Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02209906 1997-07-09
WO 96/21355 PCT/GB96/00042
pF~TICIDAT COMPOUNDS
The present invention relates to novel 1,2,3,4-~ulJ~Liluled naphthalene compounds
having utility as pesticides, particularly as insecticides, acaricides and fungicides; to
methods for plc~dlion of these compounds; to compositions co~ li "g them and to use
5 of the compounds and compositions for the conkol of pests.
US 2,572,946 discloses a composition for the control of mites and aphids in which
the active ingredient is a compound of the general formula (P 1)
~ OH (P1)
where R is a radical, cont~inin~; from 6 to 15 carbon atoms, selected from alkyl, cyclohexyl
10 and cyclohexylalkyl groups; n-alkyl, iso-alkyl, alkylcycloalkyl and aralkyl groups are
exemplified but no specific miticidal or aphicidal data being given for them.
DE 2641343 A1 generically discloses compounds of the general formula (P2)
X O ~
0--C--R2 (P2)
Y O
in which R1 is a straight, branched or cyclic Cg 14 alkyl group, R2 is a straight or branched
15 C1 17 alkyl, C2 17 alkenyl, C3 6 cycloalkyl, C1 4 alkoxy, -CH20CH3, -CH20CH2CH3
or -CH=CH-COOH group, and X and Y represent a hydrogen, fluorine, chlorine or bromine
atom or a methyl or methoxy group. These compounds are said to exhibit acaricidal and
aphicidal activity but only compounds where Rl is a linear C8 or Cl 1 14 alkyl group are
shown to have such activity.
CA 02209906 l997-07-09
WO 96/21355 PCT/GB96/00042
US 4,110,473 concerns a method for protecting plants from mites (acarids) which
comprises treating the plant with a compound of the general formula (P3)
O R2
Y O
where Y is hydrogen, fluorine, chlorine or bromine; Rl is branched, cyclic or straight
S chain Cg 14 alkyl; R2 is branched or straight chain C1 12 saturated alkyl or
C3 12 unsaturated alkyl optionally substituted by one or two chlorine, bromine, methoxy
or ethoxy substituents, or C3 6 cycloalkyl.
DE 3801743 Al generically discloses compounds ofthe general formula (P4)
~ (CH2)n--R
10 in which n is 0 to 12, Rl represents hydrogen or an optionally substituted alkyl, aralkyl,
alkylcarbonyl, (hetero)arylcarbonyl, alkoxycarbonyl, alkylsulphonyl or arylsulphonyl
group, and R2 represents a haloalkyl, optionally substituted (hetero)aryl or substituted
cycloalkyl group. These compounds are said to exhibit acaricidal and fungicidal activity.
Ten compounds of formula (P4) are specifically disclosed in which n is 0, Rl is
15 a hydrogen atom and R2 is a 4-(-butyl)cyclohexyl, 4-(trimethylsilyl)cyclohexyl,
4-(cyclohexyl)-cyclohexyl, 2-trifluoromethylcyclohexyl or 3,5-di(trifluoromethyl)-
cyclohexyl group or n is 0, Rl is an ethanoyl group and R2 is a 4-(t-butyl)cyclohexyl,
4-(cyclohexyl)cyclohexyl, 2- or 3-trifluoromethylcyclohexyl or 3,5-di(trifluoromethyl)-
cyclohexyl group. Of these, acaricidal activity is demonstrated for two compounds of
20 formula (P4) in which n is 0, R1 is a hydrogen atom and R2 is a 4-( -butyl)cyclohexyl or
4-(trimethylsilyl)-cyclohexyl group.
CA 02209906 1997-07-09
F,~ 0~\~05PE~6~Zlt
EP û077550 discloses compounds of general forrnula (P5)
O
CH3 (p
0 CH3
in which R is an alkyl group of from 1 to 10 carbon atoms and describes their use in
veterinary formulations, particularly for prophylaxis against protozoan infection.
None of the published prior art relates to insecticidal or acaricidal naphthoquinone
compounds wherein a quaternary carbon atom is linl~ed either directly to the
naphthoquinone ring or linked to it through an n- or iso-alkyl group
Capending international application No. PCT/GB95/00953 relates to naturally
occurring compounds of the general formula (P6)
~ (P6)
~ C (CH3)2--CH = CH2
o
in which R leplesellts a hydrogen atom or a hydroxyl or an ethanoyloxy group, and to their
use as pesticides, especially fungicides, insecticides and/or acaricides. These compounds
were previously disclosed as plant metabolites by Chamy et al., (1993) Bol. Soc. Chil.
Quim 38 187-190.
Fieser et al. J.A.C.S. Vol. 70, No. 6 (1948) disclose the preparation of 2-alkyl-3-
hydroxynaphthalene-1,4-diones where the alkyl groups include ql~t~ ry carbons but
ascribe no pesticidal activity to these with respect to fungi, insects or acarids. This
document is concerned with anti-protozoan activity and the quaternary compounds are
arnong the least active disclosed.
Santisopasri et al. Biosci. Biotech. Biochem. 59(10) 1999-2000 (1995) and AnnualMeeting of the Pesticide Science Society of Japan, Tokyo, March 1993, p55, describe a
naturally occurring anti-fungal napththalene- I ,4-dione where the alkyl group comprises a
--
:
CA 02209906 1997-07-09
F~1~7'021~WOSPEC'~ ')61'1d
2,6-dimethyl-2,6-octadienoxy 2,2-dimethylpropyl group and the corresponding
~,2-dimethyl 3-hydroxypropyl group metabolite
The present inventors have now developed synthetic naphthoquinones and related
compounds having advantageous pesticidal properties as compared to those already known
s in the art, particularly as applied to the treatment of specific pests of fungal, insect and/or
acarid nature. Preferred synthetic compounds of the invention have excellent pesticidal
activity against, inter alia, whitefly and/or mites and/or aphids and/or fungi; most preferred
compounds showing usefill activity against at least two, and preferably all, of these. Many
of the compounds o~the present invention also exhibit anti-feedant activity against at least
10 some insects or acarids.
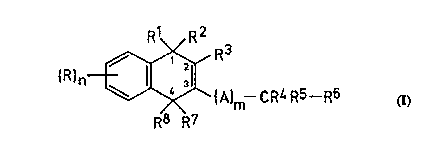
According to a first aspect of the present invention there is provided a compound
of general forrnula (I)
I 2
~"~:\~/ R3 (I)
(A)m--CR4 R5--R6
R8 R7
or a salt thereof, in ~vhich
l S n represents an integer from 0 to 4; m represents an integer 0 or 1;
each R independently represents a halogen atom or a nitro, cyano, hydroxyl, alkyl, alkenyl,
haloalkyl, haloalkenyl, alkoxy, haloalkoxy, haloalkenoxy, amino, alkylamino,
dialkylarnino, alkoxycarbonyl, carboxyl, alkanoyl, alkylthio, alkylsulphinyl,
alkylsulphonyl, carbamoyl, alkylarnido, cycloalkyl, aryl or aralkyl group; characterised in
20 that
R1 and R2 each independently represent an optionally substituted alkoxy group or together
represent a group =O, =S or -N-OR9, where R9 represents a hydrogen atom or an
optionally substituted alkyl group;
R3 represents a hydroxyl group, or a group -OL where L is a leaving group, or a group
25 which in vivo is transformed into a group oL1 where L1 is a leaving group;
R6 represents an optionally substituted alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl,
aryl, alkoxy, alkenyloxy, alkynyloxy, cycloalkyloxy, cycloalkenyloxy or aryloxy group;
-3a-
~p ~c,~
CA 02209906 1997-07-09
F \137\011~WOSPEaA~961218
R7 and R8 independently represent an optionally substituted alkoxy group or together
represent a group =O, =S or =N-OR9, where R9 is as previously defined; and
wherein R4 and RS each independently represent a halogen atom or an optionally
substituted alkyl or alkenyl group, or together with the interjacent carbon atom represent
5 an optionally substituted cycloalkyl or cycloalkenyl ring; and
A represents a straight or branched chain alkyl or alkenyl group, which may be optionally
susbtituted, preferably with halogen, an acyclic carbon chain of which links the 3 position
of the naphthalene ring shown and the moiety -CR4RSR6; ~vith the provisos that when Rl
with R2, and R7 with R8, are groups = O, n = 0, (i) when R4 and R5 are methyl m is 0 and
10 R6 is ethenyl, then R3 is not hydroxyl or ethanoyloxy, (ii) when R4 and R5 are methyl,
m is 0 or m is 1 when A is -CH2- or -(CH2)2- and R3 is hydroxyl then R6 is not methyl,
(iii) when R4 and RS are methyl, m is 1 where A is -(CH2)2- and R3 is hydroxyl then R6
is not chloro (iv) when R4 and R5 together with the interjacent carbon atom form a
cyclohexyl ring, m is I when A is -CH2- and R3 is hydroxyl R6 is not methyl and (v) when
R4 and RS are methyl, m is 1 A is -CH2-and R3 is hydroxyl R6 is not hydroxytmethyl or
the 2,6-dimethyl-2,6-octadienoate ester thereof.
CA 02209906 1997-07-09
WO 96/21355 PCTJ~1~1G/~Oa'12
When the compounds of fo~mula I contain a group defined as an alkyl, alkenyl or
alkynyl substituent otherwise undefined, this may be linear or branched and may contain
up to 12, preferably up to 6 and especially up to 4, carbon atoms. A cycloalkyl or
~ cycloalkenyl group may contain from 3 to 10, but most preferably contains 5 to 8 carbon
atoms. An aryl group may be any aromatic hydrocarbon group, especially a phenyl or
naphthyl group. An aralkyl group may be any alkyl group as defined above which is
substituted by an aryl group as defined above, especially a benzyl group optionally
substituted with an alkyl group.
When any of the foregoing substituents are ~le~ign~te~l as being optionally
substitute~1~ the substituent groups which are optionally present may be any one or more of
those customarily employed in the development of pesticidal compounds and/or themodification of such compounds to infiuence their activity, persistence, penetration or other
property. Specific examples of such substituents include, for example, halogen atoms,
nitro, cyano, hydroxyl, alkyl, alkenyl, haloalkyl, haloalkenyl, alkoxy, haloalkoxy, amino,
alkylamino, dialkylamino, alkoxycarbonyl, carboxyl, alkanoyl, alkylthio, alkylsulphinyl,
alkylsulphonyl, carbarnoyl, alkylamido, cycloalkyl, phenyl and benzyl groups. Typically,
0-3 substituents may be present. When any of the foregoing substituents represents or
contains an alkyl substituent, this may be linear or branched and may contain up to 12,
preferably up to 6, and most preferably up to 4, carbon atoms. When any of the foregoing
substitllent~ represents or contains an aryl or cycloalkyl moiety, the aryl or cycloalkyl
moiety may itself be s~lhstihltPcl by one or more halogen atoms, nitro, cyano, alkyl, alkenyl,
haloalkyl, haloalkenyl, alkoxy or haloalkoxy groups. Preferably, the aryl moiety is a
phenyl moiety and the cycloalkyl moiety contains from 3 to 8, preferably 4 to 7, carbon
atoms.
It is ple~.l~d that R, if present, represents a halogen atom or a nitro, cyano,
hydroxyl, Cl_4 alkyl, Cl 4 haloalkyl, C2 4 alkenyl, C2 4 haloalkenyl, C1 4 alkoxy,
C1 4 haloalkoxy, C1 4 alkylamino, di-CI 4 alkylamino, C1 4 alkoxycarbonyl, C1 4
alkylthio, C 1-4 alkylsulphinyl or C 1-4 alkylsulphonyl group.
More preferably, R, if present, represents a halogen atom or a C1 4 alkyl,
C1 4 haloalkyl, C2 4 alkenyl, C2 4 haloalkenyl, Cl 4 alkoxy or C1 4 haloalkoxy group.
Preferably, n is 0, l or 2 and it is especially preferred that n is 0.
CA 02209906 1997-07-09
WO 96/21355 PCT/{ib5C~ ~ JC ~2
It is also pl~r~-led that R1 and R2 each independently represent a C1 4 alkoxy,
especially a methoxy, group or together represent a group =O or =N-OR9, where R9~pic3e:llL~ a hydrogen atom or a Cl 4 alkyl, especially a methyl, group. It is especially
~.ere.-~d that Rl and R2 are both methoxy or together represent a group =O.
When R3 is a group -OL where L is a leaving group, or a group which in vivo is
transformed into a group -OL1, the leaving group may be any group customarily employed
as a leaving group. It is preferred that the leaving group is such that the PKa value of the
acid LOH in water is from 1 to 7, more preferably from 1 to 6 and especially from 1 to 5.
When R3 represents a group which in vivo is trarlsformed into a group -OL1 whereLl is a leaving group, it is preferred that the transformation is carried out in a plant to be
protected or a pest to be cnmb~t~1, preferably by action of enzymes within the plant or pest.
For instance, if R3 represents a ~-acid group, such as -O-CH2CH2CO-OH where
-CH2CH2CO-OH is not a leaving group, it may be subjected to enzymatic oxidation in vivo
to form a group -O-CO-CH2-CO-OH, eg. by a ~-oxidase, where -CO-CH2-CO-OH is a
leaving group.
Preferably, R3 Lc;~)lcs~;llLs a group -OR10 where R10 represents a hydrogen atom,
an optionally substituted alkyl, alkenyl, aryl or aralkyl group, or a group -CO-R1 1, -CO-O-
R11 SORl 1 SO2 R1 1 P(X)(oR12)(oR13)~ -P(X)(R12)(oR13)~ -P(OR12)(OR ) or
-P(R12)(oR13) where R1 1 represents a hydrogen atom, an optionally substituted alkyl,
alkenyl, aryl or aralkyl group or a group -NR12R13; R12 and R13 independently
representing a hydrogen atom or an optionally substituted alkyl group and X represents an
oxygen or sulphur atom. Where R10 or R1 1 represents an optionally substituted aryl or
aralkyl group, it is ~ r~-.ed that the aryl group is a phenyl group and that the optional
substituents are selected from halogen atoms, nitro and C1 4 alkyl groups. Substitution at
the 4-position of the phenyl ring is particularly preferred. For the purposes of R3, the term
optionally substituted includes, eg. substitution with silicon cont~ining groups, eg.
trialkylsilyl groups such as trimethylsilyl, as a substituent on R10, R11 or R12.
Preferably R3 represents a hydroxyl group or a group -O-CO-R1 1, -O-CO-OR1 1
where R11 represents a hydrogen atom or a C1 12 alkyl, C1 12 haloalkyl, C1 12
hvdroxyalkyl, C1 12 carboxylalkyl, phenyl or benzyl group.
It is particularly preferred that R3 represents a group -OH or -O-CO-R1 1, where
-6 -
CA 02209906 1997-07-09
WO 96~2~355 PCT/<;D5C~ 2
Rl 1 ~G~lesell~ a hydrogen atom or a C 1-6 alkyl, C 1-6 haloalkyl, phenyl or benzyl group.
Most pre~ll.d for Rl 1 is a methyl, ethyl, propyl or butyl group.
Preferably R6 represents a Cl 16 alkyl, C2 16 alkenyl, C1 16 haloalkyl, C2 16
~ haloalkenyl, C1 16 alkanoylalkyl, C1 16 alkoxyalkyl, C1 16 alkoxy, C1 16 haloalkoxy
5 or Cl l6 alkoxyalkoxy group. More preferably these groups are of Cl 6 in length, or C2 6
in length in the case of alkenyls.
Still more preferably, R6 represents a C1 6 alkyl, especially methyl or ethyl, C1 6
haloalkyl, eg. trifluoromethyl, difluoromethyl or monofluoromethyl group, or C2 6 alkenyl
or C2 6 haloalkenyl.
10Preferably, R7 and R8 indep~ndently represent a Cl 4 alkoxy group or together
represent a group =O or =N-OR9, where R9 represents a hydrogen atom or a C 1-4 alkyl
group, but it is especially ~lc~l~d that R7 and R8 are both methoxy or together represent
a group =O.
It will be realised by those skilled in the art that compounds wherein R1 and R2,
15 and R7 and R8 are each alkoxy, or together are =S or a group NoR9 will be potential
biological precursors for the corresponding naphthoquinones, the naphthoquinones being
the preferred compounds of the invention.
Preferably, R4 and R5 each independently represent a C 1-4 alkyl, C 1-4 haloalkyl,
C2 4 alkenyl or C2 4 haloalkenyl group or, together with the interjacent carbon atom,
20 represent an optionally substituted cycloalkyl or cycloalkenyl ring, this ring preferably
being optionally substituted with halogen, alkyl, haloalkyl, alkenyl or haloalkenyl.
The compounds of formula I may form salts, e.g. when R3 represents a hydroxyl
group. Suitable bases for forming such salts include inorganic bases, such as sodium
hydroxide, potassium hydroxide or sodium carbonate, and organic bases, for example
25 tertiary amines such as triethylamine and cyclic amines such as pyrrolidine.
It will be appreciated by those skilled in the art that many of the compounds of the
present invention will exist as different geometric isomers and diastereomers. The scope
of the present invention includes both the individual isomers and mixtures of these.
The present inventors have cletennin~d that the compounds of the present invention
30 are of particular interest in so far as they show pesticidal activity against species and strains
of pest that have developed resistance to currently commercial used pesticides. Thus the
CA 02209906 1997-07-09
W O 96/21355 PCT/~b5~l'UGC~2
compounds of the present invention will have particular application against insect, acarid
and fungal strains that are resistant to other commercially available pesticides. T h e
present inventors have determined that the characteristic ~ ly carbon atom which
links groups R4, R5 and R6 to either group A or the 3 position of the nng shown in formula
5 I may be provided in at least three optional configurations in order to provide particular
activity profiles suited to comh~tin~ of particular groups of pests.
In a first ~ler~,lled distinct group of compounds of the first aspect of the invention
the ~u~Lelll~ y carbon atom is provided imm~ t~ly a~ cçnt to the n~phth~lene nng in the
form of a group -CR4R5R6 wherein R4 and R5 independently represent a halogen or an
10 optionally substituted alkyl or alkenyl group, excluding those compounds of copending
PCT/GB95/00953 described previously and referred to in the proviso appended to the
definitions of formula I.
In this first preferred distinct group compounds of general formula (II)
Dl D2
l\ ~ ~1\
(R)n f~ (II)
CR4RS--R6
R/8 R7
15 or a salt thereof are provided
in which R, Rl, R2, R3, R6, R7 and R8 and n are as defined for formula I and R4 and R5
represent a halogen or an optionally substituted alkyl or alkenyl group .
More ~ulef~ d compounds of the general formula (II) are those where n is 0,
Rl with R2, and R7 with R8 are both =O; wherein R4 and R5 each independently
20 represent a C1 4 alkyl or C1 4 haloalkyl group and R6 represents a Cl 7 alkyl, Cl_7
haloalkyl, C1 7 alkoxyalkyl, Cl 7 alkoxy, Cl 7 alkoxyalkoxy, C2 7 alkenyl, C2-7
haloalkenyl or C2-7 alkoxyalkenyl group. R3 is preferably OH, -O-CO-R1 1 or -O-CO-O-
Rl1 wherein Rll is Cl 3 alkyl, and most preferably -OH. Still more preferably R6
represents a C 1-7 alkyl, C2 7 alkenyl or C 1-7 haloalkyl or C2 7 haloalkenyl group and
most preferably R6 represents a Cl 6 alkyl, C 1-6 haloalkyl, C2 alkenyl or C2 haloalkenyl
group. Most preferably R4 and R5 are methyl.
It is found by the inventors that compounds of this first preferred group generally
--8 -
CA 02209906 1997-07-09
WO 9612135~ PCT/~bi5Cf~ ~~2
have effective pesticidal activity against insects, acarids and fungi, and particularly against
mites and whitefly. Particularly susceptible whitefly are Bemisia species.
In a second l~r~ftll~d distinct group of compounds of the first aspect of the
~ invention the qll~tçn~ry carbon atom is provided as part of a cycloalkyl or cycloalkenyl
ring and thus this second group of preferred compounds of formula (I) are of preferred
formula (III)
R1 R2 3
(R)n~ (A) --C--R6
wherein
n, A, R, Rl, R2, R3, R6, R7 and R8 are as defined for general forrnula (I);
m represents an integer 0 to 1;
and R4 and R5 together with the interjacent carbon atom represent an optionally substituted
cycloalkyl or cycloalkenyl group.
More preferably the col~ ds of this group are of formula (III) wherein Rl with
R2, and R7 with R8 are both =O; n and m are 0; R4 and R5 together with the interjacent
carbon atom represent a fully saturated cycloalkyl ring which is optionally substituted; and
R6 ,eplese--~ a C1 16 alkyl or C2 16 alkenyl group optionally substituted by halogen.R3
is preferably OH, -O-CO-R11 or -O-CO-O-Rll wherein Rll is Cl 3 alkyl, and most
preferably -OH
Still more preferably R4 and R5 together with the interjacent carbon atom represent
a C4 8 saturated cycloalkyl ring which is optionally substituted, most preferably with
chlorine or fluorine; still more preferably being a C5 8 cycloalkyl ring~ and R6 is a C 1-6
alkyl, C2 6 alkenyl, Cl 6 haloalkyl group, C2 6 haloalkenyl group or a halogen.
Exceptionally active compounds of this group are those where R4 and R5 together with the
interjacent carbon atom represent a cyclohexyl ring and R6 is Cl 2 alkyl or C2 alkenyl.
Preferred compounds ofthis second preferred group ofthe invention are particularly
effective against mites and whitefly ~ as well as against certain fungi, with the most active
compounds having exceptional activity against whitefly, particularly of Bemisia species
CA 02209906 l997-07-09
WO 96/21355 PCTIGB96/00042
while retaining activity against mites.
In a third distinct group of compounds of the first aspect of the invention the
q1l~tern~ry carbon atom is not in a cycloalkyl or cycloalkenyl group and is provided at
between 2 and 16 carbon atoms length away from the naphthalene ring, more preferably
5 between 2 and 10. Most preferably the 4ualelll~ y carbon is between 4 and 8 carbon atoms
length away from the naphthalene ring.
Thus in this distinct group pler~ d compounds of formula (I) are of preferred
formula (IV)
Rl R2
(R~n--~ (IV)
~\ A--CR4 R5--R6
R~8 R7
1 0 wherein
n, A, R, Rl, R2, R3, R6, R7 and R8 are as defined for general formula (I),
and
R4 and R5 each independently represent a halogen or optionally substituted alkyl or alkenyl
group.
For the purpose of combatting whitefly the group A preferably has a chain of
between 3 and 7 carbon atoms between the naphthalene ring and the quaternary carbon
atom, particularly when it is an unbranched alkylene chain.
For the purpose of providing high efficacy against aphids the group A preferablyhas a chain of between 4 and 8 carbon atoms between the naphthalene ring and the20 quaternary carbon atom, particularly when it is an unbranched alkylene chain.For both these circumstances, the presence of one or more branch chains on the
linking carbon chain of A will allow peak activity to be obtained using a shorter length
chain between the naphthalene ring and q1l~tf~rn~ry carbon.
More preferred compounds of this group are of formula (IV) wherein R l with R
25 and R7 with R8 are both =O; m is 1, and A is a C3 8 alkyl or alkenyl chain, which may be
substituted by a halogen or a branch chain which may be halogenated. Preferably R4 R~
and R6 are C 1-6 alkyl or haloalkyl or C2 6 alkenyl or haloalkenyl. R3 is preferably OH.
-10-
CA 02209906 1997-07-09
WO 9612135~ PCT/<~ 2
-O-CO-Rl 1 or -O-CO-O-R1 1 wherein Rl 1 is Cl 3 alkyl, and most preferably -OH.
Preferred compounds of this group are those where A is a group -(CH2)a-, where
a is an integer of 1 to 7, or a group -(CH2)a-CH=CH-(CH2)b- where a and b are integers
which add up to 0 to 6, more preferably 0 to 5 and most preferably 0 to 4, and analogues
5 of these wherein one or more of the carbon atoms in the these groups are substituted by
alkyl, haloalkyl, alkenyl, haloalkenyl or halogen.
A second aspect of the present invention provides the use of a compound of
formula (I) as a pesticide; particularly as an insecticide, acaricide and/or fungicide,
particularly against mites, whitefly, aphids and/or fungi. Particularly susceptible whitefly
10 include those of Bemisia species. Particularly susceptible aphids include those of Myzus and
Aphis species. Particularly susceptible fungi include those of Aspergillus, Pyricularia,
Phizoctonia, Erisiphe and Botrytis species.
A preferred use of this second aspect uses a compound of formula (II) as a pesticide
against insects, acarids and/or ~ungi.
A second ~l~fe.l~ d use of this second aspect uses a compound of formula (III) as
a pesticide against mites, aphids and/or whitefly.
A third preferred use of this second aspect uses a compound of formula (IV) as apesticide against mites and/or aphids.
In addition to the direct pesticidal activity, ie. direct lethal toxic activity, exhibited
by the compounds of formula (I), (II), (III) and (IV), the present inventors have determinecl
that they also exhibit anti-feedant activity on insects of many types, particularly Diabrotica
species (Western and Eastern Corn Root Worm), Lepidoptera such as Spodoptera littoralis
and Spodoptera Jrugiperda and Beetles such as Phaedon cochleaiae Fab as well as against
those species specified above. Thus the present invention also provides use of compounds
of the present invention as pesticides operating as insect and acarid anti-feedants.
A fourth aspect of the present invention provides a method of combating pests,
particularly insect, acarid and fungal pests7 at a locus which comprises treating the locus
with a compound of the general formula (I), preferablv being of general formula (II), (III)
or (IV).
Preferably, the locus comprises the pests, ie. insects, acarids and/or fungi, per se or
environments subject to or subjected to attack by the pests. More preferably. the locus
-I 1-
CA 02209906 1997-07-09
WO 96/2135S PCTl(~b5G/C~ ~2
comprises the pests per se, stored food material, plants or ~nim~l~ subject to or subjected
to attack by pests, seeds of such plants or the medium in which such plants are growing or
are to be grown. Specifically, compounds of formula I may be used in a domestic
environment for spraying rooms to combat infestation by houseflies or other insects, acarids
5 or fungi, in a horticultural or agricultural environment for treatment of stored crops,
epecially cereals, or to spray growing crops such as cotton or rice to combat infestation by
pests, particularly whitefly and related species, and in a medical or veterinary environment,
for instance, as a cattle spray to prevent or treat infestation by insects or acarids.
In a fifth aspect the present invention also provides processes for the pl-,p~dLion of
10 compounds of formula (I) and particularly of formula (II), (III) and (IV) as defined above.
Where the compound is one in which m is 0 in formula (I) such method may
comprise reacting a compound of the general formula (V)
o
in which n, R and R3 are as defined above, R3 particularly being -OH, with a compound
15 of the general formula (VI).
R111 ~ R121
X1 C ~ \ Rs (VI)
R131
in which X represents a leaving group, preferably a hvdroxyl group or a halogen, especially
a chlorine or bromine, atom; Rll1, R121 and R131 each independently represent a
hydrogen atom or an optionally substituted alkyl group and R4 and R5 are as defined in
20 formula (I). to produce a compound of the general formula (VII)
CA 02209906 1997-07-09
WO 961213S5 PCT/<~ OOO t2
R131
(R ) n~ 'C~Rl2~ C~ RS (Vll)
in which n, R, R1 1 1, R121, R131, R4 and R5 are as defined above. When X represents a
hydroxyl group, the reaction may be carried out under Mitsunobu reaction conditions, that
is, using diethyl azodicarboxylate and triphenylphosphine in, for exarnple, tetrahydrofuran
5 at 0~C. When X represents a halogen atom, the reaction may be carried out under
alkylating conditions, that is, using a suitable solvent, such as dichloromethane, and a base,
such as triethylamine.
The compound of formula (VII) may then be heated in a suitable solvent, preferably
an alcohol such as ethanol, to effect a Claisen-type rearrangement resulting in a compound
10 of the general formula (VIII)
o
(R)n =~C~c ~C ~ R
It is also possible to react an alkyl aldehyde directly with the compound of formula
(V) in a polar organic solvent under alkaline conditions, eg. with pyrrolidine, and then heat
the product under acidic conditions, eg. p-tol~l~n~slllphonic acid in a non-polar solvent such
as benzene, to effect elimination of water to yield a 3-alkenyl substituted naphthalene ring
compound.
Compounds of formula (VIII) correspond to compounds of formula (I), (II) and (IV)
in which R6 represents an optionally substituted alkenyl group and may be converted into
other compounds of formula (I) by various derivatisation processes.
For instance, compounds of formula I in which R6 represents an optionally
substituted alkyl group may be produced by hydrogenation of a suitable compound of
formula (VIII), for exarnple. using hvdrogen gas with palladium on charcoal as a catalyst.
CA 02209906 1997-07-09
WO 96/21355 PCT/GB96/00042
Most compounds of formula (V) are available commercially, but in any case may beprepared from the eorresponding 2-hydroxybenzoquinone by, eg. Diels Alder reaction.
In an ~lt~m~tive proeess for ~r~ g eompounds of formula (I), (II), (III) and (IV),
whieh has partieular applieability in produetion of compounds of formula (I) where m is
5 1, i.e. of formula (III) or (IV), a eompound of the general formula (V)
~ (V)
in which n, R and R3 are as defined above, is reacted with a earboxylie aeid
CR4RsR6-(A)m-COOH where A, m, R4, R5 and R6 are as defined above, in the presenee
of a free radical initiator, such as ~mmt)nillm peroxysulphate and silver nitrate in a suitable
10 solvent, sueh as aqueous acetonitrile, to form a eompound of the general formula (I), (II),
(III) or (IV).
Compounds of formula (I) obtained in this manner may then be further reaeted
using the derivatisation proeesses deseribed above or eombinations thereof to obtain further
compounds of formula (I), as desired.
For use in this alternative method, in the ease where R4 and R5 together with their
interjaeent earbon atom form a cycloalkyl or cycloalkenyl ring of from 3 to 10 carbons,
many of the 1-methylcyeloalkyl and eyeloalkyleneearboxylic aeids are eommereially
available and the carboxylic aeid groups thereon may be extended by known teehniques
to give aeeess to longer carbon chain lengths, and then substituted if required using
20 techniques well known to those skilled in the art. For example the Arnst-Eistert reaction
may be used to give a -CH2- extension (see eg. Meier and Zeller (1975) Angew. Chem. Int.
Ed. Ewgl., 14, 32). Alternatively compounds where m is 1 may be ~cc~sed by the reaction
of the corresponding cycloalkanone with ethyl cyanoacetate and subsquent reaction with
a Grignard reagent, followed by hydrolysis to yield the (1 '-substituted-cycloalkyl)-acetic
25 acid (see e.g. Amsterdamsky et al (1975) Bull. Soc.Chim.Fr. (3-4 Part 2), p635-643 and
Muhs M. A. PhD Thesis, University of Washington, Diss Abst.14, 765 (1954) to increase
the carbon chain length in increments of 1.
-14-
CA 02209906 l997-07-09
WO 96J21355 PCT~GB96/aOa42
For ~ ~d~ion of compounds co~ R4 R5 incorporated within rings having
higher numbers of carbons, the corresponding monobromo-substituted cycloalkyl orcycloalkenyl compounds may be converted to the carboxylic acids by formation of the
- Grignard compound using m~gneciurn and then treating this with CO2, eg. in the form of
dry ice. The carboxylic acid so formed may be converted to the l-alkyl carboxylate by
alkylation using, eg. a compound R6-I, e.g. methyl iodide, in the presence of butyllithiurn,
where R6 is a group as defined above that is stable under these conditions.
For production of l-fluoro-cycloalkyl/cycloalkenyl carboxylic acids the method of
CA 75:17761u may be used wherein buta-1,3-diene is reacted with 1-fluoro- 1-carboxy
10 ethene in the presence of 4-hydroxy-phenol with h~ting, with subsequent reduction of the
ring unsaturation to convert the cycloalkenyl to the cycloalkyl compound. Alternatively the
corresponding 2- keto-cycloalkyl-carboxylate compound may be reacted with sodiumethoxide and fluorine gas to give the 1-fluoro-2-keto-cycloalkyl-carboxylate and the keto
group may then be reduced using (i) MeSH (ii) R~ney Nickel and (iii) potassium hydroxide
1~ base(seeJ. Org Chem.(1983)48,724-727andJ. Org Chem.(1982) 47,3242-3247.
Substitutions, e.g. alkylation, of the cycloalkyl/cycloalkylene ring at positions in
addition to the l-position to the carboxylate may be accomplished by methods known to
those skilled in the art. Starting from the ring mono-unsaturated cycloalkylene carboxylic
alkyl esters, alkylation may be directed at the l-position as previously described and then.
20 using light as initiator, reaction with a compound R20-X where R20 is alkyl or haloalkyl
and X is halogen, eg. a with a compound CF3X, allows introduction of alkyl or haloalkyl
groups, eg. CF3- groups. Thereafscer reduction using palladium-carbon catalysis conditions
allows saturation of the unsaturated bond.
Access to 1-trifluoromethyl cycloalkyl/cycloalkenyl carboxylates may be gained
25 e.g. by reacting the methyl ester of the cycloalkyl/cycloalkenyl-carboxylate with
e.g. trifluoromethyl iodide in the presence of LDA, or reacting the
1-keto cycloalkyl/cycloalkenyl-carboxylate with trifluoromethyl iodide in the presence of
triethylamine followed by reduction. Alternatively 2- trifluoromethyl acrylic acid may be
reacted with an optionally substituted buta-1,3-diene with heat in the presence of
30 4-hydroxyphenol to yield the optionally substituted l-trifluoromethyl cycloalkenyl
carboxylic acid.
-15-
CA 02209906 l997-07-09
WO 96/213SS PCT/(~b5G;~ 2
Access to compounds where R6 is ul~s~ Led may be obtained through use of the
methodology of Wood et ai. J. Chem. Soc Perkin Trans 1 ( 1985) 1645-1659 to produce a
compound (IX), wherein R141 is hydrogen or an optionally substituted alkyl, alkenyl,
alkynyl, aryl, alkoxy, alkenoxy, alkynoxy or aryloxy, which compound is reacted with the
S naphthoquinone under conditions effecting rearrangement as described above. Reduction
of such compounds allows access to iso-alkyl groups.
C
/ \ (IX)
R~JR5
In a still further process for pr~pal~lion of the compounds of the present invention
10 the compound of formula (V) is reacted with a compound of formula X-(A)m-CR4R5R6
wherein R4, RS, R6, A and m are as defined for formula I and X is a leaving group that will
leave the compound to give a charged radical +(A)m-CR4R5R6; eg. X may be a halogen
atom or tosyl group. This reaction is carried out in the presence of an acid, eg. a Lewis acid
such as aluminium chloride, using conditions broadly as described by Fieser and Gates (J.
lS Am. Chem. Soc. (1941) 63, 2943-2953.
Many other manipulations will occur to those skilled in the art for the purposes of
accessing other compounds of the general formula (I).
Compounds of formula (I) in which R3 represents a leaving group as defined abovemay be prepared by reacting a compound of formula (I) in which R3 ~ S~ ; a hydroxyl
20 group with a compound X-L, where X represents a halogen atom, in the presence of an
organic base, preferably a tertiary amine such as triethylamine. or an inorganic base such
as sodiurn carbonate. For instance compounds of formula I in which R3 represents a group
-O-CO-R11, where R11 is as defined above, may be prepared by acylation of the hydroxy
group in a suitable compound of formula V, for instance, by using an acyl chloride
25 R11-CO-CI in a suitable solvent, such as dichloromethane, in the presence of a base, such
as triethylamine. Alternatively compounds of formula I in which R3 represents a hydroxyl
group mav be reacted with an acid compound HO-L where L is as defined above and
-16-
CA 02209906 1997-07-09
WO 96121355 PCI/GB96/00042
includes the acid C=O moiety, in the presence of a dehydrating agent such as
dicyclohexylcarbodiimide. A further route to such compounds is provided by reacting
metal salt of a compound of formula (I) in which R3 represents a hydroxyl group, that is,
R3 rcpl. sents a group -OM where M is a metal ion, with a compound X-L as defined
S above.
Compounds of formula (I) in which R1 with R2 and/or R7 with R8 each
independently represent an optionally substituted alkoxy group may be prepared by
ket~ tion of one or both carbonyl groups in a suitable compound of formula (V) or
the corresponding compound of iEormula (I), for instance, by using a suitable alcohol in
10 basic or acidic conditions, such as by use of a solution of potassium hydroxide in methanol.
Compounds of formula (I) in which R1 with R2 together and/or R7 with R8
together represent a thiocarbonyl group =S may be ple~ d by treating a suitable
conl~olJl,d of formula (I), wherein Rlwith R2 and R7 with R8 are both =O, with a thiating
agent, such as Lawesson's Reagent (2,4-bis(4-methoxyphenyl)- 1 ,3-dithia-2,4-
15 diphosphetane-2,4-tli~l-lphide), using protecting groups where required.
Compounds of formula (I) in which Rl with R2 together and/or R7 with R8
togethemG~lcsclll an oxime group =N-OR9, where R9 is as defined above, may be prepared
by treating a suitable compound of formula (I), wherein Rlwith R2 and R7 with R8 are
both =O, with a hydroxylamine or alkoxylamine of formula R9O-NH2, where R9 is as20 defined above, in the presence of a base, such as pyridine.
Combinations of the above derivatisation processes may be performed to achieve
the desired compound of formula (I).
In a sixth aspect of the present invention a composition is provided which
comprises a compound of formula (I) and preferably of formula (II), (III) or (IV), as
25 defined above, in association with at least one carrier. Such a composition may contain a
single compound or a mixture of several compounds of the present invention. It is also
envisaged that different isomers or mixtures of isomers may have difr~lclll levels or spectra
of activity and thus compositions may comprise individual isomers or mixture of isomers.
The compositions ofthe invention typically contain from 0.00l to 95% by weight
30 of the active ingredient of forrnula I. Preferably the compositions contain from 0.001 to
25% by weight of the active ingredient when they are in ready-to-use form. However,
CA 02209906 1997-07-09
WO 96/21355 PCTlGB~ 2
higher concentrations, for instance, up to 95%, may be present in compositions to be sold
as conc~nlldles for dilution before use.
The compositions of the invention may be mixed with a variety of d~plo~l;ate inert
carriers such as solvents, diluents and/or surface-active agents to form dusts, granular
solids, wettable powders, mosquito coils or other solid pr~alaLions or emulsions,
em~ ifi~hle concentrates, sprays, aerosols or other liquid pl~aldlions. Suitable solvents
and diluents include water, aliphatic and aromatic hydrocarbons such as xylene or other
petroleum fractions and alcohols such as ethanol. Surface-active agents may be of an
anionic, cationic or non-ionic type. Anti-oxidants or other stabilisers may also be included
as well as perfumes and colourings. These inert carriers may be of the type and in
proportions such as are conventionally used in pesticidal compositions and thus are
conveniently inert with respect to the physiology of a plant to be treated.
Examples of carriers known to be suitable for use in compositions incorporating
naphthalene- 1,4-diones for pesticidal use include those described in the specifications, and
more specifically the Examples, of US 2572946, US 4110473, US 4970328 and JP
90/152943 (the latter to Agro-Kanesho KK).
In addition to these inert carriers, the compositions of the invention may also
contain one or more further active ingredients. These further active ingredients may be
other compounds which exhibit pesticidal activity and these other compounds may exhibit
a synergistic effect with the compounds of the present invention.
The present invention will now be described further by way of illustration only by
reference to the following non-limiting Examples and Co~ ~dLi~e Examples. Furt}le~
embodiments of the invention will occur to those skilled in the art in the light of these.
FXAMPLES
Examples 1 to 23 relate to the plepaldlion and properties of compounds of
formula (II) forming the first part of the first aspect of the invention; Examples 24 to 34
relate to the preparation and properties of the compounds of formula (III) forming the
second part of the first aspect of the invention, and Examples 35 to 50 relate to the
preparation and properties of compounds of formula (IV) forming the third part of the first
aspect of the invention together with an example of a compound of formula (II). Example
-lg-
CA 02209906 1997-07-09
WO 96~2~35~ PCT/~ 2
46, for comparison. Examples S l to 53 describe production of intermediate compounds of
formula (V) where n is i or more. Table 14 provides co~ ~d~ e data relating to
compounds where the 3-substituent is straight chain alkyl. Starting m~t~ were
- purchased from Aldrich Chemical Company.
Example 1
P~ dlion of 2-(1. I -dimethylpropyl)-3-hydroxynaphthalene- 1.4-dione
(Formula I: n and m = 0; Rl + R2 together and R7 + R8 together both represent =0,
R3=-oH; -CR4R5-=-C(CH312-; R6=-C2H5,~,
10 (a) Pl~aldlion of 2-(3-methylbut-2-enyloxy)-naphthalene-1.4-dione
To a stirred solution of 2-hydroxynaphthalene-1,4-dione (10.0 g, 57.4 mmol) and
triphenylphosphine (15.1 g, 57.4 mmol) in dry tetrahydrofuran (150 ml) at 0~C under
an atmosphere of nitrogen was added diethyl azodicarboxylate (10.0 g, 57.4 mmol).
After stirring for a further 5 minllt~s, a solution of 3-methylbut-2-enol (7.42 g, 86.1 mmol)
15 in dry tetrahydrofuran (10 ml) was added dropwise and stirring was continued
for 2 hours. The precipitate was collected, air-dried and recryst~ ecl from aqueous
methanol to yield 2-(3-methylbut-2-enyloxy)naphthalene-1,4-dione (8.3 g) as a yellow
crystalline solid, m.pt.: 138~C.
(b) Pl el)a, d~ion of 2-(1. I -dimethylprop-2-enyl!-3-hydroxy-naphthalene- 1.4-dione
20 A solution of 2-(3-methylbut-2-enyloxy)naphthalene-1,4-dione (4.27 g, 24.8 mmol)
obtained in (a) above in absolute ethanol (125 ml) was refluxed for 6 hours. Themixture was cooled and the solvent removed in vacuo. The residue was dissolved in
diethyl ether and extracted with 1 % (w/v) aqueous sodium hydroxide solution (6 x 25 ml).
The combined basic fractions were acidified to pH S with 2M hydrochloric acid and
25 extracted with diethyl ether (6 x 25 ml). The combined ethereal extracts werewashed successively with water (2 x 25 ml), saturated aqueous sodium chloride solution
(25 ml) and dried over anhydrous magnesium sulphate. Filtration and evaporation of
the solvent under reduced pressure followed by recrystallisation from aqueous methanol,
- yielded 2-(l~l-dimethylprop-2-enyl)-3-hydroxyn~rhth~lene-l~4-dione (4.27 g) as a yellow
30 crystalline solid, m.pt.: 600C.
-19-
CA 02209906 l997-07-09
WO 96/21355 PCT/GB~ 12
(c) P~ dlion of 2-(1.1 -dim~lhyl~ yl)-3-hydroxy-naphthalene-1.4-dione
A mixture of 2-(1,1-dim~lhyl~lol,-2-enyl)-3-hydroxynaphthalene-1~4-dione
(2.00 g, 8.3 mmol) obtained in (b) above and 10% palladium on charcoal (50 mg) in
absolute ethanol (30 ml) was stirred under an atmosphere of hydrogen (balloon) at room
5 temperature (about 20~C) for 1 hour. The mixture was filtered through "CELITE"(Registered Trade Mark) (acid washed, approx. 95% SiO2) and the solvent evaporated
under reduced pressure. The residue was recryst~ e~l from methanol-petrol to yield
2-(1,1-dimethylpropyl)-3-hydroxynaphthalene-1,4-dione (1.98 g) as a yellow crystalline
solid, m.pt.: 52~C.
10 F~n~ple 2
Preparation of 2-(1. I -dimethylpropvl)-3-ethanoyloxy-naphthalene- 1.4-dione
(Formula I: n and m = 0; Rl + R2 together and R7 + R8 together both represent = O;
~3=-O-CO-CH3: -CR4~5-=-C(CH3l2-: R6=-C2Hs~
To a stirred solution of 2-(1,1-dimethylpropyl)-3-hydroxynaphthalene-1,4-dione
15 (2.00 g, 8.2 mmol) obtained as in Example 1 above in dry dichloromethane (20 ml) at
0~C was sequentially added pyridine (0.5 ml) and ethanoyl chloride (2.59 g). Themixture was then stirred for 30 minutes before diluting with diethyl ether, washing with
water, saturated sodium hydrogen carbonate solution and saturated sodium chloride
solution and drying over magnesium sulphate. Filtration and evaporation of the solvents
20 under reduced pressure followed by silica gel column chromatography yielded
2-(1,1-dimethylpropyl)-3-ethanoyloxynaphthalene-1,4-dione (2.06 g) as a yellow
crystalline solid, m.pt.: 53~C.
Fx~m~le 3
Preparation of 2-(t-butyl)-3-hydroxy-naphthalene-1.4-dione
25 (Formula I: n and m = 0; Rl + R2 together and R7 and R8 together both represent = O;
~3=-oH: -CR4_5-=-C(CH3l2-: R6=-CH3l
2-Hvdrox~naphthalene-1.4-dione (1.00 g, 5.68 mmol), pivalic acid (870 mg. 8.51 mmol)
and silver nitrate (568 mg) were heated in a mixture of acetonitrile (20 ml) and water
(20 ml) at 60-65~C. A solution of ammonium peroxysulphate (1.94 g. 8.51 mmol) in30 ~ater (10 ml) was added dropwise and the mixture heated for 1 hour. The mixture was
-20-
CA 02209906 1997-07-09
WO 961~1355 PCT/GB96/00042
then cooled to room temperature (about 20~C), diluted with diethyl ether and extracted
with 1% (W/V) aqueous sodium hydroxide solution (4 x 25 ml). The combined aqueous
layers were then acidifled with 2M hydrochloric acid and extracted with diethyl ether
(3 x 25 ml). The combined ethereal extracts were then washed with water and saturated
sodium chloride solution and dried over m~gne~sium sl-lrh~te. Filtration and evaporation
of the solvent under reduced pressure and purification by silica gel column
chromatography yielded 2-(t-butyl)-3-hydroxynaphthalene-1,4-dione (450 mg) as a
yellow crystalline solid, m.pt.: 89~C.
Examples 4 to 11 and 13.
By processes similar to those described in Exarnples 1 and 2 above, further compounds
according to the invention were prepared as detailed in the Table I below. In this table, the
compounds are identified by reference to formula I.
Fx~rnple 12
Pl epal dtion of 2-(1 .1 -dimethylprop-2-enyl)-3 -methoxy-naphthalene- 1 .4-dione .
(Formula I: n and m = 0; R1 + R2 together and R7 + R8 together both represent =O,
R3 =-OCH3; m is 0; -CR4B5- =-C(CH3~2-: R6 = -CH=CH2)
To a stirred solution of 2-(1,1-dimethylprop-2-enyl)-3-hydroxy-naphthalene-1,4-dione
(50mg, 0.21 mmol) in ether (5ml) at 0~C under nitrogen was added an ethereal solution of
diazomethane (2ml). After 2 hours the solvent was removed under reduced pressure and the
residue purified by silica gel column chromatography to yield the title compound (47mg).
F~ le 14.
P~ ion of 1,1 -dimethoxy-2-( 1.1 -dimethvlprop-2-enyl)-3-hydroxynaphthalene- 1.4-
dione.
(a) 2-(1 1 -dimethvlprop-2-enyl~-3 -acetyloxv-naphthalene- 1 ~4-dione .
- 25 The standard acetylation procedure of Example 2 was repeated on compound 1 (b) ( 1 .00g,
4.1 3mmol) to give the title compound .
(b! 1~1 -dimethoxy-2-hvdroxy-3-( 1. I -dimethylprop-2-enyl)-naphthalene-4-one.
To a stirred solution of compound 14(a) (750mg, 2.63mmol) in methanol (30ml) and THF
CA 02209906 1997-07-09
WO 96/21355 PCT/GB96/00042
(Sml) was added an aqueous solution of potassium hydroxide (l .Og) in water (1 Oml). The
mixture was stirred for 1 hour before reducing to half volume and diluting with water
(20ml) and then the aqueous mixture was extracted with ether (3 x 20ml). The combined
ether fractions were washed successively with water (2 x 20ml), saturated sodium5 carbonate solution (3 x 20ml), water (2 x 20ml), saturated sodium chloride solution (20ml)
and dried over magnesium sulphate. Filtration and evaporation as solvent under reduced
pressure and silica gel column chromatography yielded the title compound (173mg).
Fxample 15
Pl ~a- aLion of 2-(1.1 -dimethylprop-2-enyl)-2-hydroxy- 1 -methoxyimino-naphthalene-4-
10 onç.
A solution of the product of Example 1 (b) (250mg, 1.03mmol) and methoxyaminehydrochloride (95mg, 1.14mmol) in pyridine (5ml) was stirred for 48 hours. The reaction
mixture was dissolved in ether (50ml) and washed with water (2 x 1 Oml), 2M hydrochloric
acid (2 x 1 Oml), water (2 x 1 Oml), saturated sodium chloride solution (1 Oml) and dried over
15 magnesium sulphate. Filtration and evaporation of the solvent under reduced pressure and
silica gel chromatography yielded the title compound (56mg).
CA 02209906 1997-07-09
WO 96/213S5 PCT/~5C~ C ~2
~ o ~ ~ ~ o~ o
~ O ~ ~ ~ ~
s:
a~
,_ o
C ~ ~ oo
o
oo
C~
~Y ~ 11 = = = = = = =
s: Y
_, 11
X X X X X X -- X
~ O ~ O ~ o, .C
a
, ~
C~ O
X ~1
'3 X
~ 1~l = = = = .~:
C~ ~
. _
o~
-- ~ loo o~ ~ _
~
-23 -
CA 02209906 1997-07-09
WO 96/213SS PcTl(~;b5clooo~2
3 ~I
o ~ o
~_ _
~'
C
o
oo
r
C~ t~ ~ _.
E , ~ C ) V
o
_, 11
~ 11 ,~ V~
¢ ~ ~ ~ ~ ~ = - O o v~
r ._
U~ ~
o
X ~ O _
R = o R = = ~r
.- ~ ~
a~
. _
~ Z ~ ~ -- -- ~ _ ~
x
zl
-24-
CA 02209906 l997-07-09
WO 9612~35~i PCT/GB96/00042
F.xamrle 19
Pesticidal Activity
- Pesticidal activity was assessed against houseflies, mustard beetles, diamond-back moths
(larvae), mites and whitefly using the following methods.
Houseflies (MD) (Musca domestica)
Female flies were tre~ted on the thorax with a one microlitre drop of test
compound dissolved in acetone. Two replicates of 15 flies were used at each dose rate
and 6 dose rates were used per compound under test. After tre~tment, the flies were
m~int~ined at a t~ GldlUl~ of 20~ ~ 1~C and kill was assessed 24 and 48 hours after
treatment. LDso values were calculated in micrograms of test compound per fly
(see Sawicki et al., Bulletin of the World Health Or~nis~tion, 35, 893 (1966) and
Sawicki et al., Entomologia and Exp. Appli 10, 253, (1967).
Mustard bçetles (PC) (Phaedon cochleariae Fab)
A one microlitre drop of an acetone solution of the test compound was applied
ventrally to adult m~l~i~d beetles using a micro drop applicator. The treated insects were
maintained for 48 hours after which time kill was assessed. Two replicates each
of 20 to 25 mustard beetles were used at each dose level and 5 dose levels were treated
comparably. LDso values were calculated as for houseflies.
Diamond-back moth (PX) (Plutella xvlostella)
Fifth instar larvae were treated with a 0.5 ,ul drop of test compound in acetone.
Three replicates of 10 larvae each were used at each dose rate and 5 dose rates were
used per compound under test. After treatment, the larvae were m~int~ined at about
22~C. and kill was assessed as failure to pupate 5 days later. LDso values were
calculated as for houseflies.
CA 02209906 lgg7-o7-o9
WO 96/21355 PCT/Gs96/00042
Mites (TU) (Tetranvchus llrticae!
25 adult female mites were immersed in 35 111 of a solution of the test compoundin a 1:4 acetone-water ~ for 30 seconds. The treated insects were m~int~inedat 21~ ~ 2~C and kill was ~ssessed 72 hours after tre~tm~nt Mites exhibiting repetitive
5 (non-reflex) movement of more than one locomotory appendage after this period were
recorded as alive. Three replicates of 25 mites each were used at each dose rate and
5 or 6 dose rates were used per compound under test. LCso values were calculated in ppm
ofthe solution of the test compound per insect. The test was carried out using a susceptible
strain of mites (GSS) supplied by Schering, AG, Berlin.
10 Whitefly (BT) (Bemisia tabaci)
Acetone solutions (0. 1 00 ml) of the test compounds were placed in 10 ml glass vials
and evaporated with rotation to deposit a film of the compound. Thirty adult
whiteflies were placed inside the vial, then after 60 mimltes, the treated insects were
transferred onto untreated cotton leaf discs which were kept moist on a bed of agar gel.
15 The temperature was m~int:~ined at 25~C and mortality assessed after 48 hours. Three
replicates were used at each of 5 to 7 dose levels per compound. LCso values (ppm
solution) were calculated by using a computer software package ("Polo-PC available from
LeOra Software, Berkeley, California) (See M.R. Cahill and B. Hackett in Procee~ing.s
Brighton Crop Protection Conference, 1992). The test was carried out using a
20 susceptible strain of whitefly (SUD-S) which was collected in Sudan in 1978 from
cotton.
The results of these tests are set out in Table 2 below. The values given are inLDso (~lg/insect) and LCso (ppm solution of test compound) unless otherwise stated.
In all the tables set out in this specification lack of detectable activity is indicated
25 by 'NA' and the absence of test data by '-'.
-26-
CA 02209906 1997-07-09
WO 96/2135~ PCTIGB96100042
TABLE 2
CompoundMD PC PX TU(GSS) BT(SUD-S)
Example No. (LD50) (LD50) (LD50) (LC50) (LC50)
<10 c. 10 - 39 19
2 <10 c.6.0 c. 10 64 4.8
3 c.2.5 c.6.0 c.8.0 81 8
4 8.2 3 .9 - 1 8 5.3
- 6.9 - 84 13
6 c. 2.0 2.1 - 27 16
7 > 20 c. 6.0 - 53 < 100
8 - c. 8.0 c. 0.1 630
9 - 0.41 - 140 13.4
>20 c.6.0 - 15 35
11 - - c. 50
12 >20 c. 12.0
13 >20 >20 c.8.0 >1000 >1000
14 c. 2.5 c. 6.0 - c. 800
- 35% * - - 97%**
16 8.8 c. 10 - 91 10
17 14 NA - 50
18 13 c. 15 - clO0 10
A >> 20 0.36 - 64 82
* % Kill at20 !lg/insect (LD50)
* * % Kill at 1000 ppm solution of test compound
Compound A = Example 1, page 5 of DE 2641343 Al which is
2-n-dodecyl-3-ethanoyloxynaphthalene- 1 ,4-dione
S (Formula I: n and m= 0; R1 + R2 together and R7 + R8 together are
both = O; R3 = -O-CO-CH3: R4 = R5 = H; R6 = nC1 lH23)
CA 02209906 1997-07-09
WO 96/21355 PCT/GB96~ 2
F,xample 20
Activity against resistant mites (TU) (Tetranvchus urticae)
The test of Example 16 (TU(GSS)) was repeated using a strain of mites which is
resistant to bifenthrin (NYR-Bif-1000). The NYR-Bif-1000 strain was provided by the
5 Department of Entomology, Cornell University, New York.
The results of this test are set out in Table 3 below. The values given are LCso(ppm solution of test compound).
TABLE 3
Compound of TU (NYR-Bif-1000)
Example No. (LC50)
84 ppm
2 83 ppm
Ex~ le 21
Activit,v against resistant whitefly (BT) (Bemisia tabaci)
15 The test of Example 16 (BT (SUD-S)) was repeated using a resistant strain of whitefly
(Ned 7). The Ned 7 strain was collected in the Netherlands in April, 1993 from hibiscus
by J. Fransen and is highly resistant to organophosphate and carbamate insecticides and
the insect growth regulator buprofezin.
The results of this test are set out in Table 4 below. The values given are LCso20 (ppm solution of test compound).
TABLE 4
Compound of BT (Ned 7)
Example No. (LC50)
21
A 470
Further tests with various resistant strains of whitefly have shown that the
compounds of Examples 1 to 15 are highly active against resistant strains.
-28-
CA 02209906 1997-07-09
WO 961213SS PCT/GBgG/000~12
Fxample 22
Aphicidal activity
Activity against resistant (R) and susceptible (S) strains of aphid (M~zus persicae) was
assessed using the following method.
Enkapment rings of fluon were painted halfway up the inside of 4 cm lengths
of glass tubing (1.5 cm diameter), and squares of insect-proof gauze were attached to
one end of each tube by elastic bands. Fifteen apterous adults were then gently
transferred into the tubes using a sable-hair brush, and the tube sealed with a second
gauze square.
Tubes cont~inin~ aphids were dipped into insecticide solutions for 10 seconds,
dried on blotting paper, and then inverted and tapped to cause keated aphids to fall to
the unimmersed end of each tube. ~n~llin~; mortality (usually zero or very slight) was
scored after 1 hour, when aphids were transferred onto chinese cabbage leaf-discs (35 mrn
diameter) on an agar bed (25 rnm in depth) in disposible plastic containers (30 mm high)
15 and confined by applying a ring of fluon to the exposed lip of the container.Containers were store upright, without lids, in a constant environrnent facility m~int~ined
at 25~C under continuous room li~;htin~. Mortality was assessed at 24, 48 and 72 hours.
Two replicates of 15 aphids each were used at each dose rate and 5 or 6 dose rates were
used per compound under test.
The test was carried out using a susceptible strain of aphids (US 1 L) collected in the
field in East Anglia, UK and an extremely resistant skain of aphids (794Jz) (R3 esterase,
sensitive AChE) collected from glasshouses in the UK.
The results of this test are set out in Table 5 below. The values given are %
mortalit~ corrected for control data. The control comprised the test solution without
25 active ingredient.
Furthertestsusingthesusceptiblestrainof Aphisgossipii 81-171Bwerealsocar~ied
out and results indiocate useful activity, particularly with compounds of formula (IV).
-29-
CA 02209906 1997-07-o9
WO 96/21355 PCT/GB96/000~2
~ ~ o o o o o o
Z ~ _. o o o o o
V ~
~ ~ g ~o ~ g o ~
a O
z
~;~3 ¢ ~ ~ O ~ l-- l
o O
E- O
o ~,
a
o . ~ ~
o
-30 -
CA 02209906 1997-07-09
WO 96(2~355 PCT~,D~G~ 2
Fxample 23
Fungicidal Activity
Fungitoxicity of coded compounds to isolates of Aspergillus niger, Pyricularia oryzae
(=Magnaporthe grisea) and Rhizoctonia solani was tested in vifro.
Each compound was incorporated into potato dextrose agar in solvent
(50/50 ethanol/acetone) at 0.5 ml solvent per 250 ml agar while the autoclaved agar was
still molten and cooled to 50~C. Each compound was tested at a single concentration
(1 00 mg 1 -1 ).
Each test, usually of two compounds, included three control treatments: (i) a
standard fungicide (carben~l~7im at 1 or 5 mg 1-1 or prochloraz at 1 mg 1-'); (ii)
ethanol/acetone only; and (iii) no additions. The fungicides used as standards may be
considered as representative of active, commercially available compounds.
Each fungus was tested on agar in four Petri dishes per treatment, with three
replicate fungal colonies per plate (one colony for R. solani). A. niger and R. solani
were incubated for 4 days at 20-25~C, and P. oryzae for 7 days. Increase in colony
diameter was then measured and used to clet~rmine activity:
The results of these tests are set out in Table 6 below. The values given are %
inhibition of growth in colony diameter in agar plates.
CA02209906 1997-07-09
WO 96/21355 PCT/~b5Gt~GG 12
TART,F, 6
Compound of Fungus Activity at Activity at Activity at
Example No. 100 mg 1~' 5 mg 1-' 1 mg 1-'
A. niger 63
2 A. niger 38
3 A. niger 61
P. oryzae100
2 P. oryzae89
3 P.oryzae 100
R solani 95
2 R. solani81
3 R.solani 92
Prochloraz A. niger 97.8
Carbçntl~7im P. oryzae 99.8 14.7
Carbendazim R.solani 82.4 3.3
In addition, tests have shown that the compounds of formula I exhibit good fungicidal
activity against a broad spectrum of fungi which cause ~ e~es in both cereal and broad
leaved crops. Particularly, good activity has been observed against fungi of the genera
Erysiphe, especially Erysiphe graminis, and Botrytis, especially Botrytis fabae and
S Botrytis cinerea, as well as the genera Rhizoctonia, Pyricularia and Aspergillus as
illustrated above.
F.x~mple 24
Pl ep~l alion of 2-hydroxy-3 -(1 '-methylcyclopentyl)-naphthalene- 1.4-dione .
(Formula III: n = O; m = O; R1 + R2 together and R7 + R8 together both represent = 0;
10 R3=-oH: -CR4~5-=cvclopentyl: R6=-CH3~
To a stirred solution of diisopropylamine (3.95 g, 39.0 mmol) in dry THF (50 ml) at -78~C
under an atmosphere of nitrogen was added n- butyllithium (2.5M, 11.7 ml, 29.3 mmol).
-32-
CA 02209906 l997-07-09
WO 96J2135S PCT~D,''OOOt2
The mixture was stirred for 10 minllte~ before methyl cyclopent~n~oczlrboxylate (Aldrich)
(2.5 g, 19.5 mmol) was added dropwise. Stirring was continl~ec~ for a further 10 minrltes
before methyl iodide (8.31 g, 58.5 mrnol) was added dropwise. The reaction mixture was
stirred at -78~C for a further 1 hour before being allowed to warm up to room temperature
5 and being stirred for a further 1 hour. The reaction mixture was poured onto an admixture
of water and ether (1:1, 100 ml) and acidified with dilute hydrochloric acid (2M). The
aqueous solution was separated and extracted further with ether (3 x 25 ml) and the
combined ether layers were washed with water (2 x 50 ml), saturated sodiurn chloride
solution (50 ml) and dried over MgSO4. Filtration and evaporation of solvent under
10 reduced pressure yielded methyl l-methylcyclopentanecarboxylate as a colourless oil
(2.38g, b.p. 106~C at lOmmHg[I~ugelrohr]).
A solution of the methyl ester (2.30 g) and potassium hydroxide (5.00 g) in a
mixture of ethylene glycol (40 ml) and water (10 ml) was refluxed for 16 hours and then
cooled to room telllpeldl~e before being diluted with ether, the aqueous layer separated,
15 acidified with dilute hydrochloric acid (2M) and extracted with ether (2 x 25 ml). The
combined ether layers were washed with water (2 x 25 ml) and then saturated sodium
chloride solution (25 ml) and dried over MgSO4. Filtration and evaporation yielded 1-
methylcyclopentanecarboxylic acid (1.69 g).
A 50% excess of this acid was added to a stirred solution of 2-hydroxynaphthalene-
20 1,4-dione (1.00 g, 5.7 mmol), and silver nitrate (600 mg) in a mixture of acetonitrile (20
ml) and water (20 ml) at 65 ~C and a solution of ammonium peroxysulphate (1.96 g, 8.6
mmol) in water (10 ml) was slowly added to that over a period of 15 minl-tes The mixture
was heated for a further hour before cooling to room temperature (about 20~C) and diluting
with diethyl ether (50 ml). The organic phase was separated and washed successively with
25 water, dilute aqueous sodium hydrogen carbonate solution, water and saturated sodium
chloride solution before drying over magnesiurn sulphate as set out in Exarnple 3.
Filtration and evaporation of the solvent under reduced pressure followed by silica gel
column chromatography using 2:1 petrol:diethyl ether as eluant gave 2-hydroxy-3-(1-
methylcyclopentyl)naphthalene-1,4-dione (mp 116-118~~).
30 Fxample 25
-33 -
CA 02209906 l997-07-09
WO 96/21355 PCT1~1~5CJ~OC12
Pl,,paldlion of 2-hydroxy-3-~1 '-methylcyclohexyl)-naphthalene-1.4-dione.
(Formula III: n = 0; m = 0; R1 + R2 together and R7 + R8 together both represent = O;
_3 = -OH; -CR4R5- = cyclohexyl: R6 = -CH3~
To a stirred solution of 2-hydroxy-naphthalene-1,4-dione (1.00 g, 5.7 rnmol),
5 1-methylcyclohexanecarboxylic acid (1.22 g, 8.6 mmol) and silver nitrate (600 mg) in a
mixture of acetonitrile (20 ml) and water (20 ml) at 65~C was slowly added a solution of
ammonium pc;~oxy~ulphate (1.96 g, 8.6 mmol) in water (10 ml) over a period of 15minutes. The mixture was heated for a further hour before cooling to room temperature
(about 20~C) and diluting with diethyl ether (50 ml). The organic phase was separated and
10 washed successively with water, dilute aqueous sodium hydrogen carbonate solution, water
and saturated sodium chloride solution before drying over m~gnesium sulphate. Filtration
and evaporation of the solvent under reduced pressure followed by silica gel column
chromatography using 2:1 petrol:diethyl ether as eluant gave 2-hydroxy-3-(1-methyl-
cyclohexyl)naphth~lene-1,4-dione (296 mg) as a yellow crystalline compound, m.pt.: 79~C.
15 Fxample 26
Pl epal dtion of 2-(1 '-ethylcyclohexyl)-3 -hydroxv-naphthalene- 1.4-dione .
The title compound was prepared by hydrogenation of the compound of Example 27,
provided as set out below, using Pd/C catalyst in ethanol (mp 56~C) using the method of
Example lc.
20 E~ml~le 27
P~ al dLionof2-(1'-ethenecyclohexyl)-3-hydroxy-naphthalene-1 4-dione.
(Formula III: n = 0; m = 0; Rl + R2 together and R7 + R8 together both represent = O;
~3 = -OH: -CR4R5- = cyclohexyl, R6 = -CH=CH2~
To a stirred solution of ethyl cyclohexylideneacetate (see route to this by Wadsworth and
25 Emmons in Org. Synth. Coll. Vol 5 547) (3.00 g, 17.8 mrnol) in dry ether (50 ml) under an
atmosphere of nitrogen at 0~C was added lithium alu,l,h,iul" hydride (407 mg, 10.7 mmol)
portionwise and the mixture stirred for 2 hours before quenching with dilute hydrochloric
acid (2M; 20 ml). The mixture was filtered and the aqueous layer was separated and
-34-
CA 02209906 1997-07-09
WQ 9612~355 PCT~5C/U ~42
extracted further with ether (2 x 25 ml) and the combined ether layers washed with water
(2 x 25 ml) and saturated sodium chloride solution (25 ml) and then dried over MgSO4.
Filtration and evaporation of the solvent and distillation of the residue gave
2-cyclohexylidene ethanol (2.16 g, bp. 112~C at 10 mrnHg [Kugelrohr]).
To a stirred solution of 2-hydroxy-naphthalene-1,4-dione (2.50 g, 14.4 mmol) andtriphenylphosphine (3.79 g, 14.4 mmol) in dry THF (50 ml) under nitrogen was added
dropwise a solution of diethyl ~odicarboxylate (2.50 g, 14.4 mmol) in THF (2 ml). The
iXlUIc; was stirred for 5 mim~t~i before the cyclohexylidene ethanol (2.00 g, 15.8 mrnol)
in THF (2 ml) was added dropwise. The mixture was stirred for 2 hours allowing the
temperature to rise to room temperature and then diluted with ether (100 ml) and washed
with 1% sodium hydroxide solution (5 x 25 ml), water (2 x 25 ml) and saturated sodiurn
chloride solution (25 ml) and dried over MgSO4. Filtration and evaporation of the solvent
yielded a brown residue which u~as dissolved in ethanol (50 ml) and refluxed for 6 hours.
The mixture was cooled, concentrated to half its volume and diluted with ether (100 ml).
The etherial solution was extracted with 1 % sodiurn hydroxide solution (6 x 25 ml) and the
combined basic fractions were acidified (2M hydrochloric acid) and extracted with ether
(6 x 25 ml) with combined ether layers being washed with water (2 x 25 ml) and saturated
sodium chloride solution (25 ml) and dried over MgSO4. Filtration and evaporation of the
solvent and purification by silica gel column chromatography yielded the title compound
as a yellow crystalline compound (153mg; mp 112-113~C).
Fxample 28
Preparation of 2-hydroxy-3 -(1 '-trifluoromethylcyclohexyl!-naphthalene- 1.4-dione
(Formula III: n = 0; m = 0; R1 + R2 together and R7 + R8 together both represent = O;
R3 --~; -CR4_5- = cyclohexyl: R6 = -CF~;L
Trifluoromethylacrylic acid (l.Sg: 10.7mmol), butadiene sulphone (1.27g: 10.7mmol) and
hydroxyquinone (1 Smg) were heated at 160~C in a pressure vessel bomb (pressure approx.
3 bar at completion) for two and a half hours. The mixture was then cooled and dissolved
in diethyl ether and extracted with 2M sodium hydroxide (3 x 25ml). The combined basic
fractions were acidified with 2M HCI and extracted with diethyl ether (5 x 25ml). The
combined ether fractions were washed with water then saturated sodium chloride solution
-35-
CA 02209906 l997-07-09
WO 96/21355 PCTl(~ , 5.'C A ~ ~2
before drying over magnesium ~lllph~te7 filtering and evaporation of sovent to yield a
brownish solid (1.267g). The crude product was purified on a silica colurnn using 1:1
petroleum ether /diethyl ether eluant yielding 927mg of the product 1-trifluoromethyl-
cyclohexyl-3-enecarboxylic acid. This compound was hydrogenated by the method of5 Example l(c) to give 1-trifluoromethylcyclohexanecarboxylic acid.
2-benzoyloxy-n~phth~lene-1,4-dione (355mg: 1.27 mmol), 1-trifluoromethyl-
cyclohexane carboxylic acid (250mg: 1.27mmol) and silver nitrate (108mg: 0.64mmol)
were heated in acetonitrile (5ml) and water (3ml) at 65-70~C. ~ solution of ammonium
peroxysulphate (436mg: l.91mmol) in water (lml) was added dropwise and the mixture
10 heated for 1 hour. The reaction ~ was cooled, diluted with water (20ml) and extracted
with ether (3 x 20ml). The combined ether layers were dried over m~gn~sium sulphate and
evaporated to dryness.
The resultant ester was hydrolysed by dissolving in a mixture of THF (20ml) and
2M aqueous KOH (1 Oml) and stirring at room temperature for 2 hours. The mixture was
15 diluted with water (20ml), washed with ether (2 x 20ml), acidified with 2M HCl and
extracted with ether (3 x 20ml). The combined ether extracts were washed with water, dried
over magnesium sulphate and the solvent evaporated under reduced pressure. The residue
was purified by silica gel column chromatography to give the product (mp 114~C).
F.x~mple 29
20 Preparation of 2-hydroxy-3-(1 '-methylcycloheptyl)-naphthalene- 1.4-dione.
(Formula III: n = 0; m = 0; R1 + R2 together and R7 + R8 together both represent = O;
_3 = -OH: -CR4_5- = cyclQheptyl; R6 = -CH3~
To a stirred solution of diisopropylamine (7.47 g, 73.8 mmol) in dry THF at -78~C under
nitrogen was added n-butyllithium(2.5M, 29.5 ml, 73.8 mmol). The mixture was stirred for
25 10 minutes before cycloheptanecarboxylic acid (2.10 g, 14.8 mmol) was added dropwise
and the reaction stirred at -78~C for a further 10 minutes before refluxing for a further 2
hours. The reaction was cooled to 0~C and methyl iodide (5.76 g, 40.6 ml) was added
dropwise before refluxing for a further 1 hour before cooling to room temperature. The
reaction mixture was poured onto an admixture of water/ether (100 ml/50 ml) and the
30 aqueous layer was separated~ acidified with dilute hydrochloric acid (2M) and extracted
-36-
CA 02209906 1997-07-09
WO 961213S5 PCT~;D5G/~,J0~2
with ether (Sx 25 ml). The combined ether layers were washed with water (2 x 50 ml) and
~hlr~t~l sodium chloride solution (50 ml) before being dried over MgS04. Filtration and
evaporation of solvent under reduced ~l~S~ yielded the 1-methylcycloheptanecarboxylic
acid which was recrystallised from hexane (2.20 g, mp 46~C). The title compound was
5 produced by the method of Example 24 using this acid in place of the
l-methylcyclopentanecarboxylic acid.
Examples 30 and 31
Further compounds of the second group of the first aspect of the invention were
10 synthesized by similar methods to those of Examples 24 to 29 and are referred to in the
Tables 9 and 10 below as Exarnples 30 to 31 where physical and activity data are set out.
Example 33
Pl c ~dl dlion of 2-acetoxv-3 -((1 '-rnethylcyclohexvl)-methyl)-naphthalene- 1.4-dione .
To a stirred solution of 2-acetoxy-naphthalne-1,4-dione (1.12g; 5.18mmol), (1-
15 methylcyclohexyl)acetic acid (prepared by the procedure of Amsterdarnsky et al Bull. Soc.Chim. Fr (1975) 3-4 part 2, 635-643) (850mg; 5.44mmol) and silver nitrate (520mg) in
acetonitrile (15ml) and water (20ml) heated at 65-70~C was added an aqueous solution of
ammonium persulphate (1.77g, 7.77mmol) in water (lOml). After heating for 1 hour the
mixture was cooled, diluted with water (50ml) and extracted with ether (3 x 40ml). The
20 combined ether fractions were washed with water (3x25ml), saturated sodium chloride
solution ~25ml) and dried over m~gn~?~ium sulphate. Filtration and evaporation of solvents
under reduced pressure and silica gel chromatography yielded the title compound as a
yellow solid (736mg).
25 Examples 32 and 34.
Preparation of 2-hydroxv-3-((1'methvlcyclohexyl)-methvl!-naphthalene-1.4-dione
(Example 32) and 1~1-dimetho~v-2-hvdroxy-3-((1'-methylcyclohexvl)-methvl)-
narhthalene-1.4-dione (Example 34).
To a stirred solution of compound of Example 33 (750mg; 2.3mmol) in an
30 admixture of THF/methanol (1:1; 30ml) was added an aqueous solution of potassium
CA 02209906 1997-07-09
WO 96/21355 PCT/~ 5G~'C~~12
hydroxide (6.45mg; 1 l.Smmol) in water (8ml) at room temperature and the reaction stirred
for 2 hours. The ~ was diluted with water (lOOml), washed with ether (20ml),acidified with 2M hydrochloric acid and extracted with ether (3 x 25ml). The combined
ether layers were washed with water (2 x 20ml), saturated sodium chloride solution (20rnl)
S and dried over m~gn~cium s~lph~te. Filtration and evaporation of the solvent under reduced
~res~ and silica gel colurnn chromatography gave the compounds of Example 32 and 34
in two bands.
Pesticidal activity was assessed against houseflies, mustard beetles, mites, aphids
10 and whitefly using the methods of Examples 19 to 22. All strains were susceptible strains
unless otherwise indicated; further tests with resistant strains of whitefly have shown that
many of the compounds of the formula III are highly active against resistant strains.
The whitefly activity test with (BT (SUD-S)) was repeated using a resistant strain
15 of whitefly (Ned 1/2). The Ned 1/2 strain is a composite collection which was collected
in the Ne~h~rl~n~l~ in 1992 from Gerbera and Bouvardia by ICI Netherlands and exhibits
high resistance to ~yl~lhloid insecticides, such as cypermethrin, organophosphate and
carbamate insecticides and the insect growth regulator buprofezin.
The results of these tests are set out in Table 7 below. The values given are LCso
(ppm solution of test compound).
TABLE 7
Compound of BT (Ned 1/2)
Example No. (LC50)
25 25 O. I
B 100
-38-
CA 02209906 1997-07-09
WO 9612135S PCT/GB96~00042
Compound B = Example 1, Table I of DE 3801743 Al which is
2-hydroxy-3-(4-t-butylcyclohexyl)-naph~h~len~- 1 ,4-dione.
The method of Example 22 was repeated using a preferred compound of Formula
III, results being shown in Table 8.
S TABLE 8
COMPOUND Aphid CONCENTRATION OF CONTROL
EXAMPLE NO. Strain ACTIVE INGREDIENT MORT-
250 PPM 100 PPM 40 PPM ALITY
S 67 23 07 0
R 79 27 10 0
-39-
CA 02209906 1997-07-09
WO 96121355 PCT/GB96100042
~,, ~ V V ~ V V ,~ ~ V V oo
-- a~ oo
,,~ O
11
~:C X ~
U V ~r ~ V v ~ c~ V V V
, I
o
x x x x c~ 3 ~, x x
~ ~ ;~
~3
~~ ¢ V V V
S3
~ ~ oooooooo~
-
X ~ X X X X X X ¢ X
C~ 0 0, 0, 0 0 0,0, 0 0, 00,
o ~
o o
~ Z
_~ ~ o X
Eo
V
-40 -
SllBSTITUTE SHEET (RULE 26)
CA 02209906 l997-07-09
WO 96/21355 PCT/GB96/00~4Z
~ E ~ ~ ~, ~ ~ '-- ~
o
.
o o o o o~
o
V
o ~ ¢ ~, ¢ ~,, o o
r
C~7
O _ O~ ~ o~t ~ "C~
a
~ a~
V ~ ~ ~ ~ V
O a
O
~ _ O
~ ~_ 3 ~ d~ 40 0 --
-41
-
CA 02209906 1997-07-09
WO 96/213S5 PCTIGB96/00042
Bycomparisonapriorartcompound,Example 1,Table 1 of DE3801743 A1 whichis2-
hydroxy-3-(4-t-butylcyclohexyl)-n~rhth~lene-l~4-dione (Formula II: n = 0; m = 0; R1 +
R2 together and R7 + R8 together both represent =O; R3 = -OH; -CR4R5- = 4-t-
butylcyclohexyl; but R6 = H therefore not covered by formula I or II), was tested against
5 these same pests and gave an MD LDso of 15.5, a PC LDso of 0.53, a TU (GSS) LCso
of 44 and a BT(SUD-S) LCso ~f 18.
Fungitoxicity of coded compounds to isolates of Aspergillus niger, Pyricularia
oryzae (= Magnaporthe grisea) and Rhizoctonia solani was tested in vitro by the methods
described in Example 23 using a preferred compound of formula (III).
The results of these tests are set out in Table 11 below. The values given are %inhibition of growth in colony diameter in agar plates.
TABLE 1 1
Compound of Fungus Activity at Activity at Activity at
Example No. 100 mg 1~1 5 mg 1-l I mg 1~
A. niger 45
P. oryzae 94
R. solani 93
Prochloraz A. niger 97.8
Carben-1~7im P. oryzae 99.8 14.7
20Carbencl~7imR.solani 82.4 3.3
In addition, tests have shown that the compounds of formula (III) exhibit good
fungicidal activity against a broad spectrum of fungi which cause diseases in both cereal
and broad leaved crops. Particularly, good activity has been observed against fungi of the
genera Erysiphe, especially Erysiphe graminis, and Botrytis, especially Botrytis fabae and
25 Botrytis cinerea, as well as the genera Rhi,octonia, Pyricularia and Aspergillus as
-4~ -
CA 02209906 1997-07-09
WO 96121355 PCT/GB~C~
illustrated above.
Fxample 35
Preparation of 2~ -dim~lhyl~)Lo~ yl)-3-hvdroxy-naphthalene-1.4-dione.
This compound was plepa~id by the general methods as described in Examples I to 15.
S Lawsone (0.15g), 3,3-dimethylbutyric acid (0.15g), and silver nitrate (O.lSg) were heated
in a mixture of acetonitrile (5ml) and water (5ml) at 60-65~C. A solution of ammoniurn
peroxysulphate (0.3g) in water (Sml) was added dropwise and the mixture heated for 1 hour
and then processed as for Example 3 to yield the title compound . The crude product was
purified on a silica gel column using 20% diethyl ether in petroleum ether eluant and
10 recrystallised from petroleum ether to yield 3 8mg of the title compound.
E~rnple 36
Preparation of 2-(3.3-dimetl~ylbutyl)-3-hydroxv-n~hth~lene-1 .4-dior e.
Sodium hydroxide solution (lM; lOOml) was added to a solution of 2-ethanoyloxy-3-(3,3-
dimethylbutyl)-n~phth~lene-1,4-dione (3.5g)(see Example 44) in THF (lOOml) at room
15 ten~ dlu.e and stirred for 4 hours. The THF was removed under vacuum and the resultant
solution was washed with diethyl ether (3x). The aqueous layer was acidified then extracted
with diethyl ether (x3), the combined extracts washed with water, dried over magnesium
sulphate then evaporated to dryness under vacuum to yield 3g title compound.
Ex~ le 37.
20 Preparationof 2-(4.4-dimethylpentyl)-3-hvdroxy-naphthalene-1~4-dione.
3,3-dimethyl butan-l-ol (1.3g) was stirred for 2 hours in dichloromethane (30ml) with
pyridinium chlorochromate (5.Sg) at room temperature, diluted with ether and filtercd.
Wittig reagent Ph3P=CH-C02C2Hs (carbethoxymethylenetriphenylphosphorane)(3.6g)
was added to the filtrate and stirred overni~ht. The mixture was evaporated under vacuum
25 and the residue purified by silica gel chromato~raphy to yield 1.25g of ethyl 5,5-
dimethylhex-2-enoate .
This product was dissolved in a mixture of THF (20ml) and 2M potassium
-43 -
CA 02209906 1997-07-09
WO 96/213S5 PCT/(,;b~ 12
hydroxide (lOml), stirred for 2 hours at room leml)cldlure, diluted with water, washed with
ether (2 x 30ml), the aqueous layer acidified then extracted with ether (2 x 30ml). The
combined extracts were washed with water, dried over m~gn.ocium sulphate and the solvent
evaporated to give S,S-dimethylhex-2-enoic acid.
5 This acid was reacted with benzoyloxynaphthalene-1,4-dione, hydrolysed by the method
of Example 28 to give 2-(4,4-dimethylpent-lenyl)-3-hydroxy-naphthalene-1,4-dione.
The product so provided was hydrogenated by the method of Example 1 (c) to give the title
compound (406mg).
Ex~rnple 38
10 Plc~ald~ion of 2-(S.S-dimethylhexyl)-3-hydroxy-naphthalene-1.4-dione.
2-(5,5-dimethylhex-2-enyl)-3-hydroxynz~phth~lene-1,4-dione(391mg) prepared in Example
45 was dissolved in ethyl acetate (lSml) and hydrogenated as in Example l(c) using
hydrogen and 100mg of Pd/C catalyst to provide 371 mg title compound.
Fxanlples 39 to 41
l S Pl~pala~ion ofthe corresponding 2-(6,6-dimethylheptyl), 2-(7,7-dimethyloctyl) and 2-(8,8-
dimethylnonyl) compounds was carried out using commercially available starting materials
by analogous methods to those of Example 48 with hydrogenation as described in Example
1 (c) using PtO2 in methanol instead of Pd/C.
F.~ample 42
20 Pl~ual~lion of 2-(3.3~dimethyl-but-1-enyl)-3-hydroxv-naphthalene-1.4- dione.
Lawsone (2-hydroxynaphthalene-1,4-dione) (1.4 g) and 3,3-dimethyl-butanal (1.0 g) were
dissolved in 20 ml THF at room temperature and 795 !11 pyrrolidine added before stirring
the reaction for a further 20 minutes. The solvent was removed under vacuum and the
residue dissolved in benzene (40ml) before addition of p-toluenesulphonic acid (2.3g).
25 The mixture was refluxed for 1 hour, cooled then diluted with ether before the organic
phase was washed with sodium bicarbonate solution, followed by one wash with dilute HCl
and one wash with water followed by drying under vacuum. The product was purified by
-44-
CA 02209906 1997-07-09
WO 9612135~i PCT/GB9G/C~~~2
chromatograpy using 10% EtOAc/petroleum ether eluant and then cryst~ e-l from
methanol to yield 260 mg; m.p. 126-128~C.
- F,xample 43
Plep~lldlion of 2-(6.6-dimethylhep-4-enyl)-3-hydroxy-naphthalene- 1.4-dione.
S A solution of 2-(7,7-dimethyloct-5-enyl)-3-hydroxynaphthalene-1,4-dione (0.lg) (see
Example 44),30% hydrogen peroxide (60,u1), aqueous sodium carbonate (36mg in lml) in
degassed dioxane (lml) was heated at 70~C for 40 minlltes under nitrogen until the
solution became colourless.20% aqueous copper (II) sulphate (30111) was added and when
bubbling ceased 25% aqueous sodium hydroxide (0.6ml) and 20% aqueous copper (II)10 sulphate (1.5ml) were added and the mixture stirred at 70~C for 30 minl]tes On cooling 2N
hydrochloric acid (Sml) was added and the product extracted with diethyl ether (3x) and
processed as for Example 48.
The crude product was purified by silica gel chromatography using l0% diethyl ether
/petrol as eluent to give 50mg of the title compound.
l S Example 44
Pl~a~dliQn of 2-(7.7-dimethyloct-5-enyl)-3-hydroxy-naphthalene-1.4-dione.
This co,mpound was prepared by use of a procedure analogous to that used in Example 48
below followed by that of Example 36.
F,xample 45
20 Preparation of 2-(5.5-dimethylhex- 1 -enyl)-3-hvdroxv-naphthalene- 1.4-dione.Ethyl 5,5-dimethylhexanoate (1.87g) ( prepared in Example 34 ~vith esterification). was
dissolved in 30ml of THF and lithiurn aluminium hydride (2g) added portionwise. The
reaction was stirred for 2 hours at 0~C before quenching with 2ml 15%NaOH and 6ml
water before diluting with dichloromethane and filtering through CELITE (RTM) and the
25 solvent evaporated to give S,S-dimethylhexanol product (1. lg) as a slightly volatile liquid.
This alcohol was dissolved in 30ml dichloromethane cont~ining pyridinium chlorochromate
and stirred at room temperature to convert it to the corresponding aldehyde, S,S-
-45-
CA 02209906 1997-07-09
WO 9612135S PCT/GB96/00042
dimethylhexanal .
The aldehyde (8.46mmol) was coupled to lawsone following the procedure set out
in Example 42 using lawsone (1.18g),THF (20ml), pyrrolidine (672~L1), benzene (40ml)
and p-toluenesulphonic acid (1.9Sg). 391mg of the title compound was isolated after
S purification.
Fxamples 46 - 47
The ethanoyloxy derivatives of the 3-(t-butyl) compound (Example 46; of formula (II))
and of the compound of Example 36 (Example 47) were prepared by the method of
Example 2.
Fxample 48
~Iternative preparation of 2-ethanoyloxy-3-(3~ 3-dimethylbutyl)-naphthalene-1.4-dione
(~xample 47).
1-Chloro-3,3-dimethylbutane (lOg) was added dropwise to m~nesium tl-ming~ (2g) with
an initiating amount of iodine crystals in dry diethyl ether (lOOml) and Grignard formation
allowed to proceed to completion over I hour. The mixture was poured onto dry ice (SOg)
very slowly and 0.5 N sodium hydroxide added and the basic aqueous layer provided
extracted with diethyl ether (x2). The basic aqueous layer was acidified and extracted with
diethyl ether which was dried over m~gnesium sulphate, filtered then evaporated under
vacuum to yield 7.2g of 4,4-dimethyl pentanoic acid.
4,4-dimethylpentanoate (0.6g) obtained above, 2-benzyloxynaphthalene-1,4-dione
(lg) and silver nitrate (0.8g) were heated in a stirred mixture of acetonitrile (25ml) and
water (25ml) at 60-65~C. A solution of ammonium peroxysulphate (l.Sg) in water (lOml)
was added dropwise and the mixture heated for an hour before being cooled to room
temperature and processed as for Example 3 to yield 0.37g title compound.
~x~ml-le 49
Pl e,t~a~ alion of 2-ethanovoxy-3 -(10.10-dimethylundecan-7-envl)-naphthalene- 1.4-dione
8-triphenylphosphonium octanoic acid bromide salt (2.43g) was prepared by reacting
triphenyl phosphine with 8-bromooctanoic acid in xylene solvent under reflux conditions,
-46-
CA 02209906 1997-07-09
WO 96121355 PCT/~D;C~ C ~2
and removing the solvent. The residue was dissolved in THF (20ml)/DMSO (2ml) andbutyllithiurn (2.5M; 4ml) in hexane (4ml) was added dropwise at 0~C. After warming to
room temperature over 30 minutes 3,3-dimethylbutanal (0.5g) in THF (5ml) was added
dropwise and the mixture stirred at room temperature for 3 hours. Water and dilute
5 hydrochloric acid were added and the mixture extracted with diethyl ether(x3). Pure
product 11,1 1-dimethyldodecan-8-enoic acid (0.4g) was provided a~er silica gel column
chromatography .
This acid (0.34g) was reacted with 2-ethanoyloxynaphthalene-1,4-dione (0.4g)
using the method of Example 47 to give 26mg title compound.
10 Further compounds in tables below were synthe~i7e-1 using these general methods.
-47-
CA 02209906 1997-07-09
WO 96/213SS PCT/(~b5G/00042
._
a v v V V ~, Ov ~, V u v
~ ~ ~D O o O O 00 0 o O ~
,~ O ~ ~ O 0 00 ~O
C~
.
O
O ~ ~ ~ X ~ X
VVVVVVVVV~)VV~V~
O V
~' v v
lol
o ~ ~ ~ ~ ~c ~c x ::c ~ ~ ~ ~ ~ ~ ~ x
ll v v v v v v v v v v v v v v v
~ l l l l l l l v v ~ l ~
~~
v v~
a~
;>
cd
LL ~ ~ ~ ~ ~C ~ ~ ~ ~ ~ ~ ~C V V V 3~
~0 ~ ooooooooooooooO
o o o
c
O O
O -- ~ ~ ~ ~ ~ ~ O
~, X
V O ~
-48 -
CA 02209906 l997-07-09
WO 96J2135S PCT/<~D~1C~ 2
c
o ') Z ~ Z
C~
~ E ~ ~ ~ ~ ~ ~ ''' ~ o o ~ ~
~ ~ ~
V
~o
o _~
C~
E ~ Z Z ~ ~, ~, Z , ~ ~ ~ ~
~) ~'~
o
.'~
_ ",
-- V ~, ~ ~ ¢ o~ , o
.~ O
~ ~ o ~ ~ ~ ~o l ~ o
o x
c~ ~
-49 -
CA 02209906 l997-07-09
WO 96/21355 PCT/GB96/00042
¢ ¢ ¢ o ~t- o~ ~ o o o
~, Z Z Z; oo _ ~ ~ o o o Z
A A A
_l
~)
o ~~o oo ~ _.
3 ~ <; ¢ ¢ o ~ ~ ~ o o ~
o
Il .~,
~ , I I ~ ' I oI ' I o
ô
Il _
Il ~:
o~
~, ¢ ¢ ¢ ¢ o ¢ o ~ ¢ ¢ ¢ ¢Z
o
v~ ,1
S~
X ~
V ~ ¢ ¢ ¢ o ~ l ~ t_ a~ ~ ¢ ¢
~ _ ~, ~ Z; Z; Z ~, '' " " o -- ~~, Z Z
V
.~ C~ X C) C~ ) ~ V V V
¢ . ~ ~ ' ~,
V V ~ V V
-50 -
CA 02209906 1997-07-09
WO 96~21355 PCT/~,;D, . /~ _ J 12
F~ ples 51-53
Synthesis of naphthalene-1 .4-diones substituted at positions 5-8 on the naphthalene ring.
xample 51
Preparation of 2-~-butyl)-3-hydroxv-6-methyl-naphthalene-1 .4-dione and 2-(t-bu+yl)-3-
5 hydroxy-7-methyl-naphthalene-1.4-dione.
(a) Preparation of 6-methyl-naphthalene-1 .4-dione.
A solution of 1,4-benzoquinone (13.9 g, 128 mmol) and isoprene (13.1 ml,
131 mmol) was stirred in glacial acetic acid (44 ml) for 68 hours at room temperature.
The mixture was diluted with water (44 ml) and refluxed for 1 l/2 hours. The mixture
10 was cooled to room temperature and acetic acid (84 ml) and chromic acid [chromium
trioxide (29.4 g) in water (30 ml)] was added sequentially, before refluxing for a further
11/2 hours. After cooling, the mi~ture was diluted with water (200 ml) and extracted
with ether (3 x 50 ml). The combined ether fractions were washed with dilute sodium
hydroxide solution (2M; 2 x 50 ml), water (2 x 50 ml), saturated sodium chloride15 solution (50 ml) and dried over magnesium sulphate. Filtration and evaporation of
solvent under reduced pressure, and repeated recryst~lli.c~tion from petroleum ether
yielded the title compound (7 g).
(b) 2-Amino-6 and 7-methyl-1.4-naphthalene-1.4-diones.
To a stirred solution of 6 methyl naphthalene-1,4-dione (2.1 g, 12 mmol) in
20 glacial acetic acid (60 ml) at room t~ c;~dlw~ was added a solution of sodium azide
(1.58 g) in water (5 ml). The mixture was stirred for 2 days before diluting with water
(200 ml) and, after stirring for a further 15 minutes, was filtered. The filtrate was
neutralised with sodi~l biea~}olla+.~ and e~tracted ~th chl~roform (3 x ~ ml~. The
combined chloroform extracts were washed with saturated sodium bicarbonate solution
25 brine and dried (CaSO4). Filtration and evaporation of solvent under reduced pressure
and silica gel chromatography yielded the title compound (100 mg) as a 3:2 mixture of
somers.
(c) 2-Hydroxy-6- and -7-methyl-naphthalene-1.4-diones.
The aminomethyl naphthalene-1,4-dione mixture from (b)(200 mg) was refluxed
30 in water (20 ml) and concentrated sulphuric acid (10 ml) for 20 minllte~. The cooled
-51-
CA 02209906 l997-07-09
WO 96/21355 PCT/GB96/00012
Ulc; was poured into ice/water (50 g) and extracted with ether (3 x 25 ml). The
combined ether extracts were washed with water, saturated NaHCO3, water, saturated
NaCl solution and dried (MgS04). Filtration and evaporation of solvent and
purification by silica gel column chromatography yielded the title compound (68 mg).
5 (d) Pl~ dldLion of 2-(~-butyl)-3-hydroxv-6 and 7-methyl-3-hydroxv-naphthalene- 1.4-dio~es.
Standard peroxysulphate/silver nitrate radical addition on the aminomethyl
compound (64 mg, 0.34 mmol), trimethylacetic acid (52 mg, 0.51 mmol), yielded the
title compound as a 3:2 mixture of isomers (12 mg).
10 Fxz~n~le 52
Preparation of 2-~-butyl)-6 and 7-dimethyl-3-hydroxy-naphthalene-1.4-diones
Steps (a) to (d) above were repeated, replacing isoprene with
2,3-dimethyl-1,3-butadiene.
Fxample 53
15 Preparation of 2-~-butyl)-3-hydroxy-5 and 8-methyl-1~4-naphthalene-1.4-dionesSteps (a) to (d) above were repeated, replacing isoprene with piperylene.
(a) Pl~dldliQnof6-methyl-l~4-naphthalene-l 4-dione.
A solution of 1,4-benzoquinone (13.9 g, 128 mmol) and isoprene
(13.1 ml, 131 mmol) was stirred in glacial acetic acid (44 ml) for 68 hours at room
20 te.,l~t;.dlul~. The mixture was diluted with water (44 ml) and refluxed for 11/2 hours.
The mixture was cooled to room t~...p~-dLIlre and acetic acid (84 ml) and chromic acid
[chromium trioxide (29.4 g) in water (30 ml)] was added sequentially, before refluxing
for a further 11/2 hours. After cooling, the mixture was diluted with water (200 ml) and
extracted with ether (3 x 50 ml). The combined ether fractions were washed with dilute
25 sodium hydroxide solution (2M; 2 x 50 ml), water (2 x 50 ml), saturated sodium
chloride solution (50 ml) and dried over m~gnecium sulphate. Filtration and
evaporation of solvent under reduced pressure, and repeated recryst~lli.c~tion from
petroleum ether yielded the title compound (7 g).
-52-
CA 02209906 1997-07-09
WO 9612135S PCT/G~;9C~ 2
Toxicity data.
In addition to the specific insecticidal and acaricidal tests described above,
compounds of the present invention were submitted for further tests relating to toxicity
to m~mm~l~ and so-called friendly species such as Chrysloperla carnea, Aleochora5 bilineata and Coccinella septempunctata.
The whole body LDso in rats for the compound of Example 25, 2-hydroxy-3-(1'-
methylcyclohexyl)-naphthalene-1,4-dione, was found to be 2786mg/Kg body weight,
indicating it to be significantly safer in m~mm~ n toxicity than many standard
insecticides currently commercially available.
LDso values of greater than lOOOppm/individual were found for several
compounds tested on Chrysloperla carnea, Aleochora bilineata and Coccinella
septempunctata.