Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
METHOD OF IMPARTING IMPACT RESISTANCE TO A PLASTIC
OPHTHALMIC LENS, COMPOSITIONS FOR USE THEREIN AND A LENS
PREPARED THEREBY
Field of the Invention
The present invention relates to ophthalmic lenses.
More particularly, it relates to a method of imparting
impact resistance to plastic lenses, compositions for use
in the method and the lenses prepared by practice of the
method.
Background of the Invention
Ophthalmic lenses of plastic have become very
popular because they are inexpensive , lighter in weight
and more resistant to shattering than glass. However, the
plastic lenses generally have less surface hardness and
wear resistance. Therefore , they are usually coated with
abrasion resistant coatings.
The surfaces of lenses made of diethylene glycol
bis(allylcarbonate) ("CR-39", a registered trademark of
PPG Industries of Pittsburgh , Pennsylvania) or high
index polyurethane (HIPU) can be made to be hard and
abrasion resistant by the use of abrasion resistant
coatings; however, their impact resistance can be greatly
reduced by these hard , abrasion resistant coatings,
especially in combination with a brittle vacuum deposited
antireflective coating. Thin lenses, with a center
thickness of about 1.0 mm, are highly desired for
cosmetic and weight considerations. However , the
thickness of a lens is related to the impact properties
of the lens. Specifically, thinner lenses have a lower
impact resistance. Plastic lenses that are sold in the
United States must meet the requirements of the Food and
Drug Administration (FDA), which has specific
requirements for the impact properties of a lens.
It would be advantageous to have plastic lenses
which are more impact resistant and a method of imparting
an impact resisting property that meets or exceed the FDA
requirements for plastic lenses.
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PC.T/EP96/00256
- 2 -
Summary of the Invention
It is an object of the present invention to disclose
a novel method of imparting impact resistance to a
plastic ophthalmic lens. it is further object to disclose novel compositions
for use in the method of the present invention.
it is a still further object to disclose a novel
lens prepared by the practice of the method of the
present invention.
The novel method of the present invention comprises
coating the back of a plastic lens with a novel impact
resistance imparting composition of multifunctional
acrylates such as tetraethylene glycol diacrylate and/or
diethylene glycol diacrylate , dissolved preferably in a
solvent mixture of an organic ketone, such as methyl
isobutyl ketone, and an alcohol , such as 1-butanol, and
then curing the composition to obtain a plastic oph-
thalmic lens with an impact resistance imparting coat on
its back.
The multinfunctional acrylates preferably comprise
at least about 40 % , by weight of the acrylates, of a
difunctional acrylate or a mixture of difunctional acry-
lates . According to another embodiment of the invention,
thr glass transition temperature of the multifunctional
acrylate is preferably less than about 60 C. This will
prevent the coating from becoming too hard, and losing
the impact resistance imparting properties.
In one embodiment of the invention the novel impact
resistance imparting composition contains a UV photo-
initiator and the coating is cured with UV light.
In another embodiment of the invention the composi-
tion also contains colloidal silica or colloidal anti-
oxide.
mony
According to another aspect of the present inven-
tion, impact resistant coatings are applied to the back
surface of lenses (usually concave), but preferably not
to the front surfaces (usually convex), by a spin-coating
SUBSTITUTE SHEET (RULE 26)
CA 02211141 2005-06-08
- 3 -
process. The lenses are then coated with an abrasion-
resistant hard coat by a dip-coating process.
It will be apparent to those skilled in the art from
the description which follows that the above objects and
further advantages can be obtained by the pactice of the
present invention.
Brief Descrintion of the Drawinas
In the drawings :
Fig. 1 is a sectional view of a portion of a plastic
ophthalmic lens with an abrasion resistant coat on the
front of said lens and an impact resistance imparting
coat on the concave back of the lens;
Fig. 2 is a sectional view like Fig. 1 in which the
lens also has antireflection coats on its front and back;
and
Fig. 3 is a sectional view like Fig. 1 in which the
lens has an impact resistance imparting coat on the con-
cave back of the lens , and an abrasion resistant hard
coat over the front and the back of the lens.
Description of the Preferred Embodiment
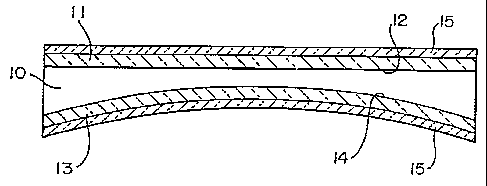
In the preferred embodiment of the invention , a
plastic lens 10, seen in Fig. 1, is provided with an
abrasion resistant hard coat 11 on its top or front 12 and
an impact resistance imparting coat 13 on its back 14.
The front of the lens is considered to be the surface
that is farthest from the eye of the wearer , and the
back of the lens is considered to be the surface that is
nearest the wearer.
The abrasion resistant hard coat 11 can be applied
by any of a variety of conventional techniques.
The impact resistance imparting coat 13 is
preferably applied to the back 14 of the lens 10 by
applying a coating of an impact resistance imparting
composition comprising a multifunctional acrylate in a
volatile solvent or reactive diluent.
CA 02211141 1997-07-22
WO 96/23243 PCTIEP96/00256
- 4 -
The coating of the impact resistance imparting
composition is then cured to provide the coat 13.
Antireflective coatings 15, seen in Fig.2, can then be
applied to the front 12 over the hard coat 11 and to the back 14 over the
impact resistance imparting coat 13.
A further embodiment illustrated in Fig. 3 has an impact resistance imparting
coat 17 on the back surface
19 of the lens 10. The impact resistance imparting coat
17 is preferably applied by a spin-coating process. An
abrasion resistant hard coat 21 is provided over the
front surface 23 and back surface 19 of the lens 10, and
is preferably formed by a dip-coating process.
A hard coat comprising .an organosilicone resin is
preferred. A typical organosilicone resin that is sui-
table for the invention has a composition comprising one
or more of the following :
(1) organosiloxane compounds with functional and/or
non-functional groups such as glycidoxypropyl tri-
methoxysilane;
(2) coreactants for functional groups of functional
organosilanes , such as organic epoxies, amines,
organic acids, organic anhydrides, imines, amides,
ketamines, acrylics , and isocyanates;
(3) colloidal--silica , sols and/or metal and non-
metal oxide sols preferably having an average
particle diameter of from about 1 to about 100
nanometers, and most preferably between about 5 and
about 40 microns;
(4) catalysts for silanol condensation, such as
dibutylin dilaurate, zinc napthenate, aluminium
acetylacetonate, zinc napthenate , zirconium
octoate, lead 2-ethylhexoate , aluminium alkoxides
and aluminium alkoxide organosilicone derivatives,
and titanium acetylacetonate; (5) catalysts for coreactants such as epoxy
catalysts and free radical catalysts;
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 5 -
(6) solvents such as water, alcohols, and ketones;
(7) surfactants, such as fluorinated surfactants
including 3M FC430 and 3M FC431 (3M Company, St.
Paul, Minnesota),DuPont FSN and DuPont FS (E.I.
Dupont de Nemours & Co., Wilmington, Delaware) or
polydimethyl siloxane surfactants , such as BYK 300
or BYK 371 (BYK Chemie U.S.A., Wallingford, Connec-
ticut);
(8) other additives, such as fillers;
In the present invention , any type of organosili-
cone hard coat can be used , but a hard coat of the type
which contains the silanol condensation product of a col-
loidal silica and organosilane containing functional
groups cured with an appropriate catalyst is preferable.
Hard coats of acrylic , urethane , melamine, and the
like may also be used. These materials, however,fre-
quently do not have the good abrasion resistant proper-
ties of organosilicone hard coatings.
Hard coats may be coated by conventional methods
such as dip coating , spray coating, spin coating, flow
coating and the like. Withdrawal rates, viscosity, and
percent solids are common methods of controlling dip
coating thickness and uniformity. Coating thickness
between 0.5 and 10 microns are preferred for abrasion and
other properties.
A single-layer anti-reflective coating can be
provided, if desired. Multi-layer anti-reflective
coatings are preferred from an optical performance
viewpoint . Examples of materials useful in forming anti-
reflective coatings includes oxides, fluorides,
silicides, borides, carbides, nitrides and sulfides of
metal and non-metals and metals.
More specifically, metal oxides which are useful for
forming anti-reflective coatings include SiO, SiOZ1ZrO2,
A1203, TiO, Ti02, Ti0203 , Y203, Yb203, MgO, Ta205 1 CeO2 and Hf02 .
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 6 -
Fluorides which can be used include MgF2,AlF31BaF2 CAFZ,
Na3AlF6, Ta205, and Na5A13F114. Metals which can be used
include Cr, W and Ta. These susbtances may also be used
in the form of mixtures.
The above mentioned substances are formed into
single layer or multi-layer anti-reflective coatings by
vacuum evaporation , deposition, sputtering , ion
plating, and ion beam assisted methods.
The present invention is further illustrated by the
Testing and Examples.
TESTING
A number of tests were performed to measure the
abrasion resistance , scratch resistance, adhesion and
impact resistance of coated and uncoated plastic lenses.
BAYER ABRASION RESISTANCE TEST
The abrasion resistance of a coating was examined by
subjecting the coated lens to a fixed cycle of oscil-
lating sand abrasion, similar to ASTM #F735-81, using
approximately 500 grams of quartz silica and sand size
6/14 supplied by Crystal Silica Company, Riverside, CA or
another suitable supplier such as Silica Sand 6/14 from
CGM, P.O. Box 413, Salem, PA 19020. The haze before and
after abrasion was measured using a Pacific Scientific
Hazemeter XL-211.
The change in the haze measurement before and after
abrasion of the uncoated and coated lenses was tested.
The ratio of the uncoated lens haze measurement to the
coated lens haze measurement determines the performance
of the test lens. The higher the ratio, the better the
performance of the coating. Results of at least 3 test
lenses are averaged for a final ratio.
STEEL WOOL SCRATCH RESISTANCE TEST
The "cutting" scratch resistance of the coatings was
examined by subjecting the coated lens to abrasion
similar to that described in U.S. Patent No.4,084,021.
The device described in the patent was modified to rock
the sample in an arch equivalent to a 600 diopter radius,
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 7 -
which matches the front curve of the test specimens.
"000" steel wool was used with the grain parallel to the
rocking motion. The amount of abrasion was quantified by
measuring the transmitted light haze of the abraded spe-
cimen, before and after abrasion, as described in the
Bayer Abrasion Resistance Test. The results of this test
are rated as Excellent, Good or Poor.
ADHESION TEST
A steel straight edge and knife with #11 blade (or
Gardco cross cut tester) was used to make eleven 1/2"
long cuts on a front surface of the coated lens. Eleven
more 1/2" cuts spaced at right angles to, and across the
first set of eleven cuts, were then made. One-inch wide
semi-transparent pressure sensitive tape (adhesion
strength of 36 + 2.6 oz./in, Ref. ASTM D-3359 American
Society of Testing and Materials, 1916 Race Street,
Philadelphia , PA 19103-1187) was then applied to the cut
areas of the coated lens. The tape was pulled vertically
at a 90 degree angle from the coated lens surface with a
quick motion.This step was performed in triplicate using
a new section of tape for each test. The lens was then
examined for any coating chips that may have been pulled
off with the tape. This entire process is repeated after
tinting in BPI black dye, available from Brainpower, Inc.
of Miami, Fl at 92 C t 2 C for three minutes. The results
of this test are rated as Excellent, Good and Poor.
IMPACT RESISTANCE
The impact resistance of the lenses was measured
directly using a Dynatup Instrument Impact tester avai-
lable from General Research Corp., Santa Barbara, CA.
This tester was modified to have a lens support tube and
a 5/8" diameter steel ball as the impactor, as specified
in CFR 801.410 (FDA) . A load cell, velocity indicator and
micro-computer were used to integrate the total energy
applied to the lens at the point of fracture. All samples
were=impacted with the equivalent velocity of a 50" free
fall. The results of this test are measured in in/lb.
SUBSTITUTE SHEET (RULE 26)
CA 02211141 2005-06-08
8
In general, impact results between about 3.01 and 10
in/lb can be regarded as good, and impact results about
above 10.01 can be regarded as excellent.
LENS PREPARATION & COATING PROCESS
The concave side of the semifinished lenses of
diethylene glycol bis(allylcarbonate) (CR-39) and high
index polyurethane (HIPU) were surfaced , a method of
cutting and removing the substrate to a preferred
thickness, and polished to 1.0 mm nomimal center
thickness. These lenses had a factory hard coat on the
convex side. The lenses were washed with a soft sponge
and a mild liquid detergent. Lenses were wiped to dryness
by using Kay DryTMpaper towels manufactured by Kimberly-
Clark.A small amount of isopropanol was used to prepare
the lens surface prior to laoding the lenses into a spin
coating machine . An impact resistance imparting coating
was applied to the lenses by a spin coating process.
The spin coating process of the invention can
utilize suitable devices such as the Clean n CoatT''' model
sold by Coburn Optical Industries, of Muskogee, Oklahoma,
or the Photo Resist model )k1-PM101D-R465 from Headway
Research,Inc. of Dallas, Texas. A coating liquid was
applied automatically in the Clean n Coat model, or
manually with a plastic pipette in the Photo Resist
model, at low spin speeds. The coating spin speeds are
preferably between about 150 rpm and 800 rpm, and most
preferably between about 500 rpm and 600 rpm. The
spinning time during the coating process is preferably
between about 5 seconds and about 20 seconds, and most
preferably between about 10 seconds and about 15 seconds.
The lenses are then spun to remove excess coating and to
dry the lenses. Spin-off and drying is preferably
performed at spin speeds of between about 700 rpm and
about 3500 rpm, and most preferably between about 800 rpm
and about 1000 rpm. The spinning time is preferably
between about 15 seconds and about 60 seconds, and most
preferably between about 20 seconds and about 40 seconds.
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 9 -
The thickness of the impact resistance imparting
coat can vary , but is preferably between about 0.1 and
about 10 microns. The thickness is most preferably
between about 0.5 and about 5 microns for impact,
abrasion, and other properties.
The coated lenses were transferred and cured in a
suitable UV curing machine (Quick. Cure III U.V. curing
unit, manufactured by Coburn Optical Industries).
Finally, an antireflective coating was applied by vacuum
deposition to both surfaces of the lens. The vacuum
deposition was done by Essilor of America,Inc.,
Reflection Free Division , of St. Petersburg, Florida.
EXAMPLE 1
Tetraethyleneglycol diacrylate 29.4 parts was added
to a premixed solvent that consisted of 51.5 parts of
methyl isobutyl ketone and 17.2 parts of 1-butanol. The
mixture was stirred and mixed for ten minutes. 1.8 parts
of photo-initiator Irgacure-500, 50:50 mixed of 1-hydro-
xycyclohexylphenyl ketone and benzophenone from Ciba
Geigy, and 0.1 part of surfactant was added to the
mixture . The final coating formulation was allowed to
mix for an additional 10 minutes. The coating was
filtered prior to the coating application.
The lens was prepared and coated as previously des-
cribed . This coating provided good adhesion to the lens
substrate. A coating of 1.7 microns was coated and cured
on the concave side of the lenses of CR-39 and HIPU.
Finally an antireflective coating was applied by vacuum
deposition to both surfaces of the lens as described
above. The final lenses were subjected to the physical
performance characterization. See Table I and Table II
for performance testing results.
COMPOSITION PARTS
Tetraethyleneglycol Diacrylate 29.4
Methyl Isobutyl Ketone 51.5
1-Butanol 17.2
Irgacure-500 (Photoinitiator) 1.8
Surfactant 0.1
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 10 -
EXAMPLE 2
Tetraethyleneglycol diacrylate 30 parts were added
to a solvent mixture that consisted of 45.3 parts of
methyl isobutyl ketone and 15.2 parts of 1-butanol. The
mixture was stirred for 10 minutes. 1.8 parts of Irga-
cure-500 and 7.6 parts of colloidal silica in methanol
(MA-ST from Nissan Chemical Industries) were added to the
mixture. Finally 0.1 part of surfactant was added and
stirred for an additional 10 minutes . The lens was
prepared , coated with a 1.7 microns coating of the
impact resistance imparting composition on the concave
side of the lens , and cured in the same manner as
described in the example 1. An antireflective coating was
applied to both surfaces of the lenses as in Example 1.
The final coated lenses were prepared for performance
testing as described above. See Table I and Table II for
performance testing results.
COMPOSITION PARTS
Tetraethyleneglycol Diacrylate 30.0
Methyl Isobutyl Ketone 45.3
1-Butanol 15.2
Irgacure-500 1.8
Colloidal Silica in methanol 7.6
Surfactant 0.1
TABLE I
Substrate Back Steel Wool Antireflec- Thickness Impact Mean
Coating tive Coat of Lens Break Point
Center
CR-39 no good no 1.10 mm 2.78 in/lb
CR-39 Ex.l excellent no 0.88 mm 17.99 in/lb
CR-39 Ex.2 excellent no 0.87 mm 13.08 in/lb
CR-39 no good yes 1.10 mm 4.52 in/lb
CR-39 EC.i excellent yes 0.88 mm 6.46 in/lb
CR-39 Ex.2 excellent yes 0.88 mm 15.38 in/lb
Note : Adhesion was excellent for all examples SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 11 -
_ TABLE II
Substrate Back Steel Wool Antireflec- Thickness Impact Mean
Coating tive Coat of Lens Break Point
Center
HIPU no poor no 1.00 mm 21.06 in/lb
HIPU Ex.1 good no 0.95 mm 29.83 in/lb
HIPU Ex.2 excellent no 0.96 mm 30.73 in/lb
HIPU no poor yes 1.10 mm 32.51 in/lb
HIPU Er..1 good yes 0.95 mm 27.78 in/lb
HIBU Ex.2 excellent yes 0.99 mm 27.05 in/lb
Note : Adhesion was excellent for all examples
Tables I and II demonstrates that the invention
provides dramatic improvement in impact resistance for
lenses without an antireflective coating. In tables I and
II, the presence of an antireflective (RF) coating is
indicated. With the HIPU lens substrate, abrasion
resistance is dramatically improved on these lenses with
no loss in impact resistance. With CR-39 lens substrates,
there is an improved impact and abrasion resistance for
lenses with an antireflective coating.
The impact resistance results demonstrated that the
back coatings of the present invention improved and pre-
served the impact resistance for CR-39 substrate. Mean-
while , it improved or maitained the impact resistance of
the HIPU substrate.
EXAMPLE 3
Diethylene glycol diacrylate 38.9 parts was added to
a solvent mixture that consisted of 40.9 parts of methyl
ethyl ketone and 17.5 parts of 1-butanol. The mixture was
stirred for approximately 10 minutes. The 2.3 part of
Irgacure-500 and 0.4 part of surfactant was added and
stirred for an additional 10 minutes. HIPU lenses was
prepared, coated with a 2.0 micron coating , and the
coating was cured in the manner described in Example 1.
See Table III for performance testing results.
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 12 -
COMPOSITION PARTS
Diethylene Glycol Diacrylate 38.9
Methyl Ethyl Ketone 40.9
1-Butanol 17.5
irgacure-500 2.3
Surfactant 0.4
EXAMPLE 4
Diethylene glycol diacrylate 26.2 parts was added to
a solvent mixture that consisted of 33.7 parts of methyl
ethyl ketone, 18.7 parts of 1-butanol and 11.2 parts of
ethanol. The mixture was stirred for approximately 10
minutes. 2.3 parts of irgacure-500 and 7.5 parts of
colloidal antimony oxide was added to the mixture and
mixed well. Finally, 0.4 parts of surfactant was added
and stirred for 10 minutes. The lenses were prepared ,
coated with a 1.5 micron coating, and cured in the manner
described in Example 1. See Table III for performance
testing results.
COMPOSITION PARTS
Diethylene Glycol Diacrylate 26.2
Colloidal Antimony Oxide 7.5
Methyl Ethyl Ketone 33.7
1-Butanol 18.7
Ethanol 11.2
Irgacure-500 2.3
Surfactant 0.4
EXAMPLE 5
Diethylene glycol diacrylate 40 parts was added to
a solvent mixture that consisted of 14.4 parts of methyl
ethyl ketone, 20.7 parts of 1-pentanol and 14.4 parts of
ethanol . The mixture was mixed for approximately 10
minutes. 1.6 parts of Irgacure-500 and 0.4 parts of
isopropylthioxanthole was added and mixed well. Finally,
0.2 part of surfactant was added and mixed for an additi-
10 minutes . The lenses were prepared , coated with
onal
a 2.0 micron coating , and cured in the manner described
in Example 1. See Table III for performance testing
results.
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 13 -
COMPOSITION PARTS
Diethylene Glycol Diacrylate 40.0
Methyl Ethyl Ketone 14.4
1-Pentanol 20.7
Ethanol 14.4
Irgacure-500 1.6
Isopropylthioxanthone 0.4
Surfactant 0.2
TABLE III
Example Substrate Steel Wool Lens Center Impact Mean
Thickness Break Point
Example 3 HIPU good 0.87 mm 22.86 in/lb
Example 4 HIPU good 1.03 mm 31.25 in/lb
Example 5 HIPU J excellent 1.01 mm 25.47 in/lb
o e: esion was exce en or a examp es.
EXAMPLE 6
It was discovered that impact resistance varies with
the UV curable back coating thickness. For a coating that
is more brittle than the substrate , better impact
resistance was achieved by a thinner coating on the
concave back of the lens. A more brittle coating would
contain trifunctional and/or higher functionality acry-
late. The following example illustrates this discovery.
Dipentaerythritol pentaacrylate , aliphatic urethane
triacrylate, tetraethylene glycol diacrylate and 1,6
henxandiol dimethacrylate were added to a solvent mixture
that consisted of methyl ethyl ketone and amyl alcohol.
The mixture was mixed for approximately 10 minutes.
Finally, Irgacure-500 and surfactant was added and mixed
for an additional 10 minutes. The lenses were prepared ,
coated and cured the same way as in Example 1 . The exact
component weights were the following
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 14 -
COMPOSITION Parts Parts
(40% solid) (30% solid)
Dipentaerythritol Pentaacrylate 59.4 parts 44.4 parts
Aliphatic urethane triacrylate 59.4 parts 44.4 parts
Tetraethylene glycol diacrylate 59.4 parts 44.4 parts
1,6 Hexandiol dimethacrylate 21.8 parts 16.4 parts
Methyl ethyl ketone 214.9 parts 254.6 parts
Amyl Alcohol 71.6 parts 84.7 parts
Irgacure-500 12.0 parts 9.0 parts
Surfactant 1.5 parts 1.5 parts
The 20% composition was made by diluting the above
40% composition with 75 parts of methyl ethyl ketone and
25 parts of amyl alcohol and 0.4 part of surfactant. The
lenses were prepared, cured and coated with these
coatings in the same way as in Example 1. See Table IV
for performance testing results on HIPU lenses.
TABLE IV
HIPU SUBSTRATE 20% SOLID 30% SOLID 40% SOLID
Avg. Center 1.36 mm 1.20 mm 1.34 mm
Thickness
Impact Mean 61.01 in-lb 47.68 in-lb 3.59 in-lb
Break Point
2 5 Coating 1 micron 2 microns 4 microns
Thickness
Steel Wool poor good excellent
Adhesion excellent excellent excellent
These results reveal that impact resistance is
excellent when the coating thickness is one or two
microns, but is not as good at four microns . The results
also reveal that compositions having as little as about
40% of difunctional acrylate, by weight of the multi-
functional acrylate, can produce lenses having improved
impact resistance.
EXAMPLE 7
When the coating is less brittle than the substrate,
then a thicker layer of coating increases the impact
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT1EP96/00256
- 15 -
resistance.Less brittle or more flexible coatings contain
monofunctional and/or difunctional acrylates.
The following example illustrates this discovery.
Diethylene glycol diacrylate is added to a solvent
mixture that consisted of methyl ethyl ketone , 1 pent-
anol and ethanol. The mixture was mixed for approximately
minutes . Finally, irgacure-500 and surfactant was
added and mixed for an additional 10 minutes. The lenses
10 were prepared, coated and cured the same way as in Exam-
ple 1. The exact component weights were the following
COMPOSITION PARTS PARTS PARTS
(18% solid) (45% solid) (55% solid)
Diethylene Glycol
Diacrylate 18.0 45.0 54.7
1-Pentanol 40.4 26.1 20.9
Ethanol 20.2 13.0 10.5
Methyl Ethyl Ketone 20.2 13.0 10.5
Irgacure-500 1.1 2.7 3.3
Surfactant 0.2 0.2 0.2
The results are reported below.
TABLE V
HIPU Subsrate 18% SOLID 45% SOLID 55% SOLID
fi
Avg.Center 1.10 mm 1.13 mm 1.13 mm
Thickness
Impact Mean Break 34.59 in-lb 38.56 in-lb 43.24 in-lb
Point
Coating Thickness 0.5 microns 2.5 microns 4.0 microns
Steel Wool good excellent excellent
Adhesion excellent excellent excellent
SUBSTITUTE SHEET (RULE 26)
CA 02211141 2005-06-08
- 16 -
TABLE VI
CR39 Substrate 181 SOLID 45% SOLID 55= SOLID
Avg.Center 0.92 mm 0.94 mm 0.95 nuo
Thickness
Impact Maan Break 2.05 in-lb 9.68 in-lb 10.45 in-lb
Point
Coating Thickness 0.5 microns 2.5 microns 4.0 microns
Stoe1 Wool excollent oxcellent excellent
Adhosion excellent excellent excellent
The above results reveal that impact resistance
increases with both HIPU and CR39 substrates with thicker
coating thicknesses.
COMPARATIVE EXAMPLES 8 AND 9
The concave side of the semifinished lenses (CR-39
and HIPU) were surfaced, a method of cutting and removing
the substrate to a preferred thickness , and polished to
1.0 mcn nominal center thickness. These lenses had a
factory hard coat on the front side. A scrubbing compound
named CR-ScrubT"' ta widely used metal oxide slurry
compound, a product of Lens Technology Inc. of Cerritos,
California) was used to prepare the concave back surface
of the lens. The scrubbing procedure was necessary to
provide a good coating adhesion to the lens. The
scrubbing compound was washed off by using a mild liquid
detergent. The lenses were wiped to dryness by using Kay
Dry paper towels.A small amount of isopropanol was used
to prepare the surface prior to loading the lenses into
a spin coating machine. A very popular commercial U.V.
curable abrasion resistant hard coating , Coburn/LTI HT-
325-B from Coburn Optical Industries of Muskogee,
Oklahoma, was used to coat the back of these lenses. The
coated lenses were transferred and cured in a W curing
machine. Finally , an antireflective coating was applied
by vacuum deposition to both surfaces of the lens. A
Dynatup impact tester was utilized for impact resistance
testing.
CA 02211141 2005-06-08
- 17 -
TABLE VII
Example Back Sub- Steal Lens Antire- Impact Nean
Coating strate Wool Center flacti- Break Point
{325B) Thickness ve Coat after anti-
reflective
coatinp
8 Yes CR-39 excellent 0.9S mm Yes 1.93 in/lbs
9 Yes HIPU exeallent 1.04 mm Yea 1.60 inJlba
The impact resistance for the comparative examples
was far less than all of the examples in Table I, II or
III.Adhesion was excellent , but only after a thorough
scrubbing with CR-ScrubTMThis difference is signifiant
because, in practice, failure to adequately perform the
scrubbing step will result in an inferior lens. The
preferred coatings of this invention do not require
scrubbing for good adhesion.
In addition to the compositions of the Examples,
other compositions can be used. Representative of the
acrylates and acrylate oligomers which may be utilized to
form the impact resistance imparting coatings of the
present invention are :
ethylene glycol dimethacrylate
diethylene glycol diacrylate
diethylene glycol dimethacrylate
triethylene glycol diacrylate
triethylene glycol dimethacrylate
tetraethylene glycol diacrylate
tetraethylene glycol dimethacrylate
trimethylol propane triacrylate
trimethylol propane trimethocrylate
1,6 henxandiol triacrylate
pentaerythritol triacrylate
ethoxylated trimethylolpropane triacrylate
propoxylated trimethylolpropane triacrylate
pentaerythritol tetracrylate
dipentaerythritol pentaacrylate
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 18 -
aliphatic urethane diacrylate
aliphatic urethane triacrylate
tetraethylene glycol diacrylate
1,6 hexandiol dimethacrylate
aliphatic urethane triacrylate
epoxy diacrylate oligomer
aromatic urethane acrylate oligomer
polyester tetraacrylate oligomer
The preferred compositions will contain about 20
percent to about 80 percent by weight of the acrylate ( s).
However, the exact amount is not critical provided it is
dissolved in the solvent mixture . Also, it is possible
that an acrylate can be used as a reactive diluent in
place of, or in addition to, a solvent. The solvent is
removed by evaporation or other suitable processes.
Reactive diluent , if used , is consumed by the reaction
and any excess is removed by suitable processes.
Although the compositions of the present invention
may contain only one type of said multifunctional
acrylate monomers, they may contain a mixture of one or
more types of multifunctional monomers, preferably a
diacrylate and/or a triacrylate. Multifunctional acrylate
oligomers are also suitable for use in the invention.
Preferably -,q.t least about 40% of the multifunctional
acrylate , by weight of the multifunctional acrylate, is
a diacrylate . More than about 10% of triacrylates, by
weight of the multifunctional acrylate, in the composi-
tion will sometimes cause the coating to become too hard.
In addition, minor amounts of mono-acrylate can be used
in particular instances, for example , to improve
adhesion.
The glass transition temperature of the multinfunc-
tional acrylate should be below about 60 C. This will
prevent the coating from becoming too hard and losing the
impact resistance imparting properties. Trifunctional
acrylate oligomers having a glass transition temperature
less than about 60 C can also be used. More than 10%, by
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 19 -
weight of the multifunctional acrylate, of such triacry-
lates could be used in the coatings of the invention. it
is further possible that such triacrylates could form a
major part of the multifunctional acrylates.
The compositions may also contain from 0 to 10% by
weight of a colloidal dispersion. of a water insoluble
dispersant of metals, non-metals, alloys, or salts
thereof, such as colloidal silica or antimony oxide. The
silica should be in the form of a silicon dioxide (Si02)
dispersed in a solvent. For example , MAST available from
Nissan Chemical Industries is a dispersion of 30% Si02
and 70% methanol, by weight. Colloidal silica is availa-
ble in basic or acidic form. Either may be utilized.
The solvent mixture for the acrylate(s) is prefera-
bly a mixture of a methyl lower a].kyl (Cl to C4) ketone,
such as methyl isobutyl ketone or methyl ethyl ketone,
and a lower alkyl alcohol (C1-C6) such as methanol,
ethanol, 1-butanol or amyl alcohol. The amount of the
solvent mixture is not critical provided it is suffi-
cient to dissolve the acrylate(s) and it can be readily
removed to leave a coat of the acrylate(s) on the lens.
The preferred coating compositions also contain a
photosensitizing amount of photoinitiator , such as
Irgacure-500, i.e., an amount effective to effect the
photocure of the coating composition. The amount will
vary with the photoinitiator used. Generally, this amount
is from about 0.01% to about 10% by weight, and
preferably from about 0.1 % to about 5% by weight of the
photocurable coating composition.
The preferred compositions also contain an effective
amount of surfactant. Silicon and fluoro surfactants are
preferred at amounts of about 0.01% to 0.50% by weight of
the total composition.
The coating compositions of the present invention
can be applied to the concave back of the lens by conven-
tional methods, such as spin coating , flowing, spraying
or dipping, to form a continuous coating. Optimum coating
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 20 -
thickness which are about 0.1 micron to about 10 microns
thick are obtained by spin coating procedures.
Prior to the composition being coated upon the back
of the lens there may optionally be included a priming
step wherein a primer, such as a thermosetting acrylic
emulsion, is first applied to the substrate. After the
coating composition is applied to the substrate or the
primed substrate, the coating may be cured thereon by an
effective amount of UV-radiation , which may be obtained
from, for example, a Hanovia 550 watt lamp or a PPG
Processor, Model QC1202
Although other forms of curing can be used.
including heat, ultraviolet light is preferred because of
its relatively low cost, ease of maintenance, and low
potential hazard to industrial users. Rapid photo-induced
polymerizations utilizing W light rather than thermal
energy for the curing of coating offer several other
significant advantages. First, faster curing coatings
offer substantial economic benefits. Furthermore, heat
sensitive materials can be safely coated and cured with
TJV light without the use of thermal energy, which could
damage the substrate.
According to another aspect of the invention, only
the back side of a lens is provided with an impact
resistance imparting coating. The impact resistance
imparting coating can be one of the improved back coating
compositions of the invention, or another impact coating
such as polyurethane or an epoxy coating. The lens is
then coated with an abrasion resistant hard coating. Such
a lens is shown in Fig.3. A lens formed in this manner
utilizes the back coatings of the invention, or another
impact coating , to impart impact resistance to the
lenses, yet has the abrasion resistance and tinting
characteristics of the hard coatings. Much of the
abrasion that a lens will encounter will be on the front
side of the lens, and the presence of the impact
resistance imparting coat on the front side can
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 P(,'T/EP96/00256
- 21 -
significantly decrease the abrasion performance of the
lens. It has been found that the application of the
impact resistance imparting coating only to the back side
of the lens will provide a lens that has acceptable
impact resistance. A lens according to this embodiment of
the invention has good scratch resistance properties and
impact resistance properties . A lens according to this
embodiment of the invention also has good tinting
characteristics.
It has further been found that the combination of
applying an impact resistant coating to the back of a
lens by a spin coating process, and applying an abrasion
resistant hard coating by a dip coating process, produces
a lens with desirable properties. The lens is impact
resistant , abrasion resistant , and light in weight.
As shown in Fig. 3, the lens 10 has an impact coating that
has been applied to the concave back side 19 of the lens
10 by a spin coating process. An abrasion resistant hard
coat 21 is applied by a dip coating process over the
front and back surfaces of the lens 10.
EXEMPLE 10
Tetraethyleneglycol diacrylate 89.4 part were added
to a premixed solvent that consisted of 153.9 parts of
-- methyl isobutyl ketone and 51 parts of 1-butanol. The
mixture was stirred and mixed for 10 minutes.
3.5 parts of photoinitiator Irgacure 500 and 0.05
part of surfactant was added to the mixture. The final
coating formulation was allowed to mix for an additional
10 minutes . The coating was filtered prior to coating
application.
Semi-finished uncoated single vision CR-39 -2.00
lenses surfaced to 1.0 mm center thickness (used for
impact testing) and uncoated finished single vision plano
CR-39 lenses (used for physical performance characteriza-
tion) were subjected to a cleaning process using 10%
aqueous sodium hydroxide solution for 3 minutes @ 60 C,
which was followed by water washing and drying. Lenses
SUBSTITUTE SHEET (RULE 26)
CA 02211141 2005-06-08
- 22 -
were then spin coated on the backside (concave) surface
and cured in the Quick Cure IIITM W cure unit for 22
seconds. These lenses with backside impact coating of 1.7
microns thickness were further dip coated with an epoxy
silane colloidal silica tintable hardcoat, and post cured
for 3 hours at 110 C. The final lense were subjected to
physical performance characterization (see Table VIII and
IX).
For impact testing, a vacuum antireflective coating
as previously described was applied to both surfaces of
the lens and impact mean break point determined by the
Dynatup procedure previously described. Antireflective
coatings , in general, dramatically lower the impact of
lenses and represent the worst case for impact.
EXAMPLE 11
In this example , the same coating as in Example 10
was applied to HIPU lenses in the same manner as Example
10, except that a higher index epoxy silane colloidal
silica tintable hardcoat was used. Coatend lenses were
postcured for 2 hours at 100 C with a 3 hour cool down.
The final lenses were subjected to physical performance
characterization as in previous examples (see Tables VIII
and IX).
TABLE VIII
Substrato Backsida Bayor Staal Wool Adhesion
Coating Abrasion
CR39 yes 1.90 Excellent Excellent
CR39 no 2.20 Excellent ESCcsllant
HIPU yes 1.79 Excellent Excall*nt
HIPU no 1.70 Excellent Sxcellont
TABLE IX
Substrate 8ack Coating Impact Maan
Break Point
CR-39 yes 5.78 in/lb
CR-39 no 1.92 in/lb
HIPU yes 25.23 in/lb
HIPU no 2.15 in/lb
CA 02211141 1997-07-22
WO 96/23243 PGT/EP96/00256
- 23 -
EXAMPLE 12
Tetraethylene glycol diacrylate 30.3 parts were
added to a premixed solvent with 45.5 parts of methyl
ethyl ketone and 15.2 pa~rts of 1-butan6l. The mixture was
stirred for 10 minutes . About 1.2 parts of Irgacure-500
and 7.6 parts of colloidal silica in methanol (MAST from
Nissan Chemical Industries) were added to the mixture .
Finally 0.15 part of surfactant was added and sirred for
an additional 10 minutes.
This backside impact coating was filtered, applied,
and cured as in Example 10. The backside coated lens thus
obtained was dip coated with an epoxy silane colloidal
silica tintable hardcoat, cured and tested as in Example
10. See Tables X and XI.
EXAMPLE 13
In this example the same coating as in Example 11
was applied to HIPU lenses in the same manner as in
Example 11, except that a higher index epoxy silane
colloidal silical tintable hardcoat was used. Also,
coated lenses were postcured for 2 hours @ 100 C with a
3 hour cool down.
The final lenses were subjected to physical perfor-
mance characterization as in previous examples. See
Tables X anc1..XI.
TABLE X
Substrate Backside Bayer Steel wool Adhesion
Coating Abrasion
CR39 yes 2.19 Excellent Excellent
CR39 no 2.07 Excellent Excellent
HIPU yes 2.10 Excellent Excellent
HIPU no 2.04 Excellent Excellent
TABLE XI
.
Substrate Back Coating Impact Mean
Break Point
CR-39 yes 11.27 in/lb
CR-39 no 1.27 in/lb
HIPU yes 49.35 in/lb
HIPU no 2.15 in/lb
SUBSTITUTE SHEET (RULE 26)
CA 02211141 1997-07-22
WO 96/23243 PCT/EP96/00256
- 24 -
These examples demonstrate that lenses which have been
backside coated with coatings of this invention and then
dip-coated provide drastically improved impact resistance
when compared to the same hardcoated lenses without
backside coating. Furthermore, the physical performance
characteristics can be as good as with the hard coat
alone.
The invention is useful with lenses formed from a
variety of different lens materials , and particularly
from a number of different polymeric plastic resins. A
common ophthalmic lens material is diethylene glycol bis
(allyl carbonate). Lens materials with higher refractive
indices are now growing in popularity. One such material
is a high index polyurethane . Other high index lens
materials are based on acrylic or allylic versions of
bisphenols or allyl phthalates and the like. Other
examples of lens materials that may be suitable for use
with the invention include other acrylics, other
allylics, styrenics, polycarbonates, vinylics,
polyesters, and the like.
It will be appreciated by those skilled in the art
that a number of modifications and changes can be made
without departing from the spirit and scope of the
invention. Therefore , it is intended that the invention
be only limited by the claims.
SUBSTITUTE SHEET (RULE 26)