Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02223~l9 l997-l2-03
W O 9C/1~379 PCT/US9~'~79~7
DESCRIPTION
Methods for Evaluation o~ Antimicrobial Targets
Backqround of the Invention
This invention relates to the fields of antimicrobial
therapy and methods for identifying and evaluating targets
for antimicrobial therapy.
The process by which bacterial pathogens and other
microorganisms cause disease involves the overt
replication of the microbe within the host, and/or the
production of both cellular and extracellular factors
which enhance the pathogenicity of the microbe. To be an
effective pathogen the invading microbe must establish
itself in the host, creating an environment in which it
can avoid destruction by the host's immune system. To
establish itself, the microbe requires a variety of
molecules which provide functions such as attachm,ent to
host tissue, penetration of anatomic barriers, disruption
or avoidance of humoral factors, and avoidance or
inactivation of phagocytic cells. Such molecules include
colonization factors, such as adhesins, and certain
proteases, endotoxins, and exotoxins. These molecules
help to secure the microbe to favorable host tissues and
to alter the local environment to allow initiation and
maintenance of infection. This process is often very
specific for a host/pathogen pair, and is essential for
the subsequent events in the pathogenic process.
Once established, the pathogen may produce a variety
of virulence factors or toxins. These microbial products
are usually genetically regulated and the microbe often
expresses these products only while within the host.
These virulence factors include a variety of toxins and
other exoproducts important for the creation of and
maintenance of the ecological niche within which the
microbe resides. For example, for the enteric pathogen,
Shigella, clinical isolates produce pili to adhere to
CA 02223~19 1997-12-03
W O 9~'~C,7Y PCT~US~'~v7~37
mucosal surfaces and toxins which enhance invasion of
mucosal cells. In Staphylococcus, a large number of
pathogene6is-related exoproducts have been identified,
including Staphylococcal lysozyme, exfoliative toxins A
and B, pyrogenic exotoxin, coagulase, hemolysins a-d, and
collagenase. In other pathogenic species other virulence
factors have been identified, but numerous virulence
factors remain to have their functions identified or fully
appreciated. All important pathogens have similar capa-
bilities. These capabilities vary among species anddepend on the environment in which the pathogen is capable
of prospering, as well as the genetic capabilities of the
microbes.
As was suggested above, virulence factors include
many molecules besides toxins and should be regarded as
including any microbial products which enhance the
capability of the microbe to create disease. So, for
example, capsular polysaccharides produced by the microbe
act as anti-phagocytic defenses to prevent the hosts'
immune system from eliminating the microbe. There are
additional virulence factors which enhance the capability
of the invading microbe by avoiding or disrupting humoral
(antibody-mediated) immunity. There are also virulence
factors which give the invading microbe properties which
overcome the hosts' anatomic barriers. All such microbial
properties which help to create or maintain a microbe~s
ability to cause disease are possible targets for novel
antimicrobial agents.
The bacteria are not alone in the microbial world in
their ability to cause disease. In addition to numerous
viruses, a number of lower eukaryotes, fungi and yeast are
being recognized as increasingly important pathogens. In
such pathogenic fungi and yeast, like in bacteria, the
organisms possess specific features which enhance the
ability to cause disease. Parasites also have a wide
variety of described and yet to be described virulence
factors.
CA 02223~19 1997-12-03
W O ~ 379 PCT/US~ 7~37
None of the above material is admitted to be prior
art to the pending claims, but is provided only to aid the
understanding of the reader.
Summarv of the Invention
This invention provides methods for evaluating
specific microbial genes and the encoded products as tar-
gets for antimicrobial therapy prior to identification of
a specific inhibitor(s) or enhancer(s) of the gene or gene
product. These methods are useful for evaluating such
genes and gene products even when information about the
function of the encoded products is incomplete. For
example, such evaluations can be performed both when the
reaction pathways involving a gene product are known to
some extent, but before the involvement of that product in
an infection process is fully appreciated, and when the
specific reaction pathways in which the gene product is
involved are known. Since, the methods utilize expressed
coding sequences, evaluations can be performed even before
the specific gene product has been characterized. Thus,
these methods provide an efficient approach t:o the
identification and evaluation of novel therapeutic
targets.
In general, these methods for eva]uating
antimicrobial targets are based on artificial control of
the level of activity of a gene product. As described
herein, such control may be achieved in different ways,
but generally involve artificially changing the
environment of a microbe (such as by contacting the
microbe with a small molecule) which causes a change in
the level of activity (or function) of a gene product.
The change in activity can be accomplished in a variety of
ways, among which are altering the transcription rate,
altering mRNA processing (for cells and genes for which
this is necessary), transport, or degradation, a]tering
the translation rate, and altering the stability of the
gene product. (Alberts et al., MOLECU~AR BIOLOGY OF THE
CA 02223~19 1997-12-03
W O 9~ S7Y PCT~US~ 7937
CELL, 3rd ed., p.403.) Any of these processes can be used
to alter the level of activity of the product of a gene,
and so could be utilized in the methods of this invention.
Along with the identification of novel targets, these
methods allow assessment of time-dependent inhibition of
a target gene product, i.e., these methods allow
assessment of the consequences of inhibiting the target
gene product at various times after infection is initiated
and/or of various dosing regimes (e.g., different
schedules of multiple dosing). Such information has
clinical relevance for the possible therapeutic
administration of any antimicrobial agent which affects
that target (such as the possible efficacy), and are
important properties to characterize for each potential
target. The differing temporal importance of various gene
products generally is primarily due to infection-related
changes in the infection host environment (includes both
global host environment and the localized site of
infection) which result from the infection.
The use of a variety of infection models also allows
evaluation of the effects of different infection sites in
relation to varying inhibition schedules. The site of
infection effects are also generally related to the
infection-related changes in the host environment and also
have clinical relevance. Such determination of infection
site differences can also be combined with temporal
evaluations as described above.
In addition, by use of various animal (or other whole
organism) or cell-based infection models, the expected
therapeutic benefit of an inhibitor of the gene, gene
product, or reaction pathway, which may later be found,
can be estimated prior to any extensive search for such an
inhibitor. This includes an assessment of the tissue-
specific effects of the inhibition of a specific target by
comparative evaluation of the effects observed in the
different infection models. In addition, certain models
can provide information on the tissue-specificity of the
:
CA 02223~19 1997-12-03
W O 9~ 9 PCT1~59''07937
infecting microbe, as well as of the pattern of clearance
of the microbe from the infected host.
Still further, these methods provide a standard for
the comparison o~ the effects of the in vivo
administration of a compound which inhibits a specific
target to affect a microbial infection. These methods
allow determination of the effects of complete loss of
function of the product of a test gene at one or at
various times after an infection i8 initiated. The
effects of complete loss of function can then be compared
with the effects of administration of an inhibitor at
similar time points after an inhibitor has been ~ound.
The capability to completely inhibit the activity of a
gene product therefore provides a baseline for evaluating
the effects of an inhibitor.
Thus, in a first aspect, this invention provides a
method for evaluating a putative pathogenesis gene or
essential gene as a target for antimicrobial treatment.
This method involves infecting a mAmm~l with a microbe
containing an artificially-created genetic alteration,
where the genetic alteration causes a change in the level
of activity of a product of the coding sequence of that
putative pathogenesis gene or essential gene in the
microbe, in response to an artificial change in the
environment of the microbe. When the environment is
changed in that way, the level of activity of a product of
the coding sequence is changed, and the method involves
determining whether the state of the infection or the
physiological condition of the mAmmAl is altered in
response to the change in the level of activity of the
product of the coding sequence. The putative pathogenesis
gene or essential gene is a target if the state of the
infection or the physiological condition of the mAmmAl is
altered in response to the change in the level of activity
of a product of the coding sequence. This method includes
the use of microbes with more than one test gene. In one
arrangement, two or more coding sequences could be linked
CA 02223519 1997-12-03
W O 96/40979 PCT~US~G/07937
together which are naturally linked (such as an operon),
or two or more coding sequences could be transcriptionally
linked by artificial manipulations known. However, the
coding sequences can also be separate. The techniques for
manipulating coding sequences and other DNA or RNA
sequences are well-known in the relevant scientific
communities.
In preferred embodiments, the artificial change of
environment is achieved by contacting the microbe with a
switching compound. As is described herein, such a
switching compound is preferably a small molecule with
well-characterized pharmacodynamics in the infection host.
An understanding of the pharmacodynamics of the switching
compound enables the selection of a switching compound
appropriate for a specific pathogenic microbe or infection
model, as well as modeling the therapeutic consequences of
the use of an inhibitor of a specific activity which has
similar pharmacodynamics (including tissue distribution
and clearing characteristics). In general, the choice of
a switching compound depends on a number of factors,
including, e.g., sufficient distribution of the compound
to the infected tissue and sufficiently long half-life of
the compound in the infected tissue to achieve effective
control of the level of activity of the test gene.
However, the choice of a switching compound and the choice
of switch design are related; selection of one of these
will often limit the possible selections of the other. It
should be clear that a switching compound as described
herein, can, in various switch designs, be any of a
variety of biologically active compounds. Some such
compounds can be referred to using other biological terms,
such as "modulators", "enhancers", "inducers",
"mediators", and "regulators." Therefore, this invention
is not limited by the specific control molecule used, nor
by the specific switch design chosen to control the level
of activity of a product of a test gene.
CA 02223~19 1997-12-03
W O 9~ 31Y PCTfU~9G~7~37
Also in certain embodiments, the methods use a
microbe which has more than one artificially-created
genetic alteration, each of which enables a change in the
level of activity of a product of a test gene. At least
two of the genetic alterations enable changes in the level
of activity of the test gene in response to different
switching compounds. One way of constructing such a
system is to have expression of a test gene blocked by
binding of a repressor molecule. Expression of the
repressor molecule can then be turned on by the p:resence
of any of several switching compounds (which then stop
expression of the test gene). Such a microbe thus allows
convenient comparison of the effects of altering the level
of activity of the test gene by the use of compounds with
a range of pharmacodynamic properties. (See Fig. 8 for
schematic depiction of an example using two di:Eferent
switching compounds.)
Similarly, in certain embodiments, the method uses a
microbe which contains more than one artificially-created
genetic alteration, and more than one test gene. The
levels of activity of at least two of the test genes can
be changed in response to different switching compounds.
Such microbes allow the convenient evaluation and
comparison of the effects of changing the levels of
activity of different gene products in the same microbial
and infection environments. Such microbes can also be
used to evaluate the effects of more than one test gene
concurrently.
The term, "artificially-created genetic alteration",
refers to a change in the DNA of an organism through the
actions of a person, but does not refer to changes
occurring through natural processes of genetic exchange,
such as conjugation. Such artificially-created genetic
alterations can be of many different types. Some of those
types include single nucleotide changes, deletion of a DNA
sequence(s), insertion of a DNA sequence(s), or
combinations of the preceding changes. Further, the
.
CA 02223~19 1997-12-03
W O ~'IC~/~ PCTrU5~ 79~7
changes may be to the DNA of the chromosome(s) or to DNA
which is extra-chromosomal. Also, the changes can be
located in coding sequences or in other sequences such as
regulatory sequences, including binding sites for
components related to transcription or translation. Such
artificially-created genetic alterations can be
transmitted through generations, and each transmission
results in a new organism. Such new organisms are also
said to have artificially-created genetic alterations.
As used herein, the term "extra-chromosomal" refers
to DNA which is part of a DNA molecule which can be
replicated within a cell, but which is not part of the
chromosome of the cell. Examples of such non-chromosomal,
replicatable DNA molecules include a large number of
plasmids, but can also include other categories of DNA
molecules, e.g., cosmids and yeast artificial chromosomes
(YACs). Since such molecules can be replicated in the
cell, copies can be passed to succeeding generations of
the cell, maintaining the presence of the extra-
chromosomal DNA.
The phrase, "level of activity of a product of thecoding sequence", refers to the level of physiologically
relevant biological function of the product of the coding
sequence being tested. For example, for an enzyme, this
refers to the level of activity of all of the molecules of
that type present. The level of activity of a product can
be altered in a variety of ways, including altering the
rate of transcription to form RNA, altering the rate of
translation of a polypeptide product from a mRNA, altering
the transport of a product to a site of action, and
altering the stability of the product to change the active
lifetime of the product. Any of these methods can be used
to alter the level of activity of the product of a coding
sequence. In the context of these methods, a "change" in
the level of activity of the product of a coding sequence
is determined by comparison with the level of the activity
in the absence of the artificial change in the
r
CA 02223~19 1997-12-03
W O 9~S'~C,/~ PCTAU~ 7~37
environment. That comparison level o~ activity may be the
same or different than the level of the same activ:ity in
a corresponding wild-type organism.
The term, "artificial change in the environment~,
refers to a change in the environment of a microbe which
is directly caused by the actions of a person. In
particular embodiments, such changes specifically include
changing the concentration of a small molecule (such as by
adding a quantity of that compound) in the vicinity of a
lo microbe. However, the term can also refer to other
changes such as exposure to light (which may be of a
specific wavelength), contact with a specific metal
ion(s), or a change in temperature.
In a related aspect, this invention provides a method
for evaluating a speci~ic putative pathogenesis gene or
essential gene, or the gene product of such a gene, or the
reaction pathway in which the gene product is an important
element, as a target for antimicrobial treatment. In this
method, recombinant microbes which contain one or more DNA
constructs are used to infect a mammal. At least one of
the DNA constructs contains a coding region of a put:ative
pathogenesis gene or essential gene, such that the
expression of that gene can be controlled by contacting
the microbes with a switching compound. The control, i.e.
the ability to switch, or change, the expression state,
mimics the effect of an antimicrobial agent, for example
the effect of an inhibitor of the product of the switched
coding region. After switching the expression state of
the putative pathogenesis or essential gene, the method
involves determining whether the state of the infection or
the physiological condition of the ~n;m~l was altered in
response to the switch of the expression state of the
gene. The gene is a target if the state of the infection
or the physiological condition of the animal is altered in
response to the switch of the expression state of the
gene.
CA 02223~19 1997-12-03
W O 3~/10379 PCTrUv9~7937
Thus, the in vivo effects (meaning effects occurring
with the microbe in the infection host environment) which
result from changes in the function of the infecting
microbe due to the administration of such an inhibitor can
be determined prior to the discovery of any such inhibi-
tor. This information provides a measure of the potential
effectiveness of such an inhibitor in treating microbial
infections, which provides valuable guidance on whether to
pursue discovery experiments searching for such an
inhibitor. One useful and measure is the "cidalness" of
the test gene, i.e., the degree to which loss of the
function of the test gene kills the infecting microbe.
In addition to providing an evaluation of the gene
and its corresponding product and reaction pathways as
potential antimicrobial targets, this method provides
additional information on the appropriateness of the
pathogenesis gene or essential gene as a practical
antimicrobial target. Such information is provided by
following the initial evaluation of whether the microbial
infection or the health of the infected animal is altered
in response to the switch of the expression state of the
pathogenesis gene or essential gene, with an evaluation of
the extent, nature, and conse~uences of the alteration of
the infection or the health of the host.
The term "method for evaluating" means a method for
determining whether a property or characteristic or effect
is present. This may, but does not necessarily, include
a measure of the size, level, or intensity of such
characteristic property or effect.
A "pathogenesis gene" is a gene whose function is
critical at least at some point in the pathogenesis
process, e . g., in the establishment, maintenance, in vivo
survival, or progression of an infection, or in the
maintenance of a latent or quiescent state of an infection
in an organism, e . g ., a mammal or other animal or a plant.
Therefore, as examples, this term includes genes for
products which allow a microbe to adhere, colonize, and
CA 02223~19 1997-12-03
W O 96/40979 PCT/U~ 79~7
survive in the host environment. These include genes for
products such as adhesion factors, which allow the microbe
to attach to a surface of the host organism, as well as
genes for products which alter the microbial environment
at the infection site and for products which reduce the
ability of the host's immune system to eliminate the
infection. In the context of this method for evaluating
a gene as a target, this implies evaluation not only of
the gene itself, but of the transcribed RNA, the
expression product, and of a reaction pathway or pathways
in which that expression is an important part.
Consequently, the term does not imply that an inhibitor
necessarily acts at the level of the DNA or transcription
process; in many cases an inhibitor would be expected to
act at the level of a reaction directly involving the
expression product of the gene (e.g., inhibition of an
enzyme).
A "putative pathogenesis gene" is a gene which is
being evaluated as a target for antimicrobial therapy.
This does not imply that it is known that the gene is a
pathogenesis gene as described above. For use in this
method, a putative pathogenesis gene has preferably, but
not necessarily, been chosen on the basis of some prior
information about the gene or a relationship of the gene
with other genes believed to be pathogenesis genes.
Examples of such other types of information are provided
below in the Description of the Preferred Embodiments.
The term "essential gene" refers to a gene whose
function is essential to the effective growth of the
microbe in a particular growth condition(s), e.g., in in
vi tro culture in a defined medium. For identificat:ion as
a possible antimicrobial target, a gene identified as
essential in vi tro is also essential in vivo, that is, in
an infection, such as of a m~mm~l, and is preferably a
gene whose function is essential to survival of the
microbe in vivo. Similar to the description of
pathogenesis genes above, the term "essential gene'~ refers
CA 02223~19 1997-12-03
WO ~ 7Y PCTrUS9G~'~7~37
not only to the DNA coding region, but also to the RNA
transcript and the expression product. It also refers to
the reaction pathways of which the expression product is
an important part. Stating that the product is an
important part of a reaction pathway means that the
absence or essentially complete inactivation of that
product substantially changes the level of a functionally
relevant activity in the microbe. This implies that there
are not alternative products or pathways to return the
relevant activity to a functionally equivalent level.
A "putative essential gene" is a gene which is being
evaluated in this method in a manner suitable for
essential genes. These genes may, for example, be
selected for testing on the basis of prior information
about their essential status, or on the basis of other
information, e.g., homology information for the DNA coding
sequence with known essential genes from other related
microbes. Prior information about the status of a gene as
essential can, for example, be provided by the isolation
of temperature sensitive mutants, mutated in a gene
corresponding to the test gene.
In the context of the DNA constructs of the claims of
this invention, the term "gene" refers to a DNA sequence
which is the same as all or a portion of the coding
sequence of a gene from a microbial genome. However, same
does not mean identical. Thus, the sequences may differ
by changing a small percentage of bases, by deleting a
small percentage of bases, by adding a small percentage of
bases, or by a combination of such differences. Such
differences are preferably less than 5 or 10~ of the full
sequence, but in some cases may be higher, e.g., 20~ or
30~. It should be recognized that the biological activity
of interest may depend on the presence of only a portion
of the natural gene product, so the product expressed by
the gene on the DNA construct should have essentially the
same biological function as the product of the native
gene. In addition, the biological activity for a fixed
j=
CA 02223~19 1997-12-03
W O ~'~0~i~ PCT~US~ 79~7
concentration of gene product i8 preferably approximately
the same for the product of the DNA construct as i-or the
product of the native gene. However, if the natural gene
product possesses multiple biological functions, the
product of the test gene need not possess all of those
activities.
As was suggested above, evaluating a gene as a target
means determining whether an alteration of the expression
of the gene alters the status or course of the microbial
infection, or the health of the infected organism (e.g.,
a m~mm~l ) . Therefore, altering the expression of the gene
can mimic the action of antimicrobial agents which may act
at the level of the DNA coding sequence itself, but this
is not essential for identifying the gene as a target. It
can equally mimic antimicrobial agents which act at the
level of the RNA transcript or directly on the expression
product, or which interfere in some manner with the
reaction pathway of which the expression product of the
gene is an important part. Therefore, in this method, a
gene is a "target" if an alteration in the expression of
that gene results in a change in the status or course of
the microbial infection, or in the health of the infected
animal.
The term "mammal" has it usual biological meaning as
referring to warm-blooded animals which nurse their young.
This includes, e . g., mice, rats, rabbits, dogs, cats,
swine, cattle, and humans.
The term "antimicrobial treatment", as used herein,
refers to the administration of a compound to interfere
with the growth, viability or effects of the presence of
a population of a microbe. To illustrate, a compound
administered as an antimicrobial treatment may kill the
microbe, or, as another possibility, the compound may
merely enhance the capability of an infected host animal
to eliminate the microbial infection.
The term "infection" refers to the presence of a
population of a microbe in an animal where the presence of
CA 02223~19 1997-12-03
WO 9~/4C37Y PCT/US9CI'~,7Y,37
14
that population is to some degree damaging to the host
animal. This effect may be due, for example, to the
presence of excess numbers of the microbe, or to the
presence of a highly virulent strain or sub-population of
a strain of the microbe, or may be due to the presence of
a population of a microbe which is not normally present in
the animal or which is present in an abnormal location in
the ~nlm~l.
The term "microbe" includes the usual biological
meaning. Therefore, the term includes, e.g., bacteria,
protozoa, fungi, and yeast, as well as viruses. A
specific example of a bacterium which is described and
utilized in the description of the preferred embodiment
and the examples is Staphylococcus aureus. It should be
recognized that species of many microbes, such as
bacteria, can exist as a variety of strains which may have
genetic differences which are significant for
pathogenesis. The term "recombinant microbe" similarly
has its usual molecular biology meaning. The term refers
to a microbe into which has been inserted, through the
actions of a person, a DNA sequence or construct which was
not previously found in that microbe, or which has been
inserted at a different location within the cell or
chromosome of that microbe. Such a term does not include
natural genetic exchange, such as conjugation between
naturally occurring organisms. Thus, for example, a
recombinant bacterium could have a DNA sequence inserted
which was obtained from a different bacterial species.
Likewise, a recombinant bacterium may contain an inserted
DNA sequence which is an altered form of a sequence
normally found in that bacterium. Another example of a
possible insert in a recombinant bacterium is the
insertion of a DNA sequence which is normally found in the
bacterium, but which is in a construct different than the
DNA structure in which the sequence was previously found
in the bacterium. An example could be a sequence on a
plasmid, when the sequence was previously chromosomally
CA 02223~19 1997-12-03
W O ~ PCT/USg~7937
located. In general then, a recombinant microbe is a
microbe into which a DNA sequence has been inserted by
artificial genetic manipulations. For most purposes it is
preferable that the inserted DNA sequence should be a
stable insertion, meaning that the secluence should be
replicated and transmitted to progeny microbes as growth
occurs.
In the context of recombinant microbes, a "DNA
construct" describes a DNA chain which contains a segment
which has been removed from its normal microbial
environment. Thus, for example, a DNA construct could
consist of a coding sequence with its normally associated
regulatory secluences, which has been isolated from its
normal microbial setting, but a DNA construct can also
include a DNA coding region which has been inserted in a
microbe in a plasmid in which it was not previously found,
or a DNA regulatory region which has been inserted into a
microbial chromosome. A variety of other such DNA
constructs are well known to, and used by, those skilled
in the art.
In the context of the coding region of a gene,
"expression state" indicates the status of the production
of the encoded product of that coding secluence. Thus, for
most genes, the expression state refers to the status of
the translation of an RNA transcript to form a polypeptide
product, but can also refer to the status o~ the
transcriptional product when that transcriptional product
has the relevant biological activity. An example of such
an active transcriptional product is RNA III, which is
related to the agr locus of Staphylococcus aureus . At the
extremes, "expression state" indicates whether detectable
gene product is being produced or not. If detectable gene
product is being produced, the expression state is "on",
if not, the expression state is "off". In addition,
however, expression state can refer to the level of
production of a gene product. Therefore, it can refer to
CA 02223~l9 l997-l2-03
W O 9~!~C37Y PCT/U~3~ 7937
16
whether the product is being produced at a low level
("off") or a high level ("on").
For a related term in the context of the expression
state of a gene, the term "switched" means that the level
of production of the gene product of the coding sequence
has been detectably altered, i . e., the expression level
has been changed. Thus, for example, the expression state
of a coding sequence has been switched if the level of
expression has been changed from a low level to a high
level, where, in some instances, the low level may be
undetectable and may therefore be referred to as no
production. Similarly, a change in the expression level
may be in the opposite direction, from a high level of
production to a low level. As indicated above, a low
level of production may be undetectable. As suggested
above, the expression state of a gene may be switched by
alteration at the transcriptional level or the
translational level. As described above, the level of
activity of the product of a gene can be changed in other
ways, e.g., by altering the stability of the product.
Therefore, more generally, "switched" or "changed" means
that the level of activity of a product of a coding
sequence of a putative pathogenesis gene or essential gene
is either higher or lower than before being switched.
Constructs and methods which allow switching or changing
in vivo are said herein to comprise an " in vivo switch".
The term "contacting", as used herein, indicates that
a concentration of a compound or other material ( e . g., an
ion such as mercury or iron ions) has been placed in the
immediate cellular environment of the microbe. Such a
compound may be, but is not necessarily, then taken up
into the interior of the microbial cell. Therefore, the
term "contacting" merely means that the exterior of the
microbial cell has been exposed to the compound. For
viruses, the term may mean that the compound has
penetrated to the interior of the host cell, or merely
CA 02223~19 1997-12-03
WO 9G/~C,/~ PCT/U~9G/07~7
mean that the exterior of the host cell has been exposed
to the compound.
In the context of the methods of this invention, a
~switching compound" is one which alters (switches) the
level of function of a product of a coding sequence, such
as by altering the amount of functional expressed product
present. In certain preferred embodiments the switching
compound alters the expression level of a coding sequence
of a test gene when the cell or the expression system
containing that coding sequence is contacted with that
switching compound. A well known example of such a
compound which operates in a number of bacterial species
is tetracycline. In certain bacteria, exposure to a low
level (sub-growth inhibitory level) of tetracycline
induces a much-elevated level of expression of a gene from
a resistance-related promoter. A switching compound may
cause an increase or a decrease in the level of activity
of functional expressed product, e.g., it may cause an
increase in the expression level of a coding sequence or
it may cause a decrease in the expression leve] of a
coding sequence.
It is pre~erable that a switching compound be a small
molecule which has well-characterized pharmacol.ogical
properties. This is useful for several reasons. One such
reason is that different compounds will distribute
differently, and have dif~erent tissue clearance rates.
This means that the effective concentration of a switching
compound at different specific infection sites can vary.
(Or conversely, that the ef~ective concentration of
different switching compounds at a specific infection site
can vary even if the average concentration of the
different compounds in the bodies of the infection hosts
is the same.) This further means that the temporal
behavior of various switching compounds will differ, with
accompanying differences in the control of the level of
activity of a gene. Therefore, a switching compound can
be selected which is appropriate for evaluating a specific
CA 02223~19 1997-12-03
W O 9614~31~ PCT/U',~ 37
test gene in a specific infection model based on suitable
pharmacodynamic properties. Also, selection of a
switching compound with specific known temporal and
distributional characteristics in vivo, for use in
evaluating a test gene, provides additional information on
the likely therapeutic effects of inhibiting the test gene
with an inhibitor which has similar characteristics, in
comparison with the use of a switching compound (or other
method of altering the level of activity of the product of
a test gene) with unknown pharmacodynamics. Such
compounds are known, for example, among the inactive (or
low activity) analogs of known antibiotics. For these
compounds, the pharmacodynamics of the class of compounds
has been previously investigated, but the inactive analog
has the advantage that it does not have significant
antimicrobial activity to complicate the evaluation of
potential targets (e.g., the ~-lactam, CBAP). Similarly,
antibiotics to which the infecting microbe is resistant
can be used to evaluate test genes unrelated to that
resistance.
In addition, the use of different switching compounds
which have varying access to different tissues can provide
additional information on the suitability of a target in
relation to dissemination of the infection, and of
microbial load in the different tissues. In certain
cases, a range of switching compounds can be used, each of
which will switch the level of activity of the product of
one coding region (in conjunction with appropriate
regulatory components) in order to evaluate the effects of
the different switching compound distributions in a single
system.
The "state of the infection" can refer to the number
of bacteria in an infection in an infection host, but can
also refer to the growth rate of an infecting bacterial
population, or to the ability of that infecting population
to expand or maintain the infected site in the host
~nlm~l, Therefore, for example, the state of an infection
CA 02223~19 1997-12-03
W O ~6~ 79 PCT,~S~61~1937
19
has been changed if the numbers (i.e., colony Eorming
units/g of tissue or ml of bodily fluids) of a specific
microbe present in the infected ~n;m~l are reduced, but
also if the rate of growth of the population of the
microbe has been reduced, even though the absolute numbers
have increased. In addition, the state of the in~ection
has been changed if the characteristics of the site of
infection have been altered so that the infecting microbe
can be more readily eliminated by the host, or so that the
microbe is more susceptible to an antimicrobial agent.
In referring to an animal which has been infected
with bacteria in the methods of this invention, the
'Iphysiological condition" of the animal refers both to the
overall health of the animal and to the tissue condition
at a localized site affected by the in~ecting microbe.
The general health of the animal may be affected, for
instance, by generalized toxins produced by the infecting
microbe which are transported throughout the animal. On
the other hand, factors produced by the microbial
population which contribute to the local death of cells or
alteration of their biological functioning also affect the
physiological condition of the animal.
In particular embodiments of the above aspect:s, the
recombinant microbe used to infect a mammal is a
recombinant bacterium. The bacterial strain used may be
any of a large number of pathogenic bacterial species and
strains, but in certain preferred embodiments the
recombinant microbe is a Staphylococcus species. These
Staphylococcus species include, in particular,
Staphylococcus aureus.
Also in particular embodiments wherein the
recombinant bacterium is Staphylococcus aureus" the
putative pathogenesis gene can be from the agr locus,
specifically the hld/RNAIII coding sequence. As this
sequence is known to be a pathogenesis gene, such an
embodiment provides a test example for the screening
system, as well as providing the capability to determine
CA 02223~19 1997-12-03
W O 9~'~J~/9 PCT~US96/07937
the effects of inhibiting the level of activity of the
product of this gene under a variety of conditions.
In other preferred embodiments the recombinant
bacterium is a Pseudomonas species.
In particular embodiments, the recombinant microbe is
a virus, where the term ~'virus" has its usual biological
meaning.
In other particular embodiments of the above aspect,
the recombinant microbe is a lower eukaryote.
The term "lower eukaryote" refers to eukaryotic
organisms which do not form multi-cellular tissues or
organs composed of specialized cells. Here, "eukaryote~
has its usual biological meaning, indicating an organism
whose cells contain a membrane-bounded nuclear compartment
distinct from the larger cytoplasm.
In particular embodiments the recombinant microbe is
a yeast, or a fungus, or a protozoan. The terms "yeast",
"fungus", and "protozoan" have their usual biological
meanings.
In certain preferred embodiments, the putative
pathogenesis gene or essential gene is derived from a
different microbial species than the microbe used for
creating the infection. For example, a yeast could
contain a gene from a protozoan.
In preferred embodiments in which the recombinant
microbe is a bacterium, a putative pathogenesis gene can
be from a different bacterial species. These embodiments
may be of particular utility for evaluating genes from
bacterial species which are difficult to culture or for
which there are not acceptable ~n;m~l infection models.
Similarly, in other preferred embodiments in which
the recombinant microbe is a bacterium, a putative
pathogenesis gene can be from a yeast, fungus, or
protozoan. These embodiments are likewise especially
useful when use of the bacterium, fungus, or protozoan
would cause technical or analytical difficulties, e.g., if
the organism is difficult to culture or if adequate ~n;m~l
I
CA 02223~l9 l997-l2-03
W O 9-'1C,7~ PCT/U',G~ 7
21
infection models using that specific organism are not
a~ailable.
In certain preferred embodiments, these methods
include the use of recombinant microbes which contain one
or more DNA constructs to infect an animal. At least one
DNA construct in those microbes contains an artif:icially
controllable promoter inserted in a chromosome. This
promoter may by transcriptionally linked with a plltative
pathogenesis gene or essential gene, such that the
expression of that gene, due to transcription from that
controllable promoter, can be changed (switched) as
described above. The putative pathogenesis or essential
gene switched by that promoter can be an endogenous copy
of the gene. Having a gene on the chromosome allows more
precise control of copy number (and thus dosage ei-fect),
which can allow these methods to more closely mimic
inhibition of a gene in its normal setting. Howe~er, in
other embodiments, the chromosomally-inserted promoter
controls another element of the switch, such as expression
of a repressor molecule. In these embodiments, the test
gene may be either chromosomally-inserted, or located in
an extra-chromosomal element, such as a plasmid.
The term "artificially controllable promoter" re~ers
to a promoter with properties such that transcription of
a transcriptionally linked coding region can be controlled
(i.e., switched) experimentally by altering the
environment of the microbial cell containing that promoter
and coding region. An example of such a change of
environment is contacting the microbe with a switching
compound. The term "promoter" is used with its usual
biological meaning to refer to a DNA sequence which
controls the initiation of transcription of a DNA sequence
into RNA. (see e.g., Watson et al., MOLECU~AR BIOI.OGY OF
THE CELL, 3rd ed., p.417). Thus, the functions of a
promoter can include locating RNA polymerase for starting
transcription.
CA 02223~19 1997-12-03
W O 95/~C~7Y PCTrUS96/07937
The term "chromosomally-inserted" means that the DNA
sequence to which the term refers has been inserted into
a chromosome of the microbe by the actions of a person,
i.e., it means that the DNA sequence was inserted in the
DNA molecule, or one of a set of coordinately replicated
DNA molecules, which contain the majority of the genetic
information of a cell of the organism. This does not
include, e.g., yeast artificial chromosomes (YACs),
plasmids, or cosmids.
The term "transcriptionally linked'~ means that
transcription controlled by a specific promoter can
proceed across a DNA sequence downstream from that
promoter in the same reading frame in which the sequence
is transcribed to form a functional RNA in the organism in
which the downstream DNA sequence is naturally found.
While the above aspects used m~mm~l ian infection
models, other animals and organisms can also be used to
evaluate test genes from pathogens of those organisms.
Thus, in another aspect, this invention provides methods
for evaluating a putative pathogenesis gene or essential
gene as a target for antimicrobial treatment by infecting
a plant with a microbe containing an artificially-created
genetic alteration. These methods are therefore similar
to the first aspect, described above, except used to
evaluate effects of changing the level of activity of a
product of a test gene in a plant infection. Particular
embodiments of these methods include the use of a
switching compound as previously described. As fungi are
common pathogens of many food plant crops, in certain
embodiments the microbe is a fungus.
In a further aspect, this invention provides
recombinant microbial strains in which the microbes
express a repressor molecule which enables artificial
control of the level of activity of the product of a test
gene. In many cases, the repressor is one not normally
expressed in the parent strain from which the recombinant
microbe was derived, but may also be an endogenous
~r
CA 02223~19 1997-12-03
W O 9''~C~79 PCT~U~ /937
repressor. The microbial strain also contains an
artificially-inserted DNA construct containing an operator
site to which the repressor molecule can bind, su~h that
binding of the repressor to the operator site blocks
expression of the test gene. In particular embodiments,
the construct is chromosomally-inserted, but i~ other
embodiments the construct is carried on an extra-
chromosomal element, e.g., a plasmid. Also in particular
embodiments, the recombinant microbial strain is a
recombinant bacterial strain, such as one derived from a
Staphyloeoeeus species, like Staphyloeoeeus aureus.
In certain preferred embodiments, the recombinant
bacterial strain expresses repressor molecules for a lac
regulatory system and a bla regulatory system, such that
expression of the lac repressor molecule is controlled by
the bla promoter. Thus, for example, in the absence of a
~-lactam which induces the bla system, the bla repressor
blocks expression from the bla promoter, which may be
linked with the coding sequence for lae repressor. The
presence of the ~-lactam releases the bla repressor,
allowing expression of the lac repressor, which can then
block expression of a test gene. (Such as if the promoter
for the test gene contains a lac operator site.) In a
particular preferred embodiment, the strain contains laeO,
laeI transcriptionally linked with Pbl~, and blaI and blaRl
transcriptionally linked with Pbl~l. In a particular
embodiment, the strain contains a DNA construct which
includes the P3 promoter from the agr locus of
Staphyloeoccus aureus, transcriptionally linked wi.th the
hld/RNAIII gene from the same locus, and including a laeO
site. Binding of a lac repressor molecule to the laeO
site blocks expression of the hld/RNAIII gene.
Other features and advantages of the invention will
be apparent from the following description of the
preferred embodiments thereof, and from the claims.
CA 02223~19 1997-12-03
W O ~OE'~D379 PCT/U',G~'~7~37
Brief Descri~tion of the Fiqures
Fig. 1 illustrates the use of an in vivo switch to
mimic the action of an inhibitor of a pathogenesis gene,
which in this illustration is agr.
Fig. 2 illustrates the working of the in vivo switch
controlling expression of the hld/RNAIII gene in a rodent
infection model.
Fig. 3 schematically shows the control of expression
of hld/RNAIII based on an exemplary selection of DNA
constructs and regulatory systems, specifically the bla
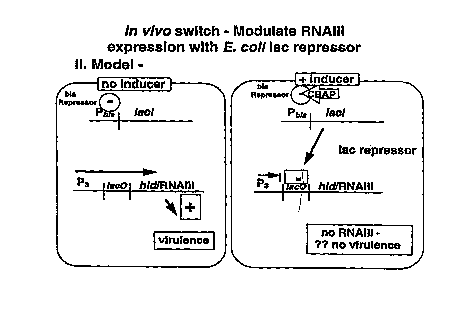
and lac regulatory systems. The "inducer" is a switching
compound which causes bla repressor to be released from
its binding site, allowing expression of lac repressor,
which then blocks expression of hld/RNAIII.
Fig. 4 schematically depicts DNA constructs suitable
for the in vivo switch system shown in Figs. 2 and 3.
These include the hld/RNAIII gene from Staphylococcus
aureus with its normal promoter, P3, but also including a
lac operator site. Also included are the coding sequences
for the bla signal receptor and bla repressor, and the bla
promoter transcriptionally linked with a coding sequence
for lac repressor.
Fig. 5 illustrates the construction of a DNA
construct containing P3, lacO, and the hld/RNAIII coding
sequence.
Fig. 6 illustrates the construction of a DNA
construct containing Pblaz transcriptionally linked with
lacI, and also containing the divergent promoter Pbl~l.
Fig. 7 illustrates the construction of a DNA
construct in which the coding sequences for blaR1 and blaI
are linked with the construct from Fig. 6.
Fig. 8 schematically depicts an embodiment of an In
vivo switch, in which two different switching compounds
can independently cause a change in the level of
transcription of a test gene. The switching compounds in
this illustration are tetracycline and the ~-lactam, CBAP.
Either of these compounds can cause the expression of the
CA 02223~19 1997-12-03
W O 9~ J79 PCT/U~,G~ 37
lac repressor, thereby causing the expression of the
hld/RNAIII gene to be switched off.
Detailed Description of the Preferred Embodiments
I. General Description of the Methods
As was described in the Summary above, this in~ention
provides methods for evaluating putative pathogene,sis and
essential genes as possible targets for antimicrobial
treatment by mimicking the effects of agents which
directly or indirectly affect the level of activity of the
expression product of such a gene. Frequently, these
methods are used to model the effect of an inhibitor of
the gene product.
In certain preferred embodiments, these methods
generally involve contacting a microbe having an
artificially-created genetic alteration with a
concentration of a molecule which causes a change in the
level of production of a second molecule, which alters the
level of activity of a product of a specific coding
region. Also in certain preferred embodiments, the
methods use recombinant microbes which contain one or more
DNA constructs, such that the expression of a test gene
can be switched by an artificial change in the environment
of the microbe. An appropriate change for many
embodiments is the addition or removal of a swltching
compound in the extracellular environment of the microbe.
As is further indicated below, genetic switches which
respond to a change in the presence of a switching
compound can be designed in a variety of ways. One such
design of a useful switch is described for use in
3 0 Staphylococcus aureus . In these recombinant microbes, at
least one DNA construct has been inserted; that construct
contains a coding region of a test gene. (A test gene is
a gene which is being evaluated, or is desired to be
evaluated, as a potential target for antimicrobial
35 therapy.) The expression of this test gene is either
directly or indirectly altered (switched) by the presence
CA 02223~19 1997-12-03
W O 95/~7Y PCT~US96/07937
of the switching compound. In certain preferred
embodiments, when expression of the test gene i8 "on", the
level of expression of that gene is under the control of
the normal cellular mechanisms of the microbe (as in the
embodiment exemplified), typically implying that the
construct including the test gene also includes the
promoter normally associated with that gene. However,
such regulation is not essential to the claimed methods.
The control over the expression state may also be
achieved using other types or other combinations of
constructs. One example is the insertion of specific
regulatory regions to artificially control the expression
of an endogenous, chromosomally-located gene.
The ability to switch the level of activity of a
product of a test gene (as by switching the expression
state) makes possible the evaluation of the effect of such
a switch in vivo (in a microbial infection). In certain
of the claimed methods, a recombinant microbe, as
generally described above, is used to infect a mammal. At
an appropriate time or times, the expression of the test
gene is switched by changing the presence of a switching
compound and the effect on the infection or the
physiological condition of the mammal is evaluated.
(Generally the change in the presence of a switching
compound is an introduction of that compound, such as by
administration to the infected mammal. However, in some
cases, the change may be a removal or a decrease in the
concentration of the switching compound.) If the switch
of the expression of the test gene produces a change in
the infection or the condition of the m~mm~l, then the
test gene is a possible target for antimicrobial therapy.
(Several types of animal infection models are described
below.) This is particularly clear if switching off the
expression of the test gene results in a significant
decrease in the number of microbes present in the
infection. In such a case, the switch is mimicking the
action of an inhibitor of the test gene or related
CA 02223~19 1997-12-03
WO.~ 79 PCT/US9GI~7937
products with a possible microcidal effect. However, it
should be clear that a demonstration of other effects can
also be useful for evaluating potential antimicrobial
targets.
Though the discussion above has primarily described
the evaluation of genes from pathogens of mammals, it
should be understood that it is also desirable to treat
microbial infections of some other organisms, speci~--ically
including plants. The frequent problems of fungal
infections of food crops has led to the widespread use of
fungicides for both active treatment of fungal disease, as
well prophylacticly. Therefore, the methods of this
invention are appropriate and useful for evaluatiny genes
of plant pathogens as targets for antimicrobial treatment
in similar manner as described for pathogens of m~mm~l S .
However, these methods are not limited to mammals and
plants, but are applicable to pathogens of many different
types of organisms. For pathogens of many non-human
organisms, the methods do not re~uire the use of infection
models, since the actual infection can be economically and
reliably used.
While many of the better-known pathogens appropriate
for use in the claimed methods are bacteria whose
molecular biology is currently reasonably well-known, it
should be recognized that other microbes can also be used.
Such use of other microbes can be of several types,
including other bacterial species, viruses, yeast, fungi,
and protozoans. Organisms which can be properly cultured
and whose genes can be suitably manipulated by mol.ecular
biology techniques can be utilized in these methods in a
manner very similar to the bacteria exemplified.
However, even microbes which are very difficult to
culture or to use for recombinant techniques can be used
as a source of test genes. Such test genes can then be
inserted into a different microbe which is more amenable
to manipulation. Thus, for example, genes whose products
are suspected of contributing to tissue damage during
CA 02223~l9 l997-l2-03
W O 9~/1C~79 PCTrUS9~ 7Y37
28
infection by a specific difficult to culture microbe can
be inserted into a bacterium which can be readily cultured
and manipulated. The differential effect of the
expression of that heterologous gene can then be evaluated
in an infection model of the recombinant bacterium.
Therefore, even though a specific microbe may not be
suitable for use as the infecting microbe in the methods
of this invention, genes associated with the pathogenesis
of that specific microbe may still be incorporated in DNA
constructs and evaluated as targets.
II. Microbial Strain Selection
Microbial strains are generally chosen for
~x~m;n~tion in the in vivo switch methodology described
herein, because of their ability to cause significant
disease in mammals (or other host organism of interest).
The organism for study can be selected from all microbial
pathogens, e.g., bacteria. However, additional factors
affect the convenience of utilizing a specific species or
strain, and so affect the choice of organisms. (Note that
an organism may still be selected despite some
difficulties in preparation or use under some
circumstances. For example, an important pathogen may be
selected for use even if genetic manipulation is
difficult.)
To allow for convenient evaluation of the effect of
expression and alteration of expression of a test gene, it
is preferable that a well-characterized infection model
for the specific microbe is available. The infection
model should be such that a researcher can distinguish
between the development of the pathogenic state and the
failure to develop or continue the pathogenic state. The
primary criterion for selection of an infection model is
the ability of the model to mimic the pathogenic process
in the organism(s) which are expected to be treated for
infections by that microbe.
CA 02223~19 1997-12-03
W O ~C/~C~7~ PCT/U',G~'~7Y37
29
Since the microbe is to be used to evaluate the
effects of a change in the level of activity of a product
of a test gene, such as the effects of a switch of the
expression o~ a test gene, it is in many embocliments
highly preferable, but not essential, that the microbe not
contain an active copy of the test gene other th.an the
test copy. Thus, the microbial strain used preferably is
one in which the normal copy of the test gene has been
inactivated or removed. Such inactivation of the normal
copy o~ the gene can occur by any of a variety of
different mechanisms. (For examples see Example 2 below.)
Of course, in some alternative switch designs, such as one
in which an arti~icially-controllable promoter is inserted
in a chromosome, thereby allowing switching of an
endogenous gene, the endogenous gene should not be
inactivated.
In addition, the microbial strain should preferably
be amenable to manipulation using molecular biological
methods, and putative pathogenesis or essential genes
(test genes) must be available for testing. In most
cases, this implies that the test gene will need to be
isolated for further manipulation. Such manipulation
generally comprise the construction of one or more DNA
constructs which include a significant portion of the test
gene, but may comprise other processes such as single base
changes of DNA sequences. Likewise, the microbial strain
which is used in the animal, or other, infection model is
preferably one in which a recombinant DNA construct can be
introduced into the microbial cell.
Once an appropriate strain is selected, it is
possible to create a set of receptor strains derived from
the initial strain which provide a "cellular cassette
system" for test genes. Such receptor strains are
particularly useful since they provide a convenient means
to provide strains to test a variety of different test
genes, or to test genes which can be switched using a
variety of different environmental changes, since the new
CA 02223~19 1997-12-03
W O ~ Y PCTAJS9G/~7~37
sequences can be inserted more readily than if new
constructs, insertion sites, and/or vectors were created
for each test. Thus, each derivative strain can contain
a specific promoter with a downstream insertion site for
test genes to be controlled by that promoter. As another
alternative, a site can be constructed for insertion for
a complete promoter/test gene construct. Such a set of
receptor strains facilitates the evaluation of potential
targets.
The use of the selection factors appropriate for
choosing a microbe to use as a recombinant microbe in the
in vivo switch methodology of this invention is described
for a bacterium, Staphylococcus aureus, in Example 1
below. It should be noted, however, that the species and
strain described, while appropriate for such use, are in
no manner limiting to the claims. Other species can
appropriately be selected, and other equally appropriate
strains of Staphylococcus aureus can be selected or
constructed using standard techniques of molecular
biology.
Exam~le 1: Selection of Recombinant Microbe
StaphYlococcus aureus
One bacterial species selected for ~X~ml n~tion is
Staphylococcus aureus. This bacterium is chosen because
of its status as a common, but often dif~icult to treat,
microbial pathogen. S. aureus has the acquired ability to
be resistant to a variety of antibiotics, and has also
adapted well to being a human pathogen, including the
ability to persist in a carrier state (as in the nasal
passages of health care workers). In some cases today,
only glycopeptides (vancomycin and, in Europe,
teicoplanin) are recommended as reasonable therapies.
This organism is genetically tractable, capable of
manipulation through the use of genetic and recombinant
technologies. The molecular biology and some elements of
the pathogenesis of this gram-positive species are
relatively well-characterized. The genetic tractability
CA 02223~19 1997-12-03
W O 9-'1Cg/~ PCTfUS~6/07937
and the partial characterization of the pathogenesis of S.
aureus means that both known pathogenesis genes as well as
test genes can be isolated and manipulated by wel:L known
molecular biology techniques. In addition, DNA constructs
can be re-introduced into Staphylococcus aureus by
currently available methods, including both c]~emical
treatment of cells to induce competence, as well as
introduction of DNA constructs by electroporation.
S. aureus is a classic representative of the eubacteria,
and understanding more about its ability to cause disease
will clearly provide a significant foundation for
understanding other bacteria.
Thus, the criteria for choosing S. aureus as an
example of a appropriate recombinant microbe include:
(1) ability to cause significant and common human disease;
(2) genetic accessibility; and (3) representative
character (for extension to other microbes).
However, within a microbial species certain strain
characteristics can be significant. The in vivo switch
provides the capability to artificially control the
expression of a test gene with a small molecule switching
compound. The recombinant construction that effect:s this
control is preferably introduced into a mutant stra.in that
lacks expression of active product from that gene (test
gene or natural homolog) apart from the control of the in
vivo switch. This allows the sole expression of the test
gene from the recombinant construction.
Staphylococcus aureus, as found in wild-type clinical
isolates, often carries extrachromosomal genetic e]ements
(both plasmids and transposons). The use of such strains
would complicate the analysis of the results of testing,
as insertions into the chromosome, or the existence of
extrachromosomal elements, is variable within clinical
isolates. Hence, a strain devoid of extrachroMosomal
elements is desirable for prototypic study.
Strain 8325-4 is such a strain. Genetic mapping has
been done on the strain; it is thought to be de~oid of
CA 02223~l9 l997-l2-03
W O ~f'1C3/~ PCTrUS9G~ 37
32
transposons, plasmids and phage. However, it is believed
to be otherwise representative of S. aureus. An in vivo
switch controlling expression of the agr-RNAIII gene uses
the S. aureus strain WA400 (CmR; Strain 8325-4 with
~hld/RNAIII 252-1472:: cat-86 (S. Arvidson et al., Ch. 30
p.419 ff in MOLECU~AR BIOLOGY OF THE STAPHYLOCOCCI, R.P.
Novick, ed., VCH, New York, New York, 1990). The WA400
Agr phenotype is readily complemented by an extra-
chromosomal copy of the agr region spanning BgIII
1-PstI 2149. The intended recombinant construction is
also expected to fully complement the Agr defect of this
mutant strain. Other such strains are available.
III. Selection of Inserted Genes
Two general types of putative pathogenesis genes are
isolated into the switch construct. First are genes
suspected of being involved in microbial pathogenesis.
These genes are identified using a variety of techniques,
including the following approaches. (1) Some pathogenesis
genes are identified by reviewing the literature. (2) A
second group is identified by using techniques which
select genes which are expressed or essential specifically
during infection. Such techniques include but are not
limited to the techniques of differential display (Liang
& Pardee, 1992, Science 257:967-971), differential
hybridization (T. Sargent, 1987, Meth. Enz. 152:423-432),
or IVET (In vivo Expression Technology) (J. Mekalanos,
1993, J. Bacteriol. 174:1-7). In each of these
techniques, cells are grown either in An;m~ls or under
conditions which mimic such in vivo growth. Conditions
which are commonly accepted as mimics of in vivo growth
include, but are not limited to, iron deprivation, late
exponential (vs. exponential) growth; conditions of oxygen
limitation and other forms of nutrient limitation. (3) A
third group of pathogenesis genes is identified by
observing differential virulence in strains in which
specific genes have been interfered with (made
CA 02223~19 1997-12-03
W O n~ 7Y PCT/US9GI'~7~37
nonfunctional or differentially functional). Using ~n;m~l
models such as described below in In vivo evaluation of
microbial virulence and pathoqenicity, lack of virulence
in such models serves to identify potential pathogenesis
genes. (4) It is becoming more commonly accepted that in
bacteria, individual pathogenesis genes may reside near
other genes which share similar functions. Such clusters
of genes involved in pathogenesis have come to be known as
"pathogenesis islands". Pathogenesis islands clearly
exist in enteric bacilli such as Salmonella. (F.A.
Groisman, 1993, EMPO J. 12:3779-37787; Galan & Curtiss,
1989, PNAS 86: 6383-6387. ) Using the assumption that an
identified pathogenesis gene may have neighbors which are
also important in pathogenesis, genes neighboring
identified pathogenesis genes are also chosen for
inclusion in switch experiments. Thus, even genes showing
no homology or very little homology to studied genes are
chosen for study using the switch based on their loc:ations
within the genome (having pathogenesis gene neighbors).
20 (5) Genes which are essential for microbial growth or
survival in vivo are also utilized. The expression of
these genes is required in vivo, but not in vi tro.
A second major collection of genes which is studied
using the in vivo switch is so-called "essential genes".
25 Essential genes are .described as genes which, if not
present in a functional state, result in the death of the
microbe. Such genes can be identified by a variety of
techniques, (S.J. Austin et al., 1971, Nature 232: 133-
136; P. Schedl & P. Primakoff, 1973, Proc. Natl. Acad.
30 Sci. 70: 2091-2095; C.M. Joyce & N.D.F. Grindley, 1984, J.
Bacteriol . 158: 636-643) but one straightforward and
proven method is through the use of temperature sensitive
mutants. Mutants, created for example by exposure to
mutagenic chemicals, are selected for their ability to
35 grow at a permissive temperature, but not a non-per~lissive
temperature. It has been demonstrated in some cases, and
is widely accepted, that such mutants have changes in
CA 02223~19 1997-12-03
WO 9G/~C~7~ PCT~U596/~ 7
34
their DNA which makes the mutated gene products
differentially active at the different temperatures. For
example, a mutant may have a functional enzyme at thirty
degrees centigrade, but a dysfunctional enzyme at 37~
centigrade. The dysfunction may be due to changes in the
enzyme brought about by the mutation in the DNA. In such
an event, if the enzyme's function is required (essential)
for the ability to grow, we have identified an essential
gene (for those growth conditions). Identification of
these essential genes in vi tro does not guarantee that the
identified genes are essential for viability in vivo, or
that disruption of the expression of an essential gene has
the same quantitative or qualitative effect in vivo.
Hence, essential genes selected in vi tro are included for
15 ~x~m; n~tion in the switch model. This e~ml n~tion allows
confirmation that the "essential gene" is, in fact,
essential for the cell's viability in vivo. Controlling
the expression of such genes while the bacteria is within
an animal provides definitive evidence for the essential
nature of such genes during infection, and as such allows
for an appreciation of the importance of the (in vitro
selected) essential gene. This allows evaluation of the
relevance of the gene as an antimicrobial target, and
therefore provides a basis for selection of appropriate
25 genes to use for the development of an antimicrobial
agent.
Genes identified as essential genes need not be
previously known, nor do the products of those genes, or
the functions of those products need to be known. The
genes may be known "only" as open reading frames, the
expression of which correlates with in vi tro microbial
viability under the tested growth conditions.
Another approach to the identification of either
pathogenesis or essential gene targets is a "shotgun
approach". While different methods could be utilized, one
such method is the integration of an artificially
controllable promoter into the microbial chromosome.
CA 02223~19 1997-12-03
W O ~"~D3/~ PCT/U~3G~'~7937
(These techniques are most developed for bacteria, both
Gram-positive and Gram-negative.) If inserted in the
proper reading frame, the artificially controllable
promoter can switch the expression of an endogenous gene,
replacing the normal cellular control of the gene. This
method is particularly appropriate for identifying genes
whose expression is deleterious to the microbe in vivo,
i.e. in an infection. Thus, a controllable promoter on a
DNA integration vehicle (e.g., insertion sequen.ce or
transposon) can be inserted in the chromosomal DN~ at a
large number of sites.
This allows the isolation of a large number of
derivative strains; in each the expression of a gene can
be artificially controlled. Artificial control over the
expression of a random set of genes then allows
determination of the effects of those gene products on the
progression of an infection. (This shotgun approach does
not rely on any prior knowledge of the probable function
of a gene.)
In summary, both pathogenesis genes and essential
genes are placed in the artificially-controllable system.
ORF's identified as neighbors of identified pathogenesis
genes or identified as essential genes are included.
Pathogenesis and essential genes which are identified from
the literature are also included as are genes which
enhance virulence (as identified in animal modeling).
Further, differentially expressed genes (genes which are
expressed specifically during infection) are also
candidates for ~mi n~tion in the switch model.
Alternatively, in the shotgun approach using
chromosomally-inserted promoters, a random set of genes
can be evaluated.
In addition to the evaluation of a single test gene,
this invention also allows (and the claims include)
evaluation of a set of genes together. For example, a
linked set of genes, such as an operon with multiple open
reading frames can be evaluated by placing the level of
CA 02223~19 1997-12-03
W O 9~/~C~79 PCT~US9G~7~37
activity of all the coding sequences under the same
control, such as transcriptional control. As another
possibility, a set of unlinked genes can be subjected to
the same control. This can be accomplished, for example,
by using the same type of operator site with each coding
sequence to bind a single type of repressor, allowing all
the test genes to be turned off at essentially the same
time.
For the microbe described in Example 1 above,
Staphylococcus aureus, a number of pathogenesis genes are
currently known. These known genes provide examples of
genes which are appropriate to evaluate as targets using
the in vivo switch methodology, and some are further
described in Example 2 below.
Exam~le 2: Staphylococcus aureus Pathoqenesis Genes
For Staphylococcus aureus, a number of pathogenesis
genes are known. These known genes include agrA, agrB,
agr-RNAIII, xpr, and sar. Of these genes, several are
part of the agr locus, which is a complex, polycistronic
locus which controls the production of most cell toxins
and many cell wall-associated proteins. The biosynthesis
of these gene products involves both positive and negative
regulation. The agr locus contains two divergent
promoters, P2 and P3, which are strongly induced during
post-exponential growth. Promoter P3 directs the
synthesis of a 514 nt transcript RNAIII which contains the
hld (delta-lysin) open reading frame. Recent evidence
indicates that the RNAIII molecule, in addition to
encoding delta-lysin, also encodes the agr-specific
regulator, since the loss of RNAIII production results in
altered regulation of exoprotein virulence factors. The
gene expression regulated by RNAIII involves virulence
factors which are up-regulated, as well as virulence
factors which are down-regulated.
CA 02223~19 1997-12-03
W O.9~'1C~1~ PCT~U~,GI'~7~7
37
IV. Inactivation of Endoqenous CopY of Test Gene
In many embodiments in which a test gene coding
region has been inserted in a microbe, expre~sion of an
endogenous gene corresponding to the test gene would
interfere with the evaluation of the test gene.
Therefore, in those embodiments, it is preferable that the
endogenous gene is inactivated. Such inac~ivation can be
achieved in various ways. Examples of such inactivation
mechanisms are, e.g., insertional disruption of function
(such as with a transposon or other insertion se~uence);
by an insertion/deletion (in which DNA has been replaced
with inserted DNA); or by a spontaneous or induced
mutation in the test gene (such as by chemically or uv-
induced mutagenesis).
A specific example of gene inactivation using
recombinant DNA methods is allelic replacement In
allelic replacement, the native chromosomal allele of a
given gene is replaced, using host cell general
recombination factors, with a nonfunctional gene copy.
Nonfunctional alleles of a given gene are created in vitro
by insertion/ disruption or insertion/ deletion within the
open reading frame of a test gene. The insertion sequence
contains both the promoter and coding region for a drug
resistance marker (eg. for tetracycline, tetM; for
chloramphenicol, cat-86; for erythromycin, ermC),, thus
enabling in vivo selection for gene replacement by
acquisition of drug resistance. Examples of the
application of this method in S. aureus are:
a) toxic shock syndrome toxin gene (tst): Sloane et
al., 1991, FEMS Microbiology Letters 78: 239-244.
b) accessory gene regulator locus (agr): Novick et
al., 1993, EWBO J. 12: 3967-3975.
c) delta hemolysin gene (hld/RNAIII): Janzon and
Arvidson, 1990, EM~O J. 9: 1391-1399.
CA 02223~19 1997-12-03
W O ~ IC3i~ PCTrUS9G~'~7~37
38
V. RequlatorY Mechanisms and Selection of Requlator~
~equences
Many genes in microbes are regulated by
transcriptional control. They are switched on and off in
a conservative fashion, being transcribed when the
products are needed, and not transcribed or transcribed at
a lower level when the products are not needed. Such
regulation is advantageous for a number of reasons,
including reducing unproductive energy usage by limiting
the synthesis of unneeded molecules, and reducing the
competition between conflicting cellular processes.
While transcriptional control is an important
mechanism, regulation of the level of activity of a gene
product in microbes can occur through a variety of
mechanisms. These include regulation of the production of
mRNA transcripts, of the intracellular longevity of the
mRNA, of the ability to translate the mRNA into protein,
of the processing of the protein into a functional form
and/or of the intracellular stability of the protein.
Each species uses some or all of these mechanism for
regulating the level of activity of gene products.
Likewise, each of these mechanisms can be used as the
basis of an in vivo switch, as disclosed herein, to
control the level of activity of the product of a test
gene. Certain preferred embodiments of the methods of
this invention achieves gene regulation by control over
synthesis of the mRNA, i.e., over transcription.
Control of the synthesis of RNAs is accomplished by
the synthesis of regulatory proteins that serve to make
the DNA template more or less accessible to the RNA
polymerase enzyme. The study of transcriptional control
in microbes, especially in bacteria, has a long history;
general features of the molecular mechanisms underlying
transcriptional control in bacteria have been under
continuous investigation since the early 1960s. Summaries
of this work can be found in any undergraduate textbook on
molecular biology ( see e . g., Watson, et al., Molecular
CA 02223~19 1997-12-03
W O 9~'4C3/~ PCT~US9G~'~19~7
39
BioloqY of the Gene). In particular, note that the
definitions of the terms "operator", "promoter",
"repressor protein", and "inducer protein~ can be found in
those textbooks. (The description immediately below is
not intended to be comprehensive, and generally discusses
bacterial gene regulation. It should be recognized that
different microbial groups may have features of gene
regulation unique to each group, and indeed, each species
with a group may have unique elements. Nonetheless, major
elements of the process are common to all the microbes.)
Regulatory proteins modify the ability o~ RNA
polymerase to synthesize the mRNA of one or more
physiologically related genes. They accomplish this task
by physically binding to the DNA in the vicinity of the
promoter site, at which RNA polymerase binds and initiates
synthesis of the mRNA. When a regulatory protein binds
and prevents or reduces the ability of RNA polymerase to
synthesize a mRNA, it is called a repressor protein. The
binding of the repressor protein to the DNA occur,s at a
site at which the repres60r protein has a particularly
high affinity. This high affinity is achieved through the
interaction of the protein with the specific sequence of
nucleic acid base pairs in the DNA duplex, typically
through a combination of H-bonding, van der Waals
interactions, hydrophobic interactions, and ionic
interactions. This binding site, called the operator site
(which is generally 8-30 bp), is specific for each
different repressor protein. Likewise, there are
regulatory proteins, inducers, that bind to a specific DNA
se~uence of base pairs that induce RNA polymerase to
synthesize a mRNA transcript.
The DNA-binding activity of repressor proteins and
inducer proteins is regulated in respon6e to change6 in
the bacterial cell physiology. In many case6, small
molecules (such as cAMP, sugars and other metabolites)
bind to the regulatory protein causing an altered affinity
of the regulatory protein for the DNA.
CA 02223~l9 l997-l2-03
W O S~'10379 PCT/U',61'079~7
The sensing mechanisms which stimulate the induction
and repression of genes include microbial capability to
accept and transmit stimuli from the outside world.
Environmental sensors can create responses from the
microbe to diverse stimuli, including presence of specific
nutrients, the lack of nutrients, metals and cofactors,
the presence o~ antibiotics and changes in the external
environment. In this last case, examples include changes
in temperature (e.g., heat shock genes) and gases (e.g.
oxygen). Two well-studied prototypic environmental
sensors are the lactose operon and the tetracycline
resistance operon.
Selection of appropriate regulatory sequences
(including those providing environmental sensor function)
to use in the methods of this invention depends on a
number of considerations. An approach to such regulatory
sequence selection with some of its associated
considerations is described in (a) through (g) below.
(a) A promoter is required that will regulate the
expression of the test gene. In the exemplified strategy,
an "off" switch has been designed. In this design, a
small molecule is introduced into the animal, and the test
gene is no longer expressed.
(b) A promoter that is regulated to turn-off when a
small molecule is introduced is the simplest mechanism to
control the expression of the test gene. However, the
best promoter provides expression at appropriate levels
in vivo, and this level of expression may differ for each
test gene. Therefore, there is good reason to provide
artificial control of the natural promoter of the test
gene. However, use of the natural promoter is not
essential. In many cases, a heterologous promoter may be
linked with the test gene, and in some cases the use of an
heterologous promoter will be preferable or even
necessary. Such situations may arise, for example, when
evaluating a test gene ~rom an organism other than the
infecting organism, particularly in those cases where
CA 02223~19 1997-12-03
W O g~ 379 PCT/US~f~7Y37
normal control of transcription relies on a number of
additional components.
(c) Regulation of the natural promoter of the test
gene can be accomplished by introducing a negative
regulatory element in cis to the test gene and promoter,
in such a position and orientation that a trans-acting
negative regulatory protein can prevent expression. An
example of such a cis/trans regulatory pair is the lacO
operator site and the lac repressor protein. (Amann, et
al., 1983, Gene, 25: 167-178; Makoff and Oxer, 1991, Nucl.
Acid Res. 19: 2417-2421). Other examples of negative
regulatory pairs are the tet operator/tet repressor; the
lambda cI repressor/lambda OL operator; (Bernard, et al.,
1979, Gene 5: 59-76; Mott, et al., 1985, Proc Natl. Acad.
Sci (USA) 82: 88-92); trp repressor/trp operator (Latta,
et al., 1990, DNA & Cell Biol . 9 : 129-137); argR
repressor/arg operator (Lim, et al., 1987, Proc. Natl.
Acad. Sci . USA 84: 6697-6701); lexA repressor/boxA
operator site (Kenyon, et al., 1982, J. Mol. Biol. 160:
445-457); the bla repressor/bla operator (Novick, et al.,
1991, EMBO J. ) . There are many others. The
operator/repressor pairs need not originate in the
pathogen under investigation, since many studies have
shown that effective regulation can occur independent of
the species of origin of the regulators. In a specific
embodiment described herein, the E. coli lac operator/lac
repressor regulatory system can be used to control the
expression of the agr-related hld/RNAIII gene of
S. aureus.
(d) In order to achieve regulation of the test gene
that has been placed under artificial control of a
negative regulatory system, artificial control of the
trans-acting repressor is required. A preferred strategy
is to control expression of the repressor protein. The
repressor protein should be synthesized in respo~se to
addition of a small molecule to the ~n; m~ 1 . This can be
achieved by a promoter that is turned on by the addition
CA 02223~19 1997-12-03
W O.~G/~C3/~ PCTAUS~G~'~7~37
42
of a small molecule switching compound. Such promoters
are either positive activators, in which the small
molecule makes a trans-acting factor allow transcription
from a promoter, or by a negative mechanism, in which the
small molecule makes a trans-acting repressor protein no
longer capable of binding to its operator site. There are
numerous examples of positive and negative regulators.
Several negative regulator systems were listed in (c);
positive acting systems include the activation of
promoters by the CAP protein in the presence of cAMP and
the activation of the ara promoter by araC protein in the
presence of arabinose. The coupling of these artificial
regulatory constructions allows a small molecule to
control the expression of a regulatory protein that
controls the expression of the test gene. In an example
described herein, a ~-lactamase system controls expression
of a lac repressor protein, and the test gene is linked
with a lac operator site in cis. Therefore, exposing a
bacterial cell containing this system to the non-
antibiotic ~-lactam CBAP causes the ~-lactamase repressor
to be released, resulting in the expression of the lac
repressor, blocking transcription of the test gene.
(e) A different strategy is to regulate expression
by an inversion mechanism. There are several examples of
site-specific inversions that occur in which the inverted
segment includes a promoter. The expression of a test
gene is on in one orientation of the promoter, and off in
the other orientation of the promoter. The inversion is
controlled by a site-specific recombinase enzyme; control
over expression of the test gene is accomplished by
controlling expression of the recombinase enzyme by a
small molecule switching compound, such as described in
(c) and (d). Addition of the small molecule results in
expression of the recombinase enzyme, which shuts off
expression of the test gene. Examples of this strategy
are tnpR resolvase-mediated inversion of ~a-res sequences,
E. coli Plcre-lox mediated inversion, and bacteriophage
CA 02223~l9 l997-l2-03
W O ~ C,/Y PCTfUS9G/~7937
43
Int-mediated inversion of att sequences. (Podhajaska et
al., 1985, Gene 40: 163-168.)
Another strategy involves antisense RNA. Numerous
genes in prokaryotes are regulated by antisense RNA
(Simons and Kleckner, 1988, Ann. Rev. Genet. 22: 567-600;
Wagner and Simons, 1994, Ann. Rev. Micorbiol . 48: 713-742) .
The expression of a test gene is down regulated by
induction of an antisense RNA complementary to th.e test
gene transcript. Expression of the antisense RNA can be
accomplished by a small molecule inducer system such as
described in (c) and (g). Alternatively, in an approach
independent of production of a regulatory molecule by the
microbial cell, antisense oligonucleotides can be
administered which are chemically modified to provide
increased biological stability, but which can bind to a
test gene transcript to down regulate expression.
(Uhlm~nn & Peyman, 1990, Chem. Rev. 90:544-584.)
Yet another strategy is to utilize ribozy~nes to
control expression by cleaving the mRNA transcribed from
the test gene. A sec~uence(s) coding for a ribozyme can be
introduced into a microbe such that expression of the
ribozyme(s) is induced by addition of a small molecule
(such as the tetracycline of ~-lactamase-inducible
promoters). In this system, expression of the ribozymes
would be off until the small molecule (switching compound)
is added. The presence of the ribozymes then reduces
expression of the test gene. Alternatively, ribozymes may
be utilized by administering modified ribozymes which are
more stable than RNA ribozymes in a form which can be
taken up by the microbe. (Eckstein et al., 1992, PCT
Application PCT/EP91/01811, Int. Publ. No. W092/07065.)
In this case, the ribozymes can act directly on the test
gene message.
(f) The promoter must respond in its expression to
a small molecule that retains bioavailability in the
relevant tissue of fluid in the ~n; m~ 1 model. The best
promoters have normal expression of the test gene in the
CA 02223~19 1997-12-03
W O 9G/~C37Y PCT/U'SG'~37
44
absence of the small molecule, and a complete cessation of
expression of the test gene in the presence of the small
molecule.
(g) The best small molecules to regulate the
promoter expression are those that have well-defined
pharmacodynamics. Such well-defined pharmacodynamics
allow assessment of the effects of the small molecule
alone (there should be very little effect, such as growth
inhibition at the doses used). In addition, the
pharmacodynamic information assures that the necessary
concentrations of the small molecule are achieved in the
tissue under investigation in the animal model, so that
the test gene is appropriately regulated. As discussed
above, different compounds will distribute differently,
and have different tropism and clearance characteristics
in various tissues, so that the effective concentration of
the compound at a specific infection site will vary. Due
to these variable properties, the temporal behavior of
various switching compounds will differ, with accompanying
differences in the control of the level of activity of a
gene. Some such compounds are known among the inactive
(or low activity) analogs of known antibiotics. For these
compounds, the pharmacodynamics of the class of compounds
has been previously investigated, but the inactive analog
does not have significant antimicrobial activity to
complicate the evaluation of targets. In addition, the
use of different switching compounds which have varying
access to different tissues can provide additional
information on the suitability of a target in relation to
dissemination of the infection, and of microbial load in
the different tissues. In certain cases, a range of
switching compounds can be used with one coding region
(and with appropriate regulatory components) in order to
evaluate the effects of the different switching compound
distributions in a single system. In general, however, a
switching compound should be chosen which has
pharmacodynamic properties appropriate for use with a
1:
CA 02223~19 1997-12-03
WO 9Cl~t9 i~ PCT/U~i3G~ 37
specific test gene and infection model. For example, the
switching compound must distribute to the infection site
with sufficient concentration and half-life to effectively
switch the level of activity of the gene.
VI. Genetic Methods and DNA Constructs
For use in evaluating a putative pathogenesis or
essential gene, one or more DNA constructs are made and
inserted in a test microbe ~ e . g., Staphylococcus aureus) .
The methods for performing these steps are well-known in
the art, and can be selected or modified from those
described in such standard references as Sambrook et al.,
MOLECULAR CLONING, 2nd ed. (1989).
Typically, but not necessarily, two constructs are
made. In one class of embodiment of this invention, the
first construct contains a regulatory region which is
controlled by the presence or absence of a small molecule,
transcriptionally linked with a coding region for a signal
molecule which can control the expression of the second
construct. The second construct includes a regulatory
region which is controlled by the normal mechanisms of the
cell, an operator region which is controlled by the
product of the coding region of the first construct, and
the coding region of a test gene.
While the paragraph above generally describes the
constructs for one class of embodiment, it does not
describe all the useful designs. It is only necessary
that the level of activity of the product of a test gene
can be switched (as by switching expression) in response
to the addition or removal of a switching compound or
other appropriate environmental change. However, as
previously indicated, in many situations it is
advantageous for the level of expression of the test gene
to be under the normal control of the microbial cell when
the expression state is on.
The example below provides a description of one
preferred embodiment of DNA construct design for
CA 02223~l9 l997-l2-03
W O 9G/~t~7Y PCT/U',GlO/9~
46
Staphylococcus aureus, but should not be regarded as
limiting even for this species. With these constructs the
small molecule CBAP (a penicillin derivative with well-
known pharmacodynamics) causes the bla repressor protein
to lose its affinity for the bla operator site. This
allows expression of the lac repressor protein, which
blocks the expression of the agr-RNAIII molecule. The
RNAIII molecule is important for the pathogenesis of S.
aureus. (M. Richmond, 1967, J. Mol. Biol., 26: 357.)
The example below describes DNA constructs which are
inserted into the bacterial chromosome, however,
chromosomal insertion is not necessary. Therefore, in
another preferred embodiment, the same switch components
are used but only one construct is inserted in the
chromosome. The Pblsz~ lacI fusion is inserted in the
chromosome, and the P3-lacO-hld/RNAIII and blaRl-blaI
sequences are placed in a plasmid which remains
independent of the chromosome. An advantage of this
embodiment is ease of construction, since plasmid
insertions are generally simpler to accomplish than
chromosomal insertions. Embodiments utilizing plasmids in
this manner require that the plasmid by stable within the
bacterial cells; if a specific plasmid is lost at an
excessive frequency then either a different plasmid or
chromosomal insertion is preferable.
~MPLE 3: DNA Constructs for Staphylococcus aureus
- aqr locus
1. Construction of hybrid S. aureus P3-lacO-RNAIII
promoter (see Fig. 5) :
(a) Subclone hld/ RNAIII 2149 bp fragment (PstI
- BglII, from pEX07; Janzon and Arvidson) into PstI
site of pALTER-1 (Promega) for oligonucleotide
mediated site-directed mutagenesis.
(b) Anneal mutagenic oligonucleotide (PIVS5) to
convert C1557 to G, creating unique BglII site at
position +13 relative to hld transcription start
point (tsp; = 1570).
CA 02223~19 1997-12-03
W O S5.'~0~9 PCT/U~ v7~37
47
(c) Insert synthetic 21 bp core lacO
oligonucleotide (PIVS6 + 7) at BglII.
(d) Select 3 lacO configurations: 1 oligo
~orward, 1 oligo reverse, and 3 tandem forward oligo
insertions.
(e) Transfer to S. aureus - E. coli shuttle
vector pMP157, to crea~e plasmids pMPswitch7/8/9,
respectively, and S. aureus integration vector pXX.
2. Construction of inducible E. coli lacI gene in S.
aureus
(a) Fuse S. aureus blaZ promoter (Pblaz) to E.
coli lacI coding sequence by polymerase chain
reaction (PCR); transfer to S. aureus - E coli
shuttle vector pMP16, to create plasmid pMPswitch3,
and to S. aureus integration vector pXXX (see Fig.
6).
(b) Subclone genes encoding beta-lactamase
repressor (blaI) and beta-lactamase signal receptor
(blaRl) into pMPswitch3, to create plasmid
pMPswitch4, and to S. aureus integration vector pXXX
(see Fig. 7).
3. Transfer plasmids containing P3-lacO-RNAIII
(pMPswitch7/8/9), and blaI-blaRl-Pblaz-lacI (pMPswitch4)
into S. aureus strain WA400 (8325-4 ~hld/RNAIII 252-
1472: :cat-86; Janzon and Arvidson) by electroporation.
VII. In Vi tro Evaluation of DNA Construct Function
Once the DNA constructs have been made for a selected
organism and test gene, it is useful to test the in vi tro
functioning of the regulation and expression of those
constructs. Such verification of function avoids wasteful
use of ~n;m~l s in infection studies, and as well can
provide greater confidence in the results of evaluation of
test genes using the constructs.
CA 02223~19 1997-12-03
W O ~f'~G~79 PCTrUS9GI'~7937
48
The exact function tests appropriate for a specific
DNA construct or set of constructs will, of course, depend
on the specific switch design, as well as on the specific
regulatory sequences, control molecules, and coding
sequences involved. However, the appropriate tests are
directly implied by the switch design, so the nature of
the appropriate testing is neither ambiguous nor
difficult. As an example, the testing of the constructs
described in Example 3 above, for use with a
Staphylococcus aureus strain, is described in Example 4
below.
Example 4: In vitro Testinq of DNA Constructs
In vitro evaluation
(1) A biochemical assay for lacO function is
performed. A gel band shift assay, employing labeled or
tagged lacO oligonucleotide and extracts derived from
induced and non-induced recombinant S. aureus containing
the Pblaz-lacI construct is performed. The sensitivity of
this method is sufficient to detect induced levels of
lacI, but low levels of the lac repressor synthesis are
generally undetectable by this method.
(2) Appropriate Agr-regulation of the P3-lacO-RNAIII
promoter.
The insertion of lacO into the agr P3 promoter must
not alter the appropriate regulation of the hld/RNAIII
transcript by the agr regulation system. The appropriate
induction of RNAIII during postexponential growth is
tested by isolation of RNA from recombinant strain
WA400:P3-lacO-RNAIII and subsequent RNA blot hybridization
analysis to identify the RNAIII transcript.
(3) Efficacy of "Off-Switch" in vitro:
The effect of lac repressor induction on RNAIII
accumulation in recombinant strain WA400: Pblaz-lacI; blaI;
blaRI; P3-lacO-RNAIII is assessed in cell culture by RNA
blot hybridization as well as by various genetic and
biochemical tests (hemolysin and lipase production;
P3-blaZ induction, etc.).
CA 02223~19 1997-12-03
W O 9~M-glY PCT~US~G~'~7937
49
VIII. In vivo evaluation of microbial virulence and
pathoqenicitY
After the genetic constructs have been placed into
their host organisms, they are evaluated in an infection
model system, e . g., in an animal, cell-based, or plant
system. (References herein to the use of an animals or
m~mm~l S should be understood to refer to particular
embodiments of this invention. As mentioned above, other
infection systems may be used for certain other
embodiments, such as to evaluate possible antimicrobial
targets of pathogens of organisms other than animals
(e.g., plants), and to embodiments employing cell-based
systems as surrogates for whole organism models.) The
criteria for evaluation include the ability of the microbe
to replicate (either with test gene expression "on" or
test gene expression "off"), the ability to produce
specific exoproducts involved in virulence o~ the
organism, and the ability to cause symptoms of disease in
the animals.
The infection models, e . g., animal infection models,
are selected primarily on the basis of the ability of the
model to mimic the natural pathogenic state of pathogen in
an organism to be treated and to distinguish the effects
produced by a change in the level of activity of a gene
product ( e . g., to a switch in the expression state of the
gene). Secondarily, the models are selected for
efficiency, reproducibility, and cost cont~'nm~nt. For
m~mm~l models, rodents, especially mice, rats, and
rabbits, are generally the preferred species.
Experimentalists have the greatest experience with these
species. Manipulations are more convenient and amount of
materials which are required are relatively small due to
the size of the rodents.
Each pathogenic microbe ( e . g., bacterium) used in
these methods will likely need to be ~mi ned using a
variety of infection models in order to adequately
CA 02223~19 l997-l2-03
W O 9''~D379 PCT/U'3U~7~37
understand the importance of the function of a particular
test gene.
A number of animal models suitable for use with
bacteria are described below. However, these models are
only examples which are suitable for a variety of
bacterial speciesi even for those bacterial species other
models may be found to be superior, at least for some
inserted genes and possibly for all. In addition,
modifications of these models, or perhaps completely
different animal models are appropriate with certain
bacteria, as well as with species of other types of
organisms, e.g., yeast, fungi, and protozoa.
Six animal models are currently used with bacteria to
appreciate the effects of switching on and off specific
genes, and are briefly described below.
Example 5: Mouse Soft Tissue Model
The mouse soft tissue infection model is a sensitive
and effective method for measurement of bacterial
proliferation. In these models (Vogelman et al., 1988, ~J.
Infect. Dis. 157: 287-298) anesthetized mice are infected
with the bacteria in the muscle of the hind thigh. The
mice can be either chemically immune compromised ( e . g.,
cytoxan treated at 125 mg/kg on days -4, -2, and 0) or
immunocompetent. The dose of microbe necessary to cause
an infection is variable and depends on the individual
microbe, but commonly is on the order of 105 - 106 colony
forming units per injection for bacteria. A variety of
mouse strains are useful in this model although Swiss
Webster and DBA2 lines are most commonly used. Once
infected the animals are conscious and show no overt ill
effects of the infections for approximately 12 hours.
After that time virulent strains cause swelling of the
thigh muscle, and the animals can become bacteremic within
approximately 24 hours. This model most effectively
measures proliferation of the microbe, and this
proliferation is measured by sacrifice of the infected
~n;m~l and counting colonies from homogenized thighs.
CA 02223~19 1997-12-03
W O ~ C379 PCTnU~9''~7~/
Example 6: Diffusion Chamber Model
A second model use~ul for assessing the virulence o~
microbes is the diffusion chamber model (Malouin et al.,
1990, Infect. IIr~nun. 58: 1247-1253; Doy et al., 1980, J.
Infect. Dis. 2: 39-51; Kelly et al., 1989, Infect. Immun.
57: 344-350. In this model rodents have a diffusion
chamber surgically placed in the peritoneal cavity. The
chamber consists o~ a polypropylene cylinder with
semipermeable membranes covering the chamber ends.
Diffusion of peritoneal fluid into and out of the chamber
provides nutrients ~or the microbes. The progression of
the "infection" can be followed by ~x~m;n1ng growth, the
exoproduct production or RNA messages. The time
experiments are done by sampling multiple chambers.
Exam~le 7: Endocarditis Model
For bacteria, an important animal model effective in
assessing pathogenicity and virulence is the endocarditis
model (J. Santoro and M.E. Levinson, 1978, Infect . Immun.
19: 915-918). A rat endocarditis model can be used to
assess colonization, virulence and proliferation. The
ability to switch specific genes on and off has clear
utility using this model.
Exam~le 8: Osteomyelitis Model
A fourth model useful in the valuation of
pathogenesis is the osteomyelitis model (Spagnolo et al.,
1993, Infect. Immun. 61: 5225-5230). Rabbits are used for
these experiments. Anesthetized animals have a small
segment of the tibia removed and microorganisms are
microinjected into the wound. The excised bone segment is
replaced and the progression of the disease is monitored.
Clinical signs, particularly inflammation and swelling are
monitored. Termination of the experiment allows histolic
and pathologic ex~m;n~tion of the infection site to
complement the assessment procedure.
Exam~le 9: Murine Septic Arthritis Model
A fifth model relevant to the study of microbial
pathogenesis is a murine septic arthritis model (Abdelnour
CA 02223~l9 l997-l2-03
W O 9''/4,/~ PCT/U~5~7Y37
52
et al., 1993, Irlfect. IIrunun. 61: 3879-3885). In this
model mice are infected intravenously and pathogenic
organisms are found to cause inflammation in distal limb
joints. Monitoring of the inflammation and comparison of
inflammation vs. inocula allows assessment of the
virulence of related strains.
E~am~le 10: Bacterial Peritonitis Model
Finally, bacterial peritonitis offers rapid and
predictive data on the virulence of strains (M.G.
Bergeron, 1978, Scand. J. Infect. Dis. Suppl. 14: 189-206;
S.D. Davis, 1975, Antimicrob. Agents Chemother. 8: 50-53).
Peritonitis in rodents, preferably mice, can provide
essential data on the importance of targets. The end
point may be lethality or clinical signs can be monitored.
Variation in infection dose in comparison to outcome
allows evaluation of the virulence of individual strains.
A variety of other in vivo models are available and
may be used when appropriate for specific pathogens or
specific genes. For example, target organ recovery assays
(Gordee et al., 1984, ~J. Antibiotics 37:1054-1065;
Bannatyne et al., 1992, Infect. 20:168-170) may be useful
for fungi and for bacterial pathogens which are not
acutely virulent to animals. For additional information
the book by Zak and Sande (EXPERIMENTAL MODELS IN
ANTIMICROBIAIJ CHEMOTHERAPY, O. Zak and M.A. Sande (eds.),
Academic Press, London (1986) is considered a standard.
It is also relevant to note that the species of
~n;m~l used for an infection model, and the specific
genetic make-up of that animal, may contribute to the
effective evaluation of the effects of altering the level
of activity of the product of a test gene. For example,
immuno-incompetent animals may, in some instances, be
preferable to immuno-competent animals. For example, the
action of a competent immune system may, to some degree,
mask the effects of altering the level of activity of the
test gene product as compared to a similar infection in an
immuno-incompetent ~n;m~l. In addition, many
-I
CA 02223~19 1997-12-03
W O 9f'~C37~ PCTrUS96/07937
opportunistic infections, in fact, occur in immuno-
compromised patients, so modeling an infection in a
similar immunological environment is appropriate.
In addition to these in vivo test systems, a variety
of ex vivo models for assessing bacterial virulence may be
employed (Falkow et al., 1992, Ann . Rev. Cell Biol . 8:333-
363). These include, but are not limited to, assays which
measure bacterial attachment to, and invasion of, tissue
culture cell monolayers. With specific regard to S.
aureus, it is well documented that this organism adheres
to and invades cultured endothelial cell monolayers (Ogawa
et al., 1985, Infect . Immun . 50: 218-224; Hamill et al.,
1986, Infect. and Imm. 54:833-836) and that the
cytotoxicity of ingested S. aureus is sensitive to the
expression of known virulence factors (Vann and Proctor,
1988, Micro. Patho. 4 :443-453) . Such ex vivo models may
afford more rapid and cost effective measurements of the
efficacy of the "Off-Switch" experiments, and may be
employed as prel; m; n~ry analyses prior to testing in one
or more of the animal models described above.
IX. Alternate Switch Desiqns and Control Mechanisms
As suggested above, a large variety of mechanisms can
be used to control the level of activity of a product of
a test gene, all within the scope of this invention. The
design exemplified above utilizes transcriptional control
based on the ability of a repressor molecule ( lac
repressor) to stop transcription from an endogenous
promoter (P3 promoter). Expression of that repressor is
then controlled by a second promoter (Pbl~). Expression
from that promoter is turned on by the release of the ~-
lactamase repressor in response to the presence of a ~-
lactam (CBAP) which does not have significant antibiotic
activity. Therefore, this system requires the production
of the blaR1 receptor molecule and the ~-lactamase
~repressor.
CA 02223~l9 l997-l2-03
W O 9C/1C,/9 PCT/Uv9
54
However, genetic switches for use in the methods of
this invention, which are conceptually ~uite similar, can
be constructed using other regulatory components with
similar functions to those described just above. In
addition, the promoter controlling transcription of the
test gene may be the promoter which normally controls the
test gene, a different endogenous promoter, or a
heterologous promoter. Some specific repressor/operator
pairs are the following:
(1) lexA protein/boxA operator. This pair could
replace the lac repressor/lacO operator. Production
of the lexA protein can be controlled by the ~-
lactamase or other control system. (Kenyon et al.,
1982.)
(2) tet repressor/ tet operator. The tet system can
clearly replace the ~-lactamase system in the
exemplified switch design.
(3 ) trp repressor/ trp operator. The trp system can
replace the ~-lactamase system in the exemplified
design. The trp repressor could be expressed
constitutively, the addition of L-tryptophan
activates the repressor causing it to bind to the
operator site. Use of this system in this manner
requires that the repressor is not significantly
activated by tryptophan from the infection host.
(4) other negative regulatory element pairs listed
in V. above.
While the above examples could be used as described, other
such regulators are known to those skilled in the art, and
the use of such regulators in the methods of this
invention is within the scope of the invention and of the
claims.
In addition to the design of a switch using two
negative regulatory elements, other switch designs can be
used to control transcription of a test gene. Among the
possible designs are the following:
j_
CA 02223~19 1997-12-03
W O ~ PCT/IJ'3G/07~37
(1) Replace the ~-lactamase repressor/operator
system with a positive activator system, such as the
ara system. The ara promoter would be
transcriptionally linked with the lacI gene (coding
for repressor) or a gene coding for another suitable
repressor. This promoter is activated by araC
protein in the presence of arabinose, so addit:ion of
arabinose would cause the production of the lac
repressor, turning off expression of the test gene.
(2) Use of a repressor which is activated by a small
molecule to turn off transcription of a test gene.
Examples of such repressor molecules are the arg and
trp repressors, which require the presence of
arginine and tryptophan, respectively, to bind to
their respective operators. In these switch designs,
the repressor can be expressed constitutively since
the repressor does not bind significantly to the
operator in the absence of the appropriate repressor
activator. Thus, if an operator which binds a
repressor of this type is placed in cis with a test
gene, addition of the appropriate small molecule will
cause the repressor to bind to the operator, blocking
transcription.
(3) Use of an "On" switch instead of an "Off"
switch. In certain evaluations, turning a test gene
on rather than off may be useful. Such an "On"
switch can readily be constructed using either
activation or derepression. For example, the ara
operator described above could be linked in ci.s with
a test gene and an appropriate promoter. If araC
protein is also produced, the addition of arabinose
will turn on expression of the test gene. (Raibaut
& Schwartz, 1984, Ann. Rev. Genet. 18:415-444.) An
example of derepression utilizes IPTG (isopropyl-~-D-
thiogalactopyranoside) and the lac repressor/operator
pair. With the lac repressor present and the lac
operator in cls with the test gene, the lac repressor
CA 02223~19 1997-12-03
w o 9r/~c37~ PCT~US~6/079
will bind to the operator. This blocks expression of
the test gene. Addition of IPTG inactivates the
repressor, releasing it from the operator and
allowing transcription of the test gene. (Note that
derepression of lac with IPTG can also be used in the
off switch design using two negative regulators. If
the lac repressor/operator pair is used to control
expression of a second repressor, the lac repressor
is released by the addition of IPTG. This then
allows expression of the second repressor, which then
binds to its respective operator, blocking
transcription of the test gene.)
(4) Use of antisense oligonucleotides (including
hybrid polymers containing nucleic acid analogs) to
block translation or transcription. As was mentioned
above, antisense molecules complementary to a portion
of an mRNA critical for translation can be introduced
into a cell to block translation. Such molecules can
be RNA oligonucleotides produced within the cell
under the control of an artificially-controllable
promoter. Alternatively, modified antisense polymers
can be used which contain nucleic acid analogs, which
are more resistant to degradation. Such molecules
can be administered in a form which can be taken up
by the infecting microbes, and which then will block
expression of the test gene by binding to the
corresponding mRNA. Antisense molecules can also be
used to inhibit transcription. Complementary
antisense molecules, under some conditions, will bind
to the sense strand of the DNA to form triplex
structures. Therefore, if the antisense sequence is
selected to be complementary to an appropriate sense
strand sequence, transcription is blocked when the
antisense molecule is present. (Uhlmann ~ Peyman,
1990.)
(5) Use of ribozymes to block translation of test
gene mRNA. Ribozymes can be provided by similar
CA 02223~19 1997-12-03
W O 9G11~3/~ PCT/USS~v/~7
approaches as discussed for antisense molecules.
All-RNA ribozymes can be produced with microbial
cells under artificial control, or modified ribozymes
containing nucleic acid analogues can be administered
in a form which will, as with antisense, be taken up
by the microbial cells. With either approach, the
ribozymes will then cut the mRNA corresponding to the
test gene, preventing (or at least significantly
reducing) expression of the test gene. (Eckstein et
al., 1992.)
In addition to the control designs and mechanisms
described above, any mechanism which provides specific
artificial control of the level of activity of a product
of a test gene is potentially useful in the methods of
this invention, and is included within the scope of this
invention.
X. EukarYotic Microbes and Test Genes
While the molecular biology of prokaryotes is, in
general, better understood than that of eukaryotes, the
use of eukaryotic microbial pathogens and test genes is
within the scope of this invention. Those skilled in the
field will recognize that there are also features of
eukaryotic biology, differing from prokaryotic biology,
which must be addressed in the practice of this invention
with eukaryotic pathogens. These additional concerns
include at least the following: (a) In diploid organisms,
two copies of a gene are present. In many cases both
copies must be inactivated to allow evaluation of an
artificially-controllable copy of a correspondin~ test
gene; (b) Control mechanisms, especially transcriptional
control mechanisms, are often more complex, involving
multiple components; (c) Chromosome structure is often
more complex and more important to gene expression; (d)
Recombinant techniques are often more difficult, due to
such factors are the large size of eukaryotic chromosomes;
CA 02223~19 1997-12-03
~ W O 9G,'4C~7~ PCTrU59~ f~37
(e) Gene expression often involves additional processing
steps, such as mRNA splicing.
However, those skilled in the field recognize that
there are control systems, even in higher eukaryotes which
can provide appropriate control for use in the methods of
this invention. For example, the steroid and thyroid
hormones (e.g., cortisol, steroid sex hormones, and
ecdysone) and retinoids are widespread, and the control
systems responding to those hormones regulate the
transcription of specific genes. Thus, such control can
be used to control specific test genes.
In addition, in the lower eukaryotes, a variety of
control systems are known which can be used in this
invention. An example is the system responding to the
mating factor peptide from Saccharomyces cerevisiae.
Further, a variety of recombinant techniques are available
for manipulating nucleic acid sequences in lower
eukaryotic microbes. For instance, the genes required for
the components of a switch can, for yeasts, be
incorporated into a YAC (yeast artificial chromosome);
similar mini-chromosomes can be constructed for some other
eukaryotic microbes as well.
XI. Viral Pathoqens
In addition to the use of the methods of this
invention to evaluate genes of pathogenic microbes which
are themselves cells, these methods are also applicable to
viruses and viral genes. While viruses utilize the host
cellular machinery to express the viral-encoded genes, at
least the products of those genes are potential
therapeutic targets. Also, for many viral pathogens, the
functions of those viral-encoded genes are not known.
Therefore, this invention is especially useful for
evaluating the viral-encoded genes as potential targets by
determining the effects of turning off the activity of the
viral-encoded gene products after infection has been
established.
r
CA 02223~19 1997-12-03
W O 9~ PCT~US95.'~7Y~7
59
In general, it is necessary to package the
artificially-controllable copy of the gene in the viral
structure by inserting that copy in the viral genome. In
some cases it i~ possible to inactivate the native copy
and insert an additional gene sequence containing the
artificially-controllable copy with the appropriate
control component(s), such as an operator site in cis. In
other cases it is necessary to replace the native copy
with the artificially-controllable copy, such as when the
amount of DNA which can be packaged in the virus is
limited. Therefore, it is preferable that other
components of the control system, not encoded by the host
cells, be minimized.
Alternatively, if a cell-based infection model is
used, the test gene and the required regulatory components
can be inserted in a plasmid and the plasmid inserted in
the cells used for the infection model, or inserted in a
chromosome of the host cell using a transposon, other
eukaryotic integration vector, or by random insertion.
It is also possible, in some cases, to insert foreign
genes in the germ line of an organism (e.g., a mouse),
producing a transgenic animal. This technique can be used
to produce an infection model host organism line which
produces the molecules needed for controlling the
expression o~ a viral gene. This obviates the packaging
problems which can be caused by increasing the size of the
viral genome.
The methods of this invention are applicable to both
DNA and RNA viruses. In either case, an artificially
controllable test gene can be inserted.
The embodiments described herein are not meant to be
limiting to the invention. Those skilled in the art will
appreciate that the invention may be practiced by using
numerous microbial strains, species, and genes, as well as
different designs of the DNA constructs, different
selections of regulatory sequences and switching
compounds, different mechanisms for control of the level
CA 02223519 1997-12-03
W O9fi'~09/~ PCTrUS9G~7~37
of activity, and various infection models for in vivo
evaluation, all within the breadth of the claims.