Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02205771 1997-OS-22
TITLE OF INVENTION
Improved Delivery of Disease Modifiers
FIELD OF INVENTION
This invention relates to novel methods for delivery of disease
modifiers using hyaluronan and to new compositions comprising the disease
modifiers and hyaluronan.
BACKGROUND OF INVENTION
Hyaluronic acid is a large, complex oligosaccaride consisting of up to
50,000 pairs of the basic disaccharide glucuronic acid-Q(1-3) N-acetylglucos-
amine Q(1-4). It is found in vivo as a major component of the extracellular
matrix. Its tertiary structure is a random coil of about 50 nm in diameter.
Hyaluronic acid appears in nature in its sodium salt form. Hyaluronic acid
and its pharmaceutically tolerable or acceptable salts (such as sodium
hyaluronate) are referred to as Hyaluronan (HA).
Hyaluronan has the ability to bind a large amount of water, which in
vivo makes it a viscous hydrated gel with viscoelastic properties. It is found
in this form in the mammalian eye, both in the vitreous and in the
extracellular matrix.
Hyaluronan (Hyaluronic Acid and pharmaceutically acceptable Salts
Thereof) have been disclosed for use with medicine and/or therapeutic
agents for the treatment of disease and/or conditions (see PCT Application,
PCT/CA 90/00306, International Publication No. WO 91/04058).
Subsequent applications taught the combination of hyaluronic acid and
pharmaceutically acceptable salts thereof for topical treatment and for
accumulation (see PCT Application, PCT/CA 93/00061, International
Publication No. WO 93/16732). It has been postulated that the medicine or
therapeutic agent for example, an NSAID, appears to be associated with the
hyaluronan as a clathrin (term is taken from clathrinida, an order of
sponges which have an asconoid structure and lack a true dermal
CA 02205771 2000-02-04
-2-
membrane or cortex), or is associated with the hyaluronan in a patient to
whom the combination is administered in association with the patient's
serum albumin which serum albumin appears to bind to the hyaluronan.
It is possible to bind hyaluronan directly to a medicine or
therapeutic agent. In this regard, see "Effects of Precipitates formed by
insulin with hyaluronic acid and mucoid from vitreous humor i n
depressing blood-sugar levels ", Science 1950; 111: 520-521 at 520; "Reaction
of Cationic Groups of Chlorpromazine with Anionic Macromolecules:
Complexes with DNA, RNA, Hyaluronic Acid and Heparin ", Acta
pharmacol. et toxicol 1974, 34, 27-32 at pages 30 to 31, and U.S. Patent
5,166,331.
PCT Application PCT/CA95/00477, International Publication No.
WO 96/06622, also owned by Hyal Pharmaceutical Corporation, teaches the
modulation of cellular activity of tissue and cells expressing a high affinity
cell-surface receptor for hyaluronic acid by the use of forms of hyaluronic
acid. These cell surface receptors comprise adhesion molecule CD44 and
adhesion molecule HARLEC (Hyaluronic Acid [Hyaluronan] Receptors
Liver Endothelial Cells) and regulatory molecule RHAMM (Receptor for
HA Mediated Motility), for binding hyaluronan. HARLEC is expressed
(produced and put on the cell surface) in liver endothelial cells. The
administration of an effective amount of a form of hyaluronic acid to bind
with the cell-surface receptors modulates cellular activity of tissues and/or
cells expressing such high affinity cell-surface receptors for hyaluronic acid
(for example, an adhesion or regulatory molecule) in the human body.
One of the reasons why the hyaluronic acid is able to be used to
transport the medicine and/or therapeutic agent is its selective binding to
the cell-surface receptors through a Hyaluronan Binding Motif.
CA 02205771 1997-OS-22
-3-
Hyaluronan Binding motif (HBM) has been identified and is identified
BX7B. See the article entitled Identification of a common hyaluronan
binding motif in the hyaluronan binding proteins RHAMM, CD44 and
link protein; The EMBO Journal, Vol. 13, No. 2 (1994) pp. 286-296.
While disease modifiers such as Cytokines, peptides mimicking
cytokines, and proteins mimicking cytokines for example, may be
administered to humans with the subject matter of PCT/CA 90/00306
(International Publication Number WO 91/04058), we have developed an
improved method of administration of these disease modifiers (cytokines,
peptides mimicking cytokines and proteins mimicking cytokines and
other proteins and peptides).
Hyaluronan interacts at sites of cell migration and proliferation via
specific hyaluronan receptors (CD44, IZHAMM, CD38, TSG-6, and extra-
cellular hyaluronan binding proteins (Versican, Aggrecan, Perlican, link-
protein, GHAP) all being examples thereof). These receptors and binding
proteins are upregulated at the cites of proliferation/migration. As a
result, addition of exogenous hyaluronan targets to these sites for example,
where injury has occurred.
It is therefore an object of this invention to provide a new method
of delivery of disease modifiers to the human using hyaluronan.
It is a further and other object of the invention to provide new
compositions for use in the new method of delivery.
These and other objects of the invention will be realized by those
skilled in the art from the following summary of the invention and
detailed description of embodiments thereof.
SUMMARY OF INVENTION
Hyaluronan binding motif (HBM) as previously stated has (have)
been identified in all hyaluronan binding proteins and receptors and is
CA 02205771 1997-OS-22
-4-
strongly conserved amongst species. HBM is identified as BX7B and is
known to persons skilled in the art. The affinity of the hyaluronan for the
HBM is enhanced if flanking basic amino acids are added or if several
internal basic amino acids are added at position 4,5. Furthermore, binding
activity is enhanced if the intervening amino acids are hydrophobic and
not acidic. Using the hyaluronan binding motif (HBM), it is now possible
to bestow hyaluronan binding properties upon a protein or peptide that
does not normally bind to hyaluronan. For example, the addition of the
HBM to casein, a milk protein that does not bind to hyaluronan bestows
binding activity on the protein.
Therefore, according to one aspect of the invention, I propose the
use of the hyaluronan binding motif (HBM) to be interposed between a
disease modifier (which may be a protein or peptide and which protein or
peptide one skilled in the art would not consider under normal conditions
to be capable of being bound effectively with hyaluronan) and hyaluronan.
By linking the components, I have found that the combination will target
the disease modifier for example, proteins such as cytokines, peptides
mimicking cytokines, and proteins mimicking cytokines to the sites of, for
example, injury or disease. The hyaluronan by binding through HBM to
the protein or peptide also protects the protein or peptide from attack from
Proteases which appear in high numbers at the sites of injury. Further, the
hyaluronan also protects protein/peptide from immune system
recognition and attack and possible destruction.
While the disease modifier includes proteins and peptides (which
may be considered drugs in the usual sense such as for example
antibiotics), they also include any other disease modifier which could be
chemically linked to the amino or carboxy terminus of the hyaluronan
binding peptide. Such disease modifiers include smyonpford. cyclosporin
CA 02205771 1997-OS-22
-5-
and other therapeutic peptides such as cytokine peptides, or cell adhesion
peptides but are not limited thereto. They also include the following:
Drug Use Route
Protein Hormones
Follicle Stimulating
Hormone (FSH)
Leutinizing Hormone amenorrhea intravenous
(LH)
Prolactin chronic renal
insufficiency
Human Growth human growth intramuscular
Hormone (GH) hormone deficiency parenteral
in
children
Adrenocorticotropin hypercalcemia intravenous
Hormone (ACTH) and inflamation
analogues (eg. diagnosis of adrenal
Leuprolide Acetate) insufficiency
Vasopressin, Lypressindiabetes insipidus intranasal
Desmopressin parenteral
injection
Oxytocin (OT) lactation intranasal, intravenous
postpartum bleedingparenteral
induction of labour
Gonadotropin Releasinginfertility, suppressionintranasal
Hormone (GnRH) of ovulation, prostateinjection
and breast tumours
Gonadorelin diagnosis of subcutaneous,
hypothalemic - pituitaryintravenous; intranasal
- gonadal dysfunction
amenorrhoea
infertility
Corticotropin Releasing
Hormone (CRH)
Thyrotropin Releasinglactation transdermal, oral
Hormone (TRH)
Leutinizing Hormone cryptorchidism intranasal
Releasing Hormone endometriosis
(LHRH)
Melanocyte - depression oral
Stimulating Hormone Tardive dyskinesia
Inhibiting Factor
(MIF)
Melanocyte - transdermal
Stimulating Hormone
Releasing Factor
(MSH)
CA 02205771 1997-OS-22
-6-
Growth Hormone transdermal
Releasing Hormone
(GHRH)
Somatostatin acromegaly, GI intranasal
tumours, intravenous
gastric ulcers
Corticotrophin diagnostic agent to intravenous
investigate
adrenocortical
insufficiency
Tetracosactide diagnostic agent to intramuscular
investigate intravenous
adrenocortical
insufficiency
Octreotide Acetategastrointestinal subcutaneous
endocrine tumours
acromegaly
Parathyroid Hormoneosteoporosis subcutaneous
(PTH)
Thyroid Stimulatingdiagnosis of thyroid injection
Hormone (TSH) / disease
Thyroid Releasing
Hormone (TRH)
Insulin diabetes mellitus intravenous
transdermal
Glucagon hypocalcemia parenteral
intravenous
intramuscular
Cholecystokinin chronic pancreatitis,intranasal, transdermal
appetite, postoperativeintravenous
paralytic ileus
Gastrin
Secretin
a1 - Antitrypsin congenital a1 - parenteral
antitrypsin deficiency
Trypsin GI disorders
debridement of wounds topical
oedema and
inflammation
associated with
infection or trauma oral
Pepsin digestive aid in pepsinoral
deficiency
gastric hypochlorhydria
dyspepsia
GI disorders
Neurotensin (NT) gastric juice secretionintravenous
CA 02205771 1997-OS-22
_7_
Calcitonin (CT) Paget's disease of intranasal,
bone
osteoporosis subcutaneous,
hypercalcemia intramuscular
gastric secretion intravenous
intractable pain oral, parenteral,
transdermal
Human Chorionic cryptorchidism intravenous, parenteral
Gonadotropin (hcG)induction of ovulation
Relaxin facilitates birth
scleroderma
Therapeutic Cytokines
Interferons
IFNa-2a Kaposi's sarcoma parenteral
IFNa-2b genital warts
Kaposi's sarcoma parenteral
AIDS-related hairy cell
leukemia
IFNa-n3 parenteral
IFN(3-1b Multiple Sclerosis
(relapsing, remitting
type)
IFN~y 1b chronic granulomatous parenteral
disease
Tumour Necrosis Factor
TNFa, TNF~i
Interleukins
protection against the parenteral
effects of radiation and
chemotherapeutic
agents
renal cell carcinoma
IL.-1, IL.-3, IL-4, IL-5, IL-6,
IL-7, IL-8
Hemotopoietic Proteins
Erythropoietin (EPO) dialysis anemia parenteral
chemotherapy-induced
anemia
chemotherapy-induced
neutropenia
CA 02205771 1997-OS-22
_8-
Granuloycyte - Colony bone marrow transplant parenteral
Stimulating Factor (G-
CSF)
Granulocyte bone marrow transplant parenteral
Macrophage - Colony
Stim. Fac. (GM-CSF)
Macrophage-Colony
Stimulating Factor (M-
CSF)
Growth Factors
Epidermal Growth corneal and cateract topical
Factor (EGF) surgeries
Transforming Growth
Factor a(TGFa)
Platelet-derived growth diabetic and decubitus
factor (PDGF) ulcers
wound healing
Transforming Growth
Factor~3 (TGF(3)
Fibroblast Growth neuropathic ulcers
Factor - basic FGF pressure sores
-
acidic FGF
Insulin-like Growthnutritional
Factor 1 (IGFl) support/metabolisn
and
type II diabetes
osteoporosis
Insulin-like Growth
Factor2 (IGF2)
Nerve Growth Factorperipheral neuropathies
Skeletal Growth
Factor
Cardiovascular
Therapeutic Proteins
Proteins of the Blood
Coagulation Pathway
Factor VIII Hemophilia A parenteral
Factor IX
Factor VII/VIIa
Factor XII
Tissue Factor (Factor
III)
Protein C
Antithrombin III
CA 02205771 1997-OS-22
-9-
Fibrinolytic
Therapeutic Proteins
Tissue Plasminogenacute myocardial parenteral
Activator infarction
Streptokinase and thromboembolism injection
Anisoylated
Streptokinase
Fibrolase myocardial infarction
Urokinase and Single-
Chain Urokinase parenteral
Kidney Plasminogen
Activator
Angiotensin-
Converting Enzyme
Inhibitors
Captopril hypertension oral
Enalapril heart failure
Vaccines Active Immunization injection or oral
Hepatitis B virus surface
antigens
Influenza virus surface
antigens
Plasmodium surface
antigens
Mycobacterium surface
antigens
Schistosoma surface
antigens
Herpes simplex virus
surface antigens
Trypanosoma surface
antigens
Streptococcus surface
antigens
Epstein - Barr virus
surface antigens
HTLV III virus surface
antigens
CA 02205771 1997-OS-22
-10-
Therapeutic and
Diagnostic Antibodies
Murine Native &
Radioimmuno-
conjugate Antibodies &
Fragments
- Muromonab-CD3 heart, kidney & liver parenteral
transplant rejection
- Staumonab pendetide detection, staging, and
follow-up of colorectal
and ovarian cancers
Murine
Chemoimmuno-
conjugate Antibodies
Murine Immunotoxin
Antibodies & Fragments
Nonmurine Polyclonal
Antibodies
Human Antibodies &
Fragments
Murine/Human
Monoclonal Antibodies
& Fragments
Opioids
(3-Endorphin cancer pain intravenous
childbirth pain intrathecal
narcotic abstinence
Dermorphin syndrome
Dynorphins
Enkephalins
Other Proteins
Cylosporine immunosuppression intravenous
in
allogenic transplants
Delta Sleep-inducingInsomnia intravenous
peptide (DSIP)
Bestatin cancer therapy oral
Bacitracin bacterial infection topical
Gramicidin bacterial infection topical
Terprotide hypertension parenteral
Serum Thymide Factorimmune deficiencies intravenous
(FTS)
CA 02205771 1997-OS-22
-11-
Crude Thymosin autoimmune disordersparenteral
collagen vascular
disease intramuscular
chemotherapy intravenous
rheumatoid arthritisintravenous
Angiogenin induces formation
of
blood vessels
Albumin & Plasma blood volume
Proteins replacement
Atrial Natriuretic fluid and electrolyte
Factor
homoeostasis
regulation of blood
pressure
Benin arterial pressure
control
Superoxide Dismutaseinflammation
rheumatoid arthritis
protection against
radiation therapy
Glucocerebrosidase Gaucher's Disease
(Algucerase)
rh DNase Cystic Fibrosis
Aprotinin haemorrhage associatedintravenous
with raised plasma
concentrations of
plasmin
Protamine neutralize effect intravenous
of
heparin
Asparaginase induction of remissionparenteral
in acute lymphoblastic
leukemia
rhBMP-2 bone and cartilage
repair
Flab) fragment Digoxin overdose injection
Other disease modifiers may also be used with this invention.
These may include:
Anti microbial Peptides
Gramicidin and Related Peptides
(eg. Gramicidin S, Tyrocidines, Gratisin)
~3-lactam and (3-lactam like Antibiotics
Sulfazecin Type (eg. Sulfazecin, Isosulfazecin)
Carbapenem Type
Cephabacin Type (eg. Chitinovorin, Cephabacin)
Norcardicin Type (eg. Formacidin)
CA 02205771 1997-OS-22
-12-
Lactivicin Type (eg. Lactivicin)
Glycopeptide Antibiotics of the Vancomycin Group
Vancomycin Type (eg. Vancomycin, Orienticin, Eremomycin)
Actinoidin Type (eg. Actinoidin, Avoparcin, Chloropolysporin)
Ristocetin Type (eg. Ristocetin, Actaplanin)
Teicoplanin Type (eg. Teicoplanin, Ardacin, Kibdelin, Parvodicin)
N-methylated Peptides and Peptolides
Linear Peptides (eg. Stenothricin)
Cyclic Peptides (eg. Ilamycins, cyclosporins)
Diketopiperazines (eg. Gliotoxin)
True Depsipeptides (eg. Enniatins, Beauvericin)
Actinomycins
20
Sideromycins (eg. Grisein, Albomycin, Desferrioxamine B)
- use iron chelation therapy for removal of excessive iron resulting from
genetic defects (primary hemochromatosis, anemias) or repeated blood
transfusions
Phleomycin - Like Antibiotics (eg. Bleomycin)
- use - squamous cell carcinomas, Hodgkin's lymphomas, testis tumour
Protease Inhibitors
Inhibitors Against Endopeptidases
Serine and Cysteine Proteinase Inhibitors
Leupeptin - use - fertilization, inflammation, chemical carcinogenesis,
burns, pancreatitis, muscular dystrophy, antoimmune diseases
Antipain - use - fertilization, inflammation, chemical carcinogenesis,
muscular dystrophy
Chymostatin - use - fertilization, inflammation
Elastatinal - use - inflammation
Ac-Leu-Argal
Lystatin
Poststatin
Aspartic Proteinase Inhibitors
Pepstatin - use - inflammation, hypertension, ascites and pleural fluid
Pepstanone
Hydroxypepstatin
Metal Proteinase Inhibitors
Phosphoramidon - use - inflammation
Steffimycins B & D
CA 02205771 1997-OS-22
-13-
Inhibitors Against Exopeptidases
Aminopeptidases Inhibitors
Amastatin
Actinonin - use - immunopotentiation, analgesia
Arphamenines A & B - use - immunopotentiation, analgesia,
autoimmune diseases
Bestatin - use - immunopotentiation, analgesia, hypertension, malignant
diseases, muscular dystrophy
Ebelactones A & B - use - immunopotentiation
Formestin
Probestin
Prostatin
Leuhistin
Dipeptidylamino Peptidase Inhibitors
Ac-Leu-Argal
Antipain
Leupeptin
Diprotins A & B - use - immunopotentiation
Octastatins A & B
30
Carboxy Peptidase Inhibitors
(S)-a-Benzylmalic acid - use - immunopotentiation
Histargin - use - immunopotentiation, hypertension
Dipeptidylcarboxy Peptidase Inhibitors
EDDS
Foroxymithine - use - immunopotentiation, hypertension
Histargin
Inhibitors Against Plasma-Membrane-Located-Enzymes
Forphenicine - use - immunopotentiation, muscular dystrophy, malignant
diseases
Forphenicinol - use - immunopotentiation, muscular dystrophy,
malignant diseases
Esterastin - use - immunopotentiation, inflammation, auto immune
diseases
Ebelactone A & B
Thus, I have provided a new composition comprising hyaluronan,
hyaluronan binding motif (HBM), for example, found in a hyaluronan
binding protein or receptor, and disease modifier (including a drug and/or
therapeutic agent) which can be chemically linked or bound by the HBM
(eg. at the amino or carboxy terminus of the hyaluronan binding peptide)
CA 02205771 1997-OS-22
-14-
to the disease modifier. Thus, the hyaluronan indirectly binds through
the hyaluronan binding motif (HBM) to the disease modifier such as a
drug which is a protein such that the hyaluronan binding motif (HBM) is
interposed between the hyaluronan and the disease modifier.
The new composition (or compound) may be used and
administered in manners previously described for example,
intravenously, interarterially, interperitoneally, intrapleurally, into the
skin, applied topically onto the skin for penetration into the skin, to the
oral mucosa, rectally or by direct injection into a tumour, abscess, or
similar disease focus or put on a patch to be secured to the skin of the
patient or administered via an enema.
Many forms of hyaluronan may be suitable although those
preferred are those discussed hereinafter:
One form of hyaluronic acid and/or pharmaceutically acceptable
salts thereof (for example sodium salt) suitable for use with my invention
is an amount having the following specifications/characteristics:
TESTS SPECIFICATIONS RESULTS
pH 5.0 to 7.0 at 25 degrees C. 6.0
Specific Gravity 0.990 to 1.010 at 25 degrees C. 1.004
Intrinsic Viscosity 4.5 to 11.0 dL/g. 7.07
Molecular Weight 178,000 to 562,000 daltons (protein 319,378 daltons
standard)
Sodium Hyaluronate 9.0 to 11.0 mg/mL. Positive 9.9 mg/ML
Assay and Identification Positive
Another such amount may comprise:
TESTS SPECIFICATIONS
1. Description White or cream odourless
powder
CA 02205771 1997-OS-22
-15-
2. Identification (IR Spectrum)Conforms to Ref. Std.
Spectrum
3. pH (1% solution) 5.0 to 7.0
4. Loss on Drying NMT 10%
5. Residue on Ignition 15.0% to 19.0%
6. Protein Content NMT 0.1%
7. Heavy Metals NMT 20 ppm
8. Arsenic NMT 2 ppm
9. Residual Solvents
a) Formaldehyde NMT 100 ppm
b) Acetone NMT 0.1%
c) Ethanol NMT 2.0%
10. Sodium Hyaluronate Assay 97.0 to 102.0%
(dried basis)
11. Intrinsic Viscosity 10.0 to 14.5 dL/g
12. Molecular Weight 500,000 to 800,000 daltons
13. Total Aerobic Microbial NMT 50 microorganisms/g
Count
(USP 23)
14. Escherichia coli (USP 23) Absent
15. Yeasts and Moulds (USP 23) NMT 50 microorganisms/g
16. Bacterial Endotoxins (LAL) NMT 0.07 EU/mg
(USP 23)
Another such amount is available
from Hyal Pharmaceuticals
Limited and comes in a 15 ml of Sodium hyaluronate 20mg/ml
vial
(300mg/vial - Lot 2F3). The
sodium hyaluronate amount is
a 2% solution
with a mean average molecular
weight of about 225,000. The
amount also
contains water q.s. which is
triple distilled and sterile
in accordance with
the U.S.P. for injection formulations.The vials of hyaluronic acid
and/or
CA 02205771 1997-OS-22
-16-
salts thereof may be carried in a Type 1 borosilicate glass vial closed by a
butyl stopper which does not react with the contents of the vial.
The amount of hyaluronic acid and/or salts thereof (for example
sodium salt) may also comprise the following characteristics:
a purified, substantially pyrogen-free amount of hyaluronic acid
obtained from a natural source having at least one characteristic selected
from the group (and preferably all characteristics) consisting of the
following:
i) a molecular weight within the range of 150,000-225,000;
ii) less than about 1.25% sulphated mucopoly-saccharides
on a total weight basis;
iii) less than about 0.6% protein on a total weight basis;
iv) less than about 150 ppm iron on a total weight basis;
v ) less than about 15 ppm lead on a total weight basis;
vi) less than 0.0025% glucosamine;
vii) less than 0.025% glucuronic acid;
viii) less than 0.025% N-acetylglucosamine;
ix) less than 0.0025% amino acids;
x) a UV extinction coefficient at 257 nm of less than about
0.275;
xi) a UV extinction coefficient at 280 nm of less than about
0.25; and
xii) a pH within the range of 7.3-7.9. Preferably, the
hyaluronic acid is mixed with sterile water and the amount of hyaluronic
acid has a mean average molecular weight within the range of 150,000-
225,000 daltons (protein standard). More preferably, the amount of
hyaluronic acid comprises at least one characteristic selected from the
CA 02205771 1997-OS-22
-17-
group (and preferably all characteristics) consisting of the following
characteristics:
i) less than about 1% sulphated mucopolysaccharides on
a total weight basis;
ii) less than about 0.4% protein on a total weight
basis;
iii) less than about 100 ppm iron on a total weight
basis;
iv) less than about 10 ppm lead on a total weight
basis;
v ) less than 0.00166% glucosamine;
vi) less than 0.0166% glucuronic acid;
vii) less than 0.0166% N-acetylglucosamine;
viii) less than 0.00166% amino acids;
x) a UV extinction coefficient at 257 nm of
less than about
0.23;
xi) a UV extinction coefficient at 280 nm of less than 0.19;
and
xii) a pH within the range of 7.5-7.7
Applicants may also use sodium hyaluronate produced and
supplied by LifeCoreTM Biomedical, Inc., having the following
specifications:
Characteristics Specification
Appearance White to cream
colored particles
Odor No perceptible odor
Viscosity Average < 750,000 Daltons
Molecular Weight
UV/Vis Scan, 190-820nm Matches reference scan
OD, 260nm < 0.25 OD units
Hyaluronidase Sensitivity Positive response
CA 02205771 1997-OS-22
-18-
IR Scan Matches reference
pH, l0mg/g solution 6.2 - 7.8
Water 8% maximum
Protein < 0.3 mcg/mg NaHy
Acetate < 10.0 mcg/mg NaHy
Heavy Metals, maximum ppm
As Cd Cr Co Cu Fe Pb Hg N i
2.0 5.0 5.0 10.0 10.0 25.0 10.0 10.0 5.0
Microbial Bioburden None observed
Endotoxin < 0.07EU/mg NaHy
Biological Safety Testing Passes Rabbit Ocular
Toxicity Test
Another amount of sodium hyaluronate
proposed to be used is sold
under the name Hyaluronan HA-M5070 Skymart Enterprises,
by Inc.
having the following specifications:
Specifications' Test Results
Lot No. HG1004
pH 6.12
Condroitin Sulfate not detected
Protein 0.05%
Heavy Metals Not more than 20 ppm
Arsenic Not more than 2 ppm
Loss on Drying 2.07%
Residue on Ignition 16.69%
Intrinsic Viscosity 12.75 dl/s (XW: 679,000)
Nitrogen 3.14%
Assay 104.1
Microbiological Counts 80/g
CA 02205771 1997-OS-22
-19-
E. coli Negative
Mold and Yeast Not more than 50/g
Other forms of hyaluronic acid and/or its salts may be chosen from
other suppliers and those described in prior art documents provided they
are suitable.
The following references teach hyaluronic acid, sources thereof, and
processes for the manufacture and recovery thereof which may be suitable.
As there is no toxicity of the form of hyaluronic acide, the form of
hyaluronic acid may be administered in doses in excess of l2mg/kg of body
weight, for example, in excess of 1000mg/70kg person and even up to
amounts of 3000mg/70kg person without adverse toxic effects. Lower
amounts may include 10-50mg of hyaluronan. Exemplary amounts of
Hyaluronan used may be 3-l0mg of hyaluronan (HA)/kg of body weight of
the patient wherein the molecular weight (protein standard) is less than
750,000 daltons.
Many forms of hyaluronan may be suitable for use herein although
those preferred are those discussed hereinafter. Particularly, molecular
weights of forms of hyaluronan between about 150,000 daltons and about
750,000 daltons (protein standard) in sterile water prepared having a
viscosity for intravenous administration are suitable.
One specific form of pharmaceutical grade is a 1% sterile sodium
hyaluronate solution (50 ml vials) provided by Hyal Pharmaceutical
Corporation which has the following characteristics:
Tests Svecifications
1. Container Description 150 mL Flint glass vial with
a red or gray rubber stopper
and an aluminum seal, 20
mm m size.
CA 02205771 1997-OS-22
-20-
2. Product Description A clear, colourless, odourless,
transparent, slightly viscous
liquid.
3. Fill Volume 50.0 to 52.0 mL
4. pH 5.0 to 7.0 at 25 degrees C.
5. Specific Gravity 0.990 to 1.010 at 25 degrees
C.
6. Intrinsic Viscosity 4.5 to 11.0 dL/g
7. Molecular Weight 178,000 to 562,000 daltons
8. Sodium Hyaluronate Assay 9.0 to 11.0 mg/mL. Positive
and Identification
9. Particulate Matter No visible Particulate Matter
10. Sterility Meets Requirements for Sterility,
USP 23
11. Bacterial Endotoxins Meets Requirements for Bacterial
(LAL)
Endotoxins, USP 23.
This pharmaceutical grade % sterile solution of hyaluronan
1 may be
made from granules/powder
having the following characteristics:
Tests Specifications
1. Description White or cream-coloured granules
or powder, odourless
2. Identification (IR Spectrum)Must conform with the Reference
Standard Spectrum.
3. pH (1% Solution) Between 5.0 and 7.0 at 25
degrees C.
4. Loss on Drying NMT 10.0% at 102 degrees C.
for
6 hours.
5. Residue on Ignition Between 15.0 and 19.0%
6. Protein Content NMT 0.10%
CA 02205771 1997-OS-22
-21-
7. Heavy Metals NMT 20 ppm (as per USP 23 p.
1727).
8. Arsenic NMT 2 ppm (as per USP 23, p.
1724).
9. Residual Solvents a) Acetone: NMT 0.1%
b) Ethanol: NMT 2.0%
c) Formaldehyde: NMT 100
ppm
10. Sodium Hyaluronate Assay 97.0 to 102.0% (dried basis)
11. Intrinsic Viscosity Between 10.0 to 14.5 deciliters
per gram.
12. Molecular Weight Between 500,000 to 800,000
daltons
(calculated using the Laurent Formula) (based Ori iritrlnciS vISCOSIty).
13. Total Aerobic Microbial Count NMT 50 microorganism/gram
(as per USP 23, p. 1684).
14. Test for Escherichia coli Escherichia coli is absent (as per
USP 23, p. 1685).
15. Yeasts & Molds NMT 50 microorganisms/gram
(as per USP 23, p. 1686).
16. Endotoxins (LAL) NMT 0.07 EU/mg (as per USP
23, p. 1696).
A topical grade of hyaluronan may, in certain circumstances be
suitable and may be made from the following granules/powder which
have the following characteristics:
Tests Svecifications
1. Description White or cream-coloured granules
or powder, odourless
CA 02205771 1997-OS-22
-22-
2. Identification (IR Spectrum) Must conform to the Reference
Standard Spectrum.
3. pH (1% Solution) Between 6.0 and 8.0 at
25
degrees C.
4. Loss on Drying NMT 10.0% at 102 degrees
C. for
6 hours.
5. Residue on Ignition Between 15.0 and 19.0%
6. Protein Content NMT 0.40%
7. Heavy Metals NMT 20 ppm (as per USP
23 p.
1727).
8. Arsenic NMT 2 ppm (as per USP
23, p.
1724).
9. Residual Solvents a) Acetone: NMT 0.1%
b) Ethanol: NMT 2.0%
c) Formaldehyde: NMT 100
ppm
10. Sodium Hyaluronate Assay 97.0 to 102.0% (dried
basis)
11. Intrinsic Viscosity Between 11.5 to 14.5 deciliters
per gram.
12. Molecular Weight Between 600,000 to 800,000
daltons
(calculated using the Laurent (based Ori lritririSiC
Formula) V1SCOSity).
13. Total Aerobic Microbial CountNMT 100 microorganism/gram
(as per USP 23, p. 1684).
14. Test for Staphylococcus aureusStaphylococcus aureus
is absent
(as per USP 23, p. 1684).
15. Test for Pseudomonas aeruginosaPseudomonas aeruginosa
is
absent (as per USP 23,
p. 1684).
CA 02205771 1997-OS-22
-23-
16. Yeasts & Molds NMT 200 CFU/gram (as per
USP 23, p. 1686).
This topical grade may then be sterilized.
Other forms of hyaluronic acid and/or its salts may be chosen from
other suppliers, for example those described in prior art documents disclosing
forms of hyaluronic acid having lower molecular weights between about
150,000 daltons and 750,000 daltons being prepared as for example, 1-2%
solutions in sterile water for intravenous administration.
The following references teach hyaluronic acid, sources thereof and
processes of the manufacture and recovery thereof.
Canadian Letters Patent 1,205,031 (which refers to United States
Patent 4,141,973 as prior art) refers to hyaluronic acid fractions having
average molecular weights of from 50,000 to 100,000; 250,000 to 350,000; and
500,000 to 730,000 and discusses processes of their manufacture
Where high molecular weight hyaluronic acid (or salts or other forms
thereof) is used, it must, prior to use, be diluted to permit administration
and
ensure no intramuscular coagulation. Recently, it has been found that large
molecular weight hyaluronic acid having a molecular weight exceeding about
1,000,000 daltons self-aggregates and thus, does not interact very well with
HA
receptors. Thus, the larger molecular weight hyaluronic acid should be
avoided.
Briefly, the methods for linking or combining the hyaluronan
targeting sequence onto other proteins using the hyaluronan binding
motif (HBM), may be the methods outlined as follows or any other
suitable methods as would be understood by persons skilled in the art:
A first method is recombinant technology which involves linking
the HBM sequence to a DNA sequence encoding a therapeutic protein.
The whole recombinant DNA is then translated by bacteria to make an
CA 02205771 1997-OS-22
-24- . ,
artificial protein that is used for therapy. In this regard, see
"Identification
of Two Hyaluronan-binding Domains in the Hyaluronan Receptor",
RHAMM, The Journal of Biological Chemistry, April 25, 1993, Vol. 268,
No.l2, pp. 8617 to 8623 previously discussed herein. In this report, the
scientists (including me) have disclosed:
"In the course of preparing RHAMM cDNAs that were defective
in binding HA to be used for genetic studies, we have identified
the carboxyl terminus as the HA binding region of RHAMM.
We sought to identify the precise motifs that contained
hyaluronan binding activity within this region.
In this report we demonstrate that the RHAMM cDNA fusion
protein retains its ability to bind to HA in two types of binding
assays including a new transblot assay using bio-tinylated HA
(20) and HA-Sepharose affinity chromatography. We have
defined the HA-binding domains) on RHAMM as a 35-amino-
acid region located near the carboxyl terminus of RHAMM. We
show that two motifs within this region, containing 11 and 10
amino acids, respectively, represent the HA binding motifs of
RHAMM. Neither of these motifs nor the entire 35-amino-acid
region containing these motifs bears any amino acid sequence
homology to other characterized hyaluronan-binding proteins."
In the Experimental Procedures, the scientists disclosed the
following:
"Construction of Recombinant RHAMM-containing
Oligonucleotides Encoding HA-Binding Peptides--PCR was used
to incorporate the HA binding regions (peptideaa401-411 and
peptideaa423-432 respectively)into a cDNA encoding the NH2
terminus of RHAMM that was prepared as a 0.71-kb fragment (aa
CA 02205771 1997-OS-22
-25-
1-238, see above and Fig. 2). The fusion protein product of this
fragment did not have the ability to bind HA (Fig. 7). The
procedure was carried out by making two PCR primers (5'TAG
AAT GAA TTC TTT CAA TTT CAC AAC ATG TTT GAT TTT
TTG TTT AAG ATC TTC TAT TTC and 5'TAG AAT GAA TTC
TTT CCT TTT AAC AAG CTG AGA TCG CAG TTT AAG ATC
TTC TAT TTC) which contained both a region mimicking the
oligonucleotides encoding either peptideaa401-411 or
peptideaa423-432 (creating an EcoRI site at the end of each
primer) and a region mimicking 18 base pairs of the 3' end cDNA
of the 0.71-kb insert. Recombinant cDNA was obtained with a
PCR reaction by using either of these two primers together with a
primer that mimicked the 5' end of the RHAMM cDNA
(nucleotide 1-22) (creating a B a m HI site) with the same
conditions described in the construction of RHAMM cDNA.
Both PCR products were digested with EcoRI and BamHI and
purified in 1% agarose gel electrophoresis. Recombinant cDNAs
were then inserted into pGEX-2T and transformed into HB101 as
above. The correct insertion of the recombinant cDNAs was
confirmed by restriction endonuclease digestion of the selected
clones and by sizing of the insert with agarose gel
electrophoresis.
The major findings of this paper are that a critical
interaction of hyaluronan with the RHAMM receptor can be
localized to a region of 35 amino acids (aa 400-434) near the
carboxyl terminus of this protein. This region contains
sequences that exhibit clusters of basic amino acids. Peptides
mimicking these sequences contain HA binding activity, and
CA 02205771 1997-OS-22
-26-
furthermore, these peptides confer HA binding activity to an
NH2-terminal fragment of RHAMM that does not bind to HA.
Collectively, these results indicate that these sequences represent
two critical HA binding motifs of RHAMM.
In the article Identification of a common Hyaluronan binding motif
in the hyaluronan binding proteins RHAMM, CD44 and link protein , The
EMBO Journal, Vol. 13, no. 2, PP. 286-296, 1994, the authors disclosed that:
We have previously identified two hyaluronan (HA) binding
domains in the HA receptor, RHAMM, that occur near the
carboxyl-terminus of this protein. We show here that these two
HA binding domains are the only HA binding regions in
RHAMM, and that they contribute approximately equally to the
HA binding ability of this receptor. Mutation of domain II using
recombinant polypeptides of RHAMM demonstrates that K423
and 8431, spaced seven amino acids apart, are critical for HA
binding activity. Domain I contains two sets of two basic amino
acids, each spaced seven residues apart, and mutation of these
basic amino acids reduced their binding to HA-Sepharose. These
results predict that two basic amino acids flanking a seven amino
acid stretch [hereafter called B(X~)B] are minimally required for
HA binding activity. To assess whether this motif predicts HA
binding in the intact RHAMM protein, we mutated all basic
amino acids in domains I and II that form part of these motifs
using site-directed mutagenesis and prepared fusion protein
from the mutated cDNA. The altered RHAMM protein did not
bind HA, confirming that the basic amino acids and their spacing
are critical for binding. A specific requirement for arginine or
lysine residues was identified since mutation of K430, 8431 and
CA 02205771 1997-OS-22
-27-
K432 to histidine residues abolished binding. Clustering of basic
amino acids either within or at either end of the motif enhanced
HA binding activity while the occurrence of acidic residues
between the basic amino acids reduced binding. The B(X7) B
motif, in which B is either R or K and X7 contains no acidic
residues and at least one basic amino acid, was found in all HA
binding proteins molecularly characterized to date.
Recombinant techniques were used to generate chimeric
proteins containing either the B(X7)B motifs present in CD44 or
link protein, with the amino-terminus of RHAMM (Amino
acids 1-238) that does not bind HA. All chimeric proteins
containing the motif bound HA in transblot analyses. Site-
directed mutations of these motifs in CD44 sequences abolished
HA binding. Collectively, these results predict that the motif of
B(X7)B as a minimal binding requirement for HA in RHAMM,
CD44 and link protein, and occurs in all HA binding proteins
described to date.
The protein -HBM may then be combined with hyaluronan and will
become bound thereto.
A second method is to prepare the HA targeting sequence by
synthesis (a standard procedure as would be understood by persons skilled
in the art) and then link to a protein via carbodiimide linkage. This is a
chemical method for linking carboxyl and amino groups together. Such a
procedure is described in Spontaneous Glycosylation of
Glycosaminoglycan Substrates of Adherent Fibroblasts, Cell, May 1979, Vol.
17, 109-115 by E.A. Turley and S. Roth. At page 114, the following
experimental procedure is found:
CA 02205771 2000-02-04
-28-
"Derivatization and Characterization of Glycosaminoglycan Dishes"
The glycosaminoglycans, chondroitin-6-sulfate (Type C, Sigma;
molecular weight 50,000), hyaluronic acid (bovine vitreous humor, Sigma;
molecular weight 1,000,000) and polygalacturonic acid (Sigma; molecular
weight 20,000-400,000), were covalently linked to 35 x lOmm polystyrene
tissue culture dishes (Falcon Plastics) by a modification of the procedure
of Keener, McDermott and Sheppard (1971). Each dish was treated for 1
hr with 1 ml of concentrated sulfuric acid at 37°C, washed extensively
with water and then treated with 1 ml of aqueous ammonium hydroxide
(30%, v/v) at room temperature for 24 hr. The resulting polysulfonamide
dishes were incubated with 1 ml of an aqueous solution of 1-ethyl-3(3-
dimethylaminopropyl) carbodiimide (25 mg/ml) and either chondroitin
sulfate (CS dishes), hyaluronic acid (HA dishes) or polygalacturonic
acid (PGA dishes) all at 5 mg/ml for 48 hr in a humidified atmosphere a t
37°C. Dishes were then boiled in 8 M urea and 10% sodium dodecylsulfate
(SDS-urea) for 20 min to remove noncovalently bound material. Dishes
were pulverized, and hydrolysis of bound sugars was achieved with 1.5
ml of 90% formic acid (v/v) at 105°C for 6-12 hr. Hydrolysates were
lyophilized and the residue was dissolved in 500 ~,1 water. This solution
was assayed for uronic acid (Dische, 194' in the case of HA and PGA
dishes, or counted in a Searle~ Mark III liquid scintillation counter in the
case of CS dishes derivatized with 3H-chondroitin sulfate (3nmole per
plate; spec. act. 1.5 x 108 cpm/mg). Aliquots of all samples were analyzed
by high voltage electro-phoresis (50 V/an for 45 min) on Whatman~
3MM paper impregnated with 1% borate (w/v). For standards, D-U-14C-
glucuronic acid (8 nmole, spec. act. 76mCi/nmole; Amerisham-Searle),
hyaluronic acid, chondroitin sulfate and polygalacturonic acid were a 11
hydrolyzed under identical conditions. The unlabeled hexosamine
CA 02205771 1997-OS-22
-29-
standards were visualized on paper using periodate-benzidine (Smith,
1969)."
Once again the protein-HBM is then combined with hyaluronan.
A third method is simply to synthesize the therapeutic peptide and
HA targeting sequence (HBM) together by standard peptide synthesis
known to persons skilled in the art. This could be done for example, if the
disease modifier (therapeutic agent) were a peptide of for example, 10-20
amino acids. This procedure would make economic sense rather than
using bacteria to make it as a small protein. The product is then combined
with hyaluronan. The new compounds formed by interposing the
hyaluronan binding motif (HBM) between the disease modifier (e.g. drug)
and hyaluronan, may be administered in the usual manner as one
administers the hyaluronan or disease modifier either together or
individually.
Because toxicity may be associated with the disease modifier, toxicity
will have to be considered in the amounts bound through the HBM to the
hyaluronan. However because of the improvement in delivery by my
invention, less of the disease modifier may be required (than would be
used normally to treat the disease or condition) and therefore toxicity
concerns are less. Further where dosages of the hyaluronan exceed 200 mg
per person (for example a 70 kg person), side effects attributed to the drug
modifier may be reduced such as gastrointestinal distress, neurological
abnormalities, depression, etc.
The invention may thus be used to bind hyaluronan through HBM
to a protein or peptide. For example the protein tissue inhibitors of
metalloproteinases (TIMPS) which break down collagen can be made
recombinantly. HA binding motif (HBM) may be added to TIMPS in one
of the many known manners and the product can be combined with
CA 02205771 1997-OS-22
-30-
hyaluronan to form the dosage amount to be administered to a patient.
The amounts of TIMPS and HA are chosen in amounts suitable for use to
treat a patient in need of treatment. When administered to the patients
(for example by injection), the HA bound TIMPS goes to the site of injury
(the pathological tissue site which expresses a surplus of HA receptors) for
treatment of the injury. The invention can be used for administration of
any protein disease modifier.
Thus the invention can be used to target disease modifiers which
are proteins such as recombinant proteins or peptides such as TIMPS,
enzymes, collagenese, cytokines, growth facts, therapeutic proteins (such as
antibiotics which may be proteins).
Figures 1, 2, 3 and 4 are provided illustrating methods of binding
proteins to hyaluronan binding motifs.
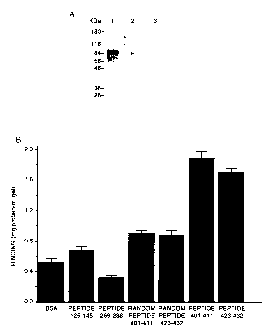
Figure 1 relates to the competition and direct binding assays of synthetic
peptides corresponding to positive charge clusters in RHAMM protein. Panel
A, transblotted RHAMM fusion protein was stained with a 1:3,000 dilution of
biotin-labeled HA that had been preincubated for 1 h with 3 mg/ml bovine
serum albumin (BSA) (lane 1), 3 mg/ml peptideaa401-411 (lane 2), or 3 mg/ml
peptideaa423-432 (lane 3). Both peptides significantly reduced the binding of
HA to RHAMM fusion protein. Panel B, HA-Sepharose affinity gel was
prepared according to the manufacturer's instruction/ RHAMM peptides
(peptideaa401-411 peptideaa423-432; randomized peptideaa401-411
(LKQKKVKKHIV); randomized peptideaa423-432 (QSKRLKKRVL);
peptideaa125-145 and peptideaa269-288 20) were applied to HA-Sepharose.
Unbound peptides were removed by washing the gel with PBS containing 0.15
M NaCI. The amounts of peptides applied and the unbound peptide removed
from the gel were determined by measuring their OD value. The results
CA 02205771 1997-OS-22
-31-
indicated that peptideaa401-411 and peptideaa423-432 bound in highest
amounts to HA-Sepharose gel.
Figure 2 relates to construction of recombinant RHAMM containing HA
binding domains. Panel A, cDNA encoding peptideaa401-411 (~) and
peptideaa423-432 (.) were, respectively, aligned by PCR to a cDNA encoding
RHAMM NH2-terminal polypeptideaal-238 that did not have the ability to
bind HA. This was carried out as described under "Experimental Procedures."
Both PCR products were digested with EcoRI and BamHI and purified with
agarose gel electrophoresis. The cDNAs were inserted into pGEX-2T opened
with BamHI and EcoRI, which were cloning sites that were followed by stop
codons, and transformed into HB101. The correct inserts were confirmed by
restriction endonuclease digestion of the selected clones and were expressed
as
glutathione S-transferase-RHAMM fusion proteins. Panel B, bacterial cell
lysates containing the glutathione S-transferase-RHAMM fusion proteins
were fractionated on SDS-PAGE, transblotted onto nitrocellulose
membrances, and visualized with either polyclonal antibody to peptideaa125-
145 (lanes 1-3) or biotin-labeled HA (lanes 4-6). The glutathione S-
transferase
fusion non-recombinant polypeptideaal-238 was used as a control (lanes 1 and
4). The linkage of either peptideaa401-411 (lanes 2 and 5) or peptideaa423-432
(lanes 3 and 6) to the NH2-terminal RHAMM polypeptideaal-238 created HA-
binding domains (lanes 5 and 6) although their antibody binding properties
remained the same (lanes 2 and 3).
Figure 3 relates to the deletion and mutation of HA binding domains in
RHAMM. (A) The HA binding domain II (aa423-432) was completely deleted
and the HA binding domain I (aa 401-411) was partially deleted. The
remaining domain (aa 401-411) was altered by mutating K405 and K409 to E.
(B) The PCR product (Figure 2A, lane 2) was ligated into the plasmid-
containing fragment (5.3 kb in lane 3) and transformed into E.coli HB101. The
CA 02205771 1997-OS-22
-32-
clone containing the correct insert (lane 4) was used to prepare RHAMM
fusion protein. (C) Cell lysates containing the complete fusion proteins
(lanes
2 and 5), deleted fusion protein (lanes 1 and 4) and HB101 lysate (lanes 3 and
6)
were prepared by sonication, then separated by SDS-PAGE and
immunoblotted. RHAMM protein was visualized using anti-RHAMM
antibody (lanes 1-3) or biotin-labelled HA (lanes 4-6). The results show that
after deletion of domains I and II, RHAMM lost its ability to bind to biotin-
labelled HA (lane 4). The bacterial lysate contains an HA binding protein of
<26 kDa that is not related to RHAMM (lanes 4-6).
Figure 4 relates to the strategy for defining the critical basic amino acids
that
determine the HA binding properties of domain II. To investigate the basic
amino acids required in domain II for HA binding, six independent
mutations were carried out and a RHAMM fusion peptide was genesuted
recombining domain II with the amino-terminus (aa 1-238) of RHAMM using
a recombinant technique. (A) The primers used to generate the altered
cDNAs. (B) The resultant amino acid sequences. Highlighted amino acids
indicate mutations. Six cDNAs, each containing site-directed mutations)
were generated in the PCRs diagrammed in panels A and B, using RHAMM
cDNA1-~20 as the template DNA and containing the oligonucleotides
encoding as 423-432 of RHAMM with different mutations. PCR products
from the six primers containing the mutated nucleotides were doubly
digested with BamHI+EcoRI, ligated into pGEX-2T and transformed into
HB101. Selected clones were confirmed to contain correct inserts by double
digestion with BamHI+EcoRI and electrophoresis on agarose gels. Fusion
proteins were prepared from clones and analyzed in Western blots with either
anti-RHAMM antibody to visualize RHAMM protein (C) or biotin-labelled
HA (D) to assay HA binding activity. The results show that the HA binding
ability of mutations I-VI (panel D) was reduced to 0, 67, 38, 21, 2 and 40%,
of
CA 02205771 2000-02-04
-33-
ability of mutations I-VI (panel D) was reduced to 0, 67, 38, 21, 2 and 40%,
of
the control (lane 1), respectively. Lane 1, control; lane 2, mutation I; lane
3,
mutation II; lane 4, mutation III; lane 5, mutation IV; lane 6, mutation V;
lane
7, mutation VI.
The amounts of disease modifiers and form of hyaluronan may be
those previously used even band together through HBM. Because of the
beneficial effects of the form of hyaluronan taking the disease modifier to
the
pathological tissue (having excess HA receptors) in need of treatment, less of
the disease modifier than would normally be expected to be used may be
useful to treat and resolve the condition/disease affecting the pathological
tissue. The amounts of the forms of hyaluronan may be those amounts
specified in W091 /04058 -- at least about l0mg of the form of hyaluronan i n
each dosage amount to in excess of 1000-1500mg of the form of hyaluronan i n
each dosage amount administered to a patient.
As many changes could be made to the examples without departing
from the scope of the invention, it is intended that all material herein be
interpreted as illustrative of the invention and not in a limiting sense.