Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
~ CA 02223044 1997-12-02
P~T~U~ 96/01798
IPEAIUS 1 6 AUG 1996
PROCESS FOR RECOVERING
IRON FROM IRON-RICH MATERIAl,
Technical Back~round
The present invention involves the recovery of metal values from metallurgical waste
materials, particularly those wastes formed in iron and steel making processes.
Background Art
In the production processes of iron and steel, waste materials are formed that contain
oxidized iron and frequently other oxidized metals. These are usually materials in the form
of dust in the gas waste streams. This waste is difficult to process because the dust usually
has a fine particle size, and merely reintroducing it into a reduction furnace to recover the
iron will usually result in it becoming again a part of the waste gas stream. Accordingly,
these fine particle size materials, even though they contain a substantial metal content, have
been essentially worthless.
There are methods of storing and stabilizing the dust into piles near the steel-making
facility, but this option is becoming increasingly unacceptable as environmental regulations
become stricter and as available space becomes more restrictive because land values increase.
The dust can also be recycled and stabilized into ceramic or building materials but not
necessarily on a cost effective basis. But these methods do not exploit the value of the
residual iron and other metals in the waste.
A waste material of common concern is dust from electric arc furnaces, commomy
referred to as EAF dust. Electric arc furnaces typically melt scrap metal through the use of
high voltage electrical current. The scrap metal may come from a variety of sources,
including; discarded railroad rails, cut sheet steel, discarded structural steel, and scrap
automobiles. The scrap metal is added to the electric arc furnaces without separating non-
~MEND~D SHEtl
t CA 02223044 1997-12-02
g~lal ~ss
ferrous metals, such as lead, zinc, and cadmium. During the operation of the electric arc
furnace, these non-ferrous metals are vaporized from the scrap, condensed into a dust from
the waste gas stream and are deposited in a bag house. In addition to these metals, the waste
gas stream deposits a large amount of recoverable iron in the bag house. Accordingly, the
iron and heavy metal, usually in an oxidized form, are combined in an amorphous EAF dust
with particle sizes commonly less than 20 microns. Such EAF dust is now classified as
hazardous waste by the EPA due to the lead and cadmium content. As such, extensive
procedures must be m~int~ined in order to protect the environment from heavy metal
cont~min~tion and meet EPA regulations. All the metals in the EAF dust have value and
can be reclaimed if an efficient way of separation and reduction of the component dust can
be achieved. Additionally, the EAF dust can be rendered non-toxic, if the trace heavy metals
can be removed from the dust.
Several processes have been applied to this problem with dif'fering degrees of success.
While these processes have been successful in removing heavy metals, they have been
inadequate in recovering the iron, and generally leave a fine iron oxide-cont~ining dust of
no value.
The most common approach is called fuming. This process utilizes the differing
boiling points of the heavy metals to obtain their separation. The dust is heated to
temperatures above the boiling points of the metals being separated, causing the metals to
evaporate. The evaporated metals are removed as a dust from the gas and condensed in a
collection device for further processing. The boiling points of these trace metals are
considerably lower than that of iron, which is the largest single component of the dust. After
the lead, zinc, and ca~lmillm are separated, the rem~ining dust consists primarily of iron in
the forrn of iron oxide. Being in a dust form, this material cannot be successfully processed
~ CA 02223044 1997-12-02
?
'. b A'JG 1
into iron and is left as a waste. Another problem with fuming, is that it is energy intensive,
and it also produces a significant amount of its own waste dust.
Another process used to treat EAF dust is electrowinning. This process combines a
leaching and precipitation operation with electrolytic deposition. The EAF dust is first
dissolved in an electrolyte to solubilize the lead, zinc, and ca~mium. The solution is filtered
and then precipitated with a zinc powder t~ capture the lead and cadmium. The resulting
zinc solution is then passed through an electrochemical recovery cell to recover the zinc.
This process recovers zinc quite well, but the leaching process does not dissolve the iron
oxides and zinc ferrite, which remain as waste materials that rnust be dried. The dried
material, once again, is in the form of a fine dust with little or no value.
EAF dust has also been processed by blending with silicate materials, such as silica
sand, clay, or cullet, and heated in a furnace to form a vitrified ceramic product. The
ceramic is useful as an abrasive, and the EAF dust is rendered nonhazardous, but the
valuable metals contained in the dust are not recovered. These metals have been processed
through an expensive refining technique just to be converted into a relatively low value
material in order to render them nonhazardous.
DISCLOSURE OF THE INVENTION
It is, therefore, an object of the invention to provide a method for the treatment of
dusts cont~inin~ iron and heavy metals that recovers both the iron and the heavy metals as
a usable product.
Further objects of the invention will become evident in thLe description below.
In brief summary, the present invention overcomes or substantially alleviates the
above-identified problems of the prior art. A method for forming a solid product, in the
~ CA 02223044 1997-12-02
, J ~ . I J _ J ~ ' J J
form of briquettes, pellets, and/or as other solid objects, is provided. The resulting product
comprises an iron-rich material, e.g. EAF dust, and a carbon source, e.g., coke breeze, coal
fines, and/or revert materials, bound together into solid shapes, such as briquettes, to
substantially prevent degradation into dust and smaller pieces. The briquettes provide a
source of iron in steel and iron-making processes and carbon i'or reduction of the iron.
Furtherrnore, heavy metals in the iron-rich material are also incorporated into the briquettes
and during the iron-reduction process are separated by being vaporized or fumed, thereby
allowing these to be recovered. This fuming method is unique in that the feed material is
first formed into a stable solid through the use of briquetting or extruding a convenient shape
or a like technique, lltili7ing the reaction product of particulate carbon and an organic binder.
The binder reaction product m~int~in~ the formed dust materials until the zinc, lead, and
c~1minm have evaporated, and the iron oxides have been reduced to elemental iron. This
method allows all the materials contained in the EAF dust to be reclaimed in one process.
The fuel for this process can be either waste coke breeze, waste coal fines, electric arc, or
natural gas, depending on which provides cost advantage.
Accordingly, the present process is for recovering iron and heavy metals from
powdered iron-rich materials. These powders, from which it has been previously not
possible to recover the iron values, can now be m~mlf~ctured into shapes that can be utilized
in iron and steel manufacture. Not only is the iron recovered, but also any heavy metals are
also recovered. Prior attempts to place carbon-cont~ining materials, such as coke breeze,
coal fines, and/or revert materials in a solid form, such as briquettes, has been largely
unsuccessful because the product does not adequately bind and is unstable, disintegrating or
retrogressively degrading back into small, fine particles during storage and h~n~ling prior
to use. However, the present invention allows a carbon and irol~ cont~ining material to be
~ CA 02223044 1997-12-02
PCTlllS 4~ ~C'-l 798
IPEA/~ G ~9~6
fonned into a solid shape that is strong and durable enough for h~nl~lling and storage, as well
as sufficient to bind the shapes in an iron-reduction process to inhibit early disintegration of
the shape such that they are carried into the waste gasses as dust.
An embodiment of the invention is a process for manufacturing shapes from fine iron-
rich material, the process may comprise;
(a) mixing the iron-rich material, and a carbon source, to form a iron-rich/carbon
mixture, the powdered material being essentially free of oils and ]n~oisture;
('n) dissolving styrene or acrylonitrile polymer resin in a hygroscopic solvent to
form a dissolved resin or conditioner;
(c) combining the dissolved resin, the iron-rich/carbon mixture, calcium
carbonate, and an alumino-silicate binder;
(d) emulsifying polyvinyl homopolymer in water, adding the emulsion to the
combination of step (c) and substantially homogenizing the resultant; and
(e) compressing the mixture from (d) into shapes.
By "fine iron-rich materials" is meant any powdered or small particle or particulate
material cont:~ining iron, iron oxides and/or other iron compounds. The powdered material
may also cont~in other metals, including heavy metals, in any form such as in metal oxides,
as well as other minerals, particularly those found in ores, waste materials from mineral
extraction, and the like.
A suitable iron-rich material is electric arc furnace dust (EAF dust) that is deposited
from waste gas streams coming from electric arc furnaces used in iron and steel production.
Other suitable iron-rich materials include other by-products from steel production, such as
mill scale, precipitated iron oxide, and dust (so-called sludge), typically collected in the filter
bag houses of oxygen furnaces.
r,D S~ tl
~ CA 02223044 1997-12-02
p,~ " r,;
~ ~ L.;ii ~ c~
The iron-rich material is normally essentially free of moisture, i.e., with a moisture
level at or below 2 wt. %, and is essentially free of non-minera.l substances, such as oils.
This can be accomplished by any suitable cleaning and drying method, preferably, by the
method that is more fully described and illustrated in the examples.
The powdered material is first mixed with a carbon source. At this point, the iron-
rich material and the carbon source material may be optionally reacted with a mineral acid,
such as hydrochloric acid. The carbon source may be any suitable source, such as a
metallurgical grade coke. The carbon source should be fine enough and in a form that allows
formation of the solid shapes, as discussed further below. In addlition, it should not contain
impurities that would illtelrel~ materially with formation of the shape or with the subsequent
iron-reduction process in which the shape is used. The carbon source is typically a fine
powdered material.
In a typical application of the invention, the powdered material and the carbon source
are mixed to form a mixture of about 15 to 35 wt. %, preferably about 25 wt. % of the carbon
source. The mixture is then reacted with hydrochloric acid. The mixture is preferably
reacted with hydrochloric acid, in an amount between about 1 and 4 wt. %, preferably about
2 wt. % acid.
After reaction with hydrochloric acid, the iron-rich~carbon mixture is then
compounded into a mixture with binders for forrnLing into one or more shapes. The reacted
mixture is mixed with calcium carbonate, an alumino-silicate binder, an organic binder, and
a polyvinyl alcohol. That may be accomplished by mixing the reacted mixture with calcium
carbonate and an alumino-silicate material. The calcium carbonate acts as a hardener and
also as a flux for removal of impurities during the reduction to iron phase. The ~IIlmin~-
silicate also functions as a hardener for the shapes, and also as a flux. The alumino-silicate
CA 02223044 1997-12-02
material may be any of such materials used in forming shapes, such as kaolin clay materials,
kaolinite. mixtures of alumina and silica, dolomite iime type clays, and the like.
An organic binder is mixed into the mixture with the calcium carbonate and alumino-
silicate. The binder is the binder described in United States Patent Application No.
08/184,099, filed January 21, 1994, which disclosure is hereby incorporated by reference.
This binder is made by dissolving styrene or acrylonitrile polymer resin in a hygroscopic
solvent, such as methyl-ethyl-ketone.
An emulsion made by emulsifying a polyvinyl polymer in water is added to the
mixture with the styrene polymer binder. The reslllt~nt is then ~ubstantially homogenized.
The polyvinyl polymer may be polyvinyl alcohol or polyvinyl acetate.
The homogenized mixture with the polyvinyl acetate or polyvinyl alcohol is then
formed into solid shapes by any suitable method, such as extrusion, molding, and/or
compression. Typically, the extrusion or molding ples~ul~:s are high, between about 15,000
and 45,000 psi, preferably about 30,000 psi, to produce dense, and fracture and abrasion
resistant product.
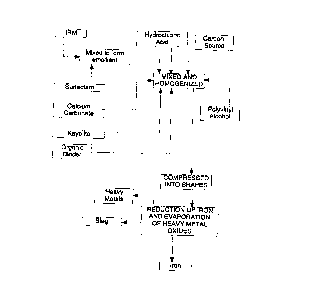
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a flow sheet illustrating an embodiment of the invention.
BEST MODE FOR CARRYING OUT THE IN''~ENTION
Example l
This example illustrates the treatment of powdered iron-rich material (IRM) feedstocks
and clllmin~tes in the production of high-grade iron metal. Referring to Figure 1, IRM is
first cleaned using a surfactant to create an emollient cont,lining the oils and other
, CA 02223044 1997-12-02
PCT,'US 9 ~ 7 9 8
cont~min~nt~ found in the IRM. The IRM is dried in a rotary kiln to vaporize the emollient
and reduce the total moisture content, preferably to below about 2 wt. %, although up to 6
wt. % may be used, depending upon the composition being processed.
The cleaned IRM is then weighted into a mixer along with approximately 25 wt. %
metallurgical grade coke and reacted with hydrochloric acid at about 2 wt. %. The IRM,
coke and hydrochloric acid is then mixed for about 5 minutes.
Aftermixing, aboutS wt.% calciumcarbonateand2.5 wt.~ Kayolite (Al~03+SIO~)
are added to the acid treated IRM and coke and mixed for aboul: 5 minutes. The calcium
carbonate and Kayolite act as hardeners in the IRM mixture and also as fluxes as the material
is reduced to metal.
After mixing, about 3 wt. % of an organic binder material is added to the batch mixer
and allowed to mix for approximately S minutes. The binder is a styrene polymer resin (10
wt.%) dissolved in a hygroscopic solvent, such as methyl-ethyl-ketone. As this binder
contains a hygroscopic solvent, any water generated in the earlier reactions is driven off with
the solvent.
After mixing, about 4 wt.% of a polyvinyl alcohol homopolymer is added to the
mixture and allowed to mix for 10 minntes. The material is then fed into a briquetting press
under high injection pressure or like machine to form an easily handled, hard shape.
The formed briquettes or other solid shapes are then heated to about 250~F to 400~F
to cure. The curing process reduces the moisture content of the briquette to less than about
2 wt.%. Once cured, the briquettes are introduced into an electric arc furnace where
reduction of the oxides takes place. Reduction of iron oxides can take place with minim~l
power penalty due to the fact that the briquette continues to be held together under the slag
layer by the blnder until such time as the reduction reaction takes place between the coke and
~ CA 02223044 1997-12-02
~lf~ ~g6Jn~ 7~8
IPEA/US .~:~ 199~
oxidized iron. The other materials added to the briquette or other solid shape act as fluxes
carrying impuri~ies into the slag layer above the liquid metal bath.
Instead of the styrene polymer, an acrylonitrile polymer may be used. A suitable
homopolymer material is 32-024 homopolymer PVA emulsion, available from National
Starch and Adhesive. The acrylonitrile polymer is preferably retaiined in a prolonged fluid
state by methyl-ethyl-ketone. Acrylonitrile polymer is available from Polymerland.
Technical grade methyl-ethyl-ketone, available from Dice Chemical Co. and Thatcher
Chemical Co., is satisfactory. Ninety percent (90%) by weight methyl-ethyl-ketone and ten
percent (10%) by weight acrylonitrile polymer is suitable, although these amounts can be
varied.
Examples II to V
These examples illustrate the treatment of powdered iron-rich material (IRM)
feedstocks and c~lmin~tes in the production of high-grade iron metal. The general procedure
for each of the examples was first to clean the IRM using a surfactant to create an emollient
cont~ining the oils and other cont~min~nt~ found in the IRM. The IRM is dried in a rotary
kiln to vaporize the emollient and reduce the total moisture content.
The cleaned IRM is then weighted into a mixer along with the particulate carbon
source reacted with hydrochloric acid at about 2 wt. %. The IRM, particulate carbon source
and hydrochloric acid are then mixed for about 5 mimltes.
After mixing, about 5 wt.% calcium carbonate and an alumino-silicate, 2.5 wt.%
Kayolite (Al203 + SIO2), are added to the acid treated IRM and palticulate carbon and mixed
for about 5 minutes.
After mixing, about 3 wt. % of an organic binder material is added to the batch mixer
and allowed to mix for approximately 5 minutes. The binder was an acrylonitrile polymer
SH~t l
~ CA 02223044 1997-12-02
PCTI~S ~ 1 79 8
~P~ 996
and was retained in a prolonged fluid state by methyl-ethyl-ketone as described above.
After mixing, about 4 wt. ~ of a polyvinyl alcohol homopolymer, such as used in
Example I, is added to the mixture and allowed to mix for 10 minutes. The material is then
fed into a briquetting press under high injection pressure or u~ing other machinery to form
an easily handled, hard, solid shape.
The formed briquettes or other solid shapes are then heated to about 250~F to 400~F
to cure. The curing process reduces the moisture content of the briquette to less than about
2 wt. %. Once cured, samples of the briquettes or other solid shapes were introduced into
an electric arc furnace where reduction of the oxides takes place Analyses of the starting
materials, and the iron and slag products resulting from the reduction were made. The
results of the tests are summarized below.
Example n
For this Example, the particulate carbon was coke breeze (10400 BTU), and the IRM
was a mixture of mill scale from a steel mill (Nucor, Plymoth, Utah), and an iron oxide
precipitate from a oxygen furnace (Gulf States, Gadston, Alabama). The analysis of the
starting materials, the briquette produced, and the reduction products (in wt. %) are shown
below in Table A. Of the mass of the briquette introduced into the reduction process,
approximately 88 % went into the iron product, and 21% into the slag (These numbers do not
add exactly to 100% because of inaccuracies in measurement and round-off errors.)
., ,; ~ .: ,.
CA 02223044 1997-12-02 ~ J ~ q ~ -
TABLE A
BASIC IRON TESTS
STARTING MATERIALS FOR REDUCTION
FORMING BRIQUETTE PRODUCTS
NUCOR GULF
DESCRIPTION COKE MILL STATES BRIQ IRON SLAG
BREEZE SCALE PRECIP
SAMPLE NO. 1/8-15-1 2/8-15-13/8-15-1 4/8-15-1 5/8-15-1 6/8-15-1
CARBON 63.30.41 6.59 18.2 3.27 0.82
SULFUR 0.540.03 0.12 0.22 0.12 0.46
IRON 73.4 51.1 45.5 83.6 6.88
MANGANESE 0.54 0.26 0.316 0.131 0.843
PHOSPHORUS 0.01 0.01 0.01 0.005 0.018
SILICON 0.32 0.84 1.15 0.35 12
COPPER 0.206 0.061 0.088 0.194 0.028
NICKEL 0.063 0.029 0.032 0.101 0.01
CHROME 0.063 0.03 0.046 0.094 0.039
MOLY 0.005 0.015 0.005 0.004 0.003
TIN 0.026 0.07 0.017 0.03 0.001
ZINC 0.008 0.426 0.143 0.008 0.001
BORON 0.01 0.01 0.01 0.03 0.01
TITANIUM 0.002 0.022 0.025 0.002 0.167
ARSENIC 0.001 0.001 0.001 0.001 0.001
EJcample lII
For this Example, the particulate carbon was coke breeze (10400 BTU), and the IRM
was a mixture of mill scale from a steel mill, and sludge from the filters from a basic oxygen
furnace (Q-BOP) (both at Geneva, Utah). The analysis of the starting materials, the briquette
produced, and the analyses of the reduction products of five reduction tests of the briquettes
(in wt. %), are shown below in Tables B-l and B-2. For tests 1 to 3 the results are for the
slag accumulated over all three tests. The percent of the mass frorn the briquettes introduced
into the reduction process that resulted in the iron product and the slag are shown in Table
C. (These numbers do not add exactly to 100% because of inaccuracies in measurement and
round-off errors.)
~ CA 02223044 1997-12-02
- ' q 8
~PE~ ' ~ 199F
TABLE B-l
BASIC IRON TESTS
STARTING MATERIALS
FOR FORMING REDUCTION PRODUCTS
BRIQUETTE
DESCRIPTION BRIQ
GENEVA GENEVA TEST 1 TE,ST 2 TEST 3TEST 1-3
MILL SLUDGE IRON IR.ON IRON SLAG
SCALE
SAMPLE/TEST# 11/8-16-212/8-16-2 13/8-16- 14/8-16- 15/8-16-16/8-17- 17/8-17-4
2 2 3 4
CARBON 2.28 19.4 7.35 1.85 2~21 1.61 0.15
SULFUR 0.07 0.28 0.09 0.08 0.06 0.07 0.28
IRON 69.3 41.5 57.5 93.2 96 97 1.22
MANGANESE0.596 0.0830.475 0.3 0.024 0.0810.94
PHOSPHORUS0.01 0.0840.007 0.007 0.008 0.0050.011
SILICON 0.01 0.014 8 0.06 0.01 0.21 0.01
COPPER 0.087 0.01 0.007 0.063 0.009 0.0310.003
NICKEL 0.032 0.0010.008 0.069 0.011 0.0220.003
CHROME 0.042 0.01 0.01 0.89 0.021 0.01190.002
MOLY 0.003 0.01 0.01 0.014 0.012 0.1570.01
TIN 0.003 0.0340.007 0.004 0.001 0.0010.29
ZINC 0.015 0.35 0.036 0.013 0.005 0.0070.005
BORON 0.01 0.25 0.26 0.01 0.01 0.01 1.45
TITANIUM 0.003 0.0340.007 0.004 0.001 0.0010.29
ARSENIC 0.003 0.0030.003 0.03 0.003 0.0030.003
ALUMINUM 18.4
MAGNESIUM 6.64
LEAD 3 77
CADMIUM 0.01
CA 02223044 1997-12-02 PCT/US 9 b / ~1 7 9 8
i PE~ o A~G ~996
TABLE B-2
BASIC IRON TESTS
STARTING
MATERIALS REDUCTION PRODUCTS
FOR FORMING
BRIQUETTE
DESCRIPTION BRIQ
GENEVA GENEVA TEST 4TEST 4 TEST 5 TEST 5
MILL SLUDGE IRON SLAG IRON SLAG
SCALE
SAMPLE/TEST# 11/8-16-212/8-16-2 13/8-16-218/8-17-5 19/8-17-520/8-21-6 21/8-21-6
CARBON 2.28 19.4 7.35 2.34 0.16 2.39 0.26
SULFUR 0.07 0.28 0.09 0.06 0.37 0.07 0.33
IRON 69.3 41.5 57.5 86.1 1.5 89.6 0.75
MANGANESE0.596 0.0830.475 0.446 1.2 0.175 0.678
PHOSPHORUS 0.010.084 0.0070.09 0.017 0.01 0.003
SILICON 0.01 0.014 8 0.05 0.01 0.193 23.5
COPPER 0.087 0.010.007 0.040.002 0.036 0.014
NICKEL 0.032 0.0010.008 0.0430.003 0.027 0.001
CHROME 0.042 0.01 0.01 0.064().01 0.029 0.001
MOLY 0.003 0.01 0.01 0.0030.003 0.01 0.01
TIN 0.003 0.0340.007 0.0580.251 0.03 0.01
ZINC 0.015 0.350.036 0.0140.00' 0.006 0.005
BORON 0.01 0.25 0.26 0.01 1.56 0.01 1.53
TITANIUM0.003 0.0340.007 0.0580.251 0.046 0.357
ARSENIC 0.003 0.0030.003 0.0030.003 0.005 0.0058
TABLE C
REDUCTION PRODUCTS
REDUCTION IRON SLAG
TEST
96.594
4.667
2 98.385
3 99.2199
4 89.379 5.342
92.637 27.4548
.M~ iG ~G SH~ I
r CA 02223044 1997-12-02
PlJT/UJ 9~ ~01 .79~J
~rL~~ A~ . J3~
Example IV
For this Example, the particulate carbon was coke breeze (10400 BTU), and the ~RM
was a mixture of iron ore from the Geneva mine near Cedar City, Utah, and sludge from the
filters from a basic oxygen furnace (Q-BOP) (Geneva Steel, Geneva, U~ah). The analysis
of the starting materials, the briquette produced, and the analyses of the reduction products
of five reduction tests of the briquettes (in wt. %), are shown below in Table D. Of the mass
from the briquettes introduced into the reduction process about 88.9% ended up in the iron
product and 22.1% ended up in the slag product for Test 1. (These numbers do not add to
100% exactly because of inaccuracies in measurement and round-off errors.) This data was
not obtained for Test 2.
TABLE D
BASIC IRON TESTS
STARTING MATERIALS
FOR l:ORMING BRIQUETTE REDUCTION PRODUCTS
BRIQ
RED SEA CEDAR TEST 1 TEST 1 TEST 2TEST 2
DESCRIPTION SLUDGE ORE IRON SLAG IRON SLAG
SAMPLE#24/8-21-8 25/8-21-826/8-21-822/8-21-723/8-21-7 27/8-21-828/8-21-8
CARBON0.82 0.24 21.6 2.29 0.86 2.69 10.7
SULFUR0.1 0.06 0.19 0.05 0.47 0.01 1.5
IRON 46.1 46.4 33.9 86 2.25 40.5 2.93
MANGANESE 0.3190.058 0.113 0.2 0.8970.084 0.013
PHOSPHORUS 0.012 0.06 0.033 0.008 0.013 0.03 0.01
SILICON1.96 3.11 6.5 0.229 15.7 0.515 3.6
COPPER0.052 0.0010.015 0.024 0.002 0.0170.006
NICKEL0.011 0.0280.014 0.025 0.001 0.0250.004
CHROME0.03 0.0060.001 0.033 0.01 0.03 0.005
MOLY 0.01 0.01 0.01 0.01 '0.01 0.01 0.01
TIN 0.02 0.02 0.01 0.03 0.01 0.01 0.01
ZINC0.386 0.0120.142 0.006 0.005 0.0050.005
BORON0.01 0.01 0.01 0.01 1.67 0.05 0.06
TITANIUM0.02 0.0050.018 0.033 0.228 0.0460.021
ARSENIC0.005 0.0050.005 0.005 ~.~~S 0.0050.005
14
., ':, ~ ~ ! i
~ CA 02223044 1997-12-02
IPEA/US - ' 1996-
E~cample V
For this Example, the particulate carbon was coke breeze ( 10400 BTU), and the ~RM
was ferric oxide dust derived as a byproduct form photographic film production. The
analysis of different batches starting materials, and a c~lm~ rive analysis of the iron
reduction products (in wt. %), are shown below in Table E.
TABLE E
BASIC IRON TESTS
STARTING IRON MATERIALS FOR FORMING BR[QUETTE IRON
PRODUCT
DESCRIPTION KMFE203KMFE203 KMFE203 K:MFE203 KMFE203
DUST DUST DUST DUST
TEST# 29.00 30.00 29.00 30.00 31.00
CARBON 0.030 0 030 0.030 0.030 3.020
SULFUR 0.020 0.025 0.020 0.025 0.022
IRON 60.500 63.600 60.500 63.600 88.200
MANGANESE 2.270 2.280 2.270 2.280 0.2~)0
PHOSPHORUS 0.003 0.003 0.003 0.003 0.032
SILICON0.570 0.610 0.570 0.610 0.330
COPPER 0.001 0.001 0.001 0.001 0.514
NICKEL 0.007 0.006 0.007 0.006 0.114
CHROME 0.048 0.047 0.048 0.047 0.275
MOLYBDENUM 0.010 0.010 0.010 0.010 0.090
TIN 0.010 0.020 0.010 0.020 0.030
ZINC 0.067 0.068 0.067 0.068 0.013
TITANIUM0.027 0.030 0.027 0.030 0.015
Example 1i7
For this Example, the particulate carbon were coal fines, and the IRM was the same
as in Example III. The analysis of the iron and slag reduction products (in wt. %), are shown
below in Table F.
CA 02223044 1997-12-02
PCT~I ~.S C~ l 7 9 8
IrEA
TABLE F
BASIC IRON TESTS
SLAG IRON
DESCRIPTION PRODUCT PRODUCT
FROM COAL FROM COAL
SAMPLE/TEST# 33/9-21-37 34/9-21-37
CARBON 3.292.74
SULFUR 0.021.05
IRON 14.0389~10
MANGANESE 0.220.55
PHOSPHORUS 0.02 0.01
SILICON 7.381.65
COPPER 0.000.45
NICKEL 0.010.06
CHROME 0.030.12
MOLYBDENUM 0.01 0.01
TIN 0.010.04
ZINC 0.010.01
TITANIUM 0.070.05
Theory
It is believed that the present invention polymerizes the carbon particles contained in
the carbon source into a new long chain polymer compound, yet unidentified, which provides
structurally superior strength of the shapes. It is known that oxides of carbon will hydrolyze
in water. This reaction leaves free carboxyl ions present in the compound.
Introduction of the doped methyl-ethyl-ketone is believed to allow for attachment of
the styrene polymer to the free carbon ions by exchange of the polymer for water which is
absorbed into the solvent.
In the next phase, polyvinyl acetate is introduced. Again the presence of the methyl-
ethyl-ketone acts as a catalyst to remove and allow the acrylonitrile or styrene to react to the
polyvinyl dcetate.
The resulting compressed shapes, such as briquettes, pellets, and/or extruded solid
16
~ CA 02223044 1997-12-02
~' 4~ J ~1 ~98
L Pl~
pieces are structurally stable and do not retrogress into fine particles during storage and
h~n-ll ing.
While this invention has been described with reference to certain specific
embodiments and examples, it will be recognized by those skilled in the art that many
variations are possible without departing from the scope and spirit of this invention, and that
the invention, as described by the claims, is intended to cover all changes and modifications
of the invention which do not depart from the spirit of the invention.
What is claimed and desired to be secured by Letters Patent is:
~jFNC'~D SH~-t~