Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02218517 1997-10-17
w 4 .. ::~'-~ T~~: ~ -
'TflAI~~.~:~:~
Method and device for the selective perfusion. of
fluids throucxh blood vessels, controlled by the
pressure in the blood vessels
The present invention relates to a method and a device for the
selective perfusion of fluids through blood vessels, controlled
by the pressure in the blood vessels. In particular, the
present invention relates, on the one hand, to the suction of a
fluid out of, and the retroinfusion of a fluid into, veins, in
particular coronary veins, controlled by the pressure in the
veins, and, on the other hand, to the perfusion of fluids
through arteries, in particular coronary arteries, controlled
by the pressure in the arteries.
The nutritive perfusion of coronary arteries and retroinfusion
of blood into coronary veins is becoming increasingly
important, especially in the area of myocardial protection
during short-term coronary artery closure in the context of a
cardiac intervention. A typical intervention of this kind is,
for example, the balloon dilation of a coronary artery which
has become narrowed as a result of arteriosclerosis. In this
method, which is also known as percutaneous transluminal
coronary angioplasty (PTCA), a balloon catheter is guided, with
radiographic monitoring, into the area of the stenosis of the
coronary artery, and the arteriosclerotic plaque is compressed
by inflating the balloon situated at the end of the catheter.
During the dilation of the balloon, there is no supply of
oxygenated blood to the tissue in the downstream area of the
artery. In most cases this does not pose any problem, as long
as the dilation lasts only a short time. However, in the case
of dilations of more than just 30 seconds' duration, functional
changes in the ischaemic area of the myocardium can be
detected, for example as ST changes on the electrocardiogram,
as a reduction in regional wall movement by echocardiography,
or else subjectively by the patient as angina pectoris
symptoms. In addition, the risk of complications in angioplasty
CA 02218517 1997-10-17
2
is higher for certain groups of patient;, for instance in
elderly patients, in cases of unstable angina, in cases of a
decreased left-ventricular ejection fraction, or in cases of
dilation of a vessel which supplies more than 40% of the left
ventricle.
Corresponding problems in protecting against myocardial
ischaemia also arise in other operations for coronary
vascularization, such as, for example, in atherectomy, coronary
endoprostheses and laser applications.
It is known to effect short-term ischaemic protection by
machine perfusion of an artery supplying the affected region of
the myocardium, for instance the actual artery which is to be
dilated, either with arterial blood, which has been taken from
the patient, at another site or with other nutritive fluids. In
doing so, however, there is a danger of overperfusion of the
myocardial tissue, which is particularly the case if the
outflow of the perfused fluid from the affected tissue is
impeded or completely blocked. In such cases, this may lead to
haemorrhagic tissue infarcts in the affected region of the
myocardium.
A further possibility for short-term protection against
ischaemia, which has been used for some time now in cases where
complications are anticipated, is the retroinfusion of arterial
blood into a vein of the area of myocardial ischaemia
concerned. The arterial blood is in this case pumped through
the corresponding vein into the nutritive capillaries of the
ischaemic area and so supplies the myocardium in this region
with oxygen and substrates.
Devices for retroinfusion of coronary veins have been known for
some years. Thus, European Patent Specification EP-B-0 297 723
describes a retroinfusion unit with which arterial blood is
taken, for example, from the femoral artery of the patient and
is conveyed to a coronary vein of the ischaemic area via a pump
system and an inflatable balloon catheter. The pumping of
CA 02218517 1997-10-17
3
arterial blood into the ~~oronary vein is in this case
synchronized with the R wave of the patient's
electrocardiogram, so that the pumping interval is adapted to
the patient's cardiac cycle. The pumping interval is in this
case predefined and begins at 45% of the R-R interval and ends
at 95% of the R-R interval. The infused blood flow is in this
case essentially constant during the pumping interval. As long
as pumping is being carried out, the balloon of the balloon
catheter is inflated and blocks the vein, thus ensuring that
arterial blood is effectively transported into the ischaemic
area during the diastole. The pumping procedure ends at the end
of the diastole and the balloon is emptied, so that the flow in
the vein is no longer blocked at this point. During the
succeeding systole, venous blood can flow off through the vein.
With the device described in EP 0 297 723, the basal metabolism
of the ischaemic area can be satisfactorily maintained during a
short-term cardiac intervention. It was furthermore observed
that the size of myocardial infarction following a coronary
artery closure was significantly reduced. However, it was also
found that the local myocardial function is not sufficiently
maintained. For example, the local myocardial function breaks
down completely in the absence of arterial collateralization of
the ischaemic area. One cause of this is to be seen primarily
in the incomplete exchange of arterial and venous blood in the
retroinfused vein system in the course of a cardiac cycle.
In order to improve this blood exchange, with the aim of better
maintaining the myocardial function in the ischaemic area,
Boekstegers et al. have recently proposed, in Cardiovascular
Research 1990, 2~: 456 - 464, and in JACC 1994, 23: 459 - 469,
a system for retroinfusion of coronary veins in which, instead
of venous blood flowing off passively during the systole, an
active suction through the retroinfusion catheter takes place.
For this purpose, the balloon of the catheter also remains
inflated in the suction interval and blocks the vein during the
systole too. When a device of this kind was used in the animal
model, improved maintenance of the myocardial function during
CA 02218517 1997-10-17
4
coronary artery closure was demonstrated.
However, with regard to clinical application in a patient, the
system still has disadvantages.
Thus, in this device, in the same way as in the device in EP 0
297 723, although the pumping volume per pump stroke can be
adjusted, the flow of the retroinfused blood cannot, however,
be influenced during a pumping interval. It has, however, been
shown that the intravenous pressure is subject to considerable
fluctuations in the course of one pumping interval as a result
of this. Even if the intravenous pressure is recorded, although
a desired mean pressure can be set in the course of several
pumping intervals, the considerable fluctuations in pressure
within one pumping interval cannot be eliminated.
There are, however, some problems associated with these
considerable changes in the coronary vein pressure. Thus, it
has been found that with a low retroinfusion flow, i.e. at a
low venous pressure, an adequate supply of oxygen to the
myocardium is not guaranteed, with the result that the
myocardial function in the ischaemic area cannot be
satisfactorily maintained. With a high infusion f low, i.e. at
too high a coronary vein pressure, there is a danger, however,
of overperfusion occurring, which does not improve the
retrograde nutritive capillary filling, but only impedes the
contraction of the myocardium and leads to an ineffective
outflow of the arterial blood into the systemic circulation. In
the case of retro-infusion at too high a coronary vein
pressure, there is also a danger of irreversible damage to the
vessel wall.
The object of the present invention is therefore to make
available a method and a device for the perfusion of blood
vessels, in particular coronary vessels, in which method the
perfusion is performed at a vascular pressure which is optimum
for nutritive capillary filling, and where this set pressure is
maintained as constant as possible during pumping. The method
CA 02218517 1997-10-17
according to the invention and the device according to the
invention are in this case intended to make it possible to
extend the application possibilities of vascular perfusion, in
particular arterial perfusion and venous retroinfusion, beyond
5 the hitherto short-term myocardial protection.
According to the invention, a method is provided for the
selective perfusion of fluids through blood vessels, controlled
by the pressure in the blood vessels, in which method a tubing
line open at the proximal end for the perfusion of fluids
through a tissue region is introduced into a patient's blood
vessel in which perfusion is to be performed, the vessel is
sealed off from the line in the area of its proximal end, and
the fluid is pumped into the vessel, the method being
characterized in that a specific set value for the internal
pressure of the vessel is predefined, the internal pressure of
the vessel is measured, and the perfused flow of fluid is
regulated during pumping in such a way that the set value for
the internal pressure of the vessel is kept as closely as
possible.
According to the invention, and in contrast to the known
perfusion methods, the flow of fluid during the pumping of
fluid is no longer determined only by the pumping volume per
pump stroke, with the effect that the volume flow pattern
during the pumping phase cannot be influenced, and instead it
is regulated in accordance with the measured vascular pressure.
General methods known in measurement and control technology may
be used as the regulating method, the actual blood pressure in
the vessel being utilized as a control variable, and the flow
of fluid actually infused being utilized as an adjustable
variable. The control function here can take account of the
typical elastic properties of a blood vessel on the basis of
predefined parameters and can adapt these parameters, if
appropriate by evaluating the dynamics of the system, to the
circumstances pertaining to the specific individual case. In
particular, the control system will extrapolate the future
pressure pattern on the basis of the current pressure pattern
CA 02218517 1997-10-17
6
and will adapt the flow of fluid at the right time. The control
system is set up in such a way that the response time is less
than 25 milliseconds.
Advantageously, the fluid is pumped periodically into the
vessel at intervals, the pumping intervals being synchronized
with the patient's heartbeat.
However, it is also possible, particularly in the case of
perfusion of arteries, to pump the fluid into the vessel
continuously.
The set value which is to be defined for the vascular pressure
is an individual value which will depend on the particular
patient, the vessel in which infusion is to be performed, and
the specific site of the perfusion in the vessel.
The set value for the pressure inside the artery is
advantageously chosen such that the nutritive perfusion is
maintained.
If nutritive fluid is retroinfused into a vein, then the fluid
is periodically infused via the tubing line into the vein and
blood is periodically suctioned from the vein via the tubing
line. The pumping and suction intervals are preferably
synchronized with the patient's heartbeat.
According to the invention, it has surprisingly been found that
it is possible to individually fix the desired set value of the
venous pressure for the particular situation in a separate
measurement before the start of the actual retroinfusion.
According to the invention, the set value for the pressure
inside the vein is defined such that, with the vein sealed off,
either the venous pressure is measured without retroinfusion,
with venous blood flow present, or, if there is no blood flow,
infusion is performed with the flow of fluid increasing at each
pumping interval and in so doing the internal pressure of the
vein is measured. It is found that the peak venous pressure
4
CA 02218517 1997-10-17
does not increase in proportion to the increasing flow of
fluid, but rather approaches a limit value (plateau pressure).
It may be assumed that the maximum retrograde nutritive
capillary filling is reached at this plateau pressure, and that
with a higher flow of fluid there is only an ineffective
outflow into the systemic circulation. According to the
invention, it is therefore proposed to predefine this plateau
pressure as the set value for the venous pressure during
retro-infusion.
In the case of protection of the myocardium from ischaemia or
of tissue protection in general, the infused or perfused fluid
is preferably an oxygen carrier. The oxygen carrier used is
preferably blood, in which case it is particularly advantageous
to use the patient's own arterial blood, which is taken, for
example, from the femoral artery and is conveyed to the vein in
which retrofusion is to be performed via a pump, a blood filter
for cleaning the blood and an air trap for freeing the blood of
air bubbles, at a pressure of preferably about 2 bar via a flow
control means. However, it is also possible to use a blood
substitute, such as, for example, a fluorocarbon or
perfluorocetyl bromide solution as oxygen carrier.
The fluid infused through the veins or perfused through the
arteries of the patient may also, however, contain therapeutic
or diagnostic active substances, such as, for example,
anticoagulants, contrast media or beta-blockers.
If the vein in which retroinfusion is to be performed is, for
example, a leg vein in which there is a thrombus, then it will
be advantageous to supplement the fluid with means for
dissolving this thrombus. In this way it is possible, for
example, to apply high concentrations of a medicament locally,
without the other physical functions being adversely affected
by this.
According to one embodiment of the method according to the
invention,
' CA 02218517 1997-10-17
8
venous blood or retroinfusate is actively suctioned between the
pumping phases. This blood is led into a reservoir via a vacuum
pump. In the case of short-term retroinfusion of a vein, the
amount of blood which is suctioned in this way is relatively
small. In the case of more prolonged retroinfusion, for example
when protecting the myocardium from ischaemia during a
prolonged coronary artery closure, it may be advantageous to
defoam the blood, which has been suctioned in one suction
interval, and to free it of air bubbles and then return it to
the patient via a vein. This effectively avoids the patient
suffering a blood loss limiting the duration of application of
the method according to the invention.
According to the invention, a device is also made available for
the selective perfusion of a fluid through blood vessels,
controlled by the pressure in the blood vessels, which device
is particularly suitable for carrying out the above-described
method. The device has a tubing line which can be introduced
into a patient's blood vessel, is open at the proximal end and
can be charged with a fluid under pressure which is to be
pumped into the blood vessel, the proximal end being provided
with an enlargeable sealing means with which the vessel can be
sealed off from the line. Means for measuring the internal
pressure of the vessel and regulating means are additionally
provided, with the aid of which a control unit regulates the
perfused flow of fluid so that a defined pressure inside the
blood vessel is kept as constant as possible during pumping.
If a vein is retroinfused using the device according to the
invention, then the line is advantageously connected to a
suction device for blood from the patient's vein, and the
control unit receives signals from the patient's heartbeat and
defines pumping and suction intervals which are synchronized
with the patient's heart cycle.
According to one preferred embodiment, the admission and
suction line is a multi-lumen catheter. In an embodiment
suitable for retroinfusion, this is an at least four-lumen vein
CA 02218517 1997-10-17
9
catheter, including an admission line for the fluid, a suction
line for the suctioned blood, a measurement line for
determining the internal pressure of the vein, and a control
line for the enlargeable sealing means. For arterial perfusion,
an at least three-lumen artery catheter is advantageously used,
including an admission line for the fluid, a measurement line
for determining the internal pressure of the artery, and a
control line for the enlargeable sealing means.
The sealing means is advantageously a pressure-controlled,
inflatable balloon, so that a balloon catheter can be used as
the catheter. The balloon is then preferably situated at the
end of the catheter that is introduced into the blood vessel.
The measurement line for determining the internal pressure of
the blood vessel communicates at one end with the inside of the
vessel and has a pressure sensor at its other end. However, it
is also possible to arrange a pressure sensor at the proximal
end of the line, which pressure sensor is connected to the
control unit by a fine cable which can be guided, for example,
through the control line for the inflatable balloon. In this
case, a three-lumen catheter would be sufficient, for example.
Moreover, the admission line and the suction line can be
designed as one line at the proximal end of the catheter, which
one line is then connected to the supply reservoir or the
suction reservoir via a switchable 3-way valve.
Depending on the task in hand, it is also possible to provide a
catheter which has additional lines, for example a glass fibre
cable for laser applications or video recordings.
In a preferred embodiment, the regulating means for the
perfused flow of fluid comprise a flow regulator which is
advantageously designed such that the admission line has an
elastically yielding tubing in the area of this flow regulator,
the flow regulator comprising a clamping member which is driven
by an electric motor and which presses the elastic tubing
together to a greater or lesser extent and so controls the
retroinfused flow of the fluid into the blood vessel. The
' CA 02218517 1997-10-17
electric motor is preferably a stepping motor controlled by the
control unit, an eccentric arranged on the axle of the stepping
motor activating the clamping member, and the clamping member
being preferably designed as a crossbar oriented essentially
5 perpendicular to the fluid admission line. The admission line
in this case lies on a rigid support.
The distal end of the admission line is preferably connected to
a fluid reservoir which is under pressure and which
10 advantageously has a pressure sensor for monitoring the
pressure in the reservoir. It is from this reservoir that the
admission line is supplied with the fluid to be infused.
In a further embodiment, the blood taken from an artery of the
patient is fed to this reservoir via a roller pump.
The suction device for the venous blood preferably has a vacuum
pump and a reservoir for the suctioned blood. If the suctioned
blood is intended to be returned to the patient, then this
blood will preferably be led into a reservoir from which it can
thereafter be conveyed via a roller pump, an air trap and a
defoaming device to a vein of the patient.
The synchronization with the patient's heart cycle is
preferahly effected via the lead of an electrocardiogram (ECG),
the R wave advantageously being used as trigger signal. The
pumping cycle advantageously begins between 15 and 50% of the
R-R interval and ends at the start of the following R wave. It
is also conceivable, however,
CA 02218517 1997-10-17
11
to vary the pumping phase within wide limits, so that, where
appropriate, it can even last beyond the following R wave of
the ECG. A fixed ratio of pumping phases to heart cycles is
normally chosen, for example 1:1, 1:2, 1:3, etc. Suction is
preferably always performed between the individual pumping
phases. It may also be advantageous, however, to provide phases
in which neither pumping nor suction is carried out.
The method according to the invention and the device for
controlling the coronary vein pressure during an individual
pumping interval has numerous advantages over the known
retroinfusion devices:
In the case of arterial perfusion, for example in antegrade
catheter perfusion of a coronary artery, the desired perfusion
pressure can be maintained both with the balloon blocked and
also with it unblocked at the catheter tip. The pressure can be
regulated within certain limits of tolerance in a narrow range
about the optimum perfusion pressure and can be kept
substantially constant. In particular, however, it is possible
to avoid overperfusion and the associated haemorrhagic tissue
infarcts even when the outflow of the perfusate in the tissue
is disrupted. The reason for this is that after an inadmissible
increase in the arterial pressure, further perfusion of fluid
only takes place again after the pressure has dropped once more
below the predefined set value.
In retroinfusion, by preventing a substantial increase in the
coronary vein pressure above a preset limit value in the course
of a pumping interval, it is possible to prevent potentially
dangerous peak pressure increases developing, which could cause
damage to the vessel wall and, in extreme cases, rupture of the
vessel.
The diastolic venous/arterial pressure gradient, which can be
adjusted constantly using the device according to the
invention, permits extremely effective retroinfusion since, on
CA 02218517 1997-10-17
12
tree one hand, the optimum pressure gradient for a nutritive
circulation is provided, and, on the other hand, overperfusion
is avoided.
Since the individually differing coronary vein pressure at
which there is an increased outflow of retroinfused arterial
blood into the systemic circulation can be determined by means
of the plateau pressure in coronary vein occlusion even before
interruption of the antegrade perfusion, the invention permits
adjustment of the optimum coronary vein pressure range for each
patient.
Above all, however, both the basal metabolism and the regional
myocardial function in the ischaemic area are markedly improved
compared to the known method. The efficiency of the myocardial
protection is markedly increased especially in patients with
poor arterial collateralization. Damage to the retroinfused
veins can to all intents and purposes be ruled out using the
method according to the invention. Initial clinical tests
suggest that the risk of complications in angioplasty
procedures can be reduced.
However, the method according to the invention and the device
according to the invention for the selective perfusion of blood
vessels, controlled by the pressure in the blood vessels, is
not limited to applications in short-term protection against
ischaemia protection. In addition to the latter, more prolonged
applications of the method are possible, for example, in
complications with persistent closure of the coronary artery,
as a stop-gap measure until surgical emergency bypass. This
more prolonged protection against ischaemia is made possible in
the first instance by the high degree of efficiency of supply
to the tissues with the method according to the invention. In
particular, when the suctioned venous blood, cleaned and
defoamed, is reinfused into a vein of the patient, a more
prolonged application of the retroinfusion method according to
the invention is also possible while maintaining the functional
metabolism.
CA 02218517 2005-10-05
65579-75
13
A further area of application lies in the
identification of chronic, but reversible, regional left-
ventricular dysfunction, also referred to as hibernating
myocardium. Here, the method according to the invention, as
a supplement to nuclear medicine techniques and NMR
techniques, may permit determination of the myocardial
metabolism, which permits differentiation of the necrotic or
scarred myocardial tissue from still potentially
metabolically active myocardial cells. In this case it is
possible, for example in order to confirm the possible
success of a bypass operation, to establish by means of
retrograde perfusion whether and to what extent the
myocardial function can be restored by improving the
nutritive perfusion.
A further possible area of application of the
invention at present is the perfusion or reinfusion of
cells, which have been genetically treated in vitro, into
the bodies of patients.
The invention may be summarized as device for the
selective perfusion of a fluid through blood vessels,
controlled by the pressure in said blood vessels,
comprising: a tubing line which can be introduced into a
patient's vein, said tubing line open at its proximal end
thereof and being chargeable with a fluid under pressure
which is to be pumped into said vein, said proximal end of
said tubing line being provided with an enlargeable sealing
means for sealing said vein off from said tubing line, a
control unit, means for measuring the internal pressure of
said vein, said measuring means being connected to said
control unit, means for regulating the perfused flow of
fluid, said regulation means being operated by said control
unit during a period when said enlargeable sealing means
CA 02218517 2005-10-05
65579-75
13a
seals off said vein such that a defined set value for the
internal pressure of the vein is maintained as constant as
possible during pumping of said fluid.
A preferred embodiment of the invention is
explained in greater detail hereinafter with reference to
the attached drawing. The illustrative embodiment concerns
the case of retroinfusion of veins, but it can also be
applied in principle to the perfusion of arteries. The
essential difference in the perfusion of arteries is that
blood is not suctioned and, accordingly, a three-lumen
catheter can also be used.
In the drawing:
Fig. 1 shows a schematic representation of a
device according to the invention for the selective suction
and retroinfusion of veins, controlled by the pressure in
the veins;
Fig. 2 shows a preferred embodiment of the vein
end of the retroinfusion line of the device according to the
invention, the line in the present case being designed as a
four-lumen balloon catheter;
Fig. 3 shows a cross-section along the line III-III
of the
CA 02218517 1997-10-17
14
catheter in Fig. 2;
Fig. 4 shows a detail of a preferred embodiment of the flow
regulator of the device in Fig. 1;
Fig. 5 shows the schematic representation of an application
of the present invention in myocardial protection
during angioplasty;
Fig. 6 shows diagrams of the time course of defined system
parameters in implementing the method according to
the invention.
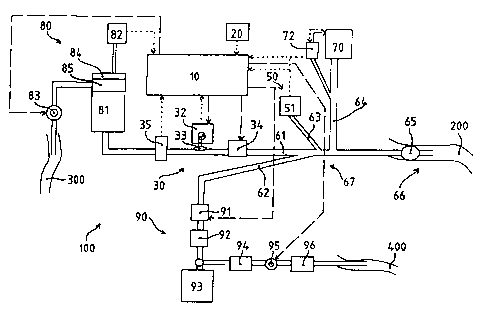
Figure 1 shows a preferred embodiment of the device 100
according to the invention for the selective suction and
retroinfusion of veins, controlled by the pressure in the
veins. The device has what in the present case is a four-lumen
tubing line 60, which is introduced as retroinfusion catheter
into the vein 200 (for example the AIV vein) in which infusion
is to be performed. Provided at the proximal end of the tubing
(vein side) there is a sealing means 65 which is advantageously
an inflatable balloon which seals off the vein 200 from the
line 60, but at the same time permits the passage of fluid from
the tubing line into the vein, or vice versa. For this purpose,
an admission line 61 in particular is provided through which
fluid can be pumped into the vein 200. To do this, the
admission line is connected at its distal end (at the opposite
end from the vein end) to a fluid supply 80. The fluid supply
80 comprises a fluid reservoir 81 which is under pressure, the
pressure in the reservoir being monitored by means of a
pressure sensor 82. When the fluid to be retroinfused is the
patient's own blood, the reservoir 81 can be connected via a
pump 83 to an artery 300 (e.g. the femoral artery) of the
patient. In this case, blood is suctioned from the artery 300
and is led into the reservoir 81, if appropriate via an air
trap and/or defoamer 84 and a blood filter 85. Means 30 for
regulating the flow of fluid are provided on the admission line
61. This flow regulator 30 has, in particular, a shut-off valve
CA 02218517 1997-10-17
34 with the aid of which the connection between reservoir 81
and vein 200 can be completely interrupted. In a particularly
simple embodiment of the f low regulator, the admission line 61
is designed as an elastically yielding tubing in the area of
5 the regulator, and the flow regulator 30 has a clamping member
31 which acts on this tubing and which is activated via an
electric motor, preferably a stepping motor 32. The
retroinfused flow of fluid is regulated by means of greater or
lesser squeezing of the tubing line. The intravenous pressure
10 in the vein 200 serves as a measure of the flow of fluid to be
infused. For this purpose, the retroinfusion catheter 60 has a
measurement line 63 which creates a communication between the
inside of the vein and a pressure sensor 51 arranged at the
distal end of the measurement line 63. However, the pressure
15 sensor can also be provided as a proximal pressure sensor 52 at
the vein end 66 of the tubing line 60. A three-lumen catheter
can also be used in this case, and the electrical connection of
the pressure sensor 52 to the means for measuring the venous
pressure 50 can be guided through one of the other lumina of
the catheter.
The intravenous pressure values thus measured are regulated by
a control unit 10 which accordingly adjusts the clamping member
via the stepping motor 32 of the flow regulator 30. An
ultrasound measurement head 35 can additionally be provided on
the admission line 61, which measurement head 35 is used, on
the one hand, for detecting air bubbles in the fluid to be
retroinfused, and, on the other hand, for determining the flow
of fluid itself. For this purpose, the ultrasound measurement
head 35 has an ultrasound transmitter and an ultrasound
receiver, the reflected ultrasound signal being used for
detecting air bubbles and the Doppler-shifted ultrasound signal
being used for determining the flow of fluid.
A further lumen of the catheter is a suction line 62 which is
used for suctioning blood or retroinfusate from the vein 200 of
the patient. The suction device 90 comprises a shut-off valve
91, a vacuum pump 92 and a container 93 for collecting the
CA 02218517 1997-10-17
16
suctioned fluid. If appropriate, provision may be made for the
suctioned fluid not to be collected and discarded, but for it
to be returned cleaned to the patient. This is particularly
useful in long-term applications of the device according to the
invention. For this purpose, an intermediate reservoir 94 is
provided, and the blood collected there is conveyed back to the
patient into another vein 400 via a roller pump 95, an air trap
96 and, if appropriate, a blood filter.
For efficient retroinfusion, it is important that the vein 200
in which infusion is to be performed should be sealed off
tightly upstream, as viewed in the infusion direction, so that
the retroinfusate flows exclusively into the tissue area to be
supplied. For this purpose, a pneumatically inflatable balloon
is provided at the proximal end 66 of the catheter 60 as a
sealing means 65. A fourth lumen of the catheter thus forms the
control line 64 which does not open into the vein 200, but
instead connects the balloon 65 to a pressure-controlled
balloon pump 70. To control the pressure, a pressure sensor 71
is provided for the balloon pressure on the line 64. If the
catheter is introduced into the vein in which retroinfusion is
to be performed, the balloon 65 can be inflated by pumping in
air, but also, if appropriate, by pumping in a liquid. It then
closes off the vein tightly at the proximal end of the tubing
line 60, while at the same time, however, the communication
from admission line 61, suction line 62 and
pressure-measurement line 63 to the inside of the vein 200
remains assured.
Figure 2 shows, on an enlarged scale, the proximal end
(introduced into the vein) of the retroinfusion catheter 60 of
the retroinfusion device 100 shown in Figure 1. The individual
lines 61, 62, 63, 64 of the four-lumen catheter 60 are welded
together in that section of the catheter which can be
introduced into the patient, and they separate into individual
lines only to the outside of the patient. In this case, for
example, the admission line 61 need not be a continuous line,
and instead it can be connected, outside the patient, and via a
CA 02218517 1997-10-17
17
coupling piece which is not shown, to a further line leading to
the supply reservoir 61.
Figure 3 shows a cross-section along the line III-III of the
proximal end of the catheter in Figure 2. The balloon is in
this case represented in the inflated state and tightly closes
off the area between line 60 and vein 200.
Figure 4 shows an enlarged detail of a preferred embodiment of
the flow regulator of the device in Figure 1. In this area, the
admission line 61 consists of an elastic tubing and is pressed
together by a clamping member 31 designed as a crossbar. The
crossbar is activated by a stepping motor 32, on whose axle an
eccentric control cam is provided, which acts on the crossbar
31. The clock frequency of the stepping motor is in this case
chosen such that a new flow of fluid can be adjusted in the
admission line 61 in less than 25 ms. The corresponding control
is effected via the control unit 10 which calculates the
required setting of the clamping member from the respectively
measured pressure and in particular from the instantaneous
pressure pattern and sends corresponding instructions to the
stepping motor. The line 61 in this case rests on a support 36
which prevents the line from moving during squeezing.
Figure 5 is a schematic representation of a typical application
of the present invention. This is when it is used for
myocardial protection during an angioplasty procedure. Here, a
coronary artery 500 which has been narrowed by arteriosclerotic
plaque is widened by means of a balloon catheter 510. As long
as the balloon 515 of the balloon catheter is inflated, the
area of the myocardium 530 normally supplied by this artery is
no longer supplied with sufficient oxygen and nutrients. For
this purpose, a retroinfusion catheter 60 of the device
according to the invention is introduced, with radiographic
monitoring, into a vein 200 draining this area of the
myocardium. Blood containing oxygen and nutrients, which has
been taken from another of the patient s arteries, is
retroinfused through the line 60 into the ischaemic area, so
CA 02218517 1997-10-17
18
that functional impairment of this area is prevented.
To implement the method according to the invention in
myocardial protection, the patient's vein in which
retroinfusion is to be performed is selected depending on the
area of the myocardium concerned, and the retroinfusion
catheter 60 of the device 100 according to the invention is
advanced, preferably with radiographic monitoring, into the
vicinity of the area of the myocardium which is to be
protected. Depending on whether venous blood flow is present or
not, the intravenous pressure is determined, or an amount of
fluid increasing with each pumping interval is infused and the
plateau pressure thus set up is measured. The desired set value
of the intravenous pressure can be determined from this during
the retroinfusion interval. The set value preferably
corresponds to the desired plateau pressure, but it may also be
chosen to be slightly higher or lower.
In the method according to the invention, the retro-infusion
with blood or another fluid is synchronized with the patient's
heartbeat. To this end, for example, an electrocardiogram is
taken using an ECG unit 20 (see Figure 1). Figure 6 shows the
time course for some important system parameters while carrying
out the method according to the invention. The control unit 10
evaluates the R waves 612 of the ECG lead 610. The
retroinfusion and suction cycles are synchronized with this R
wave of the heart cycle. The line 614 shows the trigger phase
for the retroinfusion of fluid. This phase preferably begins
after 15 to 50% of an R-R interval. The line 620 shows the
momentary setting of the flow regulator, with changes upwards
indicating an increase in flow, and with changes downwards
indicating a decrease in flow. The curve 630 shows the actual
pressure in the vein in which retrofusion is to be performed,
the horizontal line 632 representing the preselected set
pressure. The unit of pressure here is mmHg.
The line 640 shows the retrainfused fluid volume in ml/min. As
is shown in the drawing, it is possible, with the method
CA 02218517 1997-10-17
19
according to the invention, to maintain the desired intravenous
set pressure satisfactorily.
Blood or retroinfusate is suctioned from the vein 200 between
the trigger phases.
Typically, about 0.5 to 1.5 ml of fluid are infused per pumping
interval. The mean pumping quantity thus lies between 30 and
150 ml/min. However, these values are influenced by the
catheter volume used, the catheter length and the preliminary
pressure in the delivery-side high-pressure system.
Depending on the patient and on the retroinfused vein, typical
retroinfusion pressures (set values) lie between 30 and 110
mmHg.