Note : Les descriptions sont présentées dans la langue officielle dans laquelle elles ont été soumises.
CA 02223999 1997-12-08
WO 96/40436 PCTrUS95/09746
METHOD FOR REMOVlNG.lJNDESlRED PARTICLES FROM GAS
FIELD OF THE lNv~;N~l~loN
The present invention is a method and apparatus for
removing undesired particles, such as fly ash, from gas
streams. More particularly, the present invention embodies
an improved approach for removing such undesired particles
by selectively introducing adhesives into the gas stream.
BACKGROUND OF THE lNV~;N'l'lON
Environmental st~n~ds for particulate emissions by
coal-fired electrical power plants, petroleum refineries,
chemical plants, pulp and paper plants, cement plants, and
other particulate-emitting facilities are h~c ;ng
increasingly more dem~n~;ng. For example, air quality
stAn~A~ds in the United States now require power plants to
remove more than 99 percent of the fly ash produced by coal
combustion before flue gas may be discharged into the
atmosphere. As environmental st~ ~ds tighten, there is
a corresponding need for a more efficient -~nc of
particulate removal.
Electrostatic precipitators and filtration systems are
two commonly used devices for the removal of undesired
particles from the gas streams produced by plants and
refineries. As~used herein, "undesired particles" refers
to any particulate matter that is desired to be removed
from a gas stream. In electrostatic precipitators,
undesired particle-laden gases pass negatively charged
corona electrodes which impart a negative charge to the
CA 02223999 1997-12-08
W O 96/40436 PCTrUS95/09746
undesired particles. The charged particles then migrate
towards positively charged collection plates alternately
positioned between the corona electrodes. The undesired
particles accl 11 ate on the collection plates and are
removed by various t~-hn; ques, including sonic horn blasts
or rapping of the collection plates. Electrostatic
precipitators may employ one stage for both the charging
and collection of undesired particles or multiple stages
with the charging and collection being done in different
stages.
Filtration systems, such as baghouses, remove
undesired particles from gas streams by passing the gas
streams through large filters. The filters have pores
large enough to pass the gases in the gas stream but small
enough to prevent passage of undesired particles. The
filters may be of a fabric, metal, paper or ceramic
construction. The undesired particles can be removed from
a filter by many t~-hn; ques including shakers, pulse jets,
or reverse gas flow.
In both electrostatic precipitators and filtration
systems, efficiency and cost are critical considerations.
The efficiency of electrostatic precipitators is decreased
by undesired particle reentrainment into a gas stream
during the removal of undesired particles from the
collection plates. Field studies have shown that as much
as 80 percent of the particulate emissions into the
atmosphere from electrostatic precipitators result from
such reentrainment.
CA 02223999 1997-12-08
WO 96/40436 PCT~US95/09746
--3--
Filtration system efficiency is decreased by the
build-up of undesired particles on the filter. Particle
build-up clogs filter pores, h; n~l~ing the passage of the
gas stream through the filter, which causes a large
pressure drop across the filter. To reduce the pressure
drop, the filters require frequent cleaning to reduce the
build-up of undesired particles on the filter. The need to
frequently clean the filters increases not only operating
costs but also undesired particle emissions.
Numerous approaches have been proposed for increasing
the efficiency of electrostatic precipitators and
filtration systems. In one approach, ammonia gas and sulfur
trioxide may be injected into a gas stream to form ammonium
sulfates on the surfaces of undesired particles. This
approach has several drawbacks. First, a possible product
of the reaction between ~ on;a gas and sulfur gas is
ammonium bisulfate which fouls the electrostatic
precipitator or filtration system components. Such
component fouling impairs the operation of the components
and increases undesired particle emissions and unit
operating costs. Second, the use of ammonia gas in the gas
stream may require additional downstream gas purification
steps to remove unreacted ~ -n;a gas from the gas stream
prior to discharge. Ammonia gas is known to create
2S environmental damage and increase the opacity of the
discharged gas stream. Finally, the odor associated with
ammonia may also cause problems in the disposal of the
undesired particles after collection.
CA 02223999 1997-12-08
W 096/40436 PCT~US95/09746
Another approach to reduce undesired particle
emissions is to employ a wetted collection surface. In
such "wet systems," a liquid, typically water, is supplied
to the collection surface to enhance undesired particle
collection and reduce reentrA; -nt. Unless ~Yp~n-~ive
materials are employed, however, components of wet systems
can suffer high corrosion rates due to acid formation.
Other approaches to increase electrostatic
precipitator and filtration system efficiency similarly
require the addition of expensive components to new or
existing units and/or otherwise raise other operational
complications.
SUMMARY OF THE lNV~NllON
It is, therefore, an object of the present invention
to increase the efficiency of electrostatic precipitators
and filtration systems in the removal of undesired
particles from a gas stream, preferably without
significantly increasing capital and operating costs.
It is a further objective to increase electrostatic
precipitator and filtration systems efficiency without the
use of toxic additives.
It is a further objective to increase electrostatic
precipitator and filtration system efficiency by methods
and apparatus that are readily adaptable to existing
designs.
CA 02223999 1997-12-08
W O 96/40436 PCTAJS95/09746
--5--
It is a further objective to reduce undesired particle
reentr~ nt during removal of undesired particles from a
collection surface.
In one aspect of the present invention, a method for
removing undesired particles from a gas stream is provided
including the steps of (i) contacting a gas stream
cont~;n;ng undesired particles and water vapor with an
adhesive composition; (ii) collecting the undesired
particles and the adhesive composition on at least one
collection surface to form an agglomerate at a temperature
greater than the condensation temperature of the water
vapor in the gas stream; and (iii) removing the agglomerate
from the collection surface. As used herein, "undesired
particle" refers to any particle that one desires to remove
from the gas stream, regardless of the intrinsic value of
the particle. "Adhesive" refers to any substance, inorganic
or organic, natural or synthetic, that is capable of
adhering or bonding other subst~nc~c together by surface
attachment. "Agglomerate" refers to a cluster or
accumulation of undesired particles and adhesive particles.
"Condensation temperature" refers to the temperature at
which a given vapor component of a gas stream condenses
into a liquid under ambient pressure.
Preferably, the adhesive in the composition is
nontoxic and substantially odorless. An adhesive is
typically deemed "nontoxic" if the presence of the adhesive
in the resultant agglomerate does not cause the agglomerate
to be environmentally unacceptable under the st~n~rds and
CA 02223999 1997-12-08
W O 96/40436 PCTAUS95/09746 -6-
procedures set forth in the Toxicity Characteristic
Leaching Procedure ("TCLP") established by the United
States Environmental Protection Agency. The TCLP provides
analysis procedures for waste materials to detect
environmentally unacceptable levels of substances,
including inorganic elements, volatile organic compounds,
and semi-volatile organic compounds. The TCLP specifies
the ~ ~ acceptable concentration for such substances.
An adhesive is deemed to be "odorless" if the presence of
the adhesive in the agglomerate cannot be detected by the
human nose.
Preferred adhesives are selected from the group
consisting of gums, cellulose, vinyls, and derivatives and
mixtures thereof. More preferably, the adhesive should be
selected from the group consisting of xanthan gum,
carboxymethyl cellulose and mixtures thereof. As used
herein, "gum" refers to a carbohydrate high polymer that is
insoluble in alcohol and other organic solvents, but
generally soluble or dispersible in water. "Cellulose"
refers to a natural carbohydrate high polymer
(polysaccharide) con~;n;ng anhydroglucose units joined by
an oxygen linkage to form long mol~clll~r c-h~;n~. "Vinyls"
refers to a polymer having the linkage CH2 = CH- in the
polymer chain.
The adhesive composition may include a surfactant to
enhance agglomerate formation. The adhesive composition may
also include a dispersant to control agglomerate porosity,
especially in filtration plant applications. As used
CA 02223999 1997-12-08
WO 96/40436 PCT~US95/09746
herein, "surfactant" refers to any substance that alters
the surface tension of another substance. "Dispersant"
refers to any substance that influences the distance
between undesired particles in the agglomerate.
The adhesive composition is preferably introduced into
the gas stream in a dispersed and uniform manner. In this
regard, the adhesive composition can be atomized upon
introduction utilizing a "carrier fluid" component. The
carrier fluid may be a gas or liquid, such as water, that
is a solvent for the adhesive and that vaporizes in the gas
stream.
After contact with the gas stream, a substantial
portion of the carrier fluid, preferably about 90~ or more
by weight, separates from the adhesive by vaporization
prior to reaching the collection surface. Upon separation,
dispersed particles of the adhesive will remain in the gas
stream with the undesired particles. Upon contact with the
collection surface, the adhesive particles and the
undesired particles to be removed form the agglomerate.
Preferably, to yield a substantially "dry system," the
temperature of the collection surface in the collecting
step is greater than both the con~n~ation temperature of
the water vapor in the gas stream and any vaporized carrier
fluid. As used herein, a "dry system" refers to a system
that employs a substantially dry collection surface (i.e.,
having substantially no liquid in contact therewith) for
undesired particles.
CA 02223999 1997-12-08
WO 96/40436 PCT~US95/09746
The gas stream may be advantageously deflected, as may
be desired, prior to contacting the collection surface to
achieve, for example, uniform incidence of the particles on
the collection surface, thereby yielding an agglomerate of
a more uniform thickn~
After a predetermined build-up, the agglomerate of
undesired particles and adhesive particles may be ~, ved
from the collection surface, collected in a hopper and
removed from the unit. Removal may be accomplished by
vibration of the collection surface, removing the
collection surface from the collection zone, or contacting
the collection surface with a reverse gas stream having a
direction of flow substantially opposite to the gas stream.
In a related aspect of the invention, an apparatus for
undesired particle removal is disclosed that includes (i)
a housing; (ii) an inlet and outlet for the gas stream;
(iii) an injection apparatus to inject an adhesive
composition into the gas stream; and (iv) one or more
collection surfaces supportably positioned within the
housing to collect both the undesired particles to be
removed and adhesive particles which, in turn, form an
agglomerate on the collection surface. The apparatus may
include a plurality of collection surfaces and one or more
hoppers to collect the agglomerate that is removed from the
collection surface.
The adhesive injection apparatus is preferably a
plurality of dispersion devices (e.g., nozzles) positioned
within and/or across the gas stream to uniformly disperse
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746
the adhesive composition into the gas stream. The adhesive
injection apparatus may be advantageously located upstream
of the collection surface at a distance sufficient for a
substantial portion of any carrier fluid, preferably about
90% or more by weight, to separate by vaporization from the
particles before the particles contact the collection
surface. A deflecting apparatus may be provided to deflect
and uniformly distribute the gas stream prior to contacting
the collection surfaces so as to improve the uniformity of
agglomerate build-up on the collection surfaces. Such
deflecting apparatus may comprise, for example, a plurality
of selectively adjustable baffles (e.g., horizontally,
vertically, and/or angularly) disposed across the gas
stream.
In an electrostatic precipitator embodiment of the
present invention, the apparatus may include a power
supply; at least one electrode connected to the negative
terminal of the power supply and positioned relative to the
input gas stream to impart a charge to the undesired
particles to be removed and the adhesive particles; and at
least one collection surface connected to the positive
teL in~l of the power supply and positioned parallel to the
flow of the gas stream. To enhance agglomerate formation,
electrostatic injection nozzles (such as charged-fog
nozzles or those employed in many paint sprayers) may be
employed to inject the adhesive composition. That is, the
use of such nozzles may serve to impart additional charge
to adhesive composition droplets upon atomization, thereby
CA 02223999 1997-12-08
WO 96/40436 PCTAUS95/09746
--10--
increasing the collection of adhesive particles on the
collection surfaces, particularly if an anionic or nonionic
adhesive is utilized. To lower the electrical resistivity
of the agglomerate formed on the collection surface, and
thereby reduce sparkover or back corona discharge, the
carrier fluid in the adhesive composition may also be
advantageously utilized to cool the gas stream and
undesired particles contained therein. For such purposes,
the predetermined injection rate is preferably selected to
achieve a desired degree of cooling, while yet allowing for
a substantial portion of the carrier fluid to vaporize
before reaching the collection surface. It is believed that
such an approach may have particular application in low-
sulphur content coal burning facilities in view of the
~5 relatively low acid dew point of the resulting gas streams.
In a filtration system embodiment of the present
invention, the collection surface may be a filter located
transverse to the direction of flow of the gas stream to
separate the undesired particles and adhesive particles
from the gas stream. Such filter may be of ceramic, fabric,
paper or metal construction. Preferably, the adhesive
injection apparatus and/or filter are selected such that a
substantial portion of the adhesive particles dispersed
into the gas stream are larger than the pore size of the
filter.
In another aspect of the present invention, a process
for removing undesired particles from a gas stream is
provided that includes the following steps: (i) contacting
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746
--11--
a gas stream with a plurality of droplets comprising
phosphoric acid and a carrier fluid, with the droplets
having a first Sauter Mean Diameter ranging from about 20
to about 150 microns; (ii) separating the carrier fluid
from the droplets, such as by evaporation, to reduce the
droplet size to a second Sauter Mean Diameter less than
said first Sauter Mean Diameter; and (iii) collecting the
undesired particles and phosphoric acid on a collection
surface to form an agglomerate. After separation of the
carrier fluid from the phosphoric acid, the second Sauter
Mean Diameter ranges from about 1 to about 10 microns. The
preferred concentration of phosphoric acid in the gas
stream ranges from about 1 to about 10 lb/ton of undesired
particles. The residence time of the droplets in the gas
stream before the collecting step preferably ranges from
about 0.25 to about 2.00 s~co~.
In a filtration system embodiment, a process is
provided that first contacts a gas stream with a caustic
acid and/or salt or precursor thereof, preferably a
phosphorous-cont~;n;ng material, and second contacts the
undesired particles with a filtration surface to form an
agglomerate of undesired particles on the filtration
surface. The caustic acid and/or salt or precursor thereof,
and particularly the phosphorous-containing material, cause
an increase in the cohesiveness of the undesired particles
and thereby facilitate agglomerate formation. The
phosphorous-cont~;n;ng material preferably includes
phosphoric acid, elemental phosphorous, and precursors and
CA 02223999 1997-12-OX
WO 96/40~6 PCTAUS95/09746
-12-
mixtures thereof. In the first contacting step, the
phosphorous-contA; n; ~g material preferably causes the
deposition of phosphoric acid on the undesired particles.
In a hot-side electrostatic precipitating system
embodiment, a process is provided that first contacts a gas
stream with a resistivity reduction agent and collects the
undesired particles and resistivity reduction agent on a
collection surface of the electrostatic precipitating
device to form an agglomerate. The temperature of the gas
stream at the electrostatic precipitating device is no less
than about 600~F. The electrostatic precipitating device
is located upstream of the air preheater in utility
applications. The resistivity reduction agent preferably is
a caustic acid and more preferably is phosphoric acid and
precursors thereof. The preferred phosphoric acid
concentration ranges from about o.l to about 1.0% by weight
of the undesired particles in the gas stream.
The present invention has numerous advantages over
existing methods and apparatus. Electrostatic precipitator
and filtration system efficiency are increased by less
reentrainment of undesired particles. The adhesive
particles increase the force of attraction between
undesired particles in the agglomerate of undesired
particles and adhesive particles. The resulting agglomerate
of undesired particles and adhesive particles on the
collection surface, or dust cake, is not only more cohesive
but also more porous. In electrostatic precipitators, the
cohesiveness of the dust cake reduces fragmentation and
CA 02223999 1997-12-08
W 096/40436 PCT~US95/09746
undesired particle reentrainment during dust cake removal.
The resistivity of the resulting dust cake can also be
reduced through the use of phosphoric acid resulting in
better collection of the undesired particles, relative to
untreated particles. In filtration systems, the particle
cohesion produces increased porosity of the dust cake which
reduces the pressure drop across the filter (and therefore
requires less frequent filter cleanings). The cohesiveness
of the dust cake also reduces "bl~; ng~ of very fine
undesired particles through the filter pores caused by
compaction of undesired particles on the filter surface,
thus increasing efficiency.
The present invention is also particularly
advantageous as it preferably yields a "dry system," which
has numerous advantages relative to a wet system.
Additionally, the method not only employs low cost
components and additives that improve the efficiency of new
electrostatic precipitators and filtration systems but also
is readily adaptable to existing units. Further advantages
will be apparent to those skilled in the art.
BRIEF DESCRIPTION OF THE DRAWINGS
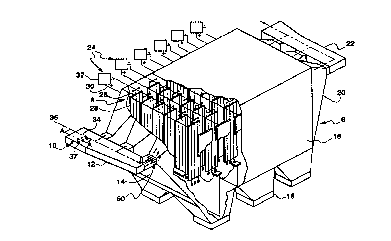
Fig. 1 is a perspective view of a first embo~; -~t of
the present invention in an electrostatic precipitator;
Fig. 2 is a cut away view along line A-A of Fig. 1
showing the adhesive injection device spraying droplets of
an adhesive composition into the gas stream;
CA 02223999 1997-12-08
W 096/40436 PCT~US95/09746
-14-
Fig. 3 is a side view of a collection plate showing an
accumulation of adhesive particles and undesired particles
on the collection plate;
Fig. 4 is a perspective view of a second r- hoA; --nt of
the present invention in a filtration system;
Fig. 5 is a cut away view along line B-B of Fig. 4
showing an accl llation of adhesive particles and undesired
particles on the filter surface;
Fig. 6 is a graph showing the relationship between
particle temperature and particle resistivity for typical
flyash particles;
Fig. 7 is a plot of relative phosphoric acid
concentration (based on a percent by weight of additive
relative to undesired particles in the gas stream) against
resistivity for a gas stream having a temperature of 300~F;
Fig 8 is a plot of relative phosphoric acid
concentration (based on a percent by weight of additive
relative to undesired particles in the gas stream) against
rate of agglomerate formation;
Fig. 9 is a plot of relative phosphoric acid
concentration (based on a percent by weight of additive
relative to undesired particles in the gas stream) against
resistivity;
Fig. 10 is a plot of phosphoric acid concentration
(based on a percent by weight of additive relative to
undesired particles in the gas stream) against resistivity;
and
CA 02223999 l997-l2-08
W O 96/40436 PCT~US95/09746
-15-
Fig. 11 is a plot of phosphoric acid conc~ntration
(based on a percent by weight of additive relative to
undesired particles in the gas stream) against resistivity
under hot-side conditions.
DETATT~n DESCRIPTION
Figs. 1 and 2 depict a first ~-ho~; -nt of the present
invention as implemented in an electrostatic precipitator
for removal of undesired particles such as fly ash from a
gas stream. The electrostatic precipitator comprises
housing assembly 6, precipitating assembly 8, and adhesive
injection assembly 10. The housing assembly 6 includes an
input duct 12, one or more input plenums 14, shell 16, one
or more hoppers 18, one or more output plenums 20, and
output duct 22.
The precipitating assembly 8 includes a plurality of
sections 24. Each section 24 includes a plurality of
alternately disposed discharge electrodes 26 and collection
plates 28, a corresponding plurality of electrical
conductors 30, and an interconne~-ted power supply 32. The
negative and positive te, ;n~l.c of the power supply 32 are
connected to the discharge electrodes 26 and collection
plates 28, respectively.
The adhesive injection assembly 10 includes a
reservoir (not shown) and an interconnected feed line 34
and plurality of nozzles 37. As will be appreciated, the
gas stream may be contacted with an adhesive composition
continuously or intermittently and by many different
CA 02223999 1997-12-08
O 96/40436 PCT~US95/09746
-16-
methods. Adhesive injection assembly 10 achieves
contacting by atomizing a composition comprising a carrier
fluid and an adhesive into the gas stream 36 in the form of
droplets 38. Atomization may be realized by a lll h~l- of
different methodologies, including spraying the composition
through a nozzle. To enhance charging of the droplets,
particularly if an anionic or nonionic adhesive is
employed, electrostatic injection nozzles may be utilized.
While preferred, a carrier fluid is not required to
disperse adhesive particles in gas stream 36. By way of
example, adhesive particles 40 may be simply dripped into
gas stream 36 by a suitable device (e.g., drip emitters).
As illustrated, adhesive injection assembly 10 should
be located upstream of the precipitating assembly 8.
Preferably, the adhesive injection ~ hly 10 is disposed
so as to provide a sufficient distance between the adhesive
injection assembly 10 and the nearest of the collection
plates 28 such that, prior to contacting the nearest
collection plate 28, a substantial portion of the carrier
fluid, preferably about 90% or more by weight, has
separated from the adhesive and a substantially uniform
dispersion of adhesive particles 40 across the gas
stream 36 has been att~i n~ . To accomplish this, adhesive
injection assembly 10 may be advantageously located in
input duct 12 with nozzles 37 evenly spaced across and
within the gas stream 36 as illustrated.
Gas stream 36 may be deflected by baffles 60 prior to
contacting collection plates 28 to achieve a more uniform
CA 02223999 l997-l2-08
W O 96/40436 PCTAUS95/09746
-17-
incidence of undesired particles 35 and adhesive particles
40 on collection plates 28, thereby yielding an agglomerate
of a more uniform thickness on collection plates 28.
Adhesives utilized in the present invention should be
nontoxic, substantially odorless, and soluble in a suitable
fluid carrier, such as water. Further, the adhesives
preferably should be organic compounds, such as polymers.
Preferred classes of polymers are gums, cellulose, vinyls
and derivatives and mixtures thereof. For polymer
adhesives, generally, the desired droplet size 38 upon
injection of the adhesive composition via nozzles 37 is
from about 10 to about 100 micrometers.
It is believed that the ionic characteristics of the
molecules of t~e adXesive utilized can impact the
performance of the present invention. That is, in
electrostatic precipitator applications, adhesives that are
anionic and nonionic may be preferable since they are
believed to more readily accept a negative charge from
electrodes 26 than cationic molecules. Consequently, it is
theorized that anionic and nonionic molecules will more
readily collect on collection plates 28 than cationic
molecules, thereby enhancing agglomerate formation.
In operation, gas stream 36 cont~;n;ng undesired
particles 35 is passed through input duct 10 and input
plenums 14 into electrostatic precipitator shell 16. Prior
to entering electrostatic precipitator shell 16, gas stream
36 passes adhesive injection assembly 10. Adhesive
CA 02223999 1997-12-08
W0 96/40436 PCTAJS95/09746
-18-
injection assembly 10 disperses droplets 38 containing
adhesive particles 40 into gas stream 36.
As noted above, the contacting of the adhesive with
the gas stream may be facilitated by use of a carrier
fluid. The carrier fluid may be any gas or liquid that is
nontoxic, substantially odorless, and capable of
transporting the adhesive over a desired distance.
Additionally, in the case of a liquid carrier fluid, the
carrier should be a solvent for the adhesive utilized.
Preferably, the carrier fluid is a liquid, such as water,
that readily vaporizes at the temperature and pressure to
which the gas stream is subjected.
The specific desired concentration of the adhesive in
the liquid carrier fluid primarily depends on the identity
of the carrier fluid, the desired size and amount of
adhesive particles 40 to be introduced into the gas
stream 36, and the size of the droplet 38 to be injected in
the gas stream 36. In general, however, the concentration
of adhesive in the carrier fluid (e.g., water) preferably
20 ranges from about .005% to about 10% by volume, and more
preferably from about .05% to about 1~ by volume. Lower
concentrations may for example be employed in low-sulphur
content coal burning applications where, in addition to
adhesive particle dispersal, the carrier fluid is
advantageously employed to cool the gas stream, thereby
reducing the resistivity of the agglomerate and the
incidence of sparkover. The adhesive composition should be
thoroughly mixed prior to injection into gas stream 36.
CA 02223999 1997-12-08
W O 96/40436 PCTrUS95/09746
The specific desired concentration of the adhesive
particles 40 to be dispersed in gas stream 36 is
established primarily based upon the concentration and size
distribution of undesired particles 35 in gas stream 36,
the tacticity of the adhesive, and the desired
concentration of undesired particles 35 in gas stream 36
after treatment. In general, however, the concentration of
adhesive particles 40 relative to undesired particles 35 in
gas stream 36 preferably ranges from about .01% to about 1%
by weight.
After the droplets 38 are injected into gas stream 36,
droplets 38 are carried downstream by gas stream 36. As the
droplets 38 are carried downstream, droplets 38 decrease in
size due to vaporization of the liquid carrier fluid and
h~-- ? smaller droplets 38a. As the liquid carrier fluid
vaporizes, adhesive particles 40 formerly cont~; ne~l in
droplets 38, 38a, will be dispersed and entrained in gas
stream 36 along with undesired particles 35. As noted,
about 90~ or more by weight of the liquid carrier fluid in
a given droplet 38 has preferably evaporated before
adhesive particles 40 contact collection plates 28.
The desired size distribution of adhesive particles 40
produced after vaporization of the liquid carrier fluid is
a function of several factors including the size
distribution of undesired particles 35, the density of the
adhesive, and the viscosity of the adhesive. Preferably,
however, the size of the adhesive particles 40 ranges from
about 1 to about 10 micrometers.
CA 02223999 1997-12-08
W 096/40436 PCT~US95/09746
-20-
The vaporization time for the liquid carrier fluid in
a droplet 38 primarily depends upon the size of droplet 38,
the volatility of the liquid carrier fluid, and the
temperature, pressure and composition of the gas stream 36.
In general, however, the preferable vaporization time for
the liquid carrier fluid should be less than about two
seconds and in most cases less than about 1 second.
After vaporization of the liquid carrier fluid, the
adhesive particles 40 contact collection plates 28. The
temperature of both the collection plate surface and the
agglomerate of undesired particles 35 and adhesive
particles 40 collected on the surface is preferably above
the condensation temperature of water vapor in gas
stream 36. Further, the temperature of both the collection
plate surface and the agglomerate is preferably above the
condensation temperature of the vaporized liquid carrier
fluid.
Gas stream 36 ContA;ning undesired particles 35 and
dispersed adhesive particles 40 enters electrostatic
precipitator shell 16. Discharge electrodes 26 impart a
negative electrical charge to undesired particles 35 and
adhesive particles 40. The negatively charged particles
adhere to the positively charged collection plates 28. As
the input gas stream moves from upstream section 24 to
downstream section 24, an increasing percentage of
undesired particles 35 and adhesive particles 40 acc~ late
on collection plates 28.
CA 02223999 l997-l2-08
W O 96/40436 PCTAJS95/09746
-21-
Fig. 3 is a side view of a portion of a collection
plate 28 that contains an agglomerate of undesired
~ particles 35 and adhesive particles 40. As depicted, after
contacting collection plate 28 adhesive particles 40 flow
into the interparticle gaps between undesired particles 40,
thereby yielding the desired agglomerate. Surfactants may
be included in the adhesive composition and, upon
contacting the collection plates, will serve to reduce the
surface tension of adhesive particles 40 and increase the
ability of the adhesive to fill the gaps between undesired
particles 35. Useful surfactants in this regard include
alkyl aryl polyether and alkyl phenyl-
hydroxypolyoxyethylene.
Fig. 3 further depicts void spaces 42 which result
from the cohesion between undesired particles 35 and
adhesive particles 40. As illustrated, the resulting dust
cake 44 is a porous agglomerate of undesired particles 35
and adhesive particles 40. The porosity of dust cake 44 may
be desirably increased by the addition of a dispersant to
the adhesive composition employed. Useful dispersants in
this regard include phosphates, such as trisodium
phosphate, tetrasodium phosphate, and sodium
hexamitaphosphate.
While not wishing to be bound by any theory, it is
believed that the bonding ?c-hAn;~ between the adhesive
particles 40 and undesired particles 35 is ?c-hAn;cal
and/or ionic in nature. Regarding ?~hAn;cal bonding, it
is believed that longer polymer chains more efficiently
CA 02223999 1997-12-08
W O 96/40436 PCTAUS95/09746
-22-
attract and entrap fine undesired particles 35 when
compared to shorter polymer ~hA; n~ in the adhesive
molecules. For this reason, higher mol~clllA~ weight
adhesive polymers more effectively form clumps of fines in
the dust cake 44 than lower molecular weight adhesive
polymers. Regarding ionic bonding, it is believed that the
polarity of the polymer impacts the ability of the adhesive
molecules to bond to undesired particles 35.
Referring to Figs. 1-3, dust cake 44 can be removed
from collection plate 28 by many t~chn;ques, including
rapping of the collection plate 28 and sonic horns. The
preferred methodology for dust cake removal involves
vibration of the collection plate 28. When collection plate
28 is vibrated, dust cake 44 separates from collection
plate 28 in large sheets and falls into hoppers 18 for
disposal. It is believed that adhesive particles 40
increase the attractive force between undesired
particles 35. The increased interparticle forces of
attraction induce a high degree of cohesiveness in dust
cake 44. The high dust cake cohesiveness is thought to
prevent the release of finer undesired particles during
dust cake removal.
Compared to the dust cakes formed in conventional
electrostatic precipitators, the cohesiveness of dust cake
44 yields many advantages. First, as noted above the
cohesiveness of dust cake 44 causes dust cake 44 to form
large, consolidated sheets during dust cake removal and
therefore r~lls~s the fragmentation of dust cake 44 and
CA 02223999 l997-l2-08
W 096/40436 PCT~US95/09746 -23-
reentrA; ent of undesired particles 35 during dust cake
removal. The decreased incidence of undesired particle
reentrA;~ nt in the present invention reduces particulate
emissions relative to conventional electrostatic
precipitators. Second, the cohesive sheets also reduce
problems of conventional electrostatic precipitators from
handling and storing loosely consolidated, fine undesired
particles. Undesired particles 35 are typically only about
10 microns in size. Finally, the vaporization of the
majority of the liquid carrier fluid before dust cake
formation produces a solid mass that avoids problems
commonly associated with slurry handling and disposal.
A second ~- hofl; ment of the present invention is a
filtration system for removal of undesired particles, such
15 as fly ash from a gas stream. Referring to Figs. 2, 4 and
5, a filtration system 45 includes a housing assembly 46,
filtrating assembly 48, and adhesive injection assembly 10.
Housing assembly 46 includes an input duct 50, filtration
shell 52, output duct 54, and hoppers 56. Filtrating
20 assembly 48 includes a plurality of filters 58 suspended
from a header (not shown). A support apparatus (not shown),
such as a cage, may be used to prevent deflation of
filters 58. Again, adhesive injection assembly 10 includes
a reservoir (not shown), feed line 34, and nozzles 37.
In operation, gas stream 36 enters the filtration
shell 52 through the input duct 50. Before gas stream 36
contacts filters 58, adhesive composition droplets 38 are
injected into gas stream 3 6. Preferably, by the time gas
CA 02223999 l997-l2-08
W 096/40436 PCT~US95/09746
-24-
stream 3 6 contacts filters 58 a substantial portion of the
liquid carrier fluid, preferably about 90% or more by
weight, in droplets 38 has vaporized, and adhesive
particles 40 are dispersed. Filters 58 pass the gaseous
components of gas stream 36 but remove undesired particles
and adhesive particles 40. As will be appreciated,
filters 58 may be of ceramic, fabric, paper or metal
construction.
As shown in Fig. 5, undesired particles 35 and
adhesive particles 40 collect on the exterior of filter 58.
As in the above discussion, void spaces 42 are formed as
undesired particles 35 and adhesive particles 40 collect on
the exterior of filter 58. t- _~~ed to conventional
filtration systems, void spaces 42 substantially reduce the
incidence of undesired particles 35 blocking the filter
pores. As noted above, such blockages cause a large
pressure drop across the filter and necessitate frequent
removal of dust cake 44 from filter 58. The porous dust
cakes 44 of the present invention reduce the pressure drop
across filters 58 and therefore filters 58 require less
frequent cl~n;ngs. Accordingly, the present invention has
a lower incidence of undesired particle reentr~; -nt in
gas stream 36 than conventional filtration plants. ~ _-~ed
to conventional filtration plants, the formation by
undesired particles 35 and adhesive particles 40 of a
cohesive agglomerate reduces the incidence of very fine
undesired particles 35 "bleeding through" filter pores and
CA 02223999 l997-l2-08
W 096/40436 PCTAJS95/09746
-25-
becoming reentrained in gas stream 36 and this results in
increased collection efficiency.
As will be appreciated, a number of methodologies
exist to remove dust cake 44 from filters 58 including
chAk~?~--cl~n;ng filters, shake-deflate filters, sonic-
horns, pulse-jet-cleaned filters, and reverse-air-cleaned
filters. In each case, a ?c-hAnism for cleaning the filters
is vibration and the dust cake 44 falls into hoppers 56 for
collection. Filters 58 may also be removably mounted to
the header so that they may be removed, cleaned, and
reinstalled.
The cohesiveness of dust cake 44 facilitates the dust
cake's removal. As a result of the increased interparticle
cohesion induced by adhesive particles 40, dust cake 44
remains in consolidated chunks after removal. The formation
of consolidated chunks of dust cake 44 simplifies the
handling and storing of undesired particles when compared
to conventional filtration systems.
After passing through filter 58, gas stream 36 flows
from the filtration shell 52 to ouL~uL duct 54 for
additional treatment or disposal.
In a third - hoA; ~nt of the present invention, the
additive is the inorganic compound, phosphoric acid, which
increases the cohesiveness of undesired particles and
decreases undesired particle resistivity. Phosphoric acid
is relatively inexpensive compared to many other additives.
It not only improves particle cohesion on the collection
plates and filters, as noted above for organic adhesives,
CA 02223999 1997-12-08
O 96/40436 PCT~US95/09746
-26-
but also reduces the resistivity of the undesired particles
35, thereby further improving the particle removal
efficiency of the electrostatic precipitator. The reduced
resistivity r~tlce~ the incidence of sparkover in an
electrostatic precipitator resulting in increased electric
field strengths and current densities. Phosphoric acid can
also be used to improve undesired particle collection
efficiencies in undesired particle collection systems
having nozzle systems already disposed in the gas stream,
such as manufacturing facilities in the metallurgical
industry.
While not wishing to bound by any theory, it is
believed that the phosphoric acid reacts with chemical
compounds in the gas stream and/or undesired particles to
form a hydrophilic material. It is believed that the
hydrophilic material reacts with water in the gas stream to
form hydrates. The hydrated compounds not only reduce
undesired particle resistivity but also increase undesired
particle cohesiveness.
The effectiveness of droplets of phosphoric acid in
reducing undesired particle emissions is surprising and
unexpected. As noted below, phosphoric acid has virtually
no vapor pressure until around 1,400 to 2,000~F and
therefore will condense at the gas stream temperatures
c~ onl y encountered in utility and other applications.
Under these conditions, one would not expect that droplets
of phosphoric acid, especially in the small concentrations
used in the present invention, would measurably impact the
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746 -27-
emissions of undesired particles because the phosphoric
acid would not vaporize and uniformly coat the undesired
particles if introduced in droplet form. Accordingly, one
would not expect that the phosphoric acid would disperse
sufficiently in the undesired particles to measurably
impact particulate emissions. This result is therefore
surprising and unexpected based upon the teachings of the
prior art. Additionally, there would be a disincentive in
introducing the phosphoric acid into the duct in the form
of droplets due to the possibility of the droplets coating
the duct, causing corrosion and gumming up equipment.
The specific desired concentration of the phosphoric
acid in the liquid carrier fluid preferably ranges from
about 0.1 to about 5.0% by volume, more preferably from
15 about .10 to about 2.0% by volume, and most preferably from
about .10 to about 1. 5% by volume. The preferred carrier
fluid has a boiling point less than the temperature of the
gas stream to ensure rapid evaporation of the carrier fluid
from the phosphoric acid as described above in connection
20 with the adhesive composition. The preferred carrier fluid
is water. The phosphoric acid is preferably thoroughly
mixed with the carrier fluid to form a fluid composition
before injection into the gas stream 36. In one embo~; -nt,
an adhesive is included in the amounts noted above.
Because phosphoric acid is a caustic acid, it is
important to utilize a small droplet size to entrain the
droplets in the gas stream and thereby substantially
;n; ;ze the contact of the acid with exposed metal
CA 02223999 1997-12-08
W O 96/40436 PCTAUS95/09746
-28-
surfaces to prevent corrosion of the surfaces. In utility
applications, the injection of the acid upstream of the air
preheater can result in undesired particle deposits on the
air preheater surfaces which can gum up the preheater and
decrease the amount of heat transferred to the boiler. The
reduced heat transfer can decrease the efficiency of the
boiler. Preferably, before separation of the carrier fluid
from the adhesive the droplets 38 have a Sauter Mean
Diameter ranging from about 20 to about 100 microns and
more preferably from about 20 to about 50 microns. After
separation of the carrier fluid from the phosphoric acid,
the droplets have a Sauter Mean Diameter preferably ranging
from about 1 to about 10 microns. Droplets in this size
range will be entrained in the gas stream and not settle
out of the gas stream on the input duct and other metal
surfaces.
To produce a small droplet size, the nozzles 37 of the
adhesive injection assembly 10 are preferably dual fluid
nozzles. In nozzles of this type, a pressurized gas, such
as air or steam, is intermixed with the fluid composition
cont~;n;ng the phosphoric acid at the injection interface
to form atomized droplets 38 of an exL~I~ ?ly small size.
The small droplets are injected a sufficient distance
upstream of the electrostatic precipitator or baghouse to
permit effective conditioning of the gas stream.
Preferably, the residence time of the droplets in the gas
stream before contacting the electrostatic precipitator or
baghouse ranges from about 0.25 to about 2.00 sGcon~.
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746
The droplets should be substantially uniformly
distributed throughout the input duct for best results.
This result is effectuated by proper placement of the
nozzles 37 in the input duct. It is also important to
position the nozzles 37 such that the droplet dispersion
pattern does not overlap with adjacent metal surfaces, such
as the input duct.
The ability of the phosphoric acid to condition the
undesired particles is not particularly temperature
dependent. For best results, however, it is preferred that
the gas stream t~ _~rature range between about 212 to about
932~F.
For electrostatic precipitators, the fluid composition
is preferably not heated before injection into the gas
stream 36. In this manner, the droplets 38 absorb thermal
energy from the gas stream 36 and thereby act as a heat
sink. Under cold-side operating conditions (e.g., at
temperatures less than about 350~F), the subsequent cooling
of the gas stream 36 can further reduce the resistivity of
the agglomerate and the incidence of sparkover for
electrostatic precipitators.
The concentration of phosphoric acid in the input duct
depends upon the type of particle removal device. For an
electrostatic precipitator, the amount of phosphoric acid
used in the gas stream preferably ranges from about 1 to
about 10 lb/ton of undesired particles. For a gas
filtration device, such as a baghouse, the amount of
CA 02223999 1997-12-08
O 96/40436 PCT~US95/09746
-30-
phosphoric acid used in the gas stream preferably ranges
from about 0.5 to about 6.0 lb/ton of undesired particles.
The phosphoric acid is preferably contacted with the
gas stream as a liquid and not as a vapor. Vaporized
phosphoric acid is likely to condense on the input duct and
other metal surfaces, causing corrosion and gumming up
equipment. As will be appreciated, phosphoric acid has
virtually no vapor pressure until around 1400 to 2000~F and
therefore will condense at the gas stream t~ ~atures
~ only encountered in utility and other applications
(e.g., 180 to 800~F). Vaporized phosphoric acid is much
more sensitive to the surface ~he ; ~try of the undesired
particles than droplets of a fluid composition containing
phosphoric acid. In contrast, droplets of the fluid
composition are not as sensitive to surface chf ;stry. When
in the agglomerate, the droplets of the fluid composition
readily spread out through the agglomerate due to capillary
forces. Another approach to contacting phosphoric acid
with the undesired particles is to combust phosphorous and
inject vaporized phosphorous into the gas stream. The
vaporized phosphorous condenses onto the undesired
particles and can form phosphoric acid. Phosphorous,
however, is highly flammable when exposed to oxygen and is
therefore dangerous to handle and costly to transport.
Additionally, vaporized phosphorous can condense in the gas
stream and form extremely small particles, e.g., less than
1 micron in size, which can bypass an electrostatic
precipitator and cause an acid fume.
CA 02223999 1997-12-08
WO 96/40436 PCTrUS95/09746 -31-
In a fourth emboA; e~t of the present invention, the
gas stream has a t~- -~ature of preferably no less than
about 350, more preferably no less than about 400, and most
preferably no less than about 450~F at an electrostatic
precipitating device and is contacted with a resistivity
reduction agent and, optionally, a carrier fluid. As used
herein, a resistivity reduction agent is any additive that
causes a reduction in undesired particle resistivity in the
surface conduction operating region. This region lies to
the left of the critical temperature as illustrated in Fig.
6. The resistivity reduction agent and precursors and
mixtures thereof preferably include caustic acids and salts
thereof, and more preferably phosphoric acid and precursors
and mixtures thereof. Preferably, the resistivity reduction
agent is substantially free of ammonia and sodium. The
preferred concentration of the phosphoric acid in the gas
stream preferably ranges from about 0.1 to about 1.0% by
weight of the undesired particles and more preferably is
about 0.5 weight percent of the undesired particles in the
gas stream.
This embodiment is particularly applicable to hot-side
electrostatic precipitating devices in utility applications
which operate at gas stream temperatures typically in
excess of 1500~F and more typically in excess of 600~F.
- 25 Such devices can exhibit back corona t~nAencies in the
agglomerate located on the collection plates. While not
wishing to be bound by any theory, it is believed that such
back corona tendencies result from the increase in
CA 02223999 1997-12-08
WO 96/40436 PCTAJS95/09746
-32-
undesired particle resistivities caused by prolonged
exposure to the gas stream. It is often difficult to remove
all of the agglomerate and, as a consequence, a portion of
the agglomerate can r~ ~;n on the collection plates for
prolonged periods. The resistivity reduction agent is
believed to reduce the resistivities in the undesired
particles in the agglomerate, thereby decreasing or
eliminating the occurrence of back corona.
The use of a resistivity reduction agent under hot-
side electrostatic precipitating conditions is surprisingly
e~fective. It is believed that under hot-side conditions,
charged carriers pass through the undesired particles on
the collection plates. This m~ch~ni~ is re~erred to as
volume conduction and the volume conduction operating
region is illustrated in Fig. 6. In this region, the
undesired particle resistivity is inversely related to
undesired particle temperature. In contrast, under cold-
side conditions (e.g., at temperatures no more than about
500~F), the charged carriers are believed to travel along
the surface of the undesired particles and not through the
undesired particles. This mcch~n;~ is known as surface
conduction and the surface conduction operating region is
illustrated in Fig. 6. In this region, the undesired
particle resistivity is directly related to undesired
particle temperature. Surprisingly, it has been discovered
that some resistivity reduction agents, especially those
described herein, are effective under hot-side conditions
in reducing resistivity. This is surprising because other
CA 02223999 1997-12-08
W 096/40436 PCTnJS95/09746
-33-
surface resistivity modifying agents, such as sulfuric
acid, have been shown to be ineffective under hot-side
operating conditions. As will be appreciated, hot-side
electrostatic precipitating devices in utility applications
are located upstream of the air preheater while cold-side
electrostatic precipitating devices are located downstream
of the air preheater.
In a fifth embodiment of the present invention, the
gas stream is contacted with an adhesive composition
including phosphoric acid, an adhesive, and a carrier
fluid. The adhesive can be any of the adhesives, preferably
organic, noted above with respect to the first ~ horl; ~rlt.
The adhesive composition is injected into the gas stream as
a plurality of droplets, with the carrier fluid separating
from the adhesive and phosphoric acid. The droplets of
adhesive and phosphoric acid contact the undesired
particles and assist in formation of the agglomerate. This
embodiment has the advantage of combining the dual
abilities of phosphoric acid to act as an adhesive and a
resistivity reducer with the cohesive nature of the
adhesive.
Example 1
Tests of sodium carboxymethylcellulose ("Adhesive A"),
and xanthan gum ("Adhesive B") were conducted using a 100-
acfm dry electrostatic precipitator drawing flue gas froma 1.5 million BTU/hr combustor. Insitec analyzers were
installed in the input and output ducts of the
electrostatic precipitator to measure undesired particle
CA 02223999 1997-12-08
W 096/40436 PCTAUS95/09746
-34-
collection efficiency. The electrostatic precipitator was
"lined-out" to operate at 10-12 kV at a current density of
0.5-1.5 mA. The adhesives were dissolved in water at a
concentration of about .1% by weight. The solutions were
injected into the gas stream through spray nozzles. The
Sauter mean diameter of the droplet,s of the sprayed
composition was about 15 microns.
Tests were run using Adhesive A at concentrations of
adhesive in the gas stream of approximately 0.05% and 2.5%
(wt additive/wt undesired particles). A decrease in
undesired particle loading in the outlet of the
electrostatic precipitator was observed for the additive at
the 0.05% concentration. An increase in the undesired
particle loading leaving the electrostatic precipitator was
observed during the injection of the 2.5% concentration.
Adhesive B was injected at a concentration of 0.05% and was
also found to decrease undesired particle loading at the
electrostatic precipitator outlet.
The electrostatic precipitator was modified by the
installation of baffles at the input and output ducts to
distribute the flow of the gas stream more uniformly
through the active portions of the electrostatic
precipitator. The modification improved electrostatic
precipitator efficiency from approximately 50% to over 70%.
After the modification, Adhesive A was injected at a rate
to produce a concentration of 0.05% twt additive/wt
undesired particles). Efficiency improved from about 73%
to about 83%. In other words, Adhesive A decreased
CA 02223999 l997-l2-08
W O 96/40436 PCT~US95/09746 -35-
undesired particle emissions by about 39%. Rapping the
collection plates established that the dust cake would
dislodge in "flakes". Undesired particle buildup did not
prove any more severe than buildup without adhesive
present.
Example 2
A mixture of phosphoric acid and corn syrup, an
organic adhesive, was injected into an experimental device
to determine the effectiveness of the additives to remove
undesired particles from a simulated flue gas stream. In
the device, the simulated flue gas stream was passed though
an additives injection ÇhA h~l- and then through a
resistivity measurement ch~ h~-~, Both undesired particles
(e.g., fly ash) and other flue gas constituents were added
dynamically to create the representative gas composition
and undesired particle content. The moisture content of
the flue gas was maintained at 10% by volume with a
temperature-controlled humidifier upstream of the additives
chamber. In the chamber, the additives were sprayed into
the flow with a fine mist atomizer. The additive volume
injected through the injection nozzle was controlled with
a precision peristaltic pump. The simulated flue gas stream
contained undesired particles that were added to the flow
by a screw feeder with the injection rate being controlled
by a variable speed motor. Entrained undesired particles
were collected downstream of the additive injection ÇhA h~
in a resistivity apparatus, e.g., a modified point-plane
electrostatic precipitator. In the resistivity cell, an
CA 02223999 1997-12-08
W O 96/40436 PCT~US9~/09746 -36-
agglomerate was collected by either electrical
precipitation or by filtering across an electrically
isolated metal frit. The resistivity of the agglomerate was
measured by first dete r ; n;ng the thickness of the
agglomerate, then by applying a measured voltage and
current across the agglomerate. Voltage/current
measurements were taken on an asc~n~;ng voltage curve with
results being calculated at a uniform field strength of
4kV/cm.
Two parameters were varied to determine the impact of
the phosphoric acid on undesired particle resistivity.
Tests were run over an extended flue gas temperature range
of 200-450~F. The phosphoric acid demonstrated dramatic
reductions in resistivity of the undesired particles over
the entire temperature range tested. At all temperatures
tested, the resistivity could be decreased to the optimal
101~ ohm-centimeter range. It is not believed that the corn
syrup impacted undesired particle resistivity, only
cohesivity.
A plot of phosphoric acid concentration and
resistivity at a constant temperature of 300~F is
illustrated in Fig. 7. The impact of the phosphoric acid on
undesired particle resistivity ranges from about 2 to over
4 orders of magnitude reduction relative to the resistivity
of untreated undesired particles. Similar results were
experienced for other temperatures of the flue gas stream.
In ~1 ~y, significant reductions in fly ash resistivity
were measured for all temperatures in the test matrix. The
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746
reduction of resistivity was demonstrated over a very broad
range of temperatures bracketing virtually all existing
> cold-side electrostatic precipitators. The measured
resistivity of additive-treated undesired particles ranged
from the low - 108 to mid - 101~ ohm-centimeters across the
spectrum of temperatures included in the test matrix. Fig.
8 plots the rate of undesired particle collection in the
resistivity apparatus against relative phosphoric acid
concentration. The rate of agglomerate formation was found
to increase with increasing phosphoric acid concentration.
This result is believed to be due to not only improved
(e.g., reduced) resistivity but also to improved cohesivity
in the agglomerate caused by the phosphoric acid and corn
syrup.
Example 3
A 300 acfm slip stream of the off gas from a trial
burn of a Powder River Basin coal in an electrostatic
precipitation pilot plant was conditioned in a large spray
contact chamber. A mixture of phosphoric acid and corn
syrup was injected into the spray contact l~.h;~r~h~r upstream
of the electrostatic precipitator. During the test, the
injection nozzle was switched between ~-hf~ h~ top and
bottom to judge the effects of residence time on additive
performance.
The pilot plant also supplied a fabric filter test
device downstream of the spray chamber. The inlet to the
fabric filter test device branched off at the electrostatic
precipitator inlet. The electrostatic precipitator was
CA 02223999 1997-12-08
WO 96/40436 PCTrUS95tO9746
-38-
instrumented to continuously monitor voltage, current,
flowrate, temperatures and outlet particulate emissions.
In addition, at each set of test conditions, both a "clean
plate" and "dirty plate" voltage/current curve was taken
manually. Resistivity was measured continuously at the
electrostatic precipitator inlet with a mea~ ~~t
frequency of less than 30 minutes. Resistivity samples were
collected by filtering across an electrically isolated
metal frit, rather than by point-plane precipitation.
After the initial baseline experiment, the mixture was
injected into the bottom of the large spray contact chamber
with a fine atomizing spray nozzle oriented downstream.
This produced a 10~F spray cooling of the flue gas stream.
Residence time to the electrostatic precipitator inlet at
this location was about 2 seconds. Gas velocity in the
~-h~h~l- was less than 1 foot per second. Conditioning with
phosphoric acid reduced the ash resistivity to 9 x 109 ohm-
centimeters. The electrical current density improved to 2
nA/cm2 with a baseline unconditioned agglomerate layer still
on the plates. The plates were rapped clean and
conditioning with phosphoric acid continued at the same
rate. Once an agglomerate had accumulated, electrostatic
precipitator current density increased to 8 nA/cm2.
Spray injection was then switched to water at the same
25 flow rate. Resistivity returned to 4 x 1011 ohm-centimeters,
essentially a repeat of the baseline measurement.
Electrostatic precipitator electrical conditions also began
to degrade. This confirmed that the improved electrical
CA 02223999 1997-12-08
W O 96/40436 PCTAJS95/09746 -39-
conditions during additive injection were not a result of
water spray cooling. Next, the mixture was injected at a
concentration 10 times lower than for the previous test.
At this rate, there was no measurable improvement in
resistivity from baseline. An intermediate concentration
between the high and low was then injected. Resistivity
was measured at 1 x 1011 ohm-centimeters for this condition.
Electrical current density increased slightly over the
baseline with water spray.
Next, the nozzle was moved to the top of the spray
chamber and the nozzle configuration was changed to a
cluster head with 5 nozzles to determine the effects of
residence time and spray droplet size on conditioning.
Residence time at this location was increased by more than
12 seconds from the previous test. Conditioning with
phosphoric acid from the spray r-h~ he~ bottom decreased fly
ash resistivity to 1.6 x 109 ohm-centimeters. However,
during this test the combustor heat ~ch~nger was beginning
to plug, which reduced the particulate loading to the spray
chamber/electrostatic precipitator. It was concluded that
the additional residence time in the spray chamber was not
required to achieve good conditioning with phosphoric acid.
Fig. 9 shows fly ash resistivity as a function of
phosphoric acid concentration at 300~F. Phosphoric acid
concentration is expressed in relative terms linearly
related to mass of the additive to the total weight of
entrained undesired particles. In the experiments, outlet
mass emissions were measured continuously by an optical
CA 02223999 1997-12-08
W O 96/40436 PCTAJS95/09746 -40-
monitor which responds proportionately to back-scattered
light from entrained undesired particles in the gas stream.
The monitor response decreased from 9~ to less than 6% due
to conditioning.
Exam~le 4
After establishing the viability of the mixture of
phosphoric acid and corn syrup in reducing particulate
emissions in the laboratory and in the pilot plant, the
effectiveness of the mixture was evaluated under typical
duct conditions. Flue gas was extracted isokinetically
from an 18 inch diameter duct supplying a particulate
control pilot plant in operation at a power plant. The slip
stream was extracted from the plant duct downstream of the
air heater and upstream of a reverse gas baghouse. An
atomizing spray nozzle manifold was inserted into the slip
stream duct approximately 40 feet in a 90 degree bend
upstream of a sample probe. Liquid feed to the nozzle
manifold was controlled with a peristaltic pump. Atomizing
air pressure was set to a constant 80 psig. The sample
probe drew flue gas isokinetically to a portable
electrostatic precipitator at a flowrate of approximately
60 acfm. In all, sample residence time from injection to
the electrostatic precipitator inlet was less than 2
seconds. Velocity in the duct was 50-60 feet per second and
the gas temperature was 250~F. The sample was reheated to
a constant 300~F at the electrostatic precipitator inlet.
The portable electrostatic precipitator was configured
as a single channel wire/plate at a 9 inch plate spacing
CA 02223999 l997-l2-08
W O 96/40436 PCTAJS95/09746
-41-
with stAn~A~d 0.1 inch diameter bare wires. Special teflon
baffles were installed around and beneath the collection
plates to ; n; ; ~e sneakage. The flowrate was set to give
an SCA of 180 square feet/kacfm.
Electrostatic precipitator voltage and current were
monitored continuously and voltage/current curves were
taken to sparking for each test condition. Multiple
resistivity measurements were taken at the electrostatic
precipitator inlet for each test condition. Outlet
particulate concentrations were monitored by a TRIBOFLOW
monitor, which produces a response proportional to the
number of charged particles contacting a probe. Fig. 10
presents the resistivity measurements taken during the
test. Baseline resistivity at 300~F was mid - 10~1 ohm-
centimeters. The additives were injected at 3 different
phosphoric acid concentrations. Conditioning with
phosphoric acid at the high concentration on the first test
day reduced the resistivity to mid - 101~ ohm-centimeters.
Conditioning on the following day at the same concentration
reduced the resistivity to mid - 109 ohm-centimeters. It is
believed that this difference is the result of a change in
nozzle alignment. At the high phosphoric acid
concentration, the condition was also repeated by doubling
liquid flow and halving additive solution concentration.
This had no apparent effect on perfol ~n~e. At the high
phosphoric acid concentration, the condition was also
repeated by doubling liquid flow to the nozzle array and
halving solution concentration. These measures had no
CA 02223999 1997-12-08
W O 96/40436 PCTAJS95/09746 -42-
apparent effect on performance. Conditioning with
phosphoric acid at the high concentration reduced the
resistivity to mid - 109 ohm-centimeters. At the high rate,
two tests showed a mid - 10~~ resistivity, while all of the
tests on the following day at the same injection rate were
an order of magnitude lower. It is believed that this
difference was a result of the change in nozzle alignment.
TRIBOFLOW monitor response was set to 0--2096full--
scale. During injection at high concentration over a 2
lo hour period, the TRIBOFLOW monitor response dropped from
2. 75% to 1.75%. After injection stopped, the response
increased to 2. 5%, indicating an increase in charged
undesired particles exiting the electrostatic precipitator.
During rapping periods the TRIBOFLOW response increased off
scale (greater than 20~). The portable electrostatic
precipitator was manually adjusted to higher power levels
twice during additive injection. Attempts to increase power
to the same power levels at baseline were spark-limited.
The change in TRIBOFLOW response and the ability to
Z0 increase power demonstrated a qualitative improvement in
electrostatic precipitator performance from baseline to
condi~ioning with phosphoric acid, consistent with the
decrease in fly ash resistivity.
This test demonstrated that the additives could be
applied as an aqueous conditioning agent through a fine
atomizing nozzle array at typical duct conditions, rather
than in a separate additive chamber. It was further
concluded that: (i) aqueous additives solutions can be
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746
-43-
successfully injected into a turbulent flue gas stream at
40--60foot per second gas velocity; (ii) liquid droplet
size for the nozzle array was less than 40 microns Sauter
Mean Diameter; (iii) a residence time of 1-2 s~con~
between injection and control device was adequate; (iv)
flue gas spray cooling is 5-10~F for the conditioning rates
required with the additives; and (v) effective spray
distribution is critical to the success of conditioning.
Based on the above-noted tests, the mixture of
additives was found to provide increased undesired particle
cohesivity and agglomerate porosity and tensile strength
relative to untreated undesired particles/agglomerates.
The phosphoric acid additive in the mixture was found to
provide more desirable undesired particle resistivities for
electrostatic precipitator applications. The tests further
indicated that undesired particle conditioning to improve
resistivity can be effective over a wider temperature range
than conventional additives, such as sulfur trioxide.
Example 5
A test was conducted to determine if the mixture of
phosphoric acid and corn syrup could alleviate
electrostatic precipitator performance problems associated
with sodium ion depletion in agglomerates deposited on hot-
side electrostatic precipitator collection plates. It was
believed that such conditioning could extend surface
conduction in undesired particles to temperatures normally
dominated by volume conduction. In the test, the
experimental device used in Example 2 above was employed.
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746 -44-
The test was conducted at a flue gas temperature of 700~F
in a 10% moisture, air environment. The undesired particles
were from an ash sample from a hot-side electrostatic
precipitator at a plant firing a western sub-bituminous
coal. As can be seen from Fig. 11, the mixture reduced
undesired particle resistivity by up to 2 orders of
magnitude. It was thus deteL ;ne~ that the amount of
resistivity reduction can be controlled by the
concentration of phosphoric acid under hot-side conditions.
Further laboratory resistivity tests were conducted
using a mixture of 75~ sio2 and 25% Al203 powder. This
synthetic undesired particle mix was created to entirely
~1; ;n~te alkali ion charge carriers from the agglomerate
and thereby present a worst-case undesired particle
condition. The mixture of refractory powders was
redispersed on the resistivity measurement frit to form a
powder layer (e.g., agglomerate) and was heated to 700~F in
a 10% moisture gas stream. The powder layer was conditioned
by injecting a liquid additive as an aqueous spray in the
spray additive chamber upstream of the resistivity
measurement cell at 700~F. Additive was brought into
contact with the powder layer by drawing sample gas through
the powder layer in the porous sample frit. Baseline tests
were also conducted with no flue gas conditioning by
injecting a water spray. Resistivity of the undesired
particles was 4 x 109 ohm-centimeters. Conditioning with
phosphoric acid successfully lowered resistivity by an
order of magnitude to 4 x 108 ohm-centimeters. While the
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746
-45-
unconditioned powder layer at this temperature exhibited
only moderate resistivity, the test demonstrated that the
phosphoric acid can effectively reduce resistivity
ind~p~n~ent of sodium or lithium ion charge carriers at
hot-side conditions.
Example 6
A fabric filter test was r-o~lcted on a flue gas
slipstream taken upstream of an existing particulate
control device at a utility power plant firing a Texas
lignite coal. The test equipment consisted of a sample
probe, an additive contact chamber, an additive injection
assembly, and a filter test device. Flue gas was extracted
isokinetically from the host plant duct through the sample
probe. The host duct temperature at the inlet extraction
15 location ranged from 360 to 400~F. The filter chamber
t~ ~~ature and the additives contact ch~ h~?- were
maintained at 400~F for all tests. In the additives contact
chamber, an aqucous solution of diluted additive was
atomized and injected into the flue gas stream using a
small, dual-fluid nozzle. Additive liquid flowrate was
metered and controlled with a precision peristaltic pump.
For the bench-scale additive tests, the filter device was
configured for reverse-gas cleaning with an 8-inch diameter
fiberglass bag of a weight and weave to match the bags
currently in use in the shake/deflate baghouse of a weight
and weave to match the bags currently in use in the
shake/deflate baghouse of the host power plant. Relative
outlet particulate emissions were measured using a
CA 02223999 1997-12-08
WO 96/40436 PCTAJS95/09746
-46-
TRIBOFLOW~ monitor installed downstream of the filter
chamber. Mass concentration measurements in the filter
chamber inlet line during the baseline tests indicated a
loading of 11 gr/acf.
The phosphoric acid/water solution was injected at a
constant rate during the test series. The phosphoric acid
solution concentration was incrementally increased, noting
the performance at each concentration for at least 4 hours.
At very low concentrations a 60% decrease in outlet
particle loading was measured. As the concentration was
increased, the cleaning frequency decreased and further
reductions in outlet emissions occurred. Table 1 ~ -~izes
the test results.
TABLE 1. Additi~e Fabric Filter Tcst Results
R~ nn in
Solution Concentration Time to pressure F~ j~ci-
weight % initiated clean % change to
additive/ash% increase to baseline baseline
20Q025 No Change No Change
0.05 No Change 60%
Q075 50-75% 60 99%
0.10 100% 60-99%
0.15 200% 80-90%
2 50.20 400% 99%
As can be seen from Table 1, even small amounts of
phosphoric acid relative to the undesired particle
concentration in the gas stream significantly reduces the
particulate emissions.
CA 02223999 1997-12-08
W O 96/40436 PCT~US95/09746
-47-
While various embodiments of the present invention
have been described in detail, it is apparent that
modifications and adaptations of those ~- hoA; ~nts will
occur to those skilled in the art. However, it is to be
expressly understood that such modifications and
adaptations are within the scope of the present invention,
as set forth in the following claims.