Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02218724 1997-10-20
WO 97131013 PCT/KR96/00281
I
l~ietabolites of ginseng saponins by human intestinal
bacteria and its preparation for an anticancer.
FIELD OF THE INVENTION
This invention relates to the metabolites of ginseng
saponins by human intestinal bacteria and its preparation
for an anticancer and more particularly, to a novel saponin
of a metabolite of Panax ginseng saponin by human bacteria
and a novel preparation of anticancer agent containing a
novel saponin of a metabolite of Panax ginseng saponin by
human bacteria, which exhibits immunopotentiating actions
including inhibitory actions on the vascularization of
tumors and extravasation of cancer cells.
DESCRIPTION OF THE RELATED ART
In recent years much of the development of novel
iv
anticancer agents has widely focused on natural sources and
synthetic compounds.
Among saponins extracted from Panax ginseng, for
example, ginsenoside Rhz L3-0-f3-D-glucopyranosyl-20(s)-
CA 02218724 1997-10-20
WO 97/31013 PCT/HIt96/00281
2
protopanaxadiol] was reported to inhibit the proliferation of
liver cancer cells (reference: Japanese Patent No. 89-28'T59).
Further, both ginsenoside Rgs [3-0-[f3-D-glucopyranosyl(1 '
--j2)-l~-D-glucopyranosyl]-20(R)-protopanaxadiol] and ginsenoside
RbzC20-0-[a-L-arabinopyranosyl(1->6)-!3-D-glucopyranosyl-3-0-[l3-D
-glucopyranosyl (1->2)-l3-D-glucopyranosyl]-20(S)-protopanaxadio
11 were reported to inhibit the vascularization of tumors and
extravasation of cancer cells including inhibitory actions
on the metastatis of cancer cells [References: Japanese
Patent No. 89-28759, Sato et al.: Biol. Pharm. Bull., 1'l. 635(1994)].
In ease of 20-0-[~3-D-glucopyranosyl]-20(S)-
protopanaxadiol, called as "compound K" and 20-0-[a-L-
arabinopyranosyl(1->6)-l3-D-glucopyranosyl]-20(S)-protopanaxadi
ol, called as "compound Y", Which were isolated from soil
l5 strains of Panax ginseng saponin and intestinal flora of
rats, their structure was already determined [References:
Yoshioka et al.: Chem. Pharm. Bull., 20, 2418(19T2), Takino et al.:
Medicinal ginseng '69 (Public Publishing Co., Ltd. 267(1989)].
Also, the structure of 20(S)-protopanaxatriol, isolated
by sapogenin of Panax ginseng saponin, was already
established (Nagai et al.: Tetrahedron, 2f, 881(1971)],
a
The pharmacological actions of these compounds, for
CA 02218724 1997-10-20
WO 97/31013 PCT/KR96/00281
3
example, inhibition of glucose transplant related to cancer
cells by the blockage of membrane protein, were merely
reported by each inventor [Hasegawa et al., Planta Med., 60,
19'l(1994)], including the report on methicillin-resistant
bacteria and the inhibition of the excretion of drugs on the
multidrugs-resistant cancer cells [Hasegawa et al.: Phytother,
Res., 9, 260(1996), Hasegawa et al., Planta Med., 61, 409(1995)].
In case of the conventional chemotherapeutics which
exhibit their therapeutic effects by attacking the cancer
cells directly, their adverse effects are quite severe.
During several decades, any antineoplastic agents with new
mode of mechanism have not yet to be on the market. Further,
in the event that Panax ginseng saponins are applied for the
treatment of some diseases, these substances are reported to
be metabolized by intestinal bacteria and said bacteria is
liable to be influenced by human's constitution and his food
pattern. Thus, any individual differences in the metabolism
of sanponins may lead to the individual differences in his
treatment.
SOMMARY OF THE INVENTION
CA 02218724 1997-10-20
WO 97/31013 PCT/HIt96/00281
4
In view of these situations, the inventors of this
invention have investigated the metabolism of ginseng
saponin associated by human intestinal bacteria and
succeeded in isolating and identifying the following
compounds, i.e., a) protopanaxadiol saponins(ginsenoside Rb~,
ginsenoside Rbz and ginsenoside Rc), b) compound K, compound Y
and 20-0-[a-L- arabinofuranosyl(1->6)-l3-D-glucopyranosyl-20(S)-
protopanaxadiol~, which are called as ginsenoside Mc,
metabolites of ginsenoside Rd, and c) 20(S)-protopanaxatriol, a
l0 metabolite of ginsenoside Rg~ and ginsenoside Re which
belongs to protopanaxatriol saponin.
The inventor ascertains that these intestinal flora
metabolites are absorbed from intestinal tracts to blood and
excreted via urine and feces. By assuring that said
l5 intestinal flora metabolites prove to be main substances of
Panax ginseng saponin, the inventor has endeavored to develop
the therapeutic dosage form containing the active ingredient
of saponin, which is not influenced by the difference of
intestinal bacteria. As a result of reviewing these
20 physiological activities, the inventor has discovered a novel
preparation of anticancer agent , which exhibits
immunopotentiating actions including inhibitory actions on
CA 02218724 1997-10-20
WO 97/31013 PCT/KR96/00281
the vascularization of tumors and extravasation of cancer
cells. Thus, this invention has finally completed.
Therefore, the object of this invention is to provide a
new compound of 20-0-[a-L-arabinofuranosyl(1-->6)-~3-D-
5 glucopyranosyl-20(S)-protopanaxadiol] having the following
formula, a novel ginseng saponin metabolite by human
intestinal bacteria(called as ginsenoside Mc) with the
following characteristics.
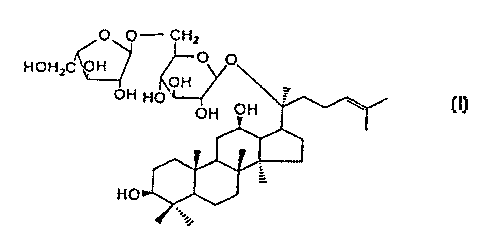
I) Structural fomula
O O- CHZ
HOHzC 'VH
OH
HO
2) Molecular formula: C~,.H~oO~z
3) The mass spectrum (Fab-MS, negative and m/z) showed
signals at 'l53[M-H]-, fi21(M-arabinofuranose-HJ-, and 459[M-
arabinofuranos-glucopyranos-HJ-.
4) The 1H-NMR spectrum(d5-pyridine) showed signals at 8
3.39(1H, t, J=10.5, 5.lHz, H-3), 0.80(1H, d, J=1l.OHz, H-5), 3.93(1H,
ddd-like, H-12), 5.32(IH, t, J=T.lHz, H-24), 0.92(3H, s, Me-I8), 0.89(3H,
CA 02218724 1997-10-20
WO 97/31013 PCT/I~1t96100281
6
s, Me-19), 1.69(3H, s; Me-21), 1.6'T(3H, s, Me-26), 1.67(3H, s, Me-27),
1.21(3H, s, Me-28), 1.01(3H, s, Me-29), 0.99(3H, s, Me-30), 5.10(IH, d,
J='T.8Hz, H-1'-20-glucopyranosyl), 5.61(1H, J=l.7Hz, H-1"-6'-
arabinofuranosyl).
5) The 13C-NMR spectrum(d5-pyridine) for aglycon moiety
showed signals at s39.5(C-1), 28.3(C-2), T8.2(C-3), 39.5(C-4), 56.5(C-5),
18.8(C-6), 35.2(C-?'), 40.2(C-8), 50.4(C-9), 37.4(C-10), 30.8(C-lI),
T0.3EC-12), 49.5(C-13), 51.5(C-14), 30.9(C-15), 26.T(C-16), 51.8(C-17)
16.3(C-18), 16.1(C-19), 83.2(C-20), 22.4(C-21), 36.2(C-22), 23.2(C-23),
l0 126.1(C-24), 131.0(C-25), 25.8(C-26), 1f.9(C-27), 28.7(C-28), 16.4(C-29),
17.5(C-30);
The 13C-NMR spectrum(d5-pyridine) for 20-glucopyranosyl
moiety showed signals at 98.1(C-1'), 75.1(C-2'), ?9.2(C-3'), f2.2(C-4'),
76.5(C-5'), 68.5(C-6');
The 13C-NMR spectrum(d5-pyridine) for 6'-arabinofuranosyl
moiety showed signals at 110(C-1"), 83.5(C-2"), f9.0(C-3"), 86.3(C-4"),
62.8(C-5").
Tn addition to ginsenoside compound Mc, a novel
intestinal bacteria metabolite of novel ginseng saponin,
another object of this invention is to provide an anticancer
CA 02218724 1997-10-20
WO 97/31013 PCT1KR96100281
7
agent containing one active ingredient selected from
intestinal bacteria metabolites of ginseng saponin such as
y compound R, compound Y, ginsenoside Mc and protopanaxatriol,
together with one or more pharmaceutically acceptable
carriers. These intestinal bacteria metabolites of ginseng
saponin exhibits remarkable antineoplastic effects in the
long run since they potentiate the inhibitory actions
against cancer ceiis in lvmphocvte and inh~bzfi t-hp
vascularization of tumors and extravasation of cancer cells.
Even though the severity of symptoms differs, the oral
dose of anticancer agent for adult according to this
invention is 1-50mg/60kg of body weight once daily or several
times per a day, preferably 3-l5mg/day /60kg of body weight.
The anticancer agent according to this invention
contains either a single ingredient, or said ingredient plus
one or more pharmaceutically acceptable carriers such as
excipients in the form of solid or liquid.
The administration method and available dosage forms
are as follows:
a) Oral forms: powders, tablets, suspensions,
emulsifiers, capsules, granules, troches, pills, suspensions,
spirits, syrups and limonades;
CA 02218724 1997-10-20
WO 97!31013 PCT/HIt96/00281
8
b) In~ectable forms, or
c) Topical forms: ointments, solids, suspensions,
powders, paps, suppositories, aerosols, cataplasmas, liniments,
lotions, enemas and emulsifiers.
According to this invention, well-known excipients in
the form of solid or liquid may be used. As mentioned in the
above, the formulation should be conducted so as to contain
the active ingredient of this invention necessary for single
dose. The several examples of excipients used in related
dosage forms are as follows:
- Excipients in powders and other oral powders: lactose,
crystalline cellulose, starch, dextrin, calcium phosphate,
calcium carbonate, synthetic and natural aluminum dioxide,
magnesium oxide, dried aluminum hydroxide, magnesium stearate,
and sodium bicarbonate;
- Excipients in topical powders: zinc oxide, talc,
starch, kaolin, borate powder, zinc stearate, magnesium
stearate, magnesium carbonate, precipitated calcium
carbonate, bismuth subgallate, and potassium aluminum sulfate
powder;
- Excipients in liquids: water, glycerin, propylene
CA 02218724 1997-10-20
WO 97/31013 PCT/KR96/00281
9
glycol, sweet-taste syrup, ethanol, fatty oil, ethylene glycol,
polyethylene glycol, and sorbitol;
- Excipients in ointments: hydrophobic or hydrophilic
base (including oil-soluble base, water-soluble base and
suspended base) prepared by mixing fat, fatty oil, lanoline,
vaseline, glycerin, wax, Japan wax, paraffin, paraffin sulfate,
resins, higher alcohols, plastics, glycols, water, or
surfactant.
EXAMPLE : Preparation of ginsenoside Mc
The suspended solution of human flora was precultured
in GAM medium overnight and then, 100mg of ginsenoside Rc was
added to said medium to the desired concentration of 2% in a
newly sterile GAM medium and then cultured at 37°C for 1 day.
The medium was extracted by 1-butanol and the extracted
solution was concentrated and purified on reversed/irreverse
phase chromatography to give 25mg of pure ginsenoside Mc.
[Formulation examplel
~ Formulation example 1 : A mixture of lactose, crystalline
cellulose and to magnesium stearate was added to 30mg of
CA 02218724 1997-10-20
WO 97/31013 PCT/Kit96/00281
compound R, an intestinal flora metabolite of ginseng
saponin, for homogenous mixing. Said mixture was tabletted by
a tabletting machine to obtain each tablet containing 200mg.
~ Formulation examples 2--4 : Based upon the same procedure
5 as described in formulation l, each preparation was obtained
containing 30mg of compound Y, ginsenoside Mc or 20(S)
protopanaxatriol, respectively, instead of 30mg of compound R.
~ Formulation example 5 : A solution of l5mg of compound K, an
intestinal flora metabolite of ginseng saponin and
10 polysorbit 80 was filled into a sterilized vial aseptically
and after removing the moisture, the preparation for
in3ection was obtained.
~ Formulation example 6~-8 : Based upon the same procedure as
described in formulation 5, each preparation was obtained
containing l5mg of compound Y, ginsenoside Mc or 20(S)
protopanaxatriol, respectively, instead of l5mg of compound R.
The physiological actions involved in the intestinal
flora metabolite of ginseng saponin of this invention are
described in the following examples but these protective
scopes are not confined to said examples.
LExperiment 1~
CA 02218724 1997-10-20
WO 97!31013 PCT/KR96/00281
lI
Antitumor activity on leukemia cell line (P388) in lymphocyte
of mice
a
a) Experimental method
Spleen lymphocytes of mice and leukemia cell line (P388)
were used for this experiment. Spleen lymphocytes (4 X 106
cells) and leukemia cells (P388) (2 X 108 cells) were
cultured in a medium (RPM! 1640 supplemented with 20 ,u M
mercaptoethanol and 10% fetal bovine serum) containing
intestinal flora metabolite of ginseng saponin (2.5 ~ M) in
5% COz saturated with steam for I6 hours.
Aside from this, same numbers of spleen lymphocytes or
leukemia cell line were cultured in a medium containing
intestinal flora metabolite of ginseng saponin in same
concentration as a control. The number of each survived
cell was purified by MTT method to calculate impaired rate
of cells on leukemia cells (P388) of lymphocytes.
b) Experimental results
0
As shown in table 1, the experimental results revealed
that all intestinal flora metabolites of ginseng saponin
in a low concentration of 2.5,~M exhibited antitumor
CA 02218724 1997-10-20
WO 97/31013 PCTlKR96/0028I
12
activities 1.6 to 2 times as higher as control group.
,.
Table 1. Antitumor activity on the cancer cells of lymphocyte
by intestinal flora metabolites of ginseng saponin
Concentration(u~/m2)Antitumor
activity
Control 3] .3 1
Compound R 1.56 51.5 1.6
Compound Y 1.86 56.6 1.8
Ginsenoside Mc 1.86 63.3 2.p
20(S)-protopanaxatriol 1.19 82.4 2.0
[Experiment 2]
Inhibition on the vascularization of tumor
(Test for the inhibition on the proliferation)
a) Experimental method
Human lymphocyte (HL), leukemia cell Line (R562) and bovine
artery endotheliocyte (BAE) were used for this experiment.
HL (1X108 cells), leukemia cell line (R562) (2X105 cells) and
BAE (5X10 cells) were cultured in a medium (HL, 8562: RPMI
1640 medium containg 10% fetal bovine, BAE: DMEM medium
containing 10% fetal bovine) containing intestinal flora
metabolites of ginseng saponin Concentrated with 2-fold
CA 02218724 1997-10-20
WO 97/31013 PCT/HIZ96/00281
13
dilution in 5~ COz saturated with steam (HL, K562: 24 hours,
BAE: 72 hours). The number of each survived cell was
purified by MTT method to calculate 50% inhibition
concentration (ICso), impaired rate of cells
(ICso(HL)/ICso(BAE) and ICso(K562)/ICso(BAE).
b) Experimental results
As shown in table 2, the experimental results revealed
that ginsenoside, Mc and 20(S)-protopanaxatriol exhibited
inhibitory activities on the proliferation of tumor cells
Table 2. Inhibitory activity on the proliferation by
intestinal flora metabolites of ginseng saponin
ICso( ICso(C)/ICso(BAE)
/~ M)
HL K562 BAE C=HL K562
Compound R 45 2fi 28 1.~ 1.6
Compound Y 83 78 32 2.& 2.6
Ginsenoside Mc 220 480 26 8.5 18
20(S)-protopanaxatriol280 49 36 'l.8 1.4
LExperiment 31
Inhibition on the vascularization of tumor
(Test for the inhibition on the migration)
CA 02218724 2000-09-11
19
a) Experimental method
Bovine artery endotheliocyte (BAE) was used for this
experiment. BAE (5X103 cells) was cultured in 6-well plate
for 24 hours and cells attached to the plate were detached
- by a razor. Said medium was replaced by a new one and of ter
one hour, a solution of intestinal flora metabolite of
ginseng saponin was added to the desired inhibitory
concentration of 10°6 and 50%, respectively for 24-hour
cultivation. After completing the cultivation, cells were
fixed with methanol, stained with Giemsa method and
counted the cells migrated from the detached line under
microscope.
b) Experimental results
18 As shown in table 3, the experimental results revealed
that each intestinal metabolite of ginseng saponin
exhibited an inhibitory activity of migration and among
them, it was noted that compound R showed more potent
inhibitory activity of migration than suramin (Wako Pure
Chem. Ind. Ltd., Japan), a control group.
CA 02218724 1997-10-20
WO 97/31013 PCT/HIt96/00281
Table 3. Migration-inhibition by intestinal flora metabolites
' of ginseng saponin
Migration-inhibition
(% control)
IClo ICSo
Snramin -3.8 37.1
Compound K 4.6 43.2
Compound Y -3.7 28_g
Ginsenoside Mc -1.6 30.0
20(S)-protopanaxatriol -1.6 30.0
LExperiment 4J
10 Inhibition on the extravasation of basement membrane
a) Experimental method
The transwell culture chamber was used for this experiment
with a haptoinvasion method (reference: Cancer Res., 4T,
1' 3239, (198?)). The lower side of a filter havi n~ a
8.0um in diameter was coated with 3u~ of matrigel for the
fabrication of matrigel/FN filter. Human adenosarcoma
tissue cell (HT1080), treated in the intestinal flora
metabolite of ginseng saponin in a concentration of 1-1000
~cM at 37 C for 30 minutes, was charged to the upper side of
each filter with 1xI05 ce11s1100uL. Then, said filters were
added to 24-well plate having 600~cL of MEM medium
CA 02218724 2000-09-11
I6
supplemented with 0.1% bovine serum albumin and them
cultured for 4-hour cultivation. After completing the
cultivation, cells were fixed with methanol and stained
with hematoxylin, a tissue staining agent. Following the
removal of cells at the upper side with a cotton pole,
cells infiltrating into the lower side were counted under
microscope.
b) Experimental results
As shown in table 4, the experimental results revealed
that each intestinal metabolite of ginseng saponin
exhibited more potent inhibitory activity of
extravasation than RGDS peptide (under development from
Glycomed Inc.:Cancer Res., 49, 3815 (1989)), a control
group and among them, 50% extravasation-inhibitory
concentration of compound K showed a potent activity in a
low concentration of 3.2 ~u NL.
Table 4. Inhibition on the extravasation of basement membrane
by intestinal flora metabolites of ginseng saponin
CA 02218724 1997-10-20
WO 97!31013 PCT/KR96/00281
17
Concentration No. of infiltratedInhibition
~c M) cancer celllf fieldrate
(i)
,
Control 11g g __
RGDS peptide 4000
61=8 48
Compound K 1 ?34 38
EDso=3.2 50
10 45 ~!- 9 62
I00 0 100 i
Control I1T ~!- 9
f
RGDS peptide 4000 51-!-IO 56
Compound Y 1 125 -!- 7
1o sz II zI
EDso=3I 50
100 242 62
1000 0 100
Control 117 -!- g
RGDS peptide 4000 5110 56
ginsenoside Mc 1 114-!-12 3
EDso=?.6 50
IO 44 -!-'T 62
100 31 g7
I5
looo o Ioo
Control g7 g
RGDS peptide 4000 494
49
20ES) protopanaxatriol1 I03 14
10 93T 4
EDso=48 50
I00 I8 4 8I
1000 I1 99
CA 02218724 1997-10-20
WO 97/31013 PCT/KIt96/00281
18
From the aforementioned results, it is noted that
intestinal flora metabolites of ginseng saponin, such as
compound K, compound Y, 2fl(S)-protopanaxatriol including ..
ginsenoside Mc of this invention, are novel types of
potential anticancer agent since they have
immunopotentiating actions including inhibitory actions on
the vascularization of tumors and extravasation of cancer
cells.
The toxicity of ginsenoside Mc, a novel compound of
l~ this invention, is nearly negligible in some animal
experiments with rats and mice and the products' stability of
each preparation based upon each formulation example is
quite effective.
1~