 PCT~S9S/15S30
--6--
dialkylaminoalkyl), or aminosulfonyl (optionally
~ substituted by alkyl); and
RS i8 hydrogen, halo or hydroxy,
as a single stereoisomer or as a mixture thereof; and the
pharmaceutically acceptable salts thereof.
Another aspect of the invention is directed to compounds
of the formula
t-B~-O ~ ,
wherein:
R2 is alkyl, aryl or heteroaryl; and
X iB a group of the formula - (CH2) m~Y~ (CH2) n~ where:
Y is O, S, or a single bond,
m is an integer from 0 to 4,
n is an integer from O to 4, and
m + n is an integer from 0 to 4;
or R2 and X together are lower alkenyl.
Another aspect of the invention is directed to processes
for synthesizing a compound of the formula
t-E~U\ J~ ~ r
o ~
wherein R2 is aryl or heteroaryl, by
SUBSTITUTE SHEET (RULE 26)
CA 02205665 1997-05-21
WO 96116027 PCT/US9S/15~i30
(a) hydrogenating a compound of the formula:
R2
t-~U~
'~
in the presence of a palladium/carbon catalyRt; or
(b) contacting a compound of the formula
R2
~,N >
o '~3
with sodium h~methyldisilazide and t-butylbromoacetate.
Other aspects of the invention are directed to compounds
of the formula
a o/ ~o
wherein R2 is aryl or heteroaryl, and a process for
synthesizing these compounds by
CA 0220~66~ 1997-05-21
WO96/16027 PCT~S9S/IS530
(a) contacting a compound of the formula:
HO~N~OBn
wherein R2 is hydrogen, aryl or heteroaryl, with an excess of
mesyl chloride in pyridine followed by refluxing under basic
conditions, and
(b) where R2 is hydrogen in step (a), reacting the
product of step (a) with an aryl halide or a heteroaryl halide
in the presence of a base and a palladium catalyst.
Another aspect of the invention is directed to methods of
inhibiting matrix metalloprotease activity in a m~mm~ l, which
methods comprise administering to the m~mm~ 1 in need thereof a
therapeutically effective amount of a compound of formula (I)
as defined above, as a single stereoisomer, or as a mixture
thereof, or a pharmaceutically acceptable salt thereof.
Another aspect of the invention iB directed to a
pharmaceutical composition useful in inhibiting matrix
metalloprotease activity in a mAmm~l, which composition
comprises a therapeutically effective amount of a compound of
formula (I) as defined above, as a single stereoisomer or as a
mixture thereof; or a pharmaceutically acceptable salt
thereof; and a pharmaceutically acceptable excipient.
DET~TT~n DESCRIPTION OF TXE lNv~N~lON
Definitions
As used in the specification and appended claims, unless
specified to the contrary, the following terms have the
meaning indicated:
"BOC" refers to t-butoxycarbonyl.
"CBZ~' refers to benzyloxycarbonyl (carbobenzyloxy).
su~nu ESHET~IE2 ;)
CA 0220~66~ 1997-0~-21
WO96116027 PCT~S9S/lSS30
"DCC" refers to N,N-dicyclohexylcarbodiimide.
"DMAP" refers to N,N-dimethylaminopyridine.
"DMF" refers to N,N-dimethylformamide.
~EDCI~ refers to N-ethyl-N'-(3-dimethylaminopropyl)-
carbodiimide.
"HOBT" refers to l-hydroxybenzotriazole.
"Hydroxy" refers to the radical -OH.
"Amino" refers to the radical -NH2.
"Acetylthio~' refers to the radical -SC(O)CH3.
"Halo" refers to bromo, chloro or fluoro.
"Carbamoyl" refers to the radical -C(O)NH2.
"Carboxy" refers to the radical -C(O)OH.
"Hydroxyamino" refers to the radical -NHOH.
"Hydroxycarbamoyl" refers to the radical -C(O)NHOH.
"N-Hydroxyformylamino" refers to the radical -N(OH)C(O)H
"Benzyloxycarbamoyl" refers to -C(O)N(H)OCH2C~s.
"Acylamino" refers to -NHC(O)Ra where Ra is alkyl.
"Mercapto" refers to the radical -SH.
"Alkyl" refers to a straight or branched chain monovalent
radical consisting solely of carbon and hydrogen, contA;n;ng
no unsaturation and having from one to ten carbon atoms, e.g.,
methyl, ethyl, n-propyl, 2-methylpropyl (iso-butyl),
l-methylethyl (i~o-propyl), n-butyl, and l,l-dimethylethyl
(t-butyl), heptyl and the like, which can be optionally
substituted by cycloalkyl, hydroxy, mercapto, alkylthio,
aralkoxy, carboxy, amino, mono- and di-alkylamino, guanidino,
N,N-dialkylguanidino, carbamoyl, aryl, and heteroaryl.
"Alkane-diyl" or "alkylene" refers to a straight chain
divalent radical consisting solely of carbon and hydrogen,
containing no unsaturation and having from one to five carbon
atoms, e.g., methylene, ethylene, propylene (or propane-1,3-
diyl) and the like.
"Lower alkenyl" refers to a straight chain univalent
hydrocarbon radical having from two to six carbon atoms and
containing at least one unsaturated bond, e.g., prop-2-enyl,
pent-4-enyl and the like.
"Alkylamino" refers to a radical of the formula -NHRa
SU~STITUTE SHEET (RULE 26)
CA 0220~66~ 1997-05-21
WO96/16027 PCT~S95/lSS30
-10 -
where Ra i8 alkyl as defined above, e.g., methylamino,
ethylamino, iso-propylamino, n-butylamino, and the like.
"Haloalkyl" refers to a radical of the formula -RaRd where
Ra is alkyl as defined above substituted by one or more halo
groups (Rd) as defined above, e.g., 2-chloroethyl,
2-bromoethyl, trifluoromethyl, and the like.
''Dialkylaminoalkylll refers to a radical of the formula
-RaN (Ra) 2 where each Ra is independently an alkyl radical as
defined above, e.g., dimethylaminoethyl, diethylamino-
n-propyl, dimethylamino-n-propyl, and the like.
"Aminosulfonyl'l refers to -S (0)2NH2.
'IAlkylsulfonylll refers to a radical of the formula
~S(O)2Ra where Ra is alkyl as defined above, e.g.,
methylsulfonyl, ethylsulfonyl, iso-propylsulfonyl, and the
like.
IlAlkylsulfinyll- refers to a radical of the formula -S(O)Ra
where Ra is alkyl as defined above.
IlAlkylthiol' refers to a radical of the formula -SRa where
Ra is optionally-substituted alkyl as defined above, e.g.,
methylthio, ethylthio, iso-propylthio, n-butylthio, and the
like.
"Alkoxy" refers to a radical of the formula ~ORa wherein
Ra is alkyl as defined above, e.g., methoxy, ethoxy, n-propoxy,
1-methylethoxy, n-butoxy, t-butoxy, and the like.
'IAlkoxycarbonylalkyl'' refers to a radical of the formula
-RaC(O)Rb where Ra is alkyl as defined above and Rb is alkoxy
as defined above, e.g., methoxycarbonylethyl,
ethoxycarbonylethyl, methoxycarbonyl-iso-propyl, and the like.
"Aryl" refers to a monovalent unsaturated aromatic
carbocyclic radical having a single ring (e.g., phenyl), two
condensed rings (e.g., naphthyl) or three condensed rings
(e.g., phenanthrenyl or fluorenyl) which can be optionally
substituted by one or more substituents independently selected
from: alkyl, hydroxy, carboxy, halo, cyano, amino, nitro,
tetrazolyl, heteroaryl, aminoalkoxy, alkylthio, haloalkyl,
alkoxy, alkoxycarbonyl, alkoxycarbonylalkyl, alkyl sulfonyl,
SUBSTIME SHEET (RULE 26~
CA 0220~66~ 1997-0~-21
WO 96116027 PCT/US9S/15S30
alkyl sulfinyl, aminosulfonyl optionally substituted by alkyl,
carbamoyl optionally substituted by alkyl or
dialkylaminoalkyl, or the substituent can be another aryl
group as defined herein (e.g., to form an optionally
~ 5 substituted biphenyl radical).
"Aryloxy" refers to a radical of the formula -ORb wherein
Rb i8 aryl as defined above, e.g., ~henoxy, quinol-2-yloxy,
naphth-1-yloxy, or naphth-2-yloxy.
"Aralkyl" refers to a radical of the formula -RaRb wherein
Ra is alkyl as defined above and Rb is aryl as defined above,
e.g., benzyl, phenylethylene, 3-phenylpropyl, and the like.
"Aralkoxy" refers to a radical of the formula -ORaRb
wherein Ra is alkyl as defined above and Rb is aryl as defined
above, e.g., benzyloxy, 3-naphth-2-ylpropoxy, and the like.
"Alkoxycarbonyl" refers to a radical of the formula
-C(O)Rb wherein Rb is alkoxy as defined above,
e.g., methoxycarbonyl, ethoxycarbonyl, t-butoxycarbonyl, and
the like.
"Aralkoxycarbonyl" refers to a radical of the formula
-C(O)RC wherein Rc is aralkoxy as defined above, e.g.,
benzyloxycarbonyl, and the like.
"Cycloalkyl" refers to a monovalent ring radical
consisting solely of carbon and hydrogen atoms, containing no
unsaturation and having from five to seven carbon atoms, e.g.,
cyclopentyl, cyclohexyl and cycloheptyl.
"Cycloalkylalkyl" refers to a radical of the formula -ReRa
where Ra is alkyl as defined above and Re is cycloalkyl as
defined above, e.g., cyclohexylmethyl, cyclohexylethyl,
cyclopentylmethyl, and the like.
"Heteroaryl" refers to a monovalent unsaturated aromatic
carbocyclic radical having a single ring or multiple condensed
rings with at least one heteroatom such as N,O,S, (e.g.,
pyridyl, quinolyl, indolyl, carbazolyl, dibenzofuranyl,
dibenzothiophenyl, phenanthridinyl), which can be optionally
substituted by one or more substituents independently selected
from: alkyl, hydroxy, carboxy, halo, cyano, amino, nitro,
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/lSS30
-12-
tetrazolyl, aryl, aminoalkoxy, alkylthio, haloalkyl, alkoxy,
alkoxycarbonyl, alkoxycarbonylalkyl, alkyl sulfonyl, alkyl
sulfinyl, aminosulfonyl optionally substituted by alkyl, and
carbamoyl optionally substituted by alkyl or
dialkylaminoalkyl.
"Heteroaralkyl" refers to a radical of the formula -RaRb
where Ra is alkyl as defined above and Rb is heteroaryl as
defined above.
"Heterocycloalkyl" refers to a monovalent saturated
carbocyclic radical having a single ring or multiple condensed
rings with at least one heteroatom such as N,O,S (e.g.,
morpholino, piperazinyl, piperidinyl, pyrrolidinyl).
~Optional" or "optionally" means that the subsequently
described event of circumstances may or may not occur, and
that the description includes instances where said event or
circumstance occurs and instances in which it does not. For
example, "optionally substituted quinol-2-yl" means that the
~uinol-2-yl radical may or may not be substituted and that the
description includes both substituted quinol-2-yl radicals and
quinol-2-yl radicals having no substitution.
"Amino-protecting group" as used herein refers to those
organic groups intended to protect nitrogen atoms against
undesirable reactions during synthetic procedures, and
includes, but is not limited to, benzyl, acyl, acetyl,
benzyloxycarbonyl (carbobenzyloxy), p-methoxybenzyloxy-
carbonyl, p-nitrobenzyloxycarbonyl, t-butoxycarbonyl,
trifluoroacetyl, and the like.
"Base" as used here includes both strong bases such as
sodium hydroxide, lithium hydroxide, ammonium hydroxide,
potassium carbonate and the like, and organic bases such as
pyridine, diisopropylethylamine, N-methylmorpholine,
triethylamine, dimethylaminopyridine and the like.
"Pharmaceutically acceptable salt" refers to those salts
which retain the biological effectiveness and properties of
the free bases or free acids and which are not biologically or
otherwise undesirable. If the compound exists as a free base,
the desired salt may be prepared by methods known to those of
SUBSTITUrE S~EET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96116027 PCT~S9S115530
ordinary skill in the art, such as ~reatment of the compound
with an inorganic acids such as hydrochloric acid, hydrobromic
acid, sulfuric acid, nitric acid, phosphoric acid and the
like; or with an organic acids such as acetic acid, propionic
acid, glycolic acid, pyruvic acid, oxalic acid, maleic acid,
malonic acid, succinic acid, fumaric acid, tartaric acid,
citric acid, benzoic acid, c; nn~mi C acid, mandelic acid,
meth~n~ulfonic acid, ethanesulfonic acid, p-toluenesulfonic
acid, salicylic acid, and the like. If the compound exists as
a free acid, the desired salt may also be prepared by methods
known to those of ordinary skill in the art, such as the
treatment of the compound with an inorganic base or an organic
base. Salts derived from inorganic bases include, but are not
limited to, the ~odium, potassium, lithium, ammonium, calcium,
magnesium, iron, zinc, copper, manganese, aluminum salts and
the like. Salts derived from organic bases include, but are
not limited to, salts of primary, secondary, and tertiary
amines, substituted amines including naturally occurring
substituted amines, cyclic amines and basic ion exchange
resins, ~uch as isopropylamine, trimethylamine, diethylamine,
triethylamine, tripropylamine, ethanolamine, 2-dimethyl-
amino ethanol, 2-diethylaminoethanol, trimet~m;ne~
dicyclohexylamine, lysine, arginine, histidine, caffeine,
procaine, hydrabamine, choline, betaine, ethylenediamine,
glucosamine, methylglucamine, theobromine, purines,
piperazine, piperidine, N-ethylpiperidine, polyamine resins
and the like.
~ mm~ includes humans and all domestic and wild
animals, including, without limitation, cattle, horses, swine,
sheep, goats, dogs, cats, and the like.
~Therapeutically effective amount" refers to that amount
of a compound of formula (I) which, when administered to a
mAmm~l in need thereof, is sufficient to effect treatment, as
defined below, for disease-states alleviated by the inhibition
of matrix metalloprotease activity, such as the activity of
stromelysin, gelatinase, matrilysin and/or collagenase. The
amount of a compound of formula (I) which constitutes a
SUBSTI~UTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/15S30
"therapeutically effective amount" will vary depending on the
compound, the disease-state and its severity, and the m~mmAl
to be treated, but can be determined routinely by one of
ordinary skill in the art having regard to his own knowledge
and to this disclosure.
"Treating" or "treatment" as used herein cover the
treatment of a disease-state in a m~mm~l, particularly in a
hl~m~n, which disease-state is alleviated by the inhibition of
matrix metalloprotease activity, such as the activity of
stromelysin, gelatinase, matrilysin and/or collagenase, and
include:
(i) preventing the disease-state from occurring in a
m~m~l ~ in particular, when such m~mm~l is predisposed to the
disease-state but has not yet been diagnosed as having it;
lS (ii) inhibiting the disease-state, i.e., arresting its
development; or
(iii) relieving the disease-state, i.e., causing
regression of the disease-state.
"Stereoisomers" refers to compounds having identical
molecular formulae and nature or sequence of bonding but
differing in the arrangement of their atoms in space.
The compounds of formula (I), or their pharmaceutically
acceptable salts, have at least two asymmetric carbon atoms in
their structure, and may therefore exist as single
stereoisomers, racemates, and as mixtures of enantiomers and
diastereomers. All such single stereoisomers, racemates and
mixtures thereof are intended to be within the scope of this
invention.
When naming the single stereoisomers of compounds of
formula (I) an absolute descriptor, R or S, may be assigned to
the chiral carbon atoms therein according to the "Sequence 4
Rule" procedure of Cahn, Ingold and Prelog.
Nomenclature
The nomenclature used herein is a modified form of
I.U.P.A.C. nomenclature wherein the compounds of the invention
are named as peptide derivatives. Where R3 of Formula (I)
Sll~llllllt ~ rRU~
CA 02205665 1997-05-21
WO g6116027 PCT~US95~1SS30
--15-
comprises the side chain of an amino acid residue, that
portion of the chemical structure which includes R3 together
with the adjacent nitrogen atom (illustrated below and named
as the N nitrogen, as opposed to the N' nitrogen) and carbonyl
- 5 group is given the name of the corresponding amino acid. The
naming and numbering of the compounds of the present invention
is illustrated below for representative compounds o~ formula
(I).
For example, the following compound of formula (I)
4 ~ 2
~ 1 N'-Cphenyi~-
~ carboxamide
~ ~ H 0
H0 lv
0 ~ S
L 1 ~ 4
r C N
L-S-[C4-cyanophenyl)methyl]-
N-[2R-carboxy-
penicill2mine
methyl-5-~bIphen-4-yl~
pentanoyl]
wherein Rl is carboxy; R2 is biphenyl; R3 is 4-
(cyano)benzylthioisopropyl; R7 is phenyl; X is propane-1,3-
diyl; and p is 0, is named
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-S-
((4-cyanophenyl)methyl)-penicillamine-N'-(phenyl)carboxamide.
Another name for this compound is N-(5-(biphen-4-yl)-2R-
carboxymethylpentanoyl)-L-S-((4-cyanophenyl)methyl)-
penicillamine-N'-(phenyl)carboxamide.
For ease of reference, the portions of the structure are
associated with their corresponding nomenclature.
SUBSTITUTE StlEET (RULE 26)
CA 0220~66~ 1997-05-21
WO 96/16027 PCT/US9SIlS530
The structures and names of several other representative
compounds o~ formula (I) follow.
HO /~ / 3
The above compound is named N- (2R-carboxymethyl-5-(biphen-4-
yl)pentanoyl) -L- t-butylglycine-N'-(pyrid-4-yl)carboxamide. The
term t-leucine can be interchanged with t-butylglycine, and
the term pyridinyl can be interchanged with pyridyl. Another
name for the above compound is: N-(5-biphen-4-yl-2R-
carboxymethylpentanoyl)-L- t-leucine -N ' - ( pyridin-4-yl)-
carboxamide.
~ \2
3 ~ H O 1~
OH ,J1~N/~ OCH3
Il,~ l
The above compound is named N-(2R-carboxymethyl-5-(7-(glycyl)-
aminofluoren-2-yl)pentanoyl)-h-leucine-N'-(4-(methoxy-
carbonyl)phenyl)carboxamide.
Another name for this compound is N-(5-t7-(glycyl)amino-
f luoren-2-yl)-2R-carboxymethylpentanoyl)-L- leucine-N'-
(4-(methoxycarbonyl)phenyl)carboxamide.
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/lSS30
-17-
~ o
~ N ~ ~ C H Z C H 3
O <~>4
CH3CH2N~ > 6
CH3CH2N
The above compound is named N- ( (2R-carboxymethyl-5-
phenyl)pentanoyl)-L-6-(N,N~-diethylguanido) lysyl-N' - (4-
(ethoxycarbonyl)phenyl)carboxamide. The term guanidino can be
used interchangeably with guanido. Another name for this
compound is N- ( (5-phenyl-2R-carboxymethyl)pentanoyl) -L-6-
(N,N'-diethylguanidino) ly5yl - N ' - ( 4 - ( ethoxycarbonyl)phenyl)-
carboxamide.
4~2
N~ O
H~ ~ \~N~
The above compound is named N-((2R-(N"-formyl-N"-
hydroxyamino)methyl) -4- ( ( (3-chloro-5-morpholino)phen-1-
yl)oxybutanoyl)-L-cyclohexylglycine-N' (4- (indol-5-yl)-
butyl)carboxamide. Another name for this compound is
N-(4-(3-chloro-5-morpholino)phen-1-yl)-2R-((N"-formyl-N"-
SUBSTITUTE SHEET (RULE 26)
CA 0220~66s 1997-0~-21
WO96/16027 Pcr/uss5llss3o
--18--
hydroxyamino)methyloxybutanoyl)-~-cyclohexylglycine-N'-
(4-(indol-5-yl)butyl)carboxamide.
Ut~lity, Testing and Administration
Utility
The compounds of formula (I) inhibit m~mm~ll ian matrix
metalloproteases, such as the stromelysins, gelatinases,
matrilysin and collagenases, and are therefore useful for
treating diseases associated with the MMP-induced excessive
degradation of matrix and connective tissue within the m~mm~ 1,
for example, arthritic diseases (rheumatoid arthritis and
osteoarthritis), bone resorptive diseases (such as
osteoporosis), the enhanced collagen destruction associated
with diabetes, periodontal disease, corneal ulceration,
ulceration of the skin, tumor invasion and metastasis, and
aberrant angiogenesis.
Te~sting
The ability of the compounds of formula (I) to inhibit
matrix metalloprotease activity, such as the activity of
stromelysin, gelatinase, matrilysin and/or collagenase may be
demonstrated by a variety of in vitro and in vivo assays known
to those of ordinary skill in the art, such as the assay
described in Anal. Biochem. (1985), Vol. 147, p. 437, and the
MMP Enzymatic Assay described in FEBS (1992), Vol. 296t3), p.
263, or modifications thereof.
Administration
Atlm; n; stration of the compounds of formula (I), or their
pharmaceutically acceptable salts, in pure form or in an
appropriate pharmaceutical composition, can be carried out via
any of the accepted modes of administration or agents for
serving similar utilities. Thus, administration can be, for
example, orally, nasally, parenterally, topically,
transdermally, or rectally, in the form of solid, semi-solid,
lyophilized powder, or liquid dosage forms, such as for
SllBSrlTUTE SHEET ~RUL~ ~6)
CA 0220~66~ l997-0~-2l
WO96/16027 PCT~S95/lSS30
--19--
example, tablets, Quppositories, pills, soft elastic and hard
gelatin capsules, powders, solutions, suspensions, or
aerosols, or the like, preferably in unit dosage forms
suitable for simple administration of precise dosages. The
compositions will include a conventional pharmaceutical
carrier or excipient and a compound of formula (I) as the/an
active agent, and, in addition, may include other medicinal
agents, pharmaceutical agents, carriers, adjuvants, etc.
Generally, depending on the intended mode of
administration, the pharmaceutically acceptable compositions
will contain about 1~ to about 99~ by weight of a compound (8)
of formula (I), or a pharmaceutically acceptable salt thereof,
and 99~ to 1~ by weight of a suitable pharmaceutical
excipient. Preferably, the composition will be about 5~ to
75~ by weigh~ of a compound(s) of formula (I), or a
pharmaceutically acceptable salt thereof, with the rest being
suitable pharmaceutical excipients.
The preferred route of administration is oral, using a
convenient daily dosage regimen which can be adjusted
according to the degree o~ severity of the disease-state to be
treated. For such oral administration, a pharmaceutically
acceptable composition containing a compound(s) of
formula (I), or a pharmaceutically acceptable salt thereof, is
formed by the incorporation of any of the normally employed
excipients, such as, for example, pharmaceutical grades of
mannitol, lactose, starch, pregelatinized starch, magnesium
stearate, sodium saccharine, talcum, cellulose ether
derivatives, glucose, gelatin, sucrose, citrate, propyl
gallate, and the like. Such compositions take the form of
solutions, suspensions, tablets, pills, capsules, powders,
sustained release formulations and the like.
Preferably such compositions will take the form of
capsule, caplet or tablet and therefore will also contain a
diluent such as lactose, sucrose, dicalcium phosphate, and the
like; a disintegrant such as croscarmellose sodium or
derivatives thereof; a lubricant such as magnesium stearate
and the like; and a binder such as a starch, gum acacia,
SUBSTIME SHEET ~RULE 26)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/15S30
-20-
polyvinylpyrrolidone, gelatin, cellulose ether derivatives,
and the like.
The compounds of formula (I), or their pharmaceutically
acceptable salts, may also be formulated into a suppository
using, for example, about 0.5~ to about 50~ active ingredient
disposed in a carrier that slowly dissolves within the body,
e.g., polyoxyethylene glycols and polyethylene glycols (PEG),
e.g., PEG 1000 (96~) and PEG 4000 (4~).
Liquid pharmaceutically administrable compositions can,
for example, be prepared by dissolving, dispersing, etc., a
compound(s) of formula (I) (about 0.5~ to about 20~), or a
pharmaceutically acceptable salt thereof, and optional
pharmaceutical adjuvants in a carrier, such as, for example,
water, saline, aqueous dextrose, glycerol, ethanol and the
like, to thereby form a solution or suspen~ion.
If desired, a pharmaceutical composition of the invention
may also contain minor amounts of auxiliary substances such as
wetting or emulsifying agents, Ph buffering agents,
antioxidants, and the like, such as, for example, citric acid,
sorbitan monolaurate, triethanolamine oleate, butylated
hydroxytoluene, etc.
Actual methods of preparing such dosage forms are known,
or will be apparent, to those skilled in this art; for
example, see Remington 's Pha~maceutical Sciences, 18th Ed.,
(Mack Publishing Company, Easton, Pennsylvania, 1990). The
composition to be administered will, in any event, contain a
therapeuticàlly effective amount of a compound of formula (I),
or a pharmaceutically acceptable salt thereof, for treatment
of a disease-state alleviated by the inhibition of matrix
metalloprotease activity in accordance with the teachings of
this invention.
The compounds of formula (I), or their pharmaceutically
acceptable salts, are administered in a therapeutically
effective amount which will vary depending upon a variety of
factors including the activity of the specific compound
employed, the metabolic stability and length of action of the
compound, the age, body weight, general health, sex, diet,
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WC~ 96J16027 PCT/US95/15530
-21--
mode and time of administration, rate of excretion, drug
combina~ion, the severity of the particular disease-state, and
the host undergoing therapy. Generally, a therapeutically
effective daily dose is from about 0.14 mg to about 14.3 mg/kg
~ 5 of body weight per day of a compound of formula (I), or a
pharmaceutically acceptable salt thereof; preferably, from
about 0.7 mg to about 10 mg/kg of body weight per day; and
most preferably, from about 1.4 mg to about 7.2 mg/kg of body
weight per day. For example, for administration to a 70 kg
person, the dosage range would be from about 10 mg to about
1.0 gram per day of a compound of formula (I), or a
pharmaceutically acceptable salt thereof, preferably from
about 50 mg to about 700 mg per day, and most preferably from
about 100 mg to about 500 mg per day.
Preferred Embodiments
Preferred are the compounds of formula (I) where X i8
alkane-diyl and where p is zero, 2 or 3.
Of the compounds where p i8 2 or 3, particularly
preferred are those compounds where R1 is carboxy, R2 i8
biphenyl, R3 is cyclohexyl, and R7 is optionally substituted
phenyl [especially 4-(aminosulfonyl)phenyl] or N-morpholino.
Of the compounds where p is zero, particularly preferred
are the group of compounds where R2 is alkyl, optionally
substituted phenyl, or a group of the formula:
R ~ ~ R10
and R7 is 4-pyridyl or optionally substituted phenyl.
Within this group, a preferred subgroup of compounds are
SllBSTlTUTE SHEET (RULE 26)
,
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/lSS30
-22-
those where Rl is carboxy, hydroxycarbamoyl, or N-hydroxy-
formylamino; R2 is phenyl, biphenyl, 4-(pyridyl)phenyl, or
2-methylpropyl; R3 i8 t-butyl, 4-~m;nohutyl, alkyl~minohut
dialkyl~m;nobutyl, 4-(N,N~diethylguanidino)butyl, propyl,
S 2-methylpropyl, 1-hydroxyisopropyl, 1-hydroxyethyl, or
cyclohexyl; and X i8 a single bond, ethylene or
propane-1,3-diyl.
Within this subgroup, a preferred class of compounds are
those where R2 is biphenyl, R3 iS t-butyl and R7 is 4-pyridyl,
particularly where Rl is carboxy, N-hydroxyformylamino, or
hydroxycarbamoyl.
Also preferred is the subgroup of compounds where R2 is a
group of the formula:
~11 R10
~ A
~
wherein A is CH2; Rl~ is H or acylamino; Rll is H; R7 is
optionally substituted phenyl; and X is propane-1,3-diyl.
Within this subgroup, preferred are the compounds where R
is carboxy, hydroxycarbamoyl, or N-hydroxyformylamino; R3 is
alkyl (especially 2-methylpropyl); and R7 is
alkoxycarbonylphenyl [especially 4-(methoxycarbonyl)phenyl].
Also within this group, preferred are the subgroup of
compounds where Rl iS carboxy; R2 is phenyl; R3 is alkyl
[especially 4-(amino)butyl and 4-(diethylguanidino)-N-butyl]
or cycloalkyl (especially cyclohexyl); and R7 iS optionally
substituted phenyl [especially 4-(ethoxycarbonyl)phenyl or
4-(dialkylaminoethylaminosulfonyl)phenyl]; and X is ethylene
or propane-1,3-diyl.
Also within this group, preferred are the subgroup of
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
W o 96116027 PC~r~S95~1553~
compounds where Rl is mercapto, carboxy, hydroxycarbamoyl, or
N-hydroxyformylamino; R2 i8 2-methylpropyl; R3 is alkyl
[e~pecially propyl, 2-methylpropyl], cycloalkyl [especially
cyclohexyl] or heteroaralkyl [especially 3-methylindolyl];
R7 is optionally substituted phenyl [e~3pecially 4-methoxy-
phenyl, 4-carboxyphenyl, 4-(methoxycarbonyl)phenyl or 4-
(dimethylaminoethylcarbamoyl)phenyl]; and X is a single bond.
Also within this group, preferred are the subgroup of
compounds where Rl is carboxy; R2 is 4-(2-hydroxyethyl~phenyl,
4-(2-hydroxypropyl)phenyl, 4-(2-hydroxybutyl)phenyl,
4-(pyridyl)phenyl, biphenyl, 4'-(aminoethoxy)biphenyl,
4'-(cyano)biphenyl, or 4'-(hydroxy)biphenyl; R3 is
2-methylpropyl; R7 is 4-(methoxycarbonyl)phenyl; and x is
propane-1,3-diyl.
Particularly preferred is the subgroup of compounds where
R2 is biphenyl, especially where R7 is optionally substituted
phenyl.
Within this particularly preferred subgroup, preferred
are the compounds where Rl is carboxy; R3 is alkyl or
cycloalkyl [especially cyclohexyl, 4-(amino)butyl, 4-
(isopropylamino)butyl, 1-hydroxyisopropyl or t-butyl]; X is
propane-1,3-diyl, and R7 is phenyl, 4-(hydroxyethyl-
aminosulfonyl)phenyl, 4-(dimethylaminoethylamino sulfonyl)-
phenyl, 4-(ethoxycarbonyl)phenyl, 4-(N-morpholinopropylamino-
sulfonyl)phenyl, 4-(methylaminosulfonyl)phenyl, 4-(hydroxy-
ethylaminosulfonyl)phenyl, or 4-(methylsulfinyl)phenyl.
Another preferred group, particularly for matrilysin
inhibition, are the compounds of formula (II), particularly
those compounds wherein Rl is mercapto or acetylthio.
Within this second group, a preferred subgroup of
compounds are those compounds wherein R2 is alkyl, aralkyl,
cycloalkylalkyl; R3 is cycloalkyl or alkyl (optionally
substituted by cycloalkyl, hydroxy, aralkoxy, alkylthio,
pyridyl or indolyl); R4 iS cyano, carboxy, hydroxy, alkoxy,
alkoxycarbonyl, alkoxycarbonylalkyl, carbamoyl (optionally
substituted by aralkylaminoalkyl), or aminosulfonyl
(optionally substituted by alkyl)i and Rs is hydrogen.
SlJBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/1S530
Within this subgroup, a preferred class of compounds are
those compounds wherein R2 is alkyl; R3 is cyclohexyl, alkyl
(optionally substituted by cyclohexyl, hydroxy, benzyloxy,
methylthio, pyridyl or indolyl); and R4 is carboxy,
alkoxycarbonyl and aminosulfonyl.
Within this class of compounds, compounds wherein R~ is
2-methylpropyl are preferred. Particularly preferred are
those compounds wherein R3 is 2-methylpropyl.
A third group preferred for matrilysin inhibition, are
the compounds of formula (II) wherein Rl iS carboxy.
Within this third group, a preferred subgroup of
compounds are those compounds wherein R2 is alkyl, aralkyl,
cycloalkylalkyl; R3 is cycloalkyl or alkyl (optionally
substituted by cycloalkyl, hydroxy, aralkoxy, alkylthio,
pyridyl or indolyl); R4 is cyano, hydroxy, alkoxy, carboxy,
alkoxycarbonyl, alkoxycarbonylalkyl, carbamoyl (optionally
substituted by aralkylaminoalkyl), or aminosulfonyl
(optionally substituted by alkyl); and Rs is hydrogen.
Within this subgroup, a preferred class of compounds are
those compounds wherein R2 iS alkyl; R3 iS cyclohexyl, alkyl
(optionally substituted by cyclohexyl, hydroxy, benzyloxy,
methylthio, pyridyl or indolyl); and R4 is carboxy,
alkoxycarbonyl and aminosulfonyl.
Within this class of compounds, preferred compounds are
those compounds wherein R2 is 2-methylpropyl. Particularly
preferred are those compounds wherein R3 i5 cyclohexyl,
2-methylpropyl, pyridin-3-ylmethyl, l-benzyloxyethyl,
l-methylpropyl, l,l-dimethylethyl, l-hydroxyethyl, and
indol-2-ylmethyl; and R4 is methoxycarbonyl.
A fourth group preferred for matrilysin inhibition, are
the compounds of formula (II) wherein R1 is hydroxycarbamoyl.
Within this fourth group, a preferred subgroup of
compounds are those compounds wherein R2 is alkyl, aralkyl,
cycloalkylalkyl; R3 iS cycloalkyl or alkyl (optionally
substituted by cycloalkyl, hydroxy, aralkoxy, alkylthio,
pyridyl or indolyl); R4 is cyano, hydroxy, alkoxy, carboxy,
alkoxycarbonyl, alkoxycarbonylalkyl, carbamoyl (optionally
SUBSlllV~t SIE~ ~RUIE26)
, , . , = = , . . .. .. .
CA 0220~66~ 1997-0~-21
WO 96/16027 PCTIUS9511S530
--25--
substituted by aralkylaminoalkyl), or aminosulfonyl
(optionally substituted by alkyl); and Rs i8 hydrogen.
Within this subgroup, a preferred class of compounds are
those compounds wherein R2 is alkyl; R3 is cyclohexyl, alkyl
(optionally substituted by cyclohexyl, hydroxy, benzyloxy,
methylthio, pyridyl or indolyl); and R4 is carboxy,
alkoxycarbonyl and aminosulfonyl.
Within this class, preferred compounds are those
compounds wherein R2 is 2-methylpropyl. Particularly preferred
are those compounds wherein R3 is cyclohexyl, 2-methylpropyl,
pyridin-3-ylmethyl, 1-benzyloxyethyl, 1-methylpropyl, 1,1-
dimethylethyl, 1-hydroxyethyl, and indol-2-ylmethyl.
Presently, the most preferred compounds of formula (I)
are the following:
N- (2R- (N' '-hydroxycarbamoyl)methyl-4-(methyl)pentanoyl)-
~-tryptophan-N'-(4-(carboxy)phenyl)carboxamide;
N-(2R-(N''-hydroxycarbamoyl)methyl-4-(methyl)pentanoyl)-
~-leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N-(2R-(N''-hydroxycarbamoyl)methyl-4-(methyl)pentanoyl)-
L-leucine-N'-(4-(carboxy)phenyl)carboxamide;
N- (2R-mercaptomethyl-4-(methyl)pentanoyl)-
L-leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N- (2R-acetylthiomethyl-4-(methyl)pentanoyl)-
L-leucine-N'-(4-methoxycarbonylphenyl)carboxamide;
N-(2R-carboxymethyl-4-(methyl)pentanoyl)-
L- leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N- (2R- (N' '-hydroxycarbamoyl)methyl-4-(methyl)pentanoyl)-
L-cyclohexylglycine-N'-(4-(methoxycarbonyl)phenyl)-
carboxamide;
N- (2R-(N''-hydroxycarbamoyl)methyl-4-(methyl)pentanoyl)-
L- t-leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N-(2R-(N-hydroxycarbamoyl)methyl-5-(biphen-4-yl)pentanoyl-
L-t-leucine-N'-(pyrid-4-yl)carboxamide;
N- (2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-t-leucine-N'-(pyridin-4-yl)carboxamide;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-t-leucine-N'-(4-((2-hydroxyethyl)aminosulfonyl)-
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95115530
phenyl)carboxamide;
N- (2R-carboxymethyl-5-(4-(pyrid-4-yl)phenyl)pentanoyl)-
L-leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N- (2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-~-hydroxyvaline-N'-(phenyl)carboxamide;
N-(N"-formyl-N"-hydroxyamino)methyl-5-(biphen-4-yl)pentanoyl)-
L- t-leucine-N'-(pyridin-4-yl)carboxamide;
N-(2R,S)-(N"-formyl-N"-hydroxyamino)methyl-4-
(methyl)pentanoyl)-
L-leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-t-leucine-N'-(4R,S-(methylsulfinyl)phenyl)carboxamide;
N- (2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-t-leucine-N'-(4-(methylaminosulfonyl)phenyl)-
carboxamide;
N- (2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-t-leucine-N'-(4-(3-(morpholin-4-yl)propylamino-
8ul fonyl)phenyl)carboxamidei
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-cyclohexylglycine-N'-(4-((2-hydroxyethyl)-
aminosulfonyl)phenyl)carboxamide;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-cyclohexylglycine-N'-(4((2-(dimethylamino)ethyl)-
aminosulfonyl)phenyl)carboxamide;
N- (2R-(N"-hydroxycarbamoyl)methyl-4-(methyl)pentanoyl)
D, L-norvaline -N ' - ( 4-(dimethylaminoethylcarbamoyl)phenyl)-
carboxamide;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L - lysine- N ' - ( 4-(ethoxycarbonyl)phenyl)carboxamide;
N-(2R-carboxymethyl-5-(phenyl)pentanoyl)-
L- (N-lysine -N ' - ( 4 - ( ethoxycarbonyl)phenyl)carboxamide;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L- (N~-isopropyl)lysine-N'-(4-(ethoxycarbonyl)-
phenyl)carboxamide;
N-(2R-carboxymethyl)-4-(phenyl)butanoyl)-
L-cyclohexylglycine-N'-(4-(N",N"-dimethyl-
aminoethylaminosulfonyl)-phenyl)carboxamide; and
CA 0220~66~ l997-0~-2l
WO96116027 Pcr~ss5/l~s3o
-27-
N-(2R-carboxymethyl-5-(phenyl)pentanoyl)-
L- (N,N'-diethylguanido)lysine-N-(4-(ethoxy-
carbonyl)phenyl)carboxamide.
~;yr,~ .SIS OF COMPOllNDS OF FORMUI-A (I)
The compounds of formula (I) are prepared as described
below, for example with reference to Reaction Schemes 1-7, in
which the ~ubstituent groups illustrated (e.g., Rl, R2, etc.)
have the ~ame me~n; ngs as described in the Summary of the
Invention, unless otherwise specified. Certain of the
reaction ~chemes illustrate structures of formula (I) where p
is zero and R7 is an optionally substituted phenyl group [the
substituents R4 and Rs having been described in connection with
formula (II) in the Summary of the Invention]. As those
skilled in the art will appreciate, while the corresponding
compounds where p is 1-4 and where R7 is as otherwise defined
can be analogously prepared, combinations of substituents
and/or variables in compounds of formula (I) and intermediates
thereof are permissible only when such combinations result in
stable compounds.
Compounds of formula (I) and their pharmaceutically
acceptable salts, as single stereoisomers or as mixtures
thereof, are peptide derivatives all or portions of which can
2S be prepared from the constituent ~-amino acid derivative(s).
Standard methods for the formation of peptide bonds are
illustrated by M. Bodanszky et al.,
The Practice of Peptide Synthesis (1984), Springer-Verlag;
M. Bodanszky, Principles of Peptide Synthesis (1984),
Springer-Verlag; J.P. Greenstein et al., Chemistry of the
Amino Acids (1961), Vol. 1-3, John Wiley and Sons Inc.; G.R.
Pettit, Synthetic Peptides (1970), Vol. 1-2, Van Nostrand
Reinhold Company.
Synthetic Reaction Parameters
The terms "solvent~ inert organic solvent" or "inert
solvent" mean a solvent inert under the conditions of the
SllBSTlTUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9SIlS530
-28-
reaction being described in conjunction therewith [including,
for example, benzene, toluene, acetonitrile, tetrahydrofuran
("THF"), dimethylformamide ("DMF"), chloroform, methylene
chloride (or dichloromethane), diethyl ether, methanol,
pyridine and the like]. Unless specified to the contrary, the
solvents used in the reactions of the present invention are
inert organic solvents.
The term "q.s." means adding a quantity sufficient to
achieve a stated function, such as to bring a solution to a
desired volume.
Unless specified to the contrary, the reactions described
herein take place at atmospheric pressure within a temperature
range from 5~C to 100~C (preferably from 10~C to 50~C; most
preferably at "room" or "ambient" temperature, e.g., 20~C).
1~ Further, unless otherwise specified, the reaction times and
conditions are intended to be approximate, e.g., taking place
at about atmospheric pressure within a temperature range of
about 5~C to about 100~C (preferably from about 10~C to about
50~C; most preferably about 20~C) over a period of about 1 to
about 10 hours (preferably about 5 hours). Parameters given
in the Examples are intended to be specific, not approximate.
Amide couplings used to form the compounds of
formula (I) are generally performed by the carbodiimide method
with reagents such as dicyclohexylcarbodiimide or N'-ethyl-
N'-(3-dimethylaminopropyl)-carbodiimide (EDCI) in the presence
of 1-hydroxybenzotriazole (HOBT) in an inert solvent such as
dimethylformamide (DMF). Other methods of forming the amide
or peptide bond include, but are not limited to synthetic
routes via an acid chloride, acyl azide, mixed anhydride or
activated ester such as nitrophenyl ester. Typically,
solution phase amide couplings with or without peptide
fragments are performed.
The selection of protecting groups for the terminal amino
or carboxy groups of compounds used in the preparation of the
compounds of formula (I) is dictated in part by the particular
amide or peptide coupling conditions, and in part by the amino
acid and/or peptide components involved in the coupling.
SllBSl ITUl~E SHEET (RULE 2~)
_
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9Sl15S30
-29-
Amino-protecting groups commonly used include those which are
well known in the art, e.g., p-methoxybenzyloxycarbonyl,
benzyloxycarbonyl (also referred to as carbobenzyloxy or CBZ),
p-nitrobenzyloxycarbonyl, t-butoxycarbonyl (BOC), and the
like. It is preferred to use either BOC or CBZ as the
protecting group for the ~-amino group because of the relative
ease of its removal by mild acids [e.g., by trifluoroacetic
acid (TFA) or hydrochloric acid in ethyl acetate] or by
catalytic hydrogenation.
I801ation and purification of the compounds and
intermediates described herein can be effected, if desired, by
any suitable separation or purification procedure such as, for
example, filtration, extraction, crystallization, column
chromatography, thin-layer chromatography or thick-layer
chromatography, or a combination of these procedures.
Specific illustrations of suitable separation and isolation
procedures can be had by reference to the examples
hereinbelow. However, other equivalent separation or
isolation procedures can, of course, also be used.
The individual stereoisomers of compounds of formula (I)
may be separated from each other by methods known to those of
ordinary skill in the art, e.g., by selective crystallization
or by chromatography, and/or by the methods disclosed herein.
Preparation o~ Formula (E)
Compounds of formula (E) are intermediates used in the
preparation of compounds of formula (I), and are prepared as
shown in Reaction Scheme 1 wherein Rl2 is mesyl or tosyl:
CA 0220~66~ l997-0~-2l
WO96/16027 PCT~S9S/15S30
-30-
Reaction Scheme 1
,.
O R2 ~~ ~ R2 Rl20 1~ R2
~ O C H2 C 113_ ~y~ ~ o c ~ 2 c ~ 3 ~ O C H2 C ~ 3
~C}~2C}~3 OCH2C~3 OCEI2Cf~3 ¦ -
~E~ ~Eb~ (Ec~
R6- 5 ~ R2 R6- S 1~ R2
CEc~ + R6_5H y~J~oC112C~3 ~P~O~
CEd~ OC~2CI13 0~1 o
~Ee) ~E)
5Starting Materials
Compounds of formula (Ea) may be prepared according to methods
known to those of ordinary skill in the art ~e.g., see
European Published Patent Application 0 276 436) or may be
prepared according to the method described in Example l below.
Compounds of formula (Ed) are commercially available or may be
prepared according to methods known to those of ordinary skill
in the art.
Formula (Eb) - In general, compounds of formula tE) are
prepared by first treating a compound of formula (Ea) in an
aprotic solvent, preferably tetrahydrofuran and methylene
chloride, at 0-15~C, preferably at OoC, in the presence of a
base, preferably diisopropylethylamine and bis- (trimethyl-
silyl)acetamide, with paraformaldehyde. The resulting
solution is brought to 25-37~C, preferably to 37~C, for 18
hours. The alcohol of formula (Eb) is then isolated by
standard methods, preferably by evaporation of solvent,
extraction and filtration.
Formula (Ec) - An alcohol of formula (Eb) in an aprotic
solvent, preferably methylene chloride, is then cooled to -
20~C to about 0~C, preferably to about -20~C, and is then
SlJBSTITUTE S~lEET ~RULE 26)
CA 0220~66~ 1997-0~-21
WO 96116027 PCT/US9S/lS~;30
--31--
esterified by the standard procedure of treating the alcohol
with at least a stoichiometric amount to about a 100~ excess
of either mesyl chloride or tosyl chloride. The
esterification takes place over an initial period of time
(preferably 15 minutes) at -20~C, followed by second period of
time (preferably 3.5 hours) at room temperature. The ester of
formula (Ec) is then isolated from the reaction mixture by
st~n~d isolation procedures, preferably by extraction,
filtration and evaporation.
Formula (Ee) - An ester of formula (Ec) in an aprotic
solvent, preferably DMF, is then reacted with a salt of a
compound of formula (Ed) (preferably the sodium salt formed
from the reaction of the compound of formula (Ed) with sodium
hydride in an aprotic solvent, preferably DMF), for about 16-
20 hours, preferably for about 18 hours, at temperatures
beginning at about 0~C and slowly warming to room temperature.
The resulting mercapto compound of formula (Ee) is isolated
from the reaction mixture by standard isolation techniques,
such as by extraction, evaporation, and flash chromatography.
Formula (E) - A compound of formula (Ee) is then
hydrolyzed under basic conditions, preferably in the presence
of sodium hydroxide, to form a compound of formula (E), which
is isolated from the reaction mixture by standard isolation
techniques.
Preparation of Formula (Ia)
Compounds of formula (Ia) are compounds of formula (I)
wherein Rl is a group of the formula
/I ~ ~ ~R5
OH
(wherein, when R6 is aryl, it is preferably naphth-1-yl,
naphth-2-yl or phenyl, and when R6 is heteroaryl, it is
preferably pyridyl or quinol-2-yl; R2 is preferably alkyl; and
Rs is preferably hydrogen) are prepared as described in
Reaction Scheme 2.
~ IIIUII~ SEEr (RUIE;~i)
CA 0220~66~ 1997-0~-21
WO 96/16027 PCT/US9511S530
--32--
Reaction Scheme 2
.~
H O
B ~I C~ ~ + ~LN ~?
( A )
H O R 4
O
H~ ~N~ ( D)
O R2
~D) + R6_5~P~OH (E)
OH o
O R2 ~ o
R6_5 ~P~ ~N~ H~ (Ia)
SUBSTITVTE SHEET (RULE 2~)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/lSS30
Starting Material~
N-protected amino acids of formula (A) and compounds of
formula (B) are commercially available or may be prepared
according to methods known to those of ordinary skill in the
art. Compounds of formula (E) are prepared as described with
reference to Reaction Scheme 1.
Formula (C) - In general, compounds of formula (Ia) are
prepared by first coupling a compound of formula (A) with a
compound of formula (B) [or with another compound of the
formula H2N-(CH2)p-R7], under standard amide coupling conditions
to form a compound of formula (~). For example, to a cold (0-
5~C) solution of the compound of formula (A) and an excess
molar amount of HOBT in DMF is added an excess molar amount of
EDCI. The resulting solution is stirred from about 1 to about
2 hours, preferably for about 1 hour, at 0-5~C, preferably at
0~C. To the cold solution is then added a solution of an
equimolar amount of a compound of formula (B) in the presence
of a base, preferably DMAP. The resulting mixture is stirred
from 12 to 24 hours, preferably for 24 hours, at room
temperature, preferably at 25~C. The compound of formula (C)
is then isolated from the reaction mixture by standard peptide
isolation techniques.
Formula (D) - The amino-protecting group of the compound
of formula (C) is then removed under mild acidic conditions,
preferably in the presence of trifluoroacetic acid, to yield a
compound of formula (D).
Alternative Preparations of Formula (D) - Another method
of preparing a compound of formula (D) particularly when R3 is
t-butyl, other ~-branched amino acid side chains, or
cyclohexyl, p is zero, and R7 is aryl or heteroaryl, employs
the intermediate (A-1), the preparation of which is
illustrated in Reaction Scheme 2A. Another alternative method
of preparing a compound of formula (D) particularly when R3 is
1-hydroxyisopropyl or another ~-hydroxy amino acid side chain,
and R7 is aryl or heteroaryl, is illustrated in Reaction Scheme
2B.
SllBSTlTUTE SHEET (RULE 26)
CA 02205665 1997-05-21
WO 96/16027 PCT/US9~/lS530
--34 -
Realction SchemQ 2A
F o r m u I a ~ A ~ t ~ O--N~ ~ B O C - N~
N-hydroxysucc i n i mi de
C A- 1~
As illustrated in Reaction Scheme 2A, a compound of formula
(A) is coupled with about one molar equivalent of
N-hydroxysuccinimide in acetonitrile at 0~C in the presence of
DCC. The reaction takes place with stirring at 0~C to 25~C,
for 8 to 16 hours to give the corresponding N-
hydroxysuccinimide ester of formula (A-1). This ester is then
reacted with a compound of formula B or another compound of
the formula H2N-(CHz)p-R7 in an inert solvent at 100~C
preferably for 3 hours; the resultant compound of formula (C)
is isolated and deprotected to yield a compound of formula (D)
as described above in Reaction Scheme 2.
Reaction Scheme 2B
C B Z - N ~ ~t C 9 Z - N ~ ~ R 5 H 2 N ~ N /~[ R S
h OH h ~ OH h
c C- 1) C C- 2) c D- 1)
S~BSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO 96tl6027 PCT/US9S/lSS30
As illustrated in Reaction Scheme 2B, a compound o~
formula (C-1) in an inert anhydrous solvent such as THF is
stirred with n-butyllithium at a temperature below 10~C,
preferably 0~C, for about 1 hour, then cooled to about -70~C
and reacted with 3 molar equivalents of acetone. A compound
of formula (C-2), as a racemate, is isolated and purified by
stAn~Ard procedures. Following hydrogenolytic removal of the
CBZ protecting group, a compound of formula (D-1) is obtained.
Formula (Ia) - As illustrated in Reaction Scheme 2, a
compound of formula (D) is coupled with a compound of formula
(E) under standard peptide coupling conditions. For example,
to a cold (0-5~C, preferably 0~C) solution of the compound of
formula (D) in an inert solvent, preferably THF, is added
1,1'-carbonyldiimidazole. The resulting mixture is stirred
from 60 to 90 minutes, preferably for 75 minutes, at 0-5~C,
preferably at 0~C, and then reacted with the compound of
formula (E) for about 12 to 17 hours, preferably for about 15
hours. The resulting compound of formula (Ia) is then
isolated from the reaction mixture by standard peptide
isolation techniques, for example, extraction and reverse
phase HPLC.
Preparation of Formula (F)
Compounds of formula (F):
O RZ
8 ll
R -OC C-OH
0
(F)
wherein R8 is t-butyl, are intermediates used in the
preparation of compounds of formula (I) as illustrated below
in Reaction Scheme 4. The compounds of formula (F) are
prepared as shown in Reaction Scheme 3.
"~ um m~
CA 0220~66~ l997-0~-2l
WO96/l6027 PCT~S9S/15530
-36-
Reaction Scheme 3
.
~C-Cl \~ O \~
O /A\o o~ O
~Fa) L-(+)-2, 10-camphor (Fb)
sul tam
0 0 R2 y
(Fb) + R8_o~Br R8_o~C-N~
~ \SJ
O~ O
( F c
R8 o ~ c-o~ and HN~
( F ~ /~
L-~+)-2, 10-camphor
sultam
Starting Material~
Compounds of formula (Fa) are commercially available or may be
prepared according to methods known to those of ordinary skill
in the art, for example, by the method described in Example 11
below. L-(+)-2~lo-rAmrhnr sultam and D-(-)-2,10-camphor sultam
SUBSTITUTE SHEET (RULE 26)
-
CA 0220~66~ 1997-0~-21
W096/16027 PCT~S95/lSS30
-37-
are commercially available, for example, from Aldrich.
Formula (Fb) - In general, compounds of formula (F)
(illustrated as one of the two isomers obtainable by this
~ynthesis) are prepared by first condensing a compound of
formula (Fa) ~where the group R2 encompasses the group "X" of
Formula (I) and can be, e.g., a biphenylpropylene or fluorenyl
propylene group] with L- (+) -2,lO-camphor sultam to form a
compound of formula (Fb).
Formula (Fc) - U~ing ~odium h~methyldisilazide to
generate the anion for l hour, the reaction is quenched with
t-butylbromoacetate to form the corresponding ester of formula
(Fc).
Formula (F) - The camphor group i8 then removed under
basic conditions, such as lithium hydroperoxide (formed in
situ from lithium hydroxide and hydrogen peroxide) initially
at reduced temperature (preferably 0~C) for 15 minutes and
warmed to room temperature for 2 hours. The mixture is cooled
back to 0~C and an aqueous mixture of sodium sulfite and
sodium bicarbonate is added with stirring, after which the
mixture is allowed to return to room temperature, and the pH
is neutralized to yield an individual stereoisomer of a
compound of formula tF) wherein the carbon to which the -X-R2
group is attached is in the (R) configuration. In a similar
manner, but substituting D- ( - ) -2,lO-camphor sultam for L- (+) -
2,lO-camphor sultam, the corresponding individual
stereoisomers in the (S) configuration can be prepared.
Alternative Preparation of Formula (F)
Another method of preparing stereoisomers of formula (F)
utilizes the commercially available chiral compound, 4S-
phenylmethyloxazolidinone, as shown below in Reaction
Scheme 3A [following the Starting Materials section, where
preparation of the compounds of starting material of formula
(Fa') are illustrated].
'~UBSTITUT~ SHEET (RULE 26)
CA 02205665 1997-05-21
WO96/16027 PCT~S95/15~30
-38-
Starting Materials
Compounds of formula (Fa') where X is -O-CH~-CH2- are prepared
as illustrated in Reaction Scheme 3A-1.
Reaction Scheme 3A-1
i3 r R 2 ~ O
l ~OEt ~ OEt OEt
Ca~ Cb) C~) Cd)
2 0 i~ 2 \O
~C i ~ OH
0
~Fa '- 1) ce,
A commercially-available alcohol (a) is reacted with
3 0 ethyl-4-bromocrotonate (b) in the presence of stoichiometric
sodium hydride in a solvent such as DMF at 0~C to room
temperature, or in the case of a phenol (a), by refluxing with
(b) in acetone in the presence of excess potassium carbonate
for several hours. The resulting unsaturated ester (c) is
converted by hydro~enation in the presence of platinum on
carbon to the saturated ester (d), which is then saponified
with aqueous sodium hydroxide in ethanol to the acid (e). The
CA 02205665 1997-05-21
WO 96~16027 PCTIUS9511S530
--39--
acid (e) is converted to the acid chloride (Fa'-1) through the
action of oxalylchloride at between room temperature and 50~C.
Compounds of formula (Fa~) where X i~ -S-CH2CH2- are
prepared as illustrated in Reaction Scheme 3A-2.
R~action Scheme 3A-2
R ~5 R ~S
R~ SH + ~ ~OH ~C I
O O
Cf) Cg) Ch~ CFa -2)
A commercially-available thiol (f) is reacted with
lithium hydride in DMF at room temperature for several hours
to form the lithium thiolate. Excess butyrolactone (g) is
added and heated to reflux under argon to give the acid (h).
Acid (h) is then converted to the acid chloride (Fa'-2) with
oxalyl chloride, as before.
Compounds of formula (Fa') where X is -CH2CH2-0- are
prepared as illustrated in Reaction Scheme 3A-3.
SlJBSTIME SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO 96/16027 PCT/US9SIlSS30
--40--
Reaction Scheme 3A-3
2 R2 R2
R2- Br R2 OH O ~O
Ci ~ ~k~ C I ~ ~OH l~f C I
c m ~ C F a - 3 )
Compounds of formula (1) and (k) are in many cases
commercially available. When not, they are prepared as
follows. Compounds of formula (j), where R2 is aryl or
heteroaryl, are converted to alkenes (k) by treatment for
2 20 several hours with vinyl-tributylst~nn~ne (commercially
available from Aldrich Chemical Co.) in the presence of
catalytic tetrakis (triphenylphosphine)palladium at reflux in
toluene. The alkenes (k) may be further converted to the
alcohols (1) by hydroboration with borane in THF at 0~C to
2 25 room temperature, over a period of several hours, followed by
oxidation with alkaline hydrogen peroxide. The alcohols (1)
are converted to the acids (m) by treatment with chloroacetic
acid and excess sodium hydride in DMF at elevated temperature,
preferably 60~C. The acids (m) are converted to the acid
chlorides (Fa'-3) with oxalyl chloride, as before.
Compounds of formula (Fa') where X is -CH2CH2-S- are
prepared according to Reaction Scheme 3A-4.
S~Er fln LE~) ,
CA 02205665 l997-05-2l
WO96/16027 PCT~S9SI15S30
-41-
Reaction Scheme 3A-4
'
Formu I a C I ) -- ~ 5 ~ S ~ S
0 o~--CH3 ~fOH ~.~CI
~n~ CP~ CFa -4
The alcohols (l) are converted to thioacetates (n) by
addition of thioacetic acid to the reagent generated from
triphosphine and diethyl azodicarboxylate in THF at 0~C. The
thioacetates (n) are converted to the acids (p) by treatment
with potassium carbonate in methanol in the presence of
chloroacetic acid. The acids (p) are converted to the acid
chloride (Fa~-4) with oxalyl chloride, as before.
CA 0220~66~ 1997-0~-21
WO 96/16027 PCT/US9S/lSS30
Reaction Scheme 3A
R
~ > R 2
O H ~ ~ "
C Fa ~ C Fb )
15Formu a ~Fb ~ ~t-BI~-O~
C F c )
R 2
20Formula CFc ) ~t-~u-O/~ OH
C F ~)
Formula (Fb') - A compound of formula (Fa') is first
condensed with 4S-phenylmethyloxazolidinone under st~n~rd
conditions to give the corresponding compound of formula
(Fb').
Formula (Fc') - An approximately equimolar amount of
sodium hexamethyldisilazide is added to a compound of formula
(Fb') in an inert solvent such as THF. The reaction takes
place at -80~C to -95~C, for about 15 minutes.
t-Butylbromoacetate is added in excess to this mixture and the
solution is stirred for about 2 hours at -90~C to -60~C to
yield a single stereoisomer of formula (Fc'), which is
purified by standard organic chemistry procedures.
SUBSTITUTE SHEET ~RULE 26)
CA 02205665 1997-05-21
W Q 96~16027 PCT~US9~/lSS30
-43-
Fo ~ (F') - The oxazoli~;no~e group of a compound of
formula (Fc') is removed under basic conditions to yield an
individual stereoisomer of formula (F'), for example as
described with reference to the preparation o~ formula (F) in
Reaction Scheme 3. The compounds of formula (F') can be u8ed
interchangeably with those of formula (F) in the syntheses
that follow.
Alternative Preparation of Formula (F) - Formula F can
also be prepared as de~cribed with reference to Reaction
Scheme 3B.
Reaction Scheme 3B
Formu I a C Fc" ~ ~ t- E~u
CFC _ 1~
For mu 1~ C Fc " 1~ t- El
'~
~ Fc" - 2)
'SUBSTITUT~ SltEET (RULE 26)
CA 0220~66~ l997-0~-2l
wos6ll6o27 PCT~S9S/15S30
-44-
R2
~ ~>
Formu I a C Fc"- 2~ t- Bu ~ ~OH
C F
Starting Materials
The compound illustrated as formula (Fc") can be prepared
analogously to the preparation of formula (Fc') as described
with reference to Reaction Scheme 3A, by substituting for the
compound of formula (Fa') the corresponding allyl compound
where the group shown as X is prop-2-enyl and R2 is H.
Fc_ la (Fcn-1) - Arylation or heteroarylation of (Fc")
is carried out in the presence of a base and a palladium
catalyst by adding aryl- or heteroaryl-halide, preferably
bromide or iodide, and heating the reaction mixture for about
2 to 4 hours, preferably 4 hours, at about 100~C to form a
compound of the formula (Fc"-l).
Formula (Fcn-2) - Catalytic hydrogenation (Pd/C) of an
allyl compound of formula (Fc"-l) yields the corresponding
alkyl compound of the formula (Fc"-2).
Formula (F) - A compound of formula (Fc"-2) is subjected
to basic conditions, such as lithium hydroperoxide (formed in
situ from lithium hydroxide and hydrogen peroxide) initially
at reduced temperature (preferably 0~C) for 15 minutes and
warmed to room temperature for 2 hours. The mixture is cooled
back to 0~C and an aqueous mixture of sodium sulfite and
sodium bicarbonate is added with stirring, after which the
mixture is allowed to return to room temperature, the pH
neutralized, and the compound of formula (F) is obtained by
standard isolation.
~:ll~llIUFE ~IE~T (RUI F 2~
CA 02205665 1997 - 05 - 21
WO96116027 PCT~S9S/lS530
-45-
Preparation of Formulae (Ib), (Ic), (Id) and (Ie)
The compounds of formulae (Ib), (Ic), (Id) and (Ie) each
represent sub-genu~es of formula I in which the R1 substituent
varies, prepared sequentially as described in Reaction Scheme
4, where R8 is t-butyl. In compounds of formula (Ib) R1 is
alkoxycarbonyl or aralkoxycarbonyl. In compoundc of formula
(Ic) R1 is carboxy. In compounds of formula (Id) R1 i5
benzyloxycarbamoyl. In compounds of formula (Ie) R1 i~
hydroxycarbamoyl.
~UBSrITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO 96116027 PCT/US95/15530
--46--
Reaction Scheme 4
O R2 H O
R e _ o C J C - O H + H~ N ~ N4~ ( D )
~F)
O R2 ~ o
lo o ~--E~ ~ Ib)
O R2 El O
o \'~H~ ~Ic)
~\ ONH2
~Ic) + ~J
O R2 ~ o
~0-N-C ~ ~ C~ ~JI\N~ ~Id)
O R2 H O
3 5 H O - N - C~ l.~ C~ N ~ N~ ( I e
CA 0220~66~ 1997-0~-21
WO 96/16027 PCTJUS95/lSS30
--47--
Starting Materials
Formula (D) is prepared as described with reference to
Reaction Schemes 2, 2A and 2B. Formula (F) is prepared as
described with reference to Reaction Sch~mPs 3 and 3A.
~ 5 O-Benzylhydroxylamine is commercially available, for example,
as the hydrochloride salt from Aldrich Co.
Formula (Ib) - A compound of formula (F) is coupled with
a compound of formula (D) under standard amide coupling
conditions to form a compound of formula (Ib). For example,
to a solution of a compound of formula (F) in an aprotic
solvent, preferably DMF, containing a slightly excess molar
amount of HOBT, is added an excess molar amount of EDCI. The
resulting mixture is stirred from 1 to 2 hours (preferably 1
hour) at 0-5~C (preferably at 0~C). To the cold solution is
then added an equimolar amount of a compound of formula (D) in
the presence of a base, preferably DMAP. The resulting
mixture i8 then stirred from 12 to 24 hours (preferably 24
hours) at room temperature (preferably at 25~C). The compound
of formula (Ib) is then isolated from the reaction mixture by
standard peptide isolation techniques, for example,
evaporation of solvents, extraction, flash chromatography
and/or HPLC.
Formula (Ic) - A compound of formula (Ib) is hydrolyzed
under mild acidic conditions, preferably with trifluoroacetic
acid, to yield a compound of formula (Ic).
Formula (Id) - A compound of formula (Ic) is then treated
with O-benzylhydroxylamine under standard amide coupling
conditions to form a compound of formula (Id). For example, a
cold (0-5~C) solution of the compound of formula (Ic) and HOBT
in an inert solvent, preferably DMF, is treated with an excess
molar amount of EDCI. After stirring the resulting mixture
for 30 minutes to an hour at 0-5~C (preferably at 0~C), an
equimolar amount of O-benzylhydroxyamine is added. The
reaction mixture is allowed to warm and remain at room
temperature overnight for 8 to 16 hours. The compound of
formula (Id) is then isolated from the reaction mixture by
standard isolation techniques, for example, by extraction and
Çt~Q..~ urrr ~ nr ~
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/15530
-48-
flash chromatography.
Formula (Ie) - The hydroxyl-protecting group (benzyl) of
a compound of formula (Id) is removed under catalytic
hydrogenation conditions (Pd/C) to yield a compound of formula
(Ie).
Alternative Preparation of Formula (Ie) - An alternative
method for preparing formula (Ie) (particularly where R4 i8 a
sulfur-containing moiety, such as alkylsulfinyl) is to treat
the corresponding compound of formula (lc) with hydroxylamine
hydrochloride and a peptide coupling reagent, preferably
benzotriazol-l-yloxy-tris(dimethylamino)phosphonium
hexafluorophosphate, in the presence of a tertiary amine base
such as N-methylmorpholine in DMF solvent. The resulting
compound of formula (le) is isolated from the reaction mixture
by standard isolation techniques, for example, by extraction
and concentration.
Alternative Preparation of Formula (Ib) - A particularly
preferred method of preparing compounds of formula (Ib) when R2
i8 an aryl or heteroaryl, and where X (not shown) is propane-
l,3-diyl and p (not shown) is zero is shown in Reaction Scheme
4A.
SU8SlllUlt slEr ~RUIE 2C)
CA 0220566s 1997-05-21
WO96/16027 PCT~S9511SS30
-49-
Reaction Scheme 4A
t Bu \OJI\ ~ OH
C Fc'')
o ~\~
For mu l a C F ~ l H2Nl ~ R t- 9u ~ ~ H
~D ~ CD -1)
R2 RZ
t-Bu ~ R7 t-Bu J~S ~ R7
C i b ~ ) ~ R 3 H O ~ D - 2 )
25Starting Materials
The compound illustrated as formula (Fc") can be prepared
analogously to the preparation of formula (Fc') as described
with reference to Reaction Scheme 3A, by substituting for the
compound of formula (Fa') the corresponding allyl compound
where R2 is prop-2-enyl. The compound of formula (D') is a
compound of formula (D) and can be as described in Reaction
Scheme 2. The halo-aryl or halo-heteroaryl reactants used in
f the preparation of compounds of formula (D'-2) are
commercially available, or can be prepared according to
methods known to those of ordinary skill in the art, e.g., as
described in Example 41C.
SU8ST1ME SHEET (RULE 26
CA 0220~66~ 1997-05-21
WO96/16027 PCT~S95/15S30
-50-
A compound of formula (F") is prepared by alkaline
hydrolysis of the oxazolidinone group from a compound of
formula (Fc"). After isolation by standard procedures, (F")
i8 coupled with a compound of formula (D') under st~n~rd
peptide coupling conditions as described above with reference
to Reaction Scheme 2, to form a compound of formula (D'-1).
Arylation or heteroarylation of (D'-l) iB accomplished by
adding aryl- or heteroaryl halide (preferably aryl- or
heteroaryl bromide, iodide or triflate) and heating the
reaction mixture for about 2 hours at about 100~C to form a
compound of the formula (D'-2). Catalytic hydrogenation
(Pd/C) of (D'-2) yields a compound of formula (Ib').
Preparation of Formula (G)
Compounds of formula (G):
x
H 3 C ~ S ~ O H
~ O O
(G)
are intermediates in the preparation of compounds of formula
(I) and are prepared as illustrated below in Reaction Scheme
6. The compounds of formula (G) are prepared as shown in
Reaction Scheme 5.
SUBSTITlJTE SHEET (P(ULE 263
CA 02205665 1997-05-21
WO96116027 PCT~S9S/15530
R-action Scheme 5
R 2 R ~ X
CH3CH20C l COCH2CH3 ~ ~ ~ OCH2CH3
O O O O
~ G a )
~ X
(Gb) , H2C/ ICIOCH2CH3 (Gc)
H C~C~OH (Gd)
2 0 lo
~Gd~ + H3C~SH H3C~S~J~ ,OH
O O O
~ G )
~ Starting Materials
Compounds of formula (Ga) and thioacetic acid are commercially
+ available, for example, from TCI America Organic Chemicals and
the Aldrich Company, respectively.
Formula (Gb) - A compound of formula (Ga) is hydrolyzed
with an equimolar amount of a base, for example, potassium
___ __ __ .
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/15S30
hydroxide, to yield a compound of formula (Gb).
~ 1 A (Gc) - A compound of formula (Gb) is deprotonated
under basic conditions, for example, in the presence of
triethylamine, at 0-5~C (preferably at O~C) and then reacted
with formaldehyde, followed by treatment with aqueous base,
preferabry-potassium carbonate, to yield a compound of formula
(Gc), which is isolated from the reaction mixture by st~n~d
isolation procedures.
Formula (Gd) - A compound of formula (Gc) is hydrolyzed
under basic conditions, preferably in the presence of lithium
hydroxide, to yield a compound of formula (Gd).
Formula (G) - A compound of formula (Gd) is reacted with
an excess molar amount of thioacetic acid at 90-100~C
(preferably at 95~C) under an inert atmosphere. The compound
of formula (G) is then isolated from the reaction mixture by
standard isolation techniques, for example, by extraction and
evaporation.
Preparation of Formulae (If) and (Ig)
The compounds of formulae (If) and (Ig) each represent
sub-genuses of formula I in which the R1 substituent is sulfur-
containing, prepared sequentially as described in Reaction
Scheme 6. In compounds of formula (If), R1 is acetylthio. In
compounds of formula (Ig), R1 is mercapto
SU8STITUTE SHEET ~RULE 26)
_ _ _ _ _ _ _ _ ,
CA 02205665 l997-05-2l
WO 96/16027 PCT/US9S/lSS30
Reaction Scheme 6
R 2
~X H O
o 3 ~1~N~RS
(G) (D)
R2~x H O
O C ~ l ~ N ~ R 5
~If )
R ~X H O
HS~
O R 3 H R S
CIg)
Formula (If)
A compound of formula (G) is coupled with a compound of
formula ~D) under standard amide coupling conditions to yield
SUB!illlUltS~ RULEa6)
CA 0220~66~ 1997-05-21
WO96/16027 PCT~S95/lSS30
-54-
a compound of formula (If). For example, to a solution of the
compound of formula (G) and HOBT in an aprotic solvent,
preferably DMF, is added an excess molar amount of EDCI.
Subsequently, the compound of formula (D) is added and the
resulting mixture is stirred overnight at room temperature.
The resulting compound of formula (If) is then isolated from
the reaction mixture by standard isolation techni~ues, for
example, by evaporation of solvent, extraction, and flash
chromatography.
Formula (Ig) - A compound of formula (If) is hydrolyzed
under basic conditions, preferably in a protic solvent such a~
methanol in the presence of ~mmo~; um hydroxide, to form a
compound of formula (Ig).
Preparation of Formula (Ih)
Compounds of formula (Ih) are a sub-genus of formula (I)
where Rl is N-hydroxyformylamino, and are prepared as shown in
Reaction Scheme 7.
SUB~IT-UTESH~TtRULE26)
CA 02205665 1997-05-21
WO 96116027 PCIIUS95/lS530
--55--
Roaction Sch~m~ 7
HO I _ HO~
C Fb~) C P- 1) C P- 2)
~ nO ~
C P- 5~ C P- 4~ C P- 3
R
I'~
CP- 5~ + D ' ~ BnO
H ~ ~N~l' N- C CHz~p_ R7
C P- 6
R~
HO H ~
H ¦ N~!~ N- C C H z ) p _ p 7
c I h)
SUBSTIME SHEET ~RULE 26~
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/lS530
-56-
8tarting Naterials
The compound illustrated as formula (Fb") can be prepared
analogously to the preparation of formula (Fb~) as described
with reference to Reaction Scheme 3A, by substituting for the
compound of formula (Fa~) the corresponding allyl compound
where R2 is prop-2-enyl.
FG 1 a (P-l)- The compound of formula (Fb") is
hydroxymethylated by incubation with titanium tetrachloride at
reduced temperature, preferably 0~C, under basic conditions
for one to three hours, preferably l hour followed by addition
o~ S-trioxane and titanium tetrachloride with continued
incubation at 0~C for 3 to 5 hours, preferably 4 hours. The
compound of formula (P-l) is then isolated by standard
methods, e.g., extraction and column chromatography.
Fo~ (P-2)- The compound of formula (P-l) is reacted
with an excess molar amount of 0-benzylhydroxylamine and of
trimethylaluminum at reduced temperature, preferably O~C. The
reaction is allowed to proceed with stirring for 5 to 7 hours,
preferably 6 hours, at 0~C under argon. The resulting
compound of formula (P-2) is isolated by standard procedures.
Fs 1 A (P-3)- Excess mesyl chloride is reacted with the
compound of formula (P-2) in pyridine at 0~C for several
hours, preferably 3 hours. The reaction mixture is cooled on
ice, organic solvent-extracted, and concentrated. The
concentrated extract is refluxed under basic conditions for
several hours, preferably 3 hours, thus yielding the
azetidinone compound of formula (P-3), which is purified by
standard procedures.
Formula (P-4)- The compound of formula (P-3) is reacted
with a desired halogenated R2 group ( e.g., an aryl- or
heteroaryl halide, preferably bromide or iodide) in an inert
solvent in the presence of a base, such as triethylamine, and
a palladium catalyst, preferably formed from palladium (II)
acetate and about 2 molar equivalents of tri-o-tolylphosphine.
After heating the reaction mixture for 15 to 20 hours,
preferably 18 hours at 100~C, the corresponding compound of
formula (P-4) is isolated and purified by standard procedures.
SU~5~111ult SHEr ~IIE ~C)
CA 0220~66~ 1997-0~-21
WO96/16027 PcT~S9SIlSS30
-57-
F~- 1 A (P-5)- Cleavage of the azetidinone ring of a
compound of formula (P-4) is carried out under basic
conditions at room temperature for 1 to 3 hours, preferably
1 hour. The resultant compound is extracted into organic
solvent, concentrated, redissolved in a base-containing
solvent (e.g., pyridine), and carboxylated with formic
anhydride at reduced temperature, preferably 0~C, for 30
minutes, to yield the corresponding compound of formula (P-5),
which i8 isolated by standard procedures.
Formula (P-6)- A compound of formula (P-5) is coupled
with a compound of formula (D') under standard amide coupling
conditions to form the corresponding compound of formula
(P-6), which is isolated by standard procedures.
Formula (Ih)- Catalytic hydrogenation of a compound of
formula (P-6) with Pd/C, followed by removal of the catalyst
by filtration yields the corresponding compound of formula
(Ih).
Preparation of Salts
In addition, all compounds of formula (I) that exist in
either the free acid or the free base form may be converted to
their pharmaceutically acceptable salts by treatment with the
appropriate inorganic or organic base or with the appropriate
inorganic or organic acid, respectively. Salts of the
compounds of formula (I) can also be converted to the free
acid or free base form or to another salt. For example, a
compound of formula (I) having a carboxylic acid moiety can be
converted to the carboxylate form by addition of 1 e~uivalent
of NaOH or KOH in an alcoholic solvent followed by evaporation
of solvent. A compound of formula (I) in the form of a free
base can be converted to the chloride salt, for example, by
addition of 1 equivalent of HCl in an organic solvent,
followed by concentration.
Preferred Synthesis and ~ast Step
In summary, compounds of formula (I) are prepared by:
SUBSTITUTE SHEET (RULE 26~
CA 0220~66~ 1997-05-21
WO 96/16027 PCT/US9S115530
--58--
(A) contacting a compound of formula (D)
.
o
H~N ~ N - ~Hz) -~7
CD~
with a compound of formula (F)
R 1 ~0H
11
c F)
wherein Rl is alkoxycarbonyl, aralkoxycarbonyl, aryl- or
heteroaryl thiomethylphosphinoyl, or acetylthio;
in the presence of a base and an amide coupling reagent to
give the corresponding compound of formula (I); or
(B) catalytically hydrogenating the corresponding
compound where X and R2 together are optionally aryl- or
heteroaryl-substituted alkenyl; or
(C) treating a compound of formula (I), where Rl is
alkoxycarbonyl or aralkoxycarbonyl, under mild acidic
conditions to give the corresponding compound of formula (I)
where Rl is carboxy; or
(D) contacting a compound of formula (I), where Rl is
carboxy, with O-benzylhydroxylamine to give the corresponding
compound of formula (I) where Rl is benzyloxycarbamoyl; or
(E) catalytically hydrogenating a compound of formula
(I), where Rl is benzyloxycarbamoyl, to give the corresponding
compound of formula (I) where Rl is hydroxycarbamoyl; or
(F) contacting a compound of formula (I), where Rl is
SUBSTITUTE SHEET (RULE 26)
CA 02205665 1997-05-21
W~ 96116027 PCr/US9S/lSS30
--59--
carboxy, with hydroxylamine to give the corresponding compound
of formula (I) where Rl is hydroxycarbamoyl; or
(G) catalytically hydrogenating a compound of the
formula
R2
H ~
H I N ~ N- C C H 2 ) p - R 7
wherein BnO is benzyloxy, to give the corresponding compound
of formula (I) where Rl is N-hydroxyformylamino; or
(H) treating a compound of formula (I), wherein Rl is
acetylthio, with ammonium hydroxide in a protic solvent to
give the corresponding compound of formula (I) where R1 is
2 0 mercapto.
A preferred method of making compounds of formula (I)
where Rl is N-hydroxyformylamino entails converting a compound
of formula (P-4)
R2
~
Y nO O
C P- 4)
wherein R2 is aryl or heteroaryl, by basic hydrolysis followed
by formylation to give a compound of formula (P-5)
u~,....c~ ~um ~n ~ M
CA 0220~66~ 1997-05-21
WO96/16027 PCT~S95/15S30
-60-
\
Bno
H l ~ O H
C P - 5 )
reacting the compound of formula (P-5) with a compound of
formula (D) to give a compound of formula (P-6)
p2
H ~
nO I I ~N- CCH2)p_ R7
O ~ R3 H
c P- 6~
and catalytically hydrogenating the compound of formula (P-6).
Compounds prepared by the above-described process of the
invention may be identified by the presence of a detectable
amount of one or more compounds of formulae (P-3), (P-4) or
(P-6). While it is well known that pharmaceuticals must meet
pharmacopoeia standards before approval and/or marketing, and
that synthetic reagents (such as O-benzylhydroxylamine) or
precursors [such as (P-3), (P-4), or (P-6)] should not exceed
the limits prescribed by pharmacopoeia standards, final
compounds prepared by a process of the present invention may
have minor, but detectable, amounts of such materials present,
for example at levels in the range of 50ppm or lower. These
SUBS~lTUrE SHEET (RULE 26)
.
0220~66~ 1997-0~-21
WO96116027 PCT~S95/lS~30
-61-
levels of (P-3) can be detected, e.g., by GC-MS, or of (P-4)
can be detected, e.g., by HPLC-MS or by HPLC with fluorescence
detection, or of (P-6) can be detected, e.g., by HPLC with
fluorescence detection. It is important to monitor the purity
r 5 of pharmaceutical compounds for the pre8ence of such
materials, which presence is additionally disclosed as a
method of detecting use of a process of the invention.
EXAMPLES
The following preparations and examples are given to
enable those skilled in the art to more clearly understand and
to practice the pre8ent invention. They should not be
considered as limiting the scope of the invention, but merely
as being illustrative and representative thereof.
Example 1
Compounds of formula (Ea)
lA. Crystalline phosphinic acid (8.4 g, 0.13 mol) was stirred
in neat triethylorthoformate (22 mL, 0.20 mL) for 90 minutes
at room temperature. This was then transferred via cannula to
a stirred solution of ethylisobutylacrylate (8 g, 0.036 mol)
and tetramethylguanidine (4.5 mL, 0.036 mol) that had been
cooled to 0~C for 10 minutes. The ice bath was removed and
the reaction stirred for 4 hours. The mixture was diluted
with 200 mL of diethyl ether and the solution washed with 1 N
HCl (100 mL), water (4xlO0 mL) brine (100 mL), and dried over
magnesium sulfate. This was rotary-evaporated to yield 8.15 g
of 2-(ethoxy)phosphinoylmethyl-4-methylpentanoic acid ethyl
ester as a slightly yellow colored oil, MS: 349 (M- H2O)+.
lB. In a similar manner, the following compounds of formula
(Ea) are prepared:
2-(ethoxy)phosphinoylmethyl-5-phenylpentanoic acid ethyl
ester;
2-(ethoxy)phosphinoylmethyl-4-phenylbutanoic acid ethyl ester;
2-(ethoxy)phosphinoylmethyl-3-phenylpropanoic acid ethyl
ester;
WBSl~lUlt S~Er ~RIJIE 26)
CA 0220~66~ 1997-05-21
Wo96/16027 PCT~S95/1SS30
-62-
2-(ethoxy)phosphinoylmethyl-3-cyclohexylpropanoic acid ethyl
ester; and
2-((ethoxy)phosphinoylmethyl)pentanoic acid ethyl ester.
Example 2
Compound of formula (Eb)
2A. Crude 2-(ethoxy)phosphinoylmethyl-4-methylpentanoic acid
ethyl ester (26 g) was dissolved in 600 mL THF/CHzCl2 (50/50)
and cooled to 0~C. Diisopropylethylamine (32 m~) and 90.8 m~
of bis(trimethylsilyl) acetamide were then added to the
solution and the resulting mixture was stirred for 20 minutes
before paraformaldehyde (5.5 g) was added. The solution was
brought to room temperature and heated at 37~C for 18 hours.
The solvent was removed by evaporation, and the resulting oil
dissolved in 200 mL ethyl acetate. The solution was washed
with 50 mL of lN HCl (2x), 50 mL of brine (2x), dried over
MgS04, filtered and evaporated to yield 19.3 g of
2-(ethoxy)(hydroxymethyl)phosphinoylmethyl-4-methylpentanoic
acid ethyl ester as a faintly yellow oil, MS: 281.2 (MH+).
2B. In a similar manner, the following compounds of formula
(Eb) are prepared:
2-(ethoxy)(hydroxymethyl)phosphinoylmethyl-5-phenylpentanoic
acid ethyl ester;
2-(ethoxy)(hydroxymethyl)phosphinoylmethyl-4-phenylbutanoic
acid ethyl ester;
2-(ethoxy)(hydroxymethyl)phosphinoylmethyl-3-phenylpropanoic
acid ethyl ester;
2-(ethoxy)(hydroxymethyl)phosphinoylmethyl-3-cyclohexyl-
propanoic acid ethyl ester; and
2-((ethoxy)(hydroxymethyl)phosphinoylmethyl)pentanoic acid
ethyl ester.
Example 3
Compounds of formula (Ec)
3A. 2-(Ethoxy)(hydroxymethyl)phosphinoylmethyl-4-methyl-
pentanoic acid ethyl ester (5 g) was dissolved in 20 mL of
CH2Cl2 and cooled to -20~C (in duplicate). Methanesulfonyl
CA 0220566~ l997-05-2l
W O 96/16027 PCTAUS9S/lSS30
-63-
chloride (1.5 mL) and triethylamine (3.0 mL) were added to
solution dropwise. After 15 minutes the bath was removed and
the reaction left at room temperature for 3~ hours. Each
Qolution was then washed with 10 mL cold 2~ HCl, 10 mL NaHC03
(sat), 10 mL brine, dried with MgS04, filtered and evaporated
to yield 12.8 g (combined yield) of 2-(ethoxy)(methane-
~ulfonyloxymethyl)phosphinoylmethyl-4-methylpentanoic acid
ethyl ester.
3B. In a similar manner, but replacing methanesulfonyl
chloride with p-toluenesul~onyl chloride, 2-(ethoxy)-
(p-toluenesulfonyloxymethyl)phosphinoylmethyl-4-methyl-
pentanoic acid ethyl ester is prepared.
3C. In a similar manner, the following compounds of formula
(Ec) are prepared:
2-(ethoxy)(methanesulfonyloxymethyl)phosphinoylmethyl-
5-phenylpentanoic acid ethyl ester;
2-(ethoxy)(methanesulfonyloxymethyl)phosphinoylmethyl-
4-phenylbutanoic acid ethyl ester;
2-(ethoxy)(methanesulfonyloxymethyl)phosphinoylmethyl-
3-phenylpropanoic acid ethyl ester;
2-(ethoxy)(methanesulfonyloxymethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid ethyl ester;
2-((ethoxy)(methanesulfonyloxymethyl)phosphinoylmethyl)-
pentanoic acid ethyl ester;
2-(ethoxy)(p-toluenesul~onyloxymethyl)phosphinoylmethyl-
5-phenylpentanoic acid ethyl ester;
2-(ethoxy)(p-toluenesulfonyloxymethyl)phosphinoylmethyl-
4-phenylbutanoic acid ethyl ester;
2-(ethoxy)(p-toluenesulfonyloxymethyl)phosphinoylmethyl-
3-phenylpropanoic acid ethyl ester;
2-(ethoxy)(p-toluenesulfonyloxymethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid ethyl ester; and
2-((ethoxy)(p-toluenesulfonyloxymethyl)phosphinoylmethyl)-
pentanoic acid ethyl ester.
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/15530
-64-
Example 4
Compounds of formula (Ee)
4A. Sodium hydride (1.52 g, (60~)) and 2-quinolinethiol (6 g)
were stirred together at 0~C in 50 mL DMF. After the initial
H2 evolution had subsided, the mixture was stirred at room
temperature for 2.5 hours. The mixture was then cooled to 0~C
and 2-(ethoxy)(methanesulfonyloxymethyl)phosphinoylmethyl-4-
methylpentanoic acid ethyl ester (12.8 g) in 10 mL DMF was
added via cannula and the resulting mixture was then stirred
for 18 hours, slowly warming to room temperature. The DMF was
removed by evaporation, the residue dissolved in 50 mL ethyl
acetate and washed with 50 mL H2O t2x), brine (50 mL), dried
with MgSO4 and evaporated to a yellow semi-solid. Purification
by flash chromatography using 10~ ethyl acetate/hexane to 80
ethyl acetate/hexane for the elution yielded 10 g of
2-(ethoxy)(quinolin-2-ylthiomethyl)phosphinoyl-methyl-4-
methylpentanoic acid ethyl ester (Rf 0.35 80~ ethyl
acetate/hexane), MS: 424.1 (MH+).
4B. In a similar manner, but replacing 2-quinolinethiol with
1-naphthalenethiol, 2-naphthalenethiol or thiophenol, the
following compounds of formula (Ee) are prepared:
2-(ethoxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoic acid ethyl ester;
2-(ethoxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoic acid ethyl ester; and
2-(ethoxy)(phenylthiomethyl)phosphinoylmethyl-
4-methylpentanoic acid ethyl ester.
4C. In a similar manner, the following compounds of formula
(Ee) are prepared:
2-(ethoxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoic acid ethyl ester;
2-(ethoxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid ethyl ester;
2-(ethoxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
3-phenylpropanoic acid ethyl ester;
SU~ uit SHEr ~IIE2C~
CA 0220~66~ 1997-05-21
WO96/16027 PCT~s95/lSS30
2-(ethoxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid ethyl ester;
2-((ethoxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl)-
pentanoic acid ethyl ester;
r 52-(ethoxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoic acid ethyl ester;
2-(ethoxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid ethyl ester;
2-(ethoxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
103-phenylpropanoic acid ethyl ester;
2-(ethoxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid ethyl ester;
2-((ethoxy~(naphth-1-ylthiomethyl~phosphinoylmethyl)-
pentanoic acid ethyl ester;
152-(ethoxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
~-phenylpentanoic acid ethyl ester;
2-(ethoxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid ethyl ester;
2-(ethoxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
203-phenylpropanoic acid ethyl ester;
2-(ethoxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid ethyl ester;
2-((ethoxy)(naphth-2-ylthiomethyl)phosphinoylmethyl)-
pentanoic acid ethyl ester;
252-(ethoxy)~phenylthiomethyl)phosphinoylmethyl-
~-phenylpentanoic acid ethyl ester;
2-(ethoxy)(phenylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid ethyl ester;
2-(ethoxy)(phenylthiomethyl)phosphinoylmethyl-
303-phenylpropanoic acid ethyl ester;
2-(ethoxy)(phenylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid ethyl ester; and
2-((ethoxy)(phenylthiomethyl)phosphinoylmethyl)-
pentanoic acid ethyl ester.
SUBSTlTl)TE StlEET (RULE 2~)
CA 0220S66~ 1997-0~-21
WO 96/16027 PCT/US95/15S30
--66-
Example 5
Compounds of formula (E)
5A. 2-(Ethoxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-4-
methylpentanoic acid ethyl ester (4.5 g) was dissolved in 100
mL THF and 12.5 m~ of 2N NaOH was added together with enough
methanol to make the solution homogeneous. After 18 hours the
THF was removed by evaporation, the residue diluted with 50 mL
H2O and washed with 50 mL ethyl acetate. The aqueous phase was
then acidified to pH 4, and the product extracted with 50 mL
ethyl acetate (2x). The ethyl acetate was washed with 20 mL
brine, dried with MgSO4 and evaporated to yield 3.8 g of
2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-4-
methylpentanoic acid as a yellow oil, MS: 368 (MH+).
5B. In a similar manner, the following compounds of
formula (E) are prepared:
2-(hydroxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoic acid;
2-(hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoic acid; and
2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
4-methylpentanoic acid.
5C. In a similar manner, the following compounds of formula
(E) are prepared:
2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoic acid;
2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid;
2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
3-phenylpropanoic acid;
2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid;
2-((hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl)-
pentanoic acid;
2-(hydroxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoic acid;
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/lS530
2-(hydroxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid;
2-(hydroxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
3-phenylpropanoic acid;
2-(hydroxy)(naphth-1-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid;
2-((hydroxy)(naphth-1-ylthiomethyl)phosphinoylmethyl)-
pentanoic acid;
2-(hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoic acid;
2-(hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid;
2-(hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
3-phenylpropanoic acid;
2-(hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid;
2-((hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl)-
pentanoic acid;
2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
5-phenylpentanoic acid;
2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
4-phenylbutanoic acid;
2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
3-phenylpropanoic acid;
2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoic acid; and
2-((hydroxy)(phenylthiomethyl)phosphinoylmethyl)pentanoic
acid.
Example 6
Resolution of a compound of formula (E)
2-(Hydroxy)(quinolin-2-ylthiomethyl)phosphinoyl-methyl-4-
methylpentanoic acid (5.3 g) was dissolved in 50 mL of warm
ethanol (abs) and 4.2 g of (-)-cinchonidine was added. After
30 minutes at room temperature the salt began to precipitate
out. The flask was covered in foil and allowed to stand for 2
days. The salt was then removed by suction filtration, and
SUBSrlTUTE SIIEET (RULE 26)
CA 0220~66s 1997-0~-21
WO96tl6027 PCT~S9511S~30
-68-
the filtrate evaporated to a yellow foam. The salt and the
filtrate were each dissolved in 100 mL ethyl acetate and
washed successively with 1~ HCl to remove the cinchonidine
while keeping the pH above 4. Both solutions were each dried
over MgSO4 and evaporated to yield 2.4 g of a single
stereoisomer, [~]24=+l0.68O (9.73 mg in methanol (2 mL)) and
2.5 g of the other single stereoisomer, [~]D4=-8.70O (9.88 mg
in methanol (2 mL)).
Example 7
Compounds of formula (B)
7A. To a cold (0~C) suspension of 4-acetamidobenzenesulfonyl
chloride (4.0 g, 17 mmol) in CH2Cl2 (40 mL) was added pyridine
(1.7 mL, 20 mmol) and DMAP (209 mg, 1.7 mmol). (A clear
solution resulted). Anhydrous methylamine was bubbled into
the solution for 1 hour at 0~C, and then the solution was
allowed to stir at 25~C for 2 hours. The solution was
e~tracted with lM NaOH (3x15 mL) and the combined extracts
were adjusted to pH 6 at 0~C with 3M HCl. The product, which
precipitated as fluffy white crystals, was filtered and washed
with cold water to afford 3.2 g (82~) of 4-acetamido-N-
methylbenzenesulphonamide: IH NMR (300 MHz, MeOH) ~ 2.35
(s,3H), 2.70 (s,3H), 7.96 (s,4H).
7B. A mixture of 4-acetamido-N-methylbenzenesulphonamide (3.2
g, 14 mmol) and 100 mL of lM HCl was refluxed under argon for
3 hours. After cooling to 25~C, CHzCl2 (lO mL) was added and
the aqueous phase was neutralized with lM NaOH at 0~C. The
aqueous phase was separated and extracted with CH2Cl2
(2x25 mL). The combined organic phases were washed with brine
(10 mL), dried (Na2SO4) and concentrated to afford 1.5 g (58~)
of a compound of formula (B) where R4 is N-methylsulfonamide as
a colorless solid: IH NMR (300 MHz, MeOH) ~ 2.46 (s,3H), 6.67-
6.72 (AAI part of AAIXXI 2H), 7.48-7.52 (XX~ part of AAIXXI, 2H).
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PcT~sss/lss30
-69-
Example 8
Compounds of formula (C)
8A. To a cold (0~C) solution of N-tert-butoxycarbonyl-
L-leucine (1.4 g, 6.3 mmol) and HOBT (1.5 g, 9.8 mmol) in DMF
~- 5 (30 mL) was added EDCI (2.5 g, 14 mmol) in portions. After
stirring for 1 hour at 0~C, the resulting ~olution wa~ treated
with methyl 4-aminobenzoate (l.o9 mL, 6.8 mmol) and DMAP
(0.32 g, 2.6 mmol). After stirring for 24 hours at 25~C, the
DMF was removed in vacuo. The residue was dissolved in CH2Cl2
and washed with saturated NaHCO3 solution, lM HCl (twice), and
brine. Drying over Na2SO4 and concentration in vacuo afforded
the crude product which was puri~ied by flash chromatography
on Sio2 (20~ ethyl acetate/hex~nes eluent). There was obtained
1.0 g (85~) of N-t-butoxycarbonyl-L-leucine-N'-(4-methoxy-
carbonylphenyl)carboxamide as a foamy solid,
MS (FAB) 363 (M-H)-.
8B. In a similar manner, the following compounds of formula
(C) were prepared:
N-t-butoxycarbonyl-L-tryptophan-N'-phenylmethylcarboxamide;
N-t-butoxycarbonyl-L-tryptophan-N'-phenylcarboxamide;
N-t-butoxycarbonyl-L-tryptophan-N'-(4-
methoxycarbonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-tryptophan-N'-(4-
ethoxycarbonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-leucine-N'-(4-(N''-
methylaminosulfonyl)phenyl)carboxamide;
N-t-butoxycarbonyl-L-alanine -N ' - (4-
methoxycarbonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-methionine-N ' - (4 -
methoxycarbonylphenyl)carboxamide;
N- t - butoxycarbonyl-L-leucine- N ' - ( 3 -
ethoxycarbonylphenyl)carboxamide;
N- t-butoxycarbonyl-L-leucine-N'-(2-
methoxycarbonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-leucine-N'-(4-(1-
methylethyloxy)carbonyl)phenyl)carboxamide;
SU~STITUTE SHEET t~ULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PcT~s95/lSS30
-70-
N- t-butoxycarbonyl-L-leucine-N'-
(aminosulfonyl)phenyl)carboxamide;
N- t-butoxycarbonyl-L-leucine-N'-(4-methoxycarbonylmethyl-
phenyl)carboxamide;
N-t-butoxycarbonyl-L-pyridin-3-ylalanine-N'-(4-methoxy- -
carbonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-cyclohexylglycine-N'-(4-methoxy-
carbonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-isoleucine-N'-(4-
methoxycarbonylphenyl)carboxamide;
N- t-butoxycarbonyl-L-O-benzylthreonine-N'-(4-methoxy-
carbonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-t-leucine-N'-(4-
methoxycarbonylphenyl)carboxamide;
N- t-butoxycarbonyl-L-leucine-N'-(4-cyanophenyl)carboxamide;
N-t-butoxycarbonyl-L-leucine-N'-(4-(N''-(2-dimethylaminoethyl-
carbamoyl)carboxamide; and
N-t-butoxycarbonyl-L-leucine-N'-(4-(N''-(3-dimethylamino-
propyl)carbamoyl)phenyl)carboxamide.
8C. In a similar m~nner~ the following compounds of formula
(C) are prepared:
N- t-butoxycarbonyl-L-tryptophan-N'-(4-nitrophenyl)carboxamide;
N- t-butoxycarbonyl-L-tryptophan-N'-(4-aminophenyl)carboxamide
N- t-butoxycarbonyl-L-leucine -N ' - (4-
methylsulfonylphenyl)carboxamide;
N-t-butoxycarbonyl-L-leucine-N'-(4-
ethylsulfonylphenyl)carboxamide; and
N- t-butoxycarbonyl -L - leucine-N'-(4-
tetrazolylphenyl)carboxamide.
Example 9
Compounds of formula (D)
9A. To a cold (0~C) solution of N-t-butoxycarbonyl-L-leucine-
N'-phenylcarboxamide (3.4 g, 11 mmol) in dry CH2Cl2 (10 mL) was
added TFA (2 mL). The solution was allowed to stir at 25~C
SUBSTITUTE SHEET (RULE 26)
-0~-2l
w~s6ll6o27 PcT~sssJ1ss30
-71-
for 6 hours and was then concentrated in vacuo. The residue
was partitioned between CH2C12 and H2O and the aqueous layer was
~a~ified at O~C with saturated K2CO3 solution. The organic
phase was separated and the aqueous layer was extracted three
times with CE2C12. The combined organic layer~ were washed
with brine and dried over Na2SO4. concentration afforded
L-leucine-N'-phenylcarboxamide.
9B. In a similar manner, the following compounds are
prepared:
L- leucine-N'-(4-methoxycarbonylphenyl)carboxamide;
L-tryptophan-N'-phenylmethylcarboxamide;
L-tryptophan-N'-phenylcarboxamide;
L-tryptophan-N'-(4-methoxycarbonylphenyl)carboxamide;
L-tryptophan-N'-(4-ethoxycarbonylphenyl)carboxamide;
L-leucine-N~-(4-(N~-methylaminosulfonyl)phenyl)carboxamide;
L- alanine -N ' - ( 4-methoxycarbonylphenyl)carboxamide;
L-methionine -N ' - ( 4-methoxycarbonylphenyl)carboxamide;
L-leucine-N'-(3-ethoxycarbonylphenyl)carboxamide;
2 0 L - leucine-N'-( 2 -methoxycarbonylphenyl)carboxamide;
L-leucine-N'-(4-(1-methylethyloxy)carbonyl)phenyl)carboxamide;
~-leucine-N'-(aminosulfonyl)phenyl)carboxamide;
L-leucine-N'-(4-methoxycarbonylmethylphenyl)carboxamidei
L-pyridin-3-ylalanine-N'-(4-methoxycarbonylphenyl)carboxamide;
2 5 L- spirocyclopentylglycine-N'-(4-methoxycarbonylphenyl)-
carboxamide;
L-cyclohexylglycine-N'-(4-methoxycarbonylphenyl)carboxamide;
L-isoleucine-N'-(4-methoxycarbonylphenyl)carboxamide;
L-O-benzylthreonine-N'-(4-methoxycarbonylphenyl)carboxamide;
L- t-leucine-N~-(4-methoxycarbonylphenyl)carboxamide;
L-leucine-N'-(4-cyanophenyl)carboxamide;
L-leucine-N'-(4-(N''-(2-dimethylaminoethyl)carbamoyl)phenyl)-
carboxamide; and
L-leucine-N'-(4-(N''-(3-dimethylaminopropyl)carbamoyl)phenyl)-
3 5 carboxamide.
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ l997-05-2l
WO 96/16027 PCT/US95/15530
9C. In a ~imilar manner, the following compounds of ~ormula
(D) are prepared: .
L-tryptophan-N'-(4-nitrophenyl)carboxamide;
L-tryptophan-N'-(4-aminophenyl)carboxamide;
~-leucine-N'-(4-methylsulfonylphenyl)carboxamide;
L-leucine-N'-(4-ethylsulfonylphenyl)carboxamide; and
L - leucine-N'-(4-tetrazolylphenyl)carboxamide.
Example 10
Compounds of formula (Ia)
10A. To a cold (0~C) solution of 2-(hydroxy)(quinolin-2-
ylthiomethyl)-phosphinoylmethyl-4-methylpentanoic acid (0.20
g, 0.54 mmol) in THF (6 mL) was added l,1'-carbonyldiimidazole
(0.12 g, 0.7 mmol). The mixture was stirred for 75 minutes at
0~C and was then treated with L-tryptophan-N'-(4-ethoxy-
carbonylphenyl)carboxamide (0.22 g, 0.62 mmol) and stirred at
25~C for 15 hours. The THF was evaporated and the residue was
dissol~ed in ethyl acetate (60 m~). The solution was washed
with H20 (10 mL), brine (10 mL), and dried over MgSO4.
Concentration was followed by reverse phase HPLC using a
gradient of acetonitrile and 50 mM NH~OAc buffer afforded 30 mg
o f N- ( 2 - ( hydroxy)~quinolin-2-ylthiomethyl)phosphinoylmethyl-4-
methylpentanoyl)-L - tryptophan-N'-(4-ethoxycarbonylphenyl)-
carboxamide as an o~f white solid, MS(FAB) 701 (M-H)+ (mixture
of diastereomers).
10B. In a similar manner, the following compounds of formula
(Ia) were prepared:
N- (2- (hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoyl)-L-tryptophan-N'-(4-methoxy-
carbonylphenyl)carboxamide,
MS(FAB) 687 (M+H)+; e
N- (2- (hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoyl)-L-alanine-N'-(4-methoxy-
carbonylphenyl)carboxamide,
MS(FAB) 572 (M+H)+;
N-(2-(hydroxy) (quinolin- 2 -ylthiomethyl)phosphinoylmethyl-
SUBSTITUTE SHEET (RULE 26)
.
CA 0220~66~ l997-0~-2l
W~96/l6027 PCT~S95/lSS30
4-methylpentanoyl)-L-methionine-Nl-(4-meth
carbonylphenyl)carboxamide,
MS(FAB) 632 (M+H)+;
N-(2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoyl)-L- leucine -N' - (4 -methoxy- -
carbonylphenyl)carboxamide,
MS(FAB) 614 (M+H)+;
N- (2- (hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoyl)-L-leucine-N'-(3-ethoxy-
carbonylphenyl)carboxamide,
IH NMR (300 MHz, MeOH) ~ 0.73-1.01 (m, 12H), 1.28-2.00 (m,
14H), 2.4-3.61 (m, 2H), 4.27-4.45 (m, 3H), 7.23-7.44 (m,
3H), 7.65-7.98 (m, 6H), 8.29 (s, 0.5H), 8.50 (s, 0.5H);
N- ( 2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoyl)-L-leucine-N'-(2-methoxy-
carbonylphenyl)carboxamide,
iH NMR (300 MHz, MeOH) ~ 0.78-0.99 (m, 13H), 1.3-2.4 (m,
7H), 2.90-3.05 (m, lH), 3.5-3.75 (m, 2H), 3.89, 3.90,
3.94 (3s, 3H total), 4.35-3.50 (m, lH), 7.05-8.10 (m,
llH), 8.32, 8.55, 8.60 (3d, J=8.7, lH);
N-(2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoyl)-L-leucine-N'-(4-(1,1-dimethyl-
ethoxycarbonylphenyl)carboxamide,
MS(FAB) 642 (MH)~;
N- (2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-methylpentanoyl)-L- leucine-N'-(4-aminosulfonyl-
phenyl)carboxamide,
IH NMR (300 MHz, MeOH) ~ 0.76 (d, J=6.5, 3H), 0.81 (d,
J=6.5, 3H), 0.85-1.1 (m, 7H), 1.2-2.1 (m, 7H), 2.92-2.95
(m, lH), 3.45-3.70 (m, 2H), 4.35-4.45 (m, lH), 7.28 (d,
J=8.7, lH), 7.45 (t, J=8.7, lH), 7.68 (t, J=8.7, lH),
7.7-7.8 (m, 3H), 7.87 (d, J=8.7, lH), 7.95-8.1 (m, 3H);
N- (2- (hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
3 5 4 -methylpentanoyl)-L- leucine- N ' - ( 4 -methoxycarbonylmethyl-
phenyl)carboxamide,
MS(FAB) 628 (MH)+.
Sl~BSlllU~t SHEr tlNlE21;)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/1SS30
-74-
lOC. In a similar m~nner~ the following compounds of formula
(Ia) are prepared: .
N- (2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoyl)-L-leucine-N~-(4-methoxycarbonyl-
phenyl)carboxamide;
N-(2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N- (2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
3-phenylpropanoyl)-L- leucine -N ' - ( 4-methoxycarbonyl-
phenyl)carboxamide;
N-(2-(hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N-(2-((hydroxy)(quinolin-2-ylthiomethyl)phosphinoylmethyl)-
pentanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N-(2-(hydroxy)(naphth-l-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoyl)-L- leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N-(2-(hydroxy)(naphth-l-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N- (2-(hydroxy)(naphth-l-ylthiomethyl)phosphinoylmethyl-
3-phenylpropanoyl)-L- leucine -N' - ( 4-methoxycarbonyl-
phenyl)carboxamide;
N- ( 2-(hydroxy)(naphth-l-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoyl)- L - leucine -N ' - ( 4-methoxycarbonyl-
phenyl)carboxamide;
N- ( 2 - ( (hydroxy)(naphth-l-ylthiomethyl)phosphinoylmethyl)-
pentanoyl)-L- leucine -N' - (4-methoxycarbonyl-
phenyl)carboxamide;
N- (2- (hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
5-phenylpentanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N- (2- (hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
4-phenylbutanoyl)-L-leucine-N'-(4-methoxycarbonyl-
SUBSlllult SllEr (RUIE 26~
CA 0220~66~ 1997-0~-21
W O 96/16027 PCTrUS9S/lSS30
- 75-
phenyl)carboxamide;
N-(2-(hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
3-phenylpropanoyl)-L-leucine-N~-(4-methoxycarbonyl-
phenyl)carboxamide;
N-(2-(hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoyl)-L- leucine -N ' - ( 4 -methoxycarbonyl-
phenyl)carboxamide;
N-(2-((hydroxy)(naphth-2-ylthiomethyl)phosphinoylmethyl)-
pentanoyl)-L- leucine -N ' - ( 4-methoxycarbonyl-
phenyl)carboxamide;
N-(2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
5-phenylpentanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N- ( 2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
4-phenylbutanoyl) -L- leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N- ( 2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
3-phenylpropanoyl)-L- leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide;
N- (2-(hydroxy)(phenylthiomethyl)phosphinoylmethyl-
3-cyclohexylpropanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide; and
N- (2-((hydroxy)(phenylthiomethyl)phosphinoylmethyl)-
pentanoyl)-L-leucine-N'-(4-methoxycarbonyl-
phenyl)carboxamide.
10D. A solution of N-(2-(hydroxy)(quinolin-2-ylthiomethyl)-
phosphinoylmethyl-4-methylpentanoyl)-L-tryptophan-N'-(4-
methoxycarbonylphenyl)carboxamide in THF (2 mL) and lM NaOH
(1 mL) was stirred for 24 hours at 25~C. The organic solvents
were evaporated, and the residue dissolved in ethyl acetate/
H20. The aqueous phase was acidified with lM HCl and the
separated aqueous phase was extracted twice with ethyl
acetate. The combined organic layers were washed with brine,
dried (MgSO4) and concentrated to 27 mg o~ N-(2-(hydroxy)-
(quinolin-2-ylthiomethyl)-phosphinoylmethyl-4-methyl-
pentanoyl)-L-tryptophan-N'-(4-carboxyphenyl)carboxamide as a
SUBSrITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9SIlSS30
-76-
yellow powder.
10E. In a similar manner, but starting with N- (2-(hydroxy)-
(quinolin-2-ylthiomethyl)phosphinoylmethyl-4-methylpentanoyl)-
L-leucine-N'-(4-methoxy-carbonylphenyl)carboxamide (30 mg,
0.048 mmol) there was obtained 10 mg of N- (2-(hydroxy)-
(quinolin-2-ylthiomethyl)phosphinoylmethyl-4-methylpentanoyl)-
~-leucine-N'-(4-carboxyphenyl)carboxamide as a semisolid after
trituration with ethyl acetate;
IH NMRI (300 MHZ, MeOH) 0.81-1.02 (m, 12H), 1.1-2.3 (m, 10H),
2.82-3.00 (m, lH), 3.49, 3.56 (2s,2H), 3.5-3.8 (m,2H), 4.45-
4.55 (m,lH), 7.09 (d, J=8.2, lH), 7.19 (d, J=8.2,1H), 7.45 (t,
J=8.2, lH) 7.45-7.6 (m, 3H), 7.65-7.80 (m, lH), 7.82-7.98 (m,
2H), 8.10-8.20 (m, lH).
Example 11
Compounds of formula (Fa)
llA. To 4-methylpentanoic acid (25 g, 0.215 mmol) in a 25~C
water bath, thionyl chloride (20.4 mL, 1.3 g) was slowly
added. Then the mixture was heated at 50~C under argon for 3
hours (until the evolution of gas had stopped). The crude
reaction mixture was distilled at atmospheric pressure to give
4-methylpentanoyl chloride (25.3 g, 87.3~), b.p. 143~C.
llB. In a similar m~nner~ but replacing 4-methylpentanoic acid
with 5-phenylpentanoic acid (5 g), 5-phenylpentanoyl chloride
was prepared (4.4 g), as a colorless liquid, b.p. 91~-93~C.
Example 12
Compounds of formula (Fb)
12A. To a suspension of 60~ NaH (836 mg, l.S eq.) in toluene
(200 mL) at room temperature under argon was added L- (+) -2,10-
c~mrhor sultam (3.0 g, 13.9 mmol) portion-wise. The mixture
was stirred vigorously at room temperature for one hour. Then
4-methylpentanoyl chloride was carefully added dropwise to the
solution at 0~C. After stirring the reaction at room
CA 0220~66~ 1997-0~-2l
W~96116027 PCT~S95/15530
-77-
temperature for 3 hours, the reaction was quenched with 10 mL
of water, and 70 mL of ether was added. The reaction mixture
was first washed with 0.5N HCl (2x50 mL), then 5~ K2CO3
(3x50 m~) and finally with brine (lx50 m~). The organic layer
wa~ dried over MgSO4, filtered and evaporated to dryness.
Purification by column chromatography (1:6 ethyl acetate/
petroleum ether a8 eluant) gave N-4-methylpentanoyl-L-(+)-
2,10-camphor ~ultam (3.39 g, 78~).
12B. In a similar manner, but replacing 4-methylpentanoyl
chloride with the appropriate chloride, the following
compounds of formula (Fb) were prepared:
N-3-phenylpropanoyl-L-(+)-2,10-camphor sultam, MS: 347 (M+);
N-5-phenylpentanoyl-L-(+)-2,10-camphor sultam, MS: 375 M+;
N-pentanoyl-L-(+)-2,10-camphor 8ultam, MS: 300 (M+H)+.
Example 13
Compounds of formula (Fc)
13A. To a solution of N-4-methylpentanoyl-L-(+)-2,10-camphor
sultam (3.39 g, 10.8 mmol) in 75 mL of dry THF at -78~C under
argon was added NaN(TMS)2 (1.0 M in THF, 11.34 mL, 1.05 eq.)
dropwise over five minutes. After stirring at -78~C for 1
hour, hexamethylphosphoramide (5 mL) was added to the mixture,
followed by t-butylbromoacetate (5.2 ml, 3 eq), then 400 mg of
tetra n-butylammonium iodide was added in one portion. The
resulting solution was kept at -78~C under argon overnight.
The next morning, the reaction was quenched with water
(100 mL), and then it was extracted with ether (3xlO0 mL).
The combined ether layers were washed with brine, then dried
over Na2SO4, filtered and concentrated. Purification by column
chromatography (5:95 ethyl acetate/petroleum ether to 10:90
ethyl acetate/petroleum ether as eluant) gave N-(4-methyl-2-t-
butoxycarbonylmethyl)pentanoyl-L-(+)-2,10-camphor sultam (4 g,
86.5~).
13B. In a similar manner, but replacing N-4-methylpentanoyl-L-
(+)-2,10-camphor sultam with the appropriate compound of
CA 0220~66~ 1997-0~-21
WO 96/16027 PCT/US95/lS530
formula (Fb), the following compounds of formula (Fc) were
prepared:
N-(3-phenyl-2-t-butoxycarbonylmethyl)propanoyl-~-(+)-2,10-
camphor sultam, MS: 461 (M+);
N-(5-phenyl-2-t-butoxycarbonylmethyl)pentanoyl-L-(+)-2,10-
camphor sultam, MS: 490.1 (M+H)+;
N- ( 2-t-butoxycarbonylmethyl)pentanoyl -L - ( + ) - 2~lo-camphor
sultam, MS: 414 (M+H)+.
Example 14
Compounds of ~ormula (F)
14A. To a stirred solution of N- (4-methyl-2-t-butoxy-
carbonylmethyl)pentanoyl-L-(+)-2,10-camphor sultam (5.45 g,
12.7 mmol) in 50~ aqueous THF (150 mL) at 0~C under argon was
added LiOHH2O crystals (2.14 g, 4 eq.) followed by 30~ H2O2
(11.5 mL). Then the ice-bath was removed and the resulting
emulaion was stirred for 3 hours before it had turned clear.
Most of the THF was removed under reduced pressure at 35~C.
Then CH2Clz (150 mL) was added and with stirring 4N HCl was
added to pH=2. After adding NaCl, the aqueous layer was
further extracted with CH2Cl2 (3x150 mL). The CH2Cl2 was
removed under reduced pressure at 35~C and then the residue
was taken up in ethyl acetate (150 mL). This solution was
then extracted with 5~ K2CO3 (3x50 mL) and the combined
extracts were washed with ether (50 mL). Then CH2Cl2 was added
to the aqueous layer and with stirring with NaCl, the aqueous
layer was extracted with CH2Cl2 (3x70 mL) and the combined
extracts were dried over Na2SO4, filtered and concentrated to
give (2R)-4-methyl-2-t-butoxycarbonylmethyl-pentanoic acid as
a colorless oil (2.95 g, quantitative yield).
14B. In a similar manner, but replacing N-(4-methyl-2-t-
butoxycarbonylmethyl)pentanoyl-L-(~)-2,10-camphor sultam with
the appropriate compound of formula (Fc), the following
compounds of ~ormula (F) were prepared:
(2R)-3-phenyl-2-t-butoxycarbonylmethyl-propanoic acid,
MS: 265 (M+H)+;
SUBSTITUTE St~EET (RULE 26)
CA 0220~66~ 1997-0~-21
WO 96/16027 PCTIUS95/lSS30
(2R)-5-phenyl-2-t-butoxycarbonylmethyl-pentanoic acid,
MS: 293.1 (M+H)+;
(2R) -2-t-butoxycarbonylmethyl-pentanoic acid,
(colorless oil, 1.09 g).
14C. (2R) -3-Phenyl-2-t-butoxycarbonylmethyl-propanoic acid (55
mg) was taken up in glacial acetic acid (20 mL) and PtO2 (25
mg) was added in acetic acid. Then the beaker was placed in a
Parr bomb, it was evacuated and charged with 100 psi of H2.
After stirring for 3 days, the mixture was suction filtered
through a 1 cm bed of Celite. The filtrate was then
concentrated to a yellow oil, (2R)-3-cyclohexyl-2-
t-butoxycarbonylmethyl-propanoic acid (56 mg),
MS: 269.5 (M-H)-.
Example 15
Compound~ of formula (Ib)
15A. To a solution of 4-methyl-2-t-butoxycarbonylmethyl-
pentanoic acid (0.28 g, 1.2 mmol) in DMF (5 mL) containing
HOBT (0.22 g, 1.8 mmol) was added EDCI (0.31 g, 1.8 mmol).
The mixture was stirred at 0~C for 1 hour and was then treated
with L- cyclohexylglycine-N'-(4-methoxycarbonylphenyl)-
carboxamide (1.2 mmol) and DMAP (27 mg, 0.24 mmol). Stirring
was continued for 24 hours at 25~C and then the DMF was
evaporated. The residue was dissolved in CH2Cl2 (20 mL) and
the solution was washed with lM HCl (10 mL), saturated NaHCO3
(10 mL), brine (10 mL) and was dried over Na2SO4.
Concentration in vacuo afforded an oil which was purified by
flash chromatography on SiO2 using 20~ ethyl acetate/hexanes as
element. There was obtained 0.22 g (22~) of N-(4-methyl-2-t-
butoxycarbonylmethyl-pentanoyl)-L-cyclohexylglycine-N'-(4-
methoxycarbonylphenyl)carboxamide as a solid, MS(FA~3) 503
(MH)+.
15B. In a similar manner, the following compounds were
prepared:
N-(4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L-pyridin-
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~Sg5/15530
-80-
3-ylalanine-N~-(4-methoxycarbonylphenyl)carboxamide;
N-(4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L- O-benzyl-
threonine -N ' - ( 4-methoxycarbonylphenyl)carboxamide;
N- (4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L-isoleucine-
N'-(4-methoxycarbonylphenyl)carboxamide;
N-(4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L-leucine-
N'- (4-methoxycarbonylphenyl)carboxamide;
N-(4-methyl-2-t-butoxycarbonylmethylpentanoyl) -L- t-leucine-
N'- (4-methoxycarbonylphenyl)carboxamide;
N- (4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L-leucine-
N ' - ( 4-cyanophenyl)carboxamide;
N- (4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L-leucine-
N'- (4- (N' '- (3-dimethylaminopropyl)carbamoyl)phenyl)-
carboxamide;
N- (4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L-leucine-
N'- (4- (N' '- (2-dimethylaminoethyl)carbamoyl)phenyl)-
carboxamide;
N- (4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L-leucine-
N'- (4- aminosulfonylphenyl)carboxamide;
N- ( 4-methyl-2-t-butoxycarbonylmethylpentanoyl)-L- leucine-
N' - (4 -methylaminosul~onylphenyl)carboxamidei
N- (2-t-butoxycarbonylmethylpentanoyl)-L- leucine-
N'- (4-methoxycarbonylphenyl)carboxamide;
N- ( 3 -phenyl-2-t-butoxycarbonylmethylpropanoyl)-L- leucine-
2 5 N ' - ( 4-methoxycarbonylphenyl)carboxamide;
N- (3 -cyclohexyl-2-t-butoxycarbonylmethylpropanoyl)-L-leucine-
N'- (4-methoxycarbonylphenyl)carboxamide;
N- (4 -phenyl-2-t-butoxycarbonylmethylbutanoyl)-L-leucine-
N'- (4-methoxycarbonylphenyl)carboxamide; and
3 0 N- ( 5 -phenyl-2-t-butoxycarbonylmethylpentanoyl)-L- leucine-
N~-(4-methoxycarbonylphenyl)carboxamide.
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ l997-0~-2l
W096/16027 PCT~S9S/15530
-81-
Example 16
Compounds of formula (Ic)
16A. To a cold (0~C) solution of N-(4-methyl-2-t-butoxy-
carbonylmethyl-pentanoyl)-~-cyclohexylglycine-N'-(4-
methoxycarbonylphenyl)carboxamide (70 mg, 0.14 mmol) in ~Cl2
(2 mL) was added TFA (0.5 mL). After stirring for 5 hours at
25~C, the solution was concentrated in vacuo and the product
was purified by reverse phase HPLC using a gradient of
acetonitrile and 50 mM NH~OAc buffer to provide 44 mg (71~) of
N- (4-methyl-2-carboxymethylpentanoyl)-L- cyclohexylglycine-N'-
(4-methoxycarbonylphenyl)carboxamide as a white solid, MS(FAB)
445 (M-H)-.
16B. In a similar m~nnerl the following compounds were
prepared:
N- ( 4-methyl-2-carboxymethylpentanoyl)-L- isoleucine-
N'- (4-methoxycarbonylphenyl)carboxamide,
MS(FAB) 419 (M-H)-;
N- (4-methyl-2-carboxymethylpentanoyl)-L-leucine-N'-(4-methoxy-
carbonylphenyl)carboxamide, MS(FAB) 419 (M-H)-;
N- (4-methyl-2-carboxymethylpentanoyl)-L-t-leucine-
N ' - ( 4-methoxycarbonylphenyl)carboxamide;
N- (4-methyl-2-carboxymethylpentanoyl)-L-leucine-N'-(4-cyano-
phenyl)carboxamide,
IH NMR (300 MHz, MeOH) ~ 0.84-0.99 (m, 12H), 1.15-1.82 (m,
6H), 2.36-2.41 (m, lH), 2.52-2.65 (m, lH), 2.8-2.95 (m,
lH), 4.49-4.54 (m, lH), 7.4-7.9 (m, 4H);
N- (4-methyl-2-carboxymethylpentanoyl)-L-leucine-N'-(4-amino-
sulfonylphenyl)carboxamide,
IH NMR (300 MHZ, MeOH) ~ 0.85-1.00 (m, 12H), 1.1-1.3 (m,
2H), 1.52-1.85 (m, 4H), 2.31-2.95 (m, 3H), 4.49-4.55 (m,
lH), 7.75-7.91 (m, 4H);
N- (4-methyl-2-carboxymethylpentanoyl)-L-leucine-N'-(4-methyl-
aminosulfonylphenyl)carboxamide, MS(FAB) 459 (M-H)-;
N- (2-carboxymethylpentanoyl)-L-leucine-N'-(4-methoxy-
carbonylphenyl)carboxamide, MS(FAB) 405 (M-H)-;
N-(3-phenyl-2-carboxymethylpropanoyl)-L-leucine-N'-(4-methoxy-
SUBSTITUI~ SHE~T (RULE 26)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/lSS30
-82-
carbonylphenyl)carboxamide, MS(FAB) 455 (M+H)+;
N-(3-cyclohexyl-2-carboxymethylpropanoyl)- L- leucine-
N'- (4-methoxycarbonylphenyl)carboxamide,
MS(FAB) 459 (M-H)-;
N- (4-phenyl-2-carboxymethylbutanoyl)-L-leucine-N'-(4-methoxy-
carbonylphenyl)carboxamide, MS(FAB) 467 (M-H)-;
N-(4-phenyl-2-carboxymethylbutanoyl)-L-cyclohexylglycine-
N'- (4-methoxycarbonylphenyl)carboxamide;
N- (4-phenyl-2-carboxymethylbutanoyl) -L- t-leucine-
N'- ( 4-methoxycarbonylphenyl)carboxamide;
N- (5-phenyl-2-carboxymethylpentanoyl)-L-leucine-N'-(4-methoxy-
carbonylphenyl)carboxamide, MS(FA~3) 481 (M-H)-; and
N-(4-methyl-2-carboxymethylpentanoyl)-L- O-benzylthreonine-
N'- (4-methoxycarbonylphenyl)carboxamide,
MS(FAB) 497 (M-H)-.
16C. In a similar manner, but triturating the crude product
with ether and then decanting the ether to yield the following
compounds as TFA salts:
N- (4-methyl-2-carboxymethylpentanoyl)-~-pyridin-3-ylalanine-
N'- (4-methoxycarbonylphenyl)carboxamide,
MS(FAB) 456 (M+H)+;
N- (4-methyl-2-carboxymethylpentanoyl)-L-leUCine-
N ' - ( 4-( N ' ' - ( 3 - dimethylaminopropyl)carbamoyl)phenyl)-
carboxamide, MS(FAB) 491 (M+H)+; and
N- (4-methyl-2-carboxymethylpentanoyl)-L-leucine-
N ' - ( ~ - ( N ' ' - ( 2 - dimethylaminoethyl)carbamoyl)phenyl)-
carboxamide, MS(FAB) 491 (M+H)+.
16D. A mixture of N-(4-methyl-2-t-butoxycarbonylmethyl-
pentanoyl)-L- O-benzylthreonine -N' - (4-methoxycarbonylphenyl)-
carboxamide (60 mg) and Pd/C in ethyl acetate/THF (l:1, 25 mL)
was hydrogenated overnight at 1 atm pressure. Filtration
through Celite, concentration of the filtrate, and trituration
of the residue with ether/hexanes produced N-(4-methyl-2-t-
butoxycarbonylmethyl-pentanoyl)-L-threonine-N'-(4-
methoxycarbonyl-phenyl)carboxamide, MS(FAB) 407 (M-H)-.
SUBSTIME S~IEET (RUEE 26)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/lSS30
-83-
16E. By following the procedure of part A and substituting N-
(4-methyl-2-t-butoxycarbonylmethyl-pentanoyl)-L-
cyclohexylglycine-NI-(4-methoxycarbonylphenyl)carboxamide with
the following:
N- (2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-
L-lysine -N ' - ( 4-(ethoxycarbonyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(phenyl)pentanoyl)-
L-ly8ine-N'-(4-(ethoxycarbonyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-
L- (N~-isopropyl)lysine-N'-(4-(ethoxycarbonyl)phenyl)-
carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-4-(phenyl)butanoyl)-
L-cyclohexylglycine-N'-(4-(N",N"-dimethylamino-
ethylaminosulfonyl)phenyl)carboxamide;
N- (2R-(t-butoxycarbonyl)methyl-5-(phenyl)pentanoyl)-
L- (N,N'-diethylguanido)lysine-N'-(4-(ethoxycarbonyl)-
phenyl)carboxamide;
N- (2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl) -L-t-
leucine-N'-(4-(methylthio)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-biphen-4-yl)pentanoyl) -L- t-
leucine- N ' - ( 3 - ( 2-hydroxyethyl)phenyl)carboxamide;
N- (2R-(t-butoxycarbonyl)methyl-5-biphen-4-yl)pentanoyl)L-S-
((4-cyanophenyl)methyl)penicillamine-N'-(phenyl)-
carboxamide;
N- (2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-
cyclohexylglycine-N'-(2-(4-aminosulfonyl)phenylethyl)-
carboxamide;
N- (2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl) -L-
cyclohexylglycine-N'-(3-(morpholin-4-yl)propyl)-
3 0 carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl) -L-t-
leucine-N'-(4-(methylaminosulfonyl)phenyl)carboxamide;
N- (2R-(t-butoxycarbonyl)methyl-5-~biphen-4-yl)pentanoyl) -L-
cyclohexylglycine-N'-(4-((2-hydroxyethyl)aminosulfonyl)-
phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl) -L-
cyclohexylglycine-N'-(4- (N",N"-
S~JBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/lS530
-84-
dimethylaminoethylaminosulfonyl) phenyl)carboxamide; and
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-
leucine-N'-(4-((3-(morpholin-4-yl)propyl)aminosulfonyl)-
phenyl)carboxamide,
there are obtained:
N-(2R,S)-(N"-formyl-N"-hydroxyamino)methyl-4-
(methyl)pentanoyl)-
L-leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide: MS:
434.2 (M-H)- 388 (M-HCO-OH);
N-(2R-(N"-hydroxycarbamoyl)methyl-4-(methyl)pentanoyl)
D, L-norvaline-N'-(4-((2-(dimethylamino)ethyl)-
carbamoyl)phenyl)carboxamide: MS: 478 (M+H)+;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-lysine -N ' - ( 4 - ( ethoxycarbonyl)phenyl)carboxamide:
MS: 588.3 (M+H)+;
N-(2R-carboxymethyl-5-(phenyl)pentanoyl)-
L-lysine-N'-(4-(ethoxycarbonyl)phenyl)carboxamide:
MS: 512.3 (M+H)+;
N- (2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L- (N~-isopropyl)lysine-N'-(4-(ethoxycarbonyl)phenyl)-
carboxamide: MS: 630 (M+H)+;
N-(2R-carboxymethyl)-4-(phenyl)butanoyl)-
L-cyclohexylglycine-N'-(4-(N",N"-dimethylaminoethyl-
aminosulfonyl)phenyl)carboxamide: MS: 586 (M+H)+;
N-(2R-carboxymethyl-5-(phenyl)pentanoyl)-
L- (N,N'-diethylguanido)lysine-N'-(4-(ethoxycarbonyl)-
phenyl)carboxamide; MS: 610.4 (M+H)+;
N- (2R-carboxymethyl)-5-(biphen-4-yl)pentanoyl)-L-t-leucine-NI-
(4-(methylthio)phenyl)carboxamide: FAB-MS (M+Na)+ calc.
569.2450; found: 569.2461i
N-(2R-carboxymethyl-5-biphen-4-yl)pentanoyl)-L-t-leucine-N'-
(3-(2-hydroxyethyl)phenyl)carboxamide: FAB-MS (M+H)+
calc. 545.3015; found: 545.3021;
N-(2R-carboxymethyl-5-biphen-4-yl)pentanoyl)L-S-((4-
cyanophenyl)methyl)penicillamine-N'-(phenyl)carboxamide:
FAB-MS (M+H)+ calc. 634.2740; found: 634.2749;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO 96116027 PCT/US9511SS30
cyclohexylglycine-N'-t2-(4-aminosulfonyl)phenylethyl)-
carboxamide: FAB-MS (M+H)+ calc. 634.2951; found:
634.2963;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-
' 5 cyclohexylglycine -N ' - ( 3-(morpholin-4-yl)propyl)-
carboxamide: FAB-MS (M+H)+ calc. 578.3594; found:
578.3583;
N- (2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-~-t-leucine-N'-
(4-(methylaminosulfonyl)phenyl)carboxamide: IH NMR (300
MHz, acetone-d6) ~ 9.73 (br,s, lH), 7.91 (d, 2H, J= 9
Hz), 7.79 (d, 2H, J= 9 Hz), 7.56 (d, 2H, J= 8 Hz), 7.25-
7.45 (m, 6H), 7.19 (d, 2H, J= 8 Hz), 4.48 (d, lH, J= 9
Hz), 2.38-3.00 (m, 9H), 1.43-1.72 (m, 4H), 1.04 (s, 9H);
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-cyclohexyl-
glycine-N'-(4-((2-hydroxyethyl)aminosulfonyl)phenyl)-
carboxamide: FAB-MS (M+Cs)+ calc. 782.1876; found:
782.1896;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-
cyclohexylglycine-N~-(4-((2-dimethylamino)ethyl)amino-
sulfonyl)phenyl)carboxamide: FAB-MS (M+ Cs)+ calc.
809.2349; found: 809.2369; and
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-N'-
(4-((3-(morpholin-4-yl)propyl)aminosulfonyl)phenyl)-
carboxamide: FAB-MS (M+H)+calc. 707.3478; found:
707.3489.
Example 17
Compounds of formula (Id)
17A. A solution of N-(4-methyl-2-carboxymethylpentanoyl) -L-
leucine-N'-(4-methoxycarbonylphenyl)carboxamide (0.28 g, 0.66
mmol) and HOBT (0.12 g) in dry DMF (20 mL) was cooled to 0~C
and treated with EDCI (0.32 g). After stirring 0.5 hours at
0~C, O-benzylhydroxylamine (0.30 m~) was added and the
reaction was allowed to warm to 25~C overnight. The DMF was
removed in vacuo and the residue was taken up in CH2Cl2 and
washed with 5~ HCl/5~ NaHCO3 and brine and the solution was
dried over Na2SO4. After concentration, the product was
SUBSTITUTE SHEET ~RULE 26)
CA 0220~66s 1997-0~-21
WO96/16027 PCT~S95/15S30
-86-
purified by flash chromatography (Sio2, R~0.6, l0~
MeOH/CH2Cl2). The product containing fractions were further
purified by trituration with CH2Cl2 to give N- (4-methyl-2- (N' '-
benzyloxycarbamoyl)methylpentanoyl)-L-leucine-N'-(4-
methoxycarbonylphenyl)carboxamide as a solid, mp 198-199~C.
17B. In a similar manner, the following compounds were
prepared:
N- (2-(N''-benzyloxycarbamoyl)methylpentanoyl)-
L-leucine-N'-(4-methoxycarbonylphenyl)carboxamide;
N- (4-phenyl-2- (N' '-benzyloxycarbamoyl)methylbutanoyl)-
L-leucine-N'-(4-methoxycarbonylphenyl)carboxamide; and
N-(4-methyl-2-(N~'-benzyloxycarbamoyl)methylpentanoyl)-
L- tryptophan-N'-(4-methoxycarbonylphenyl)carboxamide.
17C. N-(4-Methyl-2-(N''-benzyloxycarbamoyl)methylpentanoyl) -L-
leucine-N'-(4-methoxycarbonylphenyl)carboxamide (210 mg) was
hydrolyzed with lM NaOH (l.4 mL) at 50-60~C for 2 hour in THF
(20 mL) and MeOH (5 mL). The organic solvents were evaporated
and the residue was taken up in l0 mL H2O and washed with ether
(2xl0 mL). The aqueous phase was acidified to pH 2 with lO~
HCl and extracted with ethyl acetate (3xl0 mL). The combined
extracts were washed with brine dried (Na2SO4), and
concentrated to afford N-(4-methyl-2-(N''-benzyloxycarbamoyl)-
methylpentanoyl)-L-leucine-N'-(4-carboxyphenyl)carboxamide
(ll0 mg).
Example 18
Compounds of formula (Ie)
18A. To a solution of N-(4-phenyl-2-(N~'-benzyloxy-
carbamoyl)methyl-butanoyl)-L-leucine-N1-(4-methoxycarbonyl-
phenyl)carboxamide (25 mg) in 20 mL MeOH and l0 mL THF was
added lO~ Pd/C (20 mg). The suspension was hydrogenated for l
hour and then suction filtered through Celite. Concentration
afforded the product which was purified on silica (2.5
MeOH/CH2Cl2) to give 8 mg of N-(4-phenyl-2-(N''-hydroxy-
carbamoyl)methyl-butanoyl)-L-leucine-N'-(4-methoxycarbonyl-
SUBSTITVTE SHEET (RVLE 26)
0220~66~ l997-0~-2l
W096l~6027 PCT~95/lSS30
phenyl)carboxamide, MS (FA~3) 482 (M-H)-.
.
18B. In a ~imilar manner, the following compounds were
prepared:
N-(4 -methyl-2-(N''-hydroxycarbamoyl)methylpentanoyl)-
L- leucine -N'-( 4-carboxyphenyl)carboxamide;
N- (4-methyl-2- (N' '-hydroxycarbamoyl)methylpentanoyl)-
L-leucine-N'-(4-methoxycarbonylphenyl)carboxamide,
MS (FA~3) 436 (M+H) +;
N- (2- (N' '-hydroxycarbamoyl)methylpentanoyl)-
L- leucine -N'-( 4-methoxycarbonylphenyl)carboxamide,
MS (FA3) 420 (M-H)-;
N- (4-phenyl-2- tN' '-hydroxycarbamoyl)methylbutanoyl)-
L- t-leucine- N'-( 4-methoxycarbonylphenyl)carboxamide;
N-(4-phenyl-2-(N''-hydroxycarbamoyl)methylbutanoyl)-
L- cyclohexylglycine -N'-(4 -methoxycarbonyl-
phenyl)carboxamide;
N-(4-phenyl-2- (N' '-hydroxycarbamoyl)methylbutanoyl)-
L- leucine -N'-(4 -methoxycarbonylphenyl)carboxamide;
N- (4-methyl-2- (N' '-hydroxycarbamoyl)methylpentanoyl)-
L- t-leucine-N'-(4-methoxycarbonylphenyl)carboxamide;
N-( 4-methyl-2-(N''-hydroxycarbamoyl)methylpentanoyl)-
L- tryptophan-N'-(4-methoxycarbonylphenyl)carboxamide,
MS(FA~3) 507 (M-H)-; and
N- (4-phenyl-2- (N' '-hydroxycarbamoyl)methylbutanoyl)-
L-leucine-N'-(4-methoxycarbonylphenyl)carboxamide.
18C. In a ~imilar manner, the following compounds are
prepared:
N- (3-phenyl-2- (N' '-hydroxycarbamoyl)methylpropanoyl)-
L-leucine-N'-(4-methoxycarbonylphenyl)carboxamide;
N- (5-phenyl-2- (N' '-hydroxycarbamoyl)methylpentanoyl)-
L - leucine -N'-(4 -methoxycarbonylphenyl)carboxamide; and
N- (3-cyclohexyl-2- (N' '-hydroxycarbamoyl)methylpropanoyl)-
~-leucine -N'-( 4-methoxycarbonylphenyl)carboxamide.
SUBSTITUTE SHEET (~UL~ 2~)
CA 0220~66~ 1997-0~-21
W096/16027 PCT~S95/lS530
-88-
Example 19
Compounds of formula (Gb)
l9A. To a cold (0~C) solution of diethyl isobutylmalonate
(21.6 g, 0.1 mol) in 150 mL of ethanol was added a solution of
KOH (5.89 g, 0.1 mol) slowly over 30 minutes. The clear
solution was stirred at 25~C for 60 hours. The ethanol was
removed under reduced pressure and the solid residue was
dissolved in 50 mL of H20. The aqueous solution was acidified
to pH 2 with 4M HCl and extracted with ether (2x50 mL). The
combined extracts were dried over MgSO4 and evaporated to
provide 19.0 g (100~) of ethyl isobutylmalonate as a colorless
oil.
l9B. In a similar manner, the following compounds of formula
(Gb) are prepared: ethyl tert-butylmalonate; ethyl
propylmalonate; ethyl benzylmalonate; and ethyl
cyclohexymethylmalonate.
Example 20
Compounds of formulae (Gc) and (Gd)
20A. To neat ethyl isobutylmalonate (25 g, 0.13 mol) at 0~C
was slowly added ice cold diethylamine (15.1 mL, 0.15 mol).
After stirring for 15 minutes, formalin (11.1 mL of 37~
aqueous formaldehyde) was added dropwise and the mixture was
allowed to stir at 25~C for 3 days. The reaction was treated
with a solution of 20 g of K2CO3 in 40 mL of H20 and extracted
with ether (2 x 100 mL). The combined ether layers were
washed with brine, dried over MgSO4, and evaporated at 20~C on
a rotary evaporator. The crude product ethyl 4-methyl-2-
methylenepentanoate (containing some ether) was dissolved in
250 mL of absolute ethanol and treated with acetonitrile (250
mL), lM LiOH (9.7 g in 250 mL of H20, 0.23 mol). After
stirring overnight, the organic solvents were evaporated and
the aqueous residue was extracted with ethyl acetate
(2x150 mL). The combined extracts were washed with brine,
dried (MgSO4), and evaporated to afford 10.5 g of 4-methyl-2-
methylenepentanoic acid as a colorless oil.
SUBSTITUTE SHEET (RULE 26)
0220~66~ 1997-0~-21
WO96/16027 PCT~S95/15530
-89-
20B. In a similar manner, the following compounds of formula
(Gd) are prepared: 4-phenyl-2-methylenebutanoic acid; 3-
cyclohexyl-2-methylenepropanoic acid; 5-phenyl-2-
methylenepentanoic acid; 2-methylenepentanoic acid; and 3,3-
dimethyl-2-methylenebutanoic acid.
Example 21
Compounds of formula (G)
A mixture of 4-methyl-2-methylenepentanoic acid (5.0 g)
and thioacetic acid (25 mL) was heated at 95~C under argon for
3 days. The excess thioacetic acid was evaporated and the
residual oil was dissolved in ethyl acetate (40 mL) and
extracted with saturated NaHCO3 (3x40 m~). The combined NaHCO3
extracts were combined and acidified at 0~C to pH 2 with lM
HCl. The aqueous layer was extracted with CH2Cl2 (3x40 mL),
the combined organic phases were dried (MgSO4) and evaporated
to give 3.0 g of 4-methyl-2-acetylthiomethyl-pentanoic acid; IH
NMR (80 MHz, CDCL3) ~ 0.95 (d, J=8.0, 6H), 1.20-1.90 (m, 4H),
2.35 (8, 3H), 2.50-3.20 (m, 3H), 6.7 (br s, lH).
Example 22
Compounds of formula (If)
22A. To a solution of 4-methyl-2-acetylthiomethyl-pentanoic
acid (204 mg, 1.0 mmol) in dry DMF (15 mL) containing HOBT (92
mg, 0.6 mmol) and L-leucine-N'-(4-methoxycarbonylphenyl)-
carboxamide (0.6 mmol) was added EDCI (345 mg, 1.8 mmol). The
solution was stirred overnight at 25~C and then the DMF was
removed in vacuo. The residue was dissolved in ethyl acetate
(35 mL) and washed with lM HCl, lM NaOH, and brine. Drying
over MgSO4 and evaporation afforded a semisolid which was flash
chromatographed on silica gel (ethyl acetate l:petroleum ether
2) to give N-(4-methyl-2-acetylthio-methylpentanoyl) -h-
leucine-N'-(4-methoxycarbonylphenyl)carboxamide (190 mg) as a
white solid.
22B. In a similar mAnn~r~ the following compounds of formula
(If) are prepared:
SUBSTITUTE SHEET (Rl)LE 2~)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/15530
--90--
N- (5-phenyl-2-acetylthiomethylpentanoyl)-L-leucine-
N'- ( 4-methoxycarbonylphenyl)carboxamide;
N-(4-phenyl-2-acetylthiomethylbutanoyl)-L-leucine-N'-(4-
methoxycarbonylphenyl)carboxamide;
5N- ( 3-phenyl-2-acetylthiomethylpropanoyl)-L- leucine -N' -
(4-methoxycarbonylphenyl)carboxamide;
N- (3-cyclohexyl-2-acetylthiomethylpropanoyl)-L-leucine-N'-
(4-methoxycarbonylphenyl)carboxamide;
N- (2-acetylthiomethylpentanoyl)-L-leucine-N'-(4-
l0methoxycarbonylphenyl)carboxamidei
N- (5-phenyl-2-acetylthiomethylpentanoyl)-L-leucine-
N'- (4-aminocarbonylphenyl)carboxamide;
N- ( 4-phenyl-2-acetylthiomethylbutanoyl)-L - leucine -N ' -
(4-carboxyphenyl)carboxamide;
15N-(3-phenyl-2-acetylthiomethylpropanoyl)-L- leucine-N'-
(4-methylsulfonylphenyl)carboxamide;
N- (3-cyclohexyl-2-acetylthiomethylpropanoyl)-L-leucine-N'-
(4-carbamoylphenyl)carboxamide;
N- ( 2-acetylthiomethylpentanoyl)-L - leucine -N ' -
20(4-cyanophenyl)carboxamide;
N- (5-phenyl-2-acetylthiomethylpentanoyl)-L-tryptophan-
N'- (4-methoxycarbonylphenyl)carboxamide;
N- (4-phenyl-2-acetylthiomethylbutanoyl)-L-tryptophan-N'-(4-
methoxycarbonylphenyl)carboxamide;
25N-(3-phenyl-2-acetylthiomethylpropanoyl)-L-tryptophan-N'-
(4-methoxycarbonylphenyl)carboxamide;
N- (3-cyclohexyl-2-acetylthiomethylpropanoyl)-L-tryptophan-N'-
(4-methoxycarbonylphenyl)carboxamide; and
N- (2-acetylthiomethylpentanoyl)-L-tryptophan-N'-(4-
30methoxycarbonylphenyl)carboxamide.
Example 23
Compounds of formula (Ig)
23A. To a solution of N- (4-methyl-2-
35acetylthiomethylpentanoyl)-L- leucine -N ' - ( 4-methoxycarbonyl-
phenyl)carboxamide (85 mg, 0.l9 mmol) in MeOH (8 mL) at O~c
was added concentrated HN40H (0.4 mL). After stirring at O~C
SUBSTITUTE SltEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/lSS30
for 5 hour~, the methanol was evaporated and ether ~30 mL) wa~
added. The ether ~olution was washed with 0.5 M HCl, brine,
and was dried over MgSO4. Concentration afforded N- (4-methyl-
2-mercaptomethylpentanoyl)-L- leucine -N ' - ( 4 -methoxycarbonyl-
phenyl)carboxamide in quantitative yield as a white foam,
MS(FAB) 407 (M-H)-.
23B. In a similar manner, the following compounds of formula
(Ig) are prepared:
N-(5-phenyl-2-mercaptomethylpentanoyl)-L- leucine-
N' - (4 -methoxycarbonylphenyl)carboxamide;
N- ( 4 -phenyl-2-mercaptomethylbutanoyl)-L - leucine-N'-(4-
methoxycarbonylphenyl)carboxamide;
N-(3-phenyl-2-mercaptomethylpropanoyl)-L-leucine-Nr-
(4-methoxycarbonylphenyl)carboxamide;
N- (3-cyclohexyl-2-mercaptomethylpropanoyl)-L-leucine-N'-
(4-methoxycarbonylphenyl)carboxamide;
N- (2-mercaptomethylpentanoyl)-L-leucine-N'-(4-
methoxycarbonylphenyl)carboxamide;
N- (5-phenyl-2-mercaptomethylpentanoyl)-L-leucine-
N'-(4-aminocarbonylphenyl)carboxamide;
N- (4-phenyl-2-mercaptomethylbutanoyl)-L-leucine-N'-
(4-carboxyphenyl)carboxamide;
N- ( 3-phenyl-2-mercaptomethylpropanoyl)- L- leucine -N ' -
(4-methylsulfonylphenyl)carboxamide;
N- (3-cyclohexyl-2-mercaptomethylpropanoyl)-L-leucine-N'-
(4-carbamoylphenyl)carboxamide;
N- (2-mercaptomethylpentanoyl)-L-leucine-N'-
(4-cyanophenyl)carboxamide;
N- ( 5-phenyl-2-mercaptomethylpentanoyl)-L- tryptophan-
N ' - ( 4 -methoxycarbonylphenyl)carboxamide;
N- (4-phenyl-2-mercaptomethylbutanoyl)-L-tryptophan-N'-(4-
methoxycarbonylphenyl)carboxamide;
N- ( 3-phenyl-2-mercaptomethylpropanoyl)-L- tryptophan-N'-
(4-methoxycarbonylphenyl)carboxamide;
N- (3-cyclohexyl-2-mercaptomethylpropanoyl)-L-tryptophan-N'-
(4-methoxycarbonylphenyl)carboxamide; and
SUBSTITUTE SHEET (RULE 26)
.
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/15530
-92-
N-(2-mercaptomethylpentanoyl) -L- tryptophan-N'-(4-
methoxycarbonylphenyl)carboxamide.
Example 24
Formula (Fc n )
To a stirred solution of 6.48 g (25.0 mmol) of N-(4-
pentenoyl)-4S-phenylmethyl-2-oxazolidinone in 50 mL of dry THF
under argon at -95 ~C was added 27.5 mL (27.5 mmol) of 1.0 M
sodium h~methyldisilazide in THF via syringe at a rate to
maintain the reaction temperature at less than -75 ~C. After
15 min at -80 ~C to -95 ~C, 5.65 mL (6.83 g, 35 mmol) of t-
butyl bromoacetate, which had been filtered through basic
alumina immediately prior to use, was added via syringe over a
1 min period. The solution was stirred at -90 ~C to -60 ~C
for 2 h, and then partitioned between hexane (100 mL) and
dilute a~. NaHSO4. The organic layer was washed with sat. aq.
NaCl containing a little 1 M phosphate buffer (pH 7), dried
over Na2SO4, and concentrated. The residue was recrystallized
from 75 mL of hexane to give 5.56 g (60~) of N-(2R-(t-
Butoxycarbonyl)methyl-4-pentenoyl)-4S-phenylmethyl-2-
oxazolidinone as pale yellow needles: mp 75-76 ~C.
El. Anal. Calc. for C2~H27NO5: C, 67.54; H, 7.29, N, 3.75.
Found: C, 67.76; X, 7.34; N, 3.87.
~xample 25
Fo la (Fcn-1) Where R2 iB Biphenyl
To a solution of 4.75 g (12.7 mmol) of N-(2R-(t-
Butoxycarbonyl)-methyl-4-pentenoyl)-4S-phenylmethyl-2-
oxazolidinone, 3.73 g (16.0 mmol) of 4-bromobiphenyl, 0.234 g
(0.77 mmol) of tri-o-tolylphosphine, and 2.22 mL (1.62 g, 16.0
mmol) of triethylamine in 10 mL of anhydrou~ DMF under argon
was added 0.086 g (0.385 mmol) of palladium(II) acetate. The
solution was heated at 100 ~C for 4 h, cooled to room
temperature, diluted with ethyl acetate. The precipitate was
removed by filtration, and the filtrate was partitioned
between 150 mL of 2:1 ethyl acetate:hexane and 50 mL of pH 7
phosphate buffer (0.5 M) containing a little sodium sulfite.
SUBSTITUTE SHEET (F~llLE 26)
_ _ _
-
CA 0220~66~ 1997-0~-21
WO 96/16027 PCT/US9S115~i30
-93-
The organic layer was washed with 0.2 N aq. sodium bisulfate
and brine/pH 7 phosphate buffer, dried over sodium sulfate,
and concentrated. The residue was dissolved in 50 mL of ethyl
acetate, diluted with 250 mL of isooctane, and seeded with a
' 5 few crystals of the product. The solid was removed by
filtration, and recrystallized from 250 mL of 4:1 isooctane:
ethyl acetate to give 4.20 g (63~) of the product, N-(2R-(t-
Butoxycarbonyl~methyl-(5-(biphen-4-yl)-4-pentenoyl)-4S-
phenylmethyl-2-oxazolidinone as fine white needles: mp 118-
119 ~C; IH NMR (300 MHz, CDCl3) 7.25-7.60 (m, 14H), 6.47 (d,
lH, J = 16 Hz), 6.25 (dt, lH, J = 16 and 8 Hz), 4.65-4.70 (m,
lH), 4.34-4.44 (m, lH), 4.11 (dd, lH, J = 9 and 2 Hz), 4.01
(t, lH, J = 8 Hz), 3.33 (dd, lH, J = 14 and 3 Hz), 2.89 (dd,
lH, J = 17 and 11 Hz), 2.76 (dd, lH, J = 14 and 10 Hz), 2.40-
2.57 (m, 3H), 1.43 (s, 9H).
El. Anal. Calc. for C33H3sNOs: C, 75.40; H, 6.77, N, 2.66.
Found: C, 75.17; H, 6.84; N, 2.58.
Example 26
Formula (Fcn-2) Where R2 i8 Biphenyl
A solution of 5.23 g (10.00 mmol) of N-(2R-(t-
butoxycarbonyl)methyl-(5-(biphen-4-yl)-4-pentenoyl)-4S-phen
ylmethyl-2-oxazolidinone in 50 mL of ethyl acetate was
hydrogenated at 1 atm of hydrogen over 500 mg of 10% Pd/C for
2 h at room temperature. The catalyst was removed by
filtration through Celite, and the filtrate was concentrated
to about 20 mL, then diluted with about 75 mL of isooctane.
The solution was seeded with a few crystals of the product,
and the mixture was concentrated to about 50 mL, then cooled
to -20 oc. Filtration of the precipitate gave 4.91 g (94~) of
N-(2R-(t-butoxy- carbonyl)methyl-(5-(biphen-4-yl)pentanoyl)-
4S-phenylmethyl-2-oxazolidinone as a white powder: mp: 75-76
oc; 'H NMR (300 MHz, CDC13) 7.22-7.59 (m, 14H), 4.61-4.70 (m,
lH), 4.18-4.24 (m, lH), 4.14 (d, 2H, J = 5 Hz), 3.34 (dd, lH,
J = 13 and 3 Hz), 2.57-2.89 (m, 4H), 2.48 (dd, lH, J = 13 and
5 Hz), 1.65-1.83 (m, 3H), 1.50-1.60 (m, lH), 1.42 (s, 9H).
El. Anal. Calc. for C33H37NOs: C, 75.11; H, 7.07, N, 2.65.
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/lS530
-94-
Found: C, 75.34; H, 7.11; N, 2.69.
Example 27
Formula F Wh~re R2 is Biphenyl
To a solution of 4.02 g (7.62 mmol) of N-(2R-(t-
butoxycarbonyl)-methyl-(5-(biphen-4-yl)pentanoyl)-4S-
phenylmethyl-2-oxazolidinone in 60 mL of THF at 0 ~C was added
2.8 mL of 30~ aq. hydrogen peroxide followed by 8.0 mL of 2 N
aq. lithium hydroxide. The mixture was stirred vigorously at
0 ~C for 15 min, and then allowed to warm to room temperature.
After 2 h, the mixture was cooled to 0 ~C, and 20 mL of 2 N
a~. sodium sulfite and 30 mL of saturated aq. sodium
bicarbonate were added. After 10 min at 0 ~C, the mixture was
stirred an additional 1 h at room temperature and then poured
into 1 M pH 7 phosphate buffer. The aqueous phase was
acidified to pH 6 by addition of solid sodium bisulfate, and
then the mixture was extracted with 1:1 ethyl acetate:hexane
(200 mL). The organic layer was washed with brine, dried over
sodium sulfate, and concentrated. The residue was
chromatographed on 125 g of silica gel, eluting with 20~ to
30~ ethyl acetate:hexane containing 0.5~ acetic acid. The
product-containing fractions were concentrated and then
azeotroped several times with toluene to give 2.93 (~100~)
of the product, 2R-tt-butoxycarbonyl)methyl-(5-(biphen-4-
yl)pentanoic acid, as a thick syrup which slowly solidified
upon storage at -20 ~C: mp 44-45 ~C (after drying solid in
vacuo); IH NMR ~300 MHz, CDCl3) ~ 7.22-7.59 (m, 9H), 2.82-
2.90 (m, lH), 2.59-2.75 (m, 3H), 2.40 (dd, lH, J = 14 and 5
Hz), 1.55-1.80 (m, 4H), 1.42 (s, 9H).
El. Anal. Calc. for C23H28O4: C, 74.97; H, 7.66. Found: C,
75.08; H, 7.76.
.,
Example 28
Formula (A-l)
To a solution of 5.00 g (21.6 mmol) of N-(t-butoxy-
carbonyl)-L-t-leucine and 2.50 g (21.7 mmol) o~ N-hydroxy-
succinimide in 40 mL of acetonitrile at 0 ~C was added
_ _ _
-
CA 02205665 l997-05-2l
WO96/16027 PCT~S9SJl5530
-95-
dropwise a solution of 4.12 g (20 mmol) of dicyclohexyl-
carbodiimide in 40 mL of acetonitrile. The mixture was
stirred at 0 ~C to room temperature overnight, and then the
mixture was filtered to remove the precipitated
dicyclohexylurea. The filtrate was concentrated and the
residue was triturated with ethyl acetate/dichloromethane to
give 5.06 g (80~) of N-(t-Butoxycarbonyl)-L-t-leucine,
N-hydroxysuccinimide ester as a white solid: mp 136-137 ~C;
IH NMR (300 MHz, CDC13) ~ 5.07 (br d, lH), 4.43 (d, lH, J = 10
Hz), 2.84 (s, 4H), 1.46 (s, 9H), 1.10 (s, 9H).
Example 29
FG- 1 a (C) Where R3 i~ t-Butyl, and R7 i~ 4-Pyridine (in place
o~ the illustrated phenyl group)
A solution of 2.00 g (6.32 mmol) of N-(t-butoxycarbonyl)-
L-t-leucine, N-hydroxysuccinimide ester and 2.98 g (31.6 mmol)
of 4-aminopyridine in 20 mL of dioxane was heated at 100 ~C
for 3 h. The reaction was cooled to room temperature and
concentrated. The residue was purified by chromatography on
silica gel, eluting with 5~ to 10~ methanol in
dichloromethane, to give 1.06 g (54~) of N-(t-butoxycarbonyl)-
L-t-leucine-N'-(pyrid-4-yl)carboxamide as a white solid:
IH NMR (300 MHz, CDC13) ~ 8.47 (d, 2H, J = 6 Hz), 8.35 (br s,
lH), 7.50 (d, 2H, J = 6 Hz), 5.23 (broad d, lH), 4.00 (br d,
lH), 1.44 (s, 9H), 1.05 (s, 9H).
FAB-MS ((M+H)+ calculated: 308.1974 observed: 308.1970.
Example 30
30A. Formula (D) Where R3 is t-Butyl, and R7 is 4-Pyridine (in
place of the illustrated phenyl group)
To a solution of 132 mg (0.43 mmol) of N-(t-
butoxycarbonyl)-L-t-leucine-N'-(pyrid-4-yl)carboxamide in 2 mL
of dichloromethane was added 1 mL of trifluoroacetic acid.
After 1 h at room temperature, the solution was diluted with
ca. 5 mL of toluene and concentrated. Repeated dissolution in
toluene/dichloromethane/methanol and concentration eventually
provided 190 mg (100~) of L-t-leucine-N'-(pyrid-4-
u~ ~11F 2~:~
_
CA 0220~66~ 1997-0~-21
WO 96116027 PCT/US9S/15530
-96-
yl)carboxamide bis(trifluoroacetate) as a white solid: 1H NMR
~300 MHz, DMSO-d6) ~ 11.58 (br s, lH), 8.68 (d, 2H, J = 6
Hz), 8.35 (br 8, 2H), 7.91 (d, 2H, J = 6 Hz), 3.81 (s, lH),
1.04 (s, 9H).
FA~3-MS ((M+H)+ calculated: 208.1500 observed: 208.1496.
30B. Formula (D) Where R3 i8 t-Butyl, and R7 i8 4-(Methyl-
thio)phonyl
In a manner analogous to that of part A was prepared L-t-
leucine-N'-(4-(methylthio)phenyl)carboxamide: IH NMR (300 MHz,
CDCl3) ~ 9.02 (s, lH), 7.49 (d, 2H, J = 6.5 Hz), 7.23 (d, 2H,
J = 6.5 Hz), 3.23 (s, 2H), 2.44 (s, 3H), 1.03 (s, 9H).
Example 31
31A. Formula (Ib) Where R2 i8 Biphenyl (and X i8 propane-1,3-
diyl), R3 and R~ are t-Butyl, and R7 i 4-Pyridine (in
place of the illustrated phenyl group)
To a solution of 357 mg ( 0.97 mmol) of 2R-(t-
butoxycarbonyl)methyl-(5-(biphen-4-yl)pentanoic acid, 422 mg
(0.97 mmol) of L-t-leucine-N'-(4-(pyrid-4-yl)carboxamide
bis(trifluoroacetate), and 0.50 mL (3.6 mmol) of triethylamine
in 5 mL of DMF was added 442 mg (1.00 mmol) of benzotriazol-
1-yl-tris-(dimethylamino)phosphonium hexafluorophosphate.
After 4 h, the reaction was partitioned between ethyl acetate
and water. The aqueous layer was extracted twice with ethyl
acetate, and the combined organic layers were washed with
water and with brine, dried over sodium sulfate, and
concentrated. Purification of the residue by silica gel
chromatography, eluting with 25~ to 75~ ethyl acetate in
hexane, gave 360 mg (66~) of N-(2R-(t-butoxycarbonyl)methyl-5-
(biphen-4-yl)pentanoyl)-L-t-leucine-N'-(pyrid-4-yl)-
carboxamide: IH NMR (300 MHz, CDCl3) ~ 8.52 (s, lH), 8.42 (d,
2H, J = 6 Hz), 7.54 (d, 2H, J = 7Hz), 7.40-7.48 (m, 6 H), 7.32
(t, lH, J = 7 Hz), 7.13 (d, 2H, J = 8 Hz), 6.62 (d, lH, J = 9
Hz), 4.35 (d, lH, J = 9 Hz), 2.58-2.68 (m, 4H), 2.40 (dd, lH,
J = 16 and 3 Hz), 1.40-1.75 (s over m , obscured by H2O, 13 H),
1.08 (s, 9H).
SUBSlllUlt SIEr tRIllE 26)
s
CA 0220~66~ l997-0~-2l
WO96/16027 PCT~S95/lSS30
-97-
31B. Formula (Ib) Varying R7
By following the procedure of part A and substituting L-
t-leucine-N'-(4-(pyrid-4-yl)carboxamide bis(trifluoroacetate)
with the following:
~-t-leucine-N'-(4-((2-hydroxyethyl)aminosulfonyl)phenyl)-
carboxamide;
L-t-leucine-N'-(4-(methylthio)phenyl)carboxamide;
there are obtained:
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-~-t-
leucine -N'-(4-((2-hydroxyethyl)aminosulfonyl)-
phenyl)carboxamide: mp 89-92 ~C; 1H NMR (300 MHz,
methanol-d4) ~ 7.80 (s, 4H), 7.51 (d, 2H, J = 7 Hz),
7.41 (d, 2H, J = 7 Hz), 7.36 (d, 2H, J = 8 Hz), 7.28 (t,
lH, J = 7 Hz), 7.14 (d, 2H, J = 8 Hz), 4.47 (s, lH), 3.47
(t, 2H, J = 6 Hz), 2.85-2.95 (t overlapping m, 3H), 2.52-
2.62 (m, 3H), 2.32 (dd, lH, J = 16.5 and 5 Hz), 1.48-1.62
(m, 4H), 1.41 (s, 9H), 1.09 (s, 9H)
El. Anal. Calc. for C37H49N307S: C, 65.37; H, 7.26; N,
6.18; S, 4.72. Found: C, 65.13; H, 7.33; N, 6.22; S,
4.63;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-
leucine-N~-(4-(methylthio)phenyl)carboxamide: IH NMR (300
MHz, CDC13) ~ 7.79 (s, lH), 7.53 (d, 2H, J = 7 Hz), 7.31-
7.44 (m, 7H), 7.19 (d, 2H, J = 9 Hz), 7.13 (d, 2H, J = 8
Hz), 6.58 (d, lH, J = 9 Hz), 4.36 (d, lH, J = g Hz),
2.55-2.67 (m, 4H), 2.34-2.40 (s overlapping m, 4H), 1.38-
1.75 (s overlapping m, 13 H), 1.07 (s, 9H)
El. Anal. Calc. for C36H46NO4S~0.25 H2O: C, 71.19; H,
7.72, N, 4.61, S, 5.28. Found: C, 71.20; H, 7.78, N,
4.58, S, 5.28;
Example 32
32A. Formula (Ic) Where R2 is Biphenyl (and X is propane-1,3-
diyl), R3 is t-Butyl, and R7 is 4-Pyridine (in place of
the illustrated phenyl group)
To a solution of 360 mg (0.64 mmol) of N-(2R-(t-
butoxycarbonyl)-methyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/lS530
-98-
N'-(pyrid-4-yl)carboxamide in 4 mL of dichloromethane was
added 2 mL of trifluoroacetic acid. After 1 h at room
temperature, the solution was diluted with toluene and
concentrated. The residue was dissolved in ethyl acetate (15
mL) and washed with 0.5 M pH 4 citrate buffer (2 x 15 mL).
The combined aq. layers were extracted with ethyl acetate (2 x
15 mL), and the combined organic layers were washed with
brine, dried over sodium sulfate, and concentrated. The
residue was triturated with ethyl acetate/hexane to give 230
mg (71~) of N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-
L-t-leucine-N'-(pyridin-4-yl)carboxamide as a white solid:
mp 198-201 ~C; IH NMR (300 MHz, MeOH-d4) ~ 8.32 (d, 2H, J = 7
Hz), 7.64 (d, 2H, J = 5 Hz), 7.45 (d, 2H, J = 7 Hz), 7.24-7.38
(m, 7H), 7.10 (d, 2H, J = 8 Hz), 4.42 (B, lH), 2.85-3.00 (m,
lH), 2.33-2.65 (m, 4H), 1.40-1.62 (m, 4H), 1.02 (S, 9H).
El. Anal. Calc. for C3~3~304~ O . 5 H20~0.5 ethyl acetate
(solvate): C, 69.29; H, 7.27, N, 7.58. Found: C, 69.46; H,
7.09; N,7.55.
32B. Formula (Ic) Varying R7
By following the procedure of part A and substituting N-
(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-
leucine-N'-(pyrid-4-yl)carboxamide with the following:
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-
leucine -N'-(4-((2-hydroxyethyl)aminosulfonyl)-
phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-
leucine-N'-(4R/S-(methylsulfinyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(fluoren-2-yl)pentanoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(7-(glycyl)aminofluoren-2-
yl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)phenyl)-
carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(4-(pyrid-4-yl)phenyl)-
pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)phenyl)-
carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-~-
SUBSTITUTE SHEET (RU~E 26)
_ _
CA 0220~66~ 1997-0~-21
WO 96116027 ~CT/US9S/l~iS30
_99_
.
hydroxyvaline-N'-(phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-
leucine-N'-(4-(methylsulfonyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(4-(2-
~ 5 hydroxyethyl)phenyl)pentanoyl)-L-leucine-Nl-(4
(methoxycarbonyl)phenyl)carhox~m;de;
N-(2R-(t-butoxycarbonyl)methyl-5-(4'-hydroxybiphen-4-
yl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)phenyl)-
carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(4'-cyanobiphen-4-
yl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)-
phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(4'-(2-aminoethoxy)biphen-4-
yl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)-
phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(4-(pyridin-4-yl)phenyl)-
pentanoyl)-L-cyclohexylglycine-N'-(4-((2-hydroxy-
ethyl)aminosulfonyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-
threonine-N'-(4S-(methylsulfinyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(2-fluorobiphen-4-
yl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)-
phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-4-((biphen-4-yl)thio)butanoyl)-
L-t-leucine-N'-(pyridin-4-yl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(4-(2-aminopyridin-5-
yl)phenyl)pentanoyl)-L-threonine-N'-(4S-(methyl-
3 0 8ul finyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(2-hydroxybiphen-4-
yl)pentanoyl)-L-threonine-N'-(4S-(methylsulfinyl)phenyl)-
carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(4'-cyanobiphen-4-
yl)pentanoyl)-L-(trans-4-hydroxycyclohexyl)glycine-N'-
(4S-(methylsulfinyl)phenyl)carboxamide;
N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-(4-
SUBSTITUTE SHEET (RULe 26)
CA 0220~66~ 1997-0~-21
WO 96/16027 PCT/US9SI15530
-100-
hydroxytetrahydropyran-4-yl)glycine-N'-(4S-(methyl-
sulfinyl)phenyl)carboxamide; and
N-(2R-(t-butoxycarbonyl)methyl-5-(2R/S-hydroxy-3,3,3-
trifluoropropyl)phenyl)pentanoyl)-L-(cyclohexyl)glycine-
N'-(4S-(4-((2-hydroxyethyl)aminosulfonyl)phenyl)-
carboxamide,
there are obtained:
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-N'-
(4-((2-hydroxyethyl)aminosulfonyl)phenyl)carboxamide:
IH NMR (300 MHz, MeOH-d4) ~ 7.80 (s, 4H), 7.50 (d, 2H, J = 7
Hz), 7.25-7.42 (m, 5H), 7.15 (d, 1 H, J = 8 Hz), 4.47 (s, lH),
3.47 (t, 2H, J = 6 Hz), 2.89-3.00 (m, lH), 2.87 (t, 2H, J = 6
Hz), 2.49-2.70 (m, 3H), 2.39 (dd, lH, J = 16 and 5 Hz), 1.46-
1.67 (m, 4H), 1.07 (s, 9H)
El. Anal. Calc. for C33H4lN3O7S~0.5 H2O: C, 62.64; H, 6.69; N,
6.64; S, 5.07. Found: C, 62.61; H, 6.80; N, 6.31; S, 4.97;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-N'-
(4R/S-(methylsulfinyl)phenyl)carboxamide:
IH NMR (300 MHz, MeOH-d4) ~ 8.03 (d, lH, J = 9 Hz), 7.76 (d,
2H, J = 9 Hz), 7.57 (dd, 2H, J = 9 and 2 Hz), 7.44 (d, 2H, J =
7 Hz), 7.36 (t, 2H, J - 7 Hz), 7.23-7.28 (m, 3H), 7.10 (d, 2H,
J = 8 Hz), 4.45 (d, lH, J = 9 Hz), 2.85-2.98 (m, lH, J = 8
Hz), 2.44-2.64 (m, 7H), 2.35 (dd, lH, J = 16 and 5 Hz), 1.43-
1.62 (m, 4H), 1.03 (s, 9H);
N-(2R-carboxymethyl-5-(fluoren-2-yl)pentanoyl)-~-leucine-N'-
(4-(methoxycarbonyl)phenyl)carboxamide: mp 188-190 ~C;
H NMR (300 MHz, CDCl3) ~ 12.06 (s, lH), 10.30 (s, lH), 8.19
(d, lH, J = 8 Hz), 7.86 (d, 2H, J = 9 Hz), 7.68-7.77 (m, 3H),
7.62 (d, lH, 8 Hz), 7.48 (d, lH, J = 7 Hz), 7.20-7.35 (m, 3H),
7.10 (d, lH, J = 7 Hz), 4.48 (m, lH), 3.76 (s, 3H), 3.70 (s,
2H), 2.15-2.75 (m, 5H), 1.35-1.75 (m, 5H), 0.90-0.99 (m, 4H)
El. Anal. Calc. for C34H38N2O6: C, 71.56; H, 6.71, N, 4.91.
Found: C, 71.51; H, 6.97; N, 4.84;
N-(2R-carboxymethyl-5-(7-(glycyl)aminofluoren-2-yl)pentanoyl)-
L-leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide:
mp 222-224 ~C; FAB-MS (M+H)+ calculated for C40H51N407:
699.3758; observed: 699.3770;
SUB~ t SHEr ~RUIE 21;)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/15~30
-101-
N-(2R-carboxymethyl-5-(4-(pyrid-4-yl)phenyl)pentanoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide:
H NMR (300 MHz, d4-MeOH) ~ 8.52 (d, 2H, J = 5.5 Hz), 7.92
(d, 2H, J = 9.19 Hz), 7.67 (d, 2H, J = 8.82 Hz), 7.58 (d, 2H,
J = 6.25 Hz), 7.46 (d, 2H, J = 8.45 Hz), 7.20 (d, 2H, J = 8.46
Hz), 4.6-4.4 (m, lH), 3.84 (s, 3H), 2.83-2.59 (m,4H), 2.38
(dd, lH, J = 16.7 and 5 Hz), 1.71-1.57 (m,7H), 0.96 (dd, 6H,
J = 9.92 and 6.3 Hz);
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-~-
hydroxyvaline-N'-(phenyl)carboxamide:
IH NMR (300 MHz, CDCl3) ~ 8.73 (s, lH), 8.07 (d, lH, J = 8.09
Hz), 7.52-7.25 (m, llH), 7.10 (t, lH, J = 7.54 Hz), 6.97 (d,
2H, J = 8.08 Hz), 4.41(d, lH, J = 8.45 Hz), 3.02-3.00 (m, lH),
2.75 (dd, lH, J = 16.55 and 8.45 Hz), 2.53- 2.51 (m, 2H), 2.44
(dd, lH, J = 17.1 and 4.6 Hz), 1.85 1.47 (m,4H), 1.45 (s, 3H),
1.21 (s, 3H)
El. Anal. Calc. for C3H3N404: C, 71.69; H, 6.82; N, 5.57.
Found: C, 71.65; H, 6.86; N, 5.53;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-N'-
(4-(methyl-sulfonyl)phenyl)carboxamide:
IH NMR (300 MHz, MeOH-d4) ~ 8.10 (d, lH, J= 9 Hz), 7.84 (s,
4H), 7.47 (d, 2H, J= 8Hz), 7.25-7.40 (m, 5H), 7.13 (d, 2H, J=
8 Hz), 4.48 (d, lH, J= 9Hz), 2.95 (s, 3H), 2.44-2.70 (m, 4H),
2.36 (dd, lH, J= 16 and 5 Hz), 1.47-1.63 (m, 4H), 1.06
(s, 9H);
N-(2R-carboxymethyl-5-(4-(2-hydroxyethyl)phenyl)pentanoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide:
lH NMR (300 MHz, MeOH-d4) ~ 7.91 (d, 2H, J= 9Hz), 7.64 (d, 2H,
J= 9Hz), 6.96 (s, 4H), 4.47-4.51 (m, lH), 3.84 (s, 3H), 3.63
(t, 2H, J=7 Hz), 2.68 (t, 2H, J= 7 Hz), 2.46-2.75 (m, 4H),
2.37 (dd, lH, J= 16 and 5 Hz), 1.51-1.73 (m, 7H), 0.93 and
0.89 (2d, 6H, J = 7 Hz);
N-(2R-carboxymethyl-5-(4'-hydroxybiphen-4-yl)pentanoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide:
mp 195-197 ~C; FAB-MS (M+H) expected: 575.2757; observed:
595.2750;
N-(2R-carboxymethyl-5-(4'-cyanobiphen-4-yl)pentanoyl)-L-
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ lss7-0s-2l
Wos6/l6027 PCT~S95/lS530
-102-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide:
H NMR (300 MHz, MeOH-d4) ~ 10.02s, lH), 8.37 (d, lH, J=7 Hz),
7.87 (d, 2H, J=8 Hz), 7.71 td, 2H, J=8.5 Hz), 7.64 (d, 4H, J=9
Hz), 7.35 (d, 2H, J= 8 Hz), 7.16 (d, 2H, J= 8 Hz), 4.50-4.53
(m, lH, 3.81 (s, 3H), 2.49-2.78 (m, 4H), 2.35 (dd, lH, J= 16
and 5 Hz), 1.46-1.72 (m, 7H), 0.88 and 0.90 (2d, 2H, J= 7 Hz);
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-leucine-N'-(4-
(methoxycarbonyl)phenyl)carboxamide:
mp 191-193 ~C;
N-(2R-carboxymethyl-5-(4'-(2-aminoethoxy)biphen-4-yl)-
pentanoyl)-L-leucine-N~-(4-(methoxycarbonyl)phenyl)-
carboxamide:
FA~3-MS (M+H)+ calc. 618.3179; found: 618.3189;
N-(2R-carboxymethyl-5-(4-(pyridin-4-yl)phenyl)pentanoyl)-L-
cyclohexylglycine-N'-(4-((2-hydroxyethyl)aminosulfonyl)-
phenyl)carboxamide;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-threonine-N'-
(4S-(methylsulfinyl)phenyl)carboxamide;
N-(2R-carboxymethyl-5-(2-fluorobiphen-4-yl)pentanoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide;
N-(2R-carboxymethyl-4-((biphen-4-yl)thio)butanoyl)-L-t-
leucine-N'-(pyridin-4-yl)carboxamide;
N-(2R-carboxymethyl-5-(4-(2-aminopyridin-5-yl)phenyl)-
pentanoyl)-L-threonine-N'-(4S-(methylsulfinyl)phenyl)-
carboxamide;
N-(2R-carboxymethyl-5-(2-hydroxybiphen-4-yl)pentanoyl)-L-
threonine-N'-(4S-(methylsulfinyl)phenyl)carboxamide;
N-(2R-carboxymethyl-5-(4'-cyanobiphen-4-yl)pentanoyl)-L-
(trans-4-hydroxycyclohexyl)glycine-N'-(4S-
(methylsulfinyl)phenyl)carboxamide;
N-(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-(4-
hydroxytetrahydropyran-4-yl)glycine-N'-(4S-
(methylsulfinyl)phenyl)carboxamide; and
N-(2R-carboxymethyl-5-(2R/S-hydroxy-3,3,3-trifluoropropyl)-
phenyl)pentanoyl)-L-(cyclohexyl)glycine-N'-(4S-(4-((2-
hydroxyethyl)aminosulfonyl) phenyl)carboxamide.
SU~ t slEr~llE21~
CA 0220~66~ l997-0~-2l
WO96/16027 PCT~S9SIlSS30
-103-
Example 33
33A. Fs~ ~ A (Ie) Where R2 is Biphenyl (and X is propane-1,3-
diyl), R3 is t-Butyl, and R7 i8 4-Pyridine (in place of
the ~llu~trated ph~nyl group)
~ 5 To a solution of 127.4 mg (0.200 mmol) of N-(2R-carboxymethyl-
5-(biphen-4-yl)pentanoyl)-L-t-leucine-Nl-(pyridin-
4-yl)carboxamide and 40 ~L of N-methyl morpholine in 1.0 m~ of
DMF at room temperature was added 115 mg (0.26 mmol) of
benzotriazol-1-yl-tris(dimethylamino)phosphonium hexafluoro-
phosphate. After 15 min, 42 mg (0.60 mmol) of hydroxylamine
hydrochloride was added in one portion, followed by addition
of an additional 70 ~L of N-methyl morpholine. The mixture
was stirred for 24 h at room temperature, then partitioned
between 30 mL of ethyl acetate and 25 mL of 0.5 M aq. sodium
bicarbonate. The organic layer was washed with additional aq.
sodium bicarbonate and with brine/pH 7 buffer, dried over
sodium sulfate, and concentrated. Recrystallization from
ethyl acetate pro~ided 42.2 mg of N-(2R-(N-hydroxycarbamoyl)-
methyl-5-(biphen-4-yl)pentanoyl-~-t-leucine-N'-(pyrid-4-yl)-
carboxamide. Concentration of the filtrate and purification
by radial chromatography (1 mm plate, 5~ to lO~
ethanol:dichloromethane) provided, after recrystallization
from 2:1 ethyl acetate:h~ne, an additional 21.0 mg of
product. Total yield was 63.1 mg (61~) of N-(2R-(N-
hydroxycarbamoyl)methyl-5-(biphen-4-yl)pentanoyl-L-t-leucine-
N'-(pyrid-4-yl)carboxamide as a white powder: IH NMR (300 MHz,
DMSO-d6) ~ 10.45 (s, lH), 10.34 (s, lH), ~.68 (s, lH), 8.38
(d, 2H, J = 7 Hz), 8.04 (d, 1 H, J = 9 Hz), 7.25-7.60 (m, 9H),
7.12 (d, 2H, J = 7 Hz), 4.39 (d, lH, J = 9 Hz), 2.86-2.97 (m,
lH), 2.36-2.60 (m, 2H, obscured by DMSO-d5 resonance), 2.14
(dd, lH, J = 15 and 7 Hz), 2.02 (dd, lH, J = 15 and 8 Hz),
1.30-1.53 (m, 4H), 0.94 (s, 9H).
El. Anal. Calc. for C30H3~4O4~0.25 HzO: C, 69.14; H, 7.06, N,
10.75. Found: C, 69.15; H, 7.23; N, 10.56.
33B. ~ormula (Ie) Varying R2, R3, and R7
By following the procedure of part A and substituting N-
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ l997-0~-2l
WO96/16027 PCT~S9S/15530
-104-
(2R-carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-N~-
(pyridin-4-yl)carho~m;de with the following:
N-(2R-(carboxymethyl-5-(biphen-4-yl)pentanoyl)-L-threonine-N'-
(4S-(methylsulfinyl)phenyl)carboxamide;
N-(2R-(carboxymethyl-5-(4-(pyridin-4-yl)phenyl)pentanoyl)-~-
(~-hydroxy)valine-N'-(4S-
methylsulfinyl)phenylcarboxamide; and
N-(2R-(carboxymethyl-4-methylpentanoyl)-L-t-leucine-N'-
(pyridin-4-yl)carboxamide;
N-(2R-(carboxymethyl-(5-(4-(2S-
hydroxypropyl)phenyl)pentanoyl)-L-(trans-4-
hydroxycyclohexyl)glycine-N'-(pyridin-4-yl)carboxamide;
N-(2R-(carboxymethyl-5-(4-(2-methylthiazol-
4yl)phenyl)pentanoyl)- L-(~-hydroxy)valine-N'-(pyridin-
4yl)carboxamide; and
N-(2R-(carboxymethyl-5-(4-(2R/S-hydroxy-3,3,3-trifluoropropyl)
phenyl)pentanoyl)-~ -hydroxy)valine-N'(pyridin-4-
yl)carboxamide,
there are obtained:
N-(2R-(N-hydroxycarbamoyl)methyl-5-(biphen-4-yl)pentanoyl)-L-
threonine-N'-(4S-(methylsulfinyl)phenyl)carboxamide;
N-(2R-(N-hydroxycarbamoyl)methyl-5-(4-(pyridin-4-
yl)phenyl)pentanoyl)-L-(~-hydroxy)valine-N'-(4S-
methylsulfinyl)phenylcarboxamide;
N-(2R-(N-hydroxycarbamoyl)methyl-4-methylpentanoyl)-L-t-
leucine-N'-(pyridin-4-yl)carboxamide;
N-(2R-(N-hydroxycarbamoyl)methyl-(5-(4-(2S-
hydroxypropyl)phenyl)pentanoyl) L-(trans-4-
hydroxycyclohexyl)glycine-N'-(pyridin-
4-yl)carboxamide;
N-(2R-(N-hydroxycarbamoyl)methyl-5-(4-(2-methylthiazol-4-
yl)phenyl)pentanoyl)-L-(~-hydroxy)valine-N'-(pyridin-4-
yl)carboxamide;
N-(2R-(N-hydroxycarbamoyl)methyl-5-(4-(2R/S-hydroxy-3,3,3-
trifluoropropyl)phenyl)pentanoyl)-L-(~-hydroxy)valine-
N'(pyridin-4-yl)carboxamide.
N-(2R-(N-hydroxycarbamoyl)methyl(5-(4-(2S-hydroxypropyl)-
CA 0220~66~ 1997-0~-21
WO 96/1~i027 PCT/US9S/lS530
-105 -
phenyl)pentanoyl)-~-(trans-4-hydroxycyclohexyl)glycine-
N'-(pyridin-4-yl)carboxamide;
N-(2R-(N-hydroxycarbamoyl)methyl-5-(4-(2-methylthiazol-
4yl)phenyl)pentanoyl)-L-(~-hydroxy)valine-N'-(pyridin-
4yl)carboxamide; and
N-(2R-(N-hydroxycarbamoyl)methyl-5-(4-(2R/S-hydroxy-3,3,3-
trifluoropropyl)phenyl)pentanoyl)-L-(~-hydroxy)valine-
N'(pyridin-4-yl)carboxamide.
Example 34
34A. Formula (C) Where R3 in t-sutyl~ and R4 and R5 are H
A solution of 2.00 g (6.32 mmol) of the N-
hydroxysuccinimide ester of N-(t-butoxycarbonyl)-L-t-leucine
in 9 mL of distilled aniline was stirred and heated at 100 ~C
for 30 min. The mixture was allowed to cool to room
temperature, and diluted with 40 mL of ethyl acetate. The
solution was washed with 4 x 50 mL of 1 N aq. sodium
bisulfate, and the combined aqueous layers were extracted with
25 mL of ethyl acetate. The combined organic layers were
washed with brine, dried over sodium sulfate, and
concentrated. The residue was purified by chromatography on
silica gel (2~ to 10~ ethyl acetate in dichloromethane) to
give 1.36 g (74~) of N-(t-butoxycarbonyl) -L-t-leucine-N'-
phenylcarboxamide as a white solid: IH NMR (300 MHz, CDCl3)
7.49 (d, 2H, J = 8 Hz), 7.31 (t, 2H, J = 8 Hz), 7.11 (t, lH, J
= 7 Hz), 5.30-5.36 (m, lH), 3.95 (d, lH, J = 9 Hz), 1.44 (s,
9H), 1.07 (s, 9H).
SUBSTITUTE SHEET (RUEE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/lS530
-106-
34B. F~ (C) Where R3 i8 t-Butyl, R4 is 4-Methylthio,
and Rs is H
In a manner analogous to that of part A was prepared N-
(t-butoxycarbonyl)-L-t-leucine-N'-(4-(methylthio)phenyl)-
carboxamide: IH NMR (300 MHz, CDCl3) ~ 7.65 (br s, lH), 7.42
td, 2H, J = 8.5 Hz), 7.21 (d, 2H, J = 8.5 Hz), 5.32 (br d,
lH), 3.95 (d, lH, J = 8.5 Hz), 2.45 (s, 3H), 1.44 (s, 9H),
1.06 (8, 9H).
El. Anal. Calc. for Cl8H23N2O3S: C, 61.33; H, 8.01, N, 7.95,
S, 9.09. Found: C, 61.34; H, 8.06; N, 8.00, S, 9.18.
Example 35
Formula (C) ~a~ing A Trifluoroacetyl Protecting Group, Where R3
i8 t-Butyl, and R4 and R5 are H
To a solution of 1.36 g (4.4 mmol) of N-(t-butoxy-
carbonyl)-L-t-leucine-N~-phenylcarboxamide in 10 mL of
dichloromethane was added 5 mL of trifluoroacetic acid. After
45 min at room temperature, the solution was diluted with
toluene and concentrated. The residue was twice more
concentrated from toluene to remove excess trifluoroacetic
acid, then dried under vacuum (ca. 1 mm Hg). The residue was
then dissolved in 15 mL of dichloromethane and treated
successively with pyridine (0.90 mL, 11 mmol) and
trifluoroacetic anhydride (0.70 mL, 4.84 mmol). After 30 min,
the mixture was partitioned between dichloromethane (25 mL)
and 1 N aq. sodium bisulfate. The organic layer was washed
with additional aq. sodium bisulfate, brine, dried over sodium
sulfate, and concentrated to give 1.22 g (91~) of N-
(trifluoroacetyl)-L-t-leucine-N'-phenylcarboxamide as a white
solid: mp 201-203 ~C, IH NMR (300 MHz, CDCl3) ~ 7.50 (d, 2H,
J = 8 Hz), 7.36 (t, 2H, J = 8 Hz), 7.17 (t, lH, J = 7 Hz),
4.43(d, lH, J = 9 Hz), 1.10 (s, 9H).
El. Anal. Calc. for C~4H~7F3N2O2: C, 55.62; H, 5.67, N, 9.27.
Found: C, 55.57; H, 5.60; N, 9.18.
SUBSTITUTE SHEET ~RLILE 26)
CA 02205665 1997-05-21
WO 96116027 PCTlUS95/lS530
-107-
Examplo 36
Formula (C) Having A Trifluoroacetyl Protecting Group, Where R3
is t-Butyl, R4 ~ 8 4-(2-Hydroxyethyl)aminosulfonyl, and R5 is H
To a solution of 250 mg (0.83 mmol) of N-
(trifluoroacetyl) -L-t-leucine-N'-phenylcarboxamide in 5 mL of -
chloroform was added 0.4 mL (6 mmol) of chlorosulfonic acid.
The mixture was heated to reflux for 35 min and then cooled to
0 ~C and diluted with ethyl acetate. Ethanolamine (1.5 mL)
was added and the mixture was stirred at 0 ~C for 15 min. The
mixture was partitioned between water and ethyl acetate, and
the organic layer was washed with 1 N aq. sodium bisulfate,
dried over sodium sulfate, and concentrated to provide 140 mg
(40~) of N-(trifluoroacetyl) -L-t-leucine-N'-(4-((2-
hydroxyethyl)aminosulfonyl)phenyl)carboxamide: IH NMR (300
MHz, CDCl3) ~ 7.93 (s, lH), 7.84 (d, 2H, J = 9 Hz), 7.67 (d,
2H, J = 9 Hz), 7.15 (br d, lH), 4.90 (br t, lH), 4.49 (d, lH,
J = 9 Hz), 3.70 (t, 2H, J = 5 Hz), 3.11 (q, 2H, J = 5 Hz),
1.12 (s, 9H).
Example 37
Formula (D) Where * is t-Butyl, R4 is 4-(2-Hydroxyethyl)-
aminosulfonyl, and R5 is H
To a solution of 257 mg (0.6 mmol) of N-(trifluoroacetyl)
-L-t-leucine-N'-(4-((2-hydroxyethyl)aminosulfonyl)phenyl)-
carboxamide in 8 mL of ethanol was added 227 mg (6 mmol) of
sodium borohydride. The mixture was heated to 55 ~C for 15
min, allowed to cool to room temperature, and quenched with
10~ ammonium hydroxide in methanol. After 20 h at room
temperature, the mixture was filtered and the filtrate was
absorbed onto silica gel. Chromatography (dichloromethane to
90:9:1 dichloromethane:methanol:ammonium hydroxide) gave 110
mg (56~) of L-t-leucine-N'-(4-((2-hydroxyethyl)amino-
sulfonyl)phenyl)carboxamide, as an oil: IH NMR (300 MHz,
CDCl3) ~ 9.48 (br s, lH), 7.81 (d, 2H, J = 9 Hz), 7.72 (d, 2H,
J = 9 Hz), 3.67 (t, 2H, J = 5 Hz), 3.30 (s, lH), 3.08 (q, 2H,
J = 5 Hz), 1.06 (s, 9H).
FA3-MS (M+H+): expected 330.1488 observed 330.1480
!PJBS~llUlt SHEr (~E 2B)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/lS530
-108-
Example 38
Formula (Ib) Where RZ is Biphenyl (X i~ propane-1,3-diyl),
R3 is t-Butyl, R4 is 4R/S-Methylsulfinyl, and R5 is ~
To a solution of 60.3 mg (0.100 mmol) of N-(2R-(t-
butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine
N'-(4-(methylthio)phenyl)carboxamide in 2 mL of
dichloromethane at -78 ~C was added a solution of 26 mg (0.15
mmol) of m-chloroperbenzoic acid in 1 mL of dichloromethane.
The reaction was stirred at -78 ~C for 50 min, and then 0.2
mL of dimethyl sulfide was added. The mixture was allowed to
warm to room temperature, and then partitioned between
dichloromethane and sat. aq. sodium bicarbonate. The organic
layer was washed with brine, dried over sodium sulfate, and
concentrated to give 59.6 mg (96~) of N-(2R-(t-butoxy-
carbonyl)methyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine -N'-
(4R/S-(methylsulfinyl)phenyl)carboxamide, as a white solid:
IH NMR (300 MHz, CDCl3) ~ 8.46 (br s, lH), 7.32-7.68 (m, 11
H), 7.14 (d, 2H, J = 8 Hz), 6.63 (d, lH, J = 9Hz), 4.42 (d,
lH, J = 9 Hz), 2.36-2.72 (m, 8H), 1.42-1.76 (s overlapping m,
13 H), 1.09 (s, 9H).
Example 39
Formula (F")
To a solution of 2.779 g (7.50 mmol) of N-(2R-(t-
butoxycarbonyl)-methyl-4-pentenoyl)-4S-phenylmethyl-2-
oxazolidinone in 30 mL of THF at 0 ~C was added 2.55 mL (22.5
mmol) of 30~ aq. hydrogen peroxide, followed by the addition
of 7.5 mL (15 mmol) of 2.0 N aq. lithium hydroxide. The
mixture was ~tirred for 2 h at 0 ~C and for 0.5 h at room
temperature. After the mixture was recooled to 0 ~C, 15 mL of
2 M aq. sodium sulfite and 23 mL of sat. aq. sodium
bicarbonate were added. The mixture was stirred an additional
30 min at 0 ~C, and then most of the THF was removed by
concentration in vacuo. The residue was partitioned between
CH2Cl2 and H2O, and the aq. layer was extracted with additional
CH2Cl2. The combined organic layers were extracted with aq.
sodium bicarbonate, and then the combined a~. layers were
SUBSTITUTE SHEET (RULE 26)
CA 02205665 1997-05-21
WO 96/16027 PCI~/US95/15530
-109-
acidified to pH 2 with sodium bisulfate. The resulting
mixture was extracted twice with ethyl acetate, and the
combined organic layer~ were washed with sat. aq. NaCl,
diluted with 0.25 volume of hexane, dried over Na2S04, and
concentrated to provide 1.47 g (92~) of 2R-(t-butoxycarbonyl)-
methyl-4-pentenoic acid as a colorless oil: 1H NMR (300 MHz,
CDCl3) ~ 5.68-5.82 (m, lH), 5.07-5.15 (m, 2H), 2.86-2.96 tm,
lH), 2.60 (dd, lH, J = 18 and 10 Hz), 2.25-2.52 (m, 3H), 1.43
(s, 9H).
Example 40
Formula (D'-l) Where R3 is t-Butyl and R7 is 4-(methoxy-
~h~nyl ) phenyl
In a m~nner analogous to Example 31, substituting 2R-(t-
butoxycarbonyl)methyl-(5-(biphen-4-yl)pentanoic acid with
2R-(t-Butoxycarbonyl)methyl-4-pentenoic acid, and substituting
L-t-leucine-N'-(4-(pyrid-4-yl)carboxamide with L-leucine-N'-
(4-(methoxycarbonyl)phenyl)carboxamide, there was prepared N-
(2R-(t-butoxycarbonyl)methyl-4-pentenoyl)-L-leucine-N'-(4-
(methoxycarbonyl)phenyl)carboxamide: mp 118-119 ~C
(cyclohexane); IH NMR (300 MHz, CDCl3) ~ 8.96 (s, lH), 7.95
(d, 2H, J = 9Hz), 7.57 (d, 2H, J = 9 Hz), 6.32 (d, lH, J =
7Hz), 5.63-5.77 (m, lH), 4.95-5.08 (m, 2H), 4.52-4.60 (m, lH),
3.88(s, 3H), 2.36-2.77 (m, 4H), 2.14-2.27 (m, lH), 1.60-1.87
(m, 3H), 1.45 (s, 9H), 0.97 (d, 3H, J = 7 Hz), 0.91 (d, 3H, J
= 7 Hz)-
El. Anal. Calc. for C~H36N206: C, 65.20; H, 7.88, N, 6.08.
Found: C, 65.04; H, 7.80; N, 6.06.
Example 41
41A. Formula ~D'-2) Where R2 is Fluoren-2-yl (X is propane-1,3-
diyl), R3 is t-Butyl, and R7 i~ 4-(methoxycarbonyl)phenyl
To a solution of 167 mg (0.36 mmol) of N-(2R-(t-
butoxycarbonyl)-methyl-4-pentenoyl)-L-leucine-N'-(4-
(methoxycarbonyl)phenyl)carboxamide, 108 mg (0.44 mmol) of 2-
bromofluorene, 21 mg (0.07 mmol) of tri-o-tolylphosphine, and
69 ~L (50 mg, 0.50 mmol) of triethylamine in 1.0 mL of DMF
CA 0220~66~ l997-0~-2l
WO96/16027 PCT~S95/lSS30
-110-
under argon was added 8.0 mg (0.035 mmol) of palladium
diacetate. The solution was heated at 100 ~C for 2 h, cooled
to room temperature, and then partitioned between 3:1 ethyl
acetate:hexane and water. The organic layer was washed with
lN aq. sodium bisulfate and with brine/pH 7 buffer, dried over -
sodium sulfate, and concentrated. Purification by flash
chromatography (20 g silica, 5~ to 10~ t-butyl methyl ether in
dichloromethane) gave 185 mg (82~) of the product as a solid
containing trace impurities by TLC. Recrystallization from t-
butyl methyl ether/isooctane provided 115 mg (51~) of N-(2R-
(t-butoxycarbonyl)methyl-5-(fluoren-2-yl)-4E-pentenoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide as fine
white needles: mp 189-192 ~C; IH NMR (300 MHz, CDCl3)
8.32 (s, lH), 7.79 (d, 2H, J = 8 Hz), 7.72 (d, lH, J = 8 Hz),
7.57 (d, lH, J = 8 Hz), 7.45-7.53 (m, 3 H), 7.36 (t, lH, J =
7.5 Hz), 7.25-7.32 (m, 2H), 7.17 (d, lH, J = 7 Hz), 4.52-4.60
(m, lH), 3.75 (s, 5H), 2.33-2.87 (m, 5H), 1.60-1.90 (m, 3H),
1.45 (s, 9H), 0.92 (apparent t, 6H).
El. Anal. Calc. for C38H~N2O6~0.5 H2O: C, 72.01; H, 7.16, N,
4.42. Found: C, 71.87; H, 7.07; N, 4.32.
41B. Formula (D'-2) Where R2 is 7-(N-(benzyloxycarbonyl)-
glycyl)aminofluoren-2-yl (X is propane-1,3-diyl),
R3 is t-Butyl, and R7 is 4-(methoxycarbonyl)phenyl
To a solution of 600 mg (2.31 mmol) of 2-amino-7-
bromofluorene and 483 mg (2.31 mmol) of N-(benzyloxycarbonyl)-
glycine in 10 mL of anhydrous pyridine was added 442 mg (2.31
mmol) of EDC hydrochloride. The reaction was heated at 60 ~C
for 4 days, and then the solution was concentrated. The
residue was partitioned between ethyl acetate and 1 N aq.
hydrochloric acid, and the organic layer was washed with sat.
aq. sodium bicarbonate and with brine, dried over magnesium
sulfate, and concentrated to give 813 mg (78~) of N-
(benzyloxycarbonyl)glycine-N'-(7-bromofluoren-2-yl)carboxamide
as a tan solid: mp 194-195 ~C.
By following the procedure of part A and substituting 2-
bromofluorene with N-(benzyloxycarbonyl)glycine-N'-(7-
SUBSrITUTE SHEET (RULE 26)
,
CA 0220~66~ l997-0~-2l
WO96/l6027 PCT~S9S/15530
-111-
bromofluoren-2-yl)carboxamide, there is obtained N-(2R-(t-
butoxycarbonyl)methyl-5-(7-(N-(benzyloxy-carbonyl)glycyl)-
aminofluoren-2-yl)-4E-pentenoyl)-L-leucine-N'-(4-
(methoxycarbonyl)phenyl)carboxamide: mp 213-214 ~C.
FAB-MS (M+Cs)+ calculated for C48H~N4Og~Cs: 963.2945;
observed: 963.2960.
El. Anal. Calc. for C47H54N409: C, 69.40; H, 6.51, N, 6.75.
Found: C, 69.47; H, 6.51; N, 6.70.
41C. Formula ~D~-2) Where R2 is 4-(pyrid-4-yl)phenyl (X iR
propane-1,3-diyl), R3 i~ t-Butyl, and R7 i8 4-(methoxy-
carbonyl)phenyl
Aq. 2 M sodium carbonate (3 m~)was added to a suspension
of 400 mg (2.0 mmol) of 4-bromopyridine in 2 mL of benzene to
give 2 clear phases, and argon was bubbled through the
mixture for a few minutes before added 115 mg (0.10 mmol) of
palladium tetrakis(triphenylphosphine). To the resulting
mixture was added a solution of 200 mg (1.00 mmol) of 4-
bromophenylboronic acid in 1 mL of ethanol, and the mixture
was heated at reflux for 4 h. After cooling to room
temperature, the mixture was partitioned between ethyl acetate
(25 m~) and water (25 mL). The organic layer was dried over
sodium sulfate and concentrated. Purification of the residue
by silica gel chromatography, eluting with 25~ to 50~ ethyl
acetate in h~x~ne~ gave 193 mg (82~) of 4-(4-bromophenyl)-
pyridine as a white solid: mp 124-126 ~C.
By following the procedure of part A and substituting 2-
bromofluorene with 4-(4-bromophenyl)pyridine, there is
obtained N-(2R-(t-butoxy-carbonyl)methyl-5-(4-(pyrid-4-yl)-
phenyl)-4E-pentenoyl)-~-leucine-N'-(4-(methoxycarbonyl)-
phenyl)carboxamide: IH NMR (300 MHz, CDCl3) ~ 8.80 (s, lH),
8.64 (d, 2H, J = 6 Hz), 7.85 (d, 2H, J = 8.5 Hz), 7.49 (d, 2H,
J = 9 Hz), 7.45 (d, 2X, J = 6 Hz), 7.19 (d, 2H, J = 8 Hz),
6.40 (d, lH, J = 16 Hz), 6.33 (d, lH, J = 8 Hz), 6.09-6.17 (m,
lH), 4.54-4.57 (m, lH), 3.80 (s, 3H), 2.38-2.81 (m, 5H), 1.48-
1.84 (m, 3H), 1.44 (s, 9H), 0.90 and 0.94 (2 d, 6H, J = 7 Hz).
SUBSTITUTE SH~ET (RULE 26)
CA 0220~66~ l997-0~-2l
WO96/16027 PCT~S95/lSS30
-112-
Example 42
42A. Formula (Ib~) Where R2 is Fluoren-2-yl (X i~ propane-1,3-
diyl), R3 i8 t-Butyl, and R7 i8 4-(methoxycarbonyl)phenyl
A solution of 111 mg (0.177 mmol) of N-(2R-(t-
butoxycarbonyl)methyl-5-(fluoren-2-yl)-4-pentenoyl)-L-leucine-
N'-(4-(methoxycarbonyl)phenyl)carboxamide in 7 mL of 4:3 ethyl
acetate:ethanol was hydrogenated at 1 atm hydrogen pre~sure
over 30 mg of 10~ palladium on carbon for 3 h. The catalyst
was removed by filtration through Celite, and the filtrate was
concentrated. Trituration with t-butyl methyl ether gave 110
mg (99~) of N-(2R-(t-butoxycarbonyl)methyl-5-(fluoren-2-
yl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)phenyl)-
carboxamide as a white solid: mp 166-167 ~C (softening at 161
~C) .
El. Anal. Calc. for C37H~N2O6: C, 72.29; H, 7.54, N, 4.56.
Found: C, 72.32; H, 7.54; N, 4.62.
42B. Formula (Ib') Varying R2
By following the procedure of part A and substituting N-
(2R-(t-butoxycarbonyl)methyl-5-(fluoren-2-yl)-4-pentenoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide with:
N-(2R-(t-butoxycarbonyl)methyl-5-(7-(N-(benzyloxy-
carbonyl)glycyl)aminofluoren-2-yl)-4E-pentenoyl)-L-
leucine-N'-(4-(methoxycarbonyl)phenyl)carboxamide; and
N-(2R-(t-butoxycarbonyl)methyl-5-(4-(pyrid-4-yl)phenyl)-4E-
pentenoyl)-L-leucine-N'-(4-(methoxycarbonyl)phenyl)-
carboxamide,
there are obtained:
N-(2R-(t-butoxycarbonyl)methyl-5-(7-(glycyl)aminofluoren-
2-yl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)-
phenyl)carboxamide:
F~3-MS (M+H)+ calculated for C4~slN4O7: 699.3758;
observed: 699.3770; and
N-(2R-(t-butoxycarbonyl)methyl-5-(4-(pyrid-4-yl)phenyl)-
pentanoyl)-L- leucine-N'-(4-(methoxycarbonyl)-
phenyl)carboxamide: mp 174-176 ~C; IH NMR (300 MHz,
CDCl3) ~ 8.85 (s, lH), 8.63 (d, 2H, J = 5 Hz), 7.95 (d,
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S9S/15S30
-113-
2H, J = 8 Hz), 7.57 (d, 2H, J = 9 Hz), 7.41-7.47 (m, 4H),
7.07 (d, 2H, J = 8 Hz), 6.19 (d, lH, J = 7 Hz), 4.53-4.56
(m, lH), 3.85 (s, 3H), 2.37-2.6~ (m, 5H), 1.45-1.83 (m,
7H), 1.42 (s, 9H), 0.91 and 0.95 (2 d, 6H, J = 7 Hz).
Example 43
Fc_ ~- A (C-l) Where R~ and R5 are ~
To a stirred suspension of 4.18 g (20.0 mmol) of
N-(benzyloxycarbonyl)glycine, 2.73 mL (2.79 g, 30 mmol) of
aniline, and 110 mg (1.0 mmol) of 4-dimethylaminopyridine in
55 mL of dichloromethane at 0 ~C was added 6.53 g (22 mmol) of
EDC methiodide in one portion. The mixture was stirred for 18
h at room temperature, and then partitioned between 200 mL of
3:1 ethyl acetate:h~ne and water. The organic layer was
washed with 1 N aq. sodium bisulfate, sat. aq. sodium
bicarbonate, and finally with brine/pH 7 phosphate buffer,
dried over sodium sulfate, and concentrated.
Recrystallization from 1:1 ethyl acetate/isooctane gave 3.69 g
(65~) of N-(benzyloxycarbonyl)glycine-N'-phenylcarboxamide:
mp 143-144 ~C.
Example 44
FG 1~ (C-2) Where R4 and R5 are H
To a stirred solution of 1.42 g (5.00 mmol) of N-
(benzyloxycarbonyl)glycine-N'-phenylcarboxamide in 35 mL of
dry THF at -5 ~C was added by syringe 6.15 mL (16.0 mmol) of
2.6 M n-butyllithium in hexane at a rate to maintain the
reaction temperature below 10 ~C. After ca. 2/3 of the n-
butyllithium had been added, a yellow color began to persist,
and the addition was stopped for ca. 10 min and then resumed
in a dropwise fashion so as to maintain the reaction
temperature at about 0 ~C. After the addition was complete,
the orange solution was stirred at 0 ~C for 45 min, and then
cooled to -70 ~C. Acetone (1.10 mL, 15 mmol) was added in one
portion by syringe. After 10 min, the reaction was
partitioned between 1 M pH 7 phosphate buffer and 3:1 ethyl
acetate:hexane. The organic layer was washed with brine,
SU~ ult SHE~ p~UlE26)
,
CA 0220~66~ 1997-0~-21
WO 96/16027 PCT/US95/lSS30
-114-
dried over sodium sulfate, and concentrated. The residue was
purified by chromatography on 75 g of silica, eluting with 40
ethyl acetate:hexane. First to elute was pure products
fractions (pool #1), followed by fractions containing product
and the starting glycinanilide (pool #2). The residue from
pool ~2 was recrystallized from ethyl acetate:isooctane to
give nearly pure starting material as a solid, and mother
liquors containing mostly product. The residue from the
concentration of the mother li~uors was purified by radial
chromatography (4 mm plate, 30~ ethyl acetate:he~ne), and the
product fractions were combined with pool #l to give, after
trituration of the gummy residue with hexane/t-butyl methyl
ether, 423 mg (25~) of N-(benzyloxycarbonyl)-DL-,l~-
hydroxyvaline-Nl-(phenyl)carboxamide as a pale yellow solid:
mp 128-129 ~C; IH NMR (300 MHz, CDC13) ~ 8.30 (br s, lH),
7.39 (d, 2H, J = 8 Hz), 7.18-7.28 (m, 7H), 7.06 (t, lH, J = 7
Hz), 5.83 (br d, lH), 5.05 (s, 2H), 4.04 (d, lH, J = 9 Hz),
3.73 (s, lH), 1.33 (s, 3H), 1.16 (s, 3H).
El. Anal. Calc. for ClgH22N2O4: C, 66.65; H, 6.48, N, 8.18.
Found: C, 66.66; H, 6.57; N, 8.14.
Example 45
I~G la (C-2) Where R4 and R5 are H
A solution of 400 mg (1.17 mmol) of N-(benzyloxy-
carbonyl)-DL-~B-hydroxyvaline-N~-phenylcarboxamide in 10 mL of
ethyl acetate was hydrogenated over 50 mg of 10~ palladium on
carbon at 1 atm of hydrogen pressure for 1.5 h. The catalyst
was removed by filtration through Celite, and the filtrate was
concentrated to give 259 mg (>100~) of D~ -hydroxyvaline-N'-
(phenyl)carboxamide, which was used without further
purification: mp 97-99 ~C.
El. Anal. Calc. for CIlH~6N2O2: C, 63.44; H, 7.74, N, 13.45.
Found: C, 63.52; H, 7.79; N, 13.40.
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-21
WO 96116027 PCT/US95/1SS30
- l l 5 -
Example 46
Formula (Ib) W~re R2 i8 Biphenyl, R3 i~3 Hydroxy-t-butyl, and R4
and R5 are H
To a solution of 203 mg (0.55 mmol) of 2R- (t-butoxy-
carbonyl)methyl(5-(biphen-4-yl)pentanoic acid, 104 mg (0.50
mmol) of DL-~-hydroxyvaline-N'-(phenyl)carboxamide, and 90 ~L
(0.65 mmol) of triethylamine in 2.5 mL of DMF was added 265 mg
(0.60 mmol) of benzotriazol-1-yl-tris-(dimethylamino)-
phosphonium hexafluorophosphate. After 24 h, the reaction was
partitioned between 3:1 ethyl acetate:hexAne and ca. 0.2 N aq.
sodium bicarbonate. The organic layer was washed with lN aq.
sodium bisulfate and with brine/pH 7 buffer, dried over sodium
sulfate, and concentrated. The residue was purified by radial
chromatography (4 mm plate), eluting with 25~ to 30~ ethyl
acetate in hexane. First to elute was 121 mg (43~) of N-(2R-
(t-butoxycarbonyl)methyl-5-(biphen-4-yl)pentanoyl)-D-~-
hydroxyvaline-NI-(phenyl)carboxamide (diastereomer), followed
by 140 mg (50~) of N-(2R-(t-butoxycarbonyl)methyl-5-(biphen-4-
yl)pentanoyl)-L-~-hydroxyvaline-N'-(phenyl)carboxamide as a
gummy semi-solid containing, according to NMR analysis, about
1 mole-equivalent of isooctane (the solvent from which the
final sample was concentrated): ~H NMR (300 MHz, CDCl3) ~ 8.77
(s, lH), 7.24-7.56 (m, 11 H), 7.03-7.14 (m, 3H), 6.89 (d, lH,
J = 8.5 Hz), 4.46 (d, lH, J = 8.5 Hz), 4.17 (s, lH), 2.52-2.70
(m, 4H), 2.36 (br d, lH, J = 12.5 Hz), 1.50-1.70 (m, 4H), 1.43
(s, 3H), 1.40 (s, 9H), 1.25 (s, 3H).
Example 47
Formula (P-1)
To a solution of 510 mg (1.97 mmol) of N-(4-pentenoyl)-
t 4S-phenylmethyl-2-oxazolidinone in 8 mL of dichloromethane at
0 ~C was added 2.2 mL (2.2 mmol) of 1 M titanium tetrachloride
in dichloromethane. After 15 min, 0.42 mL (2.4 mmol) of
diisopropylethylamine was added to the thick slurry to give a
deep red solution. After 1 h at 0 ~C, 216 mg (2.4 mmol) of s-
trioxane in 2 mL of dichloromethane was added via cannula,
followed by an additional 2.2 mL of lM titanium tetrachloride
SUBSTtTUTE SHEET (RULE 2~)
_ _ _
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S9S/lS530
-116-
in dichloromethane. After 4 h at 0 ~C, the solution was
partitioned between aq. ammonium chloride and dichloromethane.
The organic layer washed with 1 N aq. HCl, with brine
containing pH 7 phosphate buffer, dried over sodium sulfate,
and concentrated. The residue was purified by chromatography
on 20 g silica, eluting with 30~ to 40~ ethyl acetate in
h~ne. Recrystallization of the purified product from t-
butyl methyl ether/isooctane provided 404 mg (71~) of N-(2R-
hydroxymethyl-4-pentenoyl)-4s-phenylmethyl-2-oxazolidinone:
mp 71-72 ~C; IH NMR (300 MHz, CDC13) ~ 7.22-7.37 (m, 5H), 5.78
(dddd, J = 10, 7, 4, and 3 Hz), 5.03-5.15 (m, 2H), 4.69 (dddd,
lH, J = 10, 6, 4, and 3 Hz), 4.17-4.24 (m, 2H), 4.02-4.10 (m,
lH), 3.85-3.91 (m, 2H), 3.29 (dd, lH, J = 4 and 3 Hz), 2.82
(dd, lH, J = 14 and 10 Hz), 2.44 (dt, lH, J = 14 and 7 Hz),
2.31 (dd, lH, J = 14 and 7 Hz), 2.17 (br s, lH).
Ex~mple 48
Formula (P-2)
To a suspension of 4.0 g (25.1 mmol) of O-benzyl-
hydroxylamine hydrochloride in 50 mL of THF at 0 ~C under
argon was added 11.4 mL (22.8 mmol) of 2M trimethylaluminum in
toluene. After the addition was complete, the solution was
allowed to warm to room temperature. After 15 min, this
solution was added via cannula to a solution of 2.40 g (8.30
mmol) of N-(2R-hydroxymethyl-4-pentenoyl)-4S-phenylmethyl-2-
oxazolidinone in loO mL of THF at 0 ~C under argon. The
reaction was stirred for 6 h at 0~C, and then partitioned
between lN HCl/brine and ethyl acetate/diethyl ether. The
organic layer was with lM pH 7 phosphate buffer and with
brine, dried over sodium sulfate, and concentrated. The
residue was purified by chromatography on silica, eluting
with 35~ to 45~ ethyl acetate in hexane, to give, after
elution of 4S-phenylmethyl-2-oxazolidinone, 2.01 g of the
product. Recrystallization from ethyl acetate: isooctane
provided 1.90 g (97~) of N-benzyloxy-2R-hydroxymethyl-4-
pentenamide as a white powder: mp 58-59 ~C; IH NMR (300 MHz,
CDCl3) ~ 8.40 (s, lH), 7.38 (m, 5H), 5.71 (m, lH), 5.03-5.06
SUBSTITUTE SHEET (RULE 26)
CA 0220~66~ l997-0~-2l
wos6ll6o27 PCT~S9S/1~530
-117-
(m, lH), 4.91 (dd, lH, J = 16 and 12 Hz), 3.73 (m, lH), 2.20
(m, 2H).
El. Anal. Calc. for Cl3Hl7NO3: C, 66.36; H, 7.28, N, 5.95.
Found: C, 66.15; H, 7.32; N, 5.99.
Example 49
Formula (P-3)
To a solution of 1.92 g (8.17 mmol) of N-benzyloxy-2R-
hydroxymethyl-4-pentenamide in 10 mL of anhydrous pyridine at
0~C was added 1.24 mL (16.3 mmol) of mesyl chloride. After 3
h, the reaction was poured onto ice, and the mixture was
partitioned between ethyl acetate and 1 N aq. sodium
bisulfate. The organic layer was washed with additional
sodium bisulfa~e, and the combined aqueous layers were
extracted with ethyl acetate. The combined organic layers
were dried over sodium sulfate and concentrated. The residual
oil was dissolved in 30 m~ of acetone and 3.38 g of powdered
pota~sium carbonate was added. The mixture was heated at
reflux for 3 h and then cooled to room temperature. The
precipitate was removed by filtration through Celite, and the
filter cake was washed well with ethyl acetate. The filtrate
was concentrated, and the residue was purified by
chromatography on silica, eluting with 25~ ethyl acetate in
hexane, to provide 1.64 g (93~) of N-benzyloxy-3R-(2-propen-1-
yl)-2-azetidinone as a slightly orange oil: IH NMR (300 MHz,
CDCl3) ~ 7.37-7.42 (m, 5H), 5.65-5.75 (m, lH), 5.00-5.06 (m,
lH), 4.93 (s, 2H), 3.32 (ddd, lH, J = 5, 4, and 2 Hz), 2.95
(m, 2H), 2.40-2.47 (m, lH), 2.20-2.28 (m, lH).
El. Anal. Calc. for Cl3H,sNO2: C, 71.86; H, 6.96, N, 6.45.
Found: C, 71.59; H, 6.88; N, 6.37.
Example 50
Formula (P-4) Where R2 i~ Biphenyl
A solution of 434 mg (2.00 mmol) of N-benzyloxy-3R-(2-
propen-1-yl)-2-azetidinone, 583 mg (2.5 mmol) of 4-
bromobiphenyl, 0.34 mL (2.5 mmol) of triethylamine, 35 mg
(0.11 mmol) of tri(o-tolyl)phosphine, and 14 mg (0.06 mmol) of
SIlBSIIlu~ SHEl (Rlll~ X~
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95115530
-118-
palladium diacetate in 7 mL of DMF was heated at 100 ~C for 18
h. The reaction solution was cooled to room temperature and
partitioned between ethyl acetate and water. The organic
layer was washed with water, dried over sodium sulfate, and
concentrated. The residue was chromatographed on silica,
eluting with 25~ ethyl acetate in hexane, to give slightly
impure product, which was recrystallized from ethyl
acetate/isooctane to give 315 mg (43~) of N-benzyloxy-3R-(3-
(biphen-4-yl)-2-propen-1-yl)-2-azetidinone as small white
flakes: mp 109-110 ~C; IH NMR (300 MHz, CDC13) ~ 7.31-7.61
(m, 14H), 6.43 (d, lH, J = 15 Hz), 6.18 (ddd, lH, J = 15, 9,
and 7 Hz), 4.94 (s, 2H~, 3.36 (dd, lH, J = 10 and 5 Hz), 3.00-
3.04 (m, 2H), 2.60-2.75 (m, lH), 2.20-2.50 (m, lH).
El. Anal. Calc. for C~H~NO2: C, 81.27; H, 6.28, N, 3.79.
Found: C, 81.09; H, 6.31; N, 3.71.
Example 51
Formula (P-5) Where R2 i Biphenyl
To a stirred solution of 62.0 mg (0.168 mmol) of N-
benzyloxy-3R-(3-(biphen-4-yl)-2-propen-1-yl)-2-azetidinone in
5 mL of 4:1 THF:ethanol was added 2 mL of lN aq. lithium
hydroxide. The mixture was stirred vigorously for 1 h at room
temperature, and the diluted with 10 mL of 0.5 M pH 4 citrate
buffer. The mixture was partitioned between 20 mL of t-butyl
methyl ether and brine, and the organic layer was dried, after
dilution with ca. 5 mL hexane, over sodium sulfate, and
concentrated to a residual glass. This residue was
immediately dissolved in 5 mL of dichloromethane, cooled to 0
~C, and 0.10 mL of pyridine was added, followed by 1.2 mL of a
solution of formic anhydride in dichloromethane, which was
prepared by allowing 297 mg (1.00 mmol) of EDC methiodide and
80 ~L (2.00 mmol) of formic acid in 5 mL of dichloromethane to
react at 0~C for 15 min. After 30 min, the reaction was
partitioned between dichloromethane and 0.5 M pH 3 citrate
buffer. The organic layer was dried over sodium sulfate and
concentrated. Chromatography of the residue on 5 g of silica,
eluting with a gradient of 5~ to 10~ ethanol in
S~ t s~Er ~IIE~)
CA 02205665 1997-05-21
WO 96ll6027 PCT/US95115!i30
-119-
dichloromethane, gave 58 mg (83~) of N-((N"-formyl-N"-
benzyloxyamino)methyl-5-(biphen-4-yl)-4-pentenoic acid as a
glass. IH NMR spectrum at room temperature in CDCl3 showed
broad peaks of amide rotamers.
Example 52
Formula (P-6) Where R2 i8 Biphenyl, R3 i8 t-Butyl, R7 iR 4-
Pyridyl and p i~ 2ero
To a solution of 99.6 mg (0.24 mmol) of N-((N"-formyl-N"-
benzyloxyamino)methyl-5-(biphen-4-yl)pentanoic acid, 125 mg
(0.288 mmol) of L-t-leucine-N'-(pyridin-4-yl)carboxamide
bis(trifluoroacetate), and 0.125 mL (0.90 mmol) of
triethy~amine in 4 mL of DMF was added 133 mg (O.30 mmol) of
benzotriazol-l-yl-tris-(dimethylamino)phosphonium
hexafluorophosphate. After 16 h at room temperature, the
reaction was partitioned between ethyl acetate and ca. 0.5 M
aq. sodium bicarbonate. The organic layer was washed with 1 M
pH 7 phosphate buffer and with brine, dried over sodium
sulfate, and concentrated. Purification of the residue by
chromatography, eluting with 40~ to 75~ ethyl acetate in
hexane, followed by recrystallization from ethyl
acetate/isooctane gave 97.4 mg (67~) of N-((N"-formyl-N"-
benzyloxyamino)methyl-5-(biphen-4-yl)-4-pentenoyl)-L-t-
leucine-N'-(pyridin- 4-yl)carboxamide: mp: 215-216 ~C
Example 53
53A. Formula (Ih) Where R2 iR Biphenyl, R3 i8 t-Butyl, R7 i~ 4-
Pyridyl and p i8 Zero
A solution of 87.1 mg (0.143 mmol) of N-((N"-formyl-N"-
benzyloxyamino)methyl-5-(biphen-4-yl)pentanoyl)-L-t -leucine-
N'-(pyridin-4-yl)carboxamide in 5 mL of 3:2 ethyl acetate/
ethanol was hydrogenated over 25 mg of 10~ palladium on carbon
at 1 atm of hydrogen pressure for 6 h. The catalyst was
removed by filtration through Celite, and the ~iltrate was
concentrated. Recrystallization of the residue from ethyl
acetate gave 59.0 mg (80~) of N-((N"-formyl-N"-hydroxy-
amino)methyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-N'-
SUBSTITUTE SltEET (RULE 26)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/lS530
-120-
(pyridin-4-yl)carboxamide as a white powder: mp 190-191 ~C;
H NMR (300 MHz, DMSO-d6) ~ 10.52 (br s, lH), 9.97 (br s, lH,
9.53 (br s, lH), 8.38 (d, 2H, J = 7 Hz), 7.20 (br 8, lH), 7.14
(br d, lH, J = 8 Hz), 7.23-7.60 (m, 9H), 7.12 (d, 2H, J = 7
Hz), 4.41 (d, lH, J = 9 Hz) 3.40-3.62 (m, 2H), 2.90-3.10 (m,
lH), 2.4-2.6 (m, 2H, partially obscured by DMSO-d5 resonance),
1.28-1.52 (m, 4H), 0.94 (s, 9H).
El. Anal. Calc. for C3~36N4O4~0.5 HzO: C, 68.55; H, 7.10, N,
10.66. Found: C, 68.48; H, 7.04; N, 10.63.
53B. Formula (Ih) Varying R2, R3, and R7
By following procedures analogous to Examples 50-52 there
are obtained the following compounds of formula (P-6):
N-(2R-(N"-formyl-N"-benzyloxyamino)methyl-5-(4-(2RS-hydroxy-
3,3,3-trifluoropropyl)phenyl)-4-pentenoyl)-L-t-leucine-
N'-(pyridin-4-yl)carboxamide;
N-(2R-(N"-formyl-N"-benzyloxyamino)methyl-5-(4-(imidazol-4-
yl)phenyl)-4-pentenoyl)-L-threonine-N'-((4S-
methylsulfinyl)phenyl)carboxamide;
N-(2R-(N"-formyl-N"-benzyloxyamino)methyl-5-(4-(2RS-hydroxy-
3,3,3-trifluoropropyl)phenyl)-4-pentenoyl)-L-t-leucine-
N'-(pyrid-4-yl)carboxamide;
N-(2R-(N"-formyl-N"-benzyloxyamino)methyl-5-(4-(imidazol-4-
yl)phenyl)-4-pentenoyl)-L-threonine-N'-((4S-methyl-
sulfinyl)phenyl)carboxamide;
N-(2R-(N"-formyl-N"-benzyloxyamino)methyl-5-(4-(pyridin-4-
yl)phenyl)-4-pentenoyl)-L-t-leucine-N'-(4-((2-
hydroxyethyl)-aminosulfonyl)phenyl)carboxamide;
N-(2R-(N"-formyl-N"-benzyloxyamino)methyl-5-(4-(pyridin-4-
yl)phenyl)-4-pentenoyl)-L-(~-hydroxy)valine-N'-(4S-
methylsulfinyl)phenyl)carboxamide;
N-(2R,S-(N"-formyl-N"-benzyloxyamino)methyl-(4-(methyl)-4-
pentenoyl)-L-leucine-N'-(4-(methoxycarbonyl)phenyl)-
carboxamide; and
N-(2R-(N"-formyl-N"-benzyloxyamino)methyl-5-(biphen-4-
yl)pentanoyl)-L-cyclohexylglycine-N'-(4-((2-(dimethyl-
amino)ethyl)aminosulfonyl) phenyl)carboxamide,
~ ult S~I ~mE 26)
CA 02205665 1997 - 05 - 21
WO 96/16027 PCT/US9511S530
-121 -
which, when ~ubstituted for N-(~N"-formyl-N"-benzyloxyamino)-
methyl-5-(biphen-4-yl)pentanoyl)-L-t-leucine-N'-(pyridin-4-
yl)carboxamide in the procedure of Example 53A, give the
following respective compound8:
N-(2R-(N"-formyl-N"-hydroxyamino)methyl-5-(4-(2RS-hydroxy-
3,3,3-trifluoropropyl)phenyl)pentanoyl)-L-t-leucine-N'-
(pyridin-4-yl)carboxamide;
N-(2R-(N"-formyl-N"-hydroxyamino)methyl-5-(4-(imidazol-4-
yl)phenyl)-pentanoyl)-L-threonine-N'-((4S-
methylsulfinyl)phenyl)carboxamide;
N-(2R-(N"-formyl-N"-hydroxyamino)methyl-5-(4-(pyrid-4-
yl)phenyl)pentanoyl)-L-t-leucine-N'-(4-((2-
hydroxyethyl)aminosulfonyl)- phenyl)carboxamide;
N-(2R-(N''-formyl-N'I-hydroxyamino)methyl-5-(4-(pyrid-4-
yl)phenyl)pentanoyl)-L-(~-hydroxy)valine-N'-(4S-
methyl~ulfinyl)phenyl)carboxamide;
N-(2R,S-(N"-formyl-N"-hydroxyamino)methyl-(4-
(methyl)pentanoyl)-L-leucine-N'-(4-(methoxycarbonyl)-
phenyl)carboxamide: MS (M-H)-: 434.2;(M-CO, H2O): 388; and
N-(2R-(N"-formyl-N"-hydroxyamino)methyl-5-(biphen-4-
yl)pentanoyl)-L-cyclohexylglycine-N'-(4-((2-
(dimethylamino)ethyl)aminosulfonyl)phenyl)carboxamide.
Examples 54-59
These examples illustrate the preparation of a
representative pharmaceutical formulation containing an active
compound of formula (I), e.g., N-(2R-carboxymethyl-5-(biphen-
4-yl)pentanoyl)-L-~-hydroxyvaline-N'-(phenyl)carboxamide, or
a pharmaceutically acceptable salt thereof. Other compounds
of formula (I) can be used as the active compound in
preparation of the formulations of these examples.
CA 0220~66~ 1997-0~-21
WO96/16027 PCT~S95/15S30
-122-
Example 54
This example illustrates the preparation of
representative pharmaceutical formulations for oral
administration.
A. Ingredients ~ wt./w~.
Compound of formula (I) 20.0
Lactose 79.5
Magnesium stearate 0.5~
The above ingredients are mixed and dispensed into hard-shell
gelatin capsules containing lO0 mg each, one capsule would
approximate a total daily dosage.
B. Inqredients ~ wt./wt.
Compound of formula (I) 20.0
Magnesium stearate 0.9
Starch 8.6
Lactose 79.6
PVP (polyvinylpyrrolidine)0.9~
The above ingredients with the exception of the magnesium
stearate are combined and granulated using water as a
granulating liquid. The formulation is then dried, mixed with
the magnesium stearate and formed into tablets with an
appropriate tableting machine.
C. Ingredients
Compound of formula (I)O.l g
Propylene glycol 20.0 g
Polyethylene glycol 40020.0 g
Polysorbate 80 l.0 g
Water q.s. lOO mL
The compound of formula (I) is dissolved in propylene glycol,
polyethylene glycol 400 and polysorbate 80. A sufficient
quantity of water is then added with stirring to provide lO0
mL of the solution which is filtered and bottled.
SUBSIllulk SHEr ~RUIE 26~
CA 02205665 1997-05-21
W O 96/16027 PCT~US95/lSS30
-123-
D. In~redient~ ~ wt./wt.
Compound of formula (I) 20.0
Peanut Oil 78.0
Span 60 2.0~
The above ingredients are melted, mixed and filled into ~oft
elastic capsules.
Example 55
This example illustrates the preparation of a
representative pharmaceutical formulation ~or parenteral
administration.
In~redients
Compound of formula (I) 0.02 g
Propylene glycol 20.0 g
Polyethylene glycol 400 20.0 g
Poly80rbate 80 1.0 g
0.9~ Saline Qolution q.s. 100 mL
The compound of formula (I) is dissolved in propylene glycol,
polyethylene glycol 400 and polysorbate 80. A sufficient
quantity of 0.9~ saline solution is then added with stirring
to provide 100 mL of the I.V. solution which is filtered
through a 0.2 ~ membrane filter and packaged under sterile
conditions.
Example 56
This example illustrates the'preparation of a
representative pharmaceutical composition in suppository form.
Inqredients ~ wt./wt.
Compound of ~ormula (I) 1.0
Polyethylene glycol 1000 74.5
Polyethylene glycol 4000 24.5~
The ingredients are melted together and mixed on a steam bath,
and poured into molds containing 2.5 g total weight.
SlJBSTITUTE SHEET (RULE 26)
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/15530
-124-
Example 57
This example illustrates the preparation of a
representative pharmaceutical formulation for insufflation.
Inqredients ~ wt./wt.
Micronized compound of formula (I) 1.0
Micronized lactose 99.0
The ingredients are milled, mixed, and packaged in an
insufflator equipped with a dosing pump.
Example 58
This example illustrates the preparation of a
representative pharmaceutical formulation in nebulized form.
Inqredients ~ wt./wt.
Compound of formula (I) 0.005
Water 89.995%
Ethanol 10.000
The compound of formula (I) is dissolved in ethanol and
blended with water. The formulation is then packaged in a
nebulizer equipped with a dosing pump.
Example 59
This example illustrates the preparation of a
representative pharmaceutical formulation in aerosol form.
Inqredients ~ wt./wt.
Compound of formula (I) 0.10
Propellant 11/12 98.90
Oleic acid 1.00~
The compound of formula (I) is dispersed in oleic acid and the
propellants. The resulting mixture is then poured into an
aerosol container fitted with a metering valve.
Example 60
In vi tro matrilysin assay
Matrilysin was purified from cloned m~mm~l ian cell
culture by Blue-Sepharose and zinc-chelating sepharose column
SUBSTITUTE SHEET (RULE 26)
CA 0220~665 1997-0~-21
WOg6/16~27 PCT~S9S/1SS30
-125-
followed by fast protein liguid chromatography over a MONO S
column. The enzyme was activated by incubation with l mmol
APMA for l hr at 35-37~C.
Compounds of formula (I) were dissolved in DMSO and added
to a cuvette containing 0.4 ~g matrilysin in l ml TC buffer
(20mM Tris, 5mM CaCl2, pH 7.5) (2~ DMSO final concentration).
The concentrations of the compounds of formula (I) were chosen
such that there was at least one data point for every 20~
change in activity. Enzyme and compounds were permitted to
pre-incubate 3 min at 37~C. To initiate the reaction, N-(7-
dimethylamino-4-methyl-3-coumarinyl)maleimide ("DACM") (Sigma)
and thiopeptide (Ac-Pro-Leu-Gly-S-~Leu~-Leu-Gly-OEt, sachem
Bioscience Inc.) were added to 20 ~M each. The fluorescence
increase was recorded with excitation and emission wavelengths
of 395 and 485 nm, respectively. Each data point is the
average of duplicate experiments. At least six data points,
expressed as change in fluorescence per minute versus compound
concentration were analyzed using the IC50 fit in the program,
Enzfitter.
Compounds of formula (I) exhibited the ability to inhibit
matrilysin when tested in this assay.
Example 61
In vi tro assay
This assay determines if the compounds of formula (I)
inhibit the release of ~S-labelled glycosaminoglycans (GAG's)
from cartilage explants.
Small cartilage explants (3 mm diameter) were prepared
from freshly sacrificed bovine knee joints and labeled with
35so4. 3~S-labelled glycosaminoglycans (GAG's) are released into
the culture medium in response to the addition of rhIL-l-
alpha, which induces the expression of chondrocyte matrix
metalloproteases (MMP's), including stromelysin and
collagenase. The percent inhibition of labeled GAG~s was
corrected for spontaneous release in the absence of rhlL-l-
alpha. Results for each group represent the mean i the S.E.M.
for five explants.
SUBSTITUTE SHEET (RULE 26)
.
CA 0220~66~ 1997-0~-2l
WO96/16027 PCT~S95/15530
-126-
Compounds of formula (I), when tested in this assay,
displayed the ability to inhibit the release of 35S-labelled
GAG's from cartilage explants.
Example 62
In vi tro assay
An in ~i tro fetal rat long bone model was used to study
the anti-bone resorptive effect of the compounds of
formula (I). Bovine PTH was used to induce bone resorption in
vitro. The bone resorptive effects were expressed by the
amounts of 45Ca released from the 45Ca pre-labelled fetal rat
long bones into the culture medium. The inhibitory effect of
the compounds of formula (I) against bovine PTH induced bone
resorption was expressed as mean percent inhibition i sem.
45Ca-prelabelled fetal rat long bones (from forearms) were
dissected and cultured in Linbro dishes at 37~C overnight BGJb
medium, supplemented with 1mg/ml BSA. There were five pairs
of bones in each group. The compounds of formula (I) were
dissolved in ethanol first, then diluted to various
concentrations and added simultaneously with Bovine PTH (1-34)
at lx108M on Day 1. The ethanol concentrations in the compound
solutions were less than 0. 05~ which did not interfere with
the assay. The assay was terminated on Day 6 with one media
change on Day 3.
At the end of each medium change, the 45Ca present in the
culture medium was counted. The remaining bones were digested
with O.lN HCl and the 45Ca presented in the bone digest was
also counted. The results are expressed as ~ of the total 45Ca
released from each pair of bones. Bovine PTH at lx10-8M
induces bone resorption to the maximum level which is set as
100~ and this concentration was used as standard. The level
of base line bone resorption in the presence of medium only
was set as 0~. All compound-treated groups were compared with
bovine PTH (1-34) at lx10-8M. The concentration at which a
compound inhibited bone resorption by 50~ was defined as IC50.
Compounds of formula (I) exhibited the ability to inhibit
bovine PTH-induced bone resorption in this assay.
SU~Slllult SllEr pllD.E26)
CA 0220566~ 1997-05-21
W O96116027 PCTrUS95115530
-127-
-
Exampl~ 63
In vi tro stromelysin assay
63A. Stromelysin enzymatic activity was measured by a
resonance energy transfer fluorogenic assay using the MCA
peptide substrate: 7-methoxycoumarin-4-yl-acetyl-pro-leu-gly-
leu-3-(2~4-dinitrophenyl)-L-2~3-diaminoproprionyl-ala-arg-NH2.
Cleavage of the substrate at the gly-leu bond results in the
1058 of resonance energy transfer to the 2,4-dinitrophenyl
group and an increase in fluorescence of the MCA (7-
methoxycoumarin-4-yl-acetyl) group.
The assay was performed at 37~C in buffer containing 50
mM Tricine, pH 7.5, lOmM CaCl2, 200 mM NaCl, l~ DMSO and 1.4 nM
stromelysin. The concentration of MCA sub6trate was 10 or 20
~M in a ~inal volume o~ 1.6 ml. In the absence of compounds to
be tested for inhibitory activity, or in the presence of non-
slowbinding inhibitors, fluorescence was measured with Perkin-
Elmer LS-5B and LS-50B spectrofluorimeters with ~c~
= 328 nm and ~ n = 393 nm over a 3 to 5 minute time period
and data were fitted to a straight line. For slow-binding
inhibitors, inhibition data were collected for 45 minutes to 1
hour. Steady-state rates of fluorescence change were
calculated by fitting the curve to an equation for a single
exponential decay containing a linear phase, and taking the
fitted value of the linear phase as the steady-state rate.
Compounds of formula (I) were tested and found to be active as
inhibitors of MMP activity in this assay.
63B, The MCA assay can also be used with other matrix
metalloproteinases, such as matrilysin or gelatinase A, by
substituting 0.063 nM matrilysin or 0.030 nM gelatinase A for
stromelysin.
While the present invention has been described with
reference to the specific embodiments thereof, it should be
understood by those skilled in the art that various changes
may be made and equivalents may be substituted without
departing from the true spirit and scope of the invention.
SUBSTITUTE SHEET (RULE 26)
CA 02205665 1997-05-21
WO96/16027 PCT~S95/1S530
-128-
In addition, many modifications may be made to adapt a
particular situation, material, composition of matter,
process, process step or steps, to the objective, spirit and
scope of the present invention.
SUBSTITUTE SHEET ~RULE 26)