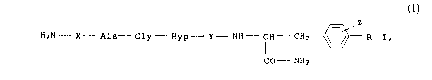Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
- - CA 02209496 1997-07-os
Novel peptides, their production and use
The present invention relates to novel peptides, a process for
5 their preparation and to their use for controlling diseases.
It is known that cardiac dysrhythmias represent one of the com-
monest causes of death in western industrialized countries. More-
over arrhythmias in particular are of great importance in connec-
lO tion with coronary heart disease, ischemia and advanced age. Themechanisms leading to arrhythmias moreover vary widely and, in
some cases, are still unelucidated. It is certain that, for exam-
ple, surviving Purkinje fibers in an area of infarct are able to
maintain arrhythmias as external pacemakers (foci). It is like-
15 wise possible within the framework of the cardiac ischemia fordepolarization of fibers to occur, owing to outflow of potassium,
and these may become in part unexcitable so that unidirectional
blocking of stimulus conduction may result. Besides a large num-
ber of other mechanisms, within the framework of the infarct
20 there are also local differences in the action potential duration
(dispersion~, which may then trigger reentry circuits. It is then
possible for ventricular flutter or fibrillation to develop from
such circulating conduction. Another mechanism which may lead to
such dispersion of the action potential duration is cellular de-
25 coupling (Circ. Res. 65 (1989) 1426) because it is then no longerpossible to compensate for potential differences between the
cells. This decoupling may arise on the one hand with increasing
age owing to, for example, connective tissue infiltration (Circ.
Res. 62 (1988) 811), or on the other hand within the framework of
30 the infarct owing to closure of the intercellular connections
(gap junction channels) because of, for example, the increasing
PCO2 and the falling pH and ATP content (Am. J. Physiol. 248,
(1985) H753-H764, Circ. Res 45, (1979) 324).
35 Antiarrhythmics used to date have been ion channel blockers which
block transmembrane ion channels (sodium channels, calcium chan-
nels and/or potassium channels) and are suitable for therapy of
acute existent cardiac dysrhythmias.
40 However, problems emerge when these substances are intended to be
used for the prophylaxis of arrhythmias. At least when adminis-
tered prophylactically, these ion channel blockers show a high
proarrhythmic risk (Drugs 29, (1985) Suppl. 4, 33-34, New England
J. Med. 324, (1991) 781). This means ~hat, paradoxically,
45 arrhythmias are provoked precisely by administration of classical
-- ~ ~ v ~
- CA 02209496 lss7-07-os
antiarrhythmics. This is why these substances are suitable only
with great restrictions for prophylaxis.
Hence there is currently a search for substances which can be ad-
5 ministered prophylactically and have novel principles of action
and which no longer show this proarrhythmic effect. One novel
principle is improvement in cellular coupling.
Clinical and experimental results with conventional anti-
lO arrhythmics showed that prophylaxis of arrhythmias is scarcely
possible because of the pronounced proarrhythmic side effects,
which have also been detectable in in vitro tests (New England J.
Med. 324, (1991) 781), Circulation 87, (1993) 617).
15 An antiarrhythmic peptide AAP10 has been proposed (Nauny
Schmiedeberg's Arch. Pharmacol. 350 (1994) 174) has been proposed
as novel principle leading to improved cellular coupling,
reducing local differences in the action potential duration and
stabilizing the epicardial conduction pattern. This substance
20 shows virtually no proarrhythmic risk in in vitro tests on
isolated rabbit hearts but is very effective for ischemia-
associated arrhythmias. The primary effect of the substance is to
reduce the dispersion of the potential duration.
25 The invention relates to the compounds of the formula I
Z
H2N- X Ala Gly - Hyp Y - NH FH - CH2 ~ R I,
CO NH2
where
35 R is H or OH,
X is Ala, Arg, Gly or Val;
Y is Pro or His and
Z is H, F, Cl, Br or I
40 but where Z is not H when X is Gly, Y is Pro and R is OH, and to
the use of these peptides for controlling diseases. Hyp in the
above formula means 4-hydroxyproline.
In formula I, X is preferably a glycinelresidue, Y is preferably
45 a proline residue, Z is particularly a halogen atom, preferably
- - iodine, which is in position 2 and preferably in position 3.
0~5~/4~YI
CA 02209496 1997-07-os
The compounds can be prepared by conventional methods of pep~ide
chemistry. Particularly suitable processes for preparing them arC
the following:
5 Solid-phase synthesis on insoluble resins by a modified
Merrifield process as described by E. Atherton ~ R.C. Sheppard
(1989; "Solid phase peptide synthesis, IRL-Press, Oxford) usir.g
the Fmoc strategy.
lO The amino acid activation can in this case take place by forma-
tion of anhydrides, 1-hydroxybenzotriazole esters or pentafluoro-
phenyl esters.
The particular effect of the novel peptides is to diminish local
15 differences in the action potential duration and irregularities
in stimulus conduction, both of which occur in the framework cf,
for example, myocardial infarcts or with increasing age.
The invention makes prophylactic therapy of ischemia-associated
20 and age-associated cardiac dysrhythmias possible. The substances
moreover show, in contrast to conventional antiarrhythmics, a
negligible proarrhythmic risk in in vitro tests. Compared with
known substances, the novel peptides show a greater potency and a
higher minimum effect which can be achieved.
Examples
1. Preparation of fluorenylmethoxycarbonyl-iodotyrosine
Tyrosine and phthalic anhydride were reacted together in gla-
cial acetic acid for 20 h, corresponding to a Gabriel synthe-
sis, and the reaction product was reacted with iodine and
HgtII) acetate to give N-phthaloyl-L-monoiodotyrosine. The
protective group was then eliminated with phenylhydrazine.
The reaction product was reacted with N-fluorenylmethoxycar-
bonyloxysuccinimide in the presence of Na2CO3, water and ace-
tone. The required Fmoc-iodotyrosine was obtained after acid-
ification with HCl.
40 2. Synthesis of the peptide (1) H2N-Gly-Ala-Gly-Hyp-Pro-3-iodo-
tyrosinamide
The synthesis protocol for the Fmoc strategy disclosed by
Atherton & Sheppard was used. 272.7 mg of Rink resin with a
loading of 0.55 mmol/g were swollen with dimethylformamide
and then the protective group was eliminated with 20~ piper-
idine in dimethylformamide (DMF). After washing with DMF,
. CA 02209496 lss7-07-os
0.9 mmol of Fmoc-iodotyrosine was added with dicyclohexylcar-
bodiimide (DCC) in DMF. After washing with DMF and methanol,
the protective group was eliminated with 20% piperidine in
DMF and, after further washing, the next amino acid was
coupled. For this purpose, Fmoc-proline-OH was reacted with
the peptide together with O-(lH-benzotriazol-l-yl)-N,N,N',N -
tetramethyluronium tetrafluoroborate (TBTU), 1-hydroxybenzo-
triazole (HOBT) and diisopropylethylamine (DIPEA) in DMF. Af-
ter washing, the protective group was once again eliminated
with piperidine and DMF and, after further washing, the reac-
tion product was reacted with Fmoc-hydroxyproline-OH as de-
scribed above. Washing and elimination were followed by
successive coupling of Fmoc-glycine-OH, Fmoc-alanine-OH and
Fmoc-glycine-OH in this way. Subsequently, the protective
group was eliminated with 20% piperidine in DMF and, after
washing, drying was carried out under 0.1 mbar for 6 h. Fi-
nally, the resin was cleaved off with trifluoroacetic acid
and 5% water for 2 h, and the product was washed, evaporated
in a rotary evaporator, dissolved in glacial acetic acid and
precipitated with diethyl ether. The precipitate was filtered
off with suction and purified by semipreparative HPLC in a
conventional way. 7.6 mg of the novel peptide were obtained
(molecular weight: 701.6).
25 3. The following peptides were obtained in a similar way to
Examples 1 and 2.
(2) H2N-Gly-Ala-Gly-Hyp-Pro-3-Fluorotyrosinamide
(3) H2N-Gly-Ala-Gly-Hyp-Pro-3-Chlorotyrosinamide
(4) H2N-Gly-Ala-Gly-Hyp-Pro-3-Bromotyrosinamide
(5) H2N-Arg-Ala-Gly-Hyp-Pro-Tyrosinamide
(6) H2N-Val-Ala-Gly-Hyp-Pro-Tyrosinamide
(7) H2N-Ala-Ala-Gly-Hyp-Pro-Tyrosinamide
(8) H2N-Gly-Ala-Gly-Hyp-His-Tyrosinamide
(9) H2N-Gly-Ala-Gly-Hyp-Pro-Phenylalaninamide
Use:
Intracoronary infusion was carried out with peptide (1) in in-
40 creasing concentrations (10-1~, 10-9, 10-8, 10-7 mol/l) on isolated
rabbit hearts perfused with Tyrode solution by the Langendorff
technique under constant pressure (70 cm H2O) and, simultaneously,
epicardial potential mapping was carried out, cf. J. Pharmacol.
Methods 22, (1989) 197, Circulation 87, (1993) 617).
-
v v J ~
CA 02209496 lss7-07-os
In these investigations, a unipolar electrocardiogram was re-
corded simultaneously at 256 points on the epicardial surface 5~
the heart so that it was possible to determine therefrom the lo-
cal epicardial action potential duration at all 256 sites. The
5 distribution of the action potential duration around the averago,
and the change in this distribution by the substance compared
with AAP10 were then examined using these data. There was found
to be with both substances an increasing leptokurtosis of the
curve, that is to say more values were near the average as the
10 concentration increased both with AAP10 and with the novel pep-
tide. Thus, under control conditions 50% ~f all values were in a
region of + 5 ms around the average, whereas this was up to a
maximum of 74% (10-8 mol/l) with AAP10 but as much as 90%
( 1 o-7 mol/l) with the novel peptide. This means that the novel
15 peptide distinctly reduces the dispersion of the epicardial
action potential duration and this reduction is more pronounced
than that achievable with AAP10.
Table 1: Concentration-dependent effect on the dispersion of the
20 epicardial potential duration with the antiarrhythmic peptide
AAP10 and the novel peptide.
log conc. AAP10 Novel peptide (1)
Control 7.B + 0.9 6.0 + l.0
-10 6.5 + 0.4 4.5 + 0.9
- 9 6.2 + 0.4 5.0 + 1.1
- 8 5.2 + 0.4 4.6 + 0.9
- 7 6.1 + 0.1 3.8 + 0.6
The better effect of the novel peptide becomes particularly clear
on examination of the number of values for the epicardial poten-
tial duration (ARI) which differ by less than + 5 ms from the av-
35 erage. The proportions of ARI values in the interval + 5 msaround the average with the novel peptide were 54% under control
conditions but 71% at 10-1~ mol/l, 73% at 10-9 mol/l, 75~ at
10-8 mol/l and 90% at 10-7 mol/l. With AAP10, values above 70%
were not reached until the concentration was above 10-8 mol/l, and
40 a maximum of 74~ was not exceeded.
The novel substance shows an effect at lower concentrations and a
greater maximum achievable effect than AAP10: 90% versus 74% for
AAP10 (% values: n% of the epicardial action potentials showed a
45 duration in the range of + 5 ms aroundithe average, corresponding
to a decrease in dispersion). This means that the novel peptide
is not only more potent but also more effective in respect of the
UU3U/ 4:~Y /
- CA 02209496 lss7-07-os
maximum effect than AAP10 and therefore represents an advance
compared with AAP10.
Proarrhythmic risk
The novel substance, like AAP10, shows a particularly low pro-
arrhythmic risk compared with conventional antiarrhythmics
(Table 2).
10 Table 2: Change in the proarrhythmic risk (the vector field simi-
larity) (for details on this parameter and the results with clas-
sical antiarrhythmics, cf. Circulation 87 (1993) 617) with AAP10,
the novel peptide, lidocaine and flecainide in usual therapeutic
concentrations (free plasma concentration~.
log conc. AAP10 AAP13TT Lidocaine Flecainide
Control 29 + 3 29 + 6 23 + 2 23 + 2
-10 24 + 3 23 + 4
20 - 9 23 + 2 18 + 5
- 8 22 + 3 17 + 5
- 7 21 + 2 17 + 6 26 + 2
- 6.3 19 + 3
25 ~ 5.698 26 + 2
- 5.824 10 + 2
- 5.3 23 + l
- 5 17 + 3
30 A substance has a greater proarrhythmic effect if the vector
fields show less similarity. All the concentrations correspond to
usual therapeutic free plasma concentrations. Of the conventional
class I antiarrhythmics, lidocaine is acknowledged to have a
relatively low proarrhythmic risk, whereas the general assessment
35 is that flecainide has a very high proarrhythmic risk.
.