Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
METHOD AND APPARATUS FOR CLEANING AMBIENT AIR BY CONTACT WITH A STATIONARY
SUBSTRATE.
RELATED APPLICATIONS
This application is a continuation-in-part of
Appl. No. 08/549,996, filed October 27, 1995, which is a
continuation-in-part of Appl. No. 08/537,206, filed
September 29, 1995, which is a continuation-in-part of
Appl. No. 08/410,445, filed March 24, 1995, which is a
continuation-in-part of Appl. No. 08/376,332, filed
January 20, 1995, all of which are incorporated herein by
reference. Appl. No. 08/549,996 is also a continuation-
in-part of Appl. No. 08/412,525, filed March 29, 1995,
which is a continuation-in-part of Appl. No. 08/410,445,
filed March 24, 1995, which is a continuation-in-part of
Appl. No. 08/376,332, filed January 20, 1995, all of
which are incorporated herein by reference.
BACKGROUND OF THE lNv~NllON
Field of the Invention
The present invention relates to a method and
apparatus for cleaning the atmosphere; and more
particularly to a stationary substrate comprising at
least one atmosphere contacting surface having a
pollutant treating material thereon.
Discussion of the Related Art
A review of literature relating to pollution
control reveals many references discussing the general
approach of cleaning waste gas streams entering the
environment. If too much of one pollutant or another is
detected as being discharged, steps are taken to reduce
the level of that pollutant, either by treating the gas
~ 30 stream or by modifying the process that produces the
pollutant. However, there has been little effort to
treat pollutants which are already in the environment;
the environment has been left to its own self cleansing
systems.
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
U.S. Patent No. 3,738,088 discloses an air
filtering assembly for cleaning pollution from the
- ambient air by utilizing a vehicle as a mobile cleaning
device. A variety of elements are used in combination
with a vehicle to clean the ambient air as the vehicle is
driven through the environment. In particular, modified
vehicles include ducting to control air stream velocity
and direct the air to a variety of filters, electronic
precipitators and catalyzed postfilters.
German Patent DE 43 18 738 C1 also discloses a
process for the physical and chemical cleaning of outside
air. Motor vehicles are used as carriers of conventional
filters and/or catalysts, which do not constitute
operational components of the vehicle but are used to
directly clean atmospheric air.
Another approach is discussed in U.S. Pat. No.
5,147,429, which is directed to a mobile airborne air
cleaning station. In particular, this patent features a
dirigible for collecting air with a plurality of
different types of air cleaning devices contained
therein. The air cleaning devices disclosed include wet
scrubbers, filtration machines, and cyclonic spray
scrubbers.
The difficulty with devices previously
disclosed for cleaning ambient air in the atmosphere is
that they re~uire new and additional equipment, and may
be required to be operated separately just to accomplish
such cleaning. For example, the modified vehicle
disclosed in U.S. Pat. No. 3,738,088 requires separate
ducting and filters, and the e~uipment laden dirigible of
U.S. Pat. No. 5,147,429 is operated solely for such
cleaning purposes.
German patent DE 40 07 965 C2 to Klaus Hager
discloses a catalyst comprising copper oxides for
converting ozone and a mixture of copper oxides and
manganese oxides for converting carbon monoxide. The
catalyst can be applied as a coating to a self heating
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96100781
radiator, oil coolers or charged-air coolers. The
catalyst coating comprises heat resistant binders which
are also gas permeable. It is indicated that the copper
oxides and manganese oxides are widely used in gas mask
filters and have the disadvantage of being poisoned by
water vapor. However, the heating of the surfaces of the
automobile during operation evaporates the water. In
this way, continuous use of the catalyst is possible
since no drying agent is necessary.
Manganese oxides are known to catalyze the
oxidation of ozone to form oxygen. Many commercially
available types of manganese compound and compositions,
including alpha manganese oxide are disclosed to ca~alyze
the reaction of ozone to form oxygen. In particular, it
is known to use the cryptomelane form of alpha manganese
oxide to catalyze the reaction of ozone to form oxygen.
Alpha manganese oxides are disclosed in
references such as O'Young, Hydrothermal Synthesis of
Manganese Oxides with Tunnel Structures, Modern
Analytical Techniques for Analysis of Petroleum,
presented at the Symposium on Advances in Zeolites and
Pillared Clay Structures before the Division of Petroleum
Chemistry, Inc. American Chemical Society New York City
Meeting, August 25-30, 1991 beginning at page 348. Such
materials are also disclosed in U.S. Patent No. 5,340,562
to O'Young, et al. Additionally, forms of ~-MnO2 are
disclosed in McKenzie, the Synthesis of Birnessite,
Cryptomelane, and Some Other Oxides and Hydroxides of
Manganese, Mineralogical Magazine, December 1971, Vol.
38, pp. 493-502. For the purposes of the present
invention, ~-MnO2 is defined to include hollandite
(~aMn8O~6.xH2O), cryptomelane (KMn8Ol6.xH2O), manjiroite
(NaMn8OI6.xH2O) and coronadite (PbMn8OI6.xH2O). O'Young
discloses these materials to have a three ~;m~n~ional
framework tunnel structure (U.S. Patent No. 5,34Q,562 and
O'Young Hydrothermal Synthesis of Manganese Oxides with
Tunnel Structures both hereby incorporated by reference).
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
For the purposes of the present invention, ~-MnO2 is
considered to have a 2 x 2 tunnel structure and to
include hollandite, cryptomelane, manjiroite and
coronadite.
Commonly assigned U.S. Pat. No. 5,422,331,
incorporated herein by reference, discloses methods and
catalyst compositions for abating noxious substances,
particularly ozone, contained in air. The treatment of
carbon monoxide, hydrogen sulfide and hydrocarbons is
also discussed. A primary focus of this patent is
methods of treating air taken into and/or circulated in
aircraft cabins, with the cabins of trains, buses and
other vehicles being mentioned as well. The patent also
indicates that the disclosed catalysts can be used to
abate ozone in equipment, such as xerographic copy
machines, which generate ozone. Further, the patent
indicates that the catalysts can be applied to surfaces
in air handling systems for residences, office and
factory buildings, public buildings, hospitals and the
like. For this method, the catalyst can be applied to
existing substrates of the air handling system, such as
fan blades in air handling fans or compressors, grills,
louvers or any other surface exposed to the air stream.
Responsive to the difficulties associated with
devices for proactively treating the atmosphere, the
Assignee herein in U.S. Appl. No. 08/410,445, filed March
24, 1995, disclosed apparatus and related methods for
treating the atmosphere by employing a moving vehicle.
In preferred embodiments a portion of the cooling system
(e.g. the radiator) is coated with a catalytic or
adsorption composition. Additionally, a fan associated
with the cooling system can operate to draw or force air
into operative contact with the radiator. Pollutants
cont~; n~ within the air such as ozone and/or carbon
m~n~; de are then converted to non-polluting compounds
(e.g. oxygen gas and carbon dioxide).
CA 02206460 1997-0~-29
W 096/22149 PCTrUS96/00781
U.S. Appl. No. 08/412,525 ('525), of which the
present application is a continuation-in-part, discloses
- methods and apparatus for treating pollutants present in
the atmosphere, by the use of a stationary substrate
coated with pollutant treating composition. The present
application is directed to particular embodiments of the
invention set forth in the '525 application, directed at
coating various surfaces which contact the atmosphere
with pollution treating compositions.
SUMMARY OF THE INVENTION
The present invention relates to apparatus,
methods and compositions to treat the atmosphere to
remove pollutants therefrom. The term "atmosphere" is
defined herein as the mass of air surrounding the earth.
The term "ambient air" shall mean the atmosphere which is
naturally or purposefully drawn or forced towards a
pollutant treating substrate. It is also intended to
include air which has been heated either incidentally or
by a heating means.
The present invention is generally directed to
a method for treating the atmosphere comprising passing
ambient air over a stationary substrate having at least
one air contacting surface having a pollutant treating
material thereon. The stationary substrate is any
substrate that can be modified, for example by coating,
to contain the pollutant treating material. For purposes
of this application, a substrate is considered stationary
when it is operatively attached to a non-moving
structure. For example, the fan or adjustable louvers of
an air handling system for a building are considered
stationary, even though the fan revolves and the louvers
~ can be moved.
In one embodiment of the present invention, the
pollutant treating substrate is a surface which already
exists on a stationary object. This includes surfaces,
as discussed above and further below, such as heat
-
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
exchange surfaces, fan blades, building exteriors, duct
surfaces, and so forth.
Preferably the surface is one which permits
periodic rejuvenating of the pollutant treating material.
Such rejuvenating may include cleaning, reactivating
and/or replacing the pollutant treating material on the
substrate, or any other process which restores the active
properties of the material. Suitable cleaning processes
include water washing, steam washing or air lancing.
Such cleaning processes can be used to remove
cont~m;n~nts from the pollutant treating material, or to
remove some or all of the material prior to applying
fresh material to the substrate. Reactivating steps
include, but are not limited to, thermal processes to
remove volatile pollutants or other cont~m;n~nts which
can be volatilized by th~rm~l treatment, and chemical
processes to restore the pollutant treating properties of
the material.
In another embodiment of the present invention,
the pollutant treating substrate can be a surface of an
additional component which can be added to a stationary
object. For example, a pollutant treating substrate can
be included in a device which is pPrm~n~ntly or removably
mounted on an existing air-handling system so as to
provide a pollutant treating substrate in the path of the
air flow without substantial alteration to the existing
equipment. The added substrate is preferably in the form
of a replaceable device, to facilitate replacement or
rejuvenation of the pollutant treating material.
Alternatively, the substrate may be pPrm~npntly mounted
in a manner which permits rejuvenating of the pollutant
treating material in place.
A key aspect of the present invention is that
it is directed to reducing levels of pollutants in the
atmosphere in general, rather than to treating an
airstream being drawn or forced into or out of a confined
space, such as a building. The ambient air may be
-
CA 02206460 l997-0~-29
W 096/22149 PCTrUS96/00781
drawn over the substrate by natural wind currents or by
the use of an air drawing means such as a fan or the like
to draw or force ambient air into operative contact with
the substrate having the pollutant treating composition
thereon.
In one embodiment of the present invention, the
pollutant treating process is carried out at or below
ordinary room temperature, which is defined for purposes
of this application as about 25~C. As discussed below,
most adsorbents and many catalysts can be used at such
temperatures. Methods and apparatus which can operate at
below ordinary ambient temperatures are desirable because
they do not require additional heating.
In another embodiment, the pollutant treating
process is carried out at temperatures above the 25~C
ordinary room temperature. Such elevated temperatures
may be necessary to activate the pollutant treating
material, particularly certain catalysts, or may simply
improve the efficiency of the treatment process. The
elevated temperatures may be provided by either heating
the air prior to its contact with the treatment surface,
by heating the treatment surface, or by heating both.
Such heating may be the result of purposefully heating
the air or the surface, or by the use of a system in
which the air or the surface is normally at a temperature
above 25~C. Furthermore, it is not necessary that the
heating be continuous, but only that the temperature at
the air contacting surface be above the desired
temperature for at least a measurable period of time, to
allow the treatment to proceed for that period of time.
For example, an exterior surface which is heated during
the daylight by the sun, could be catalytically active
just during the day, and this may be satisfactory for
treating a pollutant which is at particularly high levels
during the day. The present invention is also applicable
to processes where the ambient air or treatment surface
is heated by contact with a object which is normally
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
-- 8
heated for other purposes, either continuously or
intermittently, such as the coils of an air conditioning
- condenser.
The present invention is directed to
compositions, methods and articles to treat pollutants in
air. Such pollutants may typically comprise from 0 to
400 parts, more typically 1 to 300, and yet more
typically 1 to 200, parts per billion (ppb) ozone; 0 to
30 parts, and more typically 1 to 20, parts per million
(ppm) carbon monoxide; and 2 to 3000 ppb unsaturated
hydrocarbon compounds such as C2 to about C20 olefins and
partially oxygenated hydrocarbons such as alcohols,
aldehydes, esters, ethers, ketones and the like. Other
pollutants present may include nitrogen oxides and sulfur
oxides. The National Ambient Air Quality Standard for
ozone is 120 ppb, and for carbon monoxide is 9 ppm.
Pollutant treating compositions include
catalyst compositions useful for catalyzing the
conversion of pollutants present in the atmosphere to
non-objectionable materials. Alternatively, adsorption
compositions can be used as the pollutant treating
composition to adsorb pollutants which can be destroyed
upon adsorption, or stored for further treatment at a
later time.
Catalyst compositions can be used which can
assist in the conversion of the pollutants to harmless
compounds or to less harmful compounds. Useful and
preferred catalyst compositions include compositions
which catalyze the reaction of ozone to form oxygen,
catalyze the reaction of carbon m~no~ide to form carbon
dioxide, and/or catalyze the reaction of hydrocarbons to
form water and carbon dioxide. Specific and preferred
catalysts to catalyze the reaction of hydrocarbons are
useful for catalyzing the reaction of low molecular
weight unsaturated hydrocarbons having from two to twenty
carbons and at least one double bond, such as C2 to about
C8 mono-olefins. Such low molecular weight hydrocarbons
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
have been identified as being sufficiently reactive to
cause smog. Particular olefins which can be reacted
include propylene and butylene. A useful and preferred
catalyst can catalyze the reactions of both ozone and
carbon monoxide; and preferably ozone, carbon monoxide
and hydrocarbons.
Ozone - Useful and preferred catalyst
compositions to treat ozone include a composition
comprising manganese compounds including oxides such as
Mn2O3 and MnO2 with a preferred composition comprising ~-
MnO~, and cryptomelane being most preferred. Other useful
and preferred compositions include a mixture of MnO2 and
CuO. Specific and preferred compositions comprise
hopcalite which contains CuO and MnO2 and, more preferably
Carulite~ which contains MnO2, CuO and Al2O3 and sold by
the Carus Chemical Co. An alternative composition
comprises a refractory metal oxide support on which is
dispersed a catalytically effective amount of a palladium
component and preferably also includes a manganese
component. Also useful is a catalyst comprising a
precious metal component, preferably a platinum component
on a support of coprecipitated zirconia and manganese
oxide. The use of this coprecipitated support has been
found to be particularly effective to enable a platinum
component to be used to treat ozone. Yet another
composition which can result in the conversion of ozone
to oxygen comprises carbon, and palladium or platinum
supported on carbon, manganese dioxide, Carulite~ and/or
hopcalite. Manganese supported on a refractory oxide
such as alumina has also been found to be useful.
Carbon Monoxide - Useful and preferred catalyst
compositions to treat carbon monoxide include a
composition comprising a refractory metal oxide support
on which is dispersed a catalytically effective amount of
a platinum or palladium component, preferably a platinum
component. A most preferred catalyst composition to
treat carbon monoxide comprises a reduced platinum group
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 10
component supported on a refractory metal oxide,
preferably titania. Useful catalytic materials include
- precious metal components including platinum group
components which include the metals and their compounds.
Such metals can be selected from platinum, palladium,
rhodium and ruthenium, gold and/or silver components.
Platinum will also result in the catalytic reaction of
ozone. Also useful is a catalyst comprising a precious
metal component' preferably a platinum component on a
support of coprecipitated zirconia and manganese dioxide.
Pre~erably, this catalyst embodiment is reduced. Other
useful compositions which can convert carbon monoxide to
carbon dioxide include a platinum component supported on
carbon or a support comprising manganese dioxide.
Preferred catalysts to treat such pollutants are reduced.
Another composition useful to treat carbon monoxide
comprises a platinum group metal component, preferably a
platinum component, a refractory oxide support,
preferably alumina and titania and at least one metal
component selected from a tungsten component and rhenium
component, preferably in the metal oxide form.
Hydrocarbons - Useful and preferred catalyst
compositions to treat unsaturated hydrocarbons including
C2 to about C20 olefins and typically C2 to C8 mono-olefins
such as propylene and partially oxygenated hydrocarbons
as recited have been found to be the same type as recited
for use in catalyzing the reaction of carbon monoxide
with the preferred compositions for unsaturated
hydrocarbons comprising a reduced platinum component and
a refractory metal oxide support for the platinum
component. A preferred refractory metal oxide support is
titania. Other useful compositions which can convert
hydrocarbons to carbon dioxide and water include a
platinum component supported on carbon or a support
comprising manganese dioxide. Preferred catalysts to
treat such pollutants are reduced. Another composition
useful to convert hydrocarbons comprises a platinum group
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
metal component, preferably a platinum component, a
refractory oxide support, preferably alumina and titania
and at least one metal component selected from a tungsten
component and rhenium component, preferably in the metal
oxide form.
Ozone and Carbon Monoxide - A useful and
preferred catalyst which can treat both ozone and carbon
monoxide comprlses a support such as a refractory metal
oxide support on which is dispersed a precious metal
component. The refractory oxide support can comprise a
support component selected from the group consisting of
ceria, alumina, silica, titania, zirconia, and mixtures
thereof. Also useful as a support for precious metal
catalyst components is a coprecipitate of zirconia and
manganese oxides. Most preferably, this support is used
with a platinum component and the catalyst is in reduced
form. This single catalyst has been found to effectively
treat both ozone and carbon monoxide. Other useful and
preferred precious metal components are comprised of
precious metal components selected from palladium and
also platinum components with palladium preferred. A
combination of a ceria support with a palladium component
results in an effective catalyst for treating both ozone
and carbon ~ono~;de. Other useful and preferred
catalysts to treat both ozone and carbon monoxide include
a platinum group component, preferably a platinum
component or palladium component and more preferably a
platinum component, on titania or on a combination of
zirconia and silica. Other useful compositions which can
convert ozone to oxygen and carbon monoxide to carbon
dioxide include a platinum component supported on carbon
or on a support comprising manganese dioxide. Preferred
catalysts are reduced.
Ozone Carbon Monoxide and Hydrocarbons - A
useful and preferred catalyst which can treat ozone,
carbon monoxide and hydrocarbons, typically low molecular
weight olefins (C2 to about C20) and typically C2 to C8
-
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 12 -
mono-olefins and partially oxygenated hydrocarbons as
recited comprises a support, preferably a refractory
- metal oxide support on which is dispersed a precious
metal component. The refractory metal oxide support can
comprise a support component selected from the group
consisting of ceria, alumina, titania, zirconia and
mixtures thereof with titania most preferred. Useful and
preferred precious metal components are comprised of
precious metal components selected from platinum group
components including palladium and platinum components
with platinum most preferred. It has been found that a
combination of a titania support with a platinum
component results in the most effective catalyst for
treating ozone, carbon monoxide and low molecular weight
gaseous olefin compounds. It is preferred to reduce the
platinum group components with a suitable reducing agent.
Other useful compositions which can convert ozone to
oxygen, carbon monoxide to carbon dioxide, and
hydrocarbons to carbon dioxide include a platinum
component supported on carbon, a support comprising
manganese dioxide, or a support comprising a
coprecipitate of manganese oxides and zirconia.
Preferred catalysts are reduced.
The above compositions can be applied by
coating to at least one atmosphere contacting surface.
Particularly preferred compositions catalyze the
destruction of ozone, carbon m~noxl de and/or unsaturated
low molecular weight olefinic compounds at ambient
conditions or a-mbient operating conditions. Ambient
conditions are the conditions of the atmosphere. By
ambient operating conditions it is meant the conditions,
such as temperature, of the atmosphere contacting surface
during normal operation without the use of additional
energy directed to heating the pollutant treating
composition. Certain atmosphere contacting surfaces can
be at the same or similar temperature as the atmosphere.
It has been found that preferred catalysts which catalyze
CA 02206460 1997-05-29
W O 96/22149 PCTrUS96/00781
- 13 -
the reaction of ozone can catalyze the reaction of ozone
at ambient conditions in ranges as low as 5~C to 30~C.
~ Atmosphere contacting surfaces may have higher
temperatures than the ambient atmospheric temperatures
due to the nature of the operation of the component
underlying the surface. For example, among the preferred
atmosphere contacting surfaces are the heat transfer
surfaces of air conditioning or steam condensers due to
their high surface area and elevated temperatures during
normal operation, due to the nature of their operation.
The actual surface temperature will vary widely depending
on the type of equipment in use. Typical home air
conditioning condensers may operate at surface
temperatures which range up to about 60~C and typically
are from about 40~C to 50~C. Steam condensers may
operate over a wide range of surface temperatures,
depending on the temperature and pressure of the steam.
The temperature range of these atmosphere contacting
surfaces helps to enhance the conversion rates of the
ozone, carbon monoxide and hydrocarbon catalysts
supported on such surfaces. The catalysts useful in the
present invention are particularly ef~ective at the
higher temperatures present on the surfaces of such
equipment.
Various of the catalyst compositions can be
combined, and a combined coating applied to the
atmosphere contacting surface. Alternatively, different
surfaces or different parts of the same surface can be
coated with different catalyst compositions.
The method and apparatus of the present
invention are preferably designed so that the pollutants
can be treated at ambient conditions or at the ambient
operating conditions of the atmosphere contacting
surface. The present invention is particularly useful
for treating ozone by coating atmosphere contacting
surfaces with suitable catalysts useful to destroy such
pollutants even at ambient conditions, and at surface
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 14 -
temperatures typically from at least 0~C, preferably from
10~C to 105~C, and more preferably from 40~C to 100~C.
- Carbon monoxide is preferably treated at atmosphere
contacting surface temperatures from 20~C to 105~C. Low
molecular weight hydrocarbons, typically unsaturated
hydrocarbons having at least one unsaturated bond, such
as C2 to about C20 olefins and typically C2 to C8 mono-
olefins, are preferably treated at atmosphere contacting
surface temperatures of from 40~C to 105~C. The percent
conversion of ozone, carbon monoxide and/or hydrocarbons
depends on the temperature and space velocity of the
atmospheric air relative to the atmosphere contacting
surface, and the temperature of the atmosphere contacting
surface.
Thus, in a preferred embodiment of the present
invention, ambient levels of ozone, carbon monoxide
and/or hydrocarbon are reduced without the addition of
any mechanical features or energy source to existing
stationary substrates. The pollutant treating surface
may be one which is already present on the stationary
substrate, or one which is added as a removable or
pPrm~n~ntly mounted unit. Preferably, the catalytic
reaction takes place at the normal ambient operating
conditions experienced by the surfaces of stationary
substrate so that no changes in the construction or
method of operation are required.
While the preferred embodiments of the present
invention are directed to the destruction of pollutants
at the ambient operating temperatures of the atmosphere
contacting surface, it is also desirable to treat
pollutants which have a catalyzed reaction temperature
higher than the ambient temperature or ambient operating
temperature of the available atmosphere contacting
surface. ~uch pollutants include hydrocarbons and
nitrogen oxides and any carbon monoxide which bypasses or
is not treated at the atmosphere contacting surface.
These pollutants can be treated at higher temperatures
CA 02206460 1997-0~-29
W O96122149 PCTrUS96100781
- 15 -
typically in the range of at least 100 to 450~C. This
can be accomplished, for example, by the use of an
auxiliary heated catalyzed surface. By an auxiliary
heated surface, it is meant that there are supplemental
means to heat the surface. A preferred auxiliary heated
surface is the surface of an electrically heated
catalyzed monolith such as an electrically heated
catalyzed metal honeycomb of the type known to those
skilled in the art. Another preferred auxiliary heated
surface is one heated by a process stream, such as steam
or hot water, which may be readily available in
industrial plants or commercial facilities. Furthermore,
when the air is being forced through a heat exchanger,
then a heated fluid passing in or out of the heat
exchanger, or a side stream thereof, can be used as the
source of heat for the auxiliary surface.
The catalyst composition can be any well known
oxidation and/or reduction catalyst, preferably a three
way catalyst (TWC) comprising precious group metals such
as platinum, palladium, rhodium and the like supported on
refractory oxide supports. An auxiliary heated catalyzed
surface can be used in combination with, and preferably
downstream of, an ambient temperature atmosphere
contacting surface to further treat the pollutants.
As previously stated, adsorption compositions
can also be used to adsorb pollutants such as
hydrocarbons and/or particulate matter for later
oxidation or subsequent removal. Useful and preferred
adsorption compositions include zeolites, other molecular
sieves, carbon, and Group IIA alkaline earth metal oxides
such as calcium oxide. Hydrocarbons and particulate
matter can be adsorbed from 0~C to 110~C and subse~uently
treated by desorption followed by catalytic reaction or
incineration.
It is preferred to coat areas of the stationary
substrate that have a relatively high surface area
exposed to a large flow rate of atmospheric air. For
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 16 -
this reason, the surfaces of air-cooled heat exchangers
are particularly desirable, because they are designed for
high surface area and high exposure to air flow. When a
separate pollutant treating device is added onto existing
equipment, then such a device can desirably be modeled as
a heat exchanger, to provide maximum air contact area.
Furthermore, if a heat exchanger is used as an add-on
device, then a heating fluid can be channeled through the
heat exchanger to elevate the temperature of the
pollutant treating substrate. This may be particularly
desirable when catalysts which require elevated
temperatures are used.
The present invention also includes methods to
coat pollutant treating compositions onto atmosphere
contacting surfaces of stationary substrates.
BRIEF DESCRIPTION OF THE DRAWINGS
The following drawings in which like reference
characters indicate like parts are illustrative of
embodiments of the invention and are not intended to
limit the invention as encompassed by the claims forming
part of the application.
Figure 1 is a schematic representation of one
embodiment of an atmospheric pollutant treating device in
accordance with the present invention, wherein the device
is an air conditioning csn~enRer~
Figure 2 is a schematic representation of
another embodiment of the present invention, in which a
pollutant treating substrate is added to a commercial or
industrial air cooled heat ~ch~nger~
Figure 3 is a schematic representation of a
particular type of air cooled heat exchanger.
Figures 4-9 and 11-12 are plots of CO
conversion versus temperature using the different
catalysts of Examples 4, 9-12, 14 and 15.
Figure 10 is a plot of propylene conversion
versus temperature based on Example 14.
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
Figure 13 is a plot of ozone conversion versus
temperature based on Example 17.
Figure 14 is a schematic representation of a
test model air conditioning condenser.
Figure 15 is a graph showing ozone conversion
versus time for three catalyst test patches.
Figure 16 is an IR spectrum for cryptomelane.
Figure 17 is an XRD pattern for cryptomelane
shown as counts using a square root scale versus the
Bragg angle, 2~.
DETAILED DESCRIPTION OF THE INVENTION
The present invention is directed to apparatus
and methods for treating the atmosphere in which a
stationary substrate has a pollutant treating composition
thereon. When air is drawn or forced into contact with
the substrate the pollutants are caused to change into
non-polluting compounds. The atmosphere contacting
surface of the substrate which has the pollutant treating
composition thereon is one which is in direct contact
with the ambient air.
There are many atmosphere contacting surfaces
which can be used as the pollutant treating substrates in
accordance with the present invention. The substrate may
be part of an existing air handling systems, such as
those used in residential, commercial and industrial
buildings, as well as power plants, oil refineries,
chemical plants and any other facility in which air is
drawn or forced over coatable surfaces. Such air
handling systems include the outdoor components of
heating, ventilation and air conditioning systems
(referred to collectively as HVAC systems), industrial
cooling systems, or any other system in which ambient air
is naturally or purposefully forced into contact with a
suitable substrate.
The outdoor components of HVAC systems,
particularly condensers, include fans for blowing or
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
- 18 -
forcing ambient air over external heat transfer surfaces,
such as cooling coils or fins. In such systems, the
ambient air passes over the heat transfer surface and
returns to the atmosphere. Suitable substrates for
applying pollutant treating materials include any exposed
surfaces, such as fan blades, duct and plenum surfaces,
louvers, grills, motor housings, filtration media,
screens, and heat transfer surfaces.
As discussed above, heat transfer surfaces in
HVAC, as well as other air handling systems, include
various coils, fins, plates or other surfaces which are
designed to transfer heat to or from ambient air. Of
particular use as substrates for catalytic pollutant
treating materials are those surfaces which are at
elevated temperatures above st~n~rd ambient temperature
of about 25~C. Desirably, the substrates are of even
higher temperatures, because many catalysts are more
effective at higher temperatures. In air conditioning
systems, the coils or fins used to dissipate accumulated
heat to the atmosphere are usually at temperatures above
25~C, and often much higher, depending on the coolant and
operating parameters. Such heat dissipation apparatus
can be as small as the external coils of a window air
conditioner, or as large as the cooling towers used for
commercial buildings. In addition, surfaces downstream
from the heat transfer equipment, such as plenum or duct
walls, fan blades or grills, may also be at elevated
temperatures for improved catalytic activity.
Air handling systems are also used for many
operations other than HVAC. Cooling towers are used to
dissipate excess heat from various industrial sources.
In power plants, cooling towers dissipate heat from
relatively low temperature (but above 25~C) water or
steam from which no further useful power can be
extracted. In such systems, huge amounts of air are
blown over heat transfer surfaces, or directly over or
through the water being cooled. Suitable substrates for
CA 02206460 1997-0~-29
W O 96/22149 PCTrUS96/00781
- 19
coating in accordance with the present invention include
- fan blades, walls of cooling towers, heat transfer
surfaces and any other surfaces exposed to the flow of
air.
In chemical plants, oil refineries and the
like, there are many surfaces suitable for use as
pollutant treating substrates. Such plants include large
air handling systems and cooling towers, as generally
discussed above. For example, low temperature process
steam is cooled to condense it to water prior to
returning it to the boiler. Various operating units may
include air cooling systems, such as external fins, to
dissipate excess heat.
As discussed above, many air handling systems
provide a means, such as a fan, to circulate air ambient
air over a heated surface for the purpose of cooling that
surface. Generally the fan only circulates air over the
surface when the equipment is operating. In accordance
with a preferred embodiment of the present invention, the
fan in such an intermittently operated system can be set
to operate continuously to allow the pollutant treating
process to continue even when the equipment is not
otherwise operating. In a variation on this process, a
temperature sensor can be provided in the air handling
system, which can switch the circulating fan on or off in
response to the temperature at one or more points in the
system. Thus, even when a condenser or heat exchanger is
not operating, the latent heat can still be used to
catalyze pollutants. The operation of such a system
would depend on the desired temperature needed to treat a
particular pollutant. Alternatively, the fan can be set
to operate in response to some other external variable,
such as the level of a particular pollutant, or at
particular time intervals. In another alternative, a
m~nl~l or remote control switch could be provided to
actuate the fan of one or multiple air handling systems.
For example, all of the air circulating fans in an area
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 20 -
could be actuated simultaneously from a central
controller in response to detection of a high level of a
pollutant, or at particular times of the day.
In addition to the substrates which can be
coated in active air handling systems, there are many
surfaces which are naturally or passively exposed to a
flow of ambient air. Some of these surfaces are
particularly suitable for coating with pollutant treating
compositions in accordance with the present invention.
In this regard, sur~aces which are also at elevated
temperatures, either by contact with a source of heat or
by exposure to the sun, are especially suitable. For
example, the exterior surfaces of buildings or industrial
equipment may be suitable substrates. As air blows over
these surfaces, pollutant treating compositions can
reduce the levels of pollutants in the air. Exposed
surfaces of buildings may be at elevated temperature due
to solar heating or heat loss from the building. Process
equipment in refineries and chemical plants is often at
elevated temperatures, making them particularly suitable
for catalytic substrates.
When such passive treatment systems are used
with a pollutant treatment material which is more
effective at an elevated temperature, it is not necessary
for the surface or air to be at such elevated temperature
continuously. It is enough for the contacting surface to
attain the desired temperature for any measurable period
of time. Then, at least during that period of time, the
pollutant treating process can operate at such desired
temperature.
Roofs of buildings are of particular interest
as pollutant treating substrates, because they are
especially heated by the sun and internal heat from the
building, and are naturally exposed to ambient air flow.
Pollutant treating compositions can be incorporated into
various roofing materials, such as shingles, tar or tar
paper, or may be sprayed or painted onto existing
-
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 21 -
surfaces. In like manner, road surfaces are also an
excellent substrate to support pollutant treating
compositions. As with roofs, road surfaces are heated by
the sun and exposed to large flows of ambient air. In
addition, exhaust from the vehicles on the roads results
in localized concentrations of pollutants, rendering
treated road surfaces particularly useful and efficient
for reducing atmospheric pollution. The pollutant
treating compositions can be incorporated into the paving
materials, or applied as a topcoat to existing roadways.
Another reason why chemical plants, oil
refineries and power plants are specifically identified
for treatment by the methods of the present invention is
that these facilities are already subject to stringent
air pollution requirements, and reductions in ambient
pollution levels can translate into increased profit.
Furthermore, equipment and processes in such plants may
produce various localized areas of high pollutant
concentration, where the treatment compositions can be
most effective. For example, industrial plants include
many electric motors, some particularly large, which may
produce relatively high localized ozone levels due to
electric arcing. Ozone treating materials can be applied
to the motor casings, or to other surfaces in the
vicinity of the motors. Further, ventilation systems
exhausting air from buildings or enclosures containing
electrical equipment which produces ozone, such as motors
or transformers, can be coated to reduce ozone levels.
Furthermore, transformers may also emit other pollutants,
such as hydrocarbons, which may also be treated.
Another possible source of ozone in industrial
plants are electrostatic precipitators. These are
commonly found in dust handling equipment, such as bag
houses, in which electric fields are used to remove dust
from an air stream. In generating the electric fields,
arcing may occur, which can result in the formation of
large amounts of ozone. Treatment surfaces can be in the
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
path of the air flow, as discussed above for air-handling
systems, or can be on the exterior of the equipment or
the enclosures housing the equipment where there may be
high localized ozone concentrations.
Parent U.S. Pat. Appl. No. 08/412,525, already
incorporated by reference, also discusses applying
pollutant treating compositions to free st~n~lng objects
such as billboards or signs. More generally, any free
standing object with exposed surfaces could be used as a
substrate in accordance with the present invention. In
addition to billboards and signs, such objects may
include flagpoles, utility poles, including wires and
equipment carried thereon, transmission antennae (which
may also have localized high levels of ozone), storage
tanks, bridges or the like. The key point is that the
object include a surface which is exposed to ambient air
and can act as a substrate for carrying a pollutant
treating composition. Preferably, the surface is also
heated, either naturally or by some source of applied
heat.
Another variation on coating free st~n~;ng
objects is to erect structures specifically designed for
treating air. This can included adding baffles, wings or
other structures to buildings at the locations of
exceptional wind flow. For example, wings could extend
from the corners of buildings, taking advantage of the
geometry of the building and the prevailing ambient wind
currents. Such baffles or wings can either be solid or
porous, with porous structures offering the ability to
increase active surface area.
An advantage of the present invention is that
the atmosphere contacting surface useful to support a
pollution treating composition can be any existing
surface which lies in the path of a flow of ambient air.
Accordingly, the apparatus and method of the present
invention can be located on new components or retrofitted
onto old ones.
CA 02206460 1997-0~-29
wo96l22l4s PCT~S96/00781
- 23 -
Pollutant treating compositions include
catalyst compositions useful for catalyzing the
conversion of pollutants present in the atmosphere to
non-objectionable materials. Alternatively, adsorption
compositions can be used as the pollutant treating
composition to adsorb pollutants which can be destroyed
upon adsorption, or stored for further treatment at a
later time.
Catalyst compositions can be used which can
convert the pollutants to harmless compounds or to less
harmful compounds. Useful and preferred catalyst
compositions include compositions which catalyze the
reaction of ozone to form oxygen. The compositions can
be applied by coating at least one atmosphere contacting
surface. Particularly preferred compositions catalyze
the destruction of ozone at ambient conditions.
Various catalyst compositions can be combined,
and a combined coating applied to the atmosphere
contacting surface. Alternatively, different surfaces or
different parts of the same surface can be coated with
different catalyst compositions.
The method and apparatus of the present
invention are preferably designed so that the pollutants
can be treated at ambient conditions, requiring no
heating means or incidental heat. The present invention
is particularly useful for treating ozone by coating a
surface with suitable catalysts useful to destroy such
pollutants at ambient conditions. The percent conversion
of ozone depends on the temperature and space velocity of
the atmospheric air relative to the catalyst surface, and
- the temperature of the atmosphere contacting surface.
Accordingly, the present invention, in one
- embodiment results in at least reducing the ozone levels
present in the atmosphere without the addition of any
mechanical features or energy source to existing
substrates. Additionally, the catalytic reaction of
ozone to oxygen takes place at the normal ambient
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 24 -
conditions experienced by the surfaces of these
substrates so that m; n 1 m~ 1 changes in the construction or
method of operation are required.
While some embodiments of the present invention
are directed to the destruction of pollutants at ambient
operating temperatures, it will be noted that the ambient
air may be heated by a heating means such as a heater or
by incidental contact with a heated component of the
stationary substrate. This may allow other pollutants to
be catalyzed which require a higher reaction temperature
than the ambient temperature or ambient operating
temperature of the atmosphere contacting surface. Such
pollutants include carbon monoxide, hydrocarbons and
nitrogen oxides. These pollutants can be treated at
higher temperatures typically in the range of about 40~C
to 450~C.
It is preferred to coat areas of the substrate
that have a relatively high surface area exposed to a
large flow rate of atmospheric air. Air is drawn or
forced over the catalytic surface. The present invention
includes methods to coat pollutant treating compositions
onto ambient air contacting surfaces as described herein.
In particular, the present invention includes a method to
coat catalyst compositions onto various metallic
2 5 surfaces.
The present invention can be applied to any
stationary substrate with at least one atmosphere
contacting surface comprising a pollutant treating
composition (e.g. a catalyst or an adsorber) located
thereon. As the atmospheric air encounters the pollutant
treating composition, various pollutants including
particulate matter and/or gaseous pollutants carried in
the air can be catalytically reacted or adsorbed as the
case may be by the pollutant treating composition located
on the atmosphere contacting surface.
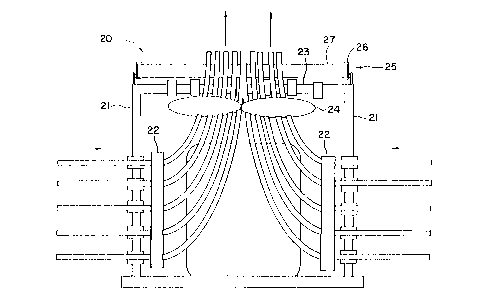
Figure 1 is a schematic representation of one
embodiment of an atmospheric pollutant treating device in
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 25 -
accordance with the present invention, wherein the device
is an air conditioning condenser. Such con~en~ers are
generally located outdoors, and are used to air cool an
air conditioning fluid which is transported through coils
in the unit. In Figure 1, ambient air which may contain
ozone enters co~n~er 20 through one or more inlet
grills 21, passes through one or more heat exchangers 22,
and exits the condenser through one or more outlet grills
23. The air is forced through the condenser by fan 24,
which is shown in this embodiment mounted to the top of
the condenser housing. It will be readily recognized by
one skilled in the art that the components of such a
condenser can be arranged in any suitable operating
configuration, provided that the ambient air passes in
operative contact with a heat exchanger and returns to
the atmosphere. Thus, the inlet can be on the sides, as
shown, or on the bottom or top of the unit, and the
outlet can be at the top as shown, or on the sides or
bottom of the unit. The heat exchangers can be next to
the inlets, as shown or next to the outlet. The fan can
be between the heat exchangers and the outlet, as shown,
or at any other location in the air stream. The
condenser unit can be of any suitable shape, such as
cubic, rectangular or cylindrical.
In accordance with one embodiment of the
invention, a pollutant treating material is applied to a
surface in the flow path of the air passing through the
con~n~er. Suitable surfaces for this material are inlet
grill 21, heat exchanger 22, outlet grill 23 or the
blades of fan 24. When the pollutant treating material
~ includes an ozone catalyst, as discussed elsewhere in the
present specification, it is generally more effective to
operate at the highest available temperature. Because
heat ~ch~nger 22 is at an elevated temperature during
normal operation of the condenser, the pollutant treating
surface is therefore preferably located on the heat
~xch~nger or down stream of the heat exchanger. In the
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 26 -
embodiment shown in Figure 1, fan 24 and outlet grill 23
are both downstream from heat exchanger 22, and therefore
would be preferred sites for the pollutant treating
surface.
In another embodiment of this invention, a
separate treatment device 25 is provided, which contains
the pollutant treating surface. Device 25 may be at any
suitable location in the airstream passing through the
condenser. In the embodiment as shown, device 25 is
mounted on the exterior of condenser 20, to receive the
air flowing out of outlet grill 23. It will be readily
seen that device 25 could be located anywhere in the
airstream passing through the condenser, such as at the
inlet, after the heat exchanger, or next to the fan. As
discussed above, when a heat sensitive catalyst is being
used, then device 25 is preferably located downstream of
heat ~xch~nger 22 to take advantage of the elevated
temperature of the air passing therethrough.
Treatment device 25 may be permanently or
removably mounted to condenser 20. Preferably, device 25
is removably mounted to permit replacement or
rejuvenation of the pollutant treating material. In a
preferred embodiment, treatment device 25 includes a
housing 26 which is attached to condenser 20 for
receiving and holding a removable treatment cartridge 27.
Treatment cartridge 27 may be of any suitable
configuration, such as a honeycomb, filter pad, frame or
screen coated with the catalyst or adsorbent material.
The cartridge can be designed to be discarded after a
single use, or to be cleaned or otherwise rejuvenated and
reused.
A dust filter (not shown) may be provided to
protect the active pollutant treating surface of
treatment cartridge 27. Such a dust filter can be
located anywhere in unit 20 or device 25 upstream of
cartridge 27, or can even be integral with cartridge 27,
upstream of the active surface.
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 27 -
In a test of a condenser such as that depicted
in Figure 1, an automobile radiator which had been coated
with ozone treating catalyst was used as treatment device
25. Such automobile radiators are described in Example 1
5 et seq. below, and are conveniently used as test devices
on the stationary apparatus of the present invention.
Figure 2 iS a schematic representation of
another embodiment of the present invention, in which a
pollutant treating substrate is added to a commercial or
industrial air cooled heat exchanger 40. Such heat
exchangers are commonly used to condense low pressure
steam into water, and may be found in industrial plants,
power plants, commercial heating systems, and other steam
handling facilities. As depicted in Figure 2, steam is
15 fed to header portion 42 (shown cut-away) of air-cooled
heat exchanger 40, which is provided with top vents 44
for removing non-condensibles. The steam circulates back
and forth through finned tube bundle 46 mounted in
channel frame 48 where it is cooled and condensed by the
air flow. The condensate is removed by bottom drains
(not shown) and carried by drain line 50 to conCl~nRate
tank 52. Air flow is provided by axial flow fan $4 which
is shown mounted above the tube bundle 46, in a plenum 56
which channels the air flow to fan ring 58. Fan 54 is
driven by electric motor 60, shown mounted below tube
bundle 46, which is connected directly or indirectly to
drive shaft 62. In operation, air is drawn by fan 54
through tube bundle 46 and out through fan ring 58.
It will be readily recognized by one skilled in
the art that the components of such a heat exchanger can
be arranged in any suitable operating configuration,
provided that the ambient air passes in operative contact
with the tube bundle and returns to the atmosphere.
Thus, tube bundle 46 may be mounted horizontally (as
shown), vertically or diagonally. Further, the tube
bundle can be of any suitable design which allows
condensing of steam passing therethrough. Fan 54 may be
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 28 -
located on the output side of tube bundle 46 (as shown),
at the inlet or even between separate heat ~ch~nge
elements. Ducting to direct the air flow, may be
included on the inlet and/or outlet sides of the heat
exchanger, as well as protective screens or grills.
In accordance with one embodiment of the
invention, the pollutant treating material may be applied
to an existing surface in the flow path of the air
passing through the heat exchanger. Suitable surfaces
for this material include tube bundle 46, the blades of
fan 54, and the inner surfaces of plenum 56 and fan ring
58. Alternatively, the material could be applied to any
other ducting, screens or grills which are in the air
flow path. As discussed elsewhere in the present
specification, when the pollutant treating material
includes an ozone catalyst or any other catalyst which is
more effective aF an elevated temperature, it is
preferred to apply the material to a surface with the
highest available temperature. Because tube bundle 46 is
at an elevated temperature during normal operation of the
condenser, the pollutant treating surface is preferably
located on the tube bundle or downstream thereof. In the
embodiment shown in Figure 2, fan 54, plenum 56 and fan
ring 58 are all downstream of tube bundle 46, and would
therefore be preferred sites for the pollutant treating
surface.
In another embodiment of this invention, a
separate treatment device (not shown) may be provided,
which contains the pollutant treating surface. Such a
device may be at any suitable location in the airstream
passing through the heat exchanger. In the embodiment as
shown, the treatment device could desirably be mounted at
the outlet of fan ring 58, or within plenum 56 between
tube bundle 46 and fan 54. The treatment device could
also be located on the inlet side of tube bundle 46.
However, as discussed above, when a heat sensitive
catalyst is being used, then the treatment device is
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 29 -
preferably located downstream of tube bundle 46 to take
advantage of the elevated temperature of the air passing
therethrough.
As with the embodiment depicted in Figure 1,
the treatment device for the present air-cooled heat
exchanger 40 may be permanently or removably mounted to
the unit. Preferably, the device is removably mounted to
permit replacement or rejuvenation of the pollutant
treating material. In a preferred embodiment, the
treatment device includes a housing which is attached to
heat exchanger 40 for receiving and holding a removable
treatment substrate. The treatment substrate may be of
any suitable configuration, and can be designed to be
discarded after a single use, or to be cleaned or
otherwise rejuvenated and reused.
For industrial applications, a high surface
area structure, such as a ceramic or metal honeycomb, can
provide an excellent substrate to support the pollutant
treating material. The high surface area structure can
also be any type of filter, screen or grill capable of
supporting the treatment material.
Alternatively, the substrate can be a separate
heat exchange~, in which a separate source of heat may be
provided to increase the working temperature of the
pollutant treating material, particularly when a catalyst
is being used. The separate source of heat can be a side
stream of the steam which is entering or the condensate
which is exiting the principal heat exchanger 40, or an
independent heated stream. Alternatively, electric or
combustion heating could be used.
As with the condenser embodiment of Figure 1,
automobile radiators which had been coated with ozone
treating catalyst in accordance with Example 1 et seq.
below, have been conveniently used as test treatment
devices on the stationary heat exchangers of the present
embodiment.
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 30 -
Figure 3 is a schematic representation of a
particular type of air cooled heat ~ch~nger 70, which is
~ in use in some power plants to con~Pn~e large volumes of
low pressure steam. Steam enters the unit at inlet 71,
and is distributed through a top-mounted plenum 72. The
steam passes through diagonally disposed heat exchange
tubes 73 mounted in housing 74, and the condensate is
collected in condensate tank 75. Air flow is provided by
bottom mounted forced draft fans 76, which direct the air
upwardly through the tube bundles and then to the
atmosphere.
In this embodiment, the diagonally disposed
heat exchange tubes may be directly coated with the
pollutant treating material. Alternatively, separate
pollutant treating devices may be permanently or
removably affixed to the inside or outside of the
diagonal surface of housing 74. In a preferred
embodiment, the treating devices are pivotably mounted to
the housing so that they can be swung out of the way to
permit cleaning and servicing of the underlying tubes 73.
As discussed above, test units of catalyzed
automobile radiators can be attached to, or simply laid
on, the exterior of such a heat ~ch~nger to test the
catalysts of the present invention.
The pollutant treating composition is
preferably a catalytic composition or adsorption
composition. Useful and preferred catalyst compositions
are compositions which can catalytically cause the
reaction of targeted pollutants at the space velocity of
the air as it contacts the surface, and at the
temperature of the surface at the point of contact.
Typically, these catalyzed reactions will be in the
temperature range at the atmosphere contacting surface of
from 0~C to 130~C, more typically 20~C to 105~C and yet
more typically from about 40~C to 100~C. There is no
limit on the efficiency of the reaction as long as some
reaction takes place. Preferably, there is at least a 1
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
conversion efficiency with as high a conversion
efficiency as possible. Useful conversion efficiencies
are preferably at least about 5~ and more preferably at
least about 10~. Preferred conversions depend on the
particular pollutant and pollutant treating composition.
Where ozone is treated with a catalytic composition on an
atmosphere contacting surface it is preferred that the
conversion efficiency be greater than about from 30~ to
40~, preferably greater than 50~, and more preferably
greater than 70~. Preferred conversion for carbon
monoxide is greater than 30~ and preferably greater than
50~. Preferred conversion efficiency for hydrocarbons
and partially oxygenated hydrocarbons is at least 10~,
preferably at least 15%, and most preferably at least
25~. These conversion rates are particularly preferred
where the atmosphere contacting surface is at ambient
operating conditions of up to about 110~C. These
temperatures are the surface temperatures typically
experienced during normal operation of atmosphere
contacting surfaces of the vehicle including the surfaces
of the radiator and air conditioning co~Pncer~ Where
there is supplemental heating of the atmosphere
contacting surface such as by having an electrically
heated catalytic monolith, grid, screen, gauze or the
like, it is preferred that the conversion efficiency be
greater than 90~ and more preferably greater than 95~.
The conversion efficiency is based on the mole percent of
the particular pollutants in the air which react in the
presence of the catalyst composition.
Ozone treating catalyst compositions comprise
manganese compounds including manganese dioxide,
including non stoichiometric manganese dioxide (e.g.,
~ MnO(~52~), and/or Mn2O3. Preferred manganese dioxides,
which are nnm~n~l ly referred to as MnO2 have a chemical
formula wherein the molar ratio of manganese to oxide is
about from 1.5 to 2.0, such as Mn8OI6. Up to 100 percent
by weight of manganese dioxide MnO2 can be used in
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
catalyst compositions to treat ozone. Alternative
compositions which are available comprise manganese
dioxide and compounds such as copper oxide alone or
copper oxide and alumina.
Useful and preferred manganese dioxides are
alpha manganese dioxides nnm;n~lly having a molar ratio
of manganese to oxygen of from 1 to 2. Useful alpha
manganese dioxides are disclosed in U.S. Patent No.
5,340,562 to O'Young, et al.; also in O'Young,
Hydrothermal Synthesis of Manganese Oxides with Tunnel
Structures presented at the Symposium on Advances in
Zeolites and Pillared Clay Structures presented before
the Division of Petroleum Chemistry, Inc. American
Chemical Society New York City Meeting, August 25-30,
1991 beginning at page 342, and in Mc~n~ie, the
Synthesis of Birnessite, Cryptomelane, and Some Other
Oxides and Hydroxides of Manganese, Mineralogical
Magazine, December 1971, Vol. 38, pp. 493-502. For the
purposes of the present invention, the preferred alpha
manganese dioxide is a 2 x 2 tunnel structure which can
be hollandite (BaMn8OI6.xH2O), cryptomelane (KMn8O~6.xH2O),
manjiroite (NaMn8OI6.xH2O) and coronadite (PbMn8Ol6.xH2O).
The manganese dioxides useful in the present
invention preferably have a surface area of greater than
150 m2/g, more preferably greater than 200 m2/g, yet more
preferably greater than 250 m2/g and most preferably
greater than 275 m2/g. The upper range of such materials
can be as high as 300 m2/g, 325 m2/g or even 350 m2/g.
Preferred materials are in the range of 200-350 m2/g,
preferably 250-325 m2/g and most preferably 275-300 m2/g.
The composition preferably comprises a binder as of the
type described below with preferred binders being
polymeric binders. The composition can further comprise
precious metal components with preferred precious metal
components being the oxides of precious metal, preferably
the oxides of platinum group metals and most preferably
the oxides of palladium or platinum also referred to as
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 33 -
palladium black or platinum black. The amount of
palladium or platinum black can range from 0 to 25~, with
useful amounts being in ranges of from about 1 to 25 and
5 to 15~ by weight based on the weight of the manganese
component and the precious component.
It has been found that the use of compositions
comprising the cryptomelane form of alpha manganese
oxide, which also contain a polymeric binder can result
in greater than 50~, preferably greater than 60~ and most
preferably from 75-85~ conversion of ozone in a
concentration range of from 0 to 400 parts per billion
(ppb) and an air stream moving across a radiator at space
velocity of from 300,000 to 650,000 reciprocal hours.
Where a portion of the cryptomelane is replaced by up to
15 25~ and preferably from 15-25~ parts by weight of
palladium black (PdO), ozone conversion rates at the
above conditions range from 95-100~ using a powder
reactor.
The preferred cryptomelane manganese dioxide
20 has a crystallite size ranging from 2 to 10 and
preferably from less than 5 nm. It can be calcined at a
temperature range of from 250~C to 550~C and preferably
below 500~C and greater than 300~C for at least 1.5 hours
and preferably at least 2 hours up to about 6 hours.
The preferred cryptomelane can be made in
accordance described in the above referenced articles and
patents to O'Young and McKen~ie. The cryptomelane can be
made by reacting a manganese salt including salts
selected from the group consisting MnCl2, Mn(NO3) 2/ MnSO4
30 and Mn(CH3COO) 2 with a permanganate compound.
Cryptomelane is made using potassium permanganate;
hollandite is made using barium permanganate; coronadite
is made using lead permanganate; and manjiroite is made
using sodium permanganate. It is recognized that the
35 alpha manganese useful in the present invention can
contain one or more of hollandite, cryptomelane,
manjiroite or coronadite compounds. Even when making
CA 02206460 1997-0~-29
W O96/22149 PCTnUS96/00781
- 34 -
cryptomelane minor amounts of other metal ions such as
sodium may be present. Useful methods to form the alpha
manganese dioxide are described in the above references
which are incorporated by reference.
The preferred alpha manganese for use in
accordance with the present invention is cryptomelane.
The preferred cryptomelane is "clean" or substantially
free of inorganic anions, particularly on the surface.
Such anions could include chlorides, sulfates and
nitrates which are introduced during the method to form
cryptomelane. An alternate method to make the clean
cryptomelane is to react a manganese carboxylate,
preferably manganese acetate, with potassium
permanganate. It has been found that the use of such a
material which has been calcined is "clean". The use of
material containing inorganic anions can result in
conversion of ozone to oxygen of up to about 60%. The
use of cryptomelane with a "clean" surface results in
conversions of up about 80~.
It is believed that the carboxylates are burned
off during the calcination process. However, inorganic
anions remain on the surface even during calcination.
The inorganic anions such as sulfates can be washed away
with an aqueous solution or a slightly acidic aqueous
solution. Preferably the alpha manganese dioxide is a
"clean" alpha manganese dioxide. The cryptomelane can be
washed at from about 60~C to lOO~C for about one-half
hour to remove a significant amount of sulfate anions.
The nitrate anions may be removed in a similar m~nner
The ~clean" alpha manganese dioxide is characterized as
having an IR spectra as illustrated in Figure 16 and in
X-ray diffraction (XRD) pattern as illustrated in Figure
17. Such a cryptomelane preferably has a surface area
greater than 200 m2/g and more preferably greater than 250
m2/g
A preferred method of making cryptomelane
useful in the present invention comprises ml ~; ng an
CA 02206460 l997-0~-29
W 096122149 PCTrUS96/00781
- 35 -
aqueous acidic manganese salt solution with a potassium
permanganate solution. The acidic manganese salt
solution has a pH of from 0.5 to 3 and can be made acidic
using any common acid, preferably acetic acid at a
concentration of from 0.5 to 5.0 normal and more
preferably from 1.0 to 2.0 normal. The mixture forms a
slurry which is stirred at a temperature range of from
50~C to 110~C. The slurry is filtered and the filtrate
is dried at a temperature range of from 75~C to 200~C.
The resulting cryptomelane crystals have a surface area
of typically in the range of from 200 m2/g to 350 m~/g.
A review of the IR spectrum for the most
preferred cryptomelane, shown in Figure 16 is
characterized by the absence of peaks assignable to
carbonate, sulfate and nitrate groups. Expected peaks
for carbonate groups appear in the range of from 1320 to
1520 wavenumbers; and for sulfate groups appear in the
range of from 950 to 1250 wavenumbers. Figure 17 is a
powder X-ray diffraction pattern for high surface area
cryptomelane prepared in Example 25. The X-ray pattern
for cryptomelane useful in the present invention is
characterized by broad peaks resulting from small
crystallite size (~5- lOnm). Approximate peak positions
(iO.15~2~) and approximate relative intensities (~5) for
cryptomelane using CuK~ radiation as shown in Figure 17
are: 2~/Relative Intensities - 12.1/9; 18/9; 28.3/10;
37.5/100; 41.8/32; 49.7/16; 53.8/5; 60.1/13; 55.7/38; and
68.0/23.
A preferred method of making cryptomelane
useful in the present invention comprises m; ~; ng an
aqueous acidic manganese salt solution with a potassium
permanganate solution. The acidic manganese salt
solution preferably has a pH of from 0. 5 to 3.0 and can
be made acidic using any common acid, preferably acetic
acid at a concentration of from 0.5 to 5.0 normal and
more preferably from 1.0 to 2.0 normal. The mixture
forms a slurry which is stirred at a temperature range of
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 36 -
from 50~C to 110~C. The slurry is filtered and the
filtrate is dried at a temperature range of from 75~C to
200~C. The resulting cryptomelane crystals have a
surface area of typically in the range of from 200 m2/g to
350 m2/g.
Other useful compositions comprise manganese
dioxide and optionally copper oxide and alumina and at
least one precious metal component such as a platinum
group metal supported on the manganese dioxide and where
present copper oxide and alumina. Useful compositions
contain up to 100, from 40 to 80 and preferably 50 to 70
weight percent manganese dioxide and 10 to 60 and
typically 30 to 50 percent copper oxide. Useful
compositions include hopcalite which is about 60 percent
manganese dioxide and about 40 percent copper oxide; and
Carulite~ 200 (sold by Carus Chemical Co.) which is
reported to have 60 to 75 weight percent manganese
dioxide, 11 to 14 percent copper oxide and 15 to 16
percent alllm~ m oxide. The surface area of Carulite~ is
reported to be about 180 m2/g. Calcining at 450~C reduces
the surface area of the Carulite~ by about fifty percent
(50~) without significantly affecting activity. It is
preferred to calcine manganese compounds at from 300~C to
500~C and more preferably 350~C to 450~C. Calcining at
550~C causes a great loss of surface area and ozone
treatment activity. Calcining the Carulite~ after ball
milling with acetic acid and coating on a substrate can
improve adhesion of the coating to a substrate.
Other compositions to treat ozone can comprise
a manganese dioxide component and precious metal
components such as platinum group metal components.
While both components are catalytically active, the
manganese dioxide can also support the precious metal
component. The platinum group metal component preferably
is a palladium and/or platinum component. The amount of
platinum group metal compound preferably ranges from
about 0.1 to about 10 weight percent (based on the weight
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96100781
- 37 -
of the platinum group metal) of the composition.
Preferably, where platinum is present it is in amounts of
from 0.1 to 5 weight percent, with useful and preferred
amounts on pollutant treating catalyst volume, based on
the volume of the supporting article, ranging from about
0.5 to about 70 g/ft3. The amount of palladium component
preferably ranges from about 2 to about 10 weight percent
of the composition, with useful and preferred amounts on
pollutant treating catalyst volume ranging from about 10
to about 250 g/ft3.
Various useful and preferred pollutant treating
catalyst compositions, especially those containing a
catalytically active component such as a precious metal
catalytic component, can comprise a suitable support
material such as a refractory oxide support. The
preferred refractory oxide can be selected from the group
consisting of silica, alumina, titania, ceria, zirconia
and chromia, and mixtures thereof. More preferably, the
support is at least one activated, high surface area
compound selected from the group consisting of alumina,
silica, titania, silica-alumina, silica-zirconia, alllm;n~
silicates, alumina zirconia, alumina-chromia and al~lm;n~-
ceria. The refractory oxide can be in suitable form
including bulk particulate form typically having particle
sizes ranging from about 0.1 to about 100 and preferably
1 to 10 ~m or in sol form also having a particle size
ranging from about 1 to about 50 and preferably about 1
to about 10 nm. A preferred titania sol support
comprises titania having a particle size ranging from
about 1 to about 10, and typically from about 2 to 5 nm.
Also useful as a preferred support is a
coprecipitate of a manganese oxide and zirconia. This
composition can be made as recited in U.S. Patent No.
5,283,041 incorporated herein by reference. Briefly,
this coprecipitated support material preferably comprises
in a ratio based on the weight of manganese and zirconium
metals from 5:95 to 95:5; preferably 10:90 to 75:25; more
CA 02206460 l997-0~-29
W O96122149 PCTrUS96/00781
- 38 -
preferably 10:90 to 50:50; and most preferably from 15: 85
to 50:50. A useful and preferred embodiment comprises a
Mn:Zr weight ratio of 20:80. U.S. Patent No. 5,283,041
describes a preferred method to make a coprecipitate of a
manganese oxide component and a zirconia component. AS
recited in U.S. Patent No. 5,283,041 a zirconia oxide and
manganese oxide material may be prepared by mixing
aqueous solutions of suitable zirconium oxide precursors
such as zirconium oxynitrate, zirconium acetate,
zirconium oxychioride, or zirconium oxysulfate and a
suitable manganese oxide precursor such as manganese
nitrate, manganese acetate, manganese dichloride or
manganese dibromide, adding a sufficient amount of a base
such as ammonium hydroxide to obtain a pH of 8-9,
filtering the resulting precipitate, washing with water,
and drying at 450~-500~C.
A useful support for a catalyst to treat ozone
is selected from a refractory oxide support, preferably
alumina and silica-alumina with a more preferred support
being a silica-alumina support comprising from about 1~
to 10~ by weight of silica and from 90~ to 99~ by weight
of alumina.
Useful refractory oxide supports for a catalyst
comprising a platinum group metal to treat carbon
mn~o~;de are selected from alumina, titania, silica-
zirconia, and manganese-zirconia. Preferred supports for
a catalyst composition to treat carbon mo~o~r; de is a
zirconia-silica support as recited in U.S. Patent No.
5,145,825, a manganese-zirconia support as recited in
U.S. Patent No. 5,283,041 and high surface area alumina.
Most preferred for treatment of carbon mnIlo~; de is
titania. Reduced catalysts having titania supports
resulted in greater carbon monoxide conversion than
corresponding non reduced catalysts.
The support for catalyst to treat hydrocarbons,
such as low molecular weight hydrocarbons, particularly
low molecular weight olefinic hydrocarbons having about
CA 02206460 1997-0~-29
wo96l22l4s PCTlUS96/0078l
- 39
from two up to about twenty carbons and typically two to
about eight carbon atoms, as well as partially oxygenated
hydrocarbons is preferably selected from refractory metal
oxides including alumina and titania. As with catalysts
to treat carbon monoxide reduced catalysts results in
greater hydrocarbon conversion. Particularly preferred
is a titania support which has been found useful since it
results in a catalyst composition having enhanced ozone
conversion as well as significant conversion of carbon
monoxide and low molecular weight olefins. Also useful
are high surface area, macroporous refractory oxides,
preferably alumina and titania having a surface area of
greater than 150 m2/g and preferably ranging from about
150 to 350, preferably from 200 to 300, and more
preferably from 225 to 275 m2/g; a porosity of greater
than 0.5 cc/g, typically ranging from 0.5 to 4.0 and
preferably about from 1 to 2 cc/g measured based on
mercury porosometry; and particle sizes range from 0.1 to
10 ~m. A useful material is Versal GL alumina having a
surface area of about 260 m2/g, a porosity of 1.4 to 1.5
cc/g and supplied by LaRoche Industries.
A preferred refractory support for platinum for
use in treating carbon monoxide and/or hydrocarbons is
titania dioxide. The titania can be used in bulk powder
form or in the form of titania dioxide sol. The catalyst
composition can be prepared by adding a platinum group
metal in a li~uid media preferably in the form of a
solution such as platinum nitrate with the titania sol,
with the sol most preferred. The obtained slurry can
then be coated onto a suitable substrate such as an
atmosphere treating surface such as a radiator, metal
monolith substrate or ceramic substrate. The preferred
platinum group metal is a platinum compound. The
platinum titania sol catalyst obtained from the above
procedure has high activity for carbon monoxide and/or
hydrocarbon oxidation at ambient operating temperature.
Metal components other than platinum components which can
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96100781
- 40 -
be combined with the titania sol include gold, palladium,
rhodium and silver components. A reduced platinum group
component, preferably a platinum component on titanium
catalyst which is indicated to be preferred for treating
carbon monoxide, has also been found to be useful and
preferred for treating hydrocarbons, particularly
olefinic hydrocarbons.
A preferred titania sol support comprises
titania having a particle size ranging from about 1 to
10 about 10, and typically from about 2 to 5 nm.
A preferred bulk titania has a surface area of
about from 25 to 120 m2/g, and preferably from 50 to 100
m2/g; and a particle size of about from 0.1 to 10 ,~4m. A
specific and preferred bulk titania support has a surface
15 area of 45-50 m2/g, a particle size of about 1 ~bm, and is
sold by DeGussa as P-25.
A preferred silica-zirconia support comprises
from 1 to 10 percent silica and 90 to 99 percent
zirconia. Preferred support particles have high surface
20 area, e.g. from 100 to 500 s~uare meters per gram (m2/g)
surface area, preferably from 150 to 450 m2/g, more
preferably from 200 to 400 m2/g, to enhance dispersion of
the catalytic metal component or components thereon. The
preferred refractory metal oxide support also has a high
25 porosity with pores of up to about 145 nm radius, e.g.,
from about 0.75 to 1.5 cubic centimeters per gram (cm3/g),
preferably from about 0.9 to 1.2 cm3/g, and a pore size
range of at least about 50~ of the porosity being
provided by pores of 5 to 100 nm in radius.
A useful ozone treating catalyst comprises at
least one precious metal component, preferably a
palladium component dispersed on a suitable support such
as a refractory oxide support. The composition comprises
from 0.1 to 20.0 weight percent, and preferably 0.5 to 15
35 weight percent of precious metal on the support, such as
a refractory oxide support, based on the weight of the
precious metal (metal and not oxide) and the support.
CA 02206460 1997-0~-29
W O 96/22149 PCTrUS96/00781
- 41 -
Palladium is preferably used in amounts of from 2 to 15,
more preferably 5 to 15 and yet more preferably 8 to 12
weight percent. Platinum is preferably used at 0.1 to
- 10, more preferably 0.1 to 5.0, and yet more preferably 2
to 5 weight percent. Palladium is most preferred to
catalyze the reaction of ozone to form oxygen. The
support materials can be selected from the group recited
above. In preferred embodiments, there can additionally
be a bulk manganese component as recited above, or a
manganese component dispersed on the same or different
refractory oxide support as the precious metal,
preferably palladium component. There can be up to 80,
preferably up to 50, more preferably from 1 to 40 and yet
more preferably 5 to 35 weight percent of a manganese
component based on the weight of palladium and manganese
metal in the pollutant treating composition. Stated
another way, there is preferably about 2 to 30 and
preferably 2 to 10 weight percent of a manganese
component. The catalyst loading is from 20 to 250 grams
and preferably about 50 to 250 grams of palladium per
cubic foot (g/ft3) of catalyst volume. The catalyst
volume is the total volume of the f; nl s~ed catalyst
composition and therefore includes the total volume of
air conditioner co~Pn~er or radiator including void
spaces provided by the gas flow passages. Generally, the
higher loading of palladium results in a greater ozone
conversion, i.e., a greater percentage of ozone
decomposition in the treated air stream.
Conversions of ozone to oxygen att~nP~ with a
palladium/manganese catalyst on alumina support
compositions at a temperature of about 40~C to 50~C have
been about 50 mole percent where the ozone concentrations
range from 0.1 to 0.4 ppm and the face velocity was about
10 miles per hour. Lower conversions were attained using
a platinum on alumina catalyst.
Of particular interest is the use of a support
comprising the above described coprecipitated product of
CA 02206460 1997-0~-29
W O96/~2149 PCTrUS96/00781
- 42 -
a manganese oxide, and zirconia which is used to support
a precious metal, preferably selected from platinum and
palladium, and most preferably platinum. Platinum is of
particular interest in that it has been found that
platinum is particularly effective when used on this
coprecipitated support. The amount of platinum can range
from 0.1 to 6, preferably 0. 5 to 4, more preferably 1 to
4, and most preferably 2 to 4 weight percent based on
metallic platinum and the coprecipitated support. The
use of platinum to treat ozone has been found to be
particularly effective on this support. Additionally, as
discussed below, this catalyst is useful to treat carbon
monoxide. Preferably the precious metal is platinum and
the catalyst is reduced.
Other useful catalysts to catalytically convert
ozone to oxygen are described in U.S. Patent Nos.
4,343,776 and 4,405,507, both hereby incorporated by
reference. A useful and most preferred composition is
disclosed in commo~ly assigned U.S. Serial No. 08/202,397
filed February 25, 1994, now U.S. Patent No. 5,422,331
and entitled, "Light Weight, Low Pressure Drop Ozone
Decomposition Catalyst for Aircraft Applications" hereby
incorporated by reference. Yet other compositions which
can result in the conversion of ozone to oxygen comprises
carbon, and palladium or platinum supported on carbon,
manganese dioxide, Carulite~, and/or hopcalite.
Manganese supported on a refractory oxide such as recited
above has also been found to be useful.
~hcln m~,n~Y~de treating catalysts preferably
comprise at least one precious metal component,
preferably selected from platinum and palladium
components with platinum components being most preferred.
The composition comprises from 0.01 to 20 weight percent,
and preferably 0. 5 to 15 weight percent of the precious
metal component on a suitable support such as refractory
oxide support, with the amount of precious metal being
based on the weight of precious metal (metal and not the
CA 02206460 1997-0~-29
W O 96/22149 PCTrUS96/00781
- 43 -
metal component) and the support. Platinum is most
preferred and is preferably used in amounts of from 0.01
to 10 weight percent and more preferably 0.1 to 5 weight
- percent, and most preferably 1.0 to 5.0 weight percent.
Palladium is useful in amounts from 2 to 15, preferably 5
to 15 and yet more preferably 8 to 12 weight percent.
The preferred support is titania, with titania sol most
preferred as recited above. When loaded onto a
monolithic structure such as a radiator or onto other
atmosphere contacting surfaces the catalyst loading is
preferably about 1 to 150, and more preferably 10 to 100
grams of platinum per cubic foot (g/ft3) of catalyst
volume and/or 20 to 250 and preferably 50 to 250 grams of
palladium per g/ft3 of catalyst volume. Preferred
catalysts are reduced. Conversions of 5 to 80 mole
percent of carbon monoxide to carbon dioxide were
attained using coated core samples from automotive
radiator having from 1 to 6 weight percent (based on
metal) of platinum on titania compositions at
temperatures from 25~ to 90~C where the carbon m~o~;de
concentration was 15 to 25 parts per million and the
space velocity was 300,000 to 500,000 reciprocal hours.
Also, conversions of 5 to 65 mole percent of carbon
monoxide to carbon dioxide were attained using 1.5 to 4.0
weight percent platinum on alumina support compositions
at a temperature of about up to 95~C where the carbon
mono~;de concentration was about 15 parts per million and
the space velocity was about 300,000 reciprocal hours.
Lower conversions have been attained with palladium on a
ceria support.
An alternate and preferred catalyst composition
to treat carbon monoxide comprises a precious metal
component supported on the above described coprecipitate
of a manganese oxide and zirconia. The coprecipitate is
formed as described above. The preferred ratios of
manganese to zirconia are 5:95 to 95:5; 10:90 to 75:25;
10:90 to 50:50; and 15:85 to 25:75 with a preferred
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 44 -
coprecipitate having a manganese oxides to zirconia of
20:80. The percent of platinum supported on the
coprecipitate based on platinum metal ranges from 0.1 to
6, preferably 0.5 to 4, more preferably 1 to 4, and most
preferably 2-4 weight percent. Preferably the catalyst
is reduced. The catalyst can be reduced in powder form
or after it has been coated onto a supporting substrate.
Other useful compositions which can convert carbon
mono~' de to carbon dioxide include a platinum component
supported on carbon or a support comprising manganese
dioxide.
Catalysts to treat hydrocarbons, typically
unsaturated hydrocarbons, more typically unsaturated
mono-olefins having from two to about twenty carbon atoms
and, in particular, from two to eight carbon atoms, and
partially oxygenated hydrocarbons of the type referred to
above, comprise at least one precious metal component,
preferably selected from platinum and palladium with
platinum being most preferred. Useful catalyst
compositions include those described for use to treat
carbon monoxide. Composition to treat hydrocarbons
comprise from 0.01 to 20 wt.~ and preferably 0.5 to 15
wt.~ of the precious metal component on a suitable
support such as a refractory oxide support, with the
amount of precious metal being based on the weight of the
precious metal, (not the metal component) and the
support. Platinum is the most preferred and is
preferably used in amounts of from 0.01 to 10 wt.~ and
more preferably 0.1 to 5 wt.~ and most preferably 1.0 to
5 wt.~. When loaded onto a monolithic structure such as
a motor vehicle radiator or on to other atmospheric
contacting surfaces, the catalyst loading is preferably
about 1 to 150, and more preferably 10 to 100 grams of
platinum per cubic foot (g/ft3) of catalyst volume. The
preferred refractory oxide support is a metal oxide
refractory which is preferably selected from ceria,
silica, zirconia, alumina, titania and mixtures thereof
CA 02206460 l997-0~-29
W O96122149 PCTrUS96/00781
- 45 -
with alumina and titania being most preferred. The
preferred titania is characterized by as recited above
with titania sol most preferred. The preferred catalyst
is reduced. Testing on a coated automotive radiator
resulted in conversions of a low molecular weight mono-
olefin such as propylene to water and carbon dioxide with
1.5 to 4 wt.~ of platinum on an alumina or titania
support have been between 15 and 25~ where the propylene
concentration was about 10 parts per million propylene
and the space velocity was about 320,000 reciprocal
hours. These catalysts were not reduced. Reduction of
the catalyst improves conversion.
Catalysts useful for the oxidation of both
carbon mnnoY;de and hydrocarbons generally include those
recited above as useful to treat either carbon monoxide
or hydrocarbons. Most preferred catalysts which have
been found to have good activity for the treatment of
both carbon monoxide and hydrocarbon such as unsaturated
olefins comprise platinum component supported on a
preferred titania support. The composition preferably
comprises a binder and can be coated on a suitable
support structure in amounts of from 0.8 to 1.0 g/in. A
preferred platinum concentration ranges from 2 to 6~ and
preferably 3 to 5~ by weight of platinum metal on the
titania support. Useful and preferred substrate cell
densities are equivalent to about 300 to 400 cells per
square inch. The catalyst is preferably reduced as a
powder or on the coated article using a suitable reducing
agent. Preferably the catalyst is reduced in the gas
stream comprising about 7~ hydrogen with the balance
nitrogen at from 200~ to 500~C or from 1 to 12 hours.
The most preferred reduction or forming temperature is
400~C for 2-6 hours. This catalyst has been found to
maintain high activity in air and humidified air at
elevated temperatures of up to 100~C after prolonged
exposure.
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
- 46 -
Useful catalysts which can treat both ozone and
carbon mnnoY~de comprise at least one precious metal
component, most preferably a precious metal selected from
palladium, platinum and mixtures thereof on a suitable
support such as a refractory oxide support. Useful
refractory oxide supports comprise ceria, zirconia,
alumina, titania, silica and mixtures thereof including a
mixture of zirconia and silica as recited above. Also
useful and preferred as a support are the above described
coprecipitates of manganese oxides and zirconia. The
composition comprises from 0.1 to 20.0, preferably 0.5 to
15, and more preferably from 1 to 10 weight percent of
the precious metal component on the support based on the
weight of the precious metal and the support. Palladium
is preferably used in amounts from 2 to 15 and more
preferably from 3 to 8 weight percent. Platinum is
preferably used in amounts of from 0.1 to 6 percent and
more preferably 2 to 5 weight percent. A preferred
composition is a composition wherein the refractory
component comprises ceria and the precious metal
component comprises palladium. This composition has
resulted in relatively high ozone and carbon monoxide
conversions. More particularly, testing of this
composition on a coated radiator has resulted in a 21~
conversion of carbon monoxide in an air stream comprising
16 ppm of carbon monoxide contacting a surface at 95~C
with a face velocity of the gas stream being 5 miles per
hour. The same catalyst resulted in a 55~ ozone
conversion where the stream cont~ne~ 0.25 ppm of ozone
and the treating surface was at 25~C with an air stream
face velocity of 10 miles per hour. Also preferred is a
composition comprising a precious metal, preferably a
platinum group metal, more preferably selected from
platinum and palladium components, and most preferably a
platinum component and the above recited coprecipitate of
manganese oxide and zirconia. This above recited
precious metal cont~n~ng catalyst in the form of a
CA 02206460 1997-0~-29
W 096/22149 PCTrUS96/00781
- 47 -
catalyst powder or coating on a suitable substrate is in
reduced form. Preferred reduction conditions include
those recited above with the most preferred condition
~ being from 250~ to 350~C for from 2 to 4 hours in a
reducing gas comprising 7~ hydrogen and 93~ nitrogen.
This catalyst has been found to be particularly useful in
treating both carbon monoxide and ozone. Other useful
compositions to convert ozone to oxygen and carbon
monoxide to carbon dioxide comprise a platinum component
supported on carbon, manganese dioxide, or a refractory
oxide support, and optionally having an additional
manganese component.
A useful and preferred catalyst which can treat
ozone, carbon m~n~Yide and hydrocarbons, as well as
partially o~yy~ated hydrocarbons, comprises a precious
metal component, preferably a platinum component on a
suitable support such as a refractory oxide support.
Useful refractory oxide supports comprise ceria,
zirconia, alumina, titania, silica and mixtures thereof
including a mixture of zirconia and silica as recited
above. Also useful is a support including the above-
recited coprecipitate of manganese oxide and zirconia.
The composition comprises from 0.1 to 20, preferably 0.5
to 15 and more preferably 1 to 10 wt.~ of the precious
metal component on the refractory support based on the
weight of the precious metal and the support. Where the
hydrocarbon component is sought to be converted to carbon
dioxide and water, platinum is the most preferred
catalyst and is preferably used in amounts of from 0.1 to
5~ and more preferably 2 to 5~ by weight. In specific
embodiments, there can be a combination of catalysts
including the above recited catalyst as well as a
catalyst which is particularly preferred for the
treatment of ozone such as a catalyst comprising a
manganese component. The manganese component can be
optionally combined with a platinum component. The
manganese and platinum can be on the same or different
CA 02206460 1997-0~-29
W 096/2214s PCTrUS96/00781
- 48 -
supports. There can be up to 80, preferably up to 50,
more preferably from 1 to 40 and yet more preferably from
10 to 35 wt.~ of the manganese component based on the
weight of the precious metal and manganese in the
pollutant treating composition. The catalyst loading is
the same at that recited above with regard to the ozone
catalyst. A preferred composition is a composition
wherein the refractory component comprises an alumina or
titania support and the precious metal component
comprises a platinum component. Testing of such a
composition coated onto a radiator has resulted in 68 to
72~ conversion of carbon monoxide, 8 to 15~ conversion of
ozone and 17 to 18~ conversion of propylene when
contacting a surface at 95~C with a face velocity of the
gas stream being about ten miles per hour (hourly space
velocity of 320,000 per reciprocal hours) with air dew
point at 35~F. Generally, as the contacting surface
temperature decreases and the space velocity or face
velocity of the atmosphere air flow over the pollutant
contacting surface increases, the percent conversion
decreases.
Catalyst activity, particularly to treat carbon
monoxide and hydrocarbons can be further enhanced by
r~ ;ng the catalyst in a forming gas such as hydrogen,
carbon monoxide, methane or hydrocarbon plus nitrogen
gas. Alternatively, the reducing agent can be in the
form of a liquid such as a hydrazine, formic acid, and
formate salts such as sodium formate solution. The
catalyst can be reduced as a powder or after coating onto
a substrate. The reduction can be conducted in gas at
from 150~-500~C, preferably 200~-400~C for 1 to 12 hours,
preferably 2 to 8 hours. In a preferred process, coated
article or powder can be reduced in a gas comprising 7
hydrogen in nitrogen at 275~-350~C for 2 to 4 hours.
An alternate composition for use in the method
and apparatus of the present invention comprises a
catalytically active material selected from the group
CA 02206460 1997-0~-29
W O 96122149 PCTrUS96/00781
- 49 -
consisting of precious metal components including
~ platinum group metal components, gold components and
silver components and a metal component selected from the
group consisting of tungsten components and rhenium
components. The relative amounts of catalytically active
material to the tungsten component and /or rhenium
component based on the weight of the metal are from 1:25,
to 15:1.
The composition cont~;n;ng a tungsten component
and/or a rhenium component preferably comprises tungsten
and/or rhenium in the oxide form. The oxide can be
obtained by forming the composition using tungsten or
rhenium salts and the composition can subsequently be
calcined to form tungsten and/or rhenium oxide. The
composition can comprise further components such as
supports including refractory oxide supports, manganese
components, carbon, and coprecipitates of a manganese
oxide and zirconia. Useful refractory metal oxides
include alumina, silica, titania, ceria, zirconia,
chromia and mixtures thereof. The composition can
additionally comprise a binder material, such as metal
sols including alumina or titania sols or polymeric
binder which can be provided in the form of a polymeric
latex binder.
In preferred compositions, there are from 0.5
to 15, preferably 1 to 10, and most preferably from 3 to
5 percent by weight of the catalytically active material.
The preferred catalytically active materials are platinum
group metals with platinum and palladium being more
preferred and platinum being most preferred. The amount
of tungsten and/or rhenium component based on the metals
ranges 1 to 25, preferably 2 to 15 and most preferably 3
to 10 weight percent. The amount of binder can vary from
0 to 20 weight percent, preferably 0.5 to 20, more
preferably 2 to 10 and most preferably 2 to 5 weight
percent. Depending on the support material a binder is
not necessary in this composition. Preferred
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 50 -
compositions comprise from 60 to 98. 5 weight percent of a
refractory oxide support, from 0. 5 to 15 weight percent
of the catalytically active material, from 1 to 25 weight
of the tungsten and/or rhenium component, and from 0 to
10 weight percent binder.
Compositions cont~ln;ng the tungsten component
and rhenium component can be calcined under conditions as
recited above. Additionally, the composition can be
reduced. However, as shown in the examples below, the
compositions need not be reduced and the presence of the
tungsten and/or rhenium component can result in
conversions of carbon monoxide and hydrocarbons
comparable to compositions cont~'n;ng platinum group
metals which have been reduced.
The pollutant treating compositions of the
present invention preferably comprise a binder which acts
to adhere the composition and to provide adhesion to the
atmosphere contacting surface. It has been found that a
preferred binder is a polymeric binder used in amounts of
from 0. 5 to 20, more preferably 2 to 10, and most
preferably to 2 to 5 percent by weight of binder based on
the weight of the composition. Preferably, the binder is
a polymeric binder which can be a therm~setting or
thPrmoplastic polymeric binder. The polymeric binder can
have suitable stabilizers and age resistors known in the
polymeric art. The polymer can be a plastic or
elastomeric polymer. Most preferred are therm~setting,
elastomeric polymers introduced as a latex into the
catalyst into a slurry of the catalyst composition,
preferably an aqueous slurry. Upon application of the
composition and heating the binder material can crosslink
providing a suitable support which enhances the integrity
of the coating, its adhesion to the atmosphere contacting
surface and provides structural stability under
vibrations encountered in motor vehicles. The use of
preferred polymeric binder enables the pollutant treating
composition to adhere to the atmosphere contacting
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
surface without the necessity of an undercoat layer. The
binder can comprise water resistant additives to improve
water resistance and improve adhesion. Such additives
can include fluorocarbon emulsions and petroleum wax
emulsions.
Useful polymeric compositions include
polyethylene, polypropylene, polyolefin copolymers,
polyisoprene, polybutadiene, polybutadiene copolymers,
chlorinated rubber, nitrile rubber, polychloroprene,
ethylene-propylene-diene elastomers, polystyrene,
polyacrylate, polymethacrylate, polyacrylonitrile,
poly~vinyl esters), poly(vinyl halides), polyamides,
cellulosic polymers, polyimides, acrylics, vinyl acrylics
and styrene acrylics, poly vinyl alcohol, thermoplastic
polyesters, thermosetting polyesters, poly(phenylene
oxide), poly(phenylene sulfide), fluorinated polymers
such as poly(tetrafluoroethylene) polyvinylidene
fluoride, poly(vinylfluoride) and chloro/fluoro
copolymers such as ethylene chlorotrifluoroethylene
copolymer, polyamide, phenolic resins and epoxy resins,
polyurethane, and silicone polymers. A most preferred
polymeric material is an acrylic polymeric latex as
described in the accompanying examples.
Particularly preferred polymers and copolymers
are vinyl acrylic polymers and ethylene vinyl acrylic
copolymers. A preferred vinyl acetate polymer is a cross
linking polymer sold by National Starch and Chemical
C~mp~ny as Xlink 2833. It is described as a vinyl
acrylic polymer having a Tg of -15~C, 45~ solids, a pH of
4.5 and a viscosity of 300 cps. In particular, it is
indicated to have vinyl acetate CAS No. 108-05-4 in a
concentration range of less than 0.5 percent. It is
indicated to be a vinyl acetate copolymer. Other
preferred vinyl acetate copolymers which are sold by the
National Starch and Chemical Company include Dur-O-Set E-
623 and Dur-O-Set E-646. Dur-O-Set E-623 is indicated to
be ethylene vinyl acetate copolymers having a Tg of 0~C,
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 52 -
52~ solids, a pH of 5.5 and a viscosity of 200 CpS. Dur-
O-Set E-646 is indicated to be an ethylene vinyl acetate
copolymer with a Tg of -12~C, 52~ solids, a pH of 5.5 and
a viscosity of 300 cps.
An alternate and useful binding material is the
use of a zirconium compound. Zirconyl acetate is
preferred zirconium compound used. It is believed that
zirconia acts as a high temperature stabilizer, promotes
catalytic activity, and improves catalyst adhesion. Upon
calcination, zirconium compounds such as zirconyl acetate
are converted to ZrO2 which is believed to be the binding
material. Various useful zirconium compounds include
acetates, hydroxides, nitrates, etc. for generating ZrO2
in catalysts. In the case of using zirconyl acetate as a
binder for the present catalysts, ZrO2 will not be formed
unless the radiator coating is calcined. Since good
adhesion has been attained at a "calcination" temperature
of only 120"C, it is believed that the zirconyl acetate
has not decomposed to zirconium oxide but instead has
formed a cross linked network with the pollutant treating
material such as Carulite~ particles and the acetates
which were formed from ball milling with acetic acid.
Accordingly, the use of any zirconium cont~; n; ng
compounds in the present catalysts are not restricted
only to zirconia. Additionally, the zirconium compounds
can be used with other binders such as the polymeric
binder recited above.
An alternate pollutant treating catalyst
composition can comprise activated carbon compo~ition.
The carbon composition comprises activated carbon, a
binder, such as a polymeric binder, and optionally
conventional additives such as defoamers and the like. A
useful activated carbon composition comprises from 75 to
85 weight percent activated carbon such as "coconut
shell~l carbon or carbon from wood and a binder such as an
acrylic binder with a defoamer. Useful slurries comprise
from 10 to 50 weight percent solids. The activated
CA 02206460 1997-0~-29
W O96/22149 PCT~US96100781
- 53 -
carbon can catalyze reduction of ozone to oxygen, as well
as adsorb other pollutants.
Pollutant treating catalyst compositions of the
present invention can be prepared in any suitable
process. A preferred process is disclosed in U.S. Patent
No. 4,134,860 herein incorporated by reference. In
accordance with this method, the refractory oxide support
such as acti~ated alumina, titania or activated silica
alumina is jet milled, impregnated with a catalytic metal
salt, preferably precious metal salt solution and
calcined at a suitable temperature, typically from about
300~C to about 600~C, preferably from about 350~C to
about 550~C, and more preferably from about 400~C to
about 500~C for from about 0. 5 to about 12 hours.
Palladium salts are preferably a palladium nitrate or a
palladium amine such as palladium tetr~m1n~ acetate, or
palladium tetraamine hydroxide. Platinum salts
preferably include platinum hydroxide solubilized in an
amine. In specific and preferred embodiments the
calcined catalyst is reduced as recited above.
In an ozone treating composition, a manganese
salt, such as manganese nitrate, can then be m;XI~A with
the dried and calcined alumina supported palladium in the
presence of deionized water. The amount of water added
should be an amount up to the point of incipient wetness.
Reference is made to the method reviewed in the above
referenced and incorporated U.S. Patent No. 4, 134, 860.
The point of incipient wetness is the point at which the
amount of liquid added is the lowest concentration at
30 which the powdered mixture is sufficiently dry so as to
absorb essentially all of the liquid. In this way a
soluble manganese salt such as Mn(NO3) 2 in water can be
f added into the calcined supported catalytic precious
metal. The mixture is then dried and calcined at a
suitable temperature, preferably 400 to 500~C for about
0.5 to about 12 hours.
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
- 54 -
Alternatively, the supported catalytic powder
(i.e., palladium supported on alumina) can be combined
with a liquid, preferably water, to form a slurry to
which a solution of a manganese salt, such as Mn(NO3) 2 iS
added. Preferably, the manganese component and palladium
supported on a refractory support such as activated
alumina, more preferably activated silica-alumina is
mixed with a suitable amount of water to result in a
slurry having from 15 to 40~ and preferable 20 to 35
weight percent solids. The combined mixture can be
coated onto a carrier such as a radiator and the radiator
dried in air at suitable conditions such as 50~C to 150~C
for 1 to 12 hours. The substrate which supports the
coating can then be heated in an oven at suitable
conditions typically from 300~C to 550~C, preferably
350~C to 500~C, more preferably 350~C to 450~C and most
preferably from 400~C and 500~C in an oxygen cont~;n;ng
atmosphere, preferably air for about 0.5 to about 12
hours to calcine the components and help to secure the
coating to the substrate atmosphere contacting surface.
Where the composition further comprises a precious metal
component, it is preferably reduced after calcining.
The method of the present invention includes
forming a mixture comprising a catalytically active
material selected from at least one platinum group metal
component, a gold component, a silver component, a
manganese component and water. The catalytically active
material can be on a suitable support, preferably a
refractory oxide support. The mixture can be milled,
calcined and optionally reduced. The calcining step can
be conducted prior to adding the polymeric binder. It is
also preferred to reduce the catalytically active
material prior to adding the polymeric binder. The
slurry comprises a carboxylic acid compound or polymer
cont~;n;ng carboxylic acid in an amount to result in a pH
of about from 3 to 7, typically 3 to 6, and preferably
from 0.5 to 15 weight percent of glacial acetic acid
-
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 55 -
based on the weight of the catalytically active material
and acetic acid. The amount of water can be added as
suited to attain a slurry of the desired viscosity. The
percent solids are typically 20 to 50 and preferably 30
to 40 percent by weight. The preferred vehicle is
deionized water (D.I.). The acetic acid can be added
upon forming the mixture of the catalytically active
material, which may have been calcined, with water.
Alternatively, the acetic acid can be added with the
polymeric binder. A preferred composition to treat ozone
using manganese dioxide as the catalyst can be made using
about 1,500 g of manganese dioxide which is mixed with
2,250 g of deionized water and 75 g or acetic acid. The
mixture is combined in a 1 gallon ballmill and ballmilled
for about 8 hours until approximately 90~ of the
particles are less than 8 micrometers. The ballmill is
drained and 150 g of polymeric binder is added. The
mixture is then blended on a rollmill for 30 minutes.
The resulting mixture is ready for coating onto a
suitable substrate such as an automobile radiator
according to the methods described below.
The pollutant treating composition can be
applied to the atmosphere contacting surface by any
suitable means such as spray coating, powder coating, or
brushing or dipping the surface into a catalyst slurry.
The atmosphere contacting surface is preferably
cleaned to remove surface dirt, particularly oils which
could result in poor adhesion of the pollutant treating
composition to the surface. Where possible, it is
preferred to heat the substrate on which the surface is
located to a high enough temperature to volatilize or
burn off surface debris and oils.
Where the substrate on which there is an
atmosphere contacting surface is made of a material which
can withstand elevated temperatures such as an aluminum
radiator, the substrate surface can be treated in such a
manner as to improve adhesion to the catalyst
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 56 -
composition, preferably the ozone carbon monoxide, and/or
hydrocarbon catalyst composition. One method is to heat
the alllm~n~lm substrate such as the radiator to a
sufficient temperature in air for a sufficient time to
form a thin layer of aluminum oxide on the surface. This
helps clean the surface by removing oils which may be
detrimental to adhesion. Additionally, if the surface is
aluminum a sufficient layer of oxidized aluminum has been
found to be able to be formed by heating the radiator in
air for from 0. 5 to 24 hours, preferably from 8 to 24
hours and more preferably from 12 to 20 hours at from
350~C to 500~C, preferably from 400 to 500~C and more
preferably 425 tO 475~C. In some cases, sufficient
adhesion without the use of an undercoat layer has been
attained where an aluminum radiator has been heated at
450~C for 16 hours in air. This method is particularly
useful when applying the coating to new surfaces prior to
assembly, either as original equipment or replacement.
Adhesion may improve by applying an undercoat
or precoat to the substrate Useful undercoats or
precoats include refractory oxide supports of the type
discussed above, with alumina preferred. A preferred
undercoat to increase adhesion between the atmosphere
contacting surface and an overcoat o~ an ozone catalyst
composition is described in commonly assigned U.S. Patent
No. 5,422,331 herein incorporated herein by reference.
The undercoat layer is disclosed as comprising a mixture
of fine particulate refractory metal oxide and a sol
selected from silica, alumina, zirconia and titania sols.
In accordance with the method of the present invention,
surfaces on existing stationary surfaces can be coated in
place. The catalyst composition can be applied directly
to the surface. Where additional adhesion is desired, an
undercoat can be used as recited above.
Where it is practical to separate the radiator
from the stationary substrate, a support material such as
activated alumina, silica-alumina, bulk titania, titanium
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 57 -
sol, silica zirconia, manganese zirconia and others as
recited can be formed into a slurry and coated on the
substrate preferably with a silica sol to improve
adhesion. The precoated substrate can subsequently be
coated with soluble precious metal salts such as the
platinum and/or palladium salts, and optionally manganese
nitrate. The coated substrate can then be heated in an
oven in air for sufficient time (0.5 to 12 hours at 350~
C to 550~C) to calcine the palladium and manganese
components to form the oxides thereof.
The present invention can comprise adsorption
compositions supported on the atmosphere contacting
surface. The adsorption compositions can be used to
adsorb gaseous pollutants such as hydrocarbons and sulfur
dioxide as well as particulate matter such as particulate
hydrocarbon, soot, pollen, bacteria and germs. Useful
supported compositions can include adsorbents such as
zeolite to adsorb hydrocarbons. Useful zeolitic
compositions are described in Publication No. WO 94/27709
published December 8, 1994 and entitled Nitrous Oxide
Decomposition Catalyst hereby incorporated by re~erence.
Particularly preferred zeolites are Beta zeolite, and
dealuminated Zeolite Y.
Carbon, preferably activated carbon, can be
formed into carbon adsorption compositions comprising
activated carbon and binders such as polymers as known in
the art. The carbon adsorption composition can be
applied to the atmosphere contact~ng surface. Activated
carbon can adsorb hydrocarbons, volatile organic
components, bacteria, pollen and the like. Yet another
adsorption composition can include components which can
adsorb SO3. A particularly use~ul SO3 adsorbent is
calcium oxide. The calcium oxide is converted to calcium
sulfate. The calcium oxide adsorbent compositions can
also contain a vanadium or platinum catalyst which can be
used to convert sulfur dioxide to sulfur trioxide which
CA 02206460 1997-0~-29
WO96/22149 PCT~S96/00781
- 58 -
can then be adsorbed onto the calcium oxide to form
calcium sulfate.
- In addition to treatment of atmospheric air
containing pollutants at ambient condition or ambient
operating conditions, the present invention contemplates
the catalytic oxidation and/or reduction of hydrocarbons,
nitrogen oxides and residual carbon monoxide using
conventional three way catalysts supported on
electrically heated catalysts such as are known in the
art. The electrically heated catalysts can be located on
an electrically heated catalyst monolith. Such
electrically heated catalyst substrates are known in the
art and are disclosed in references such as U.S. Patent
Nos. 5,308,591 and 5,317,869, both hereby incorporated by
reference. For the purposes of the present invention,
the electrically heated catalyst is a metal honeycomb
having a suitable thickness in the flow direction,
preferably of from 1/8 inch to 12 inches, and more
preferably 0.5 to 3 inches. Preferred supports are
monolithic carriers of the type having a plurality of
fine, parallel gas ~low passages extending therethrough
from an inlet face to an outlet face of the carrier so
that the passages are open to air flow entering from the
front and passing through the monolith in the direction
toward the fan. Preferably the passages are essentially
straight from their inlet to their outlet and are defined
by walls in which the catalytic material is coated as a
wash coat so that the gases flowing through the passages
contact ~he catalytic material. The flow passages of the
monolithic carrier are thin wall channels which can be of
any suitable cross-sectional shape and size such as
trapezoidal, rectangular, square, sinusoidal, hexagonal,
oval, circular or formed from metallic components which
are corrugated and flat as are known in the art. Such
structures may contain from about 60 to 600 or more gas
inlet openings ("cells") per square inch of cross
section. The monolith may be made of any suitable
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
59
material and is preferably capable of being heated upon
application of an electric current. A useful catalyst to
apply is the three way catalyst (TWC) as recited above
which can enhance the oxidation of hydrocarbons and
carbon monoxide as well as the reduction of nitrogen
oxides. Useful TWC catalysts are recited in U.S. Patent
Numbers 4,714,694; 4,738,947; 5,010,051; 5,057,483; and
5,139,992.
EXAMP~ES
The present invention is illustrated further by
the following examples which are not intended to limit
the scope of this invention.
In some of the following examples, an
automobile radiator is used as the test substrate.
Although such a substrate would not be "stationary" in
operation, the purpose of these examples is to show the
effectiveness of certain catalysts in treating particular
gaseous pollutants. In addition, a test of such
catalysts for use on stationary substrates can be
conducted by mounting such a catalyzed radiator in the
path of an air stream at a stationary location. For
example, a catalyzed radiator can be mounted on a
stationary heat exchanger or in the path of an air
handling system to determine whether the catalyst is
suitable for treating the particular gaseous pollutants
under the ambient conditions at that location.
Example 1
- A 1993 Nissan Altima radiator core (Nissan part
number 21460-lE400) was heat treated in air to 450~C for
16 hours to clean and oxidize the surface and then a
portion coated with high surface area silica-alumina
undercoat (dry loading = 0.23 g/in3) by pouring a water
slurry containing the silica-alumina through the radiator
~h~nn~l S, blowing out the excess with an air gun, drying
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 60 -
at room temperature with a fan, and then calcining to
450~C. The silica-alumina slurry was prepared by ball
- milling high surface area calcined SRS-II alumina
(Davison) with acetic acid (0.5~ based on alumina) and
water (total solids ca. 20~) to a particle size of 90~
<4 ~m. The ball milled material was then blended with
Nalco silica sol (#9lSJ06S - 28~ solids) in a ratio of
25~/75~. The SRS-II alumina is specified to have a
structure of xSiO2.yAl2O3.zH~O with 92-95~ by weight Al2O3
and 4-7~ by weight SiO2 after activation. BET surface
area is speclfied to be a m1n1mnm of 260 m2/g after
calcination.
A Pd/Mn/Al~O3 catalyst slurry ~n~ml n~ 1 ly 10~ by
weight palladium on alumina) was prepared by impregnating
high surface area SRS-II alumina (Davison) to the point
of incipient wetness with a water solution containing
sufficient palladium tetraamine acetate. The resulting
powder was dried and then calcined for 1 hour at 450~C.
The powder was subsequently mixed under high shear with a
water solution of manganese nitrate (amount equivalent to
5.5~ by weight MnO2 on the alumina powder) and sufficient
dilution water to yield a slurry of 32-34~ solids. The
radiator was coated with the slurry, dried in air using a
fan, and then calcined in air at 450~C for 16 hours.
This ozone destruction catalyst contained palladium (dry
loading = 263 g/ft3 of radiator volume) and manganese
dioxide (dry loading = 142 g/ft3) on high surface area
SRS-II alumina. The partially coated radiator was
reassembled with the coolant tanks, also referred to as
headers.
Ozone destruction performance of the coated
catalyst was determined by blowing an air stream
cont~; nl ng a given concentration of ozone through the
radiator channels at face velocities typical of driving
speeds and then measuring the concentration of ozone
exiting the back face of the radiator. The air used was
at about 20~C and had a dew point of about 35~W Coolant
-
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 61 -
fluid was circulated through the radiator at a
temperature of about 50~C. Ozone concentrations ranged
from 0.1-0.4 ppm. Ozone conversion was measured at
linear air velocities (face velocities) equivalent to
12.5 miles per hour to be 43~; at 25 mph to be 33~; at
37.5 mph to be 30~ and at 49 mph to be 24~.
Example 2 (Comparative)
A portion of the same radiator used in Example
1 which was not coated with catalyst was similarly
evaluated for ozone destruction performance (i.e. control
experiment). No conversion of ozone was observed.
Example 3
After heat treatment for 60 hours in air at
450~C, a Lincoln Town Car radiator core (part #FlVY-8005-
A) was coated sequentially in 6" X 6" square patches witha variety of different ozone destruction catalyst
compositions (i.e., different catalysts; catalyst
loadings, binder formulations, and heat treatments).
Several of the radiator patches were precoated with a
high surface area alumina or silica-alumina and calcined
to 450~C prior to coating with the catalyst. The actual
coating was accomplishea similarly to Example 1 by
pouring a water slurry containing the specific catalyst
formulation through the radiator channels, blowing out
the excess with an air gun, and drying at room
temperature with a fan. The radiator core was then dried
to 120~C, or dried to 120~C and then calcined to 400 to
450~C. The radiator core was then reattached to its
~ plastic tanks and ozone destruction performance of the
various catalysts was determined at a radiator surface
temperature of about 40~C to 50~C and a face velocity of
10 mph as described in Example 1.
Table I summarizes the variety of catalysts
coated onto the radiator. Details of the catalyst slurry
preparations are given below.
CA 02206460 l997-0~-29
W O96/221~9 PCTAUS96/00781
- 62 -
A Pt/Al203 catalyst (nom; n~3lly 2~ by weight Pt
on Al2O3) was prepared by impregnating 114 g of a platinum
salt solution derived from H2Pt(OH) 6 solubilized in an
amine, (17.9~ Pt), dissolved in 520 g of water to 1000 g
of Condea SBA-15Q high surface area (specified to be
about 150 m2/g) alumina powder. Subsequently 49. 5 g of
acetic acid was added. The powder was then dried at
110~C for 1 hour and calcined at 550~C for 2 hours. A
catalyst slurry was then prepared by adding 875 g of the
powder to 1069 g of water and 44.6 g of acetic acid in a
ball mill and milling the mixture to a particle size 90
10 ~m. (Patches 1 and 4)
The carbon catalyst slurry was a formulation
(29~ solids) purchased from Grant Industries, Inc.,
Elmwood Park, NJ. The carbon is derived from coconut
shell. There is an acrylic binder and a defoamer.
(Patches 8 and 12)
The Carulite~ 200 catalyst (CuO/MnO~) was
prepared by first ball milling 1000 g of Carulite6' 200
(purchased from Carus Chemical Co., Chicago, IL) with
1500 g of water to a particle size 90~ < 6 ,um. ~arulite~
200 is specified as containing 60 to 75 weight percent
MnO~, 11-14 percent CuO and 15-16 percent A1~03. The
resulting slurry was diluted to ca. 2 8~ solids and then
mixed with either 3~ (solids basis) of Nalco #1056 silica
sol or 2~ (solids basis) National Starch #x4260 acrylic
copolymer. (Patches 5, 9 and 10)
The Pd/Mn/Al2O3 catalyst slurry (n~m' ni~lly 10~
by weight palladium on alumina) was prepared as described
in Example 1. (Patches 2, 3 and 6)
An I.W. (incipient wetness) Pd/Mn/Al2O3
catalyst (n~m' n;3lly 8~ palladium and 5.5~ MnO, based on
alumina) was prepared similarly by first impregnating
high surface area SRS-II alumina (Davison) to the point
of incipient wetness with a water solution ccntaining
palladium tetraamine acetate. After drying and then
calcining the powder for two hours at 450~C, the powder
_
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 63 -
was reimpregnated to the point of incipient wetness with
a water solution containing manganese nitrate. Again,
after drying and calcination at 450~C for two hours, the
powder was mixed in a ball mill with acetic acid (3~ by
weight of catalyst powder) and enough water to create a
slurry of 35~ solids. The mixture was then milled until
the particle size was 90~ < 8 ~m. (Patches 7 and 11)
The SiO2/Al~03 precoat slurry was prepared as
described in Example 1. (Patches 3 and 11)
The Al703 precoat slurry was prepared by ball
milling high surface area Condea SBA-150 alumina with
acetic acid (5~ by weight based on alumina) and water
(total solids ca. 44~) ~o a particle size of 90~ < 10 ~m.
(Patches 9 and 12)
Results are summarized in Table I. The
conversion of carbon monoxide after being on the
automobile for 5,000 miles was also measured at the
conditions recited in Example 1 for patch #4. At a
radiator temperature of 50~C and a linear velocity of 10
mph no conversion was observed.
TABLE I - CATALYST SUI\~RY
PATCH #CATALYST OZONE
CONVERSION ( % )
Pt/AI~03 12
0.67 g/in3 (23 g/ft3 Pt)
No Precoat
No Calcine (120~C orlly)
Pd/Mn/Ako3 25
0.97 g/in3 (171 g/ft3 Pd)
No Precoat
Calcined 450~C
3 Pd/Mn/AI~03 24
1.19 g/in3 (209 g/ft3 Pd)
SiO"/Al~O3 Precoat (0.16 g/in3)
Calcined 450~C
CA 02206460 1997-05-29
W O96/221~9 PCTrUS96/00781
- 64 -
4 Pt/AI"03 8
0.79 g/in3(27 g/ft3 Pt)
No Precoat
Calcined 450 ~ C
5Carulite 200 50
0.4 9 g/in3
3 % SiO~ O3 Binder
No Precoat
Calcined 400 ~ C
6Pd/Mn/AI 03 28
0.39 g/in3 (70 g/ft3 Pd)
No Precoat
Calcined 450 ~ C
7I.W. Pd/Mn/AI~03 50
0.69 g/in3 (95 g/ft3 Pd)
No Precoat
No Calcine (120CC or,ly)
8 Carbon 22
0.80 g/in3
No Precoat
No Calcine (120 ~ C only)
9Carulite 200 38
0.65 g/in3
3 % SiO~ O3 Binder
Al~O3 Precoat fO.25 g/in3)
Calcined 450 ~ C
10Carulite 200 42
0.70 g/in3
'7 % Latex Binder
No Precoat
No Caicine (120~C only)
11I.W. Pd/Mn/AI~03 46
0.59 g/in3 (82 g/ft3 Pd)
SiO"/Al,O3 precoat (0.59 g/in3)
No Calcine either Coat (120~C only)
12Carbon 17
1.07 g/in3
Al103 Precoat (0.52 g/in3) calcined
to 450~C
Topcoat not calcined (120~C only)
Example 4
A 1993 Nissan Altima radiator core (Nissan part
number 21460-lE400) was heat treated in air to 400~C for
16 hours and then a portion coated with Condea high
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 65 -
surface area SBA-150 alumina (dry loading = 0.86 g/in3) by
pouring a water slurry containing the alumina through the
- radiator channels, blowing out the excess with an air
gun, drying at room temperature with a fan, and then
calcining to 400~C. The alumina precoat slurry was
prepared as described in Example 3. The radiator was
then coated sequentially in 2" x 2" s~uare patches with
seven different CO destruction catalysts (Table II).
Each coating was applied by pouring a water slurry
containing the specific catalyst formulation through the
radiator channels, blowing out the excess with an air
gun, and drying at room temperature with a fan.
The Carulite~ and 2~ Pt/Al2O3 catalysts (Patches
#4 and #6, respectively) were prepared according to the
procedure described in Example 3. The 3~ Pt/ZrO2/SiO~
catalyst (Patch #3) was made by first calcining 510 g of
zirconia/silica frit (95~ ZrO2/5~SiO~ - Magnesium Elektron
XZO678/01) for 1 hour at 500~C. A catalyst slurry was
then prepared by adding to 480 g of deionized water,
468 g of the resulting powder, 42 g of glacial acetic
acid, and 79.2 g of a platinum salt solution (18.2~ Pt)
derived from H,Pt(OH) 6 solubilized with an amine. The
resulting mixtune was milled on a ball mill for 8 hours
to a particle size of 90~ less than 3~m.
2S The 3~ Pt/TiO2 catalyst (Patch #7) was prepared
by mixing in a conventional blender 500 g of TiO2 (Degussa
P25), 500 g of deionized water, 12 g of concentrated
on;um hydroxide, and 82 g of a platinum salt solution
(18.2~ Pt) derived from H2Pt(OH)6 solubilized with an
amine. After blending for 5 minutes to a particle size
of 90~ less than 5~m, 32.7 g of Nalco 1056 silica sol and
sufficient deionized water to reduce the solids content
to ca. 22~ was added. The resulting mixture was blended
on a roll mill to mix all ingredients.
The 3~ Pt/Mn/ZrO2 catalyst slurry (Patch #5) was
prepared by combining in a ball mill 70 g of
manganese/zirconia frit comprising a coprecipitate of 20
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
- 66 -
weight percent manganese and 80 weight percent zirconium
based on metal weight (Magnesium Elektron XZO719/01), >
- 100 g of deionized water, 3.5 g of acetic acid and 11.7 g
of a platinum salt solution (18.2~ Pt) derived from
H7Pt(OH) 6 solubilized with an amine. The resulting
mixture was milled for 16 hours to a particle size 90
less than 10~m.
The 2~ Pt/CeO7 catalyst (Patch ~1) was prepared
by impregnating 490 g of alumina stabilized high surface
area ceria (Rhone Poulenc) with 54.9 g of a platinum salt
solution (18.2~ Pt) derived from H7Pt(OH)6 solubilized
with an amine and dissolved in deionized water (total
volume - 155 mL). The powder was dried at 110~C for 6
hours and caIcined at 400~C for 2 hours. A catalyst
slurry was then prepared by adding 491 g of the powder to
593 g of deionized wa~er in a ball mill and then milling
the mixture for 2 hours to a particle size of 90~ less
than 4~m. The 4.6~ Pd/CeO7 catalyst (Patch ~2) was
prepared similarly via incipient wetness impregnation
using 209.5 g (180 mL) of palladium tetraamine acetate
solution.
After all seven catalysts were applied, the
radiator was calcined for about 16 hours at 400~C. After
attaching the radiator core to the plastic tanks, CO
destruction performance of the various catalysts was
determined by blowing an air stream cont~-n'ng CO (ca.
16 ppm) through the radiator channels at a 5 mph linear
face velocity (315,000/h space velocity) and then
measuring the concentration of CO exiting the back face
of the radiator. The radiator temperature was about
95~C, and the air stream had a dew point of approximately
35~F. Results are summarized in Table II. Figure 4
illustrates CO conversion vs. temperature for Patch Nos.
3, 6 and 7.
Ozone destruction performance was measured as
described in Example 1 at 25~C, 0.25 ppm ozone, and a
linear face velocity cf 10 mph with a flow of 135.2 L/min
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 67 -
and an hourly space velocity of 640, 000/h. The air used
had a dewpoint of 35~F. Results are summarized in Table
~ II.
The catalysts were also tested for the
destruction of propylene by blowing an air stream
containing propylene (ca. 10 ppm) through the radiator
channels at a 5 mph linear face velocity, with a flow
rate of 68. 2 L/min and an hourly space velocity of
320,000/h, and then measuring the concentration of
propylene exiting the back face of the radiator. The
radiator temperature was ca. 95~C, and the air stream had
a dew point of approximately 35~F. Results are
summarized in Table II.
CA 02206460 1997-05-29
W O96t221~9 PCTrUS96/00781
- 68 -
TABLE II - CO/HC/OZONE CONVERSION SI~IMARY
PATCH # CATALYST CO OZONE PROPYLENE
CONV. (%)~CONV. (%)2CONV. (%)3
2% Pt/CeO~ 2 14 0
0.7 g/in3 (24 g/ft3 Pt)
2 4.6% Pd/CeO~ 21 55 0
0.5 g/in3 (40 g/ft3 Pd)
3 3% Pt/ZrO~!SiO~ 67 14 2
0.5 g/in3 (26 g/ft3 Pt)
4 Carulite 200 5 56 0
0.5 ~
3 % SiOIAl~O~ binder
3% Pt/Mn/ZrQ 7 41 0
0.7 g/in3 (36 ~/ft3 Pt)
6 2% Pt/Al,O3 72 8 17
0.5 ~iin3 (17 g/ft3 Pt)
7 3% Pt/TiO~ 68 15 18
0.7 g/in3 (36 g/ft3 Pt)
3% SiO~ O3 binder
~Test Cnn-litinnc 16 ppm CO; 95~C; 5 mph face velocit~; 68.2 L/min: LHSV (hourly space
velocity) = 320,000/h; Air dewpoint = 35~F
2Test Conditions: 0.25 ppm O~: 25~C; 10 mph face velocit~; 135.2 L/min; LHSV (hourly
space velocity) = 640,000/h: Air dewpoint = 35~F
3Test Conditions: 10 ppm propylene; 95~C; S mph face velocity; 68.2 L/min; LHSV (hourly
space velocit ) = 3~0.000/h: Air dewpoint = 35~F
Example 5
This example summarizes technical results from
an on-the-road vehicle test conducted in February and
March 1995 in the Los Angeles area. The purpose of the
test was to measure catalytic ozone decomposition
efficiency over a catalyzed radiator under actual driving
conditions. The Los Angeles (LA) area was chosen as the
most appropriate test site primarily due to its
measurable ozone levels during this March testing period.
In addition, specific driving routes are defined in the
LA area which are typical of AM and PM peak and off-peak
driving. Two different catalyst compositions were
CA 02206460 1997-0~-29
W O961221~9 PCT~US96/00781
- 69 -
evaluated: 1) Carulite~ 200 (CuO/MnO2/Al2O3 purchased from
~ Carus Chemical Company); and 2) Pd/Mn/Al2O3 (77 g/ft3 Pd)
prepared as described in Example 3. Both catalysts were
coated in patches onto a late model Cadillac V- 6 engine
aluminum radiator. The radiator was an all~mlnllm
replacement for the copper-brass OEM radiator which was
on the Chevrolet Caprice test vehicle. The car was
outfitted with 1/4" Teflon~ PTFE sampling lines located
directly behind each catalyst patch and behind an
uncoated portion of the radiator (control patch).
Ambient (catalyst in) ozone levels were measured via a
sampling line placed in front of the radiator. Ozone
concentrations were measured with two Dasibi Model 1003AH
ozone monitors located in the back seat of the vehicle.
Temperature probes were mounted (with epoxy) directly
onto each radiator test patch within a few inches of the
sampling line. A single air velocity probe was mounted
on the front face of the radiator midway between the two
patches. Data from the ozone analyzers, temperature
probes, air velocity probe, and vehicle speedometer were
collected with a personal computer located in the trunk
and downloaded to floppy disks.
Overall results from the test are summarized in
Table III below. For each catalyst (Carulite~ ~
Pd/Mn/Al2O3), results for cold idle, hot idle and on-the-
road driving are reported. Data were collected on two
separate trips to LA in February and March of 1995. The
first trip was cut short after only a few days due to low
ambient ozone levels. Although somewhat higher during
the second trip in March, ambient levels still only
averaged approximately ~0 ppb. The last three days of
testing (March 17-20) had the highest ozone encountered.
Peak levels were approximately 100 ppb. In general, no
trend in conversion vs. ozone concentration was noted.
Except for the cold idle results, those
reported in Table III are averages from at least eleven
different runs (the actual range of values appear in
CA 02206460 1997-0~-29
W O961221~9 PCTrUS96/00781
- 70 -
parentheses). Only data corresponding to inlet ozone
concentration greater or equal to 30 ppb were included.
Freeway data was not included since ambient levels
dropped to 20 ppb or lower. Only two runs were completed
for the cold idle tests. By cold idle refers to data
collected immediately after vehicle startup during idle
before the thermostat switches on and pumps warm coolant
fluid to the radiator. Overall, ozone conversions were
very good for both catalysts with the highest values
obtained during hot idle. This can be attributed to the
higher temperatures and lower face velocities associated
with idling. Cold idle gave the lowest conversion due to
the lower ambient temperature of the radiator surface.
Driving results were intermediate of hot and cold idle
results. Although the radiator was warm, temperature was
lower and face velocity higher than those encountered
with hot idle conditions. In general, ozone conversions
measured for Carulite~ were greater than those measured
for Pd/Mn/Al2O3 (e.g. 78.1 vs. 63.0~ while driving).
However, for the hot idle and driving runs, the average
temperature of the Carulite3 catalyst was typically 40CF
greater than the Pd/Mn/Al~O3 catalyst while the average
radiator face velocity was typically 1 mph lower.
Overall, the results indicate that ozone can be
decomposed at high conversion rates under typical driving
conditions.
CA 02206460 1997-0~-29
W O96/22149 PCT~US96100781
TABLE m - ON-ROAD OZONE CONVERSION RESULTS
OZONE TEMPERATURE FACE VEHrCLE
CONVERSION (~F) VELOCITY SPEED
(%) (mph) (mph)
Pd/Mn/AI~03
Idle Cold 48.2 70.6 9.0 0.0
(47.2-49.2) (70.s-70.8) (8.9-9.2)
Idle Hot 80.6 120.0 7.4 0.0
(70.7-89.9) (104.7-145.2) (6.1-8.4)
Driving 63.0 104.3 13.2 23.3
(55.5-69.9) (99.2-109.6) (12.2-14.9) (20.5-29.7)
Caru~te (CuO/~nO2)
Idle Cold 67.4 71.8 8.2 0.0
(67.4b7.5) (70.8-72.9) (7.5-8.9)
Idle Hot 84.5 157.1 7.5 0.0
(71.4-93.5) (134.8-I71.2) (6.7-8.2)
Driving 78.1 143.7 12.2 19.2
(72.3-83.8) (132.9-149.6) (11.2-13.5) (13.7-24.8)
* Average values. R~nges appear in parentheses.
In general, the results of motor testing are
consistent with fresh activity measured in the lab prior
to installation of the radiator. At room temperature,
20~ relative humidity (0.7~ water vapor absolute), and a
10 mph eauivalent face velocity, lab conversions for
Pd/Mn/Al2O3 and Carulite~ were 55 and 69~ respectively.
Increasing the RH to 70~ (2.3~ absolute) lowered
conversions to 38 and 52~, respectively. Since the cold
idle (70~F) conversions measured at a 9 mph face velocity
were 48 and 67~ respectively, it would appear that the
humidity levels encountered during the testing were low.
The face velocity of air entering the radiator
was low. At an average driving speed of roughly 20 mph
(typical of local driving), radiator face velocity was
only approximately 13 mph. Even at freeway speeds in
CA 02206460 l997-0~-29
W O96/221~9 PCTrUS96/00781
- 72 -
excess of 60 mph, radiator face velocity was only ca. 25
mph. The fan significantly affects control of air
flowing through the radiator. While idling, the fan
typically pulled about 8 mph.
Exam~le 6 - .
An 8 weight percent Pd on Carulite~ catalyst
was prepared by impregnating 100 g Carulite~ 200 powder
(ground up in a blender) to the point of incipient
wetness with 69.0 g of a water solution containing
palladium tetraamine acetate (12. 6~ Pd). The powder was
dried overnight at 90~C and then calcined to 450~C for 2
hours. 92 g of the resulting calcined catalyst was then
combined with 171 g of deionized water in a ball mill to
create a slurry of 35~ solids. After milling for 30
minutes to a particle size 90~ 59 ,um, 3.1 g of National
Starch X4260 acrylic latex binder (50~ solids) was added,
and the resulting mixture was milled for an additional 30
minutes to disperse the binder. Compositions containing
2,4 and 6 weight percent Pd on Carulite~ catalysts were
similarly prepared and evaluated.
The catalysts were evaluated for ozone
decomposition at room temperature and 630, 000/h space
velocity using washcoated 300 CpSi ceramic honeycombs, as
described below in Example 7. The catalyst samples were
prepared as recited above. Results are summarized in
Table IV. As can readily be seen, the 4 and 8~
Pd/Carulite~ catalysts which were calcined to 450~C gave
equivalent initial and 45 minute ozone conversions (ca.
62 and 60~, respectively). These results are equivalent
to those of Carulite~ alone under the identical test
conditions. The 2 and 4~ Pd catalysts which were
calcined to 550~C gave significantly lower conversions
after 45 minutes (47~). This is attributed to a loss in
surface area at the higher temperature of calcination.
The 6~ catalyst was also calcined to 550~C but did not
show quite as large of an activity drop.
CA 02206460 l997-0~-29
W O96t221~9 PCTrUS96/00781
- 73 -
TABLE rv OZONE RESULTS (300cpsiHol.~Jc~,l,b,630,000/hSpace VelociO
CATALYST LO~I~ING CONVERSION (%) CONVERSION (%)
(g/i113)Initial45 Minutes
Pdon Carulite200
4% Pd / Carulite (calci~ed 450~C) 1.8 64 59
8% Pd / Carulite (calcined 450~C) 2.0 61 60
2 % Pd / Carulite (calcined 550 ~ C) 2 . 1 57 48
4% Pd / Carulite (calcined 550~C) 1.9 57 46
6% Pd / Carulite (calcined 550~C) 2.3 59 53
Example 7
A series of tests were conducted to evaluate a
variety or catalyst compositions comprising a palladium
component to treat air containing 0.25 ppm ozone. The
air was at ambient conditions (23~C; 0.6~ water). The
compositions were coated onto 300 cell per inch ceramic
(cordierite) flow through honeycombs at loadings of about
2 g of washcoat per cubic inch of substrate. The coated
monoliths containing the various supported palladium
catalysts were loaded into a 1" diameter stainless steel
pipe, and the air stream was passed perpendicular to the
open face of the honeycomb at a space velocity of
630,000/h. Ozone concentration was measured inlet and
outlet of the catalyst. One alumina support used was
SRS-II gamma alumina (purchased from Davison)
characterized as described in Example 1 (surface area
approximately 300 m2/g). Also used was a low surface area
theta alumina characterized by a surface area of
approximately 58 mq/g and an average pore radius of about
80 Angstrom. E-160 alumina is a gamma alumina
characterized by a surface area of about 180 mq/g and an
average pore radius of about 47 Angstrom. Ceria used had
a surface area about 120 m-/g and an average pore radius
CA 02206460 1997-0~-29
W O96~221~9 PCTrUS96/00781
- 74 -
of about 28 Angstrom. Also used was dealuminated Beta
zeolite with a silica to alumina ratio of approximately
250 to 1 and a surface area about 430 m/g. Carbon, a
microporous wood carbon characterized with a surface area
of about 850 m2/g, was also used as a support. Finally, a
titania purchased from Rhone-Poulenc (DT51 grade) and
characterized by a surface area of approximately 110 m2/g
was used as a support. Results are summarized in Table V
which includes the relative weight percent of various
catalyst components, the loading on the honeycomb,
initial ozone conversion, and conversion after 45
minutes.
TABLE V: OZONE RESULTS - (300 cpsi Ho~ v~b, 630,000/h Space Velocity,
0.6% Water: ca. 0.25 ppm Ozone)
CATALYST LOADING CONVERSION ( % ) CONVERSION ( % )
(g/in3) Initial 45 Minutes
I.W. 8%Pd / 5% Mn / Al,O~ 1.8 60 55
I.W. 8 %Pd / 5 % Mn / Low 1.9 64 60
Surface Area Al..03
8% Pd / I~w Surface Area 1.9 56 44
Al. 03
8% Pd / E-160 A1~03 2.2 61 57
4.6% Pd / CeO~ 1.99 59 58
8% Pd / BETA Zeolite 1.9 38 32
(t~P~ min~tp~l)
5% Pd/C 0.5 63 61
8% Pd / DT-51 TiQ 1.8 39 20
Example 8
Following is a preparation of Carulite~ slurry
which includes vinyl acetate latex binder and is used in
coating radiators which results in excelle~t adhesion of
the catalyst to an aluminum radiator.
1000 g of Carulite~ 200, 1500 g of deionized
water, and 50 g of acetic acid (5~ based on Carulite~)
were combined in a 1 gallon ball mill and milled for 4
CA 02206460 1997-0~-29
W 0961221~9 PCTrUS96/00781
hours to a particle size 90~ 57 ~m. After draining the
resulting slurry from the mill, 104 g (5~ solids basis)
of National Starch Dur-O-Set E-646 cross linking EVA
copolymer (48~ solids) was added. Thorough blending of
the binder was achieved by rolling the slurry on a mill
without milling media for several hours. Following
coating of this slurry onto a piece of alllm;nl~m substrate
(e.g., radiator), excellent adhesion (i.e., coating could
not be wiped off) was obtained after drying for 30
minutes at 30~C. Higher temperatures of curing (up to
150~C) can be utilized if desired.
Example 9
Carbon monoxide conversion was tested by
coating a variety of titania supported platinum
compositions onto ceramic honeycombs as described in
Example 6. Catalyst loadings were about 2 g/in3, and
testing was conducted using an air stream having 16 ppm
carbon monoxide (dew point 35~F) at a space velocity of
315,000/h. The catalyst compositions were reduced on the
honeycomb using a forming gas having 7~ H2 and 93~ N2 at
300~C for 3 hours. Compositions containing Tio2 included
2 and 3 weight percent platinum component on P25 titania;
and 2 and 3 weight percent platinum component on DT51
grade titania. DT51 grade titania was purchased from
Rhone-Poulenc and had a surface area of about 110 m2/g.
Alternatively, DT52 grade titania, a tungsten cont~;n;ng
titania from Rhone-Poulenc which also has a surface area
of about 110 m2/g can be used. P25 grade titania was
purchased from Degussa and was characterized as having a
particle size of approximately 1 ~m and a surface area of
about 45-50 m2/g. Results are illustrated in Figure 5.
Example 10
Example 10 relates to the evaluation of CO
conversion for compositions containing alumina, ceria and
zeolite. The supports were characterized as described in
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
Example 7. Compositions evaluated included 2 weight
percent platinum on low surface area theta alumina; 2
weight percent platinum on ceria; 2 weight percent
platinum on SRS-II gamma alumina, and 2 weight percent
platinum on Beta zeolite. Results are illustrated in
Figure 6. The catalyst compositions were reduced.
Example 11
CO conversion was measured v. temperature for
compositions containing 2 weight percent platinum on SRS-
II gamma alumina and on ZSM-5 zeolite which were coated
onto a 1993 Nissan ~tima radiator as recited in Example
4 and tested using the same procedure to test CO as used
in Example ~. Results are illustrated in Figure 4.
Exam~le 12
0.659 g of a solution of amine solubilized
platinum hydroxiae solution having 17.75 weight percent
platinum (based on metallic platinum) was slowly added to
20 g of an 11.7 weight percent aqueous slurry of a
titania sol in a glass beaker and stirred with a magnetic
stirrer. A one-inch diameter by one-inch long 400 cells
per square inch (cpsi) metal monolith was dipped into the
slurry. Air was blown over the coated monolith to clear
the channels and the monolith was dried for three hours
at 110~C. At this time, the monolith was redipped into
the slurry once again and the steps of air blowing the
ch~nn~ls and drying at 110~C was repeated. The twice
coated monolith was calcined at 300~C for two hours. The
uncoated metal monolith weighed 12.36 g. After the first
dipping, it weighed 14.06 g, after the first drying
12.6 g, after the second dipping 14.38 g and after
calcination weighed 13.05 g indicating a total weight
gain of 0.69 g. The coated monolith had 72 g/ft3 of
platinum based on the metal and is designated as 72Pt/Ti.
The catalyst was evaluated in an air stream containing 20
ppm carbon monoxide at a gas flow rate of 36.6 liters per
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
minute. After this initial evaluation the catalyst core
was reduced in a forming gas having 7~ hydrogen and 93
' nitrogen at 300~C for 12 hours and the evaluation to
treat an air stream containing 20 ppm carbon monoxide was
repeated. The reduced coated monolith as designated as
72Pt/Ti/R. The above recited slurry was then evaluated
using a core from a ceramic monolith having 400 cells per
square inch (cpsi), which was precoated with 40 g per
cubic foot, of 5:1 weight ratio of platinum to rhodium
plus 2.0 g per cubic inch of ES-160 (alumina) and the
core had 11 cells by 10 cells by 0.75 inches long
monolith and designated as 33Pt/7Rh/Al was dipped into
the above recited slurry and air blown to clean the
channels. This monolith was dried at 110~C for three
hours and calcined at 300~C for two hours. The catalyst
substrate including the first platinum and rhodium layer
weighed 2.19 g. After the first dip it weighed 3.40 g
and after calcination 2.38 g showing a total weight gain
of 0.19 g which is equal to 0.90 g per cubic inch of the
platinum/titania slurry. The dipped ceramic core
contained 74 per cubic foot of platinum based on the
platinum metal and designated as 74Pt/Ti//Pt/Rh. Results
are illustrated in Figure 7.
Example 13
A platinum on titanium catalyst as described in
the above referenced Example 12 was used in an air stream
containing 4 ppm propane and 4 ppm propylene, at a space
velocity of 650,000 shsv. The platinum and titanium
catalyst had 72 g of platinum per cubic foot of total
~ 30 catalyst and substrate used. It was evaluated on the
ceramic honeycomb as recited in Example 13. The measured
~ results for propylene conversion were 16.7~ at 65~C; 19
at 70~C; 23.8~ at 75~C; 28.6~ at 80~C; 35.7~ at 85~C;
40.5~ at 95~C and 47.6~ at 105~C.
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
- 78 -
Example 14
Example 14 is an illustration of a platinum
component on a titania support. This Example illustrates
the excellent activity of platinum supported on titania
for carbon monoxide and hydrocarbon oxidation. The
evaluation was carried out using a catalyst prepared from
a colloidal titania sol to form a composition comprising
5.0 weight percent platinum component based on the weight
of the platinum metal and titania. The platinum was
added to titania in the form of amine solubilized
platinum hydroxide solution. It was added to colloidal
titania slurry or into titania powders to prepare a
platinum and titania containing slurry. The slurry was
coated onto a ceramic monolith having 400 cells per
square inch (cpsi). Samples had coating amounts varying
from 0. 8-1.0 g/in. The coated monoliths were calcined
for 300~C for 2 hours in the air and then reduced. The
reduction was carried out at 300~C in a gas containing 7
hydrogen and 93~ nitrogen for 12 hours. The colloidal
titania slurry contained 10~ by weight titania in an
aqueous media. The titania had a noml n~ 1 particle size
of 2-5 nm.
Carbon monoxide conversion was measured in an
air stream containing 20 ppm CO. The flow rate of the
carbon monoxide in various experiments range from space
velocities of 300,000 VESV to 650,000 VHSV at a
temperature between ambient to 110~C. The air used was
purified air from an air cylinder and where humidity was
added the air was passed through a water bath. Where
humidity was studied the relative humidity was varied
from 0-100~ humidity at room temperature (25~C). The
carbon monoxide containing air stream was passed through
the ceramic monolith coated with the catalyst
compositions using a space velocity of 650,000/h.
Figure 8 represents a study using air with 20
ppm CO having to measure carbon monoxide conversion v.
temperature comparing platinum supported on titania which
~ CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 79 -
has been reduced (Pt/Ti-R) at 300~C using a reducing gas
- containing 7~ hydrogen and 93~ nitrogen for 12 hours as
recited above with a non reduced platinum supported on
titania catalyst ~Pt/Ti) coating. Figure 8 illustrates a
significant advantage when using a reduced catalyst.
Figure 9 illustrates a comparison of platinum
on titania which has been reduced with varying supports
including platinum on tin oxide (Pt/Sn), platinum on zinc
oxide (Pt/Zn) and platinum on ceria (Pt/Ce) for
comparative sake. All of the samples were reduced at the
above indicated conditions. The flow rate of carbon
monoxide in the air was 650,000 shsv. As can be seen,
the reduced platinum on colloidal titania had
significantly higher conversion results than platinum on
the various other support materials.
Hydrocarbon oxidation was measured using a 6
ppm propylene/air mixture. The propylene/air stream was
passed through the catalyst monolith at a space velocity
of 300,000 vhsv at a temperature which varied from room
temperature to 110~C. Propylene concentration was
determined using a flame ionized detector before and
after the catalyst. The results are summarized in Figure
10. The support used was 5~ by weight based on the
weight of platinum metal and yttrium oxide Y203. The
comparison was between reduced and non reduced catalyst.
As shown in Figure 10 reducing the catalyst resulted in a
significant improvement in propylene conversion.
The above recited platinum supported on titania
catalyst was reduced in a forming gas cont~in'ng 7~
hydrogen and 93~ nitrogen at 500~C for 1 hour. The
conversion of carbon monoxide was evaluated in 0 percent
relative humidity air at a flow rate of 500,000 vhsv.
The evaluation was conducted to determine if the
reduction of the catalyst was reversible. Initially, the
catalyst was evaluated for the ability to convert carbon
monoxide at 22~C. As shown in Figure 11, the catalyst
initially converted about 53~ of the carbon monoxide and
CA 02206460 l997-0~-29
W O96/22119 PCTrUS96/00781
- 80 -
dropped down to 30~ after approximately 200 minutes. At
200 minutes the air and carbon monoxide was heated to
50~C and carbon monoxide conversion increased to 65~.
The catalyst was further heated to 100~C in air and
carbon monoxide and held at 100~C for one hour, and then
cooled in air to room temperature (about 25~C).
Initially, the conversion dropped to about 30~ in the
period ~rom about 225-400 minutes. The evaluation was
continued at 100~C to 1200 minutes at which time
conversion was measured at about 40~. A parallel study
was conducted at 50~C. At about 225 minutes the
conversion was about 65~. After 1200 minutes, the
conversion actually rose to about 75~. This Example
shows that reduction of the catalyst permanently improves
the catalysis activity.
Example 15
Example 15 is used to illustrate ozone
conversion at room temperature for platinum and/or
palladium components supported on a manganese
oxide/zirconia coprecipitate. This Example also shows a
platinum catalyst which catalyzes the conversion of ozone
to oxygen and, at the same time, oxidize carbon monoxide
and hydrocarbons. Manganese oxide/zirconia mixed oxide
powders were made having 1:1 and 1:4 weight based on Mn
and Zr metals. The coprecipitate was made in accordance
with the procedure disclosed in U.S. Patent No. 5,283,041
referenced above. 3~ and 6~ Pt on manganese/zirconia
catalysts (1:4 weight basis of Mn to Zr) were prepared as
described in Example 4. SBA-150 gamma alumina (10~ based
on the weight of the mixed oxide powder) was added as a
binder in the form of a 40~ water slurry containing
acetic acid (5~ by weight of alumina powder) and milled
to a particle size 90~ < 10 ~m. The 6~ weight percent Pd
catalyst was prepared by impregnating manganese/zirconia
frit (1:1 weight basis of Mn to Zr) to the point of
incipient wetne s with a water solution containing
CA 02206460 1997-0~-29
W O961221~9 PCTrUS96/00781
- 81 -
palladium tetraamine acetate. After drying and then
- calcining the powder for two hours at 450~C, the catalyst
- was mixed in a ball mill with Nalco #1056 silica sol (10
by weight of catalyst powder) and enough water to create
a slurry of approximately 35~ solids. The mixture was
then milled until the particle size was 90~ ~ 10 ~m.
Various samples were reduced using a forming gas having
7~ H2 and 93~ N2 at 300~C for 3 hours. Evaluations were
conducted to determine the conversion of ozone on coated
radiator minicores from a 1993 Altima radiator which were
approximately 1/2 inch by 7/8 inch by 1 inch deep. The
evaluation was conducted at room temperature using a one-
inch diameter stainless steel pipe as described in
Example 7 with house air (laboratory supplied air) at a
630,000/h space velocity with an inlet ozone
concentration of 0.25 ppm. Results are provided on Table
VI.
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 82 -
TABLE VI: SUMMARY OF FRESH A(~ l lVll ~ OZONE RESULTS - (39 cpsi NissanAltima core, 630,000/h Space Velocity; 25~C~ 0.25 ppm ozone; House ail - ca. 0.6% water)
CORE CATALYST LOADING CONV. (%) CONV. (%)
NO. (g/in3)Initial45 Minutes
3% Pt/MnO2/ZrO~ 4) (calcined at0.7 70.7 65.8
450~C)
'~3% Pt/MnO2/ZrO2 (1:4) (calcined at 0.7 70.5 63.7
450~C; reduced at 300~C)
36% Pt/MnO2/ZrO2 (1:4) (calcined at 0.68 68.2 62.3
450~C)
46% Pt/MnO2/ZrQ (1:4) (calcined0.66 66 55.8
450~C; reduced at 300~C)
56% Pd/MnQ/ZrO~ (1:1) w. 10% 0.39 38.3 21.1
Nalco 1056
6MnO2/ZrO~ (1:1) w. 10% Nalco 0.41 58.3 M.9
1056
7MnO~/ZrO~ (1:1) w. 10% Nalco 0.37 55.~ 41.2
1056
83% Pt/ZrO~/SiO~ (calcined 450~C) 0.79 27.4 10
93% Pt/ZrO~/SiO2 (calcined 450~C0.76 5~.2 30.1
and reduced at 300~C)
As can be seem from Table VI Cores 1 and 2
having only 3~ platinum resulted in excellent ozone
conversion initially and after 45 minutes both for
reduced and unreduced catalyst. Cores 3 and 4 having a
6~ platinum concentration also had excellent results.
Cores 5-7 illustrate a variety of other support materials
used which resulted in conversion of ozone. Core 5 had
palladium on a manganese oxide/zirconia coprecipitate and
resulted in lower than expected but still significant
ozone conversion. Cores 6 and 7 used the coprecipitate
without precious metal and also resulted in significant
ozone conversions but here again not as good as when
using platinum as a catalyst. Core 8 was platinum on a
zirconia/silica support which was calcined but not
reduced and Core 9 was platinum on zirconia/silica
support which was reduced. Both Cores 8 and 9 gave some
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 83 -
conversion but yet not as good as the conversion obtained
with platinum on the coprecipitate.
In addition, carbon monoxlde conversion was
evaluated on 39 cpsi radiator minicores, as recited, for
3~ and 6~ platinum on manganese/zirconia supports.
Reduced and unreduced samples were evaluated. For
illustrative purposes, platinum on zirconia/silica
supports and platinum on Carulite~ reduced and unreduced
are also presented. As can be seen ~rom Figure 12, the
results of 3~ reduced platinum on manganese/zirconia
support were higher when compared to the other
embodiments.
Exam~le 16 (Comparative)
Ozone conversion was measured over an uncoated
1995 Ford Contour radiator at room temperature and 80~C
by blowing an air stream containing ozone (0.25 ppm)
through the radiator channels at a 10 mph linear velocity
(630,000/h space velocity) and then measuring the
concentration of ozone exiting the back face of the
radiator. The air stream had a dew point of
approximately 35~F. Heated coolant was not circulated
through the radiator, but the air stream was heated as
necessary with heating tape to achieve the desired
radiator temperature. Additional testing was completed
with an uncoated 0.75"(L) x 0.5"(W) x 1.0"(D) Ford Taurus
radiator "mini-core" in a 1" diameter stainless steel
pipe as described in Example 7. The air stream was
heated with heating tape to achieve the desired radiator
temperature. For both tests, no decomposition of ozone
was observed up to 120~C.
~ Example 17
Ozone conversion was measured at various
temperatures for a reduced 3~ Pt/TiO~ catalyst in the
absence and in the presence of 15 ppm CO. Degussa P25
grade titania was used as the support and was
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 84 -
characterized as having a particle size of approximately
1 ~c4m and a surface area of ca. 45-50 m~/g. The catalyst
was coated onto a 300 cpsi ceramic (cordierite) honeycomb
and was reduced on the honeycomb using a forming gas
having 7~ H2 and 93~ N2 at 300~C for 3 hours. Testing was
accomplished as described previously in Example 7. The
air stream (35~F dewpoint) was heated with heating tape
to achieve the desired temperature. As can be seen in
Figure 13, an approximate 5~ enhancement in absolute
ozone conversion was observed from 25 to 80~C. The
presence of CO improves the conversion of ozone.
Exam~le 18 ~= =
To demonstrate the effectiveness of the process
of the present invention in reducing atmospheric
pollutant levels, a test model air conditioning condenser
was set up as shown schematically in Figure 14. In this
test, the catalytic conversion of ozone to molecular
oxygen was measured. However, it should be recognized
that similar catalytic conversions of other pollutants
can be similarly conducted using the appropriate
catalysts. The action o~ fan 81 draws a stream of
ambient air 82 into air conditioning condenser unit 80
through grill 83. The air passes over a condenser coil
84 before exiting through grill 85 as outlet air stream
86. A refrigerant enters coil 84 as vapor stream 87 and
exits as condensate stream 88.
Test samples of catalysts can either be applied
directly to coil 84, or applied to a separate pollutant
treating device 89 mounted downstream of the condenser
coil. Details of the actual test unit are set forth
below in Table VII.
= = --
CA 02206460 1997-0~-29
W O96/22119 PCTrUS96/00781
- 85 -
TABLE VII
Condenser Coil Equipment Specification
Nom-n~l duty rating 20 Ton
Trane model number CAUC-C20
Gross Heat rejection 301,000 BTU/h
Condenser fan data:
Number/size/type 2/26"/propeller
Fan drive direct
No. of motors/Hp each 2/1.0
Nom~n~l total CFM 12,400
Condenser coil data:
No./size (in.) 1/63x71
Metal tube/fin copper/aluminum
Face area (ft2) 31.0
Rows/fins per foot 3/168
Fin thickness (in.) 0.01
Coil depth (in.) 2.75
General data:
No. of refrig. circuits
Operating charge, R22 25 lbs.
Std. ambient range 40-115~F
Unit ~lm~n~ions 88"W x 60"D x 68"H
Test coatings of ozone destruction catalysts
were spray coated onto the coil of the con~en~er unit in
three 12" x 12" square patches. The catalysts used were
Carulite and Pd/Mn/Al O3 (see Example 3, above). Two
patches of Carulite catalyst were applied at two
different loadings, 0.3 and 0.6 g/in3, while only a single
patch of the palladium catalyst was applied at 0.3 g/in3.
The Carulite contained a proprietary latex binder from
National Starch, although other binders have since been
found to provide suitable adhesion, as discussed above.
The palladium catalyst had no binder. During operation
of the air conditioner, inlet and outlet condenser air
temperatures averaged approximately 35~C and 45~C,
respectively. Fin temperature on the outlet side of the
coil was typically only a few degrees higher than the
exhaust temperature. Air velocity on the front face of
the coil (2.5" deep) was approximateiy 300 ft/min, and
CA 02206460 l997-0~-29
W O96/221~9 PCTrUS96/00781
- 86 -
this correlates to an hourly space velocity of about
86, 400/h.
- The three catalyst patches were applied to the
csn~Pn~er after assembly, and the unit was then installed
on the roof of a building. However, the condenser was at
no time removed from its mounting frame. The unit was
stood on its end so that the coolant tubes ran vertically
and the corresponding cooling fins ran horizontally.
Prior to coating with catalyst, the fins were steam
cleaned to remove resldual oils from the surface which
could detrimentally affect washcoat adhesion. After
drying, the 12" x 12" sections to be coated with catalyst
were first spray coated with a thin precoat of alumina
(loading 0.1 g/in3 of condenser volume) to aid in adhesion
of the catalyst washcoat to the metal fin surface. After
drying with a forced air flow at about 40~C, the catalyst
coatings were then applied by spraying over the alumina
precoats. Drying was accomplished at about 40~C with
forced air flow.
Spray coating of the catalyst and alumina
precoat was accomplished using a Binks High Volume Low
Pressure (HVLP) 2.5 gallon paint spray system (Model
39-20) equipped with an Accuspray Series 10 spray gun.
The system allowed for delivery of liquid slurry to the
gun nozzle, where atomization by high velocity air
occurs. The liquid and air delivery pressures were
controlled separately. The spray pattern was adiusted to
give a vertical spray of roughly 1 inch width when the
gun was held about 3 inches from the condenser face.
Freshly mixed slurry was added to the 2.5 gallon canister
which was pressurized to 10 psig. The atomization air
line was pressurized to 70 psig. The canister was then
placed on a balance to record slurry weight loss while
spraying. In this way, a controlled amount of slurry
could be metered onto the co~en~er coils. This amount
was in turn calculated ~rom the desired catalyst loading
and the solids content of the particular slurry. One
CA 02206460 1997-0~-29
W O'~6/22149 PCTrUS96/00781
- 87 -
half of the slurry was sprayed onto the front face of the
coil, while the other half was sprayed onto the back
- face. The gun was held approximately 1-2 inches from the
face of the condenser, which allowed for good penetration
of slurry into the interior of the coil without being
blown out the back side. The slurry was applied at a
rate of 5 g every 3-5 seconds. This allowed application
of thinner, more uniform coats. Immediately after
addition of the desired amount of slurry to each side,
the patch was thoroughly air-knifed to unblock any
clogged louvers and to more evenly distribute the
catalyst in the condenser interior.
This same coating technique was used to spray
additional catalyst patches in-situ onto the condenser
coil after the unit was installed on the building roof.
In this case, the unit could not be stood on its end, and
thus the coolant tubes ran horizontally while the fins
ran vertically.
Ozone conversion vs. time for the three
catalyst patches is summarized in the graph presented as
Figure 15. For reference, the calculated mass transfer
conversion limit is approximately 90~. Ozone conversion
is expressed as percent of the ambient ozone converted to
O2. As is readily apparent, the Carulite patches gave
consistently higher ozone conversion than the Pd/Mn/Al2O3
patch. The Carulite patches also appear to have held up
better over time.
An OREC Model 03DM-100 analyzer was used to
measure ozone levels. Because the detection limit on
this analyzer is 10 ppb, the corresponding error in the
~ conversion calculations is about +10~ at 100 ppb, and
about ~20~ at 50 ppb. Despite this large error window,
it appeared that over 1300 hours some deactivation in
catalytic activity had occurred, particularly for the
palladium sample. Since no definitive correlation of
activity with absolute humidity or ozone concentration
was observed, a likely cause for the loss in activity was
CA 02206460 1997-0~-29
W O96/221~9 PCTrUS96/00781
- 88 -
the accumulation of dirt and other non-gaseous
cont~m;n~nts on the catalyst and resultant physical
masking of the catalytic sites. While the exhaust face
of the condenser still looked very clean, the inlet face
showed a significant accumulation of dirt which masked
the original color of the catalysts. Best results were
obtained using Carulite catalyst, which generally showed
a consistent conversion above 90~ during the first 700
hours, and above 70~ for the duration of the test. The
palladium catalyst showed a conversion above 70~ for the
first 700 hours, but then fell off to about 50~ at 1100
hours, and below 50~ at 14C0 hours.
At approximately 1400 hours, the test patches
on the condenser were washed with a water spray to remove
the accumulated dust. Test results showed a temporary
improvement in ozone conversion, followed by a rapid
return to pre-wash values. It is believed that the wash
procedure was not effective in removing the cont~m;n~nts
which were reducing the conversion rates. This suggests
that a better rejuvenation technique is needed, or that a
filter should be provided upstream of the catalyzed
surface to protect the surface from dust and other non-
gaseous cont~m;n~nts.
Exam~le 1g - Metal Foam Insert
As discussed above in regard to Figure 1, a
separate treatment device, such as device 25, can be used
to treat a pollutant such as ozone. Treatment cartridge
27 is described as being of any suitable material such as
a pad, frame or screen coated with a catalyst or
adsorbent material. The present example is directed to a
metal foam insert which can be used as a removable
treatment cartridge for catalyzing ozone conversion. A
one-inch diameter, 0.5 inch deep piece of alllm;nllm foam
(Duocel, 10 pores per inch) was coated with Carulite 200
by dipping the piece in a slurry cont~;n;ng Carulite 200,
5~ acetic acid (based on the weight of Carulite 200), and
CA 02206460 1997-0~-29
W O961221~9 PCT~US96/00781
- 89 -
5~ DUR-O-SET E-646 EVA polymer binder (also based on the
weight of Carulite 200). The piece was blown with an air
- knife to remove excess slurry and then dried at 90~C for
30 minutes. The dry catalyst loading was 0.22 g/in3 of
metal foam volume. The catalyst was tested for ozone
conversion at room temperature by placing the piece in a
one-inch diameter stainless steel tube and then passing
an air stream containing 0.1~ water and 0.25 ppm ozone
through it. Ozone concentration was measured before and
after the catalyst using an OREC 03DM-100 ozone analyzer.
The flow rate was 67.6 L/min which corresponds to an
hourly space velocity of 630,000/h. Ozone conversion
after 45 minutes was 27.3~.
Exam~le 20
100 g of Versal G~ alumina obtained from
LaRoche Industries Inc. was impregnated with about 2 8 g
of Pt amine hydroxide (Pt(A)salt) diluted in water to
about 80 g of solution. 5 g of acetic acid was added to
fix the Pt onto the alumina surface. After mixing for
half hour, the Pt impregnated catalyst was made into a
slurry by adding water to make about 40~ solids. The
slurry was ballmilled for 2 hours. The particle size was
measured to be 90~ less than 10 microns. The catalyst
was coated onto a 1.5" diameter by 1.0" length 400 cpsi
ceramic substrate to give a washcoat loading after drying
of about 0.65 g/in3. The catalyst was then dried at 100~C
and calcined at 550~C for 2 hours. This catalyst was
tested for C3H6 oxidation at temperatures between 60 and
100~C in dry air as described in Example 23.
Some of calcined Pt/Al2O3 sample described above
was also reduced in 7~ H2/N2 at 400~C for 1 hour. The
reduction step was carried out by ramping the catalyst
temperature from 25 to 400~C at a HJN2 gas flow rate of
500 cc/min. The ramp temperature was about 5~C/min. The
catalyst was cooled down to room temperature and the
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 90 -
catalyst was tested for C3H6 oxidation as described in
Example 23.
Example 21
6.8 g of ammonium tungstate was dissolved in 30
cc of water and the pH adjusted to 10 and the solution
impregnated onto 50 g of Versal GL alumina (LaRoche
Industries Inc.). The material was dried at 100~C and
calcined for 2 hours at 550~C. The approximately 10~ by
metal weight of W on Al2O3 was cooled to room temperature
and impregnated with 13.7 g of Pt amine hydroxide (18.3~
Pt). 2.5 g of acetic acid was added and mixed well. The
catalyst was then made into a slurry containing 35~ solid
by adding water. The slurry was then coated over a 400
cpsi, 1.5" x 1.0" diameter ceramic substrate resulting,
after drying, in having a catalyst washcoat loading of
0.79 g/in3. The coated catalyst was then dried and
calcined at 550~C for 2 hours. The catalyst was tested
calcined in C3H6 and dry air in the temperature range 60
to 100~C.
Example 22
6.8 g of perrhenic acid (36~ Re in solution)
was further diluted in water to make 10 g percent
perrhenic acid solution. The solution was impregnated
onto 25 g of Versal GL alumina. The impregnated alumina
was dried and the powder calcined at 550~C for 2 hours.
The impregnated 10 weight percent based metal of Re on
Al2O3 powder was then further impregnated with 6.85 g of
Pt amine hydroxide solution (Pt metal in solution was
18.3~). 5 g of acetic acid was added and mixed for a
half hour. A slurry was made by adding water to make 28
solid. The slurry was ballmilled for 2 hours and coated
onto 1.5" diameter x 1.0" length 400 cpsi ceramic
substrate to give a catalyst washcoat loading of 0.51
g/in3 after drying. The catalyst coated substrate was
dried at 100~C and calcined at 550~C for 2 hours. The
-
CA 02206460 1997-0~-29
W O96122149 PCTrUS96/00781
~ 91 - ,
catalyst was tested in the calcined form using 60 ppm C3H6
and dry air in the temperature range of 60 to 100~C.
Example 23
The catalyst of Examples 20, 21 and 22 were
tested in a microreactor. The size of the catalyst
samples was 0.5" diameter and 0.4" length. The feed was
composed of 60 ppm C3H6 in dry air in the temperature
range of 25 to 100~C. The C3H6 was measured at 60, 70,
80, 90 and 100~C at steady sate condition. Results are
summarized in Table VIII.
TABLE VIII - S~MMARY RESULTS OF C3H6 CONVERSION
Catalyst Pt/Al2O3 Pt/Al2O3 Pt/10~W/Al2O3 Pt/10~Re/Al2O3
Name Calcined Calcined CalcinedCalcined
~Ex. 20) and (Ex. 21) (Ex. 22)
Reduced
(Ex. 20)
~C3H6
15Conversion
@
60~C 0 10 9 11
70~C 7 22 17 27
80~C 20 50 39 45
90~C 38 70 65 64
100~C 60 83 82 83
It is clear from the Table that addition of W
or Re oxide has enhanced the activity of the Pt/Al2O3 in
the calcined form. The C3H6 conversion of the calcined
Pt/Al2O3 was enhanced significantly when catalyst was
reduced at 400~C for 1 hour. The enhanced activity was
also observed for the calcined catalyst by incorporation
of W or Re oxides.
Example 24
This is an example of preparing high surface
area cryptomelane using MnSO4.
CA 02206460 l997-0~-29
W O96/221~9 _ PCTrUS96/00781
- 92 -
Molar ratios o~ KMnO4:MnSO4:acetic acid were 1:1.43:5.72
Molarities of Mn in solutions prior to mixing were:
0.44 M KMnO4
0.50 M MnS 04
FW KMnO4 = 158.04 g/mol
FW MnS04-H20 = 169.01 g/mol
FW C2H402 = 60.0 g/mol
The following steps were conducted:
1. Made a solution of 3.50 moles (553 grams) of KMnO4
in 8. 05 L of D.I. water and heated to 68 C.
2. Made 10.5 L of 2N acetic acid by using 1260 grams of
glacial acetic acid and diluting to 10.5 L with D.I.
water. Density of this solution is 1.01 g/mL.
3. Weighed out 5.00 moles (846 grams) of manganous
sulfate hydrate (MnS04-H20) and dissolved in 10,115 g
of the above 2N acetic acid solution and heated to
40~C.
4. Added the solution from 3. to the solution from 1.
over 15 minutes while continuously stirring. After
addition was complete, began heating the slurry
according to the following heat-up rate:
1:06 pm 69.4~C
1:07 pm 71.2~C
1:11 pm 74.5~C
1:15 pm 77.3~C
1:18 pm 80.2~C
1:23 pm 83.9~C
1:25 pm 86.7~C
1:28 pm 88.9~C
5. At 1:28 pm approximately 100 mL of slurry was
removed from the vessel and promptly filtered on a
Buchner funnel, washed with 2 L of D.I. water, and
then dried in an oven at 100~C. The sample was
determined to have a BET Multi-Point surface area of
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 93 -
259.5 m2/g and Matrix (T-Plot) surface area of 254.1
m2/g .
Example 25
This is an example of preparing high surface
area cryptomelane using Mn(CH3COO) 2
Molar ratios of KMnO4:Mn(CH3CO2)2:acetic acid were
1:1.43:5.72
FW KMnO4 = 158.04 g/mol Aldrich Lot #08824MG
FW Mn(CH3CO2)2-H~O = 245.09 g/mol Aldrich Lot #08722HG
FW C2H4O2 = 60.0 g/mol
The following steps were conducted:
1. Made a solution of 2.0 moles (316 grams) of KMnO4
in 4.6 L of D.I. water and heated to 60~C by
heating on hot plates.
2. Made up 6.0 of 2N acetic acid by using 720 grams
of glacial acetic acid and diluting to 6.0 L with
D.I. water. Density of this solution is 1.01
g/mL.
3. Weighed out 2.86 moles (700 grams) of manganese
(II) acetate tetrahydrate [Mn(CH3CO2)2-4H2O] and
dissolved in 5780 g of the above 2N acetic acid
solution (in the reactor vessel). Heated to 60~C
in the reactor vessel.
4. Added the solution from 1. to the solution from 3.
while maint~;n;ng the slurry at 62-63~C. After
complete addition, gently heated the slurry
according to the following:
82.0~C at 3:58 pm
86.5~C at 4:02 pm
87.0~C at 4:06 pm
87.1~C at 4:08 pm
shut off heat
CA 02206460 l997-0~-29
W O96/22149 PCTrUS96/00781
- 94 -
then quenched the slurry by pumping 10 L of D.I.
water into the vessel. This cooled the slurry to
58~C at 4:13 pm.
The slurry was filtered on Buchner funnels. The
resulting filter cakes were reslurried in 12 L of
D.I. water then stirred overnight in a 5 gallon
bucket using a mechanical stirrer. The washed
product was refiltered in the morning then dried
in an oven at 100~C. The sample was determined to
have a BET Multi-Point surface area of 296.4 m2/g
and Matrix (T-Plot) surface area of 267.3 m2/g.
The resulting cryptomelane is characterized by the
XRD pattern of Figure 17. It is expected to have
an IR spectrum similar to that shown in Figure 16.
Example 26 ~
Following is a description of the ozone
testing method for determtntng percent ozone
decomposition used in this Example. A test apparatus
comprising an ozone generator, gas flow control
equipment, water bubbler, chilled mirror dew point
hygrometer, and ozone detector was used to measure the
percent ozone destroyed by catalyst samples. Ozone was
generated in situ utilizing the ozone generator in a
flowing gas stream comprised of air and water vapor.
The ozone concentration was measured using the ozone
detector and the water content was determined utilizing
the dew point hygrometer. Samples were tested as 25~C
using inlet ozone concentrations of 4.5 to 7 parts per
million (ppm) in a gas stream flowing at approximately
1.5 L/minute with a dew point between 15~C and 17~C.
Samples were tested as particles sized to -25/+45 mesh
held between glass wool plugs in a 1/4" I. D. Pyrex
glass tube. Tested samples filled a 1 cm portion of
the glass tube.
Sample testing generally required between 2
to 16 hours to achieve a steady state of conversion.
CA 02206460 1997-0F7-29
wos6l22l4s PCT~S96/00781
Samples typically gave close to l00~ conversion when
testing began and slowly decreased to a ~leveled off"
conversion that remained steady for extended periods of
time (48 hours). After a steady state was obtained,
conversions were calculated from the equation: ~ ozone
conversion = [(l-(ozone concentration after passing
over catalyst)/(ozone concentration before passing over
catalyst)]*l00.
Ozone destruction testing on the sample of
Example 24 showed 58~ conversion.
Ozone destruction testing on the sample of
Example 25 showed 85~ conversion.
Example 27
This example is intended to illustrate that
the method of Example 25 generated "clean" high surface
area cryptomelane for which the ozone destruction
performance was not further enhanced by calcination and
washing. A 20 gram portion of the sample represented
by Example 25 was calcined in air at 200~C for l hour,
cooled to room temperature, then washed at 100~C in 200
mL of D.I. water by stirring the slurry for 30 minutes.
The resulting product was filtered and dried at 100~C
in an oven. The sample was determined to have BET
Multi-Point surface area of 265 m2/g. Ozone
destruction testing on the sample showed 85~
conversion. A comparison to the testing of the sample
of Example 25 demonstrated that no benefit in ozone
conversion was realized from the washing and
calcination of the sample of Example 25.
Example 28
~,amples of high surface area cryptomelane
were obtained from commercial suppliers and modified by
calcination and/or washing. As received and modified
powders were tested for ozone decomposition performance
according to the method of Example 26 and characterized
CA 02206460 1997-0~-29
W O96/22149 PCTrUS96/00781
- 96 -
by powder X-ray diffraction, infrared spectroscopy, and
BET surface area measurements by nitrogen adsorption.
Example 28a
A commercially supplied sample of high
surface area cryptomelane (Chemetals, Inc., Baltimore,
MD) was washed for 30 minutes in D.I. water at 60~C,
filtered, rinsed, and oven-dried at 100~C. Ozone
conversion of the as received sample was 64~ compared
to 79~ for the washed material. Washing did not change
the surface area or crystal structure of this material
(223 m/g cryptomelane) as determined by nitrogen
adsorption and powder X-ray diffraction measurements,
respectively. However, infrared spectroscopy showed
the disappearance of peaks at 1220 and 1320 wavenumbers
in the spectrum of the washed sample indicating the
removal of sulfate group anions.
Example 28b
Commercially supplied samples of high surface
area cryptomelane (Chemetals, Inc., Baltimore, MD) were
calcined at 300~C for 4 hours and 400~C for 8 hours.
Ozone conversion of the as received material was 44
compared to 71~ for the 300~C calcined sample and 75
for the 400~C calcined sample. Calcination did not
significantly change the surface area or crystal
structure of the 300~C or 400~C samples (334 m2/g
cryptomelane). A trace of Mn2O3 was detected in the
400~C sample. Calcination causes dehydroxylation of
these samples. Infrared spectroscopy show a decrease
in the intensity of the band between 2700 and 3700
wavenumbers assigned to surface hydroxyl groups.
Example 29
The addition Pd black (containing Pd metal
and oxide) to high surface area cryptomelane is found
to significantly enhance ozone decomposition
CA 02206460 1997-0~-29
W O96/22149 PC~rUS96100781
- 97 -
performance. Samples were prepared comprising Pd black
powder physically mixed with powders of (1) a
commercially obtained cryptomelane (the 300~C calcined
sample described in Example 28b) and (2) the high
surface area cryptomelane synthesized in Example 25
calcined at 200~C for 1 hour. The samples were
prepared by mixing, in a dry state, powder of Pd black
and cryptomelane in a 1:4 proportion by weight. The
dry mixture was shaken until homogeneous in color. An
amount of D.I. water was added to the mixture in a
beaker to yield 20-30~ solids content, thus forming a
suspension. Aggregates in the suspension were broken
up mechanically with a stirring rod. The suspension
was sonicated in a Bransonic~ Model 5210 ultrasonic
cleaner for 10 minutes and then oven dried at 120-140~C
for approximately 8 hours.
The ozone conversion for the commercially
obtained cryptomelane calcined at 300~C was 71~ as
measured on the powder reactor (Example 28b). A sample
of this product was mixed with 20 weight percent Pd
black yielded 88~ conversion.
The cryptomelane sample prepared as in
Example 25 calcined at 200~C had 85~ conversion.
Performance improved to 97~ with 20 weight percent Pd
black added.