Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02207781 1999-06-14
SYSTEMS AND METHODS FOR BIODEGRADAT~ON
10 1. FIELD OF THE INVENTION
This invention is related to the aerobic degradation
of compounds such as aromatic, vitro-aromatic, halo-aromatic,
halo-vitro-aromatic, aliphatic and halo-aliphatic compounds.
These compounds are aerobically degraded by novel
microorganisms to products comprising COZ and HZO using a
variety of methods. The microorganisms are also capable of
aerobically bioremediating compositions containing these
compounds. Further, the microorganisms described herein are
capable of aerobically bioremediating vitro- and.halo-
substituted aromatic compounds to products comprising C02 and
HZO without the production of toxic intermediates or by-
products.
This invention is further related to fluid phase
systems and methods for aerobic reaction of compounds such as
aromatic,,nitro-aromatic, halo-aromatic, halo-vitro-aromatic,
aliphatic and halo-aliphatic compounds. In particular
embodiments, elastomeric solids or sludges containing such
compounds are converted to fluidized compositions suitable for
aerobic reaction. In certain embodiments, the fluidized
compositions comprise slurries for aerobic bioremediation of
waste materials containing organic compounds or mixtures
thereof .
This invention is further related to solid phase
_ systems and methods for aerobic degradation of compounds such
as aromatic, vitro-aromatic, halo-aromatic, halo-nitro
aromatic, aliphatic and halo-aliphatic compounds in solids,
sludges or soils.
75365-125
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
This invention additionally relates to a two step
process for bioremediation of waste materials containing at
least one compound selected from heavily halogenated organic
compounds, for example, polychlorinated biphenyls,
polybrominated biphenyls, etc., heavily nitrated compounds,
such as trinitrotoluene, etc., and heavily nitrated and cross-
linked polymeric compounds, e,g., nitrocellulose, etc.
According to this embodiment, the waste material is first
combined with a reagent capable of at least partially
degrading said compounds in the waste material and then
contacted with the novel microorganisms which aerobically
degrade any aromatic, substituted aromatic or aliphatic
compounds present in the treated waste material.
This invention further relates to systems for
bioremediation of gases, aerosols, and fluids including
liquids using the novel microorganisms immobilized on a solid
support.
2. BACRGROOND OF THE INVENTION
The use of microorganisms to treat waste or waste
contaminated material is well documented. At the February,
1990, symposium which preceded the "EPA-Industry Meeting on
Environmental Applications of Biotechnology" the EPA noted
that biotechnology has been successfully utilized to treat
soils and sludges from superfund sites which include
contaminants from multiple and varied sources. Economic and
environmental considerations indicate that bioprocessing
technologies offer a significant potential for the reme:diation
and treatment of waste and waste contaminated materials. The
use of ultimate disposal technologies such as incineration or
chemical fixation and encapsulation results in very large
expenditures of capital, in addition to the liability
associated with the handling and transport of these materials
to the disposal site. Biodegradation methods entail a lower
cost relative to most other approaches because they are
conducted on site and use less complicated equipment.
Furthermore, they can be conducted using a combination of
- 2 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95116364
above-ground and in situ treatments for a total treatment
approach.
V
Examples of microbial degradation or treatment of
compounds are well known in the art. For instance, United
States Patent Nos. 4,843,007 and 4,876,201 disclose the
aerobic treatment of polychlorinated biphenyls (PCBs) and
acetophenones with Alcaligenes, however, there is no
disclosure of aromatic ring cleavage, indicating that the
compounds were not degraded to the point of mineralization.
Further, U.S. Pat. Nos. 5,009,999 and 4,876,201 disclose
aerobic treatment of PCBs with Pseudomonas as well, also with
no evidence of ring cleavage. U.S. Pat. No. 4,493,895
discloses the aerobic treatment of halogenated organic
compounds with Pseudomonas cepacia, whereas U.S. Pat. No.
5,100,800 discloses treatment of the same compounds with
Pseudomonas putida strain UNK-1.
Halo-aliphatic compounds, such as trichloroethylene
or dimethylammonium chloride have also been shown to be
aerobically degraded. Specific examples are found in U.S.
Pat. Nos. 4,713,343 (trichloroethylene), 4,492,756
(dimethylammonium chloride), and 5,079,166
(trichloroethylene).
Funk et al., 1993, Appl. Environ. Microbiol. 59:7,
pp. 2171-2177 describes a two-step in situ treatment process
for soils contaminated with 2,4,6-trinitrotoluene, hexahydro
1,3,5-trinitro-1,3,5-triazine and octahydro-1,3,5,7-
tetranitro-1,3,5,7-tetraazocine. The soil is first flooded
with an aqueous buffer and starch to promote bacterial
activity. The aerobic heterotrophs in the soil or added as
3o inoculum quickly remove the oxygen from the soil creating
anaerobic conditions. Under anaerobiosis the contaminating
compounds were partially degraded by the microorganisms. They
were, however, not degraded to COz and H20, because only the
~ substituted nitro groups were reduced and the aromatic ring
was not cleaved.
Venkataramani -and Ahlert, 1984, J. WPCF, 56:11, pp.
1178-1184, disclose the use of acclimated bacteria from a
- 3 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
sewage treatment plant to aerobically degrade contaminants in
an industrial landfill leachate.
4
The bulk of the published literature, on
biodegradation, is focused on the degradation of single pure
chemical by pure cultures and not on the degradation of
complex mixtures of organic pollutants by mixed culturea or
microbial consortia. Much of the work with pure chemicals
also has been conducted at concentrations which are ordsars of
magnitude lower than those commonly encountered with
industrial wastes. For example Speitel et al., 1989, Environ.
Sci. Technol. 23:68-74) examined the degradation of phenols
(e. g. p-nitrophenol, 2,4-dinitrophenol, and pentachlorophenol)
using pure chemicals at very low levels, i.e., 1-100 ppb.
Similarly, Arcangeli and Arvin, 1992, Appl. Microbiol.
Biotechnol. 37:510-517, employed very low toluene
concentrations, less than 1 ppm to 6 ppm, in their biorE~actor.
In controlled microcosm studies, Heitkamp, et al.,
1987, Appl. Environ. Microbiol. 53:129-136), showed thai~
naphthalene, when added to selected soil microcosms at :Levels
of less than 1 ppm could be effectively mineralized within 17
to 31 days.
The degradation of methyl-substituted aromatics, in
nature, is generally regarded to occur via the meta-cleavage
pathway. However, the degradation of halo-organics, such as,
for example, chlorobenzoate, proceeds best through the ortho-
cleavage pathway. Knackmuss, (Taeger, et al., 1988, Ap~pl.
Microbiol. Biotechnol. 28:603-608; Romanov, et al., 1993,
Microbiology 62:887-896) and Pierce (Pierce, et al. 1983, Dev.
Ind. Microbiol. 24:499-507; Pierce, et al., 1984, Dev. Ind.
Microbiol. 25:597-602), have shown that microorganisms can be
enriched which are capable of degrading both methyl- and
chloro-aromatics via the ortho-pathway. Likewise, Oltmanns,
et al., 1988, Appl. Microbiol. Biotechnol. 28:609-616) :have
shown that bacteria enriched from nature can be constructed t
which~are capable of degrading 1,4-dichlorobenzene via a
modified ortho-pathway, not present in the wild-type strains.
- 4 -
CA 02207781 1997-06-13
WO 96/iS724 PCT/iTS95116364
Boronin and coworkers (Boronin et al., 1993, FEMS
Microbiol. Letters. 113:303-308) in preparing various
naphthalene plasmid constructs in P. putida have shown that
when naphthalene is the sole carbon and energy source, the
highest specific growth rates are observed with meta-pathway >
ortho-pathway > gentisate-pathway.
The degradation of mixed organic substrates, and
mixed, substituted aromatics in particular, increases
considerably the biochemical complexity of degradation, and
19 the regulatory and physiological control of these degradative
processes. A key factor in the degradation of mixed organic
substrates, particularly where pathways are inducible, is how
the cultures are originally grown (and thus, induced).
Hollander, et al., 1994, Appl. Environ. Microbiol.
60:2330-2338) have noted that Commamonas testosteroni
(previously classified as Pseudomonas testosteroni) degrades
4-chlorophenol and 4-methylphenol sequentially and not
simultaneously. This degradation occurs via the meta-pathway.
However, where multiple organic compounds were
supplied, which were degraded only via the meta-pathway,
degradation was simultaneous. Because of the prior induction
of the meta-pathway, degradation of compounds which proceed
via the ortho-pathway required additional treatment time,
because the proper enzymes had to be induced to achieve
adequate levels of degradation of these compounds. In such
cases, this requirement for increased treatment time has a
direct negative impact on treatment economics.
Recently, Grifoll et al., 1994, Appl. Environ.
Microbiol. 60:2438-2449) have isolated a Pseudomonas sp.
(strain F274) which is capable of metabolizing fluorene, and
when grown in the presence of p-hydroxybenzoate, cleaves p-
hydroxybenzoate via the ortho-pathway. This strain, however,
is incapable of utilizing toluene, naphthalene or benzene.
The same situation was observed by Pettigrew et al.,
1991, Appl. Environ. Microbiol. 57:157-162) with the
,. degradation of chlorobenzene and toluene by a Pseudomonas
strain, that until the meta-pathway was repressed/modified,
- 5 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
the simultaneous degradation of organics metabolized via the
meta-pathway and ortho-pathway was not possible.
Viliesid and Lilly, 1992, Enz. Microb. Technol..
14:561-565 have shown that the basal or induced levels of
catechol 1,2-dioxygenase (the key enzyme of the ortho-pathway) '
are directly influenced by the dissolved oxygen tension.
Based upon their observations it was necessary for the oxygen
tension to be above 4~ of saturation (at the initiation of
degradation) in order to maintain active ortho-pathway
l0 degradation.
~In the recent literature, there are examples of
cases where higher concentrations (1000 ppm) of phenol, Brown
et al., 1993, Critical Review and Case Study on Biotechnology
for Pollution Prevention, United States~EPA; Hinteragger, et
al. 1992 or xylene, Wolfram et al., 1990, NTIS Report No. EGG-
M-90407, p.17, in aqueous solutions have been successfully
degraded.
However, care should be taken to discriminate
between primary metabolism and co-metabolism or resting cell
metabolism. See, for example, Spain and Gibson (1988, Appl.
Environ. Microbiol. 54:1399-1404), which shows resting cell
metabolism of nitrophenols by toluene grown cells; and '.Caylor
and Amador, (1988, Appl. Environ. Microbiol. 54:2342-2344)
which shows resting cell metabolism of pyridine by phthalate
grown cells.
By definition, heterotrophic bacteria utilize
various forms of organic carbon as a source of carbon and
energy. In addition to a carbon source, heterotrophic
bacteria also require nitrogen and phosphorous for growth.
Most commonly, inorganic forms of nitrogen or phosphorous are
supplied to meet this requirement, though the use of organic
nitrogen in the form of amino acids (amino nitrogen) also have
been used historically. While documented in the literature,
meeting nitrogen requirements through the use of hydrocarbons
which contain nitrogen, e.g., heterocycles or nitrophenol or
the use of organic phosphorous compounds e.g., phosphinates is
,:
less practiced, Wackett, et al., 1987, J. Bacteriol 169:710-
- 6 -
CA 02207781 1997-06-13
WO 96/18724 . PCT/US95/16364
717; Schowanek and Verstraete, 1990, Appl. Environ. Microbiol.
56:895-903. Glyphosate degradation in nature is accomplished
y
by bacteria which not only utilize the organic carbon of this
pesticide for growth and energy but utilize the organic
phosphorous of glyphosate as the source of phosphorous. In
fact, glyphosate degradation in nature is suppressed if other
more available forms of inorganic phosphorous are present.
While there is considerable interest in using co-
metabolic activity to degrade selected organic wastes, such as
TCE, the use of co-metabolic processes to treat mixed wastes
is likely~to be inefficient, and therefore, ultimately more
costly. Klecka and Maier, 1988, Biotechnol. Bioeng. 31:328-
335) have shown that when degradable but non-utilizable ca~:bon
sources are added to a mixed population of pentachlorophenol
degrading bacteria, the rate of pentachlorophenol degradation
decreases. When however, utilizable forms of hydrocarbons are
added to the mixture, the overall removal rate increases.
This increase is due to an increase in biomass which results
in overall improvement in degradation.
The aerobic degradation of selected aromatics and
polyaromatic hydrocarbons (PANS) is well documented. However,
the aerobic degradation of compounds where present in
elastomeric or tarry compositions has never been reported to
the knowledge of the present inventor(s). Under conditions of
anaerobic respiration (i.e. nitrate reduction/denitrification)
the oxidative degradation of these same selected chemicals has
been reported, using nitrate as the terminal electron
acceptor, Bossert and Young, 1986, Appl. Environ. Microbiol.
52:1117-1122; Bouwer and McCarty, 1983, Appl. Environ.
Microbiol., 45:1295-1299. However, the degradation of
compounds such as naphthalene is not rapid under nitrate
respiration. Mihelcic and Luthy, 1988, Appl. Environ.
Microbiol. 54:1188-1198 demonstrated that approximately 63
days were required to degrade naphthalene at a concentration
of 1 ppm under denitrifying conditions.
Fries et al., 1994, Appl. Environ. Microbiol.,
60:2802-2810, generally indicates that biodegradation of
_ 7 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
benzene, toluene, ethylbenzene and xylenes under aerobic:
conditions is well known, although the availability of oxygen
due to its low solubility in water and low rate of transport
in soils and sediments often becomes rate limiting. Fries
describes anaerobic respiration of toluene by microorganisms
isolated from nature using < .5 ppm toluene. The
microorganisms could grow on 25 ppm toluene and could be: fed
50 ppm toluene. There has been no demonstration that these
microorganisms can degrade any higher concentrations of
l0 toluene.
Ortega-Calvo and Alexander, 1994, Appl. Environ.
Microbiol. 60:2643-2646, have speculated that two
physiologically different populations, one free-swimming and
the other at the organic interface are involved in the
degradation of compounds such as naphthalene (when supp:Lied at
concentrations of 0.1-1.0 ppm). From their observations, it
appears that the initial activity is conducted by the free-
swimming bacteria, which are dependent upon the partitioning
of naphthalene to the aqueous phase.
Recently, Hack, et al., 1994, Appl. Microbiol.
Biotechnol. 41:495-499 have shown that cells of P. putida when
grown on glucose, lost over 50~ of this activity within 90
hours when stored at 4°C.
Considerable interest has been raised lately
regarding the co-metabolism of trichloroethylene, TCE, ldy the
recombinant strain P. cepacia G4 when grown on toluene. From
the recent paper by Landa et al., 1994, Appl. Environ.
Microbiol., 60:3368-3374, several conclusions can be drawn.
It takes considerable amounts of toluene to degrade a small
amount of TCE. Approximately 64 ppm of toluene is required to
metabolize 3.2 ppm of TCE (a ratio of 20 parts toluene
degraded for each part of TCE degraded). Furthermore, when
the TCE concentration exceeds 19 ppm, competitive inhibition
of toluene degradation results in the loss of TCE co-
metabolism and the cessation of toluene degradation.
Immobilized and entrapped bacterial processes have
been established for many years (Atkinson and Movituna, 1991,
_ g _
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
Biochemical Engineering and Biotechnology Handbook: 2nd Ed.
Stockton Press, NY). These processes claim to provide
additional benefit with respect to improving the ruggedness of
the microorganisms. For example, Dickman, et al., 1990,
Bioprocess Eng'r 5:13-17, showed improved stability to oxygen
deprivation and pH shocking in an immobilized continuous
culture reactor versus free swimming bacteria. Westmeier and
Rehm, 1985, Appl. Microbiol. Biotechnol. 22:301-305 have shown
that immobilized cells of Alcaliaenes sp. degrade 4-
chlorophenol at faster rates than do free-swimming cells when
fed 4- -chlorophenol at low concentrations (i.e., < 19 ppm).
Haigler, et al., 1994, Appl. Environ. Microbiol.,
60:3466-3469, describes the isolation of a strain of
Pseudomonas (strain JS42) based upon its ability to degrade
and utilize 2-nitrotoluene (2-NT) as a sole source of carbon,
energy, and nitrogen. While this reference'shows that this
strain was able to utilize 2-nitrotoluene, Haigler
specifically states that Pseudomonas strain JS42 is incapable
of utilizing nitrobenzene. In addition, Haigler makes no
mention regarding the ability to degrade or utilize aniline or
naphthalene. While washed cells of strain JS42 grown on 2-NT
are capable of oxidizing nitrobenzene, the reference
specifically makes clear that the cells cannot utilize
nitrobenzene. Therefore, this biotransformation activity is
more correctly defined as co-metabolism.
Composting of hazardous organic wastes represents a
relatively novel application of biotreatment technology. Most
notable is the example of composting of chlorophenols (Valo
and Salkinoja- -Salonen, 1986, Appl. Environ. Microbiol. 25:68-
75). However, the time required to treat contaminated soils
using this technology is not rapid (> 4 months). Part of the
problem with the use of composting for chlorophenols is the
development of a significant level of active chlorophenol
degraders. While this problem was addressed, in part, by Valo
and Salkinoja-Salonen (Id., 1986), through the addition of
~ microbial amendments, this was only possible when the soil had
- g -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95I16364
been previously sterilized to kill-off the indigenous
microflora.
V
U.K. Patent No. 1,375,394 states generally th<~t
microorganisms of the genera Pseudomonas, Mycobacterium,
Flavobacterium or Sarcina can aerobically degrade nitro~- '
aromatic compounds. This reference states that the
microorganisms must be induced to have the ability for :such
degradative activity. However, there is no indication .3t all
regarding what concentration of vitro-aromatic should be used
for induction nor any teaching of what culture conditions
should be employed. Further, there is no indication in this
reference at all regarding what particular species of any of
the mentioned genera could be induced to have the desired
degradative activity, nor is there any indication where such
microorganisms could be found.
European Patent Publication No. 0278296 generally
describes a method for the simultaneous chemical and
biological treatment of solids and liquids containing organic
waste.
Thus, there remains a real need for microorganisms
and for systems and processes which are useful for rapid,
efficient aerobic degradation of aromatic, vitro-aromatic,
halo-aromatic, halo-vitro-aromatic, aliphatic and halo-
aliphatic compounds. There is also a real need for degrading
any or all of these compounds when present in elastomeric or
tarry materials.
Citation or identification of any reference in
Section 2 of this application shall not be construed as an
admission that such reference is available as prior art to the
present invention.
3. SUMMARY OF THE INVENTION
Novel isolated microorganisms, in pure or mixed
culture, are provided which are useful for the aerobic
degradation of aromatic, vitro-aromatic, halo-aromatic, halo
nitro-aromatic, aliphatic and halo-aliphatic compounds or
C
mixtures thereof. The microorganisms are advantageously
- 10 -
CA 02207781 1997-06-13
WO 96118724 PCT/LTS95116364
useful for the aerobic degradation of said compounds when
contained in elastomeric and/or tarry solids, sludges, or
soils as well as when contained in non-elastomeric
compositions. The microorganisms are also useful for the
" 5 degradation of such compounds or mixtures thereof in the form
of gases, aerosols or fluids, including liquids. Biofilters
comprising the microorganisms are provided.
The microorganisms can be stored for extended
periods of time, a g., at least 4 months, without loss of
degradative activities. In addition, the microorganisms can
rapidly and efficiently degrade relatively high concentrations
of said compounds or mixtures thereof. Further, the
microorganisms can tolerate a wide range of concentrations of
said compounds. The microorganisms are capable of utilizing
at least one of the compounds as a sole source of carbon and
energy. Certain of the microorganisms are capable of
utilizing at least one of the compounds as a sole source of
carbon and nitrogen.
Novel methods for fluid phase and solid systems
advantageously useful for aerobic reactions of compounds are
provided.
In a particularly advantageous embodiment of the
fluid phase systems, novel methods for the rapid and efficient
degradation of at least one compound selected from aromatic,
vitro-aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic
and halo-aliphatic compounds or mixture thereof contained in
elastomeric and/or tarry solids, sludges or soils are
provided.
In a particularly advantageous embodiment of the
solid phase systems, novel methods for the rapid and efficient
degradation of at least one compound selected from aromatic,
vitro-aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic
and halo-aliphatic compounds or mixture thereof contained in
an elastomeric and/or tarry solid, sludge or soil are
provided.
The fluid phase and solid phase systems, can be
scaled up to efficiently handle a wide variety of influent
- 11 -
CA 02207781 1997-06-13
WO 96/18724 PCT/L1S95/16364
feeds in time periods considerably shorter than conventional
methods employed for biodegradation of aromatic and/or
aliphatic compounds.
According to one embodiment of the present
invention, a method for the aerobic degradation of aromatic
and/or substituted aromatic compounds is provided. In
general, the method entails contacting an aromatic compound
with a mixed or pure culture of a microorganism, said
microorganism being a member of the group consisting of
microorganisms having ATCC Accession No. 55644, 55648, !55645,
55641, 55647, 55642, 55643, 55646, 55649, 55722, 55723, 55726,
55727, 55724, and 55725. In one mode of this embodiment, at
least one compound selected from the group of aromatic, nitro-
aromatic, halo-aromatic, halo-nitro-aromatic, aliphatic and
halo-aliphatic compounds is aerobically degraded. In another
mode of this embodiment, a mixture of at least two compounds
selected from the group consisting of aromatic, nitro-
aromatic, halo-aromatic, halo-nitro-aromatic, aliphatic and
halo-aliphatic compounds is aerobically degraded. The method
may further comprise culturing the microorganisms in contact
with said compounds) so that the aromatic compound or
compounds are degraded to products comprising COZ and H2~~.
According to another embodiment of the invention,
fluid phase systems and methods for aerobic reaction of
compounds are provided. Most generally, the fluid phase
systems entail converting an elastomeric solid or sludge into
a fluidized composition suitable for aerobic reaction of
organic compounds contained in the elastomeric solid or
sludge. The aerobic reactions for which the fluidized
compositions are useful include synthetic as well as
degradative reactions which take place preferably under
aerobic conditions.
The method for preparing a fluidized composition
suitable for aerobic reaction comprises the steps of: (a)
particularizing an elastomeric solid or sludge containing an
organic compound; and (b) contacting the particularized solid
or sludge in a vessel with a current of fluid selected from
- 12 -
- CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
the group consisting of oxygen, oxygen containing gas,
including air, water and an aqueous solution, such that the
particularized solid or sludge is suspended or dispersed in
the current of fluid to form a composition suitable for
aerobic reaction of an organic compound contained in the solid
or sludge.
The method can further comprise combining the
elastomeric solid or sludge with a detackifying agent either
simultaneously with or subsequent to step (a).
According to another embodiment of the present
invention, fluid phase systems and methods for aerobic
degradation of compounds using microorganisms are provided. A
fluid phase which is a slurry formed from, for example, a
solid, soil, and/or sludge is produced.
A fluid phase which is a slurry can be formed from
either non-elastomeric or an elastomeric solid, sludge or
soil. Such slurries are used to aerobically degrade an
aromatic or aliphatic compound or mixture thereof contained in
said solid, sludge or soil.
The method comprises (a) combining said solid or
sludge with water or an aqueous solution; and (b) imparting
energy to said solid or sludge/aqueous combination in a vessel
such that said solid or sludge is fluidized into a slurry.
Energy can be imparted, for example, by imparting
mechanical energy, e.g., by mixing; by imparting acoustic
energy; e.g., by setting up a standing acoustic wave in the
slurry materials; or by imparting an electrical or
electrostatic field.
In one alternative embodiment, the method comprises
(a) combining an elastomeric solid or sludge with water or an
aqueous solution; (b) imparting energy to said elastomeric
solid or sludge/water combination such that said solid or
sludge is fluidized into a slurry; and (c) separating said
,, slurry away from any residual elastomeric solid or sludge.
Alternatively, the method comprises (a) combining an
_ elastomeric solid or sludge with a detackifying agent to form
a solid or sludge/detackifying agent combination; (b)
- 13 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16;364
combining said solid or sludge with water or an aqueous
solution to form a solid or sludge/detackifying agent aqueous
combination; and (c) imparting energy to said solid or
sludge/detackifying agent aqueous combination such that said
detackified solid or sludge is fluidized into a slurry. This
method can further comprise mixing said solid or
sludge/detackifying agent combination to form a detackif:ied
solid or sludge. In still another alternative, the method
comprises (a) combining an elastomeric solid or sludge with a
detackifying agent and water or an aqueous solution; and (b)
imparting energy to said mixture formed in step (a) such that
said elastomeric solid or sludge is fluidized into a slurry.
According to the present invention, a method f:or
slurry phase bioremediation of solids, sludges or soils
containing at least one compound or a mixture of at leases two
compounds selected from the group consisting of aromatic,
nitro-aromatic, halo-aromatic, halo-nitro-aromatic, aliphatic
and halo-aliphatic compounds comprises (a) adjusting the pH of
a slurry towards neutrality, if necessary; and (b) contacting
said neutral slurry with microorganisms, said microorganisms
being a member of the group consisting of microorganism::
having ATCC Accession No. 55644, 55648, 55645, 55641, 5_°°>647,
55642, 55643, 55646, 55649, 55722, 55723, 55726, 55727, 55724,
and 55725. The method can further comprise culturing ssLid
microorganisms with said slurry such that the compound is
degraded to products comprising COZ and HZO.
The methods for solid phase bioremediation of
solids, sludges or soils containing at least one compound or a
mixture of at least two compounds selected from the group
consisting of aromatic, nitro-aromatic, halo-aromatic, halo-
nitro-aromatic, aliphatic and halo-aliphatic compounds
comprise (a) mixing said solid, sludge or soil with a bulking
agent such that a fluid, for example, air, can readily pass
through the bulked mixture; (b) adjusting the pH of the bulked '.
mixture towards neutrality, if necessary; and (c) contacaing
said bulked mixture with microorganisms, said microorganisms
being a member of the group consisting of microorganisms
- 14 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
having ATCC Accession No. 55644, 55648, 55645, 55641, 55647,
55642, 55643, 55646, 55649, 55722, 55723, 55726, 55727, 55724,
and 55725. The methods can further comprise culturing said
microorganisms with said bulked solid, sludge or soil such
that said compound is degraded to products comprising COZ and
HZO .
Where the solid, sludge or soil is a tarry or
elastomeric solid, sludge or soil, the methods for solid phase
bioremediation comprise: (a) mixing a tarry or elastomeric
solid, a tarry or elastomeric sludge or a tarry or elastomeric
soil with a detackifying agent such that said solid soil or
sludge forms a particularized less tarry and/or elastomeric
mixture.
Another embodiment of the present invention is a
biofilter and methods for its use. Biofilters are used in the
bioremediation of compounds in effluents such as air, vapors,
aerosols, and water or aqueous solutions.
According to yet another embodiment of the
invention, a two step method for aerobic degradation of waste
materials containing at least one compound, selected from
heavily halogenated organic compounds such as polychlorinated
biphenyls, polybrominated biphenyls, etc., heavily nitrated
compounds, such as trinitrotoluene, etc., and heavily nitrated
and cross-linked polymeric compounds, e.g., nitrocellulose,
etc. is provided. The waste materials can further comprise as
a compound selected from the group consisting of aromatic,
nitro-aromatic, halo-aromatic, halo-nitro-aromatic, aliphatic
and halo-aliphatic compounds or a mixture of such compounds.
The methods comprise: (a) combining a reagent capable of
chemically degrading, at least partially, a heavily
halogenated, a heavily nitrated or a heavily nitrated cross-
linked compound in a waste material to form a pretreated
composition; and (b) contacting said pretreated composition
A with microorganisms, said microorganisms being a member of the
group consisting of microorganisms having ATCC Accession No.
a 55644, 55648, 55645, 55641, 55647, 55642, 55643, 55646, 55649,
55722, 55723, 55726, 55727, 55724, and 55725. The method can
- 15 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
further comprise culturing said microorganisms such that: at
least one compound is degraded to products comprising CC>2 and
H20 .
The speed and efficiency afforded by the methods of
the present invention have been never before achieved for the
bioremediation of tarry or elastomeric compositions containing
either a single or a mixture of aromatic, nitro-aromatic:,
halo-aromatic, halo-nitro-aromatic, aliphatic and halo-
aliphatic compound(s).
The present invention may be understood more fully
by reference to the following definitions, detailed
description of the invention, illustrative examples of
specific embodiments of the invention and the appended figures
w in which:
4. HRIEF DESCRIPTION OF THE FIGURES
Figure la-c. A schematic illustration of a
representative fluid phase system. Figure la illustrates a
slurry formation system; Figure 1b illustrates a fluid phase
bioremediation system; and Figure lc illustrates filtration
and dewatering of treated materials.
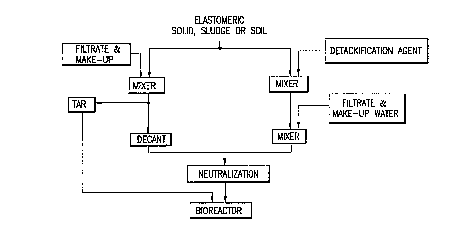
Figure 2. Schematic diagram for slurry phase
formation. Figure 2a. Formation of a slurry phase from; a
non-elastomeric solid, sludge or soil. Figure 2b. Formation
of a slurry phase from an elastomeric solid, sludge or soil.
Figure 3. A graph demonstrating the correlation
between decreasing levels of hydrocarbon compounds and
increasing levels of COz evolved. ~ Naphthalene; ~ Toluene;
o Benzene; v Coz.
Figure 4. A graph demonstrating naphthalene
degradation over 30 days in a sequencing batch bioreacto~r.
~ Naphthalene.
Figure 5. A graph demonstrating naphthalene and
benzene degradation over 30 days in a sequencing batch
bioreactor. ~ Naphthalene; ~ Benzene.
a
- 16 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
5. DEFINITIONS
As used in the present invention, the following
terms are intended to encompass the following:
AEROBIC Pertaining to or requiring oxygen wherein
- 5 the oxygen tension is 0.1% to 100% of
saturation (where, 100% saturation
corresponds to 40 mg OZ per liter based on
oxygen in water at 25°C), preferably
between 4% and 80%, more preferably
between 10% and 20%.
ALKYL A methyl, ethyl or propyl group.
ALIPHATIC An acyclic or alicyclic organic
hydrocarbon compound that can be regarded
as a derivative of methane and lacks a
cyclic conjugated six-member carbon
(benzene) ring.
AROMATIC An organic compound which is characterized
by the presence of at least one cyclic
fully conjugated six-member carbon
ZO (benzene) ring or one cyclic fully
conjugated hetero-six-member ring in which
one or more ring carbons) is replaced by
a nitrogen atom(s). This is intended to
include non-substituted aromatic compounds
as well as aromatic compounds containing
one or more of the following in place of a
hydrogen atom(s): a hydroxyl, an amine, an
alkyl, a carboxyl, or an unsubstituted or
substituted aliphatic group, in which the
substituted aliphatic group contains a
carbonyl or carboxyl group in place of a
hydrogen atom(s).
BULKING AGENT A compound or composition that when added
to a solid, sludge or soil facilitates the
flow of fluid through said solid, sludge
or soil.
- 17 -
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/16364
COMPOSTING-LIKE A process wherein organic hydrocarbon
compounds in a solid composition are
degraded by microorganisms, usually in a
closed or confined area.
DETACKIFYING AGENT A compound that when mixed with an
elastomeric or tarry substance renders the
substance less elastomeric or tarry. When
used in conjunction with the proces;a for
forming a slurry, the detackifying agent
aids in the compositions becoming
fluidizable.
ELASTOMERIC The property whereby a solid material
changes its shape and size under the
action of opposing forces, but recovers
its original configuration when the forces
are removed, provided the opposing forces
do not exceed the elastic modulus of the
solid material.
HALO-ALIPHATIC An aliphatic hydrocarbon compound
containing one or more halogen atom:a such
as, for example, chlorine, bromine or
iodine or a mixture thereof in placE= of an
hydrogen atom(s).
HALO-AROMATIC An aromatic hydrocarbon compound
containing one or more halogen atom: such
as, for example, chlorine, bromine or
iodine or a mixture thereof in placEa of a
hydrogen atom(s).
HALO-NITRO-
'~O~TIC An aromatic hydrocarbon compound
containing one or more halogen atom:a
such as, for example, chlorine,
bromine or iodine or a mixture
thereof in place of a hydrogen
atoms) and containing one or more '
nitro groups in place of a hydrogen
atom ( s ) . '
- 1s -
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/16364
FLUIDIZING A process wherein energy, such as, for
example, mechanical energy, is imparted to
suspend finely divided or particularized
solids in a fluid such as, for example,
- 5 air, water or an aqueous solution.
NITRO-AROMATIC An aromatic hydrocarbon compound
containing one or more vitro groups in
place of a hydrogen atom(s).
SLUDGE A collection of solids such as, for
example, a still-bottom, that have settled
out of a suspension.
SLURRY A suspension of finely divided or
particularized solids in a fluid or liquid
wherein energy, such as, for example,
mechanical energy, may be imparted to
maintain dispersion of the particularized
solids.
TARRY A viscous hydrocarbon containing material,
which may have the consistency and
appearance of roofing tar.
TCL Target Compound List, a designated list of
compounds analyzed using a solvent
extraction as defined in EPA, SW-846. As
used presently, the extraction, solvent is
methylene chloride: methanol (90:10).
TCLP Toxicity Characteristic Leaching
Procedure, an aqueous extraction method as
defined in EPA, SW-846, Method No. 1311.
6. DETAILED DESCRIPTION
OF THE INVENTION
6.1. NOVEL ISOLATED MICROORGANISMS
Novel mi croorganisms have been isolated from soil
and selected for the ability
to utilize specific compounds
such as aromatic, substituted aromatic and/or aliphatic
n
compounds as sole nitrogen and/or carbon and energy sources.
The microorganisms are useful for aerobic degradation of at
least one of these compounds. The selection process ensured
- 19 -
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/16364
that the biochemical activity of the microorganisms is
directed towards destructive treatment of at least one of such
compounds and that the microorganisms are capable of utilizing
at least one of the compounds as a sole nutrient source.
Although the present inventors) does not wish to be limited
to a particular mechanism of action, it is believed that: such
utilization results in mineralization of the compounds) via
primary metabolism and not co-metabolism. An important
element in the selection of the desired microorganisms i.s that
the selection process is conducted under aerobic conditions
such that the isolated microorganisms aerobically degrade the
desired compound or mixtures thereof. Further, the
microorganisms may degrade mixtures simultaneously, not
sequentially.
Additionally, although the present inventors) .
doles) not wish to be limited to a particular mechanism of
action, it is believed that the majority of the degradation
occurs via the ortho- or modified ortho-pathway. The or~tho-
or modified ortho-pathway is especially important so that
highly toxic halo-acids and/or non-metabolizable intermediates
are not produced as intermediates or end products during' the
degradation of halo-aromatic compounds or aromatic compounds
substituted with one or more methyl group(s). It is noted
that, for example, two of the microorganisms i.e. DAP 66 and
DAP 70 do possess catechol 2,3-dioxygenase activity,
indicating the ability to use meta-cleavage.
Additionally, the microorganisms are isolated such
that they are able to withstand high and/or variable
concentrations of the compounds. Moreover, the microorganisms
can degrade a high total or composite concentration of mixed
organic compounds, for example, >_ 1% (10,000 ppm). As used in
the present invention, ~~high" concentrations of aromatic,
nitro-aromatic, halo-aromatic, halo-nitro-aromatic, aliphatic
and halo-aliphatic compounds are intended to encompass the
4
following: (1) aromatic compounds: for example, benzene,
toluene, xylenes, ethylbenzene: >_ 5,000 ppm; phenol: >_ 6,000
ppm, creosol, dimethylphenol: >_ 1,000 ppm; anthracene: >_ 300
- 20 -
CA 02207781 1997-06-13
WO 96!18724 PCT/US95l16364
ppm; styrene: >_ 5,000 ppm; aniline: >_ 15o ppm; naphthalene:
>_ 1,000 ppm; 1- or 2-methylnaphthalene: >_ 200 ppm; (2) nitro-
aromatic compounds: for example, nitrobenzene: >- 150 ppm; (3)
halo-aromatic compounds: for example, chlorobenzene, 2-
chloronaphthalene: >_ 200 ppm.
The microorganisms of the present invention can also
degrade the following compounds at concentrations of at least
1000 ppm: pyrene, acenaphthylene, fluoranthene, phenanthrene,
benzo-(b)-fluoranthene, dibenzofuran, chrysene, catechol, m-
io toluic acid, cinnamyl acetate, vanillin, trans-cinnamaldehyde,
mesitylene, salicylate, 2-, 3-, or 4-chlo~otoluene, 2-, 3-, or
4-chlorobenzoate, 1,3-dichlorobenzoate, 1-chloro-3-
nitrobenzene, 1-chloro-4-nitrobenzene, 1,2-, 1,3-, or 1,4-
dinitrobenzene, melamine, cyanuric acid, hexadecane, and
d-(-)-limonene.
Preference is given in the selection process for
those microorganisms which are capable of growing/metabolizing
on solid surfaces and for those microorganisms which
chemotactically migrate towards solid surfaces.
In general, the microorganisms isolated do not
constitutively express the metabolic proteins necessary for
degradation of the desired compounds but rather have to be
induced by culturing on medium containing the relevant
compound or mixture thereof or on medium containing a compound
which induces enzymes of the pathway specific for the
degradation of the relevant compound(s). In a particular
embodiment, the medium contains at least one of nitrobenzene,
aniline, melamine and cyanuric acid; and at least one of
naphthalene, benzene, toluene, ethylbenzene and xylene.
Additionally, all of the microorganism isolates are
naturally occurring strains, i.e., none of the strains are
modified recombinantly.
Pure and mixed cultures of the novel microorganisms
of the present invention can be maintained using BACTO''" R2A
medium (Difco, Detroit, Michigan). Use of BACTO'"' R2A medium
as maintenance medium entails: inoculation of BACTO"' R2A
medium with a pure or mixed culture according to the present
- 21 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
invention, and culture of the microorganisms at room
temperature, i.e., about 25-27°C for about 48 hours. The
cultures can then be covered with a material which forma a '
barrier to passage of air and moisture, e.g., parafilm, and
stored under refrigeration, for example, at about 4°C. -
Alternatively, pure and mixed cultures of the
microorganisms of the present invention can be maintainead by
culture using Stanier's minimal medium (Stanier et al., 1966,
J. Gen. Microbiol. X3:159-271) supplemented with 5-10 mM of
the desired aromatic, vitro-aromatic, halo-aromatic, halo-
nitro-aromatic, aliphatic or halo-aliphatic compound or
mixture thereof. According to a preferred mode of this
embodiment a C:N ratio of about 10:1 to 25:1 is maintained in
the supplemented Stanier's medium. The cultures are
maintained, with aeration, for example, using pure oxygen at
100-400 ml/min and with stirring. After about 24 hours
cultured on the supplemented Stanier's, the bacterial cells
are removed from the medium by centrifugation, resuspended in
either Stanier's minimal medium (SMM) or phosphate buffered
saline (PBS), and removed from the resuspension wash by
centrifugation. The cell pellet can be stored at about 4°C.
Alternatively, mixed cultures can be maintained as
follows. A mixed culture can be inoculated into a composition
containing the following: (1) naphthalene, preferably between
about 1000-4000 ppm; (2) one or more of: benzene, toluene,
ethylbenzene and xylene at about 400-500 ppm each; (3) either
or both chloronaphthalene and/or methylnaphthalene at about
200 ppm each; and (4) aniline and/or nitrobenzene at about 30-
300 ppm each and treated using a fluid phase or a solid phase
3o system as described in Section 6.3, infra. Preferably i=he
C:N:P ratio is about 25:1:0.1 and the culture is maintained at
about room temperature for the treatment cycle. At the end of
the treatment, the contents of the slurry phase treatment can
be filtered, for example, using Whatman 1 filter or other
equivalent and the dewatered residual solid, designated
"filter cake" containing induced microorganisms can be used to
- 22 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95116364
maintain a mixed culture suitable for use according to the
present invention.
Some of the microorganisms described below are
capable of utilizing nitrobenzene aerobically as a sole source
of carbon, nitrogen and energy. In particular, microorganisms
designated DAP 111, DAP 119, DAP 622, DAP 623, DAP 626, DAP
629, DAP 632, DAP 115, DAP 120 and the mixed culture
designated DAP-2 can aerobically degrade nitrobenzene.
Microorganisms designated DAP 70, DAP 73, DAP 111, DAP 119 and
DAP 622 and the mixed culture DAP 2 can aerobically degrade
naphthalene, methylnaphthalene, chloronaphthalene or
anthracene. Microorganisms designated DAP 111, DAP 119 and
DAP 622, DAP 623, DAP 626, DAP 629, DAP 632, DAP 115, DAP 120
and the mixed culture DAP 2 can aerobically degrade aniline.
Additionally, some of the microorganisms described below are
able to utilize a wide variety of substituted and non-
substituted aromatic compounds, for example, benzene, toluene,
aniline, phenol and ethylbenzene, aerobically as a sole source
of carbon and/or nitrogen and energy. All of the pure
cultures of microorganisms described below utilize these
compounds aerobically. Although not wishing to be limited to
a single mechanism of action, the present inventors) believes
that the compounds are degraded aerobically, for the most
part, via the ortho- or modified ortho-pathway. The pure and
mixed cultures can degrade the compounds to products
comprising COZ and H20.
Some of the microorganisms described below are also
able to utilize a wide variety of substituted and non-
substituted aliphatic compounds, for example, a-(-)-limonene,
formaldehyde, chloroform and methanol, aerobically. In
addition, some of the microorganisms are also able to degrade
longer-chain aliphatic compounds. The latter ability can be
evidenced, for example, by the utilization of hexadecane as a
sole carbon and energy source.
All the microorganisms described below were observed
_ to grow better, i.e., cells more rapidly developed into larger
colonies, when cultured on low density agar medium, i.e., at
- 23 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16a64
about 3-10 gm agar per liter of medium, preferably at about 3
gm of agar per liter of medium. The microorganisms described
below can be cultured on normal density agar medium, but: '
growth is less rapid.
The motile microorganisms described below are -
induced to exhibit chemotaxis by a wide variety of compounds.
Chemotaxis is achieved by two modes of motility, namely,
flagellar and twitching. Growth conditions allow the
microorganism to exhibit either of the two modes of motility.
For example, to observe flagellar motility, the microorganisms
are grown under less viscous conditions, for example, in
liquid medium or on agar plates wherein the percentage of agar
is less than about 1%, perferrably 0.3%. To observe twitching
motility, the microorganisms are grown on a solid medium, such
as agar plates wherein the percentage of agar is about 7.%. If
the percentage of agar is too high, for example about 2%, both
phenotypes of chemotaxis are not likely to be observed.
Certain of the motile microorganisms, including DAP 111 and
DAP 119 exhibit both modes of motility under appropriate:
conditions.
Each of the pure cultures, as well as the mixs:d
culture, described below in Sections 6.1.1 - 6.1.5, including
sub-Section 6.1.5.1, were deposited with the American Type
Culture Collection (see Section 10, infra).
6.1.1 MICROORGANISM ISOhATED USING NITROBENZENE
The following microorganism was isolated from soil
using aerobic culture on a minimal medium containing only
nitrobenzene as the sole source of carbon, nitrogen and
energy.
1'~icroorganism DAP 622:
DAP 622 is a Pseudomonas sp. Gram negative motile
rod occasionally seen in pairs, and when grown on nutrient
agar the colonies appear white to creamy. Floc formation is
present and motility appears flagellar when the microorganism
is grown on flagella plates. This organism is able to x>roduce
- 24 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
yellow pigment when grown on Pseudomonas F Agar (Difco). In
addition, this organism can utilize the following: lactate,
chlorobenzene, ethylbenzene, salicylate,and succinate as
a
sole source of carbon and energy. DAP 22 is further
6
characterized as shown in Table 1.
TABLE 1
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (+)
.TRIPLE SUGAR IRON AGAR HZS is produced
GROWTH AT: 15C (+)
25 (+)
35 (+)
.. 41
ZS UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
MANNOSE (+)
2-METHYLNAPHTHALENE (+)
2 0 a-KETOGLUTARATE (+)
GLYOXYLATE (-)
GLUTAMATE (+)
ETHANOL (+)
HEXADECANE (-)
N03 -> NOZ ( + )
2 5 ARGININE DECARBOXYLASE (-)
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (-)
GELATIN HYDROLYSIS (+)
UREASE (+)
ANTIBIOTIC RESISTANCE:
3 0 HgCl~ R
AMPICILLIN R
KANAMYCIN (-)
NEOMYCIN (-)
TETRACYCLINE (-)
SPECTINOMYCIN (R)
a
35 STREPTOMYCIN (-)
- 25 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16:364
6.1.2 MICROORGANISMS ISOLATED USING CHLOROBENZENE~
The following microorganisms were isolated from soil
using aerobic culture on minimal medium containing only
chlorobenzene as the sole source of carbon and energy.
Microorctanism DAP 631:
DAP 631 is a Pseudomonas sp. Gram negative slender
motile rod seen occasionally in pairs, colonies of the
microorganism appear white on BACTO'''" R2A medium. In addition,
this organism can utilize the following: lactate,
chlorobenzene, and ethylbenzene as a sole source of carbon and
energy. DAP 631 is further characterized as shown in Table 2.
20
30
- 26 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABLE 2
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (- to weak)/(+)
CITRATE UTILIZATION (-)
TRIPLE SUGAR IRON AGAR HzS is produced
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (-)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (-)
LACTOSE (-)
MANNITOL (-)
MANNOSE (-)
2-METHYLNAPHTHALENE (-)
a-KETOGLUTARATE (-)
GLYOXYLATE (-)
GLUTAMATE (+)
ETHANOL (-)
HEXADECANE (-)
N03 -> NOZ ( + )
ARGININE DECARBOXYLASE (-)
2 O
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE ' (-)
GELATIN HYDROLYSIS (-)
UREASE (-)
ANTIBIOTIC RESISTANCE:
Hgcl= (-)
AMPICILLIN R
KANAMYCIN (-)
NEOMYCIN (-)
TETRACYCLINE (-)
SPECTINOMYCIN (-)
STREPTOMYCIN (-)
3 O
Microorganism DAP 68:
DAP 68 is a Aeromonas sp. Gram negative motile rod
~ found occasionally in pairs and appears white to creamy on
BACTO''" R2A medium. In addition, this organism can utilize the
- following: lactate, chlorobenzene, ethylbenzene, and succinate
- 27 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16~64
as a sole source of carbon and energy. DAP 68 is further
characterized as shown in Table 3.
TABhE 3
$ DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(-)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR HZS is produced
acid and gas from
glucose
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (+)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (+)
MANNITOL (+)
MANNOSE (+)
2-METHYLNAPHTHALENE (-)
a-KETOGLUTARATE (+)
GLYOXYLATE (+)
2 O GLUTAMATE (+)
ETHANOL (-)
HEXADECANE (-)
N03 -~ NOz ( + )
ARGININE DECARBOXYLASE (+)
LYSINE DECARBOXYLASE (+)
2 5 ORNITHINE DECARBOXYLASE (+)
GELATIN HYDROLYSIS (+)
UREASE (+)
ANTIBIOTIC RESISTANCE:
HgCl2 R
AMPICILLIN R
3 0 KANAMYCIN R
NEOMYCIN R
TETRACYCLINE R
SPECTINOMYCIN R
STREPTOMYCIN R
- 28 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
Microorgvanism DAP 66:
DAP 66 is a Corynebacterium sp. Gram variable,
'' large, non-motile rod seen singly and in chains. Some chains
approach filaments in size and some single rods are motile.
Floc formation is present and the cells have capsules. Growth
on twitching plates is equivocal. Colonies appear hard and
waxy when grown on BACTO'"' R2A medium. This organism tested
positive for the first enzyme in the meta-pathway, catechol-
2,3-dioxygenase (C230), according to the procedure outlined by
Bayly and Wigmore, 1973, J. Bacteriol. 113:1112-1120. In
addition, this organism can utilize the following: lactate,
chlorobenzene, m-toluic acid, ethylbenzene, and succinate as a
sole source of carbon and energy. DAP 66 is further
characterized as shown in Table 4.
a
25
35
- 29 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABLE 4
DIFFERENTIAL CHARACTERISTIC RESULT '
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (-)
TRIPLE SUGAR IRON AGAR no fermentation
GROWTH AT: 15 (-)
25 (+)
35 (+)
41 (-)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
MANNOSE (-)
2-METHYLNAPHTHALENE (-)
-~TOGLUTARATE (-)
GLYOXYLATE (-)
GLUTAMATE (-)
ETHANOL (+)
HEXADECANE (+)
N03 -> NOZ ( - )
ARGININE DECARBOXYLASE (-)
Z O
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (-)
GELATIN HYDROLYSIS (-)
UREASE (+)
ANTIBIOTIC RESISTANCE:
HgCh R
2 5
AMPICILLIN R
KANAM7CCIN R
NEOMYCIN (-)
TETRACYCLINE (-)
SPECTINOMYCIN (-)
3 0 STREPTOMYCIN (-)
6.1.3 MICROORGANISMS ISOLATED USING NAPHTHALENE
The following microorganisms were isolated from soil
35 using aerobic culture on minimal medium containing only
naphthalene as the sole source of carbon and energy.
- 30 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
Microorganism DAP 70:
DAP 70 is a Pseudomonas sp. Gram negative motile rod
where the rods are seen singly, in pairs or in long chains,
wherein some chains approach the size of filaments. When
grown on flagella plates the motility appears flagellar and
when grown on BACTO"' R2A medium the colonies appear white. In
addition, the microorganism forms large flocs. This organism
tested positive for the first enzyme in the meta-pathway,
catechol-2,3-dioxygenase (C230), according to the procedure
outlined by Bayly and Wigmore, 1973, J. Bacteriol. 113:1112-
1120. In addition, this organism can utilize the following:
lactate, chlorobenzene, ethylbenzene, and succinate as a sole
source of carbon and energy. DAP 70 is further characterized
as shown in Table 5.
20
30
- 31 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABLE 5
DIFFERENTIAL CHARACTERISTIC RESULT "
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR acid from glucose
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (-)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (+)
MANNITOL (+)
MANNOSE (-)
2-METHYLNAPHTHALENE (-)
a-KETOGLUTARATE (-)
GLYOXYLATE (+)
GLUTAMATE (-)
ETHANOL (+)
HEXADECANE (+)
N03 -> NOZ ( - )
ARGININE DECARBOXYLASE (-)
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (-)
GELATIN HYDROLYSIS (-)
UREASE (+)
ANTIBIOTIC RESISTANCE:
HgCh R
AMPICILLIN R
KANAMYCIN R
NEOMYCIN R
TETRACYCLINE R
SPECTINOMYCIN R
3 0 STREPTOMYCIN R
Microorganism DAP 73:
DAP 73 is a Zoogloea sp. Gram variable motile rod
found singly and in pairs. Growth on motility plates
S5 indicates mobility. Floc formation is present with many
finger-like projections. This organism is able to produce
yellow pigment when grown on Pseudomonas F Agar (Difco). In -
- 32 -
CA 02207781 1997-06-13
WO 96/18724 PCTIUS95/16364
addition, this organism can utilize the following: lactate,
chlorobenzene, and succinate as a sole source of carbon and
energy. DAP 73 is further characterized as shown in Table 6.
' S TABLE 6
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR HZS is produced
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (+)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
MANNOSE (+)
2-METHYLNAPHTHALENE (+)
a-KETOGLUTARATE (+)
GLYOXYLATE (-)
GLUTAMATE (+)
2 0 ETHANOL (+)
HEXADECANE (-)
N03 -> NOz ( + )
ARGININE DECARBOXYLASE (-)
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (-)
2 5 GELATIN HYDROLYSIS (+)
UREASE (+)
ANTIBIOTIC RESISTANCE:
HgCl~ R
AMPICILLIN R
KANAMYCIN (-)
3 O NEOMYCIN (-)
TETRACYCLINE (-)
SPECTINOMYCIN R
STREPTOMYCIN (-)
- 33 -
CA 02207781 1997-06-13
WO 96/18724 . PCT/US95116~364
6.1.4 MICROORGANISMS ISOLATED USING
NITROBENZENE AND NAPHTHALENE
The following microorganisms were isolated initially
from soil and aerobically cultured to pure microorganism
isolates. These pure microorganism isolates were subsequently ,
cultured aerobically together with a sludge/waste material
containing a mixture of compounds for example, naphthalene,
preferably between about 1000-4000 ppm; benzene, toluene,
ethylbenzene and xylene at about 400-500 ppm each;
1o chloronaphthalene and methylnaphthalene at about 200 ppm each;
and aniline and nitrobenzene at about 30-300 ppm each. In
addition, substituted and non-substituted aliphatic compounds
were also present in the mixture. Pure microorganism isolates
were recovered from the cultured materials using aerobi~~
culture on a minimal medium containing 150 ppm nitrobenzene
and 150 ppm naphthalene as the sole sources of carbon,
nitrogen and energy.
Microorganism DAP 111:
DAP 111 is a Pseudomonas sp. Gram negative motile
2~ rod found both in pairs and singly. The colonies appear white
on BACTO'''u R2A medium and some floc formation occurs. Motility
on both twitching and flagella plates is observed. In
addition, this organism can utilize the following: lactate,
vanillin, chlorobenzene, ethylbenzene, cyanuric acid,
salicylate, and succinate as a sole source of carbon and
energy. DAP 111 is further characterized as shown in Table 7.
35
- 34 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABLE 7
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR HzS is produced
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (+)
UTILIZATION OF: GLUCOSE (+)
, FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
MANNOSE (+)
" , 2-METHYLNAPHTHALENE (+)
a-KETOGLUTARATE (+)
GLYOXYLATE (+)
GLUTAMATE (+)
ETHANOL (-)
HEXADECANE (+)
N03 -~ NOZ ( + )
ARGININE DECARBOXYLASE (-)
2 0
LYSINE DECARBOXYLASE (+)
ORNITHINE DECARBOXYLASE (-)
GELATIN HYDROLYSIS (+)
UREASE (+)
ANTIBIOTIC RESISTANCE:
HgC 1: R
2 5
AMPICILLIN R
KANAMYCIN R
NEOMYCIN R
TETRACYCLINE R
SPECTINOMYCIN R
STREPTOMYCIN (-)
3 O
M_icrooraanism DAP 119:
DAP 119 is an Aeromonas sp. Gram negative motile
rod. The colonies appear white on BACTOT" R2A medium but
the
microorganism culture appears yellow when grown in nutrient
35
broth. Twitching motility is evidenced on
twitching plates,
and flagellar motility is evidenced by growth
on flagella
- 35 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16:364
plates. In addition, this organism can utilize the following:
lactate, vanillin, chlorobenzene, ethylbenzene, cyanuric: acid,
salicylate, and succinate as a sole source of carbon and
energy. DAP 119 is further characterized as shown in Table 8.
,
TABhE 8
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR HIS is produced
acid and gas from
glucose
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (+)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (+)
MANNITOL (+)
MANNOSE (+)
2-METHYLNAPHTHALENE (-)
2 0 a-KETOGLUTARATE (+)
GLYOXYLATE (+)
GLUTAMATE (+)
ETHANOL (+)
HEXADECANE (-)
N03 -~ N02 ( + )
2 5 ARGININE DECARBOXYLASE (+)
LYSINE DECARBOXYLASE (+)
ORNITHINE DECARBOXYLASE (+)
GELATIN HYDROLYSIS (+)
UREASE (+)
ANTIBIOTIC RESISTANCE:
3 0 HgCh R
AMPICILLIN R
KANAMYCIN R
NEOMYCIN R
TETRACYCLINE R
SPECTINOMYCIN R r
3 5 STREPTOMYCIN R
- 36 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
6.1.5 MIXED MICROORGANISM CULTORE
Over 200 separate pure microorganism isolates were
cultured from soil at the collection site. All of these pure
isolates, including those described above in Sections 6.1.1
through 6.1.4, were combined and cultured, aerobically, with a
sludge/waste material containing a mixture of aromatic, nitro-
aromatic, halo-aromatic, aliphatic and halo-aliphatic
compounds. A mixed culture of microorganisms was recovered
from the cultured material and has been maintained on BACTO"'
R2A medium (Difco, Detroit, Michigan).
The mixed culture designated DAP-2, aerobically
degrades at least the following compounds or mixtures thereof:
benzene, toluene, xylene, ethylbenzene, naphthalene,
chlorobenzene, phenol, cresol, nitrobenzene, aniline,
anthracene, dimethylphenol, styrene, halonaphthalene, 2-, 3-
or 4-chlorotoluene, 2-, 3- or 4-chlorobenzoate, 1,3-
dichlorobenzoate, 1,2-, 1,3- or 1,4-dinitrobenzene, 1-chloro-
3-nitrobenzene, 1-chloro-4-nitrobenzene, 1- or 2-
methylnaphthalene, pyrene, acenaphthylene, fluoranthene,
phenanthrene, benzo-(b)-fluoranthene, dibenzofuran, chrysene,
catechol, m-toluic acid, cinnamyl acetate, vanillin, trans-
cinnamaldehyde, mesitylene, salicylate, melamine, cyanuric
acid, s-(-)-limonene, hexadecane, methanol, formaldehyde, and
chloroform .
6.1.5.1. PORE ISOhATES FROM THE MIXED CUhTURE
The following pure cultures were isolated and
identified from the mixed culture designated DAP 2 by
isolating single colonies on BACTO"' R2A medium supplemented
with 150 ppm each of nitrobenzene, naphthalene, and toluene.
Microorctanism DAP 623:
DAP 623 is a Gram negative motile rod, generally
small single rods, though some pairs are seen. Staining can
be uneven and there is some floc formation. The colonies
appear white to creamy on BACTO''" R2A medium. In addition,
this organism can utilize the following: mesitylene, lactate,
- 37 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16~364
succinate, limonene, m-toluic acid, chlorobenzene, salicylate,
2-, 3-, and 4-chlorotoluene, 2-, 3-, and 4-chlorobenzoic acid,
and 1,3-dichlorobenzene as a sole source of carbon and csnergy.
DAP 623 is further characterized as shown in Table 8A.
,
TABLE 8A
DIFFERENTIAL. CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(-)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR acid from ghicose
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (+)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
MANNOSE (+)
2-METHYLNAPHTHALENE (-)
a-KETOGLUTARATE (+)
GLUTAMATE (+)
2 0
ETHANOL (-)
HEXADECANE (-)
N03 -~ NOZ ( + )
ARGININE DECARBOXYLASE (+)
LYSINE DECARBOXYLASE (+)
ORNITHINE DECARBOXYLASE (+)
2 5
GELATIN HYDROLYSIS (+)
UREASE (+)
ANTIBIOTIC RESISTANCE:
HgCl~ ( - )
AMPICILLIN R
KANAMYCIN (-)
30
TETRACYCLINE R
SPECTINOMYCIN R
STREPTOMYCIN (-)
r
35 Microorganism DAP 626:
DAP 626 is a Gram variable rod which vary in size y
and occur singly and in pairs. Grocath on flagella plates is
- 38 -
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/16364
seen which indicates flagellar motility. In addition, this
organism can utilize the following: mesitylene, lactate,
succinate, limonene, cinnamyl acetate, catechol, m-toluic
acid, chlorobenzene, 2-, 3-, and 4-chlorotoluene, 2-, 3-, and
4-chlorobenzoic acid, and 1,3-dichlorobenzene as a sole source
of carbon and energy. DAP 626 is further characterized as
shown in Table 8B.
TABhE 8B
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (-)
TRIPLE SUGAR IRON AGAR H~S is produced
GROWTH AT: 15° (+)
25° (+)
35° (+)
41° (+)
UTILIZATION OF: GLUCOSE (-)
FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
2 0 MANNOSE (-)
2-METHYLNAPHTHALENE (-)
a-KETOGLUTARATE (+)
GLUTAMATE (+)
ETHANOL (+)
HEXADECANE (+)
2 5 No3 -~ No2 ( - )
ARGININE DECARBOXYLASE (-)
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (-)
GELATIN HYDROLYSIS (-)
UREASE (+)
3 0 ANTIBIOTIC RESISTANCE:
HgCl~ ( - )
AMPICILLIN R
KANAMYCIN (-)
SPECTINOMYCIN (-)
STREPTOMYCIN R
- 39 -
CA 02207781 1997-06-13
WO 96/18724 PCT/U595l16364
Microorganism DAP 629:
DAP 629 is a Gram negative small motile rod, almost
cocco-bacillary. Colonies appeared white with a slight
fluorescence when grown on BACTO"' R2A agar. In addition, this
organism can utilize the following: fluoranthrene, mesitylene,
lactate, succinate, limonene, m-toluic acid, chlorobenzene, 2
3-, and 4-chlorotoluene, 2-, 3-, and 4-chlorobenzoic acid,
and 1,3-dichlorobenzene as a sole source of carbon and energy.
DAP 626 is further characterized as shown in Table 8C.
15
25
35
r
- 40 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABLE 8C
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (-)
TRIPLE SUGAR IRON AGAR no fermentation
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (-)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (-)
LACTOSE (-)
MANNITOL (-)
MANNOSE (-)
2-METHYLNAPHTHALENE (-)
a-KETOGLUTARATE (+)
GLUTAMATE (+)
ETHANOL (-)
HEXADECANE (-) ,
N03 -~ NOZ ( + )
ARGININE DECARBOXYLASE (-)
2 O LYSINE DECARBOXYLASE (+)
ORNITHINE DECARBOXYLASE (-)
GELATIN HYDROLYSIS (+)
UREASE (-)
ANTIBIOTIC RESISTANCE:
HgCl2 ( - )
2 5 AMPICILLIN R
KANAMYCIN (-)
TETRACYCLINE (-)
SPECTINOMYCIN (-)
STREPTOMYCIN (-)
Microorganism DAP 632:
DAP 632 is a Gram variable motile slender rod, seen
both singly and in pairs. Colonies appeared creamy to
yellowish when grown on BACTO''°' R2A agar. In addition, this
organism can utilize the following: fluoranthrene,
acenaphthalene, mesitylene, lactate, limonene, m-toluic acid,
chlorobenzene, 2-, 3-, and 4-chlorotoluene, 2-, 3-, and 4-
- 41 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
chlorobenzoic acid, and 1,3-dichlorobenzene as a sole source
of carbon and energy. DAP 626 is further characterized as
shown in Table 8D.
TABhE 8D "
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(-)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR no fermentation
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (+)
UTILIZATION OF: GLUCOSE (-)
FRUCTOSE (-)
LACTOSE (-)
MANNITOL (-)
MANNOSE (-)
2-METHYLNAPHTHALENE (-)
a-KETOGLUTARATE (-)
GLUTAMATE (+)
ETHANOL (-)
2 O HEXADECANE (-)
N03 -~ NOZ ( - )
ARGININE DECARBOXYLASE (-)
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (-)
GELATIN HYDROLYSIS (+)
2 5 uREASE (+)
ANTIBIOTIC RESISTANCE:
HgCl2 R
AMPICILLIN R
KANAMYCIN R
TETRACYCLINE R
3 O SPECTINOMYCIN R
STREPTOMYCIN R
Microorganism DAP 115:
r
35 DAP 115 is a Gram negative motile rod. Growth is
observed on flagella plates, indicating motility is flagellar.
Colonies appeared white when grown on BACTO'"" R2A agar, but
- 42 -
CA 02207781 1997-06-13
WO 96/18724 PCTIUS95/16364
appear yellow in nutrient broth. In addition, this organism
can utilize the following: benzo-(b)-fluoranthrene,
fluoranthrene, dibenzofuran, acenaphthalene, salicylate,
lactate, succinate, glyoxylate, mesitylene, vanillin,
S limonene, cinnamyl acetate, catechol, m-toluic acid,
chlorobenzene, 2-, 3-, and 4-chlorotoluene, 2-, 3-, and 4-
chlorobenzoic acid, and 1,3-dichlorobenzene as a sole source
of carbon and energy. DAP 115 is further characterized as
shown in Table 8E.
15
25
35
- 43 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16:364
TABLE 8E
w
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (+) ,
TRIPLE SUGAR IRON AGAR H~S is produced
acid and gas from
glucose
GROWTH AT: 15 (+/-)
25 (+)
35 (+)
41 (+)
'UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
MANNOSE ~ (+)
2-METHYLNAPHTHALENE (+)
a-KETOGLUTARATE (+)
GLUTAMATE (+)
ETHANOL (-)
HEXADECANE (+)
N03 -~ NOZ ( + )
2 0 ARGININE DECARBOXYLASE (-)
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (+)
GELATIN HYDROLYSIS (+)
UREASE C+)
ANTIBIOTIC RESISTANCE:
2 5 Hgcl~ R
AMPICILLIN R
KANAMYCIN R
TETRACYCLINE R
SPECTINOMYCIN R
STREPTOMYCIN R
Microorganism DAP 120:
DAP 120 is a Gram negative motile rod. Growth is
observed on flagella plates, indicating motility is flagellar.
In addition, this organism can utilize the following:
chrysene, pyrene, lactate, succinate, glyoxylate, salic:ylate, ,
mesitylene, vanillin, limonene, cinnamyl acetate, catec:hol, m-
- 44 -
CA 02207781 1997-06-13
WO 96118724 PCT/US95/16364
toluic acid, chlorobenzene, 2-, 3-, and 4-chlorotoluene, 2-,
3-, and 4-chlorobenzoic acid, and 1,3-dichlorobenzene
as a
sole source of carbon and energy. DAP 120 is further
characterized as shown in Table 8F.
TAHLE 8F
DIFFERENTIAL CHARACTERISTIC RESULT
CATALASE/OXIDASE (+)/(+)
CITRATE UTILIZATION (+)
TRIPLE SUGAR IRON AGAR HZS is produced
ZO
GROWTH AT: 15 (+)
25 (+)
35 (+)
41 (+)
UTILIZATION OF: GLUCOSE (+)
FRUCTOSE (+)
LACTOSE (-)
MANNITOL (+)
MANNOSE (-)
2-METHYLNAPHTHALENE (+)
a-KETOGLUTARATE (+)
GLUTAMATE (+)
2 O
ETHANOL
(-)
HEXADECANE (+)
N03 --> NOz ( + )
ARGININE DECARBOXYLASE (-)
LYSINE DECARBOXYLASE (-)
ORNITHINE DECARBOXYLASE (-)
2 5
GELATIN HYDROLYSIS (+)
UREASE (+)
ANTIBIOTIC RESISTANCE:
HgCl~ R
AMPICILLIN R
KANAMYCIN R
30
TETRACYCLTNE R
SPECTINOMYCIN (-)
STREPTOMYCIN (-)
Y
35 The following Table 8G shows that the above-
described pure cultures, isolated from the mixed culture
- designated DAP 2, are able to grow solely on Stanier's minimal
- 45 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
medium supplemented with 150 ppm each of nitrobenzene,
naphthalene, and toluene. The cultures were grown at 2!5-27°C,
colony size determined after 14 days. Values represent mean '
of five replicate colonies for each determination.
f
TABLE 8G
CULTURE GROWTH COLONY SIZE_
DAP 626 ++ 3.8 mm
DAP 115 ++/+++ 5.0 mm
DAP 632 ++/+++ 4.7 mm
DAP 623 ++ 4.0 mm
DAP 120 +/++' '
DAP 629 ++ 4.3 mm
~' Growth scored as ++++ luxuriant, +++ good, ++ fair, + modest, +~-~ scant,
- no growth
' Growth of strain DAP 120 was very thin but rapidly spreading, therefore,
precise quantitation was not possible.
The following Table 8H shows that the above-
described pure cultures, isolated from the mixed culture
designated DAP 2, are able to utilize melamine as a source of
nitrogen as determined by colony size of cultures. The
cultures were grown on Stanier's minimal medium supplemented
with 150 ppm each of naphthalene and toluene and 25 ppm of
melamine as either the sole source of nitrogen or supplemented
with ammonium sulfate, (NH4)ZS04. The cultures were grown at
25-27°C, colony size determined after 7 days. Values
represent mean of five replicate colonies for each
determination.
TABLE 8H
CULTURE WITH f NH,;1~ WITHOUT f NBA
) S~O~
3.2 mm 4.2 mm
DAP 626
DAP 115 5.2 mm 4.6 mm
DAP 632 4.9 mm 4.7 mm
DAP 623 4.1 mm 6.0 mm
DAP 120 4.3 mm 4.5 mm
DAP 629 3.9 mm 3.8 mm
r
- 46 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
6.1.6 MICROORGANISMS WHICH CANNOT DEGRADE NITROBENZENE
A number of microorganisms were isolated from
sludges or soils containing nitrobenzene and tested for the
ability to aerobically degrade this compound. The following
S strains were identified which could not degrade nitrobenzene:
(1) Pseudomonas sp. DN-1081; (2) Pseudomonas sp.~DN-1101-l;
(3) Pseudomonas sp DN-1018; (4) Pseudomonas sp. DN-1019; (5)
Pseudomonas sp. DR-1111-1; and (6) Pseudomonas sp. DR-1111-2.
6.2. METHODS FOR AEROBIC DEGRADATION OF COMPOUNDS
According to one embodiment of the present
invention, a method for the aerobic degradation of aromatic
and/or substituted aromatic compounds is provided. In
general, the method entails contacting an aromatic compound
with a mixed or pure culture of microorganisms, said
microorganisms being a member of the group consisting of
microorganisms having ATCC Accession No. 55644, 55648, 55645,
55641, 55647, 55642, 55643, 55646, 55649, 55722, 55723, 55726,
55727, 55724, and 55725. In one mode of this embodiment, at
least one compound selected from the group of aromatic, nitro-
aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic and
halo-aliphatic compounds is aerobically degraded. In another
mode of this embodiment, a mixture of at least two compounds
selected from the group consisting of aromatic, nitro-
aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic and
halo-aliphatic compounds is aerobically degraded. The method
may further comprise culturing the microorganisms in contact
with said compounds) so that the aromatic compound or
compounds are degraded to products comprising Coz and H20.
According to yet another embodiment of the invention, the
method entails using a microorganism selected from the group
consisting of microorganisms having ATCC Accession No. 55644,
55648, 55645, 55641, 55647, 55642, 55643, 55646, 55649, 55722,
55723, 55726, 55727, 55724, and 55725 to degrade at least one
aromatic, vitro-aromatic, halo-aromatic and/or halo-nitro-
aromatic compound at a total concentration of about 10 ppm to
- 47 -
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/1b~364
100,000 ppm to products comprising COZ and H20 in about 2 to 72
hours.
In a preferred embodiment, if for example, nitrogen
containing aromatic compounds are present, they are degraded
to products comprising COZ and H2o and nitrogen containing
compounds which pose little or no threat to the biosphere.
As mentioned above herein, the aromatic, nitro-
aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic and
halo-aliphatic compounds which are degraded according to the
present invention, include but are not limited to compounds
such as benzene, toluene, xylene, ethylbenzene, naphthalene,
chlorobenzene, phenol, cresol, nitrobenzene, aniline,
anthracene, dimethylphenol, styrene, halonaphthalene, 2~-, 3-
or 4-chlorotoluene, 2-, 3- or 4-chlorobenzoate, 1,3-
dichlorobenzoate, 1,2-, 1,3- or 1,4-dinitrobenzene, 1-chloro-
3-nitrobenzene, 1-chloro-4-nitrobenzene, 1- or 2-
methylnaphthalene, pyrene, acenaphthylene, fluoranthene,
phenanthrene, benzo-(b)-fluoranthene, dibenzofuran, chrysene,
catechol, m-toluic acid, cinnamyl acetate, vanillin, trans-
cinnamaldehyde, melamine, cyanuric acid, mesitylene, and
salicylate.
According to another embodiment of the present
invention, a method for the aerobic degradation of aliphatic
compounds is provided. These aliphatic compounds include but
are not limited to S-(-)-limonene, hexadecane, methanol,
formaldehyde and chloroform. In general, the method entails
contacting said aliphatic or halo-aliphatic compounds o:r a
mixture of said compounds with microorganisms, said
microorganisms being a member of the group consisting o:E
microorganisms having ATCC Accession No. 55644, 55648, 55645,
55641, 55647, 55642, 55643, 55646, 55649, 55722, 55723, 55726,
55727, 55724, and 55725. The method may further comprise
culturing said microorganisms in contact with said compound or
mixture of compounds such that said compound or mixture
thereof is degraded to products comprising COZ and H20.
- 48 -
CA 02207781 1997-06-13
WO 96118724 PCTIUS95/16364
The microorganisms can degrade high levels of the
compounds to be degraded, such that high levels do not
interfere with actual degradation.
These methods may further comprise monitoring the
S removal of the aromatic or aliphatic compound or compounds of
interest. For example, measurements of oxygen uptake or
carbon dioxide evolution can be used to monitor the
degradation of the compound or compounds of interest. In
addition, the pH and/or buffering capacity is useful to assess
the level of biological activity.
The compounds to be degraded may be in solid,
liquid, and/or gaseous form. When a compound is in the
gaseous and/or liquid form, it may be sorbed onto a material,
such as a solid.
Ideally, when the method entails a culture of the
microorganisms, culture conditions should be such that
bacterial growth is supported, for example, pH between 3.0 and
11.0, preferably between 6.0 and 8.0; temperature between 4°C
and 41°C, preferably between 15°C and 37°C; dissolved
oxygen
tension between 0.1% and 100%, preferably between 4% and 80%,
more preferably between 4% and 40% of saturation where the
oxygen may be supplied by use of an oxygen containing or
oxygen liberating composition. The oxygen containing or
oxygen liberating composition can be air, pure oxygen,
peroxide, or other peroxy chemicals which liberate oxygen or
mixtures thereof.
Further, the culture medium may be stirred or may
not be stirred, provided with positive dissolved oxygen
tension or not, and supplemental nutrients may or may not be
added to maintain an optimal Carbon: Nitrogen: Phosphorous ratio
between 10:1:0.1 and 50:1:1, preferably 25:1:0.1. In a
preferred mode, only carbon is limiting for bacterial growth.
Any method for contacting the microorganisms with a
W composition containing any one or more of the above recited
compounds or mixtures thereof can be used according to the
present invention. Such methods for contact include but are
not limited to in situ contact, for example, at a site
- 49 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
contaminated with such compound or mixture thereof, contact in
a closed vessel or container, etc.
r
6.3. FLUID PHASE BY8TEM FOR AEROBIC REACTION OF COMPOUNDS
According to another embodiment of the invention,
fluid phase systems and methods for aerobic reaction of
compounds are provided. Most generally, the fluid phase
systems entail converting an elastomeric solid or sludge into
a fluidized composition suitable for aerobic reaction of
organic compounds contained in the elastomeric solid or
sludge. The aerobic reactions for which the fluidized
compositions are useful include synthetic as well as
degradative reactions which take place preferably under
aerobic conditions.
The method for preparing a fluidized composition
suitable for aerobic reaction comprises the steps of: (a)
particularizing an elastomeric solid or sludge containing an
organic compound; and (b) contacting the particularized solid
or sludge in a vessel with a current of fluid selected from
the group consisting of oxygen, oxygen containing gas,
including air, water and an aqueous solution, such that the
particularized solid or sludge is suspended in the current of
fluid to form a fluidized composition suitable for aerobic
reaction of an organic compound contained in the solid or
sludge.
The elastomeric solid or sludge can be
particularized by mixing the elastomeric solid or sludge, for
example, in a pug mill, a plow-bladed mixer or a screw mixer.
The size of the particularized material will vary depending
upon a number of factors, including such as the size of the
blades of the mill or mixer, the clearance between the blades
and the mill or mixer wall, the amount of detackifying agent,
if added, and the degree and rate of mixing.
The method can further comprise combining the
elastomeric solid or sludge with a detackifying agent either
simultaneously with or subsequent to step (a). In one .
embodiment, the detackifying agent is selected from the group
- 50 -
CA 02207781 1997-06-13
WO 96/18724 PCT/iTS95/16364
consisting of clays, chopped, minced or otherwise finely
divided organic materials, powdered inorganic salts and rock
dust. In an alternative embodiment, the detackifying agent is
selected from the group consisting of pulverized lime,
S portland cement, bentonite clay, sawdust, diatomaceous earth,
pulverized corn cobs and mixtures thereof. The range of
detackifying agent that can be used is from about 2-100
(w/w).
In a particular mode of this embodiment of the
invention, the fluidized composition is used to partially
convert an aromatic compound to a cis-cis muconate which is
useful for the preparation of useful polymers. In an
alternative particular mode of this embodiment of the
invention, the fluidized composition comprises a composition
containing hydrocarbons such as naval stores, e.g. a-pinene
and/or ~3-pinene, and a detackifying agent. The fluidized
composition is used as an oxygenated fuel which advantageously
results in a cleaner burning fuel.
In a different particular mode of this embodiment of
the invention, the fluidized composition is used for a
reaction which comprises aerobic degradation of an organic
compound selected from the group consisting of aromatic,
vitro-aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic
and halo-aliphatic compounds. For example, a fluidized
composition comprising a particularized sludge, containing
nitrobenzene, suspended in a current of water or an aqueous
solution is contacted under aerobic conditions with
microorganisms selected from the group of microorganisms have
ATCC Accession No. 55644, 55648, 55645, 55641, 55647, 55642,
55643, 55646, 55649, 55722, 55723, 55726, 55727, 55724, and
55725, so that the nitrobenzene in the fluidized compositions
is degraded to products comprising COz and H20. Figure 1b is
an illustrative schematic of one fluid phase system useful for
,, the methods of the invention. This illustrates a system for
imparting energy in the form of mechanical energy to form the
y slurry.
- 51 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
According to another embodiment of the present:
invention fluid phase systems and methods for aerobic
degradation of compounds are provided. A fluid phase which is
a slurry formed from, for example, a solid, soil, and/or
sludge is produced. The slurries are used, for example for '
treatment of aromatic, vitro-aromatic, halo-aromatic, halo-
nitro-aromatic, aliphatic or halo-aliphatic compounds in
solids, soils and/or sludges with microorganisms which c;an act
on such compounds.
A fluid phase which is a slurry can be formed from
either non-elastomeric or an elastomeric solid, sludge or
soil. Such slurries are used to aerobically degrade an
aromatic or aliphatic compound or mixture thereof contained in
said solid, sludge or soil.
The preparation of slurries, according to the
present invention, using elastomeric solids, sludge and/or
soils is particularly advantageous for the aerobic degraidation
of aromatic or aliphatic compounds contained in such
compositions using the microorganisms disclosed in this
application.
The preparation of slurries as well as systemss and
methods for the aerobic degradation, by microorganisms, using
the slurries is described in the following sub-sections.
6.3.1. FORMATION OF ShURRY PHASES
The formation of slurry phases useful according to
this embodiment is illustrated schematically in Figure 2a-b.
Figure 2a illustrates the formation of a slurry using a non-
elastomeric solid, sludge or soil. The method comprises (a)
combining said solid or sludge with water or an aqueous
solution; and (b) imparting energy to said solid or
sludge/aqueous combination in a vessel such that said solid or
sludge is fluidized into a slurry.
Energy can be imparted, for example, by impart-ing
mechanical energy, e.g., by mixing; by imparting acoustic
energy; e.g., by setting up a standing acoustic wave in the ,
slurry materials; or by imparting an electrical or
- 52 -
CA 02207781 1997-06-13
WO 96/18724 PCT/L1S95/16364
electrostatic field. Figure la also illustrates one exemplary
mode of the invention in which mechanical energy is imparted
for example, by mixing.
As illustrated in Figure 2a, the pH of said slurry
" 5 can be adjusted towards neutrality, if necessary, for example,
if the slurry is to be contacted with microorganisms to
degrade a compound or mixture of compounds in said slurry.
Figure 2b illustrates the formation of a slurry from
an elastomeric solid, sludge or soil. In one alternative
embodiment, the method comprises (a) combining an elastomeric
solid or sludge with water or an aqueous solution; (b)
imparting energy to said elastomeric solid or sludge/water
combination such that said solid or sludge is fluidized into a
slurry; and (c) separating said slurry~away from any residual
elastomeric solid or sludge. Separation can be accomplished,
for example, by decanting the slurry from residual elastomer.
Alternatively, the method comprises (a) combining an
elastomeric solid or sludge with a detackifying agent to form
a solid or sludge/detackifying agent combination; (b)
combining said solid or sludge/detackifying agent combination
with water or an aqueous solution; and (c) imparting energy to
said solid or sludge/detackifying agent aqueous combination
such that said detackified solid or sludge is fluidized into a
slurry. The method can further comprise mixing said solid or
sludge/detackifying agent combination to form a detackified
solid or sludge. In still another alternative, the method
comprises (a) combining an elastomeric solid or sludge with a
detackifying agent and water or an aqueous solution; and (b)
imparting energy to said mixture formed in step (a) such that
said elastomeric solid or sludge is fluidized into a slurry.
Energy can be imparted, for example, by imparting
mechanical energy, e.g., by mixing; by imparting acoustic
energy; e.g., by setting up a standing acoustic wave in the
slurry materials; or by imparting an electrical or
electrostatic field. Figure la illustrates one exemplary mode
of the invention in which mechanical energy is imparted for
example, by mixing.
- 53 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
Suitable detackifying agents for producing a slurry
according to the invention include but are not limited to
clays, chopped, minced or otherwise finely divided organic
materials, powdered inorganic salts and rock dust. Additional
suitable detackifying agents include pulverized lime, portland
cement, bentonite clay, sawdust, diatomaceous earth,
pulverized corn cobs and mixtures thereof.
The aqueous solution used to make a slurry of the
invention can be the filtrate from a previously conducted
slurry phase bioremediation as described herein.
As illustrated in Figure 2b, any of the alternative
embodiments described above can further comprise adjusting the
pH of said slurry towards neutrality, if desired.
The above described methods for forming a slurry
phase from an elastomeric solid, sludge or soil containing an
aromatic or aliphatic compound or mixture thereof are
particularly advantageous because such slurries, which can
comprise about 45~ (w/w) of the original elastomeric solid or
sludge are useful in fluid phase methods for aerobic
degradation of said compounds or mixtures thereof. Prior to
the present invention, slurry phase treatments of such
elastomeric materials were not possible.
Thus, the slurries are useful for bioremediation
processes in which aromatic, vitro-aromatic, halo-aromatic,
halo-vitro-aromatic, aliphatic or halo-aliphatic compou.nd(s)
or a mixture thereof contained in a solid, sludge, soil or
other waste material are treated aerobically.
If volatile compounds are present in the solid,
sludge, soil or other waste material, they may be stripped
from the material while under going mixing with a detac:kifying
agent. Accordingly, such steps should be carried out i.n such
a way that the volatiles are trapped, for example, in a:
biofilter. Once trapped in a biofilter, the volatiles can be
treated with microorganisms as described infra in Section 6.5.
- 54 -
CA 02207781 1997-06-13
WD 96/18724 PCT/US95/16364
6.3.2. MICROORGANISMS/INOCULUM FOR SI~IJRRY PHASE DEGRADATION
A pure culture of microorganisms or a mixed culture
of microorganisms selected from those described in Section 6.1
above is used as inoculum for the slurry phase methods. The
microorganisms used are selected based on their ability to
degrade a desired compound or mixture of compounds present in
a particular slurry aerobically.
The microorganisms are induced as described in
section 6.1 above, for example, by culturing them on a medium,
which contains as the sole source of nutrients the compounds)
one wishes to degrade.
Alternatively, residual solids from a previously
performed slurry phase bioremediation, which contains already
induced microorganisms, can be used as inoculum for the slurry
phase methods. For example, after a slurry has been
bioremediated, it can be filtered. The filtrate can be used
for producing more slurry and the dewatered residual solid
residue, designated "filter cake", containing already induced
microorganisms, is added to a slurry to be bioremediated.
When using filter cake as the source of mixed
culture inoculum, between 200-600 grams of filter cake, and
preferably between 350-450 grams of filter cake are used, for
example, to start a 4 liter batch. Once the aromatic or
aliphatic compound or mixture thereof has been degraded, the
contents of a 4 liter batch can be used as the source of
inoculum for a 10 gallon batch, and this in turn can be used
to initiate a 150 gallon batch. This technique can be
extended and extrapolated to build up an inoculum for
increasingly larger reactors.
If filter cake is not available, the inoculum can be
re-established by using preserved cultures of the micro-
organisms described in Section 6.1.1 through 6.1.4 to
inoculate several plates per preserved culture. The plates
Y containing Stanier's minimal medium supplemented with
appropriate hydrocarbons) are then incubated at 25°C. When
the cultures have grown, the plates are washed with 5-10 ml of
Stanier's minimal medium. The washes are pooled and used to
- 55 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
inoculate a series of biphasic flasks with medium supplemented
with agar and the appropriate hydrocarbon(s), and 50 ml of
liquid medium of the same composition. After the
microorganism inoculated in the biphasic flasks has grown-up,
the surface of the agar layer is scraped to remove cells. The
liquid layer from 4 flasks is used to inoculate a four liter
vessel. From this point, further scale-up is identical to
that employed when filter cake is used as the source of
inoculum.
6.3.3. SLURRY PIiABE METFiODB AND BIOTREATMENT PARAME'PER8
According to the present invention, a method for
slurry phase bioremediation of solids, sludges or soils
containing at least one compound or a mixture of at least two
compounds selected from the group consisting of aromatic,
vitro-aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic
and halo-aliphatic compounds comprises (a) adjusting the pH of
a slurry towards neutrality, if necessary; and (b) contacting
said neutral slurry with microorganisms, said microorganisms
being a member of the group consisting of microorganisms
having ATCC Accession No. 55644, 55648, 55645, 55641, 55647,
55642, 55643, 55646, 55649, 55722, 55723, 55726, 55727, 55724,
and 55725. The method can further comprise culturing said
microorganisms with said slurry such that the compound .is
degraded to products comprising COZ and HZO. The method can be
accomplished in a vessel, such as bioreactor.
Another method for the slurry phase bioremedi~~tion
of solids, sludges or soils containing at least one compound
or a mixture of at least two compounds selected from the group
consisting of aromatic, vitro-aromatic, halo-aromatic, halo-
nitro-aromatic, aliphatic and halo-aliphatic compounds
comprises (a) combining said solid or sludge with water or an
aqueous solution; (b) imparting energy to said solid or
sludge/aqueous combination in a vessel such that said solid or
sludge is fluidized into a slurry; (c) adjusting the pH of
said slurry, if necessary; and (d) contacting said neutral
slurry with microorganisms, said microorganisms being a member
- 56 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
of the group consisting of microorganisms having ATCC
Accession No. 55644, 55648, 55645, 55641, 55647, 55642, 55643,
55646, 55649, 55722, 55723, 55726, 55727, 55724, and 55725.
Energy can be imparted using any of the methods mentioned
about in Section 6.3.1 for forming a slurry. The method can
further comprise culturing said microorganisms with said
slurry such that the compound is degraded to products
comprising C02 and H20.
If the solid, sludge or soil is a tarry or
elastomeric solid, sludge or soil the method comprises (a)
combining said solid or sludge with water or an aqueous
solution; (b) imparting energy to said solid or sludge/aqueous
combination in a vessel such that said solid or sludge is
fluidized into a slurry; (c) separating said slurry from any
residual elastomeric solid or sludge; (d) adjusting the pH of
said slurry, if necessary; and (e) contacting said neutral
slurry with microorganisms, said microorganisms being a member
of the group consisting of microorganisms having ATCC
Accession No. 55644, 55648, 55645, 55641, 55647, 55642, 55643,
55646, 55649, 55722, 55723, 55726, 55727, 55724, and 55725.
Energy can be imparted using any of the methods mentioned
above in Section 6.3.1 for forming a slurry. Further, the
method can also comprise gradually adding the residual
elastomeric solid or sludge to the neutral slurry in contact
with said microorganisms in step (e).
If the solid, sludge or soil to be treated in slurry
phase is a tarry or elastomeric solid, sludge or soil
containing at least one compound or a mixture of compounds
selected from the group consisting of aromatic, nitro-
aromatic, halo-aromatic, halo-vitro-aromatic, aliphatic and
halo-aliphatic compounds the method, alternatively, comprises
(a) combining said elastomeric solid or sludge with a
detackifying agent; (b) mixing said solid or
sludge/detackifying agent combination to form a detackified
solid or sludge; (c) combining said detackified solid or
. sludge with water or an aqueous solution; (d) imparting energy
to said detackified solid or sludge such that said detackified
- 57 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
solid or sludge is fluidized into a slurry; (e) adjusting the
pH of said slurry towards neutrality, if necessary; and (f)
contacting said neutral slurry with microorganisms, said
microorganisms being a member of the group consisting of
microorganisms having ATCC Accession No. 55644, 55648, 55645,
55641, 55647, 55642, 55643, 55646, 55649, 55722, 55723, 55726,
55727, 55724, and 55725. Energy can be imparted using any of
the methods mentioned about in Section 6.3.1 for forming a
slurry. This method can further comprise culturing said
microorganisms with said slurry such that the compound is
degraded to products comprising COZ and H20.
Another method for the slurry phase bioremediation
of a solid, sludge or soil where the solid, sludge or soil is
a tarry or elastomeric solid, sludge or soil containing at'
least one compound or a mixture of compounds selected from the
group consisting of aromatic, vitro-aromatic, halo-aromatic,
halo-vitro-aromatic, aliphatic and halo-aliphatic compounds
comprises (a) combining an elastomeric solid, sludge or soil
with a detackifying agent and water or an aqueous solution to
form a mixture; (b) imparting energy to said mixture formed in
step (a) such that said elastomeric solid, sludge or soil is
fluidized into a slurry; (c) adjusting the pH of said slurry
towards neutrality, if necessary; and (d) contacting said
neutral slurry with microorganisms, said microorganisms being
a member of the group consisting of microorganisms having ATCC
Accession No. 55644, 55648, 55645, 55641, 55647, 55642, 55643,
55646, 55649, 55722, 55723, 55726, 55727, 55724, and 55725.
Energy can be imparted using any of the methods mentioned
above in Section 6.3.1 for forming a slurry. The method can
further comprise culturing said microorganisms with said
slurry such that the compound is degraded to products
comprising COZ and HZo.
In any of the above methods, the tarry or
elastomeric solid, sludge or soil may be residual elast.omeric y
solid, sludge or soil formed as described according to the
methods of the invention. In each case the residual
elastomeric solid, sludge or tar may contain a very high
- 58 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
concentration of compounds which can be effectively degraded
according to the methods of the present invention.
In one embodiment, the compound contained in the
solid, sludge, soil or other waste material is selected from
benzene, toluene, xylene, ethylbenzene, naphthalene,
chlorobenzene, phenol, cresol, nitrobenzene, aniline,
anthracene, dimethylphenol, styrene, halonaphthalene, 2-, 3-
or 4-chlorotoluene, 2-, 3- or 4-chlorobenzoate, 1,3-
dichlorobenzoate, 1,2-, 1,3- or 1,4-dinitrobenzene, 1-chloro-
3-nitrobenzene, 1-chloro-4-nitrobenzene, 1- or 2-
methylnaphthalene, pyrene, acenaphthylene, fluoranthene,
phenanthrene, benzo-(b)-fluoranthene, dibenzofuran, chrysene,
catechol, m-toluic acid, cinnamyl acetate, vanillin, trans-
cinnamaldehyde, mesitylene, salicylate, melamine, cyanuric
acid, 6-(-)-limonene, hexadecane, methanol, formaldehyde, and
chloroform or a mixture of said compounds.
Suitable detackifying agents are selected from
clays, chopped, minced or otherwise finely divided organic
materials, powdered inorganic salts and rock dust.
Alternatively, detackifying agents are selected from
pulverized lime, portland cement, bentonite clay, sawdust,
diatomaceous earth, pulverized corn cobs and mixtures thereof.
In another embodiment, the compound is selected from methanol,
formaldehyde or chloroform.
According to a preferred embodiment, the
detackifying agents are selected from inorganic agents, such
as rock dust, diatomaceous earth, etc.
The slurry/microorganism mixture is maintained under
conditions which favor the growth of the bacteria and the
biodegradation of the desired compound(s). Generally, the
conditions should be such that bacterial growth is supported,
for example, pH between about 3.0 and 11.0, preferably between
6.0 and 8.0; and temperature between about 4°C and 41°C,
preferably between 15°C and 37°C. The dissolved oxygen
tension should be between about 0.1% and 100%, preferably
between 4% and 80%, more preferably between 4% and 30%. The
dissolved oxygen tension may be monitored and maintained in
- 59 -
CA 02207781 1997-06-13
WO 96/18724 PCT/L1S95/16:i64
the desired range by supplying oxygen in the form of aiz~, pure
oxygen, peroxide, and/or other peroxy compositions which
liberate oxygen. The mixture may be stirred or may not be
stirred, provided with positive dissolved oxygen tension or
not, and supplemental nutrients may or may not be added to
maintain an optimal Carbon: Nitrogen: Phosphorous ratio bsatween
about 10:1:0.1 and 50:1:1, preferably 25:1:0.1, such that only
carbon is limiting for bacterial growth. Additionally, a
water-soluble, polymeric coagulant/floculant such as
MAGNIFLOC~ 591C, a quaternary ammonium cationic polymeric with
a molecular weight of about 300 kD to 500 kD (Cytec
Industries, West Paterson, NJ) can be added to improve t:he
filterability and settling characteristics of solids in the
slurry phase bioreactor. The settled solids can be used as
inoculum for a subsequent bioremediation process.
At different time points one may remove solider or
liquids and, for example, extract them with methylene
chloride:methanol, (90:10) or by EPA approved methodology for
TCLP or TCL, and measure the concentration of selected
compounds) by gas-liquid chromatography.
6.3.4. MODES OF OPERATION
The fluid phase methods for aerobic reaction of
compounds of the present invention can be operated in a
variety of modes, including batch mode, sequencing batch mode
and continuous or semi-continuous mode. Three modes of
operation are described in below in terms of modes of
operation for slurry phase methods of aerobic degradation of
an aromatic or aliphatic compound or mixture thereof; however
the modes of operation described below can also be used for
the methods for aerobically reacting an organic compound in a
fluidized composition as described above.
In all three modes of operation, samples of tree
contents may be removed periodically to monitor degradation of
the compounds) of interest. Additionally, the agitating
and/or mixing of the reactor contents may induce foaming. In ,
these cases, an anti-foaming agent may be added to prevent
- 60 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95116364
foaming. Suitable anti-foaming agents include such as silicon
containing anti-foam emulsion (e.g., Dow ANTIFOAM-A~; a
x
silicon based anti-foaming agent).
6.3.4.1. BATCH MODE OPERATION
Batch mode operation entails placing a slurry
containing a compound or mixture of at least two compounds
selected from the group consisting aromatic, nitro-aromatic,
halo-nitro-aromatic, aliphatic and halo-aliphatic compounds
l0 into a vessel, such as a bioreactor, inoculating with induced
microorganisms as described in Section 6.1.1 through 6.1.4 and
incubating the mixture to culture the microorganisms such that
the aromatic or aliphatic compounds) is (are) degraded.
After a predetermined time period, the incubation is stopped
and the contents are removed and the solids are separated from
the liquid by filtration. Samples may then be taken from both
the solid and liquid phase and are tested, for example, by
TCLP or by gas-liquid chromatography to assess the level of
the compounds) to confirm that the compounds) has been
degraded. The reactor solids are subsequently dewatered and
may be further processed into, for example, a landfill or may
be used.as bacterial inoculum for the next batch mode. In
batch mode the dewatered solid residue is re-added at about
2%-40% by weight or volume, preferably at about 5~-zu~. ~5ee,
for example Figure lc)
Figure 1b illustrates a typical reactor set-up which
can be used in a batch mode as well as in the modes described
below. The neutralized slurry and inoculum are placed in a
bioreactor. Air or oxygen may be pumped into the reactor and
the contents agitated, mechanically in the bioreactor.
6.3.4.2. SEQUENCING BATCH MODE OPERATION
Sequencing batch mode is operated much the same as
batch mode except that after the incubation period is over,
the reactor is allowed to settle for a time, usually about 15
minutes, and the top 60%-95% of the reactor contents are
removed, leaving settled solids at the bottom as inoculum for
- 61 -
CA 02207781 1997-06-13
WO 96/18724 . PCT/US95/16364
the next batch of neutralized slurry. Preferably betwes:n 70%
and 90% of the contents are drawn off. Sequencing batch mode
is a preferred embodiment for slurry phase aerobic degradation
because the lag or acclimation phase is reduced, high levels
of biomass are retained in the reactor, variability in t:he '
composition of the waste feed is better accommodated, arid the
residual solids remaining after biotreatment are potentially
reduced.
By using residual solids as the source of inoc:ulum
for subsequent runs in both the sequencing batch and batch
modes and~by using the residual liquid or filtrate to prepare
fresh slurry, the process operates on a net loss of water.
Therefore, this results in no aqueous effluent being produced.
6.3.4.3. SEMI-CONTINUOUS MODE
Semi-continuous mode is similar to both batch and
sequencing batch modes. However, rather than stopping t:he
incubation after a predetermined time, fresh slurry is pumped
into the bioreactor in a fixed amount over a given period of
time as treated slurry is drawn out of the bioreactor. This
provides for a continuous treatment of slurry without herring
to stop the biodegradative process.
6.4. SOLID PIiASE DEGRADATION
Another embodiment of the present invention is
directed to methods for solid phase aerobic degradation of
materials. This embodiment involves methods for the treatment
of solids, sludges, including those which are tarry and~or
elastomeric in nature, as well as soils, sediments, and
sorptive materials, including but not limited to granulated
activated carbon, said materials containing least one compound
or mixture of at least two compounds selected from the croup
consisting of aromatic, vitro-aromatic, halo-aromatic, halo-
nitro-aromatic, aliphatic and halo-aliphatic compounds. ,
- 62 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
6.4.1. BOLID PHASE METHODS AND BIOTREATMENT PARAMETERS
The methods for solid phase bioremediation of
solids, sludges or soils containing at least one compound or a
mixture of at least two compounds selected from the group
consisting of aromatic, nitro-aromatic, halo-aromatic, halo-
nitro-aromatic, aliphatic and halo-aliphatic compounds
comprise (a) mixing said solid, sludge or soil with a bulking
agent such that air can readily pass through the bulked
mixture; (b) adjusting the pH of the bulked mixture towards
l0 neutrality, if necessary; and (c) contacting said bulked
mixture with microorganisms, said microorganisms being a
member of the group consisting of microorganisms having ATCC
Accession No. 55644, 55648, 55645, 55641, 55647, 55642, 55643,
55646, 55649, 55722, 55723, 55726, 55727, 55724, and 55725.
The methods can further comprise culturing said microorganisms
with said bulked solid, sludge or soil such that said compound
is degraded to products comprising COZ and H20. In one
embodiment, the compound contained in the solid, sludge, soil
or other waste material is selected from benzene, toluene,
xylene, ethylbenzene, naphthalene, chlorobenzene, phenol,
cresol, nitrobenzene, aniline, anthracene, dimethylphenol,
styrene, halonaphthalene, 2-, 3- or 4-chlorotoluene, 2-, 3- or
4-chlorobenzoate, 1,3-dichlorobenzoate, 1,2-, 1,3- or 1,4-
dinitrobenzene, 1-chloro-3-nitrobenzene, 1-chloro-4-
nitrobenzene, 1- or 2-methylnaphthalene, pyrene,
acenaphthylene, fluoranthene, phenanthrene, benzo-(b)-
fluoranthene, dibenzofuran, chrysene, catechol, m-toluic acid,
cinnamyl acetate, vanillin, traps-cinnamaldehyde, mesitylene,
salicylate, melamine, and cyanuric acid or a mixture of said
compounds. In another embodiment, the compound contained in
the solid, sludge or soil is selected from methanol,
formaldehyde, chloroform, 8-(-)-limonene, and hexadecane or a
mixture of said compounds.
Where the solid, sludge or soil is a tarry or
elastomeric solid, sludge or soil containing at least one
s compound or a mixture of compounds selected from the group
consisting of aromatic, nitro-aromatic, halo-aromatic, halo-
- 63 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16:f64
nitro-aromatic, aliphatic and halo-aliphatic compounds, the
methods for solid phase bioremediation comprise: (a) mixing a
tarry or elastomeric solid, a tarry or elastomeric sludge or a
tarry or elastomeric soil with a detackifying agent such. that
said solid soil or sludge forms a particularized mixture which
is less tarry and/or elastomeric; (b) adjusting the pH of said
mixture towards neutrality, if necessary; and (c) contacting
said mixture with microorganisms, said microorganisms being a
member of the group consisting of microorganisms having ATCC
Accession No. 55644, 55648, 55645, 55641, 55647, 55642, 55643,
55646, 55649, 55722, 55723, 55726, 55727, 55724, and 55725.
The methods can further comprise combining the particularized
tarry or elastomeric solid, tarry or elastomeric sludge or
tarry or elastomeric soil with a bulking agent either
simultaneously with or following step (a).
Suitable detackifying agents are selected from.
clays, chopped, minced or otherwise finely divided organic
materials, powdered inorganic salts and rock dust.
Alternatively, detackifying agents are selected from
pulverized lime, portland cement, bentonite clay, sawdust,
diatomaceous earth, pulverized corn cobs and mixtures thereof.
Suitable bulking agents are selected from the group
consisting of chopped, minced or otherwise finely divided
organic materials and inorganic salts. More specifically, the
bulking agents are selected from the group consisting of wood
chips, sawdust, corn cobs and mixtures thereof.
According to a preferred embodiment, the bulking
agent can also serve as a detackifying agent, for example,
including but not limited to wood chips, sawdust, corn cobs
and mixtures thereof.
6.4.2. MICROORGANISMS/INOCULUM FOR SOLID PIiASE DEGRAD7~TION
A pure culture of microorganisms or a mixed culture
of microorganisms selected from those described in Section 6.1
.
above are used as inoculum for the solid phase method. The
microorganisms used are selected based on their ability to
r
- 64 -
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/16364
degrade a desired compound or mixture of compounds present in
a particular solid aerobically.
The microorganisms are induced as described in
Section 6.1 above by growing them, for example, on a medium,
which contains as the sole source of nutrients the compounds)
one wishes to degrade.
Alternatively, residual solids from a previously
performed solid phase bioremediation, which contains already
induced microorganisms, can be used as inoculum for the solid
phase method. For example, after a pile of solids has been
bioremediated, it contains already induced microorganisms,
which can be added to another pile to be bioremediated.
If a bioremediated pile is not available, the
inoculum can be re-established by using preserved cultures of
the micro-organisms described in Section 6.1.1 through 6.1.4
to inoculate several plates per preserved culture. The plates
containing Stanier's minimal medium supplemented with
appropriate hydrocarbons) are then incubated at 25°C. When
the cultures have grown, the plates are washed with 5-10 ml of
Stanier's minimal medium. The washes are pooled and used to
inoculate a series of biphasic flasks with medium supplemented
with agar and the appropriate hydrocarbon(s), and 50 ml of
liquid medium of the same composition. After the
microorganism inoculated in the biphasic flasks has grown-up,
the surface of the agar layer is scraped to remove cells. The
liquid layer from 4 flasks can be used to inoculate a pile.
This can be scaled up to any size required as described above
in Section 6.3.2.
6.4.3 TREATMENT OF SOLIDS
After the bulked, neutralized, and inoculated solid
is placed in a composting-like pile bioreactor or vessel, it
is incubated for a predetermined time, during which, for
example, oxygen or air or a mixture thereof is passed through
the material to ensure aerobic degradation of the compound(s).
The solid material may be mixed occasionally, but this is
contraindicated for solids that have a high level of volatile
- 65 -
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/16~364
compounds. Further, as described above, the solids may be
removed from the pile bioreactor or vessel and extracted, for
example, with methylene chloride:methanol, (90:10), to measure '
the concentration of selected compounds) by gas-liquid
chromatography or by the TCLP procedure. ~
6.5. BIOFILTERS
Another embodiment of the present invention is a
biofilter and methods for its use. Biofilters are used in the
bioremediation of compounds in effluents such as air, vapors,
aerosols, and water or aqueous solutions.
The biofilters of the present invention comprise an
apparatus having microorganisms immobilized on a solid
support, said microorganisms being a member of the group
consisting of microorganisms having ATCC Accession No. 55644,
55648, 55645, 55641, 55647, 55642, 55643, 55646, 55649, 55722,
55723, 55726, 55727, 55724, and 55725. Suitable solid
supports include but are not limited to, granular activated
carbon, wood chips, alumina, ruthenium, iron oxide, ceramic or
alginate. The apparatus can have influx and efflux ori:~ices,
such that the material to be treated can flow through the
apparatus.
The biofilters can be used, for example, for
bioremediation of an effluent containing a compound selected
from the group consisting of aromatic, nitro-aromatic, halo-
aromatic, halo-nitro-aromatic, aliphatic and halo-aliphatic
compounds. The method comprises flowing said effluent 'through
a biofilter which comprises an apparatus having microorganisms
immobilized on a solid support, said microorganisms being a
member of the group consisting of microorganisms having ATCC
Accession No. 55644, 55648, 55645, 55641, 55647, 55642, 55643,
55646, 55649, 55722, 55723, 55726, 55727, 55724, and 55'725.
The method may further comprise monitoring the effluent to
determine that the compounds) have indeed been degraded.
i
- 66 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
6.6. TWO-STEP PROCESS FOR DEGRADATION
According to yet another embodiment of the
J
invention, a two step method for aerobic degradation of waste
materials containing at least one compound, selected from
heavily halogenated organic compounds such as polychlorinated
biphenyls, polybrominated biphenyls, etc., heavily nitrated
organic compounds, such as trinitrotoluene, etc., and heavily
nitrated and cross-linked polymeric compounds, e.g.,
nitrocellulose, etc. is provided. The waste materials can
further comprise a compound selected from the group consisting
of aromatic, vitro-aromatic, halo-aromatic, halo-nitro-
aromatic, aliphatic and halo-aliphatic compounds or a mixture
of such compounds. The methods comprise: (a) combining a
reagent capable of chemically degrading, at least partially, a
heavily halogenated, a heavily nitrated or a heavily nitrated
cross-linked compound in a waste material to form a pretreated
composition; and (b) contacting said pretreated composition
with microorganisms, said microorganisms being a member of the
group consisting of microorganisms having ATCC Accession No.
55644, 55648, 55645, 55641, 55647, 55642, 55643, 55646, 55649,
55722, 55723, 55726, 55727, 55724, and 55725. The method can
further comprise culturing the microorganisms such that at
least one said compound is degraded to products comprising COZ
and H20. According to one mode of this embodiment, waste
materials containing at least one compound br a mixture of
compounds selected from the group consisting of benzene,
toluene, xylene, ethylbenzene, naphthalene, chlorobenzene,
phenol, cresol, nitrobenzene, aniline, anthracene,
dimethylphenol, styrene, halonaphthalene, 2-, 3- or 4-
chlorotoluene, 2-, 3- or 4-chlorobenzoate, 1,3-
dichlorobenzoate, 1,2-, 1,3- or 1,4-dinitrobenzene, 1-chloro-
3-nitrobenzene, 1-chloro-4-nitrobenzene, 1- or 2-
methylnaphthalene, pyrene, acenaphthylene, fluoranthene,
~henanthrene, benzo-(b)-fluoranthene, dibenzofuran, chrysene,
catechol, m-toluic acid, cinnamyl acetate, vanillin, trans-
cinnamaldehyde, mesitylene, salicylate, melamine, cyanuric
acid, methanol, formaldehyde, chloroform, ~-(-)-limonene, and
- 67 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/163~64
hexadecane are degraded. The reagent can be, but is not.
limited to, Fenton's reagent, which is a mixture of ferrous
sulfate and hydrogen peroxide. Other examples include, but '~
are not limited to free radicals, UV light, metallic iron,
peroxidase enzymes such as lignin and lignin-like enzymes. '
These reagents partially degrade a recalcitrant compounds) to
a compound which the microorganisms can degrade, such that the
microorganisms can now finish the degradation of the
compound(s).
l0
7. ERAMPLE: STORAGE AND INDUCTION OF MICROORGANI81K8
Mixed cultures of the isolated microorganisms were
maintained on 1.5-2.0 ml of BACTO'"' R2A medium (Difco, Detroit,
Michigan) in 4.0 ml Wheaton vials. Cultures inoculated onto
the maintenance medium were incubated at 25-27 °C for 48.
hours. After this incubation the cultures were wrapped with
parafilm and stored at 4°C.
In one set of experiments, the mixed culture was
induced by returning the stored culture to ambient temperature
and transferring the mixed culture to 1% agar bacterial plates
with fresh BACTO"' R2A medium supplemented with 1000-4000 ppm
naphthalene, 30-300 ppm nitrobenzene, 400-500 ppm benzene,
400-500 ppm toluene, 400-50o ppm xylenes, 30-300 ppm aniline,
400-500 ppm ethylbenzene, 50-300 ppm chlorobenzene, 200 ppm 2-
methylnaphthalene and about 200 ppm 2-chloronaphthalene. The
plates were incubated for 48-96 hours at 25-27°C. The
cultures were then transferred to bacterial plates with
Stanier's minimal medium (Stanier et al., 1966, J. Gen.
Microbiol. 43:159-271) supplemented with the same hydrocarbon
compounds as listed above and incubated for an additional 48
hours at 25-27°C. After incubation, the plates were wa~:hed
with 5-10 ml Stanier's minimal medium, the washes pooled and
used to incubate biphasic flasks. The biphasic flasks
contained 75 ml of Stanier's minimal medium (liquid) in the
upper layer and 50 ml of Stanier's minimal medium with 2%
agar. Both the upper layer and the lower layer were
i
supplemented with the hydrocarbons listed above. The flasks
- 68 -
CA 02207781 1997-06-13
WO 96118724 PCT/US95/16364
were incubated at 25-27°C for 48-96 hours. The cells, now
induced, were scraped off the surface of the agar and used as
inoculum.
In another set of experiments, the mixed culture is
'' S induced by returning the stored culture to ambient temperature
and transferring the mixed culture to 0.3~ agar bacterial
plates with fresh BACTO"' R2A medium supplemented with 1000-
4000 ppm naphthalene, 30-300 ppm nitrobenzene, 400-500 ppm
benzene, 400-500 ppm toluene, 400-500 ppm xylenes, 30-30o ppm
aniline, 400-500 ppm ethylbenzene, 50-300 ppm chlorobenzene,
200 ppm 2-methylnaphthalene and about 200 ppm 2-
chloronaphthalene. The plates are incubated for 48-96 hours
at 25-27°C. The cultures are then transferred to bacterial
plates with Stanier's minimal medium (Stanier et al., 1966, J.
Gen. Microbiol. 43:159-271) supplemented with the same
hydrocarbon compounds as listed above and incubated for an
additional 48 hours at 25-27°C. After incubation, the plates
are washed with 5-10 ml Stanier's minimal medium, the washes
pooled and used to incubate biphasic flasks. The biphasic
flasks contained 75 ml of Stanier's minimal medium (liquid) in
the upper layer and 50 ml of Stanier's minimal medium with 2~
agar. Both the upper layer and the lower layer are
supplemented with the hydrocarbons listed above. The flasks
are incubated at 25-27°C for 48-96 hours. The cells, now
induced, are scraped off the surface of the agar and used as
inoculum.
8. EXAMPLE: SLURRY PIiASE DEGRADATION
8.1 EXAMPLE: BATCH MODE DEGRADATION
An elastomeric sludge containing a mixture of high
levels of aromatic, nitro-aromatic, halo-aromatic, halo-nitro-
~aromatic, aliphatic and halo aliphatic compounds was fluidized
as described in Section 6.3 above, by mixing the elastomeric
sludge with water.
Table 9 shows the average concentration in ppm for
selected compounds found in the original elastomeric sludge.
- 69 -
CA 02207781 1997-06-13
WO 96/18724 , PCT/US95/16a64
TABhE 9
COMPOUND (Averaae concentration in nm)
Chloroform 680
Benzene 720
Toluene 3,000
Chlorobenzene 130
Ethylbenzene 240
o-xylene 680
Aniline 630
Nitrobenzene 720
Naphthalene 42,000
2-Methylnaphthalene 2,800
2-Chloronaphthalene <100
m,p-xylene 2,300
After mixing, the slurry was decanted away from
residual elastomeric sludge to form an approximately 30~; (w/w)
slurry and into a conventional stirred tank vessel (B. Elraun,
Allentown, PA). The slurry was neutralized to approximately
pH 7 by the.addition of NaOH (2N) and inoculated with a 10~
(v/v) mixed culture of induced microorganisms. These
hydrocarbon compounds present in the elastomeric sludge were
the only source of carbon and energy for the microorganisms.
A 4 liter vessel containing the inoculated
neutralized slurry was stirred at about 200-700 rpm,
preferably 400 rpm, and aerated with pure oxygen at about 15
Psi, 250 ml/min at room temperature for 24 hours. The ~>lurry
was sampled before and after biological treatment of 24 hours
to determine the concentration of compounds present in t:he
slurry. The slurry was extracted using the Toxicity
Characteristic Leaching Protocol, (TCLP), and analyzed by gas-
liquid chromatography as outlined by EPA SW-846. As seE~n in
Table 10, the compounds present in slurry that were analyzed
were successfully bioremediated.
x
v
- 70 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABhE 10
30% Percent Slurry
TCL TCLP
Comvound Untreated* Treated* TCLP Limits
Chloroform 314 <1 6.0
Benzene 94 <0.5 0.5
Toluene 509 <1 ***
Chlorobenzene 18 <1 100.00
Ethylbenzene 15 <0.5 ***
o-Xylene 61 <0.5 ***
Aniline 114 <1 ***
Nitrobenzene 39 <1 2.0
Naphthalene 3249 <5 ***
* Concentrations in ppm
*** TCLP Limits not yet established.
Effluent gas, containing stripped VOC and COZ was
collected in two granular activated carbon traps and in two
alkali (2N KOH) traps, respectively. Over the 24 hour
incubation period, less than~2% of the total volatile organic
compounds present were lost due to stripping.
Further, Figure 3 shows a correlation between
decreasing amounts of compounds present and an increasing
amount of COZ produced by the microorganisms. Because the
vessel was aerated with pure oxygen, any COZ production was a
direct result of microbial aerobic utilization of the
compounds present in the slurry. Therefore, Figure 3 also
indicates that the microorganisms were able to utilize the
compounds present in the original sludge as the sole source of
carbon and energy and that these compounds were degraded to
products comprising COZ and H20.
8.2 SEQUENCING BATCH MODE DEGRADATION: ERAMPLE 1
The same elastomeric sludge used in Section 8.1 was
fluidized, neutralized and inoculated in the same manner with
a mixed culture inoculum. However, rather than stopping the
'' degradation of the compounds every 24 hours to empty and
completely re-fill the vessel for a new round of
bioremediation, only part of the contents of the reactor was
- 71 -
CA 02207781 1997-06-13
WO 96/I8724 PCT/LTS95/16a64
emptied. For a 30 day period, after each 24 hour incubation,
except over weekends, the contents of the vessel were allowed
to settle for 15 minutes. Once the solids contents of t:he
vessel settled, 80% of bioremediated slurry was removed from
the top of the vessel. An equal amount of a fresh non-
bioremediated 30~ slurry (w/w) from the same original source
was added into the vessel. The vessel contents were their
stirred and aerated with pure oxygen for another 24 hours as
described in Section 8.1.
40 ml samples of the vessel contents were takean
before and after each 24 hour incubation period, extracted
with methylene chloride: methanol (90:10) and analyzed i:or
naphthalene by gas-liquid chromatography as described in
Section 8.1. Figure 4 demonstrates that aerobic degradation
of naphthalene using the sequencing batch mode over a pe=riod
of 30 days was rapid and consistent, that the microorganisms
present tolerated large variation of naphthalene, 700 ppm to
4,700 ppm, and that these large variations had little or no
effect on the ability of the microorganism to aerobically
utilize naphthalene and degrade it to products comprising COZ
and H20.
8.3 SEOOENCING BATCH MODE DEGRADATION: EXAMPLE 2
A non-elastomeric solid was fluidized with waiver to
form a 30~ (w/w) slurry. Table 11 shows the concentrat=ion of
various,selected compounds found in the original solid :in
parts per million (ppm).
35
k
- 72 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABLE 11
COMPOUND Ranae of Concentration lnpm)
Chloroform <10
Benzene 2005-2284
Toluene 38-42
Chlorobenzene 1914-2112
Ethylbenzene 521-578
o-xylene 803-887
Aniline 301-331
Nitrobenzene 321-256
Naphthalene 37-40
2-Methylnaphthalene 654-729
2-Chloronaphthalene <10
m,p-xylene 2126-2362
The 30% (w/w) slurry produced had an alkaline pH and was
neutralized with a 30% (w/w) slurry with an acidic pH produced
from the elastomeric sludge of Section 8.1 in an 1:1 ratio.
Subsequently, 2N H2S04 acid was added to pH the combined
mixture of the two slurries to neutrality. A mixed culture of
induced microorganisms 5-20% (w/v), preferably about 10% was
2o added to the neutralized slurry and the mixture was stirred
and aerated with pure oxygen for 24 hours. After incubation,
the contents were allowed to settle for 15 minutes and then
80% of the contents were drawn off the top. Fresh neutralized
slurry produced as described above was added back and the
vessel contents were again stirred and aerated. A sample of
the vessel contents was removed before and after each 24 hour
incubation and analyzed for benzene and naphthalene. Figure 5
shows the successful bioremediation of benzene and naphthalene
present in the slurry over 30 days.
8.4 ExAMPLE: BATCH MODE DEGRADATION
A 30% (w/w) neutralized slurry produced from an
elastomeric sludge and a 33% (w/w) neutralized slurry produced
from another elastomeric sludge were mixed in a 1:1 ratio.
Table 9, above, and Table 12 show the average concentration in
parts per million of some selected compounds in each
individual elastomeric sludge.
- 73 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16;364
TABLE 12
COMPOUND Average concentration in npm
Chloroform 1,000
Benzene 68,000
Toluene 16,000
Chlorobenzene 200
Ethylbenzene 670
o-xylene 1,000
Aniline 1,500
Nitrobenzene 1,200
Naphthalene 16,000
2-Methylnaphthalene 1,300
2-Chloronaphthalene 150
m,p-xylene 3,500
The slurry mixture was added to a stirred tank vessel and
inoculated with an induced mixed culture of microorganisms.
The vessel contents were stirred and aerated with pure oxygen
at room temperature for 40 hours. Samples of the vessel
contents taken before incubation, at 16 hours and at 40 hours
were extracted with methylene chloride:methanol (90:10) and
2o analyzed by gas-liquid chromatography as described. Table 13
shows that for the compounds analyzed, the compounds were
successfully bioremediated by the microorganisms.
TABLE 13
2 5
TCL TCL TCL
Compound Untreated* t = 16 hr* t = 40 hr*
Benzene 480 <10 <10
Toluene 190 90 <10
Chlorobenzene 190 <10 <10
Ethylbenzene <10 <10 <10
m,p-Xylene 100 90 <10
3 0 Aniline 80 14 <10
Nitrobenzene 40 13 <10
Naphthalene 5100 140 50
2- 180 130 30
Methylnaphthalen
a
* Concentrations in ppm ,.
35
- 74 -
CA 02207781 1997-06-13
WO 96118724 PCTIUS95/16364
9. ERAMPLE: COMPOSTING-LIRE SOLID PHASE DEGRADATION
Solid phase degradation can be conducted in a
chamber, a constructed pile, a heap or the like.
9.1 EBAMPLE: LO88 OF VOLATILE ORGANIC COMPOUNDS
Five individual elastomeric sludges containing a
mixture of high concentrations of aromatic, vitro-aromatic,
halo-aromatic, halo-vitro-aromatic, aliphatic and halo-
aliphatic compounds were bulked individually by mixing in a
pug mill with sawdust to determine the potential for losses of
volatile organic compounds (VOC) such as, for example,
benzene, due to stripping during preparation of the material
for aerobic degradation of the compounds in the materials
according to the methods of the invention.
The elastomeric sludge and a bulking agent, sawdust,
were added to the pug mill. For sludges 1-3 and 5, the
bulking agent comprised 20% of the mixture, whereas sludge 4,
the bulking agent comprised 25% of the mixture. While mixing,
nitrogen gas was passed through the headspace of the pug mill
to prevent combustion of any flammable material present.
Samples for analysis were taken before and after mixing and
the relative amount of benzene and chlorobenzene was
determined by gas-liquid chromatography.
TABLE 14
$ Organic Retained After Pretreatment
SOLID MATERIAL
Benzene Chlorobenzene
1 60-90 80
2 90 100
3 70 85
3 0 4 75-90 80-95
5 5-25 60-75
As seen in Table 14, for 4 out of the 5 sludges
tested the loss of benzene due to stripping was only between 6
and 26%. However, for one sludge tested, the amount of
benzene lost was between 70 and 90% of the original amount of
x
benzene present. Chlorobenzene was stripped to a lesser
- 75 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/163t64
extent overall, but the sludge that lost the most benzene also
lost the most chlorobenzene. This sludge was unique in that
it had a pH >10.5, whereas the other sludges were more or less
acidic. These results demonstrate that the pH of a particular
sludge or solid can affect the degree to which volatile
organic compounds are lost during handling.
9.2 COMPOSTING-LIRE SOLID PHASE DEGRADATION: EgAMPLE 1
A soil containing a mixture of organic compounds
such as, for example, benzene, toluene, nitrobenzene,
naphthalene, chlorobenzene, chloroform, xylene, aniline and
ethylbenzene was mixed in a pug mill with a bulking agent,
i.e., sawdust. The 80% soil/20o sawdust mixture was
neutralized by the addition of NaOH. The neutralized mixture
was placed in a vessel, inoculated with an induced liquid
mixed culture of microorganisms, and the mixture was treated
for 14 days as a pile. The sealed vessels were operated under
vacuum conditions in order to draw air through the mixture.
Praper air dispersion through the mixture was effected :by
means of a network of perforated tubing which was positioned
beneath the mixture. The effluent air was passed through two
granulated activated carbon (GAC) traps to collect volatile
organic compounds (VOC). The moisture content and the air
flow were held constant during composting. Samples of the
soil before and after composting were taken and extracted with
methylene chloride:methanol (90:10) or by TCLP and analyzed by
gas-liquid chromatography for selected compounds, for example,
benzene and nitrobenzene. Table 15 shows two independent
treatments of the same material. The concentration in parts
per million of the selected compounds found in the bulked soil
before and after composting analyzed by both solvent
extraction (methylene chloride:methanol) and TCLP as well as
the percentage of VOCs both in the residual material and those
stripped and trapped in the GAC traps is shown. Solid phase
biotreatment was able to reduce the concentration of the
analyzed compounds to TCLP limits.
k
- 76 -
CA 02207781 1997-06-13
WO 96/18724 PCTlUS95/16364
TABLE 15
SOIL
Conditions: slpm air flow
inoculated,
30-45% moisture,
0.25
Compounds in
r~ material TCLP
percenta ge VOC
(solvent (acidic
organic
extractionl* aqueous
Compounds Initial Final extraction) % residual
/D m L in % volatilized
m) ID
m)
D material to GAC
D
Benzene 21.0 <10 <0.5 --- 64.0
Chlorobenzene<10 <10 <1 --- ---
Nitrobenzene <10 <10 <2 --- ---
Naphthalene 736.0 17.0 --- 2.3 1.7
SOIL
Conditions: slpm air flow
inoculated,
30-45% moisture,
0.25
Compounds in
material TCLP PerCenta Cte VOC
(solvent (acidic
organic
extraction)* aqueous
Compounds
extraction
Initial Final ) % residual
(pnm) m L in % volatilized
material to GAC
Benzene 47.0 <10 <0.5 --- 33.0
Chlorobenzene<10 <10 <1 --- ---
Nitrobenzene <10 <10 <2 --- ---
2 0 Naphthalene 1245.0 31.0 --- 2.5 0.9
*TCL
9.3 COMPOSTING-LIRE SOLID PHASE DEGRADATION: ERAMPLE 2
A tarry soil containing a mixture of organic
compounds was mixed in a pug mill with a bulking agent, i.e.,
sawdust. The tarry soil/sawdust mixture (80:20) was
neutralized by the addition of NaOH and placed in a vessel.
The neutralized mixture was inoculated with an induced liquid
mixed culture and the vessel sealed. The mixture was treated
and analyzed as described in Section 9.2. Table 16
demonstrates two independent successful biotreatments of the
tarry soil. The bulked material was successfully treated for
both benzene and chlorobenzene as evaluated by TCLP.
r
_ 77 _
CA 02207781 1997-06-13
WO 96/18724 PCT/US95116364
TABhE 16
TARRY SOIL
Conditions: lated, 30-40~
inocu moisture,
0.25 slpm
air flow
Compounds in
material TCLP Percentaoe VC~C '
(solvent (acidic
Organic extraction)* aqueous
Compounds extraction) ~ residual
Initial Final m L in ~ volat:ilized
(pnm) ~ material to GAC
Benzene 4377.0 <10 <0.5 --- 61.0
Chlorobenzene 6606.0 62.0 <1 0.1 64.3
10Nitrobenzene 74.0 70.0 <2 94.6 70
Naphthalene 4075.0 3038.0 3.0 74.6 30
TARRY SOIL
Conditions: isture, 0.25 slpm air flow
inoculated,
30-50$ mo
Compounds in
material TCLP Percentage Vc~C
15 (solvent (acidic
Organic extraction)* aqueous
Compounds extraction
) ~ residual
Initial Final m L in ~ volatilized
lppml ~ material to GAC
Benzene 4137.0 <10 NA** --- 57..4
Chlorobenzene 6065.0 30.0 0.5 6;t.9
Nitrobenzene <10 <10 --- -
2 Naphthalene 3967.0 2533.0 63.9 3.9
0
*TCL
**Not Available
A significant percentage both benzene (63%) and
of
chlorobenzene (35.5%) was
rapidly removed
by stripping
during
the first two days of treatment,
subsequently
removal occurred
25more slowly. Greater than 40% of the naphthalene was removed
during treatment little stripping (4%) indicating
with very
removal was erobic degradation of the compound
mainly to
due a
by the microorganisms nt.
prese
30 g~4 COMPOSTING-hIRE SOLID PHASE DEGRADATION: EXAMPhE 3
A tarry soil was detackified and bulked by mixing
the soil in the pug mill with sawdust. This mixture was
neutralized with NaOH and placed in a vessel. The mixture was
inoculated with an induced liquid mixed culture and the vessel
35 sealed. The inoculated mixture was treated as described in
_ 78 _
CA 02207781 1997-06-13
WO 96/18724 PCT/LTS95/16364
Section 9.2. As shown in Table 17, two successful independent
biotreatments of the tarry soil were achieved.
TABLE 17
TARRY SOIL
Conditions: culated, 40-50$ slpm air flow
ino moisture, 0.25
Compounds in
material TCLP
percentage
(solvent (acidic VOC
extractionl* aqueous
Organic extraction)~ residual
Initial Final
Compounds (DDm) (DDm) m L in ~ volatilized
material to GAC
Benzene 25320.0 <10 <0.5 --- 15.1
Chlorobenzene 77.0 <10 <1 --- 98.6
Nitrobenzene 104.0 47.0 <2 0.5 11.3
Naphthalene 10758.0 8206.0 5.0 76.3 3.3
TARRY SOIL
Conditions: slpm air flow
inoculated,
35-50$ moisture,
0.25
Compounds in
material TCLP
Percenta cte VOC
(solvent (acidic
p extraction)*
com aqueous
ounds
Initial Final
extraction)~ residual
(unm) lppml m L in ~ volatilized
m aterial to GAC
2 O Benzene 25000.0 <10 <0.5 --- 13.0
Chlorobenzene 81.0 <10 <1 --- 63.0
Nitrobenzene 87.0 48.0 <2 55.2 13.1
Naphthalene 10440.0 8905.0 8.0 85.3 3.8
*TCL
The tarry soil contained a very high concentration
of benzene (25,000 ppm) and lesser amounts of chlorobenzene
and nitrobenzene. Solid phase biotreatment was able to reduce
the concentration of these compounds to TCLP limits. The
final benzene concentration was less than 10 ppm. Rapid
removal of the compound by the microorganisms occurred in the
first 48 hours and removal to TCLP limits was achieved within
one week. However, only 20-25% of the naphthalene was removed
by the microorganisms after 14 days.
~~5 COMPOSTING-LIRE SOLID PHASE DEGRADATION: EXAMPLE 4
An elastomeric sludge was detackified and bulked by
mixing the elastomeric sludge and sawdust together in a pug
_ 79 _
CA 02207781 1997-06-13
WO 9G/18724 PCT/US95/16'~64
mill. While mixing, nitrogen gas was passed through then
headspace to prevent combustion of flammable materials. The
bulked and detackified sludge was placed in a vessel and the
pH neutralized with the addition of NaOH. The neutrali:,ed
mixture of sludge and sawdust was inoculated with an induced
liquid mixed culture and the vessel sealed. The material was
treated as a pile for 14 days as described in Section 9.2.
Table 18 shows two successful independent biotreatments of the
elastomeric sludge.
TABLE 18
SLUDGE
Conditions: sture, 0.25 air flow
inoculated, slpm
30-50~ moi
Compounds in
material TCLP Percenta ge yOC
(solvent (acidi c
extractionl* aqueous
Organic extraction) residual
~
Compounds Initial Final
m L in ~ volatilized
-Llpm) ~P~- material to GAC
Benzene 99.0 <10 <0.5 --- 93.8
Chlorobenzene40.0 <10 <1 --- 56.4
Nitrobenzene 449.0 <10 2.0 --- 16.5
2 Naphthalene 15341.0 84.0 1.0 0.5 :1.8
0
SLUDGE
Conditions: air flow
inoculated,
30-50~ moisture,
0.25 slpm
Compounds in
material TCLP Percenta ge 'iIOC
(solvent (acidi c
2 Organic extraction)* aqueous
5
Compounds extraction) residual
~
Initial Final m L in ~ volatilized
(ppml ~ material to GAC
Benzene 89.0 <10 <0.5 --- 109.0
Chlorobenzene38.0 <10 <1 --- 6.5
Nitrobenzene 454.0 <10 <1 --- 139
Naphthalene 13380.0 280.0 <1 2.1 1.6
3
0
*TCL
The elastomeric sludge was successfully treai~ed for
benzene and nitrobenzene. The final concentration of both
compounds was less than 10 ppm. In addition, naphthalene was
35 degraded to less than 330 ppm from 13,000 - 15,000 pm
initially. However, not all removal of these compounds was
due to aerobic bioremediation. More than (90%) of the benzene
- 80 -
CA 02207781 1997-06-13
WO 96/18724 . PCT/US95/16364
and approximately (15%) of the nitrobenzene were stripped from
the mixture during the first two days. On the other hand,
a
stripping was not a major removal mechanism for naphthalene
and chlorobenzene indicating that their removal was due mainly
to aerobic degradation of the compound by the microorganism
added.
9.6 COMPOSTING-hIRE 80hID PHASE DEGRADATION: EXAMPLE 5
A tarry sludge containing a mixture of high levels
of benzene, chlorobenzene, nitrobenzene and naphthalene was
bulked and made less tarry by mixing the tarry sludge with
sawdust (25~ w/w). The bulked sludge was neutralized with
NaOH and placed in a vessel. The neutralized and bulked
sludge was inoculated with a liquid mixed culture of
microorganism (2-10% w/v) and the vessel sealed. The mixture
of sludge, sawdust and microorganisms was treated as a pile
for 14 days. The mixture was successfully treated for removal
of the compounds tested. Table 19 demonstrates the successful
aerobic bioremediation of benzene, chlorobenzene, nitrobenzene
and naphthalene as measured by TCLP. 30-60% of the
chlorobenzene and 10-30% of the benzene but less than 17% of
the nitrobenzene was lost due to stripping. This indicates
that the major removal process for these compounds is by
bacterial aerobic degradation of these compounds.
30
- 81 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16;f64
TABLE 19
TARRY SLUDGE
Conditions: inoculated, air flow
30-35$ moisture,
0.25 slpm
Compounds in
material TCLP
Percenta ge V'OC
(solvent (acidi c
extractionl* aqueous
Organic extraction) residual
$
nds Initial Final m L in $ volatilized
Compou (ppm) material to GAC
Benzene 553.0 <10 <0.5 --- 11.3
Chlorobenzene 3528.0 38.0 <1 1.1 808
Nitrobenzene 5752.0 85.0 <2 1.5 7.8
Naphthalene 17670.0 357.0 <1 2.0 .3.0
TARRY SLUDGE
Conditions: inoculated, air flow
30-35$ moisture,
0.25 slpm
Compounds in
material TCLP Percentage
'VOC
(solvent (acidi c
Organic extractionl* aqueous
Compounds extraction) residual
~
Initial Final m L in ~ volatilized
lPpm) ~ material to GAC
Benzene 643.0 <10 <0.5 --- 35.2
Chlorobenzene 3905.0 644.0 <1 16.5 345
Nitrobenzene 6065.0 1587.0 <2 26.2 0.5
2 0 Naphthalene 16980.0 6110.0 <1 36.0 0.2
*TCL
9.7 COMPOSTING-LIRE SOLID PHASE DEGRADATION: EXAM~iLE 6
A non-elastomeric sludge was bulked with sawdust
(20~ w/w) as described above. The bulked sludge, which had an
alkaline pH, was neutralized with HzS04. The neutralized and
bulked sludge was placed in a vessel, inoculated with a liquid
mixed culture and treated for 14 days. Table 20 shows two
successful individual biotreatments of the same starting
material.
- 82 -
CA 02207781 1997-06-13
WO 96/18724 PCT/US95/16364
TABLE 20
SLUDGE
Conditions: pH control, no moisture
no inoculum, 50-55~
,
250 slpm air
flow
Compounds in
material TCLP
percenta cie VOC
(solvent (acidic
extraction)* aqueous
Organic extraction)~ residual
Initial Firial
Compounds ,~, L in ~ volatilized
(ppml '~~
' material to GAC
Benzene 1972.0 61.0 NA** 3.1 88.3
Chlorobenzene29.0 <10 --- 81.4
Nitrobenzene <10 <10 ___ ___
Naphthalene <10 <10 --- ___
SLUDGE
Conditions:
inoculated,
50-55~ moisture,
250 slpm
air flow
Compounds in
material TCLP
Percenta ge VOC
(solvent (acidic
Or anic extraction)* aqueous
Compounds extraction)~ residual
Initial Final
m L in ~ volatilized
(ppm) ~
material to GAC
Benzene 2135.0 55.0 NA** 10.2 90.1
Chlorobenzene30.0 <10 --- 66.4
2 0 Nitrobenzene <10 <10 --- ---
Naphthalene <10 <10 --- --_
*TCL
**Not Available
Significant amounts of both benzene and
35
25 chlorobenzene were stripped and trapped into the GAC traps.
Of the benzene and chlorobenzene stripped, greater than 95~ of
the benzene and 90% of the chlorobenzene were stripped in the
first 48 hours. This rapid removal was followed by a slow
reduction over the remaining time.
10. DEPOSIT OF MICROORGANISMS
The following microorganisms were deposited on
December 13, 1994 with the American Type Culture Collection
- 83 -
CA 02207781 1997-06-13
WO 96/18724 PCT/L1S95/16364
(ATCC), Rockville, MD, and have been assigned the indicated
Accession numbers: r
Microorganism ATCC Accession No.
Pseudomonas sp. (DAP 70) 55646
Pseudomonas sp. (DAP 111) 55645
Pseudomonas sp. (DAP 622) 55648
Pseudomonas sp. (DAP 631) 55647
Aeromonas sp. (DAP 68) 55642
Aeromonas sp. (DAP 119) 55641
Corynebacterium sp. (DAP 66) 55643
Zoogloea sp. (DAP 73) 55649
Mixed Culture Microorganisms ATCC Accession No.
DAP 2 55644
The following microorganisms were deposited on
November 30, 1995 also with the American Type Culture
Collection (ATCC), Rockville, MD, and have been assigned the
indicated Accession numbers:
Microorganism ATCC Accession No.
DAP 623 55722
DAP 626 55723
DAP 629 55726
DAP 632 55727
DAP 115 55724
DAP 120 55725
The invention described and claimed herein ins not to
be limited in scope by the specific embodiments herein
disclosed since these embodiments are intended as
illustrations of several aspects of the invention. .any
equivalent embodiments are intended to be within the scope of
this invention. Indeed, various modifications of the
invention in addition to those shown and described herein will
become apparent to those skilled in the art from the foregoing
description. Such modifications are also intended to :Fall
r
within the scope of the appended claims.
i
- 84 -
CA 02207781 1999-06-14
International Application No: PCT/
MICROORGANISMS
Optional Sheet in connection with the microorganism referred
to on page 84, lines t-25 of the description '
A. IDENTIFICATION OF DEPOSIT'
Further deposits are identified on an additional sheet
'
Name of depository irutiattion '
American Type Ctdttae Collection
Address of depository institution lincluding postal code
and country) '
12301 Perktswn Drive
Rockville. MD 20852
US
Date of deposit ' December 13, 1994 Accession Number
' 55646
B. ADDITIONAL INDICATIONS ' ((cave blurt i( mt app(raDkl.
Thu mtarnutae a canirated on a separate attaehod snit
C. DESIGNATED STATES FOR WHICH INDICATIONS ARE MADE '
tau..a~o....v~u~s~..n
D. SEPARATE FURNISHING OF INDICATIONS ' peae btant it
rot appliwDkt
The inotcanona iietad below wiH De audnittsd to tM W
ternanonel Burew iatn' (Specify the penarM nature of
the maications e.p..
'ACCesaton Number of Oeposrt'1
E. ~ 'Ibis sheet was received with the Intctnanonal application
when filed (to be checked by the receiving Office)
(Authorized Officer)
~ The date of receipt (from the applicant) by the Intetnabonal
Bureau "
was
(Authorized Officer)
orm ! ~ (January 1 11
-85-
75365=125
CA 02207781 1999-06-14
International Application No: PCTI
Form PCT/RO/134 Icont.)
American Type Culture Collection
12301 Parklawn Drive
Rockville, MD 20852
US
Accession No. Date of Deposit
55645 December 13, 1994
55648 December 13, 1994
55647 December 13, 1994
55642 December 13, 1994
55641 December 13, 1994
55643 December 13, 1994
55649 December 13, 1994
55644 " December 13, 1994
55722 November 30, 1995
55723 November 30, 1995
55726 November 30, 1995
55727 November 30, 1995
55724 November 30, 1995
55725 November 30, 1995
-86/87-
75365-125