Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
WO 96/17639 CA 02205936 1997-05-23 pCT/US95/15078
WINGED NEEDLE ASSEMBLY WITH PROTECTIVE MEMBER
FIELD OF THE INVENTION
The present invention generally relates to an intravenous (IV)
infusion set and in particular, to a winged needle assembly usable for
venipuncture that includes an integral protective member to reduce
accidental needle stick from such infusion sets.
BACKGROUND OF THE INVENTION
l0 Accidental stick from contaminated needles, syringes, and other
sharp medical equipment contaminated by contact with an infected
patient's bodily fluids pose a serious risk to healthcare workers. Proper
disposal of "sharps" significantly reduces accidental stick. For example,
standard procedures most often require used needles to be covered or
recapped prior to disposal.
A sheath is provided to protect the needle point from damage prior
to use and is often retained and refitted by the healthcare provider prior to
disposal of the used needle. However, replacing a protective sheath
requires the healthcare provider to align and push the sheath on the
2o needle. This exposes the worker's hand to the sharp end of the needle and
creates a new risk of needle stick injury. If the sheath is misplaced or lost,
the needle is often disposed of without being covered.
Recently, certain safety devices for infusion sets have been
developed to enclose or contain needles once they have been used.
However most of these devices have limitations such as excessive size or
require extra manipulation that frustrate their proper and easy use. For
example, the devices described in U.S. Patents 4,941,881, 5,069,341, and
CA 02205936 2005-06-22
-2-
5,330,438 are directed to protective sheaths that are slidably disposed on the
IV
tubing but require extra manipulation and/or are too bulky in size to be
reliably
and properly utilized.
The present invention overcomes the problems associated with prior
procedures and the recently described devices and provides new advantages.
SUMMARY OF THE INVENTION
In accordance with the invention, there is provided an intravenous needle
1 o assembly, comprising:
a hollow needle having a front portion with a sharp end and a rear portion;
a cylindrical hub for securing the rear portion of the needle so that the
front
portion of the needle projects axially forward from the cylindrical hub;
a first wing radially attached to the cylindrical hub and extending
i5 longitudinally parallel to the needle;
a hollow, longitudinally extending tubular member having a front end, a
rear end, and an axial bore for axial movement of the cylindrical hub within
the
bore;
a longitudinal slot in the hollow tubular member extending from the front
a o end to a lock-out position, the first wing engagable in the longitudinal
slot for
longitudinal movement of the first wing in the longitudinal slot to control
the axial
movement of the cylindrical hub within the bore of the hollow tubular member;
and
a second wing extending longitudinally outward from the front end of the
25 hollow tubular member, the second wing being radially perpendicular to the
longitudinal slot.
In particular, the intravenous needle assembly of the present invention
includes a hollow needle having a sharp end, a rear end, and a fluid
passageway
therebetween. A cylindrical hub circumferentially secures the rear portion of
the
3o needle so that a portion of the needle including the sharp end projects
forward
from the hub. A first wing extends longitudinally and radially outward from
the
hub. A hollow, longitudinally extending tubular member having a front end, a
rear end, and an axial bore is adapted to slidably receive the cylindrical
hub. A
CA 02205936 2005-06-22
-3-
longitudinal slot in the tubular member extends from the front edge to a lock-
out
position on the tubular member and is adapted to engage the extending first
wing
so that the longitudinal movement of the first wing in the slot controls the
axial
movement of the hub and needle within the bore of the hollow tubular member.
The slot has a length from the front end to the lock-out position that is
greater than
the portion of the needle projecting forward from the hub. Thus, when the
first
wing is at the lock-out position of the slot, the sharp end of the needle is
i o completely retracted within the hollow tubular member.
The invention also includes structure for automatically retaining the hub
and the used needle within the hollow tubular member. In a preferred
embodiment, the retaining structure includes a lateral notch at the lock-out
position of the slot that is adapted to automatically disengage the first wing
from
the slot and capture the first wing in the notch.
These and other objects, features, and advantages of this invention are
evident from the following description of a preferred embodiment of this
invention with reference to the accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
a o Figure 1 is an exploded perspective view of the winged needle assembly of
the present invention;
Figure 2 is a perspective view of an IV infusion set prior to use including
the winged needle assembly of the present invention;
Figure 3 is a perspective view of the winged needle assembly with the
a 5 sheath removed and the needle oriented for insertion into a patient's
vein;
Figure 4 is a perspective view of the winged needle assembly with the
wings spread fiat for securing to a patient during an IV infusion procedure;
Figure 4A is an enlarged cross-sectional view along line 4A-4A in Figure
4;
WO 96/17639 ~1 02205936 1997-05-23 pCT/US95115078
-4-
Figure 5 is a perspective view of the winged needle assembly
with the hub wing aligned in the. longitudinal slot and the needle
partially withdrawn into the protective member;
Figure 5A is an enlarged cross-sectional view along line 5A-5A of
Figure 5;
Figure 6 is a perspective view of the winged needle assembly after
the needle has been withdrawn from the patient and completely
retracted within the protective member and the hub wing disengaged
from the longitudinal slot;
Figure 7 is a longitudinal cross-section view along the line 7-7 in
Figure 4; and
Figure 8 is a longitudinal cross-section view along line 8-8 in
Figure 6.
~5 DETAILED DESCRIPTION
OF THE PREFERRED EMBODIMENT
While the present invention is susceptible of embodiments in
various forms, there is shown in the drawings and will hereinafter be
2o described a presently preferred embodiment, with the understanding
that the present disclosure is to be considered an exemplification of the
invention, and is not intended to limit the invention to the specific
embodiments illustrated.
An intravenous infusion set 10 having a winged needle assembly
25 12 according to the present invention is shown in Figure 2. The
infusion set also includes a section of hollow medical tubing 14, a
standard fluid connector 16 and a removable sheath 18 covering the
needle. A conventional female luer fitting is shown although other
WO 96/17639 ~ 02205936 1997-05-23 p~y[J$95/15078
-5-
standard fluid connectors such as a male adapter plug, for example, are
also suitable for fluidly connecting the set to a fluid source.
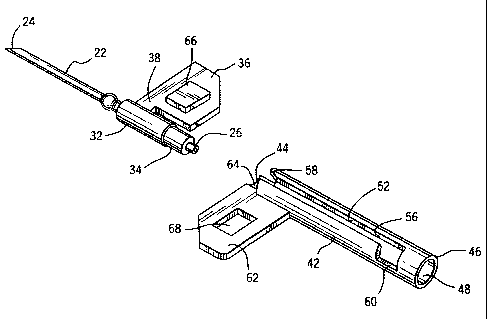
The winged needle assembly 12 of the present invention is best
understood with reference to Figure 1. The needle assembly 12 includes
a hollow needle 22 having a sharp end 24. The sharp end is preferably
beveled. The hollow needle is manufactured of stainless steel and has a
fluid passageway between the front end 24 and the rear end 26.
A cylindrical hub 32 circumferentially secures a rear portion of
the needle. The hub is preferably molded of a resilient plastic such as a
1o medical grade polypropylene or polyvinylchloride. The hub 32 may be
molded with the needle 22 in place or the needle may be secured to a
molded hub in a subsequent assembly step. The rear end of the hub is
constructed with a reduced diameter portion 34 for connecting the
medical tubing 14 to the fluid passageway of the needle 22. The medical
tubing 14 is permanently attached to the hub 32 by a solvent bond at 34
for example. The tubing 14 may form a slight outward radial shoulder
at the juncture with the hub. The purpose of this shoulder will be
pointed out in the description that follows.
A first wing 36 extends longitudinally and outward from the hub
32. The wing 36 is radially attached to the hub and is of sufficient size to
be easily grasped by a healthcare provider between the thumb and the
first finger to firmly control placement of the sharp end of the needle
extending from the hub 32. The wing is preferably connected to the hub
by a small stem portion 38 that has a longitudinal length which is less
than the length of the wing 36. By minimizing the portion of the wing
that is directly attached to the hub the overall length of the needle
assembly 12 becomes shorter, as will be apparent from the description
that follows. The stem portion 38 has generally the same thickness as
WO 96117639 ~ 02205936 1997-05-23 p~y[JS95/15078
-6-
the wing 36 to provide a solid connection of the wing 36 to the hub 32.
The wing 36 and stem 38 are preferably integrally manufactured with
the hub 32 as one piece by a plastic injection mold process for example.
A hollow tubular protective member 42 has a front end 44, a rear
end 46, and an axial bore 48. The protective member 42 is also
preferably manufactured from a moldable medical grade plastic. The
axial bore 48 of the protective member 42 is constructed with an inner
diameter of sufficient size to slidably receive the cylindrical hub 32 and
tubing 14. Thus, enough clearance is provided between the inner
1o diameter of the bore 48 and the outer diameter of the cylindrical hub 32
or tubing 14 to allow the hub and tubing to slide axially within the bore.
The protective member 42 also includes a longitudinal slot 52
that extends through the tubular surface of the hollow member and
into the axial bore 48. The slot 52 extends longitudinally from the front
end 44 of the protective member to a lock-out position 56 that is located
forward of the rear end 46.
The longitudinal slot 52, as manufactured and before further
assembly, has a predetermined slot width configuration. The
predetermined slot width is wide at the front end 44 of the tubular
2o member to facilitate entry of the first or hub wing 36 into the slot. The
predetermined slot width is narrow at the lock-out position 56 to resist
re-entry of the hub wing into the slot. Preferably, the predetermined
slot width converges inward from the front end 44 of the slot to a
minimal width at the lock-out position 56. The predetermined slot
width at the lock-out position 56 is always less than the thickness of the
engaged wing 36 or stem portion 38. Optimally, the preselected slot
width at the lock-out position 56 is zero. That is, the opposite sides of
the slot are in touching contact, and the slot closes on itself.
WO 96/17639 ~ 02205936 1997-05-23 p~/[I$95/15078
Because of the longitudinal split created by the slot 52, the tubular
protective member 42 has certain spring characteristics. These
characteristics allow the slot width as manufactured to increase to
accommodate the thickness of the wing 36 or stem portion 38.
Thus, the spring characteristics of the split plastic tubular
member 42 creates an effective slot width to accommodate the thickness
of the wing 36 or the stem portion 38. The effective slot width will not
cause the wing or stem to bind in the slot as the hub 32 moves through
the axial bore 48.
The longitudinal slot 52 has a length from the front end 44 to the
lock-out position 56 that is greater than the length of the exposed
portion of the needle 22 projecting forward from the hub 32. Thus,
when the first wing 36 is moved to the lock-out position 56 in the slot,
the sharp end 24 of the needle is completely retracted within the
protective member 42.
A lateral notch 60 is constructed at the lock-out position 56. The
lateral notch 60 automatically disengages the wing or stem portion from
the slot after the trailing edge of the wing or stem portion passes the
lock-out position 56. The lateral notch 60 is constructed with a radial
2o dimension that is greater than the thickness of the wing or stem
portion. The lateral notch 60 is also constructed with a longitudinal
length greater than the length of the wing 36 or stem portion 38. Thus,
when the trailing edge of the wing or stem to moves past the lock-out
position 56 and into the lateral notch 60, the wing or stem portion is no
longer engaged in the slot 52. The resiliency of the stretched tubular
member 32 causes the longitudinal slot 52 to spring back to the
manufactured predetermined slot width. At the lock-out position 56,
the slot contracts to the minimal preselected slot width that is less than
CA 02205936 1997-05-23
WO 9611?639 PCT/US95115078
_g_
the thickness of the wing or stem portion. The lateral notch 60 captures
the wing or stem portion and prevents either forward or rearward
movement of the wing 36 or stem 38. Thus the needle 22 is retained in
the protective member 32.
Since the longitudinal slot 52 does not extend the full length of
the protective member 32, the lateral notch 60 prevents the wing or
stem from sliding completely out the back of the protective member,
which would expose the needle point 24.
The longitudinal slot 52 also includes a tapered lead-in portion 58
l0 at the front end 44 of the protective member 42 to facilitate initially
engaging the wing 36 or stem portion 38 in the slot 52.
The protective member 42 also includes a second wing 62 that
extends longitudinally and outward along the front of the hollow
tubular member. The second wing 62 extends in the opposite direction
of the first wing 36 and is radially perpendicular to the slot 52.
The second wing 62 is positioned forwardly offset from the front
end 44 of the tubular member to create an open semi-circular arc
portion 64 at the front of the hollow member 42. The open arc portion
64 has generally the same or greater length as the wing 36 or stem
portion 38 and allows the wing or stem portion 38 to rotate freely
through an arc of 180°. Thus, the first wing 36 can rotate from
abutting
contact with the second wing 62 to a position directly opposite the fixed
orientation of the second wing. Preferably, at a perpendicular position
or approximately midway through the 180° arc, the first wing 36 rotates
into alignment with the tapered lead-in portion 58 of the longitudinal
slot 52.
The lateral notch 60 extends radially from the longitudinal
orientation of the slot in the direction of the second wing 62. The
WO 96/17639 CA 02205936 1997-05-23 p~'jy[7$95115078
_g_
lateral notch arc portion is approximately 90° and allows the first
wing
36 or stem portion 38 to be rotated into flat alignment with the second
wing 62 for disposal.
The two wings 36 and 62 also include a projection 66 and a recess
68 for temporarily securing the wings together. The wings are secured
together to increase needle placement control during the venipuncture
step described below. The wings also include diagonally opposite cut-off
portions 70 and 71 to assist in separating the temporarily secured wings.
When the winged needle assembly is initially assembled, the
unconnected tubing 14 is inserted through the rear end 46 and out the
front end 44 of the tubular member. The tubing is bonded to the
reduced diameter portion 34 of the hub. As previously described, the
tubing may have a slightly larger outer diameter at the juncture with
the hub than the hub outer diameter. This shoulder 72 is best seen in
Figure 7.
Also the hollow tubular member 42 is preferably constructed
with a reduced inner diameter annular portion 74 at the front end 44.
The reduced diameter portion initially locates the wing 36 or stem
portion 38 of the hub at the open semi-circular arc portion 64. The
2o reduced diameter portion 74 abuts against the shoulder 72 and prevents
the hub and needle from sliding axially forward out of the tubular
member from the initial position.
A venipuncture procedure that utilizes the winged needle
assembly of the present invention will now be described. The winged
needle infusion set is conventionally packaged in a sterile blister
package (not shown) with the open end sheath 18 covering the sharp
end of the needle 22, as shown in Figure 2. As previously described, a
section of medical tube 14 and a fluid connector 16 such as a female luer
WO 96/17639 ~ 02205936 1997-05-23 pCT/US95/15078
-10-
connector are attached to the rear end of the needle assembly. The two
wings 36 and 62 are initially folded and secured together. The connector
16 is removed from the packaging and attached in an aseptic manner to
a source of fluid. The wing needle infusion set is then primed with the
sheath 18 in place.
The healthcare provider then grasps the secured wings between
the thumb and first finger. As shown in Figure 3, the needle 22 is in a
bevel-up orientation and is readily inserted into a vein of the patient
after the sheath is removed.
1o As shown in Figure 4, once the needle 22 is properly positioned
in the vein, the two wings are rotated opposite to one another until
they are in a flat orientation in contact with the patient's skin. The
wings are then secured by tape to the patient's skin to minimize needle
movement.
To safely remove the winged needle infusion set from the
patient, the securing tape is removed. As shown in Figure 5, the wing
62 integral with the hollow tubular member 42 is then pressed against
the skin and the wing 36 integral with the hub is rotated into alignment
with the longitudinal slot 52. The tapered lead-in portion 58 at the
2o front end of the slot 52 facilitates alignment and entry of the wing 36 or
stem portion of the wing 36 into the slot. The medical tubing 14
connected to the winged needle assembly 12 is then pulled back, which
pulls the wing 36 or stem portion 38 through the slot 52 and withdraws
the needle 22 from the vein and into the hollow tubular protective
member 42.
When the wing or stem portion 38 reaches the lateral notch 60 at
the lock-out position of the slot, the wing or stem automatically
disengages from the slot. The resiliency of the plastic material of the
WO 96/17639 CA 02205936 1997-05-23 pCT/US95I15078
-11-
partially split tubular member 42 causes of the slot 52 to substantially
close on itself as seen at the lock-out position 56 in Figure 6. The
substantially closed slot prevents the wing or stem portion 38 from re-
engaging in the slot and thus prevents the sharp end of the needle from
moving out of the protective member 42.
As a further precaution and to reduce the profile of the used
needle assembly, the wing 36 can be rotated in the lateral notch into flat
alignment with wing 62. The winged needle infusion set 10 is now
ready for safe disposal with the needle retained in the tubular member
1o thus reducing further risk of accidental needle stick injury.
A key advantage of the present invention is that the sharp end 24
of the needle 22 is never exposed after it is inserted into the patient's
vein. The needle is pulled directly into the hollow tubular member 42
as it is withdrawn from patient's vein.
Another advantage of the present invention is that the lateral
notch 60 at the lock-out position 56 of the longitudinal slot 52
automatically disengages the wing or stem portion from the slot. The
lateral notch 60 further retains the wing or stem portion from re-
engaging in the slot, thus retaining the sharp end of the needle in the
2o protective member.
Another key advantage of the present invention is that the
protective member 42 is integrally assembled to the winged needle
infusion set. Integral attachment eliminates the possibility of the
protective member being lost or not attached and thus not used by the
healthcare provider. Also, the location of the protective member
immediately adjacent and in line with the rear portion of the needle 22
and the ease of use greatly increases the probability a contaminated
needle will be properly enclosed in the protective member prior to
WO 96/17639 ~ 02205936 1997-05-23 p~y[j$95/15078
-12-
disposal.
From the foregoing, it will be seen that numerous modification
and variation can be affected without departing the true spirit and scope
of the novel concept of the present invention. It is to be understood
that no limitation with respect to the specific embodiments is intended
or should be inferred. The disclosure is intended to cover all such
modification as fall within the scope of the appended claims.