Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02222199 2006-04-21
30754-11
1
N-Phenylpyrazole-based antiparasitic external
device for cattle, in particular ear-rings
The present invention relates to external
antiparasitic devices for cattle, in particular in the
form of a ring and more particularly an ear-ring.
The invention also relates to the use of
active compounds for the manufacture of such external
devices, as well as to a treatment process relating
thereto.
One of the main cattle parasites is a fly
known as Haematobia irritans (horn fly), the adult of
which is a permanent haematophagic parasite of cattle.
This parasite, which is encountered especially in North
America, but also in Europe, Australia and Southern
Africa, can lead to considerable economic losses, as
regards both weight gain and milk production, in
particular when the parasite density exceed 50 flies per
animal, while, in certain regions, it is not unco mon to
exceed 200 flies per animal.
At the present time, it is not known how to
control this parasite effectively. The difficulty in
controlling it results mainly from the fact that the
parasite density is constant, since this is a parasite
which possesses wings in its adult form, and can thus
pass from one hard to another and thereby permanently
rsinfsst hards for which a procedure to control this
parasite has been iaplemented. Irt addition, in certain
regions, the parasite dansity lasts throughout the year.
Breeders currently have few effective means of control
available to them, in particular in the case of rearing
in open grazing, even by ensurinQ constant control of
these parasites, that is to say regular and closely-
timed supplies of insecticides, all the more so since
flies have developed resistance to pyrathroids and to
CA 02222199 2006-04-21
30754-11
- 2 -
organophosphorus compounds.
Ear-rings intended, as their name implies,
to be attached to the animal's ear and to release an
active substance over a longer or shorter period have
also been proposed. These rings consist of a matrix,
usually a plastic matrix, which incorporates the active
substance and is able to release it over time. The aim
of these rings is thus, theoretically, to ensure long-
lasting protection.
However, despite the activity claims, in the
field rings do not display the efficacy required to
ensure that these parasites are actually eliminated. The
reason for this may be the low activity of the active
substance included in the matrix. Another reason may be
the accelerated degradation of these active substances
under the effect of climatic factors, such as light,
heat and rain. Lastly, the control of the release of the
active substance from the matrix is widely
overevaluated. The release generally proves to be
difficult and variable, and it may depend highly on the
manufacturing conditions, which may vary from one batch
to another, and on the conditions of use, in particular
climatic variations, and especially humidity and
temperature, etc. In addition, only a relatively small
amount of the active substance incorporated is actually
relpased and it proves to be difficult to be able to
control and optimize its release.
Only orQanophosphorus ccmqpounds have made it
possible to obtain a release over about 4 months, but
by means of considerable concentrations of active
substance, of about 40% by weight.
Patent applications WO-A-87/03781,
EP-A-0,295,117, EP-A-0,296,381 and EP-A-0,500,209 relate
to a family of insecticides which are N-phenylpyrazoles.
These substances are described as being active against
a very large number of parasites encountered in various
CA 02222199 2006-04-21
30754-11
- 3 -
fields, namely agriculture, public health and human and
veterinary medicine.
EP-A-0,500,209 indicates that, in the field
of public health, these compounds may be used to combat
a large number of insects, in particular flies and among
which are mentioned "hornfly" or Haematobia irritans.
However, Haematobia irritans is not mentioned in the
context of treating the animals.
These substances may be applied in different
ways, namely via the oral, parenteral, percutaneous or
topical route. Topical administration itself covers
various possibilities, namely sprays, powders, baths,
showers, jets, greases, shampoos, creams, waxes,
preparations of skin solution type (pour-on) and
external devices such as ear-rings and collars to
provide local or systemic treatment.
EP-A-0,295,117 and EP-A-0,500,209 also
propose a composition for slow release which may be in
the form of a collar or ear-rings, to control harmful
insects. Such a formulation may comprise from 0.5 to 25%
active material, from 75 to 99.5% polyvinyl chloride and
a catalytic amount of a plasticizer, dioctyl phthalate.
US-A-5,472,955 illustrates the difficult
nature of controlling Haematobia irritans and indicates
that it is necessary to make use of a combination of two
active compounds, diazinon and chlorpyrifos, in high
concen-tration in a slow-release device. That document
indicates that the compounds taken alone are not as
effective as the synergistic combination of the two
compounds. Diazinon simply affords an acceptable level
of control of Haemotobia irritans over 3 to 5 months,
whereas chlor-pyrifos appears to be incapable of this.
The said document specifies at the end that an ear-ring
containing a 40% mixture was much more active than two
ear-rings applied on the same animal and containing
CA 02222199 2006-04-21
30754-11 - 4 -
21.4% diazinon as sole active ingredient. Although the
proportion of active principle may range in theory from
8 to 40gs, it is preferably between 20 and 40%. in the
case of ear-rings, the proportion is from 10 to 50% by
weight, preferably from 30 to 45% and optimally 40% by
weight.
None of the documents cited either describes
or suggests that it is possible to effectively protect
cattle against Haematobia irritans, over a long period,
using ear-rings comprising a single active compound, for
which, in addition, a specialist might expect to
encounter conventional problems of release from the
rings and thus problems of activity.
As with the other insecticides of the prior
art, these N-phenylpyrazoles are, indeed, subject to the
same difficulties in the context of external release
devices such as ear-rings.
It therefore did not appear to be
conceivable to be able thus to effectively control
parasites liable to be found on any part of the animal,
which is all the more the case for winged parasites
which give rise to a parasite density as high and
constant as that of Haematobia irritans.
The Applicant has now found, surprisingly,
that it is actually possible to effectively eliminate
Haematobia irritans from cattle by using an ear-ring
using an N-phenylpyrazole under specific concentration
conditions. The Applicant has observed with surprise
that, despite the problems associated with release by
ear-rings, the efficacy extended throughout the animal's
body and was very lonQ-lasting and that, for example,
with rings loaded to a content of 10%, it was possible
to exceed 32 weeks at an efficacy of greater than 90%.
The active substance acts by simple contact,
the parasite becoming impregnated with it on contact
CA 02222199 2006-04-21
30754-11
- 5 -
with the hairs and the skin.
The subject of the present invention is thus
an external device such as a ring, in particular an ear-
ring, intended to eliminate cattle parasites, in
particular Haema.tobia irritans, this device being formed
of a matrix including from 0.1 to 40% by weight,
relative to the ear-ring, of an active substance which
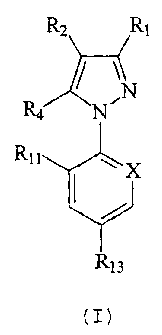
is at least one compound of formula (I) below:
R2 Ri
R4.
Rit ~X
13
in which:
R1 is CN or methyl or a halogen atomf
R, is s(O)qR, or 4,5-dicyanoiinidazol-2-yl or
haloalkyl;
R, is alkyl or haloalkyl;
R, represents a hydrogen or halogen atom; or
a radical NR,R6, 8(O)iR,, C(O) R,, C(O) O-R,, alkyl,
haloalkyl or OR, or a radical -N-C ( R9 )( Rla ) j
R, and Rs independently represent a hydrogen
atom or an alkyl, haloalkyl, C(O)alkyl, alkoxycarbonyl
or S(O) =CF3 radical; or R. and Rs may together form a
divalent alkylene radical which may be interrupted by
one or two divalent hetero atomns, such as oxygen or
sulphur;
R, represents an alkyl or haloalkyl radical;
R. represents an alkyl or haloalkyl radical
or a hydroQen atom;
R. represents an alkyl radical or a hydrogen
atom;
CA 02222199 2006-04-21
30754-11
- 6 -
Rlo represents a phenyl or heteroaryl group
optionally substituted with one or more halogen atoms or
groups such as OH, -O-alkyl, -S-alkyl, cyano or alkyl;
R11 and Rl, represent, independently of each
other, a hydrogen or halogen atom, or optionally CN or
NO2;
RI, represents a halogen atom or a haloalkyl,
haloalkoxy, S( O) QCF, or SF5 group;
M. n. q and r represent, independently of
each other, an integer equal to 0, 1 or 2;
X represents a trivalent nitrogen atom or a
radical C-R1? the other three valency positions of the
carbon atom forming part of the aromatic ring;
with the proviso that when R. is methyl,
either R3 is haloalkyl, R, is NHõ Ril is Cl, Ri, is CF3
and X is Nf or Rs is 4,5-dicyanoimidazol-2-yl, R, is Cl,
Rli iS Cl, Rl, is CF3 and X 1S -C-Cl,
this external device being designed to
ensure efficacy for more than 30 weeks.
Preferably, the compound of formula (I) is
such that:
Rl is CN or methyl j
R1 is s(O)nR,;
R, is alkyl or haloalkyl;
R, represents a hydrogen or halogen atom; or
a radical NRsRs, S(O).Rõ C(O)R,, alkyl, haloalkyl or OR,
or a radical -N=C ( Ry )( Rt, ) j
R, and R6 independently represent a hydrogen
atoaa or an alkyl, haloalkyl, C(O)alkyl or s(O)=CF,
radical; or R. and R6 may together form a divalent
alkylene radical which may be interrupted by one or two
divalent hetero atoms, such as oxygen or sulphur;
R, represents an alkyl or haloalkyl radical;
R. represents an alkyl or haloalkyl radical
or a hydroQen atoma
CA 02222199 2006-04-21
30754-11
- ~ -
R9 represents an alkyl radical or a hydrogen
atom;
Rlp represents a phenyl or heteroaryl group
optionally substituted with one or more halogen atoms or
groups such as OH, -0-alkyl, -S-alkyl, cyano or alkyl;
Rll and Rl, represent, independently of each
other, a hydrogen or halogen atom;
R1, represents a halogen atom or a haloalkyl,
haloalkoxy, S(O)aCF, or SF5 group;
M. n, q and r represent, independently of
each other, an integer equal to 0, 1 or 2;
X represents a trivalent nitrogen atom or a
radical C-Rl,, the other three valency positions of the
carbon atom forming part of the aromLatic ring;
with the proviso that when Rl is methyl, then
R, is haloalkyl, R, is IH2, Ri*i is Cl, Rl, is CF3 and X is
N.
The compounds of formula (I) in which Rl is
CN will be selected most particularly. The cospounds in
which R, is 3(O)aR,, preferably with n a 1, R3 preferably
being CF, or alkyl, for example methyl or ethyl, or
alternatively n - 0, R, preferably being CF,, as well as
those in which X - C-R,,, R11 being a halogen atom, will
also be selected. The co pounds in which R11 is a halogen
atom and those in which Rl, is haloalkyl, preferably CF3 ,
are also preferred. In the context of the present
invention, the compounds which combine two or more of
these characteristics will advantaQeously be selected.
A preferred class of compounds of
formula (I) consists of coaapounds such that Rl is CN, R,
is haloalkyl, preferably CF,, or ethyl, R, is NH,, Ri, and
R12 are, independently of each other, a halogen atom,
and/or R1, is haloalkyl.
The alkyl radicals in the definition of the
coa~pounds of formula (I) generally coanprise from 1 to 6
CA 02222199 2006-04-21
30754-11
- 8 -
carbon atoms. The ring formed by the divalent alkylene
radical representing RS and R6, as well as the nitrogen
atom to which R. and R. are attached, is generally a 5-,
6- or 7-membered ring.
A compound of formula (I) which is most
particularly preferred in the invention is
1- [2, 6-C124-CF,phenyl] 3-CN4- [SO-CF3] 5-
NH2pyrazole,
referred to hereinbelow as compound A.
Mention may also be made of two compounds
which differ from the above A by the following
characteristics:
1- nn 0, R3 - CF3
2- n - 1, R, = ethyl.
Compounds of formula (1) may be prepared
according to one or other of the processes described in
patent applications WO-A-87/3781, 93/6089, 94/21606 or
European patent application EP-A-0,295,117, or any other
process falling within the competence of a specialist
skilled in the art of chemical synthesis. For the
chemical preparation of the products of the invention,
a person skilled in the art is considered as having at
his disposal, inter alia, all the contents of "Chemical
Abstracts" and the documents which are cited therein.
However, loorv concentrations of from 1 to 15%
by weight and more particularly, especially for compound
A, from 2.5 to 10% are preferred. Under optimum
conditions, the rings according to the invention
comprise from 5 to 10% by weight of active substance, in
particular of compound A.
Within the context of the present invention,
the external devices obviously include any device
intended to be attached to the animal's body and which
allows the active substance to be released under the
efficacy conditions of the invention.
CA 02222199 2006-04-21
30754-11
- 9 -
Hy acting on the concentration of active
substance and/or on the composition, ear-rings can be
made with an efficacy towards Haematobia irritans which
can exceed 90% and even 95% for more than 30 weeks, in
particular for 32 weeks.
It is noteworthy that this total and very
long-lasting efficacy is obtained by the compound
according to the invention alone, without addition of
another insecticide.
The active substance can however comprise,
in addition to the compound of formula (I), another
insecticide, for example pyrethroids (in particular
permethrin, cypermethrin, etc.), orQanophosphorus
compounds (for example diazinon), imidacloprid and IGRa
(insect Qrowth regulators).
These active substances may be combined in
different ways. The active substances are either
combined within the same ear-ring, including a composite
ear-ring, namely one made of two parts, each part
includinQ one of the active substances, or use may be
made of two separate ear-rings, each comprising one of
the active substances and each preferably intended to be
placed on a different ear of the animal. In the latter
case, the subject of the invention is a kit comprising
these two ear-rings.
The matrix of the external devices according
to the invention may be based on polyvinyl chloride
(PVC) (see US-A-3,318,769, 3,852,416, 4,150,109,
5,437,869) and other vinyl polymers, to which additives
such as plasticizers, pigments, etc. are optionally
added. In general, the matrices usually used in the
comaon external devices of ear-ring and collar type can
be used.
The plasticizers may be chosen in particular
from adiDates, phthalates, phosphates and citrates.
CA 02222199 2006-04-21
30754-11
- 10 -
One or more plasticizers will preferably be
added to the PVC, these plasticizers being chosen, in
particular, from the following compounds:
- diethyl phthalate
- dioctyl sebacate
- dioctyl adipate
- diisodecyl phthalate
- acetyl tributyl citrate
- diethyl hexyl phthalate
- di-n-butyl phthalate
-benzyl butyl phthalate
- acetyl tributyl citrate
- tricresyl phosphate
- 2-ethylhexyl diphenyl phosphate.
Even more peferably, a PVC matrix will be
used in the presence of a first remanent plasticizer as
described above and of a second plasticizer, in
particular according to EP-A-0,539,295 and BP-A-
0,537,998.
AmonQ the secondary plasticizers, mention
may be made of the follaarinQ products:
- acetyl triethyl citrate
- triethyl citrate
- triacatin
- diethylene glycol monoethyl ether
- triphenyl phosphate.
A common stabilizer may also be added
thereto.
Another subject of the present invention is
a process for the elimination of external parasites, in
particular Haematobia irritans, from cattle, in particu-
lar cattle reared in the open air and more particularly
in open grazing, which consista in attaching to the
animal the ear-ring or -rings according to the present
invention, which ensure(s) the effective and long-
CA 02222199 2006-04-21
30754-11
- i1 -
lasting protection described above.
This process generally consists in attaching
to the animal 1 or 2 ear-rings or the like loaded with
compound according to the invention, preferably with
compound A. These rings have the specifications
mentioned above.
The process may also comprise the commbined
use of other insecticides, by the use of rings,
composite rings or separate rings as described above.
In general, the process according to the
invention envisages the animal wearing the ring or rings
throughout the year.
The aim of the method is non-therapeutic and
is in particular to cleanse the aniaaals' skin and hairs
by eliminating the parasites which are present thereon,
as well as their residues and dejections. The result of
this is that the animals are no longer stressed by the
parasites and their bites, this having positive con-
sequences, for example on their growth and on the use of
their food ration.
Another subject of the invention is a
therapeutic method using the external device according
to the invention, intended for treating and preventing
parasit-oses having pathogenic consequences.
The subject of the present invention is also
the use of a comapound corresponding to formula (I)
above, for the preparation of external devices such as
rinQs, in particular ear-rings, intended to be attached
to cattle in order to ensure elimination of Haemotobia
irritans to a high degree of efficacy and over a period
exceeding 30 weeks. The devices as above are concerned.
The use according to the invention preferably includes
the incorporation of 0.1 to 40% by weight, preferably
from 1 to 15% by weight, of the compound of formula (i)
in a snatrix intended to form the external device. Even
CA 02222199 2006-04-21
30754-11
- 12 -
more preferably, the incorporation is made in a
proportion of 2.5 to 10% by weight, and even from 5 to
10%.
Preferably, the use according to the
invention is directed towards the manufacture of ear-
rings.
Preferably, the use according to the
invention is directed towards the production of external
devices or ear-rings having an efficacy of greater than
90% or 95%.
The present invention will now be described
in greater detail by means of a non-limiting embodiment
example which refers to the drawing in which:
- Figure 1 is a graph showing the population
of Haematobia irritans in a control group, with the time
(weeks) on the X-axis and the arithmetic mean on the
Y-axis; and
- Figure 2 is a graph with the time (weeks)
on the X-axis and the arithmetic mean of the Haematobia
irritans count on the Y-axis.
The followinQ groups were prepared:
Group A: controls: 10 heifers
Group B: 10 heifers which have received ear-
rings containing 2.5% compound A. Each animal receives
one ring.
Group C: 10 heifera which have received ear-
rings containing 10% compound A. Each animal receives
one ring.
Group D: 10 heifers which have received ear-
rings containing 10% compound A. Each animal receives
two ring, one per ear.
The ear-rings are made based on polyvinyl
chloride (PVC), to which a stabilizer (Mark 1528), a
plasticizer (acetyl tributyl citrate) and a pigment
(titanium dioxide) are added. They are manufactured by
CA 02222199 2006-04-21
30754-11
- 13 -
injection-moulding.
Each group of animals is placed in a pasture
of about 100 to 150 acres (40 to 60 hectares).
The infestation with Haematobia irritans is
of natural origin.
The chronology of events is as follows:
the ear-rings are inserted on day 0. The
Haematobia irritans parasites are then counted every 6,
7 or 8 days.
An efficacy of greater than 90% and even
greater than 959s, which can be up to 100%, over long
periods was obtained with all of the formulations
according to the invention. The 2.5% couposition proved
to be greater than 90% effective for 18 weeks. When
inserted in only one ear or in both ears, the 10%
composition also affords greater than 90% protection,
over a much longer period of up to 32 weeks. The use of
two ear-rings per animal affords greater protection,
which is close to 1009s over the entire 32-week period.
The results are reported in Figures 1 and 2.