Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
CHEMICALLY MODIFIED INTERFERON
~,
The present invention relates generally to the
modification of consensus interferon and, more
particularly, to neoglycosylated analog compositions o~
recombinant consensus interferon, wherein glycosyl
ligands are conjugated to the recombinant consensus
interferon.
BACKGROUND OF THE INVENTION
Interferons are a subclass of cytokines that
exhibit both antiviral and antiproliferative activity.
On the basis of biochemical and immunological
properties, human interferons are grouped into three
classes: interferon-alpha (leukocyte), interferon-beta
(fibroblast) and interferon-gamma (immune). At least
fourteen alpha interferons (grouped into subtypes A
through H) having distinct amino acid sequences have
been identified by isolating and sequencing DNA encoding
these polypeptides. Alpha interferons have received
considerable attention as potential therapeutic agentc
due to their antiviral and antitumor growth inhibitior.
Alpha-interferon is currently approved in the
United States and other countries for the treatment o
hairy cell leukemia, venereal warts, Kaposi's Sarcoma (a
cancer commonly afflicting patients suffering from
Acquired Immune Deficiency Syndrome (AIDS)), and chror.ic
hepatitis C virus (HCV) infection. Two variants of
alpha interferon have received approval for therapeut--
use: Interferon alfa-2a, marketed under the trade nam-
Roferon~-A, and Interferon alfa-2b, marketed under th_
trade name INTRON~ A.
In addition to the labeled indications,
alpha-interferon is being used or evaluated alone or -n
conjunction with chemotherapeutic agents in a variety ~f
CA 02209~7 l997-07-04
WO 96/21468 PCT/US96/00323
other cellular proliferation disorders, including
chronic myelogenous leukemia, multiple myeloma,
superficial bladder cancer, skin cancers (basal cell
carcinoma and malignant melanoma), renal cell carcinoma,
ovarian cancer, low grade lymphocytic and cutaneous
T cell lymphoma, and glioma. Alpha-interferon may be
effective in combination with other chemotherapy agents
for the treatment of solid tumors that arise from lung,
colorectal and breast cancer (see Rosenberg et al.
NPrinciples and Applications of Biologic Therapy/' in
Cancer: Principles and Practices o~ Oncolo~y, 3rd ed.,
Devita et al., eds. pp. 301-547 (1989), Balmer DICP,
Ann Pharmacother 24, 761-768 (1990)).
Alpha-interferons are known to affect a
variety of cellular functions, including DN~ replication
and RNA and protein synthesis, in both normal and
abnormal cells. Thus, cytotoxic ef~ects of interferon
are not restricted to tumor or virus infected cells but
are also manifested in normal, healthy cells as well.
As a result, undesirable side effects arise during
interferon therapy, particularly when high doses are
required. Administration of interferon can lead to
myelosuppression resulting in reduced red blood cell,
white blood cell and platelet levels. Higher doses of
interferon commonly give rise to flu-like symptoms
(e.g., fever, fatigue, headaches and chills),
gastrointestinal disorders (e.g., anorexia, nausea and
diarrhea), dizziness and coughing.
Natural human interferon-beta is a
glycoprotein with an apparent 22-23 kDa molecular weight
and an antiviral specific activity of 2 - 5 X 108
international units (IU)/mg protein. Human IFN-~has
been clinically applied to the treatment of glioma,
melanoma, viral dermatropic diseases and hepatitisi see
Interferon, Principles and Medical Applications, 1st
CA 02209~7 1997-07-04
WO96/2l468 PCT~S96/00323
Edition (1992, Univ. of Texas Medical Branch of
Galveston) pages 107-116.
U.S. Patents Nos. 4,695,623 and 4,897,471
disclose novel interferon polypeptides having amino acid
sequences which include common or pred~mln~nt amino
acids found at each position among naturally-occurring
alpha interferon subtype polypeptides, and are referred
to as consensus interferons (IFN-con). The specific
IFN-con amino acid sequences disclosed are designated
IFN-con1, IFN-con2, and IFN-con3. The preparation of
manufactured genes encoding IFN-con and the expression
o~ said genes in E. coli are also disclosed.
A purification of IFN-con1 produced in E. coli
is described in Klein et al. (J. Chromatog. 454, 205-215
(1988)). IFN-con1 purified in this manner is reported
to have a specific activity of 3 x 109 units/mg. protein
as measured in the cytopathic effect inhibition assay
using the T98G human cell line (Fish et al. J.
Interferon Res. 9, 97-114 (1989)).
U.S. Patent No. 5,372,808 discloses methods of
treatment of diseases using consensus interferon. It is
shown that IFN-con, when used in the treatment of
diseases susceptible to treatment by alpha interferons,
does not cause the same degree of side effects in
patients as do the alpha interferons. It was further
shown that 3 to 5 times higher doses of IFN-con can be
used, leading to enhanced therapeutic benefit, with
substantially no corresponding increase in the frequency
or severity of undesirable side effects.
Because the interferon dosage form that will
achieve the greatest therapeutic effect, with reduced
adverse side effects, remains unclear, the need exists
to develop improved forms'of the interferons for
therapeutic use. The development of interferon forms
which would allow for high dosage without the
undesirable side effects associated with interferon
CA 02209~7 1997-07-04
W096/21468 PCT~S96/00323
therapy would be of great benefit. It is therefore an
object of the present invention to provide improved
forms of interferon comprising conjugates of galactose
residues to interferon, for use in treatment of
conditions that are susceptible to interferon treatment.
It is a further object of the present invention to
provide compositions which allow for selective delivery
of interferon to the liver, thereby increasing the
opportunity of interferon binding to hepatocyte cell
surface receptors, and ~imini shing the undesired binding
of interferon to other organs during the antiviral
treatment of such disease states as chronic HCV.
SUMMARY OF THE INVENTION
The present invention encompasses
neoglycosylated analogs of recombinant consensus
interferon. The invention is based on the discovery
that multiple lactose ligands can be conjugated to the
IFN-con to give neoglycosylated forms of IFN-con
(lacIFN-con). Preferably, the lactose ligands are
conjugated through the ~-NH2 of lysine and the N-terminal
amine of the IFN-con. As compared to native consensus
interferon, lacIFN-con is highly targeted to the liver.
Preferably, IFN-con is a polypeptide having
the amino acid sequence of IFN-con1, IFN-con2, or
IFN-co~3, and the conjugate will have between 1 and 10
glycosyl residues per IFM-con molecule. Most preferably,
IFN-con has the amino acid sequence of IFN-con1, and the
neoglycosylated IFN-con will have between 4 and 7
lactose residues for each IFN-con molecule.
The invention also relates to pharmaceutical
compositions comprising a therapeutically effective
amount of lacIFN-con along with suitable diluents,
adjuvants, carriers, preservatives and/or solublizers.
These compositions may provide therapeutic benefit in
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
the treatment of those conditions currently susceptible
to treatment with IFN-con.
The present invention also encompasses a
method o~ delivering an interferon directly to the liver
of a patient, comprising administering to the patient a
therapeutically effective amount of a chemically
modified interferon, wherein the chemically modified
interferon is comprised of an interferon conjugated to
at least one glycosyl ligand, wherein said ligand
comprises a terminal galactose, and wherein said
interferon is selected form the group consisting of
IFN-~, IFN-~, and IFN-con.
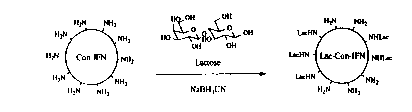
DETAILE~ ~ESCRIPTION OF THE DRAWINGS
Figure l is a schematic diagram showing the
preparation of lacIFN-con from IFN-con and lactose
through reductive amination.
Figure 2 is a graphical representation of the
percentage of injected radiolabeled IFN-con (-O-) and
lacIFN-con (-O-) accumulated in the liver of hamsters at
indicated time intervals. The bars represent standarG
deviation (n=3).
Figure 3 is a graphical representation of tre
biodistribution of radiolabeled IFN-con in rats. The
% injected dose was determined at indicated time
intervals.
Figure 4 is a graphical representation of the
biodistribution of radiolabeled lacIFN-con in rats. The
% injected dose was determined at indicated time
intervals.
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 6 -
Figure 5 is a graphical representation of
hamster serum pharmacokinetics of IFN-con (-O-) and
lacIFN-con (-O-) after intravenous administration
(300 ~g/kg ). Serum interferon concentration is plotted
versus time. The bars represent standard deviation
(n=3).
Figure 6 is a graphical representation of 2-5A
synthetase activity measured in hamster serum after
intravenous administration of IFN-con (-O-), lacIFN-con
(-O-), and phosphate buffer vehicle group (-~-) at
various time points. The bars represent standard
deviation (n=3).
Figure 7 is a graphical representation of the
survival rate of untreated EMCV-infected hamsters (-O-),
EMCV-infected hamsters preinjected with IFN-con (-O-),
or EMCV-infected hamsters pretreated with lacIFN-con
(-0-). The ~lm~l S were monitored twice daily for 27
days.
DETAILED ~ESCRIPTION OF THE INVENTIQN
As employed herein, consensus human leukocyte
interferon (IFN-con) means a nonnaturally-occurring
polypeptide, which predominantly includes those amino
acid residues that are common to all naturally-occurring
human leukocyte interferon subtype sequences and which
includes, at one or more of those positions where there
is no amino acid common to all subtypes, an amino acid
which predominantly occurs at that position and in no
event includes any amino acid residue which is not
extant in that position in at least one naturally-
occurring subtype. IFN-con encompasses but is not
limited to the amino acid sequences designated IFN-conl,
IFN-con2 and IFN-con3 which are disclosed in commonly
CA 02209S~7 1997-07-04
W O96/21468 PCTrUS96/0032
owned U.S. Patents 4,695,623 and 4,897,471, the entire
disclosures of which are hereby incorporated by
reference. DNA sequences encoding IFN-con may be
synthesized as described in the above-mentioned patents
or other standard methods.
IFN-con polypeptides are preferably the
products o~ expression o~ manufactured DNA sequences
transformed or transfected into bacterial hosts,
especially E. coli. That is, IFN-con is preferably
recombinant IFN-con. Recombinant IFN-con is preferably
produced in F., coli and is purified by procedures known
to those skilled in the art, as generally described in
Klein et al., su~ra (1988) for IFN-conl. Purified
IFN-con may comprise a mixture of isoforms, e.g.,
purified IFN-conl may comprise a mixture of methionyl
IFN-conl, des-methionyl IFN-conl and des-methionyl
IFN-conl with a blocked N-terminus (Klein et al., su~ra
(1990)). Alternatively, IFN-con may comprise a
specific, isolated isoform. Isoforms of IFN-con are
separated from each other by techniques such as
isoelectric focusing which are known to those skilled in
the art.
The chemically modified IFN-con of the present
invention will be comprised of at least one terminal
galactose-containing glycosyl ligand per protein. Such
glycosyl ligands will be selected from the group
consisting of galactose, terminal glycosyl
oligosaccharides, asialoglycopeptides, and
asialoglycoproteins. When the glycosyl ligand is a
monosaccharide, it will generally be galactose. The
oligosaccharides of this invention are generally those
having at least two sugar moieties, wherein, in each
case, galactose residue(s) will be attached to the
terminal end(s) of the oligosaccharide. Some
representative oligosaccharides useful for attachment to
IFN-con are lactose, N-acetyllactosamine and galactan.
CA 02209~7 1997-07-04
WO96/2l468 PCT~S96/00323
Preferably, the neoglycosylated IFN-con analog will
contain four to seven ligands and the glycosyl ligand
will be an oligosaccharide. Most preferably, the
neoglycosylated IFN-con analog will contain four to
seven ligands and the glycosyl ligand will be lactose.
The multiple ligands are preferably identical, but
mixtures of ligands are also encompassed by this
nvent lon .
The neoglycosylated IFN-con analogs of the
present invention can be obtained by a number of
conventional methods. Such methods have been very well
summarized; see Kataoka and Tavassoli, ~our. of
Histochem. and Cytochemistry, 32: 1091-1098 (1984);
Chipowsky and Lee, Carbohy. Research, 31: 339~346 (1973)
and references cited therein. Preferably, the
neoglycosylated IFN-con analogs are obtained by a method
which comprises conjugating multiple glycosyl ligands to
the protein backbone through active amino acid
residue(s) such as lysine, cysteine, serine, threonine,
or asparagine. Most preferably, the neoglycosylated
IFN-con analogs are obtained by a method which comprises
conjugating multiple glycosyl ligands through the ~-NH2
of lysine and/or the N-terminal amine of the IFN-con.
This chemical modification is achieved by reacting
glycosyl ligands with IFN-con ~under certain reaction
conditions, pre~erably non-denaturing conditions, in
sufficient amounts such that the amino groups are
accessible for the reductive amination. In a preferred
embodiment, the glycosyl ligand is lactose and the
reactions are carried out at pH 7.0, require the
addition of a reducing agent, e.g. sodium
cyanoborohydride, in large molar excess, and are
controlled such that the analog obtained contains four
to seven lactose ligands per protein. The means used to
control such reactions are readily determined by those
skilled in the art.
CA 02209~7 lgg7-07-04
wos6l2l468 PCT~S96/00323
Because the methods of this invention provide
a substantially homogeneous modified IFN-con
preparation, the invention also encompasses
pharmaceutical compositions which comprise a
therapeutically effective amount of lacIFN-con in a
mixture with a pharmaceutically acceptable carrier,
diluent, preservative, solublizer, adjuvants and/or
emulsi~ier; see Remington's Pharmaceutical Sciences,
18th Edition (1990, Mack Publishing Co., Easton, PA
18042) pages 1435-1712 which are herein incorporated by
reference. Il Substantially homogenous~' as used herein
means that the only neoglycosylated IFN-con analogs
observed are those having four to seven ligands attached
per protein. The preparation may contain unreacted
(i.e., lacking glycosyl ligands) protein. Preferably,
the chemically modified IFN-con is at least 90% one
product (as in the working example below) and most
preferably, the chemically modified IFN-con is 298% one
product.
The chemically modified IFN-con contemplated
by the present invention are those analogs which are
active in a biological assay such as that described i-
Example 1. The analogs will have activity of at leas_
50% compared to that of native IFN-con. Preferably, the
analogs will have activity of at least 60~. Those
skilled in the art will be able to readily evaluate such
analogs and determine whether they demonstrate activi;y
in such assays.
In general, the compositions of the presen~_
invention can be used in the same manner as that
described previously for IFN-con and it is contemplated
that the compositions will be used for treating those
~ conditions treatable with a consensus interferon.
Exemplary conditions include, but are not limited to,
cell proliferation disorders and viral infections.
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 10 -
Viral conditions treatable by IFN-con include,
but are not limited to, hepatitis A, hepatitis C, other
non-A, non-B hepatitis, hepatitis B, herpes virus (EB,
CML, herpes simplex), papilloma, poxvirus, picorna
virus, adenovirus, rhino virus, HTLV I, HTLV II, and
human rotavirus. IFN-con is also effective in treating
cell proliferation disorders frequently associated with
cancer. Such disorders include, but are not limited to,
hairy cell leukemia and Kaposi's Sarcoma. Preferably,
the conditions to be treated are hepatic disorders.
IFN-con may be used alone or in combination
with one or more factors that stimulate myeloid cell
proliferation or differentiation, such as granulocyte
colony stimulating factor (G-CSF), granulocyte/
macrophage colony stimulating factor (GM-CSF),
interleukin-1 (IL-1), interleukin-3 (IL-3),
interleukin-6 (IL-6), erythropoietin, stem cell factor
(SCF), and megakaryocyte growth and development factor
(MGDF).
It is contemplated by the present invention
that treatment with chemically modified IFN-con will
provide improved efficacy with substantially reduced or
eliminated side effects as compared to treatment with
alpha interferon. The reduction or elimination of side
effects is expected to be demonstrated regardless of the
condition being treated. More particularly, because
IFN-con modified with ligands containing terminal
galactose residues will be selectively bound to
asialoglycoprotein-binding receptors on the hepatocyte
cell surface, it is further contemplated that the
modified IFN-con will be particularly useful in the
treatment of hepatic disorders such as HCV.
Because the chemically modified interferons of
the present invention are directed to the liver, the
present invention also contemplates a method of
delivering an interferon directly to the liver of a
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
patient, comprising administering to the patient a
therapeutically effective amount of a chemically
modified interferons of the present invention.
Interferons contemplated ~or use in such methods include
IFN-a, IFN-~, and IFN-con. IFN-a and IFN-~contain
lysine residues which allow for chemlcal modification by
glycosyl ligands in a similar manner as that described
for IFN-con in the detailed examples.
The amount of the chemically modified
interferon that will be effective in the treatment of a
particular disorder will depend on the nature of the
disorder, and other factors, and can be det~rm;ned by
standard clinical techniques or based on dosage amounts
or regimens already established for interferon.
The following examples will illustrate in more
detail the various aspects of the present invention.
EXAMPL~ 1
This example demonstrates chemically modified
human recombinant consensus interferon. More
specifically, this example demonstrates a method of
preparing a homogeneous preparation of neoglycosylated
IFN-conl, and characterization of the preparation.
A. Pre~aration of Consensus Interferon
IFN-conl as described in Figure 2 of U.S.
30 Patent No. 4,695,623, which is incorporated by reference
in its entirety, was used for preparation of the
neoglycosylated human recombinant consensus interferon.
~ The IFN-conl was produced by expression of exogenous DNA
in bacteria, and contained a methionyl residue at the
N-terminus.
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
B. Pre~aration of ~acIFN-con
LacIFM-con was prepared by conjugating
multiple lactose ligands through the ~-NH2 of lysine and
the N-terminal amine of the IFN-con as depicted in
Figure 1. In general, the conjugation reaction can be
described as follows: ~-D-Lactose (Sigma, St. Louis, MO)
was dissolved in phosphate buffer saline (PBS)
containing IFN-con1 at various lactose:IFN-con molar
ratios. The reaction was stirred at room temperature
for various lengths of time, and sodium cyanoborohydride
(NaBH3CN)(Sigma, St. Louis, MO) was added to the
reaction mixture twice a day. The progression of the
reactions was monitored on 16% SDS-PAGE. The reaction
is then stopped by the passing the mixture through a
Sephadex G-25 column (Sigma, St. Louis, MO) eluted with
PBS to remove unreacted lactose and NaBH3CN.
The proteins were further purified on FPLC
with a Pharmacia HiLoad Superdex 75pg 26/60 column
(Piscataway, NJ) eluted with PBS at 1.2 ml~min flow rate
and monitored for absorption at 280 nm. Fractions of
2 ml were collected and the protein ~ractions pooled.
The purified lacIFN-con preparations were analyzed on
gel filtration HPLC with a Pharmacia Superose 12 H/R
10/30 column (Piscataway, NJ) or a Phenomenex BioSep
SEC-2000 column (Torrance, CA) at 0.5 ml/min eluted with
PBS. The derivatized proteins were characterized using
16% SDS-PAGE gels (Novex, San Diego, CA) and pH 3-10
isolectric focusing gels (Novex, San Diegeo, CA) and
mass spectroscopy.
The reaction condition was optimized by
studying various factors such as the reaction pH,
reaction time, and reagent concentration. The pH effect
was studied by conducting reaction in pH 4.0, 4.5, 5.0,
5.5, 6.0, 6.5, and 7.0 PBS at room temperature for 3:
days. The progression of the reactions was monitored on
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 13 -
16% SDS-PAGE. The degree of derivatization was greater
at higher pH as the amino groups were less protonated
and more accessible for the nucleophilic attack to the
aldose form of lactose. Based on results from gel
filtration HPLC, no more than 10% of the derivatized
proteins were found as dimer or aggregate at all
reaction conditions except for the one conducted at pH
5.5, since the pI for IFN-con is 5.7.
The amount of reducing agent added to the
reactions was found to directly affect the reaction
progres~ion and the degree of protein denaturation.
Sodium cyanoborohydride was dissolved in water into a
10M solution just prior to addition to the reaction i~
order to preserve protein stability. As high as 20,000
molar excess reducing agent was added twice a day during
the reaction to facilitate the reaction progression
while minimum amount of aggregation was detected under
this treatment.
C. Characterization of LacIFN-con
The degree of glycosylation, and in vi tro
bioactivity of the derivatized proteins were examined.
The lacIFN-con used for this analysis was prepared as
follows: one gram of ~-D-Lactose was dissolved in 5 m
PBS containinq 12.5 mg IFN-con1. The reaction was
stirred at room temperature for four days and 50 mg c-
sodium cyanoborohydride (NaBH3CN) was added to the
reaction mixture twice a day.
The molecular weight of lactose-modified
IFN-con was determined by mass spectroscopy using Kra=os
Kompact MALDI-TOF mass spectrometer. The number of
lactose attached to IFN-con was calculated by
subtracting 19,516 from the analog molecular weight, and
then dividing that number by 326 (lactose - oxygen).
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 14 -
The in vi tro bioactivity was determined by
measurement of the inhibition of viral replication in a
cultured cell line. HeLa cells were plated into 96-well
plates at 15,000 cells/well and incubated for twenty
four hours at 37~C under 5% carbon dioxide in base
medium (Dulbecco's modified Eagles medium (DMEM),
containing 100 units/ml of penicillin, 100 mg/ml of
streptomycin, 2 mM L-glutamine, 1% by weight of non-
essential amino acids, 0.1% by weight of gentamicin
sulfate and 1% HEPES buffer), with 10% FBS. IFN-con and
lacIFN-con was prepared at multiple dilutions in base
medium and 0.2% FBS. One hundred microliters of each
standard and appropriately diluted IFN-con and lacIFN-
con were added to each well. After further incubation
for 19-23 hours, the medium was aspirated and replaced
with 100 microliters of the challenge virus, i.e.,
Encephalomyocarditis virus (EMCV), at a dilution e~ual
to 100-1000 tissue culture infected dose (TCID) units in
DMEM with 1% FBS. The plates were further incubated for
twenty two hours, the medium removed, and the cells were
fixed with 200 microliters of anhydrous methyl alcohol
for five minutes. The fixative is removed and the cells
are stained for thirty minutes in 0.5% Gentian dye, then
rinsed free of dye and air-dried for one to two hours.
The dye was eluted with two hundred microliter of'
ethylene glycol monoethyl ether and shaken for thirty
minutes. The absorbance of each well at 650 nm was
determined in a Vmax Kinetic Microplate Reader, model
88026 (Molecular Devices). The results for the standard
were graphed as the log concentration of IFN-con versus
the percentage of dye uptake, and the bioactivity of the
IFN-con and lacIFN-con determined.
The results of the above analysis on four
separate preparations is shown in Table I. Potency was
compared using IFN-con activity = 1.
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
Table 1
Pre~aration # of lac Potenev
IFN-eon 0
lacIFN-con ~1 4 .55
lacIFN-con #2 5 .60
lacIFN-eon #3 6 .62
lacIFN-con $~4 7 .55
The Table I data indicates that the optimum
reaction condition gave a lacIFN-con with an average of
~ive-six lactose residues attached to IFN-con. These
lacIFN-con analogs had an average in vitro bioactivity
of 6.4 X 108 U/mg as compared to 11 X 108 U/mg for IFN-
con.
EXAMPLE 2
This example relates to biodistribution
studies using IFN-con and lacIFN-con as prepared in
Example 1. The tissue-targeting ability of the lactose
conjugated IFN-con was determined by studying the total
body distribution of the 125I-labeled protein in hamsters
and in rats.
A. Iodination of IFN-con and lacIFN-con
The iodination procedure was slightly modified
~rom that described in Fraker and Speck, Biophys. Res.
Comm., 80: 849-857 (1978). In a 12 x 75 mm borocilicate
test tube was weighed 3 mg of IODO-GEN (Pierce) which
~ was then dissolved in 100 ~L CHCl3 and dried under a
gentle stream of N2 to form a thin film in the tube.
(125I) sodium iodine (12 ~L, 1.2 mCi)(New England
Nuclear) and 600 ~g protein in PBS were added to the
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 16 -
tube and the reaction was incubated for 10 min on ice.
The reaction was transferred to a 1.5 mL eppendorf tube
containing 20 ~L of parahydroxybenzoate (110 nmol) and
incubated for 10 min on ice. The iodinated protein was
purified with PD-10 columns (Pharmacia) pre-equilibrated
stepwisely with 25 mL of PBS containing 1% BSA and 0.05%
Triton X100, and 25 mL of PBS. Fractions of 0.5 mL were
collected and counted for radioactivity. The fractions
containing the iodinated proteins were pooled and the
contamination due to unbound 125I was determined by
precipitation with a 6% final solution of trichloric
acid.
B. Biodistribution ~nalvsis in hamsters
Male Syrian-Golden hamsters (85-130 g) were
obtained from Charles River Laboratories and maintained
on a 12/12 light dark cycle in a controlled environment
of 21~C. A diet of Purina Chow and water was available
ad libitum. The animals were caged in groups of two and
five, and were allowed one week to acclimate to the
facility after arrival. Three hamsters (85-130 g) per
group were given the l25I-labeled proteins at a dose of 2
mg/kg (2 x 107 cpm/hamster) intravenously through the
penile vein. At 3, 15, 30, 60 and 120 minutes, the
animals were sacrificed and the radioactivity associated
with the organs was determined. Percent injected dose
per gram of organ weight was measured at various time
points. The data is summarized in Table 2.
CA 02209~7 1997-07-04
wos6l2l468 PCT~S96/00323
T~bl~ 2
Biodistribution o~ IFN-con and lacIFN-con
in hamstersl
Tissue 3 min 15 min 30 min 60 min 20 min
blood2 1.84(1.18) 4.23(0.52) 2.63(0.25) 1.30(0.13) 1.14(0.33)
4.94(0.8) 2.37(0.34 1.99(0.2) 1.15(0.0) 0.90(0.2)
liver3 1.58(0.76) 1.42(0.18) 0.95(0.11) 0.75(0.51) 0._3(0.06)
4.71(0.4) 4.18(0.2) 2.04(0.1) 0.72(0.0) 0.48(0.0)
kidney4 25.80(9.6) 67.54(11) 23.08(2.5) 13.28(0.8) 5.09(0.8)20.9(3.6) 36.8(3.9) 23.2(5.2) 11.1(0.2) 5.28(0.2)
spleen 0.58(0.18) 0.44(0.18) 0.29(0.11) 0.28(0.01) 0.14(0.03)
0.36(.05) 0.62(.04) 0.19(.02) 0.26(.08) 0.18(.02)
lung 0.21(0.02) 0.24(0.01) 0.26(0.00) 0.16(0.05) 0.38(0.16)
0.19(.01) 0.20(.11) 0.20(.02) 0.08(.01) 0.06(.01)
duodenum5 0.66(0.02) 0.59(0.05) 0.70(0.00) 1.04(0.13) 0.~'5(0.11)
0.36(.04) 0.49(.02) 0.78(.09) 0.48(.13) 0.69(.37)
brain 2.05(0.84) 1.11(0.06) 0.77(0.0g) 0.76(0.04) 0.65(0.06)
0.75(.11) 0.34(.01) 0.57(.03) 0.25(.00) 0.38(.28)
heart 1.75(0.29) 0.97(0.14) 0.99(0.19) 0.42(0.03) 0.47!~.02)
1.29(.34) 0.68(.15) 0.61(.02) 0.43(.05) 0.27(.05)
1 The numbers for IFN-con are on top and the numbers for lacIF''-
con are on the bottom in bold. Number in parentheses are S.
(n = 3).
2 Calculated from collected blood and assumin~ that 7.3 % of ~he
total body weight is blood
3 Radioactivity in one lobe of liver was measured and calcula_ed
for whole organ.
4 Radioactivity in one kidney was measured and doubled for bo_-
kidney.5 Radioactivity in 10 cm of duodenum was measured
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
As depicted in Table 2 and in Figure 2,
intravenous injection gave an instantaneous distribution
of the drugs in the systemic circulation, and the
lacIFN-con rapidly accumulated in the liver after 3-5
minutes. The amount of native IFN-con accumulated in
the liver was less than one third as compared to lacIFN-
con. Furthermore, the accumulation remained higher than
that of native IFN-con for at least 30 minutes.
C. Biodistribution analvsis in rats
Male Sprague-Dawley rats (250-300 g) were
obtained from Charles River Laboratories and maintained
on a 12/12 light dark cycle and in a controlled
environment of 21~C. A diet of Purina Chow and water
was available ad libitum. The ~nim~l s were caged in
groups of two and five. Three rats (250-300 g) per
group were given the 125I-labeled proteins at a dose of
12 ~g/kg (2 x Io6 cpm/rat) intravenously through the
penile vein. At 5, 60 and 360 minutes, the ~ni~l S were
sacrificed and the whole organs were removed to
determine the total associated radioactivity. Percent
injected dose per gram of organ weight was measured at
various time points. The data is summarized in Table 3.
CA 02209~S7 l997-07-04
W O96/21468 PCTrUS96/00323
-- 19 --
Table 3
Biodistribution of IFN-con and lacIFN-con
in rats1
A~ .
Tissue 5 min60 min 360 min .
blood2 29.92(1.61)6.67(0.21)2.69(0.05)
5.96(0.16)4.56(0.27)2.29(0.13)
liver3 11.01(0.45)3.30(0.20)1.46(0.06)
40.14 (1.73) 4.85(0.37) 1.64 (0.12)
kidney4 18.81(2.11)2.42(1.91)0.68(0.02)
6.69(0.33)1.50(0.34)0.37 (0.02
spleen 0.66(0.03)0.18(0.02)0.06(0.01)
0.15(0.02)0.11(0.02)0.05(0.01)
lung 0.70(0.05)0.32(0.04)0.10(0.00)
0.21(0.03)0.45(0.05)0.84 (0.01)
duodenum5 0.31(0.01)0.43(0.01)0.11(0.01)
0.13 (0.06)0.22(0.02)0.18(0.04)
1 The numbers for IFN-con are on top and the numbers for lacIFN-
con are on the bottom in bold. Number in parentheses are S.D
(n = 3).
2 Calculated from collected blood and assuming that 7.3 % of the
total body weight is blood.
3 Radioactivity in one lobe of liver was measured and calculated
for whole organ.
4 Radioactivity in one kidney was measured and doubled for both
kidney.
5 Radioactivity in 10 cm of duodenum was measured.
Again, as with the hamster study, the
lacIFM-con rapidly accumulated in the liver a~ter 3-5
minutes, and the amount of native IFN-con accumulated in
the liver was less than one third as compared to lacIFN-
con (see also Figures 3 and 4). Furthermore, less than
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 20 -
1% dose was accumulated in the spleen, duodenum, brain
and heart, and the amount of IacIFN-con found in the
serum was 40~ the amount of native IFN-con. Therefore,
despite the fact that the in vitro bioactivity of
lacIFN-con is lower (see Example l), the lacIFN-con is
still advantageous in that it is targeted specifically
to the liver and may provide improved treatment of
certain hepatic disorders. The in vitro assay in
Example l used EMCV as a marker and EMCV is not a liver
virus.
E~AMPT,~ 3
This example relates to pharmacokinetic
analysis in hamsters using IFN-con and lacIFN-con as
prepared in Example l. IFN-con and lacIFN-con was
administered intravenously through the penile vein to
male Syrian Golden hamsters (85-130 g) at a dose of
300 ~g/kg. At 0.05, 0.17, 0.25, 0.5, l, 2, 4, 6 and
8 hr, groups of three animals were sacrificed and blood
samples (lO0 ~L) were collected by cardiac puncture.
Serum samples were obtained using serum separation tubes
(Becton Dickinson).
The serum samples were analyzed with a
sandwich-type ELISA assay. The primary, immobilizing
antibody (polyclonal rabbit derived anti-CIFN antibody),
and the secondary antibody (mouse-derived monoclonal
anti-IFN IgGl), were generated at Amgen Inc. Three
different batches of rabbit polyclonal anti-IFN antibody
were screened. The detection antibody was goat-derived
anti-mouse IgGl conjugated with horse radish peroxidase
(Boehringer Mannheim), and the color development from
the treatment of 3,3',5,5'-tetramethylethylenediamine
(TMB) peroxidase substrate (Boehringer Mannheim) was
used to determine the concentration of CIFN and lac-CIFN
in the serum samples. The sensitivity of this assay has
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 21 -
a linear correlation for consensus interferon
concentration between 0.156 to 10 ng/mL.
Both native and lactose conjugated IFN-con
were able to bind to anti-IFN antibody but with
different degrees of affinity LacIFN-con has a 25%
binding affinity compared to IFN-con to all of the
polyclonal antibodies, and a linear correlation was
obtained for the protein concentration between
0.156 - 2.0 ng/mL. IFN-con and lacIFN-con showed a
similar biphasic clearance mechanism and no significar-
difference in the T1/2 was found (see Figure 5). When
given the same dose, lacIFN-con concentration in hams~er
serum was quickly dropped to about 50 % that of IFN-ccn
3 min after the injection and then maintained about the
same ratio throughout the 8 hr perlod. The initial
distribution half life (alpha-T1/2) for both proteins .~Jas
~11 minutes and the terminal clearance half life (beta-
T1/2) for IFN-con and lacIFN-con was 1.3 and 1.0 hr
respectively. The area under the curve (AUC) and the
apparent volume of distribution (Vss) for IFN-con and
lacIFN-con, however, were very dif~erent. The AUC for
IFN-con and lacIFN-con was 1817 and 691 ng-hr/mL, and
Vss was 198 and 434 mL/kg respectively.
~x~MPLE 4
In this example the in vivo bioactivity of
IFN-con and lacIFN-con was determined in hamsters by
measuring the plasma level of 2',5'-oligoadenylate
synthetase (2-5A synthetase). 2-5A synthetase is an
enzyme produced as a direct response of interferon
binding to its receptor and is believed to be the fir__
step in the mechanism of antiviral activity; see
Interferon, Principles and Medical Applications, 1st
Edition (1992, Univ. of Texas Medical Branch of
Galveston) pages 225-237.
CA 02209~7 1997-07-04
WO96/21468 PCT~S96/00323
- 22 -
Three male Syrian Golden hamsters (85-130 g)
per group were intravenously administered through the
penile vein with 300 ~g/kg of IFN-con and lacIFN-con.~
The vehicle groups were dosed with 100 ~L of PBS. The
~n;m~l S were sacrificed at 6, 12, 24, 48, 72 and 96 hr
and blood samples were obtained by cardiac puncture.
The 2-5A synthetase activity in the serum was assayed
using the 2-5A RIA kit (Eiken, Kitaku, Japan) using the
method described by Sawai et al., Biomed. Res., 9: 59-66
(1988).
The 2-5A synthetase produced in hamster serum
after intravenous administration of IFN-con and lacIFN-
con were compared (see Figure 6). Minimum amounts of
2-5A synthetase activity was found in the hamster
vehicle groups and the time zero group, indicating the
injection procedure induced very low background level of
enzyme production of hamsters. When treated with IFN-
con, the enzyme activity was found elevated in a
biphasic manner. The first peak was found at 24 hour
after the injection and the enzyme activity was found to
be seven times higher than that of the vehicle group.
The enzyme activity was decreased down to base-line
level at 48 hours, and elevated again at 72 hours to
four times higher than that of the vehicle group. At 96
hours, the enzyme activity was lowered down to base-line
level.
For lacIFN-con, the enzyme activity was found
greatly increased at 48 hours and lasted to 96 hours.
The elevation of the 2-5A synthetase activity was ten
times higher than that of the vehicle group at 48 hours.
These results indicate a different enzyme production
pattern for IFN-con and lacIFN-con, suggesting that
lacIFN-con might stimulates very different antiviral
activity as compared to IFN-con, and possibly related to
the different biodistribution profiles of the two
proteins.
CA 02209~7 1997-07-04
WO 96/21468 PCT/US96100323
-- 2-3
EXAMPLE 5
In this example, the in vivo antiviral
activity of IFN-con and lacIFN-con were studied by
challenging the hamsters receiving the proteins with
Encephalomyocarditis virus (EMCV) and then comparing the
survival rates.
Ten male Syrian-Golden hamsters (85-130 g) per
group were injected intraperitoneally with IFN-con and
lacIFN-con at dose of 10 ~g/kg. Six hours later, each
hamster was then injected intraperitoneally with 100 ~L
(2E+4 pfu/mL) of EMCV. The ~nim~l S were monitored twice
daily, and the end point was the sign of hind quarter
paralysis. Hamsters which survived through 27 days were
considered protected ~rom the virus. When the
interferon administered hamsters were challenged by
EMCV, IFN-con protected 86% and lacIFN-con protected S7%
of the animals as observed through 27 days after the
challenge (see Figure 7 ) . The control group which did
not receive inter~eron all died on or be~ore the ~ourth
day after the EMCV challenge.